Abstract
Background
Low dual-task gait performance (the slowing of gait speed while performing a demanding cognitive task) is associated with low cognitive performance and an increased risk of progression to dementia in older adults with mild cognitive impairment. However, the reason for this remains unclear. This study aimed to examine the relationship between dual-task cost and regional brain volume, focusing on the hippocampus, parahippocampal gyrus, entorhinal cortex, and motor and lateral frontal cortices in older adults with mild cognitive impairment.
Methods
Forty older adults with mild cognitive impairment from the “Gait and Brain Study” were included in this study. Gait velocity was measured during single-task (ie, walking alone) and dual-task (ie, counting backwards, subtracting serial sevens, and naming animals, in addition to walking) conditions, using an electronic walkway. Regional brain volumes were derived by automated segmentation, using 3T magnetic resonance imaging.
Results
Partial rank correlation analyses demonstrated that a smaller volume of the left entorhinal cortex was associated with higher dual-task costs in counting backwards and subtracting serial sevens conditions. Subsequent logistic regression analyses demonstrated that a smaller volume of the left entorhinal cortex was independently associated with higher dual-task cost (slowing down >20% when performing cognitive task) in these two conditions. There were no other significant associations.
Conclusions
Our results show that lower dual-task gait performance is associated with volume reduction in the entorhinal cortex. Cognitive and motor dysfunction in older adults with mild cognitive impairment may reflect a shared pathogenic mechanism, and dual-task-related gait changes might be a surrogate motor marker for Alzheimer’s disease pathology.
Keywords: Dual task, Entorhinal cortex, Hippocampus, Gait
Mild cognitive impairment (MCI) is typically described as a clinical state between normal cognition in aging and very early dementia. Although older adults with MCI have an increased risk of progressing to Alzheimer’s disease (AD) and other dementias, almost one third of the older adults with MCI remain clinically stable or even revert to normal (1). Early detection of the risk factors associated with progression to dementia among older adults with MCI is, therefore, necessary for the appropriate targeting of prompt treatments.
Numerous studies have shown that slow gait is a risk factor associated with the development of MCI, dementia, and a more rapid rate of cognitive decline (2,3). This may be because gait control, which relies on executive function (4,5), shares common brain networks with the cognitive processes essential for the planning and monitoring of goal-directed behavior. Previous studies have elucidated this cognition-motor interaction using the dual-task gait paradigm (walking while performing a cognitive task) (4,6–8). These studies have revealed an association between slowing gait while performing dual tasking and lower level of cognitive function (9,10). It has been suggested that the dual-task gait paradigm examines the cognitive component of locomotion and can provide a window to the mechanism of gait control and cognitive performance (5,11). However, the longitudinal association between slowing gait while performing dual tasking and the risk of progression to dementia among older adults with MCI was, until recently, unknown.
A recent prospective study answered this question and demonstrated that a high dual-task gait cost (slowing down >20% when dual tasking compared with single tasking), which adjusts for an individual’s baseline gait velocity, was associated with a twofold to threefold increased risk of progression to dementia, whereas slow single-task gait velocity was not associated with progression to dementia (12). These results suggest that, among older adults with MCI, using solely a slow gait velocity threshold may be insufficient to identify individuals at higher risk of progression to dementia. In contrast, the dual-task paradigm could be applied as a clinical motor marker for early detection of older adults with MCI at higher risk of progression (12).
Considering the close association between high dual-task gait cost and progression to dementia, the pathways involved in these two processes may share the same neural pathology, including atrophy in the hippocampus, parahippocampal gyrus, and/or entorhinal cortex (13–17). Although the neural pathways underlying dual-task gait performance are not well established, for single-task gait, a slow gait speed has been related to specific cognition-related cerebral metabolic and structural abnormalities (18–22), and AD neuropathology (23). For example, studies using positron emission tomography showed that lower gait velocity was associated with lower glucose metabolism in the posterior cingulate cortex of older adults without a neurologic disease, which is an early sign of AD (20,21). More recently, a longitudinal study found that the association between gait slowing and cognitive impairment is supported by a shared neural substrate that includes a decreased volume of the right hippocampus (22).
Understanding the association between dual-task gait performance and regional brain volumes, vulnerable to AD pathology in older adults with MCI, may provide a neurological explanation for a high dual-task gait cost being associated with an increased risk of progression to dementia. The purpose of this study was to examine the neural substrate of dual-task gait cost, focusing on the medial temporal areas, particularly the hippocampus, parahippocampal gyrus, and entorhinal cortex, which are vulnerable regions in AD. We also introduced control regions of interest thought to be involved in motor control and dual tasking, including the motor and lateral frontal cortices, which completely overlap with the dorsolateral prefrontal cortex, and other neighboring regions such as the ventrolateral prefrontal and premotor cortices (24–26).
Methods
Participants
The Gait and Brain Study is a prospective cohort study designed to elucidate whether quantitative gait impairments can predict incident cognitive and mobility decline as well as progression to dementia among community-dwelling older adults without dementia at baseline (10). The experimental design and rationale have been described elsewhere (10,12). Participants were included in this study if they fulfilled MCI criteria and had 3T magnetic resonance imaging (MRI) of the brain performed during baseline assessment, introduced in the second wave of the “Gait and Brain Study” in 2010.
We defined MCI by a score of 0.5 on the clinical dementia rating scale and fulfillment of the following criteria: (a) subjective cognitive complaints; (b) objective cognitive impairment in at least one of the following cognitive domains: memory, executive function, attention, and language (27,28); (c) preserved daily activities, confirmed by clinician interviews; and (d) the absence of dementia, on the basis of criteria from the Diagnostic and Statistical Manual of Mental Disorders, fourth edition revised.
The exclusion criteria included diagnosis of a terminal illness, a life expectancy of less than 12 months, pending nursing home placement, hip or knee joint arthroplasty within the preceding 6 months, inability to walk 10 m independently without any gait aid, and the diagnosis of dementia.
Written informed consent was obtained from all participants prior to the study. The study was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki, and the research protocol was approved by the University of Western Ontario Health Sciences Research Ethics Board (clinicaltrials.gov identifier: NTC03020381).
Assessments
Comprehensive assessments, including gait performance, medical, and cognitive evaluations, were conducted at the Gait and Brain Lab, at Parkwood Institute, and brain MRI were performed at the Centre for Functional and Metabolic Mapping at the Robarts Research Institute using a 3T Tim Trio and 3T Magnetom Prisma MRI scanners (Siemens). Both centers are affiliated to the University of Western Ontario.
Quantitative gait assessment
Gait velocity (centimeter per second) was assessed during single and dual tasks using an electronic walkway (GaitRite Systems, 600 cm long and 64 cm wide; CIR Systems, Inc., Sparta, NJ). Start and end points were marked on the floor, 1 m from both the walkway start and end point, to avoid recording the participants’ acceleration and deceleration phases.
The single-task trial consisted of walking the length of the walkway at the participant’s usual pace. For the dual-task trials, participants walked at their usual pace with no instruction to prioritize the gait or cognitive task while performing the following cognitive tasks aloud: (a) counting backwards from one hundred by ones, (b) subtracting serial sevens from one hundred, or (c) naming animals. To balance and minimize the effects of learning and fatigue, the order of the single and dual tasks was randomized. The rationale and reliability behind the use of this protocol in people with MCI have been described elsewhere (29). To examine the effect of the addition of a cognitive task on gait performance, gait velocity in dual-task condition was compared with single-task condition (ie, the dual-task gait cost) using the following formula: ([single-task gait velocity − dual-task gait velocity]/single-task gait velocity) × 100. Participants who had a dual-task cost of more than 20% were considered to have a high dual-task cost (12). The gait assessments were conducted in a quiet, well-lit room, and participants wore comfortable footwear.
MRI scanning protocol and image processing
The MRI protocol included 3D T1-weighted magnetization-prepared rapid acquisition gradient echo images, with matrix size, 256 × 256 × 160; field of view 256 × 256 × 192 mm, repetition time = 2.3 ms, and echo time = 2.9 ms. All 3D T1-weighted MRI scans were visually inspected for abnormalities.
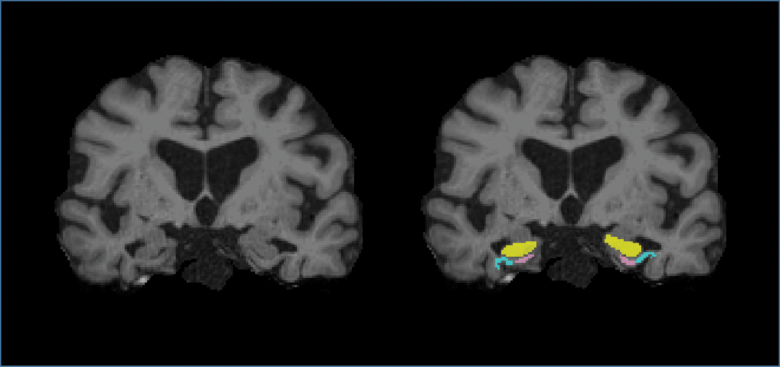
Subcortical structures including the hippocampus, parahippocampal gyrus, and entorhinal cortex (Figure 1), and regions of the motor and lateral frontal cortices were demarcated using the FreeSurfer software (version 5.3.0, available at http://surfer.nmr.mgh.harvard.edu/). A composite volume of the lateral frontal cortex was calculated by adding the volumes of superior, middle, and inferior frontal gyri, excluding the frontopolar and orbitofrontal regions of each hemisphere (30).
Figure 1.
Coronal T1-weighted magnetic resonance imaging after skull stripping in one participant showing example masks of the segmented hippocampus (yellow), parahippocampal gyrus (light blue), and entorhinal cortex (pink). Full color version is available within the online issue.
The initial processing of the 3D T1-weighted images included the following steps: removal of nonbrain tissue, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, intensity normalization, tessellation of the gray matter/white matter boundary, automated topology correction, and surface deformation to optimally place the gray matter/white matter and gray matter/cerebrospinal fluid boundaries. Subsequently, all participants’ data were visually evaluated to ensure that the target areas were correctly segmented. If errors were detected, the participants’ data were processed to correct misidentified regions using a statistical parametric mapping and checked visually for a second time.
Medical and cognitive assessments
Participants were interviewed on relevant sociodemographic and clinical variables. Global cognitive function was assessed by the Mini–Mental State Examination (31) and the Montreal Cognitive Assessment (MoCA) test (32), which examines six domains of overall cognitive function. Both tests have a maximum score of 30 points, with higher scores indicating higher overall cognitive function. Executive function and episodic memory were evaluated using the Trail-Making Test (TMT) (33) and the Rey Auditory Verbal Learning Test (RAVLT) (34). The TMT consists of parts A and B; TMT-A assesses visual search, motor speed skills, and attention, and TMT-B evaluates working memory and task shifting.
Data Analysis
The participants’ characteristics were summarized using means and SD or frequencies and percentages, as appropriate (Table 1). To assess associations between cerebral regions (hippocampus, parahippocampal gyrus, entorhinal cortex, and motor and lateral frontal cortices of each hemisphere) and gait variables (single- and dual-task gait velocity and dual-task cost), partial correlation analyses adjusted for intracranial volume were performed. In the case of dual-task costs, partial rank correlation analyses were used for all three conditions, due to their nonparametric distribution. A Bonferroni correction was applied to avoid type 1 error because correlation analyses were repeated 10 times for each gait variable (p = .05/10 = .005). Multiple regression for parametric variables (ie, gait velocity) and logistic regression analyses for nonparametric variables (ie, dual-task costs) were then performed to examine respective associations among regional brain volumes and gait variables on the basis of all resultant significant correlation coefficients. For the logistic regression analysis, dual-task cost > 20% was used as the dependent variable (12). The regression analyses were adjusted for age, sex, intracranial volume, education level, and MoCA score. Statistical analyses were conducted using SPSS 21.0 (IBM Corporation, Chicago, IL).
Table 1.
Participants’ Characteristics
| Variables | Participants in this Study (n = 40) |
|---|---|
| Age (y), mean (SD) | 74.2 (6.0) |
| Females, n (%) | 17 (42.5) |
| Education (y), mean (SD) | 13.3 (2.6) |
| Total number of medications taken | 8.0 (4.3) |
| Total number of comorbidities | 5.8 (2.8) |
| Previous fall, n (%) | 8 (20.0) |
| GDS, mean (SD) | 2.3 (2.2) |
| MMSE, mean (SD) | 27.7 (2.4) |
| MoCA, mean (SD) | 23.7 (3.7) |
| Rey auditory verbal learning (/15), mean (SD)a | 6.8 (2.0) |
| Rey auditory verbal learning (/45), mean (SD)b | 16.8 (5.1) |
| TMT-A (s), mean (SD) | 44.5 (15.5) |
| TMT-B (s), mean (SD) | 142.9 (151.9) |
| Amnestic-MCI single domain, n (%) | 15 (37.5) |
| Amnestic-MCI multiple domains, n (%) | 4 (10.0) |
| Gait velocity (cm/s), mean (SD) | |
| Single gait | 110.1 (20.5) |
| Counting gait | 106.3 (26.2) |
| Serial sevens gait | 94.7 (31.3) |
| Naming animals gait | 96.1 (28.1) |
Note: GDS = Geriatric Depression Scale; MCI = mild cognitive impairment; MMSE = Mini–Mental State Examination; MoCA = Montreal Cognitive Assessment; TMT = Trail-Making Test.
aFinal score is the number of words remembered out of a list of 15 in trial 3 (delayed recall). bFinal score is the total number of words remembered for trials 1–3.
Results
Although none of the participants met the exclusion criteria, two participants were excluded due to anatomical abnormalities causing mis-segmentation of target areas during image processing. Thus, a total of 40 Caucasian older adults with MCI were included in these analyses. Demographic and clinical characteristics are presented in Table 1. The mean age was 74.2 ± 6.0 years, and 42.5% of the participants were women. Performance during cognitive testing revealed that half of the participants exhibited amnestic-MCI, single and multiple domains (37.5% and 10%, respectively). Mean score of the RAVLT in the delayed recall was 6.8 out of 15. Aligned with the good functionality of this cohort, the mean single-task gait velocity was 110.1 cm/s, above the accepted cutoff for impairment (35).
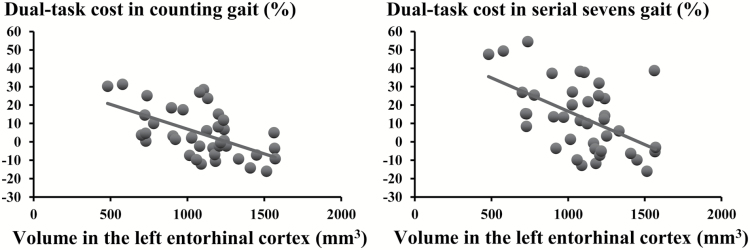
The results of the partial correlation analyses for regional brain volumes and gait variables are presented in Table 2. A lower volume of the left entorhinal cortex was significantly associated with slower dual-task gait velocity under all three conditions and a higher dual-task gait cost in counting backwards and subtracting serial sevens conditions (Figure 2). There were no significant associations between gait variables and other regional brain volumes.
Table 2.
Correlation Coefficient Matrix of Gait Variables and Regional Brain Volumes
| Left Hemisphere | Right Hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | PHG | EC | MC | LFC | HC | PHC | EC | MC | LFC | |
| Gait velocitya | ||||||||||
| Single gait | .05 | .36 | .43 | .10 | .33 | .09 | .32 | .29 | .23 | .19 |
| Counting gait | .14 | .32 | .56* | −.02 | .26 | .17 | .36 | .40 | .12 | .13 |
| Serial sevens gait | .09 | .29 | .48* | −.08 | .24 | .13 | .27 | .33 | .18 | .18 |
| Naming animals gait | .13 | .23 | .55* | −.10 | .22 | .18 | .23 | .34 | .19 | .12 |
| Dual-task costb | ||||||||||
| Counting gait | −.21 | −.19 | −.49* | .21 | −.07 | −.27 | −.17 | −.37 | .12 | .02 |
| Serial sevens gait | −.16 | −.28 | −.46* | .20 | −.10 | −.21 | −.10 | −.27 | .01 | −.02 |
| Naming animals gait | .01 | −.01 | −.30 | .21 | .04 | −.11 | .08 | −.21 | −.08 | −.02 |
Note: EC = entorhinal cortex; HC = hippocampus; LFC = lateral frontal cortex; MC = motor cortex; PHG = parahippocampal gyrus. Correlation coefficients were adjusted for intracranial volume.
aPartial correlation analysis. bPartial rank correlation analysis.
*p < .005.
Figure 2.
Scatter graphs of association between dual-task cost in counting backwards and subtracting serial sevens conditions and volume in the left entorhinal cortex.
When regression analyses were performed on all significant correlation coefficients, results remained the same: The lower left entorhinal volume was associated with slower gait velocities on all dual tasks and a higher dual-task gait cost in counting backwards and subtracting serial sevens. This association remained significant after adjusting for MoCA score, a measure of global cognition (Table 3).
Table 3.
Results of the Multiple Linear Regression and Logistic Regression Analyses for Gait Variables and Volume of the Entorhinal Cortex
| Dependent Variable | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β | p (95% CI) | β | p (95% CI) | |
| Gait velocitya | ||||
| Counting gait velocity | 0.05 | .01 (0.02–0.09) | 0.05 | .01 (0.02–0.09) |
| Serial sevens velocity | 0.06 | .02 (0.01–0.10) | 0.05 | .03 (0.01–0.10) |
| Naming animals velocity | 0.06 | .01 (0.02–0.10) | 0.06 | .01 (0.02–0.10) |
| OR | p (95% CI) | OR | p (95% CI) | |
| Dual-task costb | ||||
| Counting gait velocity (reference, DTC < 20%) | 1.04 | .02 (1.01–1.02) | 1.02 | .02 (1.00–1.04) |
| Serial sevens velocity (reference, DTC < 20%) | 1.03 | .02 (1.01–1.03) | 1.01 | .02 (1.00–1.03) |
Note: CI = confidence interval; DTC = dual-task cost; OR = odds ratio. Model 1: adjusted for age, sex, and intracranial volume; Model 2: adjusted for age, sex, intracranial volume, years of education, and Montreal Cognitive Assessment.
aMultiple linear regression analysis. bLogistic regression analysis.
Discussion
Our findings reveal that, in a well-characterized cohort of older adults with MCI, a lower volume of the left entorhinal cortex is associated with slow dual-task velocity and higher dual-task gait cost, but not with single-task slow gait. This brain region may be key in explaining the link between dual-task gait performance and progression to dementia.
Our results are aligned with the recent finding that increased dual-task gait cost is associated with an increased risk of progression to dementia in older adults with MCI (12). Recently, lower hippocampal volume was associated with slowing gait in a large-population cohort study (22). This may suggest that dual-task gait performance could be an early motor manifestation of entorhinal cortical atrophy, and when the hippocampal volume is involved, slowing of single gait may become subsequently evident.
Although it is well recognized that both the entorhinal cortex and the hippocampus are affected early in AD pathology (13,14) and that MRI shows abnormalities at very early stages (15–17), a growing body of literature indicates that entorhinal cortex atrophy precedes hippocampal atrophy in pathological aging (36–39). Furthermore, in older adults with MCI, the entorhinal cortex had greater volume loss than the hippocampus (38), and both the volume and rate of atrophy in the entorhinal cortex, but not the hippocampus, were associated with progression to AD (39). Because volume reduction in the entorhinal cortex may be an early sign of AD pathology, our findings may provide an anatomical substrate to the recent finding that lower dual-task gait performance is associated with progression from MCI to dementia (12). Although the association of lower volume in the entorhinal cortex with higher dual-task gait cost was evident in MCI cases, caution should be exercised when drawing conclusions because this association might only be observed in those with a higher risk or progression to dementia.
We found that the relationship between lower volume in the entorhinal cortex and higher dual-task gait cost was observed in the left hemisphere only. It has been suggested that the left entorhinal cortex plays an important role in memory performance (40,41), which would support the concept that other cognitive domains beyond executive function (eg, memory) are involved in controlling and maintaining a safe gait in MCI. A previous study has shown a close association between dual-task gait cost and performance in delayed recall among MCI participants (10). Therefore, lower volume of the entorhinal cortex might affect dual-tasking ability through memory decline.
In the present study, single-task gait velocity did not show any association with regional brain volumes. This is inconsistent with previous findings that slower gait velocity was associated with reduced gray matter volume in the prefrontal regions and medial temporal area (18,19,22). A possible explanation is that dual-task gait may uncover valuable subtleties regarding the role of cognitive control in a participant’s gait, which gait velocity alone may not be able to capture, particularly in high-functioning older adults, as in our sample (12).
Although our study provides novel evidence of a potential mechanism underlying the relationship between the aging brain and dual-task gait performance among older adults with MCI, some studies have raised the possibility that brain areas other than the entorhinal cortex are involved in controlling dual tasking in MCI populations. A study using magnetic resonance spectroscopy and MRI showed that the metabolite ratios and volume of the primary motor cortex were associated with gait performance during the dual-task condition (26). More recently, it was shown that in older adults with MCI a slower gait velocity during the dual-task condition was associated with a decreased volume in several cerebral regions, including the medial frontal gyrus, superior frontal gyrus, and cingulate (42). Future studies are needed to examine the interactive effects of atrophy in each brain region on the progression to dementia among older adults with MCI, focusing on its association with dual-task gait performance.
The strength of this study is that it is the first to assess the relationship between dual-task gait velocity cost and volume in the medial temporal areas, which are vulnerable regions in AD pathology, in a well-characterized MCI cohort with detailed evaluations. However, there are limitations that need to be considered when interpreting the results. First, the cross-sectional study design precludes our ability to assess causality. Second, we included a relatively small number of participants, which may affect the statistical power of the associations between additional brain regions and dual-task gait performance. Furthermore, our sample was composed of relatively well-educated and high-functioning older adults (ie, mean gait velocity > 100 cm/s), which limits the generalizability of our results. Third, because the present study investigated the association of dual-task cost with regional brain volume, specifically, medial temporal area, we did not perform whole-brain analysis including ventricular volume (43,44), which raises the possibility of type 1 error. Fourth, having the cost in the cognitive component of dual tasking in our cohort could have improved our findings by considering cognitive errors on dual tasking. Finally, cross-validation of our findings may be required from other MCI cohorts.
Conclusion
The present study reveals that a lower volume of the entorhinal cortex is associated with poor dual-task gait performance in older adults with MCI. Cognitive and motor dysfunction in older adults with MCI may reflect a shared pathogenic mechanism at the brain level, and dual-task-related gait changes may be a surrogate motor marker for AD pathology, such as entorhinal atrophy. Further longitudinal studies are needed to confirm our results in a larger population.
Funding
This article is funded by a project grant from Canadian Institutes of Health and Research-Institute of Aging (CIHR; PJT 153100).
Conflict of Interest
None reported.
Acknowledgments
The authors thank Samaneh Kazemifar for her help in postprocessing brain MRI data and Dr. Yanina Sarquis-Adamson for her help in proof-editing this manuscript. Dr. Ryota Sakurai holds a scholarship award from the Japan Society for the Promotion of Science (JSPS) while conducting postdoctoral research at the Gait and Brain Lab, University of Western Ontario. Dr. Montero-Odasso’s program in “Gait and Brain Health” is supported by grants from the Canadian Institute of Health and Research (CIHR), the Ontario Ministry of Research and Innovation, the Ontario Neurodegenerative Diseases Research Initiative (ONDRI), the Canadian Consortium on Neurodegeneration in Aging (CCNA), and the Department of Medicine Program of Experimental Medicine (POEM) Research Award, University of Western Ontario. He is the first recipient of the Schulich Clinician-Scientist Award and holds the CIHR Investigator Award.
References
- 1. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi:10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 2. Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50:1496–1498. doi:10.1212/WNL.50.5.1496 [DOI] [PubMed] [Google Scholar]
- 3. Verghese J, Annweiler C, Ayers E et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83:718–726. doi:10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi:10.1007/s00221-005-2280-3 [DOI] [PubMed] [Google Scholar]
- 5. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9:41. doi:10.1186/1471-2318-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93:293–299. doi:10.1016/j.apmr.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 8. Beauchet O, Launay CP, Sekhon H et al. Association of increased gait variability while dual tasking and cognitive decline: results from a prospective longitudinal cohort pilot study. GeroScience. 2017. [Epub ahead of print]. doi:10.1007/s11357-017-9992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doi T, Shimada H, Makizako H et al. Cognitive function and gait speed under normal and dual-task walking among older adults with mild cognitive impairment. BMC Neurol. 2014;14:67. doi:10.1186/1471-2377-14-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montero-Odasso M, Oteng-Amoako A, Speechley M et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–1421. doi:10.1093/gerona/glu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hausdorff JM, Buchman AS. What links gait speed and MCI with dementia? A fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci. 2013;68:409–411. doi:10.1093/gerona/glt002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montero-Odasso MM, Sarquis-Adamson Y, Speechley M et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol. 2017;74:857–865. doi:10.1001/jamaneurol.2017.0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi:10.1126/science.6474172 [DOI] [PubMed] [Google Scholar]
- 14. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi:10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- 15. Dickerson BC, Goncharova I, Sullivan MP et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. doi:10.1016/S0197-4580(01)00271-8 [DOI] [PubMed] [Google Scholar]
- 16. Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59:673–681. doi:10.1002/ana.20799 [DOI] [PubMed] [Google Scholar]
- 17. Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging. 2011;32:1733–1741. doi:10.1016/j.neurobiolaging.2009.11.008 [DOI] [PubMed] [Google Scholar]
- 18. Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi:10.1093/gerona/63.12.1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62:1048–1055.doi:10.1093/gerona/62.9.1048 [DOI] [PubMed] [Google Scholar]
- 20. Sakurai R, Fujiwara Y, Yasunaga M et al. Regional cerebral glucose metabolism and gait speed in healthy community-dwelling older women. J Gerontol A Biol Sci Med Sci. 2014;69:1519–1527. doi:10.1093/gerona/glu093 [DOI] [PubMed] [Google Scholar]
- 21. Sakurai R, Ishii K, Yasunaga M et al. The neural substrate of gait and executive function relationship in elderly women: a PET study. Geriatr Gerontol Int. 2017;17:1873–1880. doi:10.1111/ggi.12982 [DOI] [PubMed] [Google Scholar]
- 22. Rosso AL, Verghese J, Metti AL et al. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology. 2017;89:336–342. doi:10.1212/WNL.0000000000004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wennberg AMV, Lesnick TG, Schwarz CG et al. Longitudinal association between brain amyloid beta and gait in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2017. [Epub ahead of print]. doi:10.1093/gerona/glx240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mirelman A, Maidan I, Bernad-Elazari H et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. doi:10.1186/ 1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66:879–887. doi:10.1093/gerona/glr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Annweiler C, Beauchet O, Bartha R et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain. 2013;136:859–871. doi:10.1093/brain/aws373 [DOI] [PubMed] [Google Scholar]
- 27. Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi:10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 28. Winblad B, Palmer K, Kivipelto M et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi:10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- 29. Montero-Odasso M, Casas A, Hansen KT et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. doi:10.1186/1743-0003-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gautam P, Cherbuin N, Sachdev PS, Wen W, Anstey KJ. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: the PATH through life study. Neuroimage. 2011;55:845–855. doi:10.1016/j.neuroimage.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 31. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198.doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 32. Nasreddine ZS, Phillips NA, Bédirian V et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi:10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 33. Spreen O, Strauss E.. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1998:533–547. [Google Scholar]
- 34. Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Association; 1996. [Google Scholar]
- 35. Studenski S. Bradypedia: is gait speed ready for clinical use?J Nutr Health Aging. 2009;13:878–880. doi:10.1007/s12603-009-0245-0 [DOI] [PubMed] [Google Scholar]
- 36. Killiany RJ, Hyman BT, Gomez-Isla T et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi:10.1212/WNL.58.8.1188 [DOI] [PubMed] [Google Scholar]
- 37. deToledo-Morrell L, Stoub TR, Bulgakova M et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–1203. doi:10.1016/j.neurobiolaging.2003.12.007 [DOI] [PubMed] [Google Scholar]
- 38. Pennanen C, Kivipelto M, Tuomainen S et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi:10.1016/S0197-4580(03)00084-8 [DOI] [PubMed] [Google Scholar]
- 39. Stoub TR, Bulgakova M, Leurgans S et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi:10.1212/01.WNL.0000160089.43264.1A [DOI] [PubMed] [Google Scholar]
- 40. McDonald CR, Gharapetian L, McEvoy LK et al. ; Alzheimer’s Disease Neuroimaging Initiative Relationship between regional atrophy rates and cognitive decline in mild cognitive impairment. Neurobiol Aging. 2012;33:242–253. doi:10.1016/j.neurobiolaging.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eustache F, Desgranges B, Giffard B, de la Sayette V, Baron JC. Entorhinal cortex disruption causes memory deficit in early Alzheimer’s disease as shown by PET. Neuroreport. 2001;12:683–685. doi:10.1097/00001756-200103260-00013 [DOI] [PubMed] [Google Scholar]
- 42. Doi T, Blumen HM, Verghese J et al. Gray matter volume and dual-task gait performance in mild cognitive impairment. Brain Imaging Behav. 2017;11:887–898. doi:10.1007/s11682-016-9562-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Annweiler C, Beauchet O, Bartha R, Montero-Odasso M. Slow gait in MCI is associated with ventricular enlargement: results from the Gait and Brain Study. J Neural Transm. 2013;120:1083–1092. doi:10.1007/s00702-012-0926-4 [DOI] [PubMed] [Google Scholar]
- 44. Annweiler C, Montero-Odasso M, Bartha R, Drozd J, Hachinski V, Beauchet O. Association between gait variability and brain ventricle attributes: a brain mapping study. Exp Gerontol. 2014;57:256–263. doi:10.1016/j.exger.2014.06.015 [DOI] [PubMed] [Google Scholar]