Conspectus
Biocompatible and bioorthogonal conjugation reactions have proven to be powerful tools in biological research and medicine. While the advent of bioorthogonal conjugation chemistries greatly expand our capacity to interrogate specific biomolecules in situ, biocompatible reactions that target endogenous reactive groups have given rise to a number of covalent drugs as well as a battery of powerful research tools. Despite the impressive progress, limitations do exist with the current conjugation chemistries. For example, most known bioorthogonal conjugations suffer from slow reaction rates and imperfect bioorthogonality. On the other hand, covalent drugs often display high toxicity due to off-target labeling and immunogenicity. These limitations demand continued pursuit of conjugation chemistries with optimal characteristics for biological applications. A spate of papers appearing in recent literature report the conjugation chemistries of 2-formyl and 2-acetyl phenylboronic acids (abbreviated as 2-FPBA and 2-APBA, respectively). These simple reactants are found to undergo fast conjugation with various nucleophiles under physiological conditions, showing great promise for biological applications.
The versatile reactivity of 2-FPBA and 2-APBA manifests in dynamic conjugation with endogenous nucleophiles as well as conjugation with designer nucleophiles in a bioorthogonal manner. 2-FPBA/APBA conjugates with amines in biomolecules, such as lysine side chains and aminophospholipids, in a highly dynamic manner to give iminoboronates. In contrast to typical imines, iminoboronates enjoy much improved thermodynamic stability, yet are kinetically labile for hydrolysis due to the boronic acid activation of the imine. The dynamic conjugation presents a novel binding mechanism analogous to hydrogen bonding and electrostatic interactions. Implementation of this covalent binding mechanism has yielded reversible covalent probes of prevalent bacterial pathogens. It has also resulted in reversible covalent inhibitors of a therapeutically important protein Mcl-1. Such covalent probes/inhibitors with 2-FPBA/APBA warheads avoid permanent modification of their biological target, potentially able to mitigate off-target labeling and immunogenicity of covalent drugs. The dynamic conjugation of 2-FPBA/APBA has been recently extended to N-terminal cysteines, which can be selectively targeted via formation of a thiazolidino boronate (TzB) complex. The dynamic TzB formation expands the toolbox for site-specific protein labeling and the development of covalent drugs. On the front of bioorthogonal conjugation, 2-FPBA/APBA has been found to conjugate with α-nucleophiles under physiologic conditions with rate constant (k2) over 1,000 M−1s−1, which overcomes the slow kinetics problems and rekindles the interest of using the conjugation of α-nucleophiles for biological studies. With fast kinetics being a shared feature, this family of conjugation chemistries gives a remarkable range of product structure depending on the choice of nucleophile. Importantly, both dynamic and irreversible conjugations have been developed, which we believe will enable a wide array of applications in biological research.
In this Account, we collectively examine this rapidly expanding family of conjugation reactions, seeking to elucidate the unifying principles that would guide the further development of novel conjugation reactions, as well as their applications in biology.
Graphical Abstract
Introduction
Living systems present awe-inspiring complexity to chemists, with numerous molecules undergoing various chemical transformations in a well-orchestrated manner. Unraveling the intricacies of these biological systems requires molecular probes that selectively target and perturb endogenous molecules individually or in combination. While genetic approaches and noncovalent inhibitors are traditionally used to interrogate the function of biomolecules, innovative technologies have been developed to covalently label and modify a biomolecule of interest in live cells and organisms. A central piece of this endeavor is to develop chemoselective reactions that readily proceed in the biological milieu. Some of these biocompatible reactions target the reactivities of endogenous functional groups, such as nucleophilic side chains of proteins.1 Indeed, clever incorporation of a reactive warhead in a noncovalent scaffold has yielded a number of clinically applied drugs that inactivate their target protein via covalent modification 2 On a different front, various bioorthogonal conjugation reactions have been developed to join unnatural reactive partners that are inert to endogenous molecules. 3,4. An example of such reactions is the azide-alkyne cycloaddition reaction, often referred to as “Click” chemistry.5 Such bioorthogonal reactive groups can be engineered into a biomolecule via chemical or metabolic processes,4 thus serving as an anchor to allow specific labeling and manipulation of the target in its native environment.
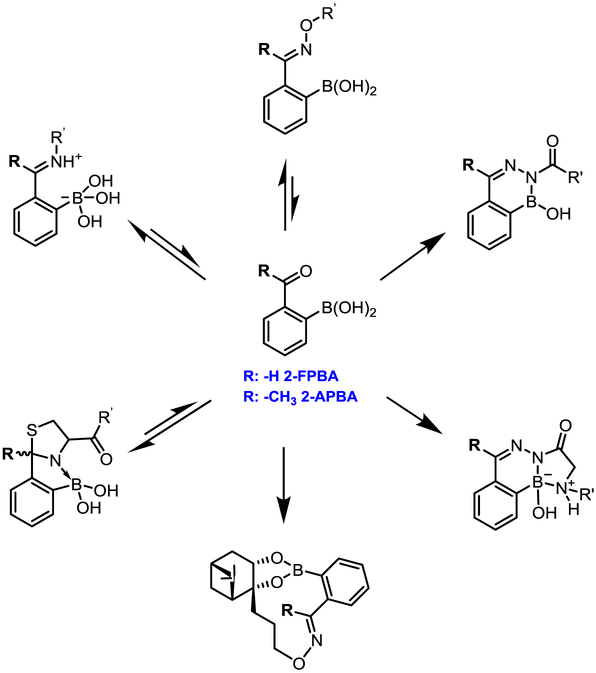
Much interest remains in the pursuit of novel biocompatible conjugation reactions, which is to a large extent stimulated by the limitations of the known biocompatible conjugation chemistries. For example, the development of covalent drugs is often plagued with idiosyncratic toxicity, presumably due to off-target labeling and unintended immune responses triggered by irreversible covalent modification of proteins.6 On the other hand, most of the known bioorthogonal conjugation reactions exhibit slow kinetics and side reactions with endogenous molecules.4 The recent literature has presented a new series of biocompatible conjugation reactions, which exploits the unique reactivities of aryl ketones and aldehydes with an ortho-boronyl substituent. Specifically, 2-formyl and 2-acetyl phenylboronic acid (abbreviated as 2-FPBA and 2-APBA respectively, Figure 1) have been found to conjugate with various amines and α-nucleophiles with rate constants over 103 M−1 s−1, which are orders of magnitude greater than that of the azide-alkyne click chemistry.4 Importantly, depending on the reactive partner used, a conjugation reaction of 2-FPBA/APBA can yield diverse product structures with distinct thermodynamic and kinetic stability. The remarkable variation of reaction profiles makes this family of conjugation reactions unique and applicable to multiple areas of chemical biology. We note that various boronic acids have been explored for biomolecular recognition and protein labeling, which are summarized in a recent review article by Hall and Akgun 7 This Account focuses on the unique reactivity of 2-FPBA and 2-APBA, seeking to provide a structure-reactivity relationship to guide their applications in biology.
Figure 1.
Diverse conjugation chemistries of 2-FPBA/APBA displaying varied product stability and reaction reversibility. The conjugation partners include both important functional groups of biomolecules and non-natural nucleophiles designed for bioorthogonal conjugation.
Dynamic Conjugation with Endogenous Nucleophiles
The reactivity of endogenous nucleophiles (e.g., thiols and amines) has been routinely exploited for labeling biomolecules. Such labeling, however, has been largely limited to purified samples instead of complex mixtures due to a lack of selectivity. One way to acquire selectivity is to install a latent reactive group onto a nonreactive ligand, which directs the reactive group to an intended target. Realization of this strategy in medicinal chemistry, both by design and serendipity, has led to a number of FDA approved drugs that inactivate their targets via covalent conjugation.2 Covalent drugs, while enjoying superior potency, often exhibit high toxicity due to off-target labeling and possible immunogenic properties.8 These problems have stimulated interest in the development of dynamic conjugation reactions of endogenous nucleophiles.9-12 Towards this end, the iminoboronate chemistry of 2-FPBA/APBA has emerged as a powerful reaction to target amine-presenting biomolecules.
Dynamic conjugation with biological amines
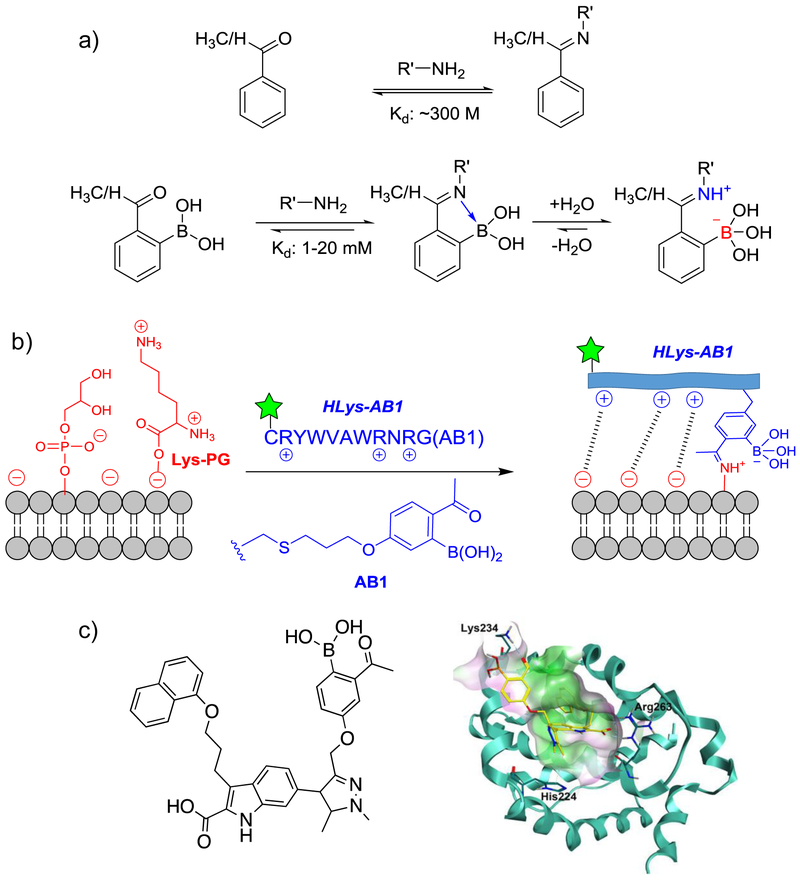
Despite the fundamental importance of imines in enzyme catalysis, typical imine formation is thermodynamically unfavorable under physiological conditions. For example, the imine formation of glycine with acetone is estimated to give a dissociation constant (Kd) of 300 M (Ka: 3 × 10−3 M−1), which means the imine conjugate exists only at parts per million level, even with mM reactants.13 In comparison, an iminoboronate enjoys much greater thermodynamic stability. Several groups 14-17 including our own18,19 have reported iminoboronate formation of both 2-FPBA and 2-APBA with low millimolar concentrations of reactants in neutral aqueous media. Interestingly, 2-APBA is selective for unhindered amines such as lysine side chains,18 while 2-FPBA is more tolerant of steric hindrance and does conjugate with the α-amine of peptides at higher concentrations.14 The thermodynamic stabilization can be potentially rationalized by the formation of an N-B dative bond (Figure 2a). However, a recent 11B-NMR study 20 shows that iminoboronate formation of 2-FPBA in methanol predominantly gives a solvent-inserted, zwitterionic structure (Figure 2a), indicating that the ion-pair interaction underlies the superior stability of iminoboronates. Solvent-inserted structures were also observed with o-aminomethyl phenylboronic acid in methanol.21,22 It is likely that, in aqueous media, an iminoboronate primarily adopts the solvent-inserted structure as well, although 11B-NMR studies of iminoboronates in water has been elusive. Importantly, the improved thermodynamic stability does not compromise the reversibility of the reaction: Gois and coworkers first described that iminoboronate formation can be reversed by the addition of competing molecules such as fructose, dopamine and glutathione.14 We later showed that an iminoboronate complex dissociates instantaneously upon dilution, indicating that iminoboronate formation is under thermodynamic control in physiologic conditions.18 The rapid reversibility can be rationalized by the N-B coordination or imine protonation resulting from solvent insertion (Figure 2a). Nevertheless, the ortho-boronyl substituent, while providing thermodynamic stabilization of the product, also accelerates the forward and the backward reaction of iminoboronates.
Figure 2.
Boronic acid mediated dynamic conjugation of aryl aldehydes and ketones with biological amines. (a) Schematic comparison of aryl aldehydes/ketones with and without the ortho-boronic acid substituent in their conjugation reaction with primary amines. The much reduced Kd values highlights the superior thermodynamic stability of iminoboronates. (b) Reversible covalent labeling of gram-positive bacteria enabled by the iminoboronate formation of Lys-PG that synergizes with electrostatic interactions. (c) Reversible covalent inhibition of Mcl-1 achieved by installing the 2-APBA warhead onto a noncovalent ligand of the target protein. The inhibitor structure is shown on the left and a structural model of the inhibitor-protein complex is shown on the right with the iminoboronate-forming Lys residue highlighted (details can be found in ref. 16).
The thermodynamic stability and quick reversibility make iminoboronate formation analogous to hydrogen bonds, except that the iminoboronate-mediated binding is specific for primary amines and stronger: iminoboronate formation in neutral buffer gives low mM Kd’s, which correspond to 3–4 kcal/mol in free energy gain.18, 23In contrast, hydrogen bonds typically gives 1–2 kcal/mol in free energy gain as estimated from protein stability studies. 24 Incorporation of this dynamic covalent binding mechanism in ligand design could lead to reversible covalent probes and inhibitors that bind a specific target in complex biological systems. Indeed, by conjugating 2-APBA to a cationic peptide, we have devised a peptide probe (Hlys-AB1) that selectively labels Staphylococcus aureus in blood serum, yet bypasses mammalian cells.18 As a mechanism to evade host defense surveillance, Staphylococcus aureus is known to overexpress lysyl phosphoglycerol (Lys-PG, Figure 2b), which is amenable to iminoboronate formation. The bacterial staining by Hlys-AB1 in serum is remarkable considering the high abundance of serum proteins that could compete for iminoboronate formation. This bacterial selectivity originates from the synergy of iminoboronate formation of Lys-PG and the electrostatic attraction between the cationic peptide and the anionic membrane. More recently, we have incorporated 2-APBA into peptide libraries on phage, which allows facile identification of reversible covalent probes of diverse bacterial pathogens.25
Similar to our report on bacterial lipid labeling, iminoboronate chemistry has been adapted to develop reversible covalent inhibitors of proteins. Modification of protein surface lysines via iminoboronate chemistry was first reported by Gois and coworkers,14, 17 in which protein labeling was performed with purified proteins and not intended to be site-specific. As mass spectrometry was used as the primary method for characterization, the stability of the conjugate, or lack thereof, was not fully assessed. More recently, Su and coworkers described targeted covalent inhibition of the important protein Mcl-1 by grafting an iminoboronate warhead onto a noncovalent ligand (Figure 2c).16 Compared to control molecules, the iminoboronate-capable inhibitors showed 20–50 times better binding affinity. The use of 2-FPBA or 2-APBA warhead yielded comparable binding affinity to the target protein. The protein-inhibitor adduct was directly observed via LC-MS analysis, while no covalent conjugation was observed with the 2-APBA warhead alone, which is expected considering the rapid reversibility of iminoboronate formation. However, for the Mcl-1 inhibitor, the iminoboronate linkage is further stabilized by additional noncovalent interactions between the inhibitor and the protein. Importantly, the covalent linkage breaks apart upon unfolding or degradation of the protein, which abolishes the noncovalent interactions. Reversible covalent inhibitors have been previously described for targeting alcohols26 and thiols10 as exemplified by Velcade® and PRN1008 (currently in clinical trials).27 The work by Su and coworkers adds iminoboronate chemistry to the design of reversible covalent inhibitors, which enjoys the benefits of covalent inhibitors in general (high potency, long residence time), yet able to avoid permanent modification of proteins.
Dynamic conjugation with cysteine and analogues
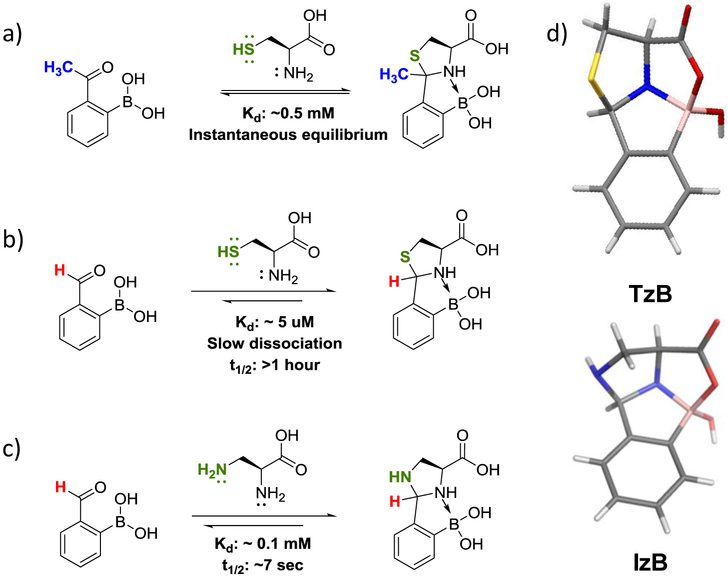
Iminoboronate formation favors unhindered amines such as lysine side chains. In contrast, an N-terminal amine of proteins is less prone to form iminoboronates. We found that a free or N-terminal cysteine, however, reversibly conjugates with 2-APBA at sub-millimolar concentrations (Kd: ~0.5 mM, Figure 3a).28 In contrast, alanine does not conjugate with 2-APBA below molar concentrations (unpublished data). The distinct reactivity of cysteine results from the side-chain thiol attacking the iminoboronate to form a thiazolidino boronate (TzB) complex. The TzB formation was also described for 2-FPBA by our group29 and the Gois group30 independently. In contrast to 2-APBA, 2-FPBA conjugates with free cysteine even at low µM concentrations (Figure 3b). Kinetic studies monitored with UV-vis spectroscopy revealed that the conjugation proceeds with rate constants over 103 M−1 s−1, in sharp contrast to the conjugation of cysteine to unsubstituted benzaldehyde, which takes days even under acidic conditions.31 The fast kinetics of TzB formation is presumably due to the boronic acid activation of the imine intermediate formed between cysteine and the aldehyde. Similar rate acceleration has been reported for 2-FPBA conjugation with 2-aminothiophenol, which rapidly gives a thiazolidine intermediate that further oxidizes into a benzothiazole final product.32
Figure 3.
Conjugation of 2-FPBA/APBA to cysteine and related analogues. (a) 2-APBA conjugates with free cysteine at sub-millimolar concentrations to give a TzB complex. The TzB complex formation of 2-APBA proceeds in a highly dynamic manner. (b) 2-FPBA rapidly conjugates with free cysteine even at low micromolar concentrations, yielding a TzB complex of much improved stability. (c) 2-FPBA conjugates with 1,2-diaminopropionic acid (Dap) to give an IzB complex, analogous to TzB formation. The IzB formation of 2-FPBA is quickly reversible under physiologic conditions. (d) X-ray crystal structures of the TzB and IzB conjugates (gray= C, yellow= S, red=O, blue=N, pink=B). The crystals were obtained from solutions of the compounds in MeOH/H2O (1:1). The crystal structures, in comparison to the chemdraw structures in a-c, exhibit an additional ring that originates from the mixed anhydride formation between the -COOH and -B(OH)2 under mildly acidic conditions.
Interestingly, 2-FPBA and 2-APBA show dramatic differences in their reaction profiles of TzB formation. 2-APBA conjugates with cysteine at sub-millimolar concentrations in a rapidly reversible manner, yet 2-FPBA forms a more stable TzB conjugate that only dissociates on the time scale of hours as revealed by a TzB-cysteine exchange experiment. It has not been possible to directly measure the dissociation rate due to the high stability of the conjugate. Importantly, the TzB formation of 2-FPBA is highly specific to free or N-terminal cysteines, showing little interference by endogenous molecules including glutathione, cysteine, serine and lysine. These features make 2-FPBA suitable for labeling proteins with cysteine on the N-terminus. Indeed, we showed that such protein labeling can be accomplished with low µM concentrations of 2-FPBA within minutes.29 On the other hand, the dynamic conjugation of 2-APBA with cysteine, similar to the iminoboronate formation, is appealing to medicinal chemistry efforts toward the development of reversible covalent inhibitors. Finally, given the distinct behavior of 2-FPBA and 2-APBA, the TzB conjugation should be tunable both in thermodynamic stability and in reaction kinetics by using analogues of 2-FPBA/APBA.
Serine, although structurally similar to cysteine, does not conjugate with 2-FPBA to give a TzB-like complex. In contrast, 1,2-diaminoproprionic acid (Dap) does react with 2-FPBA to give an imidazolidino boronate (IzB) complex (Figure 3c, d).33 The IzB complex formation is quickly reversible with an apparent Kd of 0.1 mM. In comparison to a TzB complex, the corresponding IzB complex exhibits lower stability and faster dissociation kinetics: IzB dissociation occurs on the time scale of seconds, while the TzB complex of 2-FPBA is stable for hours. Similar to TzB formation, the conjugation of Dap and 2-FPBA displays high bioorthogonality: the reaction proceeds with similar rate and efficiency in the presence and absence of abundant endogenous molecules except free cysteine, which converts an IzB into a TzB complex. Taking advantage of the dynamic IzB/TzB exchange, we have devised peptide probes that are able to report cysteine concentration changes in blood serum in real time.33
Described above are several conjugation reactions of 2-FPBA/APBA with prevalent nucleophilic groups of biomolecules. The dynamic iminoboronate formation presents a powerful mechanism for binding biological amines, the potential of which has been convincingly demonstrated with the covalent Mcl-1 inhibitors and bacterial probes. The repertoire of biomolecules that can be targeted by this “covalent binding” mechanism has been further expanded to cysteine and analogues, the application of which are being explored with our ongoing research. These dynamic conjugation reactions greatly expand the toolbox for molecular design towards the development of reversible covalent probes and inhibitors.
Bioorthogonal Conjugation with Designer Nucleophiles
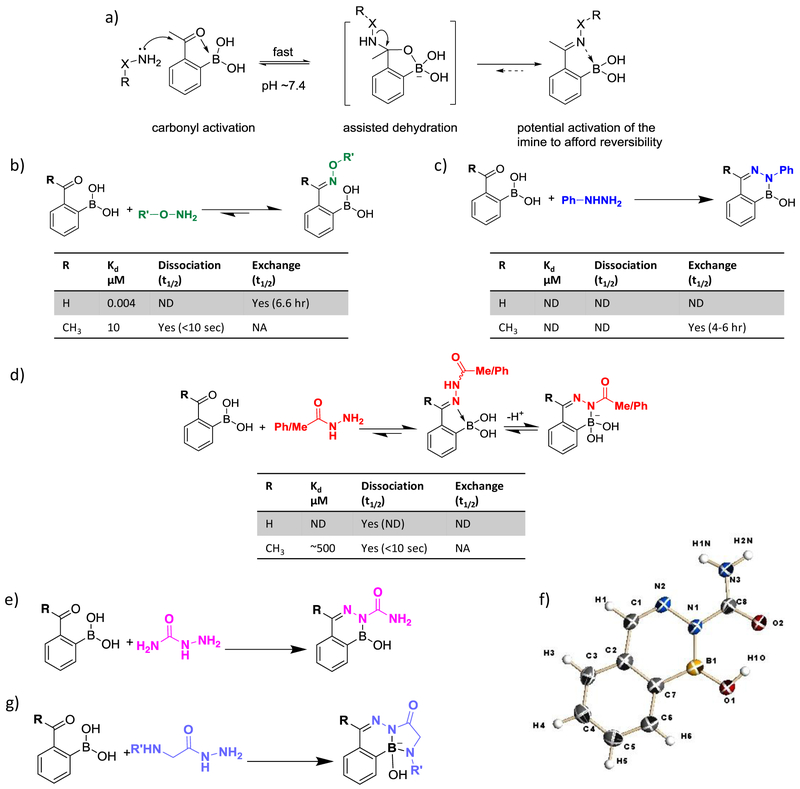
Mechanistically similar to the imine formation, α-nucleophiles are known to conjugate with a carbonyl to yield oximes and hydrazones. The oxime/hydrazone formation is among the earliest known bioorthogonal reactions. However, such conjugation reactions typically suffer from slow kinetics, even with acid or aniline catalysis.34 For example, with aniline at a concentration of 100 mM, benzaldehyde and phenylhydrazine conjugate with a rate constant of < 1 M−1 s-1.35 Analogous to iminoboronate formation, incorporation of an ortho-boronyl substituent greatly accelerates the conjugation of 2-APBA/FPBA to α-nucleophiles via carbonyl activation and assisted dehydration to give the carbon-nitrogen double bond (Figure 4a). Such conjugation reactions typically display second order rate constants on the order of 103 – 104 M−1 s−1 (see later discussion for details), which places them among the fastest bioorthogonal reactions known to date.4 With fast kinetics being a universal characteristic, the reaction profiles of 2-APBA/2-FPBA and α-nucleophiles vary dramatically in terms of reversibility and product stability. Here we compare the various reactant combinations to reveal the landscape of this family of bioorthogonal conjugation reactions.
Figure 4.
Fast bioorthogonal conjugation of 2-FPBA/APBA with α-nucleophiles. (a) Mechanistic illustration of the boronic acid-accelerated conjugation of 2-FPBA/APBA with α-nucleophiles. (b) Oxime formation of 2-FPBA and 2-APBA yielding distinct product stability. ND: Not Determined; NA: Not Applicable. Exchange is considered Not Applicable to highly dynamic reactions. (c) Stable diazaborine formation of 2-FPBA/APBA upon conjugation with phenylhydrazine. (d) Dynamic conjugation of 2-FPBA/APBA to carbohydrazides. (e) Stable diazaborine formation of 2-FPBA/APBA with semicarbazide. (f) Crystal structure of a diazaborine conjugate of 2-FPBA and semicarbazide. (g) Improving the conjugate stability of carbohydrazide with an α-amino group that forges additional B-N bond.
Conjugation with oxyamines
The fast conjugation of oxyamines to 2-FPBA and 2-APBA was independently reported by the Gillingham group36 and our own 23 in 2015 (Figure 4b). In the Gillingham report, O-benzylhydroxylamine was found to conjugate to 2-FPBA with a rate constant of 11.1 × 103 M−1 s-1. The reaction kinetics was measured via a fluorescence-quenching assay with fluorophore/quencher-labeled reactants. The conjugation proceeds with only a slight reduction of yield in blood serum, showcasing its bioorthogonality. Although 2-FPBA (2-APBA alike) is known to conjugate with serum proteins via iminoboronate formation, the protein conjugation is highly dynamic and affords no interference to its conjugation with oxyamines. The dissociation of the oxime product was assessed through an oxime-oxyamine exchange experiment, which yielded a dissociation rate constant (k-1) of 4.2 × 10−5 s−1 (t½: 6.6 hrs). The dissociation rate is just slightly higher than that of oximes without the boronyl substituent.37 In contrast, we found the conjugation of 2-APBA with an alkoxyamine to be instantaneously reversible according to a dilution experiment.23 We further estimated the equilibrium dissociation constant (Kd) to be 1.4 × 10−5 M, which is three orders of magnitude larger than that of 2-FPBA (Kd: ~ 10−8 M). 11B-NMR analysis of the 2-APBA oxime conjugate revealed a broad and slightly upfield shift of the boron peak, indicating partial formation of the N-B dative bond, which accelerates the hydrolysis of the conjugate. The distinct stability is remarkable given that 2-FPBA and 2-APBA only differ by a methyl group. While the dynamic conjugation of 2-APBA may be used for dynamic combinatorial chemistry applications, the more stable oxime of 2-FPBA has shown promise for biomolecular labeling in complex mixtures. 36
Conjugation with hydrazines
Alongside our studies of oxyamine conjugation to 2-APBA, we investigated the reactivity of phenylhydrazine (Phz) as well.23 Interestingly, 2-APBA was found to conjugate with phenylhydrazine with a rate constant over 103 M−1 s−1 and give a highly stable product, as no dissociation was observed with NMR or UV-vis based analysis. To probe the kinetic stability of the conjugate, we performed an exchange experiment by mixing a pre-formed 2-APBA-Phz with a Phz derivative. The exchange reached equilibrium after 4–6 hours, indicating that the 2-APBA-Phz conjugate dissociates very slowly (k-1 < 10−4 s−1), if at all. We note that our estimate of k-1 assumes a dissociative mechanism (dissociation and then reconjugation) for the exchange. It is possible that the exchange proceeds with an associative mechanism instead, whereby the Phz derivative attacks the 2-APBA-Phz conjugate to initiate exchange. Therefore, the estimated k-1 value should be considered as an upper limit. The superior stability of the 2-APBA-Phz conjugate over the corresponding oxime is counterintuitive as oximes are typically more stable than hydrazones.37 Shortly after our report, the Bane group 38 as well as the Gillingham group 39described that the conjugation of 2-FPBA and phenylhydrazine gives a diazaborine product (Figure 4c). We suspect that the 2-APBA conjugation with Phz gives a diazaborine product as well, which may explain the observed stability. However, structural confirmation of the 2-APBA-Phz conjugate has been elusive due to the lack of crystal structure as well as the water loss problem of boronic acids in mass spectrometry.40 Proof-of-concept demonstrations by the Bane group38 and our own23 showed that Phz conjugation with 2-FPBA/APBA can be used to label proteins under physiologic conditions. Similar to the oxime conjugation chemistry, the 2-FPBA/APBA-Phz conjugation readily proceeds without interference of endogenous small molecules or serum proteins, again owing to the instantaneous reversibility of the 2-FPBA/APBA conjugation with endogenous amines.
Conjugation with hydrazides
In comparison to phenylhydrazine, which is prone to oxidation and cytotoxic,20 hydrazides present more attractive reagents for bioorthogonal conjugations.41 In contrast to phenylhydrazine, the conjugation acethydrazide or benzhydrazide with 2-APBA gives acylhydrazone products, which undergo rapid hydrolysis to establish an equilibrium. These dynamic conjugation reactions yield Kd values in the sub-millimolar range (Figure 4d) as revealed by titration experiments monitored by 1H-NMR. 2-FPBA also fails to give a stable conjugate with carbohydrazides: although the conjugation of 2-FPBA and acethydrazide yields a B-N heterocycle, this heterocycle, unlike the diazaborines, displays a nonplanar structure with boron existing in a tetrahedral geometry as shown by crystallography data (Figure 4d).42 This nonplanar heterocycle undergoes rapid hydrolysis presumably due to the poor conjugation between boron and the α-nitrogen of acethydrazide.
Our recent studies show that the conjugate stability of carbohydrazides can be improved by tuning the electronic properties of the hydrazide. Specifically, semicarbazide (Figure 4e, f), in contrast to acethydrazide, reacts with either 2-FPBA or 2-APBA to give a stable diazaborine conjugate.28 Crystallographic characterization revealed the existence of a planar B-N heterocycle of the diazaborine conjugates (Figure 4f). As shown by 1H-NMR data, LC-MS analysis and UV-vis based kinetic analysis, the diazaborine formation of semicarbazide with 2-FPBA proceeds at rate constants over 103 M−1 s−1, and gives quantitative yields. The conjugation with 2-APBA is comparably fast, but with somewhat reduced yield, possibly due to product inhibition. This hypothesis is supported by the 1H-NMR data showing complex formation between the diazaborine product and the reactants.28 Given the structural similarity, it is remarkable that semicarbazide and acethydrazide yield distinct conjugation products. The fact that semicarbazide gives a planar diazaborine is presumably due to its α-N bearing higher electron density to favor conjugation with boron. In contrast, the α-N of acethydrazide is electron deficient due to conjugation with the carbonyl. The diazaborine formation of 2-APBA shows excellent bioorthogonality and proceeds without interference by blood serum or cell lysates. In comparison, the conjugation of 2-FPBA and semicarbazide displays slowed kinetics in cell lysate due to the competitive reaction with cysteine or cysteine derivatives.29 To demonstrate the potential of the diazaborine chemistry for biological applications, we synthesized an unnatural amino acid D-AB3, which displays a 2-APBA moiety as its side chain.20 D-AB3 readily incorporates into the cell envelope of bacteria by hijacking the peptidoglycan remodeling mechanisms,43 and then serves as an anchor for fluorescent labeling. Owing to its excellent bioorthogonality, the diazaborine conjugation of D-AB3 and semicarbazide allows facile labeling of pathogenic bacteria in blood serum. More recently, we reported fluorogenic versions of the diazaborine conjugation chemistry, which reduce the background fluorescence to allow even better visualization of bacterial pathogens.44
An alternative strategy for stabilizing the carbohydrazide conjugate of 2-FPBA was recently reported by the Bane group,42 in which an amine substituent is introduced onto the α-carbon of acethydrazide to forge an additional B-N bond in the conjugate (Figure 4g). Bane and coworkers demonstrated the utility of this conjugation reaction by chemically installing the α-amino acethydrazide onto a protein surface, followed by conjugation with a fluorophore-labeled 2-FPBA. The labeled protein survives SDS-PAGE analysis and remains intact in a gel for weeks. Conjugation of α-amino acethydrazide with 2-APBA yields a similar structure; however, the stability of this 2-APBA conjugate was not assessed explicitly.
Multicomponent and bifunctional conjugation reactions
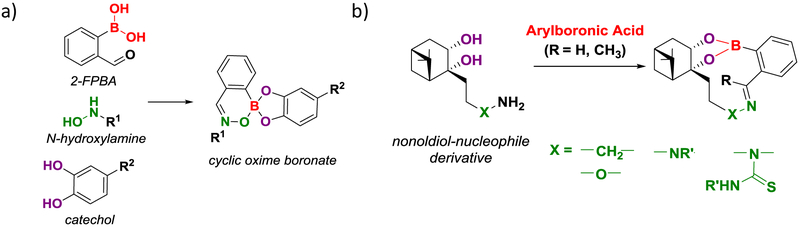
2-FPBA and 2-APBA are intrinsically bifunctional with the carbonyl and the boronic acid groups installed onto the same scaffold. In addition to promoting imine formation as described above, the boronic acid moiety is well known to conjugate with a diol to form boronate esters.45 Anslyn and coworkers recently reported a three-component condensation reaction in which 2-FPBA conjugates in tandem with a catechol derivative, such as L-DOPA, and an N-hydroxylamine (Figure 5a).46 In contrast to the O-substituted hydroxylamines, N-hydroxylamines conjugate with 2-FPBA to form a cyclic oxime boronate in aqueous solution. The N-hydroxyamine condensation proceeds equally well in the presence of various catechols, which quickly equilibrate with 2-FPBA to give the corresponding boronate esters. The three component condensation gives a stable product, which survives acidic (pH 1) and basic (pH 13) conditions, as well as heat (50 °C) for over 24 hours. By introducing L-DOPA into synthetic peptides, the authors showed that peptide labeling can be accomplished through this three-component condensation reaction. Additionally, dual labeling of the peptides was demonstrated by incorporating L-DOPA and another aldehyde side chain, which elicit N-hydroxyamine/2-FPBA condensation and canonical oxime formation, respectively. A less optimal aspect of this three-component condensation reaction is the requirement for high reagent concentrations: the peptide labeling experiment was performed with millimolar concentrations of 2-FPBA for 4 hours.
Figure 5.
Multicomponent and bifunctional conjugation reactions of 2-FPBA and 2-APBA. (a) Three-component condensation reaction with 2-FPBA conjugation to a catechol derivative and an N-hydroxylamine in tandem. R1, R2 denote variable substituents. (b) Conjugation of 2-FPBA/APBA with nopoldiol-based bifunctional scaffolds incorporating an amine or α-nucleophile (e.g., oxyamine, hydrazine and thiosemicarbazide). R’ denotes a variable substituent. Stable products are obtained from conjugation of such bifunctional scaffolds.
Following a similar rationale, Hall and coworkers have developed a new series of bifunctional reagents that install an α-nucleophile onto a nopoldiol scaffold (Figure 5b).47 An earlier paper of the Hall group reports the high propensity of nopoldiol to conjugate with aryl boronic acids.48 More specifically, nopoldiol derivatives were found conjugate with an arylboronic acid at micromolar concentrations, and this conjugation is dynamic with an estimated Kd of ~10−5 M, which is about 100 times lower than that of catechol. Despite the improved thermodynamic stability, the boronate ester of nopoldiol dissociates on the time scale of hours to minutes. To further stabilize the boronate conjugate, Hall and coworkers derivatized nopoldiol with a second reactive group to react with the carbonyl. Specifically, nopoldiol derivatives were synthesized to incorporate oxyamine, hydrazines and (thio)semicarbazides respectively. These bifunctional compounds were found to conjugate with both 2-FPBA and 2-APBA in neutral aqueous media with second order rate constants close to 10 M−1 s-1. The kinetics of conjugation is on par with the boronate ester formation of nopoldiol without an α-nucleophile. However, in contrast to nopoldiol alone, the bifunctional reagents give stable conjugation products, which show no exchange with newly added 2-APBA derivatives. In addition, the conjugates are shown to be stable under acidic (pH 3) and basic (pH 9) conditions. To demonstrate the potential of this bioorthogonal conjugation, the authors performed live cell imaging experiments in which a 2-APBA moiety was introduced into β2-adrenergic receptor (ADRβ2) through a SNAP tag. Application of a fluorophore labeled nopoldiol-thiosemicarbazide then allowed facile fluorescent labeling and visualization of cells expressing the SNAP-ADRβ2 fusion protein.
Conclusion
Summarized above are recently reported bioorthogonal conjugation reactions that exploit the unique reactivity of 2-FPBA and 2-APBA. The judicious placement of a boronic acid at the ortho position of an aryl ketone or aldehyde greatly accelerates the addition-elimination chemistry of the carbonyl group. Importantly, the imine or imine-like products can be thermodynamically stabilized, yet kinetically activated by the boronic acid. The seemingly opposing effects of the boronyl substituent give rise to a range of reactivity profiles in terms of product stability and reversibility depending on the applied nucleophiles. Both 2-FPBA and 2-APBA conjugate with amines in a dynamic manner to form iminoboronates. Given the abundance of amines in biomolecules, the dynamic iminoboronate formation serves as a powerful mechanism to enable reversible covalent ligands and inhibitors for various biological targets. The dynamic conjugation of 2-FPBA and 2-APBA extends to other endogenous nucleophiles including the 1,2-aminothiol moiety presented by free or N-terminal cysteines. On a different front, the kinetic acceleration of the boronic acid substituent has yielded fast conjugation reactions of α-nucleophiles. The lauded oxime/hydrozone formation has been plagued by the slow kinetics; yet the ortho-boronic acid substituent enables 2-FPBA/APBA to conjugate with α-nucleophiles with rate constants over 103 M−1 s-1. Depending on the choice of α-nucleophiles, both dynamic and irreversible conjugation reactions have been reported. The irreversible conjugation chemistries display excellent bioorthogonality and have been adapted to label molecules of both bacterial and mammalian cells. The dynamic conjugation reactions show fast reaction kinetics and tunable product stabilities. These characteristics make them appealing for applications in dynamic combinatorial chemistry,49 although this direction has not been fully explored. Finally, it is interesting to note that essentially all reactions discussed herein appeared within the past three years with just two chemotypes, namely 2-FPBA and 2-APBA, whose reactivity originates from the ortho-arrangement of a carbonyl and a boronic acid. It is exciting to speculate on the potential expansion of reactivities when we venture beyond this particular combination of functional groups on a preorganized scaffold.
Acknowledgements
We acknowledge the National Institutes of Health for providing financial support of our work (Grant GM102735 to J.G.).
Biography
Samantha Cambray
Samantha Cambray received her B.S. in Biochemistry from Villanova University in 2014. She is currently a Ph.D. student in Professor Jianmin Gao’s group at Boston College where she studies the design and application of chemoselective biocompatible chemical probes towards detection of bacterial pathogens and other clinically relevant biological targets.
Jianmin Gao
Jianmin Gao is currently an Associate Professor of Chemistry at Boston College. He received his PhD from Stanford University under the supervision of Professor Eric Kool and performed his post-doctoral training with Professor Jeffery Kelly at Scripps. His group at Boston College explores novel strategies of molecular recognition toward the goal of targeting and controlling important biomolecules, particularly those involved in antibiotic resistance.
References
- (1).Shannon DA; Weerapana E Covalent Protein Modification: The Current Landscape of Residue-Specific Electrophiles. Current Opinion in Chemical Biology 2015, 24, 18–26. [DOI] [PubMed] [Google Scholar]
- (2).Singh J; Petter RC; Baillie TA; Whitty A The Resurgence of Covalent Drugs. Nat. Rev. Drug. Discov. 2011, 10, 307–317. [DOI] [PubMed] [Google Scholar]
- (3).Bertozzi CR A Decade of Bioorthogonal Chemistry. Acc. Chem. Res. 2011, 44, 651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).McKay CS; Finn MG Click Chemistry in Complex Mixtures: Bioorthogonal Bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kolb HC; Finn MG; Sharpless KB Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [DOI] [PubMed] [Google Scholar]
- (6).Johnson DS; Weerapana E; Cravatt BF Strategies for Discovering and Derisking Covalent, Irreversible Enzyme Inhibitors. Future Medicinal Chemistry 2010, 2, 949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hall DG; Akgun B Boronic Acids as Bioorthogonal Probes in Site-Selective Labeling of Proteins. Angew. Chem. Int. Ed. Engl. 2018, DOI: 10.1002/anie.201712611 [DOI] [PubMed] [Google Scholar]
- (8).Uetrecht J Idiosyncratic Drug Reactions: Past, Present, and Future. Chem. Res. Toxicol. 2008, 21, 84–92. [DOI] [PubMed] [Google Scholar]
- (9).Bandyopadhyay A; Gao J Targeting Biomolecules with Reversible Covalent Chemistry. Curr. Opin. Chem. Biol. 2016, 34, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bradshaw JM; McFarland JM; Paavilainen VO; Bisconte A; Tam D; Phan VT; Romanov S; Finkle D; Shu J; Patel V; Ton T; Li X; Loughhead DG; Nunn PA; Karr DE; Gerritsen ME; Funk JO; Owens TD; Verner E; Brameld KA; Hill RJ; Goldstein DM; Taunton J Prolonged and Tunable Residence Time Using Reversible Covalent Kinase Inhibitors. Nat. Chem. Biol. 2015, 11, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Krishnan S; Miller RM; Tian B; Mullins RD; Jacobson MP; Taunton J Design of Reversible, Cysteine-Targeted Michael Acceptors Guided by Kinetic and Computational Analysis. J. Am. Chem. Soc. 2014, 136, 12624–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Serafimova IM; Pufall MA; Krishnan S; Duda K; Cohen MS; Maglathlin RL; McFarland JM; Miller RM; Frodin M; Taunton J Reversible Targeting of Noncatalytic Cysteines with Chemically Tuned Electrophiles. Nat. Chem. Biol. 2012, 8, 471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Crugeiras J; Rios A; Riveiros E; Amyes TL; Richard JP Glycine Enolates: The Effect of Formation of Iminium Ions to Simple Ketones on Alpha-Amino Carbon Acidity and a Comparison with Pyridoxal Iminium Ions. J. Am. Chem. Soc. 2008, 130, 2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cal PM; Vicente JB; Pires E; Coelho AV; Veiros LF; Cordeiro C; Gois PM Iminoboronates: A New Strategy for Reversible Protein Modification. J. Am. Chem. Soc. 2012, 134, 10299–10305. [DOI] [PubMed] [Google Scholar]
- (15).Gutierrez-Moreno NJ; Medrano F; Yatsimirsky AK Schiff Base Formation and Recognition of Amino Sugars, Aminoglycosides and Biological Polyamines by 2-Formyl phenylboronic Acid in Aqueous Solution. Organic & Biomolecular Chemistry 2012, 10, 6960–6972. [DOI] [PubMed] [Google Scholar]
- (16).Akcay G; Belmonte MA; Aquila B; Chuaqui C; Hird AW; Lamb ML; Rawlins PB; Su N; Tentarelli S; Grimster NP; Su Q Inhibition of Mcl-1 Through Covalent Modification of a Noncatalytic Lysine Side Chain. Nat. Chem. Biol. 2016, 12, 931–936. [DOI] [PubMed] [Google Scholar]
- (17).Cal PM; Frade RF; Cordeiro C; Gois PM Reversible Lysine Modification on Proteins by Using Functionalized Boronic Acids. Chemistry 2015, 21, 8182–8187. [DOI] [PubMed] [Google Scholar]
- (18).Bandyopadhyay A; McCarthy KA; Kelly MA; Gao J Targeting Bacteria via Iminoboronate Chemistry of Amine-Presenting Lipids. Nat. Comm. 2015, 6, 6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bandyopadhyay A; Gao J Iminoboronate-Based Peptide Cyclization That Responds to pH, Oxidation, and Small Molecule Modulators. J. Am. Chem. Soc. 2016, 138, 2098–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chapin BM; Metola P; Lynch VM; Stanton JF; James TD; Anslyn EV Structural and Thermodynamic Analysis of a Three-Component Assembly Forming ortho-Iminophenylboronate Esters. The Journal of Organic Chemistry 2016, 81, 8319–8330. [DOI] [PubMed] [Google Scholar]
- (21).Ni W; Kaur G; Springsteen G; Wang B; Franzen S Regulating the Fluorescence Intensity of an Anthracene Boronic Acid System: A B-N bond or a Hydrolysis Mechanism? Bioorg Chem 2004, 32, 571–581. [DOI] [PubMed] [Google Scholar]
- (22).Collins BE; Sorey S; Hargrove AE; Shabbir SH; Lynch VM; Anslyn EV Probing Intramolecular B–N Interactions in Ortho-Aminomethyl Arylboronic Acids. The Journal of Organic Chemistry 2009, 74, 4055–4060. [DOI] [PubMed] [Google Scholar]
- (23).Bandyopadhyay A; Gao J Iminoboronate Formation Leads to Fast and Reversible Conjugation Chemistry of Alpha-Nucleophiles at Neutral pH. Chemistry 2015, 21, 14748–14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Myers JK; Pace CN Hydrogen bonding stabilizes globular proteins. Biophys. J. 1996, 71, 2033–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).McCarthy KA; Kelly MA; Li K; Cambray S; Hosseini AS; van Opijnen T; Gao J Phage Display of Dynamic Covalent Binding Motifs Enables Facile Development of Targeted Antibiotics. J. Am. Chem. Soc. 2018, 140, 6137–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Groll M; Berkers CR; Ploegh HL; Ovaa H Crystal Structure of the Boronic Acid-Based Proteasome Inhibitor Bortezomib in Complex with the Yeast 20S Proteasome. Structure 2006, 14, 451–456. [DOI] [PubMed] [Google Scholar]
- (27).F., S. P.; Janakan K; A., N. P.; J., H. R.; Dane K; D., T.; Mohammad M; O., F. J.; G., G. S. “A phase I trial of PRN1008, a novel reversible covalent inhibitor of Bruton’s tyrosine kinase, in healthy volunteers” British Journal of Clinical Pharmacology 2017, 83, 2367–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bandyopadhyay A; Cambray S; Gao J Fast Diazaborine Formation of Semicarbazide Enables Facile Labeling of Bacterial Pathogens. J. Am. Chem. Soc. 2017, 139, 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bandyopadhyay A; Cambray S; Gao J Fast and Selective Labeling of N-Terminal Cysteines at Neutral pH via Thiazolidino Boronate Formation. Chemical Science 2016, 7, 4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Faustino H; Silva MJSA; Veiros LF; Bernardes GJL; Gois PMP Iminoboronates are Efficient Intermediates for Selective, Rapid and Reversible N-terminal Cysteine Functionalisation. Chemical Science 2016, 7, 5052–5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Bernardes GJ; Steiner M; Hartmann I; Neri D; Casi G Site-Specific Chemical Modification of Antibody fragments using Traceless Cleavable Linkers. Nat. Protoc. 2013, 8, 2079–2089. [DOI] [PubMed] [Google Scholar]
- (32).Draganov AB; Wang K; Holmes J; Damera K; Wang D; Dai C; Wang B Click with a Boronic Acid Handle: A Neighboring Group-Assisted Click Reaction that Allows Ready Secondary Functionalization. Chem. Commun. (Camb) 2015, 51, 15180–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Li K; Weidman C; Gao J Dynamic Formation of Imidazolidino Boronate Enables Design of Cysteine-Responsive Peptides. Org. Lett. 2018, 20, 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kölmel DK; Kool ET Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chemical Reviews 2017, 117, 10358–10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Dirksen A; Dawson PE Rapid Oxime and Hydrazone Ligations with Aromatic Aldehydes for Biomolecular Labeling. Bioconjug. Chem. 2008, 19, 2543–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Schmidt P; Stress C; Gillingham D Boronic Acids Facilitate Rapid Oxime Condensations at Neutral pH. Chem. Sci. 2015, 6, 3329–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kalia J; Raines RT Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. Engl. 2008, 47, 7523–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Dilek O; Lei Z; Mukherjee K; Bane S Rapid Formation of a Stable Boron-Nitrogen Heterocycle in Dilute, Neutral Aqueous Solution for Bioorthogonal Coupling Reactions. Chem. Commun. (Camb) 2015, 51, 16992–16995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Stress CJ; Schmidt PJ; Gillingham DG Comparison of Boron-Assisted Oxime and Hydrazone Formations Leads to the Discovery of a Fluorogenic Variant. Org. Biomol. Chem. 2016, 14, 5529–5533. [DOI] [PubMed] [Google Scholar]
- (40).Wang L; Dai C; Burroughs SK; Wang SL; Wang B Arylboronic Acid Chemistry Under Electrospray Conditions. Chemistry 2013, 19, 7587–7594. [DOI] [PubMed] [Google Scholar]
- (41).Zhang Z; Smith BAC; Wang L; Brock A; Cho C; Schultz PG A New Strategy for the Site-Specific Modification of Proteins in Vivo. Biochemistry 2003, 42, 6735–6746. [DOI] [PubMed] [Google Scholar]
- (42).Gu H; Chio TI; Lei Z; Staples RJ; Hirschi JS; Bane S Formation of hydrazones and stabilized boron-nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids. Organic & Biomolecular Chemistry 2017, 15, 7543–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Lam H; Oh DC; Cava F; Takacs CN; Clardy J; de Pedro MA; Waldor MK D-Amino Acids Govern Stationary Phase Cell Wall Remodeling in Bacteria. Science 2009, 325, 1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Cambray S; Bandyopadhyay A; Gao J Fluorogenic Diazaborine Formation of Semicarbazide with Designed Coumarin Derivatives. Chemical Communications 2017, 53, 12532–12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).James TD; Sandanayake KRAS; Shinkai S Saccharide Sensing with Molecular Receptors Based on Boronic Acid. Angewandte Chemie-International Edition 1996, 35, 1910–1922. [Google Scholar]
- (46).Meadows MK; Roesner EK; Lynch VM; James TD; Anslyn EV Boronic Acid Mediated Coupling of Catechols and N-Hydroxylamines: A Bioorthogonal Reaction to Label Peptides. Org. Lett. 2017, 19, 3179–3182. [DOI] [PubMed] [Google Scholar]
- (47).Akgun B; Li C; Hao Y; Lambkin G; Derda R; Hall DG Synergic “Click” Boronate/Thiosemicarbazone System for Fast and Irreversible Bioorthogonal Conjugation in Live Cells. J. Am. Chem. Soc. 2017, 139, 14285–14291. [DOI] [PubMed] [Google Scholar]
- (48).Akgun B; Hall DG Fast and Tight Boronate Formation for Click Bioorthogonal Conjugation. Angewandte Chemie International Edition 2016, 55, 3909–3913. [DOI] [PubMed] [Google Scholar]
- (49).Cougnon FB; Sanders JK Evolution of Dynamic Combinatorial Chemistry. Acc. Chem. Res. 2012, 45, 2211–2221. [DOI] [PubMed] [Google Scholar]