Abstract
The use of targeted oral anticancer medications (OAMs) is becoming increasingly prevalent in cancer care. Approximately 25–30% of the oncology drug pipeline involves oral agents and there are now over 50 OAMs approved by the Food and Drug Administration. This change represents a major shift in management of patients with cancer from directly observed, intermittent intravenous therapy to self-administered, oral chronic therapy. The increased prevalence of OAMs raises the issue of adherence in oncology, including understanding the challenges of adherence to OAMs. This review focuses on studies of adherence for patients taking molecularly targeted OAMs for breast cancer, chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GIST), non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC). We then discuss barriers to adherence and studies performed to date testing interventions for improving adherence. Finally, we discuss future areas of investigation needed to define and improve adherence to OAMs in targeted therapy for cancer.
Introduction
Over the last decade, the rise in the use of targeted oral anticancer medications (OAMs) represents a major shift in management of patients with cancer from directly observed, intermittent intravenous therapy to self-administered, oral chronic therapy (Gebbia et al., 2012; Partridge et al., 2002; Ruddy et al., 2009). Through recent understanding of genetic, genomic, and molecular changes involved in tumor progression, many oral anticancer therapies have been developed to target abnormal proteins and signaling pathways specific to cancer cells. In 2008, it was estimated that 25–30% of the oncology drug pipeline involved oral agents, most of them targeted, with approximately 40% of all OAMs having been approved within the last seven years (Weingart et al., 2008). Table 1 lists recently approved targeted OAMs with many more in clinical development.
Table 1.
Selected FDA Approved Targeted Oral Anticancer Medications.
| Generic Name | Trade Name | Year Approved* | Target Gene or Receptor | Indication |
|---|---|---|---|---|
| Dasatinib | Sprycell | 2006 | BCR-ABL | Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) |
| Imatinib | Gleevec | 2001 | BCR-ABL | Ph+ CML Gastrointestinal stromal tumors (GIST) |
| Nilotinib | Tasigna | 2007 | BCR-ABL | Ph+ CML |
| Bosutinib | Bosulif | 2012 | BCR-ABL, Src | Ph+ CML |
| Ponatinib | Iclusig | 2012 | BCR-ABL | ALL and CML |
| Vemurafenib | Zelboraf | 2012 | BRAF V600E | Melanoma |
| Vismodegib | Erivedge | 2012 | SMO | Basal cell carcinoma |
| Ruxolitinib | Jakafi | 2011 | JAK1/2 | Myelofibrosis |
| Gefinitib | Iressa | 2003 | EGFR | Non-small cell lung cancer (NSCLC) |
| Erlotinib | Tarceva | 2004 | EGFR | NSCLC and pancreatic cancer |
| Crizotinib | Xalkori | 2011 | EML4-ALK | NSCLC |
| Abiraterone | Zytiga | 2011 | CYP17A1 | Prostate cancer |
| Enzalutamide | Xtandi | 2012 | AR | Prostate cancer |
| Regorafenib | Stivarga | 2012 | VEGFR2-TIE2 | Colon cancer |
| Lenalidomide | Revlimid | 2006 | Anti-tumor, immunomodulatory | Multiple myeloma |
| Lapatinib | Tykerb | 2007 | EGFR, HER2/neu | Breast cancer |
| Sunitinib | Sutent | 2006 | PDGFR, VEGF, cKIT, RET, CSF-1R, flt3 | GIST Renal cell carcinoma (RCC) |
| Sorafenib | Nexavar | 2006 | VEGFR, PDGFR, C-Raf, B-Raf, MAP Kinase, cKIT | RCC Hepatocellular carcinoma |
| Pazopanib | Votrient | 2009 | VEGF, c-kit, PDGFR | RCC Soft tissue sarcoma |
| Axitinib | Inlyta | 2012 | VEGFR1–3, PDGFR, cKIT | RCC |
| Everolimus | Afinitor | 2009 | mTOR | Breast, RCC, soft tissue sarcoma and renal angiomyolipoma |
Abbreviations: BCR-ABL, fusion of Abelson (Abl) tyrosine kinase gene at chromosome 9 and break point cluster (Bcr) gene at chromosome 22; EGFR, epidermal growth factor receptor; EML4-ALK, rearrangement of echinoderm microtubule-associated protein-like 4anaplastic lymphoma kinase; HER2/neu, one of four membrane proteins in EGFR family; PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor; RET, proto-oncogene, encodes receptor tyrosine kinase for the neurotrophic factor family; CSF-1R, colony stimulating factor 1; flt3, encodes receptor tyrosine kinase that regulates hematopoiesis; MAP Kinase, family of serine/threonine proteins responsible for regulating cellular activities, such as apoptosis; c-kit, tyrosine kinase stem cell factor receptor; SMO, Smoothened, a transmembrane protein involved in Hedgehog signal transduction; mTOR, mammalian target of rapamycin inhibitor; BRAF, gene encoding for B-Raf, member of Raf kinase family
year approved for initial indication.
Patients prefer oral medications over intravenous therapy. A study addressing self-reported patient preference for oral drugs versus intravenous palliative chemotherapy found that 92 of 102 assessable patients preferred oral chemotherapy, most importantly due to convenience and with the understanding that efficacy would not be sacrificed (Liu et al., 1997). Oncologists are also amenable to targeted OAMs, particularly in the palliative and adjuvant disease setting where quality of life is paramount (Benjamin et al., 2012). The rise in use of targeted OAMs has brought medication adherence to the forefront in oncology; for example, suboptimal adherence to targeted OAMs has already been shown to lead to worsened event free survival (EFS) in patients with chronic myelogenous leukemia (CML) (Ganesan et al., 2011). This review will summarize studies of adherence to targeted OAMs, barriers to adherence to targeted OAMs, and interventions to improve adherence in cancer patients.
Definition and Assessment of Adherence
Adherence, defined as the degree to which one conforms to recommendations about day-to-day treatment by the provider with respect to the timing, dosage, and frequency (Cramer et al., 2008), has long been an important issue for patients with other chronic diseases including diabetes (Skelly et al., 2009), hypertension (Bosworth et al., 2009), heart disease (Murphy et al., 2009), asthma (Horne, 2006), and HIV/AIDS (Gardner et al., 2010; Lima et al., 2009). Costs to the U.S. health-care system of nonadherence for patients with chronic disorders are estimated to be $300 billion/year (DiMatteo, 2004). Furthermore, multiple studies have shown that good adherence is associated with a reduction in healthcare costs (Sokol et al., 2005).
Techniques to quantify adherence are imperfect (Osterberg and Blaschke, 2005), and there is no consensus for a “gold standard” measurement of adherence (Table 2) (Font et al., 2012). Furthermore, no single standard definition of an acceptable adherence threshold exists in the literature, although a widely used one has been 80% (Banning, 2012; Spoelstra and Given, 2011). However, in an era of molecularly targeted drugs, 80% adherence may not be an appropriate benchmark because even small deviations from full (100%) adherence may result in resistance and treatment failure, as evidenced by studies of adherence in individuals with CML (Gater et al., 2012).
Table 2.
Measures of Adherence.
| Measure | Pros | Cons |
|---|---|---|
| Direct | ||
| Direct observation | Most accurate | Not feasible in real-world practice |
| Serum drug levels | Objective measure of recent exposure to drug | Can be manipulated; acceptable ranges often unknown; assays not widely available |
| Indirect | ||
| Pill counts | Inexpensive | Difficult in real-world practice; easy to manipulate; may overestimate adherence; demeaning |
| MEMS (microelectronic event monitoring system) | Accurate data on when one opens the bottle; may be combined with reminder systems | Not easily feasible in real-world practice; expensive |
| Refill records | Objective higher level data; good for research purposes | Report fill rate and not actual intake; impractical for daily use |
| Biomarkers | May be important intermediaries to outcomes (e.g., hypertension with TKI use) | Few developed and validated |
| Outcomes | Most important variable | Difficult to discern nuances of adherence outside of clear extremes |
| Indirect and Subjective | ||
| Self-report | Quick; can use past validated instruments; does not require clinician time | Subject to significant bias such as the Hawthorne effect and overestimates adherence |
| Assessment by others | Inexpensive; allows for a dialogue | Hawthorne effect; time consuming |
| Diaries | Inexpensive; actively involves the patient | Subject to manipulation; demeaning; time consuming |
Adherence to Targeted OAMs
In oncology, adherence to OAMs has been evaluated in a broad range of cancers (Mazzeo et al., 2011; Waterhouse et al., 1993) with adherence rates ranging from less than 20% to 100% (Partridge et al., 2003). The most robust data come from studies of women with breast cancer taking oral hormonal therapy (Banning, 2012) and patients with CML (Jabbour et al., 2012) taking imatinib. Seminal work in breast cancer (Fink et al., 2004; Hershman et al., 2010; Lash et al., 2006; Partridge et al., 2008; 2003; Waterhouse et al., 1993) has provided the basis for research examining adherence to targeted OAMs, particularly for “chronically critically ill” individuals with cancer (e.g., lung cancer, renal cell carcinoma). Research concerning adherence for women with breast cancer taking oral hormonal therapies has been well summarized; therefore, in this review, we focus on studies of adherence to molecularly targeted OAMs for persons with cancer (Table 3).
Table 3.
Salient Studies of Adherence to Targeted Oral Anticancer Medications.
| Cancer | # of Patients | Oral Therapy | Adherence Measure | Adherence Definition | Outcome | Study |
|---|---|---|---|---|---|---|
| CML | 267 | Imatinib | MPR | Continuous | 77.7% over 1 year; lower MPR associated with higher cost | Darkow et al., 2007 |
| CML | 169 | Imatinib | BAAS; PC | Number of positive answers out of 4 | 14% perfectly adherent; 32.7% with at least one positive answer over 90 days | Noens et al., 2009 |
| CML | 592 | Imatinib | MPR | Continuous | 79% mean MPR over 12 months with 41% MPR <85% | Wu et al., 2010 |
| CML | 516 | Imatinib | Patients’ clinic records | Interrupt >1 week | 29.6% nonadherence rate; better survival in those adherent (5 year EFS 76.7% vs. 59.8%) | Ganesan et al., 2011 |
| CML | 87 | Imatinib | MEMS x3 months | Continuous | Median adherence=97.6% (range 24–104%); 26% with <90%; 21% ≤85% →18 fold higher rate of losing CCyR | Marin et al., 2010 Ibrahim et al.,2011 |
| CML | 430 | Imatinib | MPR | Continuous | 60% adherent based on 85% threshold. | St. Charles et al., 2009 |
| CML | 328 550 | Nilotinib Dasatinib | MPR from two claims databases | Continuous | Dasatinib users (73.9%) were less adherent than nilotinib (80.0%) users. | Guerin et al., 2012 |
| CML | 53 197 | Nilotinib Dasatinib | MPR from research database | Continuous | Nilotinib users were almost 2× more likely to have poor adherence than dasatinib users. | Yood et al., 2012 |
| NSCLC | 65 | Erlotinib | Self-report, MEMS, plasma erlotinib levels | Continuous | Ongoing | Timmers et al., 2011 |
| NSCLC | 30 | Erlotinib | Self-report | Continuous | Individuals had mostly medium (n=11) to high (n=14) adherence rates. | Lucca et al., 2012 |
| NSCLC | 200 | Erlotinib | BAAS, VAS, PC, missed appointments | Continuous | Disease control was higher in the intervention cohort (63%) compared with control (44%). The number of adverse events and patient-reported adherence were highly correlated (r=0.105; p=0.0001). | Gebbia et al., 2013 |
| RCC | 1080 | Various | MPR from | Continuous | 81% had adherence rates ≥80% | Hess et al., 2011 |
| RCC | 49 | Various | MEMS | Continuous | Adherence=98.9% | Wolter et al., 2012 |
| GIST | 28 | Imatinib | BAAS | No positive answers out of 4 | 24–29% at 90 days | Mazzeo et al., 2011 |
| CML & GIST | ~4,000 | Imatinib | MPR | Continuous | MPR in GIST was 73%. | Tsang et al., 2006 |
Abbreviations: MPR, medication possession ratio; NA, not available; D/C rate, rate of complete medication discontinuation; PC, pill count; BAAS, Basel Assessment of Adherence Scale with Immunosuppressive Medications; MEMS, Medication Event Monitoring System; CCyR, complete cytogenetic response; VAS, Visual Analog Scale.
Breast cancer
Two targeted OAMs approved for women with breast cancer are lapatinib and everolimus. Lapatinib is a dual inhibitor of the epidermal growth factor receptor (EGFR) family and is approved for combination use in women with metastatic breast cancer (Opdam et al., 2012). The most common side effects of lapatinib include diarrhea, nausea, vomiting, palmar plantar erythrodesthesia, and rash. Everolimus is a mammalian target of the rapamycin (mTOR) inhibitor and its most common side effects are stomatitis, hypertension, edema, fatigue, and cytopenias (Vinayak and Carlson, 2013). Although no published studies of adherence to everolimus in breast cancer were found, two studies examined adherence to lapatinib. In a study of 69 women, adherence was assessed using self-report, medication diaries, and pharmacy controlled drug boxes (Addeo et al., 2011). Adherence was 82%, with a 65% rate of dosing violations (not further specified). In a second retrospective study using MarketScan® (a large database of pharmacy records for individuals with private insurance) data, refill records were used as a surrogate marker for adherence to lapatinib (Kartashov et al., 2012). Adherence measured by medication possession ratio (MPR), the proportion of days in the measured period covered by prescription claims, was 87% (n=666), with 22% of patients having an MPR <80%. Nonadherence was associated with concurrent IV chemotherapy use and more physician visits, with a trend toward higher cost.
Chronic myelogenous leukemia (CML)
Perhaps the best example of the profound shift to use of targeted OAMs is CML and imatinib mesylate (imatinib), a tyrosine kinase inhibitor (TKI) that blocks the adenosine triphosphate-binding site of the BCR-ABL tyrosine kinase. Imatinib has been shown to be effective in treating chronic and accelerated phases of CML as well as blast crisis (Jabbour and Kantarjian, 2012). Common side effects of imatinib include edema/fluid retention, fatigue, rash, nausea, anemia and leukopenia, muscle cramps, and transaminitis (Breccia et al., 2012).
In the last five years, many reports examining adherence to imatinib have been published with adherence rates ranging from 60% (St Charles et al., 2009) to 97% (Noens et al., 2009). Generally, adherence was determined by MPR, but thresholds for defining “adequate adherence” varied (Darkow et al., 2007). The most commonly used thresholds were 85% (0–100% scale) (St Charles et al., 2009; Wu et al., 2010) and 90% (Ibrahim et al., 2011; Marin et al., 2010). Predictors of adherence to imatinib in CML such as gender, age, number of concomitant medications, dose of imatinib, time since diagnosis, living alone, time on therapy, physician characteristics and interaction with the patient, patient knowledge and self-beliefs about their disease, prescription co-payment amount, and selfreported functional status and quality of life have been mixed between studies and well summarized previously (Gater et al., 2012).
Importantly, adherence to imatinib has been found to correlate to response to treatment. In a retrospective study in India of patients received imatinib free of charge, 29.6% were nonadherent (defined as not returning to the clinic for one week or more to receive drug) and had a decreased 5-year EFS (combination of cytogenetic and hematologic milestones, including death) compared to their adherent counterparts (p=0.011) (Ganesan et al., 2011). Univariate analysis demonstrated that prolonged symptom duration before diagnosis, treatment with hydroxyurea for more than one month prior to imatinib therapy, and nonadherence were associated with worse EFS, but only nonadherence was significant in the multivariate analysis (HR 1.6; p=0.048). Another study found a strong correlation between adherence measured by a medication event monitoring system (MEMS) (≤90% or >90%) and 6-year probability of a major molecular response (p=0.001) and complete molecular response (p=0.002) (Marin et al., 2010). Others have found similar results with Ibrahim et al. (2011) showing adherence rate and failure to achieve molecular response being the only predictors for loss of complete cytogenetic response and discontinuation of imatinib therapy in CML.
Dasatinib and nilotinib are second generation tyrosine kinase inhibitors that primarily target BCR-ABL. Nilotinib is approved for treatment of drug resistant CML and dasatinib is approved for treatment of CML after imatinib therapy and for individuals with Philadelphia chromosome-positive acute lymphocytic leukemia. Both dasatinib and nilotinib have side effect profiles similar to imatinib, with a few important rare exceptions (Wei et al., 2010). When examining real-world adherence to second-line therapies with dasatinib and nilotinib, one study found that patients taking nilotinib were two times more likely to be nonadherent than patients taking dasatinib 100 mg daily (Yood et al., 2012). However, a second study found a higher mean MPR for individuals taking nilotinib (adjusted difference=0.061; p=0.002) compared to dasatinib, regardless of dose (Guerin et al., 2012). Both studies measured adherence using an MPR calculated from large claims databases and it is possible that the data may not reflect actual consumption of medication, skipped doses, or division of doses (i.e., 100 mg in two 50 mg doses).
Gastrointestinal stromal tumors (GIST)
GISTs are mesenchymal neoplasms originating in the gastrointestinal tract that share molecular activation of either the KIT or PDGFRA proto-oncogenes. As a result, TKIs have been used in the metastatic and adjuvant settings, leading to improved progression free survival and overall survival. Imatinib is approved in the adjuvant and metastatic settings and sunitinib in the metastatic setting only. Duration of therapy is three years for individuals completing therapy after surgical resection and indefinite for individuals with metastatic disease (Dasanu, 2012).
Previous work has shown that for individuals with advanced GIST, imatinib trough plasma levels correlate with time to progression and objective response (Demetri et al., 2009; von Mehren and Widmer, 2011), supporting the importance of adherence. Tsang et al. (2006) found the overall MPR for patients with GIST was 73% and by 14 months of treatment, only 23% of CML and GIST patients were 100% persistent with their therapy. In the companion ADAGIO study (GIST), adherence was prospectively evaluated over 90 days in patients with GIST (N=28) taking imatinib (Mazzeo et al., 2011). There was a 29% nonadherent rate four weeks prior to baseline and 24% at follow-up. Further supporting the importance of persistence of therapy, Le Cesne et al. (2010) randomized 50 patients with advanced GIST after three years of no progression on imatinib therapy to either continue or interrupt treatment. The primary endpoint was progression free survival (PFS) and after a median follow-up of 35 months post-randomization, two-year PFS was 80% in the continual arm and 16% in the interruption arm (p<0.0001).
Several descriptive studies of patients with GIST have examined the role of toxicity on medication use and adherence. In one survey (N=173), decreasing toxicity from severe to moderate levels was most important to persons taking imatinib rather than completely eliminating all toxicity. Reducing heart failure from moderate to mild and diarrhea from severe to moderate had the largest effects on subjects’ evaluation of adherence (Hauber et al., 2011). An ethnographic study (N=50) revealed the most common strategies for remaining adherent included obtaining family support, setting reminders, taking medicine at routine times, and storing medicine in prominent places (Macdonald et al., 2012).
Non-small cell lung cancer (NSCLC)
Erlotinib and gefitinib (no longer approved for use in the U.S.) were developed to inhibit EGFR over-expression in individuals with NSCLC. Erlotinib is approved for second- and third-line treatment of advanced NSCLC and maintenance therapy for advanced stage NSCLC after initial treatment of chemotherapy. Erlotinib is also recommended for first-line therapy for individuals with EGFR mutations (Majem and Pallares, 2013). Common side effects include rash, diarrhea, fatigue, and anorexia (Hotta and Kiura, 2011).
Three studies examined adherence to erlotinib for patients with NSCLC and two of those studies examined interventions to improve adherence to erlotinib. Gebbia et al. (2013) evaluated the impact of a treatment-monitoring intervention on adherence for patients with advanced NSCLC receiving erlotinib as second-line therapy in two cohorts: 1) a retrospective non-interventional phase monitoring 50 participants without a treatment management strategy; and, 2) a prospective interventional phase following 150 participants who received a treatment-management program, including identification of a caregiver, patient/caregiver education and training about treatment and side effects of therapy, a calendar for follow-up visits, and a dedicated facsimile phone line to receive instructions or use of a fast-track visit system. Adherence was measured using multiple methods and generally patient self-reported adherence was higher than adherence measured by pill counts. Disease control rate (complete response plus partial response plus stable disease) was 44% in the first cohort and 63% in the second cohort (p=0.0368). Also, a significant correlation was found between the number of adverse events and adherence (r=0.176, p=0.035).
Lucca et al. (2012) tested an educational intervention to enhance knowledge and adherence to erlotinib while monitoring for side effects in 30 patients. Adherence behaviors were measured with the 8-item Morisky Medication Adherence Scale (MMAS-8); however, it is unclear if the tool was adapted for patients with cancer. MMAS-8 adherence scores were medium to high, and the mean number of erlotinib adverse events was 2.48 per patient; 22% reported four or more side effects. An ongoing prospective observational cohort study (Timmers et al., 2011) is examining adherence over 16 weeks for patients with NSCLC. Adherence is measured using MEMS, several questionnaires, and plasma levels of erlotinib. Findings from this study will provide information about adherence and short-term persistence to erlotinib.
Renal cell carcinoma (RCC)
For persons with metastatic RCC, four oral VEGF inhibitors (sorafenib, sunitinib, pazopanib, axitinib) and one oral mTOR inhibitor (everolimus) are available for use with more in clinical trials. Common side effects include hypertension and hand-foot syndrome (Mendez-Vidal et al., 2012). Complete responses, although rare, have been noted and some patients are now living for over three years with sequential treatments (Albiges et al., 2012; Posadas and Figlin, 2012; Sonpavde et al., 2012). At the same time, cost of these medications for patients and the medical community can be high, totaling more than tens of thousands of dollars per year per patient (Shih et al., 2011). Furthermore, ongoing trials are examining the role of targeted OAMs as adjuvant treatment for non-metastatic RCC, possibly leading to significant expansion of the use of these agents in the future (ClinicalTrials.gov, NCT01235962).
Few studies have investigated adherence for patients with metastatic RCC. Hess et al. (2011) examined adherence to sorafenib, sunitinib, and everolimus and found 81% had adherence rates of 80% or higher. Limitations of this study involve the inclusion of infused agents such as temsirolimus, bevacizumab, and interferon; use of medication fill rates via claims records rather than actual patient reported intake; and, the majority of patients had commercial healthcare insurance. At the 2012 ASCO Annual Meeting, Wolter et al. (2012) presented preliminary data of a prospective observational study measuring adherence using MEMS technology. Adherence was 98.9% and while the described adherence rate may be high, several significant limitations of the above study are worth noting, including a small sample size, free medications supplied to patients throughout the study, a European only cohort, and limited qualitative information. With a presumed transition to perceiving RCC as a chronic disease (Escudier, 2012), it becomes vitally important to identify at-risk populations and factors leading to nonadherence so targeted interventions can be developed.
Barriers to Adherence
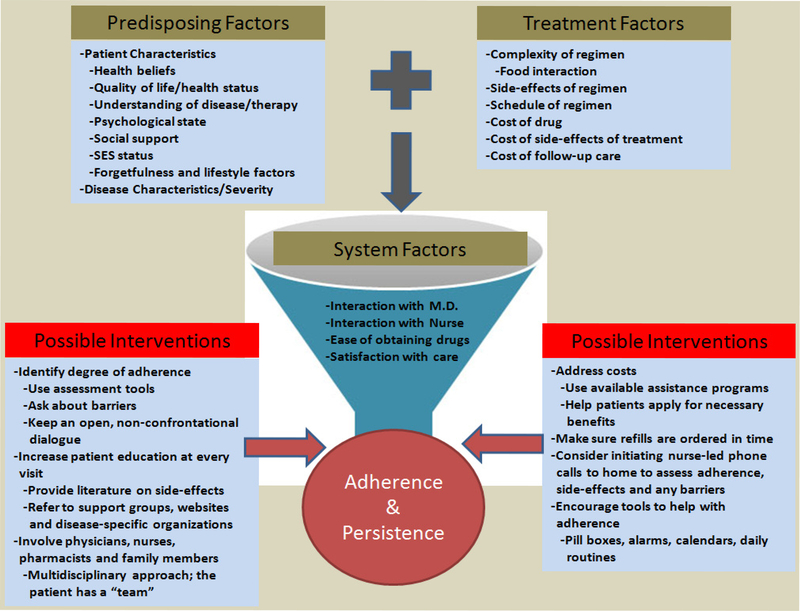
Potential barriers to adherence to OAMs in general have been identified and are summarized in Figure 1 as well as in previous reviews (Gater et al., 2012; Ruddy et al., 2009). Barriers to adherence specifically for targeted OAMs are still being elucidated, but in particular may include cost, side effects, and timing with food.
Figure 1.
Schematic of factors and barriers involved in adherence to targeted OAMs and possible ways to improve adherence.
Patients with advanced cancer are living longer and thus the expense of long-term targeted OAMs may lead to a substantial burden, in particular for older adults. Targeted OAMs routinely cost thousands of dollars per month with variable levels of co-payments and premiums incurred by patients (Shih et al., 2011; Weingart et al., 2008; Winkeljohn, 2007). Findings from a grounded theory study (n=13) examining the process of medication-taking for individuals with NSCLC taking erlotinib suggest that increased cost can lead to premature therapy discontinuation as nearly all participants referenced the high cost of erlotinib (Wickersham et al., 2012). Examining the association of OAMs’ cost and adherence rates in Canada, Ebrahim et al. (2012) surveyed 453 patients and found self-reported adherence to be 80% with 51% of patients having costs of ≥$100/month.
The interaction of food with targeted OAMs is important. Most OAMs are labeled to be taken without food because they often have large positive food effects with much greater bioavailability when taken with food (Szmulewitz and Ratain, 2013). This can lead to complex dosing schedules (pill needs to be taken some hours before or after a meal) which are difficult to sustain over a long period of time. A prospective study of 77 patients taking OAMs for various malignancies showed that 43% of those taking an OAM with a significant food-drug effect did not know last time they ate before taking their OAM, 23% did not know that their OAM had a food-drug effect, 21% intentionally skipped or cut back on their OAM with 38% of those not informing their physicians and 20% had some difficulty understanding the directions on the bottle (Muluneh et al., 2012).
Finally, side effects are also common with targeted OAMs and vary depending on the drug. In the same grounded theory study above, all participants referenced side effects with the most common being rash and diarrhea; in one case, these side effects were referred to as “social inhibitors” (Wickersham et al., 2012). Another qualitative study examined reasons for nonadherence for individuals with CML and found both intentional (e.g., side effects) and unintentional (e.g., forgetfulness) reasons for nonadherence (Eliasson et al., 2011). In general, a conceptual model of adherence developed in the setting of tyrosine kinase inhibitor use in persons with CML involves an interplay of predisposing factors (patient, disease, treatment, and physician characteristics), patient interactions with their physician, and patients’ knowledge and beliefs, which can be expanded further to other targeted OAMs (Gater et al., 2012).
Interventions to Improve Medication Adherence in Persons with Cancer
Several studies examined interventions to improve adherence to OAMs with others underway. Spoelstra et al. (2013) developed a nursing intervention to improve adherence using a Symptom Management Toolkit®, based on a modified health belief model approach, and an automated voice response (AVR) reminder system. Participants were randomized to one of three groups: (AVR) system alone (n=40), AVR with strategies to manage symptoms and adherence (n=40), or AVR with strategies to manage adherence (n=39). Adherence was measured using medical record audit, patient self-report, and pharmacy report. Participants had primarily breast, colon, or lung cancers, received non-hormonal agents for cancer treatment, and adherence was defined as 80–100% over the past seven days. Findings showed 42% were nonadherent, with missed doses increasing with regimen complexity. Symptom severity declined over time in all groups and no difference was found in adherence rates; higher adherence was related with lower symptom severity across groups.
Another study reported preliminary results of an OAM management clinic for patients with breast, colon, rectal, and lung cancer (Wong et al., 2012). Thirty patients enrolled into the clinic by their oncologist were retrospectively analyzed for variables such as depression, adherence, and persistence to cancer treatment. The most common drug prescribed was capecitabine and patients on average had 12.7 concurrent medications. Interventions to improve adverse drug events, nonadherence, drug interactions, and medications errors decreased over time with complete resolution or improved response seen in 67% of patients.
An Irish study of 101 patients examined perceptions of education and safety of OAMs (Graham et al., 2012). Fifteen percent of patients took targeted OAMs and the rest received conventional chemotherapy. When starting OAMs, 17% did not understand the medication; this was improved by physician (p=0.03) or hospital-based nurse (p=0.04) and provision of information booklets (p=0.04). Patients were unaware of drug-drug interactions in 30% of cases and 20% were not aware of any safety issues. Patients who had been given information leaflets were significantly more aware of safety including careful handling (p<0.001), storage conditions (p=0.02), and safe disposal (p<0.001). Patients attending nurse-led oral chemotherapy clinics were significantly more aware of safety issues (p=0.04) and had improved adherence.
Finally, Sommers et al. (2012) conducted a feasibility study of a telephone education guide to improve adherence in 30 patients with gastrointestinal cancer. Participants received oral chemotherapy regimens, including therapy with targeted OAMs. The intervention included physician and nurse-delivered education in the clinic followed by telephone support from clinic nurses. Adherence was measured using the MMAS-8 adapted for oncology and a medication diary. Findings showed that the adapted MMAS-8 was a feasible measure of adherence with high self-reported rates (mean 7.89 on 0–8 scale) consistent with medication diaries. Figure 1 summarizes some other possible interventions for patients on targeted OAMs.
Conclusion and Future Directions
Patient adherence to medication is essential to optimize clinical outcomes, minimize toxicity, decrease bias in clinical trials, and reduce healthcare costs. The field of oncology is rapidly evolving, with oral anticancer medications becoming a common treatment modality. Given the often narrow therapeutic margins of some targeted OAMs, their enormous costs, and significant side effects, it is critical to understand barriers to adherence for individuals taking these drugs and ways to maximize adherence. To date, studies examining adherence to OAMs have provided valuable information, but most of the work has been conducted in patients with CML. Future research will need to expand to include individuals with other cancers such as NSCLC, RCC, prostate cancer, GIST, breast cancer, melanoma, and others for which oral drugs are being developed. Future directions may include development of oncology-specific adherence tools, defining an optimal threshold for adherence for persons with cancer taking targeted OAMs, and development of interventions for improving adherence and the implementation of systematic programs for all patients, including the rapidly growing older adult population.
Acknowledgments
Dr. Geynisman gratefully acknowledges funding from the Ruth F. Kirschstein National Research Service Award (NIH T32 CA 9566–24) as well as helpful input from Drs. Daugherty, Hlubocky, and Stadler from the University of Chicago. Dr. Wickersham gratefully acknowledges Susan G. Dorsey, Ph.D., R.N., F.A.A.N., and her Dissertation Committee: Judith A. Erlen, Ph.D., R.N., F.A.A.N., Mary Beth Happ, Ph.D., R.N., F.A.A.N., Catherine M. Bender, Ph.D., R.N., F.A.A.N., Sandra J. Engberg, Ph.D., R.N., C.R.N.P., F.A.A.N., and Ahmad Tarhini, M.D., Ph.D., M.Sc. Funding for Dr. Wickersham was provided through the National Institute of Nursing Research (F31NR011261, PI: Wickersham; T32 NR008857, PI: Erlen; NR012686, PI: Dorsey); Sigma Theta Tau International, Eta Chapter Research Award; and the American Cancer Society (DSCN-11–193-01).
Footnotes
Disclosure
The authors report no conflicts of interest.
Contributor Information
DANIEL M. GEYNISMAN, Section of Hematology/Oncology, Department of Medicine, University of Chicago Medical Center, 5841 S. Maryland Ave., Chicago, Illinois 60637, USA.
KAREN E. WICKERSHAM, School of Nursing, University of Maryland, Baltimore, 655 West Lombard St., Baltimore, Maryland 21201, USA.
References
- Addeo R, Vincenzi B, Riccardi F, Febbraro A, Maiorino L, Incoronato P, Mabilia R, Bianco M, Russo E, Pisano A, DP S. Multicenter observational study on adherence and acceptance of lapatinib treatment in patients with HER2+ metastatic breast cancer. J Clin Oncol 29(Suppl):abstr #e11102, 2011. [Google Scholar]
- Albiges L, Oudard S, Negrier S, Caty A, Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, Laguerre B, Legouffe E, Kohser F, Dietrich PY, Theodore CA, Escudier B. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol 30(5):482–487, 2012. [DOI] [PubMed] [Google Scholar]
- Banning M Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care (Engl) 21(1):10–19, 2012. [DOI] [PubMed] [Google Scholar]
- Benjamin L, Cotte FE, Philippe C, Mercier F, Bachelot T, Vidal-Trecan G. Physicians’ preferences for prescribing oral and intravenous anticancer drugs: a Discrete Choice Experiment. Eur J Cancer 48(6):912–920, 2012. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, Dolor RJ, Oddone EZ. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 151(10):687–695, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breccia M, Tiribelli M, Alimena G. Tyrosine kinase inhibitors for elderly chronic myeloid leukemia patients: a systematic review of efficacy and safety data. Crit Rev Oncol Hematol 84(1):93–100, 2012. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health 11(1):44–47, 2008. [DOI] [PubMed] [Google Scholar]
- Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, Hatfield A, Cortes J. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. PharmacoEconomics 25(6):481–496, 2007. [DOI] [PubMed] [Google Scholar]
- Dasanu CA. Length of adjuvant imatinib therapy in GIST: weighing benefits, side effects and costs. J Oncol Pharm Pract 18(3):379–380, 2012. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, Joensuu H, Von Mehren M. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol 27(19):3141–3147, 2009. [DOI] [PubMed] [Google Scholar]
- Dimatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 42(3):200–209, 2004. [DOI] [PubMed] [Google Scholar]
- Ebrahim J, Han D, Hogeveen S, Trinkaus M, Fadhel E, Hon H, Kassam Z, Liu G, Simmons CE. Does drug cost drive drug adherence? The designer drug phenomenon. J Clin Oncol 30(Suppl):abstr #6084, 2012. [Google Scholar]
- Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res 35(5):626–630, 2011. [DOI] [PubMed] [Google Scholar]
- Escudier B Metastatic RCC: moving towards a chronic disease. Oncology (Williston Park) 26(3):304–306, 2012. [PubMed] [Google Scholar]
- Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol 22(16):3309–3315, 2004. [DOI] [PubMed] [Google Scholar]
- Font R, Espinas JA, Gil-Gil M, Barnadas A, Ojeda B, Tusquets I, Segui MA, Margeli M, Arcusa A, Prat A, Garcia M, Borras JM. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. Br J Cancer 107(8):1249–1256, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, Nandennavar M. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol 86(6):471–474, 2011. [DOI] [PubMed] [Google Scholar]
- Gardner EM, Hullsiek KH, Telzak EE, Sharma S, Peng G, Burman WJ, Macarthur RD, Chesney M, Friedland G, Mannheimer SB. Antiretroviral medication adherence and class- specific resistance in a large prospective clinical trial. AIDS 24(3):395–403, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater A, Heron L, Abetz-Webb L, Coombs J, Simmons J, Guilhot F, Rea D. Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leuk Res 36(7):817–825, 2012. [DOI] [PubMed] [Google Scholar]
- Gebbia V, Bellavia G, Ferrau F, Valerio MR. Adherence, compliance and persistence to oral antineoplastic therapy: a review focused on chemotherapeutic and biologic agents. Expert Opin Drug Saf 11(Suppl 1):S49–S59, 2012. [DOI] [PubMed] [Google Scholar]
- Gebbia V, Bellavia M, Banna GL, Russo P, Ferrau F, Tralongo P, Borsellino N. Treatment monitoring program for implementation of adherence to second-line erlotinib for advanced non-small-cell lung cancer. Clin Lung Cancer, epub ahead of print, January 9, 2013. [DOI] [PubMed] [Google Scholar]
- Graham DM, Bambury RM, Ismail JRM, O’Keefe M, Drake C, O’Shea A, Moylan EJ, Power DG, O’Reilly S. Oral anticancer therapy: Does the patient understand? J Clin Oncol 30(Suppl):abstr #e16506, 2012. [Google Scholar]
- Guerin A, Chen L, Wu EQ, Ponce De Leon D, Griffin JD. A retrospective analysis of therapy adherence in imatinib resistant or intolerant patients with chronic myeloid leukemia receiving nilotinib or dasatinib in a real-world setting. Curr Med Res Opin 28(7):1155–1162, 2012. [DOI] [PubMed] [Google Scholar]
- Hauber AB, Gonzalez JM, Coombs J, Sirulnik A, Palacios D, Scherzer N. Patient preferences for reducing toxicities of treatments for gastrointestinal stromal tumor (GIST). Patient Prefer Adherence 5:307–314, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess GP, Chi-Chang C, Hill JW, Liu Z, Gesme DH, Agarwala SS. Metastatic renal cell carcinoma: patient characteristics, treatment patterns, and schedule compliance in clinical practice. Kidney Cancer J 9(3):84–89, 2011. [Google Scholar]
- Horne R Compliance, adherence, and concordance: implications for asthma treatment. Chest 130(1 Suppl):65S–72S, 2006. [DOI] [PubMed] [Google Scholar]
- Hotta K, Kiura K. Safety profiles of erlotinib therapy in patients with advanced non-small-cell lung cancer. Expert Rev Anticancer Ther 11(7):991–997, 2011. [DOI] [PubMed] [Google Scholar]
- Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, Mahon FX, Kozlowski K, Paliompeis C, Foroni L, Khorashad JS, Bazeos A, Molimard M, Reid A, Rezvani K, Gerrard G, Goldman J, Marin D. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 117(14):3733–3736, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol 87(11):1037–1045, 2012. [DOI] [PubMed] [Google Scholar]
- Jabbour EJ, Kantarjian H, Eliasson L, Megan Cornelison A, Marin D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol 87(7):687–691, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartashov A, Delea TE, Sharma PP. Retrospective study of predictors and consequences of nonadherence with lapatinib (LAP) in women with metastatic breast cancer (MBC) who were previously treated with trastuzumab. J Clin Oncol 30(Suppl):abstr #e11067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99(2):215–220, 2006. [DOI] [PubMed] [Google Scholar]
- Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Cioffi A, Emile JF, Chabaud S, Perol D, Blay JY, French Sarcoma G. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol 11(10):942–949, 2010. [DOI] [PubMed] [Google Scholar]
- Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, Yip B, Montaner JS. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr 50(5):529–536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15(1):110–115, 1997. [DOI] [PubMed] [Google Scholar]
- Lucca JV, Hooper CL, Boucher J, Pedulla LV, Berry DL, Marcoux JP. A pilot study of direct care nurse (DCN) education to improve adherence and knowledge of erlotinib in patients with non-small cell lung cancer. J Clin Oncol 30(Suppl):abstr #e19637, 2012. [Google Scholar]
- Macdonald N, Shapiro A, Bender C, Paolantonio M, Coombs J. Experiences and perspectives on the GIST patient journey. Patient Prefer Adherence 6:253–262, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majem M, Pallares C. An update on molecularly targeted therapies in second- and third-line treatment in non-small cell lung cancer: focus on EGFR inhibitors and anti-angiogenic agents. Clinical Transl Oncol 15(5):343–357, 2013. [DOI] [PubMed] [Google Scholar]
- Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K, Paliompeis C, Latham V, Foroni L, Molimard M, Reid A, Rezvani K, De Lavallade H, Guallar C, Goldman J, Khorashad JS. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 28(14):2381–2388, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo F, Duck L, Joosens E, Dirix L, Focan C, Forget F, De Geest S, Muermans K, Ma VaNL, Macdonald K, Abraham I, De Greve J. Nonadherence to imatinib treatment in patients with gastrointestinal stromal tumors: the ADAGIO study. Anticancer Res 31(4):1407–1409, 2011. [PubMed] [Google Scholar]
- Mendez-Vidal MJ, Martinez Ortega E, Montesa Pino A, Perez Valderrama B, Viciana R. Management of adverse events of targeted therapies in normal and special patients with metastatic renal cell carcinoma. Cancer Metastasis Rev 31(Suppl 1):S19–S27, 2012. [DOI] [PubMed] [Google Scholar]
- Muluneh B, Alexander M, Deal AM, Deal M, Markey J, Neal J, Bernard SA, Valgus V, Dressler LG. Prospective evaluation of perceived barriers to medication adherence by patients on oral antineo-plastics. J Clin Oncol 30(Suppl):abstr #6042, 2012. [Google Scholar]
- Murphy AW, Cupples ME, Smith SM, Byrne M, Byrne MC, Newell J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ 339:b4220, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noens L, Van Lierde MA, De Bock R, Verhoef G, Zachee P, Berneman Z, Martiat P, Mineur P, Van Eygen K, Macdonald K, De Geest S, Albrecht T, Abraham I. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood 113(22):5401–5411, 2009. [DOI] [PubMed] [Google Scholar]
- Opdam FL, Guchelaar HJ, Beijnen JH, Schellens JH. Lapatinib for advanced or metastatic breast cancer. Oncologist 17(4):536–542, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 353(5):487–497, 2005. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Avorn J, Wang PS, Winer EP. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94(9):652–661, 2002. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Lafountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26(4):556–562, 2008. [DOI] [PubMed] [Google Scholar]
- Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606, 2003. [DOI] [PubMed] [Google Scholar]
- Posadas EM, Figlin RA. Systemic therapy in renal cell carcinoma: advancing paradigms. Oncology (Williston Park) 26(3):290–301, 2012. [PubMed] [Google Scholar]
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59(1):56–66, 2009. [DOI] [PubMed] [Google Scholar]
- Shih YC, Chien CR, Xu Y, Pan IW, Smith GL, Buchholz TA. Economic burden of renal cell carcinoma: Part I–an updated review. PharmacoEconomics 29(4):315–329, 2011. [DOI] [PubMed] [Google Scholar]
- Skelly AH, Carlson J, Leeman J, Soward A, Burns D. Controlled trial of nursing interventions to improve health outcomes of older African American women with type 2 diabetes. Nurs Res 58(6):410–418, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol MC, Mcguigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 43(6):521–530, 2005. [DOI] [PubMed] [Google Scholar]
- Sommers RM, Miller K, Berry DL. Feasibility pilot on medication adherence and knowledge in ambulatory patients with gastrointestinal cancer. Oncol Nurs Forum 39(4):E373–E379, 2012. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Choueiri TK, Escudier B, Ficarra V, Hutson TE, Mulders PF, Patard JJ, Rini BI, Staehler M, Sternberg CN, Stief CG. Sequencing of agents for metastatic renal cell carcinoma: can we customize therapy? Eur Urol 61(2):307–316, 2012. [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given BA, Given CW, Grant M, Sikorskii A, You M, Decker V. An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nurs 36(1):18–28, 2013. [DOI] [PubMed] [Google Scholar]
- Spoelstra SL, Given CW. Assessment and measurement of adherence to oral antineoplastic agents. Semin Oncol Nurs 27(2):116–132, 2011. [DOI] [PubMed] [Google Scholar]
- St Charles M, Bollu VK, Hornyak E, Coombs J, Blanchette CM, Deangelo DJ. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. ASH Annual Meeting Abstracts 114(22):2209, 2009. [Google Scholar]
- Szmulewitz RZ, Ratain MJ. Playing Russian roulette with tyrosine kinase inhibitors. Clin Pharmacol Ther 93(3):242–244, 2013. [DOI] [PubMed] [Google Scholar]
- Timmers L, Boons CC, Mangnus D, Moes JE, Swart EL, Boven E, Smit EF, Hugtenburg JG. The use of erlotinib in daily practice: a study on adherence and patients’ experiences. BMC Cancer 11:284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J, Rudychev I, Pescatore SL. Prescription compliance and persistency in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM). J Clin Oncol 24(18S):6119, 2006. [Google Scholar]
- Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology (Williston Park) 27(1):38–44, 46, 48 passim, 2013. [PubMed] [Google Scholar]
- Von Mehren M, Widmer N. Correlations between imatinib pharmacokinetics, pharmacodynamics, adherence, and clinical response in advanced metastatic gastrointestinal stromal tumor (GIST): an emerging role for drug blood level testing? Cancer Treat Rev 37(4):291–299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol 11(6):1189–1197, 1993. [DOI] [PubMed] [Google Scholar]
- Wei G, Rafiyath S, Liu D. First-line treatment for chronic myeloid leukemia: dasatinib, nilotinib, or imatinib. J Hematol Oncol 3:47, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart SN, Brown E, Bach PB, Eng K, Johnson SA, Kuzel TM, Langbaum TS, Leedy RD, Muller RJ, Newcomer LN, O’brien S, Reinke D, Rubino M, Saltz L, Walters RS. NCCN Task Force Report: Oral chemotherapy. J Natl Compr Canc Netw 6(Suppl 3):S1–S14, 2008. [PubMed] [Google Scholar]
- Wickersham K, Happ MB, Bender CM, Engberg SJ, Tarhini A, Erlen JA. Oncology Nursing Society’s Connections: Advancing care through science poster abstracts (Abstract 1407539). Oncology Nursing Forum 39(6):E511–E547, 2012. [Google Scholar]
- Winkeljohn DL. Oral chemotherapy medications: the need for a nurse’s touch. Clin J Oncol Nurs 11(6):793–796, 2007. [DOI] [PubMed] [Google Scholar]
- Wolter P, Hendrickx T, Renard V, Mebis J, Debruyne PR, Wynendaele W, Schallier DCC, Vermeij J, Nielander A, Machiels JPH, Rottey S, Delande S, Goeminne JC, Schoffski P, Coster SD, Lacour VVF. Adherence to oral anticancer drugs (OAD) in patients (pts) with metastatic renal cancer (mRCC): First results of the prospective observational multicenter IPSOC study (Investigating Patient Satisfaction with Oral Anti-cancer Treatment. J Clin Oncol 30(Suppl):abstr #4622, 2012. [Google Scholar]
- Wong SF, Nguyen CP, Bounthavong M, Bechtoldt K, Hernandez E. Outcome assessment of an oral chemotherapy management clinic: A preliminary report. J Clin Oncol 30(Suppl 34):abstr #105, 2012. [Google Scholar]
- Wu EQ, Johnson S, Beaulieu N, Arana M, Bollu V, Guo A, Coombs J, Feng W, Cortes J. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin 26(1):61–69, 2010. [DOI] [PubMed] [Google Scholar]
- Yood MU, Oliveria SA, Cziraky M, Hirji I, Hamdan M, Davis C. Adherence to treatment with second-line therapies, dasatinib and nilotinib, in patients with chronic myeloid leukemia. Curr Med Res Opin 28(2):213–219, 2012. [DOI] [PubMed] [Google Scholar]