Abstract
Background
Frailty is an important geriatric syndrome, but little is known about its development in the years preceding onset of the syndrome. The aim of this study was to examine the progression of frailty and compare the trajectories of each frailty component prior to frailty onset.
Methods
Repeat data were from two cohort studies: the Longitudinal Aging Study Amsterdam (n = 1440) with a 15-year follow-up and the InCHIANTI Study (n = 998) with a 9-year follow-up. Participants were classified as frail if they had >3 frailty components (exhaustion, slowness, physical inactivity, weakness, and weight loss). Transitions between frailty components were examined with multistate modeling. Trajectories of frailty components were compared among persons who subsequently developed frailty to matched nonfrail persons by using mixed effects models.
Results
The probabilities were 0.43, 0.40, and 0.36 for transitioning from 0 to 1 frailty component, from 1 component to 2 components, and from 2 components to 3–5 components (the frail state). The transition probability from frail to death was 0.13. Exhaustion separated frail and nonfrail groups already 9 years prior to onset of frailty (pooled risk ratio [RR] = 1.53, 95% confidence interval [CI] 1.04–2.24). Slowness (RR = 1.94, 95% CI 1.44–2.61), low activity (RR = 1.59, 95% CI 1.19–2.13), and weakness (RR = 1.39, 95% CI 1.10–1.76) separated frail and nonfrail groups 6 years prior to onset of frailty. The fifth frailty component, weight loss, separated frail and nonfrail groups only at the onset of frailty (RR = 3.36, 95% CI 2.76–4.08).
Conclusions
Evidence from two cohort studies suggests that feelings of exhaustion tend to emerge early and weight loss near the onset of frailty syndrome.
Keywords: Frailty, Exhaustion, Walking speed, Physical activity, Muscle strength
Introduction
Frailty increases the risk of disability, falls, fractures, institutionalization, and death (1–5) and is an important public health concern as populations are aging (6). A consensus group consisting of international experts has defined frailty as “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiologic function that increases an individual’s vulnerability for developing increased dependency and/or death” (7). Although often accompanied with disabilities and multimorbidity, frailty is its own entity designated as a state of increased vulnerability due to impairments in multiple systems (7–11). In many studies, frailty is adjudicated according to the phenotypic criteria developed by Fried and colleagues, which include five components: weight loss, exhaustion, low physical activity, weakness, and slowness (1). Presence of three or more of these components defines frailty, whereas one or two components denote prefrailty, and none indicates no frailty.
Much research on frailty has focused on the causes and risk of adverse outcomes associated with this condition. In contrast, comparatively little is known about the typical sequence by which frailty components emerge and cumulate prior to frailty onset. Previous studies have examined the dynamic nature of frailty in terms of progression from no frailty to prefrailty and to frailty and shown that frailty is characterized by recurrent transitions between frailty states over time (12–16). Most changes between frailty states occurred gradually, and some participants improved and reversed some of the components (13). However, to date, we do not know whether and when there are specific components that are more likely to emerge prior to frailty development. This information is essential to understand the natural course of frailty development in people who develop frailty syndrome and would help in early identification of individuals at risk who are more likely to benefit from preventive interventions.
In this study, we sought to characterize prospectively the emergence of the frailty components based on the phenotypic model by modeling transitions between frailty states over a 9- to 15-year follow-up. We also examined in a retrospective setting the differences in longitudinal trajectories of each frailty component prior to frailty onset between people developing frailty compared with matched controls who remained free of frailty.
Methods
Study Populations
Data were from two long-term cohort studies, namely the Longitudinal Aging Study Amsterdam (LASA) from the Netherlands and the Invecchiare in Chianti, aging in the Chianti area (InCHIANTI) study from Italy, in which information on all frailty components based on the phenotypic model to frailty has been collected repeatedly at 3-year intervals.
LASA is an ongoing study on physical, emotional, cognitive, and social functioning of Dutch older adults. The sampling procedure and data collection of this study have been described elsewhere (17,18). In brief, a nationally representative survey was conducted in 1992–1993 among 3,107 respondents aged 55–84 years. Follow-up measurements were collected approximately every 3 years. In each wave, data were collected by trained interviewers who visited the respondents at home. For the current study, we used data from six waves (1995–1996, 1998–1999, 2001–2002, 2005–2006, 2008–2009, 2011–2012) consisting of measures of all frailty components. From the 2,545 LASA respondents at wave 1995–1996 those aged 65 and over were invited for the medical interview (n = 1,722) and of which 1,506 participated.
The InCHIANTI Study is an epidemiologic study of risk factors of mobility disability in old age. The study design and data collection have been previously described in detail (19). In brief, out of the random sample of 1,260 persons aged 65 years and older, 1,155 agreed to participate in the study at baseline in 1998–2000. Thereafter, participants have been followed up every 3 years. For the current study, data were used from all four measurement waves available (1998–2000, 2001–2003, 2004–2006 and 2007–2008).
For the prospective part of our analyses, we included participants who had information on frailty status available at least in two study waves and those who had frailty status available once and had died by the end of year 2015 (n = 1,440 in LASA and n = 998 in InCHIANTI). For the retrospective part of the study, we included participants if they were not classified as frail (three of more criteria) at baseline and had at least one other frailty measurement available in the following measurement waves (n = 981 in LASA and n = 765 in InCHIANTI). Those who developed frailty during the follow-up were categorized as frailty (n = 301 in LASA and n = 214 in InCHIANTI), and we then randomly selected 1–2 nonfrail controls for each frailty case separately in both studies (n = 515 in LASA and n = 339 in InCHIANTI) matching for sex, age group at the end of follow-up (5-year bands) and education.
Both the LASA and InCHIANTI studies are conducted in line with the Declaration of Helsinki, and participants in both studies received an extensive description of the study and participated after providing written informed consent. LASA was approved by the medical ethics committee of the VU University Medical Center, and the InCHIANTI study was approved by the ethical committee of the Italian National Institute of Research and Care on Aging.
Measurement of Frailty
Frailty was ascertained using five components and criteria proposed by Fried colleagues (1), namely exhaustion, slowness, low physical activity, weakness, and weight loss. All components and the frailty summary score were measured at each of the study waves in the LASA and InCHIANTI studies. The detailed description of the measurements is presented in Supplementary Table 1. A total frailty score at each study wave was calculated by allocating a value of 1 to each of the above components if present (range 0 to 5). When a participant met at least three of five of the frailty components, s/he was defined “frail” and information on individual frailty criteria from the preceding waves was used in the analyses (1). If a participant did not develop three or more frailty components over the follow-up, s/he was categorized into “nonfrail” group and information on individual frailty components from all available waves was used in the analyses.
Covariates
Age and sex were used as covariates in both study settings. In the prospective part of the study, age was modeled as time-varying continuous variable, whereas in the retrospective part, age as a continuous variable at the last measurement point was used. In the retrospective part, the highest level of education was obtained from the baseline and was further categorized into low (<9 years), middle (9–12 years), and high (≥ 13 years) in both studies.
Statistical Analysis
We reported characteristics of the study population in prospective and retrospective modeling as mean and standard deviation (SD) for continuous variables and percentages for categorical variables in both cohorts. In the retrospective part, differences across frailty groups were examined with t-test for continuous variables and chi-square for categorical variables. There were six data waves in the 15-year follow-up of LASA study and four data waves in the 9-year follow-up of the InCHIANTI study.
In prospective analyses, we used the msm package for R for prospective multistate modeling to estimate transitions between frailty states (0, 1, 2, 3–5 components) and death (20). In these analyses, data from the LASA and InCHIANTI studies were pooled into a single dataset and analysis were adjusted for sex, age, and cohort.
In the retrospective part, we divided participants into two groups: those who developed (cases) and those who did not develop frailty (controls matched by sex, age group in 5-year bands at the end of follow-up, and education) during the follow-up period. We used a backward timescale, such that year = 0 in the analysis was the year of frailty onset for frailty cases and the end of follow-up for nonfrail controls that remained free of frailty during the follow-up. Participants were then traced backward to their first measurement wave. Data at each wave during this retrospective observation period were collated to build trajectories for each frailty component (exhaustion, slowness, low physical activity, weakness, and weight loss) and the total number of frailty components separately for those in the “frail” and “nonfrail” groups. This was done by using mixed effects models (21) with the intercept as random effects and a backward timescale. Normal distribution was used for continuous and Poisson distribution for dichotomous outcomes (22,23). The analyses were adjusted for sex, age, and education. We used contrast statements to detect when the prevalence of an individual component and the total number of frailty components diverged significantly between the two groups. Results were presented as risk ratios (RRs), and their 95% confidence intervals (CI) at each wave with the “nonfrail” group as the reference.
We examined interaction effects of sex and frailty status (at the end of follow-up) on development of each frailty component by using mixed effects models but found them to be nonsignificant, and thus, results are presented for men and women combined and adjusting for sex. After separate analyses in both cohorts, we used fixed-effects meta-analysis (24) to pool the cohort-specific results into summary estimates. In addition, we conducted a sensitivity analysis to examine the potential effect of attrition to our results by including only participants who had at least a 9-year follow-up available. Trajectories of individual frailty components and the total number of components were plotted separately for those in the “frail” and “nonfrail” groups, and the cohort-specific results were pooled by using fixed-effects meta-analysis into summary estimates.
Statistical analyses were performed by using SAS 9.4 Statistical Package (SAS Institute Inc., Cary, NC) and the R 3.3.1 statistical package.
Results
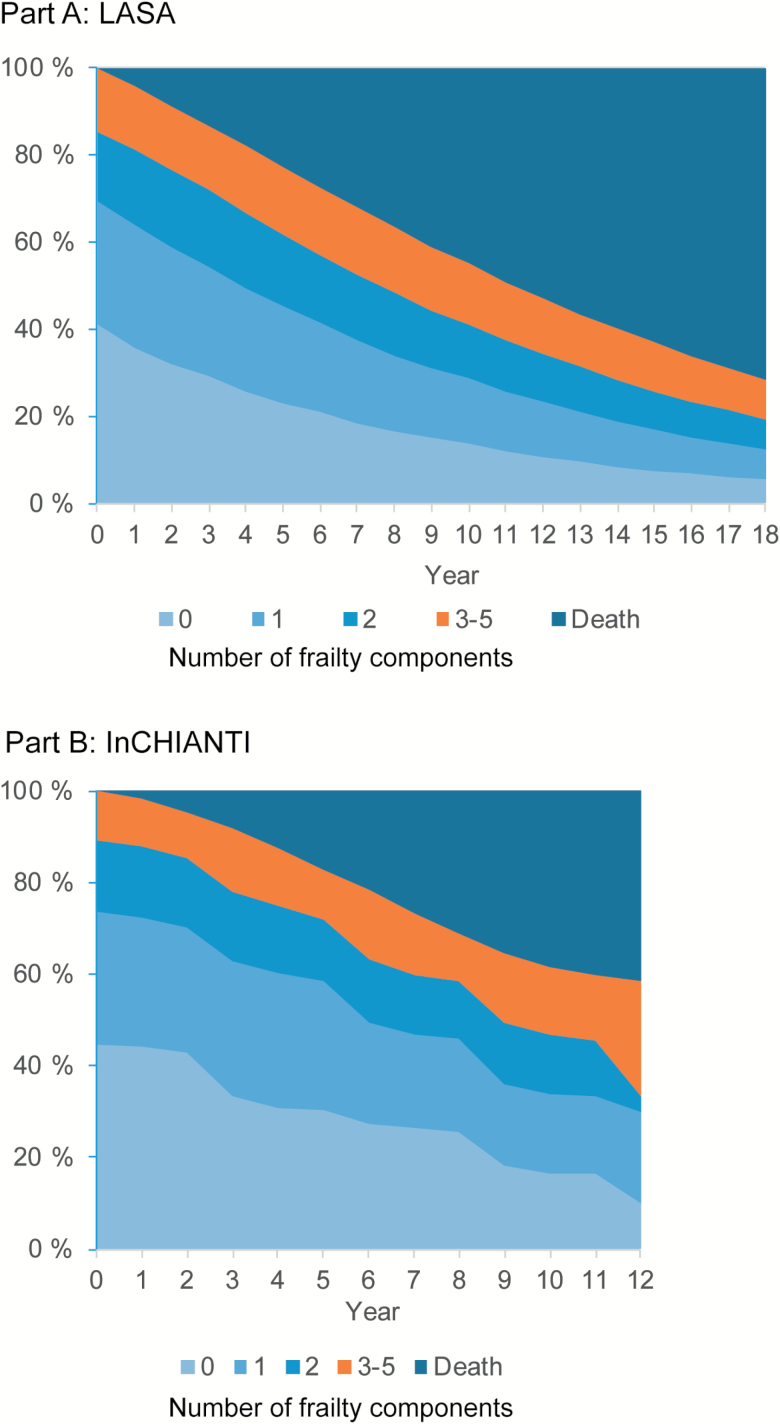
In the prospective study setting for multistate modeling, 51% were women in LASA and 55% in InCHIANTI, and mean age of the participants at baseline was 75.7 years in LASA and 74.8 years in InCHIANTI. Of the participants, 41% in LASA and 45% in InCHIANTI had no frailty components, 44% and 45% had one or two frailty components, and 15% and 11% had three or more frailty components and were classified as frail at baseline. Prevalence of frailty was relatively constant over the follow-up with the amount of nonfrail participants transitioning to frailty being equal to the amount frail people who died (Figure 1).
Figure 1.
Observed prevalence of the number of frailty components and death in LASA study with 18-year follow-up (A) and the InCHIANTI study with 12-year follow-up (B).
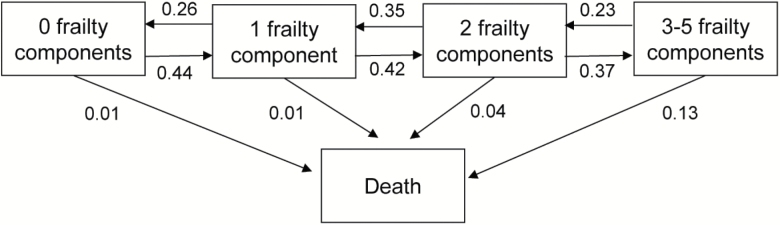
Figure 2 provides transition probabilities between frailty states and death during the 9–15 years of follow-up in the two cohorts combined. The probabilities were 0.44, 0.42, and 0.37 for transitioning from 0 to 1 frailty component, from 1 component to 2 components, and from 2 components to 3–5 components (the frail state). The corresponding transition rate from frail to death was 0.13. As expected, accumulating frailty components over time was more common than reducing frailty components. The hazard ratio of transitioning from a less advantaged state to a more advantaged state compared with transitioning from a more advantaged state to a less advantaged state was 1.68 (95% CI 1.50–1.88) between states of 0 and 1 components, 1.21 (95% CI 1.08–1.37) between one and two components, 1.61 (95% CI 1.38–1.89) between two components and being frail (ie, having three or more components).
Figure 2.
Transition probabilities between the number of frailty components and death. Pooled study populations based on the LASA and InCHIANTI studies (n = 1,438). Analysis adjusted for age, sex, and study cohort.
After matching frail and nonfrail participants for the retrospective analysis, the average age for onset of frailty was 83.7 years in LASA and 82.1 years in InCHIANTI (Supplementary Table 2). The respective ages for nonfrail participants at the end of follow-up were 83.0 and 80.6 years. Frailty was more common in women than in men in both study cohorts, proportion of women being 57% in LASA and 64% in InCHIANTI. In the LASA study, participants who did not develop frailty during the follow-up provided data from 3.8 (SD 1.5) and those who developed frailty participated in 3.5 (SD 1.4) of the possible six study waves. In the InCHIANTI study, the corresponding mean numbers were 3.5 (SD 0.8) and 3.1 (SD 0.8) of the possible four study waves.
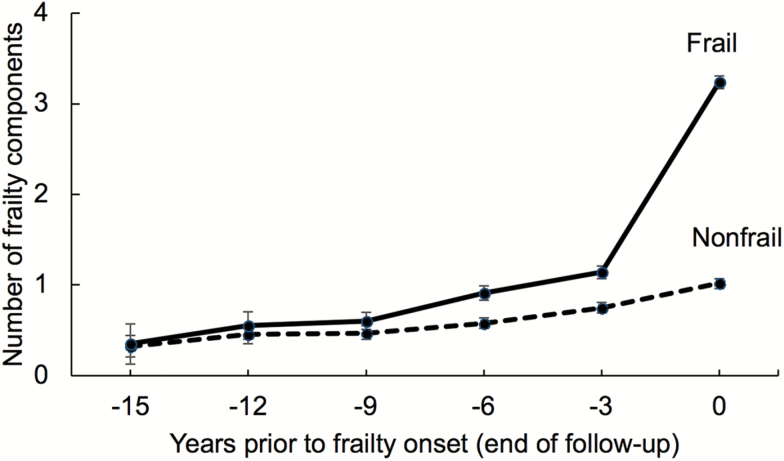
Overall, the development of frailty components was very similar in the two cohorts, and accumulation of frailty components increased gradually over the years toward the onset of frailty (Figure 3, Supplementary Figure 1). On average, the number of frailty components was 1.2 in LASA and 1.0 in InCHIANTI 3 years before onset of frailty. The corresponding numbers for participants who did not develop frailty during the follow-up were 0.8 in LASA and 0.6 in InCHIANTI, 3 years before the end of follow-up. Of the frailty components, weakness was most prevalent and weight loss least prevalent in LASA and exhaustion most prevalent and weight loss least prevalent in InCHIANTI (Supplementary Figure 2).
Figure 3.
Number of frailty components (mean and 95% confidence intervals) among frail and matched nonfrail participants during the 15 years prior to onset of frailty (year 0). For nonfrail participants, year 0 is the last available measurement wave. Pooled analysis of the LASA and InCHIANTI studies. Values from years −15 to −12 are from the LASA study only. Models adjusted for age (year 0), sex, and education.
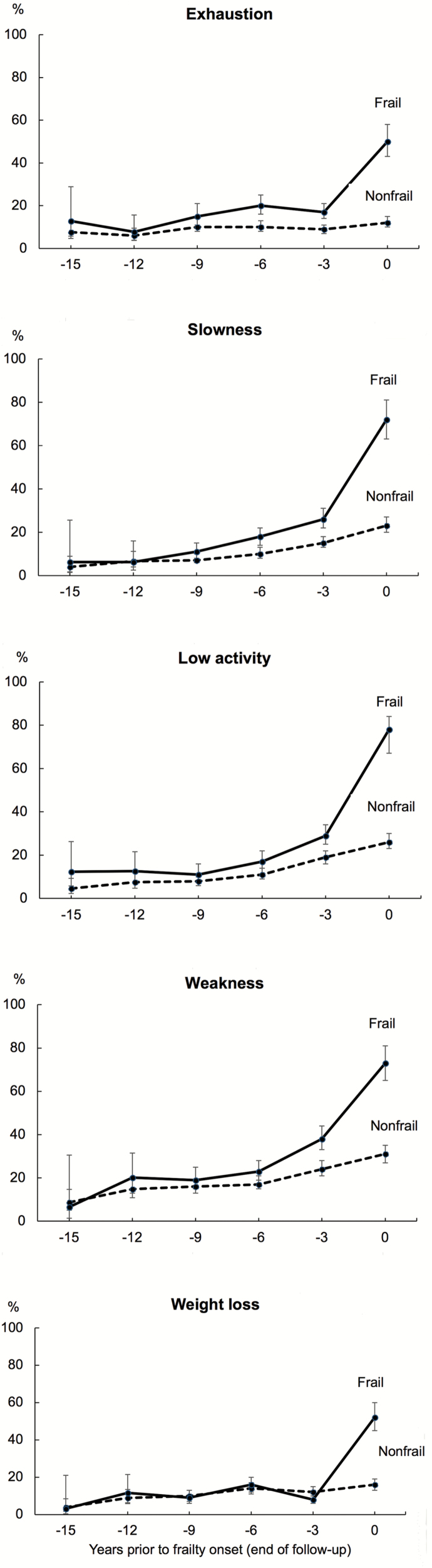
Estimated prevalence of each of the frailty components among frail and nonfrail participants during 9- to 15-year time window prior to onset of frailty are shown in Figure 4, and comparison between frail and nonfrail participants at different time points are shown in Table 1. These results are based on pooled analyses (cohort-specific results are shown in Supplementary Figure 2). Among the five frailty components, exhaustion separated frail and nonfrail groups already 9 years prior to onset of frailty, the pooled RR being 1.53 (95% CI 1.04–2.24) at that time point. The prevalence of exhaustion remained relatively constant in frail and nonfrail groups over the years but increased markedly in the frailty group during the last 3 years of follow-up. At the end of the follow-up, the prevalence of that component was four times higher in frailty participants (pooled RR 3.96, 95% CI 3.27–4.79) than nonfrail participants.
Figure 4.
Estimated prevalence and 95% confidence intervals of frailty components among frail and matched nonfrail participants during the 15 years prior to onset of frailty (year 0). For nonfrail participants, year 0 is the last available measurement wave. Pooled analysis of the LASA and InCHIANTI studies. Values from years −15 to −12 are from the LASA study only. Models adjusted for age (year 0), sex, and education.
Table 1.
Risk Ratios (RRs) and Their 95% Confidence Intervals (CI) for Each Frailty Components Comparing Frail and Matched Nonfrail at Different Time Points Prior the Onset of Frailty (Year 0) in the LASA (N = 816) and InCHIANTI (N = 553) Studies
| Years Prior to Frailty Onset | LASA | InCHIANTI | Pooled Summary Estimates | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | ||||
| Exhaustion | |||||||||
| −15 | 1.47 | 0.56 | 3.83 | 1.47 | 0.56 | 3.83 | |||
| −12 | 1.15 | 0.48 | 2.75 | 1.15 | 0.48 | 2.75 | |||
| −9 | 1.82 | 0.96 | 3.44 | 1.43 | 0.88 | 2.30 | 1.53 | 1.04 | 2.24 |
| −6 | 2.18 | 1.29 | 3.69 | 1.95 | 1.38 | 2.75 | 2.02 | 1.51 | 2.69 |
| −3 | 1.71 | 1.11 | 2.63 | 2.13 | 1.53 | 2.96 | 1.96 | 1.51 | 2.55 |
| 0 | 5.03 | 3.76 | 6.73 | 3.31 | 2.57 | 4.26 | 3.96 | 3.27 | 4.79 |
| Slowness | |||||||||
| −15 | 1.36 | 0.41 | 4.50 | 1.36 | 0.41 | 4.50 | |||
| −12 | 1.00 | 0.43 | 2.29 | 1.00 | 0.43 | 2.29 | |||
| −9 | 1.31 | 0.78 | 2.19 | 1.68 | 0.81 | 3.47 | 1.42 | 0.93 | 2.17 |
| −6 | 1.59 | 1.11 | 2.26 | 3.07 | 1.79 | 5.27 | 1.94 | 1.44 | 2.61 |
| −3 | 1.60 | 1.24 | 2.07 | 3.09 | 2.07 | 4.60 | 1.94 | 1.56 | 2.41 |
| 0 | 2.87 | 2.38 | 3.46 | 5.39 | 3.93 | 7.38 | 3.39 | 2.88 | 3.98 |
| Low activity | |||||||||
| −15 | 2.29 | 0.85 | 6.15 | 2.29 | 0.85 | 6.15 | |||
| −12 | 1.57 | 0.81 | 3.07 | 1.57 | 0.81 | 3.07 | |||
| −9 | 1.44 | 0.85 | 2.46 | 0.87 | 0.36 | 2.07 | 1.26 | 0.8 | 1.98 |
| −6 | 1.53 | 1.04 | 2.23 | 1.68 | 1.07 | 2.64 | 1.59 | 1.19 | 2.13 |
| −3 | 1.58 | 1.21 | 2.06 | 1.43 | 1.10 | 1.87 | 1.5 | 1.24 | 1.81 |
| 0 | 3.15 | 2.58 | 3.86 | 2.39 | 1.97 | 2.89 | 2.73 | 2.37 | 3.13 |
| Weakness | |||||||||
| −15 | 0.77 | 0.32 | 1.84 | 0.77 | 0.32 | 1.84 | |||
| −12 | 1.28 | 0.84 | 1.95 | 1.28 | 0.84 | 1.95 | |||
| −9 | 1.36 | 0.96 | 1.92 | 0.89 | 0.50 | 1.59 | 1.21 | 0.9 | 1.63 |
| −6 | 1.30 | 1.00 | 1.70 | 1.81 | 1.07 | 3.07 | 1.39 | 1.1 | 1.76 |
| −3 | 1.61 | 1.33 | 1.95 | 1.28 | 0.85 | 1.94 | 1.55 | 1.3 | 1.84 |
| 0 | 2.31 | 1.97 | 2.70 | 2.61 | 2.01 | 3.37 | 2.39 | 2.09 | 2.73 |
| Weight loss | |||||||||
| −15 | 0.96 | 0.13 | 7.18 | 0.96 | 0.13 | 7.18 | |||
| −12 | 1.36 | 0.64 | 2.90 | 1.36 | 0.64 | 2.90 | |||
| −9 | 1.09 | 0.61 | 1.97 | 0.50 | 0.19 | 1.33 | 0.84 | 0.5 | 1.39 |
| −6 | 1.33 | 0.87 | 2.03 | 0.91 | 0.58 | 1.42 | 1.11 | 0.81 | 1.51 |
| −3 | 0.62 | 0.40 | 0.97 | 0.77 | 0.46 | 1.29 | 0.68 | 0.49 | 0.95 |
| 0 | 3.05 | 2.41 | 3.87 | 4.13 | 2.91 | 5.86 | 3.36 | 2.76 | 4.08 |
For nonfrail participants, year 0 is the last available measurement wave.
Note: Models adjusted for age (year 0), sex, and education.
Slowness, low activity, and weakness separated frail and nonfrail groups 6 years prior to onset of frailty. The pooled RRs for these components were 1.94 (95% CI 1.44–2.61), 1.59 (95% CI 1.19–2.13), and 1.39 (95% CI 1.10–1.76), respectively. In contrast to exhaustion, prevalence of slowness, low activity, and weakness increased gradually with increasing age both in the frail and nonfrail groups. At the end of the follow-up, frail participants reported 3.4 times (pooled RR 3.39, 95% CI 2.88–3.98) more often slowness, 2.7 times (pooled RR 2.73, 95% CI 2.37–3.13) more often low activity, and 2.4 times (pooled RR 2.39, 95% CI 2.09–2.73) more often weakness than nonfrail participants.
Weight loss did not separate frail and nonfrail groups before onset of frailty. However, when comparing prevalence of weight loss at the end of the follow-up, the prevalence of weight loss was more than three times higher in frail participants compared with those who did not develop frailty (pooled RR 3.36, 95% CI 2.76–4.08).
In a sensitivity analysis including only participants with at least 9-year follow-up data (LASA, n = 397 and InCHIANTI, n = 307), the overall development of frailty components was very similar to that seen in the main results (Supplementary Figure 3). In terms of the individual frailty components (Supplementary Figure 4 and Supplementary Table 3), exhaustion separated frail and nonfrail groups 9 years and slowness and low activity 6 years prior to onset of frailty, as in the main results. Weakness showed moderate evidence in separating frail and nonfrail groups 3 years prior to onset of frailty, which was 3 years later than in the main results. Weight loss did not separate frail and nonfrail groups before onset of frailty as in the main results.
Discussion
This analysis, based on two independent well-characterized cohort studies, described the progression of frailty and characterized the sequence of emergence of its components over 9–15 years before onset of frailty. We found that participants who developed frailty, reported more often exhaustion already 9 years and slowness, low activity, and weakness 6 years prior to onset of frailty than those who remained free of frailty during the follow-up. In contrast, the fifth frailty component, weight loss, did not separate frail and nonfrail groups before onset of frailty. The findings were similar in the two cohorts.
The main strength of this prospective study is repeated measurements of all five frailty components up to 9 to 15 years and the use of data from two large, independent cohorts from Europe. This allowed us to identify the time points when prevalence of different frailty criteria begins to diverge between those who subsequently developed frailty and those who remained free of frailty. Another strength was the possibility to assess the similarities and differences of the observations between two different cohorts, an important point regarding the generalizability of our findings to other countries. The participation rate was high, and on average, the participants provided data on more than three time points. Finally, the similar study design and nearly identically measured frailty criteria allowed us to pool the results from two different cohorts to obtain more robust results.
The finding that exhaustion was the first frailty indicator that separated persons who subsequently developed frailty and those who remained free of frailty is novel. To our knowledge, only one previous study (the Women’s Health and Aging Study II, WHAS II) has attempted to examine manifestation of individual frailty components before onset of frailty (16). In that study, based on relatively small sample of incident frailty cases, exhaustion was found to be among the last components to occur. Direct comparison between our and WHAS II findings is not, however, meaningful because InCHIANTI and LASA are population-based studies, whereas WHAS II participants were initially relatively high-functioning women. The discrepancy to our findings may also lie on different measures of exhaustion. The WHAS II study defined exhaustion by low energy level or feeling tiredness and weakness most of the time. In our study, exhaustion was measured with two items drawn from the Center for Epidemiology Studies-Depression (CES-D) scale (25): “I felt that everything I did was an effort in the last week” and “I could not get going in the last week.” Both items have previously also been used as general measures of fatigue (26). Fatigue is complex and multidimensional concept involving a sensation of ‘‘low energy” and disturbed energy balance (27). Given this, our finding is biologically plausible. Perceived global fatigue, an overall subjective lack of physical or mental energy, may limit physical activity and lead to sedentary behavior, which further deteriorates physical functioning and, over time, increases the risk of functional limitation and mortality (27–30). As a distal risk factor in the causal chain, perception of exhaustion or fatigue has been hypothesized to be one of the early markers identifying people at frailty risk (31). Our findings, supporting this hypothesis, suggest that early identification of exhaustion and fatigue in apparently physically well-functioning older adults might help to identify persons in the initial stages of frailty allowing timely interventions to prevent development of frailty.
Other frailty components that related to early differences between the frail and nonfrail were slowness, low activity, and weakness. These criteria mostly reflect the physical determinants of frailty and are quite interrelated with each other. Distinctive in these frailty criteria compared with exhaustion and weight loss criteria was that the prevalence increased gradually with time among both frail and nonfrail participants, but the increment became greater in the frailty group 6 years before onset of frailty. Walking speed, physical activity, and muscle strength all decline with age, and therefore, it is understandable that also nonfrail participants show decline in these conditions, although to a lesser extent than individuals who develop frailty later. Prevalence of slowness, low activity, and weakness was about 70% at the time of onset of frailty suggesting that these physical characteristics commonly characterize people with frailty. Our finding is in agreement with previous study showing that weakness often co-occurs with slowness or low physical activity (16).
Weight loss is often seen as an important component of the frailty syndrome resulting possibly from energy dysregulation, reduction of appetite, and food intake (32). That weight loss differentiated frail and nonfrail persons only at the onset of frailty (ie, the last measurement point) suggests weight loss is a late component in the process leading to development of frailty and thus not necessarily informative for early detection of risk groups. Similarly, with our study, Xue and colleagues observed that weight loss was the last frailty component to emerge before onset of frailty (16). However, based on our findings, it is also possible that weight loss is a consequence of frailty rather than a causal factor. Future research is needed to disentangle the temporal order of weight loss and frailty.
Our findings suggest that screening for exhaustion and other components of frailty in persons aged 70 years and older could be warranted. Fried’s phenotypic model (1), which was used in the current study, is suggested to be one suitable screening test. Identification of frailty components on time is pivotal for implementing preventive interventions to stop or slow down the development of frailty. These findings also suggest that a sense of exhaustion may be the trigger to behavioral changes, such as being less physically active, which predisposes a vicious cycle that eventually leads to frailty. Interventions aimed at counteracting such behavioral changes in people who already feel exhausted may slow down this process and possibly prevent frailty.
This study has some limitations that warrant discussion. Due to the 3-year measurement interval, we were unable to define the precise year when frailty emerged. Thus, for some people −3 year could in fact be −1 (before frailty onset), and therefore, the differences found in time point −3 would be overestimated. However, the main interest in this study was the whole 15 years prior to onset of frailty, and we feel that the findings related to early differentiation among frail and nonfrail are relatively accurate. It is also worth noting that people in the “nonfrail” group may have developed frailty after the end of the follow-up and thus were already prefrail during the examined study period. This, however, rather underestimates than overestimates the observed differences between frail and nonfrail at different time points. Attrition is typical in longitudinal studies, including LASA and InCHIANTI in which about half of the participants remained in the study at least for 9 years. To explore the effect of attrition to our findings, we conducted sensitivity analyses and were able to replicate the main findings, suggesting attrition is not a major source of bias in our analyses. Finally, multiple instruments have been developed to measure frailty, but frailty does not yet have an internationally recognized standard definition (33). In this study, Fried’s phenotypic model was used and both studies had measured the required five components shown to predict adverse health outcomes such as falls, disability, and death (4,5). The advantage of Fried’s phenotypic model is that it is mostly based on objective testing compared with some other tools relying on subjective evaluations.
In conclusion, to the best of our knowledge, this is the first large-scale study based on representative population samples to examine longitudinally the development of frailty criteria over 9 to 15 years of follow-up among initially nonfrail or prefrail participants. We found evidence suggesting that older adults who develop frailty tend to report exhaustion quite early, approximately 9 years prior to onset of frailty compared with those who remain free of frailty. In addition, slowness, low activity, and weakness differentiated frailty and nonfrail groups from each other already 6 years before onset of frailty. In contrast, a typical feature of frailty, weight loss, was observed to take place only in the last years preceding onset of frailty. Further research is needed to examine the generalizability of our findings and to evaluate whether routine screening of frailty components could improve cost-effectiveness of interventions to prevent or delay frailty by targeting earlier those individuals who are at the greatest risk.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by Academy of Finland (grant number 286294 and 294154 to S.S. and 311492 to M.K.); Medical Research Council (grant number K013351 to M.K.); NordForsk, the Nordic Programme for Health and Welfare (grant number 75021 to M.K.), and a Helsinki Institute of Life Science fellowship (M.K.). The Longitudinal Aging Study Amsterdam (LASA) is largely supported by a grant from the Dutch Ministry of Health, Welfare and Sports, Directorate of Long-Term Care. The InCHIANTI study baseline (1998–2000) was funded by the Italian Ministry of Health (ICS110.1/RF97.71) and in part by the U.S. National Institute on Aging, Bethesda, Maryland (contracts 236 MD 916413 and 236 MD 821336). The InCHIANTI follow-up 1 (2001–2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI follow-up 2 and 3 studies (2004–2010) were financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002).
Conflict of interest statement
S.S., L.F., and J.G. currently serve on the editorial board for the Journal of Gerontology: Medical Sciences. All other authors have no conflicts of interest to disclose.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 2. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 3. Abizanda P, Romero L, Sánchez-Jurado PM, Martínez-Reig M, Gómez-Arnedo L, Alfonso SA. Frailty and mortality, disability and mobility loss in a Spanish cohort of older adults: the FRADEA study. Maturitas. 2013;74:54–60. doi: 10.1016/j.maturitas.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 4. Ensrud KE, Ewing SK, Cawthon PM, et al. ; Osteoporotic Fractures in Men Research Group A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Prince M, Thiyagarajan JA, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. 2016;17:188–192. doi: 10.1016/j.jamda.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 7. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 10. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. [DOI] [PubMed] [Google Scholar]
- 11. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- 13. Fallah N, Mitnitski A, Searle SD, Gahbauer EA, Gill TM, Rockwood K. Transitions in frailty status in older adults in relation to mobility: a multistate modeling approach employing a deficit count. J Am Geriatr Soc. 2011;59:524–529. doi: 10.1111/j.1532-5415.2011.03300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Dir Assoc. 2014;15:281–286. doi: 10.1016/j.jamda.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 15. Trevisan C, Veronese N, Maggi S, et al. Factors influencing transitions between frailty states in elderly adults: the Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc. 2017;65:179–184. doi:10.1111/jgs.14515 [DOI] [PubMed] [Google Scholar]
- 16. Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008;63:984–990. [DOI] [PubMed] [Google Scholar]
- 17. Huisman M, Poppelaars J, van der Horst M, et al. Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol. 2011;40:868–876. doi: 10.1093/ije/dyq219 [DOI] [PubMed] [Google Scholar]
- 18. Hoogendijk EO, Deeg DJ, Poppelaars J, et al. The Longitudinal Aging Study Amsterdam: cohort update 2016 and major findings. Eur J Epidemiol. 2016;31:927–945. doi: 10.1007/s10654-016-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 20. Jackson C. Multi-state modelling with R: the msm package. Version 1.6.6. February 1, 2018. https://cran.r-project.org/web/packages/msm/index.html. Accessed May 10, 2018. [Google Scholar]
- 21. Goldstein H. Multilevel Statistical Models. London, UK: Arnold; 1995. [Google Scholar]
- 22. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 23. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174:984–992. doi: 10.1093/aje/kwr183 [DOI] [PubMed] [Google Scholar]
- 24. Hedges L, Vevea J. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 25. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 26. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010;58:967–975. doi:10.1111/j.1532-5415.2010.02811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:887–895. doi: 10.1093/gerona/glq064 [DOI] [PubMed] [Google Scholar]
- 29. Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hardy SE, Studenski SA. Fatigue predicts mortality in older adults. J Am Geriatr Soc. 2008;56:1910–1914. doi: 10.1111/j.1532-5415.2008. 01957.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avlund K. Fatigue in older adults: an early indicator of the aging process?Aging Clin Exp Res. 2010;22:100–115. [DOI] [PubMed] [Google Scholar]
- 32. Sanford AM. Anorexia of aging and its role for frailty. Curr Opin Clin Nutr Metab Care. 2017;20:54–60. doi: 10.1097/MCO.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 33. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.