Abstract
Introduction
Levels of complement proteins (CPs) in plasma astrocyte-derived exosomes (ADEs) that are abnormal in Alzheimer's disease (AD) have not been assessed in mild cognitive impairment (MCI).
Methods
Participants (n = 20 per group) had either MCI converting to dementia within 3 years (MCIC), MCI remaining stable over 3 years (MCIS), Alzheimer's disease, or were controls. CPs of ADEs isolated from plasmas by anti-human glutamine aspartate transporter antibody absorption were quantified by ELISAs.
Results
ADE levels of C1q and C4b of the classical pathway, factor D and fragment Bb of the alternative pathway, and C5b, C3b, and C5b-C9 of both pathways were significantly higher in patients with MCIC than those with MCIS. ADE levels of inhibitory CPs decay-accelerating factor, CD46, CD59, and type 1 complement receptor were significantly lower in patients with MCIC than those with MCIS.
Discussion
ADE CPs are components of neurotoxic neuroinflammation that may be predictive biomarkers of MCI conversion to Alzheimer's disease.
Keywords: Neuroinflammation, Neurodegeneration, Biomarkers, Cognitive loss, Complement regulators
1. Introduction
Diverse homeostatic functions of central nervous system (CNS) astrocytes support neurons in health, but the multicellular changes in CNS degenerative diseases include an increased total number of astrocytes and a greater extent of differentiation into neurotoxic A1 inflammatory than neuroprotective A2 ischemia-related types [1], [2], [3], [4]. Proteins of the classical, alternative, and lectin complement systems provide protection against infections and toxins, regulate immunity especially that involving B cells, and mediate tissue damage in myriad autoimmune and other inflammatory diseases. Several lines of experimental evidence suggest that complement system proteins may mediate the capacity of type A1 astrocytes and microglia to damage neurons [4], [5], [6]. Furthermore, astrocytes expressing type A1 markers in areas of postmortem brain tissues affected by neurodegenerative or neuroinflammatory diseases have high levels of complement component 3 (C3), whereas C3 is absent from type A2 astrocytes [7].
Astrocyte-derived exosomes (ADEs) have been recovered by immunoabsorption from plasma of patients with Alzheimer's disease (AD) and matched controls [8]. Plasma ADE levels of numerous classical and alternative pathway complement effector proteins were significantly higher in patients with AD than controls, including opsonizing C3b and the cellular attack complex C5b-C9 [9]. Furthermore, ADE complement levels were 6- to 50-fold higher than corresponding values in neuron-derived exosomes. Plasma ADE complement effector protein levels were not significantly elevated in preclinical AD at 5 to 12 years before cognitive losses, but some complement regulatory proteins were decreased at these early preclinical stages and were further decreased in manifest AD when complement effector proteins became elevated [9].
The establishment of criteria for delineation of progressive stages of preclinical AD, which correlate with an increased risk of development of dementia proportional to the stage of preclinical AD, has been challenging and is still in evolution. Variables that delineate each stage of preclinical AD have included subtle cognitive changes, abnormal cerebrospinal fluid levels of putative pathogenic proteins, and MRI and PET bioimaging data [10], [11], [12], [13]. Models for predicting cognitive trajectory in mild cognitive impairment (MCI) have proven to be especially challenging and required application of complex multiple domain neuropsychological testing and evaluation by MRI of functional connectivity of CNS network components [14], [15], [16], [17]. Blood-based biochemical approaches to prediction of the courses of MCI and AD have improved in sensitivity and accuracy, but it is yet to be determined which of a wide range of analytes are most useful for each purpose [8], [18], [19], [20], [21], [22], [23].
The present study of plasma ADE complement effector and regulatory proteins was designed to document abnormalities of this component of neurotoxic neuroinflammation in the common predementia condition of MCI and to determine differences between the complement profiles of those who would convert from MCI to dementia after 3 years and those who would remain stable at the stage of MCI without progressive cognitive loss. A well-characterized and previously reported series of participants is the basis for the investigation [23]. Differences in plasma ADE complement levels between MCI converters and those with stable MCI were significant and were as clearly distinct as those between patients with AD and their controls.
2. Methods
2.1. Study design, participant characterization, and blood collection
Participants in four distinct groups were evaluated clinically and by mental status testing at the time of entry into the study and then annually for 36 months: patients with an established diagnosis of mild-to-moderate AD (AD, n = 20), cognitively normal controls who were age- and gender-matched to the AD group (controls, n = 20), patients with stable MCI that did not change during the 36 month study (MCIS, n = 20), and patients who converted within 3 years from MCI to AD dementia (MCIC, n = 20) (Table 1). All participants in the MCIS and MCIC groups had been identified through the Alzheimer Disease Cooperative Study (ADCS) Biomarker Core at University of California, San Diego (UCSD). Participants in the AD and control groups were from the Jewish Home of San Francisco (JHSF). Cognitive normality of controls was based on Mini–Mental State Examination, level of activities of daily living, and capacity to provide a coherent history and list of medications. Comorbidities in the four groups were principally hypertension and type 2 diabetes mellitus and were equally distributed in the MCIS and MCIC groups.
Table 1.
Demographic and cognitive characteristics of the study participants
| Diagnosis | Total number | Male/Female | Age (mean + S.D.) | MMSE, entry | MMSE, end (3 years) |
|---|---|---|---|---|---|
| Control | 20 | 12/8 | 70.8 ± 5.34 | 29.2 ± 0.45 | NA |
| MCIS | 20 | 13/7 | 68.7 ± 7.76 | 29.0 ± 0.26 | 28.8 ± 0.33 |
| MCIC | 20 | 11/9 | 75.4 ± 6.82 | 27.4 ± 0.29 | 17.7 ± 0.70* |
| AD | 20 | 12/8 | 71.1 ± 6.90 | 23.9 + 0.75* | NA |
Abbreviations: AD, Alzheimer's disease; MMSE, Mini–Mental State Examination on entry into the study and at the 3-year end of the study, mean ± SEM; MCIS, subjects with mild cognitive impairment that was stable over the 3-year study; and MCIC, subjects with mild cognitive impairment on entry into the study that converted to dementia by the end of the three-year study.
NOTE. The significance of differences in MMSE between the MCIS and MCIC groups and between the control and AD groups was calculated with an unpaired t-test; *P < .001. NA = not applicable to the control and AD groups where cognitive evaluation was conducted once on entry into the study.
Mental status testing was conducted with the Mini–Mental State Examination as described in the study by Goetzl et al. [9]. Study patients with MCI or mild-to-moderate dementia with high probability of AD had a Clinical Dementia Rating global score of 0.5 or 1.0 according to the NIA–Alzheimer's Association and International Working Group-2 criteria [24]. All patients with MCI or AD had an abnormal cerebrospinal fluid level of amyloid β-peptide (Aβ) 1–42, which supported their diagnosis [25].
All participants with MCI donated blood for exosome analyses at the time of entry into the UCSD study and those with AD and their controls had donated blood over the same period of time at JHSF. Five mL of venous blood was drawn by syringe into 0.5 mL of saline with EDTA, incubated for 10 minutes at room temperature, and centrifuged for 15 minutes at 2500× g. Plasma samples were stored in 0.25 mL aliquots at −80°C.
The protocol and procedures of this study received prior approval by the Institutional Review Boards of the University of California, San Francisco (for JHSF) and the UCSD. Informed consent was obtained from each participant and often also from a family representative.
2.2. Enrichment of plasma ADEs for extraction and ELISA quantification of proteins
Aliquots of 0.25 mL plasma were incubated with 0.1 mL thromboplastin D (Thermo Fisher Scientific, Inc., Waltham, MA, USA), followed by addition of 0.15 mL of calcium- and magnesium-free Dulbecco's balanced salt solution (DBS−2) with protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Inc.; DBS++), as described [8]. After centrifugation at 3000 × g for 30 minutes at 4°C, total exosomes were precipitated from resultant supernatants with 126 μL per tube of ExoQuick (System Biosciences, Mountain View, CA, USA) and centrifugation at 1500 × g for 30 minutes at 4°C. To enrich ADEs, total exosomes were resuspended in 0.35 mL of DBS−2 and incubated for 60 minutes at room temperature with 1.5 μg of mouse anti-human glutamine aspartate transporter (GLAST) (ACSA-1) biotinylated antibody (Miltenyi Biotec, Inc., Auburn, CA) in 50 μL of 3% BSA (1:3.33 dilution of Blocker BSA 10% solution in DBS−2; Thermo Fisher Scientific, Inc.) per tube with mixing, followed by addition of 10 μL of streptavidin-agarose UltraLink resin (Thermo Fisher Scientific, Inc.) in 40 μL of 3% BSA and incubation for 30 minutes at room temperature with mixing. After centrifugation at 800× g for 10 minutes at 4°C and removal of the supernate, each pellet was resuspended in 100 μL of cold 0.05 M glycine-HCl (pH 3.0) by gentle mixing for 10 seconds and centrifuged at 4000× g for 10 minutes, all at 4°C. Supernatants then were transferred to clean tubes containing 25 μL of 10% BSA and 10 μL of 1 M Tris-HCl (pH = 8.0) and mixed before addition of 365 μL of mammalian protein extraction reagent (M-PER) (Thermo Fisher Scientific) with protease and phosphatase inhibitors. Resultant 0.5 mL lysates of ADEs were stored at −80°C.
ADE proteins found to be abnormal in AD, previously [23], were quantified by ELISA kits for human tetraspanning exosome marker CD81, complement fragment C4b, DAF (CD55) (American Research Products–Cusabio, Waltham, MA, USA), membrane cofactor protein (CD46), CR1 (American Research Products-Cloud-Clone Corp., Waltham, MA), complement fragment C3b, C1q portion of the C1 complement complex (Abcam, Inc., Cambridge, MA), Bb fragment of complement factor B (Quidel-Microvue, San Diego, CA), complement fragment C5b and terminal complement complex C5b-C9 (Elabscience, Bethesda, MD), CD59, mannose-binding lectin (MBL) (Ray Biotech, Inc., Norcross, GA), and complement factor D (Thermo Fisher–Invitrogen, Lafayette, CO). The mean value for all determinations of CD81 in each assay group was set at 1.00, and relative values of CD81 for each sample were used to normalize their recovery.
2.3. Statistical analyses
The Shapiro-Wilk test showed that data in all sets were distributed normally. Statistical significance of differences between means for cross-sectional groups AD and control, MCIS and control, MCIC and MCIS, and MCIC and AD were determined with an unpaired Student's t-test, including a Bonferroni correction (Prism 6; GraphPad Software, La Jolla, CA, USA). ROC analyses were conducted to assess the sensitivity of complement proteins (CPs) in distinguishing among the subgroups of participants (Prism 6; GraphPad Software).
3. Results
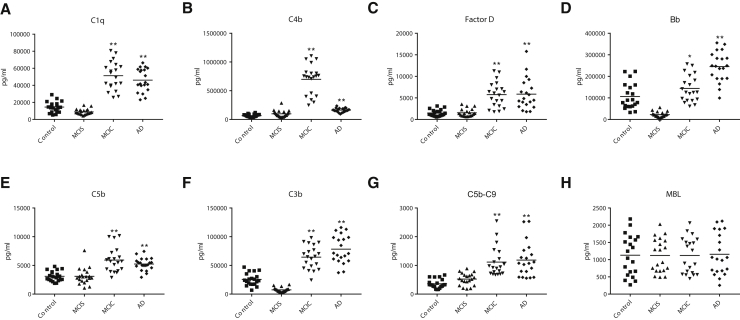
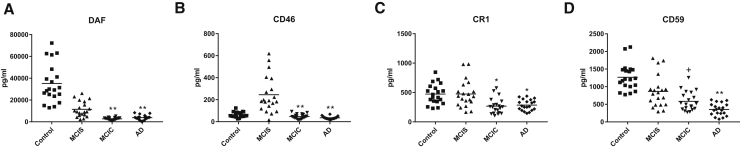
The mean ADE levels of all complement effector proteins for the patients with MCIC who converted to dementia after 3 years were significantly higher than those for the patients with MCIS who remained cognitively stable at the level of MCI after 3 years (Table 1, Fig. 1). Complement effector proteins with elevated levels in patients with MCIC included C1q and C4b of the classical pathway, factor D, and fragment Bb of the alternative pathway and C5b, C3b, and C5b-C9 terminal complex of both pathways. Levels of four of the ADE complement effector proteins show no overlap between the two groups of patients with MCI. The exception was MBL of the lectin complement pathway, where there was no difference between ADE levels of the two MCI groups. Inversely, mean ADE levels of all membrane-localized complement inhibitory proteins for the patients with MCIC were significantly lower than those for the patients with MCIS (Fig. 2). Significant differences in mean ADE levels of complement effector and inhibitory proteins separate patients with MCIC from cognitively normal controls (Figs. 1 and 2). Similar differences between patients with AD and their controls in ADE levels of all effector and regulatory proteins of the complement system, except MBL, were significant, as has been reported for AD [9].
Fig. 1.
ADE levels of complement effector proteins in cross-sectional control, MCI, and AD groups. Each point represents the value for a control or patient, and the horizontal line in point clusters is the mean level for that group. Mean ± SEM for control, stable MCI (MCIS), MCI that converted to dementia (MCIC), and AD patient values, respectively, are 14,560 ± 1423, 8845 ± 744, 51,274 ± 3706, and 46,135 ± 3001 pg/mL for C1q; 66,936 ± 5660, 98,464 ± 13,248, 698,662 ± 57,983, and 162,747 ± 8076 pg/mL for C4b; 1478 ± 171, 1539 ± 200, 5763 ± 653, and 5875 ± 816 pg/mL for factor D; 106,508 ± 13,449, 22,503 ± 2908,144,103 ± 13,792, and 245,094 ± 15,609 pg/mL for factor B fragment Bb; 3105 ± 198, 3109 ± 319, 5937 ± 478, and 5238 ± 244 pg/mL for C5b; 25,571 ± 2412, 7453 ± 876, 64,389 ± 4593, and 78,256 + 5499 pg/mL for C3b; 358 ± 35.0, 523 ± 47.2, 1117 ± 117, and 1187 ± 134 pg/mL for C5b-C9 TCC; and 1130 ± 131, 1122 ± 110, 1123 + 116, and 1157 ± 134 pg/mL for MBL. The significance of differences between values for controls and AD patients, and between values for MCIS and MCIC patients, was calculated by an unpaired Student's t-test and shown over the last two bars of each set; * = P < .01 and ** = P < .0001. Abbreviations: ADE, astrocyte-derived exosome; AD, Alzheimer's disease; MCI, mild cognitive impairment; MBL, mannose-binding lectin.
Fig. 2.
ADE levels of complement regulatory proteins in cross-sectional control, MCI, and AD groups. Each point represents the value for a control or patient, and the horizontal line in point clusters is the mean level for that group. Mean ± SEM for control, MCIS, MCIC, and AD patient values, respectively, are 35,166 ± 3981, 11,486 ± 1581, 2796 ± 230, and 3825 ± 499 pg/mL for DAF; 62.4 ± 5.48, 245 ± 37.3, 47.1 ± 4.78, and 35.3 ± 2.91 pg/mL for CD46; 473 ± 38.2, 475 ± 51.9, 268 ± 29.9, and 286 ± 20.6 pg/mL for CR1; and 1268 ± 84.1, 865 ± 103, 584 ± 60.6, and 353 ± 38.5 pg/mL for CD59. The significance of differences between values for controls and AD patients, and between values for MCIS and MCIC patients, was calculated by an unpaired Student's t-test and shown over the last two bars of each set; + = P < .05, * = P < .01, and ** = P < .0001. Abbreviations: ADE, astrocyte-derived exosome; AD, Alzheimer's disease; MCI, mild cognitive impairment; MCIC, MCI that converted to dementia; MCIS; stable MCI.
There were nearly universally significant differences in ADE CP levels between participants with MCIC and MCIS and between patients with AD and controls (Figs. 1 and 2, Table 2). Although ADE levels of seven CPs reliably distinguished MCIS participants from controls, ADE levels of only C4b, Bb, and CD59 significantly distinguished patients with AD from those with MCIC, who are at an early stage of AD and converted to AD within 3 years. The CPs that most consistently distinguished among groups were Bb, C3b, C4b, DAF, and CD46 (Table 2). C5b, factor D, and CR1 all failed to distinguish patients with MCIS from controls or patients with AD from patients with MCIC (Table 2). Overall, Bb was the best performer, and factor D and CR1 were the least helpful.
Table 2.
Receiver operating characteristic (ROC) evaluation of complement protein sensitivity in distinguishing clinical subgroups
| Complement protein | Sensitivity, % (mean ± SEM) |
|||
|---|---|---|---|---|
| MCIS vs. control | MCIC vs. MCIS | AD vs. control | AD vs. MCIC | |
| Bb | 97.8 ± 0.018 | 100 | 93.0 ± 0.038 | 85.5 ± 0.059 |
| C3b | 96.3 ± 0.028 | 100 | 98.0 ± 0.017 | 65.8 ± 0.088 |
| C1q | 78.3 ± 0.075 | 100 | 99.3 ± 0.009 | __ |
| C4b | 66.3 ± 0.087 | 99.8 ± 0.004 | 99.0 ± 0.011 | 100 |
| C5b | __ | 89.3 ± 0.052 | 93.8 ± 0.036 | __ |
| TCC | 72.6 ± 0.082 | 92.8 ± 0.039 | 95.0 ± 0.031 | __ |
| Factor D | __ | 94.3 ± 0.033 | 95.3 ± 0.029 | __ |
| DAF | 93.4 ± 0.036 | 93.0 ± 0.045 | 100 | 65.9 ± 0.092 |
| CD46 | 92.0 ± 0.052 | 94.3 ± 0.049 | 85.3 ± 0.061 | 66.3 ± 0.089 |
| CD59 | 77.5 ± 0.079 | 69.0 ± 0.084 | 100 | 75.5 ± 0.075 |
| CR1 | __ | 79.3 ± 0.072 | 83.0 ± 0.064 | __ |
Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment; MCIC, MCI that converted to dementia; MCIS, stable MCI.
NOTE. All values of sensitivity less than 60% are represented by dashes. All values for MBL were lower than 60%.
4. Discussion
Levels of ADE cargo proteins of the classical and alternative effector pathways, as well as of several distinct complement regulatory systems, differed with the nature and stage of CNS degeneration (Figs. 1 and 2). The elevated levels of ADE C3b and C5b-C9 terminal complex are of special interest as C3b opsonization of neurons may enhance attraction and neuronal toxicity of microglia, while the C5b-C9 complex may injure neurons directly. It is not yet known which profile of ADE proteins will most significantly distinguish stable MCI (MCIS) from that poised to convert to AD dementia (MCIC) at each stage of the course. However, it is clear that the differences between ADE levels of each protein of the classical and alternative complement pathways were as great when comparing the MCIC group with the MCIS group as comparing AD to controls (Figs. 1 and 2). Diminished ADE levels of complement regulatory proteins CD59 and DAF were found previously at the preclinical stage of AD [9], when levels of complement effector proteins were still normal, and the early appearance of abnormalities of similar magnitude now was detected in CD59 and DAF in the patients with MCIC as well (Fig. 2). The capacity to characterize differences in distinct sets of MCI may prove to be most useful, as there are fewer predictive markers for MCI conversion to AD than for staging the course and severity of AD.
Any potential value of ADE CPs for prediction of conversion and other aspects of the pathophysiology of MCI will be dependent on considerable further validating studies of many more patients of different age ranges, ethnicities, and comorbidities. This is especially important as our AD and control groups were in a long-term care facility, whereas the participants with MCIS and MCIC were principally community based. Perhaps, these investigations will also provide insights as to any avenues for exploitation of complement systems as therapeutic targets in MCI, AD, or other proteinopathic neurodegenerative diseases.
Research in Context.
-
1.
Systematic review: The authors have reviewed with PubMed reports of blood-based protein markers in Alzheimer's disease (AD). Inflammatory-type astrocytes now are recognized as neurotoxic in AD. Our recent publication [9] reports abnormal levels of complement proteins in plasma astrocyte-derived exosomes (ADEs), including low levels of complement inhibitors 5-12 years before and high levels of complement effectors at the onset of AD. Here, we address whether ADE levels of complement proteins in mild cognitive impairment can predict risk of conversion to AD within 3 years.
-
2.
Interpretation: New findings are significant elevations of ADE complement effectors and significant depressions of complement inhibitors in patients with mild cognitive impairment who convert to dementia 3 years later.
-
3.
Future directions: Analyses of ADE complement proteins in a large set of patients with mild cognitive impairment will be needed to define their usefulness for prediction of risk and course of conversion to AD.
Acknowledgments
These studies were supported by grants AG057459, AG057469, AG051848, and AG018440 from the National Institute on Aging, and by VA Merit Awards BX003040 and BX004312 to Dr. Robert A. Rissman. The authors are grateful to Judith H. Goetzl (JHSF) for preparation of the graphic illustrations.
Authors' contributions: C.N.W. designed the study, evaluated patients, and edited the manuscript; E.J.G. designed the study, performed laboratory work, and wrote the manuscript; J.B.S. evaluated patients and edited the manuscript; F.M.E. performed laboratory work; and R.A.R. designed the study and edited the manuscript.
References
- 1.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamanian J.L., Xu L., Foo L.C., Nouri N., Zhou L., Giffard R.G. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson M.A., Burda J.E., Ren Y., Ao Y., O'Shea T.M., Kawaguchi R. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetzl E.J., Miller B.L. Multicellular hypothesis for the pathogenesis of Alzheimer's disease. Faseb J. 2017;31:1792–1795. doi: 10.1096/fj.201601221R. [DOI] [PubMed] [Google Scholar]
- 5.Ben Haim L., Carrillo-de Sauvage M.A., Ceyzeriat K., Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9:278. doi: 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liddelow S.A., Barres B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Goetzl E.J., Mustapic M., Kapogiannis D., Eitan E., Lobach I.V., Goetzl L. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer's disease. Faseb J. 2016;30:3853–3859. doi: 10.1096/fj.201600756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetzl E.J., Schwartz J.B., Abner E.L., Jicha G.A., Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544–552. doi: 10.1002/ana.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knopman D.S., Jack C.R., Jr., Wiste H.J., Weigand S.D., Vemuri P., Lowe V. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roe C.M., Fagan A.M., Grant E.A., Hassenstab J., Moulder K.L., Maue Dreyfus D. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–1791. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvatore C., Cerasa A., Castiglioni I. MRI Characterizes the Progressive Course of AD and Predicts Conversion to Alzheimer's Dementia 24 Months Before Probable Diagnosis. Front Aging Neurosci. 2018;10:135. doi: 10.3389/fnagi.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.J., Ritchie C.S., Yaffe K., Stijacic Cenzer I., Barnes D.E. A clinical index to predict progression from mild cognitive impairment to dementia due to Alzheimer's disease. PLoS One. 2014;9:e113535. doi: 10.1371/journal.pone.0113535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang H., Ye B.S., Woo S., Kim S.W., Chin J., Choi S.H. Prediction model of conversion to dementia risk in subjects with amnestic mild cognitive impairment: a longitudinal, multi-center clinic-based study. J Alzheimers Dis. 2018;61:825. doi: 10.3233/JAD-179010. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.S., Cho S.K., Kim H.J., Kim Y.J., Park K.C., Lockhart S.N. Prediction models of cognitive trajectories in patients with nonamnestic mild cognitive impairment. Sci Rep. 2018;8:10468. doi: 10.1038/s41598-018-28881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapogiannis D., Boxer A., Schwartz J.B., Abner E.L., Biragyn A., Masharani U. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer's disease. Faseb J. 2015;29:589–596. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl E.J., Boxer A., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology. 2015;85:40–47. doi: 10.1212/WNL.0000000000001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetzl E.J., Boxer A., Schwartz J.B., Abner E.L., Petersen R.C., Miller B.L. Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Ann Clin Transl Neurol. 2015;2:769–773. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetzl E.J., Kapogiannis D., Schwartz J.B., Lobach I.V., Goetzl L., Abner E.L. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer's disease. Faseb J. 2016;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetzl E.J., Abner E.L., Jicha G.A., Kapogiannis D., Schwartz J.B. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer's disease. Faseb J. 2018;32:888–893. doi: 10.1096/fj.201700731R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winston C.N., Goetzl E.J., Akers J.C., Carter B.S., Rockenstein E.M., Galasko D. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw L.M., Vanderstichele H., Knapik-Czajka M., Clark C.M., Aisen P.S., Petersen R.C. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]