Abstract
Background:
Carvedilol and metoprolol are the beta-blockers most commonly prescribed to U.S. hemodialysis patients, accounting for approximately 80% of beta-blocker prescriptions. Despite well-established pharmacologic and pharmacokinetic differences between the two medications, little is known about their relative safety and efficacy in the hemodialysis population.
Study design:
A retrospective cohort study using a new-user design.
Setting & participants:
Medicare-enrolled hemodialysis patients treated at a large U.S. dialysis organization who initiated carvedilol or metoprolol therapy from 01/01/2007 through 12/30/2012.
Predictor:
Carvedilol versus metoprolol initiation.
Outcomes:
All-cause mortality, cardiovascular mortality and intradialytic hypotension (systolic blood pressure drop ≥20 mmHg during hemodialysis plus intradialytic saline administration) during a 1-year follow-up period.
Measurements:
Survival models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) in mortality analyses. Poisson regression was used to estimate incidence rate ratios (IRR) and 95% CIs in intradialytic hypotension analyses. Inverse probability of treatment weighting was used to adjust for several demographic, clinical, laboratory and dialysis treatment covariates in all analyses.
Results:
27,064 individuals receiving maintenance hemodialysis were included: 9,558 (35.3%) carvedilol initiators and 17,506 (64.7%) metoprolol initiators. Carvedilol (versus metoprolol) initiation was associated with greater all-cause mortality (adjusted HR, 1.08; 95% CI, 1.02–1.16) and cardiovascular mortality (adjusted HR, 1.18; 95% CI, 1.08–1.29). In subgroup analyses, similar associations were observed among patients with hypertension, atrial fibrillation, heart failure, and a recent myocardial infarction, the main cardiovascular indications for beta-blocker therapy. During follow-up, carvedilol (versus metoprolol) initiators had a higher rate of intradialytic hypotension (adjusted IRR, 1.10; 95% CI, 1.09–1.11).
Limitations:
Residual confounding may exist.
Conclusions:
Relative to metoprolol initiation, carvedilol initiation was associated with higher 1-year all-cause and cardiovascular mortality. One potential mechanism for these findings may be the increased occurrence of intradialytic hypotension after carvedilol (versus metoprolol) initiation.
Index words: beta-blocker, cardiovascular, carvedilol, hemodialysis, hypotension, metoprolol, mortality, blood pressure, intradialytic hypotension (IDH), dialyzability, end-stage renal disease (ESRD), pharmacoepidemiology
Graphical Abstract
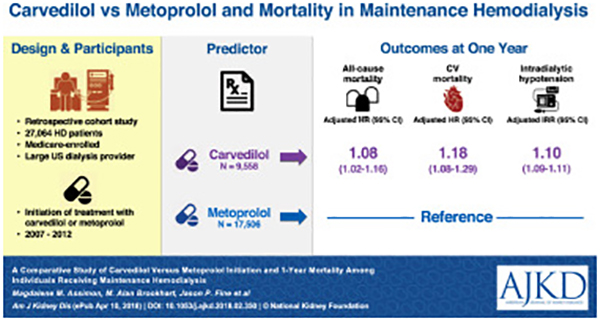
Individuals receiving maintenance hemodialysis have cardiovascular mortality rates that exceed those of the general population by 5 to 7-fold.1 Cardioprotective medications such as beta-blockers, among others, are often prescribed to reduce cardiovascular risk. However, clinical trials establishing the cardioprotective nature and safety of beta-blockers largely excluded individuals with end-stage renal disease (ESRD).2,3 Approximately 65% of the United States (U.S.) hemodialysis population is treated with a beta-blocker.4 Despite widespread use, surprisingly little is known about the relative safety and efficacy of different beta-blockers in hemodialysis patients, a population with special drug dosing considerations.
Within the beta-blocker class, individual medications possess different pharmacologic and pharmacokinetic properties. Pharmacologically, beta-blockers differ with respect to their beta-adrenergic receptor selectivity and vasodilatory capabilities. Kinetically, physiochemical factors, such as molecular size, hydrophilicity, plasma protein binding, and volume of distribution influence the extent of beta-blocker clearance by the hemodialysis procedure (i.e. dialyzablity). These key differences may plausibly alter the hemodynamic and antiarrhythmic risk-benefit profiles of individual beta-blockers in the setting of ESRD.
In fact, observational data suggests that the potential survival benefit conferred by beta-blockers may differ across agents. In a Canadian cohort, Weir et al. found that the risk of all-cause death was significantly higher among hemodialysis patients treated with high dialyzability beta blockers (acebutolol, atenolol, metoprolol tartrate) as compared to patients treated with low dialyzablity beta-blockers (bisoprolol and propranolol).5 However, carvedilol and metoprolol succinate, two commonly prescribed beta-blockers in the U.S.,4 were not considered due to Canadian provincial prescription formulary restrictions. Carvedilol is a non-selective beta-blocker with alpha-blocking effects and is minimally cleared by hemodialysis. Metoprolol (tartrate and succinate) is a cardioselective beta-blocker and is extensively cleared by hemodialysis. The marked pharmacologic and pharmacokinetic heterogeneity between carvedilol and metoprolol may differentially influence clinical outcomes and safety among individuals receiving maintenance hemodialysis and warrants further study.
While a head-to-head randomized clinical trial would be the ideal approach to investigate the comparative safety and efficacy of carvedilol and metoprolol in the dialysis population, a recent feasibility study suggests that recruitment for such a trial may be challenging.6 Well-designed pharmacoepidemiologic studies are thus needed to inform clinical decision-making. We undertook this study to investigate the association between carvedilol versus metoprolol initiation and 1-year mortality in a cohort of prevalent hemodialysis patients treated at a large U.S. dialysis organization.
METHODS
This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board (#15–2651). A waiver of consent was granted due to the study’s large size, data anonymity, and retrospective nature.
Data source
The study data were extracted from the clinical database of a large U. S. dialysis organization and the U.S. Renal Data System (USRDS). Data were linked at the patient level. The dialysis organization operates over 1,500 outpatient dialysis clinics throughout the nation. Its database captures detailed demographic, clinical, laboratory, and dialysis treatment data. Laboratory data were measured on a biweekly or monthly basis. Hemodialysis treatment parameters were recorded on a treatment-to-treatment basis. The USRDS is a national ESRD surveillance system that includes: the Medical Evidence and ESRD Death Notification forms, the Medicare Enrollment database (a repository of Medicare beneficiary enrollment and entitlement data), and Medicare standard analytic files (final action administrative claims data including Medicare Parts A, B and D).
Study design and population
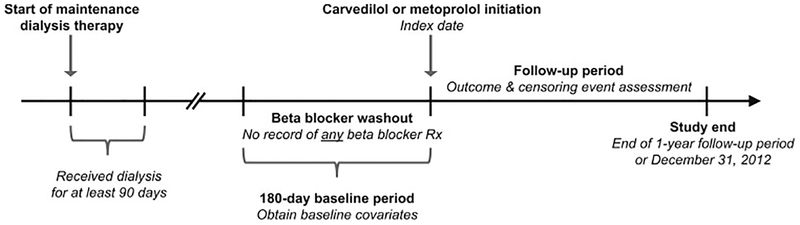
We conducted a retrospective cohort study using an active comparator new-user design,7 the observational analog to a head-to-head randomized controlled trial, to investigate the association between carvedilol versus metoprolol initiation and 1-year all-cause and cardiovascular mortality (separately) among individuals receiving maintenance hemodialysis. Employing a new-user study design to evaluate the comparative safety and/or effectiveness of medications in retrospective investigations helps to mitigate biases common to observational studies of prescription drugs, such as selection and immortal time biases. Figure 1 displays the study design. First, using Medicare Part D claims, we identified dialysis patients treated at the large dialysis organization who initiated oral beta-blocker therapy from January 1, 2007 to December 30, 2012 following a 180-day baseline period free of any documented oral beta-blocker use (i.e. a beta-blocker washout period). We then applied the following exclusion criteria: 1) age <18 years old at the start of the baseline period, 2) dialysis vintage ≤90 days at the start of the baseline period (to ensure all potential study patients were eligible for Medicare coverage regardless of their age), 3) lack of continuous Medicare Part A, B and D coverage during the entire baseline period, 4) receipt of home hemodialysis or peritoneal dialysis during the baseline period, 5) receipt of <6 center-based hemodialysis treatments in the last 30 days of the baseline period, 6) receipt of hospice care during the baseline period, 7) missing demographic or laboratory data, and 8) initiation of an oral beta-blocker other than carvedilol or metoprolol. The study cohort consisted of prevalent, center-based hemodialysis patients who were carvedilol or metoprolol new-users.
Figure 1. Study design.
Carvedilol and metoprolol initiators were defined as hemodialysis patients who had no record of a beta-blocker prescription in the previous 180 days (beta-blocker washout period). Among these patients, the index date was defined as the date of carvedilol or metoprolol initiation. Baseline covariates were identified in the 180-day period prior to the index date. Study follow-up began immediately after the index date. To ensure all potential study patients were eligible for Medicare coverage regardless of their age, individuals needed to have a dialysis vintage > 90 days at the start of the baseline period.
Abbreviations: Rx, prescription
Study exposure, outcomes, and censoring events
The exposures of interest were carvedilol and metoprolol initiation. The index date was designated as the date of the first carvedilol or metoprolol prescription after the washout period. Primary study outcomes were 1-year all-cause and cardiovascular mortality (assessed separately). Secondary outcomes were all-cause and cardiovascular hospitalizations (assessed separately) during the 1-year follow-up period. Mortality and hospitalization outcomes were defined using established USRDS definitions (Table S1).8 Censoring events included: kidney transplantation, dialysis modality change, recovery of renal function, loss of Medicare Part A, B or D coverage, being lost to follow-up, reaching 1-year of follow-up post-index date, or study end (December 31, 2012).
Baseline covariate determination
Baseline covariates included potential confounders and variables known to be strong risk factors for death in the hemodialysis population.9 Similar to previous pharmacoepidemiologic analyses using USRDS data,10–13 covariates were identified in the 180 days prior to the index date and included: patient demographics, comorbid conditions, laboratory data, dialysis treatment parameters, and prescription medication use (] Table S2). Use of a 180-day baseline period enabled us to maximize cohort generalizability and facilitated capture of patient characteristics that: 1) occurred close to study medication initiation that may have influenced beta-blocker prescribing decisions,14 and 2) are highly predictive of the study outcomes.15
Statistical analysis
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC). Baseline characteristics were described across carvedilol and metoprolol initiators as count (%) for categorical variables and mean ± standard deviation for continuous variables. Baseline covariate distributions were compared using standardized differences. A standardized difference >0.1 represents meaningful imbalance between treatment groups.16
In primary analyses, we used an intent-to-treat approach to evaluate the association between carvedilol (versus metoprolol) initiation and 1-year all-cause and cardiovascular mortality. Individuals were followed forward in historical time from the index date to the first occurrence of a study outcome or censoring event. Cox proportional hazards models were used to assess the study beta-blocker—all-cause mortality association. Fine and Gray proportional subdistribution hazards models17 that treated non-cardiovascular death as a competing risk were used to assess the study beta-blocker—cardiovascular mortality association. Both models estimate hazard ratios (HRs) and their 95% confidence intervals (CIs). Robust variance estimation was used in all analyses.18 Inverse probability of treatment (IPT) weighting was used to control for confounding. We used multivariable logistic regression to calculate the predicted probability (i.e. propensity score) of receiving carvedilol (versus metoprolol) as a function of baseline covariates. Propensity scores were used to generate IPT weights.19,20 We estimated adjusted HRs by applying IPT weights in regression models.
We conducted several sensitivity analyses to assess the robustness of our primary results. First, since the effect of metoprolol (versus carvedilol) on all-cause mortality may differ by metoprolol formulation,21 we repeated primary analyses and separately compared: 1) carvedilol versus metoprolol tartrate (the immediate release formulation), and 2) carvedilol versus metoprolol succinate (the controlled/extended release formulation). Second, we repeated primary analyses using an on-treatment (i.e. per-protocol) approach. In these analyses, index beta-blocker discontinuation and switching to a non-index beta-blocker during follow-up were considered as additional censoring events. Third, to further minimize the influence of potential confounding by indication (i.e. indication bias), we evaluated the association between carvedilol (versus metoprolol) initiation and 1-year mortality among individuals who did not experience a cardiovascular hospitalization during the last 30 days of the baseline period. Fourth, we tested the specificity of our findings by examining the association between carvedilol (versus metoprolol) initiation and hospitalized bowel obstruction, a tracer (i.e. negative control) outcome that we did not expect to be influenced by the utilization of either of the study medications.
In secondary analyses, we evaluated the study beta-blocker–mortality associations within clinically relevant subgroups. We assessed the association between carvedilol (versus metoprolol) initiation and 1-year morality among individuals with hypertension, atrial fibrillation, heart failure and a recent myocardial infraction, the main cardiovascular indications for beta-blocker therapy. In additional analyses, we assessed the associations between carvedilol (versus metoprolol) initiation and the occurrence of hospitalizations during the 1-year follow-up by estimating incidence rate ratios (IRRs) and their 95% CIs using Poisson regression.
We also conducted post hoc analyses to evaluate potential mechanistic explanations for our study findings. We assessed the association between carvedilol (versus metoprolol) initiation and the occurrence of intradialytic hypotension during the 1-year follow-up period by estimating IRRs and their 95% CIs using Poisson regression. Episodes of intradialytic hypotension were identified using two different definitions: 1) a systolic blood pressure drop ≥20 mmHg during hemodialysis plus intradialytic saline administration (a guideline-based definition);22–24 and 2) an intradialytic nadir systolic blood pressure <90 mmHg (a definition shown to associate with mortality).25 We also evaluated study beta-blocker–mortality associations among patients with and without a recent history of frequent intradialytic hypotension. Patients were classified as having a recent history of frequent intradialytic hypotension if they experienced an episode of intradialytic hypotension (defined both ways, separately) in at least 30% of outpatient hemodialysis treatments during the last 30 days of the baseline period.25
RESULTS
Study cohort characteristics
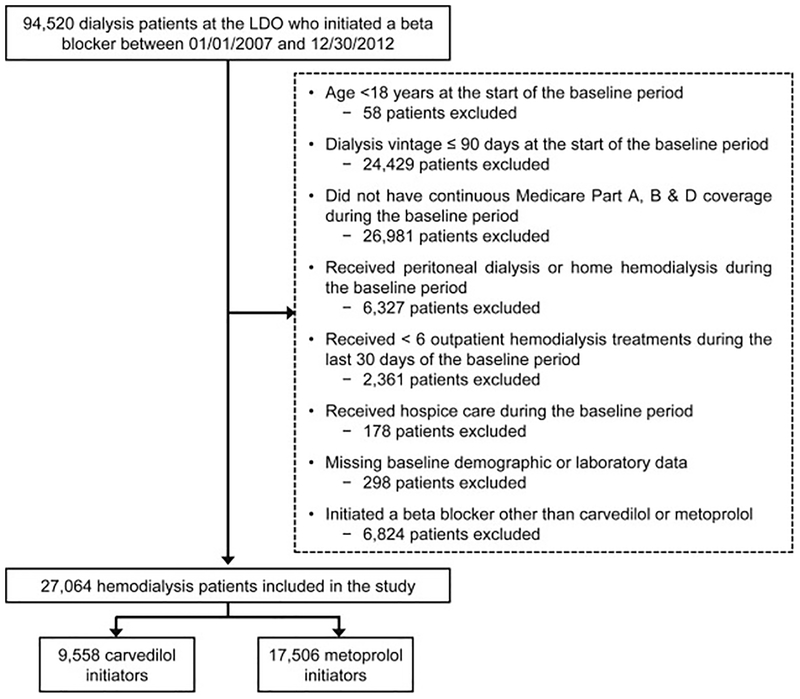
Figure 2 displays a flow diagram of study cohort selection. A total of 27,064 individuals receiving maintenance hemodialysis were included in the study: 9,558 (35.3%) carvedilol initiators and 17,506 (64.7%) metoprolol initiators. Overall, study patients had an average age of 59.6 ± 14.7 years, 46.7% were female, 42.9% were black, 19.5% were Hispanic and the most common ESRD cause was diabetes (49.0%). Cardiovascular comorbidities were common; 13.9% of the cohort had atrial fibrillation, 29.9% had coronary atherosclerosis, 72.7% had hypertension, 34.6% had heart failure, 6.6% had a recent myocardial infarction, and 21.7% had peripheral arterial disease.
Figure 2. Flow diagram depicting the assembly of the study cohort.
Abbreviations: LDO, large dialysis organization
The propensity score distribution of carvedilol and metoprolol initiators exhibited substantial overlap (Figure S1), indicating that the study groups were highly comparable. Patient baseline characteristics stratified by study beta-blocker are presented in Table 1. Before IPT weighting, baseline covariates were generally well-balanced between treatment groups (standardized differences ≤0.1), with a few exceptions (year of index carvedilol or metoprolol initiation, heart failure and an ESRD cause of diabetes). After IPT weighting all baseline covariates were well-balanced between treatment groups.
Table 1.
Baseline characteristics of study patients initiating carvedilol and metoprolol
| Characteristic | Unweighted | Weighted | ||||
|---|---|---|---|---|---|---|
| Carvedilol n = 9,558 | Metoprolol n = 17,506 | Std diffa | Carvedilol n = 9,533 | Metoprolol n = 17,521 | Std diffa | |
| Age (y) | 59.8 ±14.4 | 59.5 ±14.9 | 0.026 | 59.8 ± 14.4 | 59.5 ± 14.9 | 0.026 |
| Female sex | 4,314 (45.1%) | 8,316 (47.5%) | 0.048 | 4,444 (46.6%) | 8,183 (46.7%) | 0.002 |
| Race | ||||||
| White | 4,848 (50.7%) | 9,054 (51.7%) | 0.020 | 4,881 (51.2%) | 8,991 (51.3) | 0.002 |
| Black | 4,186 (43.8%) | 7,419 (42.4%) | 0.029 | 4,103 (43.0%) | 7,524 (42.9%) | 0.002 |
| Other | 524 (5.5%) | 1,033 (5.9%) | 0.018 | 549 (5.8%) | 1,006 (5.7%) | 0.001 |
| Hispanic ethnicity | 1,925 (20.1%) | 3,351 (19.1%) | 0.025 | 1,874 (19.7%) | 3,428 (19.6%) | 0.002 |
| Low-income subsidy | 7,259 (75.9%) | 13,524 (77.3%) | 0.031 | 7,328 (76.9%) | 13,463 (76.8%) | 0.001 |
| Year index beta-blocker was prescribed | ||||||
| 2007 | 1,339 (14.0%) | 3,364 (19.2%) | 0.140 | 1,631 (17.1%) | 3,034 (17.3%) | 0.005 |
| 2008 | 1,385 (14.5%) | 3,011 (17.2%) | 0.074 | 1,534 (16.1%) | 2,833 (16.2%) | 0.002 |
| 2009 | 1,440 (15.1%) | 2,561 (14.6%) | 0.012 | 1,406 (14.8%) | 2,588 (14.8%) | 0.000 |
| 2010 | 1,524 (15.9%) | 2,696 (15.4%) | 0.015 | 1,497 (15.7%) | 2,736 (15.6%) | 0.002 |
| 2011 | 1,804 (18.9%) | 2,852 (16.3%) | 0.068 | 1,665 (17.5%) | 3,029 (17.3%) | 0.005 |
| 2012 | 2,066 (21.6%) | 3,022 (17.3%) | 0.110 | 1,801 (18.9%) | 3,302 (18.8%) | 0.001 |
| Cause of ESRD | ||||||
| Diabetes | 5,027 (52.6%) | 8,227 (47.0%) | 0.112 | 4,703 (49.3%) | 8,606 (49.1%) | 0.004 |
| Hypertension | 2,563 (26.8%) | 5,051 (28.9%) | 0.045 | 2,686 (28.2%) | 4,927 (28.1%) | 0.001 |
| Glomerular disease | 909 (9.5%) | 1,936 (11.1%) | 0.051 | 982 (10.3%) | 1,828 (10.4%) | 0.004 |
| Other | 1,059 (11.1%) | 2,292 (13.1%) | 0.062 | 1,163 (12.2%) | 2,160 (12.3%) | 0.004 |
| Body mass index | ||||||
| < 18.5 kg/m2 | 474 (5.0%) | 844 (4.8%) | 0.006 | 464 (4.9%) | 854 (4.9%) | 0.000 |
| 18.5 – 24.9 kg/m2 | 3,555 (37.2%) | 6,285 (35.9%) | 0.027 | 3,475 (36.5%) | 6,371 (36.4%) | 0.002 |
| 25.0 – 29.9 kg/m2 | 2,761 (28.9%) | 4,978 (28.4%) | 0.010 | 2,719 (28.5%) | 5,005 (28.6%) | 0.001 |
| ≥ 30.0 kg/m2 | 2,768 (29.0%) | 5,399 (30.8%) | 0.041 | 2,875 (30.2%) | 5,292 (30.2%) | 0.001 |
| History of prior kidney transplantation | 502 (5.3%) | 1,204 (6.9%) | 0.068 | 594 (6.2%) | 1,103 (6.3%) | 0.003 |
| Dialysis vintage | ||||||
| 0.7 – 0.9 years | 595 (6.2%) | 935 (5.3%) | 0.038 | 536 (5.6%) | 988 (5.6%) | 0.001 |
| 1.0 – 1.9 years | 2,118 (22.2%) | 3,705 (21.2%) | 0.024 | 2,053 (21.5%) | 3,778 (21.6%) | 0.001 |
| 2.0 – 2.9 years | 1,668 (17.5%) | 2,778 (15.9%) | 0.042 | 1,556 (16.3%) | 2,875 (16.4%) | 0.002 |
| ≥ 3.0 years | 5,177 (54.2%) | 10,088 (57.6%) | 0.070 | 5,388 (56.5%) | 9,881 (56.4%) | 0.003 |
| CV admission during the last 30 d of baseline | 1,801 (18.8%) | 2,815 (16.1%) | 0.073 | 1,618 (17.0%) | 2,989 (17.1%) | 0.002 |
| Atrial fibrillation | 1,236 (12.9%) | 2,525 (14.4%) | 0.043 | 1,300 (13.6%) | 2,426 (13.8%) | 0.006 |
| Other arrhythmia | 930 (9.7%) | 1,630 (9.3%) | 0.014 | 906 (9.5%) | 1,657 (9.5%) | 0.002 |
| Angina | 210 (2.2%) | 302 (1.7%) | 0.034 | 182 (1.9%) | 334 (1.9%) | 0.000 |
| Cancer | 312 (3.3%) | 661 (3.8%) | 0.028 | 335 (3.5%) | 627 (3.6%) | 0.003 |
| Conduction disorder | 367 (3.8%) | 496 (2.8%) | 0.056 | 304 (3.2%) | 559 (3.2%) | 0.000 |
| COPD/asthma | 1,704 (17.8%) | 2,795 (16.0%) | 0.050 | 1,601 (16.8%) | 2,922 (16.7%) | 0.003 |
| Coronary atherosclerosis | 3,126 (32.7%) | 4,960 (28.3%) | 0.095 | 2,867 (30.1%) | 5,251 (30.0%) | 0.002 |
| Diabetes | 5,473 (57.3%) | 9,286 (53.0%) | 0.085 | 5,236 (54.9%) | 9,586 (54.7%) | 0.004 |
| GI bleed | 471 (4.9%) | 932 (5.3%) | 0.018 | 503 (5.3%) | 911 (5.2%) | 0.004 |
| Heart failure | 4,107 (43.0%) | 5,251 (30.0%) | 0.272 | 3,332 (34.9%) | 6,087 (34.7%) | 0.004 |
| Hypertension | 7,021 (73.5%) | 12,652 (72.3%) | 0.027 | 6,960 (73.0%) | 12,763 (72.8%) | 0.004 |
| Liver disease | 421 (4.4%) | 783 (4.5%) | 0.003 | 434 (4.6%) | 784 (4.5%) | 0.004 |
| Myocardial infarction | 642 (6.7%) | 1,151 (6.6%) | 0.006 | 644 (6.8%) | 1,171 (6.7%) | 0.003 |
| Peripheral artery disease | 2,149 (22.5%) | 3,729 (21.3%) | 0.029 | 2,095 (22.0%) | 3,820 (21.8%) | 0.004 |
| Stroke | 975 (10.2%) | 1,876 (10.7%) | 0.017 | 1,030 (10.8%) | 1,861 (10.6%) | 0.006 |
| Valvular disease | 904 (9.5%) | 1,337 (7.6%) | 0.065 | 795 (8.3%) | 1,457 (8.3%) | 0.001 |
| History of treatment nonadherenceb | 594 (6.2%) | 1,021 (5.8%) | 0.016 | 581 (6.1%) | 1,051 (6.0%) | 0.004 |
| Vascular access | ||||||
| Fistula | 5,645 (59.1%) | 10,054 (57.4%) | 0.033 | 5,516 (57.9%) | 10,150 (57.9%) | 0.001 |
| Graft | 2,428 (25.4%) | 4,451 (25.4%) | 0.001 | 2,448 (25.7%) | 4,470 (25.5%) | 0.004 |
| Catheter | 1,485 (15.5%) | 3,001 (17.1%) | 0.043 | 1,570 (16.5%) | 2,902 (16.6%) | 0.003 |
| Interdialytic weight gain ≥ 3 kg | 2,377 (24.9%) | 4,196 (24.0%) | 0.021 | 2,310 (24.2%) | 4,253 (24.3%) | 0.001 |
| Delivered dialysis treatment time < 240 min | 7,657 (80.1%) | 13,940 (79.6%) | 0.012 | 7,628 (80.0%) | 13,989 (79.8%) | 0.004 |
| Pre-dialysis systolic BP | ||||||
| < 130 mmHg | 1,384 (14.5%) | 2,159 (12.3%) | 0.063 | 1,241 (13.0%) | 2,289 (13.1%) | 0.001 |
| 130 – 149 mmHg | 2,696 (28.2%) | 4,744 (27.1%) | 0.025 | 2,621 (27.5%) | 4,808 (27.4%) | 0.001 |
| 150 – 169 mmHg | 3,175 (33.2%) | 6,084 (34.8%) | 0.032 | 3,253 (34.1%) | 5,997 (34.2%) | 0.002 |
| ≥170 mmHg | 2,303 (24.1%) | 4,519 (25.8%) | 0.040 | 2,419 (25.4%) | 4,427 (25.3%) | 0.002 |
| Recent history of frequent IDHc | 1,349 (14.1%) | 2,363 (13.5%) | 0.018 | 1,321 (13.9%) | 2,415 (13.8%) | 0.002 |
| Albumin | ||||||
| ≤ 3.0 g/dL | 468 (4.9%) | 883 (5.0%) | 0.007 | 483 (5.1%) | 877 (5.0%) | 0.003 |
| 3.1 – 4.0 g/dL | 6,221 (65.1%) | 11,057 (63.2%) | 0.040 | 6,092 (63.9%) | 11,191 (63.9%) | 0.001 |
| > 4.0 g/dL | 2,869 (30.0%) | 5,566 (31.8%) | 0.038 | 2,959 (31.0%) | 5,453 (31.1%) | 0.002 |
| Calcium | ||||||
| < 8.5 mg/dL | 1,338 (14.0%) | 2,497 (14.3%) | 0.008 | 1,352 (14.2%) | 2,488 (14.2%) | 0.001 |
| 8.5 – 10.2 mg/dL | 7,756 (81.1%) | 14,159 (80.9%) | 0.007 | 7,714 (80.9%) | 14,180 (80.9%) | 0.000 |
| > 10.2 mg/dL | 464 (4.9%) | 850 (4.9%) | 0.000 | 467 (4.9%) | 853 (4.9%) | 0.002 |
| Phosphorus | ||||||
| < 3.5 mg/dL | 1,088 (11.4%) | 1,907 (10.9%) | 0.016 | 1,050 (11.0%) | 1,936 (11.0%) | 0.001 |
| 3.5 – 5.5 mg/dL | 5,224 (54.7%) | 9,431 (53.9%) | 0.016 | 5,175 (54.3%) | 9,495 (54.2%) | 0.002 |
| > 5.5 mg/dL | 3,246 (34.0%) | 6,168 (35.2%) | 0.027 | 3,309 (34.7%) | 6,091 (34.8%) | 0.001 |
| Potassium | ||||||
| < 4.0 mEq/L | 1,064 (11.1%) | 1,918 (11.0%) | 0.006 | 1,047 (11.0%) | 1,931 (11.0%) | 0.001 |
| 4.0 – 6.0 mEq/L | 8,152 (85.3%) | 14,915 (85.2%) | 0.003 | 8,127 (85.2%) | 14,934 (85.2%) | 0.000 |
| > 6.0 mEq/L | 342 (3.6%) | 673 (3.8%) | 0.014 | 360 (3.8%) | 656 (3.7%) | 0.002 |
| Hemoglobin | ||||||
| < 9.5 g/dL | 663 (6.9%) | 1,166 (6.7%) | 0.011 | 650 (6.8%) | 1,185 (6.8%) | 0.002 |
| 9.5 – 12.0 mg/dL | 6,164 (64.5%) | 10,709 (61.2%) | 0.069 | 5,972 (62.6%) | 10,942 (62.4%) | 0.004 |
| > 12.0 mg/dL | 2,731 (28.6%) | 5,631 (32.2%) | 0.078 | 2,912 (30.5%) | 5,394 (30.8%) | 0.005 |
| Equilibrated Kt/V < 1.2 | 2,235 (23.4%) | 3,850 (22.0%) | 0.033 | 2,145 (22.5%) | 3,944 (22.5%) | 0.000 |
| Number of medications in last 30 days of baseline | 5.5 ± 3.8 | 5.5 ± 3.9 | 0.014 | 5.5 ± 3.9 | 5.5 ± 3.9 | 0.014 |
| Alpha blocker | 63 (0.7%) | 168 (1.0%) | 0.034 | 83 (0.9%) | 151 (0.9%) | 0.001 |
| ACE inhibitor | 2,232 (23.4%) | 4,040 (23.1%) | 0.006 | 2,224 (23.3%) | 4,070 (23.2%) | 0.002 |
| Angiotensin receptor blocker | 1,212 (12.7%) | 1,848 (10.6%) | 0.066 | 1,103 (11.6%) | 2,004 (11.4%) | 0.004 |
| Calcium channel blocker | 3,060 (32.0%) | 5,959 (34.0%) | 0.043 | 3,195 (33.5%) | 5,853 (33.4%) | 0.002 |
| Central alpha agonist | 1,272 (13.3%) | 2,486 (14.2%) | 0.026 | 1,339 (14.0%) | 2,446 (14.0%) | 0.003 |
| Diuretic | 1,239 (13.0%) | 1,845 (10.5%) | 0.075 | 1,095 (11.5%) | 2,010 (11.5%) | 0.000 |
| Vasodilator | 997 (10.4%) | 1,916 (10.9%) | 0.017 | 1,030 (10.8%) | 1,893 (10.8%) | 0.000 |
| Statin | 2,578 (27.0%) | 4,509 (25.8%) | 0.028 | 2,512 (26.4%) | 4,606 (26.3%) | 0.001 |
| Other cholesterol medicationd | 394 (4.1%) | 717 (4.1%) | 0.001 | 394 (4.1%) | 720 (4.1%) | 0.001 |
| Digoxin | 258 (2.7%) | 332 (1.9%) | 0.054 | 205 (2.2%) | 382 (2.2%) | 0.002 |
| Long-acting nitrate | 845 (8.8%) | 1,216 (6.9%) | 0.070 | 733 (7.7%) | 1,344 (7.7%) | 0.001 |
| Antiplatelet medication | 1,280 (13.4%) | 2,065 (11.8%) | 0.048 | 1,202 (12.6%) | 2,187 (12.5%) | 0.004 |
| Anticoagulant medication | 711 (7.4%) | 1,458 (8.3%) | 0.033 | 754 (7.9%) | 1,401 (8.0%) | 0.003 |
| Midodrine | 192 (2.0%) | 350 (2.0%) | 0.001 | 192 (2.0%) | 352 (2.0%) | 0.000 |
| Use of ≥ 1 potent inhibitor of CYP2D6e | 2,690 (29.5%) | 5,162 (28.1%) | 0.030 | 2,767 (29.0%) | 5,090 (29.0%) | 0.001 |
Abbreviations: Std diff, standardized difference; CV, cardiovascular; IDH, intradialytic hypotension; BP, blood pressure.
All-covariates were measured during the baseline period prior to carvedilol or metoprolol initiation. Values for categorical variables are given as number (%) and as mean ± standard deviation for continuous variables. The weighted cohort is the pseudo-population that was generated by the inverse probability of treatment weighting process.
A standardized difference > 0.1 represents meaningful imbalance between groups.16
Claims-based definition of nonadherernce included ICD-9 discharge diagnosis codes V15.81 (personal history of noncompliance with medical treatment, presenting hazards to health) and V45.12 (noncompliance with renal dialysis).
Patients were considered as having a recent history of frequent IDHBP if they had an intradialytic nadir systolic BP < 90 mmHg in at least 30% of outpatient hemodialysis treatments during the last 30 days of the baseline period.25
Other cholesterol medications included the following non-statin cholesterol medications: bile acid sequestrants, cholesterol absorption inhibitors, fibrates and niacin.
Both carvedilol and metoprolol are metabolized by cytochrome P450 2D6. Concomitant use of medications that are potent inhibitors cytochrome P450 2D6 of may increase serum concentrations of both carvedilol and metoprolol, putting patients at increased risk for beta-blocker—related adverse events such as hypotension. Cytochrome P450 2D6 inhibitors included: amiodarone, bupropion, chloroquine, cinacalcet, diphenhydramine, fluoxetine, haloperidol, imatinib, paroxetine, propafenone, propoxyphene, quinidine, terbinafine and thioridazine.
Abbreviations: COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; GI, gastrointestinal
Primary analyses
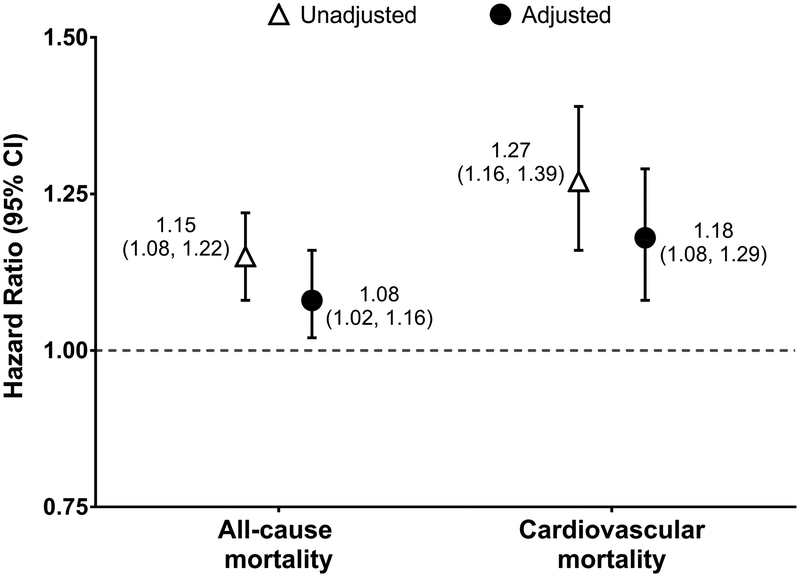
Under the intent-to-treat paradigm, the study cohort was followed for a total of 20,863 person-years (7,219 person-years for carvedilol initiators and 13,644 person-years for metoprolol initiators). The average duration of follow-up was 276 days for carvedilol initiators and 285 days for metoprolol initiators. During follow-up 4,296 all-cause deaths (1,625 in the carvedilol group and 2,671 in the metoprolol group) and 1,943 cardiovascular deaths (782 in the carvedilol group and 1,161 in the metoprolol group) occurred. Figure 3 displays the associations between carvedilol (versus metoprolol) initiation and 1-year all-cause and cardiovascular mortality. Compared to individuals initiating metoprolol, individuals initiating carvedilol had a higher rate of all-cause mortality (225.1 versus 195.8 events/1,000 person-years; adjusted HR, 1.08 [95% CI, 1.02–1.16]) and cardiovascular mortality (108.3 versus 85.1 events/100 person-years; adjusted HR, 1.18 [95% CI, 1.08–1.29]), Figure 3 and Figure S2.
Figure 3. Association between carvedilol versus metoprolol initiation and 1-year mortality: intent-to-treat analysis.
An intent-to-treat design was employed in all analyses. Cox proportional hazards models were used to estimate the association between carvedilol (versus metoprolol) initiation and 1-year all-cause mortality. Fine and Gray proportional subdistribution hazards models were used to estimate the association between carvedilol (versus metoprolol) initiation and 1-year cardiovascular mortality. In cardiovascular mortality analyses, non-cardiovascular death was treated as a competing risk. Inverse probability of treatment weighting was used in adjusted analyses to control for all the baseline covariates listed in Table 1.
Abbreviations: CI, confidence interval; HR, hazard ratio; ref., referent
Secondary analyses
Secondary analyses assessing associations between carvedilol (versus metoprolol) initiation and mortality among individuals with hypertension, atrial fibrillation, heart failure or a recent myocardial infarction produced results analogous to primary study findings (Table 2, Table S3).
Table 2.
Association between carvedilol versus metoprolol initiation and 1-year mortality among clinically relevant subgroups: intent-to-treat analysisa
| 1-year all-cause mortalityb | 1-year cardiovascular mortalityc | |||||
|---|---|---|---|---|---|---|
| Beta-blocker | N | Rate per 1,000 p-y | Adjusted HR (95% CI) | Rate per 1,000 p-y | Adjusted HR (95% CI) | |
| Patients with hypertension (n = 19,673) | ||||||
| Metoprolol | 12,652 | 234.7 | 1.00 (ref.) | 100.7 | 1.00 (ref.) | |
| Carvedilol | 7,021 | 266.0 | 1.09 (1.02, 1.17) | 126.1 | 1.18 (1.07, 1.31) | |
| Patients with atrial fibrillation (n = 3,761) | ||||||
| Metoprolol | 2,525 | 406.1 | 1.00 (ref.) | 174.1 | 1.00 (ref.) | |
| Carvedilol | 1,236 | 458.4 | 1.08 (0.94, 1.23) | 215.9 | 1.12 (0.94, 1.35) | |
| Patients with heart failure (n = 9,358) | ||||||
| Metoprolol | 5,251 | 336.7 | 1.00 (ref.) | 144.9 | 1.00 (ref.) | |
| Carvedilol | 4,107 | 335.8 | 1.02 (0.94, 1.11) | 157.6 | 1.09 (0.96, 1.23) | |
| Patients with a recent MI (n = 1,793) | ||||||
| Metoprolol | 1,151 | 395.6 | 1.00 (ref.) | 187.1 | 1.00 (ref.) | |
| Carvedilol | 642 | 443.6 | 1.02 (0.84, 1.23) | 244.7 | 1.19 (0.92, 1.53) | |
An intent-to-treat design was employed in all analyses. Adjusted analyses controlled for baseline covariates listed in Table 1 using inverse probability of treatment weighting. Subgroups of interest were excluded the corresponding propensity score models. For example, in subgroup analyses of patients with hypertension, the hypertension covariate was excluded from the propensity score model.
Presented patient counts and outcome event rates are based on the unweighted cohort.
Cox proportional hazards models were used to estimate the associations between carvedilol (versus metoprolol) initiation and 1-year all-cause mortality.
Fine and Gray proportional subdistribution hazards models were used to estimate the associations between carvedilol (versus metoprolol) initiation and 1-year cardiovascular mortality. Non-cardiovascular death was treated as a competing risk.
Abbreviations: CI, confidence interval; HR, hazard ratio; no., number; p-y, person-year; MI, myocardial infarction
In secondary analyses evaluating the associations between study beta-blockers and hospitalizations, individuals who initiated carvedilol (versus metoprolol) had similar rates of all-cause hospitalizations (2,383.8 versus 2,270.3 events/1,000 person-years; adjusted IRR, 1.00 [95% CI, 0.97–1.04]) and higher rates of cardiovascular hospitalizations (827.1 versus 726.5 events/1,000 person-years; adjusted IRR, 1.06 [95% CI, 1.01–1.12]) during the 1-year follow-up period.
Sensitivity analyses
Sensitivity analyses comparing carvedilol initiators to metoprolol tartrate and metoprolol succinate initiators (separately) generated results similar to primary analyses. Treatment with carvedilol (versus metoprolol) was associated greater 1-year all-cause and cardiovascular mortality, regardless of the comparator metoprolol formulation (Table S4).
In sensitivity analyses using an on-treatment analytic paradigm, the study cohort was followed for a total of 14,460 person-years (5,127 person-years for carvedilol-treated patients and 9,333 person-years for metoprolol-treated patients). During follow-up there were 2,941 all-cause deaths (1,117 in the carvedilol group and 1,824 in the metoprolol group) and 1,341 cardiovascular deaths (554 in the carvedilol group and 797 in the metoprolol group). A total of 11,110 individuals discontinued index beta-blocker therapy and 1,662 switched to a different beta-blocker during follow-up. The average duration of continuous index medication use was 195 days for both carvedilol initiators metoprolol initiators. Individuals who remained on carvedilol (versus metoprolol) treatment had nominally higher rates of all-cause mortality (217.9 versus 195.4 events/1,000 person-years; adjusted HR, 1.06 [95%, 0.98–1.14]) and had higher rates cardiovascular mortality (106.3 versus 85.4 events/1,000 person-years; adjusted HR, 1.15 [95% CI, 1.03–1.28]).
Sensitivity analyses assessing beta-blocker–mortality associations among individuals who did not experience a cardiovascular hospitalization in the last 30 days of the baseline period produced results analogous to primary study findings. Carvedilol (versus metoprolol) initiation was associated with higher 1-year all-cause and cardiovascular mortality in this patient subgroup (Table S5). In sensitivity analyses evaluating the study beta-blocker–tracer outcome association, carvedilol (versus metoprolol) initiation was not associated with the occurrence of hospitalized bowel obstruction (rate of 30.3 versus 28.7 events/1,000 person-years; adjusted HR, 1.02 [95% CI, 0.86–1.20]).
Post hoc analyses
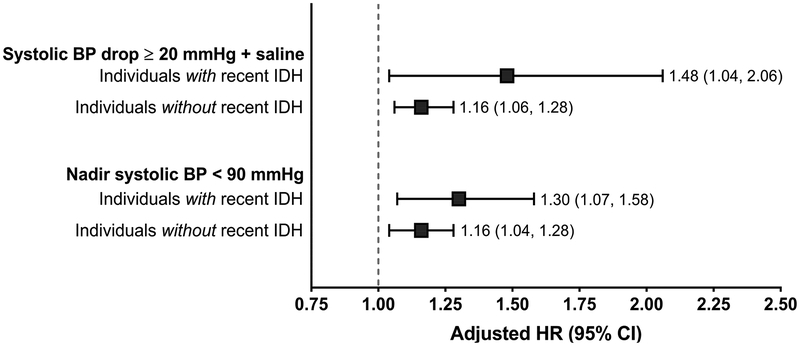
The rate of intradialytic hypotension (a systolic blood pressure drop ≥20 mmHg during hemodialysis plus intradialytic saline administration) during study follow-up was higher among carvedilol (versus metoprolol) initiators (57.5 versus 55.2 episodes/1,000 person-treatments; adjusted IRR, 1.10 [95% CI, 1.09–1.11]). Similar findings were observed when an episode of intradialytic hypotension was defined as an intradialytic nadir systolic blood pressure <90 mmHg (comparing carvedilol to metoprolol initiators: rate of 144.4 versus 136.5 episodes/1,000-person-treatments; adjusted IRR, 1.02 [95% CI, 1.01–1.03]). In additional post hoc analyses, all-cause and cardiovascular mortality rates were higher among individuals with vs without a recent history of frequent intradialytic hypotension (Figure 4, Table S6).
Figure 4. Association between carvedilol versus metoprolol initiation and 1-year cardiovascular mortality among individuals with and without a recent history of intradialytic hypotension: intent-to-treat analysis.
An intent-to-treat design was employed in all analyses. Fine and Gray proportional subdistribution hazards models were used to estimate the association between carvedilol (versus metoprolol) initiation and 1-year cardiovascular mortality. In these analyses, non-cardiovascular death was treated as a competing risk. Inverse probability of treatment weighting was used in adjusted analyses to control for all the baseline covariates listed in Table 1.
Abbreviations: CI, confidence interval; HR, hazard ratio; ref., referent
DISCUSSION
This observational study evaluated the comparative mortality risk of carvedilol and metoprolol initiation among individuals receiving maintenance hemodialysis. We found evidence that carvedilol (versus metoprolol) initiation was associated with greater 1-year all-cause and cardiovascular mortality. The associations were consistent within clinically relevant subgroups and robust across sensitivity analyses. We also found that carvedilol initiators experienced higher rates of intradialytic hypotension during follow-up compared to metoprolol initiators. In addition, the observed study beta-blocker–mortality associations were more pronounced among individuals with vs without a recent history of frequent intradialytic hypotension.
To date, there have been no randomized clinical trials comparing the efficacy and safety of individual beta-blockers in the dialysis population. Prior beta-blocker clinical trials were either placebo-controlled6,26 or compared beta-blockers to other antihypertensive medication classes (e.g. angiotensin-converting enzyme inhibitors).27 Existing observational investigations of beta-blockers have predominantly focused on comparing beta-blocker users to non-users,28–34 and only two observational studies have considered head-to-head beta-blocker comparisons. Weir et al. assessed the association between beta-blocker dialyzability and 180-day mortality in a cohort of 6,588 elderly, Canadian hemodialysis patients.5 Initiation of a highly versus a minimally dialyzable beta-blocker was associated with higher all-cause death. This study provided initial evidence that beta-blocker heterogeneity may differentially impact clinical outcomes in the hemodialysis population, but, carvedilol (a minimally dialyzable beta-blocker) and metoprolol succinate (a highly dialyzable beta-blocker) were not considered. In the U.S., carvedilol and metoprolol succinate account for 50% of all beta-blocker prescriptions.
In a second epidemiologic study, Shireman et al. evaluated the association between beta-blocker selectivity and mortality in a cohort of 4,398 incident U.S. hemodialysis and peritoneal dialysis patients with dual Medicare/Medicaid coverage and hypertension.35 Initiation of a cardioselective beta-blocker (atenolol, metoprolol) versus a non-selective beta-blocker (carvedilol, labetalol) was associated with greater survival. However, the relative contributions of carvedilol and metoprolol to the observed association are unclear, and this investigation relied on data from 2000–2005. In the last decade, carvedilol use has risen,4,36 rendering a contemporary analysis important. In fact, international guideline bodies have called for additional comparative effectiveness research on putative cardioprotective drugs such as beta-blockers in the hemodialysis population.37
To begin to address this evidence gap, we performed a head-to-head comparison of the two most commonly prescribed beta-blockers in the U.S., carvedilol and metoprolol. We found that carvedilol (versus metoprolol) initiation was associated with higher 1-year all-cause and cardiovascular mortality. Results were consistent among individuals with hypertension, atrial fibrillation, heart failure, and a recent myocardial infarction. Furthermore, the observed study beta-blocker–mortality association was robust across sensitivity analyses comparing carvedilol to immediate-release metoprolol tartrate and extended/controlled-release metoprolol succinate (separately). In post hoc analyses, we found that the association between carvedilol (versus metoprolol) initiation and mortality was more potent among individuals with a recent history of frequent intradialytic hypotension. In addition, the occurrence of intradialytic hypotension (defined two ways) was more common after carvedilol (versus metoprolol) initiation. Given that recurrent intradialytic hypotension is associated with increased morbidity and mortality in the hemodialysis population,25,38–40 the results from our post hoc analyses support the notion that hemodynamic instability may play a mechanistic role in the observed association between carvedilol (versus metoprolol) initiation and greater mortality.
Pharmacologic and kinetic differences between carvedilol and metoprolol may plausibly explain the observed differences in mortality and intradialytic hypotension. First, the extent to which a beta-blocker is removed from circulation by hemodialysis may impact intradialytic blood pressure. Carvedilol is minimally dialyzed, and metoprolol is highly dialyzed. As a result, carvedilol’s antihypertensive effects are likely maintained over the course of dialysis, whereas metoprolol’s antihypertensive effects may be diminished as serum drug concentrations fall during treatment. Second, carvedilol and metoprolol differ with respect to their beta-adrenergic receptor selectivity and vasodilatory capabilities. Carvedilol is a non-selective beta-blocker (a β1 and β2 adrenergic receptor antagonist) with additional alpha-blocking activity (an α1 adrenergic receptor antagonist). In contrast, metoprolol is a cardioselective beta-blocker with high β1 adrenergic receptor affinity. Both medications reduce heart rate and cardiac contractility, but due to its alpha-blocking effects, carvedilol is also a vasodilator. It is plausible that carvedilol-induced alpha-blockade may blunt compensatory sympathetic nervous system-mediated peripheral vasoconstriction during ultrafiltration, increasing the risk of intradialytic hemodynamic instability. These proposed clinical mechanisms likely act in concert in carvedilol-treated patients.
Ultimately, randomized controlled clinical trials are needed to definitively determine the relative safety and efficacy of carvedilol and metoprolol in the hemodialysis population. However, in the interim, our results suggest that the potential adverse hemodynamic effects of carvedilol (versus metoprolol) require consideration when prescribing beta-blockers to hemodialysis patients, particularly among individuals with a history of intradialytic hemodynamic instability. For example, it may be reasonable to: 1) consider metoprolol over carvedilol among individuals at higher risk for intradialytic hypotension; or 2) recommend that patients hold carvedilol doses prior to hemodialysis treatments to minimize potential intradialytic hypotensive effects. However, such decisions must be made carefully on an individual basis with consideration of comorbid cardiovascular conditions, historical blood pressure patterns, and concomitant antihypertensive medication use and dosing.
Our study has several strengths. First, we used a modern pharmacoepidemiologic study design to evaluate the comparative 1-year mortality risks associated with carvedilol and metoprolol treatment. To minimize the influence of bias due to confounding by indication or disease severity, we selected study medications with similar indications and therapeutic roles.41 Notably, the carvedilol and metoprolol initiators were highly comparable, and all baseline covariate imbalances between treatment groups were diminished after IPT weighting. Additionally, we chose to study the two most commonly prescribed beta-blockers to closely mirror a real-world clinical practice decision.41 Second, unlike previous claims-based studies, we utilized a linked data set with detailed clinical data that enabled us to account for many important biochemical indices and dialysis treatment parameters in our analyses. Finally, we performed multiple sensitivity analyses to test the robustness of our findings.
However, these results should be considered within the context of study limitations. Because our study was observational, there may be residual confounding. However, we controlled for variables including albumin, phosphorus, and a history of non-adherence to treatment as a way to minimize confounding from difficult-to-measure factors such as ambient health status. Reassuringly, carvedilol (versus metoprolol) initiation was not associated with the occurrence of the tracer outcome, hospitalized bowel obstruction. Second, while our linked data source was comprised of detailed administrative and clinical data, information on some potentially important factors, such as the timing of medication dosing, subspecialty of the index beta-blocker prescriber, and cardiac status (e.g. ejection fraction, left ventricular hypertrophy) were not available. In particular, it is possible that a clinician’s decision to prescribe carvedilol over metoprolol was influenced by left ventricular hypertrophy severity or other markers of cardiac function. As such, it is possible that residual confounding by indication (i.e. indication bias)41 may have influenced results. Third, comorbid condition designations were based upon International Classification of Diseases, 9th Revision, diagnostic codes. Administrative claims data are generated for reimbursement and billing purposes. These data may not always reflect clinical subtleties and may not include all patient characteristics, potentially affecting the accuracy of claims-identified comorbid conditions. For example, only a limited number of discharge diagnoses can be coded for each billable health care encounter, possibly reducing comorbidity ascertainment. In addition, comorbidities not requiring a healthcare encounter during the 180-day baseline period may have been missed. Reassuringly, our approach facilitated capture of the most severe conditions, and thus strongest potential confounders.15,42 Fourth, our study population was comprised of prevalent ESRD patients receiving in-center hemodialysis. Our results may not be generalizable to excluded populations such as incident hemodialysis, home hemodialysis or peritoneal dialysis patients. Understanding the relative risk-benefit profiles of carvedilol and metoprolol in these excluded patient populations is an area for future inquiry. Finally, our study evaluated a cohort of U.S. hemodialysis patients. Our results may not apply to other countries where national or regional prescription formularies limit metoprolol and/or carvedilol prescribing.
In conclusion, we observed that carvedilol (versus metoprolol) initiation was associated with higher 1-year all-cause and cardiovascular mortality in a cohort of prevalent U.S. hemodialysis patients. Data from our post hoc analyses suggest that one potential mechanism for the observed mortality associations may be an increased rate of intradialytic hypotension after carvedilol (versus metoprolol) initiation. Given the unique pharmacokinetic and hemodynamic considerations in the ESRD population, additional study of the efficacy and safety of beta-blockers, as well as other cardioprotective medications with antihypertensive properties is needed.
Supplementary Material
Figure S1. Propensity score distribution of patients treated with carvedilol and metoprolol.
Figure S2. The 1-year cumulative incidence of all-cause and CV mortality among carvedilol and metoprolol initiators: intent-to-treat analysis.
Table S1. Outcome definitions.
Table S2. Baseline covariate definitions.
Table S3. Association between carvedilol versus metoprolol initiation and 1-year mortality among clinically relevant subgroups: intent-to-treat analysis.
Table S4. Association between the initiation of carvedilol versus the initiation of the different metoprolol formulations and 1-year mortality: intent-to-treat analysis.
Table S5. Association between carvedilol versus metoprolol initiation and 1-year mortality among individuals who did not have a CV hospitalization during the last 30 days of the baseline period: intent-to-treat analysis.
Table S6. Association between carvedilol versus metoprolol initiation and 1-year mortality among individuals with and without a recent history of frequent IDH: intent-to-treat analysis.
ACKNOWLEDGMENTS
Authors’ Contributions: Research idea and study design: MMA, MAB and JEF; data acquisition: MMA, MAB and JEF; data analysis/interpretation: MMA, MAB, JPF, GH, JBL and JEF; statistical analysis: MMA; and supervision or mentorship: MAB, JEF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support: MMA was supported by grant F32 DK109561 and JEF by grant K23 DK109401, both awarded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The funders of this study had no role in: study design; collection, analysis, or interpretation of data; writing the manuscript; or the decision to submit the report for publication.
Financial Disclosure: MMA and JEF have received investigator-initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America. MAB has received research support from Amgen and AstraZeneca; he has served as a scientific advisor for Merck, Amgen, Genentech, and RxAnte; and he also owns equity in NoviSci, LLC, a data sciences company. JBL was formerly an employee of UNC were he received salary support from the Center for Pharmacoepidemiology of the University of North Carolina Department of Epidemiology (Center member companies included GlaxoSmithKline, Merck, and UCB Biosciences); he is currently an employee of RTI International, an independent research organization which does work for government and pharmaceutical companies. JEF has received speaking honoraria from Dialysis Clinic Incorporated, Renal Ventures, American Renal Associates, American Society of Nephrology, Baxter, National Kidney Foundation, and multiple universities. The other authors declare that they have no relevant financial interests.
Disclaimer: Some of the data reported here have been supplied by DaVita Clinical Research. DaVita Clinical Research had no role in the design or implementation of this study or the in the decision to publish. Additionally, some of the data reported here have been provided by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the United States government.
Peer Review: Received September 22, 2017. Evaluated by three external peer reviewers, with direct editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form February 4, 2018.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ. 2015;350:h1059. [DOI] [PubMed] [Google Scholar]
- 2.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296(11):1377–1384. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinidis I, Nadkarni GN, Yacoub R, et al. Representation of Patients With Kidney Disease in Trials of Cardiovascular Interventions: An Updated Systematic Review. JAMA Intern Med. 2016;176(1):121–124. [DOI] [PubMed] [Google Scholar]
- 4.St Peter WL, Sozio SM, Shafi T, et al. Patterns in blood pressure medication use in US incident dialysis patients over the first 6 months. BMC Nephrol. 2013;14:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir MA, Dixon SN, Fleet JL, et al. b-blocker dialyzability and mortality in older patients receiving hemodialysis. J Am Soc Nephrol. 2015;26(4):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MA, Pilmore HL, Ierino FL, et al. The beta-Blocker to Lower Cardiovascular Dialysis Events (BLOCADE) Feasibility Study: A Randomized Controlled Trial. Am J Kidney Dis. 2016;67(6):902–911. [DOI] [PubMed] [Google Scholar]
- 7.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. [DOI] [PubMed] [Google Scholar]
- 8.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3)(suppl 1):S1–S688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol. 2013;24(7):1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA. The comparative short-term effectiveness of iron dosing and formulations in US hemodialysis patients. Am J Med. 2013;126(6):541 e541–541 e514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf AA, Weinhandl ED, St Peter WL. Comparative effectiveness of calcium acetate and sevelamer on clinical outcomes in elderly hemodialysis patients enrolled in Medicare part D. Am J Kidney Dis. 2014;64(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinhandl ED, Nieman KM, Gilbertson DT, Collins AJ. Hospitalization in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. Am J Kidney Dis. 2015;65(1):98–108. [DOI] [PubMed] [Google Scholar]
- 14.Brookhart MA. Counterpoint: the treatment decision design. Am J Epidemiol. 2015;182(10):840–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbertson DT, Bradbury BD, Wetmore JB, et al. Controlling confounding of treatment effects in administrative data in the presence of time-varying baseline confounders. Pharmacoepidemiol Drug Saf. 2016;25(3):269–277. [DOI] [PubMed] [Google Scholar]
- 16.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38(6):1228–1234. [Google Scholar]
- 17.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 18.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6(5):604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briasoulis A, Palla M, Afonso L. Meta-analysis of the effects of carvedilol versus metoprolol on all-cause mortality and hospitalizations in patients with heart failure. Am J Cardiol. 2015;115(8):1111–1115. [DOI] [PubMed] [Google Scholar]
- 22.K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(4 Suppl 3):S1–153. [PubMed] [Google Scholar]
- 23.Kooman J, Basci A, Pizzarelli F, et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22 Suppl 2:ii22–44. [DOI] [PubMed] [Google Scholar]
- 24.Mactier R, Hoenich N, Breen C. UK Renal Association clinical practice guidelines: haemodialysis. http://www.renal.org/guidelines/modules/haemodialysis#sthash.eBdbSrRk.dpbs, 2009. [Accessed 23 Dec 2017]. [DOI] [PubMed] [Google Scholar]
- 25.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survival in dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014;29(3):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley RN, Herzog CA, Collins AJ, United States Renal Data S. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62(5):1784–1790. [DOI] [PubMed] [Google Scholar]
- 29.Griffith TF, Chua BS, Allen AS, Klassen PS, Reddan DN, Szczech LA. Characteristics of treated hypertension in incident hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. 2003;42(6):1260–1269. [DOI] [PubMed] [Google Scholar]
- 30.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. beta-Blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164(22):2465–2471. [DOI] [PubMed] [Google Scholar]
- 31.Ishani A, Herzog CA, Collins AJ, Foley RN. Cardiac medications and their association with cardiovascular events in incident dialysis patients: cause or effect? Kidney Int. 2004;65(3):1017–1025. [DOI] [PubMed] [Google Scholar]
- 32.Nakao K, Makino H, Morita S, et al. Beta-blocker prescription and outcomes in hemodialysis patients from the Japan Dialysis Outcomes and Practice Patterns Study. Nephron Clin Pract. 2009;113(3):c132–139. [DOI] [PubMed] [Google Scholar]
- 33.Tangri N, Shastri S, Tighiouart H, et al. beta-Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO Study. Am J Kidney Dis. 2011;58(6):939–945. [DOI] [PubMed] [Google Scholar]
- 34.Kitchlu A, Clemens K, Gomes T, et al. Beta-blockers and cardiovascular outcomes in dialysis patients: a cohort study in Ontario, Canada. Nephrol Dial Transplant. 2012;27(4):1591–1598. [DOI] [PubMed] [Google Scholar]
- 35.Shireman TI, Mahnken JD, Phadnis MA, Ellerbeck EF. Effectiveness comparison of cardio-selective to non-selective beta-blockers and their association with mortality and morbidity in end-stage renal disease: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetmore JB, Mahnken JD, Mukhopadhyay P, et al. Geographic variation in cardioprotective antihypertensive medication usage in dialysis patients. Am J Kidney Dis. 2011;58(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin NW, Kotanko P, Eckardt KU, et al. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010;77(4):273–284. [DOI] [PubMed] [Google Scholar]
- 38.Stefansson BV, Brunelli SM, Cabrera C, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9(12):2124–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou JA, Streja E, Nguyen DV, et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant. 2017,33(1):149–159.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang TI, Paik J, Greene T, et al. Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol. 2011;22(8):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.In: Velentgas P, Dreyer NA, Nourjah P, Smith SR, Torchia MM, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville (MD) 2013. [PubMed] [Google Scholar]
- 42.Goldstein BA, Pencina MJ, Montez-Rath ME, Winkelmayer WC. Predicting mortality over different time horizons: which data elements are needed? J Am Med Inform Assoc. 2017;24(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Propensity score distribution of patients treated with carvedilol and metoprolol.
Figure S2. The 1-year cumulative incidence of all-cause and CV mortality among carvedilol and metoprolol initiators: intent-to-treat analysis.
Table S1. Outcome definitions.
Table S2. Baseline covariate definitions.
Table S3. Association between carvedilol versus metoprolol initiation and 1-year mortality among clinically relevant subgroups: intent-to-treat analysis.
Table S4. Association between the initiation of carvedilol versus the initiation of the different metoprolol formulations and 1-year mortality: intent-to-treat analysis.
Table S5. Association between carvedilol versus metoprolol initiation and 1-year mortality among individuals who did not have a CV hospitalization during the last 30 days of the baseline period: intent-to-treat analysis.
Table S6. Association between carvedilol versus metoprolol initiation and 1-year mortality among individuals with and without a recent history of frequent IDH: intent-to-treat analysis.