Abstract
Aims
The prevalence of type 2 diabetes is increasing worldwide but little known about the status in the Faroe Islands. The aim was therefore to determine the prevalence of type 2 diabetes mellitus and prediabetes in two non-random populations aged 44–77 years.
Methods
This cross-sectional survey was conducted between 2011 and 2012 and included two sub-populations, namely 518 Septuagenarians aged 74–77 years (84% of the invited) and 401 Mark aged 44–73 years (87% of the invited). Subjects were screened for glycosylated haemoglobin, type A1c, non-fasting random plasma glucose, fasting plasma glucose followed by an oral glucose tolerance test. The screening was based on a diagnostic algorithm that included screening, diagnostic and confirmatory steps.
Results
Each group was analysed separately. In the Septuagenarian group 20.4% had type 2 diabetes, with 5.2% being newly detected and a total of 59% had prediabetes. In the Mark group 4.1% had diabetes, with 2.1% being newly detected and 22.3% had prediabetes. Diabetes increased with age and was significantly more prevalent among men. Women had lower mean fasting plasma glucose concentrations and men had lower values for 2-hours plasma glucose. Significant predictors associated with diabetes mellitus included obesity (BMI ≥ 30, abnormal waist/hip ratio and vegetable consumption.
Conclusions
Among the Faroese populations studied, the prevalence of type 2 diabetes increased with age and was more prevalent among men. The detected prevalence was comparable to other Nordic countries for all age-groups.
Abbreviations: BMI, body mass index; CDC, Centers for Disease Control and Prevention; FPG, fasting plasma glucose; HbA1c, glycosylated heamoglobin type A1c; IFG, impaired fasting glycaemia; IGT, impaired glucose tolerance; K-T2D, Previously Known Type 2 Diabetes; M, Mark; NGT, normal glucose tolerance; N-T2D, newly diagnosed diabetes; OCP, organochlorine pollutant; OGTT, oral glucose tolerance test; RPG, random plasma glucose; S, Septuagenarians; SD, standard deviation; T2D, type 2 diabetes; WHO, World Health Organization; WHR, waist/hip ratio; 2hPG, 2-hour plasma glucose
Keywords: Type 2 diabetes mellitus, Impaired glucose regulation, Prediabetes, Faroe Islands, Prevalence, Diagnostic criteria
Introduction
The prevalence of diabetes is increasing worldwide. In the IDF Diabetes Atlas from 2017 it is estimated that 8.8% of the world population lives with type 2 diabetes (T2D) [1] compared to 4.7% in 1980 and an aging population is not the sole explanation for this increase according to The World Health Organization (WHO) [2]. In Alaska, the prevalence among the Native population living with T2D is estimated to be 15% [3]. Numbers from Canada exhibit some variation as First-Nation members (on-reserve) had an age-standardized prevalence of T2D of 17.2% and the Inuit population a crude prevalence of 4% [4], although Singh and Chan [4] found an average prevalence of 5.7% among the Inuit population in Arctic Canada, with a variation from 3.9% to 8.7% in different settlements [5]. Within the Nordic countries, Greenland mirrors Native Alaska and Northern Canada with a prevalence of 9% among persons aged 18 years and older [6], whereas the prevalence in Iceland and Norway are lower; 4% for people aged 45–64 years and about 6% for people aged 40–77 years, respectively [7], [8].
Until recently, little was known about the status of T2D in the Faroe Islands. The Faroe Islands are located in the North Atlantic Ocean with a total population of just above 51,000 individuals [9]. The islands belong to the Nordic countries and have similarities in terms of welfare system, language and lifestyle.
In 2011 a large population-based study was conducted in the Faroe Islands, comprising three sub-populations encompassing 13% of the entire population in the age-group 40–77 years. A link between high concentrations of environmental contaminants and increased T2D risk was hypothesized [5], [10] but the detailed screening for glycemic status and the sample size permitted an investigation of the prevalence of T2D for this particular age-group. The rationale for the age-span was that the risk of T2D increases with age and the prevalence is low before the age of 40 years. From the study, a random sample aged 40–74 years constituted one sub-group by which the prevalence is estimated to be 9.5% [11]. Thus, the aim of this study is to determine the prevalence of T2D in two non-random populations in the Faroese aged 44–77 years and to identify cases of prediabetes, as defined by impaired fasting glycaemia (IFG), impaired glucose tolerance (IGT), the combination of the two and normal glucose tolerance (NGT).
Material and methods
Study population
This study is a cross-sectional survey conducted in 2011–2012 and the original study including three different sub-populations, with one random sample, which is published elsewhere [11] and two subsequent populations, the basis for this paper, namely the Septuagenarians (S) and Mark (M) groups.
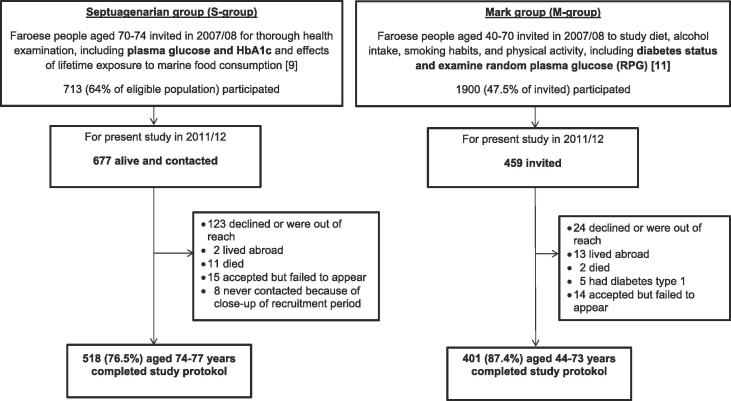
For the S group, all Faroese citizens aged 70–74 years were originally invited to participate in a study (The Septuagenarian study) in 2007–2008 to examine cardiovascular and neurobehavioral effects of lifetime exposure to marine food consumption. A total of 713 individuals (64% of the eligible population) participated. All members from the Septuagenarian study, that were still alive, were invited to participate in this present study (n = 677), now aged 74–77 years, and 518 (76.0%) completed the study protocol, see Fig. 1.
Fig. 1.
Protocol flow chart of the study population.
In the M group, subjects aged 40–70 years (N = 1900) were invited to participate in a cross-sectional population-based study in 2007–2008 according to a protocol identical to the Danish KRAM study [12], and a selected group was invited for this diabetes study, see Fig. 1.
All invited participants received a letter of invitation and subsequently a phone call to arrange the examination details. The study was approved by the local ethical review committee, with participation on a voluntary basis as documented by written informed consent.
Diagnostic algorithm
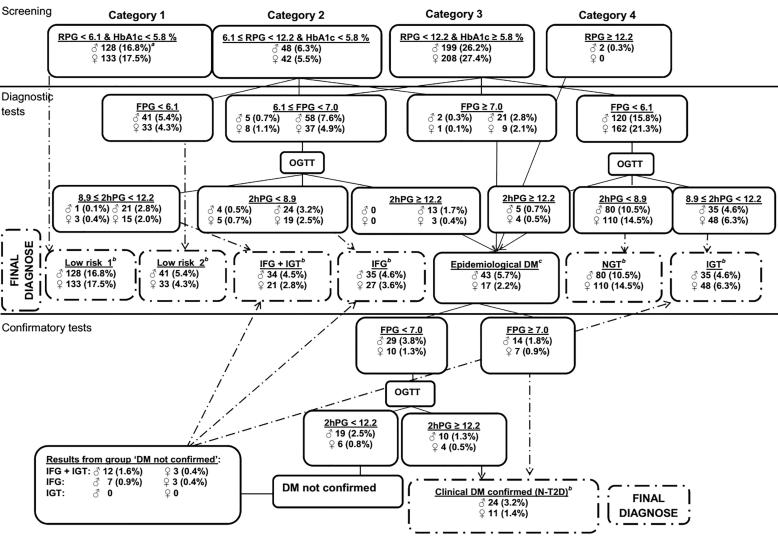
The screening program was based on a diagnostic algorithm (Fig. 2) designed by the ADDITION study group [13] and a detailed description of the program has been published previously [11]. In short, this program consisted of a three step testing with an initial screening test followed by a diagnostic and a confirmatory test before a final diagnoses of either type 2 diabetes or prediabetes, classified as newly diagnosed diabetes (N-T2D) defined as fasting plasma glucose (FPG) ≥ 7.0 mmol/L & 2-hour plasma glucose (2hPG) ≥ 12.2 mmol/L; IFG, (6.1 mmol/L ≤ FPG < 7.0 mmol/L & 2hPG < 8.9 mmol/L); IGT, (FPG < 6.1 mmol/L & 8.9 mmol/L ≤ 2hPG < 12.2 mmol/L); the combination of both, (6.1 mmol/L ≤ FPG < 7.0 mmol/L & 8.9 ≤ 2hPG < 12.2 mmol/L); or NGT, (FPG < 6.1 mmol/L & 2hPG < 8.9 mmol/L but note that the last group had an initial HbA1c level ≥5.8% and is therefore considered as having prediabetes in this study). Participants were considered to be at Low risk if RPG < 6.1 mmol/L & HbA1c < 5.8%, or if 6.1 mmol/L ≤ RPG < 12.2 mmol/L & HbA1c < 5.8% as well as FPG < 6.1 mmol/L. The intention with the algorithm was to find as many subjects as possible with prior unknown T2D, while minimizing the number of unnecessary oral glucose tolerance tests (OGTTs). Non-fasting RPG, fasting plasma glucose (FPG) and HbA1c were screened using finger capillary blood. The cut-off values for capillary blood vs. venous blood for random and 2-hours plasma glucose (2hPG) were aligned with the conversion factor of 1.1 [14]. The algorithm has proven reliable in a large Danish study [15].
Fig. 2.
The screening algorithm used in the Faroese Diabetes Study. aPercent were calculated from the entire screening group (n = 760). bEpidemiological DM was defined as a preliminary diagnosis of T2D. According to the screening program an initial screening confirmed the final diagnoses whether the subjects were diagnosed as being at low risk, having prediabetes or T2D (see boxes with broken lines).
Assessment of blood glucose
The blood glucose concentrations were analysed using a HemoCue Glucose 201 RT Analyzer (Axis-Shield Poc AS, Ängelholm, Sweden), which has a coefficient of variation (CV) of 1,6% in the operating range of 4–8 mmol/L (plasmaglucose). Two blood samples were taken for all tests and the average of the two results was used to minimize the measurement error. If the discrepancy was ≥0.5 mmol/L a third blood sample was taken to calculate the mean. An Afinion AS100 Analyzer (HemoCue AB, Oslo Norway) was used to analyse HbA1c. The CV for the Afinion (HbA1c) is 1.7–2.6% (a precision of <3% is expected in a controlled laboratory setting). Detailed description of sample procedure and quality control is published previously [11]. In short, all participants had minimum two blood samples taken for each round and a monthly quality control was performed.
Anthropometric measures
The anthropometric measurements were available for all individuals who underwent the ‘Diagnostic’ step of the algorithm. These include body weight (in kg), and height, waist and hip circumference (in cm).
Questionnaire
Individuals who proceeded to the second step of the screening (n = 423) (diagnostic test, Fig. 1), as well as a selected group from the low risk category and K-T2D (n = 236), subsequently answered a questionnaire. Four individuals failed to hand in the questionnaire. A total of 659 questionnaires were employed in the statistical analyses.
The questionnaire included medical history regarding T2D; family history of T2D for first-relatives only; cardiovascular conditions; present medication use; smoking habits and alcohol intake; dietary information, including local traditional food items like whale meat and blubber, sea birds and fish; educational level and employment; physical activity at work and during leisure time.
Statistical methods
The statistical analyses were performed using the IMB SPSS Statistics for Windows (version 24.0; SPSS Inc., Chicago, IL, USA). Descriptive results are presented with mean and standard deviations (SD) for continuous variables and as a percentage for categorical variables. The prevalence date was fixed at January 1st, 2012 and the age was determined as of this date. Age was standardized to the 2012 Faroese Census population by using the age-span 74, 75, 76 and 77 for the S-group and age 44–73 years for the M-group, in six age-groups (44–48, 49–53, 54–58, 59–63, 64–68, 69–73) [16].
When possible and relevant, analyses were controlled for sex and age or as 10-year age-group. However, when the subdivision caused too small groups for statistical analyses age-groups and/or male/female groups were merged.
Student’s t-test or Mann-Whitney test for continuous variables was used, after normality was assessed by visual inspection of plots and by Kolmogorov-Smirnov test. Associations between the various prediabetes, N-T2D and K-T2D groups were analysed against the low risk group according to risk factors, such as age; body mass index (BMI); abnormal waist/hip ratio (abnormal-(WHR)), defined as WHR > 1 for men and >0.85 for women; hypertension and vascular attack (heart attack and/or stroke); family history of T2D; and intake of vegetables by logistic regression analyses, controlling for age, sex and BMI when stated. Variables not demonstrating a significant association were not included in the tables. Reported p-values were judged on the basis of a statistical significance limit of 0.05.
Results
The two study populations, that were analysed according to the algorithm, were comprised of (S) 254 women and 264 men & (M) 190 women and 211 men, with a mean age of 75.7 (SD = 1.1, range 74–77) and 58.0 (SD = 7.9, range 44–73), respectively. No difference was observed between the participants and non-participants in terms of sex, age and place of residence.
Major findings
Regarding the S-group: A total of 105 subjects had K-T2D at study entrance and the crude prevalence for the entire group (n = 677) was 15.5% and age-adjusted 15.2%. Based on the study group (n = 518), the age-adjusted prevalence for the N-T2D was 5.2%, IFG, 7.3%; IGT, 13.2%; the combination of both 7.7%, NGT, 30.6% (the NGT group had HbA1c ≥ 5.8% (40 mmol/mol) in the screening step and was therefore regarded as having prediabetes); and Low risk, 16.3%. The crude prevalence for each age is presented in Table 1.
Table 1.
S-group: Crude age prevalence (in %) for each diagnostic group.
| Age (years) | Low riskb | NGTa,c | IFGc | IGTc | IFG + IGT | N-T2Dc | K-T2Dc,d |
|---|---|---|---|---|---|---|---|
| n = 88 | n = 155 | n = 36 | n = 69 | n = 39 | n = 26 | n = 105 | |
| 74 | 7.4 | 38.3 | 10.6 | 13.8 | 8.5 | 6.4 | 10.4 |
| 75 | 26.6 | 23.1 | 4.2 | 10.5 | 6.3 | 4.2 | 19.4 |
| 76 | 22.1 | 26.7 | 6.9 | 12.2 | 9.2 | 6.1 | 11.5 |
| 77 | 9.3 | 34.0 | 7.3 | 16.7 | 6.7 | 4.0 | 19.5 |
| Overall | 17.0 | 29.9 | 6.9 | 13.3 | 7.5 | 5.0 | 15.4 |
Note that the NGT group had HbA1c ≥ 5.8% (40 mmol/mol) in the screening step of the standard algorithm and will therefore be regarded as having prediabetes.
Low risk, defined as having RPG < 6.1 mmol/L & HbA1c < 5.8% or 6.1 mmol/L ≤ RPG < 12.2 mmol/L & HbA1c a 5.8% followed by FPG < 6.1 mmol/L (see Fig. 2).
NGT, Normal Glucose Tolerance; IFG, Impaired Fasting Glucose; IGT, Impaired Glucose Tolerance; N-T2D, Newly Diagnosed Type 2 Diabetes Mellitus; K-T2D, Previously Known Type 2 Diabetes Mellitus.
The prevalence is calculated from the entire group alive: age 74, n = 135; 75, n = 186; 76, n = 192; 77, n = 169.
Regarding the M-group: A total of 54 participants were diagnosed with K-T2D at study entrance, from this group 16 participants were not diagnosed with diabetes in the baseline study (see selection criteria described earlier). The age-adjusted prevalence of this group (n = 16) was 4.0%; for N-T2D, 2.1%; IFG, 6.3%; IGT, 3.7%; the combination of both, 3.6%; NGT 8.7%; and Low risk, 62.8%. The crude prevalence for six age-groups is presented in Table 2.
Table 2.
M-group: Crude age prevalence (in %) for each diagnostic group.
| Age-group | Low riskb | NGTa,c | IFGc | IGTc | IFG + IGT | N-T2Dc | K-T2Dc,d |
|---|---|---|---|---|---|---|---|
| n = 247 | n = 35 | n = 26 | n = 14 | n = 16 | n = 9 | n = 16 | |
| 44–48 | 75.0 | 9.6 | 5.8 | 5.8 | 0 | 0 | 3.8 |
| 49–53 | 80.5 | 10.4 | 3.9 | 0 | 2.6 | 1.3 | 1.3 |
| 54–58 | 68.9 | 8.2 | 6.6 | 4.9 | 3.3 | 4.9 | 3.3 |
| 59–63 | 65.0 | 11.3 | 10.0 | 2.5 | 6.3 | 2.5 | 2.5 |
| 64–68 | 53.7 | 9.3 | 7.4 | 3.7 | 7.4 | 3.7 | 14.8 |
| 69–73 | 59.0 | 7.7 | 10.3 | 10.3 | 7.7 | 2.6 | 2.6 |
| Overall | 68.0 | 9.6 | 7.2 | 3.9 | 4.4 | 2.5 | 4.4 |
The NGT group had HbA1c ≥ 5.8% (40 mmol/mol) in the screening step of the standard algorithm and will therefore be regarded as having prediabetes.
Low risk, defined as having RPG < 6.1 mmol/L & HbA1c < 5.8% or 6.1 mmol/L ≤ RPG < 12.2 mmol/L & HbA1c a 5.8% followed by FPG < 6.1 mmol/L (see Fig. 2).
NGT, Normal Glucose Tolerance; IFG, Impaired Fasting Glucose; IGT, Impaired Glucose Tolerance; N-T2D, Newly Diagnosed Type 2 Diabetes Mellitus; K-T2D, Previously Known Type 2 Diabetes Mellitus.
Included are subjects that have been diagnosed with T2D subsequently to the baseline study.
For both study groups, T2D was more prevalent among men. For the S-group: 26.2% among men vs. 17.7% for women and for the M-group: 10.2% among men vs. 3.4% among women. Despite the narrow age-span in the old group, we did observe an increase in prevalence with age: 19.6% respectively 15.3% for men and women aged 74 years and 29.9% and 19.1% 77 years. This was also the case for the M-group, as the youngest men and women (aged 44–48 years) had a prevalence of 3.6% and 4.2%, respectively vs. 27.8% and 16% aged ≥64 years.
Relationship between sex and FPG and 2hPG concentrations
Women had generally lower median FPG across all age-groups compared to men, with a significant difference only in the oldest age-group 5.60 mmol/L vs. 5.70 mmol/L, p = 0.02.
The opposite relationship was observed for 2hPG up to the age of 70 years, with women having higher concentrations, although not significantly different. In the oldest age-group (70–77 years), men had a median value of 8.40 mmol/L and women 8.08 mmol/L (p = 0.09).
Predictors of T2D
The frequency of obesity and an abnormal WHR was significantly higher in the groups with diabetes, both newly detected and previously known compared to the NGT and low risk groups, as presented in Table 3. In particular, subjects with N-T2D tended to be obese (OR 4.4 [95% CI: 2.1; 9.1]), have an abnormal WHR (OR 2.5 [95% CI: 1.2; 5.2]) and consume less vegetables (OR 0.3 [95% CI: 0.11; 0.58]). A history of vascular attack and a family history of T2D were significantly more frequent only in the groups having diabetes and hypertension in the K-T2D group only. Smoking on the other hand, was rather evenly distributed in all diagnostic groups and leisure activity as less common among the groups having diabetes, especially the newly detected (data not shown).
Table 3.
Association between low risk and impaired glucose regulation categories according to various risk factors.
| Low risk | NGTa | IFGa | IGTa | IFG + IGT | N-T2Da | K-T2Da | p value for trend | ||
|---|---|---|---|---|---|---|---|---|---|
| n | 335 | 190 | 62 | 83 | 55 | 35 | 159 | ||
| Age, mean | 61.7 (10.6) (ref.) | 72.3 (7.7) | 68.8 (9.5) | 73.2 (7.0) | 71.7 (7.1) | 71.6 (7.6) | 70.9 (8.1) | <0.001f | |
| Obesityb | % | 24.5 (ref.) | 31.1 | 54.1 | 34.9 | 52.7 | 60.0 | 49.1 | |
| OR | 1 | 1.28 (0.83; 1.97) | 3.48*** (1.96; 6.17) | 1.52 (0.88; 2.62) | 3.23*** (1.76; 5.92) | 4.37*** (2.08; 9.14) | 2.83*** (1.78; 4.92) | <0.001g | |
| Abnormal WHRc | % | 29.9 (ref.) | 31.9 | 33.3 | 37.7 | 45.5 | 48.6 | 48.6 | |
| OR | 1 | 0.90 (0.59; 1.39) | 1.16 (0.63; 2.15) | 1.15 (0.67; 1.99) | 2.03* (1.10; 3.78) | 2.49* (1.18; 5.23) | 2.30** (1.43; 3.69) | 0.001g | |
| Hypertension | % | 70.5 (ref.) | 55.6 | 69.8 | 72.2 | 78.3 | 78.1 | 89.5 | |
| OR | 1 | 0.53* (0.32; 0.89) | 0.98 (0.47; 2.02) | 1.08 (0.55; 2.10) | 1.34 (0.59; 3.03) | 1.22 (0.48; 3.12) | 3.37** (1.63; 6.99) | <0.001h | |
| Vascular attack | % | 39.7 (ref.) | 31.9 | 34.6 | 44.4 | 45.7 | 65.6 | 55.8 | |
| OR | 1 | 0.73 (0.44; 1.22) | 0.98 (0.49; 1.99) | 1.21 (0.66; 2.23) | 1.31 (0.64; 2.65) | 2.99* (1.28; 6.70) | 2.20** (1.28; 3.78) | 0.001h | |
| Hereditary T2Dd | % | 32.8 (ref.) | 31.0 | 40.0 | 36.8 | 34.0 | 53.6 | 60.8 | |
| OR | 1 | 0.85 (0.51; 1.42) | 1.32 (0.67; 2.59) | 1.10 (0.59; 2.03) | 1.05 (0.52; 2.12) | 2.40* (1.03; 5.59) | 3.12*** (1.77; 5.49) | <0.001h | |
| Cooked vegetablese | % | 81.5 (ref.) | 830. | 73.3 | 78.0 | 70.9 | 52.9 | 78.6 | |
| OR | 1 | 1.04 (0.57; 1.92) | 0.53 (0.25; 1.13) | 0.78 (0.38; 1.59) | 0.55 (0.26; 1.17) | 0.25* (0.11; 0.58) | 0.76 (0.39; 1.47) | 0.02h |
NGT, Normal Glucose Tolerance; IFG, Impaired Fasting Glucose; IGT, Impaired Glucose Tolerance; N-T2D, Newly Diagnosed Type 2 Diabetes Mellitus; K-T2D, Previously Known Type 2 Diabetes Mellitus.
BMI ≥ 30 kg/m2.
WHR > 1 for men and >0.85 for women.
First relative only.
Consumed once a week or more.
Tested with ANOVA (Bonferroni) and difference in mean age between men and women by Mann-Whitney test, none were significant.
Adjusted for sex and age.
Adjusted for sex, age and BMI.
p < 0.05.
p < 0.001.
p < 0.0001.
Cut-off limits
According to the standard algorithm the cut-off limit for HbA1c was ≥5.8% (40 mmol/mol) without any upper limit. A total of 32 participants had HbA1c ≥ 6.5% (48 mmol/mol) (present limit for diagnosing diabetes), from this group 10 subjects were diagnosed to have N-T2D, while the remaining individuals had IFG (N = 5), IGT (N = 4), IFG + IGT (N = 10) and NGT (N = 3). If these latter subjects (n = 22) were included in the N-T2D group the age-adjusted prevalence would increase from 2.4% to 3.3%.
Further, at the screening level according to the standard algorithm, Category 3 included 409 participants (53.6%) from the total study population. If the HbA1c limit was lowered to 5.7% (39 mmol/mol) [17] and RPG < 12.2 mmol/L, 45 (5.9%) subjects would be added to the category. Of these, 28 (3.7%) were originally in Category 1 and 17 (2.2%) were in Category 2.
The HbA1c levels increased with age and close to two thirds of the subjects with NGT were in the oldest age category. Thus, 78.8% of the subjects above age 70 had elevated HbA1c as compared to 21.2% for those below 70 years (data not shown) with a median concentration of 5.5% (36 mmol/mol) below the age of 70 years and 6.0% (42 mmol/mol) above the age of 70 years, p ≤ 0.001.
Discussion
This extensive study including two non-random populations contributes to the overall estimation of the prevalence of prediabetes and T2D in the Faroe Islands [11].
The age-adjusted prevalence of T2D for the S-group was 20.4%, of whom 5.2% were diagnosed with N-T2D and for the M-group 6.1%, with 2.1% N-T2D. The relatively small proportions of new cases (25% in the older group and 34% in the younger) [18] may indicate that health-care visits increase with age and a well-functioning referral system between the primary and secondary care sectors as the diabetes unit at the National Hospital arranges regular workshops for personnel in the primary care sector.
Similar to previous findings, the prevalence of T2D increased with age, particularly among men [19], [20] and men were also more likely to have high blood sugars when tested by FPG and women by 2hPG [21]. Yet, the prevalences of prediabetes and diabetes depend on the screening method and cut-off levels for glycaemic values [17], [22]. The profile of the NGT group (see Table 3) was comparable to or had better outcomes (abnormal WHR, hypertension and vascular attack) compared to the low risk group, despite being 10 years older. One explanation can be that HbA1c increases with age [23] but not FPG and 2hPG and if only the two latter values were investigated subjects would be regarded as being at low risk.
Conditions known to be related to T2D such as obesity (BMI ≥ 30.0), abnormal WHR (WHR > 1 for men and >0.85 for women) and intake of vegetables (consumed more than once a week) were also identified as associated factors in this study. Persons with N-T2D were more than four times as likely to be obese compared to persons from the low risk group (OR, 4.4; p < 0.001), more than two times as likely to have an abnormal WHR (OR, 2.5; p < 0.05) and less likely to consume vegetables (OR, 0.3; p < 0.05). Of anthropometric measures waist and/or hip circumference or its ratio is often considered better predictors of T2D compared to BMI, especially among people with low or normal weight, due to risk of central fat deposits [24], [25]. This was not the case in our study and in agreement with findings presented by Hardy and colleagues [26]. Other risk factors associated to T2D were hypertension, vascular attack and hereditary T2D.
Close to one third of the entire S-group was diagnosed with NGT whereas this was less than 10% in the M-group. The NGT group had at the diagnostic step an initial elevated HbA1c level. This can be ascribed to the increasing HbA1c levels with age [23], as we found close to 80% having elevated HbA1c levels at the age above 70 years and only 21.2% for those below the age of 70 years. The benefit of lowering HbA1c levels in elderly is being discussed as elderly with HbA1c above 8% (64 mmol/mol) had better functional outcome compared to levels below 8% [27], [28]. This can also be one explanation to the relatively lower proportion of hypertension in the NGT group despite them being 10 years older than the low-risk group.
For women in the S-group the age-adjusted prevalence of T2D was 13% and for men 17%, respectively. This is in the same range as reported by other Nordic countries. In Stockholm’s county of Sweden, the self-reported prevalence of diabetes was 13% for women and 18% for men aged 65–84 years [29]. In Denmark, over 160,000 persons participated in a questionnaire-survey conducted by the National Board of Health and 13% of the women and 16% of the men aged ≥75 years reported to have diabetes [30]. Strøm and colleagues [31] analysed the Norwegian Prescription Database for the use of blood glucose-lowering drugs. Their findings indicated that, the diabetes prevalence increased with age and was more frequent among men with peak prevalence at age 76 years (12.4%) and in women at age 80 years (9.9%) [31] A consensus report from the US reported the prevalence of diabetes among adults aged ≥65 years to vary from 22 to 33% depending on the diagnostic criteria used [32], while, the U.S. Centres for Disease Control and Prevention (CDC) estimated that 20.8% aged ≥65 years were diagnosed with diabetes [3].
The age-adjusted prevalence in the M-group was 6.1%, which was more than two and a half times less compared to the S-group’s findings. Yet, the overall prevalence in the Faroe Islands may be even lower, as the age-group 20–43 were not included. On the other hand, subjects 80+ were also not included. In Denmark the overall prevalence of diabetes in 2013 was 5.2%, an increase of 6% since 2010 and the age-related increase was from 1% to 16% for men aged 25–34 to ≥75 years and from 1% to 13% for women for same age-groups [30]. In Iceland approximately 4% in the age-group 45–64 years were diagnosed with T2D and this increased to 11% for obese men (BMI > 30) and 7% for obese women [7]. In Norway in 2011, 3.2% of the population were prescribed blood glucose-lowering drugs and the prevalence of diabetes was <2% for men below the age of 40 years and for women below the age of 45 [31]. In Sweden, the prevalence of T2D is reported to be between 2.5% and 4.5%, and the variation is likely due to differences in methods rather than to actual prevalence differences [33]. The occurrence increases with aged and the prevalence is between 10 and 20% for people >65 years [33]. On the other hand, only roughly 1% has diabetes below the age of 45 [29].
This was a comprehensive project. The strength lies in the sample size with 13% of the entire population in the respective age-group completing the study protocol, when the random group is included [11]. When all three sub-groups are included the overall age-adjusted prevalence is 11.6%. As overall country prevalence, this is higher than in the other Nordic countries but not when considering individual age-groups [19]. Furthermore, a national prevalence generally includes age-groups from 18 or 20 and upwards which is not the case in our study as the participants were from the age of 40 years. For this reason, we deduce that the prevalence is comparable to other population-based studies based on screening [6], [7], [34], [35]. Prior to implementation detailed planning, close follow-up and an extensive screening procedure with two rounds including HbA1c, RPG, FPG and 2 h-PG concentrations as well as a high participation rate, namely 84% and 87% for the two sub-groups and nearly 83% for the entire study population, and minor punching error all strengthened both an acceptable external and internal validity.
However, we cannot rule-out some limitations. Determining blood glucose from capillary blood, rather than venous blood samples, may involve uncertainties unless standard procedures are followed strictly, such as warm fingers, using cleaning swabs that do not affect the analyses, lack of verification if only one blood sample is taken, delay from sampling time to analysis of blood sample. These were all details taken into account at the participants’ attendance in this project. Pursuing the goal to determine the prevalence of T2D and prediabetes in the Faroe Islands we found the screening procedure developed by the Addition group (13) suitable for the Faroese setting. The comprehensiveness of this procedure imposed a participation burden. To overcome this, screenings were undertaken at local health care centers around the Faroes. If venous blood samples were used this would require a portable centrifuge and freezer as well as strict procedures for handling the samples. We believe that using the point-of-care devices with inbuilt fixed calibration and a minimum of two blood samples from each subject has produced valid results. It is to be noted, that monthly quality controls of the devices were performed by the laboratory at the National Hospital in the Faroe Islands, as described previously (11).
The rationale for the age-span presented in the introduction might alter the national prevalence of T2D from the age of 20 and onwards [17]. Also, the overall prevalence in the Faroes may be lower if the age-group 20–39 was included in the study, as the prevalence is low in this group and the proportion of this age-group in the national statistics is relatively larger compared to older age-groups [16]. Despite the non-random study groups, our results are in agreement with the prevalence increasing with age, especially among men although, we cannot rule-out and over or underestimation [6], [20]. Additionally, our low risk group did not undergo OGTT, the sole diagnostic criterion in many studies based on screening [6], [7] and again, may underestimate undiagnosed T2D, especially among women, as they are more prone to high blood sugars when tested by OGTT rather than FPG alone [21]. There is a risk of selection bias, especially in the M-group as the participants in the baseline study may be selected due to the investigated indicators: diet, physical exercise, tobacco and alcohol. The initial participants may represent a healthy profile in the community and thus a lower prevalence of T2D, although the high prevalence we detected among men aged ≥64 years old can be explained by the uncertainties in the small number with K-T2D. The S-group included 65% of the total population age 70–74 years in the baseline study and may, at first glance, be selected by healthy participants. However, as the initial aim of the study was to investigate neurobehavioral effects of lifetime exposure to marine contaminants, an issue very well implemented in the Faroese society with ongoing studies for the past 30 years, this is not believed to be the case. We did find similar trends in the prevalence for men and women as well as for the different age-groups, and with the same increasing trend. The screening procedure (see Fig. 2) may have burdened the participants introducing a selection bias for a healthier population, yet, no significant difference between participants and non-participants in terms of age, sex and place of living was found (data not shown).
Conclusion
In conclusion, 20.4% of the older group had diabetes and 6.2% of the younger group and only 25% and 34% were new cases, respectively. This finding may indicate a well-functioning primary and secondary health care system. Also, we found that T2D was more prevalent among men and increased with age and was similar to the prevalence in other Nordic countries. Significant predictors related to the risk of T2D were obesity, and, although significant to a lesser extent, abnormal WHR and vegetable consumption.
Acknowledgments
Acknowledgements
The authors would like to thank all the participants for their contribution, some participating more than once. Hildigunn Steinhólm and Marita Hansen for administrating the logistics and Nanna Kallsberg for assisting contribution, laboratory technician Sólrun Wardum, and laboratory staff members at the National Hospital.
Funding
This study was supported by the Faroese Research Council; Ministry of Environment and Food of Denmark, The Danish Environmental Protection Agency; Statoil Faroe Islands.
Contribution statement
The study was conceived by PW and conducted by PW, JH, and JA; data preparation was carried out by ASV; statistical analyses and writing the draft was carried out by ASV; all authors contributed to the interpretation of the findings and the paper’s critical revision; all authors have approved the final version; PW is guarantor for the work as a whole.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.IFD . International Diabetes Federation; 2017. IDF diabetes atlas. Eighth edition 2017.file:///C:/Users/ln47670/Downloads/IDF_DA_8e-EN-final.pdf [accessed 14. March 2019] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; 2016. Global report on diabetes.http://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=6C39C8422B5F17D2086ED16B4217EADC?sequence=1 [accessed 14. March 2019] [Google Scholar]
- 3.CDC . Centers for Disease Control and Prevention; 2017. National diabetes statistics report, 2017. Estimates of diabetes and its burden in the United State.https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf [accessed 14. March 2019] [Google Scholar]
- 4.PHAC . Public Health Agency of Canada; 2011. Diabetes in Canada: facts and figures from a public health perspective.http://passthrough.fw-notify.net/download/662510/http://www.phac-aspc.gc.ca/cd-mc/publications/diabetes-diabete/facts-figures-faits-chiffres-2011/pdf/facts-figures-faits-chiffres-eng.pdf [accessed 14. March 2019] [PubMed] [Google Scholar]
- 5.Singh K., Chan H.M. Persistent organic pollutants and diabetes among Inuit in the Canadian Arctic. Environ Int. 2017;101:183–189. doi: 10.1016/j.envint.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen M.E., Borch-Johnsen K., Stolk R., Bjerregaard P. Fat distribution and glucose intolerance among Greenland Inuit. Diabetes Care. 2013;36:2988–2994. doi: 10.2337/dc12-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergsveinsson J., Aspelund T., Guðnason V., Benediktsson R. Algengi sykursýki af tegund tvö á Íslandi 1967–2002 (Prevalence of type 2 diabetes mellitus in Iceland 1967–2002) Laeknabladid. 2007;93:397–402. [PubMed] [Google Scholar]
- 8.FHI . Folkehelse instituttet (FHI) (National Institute of Public Health, Norway); 2018. Folkehelserapporten – kortversjon. Helsetilstanden i Norge 2018 (Public Health Report – short version. Health condition in Norway 2018)https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2018/helsetilstanden-i-norge-2018.pdf [accessed 14. March 2019] [Google Scholar]
- 9.Hagstova Føroya . Hagstova Føroya (Statistics Faroe Islands); 2019. Key figures.http://www.hagstova.fo/fo [accessed 14. March 2019] [Google Scholar]
- 10.Grandjean P., Henriksen J.E., Choi A.L., Petersen M.S., Dalgård C., Nielsen F. Marine food pollutants as a risk factor for hypoinsulinemia and type 2 diabetes. Epidemiology. 2011;22:410–417. doi: 10.1097/EDE.0b013e318212fab9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veyhe A.S., Andreassen A., Halling J., Grandjean P., Petersen M.S., Weihe P. Prevalence of type 2 diabetes and prediabetes in the Faroe Islands. Diabeets Res Clin Pract. 2018;140:162–173. doi: 10.1016/j.diabres.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Holmberg T., Bech M., Curtis T., Juel K., Grønbæk M., Brixen K. Association between passive smoking in adulthood and phalangeal bone mineral density: results from the KRAM study–the Danish Health Examination Survey 2007–2008. Osteoporos Int. 2011;22:2989–2999. doi: 10.1007/s00198-010-1506-9. [DOI] [PubMed] [Google Scholar]
- 13.Christensen J.O., Sandbæk A., Lauritzen T., Broch-Johnsen K. Population-based stepwise screening for unrecognised Type 2 diabetes is ineffective in general practice despite reliable algorithms. Diabetologia. 2004;47:1566–1573. doi: 10.1007/s00125-004-1496-2. [DOI] [PubMed] [Google Scholar]
- 14.Haeckel R., Brinck U., Colic D., Janka H.U., Püntmann I., Schneider J. Comparability of blood glucose concentrations measured in different sample systems for detecting glucose intolerance. Clin Chem. 2002;48:936–939. [PubMed] [Google Scholar]
- 15.Sandbæk A., Lauritzen T., Borch-Johnsen K., Mai K., Christiansen J.S. The comparison of venous plasma glucose and whole blood capillary glucose in diagnoses of Type 2 diabetes: a population-based screening study. Diabet Med. 2005;22:1173–1177. doi: 10.1111/j.1464-5491.2005.01491.x. [DOI] [PubMed] [Google Scholar]
- 16.Hagstova Føroya . Hagstova Føroya (Statistics Faroe Islands); 2017. Fólkið skift á kyn, aldur og bygd 1. januar (1985-2018) (Population by sex, age and village/city, 1th January (1985-2018))http://statbank.hagstova.fo/pxweb/en/H2/H2__IB__IB01/fo_aldbygd.px/?rxid=2ba38fb9-f9bc-4b57-a040-83ee674c6ad8 [accessed 14. March 2019] [Google Scholar]
- 17.James C., Bullard K.M., Rolka D.B., Geiss L.S., Williams D.E., Cowie C.C. Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care. 2011;34:387–391. doi: 10.2337/dc10-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beagley J., Guariguata L., Weil C., Motala A.A. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2014;103:150–160. doi: 10.1016/j.diabres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Ringborg A., Lindgren P., Martinell M., Yin D.D., Schön S., Stålhammar J. Prevalence and incidence of Type 2 diabetes and its complications 1996–2003–estimates from a Swedish population-based study. Diabet Med. 2008;25:1178–1186. doi: 10.1111/j.1464-5491.2008.02541.x. [DOI] [PubMed] [Google Scholar]
- 20.FHI . Folkehelseinstituttet (FHI) (National Institute of Public Health, Norway); 2014. Folkehelserapporten 2014 (Public Health Report 2014)https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2014/folkehelserapporten-2014-pdf.pdf [accessed 14. March 2019] [Google Scholar]
- 21.DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care. 2003;26:61–69. doi: 10.2337/diacare.26.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Bernal-Lopez M.R., Santamaría-Fernandez S., Lopez-Carmona D., Tinahones F.J., Mancera-Romero J., Peña-Jimenez D. HbA(1c) in adults without known diabetes from southern Europe. Impact of the new diagnostic criteria in clinical practice. Diabet Med. 2011;28:1319–1322. doi: 10.1111/j.1464-5491.2011.03317.x. [DOI] [PubMed] [Google Scholar]
- 23.Roth J., Müller N., Lehmann T., Heinemann L., Wolf G., Müller U.A. HbA1c and age in non-diabetic subjects: an ignored association? Exp Clin Endocrinol Diabetes. 2016;10:637–642. doi: 10.1055/s-0042-105440. [DOI] [PubMed] [Google Scholar]
- 24.Hartwig S., Kluttig A., Tiller D., Fricke J., Müller G., Schipf S. Anthropometric markers and their association with incident type 2 diabetes mellitus: which marker is best for prediction? Pooled analysis of four German population-based cohort studies and comparison with a nationwide cohort study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulze M.B., Thorand B., Fritsche A., Häring H.U., Schick F., Zierer A. Body adiposity index, body fat content and incidence of type 2 diabetes. Diabetologia. 2012;55:1660–1667. doi: 10.1007/s00125-012-2499-z. [DOI] [PubMed] [Google Scholar]
- 26.Hardy D.S., Stallings D.T., Garvin J.T., Xu H., Racette S.B. Best anthropometric discriminators of incident type 2 diabetes among white and black adults: a longitudinal ARIC study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau C.K., Eng C., Cenzer I.S., Boscardin W.J., Rice-Trumble K., Lee S.J. Glycosylated haemoglobin and functional decline in community-dwelling nursing home-eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60:1215–1221. doi: 10.1111/j.1532-5415.2012.04041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quartuccio M., Buta B., Kalyani R.R. Comparative effectiveness for glycemic control in older adults with diabetes. Curr Geriatr Rep. 2017;6:175–186. doi: 10.1007/s13670-017-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lager A., Backhans M., Forsberg J.S. Stockholm läns landsting (Stockholm County Council); 2015. Folkhälsorapport 2015. Folkälsan in Stockholms län (Public Health Report 2015.Public Health in Stockholm County)http://dok.slso.sll.se/CES/FHG/Folkhalsoarbete/Halsa%20Stockholm/Folkhalsorapport_2015.pdf [accessed 14. March 2019] [Google Scholar]
- 30.Sundhedsstyrelsen . Sundhedsstyrelsen (National Board of Health); 2017. Danskernes Sundhed – Den Nationale Sundhedsprofil 2017 (Danes’ Health – National Health Profile 2017)https://www.sst.dk/~/media/EAB50E1A9DD84D1D822308CE397AD19D.ashx [accessed 14. March 2019] [Google Scholar]
- 31.Strøm H., Selmer R., Birkeland K.I., Schirmer H., Bert T.J., Jenum A.K. No increase in new users of blood glucose-lowering drugs in Norway 2006–2011: a nationwide prescription database study. BMC Public Health. 2014;14:520. doi: 10.1186/1471-2458-14-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkman M.S., Briscoe V.J., Clark N., Florez H., Haas L.B., Halter J.B. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Östenson C.G. Miljö och arv i samspel bestämmer vem som får diabetes (The interplay between environment and genetics determines who gets diabetes) Lakartidningen. 2010;107:2792–2795. [PubMed] [Google Scholar]
- 34.Bjerregaard P., Curtis T., Borch-Johnsen K., Mulvad G., Becker U., Andersen S. Inuit health in Greenland: a population survey of life style and disease in Greenland and among Inuit living in Denmark. Int J Circumpolar Health. 2003;62(Suppl 1):3–79. doi: 10.3402/ijch.v62i0.18212. [DOI] [PubMed] [Google Scholar]
- 35.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Rec Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]