Abstract
Aims
Left ventricular (LV) ejection fraction (LVEF) is an extensively utilized marker of LV function that is often interpreted without recourse to alterations in LV geometry and hypertrophy. LV global function index (LVGFI) is a novel marker that incorporates LV structure in the assessment of LV cardiac performance. We evaluated the prognostic utility of LVGFI from young adulthood into middle age for incident heart failure (HF) and cardiovascular disease (CVD) in comparison to LVEF.
Methods and results
Included were 4107 CARDIA participants with echocardiograms in Year-5 (1990–1991). LVGFI was defined as LV stroke volume/LV global volume*100, where LV global volume was the sum of the LV mean cavity volume ((LV end-diastolic volume + LV end-systolic volume)/2) and myocardial volume (LV mass/density). Adjusted Cox proportional hazard models were utilized to predict incident HF and CVD outcomes. Mean age of participants was 29.8 ± 3.7 years, 55% female, and 48.7% black. Higher body mass index [beta coefficient (B) = −0.11 standard error (SE) = 0.02, P < 0.001], higher blood pressure (B = −0.04, SE = 0.01, P < 0.01), smoking (B = −0.82, SE = 0.22, P < 0.001), male sex (P < 0.001), and black race (P < 0.001) were associated with worse LVGFI. A total of 207 incident CVD events were observed over the course of 98 035 person-years at risk. Higher LVGFI was associated with HF, hazard ratio (HR) = 0.70, 95% confidence interval (CI) (0.54–0.91), hard CVD HR = 0.83, 95% CI (0.71–0.96), and all CVD HR = 0.83, 95% CI (0.72–0.96). For HF outcomes, Harrell’s C-statistic for LVGFI (0.80) was greater than LVEF (0.66).
Conclusion
LVGFI is a strong, independent predictor of incident HF and CVD that provides incremental prognostic value compared with LVEF. Male sex, black race, obesity, hypertension, and smoking are associated with worse LVGFI in the early adult lifespan.
Keywords: early adulthood, left ventricular systolic function, left ventricular ejection fraction, cardiac function, cardiac structure, heart failure
Introduction
Left ventricular (LV) ejection fraction (LVEF) is a putative measure of LV function that is widely utilized to guide research, clinical decision-making for individuals with cardiovascular disease (CVD), and risk stratification in the general population.1–3 LVEF was conceptualized in the mid 20th century as a mathematical expression of ejected stroke volume to LV volume at the end of diastole and interpreted as a surrogate for LV systolic pump function.4 LV systolic dysfunction determined by LVEF is a strong predictor of adverse outcomes in the aftermath of myocardial infarction as well as in asymptomatic community-dwelling individuals.1,2,5 Beyond alterations in systolic function, aging and cardiovascular risk factors can also induce LV geometric alterations and myocardial hypertrophy,6,7 which provide additional predictive value for adverse cardiovascular outcomes.7–9 As standardization of LV assessment with functional indices such as strain by speckle tracking echocardiography continues to progress,10,11 it is reasonable to explore approaches to optimize readily available tools already at our disposal. LV functional indices that incorporate structural components of adverse myocardial remodelling may provide incremental prognostic value compared with LVEF.
LV global function index (LVGFI) is a cardiac magnetic resonance (CMR) validated measure of LV cardiac performance that integrates LV structure into LV functional assessment, defined as LV stroke volume/LV global volume*100 where LV global volume was calculated as the sum of the LV mean cavity volume ((LV end-diastolic volume + LV end-systolic volume)/2) and myocardial volume (LV mass/density).12 LVGFI by CMR was associated with CVD outcomes in a cohort of middle-aged-to-elderly individuals. We explored the hypothesis that among young adults, echocardiography defined LVGFI would be associated with cardiovascular risk burden, incident heart failure (HF), and CVD later in life. We examined these relationships in the Coronary Artery Risk Development in Young Adults (CARDIA) study—a community-based prospective cohort study that provided a unique opportunity to explore these changes over the early adult lifespan.
Methods
Study design and participant selection
The objectives and study design of CARDIA have been described in detail elsewhere.13 Briefly, CARDIA is a National Heart, Lung, and Blood Institute multicentre community-based prospective cohort study that enrolled 5115 men and women from four US field centres (Birmingham, AL; Oakland, CA; Chicago, IL; and Minneapolis, MN), aged 18–30 years, who were free of CVD at study inception [Year-0 exam (1985–1986)] with 30 years of follow-up in eight subsequent visits up until 2015–2016 (Year-30 examination). Baseline for this current study was specified as the CARDIA Year-5 exam (1990–1991). Out of a total of 4352 CARDIA participants who attended the Year-5 exam, 109 had no available echocardiograms, one participant withdrew consent, and 135 had missing or suboptimal images for one or more LV parameter. The CARDIA Year-5 echocardiography examination was focused on the acquisition of 2D-guided M-mode,14 thus fewer numbers of two-dimensional (2D) images were available. This resulted in an analytical sample of 4107 study participants with 2D guided M-mode derived LV parameters and a subset of 1868 study participants with 2D LV data. Study was conducted in accordance with the Declaration of Helsinki. All study participants gave written informed consent, and the institutional review board of each participating institution approved the study.
Echocardiography
CARDIA Year-5 study participants underwent echocardiographic assessments performed on an Acuson cardiac ultrasound system (Siemens), fitted with 2.5 and 3.75 MHz phased-array transducers.15 An echocardiography-reading centre was located at the University of California, Irvine, and a quality control and co-ordinating centre at the University of Alabama in Birmingham. Trained sonographers, across all four-field centres, acquired images following standardized quality control protocols available on the CARDIA website (https://www.cardia.dopm.uab.edu/exam-materials2). Standardized training of readers, periodic reader review sessions, phantom studies on ultrasound equipment, quality control audits, and blind duplicate readings were executed to achieve acceptable inter- and intra-reader measurement variability. The reproducibility of LV mass and volume parameters from, which LVGFI was calculated has been previously published.2,15
Studies were recorded on super-VHS videotapes and measurements were made from digitized images using a Dextra D-200 off-line analysis system. LV volumes and LVEF were obtained using Teichholz method. Simpson’s method was also used in 1868 participants with 2D echocardiograms. LV mass was measured from 2D-guided M-mode short-axis views using the leading-edge-to-leading-edge technique in accordance with recommendations by the American Society of Echocardiography.16
Left ventricular global function index
LVGFI (%) was defined as LV stroke volume/LV global volume*100 where LV global volume was calculated as the sum of the LV mean cavity volume ((LV end-diastolic volume + LV end-systolic volume)/2) and myocardium volume (LV mass/density). Density of LV was specified as 1.05 g/mL.12 A higher LVGFI reflects better LV cardiac performance.
Cardiovascular risk factors
Risk factor assessments in the CARDIA study Year-5 exam have been previously described.9,17 In brief, three resting seated blood pressure measurements were obtained using a random-zero sphygmomanometer. The mean of the second and third blood pressure readings was utilized. Smoking status and use of anti-hypertensive medications were self-reported using validated questionnaires.17,18 Total cholesterol, high-density lipoprotein cholesterol, and triglycerides were determined using enzymatic assays.17
Cardiovascular outcomes
The CARDIA study outcomes ascertainment protocols have been described in detail elsewhere.2,8 Cardiovascular endpoints of interest were HF, hard CVD, and all CVD events. HF required admission for new-onset decompensated HF and classification was based on a constellation of clinical symptoms, signs, and imaging. Hard CVD included fatal or non-fatal ST-elevation myocardial infarction, non-ST elevation acute coronary syndrome, stroke, HF, coronary artery disease, peripheral artery, and carotid artery disease. All CVD included hard CVD as well as cardiac revascularization and transient ischaemic attacks. Routine reviews of the National Death Index were conducted. Surveillance continued through 31 August 2015, or last contact in those without an event.
Statistical analysis
Baseline characteristics of participants were presented as mean ± standard deviation for continuous variables and frequency with proportions for categorical variables. The goals of the analyses were (i) To assess the association between CVD risk factors and LVGFI. (ii) Explore the associations between LVGFI and incident HF, hard CVD, and all CVD events in comparison to LVEF. (iii) Examine the comparative discriminative ability between LVGFI and LVEF. Accordingly, adjusted multivariable linear regression models, using LVGFI as the dependent variable, were used to assess the association between cardiovascular risk factors and LVGFI. Models were adjusted for age, sex, race, heart rate, body mass index (BMI), systolic blood pressure, diastolic blood pressure, anti-hypertension medication use, high-density lipoprotein cholesterol, total cholesterol, diabetes, and smoking status, as appropriate. Linear assumptions and goodness of fit were verified. Nelson–Aalen cumulative hazard estimates for LVGFI quartiles and HF and all CVD were generated and plotted. Cox proportional hazard regression models using LV function parameters as the explanatory variable and adjusted for confounders were used to determine relative hazards with 95% confidence intervals (CIs) for incident HF, hard CVD, and all CVD events. For survival analysis, proportional hazards assumption was met based on Schoenfeld residuals. Harrell’s C-statistics were derived to examine the discrimination between LV indices and CVD outcomes. The best fit for each LV indicator was assessed using Akaike information criterion. Statistical significance was set at P < 0.05. All analyses were performed using Stata (StataCorp, version 15; StataCorp, College Station, TX, USA).
Results
Baseline characteristics
Table 1 displays the baseline characteristics of our study sample of 4107 young adults, stratified by CVD outcomes. Figure 1 shows the normal distribution of LVGFI. Mean age of participants with no cardiovascular events was 29.9 ± 3.6 years in contrast to 31.2 ± 3.5 years for individuals with CVD. Compared with those who did not develop HF or CVD, young adults with subsequent HF and CVD were more likely to be males, blacks and had higher mean systolic and diastolic blood pressures. A higher proportion of individuals with HF were current smokers and diabetics compared with event-free study subjects. Mean LVGFI was 34.8 ± 6.1 in event-free individuals. All LV functional parameters were lower (worse) in individuals with CVD compared with those without CVD.
Table 1.
Baseline characteristics for Year-5 CARDIA participants with and without cardiovascular events
| Characteristics | No events (N = 3900) | All CVD (N = 207) | Hard CVD (N = 185) | Heart failure (N = 59) |
|---|---|---|---|---|
| Age (years) | 29.9 ± 3.6 | 31.2 ± 3.5 | 31.09 ± 3.6 | 30.7 ± 3.8 |
| Male sex | 1720 (44.1%) | 128 (61.8%) | 115 (62.2%) | 36 (61.0%) |
| Black | 1873 (48%) | 127 (61.4%) | 120 (64.9%) | 49 (83.1%) |
| Systolic BP (mmHg) | 107.3 ± 11.2 | 114.1 ± 14.7 | 114.8 ± 15.0 | 116.3 ± 15.2 |
| Diastolic BP (mmHg) | 68.9 ± 9.7 | 74.3 ± 12.0 | 74.8 ± 12.1 | 76.7 ± 12.6 |
| Diabetes | 60 (1.5%) | 13 (6.3%) | 12 (6.5%) | 1 (1.7%) |
| Total cholesterol (mg/dL) | 177.2 ± 33.8 | 192.8 ± 39.7 | 193 ± 40.4 | 193.0 ± 40.3 |
| HDL-C (mg/dL) | 53.6 ± 14.2 | 48.7 ± 14.3 | 49.1 ± 14.4 | 51.7 ± 14.6 |
| Current smoker | 1064 (27.3%) | 98 (47.8%) | 92 (50.3%) | 26 (44.1%) |
| Anti-HTN medication use | 51 (1.3%) | 14 (6.8%) | 13 (7%) | 4 (6.8%) |
| LVEF (M-mode) (%) | 64.6 ± 7.9 | 63.4 ± 9.6 | 63.3 ± 9.7 | 60.9 ± 11.9 |
| LVEF (2D) (%) | 63.3 ± 11.5 | 61.4 ± 6.0 | 61.2 ± 5.9 | 59.8 ± 6.6 |
| LV mass (g) | 148.2 ± 44.0 | 174.2 ± 50.2 | 176.8 ± 51.9 | 189.5 ± 63.6 |
| LVGFI (M-mode) (%) | 34.8 ± 6.1 | 32.4 ± 6.9 | 32.2 ± 7.0 | 30.9 ± 8.0 |
| LVGFI (2D) (%) | 34.6 ± 6.4 | 31.4 ± 6.3 | 31.3 ± 6.4 | 27.3 ± 6.6 |
| LV stroke volume (2D) (mL) | 77.5 ± 20.4 | 79.0 ± 21.3 | 79.1 ± 21.2 | 77.4 ± 20.4 |
| LV EDV (2D) (mL) | 122.4 ± 30.3 | 127.9 ± 33.3 | 128.5 ± 33.7 | 129.6 ± 40.4 |
| LV ESV (2D) (mL) | 44.9 ± 14.2 | 48.9 ± 15.2 | 49.4 ± 15.6 | 51.0 ± 17.2 |
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LVEF, left ventricular ejection fraction; LV, left ventricular; LVGFI, left ventricular global function index; EDV, end-diastolic volume; ESV, end-systolic volume.
Data are expressed as median (interquartile range), mean ± standard deviation, or frequency (proportion). Two-dimensional LVEF was derived from 1868 participants using Simpson’s method.
Figure 1.
Normal distribution of left ventricular global function index, percentage for M-mode and 2D echocardiography.
Cardiovascular risk factors and LVGFI
The association between baseline cardiovascular risk factors and LVGFI is shown in Table 2. In adjusted multivariable linear regression analysis, higher BMI [beta coefficient (B) = −0.11, standard error (SE) 0.02, P < 0.001], higher diastolic blood pressure (B = −0.04, SE 0.01, P < 0.01), current smokers (B = −0.82, SE 0.22, P < 0.001), male sex (P < 0.001), and black race (P < 0.001) were associated with worse LVGFI. There was no relationship between blood pressure, smoking status, or use of anti-hypertensive medications and LVEF (see Supplementary data online, Table S1).
Table 2.
Multivariable linear regression showing the relationship between cardiovascular risk factors at CARDIA Year-5 and left ventricular global function index
| Cardiovascular risk factors | Univariables | Multivariables |
|---|---|---|
| Β-Coefficient (SE) | Β-Coefficient (SE) | |
| Age (years) | 0.03 (0.03) | 0.02 (0.03) |
| Female | 3.23 (0.19)*** | 3.23 (0.22)*** |
| White | 1.53 (0.19)*** | 1.20 (0.20)*** |
| Resting heart rate (beats per 30 s) | 0.02 (0.02) | −0.03 (0.02) |
| Body mass index (kg/m2) | −0.14 (0.02)*** | −0.11 (0.02)*** |
| Systolic BP (mmHg) | −0.08 (0.01)*** | 0.01 (0.01) |
| Diastolic BP (mmHg) | −0.09 (0.01)*** | −0.04 (0.01)** |
| Anti-hypertensive medication use | −0.85 (0.8) | 0.52 (0.78) |
| HDL-C (mg/dL) | 0.05 (0.01)*** | 0.004 (0.007) |
| Total cholesterol (mg/dL) | −0.006 (0.003)* | 0.0004 (0.003) |
| Diabetes | 0.23 (0.22) | 0.28 (0.21) |
| Smoking status | ||
| Never | Ref | Ref |
| Former | 0.37 (0.29 | −0.012 (0.28) |
| Current | −1.05 (0.22)*** | −0.82 (0.22)*** |
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; SE, standard error.
Multivariate models showing coefficients and standard errors (in parentheses) per 1% change in LVGFI.
Multivariable linear regression models showing unadjusted and adjusted coefficients and standard errors for LVGFI as the outcome and the above listed cardiovascular risk factors.
Models are adjusted for age, sex, race, heart rate, body mass index, systolic blood pressure, diastolic blood pressure, anti-hypertension medication use, HDL-C, total cholesterol, diabetes, and smoking status, as appropriate.
P < 0.001.
P < 0.01.
P < 0.05.
LVGFI and heart failure and cardiovascular disease outcomes
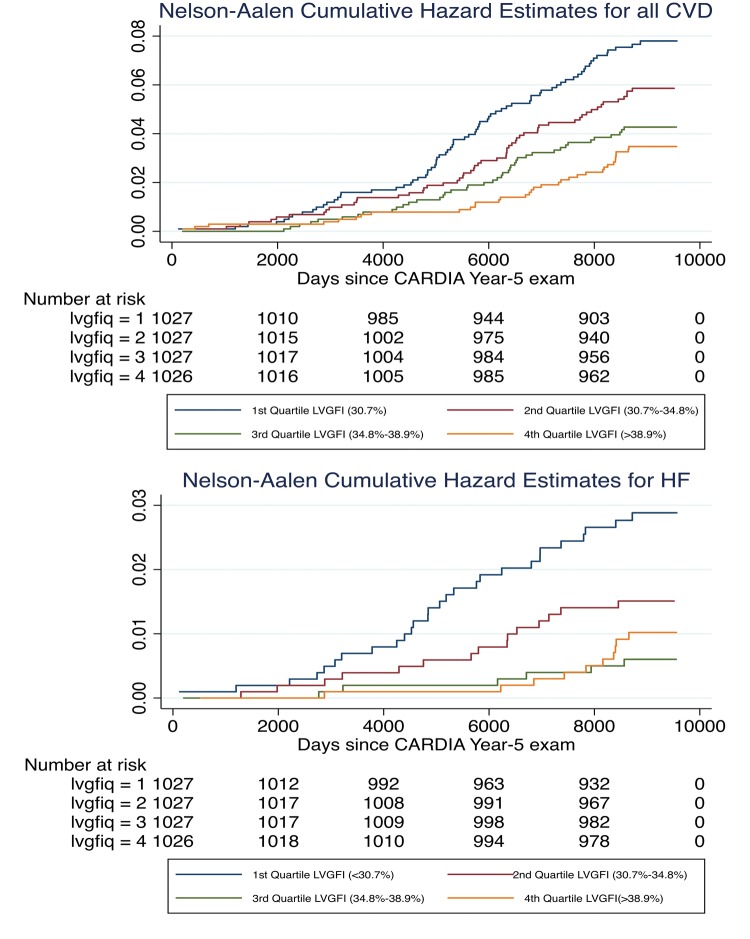
During a median longitudinal follow-up of 24.9 years, a total of 207 incident CVD events were observed over the course of 98 035 person-years at risk. Over the period of follow-up, HF and hard CVD developed in 1.4% and 4.5% of the study cohort, respectively. In ascending order, 1st–4th quartiles of LVGFI were (<30.7%, 30.7–34.8%, 34.8–38.9%, >38.9%).
Nelson–Aalen cumulative hazard estimates according to LVGFI quartiles were displayed (Figure 2). The 1st quartile of LVGFI (<30.7%) had a significantly higher risk of cardiovascular events compared with the 4th quartile of LVGFI (>38.9%) for both HF and all CVD (log rank P < 0.01 for all). In adjusted Cox proportional hazard models (Table 3), higher LVGFI by 2D was associated with incident HF HR = 0.49, 95% CI (0.34–0.68), hard CVD HR = 0.68, 95% CI (0.54–0.86), and all CVD HR = 0.66, 95% CI (0.46–0.93). Similarly, higher LVGFI by M-mode was associated with a lower hazard ratio (HR) of incident HF = 0.70, 95% CI (0.54–0.91), hard CVD HR = 0.83, 95% CI (0.71–0.96), and all CVD HR = 0.83, 95% CI (0.72–0.96).
Figure 2.
Nelson–Aalen cumulative hazard estimates for all cardiovascular disease (above) and heart failure, respectively by quartiles of LVGFI (%). Lower LVGFI represents worse function. LVGFI, left ventricular global function index.
Table 3.
Cox proportional hazard models showing the relationship between left ventricular global function index and incident cardiovascular disease events
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Heart failure | ||
| LVGFI (M-mode) 59/4107 | 0.54 (0.42–0.69) | 0.70 (0.54–0.91) |
| LVGFI (2D) 21/1868 | 0.43 (0.33–0.57) | 0.48 (0.34–0.68) |
| LVEF (M-mode) 59/4107 | 0.65 (0.51–0.81) | 0.69 (0.55–0.86) |
| LVEF (2D) 21/1868 | 0.55 (0.35–0.87) | 0.51 (0.30–0.86) |
| Hard cardiovascular disease | ||
| LVGFI (M-mode) 185/4107 | 0.66 (0.58–0.77) | 0.83 (0.71–0.96) |
| LVGFI (2D) 78/1868 | 0.62 (0.51–0.76) | 0.68 (0.54–0.86) |
| LVEF (M-mode) 185/4107 | 0.85 (0.74–0.98) | 0.86 (0.75–0.99) |
| LVEF (2D) 78/1868 | 0.67 (0.50–0.91) | 0.64 (0.45–0.91) |
| All cardiovascular disease | ||
| LVGFI (M-mode) 207/4107 | 0.68 (0.59–0.78) | 0.83 (0.72–0.96) |
| LVGFI (2D) 84/1868 | 0.63 (0.52–0.76) | 0.68 (0.54–0.86) |
| LVEF (M-Mode) 207/4107 | 0.86 (0.75–0.98) | 0.87 (0.76–0.99) |
| LVEF (2D) 84/1868 | 0.68 (0.51–0.92) | 0.66 (0.46–0.93) |
CI, confidence interval; 2D, two-dimensional; HR, hazard ratio; LVEF, left ventricular ejection fraction; LVGFI, left ventricular global function index.
Estimated relative hazards are per 1 standard deviation increase in each LV parameter.
Cox proportional hazard models showing HRs and 95% CIs for the relationships between left ventricular parameters and incident cardiovascular disease events.
Models adjusted for age, sex, race, resting heart rate, body mass index, systolic and diastolic blood pressure, anti-hypertensive medications, diabetes status, high-density lipoprotein cholesterol, total cholesterol, and smoking status.
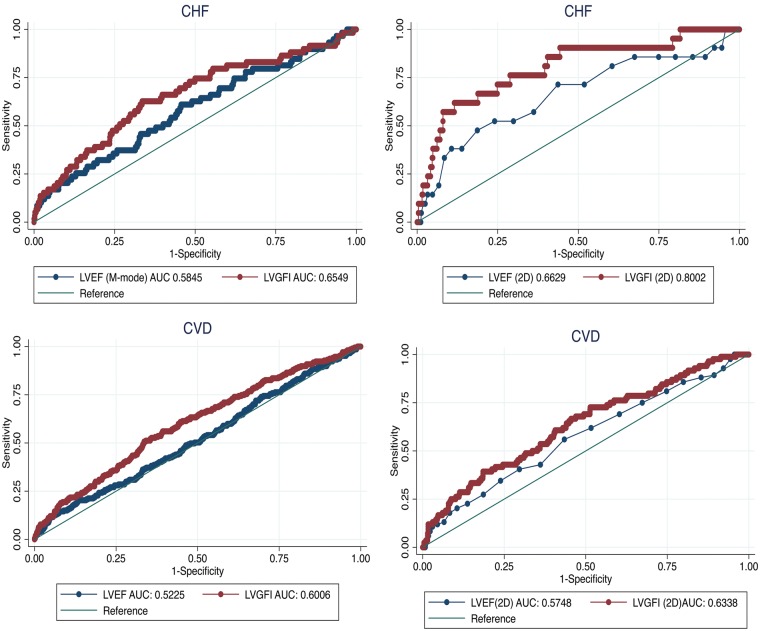
Comparison of model performance
For Harrell’s C-statistic, as shown in Table 4 and Figures 3, the area under the curve’s for survival analysis of unadjusted cardiovascular outcomes of interest were greater for LVGFI compared with LVEF. With respect to HF events, Harrell’s C-statistic for LVGFI (0.80) was greater than LVEF (0.66). Similarly, for all CVD, Harrell’s C-statistic for LVGFI (0.63) was significantly higher compared with LVEF (0.57).
Table 4.
Harrell C-statistic for the predictive power of LV parameters and CVD outcomes determined from non-adjusted cox regression models
| LV parameters | C-statistic/AUC | 95% CI |
|---|---|---|
| Heart failure | ||
| LVGFI (2D) | 0.80 | 0.69–0.91 |
| LVEF (2D) | 0.66 | 0.53–0.80 |
| LVGFI | 0.65 | 0.58–0.73 |
| LVEF | 0.58 | 0.51–0.66 |
| Hard CVD | ||
| LVGFI (2D) | 0.64 | 0.58–0.71 |
| LVEF (2D) | 0.58 | 0.52–0.65 |
| LVGFI | 0.61 | 0.56–0.65 |
| LVEF | 0.52 | 0.48–0.56 |
| ALL CVD events | ||
| LVGFI (2D) | 0.63 | 0.57–0.70 |
| LVEF(2D) | 0.57 | 0.51–0.64 |
| LVGFI | 0.60 | 0.56–0.64 |
| LVEF | 0.52 | 0.48–0.56 |
AUC, area under the curve; CI, confidence interval; 2D, two-dimensional; LV, left ventricular; LVEF, left ventricular ejection fraction; LVGFI, left ventricular global function index.
Figure 3.
Receiver-operating characteristics showing the area under the curves for heart failure (above) and all cardiovascular disease (below), using M-mode and 2D-echocardiography. Blue line represents left ventricular ejection fraction and red line represents left ventricular global function index.
For all CVD outcomes in study as shown in Table 5, Akaike information criterion for LVGFI was consistently lower (better model fit) compared with LVEF.
Table 5.
Akaike information criterion for LV functional indices and CV outcomes
| ALL CVD | Hard CVD | CHF | |
|---|---|---|---|
| LV functional indices | AIC | AIC | AIC |
| LVEF (M-mode) | 3418.7 | 3056.3 | 966.4 |
| LVGFI (M-mode) | 3392.1 | 3029.8 | 955.8 |
| LVEF (2D) | 1253.0 | 1163.2 | 312.4 |
| LVGFI (2D) | 1238.4 | 1149 | 294.0 |
AIC, Akaike information criterion; CV, cardiovascular; 2D, two-dimensional; LV, left ventricular; LVEF, left ventricular ejection fraction; LVGFI, left ventricular global function index.
Discussion
In this large, well-characterized prospective cohort study of young adults followed for nearly 25 years, our findings demonstrate that LVGFI is associated with early cardiovascular risk factors such as male sex, black race, higher blood pressure, higher BMI, and cigarette smoking. Importantly, we establish that in relatively healthy adults, LVGFI is a strong, independent, antecedent predictor of incident HF and CVD that provides incremental prognostic value compared with LVEF. Our findings underscore the benefit of utilizing LV functional indices that integrate LV structural components of cardiac remodelling in the assessment of LV cardiac performance and prognosis over long periods of time.
LVGFI is a promising LV function index for screening, surveillance in individuals with CVD, and risk-stratification in the general population.12,19 Prior studies have shown that in comparison to LVEF, LVGFI by CMR was not only associated but also provided incremental prognostic value for incident HF and CVD in community-dwelling individuals as well as mortality following coronary reperfusion after myocardial infarction.12,19 Our study results are consistent with these findings, and importantly extend them to the early adult lifespan and the use of echocardiography. To our knowledge, this is the first comprehensive study evaluating the relationship between echocardiography determined LVGFI, cardiovascular risk burden and, future cardiovascular events.
In comparison to CMR, echocardiography is more routinely utilized in clinical and research settings. The present study includes community-dwelling individuals in early adulthood, which represents an important period that allows for lifestyle modification or medical therapy that can mitigate the effects of early modifiable risk factors for cardiac remodelling. Antecedent cardiac remodelling can precede overt CVD by decades.2 Our study results showed a relationship between LVGFI and modifiable and non-modifiable risk factors even at an early age. These findings have important implications for public health and highlight the need for enhanced and sustained efforts for preventing, identifying, and treating CVD risk factors from early adulthood.
In a multicentre randomized clinical trial of individuals with coronary reperfusion for ST-elevation myocardial infarction, lower LVGFI was associated with longer door-to-balloon time, presence of left anterior descending artery culprit lesions, larger infarct size, and microvascular obstruction assessed by CMR.19 In this clinical trial, LVGFI provided incremental predictive value for mortality in comparison to LVEF.19 Similarly, in the Multi-Ethnic Study of Atherosclerosis, LVGFI provided incremental value over LVEF for a wide spectrum of CVD.12 In the present study, we demonstrate strong associations between LVGFI and CVD events including HF. These associations persisted even after accounting for confounding cardiovascular risk factors.
It is increasingly clear that beyond alterations in systolic function, cardiac remodelling encompasses a broad range of pathophysiological alterations that can occur independently or concomitantly with systolic dysfunction.20 Aging-related risk factor exposure and neurohormonal activation can induce a maladaptive sequence of molecular, cellular and interstitial effects in the setting of elevated arterial loading conditions. These changes induce adverse alterations in LV stiffness, LV filling pressures, diastolic function, LV geometry, and hypertrophy leading to the manifestation of morphological phenotypes such as concentric remodelling, concentric and eccentric hypertrophy.21–25
In concentric LV phenotypes—with smaller LV volumes and thicker walls—a higher LVEF does not necessarily result in the production of a metabolically sufficient cardiac output. The prevalence of HFpEF is increasing,26 this may be related to changing trends in demographics, risk factors, and epigenetic effects, but also at least in part, to the greater propensity for concentric remodelling and hypertrophy with senescence and increasing life expectancy.27
Despite increasing recognition for the role of LV hypertrophy and geometric alterations for adverse cardiovascular outcomes,8,28,29 interpretations of LVEF are often made without recourse to structural components of cardiac remodelling.
The incremental prognostic and discriminatory performance of LVGFI compared with LVEF clearly highlights the utility of LVGFI as a LV function index that under the influence of aging-related exposure to cardiovascular risk factors—captures LV cardiac performance beyond systolic alterations alone. Apart from the benefit of inclusion of structural aspects of cardiac remodelling, another contributory factor for the improved performance of LVGFI may be related to the tendency of LVEF to overestimate LV systolic function in concentric LV phenotypes,12 which is frequently the prevailing phenotypic expression in HFpEF. LVGFI was more closely associated with cardiovascular risk factors such as hypertension and smoking when compared with LVEF. The increased sensitivity of LVGFI to cardiovascular risk factors in comparison to LVEF, partly explains its superior predictive and discriminatory ability.
Observational studies have suggested that HFpEF is more frequently identified in the elderly.27 In our study of young adults, LVEF was a strong predictor of HF and CVD. However, even in this presumably predominant HF with reduced ejection fraction subgroup (HFrEF),2 LVGFI consistently showed robust, incremental prognostic value over LVEF for incident HF as well as CVD. Our study findings, therefore, highlight the prognostic utility of LVGFI in the first half of the human life span across a broad spectrum of CVDs. Thus, they underscore the incremental benefit of integrating structural components of adverse cardiac remodelling in the assessment of LV cardiac performance. Further studies are however needed to elucidate the utility of LVGFI in various clinical and research settings.
Strengths and limitations
The unavailability of ejection fraction at the time of HF precluded sub-analysis of HFrEF, HFmrEF, or HFpEF. Absolute numbers of CVD events were not high, but cohort CVD events incidence rates are in accordance with the expected incidence among young healthy adults.2
Despite the relatively small number of events, which can serve as a conservative bias, we found that antecedent impairment in LVGFI remained a strong marker of future HF and CVD. Strain by speckle tracking echocardiography and tissue Doppler was not available at the time of image acquisition in 1990. The clear strength of this study was the large, well-phenotyped cohort of young adults with good echocardiographic quality control procedures and reproducibility profile.14,30
Clinical perspective
Our findings establish a relationship between LVGFI and modifiable and non-modifiable risk factors even at an early age in a biracial population. These findings have important implications for sustained efforts for preventing, identifying, and treating CVD risk factors from early adulthood. We demonstrate the strong predictive value of LVGFI across a broad spectrum of CVDs, underscoring the incremental benefit of integrating structural components of adverse cardiac remodelling in the assessment of LV cardiac performance for individuals with CVD and for risk stratification in the general population.
Funding
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005).
Conflict of interest: none declared.
Supplementary Material
References
- 1. Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, Carr JJ. et al. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA). Circulation 2012;126:2713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A. et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009;360:1179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE.. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Packer M. Heart failure with a mid-range ejection fraction: a disorder that a psychiatrist would love. JACC Heart Fail 2017;5:805–7. [DOI] [PubMed] [Google Scholar]
- 5. Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE. et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 2011;124:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang CN, Gerdts E, Aurigemma GP, Boman K, de Simone G, Dahlof B. et al. Four-group classification of left ventricular hypertrophy based on ventricular concentricity and dilatation identifies a low-risk subset of eccentric hypertrophy in hypertensive patients. Circ Cardiovasc Imaging 2014;7:422–9. [DOI] [PubMed] [Google Scholar]
- 7. Ambale-Venkatesh B, Yoneyama K, Sharma RK, Ohyama Y, Wu CO, Burke GL. et al. Left ventricular shape predicts different types of cardiovascular events in the general population. Heart 2017;103:499–507. [DOI] [PubMed] [Google Scholar]
- 8. Armstrong AC, Jacobs DR Jr, Gidding SS, Colangelo LA, Gjesdal O, Lewis CE. et al. Framingham score and LV mass predict events in young adults: CARDIA study. Int J Cardiol 2014;172:350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gidding SS, Liu K, Colangelo LA, Cook NL, Goff DC, Glasser SP. et al. Longitudinal determinants of left ventricular mass and geometry: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging 2013;6:769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moreira HT, Nwabuo CC, Armstrong AC, Kishi S, Gjesdal O, Reis JP. et al. Reference ranges and regional patterns of left ventricular strain and strain rate using two-dimensional speckle-tracking echocardiography in a healthy middle-aged black and white population: the CARDIA study. J Am Soc Echocardiogr 2017;30:647–58.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R. et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 2015;16:1–93. [DOI] [PubMed] [Google Scholar]
- 12. Mewton N, Opdahl A, Choi EY, Almeida AL, Kawel N, Wu CO. et al. Left ventricular global function index by magnetic resonance imaging—a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertension 2013;61:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr. et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 14. Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T. et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation 1995;92:380–7. [DOI] [PubMed] [Google Scholar]
- 15. Gardin JM, Brunner D, Schreiner PJ, Xie X, Reid CL, Ruth K. et al. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol 2002;40:529–35. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong AC, Gidding SS, Colangelo LA, Kishi S, Liu K, Sidney S. et al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20-year follow-up period: the CARDIA study. BMJ Open 2014;4:e004001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Appiah D, Schreiner PJ, Gunderson EP, Konety SH, Jacobs DR Jr, Nwabuo CC. et al. Association of gestational diabetes mellitus with left ventricular structure and function: the CARDIA study. Diabetes Care 2016;39:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eitel I, Poss J, Jobs A, Eitel C, de Waha S, Barkhausen J. et al. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J Cardiovasc Magn Reson 2015;17:62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gjesdal O, Bluemke DA, Lima JA.. Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat Rev Cardiol 2011;8:673–85. [DOI] [PubMed] [Google Scholar]
- 21. Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S. et al. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging 2014;7:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaasch WH, Zile MR.. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol 2011;58:1733–40. [DOI] [PubMed] [Google Scholar]
- 23. Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA.. Controversies in ventricular remodelling. Lancet 2006;367:356–67. [DOI] [PubMed] [Google Scholar]
- 24. Ohyama Y, Ambale-Venkatesh B, Noda C, Chugh AR, Teixido-Tura G, Kim JY. et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2016;9:e004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nwabuo CC, Moreira HT, Vasconcellos HD, Ambale-Venkatesh B, Yoneyama K, Ohyama Y. et al. Association of aortic root dilation from early adulthood to middle age with cardiac structure and function: the CARDIA study. J Am Soc Echocardiogr 2017;30:1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S. et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation 2018;137:e493–2. [DOI] [PubMed] [Google Scholar]
- 27. Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S. et al. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging 2018;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL. et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP.. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K. et al. Quality control and reproducibility in M-mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography 2015;32:1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.