Abstract
Type 1 diabetes studies consistently generate data showing islet β-cell dysfunction and T cell–mediated anti-β-cell–specific autoimmunity. To explore the pathogenesis, we interrogated the β-cell transcriptomes from donors with and without type 1 diabetes using both bulk-sorted and single β-cells. Consistent with immunohistological studies, β-cells from donors with type 1 diabetes displayed increased Class I transcripts and associated mRNA species. These β-cells also expressed mRNA for Class II and Class II antigen presentation pathway components, but lacked the macrophage marker CD68. Immunohistological study of three independent cohorts of donors with recent-onset type 1 diabetes showed Class II protein and its transcriptional regulator Class II MHC trans-activator protein expressed by a subset of insulin+CD68− β-cells, specifically found in islets with lymphocytic infiltrates. β-Cell surface expression of HLA Class II was detected on a portion of CD45−insulin+ β-cells from donors with type 1 diabetes by immunofluorescence and flow cytometry. Our data demonstrate that pancreatic β-cells from donors with type 1 diabetes express Class II molecules on selected cells with other key genes in those pathways and inflammation-associated genes. β-Cell expression of Class II molecules suggests that β-cells may interact directly with islet-infiltrating CD4+ T cells and may play an immunopathogenic role.
Introduction
The immune system plays a critical role in human type 1 diabetes pathogenesis. Varying proportions of T-cell subsets (CD8+ and CD4+) and B cells infiltrate the pancreatic islets (1) and target β-cells by recognizing type 1 diabetes–associated autoantigens (2,3). The immunological mechanisms recruiting these cells to the islets had remained incompletely understood because, until recently, islets from donors with type 1 diabetes were not available for study. Antigen presentation to T cells is mediated by antigen-presenting cells (APCs) via two classes of HLA molecules: HLA Class I, recognized by CD8+-expressing T cells (Class I is present on nearly all nucleated cells), and HLA Class II, recognized by CD4+-expressing T cells. Class II has a more limited tissue expression pattern, being present under most circumstances only on “professional” APCs, such as dendritic cells and macrophages (4).
Immunohistochemical studies of pancreas samples from donors with type 1 diabetes obtained at autopsy or by surgical biopsy (2,5–9) have consistently reported islet cell “hyperexpression” of Class I molecules relative to that observed in nondiabetic donor pancreas sections. Even so, it was surprising when Bottazzo et al. (10), Foulis and Farquharson (11), and others suggested that some β-cells from patients with type 1 diabetes also expressed HLA-DR Class II molecules (11–16). This pattern of expression by β-cells was termed “aberrant” as its expression is absent from β-cells in persons without type 1 diabetes. These reports raised the intriguing possibility that in individuals with type 1 diabetes, β-cell HLA Class II expression might allow direct presentation of autoantigens to infiltrating CD4+ T cells (17).
Several questions have been raised about these observations, not least because the identity of the islet cell subtypes affected was equivocal (18,19). Some suggested that the cellular colocalization of insulin and Class II molecules might reflect β-cells phagocytosed by scavenger immune cells (e.g., macrophages). Moreover, the functional significance of any expressed Class II molecule was questioned since β-cells may not have the capacity to process and load these molecules with antigens. The immunological consequences of β-cell Class II molecule expression have been debated (20,21) and remain unclear (22).
Using isolated human islets from donors with and without type 1 diabetes, we performed RNA sequencing (RNA-Seq) of bulk-sorted cells, single-cell RNA-Seq of islet cells, immunostaining of pancreatic islet sections, and flow cytometry of dispersed islets to examine the β-cell–specific expression of Class I and II molecules and associated regulatory genes. We now confirm that a subset of β-cells from donors with type 1 diabetes express Class II mRNA and cell surface protein and coexpresses Class II MHC trans-activator (CIITA), many other genes in the Class II pathway, and inflammation-associated gene products. The results indicate that β-cells can, when in a proinflammatory environment, upregulate Class II molecules in vivo. Overall, these results raise the possibility that β-cells may become involved in autoantigen presentation to CD4+ T cells during the pathogenic process culminating in type 1 diabetes.
Research Design and Methods
Islets and Tissue
Isolated human islets from anonymous organ donors (exempt from Institutional Review Board review) were obtained through the Network of Pancreatic Organ Donors with Diabetes (nPOD), the Integrated Islet Distribution Program or Prodo Laboratories, Inc., or through the efforts of Vanderbilt University via either the National Disease Research Interchange or the International Institute for the Advancement of Medicine. Supplementary Table 1 lists all donors with demographic information. Some samples from donors with type 1 diabetes were processed as described previously (3). For some islets samples, aliquots of islets were cryopreserved locally after receipt (∼200–600 islet equivalents/donor). These were used for the flow cytometry experiments described below (also see Supplementary Table 1).
Islets were isolated for RNA-Seq from 13 donors without diabetes (9 males, 4 females; age range 10–30 years; mean ± SEM age 16.1 ± 1.6 years) and from four donors with type 1 diabetes (3 males and 1 female; age range 12–24 years; mean age 20.3 ± 2.6 years) with disease durations of 0.42, 2, 3, and 7 years.
Formalin-fixed paraffin-embedded pancreas sections from donors with and without type 1 diabetes were from the Exeter Archival Diabetes Biobank (EADB) (http://foulis.vub.ac.be/), the Diabetes Virus Detection (DiViD) Study (8), and nPOD. The four EADB donors without diabetes included at least three males (the sex of the fourth donor was not reported) ranging in age from 5 to 47 years (mean age 24.8 ± 10.9 years). The 18 donors with type 1 diabetes (from all three cohorts) studied using immunohistology included 11 males and 7 females ranging in age from 1 to 42 years (mean age 19.7 ± 2.8 years) and with a range of disease duration of <1 week to 6 years (mean duration 0.61 ± 0.34 year). Full ethical approval was available for all samples studied, and all six DiViD Study participants provided written consent.
Dissociation, Fixation, Staining, and FACS
Dissociation, fixation, and staining were performed as described previously (23), using the following stains: Zombie Violet Fixable Viability dye (BioLegend), anti-insulin Alexa Fluor 647 (C27C9; Cell Signaling Technology), anti-glucagon (K79bB10; Sigma-Aldrich) labeled with Zenon Alexa Fluor 568, and anti-somatostatin (7G5) labeled with Zenon Alexa Fluor 488 (Thermo Fisher Scientific). Islet cell subsets (α-cells and β-cells) were sorted using a BD Biosciences FACSAria with the following gating strategy. Live cells were selected based upon Zombie Violet dye exclusion. These cells were then selected based upon the presence or absence of an insulin+ signal. Insulin+ cells were further gated for single cells (forward scatter height vs. forward scatter area) prior to sorting. All insulin-negative cells were first gated for single cells and then for glucagon and somatostatin signals prior to sorting.
Locally cryopreserved nondiabetic donor islets (14149, 14128, 14219, and 14223) and islets from donors with type 1 diabetes (nPOD donors 6414, 6480, and 6323 and T1D.13, T1D.18, and T1D.19) were thawed (100–300 islet equivalents/aliquot) dispersed with Accutase (Stemcell Technologies), stained for viability (LIVE/DEAD Fixable Aqua Dead Cell Stain Kit; Thermo Fisher Scientific), blocked with 50% human sera, and surface stained for CD45 (clone 2D1; BioLegend) with or without anti-HLA-DR (clone L243; BioLegend). After washing, cells were fixed, permeabilized, and stained for intracellular insulin as described above. For treatment of fresh islets with proinflammatory cytokines, islets were cultured with interferon-γ (IFN-γ), interleukin (IL-1β), and tumor necrosis factor (TNF)-α (R&D Systems) for 48 h, dispersed with enzyme, and stained for viability before staining with anti-CD45 and anti–HLA-DR. Cells were washed, fixed/permeabilized, and stained intracellularly for insulin and glucagon, as described. FMO (Fluorescence Minus One) controls were used to determine gates for each stain. Cells were run on a BD LSRII cytometer (BD Biosciences) and analyzed with FlowJo (version 10.3) software.
RNA-Seq Library Construction, Sequencing, Gene Expression, and Statistical Analysis
Sorted insulin+ and glucagon+ cells were pelleted and resuspended in Digestion Buffer and treated with Proteinase K for 3 h at 50°C (RecoverAll; Thermo Fisher Scientific). The RecoverAll protocol for RNA isolation was followed for samples with at least 75,000 cells. For samples with <75,000 cells, nucleic acids were purified using Agencourt RNAClean XP beads (Beckman Coulter) and digested with TURBO DNase (Thermo Fisher Scientific), and intact RNA was isolated with Agencourt RNAClean XP beads. RNAs were quantified using Qubit HS RNA (Thermo Fisher Scientific) and the Agilent Technologies Bioanalyzer RNA Pico chip was used to assess purity. Based upon RNA concentration and RNA integrity number, libraries were constructed using either the Ovation Human FFPE (formalin-fixed paraffin-embedded) RNA-Seq Library System (NuGen) or the SMARTer Stranded Total RNA-Seq Kit (Pico Input Mammalian; Takara) and sequenced as 42–base pair paired-end reads on a NextSeq 500 Sequencing System (Illumina). We processed sequence data essentially as described in the study by Conesa et al. (24) using FastQC 0.10.1 for quality control analysis and FASTX-Toolkit version 0.0.13 to trim the low-quality ends of each read. Bowtie2 version 2.1.0 removed rRNA and small nuclear RNA, Tophat2 version 2.0.14 mapped reads to the genome and transcriptome, and RSEM version 1.2.28 and Bowtie version 0.12.9 quantified gene abundance. Harman 1.2.0 (25) was used to normalize batch effects in transcripts per kilobase million count data to account for technical differences in library construction while maintaining biological variability. To normalize for gene length, transcripts per kilobase million counts were converted to expected counts, which we submitted to DESeq2 version 1.10.2 with parametric fitting and a minimum row sum of 10 for differential gene expression analysis. For statistical analysis, the Wald test with Benjamini-Hochberg correction was used. All bulk and single-cell RNA-Seq data are available in the Gene Expression Omnibus repository (DataSet Identifier GSE121863).
inDrop Single-Cell RNA-Seq Gene Expression and Statistical Analysis
Dissociated islets from selected preparations were droplet encapsulated, and single-cell RNA-Seq libraries were generated as described previously (26). Sequence output from a NextSeq 500 System was analyzed as described previously (27), except that an additional filtering step was added to the End Sequence Analysis Toolkit output, where the unique molecular identifier for each “singleton” (gene transcripts represented by only one read) was compared with the unique molecular identifiers of all transcripts for that gene. If any transcript with at least two reads was only one base different from the singleton, the singleton was merged with the multiread transcript.
The islet cell subsets (α-cells and β-cells) were identified using a multistage process. In the initial stage, transcript count data were subjected to TMM (trimmed mean of M-values) normalization (DESeq2), and principal component analysis (prcomp) was performed on the normalized data to identify genes that were most responsible for the variance in the data. Independent component analysis (fastICA) was applied for dimensionality reduction, followed by t-distributed stochastic neighbor embedding (t-SNE [Rtsne]) and density-based clustering. t-SNE groups cells into clusters based on the similarity of their entire transcriptomes, such that cells of a similar type form distinct clusters. The resulting clusters were examined to find the genes that were most differentially expressed in each cluster compared with all other cells. These differentially expressed genes were used to identify and remove cell clusters that were not α- or β-cells: we identified acinar (where the majority of the cells in the cluster showed relatively high expression of CPA1, PRSS1, and PNLIP), ductal (KRT19), stellate (PDGFRB, COL1A1, ACTA2), vascular (PECAM1, ESM1, FLT1), professional APC (high HLA-DR), δ-cells (somatostatin), and PP (pancreatic polypeptide Y) cells. The remaining cells were then renormalized and clustered using the steps described above, resulting in five clusters. The cells in one of the clusters were mainly from the donor with type 1 diabetes, and showed high expression of both insulin and glucagon RNA. These rare events likely represent “doublets” (i.e., droplets containing more than one cell). The remaining cells formed four distinct clusters composed mainly of β-cells (high insulin mRNA expression) and α-cells (high glucagon mRNA expression) from the donor without diabetes and the donor with type 1 diabetes. For differential expression analysis, we used the clusters to divide the cells into α- and β-cells, and used the sample label to distinguish between the donor without diabetes and the donor with type 1 diabetes. Genes with a Benjamini-Hochberg–adjusted P value of <0.05 were selected as differentially expressed, regardless of the log2 fold-change difference.
Immunostaining
A standard immunoperoxidase approach was used to examine single antigens on formalin-fixed paraffin-embedded tissue sections (28). To examine multiple antigens within the same tissue, an immunofluorescence approach was used in which antisera were applied sequentially (Supplementary Table 2). For some antibodies, the fluorescence signal was enhanced by tyramide signal amplification according the manufacturer instructions (Thermo Fisher Scientific). Images were captured using either an AF6000 Fluorescence Microscope (Leica) or a SP8 Confocal Microscope (Leica) with a PL APO ×40/1.25 numerical aperture lens and 488- and 561-nm laser lines. Analysis of the images was performed using either LAS AF software (Leica) or ImageJ version 1.50b Java download 1.8.0.77. In some cases, Huygens deconvolution software was used to take high-resolution confocal images (SVI).
Statistical Analysis
When two groups were compared, Student t test or Wilcoxon signed rank test was used. Where more than two groups were compared, a one-way ANOVA was used with a Tukey post hoc test to determine the statistical significance. Results were considered statistically significant at P < 0.05.
Results
Bulk-Sorted β-Cells From Donors With Type 1 Diabetes Express Class I and Class I–Associated mRNA Transcripts
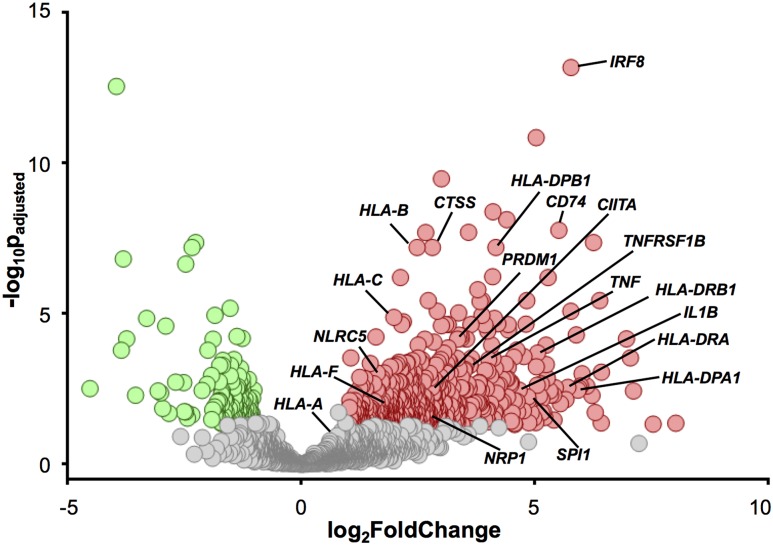
Human isolated islets (Supplementary Table 1) were dissociated, and insulin+ β-cells obtained by FACS were used to create RNA libraries. RNA-Seq was performed to determine β-cell gene expression profiles from donors without diabetes (N = 12; donor 17181 was only analyzed using inDrop single-cell RNA-Seq) or those in whom type 1 diabetes had been diagnosed (N = 4). Using a greater than twofold change and a P value <0.05 as a cutoff to define differential expression, we found 650 differentially expressed genes in β-cells isolated from donors with type 1 diabetes (Fig. 1). A total of 504 genes were upregulated (red), whereas 146 others were downregulated (green). Class I and Class II pathway genes and proinflammatory-associated genes upregulated in β-cells from the donor with type 1 diabetes are labeled in Fig. 1. Bulk FACS-sorted β-cells from donors with type 1 diabetes (relative to donors without diabetes) heterogeneously displayed upregulated mRNA expression of HLA-A, HLA-B, HLA-C, and HLA-F Class I mRNA transcripts as well as the Class I transactivator mRNA NLRC5 (29). Gene expression levels ranged from 1.9- to 5.6-fold higher in the β-cells of the cohort of donors with type 1 diabetes, with significant P values ranging from 0.02 to 6.3 × 10−8 (Supplementary Fig. 1). These results were consistent with immunohistochemical studies on pancreata from donors with type 1 diabetes (5,6,9,29). In addition, genes for proinflammatory cytokines and associated factors were also found to be differentially expressed by β-cells (IRF8, TNFRSF1B, TNF, and IL1β) (Fig. 1).
Figure 1.
Volcano plot shows differential gene expression from bulk β-cell populations and highlights the upregulation of immune response–specific genes in β-cells from donors with type 1 diabetes. β-cells were isolated using FACS based on insulin expression from donors without diabetes (N = 12) and with type 1 diabetes (N = 4). RNA was isolated and libraries were sequenced. The volcano plot shows the 650 genes that are differentially (P < 0.05 and fold change >2) upregulated (red circles, 504 genes) and downregulated (green circles, 146 genes) in the donors with type 1 diabetes. The lines point to individual circles and identify the −log10Padjusted and log2FoldChange for specific genes.
Bulk-Sorted β-Cells From Donors With Type 1 Diabetes Express Class II and Class II–Related Genes
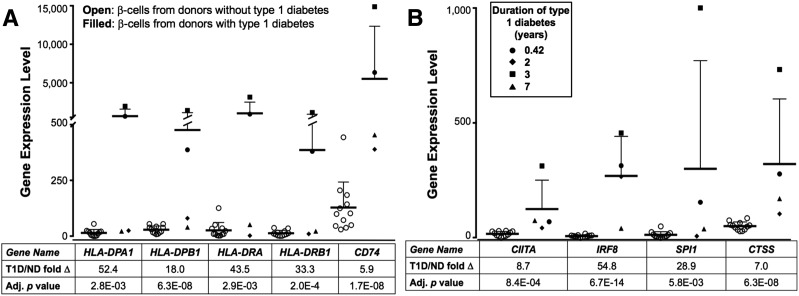
Class II genes HLA-DPA1, DPB1, DRA, and DRB1 were also significantly upregulated in β-cells from donors with type 1 diabetes. Gene expression levels ranged from 18- to 52.4-fold higher than in donors without diabetes at statistically significant P values ranging from 2.8 × 10−3 to 6.3 × 10−8 (Figs. 1 and 2A), highlighting the large donor-to-donor variability in gene expression and the heterogeneous nature of HLA Class II pathway upregulation. β-Cells from donors with type 1 diabetes differentially expressed additional genes in the Class II pathway, including the Class II–invariant chain CD74 and CIITA, the transcriptional regulator controlling Class II gene expression (Fig. 2A and B). We were not able to correlate the expression of these factors and disease duration due to the variability seen in the expression in these genes from the four donors with type 1 diabetes. Genes for two upstream CIITA transcriptional transactivators, IRF8 and SPI1, and the downstream CTSS (cathepsin S enzyme that cleaves CD74 to yield the CLIP fragment) were also expressed at higher levels in β-cells from donors with type 1 diabetes relative to donors without diabetes (gene expression levels were increased by 5.9-fold to 54.8-fold with P values between 5.8 × 10−3 and 6.7 × 10−14). These findings show that the mRNA for Class II HLA and other Class II molecules and associated factors are upregulated in the β-cells from donors with type 1 diabetes.
Figure 2.
Differentially expressed RNA transcripts from sorted β-cell populations from donors with type 1 diabetes displaying increased gene expression of Class II, upstream regulatory genes for the Class II pathway, and downstream response element genes. Gene expression levels from donors without diabetes (ND) (N = 12, open circles) and with type 1 diabetes (T1D) (N = 4, filled shapes; circle nPOD donor 6414; diamond nPOD donor 6367; square nPOD donor 6268; triangle T1D.6) are shown on the ordinate (as expected counts of raw values calculated using relative SEM), and the gene name, expression fold change, and adjusted P value are shown below the abscissa. Increased Class II–associated gene expression is shown in A, i.e., mRNA for four Class II chain proteins (HLA-DP α- and β-chains, HLA-DR α- and β-chains) and the Class II invariant chain CD74 in type 1 diabetes donor β-cells, and in B, CIITA and its positive regulators IRF8 and SPI1 and the mRNA for the CLIP cleavage protein CTSS, which plays a critical role in antigen loading on Class II. Bars indicate the mean values ± SEM. Adj., adjusted.
Single-Cell RNA-Seq Reveals β-Cell Gene Expression Differences Between a Donor With Type 1 Diabetes and a Donor Without Diabetes
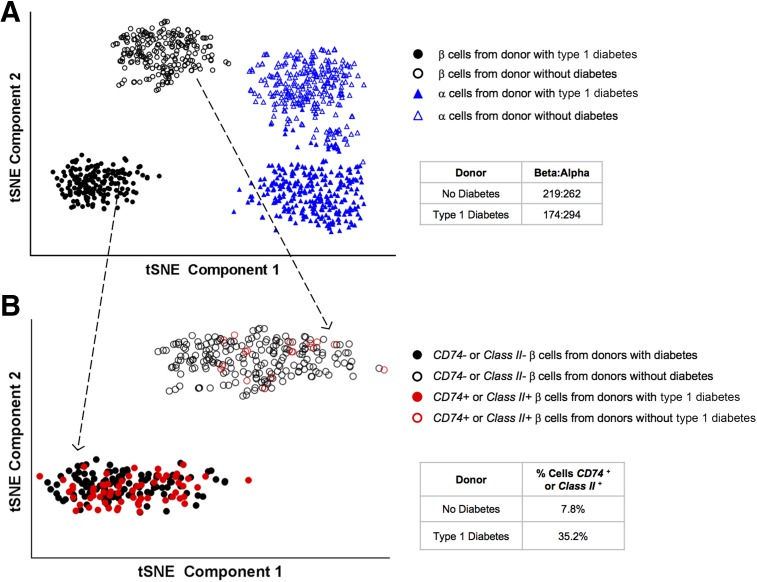
Applying a complementary methodology to study the β-cell population, single cells from dissociated islets were encapsulated using the inDrop system and prepared for RNA sequencing. Single islet cells (1,167 in total) from a 16-year-old donor without diabetes (donor 17181) and from a 23-year-old donor in whom type 1 diabetes had been diagnosed 5 months before death (nPOD donor 6414; 1,235 cells in total) were collected and sequenced. α-Cells and β-cells were identified based on the absence of endothelial, stellate, ductal, and other nonendocrine cell markers, and were validated by the presence of glucagon and insulin hormone expression. t-SNE plots clustered the cells into unique subpopulations based on their gene expression patterns. The α-cells and β-cells separated into two subpopulations, predominantly based on the diabetes status of the donor (Fig. 3A). In the donor without diabetes, 481 of the 1,167 cells identified were α-cells or β-cells, and there were ∼0.84 β-cells for every α-cell. From the donor with type 1 diabetes, 468 of the 1,235 were α-cells or β-cells, with ∼0.59 β-cells for every α-cell. Consistent with the observations from the bulk sequencing of β-cells from donors with type 1 diabetes, the β-cells from the donor with type 1 diabetes were more frequently positive for Class II and CD74 transcripts (Fig. 3B) with 35.2% of the β-cells from the donor with type 1 diabetes expressing Class II and/or CD74 genes versus 7.8% of the β-cells from the donor without diabetes, a 4.5-fold difference.
Figure 3.
Single-cell transcriptome using inDrop single-cell RNA-Seq analysis to separate and identify α-cells (triangles) and β-cells (circles) from two donors, a 16-year-old male without diabetes (open shapes; donor ID 17181) and a 23-year-old donor male with 0.42 years duration of type 1 diabetes (filled shapes; nPOD 6414). Single α- and β-cells were identified as described in the Research Design and Methods and plotted by t-SNE into the observed subpopulations. A: α- and β-cells show two distinct subpopulations, depending on donor diabetes status. B: Focusing only on the β-cell subpopulation (indicated by the dotted arrows), CD74 and/or Class II+ β-cells (red circles) are 4.5 times more prevalent in the donor with type 1 diabetes (filled circles) than the donor without diabetes (unfilled circles).
Some β-Cells in Islets From a Donor With Type 1 Diabetes Coexpress Class II and CIITA
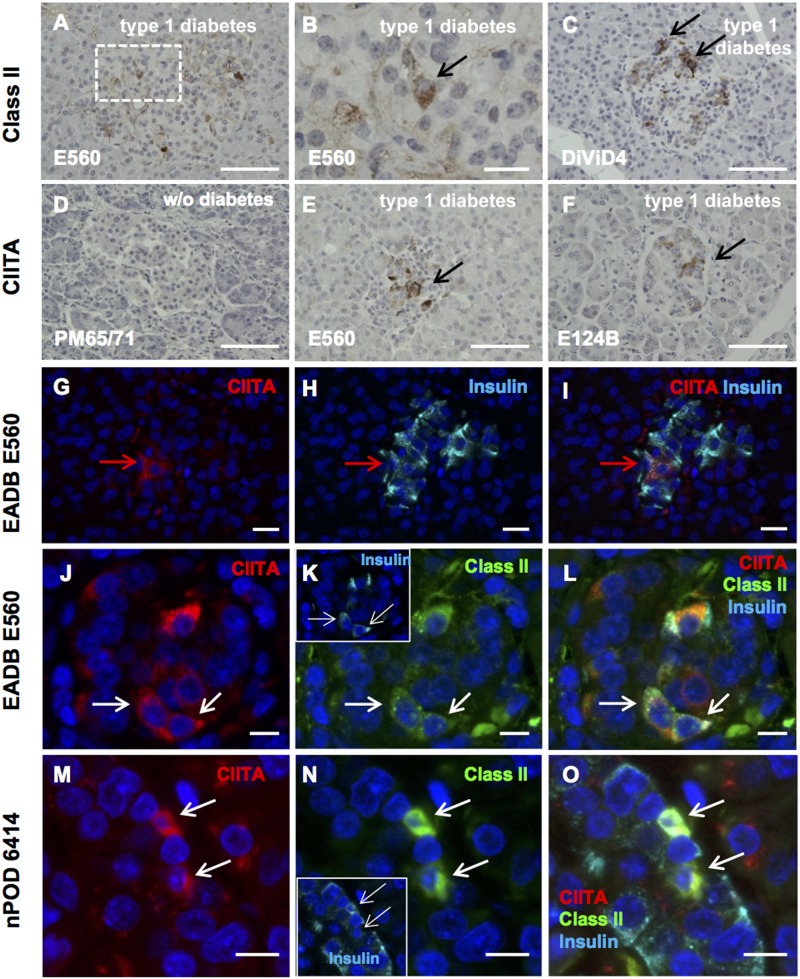
Immunostaining pancreas sections from donors with type 1 diabetes revealed that Class II was expressed on some cells within a subset of islets (Fig. 4A–C). Class II expression was never observed in the pancreatic islet cells from a donor without diabetes (Supplementary Table 3). Since the tissue used in these studies was recovered at autopsy, we also examined pancreas tissue collected by biopsy from six DiViD Study living donors to obviate postmortem artifacts. In five of six DiViD case patients, islet cells expressed Class II, although the proportion of Class II–immunopositive islets was variable, ranging from <10% in two cases to ∼70% in another. From four pancreata from donors without diabetes (each containing between 50 and 100 islets), we found no islet CIITA expression (a representative image from one pancreas from a donor without diabetes is shown in Fig. 4D). However, in pancreata from 8 of 12 (67%) age-matched donors with type 1 diabetes, strong CIITA immunostaining was detected in at least one islet on each section examined, (Fig. 4E–G, I, J, L, M, and O and Supplementary Table 3). Most sections from donors with type 1 diabetes contained multiple islets staining for CIITA, and this was largely, but not exclusively, restricted to β-cells (Fig. 4G–I). Of 20 islets from four donors with type 1 diabetes, 74.9% of CIITA+ cells costained for insulin. Even though CIITA exerts its effect in the nucleus, the cytoplasmic staining pattern for CIITA was also seen in human tonsil tissue (Supplementary Fig. 2), which is similar to the cytoplasmic CIITA staining seen in islet β-cells. Pancreas sections from two donors with type 1 diabetes (nPOD donors 6414 and 6367) with mRNA for Class II and Class II–associated mRNA species (like CIITA) also showed immunoreactive Class II and CIITA protein expression in insulin+ β-cells (nPOD donor 6414) (Fig. 4M–O) and in certain insulin-negative islet cells (nPOD donor 6367) (Supplementary Fig. 3A–D).
Figure 4.
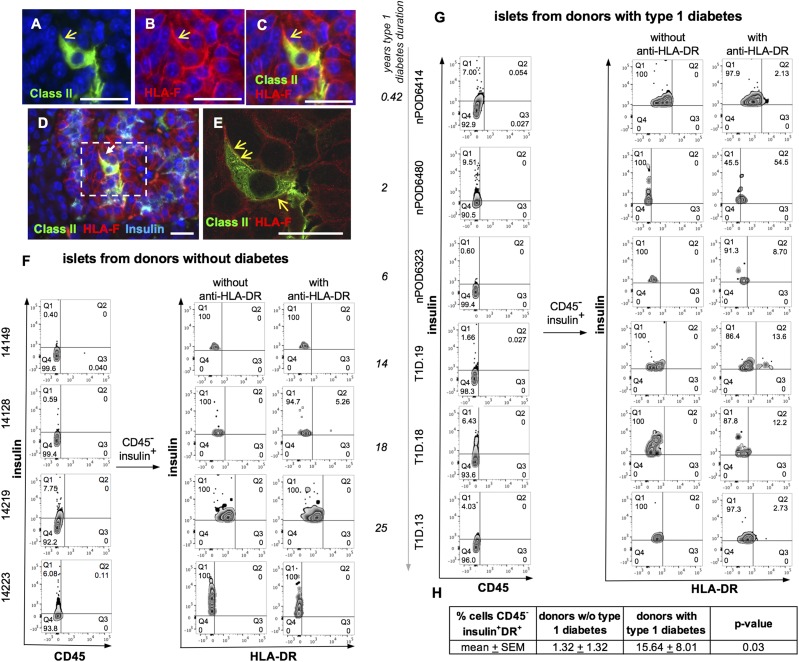
Class II and CIITA are expressed in discrete islet endocrine cells in donors with type 1 diabetes. Representative images of islets from donors with type 1 diabetes from the EADB (A, B, and E–L; E560 or E124B), DiViD (DiViD 4) (C), or nPOD (M–O; donor 6414) cohorts. Sections were immunostained with an antiserum raised against Class II protein (A–C, K, L, N, and O), CIITA (D–G, I, J, L, M, and O), and/or insulin (H, I, K, L, N, and O) using either an immunoperoxidase (A–F) or immunofluorescence (G–O) approach. Representative images of pancreas sections probed with an antiserum against CIITA from either a healthy control individual (D, PM65/71) or from donors with type 1 diabetes (E and F; E560, E124B). Black arrows indicate cells stained positively for Class II (B and C) or CIITA (E and F). Representative immunofluorescence images from a pancreas from a donor with type 1 diabetes (E560) probed for CIITA (G; red) and insulin (H; light blue). The merged image is shown (I). The red arrow indicates a cell positive for both CIITA and insulin. Representative images of pancreas sections from a donor with type 1 diabetes from EADB (J–L; E560) and nPOD (M–O; 6414) were probed for CIITA (J and M; red), Class II protein (K and N; green), and insulin (K [inset], L, N [inset], and O; light blue). Merged images are shown (L and O). Class II+ insulin+CIITA+ cells are denoted by the white arrow in L and O. The nPOD donor examined was part of the cohort from which RNA transcriptome data are shown in Figs. 2 and 3. Scale bars: A and C–F, 50 µm; B and J–O, 10 µm; G–I, 20 μm.
Surface expression of HLA Class II on insulin+ β-cells was detected by colocalization with upregulated HLA-F expression on pancreas sections from donor E560 with type 1 diabetes (Fig. 5A–E and Supplementary Fig. 3E–G, and z-stack staining shown in Supplementary Video). Dispersed islets were analyzed for surface expression of CD45 and HLA-DR and intracellular expression of insulin by flow cytometry. Examples of CD45− and CD45+ cells from islets are shown in Supplementary Fig. 3H–J. From donors with type 1 diabetes (N = 6), 15.64 ± 8.01% of CD45−insulin+ islet cells had detectable surface HLA-DR compared with those cells from donors without diabetes (N = 4, 1.32 ± 1.32%) (Fig. 5F–H). Islet cells (CD45−insulin+) from a donor without diabetes that were treated with proinflammatory cytokines upregulated surface HLA-DR (Supplementary Fig. 3K and L) (30).
Figure 5.
β-cell expression of HLA-Class II (HLA-DR) on the surface of a portion of CD45−insulin+ cells from donors with type 1 diabetes. Representative image of a pancreas section from a single donor with type 1 diabetes (E560) stained for Class II (A and C–E; green), HLA-F (B–E; red), and insulin (D; light blue), with merged images shown (C–E). An enlargement of the highlighted area in panel D (dotted white box) is presented in A–C and E. A high-resolution image of the same cells is shown in Supplementary Fig. 3 and Supplementary Video. Images were captured at ×400 magnification unless otherwise stated. Scale bars: A–E, 25 μm. Cryopreserved, non–hand-picked islets (100–300 islet equivalents/stain) were thawed, washed, dispersed with enzyme, washed, stained for viability, washed, and blocked with 50% human serum, and then surface stained for CD45 with and without anti-HLA-DR (L243 monoclonal antibody). Cells were washed and stained for intracellular insulin as described in the Research Design and Methods. Single viable cells were analyzed for surface CD45 and intracellular insulin (left panels of each group). CD45−insulin+ cells were then analyzed for HLA-DR expression (middle and right panels of each group). F: Islets from donors without diabetes (donors 14223, 14219, 14128, and 14149). G: Islets from donors with type 1 diabetes (T1D.13, T1D.18, T1D.19, nPOD 6323, nPOD 6414, and nPOD 6480; disease duration is indicated to the left of the sample identifier). Samples from donors 14223 and nPOD 6480 were prepared and analyzed as a set; 14219 and nPOD 6414 were prepared and analyzed as a set; and the remainder of the samples were prepared and analyzed as one set. Gates were set for FMO (Fluorescence Minus One) stains for each marker. For HLA-DR staining, this is shown for each sample. The percentage of CD45−insulin+HLA-DR+ cells for each sample are shown in Q2 for each sample (far right columns in F and G). A comparison of the frequencies of CD45−insulin+HLA-DR+ cells for donors without diabetes and donors with type 1 diabetes is shown in H by Wilcoxon signed rank test. Donor samples nPOD6414, nPOD6367, and 14223 were analyzed for RNA transcriptome (Figs.1 and 2 and Supplementary Fig. 1).
Islet CIITA Expression Correlates With Class II, but Not Class I or Enterovirus VP1
Using immunohistology to study a range of pancreata from donors without diabetes, we found no evidence of cells expressing CIITA or Class II in islets. Conversely, in all cases with type 1 diabetes studied in whom CIITA+ islets were detected, islets expressing Class II were also present (Supplementary Table 3). We next used coimmunofluorescence staining to localize CIITA and Class II within islets and found that all islet endocrine cells expressing Class II also coexpressed CIITA (Fig. 4J–O), while occasional CIITA+ islet cells did not stain positively for Class II.
In contrast to Class II mRNA species, the transcription of Class I genes is regulated by NLRC5 in many cell types. Earlier studies (9,31) did not find elevated NLRC5 levels by immunostaining or RNA array analysis in islets from donors with type 1 diabetes that hyperexpressed Class I. Using methods with higher sensitivity, we observed clear evidence of upregulated NLRC5 mRNA in purified β-cells from the donors with type 1 diabetes (Supplementary Fig. 1). We examined whether some Class I–expressing cells might have elevated CIITA expression. As expected, numerous insulin-containing islets from donors with type 1 diabetes hyperexpressed Class I in all endocrine cell subtypes (Supplementary Fig. 4A–C). CIITA was not found in islets having normal levels of Class I expression, but was present in a few cells in some islets with upregulated Class I. Many islets with elevated Class I expression had no detectable CIITA. In common with Class II, the enteroviral capsid protein VP1 has also been detected in a small subset of β-cells from donors with type 1 diabetes (32). CIITA was occasionally expressed in islets that also contained VP1+ cells but, among a total of 29 VP1+ cells in 12 islets, the two antigens were never seen in the same cells (Supplementary Fig. 4D–F). When pancreas sections were costained with antisera directed against Class I and Class II, all Class II+ β-cells had Class I hyperexpression, but many Class I+ islet cells had no detectable Class II (Supplementary Fig. 5).
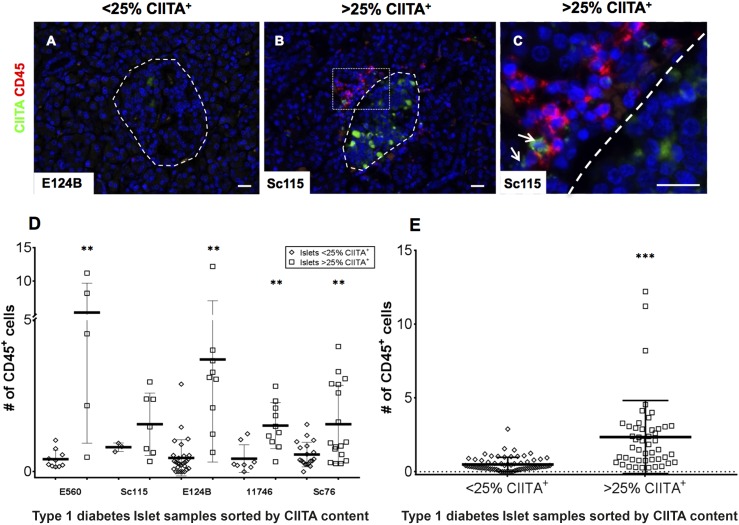
Islet CIITA Expression Correlates With the Number of Islet-Associated CD45+ Cells
Activated CD8+ T cells secrete proinflammatory cytokines like IFN-γ, which is reported to mediate CIITA (and thereby Class II) expression in certain nonhematopoietic cells (33,34). Our immunohistological analysis suggested that islets with infiltrating or peri-islet CD45+ lymphocytes contained more CIITA+ islet cells. In general, islets with >25% of endocrine cells expressing CIITA also had large numbers of lymphocytes within, or in contact with, the islet boundary (Fig. 6A–C). Thus, CIITA-expressing islets display greater numbers of infiltrating CD45+ cells, as shown within individual donor sections (Fig. 6D), or when all data for islets from donors with type 1 diabetes were pooled (Fig. 6E). As expected, certain immune cells located either at the islet periphery or in other pancreas section areas, also stained for CIITA and Class II (Fig. 6C and Supplementary Fig. 4D and F).
Figure 6.
The number of CIITA+ islet cells in sections of pancreas from individuals with type 1 diabetes correlates with the presence of infiltrating immune cells. A–C: Representative images of pancreas sections from five donors with type 1 diabetes stained for CD45 (red) or CIITA (green). Islets are highlighted with dashed lines, and the images depict islets with either <25% of CIITA+ cells (A) or those containing >25% CIITA+ cells (B). C: An enlargement of the highlighted area (dotted white box) is also presented. Arrows indicate cells among the immune cell infiltrate positive for CIITA. Images were captured at ×200 magnification. Multiple <25% and >25% CIITA+ islets were imaged in each of five pancreata from donors with type 1 diabetes and the number of CD45+ cells within (or immediately adjacent to) each islet was counted. Data are shown for each individual case studied (D) and for all cases combined (E). Bars indicate the mean values ±SEM. Scale bars, 20 μm. **P < 0.01, ***P < 0.001.
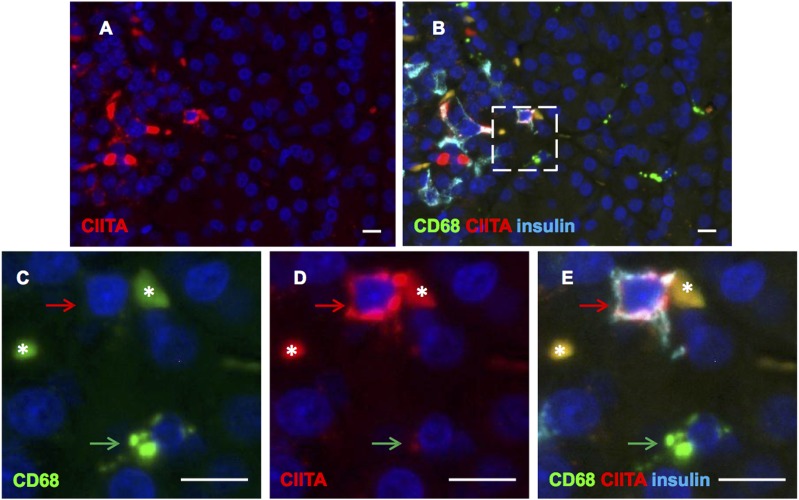
CIITA and Insulin Costained Cells Are Not Macrophages That Have Phagocytosed β-Cells
Since Class II and insulin expression within the same cell could be due to a professional APC engulfing a dead or damaged β-cell, we costained for CIITA, CD68 (a macrophage marker), and insulin. We found no cells stained by all three antisera. In fact, we found no cells that were stained for both insulin and CD68, although multiple CD68+ cells were found across the sections (Fig. 7A–E). As expected, CD68+ cells expressed CIITA, albeit at much lower levels than were seen in those islet endocrine cells where this transcription factor was detected.
Figure 7.
Islet cells staining for CIITA and insulin are not CD68 immunopositive. Immunofluorescence staining of a pancreas section from a donor with type 1 diabetes, probed for CIITA (A, B, D, and E, red), CD68 (B, C, and E, green), and insulin (B and E, light blue). Enlargement of the highlighted area (dotted white box) in the top right panel is shown (C, D, and E). The red arrow indicates an insulin+ cell that stained positively for CIITA but not CD68. The green arrow highlights a nearby CD68-immunopositive cell, which is also positive for CIITA, but is negative for insulin. The asterisks indicate areas of autofluorescence. Images captured at ×400 magnification. Scale bars, 10 μm.
Discussion
Several lines of evidence have suggested that β-cells are not simply passive victims of a T cell–mediated destructive process in type 1 diabetes. We now report data obtained using multiple methodologies confirming that Class II molecules and transcripts, along with other molecules in the Class II pathway, are expressed by a population of endocrine cells from donors with type 1 diabetes. The Class II–expressing cells were predominantly β-cells, although islet cells that did not stain for insulin were also seen to express Class II. In addition to other islet endocrine cells, these cells might include degranulated, dysfunctional, and dedifferentiating β-cells. Our conclusions are supported by immunohistological, flow cytometric, and mRNA expression assays, using both bulk-sorted and single β-cells isolated from donors with type 1 diabetes. The transcriptomic analysis revealed that the β-cells display increased levels of genes associated with both Class II expression (including CIITA, IRF8, and SPI1) and function (CD74 and CTSS, gene for cathepsin S), supporting the conclusion that β-cell Class II expression is a bona fide phenomenon in human type 1 diabetes. Immunohistological analysis verified that the small complement of β-cells expressing Class II also stain for CIITA, which was mainly located in the cytoplasm rather than in the nucleus. Nuclear CIITA has been visualized using green fluorescent protein or FLAG-tagged CIITA (35,36), or by using sufficient nuclear protein for Western blot detection. However, we were unable to detect CIITA protein in the nucleus of endocrine or immune cells in pancreas tissue sections, in human tonsillar tissue sections (Supplementary Fig. 2), or in cell lines with confirmed surface HLA-DR Class II expression, including human mature monocyte-derived dendritic cells, two B-lymphoblastoid cell lines, and a cell line transduced to express CIITA, CIITA-SupT1 (37) (data not shown). This is likely due to the blocking of the binding site of the anti-CIITA antibody in the N-terminal region of the protein, which overlaps with domains that bind to basal transcriptional machinery (38). We were not able to detect CIITA by Western blot of the nuclear fraction of CD45−Class II+ cells sorted from proinflammatory cytokine–treated islets from donors without diabetes, most likely due to the insufficient quantity of nuclear protein recovered from the small number of cells sorted from dissociated islets, though we detected nuclear CIITA from B-lymphoblastoid cell lines (data not shown). Even so, the detection of Class II transcripts and proteins strongly indicates that CIITA is performing its Class II transcriptional transactivation function in the nucleus. We also show that Class II and CIITA are absent from islet endocrine cells in individuals who do not have diabetes. The β-cell Class II upregulation appears to be unique to type 1 diabetes since it is not seen in people with type 2 diabetes or in other diseases of the pancreas such as cystic fibrosis or autoimmune pancreatitis (11).
Autoreactive CD8+ T cells within islets of donors with type 1 diabetes (2,3,39), Class I hyperexpression, CD8-mediated β-cell killing, and in vitro hyperexpression of Class I on β-cells in in vitro models are well established (40,41). However, surface expression of Class II on β-cells implies that β-cells may serve as APC and may directly play a role in the autoimmune response. As has been reported (30), in vitro treatment of islets with proinflammatory cytokines upregulated Class II expression on the surface of some β-cells, detected by flow cytometry (Supplementary Fig. 3K and L). Colocalized Class II and HLA-F (9), an upregulated gene product in the β-cells of donors with type 1 diabetes (Supplementary Fig. 1), were detected by immunofluorescence on the surface of β-cells from a donor with type 1 diabetes (Fig. 5) in a pattern also seen on dendritic cells (42). In the dendritic cell study, 5% of total Class II expression was estimated to be on the cell surface. From cryopreserved islets of six donors with type 1 diabetes, compared with those from four donors without diabetes, by flow cytometry, we detected HLA-DR expression, albeit at a low intensity, on the surface of a portion (15.64 ± 8.01%) of CD45−insulin+ cells despite known decreased insulin expression from cryopreserved human islets (43).
In order to address the possibility that the colocalization of Class II with insulin immunopositivity in islets might reflect the phagocytosis of dead or dying β-cells by professional APCs (44) rather than by the β-cells per se, we stained sections with an anti-CD68 antibody to detect resident macrophages (45,46). As expected, the islet macrophages expressed Class II, but, interestingly, these were readily distinguished from Class II–expressing β-cells as the CD68+ cells displayed less intense CIITA immunostaining. We found no human islet macrophages that stained positively for CD68 and Class II and that also stained for insulin.
We also show that CIITA expression is closely correlated with inflammation in the islets from donors with type 1 diabetes, as judged by the presence of immune cells close to, or within, the islet boundary. Various lines of evidence suggest that certain proinflammatory cytokines (principally, IFN-γ) can promote the expression of CIITA and Class II on nonhematopoietic cells, and this has also been shown in islet cells (14,47–50). Hence, it is plausible that the local IFN-γ associated with islet inflammation may drive β-cell Class II expression in type 1 diabetes. Although this conclusion conflicts with those of other reports (11,15), one potential explanation is the improved sensitivity of contemporary methods.
CD8+ T cells represent the most abundant cell type in the inflammatory infiltrates in individuals with type 1 diabetes (51–53), and, since they can secrete IFN-γ, it seems likely that infiltrating CD8+ T cells may promote β-cell Class II expression. It is unclear why the response is restricted to only a few scattered β-cells within any given islet, since the islet infiltrating lymphocyte-secreted cytokines should bathe most islet cells. One possibility is that the differential response may reflect the known heterogeneity among β-cells in vivo (53–55). Interestingly, several inflammatory-associated genes were found to be upregulated in β-cells from donors with type 1 diabetes (IRF8, TNFRSF1B, TNF, and IL-1β), some of which are downstream of signaling by CXCL10, which has been reported to be pivotal in recruiting immune cells to islets (56–58).
The β-cell CIITA and Class II expression now reported contrasts markedly with that reported for Class I, which appears to be independent of inflammation (59). In support of a differential response, Class I hyperexpression was readily detected in islet cells that did not stain for CIITA and where no Class II was seen. Class I expression is often driven by the NLRC5 complex (Supplementary Fig. 6), and, in the present transcriptomic analysis, we found increased NLRC5 mRNA in the β-cells of donors with type 1 diabetes. This result stands in contrast to earlier work where no increase in NLRC5 was detected either by RNA array analysis of laser capture microdissected islets from nPOD donors with type 1 diabetes or by immunostaining of islets in pancreas sections (9). The present data reinforce the conclusion that Class I is hyperexpressed on β-cells from donors with type 1 diabetes, and now suggest a role for NLRC5 in addition to STAT1, as previously shown (9).
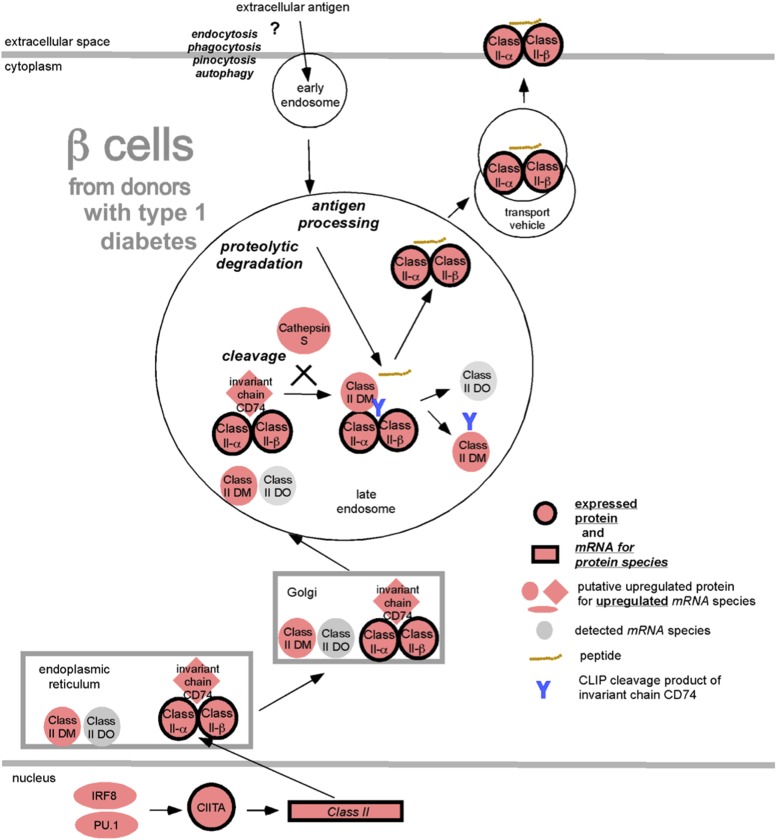
The role of Class II–expressing professional APCs is to take up and process exogenous proteins, then present peptides in the context of Class II to CD4+ T cells. In the Class II pathway (Fig. 8), we have depicted this as a “question mark” since the ability of β-cells to take up exogenous antigen in this manner or present endogenous peptides is not known. A key question arising from this work is whether the β-cell Class II expression enables the cells to act within islets as surrogate APCs to CD4+ T cells. Neither CD80 nor CD86 (costimulation required for effective presentation to naive T cells, but not memory T cells) could be detected on the β-cells of patients with recent-onset type 1 diabetes (60), and we found no such staining in the current study (data not shown). Whether other proteins could fulfill this costimulatory role is unknown.
Figure 8.
Upregulated protein and mRNA species in β-cells from donors with type 1 diabetes from the Class II processing and presentation pathway. The observed upregulated proteins and mRNA species from the Class II processing and presentation pathway of β-cells from donors with type 1 diabetes are displayed according to their intracellular locations and with their functions. Depicted by symbols with black borders are upregulated proteins (red circles) and mRNA species (red rectangles), whereas putative upregulated proteins identified by upregulated mRNA species are shown with red ovals, diamonds, or elongated ovals. Gray ovals depict mRNA species that were detected but not upregulated in the β-cells from islets of donors with type 1 diabetes.
Taken as a whole, this study clarifies the much debated suggestion that some β-cells from individuals with type 1 diabetes express Class II, as well as associated pathway genes and inflammatory pathway genes. Further work is needed to determine whether, and how, β-cell Class II expression contributes to type 1 diabetes pathogenesis.
Supplementary Material
Article Information
Acknowledgments. The authors thank the University of Massachusetts for support from the Diabetes Center of Excellence and, in particular, Susanne Pechhold from the UMass Flow Cytometry Flow Core Facility; Dr. Rita Bortell, Dr. Laura Alonso, and Dr. Dale Greiner for helpful comments during the study progress and data analysis; Megan E. DeNicola and Juanita Campos Rivera for technical assistance; and Patricia Cannon and Lisa Hubacz for administrative support. The authors also thank Lawrence Stern (University of Massachusetts Medical School) for the SupT1 and CIITA-SupT1 cell lines. In addition, the authors thank Dr. Alberto Pugliese, Dr. Francesco Vendrame, and Dr. George Burke III (all from Diabetes Research Institute, University of Miami) for helpful advice and discussions. The authors additionally thank Dr. Alan Foulis (University of Glasgow) for helpful discussion and access to samples. K.D.-J. is principal investigator of the DiViD Study. Also, the authors thank the surgeons Bjørn Edwin and Trond Buanes and specialist nurse Trine Roald, Oslo University Hospital (Oslo, Norway), for invaluable efforts that were essential to the success of the DiViD Study. Finally, the authors thank the Organ Procurement Organizations partnering with the International Institute for Advancement of Medicine and the National Disease Research Interchange and the families of the donors.
Funding. This research was performed with the support of the nPOD (Research Resource Identifier [RRID]: SCR-014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD grant 5-SRA-2018-557-Q-R) and The Leona M. and Harry B. Helmsley Charitable Trust (grant 2018PG-T1D053). Organ Procurement Organizations partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org//for-partners/npod-partners/. This research was performed using resources and/or funding provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)–supported Human Islet Research Network (RRID: SCR-014393 [https://hirnetwork.org ] with grants to A.C.P. [UC4DK-104211, DK-108120, DK-112232], DK-106755, the Vanderbilt Diabetes Research and Training Center grant, DK-20593, and the Department of Veterans Affairs). This research was supported by DK-116284 (to S.C.K.) and DK-105837 (to D.M.H.). This research was supported by grants from JDRF and The Leona M. and Harry B. Helmsley Charitable Trust (to A.C.P. and D.M.H.). This research was supported by Diabetes UK grant 16/0005480 (to S.J.R. and N.G.M.), a JDRF Career Development Award (5-CDA-2014-221-A-N, to S.J.R.), and a JDRF Postdoctoral Fellowship (3-PDF-2017-374-A-N, to J.A.B.B.). D.M.B. received support from the Babson Faculty Research Fund. The DiViD Study was funded by grants from South-Eastern Norway Regional Health Authority (to K.D.-J.), The Novo Nordisk Foundation (to K.D.-J.), and through the PEVNET Study Group funded by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 261441 PEVNET (http://www.uta.fi/med/pevnet/publications.html).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.A.R. collected data, designed and analyzed the data from the immunohistology studies, helped to draft the manuscript, and reviewed and approved the manuscript. S.D.R. designed and performed the bulk-sorted human islet cell studies and the single-cell studies for the RNA transcriptome work, provided biological insight for the RNA-Seq analysis, performed experiments with cryopreserved and fresh islets for flow cytometry, helped to draft the manuscript, and reviewed and approved the manuscript. D.M.B. and D.M.H. designed and performed the bulk-sorted human islet cell studies for the RNA transcriptome work, provided biological insight for the RNA-Seq analysis, helped to draft the manuscript, and reviewed and approved the manuscript. S.J.R. and N.G.M. designed the immunohistology studies and analyzed those data, helped to draft the manuscript, and reviewed and approved the manuscript. P.L. collected data, helped to draft the manuscript, and reviewed and approved the manuscript. L.K. and K.D.-J. provided patient material from the DiViD Study, helped to draft the manuscript, and reviewed and approved the manuscript. R.B., M.B., J.M.S., R.H., and A.C.P. procured or analyzed some patient material and data, helped to draft the manuscript, and reviewed and approved the manuscript. J.A.B.B. performed experiments with cryopreserved and fresh islets for flow cytometry, helped to draft the manuscript, and reviewed and approved the manuscript. C.Y. performed the single-cell studies for the RNA transcriptome work, provided biological insight for the RNA-Seq analysis, helped to draft the manuscript, and reviewed and approved the manuscript. S.C.K. provided biological insight for the RNA-Seq analysis, designed and performed the flow cytometry experiments, helped to draft the manuscript; and reviewed and approved the manuscript. A.G.D., A.K., and M.G.G. were primarily responsible for the RNA-Seq analysis, helped to draft the manuscript, and reviewed and approved the manuscript. N.G.M. and D.M.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 2018 JDRF nPOD 10th Annual Scientific Meeting, Hollywood, FL, 20–23 February 2018.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0686/-/DC1.
References
- 1.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes 2016;65:719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babon JA, DeNicola ME, Blodgett DM, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016;22:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 2005;23:975–1028 [DOI] [PubMed] [Google Scholar]
- 5.Foulis AK, Farquharson MA, Hardman R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1987;30:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 1993;92:2313–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 8.Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015;64:1682–1687 [DOI] [PubMed] [Google Scholar]
- 9.Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia 2016;59:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med 1985;313:353–360 [DOI] [PubMed] [Google Scholar]
- 11.Foulis AK, Farquharson MA. Aberrant expression of HLA-DR antigens by insulin-containing beta-cells in recent-onset type I diabetes mellitus. Diabetes 1986;35:1215–1224 [DOI] [PubMed] [Google Scholar]
- 12.Bottazzo GF, Pujol-Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet 1983;2:1115–1119 [DOI] [PubMed] [Google Scholar]
- 13.Hanafusa T, Miyazaki A, Miyagawa J, et al. Examination of islets in the pancreas biopsy specimens from newly diagnosed type 1 (insulin-dependent) diabetic patients. Diabetologia 1990;33:105–111 [DOI] [PubMed] [Google Scholar]
- 14.Pujol-Borrell R, Todd I, Doshi M, et al. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature 1987;326:304–306 [DOI] [PubMed] [Google Scholar]
- 15.Pujol-Borrell R, Todd I, Doshi M, Gray D, Feldmann M, Bottazzo GF. Differential expression and regulation of MHC products in the endocrine and exocrine cells of the human pancreas. Clin Exp Immunol 1986;65:128–139 [PMC free article] [PubMed] [Google Scholar]
- 16.Somoza N, Vargas F, Roura-Mir C, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol 1994;153:1360–1377 [PubMed] [Google Scholar]
- 17.Bottazzo GF. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabet Med 1986;3:119–130 [DOI] [PubMed] [Google Scholar]
- 18.Hänninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest 1992;90:1901–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lernmark A, Klöppel G, Stenger D, et al. Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch 1995;425:631–640 [DOI] [PubMed] [Google Scholar]
- 20.Markmann J, Lo D, Naji A, Palmiter RD, Brinster RL, Heber-Katz E. Antigen presenting function of class II MHC expressing pancreatic beta cells. Nature 1988;336:476–479 [DOI] [PubMed] [Google Scholar]
- 21.Reich EP, Sherwin RS, Kanagawa O, Janeway CA Jr. An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature 1989;341:326–328 [DOI] [PubMed] [Google Scholar]
- 22.Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest 2017;127:2881–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blodgett DM, Nowosielska A, Afik S, et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 2015;64:3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conesa A, Madrigal P, Tarazona S, et al. A survey of best practices for RNA-seq data analysis. Genome Biol 2016;17:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oytam Y, Sobhanmanesh F, Duesing K, Bowden JC, Osmond-McLeod M, Ross J. Risk-conscious correction of batch effects: maximising information extraction from high-throughput genomic datasets. BMC Bioinformatics 2016;17:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein AM, Mazutis L, Akartuna I, et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 2015;161:1187–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derr A, Yang C, Zilionis R, et al. End Sequence Analysis Toolkit (ESAT) expands the extractable information from single-cell RNA-seq data. Genome Res 2016;26:1397–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014;63:3835–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kronenberg-Versteeg D, Eichmann M, Russell MA, et al. Molecular pathways for immune recognition of preproinsulin signal peptide in type 1 diabetes. Diabetes 2018;67:687–696 [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Scott NA, Quah HS, et al. Mouse pancreatic beta cells express MHC class II and stimulate CD4(+) T cells to proliferate. Eur J Immunol 2015;45:2494–2503 [DOI] [PubMed] [Google Scholar]
- 31.Skog O, Korsgren S, Wiberg A, et al. Expression of human leukocyte antigen class I in endocrine and exocrine pancreatic tissue at onset of type 1 diabetes. Am J Pathol 2015;185:129–138 [DOI] [PubMed] [Google Scholar]
- 32.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 33.Thelemann C, Eren RO, Coutaz M, et al. Interferon-γ induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS One 2014;9:e86844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giroux M, Schmidt M, Descoteaux A. IFN-gamma-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-alpha. J Immunol 2003;171:4187–4194 [DOI] [PubMed] [Google Scholar]
- 35.Chiu E, Gold T, Fettig V, LeVasseur MT, Cressman DE. Identification of a nuclear export sequence in the MHC CIITA. J Immunol 2015;194:6102–6111 [DOI] [PubMed] [Google Scholar]
- 36.Towey M, Kelly AP. Nuclear localisation of CIITA is controlled by a carboxy terminal leucine-rich repeat region. Mol Immunol 2002;38:627–634 [DOI] [PubMed] [Google Scholar]
- 37.Porter KA, Kelley LN, Nekorchuk MD, et al. CIITA enhances HIV-1 attachment to CD4+ T cells leading to enhanced infection and cell depletion. J Immunol 2010;185:6480–6488 [DOI] [PubMed] [Google Scholar]
- 38.Fontes JD, Jiang B, Peterlin BM. The class II trans-activator CIITA interacts with the TBP-associated factor TAFII32. Nucleic Acids Res 1997;25:2522–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michels AW, Landry LG, McDaniel KA, et al. Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes 2017;66:722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronenberg D, Knight RR, Estorninho M, et al. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells. Diabetes 2012;61:1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marroqui L, Dos Santos RS, Op de Beeck A, et al. Interferon-α mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 2017;60:656–667 [DOI] [PubMed] [Google Scholar]
- 42.Pierre P, Turley SJ, Gatti E, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 1997;388:787–792 [DOI] [PubMed] [Google Scholar]
- 43.Manning Fox JE, Lyon J, Dai XQ, et al. Human islet function following 20 years of cryogenic biobanking. Diabetologia 2015;58:1503–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pipelleers DG, in ’t Veld PA, Pipeleers-Marichal MA, Gepts W, van de Winkel M. Presence of pancreatic hormones in islet cells with MHC-class II antigen expression. Diabetes 1987;36:872–876 [DOI] [PubMed] [Google Scholar]
- 45.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia 2009;52:1686–1688 [DOI] [PubMed] [Google Scholar]
- 46.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007;56:2356–2370 [DOI] [PubMed] [Google Scholar]
- 47.Campbell IL, Oxbrow L, West J, Harrison LC. Regulation of MHC protein expression in pancreatic beta-cells by interferon-gamma and tumor necrosis factor-alpha. Mol Endocrinol 1988;2:101–107 [DOI] [PubMed] [Google Scholar]
- 48.Jackson AM, Connolly JE, Matsumoto S, et al. Evidence for induced expression of HLA class II on human islets: possible mechanism for HLA sensitization in transplant recipients. Transplantation 2009;87:500–506 [DOI] [PubMed] [Google Scholar]
- 49.Kim KA, Kim S, Chang I, et al. IFN gamma/TNF alpha synergism in MHC class II induction: effect of nicotinamide on MHC class II expression but not on islet-cell apoptosis. Diabetologia 2002;45:385–393 [DOI] [PubMed] [Google Scholar]
- 50.Pavlovic D, van de Winkel M, van der Auwera B, et al. Effect of interferon-gamma and glucose on major histocompatibility complex class I and class II expression by pancreatic beta- and non-beta-cells. J Clin Endocrinol Metab 1997;82:2329–2336 [DOI] [PubMed] [Google Scholar]
- 51.Foulis AK, McGill M, Farquharson MA. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man--macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol 1991;165:97–103 [DOI] [PubMed] [Google Scholar]
- 52.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes 2016;65:1362–1369 [DOI] [PubMed] [Google Scholar]
- 53.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorrell C, Schug J, Canaday PS, et al. Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pipeleers DG. Heterogeneity in pancreatic beta-cell population. Diabetes 1992;41:777–781 [DOI] [PubMed] [Google Scholar]
- 56.Bender C, Christen S, Scholich K, et al. Islet-expressed CXCL10 promotes autoimmune destruction of islet isografts in mice with type 1 diabetes. Diabetes 2017;66:113–126 [DOI] [PubMed] [Google Scholar]
- 57.Coppieters KT, Amirian N, Pagni PP, et al. Functional redundancy of CXCR3/CXCL10 signaling in the recruitment of diabetogenic cytotoxic T lymphocytes to pancreatic islets in a virally induced autoimmune diabetes model. Diabetes 2013;62:2492–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roep BO, Kleijwegt FS, van Halteren AG, et al. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 2010;159:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK. Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 2011;33:9–21 [DOI] [PubMed] [Google Scholar]
- 60.Imagawa A, Hanafusa T, Itoh N, et al. Islet-infiltrating t lymphocytes in insulin-dependent diabetic patients express CD80 (B7-1) and CD86 (B7-2). J Autoimmun 1996;9:391–396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.