Abstract
Accrual of metastatic pulmonary carcinoid patients for therapy is usually relied on clinical and histologic characterization, with no role for the proliferation activity as defined by Ki-67 labelling index (LI). A total of 14 carcinoid patients with tumour primaries (TP) and 19 corresponding tumour metastases (TM) were blindly reviewed by 2 different pathologists for necrosis, mitotic count, and Ki-67 LI. Ki-67 LI outperformed histologic subtyping, mitotic count, and necrosis with good to almost excellent (0.40-0.75) inter-observer agreement. About 10% cut-off Ki-67 LI predicted survival better than histology for TP and TM for both observers. The TM patients survived differently according to diverse treatments (somatostatin analogues [SSAs], analogues plus additional treatments except for platinum; platinum-based chemotherapy) in close correlation with <10%, 10% to 20%, and >20% cut-off thresholds of Ki-67 LI, respectively. There was also a trend for an increase in Ki-67 LI in TM as compared with TP. This is the first proof of concept in which a clinical potential is preliminarily suggested for Ki-67 LI to better stratify pulmonary metastatic carcinoid patients for treatment according to a criterion of histology-independent biological aggressiveness.
Keywords: carcinoid, therapy, histology, metastasis, Ki-67 labelling index
Introduction
The current classification of lung neuroendocrine tumours groups 4 histologic variants, namely, typical carcinoid (TC), atypical carcinoid (AC), large-cell neuroendocrine carcinoma, and small-cell lung carcinoma (SCLC), which are hallmarked on resection specimens by assessing morphology, necrosis, and mitoses.1 In the clinical handling of carcinoid patients, however, an important source of uncertainty may derive from the metastatic disease, especially in the setting of AC,2 as defining criteria may be challenging to reproduce.3,4 The evaluation of Ki-67 antigen expressed as labelling index (henceforth simply Ki-67 LI) on immunohistochemistry may help to separate carcinoids from SCLC5 and may have prognostic and grading potential,6,7 but it is worthwhile deciphering whether this marker may highlight different subsets of lung carcinoid patients diversely behaving or responding to treatments in a more effective way than using morphology as thus far outlined in most clinical trials.8
This study was aimed as a preliminary one to generate a proof of concept that could be instrumental to activate clinical trials in the future.
Materials and Methods
Study design
This study aimed to generate a proof of concept on the clinical usefulness of the Ki-67 LI in carcinoid patients treated with diverse treatments and experiencing a range of outcomes when using small-sized diagnostic material. The proliferation rate progression at metastatic sites was also evaluated. This is a retrospective study correlating the Ki-67 LI with the diverse treatments being administered and the observed relevant outcomes.
Patients and tumours
A total of 16 consecutive carcinoid patients (9 men and 7 women), aged between 35 and 73 years, were retrospectively abstracted from the pathology archives of the University of Turin during the time frame January 1996 to May 2017 (6.7% of 239 consecutive lung carcinoid resections) to have comparable therapy schedules. All consecutive cases of carcinoids in this time frame were included to minimize selection biases. Of the 16 patients, 14 under evaluation were lung resected (henceforth simply tumour primaries or TP), whereas 2 patients had been diagnosed on metastases only (1 by liver biopsy and 1 by supraclavicular lymph node FNAC [fine needle aspiration cytology]). Overall, 17 paired metastases in either loco-regional or distant sites and these 2 metastatic foci made up the group of tumour metastases that entered the study (henceforth simply TM).
The TP comprised 13 lung surgical procedures and 1 pulmonary FNAC sample, whereas TM included lymph node, parotid gland, liver, bronchus, and skin specimens obtained from resection, biopsy, or FNAC. First-, second- and third-line therapies were offered for 13, 10, and 4 patients, respectively. Treatments included lanreotide (a long-acting SSA) in 8 and 2 patients in the first and second treatment line, respectively; everolimus (an inhibitor of mammalian target of rapamycin [mTOR]) with or without SSA in 2, 6, and 2 patients in the first, second, and third line, respectively; platinum-based chemotherapy (CT) (cisplatin, carboplatin, etoposide) in 3 patients at the first line; and non-platinum-based CT (temozolomide, taxanes) in 2 and 1 patient in the second and third line, respectively (some patients received more treatment lines over time). Peptide receptor radionuclide therapy (PRRT) was administered in 1 patient in the third line of treatment. In all, 6 patients died of disease, 7 were alive with persisting disease, and 3 were alive and well (due to loco-regional lymph node metastases that were excised at the time of the initial surgery, thus without residual disease).
All original slides were blindly revised by 2 pathologists (F.M. and G.G.), independent of each other and blinded to patient identity and clinicopathologic characteristics including Ki-67 activity, who accomplished mitotic count on 2 mm2 and necrosis assessment in keeping with the 2015 World Health Organization (WHO) classification.1 Ki-67 LI was quantified on 2000 cells or 2 mm2 on both resection specimens and biopsy samples as recently detailed.9 In TP, the original diagnosis was TC, AC and carcinoid not otherwise specified/NOS (on FNAC sample) in 1, 12, and 1 case, respectively. In TM, AC was recognizable in 10 of the 19 metastatic foci, with the remaining 9 cases being labelled as carcinoid NOS (4 of them were FNAC samples).
Statistical analysis
All analyses (Fisher exact test for contingency tables, Kaplan-Meier/log-rank method for survival curves and weighted kappa statistics using the Cicchetti-Allison weights for inter-observer agreement) were performed on the SAS statistical software version 9.2 (SAS Institute, Inc., Cary, NC, USA), considering 2-sided P values <.05 as statistically significant.
Results
A clinicopathologic summary of the tumour series under evaluation is presented in Table 1. While an inconsistent association was found with tumour subtyping (TC vs AC), histopathologic characteristics, and Ki-67 LI distribution according to pathologists and types of material (TP or TM) (Tables 2 and 3), the best inter-observer agreement was observed for Ki-67 LI and mitotic count, whereas necrosis failed to a larger extent. As necrosis is one of the defining criteria for AC, this caused a less reproducible separation of TC or AC to be obtained in either TP or TM sample (Table 4). While no or only marginal differences in survival were observed according to histology in either TP or TM, a 10% threshold was found to best separate diversely behaving patients in both TP and TM (Supplemental Figure 1A and B). The lack of association between Ki-67 LI in metastases and histology for 1 of the 2 pathologists confirmed that histology was not the best approach for describing these tumours in both TP and TM samples (Table 3).
Table 1.
Clinicopathologic characteristics.
| Patient characteristics | No. (%) |
|---|---|
| All patients | 16 (100) |
| Sex | |
| Male | 9 (56.2) |
| Female | 7 (43.8) |
| Age | |
| Median (range) | 60 (35-73) |
| <50 | 3 (18.8) |
| 50-59 | 5 (31.3) |
| 60-69 | 6 (37.5) |
| 70+ | 2 (12.5) |
| Therapy | |
| SSA or FU | 11 (68.7) |
| SSA + everolimus | 2 (12.5) |
| Chemotherapy | 3 (18.8) |
| Follow-up, y | |
| Median (range) | 3.8 (1.7-15.9) |
| Person-years | 81 |
| Deaths | 6 |
| Annual death rate | 7.4/100 y |
Abbreviations: FU, follow-up only; SSA, somatostatin analogues.
Table 2.
Histopathologic characteristics according to pathologist and tissue.
| Primary tumoursa |
Primary tumoursa |
Metastases |
Metastases |
|
|---|---|---|---|---|
| First pathologist | Second pathologist | First pathologist | Second pathologist | |
| Histology | ||||
| TC | 3 | 7 | 7 | 10 |
| AC | 11 | 7 | 12 | 9 |
| Mitosis | ||||
| Median (range) | 2 (0-8) | 1 (0-8) | 2 (0-5) | 1 (0-8) |
| 0-1 | 5 | 8 | 9 | 11 |
| 2-4 | 7 | 5 | 9 | 6 |
| 5-9 | 2 | 1 | 1 | 2 |
| Necrosis | ||||
| Absent | 4 | 11 | 9 | 15 |
| Present | 10 | 3 | 10 | 4 |
| Ki-67 | ||||
| Median (range) | 7 (1-30) | 7 (1-32) | 7 (1-60) | 8 (1-62) |
| 0-4 | 2 | 5 | 2 | 5 |
| 5-9 | 8 | 6 | 8 | 5 |
| 10+ | 4 | 3 | 9 | 9 |
Missing for 2 patients.
Table 3.
Association between Ki-67 labelling index in metastases and histology.
| All | Typical carcinoid | Atypical carcinoid | P valuea | |
|---|---|---|---|---|
| First pathologist | ||||
| Ki-67 (metastasis, %) | ||||
| 0-4 | 2 | 2 | 0 | |
| 5-9 | 8 | 4 | 4 | |
| 10+ | 9 | 1 | 8 | .03 |
| Second pathologist | ||||
| Ki-67 (metastasis, %) | ||||
| 0-4 | 5 | 3 | 2 | |
| 5-9 | 5 | 4 | 1 | |
| 10+ | 9 | 3 | 6 | .32 |
P value calculated using the Fisher exact test.
Table 4.
Agreement between 2 pathologists for the assessment of necrosis, histology, Ki-67, and mitotic count on primary tumour and metastasis.
| Primary tumours |
Metastases |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second pathologist | Second pathologist | |||||||||
| Histology | TC | AC | TC | AC | ||||||
| First pathologist | TC | 3 | 0 | Kappa (95% CI) | TC | 7 | 0 | Kappa (95% CI) | ||
| AC | 4 | 7 | 0.43 (0.04-0.82) | AC | 3 | 9 | 0.69 (0.38-0.99) | |||
| Necrosis | No | Yes | No | Yes | ||||||
| First pathologist | No | 4 | 0 | Kappa (95% CI) | No | 9 | 0 | Kappa (95% CI) | ||
| Yes | 7 | 3 | 0.20 (0.00-0.45) | Yes | 6 | 4 | 0.39 (0.07-0.71) | |||
| Mitoses | 0-1 | 2-4 | 5-9 | 0-1 | 2-4 | 5-9 | ||||
| First pathologist | 0-1 | 5 | 0 | 0 | Kappa (95% CI) | 0-1 | 8 | 0 | 1 | Kappa (95% CI) |
| 2-4 | 2 | 5 | 0 | 0.65 (0.31-0.98) | 2-4 | 3 | 5 | 1 | 0.45 (0.13-0.76) | |
| 5-9 | 1 | 0 | 1 | 5-9 | 0 | 1 | 0 | |||
| Ki-67 LI | 0-1 | 2-4 | 5-9 | 0-1 | 2-4 | 5-9 | ||||
| First pathologist | 0-4 | 2 | 0 | 0 | Kappa (95% CI) | 0-4 | 2 | 0 | 0 | Kappa (95% CI) |
| 5-9 | 3 | 5 | 0 | 0.56 (0.20-0.91) | 5-9 | 3 | 5 | 0 | 0.75 (0.52-0.98) | |
| 10+ | 0 | 1 | 3 | 10+ | 0 | 0 | 9 | |||
Abbreviations: AC, atypical carcinoid; CI, confidence interval; TC, typical carcinoid.
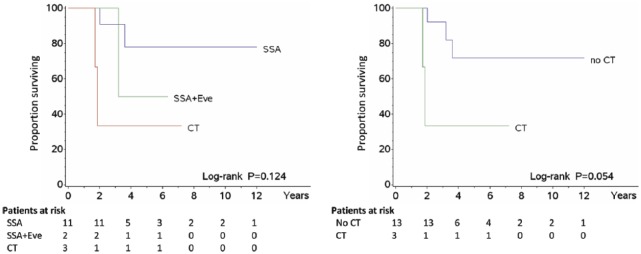
When TM were stratified by first-line treatment, a trend was found for patients treated with SSAs to run a longer survival than those receiving also everolimus or treated with CT alone (Figure 1), where the relevant Ki-67 LI averaged 8.6%, 12.8%, and 28%, respectively. In second and third line, SSAs and/or everolimus and/or PRRT was administered in 9 patients, whereas non-platinum-based CT (taxanes or temozolomide) was continued in 2 out of 3 patients undergoing this treatment (P = .039). In these instances, the mean Ki-67 LI was 3.5% for SSA patients, 14.1% for those also treated with everolimus and/or PRRT, and 23.5% for CT-only patients. Accordingly, 3 patient subsets could be thus identified using the same cut-off values of <10% (SSA), 10% to 20% (SSA ± everolimus ± PRRT), and >20% (non-platinum-based CT) emerging from such a therapy-related distribution model, which resulted in different histology-independent survival curves (Figure 2). Finally, a trend towards an average increase in Ki-67 LI from TP to TM was found in all patients under evaluation (Table 5).
Figure 1.
Survival by therapy – Overall survival of patients according to the type of treatment. SSA indicates somatostatin analogues; Eve, everolimus; CT, chemotherapy. The mean Ki-67 labelling index of tumour metastases was 8.6% for SSA, 12.5% for Eve, and 28% for CT.
Figure 2.
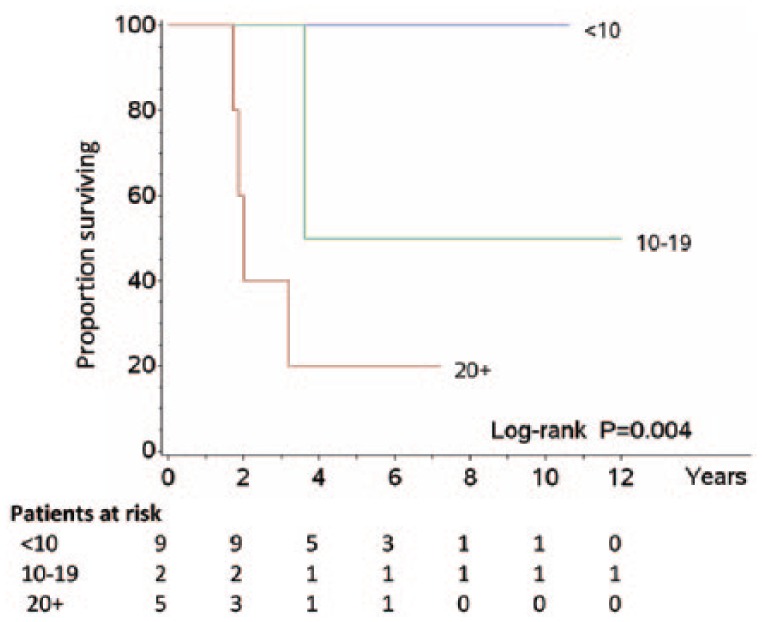
Survival by Ki-67 – Overall survival of patients according to tripartite division of Ki-67 labelling index (<10%, between 10% and 19%, ⩾20%). This distribution looked like that of type of therapy, with which shared comparable mean values of proliferation.
Table 5.
Difference in Ki-67 labelling index between primary tumours and metastases.
| First pathologist | Ki-67 in metastases, % |
||||
|---|---|---|---|---|---|
| 0-4 | 5-9 | 10+ | |||
| Ki-67 in primary tumoursa, % | 0-4 | 0 | 2 | 0 | Kappa (95% CI) |
| 5-9 | 1 | 5 | 2 | 0.31 (0.0-0.66) | |
| 10+ | 1 | 1 | 5 | Fisher P = .13 | |
| Second pathologist | Ki-67 in metastases, % |
||||
| 0-4 | 5-9 | 10+ | |||
| Ki-67 in primary tumoursa, % | 0-4 | 2 | 2 | 1 | Kappa (95% CI) |
| 5-9 | 3 | 2 | 1 | 0.29 (0.0-0.64) | |
| 10+ | 0 | 1 | 5 | Fisher P = .14 | |
Abbreviation: CI, confidence interval.
There were 2 patients with metastatic tumours only.
Discussion
This study makes up a preliminary proof of concept on the clinical usefulness of the Ki-67 LI in individual carcinoid patients benefitting from diverse treatment options on small-sized diagnostic material. Proliferation rate progression at metastatic sites was also investigated in the light of recent data highlighting the concept of transition or evolution of carcinoids to neuroendocrine carcinomas.10,11
At variance with gastrointestinal tract neuroendocrine neoplasms, where clinical decisions are dictated on an effective grading system incorporating Ki-67 LI,12,13 the treatment of pulmonary metastatic carcinoids is often challenging and still based on morphology as the sole interpretation cue.2,8 A patient selection through Ki-67 LI has not been thus far playing any decisional role in most clinical trials dealing with metastatic lung carcinoids,2,8 including the recent LUNA trial, which was specifically designed for lung and thymus well-differentiated neuroendocrine tumours.14 Although ongoing clinical trials suggest pulmonary carcinoid patient selection is likely to be clinically warranted,8 we herein preliminarily suggest that different cut-off thresholds of Ki-67 LI could help to stratify histology-independent patient subsets benefitting from diverse treatments on the basis of their own tumour aggressiveness. In other words, Ki-67 LI would not act as a surrogate of diagnosis (for which it was not designed in whatever context is dealt with1,7) but rather constitutes an innovative key player in the clinical decision-making process on metastatic pulmonary carcinoid patients as previously proposed for resection specimens.15 The variable association between Ki-67 LI and histology we documented between observers supported even more its independent value of traditional morphology, whereas the substantial inter-observer agreement of Ki-67 LI on both types of material – with Cohen kappa 0.56 in TP and 0.75 in TM – confirmed its reliability as decision-making parameter outperforming histologic subtyping, which in turn suffered from lower reproducibility of necrosis and, to some extent, mitotic count.4,16 Strict counting guidelines of Ki-67 LI were likely to account for this improved inter-observer and inter-material variability on both resection and biopsy samples.9 This parameter was also superior to histology to predict patient survival in TM when adopting a 10% cut-off value, whereas a steady increase in Ki-67 LI was revealed at metastatic sites indicative of malignancy progression.17
In our study, decision on the treatment type of metastatic pulmonary carcinoid patients had been guided on the basis of the individual clinical balance and performance status, although Ki-67 LI was originally available in all cases, whereas histologic diagnosis had remained carcinoid NOS in almost 50% of instances on revision because of challenging material (4 samples were FNAC and other 5 missed defining criteria due to small size and/or crushing).5,9 Of note, patients treated in first line with SSAs showed a trend for longer survival compared with those also receiving everolimus or treated with CT, in whom the relevant Ki-67 LI averaged 8.6%, 12.8%, and 28%, respectively. These differences among the first-line patient groups were likely to be the cause rather than the consequence of the diverse therapies, inasmuch as precise guidelines on the use of Ki-67 LI in metastatic lung carcinoids are still lacking.2,8 In second and third line, SSA patients showed an average 3.5% Ki-67 LI, whereas those also receiving everolimus and/or PRRT or further CT courses averaged 14.1% and 23.5% Ki-67 LI, respectively. Interestingly, 10% and 20% cut-off levels have recently been proposed in primary AC to identify diversely behaving tumours15,18 and as a tool to avoid misdiagnosing AC as SCLC.5,9
Accordingly, we devised Ki-67 LI cut-off levels <10%, between 10% and 20%, and >20% to stratify different patient subsets as a function of such a therapy-related distribution model (Figure 2). More specifically, metastatic lung carcinoids with a lower proliferation activity (Ki-67 LI <10%) could be amenable of SSAs; patients with an intermediate proliferation activity (10% ⩽ Ki-67 ⩽ 20%) could also undergo mTOR inhibitors and/or PRRT; and patients with a higher proliferation rate (Ki-67 >20%) could benefit from alkylating agents, antiangiogenic drugs, or capecitabine/temozolomide much better than platinum-based CT. Interestingly, all these treatments have been proposed in the therapy of metastatic lung carcinoids,2,14 although biological pre-selection strategies according to tumour aggressiveness have often been missed. We found that there was a trend towards an average increase in Ki-67 LI from TP to TM in all patients under evaluation; this remarks the concept of transition or evolution of carcinoids to neuroendocrine carcinomas as recently highlighted.10,11
Limitations to our study were its retrospective character, the relatively small number of patients (despite the rarity of well-documented metastatic carcinoids as investigated in literature), not complete uniformity in the treatments, and the lack of an independent validation set.
Conclusions
Our results make up the first proof of concept on the potential strategic use of Ki-67 LI in the clinical handling of metastatic lung carcinoids. The assessment of Ki-67 LI is likely destined to become a backbone in the clinical handling of metastatic lung carcinoids in virtue of the close relationship among its expression, clinical behaviour, and, potentially, therapy options as preliminarily suggested in the current investigation. The current view still emphasizes the preeminence of morphology, but it is also essential to implement innovative algorithms based on the use of Ki-67 LI in the decision-making process on lung carcinoids as recently emphasized.19 Further improvements in the quantification criteria of this marker in biopsy samples will increase even more its clinical utility.9
Supplemental Material
Supplemental material, Supplemental_Material for Ki-67 Evaluation for Clinical Decision in Metastatic Lung Carcinoids: A Proof of Concept by Giuseppe Pelosi, Federica Massa, Gaia Gatti, Luisella Righi, Marco Volante, Nadia Birocco, Patrick Maisonneuve, Angelica Sonzogni, Sergio Harari, Adriana Albini and Mauro Papotti in Clinical Pathology
Acknowledgments
This work is dedicated to the memory of Carlotta, an extraordinarily lively girl who died untimely of cancer in the prime of her life.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.V. was partially supported by grants from the Italian Association for Cancer Research, IG19238. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, which are responsibilities of the authors only.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: GP conceived, designed, wrote and finalized the manuscript; FM and GG reviewed all histological slides and contributed to write the manuscript; LR, MV, AS, SH, AA and MP contributed to write and critically evaluate the manuscript; NB provided clinical information and critically revised the manuscript; PM performed all statistical analysis. All authors approved the submitted version.
Ethical Approval: The study was approved by the Internal Review Board of participants’ institutions.
ORCID iD: Giuseppe Pelosi  https://orcid.org/0000-0003-4725-4692
https://orcid.org/0000-0003-4725-4692
References
- 1. Travis W, Brambilla E, Burke A, Marx A, Nicholson A. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2015. [DOI] [PubMed] [Google Scholar]
- 2. Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015;26:1604-1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
- 3. Derks JL, Speel EJ, Thunnissen E, et al. Neuroendocrine cancer of the lung: a diagnostic puzzle. J Thorac Oncol. 2016;11:e35–e38. doi: 10.1016/j.jtho.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 4. Swarts DR, van Suylen RJ, den Bakker MA, et al. Interobserver variability for the WHO classification of pulmonary carcinoids. Am J Surg Pathol. 2014;38:1429-1436. doi: 10.1097/PAS.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 5. Pelosi G, Rodriguez J, Viale G, Rosai J. Typical and atypical pulmonary carcinoid tumor overdiagnosed as small-cell carcinoma on biopsy specimens: a major pitfall in the management of lung cancer patients. Am J Surg Pathol. 2005;29:179-187. [DOI] [PubMed] [Google Scholar]
- 6. Pelosi G, Pattini L, Morana G, et al. Grading lung neuroendocrine tumors: controversies in search of a solution. Histol Histopathol. 2017;32:223-241. doi: 10.14670/HH-11-822. [DOI] [PubMed] [Google Scholar]
- 7. Pelosi G, Rindi G, Travis WD, Papotti M. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. J Thorac Oncol. 2014;9:273-284. [DOI] [PubMed] [Google Scholar]
- 8. Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12:425-436. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 9. Fabbri A, Cossa M, Sonzogni A, et al. Ki-67 labeling index of neuroendocrine tumors of the lung has a high level of correspondence between biopsy samples and surgical specimens when strict counting guidelines are applied. Virchows Arch. 2017;470:153-164. doi: 10.1007/s00428-016-2062-2. [DOI] [PubMed] [Google Scholar]
- 10. Pelosi G, Bianchi F, Dama E, et al. Most high-grade neuroendocrine tumours of the lung are likely to secondarily develop from pre-existing carcinoids: innovative findings skipping the current pathogenesis paradigm. Virchows Arch. 2018;472:567-577. doi: 10.1007/s00428-018-2307-3. [DOI] [PubMed] [Google Scholar]
- 11. Pelosi G, Sonzogni A, Harari S, et al. Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 2017;6:513-529. doi: 10.21037/tlcr.2017.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bosman F, Carneiro F, Hruban R, Theise N. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. [Google Scholar]
- 13. Lloyd R, Osamura R, Klöppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2017. [Google Scholar]
- 14. Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of pasireotide LAR or everolimus alone, or in combination in patients with advanced carcinoids (NET) of the lung/thymus: results from the randomized, phase 2 LUNA study. Ann Oncol. 2016;27:vi136-vi148. [Google Scholar]
- 15. Rindi G, Klersy C, Inzani F, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer. 2014;21:1-16. [DOI] [PubMed] [Google Scholar]
- 16. Warth A, Fink L, Fisseler-Eckhoff A, et al. Interobserver agreement of proliferation index (Ki-67) outperforms mitotic count in pulmonary carcinoids. Virchows Arch. 2013;462:507-513. doi: 10.1007/s00428-013-1408-2. [DOI] [PubMed] [Google Scholar]
- 17. Desmeules P, Sabari J, Santos-Zabala ML, et al. Metastatic lung carcinoid tumors: evidence of proliferation rate progression at metastatic sites. Mod Pathol. 2017;130:475A. [Google Scholar]
- 18. Marchiò C, Gatti G, Massa F, et al. Distinctive pathological and clinical features of lung carcinoids with high proliferation index. Virchows Arch. 2017;471:713-720. doi: 10.1007/s00428-017-2177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchevsky AM, Hendifar A, Walts AE. The use of Ki-67 labeling index to grade pulmonary well-differentiated neuroendocrine neoplasms: current best evidence. Mod Pathol. 2018;31:1523-1531. doi: 10.1038/s41379-018-0076-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Ki-67 Evaluation for Clinical Decision in Metastatic Lung Carcinoids: A Proof of Concept by Giuseppe Pelosi, Federica Massa, Gaia Gatti, Luisella Righi, Marco Volante, Nadia Birocco, Patrick Maisonneuve, Angelica Sonzogni, Sergio Harari, Adriana Albini and Mauro Papotti in Clinical Pathology