Abstract
Background
No established strategy for household tuberculosis (TB) contact investigation (HTCI) exists in Ethiopia. We implemented integrated, active HTCI model into two hospitals and surrounding community health services to determine yield of active HTCI of all forms of TB and explore factors associated with active TB diagnosis in household contacts (HHCs).
Methods
Case managers obtained HHC information from index cases at TB/DOTS clinic and liaised with health extension workers (HEWs) who screened HHCs for TB at household and referred contacts under five and presumptive cases for diagnostic investigation.
Results
From 363 all forms TB index cases, 1509 (99%) HHCs were screened and 809 (54%) referred, yielding 19 (1.3%) all forms TB cases. HTCI of sputum smear-positive pulmonary TB (SS + PTB) index cases produced yield of 4.3%. HHCs with active TB were more likely to be malnourished (OR: 3.39, 95%CI: 1.19–9.64), live in households with SS + PTB index case (OR: 7.43, 95%CI: 1.64–33.73) or TB history (OR: 4.18, 95%CI: 1.51–11.55).
Conclusion
Active HTCI of all forms of TB cases produced comparable or higher yield than reported elsewhere. HTCI contributes to improved and timely case detection of Tuberculosis among population who may not seek health care due to minimal symptoms or access issues. Active HTCI can successfully be implemented through integrated approach with existing community TB programs for better coordination and efficiency. Referral criteria should include factors significantly associated with active disease.
Keywords: Yield, Active case finding, Health extension workers, Implementation
Background
Ethiopia is among 30 post-2015 high-burden countries for tuberculosis (TB) [1]. National prevalence rates have halved since 1990 to an estimated 200/100,000 population in 2014 [1]. However, in 2010–2014, case detection rates decreased from 66 to 60% [1]. Lack of routine access to better diagnostics such as Xpert MTB/RIF and culture as well as quality control contribute to low detection rates [1–4]. Lack of access to and coordination across TB services and facilities delays case detection and treatment, increasing transmission risk [5–9].
The World Health Organization (WHO) [10] and STOP TB Strategy [11] recommend contact investigation to increase case finding. The yield of new active TB cases is greater among household contacts (HHCs) than the general population [12, 13]. Recent meta-analyses of household TB contact investigation (HTCI) in low- and middle-income countries revealed a prevalence of 3.1% all forms TB [12] and 1.5% bacteriologically confirmed TB [14] among HHCs. However, implementation in low-income countries remains limited with significant heterogeneity in policies, procedures, and results [12–15]. In Ethiopia, no established implementation strategies exist for HTCI [16]. Recent studies of HTCI in routine settings have focused on passive investigation [17–21]. A 2013 study in southern Ethiopia [22] utilizing health extension workers (HEWs) to actively screen and collect sputum from symptomatic HHCs at home yielded 5.3% SS + PTB cases among those tested; however, training, laboratory, and supervision components may impact feasibility of scale-up.
This paper presents results from a study of active HTCI integrated into routine facility- and community-based TB services in northern Ethiopia. The purpose of the study was to determine HTCI yield from two high TB caseload hospitals, compare findings across three operational index case definitions, and explore factors associated with active TB in HHCs to inform policies and procedures necessary to facilitate scaled active HTCI implementation in Ethiopia.
Methods
This cross-sectional study evaluates an integrated model of active HTCI implemented across facility-based TB/DOTS services and the community-based 1HEW programme in northern Ethiopia. Study sites were Felegehiwot Referral and Mekelle hospitals, two high TB caseload public hospitals in Amhara and Tigray regions, respectively. The study population included patients with new, relapse, retreatment, and return after default all forms TB living in 34 woredas (districts) located within 100 km radius of each study hospitals, and their HHCs. We excluded multi-drug resistant (MDR) cases given lack of routine access to MDR-TB diagnostic services. No additional selection criteria were applied. Study period lasted from February 04, 2015 to July 28, 2015 for 5 months.
Terms and definitions
Index case: first TB patient identified in the household registered at a TB/DOTS clinic, with at least one HHC, not necessarily source cases. Four operational definitions specify form of TB: all forms TB, Clinically diagnosed Pulmonary Tuberculosis (Clinically diagnosed PTB), Extra Pulmonary TB (EPTB) and SS + PTB.
Household contact (HHC): person living in the same house with an index case at registration and have lived for at least seven consecutive days in a month within the past 3 months and, not on TB treatment.
Malnutrition was assessed using a WHO adopted standard mean arm circumference (MUAC) tape with age-appropriate classification of nutritional status.
Tuberculosis case definitions align with WHO recommendations [1].
Diagnosis of active TB disease followed national guidelines [16]. PTB was diagnosed by sputum smear positive (SS+) result for acid-fast bacilli (AFB) examined using Ziehl-Neelsen or fluorescent sputum smear microscopy (SSM) or, sputum smear negative (SS-) or no smear with abnormal chest x-ray (CXR) at clinician discretion. Xpert MTB/RIF and culture are not routine diagnostics and were performed only at clinician discretion. Extrapulmonary TB (EPTB) was diagnosed by suggestive finding from histology, radiology/imaging or other body fluid analyses at clinician discretion. In contacts less than five, TB was diagnosed based on clinical signs and symptoms and abnormal CXR or suggestive tissue/fluid analysis. Tuberculin test was not performed, as it was not part of the national guideline.
Yield: proportion screened HHCs newly diagnosed with all forms active TB.
Number needed to be screened (NNS): Number of HHCs needed to be screened to detect one active TB case, calculated as 100/% yield [14, 23].
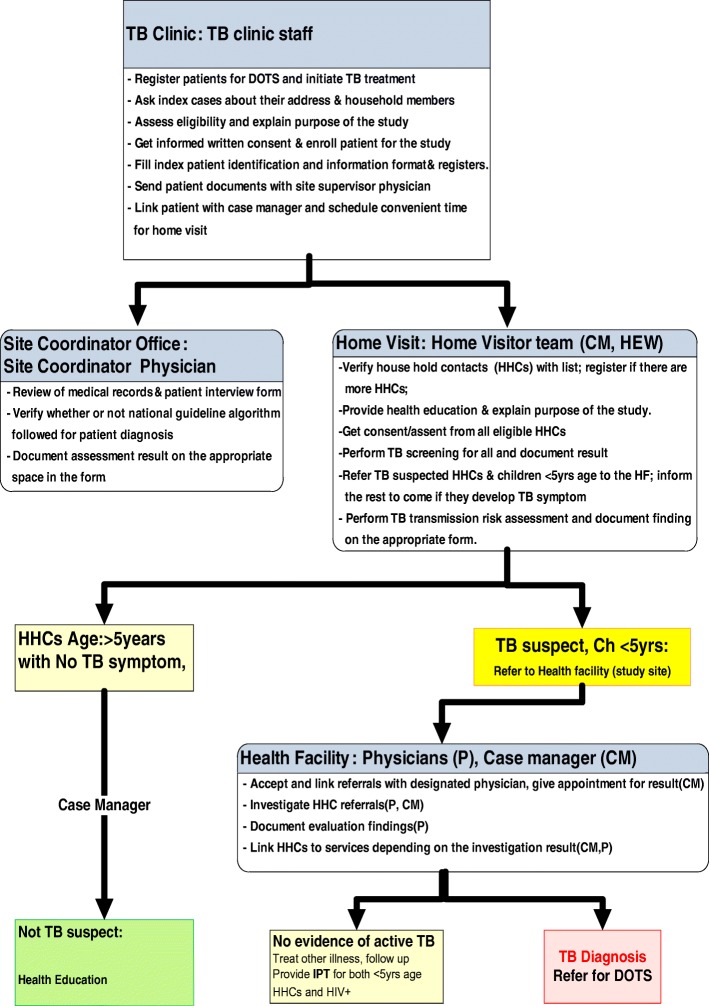
Integrated active HTCI model
Our model aligns with WHO HTCI recommendations [10]. TB focal persons in study hospitals enrolled eligible TB patients as index cases. Trained HTCI case managers recorded household location and number, age, and sex of HHCs. They provided information to Woreda/city TB focal persons and coordinated with HEWs to conduct household visits. At the household, HEWs enrolled and screened eligible HHCs using a TB screening tool adapted from WHO [24] and referred presumptive TB cases (one or more symptoms) and all under-five contacts to the study hospital for clinical investigation. If necessary, HEWs conducted one additional screening visit. They asked referred HHCs to present for investigation at the study hospital within 2 weeks and reminded those not presenting in time by phone (Please see Fig. 1). A physician conducted clinical examination, diagnostic testing, diagnosis and TB management including TB preventive therapy (TPT) as per the national guidelines [16].
Fig. 1.
Participant enrollment and contact investigation workflow
The study provided phone cards and, if appropriate, travel reimbursement per completed household visit for the case manager, TB focal person and HEW and, reimbursement for transportation, per diem, and accommodation costs for referred HHCs and one parent/guardian.
Data collection and analysis
Clinical data for index cases and clinically investigated HHCs were abstracted from patient files. We collected sociodemographic and risk factor data per participant and, household conditions data per household using piloted structured questionnaires in local languages. Data was entered into Epi Info (Version 7.1.5, CDC: Atlanta, GA, USA) in English. Data quality assurance methods included routine randomized review of electronic data against paper-based forms and validity checks between related variables across the electronic database. Co-investigators trained study and hospital staff involved in HTCI implementation and data collection and management. While HEWs have basic training in TB screening and prevention, trained HTCI case manager accompanied each HEW at least once to train and supervise data collection.
Data were extracted and analyzed using IBM SPSS Statistics (Version 19.0, IBM Corp) and Stata (Release 12, StataCorp LP). We analyzed HTCI flow, yield and NNS by region and operational index case definition. Yields are reported as proportions and per 100,000 with 95% confidence intervals (CI) using exact binomial method.
We described characteristics of index cases and HHCs using frequencies. We explored factors associated with active TB among HHCs using a multivariate generalized estimating equation (GEE) model composed of known active TB [8] risk factors found significant at p < 0.1 in bivariate analysis. Results are reported as odds ratios (OR) with 95% CI, statistically significant at p < 0.05.
Ethical considerations
The University of Washington, Ethiopian National Ethics Review Committee, and U.S. Centers for Disease Control and Prevention granted ethical approval. Per Ethiopian guidelines, written informed consent for all persons 18 and older, parental consent for all children under 18, and assent for children 12–17 years old were obtained before enrolment. Household visits began with permission of household head or equivalent. Persons refusing participation were advised to visit the nearest facility for TB screening.
Results
Table 1 details HTCI flow, yield and NNS by index case definition. In total, 363 index cases were enrolled. Of 1528 HHCs identified, 1509 (99%) were screened at first (97%) or second (3%) household visits. The total time needed to complete these HHCI for the 1509 HHCs of 363 index cases was 5 months. Of these, 809 (54%) were referred to study hospitals for further clinical investigation. Of referred HHCs, 763 (94%) presented for investigation where 598 (78%) received diagnostic testing. Nineteen HHCs were diagnosed with active TB with overall yield of 1.3% and 79 NNS. Yields were highest and NNS lowest with HTCI of SS + PTB index cases (yield = 4.3%; NNS = 23) compared to HTCI of EPTB Index cases (yield = 1.2%; NNS = 85), and all forms of TB index cases (yield = 1.3%; NNS = 79). Clinically diagnosed PTB has the lowest yield (yield = 0.3%; NNS = 348.)
Table 1.
Yield of household TB contact investigation by TB diagnosis of index
| All Forms TB | EPTB | Clinically Dx PTB | SS+ PTB | |
|---|---|---|---|---|
| Index cases | 363 | 232 | 88 | 43 |
| HHCs identified | 1528 | 1031 | 357 | 140 |
| HHCs screeneda (% of identified) | 1509 (99) | 1021 (99) | 348 (97) | 140 (10) |
| Referreda (% of screened) | 809 (54) | 594 (58) | 154 (44) | 61 (44) |
| < 5, no symptoms (% of referred) | 78 (10) | 60 (10) | 17 (11) | 1 (2) |
| Symptoms (% of referred) | 731 (90) | 534 (90) | 137 (89) | 60 (98) |
| Investigateda (% of referred) | 763 (94) | 557 (94) | 151 (98) | 55 (90) |
| Testeda (% of investigated) | 598 (78) | 425 (76) | 125 (83) | 48 (87) |
| SSMb(% of tested) | 175 (29) | 100 (24) | 52 (42) | 23 (48) |
| GeneXpertb(% of tested) | 7 (1) | 7 (1) | 0 (0) | 0 (0) |
| Otherc(% of tested) | 416 (70) | 318 (75) | 73 (58) | 25 (52) |
| Diagnoseda (% Yieldd) | 19 (1.3) | 12 (1.2) | 1 (0.3) | 6 (4.3) |
| Yield per 100,000 (95% CIe) | 1259 (759–1959) | 1175 (609–2044) | 287 (7–1591) | 4286 (1589–9095) |
| NNS (95% CI) | 79 (55–132) | 85 (49–164) | 348 (63–1375) | 23 (11–63) |
Abbreviations: TB tuberculosis, EPTB Extra pulmonary TB, PTB Pulmonary TB, DX Diagnosed, SS+ sputum smear positive, SSM sputum smear microscopy, NNS number needed to screen to detect one TB case
aPresented as n (%) unless otherwise indicated
bIndicated test ± any test indicated in the subsequent rows
cOther tests included histopathology (FNAc), imaging (CXR, ultrasound), or other body fluid analysis
dPercent (%) yield calculated as number diagnosed with TB divided by the number screened
eYield per 100,000 with 95% confidence interval using exact binomial method
Females represented 51% of index cases and 50% of HHCs. 90% of index cases and 52% of their HHCs were aged 15 years and older (See Table 2). Moreover, 8% of index cases were HIV positive and 12% had SS + TB. Among HHCs, 56% shared bed with index cases and 96% were exposed to the index case for more than 31 days (See Table 2).
Table 2.
Demographic, clinical, and exposure characteristics of index cases and household TB contacts
| Characteristics | Index Cases | Household TB contacts | ||
|---|---|---|---|---|
| N = 363 | % | N = 1509 | % | |
| Sex | ||||
| Male | 179 | 49% | 750 | 50% |
| Female | 184 | 51% | 759 | 50% |
| Age group | ||||
| 0–4 | 2 | 1% | 192 | 13% |
| 5–14 | 33 | 9% | 535 | 35% |
| 15+ | 328 | 90% | 782 | 52% |
| HIV statusa | ||||
| HIV-negative | 275 | 76% | 1503 | 99.6% |
| HIV-positive | 31 | 8% | 6 | 0.4% |
| Unknown status | 57 | 16% | – | – |
| Cough in index case | ||||
| No | 199 | 54% | 868 | 57.5% |
| Yes | 167 | 46% | 641 | 42.5% |
| TB diagnosis in index case | ||||
| EPTB | 232 | 64% | 1021 | 68% |
| Clinically Dx PTB | 88 | 24% | 348 | 23% |
| SS+ PTB | 43 | 12% | 140 | 9% |
| Relationship to index case | ||||
| Other | – | – | 611 | 40% |
| Offspring/spouse | – | – | 898 | 60% |
| Sleep area of index case | ||||
| Alone in separate rm. | – | – | 184 | 12% |
| Shared bed | – | – | 847 | 56% |
| Separate bed, shared rm. | – | – | 478 | 32% |
| Duration of exposure | ||||
| 7–30 days | – | – | 45 | 4% |
| 31+ days | – | – | 1455 | 96% |
Abbreviations: TB tuberculosis, EPTB extra pulmonary tuberculosis, Dx diagnosed, SS+ sputum spear positive, PTB pulmonary tuberculosis, rm room
aHousehold TB contact HIV status is reported by self or parent/guardian at household visit
Factor analysis (Table 3) revealed HHCs with active TB were more likely to be malnourished (OR: 3.39, 95%CI 1.19–9.64), and live in households with a SS + PTB index case (OR: 7.43, 95%CI 1.64–33.73) and history of active TB (OR:4.18, 95%CI 1.51–11.55). While statistically significant in bivariate analysis, having an immunodeficiency condition, relationship to index case and sleeping in the same room with livestock were not statistically significant in the multivariate model. Age, sex, marital status, sleeping area of index case and crowding were not significant in bivariate analysis (data not shown). Data were insufficient to analyze HIV status and ventilation.
Table 3.
Factors associated with active TB diagnosis among HHCs in Amharaa
| No TB | TB | OR (CI)b | Adjusted OR (CI)c | |
|---|---|---|---|---|
| Host risk factors | ||||
| Nutritional status | ||||
| No malnutrition | 1083 (73) | 8 (42) | 1.0 (ref) | 1.0 (ref) |
| Malnutrition | 404 (27) | 11 (58) | 3.69 (1.19–11.44) | 3.39 (1.19–9.64) |
| Immuno-deficiencyd | ||||
| Absent | 1450 (97) | 16 (84) | 1.0 (ref) | 1.0 (ref) |
| Present | 40 (3) | 3 (16) | 6.80 (1.90–24.34) | 3.70 (0.86–15.87) |
| Environmental risk factors | ||||
| TB diagnosis in index case | ||||
| EPTB | 1009 (68) | 12 (63) | 1.0 (ref) | 1.0 (ref) |
| SS+ TB | 134 (9) | 6 (32) | 3.77 (1.06–13.34) | 7.43 (1.64–33.73) |
| Clinically Dx TB | 347 (23) | 1 (5) | 0.24 (0.03–1.87) | 0.43 (0.05–3.68) |
| HHC relationship to index case | ||||
| Other | 598 (40) | 13 (68) | 1.0 (ref) | 1.0 (ref) |
| Offspring/spouse | 892 (60) | 6 (32) | 0.31 (0.11–0.89) | 0.39 (0.15–1.02) |
| Household sleeps in same room as livestock | ||||
| No | 1085 (73) | 9 (47) | 1.0 (ref) | 1.0 (ref) |
| Yes | 405 (27) | 10 (53) | 2.98 (1.05–8.48) | 3.36 (0.92–12.28) |
| TB history in household | ||||
| No | 1336 (90) | 12 (63) | 1.0 (ref) | 1.0 (ref) |
| Yes | 154 (10) | 7 (37) | 5.06 (1.77–14.44) | 4.18 (1.51–11.55) |
Abbreviations: TB tuberculosis, EPTB extra-pulmonary TB, SS+ PTB sputum smear positive pulmonary TB, Dx diagnosed
aPresented as n (%) unless otherwise indicated
bBivariate GEE analysis, bold indicates statistical significance at p < 0.1
cMultivariate GEE analysis, bold indicates statistical significance at p < 0.05
dSelf-reported; includes HIV, severe kidney disease, diabetes, previous or current cancer treatment, previous TB disease, and symptoms/signs of primary immunodeficiency (e.g., recurrent or chronic infections)
Discussion
Our study shows that integrated active HTCI can achieve high rates of screening and referral completion to yield new TB cases in two regions of northern Ethiopia. Consistent with other studies, results demonstrate that HEWs can successfully contribute to active case finding by screening HHCs and referring presumptive cases, and improve access to TB services by screening and referring at the household-level [22, 25–27]. This study contributes to evidence recommending HTCI for SS + PTB index cases to yield the highest proportion of newly diagnosed TB cases.
Yield comparison across studies is challenging given wide variation in index case, HHC and yield definitions. In this study (Table 1), HTCI of SS + PTB index cases generated a higher yield of all forms TB cases among screened HHCs (4.3%: 4286 per 100,000) than found in recent meta-analyses in low-income, high TB-burden countries (3.1% [12], 1.8% [14]), active HTCI studies in Ethiopia (2.5% [17], 0.9% [22]) and estimated national TB prevalence (200 per 100,000). When considering only bacteriologically confirmed TB cases among screened HHCs, it yielded 1.4% (data not shown), which is comparable to recent studies (1.2% [12], 1.5% [14], 0.76% [17], 0.8% [22]). In addition, HTCI of all forms of TB index cases yielded (1.3%; 1259 per 100,000) cases of TB and HTCI of EPTB index cases yielded (1.2%; 1175 per 100,000) cases (See Table 1), a six-fold increase compared to the general population prevalence. This supports the definition in our and other studies of index cases as the first patient identified within the household to initiate HTCI and not necessarily being source cases [28]. Studies of passive HTCI have observed higher yields of all forms TB cases among HHCs receiving facility-based screening or testing (6.5% from PTB index cases [19]; 10.1% from all forms index cases [18]) possibly due to appropriate self-referral. HTCI of clinically diagnosed PTB index cases generated a lower yield of all forms TB cases among screened HHCs (yield = 0.3%; 287 per 100,000) (Table 1). This could be due to use of clinical criteria (sign and symptom complex, non-response to antibiotic regimen, CXR, low quality smear etc.) to make clinical diagnosis of PTB may result in incorrect TB diagnosis thus contact investigation may not have been needed in the first place. However further study may be needed using multiple supportive diagnostic techniques with better specificity (such as imaging techniques) for establishing firm diagnosis in the absence of bacterial confirmation before concluding that these group are indeed low yield groups.
Factor analysis results (Table 3) suggest a more sensitive screening tool for the Ethiopian context could include household history of TB, malnutrition, and possibly immunodeficiency signs/conditions as referral criteria. Replicable, low-cost design elements including a dedicated HTCI case manager and household screening and follow-up via HEWs [22]; use of trusted persons in the community speaking the local language [10]; provision of referral slips [29]; household-specific TB education [19]; and a dedicated process for receiving and investigating referred HHCs at facilities to reduce opportunity costs [30–32] may have contributed to the model’s high screening and referral completion rates. Notably, this model introduced HTCI case manager as the only new cadre to the workforce. The 16 health packages HEWs address includes TB; however, workload is a concern given competing priorities and shortages in rural areas [33]. Reimbursement of transportation and diagnostic costs likely increased referral completion [31], which may still be achieved without reimbursement if investigation is decentralized. Health centers provide sputum examination at no-cost. Lack of chest x-ray and other more sensitive diagnostics may reduce yield particularly if HHCs cannot travel to hospitals for further investigation.
Given people recently infected with Mycobacterium tuberculosis are at increased risk for the development of active TB within 1–2 years after the acquisition of the infection [28], HTCI guidance should consider timing for screening, inclusion of risk-based referral criteria, and follow-up of asymptomatic HHCs. Disparate rates of diagnostic testing performed suggest that clinical screening algorithms and diagnostic guidelines specific to HTCI are necessary to guide routine implementation. While the least restrictive criteria (i.e., diagnostic testing for all contacts) is associated with increased yield [14, 34], guidelines must consider cost to the health system as this strategy may not be as effective in preventing transmission.
This study has several limitations. While consistent with operational research, non- randomization limits generalization of findings. Our small sample size reduces precision of point estimates and may be insufficient in detecting any but the largest statistical differences. Difficulty of TB diagnosis in the Ethiopian context, particularly in early stages of manifestation [35], could have resulted in undiagnosed cases in both regions. Conversely, TB may be clinically over-diagnosed including the eight cases with pleural fluid and FNAC results suggestive of TB, in the absence of routine access to Xpert MTF/RIF and culture for TB for both index cases and HHCs (Table 4). High proportion of EPTB cases observed in this study requires further investigation. While we cannot establish the contribution of our model to overall case notification in the region, relative contribution to notification is associated with increased index case coverage [14] for which we achieved 98% in this study. Financial and temporal constraints prevented cost analyses, sensitivity analyses, and comparison with passive and other models of active HTCI.
Table 4.
Age, sex, health status and clinical details of HHCs diagnosed with active TB disease
| HHC | Age | Sex | Symptoms at screening | TB Dx in index case | Mal-nutrition | Immuno-deficiencyd | Active TB diagnosis in HHCse | TB Site(s) | Diagnostic resultsf |
|---|---|---|---|---|---|---|---|---|---|
| Amhara Region | |||||||||
| 1 | 58 | M | Cough, night sweats, weight loss, appetite loss, fatigue | SS + PTB | No | No | EPTB | Cervical | AFB-negative SSM, normal CXR, suggestive FNAc |
| 2 | 53 | M | Cough, night sweats, fatigue | EPTB | No | No | EPTB | Pleural | Abnormal CXR, suggestive body fluid analysis |
| 3 | 2 | M | Fever, appetite loss | SS + PTB | Yes | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| 4a | 11 | M | Fever, night sweats, weight loss, appetite loss | SS + PTB | No | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| 5a | 23 | F | Cough, fever, night sweats, weight loss, appetite loss, fatigue | SS + PTB | No | No | SS + PTB | Pulmonary, cervical | AFB-positive SSM, abnormal CXR, suggestive FNAc |
| 6a | 40 | F | Cough, fever, night sweats, weight loss, appetite loss, fatigue | SS + PTB | No | No | EPTB | Pleural | AFB-negative SSM, abnormal CXR, suggestive FNAc, suggestive U/S |
| 7 | 14 | F | Cough, neck swelling | EPTB | Yes | No | EPTB | Cervical | Suggestive FNAc |
| 8 | 55 | M | Cough, fever | SS-PTB | Yes | Yes | SS-PTB | Pulmonary | AFB-negative SSM, abnormal CXR |
| 9 | 14 | F | Cough, fever, night sweats, haemoptysis | EPTB | Yes | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| 10 | 5 | M | Cough, fever, appetite loss | EPTB | No | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| 11 | 28 | F | Fever, night sweats | EPTB | Yes | No | EPTB | Hilar lymph-adenopathy | Abnormal CXR |
| 12 | 30 | F | Cough, fever, fatigue | EPTB | Yes | Yesc | EPTB | Axillary lymph-adenopathy | Normal CXR, suggestive FNAc |
| 13 | 8 | M | Night sweats | EPTB | No | No | PTB, no SSM | Pulmonary, cervical | Abnormal CXR, suggestive FNAc |
| 14b | 60 | F | Cough, fever, night sweats, weight loss, appetite loss, fatigue | EPTB | Yes | No | EPTB | Pleural, cervical | AFB-negative SSM, abnormal CXR, suggestive FNAc |
| 15b | 20 | F | Cough, fever, night sweats | EPTB | Yes | Yes | PTB, no SSM | Pulmonary | Abnormal CXR |
| 16 | 10 | M | Cough, fever, night sweats, appetite loss | EPTB | Yes | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| 17 | 15 | F | Cough, fever, night sweats, weight loss, neck swelling, fatigue | EPTB | Yes | No | PTB, no SSM | Pulmonary | Abnormal CXR, suggestive FNAc |
| 18 | 4 | F | Fever, night sweats | EPTB | No | No | PTB, no SSM | Pulmonary | Abnormal CXR |
| Tigray Region | |||||||||
| 19 | 14 | F | Cough, weight loss | SS + PTB | Yes | No | SS + PTB | Pulmonary | AFB-positive SSM, abnormal CXR |
a, b Observed clustering (HHCs within the same household)
cOnly HHC diagnosed with active TB disease with a positive HIV status
dSelf-reported; includes HIV, kidney disease, diabetes, previous/current cancer treatment, previous TB, signs of primary immunodeficiency (e.g., recurrent or chronic infections)
eSS-PTB Smear-negative pulmonary TB, SS + PTB smear-positive pulmonary TB, PTB pulmonary TB, no SSM sputum smear microscopy not performed, EPTB extra pulmonary TB
fAFB acid-fast bacilli, SSM sputum smear microscopy, CXR chest x-ray, FNAc fine needle aspiration cytology, U/S ultrasound
Conclusion
This program evaluation finding demonstrated that active HTCI integrated into the existing health system produced high rates of HHC coverage, screening and referral completion. HHCI intervention from index cases with all forms of TB has a considerable high yield compared to the estimated prevalence in the general population. Therefore we recommend that HHCI should be done for all forms of TB index cases. However, where there is resource limitation priority should be given to HHCI of SS+ followed by EPTB index cases. It is also our recommendation that HTCI be done integrated with the existing community TB control where the structure exists. Standard protocols and procedures must be developed to improve efficiency and consistency. In addition, screening and investigation algorithms that include factors highly associated with increased risk of TB can further increase yield. Implementation must be coupled with efforts to improve passive case finding and access to rapid and sensitive diagnostic services. Routine monitoring and evaluation of HTCI is necessary and its contribution to case notification and cost-effectiveness should be further investigated.
Acknowledgements
The authors thank T. Antifie and Z. Feleke for their support in data collection, data management, and monitoring process, J. Stern in assisting with data analysis, as well as P. Drain and K. Holmes for their valuable feedback. They give special thanks to TB focal persons at Felegehiwot Referral Hospital, Mekelle Regional Hospital, health centers, Woreda, and city administration; case managers and health extension workers; clinicians and hospital staff. They sincerely thank Amhara and Tigray Regional Health Bureaus for their support. This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through U.S. Department of Health and Human Services - Health Resources and Services Administration (HHS-HRSA) under the terms of Cooperative Agreement (U91HA06801). This study also received statistical support from the University of Washington/Fred Hutchinson Center for AIDS Research (NIH P30 AI027757). The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Funding
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through U.S. Department of Health and Human Services - Health Resources and Services Administration (HHS-HRSA) under the terms of Cooperative Agreement (U91HA06801) through the U.S Centers for Disease Control and Prevention (CDC). This study received statistical support by University of Washington/Fred Hutchinson Center for AIDS Research (NIH P30 AI027757). It also received support from CDC Ethiopia during concept and design of the study as well as manuscript writing. U.S. Centers for Disease Control and Prevention granted ethical approval.
The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official position of the funding agencies and that of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
Availability of data and materials
The data set used or analyzed during current study are available from the corresponding author on a reasonable request.
Abbreviations
- AFB
Acid fast bacilli
- CXR
Chest x-ray
- EPTB
Extra pulmonary TB
- GEES
Generalized estimating equation
- HEWs
Health extension workers
- HHC
Household contacts
- HTCI
Household tuberculosis contact investigation
- MUAC
Mean arm circumference
- NNS
Number needed to be screened
- SS-
Smear negative
- SS+
Smear positive
- SSM
Sputum smear microscopy
- TB
Tuberculosis
- TB/DOTS
Tuberculosis directly observed therapy short course
- WHO
World Health Organization
Authors’ contributions
Concept and design: FT, GF, BF, AS, DA, NH, GO, Data collection, analysis and manuscript: AS, GB, Manuscript writing: FT, GB, BF, DA, NH, GO, GF. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The University of Washington, Ethiopian National Ethics Review Committee, and U.S. Centers for Disease Control and Prevention granted ethical approval. Per Ethiopian guidelines, written informed consent for all persons 18 and older, parental consent for all children under 18, and assent for children 12–17 years old were obtained before enrolment. Household visits began with permission of household head or equivalent. Persons refusing participation were advised to visit the nearest facility for TB screening. Additional data set (other than those presented in the manuscript) generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
HEWs are women aged 18 years or older with at least 10th grade education who are nominated by the local community, representatives in which they reside in order to ensure acceptance by community members. They are government salaried community health workers and implementing The Extension Program (HEP) which is a defined package of basic and essential promotive, preventive and selected high impact curative health services including community TB program targeting households.
Contributor Information
Fana Tefera, Email: obi3@cdc.gov.
Gena Barnabee, Email: genab@uw.edu.
Anjali Sharma, Email: Anjali_sharma@hotmail.com.
Beniam Feleke, Email: hmz8@cdc.gov.
Daniel Atnafu, Email: discussdan101@yahoo.com.
Negasi Haymanot, Email: negassi2012@yahoo.com.
Gabrielle O’Malley, Email: gomalley@uw.edu.
Getachew Feleke, Email: gfeleke@gmail.com.
References
- 1.World Health Organization . Global tuberculosis report 2015. 20th. Geneva: WHO; 2015. [Google Scholar]
- 2.Federal Democratic Republic of Ethiopia Ministry of Health . Health sector development program IV annual performance report. In: HSDP, editor. Addis Ababa: FMOH; EFY; 2003. [Google Scholar]
- 3.Shiferaw MB, Hailu HA, Fola AA, Derebe MM, Kebede AT, Kebede AA, et al. Tuberculosis laboratory diagnosis quality assurance among public health facilities in West Amhara region, Ethiopia. PLoS One. 2015;10(9):e0138488. doi: 10.1371/journal.pone.0138488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addisu MD, Balew ZT. Laboratory diagnostic systems used in the diagnosis of tuberculosis in Ethiopia: a systematic review. J Med Lab Diagn. 2014;5(2):14–21. doi: 10.5897/JMLD2013.0086. [DOI] [Google Scholar]
- 5.Belay M, Bjune G, Ameni G, Abebe F. Diagnostic and treatment delay among tuberculosis patients in Afar region, Ethiopia: a cross-sectional study. BMC Public Health. 2012;12:369. doi: 10.1186/1471-2458-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: a cross sectional study. BMC Infect Dis. 2005;5:112. doi: 10.1186/1471-2334-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussen A, Biadgilign S, Tessema F, Mohammed S, Deribe K, Deribew A. Treatment delay among pulmonary tuberculosis patients in pastoralist communities in bale zone, Southeast Ethiopia. BMC Res Notes. 2012;5:320. doi: 10.1186/1756-0500-5-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013:828939. doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golub JE, Bur S, Cronin WA, Gange S, Baruch N, Comstock GW, et al. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis. 2006;10(1):24–30. [PubMed] [Google Scholar]
- 10.World Health Organization . Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. In: WHO/HTM/TB, editor. 9. Geneva: WHO; 2012. [PubMed] [Google Scholar]
- 11.World Health Organization . Documentation for the sixty-seventh world health assembly: global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: WHO; 2014. [Google Scholar]
- 12.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(1):140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(6):359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 14.Blok L, Sahu S, Creswell J, Alba S, Stevens R, Bakker MI. Comparative meta-analysis of tuberculosis contact investigation interventions in eleven high burden countries. PLoS One. 2015;10(3):e0119822. doi: 10.1371/journal.pone.0119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang TJ, Ottmani S, Uplekar M. A rapid assessment of prevailing policies on tuberculosis contact investigation. Int J Tuberc Lung Dis. 2011;15(12):1620–1623. doi: 10.5588/ijtld.11.0222. [DOI] [PubMed] [Google Scholar]
- 16.Federal Democratic Republic of Ethiopia Ministry of Health . Guidelines for clinical and programmatic management of TB, leprosy and TB/HIV in Ethiopia. 5. Addis Ababa: FMOH; 2013. [Google Scholar]
- 17.Jerene D, Melese M, Kassie Y, Alem G, Daba SH, Hiruye N, et al. The yield of a tuberculosis household contact investigation in two regions of Ethiopia. Int J Tuberc Lung Dis. 2015;19(8):898–903. doi: 10.5588/ijtld.14.0978. [DOI] [PubMed] [Google Scholar]
- 18.Ramos JM, Biru D, Tesfamariam A, Reyes F, Górgolas M. Screening for tuberculosis in family and household contacts in a rural area in Ethiopia over a 20-month period. Int J Mycobacteriol. 2013;2(4):240–243. doi: 10.1016/j.ijmyco.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Gebregergs GB, Alemu WG. Household contact screening adherence among tuberculosis patients in northern Ethiopia. PLoS One. 2015;10(5):e0125767. doi: 10.1371/journal.pone.0125767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assefa D, Klinkenberg E, Yosef G. Cross sectional study evaluating routine contact investigation in Addis Ababa, Ethiopia: a missed opportunity to prevent tuberculosis in children. PLoS One. 2015;10(6):e0129135. doi: 10.1371/journal.pone.0129135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abebe M, Wassie L, Demissie A, Mihret A, Engers H, Aseffa A, et al. TB case detection: can we remain passive while the process is active? Pan Afr Med J. 2012;11:50. [PMC free article] [PubMed] [Google Scholar]
- 22.Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in southern Ethiopia. PLoS One. 2013;8(5):e63174. doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 1998;317(7154):307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . Systematic screening for active tuberculosis: principles and recommendations. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 25.Datiko DG, Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4(5):e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yimer A, Holm-Hansen C, Yimaldu T, Bjune G. Evaluating an active case-finding strategy to identify smear-positive tuberculosis in rural Ethiopia. Int J Tuberc Lung Dis. 2009;13(11):1399–1404. [PubMed] [Google Scholar]
- 27.Tadesse T, Demissie M, Berhane Y, Kebede Y, Abebe M. Two-thirds of smear-positive tuberculosis cases in the community were undiagnosed in Northwest Ethiopia: population based cross-sectional study. PLoS One. 2011;6(12):e28258. doi: 10.1371/journal.pone.0028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fair E, Miller CR, Ottmani SE, Fox GJ, Hopewell PC. Tuberculosis contact investigation in low- and middle-income countries: standardized definitions and indicators. Int J Tuberc Lung Dis. 2015;19(3):269–272. doi: 10.5588/ijtld.14.0512. [DOI] [PubMed] [Google Scholar]
- 29.Kozuki N, Guenther T, Vaz L, Moran A, Soofi SB, Kayemba CN, et al. A systematic review of community-to-facility neonatal referral completion rates in Africa and Asia. BMC Public Health. 2015;15(1):989. doi: 10.1186/s12889-015-2330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauch V, Bonsu F, Gyapong M, Awini E, Suarez P, Marcelino B, et al. Free tuberculosis diagnosis and treatment are not enough: patient cost evidence from three continents. Int J Tuberc Lung Dis. 2013;17(3):381–387. doi: 10.5588/ijtld.12.0368. [DOI] [PubMed] [Google Scholar]
- 31.Barter DM, Agboola SO, Murray MB, Barnighausen T. Tuberculosis and poverty: the contribution of patient costs in sub-Saharan Africa--a systematic review. BMC Public Health. 2012;12:980. doi: 10.1186/1471-2458-12-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nezenega ZS, Gacho YH, Tafere TE. Patient satisfaction on tuberculosis treatment service and adherence to treatment in public health facilities of Sidama zone, South Ethiopia. BMC Health Serv Res. 2013;13:110. doi: 10.1186/1472-6963-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teklehaimanot HD, Teklehaimanot A. Human resource development for a community-based health extension program: a case study from Ethiopia. Hum Resour Health. 2013;11:39. doi: 10.1186/1478-4491-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tulloch O, Theobald S, Morishita F, Datiko DG, Asnake G, Tesema T, et al. Patient and community experiences of tuberculosis diagnosis and care within a community-based intervention in Ethiopia: a qualitative study. BMC Public Health. 2015;15:187. doi: 10.1186/s12889-015-1523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guwatudde D. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158(9):887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used or analyzed during current study are available from the corresponding author on a reasonable request.