Abstract
Pulmonary venous (PV) obstruction is associated with a poor prognosis, as well as a high risk of recurrence, following surgical treatment. It can also interfere with the successful completion of Fontan circulation in patients with complex congenital heart disease. A case of a patient who had right isomerism (also known as asplenia syndrome), total anomalous pulmonary venous connection (TAPVC), and a single right ventricle is presented. Although bilateral total occlusion of the inferior PVs was identified postoperatively, the formation of the anastomosis and collateral vessels into the superior and middle PVs enabled successful completion of Fontan circulation. Anastomoses and collateral flow of the PVs were found largely in the interlobar pleura and not in the lung parenchyma.
Keywords: Fontan procedure, pulmonary vein, collateral circulation, angiogenesis, total anomalous pulmonary venous connection
Case report
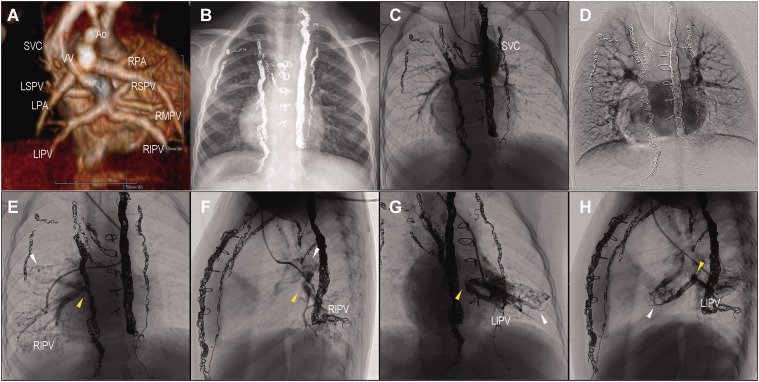
A full-term male newborn weighing 2540 g was evaluated for suspected congenital heart disease on the day of birth. Echocardiography and computed tomographic angiography showed heterotaxia, dextrocardia, single right ventricle, and type Ib total anomalous pulmonary venous connection (TAPVC), with two left and three right pulmonary veins (PVs) entering the confluence. Figure 1a depicts the TAPVC from his back. Transcatheter stenting of the vertical vein was performed on day 0 after birth; TAPVC repair and pulmonary artery banding were performed on day 8. A bidirectional cavopulmonary connection and repair of PV stenosis were performed when the patient reached the age of 10 months; another repair of PV stenosis was performed by sutureless repair when the patient reached the age of one year and five months because of accelerated PV flow. A chest X-ray did not show pulmonary congestion (Fig. 1b). Cardiac catheterization was performed at the age of 2.5 years before the Fontan procedure. Superior vena cavography showed a smooth stream to bilateral pulmonary arteries, although bilateral inferior PV returns were not clearly defined (Fig. 1c and d, Video 1). Selective right inferior (Fig. 1e and f, Video 2) and left inferior (Fig. 1g and h, Video 3) pulmonary wedge angiography showed complete obstruction (yellow arrowheads) and the development of intrapulmonary venous anastomosis to the superior and middle PVs (white arrowheads). At the site of occlusion on each side, the inferior PV blood flow returned to the superior-middle PVs through PV–PV shunts and into the atrium without congestion. The patient had a mean pulmonary arterial pressure (mPAP) 9 mmHg, atrial pressure of 7 mmHg, and pulmonary vascular resistance (PVR) of 1.0 Wood U m2. This pulmonary vascular condition was demonstrated again at the age of three years. Based on these findings, the Fontan procedure was considered safe for the patient and was performed when the patient was aged three years and one month. The patient recovered well and is being followed up for 1.5 years.
Fig. 1.
(a) CT angiography, (b) chest X-ray, (c, d) superior vena cavography, (e, f) right pulmonary wedge angiography showing right inferior pulmonary venous (RIPV) obstruction and anastomosis to superior and middle pulmonary vein. (g, h) Left pulmonary wedge angiography showing left inferior pulmonary venous (LIPV) obstruction and anastomosis to superior pulmonary vein. Ao, aorta; LIPV, left inferior pulmonary vein; LPA, left pulmonary artery; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RMPV, right middle pulmonary vein; RPA, right pulmonary artery; RSPV, right superior pulmonary vein; SVC, superior vena cava; VV, vertical vein.
Discussion
PV obstruction after TAPVC repair is a well-described phenomenon in heterotaxia patients. It can be a devastating factor for Fontan circulation.1,2 The patient in the present study had developed bilateral collateral vessels, thus maintaining circulation into the patent superior and middle PVs. PAP and PVR were within an acceptable range to proceed with a Fontan procedure. This is the first case report in which the Fontan operation was indicated and successfully performed with the bilateral PV obstruction associated with full intrapulmonary compensation.
In the present study, anastomoses and collateral flow of the PVs were found largely in the interlobar pleura and not in the lung parenchyma. Thus, due to the occlusion of the left inferior PV, the blood flow returned into the left superior PV through the PV–PV shunt in the fissures between the lobes. Similarly, due to the occlusion of the right inferior PV, the blood flow returned into the right superior and middle PVs through the PV–PV shunt, which formed anastomotic capillaries in the interlobular space. In both PVs, collateral vessels were found in the visceral pleura into the fissures between the lobes and formed PV anastomoses.
Studies have demonstrated that blood flow into the visceral pleura is maintained by the bronchial artery and branches of the pulmonary artery before returning to the PV, while blood flow into the parietal pleura is maintained by the intercostal artery and pericardiacophrenic artery before returning to the PV.3 Thus, blood flow into the pleura is supplied by either the aorta or pulmonary artery and returns to the PV. The present findings suggest that the existing vascular supply and angiogenesis in the pleura contributed to the anastomoses and collateral vessel formation between PVs. The collateral vessels development after PV obstruction has been reported in several adult patients.4,5 However, there have been no studies demonstrating the mechanism by which collateral vessels form. Formation of collateral vessels may be influenced by individual predispositions, as well as the presence of vascular growth factors. An increase in the systemic collateral vessels from the aorta has been demonstrated in cyanotic congenital heart disease patients with reduced pulmonary blood flow, cyanosis, and hypoxemia.6,7 Similarly, the formation of collateral vessels in patients with PV occlusion may be attributed to tissues with systemic vessels, rather than through angiogenesis from pulmonary vessels. PV obstruction after TAPVC repair is associated with a poor prognosis. Our case underwent Fontan procedure successfully with full intrapulmonary compensation. It is desirable to elucidate the mechanisms of PV–PV collateral development and anastomosis to maintain the pulmonary circulation in complex congenital heart disease patients.
Conclusion
Fontan circulation was completed successfully due to the formation of collateral vessels in PVs in a patient who had bilateral occlusion of the inferior PVs following a bidirectional cavopulmonary connection procedure.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Serraf A, Bensari N, Houyel L, et al. Surgical management of congenital heart defects associated with heterotaxy syndrome. Eur J Cardiothorac Surg 2010; 38(6): 721–727. [DOI] [PubMed] [Google Scholar]
- 2.Hoashi T, Kagisaki K, Oda T, et al. Long-term results of treatments for functional single ventricle associated with extracardiac type total anomalous pulmonary venous connection. Eur J Cardiothorac Surg 2013; 43(5): 965–970. [DOI] [PubMed] [Google Scholar]
- 3.Abdalla MA, King AS. The functional anatomy of the bronchial circulation of the domestic fowl. J Anat 1976; 121(Pt 3): 537–550. [PMC free article] [PubMed] [Google Scholar]
- 4.Saida Y, Eguchi N, Mori K, et al. Isolated pulmonary vein stenosis associated with full intrapulmonary compensation. AJR Am J Roentgenol 1999; 173(4): 961–962. [DOI] [PubMed] [Google Scholar]
- 5.Lee HN, Kim YT, Cho SS. Individual pulmonary vein atresia in adults: report of two cases. Korean J Radiol 2011; 12(3): 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davie NJ, Crossno JT, Jr, Frid MG, et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 2004; 286(4): L668–678. [DOI] [PubMed] [Google Scholar]
- 7.Fadel E, Michel RP, Eddahibi S, et al. Regression of postobstructive vasculopathy after revascularization of chronically obstructed pulmonary artery. J Thorac Cardiovasc Surg 2004; 127(4): 1009–1017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.