Abstract
Aims
To update pretest probabilities (PTP) for obstructive coronary artery disease (CAD ≥ 50%) across age, sex, and clinical symptom strata, using coronary computed tomography angiography (CTA) in a large contemporary population of patients with stable chest pain referred to non-invasive testing.
Methods and results
We included patients enrolled in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial and randomized to CTA. Exclusively level III-certified readers, blinded to demographic and clinical data, assessed the prevalence of CAD ≥ 50% in a central core lab. After comparing the recent European Society of Cardiology-Diamond and Forrester PTP (ESC-DF) with the actual observed prevalence of CAD ≥ 50%, we created a new PTP set by replacing the ESC-DF PTP with the observed prevalence of CAD ≥ 50% across strata of age, sex, and type of angina. In 4415 patients (48.3% men; 60.5 ± 8.2 years; 78% atypical angina; 11% typical angina; 11% non-anginal chest pain), the observed prevalence of CAD ≥ 50% was 13.9%, only one-third of the average ESC-DF PTP (40.6; P < 0.001 for difference). The PTP in the new set ranged 2–48% and were consistently lower than the ESC-DF PTP across all age, sex, and angina type categories. Initially, 4284/4415 (97%) patients were classified as intermediate-probability by the ESC-DF (PTP 15–85%); using the PROMISE-PTP, 50.2% of these patients were reclassified to the low PTP category (PTP < 15%).
Conclusion
The ESC-DF PTP overestimate vastly the actual prevalence of CAD ≥ 50%. A new set of PTP, derived from results of non-invasive testing, may substantially reduce the need for non-invasive tests in stable chest pain.
Keywords: Diamond and Forrester, computed tomography angiography, coronary stenosis, obstructive coronary artery disease, stable chest pain
Introduction
In 1979, 18 years after the first publication of major cardiovascular risk factors by the Framingham Heart Study1 and 2 years after the introduction of balloon angioplasty, Diamond and Forrester (DF) proposed a set of age, sex, and symptom-based pretest probabilities (PTP) to estimate the presence of obstructive coronary artery disease (CAD ≥ 50%).2 Notably, these PTP were derived from the observed prevalence of CAD ≥ 50% in invasive coronary angiography (ICA) or autopsy.
There are several lines of evidence suggesting that these PTP may overestimate the presence of CAD in patients referred to non-invasive testing. For instance in CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry), Cheng et al.3 have reported an overestimation across all clinical categories as detected in computed tomography angiography (CTA) site reads.
Along the major American societies,4,5 the European Society of Cardiology (ESC) has updated the PTP tables as presented in the current (2013) guidelines.6,7 Even though the update used more recent populations, it was based on ICA results, in general data generated in a population with increased probability to present with obstructive CAD. Thus, further studies are needed to investigate rather the revised version of the ESC-DF PTP still overestimates the actual prevalence of obstructive disease in patients referred to non-invasive testing. Furthermore, a re-evaluation of the PTP for obstructive CAD in a population referred to non-invasive tests is needed to address the methodological issues (e.g. invasive test results as reference).
Such opportunity was offered through the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial (NCT01174550 ClinicalTrials.gov), a prospective randomized multicentre trial including a protocol-defined capture of the pre-test data and state of the art CTA data for detection of the prevalence of CAD ≥ 50%.
Therefore, the goal of this research was to (i) compare the most recent version of the ESC-DF PTP with the observed prevalence of CAD ≥ 50% in a large contemporary population of symptomatic patients referred to non-invasive diagnostic testing (CTA), (ii) derive a new set of PTP, based on the non-invasive test results, and (iii) determine potential implications for primary non-invasive and downstream invasive tests and interventions.
Methods
Study population
The PROMISE trial is a pragmatic randomized comparative effectiveness trial that enrolled 10 003 patients (men ≥45 years and women ≥50 years of age) from 193 North American sites, who required non-invasive testing (i.e. intermediate PTP category) to determine presence of obstructive CAD or myocardial ischaemia. Patients were randomized 1:1 to receive functional or anatomical (i.e. CTA) diagnostic testing. Those with known CAD, acute or unstable presentation, or contraindications for CTA were excluded.
For this research, we included patients who underwent coronary CTA as their first diagnostic test. Patients who did not undergo any diagnostic test, received an examination other than coronary CTA, received non-contrast calcium scoring testing only, or those for whom coronary CTA datasets were unavailable or non-diagnostic were excluded (Figure 1). Local or central institutional review boards approved the trial, and all patients provided written informed consent.
Figure 1.
CONSORT diagram. CTA, computed tomography angiography; PROMISE, Prospective Multicenter Imaging Study for Evaluation of Chest Pain.
Demographic and clinical data
The capture of all demographic and clinical data was defined prospectively, as previously described in the PROMISE trial rationale/design publication and corresponding trial protocol.8,9 Apart from age and sex, the collected data also included the type of chest pain at presentation, defined as typical angina, atypical angina, or non-anginal chest pain, according to current European Society of Cardiology (ESC) and American College of Cardiology/American Heart Association (ACC/AHA) guidelines.4,6 Adhering to these guidelines, the presence of dyspnoea was not a part of the chest pain classification.
Prevalence of the obstructive CAD based on standardized expert core lab reads of coronary CTA
Coronary CTA images were acquired using standard electrocardiogram-gated or triggered protocols according to the Society of Cardiac Computed Tomography (SCCT) guidelines.10 In a central core lab, six level-III-certified readers were randomly assigned CTA datasets for analysis. The datasets were evaluated for the presence of obstructive CAD in every coronary segment.11 All readers were blinded to the demographic and clinical data. All patients presenting with at least one coronary stenosis ≥50%, in any vessel with a caliber of >2 mm, were considered as having CAD ≥ 50%. In a sensitivity analysis, we assessed CAD ≥ 70%, defined as the presence of at least one ≥70% stenosis or ≥50% left main stenosis. In a Supplementary data online, Table, we list the prevalence of non-obstructive CAD (1–49% stenosis12) across age, sex, and clinical symptom strata. The readers reached a good agreement, as determined in a random subset of 50 CTA datasets (Kappa = 0.69).13
Creating a new PROMISE-PTP set based on the CTA results
Using the prospectively captured demographic and clinical pre-test data and analogous to the updated (2013) ESC-DF tables, we assigned patients to individual groups across age, sex, and symptom strata. After assignment, we used the core lab-observed prevalence of CAD ≥ 50% in individual groups as the new PROMISE-PTP. Of note, we pooled the ESC-DF age classes 70–79 and ≥80 years due to a small number of patients ≥80 years of age.
According to the ESC guideline recommendations, we categorized patients with PROMISE-PTP < 15% as low, 15–85% as intermediate, and >85% as a high probability for having CAD ≥ 50%.6 These guidelines suggest that patients with low probability do not need further diagnostic testing or ICA, while those with intermediate probability should undergo non-invasive testing, and individuals in the high-probability category should be referred directly to ICA.6
The ESC-DF PTP for obstructive CAD as a comparator
We assigned a ESC-DF PTP value to each patient and categorized all patients to low, intermediate, and high PTP (<15%, 15–85%, and >85%, respectively).6 In a supplemental analysis, we also provided data on the DF/Coronary Artery Surgery Study risk score (DF/CASS) method (Supplementary data online, Table S1), which is recommended by the American College of Cardiology/American Heart Association (ACC/AHA) and the Society of Cardiac Computed Tomography (SCCT) guidelines.4,14
Potential implications of incorporating the PROMISE-PTP into clinical decision making
In an exploratory analysis, we investigated the presence of high-risk anatomy in the patients who were down classified to the low-PTP category as well as the potential impact of the corrected PROMISE-PTP on resource utilization (i.e. downstream ICA, revascularization) and the 2-year event rate. High-risk anatomy was defined as the presence of three-vessel disease (≥50% in all three major territories) or a left main stenosis ≥50% in the coronary CTA. We also provide data on high-risk anatomy which additionally includes the proximal left anterior descending. Events were defined as the composite of time to death from cardiovascular cause, or non-fatal myocardial infarction.8
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range) as appropriate. The primary statistical strategy consisted of a tabulation of observed prevalence of obstructive CAD across age, sex, and clinical symptom strata. All prevalence values included 95% confidence intervals (CIs) to account for the sample size in individual groups (Supplementary data online, Table S2). The differences between ESC-DF PTP and the observed prevalence of obstructive CAD within individual patient groups were quantified as ratios (i.e. ESC-DF PTP/observed prevalence), testing for differences using Kruskal–Wallis. We used Stata 14.0 (StataCorp LP, College Station, TX, USA) for all analyses and considered two-sided P-values of <0.05 as statistically significant.
Results
Patient demographics
Out of the 10 003 patients enrolled in PROMISE, 4996/10 003 (50%) were randomized to the anatomical testing arm, of whom 4686/4996 (93.8%) underwent CTA as the first test, and 4415/4686 (94.2%) were available for the analysis in the central core lab (Consort diagram, Figure 1). Within the final analysis cohort of 4415 patients [average age 60.5 ± 8.2 years; 2283/4415 (51.7%) women] 969/4415 (22.0%) patients self-reported themselves as a racial or ethnic minority. The most common presenting symptom was atypical angina [3440/4415 (77.9%)], and dyspnoea was recorded in 625/4412 (14.2%) patients and was most frequently seen in patients with atypical angina (81.9%) (Table 1).
Table 1.
Patient characteristics
| Clinical/ demographic characteristic | (n = 4415) |
|---|---|
| Age (years), mean ± SD | 60.5 ± 8.2 |
| Men, n (%) | 2283 (51.7) |
| Clinical symptoms, n (%) | |
| Non-anginal chest pain | 472 (10.7) |
| Atypical angina | 3440 (77.9) |
| Typical angina | 503 (11.4) |
| Risk factors | |
| Framingham Risk Score, mean ± SD | 21.2 ± 14.8 |
| Body-mass-index, n (%) | 30.4 (5.9) |
| Hypertension, n (%) | 2829 (64.1) |
| Diabetes mellitus, n (%) | 908 (20.6) |
| Dyslipidaemia, n (%) | 2965 (67.2) |
| Family CAD history, n (%) | 1441 (32.7) |
| PAD or cerebrovascular disease, n (%) | 221 (5.0) |
| Metabolic syndrome, n (%) | 1630 (36.9) |
| Smoker (current or past), n (%) | 2256 (51.1) |
| Sedentary lifestyle, n (%) | 2280 (51.8) |
| Depression, n (%) | 868 (19.7) |
| ECG abnormalitiesa, n (%) | 340 (7.9) |
| Racial or ethnic minority groupb, n (%) | 969 (22.0) |
| Relevant medication, n (%) | |
| Beta-blocker | 1041 (24.7) |
| ACE inhibitor or ARB | 1811 (42.9) |
| Statin | 1926 (45.6) |
| Aspirin | 1905 (45.1) |
Includes left branch bundle block, Q-wave, ST-depression, left-ventricular hypertrophy signs. Information available in 4282 patients.
Racial or ethnic minority group was self-reported, with the status of ‘minority’ being defined by the patient (available in 4370 patients).
ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; BMI, body mass index; CAD, coronary artery disease; CTA, computed tomography angiography; ECG, electrocardiogram; PAD, peripheral arterial disease.
The observed prevalence of obstructive CAD and the new PROMISE-PTP set
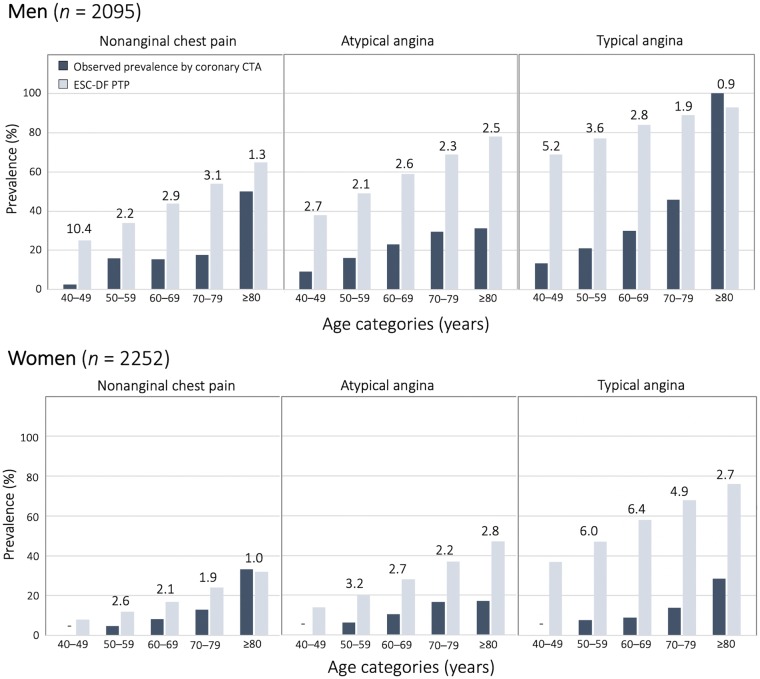
Based on the standardized expert assessment of coronary CTA, the average observed prevalence of CAD ≥ 50% within the population was 13.9% (615/4415). The prevalence increased with age in both men and women and was twice as high in men compared with women [18.7%; 398/2132 vs. 9.5%; 217/2283 (P < 0.001)]. In men, the prevalence of CAD ≥ 50% increased significantly from non-anginal to atypical to typical angina symptom categories (P = 0.002), while there was no difference based on the clinical presentation in women (P = 0.694). In the subanalysis investigating obstructive disease 70%, the observed prevalence of CAD ≥ 70% was 6.1% (270/4415), and the overestimations were observed across all individual patient groups similar to the CAD ≥ 50%.
Reflecting the observed prevalence of CAD ≥ 50%, the PTP within the groups in the new PROMISE-PTP set ranged between 2% and 48% across age, sex, and symptom-based strata (Table 2). Applying the ESC recommended PTP thresholds (i.e. <15%, 15–85%, >85%) in the new PROMISE-PTP table, 2258/4415 (51.1%) patients were classified as low-probability, 2082/4415 (48.9%) as intermediate-probability, and there were no patients in the high-probability category (Table 2). Of note, all men under the age of 50 years and all women under the age of 70 years were categorized as low-probability (<15%) regardless of symptoms. Those >50 years and >70 years were classified as intermediate-probability, while no group of patients had a PTP >85% and hence there was no high-probability category.
Table 2.
Overview of the traditional ESC-DF PTP and the corrected PROMISE PTP based on the observed prevalence in patients referred to non-invasive testing
| Sex/age (years) | Non-anginal chest pain (%) | Atypical angina (%) | Typical angina (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESC-DF PTP | |||||||||||
| Men | |||||||||||
| 30–39 | 18 | 29 | 59 | ||||||||
| 40–49 | 25 | 38 | 69 | ||||||||
| 50–59 | 34 | 49 | 77 | ||||||||
| 60–69 | 44 | 59 | 84 | ||||||||
| 70–79 | 54 | 69 | 89 | ||||||||
| ≥80 | 65 | 78 | 93 | ||||||||
| Women | |||||||||||
| 30–39 | 5 | 10 | 28 | ||||||||
| 40–49 | 8 | 14 | 37 | ||||||||
| 50–59 | 12 | 20 | 47 | ||||||||
| 60–69 | 17 | 28 | 58 | ||||||||
| 70–79 | 24 | 37 | 68 | ||||||||
| ≥80 | 32 | 47 | 76 | ||||||||
| PROMISE PTP CAD ≥ 50% | |||||||||||
| Men | |||||||||||
| 30–39a | |||||||||||
| 40–49 | 2 (0.1–12.6) | 9 (5.9–13.1) | 13 (3.8–30.7) | ||||||||
| 50–59 | 16 (9.1–24.7) | 16 (13.5–18.9) | 21 (14.1–29.9) | ||||||||
| 60–69 | 15 (7.6–26.5) | 23 (19.4–27.0) | 30 (20.5–41.2) | ||||||||
| ≥70 | 21 (6.1–45.6) | 30 (22.7–37.5) | 48 (27.8–68.7) | ||||||||
| Women | |||||||||||
| 30–39a | |||||||||||
| 40–49a | |||||||||||
| 50–59 | 5 (1.5–10.7) | 6 (4.7–8.3) | 8 (3.4–14.9) | ||||||||
| 60–69 | 8 (3.9–15.2) | 11 (8.3–13.0) | 9 (4.2–16.4) | ||||||||
| ≥70 | 16 (6.2–32.0) | 17 (12.6–21.6) | 16 (7.2–29.1) | ||||||||
| PROMISE PTP CAD ≥ 70% | |||||||||||
| Men | |||||||||||
| 30–39a | |||||||||||
| 40–49 | 0 (0.0–8.4)a | 5 (2.5–7.9) | 10 (2.1–26.5) | ||||||||
| 50–59 | 5 (1.7–11.9) | 7 (5.1–8.9) | 9 (4.3–15.7) | ||||||||
| 60–69 | 6 (1.7–15.0) | 11 (8.6–14.3) | 19 (11.4–29.4) | ||||||||
| ≥70 | 5 (0.1–26.0) | 13 (7.9–18.9) | 40 (21.1–61.3) | ||||||||
| Women | |||||||||||
| 30–39a | |||||||||||
| 40–49a | |||||||||||
| 50–59 | 2 (0.2–6.7) | 3 (1.9–4.3) | 0 (0.0–3.6)a | ||||||||
| 60–69 | 3 (0.6–7.9) | 4 (2.9–6.0) | 1 (0.0–5.4) | ||||||||
| ≥70 | 8 (1.7–21.9) | 6 (3.5–9.3) | 8 (2.2–19.2) | ||||||||
The PROMISE PTP reflect the observed prevalence of CAD ≥ 50% and CAD ≥ 70% by coronary CTA. Values as percentages (95% CI). Colour-coding: low- (<15%, green), intermediate- (15–85%, yellow), and high-probability categories (>85%, orange).6
Women <50 years of age or men <45 years of age were not included in PROMISE trial. However, these patients were likely to have had low PTP, which is emphasized by the fact that the PTP of obstructive CAD in the next higher age group were low (men: 2–13%; women: 5–8%). Notably, in the PROMISE PTP, all men <50 years and women <70 years of age were categorized as low-probability (<15%) regardless of symptoms.
CAD, coronary artery disease; DF, Diamond and Forrester; ESC, European Society of Cardiology; PTP, pretest probability.
In a supplementary analysis, 2275/4415 (51.5%) patients presented with non-obstructive CAD (1–49% stenosis). Similar to the obstructive disease, the prevalence increased with age in both men and women regardless of clinical symptoms (Supplementary data online, Table S3).
Difference between the ESC-DF PTP and the observed prevalence of obstructive CAD
The mean ESC-DF PTP was 40.6 ± 18.11% (range across groups 12–93%) (Table 2). Using the ESC-DF PTP, 4284/4415 (97%) patients were classified to intermediate-PTP category (PTP 15–85%) (Table 3). Across all ESC-DF groups, ESC-DF PTP overestimated substantially the observed prevalence of CAD ≥ 50% by nearly three-fold (range 2.2–4.5 for PTP/observed prevalence ratio). This overestimation was seen across all age, sex, and symptom-based strata but was higher in younger patients (4.5 vs. 2.2 in 40–49 vs. ≥80 years old patients, respectively) and patients with typical angina (3.8 vs. 2.8 vs. 2.4 in typical, atypical angina, and non-anginal chest pain, respectively). Younger women with typical angina presented with the largest differences between ESC-DF PTP and observed prevalence of CAD ≥ 50% (ratios up to 6.4) (Figure 2). Notably, overall, the overestimation did not differ between women and men (3.0 vs. 2.9) or between racial and ethnic groups (3.0 vs. 2.9). In the supplemental analysis using the PTP values from the American AHA/ACC DF/CASS guidelines,4 the results were similar (Supplementary data online, Figure S1).
Table 3.
Patient reclassification by the corrected PROMISE PTP.
| ESC-DF PTP categories |
||||
|---|---|---|---|---|
| PROMISE PTP categories | Low (<15%) | Intermediate (15–85%) | High (>85%) | Total |
| Low (<15%) | 106/106 (100.0) | 2152/4284 (50.2) | 0/0 (–) | 2258/4415 (51.1) |
| Intermediate (15–85%) | 0/0 (–) | 2132/4284 (49.8) | 25/25 (100.0) | 2157/4415 (48.9) |
| High (>85%) | 0/0 (–) | 0/0 (–) | 0/0 (–) | 0/4415 (–) |
| Total | 106/4415 (3.6) | 4284/4415 (97.0) | 25/4415 (0.6) | 4415/4415 (100.0) |
Categorization using PTP values for the probability thresholds recommended by the ESC guidelines.6
Values as n/N (%).
DF, Diamond and Forrester; ESC, European Society of Cardiology; PTP, pretest probability.
Figure 2.
Head-to-head comparison of the observed prevalence of CAD ≥ 50% by coronary CTA with the ESC-DF PTP. The observed prevalence of CAD ≥50% (dark blue) was lower than the ESC-DF PTP (light blue) across majority of the DF groups. The differences were especially striking in younger women with typical angina. Numbers above the columns represent the overestimation factor defined as a ratio: PTP/observed prevalence of CAD ≥50%. Here, patients with available core lab data were included (N = 4415). CTA, computed tomography angiography; DF, Diamond and Forrester; ESC, European Society of Cardiology; PTP, pretest probability.
Comparison of the ESC-DF PTP with the PROMISE-PTP set
Applying the PTP thresholds (i.e. <15%, 15–85%, >85%) as recommended by the ESC guidelines in the PROMISE-PTP, 2152/4284 (50.2%) patients within the initially intermediate ESC-DF PTP category were reclassified to the low-probability category, leaving only 2132/4284 (49.8%) in the intermediate category. Furthermore, all 25 patients with high probability for having CAD ≥ 50% by the ESC-DF PTP were reclassified into the intermediate-probability category (Table 3).
Potential implications of incorporating the PROMISE PTP into decision making
In the PROMISE, subsequent decision making was based on site interpretation of the CTA available in 4686 and functional test results in 4692 patients. Among these patients 1739/4686 (37.1%) and 3588/4692 (76.5%) presented with normal results in the CTA and functional arm, respectively.
The application of the PROMISE-PTP would reclassify 50.2% (CTA arm) and 50.7% (functional arm) of these patients to the low PTP category, a group without a need for further testing (i.e. no testing group). As expected, the number of normal tests in the no testing group was higher compared with the testing group; 48.8 vs. 25.2% in the CTA and 79.8 vs. 73.1% in the functional arm. Vice versa, regardless of the randomization, the percentages of ICA, revascularizations were lower in the no testing group compared with the testing group (P < 0.001 for both). Moreover, in the CTA arm, the high-risk anatomy was over three times more frequent in the testing group compared with the no testing group (1.8% vs. 0.5%). In a supplemental analysis including the CAD ≥ 50% in pLAD as high-risk anatomy, the high-risk anatomy was still two times more frequent in the testing group compared to the no testing group (6% vs. 2.8%) (Supplementary data online, Table S4).
In the CTA arm among the 2367 patients reclassified to the low PTP category by the PROMISE-PTP, and thus not recommended by guidelines for non-invasive testing, 17/2367 (0.7%) did experience events in the median follow-up of 26.1 (18.0–34.4) months. Of these, 10/17 (59%) patients died, and 7/17 (41%) had a non-fatal myocardial infarction. By CTA, none of the patients had high-risk-anatomy, and only 2/17 (12%) had CAD > 50% in coronary CTA (Supplementary data online, Table S5).
Discussion
Our data outlines the vast overestimation of the ESC-DF PTP for having obstructive CAD. Moreover, our study provides a new set of PTP for obstructive CAD (PROMISE-PTP), derived from a large, community-based population of symptomatic patients referred for non-invasive diagnostic testing. In the PROMISE-PTP table, the traditional ESC-DF PTP values were replaced by the observed prevalence of CAD ≥ 50% across age, sex, and clinical symptom strata. The prevalence of obstructive CAD was assessed in coronary CTA expert core lab reads, a non-invasive test appropriate for stable chest pain patients with intermediate PTP. Applying the PTP thresholds, as recommended by the ESC guidelines, to the PROMISE-PTP table, 50.2% of otherwise intermediate probability patients would be reclassified to the low probability category; a category without a need for further testing and low risk for subsequent events. Thus, the use of the PROMISE-PTP has the potential to substantially and safely reduce the number of non-invasive diagnostic tests.
Current clinical practice data suggest that selection of patients with stable chest pain, for both invasive and non-invasive testing, is generally inefficient. In fact, over >90% of patients referred to stress nuclear testing in one centre had no inducible ischaemia, and 75–86% of patients referred to coronary CTA as well as 42–60% of patients referred to elective ICA had no obstructive CAD.7,15–18 The low yield may be a result of a change in patient demographics, risk factors, clinical presentation, with a decreasing typicality of clinical symptoms, and changing natural history of CAD as well as changing referral patterns.19 While lower rates of smoking, and availability of statin treatment have decreased cardiovascular risk, and frequency of typical chest pain clinical presentations,19 a rising prevalence of diabetes mellitus and obesity may have increased the risk and altogether altered the prevalence of CAD.3,18
To consider these changes, studies have reassessed PTP in contemporary populations. For example, the 2011 European multicentre CAD Consortium study assessed PTP in 2260 elderly patients referred for ICA, finding an overestimation of PTP by DF in all patients, but especially in women.7 The observed lower prevalence of obstructive CAD in this study was the basis of the revised ESC-DF PTP table in the 2013 ESC guidelines.6 Although this adjusted PTP set reflects some of the changes in patient characteristics, it also applies to patients referred to ICA. This population is highly preselected, at likely much higher risk, and thus the ESC-DF PTP may not be readily generalizable to populations referred to non-invasive testing.
The observed prevalence of CAD ≥ 50% in PROMISE was substantially lower when compared with the predicted prevalence by the ESC-DF PTP (13.9% vs. 40.6%). This difference was consistently seen across age, sex, and presenting symptom strata. The low observed prevalence of CAD ≥ 50% in PROMISE (13.9%) was consistent with data from other contemporary cohorts referred for non-invasive testing from Europe and the US (range 14–18%).3,18 Not surprisingly, the prevalence of CAD ≥ 50% was considerably lower overall in these cohorts when compared with the prevalence in populations referred to ICA (58%).7
Applying the PROMISE-PTP may have clinical implications. In general, European and the US guidelines recommend non-invasive testing in the intermediate PTP category.4,6 Almost all PROMISE patients (97%) fell into this intermediate category by use of the ESC-DF PTP, reflecting the inclusion criteria of the trial. Applying the PROMISE-PTP, however, would reclassify 50.2% patients from intermediate to a low-probability category, with the implication that further testing may not be necessary. Thus, once validated, the use of the PROMISE-PTP may reduce the number of unnecessary primary non-invasive and subsequent downstream invasive diagnostic tests as well as interventions, thereby potentially addressing a major concern of coronary CTA guided management. Future studies should investigate if the application of the PROMISE-PTP in a daily practice leads to a reduced referral to non-invasive testing and also explore if the PROMISE-PTP could be used to estimate prevalence of inducible ischaemia in functional testing.
Interestingly, using the PROMISE-PTP, all men under the age of 50 years and all women under the age of 70 years were categorized as low-probability (<15%) regardless of their symptoms. In particular in women, the observed prevalence did not change with symptoms. This observation underlines the questionable value of the symptom classification in this patient group as previously reported in a sub-analysis of the EVINCI study,20 and it confirms the Bypass Angioplasty Revascularization Investigation 2 Diabetes Trial (BARI 2D) which revealed that women presented more often with angina but in general lower prevalence of obstructive CAD.21 These observations suggest that the clinical symptoms may be influenced by other factors than obstructive CAD and raise the question if the current age, sex, and symptom-based stratification is sufficient enough. Future models may need to consider substituting the symptom type or add variables with a higher discriminatory value to detect obstructive CAD. For instance, Aspartate aminotransferase, hsCRP, and HDL have been described as independent predictors of obstructive CAD beyond the traditional model using age, sex and chest pain type.22
PROMISE as a pragmatic multicentre trial at 193 clinical sites enrolled a representative sizeable contemporary cohort of patients with stable chest pain referred to non-invasive testing (i.e. frequently female, predominantly presenting with atypical angina and receiving statins in 46%).9 The detection of obstructive CAD was performed in a central core lab by level III-certified readers, blinded to patient characteristics using standardized methods.8,9 It is essential to understand the significance of using core lab evaluation as opposed to site reads. As previously reported in PROMISE, core lab reads not only render a much lower prevalence of CAD > 50% (14% vs. 25%, relative difference 41%) as compared with site assessments but also represent a better gold standard as the agreement with ICA is significantly higher.13 Consequently, the overestimation of PTP was in general higher than the one described in other studies using site reads (e.g. CONFIRM).3 Finally, in the present analysis, uniform and complete data were available for all patients, with prospective definitions operationalized within the trial sites, leading to substantially complete data acquisition.2
Limitations
Our analysis has limitations. The PROMISE trial did not include women <50 or men <45 years of age and thus not all pre-specified patient groups within the PTP table could be populated. However, the excluded patients were likely to have had low PTP, which is emphasized by the fact that the observed prevalence in the next higher age group was low (PTP range in women: 5–8%; men: 2–13%). The trial population also did not include patients with initially low (<15%) or high (>85%) probability for CAD ≥ 50%, as PROMISE sought to enroll those with intermediate probability. Nonetheless, among the modest-sized group of patients who were included in PROMISE and were identified by this analysis as having high ESC-DF PTP, the prevalence of obstructive CAD was not high. Of note, the majority of the included patients presented with atypical angina and some PTP groups (e.g. elderly patients with typical or non-anginal chest pain) were underpopulated leading to relatively large CIs in the PROMISE-PTP table. The small proportion of patients who received only CAC score [97/4686 (2%)] presented with high CAC score values (>400) and increased cardiovascular risk profile; thus, these patients may have had a high prevalence of obstructive CAD which is not reflected in the PROMISE-PTP. The proposed PROMISE-PTP set requires a validation in a large, prospective cohort, preferably in a non-preselected population of patients presenting with stable chest pain (i.e. all comers) electively referred to non-invasive testing in which the reads are evaluated in a central core lab.
Conclusion
In conclusion, the ESC-DF PTP overestimate vastly the actual prevalence of CAD ≥ 50%. A new set of PTP, like the PROMISE-PTP, derived from results of non-invasive testing in patients initially categorized as intermediate PTP, may substantially reduce the need for non-invasive tests in stable chest pain.
Funding
This work was supported by National Heart, Lung, and Blood Institute (R01HL098237, R01HL098236, R01HL98305, and R01HL098235; 5T32HL076136 to D.B.; K24HL113128 to U.H.); and German Research Foundation (DFG) project 290004377 (FO 993/1) to B.F. Fulbright Program Student Grant (E0583118) to J.K.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J.. Factors of risk in the development of coronary heart disease—six-year follow-up experience: the Framingham study. Ann Intern Med 1961;55:33.. [DOI] [PubMed] [Google Scholar]
- 2. Diamond GA, Forrester JS.. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–8. [DOI] [PubMed] [Google Scholar]
- 3. Cheng VY, Berman DS, Rozanski A, Dunning AM, Achenbach S, Al-Mallah M. et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation 2011;124:2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP. et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; American College of Physicians; American Association for Thoracic Surgery. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–164. [DOI] [PubMed] [Google Scholar]
- 5. Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM. et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;63:380–406. [DOI] [PubMed] [Google Scholar]
- 6.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 7. Genders TSS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K. et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J 2011;32:1316–30. [DOI] [PubMed] [Google Scholar]
- 8. Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K. et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796–803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B. et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L. et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190–204. [DOI] [PubMed] [Google Scholar]
- 11. Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ. et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2014;8:342–58. [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR. et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu MT, Meyersohn NM, Mayrhofer T, Bittner DO, Emami H, Puchner SB. et al. Central core laboratory versus site interpretation of coronary CT angiography: agreement and association with cardiovascular events in the PROMISE trial. Radiology 2017;287:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P. et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr 2010;4:407.e1–33. [DOI] [PubMed] [Google Scholar]
- 15. Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LEJ. et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61:1054–65. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV. et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 18. Ferreira AM, Marques H, Tralhão A, Santos MB, Santos AR, Cardoso G. et al. Pre-test probability of obstructive coronary stenosis in patients undergoing coronary CT angiography: comparative performance of the modified Diamond-Forrester algorithm versus methods incorporating cardiovascular risk factors. Int J Cardiol 2016;222:346–51. [DOI] [PubMed] [Google Scholar]
- 19. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE. et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 2007;356:2388–98. [DOI] [PubMed] [Google Scholar]
- 20. Rovai D, Neglia D, Lorenzoni V, Caselli C, Knuuti J, Underwood SR.. Limitations of chest pain categorization models to predict coronary artery disease. Am J Cardiol 2015;116:504–7. [DOI] [PubMed] [Google Scholar]
- 21. Tamis-Holland JE, Lu J, Bittner V, Magee MF, Lopes N, Adler DS. et al. Sex, clinical symptoms, and angiographic findings in patients with diabetes mellitus and coronary artery disease (from the Bypass Angioplasty Revascularization Investigation [BARI] 2 Diabetes trial). Am J Cardiol 2011;107:980–5. [DOI] [PubMed] [Google Scholar]
- 22. Caselli C, Rovai D, Lorenzoni V, Carpeggiani C, Teresinska A, Aguade S. et al. A new integrated clinical-biohumoral model to predict functionally significant coronary artery disease in patients with chronic chest pain. Can J Cardiol 2015;31:709–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.