Abstract
Background:
Indigenous youth with type 2 diabetes (T2D) are disproportionately affected by early onset albuminuria and are at high risk of kidney failure in early adulthood. Traditional biological approaches have failed to fully explain the renal morbidity seen in this population. The improving renal Complications in Adolescents with type 2 diabetes through REsearch cohort (iCARE) study was therefore designed in collaboration with patients, to more holistically evaluate risk factors for renal morbidity. We hypothesize that both biological factors and mental health influence renal outcomes, mediated via inflammatory pathways.
Objective:
The objective of this study was to evaluate the iCARE analytic framework which evaluates relationships between biological factors, mental health, inflammation, and albuminuria utilizing a structural equation modeling (SEM) approach.
Methods:
The first 187 youth with T2D (10-25 years) from the Manitoba iCARE cohort are presented here to evaluate our theoretical and analytic framework. An SEM was chosen to evaluate the statistical significance of proposed associations. The primary outcome was a nonorthostatic urine albumin:creatinine ratio ≥2 mg/mmol. Main exposures (ie, latent factors) included psychological health (distress, perceived stress, positive mental health and resilience), hypertension (24 hour monitored), and inflammatory markers (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], fibrinogen). Hemoglobin A1c (HbA1c) and duration of diabetes were covariates.
Results:
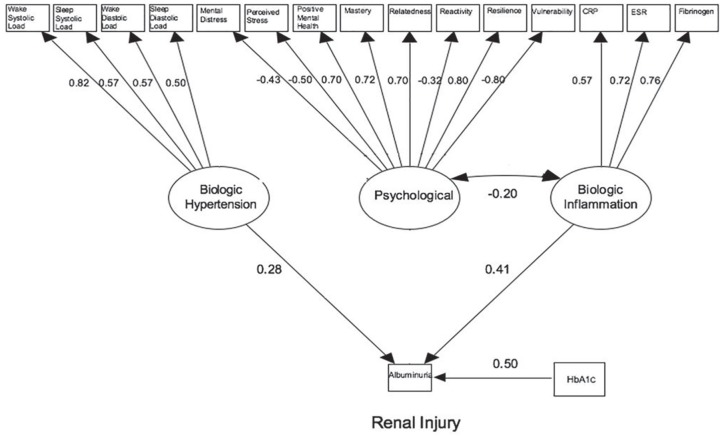
Within the initial cohort (median age = 15 years, duration of diabetes = 2.3 years, 66.8% female), 30.5% (n = 57) had nonorthostatic albuminuria (ALB), and the majority of ALB was persistent (confirmed in 2/3 samples over a 6-month period; n = 47). Youth with ALB had higher HbA1c (10.9% vs 8.9%; P < .001), more hypertension (94.2% vs 78·2%; P = .02), longer duration of diabetes (3.4 vs 2.4 years; P = .01), higher distress (9.2 vs 7.3; P = .02), and stress scores (28.7 vs 26.4; P = .03), and elevated inflammatory markers (CRP: 4.9 vs 3.1 mg/L; P = .01, fibrinogen: 3.7 vs 3.3 µmol/L; P = .02). Factors directly associated with ALB in the SEM were hypertension (0.28; P = .001), inflammation (0.41; P < .001), and HbA1c (0.50; P < .001). Psychological health was independently associated with inflammation (−0.20; P < .001) but not directly associated with ALB.
Conclusions:
Albuminuria is highly prevalent in Indigenous youth with T2D. This preliminary analysis supports a theoretical framework linking glycemic control, hypertension, and inflammation, potentially mediated by psychological factors with albuminuria. These data support the need for more holistic models of evaluation and care for youth with T2D and multifactorial interventions to prevent complications.
Keywords: type 2 diabetes, youth, albuminuria, structural equation model, holistic
Abrégé
Contexte:
L’albuminurie à déclenchement précoce affecte de façon disproportionnelle les jeunes autochtones atteints de diabète de type 2 (T2D). Ces derniers présentent également un risque plus élevé d’insuffisance rénale au début de l’âge adulte. Les approches biologiques traditionnelles n’ont pas été en mesure d’expliquer entièrement la morbidité rénale observée dans cette population. Ainsi, l’étude de cohorte iCARE (improving renal Complications in Adolescents with type 2 diabetes through REsearch) a été conçue en collaboration avec les patients pour évaluer de façon plus globale les facteurs de risque de morbidité rénale. Nous posons l’hypothèse que les résultats rénaux sont influencés à la fois par la santé mentale du patient et des facteurs biologiques, avec médiation par les voies inflammatoires.
Objectif:
Évaluer le cadre d’analyse iCARE qui examine les liens entre les facteurs biologiques, la santé mentale, l’inflammation et l’albuminurie à l’aide d’une approche de modélisation par équation structurelle (SEM).
Méthodologie:
Les 187 premiers jeunes autochtones atteints de T2D (âgés de 10 à 25 ans) de la cohorte manitobaine iCARE sont présentés ici pour évaluer notre cadre théorique et analytique. Une SEM a été choisie pour évaluer la pertinence statistique des associations suggérées. Le résultat principal était un rapport urinaire albumine/créatinine non orthostatique d’au moins 2 mg/mmol. Les principaux risques (c.-à-d. les facteurs latents) comprenaient la santé mentale (détresse, stress perçu, bien-être mental et résilience), l’hypertension (suivie sur 24 heures) et les taux de marqueurs inflammatoires (CRP, ESR, fibrinogène). L’hémoglobine A1c (HbA1c) et la période depuis l’apparition du diabète constituaient les covariables.
Résultats:
Les sujets retenus (66,8 % de sujets féminins) avaient 15 ans d’âge médian et étaient diabétiques depuis 2,3 ans. Dans cette cohorte, 30,5 % (n = 57) présentaient une albuminurie non orthostatique (confirmée dans 2/3 des échantillons sur une période de six mois) qui s’est avérée persistante dans la majorité des cas (n = 47). Les jeunes souffrant d’albuminurie présentaient des taux plus élevés d’HbA1c (10,9 c. 8,9 %; P < ,001), davantage d’hypertension (94,2 c. 78,2 %; P = ,02), étaient diabétiques depuis plus longtemps (3,4 c. 2,4 ans; P = ,01), vivaient davantage de détresse (9,2 c. 7,3; P = ,02), et présentaient des scores pour le stress (28,7 c. 26,4; P = ,03) et des taux de marqueurs inflammatoires plus élevés (CRP : 4,9 c. 3,1 mg/L; P = ,01, fibrinogène : 3,7 c. 3,3 µmol/L; P = ,02). Avec la SEM, les facteurs directement associés à l’albuminurie étaient l’hypertension (0,28; P = ,001), l’inflammation (0,41; P < ,001) et l’HbA1c (0,50; P < ,001). La santé psychologique a été associée à l’inflammation de manière indépendante (−0,20; P < ,001), mais n’a pas été directement associée à l’albuminurie.
Conclusion:
L’albuminurie est très répandue chez les jeunes autochtones atteints de T2D. Cette analyze préliminaire vient étayer un cadre théorique qui établit un lien entre l’albuminurie et le contrôle de la glycémie, l’hypertension et l’inflammation; lien potentiellement médié par des facteurs psychologiques. Ces données appuient la nécessité d’avoir des modèles plus holistiques d’évaluation et de prise en charge des jeunes atteints de T2D, et des interventions multifactorielles visant à prévenir les complications.
What was known before
Indigenous youth with type 2 diabetes are at very high risk of early and progressive renal complications. Traditional risk factors do not fully explain the renal morbidity seen in this population. Indigenous youth endorse mental health as important; however, its role in renal complications is unknown.
What this adds
This study supports the iCARE theoretical framework, which identifies the potential multifactorial pathogenesis of early kidney injury in this population. In this preliminary analysis, glycemic control and hypertension are associated with albuminuria, while psychological stress and distress may mediate their effects on albuminuria via inflammatory pathways.
Introduction
Pediatric diabetes is increasing, now affecting close to 1% of children in North America.1 Rates of type 2 diabetes are increasing disproportionately in minority ethnic groups such as Indigenous populations, and in some areas of the world including the Canadian province of Manitoba, new cases of type 2 diabetes now surpass new cases of type 1 diabetes. This is of significant concern because Indigenous populations have consistently been shown to be at an increased risk of chronic kidney disease (CKD) and progress more quickly throughout each stage of CKD.2 Similarly, youth onset type 2 diabetes has also consistently been shown to be associated with significantly higher rates of early kidney complications, initially presenting with albuminuria as an early marker of kidney injury.3 While albuminuria can regress in traditional diabetic nephropathy, it has been shown in Indigenous populations that it is less likely to do so and predicts progression to end-stage kidney disease in young adulthood.4 Population-based studies have attempted to explain the excessive burden of kidney injury in youth with type 2 diabetes; however, in the absence of rigorous risk factor phenotyping and sufficient sample sizes, 80% of the increased renal burden among youth with type 2 diabetes remains unexplained.5
Our group and others have documented the clinical characteristics that distinguish type 2 diabetes in youth from youth with type 1 diabetes that could explain the higher burden of kidney disease. These include greater adiposity and increased cardiometabolic risk burden.6 In addition, youth with type 2 diabetes tend to live in lower socioeconomic households and are predominantly from minority ethnic groups including Indigenous communities.7 Finally, compared with youth with type 1 diabetes, youth with type 2 diabetes display a low quality of life and a greater risk for depression and anxiety.7,8 These factors are particularly relevant to Indigenous youth worldwide, who suffer from some of the highest rates of poor quality of life, suicide, and mental illness. To date, a comprehensive study of the impact of this clustering of interrelated risk factors on kidney injury in Indigenous youth with type 2 diabetes has yet to be performed.
The improving renal Complications in Adolescents with type 2 diabetes through REsearch (iCARE) cohort9 was designed to evaluate the most relevant risk factors for early onset albuminuria in youth with type 2 diabetes. We have created a theoretical framework in collaboration with our patient advisory group which includes biological factors such as glycemic control and hypertension as well as psychological factors such as perceived stress and mental distress. We hypothesize that psychological pathways influence renal outcomes via metabolic hormone stimulation and subsequent influence on inflammatory pathways. Here, we present a preliminary evaluation of the planned structural equation modeling (SEM) approach.
Patients and Methods
The iCARE study rationale, design, and methods have previously been reported.9 In brief, iCARE is a prospective cohort study designed to discover novel risk factors and therapeutic targets for the prevention and treatment of albuminuria and progression of CKD in youth with type 2 diabetes. The study began as a single-center study in Winnipeg, Manitoba, and has now been funded to expand to a multicenter study to test a holistic bio-psycho-social (BPS) model of renal health trajectories in youth with type 2 diabetes of broader ethnic diversity with a planned sample size of 400 youth. This initial cohort was predominantly Indigenous and recruited from Manitoba and Northwestern Ontario. The study protocol was approved by the biomedical research board at the University of Manitoba, in accordance with the Declaration of Helsinki. All patients or their guardians (if less than 18 years of age) provided informed consent and youth less than 18 years provided assent. In addition, our study is guided by a patient and parent advisory group and an advisory board with representation from community and government-based organizations (First Nations Health and Social Secretariat of Manitoba, the regional CIHR-funded Partners for Engagement and Knowledge Exchange, and primary care and community-based care representatives).
Population
Participants in the Manitoba iCARE cohort consist of 10- to 25-year-old adolescents and young adults diagnosed with type 2 diabetes prior to 18 years of age. The first 187 recruited in the study were included in this preliminary analysis. The diagnosis of type 2 diabetes was made according to the Canadian Diabetes Association guidelines,10 consistent with those of the American Diabetes Association. The diagnosis of type 2 diabetes was based on clinical criteria including the presence of obesity, other evidence of insulin resistance, family history of type 2 diabetes, intrauterine exposure to hyperglycemia, and family heritage from a high-risk ethnic group and the absence of insulin and glutamic acid decarboxylase antibodies. Exclusion criteria were medication or surgery induced diabetes, ever being diagnosed with cancer, current treatment with immunosuppressant medications, a concomitant chronic inflammatory condition, currently being pregnant, or unable/unwilling to give consent.
Exposures and Outcome Variables
Biological Factors
To generate a continuous measure of blood pressure that was standardized to age and sex, daytime and nocturnal systolic and diastolic blood pressure loads were chosen for this initial analysis (ie, the percentage of time spent above the 95th percentile for sex and height), measured with 24-hour ambulatory blood pressure monitoring (SpaceLabs, Snoqualmie, Washington). The daytime parameter of 140/90 mm Hg and nocturnal parameter of 120/80 mm Hg were utilized for participants 18 years of age or older. Any hypertension was defined as ≥1 blood pressure parameter (daytime or nocturnal systolic or diastolic) above the 95th percentile for sex and height more than 25% of the time for youth <18 years of age.11 Glycemic control was quantified using hemoglobin A1c (HbA1c) analyzed on a Roche Cobas Integra 800 CTS (Diagnostic Services Manitoba laboratory, Health Sciences Center, Winnipeg). Smoking status was self-reported as cigarettes per day. Nonsmokers were coded as 0 cigarettes per day. Angiotensin-converting enzyme inhibitor (ACEi) and angiotensin receptor blocker (ARB) use was determined from self-report.
Psychological Factors
The following validated questionnaires were utilized to evaluate various aspects of mental health. The Kessler Psychological Distress Scale (K6) was utilized to capture symptoms of depression and anxiety. This measure has a receiver operating characteristic curve (ROC) of 0.87 to 0.88 for disorders of Global Assessment Functioning and has been used in the US Mental Health and Canadian Community Health Surveys (CCHS) for youth >12 years of age and young adults and has been validated in many populations including Indigenous.12 A score ≥13 is consistent with a mental health problem. The Perceived Stress Scale 14 (PSS-14) was used to assess perceived level of stress.13,14 Higher scores reflect more perceived stress (up to 56). There are no set cutoffs. The Mental Health Continuum was used to assess positive mental health, has excellent discriminant validity in adolescents, and was also utilized in the CCHS for youth and young adults.15 This scale can be scored between 0 and 70 where a higher score reflects higher levels of emotional well-being. Finally, the Resilience Scale for Children and Adolescents (RSCA), a measure specifically developed and validated in children, was used to assess the resiliency constructs of mastery, relatedness, and emotional reactivity as well as calculated resourcefulness and vulnerability scores.16 We present the mean scores with standard deviation for each subscale. Higher scores reflect more resilience except for the vulnerability score which reflects more vulnerability.
Inflammatory Markers
C-reactive protein (CRP) was quantified using a particle-enhanced immunoturbidimetric assay. Erythrocyte sedimentation rate (ESR) was quantified using Alifax Test 1 assay. Fibrinogen was assessed using the Clauss instrument coagulation instruments (from Instrumentation Laboratory). All samples were run at the Diagnostic Services Manitoba laboratory at the Health Sciences Center in Winnipeg.
Demographic Variables
Current age, sex, self-reported ethnicity, and duration of diabetes were included as demographic variables. Height and weight were measured directly in duplicate using a calibrated stadiometer and electronic scale. The mean values were used to calculate body mass index (BMI) and BMI z-scores.
Primary Outcome
The main outcome of interest was nonorthostatic albuminuria as defined by a urine albumin to creatinine ratio (ACR) greater than 2·0 mg/mmol on an overnight urine collection (preferred), or first morning sample (if overnight sample not available). Persistent albuminuria was defined as an ACR >2.0 mg/mmol on 2/3 samples over a 6-month period as per national guidelines.
Statistical Analysis
Univariate analyses were initially conducted to inform the SEM. An SEM was chosen for this analysis as it is a powerful statistical modeling technique for observational data that can be used to statistically confirm a hypothesized model by evaluating the observed covariance structure of the data. It also has the ability to include latent variables, which are underlying unobservable phenomena assessed by clustering of measured indicator variables. This was especially important for our study for the inclusion of “psychological health” for example, which is not directly observable, but rather made up of a series of measurable variables like anxiety, depression, resilience, and stress, utilizing standardized tools, as described below. SEM describes a class of causal linear models representing a mix of confirmatory factor analysis (measurement model) and path analysis (structural model). Conceptually, the measurement model is first estimated and the correlation/covariance matrix used as input to estimate the structural coefficients (eg, regression betas). In practice, both models are estimated simultaneously with SEM software.
We took 2 preliminary steps to inform the final SEM. First, differences in predictor variables and confounders between groups with and without albuminuria were evaluated. T tests, for continuous variables, and Pearson chi-square tests, for categorical variables, were used to compare differences in exposures between youth stratified by the presence of albuminuria. A P value of <.05 was considered significant for these analyses.
Subsequently, a principal component analysis (PCA) was performed. Polychoric/tetrachoric correlations were used and fitted with weighted least squares for the exploratory model without imputation. ACEi/ARB use was not included in the modeling as it is a confounder by indication.3
Based on the exploratory PCA, we created a measurement model with three latent factors (hypertension, psychological wellness, and inflammation). The validity of the latent factor model was assessed by confirmatory factor analysis. Overall model fit was assessed using chi-square analysis and root mean square error approximation (RMSEA). R software 3.2 was utilized for this analysis.
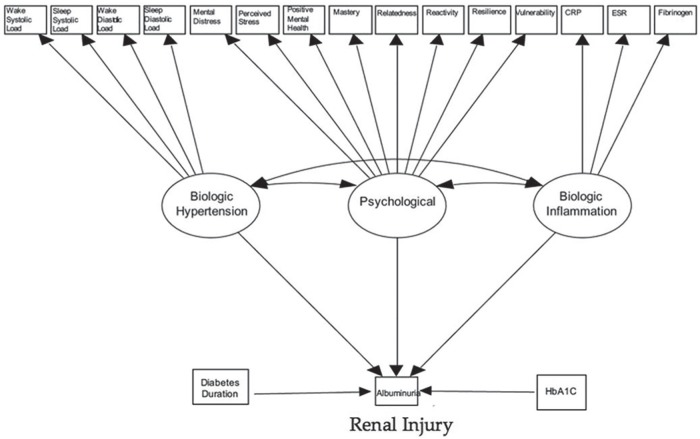
Lavaan 0.5-18 (LAtend VAriable ANalysis; Rosseel, 2012)17 was then used to fit the SEM to the study participants using diagonally weighted least squares, to accommodate 15 continuous indicators and the primary outcome, albuminuria. With item factor analysis, a continuous underlying variable was assumed to be related to albuminuria and its threshold estimated as part of the model fit. Three latent factors were included: hypertension (4 indicators = wake systolic and diastolic blood pressure loads and sleep systolic and diastolic blood pressure loads), inflammation (3 indicators = CRP, ESR, fibrinogen), and psychological wellness (indicators = distress [K6 score], perceived stress [PSS-14 score], positive mental health [MHC score], and resiliency measures: mastery, relatedness, reactivity, resourcefulness, and vulnerability). All latent factors were assumed to covary and each was independently allowed to causally influence albuminuria status. HbA1c and diabetes duration were treated as independent covariates with causal paths leading directly to the outcome. Model fit was assessed by chi-square, RMSEA, and comparative fit indices (Tucker–Lewis index [TLI]). Scale was imposed on the latent factors using the standardization method (mean = 0, SD = 1; all loadings freely estimated), and conditional local independence was assumed for all manifest variables. Missing data were imputed using multiple chained equations with 20 complete datasets. To facilitate comparison and interpretation, all parameter estimates are based on standardized variables and interpretable as correlations. The a priori theoretical model was developed by the iCARE investigators based on exploratory factor analysis, review of the literature, and input from our patient advisory group with post hoc assessment of the assumed structural form based on residual correlations and modification indices.
Results
Of the 187 youth with type 2 diabetes included in this analysis, 30.5% (57/187) presented with nonorthostatic albuminuria and the majority of these cases also had persistent albuminuria (82.5%; n = 47). The median age was 15 years (IQR: 13.3-16.8), duration of type 2 diabetes was 2.3 years (IQR: 0.9-4.1), and 66.8% were female. ACEi/ARB use was identified in 24.1% of the group with albuminuria and only 3.1% of the group without it (P < .001).
In the univariate analysis (Table 1), youth with albuminuria had longer duration of diabetes (3.4 vs 2.4 years; P = .01), higher HbA1c (10.9% vs 8.9%; P < .001), higher blood pressure loads, and were more likely to be hypertensive (94.2% vs 78.2%; P = .02), relative to youth without albuminuria. Age, sex, and smoking habits were not different between groups. The mental health outcomes and inflammatory markers are presented in Table 2. Youth with albuminuria had higher distress scores (9.2 vs 7.3; P = .02), perceived stress scores (28.7 vs 26.4; P = .03), and some markers of inflammation (CRP: 4.9 vs 3.1 mg/L; P = .01, fibrinogen: 3.7 vs 3.3 µmol/L; P = .02). Positive mental health and determinants of resilience were not different between groups.
Table 1.
Demographic and Clinical Characteristics of the Manitoba iCARE Cohort.
| Albuminuria group N = 57 (30.5%) |
No albuminuria N = 130 (69.5%) |
P value | |
|---|---|---|---|
| Age (years) | 15.7 ± 2.4 | 14.9 ± 2.4 | .07 |
| Sex (% female) | 73.7 | 63.8 | .30 |
| Duration of diabetes (years) | 3.4 ± 2.4 | 2.4 ± 2.1 | .01 |
| Indigenous ethnicity (%) | 98.2% | 94.6% | .40 |
| BMI Z-score | 2.4 ± 1.2 | 2.4 ± 1.0 | .9 |
| HbA1c (%) (mmol/mol) |
10.9 ± 2.3 (96 ± 25.1) |
8.9 ± 2.5 (74 ± 27.3) |
<.001 |
| Smoking (Cigarettes per day) |
0 (IQR: 0-12) |
0 (IQR: 0-13) |
.08 |
| Wake systolic blood pressure load (%) | 45.8 (IQR: 10.5-94.1) |
22.9 (IQR: 17.8-64.8) |
.003 |
| Wake diastolic blood pressure load (%) | 18.3 (IQR: 9.8-30.4) |
10.6 (IQR: 5.4-18.7) |
.009 |
| Nocturnal systolic blood pressure load (%) | 63.2 (IQR: 42.0-85.9) |
47.7 (IQR: 12.6-68.2) |
.004 |
| Nocturnal diastolic blood pressure load (%) | 63.2 (IQR: 42-100) |
47.7 (IQR: 42-85.9) |
.05 |
| Any hypertension (%) | 94.2 | 78.2 | .02 |
| ACE inhibitor/angiotensin receptor blocker (%) | 24.1 | 3.1 | <.001 |
Note. iCARE = improving renal Complications in Adolescents with type 2 diabetes through REsearch; BMI = body mass index; HbA1c = hemoglobin A1c; IQR = interquartile range; ACE = angiotensin-converting enzyme.
Table 2.
Psychological and Inflammatory Measures of the Manitoba iCARE Cohort.
| Albuminuria group N = 57 (30.5%) |
No albuminuria N = 130 (69.5%) |
P value | |
|---|---|---|---|
| Distress (Kessler 6 score) |
9.2 ± 5.1 | 7.3 ± 4.1 | .02 |
| Perceived Stress Score (PSS-14 score) |
28.7 ± 6.5 | 26.4 ± 5.6 | .03 |
| Positive Mental Health (MHC score) |
39.4 ± 17.2 | 44.1 ± 13.2 | .08 |
| Mastery | 39.5 ± 10.9 | 40.6 ± 11.4 | .6 |
| Relatedness | 36.2 ± 12.7 | 39.7 ± 11.6 | .1 |
| Reactivity | 52.8 ± 12.4 | 52.4 ± 10.22 | .8 |
| Resilience | 36.6 ± 12.0 | 39.2 ± 11.8 | .2 |
| Vulnerability | 59.8 ± 11.3 | 58.2 ± 10.7 | .4 |
| CRP (mg/L) | 4.9 (IQR: 2.4-8.0) |
3.1 (IQR: 1.7-5.5) |
.01 |
| ESR (mm/h) | 19.0 (IQR: 8.0-30.0) |
14.0 (IQR: 6.0-25.8) |
.2 |
| Fibrinogen (µmol/L) | 3.7 ± 0.9 | 3.3 ± 0.7 | .02 |
Note. iCARE = improving renal Complications in Adolescents with type 2 diabetes through REsearch; PSS = Perceived Stress Scale; MHC = Mental Health Continuum; CRP = C-reactive protein; IQR = interquartile range; ESR = erythrocyte sedimentation rate.
The PCA identified 4 principal components, which explained 54% of the variance in the prediction of albuminuria. Based on indicators with loadings >0.5, the latent factors were defined as psychological factors (PC1), hypertension (PC2), inflammation (PC3), and other (PC4; age and duration of diabetes). Psychological factors explained 22%, hypertension explained 12%, inflammation explained 11%, and other factors explained 9% of the variability in albuminuria (54% total variability explained) within the cohort.
The SEM is presented in Figure 1. Figure 2 presents a revised model which includes only the statistically significant loadings. The coefficients presented in both Table 3 and Figure 2 can be interpreted as correlation coefficients measuring the linear relationship between the latent variable or covariate and albuminuria on a scale of −1 to 1. For example, the correlation between HbA1c and albuminuria is 0.499, whereas the correlation between hypertension and albuminuria is only 0.282. As a result, 24.9% (ie, 0.499)2 of the variation in albuminuria is explained by HbA1c vs 8.0% explained by hypertension. The P values <.05 suggest a statistically significant result. Overall model fit was good with a chi-square P value = .11 and RMSEA = 0.032 (0-0.049, P = .96) (ie, both P > .05, therefore null hypothesis of good fit not rejected). The patient advisory group also developed a lay version of the findings which is presented as an infographic in Figure 3.
Figure 1.
Structural equation model developed to evaluate associations between latent factors and covariates with albuminuria.
Note. Variances or residual error terms not shown for clarity. CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; HbA1c = hemoglobin A1c.
Figure 2.
Structural equation model showing statistically significant factor loadings.
Note. Factor loadings can be interpreted as correlations. CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; HbA1c = hemoglobin A1c.
Table 3.
Regression Analyses Evaluating Relationships Between Latent Factors, Covariates, and Albuminuria in the Structural Equation Model.
| Variables | Standardized loadings | SE | P value |
|---|---|---|---|
| By albuminuria status | |||
| Hypertension | .282 | .086 | .001 |
| Psych Factors | −.095 | .066 | .148 |
| Inflammation | .407 | .086 | <.001 |
| Hemoglobin A1c | .499 | .117 | <.001 |
| Duration of Diabetes | .169 | .113 | .136 |
| Covariances | |||
| Hypertension | |||
| Psych Factors | .049 | .050 | .318 |
| Inflammation | .061 | .040 | .311 |
| Psych Factors | |||
| Inflammation | −.198 | .040 | <.001 |
Note. Coefficients can be interpreted as correlations.
Figure 3.
Infographic of preliminary results from the iCARE study (designed by iCARE Participant Advisory Group (PAG)).
Table 3 describes results from the regression analyses of the association between latent factors, covariates, and albuminuria status. Significant associations were seen with coefficients for hypertension (β = 0.28; P = .001), inflammation (β = 0.41; P < .001), and HbA1c (β = 0.50; P < .001) in the structural model for albuminuria. Psychological factors were not directly associated with albuminuria; however, there was a significant association between the psychological latent factor and inflammation (β = −0.20; P < .001). This model explained 44.0% of the variance in the albuminuria status.
Discussion
Indigenous youth with type 2 diabetes have a disproportionate burden of early kidney complications. In this article, we present support for a novel theoretical framework which links biological and psychological risk factors via systemic inflammatory markers with albuminuria in this population. While this analysis is preliminary, it presents the importance of a broad approach to risk evaluation in this population including gold standard measures of biological risk assessment including 24-hour ambulatory blood pressure monitoring, as well as validated mental health assessment tools.
The preliminary albuminuria rates in the iCARE cohort suggest that our cohort may be at particularly high risk. Previously reported rates of albuminuria among youth with type 2 diabetes had been quite variable. In the TODAY trial, the prevalence of albuminuria was only 6.3% among the 699 youth with type 2 diabetes at randomization and increased 2.5 fold to 16.6% over the 3.9 years of follow-up.18 National surveillance studies in Canada and the United States revealed rates of albuminuria in youth with type 2 diabetes of 5.1% (Canadian Pediatric Surveillance Program)19 and 22.2% (SEARCH),5 respectively. Of note, albuminuria has been identified at diagnosis in many cases and therefore suggests preexisting renal injury of other causes. Importantly, all studies ensured urine samples were collected first morning or overnight to exclude nonorthostatic proteinuria. As age and duration of diabetes are similar across studies, the elevated rates of renal injury in youth within this cohort may be explained by the higher rates of metabolic decompensation (mean HbA1c = 9.9%), hypertension (>80%), and poor mental health. It may be alarming to some readers that the glycemic control in this population is so poor. While this is an observational study, all participants are also seen clinically in the Diabetes Education Resource for Children and Adolescents (DER-CA) and receive the current education and treatment that is standard of care. The high HbA1c reflect the significant barriers that exist for these youth grounded in the past and current colonial actions that adversely affect indigenous children worldwide.20 Certainly more efforts are required to identify better treatment strategies that address the existing barriers. In essence, these data reinforce calls both for tight multifactorial risk factor control and a broader, holistic approach to preventing albuminuria in Indigenous youth with type 2 diabetes.
Strict glycemic control is the current cornerstone in the prevention of complications in type 2 diabetes in adults21 and youth.3,5,18 While the important role of hypertension is also clear in the adult literature,22 previous observational studies in children have not consistently shown hypertension to be associated with albuminuria.3 The current study expands on one, relatively small (n = 24), previous report that found a positive association between hypertension, derived from using 24-hour ambulatory monitoring, and albuminuria in youth with type 2 diabetes.23 Using the same methodology in a larger cohort, we found that all types of hypertension (systolic, diastolic, daytime and nocturnal) were associated with albuminuria. These factors collectively predicted ~12% of the variance in albuminuria status among youth with type 2 diabetes. This is a novel finding among youth with type 2 diabetes, as hypertension was not associated with albuminuria in either of the TODAY18 or SEARCH5 studies; however, importantly they did not use ambulatory monitoring to quantify blood pressure in these studies. This observation reinforces the benefits of routine utilization of 24-hour ambulatory blood pressure monitoring in youth with type 2 diabetes in clinical care. Future analyses will include the inclusion of mean blood pressure as well, which is now considered the gold standard for blood pressure assessment.
There is an increasing awareness of the potential interactions between mental and physical health among adolescents.24 In the context of a chronic disease such as type 2 diabetes, psychological factors can impact quality of life25 and play an important role in self-management and the adoption of healthy lifestyle behaviors.26,27 Quality of life is lower8 and rates of depression28 are higher among youth with type 2 diabetes, relative to peers with type 1 diabetes. Indigenous youth are even more susceptible to poor mental health in part due to the combined social factors that Indigenous youth face (poverty, and racism) and the ongoing effects of multigenerational trauma.20 This is the first study in Indigenous youth with type 2 diabetes to determine whether various aspects of mental health are associated with albuminuria. Although the SEM did not reveal a direct association between the cluster of mental health variables and albuminuria, the mental health latent factor was associated with elevated inflammatory biomarkers, which were themselves associated with albuminuria. Negative mental health factors can manifest in elevated stress, which activates the hypothalamic-pituitary axis and the production of cortisol.29 This is a maladaptive process and can lead to systemic inflammation.30 Many recent studies have identified important associations between higher levels of inflammatory cytokines and diabetes complications,31 and there is also evidence that many anti-diabetic treatments have anti-inflammatory properties that are independent of their hypoglycemic effects.32 Evidence is starting to emerge in adult populations that integrative care models which address biomedical, psychological, and social diabetes care lead to both improved mental health and physical health.33 At this point, the impact of this type of treatment on systemic inflammation is unknown but does warrant future study. Our study does support the importance of screening for and managing poor mental health in the clinical management of Indigenous youth with type 2 diabetes.
The main strength of this study is the extensively phenotyped patient population of Indigenous youth with type 2 diabetes using gold standard methods for quantifying exposures and outcomes, including 24-hour blood pressure monitoring and overnight urine collections. We have also included validated measures of mental health to allow for inclusion of both biological and psychological determinants of risk in our analysis. The findings from the structural model revealed standardized loadings in the expected direction, and robust model fit statistics, confirming our theoretical framework. Despite these strengths, there are some limitations. First, this is a cross-sectional analysis, and therefore the associations observed cannot be considered causal. In the absence of prospective data, it is unclear whether the factors identified here will predict incident albuminuria or worsening of renal injury in youth with type 2 diabetes over time. As the iCARE cohort is a unique population, consisting largely of Indigenous youth, the generalizability of these findings in other cohorts needs to be evaluated. Finally, sample size is always an issue for complicated SEMs. Care was taken to respect the usual rule of thumb for endogenous categorical outcomes, namely 5 events (here albuminuria) for each direct parameter estimated for variables that point directly to the outcome (including slopes, variances, and covariances).34
Conclusion
Indigenous youth with type 2 diabetes have high rates of albuminuria. This study supports a multifactorial theoretical model linking glycemic control, hypertension, and inflammation in the pathogenesis of albuminuria in youth with type 2 diabetes. Psychological factors appear to play an important but indirect role in the pathophysiology of renal injury associated with type 2 diabetes in youth, potentially by mediating its effects through inflammatory pathways. These data support the need for more holistic models of evaluation and care for youth with type 2 diabetes and multifactorial interventions to prevent complications.
Acknowledgments
We acknowledge the iCARE patient advisory group for their guidance in developing this study.
Footnotes
Ethics Approval and Consent to Participate: The study protocol was approved by the biomedical research board at the University of Manitoba, in accordance with the Declaration of Helsinki. All patients or their guardians (if less than 18 years of age) provided informed consent and youth less than 18 years provided assent. In addition, our study is guided by a patient and parent advisory group and an advisory board with representation from community and government-based organizations (First Nations Health and Social Secretariat of Manitoba, the regional CIHR-funded Partners for Engagement and Knowledge Exchange, and primary care and community-based care representatives).
Consent for Publication: All authors read and approved the final version of this manuscript.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author.
Author Contributions: Allison Dart drafted the initial manuscript and assisted with participant enrollment and data collection. She is the guarantor and takes full responsibility for the contents of the article. Brandy Wicklow and Elizabeth A. C. Sellers made critical revisions of the manuscript for important intellectual content and assisted with participant enrollment and data collection. Tom D. Blydt-Hansen, Dan Chateau, Sayma Malik, and Jonathan M. McGavock made critical revisions of the manuscript for important intellectual content. Atul Sharma made critical revisions of the manuscript for important intellectual content and performed the statistical analysis. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the Canadian Diabetes Association #OG-3-11-3354-AD (Diabetes Canada) and the Manitoba Health Research Council #1475 (Research Manitoba) for the initial cohort creation. Additional funding has now been obtained from CIHR and the Can-SOLVE CKD SPOR network including partnership funding from Research Manitoba and the Children’s Hospital Research Institute of Manitoba.
ORCID iD: Allison B. Dart  https://orcid.org/0000-0002-4718-9408
https://orcid.org/0000-0002-4718-9408
References
- 1. Menke A, Casagrande S, Cowie CC. Prevalence of diabetes in adolescents aged 12 to 19 years in the United States, 2005-2014. JAMA. 2016;316(3):344-345. [DOI] [PubMed] [Google Scholar]
- 2. Samuel SM, Palacios-Derflingher L, Tonelli M, et al. Association between First Nations ethnicity and progression to kidney failure by presence and severity of albuminuria. CMAJ. 2014;186(2):E86-E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296(4):421-426. [DOI] [PubMed] [Google Scholar]
- 5. Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30(10):2593-2598. [DOI] [PubMed] [Google Scholar]
- 6. Amed S, Dean HJ, Panagiotopoulos C, et al. Type 2 diabetes, medication-induced diabetes, and monogenic diabetes in Canadian children: a prospective national surveillance study. Diabetes Care. 2010;33(4):786-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allan CL, Flett B, Dean HJ. Quality of life in First Nation youth with type 2 diabetes. Matern Child Health J. 2008;12(suppl 1):103-109. [DOI] [PubMed] [Google Scholar]
- 9. Dart AB, Wicklow BA, Sellers EA, et al. The Improving Renal Complications in Adolescents With Type 2 Diabetes Through the Research (iCARE) Cohort Study: rationale and Protocol. Can J Diabetes. 2014;38(5):349-355. [DOI] [PubMed] [Google Scholar]
- 10. Ball BW, Sellers EA, Wicklow BA, Dean HJ, Canadian Task Force on Preventive Health Care. Diabetes guidelines. CMAJ. 2013;185(3):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):1-74. [DOI] [PubMed] [Google Scholar]
- 12. Hood KK, Lawrence JM, Anderson A, et al. Metabolic and inflammatory links to depression in youth with diabetes. Diabetes Care. 2012;35(12):2443-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreou E, Alexopoulos EC, Lionis C, Varvogli L, Gnardellis C, Chrousos GP, Darviri C. Perceived Stress Scale: reliability and validity study in Greece. Int J Environ Res Public Health. 2011;8(8):3287-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184-189. [DOI] [PubMed] [Google Scholar]
- 15. Lamers SM, Westerhof GJ, Bohlmeijer ET, tenKlooster PM, Keyes CL. Evaluating the psychometric properties of the Mental Health Continuum-Short Form (MHC-SF). J Clin Psychol. 2011;67(1):99-110. [DOI] [PubMed] [Google Scholar]
- 16. Prince-Embury S. Assessing personal resiliency in the context of school settings: using the resiliency scales for children and adolescents. Psychol Schools. 2011;48:672-685. [Google Scholar]
- 17. Rosseel Y. Lavaan: An R Package for structural equation modeling. J Stat Softw. 2012;48(2):1-36. [Google Scholar]
- 18. Group TS. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sellers EA, Hadjiyannakis S, Amed S, et al. Persistent albuminuria in children with type 2 diabetes: a Canadian Paediatric Surveillance Program study. J Pediatr. 2016;168:112-117. [DOI] [PubMed] [Google Scholar]
- 20. Hackett C, Feeny D, Tompa E. Canada’s residential school system: measuring the intergenerational impact of familial attendance on health and mental health outcomes. J Epidemiol Community Health. 2016;70(11):1096-1105. [DOI] [PubMed] [Google Scholar]
- 21. DCCT/EDIC Research Group, de Boer IH, Sun W, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35(10):2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147(1):67-73. [DOI] [PubMed] [Google Scholar]
- 24. Keyes CL. Mental health in adolescence: is America’s youth flourishing. Am J Orthopsychiatry. 2006;76(3):395-402. [DOI] [PubMed] [Google Scholar]
- 25. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289(14):1813-1819. [DOI] [PubMed] [Google Scholar]
- 26. Hood KK, Beavers DP, Yi-Frazier J, Bell R, Dabelea D, Mckeown RE, Lawrence JM. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health. 2014;55(4):498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kongkaew C, Jampachaisri K, Chaturongkul CA, Scholfield CN. Depression and adherence to treatment in diabetic children and adolescents: a systematic review and meta-analysis of observational studies. Eur J Pediatr. 2014;173(2):203-212. [DOI] [PubMed] [Google Scholar]
- 28. Anderson BJ, Edelstein S, Abramson NW, et al. Depressive symptoms and quality of life in adolescents with type 2 diabetes: baseline data from the TODAY study. Diabetes Care. 2011;34(10):2205-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, vanRossum EF. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220-1235. [DOI] [PubMed] [Google Scholar]
- 30. Pervanidou P, Chrousos GP. Stress and obesity/metabolic syndrome in childhood and adolescence. Int J Pediatr Obes. 2011;6(suppl 1):21-28. [DOI] [PubMed] [Google Scholar]
- 31. Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19(3):433-442. [DOI] [PubMed] [Google Scholar]
- 32. Yaribeygi H, Atkin SL, Pirro M, Sahebkar A. A review of the anti-inflammatory properties of antidiabetic agents providing protective effects against vascular complications in diabetes. J Cell Physiol. 2019;234:8286-8294. [DOI] [PubMed] [Google Scholar]
- 33. Ismail K, Stewart K, Ridge K, et al. A pilot study of an integrated mental health, social and medical model for diabetes care in an inner-city setting: three Dimensions for Diabetes (3DFD) [published online ahead of print February 1, 2019]. Diabet Med. doi: 10.1111/dme.13918. [DOI] [PubMed] [Google Scholar]
- 34. Vittinghoff EMCE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710-718. [DOI] [PubMed] [Google Scholar]