Abstract
Objectives:
Ewing sarcoma is a rare tumor of the head and neck. Previous efforts to characterize Ewing sarcoma of the head and neck (ES-HN) have been limited to small retrospective series. The objective of this study was to analyze the demographic, clinicopathologic, treatment, and survival characteristics of ES-HN compared to Ewing sarcoma at other locations (ES-other).
Methods:
Using the Surveillance, Epidemiology, and End Results (SEER) database, we compared 183 patients with ES-HN to 3177 patients with ES-other. Patient characteristics were analyzed with chi-square or t test. Ten-year disease-specific survival (DSS) and overall survival (OS) were estimated via the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox regression analysis was used to determine if HN location was an independent prognosticator.
Results:
The ES-HN displayed a lower tumor size (P < .001) and metastatic rate (P < .001) compared to ES-other. The ES-HN had a better 10-year DSS and OS than ES-other (P = .001, P = .015, respectively). The HN location did not achieve statistical significance on multivariate Cox regression analysis (P = .88).
Conclusion:
ES-HN does not appear to be a separate clinical entity compared to ES-other; rather, its associated improved prognosis is likely secondary to its smaller size and lower metastatic rate compared to ES-other.
Keywords: Ewing sarcoma, head and neck, SEER, survival
Introduction
Ewing sarcoma is a highly malignant tumor of bone and soft tissues that primarily occurs in children and young adults. Ewing sarcoma is most commonly located in the pelvis, axial skeleton, and extremities; however, Ewing sarcoma involving the head and neck has been reported to account for 3% to 9% of all Ewing tumors.1–4 Because Ewing sarcoma of the head and neck (ES-HN) accounts for such a small proportion of Ewing tumors, few analyses specific to this region have been performed. To date, the largest series of ES-HN was 51 patients.2 Previous studies have observed improved outcomes for patients with ES-HN compared to Ewing sarcoma at other locations (ES-other).1,3–6 However, these studies’ conclusions have been limited by small sample size and short follow-up times. Thus, the full extent to which ES-HN is similar or different to ES-other is unknown.
We hypothesized that ES-HN has different clinicopathologic characteristics, treatment approaches, and survival outcomes compared with ES-other. Using the Surveillance, Epidemiology, and End Results (SEER) database, we have collected the largest series of ES-HN to date, thereby allowing us to better understand this rare cancer and its relationship to ES-other.
Methods
Data Source and Patient Selection
The SEER database consisting of 18 patient registries for years 1993 to 2013 was used for this study. Because SEER data are de-identified and publicly available, this study was exempt from formal review by the Medical University of South Carolina Institutional Review Board. Patients were selected using the International Classification of Diseases for Oncology, third edition (ICD-O-3) codes for Ewing sarcoma (9260/3) and peripheral neuroectodermal tumor (9364/3). Patients with peripheral neuroectodermal tumor (PNET) were included because these tumors are recognized as a subgroup of Ewing sarcoma.7,8 Cases without a histologically confirmed diagnosis as well as those with unknown primary sites (C80.9) were excluded. Patients were divided into 2 groups based on anatomic location of the primary tumor: ES-HN and ES-other. The ICD-O-3 topography codes corresponding to ES-HN are listed in Table 1. All other cases were classified as ES-other.
Table 1.
Head and Neck Primary Sites.
| Primary Site | No. | % | ICD-O-3 Code |
|---|---|---|---|
| Total | 183 | ||
| Bones of skull and face and associated joints |
73 | 40 | C41.0 |
| Connective, subcutaneous, other soft tissue: head, face, neck |
55 | 30 | C49.0 |
| Mandible | 17 | 9 | C41.1 |
| Peripheral nerves and autonomic nervous system: head, face, neck |
5 | 3 | C47.0 |
| Ethmoid sinus | 4 | 2 | C31.1 |
| Maxillary sinus | 4 | 2 | C31.0 |
| Parotid gland | 3 | 2 | C07.9 |
| Orbit, NOS | 3 | 2 | C69.6 |
| Middle ear | 2 | 1 | C30.1 |
| Nasopharynx, NOS | 2 | 1 | C11.9 |
| Skin of scalp and neck | 2 | 1 | C44.4 |
| Other | 13 | 7 | C00.1, C01.9, C02.0, |
| C02.3, C04.9, C10.9, | |||
| C15.3, | |||
| C30.0, C31.2, C44.2, | |||
| C44.3, C73.9, C75.1 | |||
Abbreviation: NOS, not otherwise specified.
Study Variables
Patient variables of interest included age at diagnosis, sex, and race. Race was categorized as white, black, Hispanic, Asian/Pacific Islander, or American Indian/Alaskan. Tumor factors of interest included tumor size and extent of disease. Tumor size was categorized as ≤5 cm, >5 to 10 cm, or >10 cm. Extent of disease was categorized as localized/regional or distant. Treatment modality was categorized as surgery, radiotherapy, or surgery and radiotherapy. Relevant disease-specific survival (DSS) and overall survival (OS) variables were extracted for analysis. All data were obtained using SEER*Stat version 8.3.2 software (http://seer.cancer.gov/seerstat/).
Statistical Methods
Differences in demographic, clinicopathologic, and treatment characteristics between ES-HN and ES-other were compared using the chi-square test (categorical variables) and the t test (continuous variables). Kaplan-Meier survival analyses and log-rank test were used to compare 10-year DSS and OS between ES-HN and ES-other. Ten-year DSS data for ES-HN versus ES-other stratified by tumor size, extent of disease, and treatment modality were determined and compared using the life-tables function. Multivariate Cox regression analysis was used to assess the effect head and neck location has on 10-year DSS while controlling for known prognosticators. A P value <.05 was considered statistically significant for all test. All statistical analyses were performed using SPSS statistical software version 23 (IBM Corp, Armonk, New York, USA).
Results
Patient Characteristics and Treatment
A total of 183 patients with ES-HN and 2558 patients with ES-other were identified. Ewing sarcoma of the head and neck was most commonly found in bones of the skull and face (40%), followed by connective and soft tissues of the head and neck (30%) and the mandible (9%). The full distribution of ES-HN primary sites is listed in Table 1.
Table 2 shows demographic, clinicopathologic, and treatment differences between ES-HN and ES-other. ES-HN tumors were more commonly ≤5 cm compared to ES-other (60.5% vs 23.9%; P < .001) and less likely to be 5 to 10 cm and >10 cm compared to ES-other (38.8% vs 42.8% and 0.8% vs 33.4%, respectively; P < .001). Ewing sarcoma of the head and neck presented more commonly with local/regional disease compared to ES-other (82.1% vs 65.6%; P < .001) and likewise were less likely to present with metastasis at presentation (17.9% vs 34.4%; P < .001). Regarding treatment modality, ES-HN was less likely to be treated by single modality therapy and was more commonly treated with both surgery and radiotherapy (47.5% vs 30.5%; P < .001). There were no statistically significant differences for mean age at diagnosis, sex, race, and histology between the 2 cohorts.
Table 2.
Demographics, Clinicopathologic, and Treatment Characteristics of Ewing Sarcoma.a
| Head and Neck ES |
Other ES |
||||
|---|---|---|---|---|---|
| No. | % | No. | % | P Value | |
| Total | 183 | 2558 | |||
| Mean age at diagnosis (SD) | 25.5 (20.1) | 23.4 (16.6) | .093 | ||
| Sex | .074 | ||||
| Male | 96 | 52.5 | 1514 | 59.2 | |
| Female | 87 | 47.5 | 1044 | 40.8 | |
| Race | .77 | ||||
| White | 113 | 62.1 | 1723 | 67.6 | |
| Hispanic | 46 | 25.3 | 544 | 21.4 | |
| Asian or Pacific Islander | 13 | 7.1 | 154 | 6 | |
| Black | 8 | 4.4 | 100 | 3.9 | |
| American Indian/Alaska Native | 2 | 1.1 | 27 | 1.1 | |
| Unknown | 1 | 10 | |||
| Tumor size | <.001 | ||||
| ≤5 cm | 78 | 60.5 | 400 | 23.9 | |
| 5–10 cm | 50 | 38.8 | 717 | 42.8 | |
| >10 cm | 1 | 0.8 | 560 | 33.4 | |
| Unknown | 54 | 881 | |||
| Extent of disease | <.001 | ||||
| Local/regional | 138 | 82.1 | 1549 | 65.6 | |
| Distant | 30 | 17.9 | 811 | 34.4 | |
| Unknown | 15 | 182 | |||
| Histology | .46 | ||||
| PNET | 10 | 5.5 | 110 | 4.3 | |
| Ewing Sarcoma | 173 | 94.5 | 2448 | 95.7 | |
| Treatment modality | <.001 | ||||
| Surgery | 47 | 29.7 | 933 | 45.5 | |
| Radiotherapy | 36 | 22.8 | 491 | 24 | |
| Surgery and radiotherapy | 75 | 47.5 | 625 | 30.5 | |
| None/unknown | 25 | 509 | |||
The P values in bold represent statistically significant difference. ES, Ewing Sarcoma; PNET, primitive neuroectodermal tumor; SD, standard deviation.
Survival Analysis
DSS and OS were superior for ES-HN compared to ES-other (69% vs 54%; P = .001 and 57% vs 49%; P = .015, respectively) (Figures 1 and 2). Table 3 shows 10-year DSS for ES-HN versus ES-other stratified by tumor size, extent of disease, and treatment modality. Disease-specific survival was superior for ES-HN compared to ES-other only when stratified by treatment modality: both surgery and radiation (P = .018). Otherwise, there were no statistically significant differences in 10-year DSS between the 2 cohorts when stratified by other variables.
Figure 1.
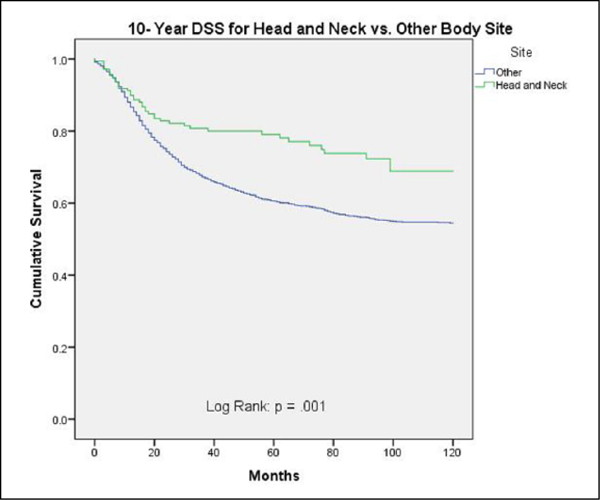
Ten-year disease-specific survival for head and neck versus other body sites.
Figure 2.
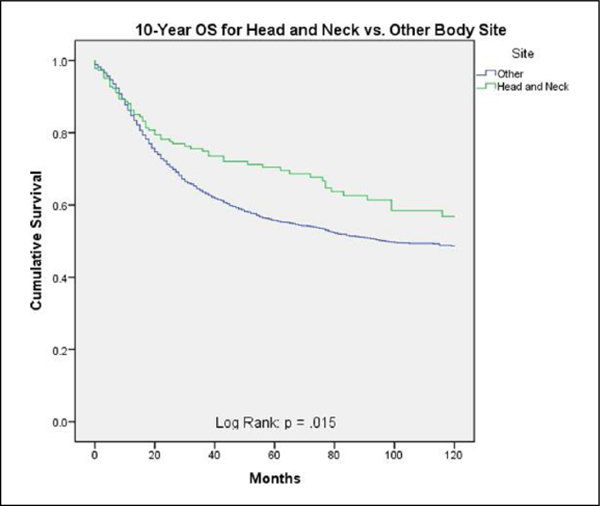
Ten-year overall survival for head and neck versus other body sites.
Table 3.
Ten-Year Disease-Specific Survival for Head and Neck Versus Other Ewing Sarcoma.a
| Head and Neck ES |
Other ES |
P Value | |
|---|---|---|---|
| 10-Year DSS (%) |
10-Year DSS (%) |
||
| Overall | 69.0 | 54.0 | .001 |
| Tumor size | |||
| ≤5 cm | 76.0 | 71.0 | .21 |
| 5–10 cm | 55.0 | 60.0 | .29 |
| >10 cm | 100.0 | 38.0 | .48 |
| Extent of disease | |||
| Local/regional | 76.0 | 67.0 | .063 |
| Distant | 40.0 | 26.0 | .63 |
| Treatment modality | |||
| Surgery | 76.0 | 67.0 | .24 |
| Radiation | 55.0 | 41.0 | .25 |
| Surgery and radiation | 75.0 | 61.0 | .018 |
The P values in bold represent statistically significant difference. DSS, disease-specific survival; ES, Ewing sarcoma.
In order to evaluate the survival difference between ES-HN and ES-other further, a multivariate Cox regression analysis was performed controlling for age, sex, race, tumor size, and extent of disease (Table 4). This analysis found age, black race, Hispanic race, large tumor size, and distant disease to be independent predictors of worse 10-year DSS. Head and neck location did not achieve statistical significance on multivariate Cox regression analysis (Table 4, Figure 3).
Table 4.
Multivariate Cox Regression Analysis.a
| HR (95% CI) | P Value | |
|---|---|---|
| Age (y) | 1.02 (1.01–1.03) | <.001 |
| Sex | ||
| Female | Referent | |
| Male | 1.13 (0.96–1.34) | .155 |
| Race | ||
| White | Referent | |
| American Indian/Alaska Native | 1.59 (0.82–3.10) | .17 |
| Asian or Pacific Islander | 0.99 (0.72–1.39) | .98 |
| Black | 1.60 (1.09–2.35) | .02 |
| Hispanic | 1.29 (1.05–1.57) | .013 |
| Tumor size | ||
| ≤5 cm | Referent | |
| 5–10 cm | 1.36 (1.06–1.73) | .014 |
| >10 cm | 2.26 (1.77–2.90) | <.001 |
| Extent of disease | ||
| Local/regional | Referent | |
| Distant | 3.10 (2.62–3.67) | <.001 |
| Site | ||
| Other | Referent | |
| Head and neck | 0.97 (0.65–1.44) | .88 |
The P values in bold represent statistically significant difference. CI, confidence interval; HR, hazard ratio.
Figure 3.
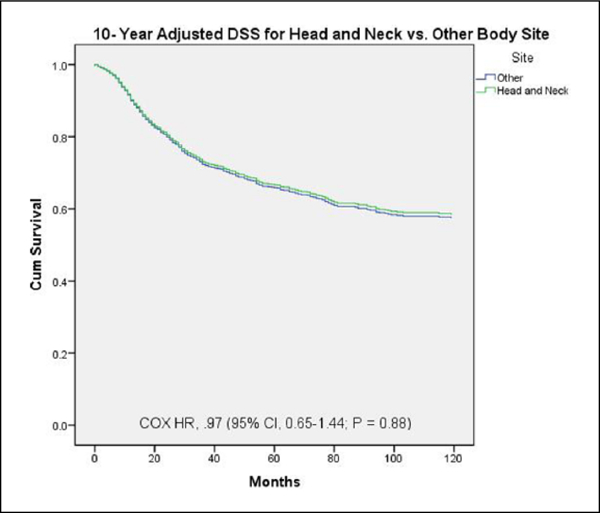
Ten-year Cox multivariate survival analysis of all patients. Disease-specific survival comparison by tumor site (head and neck vs other) adjusted for age, sex, race, tumor size, and extent of disease.
Discussion
The purpose of this study was to analyze the clinicopathologic characteristics, treatment modalities, and survival outcomes of ES-HN with direct comparison to ES-other. Our results moderately agree with our hypothesis in that ES-HN does demonstrate different clinicopathologic characteristics and treatment modalities compared to ES-other yet has remarkably similar survival outcomes when controlling for confounders. We found that ES-HN displays a smaller tumor size and lower rate of metastasis at presentation compared to ES-other. We also found that ES-HN is more commonly treated with dual therapy (surgery and radiotherapy) compared to ES-other. In addition, ES-HN was associated with an improved 10-year OS and DSS; however, this finding did not hold up on multivariate analysis.
Smaller prior studies have suggested better survival rates for ES-HN compared to ES-other. A series of 24 patients with ES-HN observed a 5-year disease-free survival of 30% compared to a x5% for ES-other.1 However, their follow-up time was limited to a mean of 3.4 years, and no statistical analysis was included in the manuscript showing that the survival rates were statistically significant. Likewise, a series of 29 patients with ES-HN observed a better survival for ES-HN compared to ES-other; however, of the 29 patients with ES-HN, only 10 were followed for more than 5 years.4 Our findings partially agree with these studies yet offer more accurate conclusions due to our increased numbers, longer follow-up times, and ability to control for confounders. We found ES-HN to be associated with an improved prognosis compared to ES-other. However, this finding was only an association on univariate analysis and did not persist on our multivariate model when controlling for confounders.
Our study showed that ES-HN was more likely to present as a smaller tumor size and with local/regional disease (vs distant disease), both of which likely explain the associated improved survival for ES-HN compared to ES-other.The 10-year DSS was superior for ES-HN as illustrated in Figure 1; however, when stratified by tumor size and extent of disease (Table 3), there were no statistically significant differences in DSS between the 2 cohorts. Furthermore, on multivariate analysis, tumors 5 to 10 cm and >10 cm as well as distant disease were independent negative prognosticators for all Ewing sarcomas. This finding is in agreement with the literature that shows that the presence or absence of metastasis is the key prognostic factor for Ewing sarcoma.9–11 In addition, patients with large tumors have been shown to fare worse than those with small tumors.9–12 Our findings strongly suggest that the associated improved survival observed in ES-HN is due to the reduced disease burden at presentation rather than an intrinsic improved prognosis due to head and neck location.
The reason for ES-HN’s smaller tumor size and lower metastatic rate at diagnosis is unclear. It is possible that the disruption in speech and swallowing, cosmetic changes, or neurologic impairment, which occurs with tumors of the head and neck because of the anatomical constraints, leads to the early detection of Ewing tumors, as has been postulated for head and neck liposarcomas and chondrosarcomas.13,14 Likewise, tumor size has been shown to be independently related to metastatic rate for Ewing tumors at all locations.15 Hence, the small tumor size and low metastatic rate for our ES-HN cohort are likely correlated.
Interestingly, we found significant treatment differences between our 2 cohorts. Ewing sarcoma of the head and neck was less likely to receive surgery or radiotherapy alone and more likely to undergo both surgery and radiotherapy compared to ES-other. This is likely related to the difficulty in achieving negative surgical margins within the head and neck due to the anatomical constraints and cosmetic concerns, consequently warranting the use of postoperative radiotherapy for residual disease. Previous studies have reported 6 of 8 (75%), 21 of 34 (62%), 7 of 14 (50%), and 3 of 6 (50%) rates of incomplete excision for ES-HN undergoing surgery, illustrating that these tumors are frequently unamenable to complete surgical resection.1,2,4,16 Since there are no randomized controlled trials comparing the role of surgery and/or radiotherapy for Ewing sarcoma, the optimal local treatment strategies for these tumors are unknown.
Several limitations to this study are important to note. Our analysis was limited to data included in the SEER registries, and thus data regarding tumor size, extent of disease, and treatment modality were missing for many patients. In addition, the SEER database does not contain information regarding disease recurrence, disease-free survival, or chemotherapy use. Despite these limitations, the SEER database provides significant advantages. Most importantly, the SEER database allows for inclusion of a large cohort of patients with a rare tumor. Without such a database, this study would not be possible.
Conclusion
This study is the largest series of ES-HN to date and is the first to directly compare ES-HN to ES-other. Our results show that ES-HN displays a lower tumor size and metastatic rate compared to ES-other. ES-HN does not appear to be a separate clinical entity compared to ES-other; rather, its associated improved prognosis is likely secondary to its smaller size and lower metastatic rate compared to ES-other. Future studies are needed to clarify the optimal local treatment strategies for ES-HN.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ayman Allam, El-Husseiny G, Khafaga Y, et al. Ewing’s sarcoma of the head and neck: a retrospective analysis of 24 cases. Sarcoma. 1999;3:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grevener K, Haveman LM, Ranft A, et al. Management and outcome of Ewing sarcoma of the head and neck. Pediatr Blood Cancer. 2016;63:604–610. [DOI] [PubMed] [Google Scholar]
- 3.Razek A, Perez CA, Tefft M, et al. Intergroup Ewing’s sarcoma study. Local control related to radiation dose, volume, and site of primary lesion in Ewing’s sarcoma. Cancer. 1980;46:516–521. [DOI] [PubMed] [Google Scholar]
- 4.Siegal GP, Oliver WR, Reinus WR, et al. Primary Ewing’s sarcoma involving the bones of the head and neck. Cancer. 1987;60:2829–2840. [DOI] [PubMed] [Google Scholar]
- 5.Desai KI, Nadkarni TD, Goel A, et al. Primary Ewing’s sarcoma of the cranium. Neurosurgery. 2000;46:62–69. [PubMed] [Google Scholar]
- 6.Watanabe H, Tsubokawa T, Katayama Y, et al. Primary Ewing’s sarcoma of the temporal bone. Surg Neurol. 1992;37:54–58. [DOI] [PubMed] [Google Scholar]
- 7.Dehner LP Neuroepithelioma (primitive neuroectodermal tumor) and Ewing’s sarcoma. At least a partial consensus. Arch Pathol Lab Med. 1994;118:606. [PubMed] [Google Scholar]
- 8.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. [DOI] [PubMed] [Google Scholar]
- 9.Cotterill S, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. [DOI] [PubMed] [Google Scholar]
- 10.Duchman KR, Yubo G, Miller BJ Prognostic factors for survival in patients with Ewing’s sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39:189–195. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Hoang BH, Ziogas A, Zell JA Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010;116:1964–1973. [DOI] [PubMed] [Google Scholar]
- 12.Paulussen M, Ahrens S, Dunst J, et al. Localized Ewing tumor of bone: final results of the cooperative Ewing’s sarcoma study CESS 86. J Clin Oncol. 2001;19:1818–1829. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MA, Gerry DR, Byrd JK Head and neck chondrosarcomas: analysis of the Surveillance, Epidemiology, and End Results database. Head Neck. 2016;38(9):1359–1366. [DOI] [PubMed] [Google Scholar]
- 14.Gerry D, Fox NF, Spruill LS, Lentsch EJ Liposarcoma of the head and neck: analysis of 318 cases with comparisons to non-head and neck sites. Head Neck. 2014;36;393–400. [DOI] [PubMed] [Google Scholar]
- 15.Hense HW, Ahrens S, Paulussen M, Lehnert M, Jürgens H Factors associated with tumor volume and primary metastases in Ewing tumors: results from the (EI) CESS studies. Annals Oncol. 1999;10:1073–1077. [DOI] [PubMed] [Google Scholar]
- 16.Whaley JTaylor Indelicato DJ, Morris CG, et al. Ewing tumors of the head and neck. Am J Clin Oncol. 2010;33:321–326. [DOI] [PubMed] [Google Scholar]


