Abstract
Background
High altitude illness (HAI) is a term used to describe a group of mainly cerebral and pulmonary syndromes that can occur during travel to elevations above 2500 metres (˜ 8200 feet). Acute mountain sickness (AMS), high altitude cerebral oedema (HACE), and high altitude pulmonary oedema (HAPE) are reported as potential medical problems associated with high altitude ascent. In this, the third of a series of three reviews about preventive strategies for HAI, we assessed the effectiveness of miscellaneous and non‐pharmacological interventions.
Objectives
To assess the clinical effectiveness and adverse events of miscellaneous and non‐pharmacological interventions for preventing acute HAI in people who are at risk of developing high altitude illness in any setting.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) in January 2019. We adapted the MEDLINE strategy for searching the other databases. We used a combination of thesaurus‐based and free‐text search terms. We scanned the reference lists and citations of included trials and any relevant systematic reviews that we identified for further references to additional trials.
Selection criteria
We included randomized controlled trials conducted in any setting where non‐pharmacological and miscellaneous interventions were employed to prevent acute HAI, including preacclimatization measures and the administration of non‐pharmacological supplements. We included trials involving participants who are at risk of developing high altitude illness (AMS or HACE, or HAPE, or both). We included participants with, and without, a history of high altitude illness. We applied no age or gender restrictions. We included trials where the relevant intervention was administered before the beginning of ascent.
Data collection and analysis
We used the standard methodological procedures employed by Cochrane.
Main results
We included 20 studies (1406 participants, 21 references) in this review. Thirty studies (14 ongoing, and 16 pending classification (awaiting)) will be considered in future versions of this suite of three reviews as appropriate. We report the results for the primary outcome of this review (risk of AMS) by each group of assessed interventions.
Group 1. Preacclimatization and other measures based on pressure
Use of simulated altitude or remote ischaemic preconditioning (RIPC) might not improve the risk of AMS on subsequent exposure to altitude, but this effect is uncertain (simulated altitude: risk ratio (RR) 1.18, 95% confidence interval (CI) 0.82 to 1.71; I² = 0%; 3 trials, 140 participants; low‐quality evidence. RIPC: RR 3.0, 95% CI 0.69 to 13.12; 1 trial, 40 participants; low‐quality evidence). We found evidence of improvement of this risk using positive end‐expiratory pressure (PEEP), but this information was derived from a cross‐over trial with a limited number of participants (OR 3.67, 95% CI 1.38 to 9.76; 1 trial, 8 participants; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Group 2. Supplements and vitamins
Supplementation of antioxidants, medroxyprogesterone, iron or Rhodiola crenulata might not improve the risk of AMS on exposure to high altitude, but this effect is uncertain (antioxidants: RR 0.58, 95% CI 0.32 to 1.03; 1 trial, 18 participants; low‐quality evidence. Medroxyprogesterone: RR 0.71, 95% CI 0.48 to 1.05; I² = 0%; 2 trials, 32 participants; low‐quality evidence. Iron: RR 0.65, 95% CI 0.38 to 1.11; I² = 0%; 2 trials, 65 participants; low‐quality evidence. R crenulata: RR 1.00, 95% CI 0.78 to 1.29; 1 trial, 125 participants; low‐quality evidence). We found evidence of improvement of this risk with the administration of erythropoietin, but this information was extracted from a trial with issues related to risk of bias and imprecision (RR 0.41, 95% CI 0.20 to 0.84; 1 trial, 39 participants; very low‐quality evidence). Regarding administration of ginkgo biloba, we did not perform a pooled estimation of RR for AMS due to considerable heterogeneity between the included studies (I² = 65%). RR estimates from the individual studies were conflicting (from 0.05 to 1.03; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Group 3. Other comparisons
We found heterogeneous evidence regarding the risk of AMS when ginkgo biloba was compared with acetazolamide (I² = 63%). RR estimates from the individual studies were conflicting (estimations from 0.11 (95% CI 0.01 to 1.86) to 2.97 (95% CI 1.70 to 5.21); low‐quality evidence). We found evidence of improvement when ginkgo biloba was administered along with acetazolamide, but this information was derived from a single trial with issues associated to risk of bias (compared to ginkgo biloba alone: RR 0.43, 95% CI 0.26 to 0.71; 1 trial, 311 participants; low‐quality evidence). Administration of medroxyprogesterone plus acetazolamide did not improve the risk of AMS when compared to administration of medroxyprogesterone or acetazolamide alone (RR 1.33, 95% CI 0.50 to 3.55; 1 trial, 12 participants; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Authors' conclusions
This Cochrane Review is the final in a series of three providing relevant information to clinicians, and other interested parties, on how to prevent high altitude illness. The assessment of non‐pharmacological and miscellaneous interventions suggests that there is heterogeneous and even contradictory evidence related to the effectiveness of these prophylactic strategies. Safety of these interventions remains as an unclear issue due to lack of assessment. Overall, the evidence is limited due to its quality (low to very low), the relative paucity of that evidence and the number of studies pending classification for the three reviews belonging to this series (30 studies either awaiting classification or ongoing). Additional studies, especially those comparing with pharmacological alternatives (such as acetazolamide) are required, in order to establish or refute the strategies evaluated in this review.
Plain language summary
Diverse strategies for preventing high altitude illness
Background
The term high altitude illness (HAI) is used to describe a group of brain and lung conditions that can occur when people travel to altitudes above approximately 2500 metres (approximately 8200 feet). Individuals can respond to high altitudes in different ways and experience a variety of symptoms. These include HAI‐related headache, nausea, vomiting and tiredness, often called acute mountain sickness. Drowsiness, confusion or unconsciousness can occur when the brain is particularly affected (high altitude cerebral oedema or HACE), and cough or breathlessness when it is the lungs (high altitude pulmonary oedema or HAPE). A number of different strategies are used to prevent HAI. In this review we assessed the evidence from randomized controlled trials on whether various approaches could prevent the onset of high altitude illness, with a focus on non‐drug approaches, herbs and natural supplements.
Study characteristics
The evidence is current to January 2019. We included 20 randomized controlled studies involving 1406 participants. The studies looked at diverse approaches to HAI prevention. These approaches included strategies to acclimatize to high altitudes by mimicking quick ascents by reducing levels of oxygen in the air that participants are breathing, and herbal products or vitamin supplements available without a prescription.
The participants ranged in age between 17 and 65 years. Only one study included people at high risk of developing HAI as they had a history of HAI. Four trials provided the intervention between one to three days before making the ascent (20% of the studies), and eight between four to 30 days before departure for the ascent (40% of the studies). The participants in all these studies reached a final altitude of between 3500 and 5500 metres above sea level. Most of the studies did not provide clear information on how they were funded (55% of studies). Thirty additional studies were classified as either ongoing (14 studies), or awaiting classification (16 studies), and they will be considered in future versions of this suite of three reviews as appropriate.
Key results
The evidence for any benefit of the various strategies is inconclusive, and even contradictory among the included studies.
In three studies comparing normal levels of oxygen with low oxygen levels as a way of acclimatization before leaving for high altitudes, we found no differences in the risk of developing acute mountain sickness (3 trials, 140 participants; low‐quality evidence). Adverse events were not reported, nor were high altitude cerebral oedema (HACE) or pulmonary oedema (HAPE).
Ginkgo biloba was compared with taking an inactive placebo in seven studies (523 participants) looking at acute mountain sickness. There was no difference between ginkgo biloba and placebo in terms of the risk of developing HACE (3 studies, 371 participants), or in the risk of developing tingling or pricking, often described as 'pins and needles', as a side effect of treatment (2 studies, 352 participants). No HAPE events were reported (3 studies, 371 participants).
Ginkgo biloba was compared with acetazolamide, which is a drug used to prevent acute mountain sickness, in four studies (397 participants). The findings differed between the studies, and no conclusions could be drawn. Acetazolamide increased the risk of developing pins and needles in two studies (354 participants). No HAPE or HACE events were reported. Overall, the limited information on the safety of the various interventions means that their safety remains unclear.
Quality of the evidence
The quality of the evidence was low to very low. We could not obtain the full text reports of some of the studies we had identified, which limited the number of studies included in the review. Many of the studies had small numbers of participants; and for some outcomes few events occurred so that any findings were uncertain. Additional research is needed to clarify the effectiveness and safety of the various strategies to reduce HAI.
Summary of findings
Background
High altitude illness (HAI) is a term used to describe a group of cerebral and pulmonary syndromes that can occur during travel to elevations above 2500 metres (m) (˜ 8200 feet). HAI is commonly classified as high (1500 m to 3500 m), very high (above 3500 m to 5500 m), and extreme (above 5500 m) (Flaherty 2016; Kayser 2012; Khodaee 2016; Low 2012; Paralikar 2010; Zafren 2014). Because of the large number of people who ascend rapidly to between 2500 m and 3500 m, high altitude illness is common in this height range as a result of hypoxia (Davis 2017; Paralikar 2010). Although the proportion of oxygen remains unchanged at 20.93%, increases in altitude result in lower partial pressure of oxygen in the inspired air (Anonymous 1892; Wilson 2009). This reduction in the driving pressure of oxygen along the oxygen cascade from the lungs to the tissues can compromise the supply of oxygen to the tissues (Wilson 2009), especially the cardiovascular and pulmonary systems (Leissner 2009). The physiological responses to hypoxia and acclimatization related to HAI include hyperventilation (increased depth and rate of breathing); elevation of systemic blood pressure; and tachycardia (elevations of heart rate) (Leissner 2009; Naeije 2010). However, in many instances, these physiological changes may be inadequate, so that the ascent to high altitude and the attendant hypoxia are complicated by altitude‐associated medical illness (Luks 2017; Palmer 2010), which is also known as high altitude illness (HAI).
Description of the condition
High altitude illness (HAI)
As mentioned earlier, HAI is a term used to describe a group of mainly cerebral and pulmonary syndromes that can occur during travel to elevations above 2500 metres. There are two types of mountain sickness: acute mountain sickness; and chronic mountain sickness (CMS), also called Monge's disease (Monge 1942). CMS prevention is not included in this review. Acute hypoxia, acute mountain sickness (AMS), high altitude cerebral oedema (HACE), high altitude pulmonary oedema (HAPE), cerebrovascular syndromes, peripheral oedema, retinopathy, thromboembolism, sleep disorders and periodic breathing, high altitude pharyngitis and bronchitis, ultraviolet exposure and keratitis (snow blindness), and exacerbation of pre‐existing illness are reported as potential medical problems associated with high altitude ascent (CATMAT 2007; Kayser 2012; Khodaee 2016; Palmer 2010; Schoene 2008). Factors such as the rate of ascent, the absolute change in altitude, and individual physiology are factors usually implicated in the development of these conditions (Flaherty 2016; Leissner 2009; Low 2012; Luks 2017; Palmer 2010; Zafren 2014). The risk categories for acute mountain sickness are shown in Appendix 1 (Luks 2010).
In the 19th century Dr Daniel Vergara, a Mexican physiologist, pioneered studies on high altitude physiology and the physiological and anatomical mechanisms of adaptation to high elevations. Forty years later Dr Carlos Monge, a Peruvian physiologist, reported his ideas on this issue. The work of these pioneers was summarized early this century (Rodríguez de Romo 2002). Both the physiology and pathophysiology of high altitude have recently been widely reviewed (Bärtsch 2007; Davis 2017; Leissner 2009; Luks 2017; Palmer 2010; Paralikar 2010). In brief, these reviews confirm both the increase in respiratory rate and increase in haemoglobin concentration on exposure to low oxygen pressure. They identify the rate of ascent, the absolute change in altitude and individual variation in physiology as the primary determinants of whether HAI will develop or not (Palmer 2010). In addition, HAI is considered an important cause of mountain mortality (Windsor 2009).
Acute mountain sickness (AMS) or high altitude cerebral oedema (HACE)
AMS is a disorder with prominent neurological features, characterized by headache, anorexia, nausea and sometimes vomiting, light‐headedness, insomnia, and fatigue (Bailey 2009a; Leissner 2009; Palmer 2010). Headache is the most prevalent symptom of acute mountain sickness. In contrast, HACE is a potentially fatal neurological disorder, and it is characterized by altered consciousness or ataxia (Bailey 2009a; Hackett 2004; Imray 2010), or both, in an individual with AMS. If left untreated, HACE can result in death due to cerebral oedema (Bailey 2009a; Bailey 2009b). HACE is widely viewed as the end stage of AMS and is normally preceded by symptoms of AMS, which suggest a similar pathophysiological process (Bailey 2009a; Imray 2010; Palmer 2010). It has been suggested that both syndromes could share a common pathophysiology linked by intracranial hypertension (Bailey 2009a; Bailey 2009b; Davis 2017; Kallenberg 2007; Luks 2017; Schoonman 2008; Wilson 2009). The severity of AMS can be scored using questionnaires such as the Lake Louise Questionnaire, Environmental Symptoms Questionnaire, or by the use of a simple analogue scale (Imray 2010). Headache is a very common symptom at altitude, and some authors have suggested it could be viewed as a distinct clinical entity.
High altitude pulmonary oedema (HAPE)
HAPE is a non‐cardiogenic pulmonary oedema (Luks 2008a; Schoene 2004; Stream 2008). It is characterized by cough, progressive dyspnoea with exertion, and decreased exercise tolerance, generally developing within two to four days after arrival at high altitude (Palmer 2010; Stream 2008). It is rare after one week of acclimatization at a particular altitude (Maggiorini 2010; Palmer 2010). Hypoxia is the trigger that results in a complex cascade of events leading to HAPE (Stream 2008). Essentially, HAPE is due to a "persistent imbalance between the forces that drive water into the airspace and the biologic mechanisms for its removal" (Scherrer 2010); and the hallmark of this condition is hypoxic pulmonary hypertension. The hypertension may be mediated via at least four potential mechanisms: defective pulmonary nitric oxide synthesis; exaggerated endothelin‐1 synthesis; exaggerated sympathetic activation; and a defect in alveolar transepithelial sodium transport (Scherrer 2010). An extensive review of pulmonary hypertension induced by HAI is reported by Pasha 2010.
Epidemiology of acute HAI
It has been estimated that 84% of people who fly directly to 3860 m are affected by AMS (Murdoch 1995). The risk of HACE and HAPE is much lower than for AMS, with estimates in the range of 0.1% to 4.0% (Basnyat 2003). The rate of ascent, altitude reached (especially the sleeping altitude), and individual susceptibility has been proposed as the most important risk factors for the development of HAI conditions (Basnyat 2003; Schneider 2002). Other presumptive risk factors are a history of HAI and permanent residence lower than 900 m, exertion in children and adults (Basnyat 2003), obesity (Ri‐Li 2003), and coronary heart disease (Dehnert 2010). It is advisable that those with asthma make sure that their condition is well controlled before they undertake exertion at altitude (CATMAT 2007).
See Appendix 2 for other medical terms.
Description of the intervention
The risk of high altitude illness (HAI) begins with a non‐acclimatized individual ascending to an altitude higher than 2500 metres (Flaherty 2016; Kayser 2012; Khodaee 2016; Low 2012; Paralikar 2010). However, a susceptible individual may develop acute mountain sickness (AMS) at intermediate altitude such as 2100 metres (Davis 2017). Several interventions to prevent HAI conditions, especially AMS, have been described, compiled, and published in guidelines and consensus statements (CATMAT 2007; Flaherty 2016; Kayser 2012; Khodaee 2016; Low 2012; Luks 2010; Ritchie 2012; Seupaul 2012; Zafren 2014). Interventions for HAI prevention can be classified as pharmacological and non‐pharmacological or miscellaneous (Bärtsch 1992; Luks 2008b; Luks 2010; Wright 2008). The Committee to Advise on Tropical Medicine and Travel proposed a consensus for HAI in 2007, describing prevention and treatment approaches among several topics regarding this medical condition (CATMAT 2007).
In 2014 the Wilderness Medical Society (WMS) published an update of their 2010 guidelines, detailing prevention and treatment directives for HAI (AMS, HACE, HAPE) (Luks 2010; Luks 2014). This guideline was developed by an expert panel that compiled and classified all available evidence on HAI prevention and treatment (Luks 2014). For AMS and HACE, the experts proposed a risk classification where low‐risk participants are discarded for prevention interventions; for HAPE, pharmacological prophylaxis is recommended for participants with a previous diagnosis of HAI (Luks 2014).
These previous reviews have not given a clear indication as to which preventative strategies (whether pharmacological or non‐pharmacological) are of most use, nor how one might modify the approach in different situations. For example, while CATMAT 2007 suggests that in general the safest method of prevention is graded ascent, it is not always clear which of the alternative strategies is to be preferred if, for some reason, this is not possible, nor what the major adverse effects of combined approaches might be.
Previously, we assessed 11 groups of pharmacological interventions for the prevention of HAI (Nieto 2017; Gonzalez 2018). In this Cochrane Review, we assessed non‐pharmacological and miscellaneous interventions (that is, those strategies not based on the administration of drugs) recommended for this condition. Those interventions can be classified into two groups:
preacclimatization and other measures based on pressure: include use of hypobaric air breathing to simulate altitude, positive end‐expiratory pressure and remote ischaemic preconditioning (Berger 2017; Burse 1988; Dehnert 2014; Launay 2004; Schommer 2010);
supplements: include provision of herbal extracts (such as ginkgo biloba and R crenulata), minerals (iron), antacids and hormonal agents (medroxyprogesterone and erythropoietin) (Bailey 2001; Chiu 2013; Chow 2005; Gertsch 2004; Heo 2014; Ke 2013; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007; Ren 2015; Roach 1983; Roncin 1996; Talbot 2011; Wright 2004a; Wright 2004b).
How the intervention might work
Extensive reviews for prophylaxis of HAI have recently been published (Maggiorini 2010; Wright 2008). Below is a brief description of the non‐pharmacological approaches that have been suggested to date.
Preacclimatization measures: in general, graded ascent has been suggested as the main prophylactic measure to prevent HAI (CATMAT 2007; Paralikar 2010). Key elements in acclimatization are aimed at securing the oxygen supply to tissues and organs of the body with an optimal oxygen tension of the arterial blood (Bärtsch 2008). Graded ascent means that individuals, especially persons without altitude experience, avoid rapid ascent to sleeping altitudes above 3000 m, spend 2 to 3 nights at 2500 m to 3000 m before going higher, and spend an extra night for acclimatization every 600 m to 900 m if continuing ascent. Day trips to higher altitude, with a return to lower altitude for sleep, aid in acclimatization (CATMAT 2007). Due to acclimatization requiring additional investment in time, transportation and staging locations, those strategies that mimic its effects could be attractive and widely accepted for high altitude climbers (Burse 1988), including use of devices or chambers that modified the levels of fractional inspired oxygen (FIO₂) or positive end‐expiratory pressure (PEEP) (Burse 1988; Dehnert 2014; Launay 2004). In addition, interventions based on remote ischaemia to protect the brain (i.e. episodes of ischaemia–reperfusion induced in the extremities, typically with an inflated blood pressure cuff) could ameliorate damage from subsequent ischaemic insults, due to its effects on vasoactive and inflammatory pathways (Berger 2017; Perez‐Pinzon 1997).
Supplements: over‐the‐counter herbal supplements, such as ginkgo biloba leaves, have a potent antioxidant effect and induce arterial vasodilation, suggesting a relationship with nitric oxide (NO) and potential in haemodynamic disorders decreasing free radicals produced during exposure to hypoxia (Kleijnen 1992). Components of R crenulata have been involved in the prevention of hypoxia‐mediated Na/K‐ATPase endocytosis due to its effects in maintaining the integrity of the alveolar‐capillary barrier and pulmonary sodium transportation (Lee 2013). In addition, iron supplements can have an impact on pathological and physiological responses to hypoxia, especially those caused by iron deficiency (Ren 2015). Hormonal supplements can increase hypoxic ventilatory responses with an improvement in oxygen saturation and a reduction in haematocrit levels (Kryger 1978), as well as stimulate red blood cell production (Heo 2014; Milledge 1985).
Why it is important to do this review
It is important to conduct this systematic review for several reasons.
Many people travel to recreational areas located at high altitude, putting themselves at an increased risk of developing acute HAI. HAI may be severe and life‐threatening, so effective prevention is likely to be of great value both to these visitors to high altitude areas and to those responsible for their treatment and rescue when required. At the other end of the spectrum, reliable prevention of minor degrees of AMS would greatly enhance the experience of many travellers. Travel to high altitudes may also aggravate underlying illnesses, particularly cardiopulmonary diseases (CATMAT 2007).
The true role of the approaches for preventing acute HAI is uncertain (Adams 2004; Bärtsch 2004; CATMAT 2007; Elphick 2004), meaning that their clinical effectiveness and safety must be assessed.
It is necessary to answer questions such as: are all these interventions equally useful regardless of the type of HAI? Is there reason to believe that some forms are more appropriate for some patients (persons at risk) than others?
An updated meta‐analysis on AMS prevention needs to be produced (Dumont 2000;Kayser 2012; Low 2012; Ritchie 2012).
Finally, a systematic review including a rigorous assessment of the risk of bias of the most up‐to‐date evidence will help clinicians make informed decisions regarding the use of non‐pharmacological and pharmacological interventions for preventing acute HAI. At present, this kind of assessment is available for pharmacological prophylactic strategies (Gonzalez 2018; Nieto 2017), and treatment of HAI (Simancas‐Racines 2018), but information about non‐pharmacological approaches is still needed. The protocol of this review included all agents to prevent high altitude illness (Martí‐Carvajal 2012), but we decided to split that review into a series of three publications about the prevention of this condition: Part 1: Commonly used drugs (Nieto 2017); Part 2: Less commonly used drugs (Gonzalez 2018); and Part 3: Miscellaneous and non‐pharmacological interventions (this review).
Objectives
To assess the clinical effectiveness and adverse events of miscellaneous and non‐pharmacological interventions for preventing acute HAI in people who are at risk of developing high altitude illness in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) irrespective of publication status (unpublished trials or published as articles, abstracts, or letters), language and country. We applied no restrictions with respect to periods of follow‐up.
We excluded quasi‐randomized studies, and prospective observational studies for evaluating clinical effectiveness.
Types of participants
We included trials involving participants who are at risk of developing high altitude illness (such as AMS or HACE, or HAPE, or both). We included participants with, and without, a history of high altitude illness. We applied no age or gender restrictions.
Types of interventions
The published protocol of this review included all agents to prevent high altitude illness (Martí‐Carvajal 2012). However, we decided to split the topic into a series of three publications about the prevention of this condition (See Differences between protocol and review section). This is the third of three reviews and includes non‐pharmacological and miscellaneous interventions to prevent acute HAI.
Interventions
Preacclimatization and other measures based on pressure: include hypobaric air breathing to simulate altitude conditions, positive end‐expiratory pressure and remote ischaemic preconditioning.
Supplements: include provision of herbal extracts (such as ginkgo biloba and Rhodiola crenulata), minerals (iron), antacids and hormonal agents (medroxyprogesterone and erythropoietin).
We included trials where the intervention was administered before the beginning of ascent. We excluded trials using these drugs during ascent only or after ascent.
Types of outcome measures
The following outcome measures were modified from the published protocol (Martí‐Carvajal 2012). This is a change to the protocol and is explained in the Differences between protocol and review section.
Primary outcomes
Risk of acute mountain sickness (AMS ‒ as defined by each study) at any time.
Secondary outcomes
Risk of high altitude pulmonary oedema (HAPE ‒ as defined by each study) at any time.
Risk of high altitude cerebral oedema (HACE ‒ as defined by each study), at any time.
Risk of adverse events in general, including paraesthesia, at any time.
Differences in HAI or AMS scores at high altitude. We analysed the differences between groups in any measure of AMS severity and between the first to the 48th hour at high altitude.
Search methods for identification of studies
The same search methods were used for the identification of potential studies and are common for the three reviews included in this set.
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply restrictions to language or publication status. The evidence is current to 18 January 2019.
We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 12) in the Cochrane Library;
MEDLINE (Ovid SP, 1966 to January 2019);
Embase (Ovid SP, 1988 to January 2019);
LILACS (BIREME, 1982 to January 2019).
We developed a subject‐specific search strategy in MEDLINE, and used that as the basis for the search strategies in the other databases listed. Where appropriate, the search strategy was expanded with search terms for identifying RCTs. All search strategies can be found in Appendices 3 to 7 (Appendix 3; Appendix 4; Appendix 5; Appendix 6). In addition, we scanned the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) for ongoing and unpublished trials (January 2019; Appendix 7). The search strategy was developed in consultation with the Information Specialist from Cochrane Anaesthesia, and Cochrane Emergency and Critical Care.
Searching other resources
We scanned the reference lists and citations of included trials and any relevant systematic reviews that we identified for further references to additional trials.
Data collection and analysis
Data collection and analysis methods were common for the three reviews included in this series.
Selection of studies
Two review authors independently assessed each reference identified by the search against the inclusion criteria. We resolved any disagreements by discussion; a third author was consulted as an arbiter if we could not reach an agreement. We retrieved in full those references which appeared to meet the inclusion criteria for further independent assessment by the same three review authors.
Data extraction and management
We used a pre‐defined form to extract the following data, among others: eligibility criteria, demographics (age, gender, country), rate of ascent (m/h), final altitude reached (m), AMS scale, design study, history of HAI, type of HAI, proposed intervention and outcomes; (see Appendix 8 for details of the data extraction form). For eligible studies, two review authors extracted the data using the selected form. We resolved disagreements through discussion or, if required, we involved a third author of this review. We entered data into Review Manager 5 (RevMan 5) software and checked it for accuracy (Review Manager 2014).
Assessment of risk of bias in included studies
Three review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. We judged the methodological quality of each study using Cochrane’s tool for assessing risk of bias, a two‐part tool that addresses the following six specific domains: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective reporting; and other bias (Higgins 2011). The first part describes the risk of bias; the second part provides criteria for making judgements about the risk of bias from each of the six domains in the tool (Appendix 9). Based on this tool we implemented a 'Risk of bias' worksheet to be filled out for each study. Two authors assessed the risk of bias independently. We resolved any disagreement through consultation with a third author. We displayed the results by creating a 'Risk of bias' graph and a 'Risk of bias' summary figure using RevMan 5 software, if appropriate (Review Manager 2014). We present the risk of bias in the 'Results' section. Likewise, we provide summary assessments of the risk of bias for each outcome within and across studies.
Measures of treatment effect
For dichotomous outcomes (such as risk of AMS or HAPE), we show results as summary risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes, (such as differences in AMS scores), we present the results as summary mean differences (MD), or standardized mean differences (SMD) as appropriate, with 95% CI. if needed, we used the CS command in STATA 14.0 (www.stata.com/stata14), for estimation of risk ratios with the corresponding 95% CI. This is a change to the protocol (Martí‐Carvajal 2012); it is explained in the Differences between protocol and review section. In addition, because we identified a considerable number of cross‐over trials concerning assessed interventions, we included these studies separately, and analysed this information using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 16.4 (Elbourne 2002; Higgins 2011; Stedman 2011), specially related to estimation of the Mantel‐Haenszel odds ratio (OR) for paired outcomes.
Unit of analysis issues
Martí‐Carvajal 2012 (the published protocol) did not include considerations about any unit of analysis issues. However, we identified two cross‐over studies in our search strategies, and they were included in the analyses (Chiu 2013; Launay 2004), but separate from the parallel studies. In brief, we used the methods recommended by Elbourne (Elbourne 2002; Stedman 2011). This is a change to the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Dealing with missing data
For all outcomes we carried out analyses on an intention‐to‐treat (ITT) basis as far as possible (i.e. we attempted to include all randomized participants in the denominator of the assessed groups in the analyses). Due to the fact that we included studies with missing information (especially standard deviations), or data not suitable for planned analyses, we followed the methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 16.1.3 (Higgins 2011). In brief, we transformed median values and their interquartile ranges or range extracted from included studies to means and standard deviations according to Wan and colleagues (Hozo 2005; Wan 2014). This is a change to the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Assessment of heterogeneity
We used the I² statistic to measure statistical heterogeneity among the trials in each analysis. When we identified substantial heterogeneity, we explored it by prespecified subgroup analysis. The I² statistic describes the percentage of total variation across trials due to heterogeneity rather than sampling error (Higgins 2003). We considered there to be significant statistical heterogeneity if I² was greater than 50% (Higgins 2011). We assessed clinical and methodological diversity of the included studies in a comparison for sufficient homogeneity before choosing to estimate summary effect sizes.
Assessment of reporting biases
We planned to assess whether the review is subject to publication bias by using a funnel plot to illustrate variability between trials graphically. If asymmetry had been detected, we planned to explore causes other than publication bias. We planned to perform a funnel plot if we included 10 or more RCTs for comparison. However, due to the scarcity of information we were not able to perform this analysis. This is a change to the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Data synthesis
We summarized the findings using the random‐effects (DerSimonian–Laird) model. We carried out statistical analyses using RevMan 5 (Review Manager 2014). We accepted important differences where the effect size 95% confidence limits do not cross the value of no difference between groups. We planned to apply trial sequential analysis (TSA), as cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Brok 2009; Lan 1983; Thorlund 2009; Wetterslev 2008; Wetterslev 2017). However, due to the scarcity of data for the assessed comparisons in this review, we decided not to report the TSA results in this case (all of them having only one study). This is a change from the published protocol (Martí‐Carvajal 2012); (see the details in the Differences between protocol and review section).
Subgroup analysis and investigation of heterogeneity
We investigated heterogeneity by an informed clinical evaluation of each outcome, combining data only when clinically appropriate. We also investigated statistical heterogeneity using the I² statistic as described above. For the primary outcome, we considered subgroup analysis for the following factors, as appropriate.
Extreme altitude exposure versus high or very high exposure (high: 1500 m to 3500 m; very high: 3500 m to 5500 m; and extreme: above 5500 m) (Paralikar 2010).
Presence or absence of people at high risk of HAI.
Presence or absence of significant pre‐existing disease: cardiovascular diseases, chronic obstructive pulmonary disease (COPD), diabetes mellitus.
However, due to the scarcity of information, we were not able to perform the planned analysis in most of the cases. This is a change to the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
Sensitivity analysis
We performed a sensitivity analysis comparing the general results versus RCTs with high methodological quality (studies classified as having a 'low risk of bias' (Higgins 2011)). We chose only three core domains: generation of allocation sequence, incomplete outcome data, and selective reporting bias. However, due to only one trial being considered as having low risk of bias (Ke 2013), we were not able to perform the planned analysis in most of the cases. This is a change to the protocol (Martí‐Carvajal 2012), and is explained in the Differences between protocol and review section.
'Summary of findings' tables and GRADE
We developed 'Summary of findings' tables for the following groups:
Preacclimatization and other measures based on pressure (Table 1).
Supplements and vitamins (Table 2).
Other comparisons (Table 3)
Summary of findings for the main comparison. Summary of findings group 1: pre‐acclimatization and other measures based on pressure.
| Group 1: pre‐acclimatization and other measures based on pressure | ||||||
|
Patient or population: participants at risk of high altitude illness Settings: high altitude (including simulated; Austria, France, Germany, Italy, USA) Intervention: simulated altitude conditions, positive end‐expiratory pressure (PEEP), remote ischaemic preconditioning (RIPC) Comparison: normal conditions, placebo, no measures | ||||||
| Comparison: outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control group | Intervention group | |||||
| Normal versus simulated altitude conditions: risk of AMS | 397 per 1000 | 469 per 1000 (326 to 679) | RR 1.18 (0.82 to 1.71) | 140 (3 studies) | ⊕⊕⊝⊝ Low1,2 | No studies reported on adverse effects, or risk of HAPE or HACE |
| Positive end‐expiratory pressure (PEEP) versus nothing: risk of AMS | Not estimable | Not estimable |
OR 3.67 (1.38 to 9.76) |
8 (1 study) |
⊕⊕⊝⊝ Low1,2 | Cross‐over trial. The study did not report on adverse effects, or risk of HAPE or HACE |
| Remote ischaemic preconditioning (RIPC) versus placebo: risk of AMS | 100 per 1000 |
300 per 1000 (69 to 1000) |
RR 3.00 (0.69 to 13.12) |
40 (1 study) |
⊕⊕⊝⊝ Low2,3 | No studies reported on adverse effects, or risk of HAPE or HACE |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; AMS: acute mountain sickness; HAPE: high altitude pulmonary oedema; HACE: high altitude cerebral oedema. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Quality of evidence downgraded by one level due to unclear/high selection and performance bias 2 Quality of evidence downgraded by one level for imprecision: optimal information size not reached
3 Quality of evidence downgraded by one level due to unclear/high selection and detection bias
Summary of findings 2. Summary of findings group 2: supplements and vitamins.
| Group 2: supplements and vitamins | ||||||
|
Patient or population: participants at risk of high altitude illness Settings: high altitude (including simulated; China, Chile, France, Nepal, Peru, Taiwan, USA) Intervention: antioxidants, ginkgo biloba, erythropoietin, medroxyprogesterone, iron supplementation, R crenulata Comparison: placebo | ||||||
| Comparisons: outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo group | Intervention group | |||||
| Antioxidants versus placebo: risk of AMS | 1000 per 1000 | 580 per 1000 (320 to 1000) |
RR 0.58 (0.32 to 1.03) |
18 (1 study) |
⊕⊕⊝⊝ Low1,2 | No studies reported on adverse effects, or risk of HAPE or HACE |
| Ginkgo biloba versus placebo: risk of AMS | Not estimable | Not estimable |
RR ranged from 0.05 to 1.033 |
504 (6 studies) | ⊕⊕⊝⊝ Low4 | 2 studies reported 22 adverse events: paraesthesia: 10/178 (5.6%) with ginkgo biloba versus 12/174 (6.8%) with placebo (RR 0.80, 95% CI 0.36 to 1.80). No events of HAPE occurred in 3 studies. 1 event of HACE occurred in 3 studies (ginkgo biloba: 0/188 (0%); placebo 1/183 (0.5%); RR 0.36, 95% CI 0.02 to 8.47) |
| Erythropoietin versus placebo: risk of AMS | 737 per 1000 | 302 per 1000 (147 to 619) |
RR 0.41 (0.20 to 0.84) |
39 (1 study) |
⊕⊝⊝⊝ Very Low2,5 | No adverse events occurred in the study. 4 events of HAPE occurred in the study (erythropoietin: 1/20 (5%); placebo: 3/19 (15.7%); RR 0.32, 95% CI 0.04 to 2.79). 3 events of HACE occurred in the study (erythropoietin: 1/20 (5%); placebo: 2/19 (10.5%); RR 0.48, 95% CI 0.05 to 4.82) |
| Medroxyprogesterone versus placebo: risk of AMS | 938 per 1000 | 666 per 1000 (450 to 984) |
RR 0.71 (0.48 to 1.05) |
32 (2 studies) |
⊕⊕⊝⊝ Low2,6 | No studies reported on adverse effects, or risk of HAPE or HACE |
| Iron supplementation versus placebo: risk of AMS | 563 per 1000 | 366 per 1000 (214 to 624) |
RR 0.65 (0.38 to 1.11) |
65 (2 studies) |
⊕⊕⊝⊝ Low2,6 | No adverse events occurred in 1 study. No studies reported on risk of HAPE or HACE |
| Rhodiola crenulataversus placebo: risk of AMS | Not estimable | Not estimable |
OR 1.00 (0.78 to 1.29) |
125 (1 study) |
⊕⊕⊝⊝ Low2,7 | Cross‐over trial. Adverse events were "rare" according with the narrative findings from 1 study. No studies reported on risk of HAPE or HACE |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Quality of evidence downgraded by one level due to unclear risk of detection and other biases
2 Quality of evidence downgraded one level for imprecision: optimal information size not reached
3 A pooled analysis of these data reported an I² of 65% and this could not be explained by any of our planned subgroup analyses. We have therefore not pooled the results of these trials
4 Quality of evidence downgraded two levels for unclear risk of selection, performance, detection and other biases, as well as inconsistency.
5 Quality of evidence downgraded two levels for high risk of detection and performance bias
6 Quality of evidence downgraded one level due to unclear risk of selection, performance and detection bias
7 Quality of evidence downgraded one level due to unclear risk of detection and other biases
Summary of findings 3. Summary of findings group 3: other comparisons.
| Group 3: other comparisons | ||||||
|
Patient or population: participants at risk of high altitude illness Settings: high altitude (including simulated; Chile, China, Nepal, USA) Intervention: ginkgo biloba, acetazolamide + ginkgo biloba, acetazolamide, acetazolamide+ medroxyprogesterone Comparison: acetazolamide, ginkgo biloba, medroxyprogesterone | ||||||
| Comparison: outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| control group | Intervention group | |||||
| Ginkgo biloba versus acetazolamide: risk of AMS | Not estimable | Not estimable | RR ranged from 0.11 to 2.97 | 378 (3 studies) | ⊕⊕⊝⊝ Low1 | 2 studies reported 102 events of paraesthesia: 92/176 (52.2%) with acetazolamide versus 10/178 (5.6%) with ginkgo biloba (RR 0.11, 95% CI 0.06 to 0.20). 1 study reported one event of polyuria: 1/9 (11.1%) with acetazolamide versus 0/10 (0%) with ginkgo biloba (RR 3.30, 95% CI 0.15 to 72.08). No events of HAPE or HACE occurred in 3 studies. |
| Acetazolamide + ginkgo biloba versus ginkgo biloba: risk of AMS | 274 per 1000 | 118 per 1000 (71 to 194) |
RR 0.43 (0.26 to 0.71) |
311 (1 study) |
⊕⊕⊝⊝ Low2 | 1 study reported 103 events of paraesthesia: 93/154 (60.3%) with acetazolamide plus ginkgo biloba versus 10/157 (6.3%) with ginkgo biloba alone (RR 9.48, 95% CI 5.14 to 17.51). No events of HAPE or HACE occurred in 3 studies. |
| Acetazolamide + ginkgo biloba versus acetazolamide: risk of AMS | 92 per 1000 | 117 per 1000 (60 to 227) |
RR 1.27 (0.65 to 2.46) |
306 (1 study) |
⊕⊕⊝⊝ Low2 | 1 study reported 178 events of paraesthesia: 93/154 (60.3%) with acetazolamide plus ginkgo biloba versus 85/152 (55.9%) with acetazolamide alone (RR 1.08, 95% CI 0.89 to 1.31). No events of HAPE or HACE occurred in 3 studies. |
| Acetazolamide versus medroxyprogesterone: risk of AMS | 500 per 1000 | 500 per 1000 (160 to 1000) |
RR 1.00 (0.32 to 3.10) |
12 (1 study) |
⊕⊕⊝⊝ Low3,4 | No studies reported on adverse effects, or risk of HAPE or HACE |
| Acetazolamide + medroxyprogesterone versus medroxyprogesterone: risk of AMS | 500 per 1000 | 665 per 1000 (250 to 1000) |
RR 1.33 (0.50 to 3.55) |
12 (1 study) |
⊕⊕⊝⊝ Low3,4 | No studies reported on adverse effects, or risk of HAPE or HACE |
| Acetazolamide + medroxyprogesterone versus acetazolamide: risk of AMS | 500 per 1000 | 665 per 1000 (250 to 1000) |
RR 1.33 (0.50 to 3.55) |
12 (1 study) |
⊕⊕⊝⊝ Low3,4 | No studies reported on adverse effects, or risk of HAPE or HACE |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Quality of evidence downgraded by two levels due to unclear or high risk of performance and detection bias, as well as inconsistency
2. Quality of evidence downgraded by two levels due to unclear or high risk of performance, detection and attrition bias
3. Quality of evidence downgraded by one level due to unclear selection, performance, detection and other bias
4 Quality of evidence downgraded by one level for imprecision: optimal information size not reached
We highlighted the quality of evidence for the primary outcome only (risk of AMS). We used the five GRADE criteria (study limitations; consistency of effect; imprecision; indirectness; and publication bias) to assess the quality of evidence relating to the studies that contributed data to the analyses for each of these four outcomes. When we identified an issue that we considered to be serious in each of the five GRADE criteria, we downgraded the quality of evidence by one level; and when we considered the issue to be very serious, we downgraded the quality of evidence by two levels (Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h). Whenever we decided to downgrade the quality of evidence from the default high quality, we justified our decisions and described the level of downgrade in the footnotes of the table. We developed the 'Summary of findings' table using a web‐based version of the GRADEpro software(www.guidelinedevelopment.org), according to the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our searches in January 2019 identified a total of 6169 references. After reviewing the references by title and abstract, we selected 180 of the citations to review as full texts (see Figure 1). After reading the articles, we included in this review 20 studies (21 records, 1406 participants). We excluded 42 references, and classified a further 14 studies as ongoing, and another 16 studies as awaiting assessment (due to the full text not yet being available, or due to the study assessing an intervention addressed in a previously published Cochrane Review). A further 87 studies were not included in the present review: this is because they are included in the other two reviews in this series of three reviews.
1.

Study flow diagram.
Included studies
We included 20 studies (1406 participants; 21 references) in this review (Bailey 2001; Berger 2017; Burse 1988; Chiu 2013; Chow 2005; Dehnert 2014; Gertsch 2004; Heo 2014; Ke 2013; Launay 2004; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007; Ren 2015; Roach 1983; Roncin 1996; Schommer 2010; Talbot 2011; Wright 2004a; Wright 2004b). Eighteen out of 20 of the included studies were parallel trials, while the remaining two trials were cross‐over trials (Chiu 2013; Launay 2004). Two studies were performed at sea level using special chambers or rooms simulating altitude (Burse 1988; Dehnert 2014), and the remaining studies were developed at high altitude. One study did not provide information about any of the assessed outcomes in this review (Roach 1983).
Participants
The participants' ages ranged between 17 and 65 years. Eight out of 20 studies included only men (40%; Burse 1988; Dehnert 2014; Ke 2013; Launay 2004; Moraga 2007; Ren 2015; Roncin 1996; Talbot 2011). Only Heo 2014 included people with a history of AMS.
Setting
Five studies were undertaken in the USA (25%; Burse 1988; Chow 2005; Leadbetter 2009a; Leadbetter 2009b; Roach 1983). The remaining studies were carried out in Asia (35%; Bailey 2001; Chiu 2013; Gertsch 2004; Heo 2014; Ke 2013; Ren 2015; Wright 2004b); and in Europe or South America (40%; Berger 2017; Dehnert 2014; Launay 2004; Moraga 2007; Roncin 1996; Schommer 2010; Talbot 2011; Wright 2004a).
Administration of intervention to prevent HAI
Four out of 20 studies provided the intervention less than, or up to 24 hours prior to, the ascent (20%; Berger 2017; Moraga 2007; Ren 2015; Talbot 2011), four studies between one to three days prior (20%; Gertsch 2004; Ke 2013; Launay 2004; Leadbetter 2009b), and eight studies between 4 to 30 days before departure (40%; Bailey 2001; Burse 1988; Chiu 2013; Chow 2005; Dehnert 2014; Heo 2014; Leadbetter 2009a; Schommer 2010). Four trials did not provide information about this issue (Roach 1983; Roncin 1996; Wright 2004a; Wright 2004b). In 22% of the trials in high mountains, the participants hiked (trekked) to endpoint altitude (Bailey 2001; Gertsch 2004; Roncin 1996; Schommer 2010); and in the remaining studies in high altitude, the participants used a combination of means of transportation, including cars, trains, and cable cars (70%).
Altitude
All of the included studies reached a very high altitude (between 3500 m and 5500 m) above sea level. The difference between the endpoint and the baseline altitude ranged from 648 m (Gertsch 2004), to 4700 m (Launay 2004). The most frequent durations for ascent were two hours (five studies; Berger 2017; Chow 2005; Leadbetter 2009a; Leadbetter 2009b; Ren 2015). Two studies did not provide any information about these issues (Burse 1988; Gertsch 2004).
Scale used to assess AMS
The most commonly used scale used was the Lake Louise Score (60%; Bailey 2001; Berger 2017; Chiu 2013; Chow 2005; Dehnert 2014; Gertsch 2004; Heo 2014; Launay 2004; Ren 2015; Talbot 2011; Wright 2004a; Wright 2004b), and the criterion to define AMS onset was a score of three points or more in six trials (Bailey 2001; Chiu 2013; Heo 2014; Launay 2004; Wright 2004a; Wright 2004b).
Funding
Eleven out of 20 studies did not provide clear information about the source of funding (55%; Bailey 2001; Berger 2017; Burse 1988; Chiu 2013; Chow 2005; Dehnert 2014; Ke 2013; Leadbetter 2009a; Leadbetter 2009b; Roncin 1996; Talbot 2011). Eight studies declared their possible conflicts of interests (40%).
For further information refer to the table 'Characteristics of included studies'.
Excluded studies
We excluded 42 studies from this series of three reviews (Agostoni 2013; Baillie 2009; Bartsch 1993; Bartsch 1994; Bilo 2015; Bloch 2009; Broome 1994; Cain 1966; Debevec 2015; Dumont 1999; Forster 1982; Forwand 1968; Fulco 2011; Gertsch 2002; Gray 1971; Harris 2003; Johnson 1988; Jonk 2007; Kayser 1993; Kotwal 2015; Lalande 2009; Lawley 2012; Levine 1989; Liu 2013; Mairer 2012; McIntosh 1986; Modesti 2006; Purkayastha 1995; Reinhart 1994; Sandoval 2000; Savourey 1998; Scalzo 2015; Serra 2001; Siebenmann 2011; Silva‐Urra 2011; Singh 1969; Solís 1984; Suh 2015; Teppema 2007; Vuyk 2006; White 1984; Wright 1988). Thirty (71%) out of the 42 studies were excluded because they did not focus on HAI prevention. In eight of the excluded studies, the authors reported results for the treatment of HAI conditions (21%). The remaining references were excluded for other reasons.
For further information refer to the table Characteristics of excluded studies.
Studies awaiting classification
We classified 16 studies as awaiting assessment for this series of three reviews (Burns 2018; Dugas 1995; Ellsworth 1987; Furian 2018; Hefti 2014; Kanaan 2017; Kasic 1991; Lee 2011; Lipman 2018; Menz 2018; Pun 2014; Swenson 1997; Utz 1970; Wang 1998; Warner 2018; Xiangjun 2014). Most of these studies were excluded because we were unable to obtain the full texts from the authors, the Cochrane Emergency and Critical Care Group, or the Iberoamerican Cochrane Centre. In addition, some studies address an intervention previously assessed in our Cochrane series about prevention of HAI; these studies will be considered in future updates of these reviews.
For further information refer to the table Characteristics of studies awaiting classification.
Ongoing studies
We considered an additional 14 studies as ongoing for this series of three reviews as we were only able to find them cited in trial registers, but we considered that they could be due for publication shortly (ChiCTR‐TRC‐13003319; ChiCTR‐TRC‐13003590; NCT00886912; NCT01606527; NCT01682551; NCT01794078; NCT01993667; NCT02244437; NCT02450968; NCT02811016; NCT02941510; NCT03424226; NCT03552263; NCT03561675).
For further information refer to the table Characteristics of ongoing studies.
Risk of bias in included studies
The risk of bias for the studies was assessed in seven categories. We provide a summary of our assessment of the methodological quality of included studies in Figure 2 and Figure 3.
2.
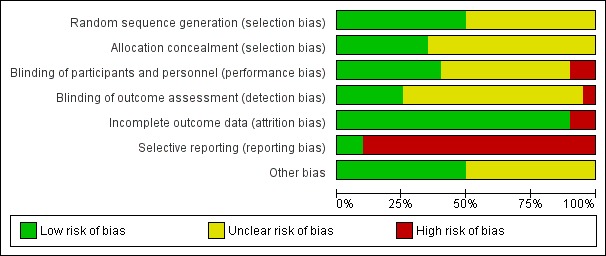
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
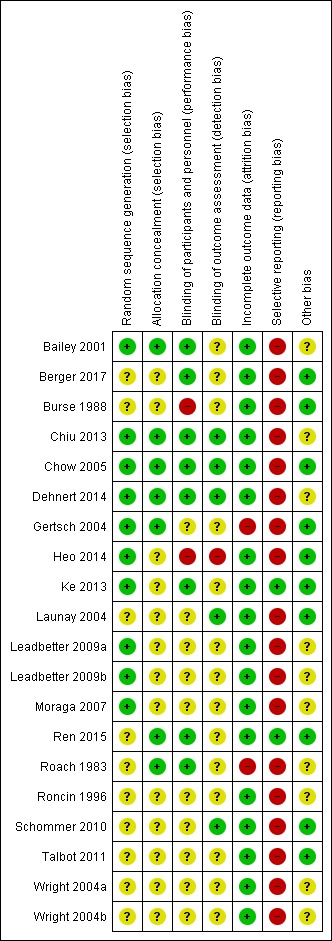
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 10 studies, the authors reported a valid method of randomization, such as a table of random numbers or a computerized random assignment (Bailey 2001; Chiu 2013; Chow 2005; Dehnert 2014; Gertsch 2004; Heo 2014; Ke 2013; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007), whereas this information was not clearly reported in the remaining studies (50%). Similarly, only seven studies undertook and reported random allocation concealment (Bailey 2001; Chiu 2013; Chow 2005; Dehnert 2014; Gertsch 2004; Ren 2015; Roach 1983), and the information was absent in the remaining included studies (65%).
Blinding
Eight studies reported adequate blinding of participants and personnel (Bailey 2001; Berger 2017; Chiu 2013; Chow 2005; Dehnert 2014; Ke 2013; Ren 2015; Roach 1983). In two studies we assessed blinding to be at high risk of bias due to single or no blinding (Burse 1988; Heo 2014). In the remaining studies, we classified this domain as unclear.
Regarding detection bias, we considered the risk as low in only five studies (Chiu 2013; Chow 2005; Dehnert 2014; Launay 2004; Schommer 2010), whereas we considered this risk of bias as high in one study (Heo 2014). In three of the studies, we classified the risk of bias as low for both blindings (Chiu 2013; Chow 2005; Dehnert 2014).
Incomplete outcome data
Significant numbers of participants were lost or excluded from the final analysis of two studies (Gertsch 2004; Roach 1983). In the remaining five studies, we classified the risk of bias as low (90%).
Selective reporting
All studies but two did not report adverse events associated with the interventions suggested for prevention of HAI.
Other potential sources of bias
In 10 studies, we found additional sources of bias. Interventions were administered before and during the ascent in five studies (Bailey 2001; Chiu 2013; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007). Four additional trials were unclear in the administration time for the intervention (Roach 1983; Roncin 1996; Wright 2004a; Wright 2004b). Other issues were detected in Dehnert 2014. We identified no additional sources of risk in the remaining studies.
Effects of interventions
See: Table 1; Table 2; Table 3
See Table 1
Group 1: preacclimatization and other measures based on pressure
Comparison 1. Normal versus simulated altitude conditions
Three studies compared different approaches to simulate altitude (i.e. hypobaric air breathing), including a lightweight device (Burse 1988), and hypoxia rooms (Dehnert 2014: Schommer 2010). We analysed the information from the three studies with a total of 140 participants (Burse 1988; Dehnert 2014; Schommer 2010). The fraction of inspired oxygen (FIO₂) in the simulated altitude arm ranged from 0.12 to 0.16. Only one study defined the FIO₂ for the normal conditions group (Dehnert 2014; FIO₂ = 0.21). One study involved a training programme on a bicycle ergometer (Schommer 2010), while the remaining studies assessing the intervention while the participants slept (Dehnert 2014), or remained in rest (Burse 1988). The studies were carried out in the USA (Burse 1988), Germany (Dehnert 2014), and Italy (Schommer 2010). All three studies reached altitudes of 4500 m or more. Preacclimatization lasted from 10 days (Burse 1988), to 21 days (Schommer 2010).
Primary outcome 1: risk of acute mountain sickness (AMS)
All three studies provided information about this outcome (Burse 1988; Dehnert 2014; Schommer 2010), registering a total of 62 events of acute mountain sickness (35/72 (48.6%) of those under normal conditions versus 27/68 (39.7%) of those under simulated altitude conditions). The risk ratio (RR) for acute mountain sickness, comparing normal versus simulated altitude conditions, was 0.85 (95% confidence interval (CI) 0.58 to 1.23; I² = 0%; 3 trials, 140 participants; Analysis 1.1). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 1). Because of the very low heterogeneity, we did not consider subgroup analysis.
1.1. Analysis.
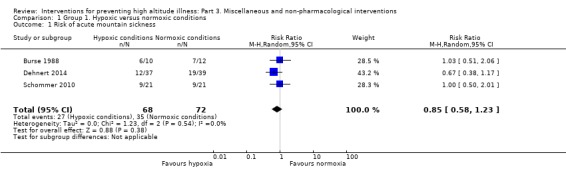
Comparison 1 Group 1. Hypoxic versus normoxic conditions, Outcome 1 Risk of acute mountain sickness.
We were unable to perform subgroup and sensitivity analysis. This is because all three studies reached final altitudes considered as 'very high' (two studies' participants climbed to 4500 m and one group to 4559 m), none of them included groups at high risk of AMS, and none of them was considered at 'low risk' (all of them have high risk of selective reporting bias).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included studies.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included studies.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included studies.
Secondary outcome 4: differences in HAI or AMS scores
Two studies provided information about AMS scores, including clinical criteria and Lake Louise AMS scores (Burse 1988; Schommer 2010). A pooled analysis of these data reported an I² of 75%, and this could not be explained by any of our planned subgroup analyses. We have therefore not pooled the results of these trials. The two trials reported conflicting results for this outcome. Burse 1988 reported benefits in terms of reduction of symptoms with the use of a lightweight device (MD −0.60, 95% CI −0.94 to −0.26). On the contrary, Schommer 2010 did not find benefits in terms of the number of symptoms after exercise in a hypoxic room (MD −1.00, 95% CI −3.19 to 1.19).
Comparison 2. Positive end‐expiratory pressure (PEEP) versus nothing
For this comparison, we analysed the information from one cross‐over study with a total of eight participants (Launay 2004). This study was carried out in France comparing the administration of positive end‐expiratory pressure (PEEP) 5 cm H₂O, versus no PEEP provision. Participants reached an altitude of 4100 to 4810 metres. PEEP was performed at low altitude and was completed two days before ascent.
Primary outcome 1: risk of acute mountain sickness (AMS)
Launay 2004 reported a total of seven events of acute mountain sickness for the two ascents. The OR for acute mountain sickness, comparing PEEP versus no PEEP, was 3.67 (95% CI 1.38 to 9.76; 1 cross‐over trial, 8 participants). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 1).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Launay 2004 provided information about Lake Louise AMS‐C scores. There was no improvement in scores with the use of PEEP (MD −0.25, 95% CI −2.79 to 2.29; 1 cross‐over trial; 8 participants).
Comparison 3. Remote ischaemic preconditioning (RIPC) versus placebo
For this comparison, we analysed the information from one study with a total of 40 participants (Berger 2017). This study was carried out in Austria comparing four cycles of lower limb ischaemia, induced by inflation of two thigh cuffs to 200 mmHg versus 20 mmHg in the control group. Cuffs were left inflated for five minutes, followed by five minutes of deflation. Participants reached an altitude of 3450 m. RIPC was performed at low altitude and was completed approximately 30 minutes before ascent.
Primary outcome 1: risk of acute mountain sickness (AMS)
Berger 2017 reported a total of 8 events of acute mountain sickness (6/20 (30%) of those receiving RIPC versus 2/20 (10%) of those receiving placebo). The RR for acute mountain sickness, comparing RIPC versus placebo, was 3.00 (95% CI 0.69 to 13.12; 1 trial, 40 participants). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 1).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Berger 2017 provided information about Lake Louise AMS scores. The mean difference for these scores, comparing RIPC versus placebo, was 0.50 (95% CI −0.98 to 1.98; 1 trial; 40 participants).
Group 2: supplements and vitamins
Comparison 1. Antacids versus placebo
For this comparison, we identified one study with a total of 45 participants (Roach 1983). This study was carried out in the USA, comparing administration of dihydroxy aluminium sodium carbonate (12 g every eight hours) versus placebo capsules. Participants reached an altitude of 4392 m. Duration of this supplementation was unclear. None of the outcomes predefined by our review was assessed in this study.
Comparison 2. Antioxidants versus placebo
For this comparison, we analysed the information from one study with a total of 18 participants (Bailey 2001). This study was carried out in India comparing a combination of L‐ascorbic acid (250 mg), dl‐alpha‐tocopherol acetate (100 UI) and alpha‐lipoic acid (150 mg) versus placebo capsules. Participants reached an altitude of 5180 m. Antioxidant supplementation lasted 21 days at sea level.
Primary outcome 1: risk of acute mountain sickness (AMS)
Bailey 2001 reported a total of 14 events of acute mountain sickness (5/9 (55.5%) of those taking antioxidants versus 9/9 (100%) of those taking placebo). The RR for acute mountain sickness, comparing antioxidants versus placebo, was 0.58 (95% CI 0.32 to 1.03; 1 trial, 18 participants). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 2).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Bailey 2001 provided information about Lake Louise AMS scores. The mean difference for these scores, comparing antioxidants versus placebo, was −1.64 (95% CI −2.75 to −0.54; 1 trial; 18 participants).
Comparison 2. Ginkgo biloba versus placebo
For this comparison, we analysed the information from seven studies performed in high mountains with a total of 523 participants (Chow 2005; Gertsch 2004; Ke 2013; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007; Roncin 1996). Three studies were developed in the USA (Chow 2005; Leadbetter 2009a; Leadbetter 2009b), and maximum altitude reached ranged from 3658 m (Ke 2013), to 4928 m (Gertsch 2004). Three studies only included men (Ke 2013; Moraga 2007; Roncin 1996). Dosages of ginkgo biloba ranged from 160 mg (Moraga 2007; Roncin 1996), to 240 mg (Chow 2005; Gertsch 2004; Ke 2013; Leadbetter 2009a; Leadbetter 2009b). Ginkgo biloba administration lasted from one day to five days (Moraga 2007 and Chow 2005 respectively). Leadbetter and colleagues reported two sets of data in a single reference and these data were analysed in a separate way (Leadbetter 2009a; Leadbetter 2009b). Data from Chow 2005 about AMS scores were provided as medians and ranges, and these statistical measures were transformed to be included in the main analysis (See Appendix 10).
Primary outcome 1: risk of acute mountain sickness (AMS)
Six studies provided information about the incidence of acute mountain sickness (Chow 2005; Gertsch 2004; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007; Roncin 1996). They found a total of 156 events (65/255 (25.4%) of those taking ginkgo biloba versus 91/249 (36.5%) of those taking placebo). A pooled analysis of these data reported an I² of 65% and this could not be explained by any of our planned subgroup analyses. We have therefore not pooled the results of these trials. RRs ranged from 0.05 (Roncin 1996), to 1.03 (Gertsch 2004), with two studies out of six finding a reduction of AMS with administration of ginkgo biloba (Leadbetter 2009a; Roncin 1996). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and inconsistency (See Table 2).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
In three studies the researchers assessed the risk of altitude pulmonary oedema, but did not find events to report (Chow 2005; Gertsch 2004; Ke 2013), (Analysis 2.2).
2.2. Analysis.
Comparison 2 Group 2. Ginkgo biloba versus placebo, Outcome 2 Risk of high altitude pulmonary oedema.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
In three studies (Chow 2005; Gertsch 2004; Ke 2013), the researchers assessed the risk of altitude cerebral oedema, and found one event in the placebo arm (0/188 (0%) of those taking ginkgo biloba versus 1/183 (0.5%) of those taking placebo). The estimated RR for HACE, comparing ginkgo biloba versus placebo, was 0.36 (CI 95% 0.02 to 8.47; three studies, 371 participants; Analysis 2.3).
2.3. Analysis.
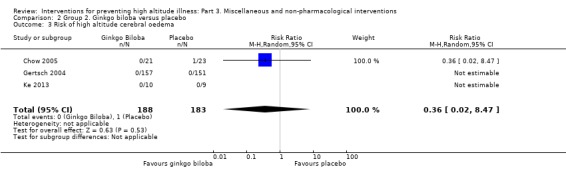
Comparison 2 Group 2. Ginkgo biloba versus placebo, Outcome 3 Risk of high altitude cerebral oedema.
Secondary outcome 3: risk of adverse events
Two studies assessed the incidence of paraesthesias (Chow 2005; Gertsch 2004). They found a total of 22 adverse events (10/178 (5.6%) of those taking ginkgo biloba versus 12/174 (6.8%) of those taking placebo). The estimated RR for paraesthesia, comparing ginkgo biloba versus placebo was 0.80 (95% CI 0.36 to 1.80; 2 studies, 352 participants; Analysis 2.4).
2.4. Analysis.
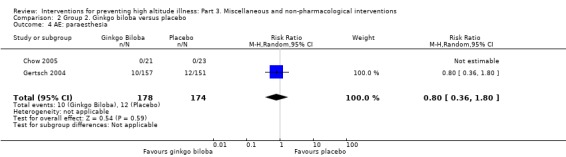
Comparison 2 Group 2. Ginkgo biloba versus placebo, Outcome 4 AE: paraesthesia.
Secondary outcome 4: differences in HAI or AMS scores
Three studies provided information about Lake Louise AMS Scores (Chow 2005; Leadbetter 2009a; Leadbetter 2009b; Moraga 2007). A pooled analysis of these data reported an I² of 90%, and this could not be explained by any of our planned subgroup analyses. We have therefore not pooled the results of these trials. SMD ranged from −2.99 (Leadbetter 2009a), to −0.26 (Chow 2005), with three studies out of four finding a reduction of AMS scores with administration of ginkgo biloba (Leadbetter 2009a;Leadbetter 2009b; Moraga 2007).
Comparison 3. Hormonal supplementation: erythropoietin versus placebo
For this comparison, we analysed the information from one study with a total of 39 participants (Heo 2014). This study was carried out in Nepal and compared administration of 10,000 IU epoetin alpha subcutaneous injections once per week for four consecutive weeks versus an unspecified control. Participants reached an altitude of 4130 m. Erythropoietin supplementation lasted four weeks before departure.
Primary outcome 1: risk of acute mountain sickness (AMS)
Heo 2014 reported a total of 20 events of acute mountain sickness (6/20 (30%) of those taking erythropoietin versus 14/19 (73.6%) of those taking placebo). The RR for acute mountain sickness, comparing erythropoietin versus placebo, was 0.41 (95% CI 0.20 to 0.84; 1 trial, 39 participants). We downgraded the quality of evidence from high to very low, due to risk of bias and imprecision issues (Table 2).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
Heo 2014 reported a total of four events of HAPE (1/20 (5%) of those taking erythropoietin versus 3/19 (15.7%) of those taking placebo). The RR for HAPE, comparing erythropoietin versus placebo, was 0.32 (95% CI 0.04 to 2.79; 1 trial, 39 participants).
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
Heo 2014 reported a total of three events of HACE (1/20 (5%) of those taking erythropoietin versus 2/19 (10.5%) of those taking placebo). The RR for HACE, comparing erythropoietin versus placebo, was 0.48 (95% CI 0.05 to 4.82; 1 trial, 39 participants).
Secondary outcome 3: risk of adverse events
Heo 2014 assessed the incidence of adverse events in general, but found no events to report.
Secondary outcome 4: differences in HAI or AMS scores
Heo 2014 provided information about Lake Louise AMS Scores, finding a SMD of −1.66 (95% CI −2.40 to −0.92).
Comparison 4. Hormonal supplementation: medroxyprogesterone versus placebo
For this comparison, we analysed the information from two studies with a total of 32 participants (Wright 2004a; Wright 2004b). These studies were carried out in Chile and Nepal, respectively, and compared administration of medroxyprogesterone 30 mg twice daily versus placebo capsules of ascorbic acid. Participants reached an altitude of 4680 and 5200 metres, respectively. Duration of medroxyprogesterone supplementation was unclear.
Primary outcome 1: risk of acute mountain sickness (AMS)
Two studies provided information about the incidence of acute mountain sickness (Wright 2004a; Wright 2004b). They found a total of 25 events (10/16 (62.5%) of those taking medroxyprogesterone versus 15/16 (93.7%) of those taking placebo). The estimated RR for AMS, comparing medroxyprogesterone versus placebo, was 0.71 (95% CI 0.48 to 1.05; I² = 0%; 2 studies; 32 participants; Analysis 3.1). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 2).
3.1. Analysis.
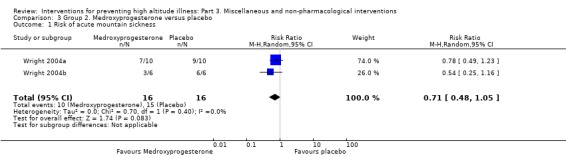
Comparison 3 Group 2. Medroxyprogesterone versus placebo, Outcome 1 Risk of acute mountain sickness.
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included studies.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included studies.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included studies.
Secondary outcome 4: differences in HAI or AMS scores
Both studies provided information about differences in Lake Louise AMS Scores (Wright 2004a; Wright 2004b), finding a SMD of −0.61 (95% CI −1.32 to 0.11; Analysis 3.2).
3.2. Analysis.
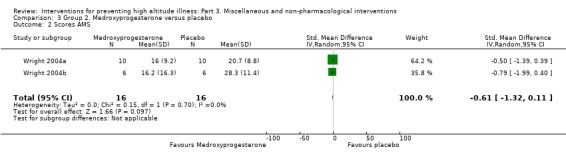
Comparison 3 Group 2. Medroxyprogesterone versus placebo, Outcome 2 Scores AMS.
Comparison 5. Iron supplementation versus placebo
For this comparison, we analysed the information from two studies with a total of 65 participants (Ren 2015; Talbot 2011). These studies were carried out in Chile and Nepal, respectively, and compared intravenous iron hydroxide‐sucrose 200 mg, unique doses, versus placebo. Participants reached an altitude of 3650 and 4340 metres, respectively. Duration of iron supplementation was one day.
Primary outcome 1: risk of acute mountain sickness (AMS)
Two studies provided information about the incidence of acute mountain sickness (Ren 2015; Talbot 2011), and they found a total of 30 events (12/33 (36.3%) of those taking iron versus 18/32 (56.2%) of those taking placebo). The estimated RR for AMS, comparing iron supplementation versus placebo was 0.65 (95% CI 0.38 to 1.11; I² = 0%; 2 studies, 65 participants; Analysis 4.1). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 2).
4.1. Analysis.
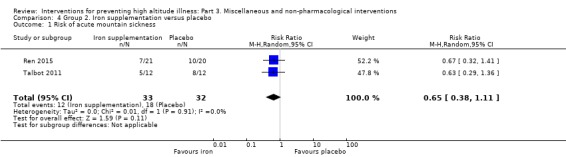
Comparison 4 Group 2. Iron supplementation versus placebo, Outcome 1 Risk of acute mountain sickness.
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included studies.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included studies.
Secondary outcome 3: risk of adverse events
Talbot 2011 assessed the incidence of adverse events in general, but found no events to report.
Secondary outcome 4: differences in HAI or AMS scores
Talbot 2011 provided information about Lake Louise AMS Scores, finding an SMD of −0.55 (95% CI −1.37 to 0.26).
Comparison 6. Rhodiola crenulata versus placebo
For this comparison, we analysed the information from one cross‐over study with a total of 125 participants (Chiu 2013). This study was carried out in Taiwan and compared administration of R crenulata 800 mg in capsules daily for nine days versus placebo capsules. Participants reached an altitude of 3421 metres. Administration of R crenulata lasted seven days before departure.
Primary outcome 1: risk of acute mountain sickness (AMS)
Chiu 2013 found a total of 124 events of acute mountain sickness. The odds ratio (OR) for acute mountain sickness, comparing R crenulata extract versus placebo, was 1.00 (95% CI 0.78 to 1.29; 1 trial, 125 participants). We downgraded the quality of evidence from high to low, due to risk of bias and imprecision issues (Table 2).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
Chiu 2013 reported in a narrative way that "During the 7‐day period prior to ascent, adverse events were rare. All were rare, mild and lasted less than two days; therefore, no participant needed to stop taking the study drugs prior to ascent" (Page 6).
Secondary outcome 4: differences in HAI or AMS scores
Chiu 2013 provided information about Lake Louise AMS Scores, finding an SMD of 0.07 (95% CI −0.55 to 0.69).
Group 3: other comparisons
Comparison 1. Ginkgo biloba versus acetazolamide
For this comparison, we analysed information from four studies performed in high mountains with a total of 397 participants (Chow 2005; Gertsch 2004; Ke 2013; Moraga 2007). In all but one study (Ke 2013), investigators used 500 mg of acetazolamide/day; and in all studies but one (Moraga 2007), investigators used 240 mg of ginkgo biloba. All studies reached very high altitudes (3500 to 5500 meters) and all but one had between three to five days of prophylaxis (Chow 2005; Ke 2013; Moraga 2007). Data for Chow 2005 related to scores of AMS should be transformed to mean and standard deviation (See Appendix 10).
Primary outcome 1: risk of acute mountain sickness (AMS)
Three studies assessed the risk of AMS (Chow 2005; Gertsch 2004; Moraga 2007), and they reported a total of 78 events (24/188 (12.7%) of those taking acetazolamide versus 54/190 (28.4%) of those taking ginkgo biloba). Pooled estimation of these data present a considerable heterogeneity (95% CI 0.21 to 1.35; I² = 63%; three studies, 378 participants; Analysis 5.1). We have therefore not pooled the results of these trials. RRs ranged from 0.11 (Moraga 2007), to 2.97 (Gertsch 2004), with one study out of three finding an increase of AMS with administration of ginkgo biloba (Gertsch 2004). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and inconsistency (Table 3).
5.1. Analysis.
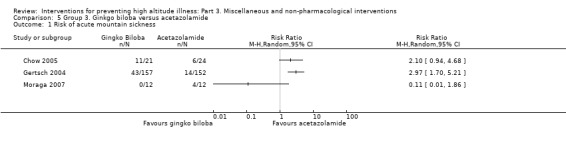
Comparison 5 Group 3. Ginkgo biloba versus acetazolamide, Outcome 1 Risk of acute mountain sickness.
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
Based on information from three studies (Chow 2005; Ke 2013; Gertsch 2004), and 375 participants, we did not find events of high altitude pulmonary oedema (Analysis 5.2).
5.2. Analysis.
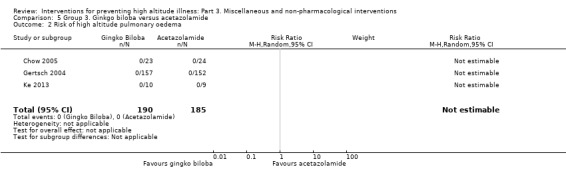
Comparison 5 Group 3. Ginkgo biloba versus acetazolamide, Outcome 2 Risk of high altitude pulmonary oedema.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
Based on information from three studies (Chow 2005; Gertsch 2004; Ke 2013), and 373 participants, we did not find events of high altitude cerebral oedema (Analysis 5.3).
5.3. Analysis.
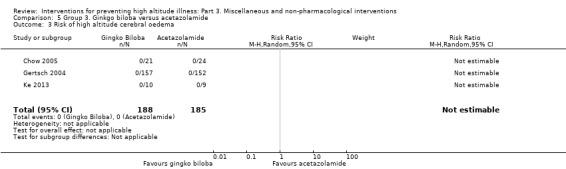
Comparison 5 Group 3. Ginkgo biloba versus acetazolamide, Outcome 3 Risk of high altitude cerebral oedema.
Secondary outcome 3: risk of adverse events
Two studies reported a total of 102 events of paraesthesias for this comparison (92/176 (52.2%) of those taking acetazolamide versus 10/178 (5.6%) of those taking ginkgo biloba) (Chow 2005; Gertsch 2004). The estimated RR for paraesthesias, comparing ginkgo biloba versus acetazolamide, was 0.11 (95% CI 0.06 to 0.20; I² = 0%; two studies, 354 participants; Analysis 5.4). Likewise, Ke 2013 reported one event of polyuria (1/9 (11.1%) of those taking acetazolamide versus 0/10 (0%) of those taking ginkgo biloba). The estimated RR for polyuria, comparing acetazolamide versus ginkgo biloba, was 3.30 (95% CI 0.15 to 72.08; one study, 19 participants).
5.4. Analysis.
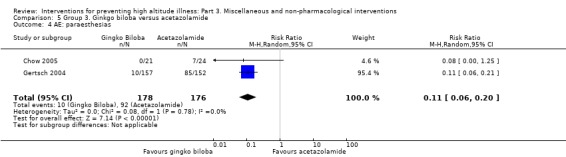
Comparison 5 Group 3. Ginkgo biloba versus acetazolamide, Outcome 4 AE: paraesthesias.
Secondary outcome 4: differences in HAI or AMS scores
Chow 2005 reported information about Lake Louise AMS scores. The estimated mean difference between acetazolamide versus ginkgo biloba was −1.38 (95% CI −2.03 to −0.72).
Comparison 2. acetazolamide and ginkgo biloba versus ginkgo biloba
For this comparison, we analysed information from one study with a total of 311 participants for this comparison (Gertsch 2004). In Gertsch 2004, investigators administered 500 mg of acetazolamide/day and 240 mg of ginkgo biloba/day. This study was developed in Nepal and participants reached a maximum altitude of 4928 metres. They took a minimum of three or four doses of the study drugs at baseline altitude before proceeding on their trek.
Primary outcome 1: risk of acute mountain sickness (AMS)
Authors of Gertsch 2004 found 61 events of acute mountain sickness for this comparison (18/154 (11.6%) of those taking acetazolamide plus ginkgo biloba versus 43/157 (27.3%) of those taking ginkgo biloba alone). The estimated RR for AMS, comparing acetazolamide plus ginkgo biloba versus ginkgo biloba alone was 0.43 (95% CI 0.26 to 0.71; one study; 311 participants). We downgraded the quality of the evidence from high to low due to issues related to risk of bias (Table 3).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
Authors of Gertsch 2004 did not find events of high altitude pulmonary oedema.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
Authors of Gertsch 2004 did not find events of high altitude cerebral oedema.
Secondary outcome 3: risk of adverse events
Authors of Gertsch 2004 found 103 events of paraesthesia (93/154 (60.3%) of those taking acetazolamide plus ginkgo biloba versus 10/157 (6.3%) of those taking ginkgo biloba alone). The estimated RR for paraesthesia for this comparison was 9.48 (95% CI 5.14 to 17.51; one study; 311 participants).
Secondary outcome 4: differences in HAI or AMS scores
We found no information about this outcome in the included study.
Comparison 3. acetazolamide and ginkgo biloba versus acetazolamide
For this comparison, we analysed information from one study with a total of 306 participants (Gertsch 2004). In Gertsch 2004, investigators administered 500 mg of acetazolamide/day and 240 mg of ginkgo biloba/day. This study was developed in Nepal, and reached a maximum altitude of 4928 metres.
Primary outcome 1: risk of acute mountain sickness (AMS)
Gertsch 2004 found 32 events of acute mountain sickness for this comparison (18/154 (11.6%) of those taking acetazolamide plus ginkgo biloba versus 14/152 (9.2%) of those taking acetazolamide alone). The estimated RR for AMS, comparing acetazolamide plus ginkgo biloba versus acetazolamide, was 1.27 (95% CI 0.65 to 2.46; one study; 306 participants). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and imprecision (Table 3).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
Gertsch 2004 did not find events of high altitude pulmonary oedema.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
Gertsch 2004 did not find events of high altitude cerebral oedema.
Secondary outcome 3: risk of adverse events
Gertsch 2004 found 178 events of paraesthesia (93/154 (60.3%) of those taking acetazolamide plus ginkgo biloba versus 85/152 (55.9%) of those taking acetazolamide alone). The estimated RR for paraesthesia for this comparison was 1.08 (95% CI 0.89 to 1.31; one study; 306 participants).
Secondary outcome 4: differences in HAI or AMS scores
We found no information about this outcome in the included study.
Comparison 4. acetazolamide versus medroxyprogesterone
For this comparison, we analysed the information from one study with a total of 12 participants (Wright 2004b). This study was carried out in Nepal, and compared administration of medroxyprogesterone 30 mg twice daily versus acetazolamide 250 mg twice daily. Participants reached an altitude of 5200 metres. Duration of administration was unclear.
Primary outcome 1: risk of acute mountain sickness (AMS)
Wright 2004b reported a total of six events of acute mountain sickness (3/6 (50%) of those taking acetazolamide versus 3/6 (50%) of those taking medroxyprogesterone). The RR for acute mountain sickness, comparing acetazolamide versus medroxyprogesterone, was 1.00 (95% CI 0.32 to 3.10; 1 trial, 12 participants). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and imprecision (Table 3).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Wright 2004b provided information about Lake Louise AMS Scores, finding a SMD of 0.58 (95% CI −0.59 to 1.74; one study; 12 participants).
Comparison 5. Acetazolamide and medroxyprogesterone versus medroxyprogesterone
For this comparison, we analysed the information from one study with a total of 12 participants (Wright 2004b). This study was carried out in Nepal, and compared administration of medroxyprogesterone 30 mg twice daily versus acetazolamide 250 mg twice daily. Participants reached an altitude of 5200 metres. Duration of administration was unclear.
Primary outcome 1: risk of acute mountain sickness (AMS)
Wright 2004b reported a total of seven events of acute mountain sickness (4/6 (66.6%) of those taking acetazolamide plus medroxyprogesterone versus 3/6 (50%) of those taking medroxyprogesterone alone). The RR for acute mountain sickness, comparing acetazolamide and medroxyprogesterone versus medroxyprogesterone alone, was 1.33 (95% CI 0.50 to 3.55; one trial, 12 participants). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and imprecision (Table 3).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Wright 2004b provided information about Lake Louise AMS Scores, finding an SMD of 0.06 (95% CI −1.07 to 1.19; one study; 12 participants).
Comparison 6. Acetazolamide and medroxyprogesterone versus acetazolamide
For this comparison, we analysed the information from one study with a total of 12 participants (Wright 2004b). This study was carried out in Nepal, and compared administration of medroxyprogesterone 30 mg twice daily versus acetazolamide 250 mg twice daily. Participants reached an altitude of 5200 metres. Duration of administration was unclear.
Primary outcome 1: risk of acute mountain sickness (AMS)
Wright 2004b reported a total of seven events of acute mountain sickness (4/6 (66.6%) of those taking acetazolamide plus medroxyprogesterone versus 3/6 (50%) of those taking acetazolamide alone). The RR for acute mountain sickness, comparing acetazolamide plus medroxyprogesterone versus medroxyprogesterone alone, was 1.33 (95% CI 0.50 to 3.55; 1 trial, 12 participants). We downgraded the quality of the evidence from high to low due to issues related to risk of bias and imprecision (Table 3).
Secondary outcome 1: risk of high altitude pulmonary oedema (HAPE)
We found no information about this outcome in the included study.
Secondary outcome 2: risk of high altitude cerebral oedema (HACE)
We found no information about this outcome in the included study.
Secondary outcome 3: risk of adverse events
We found no information about this outcome in the included study.
Secondary outcome 4: differences in HAI or AMS scores
Wright 2004b provided information about Lake Louise AMS Scores, finding an SMD of −0.68 (95% CI −1.86 to 0.50; one study; 12 participants).
Discussion
Summary of main results
We included 20 studies (1406 participants, 21 references) in this review. Thirty studies (14 ongoing and 16 awaiting) will be considered in future versions of this suite of three reviews as appropriate. We report the results for the primary outcome of this review (Risk of AMS) by each group of assessed interventions:
Group 1. Preacclimatization and other measures based on pressure
Use of simulated altitude or remote ischaemic preconditioning (RIPC) might not improve the risk of AMS on subsequent exposure to altitude, but this effect is uncertain (simulated altitude: RR 1.18, 95% CI 0.82 to 1.71; I² = 0%; 3 trials, 140 participants; low‐quality evidence. RIPC: RR 3.0, 95% CI 0.69 to 13.12; 1 trial, 40 participants; low‐quality evidence). We found evidence of benefits using positive end‐expiratory pressure (PEEP), but this information was derived from a cross‐over trial with a limited number of patients (OR 3.67, 95% CI 1.38 to 9.76; 1 trial, 8 participants; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Group 2. Supplements and vitamins
Supplementation of antioxidants, medroxyprogesterone, iron or Rhodiola crenulata might not improve the risk of AMS on exposure to high altitude, but this effect is uncertain (antioxidants: RR 0.58, 95% CI 0.32 to 1.03; 1 trial, 18 participants; low‐quality evidence. Medroxyprogesterone: RR 0.71, 95% CI 0.48 to 1.05; I² = 0%; 2 trials, 32 participants; low‐quality evidence. Iron: RR 0.65, 95% CI 0.38 to 1.11; I² = 0%; 2 trials, 65 participants; low‐quality evidence. R crenulata: RR 1.00, 95% CI 0.78 to 1.29; 1 trial, 125 participants; low‐quality evidence). We found evidence of improvement of this risk with the administration of erythropoietin, but this information was extracted from a trial with issues related to risk of bias and imprecision (RR 0.41, 95% CI 0.20 to 0.84; 1 trial, 39 participants; very low‐quality evidence). Regarding administration of ginkgo biloba, a pooled estimation of RR for AMS was not performed due to considerable heterogeneity between the included studies (I² = 65%). RR estimates from the individual studies was conflicting (from 0.05 to 1.03; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Group 3. Other comparisons
We found heterogeneous evidence regarding the risk of AMS when ginkgo biloba was compared with acetazolamide (I² = 63%). RR estimates from the individual studies were conflicting (estimations from 0.11 (95% CI 0.01 to 1.86) to 2.97 (95% CI 1.70 to 5.21); low‐quality evidence). We found evidence of benefits when ginkgo biloba was administered along with acetazolamide, but this information was derived from a single trial with issues associated to risk of bias (compared to ginkgo biloba alone: RR 0.43, 95% CI 0.26 to 0.71; 1 trial, 311 participants; low‐quality evidence). Administration of medroxyprogesterone plus acetazolamide did not improve the risk of AMS when compared to administration of medroxyprogesterone or acetazolamide alone (RR 1.33, 95% CI 0.50 to 3.55; 1 trial, 12 participants; low‐quality evidence). We found scarcity of evidence about the risk of adverse events for these interventions.
Overall completeness and applicability of evidence
We identified a limited number of studies addressing the effectiveness and safety of the non‐pharmacological and miscellaneous interventions for the prevention of HAI, with almost all the evidence being specifically about AMS. We included 20 studies in this review (1406 participants), but most of the assessed comparisons were only reported by single studies. Few of the included studies reported the incidence of adverse events, which was one of the secondary outcomes of our review.
Quality of the evidence
We used the GRADE system to assess the quality of the body of evidence associated with primary and secondary outcomes. (See Table 1, Table 2 and Table 3, for complete assessments and the rationale for ratings.) Risk of bias and imprecision were the GRADE considerations most affected in the assessment of the quality of the evidence in our review. Finally, presence of considerable heterogeneity of findings was a decisive factor to avoid the pooled estimations of AMS in critical comparisons.
Potential biases in the review process
In all cases, we followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, we had to make extensive modifications to the published protocol (Martí‐Carvajal 2012), due to the need to update the methods to the current Methodological Expectations of Cochrane Intervention Reviews (MECIR) (Higgins 2016). The MECIR guidelines were published after publication of this review's protocol (Martí‐Carvajal 2012), and some sections required major post hoc modifications. At that point we had some knowledge about the results of our search, and this could have introduced bias in these modifications. All modifications were approved by the Cochrane Emergency and Critical Care (previously Cochrane Anaesthesia, Critical and Emergency Care (ACE)) editors in collaboration with clinical and statistical experts, and we believe the risk of bias was reduced as far as possible. In addition, one major change was the decision to split the review into three parts, considering the numerous interventions assessed for HAI prevention. This decision was guided by the search results submitted in a first draft of the review, and because the ACE editors considered that the readability of the information could be adversely affected without this division. We believe the subgroups help readers to understand the heterogeneity and variability of interventions in this field, as well as allow the authors to present critical information in a clearer manner. We also suggest all these interventions should be analysed in a network meta‐analysis, in order to determine which interventions are more effective in avoiding the onset of new cases of this condition. Please see Differences between protocol and review for the full list of the modifications undertaken for this series of reviews about the prevention of HAI.
Sixteen potentially eligible studies were classified as 'awaiting', most of them because they were published only as conference proceedings, or because we did not have access to the full texts when we were completing this review. We also considered 14 additional studies as 'ongoing' because they were published only as protocols. These references will be considered in future updates of the three reviews belonging to this series.
An additional potential bias in our review was the difficulty we had in contacting trial authors to request additional information. We were unable to undertake this task due to, in most cases, no clear contact information being supplied in the publication. In addition, at least half of the included studies were published more than two decades ago. Trial authors might have been a potential source of information to document the rate of adverse events related to assessed interventions. We found that most of the studies did not report adverse events associated with the administration or use of these strategies. This constitutes a lack of information about the safety profile of the drugs in question.
In addition, we did not expect to encounter any unit of analysis issues as we did not expect to find cross‐over studies. However, we identified in this review two cross‐over study (12 cross‐over studies in total for this series of reviews) in our search strategies. In order to avoid bias in the development of our review, we analysed those studies separately.
Agreements and disagreements with other studies or reviews
Most of the published reviews recommend graded and slow ascent for the prevention of this condition (CATMAT 2007; Flaherty 2016; Kayser 2012; Khodaee 2016; Low 2012; Luks 2017; Ritchie 2012; Seupaul 2012; Zafren 2014). For CATMAT 2007 authors, the use of hyperbaric chambers is only reserved for treatment of acute mountains sickness. For Bartsch 2013, published clinical trials assessing normobaric hypoxic conditions have failed to show a significant reduction in the incidence of HAI, as well as their severity. Recently, Davis and colleagues discussed current advances in the prevention and treatment of HAI (Davis 2017). The authors stated that prophylaxis of HAI has as a main goal optimal acclimatization to prevent these conditions, so pharmacological interventions such as acetazolamide remain as the major strategy in AMS and HAPE prevention. Authors include preacclimatization strategies, remote ischaemic preconditioning and supplementation of oxygen as non‐pharmacological alternatives for the prevention of HAI, although they recognise that these methods are not fully supported by the literature (Davis 2017). Likewise, Luks and colleagues stated that normobaric or hyperbaric exposures have conflicting results in the prevention of HAI, due to the variability of studies in terms of duration and magnitude of assessed exposures (Luks 2017).
Authors' conclusions
Implications for practice.
The assessment of non‐pharmacological and miscellaneous interventions suggest that there is heterogeneous and even contradictory evidence related to the effectiveness of these prophylactic strategies. Safety of these interventions remains as an unclear issue due to lack of assessment. Overall, the evidence is limited due to its quality (low to very low) and the number of studies pending classification (30 studies ongoing or awaiting classification for this suite of three reviews about prevention of HAI).
Implications for research.
There is a lack of large and multi‐centre studies of most of the non‐pharmacological agents evaluated in this review. For most of the comparisons evaluated, small sample sizes and lack of reporting of important outcomes, such as adverse events, affect the quality of results. Further studies should also evaluate combinations of pharmacological and non‐pharmacological strategies to prevent HAI. Design of future trials might be improved by the following suggestions.
Refining the operative definition of HAI conditions by selecting a unified scale and threshold.
Improving the reporting of statistical data related to important outcomes in order to avoid missing data, and inclusion of information about elevation where HAI occurs.
Adding adverse events as an important endpoint in assessment of these preventive strategies.
Comparing potential non‐pharmacological or miscellaneous strategies against interventions of well‐known effectiveness (such as acetazolamide, an intervention assessed in the first part of this series of reviews).
Finally, we suggest performing a network meta‐analysis of all interventions (pharmacological and non‐pharmacological) used for high altitude illness prevention, in order to determine which interventions are more effective in avoiding the onset of new cases of this condition.
History
Review first published: Issue 4, 2019
| Date | Event | Description |
|---|---|---|
| 17 April 2012 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Mike Bennett (content editor), Cathal Walsh (statistical editor), Jeremy Windsor and Matiram Pun (peer reviewers), Janet Wale (consumer editor), and Harald Herkner (Co‐ordinating Editor) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Risk categories for acute mountain sickness
| Risk categories | Description |
| Low | Individuals with no prior history of altitude illness and ascending to ≤ 2800 m (˜ 9200 feet). |
| Low | Individuals taking ≥ 2 days to arrive at 2500 m to 3000 m (˜ 8200 to ˜ 9850 feet) with subsequent increases in sleeping elevation < 500 m by day/˜ 1650 feet by day. |
| Moderate | Individuals with prior history of AMS and ascending to 2500m to 2800 m (˜ 8200 to ˜ 9200 feet) in 1 day. |
| Moderate | No history of AMS and ascending to > 2800 m (˜ 9200 feet) in 1 day |
| Moderate | All individuals ascending > 500 m/d (˜ 1650 feet) (increase in sleeping elevation) at altitudes above 3000 m/˜ 9850 feet. |
| High | History of AMS and ascending to ≥ 2800 m/˜ 9200 feet in 1 day |
| High | All individuals with a prior history of HAPE or HACE. |
| High | All individuals ascending to > 3500 m/˜ 11,500 feet in 1 day. |
| High | All individuals ascending > 500 m/d (˜ 1650 feet/d) increase in sleeping elevation above > 3500 m/˜ 11,500 feet. |
| High | Very rapid ascents (e.g. Mt Kilimanjaro). |
Appendix 2. Medical terms glossary
| Term | Definition | Source |
| Anorexia | The lack or loss of appetite accompanied by an aversion to food and the inability to eat. | www.ncbi.nlm.nih.gov/mesh |
| Ataxia | Impairment of the ability to perform smoothly coordinated voluntary movements. | http://www.ncbi.nlm.nih.gov/mesh |
| Dyspnoea | Difficult or laboured breathing. | www.ncbi.nlm.nih.gov/mesh |
| Dizziness | An imprecise term which may refer to a sense of spatial disorientation, motion of the environment, or lightheadedness. | www.ncbi.nlm.nih.gov/mesh |
| Endothelium | A layer of epithelium that lines the heart, blood vessels (endothelium vascular), lymph vessels (endothelium lymphatic), and the serous cavities of the body. | www.ncbi.nlm.nih.gov/mesh |
| Fatigue | The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. | www.ncbi.nlm.nih.gov/mesh |
| Hallucination | Subjectively experienced sensations in the absence of an appropriate stimulus, but which are regarded by the individual as real. | www.ncbi.nlm.nih.gov/mesh |
| Headache | The symptom of pain in the cranial region. | www.ncbi.nlm.nih.gov/mesh |
| Herniation | Protrusion of tissue, structure, or part of an organ through the bone, muscular tissue, or the membrane by which it is normally contained. | www.ncbi.nlm.nih.gov/mesh |
| Hypoxia | A disorder characterized by a reduction of oxygen in the blood. | www.ncbi.nlm.nih.gov/mesh |
| Insomnia | Disorders characterized by impairment of the ability to initiate or maintain sleep. | www.ncbi.nlm.nih.gov/mesh |
| Lightheadedness | See dizziness. | www.ncbi.nlm.nih.gov/mesh |
| Nausea | An unpleasant sensation in the stomach usually accompanied by the urge to vomit. | www.ncbi.nlm.nih.gov/mesh |
| Pulmonary oedema | Excessive accumulation of extravascular fluid in the lung, an indication of a serious underlying disease or disorder. Pulmonary oedema prevents efficient pulmonary gas exchange in the pulmonary alveoli, and can be life‐threatening. | www.ncbi.nlm.nih.gov/mesh |
| Pulmonary alveoli | Small polyhedral outpouchings along the walls of the alveolar sacs, alveolar ducts and terminal bronchioles through the walls of which gas exchange between alveolar air and pulmonary capillary blood takes place. | www.ncbi.nlm.nih.gov/mesh |
| Seizures | Clinical or subclinical disturbances of cortical function due to a sudden, abnormal, excessive, and disorganized discharge of brain cells. Clinical manifestations include abnormal motor, sensory and psychic phenomena. | www.ncbi.nlm.nih.gov/mesh |
Appendix 3. MEDLINE (Ovid SP) search strategy
exp Brain Edema/ or exp Pulmonary Edema/ or Altitude Sickness/ or ((edema* or oedema*) adj3 (highaltitude or altitude or cerebral or pulmonary or brain or lung)).mp. or ((mountain or highaltitude or altitude) adj3 (sickness or illness or disease*)).mp. or (high altitude or highaltitude).ti,ab.
exp Secondary Prevention/ or exp Primary Prevention/ or exp Drug Therapy/ or (drug therap* or prevent* or acclimati?ation or nifedipine or dexamethasone or taladafil or sildenafil or theophylline or salmeterol or acetazolamide or aspirin* or sumatriptan or gabapentin or phenytoin or magnesium or ginkgo biloba or ascorbic acid or alpha‐tocopherol acetate or alpha‐lipoic acid or beta‐carotene or selenium or zinc or bosentan or calcium channel blockers or selective inhibitor of phosphodiesterase type or nonsteroidal anti‐inflammatory drug* or NSAID* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase inhibitor* or carbonic anhydrase inhibitor* or beta agonist* or 5‐HT1 receptor agonist* or N‐methyl‐D‐aspartate antagonist* or antioxidant* or vitamin* or mineral* or endothelin antagonist* or iron).mp.
((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
1 and 2 and 3
Appendix 4. Embase (Ovid SP) search strategy
brain edema/ or lung edema/ or altitude disease/ or ((edema* or oedema*) adj3 (highaltitude or altitude or cerebral or pulmonary or brain or lung)).mp. or ((mountain or highaltitude or altitude) adj3 (sickness or illness or disease*)).mp. or (high altitude or highaltitude).ti,ab.
secondary prevention/ or primary prevention/ or drug therapy/ or (drug therap* or prevent* or acclimati?ation or nifedipine or dexamethasone or taladafil or sildenafil or theophylline or salmeterol or acetazolamide or aspirin* or sumatriptan or gabapentin or phenytoin or magnesium or ginkgo biloba or ascorbic acid or alpha‐tocopherol acetate or alpha‐lipoic acid or beta‐carotene or selenium or zinc or bosentan or calcium channel blockers or selective inhibitor of phosphodiesterase type or nonsteroidal anti‐inflammatory drug* or NSAID* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase inhibitor* or carbonic anhydrase inhibitor* or beta agonist* or 5‐HT1 receptor agonist* or N‐methyl‐D‐aspartate antagonist* or antioxidant* or vitamin* or mineral* or endothelin antagonist* or iron).ti,ab,hw.
(placebo.sh. or controlled study.ab. or random*.ti,ab,sh. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animal* not human*).sh.
1 and 2 and 3
Appendix 5. CENTRAL search strategy
#1 MeSH descriptor: [Brain Edema] explode all trees #2 MeSH descriptor: [Pulmonary Edema] explode all trees #3 MeSH descriptor: [Altitude Sickness] explode all trees #4 ((edema* or oedema*) NEAR/3 (highaltitude or altitude or cerebral or pulmonary or brain or lung)) or ((mountain or highaltitude or altitude) NEAR/3 (sickness or illness or disease*)) or (high altitude or highaltitude) (Word variations have been searched) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Secondary Prevention] explode all trees #7 MeSH descriptor: [Primary Prevention] explode all trees #8 drug therap* or prevent* or acclimatization or acclimatisation or acclimation or acclimatation or nifedipine or dexamethasone or taladafil or sildenafil or theophylline or salmeterol or acetazolamide or aspirin* or sumatriptan or gabapentin or phenytoin or magnesium or ginkgo biloba or ascorbic acid or alpha‐tocopherol acetate or alpha‐lipoic acid or beta‐carotene or selenium or zinc or bosentan or calcium channel blockers or selective inhibitor of phosphodiesterase type or nonsteroidal anti‐inflammatory drug* or NSAID* or steroid* or glucocorticosteroid* or corticosteroid* or non‐selective phosphodiesterase inhibitor* or carbonic anhydrase inhibitor* or beta agonist* or "5‐HT1 receptor agonist*" or "N‐methyl‐D‐aspartate antagonist*" or antioxidant* or vitamin* or mineral* or endothelin antagonist* or iron (Word variations have been searched) #9 #6 or #7 or #8 #10 #5 and #9 #11 #10 in Trials (Word variations have been searched)
Appendix 6. Search strategy for LILACS via BIREME interface
"EDEMA CEREBRAL" or "edema pulmonary$" or "mountain sickness" or "high altitude" or "montaña enfermedad$" or "mal da montanha$" or "doença de alta altitude$" or "mal de altura$"
Appendix 7. WHO International Trials Registry Portal search
Advanced search
high‐altitude pulmonary oedema (in the title field)
Appendix 8. Study eligibility screening and data extraction form.
Intervention for preventing high altitude illness
Study selection, quality assessment and data extraction form
| First author | Journal/Conference Proceedings etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after 3 attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan 5.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| The paper listed above | |||
| Further papers | |||
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Country | |
| Other | |
| Rate of ascent (m/h) | |
| Final altitude reached (meters) | |
| AMS scale | |
| History of HAI | |
| Type of HAI reported | |
Intervention characteristics
| Intervention characteristics | |
| Further details | |
| Name | |
| Doses | |
| Administration route | |
| Time to administration | |
| Duration | |
If RCT included a combination:
| Intervention characteristics | |
| Further details | |
| Name | |
| Doses | |
| Administration route | |
| Time to administration | |
| Duration | |
If RCT included acclimatization:
| Intervention characteristics | |
| Rate of ascent (m/h) | Further details |
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Low risk of bias (random) |
| High risk of bias (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Low risk of bias | |
| High risk of bias | |
| Unclear | |
| Blinding | |
| Person responsible for participants care | Yes / No |
| Participant | Yes / No |
| Outcome assessor | Yes / No |
| Other (please specify) | Yes / No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
| Free selective report | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Low risk of bias |
| High risk of bias | |
| Unclear | |
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| Incidence of AMS (headache, nausea, insomnia, dizziness, and sleep disorder) | Yes / No |
| Incidence of HACE. | Yes / No |
| Incidence of HAPE. | Yes / No |
| Safety of adverse events | Yes / No |
| Safety (adverse drug reaction) | Yes / No |
| For dichotomous data | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| A | Incidence of AMS ((headache, nausea, insomnia, dizziness, and sleep disorder) | ||
| Incidence of HACE. | |||
| Incidence of HAPE | |||
| Safety of adverse events | |||
| Safety (adverse drug reaction) | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
| Trial characteristics | |
| Further details | |
| Single centre / Multicentre | |
| Country / Countries | |
| How was participant eligibility defined? |
|
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose / frequency of administration | |
| Duration of treatment (State weeks / months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
Appendix 9. Assessment of risk of bias in included studies.
We will assess the following domains as 'low risk of bias', 'unclear risk of bias' or 'high risk of bias'.
Random sequence generation
Allocation concealment
Blinding (of participants, personnel and outcome assessors)
Incomplete outcome data
Selective reporting
Free of other bias (baseline imbalance, early stopping, academic fraud, drug company involvement)
We will use the following definitions.
(1) Sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear , if the trial was described as randomized, but the method used for the allocation sequence generation was not described.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear, if the trial was described as randomized, but the method used to conceal the allocation was not described.
(3) Blinding or masking (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will judge studies at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low risk, high risk or unclear for participants;
low risk, high risk or unclear for personnel;
low risk, high risk or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Low risk, the numbers and reasons for drop‐outs and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, the report gave the impression that there had been no drop‐outs or withdrawals, but this was not specifically stated.
High risk, the number or reasons for drop‐outs and withdrawals were not described.
We will further examine the percentages of drop‐outs overall in each trial and per randomization arm and we will evaluate whether intention‐to‐treat analysis has been performed or could be performed from the published information.
Were all randomized participants analysed in the group to which they were allocated? (intention‐to‐treat (ITT) analysis)
Low risk of bias: specifically reported by authors that ITT was undertaken and this was confirmed on study assessment, or not stated but evident from study assessment that all randomized participants are reported or analysed in the group they were allocated to for the most important time point of outcome measurement irrespective of non‐compliance and co‐interventions.
High risk of bias: lack of ITT confirmed on study assessment (patients who were randomized were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation) regardless of whether ITT reported or not.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation.
Unclear: described as ITT analysis, but unable to confirm on study assessment, or not reported and unable to confirm by study assessment.
(5) Selective reporting bias
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear: not all pre‐defined, or clinically relevant and reasonably expected outcomes were reported on, or were not reported fully, or it was unclear whether data on these outcomes were recorded or not.
(6) Free of other bias
We will describe for each included study any important concerns we have about other possible sources of bias.
Low risk of bias; the trial appears to be free of other components that could put it at risk of bias.
Unclear; the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias; there are other factors in the trial that could put it at risk of bias, e.g., no sample size calculation made, early stopping.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to points 1 to 6 above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses; see Sensitivity analysis.
Appendix 10. Parallel studies ‐ transformation of numerical data
| Outcome | Study | Original data | Transformed data | Original data | Transformed data | Original data | Transformed data | Original data | Transformed data | Original data | Transformed data | Original data | Transformed data |
| Scores AMS | Chow 2005 | Median: 2 | Mean = 2.25 | Range = 0 to 5 | DS = 1.28 | Median = 4 | Mean = 5. 5 | Range = 1 to 13 | DS = 3.11 | Median = 4 | Mean = 4.75 | Range = 1 to 10 | DS = 2.38 |
Data and analyses
Comparison 1. Group 1. Hypoxic versus normoxic conditions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of acute mountain sickness | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.23] |
| 2 Scores AMS | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.2. Analysis.
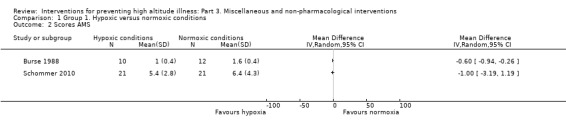
Comparison 1 Group 1. Hypoxic versus normoxic conditions, Outcome 2 Scores AMS.
Comparison 2. Group 2. Ginkgo biloba versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of acute mountain sickness | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Risk of high altitude pulmonary oedema | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Risk of high altitude cerebral oedema | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.47] |
| 4 AE: paraesthesia | 2 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.80] |
| 5 Scores AMS | 4 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected |
2.1. Analysis.
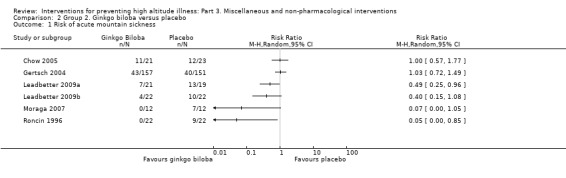
Comparison 2 Group 2. Ginkgo biloba versus placebo, Outcome 1 Risk of acute mountain sickness.
2.5. Analysis.
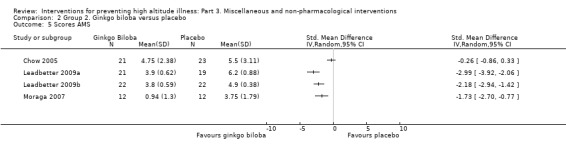
Comparison 2 Group 2. Ginkgo biloba versus placebo, Outcome 5 Scores AMS.
Comparison 3. Group 2. Medroxyprogesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of acute mountain sickness | 2 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.48, 1.05] |
| 2 Scores AMS | 2 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.32, 0.11] |
Comparison 4. Group 2. Iron supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of acute mountain sickness | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.38, 1.11] |
Comparison 5. Group 3. Ginkgo biloba versus acetazolamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Risk of acute mountain sickness | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Risk of high altitude pulmonary oedema | 3 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Risk of high altitude cerebral oedema | 3 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 AE: paraesthesias | 2 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.06, 0.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bailey 2001.
| Methods | Design: parallel (2 arms) Country: India Multisite: no International: no Treatment duration: 31 days Follow‐up: unclear Rate of ascent (m/h): 338 m/day average Final altitude reached: 5180 m AMS scale: Lake Louise Consensus scoring system |
|
| Participants |
|
|
| Interventions |
Antioxidant group (intervention): L‐ascorbic acid 250 mg, dl‐alpha‐tocopherol acetate 100 UI, alpha‐lipoic acid 150 mg, 4 vegetable–based capsules per 3 weeks at sea level and 10 days until the first morning following arrival at 5180 m Placebo group (control): 4 capsules of identical external appearance, taste and smell, each contained an equal quantity of plant cellulose extract, per 3 weeks at sea level and 10 days until the first morning following arrival at 5180 m. |
|
| Outcomes |
Outcomes were not pre‐specified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "...a computerized pseudo‐random number generator" (page 23). Quote "...an investigator who was unaware of the aims of the study and unrelates to data collection or analysis...". |
| Allocation concealment (selection bias) | Low risk | Quote "...an investigator who was unaware of the aims of the study and unrelates to data collection or analysis..." (page 23) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the placebo group ingested four capsules of identical external appearance, taste, and smell that each contained an equal quantity of plant cellulose extract." (Page 23) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote "all subjects successfully completed the study..." (Page 23) |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear impact of administration of intervention during the ascent (additional to prophylaxis) Quote: "supplementation was enforced for 3 weeks at sea level and during a 10‐day ascent to Mt. Everest base camp (˜5180 m)." Page 21 |
Berger 2017.
| Methods | Design: parallel (2 arms) Country: Austria Multisite: no International: no Treatment duration: not stated Follow‐up: 48 hours Rate of ascent (m/h): unclear Final altitude reached: 3450 m AMS scale: Lake Louise Consensus Symptoms score (LLS) + AMS‐C Score |
|
| Participants |
|
|
| Interventions |
RIPC group (intervention): 4 cycles of lower limb ischaemia, induced by inflation of 2 thigh cuffs to 200 mmHg. Cuffs were left inflated for 5 minutes, followed by 5 minutes of deflation. Sham group (control): 4 cycles of sham ischaemia, induced by inflation of 2 thigh cuffs to 20 mmHg. Cuffs were left inflated for 5 minutes, followed by 5 minutes of deflation. Both protocols were performed al low altitude (750 m) and were completed approximately 30 minutes before ascent. |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "subjects were randomly assigned to undergo either RIPC or sham preconditioning (...)" Page 5 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "subjects were informed that the aim of the study was to explore differences between preconditioning by ischaemia of the arterial plus venous versus the venous vasculature alone. The investigator who performed the preconditioning procedure was excluded from the assessment of other study data" Page 6 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients (RIPC group) were excluded from analysis after 25 hours of follow‐up. However, these exclusions did not affect the estimation of AMS cases and severity (measured at 24 hours) |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
Burse 1988.
| Methods | Design: parallel design (2 arms) Country: USA Multisite: no International: no Treatment duration: 10 days Follow‐up: 12 days Final altitude reached: 4500 m (simulated) AMS scale: environmental symptoms questionnaire |
|
| Participants |
|
|
| Interventions |
Experimental group (intervention): participants breathed from a lightweight device for 7.5 to 8 hours each day for 10 successive days, with an average hypoxic stimulus to 13.8 ± 0.9% (PO₂ equivalent to 3370 ± 517 m altitude Placebo group (control): participants breathed normoxic air from a placebo device On day 10, both groups were exposed for the next 2 days to simulated 4500m altitude in a hypobaric chamber. |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: “subjects numbers were randomly to the experimental and control groups by lot” Page 943 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: “subjects numbers were randomly to the experimental and control groups by lot” Page 943 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only participants were blinded to treatment assigned. Quote: “the experiment was of single‐blind design; only the experimenters knew which individuals were assigned to the experimental and placebo devices” Page 944 “A placebo simulator, used by the control subjects, was the same breathing device altered to vent all expired…” Page 943 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No additional biases were identified |
Chiu 2013.
| Methods | Design: cross‐over (2 arms) Country: Taiwan Multisite: no International: no Treatment duration: 9 days Follow up: unclear Rate of ascent (m/h): 712 m/h and 214 m/h Final altitude reached: 3421 m AMS scale: Lake Louise Score |
|
| Participants |
|
|
| Interventions |
Group A (intervention): participants received R crenulata 800 mg in capsules daily for 9 days, beginning 7 days before an ascent. Group B (control): participants received identical placebo capsules at the same doses. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random numbers were generated by using the computer, using block randomization with a block size of 2 or 4” Page 2 |
| Allocation concealment (selection bias) | Low risk | Quote: “the random numbers were placed in sealed envelopes, and a serial number was assigned to each envelope according to the sequence of allocation of the randomized number. Each envelope was then opened sequentially, according to the admission sequence of the participants at the study center. The number inside the envelope determined the treatment sequence that each participant was allocated to (Rhodiola‐placebo or placebo‐Rhodiola).” Page 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “both investigators and participants were blinded” Page 2 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “both investigators and participants were blinded” Page 2 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage of patients lost at follow‐up were up to 14% in both arms |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Unclear risk | It is unclear if previous events of HAI (specifically those from phase 1) affects the probability of new events in second phase of cross‐over trials. Unclear impact of administration of intervention during the ascent (additional to prophylaxis) Quote: "moreover, the participants were requested to take capsules every morning of their 2‐day mountaineering trip" Page 3 |
Chow 2005.
| Methods | Design: parallel design (3 arms) Country: USA Multisite: no International: no Treatment duration: 5 days Follow up: 1 day Rate of ascent (m/h): 1285 m/h Final altitude reached: 3800 m AMS scale: Lake Louise (LLS) acute mountain sickness scoring system |
|
| Participants |
|
|
| Interventions |
Acetazolamide group (Intervention A): administration of placebo 4 days before ascent and acetazolamide oral 250 mg twice a day, 1 day before ascent Ginkgo biloba group (Intervention B): administration of ginkgo biloba oral 120 mg twice a day, 5 days before ascent Placebo group (control): administration of identical‐appearing capsules of placebo 5 days before ascent |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we developed a randomization sequence by drawing cards out of a hat, using 25 labelled cards for each group" Page 297 |
| Allocation concealment (selection bias) | Low risk | Quote: "study medications were prepared (...) with enclosed administration instructions and fixed with serial numbers" Page 297 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "to maintain blinding, subjects in acetazolamide group started taking placebo 5 days before ascent and switched to a typical dosis for AMS prophylaxis" Page 297 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "in the event of an emergency, an investigator had access to the study key, which was stored within a sealed envelope" Page 297 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total percentage of participants lost at follow‐up: 16.1% |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Low risk | No other biases were identified |
Dehnert 2014.
| Methods | Design: parallel design (2 arms) Country: Germany Multisite: no International: no Treatment duration: 14 days Follow up: 4 days Rate of ascent (m/h): unclear Final altitude reached: 4500 m AMS scale: Lake Louise Score and the AMS‐C subscore of the Environmental Symptom Questionnaire |
|
| Participants |
|
|
| Interventions |
Hypoxic group (intervention): participants slept for 14 consecutive nights at home under a tent that was ventilated by and 4 nights at a fractional inspired oxygen (FIO₂) of 0.14 to 0.15. Normoxic group (control): participants slept for 14 consecutive nights at home under a tent that was ventilated by and 4 nights at a fractional inspired oxygen (FIO₂) of 0.209 Participants were asked to sleep for 8 hours each night, starting with an altitude of 2500 m (15.4% O₂) and increasing the altitude every night by about 100 m (decrease O₂ by 0.2%) until 3300 m (14% O₂) was reached. This altitude was kept constant for the last 7 days, resulting in an overall average exposure of 3043 m per night. |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned in blocks of 6 to normoxic or hypoxic treatment (...)" Page 264 |
| Allocation concealment (selection bias) | Low risk | Quote: "the person responsible for distributing the nitrogen generators and setting up the devices in the homes of the subjects was in charge of randomization and was not involved in clinical testing" Page 266 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "subjects had no information regarding the level of hypoxia because the display on the control unit showing the ambient O2 concentration was hidden while the display of the altitude remained visible for selection of the altitude" Page 266 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiners supervising subjects and performing measurements were blinded with regard to treatment and were therefore not involved in distributing the hypoxic tents" Page 266 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "three dropped out during the study and 73 finished the study protocol" Page 264 Intention‐to‐treat and per‐protocol analyses were reported |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Unclear risk | Quote: "when analyzing the data of the first 40 subjects, we discovered that many subjects had not been exposed to the intended degree of hypoxia because of technical problems discussed in the section describing the devices. Avoiding the identified causes for failure to reach sufficient hypoxia, the study was repeated in another group of 40 subjects" Page 265 Unclear impact of this issue in the measurement of treatment effect. |
Gertsch 2004.
| Methods | Design: parallel (4 arms) Country: Nepal Multisite: no International: no Treatment duration: 2 days Follow up: unclear Rate of ascent (m/h): unclear Final altitude reached: 4928 m AMS scale: Lake Louise score |
|
| Participants |
|
|
| Interventions |
Acetazolamide group (Intervention A) = Acetazolamide 250 mg, twice daily Ginkgo group (Intervention B) = Ginkgo biloba (standardized ginkgo extract GK 501) 120 mg, twice daily Acetazolamide and ginkgo group (Intervention C) = Combined Ginkgo 120 mg and acetazolamide 250 mg, twice daily Placebo group (Control group) = administered twice daily. No additional data provided Participants took a minimum of 3 or 4 doses of the study drugs at baseline altitude before proceeding on their trek without any influence from study administrators. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the randomisation code was computer generated by Deurali‐Janta Pharmaceuticals (Kathmandu, Nepal) and held by an independent physician.” Page 2 |
| Allocation concealment (selection bias) | Low risk | Quote: "the randomisation code was computer generated by Deurali‐Janta Pharmaceuticals (Kathmandu, Nepal) and held by an independent physician.” Page 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “the 127 participants (20.7%) lost to follow up…” Page 2 |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
Heo 2014.
| Methods | Design: parallel (2 arms) Country: Nepal Multisite: no International: no Treatment duration: 30 days Follow up: unclear Rate of ascent (m/h): 712 m/h, 214 m/h Final altitude reached: 4130 m AMS scale: Lake Louise Score |
|
| Participants |
|
|
| Interventions |
EPO group (Intervention): 10,000 IU epoetin alpha subcutaneous injections once per week for 4 consecutive weeks, starting 5 weeks before departure. The last injection was given 7 days before departure. Control group (control): unclear Cointerventions
|
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The randomization sequence was generated by computer at the Asan Medical Center. Block randomization to ensure gender or age equivalence between groups was not performed" Page 417 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote “First, our study was not blinded, which may affect the results …” Page 421 |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote “First, our study was not blinded, which may affect the results …” Page 421 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Low risk | No other biases were identified |
Ke 2013.
| Methods | Design: parallel design (3 arms) Country: China Multisite: no International: no Treatment duration: 4 days Follow up: unclear Rate of ascent (m/h): none Final altitude: 3658 m AMS scale: Lake Louis Score |
|
| Participants |
|
|
| Interventions |
Acetazolamide group (Intervention A): acetazolamide 125 mg twice a day, starting 3 days before ascent until 1 day at base camp (Lhasa) Ginkgo biloba group (Intervention B): ginkgo biloba 120 mg twice a day, starting 3 days before ascent until 1 day at base camp (Lhasa) Placebo group (Control): placebo capsules twice a day, starting 3 days before ascent until 1 day at base camp (Lhasa) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the participants were randomized into three groups according to random numbers generated by using a software package with nine in the acetazolamide group, ten in the gingko biloba group and nine in the placebo group.” Page 163 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "(...) and placebo (provided by the Institute of Pharmaceuticals of the Fourth Military Medical University) were packaged in visually identical capsules at the Institute of Pharmaceuticals of the Fourth Military Medical University (...)" Page 163 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | Low risk | No selective reporting bias detected |
| Other bias | Low risk | No other biases were identified |
Launay 2004.
| Methods | Design: cross‐over design Country: France Multisite: no International: no Treatment duration: 2 days Follow up: unclear Rate of ascent (m/h): not reported Final altitude: 4100 to 4810 m AMS scale: Lake Louis Score |
|
| Participants |
|
|
| Interventions |
PEEP group (Intervention A): participants were equipped with positive end‐expiratory pressure (PEEP) device of 5‐cm H₂O and was attached to a Hans Rudolf face mask with a low dead space (Hans Rudolph Inc, Kansas City, USA) Placebo group (Control): participants were no equipped with any device |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "the eight subjects climbed Mount Blanc twice: once with the 5‐cm H₂O PEEP (PEEP‐5) and once without it (w‐PEEP), according to a simple randomized order, determined before the experiment" Page 323 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the Lake Louise scoring consensus for acute mountain sickness was used by a physician who had been familiarized with the assessment of acute mountain sickness and who had no interest in this study" Page 323 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Low risk | No other biases were identified |
Leadbetter 2009a.
| Methods | Design: parallel (2 arms) Country: USA Multisite: no International: no Treatment duration: 5‐days' pretreatment and 4‐days' treatment Follow up: 24 hours Rate of ascent (m/h): 2000 to 4300 in 2 hours Final altitude reached: 4300 m AMS scale: Environmental Symptoms Questionnaire‐III and Lake Louise AMS. |
|
| Participants |
|
|
| Interventions |
Ginkgo biloba group (intervention): oral dose of 120 mg of ginkgo biloba self‐administered twice per day (morning and evening), for 4 days prior to ascent and during 24 hours at altitude Placebo group (control): capsules of lactulose without differences in appearance to ginkgo biloba capsules |
|
| Outcomes |
Primary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants in each study were matched by age, gender, height, weight, and body mass index, and each pair was randomized to either GBE or placebo treatments using a random number assignment program" Page 67 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of participants were excluded for analyses |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear impact of administration of intervention during the ascent (additional to prophylaxis) Quote: "an oral dose of 120 mg of GBE or placebo was self‐administered by participants twice per day, morning and evening, for 4 days (study 1) or 3 days (study 2) prior to ascent and during 24 hours at altitude, for a total treatment time of 5 days in study 1 and 4 days in study 2" Page 67 |
Leadbetter 2009b.
| Methods | Design: parallel (2 arms) Country: USA Multisite: no International: no Treatment duration: 4 days pretreatment and 3 days treatment Follow up: 24 hours Rate of ascent (m/h): 2000 to 4300 in 2 hours Final altitude reached: 4300 m AMS scale: Environmental Symptoms Questionnaire‐III and Lake Louise AMS. |
|
| Participants |
|
|
| Interventions |
Ginkgo biloba group (intervention): oral dose of 120 mg of ginkgo biloba self‐administered twice per day (morning and evening) for 3 days prior to ascent and during 24 hours at altitude Placebo group (control): capsules of lactulose without differences in appearance to ginkgo biloba capsules. |
|
| Outcomes |
Primary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants in each study were matched by age, gender, height, weight, and body mass index, and each pair was randomized to either GBE or placebo treatments using a random number assignment program" Page 67 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.5% of participants were excluded for analyses |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear impact of administration of intervention during the ascent (additional to prophylaxis) Quote: "an oral dose of 120 mg of GBE or placebo was self‐administered by participants twice per day, morning and evening, for 4 days (study 1) or 3 days (study 2) prior to ascent and during 24 hours at altitude, for a total treatment time of 5 days in study 1 and 4 days in study 2" Page 67 |
Moraga 2007.
| Methods | Design: parallel design (3 arms) Country: Chile Multisite: no International: yes Treatment duration: 4 days Follow up: 4 days Rate of ascent (m/h): began 830 hours from Antofagasta (sea level) via highway. Arrival to Calama (2400 m) at 1230 hours was followed by a 1‐hour stop, and arrival at Ollagüe was 1700 hours. Travel time was approximately 8.5 hours Final altitude reached: 3696 meters AMS scale: The Lake Louise Questionnaire |
|
| Participants |
|
|
| Interventions |
Ginkgo biloba group (Intervention A): ginkgo biloba extract (Egb761) 80 mg/12 hours, by 24 hours before ascending and continued for 3 days Acetazolamide group (Intervention B): acetazolamide 250 mg/12 hours, by 24 hours before ascending and continued for 3 days Placebo group (control): unclear |
|
| Outcomes |
Primary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "randomization was computer generated" Page 252 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear impact of administration of intervention during the ascent (additional to prophylaxis) Quote: "each group was evaluated under 2 conditions (...) 2) at high altitude, where the same participants received placebo, acetazolamide, or G biloba 24 hours before ascending and continued for 3 days" Page 252 |
Ren 2015.
| Methods | Design: parallel (2 arms) Country: China Multisite: no International: no Treatment duration: 1 dose Follow up: 5 days Rate of ascent (m/h): unclear Final altitude reached: 3650 m AMS scale: Lake Louise score |
|
| Participants |
|
|
| Interventions |
Iron group (Intervention A) = intravenous iron hydroxide sucrose dose of 200 mg in 100 ml saline (Venofer, Impfstoffwerk Dessau‐Tornau GmbH, Germany), at Day 0 Placebo group (Control group) = 100 ml normal saline at Day 0 |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "this was a perspective, randomized, double‐blinded, placebo controlled study" Page 2051 |
| Allocation concealment (selection bias) | Low risk | Quote: "each participant was attributed a computer‐generated 4‐digit serial number with the grouping information hidden in the third digit, which was blinded to both participants and researchers" Page 2051 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "drug administration was carried out by nurses blinded to the study, and iron supplement and placebo were injected in separate rooms. Intravenous fluids containing drug or saline were labelled with the serial number; because the drug solution was not clear, a brown light‐shading infusion apparatus (Weigao Medical Group, Weihai, China) was used to mask the grouping. Nurses performed injections according to serial number of participants and I.V. fluid labels" Page 2051 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients were lost at follow‐up (7%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting was identified |
| Other bias | Low risk | No other biases were identified |
Roach 1983.
| Methods | Design: parallel (2 arms) Country: USA Multisite: no International: no Treatment duration: 48 hours Follow up: unclear Rate of ascent (m/h): 91.5 m/h Final altitude reached: 4392 m AMS scale: authors developed a Symptoms Questionnaire Score |
|
| Participants |
|
|
| Interventions |
Antacid group: encapsulated dosages of antacid (dihydroxy aluminium sodium carbonate) 12 gm, each 8 hours Placebo group: encapsulated sucrose administered each 8 hours |
|
| Outcomes | Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "subjects were then randomly assigned to the treatment...by a person not directly involved in the field study" Page 398 |
| Allocation concealment (selection bias) | Low risk | Quote: "subjects were then randomly assigned to the treatment...by a person not directly involved in the field study" Page 398 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “the capsules appeared identical and neither the climbers nor the investigators knew the content of the capsules.” Page 398 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants (31%) were excluded for analyses |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear if intervention was administered before or during the ascent, or both |
Roncin 1996.
| Methods | Design: parallel (2 arms) Country: France Multisite: no International: no Treatment duration: 15 days Follow up: 30 days Rate of ascent (m/h): unclear Final altitude reached: 4900 m AMS scale: AMS‐C and AMS‐R scores from the Environmental Symptoms Questionnaire (ESQ). |
|
| Participants |
|
|
| Interventions |
EGb 71 group: administration of ginkgo biloba extract (EGb 761), 2 tablets 80 mg, morning and evening Placebo group: administration of placebo (no further details provided), 2 tablets 80 mg, morning and evening |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "the treatments were assigned in strictly numerical order with the assistance of a Nepalese doctor" Page 446 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear if intervention was administered before or during the ascent, or both |
Schommer 2010.
| Methods | Design: parallel design (2 arms) Country: Italy Multisite: no International: no Treatment duration: 4 weeks Follow up: unclear Rate of ascent (m/h): mean 169.4 m/h Final altitude reached: 4559 m AMS scale: Lake Louise score |
|
| Participants |
|
|
| Interventions |
Hypoxic group (intervention): participants exercised during week 1 at a FIO₂ = 0.16; In week 2, FIO₂ was 0.15, In week 3, FIO₂ was 0.14 In week 4, subjects rested in the hypoxic room (FIO₂ = 0.12) Normoxic group (control): participants exercised in normoxia for 3 weeks training sessions, and In week 4 subjects rested with FIO₂ = 0.21 For both groups, subject exercised 3 times a week for the first 3 weeks. In week 4 they rested in the room for 90 minutes without exercise. |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
Physiological variables: heart rate, SpO₂ |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were enrolled in the study and randomized to exercise" Page 20 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the physicians assessing AMS during the field study were not involved in training at low altitude and had no information about the group allocation of the subjects" Page 20 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants (2/42 = 4.7%) were excluded from the analysis due to violations of protocol |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported. |
| Other bias | Low risk | No other biases were identified |
Talbot 2011.
| Methods | Design: parallel design (2 arms) Country: Peru Multisite: no International: yes Treatment duration: 1 day Follow‐up: 3 days Rate of ascent (m/h): 542.5 m/h Final altitude reached: 4340 m AMS scale: Lake Louise AMS score |
|
| Participants |
|
|
| Interventions |
Iron group (intervention) = administration of iron (III)‐ hydroxide sucrose, 200 mg in 100 mL, intravenous infusion for 30 minutes before ascending Placebo group (control) = administration of saline solution, 100 ml intravenous infusion for 30 minutes before ascending |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote "...volunteers were block randomized (...)" Page 266 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Low risk | No other biases were identified |
Wright 2004a.
| Methods | Design: parallel (2 arms) Country: Chile Multisite: no International: no Treatment duration: unclear Follow‐up: unclear Rate of ascent (m/h): unclear Final altitude reached: 4680 m AMS scale: Lake Louise self‐reporting AMS questionnaire |
|
| Participants |
|
|
| Interventions |
Medroxyprogesterone group (intervention): administration of medroxyprogesterone 30 mg twice daily Placebo group (control): administration of 30 mg ascorbic acid twice daily |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "...were randomly allocated (...) Randomization was performed independently by the hospital pharmacy" Page 26 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomization was performed independently by the hospital pharmacy" Page 26 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost at follow‐up |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear if intervention was administered before or during the ascent, or both |
Wright 2004b.
| Methods | Design: parallel (4 arms) Country: Nepal Multisite: no International: no Treatment duration: unclear Follow up: unclear Rate of ascent (m/h): unclear Final altitude reached: 5200 m AMS scale: Lake Louise self‐reporting AMS questionnaire |
|
| Participants |
|
|
| Interventions |
Medroxyprogesterone group (intervention A): administration of medroxyprogesterone, 3 tablets of 10 mg twice daily Acetazolamide group (intervention B): administration of acetazolamide 250 mg twice daily + placebo, 3 tablets twice daily Acetazolamide and medroxyprogesterone group (intervention C): administration of acetazolamide 250 mg twice daily + medroxyprogesterone 3 tablets of 10 mg twice daily Placebo group (control): administration of ascorbic acid, 3 tablets of 50 mg twice daily |
|
| Outcomes |
Outcomes were not pre‐defined as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "study medications were randomized via computer‐generated code" Page 237 |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was lost at follow‐up and not included in main analysis |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes, such as adverse events, were not reported |
| Other bias | Unclear risk | Unclear if intervention was administered before or during the ascent, or both |
ACTH = adrenocorticotropic hormone; AMS = acute mountain sickness; AMS‐C = acute mountain sickness score‐ cerebral subscale; AMS‐R = acute mountain sickness score‐ respiratory subscale;BP = blood pressure; COPD = chronic obstructive pulmonary disease; Egb761 = ginkgo biloba extract EGb 761; EPO = erythropoietin; ESQ scores = environmental symptom questionnaire; FVC = forced vital capacity; GBE = Ginkgo biloba extract; g/dL = grams/decilitre; GHAQ = generalized high atitude questionnaire; HACE = high altitude cerebral oedema; HAH = high altitude headache; HAI = high altitude illness; HAPE = high altitude pulmonary oedema; ITT = intention‐to‐treat; IV = intravenous; kg = kilograms; LLS = Lake Louise scoring system;m = metres; MAP = mean artery pressure; mg = milligrams; m/h = metres/hour; NSAIDs = non‐steroidal anti‐inflammatory drugs; PASP = pulmonary artery systolic pressure; PEEP = positive end‐expiratory pressure; PEEP‐5 = 5‐cm H₂O positive end‐expiratory pressure; PEF = peak expiratory flow; PH = degree of acidity or alkalinity of a solution; RCT = randomized controlled trial; RIPC = remote ischaemic preconditioning; SD = standard deviation; SE = standard error; SEM = standard error of the mean; VAS = visual analogue scale; w‐PEEP = without PEEP
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agostoni 2013 | This study is not focused on prevention of high altitude illness |
| Baillie 2009 | The intervention was administered during or after the ascent, or both Quote: "treatment commenced on the day of travel to high altitude, and continued for 14 days after ascent" Page 342 |
| Bartsch 1993 | The study is focused on treatment of high altitude illness |
| Bartsch 1994 | The study is focused on treatment of high altitude illness |
| Bilo 2015 | This study is not focused on prevention of high altitude illness |
| Bloch 2009 | Non‐randomized clinical trial |
| Broome 1994 | The study is focused on treatment of high altitude illness |
| Cain 1966 | This study is not focused on prevention of high altitude illness |
| Debevec 2015 | This study is not focused on prevention of high altitude illness |
| Dumont 1999 | This study is not focused on prevention of high altitude illness |
| Forster 1982 | This study is not focused on prevention of high altitude illness |
| Forwand 1968 | This study is not focused on prevention of high altitude illness |
| Fulco 2011 | This study is not focused on prevention of high altitude illness |
| Gertsch 2002 | This study is not focused on prevention of high altitude illness |
| Gray 1971 | The study is focused on treatment of high altitude illness |
| Harris 2003 | The study is focused on treatment of high altitude illness |
| Johnson 1988 | This study is not focused on prevention of high altitude illness |
| Jonk 2007 | This study is not focused on prevention of high altitude illness |
| Kayser 1993 | The intervention was administered during or after the ascent, or both Quote: "subjects had ascended on the same day from an altitude of 1030 metres to 2350 by train, followed by an 8.5 hours climb to 4360 metres" Page 929 |
| Kotwal 2015 | This study is not focused on prevention of high altitude illness |
| Lalande 2009 | This study is not focused on prevention of high altitude illness |
| Lawley 2012 | The study is focused on treatment of high altitude illness |
| Levine 1989 | This study is not focused on prevention of high altitude illness |
| Liu 2013 | This study is not focused on prevention of high altitude illness |
| Mairer 2012 | This study is not focused on prevention of high altitude illness |
| McIntosh 1986 | This study is not focused on prevention of high altitude illness |
| Modesti 2006 | The study is focused on treatment of high altitude illness |
| Purkayastha 1995 | This study is not focused on prevention of high altitude illness |
| Reinhart 1994 | This study is not focused on prevention of high altitude illness |
| Sandoval 2000 | This study is not focused on prevention of high altitude illness |
| Savourey 1998 | The intervention was administered during or after the ascent, or both Quote: "each subject was submitted in randomized order to a run with a 5‐cm H₂O PEEP and to a run without PEEP during an 8‐h hypoxic exposure" Page 33 |
| Scalzo 2015 | This study is not focused on prevention of high altitude illness |
| Serra 2001 | This study is not focused on prevention of high altitude illness |
| Siebenmann 2011 | This study is not focused on prevention of high altitude illness |
| Silva‐Urra 2011 | The intervention was administered during or after the ascent, or both Quote: "the second 2‐kilometres walk at 5050 metres (Walk 3) was performed on the following day carrying the system and breathing the supplementary oxygen" Page 252 |
| Singh 1969 | The study is focused on treatment of high altitude illness |
| Solís 1984 | This study is not focused on prevention of high altitude illness |
| Suh 2015 | Non‐randomized clinical trial |
| Teppema 2007 | This study is not focused on prevention of high altitude illness |
| Vuyk 2006 | This study is not focused on prevention of high altitude illness |
| White 1984 | This study is not focused on prevention of high altitude illness |
| Wright 1988 | This study is not focused on prevention of high altitude illness |
PEEP = positive end‐expiratory pressure
Characteristics of studies awaiting assessment [ordered by study ID]
Burns 2018.
| Methods | Prospective, double‐blind, randomized, non‐inferiority trial |
| Participants | 92 volunteers were recruited through e‐mail lists with local and national distribution. The exclusion criteria were residence at > 1240 m, inability to complete a moderate hike at high altitude, younger than 18 years or older than 65 years, pregnant, having lived or slept at altitudes of > 1240 m in the preceding week, allergies to the study medications, or having ingested similar medicines or steroids in the preceding week |
| Interventions | Ibuprofen (600 mg, 3 times daily, starting 4 hours before ascent) and visually identical placebo; or acetazolamide (125 mg, twice daily, started the night before ascent); or placebo |
| Outcomes | The main outcome measure was acute mountain sickness incidence, using the Lake Louise Questionnaire (LLQ), with a score of > 3 with headache. Sleep quality and headache severity were measured with the Groningen Sleep Quality Survey (GSQS). |
| Notes | Related to systematic review Nieto 2017 |
Dugas 1995.
| Methods | Double‐blind randomized study |
| Participants | 20 healthy volunteers received 5 mg of isradipine (n = 6) or placebo (n = 6) for 8 days. After 5 days of treatment in normoxia, the subjects were rapidly transported to an altitude of 4350 m |
| Interventions | Israpadine (calcium channel blocker) and placebo |
| Outcomes | AMS symptom score, haemodynamic parameters and renal function |
| Notes | Full text not available (January 2016) |
Ellsworth 1987.
| Methods | Double‐blind randomized study |
| Participants | 47 individuals participated in this double‐blind, randomized trial comparing acetazolamide 250 mg, dexamethasone 4 mg, and placebo every 8 hours as prophylaxis for acute mountain sickness during rapid, active ascent of Mount Rainier (elevation 4392 m). 42 subjects (89.4%) achieved the summit in an average of 34.5 hours after leaving sea level |
| Interventions | Acetazolamide 250 mg, dexamethasone 4 mg, and placebo every 8 hours |
| Outcomes | Acute mountain sickness, symptoms reported |
| Notes | Full text not available (January 2016) |
Furian 2018.
| Methods | Double‐blind randomized, placebo‐controlled trial |
| Participants | 118 people with COPD were studied in Bishkek (760 m), Kyrgyz Republic; and after travelling within 6 hours to Tuja Ashu clinic (3200 m) stayed there for 3 days. |
| Interventions | Participants received dexamethasone (2 × 4 mg/d) or placebo before ascent and during stay at 3200 m |
| Outcomes | Cumulative risk of 1 of the following: AMS (AMS environmental symptom cerebral score ≥ 0.7); severe hypoxaemia (SpO₂ < 75% for > 30 min); or discomfort requiring descent to low altitude |
| Notes | Related to systematic review Nieto 2017 |
Hefti 2014.
| Methods | Double‐blind, placebo‐controlled trial |
| Participants | 29 participants were assigned into a treatment group (14) receiving 800 IU vitamin E, 1000 mg vitamin C, 200,000 IU vitamin A, and 600 mg N‐acetylcystein daily, starting 2 months prior to the expedition, and a placebo group (15) |
| Interventions | Vitamin group and placebo |
| Outcomes | AMS scores; levels of endothelial micro particles |
| Notes | Full text not available (January 2016) |
Kanaan 2017.
| Methods | Double‐blind, randomized trial |
| Participants | 332 non‐Nepali volunteers aged 18 to 65 years were recruited at Pheriche (4371 m) and Dingboche (4410 m) along the Everest trekking route in the Khumbu region of Nepal. Subjects were recruited with flyers and door‐to‐door recruitment at the guesthouse hotels in which they stayed in Pheriche and Dingboche |
| Interventions | Ibuprofen 600 mg or acetaminophen 1000 mg |
| Outcomes | The primary outcome was AMS incidence measured by the Lake Louise Questionnaire score |
| Notes | Related to systematic review Nieto 2017 |
Kasic 1991.
| Methods | Randomized study |
| Participants | 24 people who presented with acute mountain sickness |
| Interventions | A simulated descent of 1432 m (4600 ft) was attained by placing the participants in a fabric hypobaric chamber and pressurizing the chamber to 120 mm Hg above ambient pressure. Participants were randomly assigned to either the hypobaric treatment or treatment with 4 litres of oxygen given by facemask; both treatments lasted for 2 hours |
| Outcomes | Mean arterial oxygen saturation (SaO₂); symptoms of acute mountain sickness |
| Notes | Full text not available (January 2016) |
Lee 2011.
| Methods | Randomized trial |
| Participants | Nineteen adolescents aged 13 to 18 years attempting an ascent of Kala Patthar (5500 m) |
| Interventions | Acetazolamide, methazolamide |
| Outcomes | Risk of AMS, oxygen saturation and pulse rate |
| Notes | Full text not available (January 2017) |
Lipman 2018.
| Methods | Double‐blind, randomized, placebo‐controlled trial |
| Participants | 103 healthy participants, residing at low altitude and able to complete a moderately strenuous hike at high altitude, were included. Exclusion criteria included participants younger than 18 years or older than 65 years; pregnant or thought to be pregnant; having lived or slept at altitudes > 1240 m (4100 ft) in the past week; having taken diuretics, steroids, acetazolamide or non‐steroidal anti‐inflammatory drugs the week before the study; allergy to acetazolamide, sulfa medication, or corticosteroids; or a hazardous condition that precluded the ability to hike to high altitude, including sickle cell anaemia, severe asthma or chronic obstructive pulmonary disease, severe anaemia, or severe coronary artery disease |
| Interventions | Budesonide (180 g, twice daily, dry powder inhaler; AstraZeneca) and lactulose placebo pill (oral twice daily); visually matched acetazolamide (125 mg twice daily; Advantage Pharmaceuticals, Rocklin, CA) and indistinguishable empty inhaler twice daily; or inhaled and oral placebo (both twice daily) |
| Outcomes | The primary outcome was incidence of acute mountain sickness as calculated on the Lake Louise Questionnaire (LLQ), a widely used and validated self‐reported symptom‐based questionnaire. Presence of acute mountain sickness was defined by a LLQ score of ≥ 3 with the presence of a headache and 1 other symptom. Secondary outcome measures included incidence of severe acute mountain sickness (LLQ ≥ 5), SpO₂, and EtCO₂. |
| Notes | Related to systematic review Nieto 2017 |
Menz 2018.
| Methods | Double‐blind, placebo‐controlled trial |
| Participants | 80 healthy and physically fit participants (age 24 (22 to 28)) |
| Interventions | 12 hours in a normobaric hypoxia chamber |
| Outcomes | Blood pressure and heart rate measurements measured after 30 min, 3, 6, 9 and 12 hours. AMS scores |
| Notes | Conference proceeding (January 2019) |
Pun 2014.
| Methods | Prospective double‐blind placebo‐controlled randomized trial |
| Participants | 358 pilgrims were recruited at Dhunche (1950 m) and followed up at Chandanbari (3350 m) and up to the sacred Lake Gosaikunda. Most of these pilgrims ascended from Dhunche to the lake in 2 to 3 days |
| Interventions | Low‐dose acetazolamide (125 mg) and placebo |
| Outcomes | Lake Louise score (LLS) for AMS measurement, arterial oxygen saturation (SpO₂) and heart rate (HR) |
| Notes | Full text not available (January 2016) |
Swenson 1997.
| Methods | Randomized trial |
| Participants | 19 healthy volunteers were assessed, who ingested in randomized order both a high‐carbohydrate (68% CHO) or normal‐carbohydrate (45% CHO) diet for 4 days. On the 4th day, subjects were exposed to 8 h of 10% normobaric oxygen |
| Interventions | High‐carbohydrate (68% CHO) or normal‐carbohydrate (45% CHO) diet for 4 days |
| Outcomes | Lake Louise Consensus Questionnaire, interleukins 1 beta, 6 and 8 (IL‐1 beta, IL‐6, IL‐8) and tumour necrosis factor alpha (TNF‐alpha) |
| Notes | Full text not available (January 2016) |
Utz 1970.
| Methods | None known |
| Participants | None known |
| Interventions | None known |
| Outcomes | None known |
| Notes | Full text not available (January 2016) |
Wang 1998.
| Methods | Randomized trial |
| Participants | 65 men |
| Interventions | Conventional therapy group received oxygen, intravenous furosemide, aminophylline and dexamethasone; nifedipine group received oral nifedipine (10 mg, 3 × daily) in addition to conventional therapy; and participants in the nitric oxide group received nitric oxide (10 ppm) inhalation for 30 min, in addition to oral nifedipine |
| Outcomes | Pulmonary rales on auscultation and shadows on chest radiograph |
| Notes | Full text not available (January 2016) |
Warner 2018.
| Methods | Participants were randomized to ibuprofen 600 mg, 3 times daily, starting 4 hours before ascent, or acetazolamide 125mg, twice daily, started the night before rapid ascent from 1240 m to 3810 m during summer 2017 in the White Mountains of California |
| Participants | Healthy adult volunteers living at low altitude |
| Interventions | ibuprofen 600 mg, 3 times daily, starting 4 hours before ascent; or acetazolamide 125 mg, twice daily, started the night before rapid ascent |
| Outcomes | The main outcome measure was AMS incidence the night after ascent, measured by the Lake Louise Questionnaire (LLQ), with a score of > 3 with headache and 1 other symptom. Sleep quality was assessed with the Groningen Sleep Quality Survey (GSQS) and headache severity through a modified visual analogue scale (mVAS) |
| Notes | Related to systematic review Nieto 2017 . Conference proceeding only (January 2019) |
Xiangjun 2014.
| Methods | Randomized trial |
| Participants | 80 healthy young male plain residents (17 to 33 years old) |
| Interventions | Inhalation of budesonide (200 μg, twice daily), procaterol tablet (25 μg, twice daily), inhalation of budesonide/formoterol (160 μg/4.5 μg, twice daily) or placebo (1 tablet, twice daily) |
| Outcomes | Lake Louis AMS questionnaire, blood pressure, heart rate, and oxygen saturation |
| Notes | Full text not available (January 2017) |
AMS = acute mountain sickness; CHO = carbohydrate; COPD = chronic obstructive pulmonary disease; EGb 761 = extract of ginkgo biloba 761; ESQ = environmental symptom questionnaire; HR = heart rate; IL = interleukin; LLS = Lake Louise score; m = metres; mg = milligrams; min = minutes; ppm = parts per million; TNF = tumour necrosis factor.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐13003319.
| Trial name or title | Oral zolpidem for improving sleep and then prevention of acute mountain sickness: a single centre, randomised, double‐blind, controlled, prospective trial |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 18 years old Age maximum: 35 years old Gender: both |
| Interventions | Experimental: oral zolpidem (10 mg, daily, oral) Control: oral placebo, the same dosage as oral zolpidem |
| Outcomes | Lake Louise Score |
| Starting date | 30 June 2013 |
| Contact information | Huang Lan |
| Notes | Recruiting |
ChiCTR‐TRC‐13003590.
| Trial name or title | The meaning of intravenous iron supplementation in acute mountain sickness: a randomised, double‐blinded, placebo‐controlled trial |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 18 years old Age maximum: 65 years old Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | 30 July 2013 |
| Contact information | Ren Xuewen |
| Notes | Recruiting |
NCT00886912.
| Trial name or title | Prevention of acute mountain sickness by intermittent hypoxic training |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 18 years old Age maximum: 55 years old Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2008 |
| Contact information | Kai Schommer, MD |
| Notes | Recruiting |
NCT01606527.
| Trial name or title | Prospective, double‐blind, randomized, placebo‐controlled trial of ibuprofen versus placebo for prevention of neurologic forms of altitude sickness |
| Methods | Prospective, randomized, double‐blind, placebo‐controlled clinical trial evaluating ibuprofen and placebo for the prevention of neurological forms of altitude illness (including high altitude headache (HAH), acute mountain sickness (AMS), high altitude cerebral oedema (HACE) and high altitude anxiety). |
| Participants | The study will take place in the spring and summer of 2012 at the Marine Corps Mountain Warfare Training Center in the Eastern Sierras near Bridgeport, California. US Marines from near sea level will participate in battalion‐level training exercises at between 8500 and 11,500 feet, where some altitude illness is expected. |
| Interventions | Ibuprofen 600 mg orally 3 times daily |
| Outcomes |
|
| Starting date | July 2012 |
| Contact information | Jeffrey Gertsch MD, Naval Health Research Center |
| Notes | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than 2 years |
NCT01682551.
| Trial name or title | Evaluation of the prevention and treatment effects of Chinese medicine on high altitude illness |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 20 years Age maximum: 70 years Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2012 |
| Contact information | Not stated |
| Notes | Not yet recruiting |
NCT01794078.
| Trial name or title | A randomised, 4‐sequence, double‐blind study to test the safety of combined dosing with aminophylline and ambrisentan in exercising healthy human volunteers at simulated high altitude |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 18 years old Age maximum: 50 years old Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | September 2013 |
| Contact information | Claude A Piantadosi, MD |
| Notes | Active, not recruiting |
NCT01993667.
| Trial name or title | Acetazolamide for the prevention of high altitude illness: a comparison of dosing |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Drug: acetazolamide |
| Outcomes |
|
| Starting date | March 2012 |
| Contact information | Scott McIntosh, MD |
| Notes | Recruiting |
NCT02244437.
| Trial name or title | Ibuprofen versus acetaminophen in the prevention of acute mountain sickness: a double blind, randomised controlled trial |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 18 years old Age maximum: 65 years old Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | October 2014 |
| Contact information | Nicholas C Kanaan, MD |
| Notes | Active, not recruiting |
NCT02450968.
| Trial name or title | Dexamethasone for prophylaxis of acute mountain sickness in patients with chronic obstructive pulmonary disease travelling to altitude |
| Methods | Interventional |
| Participants |
Inclusion criteria
Exclusion criteria
Age minimum: 20 years old Age maximum: 75 years old Gender: both |
| Interventions |
|
| Outcomes |
|
| Starting date | May 2015 |
| Contact information | Talant M Sooronbaev, MD |
| Notes | Recruiting |
NCT02811016.
| Trial name or title | Effect of inhaled budesonide on the incidence and severity of acute mountain sickness at 4559 m |
| Methods | Prospective, controlled, single‐centre study on 51 healthy volunteers at 4559 m |
| Participants | 51 healthy volunteers |
| Interventions |
|
| Outcomes |
|
| Starting date | June 2016 |
| Contact information | Marc Berger, Salzburger Landeskliniken |
| Notes | This study has been completed |
NCT02941510.
| Trial name or title | Inhaled budesonide for altitude illness prevention |
| Methods | Randomized, double‐blinded study administering budesonide, a medication to reduce inflammation in the lungs, to healthy volunteers to examine effects on altitude illness prevention by spending 18 hours overnight at 14,000 ft elevation |
| Participants | Participants will be recruited from the Denver community and prescreened for eligibility via phone. 100 participants, after consenting, will have baseline data and blood collected and will begin budesonide therapy 72 hours prior to being taken from Denver to Pikes Peak, where they will be observed at altitude for 18 hours. Participants will have the opportunity to withdraw consent at any time and will be monitored continuously by physician‐researchers |
| Interventions | Budenoside; placebo |
| Outcomes |
Primary outcome measures
|
| Starting date | April 2017 |
| Contact information | University of Colorado, Denver |
| Notes | This study is not yet open for participant recruitment |
NCT03424226.
| Trial name or title | Sickness evaluation at altitude with acetazolamide at relative dosages (SEAWARD) |
| Methods | To determine whether acetazolamide started the day of ascent is inferior to the standard night‐before‐ascent dose of acetazolamide for the prevention of acute mountain sickness (AMS) in travellers to high altitude. Acetazolamide has been examined in over 200 high altitude studies over the past 50 years, and is the most commonly used drug for AMS prevention in the high mountains of Nepal, Western Europe, and Africa. Current Wilderness Medical Society Practice Guidelines recommend a 125 mg dose of acetazolamide daily started the day or evening prior to ascent. However, day of ascent dosage has recently been found to be effective prophylaxis for severe AMS compared to placebo, but efficacy of day‐of‐ascent dosage has not been confirmed versus standard acetazolamide dosage. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Day of acetazolamide (acetazolamide 125 mg twice a day, started morning of ascent) or night before acetazolamide (acetazolamide 125 mg twice a day, started evening before ascent) |
| Outcomes |
Primary outcome measures
|
| Starting date | 4 August 2018 |
| Contact information | Grant S Lipman, Associate Professor Department of Emergency Medicine, Stanford University, Stanford University |
| Notes | This study is not yet open for participant recruitment |
NCT03552263.
| Trial name or title | Safety and efficacy of T89 in prevention and treatment of adults with acute mountain sickness (AMS) |
| Methods | Prospective, double‐blind, randomized, placebo‐controlled phase 2 clinical trial having 3 arms including T89 low‐dose, T89 high‐dose and a placebo controlled group. |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 7 June 2018 |
| Contact information | Jeffrey W Sall, PhD, MD |
| Notes | Recruitment status: recruiting |
NCT03561675.
| Trial name or title | Effect of acetazolamide on acute mountain sickness in lowlanders older than 40 years |
| Methods | Randomized, placebo‐controlled, double‐blind parallel trial evaluating the efficacy of acetazolamide prophylaxis in reducing the incidence of acute mountain sickness (AMS) in lowlanders older than 40 years travelling to altitude |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | 1 June 2018 |
| Contact information | Konrad E Bloch, MD, University Hospital, Zürich |
| Notes | Recruitment Status: recruiting |
a.m = ante meridiem; AMS = acute mountain sickness; BMI = body mass index; BSI‐12 = brief symptom Iiventory‐12; COPD = chronic obstructive pulmonary disease; CYP = cytochrome P450 enzymes; dL = decilitre; ECG = electrocardiogram; FEV1 = forced expiratory volume in 1 second; FSH = follicle‐stimulating hormone; ft = feet; FVC = forced expiratory vital capacity; GAD‐2 = generalized anxiety disorder scales‐2; GOLD = global initiative for chronic obstructive lung disease criteria; HACE = high altitude cerebral oedema; HAH = high altitude headache; HR = heart rate; kg = kilograms; IUD = intrauterine device; IUS = intrauterine system; LNG 20 = levonorgestrel 20 ɥg/day; m = metres; ml = millilitres; mg = milligrams; NSAIDs = non‐steroidal anti‐inflammatory drugs; OTC = over‐the‐counter; PEFR = peak expiratory flow rate; p.m. = post meridiem; TM = morning‐after pill; VAS = visual analogue scale; VO₂ = maximal oxygen consumption.
Differences between protocol and review
Given that the original protocol was published in 2012, several sections needed updating to fulfil the current methodological guidelines for Cochrane Reviews (Higgins 2016).
We made the following changes to the published protocol (Martí‐Carvajal 2012).
Considering the numerous interventions assessed for HAI prevention, and on the recommendation of the ACE editors, we split the review into three parts. This current review is the third in a series of three, and focuses on miscellaneous and non‐pharmacological interventions to prevent this condition. This change has implications in the title, scope and objective of this review, and in the other reviews belonging to this series (Gonzalez 2018; Nieto 2017).
The Background was updated with new references to reflect current evidence about the target condition, as well as the scope on less commonly used interventions to prevent HAI.
-
The Primary outcomes and Secondary outcomes presented in the protocol (Martí‐Carvajal 2012), were modified to follow the MECIR guidelines (Higgins 2016), and improve their understanding. In particular, we made the following changes.
We removed 'All‐cause mortality (by all causes or specific)' as a primary outcome of this review. This is because the risk of mortality is low in the general population, and it is not the primary goal for prevention.
We removed the outcome 'Combined incidence of AMS, HAPE or HACE (any of these alone or in combination)'. This is because this outcome is not often reported in studies, and this information can be easily calculated by the separate reporting of AMS, HAPE and HACE.
Previously the 'Risk of AMS' was a secondary outcome. It is a primary event to assess in prevention trials of HAI. We therefore moved this outcome from the list of secondary outcomes to the primary outcomes in this series of reviews. The risk of HAPE, HACE and adverse events are also important outcomes and they were included as secondary outcomes.
We included a new secondary outcome 'Difference in HAI or AMS scores at high altitude'. This is because it is frequently reported in studies, reflecting the severity of the disease.
We limited the Types of studies included to randomized controlled trials. We excluded quasi‐randomized studies, and prospective observational studies for evaluating clinical effectiveness, even if they reported adverse events. This was due to the high risk of bias involved in these types of studies. In addition, we included studies in which participants receive the intervention before the ascent AND during the climbing.
Despite the fact that the protocol — Martí‐Carvajal 2012 — did not include considerations about any unit of analysis, we identified one cross‐over study for this review. It was included in our review to favour the full report of all evidence, and it was analysed separately from parallel studies.
We stated in the protocol that we would contact trial authors in case of missing data or selective reporting (Martí‐Carvajal 2012). However we were unable to undertake this task because in most cases no contact information was supplied in the publication.
We introduced several modifications in the Dealing with missing data section, in order to clarify the 'intention to treat' (ITT) analysis performed, and to present the methods to impute missing information (mostly related to standard deviations).
Under Data synthesis we added a method named trial sequential analyses (TSA). However, due to the scarcity of data for the assessed comparisons in this review, and following the advice of ACE Editors, we decided not to report the TSA results in this case (all of them having only one study). At the next update, we will revisit our decision to use TSA, as this method is not currently recommended by the Cochrane Scientific Committee.
We also made extensive modifications to the Subgroup analysis and investigation of heterogeneity section, and we selected only three variables to analyse. However, we were unable to find information about significant pre‐existing disease in included trials.
Due to scarcity of information we were not able to perform the planned sensitivity and subgroup analyses, as well as exploration of risk of reporting bias.
Contributions of authors
Daniel Molano Franco (DMF), Víctor Nieto Estrada (VNE), Alejandro Gonzalez Garay (AGG), Arturo J Martí‐Carvajal (AMC), Ingrid Arevalo‐Rodriguez (IAR)
Conceiving the review: AMC
Co‐ordinating the review: DMF, AMC, IAR
Undertaking manual searches: VNE, AGG and IAR
Screening search results: AGG, DMF and IAR
Organizing retrieval of papers: AGG, DMF and IAR
Screening retrieved papers against inclusion criteria: VNE, DMF and IAR
Appraising quality of papers: VNE, DMF, AGG and IAR
Abstracting data from papers: VNE, DMF, AGG and IAR
Providing additional data about papers: VNE, DMF, AGG and IAR
Obtaining and screening data on unpublished studies: VNE, DMF, AGG and IAR
Data management for the review: IAR
Entering data into Review Manager 5 (RevMan 5) (Review Manager 2014): IAR
RevMan 5 statistical data: IAR and AMC
Other statistical analysis not using RevMan 5: AMC and IAR
Interpretation of data: VNE, DMF, AGG, AMC and IAR
Statistical inferences: VNE, DMF, AGG, AMC and IAR
Writing the review: VNE, DMF, AGG, AMC and IAR
Securing funding for the review: VNE, DMF, AGG, AMC and IAR
Guarantor for the review (one author): AGG
Person responsible for reading and checking review before submission: IAR
Sources of support
Internal sources
-
Facultad de Ciencias de la Salud Eugenio Espejo, Universidad Tecnológica Equinoccial, Ecuador.
Academic
-
Methodology Research Unit/Neonatology, Instituto Nacional de Pediatria, Mexico.
Academic
-
Instituto de Investigaciones Clinicas, Facultad de Medicina, Universidad de Colombia, Colombia.
Academic
External sources
-
Iberoamerican Cochrane Center, Spain.
Academic.
-
Cochrane Anaesthesia Group, Denmark.
Academic.
Declarations of interest
Daniel Molano Franco: nothing to declare.
Victor H Nieto Estrada: nothing to declare.
Alejandro Gonzalez Garay: nothing to declare.
Arturo Marti Carvajal: nothing to declare.
Ingrid Arevalo‐Rodriguez: nothing to declare.
New
References
References to studies included in this review
Bailey 2001 {published data only}
- Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Altitude Medicine & Biology 2001;2(1):21‐9. [PUBMED: 11252695] [DOI] [PubMed] [Google Scholar]
Berger 2017 {published data only}
- Berger MM, Macholz F, Lehmann L, Dankl D, Hochreiter M, Bacher B, et al. Remote ischemic preconditioning does not prevent acute mountain sickness after rapid ascent to 3,450 m. Journal of Applied Physiology 2017;123(5):1228‐34. [PUBMED: 28798201] [DOI] [PubMed] [Google Scholar]
Burse 1988 {published data only}
- Burse RL, Forte VA. Acute mountain sickness at 4500 m is not altered by repeated eight‐hour exposures to 3200‐3550 m normobaric hypoxic equivalent. Aviation, Space, and Environmental Medicine 1988;59(10):942‐9. [PUBMED: 3142454] [PubMed] [Google Scholar]
Chiu 2013 {published data only}
- Chiu TF, Chen LL, Su DH, Lo HY, Chen CH, Wang SH, et al. Rhodiola crenulata extract for prevention of acute mountain sickness: a randomized, double‐blind, placebo‐controlled, crossover trial. BMC Complementary and Alternative Medicine 2013;13:298. [PUBMED: 24176010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chow 2005 {published data only}
- Chow T, Browne V, Heileson HL, Wallace D, Anholm J, Green SM. Ginkgo biloba and acetazolamide prophylaxis for acute mountain sickness: a randomized, placebo‐controlled trial. Archives of Internal Medicine 2005;165(3):296‐301. [PUBMED: 15710792] [DOI] [PubMed] [Google Scholar]
Dehnert 2014 {published data only}
- Dehnert C, Bohm A, Grigoriev I, Menold E, Bartsch P. Sleeping in moderate hypoxia at home for prevention of acute mountain sickness (AMS): a placebo‐controlled, randomized double‐blind study. Wilderness & Environmental Medicine 2014;25(3):263‐71. [PUBMED: 24931591 ] [DOI] [PubMed] [Google Scholar]
Gertsch 2004 {published data only}
- Gertsch JH, Basnyat B, Johnson EW, Onopa J, Holck PS. Randomised, double blind, placebo controlled comparison of ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: the prevention of high altitude illness trial (PHAIT). BMJ (Clinical Research Ed.) 2004;328(7443):797. [PUBMED: 15070635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heo 2014 {published data only}
- Heo K, Kang JK, Choi CM, Lee MS, Noh KW, Kim SB. Prophylactic effect of erythropoietin injection to prevent acute mountain sickness: an open‐label randomized controlled trial. Journal of Korean Medical Science 2014;29(3):416‐22. [PUBMED: 24616593 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SB, Shin EH. Ability of prophylactic erythropoietin injections to prevent acute mountain sickness: an open‐label randomized controlled trial. High Altitude Medicine & Biology 2014;15:A261. [EMBASE: 71529132] [Google Scholar]
Ke 2013 {published data only}
- Ke T, Wang J, Swenson ER, Zhang X, Hu Y, Chen Y, et al. Effect of acetazolamide and gingko biloba on the human pulmonary vascular response to an acute altitude ascent. High Altitude Medicine & Biology 2013;14(2):162‐7. [PUBMED: 23795737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Launay 2004 {published data only}
- Launay JC, Nespoulos O, Guinet‐Lebreton A, Besnard Y, Savourey G. Prevention of acute mountain sickness by low positive end‐expiratory pressure in field conditions. Scandinavian Journal of Work, Environment & Health 2004;30(4):322‐6. [PUBMED: 15458016] [DOI] [PubMed] [Google Scholar]
Leadbetter 2009a {published data only}
- Leadbetter G, Keyes LE, Maakestad KM, Olson S, Tissot van Patot MC, Hackett PH. Ginkgo biloba does‐‐and does not‐‐prevent acute mountain sickness. Wilderness & Environmental Medicine 2009;20(1):66‐71. [PUBMED: 19364166] [DOI] [PubMed] [Google Scholar]
Leadbetter 2009b {published data only}
- Leadbetter G, Keyes LE, Maakestad KM, Olson S, Tissot van Patot MC, Hackett PH. Ginkgo biloba does‐‐and does not‐‐prevent acute mountain sickness. Wilderness & Environmental Medicine 2009;20(1):66‐71. [PUBMED: 19364166] [DOI] [PubMed] [Google Scholar]
Moraga 2007 {published data only}
- Moraga FA, Flores A, Serra J, Esnaola C, Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollague (3696 m) in northern Chile. Wilderness & Environmental Medicine 2007;18(4):251‐7. [PUBMED: 18076292] [DOI] [PubMed] [Google Scholar]
Ren 2015 {published data only}
- Ren X, Zhang Q, Wang H, Man C, Hong H, Chen L, et al. Effect of intravenous iron supplementation on acute mountain sickness: a preliminary randomized controlled study. Medical Science Monitor 2015;21:2050‐7. [PUBMED: 26175087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roach 1983 {published data only}
- Roach RC, Larson EB, Hornbein TF, Houston CS, Bartlett S, Hardesty J, et al. Acute mountain sickness, antacids, and ventilation during rapid, active ascent of Mount Rainier. Aviation, Space, and Environmental Medicine 1983;54(5):397‐401. [PUBMED: 6347173] [PubMed] [Google Scholar]
Roncin 1996 {published data only}
- Roncin JP, Schwartz F, Darbigny P. Egb 761 in control of acute mountain sickness and vascular reactivity to cold exposure. Aviation, Space, and Environmental Medicine 1996;67(5):445‐52. [PUBMED: 8725471] [PubMed] [Google Scholar]
Schommer 2010 {published data only}
- Schommer K, Wiesegart N, Menold E, Haas U, Lahr K, Buhl H, et al. Training in normobaric hypoxia and its effects on acute mountain sickness after rapid ascent to 4559 m. High Altitude Medicine & Biology 2010;11(1):19‐25. [PUBMED: 20367484] [DOI] [PubMed] [Google Scholar]
Talbot 2011 {published data only}
- Talbot NP, Smith TG, Privat C, Nickol AH, Rivera‐Ch M, Leon‐Velarde F, et al. Intravenous iron supplementation may protect against acute mountain sickness: a randomized, double‐blinded, placebo‐controlled trial. High Altitude Medicine & Biology 2011;12(3):265‐9. [PUBMED: 21962070] [DOI] [PubMed] [Google Scholar]
Wright 2004a {published data only}
- Wright AD, Beazley MF, Bradwell AR, Chesner IM, Clayton RN, Forster PJ, et al. Medroxyprogesterone at high altitude. The effects on blood gases, cerebral regional oxygenation, and acute mountain sickness. Wilderness & Environmental Medicine 2004;15(1):25‐31. [PUBMED: 15040503] [DOI] [PubMed] [Google Scholar]
Wright 2004b {published data only}
- Wright AD, Beazley MF, Bradwell AR, Chesner IM, Clayton RN, Forster PJ, et al. Medroxyprogesterone at high altitude. The effects on blood gases, cerebral regional oxygenation, and acute mountain sickness. Wilderness & Environmental Medicine 2004;15(1):25‐31. [PUBMED: 15040503] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agostoni 2013 {published data only}
- Agostoni P, Swenson ER, Fumagalli R, Salvionia E, Cattadoria G, Farina S, et al. Acute high‐altitude exposure reduces lung diffusion: data from the HIGHCARE Alps project. Respiratory Physiology & Neurobiology 2013;188(2):223‐8. [PUBMED: 23619193] [DOI] [PubMed] [Google Scholar]
Baillie 2009 {published data only}
- Baillie JK, Thompson AA, Irving JB, Bates MG, Sutherland AI, Macnee W, et al. Oral antioxidant supplementation does not prevent acute mountain sickness: double blind, randomized placebo‐controlled trial. QJM : Monthly Journal of the Association of Physicians 2009;102(5):341‐8. [PUBMED: 19273551] [DOI] [PubMed] [Google Scholar]
Bartsch 1993 {published data only}
- Bartsch P, Merki B, Hofstetter D, Maggiorini M, Kayser B, Oelz O. Treatment of acute mountain‐sickness by simulated descent ‐ a randomized controlled trial. BMJ 1993;306(6885):1098‐101. [PUBMED: 8495155] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartsch 1994 {published data only}
- Bartsch P, Maggi S, Kleger GR, Ballmer PE, Baumgartner RW. Sumatriptan for high‐altitude headache. Lancet 1994;344(8934):1445. [PUBMED: 7968111] [DOI] [PubMed] [Google Scholar]
Bilo 2015 {published data only}
- Bilo G, Lombardi C, Revera M, Diazzi E, Giuliano A, Faini A, et al. Acetazolamide counteracts ambulatory blood pressure increase under acute exposure to high altitude. High Blood Pressure & Cardiovascular Prevention 2011;18(3):118. [EMBASE: 70547750] [Google Scholar]
- Bilo G, Villafuerte FC, Faini A, Anza‐Ramírez C, Revera M, Giuliano A, et al. Ambulatory blood pressure in untreated and treated hypertensive patients at high altitude: the High Altitude Cardiovascular Research‐Andes study. Hypertension 2015;65(6):1266‐72. [PUBMED: 25895588] [DOI] [PubMed] [Google Scholar]
- Bilo, G, Villafuerte F, Anza C, Revera, Giuliano A, Faini A, et al. Combined antihypertensive treatment and blood pressure responses to acute high altitude exposure in patients with hypertension. HIGHCARE‐ANDES Lowlanders Study. European Heart Journal. Conference: European Society of Cardiology, ESC Congress 2013. Amsterdam Netherlands. 2011;34(Suppl 1):269‐70. [EMBASE: 71258517] [Google Scholar]
Bloch 2009 {published data only}
- Bloch KE, Turk AJ, Maggiorini M, Hess T, Merz T, Bosch MM, et al. Effect of ascent protocol on acute mountain sickness and success at Muztagh Ata, 7546 m. High Altitude Medicine & Biology 2009;10(1):25‐32. [PUBMED: 19326598] [DOI] [PubMed] [Google Scholar]
Broome 1994 {published data only}
- Broome JR, Stoneham MD, Beeley JM, Milledge JS, Hughes AS. High altitude headache: treatment with ibuprofen. Aviation, Space, and Environmental Medicine 1994;65(1):19‐20. [PUBMED: 8117220] [PubMed] [Google Scholar]
Cain 1966 {published data only}
- Cain SM, Dunn JE. Low doses of acetazolamide to aid accommodation of men to altitude. Journal of Applied Physiology 1966;21(4):1195‐200. [PUBMED: 5916650] [DOI] [PubMed] [Google Scholar]
Debevec 2015 {published data only}
- Debevec T, Pialoux V, Saugy J, Schmitt L, Cejuela R, Mury P, et al. Prooxidant/Antioxidant balance in Hypoxia: a cross‐over study on normobaric vs. Hypobaric "live high‐train low". PLoS ONE 2015;10(9):e0137957. [PUBMED: 26368280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumont 1999 {published data only}
- Dumont L, Mardirosoff C, Soto‐Debeuf G, Tassonyi E. Magnesium and acute mountain sickness. Aviation, Space, and Environmental Medicine 1999;70(6):625. [PUBMED: 10373058] [PubMed] [Google Scholar]
Forster 1982 {published data only}
- Forster P. Methazolamide in acute mountain sickness. Lancet 1982;1(8283):1254. [PUBMED: 6123014] [DOI] [PubMed] [Google Scholar]
Forwand 1968 {published data only}
- Forwand SA, Landowne M, Follansbee JN, Hansen JE. Effect of acetazolamide on acute mountain sickness. New England Journal of Medicine 1968;279(16):839‐45. [PUBMED: 4877992] [DOI] [PubMed] [Google Scholar]
Fulco 2011 {published data only}
- Fulco CS, Muza SR, Beidleman BA, Demes R, Staab JE, Jones JE, et al. Effect of repeated normobaric hypoxia exposures during sleep on acute mountain sickness, exercise performance, and sleep during exposure to terrestrial altitude. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 2011;300(2):R428‐36. [PUBMED: 21123763] [DOI] [PubMed] [Google Scholar]
Gertsch 2002 {published data only}
- Gertsch JH, Seto TB, Mor J, Onopa J. Ginkgo biloba for the prevention of severe acute mountain sickness (AMS) starting one day before rapid ascent. High Altitude Medicine & Biology 2002;3(1):29‐37. [PUBMED: 12006162] [DOI] [PubMed] [Google Scholar]
Gray 1971 {published data only}
- Gray GW, Bryan AC, Frayser R, Houston CS, Rennie ID. Control of acute mountain sickness. Aerospace Medicine 1971;42(1):81‐4. [PUBMED: 4925130] [PubMed] [Google Scholar]
Harris 2003 {published data only}
- Harris NS, Wenzel RP, Thomas SH. High altitude headache: efficacy of acetaminophen vs. ibuprofen in a randomized, controlled trial. Journal of Emergency Medicine 2003;24(4):383‐7. [PUBMED: 12745039] [DOI] [PubMed] [Google Scholar]
Johnson 1988 {published data only}
- Johnson TS, Rock PB, Young JB, Fulco CS, Trad LA. Hemodynamic and sympathoadrenal responses to altitude in humans: effect of dexamethasone. Aviation, Space, and Environmental Medicine 1988;59(3):208‐12. [PUBMED: 3355474] [PubMed] [Google Scholar]
Jonk 2007 {published data only}
- Jonk AM, Berg IP, Olfert IM, Wray DW, Arai T, Hopkins SR, et al. Effect of acetazolamide on pulmonary and muscle gas exchange during normoxic and hypoxic exercise. Journal of Physiology 2007;579(Pt 3):909‐21. [PUBMED: 17218362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayser 1993 {published data only}
- Kayser B, Jean D, Herry JP, Bartsch P. Pressurization and acute mountain sickness. Aviation, Space, and Environmental Medicine 1993;64(10):928‐31. [PUBMED: 8240197] [PubMed] [Google Scholar]
Kotwal 2015 {published data only}
- Kotwal J, Kotwal A, Bhalla S, Singh PK, Nair V, Author A, et al. Effectiveness of homocysteine lowering vitamins in prevention of thrombotic tendency at high altitude area: a randomized field trial. Thrombosis Research 2015;136(4):758‐62. [PUBMED: 26319423] [DOI] [PubMed] [Google Scholar]
Lalande 2009 {published data only}
- Lalande S, Snyder EM, Olson TP, Hulsebus ML, Orban M, Somers VK, et al. The effects of sildenafil and acetazolamide on breathing efficiency and ventilatory control during hypoxic exercise. European Journal of Applied Physiology 2009;106(4):509‐15. [PUBMED: 19337745] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawley 2012 {published data only}
- Lawley JS, Oliver SJ, Mullins P, Morris D, Junglee NA, Jelleyman C, et al. Optic nerve sheath diameter is not related to high altitude headache: a randomized controlled trial. High Altitude Medicine & Biology 2012;13(3):193‐9. [PUBMED: 22994519] [DOI] [PubMed] [Google Scholar]
Levine 1989 {published data only}
- Levine BD, Yoshimura K, Kobayashi T, Fukushima M, Shibamoto T, Ueda G. Dexamethasone in the treatment of acute mountain sickness. New England Journal of Medicine 1989;321(25):1707‐13. [PUBMED: 2687688] [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu C, Croft QP, Kalidhar S, Brooks JT, Herigstad M, Smith TG, et al. Dexamethasone mimics aspects of physiological acclimatization to 8 hours of hypoxia but suppresses plasma erythropoietin. Journal of Applied Physiology 2013;114(7):948‐56. [PUBMED: 23393065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mairer 2012 {published data only}
- Mairer K, Gobel M, Defrancesco M, Wille M, Messner H, Loizides A, et al. MRI evidence: acute mountain sickness is not associated with cerebral edema formation during simulated high altitude. PloS One 2012;7(11):e50334. [PUBMED: 23226263] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 1986 {published data only}
- McIntosh IB, Prescott RJ. Acetazolamide in prevention of acute mountain sickness. Journal of International Medical Research 1986;14(5):285‐7. [PUBMED: 3533677] [DOI] [PubMed] [Google Scholar]
Modesti 2006 {published data only}
- Modesti PA, Vanni S, Morabito M, Modesti A, Marchetta M, Gamberi T, et al. Role of endothelin‐1 in exposure to high altitude: acute mountain sickness and endothelin‐1 (ACME‐1) study. Circulation 2006;114(13):1410‐6. [PUBMED: 16982943] [DOI] [PubMed] [Google Scholar]
Purkayastha 1995 {published data only}
- Purkayastha SS, Ray US, Arora BS, Chhabra PC, Thakur L, Bandopadhyay P, et al. Acclimatization at high altitude in gradual and acute induction. Journal of Applied Physiology 1995;79(2):487‐92. [PUBMED: 7592207] [DOI] [PubMed] [Google Scholar]
Reinhart 1994 {published data only}
- Reinhart WH, Goerre S, Bartsch P. Acetazolamide reduces the erythropoietin response to hypoxia at high altitude in humans. Journal of Wilderness Medicine 1994;5(3):312‐7. [EMBASE: 24320364] [Google Scholar]
Sandoval 2000 {published data only}
- Sandoval M, Silva J, Parra M, Cifuentes F, Maluenda R, Escobar J. Effects of consumption of antioxidants in altitude exposure [Efectos del consumo de antioxidantes en exposición a altitud]. Boletin Cientifico de la Asociación Chilena de Seguridad 2000;2(3):67‐73. [lil‐318088] [Google Scholar]
Savourey 1998 {published data only}
- Savourey G, Caterini R, Launay JC, Guinet A, Besnard Y, Hanniquet AM, et al. Positive end expiratory pressure as a method for preventing acute mountain sickness. European Journal of Applied Physiology and Occupational Physiology 1998;77(1‐2):32‐6. [PUBMED: 9459518] [DOI] [PubMed] [Google Scholar]
Scalzo 2015 {published data only}
- Scalzo RL, Binns SE, Klochak AL, Giordano GR, Paris HLR, Sevits KJ, et al. Methazolamide plus aminophylline abrogates hypoxia‐mediated endurance exercise impairment. High Altitude Medicine & Biology 2015;16(4):331‐42. [PUBMED: 26680684] [DOI] [PubMed] [Google Scholar]
Serra 2001 {published data only}
- Serra J. Chachacoma: An alternative treatment for the prevention of acute mountain sickness? [Chachacoma ¿un tratamiento alternativo para la prevención del mal agudo de montaña?]. Revista de Ciencias de la Salud 2001;5(1):36‐42. [lil‐498122] [Google Scholar]
Siebenmann 2011 {published data only}
- Siebenmann C, Bloch KE, Lundby C, Nussbamer‐Ochsner Y, Schoeb M, Maggiorini M. Dexamethasone improves maximal exercise capacity of individuals susceptible to high altitude pulmonary edema at 4559 m. High Altitude Medicine & Biology 2011;12(2):169‐77. [PUBMED: 21718165] [DOI] [PubMed] [Google Scholar]
Silva‐Urra 2011 {published data only}
- Silva‐Urra JA, Urizar C, Basualto‐Alarcon C, Torrella JR, Pages T, Behn C, et al. Effects of oxygen supplementation on acute mountain sickness symptoms and functional capacity during a 2‐kilometer walk test on Chajnantor plateau (5050 meters, Northern Chile). Wilderness & Environmental Medicine 2011;22(3):250‐6. [PUBMED: 21962052] [DOI] [PubMed] [Google Scholar]
Singh 1969 {published data only}
- Singh I, Khanna PK, Srivastava MC, Lal M, Roy SB, Subramanyam CS. Acute mountain sickness. New England Journal of Medicine 1969;280(4):175‐84. [PUBMED: 5782719] [DOI] [PubMed] [Google Scholar]
Solís 1984 {published data only}
- Solís JV. Acetazolamide: Effects on the "acute mountain sickness", aggravated by physical activity. Boletín de la Fundación Jiménez Díaz 1984;11(3):117‐20. [Google Scholar]
Suh 2015 {published data only}
- Suh KS, Kim T, Yi NJ, Hong G. Preparation for high altitude expedition and changes in cardiopulmonary and biochemical laboratory parameters with ascent to high altitude in transplant patients and live donors. Clinical Transplantation 2015;29(11):1013‐20. [PUBMED: 26331794] [DOI] [PubMed] [Google Scholar]
Teppema 2007 {published data only}
- Teppema LJ, Balanos GM, Steinback CD, Brown AD, Foster GE, Duff HJ, et al. Effects of acetazolamide on ventilatory, cerebrovascular, and pulmonary vascular responses to hypoxia. American Journal of Respiratory and Critical Care Medicine 2007;175(3):277‐81. [PUBMED: 17095745] [DOI] [PubMed] [Google Scholar]
Vuyk 2006 {published data only}
- Vuyk J, Bos J, Terhell K, Bos R, Vletter A, Valk P, et al. Acetazolamide improves cerebral oxygenation during exercise at high altitude. High Altitude Medicine & Biology 2006;7(4):290‐301. [PUBMED: 17173514] [DOI] [PubMed] [Google Scholar]
White 1984 {published data only}
- White AJ. Cognitive impairment of acute mountain sickness and acetazolamide. Aviation, Space, and Environmental Medicine 1984;55(7):598‐603. [PUBMED: 6466255] [PubMed] [Google Scholar]
Wright 1988 {published data only}
- Wright AD, Bradwell AR, Jensen J, Lassen N. Cerebral blood flow in acute mountain sickness and treatment with acetazolamide. Clinical Science 1988;74 Suppl 18:1P. [DOI: 10.1042/cs074001P] [DOI] [Google Scholar]
References to studies awaiting assessment
Burns 2018 {published data only}
- Burns P, Lipman G S, Warner K, Jurkiewicz C, Phillips C, Sanders L, et al. Altitude sickness prevention with ibuprofen relative to acetazolamide. American Journal of Medicine 2018;18:S0002‐9343. [PUBMED: 30419226] [DOI] [PubMed] [Google Scholar]
Dugas 1995 {published data only}
- Dugas L, Dubray C, Herry JP, Olsen NV, Court‐Payen M, Hansen JM, et al. Cardiovascular effects of a calcium channel blocker in hypoxia caused by altitude [Effets cardiovasculaires d'un bloqueur calcique en hypoxie d'altitude.]. Presse Medicale 1995;24(16):763‐8. [PUBMED: 7784415] [PubMed] [Google Scholar]
Ellsworth 1987 {published data only}
- Ellsworth AJ, Larson EB, Strickland D. A randomized trial of dexamethasone and acetazolamide for acute mountain sickness prophylaxis. American Journal of Medicine 1987;83(6):1024‐30. [PUBMED: 3332564] [DOI] [PubMed] [Google Scholar]
Furian 2018 {published data only}
- Furian M, Lichtblau M, Aeschbacher S S, Estebesova B, Emilov B, Sheraliev U, et al. Efficacy of dexamethasone in preventing acute mountain sickness in COPD patients: randomized trial. Chest 2018;154(4):788‐97. [PUBMED: 29909285] [DOI] [PubMed] [Google Scholar]
Hefti 2014 {published data only}
- Hefti JP, Stutz M, Geiser T, Hefti U, Merz T, Huber A. Effect of antioxidant supplements on AMS and endothelial function during a high altitude expedition: a prospective randomized double‐blind trial. High Altitude Medicine & Biology 2014;15(2):A262. [EMBASE: 71529135] [Google Scholar]
Kanaan 2017 {published data only}
- Kanaan NC, Peterson AL, Pun M, Holck PS, Starling J, Basyal B, et al. Prophylactic acetaminophen or ibuprofen results in equivalent acute mountain sickness incidence at high altitude: a prospective randomized trial. Wilderness & Environmental Medicine 2017;28(2):72‐78. [PUBMED: 28479001] [DOI] [PubMed] [Google Scholar]
Kasic 1991 {published data only}
- Kasic JF, Yaron M, Nicholas RA, Lickteig JA, Roach R. Treatment of acute mountain‐sickness ‐ hyperbaric versus oxygen‐therapy. Annals of Emergency Medicine 1991;20(10):1109‐12. [EMBASE: 21312027] [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee JH, Choi PC. Comparison of methazolamide and acetazolamide for prevention of acute mountain sickness in adolescents. Journal of the Korean Society of Emergency Medicine 2011;22(5):523‐30. [Google Scholar]
Lipman 2018 {published data only}
- Lipman GS, Pomeranz D, Burns P, Phillips C, Cheffers M, Evans K, et al. Budesonide versus acetazolamide for prevention of acute mountain sickness. American Journal of Medicine 2018;131(2):200.e9‐16. [PUBMED: 28668540] [DOI] [PubMed] [Google Scholar]
Menz 2018 {published data only}
- Menz V, Niebauer J, Burtscher M. Normobaric hypoxia‐induced responses in heart rate and blood pressure correlate with symptoms of acute mountain sickness. Wiener Klinische Wochenschrift 2018;130((2 Supplement 1)):S91. [Google Scholar]
Pun 2014 {published data only}
- Pun M, Neupane M, Lohani A, Thapa GB, Yadav S, Holck PS, et al. Role of low‐dose acetazolamide (125 mg BID) in prevention of acute mountain sickness in pilgrims ascending rapidly: a prospective double‐blind placebo controlled randomized trial. High Altitude Medicine & Biology 2014;15(2):A233. [Google Scholar]
Swenson 1997 {published data only}
- Swenson ER, Macdonald A, Vatheuer M, Maks C, Treadwell A, Allen R, et al. Acute mountain sickness is not altered by a high carbohydrate diet nor associated with elevated circulating cytokines. Aviation, Space, and Environmental Medicine 1997;68(6):499‐503. [PUBMED: 9184737] [PubMed] [Google Scholar]
Utz 1970 {published data only}
- Utz G, Schlierf G, Barth P, Linhart P, Wollenweber J. Prevention of acute mountain sickness using acetazolamide [Prophylaxe der akuten Hohenkrankheit mit Acetazolamid]. Munchener Medizinische Wochenschrift (1950) 1970;112(23):1122‐4. [PUBMED: 5467747] [PubMed] [Google Scholar]
Wang 1998 {published data only}
- Wang W, Zhang X, Ma Y. Low‐concentration nitrous oxide inhalation in the treatment of high‐altitude pulmonary edema. Chinese Journal of Tuberculosis and Respiratory Diseases 1998;21:212‐4. [PubMed] [Google Scholar]
Warner 2018 {published data only}
- Warner K, Burns P, Lipman G, Jurkiewicz C, Sanders L, Phillips C, et al. Acute mountain sickness prevention with ibuprofen relative to acetazolamide. Academic Emergency Medicine 2018;25(Supplement 1):S90. [DOI] [PubMed] [Google Scholar]
Xiangjun 2014 {published data only}
- Xiangjun L, Lan H. Inhaled budesonide for the prevention of acute mountain sickness in unacclimatization young men: a double‐blind randomized controlled trial. Journal of the American College of Cardiology 2014;64(16 SUPPL. 1):C69. [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐13003319 {published data only}
- ChiCTR‐TRC‐13003319. Oral zolpidem for improving sleep and then prevention of acute mountain sickness: a single center, randomized, double‐blind, controlled, prospective trial [Oral zolpidem for Improving Sleep and then prevention of acute mountain sickness]. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐13003319 (first received 29 June 2013).
ChiCTR‐TRC‐13003590 {published data only}
- ChiCTR‐TRC‐13003590. The meaning of intravenous iron supplementation In acute mountain sickness: a randomized, double‐blinded, placebo‐controlled trials. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐13003590 (first received 23 June 2013).
NCT00886912 {published data only}
- NCT00886912. Training in hypoxia to prevent acute mountain sickness [Prevention of acute mountain sickness (AMS) by intermittent hypoxic training]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT00886912 (first received 21 April 2009).
NCT01606527 {published data only}
- NCT01606527. NSAID RCT for prevention of altitude sickness [Prospective, double‐blind, randomized, placebo‐controlled trial of ibuprofen versus placebo for prevention of neurologic forms of altitude sickness]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01606527 (first received 13 April 2012).
NCT01682551 {published data only}
- NCT01682551. Evaluation of the prevention and treatment effects of chinese medicine on high altitude illness. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01682551 (first received 5 September 2012).
NCT01794078 {published data only}
- NCT01794078. A study to test the safety of combined dosing with aminophylline and ambrisentan in exercising healthy human volunteers at simulated high altitude GQ02 [A randomized, 4‐sequence, double‐blind study to test the safety of combined dosing with aminophylline and ambrisentan in exercising healthy human volunteers at simulated high altitude]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01794078 (first received 10 February 2013).
NCT01993667 {published data only}
- NCT01993667. Acetazolamide for the prevention of high altitude illness: a comparison of dosing. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01993667 (first received 12 November 2013).
NCT02244437 {published data only}
- NCT02244437. Ibuprofen vs acetaminophen for AMS prevention [Ibuprofen vs acetaminophen in the prevention of acute mountain sickness: a double blind, randomized controlled trial]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02244437 (first received 14 September 2014).
NCT02450968 {published data only}
- NCT02450968. Altitude related illness in patients with respiratory disease [Dexamethasone for prophylaxis of acute mountain sickness in patients with chronic obstructive pulmonary disease travelling to altitude]. clinicaltrials.gov/ct2/show/NCT02450968 (first received 21 May 2015).
NCT02811016 {published data only}
- NCT02811016. Inhaled budesonide and acute mountain sickness [Effect of inhaled budesonide on the incidence and severity of acute mountain sickness at 4559 m]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02811016 (first received 14 June 2016).
NCT02941510 {published data only}
- NCT02941510. Inhaled budesonide for altitude illness prevention [Inhaled budesonide for altitude illness prevention]. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT02941510 (first received 19 October 2016).
NCT03424226 {published data only}
- NCT03424226. Sickness evaluation at altitude with acetazolamide at relative dosages [Sickness evaluation at altitude with acetazolamide at relative dosages]. clinicaltrials.gov/ct2/show/NCT03424226?term=nct03424226&rank=1 (first received 6 February 2018).
NCT03552263 {published data only}
- NCT03552263. Safety and efficacy of T89 in prevention and treatment of adults with acute mountain sickness (AMS) [A double‐blind, randomized and placebo‐controlled phase 2 study to evaluate the safety and efficacy of T89 in prevention and treatment of acute mountain sickness (AMS) during rapid ascent]. clinicaltrials.gov/show/nct03552263 (first received 11 June 2018).
NCT03561675 {published data only}
- NCT03561675. Effect of acetazolamide on acute mountain sickness in lowlanders older than 40 years [Acetazolamide for prevention of acute mountain sickness in healthy lowlanders older than 40 years. Randomized trial]. clinicaltrials.gov/show/nct03561675 (first received 19 June 2018).
Additional references
Adams 2004
- Adams J. Ginkgo biloba and acetazolamide for acute mountain sickness: exclusion of high risk, low status groups perpetuates discrimination and inequalities. BMJ 2004;329(7458):171. [PUBMED: 15258082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1892
- Anonymous. Mountain sickness. British Medical Journal 1892;1(1633):829. [PUBMED: 20753647] [PMC free article] [PubMed] [Google Scholar]
Bailey 2009a
- Bailey DM, Bärtsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high‐altitude cerebral edema: from the molecular to the morphological. Cellular and Molecular Life Sciences 2009;66(22):3583‐94. [PUBMED: 19763397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bailey 2009b
- Bailey DM, Taudorf S, Berg RM, Lundby C, McEneny J, Young IS, et al. Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness?. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 2009;297(5):1283‐92. [PUBMED: 19726713] [DOI] [PubMed] [Google Scholar]
Bartsch 2013
Basnyat 2003
- Basnyat B, Murdoch DR. High‐altitude illness. Lancet 2003;361(9373):1967‐74. [PUBMED: 12801752] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Bärtsch 1992
- Bärtsch P. Treatment of high altitude diseases without drugs. International Journal of Sports Medicine 1992;13 Suppl 1:71‐4. [PUBMED: 1483799] [DOI] [PubMed] [Google Scholar]
Bärtsch 2004
- Bärtsch P, Bailey DM, Berger MM, Knauth M, Baumgartner RW. Acute mountain sickness: controversies and advances. High Altitude Medicine and Biology 2004;5(2):110‐24. [PUBMED: 15265333] [DOI] [PubMed] [Google Scholar]
Bärtsch 2007
- Bärtsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation 2007;116(19):2191‐202. [PUBMED: 17984389] [DOI] [PubMed] [Google Scholar]
Bärtsch 2008
- Bärtsch P, Saltin B. General introduction to altitude adaptation and mountain sickness. Scandinavian Journal of Medicine & Science in Sports 2008;18 Suppl 1:1‐10. [PUBMED: 18665947] [DOI] [PubMed] [Google Scholar]
CATMAT 2007
- Committee to Advise on Tropical Medicine and Travel (CATMAT). Statement on high‐altitude illnesses. An Advisory Committee Statement (ACS). Canada Communicable Disease Report = Relevé des maladies transmissibles au Canada 2007;33(ACS‐5):1‐20. [PUBMED: 17520777] [PubMed] [Google Scholar]
Davis 2017
- Davis C, Hackett P. Advances in the prevention and treatment of high altitude illness. Emergency Medicine Clinics of North America 2017;35(2):241‐60. [PUBMED: 28411926] [DOI] [PubMed] [Google Scholar]
Dehnert 2010
- Dehnert C, Bartsch P. Can patients with coronary heart disease go to high altitude?. High Altitude Medicine & Biology 2010;11(3):183‐8. [PUBMED: 20919884] [DOI] [PubMed] [Google Scholar]
Dumont 2000
- Dumont L, Mardirosoff C, Tramèr MR. Efficacy and harm of pharmacological prevention of acute mountain sickness: quantitative systematic review. BMJ 2000;321(7256):267‐72. [PUBMED: 10915127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Elphick 2004
- Elphick HL, Elphick DA. Ginkgo biloba and acetazolamide for acute mountain sickness: bias in participants may underestimate effectiveness of agents. BMJ 2004;329(7458):172. [PUBMED: 15258084 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flaherty 2016
- Flaherty GT, Kennedy KM. Preparing patients for travel to high altitude: advice on travel health and chemoprophylaxis. British Journal of General Practice : the Journal of the Royal College of General Practitioners 2016;66(642):e62‐4. [PUBMED: 26719484] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzalez 2018
- Gonzalez Garay A, Molano Franco D, Nieto Estrada VH, Marti‐Carvajal AJ, Arevalo‐Rodriguez I. Interventions for preventing high altitude illness: Part 2. Less commonly‐used drugs. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012983; PUBMED: 29529715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Hackett 2004
- Hackett PH, Roach RC. High altitude cerebral edema. High Altitude Medicine & Biology 2004;5(2):136‐46. [PUBMED: 15265335] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2016
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version 1.05, 2016.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imray 2010
- Imray C, Wright A, Subudhi A, Roach R. Acute mountain sickness: pathophysiology, prevention, and treatment. Progress in Cardiovascular Diseases 2010;52(6):467‐84. [PUBMED: 20417340] [DOI] [PubMed] [Google Scholar]
Kallenberg 2007
- Kallenberg K, Bailey DM, Christ S, Mohr A, Roukens R, Menold E, et al. Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. Journal of Cerebral Blood Flow and Metabolism 2007;27(5):1064‐71. [PUBMED: 17024110] [DOI] [PubMed] [Google Scholar]
Kayser 2012
- Kayser B, Dumont L, Lysakowski C, Combescure C, Haller G, Tramer MR. Reappraisal of acetazolamide for the prevention of acute mountain sickness: a systematic review and meta‐analysis. High Altitude Medicine & Biology 2012;13(2):82‐92. [PUBMED: 22724610] [DOI] [PubMed] [Google Scholar]
Khodaee 2016
- Khodaee M, Grothe HL, Seyfert JH, VanBaak K. Athletes at high altitude. Sports Health 2016;8(2):126‐32. [PUBMED: 26863894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleijnen 1992
- Kleijnen J, Knipschild P. Ginkgo biloba. Lancet (London, England) 1992;340(8828):1136‐9. [PUBMED: 1359218] [DOI] [PubMed] [Google Scholar]
Kryger 1978
- Kryger M, McCullough RE, Collins D, Scoggin CH, Weil JV, Grover RF. Treatment of excessive polycythemia of high altitude with respiratory stimulant drugs. American Review of Respiratory Disease 1978;117(3):455‐64. [PUBMED: 629480] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, Demets D. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63. [DOI: 10.1093/biomet/70.3.659] [DOI] [Google Scholar]
Lee 2013
- Lee SY, Shi LS, Chu H, Li MH, Ho CW, Lai FY, et al. Rhodiola crenulata and its bioactive components, salidroside and tyrosol, reverse the hypoxia‐induced reduction of plasma‐membrane‐associated Na,K‐ATPase expression via inhibition of ROS‐AMPK‐PKC xi pathway. Available at www.hindawi.com/journals/ecam/2013/284150. [PUBMED: 23840253] [DOI] [PMC free article] [PubMed]
Leissner 2009
- Leissner KB, Mahmood FU. Physiology and pathophysiology at high altitude: considerations for the anesthesiologist. Journal of Anesthesia 2009;23(4):543‐53. [PUBMED: 19921365 ] [DOI] [PubMed] [Google Scholar]
Low 2012
- Low EV, Avery AJ, Gupta V, Schedlbauer A, Grocott MP. Identifying the lowest effective dose of acetazolamide for the prophylaxis of acute mountain sickness: systematic review and meta‐analysis. BMJ (Clinical Research Ed.) 2012;345:e6779. [PUBMED: 23081689] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luks 2008a
- Luks AM. Do we have a "best practice" for treating high altitude pulmonary edema?. High Altitude Medicine & Biology 2008;9(2):111‐4. [PUBMED: 18578641] [DOI] [PubMed] [Google Scholar]
Luks 2008b
- Luks AM, Swenson ER. Medication and dosage considerations in the prophylaxis and treatment of high‐altitude illness. Chest 2008;133(3):744‐55. [PUBMED: 18321903] [DOI] [PubMed] [Google Scholar]
Luks 2010
- Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, et al. Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness. Wilderness & Environmental Medicine 2010;21(2):146‐55. [PUBMED: 20591379] [DOI] [PubMed] [Google Scholar]
Luks 2014
- Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness: 2014 update. Wilderness and Environmental Medicine 2014;25(4 Suppl):S4‐14. [PUBMED: 25498261] [DOI] [PubMed] [Google Scholar]
Luks 2017
- Luks AM, Swenson ER, Bartsch P. Acute high‐altitude sickness. European Respiratory Review : an Official Journal of the European Respiratory Society 2017;26(143):160096. [PUBMED: 28143879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maggiorini 2010
- Maggiorini M. Prevention and treatment of high‐altitude pulmonary edema. Progress in Cardiovascular Diseases 2010;52(6):500‐6. [PUBMED: 20417343] [DOI] [PubMed] [Google Scholar]
Milledge 1985
- Milledge JS, Cotes PM. Serum erythropoietin in humans at high altitude and its relation to plasma renin. Journal of Applied Physiology (Bethesda, Md. : 1985) 1985;59(2):360‐4. [PUBMED: 3897179] [DOI] [PubMed] [Google Scholar]
Monge 1942
- Monge C. Life in the Andes and chronic mountain sickness. Science 1942;95(2456):79‐84. [PUBMED: 17757318] [DOI] [PubMed] [Google Scholar]
Murdoch 1995
- Murdoch DR. Altitude illness among tourists flying to 3740 meters elevation in the Nepal Himalayas. Journal of Travel Medicine 1995;2(4):255‐6. [PUBMED: 9815403] [DOI] [PubMed] [Google Scholar]
Naeije 2010
- Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Progress in Cardiovascular Diseases 2010;52(6):456‐66. [PUBMED: 20417339] [DOI] [PubMed] [Google Scholar]
Nieto 2017
- Nieto Estrada VH, Molano Franco D, Medina RD, Gonzalez Garay AG, Martí‐Carvajal AJ, Arevalo‐Rodriguez I. Interventions for preventing high altitude illness: Part 1. Commonly‐used classes of drugs. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD009761.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2010
- Palmer BF. Physiology and pathophysiology with ascent to altitude. American Journal of the Medical Sciences 2010;340(1):69‐77. [PUBMED: 20442648] [DOI] [PubMed] [Google Scholar]
Paralikar 2010
- Paralikar SJ, Paralikar JH. High‐altitude medicine. Indian Journal of Occupational and Environmental Medicine 2010;14(1):6‐12. [PUBMED: 20808661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasha 2010
- Pasha MA, Newman JH. High‐altitude disorders: pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest 2010;137(6 Suppl):13S‐19S. [PUBMED: 20522576] [DOI] [PubMed] [Google Scholar]
Perez‐Pinzon 1997
- Perez‐Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism 1997;17(2):175‐82. [PUBMED: 9040497] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ri‐Li 2003
- Ri‐Li G, Chase PJ, Witkowski S, Wyrick BL, Stone JA, Levine BD, et al. Obesity: associations with acute mountain sickness. Annals of Internal Medicine 2003;139(4):253‐7. [PUBMED: 12965980] [DOI] [PubMed] [Google Scholar]
Ritchie 2012
- Ritchie ND, Baggott AV, Andrew Todd WT. Acetazolamide for the prevention of acute mountain sickness‐‐a systematic review and meta‐analysis. Journal of Travel Medicine 2012;19(5):298‐307. [PUBMED: 22943270] [DOI] [PubMed] [Google Scholar]
Rodríguez de Romo 2002
- Rodríguez de Romo AC. Daniel Vergara Lope and Carlos Monge Medrano: two pioneers of high altitude medicine. High Altitude Medicine & Biology 2002;3(3):299‐309. [PUBMED: 12396886] [DOI] [PubMed] [Google Scholar]
Scherrer 2010
- Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. New insights in the pathogenesis of high‐altitude pulmonary edema. Progress in Cardiovascular Diseases 2010;52(6):485‐92. [PUBMED: 20417341] [DOI] [PubMed] [Google Scholar]
Schneider 2002
- Schneider M, Bernasch D, Weymann J, Holle R, Bartsch P. Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Medicine and Science in Sports and Exercise 2002;34(12):1886‐91. [PUBMED: 12471292] [DOI] [PubMed] [Google Scholar]
Schoene 2004
- Schoene RB. Unraveling the mechanism of high altitude pulmonary edema. High Altitude Medicine & Biology 2004;5(2):125‐35. [PUBMED: 15265334] [DOI] [PubMed] [Google Scholar]
Schoene 2008
- Schoene RB. Illnesses at high altitude. Chest 2008;134(2):402‐16. [PUBMED: 18682459] [DOI] [PubMed] [Google Scholar]
Schoonman 2008
- Schoonman GG, Sandor PS, Nirkko AC, Lange T, Jaermann T, Dydak U, et al. Hypoxia‐induced acute mountain sickness is associated with intracellular cerebral edema: a 3 T magnetic resonance imaging study. Journal of Cerebral Blood Flow and Metabolism 2008;28(1):198‐206. [PUBMED: 17519973] [DOI] [PubMed] [Google Scholar]
Seupaul 2012
- Seupaul RA, Welch JL, Malka ST, Emmett TW. Pharmacologic prophylaxis for acute mountain sickness: a systematic shortcut review. Annals of Emergency Medicine 2012;59(4):307‐17.e1. [PUBMED: 22153998] [DOI] [PubMed] [Google Scholar]
Simancas‐Racines 2018
- Simancas‐Racines D, Arevalo‐Rodriguez I, Osorio D, Franco JV, Xu Y, Hidalgo R. Interventions for treating acute high altitude illness. Cochrane Database of Systematic Reviews 2018, Issue 6. [DOI: 10.1002/14651858.CD009567.pub2; PUBMED: 29959871] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732‐4. [PUBMED: 20026595] [DOI] [PubMed] [Google Scholar]
Stream 2008
- Stream JO, Grissom CK. Update on high‐altitude pulmonary edema: pathogenesis, prevention, and treatment. Wilderness & Environmental Medicine 2008;19(4):293‐303. [PUBMED: 19099331] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PUBMED: 25524443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [PUBMED: 28264661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2009
- Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurology 2009;8(2):175‐91. [PUBMED: 19161909] [DOI] [PubMed] [Google Scholar]
Windsor 2009
- Windsor JS, Firth PG, Grocott MP, Rodway GW, Montgomery HE. Mountain mortality: a review of deaths that occur during recreational activities in the mountains. Postgraduate Medical Journal 2009;85(1004):316‐21. [PUBMED: 19528307] [DOI] [PubMed] [Google Scholar]
Wright 2008
- Wright A, Brearey S, Imray C. High hopes at high altitudes: pharmacotherapy for acute mountain sickness and high‐altitude cerebral and pulmonary oedema. Expert Opinion on Pharmacotherapy 2008;9(1):119‐27. [PUBMED: 18076343] [DOI] [PubMed] [Google Scholar]
Zafren 2014
- Zafren K. Prevention of high altitude illness. Travel Medicine and Infectious Disease 2014;12(1):29‐39. [PUBMED: 24393671] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martí‐Carvajal 2012
- Martí‐Carvajal AJ, Hidalgo R, Simancas‐Racines D. Interventions for preventing high altitude illness. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009761] [DOI] [Google Scholar]


