Abstract
Calprotectin, the major neutrophil protein, is a critical alarmin that modulates inflammation and plays a role in host immunity by strongly binding trace metals essential for bacterial growth. It has two cysteine residues favourably positioned to act as a redox switch. Whether their oxidation occurs in vivo and affects the function of calprotectin has received little attention. Here we show that in saliva from healthy adults, and in lavage fluid from the lungs of patients with respiratory diseases, a substantial proportion of calprotectin was cross-linked via disulfide bonds between the cysteine residues on its S100A8 and S100A9 subunits. Stimulated human neutrophils released calprotectin and subsequently cross-linked it by myeloperoxidase-dependent production of hypochlorous acid. The myeloperoxidase-derived oxidants hypochlorous acid, taurine chloramine, hypobromous acid, and hypothiocyanous acid, all at 10 μM, cross-linked calprotectin (5 μM) via reversible disulfide bonds. Hypochlorous acid generated A9-A9 and A8-A9 cross links. Hydrogen peroxide (10 μM) did not cross-link the protein. Purified neutrophil calprotectin existed as a non-covalent heterodimer of A8/A9 which was converted to a heterotetramer - (A8/A9)2 - with excess calcium ions. Low level oxidation of calprotectin with hypochlorous acid produced substantial proportions of high order oligomers, whether oxidation occurred before or after addition of calcium ions. At high levels of oxidation the heterodimer could not form tetramers with calcium ions, but prior addition of calcium ions afforded some protection for the heterotetramer. Oxidation and formation of the A8-A9 disulfide cross link enhanced calprotectin's susceptibility to proteolysis by neutrophil proteases. We propose that reversible disulfide cross-linking of calprotectin occurs during inflammation and affects its structure and function. Its increased susceptibility to proteolysis will ultimately result in a loss of function.
Keywords: S100A8, S100A9, Neutrophil, Myeloperoxidase, Hypochlorous acid, Hypothiocyanous acid
Abbreviations: A12, S100A12; A8, S100A8; A9, S100A9; AREST, Australian Respiratory Early Surveillance Team; AUC, area under the curve; BAL, bronchoalveolar lavage; CF, cystic fibrosis; CID, collision-induced dissociation; DPI, diphenyleneiodonium chloride; fMLP, N-formyl-methionylleucylphenylalanine; HBSS, Hank's balanced saline solution; IAM, iodoacetamide; IBD, inflammatory bowel disease; LPO, lactoperoxidase; MPO, myeloperoxidase; NEM, N-ethylmaleimide; NET, neutrophil extracellular trap; NGE, neutrophil granule extract; PMA, phorbol 12-myristate 13-acetate; PVDF, polyvinylidene difluoride; SOD, superoxide dismutase; TBS, Tris-buffered saline; TX1, 3-isobutyl-2-thioxo-7H-purin-6-one
Graphical abstract
Highlights
-
•
Disulfide cross-linking of calprotectin subunits occurs in vivo.
-
•
Extensively oxidized calprotectin is less effective at forming the heterotetramer.
-
•
A8-A9 disulfide cross-linking specifically enhances susceptibility to proteolysis.
-
•
Proteolysis will lead to a loss of protein function at inflammatory sites.
1. Introduction
Calprotectin, a non-covalent heterodimer of S100A8 (A8) and S100A9 (A9) subunits, is an abundant cytosolic neutrophil protein [1]. It is released from neutrophils when they are activated, disrupted, or when they die [[2], [3], [4]]. Calprotectin plays an important role in the innate immune response of humans by strongly chelating trace metal ions that are essential for bacterial growth [[5], [6], [7], [8]]. It can also act as an alarmin by modulating the inflammatory response after its release [9]. Extracellular levels of calprotectin increase during infection and inflammation, and are used as a biomarker of neutrophil activation in many inflammatory diseases [[10], [11], [12], [13], [14], [15], [16], [17]].
The calprotectin subunits, A8 and A9, are S100 proteins. This family of proteins is composed of two EF-hand motifs. The N-terminal motif, comprising helices I and II, and the C-terminal motif, with helices III and IV, are separated by a flexible linker and provide two calcium-binding sites [18]. A8 and A9 subunits typically assume a non-covalent heterodimeric structure (A8/A9) in vivo [19], but at increased calcium concentrations the (A8/A9)2 heterotetramer is specifically formed [20]. In addition to the calcium-binding sites, there are two binding sites for transition metal ions at the A8/A9 heterodimer interface. Site one is the His3Asp motif which is comprised of His83 and His87 of the A8 monomer, and His20 and Asp30 of the A9 monomer. Site two is a hexahistidine (His6) motif comprising A8 residues His17 and His27, and A9 residues His91 and His95. The His103 and His105 of the A9 C-terminal tail complete an octahedral coordination sphere. Several studies have demonstrated that calprotectin binds various first row transition metal ions such as Mn(II) [21], Zn(II) [8], Cu(II) [5], Ni(II) [6], Fe(II) [7], and Co(II) [8] with strong affinity, ultimately leading to inhibition of bacterial growth. In addition, under conditions of high calcium, tetramer formation further enhances the affinity for transition metals [8,21].
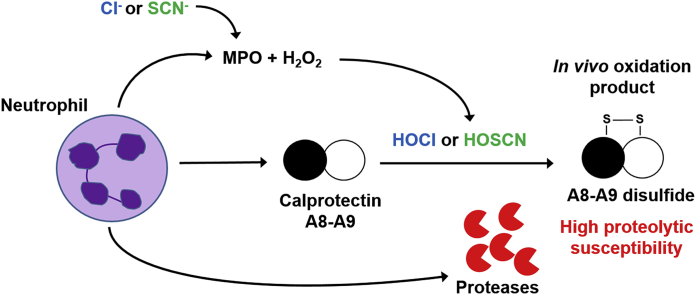
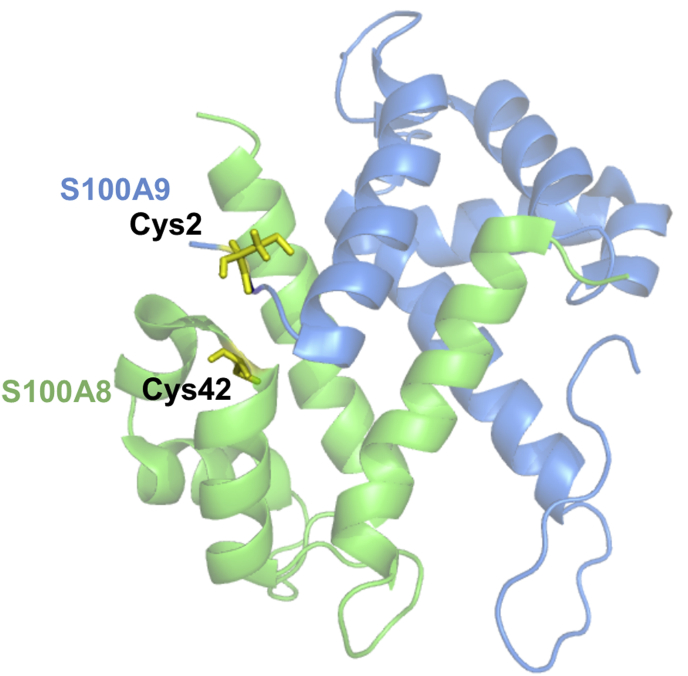
Each calprotectin subunit contains a single cysteine residue, which in the heterodimer are in close proximity (Fig. 1) [22]. A disulfide-mediated cross-link between A8 and A9 subunits may occur at inflammatory sites when neutrophils produce oxidants. Neutrophils generate superoxide, hydrogen peroxide, and hypochlorous acid as an essential part of the innate immune response to invading pathogens [23]. The neutrophil's NADPH oxidase reduces molecular oxygen to superoxide, which dismutates to hydrogen peroxide, and in turn is converted to hypochlorous acid by myeloperoxidase (MPO) [24]. Neutrophil-derived MPO also uses hydrogen peroxide to catalyse the oxidation of other halides such as bromide, iodide, and the pseudohalide thiocyanate to generate their respective hypohalous acids [25,26]. As well as aiding in killing of phagocytosed pathogens, hypochlorous acid reacts with a wide range of biological molecules such as proteins, carbohydrates, lipids, and nucleic acids [27,28]. As a result, excessive or misplaced activation of neutrophils damages host tissues. MPO-derived oxidants contribute to the pathogenesis of various inflammatory diseases such as inflammatory bowel disease (IBD) [29], cystic fibrosis (CF) [30], rheumatoid arthritis [31], cardiovascular disease [32], and chronic obstructive pulmonary disease [33].
Fig. 1.
Calprotectin heterodimer of A8 and A9 subunits. Cysteine residues on A8 (green) and A9 (blue) are highlighted in yellow. Image produced using PyMOL and Protein Data Bank ID: 4XJK. Thr1 and Cys2 were manually added to the A9 N-terminal as this data was not available. In addition, the Ser42 of A8 was changed to a Cys using the mutagenesis function in PyMOL. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Calprotectin is a likely target for oxidation due to its co-localisation with neutrophil-derived oxidants at sites of infection and inflammation. Indeed, in clinical samples from patients with asthma or CF, oxidation-sensitive cysteine and methionine residues were found to be oxidized to cysteine sulfinic acid and sulfonic acid, and methionine sulfoxide and dehydromethionine, respectively [34,35]. Cysteine residues are especially susceptible to oxidation by hypochlorous acid [36], resulting in formation of sulfenic acid, which readily reacts with nearby thiol groups to form disulfides [37,38]. Alternatively, at high oxidant concentrations over-oxidation of cysteine residues produces the corresponding sulfinic (RSO2H) or sulfonic acids (RSO3H) [28,37]. We previously demonstrated that reversible cross-linking of calprotectin subunits occurs when calprotectin is treated with low doses of reagent hypochlorous acid (one or two-fold molar excess) [34]. The specific residues involved in the cross links were not characterized. When oxidized by hypochlorous acid, calprotectin also lost its ability to inhibit bacterial growth [34]. Consequently, the impact of hypochlorous acid-mediated oxidation and disulfide-bond formation on calprotectin's function at sites of inflammation warrants further investigation.
Our objectives for this study were to investigate whether reversible cross-linking of calprotectin occurs at sites of infection and inflammation, characterize the chemical nature of cross-linking by neutrophil-derived oxidants, and assess whether cross-linking affects the structure and function of calprotectin.
2. Methods
2.1. Materials
Staphylococcus (S.) aureus strain 502a (ATCC 27217) was obtained from the New Zealand Communicable Disease Centre. Hypochlorous acid (ε292 = 350 M−1 cm−1 at pH 12 [39]) was purchased as commercial chlorine bleach from Sara Lee Household and Body Care NZ Ltd (Auckland, New Zealand). Hydrogen peroxide (ε240 = 43.6 M−1 cm−1 [40]) was purchased from BioLab (Victoria, Australia). Diphenyleneiodonium chloride (DPI), superoxide dismutase (SOD) from bovine erythrocytes, N-ethylmaleimide (NEM), iodoacetamide (IAM), l-methionine, N-formyl-methionylleucylphenylalanine (fMLP), phorbol 12-myristate 13-acetate (PMA), cytochalasin B, lactoperoxidase (LPO) from bovine milk, catalase from bovine liver, and protease inhibitors were purchased from Sigma (St Louis, MO, USA). 3-Isobutyl-2-thioxo-7H-purin-6-one (TX1) was a gift from AstraZeneca (Uppsala, Sweden). Ficoll-Paque, from GE Healthcare (Buckinghamshire, United Kingdom) and dextran from Leuconostoc mesenteroides, av. mol. wt. 150,000 from Sigma, were used for isolation of neutrophils. Chelex-100 Resin was from Bio-Rad Laboratories (Hercules, CA, USA). Dynabeads® Protein G were purchased from Invitrogen (Norway). Enhanced chemiluminescence reagent, ECL™ plus was purchased from GE Healthcare (Buckinghamshire, United Kingdom). All other chemicals were of analytical grade. For neutrophil preparations buffy coats were purchased from the New Zealand Blood Service, or blood was obtained from healthy human volunteers with informed consent and with ethical approval from the Southern Health and Disability Ethics Committee, New Zealand (reference: URA/06/12/083/AM05).
2.2. Calprotectin-specific immunoblot analysis of clinical samples
Bronchoalveolar lavage (BAL) fluid samples were obtained from children with CF by the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF, Perth, WA, Australia). Children underwent an annual assessment including clinical evaluation, measurement of lung function, BAL for assessing pulmonary inflammation and infection, and markers of oxidative stress [41]. The AREST CF surveillance program was approved by the local ethics committee and parents consented to each aspect of the program separately. The details of the AREST CF surveillance program have been previously published [41]. Saline washings from the lungs of adults undergoing routine bronchoscopy at Christchurch Hospital for diagnosis of a lung infection or lung cancer were collected and analyzed with the approval of the Health and Disability Ethics Committee, New Zealand (reference: 17/NTA/5). During the routine procedure, 10–20 ml of saline was passed down the bronchoscope tube into the parts of the lung of interest and was sucked back through the bronchoscope into a specimen jar. Saliva samples were also collected from healthy volunteers with verbal consent. The saliva sample was collected into a sterile 50 ml centrifuge tube after a single 20 sec mouth wash with 20 ml of water. Clinical details of the healthy salvia donors and patients with respiratory disease are given in Supplementary Table 1. None of the subjects had diabetes.
The alkylating reagent NEM (20 mM) was immediately added to an aliquot of all clinical samples to block thiol residues and prevent their oxidation ex vivo. Samples were centrifuged and the supernatants stored at −80 °C until analysis. The protein concentration of each sample was measured using Direct Detect™ (Merck, Darmstadt, Germany). All of the clinical samples were resolved by 4–20% gradient SDS-PAGE, with equal amounts of total protein loaded per lane, in the absence or presence of 100 mM DTT. Proteins were then transferred electrophoretically to a 0.45 μm polyvinylidene difluoride (PVDF) membrane at 100 V for 60 min. The membrane was blocked with 5% (w/v) fat free milk powder in Tris-buffered saline (TBS)/0.05% (v/v) Tween-20, and then immunoblotted with antibodies to calprotectin. Protein bands were developed using enhanced chemiluminescence reagent (GE Healthcare, Buckinghamshire, United Kingdom) following manufacturer's instructions and visualized using the UVITEC Cambridge Alliance Western blot documentation system (Cambridge, United Kingdom).
Polyclonal rabbit anti-calprotectin serum was raised in the Canterbury Research Laboratories according to a previous protocol for production of an anti-MPO serum [42]. Calprotectin antibody was purified from an ammonium sulfate precipitation of rabbit anti-serum using Dynabeads® Protein G.
2.3. Preparation of S. aureus for phagocytosis experiments
S. aureus was cultured overnight in 3% (w/v) trypticase soy broth, harvested by centrifugation, washed with PBS, and resuspended in Hank's balanced saline solution (HBSS). S. aureus cell density was measured spectrophotometrically at 550 nm and bacterial cell numbers were calculated using a standard curve based on colony forming unit counts. All bacteria were opsonised with 10% autologous human serum and slowly rotated (end-over-end) for 20 min at 37 °C. Opsonised bacteria were washed in PBS, resuspended in HBSS and immediately added to human neutrophils.
2.4. Isolation of human neutrophils
Neutrophils were isolated from heparinized peripheral blood of healthy adult donors, obtained with informed consent and the approval of the Southern Health and Disability Ethics Committee, New Zealand. Neutrophil isolation was performed at room temperature using the modified method of Boyum et al. [43]. Modification of the method included dextran (final concentration of 1%) sedimentation of erythrocytes followed by Ficoll density gradient separation of white blood cells. Contaminating red cells were removed by hypotonic lysis. Neutrophils were resuspended in HBSS. Percentages of dead cells and contaminating peripheral blood mononuclear cells were ≤5%, as routinely tested by flow cytometry.
2.5. Oxidation of calprotectin by stimulated neutrophils
Neutrophils (107/ml) were pre-incubated in the absence or presence of 1 mM methionine, 10 μM DPI, 10 μM TX1, 20 μg/ml catalase, or 20 μg/ml SOD for 5 min at room temperature prior to stimulation. An equivalent volume of opsonised S. aureus (2 × 108/ml) in HBSS or HBSS alone (control) was added to the neutrophils. All samples were slowly rotated end-over-end for 1 h at 37 °C. Neutrophils were then pelleted by centrifugation at 450g. Protease inhibitors and 20 mM NEM were added to the supernatant (extracellular fraction). The cell pellet was washed and resuspended in PBS containing 20 mM NEM and protease inhibitors. Neutrophil cells were lysed using three freeze-thaw lysis cycles, with vortexing between each cycle. The supernatant (intracellular fraction) was collected after centrifugation at 20,000 g for 10 min at 4 °C to remove any cell debris. Extracellular and intracellular proteins were separated by 4–20% gradient SDS-PAGE in the absence or presence of 100 mM DTT and immunoblotted with antibodies to calprotectin.
2.6. Purification of calprotectin from human neutrophils
Calprotectin was purified from the cytosol of human neutrophils obtained by nitrogen cavitation using a two-step ion exchange method as described previously [44]. Purified protein was characterized using SDS-PAGE and immunoblotting with antibodies to calprotectin, and LC-MS.
2.7. Preparation of oxidants
Hypochlorous acid (HOCl) was prepared by adding 50 μl of chlorine bleach to 950 μl of deionised water while vortexing. The concentration of hypochlorous acid was determined by 1:10 dilution in 200 mM potassium hydroxide and measurement of its absorbance at 292 nm (extinction coefficient (ε) of OCl− at 292 nm = 350 M−1 cm−1). The stock solution was diluted in PBS before use. Hypothiocyanous acid (HOSCN) was prepared enzymatically using bovine LPO at 4 °C. LPO (2 μM) was added to sodium thiocyanate (NaSCN) (7.5 mM) in 10 mM potassium phosphate buffer (pH 6.6), and hydrogen peroxide (0.75 mM) was added four times, at 1 min intervals, to generate approximately 2 mM HOSCN. The concentration of HOSCN was determined immediately using 5-thio-2-nitrobenzoic acid (TNB) as described previously [45] and used within half an hour of preparation.
2.8. Oxidation of calprotectin by neutrophil-derived oxidants
Calprotectin (5 μM) was treated with equal volumes of various concentrations of hypochlorous acid while vortexing. Samples were incubated at room temperature for 10 min and reactions were stopped by addition of excess methionine (1 mM). Similarly, calprotectin (5 μM) was treated with 10 μM hydrogen peroxide (H2O2), 2.5 mM taurine, 10 μM hypochlorous acid (HOCl), 10 μM taurine chloramine, or 50 nM MPO and 10 μM H2O2 in the presence of either 140 mM sodium chloride (NaCl), 5 mM sodium bromide (NaBr) or 5 mM NaSCN for 20 min at room temperature. Reactions were stopped by the addition of 0.5 μg/ml catalase. Proteins were separated by 4–20% gradient SDS-PAGE in the absence or presence of 100 mM DTT and stained with Coomassie Blue.
2.9. Molecular mass determination of cross-linked calprotectin by LC-MS
Calprotectin (10 μM) and calprotectin oxidized with a two or ten-fold molar excess of hypochlorous acid or hypothiocyanous acid were analyzed by LC-MS using a Dionex UltiMate 3000 HPLC system coupled inline to an electrospray ionization source of a Velos Pro mass spectrometer (Thermo Scientific, Waltham, MA, USA). Samples containing 0.65 μg calprotectin were injected onto an Accucore-150-C4 HPLC column (50 mm × 2.1 mm, 2.6 μm; Thermo Scientific) and were analyzed by intact protein LC-MS as described previously [46]. Briefly, mass spectra were acquired between m/z 410 and 2000 in positive-ion mode. Spectra were averaged over the full length of each protein peak and deconvoluted to yield the molecular masses using ProMass for Xcalibur (Version 2.8; Novatia LLC). The accuracy of the deconvoluted masses was observed to be ±5 Da when compared with the theoretical mass.
2.10. Characterization of peptides involved in reversible calprotectin cross-linking by LC-MS/MS
Calprotectin (10 μM) was treated with or without a two or ten-fold molar excess of hypochlorous acid. Iodoacetamide (20 mM) was added to block thiols and incubated for 20 min at room temperature and protected from light. Then trypsin (0.5 μg) was added and samples were incubated overnight at 37 °C. For analysis of tryptic peptides, samples were injected onto a Hypercarb column (100 mm × 2.1 mm, 5 μm; Thermo Scientific) and samples were analyzed by LC-MS/MS as described previously [46]. Chromatograms were post-acquisition filtered for the most intense ion representing the A8 and A9 tryptic peptides (Supplementary Table 2). The resulting peaks were integrated to obtain the area under the curve (AUC). The AUC of each peak was normalized to the AUC of a peptide that was unaffected by hypochlorous acid treatment. The normalizing peptides for A8 and A9 were YSLIK ([M+2H]2+ = 312.19 m/z) and LGHPDTLNQGEFK ([M+2H]2+ = 728.68 m/z), respectively. Collision-induced dissociation (CID)-MS/MS spectra confirmed the amino acid sequence of calprotectin tryptic peptides. The predicted mass of the AcTCK-AcTCK cross link (782.32 Da) was determined, and the singly, doubly and triply charged ions ([M + H]+ = 783.32 m/z, [M + 2H]2+ = 392.16 m/z, [M + 3H]3+ = 261.77 m/z, respectively) were searched for manually. The doubly charged precursor ion [M + 2H]2+ with an m/z of 392.16 was detected and subjected to CID fragmentation. The predicted mass of the AcTCK-LLETECPQYIR cross link (1753.84 Da) was also determined, and the singly, doubly and triply charged ions ([M + H]+ = 1754.84 m/z, [M + 2H]2+ = 877.92 m/z, [M + 3H]3+ = 585.61 m/z, respectively) were searched for manually. The triply charged precursor ion [M + 3H]3+ with an m/z of 585.61 was detected and subjected to CID fragmentation. The AUC of each cross-link peak was determined and normalized as described above.
2.11. Estimation of the rate constant for reaction of hypochlorous acid reacting with cysteine residues on calprotectin
The rate constant for reaction of hypochlorous acid with the cysteine residue on A9 of calprotectin was estimated by determining the extent to which free methionine inhibited cross-linking of calprotectin. Calprotectin (10 μM) was incubated with a range of methionine concentrations (0–160 μM) followed by addition of 5 μM hypochlorous acid while vortexing. Samples were incubated at room temperature for 10 min before being resolved by SDS-PAGE and stained with Coomassie Blue. Densitometry analysis of dimeric bands was carried out using UVIBand Max software from UVITEC Cambridge (Cambridge, United Kingdom). The methionine concentrations required to inhibit A9-A9 and A8-A9 dimers by 50% were determined. At these concentrations it was assumed that hypochlorous acid reacted equally with Cys2 and free methionine such that
| (1) |
2.12. Characterization of the quaternary structure by analytical ultracentrifugation
Analytical ultracentrifugation experiments were conducted in a Beckman model XL-I instrument (Indiana, USA) at 20 °C. The protein samples were analyzed at a concentration of 0.11 mg/ml in PBS, pH 7.4. Sample (380 μl) and PBS (400 μl) were loaded into separate sectors of the same quartz cell and mounted in a Beckman 8-hole An-50 Ti rotor. Samples were centrifuged at a rotor speed of 50,000 rpm. Data were collected at a single wavelength (226 nm) in continuous mode, using a step-size of 0.003 cm without averaging. Sedimentation velocity data at multiple time points were fitted to a continuous sedimentation-coefficient model [47,48] using the program SEDFIT, which is available from www.analyticalultracentrifugation.com. The calculated sedimentation coefficient was then used to estimate the molecular mass of the species using SEDFIT software. Where multiple species were present, the frictional ratio for each was assumed to be the same. The parameters for solvent density (1.006 g/ml at 20 °C) and viscosity (0.01002 p), and the partial specific volume of the protein (0.73 ml/g) were calculated using SEDNTERP [49].
2.13. Isolation of neutrophil granule extract
Isolated neutrophils (2 × 107/ml) in HBSS were treated with 10 μM DPI and 5 μg/ml cytochalasin B and incubated for 10 min at 37 °C while rotating. Following incubation, 1 μM fMLP was added and the neutrophil suspension was incubated for a further 20 min at 37 °C. The cell pellet was harvested by centrifugation at 500g for 5 min at 4 °C. The supernatant containing the neutrophil granule proteins was collected and the protein content was determined by Direct Detect™. The neutrophil granule extract (NGE) was stored at −80 °C.
2.14. Protein digestion by a neutrophil granule extract
Protein samples were digested with NGE using a ratio of 1 μg NGE protein:20 μg calprotectin for 0–4 h at 37 °C. Digestion was stopped by the addition of 0.6% formic acid. Samples containing 0.65 μg calprotectin were subjected to intact protein LC-MS as described above. The AUC of the protein peak was determined and expressed relative to the AUC at the zero time point for each treatment. Protein species were not resolved with this chromatographic method and the protein peak therefore represented total undigested calprotectin. Undigested species eluting in this peak were determined by deconvolution as described in section 2.9 and shown in Fig. 5.
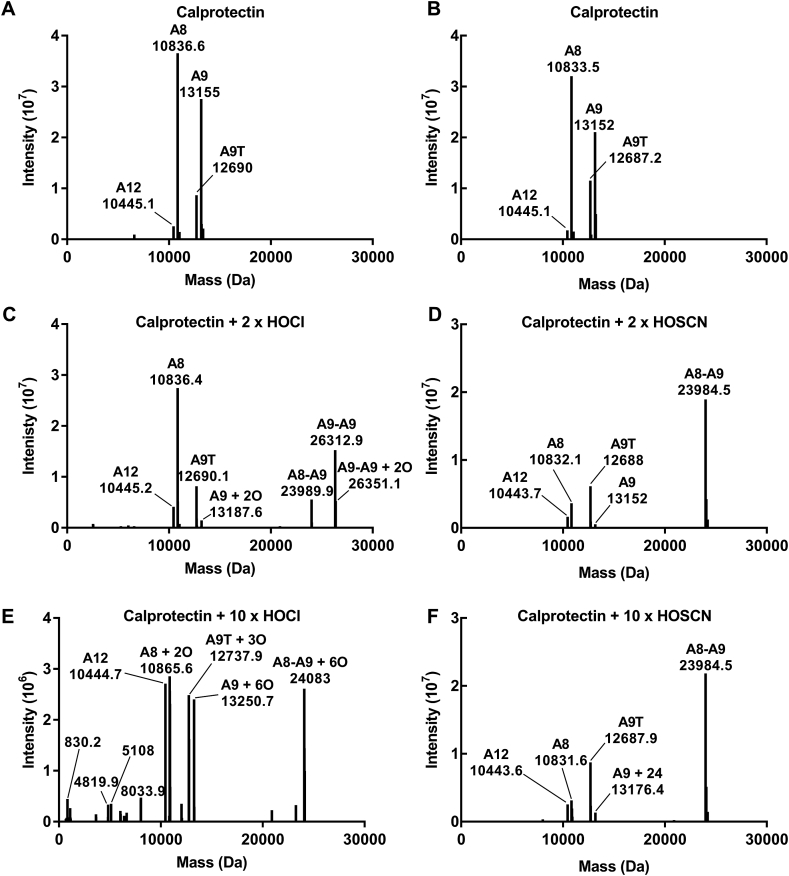
Fig. 5.
Molecular masses of calprotectin cross-linked by hypochlorous acid or hypothiocyanous acid. Calprotectin (10 μM) was treated with a 2 or 10 x molar excess of hypochlorous acid (HOCl) or hypothiocyanous acid (HOSCN) for 20 min at room temperature. Aliquots of each treatment were injected for analysis by LC-MS. Spectra recorded over the full width of the protein peak were averaged and deconvoluted. A representative deconvoluted spectrum is shown for (A, B) calprotectin, and calprotectin treated with (C) 2 x HOCl, (D) 2 x HOSCN, (E) 10 x HOCl or (F) 10 x HOSCN. A8 = S100A8, A9 = S100A9, A9T = truncated S100A9, A12 = S100A12, O = oxygen. Data are representative of at least three separate experiments.
2.15. Statistics
Graphs were plotted and statistical analysis was performed using GraphPad Prism version 7.00 (GraphPad Software, La Jolla, CA, USA). Differences between groups were determined using one-way ANOVA with Dunnett's multiple comparison test with * indicating a significant difference (p < 0.05) when compared to the control.
3. Results
3.1. Reversible calprotectin cross-linking occurs in physiological and pathological situations
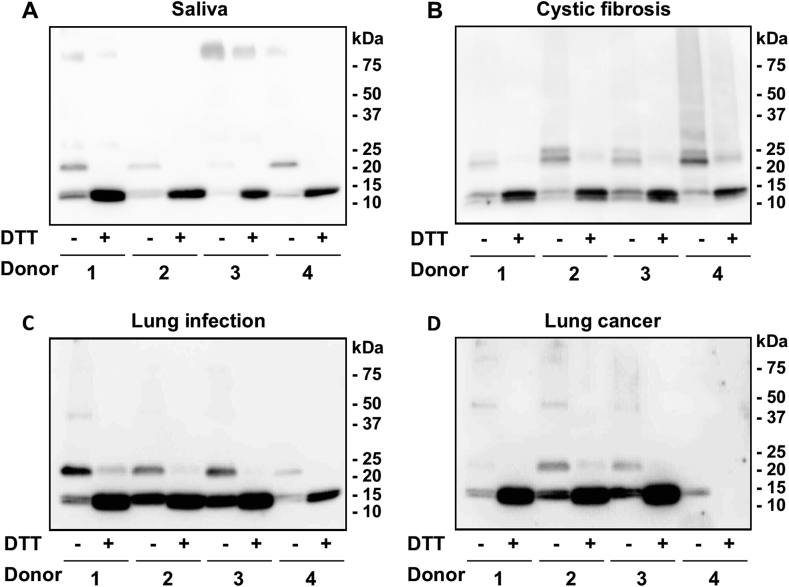
To determine whether calprotectin is reversibly cross-linked in vivo, human saliva samples and samples containing activated neutrophils were analyzed by SDS-PAGE in the absence or presence of the reducing agent DTT, and immunoblotted with antibodies to calprotectin (Fig. 2). Immunoblots were overexposed so that the A9-A9 and A8-A9 dimer bands could be seen clearly. This resulted in poor resolution of the A8 and A9 monomeric bands, due to their higher concentration and the higher affinity of the antibody for the monomeric subunits. The samples analyzed included saliva from healthy adults (Fig. 2A), BAL from children with CF (Fig. 2B), and saline washings from the lungs of adults with respiratory infections (Fig. 2C) or lung cancer (Fig. 2D). The calprotectin monomeric subunits A8 (10.8 kDa) and A9 (13.2 kDa) were clearly detected in all but one of the samples analyzed (Fig. 2D, donor 4). Under non-reducing conditions, bands corresponding to covalently bound A8-A9 (24 kDa) dimers were detected in the majority of samples. Saliva contains high levels of lactoperoxidase and thiocyanate. As a result, hypothiocyanous acid is the predominant oxidant formed in saliva [[50], [51], [52]]. Thus, the cross-linking detected in the saliva samples was most likely mediated by hypothiocyanous acid produced by lactoperoxidase. Alternatively, hypochlorous acid, generated by activated neutrophils was likely to promote cross-linking in the other samples analyzed. Bands corresponding to A9-A9 (26 kDa) dimers were clearly evident only in the CF samples (Fig. 2B). These dimers were lost when the samples were reduced with DTT, and there was an associated increase in intensity of the bands corresponding to the monomeric calprotectin subunits. In addition, calprotectin was attached to other higher molecular mass proteins, which, like the dimers, were predominantly lost upon reduction with DTT. The variation in band intensities between the non-reduced and reduced forms of the protein is most likely explained by the differing affinity of the antibody for the various calprotectin species. However, we can conclude that reversibly cross-linked calprotectin was present in the clinical samples, supporting the involvement of a disulfide bond in the cross links.
Fig. 2.
Calprotectin is reversibly cross-linked in vivo. Anti-calprotectin immunoblots of (A) saliva samples (50 μg total protein per lane) from healthy adults, (B) bronchoalveolar lavage fluid samples (4 μg total protein per lane) from children with cystic fibrosis, (C) saline washings (10 μg total protein per lane) from the lungs of adults with respiratory infections, and (D) saline washings (10 μg total protein per lane) from the lungs of adults with lung cancer. All samples were treated with NEM to prevent artefactual oxidation at the time of sampling and resolved by SDS-PAGE using 4–20% gradient pre-cast gels in the absence or presence of 100 mM DTT prior to immunoblotting with antibodies to calprotectin. Immunoblots were overexposed to enable clear visualization of the dimer bands. These are representative images of at least two separate experiments.
3.2. Reversible cross-linking of calprotectin occurs after its release from neutrophils and is dependent on NADPH-oxidase and myeloperoxidase
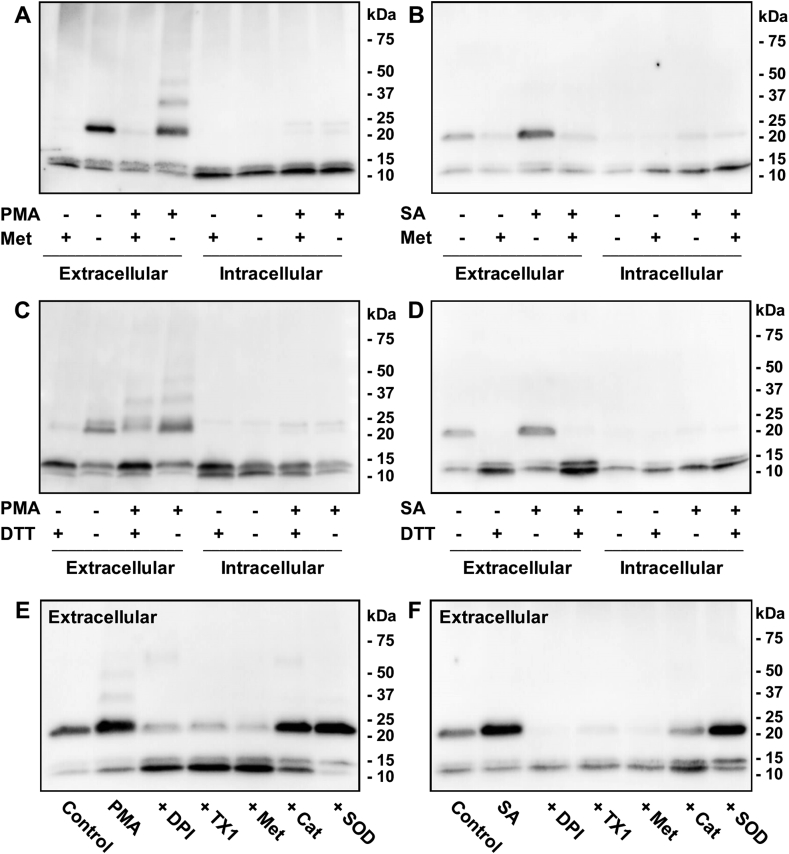
To test whether calprotectin cross-linking occurs within neutrophils or after it's released it into the extracellular medium neutrophils were stimulated and separated into intracellular and extracellular fractions, which were then resolved by SDS-PAGE and immunoblotted with antibodies to calprotectin. Cross-linked calprotectin was clearly observed in the extracellular samples, shown by detection of a band corresponding to the A8-A9 heterodimer (24 kDa) (Fig. 3A and B). No dimers were detected in the presence of methionine, an extracellular scavenger of oxidants, which suggested that cross-linking was mediated by an oxidant. In comparison, minimal cross-linked calprotectin was observed in the intracellular samples. The heterodimeric band was also detected in the extracellular samples from untreated neutrophils (Fig. 3A), suggesting that some background stimulation occurred due to the purification or the conditions of the experiment, i.e. incubation of the neutrophils at 37 °C for 1 h with end-over-end rotation. However, PMA promoted more extensive extracellular cross-linking as shown by detection of additional higher molecular mass bands above 25 kDa. Reduction of the samples with DTT indicated that a substantial proportion of PMA-stimulated extracellular calprotectin cross-linking was reversible but some irreversible cross-linked bands remained after reduction (Fig. 3C). When neutrophils phagocytosed S. aureus, all of the dimers were reduced by DTT (Fig. 3D).
Fig. 3.
Reversible cross-linking of calprotectin released by neutrophils is dependent on NADPH-oxidase and myeloperoxidase. Neutrophils (107/ml) were stimulated with 100 ng/ml PMA (A, C, E) or S. aureus (SA) (2 × 108/ml) (B, D, F) for 1 h at 37 °C. (A, B) Neutrophils were pre-treated with or without 1 mM methionine prior to stimulation. (C, D) Samples were resolved with or without 100 mM DTT after incubation. (E, F) Neutrophils were pre-treated with 10 μM DPI, 10 μM TX1, 1 mM methionine (Met), 20 μg/ml catalase (Cat), or 20 μg/ml SOD prior to stimulation. Samples were resolved by SDS-PAGE using 4–20% gradient pre-cast gels and immunoblotted with antibodies to calprotectin. All immunoblots are representative images of three separate experiments.
To determine whether calprotectin cross-linking was dependent on hypochlorous acid generated via MPO, neutrophils were pre-treated with a range of inhibitors prior to stimulation with PMA (Fig. 3E) or phagocytosis of S. aureus (Fig. 3F). DPI was used to inhibit the NADPH-oxidase, TX1 to inactivate MPO, methionine to scavenge hypochlorous acid, catalase to degrade hydrogen peroxide, and SOD to scavenge superoxide. DPI, TX1 and methionine all inhibited extracellular calprotectin cross-linking, indicating that MPO-derived production of hypochlorous acid promoted cross-linking of calprotectin. Catalase inhibited cross-linking back to control levels when neutrophils phagocytosed S. aureus (Fig. 3F). However, significant cross-linking was still detected when PMA-stimulated neutrophils were pre-treated with catalase (Fig. 3E). This result suggests that only low concentrations of oxidants are required to cross-link extracellular calprotectin. SOD failed to inhibit calprotectin cross-linking in both instances (Fig. 3E and F). Together these results confirm that calprotectin was cross-linked reversibly when it was released from neutrophils, and it required extracellular production of hypochlorous acid.
3.3. Hypochlorous acid and other reactive halogen species promote cross-linking of calprotectin
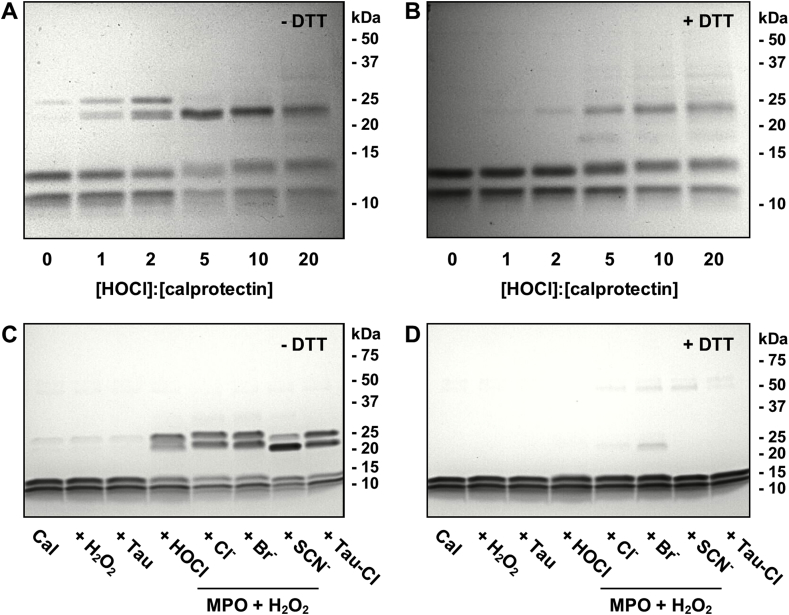
To determine the susceptibility of calprotectin to cross-linking by hypochlorous acid, the purified protein was treated with increasing doses of reagent hypochlorous acid. The oxidized protein was then analyzed by SDS-PAGE (Fig. 4A and B). Cross-linking was observed with as little as 1 mol of hypochlorous acid per mole of calprotectin. Increasing doses of hypochlorous acid promoted increasing degrees of cross-linking (Fig. 4A and B). Under non-reducing conditions and lower doses of hypochlorous acid (≤2 x molar excess), two bands were observed at approximately 26 kDa and 24 kDa (Fig. 4A). As the concentration of hypochlorous acid increased, the 24 kDa band predominated. The 26 kDa band was also present with no hypochlorous acid treatment, which suggested that some of the purified protein was already oxidized. Molecular masses of 26 kDa and 24 kDa can be attributed to A9-A9 homodimers and A8-A9 heterodimers, respectively. The A9-A9 and A8-A9 bands were predominantly reducible at lower doses of hypochlorous acid (≤2 x molar excess) but irreversibly cross-linked bands were apparent at a 5:1 or higher molar ratio of hypochlorous acid to calprotectin (Fig. 4B).
Fig. 4.
Reactive halogen species promote cross-linking of calprotectin. (A, B) Calprotectin (5 μM) was treated with increasing concentrations of hypochlorous acid (HOCl) (0–100 μM) in PBS for 10 min at room temperature. Reactions were stopped by the addition of 1 mM methionine. Proteins were separated by 15% SDS-PAGE gels (made in-house) in the (A) absence or (B) presence of 100 mM DTT and stained with Coomassie Blue. (C, D) Calprotectin (Cal) (5 μM) was treated with or without 10 μM hydrogen peroxide (H2O2), 2.5 mM taurine, 10 μM HOCl, 10 μM taurine chloramine, or 50 nM MPO and 10 μM H2O2 in the presence of either 140 mM sodium chloride (Cl−), 5 mM sodium bromide (Br−) or 5 mM sodium thiocyanate (SCN−) in 50 mM phosphate buffer for 20 min at room temperature. Proteins were separated by 4–20% gradient SDS-PAGE pre-cast gels in the (C) absence or (D) presence of 100 mM DTT and stained with Coomassie Blue. All SDS-PAGE gels are representative images of three separate experiments.
Next we tested whether other neutrophil-derived oxidants promote calprotectin cross-linking. Hydrogen peroxide alone did not promote cross-linking of calprotectin at the concentrations used in this study (Fig. 4C). A two-fold molar excess of hypochlorous acid, hypobromous acid, and taurine chloramine promoted formation of two separate dimeric cross links corresponding to A9-A9 homodimers (26 kDa) and A8-A9 heterodimers (24 kDa) in approximately equal amounts. Interestingly, treatment of calprotectin with a two-fold molar excess of hypothiocyanous acid promoted formation of two cross links that ran slightly lower on the SDS-PAGE gel than the dimers formed by the other oxidants. Also, in contrast to the other oxidants, the lower dimer was the major product. The position of the two bands formed with hypothiocyanous acid suggested that the cross links had lower molecular masses than those generated by the other oxidants. All of the cross-linking observed was predominantly reversible (Fig. 4D) indicating that the cross links were formed by disulfide bonds.
Intact protein LC-MS was used to establish the molecular masses of cross-linked calprotectin induced by hypochlorous acid or hypothiocyanous acid (Fig. 5). A representative deconvoluted mass spectrum is shown for untreated calprotectin (Fig. 5A and B), and calprotectin oxidized with hypochlorous acid (Fig. 5C and E) or hypothiocyanous acid (Fig. 5D and F). Untreated calprotectin contained two major molecular mass species corresponding to A8 (10,836.6 Da) and full length A9 (13,155 Da). The two minor peaks correspond to the truncated A9 isoform (12,690 Da) and S100A12 (A12) (10,445.1 Da). In accordance with the SDS-PAGE results, treatment of calprotectin with a two-fold molar excess of hypochlorous acid showed formation of both A9-A9 homodimers (26,312.9 Da) and A8-A9 heterodimers (23,989.9 Da) (Fig. 5C). Calprotectin treated with a ten-fold molar excess of hypochlorous acid formed predominantly the A8-A9 heterodimer, as well as further oxidation of calprotectin monomers and dimers shown by addition of oxygen atoms (Fig. 5E). Fragmentation of the protein was also observed, as indicated by detection of several lower molecular mass species (<10,000 Da). Calprotectin treated with a two or ten-fold molar excess of hypothiocyanous acid formed the A8-A9 heterodimer without other discernible modifications (Fig. 5D and F). Despite the dimer band running lower on the SDS-PAGE gel (Fig. 4C), the LC-MS results confirmed that band was in fact the A8-A9 heterodimer (Fig. 5D and F). When the concentration of calprotectin was four-fold greater than that of hypothiocyanous acid, the A9-A9 cross-link predominated (data not shown).
Collectively, these results demonstrate that hypohalous acids reversibly cross-link calprotectin via disulfide bonds between A8 and A9. A9-A9 cross links predominate at low oxidant to protein ratios but give way to mainly A8-A9 cross links at high levels of oxidation.
3.4. Reversible calprotectin cross-linking occurs between cysteine residues
Residues involved in the calprotectin cross links were identified by digesting non-oxidized and oxidized protein with trypsin and then using LC-MS/MS to compare their tryptic peptide profiles. The possible peptides involved in the cross links were determined by identifying those that underwent significant loss with hypochlorous acid treatment compared to the control (Supplementary Table 3). A significant loss was identified for the LLETECPQYIR peptide from A8, and the N-terminal TCK peptide from A9 (p < 0.05) (Fig. 6A). These two peptides were completely recovered when calprotectin was treated with a two-fold molar excess of hypochlorous acid, then reduced with DTT (Fig. 6A). This result suggested involvement of a cysteine disulfide in A9-A9 homodimer and A8-A9 heterodimer formation. At a ten-fold molar excess of hypochlorous acid, less than 50% of the TCK and LLETECPQYIR peptides were recovered after the oxidized protein was reduced with DTT. There was no formation of the non-reducible oxidation states of cysteine, i.e. cysteine sulfinic or sulfonic acid on these peptides, therefore, it is likely that these peptides were also involved in irreversible cross-linking. Consistent with this result, non-reducible cross-linking was also observed by SDS-PAGE (Fig. 4B) and has previously been identified to contain a cysteine-lysine cross link [53]. A loss of methionine-containing peptides with concomitant appearance of the methionine sulfoxide-containing peptides was also detected upon treatment with hypochlorous acid (Supplementary Table 3). Albeit slower than cysteine and methionine; lysine, histidine, tyrosine and tryptophan residues also react with hypochlorous acid [[54], [55], [56], [57]]. Apart from the A9 peptide LTWASHEK no other peptides containing these reactive residues showed significant loss (Supplementary Table 3), suggesting oxidative modifications on these residues were not major products. Tryptic peptides detected in untreated calprotectin covered the majority of the protein sequence, i.e. 73% and 63% of the A8 and A9 sequence, respectively (Supplementary Fig. 1). It is possible that oxidation occurred on reactive residues in regions that were not detected by our method.
Fig. 6.
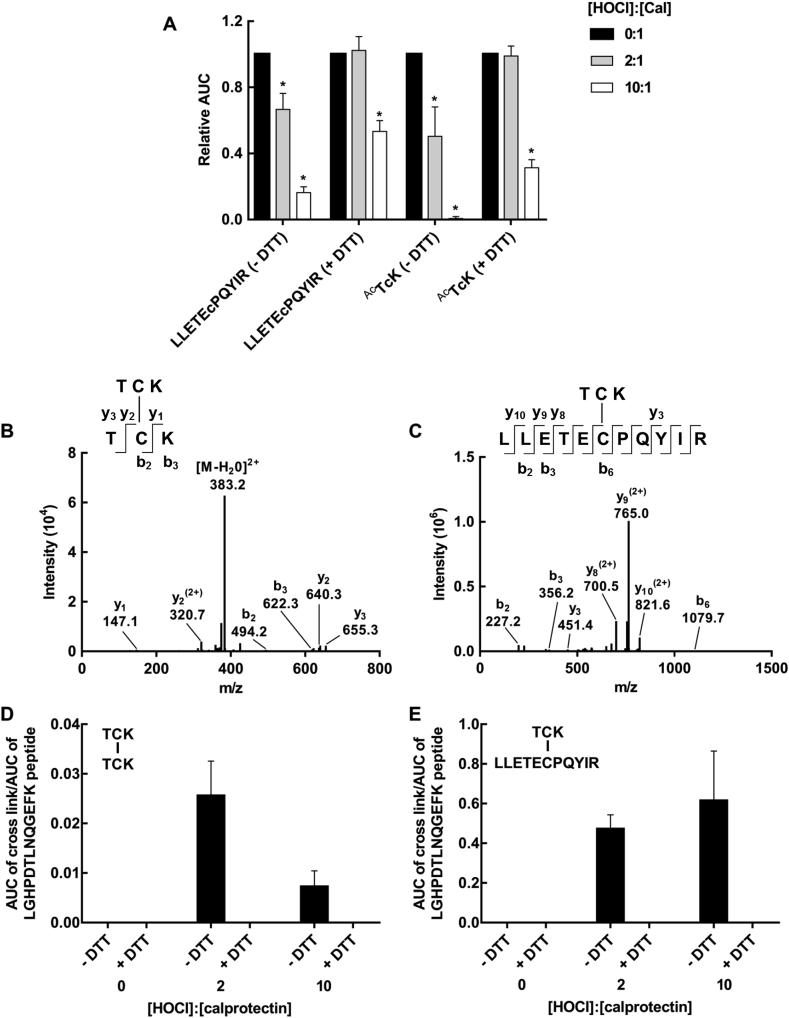
Characterization of calprotectin cross-linking after treatment with hypochlorous acid. Calprotectin (10 μM) was treated with a 2 or 10 x molar excess of hypochlorous acid (HOCl). Samples were treated with 100 mM DTT, alkylated with IAM, digested with trypsin overnight at 37 °C and analyzed by LC-MS/MS. (A) The AUC for the LLETECPQYIR and TCK cysteine containing peptides (represented by ions with m/z values of 585.07 and 392.16, respectively) was determined and expressed relative to the untreated control. Differences between groups were determined using one-way ANOVA with Dunnett's multiple comparison test, * indicates a significant difference (p < 0.05) when compared to untreated calprotectin. (B) CID-MS/MS spectrum for the [M + 2H]2+ ion of the TCK-TCK disulfide-bonded peptide (392.16 m/z). (C) CID-MS/MS spectrum for the [M + 3H]3+ ion of the TCK-LLETECPQYIR disulfide-bonded peptide (585.07 m/z). Peptides in B and C were generated by treatment with 2 x HOCl, and a representative spectrum of three separate experiments is shown. The AUC of the peaks corresponding to the disulfide-bonded peptides (D) TCK-TCK and (E) TCK-LLETECPQYIR were determined and normalized to the AUC of the LGHPDTLNQGEFK peptide that was unaffected by hypochlorous acid treatment. Each bar represents the mean ± SD of three separate experiments.
To confirm that TCK-TCK (A9-A9) and TCK-LLETECPQYIR (A8-A9) disulfide cross links were formed, LC-MS/MS was used to specifically identify the cross-linked peptides in hypochlorous acid-treated calprotectin (Fig. 6B and C). Two tryptic peptides from the oxidized protein were detected that corresponded to the doubly charged parent ion of TCK-TCK ([M + 2H]2+; m/z of 392.16) and the triply charged parent ion of TCK-LLETECPQYIR ([M + 3H]3+; m/z of 585.61). Collision-induced dissociation (CID) fragmentation of these ions resulted in partial y and b ion series that confirmed the identity of the cross-linked peptides (Fig. 6B and C).
The abundance of TCK-TCK (392.16 m/z) and TCK-LLETECPQYIR (585.61 m/z) present in tryptic digests were determined for calprotectin, and calprotectin treated with a two, or ten-fold molar excess of hypochlorous acid (Fig. 6D and E). In the absence of hypochlorous acid, no cross-linking was detected. Conversely, TCK-TCK and TCK-LLETECPQYIR cross links were observed upon treatment with a two-fold molar excess of hypochlorous acid. After treatment with a ten-fold molar excess of hypochlorous acid formation of the TCK-TCK cross link decreased (Fig. 6D), while formation of the TCK-LLETECPQYIR cross link increased (Fig. 6E). These findings matched the SDS-PAGE results, where increasing doses of hypochlorous acid produced more A8-A9 heterodimer at the expense of the A9-A9 homodimer. When the oxidized protein was reduced with DTT, the cross links were no longer detected, further confirming that the cross links were joined by disulfide bonds.
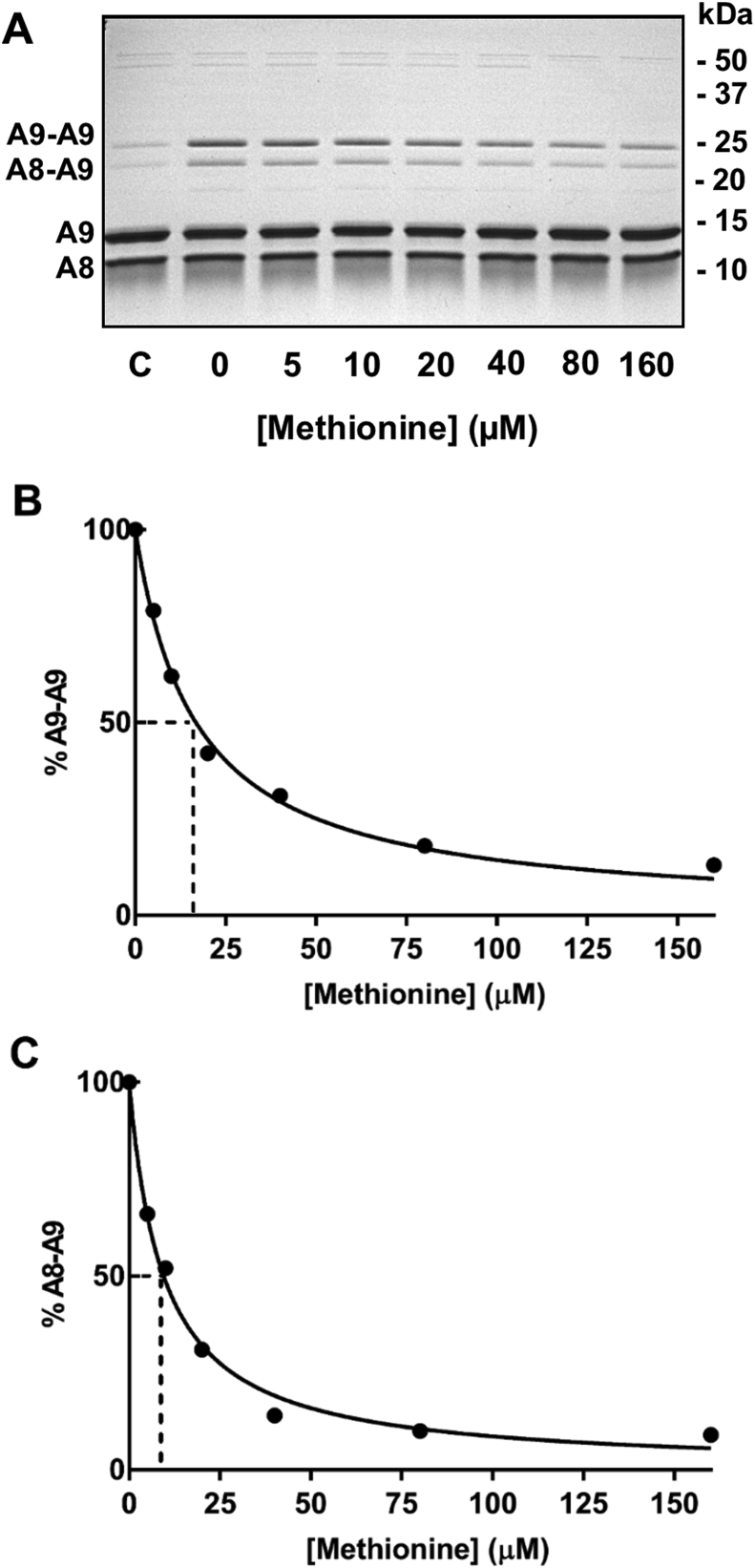
The rate constant for reaction of hypochlorous acid with the thiol residues on calprotectin was estimated using competition kinetics studies whereby methionine was used to scavenge hypochlorous acid and prevent it from producing reversible dimers. The extent of dimer formation with increasing concentrations of added methionine was assessed by SDS-PAGE (Fig. 7A). Under the reaction conditions, we assumed that, in the absence of methionine, hypochlorous acid reacts almost exclusively with Cys2 on A9. This assumption is based on our finding that at sub-stoichiometric concentrations of oxidant A9-A9 cross links predominated. Hypochlorous acid would convert Cys2 to a sulfenyl chloride which would then react either with another Cys2 to form A9-A9 dimers or with an adjacent A8 cysteine to form an A8-A9 dimer. To support this assumption, at a two-fold molar excess of hypochlorous acid there was minimal oxidation of methionine residues (Supplementary Table 3), no evidence of alternative cysteine oxidation products or formation of irreversible cross links (Fig. 4). Added methionine would compete with the thiol residues for oxidation by hypochlorous acid, and the amount of dimer formed should reflect the amount of thiol oxidized. Dimer formation should be independent of the subsequent slow steps involved in coupling of a non-oxidized cysteine residue with an oxidized cysteine. In the absence of added methionine, hypochlorous acid caused formation of A9-A9 and A8-A8 cross links, with the former predominating (Fig. 7A). Adding increasing concentrations of methionine to the reaction mixture decreased the formation of both dimers. When dimer formation was quantified by densitometry, it was apparent that methionine caused progressive inhibition of the cross links (Fig. 7B and C). Formation of A9-A9 and A8-A9 was inhibited by 50% at methionine concentrations of 16.8 μM and 9.5 μM, respectively. Using equation (1) and the known rate constant for the reaction of methionine with hypochlorous acid (3.4 × 107 M−1 s−1) [36], the rate constant for reaction of hypochlorous acid with Cys2 was calculated to be either 6 × 107 M−1 s−1 (±0.3 × 107 M−1 s−1) or 3 × 107 M−1 s−1 (±0.2 × 107 M−1 s−1), respectively. Given the complexity of dimer formation and the limited resolving power of SDS gels, our values are best described as estimates of the true rate constant. They should be used only to give an indication as to whether the reactions are fast enough to be physiologically relevant. We conclude that the rate of Cys2 oxidation and subsequent dimer formation is fast and comparable to that for reactions of hypochlorous acid with typical cysteine residues.
Fig. 7.
Estimation of the rate constant for oxidation of A9 Cys2 by hypochlorous acid. (A) Control (C) is untreated calprotectin, with the remaining lanes containing calprotectin (10 μM) treated with increasing concentrations of methionine (0–160 μM), followed by addition of hypochlorous acid (HOCl) (5 μM). Proteins were separated by 15% SDS-PAGE gels (made in-house) under non-reducing conditions and stained with Coomassie Blue. Densitometry was used to estimate the percent loss of the (B) A9-A9, and (C) A8-A9 dimers with increasing concentrations of methionine relative to the 0 μM methionine control. Data are fitted by a line of best fit and are representative of two separate experiments.
Overall, we conclude that the reversible cross-links formed when calprotectin is oxidized by hypohalous acids involve the cysteine residues on A8 and A9. These reactions are fast enough to compete with other reactions of hypochlorous acid at sites of inflammation.
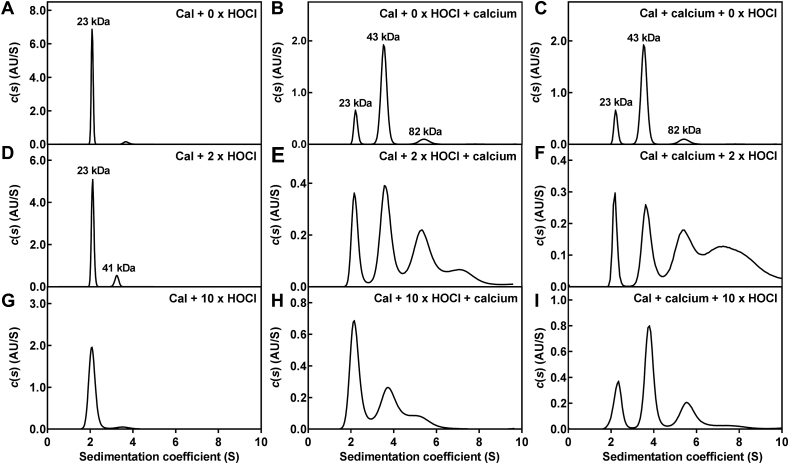
3.5. Hypochlorous acid-induced oxidation affects the quaternary structure of calprotectin
Next we wanted to determine how oxidative cross-linking of calprotectin affected the proteins quaternary structure (Fig. 8). To do this, analytical ultracentrifugation was used to estimate the molecular mass of the species in solution taking into account both non-covalent and covalent protein interactions. Purified calprotectin was present predominantly as a single 23 kDa species in solution (Fig. 8A, peak at 2.05 S), consistent with that of a non-covalent heterodimer of A8 (10.8 kDa) and A9 (13.2 kDa). Treatment of calprotectin with excess calcium to saturate the calcium binding sites has been shown to promote tetramer formation [18,20,58]. Our results were consistent with previous studies as treatment of calprotectin with excess calcium caused the protein to form a tetramer (43.0 kDa), and possibly an octamer (81.6 kDa) (Fig. 8B and C).
Fig. 8.
Sedimentation coefficient distributions of calprotectin and oxidized calprotectin treated with excess calcium before or after oxidation. Sedimentation coefficient distributions were obtained for (A) calprotectin (Cal) (0.1 mg/ml), (B, C) calprotectin (0.1 mg/ml) treated with 1 mM CaCl2, (D) calprotectin (0.1 mg/ml) treated with 2 x molar excess of hypochlorous acid (HOCl), calprotectin (0.1 mg/ml) treated with 2 x HOCl (E) before or (F) after addition of 1 mM CaCl2, (G) calprotectin (0.1 mg/ml) treated with 10 x HOCl, calprotectin (0.1 mg/ml) treated with 10 x HOCl (H) before or (I) after addition of 1 mM CaCl2 by fitting sedimentation data to a c(s) model in SEDFIT. A representative trace of two separate experiments is shown for A-D and G. A trace of one experiment is shown for E, F, H and I.
When calprotectin was oxidized with a two-fold molar excess of hypochlorous acid the quaternary structure appeared to be largely unaffected, with the heterodimer (2.05 S, 23 kDa) still the dominant species in solution (Fig. 8D). A small proportion of a higher molecular mass species was also detected at 3.2 S, with a molecular mass of approximately 41 kDa, indicating possible tetramer formation. When calcium was added either after (Fig. 8E) or before (Fig. 8F) hypochlorous acid, substantial changes were apparent in the structure of calprotectin. Tetramer formation occurred (peak at 3.6 S), but about a third to a half of the protein formed additional higher molecular mass structures (peaks from 4 to 10 S). There was also considerable broadening of the peaks compared to the non-oxidized protein (Fig. 8B).
Calprotectin oxidized with a ten-fold molar excess of hypochlorous acid was present predominantly as a single species in solution (Fig. 8G), with a mass consistent with that of the heterodimer (23 kDa). However, the peak was much broader than the non-oxidized protein, suggesting the presence of increased heterogeneity that may be caused by local unfolding of the protein with higher levels of oxidation. Calprotectin oxidized with a ten-fold molar excess of hypochlorous acid was less effective at forming the tetramer in the presence of excess calcium (Fig. 8H), which is also consistent with local unfolding of the heterodimer that blocks tetramer formation. Pre-treatment of calprotectin with calcium to promote tetramer formation appeared to protect calprotectin from structural destabilisation (i.e. local unfolding) caused by oxidation, although there was still considerable broadening of the peaks (Fig. 8I). Thus, high levels of oxidation can be expected to alter the structure of calprotectin but more so in the absence than the presence of calcium.
Collectively, these results demonstrate that even low levels of oxidation will substantially modify the quaternary structure of calprotectin. High levels of oxidation will damage the protein but this may be tempered by prior binding of calcium ions.
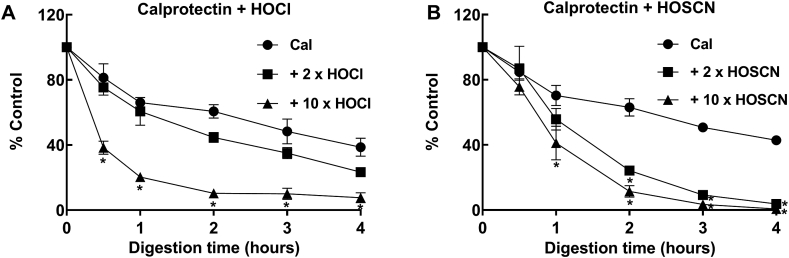
3.6. Oxidized calprotectin is more susceptible to proteolysis by neutrophil granule enzymes
Low levels of protein oxidation can result in enhanced degradation compared with native proteins [59]. Since neutrophils release calprotectin along with several proteases such as elastase, proteinase K, and cathepsin G, we determined whether oxidized calprotectin had an increased susceptibility to proteolysis by neutrophil proteases. Calprotectin and oxidized calprotectin were digested with a neutrophil granule extract (NGE) and loss of intact protein was monitored over time by LC-MS (Fig. 9). There was no significant difference in the rate of digestion between untreated calprotectin and calprotectin treated with a two-fold molar excess of hypochlorous acid. Calprotectin oxidized with a ten-fold molar excess of hypochlorous acid however, was significantly more susceptible to proteolysis at each of the time points, with almost complete digestion after 2 h incubation (Fig. 9A). A ten-fold excess formed predominantly the A8-A9 cross link, as well as further oxidation of calprotectin monomers and dimers shown by addition of oxygen atoms (Fig. 5E). Because the loss of the protein peak containing all of these species was monitored, the increased susceptibility could not be ascribed to one of the modified species in particular. However, treatment of calprotectin with a two or ten-fold molar excess of hypothiocyanous acid resulted in formation of the A8-A9 cross link exclusively (Fig. 5D and F), and rendered calprotectin significantly more prone to digestion indicating that the A8-A9 cross link is responsible for the increased susceptibility to proteolysis (Fig. 9B). Native calprotectin and calprotectin oxidized with a two or ten-fold molar excess of hypochlorous acid were stable over 4 h at 37 °C in the absence of neutrophil proteases (data not shown). Since hypothiocyanous acid produces predominantly A8-A9 cross links and little observable protein oxidation, we conclude that at moderate levels of oxidation of calprotectin where A8-A9 cross-links form, the protein becomes susceptible to proteolysis.
Fig. 9.
Susceptibility of calprotectin and oxidized calprotectin to proteolysis by a neutrophil granule extract. Calprotectin (Cal) (10 μM), or calprotectin treated with a 2 or 10 x molar excess of (A) hypochlorous acid (HOCl) or (B) hypothiocyanous acid (HOSCN) was digested with a neutrophil granule extract (NGE) for 0–4 h at 37 °C. Reactions were stopped by the addition of 0.6% formic acid. An aliquot of each sample was injected for analysis by LC-MS. The AUC of the intact protein peak was determined and normalized to the zero-time point. Each data point represents the mean ± SD from three separate experiments. Differences between groups were determined using one-way ANOVA with Dunnett's multiple comparison test, * indicates a significant difference (p < 0.05) when compared to untreated calprotectin for each time point.
4. Discussion
We propose that at sites of inflammation and infection, activated neutrophils release their calprotectin into the extracellular milieu where it binds calcium ions and forms mainly heterotetramers (A8/A9)2. When calprotectin is oxidized by hypochlorous acid, hypothiocyanous acid, or chloramines at low oxidant to protein ratios, calprotectin will be converted to a mixture of structures including dimers, tetramers and oligomers. Some of these structures will contain A8-A9 and A9-A9 disulfide bonds. In particular, the oligomers are likely to be composed of tetramers linked via A9 cysteine residues. It is possible that these modified forms of calprotectin differ in function. At high oxidant to calprotectin ratios, the tetramers will predominate and contain internal cross links between adjacent A8 and A9 monomers. Internally cross-linked calprotectin will be degraded by co-released neutrophil proteases. Proteolysis will lead to a loss of metal binding capacity and release of calprotectin peptides.
In support of our proposal, substantial proportions of calprotectin were present as reversibly cross-linked dimers in saliva of healthy individuals, or lavage fluid from the lungs of patients with respiratory infections, cystic fibrosis or cancer. Interestingly, A9-A9 cross links were obvious only in calprotectin from the airways of children with cystic fibrosis. In our in vitro work with purified calprotectin, A9-A9 cross links were formed only at low oxidant to protein ratios. Hence, it is likely that the concentration of calprotectin exceeded that of oxidants only in cystic fibrosis. Indeed, it is known that large amounts of calprotectin are released by neutrophils in cystic fibrosis. In fact, calprotectin was originally called the cystic fibrosis antigen due to its extremely high concentration in serum from cystic fibrosis patients compared to healthy individuals [60].
Previously, our group detected irreversible cross-linking between A8 and A9 calprotectin subunits in the BAL fluid from patients with cystic fibrosis, and in stool samples from patients with IBD [53]. Similar studies have detected irreversible complexes of A9 in brain extracts from patients with Alzheimer's disease [61], and of A8 and A9 in extracts from human atherosclerotic arteries [62]. Although we found that reversible cross-linking of calprotectin subunits made up the majority of the total cross-linked calprotectin in vivo, we also observed cross-linking of calprotectin to other proteins, as shown by detection of higher molecular mass bands reacting to calprotectin antibodies. Lim et al. reported disulfide bond formation of A9 with the small molecular mass thiol glutathione, and showed this modification regulated inflammatory processes of calprotectin by decreasing neutrophil binding to the extracellular matrix [63]. Together, these results highlight the importance of future work to elucidate the functional consequences of reversible calprotectin cross-linking to itself, other proteins, and low molecular mass thiols.
Our finding that neutrophils cross-link calprotectin mostly after its release into the extracellular medium has important implications for how the physiological functions of calprotectin are affected by oxidation, which will be strongly influenced by the local concentration of calcium ions. Calprotectin is reported to be present in vivo predominantly as a non-covalent heterodimer of A8 and A9 (∼24 kDa) [19], and forms heterotetramers (∼48 kDa) in the presence of excess calcium [20,58,64]. Within the cytosol of neutrophils, where the calcium ion concentration is low (∼0.1 μM) [65], calprotectin will exist as a non-covalent heterodimer of A8 and A9. When neutrophils are stimulated, intracellular calcium ion concentrations rise approximately 100-fold [65]. This increase in calcium will cause a small amount of calprotectin to form heterotetramers. Our data suggests that intracellular oxidation of calprotectin occurs to a limited extent but, if it does occur, the protein's heterodimeric form will be modified. However, once released from neutrophils into the extracellular milieu, where calcium ion concentrations are in the millimolar range, essentially all the calprotectin will be present as heterotetramers. Thus, oxidants produced by MPO outside of neutrophils can be expected to modify calprotectin heterotetramers. Hydrogen peroxide likely diffuses across the cell membrane, while MPO may be exported from neutrophils by degranulation, via microvesicles [66], bound to neutrophil extracellular traps (NETs) [67] or as a result of passive release from necrotic neutrophils.
Under physiological conditions hypochlorous acid, hypothiocyanous acid and chloramines can be expected to be the main oxidants that reversibly modify calprotectin. Stephan et al. reported that recombinant calprotectin was susceptible to hydrogen peroxide modification, however, the concentration used was 100 μM and required several hours of incubation to afford appreciable formation of A8-A9 and A9-A9 [68]. Hydrogen peroxide is unlikely to promote cross-linking in vivo because it reacts comparatively slowly with most thiols and its concentration will at most be in the low micromolar range [69]. A9-A9 cross-links formed at low oxidant to protein ratios, but these cross links declined as the oxidant to protein ratio increased, and A8-A9 cross links became increasingly dominant. The changing pattern of cross links with the degree of oxidation is best explained by the relative accessibility of the cysteine residues involved in the cross links. The Cys2 residue on A9 is freely exposed on a flexible strand whereas the Cys42 residue on A8 is located between α helices and would be less accessible to oxidants (Fig. 1). At low ratios of oxidant, some of the Cys2 will be oxidized, presumably to a sulfenyl halide intermediate. This oxidized residue must react preferentially with another Cys2 residue to form the A9-A9 cross link. At higher ratios of oxidant, all the Cys2 residues can be expected to form sulfenyl halides so they can then react only with the adjacent Cys42 on A8 to form A8-A9 cross links. In addition to cysteine, hypochlorous acid also reacts with other amino acid residues, in particular methionine [36]. Because calprotectin contains seven methionine residues, some of which are surface exposed, a proportion of the hypochlorous acid will promote methionine sulfoxide formation instead of cross-linking, as shown by LC-MS (Supplementary Table 3). In contrast to hypochlorous acid, hypothiocyanous acid reacts very slowly with methionine (k < 103 M−1 s−1) and is selective for cysteine residues [70,71]. Therefore, a two-fold molar excess of hypothiocyanous acid was sufficient to promote the A8-A9 cross link exclusively. There would be a near complete oxidation of the Cys2 residues even at low oxidant ratios, which would then rapidly couple with adjacent Cys42 residues to form the A8-A9 cross link. Analysis of oxidized calprotectin by mass spectrometry confirmed that the dimers observed on SDS gels were in fact cross links of A9-A9 or A8-A9, and established that the reversible cross links involved the Cys2 and Cys42 residues. Also, our estimates of the rate constants for formation of the cross links, although imprecise, established that these reactions are fast and are likely to occur in vivo where calprotectin is present in relatively high concentrations compared to other targets for hypohalous acids.
Structural analysis of calprotectin confirmed results of previous studies that at millimolar concentrations of calcium ions, calprotectin exists mainly as the heterotetramer (A8/A9)2. Calcium-induced tetramer formation has been shown to be essential for the biological function of calprotectin. Specifically, lack of tetramer formation was associated with inability of calprotectin complexes to promote formation of microtubules [64]. We found that at low oxidant to protein ratios the heterotetramer readily formed oligomers. Under the same reaction conditions, calprotectin was seen as A9-A9 dimers on SDS gels and by whole protein mass spectrometry, and the linkage was characterized as a disulfide bond between two Cys2 residues. In support of our current work, we previously showed that the Cys2 of A9 is the residue in calprotectin that is most susceptible to oxidation [34,72]. Thus, it is likely that the Cys2 residues on a calprotectin heterotetramer can link neighbouring heterotetramers via disulfide bonds to form chains of calprotectin. This amalgamation of calprotectin heterotetramers would be expected to occur at low oxidant fluxes where some of the Cys2 residues are oxidized and then couple with adjacent non-oxidized Cys2 residues. Functionally, it is possible that these calprotectin chains would concentrate the metal binding capacity of the protein so that it competes more effectively with bacterial siderophores for essential metal ions.
However, when neutrophils generate high fluxes of oxidants, the function of calprotectin is expected to change because A8-A9 cross links will be favoured. Intermolecular cross-linking between A8 and A9 monomers is facilitated by the non-covalent heterodimeric and heterotetrameric structures of calprotectin. Cross-linking between A8 and A9 cysteine residues is unlikely to dramatically alter the quaternary structure of calprotectin, due to the close proximity of cysteine residues and flexible nature of the A9 cysteine close to the N-terminal (Fig. 1). The close proximity of A8 and A9 cysteine residues within the heterodimer, and their reactivity with hypochlorous acid in vivo, suggests that formation of A8-A9 disulfides may act as a redox switch, regulating the function of calprotectin at sites of infection and inflammation.
High oxidant fluxes will also facilitate proteolysis of calprotectin. We found that formation the A8-A9 cross links was sufficient to increase the susceptibly of calprotectin to proteolysis. This was apparent when comparing the effects of hypochlorous acid and hypothiocyanous acid on proteolysis. Hypochlorous acid increased susceptibility to proteolysis only at high oxidant ratios whereas a two-fold molar excess of hypothiocyanous acid to protein was sufficient to significantly increase protein degradation by the NGE. Hypochlorous acid reacts with a wide range of amino acid residues, and promoted formation of various oxidation products, such as A9-A9 and A8-A9 cross links, as well as oxidation and fragmentation of the A8 and A9 calprotectin subunits (Fig. 5). In contrast, hypothiocyanous acid reacts selectively with cysteine residues, and promoted formation of the A8-A9 cross link exclusively. The increased susceptibility of calprotectin oxidized with hypothiocyanous acid to proteolysis therefore suggests that the A8-A9 cross link alone is sufficient to enhance proteolysis. This could possibly be mediated by local unfolding of the protein as demonstrated by analytical ultracentrifugation showing broadening of the peak after treatment with a ten-fold molar excess of hypochlorous acid (Fig. 8G).
As a consequence of proteolysis, the metal ion binding capacity of calprotectin will be lost as it is slowly degraded. Calprotectin cleavage products may themselves have novel functions that warrant further investigation. For example, the A8 N-terminal peptide MLTELEKALNSIID has been detected in pancreatic cancer tissue and can impair glucose metabolism [73]. Calprotectin levels are currently monitored as a marker of neutrophil activation during inflammation, particularly as a fecal marker in IBD [[74], [75], [76], [77], [78]]. The increased susceptibility of oxidized calprotectin to proteolysis reported in this study is an important consideration when interpreting calprotectin levels measured by commercial ELISA. Extensive proteolysis of calprotectin at sites of inflammation, or during storage of clinical samples, will lower the calprotectin concentration so that its measurement will under-estimate the true level of inflammation.
The extent of cross-linking of calprotectin detected in vivo may depend on the amount of truncated A9 present. A9 exists as two isoforms, the full length and the truncated form. Full-length A9 accounts for approximately 72% of total A9 [63] and it is possible that the relative amount of truncated A9 may vary between individuals. The truncated A9 isoform has an alternate translation start site at the codon for Met4 and accordingly does not contain the Cys2 residue [79]. Truncated A9 would therefore be unable to cross-link with other A8 or A9 monomers via disulfide bonds. Calprotectin cross-linking or over-oxidation of the cysteine residue to sulfinic or sulfonic acid may result in either a loss or gain in protein function. Future studies assessing the proportions of truncated A9 in individuals would be interesting, as a higher proportion of truncated A9 may be advantageous or detrimental depending on how protein function is altered by oxidation.
A major strength of our work was that we used physiologically relevant calprotectin, oxidants and proteases. We used calprotectin purified from human neutrophils, which consisted of A8, full-length A9, and the truncated A9 isoform, employed the dominant neutrophil-derived oxidants, hypochlorous acid and hypothiocyanous acid, and investigated proteolysis using human neutrophil proteases. Our results are in-line with a just published study showing that the A8-A9 disulfide was more susceptible to proteolysis likely due to protein destabilisation [68]. However, Stephan et al. [68] used recombinant human calprotectin under non-physiological conditions, i.e. the protein was oxidized with 100 μM hydrogen peroxide and digested with trypsin. It is also important to note that in several previous studies recombinant calprotectin was used with mutated cysteine residues to avoid artefactual oxidation and its potential impact on function [[80], [81], [82]]. We propose that future studies should consider that calprotectin is readily oxidized at sites of infection and inflammation. Studying only the function of non-oxidized calprotectin may provide an incomplete picture of all physiologically relevant functions of this protein.
In conclusion, we have shown that the cysteine residues of calprotectin are readily oxidized by hypohalous acids and form cross-links in vivo. Formation of disulfide bonds between the subunits of calprotectin modifies the protein's structure and increases its susceptibility to proteolysis. Future work should be aimed at determining whether oligomers of calprotectin heterotetramers (A8/A9)2 are produced during inflammation, whether the A8-A9 cross link affects the metal binding capacity of calprotectin, and if the proteolytic peptides of calprotectin play a role in host defence.
Declarations of interest
None.
Funding
This research was supported by the Health Research Council of New Zealand (15/333). AREST CF is supported by grants from the National Health and Medical Research Council, Australia (403911, 458513, 1002035, 1102590), the Cystic Fibrosis Foundation, USA (CFFT SLY4AO and STICK09AO), and Cystic Fibrosis Australia (1021316).
Acknowledgements
We acknowledge Dr Louisa Ashby and Dr Heather Parker (Centre for Free Radical Research, University of Otago Christchurch) for preparing the neutrophil granule extract and S. aureus cultures, respectively. We also thank AstraZeneca (Uppsala, Sweden) for the gift of TX1.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101202.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Teigelkamp S. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J. Biol. Chem. 1991;266(20):13462–13467. [PubMed] [Google Scholar]
- 2.Striz I., Trebichavsky I. Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiol. Res. 2004;53(3):245–253. [PubMed] [Google Scholar]
- 3.Rammes A. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 1997;272(14):9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 4.Hetland G., Talgo G.J., Fagerhol M.K. Chemotaxins C5a and fMLP induce release of calprotectin (leucocyte L1 protein) from polymorphonuclear cells in vitro. Mol. Pathol. 1998;51(3):143–148. doi: 10.1136/mp.51.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besold A.N. Role of calprotectin in withholding zinc and copper from Candida albicans. Infect. Immun. 2017;86(2):1–16. doi: 10.1128/IAI.00779-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashige T.G. Nickel sequestration by the host-defense protein human calprotectin. J. Am. Chem. Soc. 2017;139(26):8828–8836. doi: 10.1021/jacs.7b01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakashige T.G. Human calprotectin is an iron-sequestering host-defense protein. Nat. Chem. Biol. 2015;11(10):765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brophy M.B., Hayden J.A., Nolan E.M. Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin. J. Am. Chem. Soc. 2012;134(43):18089–18100. doi: 10.1021/ja307974e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pruenster M. S100A8/A9: from basic science to clinical application. Pharmacol. Ther. 2016;167:120–131. doi: 10.1016/j.pharmthera.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Golden B.E. Calprotectin as a marker of inflammation in cystic fibrosis. Arch. Dis. Child. 1996;74(2):136–139. doi: 10.1136/adc.74.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray R.D. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J. Cyst. Fibros. 2010;9(3):193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Abildtrup M., Kingsley G.H., Scott D.L. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J. Rheumatol. 2015;42(5):760–770. doi: 10.3899/jrheum.140628. [DOI] [PubMed] [Google Scholar]
- 13.Jensen L.J. Plasma calprotectin levels reflect disease severity in patients with chronic heart failure. Eur. J. Prev. Cardiol. 2012;19(5):999–1004. doi: 10.1177/1741826711421078. [DOI] [PubMed] [Google Scholar]
- 14.Berg-Hansen P. Calprotectin levels in the cerebrospinal fluid reflect disease activity in multiple sclerosis. J. Neuroimmunol. 2009;216(1–2):98–102. doi: 10.1016/j.jneuroim.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Muller F. Elevated serum calprotectin levels in HIV-infected patients: the calprotectin response during ZDV treatment is associated with clinical events. J. Acquir. Immune Defic. Syndr. 1994;7(9):931–939. [PubMed] [Google Scholar]
- 16.Kristinsson J. Fecal excretion of calprotectin in colorectal cancer: relationship to tumor characteristics. Scand. J. Gastroenterol. 2001;36(2):202–207. doi: 10.1080/003655201750065979. [DOI] [PubMed] [Google Scholar]
- 17.Blanco-Prieto S. Serum calprotectin, CD26 and EGF to establish a panel for the diagnosis of lung cancer. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santamaria-Kisiel L., Rintala-Dempsey Anne C., Shaw Gary S. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006;396(Pt 2):201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Propper C. Analysis of the MRP8-MRP14 protein-protein interaction by the two-hybrid system suggests a prominent role of the C-terminal domain of S100 proteins in dimer formation. J. Biol. Chem. 1999;274(1):183–188. doi: 10.1074/jbc.274.1.183. [DOI] [PubMed] [Google Scholar]
- 20.Strupat K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 are confirmed by electrospray ionization-mass analysis. J. Am. Soc. Mass Spectrom. 2000;11(9):780–788. doi: 10.1016/S1044-0305(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 21.Hayden J.A. High-affinity manganese coordination by human calprotectin is calcium-dependent and requires the histidine-rich site formed at the dimer interface. J. Am. Chem. Soc. 2013;135(2):775–787. doi: 10.1021/ja3096416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagnon D.M. Manganese binding properties of human calprotectin under conditions of high and low calcium: X-ray crystallographic and advanced electron paramagnetic resonance spectroscopic analysis. J. Am. Chem. Soc. 2015;137(8):3004–3016. doi: 10.1021/ja512204s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampson A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy. 2000;30(Suppl 1):22–27. doi: 10.1046/j.1365-2222.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 24.Winterbourn C.C. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 2006;281(52):39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 25.Kettle A.J., Winterbourn C.C. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3(1):3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 26.Kettle A.J. Spectral and kinetic evidence for reaction of superoxide with compound I of myeloperoxidase. Free Radic. Biol. Med. 2011;51(12):2190–2194. doi: 10.1016/j.freeradbiomed.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Winterbourn C.C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim. Biophys. Acta. 1985;840(2):204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 28.Pattison D.I., Davies M.J., Hawkins C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012;46(8):975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 29.Zhou G.X., Liu Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017;18(9):495–503. doi: 10.1111/1751-2980.12540. [DOI] [PubMed] [Google Scholar]
- 30.Dickerhof N. Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radic. Biol. Med. 2017;113:236–243. doi: 10.1016/j.freeradbiomed.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Pillinger M.H., Abramson S.B. The neutrophil in rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 1995;21(3):691–714. [PubMed] [Google Scholar]
- 32.Nicholls S.J., Hazen S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 33.Wright H.L. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49(9):1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 34.Magon N.J. Oxidation of calprotectin by hypochlorous acid Prevents chelation of essential metal Ions and Allows bacterial growth: relevance to Infections in cystic fibrosis. Free Radic. Biol. Med. 2015:133–144. doi: 10.1016/j.freeradbiomed.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Gomes L.H. S100A8 and S100A9—oxidant scavengers in inflammation. Free Radic. Biol. Med. 2013;58(0):170–186. doi: 10.1016/j.freeradbiomed.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Storkey C., Davies M.J., Pattison D.I. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 2014;73:60–66. doi: 10.1016/j.freeradbiomed.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 37.Fu X., Mueller D.M., Heinecke J.W. Generation of intramolecular and intermolecular sulfenamides, sulfinamides, and sulfonamides by hypochlorous acid: a potential pathway for oxidative cross-linking of low-density lipoprotein by myeloperoxidase. Biochemistry. 2002;41(4):1293–1301. doi: 10.1021/bi015777z. [DOI] [PubMed] [Google Scholar]
- 38.Peskin A.V., Winterbourn C.C. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic. Biol. Med. 2001;30(5):572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 39.Morris J.C. The acid ionization constant of HOCl from 5 to 35°. J. Phys. Chem. 1966;70(12):3798–3805. [Google Scholar]
- 40.Beers R.F., Jr., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195(1):133–140. [PubMed] [Google Scholar]
- 41.Sly P.D. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 42.Khalilova I.S. A myeloperoxidase precursor, pro-myeloperoxidase, is present in human plasma and elevated in cardiovascular disease patients. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0192952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 44.Yousefi R. Human calprotectin: effect of calcium and zinc on its secondary and tertiary structures, and role of pH in its thermal stability. Acta Biochim. Biophys. Sin. (Shanghai) 2007;39(10):795–802. doi: 10.1111/j.1745-7270.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 45.Bozonet S.M. Hypothiocyanous acid is a potent inhibitor of apoptosis and caspase 3 activation in endothelial cells. Free Radic. Biol. Med. 2010;49(6):1054–1063. doi: 10.1016/j.freeradbiomed.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Dickerhof N. Potent inhibition of macrophage migration inhibitory factor (MIF) by myeloperoxidase-dependent oxidation of epicatechins. Biochem. J. 2014;462(2):303–314. doi: 10.1042/BJ20140612. [DOI] [PubMed] [Google Scholar]
- 47.Perugini M.A., Schuck P., Howlett G.J. Self-association of human apolipoprotein E3 and E4 in the presence and absence of phospholipid. J. Biol. Chem. 2000;275(47):36758–36765. doi: 10.1074/jbc.M005565200. [DOI] [PubMed] [Google Scholar]
- 48.Schuck P. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys. J. 2002;82(2):1096–1111. doi: 10.1016/S0006-3495(02)75469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laue T. Computer-aided interpretation of sedimentation data for proteins. In: Harding S.E., editor. Analytical Ultracentrifugation in Biochemistry and Polymer Sciences. 1992. [Google Scholar]
- 50.Thomas E.L., Bates K.P., Jefferson M.M. Peroxidase antimicrobial system of human saliva: requirements for accumulation of hypothiocyanite. J. Dent. Res. 1981;60(4):785–796. doi: 10.1177/00220345810600040401. [DOI] [PubMed] [Google Scholar]
- 51.Ihalin R., Loimaranta V., Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch. Biochem. Biophys. 2006;445(2):261–268. doi: 10.1016/j.abb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Seidel A. Uric acid and thiocyanate as competing substrates of lactoperoxidase. J. Biol. Chem. 2014;289(32):21937–21949. doi: 10.1074/jbc.M113.544957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magon N.J. Department of Pathology and Biomedical Science. University of Otago; 2013. Oxidative modification of calprotectin during inflammation: development as a biomarker. [Google Scholar]
- 54.Pattison D.I., Davies M.J. Kinetic analysis of the role of histidine chloramines in hypochlorous acid mediated protein oxidation. Biochemistry. 2005;44(19):7378–7387. doi: 10.1021/bi0474665. [DOI] [PubMed] [Google Scholar]
- 55.Hawkins C.L., Davies M.J. Hypochlorite-induced damage to proteins: formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem. J. 1998;332(Pt 3):617–625. doi: 10.1042/bj3320617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Domigan N.M. Chlorination of tyrosyl residues in peptides by myeloperoxidase and human neutrophils. J. Biol. Chem. 1995;270(28):16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 57.Szuchman-Sapir A.J. Site-specific hypochlorous acid-induced oxidation of recombinant human myoglobin affects specific amino acid residues and the rate of cytochrome b5-mediated heme reduction. Free Radic. Biol. Med. 2010;48(1):35–46. doi: 10.1016/j.freeradbiomed.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 58.Vogl T. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1999;10(11):1124–1130. doi: 10.1016/s1044-0305(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 59.Davies M.J. Protein oxidation and peroxidation. Biochem. J. 2016;473(7):805–825. doi: 10.1042/BJ20151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson G.B., Fudenberg H.H., Jahn T.L. Studies on cystic fibrosis using isoelectric focusing. I. An assay for detection of cystic fibrosis homozygotes and heterozygote carriers from serum. Pediatr. Res. 1975;9(8):635–640. doi: 10.1203/00006450-197508000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Shepherd C.E. Inflammatory S100A9 and S100A12 proteins in Alzheimer's disease. Neurobiol. Aging. 2006;27(11):1554–1563. doi: 10.1016/j.neurobiolaging.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 62.McCormick M.M. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J. Biol. Chem. 2005;280(50):41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 63.Lim S.Y. S-glutathionylation regulates inflammatory activities of S100A9. J. Biol. Chem. 2010;285(19):14377–14388. doi: 10.1074/jbc.M109.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leukert N. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J. Mol. Biol. 2006;359(4):961–972. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 65.Krause K.H. The calcium signal and neutrophil activation. Clin. Biochem. 1990;23(2):159–166. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 66.Pitanga T.N. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014;15:21. doi: 10.1186/1471-2121-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker H. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 2012;91(3):369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 68.Stephan J.R. Oxidative post-translational modifications accelerate proteolytic degradation of calprotectin. J. Am. Chem. Soc. 2018 Oct 31 doi: 10.1021/jacs.8b06354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4(5):278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 70.Nagy P., Jameson G.N.L., Winterbourn C.C. Kinetics and mechanisms of the reaction of hypothiocyanous acid with 5-Thio-2-nitrobenzoic acid and reduced glutathione. Chem. Res. Toxicol. 2009;22(11):1833–1840. doi: 10.1021/tx900249d. [DOI] [PubMed] [Google Scholar]
- 71.Skaff O., Pattison D.I., Davies M.J. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: absolute rate constants and assessment of biological relevance. Biochem. J. 2009;422(1):111–117. doi: 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- 72.Wilkie-Grantham R.P. Myeloperoxidase-dependent lipid peroxidation promotes the oxidative modification of cytosolic proteins in phagocytic neutrophils. J. Biol. Chem. 2015;290(15):9896–9905. doi: 10.1074/jbc.M114.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basso D. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin. Chim. Acta. 2006;372(1–2):120–128. doi: 10.1016/j.cca.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 74.Limburg P.J. Fecal calprotectin levels predict colorectal inflammation among patients with chronic diarrhea referred for colonoscopy. Am. J. Gastroenterol. 2000;95(10):2831–2837. doi: 10.1111/j.1572-0241.2000.03194.x. [DOI] [PubMed] [Google Scholar]
- 75.Diamanti A. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm. Bowel Dis. 2008;14(9):1229–1235. doi: 10.1002/ibd.20472. [DOI] [PubMed] [Google Scholar]
- 76.Kawashima K. Fecal calprotectin level correlated with both endoscopic severity and disease extent in ulcerative colitis. BMC Gastroenterol. 2016;16:47. doi: 10.1186/s12876-016-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Konikoff M.R., Denson L.A. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2006;12(6):524–534. doi: 10.1097/00054725-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 78.Gisbert J.P., McNicholl A.G. Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig. Liver Dis. 2009;41(1):56–66. doi: 10.1016/j.dld.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Kerkhoff C., Klempt M., Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim. Biophys. Acta Mol. Cell Res. 1998;1448(2):200–211. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- 80.Hunter M.J., Chazin W.J. High level expression and dimer characterization of the S100 EF-hand proteins, migration inhibitory factor-related proteins 8 and 14. J. Biol. Chem. 1998;273(20):12427–12435. doi: 10.1074/jbc.273.20.12427. [DOI] [PubMed] [Google Scholar]
- 81.Vogl T. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007;13(9):1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 82.Stephan J.R., Nolan E.M. Calcium-induced tetramerization and zinc chelation shield human calprotectin from degradation by host and bacterial extracellular proteases. Chem. Sci. 2016;7(3):1962–1975. doi: 10.1039/c5sc03287c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.