Abstract
Background
Studies have suggested that increasing whole body blood flow and oxygen delivery around the time of surgery reduces mortality, morbidity and the expense of major operations.
Objectives
To describe the effects of increasing perioperative blood flow using fluids with or without inotropes or vasoactive drugs. Outcomes were mortality, morbidity, resource utilization and health status.
Search methods
We searched CENTRAL (The Cochrane Library 2012, Issue 1), MEDLINE (1966 to March 2012) and EMBASE (1982 to March 2012). We manually searched the proceedings of major conferences and personal reference databases up to December 2011. We contacted experts in the field and pharmaceutical companies for published and unpublished data.
Selection criteria
We included randomized controlled trials with or without blinding. We included studies involving adult patients (aged 16 years or older) undergoing surgery (patients having a procedure in an operating room). The intervention met the following criteria. 'Perioperative' was defined as starting up to 24 hours before surgery and stopping up to six hours after surgery. 'Targeted to increase global blood flow' was defined by explicit measured goals that were greater than in controls, specifically one or more of cardiac index, oxygen delivery, oxygen consumption, stroke volume (and the respective derived indices), mixed venous oxygen saturation (SVO2), oxygen extraction ratio (02ER) or lactate.
Data collection and analysis
Two authors independently extracted the data. We contacted study authors for additional data. We used Review Manager software.
Main results
We included 31 studies of 5292 participants. There was no difference in mortality: 282/2615 (10.8%) died in the control group and 238/2677 (8.9%) in the treatment group, RR of 0.89 (95% CI 0.76 to 1.05, P = 0.18). However, the results were sensitive to analytical methods and the intervention was better than control when inverse variance or Mantel–Haenszel random‐effects models were used, RR of 0.72 (95% CI 0.55 to 0.95, P = 0.02). The results were also sensitive to withdrawal of studies with methodological limitations. The rates of three morbidities were reduced by increasing global blood flow: renal failure, RR of 0.71 (95% CI 0.57 to 0.90); respiratory failure, RR of 0.51 (95% CI 0.28 to 0.93); and wound infections, RR of 0.65 (95% CI 0.51 to 0.84). There were no differences in the rates of nine other morbidities: arrhythmia, pneumonia, sepsis, abdominal infection, urinary tract infection, myocardial infarction, congestive cardiac failure or pulmonary oedema, or venous thrombosis. The number of patients with complications was reduced by the intervention, RR of 0.68 (95% CI 0.58 to 0.80). Hospital length of stay was reduced in the treatment group by a mean of 1.16 days (95% CI 0.43 to 1.89, P = 0.002). There was no difference in critical care length of stay. There were insufficient data to comment on quality of life and cost effectiveness.
Authors' conclusions
It remains uncertain whether increasing blood flow using fluids, with or without inotropes or vasoactive drugs, reduces mortality in adults undergoing surgery. The primary analysis in this review (mortality at longest follow‐up) showed no difference between the intervention and control, but this result was sensitive to the method of analysis, the withdrawal of studies with methodological limitations, and is dominated by a single large RCT. Overall, for every 100 patients in whom blood flow is increased perioperatively to defined goals, one can expect 13 in 100 patients (from 40/100 to 27/100) to avoid a complication, 2/100 to avoid renal impairment (from 8/100 to 6/100), 5/100 to avoid respiratory failure (from 10/100 to 5/100), and 4/100 to avoid postoperative wound infection (from 10/100 to 6/100). On average, patients receiving the intervention stay in hospital one day less. It is unlikely that the intervention causes harm. The balance of current evidence does not support widespread implementation of this approach to reduce mortality but does suggest that complications and duration of hospital stay are reduced.
Plain language summary
Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery
Death and serious complications commonly occur following major surgery and are a significant public health problem. These outcomes might be prevented by using fluids and drugs to maintain the supply of oxygen and other nutrients to vital organs. Global blood flow, adjusted to maintain specific targets, might serve as a proxy in determining whether administered fluid and drugs maintain critical nutrient supply. In this Cochrane review of 31 studies conducted in 5292 patients undergoing major surgery, the use of fluids, with or without additional drugs, to achieve defined targets associated with increased total blood flow did not reduce mortality. There was a reduction in the number of patients with complications and the length of time patients stayed in hospital (by 1.2 days). However, the quality of the studies in this area was mediocre.
Summary of findings
Summary of findings for the main comparison. Protocol to increase global blood flow compared to control for surgical patients.
| Protocol to increase global blood flow compared to control for surgical patients | ||||||
| Patient or population: Surgical patients Settings: Hospital Intervention: Protocol to increase global blood flow Comparison: Control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Protocol to increase global blood flow | |||||
| Mortality (Longest follow‐up) | 11 per 100 | 10 per 100 (8 to 11) | RR 0.89 (0.76 to 1.05) | 5292 (31 studies) | ⊕⊕⊝⊝ low1,2 | P=0.18 |
| Mortality (Hospital or 28‐day) | 7 per 100 | 6 per 100 (5 to 7) | RR 0.81 (0.65 to 1.00) | 5292 (31 studies) | ⊕⊕⊝⊝ low1,2 | P=0.06 |
| Number of patients with complications | 40 per 100 | 27 per 100 (23 to 32) | RR 0.68 (0.58 to 0.80) | 1841 (17 studies) | ⊕⊕⊝⊝ low1,2 | P<0.00001 |
| Length of hospital stay | The mean length of hospital stay in the intervention groups was 1.16 lower (1.89 to 0.43 lower) | 4729 (27 studies) | ⊕⊕⊝⊝ low1,2 | P=0.002 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Majority of studies are unblinded due to the nature of the intervention and hence we have suggested "unclear risk for most of the studies". 2 Most studies had small number of patients.
Background
Description of the condition
It has been known for many years that patients undergoing surgery are more likely to have serious complications or die if they have limited physiological reserve (Boyd 1959; Clowes 1960). Post hoc analysis of patients undergoing major surgery revealed that survivors had a higher cardiac index and lower systemic vascular resistance than those who died (Shoemaker 1972; Shoemaker 1973). Commonly monitored vital signs (heart rate, arterial blood pressure, central venous pressure, temperature, haemoglobin concentration) were found to be poor predictors of mortality when compared with the flow related variables cardiac output and total body oxygen delivery (DO2) (Shoemaker 1979; Shoemaker 1993). In particular, survivors of major surgical procedures were found to have higher values for cardiac output or DO2. More recent studies have shown mixed results for the impact of oxygen transport on postoperative morbidity and mortality (Kusano 1997; Peerless 1998; Polonen 1997).
Description of the intervention
New therapeutic options and monitoring techniques that became available in the 1970s, particularly the introduction of the pulmonary artery flow directed catheter (PAC) (Ganz 1971; Swan 1970), opened up the possibility of measuring and then manipulating an individual's cardiovascular system. It was hypothesized that targeting goals for cardiac output and DO2 in all patients to the values manifested by the survivors of surgery would improve outcome (Bland 1978).
How the intervention might work
An important principle of this intervention is that the perioperative manipulation to augment cardiac output and DO2 would lead to an improved tissue perfusion and oxygenation. This physiological improvement would lead to better survival and fewer postoperative complications in patients undergoing major surgery.
Why it is important to do this review
It is almost 30 years since the initial uncontrolled data were presented suggesting that perioperative manipulation of flow related cardiovascular variables might improve outcomes in higher risk surgical patients (Shoemaker 1982). Since then, a number of randomized trials have been undertaken in patients in the perioperative period which have investigated this issue. However, these trials differ in:
the case mix of the patients recruited (different operation severities, comorbidities and, therefore, expected mortalities);
the techniques used to measure cardiac output (pulmonary artery catheter (PAC) thermodilution, Doppler velocimetry);
the specific goals targeted (cardiac output, DO2, maximum stroke volume);
the techniques used to achieve the goals (fluids, fluids plus inotropes or vasoactive drugs);
the management of the control arm.
In addition, some of the studies were not blinded and many had small sample sizes leading to limited statistical power. Despite this, a number of non‐systematic reviews have attempted to combine studies in order to draw general conclusions from the studies (Boyd 1996; Boyd 1999; Forst 1997; Ivanov 1997; Leibowitz 1997). However, these reviews have identified varying numbers of trials and have not been undertaken systematically, using scientifically rigorous techniques for literature searching or for abstraction and analyses of data. Three previous systematic reviews have addressed this question (Heyland 1996; Kern 2002; Poeze 2005) and reported improved outcomes. They do not include recently published studies and did not focus exclusively on perioperative data. Among recent systematic reviews and meta‐analyses, one study included patients with trauma and sepsis (Hamilton 2011) while other studies analysed renal function (Brienza 2009) and gastrointestinal complications (Giglio 2009) as primary outcomes.
The time is now ripe for a systematic review of the literature to address the important question: does perioperative administration of fluids, with or without vasoactive drugs, targeted to increase global blood flow in adults undergoing surgery reduce mortality, morbidity and resource utilization?
Objectives
To describe the effects of perioperative (24 hours before surgery up to six hours after surgery) administration of fluids, with or without vasoactive drugs, that were targeted to increase global blood flow (relative to control) as defined by explicit measured goals on outcomes following surgery (mortality, morbidity, resource utilization and health status).
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), with or without blinding, that were available as full published papers. We applied no language restrictions .
Types of participants
We included adults (aged 16 years or older) undergoing surgery in an operating theatre.
Types of interventions
Perioperative administration (initiated within 24 hours before surgery and lasting up to six hours after surgery) of fluids, with or without inotropes or vasoactive drugs, to increase blood flow (relative to control) against explicit measured goals: cardiac output (CO), cardiac index (CI), oxygen delivery (DO2) or oxygen delivery index (DO2I), oxygen consumption or oxygen consumption index (VO2), stroke volume (SV) or stroke volume index, mixed venous oxygen saturation (SVO2), oxygen extraction ratio (O2ER) and lactate.
Types of outcome measures
Primary outcomes
Mortality (at longest available follow‐up)
Secondary outcomes
1. Mortality: all reported time frames e.g. hospital or 28 day, six months.
2. Morbidity: 2.1. rates of overall complications; 2.2. rates of renal impairment, arrhythmia, respiratory failure or acute respiratory distress syndrome (ARDS ), infection, myocardial infarction, congestive heart failure or pulmonary oedema, and venous thrombosis.
3. Resource utilization: length of intensive care (ICU) stay, length of hospital stay, cost.
4. Health status: e.g., six month functional health status, quality of life scores.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 1), see Appendix 1; MEDLINE via OvidSP (1966 to March 2012), see Appendix 2; and EMBASE via OvidSP (1982 to March 2012), see Appendix 3. For searching in MEDLINE we combined our topic‐specific key words with the Cochrane highly sensitive search strategy for identifying RCTs (Higgins 2011). We modified this filter for use in EMBASE. We used specific keywords to identify potential studies (Appendix 4).
Searching other resources
We searched the proceedings of the following major, relevant European and North American conferences from the year 2011 backwards, without finding eligible studies.
American College of Surgeons (2011 to 1996).
American Society of Anesthesiologists (2011 to 1995).
American Thoracic Society (2011 to 1997*) (* = not available for searching prior to 1997).
Association of Surgeons of Great Britain and Ireland (2011 to 1996).
European Society of Anaesthesiologists (2011 to 1995).
European Society of Intensive Care Medicine (2011 to 1983).
International Anesthesia Research Society (2011 to 1994).
Society of Critical Care Medicine (2011 to 1986).
We checked the reference lists of potentially eligible studies and previously published systematic reviews. We also searched the personal reference databases of the authors and the Steering Group for this review. We contacted experts in the field and relevant pharmaceutical companies and asked for published and unpublished reports.
Data collection and analysis
Selection of studies
Two independent authors (MG and MH for 2001 to 2006, AD and AV for 2006 to 2012) identified titles and abstracts of potentially eligible studies. We resolved any disagreement by discussion. We obtained the full texts of potentially eligible studies. We abstracted the study characteristics including: study design; patient population; interventions; and outcomes (see Appendix 5; Appendix 6; Table 2). Review authors were not involved in the selection of studies they had authored.
1. Study outcomes.
| Study | Mortality | Morbidity | Resource use | Cost |
| Bender 1997 | Hospital | Pulmonary oedema, acute myocardial infarction, arrhythmia, acute renal failure, wound infection, haemorrhage, sepsis, graft thrombosis or infection, groin haematoma. | HLOS, ICULOS | Cost |
| Berlauk 1991 | Hospital | Acute renal failure, congestive cardiac failure, graft thrombosis, acute myocardial infarction, arrhythmia, | HLOS, ICULOS | Cost |
| Bonazzi 2002 | Hospital | Arrhythmias, myocardial infarction, congestive heart failure, renal failure | HLOS | None |
| Boyd 1993 | 28 day | Respiratory failure, acute renal failure, sepsis, cardiorespiratory arrest, pulmonary oedema, pleural fluid, wound infection, disseminated intravascular coagulation, acute myocardial infarction, abdominal abscess, haemorrhage, gastric outlet obstruction, cerebrovascular accident, pulmonary embolism, chest infection, psychosis, distal ischaemia | HLOS, ICULOS | Reported separately |
| Cecconi 2011 | 28 day | Infections, hypotension, anaemia, pneumonia, pulmonary embolism, tachyarrhythmias, acute coronary syndrome, acute renal failure | HLOS | None |
| Challand 2012 | 30 days 90 days |
Serious postoperative complications, renal complications, creatinine increase, critical care admission | HLOS | None |
| Conway 2002 | Hospital | Tolerating oral diet | HLOS, ICULOS | None |
| Donati 2007 | Hospital | Organ failures | HLOS | None |
| Gan 2002 | Hospital | Acute renal dysfunction (urine output <500mls), respiratory support for > 24 hours, cardiovascular (hypotension, pulmonary oedema, arrhythmia), chest infection (clinical diagnosis), severe postoperative nausea and vomiting requiring rescue antiemetic, coagulopathy, wound infection, toleration of oral solid diet. | HLOS | None |
| Jerez 2001 | Hospital | Organ failures | ICULOS | None |
| Jhanji 2010 | Hospital | Cardiac complications, infections, acute kidney injury | HLOS, ICULOS |
None |
| Kapoor 2007 | Hospital | arrhythmia, renal dysfunction, low cardiac output | HLOS, ICULOS |
None |
| Lobo 2000 | 28 day, 60 day | Sepsis, shock, septic shock, cardiogenic shock, nosocomial infection, acute pancreatitis, postoperative fistula, arrhythmia, cerebrovascular accident, deep vein thrombosis, gastrointestinal bleeding, hypothermia, sepsis related organ failure assessment (SOFA) score, bronchopneumonia, urinary tract infection, wound infection. ventilator days, organ dysfunction | HLOS, ICULOS | None |
| Mayer 2010 | Hospital | Infection (pneumonia, abdominal, urinary tract, wound), respiratory (PE, respiratory support), cardiovascular (pulmonary oedema, arrhythmia, hypotension, acute myocardial infarction, stroke), abdominal (bowel obstruction, gastrointestinal bleeding, anastomotic leak), renal ( urine output ,500ml/day or required dialysis for acute renal failure), post operative haemorrhage | HLOS, ICULOS | None |
| Mckendry 2004 | Hospital mortality | Atrial fibrillation requiring treatment, pneumothorax, cerebrovascular accident, chest infection or sternal wound infection, GI bleed, acute renal failure, pleural effusion, infected leg wound, aortic regurgitation | HLOS, ICULOS | None |
| Mythen 1995 | Hospital | Knaus organ failure criteria, chest infection, pleural effusion, disorientation, respiratory failure, nausea and vomiting, cerebrovascular accident, paralytic ileus, pericardial effusion. | HLOS, ICULOS | Reported separately |
| Noblett 2006 | Hospital | surgical fitness for discharge, return of gastrointestinal function, flatus, bowel movement, food tolerance, readmission rate, cytokine markers of the systemic inflammatory response | HLOS, ICULOS | None |
| Pearse 2005 | Hospital, 28 and 60 day mortality | Number of patients with complications, infection (pneumonia, abdominal, urinary tract, CVC, wound), respiratory (pleural effusion, pneumothorax, pulmonary embolism, ARDS), cardiovascular (arrhythmia, pulmonary oedema, MI, stroke), abdominal (C. Diff, diarrhoea, acute bowel obstruction, upper GI bleed, paralytic ileus, anastomotic leak, intra‐abdominal hypertension), postoperative massive haemorrhage. | HLOS, ICUOS | None |
| Pillai 2011 | None | Nausea and vomiting, wound dehiscence, wound infection, ileus | HLOS | None |
| Pölönen 2000 | 28 day, 6 month, 12 month | Organ dysfunctions: central nervous system (hemiplegia, stroke, Glasgow coma scale (GCS <10), circulatory (vasoactive medication or intraaortic counterpulsation to treat hypotension or low cardiac output), respiratory (need for mechanical or assisted ventilation), renal (low urine output or increased creatinine), hepatic (increased liver enzymes or bilirubin), gastrointestinal (macroscopic bleeding or paralytic ileus), haematological (low white cell or platelet count), ICU readmission. | HLOS, ICULOS | None |
| Sandham 2003 | Hospital, 6 month, 12 month | Myocardial infarction, congestive heart failure, supraventricular tachycardia, pulmonary embolism, renal insufficiency, hepatic insufficiency, sepsis from central venous catheter (CVC) or pulmonary artery catheter (PAC), wound infection, pneumonia, adverse events related to PAC or CVC: pulmonary infarction, haemothorax, pulmonary haemorrhage, pneumothorax, arterial puncture. | HLOS | None |
| Senagore 2009 | Hospital | Complications: Gastrointestinal failure, blood pressure lability, arrhythmia, dehydration, electrolyte imbalance, hyperglycaemia, wound/infectious complications, sepsis, DVT/PE, intraoperative hypothermia, urinary dysfunction,respiratory dysfunction, abdominal pain, chest pain, bleeding, anaemia, altered mental status. | HLOS | None |
| Shoemaker 1988 | Hospital | Respiratory failure, renal failure, sepsis and septic shock, hepatic failure, cardiac arrest, pulmonary edema, pleural effusion, wound infection, disseminated intravascular coagulation (DIC), acute myocardial infarction, evisceration, abdominal abscess, haemorrhage, pancreatitis, gastric outlet obstruction, urinary tract infection, cerebral infarct, pulmonary embolism, ventilator days | HLOS, ICULOS | Cost |
| Sinclair 1997 | Hospital | None, "time declared fit for medical discharge" | HLOS | None |
| Ueno 1998 | Hospital | Bleeding, peritoneal infection, adult respiratory distress syndrome, hyperbilirubinaemia, liver failure | None | None |
| Valentine 1998 | Hospital | Myocardial infarction, arrhythmia, congestive heart failure, pneumonia, non‐cardiogenic pulmonary insufficiency, acute renal insufficiency, catheter sepsis. ventilator days | HLOS, ICULOS | None |
| Van der Linden 2010 | Hospital | Blood loss, infection | HLOS | None |
| Venn 2002 | Hospital | "Time to medical fitness for discharge", deep haemorrhage requiring >2 unit blood transfusion, haematemesis, chest infection, wound infection, cellulitis, pancreatitis, pulmonary embolus, cerebrovascular accident, myocardial infarction, cardiac failure, rapid atrial fibrillation, hypotension, impaired renal function, pseudo‐obstruction. | HLOS | None |
| Wakeling 2005 | hospital and 6 month mortality | Time until fit for discharge, bowel recovery (flatus, bowels opening, full diet), quality of recovery score, postoperative morbidity survey (POMS), quality of life questionnaires (European Organisation for the Research and Treatment of Cancer (EORTC) ‐ QLQ‐C30 and QLQ‐CR38) | HLOS | None |
| Wilson 1999 | Hospital | Respiratory (prolonged weaning, adult respiratory distress syndrome (ARDS), pleural effusion, secondary ventilation, sputum retention), cardiovascular (myocardial infarction, arrhythmia, cardiac arrest, pulmonary embolus, cerebrovascular accident, transient ischaemic attack, cardiac failure), gastrointestinal (infarction, haemorrhage), acute renal failure, coagulopathy, infection (bacteraemia, sepsis syndrome, septic shock, respiratory sepsis, urinary sepsis, abdominal sepsis, wound sepsis, line sepsis, other sepsis), surgical (anastomotic breakdown, deep haemorrhage, wound haemorrhage) | HLOS, ICULOS | Reported separately |
| Ziegler 1997 | Hospital | Hypotension, congestive heart failure, myocardial infarction, arrhythmia, oliguria, graft thrombosis, cerebrovascular accident | ICULOS | None |
Data extraction and management
Two authors (MG and MH for 2001 to 2006, AD and AV for 2006 to 2012) independently extracted data. We achieved consensus by resolving any disparity in data collection by discussion. In the absence of appropriate published data, we made at least three attempts to contact authors of eligible studies to obtain any required data. Some studies were conducted by the authors of this review (MGM). They were not involved in the data extraction or risk of bias assessment.
Assessment of risk of bias in included studies
We performed the risk of bias assessment according to the Cochrane risk of bias tool (Higgins 2011). From this tool, we used the following seven domains to assess the methodological quality of included studies. This is summated in a graph and a summary table.
1. Random sequence generation (selection bias): describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
2. Allocation concealment (selection bias): describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
3. Blinding of participant and personnel (performance bias): describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective.
4. Blinding of outcome assessment (detection bias): describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blindness was effective.
5. Incomplete outcome data (attrition bias): describe the completeness of the outcome data for each main outcome, including attrition and exclusion from the analysis. State whether attrition and exclusions were reported, the number in each intervention group (compared with total randomized participants), reasons for attrition and exclusions, and any re‐inclusions in analysis performed by the review authors.
6. Selective reporting (reporting bias): state how the possibility of selective outcome reporting was examined by the review authors, and what was found.
7. Other sources of bias: state any important concerns about bias not addressed in the other domains in the tool.
Measures of treatment effect
We based analyses of outcomes on intention‐to‐treat. We calculated a weighted treatment effect across all RCTs using Review Manager (RevMan 5.1). We expressed measures of treatment effect, such as mortality and complications, as relative risks (RR) and 95% confidence intervals (CI). We used mean differences (standard deviation) for continuous variables such as length of hospital or ICU stay. We explored the robustness of these estimates by comparing both fixed‐effect and random‐effects models for the primary outcome.
Unit of analysis issues
We included studies with different treatment groups, interventions and outcomes. Consequently, we performed subgroup analyses of these differences. Many studies reported the number of complications, arrhythmias and infections as total numbers, leaving unclear what the denominators were for these episodes. We have not analysed variables for which the denominator was unknown.
Dealing with missing data
We contacted the authors of the studies for further information and the analysis was performed with the best available information when there was no response.
Assessment of heterogeneity
We assessed inconsistencies and variability in the outcomes among the studies by the I2 statistic. Variations of > 40% in the outcomes may not be explained by sampling variation. We assumed substantial heterogeneity when the I2 statistic exceeded 40% (Higgins 2011).
Assessment of reporting biases
We assessed graphical evidence of reporting biases using contour enhanced funnel plots with a subsequent Harbord or Egger's test (Egger 1997; Harbord 2006).
Data synthesis
We performed statistical analysis using Review Manager 5.1 (RevMan 5.1). We applied the intention‐to‐treat method for all analyses. We used both fixed‐effect and random‐effects models for the primary outcome analysis and the fixed‐effect model for the secondary outcomes. We used relative risks (95% CI) for dichotomous outcomes and mean difference (standard deviation (SD) of the mean or 95% CI) for continuous variables.
Subgroup analysis and investigation of heterogeneity
Due to the heterogeneous nature of the selected studies, we conducted subgroup analyses in the following areas.
The urgency of surgery (elective or emergency).
The type of surgery (general, vascular, cardiac, other).
The timing of the intervention (perioperative, intraoperative, postoperative).
The type of intervention (fluids, fluids with vasoactive agents).
The intervention goals (CO, SV, oxygen indices).
Sensitivity analysis
We analysed mortality, both over the longest follow‐up and hospital or 28 day mortality, with fixed‐effect and random‐effects models. In addition, we excluded studies with fewer than 100 participants. The intervention in the protocol group varied. The control group in some studies had explicit blood flow goals to standardize care. Further, some studies did not fully control for co‐interventions, for instance admission to critical care. We performed sensitivity analysis excluding these studies.
Results
Description of studies
All included studies were RCTs of surgical participants. Compared to controls, the intervention group had a separate protocol to optimise global blood flow, measured by cardiac output (CO) or oxygen delivery.
Results of the search
The initial electronic search identified 18,951 potential studies (Figure 1). After removal of duplicated studies, the search yielded 10,462 studies. No additional studies were identified by contacting experts in the field or relevant pharmaceutical companies or by searching the personal reference databases of the authors or Steering Group. No additional studies were identified following screening of reference lists of potentially eligible studies and previously published systematic reviews.
1.
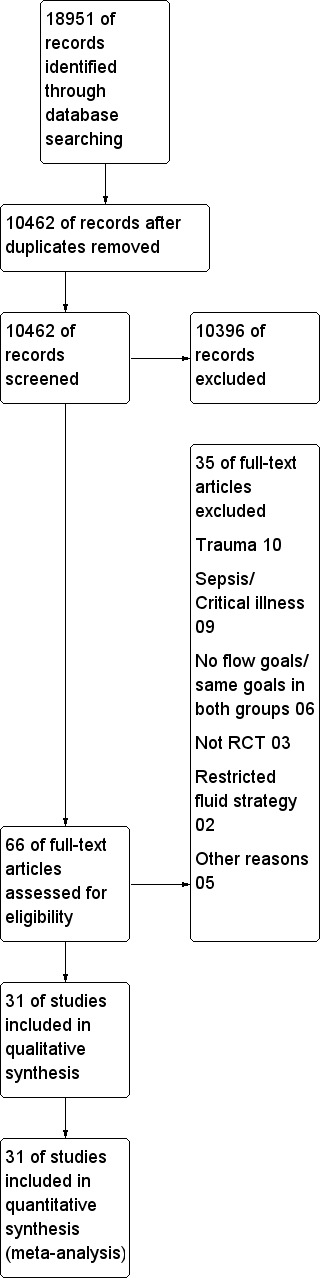
Study flow diagram.
We identified 66 potentially eligible studies following screening of the abstracts of studies. Of those 66 studies, 35 potentially eligible studies did not meet the study inclusion criteria for the reasons summarized in Characteristics of excluded studies.
The remaining 31 fully published studies (5292 participants) met the inclusion criteria. We have summarized the Included studies in Characteristics of included studies; Appendix 5; and Appendix 6. We contacted study authors for additional data where necessary.
Included studies
We included 31 studies in the review (see Characteristics of included studies). The studies were conducted in Europe (20), USA (seven), India (one), Brazil (one), Japan (one) and Canada (one). Most studies (24 studies) recruited participants having elective surgery. The studies were published between 1988 and 2011 (Appendix 5; Appendix 6).
Excluded studies
We excluded 35 studies (Characteristics of excluded studies). We excluded studies that included trauma patients (10) and septic or critically ill patients (nine) unless all patients underwent surgery.
Risk of bias in included studies
We evaluated the risk of bias of included studies with the Cochrane tool (Higgins 2011). This was performed by two authors (AD, AV) independently and we resolved any disparity by discussion and the involvement of a third person (MG). We present the methodological quality in a summary table and a graph (Figure 2; Figure 3).
2.
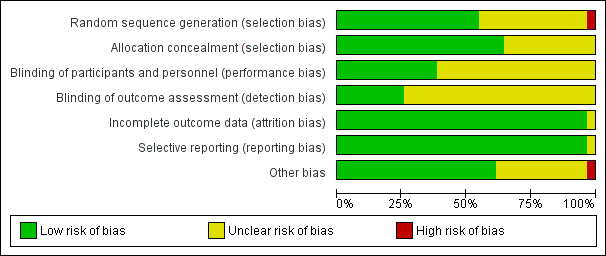
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
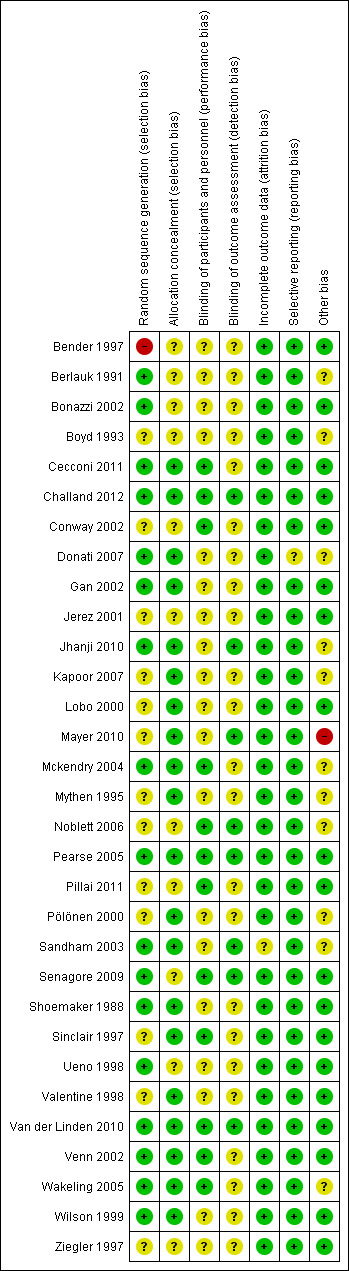
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies randomly allocated participants. The method of randomization was described in 25 studies (80%): (Bender 1997; Berlauk 1991; Bonazzi 2002; Cecconi 2011; Challand 2012; Donati 2007; Gan 2002; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Pearse 2005; Pölönen 2000; Sandham 2003; Senagore 2009; Shoemaker 1988; Sinclair 1997; Ueno 1998; Valentine 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999). However, in seven studies (Kapoor 2007; Lobo 2000; Mayer 2010; Mythen 1995; Pölönen 2000; Sinclair 1997; Valentine 1998) it was unclear whether the sealed envelope technique allocated participants sequentially. In one study (Bender 1997) participants were allocated by a surgical intensivist, which may have introduced selection bias. We assessed as adequate the random allocation in 17 studies (55%) (Berlauk 1991; Bonazzi 2002; Cecconi 2011; Challand 2012; Donati 2007; Gan 2002; Jhanji 2010; Mckendry 2004; Pearse 2005; Sandham 2003; Senagore 2009; Shoemaker 1988; Ueno 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999).
We assessed the methods of allocation concealment as adequate for 20 studies (65%) (Cecconi 2011; Challand 2012; Donati 2007: Gan 2002; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Pearse 2005; Pölönen 2000; Sandham 2003; Shoemaker 1988; Sinclair 1997; Valentine 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999).
Blinding
We assessed blinding of personnel or participants as adequate in only 12 studies (39%), reflecting the nature of the intervention (Cecconi 2011; Challand 2012; Conway 2002; Mckendry 2004; Noblett 2006; Pearse 2005; Pillai 2011; Senagore 2009; Sinclair 1997; Van der Linden 2010; Venn 2002; Wakeling 2005). We assessed blinding of outcome assessment as adequate in eight studies (26%) (Challand 2012; Jhanji 2010; Mayer 2010; Noblett 2006; Pearse 2005; Sandham 2003; Senagore 2009; Van der Linden 2010).
Incomplete outcome data
Attrition bias was detected in one study (Sandham 2003) where a large number of participants were lost to follow‐up, which may have introduced attrition bias.
Selective reporting
All anticipated outcomes were reported by the included studies.
Other potential sources of bias
In Mayer 2010, the second author has been found to have fabricated results in some clinical studies. We recognized this as a potential high risk.
Exclusion of participants after randomization was noted in seven studies (Berlauk 1991; Kapoor 2007; Mayer 2010; Mckendry 2004; Noblett 2006; Pölönen 2000; Wakeling 2005), which may have induced selection bias.
To test the effect of publication bias, we produced a contour‐enhanced funnel plot for the primary outcome (Figure 4), the subsequent Harbord test showing a significant small‐studies effect: regression bias ‐0.72 (95% CI ‐0.08 to ‐1.39) (Figure 5). Similarly, the rate of complications (Figure 6; Figure 7) showed evidence of a small studies effect. No other outcome demonstrated small study effects (Harbord 2006).
4.
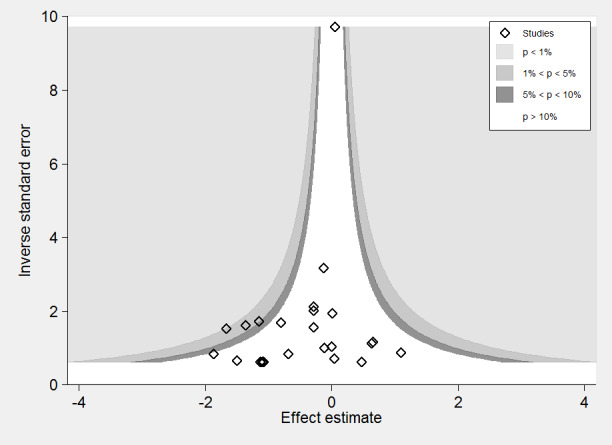
Contour‐enhanced funnel plot: mortality.
5.
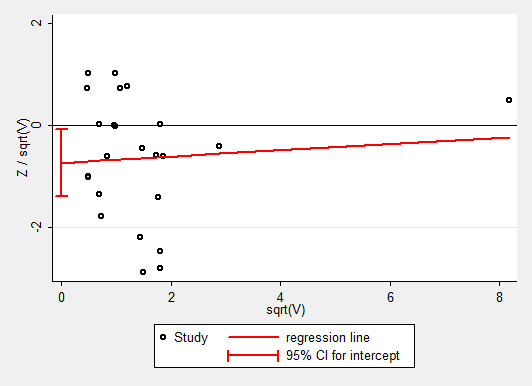
Galbraith plot, Harbord analysis, mortality. The regression slope is ‐0.72 (‐0.08 to ‐1.39).
6.
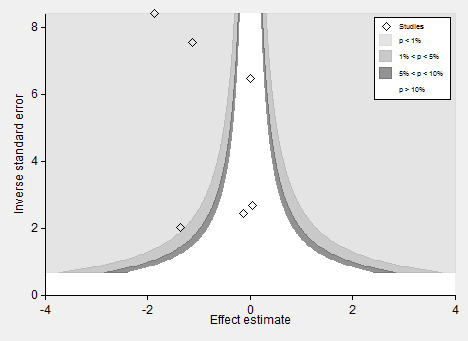
Contour‐enhanced funnel plot for rate of complications.
7.
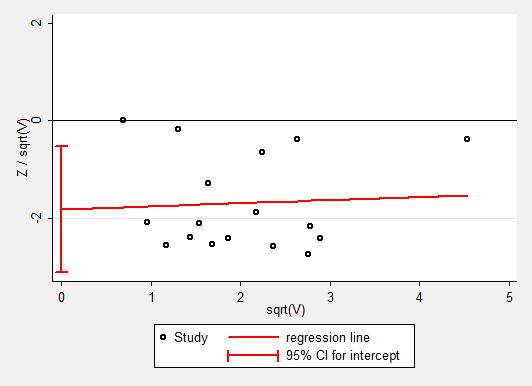
Galbraith plot of Harbord analysis for rate of complications.
Effects of interventions
See: Table 1
Data Synthesis
Mortality
1.1 Long‐term mortality
Thirty studies reported mortality data and further information was obtained from authors for one study (Pillai 2011). A number of different definitions were used and some papers reported more than one definition. Using data from the longest reported follow‐up, the overall mortality was 238/2677 (8.9%) in the intervention group and 282/2615 (10.8%) in the control group, RR of 0.89 (95% CI 0.76 to 1.05, P = 0.18, I2 = 15%) (Analysis 1.1). The results were sensitive to analytical methods, becoming statistically significant with two methods: the inverse variance random‐effects model, RR of 0.72 (95% CI 0.55 to 0.95, P = 0.02, I2 = 15%); the Mantel–Haenszel random‐effects model, RR of 0.72 (95% CI 0.55 to 0.95, P = 0.02, I2 = 16%) (Appendix 7).
1.1. Analysis.
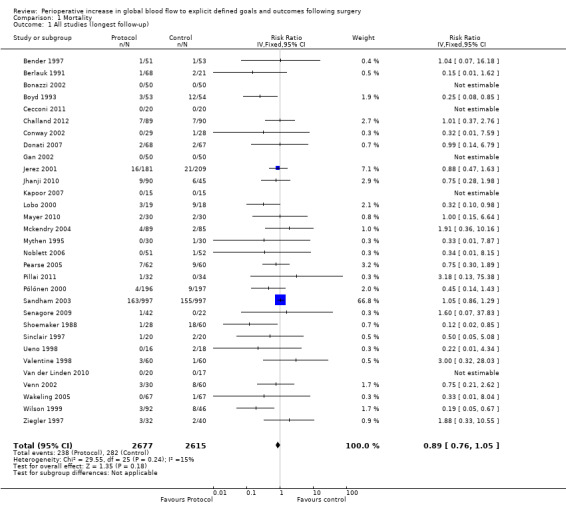
Comparison 1 Mortality, Outcome 1 All studies (longest follow‐up).
1.2 Hospital or 28 day mortality
Hospital or 28 day mortality was reported in 30 studies and further information was obtained from one study (Pillai 2011). Pooled hospital or 28 day mortality was 146/2677 (5.4%) in the intervention group and 192/2615 (7.3%) in the control group, RR of 0.81 (95% CI 0.65 to 1.00, P = 0.06, I2 = 1%) (Analysis 1.2). The results were sensitive to analytical methods, becoming significant with three methods: the inverse variance random‐effects model, RR of 0.79 (95% CI 0.63 to 0.99, P = 0.04, I2 = 1%); the Mantel–Haenszel fixed‐effect model, RR of 0.77 (95% CI 0.63 to 0.95, P = 0.01, I2 = 2%) and random‐effects model, RR of 0.78 (95% CI 0.62 to 0.99, P = 0.04, I2 = 2%) (Appendix 8).
1.2. Analysis.
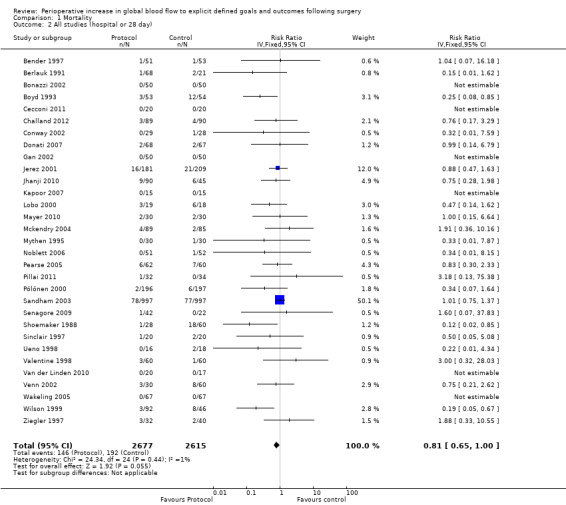
Comparison 1 Mortality, Outcome 2 All studies (hospital or 28 day).
Morbidity
We analysed seven categories of morbidity using the investigators' definitions. No two studies used the same list of morbidities following surgery (Table 2). In most cases no specific criteria were listed for morbidities. No two studies used the same criteria.
2.1 Renal impairment
We accepted the rate of renal impairment reported by study authors: we did not apply a single definition across studies. Data on renal impairment were available for 21 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Challand 2012; Donati 2007; Gan 2002; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Pölönen 2000; Sandham 2003; Shoemaker 1988; Valentine 1998; Venn 2002; Wakeling 2005; Wilson 1999). The intervention reduced the rate of renal impairment, RR of 0.71 (95% CI 0.57 to 0.90, P = 0.004, I2 = 20%) (Analysis 2.1).
2.1. Analysis.
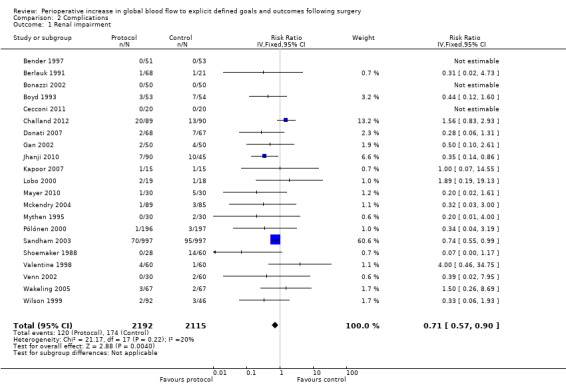
Comparison 2 Complications, Outcome 1 Renal impairment.
2.2 Arrhythmia
Arrhythmia was reported in 16 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Cecconi 2011; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Pearse 2005; Sandham 2003; Senagore 2009; Shoemaker 1988; Valentine 1998; Venn 2002; Wilson 1999; Ziegler 1997). However, we excluded three studies for which there were unit‐of‐analysis issues: two studies (Bender 1997; Berlauk 1991) reported the number of events; one of these studies and one other (Bender 1997; Valentine 1998) reported for both the intraoperative and postoperative periods. One study (Shoemaker 1988) reported transient dysrhythmias ("almost always premature ventricular complexes") during insertion of pulmonary artery (PA) catheters. This was reported as a combined percentage (12%) for both control and protocol PA catheter groups. We were unable to identify the exact rate of arrhythmias for each group separately and therefore excluded this study from the analysis. For the 12 studies (Bonazzi 2002; Cecconi 2011; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Pearse 2005; Sandham 2003; Senagore 2009; Venn 2002; Wilson 1999; Ziegler 1997) that we were able to analyse, there was no significant difference between groups in development of an arrhythmia, RR of 0.84 (95% CI 0.67 to 1.06, P = 0.14, I2 = 0%) (Analysis 2.2).
2.2. Analysis.
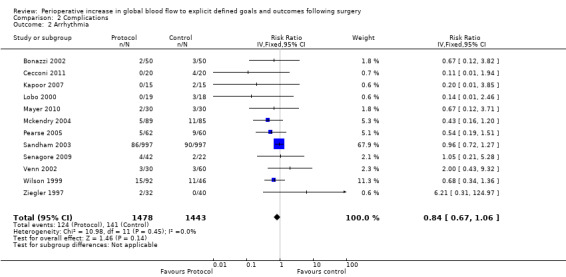
Comparison 2 Complications, Outcome 2 Arrhythmia.
2.3 and 2.4 Infection
Infections were reported several ways in 20 studies (Bender 1997; Boyd 1993; Cecconi 2011; Gan 2002; Jhanji 2010; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Pearse 2005; Pillai 2011; Sandham 2003; Senagore 2009; Shoemaker 1988; Sinclair 1997; Valentine 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999). The number of participants who had infections was reported in nine studies (Bender 1997; Jhanji 2010; Lobo 2000; Mythen 1995; Pillai 2011; Sinclair 1997; Valentine 1998; Van der Linden 2010; Wakeling 2005). The number of participants with infections was unaffected by the intervention, RR of 0.88 (95% CI 0.69 to 1.12, P = 0.29, I2 = 0%) (Analysis 2.3).
2.3. Analysis.
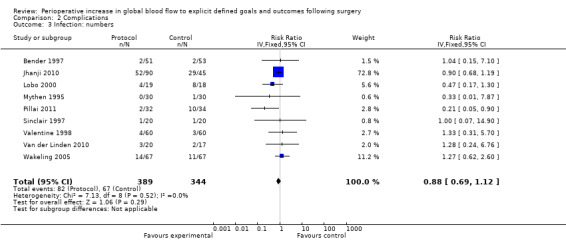
Comparison 2 Complications, Outcome 3 Infection: numbers.
The types of infection (such as pneumonia) were reported separately in 15 studies (Bender 1997; Boyd 1993; Cecconi 2011; Gan 2002; Lobo 2000; Mayer 2010; Mythen 1995; Pillai 2011; Sandham 2003; Shoemaker 1988; Sinclair 1997; Valentine 1998; Van der Linden 2010; Venn 2002; Wilson 1999). Nine studies (Boyd 1993; Cecconi 2011; Gan 2002; Mayer 2010; Pearse 2005; Sandham 2003; Shoemaker 1988; Venn 2002; Wilson 1999) reported more than one infective complication per participant. It was not possible to add the total number of infections as the exact denominator was unknown. We therefore analysed each infection separately. There was no difference in the rates of: pneumonia, RR of 0.78 (95% CI 0.61 to 1.00, P = 0.05, I2 = 0%); sepsis, RR of 0.68 (95% CI 0.26 to 1.77, P = 0.43, I2 = 6%); abdominal infections, RR of 0.53 (95% CI 0.23 to 1.22, P = 0.14, I2 = 0%); or urinary tract infections, RR of 0.54 (95% CI 0.26 to 1.15, P = 0.11, I2 = 0%). The intervention significantly reduced the rate of wound infections, RR of 0.65 (95% CI 0.50 to 0.84, P = 0.001, I2 = 22%) (Analysis 2.4). Two studies (Mckendry 2004; Senagore 2009) reported on the total number of infections and we were unable to include these studies due to unit‐of‐analysis issues.
2.4. Analysis.
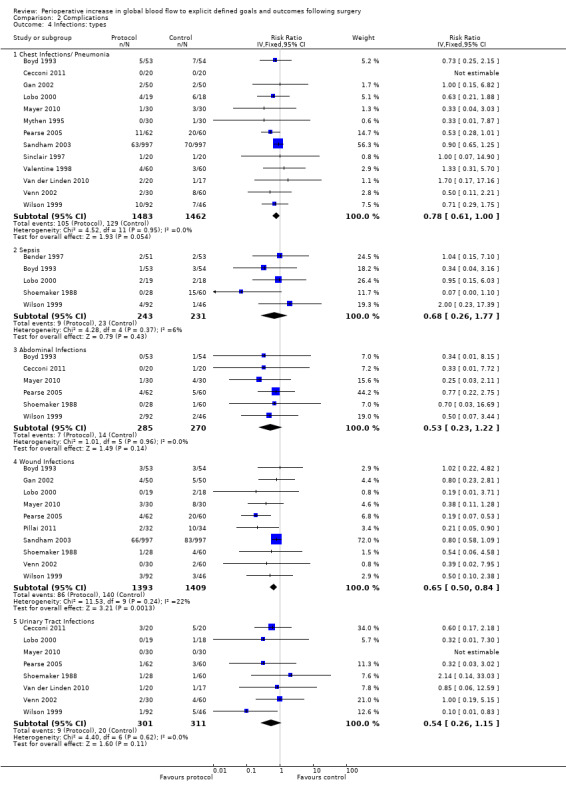
Comparison 2 Complications, Outcome 4 Infections: types.
2.5 Respiratory failure or acute respiratory distress syndrome (ARDS)
Respiratory failure or ARDS was reported in nine studies (Boyd 1993; Donati 2007; Gan 2002; Mayer 2010; Mythen 1995; Pearse 2005; Shoemaker 1988; Ueno 1998; Wilson 1999). One study (Wilson 1999) also included the number of participants with prolonged ventilation, which we were unable to analyse due to unit‐of‐analysis issues. The intervention significantly reduced the rate of respiratory failure, RR of 0.51 (95% CI 0.28 to 0.93, P = 0.03, I2 = 0%) (Analysis 2.5).
2.5. Analysis.
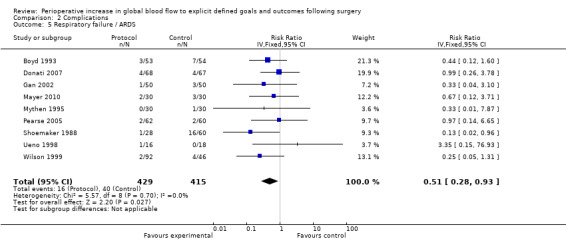
Comparison 2 Complications, Outcome 5 Respiratory failure / ARDS.
2.6 Myocardial infarction
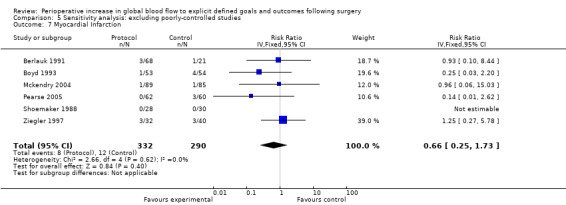
Myocardial infarction was reported in 15 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Kapoor 2007; Mayer 2010; Mckendry 2004; Pearse 2005; Sandham 2003; Shoemaker 1988; Valentine 1998; Venn 2002; Wilson 1999; Ziegler 1997). There was no significant difference in myocardial infarction, RR of 1.01 (95% CI 0.71 to 1.45, P = 0.95, I2 = 0%) (Analysis 2.6).
2.6. Analysis.
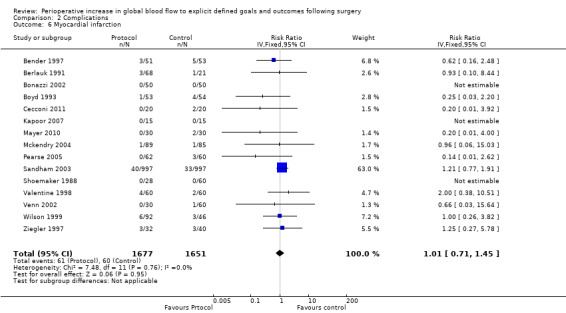
Comparison 2 Complications, Outcome 6 Myocardial infarction.
2.7 Congestive cardiac failure or pulmonary oedema
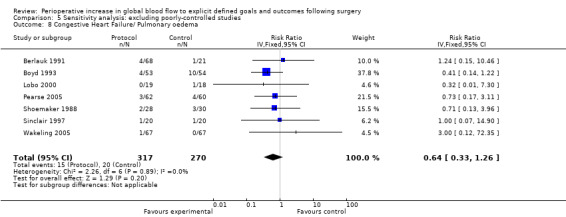
Congestive heart failure or pulmonary oedema was reported in 14 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Lobo 2000; Mayer 2010; Pearse 2005; Sandham 2003; Shoemaker 1988; Sinclair 1997; Valentine 1998; Venn 2002; Wakeling 2005; Wilson 1999). There was no significant difference, RR of 1.00 (95% CI 0.81 to 1.24, P = 0.98, I2 = 0%) (Analysis 2.7).
2.7. Analysis.
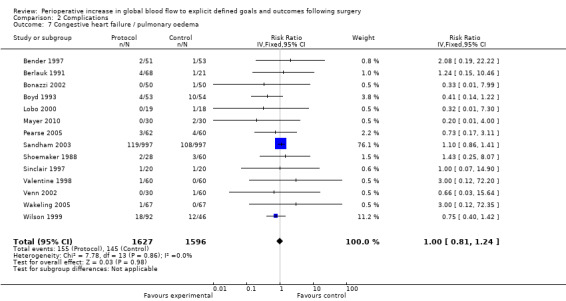
Comparison 2 Complications, Outcome 7 Congestive heart failure / pulmonary oedema.
2.8 Venous thrombosis
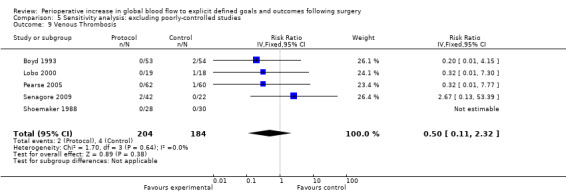
Venous thrombosis was reported in 10 studies (Boyd 1993; Cecconi 2011; Lobo 2000; Mayer 2010; Pearse 2005; Sandham 2003; Senagore 2009; Shoemaker 1988; Venn 2002; Wilson 1999). There was no difference, RR of 1.04 (95% CI 0.39 to 2.77, P = 0.93, I2 = 0%) (Analysis 2.8).
2.8. Analysis.
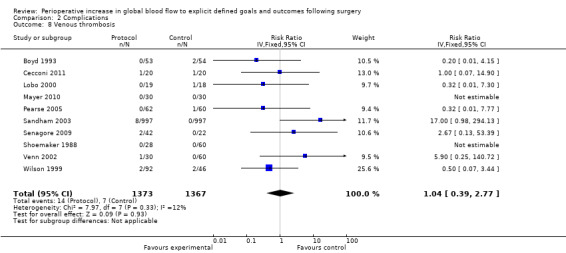
Comparison 2 Complications, Outcome 8 Venous thrombosis.
2.9 Complications
More than one method was used to pool complications. The number of participants with complications was reported by 19 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Jerez 2001; Jhanji 2010: Lobo 2000; Mayer 2010; Mythen 1995; Noblett 2006; Pearse 2005; Shoemaker 1988; Sinclair 1997; Ueno 1998; Wakeling 2005; Wilson 1999). The number of complications per participant was reported by three studies (Boyd 1993; Jerez 2001; Shoemaker 1988). The number of participants with individual complications or the number of individual complications was reported by 27 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Gan 2002; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Noblett 2006; Pearse 2005; Pölönen 2000; Sandham 2003; Senagore 2009; Shoemaker 1988; Ueno 1998; Valentine 1998; Venn 2002; Wakeling 2005; Wilson 1999; Ziegler 1997).
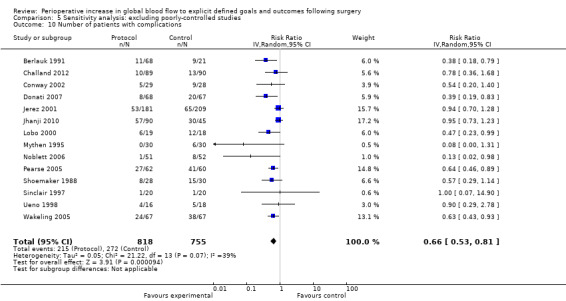
We did not pool data for the number of complications because of this variation and the associated unit‐of‐analysis issues. Further, six studies (Bonazzi 2002; Boyd 1993; Gan 2002; Kapoor 2007; Mckendry 2004; Pillai 2011) that reported the number of participants with complications also reported the individual complications separately, therefore pooling of these would again lead to unit‐of‐analysis issues. Two studies (Bender 1997; Valentine 1998) reported the number of participants with complications separately for the intraoperative and postoperative periods. We were unable to combine these outcomes due to unit‐of‐analysis issues. We therefore pooled 17 studies (Berlauk 1991; Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Jerez 2001; Jhanji 2010; Lobo 2000; Mayer 2010; Mythen 1995; Noblett 2006; Pearse 2005; Shoemaker 1988; Sinclair 1997; Ueno 1998; Wakeling 2005; Wilson 1999). The number of participants with complications was reduced by the intervention, RR of 0.68 (95% CI 0.58 to 0.80, P < 0.00001, I2 = 34%) (Analysis 2.9).
2.9. Analysis.
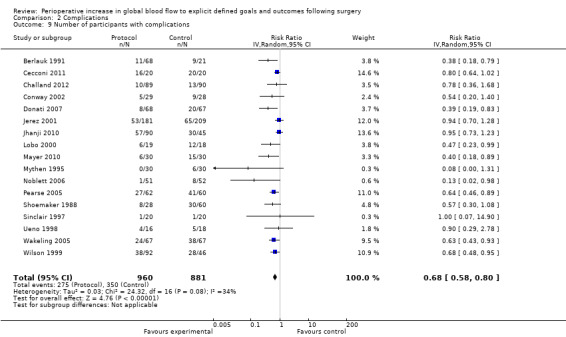
Comparison 2 Complications, Outcome 9 Number of participants with complications.
Health status
No study reported health status.
Resource use
3.1 Postoperative hospital stay
Postoperative length of hospital stay was reported in 28 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Gan 2002; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Noblett 2006; Pearse 2005; Pillai 2011; Pölönen 2000; Sandham 2003; Senagore 2009; Shoemaker 1988; Sinclair 1997; Valentine 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999). This was reported as the mean (SD) by seven studies (Bender 1997; Berlauk 1991; Donati 2007; Gan 2002; Kapoor 2007; Pearse 2005; Shoemaker 1988), mean (range) by one study (Mythen 1995), mean (95% CI) by two studies (Pillai 2011; Venn 2002), mean (SEM) by one study (Valentine 1998) and median (range or interquartile range (IQR)) by 15 studies (Bonazzi 2002; Boyd 1993; Cecconi 2011; Challand 2012; Conway 2002; Jhanji 2010; Lobo 2000; Mayer 2010; Mckendry 2004; Noblett 2006; Pölönen 2000; Sandham 2003; Sinclair 1997; Van der Linden 2010; Wakeling 2005). We excluded one study from this analysis (Senagore 2009), for which we were unable to get further information. We obtained additional details for five studies (Jhanji 2010; Mythen 1995; Noblett 2006; Wakeling 2005; Wilson 1999). We used the statistical equation by Hozo 2005 to convert the median (range/IQR) to mean (SD). We estimated the SD as IQR/1.35, SEM × √(n) or 95% CI / 1.96. Four studies (Berlauk 1991; Jhanji 2010; Shoemaker 1988; Venn 2002) had two groups in either of the intervention or control groups and these were numerically combined using equation 7.7a in Higgins 2011. The intervention significantly reduced the postoperative length of hospital stay, mean 1.16 days (95% CI 0.43 to 1.89, P = 0.002). We used the random‐effects model as the I2 = 87% (Analysis 3.1).
3.1. Analysis.
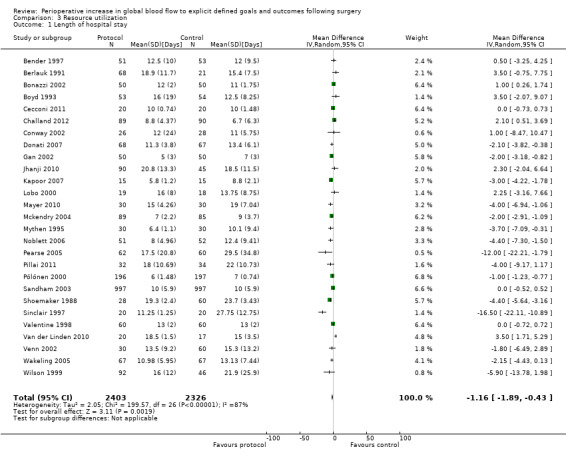
Comparison 3 Resource utilization, Outcome 1 Length of hospital stay.
3.2 Postoperative intensive care stay
Postoperative length of critical care stay was reported by 14 studies (Bender 1997; Berlauk 1991; Boyd 1993; Jerez 2001; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mythen 1995; Pearse 2005; Pölönen 2000; Shoemaker 1988; Valentine 1998; Wilson 1999). This was reported as the mean (SD) by six studies (Bender 1997; Berlauk 1991; Jerez 2001; Kapoor 2007; Mayer 2010; Shoemaker 1988), mean (range) by one study (Mythen 1995), mean (SEM) by one study (Valentine 1998) and median (range/IQR) by five studies (Boyd 1993; Jhanji 2010; Lobo 2000; Pearse 2005; Pölönen 2000). We were able to obtain additional information for three studies (Jhanji 2010; Mythen 1995; Wilson 1999). Numerical conversion to mean (SD) was performed according to the previous paragraph. There was no difference in postoperative length of critical care stay, mean difference of 0.45 days (95% CI ‐0.03 to 0.94, P = 0.06). We used the random‐effects model as the I2= 87% (Analysis 3.2).
3.2. Analysis.
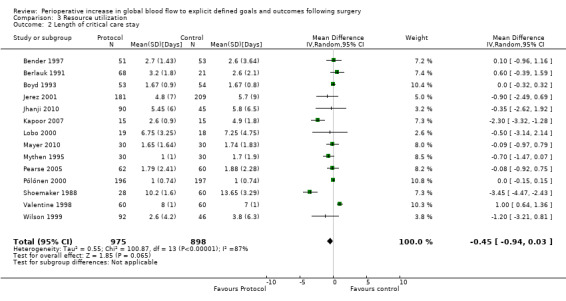
Comparison 3 Resource utilization, Outcome 2 Length of critical care stay.
Three studies (Bender 1997; Berlauk 1991; Shoemaker 1988) reported cost (USD), none of which found a statistical difference. Three other studies (Boyd 1993; Mythen 1995; Wilson 1999) reported cost in separate publications from the original report (two reported GBP (Guest 1997; Mythen 1994); one reported EUR (Fenwick 2002)). Two of these (Fenwick 2002; Guest 1997) reported that the intervention significantly reduced cost. The third (Mythen 1994) reported cost for a subgroup of patients included in the trial and these data were not analysed by treatment groups. Only one study reported means and SDs (Berlauk 1991) and only one study reported means and SEMs (Bender 1997) for cost data. In view of the variety of currencies and statistical descriptors we did not attempt to pool these data.
Subgroup mortality analyses
Timing of intervention
The intervention was commenced in the preoperative period in nine studies (Bender 1997; Berlauk 1991 ; Bonazzi 2002; Boyd 1993; Sandham 2003; Shoemaker 1988; Valentine 1998; Wilson 1999; Ziegler 1997), in the intraoperative period in 15 studies (Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Gan 2002; Lobo 2000; Mayer 2010; Mythen 1995; Noblett 2006; Pillai 2011; Senagore 2009; Sinclair 1997; Van der Linden 2010; Venn 2002; Wakeling 2005) and in the postoperative period in nine studies (Boyd 1993; Jerez 2001; Jhanji 2010; Kapoor 2007: Mckendry 2004; Pearse 2005; Pölönen 2000; Ueno 1998). In one study (Berlauk 1991) participants were randomized to two intervention groups (a preoperative and an intraoperative group) with a shared control group. In another study (Boyd 1993) the intervention was initiated either preoperatively or postoperatively depending on when the participants came to the attention of the investigators. There was no evidence that this had any effect on the chances of being recruited into the study and therefore we did not consider that this had potential to confound the randomization process. Further, one study (Lobo 2000) had both intraoperative and postoperative interventions. Timing of the intervention did not interact with mortality: preoperative, RR of 0.96 (95% CI 0.79 to 1.17, P = 0.69, I2 = 63%); intraoperative, RR of 0.67 (95% CI 0.40 to 1.13, P = 0.13, I2 = 0%); postoperative, RR of 0.73 (95% CI 0.50 to 1.06, P = 0.10, I2 = 0%) (Analysis 1.4).
1.4. Analysis.
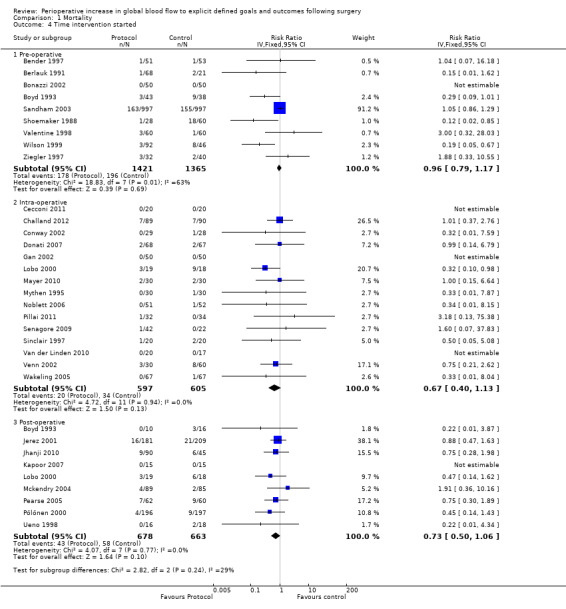
Comparison 1 Mortality, Outcome 4 Time intervention started.
Type of intervention
The intervention involved fluids alone in 10 studies (Challand 2012; Conway 2002; Gan 2002; Mythen 1995; Noblett 2006; Pillai 2011; Senagore 2009: Sinclair 1997; Venn 2002; Wakeling 2005) and fluids in combination with vasoactive drugs in 20 studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Donati 2007; Jerez 2001; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Pearse 2005; Pölönen 2000; Sandham 2003; Shoemaker 1988; Ueno 1998; Valentine 1998; Van der Linden 2010; Wilson 1999; Ziegler 1997). One study (Jhanji 2010) had two intervention groups; one group had fluid alone and the other had fluids and dopexamine. These groups were analysed separately. There was no difference in mortality between groups according to the intervention provided: fluids alone, RR of 0.80 (95% CI 0.46 to 1.39, P = 0.43, I2 = 0%); fluids in combination with vasoactive drugs, RR of 0.90 (95% CI 0.76 to 1.07, P = 0.23, I2 = 41%) (Analysis 1.5).
1.5. Analysis.
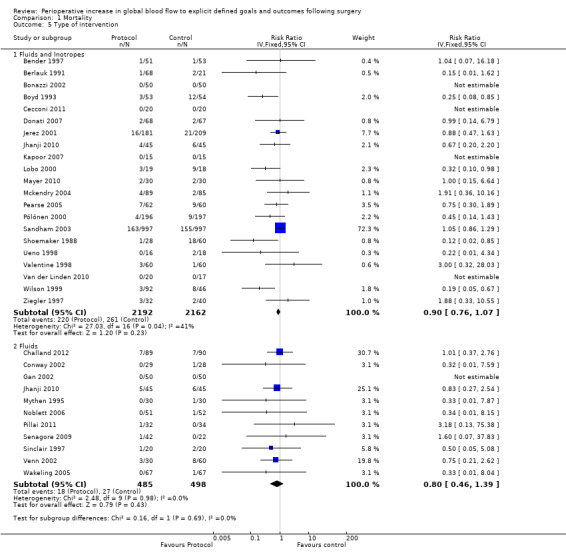
Comparison 1 Mortality, Outcome 5 Type of intervention.
Type of goal
Fourteen studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Kapoor 2007; Lobo 2000; Mayer 2010; Pearse 2005; Sandham 2003; Shoemaker 1988; Ueno 1998; Valentine 1998; Van der Linden 2010; Wilson 1999) used CO and oxygen transport goals; four studies (Donati 2007; Jerez 2001; Pölönen 2000; Ziegler 1997) used mixed venous oxygen saturation, oxygen extraction and lactate; and 13 studies (Cecconi 2011; Challand 2012; Conway 2002; Gan 2002; Jhanji 2010; Mckendry 2004; Mythen 1995; Noblett 2006; Pillai 2011; Senagore 2009; Sinclair 1997; Venn 2002; Wakeling 2005) used stroke volume (SV) goals. Mortality was not reduced for any of the three subgroups: CO and oxygen transport, RR of 0.91 (95% CI 0.75 to 1.09, P = 0.91, I2 = 58%); mixed venous oxygen saturations, oxygen extraction and lactate, RR of 0.83 (95% CI 0.50 to 1.38, P = 0.47, I2 = 0%); SV, RR of 0.84 (95% CI 0.51 to 1.41, P = 0.51, I2 = 0%) (Analysis 1.6).
1.6. Analysis.
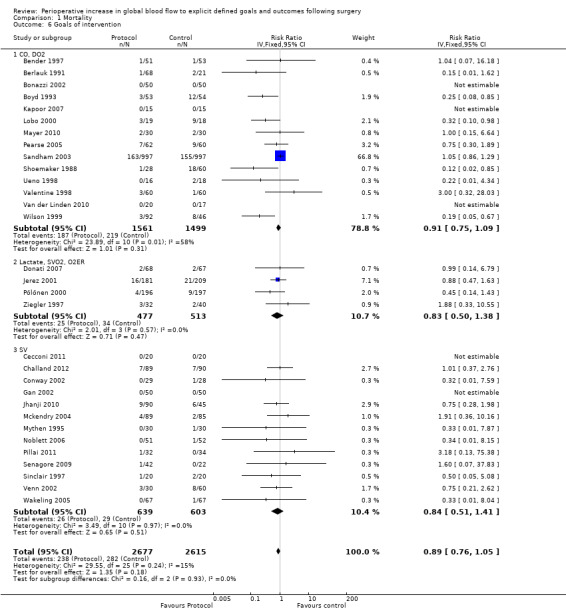
Comparison 1 Mortality, Outcome 6 Goals of intervention.
Mode of surgery
Twenty‐four studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Gan 2002; Jhanji 2010; Jerez 2001; Kapoor 2007; Lobo 2000; Mayer 2010; Mythen 1995; Noblett 2006; Pillai 2011; Pölönen 2000; Senagore 2009; Ueno 1998; Valentine 1998; Van der Linden 2010; Wakeling 2005; Wilson 1999; Ziegler 1997) recruited participants having only elective procedures; two studies were exclusively of urgent or emergency surgery (Sinclair 1997; Venn 2002) and five had a mix of urgent or emergency and elective operations (Boyd 1993; Mckendry 2004; Pearse 2005; Sandham 2003; Shoemaker 1988). None of the studies in this latter group were able to provide separate data to allow comparison between elective and urgent or emergency groups. Intervention significantly reduced the mortality of participants in RCTs of elective surgery, RR of 0.68 (95% CI 0.48 to 0.94, P = 0.02, I2 = 0%); mortality was unchanged for emergency or urgent operations, RR 0.of 68 (95% CI 0.23 to 2.06, P = 0.50, I2 = 0 %) (Analysis 1.7).
1.7. Analysis.
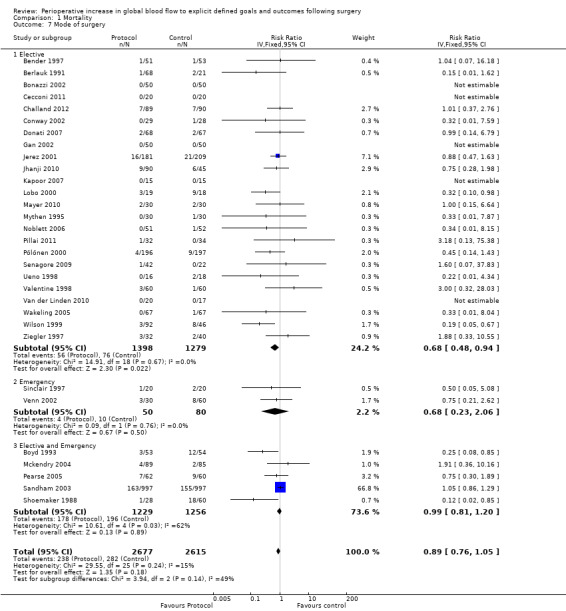
Comparison 1 Mortality, Outcome 7 Mode of surgery.
Type of surgery
Six studies (Bender 1997; Berlauk 1991; Bonazzi 2002; Valentine 1998; Van der Linden 2010; Ziegler 1997) were exclusively of participants undergoing vascular surgery. Five additional studies (Boyd 1993; Lobo 2000; Pearse 2005; Sandham 2003; Wilson 1999) included participants undergoing vascular surgery, but in only one of these were group‐specific mortality data available (Boyd 1993). Five studies were of patients undergoing cardiac surgery (Jerez 2001; Kapoor 2007; Mckendry 2004; Mythen 1995; Pölönen 2000). Fifteen studies were exclusively of patients undergoing general (non‐vascular, non‐cardiac) surgery (Cecconi 2011; Challand 2012; Conway 2002; Donati 2007; Gan 2002; Jhanji 2010; Mayer 2010; Noblett 2006; Senagore 2009; Shoemaker 1988; Sinclair 1997; Ueno 1998; Venn 2002; Wakeling 2005). Five additional studies included patients undergoing general surgery (Boyd 1993; Lobo 2000; Pearse 2005; Sandham 2003; Wilson 1999) but in only one of these were group‐specific mortality data available (Boyd 1993). There was no interaction between type of surgery and the intervention; vascular, RR of 0.78 (95% CI 0.34 to 1.79, P = 0.56, I2 = 18%); cardiac, RR of 0.81 (95% CI 0.48 to 1.35, P = 0.42, I2 = 0%); and general surgery, RR of 0.66 (95% CI 0.41 to 1.07, P = 0.09, I2 = 0%) (Analysis 1.8).
1.8. Analysis.
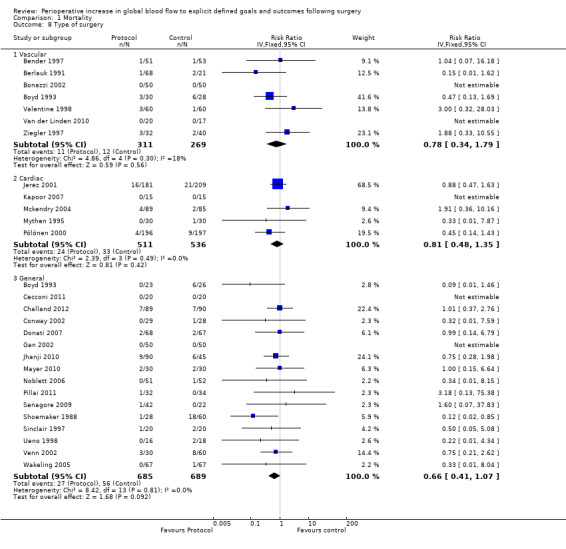
Comparison 1 Mortality, Outcome 8 Type of surgery.
Sensitivity analyses
We performed sensitivity analyses of the analysis method used to generate relative risks for mortality (Appendix 7; Appendix 8). The results were dependant upon both the analytical method and whether a random‐effects model or fixed‐effect model was used. There was no difference in mortality when small studies (fewer than 100 participants) were excluded (Analysis 1.3), consistent with Analysis 1.1. The effect of small studies was significant in the Harbord analysis, with a regression slope of ‐0.72 (95% CI ‐0.08 to ‐1.39). Participants were more likely to die in studies that recruited fewer than 100 participants, RR of 1.84 (95% CI 1.02 to 3.33, P = 0.04).
1.3. Analysis.
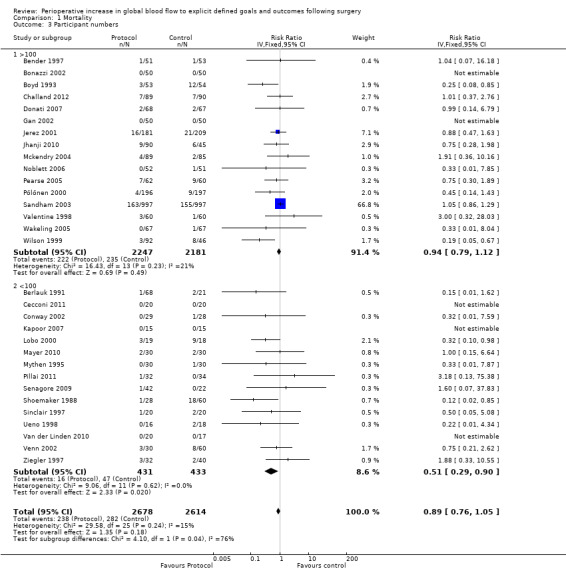
Comparison 1 Mortality, Outcome 3 Participant numbers.
In some studies the fluid and drug management in the control group was comparable with the intervention in other studies. For instance, four studies (Jerez 2001; Lobo 2000 ; Pölönen 2000; Ueno 1998) had fluid and inotropes administered in response to measures of blood flow (CI or DO2I) in the control groups. Shoemaker 1988 had one control group with DO2I driven measures. We performed a sensitivity analysis excluding these studies and the control group from Shoemaker 1988 (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7; Analysis 4.8; Analysis 4.9; Analysis 4.10; Analysis 4.11; Analysis 4.12). The findings were consistent with the primary analyses (Analysis 1.1; Analysis 1.2; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 3.1; Analysis 3.2).
4.1. Analysis.
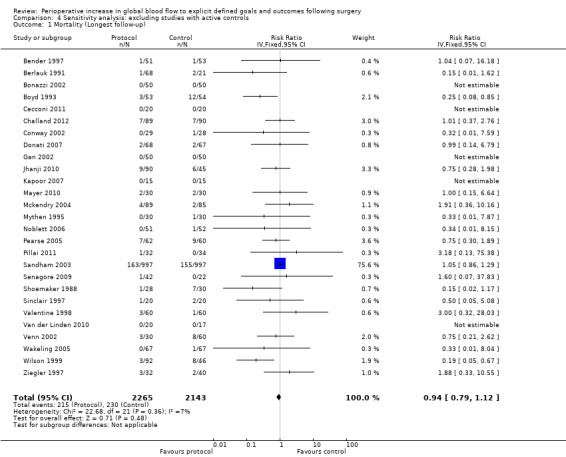
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 1 Mortality (Longest follow‐up).
4.2. Analysis.
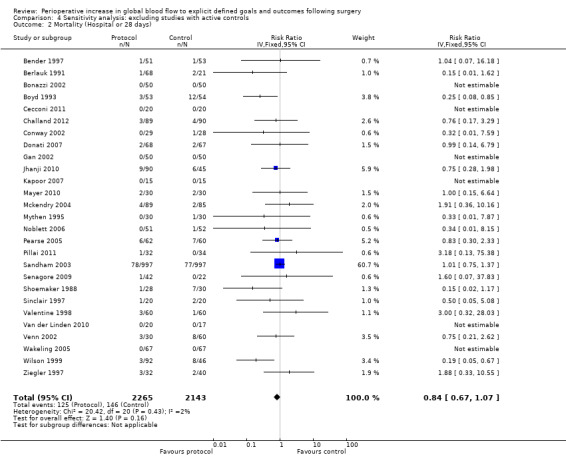
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 2 Mortality (Hospital or 28 days).
4.3. Analysis.
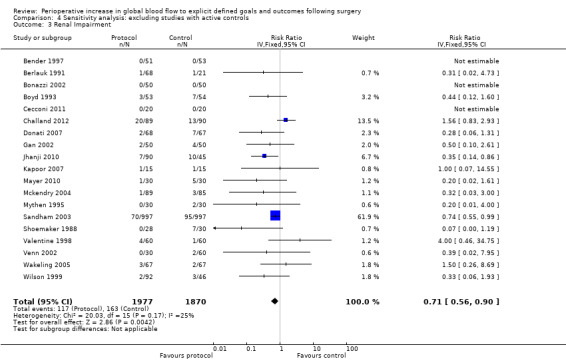
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 3 Renal Impairment.
4.4. Analysis.
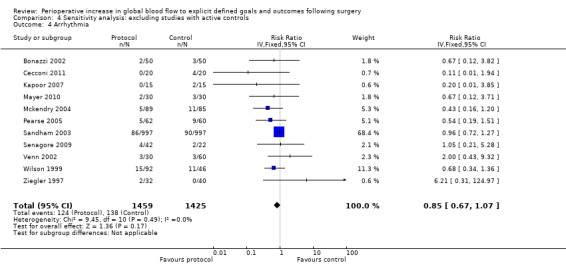
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 4 Arrhythmia.
4.5. Analysis.
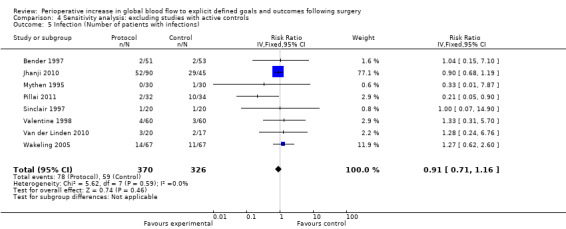
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 5 Infection (Number of patients with infections).
4.6. Analysis.
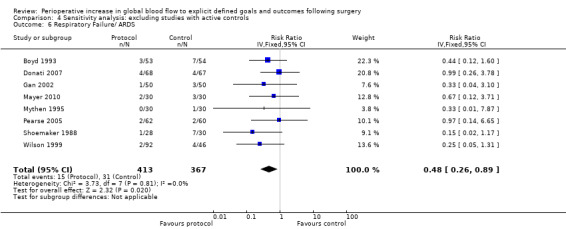
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 6 Respiratory Failure/ ARDS.
4.7. Analysis.
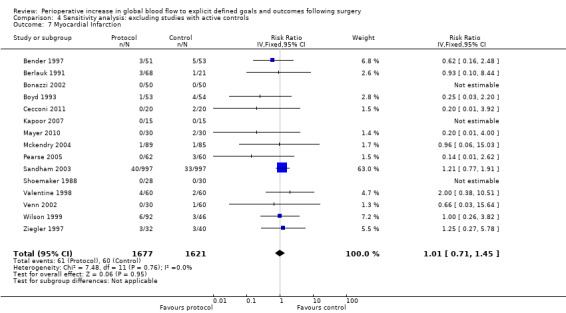
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 7 Myocardial Infarction.
4.8. Analysis.
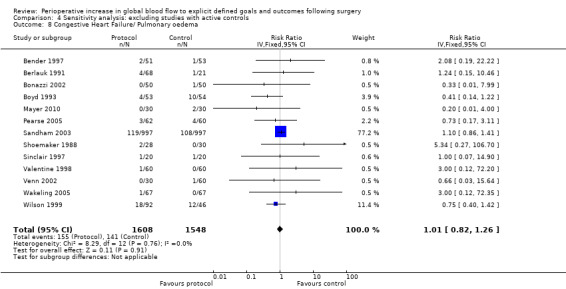
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 8 Congestive Heart Failure/ Pulmonary oedema.
4.9. Analysis.
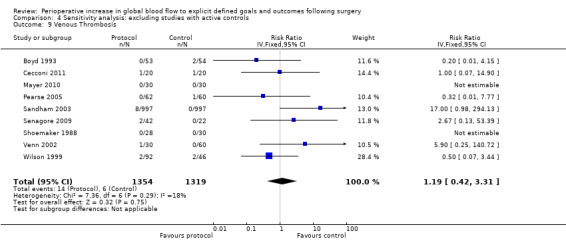
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 9 Venous Thrombosis.
4.10. Analysis.
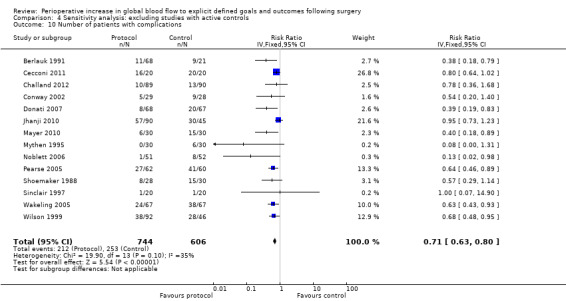
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 10 Number of patients with complications.
4.11. Analysis.
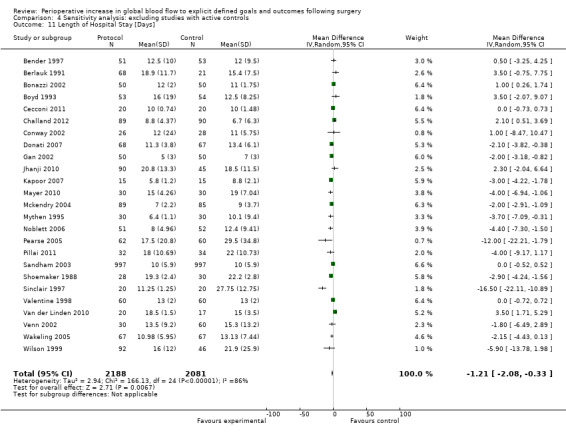
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 11 Length of Hospital Stay [Days].
4.12. Analysis.
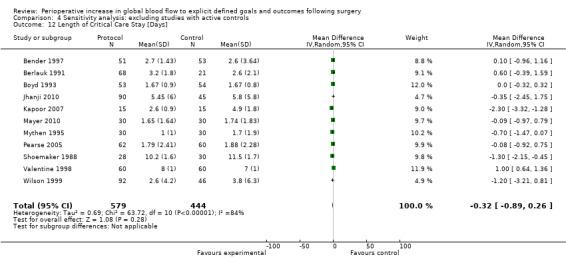
Comparison 4 Sensitivity analysis: excluding studies with active controls, Outcome 12 Length of Critical Care Stay [Days].
In some studies, fluid and inotrope administration were not the only systematic differences between the control and intervention groups. Five studies (Bender 1997; Bonazzi 2002; Sandham 2003; Valentine 1998; Wilson 1999) did not control for the insertion and presence of a pulmonary artery flow catheter. Three studies (Cecconi 2011; Kapoor 2007; Mayer 2010) did not control for the presence of other flow sensors (FloTrac or Vigileo) and one study (Venn 2002) did not control for the insertion or presence of an oesophageal doppler probe. In one study (Shoemaker 1988) one control group was not matched for the insertion and presence of a pulmonary artery flow catheter. We also performed sensitivity analyses excluding these studies for all outcome measures (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.9; Analysis 5.10; Analysis 5.11; Analysis 5.12). With these studies excluded the intervention reduced mortality (longest follow‐up), RR of 0.65 (95% CI 0.48 to 0.89, P = 0.007, I2 = 0%) (Analysis 5.1) and hospital or 28 day mortality, RR of 0.66 (95% CI 0.47 to 0.92, P = 0.01, I2 = 0%) (Analysis 5.2). The rates of renal failure and ARDS were no longer significantly different. The number of participants with complications and their length of hospital stay were not altered in this analysis, remaining significantly different between groups.
5.1. Analysis.
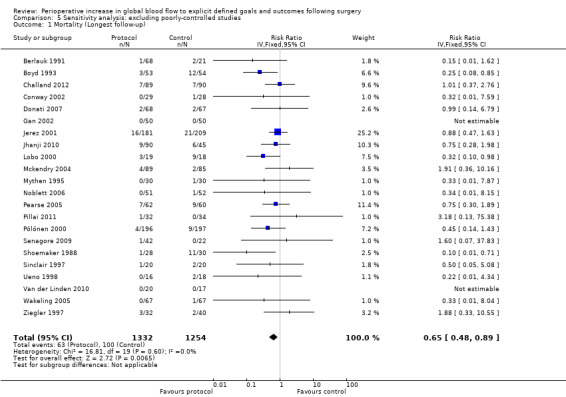
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 1 Mortality (Longest follow‐up).
5.2. Analysis.
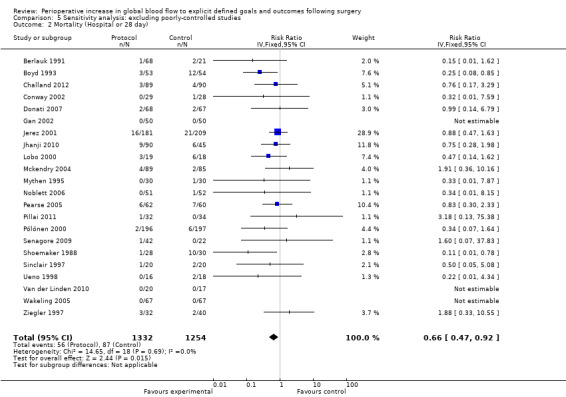
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 2 Mortality (Hospital or 28 day).
5.3. Analysis.
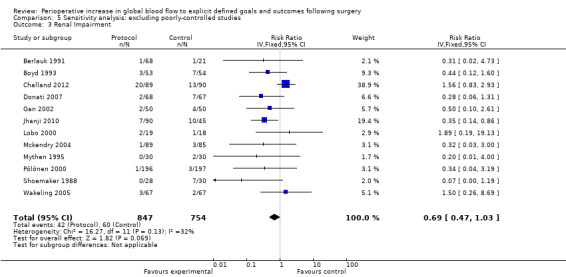
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 3 Renal Impairment.
5.4. Analysis.
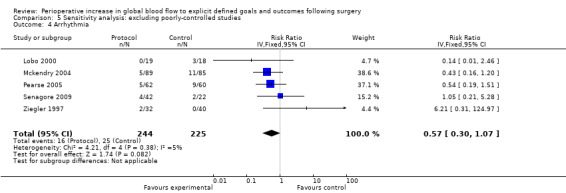
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 4 Arrhythmia.
5.5. Analysis.
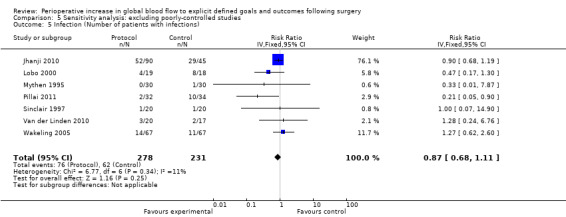
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 5 Infection (Number of patients with infections).
5.6. Analysis.
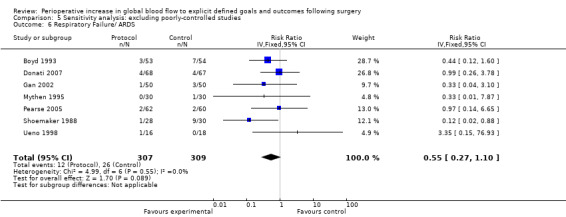
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 6 Respiratory Failure/ ARDS.
5.7. Analysis.
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 7 Myocardial Infarction.
5.8. Analysis.
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 8 Congestive Heart Failure/ Pulmonary oedema.
5.9. Analysis.
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 9 Venous Thrombosis.
5.10. Analysis.
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 10 Number of patients with complications.
5.11. Analysis.
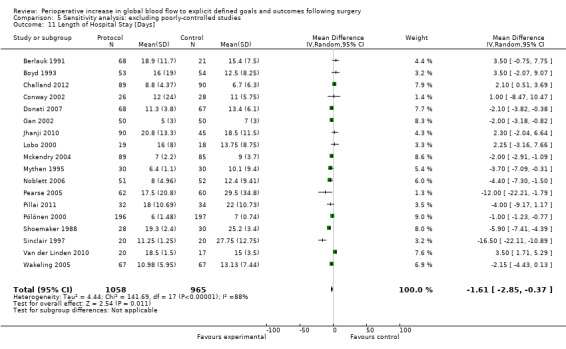
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 11 Length of Hospital Stay [Days].
5.12. Analysis.
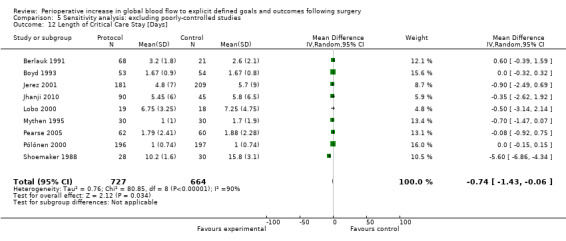
Comparison 5 Sensitivity analysis: excluding poorly‐controlled studies, Outcome 12 Length of Critical Care Stay [Days].
This meta‐analysis was dominated by one study (Sandham 2003). In this study a large number of participants were lost to follow‐up. We performed sensitivity analyses for the outcomes of mortality (longest follow‐up and hospital or 28 day mortality) excluding this study and assuming the possibility that all patients who were lost to follow‐up died. The results were not sensitive to these analyses (Analysis 6.1; Analysis 6.2).
6.1. Analysis.
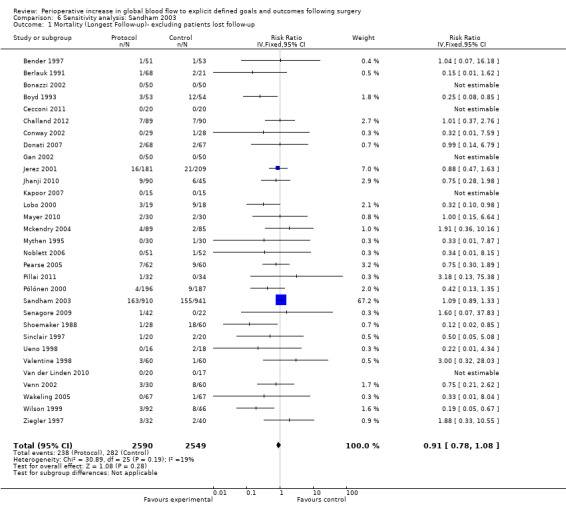
Comparison 6 Sensitivity analysis: Sandham 2003, Outcome 1 Mortality (Longest Follow‐up)‐ excluding patients lost follow‐up.
6.2. Analysis.
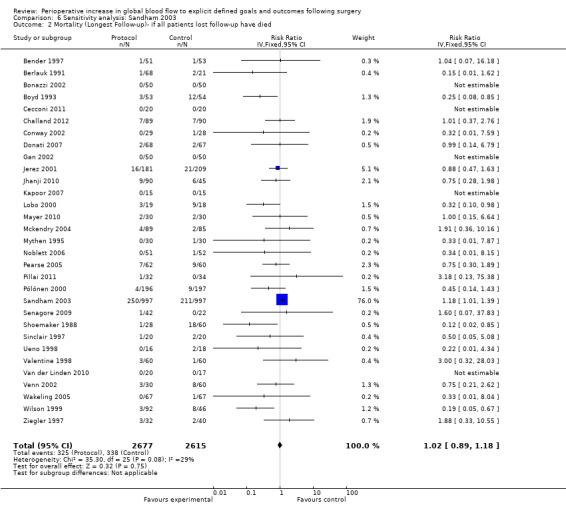
Comparison 6 Sensitivity analysis: Sandham 2003, Outcome 2 Mortality (Longest Follow‐up)‐ if all patients lost follow‐up have died.
Discussion
Summary of main results
The key finding of this review is that the perioperative administration of fluids, with or without vasoactive drugs, targeted to increase global blood flow defined by explicit measured goals reduced postoperative complications and length of stay but did not reduce mortality, using the inverse variance method. The exclusion of larger studies (> 100 participants) resulted in mortality being reduced by the intervention (RR 0.51, 95% CI 0.29 to 0.90, P = 0.02). Mortality was also significantly reduced when we used random‐effects models (Mantel‐Haenszel and inverse variance, RR 0.72, 95% CI 0.55 to 0.95, P = 0.02), but not fixed‐effect models (Mantel‐Haenszel RR 0.85, 95% CI 0.73 to 1.00, P = 0.05; inverse variance RR 0.89, 95% CI 0.76 to 1.05, P = 0.18; or Peto odds ratio 0.83, 95% CI 0.69 to 1.00, P = 0.05). We calculated similar results for hospital and 28 day mortality. When control group care was managed using a protocol that included explicit goals less than the intervention group (in contrast to 'usual care'), mortality was not reduced (longest follow‐up inverse variance RR 0.94, 95% CI 0.79 to 1.12, P = 0.45; hospital or 28 day inverse variance RR 0.84, 95% CI 0.67 to 1.07, P = 0.14). When studies with intervention groups that were less well controlled for the intervention (for example pulmonary artery catheters were not matched to intervention groups) were excluded, there was a significant reduction in mortality at the longest follow‐up (inverse variance RR 0.65, 95% CI 0.48 to 0.89, P = 0.007) and hospital or 28 day mortality (inverse variance RR 0.66, 95% CI 0.47 to 0.92, P = 0.01). It is notable that the sensitivity analyses are of limited value as they tend to reflect the inclusion or exclusion of the single largest study (Sandham 2003).
The limited data indicate that for every 100 patients exposed to treatment, one can expect 13 in 100 (from 40/100 to 27/100) to avoid a complication, 2/100 to avoid renal impairment (from 8/100 to 6/100), 5/100 to avoid respiratory failure (from 10/100 to 5/100), and 4/100 to avoid postoperative wound infection (from 10/100 to 6/100), with no effect on other types of morbidity (myocardial infarction, arrhythmia, congestive cardiac failure or pulmonary oedema, venous thrombosis, and the number of patients with infections). These results were unchanged following sensitivity analyses that excluded studies where the control group care was managed using a protocol that included explicit goals that were less than the intervention group (in contrast to 'usual care'). When studies using intervention groups that were less well controlled (control groups not matched to intervention groups) were excluded only the number of patients with complications was reduced, by 12/100 (from 36/100 to 24/10).
The hospital length of stay was reduced by about one day, from 12.4 to 11.2 days, and was not sensitive to exclusion of studies where the control group care was managed using a protocol that included explicit goals that were less than the intervention group (in contrast to 'usual care') or studies using intervention groups that were less well controlled. There was no difference in critical care stay in the intervention group. This was sensitive to exclusion of studies using intervention groups that were less well controlled and the reduction was less than a day (from 4 to 3.3 days). There were insufficient data to conduct a meta‐analysis of cost and no data available describing quality of life.
A stratified meta‐analysis to address secondary hypotheses, determined a priori, suggested that mortality was reduced in the intervention group when study participants underwent elective surgery.
The predefined analysis plan, using mortality from the longest available follow‐up, increased the weight attributed to the two largest studies that both reported one‐year follow‐up. Only one other study reported follow‐up beyond 60 days. In this group of studies a proportion of the operations were for cancer resection, therefore introducing a possible competing cause of mortality.
Overall completeness and applicability of evidence
Our systematic review pooled data from 31 studies with 5292 participants (Bender 1997; Berlauk 1991; Bonazzi 2002; Boyd 1993; Cecconi 2011; Challand 2012; Donati 2007: Conway 2002; Gan 2002; Jerez 2001; Jhanji 2010; Kapoor 2007; Lobo 2000; Mayer 2010; Mckendry 2004; Mythen 1995; Noblett 2006; Pearse 2005; Pillai 2011; Pölönen 2000; Sandham 2003; Senagore 2009; Shoemaker 1988; Sinclair 1997; Ueno 1998; Valentine 1998; Van der Linden 2010; Venn 2002; Wakeling 2005; Wilson 1999; Ziegler 1997). Study inclusion criteria were tightly defined and the meta‐analysis was rigorously conducted according to a predefined analysis plan addressing specific hypotheses. The meta‐analysis combined data from a group of predominantly underpowered single centre studies. However, the included studies reflect international practice, although the majority of included studies are from major teaching centres. The pooled studies included adults (age > 16 years) undergoing several types of surgery, including abdominal, urology, gynaecology, orthopaedic, cardiac, thoracic and vascular. Therefore, the included studies represent the population for whom the intervention might be considered.
Quality of the evidence
The quality of outcome data reporting in the included studies was variable. Mortality was reported over a variety of time frames and other outcomes were either limited or inconsistent between studies, precluding meaningful analyses in many cases. Diverse criteria and descriptions for morbidities, along with infrequent use of validated metrics, limited the precision of treatment effect estimates and the confidence that can be attached to them. Furthermore, pooling of different types of morbidity was inconsistent, limiting assessment of the overall 'morbidity load'.
Most studies tested a complex package of care (for example fluids, inotropes, monitor, goals, critical care environment) rather than a single clearly‐defined intervention. Heterogeneity in the components of such a complex intervention may contribute to study heterogeneity within a systematic review. Study heterogeneity may reduce the precision of treatment effect estimates and reduce the generalizability of the results of meta‐analyses (Louis 1991). By definition, it is not easy to define precisely the 'active ingredients' of a complex intervention (MRC 2000). However, hypothesis‐generating subgroup analyses indicated that there were insufficient data to distinguish statistically between many of the prespecified subgroups, and highlighted the limited quantity of data in some areas for example emergency surgery.
Several possible sources of bias arose in this meta‐analysis. The primary analysis was sensitive to the analytical methods used, the exclusion of larger studies, and the exclusion of studies that inadequately controlled for the intervention. Larger studies are less likely to be affected by bias (Kjaergard 2001) and the inclusion of lower quality studies can alter the interpretation of the benefit of interventions in meta‐analysis (Moher 1998).
Statistical heterogeneity was generally absent (I2 less than 40%). Except for some analyses such as hospital length of stay, there was evidence of significant statistical heterogeneity. We used random‐effects models in all cases where I2 exceeded 40%. In all analyses of mortality, the point estimate of effect was less than 0.90, suggesting that the intervention was probably not harmful.
The sensitivity of our results to the methods of analysis indicates that the results of this study are far from clear‐cut. Further research is essential in this area both to address the overall objective of this review and to focus on specific questions.
The studies included in this review are typical of studies in critical care research in general in that the majority of studies are underpowered and from single centres (Langham 2002) and about half the studies are small (< 100 participants). Future studies in this area should test an explicitly framed hypothesis, be adequately powered, methodologically rigorously and blinded (where possible). Reporting of outcomes should be standardized (to allow comparison between studies and to facilitate the conduct of future meta‐analyses) and inclusive (morbidity, health status, resource usage).
Potential biases in the review process
The possibility of publication bias cannot be excluded. We found no evidence of this from contact with experts and industry but some of the identified published abstracts have yet to be published as full peer‐reviewed papers. Harbord's regression test was significant at P = 0.03, suggesting small study effects. Language bias is possible because of the electronic databases and conferences we searched. Flaws in the original study designs are a significant potential source of bias. The meta‐analysis includes 5292 participants but the unit of analysis is the study (or study subgroup) and the sample size (31 studies) is relatively small. The results of the subgroup analyses should be considered as hypothesis‐generating only and are largely influenced by inclusion or exclusion of a single study (Sandham 2003).
Agreements and disagreements with other studies or reviews
This review represents the best up‐to‐date summary of the literature. We framed a tightly defined question and used explicit inclusion criteria for studies and a predefined analysis plan. Our primary result does not agree with previous reviews (Boyd 1999; Brienza 2009; Giglio 2009; Hamilton 2011; Heyland 1996; Ivanov 1997Kern 2002; Poeze 2005), which have been uniformly supportive of this intervention. This may be explained by the precision of the question we addressed (for example other reviews included trauma patients not having surgery) and the analytical methods used. The results of our systematic review do, however, agree with the results of the largest study in this area (Sandham 2003). However, it is of concern that the Sandham study dominates the review primary analysis both in terms of number of patients (1994/5292) and weight (67%), and that the Sandham study was one of the studies where the intervention group was less well controlled (control groups not matched to intervention groups).
Research should focus on answering these questions. Sandham et al showed that a large multicentre study can be conducted in this area and several such studies are currently ongoing, in particular a large multicentre study anticipated to recruit about 700 participants (Pearse 2009). Future research will hopefully disentangle the complex package of care that forms the intervention (for example fluids, inotropes or vasoactive agents, monitor, goals, critical care environment) and thereby identify which components are effective in different clinical contexts.
Authors' conclusions
Implications for practice.
Clinicians should base their decision whether to manipulate perioperative global blood flow on the magnitude of reductions in postoperative morbidities and length of hospital stay rather than upon the assumption that mortality will be reduced. For every 100 patients exposed to the intervention one can expect 13/100 to avoid having complications (from 40 to 27 per 100); 2/100 to avoid renal impairment (from 8 to 6 per 100); 5/100 to avoid respiratory failure (from 10 to 5 per 100); and 4/100 to avoid postoperative wound infection (from 10 to 6 per 100). Patients remain in hospital about one day less and there is no increase in harm. This intervention should be considered where the relevant resources are available and implementation will not otherwise harm the patient (for example delay in definitive care).
Implications for research.
A specific limitation of this review is the large number of studies that were published more than 10 years and the limited amount of data that represent current practice and outcomes. A specific group that particularly merits further study, in view of the high incidence of mortality and morbidity and limited available data, is patients undergoing emergency surgery.
Future studies in this area should test an explicitly framed hypothesis, be adequately powered (and preferably multicentre), methodologically rigorous, and include blinded interventions where possible. Reporting of outcomes should be standardized (to allow comparison between studies and to facilitate the conduct of future meta‐analyses) and inclusive (morbidity, health status, resource usage).
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2016 | Amended | New entry created in Published notes regarding status of Mayer 2010 study |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 11, 2012
| Date | Event | Description |
|---|---|---|
| 1 July 2013 | Amended | Journal version of review (Grocott 2013) cited in ‘Other published versions of this review’. |
| 25 October 2012 | Amended | Amendment to acknowledgment section: acknowledging University Hospital Southampton NHS Foundation Trust‐University of Southampton Respiratory Biomedical Research Unit and University College London Hospital–University College London Comprehensive Biomedical Research Centre. |
| 1 February 2006 | Amended | February 2006: The authors appealed against the original decision of the Cochrane Funders Arbiter. Their appeal was recently upheld by the Co‐chairs of the Steering Group and the decision of the funding arbiter over turned. The “Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery" will be republished in The Cochrane Library in issue 2, 2006. The authors are working on the draft review. |
| 1 February 2005 | Amended | February 2005: "Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery" was withdrawn from The Cochrane Library in issue 2, 2005 on the advice of the Cochrane Funders Arbiter. This was because the authors received funding from a commercial source (Elan Pharma) for the preparation of this review. This contravenes the current Cochrane policy on sponsorship. |
Notes
October 2016
This review includes the following study Mayer 2010.
Joachim Boldt is a co‐author of this study. Many publications by Joachim Boldt have been retracted or are being investigated; Mayer 2010 has not been retracted and future publication status is unknown. If the Mayer study is retracted in the future, then the study will be excluded in the updated version of this review.
Acknowledgements
We would like to thank John Carlisle (Content Editor) and Nathan Pace (Statistical Editor) for their significant input for the preparation of this review.
We would like to thank Karen Hovhannisyan (Trial Search Co‐ordinator) for performing the search for this review and Jane Cracknell (Managing Editor) for her patience, support and advice.
We acknowledge the advice and support provided by the late Professor David Bennett in conceiving and performing previous work that has formed the foundation for the present study.
We would like to thank Dr Martha Delgado, Dr Helen Higham and Prof Pierre Foex for their help and editorial advice during the preparation of the protocol for this systematic review. We would also like to thank the following.
Dr Jonathan Hyam, Dr Shahzad Shaefi and Dr Aanand Vibhakar (AV) for helping with literature searching and identification of potential studies.
The 'Optimisation Systematic Review' Steering Group for advice and guidance: Dr Richard Beale, Professor David Bennett, Dr Owen Boyd, Professor Mark Emberton, Dr Julia Langham, Professor Ian Roberts, Dr Jonathan Thompson.
Professor Stephen Senn (UCL, London) for statistical comments.
Dr Tony Brady for comments on an earlier draft of this paper.
Dr Lorenzo Grazioli for help with translation of Spanish text.
Some of this work was undertaken at the University Hospital Southampton NHS Foundation Trust‐University of Southampton Respiratory Biomedical Research Unit, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Units funding scheme. Some of this work was undertaken at University College London Hospital–University College London Comprehensive Biomedical Research Centre, which received a proportion of funding from the United Kingdom Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
Appendices
Appendix 1. Search strategy for CENTRAL , The Cochrane Library
#1 (Vasoactive or Fluid* or Drug Administration or fluid therapy or starch or gelatin* or crystalloid* or colloid* or splanchnic* or pulmonary artery flotation or catheter* or PAFC or Swan Ganz or Doppler):ti,ab #2 ((fluid* near (load* or administrat*)) or (perfusion near (renal or tissue))) #3 base near (acid or excess or deficit) #4 Venous near (Oxygen Saturation) #5 ((Stroke Volume Index) or (Oxygen Consumption Index)):ti,ab #6 (oxygen near (delivery or consumption or saturation)):ti,ab #7 (cardiac near (output or index)):ti,ab #8 (lactat* or CVP or pHi or PCO2 or SvO2 or VO2 or DO2 or Tonometry):ti,ab #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 ((surg* or operat*) near (general or high?risk or vascular or cardiac or cancer or trauma* or emergency or orthopaed*)):ti,ab #11 (peri?operativ* or post?operativ* or intra?operativ* or optimi?ation or goal?directed or supra?normal or aneurysm):ti,ab #12 (#10 OR #11) #13 (#9 AND #12)
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. Fluid‐Therapy/ or Body‐Fluids/ or Catheterization‐Swan‐Ganz/ or Catheterization/ or Heart‐Catheterization/ 2. (Vasoactive or Fluid* or Drug Administration or fluid therapy or starch or gelatin* or crystalloid* or colloid* or splanchnic* or pulmonary artery flotation or catheter* or PAFC or Swan Ganz or Doppler).ti,ab. 3. ((fluid* adj3 (load* or administrat*)) or (perfusion adj3 (renal or tissue))).mp. 4. Blood‐Volume/ or Oxygen‐Consumption/ or Central‐Venous‐Pressure/ or Stroke‐Volume/ or Cardiac‐Output/ or Echocardiography/ or Echocardiography‐Doppler/ 5. ((base adj3 (acid or excess or deficit)) or ((Venous adj3 Oxygen Saturation) or Stroke Volume Index or Oxygen Consumption Index)).mp. or ((oxygen adj3 (delivery or consumption or saturation)) or (cardiac adj3 (output or index)) or lactat* or CVP or pHi or PCO2 or SvO2 or VO2 or DO2 or Tonometry).ti,ab. 6. 1 or 2 or 3 or 4 or 5 7. Perioperative‐Care/ or Intraoperative‐Period/ or Postoperative‐Period/ or Aneurysm/ or Vascular‐Surgical‐Procedures/ or Thoracic‐Surgery/ or Emergency‐Treatment/ or Specialties‐Surgical/ or Orthopedics/ or Surgical‐Procedures‐Operative/ 8. ((surg* or operat*) adj3 (general or high?risk or vascular or cardiac or cancer or trauma* or emergency or orthopaed*)).ti,ab. 9. (peri?operativ* or post?operativ* or intra?operativ* or optimi?ation or goal?directed or supra?normal or aneurysm).ti,ab. 10. 8 or 7 or 9 11. 6 and 10 12. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 13. 11 and 12
Appendix 3. Search strategy for EMBASE (OvidSP)
1. fluid therapy/ or body fluid/ or Swan Ganz catheter/ or heart catheterization/ or blood volume/ or oxygen consumption/ or central venous pressure/ or heart stroke volume/ or heart output/ or Doppler echocardiography/ or echocardiography/ 2. (Vasoactive or Fluid* or Drug Administration or fluid therapy or starch or gelatin* or crystalloid* or colloid* or splanchnic* or pulmonary artery flotation or catheter* or PAFC or Swan Ganz or Doppler).ti,ab. or ((fluid* adj3 (load* or administrat*)) or (perfusion adj3 (renal or tissue))).mp. 3. ((base adj3 (acid or excess or deficit)) or ((Venous adj3 Oxygen Saturation) or Stroke Volume Index or Oxygen Consumption Index)).mp. or ((oxygen adj3 (delivery or consumption or saturation)) or (cardiac adj3 (output or index)) or lactate* or CVP or pHi or PCO2 or SvO2 or VO2 or DO2 or Tonometry).ti,ab. 4. 1 or 2 or 3 5. perioperative period/ or postoperative period/ or aneurysm/ or vascular surgery/ or thorax surgery/ or emergency treatment/ or orthopedics/ or surgery/ 6. ((surg* or operat*) adj3 (general or high?risk or vascular or cardiac or cancer or trauma* or emergency or orthopaed*)).ti,ab. 7. (peri?operativ* or post?operativ* or intra?operativ* or optimi?ation or goal?directed or supra?normal or aneurysm).ti,ab. 8. 6 or 7 or 5 9. 8 and 4 10. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 11. 10 and 9
Appendix 4. Keywords used in search strategy
We used the following topic specific key words :
Population:high‐risk surgery, peri‐operative, pre‐operative, post‐operative, intra‐operative,aneurysm, vascular surgery, cardiac surgery, cancer surgery, trauma surgery, emergency surgery, orthopaedic surgery
Intervention: Optimisation, optimization, goal‐directed, supra‐normal, Vasoactive, fluids,starch, gelatin, blood product, crystalloid, colloid, fluid therapy,fluid loading, fluid administration, body fluid
Comparison: Oxygen delivery, lactate, acid base, oxygen consumption, base excess, base deficit, blood volume,central venous pressure, CVP, cardiac output, cardiac index, pulmonary artery flotation catheter, PAFC, right‐heart catheter, Swan Ganz, Doppler, pHi, tonometry, PCO2 gap, echocardiography, stroke volume, SvO2, mixed venous oxygen saturation.
Outcomes: Splanchnic, renal perfusion, tissue perfusion, blood flow.
Appendix 5. Included studies
| Study and Year of Publication | Country | Journal |
| Bender 1997 | USA | Annals of Surgery |
| Berlauk 1991 | USA | Annals of Surgery |
| Bonazzi 2002 | Italy | Eurpoean Journal of Vascular Surgery |
| Boyd 1993 | UK | JAMA |
| Cecconi 2011 | Italy | Critical Care |
| Challand 2012 | UK | British Journal of Anaesthesia |
| Conway 2002 | UK | Anaesthesia |
| Donati 2007 | Italy | Chest |
| Gan 2002 | USA | Anesthesiology |
| Jerez 2001 | Spain | Medicina Intensiva |
| Jhanji 2010 | UK | Critical Care |
| Kapoor 2007 | India | Annals of Cardiac Anaesthesia |
| Lobo 2000 | Brazil | Critical Care Medicine |
| Mayer 2010 | Germany | Critical Care |
| Mckendry 2004 | UK | BMJ |
| Mythen 1995 | UK | Archives of Surgery |
| Noblett 2006 | UK | The British Journal of Surgery |
| Pearse 2005 | UK | Critical Care |
| Pillai 2011 | UK | The Journal of Urology |
| Pölönen 2000 | Finland | Anesthesia and Analgesia |
| Sandham 2003 | Canada | New England Journal of Medicine |
| Senagore 2009 | USA | Diseases of Colon and Rectum |
| Shoemaker 1988 | USA | Chest |
| Sinclair 1997 | UK | BMJ |
| Ueno 1998 | Japan | Surgery |
| Valentine 1998 | USA | Journal of Vascular Surgery |
| Van der Linden 2010 | Belgium | European Journal of Anaesthesiology |
| Venn 2002 | UK | British Journal of Anaesthesia |
| Wakeling 2005 | UK | British Journal of Anaesthesia |
| Wilson 1999 | UK | BMJ |
| Ziegler 1997 | USA | Surgery |
Footnotes
Appendix 6. Characteristics of eligible studies
| Study | Design | Study Population | Intervention | |||
| Patients | Modee of surgery | Type of surgery | Timing | Fluids +/‐ Inotropes | Goals | |
| Bender 1997 | 104 | elective | vascular | pre | fluids and inotropes | CI |
| Berlauk 1991 | 89 | elective | vascular | pre | fluids and inotropes | CI |
| Bonazzi 2002 | 100 | elective | vascular | pre | fluids and inotropes | CI, DO2I |
| Boyd 1993 | 107 | elective, emergency | general, vascular | pre, post | fluids and inotropes | DO2I |
| Cecconi 2011 | 40 | elective | orthopaedic | intra | fluids and inotropes | SV |
| Challand 2012 | 179 | elective | gastrointestinal | intra | fluids | SV |
| Conway 2002 | 57 | elective | general | intra | fluids | SV,FTc |
| Donati 2007 | 135 | elective | major abdominal surgery | intra | fluids and inotropes | 02ER |
| Gan 2002 | 100 | elective | general | intra | fluids | SV, FTc |
| Jerez 2001 | 390 | elective | cardiac | post | fluids and inotropes | SVO2, CI |
| Jhanji 2010 | 135 | elective | gastrointestinal surgery | post | fluids and inotropes | SV |
| Kapoor 2007 | 30 | elective | cardiac | post | fluids and inotropes | CI, SVV |
| Lobo 2000 | 37 | elective | general, vascular | pre | fluids and inotropes | DO2I |
| Mayer 2010 | 60 | elective | major abdominal surgery | intra | fluids and inotropes | CI, SV |
| Mckendry 2004 | 174 | elective, emergency | cardiac | post | fluids and inotropes | SVI |
| Mythen 1995 | 60 | elective | cardiac | intra | fluids | SV |
| Noblett 2006 | 103 | elective | general | intra | fluids | SV, FTc |
| Pearse 2005 | 122 | elective, emergency | vascular, general, urology | post | fluids and inotropes | DO2I |
| Pillai 2011 | 66 | elective | urology | intra | fluids | SV, FTc |
| Pölönen 2000 | 393 | elective | cardiac | post | fluids and inotropes | SvO2, lactate |
| Sandham 2003 | 1994 | elective, emergency | general, vascular, thoracic, hip fracture | pre | fluids and inotropes | DO2I, CI |
| Senagore 2009 | 64 | elective | Laparoscopic colectomy | intra | fluids | SV |
| Shoemaker 1988 | 58 | elective, emergency | general, vascular | pre | fluids and inotropes | CI, DO2I, VO2I |
| Sinclair 1997 | 40 | emergency | hip fracture | intra | fluids | SV, FTc |
| Ueno 1998 | 34 | elective | liver | post | fluids and inotropes | CI, DO2I, VO2I |
| Valentine 1998 | 120 | elective | vascular | pre | fluids and inotropes | CI |
| Van der Linden 2010 | 57 | elective | vascular | intra | fluids and inotropes | CI |
| Venn 2002 | 59 | emergency | hip fracture | intra | fluids | SV, FTc |
| Wakeling 2005 | 134 | elective | general | intra | fluids | SV |
| Wilson 1999 | 138 | elective | general, vascular | pre | fluids and inotropes | DO2I |
| Ziegler 1997 | 72 | elective | vascular | pre | fluids and inotropes | SvO2 |
Footnotes
Appendix 7. Sensitivity analysis using analytical methods for the primary outcome (mortality for the longest follow‐up)
| Analytical method | Results |
| Inverse variance Relative Risk Fixed‐effect model | 0.89 (95% CI 0.76 to 1.05), P = 0.18, I2 = 15% |
| Inverse variance Relative Risk Random‐effects model | 0.72 (95% CI 0.55 to 0.95), P = 0.02, I2 = 15% |
| Inverse variance Odds Ratio Fixed‐effects model | 0.87 (95% CI 0.72 to 1.05), P = 0.14, I2 = 20% |
| Inverse variance Odd's Ratio Random‐effects model | 0.67 (95% CI 0.49 to 0.92), P = 0.01, I2 = 20% |
| Peto Odd's Ratio | 0.83 (95% CI 0.69 to 1.00), P = 0.05, I2 = 37% |
| MH Odd's ratio Fixed‐effect model | 0.83 (95% CI 0.69 to 1.00), P = 0.05, I2 = 21% |
| MH Odd's ratio Random‐effects model | 0.67 (95% CI 0.49 to 0.92), P = 0.01, I2 = 21% |
| MH Relative Risk Fixed‐effect model | 0.85 (95% CI 0.73 to 1.00), P = 0.05, I2 = 16% |
| MH Relative Risk Random‐effects model | 0.72 (95% CI 0.55 to 0.95), P = 0.02, I2 = 16% |
Appendix 8. Sensitivity analysis using analytical methods for hospital or 28 day mortality
| Analytical method | Results |
| Inverse variance Relative Risk Fixed‐effect model | 0.81 (95% CI 0.65 to 1.00), P = 0.06, I2 = 1% |
| Inverse variance Relative Risk Random‐effects model | 0.79 (95% CI 0.63 to 0.99), P = 0.04, I2 = 1% |
| Inverse variance Odds Ratio Fixed‐effect model | 0.79 (95% CI 0.63 to 1.00), P = 0.05, I2 = 7% |
| Inverse variance Odd's Ratio Random‐effects model | 0.72 (95% CI 0.54 to 0.96), P = 0.02, I2 = 7% |
| Peto Odd's Ratio | 0.75 (95% CI 0.60 to 0.94), P = 0.01, I2 = 26% |
| MH Odd's ratio Fixed‐effect model | 0.76 (95% CI 0.60 to 0.95), P = 0.01, I2 = 8% |
| MH Odd's ratio Random‐effects model | 0.72 (95% CI 0.54 to 0.96), P = 0.02, I2 = 8% |
| MH Relative Risk Fixed‐effect model | 0.77 (95% CI 0.63 to 0.95), P = 0.01, I2 = 2% |
| MH Relative Risk Random‐effects model | 0.78 (95% CI 0.62 to 0.99), P = 0.04, I2 = 2% |
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies (longest follow‐up) | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 2 All studies (hospital or 28 day) | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 0.81 [0.65, 1.00] |
| 3 Participant numbers | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 3.1 >100 | 16 | 4428 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 3.2 <100 | 15 | 864 | Risk Ratio (IV, Fixed, 95% CI) | 0.51 [0.29, 0.90] |
| 4 Time intervention started | 31 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Pre‐operative | 9 | 2786 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.79, 1.17] |
| 4.2 Intra‐operative | 15 | 1202 | Risk Ratio (IV, Fixed, 95% CI) | 0.67 [0.40, 1.13] |
| 4.3 Post‐operative | 9 | 1341 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.50, 1.06] |
| 5 Type of intervention | 31 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Fluids and Inotropes | 21 | 4354 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.76, 1.07] |
| 5.2 Fluids | 11 | 983 | Risk Ratio (IV, Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 6 Goals of intervention | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 6.1 CO, DO2 | 14 | 3060 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 6.2 Lactate, SVO2, O2ER | 4 | 990 | Risk Ratio (IV, Fixed, 95% CI) | 0.83 [0.50, 1.38] |
| 6.3 SV | 13 | 1242 | Risk Ratio (IV, Fixed, 95% CI) | 0.84 [0.51, 1.41] |
| 7 Mode of surgery | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 0.89 [0.76, 1.05] |
| 7.1 Elective | 24 | 2677 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.48, 0.94] |
| 7.2 Emergency | 2 | 130 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.23, 2.06] |
| 7.3 Elective and Emergency | 5 | 2485 | Risk Ratio (IV, Fixed, 95% CI) | 0.99 [0.81, 1.20] |
| 8 Type of surgery | 27 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Vascular | 7 | 580 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.34, 1.79] |
| 8.2 Cardiac | 5 | 1047 | Risk Ratio (IV, Fixed, 95% CI) | 0.81 [0.48, 1.35] |
| 8.3 General | 16 | 1374 | Risk Ratio (IV, Fixed, 95% CI) | 0.66 [0.41, 1.07] |
Comparison 2. Complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Renal impairment | 21 | 4307 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.57, 0.90] |
| 2 Arrhythmia | 12 | 2921 | Risk Ratio (IV, Fixed, 95% CI) | 0.84 [0.67, 1.06] |
| 3 Infection: numbers | 9 | 733 | Risk Ratio (IV, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 4 Infections: types | 16 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Chest Infections/ Pneumonia | 13 | 2945 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 4.2 Sepsis | 5 | 474 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.26, 1.77] |
| 4.3 Abdominal Infections | 6 | 555 | Risk Ratio (IV, Fixed, 95% CI) | 0.53 [0.23, 1.22] |
| 4.4 Wound Infections | 10 | 2802 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.50, 0.84] |
| 4.5 Urinary Tract Infections | 8 | 612 | Risk Ratio (IV, Fixed, 95% CI) | 0.54 [0.26, 1.15] |
| 5 Respiratory failure / ARDS | 9 | 844 | Risk Ratio (IV, Fixed, 95% CI) | 0.51 [0.28, 0.93] |
| 6 Myocardial infarction | 15 | 3328 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.71, 1.45] |
| 7 Congestive heart failure / pulmonary oedema | 14 | 3223 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 8 Venous thrombosis | 10 | 2740 | Risk Ratio (IV, Fixed, 95% CI) | 1.04 [0.39, 2.77] |
| 9 Number of participants with complications | 17 | 1841 | Risk Ratio (IV, Random, 95% CI) | 0.68 [0.58, 0.80] |
Comparison 3. Resource utilization.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Length of hospital stay | 27 | 4729 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐1.89, ‐0.43] |
| 2 Length of critical care stay | 14 | 1873 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.94, 0.03] |
Comparison 4. Sensitivity analysis: excluding studies with active controls.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (Longest follow‐up) | 27 | 4408 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 2 Mortality (Hospital or 28 days) | 27 | 4408 | Risk Ratio (IV, Fixed, 95% CI) | 0.84 [0.67, 1.07] |
| 3 Renal Impairment | 19 | 3847 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.56, 0.90] |
| 4 Arrhythmia | 11 | 2884 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.67, 1.07] |
| 5 Infection (Number of patients with infections) | 8 | 696 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.71, 1.16] |
| 6 Respiratory Failure/ ARDS | 8 | 780 | Risk Ratio (IV, Fixed, 95% CI) | 0.48 [0.26, 0.89] |
| 7 Myocardial Infarction | 15 | 3298 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.71, 1.45] |
| 8 Congestive Heart Failure/ Pulmonary oedema | 13 | 3156 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.82, 1.26] |
| 9 Venous Thrombosis | 9 | 2673 | Risk Ratio (IV, Fixed, 95% CI) | 1.19 [0.42, 3.31] |
| 10 Number of patients with complications | 14 | 1350 | Risk Ratio (IV, Fixed, 95% CI) | 0.71 [0.63, 0.80] |
| 11 Length of Hospital Stay [Days] | 25 | 4269 | Mean Difference (IV, Random, 95% CI) | ‐1.21 [‐2.08, ‐0.33] |
| 12 Length of Critical Care Stay [Days] | 11 | 1023 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.89, 0.26] |
Comparison 5. Sensitivity analysis: excluding poorly‐controlled studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (Longest follow‐up) | 22 | 2586 | Risk Ratio (IV, Fixed, 95% CI) | 0.65 [0.48, 0.89] |
| 2 Mortality (Hospital or 28 day) | 22 | 2586 | Risk Ratio (IV, Fixed, 95% CI) | 0.66 [0.47, 0.92] |
| 3 Renal Impairment | 12 | 1601 | Risk Ratio (IV, Fixed, 95% CI) | 0.69 [0.47, 1.03] |
| 4 Arrhythmia | 5 | 469 | Risk Ratio (IV, Fixed, 95% CI) | 0.57 [0.30, 1.07] |
| 5 Infection (Number of patients with infections) | 7 | 509 | Risk Ratio (IV, Fixed, 95% CI) | 0.87 [0.68, 1.11] |
| 6 Respiratory Failure/ ARDS | 7 | 616 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.27, 1.10] |
| 7 Myocardial Infarction | 6 | 622 | Risk Ratio (IV, Fixed, 95% CI) | 0.66 [0.25, 1.73] |
| 8 Congestive Heart Failure/ Pulmonary oedema | 7 | 587 | Risk Ratio (IV, Fixed, 95% CI) | 0.64 [0.33, 1.26] |
| 9 Venous Thrombosis | 5 | 388 | Risk Ratio (IV, Fixed, 95% CI) | 0.50 [0.11, 2.32] |
| 10 Number of patients with complications | 14 | 1573 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.53, 0.81] |
| 11 Length of Hospital Stay [Days] | 18 | 2023 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐2.85, ‐0.37] |
| 12 Length of Critical Care Stay [Days] | 9 | 1391 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.43, ‐0.06] |
Comparison 6. Sensitivity analysis: Sandham 2003.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (Longest Follow‐up)‐ excluding patients lost follow‐up | 31 | 5139 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.78, 1.08] |
| 2 Mortality (Longest Follow‐up)‐ if all patients lost follow‐up have died | 31 | 5292 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bender 1997.
| Methods | RCT Single centre |
|
| Participants | 104 patients Elective surgery Vascular surgery |
|
| Interventions | Two groups Preoperative Fluids and Inotropes Pulmonary artery catheter Goals = CI |
|
| Outcomes | Hospital mortality Hospistal length of stay (HLOS) ICU length of stay (ICULOS) Cost Pulmonary oedema Acute myocardial infarction Arrhythmia Acute renal failure Wound infection Haemorrhage Sepsis Graft thrombosis or infection Groin haematoma |
|
| Notes | Number of arrhythmia episodes were reported in both intra and postoperative periods, but not clear it occurred in same patients or different patients. Number of patients with complications only reported in postoperative period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were "assigned randomly to one of two groups by the surgical intensivist" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data are reported |
| Selective reporting (reporting bias) | Low risk | Published report included main outcome measures |
| Other bias | Low risk | No other specific bias identified |
Berlauk 1991.
| Methods | RCT Single centre |
|
| Participants | 89 patients Elective surgery Vascular surgery |
|
| Interventions | 3 groups Preoperative Fluids and inotropes Pulmonary artery catheter Goals = CI |
|
| Outcomes | Hospital mortality HLOS ICULOS Cost Acute renal failure Congestive cardiac failure Graft thrombosis Acute myocardial infarction Arrhythmia |
|
| Notes | 3 Groups, where group‐1 had PA catheter driven protocol initiated in surgical intensive care unit (SICU) 12 hours before surgery, group‐2 had PA catheter driven protocol initiated 3 hours before surgery and group‐3 control group without protocol. Arrhythmia episodes were reported, not clear of denominator. The analysis is combined for groups 1 and 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization for all study groups was generated by a random number generator (Statworks)" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Report included all expected outcomes |
| Other bias | Unclear risk | Two patients were excluded from group 1 due to not having surgery. This may have induced selection bias. |
Bonazzi 2002.
| Methods | RCT Single centre |
|
| Participants | 100 patients Elective surgery Vascular surgery |
|
| Interventions | 2 groups Preoperative Fluids and inotropes Pulmonary artery catheter Goals = CI, DO2I |
|
| Outcomes | Hospital mortality HLOS Arrythmias Myocardial infarction Congestive heart failure Renal failure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated random number was obtained by phone call to the statistical centre of the hospital" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all expected outcomes |
| Other bias | Low risk | No other sources of bias identified |
Boyd 1993.
| Methods | RCT Single centre |
|
| Participants | 107 patients 2 groups Elective and emergency surgery General surgery Vascular surgery |
|
| Interventions | Preoperative and postoperative Fluids and inotropes Pulmonary artery catheter Goals = DO2I |
|
| Outcomes | 28 day mortality HLOS ICULOS (Cost reported separately) Respiratory failure, acute renal failure, sepsis, cardiorespiratory arrest, pulmonary oedema, pleural fluid, wound infection, disseminated intravascular coagulation, acute myocardial infarction, abdominal abscess, haemorrhage, gastric outlet obstruction, cerebrovascular accident, pulmonary embolism, chest infection, psychosis, distal ischaemias |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to a protocol or control limb of the study". No information provided regarding random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not presented in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Neither the anaesthesiologist nor the surgeon was aware of a patient's allocation". However its not clear if the investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not sure, see quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Unclear risk | The study was funded by a pharmaceutical company |
Cecconi 2011.
| Methods | RCT Single centre |
|
| Participants | 2 groups 40 patients Elective Total hip arthroplasty under regional anaesthesia |
|
| Interventions | Intraoperative Fluids and inotropes FloTrac sensor/Vigileo Goal = SV, DO2I |
|
| Outcomes | Mortality Complications Renal failure Arrhythmias Infections Anaemia Pulmonary embolism |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized with concealed envelope technique" |
| Allocation concealment (selection bias) | Low risk | Concealed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients enrolled and the clinical teams caring for the patients were blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Low risk | No other bias identified |
Challand 2012.
| Methods | RCT Single centre |
|
| Participants | 2 Groups 179 patients Elective Major colorectal surgery |
|
| Interventions | Intraoperative Fluids Oesophageal doppler Goal = SV |
|
| Outcomes | Surgical readiness for discharge Total postop days flatus passed, bowel movement, tolerance of food Any deviation from postoperative course Serious postop complications Critical care admission Readmission <30 days Mortality |
|
| Notes | Patients were initially screened with CPET and was classified into two groups (aerobically fit and unfit as defined by anaerobic threshold) with subsequent randomization into control and intervention arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by "random block allocation using sequentially numbered opaque sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | As per quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Group allocation, ODM readings and algorithm ‐guided colloid administration were concealed from other staff in the operating theatre by screens" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator had no involvement in perioperative decision‐making or postoperative care" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Conway 2002.
| Methods | RCT 2 centres |
|
| Participants | 2 groups 57 patients Elective surgery General surgery |
|
| Interventions | Intraoperative Fluids Oesophageal Doppler Goals = SV, FTc |
|
| Outcomes | Hospital mortality HLOS ICULOS Tolerating food |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prior to induction of anaesthesia, patients were individually randomized into doppler or control" . Although the study was randomized, the method of random sequence generation was not presented |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not mentioned in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Medical and nursing staff were unaware of randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See quote above, blinding of outcome assessment was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all expected outcomes |
| Other bias | Low risk | No other source of bias identified |
Donati 2007.
| Methods | RCT Multicentre study |
|
| Participants | 135 patients Elective abdominal surgery |
|
| Interventions | Intraoperative Fluids and inotropes Goals = Oxygen extraction (02ER) |
|
| Outcomes | New postoperative organ failure Number of organ failures during ICU stay ICULOS HLOS Hospital mortality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on a permuted‐ block algorithm" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to one of the two groups of treatment by a telephone system" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not explained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | All anticipated outcomes were reported |
| Other bias | Unclear risk | No other sources of bias identified |
Gan 2002.
| Methods | RCT Single centre |
|
| Participants | 100 patients Elective surgery General surgery |
|
| Interventions | Intraoperative Fluids Oesophageal Doppler Goals = SV, FTc |
|
| Outcomes | Hospital mortality HLOS Cost renal dysfunction, respiratory support for >24 hours, cardiovascular (hypotension, pulmonary oedema, arrhythmia), chest infection, severe PONV requiring rescue antiemetics, coagulopathy, wound infection, food tolerance |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized into either protocol or control group using a random number generator in sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "See quote above" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "While the data were collected by independent dedicated research personnel not involved in the intra‐operative management of patients, we were unable to blind the anaesthesiologists as to the treatment group" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The anticipated outcomes were published |
| Other bias | Low risk | The study appears free from other sources of bias |
Jerez 2001.
| Methods | RCT Single centre |
|
| Participants | 390 patients Elective surgery Cardiac surgery |
|
| Interventions | Postoperative Fluids and Inotropes Pulmonary artery catheter Goals = SVO2, CI |
|
| Outcomes | Hospital mortality Organ failures |
|
| Notes | Published in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Se trata de un estudio prospectivo, intervencional, aleatorizado y controlado" "This is a prospective, interventional, randomized controlled study". However the method of sequence generation was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Published report include all expected outcomes |
| Other bias | Low risk | The study seems free from other sources of bias |
Jhanji 2010.
| Methods | RCT Single centre |
|
| Participants | 135 patients (3 arms) 45 patients in each arm Elective Gastrointestinal surgery |
|
| Interventions | Postoperative 3 arms to the study (arm‐1 CVP group fluid optimisation according to CVP, arm‐2 SV fluid optimization according to SV, third arm had fluids and continuous dopexamine infusion) LidCO Goals = SV |
|
| Outcomes | Microvascular flow and oxygenation Complications Infections Acute kidney injury Cardiac complications Critical care free days HLOS Hospital mortality |
|
| Notes | The 3rd arm with continuous dopexamine infusion is not included in the analysis as the control group was not matched | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated to one of three treatment groups by computer‐generated random sequence in blocks of nine" |
| Allocation concealment (selection bias) | Low risk | "The study group allocations were placed in serially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Only the member of the research team who delivered the intervention was aware of the study group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Clinical outcomes data for each patient were collected by a member of the research team who was unaware of study group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes |
| Other bias | Unclear risk | Study was supported by pharmaceutical company |
Kapoor 2007.
| Methods | RCT Single centre |
|
| Participants | 30 patients 2 groups Coronary artery bypass surgery |
|
| Interventions | Postoperative Fluids and Inotropes FloTrac Goals = CI, SVV |
|
| Outcomes | Hospital mortality HLOS ICULOS Duration of ventilation Duration of inotropic days Arrhythmia Renal failure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were divided randomly into two groups, namely control and EGDT groups, by sealed envelope technique" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Report included all expected outcomes |
| Other bias | Unclear risk | Three patients excluded after allocation. There is a possibility of selection bias |
Lobo 2000.
| Methods | RCT Single centre |
|
| Participants | 37 patients 2 groups Elective surgery General surgery Vascular surgery |
|
| Interventions | Intra and postoperative Fluids and inotropes Pulmonary artery catheter Goals = DO2I |
|
| Outcomes | 28 day and 60 day mortality HLOS ICULOS Sepsis, shock, septic shock, cardiogenic shock, nosocomial infection, acute pancreatitis, postoperative fistula, arrhythmia, cerebrovascular accident, deep vein thrombosis, gastrointestinal bleeding, hypothermia, sepsis‐related organ failure assessment (SOFA) score, bronchopneumonia, urinary tract infection, wound infection. ventilator days, organ dysfunction |
|
| Notes | Both groups had PAC and protocol group aimed to achieve supranormal DO2I | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized at the preoperative stage by means of sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all expected outcomes |
| Other bias | Low risk | No other potential source of bias identified |
Mayer 2010.
| Methods | RCT Single centre |
|
| Participants | 60 patients 2 groups Elective surgery Major abdominal surgery |
|
| Interventions | Intraoperative Fluid and Inotropes FloTrac/Vigileo Goal = CI, SV (SVI,SVV) |
|
| Outcomes | HLOS Complications ICULOS Mortality |
|
| Notes | The second author for this study is currently under investigation for research misconduct in relation to published studies of fluid therapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized preoperatively either into a standard protocol group or an enhanced goal directed haemodynamic monitoring group using a closed envelope system" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Both groups were managed by the same physicians on the same wards who were not involved in the intraoperative management, data collection or group allocation of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All data collected by a study nurse blinded to the study design and group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missed outcome data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes are reported |
| Other bias | High risk | The second author for this study is currently under investigation for research misconduct in relation to published studies of fluid therapy. |
Mckendry 2004.
| Methods | RCT Single centre |
|
| Participants | 174 patients Elective surgery Emergency surgery Cardiac surgery |
|
| Interventions | Postoperative Fluids and inotropes Oesophageal Doppler Goals = SVI |
|
| Outcomes | Hospital mortality HLOS ICULOS Atrial fibrillation requiring treatment, pneumothorax, Cerebrovascular accident, chest infection or sternal wound infection, GI bleed, acute renal failure, pleural effusion, infected leg wound, aortic regurgitation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized by a priori computer generated sequence" |
| Allocation concealment (selection bias) | Low risk | "The study nurse opened the serially numbered, sealed opaque envelopes on arrival of patients" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both patients and staff on the general wards to which patients were sent after discharge from intensive care were unaware of the group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report includes all expected outcomes |
| Other bias | Unclear risk | Possibility of selection bias as 4 patients were excluded after allocation |
Mythen 1995.
| Methods | RCT Single centre |
|
| Participants | 60 patients 2 groups Elective surgery Cardiac surgery |
|
| Interventions | Intraoperative Fluids Oesophageal Doppler Goals = SV |
|
| Outcomes | Hospital mortality HLOS ICULOS (cost reported separately) Knaus organ failure criteria, chest infection, pleural effusion, disorientation, respiratory failure, nausea and vomiting, cerebrovascular accident, paralytic ileus, pericardial effusion. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized according to the contents of a sealed envelope to either a control group or a protocol group" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes are reported |
| Other bias | Unclear risk | The study was funded by a pharmaceutical company |
Noblett 2006.
| Methods | RCT Single centre |
|
| Participants | 103 patients 2 groups Elective surgery General surgery |
|
| Interventions | Intraoperative Fluids Goals = SV, FTc |
|
| Outcomes | Hospital mortality HLOS ICULOS Surgical fitness for discharge, return of gastrointestinal function, flatus, bowel movement, food tolerance, readmission rate, cytokine markers of the systemic inflammatory response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Double blind randomized controlled trial"; the method of randomization not mentioned |
| Allocation concealment (selection bias) | Unclear risk | See above quote, the method of allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Doppler probe insertion and monitoring, and trial fluid administration were carried out by a medically qualified researcher who had no involvement in postoperative patient care or decision making" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes data were presented |
| Other bias | Unclear risk | Five patients excluded after allocation may have induced selection bias |
Pearse 2005.
| Methods | RCT Single centre |
|
| Participants | 122 patients Elective surgery Emergency surgery Vascular surgery General surgery Urology surgery |
|
| Interventions | Postoperative Fluids and inotropes LidCO Goals = DO2I |
|
| Outcomes | Hospital, 28 and 60 day mortality HLOS ICULOS Number of patients with complications, infection (pneumonia, abdominal, urinary tract, CVC, wound), respiratory (pleural effusion, pneumothorax, pulmonary embolism, ARDS), cardiovascular (arrhythmia, pulmonary oedema, MI, stroke), abdominal (C. diff, diarrhoea, acute bowl obstruction, upper GI bleed, paralytic ileus, anastomotic leak, intra‐abdominal hypertension), postoperative massive haemorrhage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to GDT or control groups by computer‐generated random sequence" |
| Allocation concealment (selection bias) | Low risk | "Study group assignments were placed in serially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "These treatments were administered by a member of the research team who was the only individual aware of study group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All potential outcomes data were presented |
| Other bias | Low risk | No other sources of bias |
Pillai 2011.
| Methods | Randomized controlled study | |
| Participants | 66 patients Patients undergoing radical cystectomy |
|
| Interventions | 2 groups Oesophageal Doppler Goal = SV, FTc |
|
| Outcomes | Length of hospital stay ICU admission Wound dehiscence wound infection GI function (ileus, time to flatus, bowel opening) Postop nausea and vomiting |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All patients were randomized into a control arm, which received standard intraoperative fluids at the discretion of the consultant anaesthetist, and an intervention arm which received additional fluid from a researcher via a oesophageal doppler determined protocol" . However the method of random sequence was not mentioned |
| Allocation concealment (selection bias) | Unclear risk | See above quote, allocation concealment was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Anaesthetic and surgical teams were blinded to the randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All potential outcomes data were presented |
| Other bias | Low risk | No other sources of bias |
Pölönen 2000.
| Methods | RCT Single centre |
|
| Participants | 393 patients 2 groups Elective surgery Cardiac surgery |
|
| Interventions | Postoperative Fluids and Inotropes Pulmonary artery catheter Goals = SVO2, lactate |
|
| Outcomes | 28 day, 6 month and 12 month mortality HLOS ICULOS Organ dysfunctions: central nervous system (haemiplegia, stroke, Glasgow coma scale (GCS <10), circulatory (vasoactive medication or intraaortic counterpulsation to treat hypotension or low cardiac output), respiratory (need for mechanical or assisted ventilation), renal (low urine output or increased creatinine), hepatic (increased liver enzymes or bilirubin), gastrointestinal (macroscopic bleeding or paralytic ileus), haematological (low white cell or platelet count) ICU readmission |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized the day before surgery by sealed envelope technique" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Although study was randomized, it was not blinded and the data of the oxygen transport measurements of both groups were open to those taking care of patients during the study period" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Published report included all expected outcomes |
| Other bias | Unclear risk | 10 patients were excluded after allocation may have induced selection bias |
Sandham 2003.
| Methods | RCT Multicentre |
|
| Participants | 1994 patients Elective surgery Emergency surgery General surgery Thoracic surgery Vascular surgery Hip fracture surgery |
|
| Interventions | Preoperative Fluids and inotropes Pulmonary artery catheter Goals = DO2I, CI |
|
| Outcomes | Hospital, 6 month and 12 month mortality HLOS Myocardial infarction, congestive heart failure, supraventricular tachycardia, pulmonary embolism, renal insufficiency, hepatic insufficiency, sepsis from central venous catheter (CVC) or pulmonary artery catheter (PAC), wound infection, pneumonia, adverse events related to PAC or CVC: pulmonary infarction, haemothorax, pulmonary haemorrhage, pneumothorax, arterial puncture |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out by computer‐generated sequence, stratified according to type of surgery and according to ASA class and blocked according to centre" |
| Allocation concealment (selection bias) | Low risk | "Assignments were concealed in opaque sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded as it was not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss of patients follow‐up at 6 months in both groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Supported by a healthcare company |
Senagore 2009.
| Methods | RCT Single centre |
|
| Participants | 64 patients 3 arms study Elective Laparoscopic colectomy |
|
| Interventions | Intraoperative Fluids Oesophageal doppler Goals = SV increase 10% |
|
| Outcomes | Hospital Mortality HLOS Arrhythmia infections |
|
| Notes | This is a 3‐arm study with a control group in the first arm and goal directed groups in arms 2 and 3, where patients received lactated Ringer's solution and 6% HES | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed after EDM insertion via a established random number table" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A separate anaesthesia team monitored the EDM readings and administered boluses, whereas the clinical anaesthesia was blinded to this process" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Shoemaker 1988.
| Methods | RCT Single centre |
|
| Participants | 88 patients Elective surgery Emergency surgery General surgery |
|
| Interventions | Preoperative Fluids and inotropes Pulmonary artery catheter Goals = CI, DO2I |
|
| Outcomes | Hospital mortality HLOS ICULOS Cost Respiratory failure, renal failure, sepsis and septic shock, hepatic failure, cardiac arrest, pulmonary oedema, pleural effusion, wound infection, disseminated intravascular coagulation (DIC), acute myocardial infarction, evisceration, abdominal abscess, haemorrhage, pancreatitis, gastric outlet obstruction, urinary tract infection, cerebral infarct, pulmonary embolism, ventilator days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The remaining 88 patients were prospectively allocated to one of the three groups designated by cards arranged according to a random numbers table by an outside person and placed in opaque sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Sinclair 1997.
| Methods | RCT Single centre |
|
| Participants | 40 patients Emergency surgery Hip fracture surgery |
|
| Interventions | Intraoperative Fluids oesophageal Doppler Goals = SV |
|
| Outcomes | Hospital mortality HLOS None, "time declared fit for medical discharge" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "After consent had been obtained, the patients were individually randomized before induction of anaesthesia by a sealed envelope technique to either protocol or control groups" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The anaesthetist was blinded to the doppler measurements but was aware of the fluid volumes given as fluid challenges to the protocol group" " postoperative management was carried out on the orthopaedic ward with both medical and nursing staff blinded to the randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Ueno 1998.
| Methods | RCT Single centre |
|
| Participants | 34 patients Elective surgery Liver surgery |
|
| Interventions | Postoperative Fluids and inotropes Pulmonary artery catheter Goals = CI, DO2I, VO2I |
|
| Outcomes | Hospital mortality Bleeding, peritoneal infection, adult respiratory distress syndrome, hyperbilirubinaemia, liver failure. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomly assigned to either group N or group S by the stratified block randomization method" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All measurements were obtained and recorded by one of the authors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Valentine 1998.
| Methods | RCT Single centre |
|
| Participants | 120 patients Elective surgery Vascular surgery |
|
| Interventions | Preoperative Fluids and inotropes Pulmonary artery catheter Goals = CI |
|
| Outcomes | Hospital mortality HLOS ICULS Myocardial infarction, arrhythmia, congestive heart failure, pneumonia, non‐cardiogenic pulmonary insufficiency, acute renal insufficiency, catheter sepsis. ventilator days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomized into two groups by means of a sealed envelope" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were published |
| Other bias | Low risk | No other sources of bias identified |
Van der Linden 2010.
| Methods | RCT Single centre |
|
| Participants | 37 patients 3‐arm study High risk (ASA II‐III) Peripheral artery bypass grafting |
|
| Interventions | Intraoperative Fluids and Inotropes (dobutamine) FloTrac Vigileo monitor Goal = CI |
|
| Outcomes | Mortality HLOS Complications (infections) |
|
| Notes | The study comprised of 3 groups. Group 1: controls, Group 2: goal directed therapy and Group 3: goal directed therapy with different anaesthetic compared to group 1 and 2. As Group 3 was not matched with controls this was not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to three groups using computer generated random code" |
| Allocation concealment (selection bias) | Low risk | "The assignment was concealed in an envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The attending anaesthesiologist was blinded for the data displayed by the Vigileo monitor. Cardiac index data in these patients were recorded by an independent observer who was not involved in the patients treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data were analysed blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other potential sources of bias identified |
Venn 2002.
| Methods | RCT Single centre |
|
| Participants | 90 patients Emergency surgery Hip fracture surgery |
|
| Interventions | Intraoperative Fluids Oesophageal Doppler Goals = SV |
|
| Outcomes | Hospital mortality HLOS Time to medical fitness for discharge, deep haemorrhage requiring >2 unit blood transfusion, haematemesis, chest infection, wound infection, cellulitis, pancreatitis, pulmonary embolus, cerebrovascular accident, myocardial infarction, cardiac failure, rapid atrial fibrillation, hypotension, impaired renal function, pseudo‐obstruction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were individually randomized into 3 groups, through the use of a set of computer generated random numbers and an opaque sealed envelope technique" |
| Allocation concealment (selection bias) | Low risk | See above quote |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The anaesthetist was unaware of the central venous pressure or oesophageal doppler ultrasonography measurements" "Post operative management was performed by the orthopaedic medical team and nursing staff who were all unaware of the patient's randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above quote |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were presented |
| Other bias | Low risk | No other potential sources of bias identified |
Wakeling 2005.
| Methods | RCT Single centre |
|
| Participants | 134 patients Elective surgery General surgery |
|
| Interventions | Intraoperative Fluids Oesophageal Doppler Goals = SV |
|
| Outcomes | Hospital and 6 month mortality HLOS Time until fit for discharge, bowel recovery (flatus, bowels opening, full diet), quality of recovery score, postoperative morbidity survey (POMS), quality of life questionnaires (European organisation for the research and treatment of cancer (EORTC) ‐ QLQ‐C30 and QLQ‐CR38) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized and allocated according to the sequentially numbered, sealed opaque envelope technique" |
| Allocation concealment (selection bias) | Low risk | See quote above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The surgical teams, nursing staff and patients themselves were blinded" "The anaesthetists was blinded to the oesophageal doppler measurements" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes were reported |
| Other bias | Unclear risk | Three patients in each group did not receive allocated intervention, this may have induced selection bias |
Wilson 1999.
| Methods | RCT single centre |
|
| Participants | 138 patients 3 groups Elective surgery General surgery Vascular surgery |
|
| Interventions | Preoperative Fluids and inotropes Pulmonary artery catheter Goals = DO2I |
|
| Outcomes | Hospital mortality HLOS ICULOS (Costs reported separately) Respiratory (prolonged weaning, adult respiratory distress syndrome (ARDS), pleural effusion, secondary ventilation, sputum retention), cardiovascular (myocardial infarction, arrhythmia, cardiac arrest, pulmonary embolus, cerebrovascular accident, transient ischaemic attack, cardiac failure), gastrointestinal (infarction, haemorrhage), acute renal failure, coagulopathy, infection (bacteraemia, sepsis syndrome, septic shock, respiratory sepsis, urinary sepsis, abdominal sepsis, wound sepsis, line sepsis, other sepsis), surgical (anastomotic breakdown, deep haemorrhage, wound haemorrhage) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random sequence was generated from a Unix computer program" |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed until trial entry by sealed opaque envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | " We were unable to effect true blinding between patients in the control and treatment groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See quote above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All anticipated outcome data were reported |
| Other bias | Low risk | No other potential sources of bias |
Ziegler 1997.
| Methods | RCT Single centre |
|
| Participants | 72 patients Elective surgery Vascular surgery |
|
| Interventions | Preoperative Fluids and inotropes Pulmonary artery catheter Goals = SVO2 |
|
| Outcomes | Hospital mortality ICULOS Hypotension, congestive heart failure, myocardial infarction, arrhythmia, oliguria, graft thrombosis, cerebrovascular accident. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence was not presented |
| Allocation concealment (selection bias) | Unclear risk | This was not presented |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not detailed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not detailed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No other sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alia 1999 | Severe sepsis, septic shock |
| Balogh 2003 | Trauma |
| Benes 2010 | Patients not had surgery also included in the analysis |
| Bishop 1995 | Trauma |
| Blow 1999 | Trauma |
| Chang 2000 | Trauma, not RCT |
| Chytra 2007 | Trauma |
| Durham 1996 | Established critical illness |
| Flancbaum 1998 | Retrospective, not RCT |
| Fleming 1992 | Trauma |
| Forget 2010 | Pulse pressure variation is not a surrogate of global blood flow |
| Gattinoni 1995 | Established critical illness |
| Gutierrez 1992 | pHi guided |
| Hayes 1994 | Established critical illness |
| Ivatury 1996 | Trauma |
| Jammer 2010 | Restricted goal directed fluid administration |
| Jorgensen 2009 | Not RCT |
| Lobo 2006 | Same flow goal in each group |
| Miller 1998 | Trauma |
| Muller 1999 | No explicit flow goal |
| Pargger 1998 | pHi guided |
| Rivers 2001 | Severe sepsis and septic shock |
| Scalea 1990 | Trauma |
| Schilling 2004 | Same flow goal in each group |
| Schultz 1985 | No explicit flow goals |
| Stewart 2009 | Retrospective study |
| Stone 2003 | No explicit flow goals |
| Szakmany 2005 | Intrathoracic blood volume goal |
| Takala 2000 | No explicit flow goals |
| Tuchschmidt 1992 | Septic shock |
| Velmahos 2000 | Trauma |
| Wenkui 2010 | Restricted fluid regimen |
| Yu 1993 | Established critical illness |
| Yu 1995 | Established critical illness |
| Yu 1998 | Established critical illness |
Characteristics of ongoing studies [ordered by study ID]
Pearse 2009.
| Trial name or title | Optimization of perioperative cardiovascular management to improve surgical outcome |
| Methods | Randomized controlled study |
| Participants | Adults undergoing major abdominal surgery |
| Interventions | |
| Outcomes | 28 day mortality, postoperative complications, postoperative morbidity survey score, LOHS, critical care free days, cost |
| Starting date | 2009 |
| Contact information | ISRCTN04386758 |
| Notes | Ongoing study |
Differences between protocol and review
The search strategy was changed after December 2000, after consultation with Cochrane search editors.
Contributions of authors
Co‐ordinating the review: Mike Grocott (MG), Mark Hamilton (MH), Ahilanandan Dushianthan (AD)
Undertaking manual searches: MG, Jonathan Hayam (JH) and Shahzad Shaefi (SS), AD
Screening search results: MG, MH, Michael Mythen (MM), AD, Anand Vibhakar (AV)
Organizing retrieval of papers: MG, MH, AD, AV
Screening retrieved papers against inclusion criteria: MG, MH, AD
Appraising quality of papers: MG, MH, AD
Abstracting data from papers: MG, MH, AD
Writing to authors of papers for additional information: MG, MH, AD
Providing additional data about papers: authors of papers.
Obtaining and screening data on unpublished studies: NA
Data management for the review: MG, MH, David Harrison (DH), AD
Entering data into Review Manager (RevMan 5.1): MG, MH, AD
RevMan statistical data: MG, MH, AD
Other statistical analysis not using RevMan: DH, John Carlisle (JC)
Double entry of data: NA
Interpretation of data: MG, MH, AD, Kathy Rowan (KR)
Statistical inferences: MG, MH, AD, KR
Writing the review: MG, MH, AD, KR
Securing funding for the review: NA
Guarantor for the review (one author): MG
Person responsible for reading and checking review before submission: MG, AD
The Optimisation Systematic Review Steering Group are, in alphabetical order: Beale R, Boyd O, Emberton M, Langham J, Roberts I, Thompson J.
Sources of support
Internal sources
Special trusties of the Middlesex Hospital, UK.
External sources
No sources of support supplied
Declarations of interest
Dr Boyd and Professor Mythen are authors of included studies.
None known: Beale R, Emberton M, Langham J, Roberts I, Thompson J, Dushianthan A.
Received funding from industry for studies and lecturing in the area: Boyd O, Mythen M, Grocott M, Hamilton MA.
Pre‐existing views
None: Emberton M, Langham J, Rowan K, Dushianthan A
Against: no‐one.
Uncertain: Beale R, Roberts I, Thompson J.
In favour: Boyd O, Mythen M, Grocott M, Hamilton MA.
Professor Mike Grocott has received unrestricted grant funding (paid to his institution) not related to this review from John Caudwell, British Oxygen Company (Linde Gas Therapeutics), Smiths Medical Ltd., Ely‐Lilly Critical Care Ltd., Deltex Medical Ltd., The London Clinic, UCL Business, Rolex Ltd., Association of Anaesthetists of Great Britain and Ireland, The Intensive Care Foundation, National Institute of Health Research, National Institute of Academic Anaesthesia, The Sir Halley‐Stuart Trust, The Frances and Augustus Newman Trust and The Down Syndrome Trust. Mike Grocott has also received fees for lecturing, unrelated to this review, from Fresenius Kabi, Edwards Lifesciences and Cortex GmBH.
Professor Mythen has received honoraria for speaking/consultation and/or travel expenses from Baxter, BBraun, Covidien, Fresenius‐Kabi, Hospira, LidCo and as a consultant to AQIX (start up company with a novel crystalloid solution – pre‐clinical). He is a director of Medical Defence Technologies LLC (“Gastrostim” patented) and a co‐Inventor of “QUENCH” IP being exploited by UCL Business. Professor Mythen's chair at UCL is endowed by Smiths Medical Ltd and this company provide charitable donations to the department on an annual basis. Deltex Medical also provide unrestricted grant funds to Prof Mythen's Department. Professor Mythen is a member of the IV Fluids Guideline Development Group for the National Institute of Clinical Excellence (NICE) and he is a co‐Author of the GIFTASUP fluid guidelines.
Mark Hamiltion has received lecture fees from Edwards Life Sciences for two lectures related to optimization.
Professor Kathy Rowan is ICNARC CTU Director and the ICNARC CTU is managing a trial (evaluating the effectiveness of perioperative goal directed haemodynamic therapy in high‐risk patients undergoing major abdominal surgery involving the gastrointestinal tract) part‐funded via a National Institute for Health Research (NIHR) Clinician Scientist Award given to Dr Rupert Pearse.
All other authors': none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bender 1997 {published data only}
- Bender JS, Smith‐Meek MA, Jones CE. Routine pulmonary artery catheterization does not reduce morbidity and mortality of elective vascular surgery: results of a prospective, randomized trial. Annals of Surgery 1997;226(3):229‐36. [PUBMED: 9339929 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berlauk 1991 {published data only}
- Berlauk JF, Abrams JH, Gilmour IJ, O'Connor SR, Knighton DR, Cerra FB. Preoperative optimization of cardiovascular haemodynamics improves outcome in peripheral vascular surgery. A prospective, randomized clinical trial. Annals of Surgery 1991;214(3):289‐97. [PUBMED: 1929610] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonazzi 2002 {published data only}
- Bonazzi M, Gentile F, Biasi GM, Migliavacca S, Esposti D, Cipolla M, et al. Impact of perioperative haemodynamic monitoring on cardiac morbidity after major vascular surgery in low risk patients. A randomised pilot trial. European Journal of Vascular and Endovascular Surgery 2002;23(5):445‐51. [PUBMED: 12027474] [DOI] [PubMed] [Google Scholar]
Boyd 1993 {published data only}
- Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high‐risk surgical patients. JAMA 1993;270:2699‐707. [PUBMED: 7907668 ] [PubMed] [Google Scholar]
Cecconi 2011 {published data only}
- Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, et al. Goal‐directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Critical Care 2011;15:R132. [PUBMED: 21624138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Challand 2012 {published data only}
- Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, et al. Randomized controlled trial of intraoperative goal‐directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. British Journal of Anaesthesia 2012;108(1):53‐62. [DOI: 10.1093/bja/aer273] [DOI] [PubMed] [Google Scholar]
Conway 2002 {published and unpublished data}
- Conway DH, Mayall, R, Abdul‐LatifMS, GilliganS, Tackaberry C. Randomised controlled trial investigating the influence of intravenous fluid titration using oesophageal Doppler monitoring during bowel surgery. Anaesthesia 2002;57(9):845‐9. [PUBMED: 12190747] [DOI] [PubMed] [Google Scholar]
Donati 2007 {published data only}
- Donati A, Loggi S, Preiser JC, Orsetti G, Munch C, Gabbanelli V, et al. Goal‐directed intraoperative therapy reduces morbidity and length of hospital stay in high‐risk surgical patients. Chest 2007;132(6):1817‐24. [PUBMED: 17925428] [DOI] [PubMed] [Google Scholar]
Gan 2002 {published data only}
- Gan TJ, Soppitt A, Maroof M, Moalem H, Robertson KM, Moretti E, et al. Goal‐directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002;97(4):820‐6. [PUBMED: 12357146] [DOI] [PubMed] [Google Scholar]
Jerez 2001 {published data only}
- Jerez Gomez Coronado V, Robles Marcos M, Perez Civantos D, Tejada Ruiz J, Jimeno Torres B, Barragan Gomez Coronado I, et al. Hemodynamic optimization and morbimortality after heart surgery. Medicina Intensiva (Madrid. Ed. impresa) 2001;25(8):297‐302. [CENTRAL: CN‐00425435; EMBASE: 2002008255] [Google Scholar]
Jhanji 2010 {published data only}
- Jhanji S, Vivian‐Smith A, Lucena‐Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Critical Care 2010;14:R151. [PUBMED: 20698956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapoor 2007 {published data only}
- Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy R, Kiran U. Early goal‐directed therapy in moderate to high‐ risk cardiac surgery patients. Annals of Cardiac Anaesthesia 2008;11(1):27‐34. [PUBMED: 18182756] [DOI] [PubMed] [Google Scholar]
Lobo 2000 {published data only}
- Lobo SM, Salgado PF, Castillo VG, Borim AA, Polachini CA, Palchetti JC, et al. Effects of maximizing oxygen delivery on morbidity and mortality in high‐risk surgical patients. Critical Care Medicine 2000;28:3396‐404. [PUBMED: 11057792 ] [DOI] [PubMed] [Google Scholar]
Mayer 2010 {published data only}
- Mayer J, Boldt J, Mengistu AM, Rohm KD, Suttner S. Goal‐directed intraoperative therapy based on auto calibrated arterial pressure waveform analysis reduces hospital stay in high‐risk surgical patients: a randomized, controlled trial. Critical Care 2010;14:R18. [PUBMED: 20156348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mckendry 2004 {published and unpublished data}
- McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomised controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimisation of circulatory status after cardiac surgery. BMJ 2004;329(7460):258‐61. [PUBMED: 15242867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mythen 1995 {published and unpublished data}
- Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Archives of Surgery 1995;130:423‐9. [PUBMED: 7535996 ] [DOI] [PubMed] [Google Scholar]
Noblett 2006 {published and unpublished data}
- Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler‐optimized fluid management on outcome after elective colorectal resection. The British Journal of Surgery 2006;93(9):1069‐76. [PUBMED: 16888706 ] [DOI] [PubMed] [Google Scholar]
Pearse 2005 {published data only}
- Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal‐directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Critical Care 2005;9(6):R687‐93. [PUBMED: 16356219 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillai 2011 {published data only}
- Pillai P, McEleavy, Gaughan M, Snowden C, Nesbitt I, Durkan G, et al. A double‐blind randomized controlled trial to assess the effect of doppler optimized intraoperative fluid management on outcome following radical cystectomy. The Journal of Urology 2011;186:2201‐6. [PUBMED: 22014804] [DOI] [PubMed] [Google Scholar]
Pölönen 2000 {published data only}
- Pölönen P, Ruokonen E, Hippeläinen M, Pöyhönen M, Takala J. A prospective, randomized study of goal‐oriented haemodynamic therapy in cardiac surgical patients. Anesthesia and Analgesia 2000;90:1052‐9. [PUBMED: 10781452 ] [DOI] [PubMed] [Google Scholar]
Sandham 2003 {published data only}
- Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary‐artery catheters in high‐risk surgical patients. The New England Journal of Medicine 2003;348(1):5‐14. [PUBMED: 12510037] [DOI] [PubMed] [Google Scholar]
Senagore 2009 {published data only}
- Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid management for laparoscopic colectomy: A prospective, randomized assessment of goal‐directed administration of balanced salt solution or hetastarch coupled with enhanced recovery program. Diseases of the Colon and the Rectum 2009;52:1935‐40. [PUBMED: 19934912] [DOI] [PubMed] [Google Scholar]
Shoemaker 1988 {published data only (unpublished sought but not used)}
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high‐risk surgical patients. Chest 1988;94:1176‐86. [PUBMED: 3191758] [DOI] [PubMed] [Google Scholar]
Sinclair 1997 {published and unpublished data}
- Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ 1997;315:909‐12. [PUBMED: 9361539] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueno 1998 {published data only}
- Ueno S, Tanabe G, Yamada H, Kusano C, Yoshidome S, Nuruki K, et al. Response of patients with cirrhosis who have undergone partial hepatectomy to treatment aimed at achieving supranormal oxygen delivery and consumption. Surgery 1998;123:278‐86. [PUBMED: 9526519] [PubMed] [Google Scholar]
Valentine 1998 {published data only}
- Valentine RJ, Duke ML, Inman MH, Grayburn PA, Hagino RT, Kakish HB, et al. Effectiveness of pulmonary artery catheters in aortic surgery: a randomized trial. Journal of Vascular Surgery 1998;27:203‐11. [PUBMED: 9510275 ] [DOI] [PubMed] [Google Scholar]
Van der Linden 2010 {published data only}
- Linden PJ, Dierick A, Wilmin S, Bellens B, Hert SG. A randomised controlled trial comparing an intraoperative goal‐directed strategy with routine clinical practice in patients undergoing peripheral arterial surgery. European Journal of Anaesthesiology 2010;27:788‐93. [PUBMED: 20613538] [DOI] [PubMed] [Google Scholar]
Venn 2002 {published and unpublished data}
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. British Journal of Anaesthesia 2002;88(1):65‐71. [PUBMED: 11881887 ] [DOI] [PubMed] [Google Scholar]
Wakeling 2005 {published and unpublished data}
- Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. British Journal of Anaesthesia 2005;95(5):634‐42. [PUBMED: 16155038 ] [DOI] [PubMed] [Google Scholar]
Wilson 1999 {published data only}
- Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999;318:1099‐103. [PUBMED: 10213716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziegler 1997 {published data only}
- Ziegler DW, Wright JG, Choban PS, Flancbaum L. A prospective randomized trial of preoperative "optimization" of cardiac function in patients undergoing elective peripheral vascular surgery. Surgery 1997;122:584‐92. [PUBMED: 9308617] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alia 1999 {published data only}
- Alia I, Esteban A, Gordo F, Lorente JA, Diaz C, Rodriguez JA, et al. A randomized and controlled trial of the effect of treatment aimed at maximizing oxygen delivery in patients with severe sepsis or septic shock. Chest 1999;115:453‐61. [PUBMED: 10027447] [DOI] [PubMed] [Google Scholar]
Balogh 2003 {published data only}
- Balogh Z, McKinley BA, Cocanour CS, Kozar RA, Valdivia A, Sailors RM, et al. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Archives of Surgery 2003;138(6):637‐43. [PUBMED: 12799335 ] [DOI] [PubMed] [Google Scholar]
Benes 2010 {published data only}
- Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients:results of a prospective randomised study. Critical Care 2010;14:R118. [PUBMED: 20553586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bishop 1995 {published data only}
- Bishop MH, Shoemaker WC, Appel PL, Meade P, Ordog GJ, Wasserberger J, et al. Prospective, randomized trial of survivor values of cardiac index, oxygen delivery, and oxygen consumption as resuscitation endpoints in severe trauma. The Journal of Trauma 1995;38:780‐7. [PUBMED: 7760409 ] [DOI] [PubMed] [Google Scholar]
Blow 1999 {published data only}
- Blow O, Magliore L, Claridge JA, Butler K, Young JS. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. The Journal of Trauma 1999;47:964‐9. [PUBMED: 10568731] [DOI] [PubMed] [Google Scholar]
Chang 2000 {published data only}
- Chang MC, Meredith JW, Kincaid EH, Miller PR. Maintaining survivors' values of left ventricular power output during shock resuscitation: a prospective pilot study. The Journal of Trauma 2000;49:26‐33. [PUBMED: 10912854 ] [DOI] [PubMed] [Google Scholar]
Chytra 2007 {published data only}
- Chytra I, Pradl R, Bosman R, Pelnar P, Kasal E, Zidkova A. Esophageal doppler‐guided fluid management decreases blood lactate levels in multiple‐trauma patients: a randomized controlled trial. Critical Care 2006;11:R24. [PUBMED: 17313691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durham 1996 {published data only}
- Durham RM, Neunaber K, Mazuski JE, Shapiro MJ, Baue AE. The use of oxygen consumption and delivery as endpoints for resuscitation in critically ill patients. The Journal of Trauma 1996;41:32‐9. [PUBMED: 8676421] [DOI] [PubMed] [Google Scholar]
Flancbaum 1998 {published data only}
- Flancbaum L, Ziegler DW, Choban PS. Preoperative intensive care unit admission and haemodynamic monitoring in patients scheduled for major elective noncardiac surgery: a retrospective review of 95 patients. Journal of Cardiothoracic and Vascular Anesthesia 1998;12:3‐9. [PUBMED: 9509349 ] [DOI] [PubMed] [Google Scholar]
Fleming 1992 {published data only}
- Fleming A, Bishop M, Shoemaker W, Appel P, Sufficool W, Kuvhenguwha A, et al. Prospective trial of supranormal values as goals of resuscitation in severe trauma. Archives of Surgery 1992;127:1175‐9. [PUBMED: 1417482] [DOI] [PubMed] [Google Scholar]
Forget 2010 {published data only}
- Forget P, Lois F, Kock M. Goal‐directed fluid management based on the pulse oximeter‐derived pleth variability index reduces lactate levels and improves fluid management. Anesthesia and Analgesia 2010;111:910‐4. [PUBMED: 20705785] [DOI] [PubMed] [Google Scholar]
Gattinoni 1995 {published data only}
- Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, et al. A trial of goal‐oriented haemodynamic therapy in critically ill patients. SvO2 Collaborative Group. The New England Journal of Medicine 1995;333(16):1025‐32. [PUBMED: 7675044] [DOI] [PubMed] [Google Scholar]
Gutierrez 1992 {published data only}
- Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, et al. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 1992;339:195‐9. [PUBMED: 1346170] [DOI] [PubMed] [Google Scholar]
Hayes 1994 {published data only}
- Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. The New England Journal of Medicine 1994;330:1717‐22. [PUBMED: 7993413 ] [DOI] [PubMed] [Google Scholar]
Ivatury 1996 {published data only}
- Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ specific gastric mucosal pH. Journal of the American College of Surgery 1996;183:145‐54. [PUBMED: 8696546] [PubMed] [Google Scholar]
Jammer 2010 {published data only}
- Jammer IB, Ulvik A, Erichsen C, Lodemel O, Ostgaard G. Does central venous oxygen saturation‐ directed fluid therapy affect postoperative morbidity after colorectal surgery. Anesthesiology 2010;113:1072‐80. [PUBMED: 20885291] [DOI] [PubMed] [Google Scholar]
Jorgensen 2009 {published data only}
- Jorgensen CC, Bundgaard‐Nielsen M, Stovgaard LT, Secher NH, Kehlet H. Stroke volume averaging for individualized goal‐directed fluid therapy with oesophageal doppler. Acta Anaesthesiologica Scandinavica 2008;53:34‐8. [PUBMED: 19032566] [DOI] [PubMed] [Google Scholar]
Lobo 2006 {published data only}
- Lobo SM, Lobo FR, Polachini CA, Patini DS, Yamamoto AE, Oliveira NE, et al. Prospective, randomized trial comparing fluids and dobutamine optimization of oxygen delivery in high‐risk surgical patients. Critical Care 2006;10(3):R72. [PUBMED: 16696864 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1998 {published data only}
- Miller PR, Meredith JW, Chang MC. Randomized, prospective comparison of increased preload versus inotropes in the resuscitation of trauma patients: effects on cardiopulmonary function and visceral perfusion. The Journal ofTrauma 1998;44:107‐13. [PUBMED: 9464757 ] [DOI] [PubMed] [Google Scholar]
Muller 1999 {published data only}
- Muller M, Boldt J, Schindler E, Sticher J, Kelm C, Roth S, et al. Effects of low‐dose dopexamine on splanchnic oxygenation during major abdominal surgery. Critical Care Medicine 1999;27:2389‐93. [PUBMED: 10579253] [DOI] [PubMed] [Google Scholar]
Pargger 1998 {published data only}
- Pargger H, Hampl KF, Christen P, Staender S, Scheidegger D. Gastric intramucosal pH‐guided therapy in patients after elective repair of infrarenal abdominal aneurysms: is it beneficial?. Intensive Care Medicine 1998;24:769‐76. [PUBMED: 9757919 ] [DOI] [PubMed] [Google Scholar]
Rivers 2001 {published data only}
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. The New England Journal of Medicine 2001;345(19):1368‐77. [PUBMED: 11794169 [] [DOI] [PubMed] [Google Scholar]
Scalea 1990 {published data only}
- Scalea TM, Simon HM, Duncan AO, Atweh NA, Sclafani SJ, Phillips TF, et al. Geriatric blunt multiple trauma: improved survival with early invasive monitoring. The Journal of Trauma 1990;30:129‐34. [PUBMED: 2304107] [PubMed] [Google Scholar]
Schilling 2004 {published data only}
- Schilling T, Grundling M, Strang CM, Moritz KU, Siegmund W, Hachenberg T. Effects of dopexamine, dobutamine or dopamine on prolactin and thyreotropin serum concentrations in high‐risk surgical patients. Intensive Care Medicine 2004;30(6):1127‐33. [PUBMED: 15138671 ] [DOI] [PubMed] [Google Scholar]
Schultz 1985 {published data only}
- Schultz RJ, Whitfield GF, LaMura JJ, Raciti A, Krishnamurthy S. The role of physiologic monitoring in patients with fractures of the hip. The Journal of Trauma 1985;25:309‐16. [PUBMED: 3989888 ] [DOI] [PubMed] [Google Scholar]
Stewart 2009 {published data only}
- Stewart RM, Park PK, Hunt JP, McIntyre Jr RC, McCarthy J, Zarzabal LA, et al. Less is more: improved outcomes in surgical patients with conservative fluid administration and central venous catheter monitoring. Journal of American College of Surgeons 2009;208:725‐37. [PUBMED: 19476825] [DOI] [PubMed] [Google Scholar]
Stone 2003 {published data only}
- Stone MD, Wilson RJT, Cross J, Williams BT. Effect of adding dopexamine to intraoperative volume expansion in patients undergoing major elective abdominal surgery. British Journal of Anaesthesia 2003;91(5):619‐24. [PUBMED: 14570781] [DOI] [PubMed] [Google Scholar]
Szakmany 2005 {published data only}
- Szakmany T, Toth I, Kovacs Z, Leiner T, Mikor A, Koszegi T, et al. Effects of volumetric vs. pressure‐guided fluid therapy on postoperative inflammatory response: a prospective, randomized clinical trial. Intensive Care Medicine 2005;31(5):656‐63. [PUBMED: 15812629 ] [DOI] [PubMed] [Google Scholar]
Takala 2000 {published data only}
- Takala J, Meier‐Hellmann A, Eddleston J, Hulstaert P, Sramek V. Effect of dopexamine on outcome after major abdominal surgery: a prospective, randomized, controlled multicenter study. European Multicenter Study Group on Dopexamine in Major Abdominal Surgery. Critical Care Medicine 2000;28:3417‐23. [PUBMED: 11057795] [DOI] [PubMed] [Google Scholar]
Tuchschmidt 1992 {published data only}
- Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest 1992;102:216‐20. [PUBMED: 1623756 [] [DOI] [PubMed] [Google Scholar]
Velmahos 2000 {published data only}
- Velmahos GC, Demetriades D, Shoemaker WC, Chan LS, Tatevossian R, Wo CC, et al. Endpoints of resuscitation of critically injured patients: normal or supranormal? A prospective randomized trial. Annals of Surgery 2000;232:409‐18. [PUBMED: 10973391 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenkui 2010 {published data only}
- Wenkui Y, Ning L, Jianfeng G, WeiQin L, ShaoQiu T, Zhihui T, et al. Restricted peri‐operative fluid administration adjusted by serum lactate level improved outcome after major elective surgery for gastrointestinal malignancy. Surgery 2010;147:542‐52. [PUBMED: 20004445] [DOI] [PubMed] [Google Scholar]
Yu 1993 {published data only}
- Yu M, Levy MM, Smith P, Takiguchi SA, Miyasaki A, Myers SA. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: a prospective, randomized, controlled study. Critical Care Medicine 1993;21:830‐8. [PUBMED: 8504649 ] [DOI] [PubMed] [Google Scholar]
Yu 1995 {published data only}
- Yu M, Takanishi D, Myers SA, Takiguchi SA, Severino R, Hasaniya N, et al. Frequency of mortality and myocardial infarction during maximizing oxygen delivery: a prospective, randomized trial. Critical Care Medicine 1995;23:1025‐32. [PUBMED: 774212] [DOI] [PubMed] [Google Scholar]
Yu 1998 {published data only}
- Yu M, Burchell S, Hasaniya NW, Takanishi DM, Myers SA, Takiguchi SA. Relationship of mortality to increasing oxygen delivery in patients > or = 50 years of age: a prospective, randomized trial. Critical Care Medicine 1998;26:1011‐9. [PUBMED: 9635648 ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Pearse 2009 {unpublished data only}
- Pearse R. Optimization of perioperative cardiovascular management to improve surgical outcome. Not published.
Additional references
Bland 1978
- Bland R, Shoemaker WC, Shabot MM. Physiologic monitoring goals for the critically ill patient. Surgery, Gynecology & Obstetrics 1978;147(6):833‐41. [PUBMED: 31006] [PubMed] [Google Scholar]
Boyd 1959
- Boyd AR, Tremblay RE, Spencer FC, Bahnson HT. Estimation of cardiac output soon after cardiac surgery with cardiopulmonary bypass. Annals of Surgery 1959;150:613‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 1996
- Boyd O, Bennett ED. Enhancement of perioperative tissue perfusion as a therapeutic strategy for major surgery. New Horizons 1996;4(4):453‐65. [PUBMED: 8968978 ] [PubMed] [Google Scholar]
Boyd 1999
- Boyd O, Hayes M. The oxygen trail: the goal. British Medical Bulletin 1999;55(1):125‐39. [PUBMED: 10695083] [DOI] [PubMed] [Google Scholar]
Brienza 2009
- Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative haemodynamic optimization protect renal function in surgical patients? A meta‐analytic study. Critical Care Medicine 2009;37:2079‐90. [PUBMED: 19384211] [DOI] [PubMed] [Google Scholar]
Clowes 1960
- Clowes GH Jr, Guercio LR. Circulatory response to trauma of surgical operations. Metabolism 1960;9(1):67‐81. [PUBMED: 13810744] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenwick 2002
- Fenwick E, Wilson J, Sculpher M, Claxton K. Pre‐operative optimisation employing dopexamine or adrenaline for patients undergoing major elective surgery: a cost‐effectiveness analysis. Intensive Care Medicine 2002;28(5):599‐608. [PUBMED: 12029409] [DOI] [PubMed] [Google Scholar]
Forst 1997
- Forst H. [Maximizing O2‐transport in critical illness. A rational therapeutic concept?]. Anaesthesist 1997;46(1):46‐52. [PUBMED: 9082869] [DOI] [PubMed] [Google Scholar]
Ganz 1971
- Ganz W, Donoso R, Marcus HS, Forrester JS, Swan HJ. A new technique for measurement of cardiac output by thermodilution in man. American Journal of Cardiology 1971;27(4):392‐6. [PUBMED: 4929422 ] [DOI] [PubMed] [Google Scholar]
Giglio 2009
- Giglio MT, Marucci M, Testini M, Brienza N. Goal‐ directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta‐analysis of randomized controlled trials. British Journal of Anaesthesia 2009;103:637‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guest 1997
- Guest JF, Boyd O, Hart WM, Grounds RM, Bennett ED. A cost analysis of a treatment policy of a deliberate perioperative increase in oxygen delivery in high risk surgical patients. Intensive Care Medicine 1997;23(1):85‐90. [PUBMED: 9037645] [DOI] [PubMed] [Google Scholar]
Hamilton 2011
- Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta‐analysis on the use of preemptive haemodynamic intervention to improve post operative outcomes in moderate and high risk surgical patients. Anesthesia and Analgesia 2011;112:1392‐402. [PUBMED: 20966436] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038 ] [DOI] [PubMed] [Google Scholar]
Heyland 1996
- Heyland DK, Cook DJ, King D, Kernerman P, Brun‐Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence [see comments]. Critical Care Medicine 1996;24(3):517‐24. [PUBMED: 8625644 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (Editors). Chapter 8. Assessing risk of bias in included studies. In Higgins JPT; Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivanov 1997
- Ivanov RI, Allen J, Sandham JD, Calvin JE. Pulmonary artery catheterization: a narrative and systematic critique of randomized controlled trials and recommendations for the future. New Horizons 1997;5(3):268‐76. [PUBMED: 9259342] [PubMed] [Google Scholar]
Kern 2002
- Kern JW, Shoemaker WC. Meta‐analysis of haemodynamic optimization in high‐risk patients. Critical Care Medicine 2002;30(8):1686‐92. [PUBMED: 12163777] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Kusano 1997
- Kusano C, Baba M, Takao S, Sane S, Shimada M, Shirao K, et al. Oxygen delivery as a factor in the development of fatal postoperative complications after oesophagectomy. British Journal of Surgery 1997;84(2):252‐7. [PUBMED: 9052449] [PubMed] [Google Scholar]
Langham 2002
- Langham J, Thompson E, Rowan K. Randomised controlled trials from the critical care literature: identification and assessment of quality. Clinical Intensive Care 2002;13:73‐83. [Google Scholar]
Leibowitz 1997
- Leibowitz AB, Beilin Y. Pulmonary artery catheters and outcome in the perioperative period. New Horizons 1997;5(3):214‐21. [PUBMED: 9259333 ] [PubMed] [Google Scholar]
Louis 1991
- Louis TA. Assessing, accommodating, and interpreting the influences of heterogeneity. Environmental Health Perspectives 1991;90:215‐22. [PUBMED: 2050064 [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
MRC 2000
- MRC (UK). A framework for development and evaluation of RCTs for complex interventions to improve health. MRC Publications http://www.mrc.ac.uk/Utilities/Documentrecord/index.htm?d=MRC003372 (assessed September 2012) 2000.
Mythen 1994
- Mythen MG, Webb AR. Intra‐operative gut mucosal hypoperfusion is associated with increased post‐operative complications and cost. Intensive Care Medicine 1994;20(2):99‐104. [PUBMED: 8201106] [DOI] [PubMed] [Google Scholar]
Peerless 1998
- Peerless JR, Alexander JJ, Pinchak AC, Piotrowski JJ, Malangoni MA. Oxygen delivery is an important predictor of outcome in patients with ruptured abdominal aortic aneurysms. Annals of Surgery 1998;227(5):726‐32. [PUBMED: 9605664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poeze 2005
- Poeze M, Greve JW, Ramsay G. Meta‐analysis of haemodynamic optimization: relationship to methodological quality. Critical Care 2005;9(6):R771‐9. [PUBMED: 16356226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Polonen 1997
- Polonen P, Hippelainen M, Takala R, Ruokonen E, Takala J. Relationship between intra‐ and postoperative oxygen transport and prolonged intensive care after cardiac surgery: a prospective study. Acta Anaesthesiologica Scandinavica 1997;41(7):810‐7. [PUBMED: 9265921] [DOI] [PubMed] [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shoemaker 1972
- Shoemaker WC. Cardiorespiratory patterns of surviving and nonsurviving postoperative patients. Surgery, Gynecology & Obstetrics 1972;134(5):810‐4. [PUBMED: 5031495] [PubMed] [Google Scholar]
Shoemaker 1973
- Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Archives of Surgery 1973;106(5):630‐6. [PUBMED: 4701410] [DOI] [PubMed] [Google Scholar]
Shoemaker 1979
- Shoemaker WC, Czer LS. Archives of Surgery. Critical Care Medicine 1979;7(9):424‐31. [DOI] [PubMed] [Google Scholar]
Shoemaker 1982
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors' cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Critical Care Medicine 1982;10(6):398‐403. [PUBMED: 7042206] [DOI] [PubMed] [Google Scholar]
Shoemaker 1993
- Shoemaker WC, Appel PL, Kram HB. Hemodynamic and oxygen transport responses in survivors and non survivors of high‐risk surgery [see comments]. Critical Care Medicine 1993;21(7):977‐90. [PUBMED: 8319478 ] [DOI] [PubMed] [Google Scholar]
Swan 1970
- Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow‐directed balloon‐tipped catheter. The New England Journal of Medicine 1970;283(9):447‐51. [PUBMED: 5434111 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Grocott 2013
- Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K, Optimisation Systematic Review Steering Group. Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane Systematic Review. British Journal of Anaesthesia 2013;May 9:[Epub ahead of print]. [PUBMED: 23661403] [DOI] [PubMed] [Google Scholar]


