Abstract
Introduction:
In 1988, an estimated 350,000 children were paralyzed by polio and 125 countries reported polio cases, the World Health Assembly passed a resolution to achieve polio eradication by 2000, and the Global Polio Eradication Initiative (GPEI) was established as a partnership focused on eradication. Today, following eradication efforts, polio cases have decreased >99% and eradication of all three types of wild polioviruses is approaching. However, since polio resources substantially support disease surveillance and other health programs, losing polio assets could reverse progress toward achieving Global Vaccine Action Plan goals.
Areas covered:
As the end of polio approaches and GPEI funds and capacity decrease, we document knowledge, experience, and lessons learned from 30 years of polio eradication.
Expert commentary:
Transitioning polio assets to measles and rubella (MR) elimination efforts would accelerate progress toward global vaccination coverage and equity. MR elimination feasibility and benefits have long been established. Focusing efforts on MR elimination after achieving polio eradication would make a permanent impact on reducing child mortality but should be done through a ‘diagonal approach’ of using measles disease transmission to identify areas possibly susceptible to other vaccine-preventable diseases and to strengthen the overall immunization and health systems to achieve disease-specific goals.
Keywords: Elimination, eradication, immunizations, measles, polio, rubella, vaccine-preventable, diseases
1. Introduction
Following the end of World War II, the United Nations was established in 1945 as an intergovernmental organization to promote international co-operation through its multilateral agencies including the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) [1]. Global partnerships along with scientific advancements led to the golden age of the use of vaccines in global public health [2–4]. In 1966, the WHO intensified smallpox eradication program was launched, and the United States Centers for Disease Control and Prevention (CDC) played a major leadership role. The goal was to achieve zero incidence of smallpox. The eradication program focused efforts to increase vaccination coverage, particularly through mass immunization campaigns. With accumulating knowledge, however, the strategy was later revised to focus on surveillance and containment in which (1) cases were identified and isolated to reduce transmission, and (2) contacts of the cases as well as contacts of the contacts were identified and vaccinated to prevent spread. This globally coordinated eradication program demonstrated the value of consensus-building, focused efforts to establish a measurable goal, and bringing the assets to bear needed to achieve program targets that reduced morbidity and mortality globally, as well as the importance of surveillance and evaluation in determining the optimal eradication strategy. The achievement of smallpox eradication, along with evidence showing the beneficial impact of economic investments in vaccines, led the World Health Assembly (WHA) to request WHO to establish the Expanded Programme on Immunization (EPI) in 1974 [5].
Following a global campaign focused on surveillance and vaccination, smallpox was declared eradicated by the WHA in 1980 (with the last naturally occurring case detected by surveillance in Somalia in 1977), and there was vigorous public health debate over whether measles or polio should be the next disease target for eradication [6–9]. Although many argued that the case for the technical feasibility of eradication was stronger for measles than for polio, the bold commitment of Rotary International and partners to fund polio eradication ultimately drove the final decision and advocacy for the WHA resolution passed in 1988 to achieve polio eradication by 2000. A major factor in the decision to focus on polio was the fact that the eradication ‘proof of principle’ had been clearly demonstrated, with elimination of indigenous wild poliovirus (WPV) transmission in many industrialized countries. In contrast, by the mid-1980s, no country had achieved and sustained elimination of measles; at that time, most countries had only a one-dose MCV schedule, since WHO had not yet recommended a two-dose primary series for measles immunization.
In 1988, the Global Polio Eradication Initiative (GPEI) was established through a partnership between the WHO, Rotary International, CDC, and UNICEF [10]. At that time, an estimated 350,000 children were paralyzed by polio worldwide and 125 countries reported polio cases. Following eradication efforts, by 2000, reported polio cases decreased 99% to <3500 annually, <33% of the world’s population lived in a polio endemic country, and 2 of the 6 WHO regions were certified as polio-free [11]. In 2000, Gavi, the Vaccine Alliance (Gavi), was established with support from donor countries and global partners, including the Bill & Melinda Gates Foundation (BMGF), to strengthen health systems and support EPI efforts to increase routine vaccination coverage in eligible countries based on income level [12]. By 2012, annual polio incidence decreased 99.9% and WPV elimination was achieved in all countries except Afghanistan, Pakistan, and Nigeria. Despite this enormous progress, the program faced several technical and programmatic challenges and cynicism within the public health community. In 2012, BMGF ramped up funding and advocacy for GPEI, and the WHA declared the completion of polio eradication a programmatic emergency. Today, low-level WPV transmission persists only in Afghanistan, Nigeria, and Pakistan [13].
In 2010, an expert advisory panel convened by WHO concluded that measles can and should be eradicated [14], and the WHO Strategic Advisory Group of Experts (SAGE) on Immunization endorsed these conclusions. In January 2011, the WHA Executive Board endorsed the SAGE recommendations. In 2012, the WHA endorsed the Global Vaccine Action Plan (GVAP), developed by global partners through the Decade of Vaccines, largely funded by BMGF, and launched at the 2010 World Economic Forum. GVAP aims to extend the full benefits of EPI to all by 2020, and it set a target to eliminate measles and rubella in five of the six WHO regions by 2020. In 2012, the Measles & Rubella Initiative (M&RI), a global partnership established in 2001 by five core partners, the American Red Cross, CDC, the United Nations Foundation, UNICEF, and WHO, launched the 2012–2020 Global Measles and Rubella Strategic Plan, with goals aligned to the GVAP [14,15]. In addition to the global GVAP goal for MR elimination, as of September 2013, all six regions have established regional goals for measles elimination by 2020 or earlier.
As noted earlier, measles and rubella eradication feasibility and benefits have long been established [6–9,16]; however, measles remains a major cause of child mortality, and rubella is the leading cause of birth defects among all infectious diseases globally, the recent Zika virus outbreaks notwithstanding [3,17–19]. Eradication of both diseases can be done together with integrated strategies, because inexpensive, highly effective measles and rubella vaccines can be easily coadministered as a combined vaccine, and both diseases can be detected through rash-fever case-based surveillance. The basic reproductive number (R0) for rubella is 6–7 and for measles is 9–18; with corresponding calculated herd immunity thresholds of 83–85% for rubella and 89–94% for measles. However, R0 estimates vary by setting and are dependent on context-specific determinants like birth rate, population density, and age-specific contact rates; setting-specific estimates can help determine the level of herd immunity needed to achieve local elimination [20]. Because of vaccine effectiveness (VE) and the vaccination coverage levels likely to be achieved, the herd immunity thresholds can be reached with one dose of vaccine for rubella and with two doses for measles. Therefore, the biggest technical challenge driving a combined ‘two-for-one’ eradication effort is the high transmissibility of measles virus [14–16,21,22].
The strategies for measles and rubella elimination are similar to those for polio eradication, and, include the following: (1) achieving and maintaining high levels of population immunity by providing high vaccination coverage, ideally through routine immunization systems or, when needed, supplemented by mass immunization campaigns called supplemental immunization activities (SIAs); (2) monitoring disease using effective surveillance and evaluating programmatic efforts to ensure progress; (3) developing and maintaining outbreak preparedness, responding rapidly to outbreaks, and managing cases appropriately; (4) communicating and engaging to build public confidence and demand for immunization; and (5) implementing research and innovation to improve the program [15]. With common features and similar strategies, direct carry-over of processes from polio can be made to measles and rubella elimination. For example, the technical training, skills, and experience needed for vaccinators, epidemiologists, laboratorians, and surveillance officers often overlap, and the same staff become involved in surveillance, case investigations, outbreak response, SIA planning and implementation for both polio eradication and measles and rubella elimination. In fact, measles and rubella elimination efforts rely heavily on GPEI assets, including support for EPI and surveillance staff and activities [21,23].
However, important differences in the diseases complicate matters and not every lesson learned from polio will be directly applicable. For example, the measles virus is highly transmissible via the airborne route, making close contacts in closed settings important transmission pathways [16]. Unlike polio, there is no silent transmission, as all measles cases have a universal clinical presentation of rash and fever. Also, in contrast to polio vaccine use in developing countries where many doses are needed to achieve herd immunity thresholds, there are very low rates of measles vaccine failure both in industrialized and developing countries after two doses [24]. Furthermore, polio vaccine viruses can mutate and take on the phenotypic neurovirulence properties of wild viruses. This does not happen with measles vaccine viruses. The very high measles VE allows measles epidemiology to accurately reflect measles-susceptibility in the population. Measles is frequently the first vaccine-preventable disease (VPD) detected, when weaknesses in immunization service delivery occur, thereby exposing areas of low vaccination coverage. Therefore, measles is often referred to as the ‘canary in the coalmine’ for EPI and as such, can be effectively used as a signal and driver for overall immunizations systems strengthening. The WHA resolution for polio eradication in 1988 emphasized that polio eradication should be designed to strengthen the overall EPI program; however, over time, the program became primarily ‘vertical,’ focusing heavily on frequent mass vaccination campaigns. In contrast to polio eradication strategies, MR elimination can be achieved with high two-dose coverage through routine immunization service delivery because of the very high VE of measles and rubella vaccines, or, in settings with suboptimal routine coverage, with periodic SIAs implemented every 3–5 years. Therefore, multiple repeated rounds of mass vaccination targeting the same age groups, needed with using oral polio vaccine (OPV) for polio eradication, will not be required. Thus, MR elimination can move forward with a ‘diagonal approach,’ using measles surveillance data to identify areas missed by vaccination and using efforts to achieve high immunization coverage to strengthen health systems [25,26].
There are challenges facing ongoing efforts to achieve the established global and regional measles and rubella elimination goals. Although the M&RI has existed since 2000, it is perennially under-resourced, relative to the financial resource requirements and program capacity needed to achieve the established goals. Currently, there is a stated ‘lack of appetite’ among some large donor agencies for committing to the M&RI vision of achieving a world without measles and rubella, or providing the resources needed to achieve the GVAP goals for measles and rubella elimination. In 2016, a mid-term review of the Global Measles and Rubella Strategic Plan 2012–2020 concluded that the basic strategic approaches articulated in the plan are valid to achieve the goals, but the strategies have not been fully implemented, largely due to lack of global political will reflected in inadequate resources and in some cases, a lack of country ownership. To build country commitment, SAGE recommended that each country establish a national verification committee, and that each region establish a regional verification commission to determine progress toward measles and rubella elimination goals. However, to achieve the goals, there is still a need for advocacy, political will, and provision of adequate resources. As the end of polio approaches and GPEI funds and capacity decrease, we aim to document lessons learned from polio eradication and describe key aspects of the diagonal approach to measles and rubella elimination.
2. Key lessons learned from polio eradication
2.1. Importance of high-quality data and surveillance
Any disease eradication or elimination effort begins and ends with the need for high-quality data and surveillance. High-quality vaccination coverage data for all birth cohorts, and identification and knowledge of susceptible subpopulations is critical for targeting tailored immunization strategies. However, coverage survey information alone, even at the subnational level, may not be adequate to detect subpopulations with low immunization uptake and high susceptibility. This is where detecting cases through surveillance can help. Surveillance data are needed to provide evidence for documenting disease burden, making program decisions, setting policies, refining strategies, demonstrating impact, and verifying the absence of disease. The programs for eradication and elimination of VPDs have instilled a data-driven approach within EPI to optimize vaccination coverage and equity necessary for reaching herd immunity thresholds to achieve and maintain interruption of virus transmission [27]. Working in partnership with countries, GPEI and M&RI have provided funding to establish nationwide case-based surveillance systems for acute flaccid paralysis (AFP) to detect polioviruses and rash-fever illness to detect measles and rubella viruses. These two systems are supported by the Global Polio Laboratory Network (GPLN) and the Global Measles and Rubella Laboratory Network (GMRLN) and coordinated by WHO [28–30]. The surveillance infrastructure includes large networks of surveillance officers, systems for specimen collection and transport to participating laboratories, standardized and quality-controlled laboratory testing, and data management and analysis systems to enable rapid data-driven outbreak response. The GMRLN is the largest global laboratory network, with 703 participating laboratories supporting surveillance in 191 countries; during 2010–2015, 742,187 serum specimens were tested by GMRLN [28]. Following the GPLN experience, GMRLN provides hands-on training and capacity building in participating laboratories and has established and currently maintains a rigorous quality control program with proficiency testing and a laboratory accreditation process [29].
Molecular epidemiology using virus sequencing, established nomenclature, and phylogenetic analysis has proven highly valuable for identifying poliovirus transmission pathways, sources, and patterns of virus importations (e.g. determining whether the virus is genetically related to other indigenous viruses or represents an importation; and, if so, what country is the likely source), and areas with poor surveillance [31–33]. Following the polio experience, the WHO has established standard nomenclature for measles and rubella viruses, and the GMRLN has established global molecular surveillance for measles and rubella viruses and databases for sharing sequences [34]. During 2010–2015, 27,023 measles virus sequences and 809 rubella virus sequences were submitted to GMRLN [35]. GMRLN established standard protocols for monitoring global genotype distribution and tracking transmission of measles and rubella viruses [36]. During 2010–2015, 7 of the 24 recognized measles virus genotypes and 5 of the 13 recognized rubella virus genotypes were detected [35]. The decreasing number of circulating genotypes indicates tremendous progress toward elimination with the interruption of historic chains of virus transmission. However, the narrowing of genomic diversity necessitates expanding the capacity for viral sequencing, molecular data management, and developing advanced methods for higher resolution analyses to monitor global transmission patterns of defined lineages within the remaining circulating virus genotypes [36]. Furthermore, because measles and rubella viruses are more stable and have lower mutation rates than polioviruses, high throughput sequencing of expanded windows of the genome and whole genomes is becoming increasingly important for measles and rubella elimination [31]. Sequencing of expanded windows will help track transmission pathways; however, there is a need to increase the number of specimens collected to be able to identify virus origins and spread.
The polio program established performance indicators and targets for AFP surveillance that proved highly effective for program monitoring. Based on this experience, measles-rubella case-based surveillance indicators were established and are closely monitored with weekly data reported by countries to WHO. High quality surveillance data, along with vaccination coverage data, are needed for evidence-based risk assessments to guide prioritization of programmatic strengthening and are critical for the process of verification of elimination [37–40].
Existing polio information systems and resources for surveillance could be easily retooled and transitioned for measles and rubella elimination. Polio cases are confirmed and analyzed by age, geographic location, and vaccination status; adjustments to the program (e.g. SIAs, focus on specific population groups and geographic areas) are made based on these analyses [41]. Similar resources should be devoted to measles and rubella surveillance. Each case of measles or rubella should be evaluated to determine whether it is a program failure (i.e. occurring in someone who should have been vaccinated but was not) or a strategy failure (e.g. a case in a child too young for vaccination according to the immunization schedule in place in a given country). A program failure should result in efforts to achieve increased immunization coverage, including strategies for communications to overcome vaccination hesitancy and increase demand for immunization services. A strategy failure should result in evaluation of whether changes in the strategy are needed, such as adjusting the age for vaccination.
Expanding global capacity to conduct high-quality measles-rubella case-based surveillance with information systems and resources available at the level of the polio eradication infrastructure will require investments. A polio transition plan is critical, particularly for measles-rubella surveillance that is currently heavily dependent on the approximately US$140 million provided annually by GPEI to support surveillance activities, personnel, and operational costs [42]. Critically, the global Ebola emergency response in West Africa relied heavily on GPEI and existing systems and infrastructure to identify, test, and confirm cases, and implement contact-tracing to contain the outbreak, particularly in Nigeria [43]. In addition, GPEI support for surveillance allows for case detection, confirmation, and response for Zika, Chikungunya, and Dengue [44]. Polio eradication infrastructure also has been used to detect and contain outbreaks of yellow fever, cholera, and meningitis.
2.2. Data-driven outbreak response
GPEI established global guidelines and monitoring indicators to ensure rapid outbreak response and enhanced surveillance [45]. Context-specific strategies were developed, for example, a ‘hot case’ definition in India to trigger more rapid response activities while waiting for laboratory confirmation. GPEI methods for defining target age groups and geographic scope for outbreak response immunization (ORI) activities, using population susceptibility profiles, surveillance data, modeling, and risk assessments are now being used for measles and rubella ORI. Additionally, polio eradication demonstrated the great value of implementing coordinated multi-country synchronized campaigns to target contiguous epidemiological blocks crossing national borders, defined by population dynamics, ethnography, and migration patterns; this strategy should be adopted to interrupt measles and rubella virus transmission.
Robust capacity for rapid outbreak response is necessary and effective to reduce morbidity and contain transmission, particularly in post-elimination and near elimination settings. However, experience from GPEI cautions against focusing efforts to ‘put out fires’ in periodic outbreaks following importations that can lead to diversion of resources in the program and suggests that early eradication efforts should concentrate program resources and efforts on interrupting virus transmission in known persistent ‘sanctuaries’ that are root sources of virus importations into other settings. This strategy of concentration on persistent reservoirs appears to be particularly important for measles elimination since the virus is so highly contagious and can easily travel from endemic settings to post-elimination or near elimination countries. Even though outbreaks in these settings remain small and limited, and the response can be well-implemented by the local program, the costs of controlling such outbreaks are substantial [46]. No matter the size, every outbreak requires a rapid response and should be used as an opportunity to continue advocacy for eradication and to spur action in countries where virus transmission is sustained.
2.3. Building acceptance and demand for vaccines
Polio eradication has faced challenges of vaccine hesitancy because of rumors and misconceptions, particularly among marginalized communities. GPEI developed a robust capacity for identifying communities with vaccine hesitancy, developed context-specific communications strategies, and forged local partnerships with traditional, religious, and community leaders. Engaging communities and identifying those not accessing immunization services, improving access for all, and developing innovative social mobilization methods were important tools for GPEI. Underserved mobile and migratory populations and communities in areas of insecurity were found to play a very important role in sustaining poliovirus transmission [47–49]. The M&RI has adopted GPEI approaches, formed a communications working group, and broadened the partnership to include civil society organizations that can help build acceptance and demand for vaccines.
In countries with low incidence of measles and rubella, there is a lack of fear and understanding of the seriousness of disease, despite well-established risks of measles infection, measles complications, and congenital rubella syndrome [50]. Additionally, there is an underappreciation of the contagiousness of measles virus, the household and economic costs of measles infection, and the role individuals play in sustaining virus transmission within households and communities. Misinformation about the safety of vaccines has exacerbated vaccine hesitancy, particularly in some post-elimination settings [51–53]. For example, in the United States, endemic measles was eliminated in 2000 [54], but sporadic outbreaks continue to occur following measles virus importations from other countries, usually among vaccine-hesitant and unvaccinated US travelers who become measles-infected during international travel and return home with the virus.
As has been the experience with polio eradication, pediatric societies and health-care providers play key roles in ensuring people understand the seriousness and risks of acquiring measles and rubella virus infections and the benefits and importance of receiving on-time vaccination. In addition, there is a need to move beyond traditional immunizations communication strategies of printing ‘posters and banners’ to electronic communications methods, including mobile phone reminders and social media platforms.
2.4. Research and innovation
Building and maintaining a capacity for research, innovation, and epidemiological studies is essential for having critical evidence to establish policy and strategies for EPI strengthening and disease elimination. For polio eradication, development and use of type-specific monovalent OPVs starting in 2005 and bivalent OPVs containing types 1 and 3 starting in 2009 helped address vexing vaccine failures that impeded progress, particularly in northern India. In India, multiple (≥7) doses of OPV were needed in high population density areas, and type-specific OPV and IPV played important roles in boosting immunity. IPV provides protection against type 2 poliovirus and is needed to reduce the risk of emerging type 2 vaccine-derived poliovirus (VDPV), following the switch from trivalent OPV (tOPV) that protected against poliovirus types 1, 2, and 3, to bivalent OPV (bOPV) that protects only against types 1 and 3 [55]. The innovative development and evaluation of intradermal fractional IPV overcame the cost per dose of IPV that long made it expensive for program use and allowed vaccine in temporary limited supply to be extended in reaching the target population [56]. Despite these lessons, the availability of these innovations appeared late in the program. Nevertheless, they proved to be game-changers for polio eradication [57].
Today, a similar potential game-changer for measles and rubella elimination is the microneedle patch that would greatly simplify logistics for delivery of vaccines, but it needs investments and expeditious development and licensure [58]. Although highly effective, the currently available MR vaccines are administered by subcutaneous injection using reconstituted, lyophilized vaccine that must be meticulously kept in a cold chain. Skilled health professionals are required for safe hypodermic injection and vaccine handling. Reconstitution errors and lack of adherence to strict vaccine handling requirements have led to adverse events and deaths, eroding trust in vaccines. Additionally, injection pain caused by hypodermic needles can be a deterrent for vaccination acceptance. Unlike currently available vaccination methods, microneedle patches cause little or no pain and are easily administered, permitting vaccination by minimally trained personnel. Microneedle patches require minimal storage and disposal capacity, are easily transported, do not require reconstitution with diluent, cannot be reused because they dissolve in the skin, and do not generate sharps waste. These advantages over currently available needle-and-syringe MR vaccines would make house-to-house vaccination campaigns possible, a strategy that proved to be a key to success for the smallpox and current polio eradication efforts. GPEI experience emphasizes the importance of investing in innovation to add new technologies to overcome eradication impediments and strongly supports expedited development and licensure of microneedle patches [57]. These recommendations were made by the International Task Force for Disease Eradication and the WHO SAGE in 2016 [59]. Partner action will be needed to allow innovations to compete with currently available technologies and gain market share, since there might be little impetus for manufacturers to change from their established products.
Mathematical modeling, particularly when high-quality surveillance data are available, is useful for strategic decision-making in elimination and eradication programs. For example, the global switch from tOPV to bOPV and IPV introduction in EPI were guided by modeling results [55,60]. Additionally, evaluations showing the effectiveness of intradermal fractional dose IPV paved the way for an essential tool now being used for the polio end-game [57]. The use of fractional dose IPV was recently recommended by SAGE to overcome global IPV supply shortages and disruptions [10]. In addition to research supporting novel vaccination delivery methods, AFP surveillance performance was supplemented by the development of environmental sampling of sewage systems and by refined protocols for virus culture and intra-typic differentiation of polioviruses (e.g. vaccine, vaccine-derived, or wild) that decreased time for case classification and implementation of outbreak response [30].
To support polio research, the Polio Research Committee (PRC) was established in 2008 [61]; it is primarily supported by Rotary International and BMGF and is coordinated by WHO. The PRC provides guidance on the strategic direction of the GPEI research program, identifies knowledge gaps, and determines research priorities. The PRC publicizes current research priorities, encourages submission of research proposals, reviews the proposals, and recommends selected projects to be funded by GPEI. This process has led to testing and licensing of new formulations of polio vaccines, modified vaccine schedules to improve mucosal and humoral immunity, new diagnostic tests and algorithms, improved strategies to reach underserved and migrant population groups, and development of lot quality assurance sampling for rapid independent assessments of vaccination coverage in specific areas. The PRC convenes semi-annual meetings for sharing polio research findings, proposed studies, and coordinating research activities with partners. M&RI partners have established the global research priorities for measles and rubella [62], and are building capacity similar to the PRC. In 2015, the M&RI established the Research and Innovation Working Group (RIWG) to facilitate prioritizing and implementing research projects [63]. However, the RIWG is early in its development and resources will be needed to fully establish a PRC-like body and program of systematically prioritizing and funding research projects similar to that done by GPEI.
2.5. Program management, governance, oversight, and accountability
Disease elimination and eradication requires a broad partnership of diverse agencies with highly motivated, technically skilled people working in a well-designed system with established strategic plans, program management, oversight, accountability, and an ability to adapt and evolve. In this regard, there are several lessons learned from GPEI. For example, the GPEI Strategy Committee was established to oversee management and execution of the GPEI strategic plan [64]. GPEI program management uses six management groups that report to the Strategy Committee, and develop multiyear planning and budgeting cycles, and strategic plans for specific phases of eradication to ensure predictable funding.
To support eradication, a framework for polio eradication certification was established, including the formation of national certification committees and regional and global certification commissions. These bodies operate independently from the national programs and provide another layer of accountability for implementation of the eradication strategies. Similarly, regional verification commissions and national verification committees for measles and rubella elimination have been established. Oversight of GPEI operations is the responsibility of the Polio Oversight Board (POB), composed of the heads of agencies of core GPEI partners. The POB meets quarterly to ensure high-level accountability across the GPEI partnership. To increase advocacy and transparency, GPEI donor agencies can participate in POB meetings [64]. Having established strong program management, governance, oversight, and accountability allows for great achievements. For example, the GPEI accomplished an unprecedented globally coordinated effort strengthening immunization service delivery: as wild poliovirus type 2 was declared eradicated in 2015, all 155 countries using OPV switched from tOPV to bOPV during April–May 2016 to reduce the disease burden of type 2 VDPVs [60].
Bold goals, including those for disease eradication, require high optimism; however, a process for injecting pragmatic realism, ensuring forthright situational assessments and unvarnished recommendations is helpful to encourage dynamic program evolution. GPEI established an Independent Monitoring Board (IMB) in 2010 to monitor and guide its progress toward stopping polio transmission globally [57]. The IMB negotiates partnership issues related to data sharing, territorialism, and perceived or real power imbalances, by making recommendations for greater collaborations, closer working relationships of people at operational and management levels, and regular meetings of heads of agencies. The IMB meets with senior program officials approximately every 6 months and generates a report providing a situational assessment, critical analysis, specific recommendations about individual polio-affected countries, and guidance for the overall strategic direction of the program [57]. For example, the IMB recommended putting forward the WHA resolution that declared the completion of polio eradication a programmatic emergency for global public health in May 2012. This resolution escalated advocacy for polio eradication, strengthened focus on the remaining poliovirus ‘sanctuaries,’ spurred on efforts to improve the quality of vaccination activities to reach every child, and encouraged further innovation.
Two key essential qualities of the IMB are its high level, effective leadership, and its staunch independence including an independent secretariat. These qualities have given a powerful voice for evidence-based IMB recommendations that are often quite critical, direct, specific, and push for accountability of GPEI partners. The IMB led to the formation of the POB to determine how to operationalize IMB recommendations and guide the evolution of the program to achieve its objectives. For example, based on IMB recommendations and POB actions, each polio-endemic country established a polio eradication task force, chaired by the president or prime minister to provide oversight at country level. The IMB was created at the global level to serve that role, and it is a unique body among large global health multilateral programs [57]. Although the structure for polio eradication was somewhat complex, a ramp up of capacity among the M&RI partners, including high-level oversight bodies, similar to that for polio eradication, is needed to achieve the established elimination goals, and eventual eradication.
2.6. International health regulations, global health security, and travel requirements for vaccination
Polio is a notifiable disease through the International Health Regulations (IHR) established in 2005 [65]. To ensure full implementation of IHR and to promote global health security, particularly related to threats from infectious diseases, the Global Health Security Agenda (GHSA), a broad partnership of governments, UN agencies, and civil society organizations, was launched in 2014 [66,67]. GHSA provides support to priority countries through a framework of ‘Prevent-Detect-Respond’ that includes measles vaccination coverage as a GHSA performance indicator. IHR and GHSA both provide important opportunities for achieving disease elimination and eradication. For example, when an event occurs that is preliminarily determined to be a public health emergency of international concern (PHEIC) by a WHO emergency committee, the WHO Director-General seeks IHR Emergency Committee temporary recommendations that are reviewed every 3 months during the emergency to respond to that event. In May 2014, following multiple new polio outbreaks due to international travel, the international spread of WPV was determined to be a PHEIC under IHR [68,69]. At the 13th meeting of the polio emergency committee on 24 April 2017, committee members reviewed current polio surveillance data for WPV1 and circulating VDPV (cVDPV) and determined the events relating to poliovirus continue to constitute a PHEIC. The committee also made recommendations that were endorsed by the WHO Director General, to categorize countries as ‘States infected with WPV1, cVDPV1 or cVDPV3 with potential risk for international spread,’ ‘States infected with cVDPV2,’ and ‘States no longer infected by WPV1 or cVDPV, but which remain vulnerable to re-infection by WPV or cVDPV,’ and to extend revised temporary recommendations, including vaccination of all travelers from some countries, to reduce the risk of international spread of poliovirus [70]. For measles, once the GVAP goal of elimination in 5 regions gets closer, international spread of measles virus via international travelers could be considered a PHEIC, allowing for important measures that could be recommended under IHR, to interrupt measles virus transmission and to protect global public health [71].
2.7. Global partnerships, shared vision, and political will
Global partnerships that include implementing partners, technical agencies, and donors are essential in creating and holding a shared vision for achieving bold goals for disease elimination and eradication. Establishing the targeted disease burden, estimating economic costs, and making an investment case for elimination or eradication are important for generating sufficient political will and sustaining advocacy to set and achieve a global goal, as was done for polio eradication (even if the investment case was created much later than the WHA resolution).
Disease elimination and eradication rely on adequate, predictable, and sustained funding to operate for success. Despite established partnerships and global goals for polio eradication and GVAP measles and rubella elimination goals, chronic funding shortfalls have hampered efforts to achieve them. To avoid disruptions in funding, GPEI established the Finance and Accountability Committee that is composed of major donors, chaired by a member of the POB, and meets quarterly to address financial needs, identify funding sources, and provide financial advice to the POB. The M&RI would benefit from a similar structure and process. Since 2001, the M&RI has developed a strategic plan and framework for program management, oversight, and accountability. The M&RI continues to transform, following GPEI experience and recommendations made by an external review in 2013 and mid-term review in 2016; however, investments have limited M&RI growth and capacity relative to GPEI [23].
3. Moving forward with a diagonal approach to measles and rubella elimination
As the global community approaches polio eradication, attention is being directed to maintaining a polio-free world, continuing health systems strengthening and working toward measles and rubella elimination. Although strategies for measles and rubella elimination have similarities with those for polio eradication, a vertical approach can be avoided. A ‘diagonal approach’ has been developed that will strengthen health systems to ensure on-time delivery of the first and second doses of a measles-containing vaccine (MCV1 and MCV2), followed by SIAs when routine coverage is suboptimal, and use of surveillance data to identify areas and populations where immunization services are weak or challenging (Table 1).
Table 1.
Key lessons learned and components for moving forward a diagonal approach to measles and rubella elimination.
| High-quality data and surveillance | • In 2016, the midterm review of the Global Measles and Rubella Strategic Plan 2012–2020 concluded that the basic strategic approaches articulated in the plan are valid to achieve the goals, and encouraged a greater focus on using measles incidence as a key indicator, along with vaccination coverage, to guide elimination efforts. |
| • Having high-quality vaccination coverage and surveillance data, and identification and knowledge of susceptible subpopulation is critical for targeting tailored immunization strategies for achieving elimination. | |
| • Measles-rubella case-based surveillance indicators should be closely monitored with weekly data reported by countries to WHO. | |
| • The narrowing of genomic diversity necessitates expanding the capacity for viral sequencing, molecular data management, and developing advanced methods for higher resolution analyses to monitor global transmission patterns of defined lineages within the remaining circulating virus genotypes. Sequencing of expanded windows will help track transmission pathways; however, there is a need to increase the number of specimens collected to be able to identify virus origins and spread. | |
| • Expand global capacity to analyze high-quality measles-rubella case-based surveillance with information systems for better strategic use of data for guiding elimination. Cases should be categorized as program or strategy failures. Program failures indicate the need to better reach persons for whom vaccine is recommended. Strategy failures should prompt evaluations of the strategy such as whether the recommended ages for vaccine doses are optimal. | |
| Data-driven outbreak response | • Establish global guidelines and monitoring indicators for rapid outbreak response and enhanced surveillance. |
| • Implement coordinated multi-country synchronized campaigns to target contiguous epidemiological blocks crossing national borders, defined by population dynamics, ethnography, and migration patterns. | |
| Building acceptance and demand for vaccines | • Develop a robust capacity for identifying communities with vaccine hesitancy, develop context-specific communications strategies, and forge local partnerships with traditional, religious, and community leaders to engage communities to improve access for all, and increase coverage and equity for all vaccines. |
| • Ensure an appreciation of the contagiousness of measles virus, the severity of disease and its complications, the household and economic costs of measles infection, and the role individuals play in sustaining virus transmission within households and communities | |
| • Move beyond traditional immunizations communication strategies of printing ‘posters and banners’ to electronic communications methods, including mobile phone reminders and social media platforms | |
| Research and innovation | • Build and maintain a capacity for research, innovation, and epidemiological studies that provides critical evidence to establish policy and strategies for EPI strengthening and disease elimination. |
| • Fully resource the Measles & Rubella Initiative (M&RI) Research and Innovation Working Group to establish current research priorities, solicit and recommend selected projects to be funded by M&RI, and convene semi-annual meetings for sharing research findings, proposed studies, and coordinating research activities with partners. | |
| • Investments in research and innovation would spur progress; and specifically, expeditious development and licensure of the MR microarray patch for vaccination, a potential gamechanger for elimination, should be of highest priority. | |
| • Build capacity for rapid mathematical modeling, using high-quality surveillance data, for strategic decision-making. | |
| Program management, governance, oversight, and accountability | • Ramp-up M&RI capacity, with a structure similar to the Global Polio Eradication Initiative (GPEI) for polio eradication, including high level oversight bodies. |
| • Further develop and broaden the M&RI partnership with highly motivated, technically skilled people working to establish strategic plans, program management, oversight, accountability, and an ability to adapt and evolve. | |
| • Strengthen program management by using multiyear planning and budgeting cycles, and developing strategic plans for specific phases of elimination. | |
| • Establish predictable, sustained funding. | |
| • Further develop operational oversight and high-level accountability, by forming an oversight board composed of the heads of agencies of core M&RI partners, and an independent monitoring board to assess and guide progress toward interrupting measles and rubella virus transmission globally. | |
| International Health Regulations (IHR), Global Health Security, and travel requirements for vaccination | • International spread of measles virus via international travelers could be considered a public health emergence of international concern, allowing for important measures that could be recommended under IHR, to interrupt measles virus transmission and to protect global public health. |
| Global partnerships, shared vision, and political will | • The midterm review of the Global Measles and Rubella Strategic Plan 2012–2020 concluded that the strategies have not been fully implemented, largely due to lack of global political will, that is reflected in inadequate resources and in some cases, a lack of country ownership. |
| Strengthen global partnership that has a shared vision by all partners for a world free of measles, rubella, and CRS. | |
| • Build political will by all partners and countries to achieve the Global Vaccine Action Plan and regional elimination goals. | |
| • Establish National Verification Committees and Regional Verification Commissions to build country ownership and accountability. | |
| Providing benefits for immunization systems strengthening | • A ‘diagonal approach’ will strengthen health systems to ensure on-time routine delivery of all recommended immunizations, strengthening overall EPI, followed by SIAs when routine coverage is suboptimal, and using measles surveillance data with a ‘canary in the coalmine’ approach to identify areas and populations where overall immunization services are weak or challenging. |
| • MCV2 delivery, often given last in the childhood series during the second-year of life, is a major opportunity to catch up on any missed vaccines and improve coverage with all recommended vaccines, and to deliver other child health interventions. MCV2 coverage should be used as a key performance indicator for EPI. | |
| • School entry vaccination checks and laws supporting vaccination requirements have proven to be a highly effective for increasing vaccination coverage and achieving measles elimination. Global guidelines should be developed to establish the necessary legislation and design a strategy for implementing school-entry immunization checks or other requirements to ensure receipt of the recommended two doses of MCV and all other recommended vaccines. | |
| Improving infection prevention and control | • Strengthen infection prevention and control practices, particularly during outbreaks, with clear guidance for appropriate case referral, effective triage and isolation facilities, and procedures to reduce the risk of nosocomial exposures and transmission. |
| • Measles and rubella elimination efforts would improve overall infection prevention and control systems in health-care facilities, particularly for other airborne pathogens. | |
| Polio transition and going forward | • It is essential to transition GPEI assets, eradication infrastructure, and lessons learned to support MR elimination. |
| • A global measles and rubella eradication goal established prior to GPEI termination would minimize potential losses of polio eradication technical expertise, institutional memory, experience, and know-how. | |
| • Global measles and rubella eradication would be cost-effective and save an estimated US$8 billion per year that is currently spent for treatment costs and US$88 billion per year in losses due to disability-adjusted life-years that is currently caused by measles and rubella infections. | |
| • Although a true eradication-level effort will likely not occur until a WHA resolution formally sets an eradication goal and adequate predictable, sustained funding is made available, the optimal strategy would be to ‘go big and go fast’ to best manage population immunity and interrupt virus transmission, by preventing accumulation of susceptibility spread across many age groups and large geographic areas. | |
| • Although a true eradication-level effort will likely not occur until a WHA resolution formally sets an eradication goal and adequate predictable, sustained funding is made available, the optimal strategy would be to ‘go big and go fast’ to best manage population immunity and interrupt virus transmission, by preventing accumulation of susceptibility spread across many age groups and large geographic areas. | |
| • Concentrate resources for focused efforts to interrupt virus transmission in known persistent reservoirs, to avoid the diversion of resources in the program caused by sporadic outbreaks following virus importations in post-elimination settings. | |
| • The 2016 Mid-term Review of the Global Measles and Rubella Strategic Plan 2012–2020 concluded that by no later than 2020, a comprehensive evaluation should be undertaken to determine if an eradication goal with a timeframe should be set. The review concluded that elimination strategies were sound and what is needed most is better implementation with adequate predictable sustained investments. |
This preferred strategic approach, capitalizing on investments and learning from the lessons of polio eradication, is feasible based on unique characteristics of measles and rubella viruses and existing tools to eliminate them. For example, in contrast to poliovirus, nearly all measles cases have symptoms detectable through surveillance, so, fortunately, there is no ‘silent’ transmission from asymptomatic infections. There is also no emergence of vaccine-derived viruses [22]. In addition, the VE of OPV is suboptimal and type-specific, and multiple doses of vaccine must often be administered in multiple rounds of mass vaccination campaigns to interrupt poliovirus transmission, with a carefully chosen strategy of using either tOPV, type-specific bOPV, type-specific monovalent OPV or inactivated polio vaccine (IPV), depending on the type of circulating WPVs or VDPVs. In contrast, there is only one serotype each for measles and rubella viruses, and measles and rubella vaccine VE is very high and equivalent for the combination vaccines. The VE for a single dose of MCV is 93– 95% and 97% for 2 doses when administered to persons aged ≥12 months; VE for a single dose of rubella-containing vaccine (RCV) is approximately 95% among infants 9 months of age; and both MCV and RCV provide long-lasting, likely lifelong protection. Finally, volunteers in house-to-house campaigns can easily administer OPV; however, measles- and rubella-containing vaccine is an injectable vaccine with complicated cold chain & logistics that must be administered by trained health workers, primarily at fixed sites and clinics.
Therefore, measles and rubella elimination efforts must have a greater focus on strengthening access to and demand for routine immunization service delivery and on investing in innovations to improve vaccine delivery methods. Increasing routine immunization coverage as an essential component of measles and rubella elimination efforts constitutes the ‘diagonal approach’ to strengthen overall EPI vaccine delivery systems, in which all recommended vaccines can be delivered. In contrast, polio eradication became heavily dependent on frequent rounds of mass vaccination campaigns, usually focused on providing OPV as the only vaccine [25].
3.1. Providing benefits for immunization systems strengthening
One potential advantage of measles contagiousness is the fact that where immunization systems are weak, measles is likely to be the first disease recognized. And real disease, disability, and death may be more effective motivators for generating political will compared with warnings or predictions of problems to come based on low immunization coverage [23]. For example, the United States built its overall immunization program around efforts to eliminate measles over a period of 34 years (1966–2000). As elimination efforts progressed, the immunization system created to achieve the goal incorporated important policies such as eliminating financial barriers to access of vaccines, measuring immunization coverage in every state to help hold states accountable, and enacting and enforcing school immunization requirements for school attendance [72].
Measles is an ideal tracer for EPI performance, since measles outbreaks visibly signal areas that have suboptimal immunization service delivery and can drive prioritization of targeted interventions to improve program performance and ensure accountability [22,25]. Measles is increasingly concentrated in areas with the lowest vaccination coverage [12,18]. The recent WHO Measles and Rubella Global Strategic Plan Midterm Review emphasized the limits of MCV coverage data as an indicator and recommended, with SAGE endorsement, using measles incidence as another indicator for EPI program performance and to guide elimination efforts [23].
In 2009, WHO recommended that two doses of MCV be provided through routine immunization services. During 2000–2015, the number of countries providing MCV2 nationally increased from 97 (51%) to 160 (82%), and estimated global MCV2 coverage increased from 15% to 61% [18]. MCV2 introduction creates new opportunities to deliver other child health interventions beyond the first year of life, and it can provide an opportunity to catch up on any missed vaccines and improve coverage with all recommended vaccines [73]. MCV2 coverage should be used as a key performance indicator for EPI.
School entry vaccination checks and laws supporting vaccination requirements have proven to be a highly effective strategy for increasing vaccination coverage and preventing outbreaks and were critical to strategies that achieved measles elimination in the United States [74,75]. School-based vaccination strategies were also effective in South Korea, where measles elimination efforts led to enhanced VPD surveillance and the start of vaccination requirements for school entry [76,77]. In China, measles elimination strategies led to a school entry vaccination check law established in 2005; although not enforced from the central level, a province-level demonstration project showed that a school entry vaccination check led to an increase in vaccination coverage [78]. In the Philippines, following a large measles outbreak in 2014, the government established a school entry vaccination check [79]. In 2017, after battling sporadic measles outbreaks following multiple importations, including cases among school-aged children, both Italy and France established laws requiring children to be fully vaccinated before they can enroll in school [80,81]. To facilitate the process in other countries, global guidelines should be developed to establish the necessary legislation and design a strategy for implementing school-entry immunization checks or other requirements to ensure receipt of the recommended two doses of MCV and all vaccines.
3.2. Improving infection prevention and control
Because of the high transmissibility of measles virus via the airborne route, nosocomial transmission often plays an important role in the amplification of measles outbreaks and sustaining measles virus transmission, even in settings with high vaccination coverage [82,83]. Measles and rubella elimination efforts will require attention to health-care facility (HCF) infection prevention and control practices, particularly during outbreaks, with provision of clear guidance for appropriate case referral, effective triage and isolation facilities, and procedures to reduce the risk of measles and rubella virus transmission through nosocomial exposures [84]. Strategies to limit nosocomial transmission should emphasize documented measles and rubella immunity among all HCF personnel, primary care and home-based case management for uncomplicated measles, specific triage procedures with designated triage areas for suspected cases, well-equipped isolation rooms with appropriate ventilation or negative pressure flow, supplies to exercise respiratory precautions, and vaccination screening of children attending HCFs [82,83,85,86]. Measles and rubella elimination efforts would thus focus efforts to improve overall infection prevention and control systems in HCFs.
4. Conclusions
Through the GPEI partnership, millions of volunteers, social mobilizers, and health workers have been trained and mobilized for disease eradication; neglected households in underserved communities not routinely reached by health services have been identified, mapped, and accessed with vaccines and other child survival interventions; and a quality-controlled real-time global disease surveillance system with rapid outbreak response capacity has been established during 30 years of operations [47,87,88]. As the end of polio eradication approaches, to maximize returns on investments, it is essential to transition GPEI assets, eradication infrastructure, and lessons learned to support other disease elimination or control programs. There is a risk that polio funding currently supporting measles and rubella elimination efforts may dry up, if these funds are not replaced. Current funding and other resources, technical expertise, and workforce could be maintained and shifted to MR elimination efforts, as they become no longer needed for polio. Measles and rubella elimination programs are most closely aligned and also present an opportunity to use a diagonal approach to strengthen overall health and immunizations systems [89]. A global measles and rubella eradication goal established prior to GPEI termination would minimize potential losses of polio eradication technical expertise, institutional memory, experience, and know-how.
5. Expert commentary
GPEI received US$14.3 billion in funding and commitments during 1988–2019; however, annual funding levels are decreasing rapidly as eradication approaches [90]. Following eradication of polio, GPEI efforts will be phased out. The phasing-out of GPEI presents widespread risks of reversing progress made in immunizations and the prevention of VPDs [88,89]. In 2016, 25% of all money spent by WHO came from polio funding [91]. Currently, in the African Region, approximately 90% of WHO-funded immunization staff and infrastructure are supported by GPEI funding. In the African and South-East Asian Regions combined, >1000 WHO staff positions and >6000 non-staff workers (e.g. community health workers) are paid using polio funds. For measles-rubella surveillance globally, >2500 workers are paid using polio funds, and approximately US$77 million (70% of the overall cost) annually would be needed to replace the GPEI resources that support measles-rubella surveillance [91].
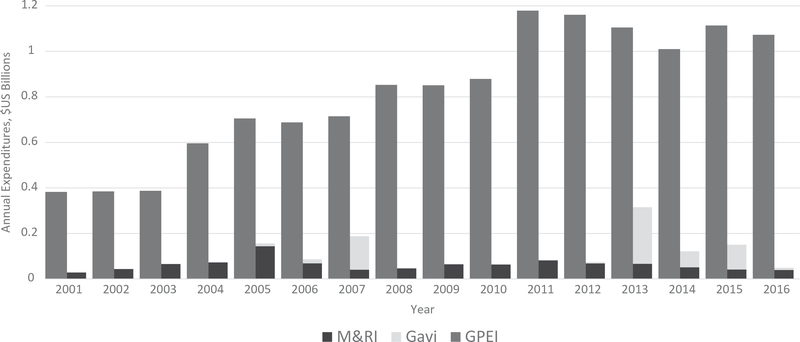
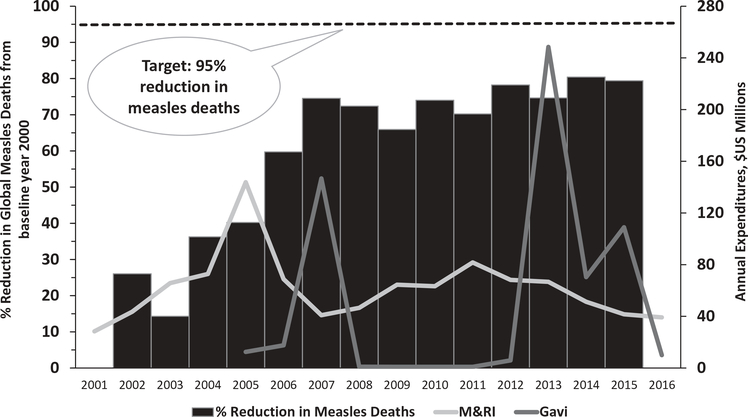
The M&RI, which has a vision of a world free of measles and rubella, has received US$1.1 billion in funding during 2001–2016; however, annual expenditures on MR elimination have been dwarfed by the funds made available for polio eradication (Figure 1). During 2000–2015, the number of estimated annual measles deaths decreased 79%, from 651,600 to 134,200, preventing an estimated 20.3 million deaths during that period (Figure 2). Despite this progress and strategy of high control, current costs include several billion dollars annually for ongoing vaccination activities, and treatment costs, disability-adjusted life-years and productivity losses due to the significant numbers of infections that occur annually. Compared with high control, global measles and rubella eradication would be cost-effective. Estimates for 2013 suggest current global measles and rubella control costs approximately US$58 billion per year, the bulk of which could be saved each year by eradication [8,92–94].
Figure 1.
Annual expenditures of the Global Polio Eradication Initiative (GPEI), Measles & Rubella Initiative (M&RI) and Gavi, the Vaccine Alliance* (Gavi), 2001–2016.
Data sourced from: http://polioeradication.org/Financing. http://measlesrubellainitiative.org/. Midterm Review of the Measles and Rubella Global Strategic Plan, 2012–2020.
*Represents measles-related Gavi expenditures. Not shown here, approximately US$800 million available to Gavi-eligible countries during 2016–2020, approved by the Gavi Board in December 2015, as part of a new Gavi comprehensive measles and rubella strategy.
Figure 2.
Global reduction in estimated measles deaths* and annual expenditures of the Measles & Rubella Initiative (M&RI) and Gavi, the Vaccine Alliance** (Gavi), 2001–2016.
Data sourced from: MMWR Morb Mortal Wkly Rep. 2016;65:1228–33. Midterm Review of the Measles and Rubella Global Strategic Plan 2012–2020.
*In 2010, the World Health Assembly established three milestones for measles control by 2015: 1) increase routine coverage with the first dose of measles-containing vaccine for children aged 1 year to ?90% nationally and ?80% in every district; 2) reduce global annual measles incidence to <5 cases per million population; and 3) reduce global measles mortality by 95% from the 2000 estimate.
**Represents measles-related Gavi expenditures. Not shown here, approximately US$800 million available to Gavi-eligible countries during 2016–2020, approved by the Gavi Board in December 2015, as part of a new Gavi comprehensive measles and rubella strategy.
Now that all six WHO regions have set measles elimination goals, in effect establishing an informal global eradication goal, if adequate predictable funding became available, then the optimal approach to strategy implementation would be to ‘go big and go fast’ [95]. This approach would best manage population immunity and interruption of virus transmission, by preventing accumulation of susceptibility spread across many older birth cohorts and avoiding susceptibility in large geographic areas that could serve as virus reservoirs. It would also minimize the emergence of vaccine refusals that can occur when low disease incidence over time reduces perceptions of risks to disease and the seriousness of its complications. GPEI demonstrated the utility of a strategy that concentrated resources for focused efforts to interrupt virus transmission in known persistent virus ‘sanctuaries,’ to avoid the diversion of resources in the program caused by sporadic outbreaks following virus importations in post-elimination settings.
At this point in global measles elimination efforts, discernible chronic measles virus reservoirs are coming to light based on surveillance data. For example, Ethiopia, Democratic Republic of the Congo, India, Indonesia, Nigeria, and Pakistan accounted for more than half of the 20 million infants who missed measles vaccination in 2015 and 75% of all estimated measles deaths worldwide. In the African region, 84% of reported measles cases occurred in the four countries that accounted for half of children who missed MCV1 during 2013–2016: Nigeria (44%), Ethiopia (22%), Angola (10%), and DRC (8%). Recognizing concentration of measles burden, the M&RI and Gavi recently joined forces to coordinate efforts toward measles elimination in the countries with the largest numbers of unvaccinated (for measles) children. However, a true eradication-level effort will likely not occur until and unless a WHA resolution is passed that formally sets an eradication goal and adequate sustained funding is made available. Measles will not simply ‘burn itself out’ over time without a concentrated effort to interrupt virus transmission, particularly in safe havens among the world’s most vulnerable communities. Without a formal measles and rubella eradication goal, the current status quo of maintaining high control, allowing >130,000 estimated measles deaths and >100,000 CRS cases annually and costing governments and donors US$58 billion per year, will continue in perpetuity [17,18].
The economic benefits of investing in vaccines, particularly measles-rubella vaccines, are well established [96–98]. Economic benefits vary by setting and depend on the amount of resources spent on treatment costs, and response activities such as contact tracing. When accounting for broad economic benefits, vaccines have an overall estimated return on investment of 44 times the cost (uncertainty range: 27–67) [96]. Of all the recommended vaccines, the highest return on investment is for measles vaccine, at 58 times the cost (uncertainty range: 28–105) through provision of two routine immunization doses and outreach campaigns [96]. Measles and rubella disease burden is preventable with one of the least expensive vaccines available and is increasingly concentrated in the most vulnerable communities in the world; measles deaths occur almost exclusively in countries with the lowest sociodemographic index, a measure of development consisting of income per capita, average years of education, and total fertility rates [99]. In 2015, world leaders agreed to a new set of Sustainable Development Goals (SDGs); expanding access to immunizations is crucial to achieving the SDGs [100]. Since the global EPI started in 1974, investments in vaccinations have become widely recognized as critical to preventing disease morbidity and mortality, and fundamental to achieving broader economic and development goals. Moreover, since measles and rubella increasingly disproportionately affect those most vulnerable among us, achieving global measles and rubella eradication ultimately would be a major achievement of global health equity.
6. Five-year view
The 2016 Mid-term Review of the Global Measles and Rubella Strategic Plan 2012–2020 stated that the failure to achieve any of the midterm goals indicated it was premature to set a measles and rubella eradication goal at this time [23]. However, no later than 2020, a comprehensive evaluation should be undertaken to determine if an eradication goal with a timeframe should be set. The review concluded that elimination strategies were sound and what is needed most is better implementation. Efforts should focus on improving immunization systems and reorienting the program to increase emphasis on surveillance strengthening, using surveillance data to drive programmatic decisions and actions. The report made recommendations for (1) improving surveillance, (2) achieving high levels of population immunity with two doses of measles and rubella containing vaccines, (3) detecting and responding to outbreaks, (4) communicating to build public confidence and demand for immunization, (5) performing research and development to support cost-effective operations and improve vaccination and diagnostic tools, (6) building on the polio transition, (7) assuring there is effective governance of the initiative, and (8) advocating and mobilizing resources. In the meantime, the Mid-term Review stated all WHO regions and partners should work toward achieving the GVAP goals and regional measles elimination goals by 2020.
Key issues.
During 2000–2015, the number of estimated annual measles deaths decreased 79%, from 651,600 to 134,200, preventing an estimated 20.3 million deaths during that period.
Achieving measles and rubella (MR) elimination after polio would make a permanent impact on reducing child mortality, but must be done with a ‘diagonal approach’ focused on using measles surveillance data to strengthen immunization and health systems.
The M&RI, with a vision of a world free of measles and rubella, received US$1.1 billion in funding during 2001–2016; but lacks predictable sustained investments for operations and to build capacity.
Investments in research and innovation would spur progress, including expeditious development and licensure of the MR microneedle patch for vaccination, a potential game-changer for elimination.
Although all six WHO regions now have measles elimination goals, without a formal measles and rubella eradication goal and adequate predictable funding, the current status quo of maintaining high control, costing governments and donors US$58 billion per year, will continue in perpetuity.
The 2016 Mid-term Review of the Global Measles and Rubella Strategic Plan 2012–2020 stated no later than 2020, a comprehensive evaluation should be undertaken to determine if a measles eradication goal with a timeframe should be set.
Acknowledgments
Funding
The manuscript was not funded.
Footnotes
Declaration of interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.United Nations. Charter of the United Nations. [cited 2017 May 20]. Available from: www.un.org
- 2.United Nations. Sustainable development goals. 2016. [cited 2017 May 10]. Available from: http://www.un.org/sustainabledevelopment/sustainable-development-goals/
- 3.United Nations General Assembly. United Nations millennium declaration. New York (NY): United Nations General Assembly; 2000. [cited 2017 May 10]. Available from: http://www.un.org/millenniumgoals/ [Google Scholar]
- 4.United Nations. Millennium development goals indicators. 2008. [cited 2017 Jul 24]. Available from: https://mdgs.un.org/unsd/mdg/Host.aspx?Content=Indicators/OfficialList.htm
- 5.Okwo Bele J-M, Cherian T. The expanded programme on immunization: a lasting legacy of smallpox eradication. Vaccine. 2011;29 (Suppl 4):D74–D9. [DOI] [PubMed] [Google Scholar]
- 6.Robbins FC. Prospects for worldwide control of measles: discussion I. Rev Infect Dis. 1983;5:619–620. [Google Scholar]
- 7.Dowdle W, Cochi S. The principles and feasibility of disease eradication. Vaccine. 2011;29(Suppl 4):D70–D73. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins DR, Hinman AR, Koplan JP, et al. The case for global measles eradication. Lancet. 1982;1(8286):1396–1398. [DOI] [PubMed] [Google Scholar]
- 9.International Task Force for Disease Eradication. Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep. 1993;42:1–38. [PubMed] [Google Scholar]
- 10.Strategic Advisory Group of Experts. Meeting of the strategic advisory group of experts on immunization, April 2017 - conclusions and recommendations. Wkly Epidemiol Rec. 2017;92:301–320. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Progress toward global poliomyelitis eradication, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:320–2, 31. [PubMed] [Google Scholar]
- 12.Casey R, Dumolard L, Danovaro Holliday MC, et al. Global routine vaccination coverage, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1270–1273. [DOI] [PubMed] [Google Scholar]
- 13.Morales M, Tangermann RH, Wassilak SG. Progress toward polio eradication - worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep. 2016;65:470–473. [DOI] [PubMed] [Google Scholar]
- 14.Strebel PM, Cochi SL, Hoekstra E, et al. A world without measles. J Infect Dis. 2011;204:S1–S3. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO). Global measles and rubella strategic plan 2012–2020. 2012. [cited 2017 Mar 7]. Available from: http://apps.who.int/iris/bitstream/10665/44855/1/9789241503396_eng.pdf• The Global Measles and Rubella Strategic Plan provides the vision and strategies to achieve a world free of measles and rubella, and set targets and goals aligned with the Global Vaccine Action Plan.
- 16.Peter MS, Papania MJ, Gastañaduy PA, et al. Vaccines 7th ed. Philadelphia, PA: Elsevier; 2018. Chapter 38, Measles Vaccines. [Google Scholar]
- 17.Vynnycky E, Adams E, Cutts F, et al. Using seroprevalence and immunisation coverage data to estimate the global burden of congenital rubella syndrome, 1996–2010: a systematic review. PLoS One. 2016;11:e0149160–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel M, Gacic Dobo M, Strebel P, et al. Progress toward regional measles elimination - worldwide, 2000–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1228–1233. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Global vaccine action plan 2011–2020. Geneva: World Health Organization; 2012. [cited 2017 September 20]. Available from: http://www.who.int/immunization/global_vaccine_action_plan/en/.• The GVAP, signed by heads of agencies and approved by WHA in 2012, is the key document guiding all global immunization efforts, that set a goal to achieve measles and rubella elimination in five of the six WHO Regions by 2020.
- 20.Guerra FM, Bolotin S, Lim G, et al. The basic reproduction number(R0) of measles: a systematic review. Lancet Infect Dis. 2017. July 27 pii: S1473–3099(17)30307–9. doi: 10.1016/S1473-3099(17)30307-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.WHO. Meeting of the International Task Force for Disease Eradication, November 2015. Wkly Epidemiol Rec. 2016;91:61–71. [PubMed] [Google Scholar]
- 22.Rota P, Moss W, Takeda M, et al. Measles. Nat Rev Dis Primers. 2016;2:16049.•• Important comprehensive review of measles disease in the pre- and postvaccination era, and the outlook for achieving measles eradication.
- 23.Orenstein WA, Nkowane B, Olive JM, et al. Measles and rubella global strategic plan 2012–2020 midterm review. 2016. [cited 2017 Sep 20]. Available from: http://www.who.int/immunization/sage/meetings/2016/october/1_MTR_Report_Final_Color_Sept_20_v2.pdf?ua=1• Concluded to elimination strategy implementation should focus on improving immunization systems, increasing emphasis on surveillance strengthening, using surveillance data to drive programmatic decisions and actions, and that a comprehensive evaluation should be undertaken no later than 2020, to determine if an eradication goal with a timeframe should be set.
- 24.Roland WS, Kew OM, Cochi SL, et al. Vaccines 7th ed. Philadelphia, PA: Elsevier; 2018. Chapter 49, Poliovirus Vaccine – Live. [Google Scholar]
- 25.Orenstein W, Seib K. Beyond vertical and horizontal programs: adiagonal approach to building national immunization programs through measles elimination. Expert Review of Vaccines. 2016;15:791–793. [DOI] [PubMed] [Google Scholar]
- 26.Kretsinger K, Strebel P, Kezaala R, et al. Transitioning lessons learned and assets of the global polio eradication initiative to global and regional measles and rubella elimination. J Infect Dis. 2017;216:S308–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulders MN, Serhan F, Goodson JL, et al. Expansion of surveillance for vaccine-preventable diseases: building on the global polio laboratory network and the global measles and rubella laboratory network platforms. J Infect Dis. 2017;216:S324–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulders MN, Rota PA, Icenogle JP, et al. Global measles and rubella laboratory network support for elimination goals, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:438–442. [DOI] [PubMed] [Google Scholar]
- 29.Featherstone DA, Rota PA, Icenogle J, et al. Expansion of the global measles and rubella laboratory network 2005–09. J Infect Dis. 2011;204:S491–S8. [DOI] [PubMed] [Google Scholar]
- 30.Maes EF, Diop OM, Jorba J, et al. Surveillance systems to track progress toward polio eradication - worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep. 2017;66:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rota P, Bankamp B. Whole-genome sequencing during measles outbreaks. J Infect Dis. 2015;212:1529–1530. [DOI] [PubMed] [Google Scholar]
- 32.Rota PA, Brown K, Mankertz A, et al. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204:S514–S23. [DOI] [PubMed] [Google Scholar]
- 33.Kidd S, Goodson J, Aramburu J, et al. Poliomyelitis outbreaks in Angola genetically linked to India: risk factors and implications for prevention of outbreaks due to wild poliovirus importations. Vaccine. 2011;29:3760–3766. [DOI] [PubMed] [Google Scholar]
- 34.WHO. Measles virus nomenclature update: 2012. Wkly EpidemiolRec. 2012;87:73–81. [PubMed] [Google Scholar]
- 35.WHO. Global measles and rubella laboratory network–update. Wkly Epidemiol Rec. 2005;80:384–388. [PubMed] [Google Scholar]
- 36.WHO. Genetic diversity of wild-type measles viruses and the globalmeasles nucleotide surveillance database (MeaNS). Wkly Epidemiol Rec. 2015;90:373–380. [PubMed] [Google Scholar]
- 37.Lam E, Schluter WW, Masresha BG, et al. Development of a districtlevel programmatic assessment tool for risk of measles virus transmission. Risk Analysis. 2017;37:1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kriss J, Stanescu A, Pistol A, et al. The World Health Organization measles programmatic risk assessment tool-Romania, 2015. Risk Analysis. 2017;37:1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goel K, Naithani S, Bhatt D, et al. The World Health Organization measles programmatic risk assessment tool-pilot testing in India, 2014. Risk Analysis. 2017;37:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriss JL, De Wee RJ, Lam E, et al. Development of the World Health Organization measles programmatic risk assessment tool using experience from the 2009 measles outbreak in Namibia. Risk Analysis. 2017;37:1072–1081. [DOI] [PubMed] [Google Scholar]
- 41.Wassilak SGF, Williams CL, Murrill CS, et al. Using acute flaccid paralysis surveillance as a platform for vaccine-preventable disease surveillance. J Infect Dis. 2017;216:S293–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Global Polio Initiative Eradication (GPEI). Global polio eradication initiative. Financial Resource Requirements. 2016. [cited 2017 Sept 20. Available from: http://polioeradicationorg/wp-content/uploads/2016/10/FRR2013-2019_April2016_EN_A4pdf
- 43.Shuaib F, Gunnala R, Musa EO, et al. Ebola virus disease outbreak Nigeria, July-September 2014. MMWR Morb Mortal Wkly Rep. 2014;63:867–872. [PMC free article] [PubMed] [Google Scholar]
- 44.Kouadio K, Okeibunor J, Nsubuga P, et al. Polio infrastructure strengthened disease outbreak preparedness and response in the WHO African Region. Vaccine. 2016;34:5175–5180. [DOI] [PubMed] [Google Scholar]
- 45.GPEI. Tools, protocols, and guidelines – outbreak preparedness and response. [cited 2017 May 23]. Available from: http://polioeradication.org/tools-and-library/resources-for-polio-eradicators/gpeitools-protocols-and-guidelines/
- 46.Ortega-Sanchez IR, Vijayaraghavan M, Barskey AE, et al. The economic burden of sixteen measles outbreaks on United States public health departments in 2011. Vaccine. 2014;32:1311–1317. [DOI] [PubMed] [Google Scholar]
- 47.Cochi S, Freeman A, Guirguis S, et al. Global polio eradication initiative: lessons learned and legacy. J Infect Dis. 2014;210(Suppl 1):S540–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine. 2011;29(Suppl 4):D80–D85. [DOI] [PubMed] [Google Scholar]
- 49.Habib MA, Soofi S, Cousens S, et al. Community engagement and integrated health and polio immunisation campaigns in conflict-affected areas of Pakistan: a cluster randomised controlled trial. Lancet Glob Health. 2017;5:e593–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodson J, Seward J. Measles 50 years after use of measles vaccine. Infect Dis Clin North Am. 2015;29:725–743. [DOI] [PubMed] [Google Scholar]
- 51.Hyle EP, Rao SR, Jentes ES, et al. Missed opportunities for measles, mumps, rubella vaccination among departing U.S. adult travelers receiving pretravel health consultations. Ann Intern Med. 2017;167 (2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dyer O Measles outbreak in Somali American community follows anti-vaccine talks. BMJ. 2017;357:j2378. [DOI] [PubMed] [Google Scholar]
- 53.Parker AA, Staggs W, Dayan GH, et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355:447–455. [DOI] [PubMed] [Google Scholar]
- 54.Papania MJ, Wallace GS, Icenogle JP, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatrics. 2014;168:148–155. [DOI] [PubMed] [Google Scholar]
- 55.CDC. Introduction of inactivated poliovirus vaccine and switch from trivalent to bivalent oral poliovirus vaccine - worldwide, 2013–2016. MMWR Morb Mortal Wkly Rep. 2015;64:699–702. [PMC free article] [PubMed] [Google Scholar]
- 56.Bahl S, Verma H, Bhatnagar P, et al. Fractional-dose inactivated poliovirus vaccine immunization campaign - Telangana State, India, June 2016. MMWR Morb Mortal Wkly Rep. 2016;65:859–863. [DOI] [PubMed] [Google Scholar]
- 57.Rutter PD, Donaldson LJ. Oversight role of the independent monitoring board of the global polio eradication initiative. J Infect Dis. 2014;210(Suppl 1):S16–22. [DOI] [PubMed] [Google Scholar]
- 58.Durrheim DN, Goodson JL. Time for an immunisation paradigm shift. Trans R Soc Trop Med Hyg. 2017;111:41–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO. Meeting of the strategic advisory group of experts on immunization, October 2016 - conclusions and recommendations. Wkly Epidemiol Rec. 2016;91:561–582. [PubMed] [Google Scholar]
- 60.Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine - worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:934–938. [DOI] [PubMed] [Google Scholar]
- 61.GPEI. Polio Research Committee. 2008. [cited 2017 May 23]. Available from: http://polioeradication.org/tools-and-library/research-innovation/polio-research-committee/
- 62.Goodson JL, Chu SY, Rota PA, et al. Research priorities for global measles and rubella control and eradication. Vaccine. 2012;30 (32):4709–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Measles & Rubella Initiative (M&RI). M&RI Research and Innovation Working Group; [cited 2017 May 23]. Available from: http://measlesrubellainitiativeorg/research-innovation/. [Google Scholar]
- 64.GPEI. Governance. [cited 2017 May 24] http://polioeradication.org/who-we-are/governance/.
- 65.WHO. Case definitions for the four diseases requiring notification in all circumstances under the International Health Regulations. 2005. [cited 2017 May 23]. Available from: http://www.who.int/ihr/Case_Definitions.pdf
- 66.Frieden T, Tappero J, Dowell S, et al. Safer countries through global health security. Lancet. 2014;383:764–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.WHO. The International Health Regulations (IHR) - 10 years of global public health security. Wkly Epidemiol Rec. 2017;92:321–323. [PubMed] [Google Scholar]
- 68.WHO. WHO statement on the second meeting of the International Health Regulations Emergency Committee concerning the international spread of wild poliovirus. Saudi Med J. 2014;35:920–922. [Google Scholar]
- 69.Rutter PD, Donaldson LJ. Mandatory polio vaccination for travellers: protecting global public health. Lancet. 2014;383:1695–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO. Statement of the 13th IHR Emergency Committee regarding the international spread of poliovirus. 2017. [cited 2017 May 23]. Available from: http://www.who.int/mediacentre/news/statements/2017/13th-ihr-polio/en/
- 71.Simons H, Patel D. International Health Regulations in practice: focus on yellow fever and poliomyelitis. Hum Vaccin Immunother. 2016;12:2690–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Orenstein WA. The role of measles elimination in development of a national immunization program. Pediatr Infect Dis J. 2006;25:1093–1101. [DOI] [PubMed] [Google Scholar]
- 73.WHO. Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84:349–360. [PubMed] [Google Scholar]
- 74.Orenstein WA, Hinman AR. The immunization system in the United States - the role of school immunization laws. Vaccine. 1999;17:S19–S24. [DOI] [PubMed] [Google Scholar]
- 75.Jiles RB, Fuchs C, Klevens RM. Vaccination coverage among children enrolled in Head Start programs or day care facilities or entering school. MMWR CDC Surveill Summ. 2000;49:27–38. [PubMed] [Google Scholar]
- 76.Vandelaer J, Olaniran M. Using a school-based approach to deliver immunization—global update. Vaccine. 2015;33:719–725. [DOI] [PubMed] [Google Scholar]
- 77.Centers for Disease Control and Prevention. Elimination of measles–South Korea, 2001–2006. MMWR Morb Mortal Wkly Rep. 2007;56:304–307. [PubMed] [Google Scholar]
- 78.Zuo S, Cairns L, Hutin Y, et al. Accelerating measles elimination and strengthening routine immunization services in Guizhou Province, China, 2003–2009. Vaccine. 2015;33:2050–2055. [DOI] [PubMed] [Google Scholar]
- 79.Takashima Y, Schluter WW, Mariano KM, et al. Progress toward measles elimination-Philippines, 1998–2014. MMWR Morb Mortal Wkly Rep. 2015;64:357–362. [PMC free article] [PubMed] [Google Scholar]
- 80.Magurano F, Baggieri M, Filia A, et al. Towards measles elimination in Italy: virological surveillance and genotypes trend (2013–2015). Virus Res. 2017;236:24–29. [DOI] [PubMed] [Google Scholar]
- 81.Arie S Compulsory vaccination and growing measles threat. BMJ British Med J. 2017;358:j3429–j. [DOI] [PubMed] [Google Scholar]
- 82.Marshall TM, Hlatswayo D, Schoub B. Nosocomial outbreaks–a potential threat to the elimination of measles? J Infect Dis. 2003;187(Suppl 1):S97–101. [DOI] [PubMed] [Google Scholar]
- 83.Botelho-Nevers E, Gautret P, Biellik R, et al. Nosocomial transmission of measles: an updated review. Vaccine. 2012;30:3996–4001. [DOI] [PubMed] [Google Scholar]
- 84.Fiebelkorn AP, Seward JF, Orenstein WA. A global perspective of vaccination of healthcare personnel against measles: systematic review. Vaccine. 2014;32:4823–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gohil SK, Okubo S, Klish S, et al. Healthcare workers and post-elimination era measles: lessons on acquisition and exposure prevention. Clin Infect Dis. 2016;62:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.WHO. Meeting of the strategic advisory group of experts on immunization, November 2013 – conclusions and recommendations. Wkly Epidemiol Rec. 2014;89:1–20. [PubMed] [Google Scholar]
- 87.Andrus J, Cochi S, Cooper L, et al. Combining global elimination of measles and rubella with strengthening of health systems in developing countries. Health Aff. 2016;35:327–333. [DOI] [PubMed] [Google Scholar]
- 88.Cochi S, Hegg L, Kaur A, et al. The global polio eradication initiative: progress, lessons learned, and polio legacy transition planning. Health Aff. 2016;35:277–283. [DOI] [PubMed] [Google Scholar]
- 89.Rutter PD, Hinman AR, Hegg L, et al. Transition planning for after polio eradication. J Infect Dis. 2017;216:S287–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.GPEI. Financing. [cited 2017 July 21]. Available from: http://polioeradicationorg/financing/
- 91.WHO. Polio transition planning. Seventieth World Health AssemblyA70/14 Add1, Provisional agenda item 123 2017. [cited 2017 Sep 20]. Available form: http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_14Add1-en.pdf [Google Scholar]
- 92.Thompson K, Badizadegan N. Modeling the transmission of measles and rubella to support global management policy analyses and eradication investment cases. Risk Anal. 2017;37(6):1109–1131.•• Provides foundational evidence for establishing the eradication investment case for measles and rubella.
- 93.Thompson KM, Odahowski CL, Goodson JL, et al. Synthesis of evidence to characterize national measles and rubella exposure and immunization histories. Risk Analysis. 2016;36:1427–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson KM, Strebel PM, Dabbagh A, et al. Enabling implementation of the global vaccine action plan: developing investment cases to achieve targets for measles and rubella prevention. Vaccine. 2013;31(Suppl 2):B149–B156. [DOI] [PubMed] [Google Scholar]
- 95.Omer SB, Orenstein WA, Koplan JP. Go big and go fast–vaccine refusal and disease eradication. N Engl J Med. 2013;368:1374–1376. [DOI] [PubMed] [Google Scholar]
- 96.Ozawa S, Clark S, Portnoy A, et al. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Aff. 2016;35:199–207.•• Provides estimates on the return of investment for vaccines; the highest is for measles vaccine, at 58 times the cost.
- 97.Lee LA, Franzel L, Atwell J, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011–2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;31(Suppl 2):B61–72. [DOI] [PubMed] [Google Scholar]
- 98.Ozawa S, Clark S, Portnoy A, et al. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001–2020. WHO Bullet World Health Organ. 2017;95:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.GBD 2015 Healthcare Access and Quality Collaborators. Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet. 2017;390(10091):231–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chan M, Elias C, Fauci A, et al. Reaching everyone, everywhere with life-saving vaccines. Lancet. 2017;389:777–779. [DOI] [PubMed] [Google Scholar]