Abstract
We investigated the effects of continuous low-dose radiation on proliferation, clonogenicity, radiosensitivity, and repair of DNA double-strand breaks (DSBs) in human salivary gland (HSG) tumor cells. Human salivary gland cells were cultured on acrylic boards above very-low-dose (4.3 μSv/h) or low-dose (27 μSv/h) radiation-emitting sheets or without sheets. Total cell numbers and plating efficiencies were compared among the 3 groups every 1 or 2 weeks until 6 weeks after starting culture. At 2, 4, and 6 weeks, surviving fractions of HSG cells after irradiation at 2 to 8 Gy cultured on the very-low-dose or low-dose sheets were compared to those of the control. At 4 weeks, HSG cells irradiated at 2 Gy were assessed for phosphorylated histone (γH2AX) foci formation, and DSBs were evaluated. No significant differences were observed in total cell number or plating efficiencies with or without low-dose-emitting sheets. The surviving fractions after irradiation of the very-low-dose group at 2 to 6 weeks and those of the low-dose group at 2 to 4 weeks were higher than those of the control (P < .01). Thus, a radioadaptive response was clearly demonstrated. From the γH2AX foci quantification, the adaptive responses were considered to be associated with the efficient repair of DSB, especially slow repair, in this cell line.
Keywords: low-dose radiation, hormesis, adaptive response, double strand break (DSB), γH2AX
Introduction
High-dose radiation is harmful and can induce cancer, whereas the effects of low-dose radiation (≤ 200 mGy) remain controversial. The linear no-threshold (LNT) theory has been applied to the dose response of radiation effects, but some investigators have questioned the reliability of this hypothesis.1-4 It is now known that low-dose irradiation can induce the radiation hormesis phenomenon first described by Luckey et al5 in 1982 and the adaptive response first reported by Olivieri et al6 in 1984. In support of these initial observations, many recent investigations revealed the beneficial effects of low-dose radiation.7-10
Our group has investigated the effects of low-dose, radiation-emitting sheets on the growth of silkworm (Bombyx mori) larvae and young mice and tumor transplantability in mice.11,12 Silkworms grown on low-dose-emitting sheets became larger than those grown on control sheets, and the time to tumor development was prolonged in mice bred on the radiation-emitting sheets. Along with these investigations, we have been investigating the effects of the low-dose-emitting sheets on the growth, clonogenicity, and radiosensitivity of cultured cells. One of the purposes of this in vitro study was to investigate whether the promotion of proliferation similarly occurs in cultured cells, and herein, we report the results of our in vitro studies. To investigate the relationship between DNA repair and the radioadaptive response, DNA double-strand breaks (DSBs) after X-ray irradiation were also investigated in cells cultured with or without the sheets.
Materials and Methods
Low-Dose Radiation-Emitting Sheets
The sheets were manufactured at Aoyama Stein Co, Ltd (Kobe, Japan). Details of the sheets were described in detail previously.11,12 Briefly, monozites containing 228Ac and 77Br were rubbed into sheets made of polyvinyl chloride materials. Two kinds of sheets containing different amounts of isotopes were made (a “very-low-dose” sheet and a “low-dose” sheet). The radiation dose rates emitted from the sheets were measured with a Geiger-Mueller (GM) counter Inspector USB (for α, β, and γ rays; Measure Works, Tokyo, Japan). The dose rates were approximately 4.3 μSv/h on the “very-low-dose-rate” sheet and 27 μSv/h on the “low-dose-rate” sheet at a point 5 mm above the sheet, simulating the cell position and including an acrylic board of 2-mm thickness and the bottom of a culture dish. Under these conditions, α-rays did not contribute to the dose, and the contribution of β-rays was also small.
Human Salivary Gland Tumor Cells
Human salivary gland (HSG; JCRB1070: HSGc-C5) cells were used. Characteristics of the cell line were previously described.13 These cells are contaminated with HeLa S3 cells, and differentiation from HeLa cells is difficult.14,15 The cells were cultured in Eagle minimum essential medium (Sigma-Aldrich, Inc, Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) and were incubated under a humidified atmosphere with 5% CO2 and 95% air at 37°C.
Cell Number Determination and Colony Assay
A frozen stock of HSG cells was thawed, and the cells were divided into 3 groups; 5 × 104 HSG cells were plated onto 10-cm plastic dishes in each group. During cell culture, the dishes were placed on 2-mm-thick acrylic boards above the very-low-dose or low-dose sheets or on the acrylic boards without the sheet. The acrylic boards were used to stabilize the cell culture. The total numbers of HSG cells cultured with or without the radiation-emitting sheets were counted using a Coulter counter and compared once a week for 6 weeks. After counting, 104 cells were plated onto new dishes, and culture was continued thereafter. The medium was changed once between the subcultures. In addition, the colony-forming ability (plating efficiency) was compared every other week for 6 weeks.
Radiation dose–survival curves were obtained at 2, 4, and 6 weeks of culture for all 3 groups of cells cultured with or without the sheets. The cells were trypsinized to single cells, plated onto 6-cm culture dishes in appropriate numbers, and irradiated at doses of 2, 4, 6, and 8 Gy at a dose rate of 1.6 Gy/min using an X-ray machine CAX-210 (Chubu Medical Co., Ltd., Yokkaichi, Japan; 210 kVp, 10 mA, 2-mm Al filter) as described previously.16 Plating efficiencies and surviving cell fractions were determined by the standard colony assay, as described previously.16 Colonies containing 50 or more cells were stained and counted 7 days later.
Quantification of Phosphorylated Histone Foci per Nucleus
After HSG cells had been cultured for 4 weeks with subculturing at 1-week intervals, 3 groups of cells cultured on the very-low-dose sheet, on the low-dose sheet, and without the sheet were trypsinized to single cells and plated onto 96-well plates. The plates were incubated on the very-low-dose or low-dose sheet or without the sheet for 24 hours, and then the plates were irradiated at a dose of 2 Gy using the same machine as mentioned above. At 1, 6, and 24 hours after 2-Gy irradiation or without irradiation, the 3 groups of cells were fixed in 3.7% formaldehyde, permeabilized in 90% methanol, and blocked in 1% blocking/antibody incubation buffer (1% bovine serum albumin/phosphate-buffered saline). All groups of cells were stained and mounted with 4’,6-diamidino-2-phenylindole (DAPI; Thermo Scientific, Waltham, Massachusetts), and phosphorylated histone (γH2AX) foci were detected with the primary antibody anti-phospho-histone H2AX (×100, Ser 139, OxiSelect DNA DSB Staining Kit; Cell Biolabs, Inc, San Diego, California) and the secondary antibody fluorescein isothiocyanate-conjugated goat antimouse IgG antibody (×100, OxiSelect DNA DSB Staining Kit). The γ-H2AX foci in each group were scored in at least 100 cells with In Cell Analyzer 6000 (GE Healthcare UK Ltd, Little Chalfont, United Kingdom).
Statistical Analysis
Total numbers of HSG cells, surviving fractions, and γ-H2AX foci/nucleus scores were compared among the 3 groups using factorial analysis of variance followed by Dunnett post hoc test. Plating efficiencies were compared using Student t test. The relative surviving fractions of the very-low-dose and low-dose groups compared to those of the control groups were calculated at each point, and then the relative surviving fractions were compared between the very-low-dose and the low-dose groups and also among the different periods of culture on the sheet (2 vs 4 vs 6 weeks) at the same radiation doses using the t test or factorial analysis of variance followed by the Bonferroni-Dunn post hoc test. All statistical analyses were performed using StatView 5.0 (SAS Institute Inc, Cary, North Carolina).
Results
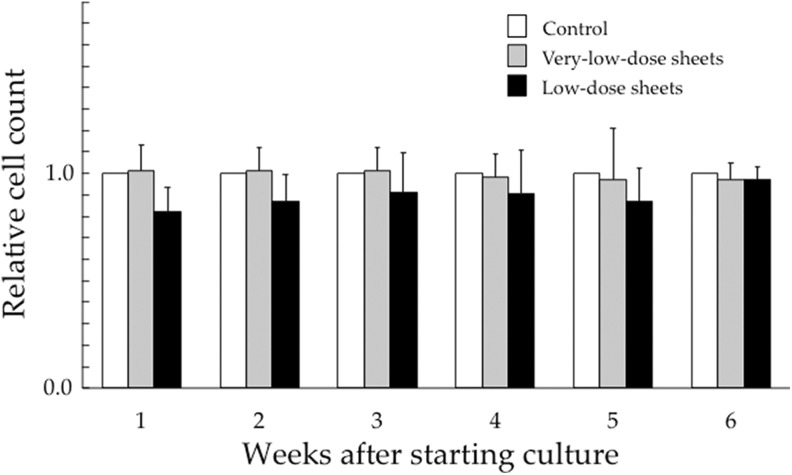
Figure 1 shows relative total numbers of HSG cells cultured for up to 6 weeks on the very-low-dose and low-dose sheets compared to those of the control groups. In the very-low-dose sheet experiments, the mean total cell numbers (standard deviation, SD) in the control groups were 5.88 (3.10) × 106 at 1 week, 1.01 (0.13) × 106 at 2 weeks, 0.72 (0.09) × 106 at 3 weeks, 0.91 (0.08) × 106 at 4 weeks, 0.77 (0.11) × 106 at 5 weeks, and 1.03 (0.14) × 106 at 6 weeks. In the low-dose sheet experiments, the total cell numbers in the control groups were 2.61 (1.82), 1.20 (0.26), 1.02 (0.10), 1.24 (0.29), 1.12 (0.19), and 1.22 (0.24) × 106 at 1 to 6 weeks, respectively. No significant differences were observed in the total number of HSG cells cultured for up to 6 weeks on the very-low-dose sheets, on the low-dose sheets, or without the sheets.
Figure 1.
Relative total numbers of human salivary gland (HSG) cells when compared to the control group cultured for up to 6 weeks on very-low-dose (4.3 μSv/h) and low-dose sheets (27 μSv/hour). Data represent the mean and standard deviation of 3 experiments.
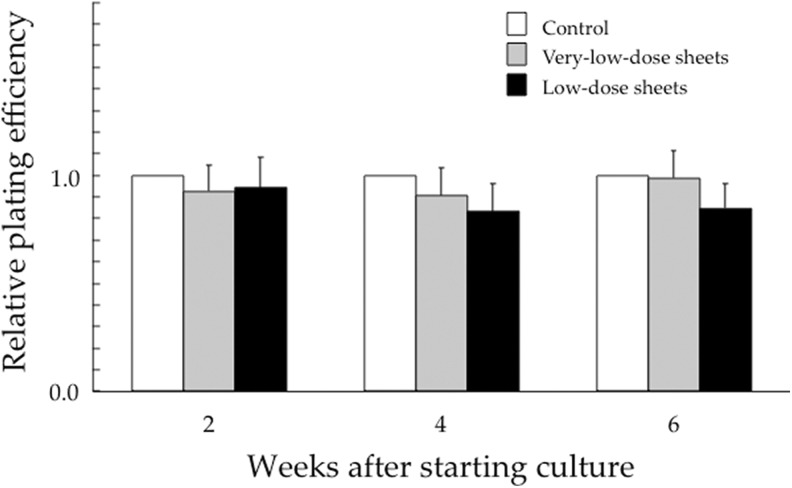
Figure 2 shows relative plating efficiencies of HSG cells cultured for up to 6 weeks on the very-low-dose and low-dose sheets, compared to those of the control groups. In the very-low-dose sheet experiments, the mean plating efficiencies (SD) in the control groups were 73% (16%) at 2 weeks, 74% (18%) at 4 weeks, and 71% (17%) at 6 weeks. In the low-dose sheet experiments, the plating efficiencies in the control groups were 75% (9%), 79% (11%), and 75% (8%) at 2, 4, and 6 weeks, respectively. No significant differences were noted in control plating efficiencies among HSG cells cultured for 2, 4, and 6 weeks on the very-low-dose sheets, on the low-dose sheets, or without the sheets (Figure. 2).
Figure 2.
Relative plating efficiencies of human salivary gland (HSG) cells when compared to the control group cultured for 2, 4, and 6 weeks on the very-low-dose and low-dose sheets. Data represent the mean and standard deviation of 3 experiments.
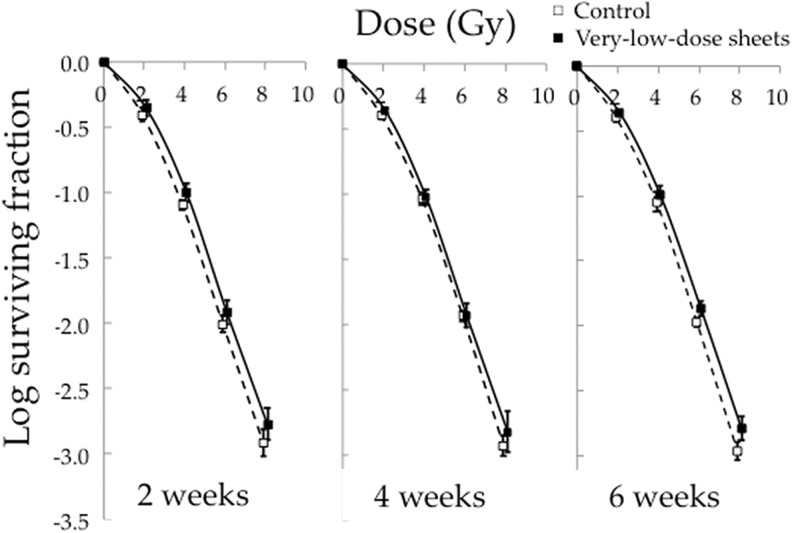
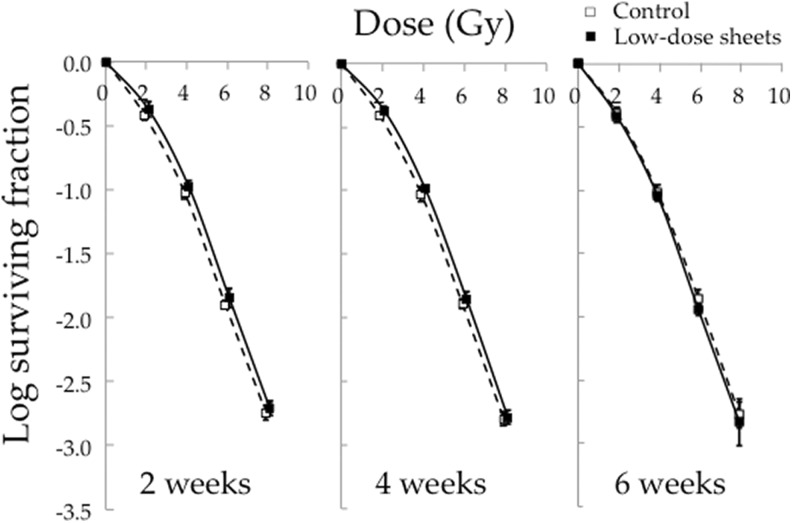
Figure 3 shows the surviving fractions after irradiation of HSG cells cultured for 2, 4, and 6 weeks on the very-low-dose sheets or without the sheets. All cell survival curves of the very-low-dose group were above those of the cells cultured without the sheets (P < .01). Figure 4 shows the surviving fractions after irradiation of HSG cells cultured on the low-dose sheets for 2 to 6 weeks or without the sheets. The cell survival curves of the cells cultured for 2 and 4 weeks on the low-dose sheets were above those of the cells cultured without the sheets (P < .01).
Figure 3.
Surviving fractions after irradiation at 2 to 8 Gy of human salivary gland (HSG) cells cultured for 2, 4, and 6 weeks on the very-low-dose sheets or without the sheets. Data represent the mean and standard deviation of 3 experiments.
Figure 4.
Surviving fractions after irradiation at 2 to 8 Gy of human salivary gland (HSG) cells cultured for 2, 4, and 6 weeks on the low-dose sheets or without the sheets. Data represent the mean and standard deviation of 3 experiments.
The experiments shown in Figures 3 and 4 were carried out separately, and so the relative surviving fractions of the very-low-dose and low-dose groups compared to the control groups were calculated at each point (Table 1). As a result, the cells cultured for 6 weeks on the very-low-dose sheets had higher relative surviving fractions than those cultured on the low-dose sheets for 6 weeks at doses of 4 to 8 Gy. The cells cultured for 2 or 4 weeks on the low-dose sheets had higher relative surviving fractions than those cultured for 6 weeks on the low-dose sheets at 6 Gy.
Table 1.
Relative Surviving Fractions Compared to Controls in Radioadaptive Response Experiments.
| Type of Sheet | Very Low Dose | Low Dose | ||||
|---|---|---|---|---|---|---|
| Culture duration, weeks | 2 | 4 | 6 | 2 | 4 | 6 |
| Radiation dose, Gy | ||||||
| 2 | 1.14 (0.20) | 1.06 (0.11) | 1.08 (0.08) | 1.12 (0.18) | 1.12 (0.13) | 0.95 (0.14) |
| 4 | 1.26 (0.19) | 1.02 (0.18) | 1.14 (0.16)a | 1.13 (0.21) | 1.11 (0.21) | 0.94 (0.10)a |
| 6 | 1.24 (0.17) | 1.08 (0.34) | 1.29 (0.18)b | 1.14 (0.20)c | 1.11 (0.17)d | 0.83 (0.15)b,c,d |
| 8 | 1.46 (0.53) | 1.37 (0.57) | 1.56 (0.48)a | 1.08 (0.18) | 1.04 (0.18) | 0.94 (0.36)a |
a P = .01, by t-test.
b P = .0001, by t-test.
c P = .01, by factorial analysis of variance followed by the Bonferroni-Dunn post hoc test.
d P = .02, by factorial analysis of variance followed by the Bonferroni-Dunn post hoc test.
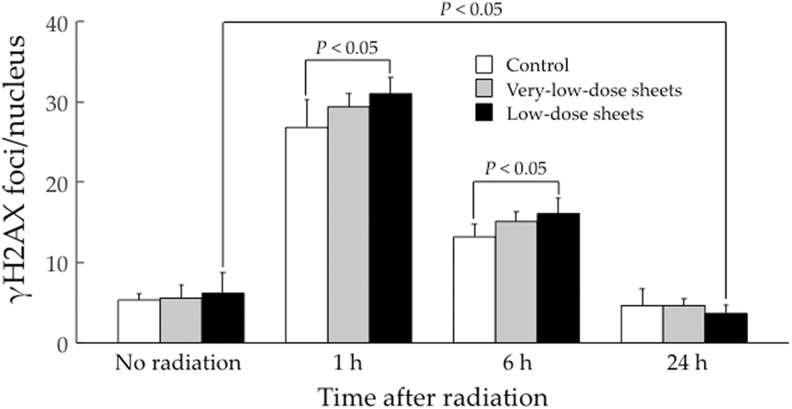
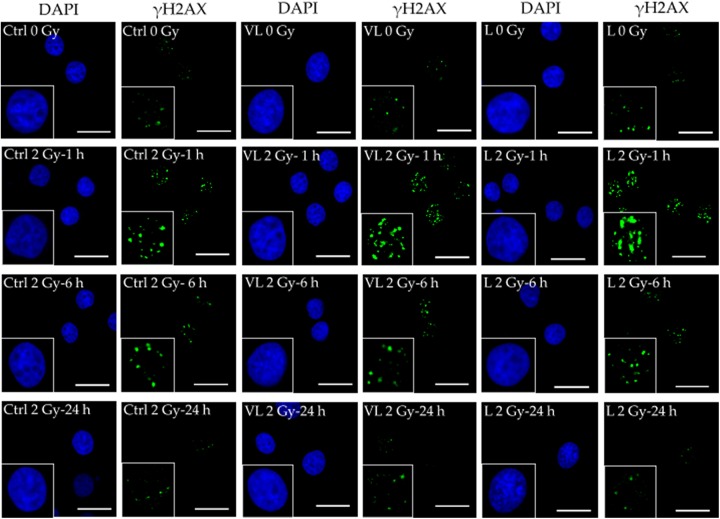
Figure 5 shows the results of γ-H2AX foci/nucleus quantification. Without irradiation, there were no differences in the γ-H2AX foci/nucleus scores among the 3 groups. At 1 and 6 hours after 2-Gy irradiation, the score was significantly higher in the low-dose group than in the control group (P < .05). At 24 hours, however, there were no differences in the γ-H2AX foci/nucleus score among the 3 groups, and the score in the low-dose group was significantly lower than that without 2-Gy irradiation in the same group (P < .05). Figure 6 shows representative photomicrographic images of DAPI and γH2AX staining in each cell group; the trends in Figure 5 can be noted.
Figure 5.
Phosphorylated histone (γH2AX) foci/nucleus fixed at 1, 6, and 24 hours after irradiation at 2 Gy of human salivary gland (HSG) cells cultured on the radiation-emitting sheets for 4 weeks. Data represent the mean and standard deviation of 6 determinations.
Figure 6.
Representative photomicrographs of 4’,6-diamidino-2-phenylindole (DAPI) and phosphorylated histone (γH2AX) staining in human salivary gland (HSG) cells cultured without the radiation-emitting sheets (control, Ctrl: left 2 panels) on the very-low-dose sheets (VL: center 2 panels) and on the low-dose sheets (L: right 2 panels). The bar corresponds to 25 µm. The γH2AX index in the cells cultured on the low-dose sheets was lower at 24 hours after 2-Gy irradiation (bottom right corner) than without 2-Gy irradiation (top right corner).
Discussion
In our previous study, we observed enhanced growth of silkworm larvae bred on low-dose-emitting sheets, but the mechanism was unclear. Growth promotion in other living organisms by low-dose radiation has also been reported.10,17 Stimulation of cell proliferation through some signaling pathways, increase in growth hormone-like substances, and stimulation of silkworm appetite may be plausible mechanisms, and we investigated the first hypothesis in this in vitro study. Human salivary gland is a standard reference cell line to compare the relative biological effectiveness of carbon ion and proton beams in Japanese institutions,13 and it is also used in other countries including Germany and Korea. We chose this cell line for the present study because of its relatively low plating efficiency.13 Although we did not observe accelerated cell proliferation, we only used one cell line, and biochemical analyses were not performed. Therefore, the possibility of growth stimulation by low-dose radiation at a cellular level cannot be ruled out, but other in vivo mechanisms to stimulate growth may be more likely. We also did not detect an increase in the clonogenicity of HSG cells by low-dose radiation, but more studies under various conditions are needed.
Previously, we did not observe a radioadaptive response after a single radiation dose of 50 mGy in 4 cell lines cultured in vitro.18 One of the cell lines was HeLa S3; HSG cells are contaminated with HeLa S3 cells and are considered to be the same as HeLa.14,15 We observed a radioadaptive response after continuous low-dose irradiation, and the total dose during the 2- to 6-week culture was 1.4 to 4.3 mGy on the very-low-dose sheet and 9.1 to 27 mGy on the low-dose sheet, which was lower than 50 mGy used in our previous single-shot radiation study. Thus, continuous low-dose radiation may be more likely to induce an adaptive response than single low-dose irradiation. Culture on the very-low-dose sheets for 6 weeks appeared to be a more suitable condition for inducing an adaptive response than culture on the low-dose sheets for 6 weeks, and culture on the low-dose sheets for 2 or 4 weeks appeared to be more suitable than culture on the low-dose sheets for 6 weeks (Table 1). Detecting the adaptive and hormetic responses depends on various conditions of the cells and environment,19,20 and absence of such effects does not suggest the absence of beneficial effects of low-dose radiation. Subtle positive responses at cellular levels may not result in an adaptive or hormetic response.10
To investigate the possible mechanism of the radioadaptive response in HSG cells, we quantified DNA DSBs with the γ-H2AX foci/nucleus score. Because phosphorylation of the H2AX protein occurs at an early step in the DSB repair pathway,20,21 γ-H2AX foci scoring is frequently used to assess DSB. At 1 and 6 hours after 2-Gy irradiation, the γ-H2AX foci/nucleus score was higher in the low-dose-sheet group than in the control group (Figure. 5). It was reported that low-dose radiation around 10 mGy increased DSB,22 and the transient increase in the score may result from conditioning low doses from the low-dose sheet (18 mGy over 4 weeks). Double-strand break repair was delayed in the low-dose group, but the score markedly decreased at 24 hours after 2-Gy irradiation, and the score at 24 hours in the low-dose group was lower than the score without irradiation. Since this reduction at 24 hours in the γ-H2AX score was not observed in the very-low-dose group, we could not clearly state that continuous low-dose radiation decreased the DSB, but it was suggested that DSBs were efficiently repaired after 6 hours in the cells cultured on the radiation-emitting sheets. When DSB and chromosome damage are evaluated immediately or shortly after low-dose (<100 mGy) radiation, increases in the indices may be observed,22,23 but the damage may be efficiently repaired thereafter or damaged cells may be eliminated by apoptosis and replaced with new cells by the effect of low-dose radiation. Overall, low-dose radiation may enhance the DNA repair capacity and contribute to repair of DSB following subsequent exposure to a high dose of radiation.24-26
Thus, the adaptive response observed in our HSG cells may be in part related to the efficient repair of DSB after 6 hours of irradiation. A previous study showed that DSBs were quickly repaired (at around 2 hours) by non-homologous end-joining (NHEJ) and slowly repaired (at around 12 hours) by homologous recombination (HR).26 In our study, therefore, the adaptive responses induced by low-dose radiation were considered to be mainly derived from enhancement of the slow repair of DSB observed after 6 to 24 hours by HR. Also, in another study, marked enhancement of DSB repair from HR and weak enhancement of DSB repair from NHEJ were observed after low-dose irradiation.27 However, these results including ours contradict those reported by Zhao et al.28 In their study, γ-H2AX foci were fewer in the group preirradiated at 25 to 100 mGy when assessed at 0.5 to 1 hour after 2-Gy irradiation, so fast repair by NHEJ might have been enhanced in their cell lines. These results suggest that the mechanisms of adaptive responses are not uniform and can vary with the cell lines. Further studies on this issue may be warranted.
The limitations of this study include not investigating the mechanisms of the adaptive response further except for γ-H2AX foci staining. Another limitation is that we only used 2 types of sheets, and an optimal dose rate could not be determined. The total dose from the low-dose sheet was 27 mGy over 6 weeks. Therefore, the mechanisms of low-dose radiation effects and the effects of higher dose rates should be investigated in future studies because similar sheets with higher dose rates are now available.12
In conclusion, the adaptive response by continuous low-dose radiation was demonstrated using low-dose-radiation-emitting sheets. One reason for the adaptive response may be efficient DSB repair through HR.
Acknowledgments
The authors would like to thank Drs Masato Ito, Tatsuya Kawai, and Masayuki Matsuo for their helpful advice on this research and Mr Natsuto Aoyama for providing the radiation-emitting sheets.
Footnotes
Declaration of Conflicting Interests: The author(s) declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and a research grant for manufacturing from Hyogo Prefecture, Japan.
References
- 1. Calabrese EJ. On the origins of the linear no-threshold (LNT) dogma by means of untruths, artful dodges and blind faith. Environ Res. 2015;142:432–442. doi:10.1016/j.envres.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 2. Sacks B, Siegel JA. Preserving the anti-scientific linear no-threshold myth: authority, agnosticism, transparency, and the standard of care. Dose Response. 2017;15(3). doi:10.1177/1559325817717839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ulsh BA. A critical evaluation of the NCRP COMMENTARY 27 endorsement of the linear no-threshold model of radiation effects. Environ Res. 2018;167:472–487. doi:10.1016/j.envres.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 4. Calabrese EJ. The mistaken birth and adoption of LNT: an abridged version. Dose Response. 2017;15(4). doi:10.1177/1559325817735478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luckey TD. Physiological benefits from low levels of ionizing radiation. Health Phys. 1982;43(6):771–789. [DOI] [PubMed] [Google Scholar]
- 6. Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223(4636):594–597. doi:10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 7. Pollycove M, Feinendegen LE. Biologic responses to low doses of ionizing radiation: detriment versus hormesis. part 2. dose responses of organisms. J Nucl Med. 2001;42(9):26N–32 N, 37 N. [PubMed] [Google Scholar]
- 8. Luckey TD, Lawrence KS. Radiation hormesis; the good, the bad, and the ugly. Dose Response. 2006;4(3):169–190. doi:10.2203/dose-response.06-102.Luckey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baldwin J, Grantham V. Radiation hormesis: historical and current perspective. J Nucl Med Technol. 2015;43(4):242–246. doi:10.2967/jnmt.115.166074. [DOI] [PubMed] [Google Scholar]
- 10. Shibamoto Y, Nakamura H. Overview of biological, epidemiological, and clinical evidence of radiation hormesis. Int J Mol Sci. 2018;19(8):2387 doi:10.3390/ijms19082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shibamoto Y, Kamei Y, Kamei K, Tsuchiya T, Aoyama N. Continuous low-dose-rate irradiation promotes growth of silkworms. Dose Response. 2017;15(4). doi:10.1177/1559325817735252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakashima M, Sugie C, Wang Z, et al. Biological effects of continuous low-dose-rate irradiation in silkworms and mice: growth promotion and tumor transplantability. Dose Response. 2018;16(4). doi:10.1177/1559325818811753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwata H, Ogino H, Hashimoto S, et al. Spot scanning and passive scattering proton therapy: relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int J Radiat Oncol Biol Phys. 2016;95(1):95–102. doi:10.1016/j.ijrobp.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 14. Capes-Davis A, Theodosopoulos G, Atkin I, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127(1):1–8. doi:10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto Y, Matsuura T, Wada M, Egashira Y, Nishio T, Furusawa Y. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res. 2014;55(4):816–822. doi:10.1093/jrr/rrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shibamoto Y, Ito M, Sugie C, Ogino H, Hara M. Recovery from sublethal damage during intermittent exposures in cultured tumor cells: implications for dose modification in radiosurgery and IMRT. Int J Radiat Oncol Biol Phys. 2004;59(5):1484–1490. doi:10.1016/j.ijrobp.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 17. Araujo SS, Paparella S, Dondi D, Bentivoglio A, Carbonera D, Balestrazzi A. Physical methods for seed invigoration: advents and challenges in seed technology. Front Plant Sci. 2016;7:646 doi:10.3389/fpls.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyamoto A, Shibamoto Y, Sugie C, Ito M, Ayakawa S. Absence of radioadaptive responses in four cell-lines in vitro as determined by colony formation assay. Kurume Med J. 2006;53(1-2):1–5. doi:10.2739/kurumemedj.53.1. [DOI] [PubMed] [Google Scholar]
- 19. Jiang H, Xu Y, Li W, Ma K, Cai L, Wang G. Low-dose radiation does not induce proliferation in tumor cells in vitro and in vivo. J Radiat Res. 2008;170(4):477–487. doi:10.1667/RR1132.1. [DOI] [PubMed] [Google Scholar]
- 20. Yang G, Yu D, Li W, et al. Distinct biological effects of low-dose radiation on normal and cancerous human lung cells are mediated by ATM signaling. Oncotarget. 2016;7(44):71856–71872. doi:10.18632/oncotarget.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laia H, Mariona T, Marta M, Laura T, Anna G. Highly sensitive automated method for DNA damage assessment: gamma-H2AX foci counting and cell cycle sorting. Int J Mol Sci. 2013;14(8):15810–15826. doi:10.3390/ijms140815810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moroni M, Maeda D, Whitnall MH, Bonner WM, Redon CE. Evaluation of the gamma-H2AX assay for radiation biodosimetry in a swine model. Int J Mol Sci. 2013;14(7):14119–14135. doi:10.3390/ijms140714119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi L, Fujioka K, Sakurai-Ozato N, et al. Chromosomal abnormalities in human lymphocytes after computed tomography scan procedure. Radiat Res. 2018;190(4):424–432. doi:10.1667/RR14976.1. [DOI] [PubMed] [Google Scholar]
- 24. Andreyan NO, Anna G, Margarita P, et al. Activation of homologous recombination DNA repair in human skin fibroblasts continuously exposed to X-ray radiation. Oncotarget. 2015;6(29):26876–26885. doi:10.18632/oncotarget.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchel REJ. Low doses of radiation are protective in vitro and in vivo: evolutionary origins. Dose Response. 2006;4(2):75–90. doi:10.2203/dose-response.04-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shibata A, Conrad S, Birraux J, et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011;30(6):1079–1092. doi:10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yatagai F, Sugasawa K, Enomoto S, Honma M. An approach to estimate radioadaptation from DSB repair efficiency. J Radiat Res. 2009;50(5):407–413. doi:10.1269/jrr.09050. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y, Zhong R, Sun L, Jia J, Ma S, Liu X. Ionizing radiation-induced adaptive response in fibroblasts under both monolayer and 3-dimensional conditions. Plos One. 2015;10(3):e0121289 doi:10.1371/journal.pone.0121289. [DOI] [PMC free article] [PubMed] [Google Scholar]