Abstract
Objective: To evaluate if patterned electroceutical dressing (PED) is safe for human chronic wounds treatment as reported by wound care providers.
Approach: This work reports a pilot feasibility study with the primary objective to determine physically observable effects of PED application on host tissue response from a safety evaluation point of view. For this pilot study, patients receiving a lower extremity amputation with at least one open wound on the part to be amputated were enrolled. Patients were identified through the Ohio State University Wexner Medical Center (OSUWMC) based on inclusion and exclusion criteria through prescreening through the Comprehensive Wound Center's (CWC) Limb Preservation Program and wound physicians and/or providers at OSUWMC. Wounds were treated with the PED before amputation surgery.
Results: The intent of the study was to identify if PED was safe for clinical application based on visual observations of adverse or lack of adverse events on skin and wound tissue. The pilot testing performed on a small cohort (N = 8) of patients showed that with engineered voltage regulation of current flow to the open wound, the PED can be used with little to no visually observable adverse effects on chronic human skin wounds.
Innovation: The PED was developed as a second-generation tunable electroceutical wound care dressing, which could potentially be used to treat wounds with deeper infections compared with current state of the art that treats wounds with treatment zone limited to the surface near topical application.
Conclusion: Technology advances in design and fabrication of electroceutical dressings were leveraged to develop a tunable laboratory prototype that could be used as a disposable low-cost electroceutical wound care dressing on chronic wounds. Design revisions of PED-1 (1 kΩ ballast resistor) circumvented previously observed adverse effects on the skin in the vicinity of an open wound. PED-10 (including a 10 kΩ ballast resistor) was well tolerated in the small cohort of patients (N = 8) on whom it was tested, and the observations reported here warrant a larger study to determine the clinical impact on human wound healing and infection control.
Keywords: electroceutical, wound care dressing, clinical, safety, chronic wound
Chandan K. Sen, PhD.

Introduction
In the United States, 6.5 million patients are affected by chronic wounds adding an estimated US$25 billion annually to health care costs.1 A recent meta-analysis of 9 chronic wound studies involving 185 wounds reported >75% prevalence of biofilms in chronic wounds.2 In addition to impaired cellular wound healing processes, the presence of biofilm infection derails natural wound healing.3–14 Innovative strategies to combat wound biofilm infection, while promoting healing of hard-to-heal wounds, are urgently needed.
The human skin has an intrinsic transepithelial potential (TEP) generated and maintained by ion flow.15 After injury, when the skin continuity is disrupted, a wound electrical current (EC) is momentarily generated followed by establishment of a lateral electric field (EF).16–19 There is growing research to suggest that this transverse EF promotes wound healing by stimulating the (1) migration of neutrophils and macrophages,20,21 (2) activity of fibroblasts,22 and (3) blood flow.23–30 In the United States, electrical stimulation (ES) devices have been used for the treatment of chronic wounds since 2002.19,31,32 Such treatment has also been reimbursed by Medicare in wounds that have failed to heal using standard wound therapies.32 Multiple clinical studies have demonstrated that ES together with standard of care (SoC) is more effective than SoC alone.19
The concept of smart electrical bandages is slowly gaining momentum33–37; the idea of providing engineered, controllable EC and EF through disposable low-cost electroceutical wound care dressing is innovative and not currently available. The first-generation technology, a wireless electroceutical dressing (WED), is available commercially (Procellera®) with FDA clearance for burn care dressings.38–42 This textile-based dressing is wireless and does not need any external power supply, making it well suited for application as disposable antimicrobial wound care dressing. We reported that EF generated by the WED due to the silver-zinc (Ag/Zn) redox chemistry is effective as prohealing38 and antibiofilm.39,40
We have recently developed the next-generation disposable dressing (patterned electroceutical dressing or PED) that can deliver controlled EC to wounds. Notably, the PED operates with a constant input voltage source, currently provided by a 6 V battery, making it an active dressing as opposed to the WED, which is a passive dressing. Based on our unpublished in vitro and preclinical porcine biofilm infection data on burn wounds, we designed a clinical study to test PED in human chronic wounds. To test clinical prototype design viability, we developed an in vitro biofilm assay.43 Following multiple iterations of designs to evaluate size, spacing, impact of electrode geometry patterns, we observed that maximal biofilm inhibition was observed on the anode.43 Based on this observation, a novel interdigitated electrode pattern with a broader anode interwoven with a narrower cathode (Fig. 1) was developed for the clinical prototype. The purpose behind this choice of design was to provide minimal internal resistance to flow of electric current as it flows from anode to cathode while providing high contact area for the anode with respect to the open wound area. PED treatment was applied to infected porcine wounds for 14 days with intermittent dressing changes. Importantly, initial host response of wound repair (epithelialization and granulation tissue) to PED was noted to be not impaired, establishing that the treatment was safe for host tissue. These results prompted this study, which was designed as a feasibility study with the primary objective to determine if PED treatment for human chronic wounds is safe to use in a clinical setting based on visual reporting by wound care providers on the lack of adverse events (AEs) after treatment with PED.
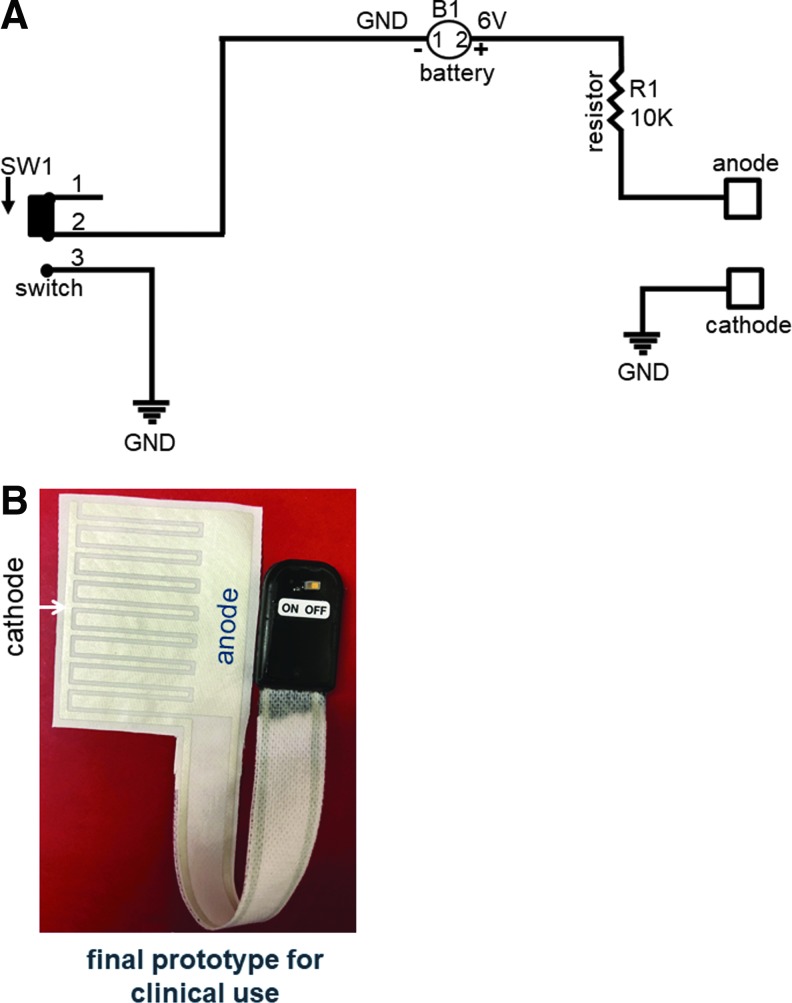
Figure 1.
PED clinical prototype. (A) Schematic for PED is shown. The electroceutical dressing consists of an interdigitated silver ink pattern on silk fabric; the dressing is powered by a 6 V battery (B) (Model No. 36-3025-2-ND) with a 10 kΩ resistor (R) that limits the current in the active circuit. GND, ground; SW, switch (Model No. 563-1151-ND). (B) Fully assembled PED-10 for clinical use. PED, patterned electroceutical dressing; PED-10, dressing with 10 kΩ ballast resistor.
Clinical Problem Addressed
Chronic wounds are an ongoing challenge for clinicians and a serious public health burden due to the high cost of care of the ulcers themselves and postulceration complications such as amputations. The identification of an alternate effective therapy to either complement SoC or work independently than the current SoC, promoting wound healing while being safe for application, is a key clinical need.
ES as wound therapy is not a new technology, and has been supported by numerous preclinical and clinical studies. However, the lack of clearly defined, standardized methods for using the available electrical devices has limited their widespread application in the wound care arena. Moreover, a priority with using such devices is to ensure that it is safe for use on the human skin.
Materials and Methods
PED Design and Quality Control
The clinical PED prototype reported in this article (Fig. 1) was engineered through an iterative design process with significant in vitro experiments.44 The clinic-ready PED prototypes were mass produced by professional engineering staff at the Center for Design and Manufacturing Excellence (CDME) at The Ohio State University (OSU) using highly specific medical-grade silk material, silver ink, and medical tape backing. The concept, design, laboratory-scale development, and initial testing of the laboratory-scale prototypes, including simplified setups to evaluate mechanisms of operation for the PED, have been reported previously44 (and unpublished observations: Stoodley P, The Ohio State University; Prakash S, The Ohio State University). The circuit and batteries attached to the dressing were encased in a fluid-resistant injection molded encasing. For insulating patients from electrical exposure, the circuit includes a ballast resistor connected in series with the battery to limit current below the approved FDA standards (in compliance with IEC 60601). The first version of clinical PED named as dressing with 1 kΩ ballast resistor (PED-1) included a 1 kΩ ballast resistor, the subsequent version was named as dressing with 10 kΩ ballast resistor (PED-10) and comprises a 10 kΩ ballast resistor to reduce the amount of current to wounds compared with the 1 kΩ dressing, and scaled by Ohm's law.44 These dressings were sterilized at a commercial sterilization facility. All sterile units of PED-10 were individually packed and identified using a unique serial number ID. As part of quality control (QC), each unit was individually tested electrically before sterilization by CDME staff to meet the specification of delivering ≤0.6 mA to the wound.
Biological QC of PED: in vitro agar biofilm assay
We utilized an in vitro agar biofilm assay45 to test the biological activity of clinical PED. To perform biological QC of every lot of the CDME manufactured PED, 5% of the dressings were randomly selected and subjected to an agar Pseudomonas aeruginosa (PA) biofilm assay (Fig. 2A). This bacterium is a known pathogen in wound infections,2,3,6–8,10,12,39,40,46 and has been previously used as a model organism to test antibiofilm effects of electroceuticals and other treatments.39,40,47 The strain of Pseudomonas used for this assay is a bioluminescent strain of P. aeruginosa (Xen41; base strain PA01; PerkinElmer).6,39,40 This strain contains an integrated plasmid carrying a luciferase gene that is constitutively expressed. P. aeruginosa strains were cultured in low-salt Luria broth (LB) for planktonic culture and the respective agar formats for biofilm studies (Thermo Fisher Scientific). Bioluminescent bacteria only produce light when they are metabolically active. Thus, we used light emission as detected with the in vivo imaging system (IVIS) spectrum system (PerkinElmer), as an indicator of bacterial activity.47
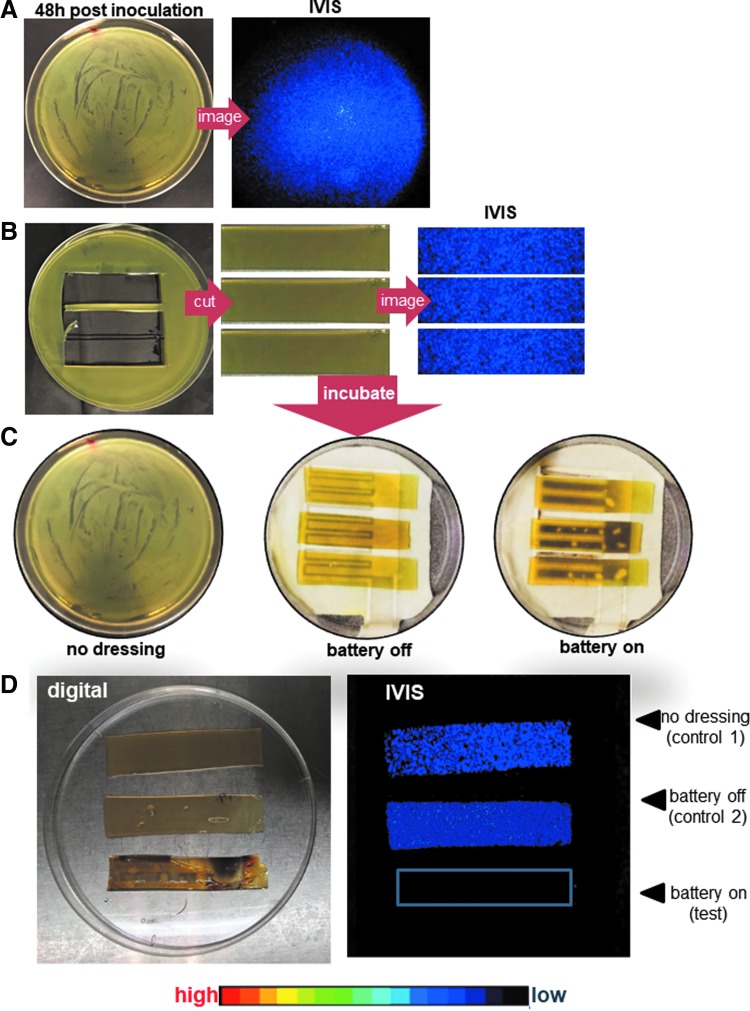
Figure 2.
Biological testing of clinical PED-10 using agar biofilm bioluminescent assay. (A) Forty-eight-hour mature Pseudomonas aeruginosa (PA Xen41) biofilms on agar strips were placed on culture plates with or without PED-10. The experiment setup of the agar biofilm bioluminescence assay for clinical PED biological testing. Top panels show the appearance of the agar biofilms 48 h postinoculation with Xen41. (B) Agar lawn biofilms were confirmed using IVIS imaging for metabolic activity, followed by excising strips of agar containing mature biofilm from plated. Bottom panels show the excised strips that were tested again for metabolic activity of biofilm by IVIS imaging before transfer onto control or test plates. (C) Plates (n = 3 agar slices/plate) included control 1 (no dressing), control 2 (PED-battery OFF), and test (PED-battery ON). (D) Representative digital and corresponding IVIS image of Xen41 exposed to controls or test treatment. Also included is a heat map showing intensity ranges. The intensity of blue signal shows that bacteria in both control groups show detectable metabolic activity, whereas the test group (battery ON) showed no activity indicating absence of viable bacteria. IVIS, in vivo imaging system.
Static biofilms were developed in vitro using an agar biofilm model and Xen41. Grown overnight in LB media at 37°C, bacteria were cultured on LB agar plates, and allowed to form a mature biofilm for 48 h (Fig. 2A). The bioluminescence imaging confirmed presence of a thick biofilm (Fig. 2B). Interestingly, PA biofilms could not be grown on the agar directly formed on top of the PED. Therefore, agar sections containing mature biofilms were transferred to a new culture plate, and placed either directly on the plate (control 1: no dressing, Fig. 2C) or on a PED placed in the agar plate (Fig. 2C). All dressings were connected to batteries that were turned either OFF (control 2, Fig. 2D) or ON (test, Fig. 2D). Agar biofilms with treatments were incubated at 37°C for 24 h in a moist environment to prevent dehydration of the agar sections. After incubation, agar sections with biofilms were subject to IVIS imaging.
Clinical study: human subjects and study design
This study was designed as a pilot feasibility study with the primary objective to determine effects of PED application on host tissue response from a safety evaluation point of view. The study was approved by the OSU Institutional Review Board (IRB 2015H0408). The Declarations of Helsinki protocols were followed, and the patients gave their written informed consent. For this pilot study, patients receiving a lower extremity amputation with at least one open wound on the part to be amputated were enrolled. Our treatment was not with the intent of limb salvage but to determine safety and experience of PED use in the clinic. Patients were identified and enrolled through the Ohio State University Wexner Medical Center (OSUWMC). The patients were selected based on inclusion and exclusion criteria through prescreening (partial HIPAA wavier) through the Comprehensive Wound Center's (CWC) Limb Preservation Program and wound physicians/providers at OSUWMC. The decision of whether to amputate was made independently of the study, and was solely made by the patients and their physician/provider. The provider decides based on the severity of the condition if or when the limb should be amputated and scheduled the surgery based on current clinical SoC. Patients enrolled in the study wore the PED on the wound(s) for a minimum of 24 h or a maximum of 3 weeks or until amputation, whichever came first. Patients were consented on their first visit to the CWC. The patients were instructed to change the PED every second day or more frequently if excessive drainage was observed as advised by the treating physician and/or medical provider. Patients were advised to report any adverse indications visually observed during the dressing changes. Observation of any adverse indications was corroborated by the attending physician, and detailed reports were filed per IRB requirements. PED risks: the use of the current limiter served as a fail-safe mechanism, and eliminated the possibility of excessive and uncontrolled current flow into the wound area. Thermal imaging confirmed that the heat generated by PED is minimal and negligible as determined by negligible temperature rise across the agar gel plates.44
Results
PED Clinical Prototype Fabrication and Design
The PED comprises a biocompatible and commercially available silk fabric, medical-grade Ag/AgCl ink, and a waterproof medical tape backing (Fig. 1). The power source includes a 1 kΩ (PED-1) or 10 kΩ (PED-10) resistor to limit current flow, an ON/OFF switch, and a battery (6 V) with positive and negative leads that attach to the dressing, all enclosed in an epoxy resin molded waterproof casing to minimize direct contact of the active electric circuit components with the wound fluid. The power source for the PED, that is, 6 V batteries, is comparable with smaller devices using 9 V batteries that are approved by the FDA for human use, such as the TENS 7000 pain management unit. In addition, manual control elements have been included in the PED design to limit current flow, which allows nominal estimates based on the geometric area of the electrodes, and to permit deposition of power density to tissue within FDA limits. The estimates for power density were conducted by the CDME staff by corresponding current flow measurements in saline. The manual control features include (1) an ON/OFF switch for the patient to switch off the device in case of perceived or felt discomfort—none of the N = 8 patients reported any discomfort with the application of powered ON PED; (2) a ballast resistor, connected in series between the positive lead of the battery and the positive lead of the dressing to limit current flow through the dressing. The maximum power density measured with this electroceutical wound care dressing and the 6 V battery was 60.3 μW/cm2 at the anode and 753.6 μW/cm2 at the cathode, well below the FDA power density limit (0.25 W/cm2)44 with measurements on an agar gel plate without a bacterial lawn. The calculations took the nominal, geometric conductive surface area, maximum current measured under steady current flow conditions, and voltage from the power source into consideration during in vitro measurements. The maximum power density was based on the maximum current measured from a dressing coated with hydrogel, as used with pig models and consented patients (Fig. 1).
The last PED applied to the patient during the feasibility study protocol and subsequently taken off in the operating room (OR) was checked for voltage and current flow levels using a multimeter (Amprobe 34XR-A True-rms Digital Multimeter). Results from patients postapplication for five of the eight dressings tested are presented in Table 1. Before use on the subject's wound, unused PED-10 exhibited a consistently stable voltage of 6.4 V and maximum current of 600 μA. The measurements performed on the used dressings indicated that no stable voltage or current could be detected postapplication. The study did not monitor the compliance of the subject for changing dressing every 48 h. None of the patients reported any physical discomfort, pain, or irritation post-PED-10 application.
Table 1.
Testing of electrical activity/parameters of PED-10 before and after clinical application
| Subject ID | Anode to Cathode Resistance (MΩ) | Voltage Across PED (V) | Current Through PED (μA) | |||
|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | |
| PED 004 | open (>40) | Open (>40) | 6.44 | 0 | 600 | 0.0 |
| PED 005 | open (>40) | Open (>40) | 6.48 | 2.85 | 607 | 0.0 |
| PED 006 | open (>40) | Open (>40) | 6.43 | 0 | 607 | 0.0 |
| PED 007 | open (>40) | Open (>40) | 6.44 | 0 | 605 | 0.2 |
| PED 008 | open (>40) | Open (>40) | 6.45 | 0 | 607 | 0.2 |
Electrical parameters such as resistance between electrodes, voltage, and current across the dressing were measured using a multimeter. “PRE” values represent measurements before application on subject wounds. “POST” values represent readings that were taken after dressing removal from subject wounds.
PED, patterned electroceutical dressing; PED-10, dressing with 10 kΩ ballast resistor; POST, postapplication; PRE, preapplication.
PED Inhibits Biofilm Formation In Vitro
An agar biofilm assay45 was utilized to test the biological efficacy of PED against biofilm growth in vitro (Fig. 2A). Preformed (48 h) biofilm treated with PED-10 with battery turned ON resulted in partial loss of bioluminescence signal as imaged using IVIS. This loss of signal is indicative of inhibition in the metabolic activity of PA biofilm, and was confirmed by monitoring colony-forming units. Such inhibition was not evident in the absence of an active EC (control 2 or no PED treated control 1, Fig. 2B). As a standard QC criterion, from every lot of CDME-fabricated PED-10, the biological test is performed on 5% from a lot of 100 of the dressings (based on random selection) to confirm the biological efficacy of the fabricated dressings.
Pilot Clinical Testing with PED-10 Indicated Safe Application for Chronic Wound Use
Demographics and wound etiologies of eight enrolled subjects in the study are presented in Table 2. Subjects were all diagnosed with chronic nonhealing wounds that required amputation. The primary objective of this study was not to save the lower extremity part or determine PED efficacy but to determine if PED application could be potentially safe for chronic wound use as reported by the patients based on their personal level of comfort and/or visual changes to the wound environment. Of the eight subjects enrolled, only PED001 was treated with PED-1 (with the 1 kΩ ballast resistor). During the first dressing change 48 h postinitial treatment, the patient's care provider noted skin irritations and some skin discoloration that was photodocumented, and reported to the study team including the physician immediately (Fig. 3). The event was deemed as an AE that was anticipated and reported as a nonserious AE to IRB. At that point, the study team met and decided to limit the amount of current delivered to the wound by increasing the electrical resistance in the circuit by changing the ballast to 10 kΩ (PED-10). All subsequent subjects were treated with PED-10, and no further similar AE were noted or reported. In one other case (PED006), a minor discoloration of the area of application at the amputation visit was noted and was deemed by the study physician as a standard response to silver dressings, and therefore no changes to the PED-10 or testing protocols were made. The photographs of the wounds before and immediately after the application of the first PED at clinic are also presented (Fig. 4). Any other images of the wound could not be obtained because of challenges in obtaining images within the OR. For PED001, the PED-1 dressing was used, and for all other subjects the PED-10 dressing was used.
Table 2.
Subject demographics and wound etiology
| Subject ID | Age | Gender | Race | Smoker | Diabetes | Wound Location | Days of PED Use | Amputation Cause |
|---|---|---|---|---|---|---|---|---|
| PED001 | 43 | Male | Caucasian | N | Yes | Right foot | 2 | Charcot foot |
| PED002 | 62 | Female | Caucasian | N | Yes | Right foot | 4 | Uncontrolled infection |
| PED003 | 59 | Female | African American | N | Yes | Left foot | 10 | Osteomyelitis |
| PED004 | 52 | Male | Caucasian | N | Yes | Left foot | 8 | Osteomyelitis |
| PED005 | 63 | Male | Caucasian | N | Yes | Toe and left plantar | 10 | Osteomyelitis |
| PED006 | 62 | Male | Caucasian | N | Yes | Right great toe | 18 | Great toe ulceration with concern for osteomyelitis |
| PED007 | 51 | Male | Caucasian | Y | Yes | Left heel | 21 | Bone infection |
| PED008 | 59 | Male | Caucasian | Y | Yes | Right plantar | 1 | Chronic osteo/Charcot foot |
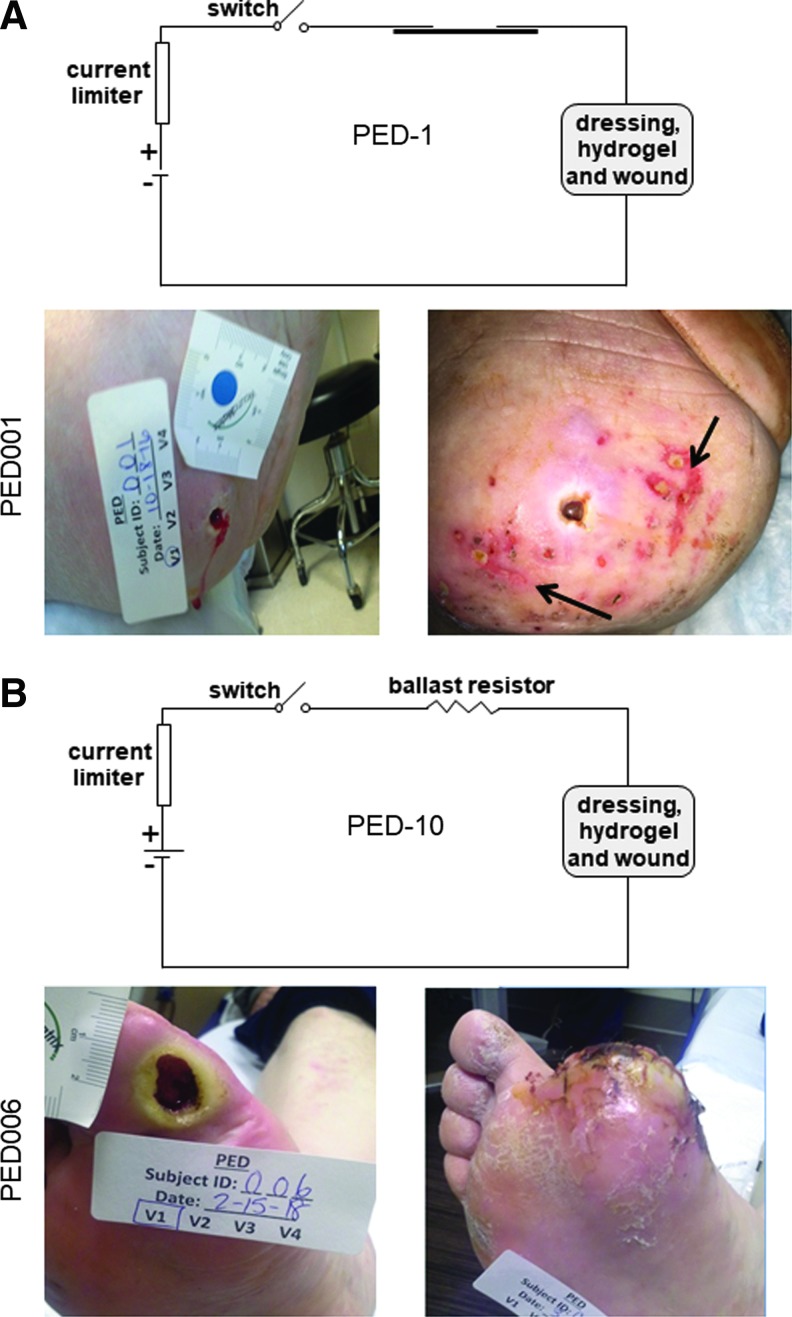
Figure 3.
Mild AEs associated with clinical PED-1 treatment application. (A) The first subject with PED-1 application reported AE associated with the PED treatment. This report described that during the first dressing change, the nurse noted a skin discoloration and minor irritation in skin surrounding the ulcer that was documented in the attached photograph (right). The treatment of PED-1 was immediately discontinued, and the event reported to IRB as unanticipated nonserious AE. At this stage, the study was put on a hold, and clinical PED was redesigned to replace 1 kΩ with 10 kΩ ballast resistor to reduce current to wounds and surrounding tissue. This PED was named as PED-10. Subsequently all subjects received PED-10. (B) Representative image of a wound treated with PED-10. AEs, adverse events; IRB, Institutional Review Board; PED-1, dressing with 1 kΩ ballast resistor.
Figure 4.
Clinical studies with PED. Digital images of wounds of all consented subjects before and immediately after the PED dressing application in clinic were obtained. The subjects did not come back to CWC until their amputation visit. (A) Wounds treated with PED-1 (1 kΩ ballast resistor). (B) Wounds treated with PED-10 (10 kΩ ballast resistor). CWC, Comprehensive Wound Center.
Discussion
Bacterial biofilms are complex aggregations of bacteria and extrapolymeric substances.3,46,48 The composition and organization of biofilms and their inherent resistance to antibiotics and host immune cells make these structures particularly challenging to treat.4,5,49–51 The prevalence of biofilm infection in chronic wounds is exceedingly high (>75%).2 Several studies, including those from our own laboratories, have indicated that biofilm infections impair wound healing.2,6–8,10,11,13,14 Therefore, novel strategies of dismantling biofilms are a primary and immediate need in wound care.
ES therapy of chronic wounds has emerged as a viable therapeutic option nationally and internationally.52 In the United States, since 2002, ES has been reimbursed by Medicare in the context of nonhealing leg ulcers and pressure ulcers.32 The rationale for ES use arises from the existence of an intrinsic TEP that promotes the migration of neutrophils and macrophages, stimulates fibroblasts, and improves blood flow.23,25–30 The strength of EFs present during normal wound healing varies throughout the healing process—starting high and dissipating at the end of the healing process.25 The majority of studies found that ES stimulated the efficacy of antibiotics against the biofilms.51,53–56 Among the earliest methods that showed great promise against biofilms included those that utilized either direct or alternating current with or without antibiotics.51,53–61 The term “bioelectric effect (BE)” was specifically used in the context of EC enhancing antibiotic efficacy.51,54,56 Details of the fundamental mechanisms of the BE or treatments using EC alone are still unclear, and could be attributed to diversity in applied electrical parameters and methods for treatment purposes5,33,51,53–57,59–67 and no standardized approach to conduct clinical evaluations. Low-intensity direct current (LIDC) and high-voltage pulse current (HVPC) have both been used to demonstrate antimicrobial effect. Our laboratory initially showed P. aeruginosa biofilm disruption in vitro using WED, which generates weak EFs. Recently, we published preclinical porcine studies showing the efficacy of WED in treating biofilm-infected wounds.38–40 In this study, PED embedded in agar inhibited initial establishment of biofilm (data not shown) likely because of the release of the silver from the dressing that inhibited/killed planktonic bacteria,43 therefore to study the effects of PED on biofilm disruption, mature biofilms were first allowed to develop followed by placing the agar pieces containing mature biofilm on PED. Although PED inhibited metabolic activity of the P. aeruginosa biofilm identified using IVIS imaging, this study does not address if the effect was due to bactericidal or static effects.
The effects of electrical therapy such as LIDC are thought to be antibacterial due to (1) changes in pH conditions near the electrode capable of killing pathogens68,69; (2) electrolytic generation of toxic compounds (H2O2 and chlorinated species such as HOCl)70 (and unpublished observations: Stoodley P, The Ohio State University); (3) disruption of bacterial cell division71; (4) overstimulation of cell membranes due to ion excitation leading to leakage72 and in some cases; and (5) interfering with adhesion and cohesion of biofilm structures resulting in a planktonic phenotype.63 Similar observations have been made with HVPC treatments.69,73–75
PED has been developed with the intent of providing a disposable device that can provide controlled EC to wounds to achieve elimination of biofilm infection. The differences in battery usage by wounds in different patients noted in this study are likely due to differences in electrical resistances in these wounds. Future studies mapping current and field in chronic wounds may help inform the frequency of PED-10 dressing change to obtain maximal efficacy against wound biofilm infection. In this feasibility study, the EC was adjusted based on AE reported by the first subject treated with PED-1. None of the other seven subjects on whom PED-10 was used reported any significant AEs, suggesting that PED-10 was well tolerated and was deemed safe for use in a larger clinical study based on visual observations of the local wound area where the efficacy of this dressing against chronic wound biofilm infection can be explored.
Innovation
We developed a novel electroceutical treatment for clinical wound biofilm infection that was tested for topical application as determined by patient physical sensation and visual changes in the region on and around chronic human wounds. While the concept of electrically active bandages is gaining momentum, the idea of providing controlled electric current through disposable electroceutical wound care dressings is innovative and not currently available. The PED reported here presents a platform that is tunable for current control, and can likely be readily adapted to unique wound and patient needs. The ability to dial up or down the current output of PED for a given, constant electric potential with minimal physically observable AEs under topical use on open chronic wounds is an innovation of the device. The pilot study as reported here demonstrated the ability to apply the device on human wounds.
Key Findings.
PED is an electroceutical wound care dressing that can be tuned to minimize AEs on chronic wounds.
PED-10 is a disposable device that provides low-level electric current to wounds.
Pilot feasibility studies enabled the determination of appropriate dose of electric current to mitigate observed adverse side effects.
PED-10 was well tolerated in the small cohort of patients (7) tested, and these observations warrant a larger study to determine impact on wound healing and infection control.
Acknowledgments and Funding Sources
This article is supported by NIH grants NR015676 and GM108014 to C.K.S., U01DK119099 to C.K.S., S.R., and G.M.G., R01DK114718 to S.R., R01GM095657 to G.M.G., UL1TR001070 from the National Center for Advancing Translational Sciences L-pilot award to S.R. and V.V.S., and the Public Health Preparedness for Infectious Disease (PHPID) transdisciplinary team grant (OSU-Infectious Disease Institute).
ABBREVIATIONS AND ACRONYMS
- AE
adverse event
- CDME
Center for Design and Manufacturing Excellence
- CWC
Comprehensive Wound Center
- EC
electrical current
- EF
electric field
- ES
electrical stimulation
- HVPC
high-voltage pulse current
- IRB
Institutional Review Board
- IUH
Indiana University Health
- IVIS
in vivo imaging system
- LB
Luria-Bertani
- LIDC
low-intensity direct current
- MAE
Mechanical and Aerospace Engineering
- OR
operating room
- OSUWMC
The Ohio State University Wexner Medical Center
- PED
patterned electroceutical dressing
- PED-1
dressing with 1 kΩ ballast resistor
- PED-10
dressing with 10 kΩ ballast resistor
- QC
quality control
- SoC
standard of care
- TEP
transepithelial potential
- WED
wireless electroceutical dressing
Author Disclosure and Ghostwriting
The authors declare no competing financial interests or other conflicts of interest. No ghostwriters were involved in the preparation of this article.
About the Authors
Sashwati Roy, PhD, is professor of surgery and director of clinical research at Indiana University Health (IUH) CWC. Her research interests include wound inflammation, mechanisms of resolution of diabetic wound infection, tissue repair, and cellar plasticity. Shaurya Prakash, PhD, is an associate professor in the Department of Mechanical and Aerospace Engineering (MAE) at the OSU with expertise in microfabrication and nanofabrication, and thermal-fluid transport phenomena that enable applications in medical devices and instrumentation, water purification, and alternate and renewable energy. Shomita S. Mathew-Steiner, PhD, is a research scientist at Indiana University with expertise in microbial biofilms and wound healing. Piya Das Ghatak is a PhD student at the Indiana University School of Medicine. Her research interests are in the fields of immunology and microbiology. Varun Lochab is a PhD student in the Department of MAE at the OSU. His research interests include microfabrication and nanofabrication, particle–fluid interactions, and other fluid mechanical applications in biology and alternative energy. Travis H. Jones is a PhD student in the Applied Physics Laboratory in the Department of MAE at the OSU. His research is focused on the interactions of electromagnetic fields with biological systems, including cancer cell behavior, tissue imaging, and biofilm inhibition. Prashanth Mohana Sundaram is a PhD student in mechanical engineering at the OSU. His research interests include micro–nano fabrication, micro–nano fluidics, and angiogenesis. Gayle M. Gordillo, MD, is chair of the Department of Plastic Surgery and Medical Director of the CWC at Indiana University. Vish V. Subramaniam, PhD, is professor and chair of the Department of MAE, and professor in the Chemical Physics Program at the OSU. His expertise is in the interaction between electromagnetic fields and currents with biological systems, plasma physics, and synthesis of novel materials from nonequilibrium environments. He leads the Applied Physics Laboratory in MAE. Chandan K. Sen, PhD, is the J. Stanley Battersby Chair and Professor of Surgery at Indiana University School of Medicine, Director, Indiana Center for Regenerative Medicine and Engineering (ICRME), Executive Director IUH CWC and Indiana CTSI. His expertise covers the biology of wound healing and biofilm infection.
References
- 1. Sen CK, Gordillo GM, Roy S, et al. . Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malone M, Bjarnsholt T, McBain AJ, et al. . The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care 2017;26:20–25 [DOI] [PubMed] [Google Scholar]
- 3. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 4. Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev Anti Infect Ther 2003;1:667–683 [DOI] [PubMed] [Google Scholar]
- 5. del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther 2007;82:204–209 [DOI] [PubMed] [Google Scholar]
- 6. Roy S, Elgharably H, Sinha M, et al. . Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol 2014;233:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper RA, Bjarnsholt T, Alhede M. Biofilms in wounds: a review of present knowledge. J Wound Care 2014;23:570., 572–574, 576–580 passim. [DOI] [PubMed] [Google Scholar]
- 8. Thomson CH. Biofilms: do they affect wound healing? Int Wound J 2011;8:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinton A, Carter T. Chronic wound biofilms: pathogenesis and potential therapies. Lab Med 2015;46:277–284 [DOI] [PubMed] [Google Scholar]
- 10. Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care (New Rochelle) 2015;4:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krom BP, Oskam J. Microbial biofilms and wound healing: an ecological hypothesis. Phlebology 2014;29(1 Suppl):168–173 [DOI] [PubMed] [Google Scholar]
- 12. Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 2012;20:647–657 [DOI] [PubMed] [Google Scholar]
- 13. Scali C, Kunimoto B. An update on chronic wounds and the role of biofilms. J Cutan Med Surg 2013;17:371–376 [DOI] [PubMed] [Google Scholar]
- 14. Gompelman M, van Asten SA, Peters EJ. Update on the role of infection and biofilms in wound healing: pathophysiology and treatment. Plast Reconstr Surg 2016;138(3 Suppl):61s–70s [DOI] [PubMed] [Google Scholar]
- 15. Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol 1984;2:34–44 [DOI] [PubMed] [Google Scholar]
- 16. Illingworth CM, Barker AT. Measurement of electrical currents emerging during the regeneration of amputated finger tips in children. Clin Phys Physiol Meas 1980;1:87 [Google Scholar]
- 17. Barker AT, Jaffe LF, Vanable JW., Jr The glabrous epidermis of cavies contains a powerful battery. Am J Physiol 1982;242:R358–R366 [DOI] [PubMed] [Google Scholar]
- 18. Barnes TC, Karasic J, Amoroso MD. Further studies of the rate of healing of human skin measured by the electrical wound potential of experimental abrasions. Am J Surg 1951;82:720–726 [DOI] [PubMed] [Google Scholar]
- 19. Kloth LC. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 2005;4:23–44 [DOI] [PubMed] [Google Scholar]
- 20. Brown SB, Dransfield I. Electric fields and inflammation: may the force be with you. ScientificWorldJournal 2008;8:1280–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoare JI, Rajnicek AM, McCaig CD, Barker RN, Wilson HM. Electric fields are novel determinants of human macrophage functions. J Leukoc Biol 2016;991141–1151 [DOI] [PubMed] [Google Scholar]
- 22. Rouabhia M, Park H, Meng S, Derbali H, Zhang Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PloS One 2013;8:e71660–e71660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eberhardt A, Szczypiorski P, Korytowski G. Effect of transcutaneous electrostimulation on the cell composition of skin exudate. Acta Physiol Pol 1986;37:41–46 [PubMed] [Google Scholar]
- 24. Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen 1999;7:495–503 [DOI] [PubMed] [Google Scholar]
- 25. Cruz NI, Bayron FE, Suarez AJ. Accelerated healing of full-thickness burns by the use of high-voltage pulsed galvanic stimulation in the pig. Ann Plast Surg 1989;23:49–55 [PubMed] [Google Scholar]
- 26. Orida N, Feldman JD. Directional protrusive pseudopodial activity and motility in macrophages induced by extracellular electric fields. Cell Motil 1982;2:243–255 [DOI] [PubMed] [Google Scholar]
- 27. Alvarez OM, Mertz PM, Smerbeck RV, Eaglstein WH. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol 1983;81:144–148 [DOI] [PubMed] [Google Scholar]
- 28. Bourguignon GJ, Bourguignon LY. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1987;1:398–402 [DOI] [PubMed] [Google Scholar]
- 29. Hecker B, Carron H, Schwartz DP. Pulsed galvanic stimulation: effects of current frequency and polarity on blood flow in healthy subjects. Arch Phys Med Rehabil 1985;66369–371 [PubMed] [Google Scholar]
- 30. Kaada B. Vasodilation induced by transcutaneous nerve stimulation in peripheral ischemia (Raynaud's phenomenon and diabetic polyneuropathy). Eur Heart J 1982;3:303–314 [DOI] [PubMed] [Google Scholar]
- 31. Isseroff RR, Dahle SE. Electrical stimulation therapy and wound healing: where are we now? Adv Wound Care (New Rochelle) 2012;1:238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. CMS. Centers for Medicare and Medicaid Services. Electrostimulation for wounds: decision memorandum (No. CAG-0068N). 2002. www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=27&NcaName=Electrostimulation+for+Wounds&TAId=13&ver=13&DocID=CAG-00068N&from2=viewtechassess.asp&id=27&bc=gAAAAAgAAgEAAA%3D%3D& (last accessed May23, 2018)
- 33. Ashrafi M, Alonso-Rasgado T, Baguneid M, Bayat A. The efficacy of electrical stimulation in lower extremity cutaneous wound healing: a systematic review. Exp Dermatol 2017;26:171–178 [DOI] [PubMed] [Google Scholar]
- 34. Ashrafi M, Alonso-Rasgado T, Baguneid M, Bayat A. The efficacy of electrical stimulation in experimentally induced cutaneous wounds in animals. Vet Dermatol 2016;27:e235–e257 [DOI] [PubMed] [Google Scholar]
- 35. Ashrafi M, Novak-Frazer L, Morris J, Baguneid M, Rautemaa-Richardson R, Bayat A. Electrical stimulation disrupts biofilms in a human wound model and reveals the potential for monitoring treatment response with volatile biomarkers. Wound Repair Regen 2019;27:5–18 [DOI] [PubMed] [Google Scholar]
- 36. Sebastian A, Syed F, Perry D, et al. . Acceleration of cutaneous healing by electrical stimulation: degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen 2011;19:693–708 [DOI] [PubMed] [Google Scholar]
- 37. Swisher SL, Lin MC, Liao A, et al. . Impedance sensing device enables early detection of pressure ulcers in vivo. Nat Commun 2015;6:6575. [DOI] [PubMed] [Google Scholar]
- 38. Banerjee J, Das Ghatak P, Roy S, et al. . Improvement of human keratinocyte migration by a redox active bioelectric dressing. PLoS One 2014;9:e89239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Banerjee J, Ghatak PD, Roy S, et al. . Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PloS One 2015;10:e0119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barki KG, Das A, Dixith S, et al. . Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann Surg 2019;269:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghatak PD, Schlanger R, Ganesh K, et al. . A wireless electroceutical dressing lowers cost of negative pressure wound therapy. Adv Wound Care (New Rochelle) 2015;4:302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. FDA. 510(k) Premarket notification—Procellera antimicrobial wound dressing. 2016. www.accessdata.fda.gov/cdrh_docs/pdf16/K160783.pdf (last accessed October15, 2018)
- 43. Dusane DH, Lochab V, Jones T, et al. . Electroceutical treatment of Pseudomonas aeruginosa biofilms. Sci Rep 2019;9:2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bennett M. Design, Fabrication, and Characterization of Electroceutical Bandages for Treatment of Chronically Infected Wounds [Masters]. Columbus, OH: Mechanical Engineering, The Ohio State University, 2016 [Google Scholar]
- 45. Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother 2015;59:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ghannoum M, O'Toole GA. Microbial Biofilms. Washington, DC: ASM Press, 2004 [Google Scholar]
- 47. Das Ghatak P, Mathew-Steiner SS, Pandey P, Roy S, Sen CK. A surfactant polymer dressing potentiates antimicrobial efficacy in biofilm disruption. Sci Rep 2018;8:873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004;350:1422–1429 [DOI] [PubMed] [Google Scholar]
- 49. Characklis WG, James D, Bryers IB. Bioengineering report: fouling biofilm development: a process analysis. Biotechnol Bioeng 1981;23:1923–1960 [DOI] [PubMed] [Google Scholar]
- 50. Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol 2002;56:187–209 [DOI] [PubMed] [Google Scholar]
- 51. Costerton JW, Ellis B, Lam K, Johnson F, Khoury AE. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother 1994;38:2803–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herberger K, Debus E, Larena-Avellaneda A, Blome C, Augustin M. Effectiveness, tolerability, and safety of electrical stimulation of wounds with an electrical stimulation device: results of a retrospective register study. Wounds 2012;24:76–84 [PubMed] [Google Scholar]
- 53. del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2009;53:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms. A systematic review. Int J Artif Organs 2008;31:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim YW, Subramanian S, Gerasopoulos K, et al. . Effect of electrical energy on the efficacy of biofilm treatment using the bioelectric effect. NPJ Biofilms Microbiomes 2015;1:15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wellman N, Fortun SM, McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother 1996;40:2012–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Caubet R, Pedarros-Caubet F, Chu M, et al. . A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob Agents Chemother 2004;48:4662–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim H, Makin I, Skiba J, et al. . Antibacterial efficacy testing of a bioelectric wound dressing against clinical wound pathogens. Open Microbiol J 2014;8:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shirtliff ME, Bargmeyer A, Camper AK. Assessment of the ability of the bioelectric effect to eliminate mixed-species biofilms. Appl Environ Microbiol 2005;71:6379–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stewart PS, Wattanakaroon W, Goodrum L, Fortun SM, McLeod BR. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother 1999;43:292–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stoodley P, deBeer D, Lappin-Scott HM. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob Agents Chemother 1997;41:1876–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sandvik EL, McLeod BR, Parker AE, Stewart PS. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS One 2013;8:e55118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van der Borden AJ, van der Werf H, van der Mei HC, Busscher HJ. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl Environ Microbiol 2004;70:6871–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Blenkinsopp SA, Khoury AE, Costerton JW. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol 1992;58:3770–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother 2009;53:41–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McLeod BR, Fortun S, Costerton JW, Stewart PS. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol 1999;310:656–670 [DOI] [PubMed] [Google Scholar]
- 67. Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices. ASAIO J 1992;38:M174–M178 [DOI] [PubMed] [Google Scholar]
- 68. Barranco SD, Spadaro JA, Berger TJ, Becker RO. In vitro effect of weak direct current on Staphylococcus aureus. Clin Orthop Relat Res 1974:250–255 [PubMed] [Google Scholar]
- 69. Merriman HL, Hegyi CA, Albright-Overton CR, Carlos J, Jr., Putnam RW, Mulcare JA. A comparison of four electrical stimulation types on Staphylococcus aureus growth in vitro. J Rehabil Res Dev 2004;41:139–146 [DOI] [PubMed] [Google Scholar]
- 70. Liu WK, Brown MR, Elliott TS. Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 1997;39:687–695 [DOI] [PubMed] [Google Scholar]
- 71. Abo-Neima SE KY, Kotb MM, Elhoseiny A, Motaweh HA. Control of metabolic activities of E. coli and S. aureus bacteria by electric field at resonance frequency in vitro study. Int J Engg Sci 2016;6:13–25 [Google Scholar]
- 72. Laatsch L OP, Kloth L. In vitro effects of two silver electrodes on select wound pathogens. J Clin Electrophysiol 1995;7:10–15 [Google Scholar]
- 73. Kincaid CB, Lavoie KH. Inhibition of bacterial growth in vitro following stimulation with high voltage, monophasic, pulsed current. Phys Ther 1989;69:651–655 [DOI] [PubMed] [Google Scholar]
- 74. Szuminsky NJ, Albers AC, Unger P, Eddy JG. Effect of narrow, pulsed high voltages on bacterial viability. Phys Ther 1994;74:660–667 [DOI] [PubMed] [Google Scholar]
- 75. Kuykendall CHT, Bloedaum A. Effects of high voltage pulsed current on bacterial viability: an in vitro study. Wound Repair Regen; 2004;12:A29 [Google Scholar]