Abstract
Purpose of Review:
Neurodevelopmental disorders disproportionately affect males. The mechanisms underlying male vulnerability or female protection are not known and remain understudied. Determining the processes involved is crucial to understanding the etiology and advancing treatment of neurodevelopmental disorders. Here, we review current findings and theories that contribute to male preponderance of neurodevelopmental disorders, with a focus on autism.
Recent Findings:
Recent work on the biological basis of the male preponderance of autism and other neurodevelopmental disorders includes discussion of a higher genetic and symptomatic burden in females and sex-specific gene mutations or epigenetic changes that differentially confer risk to males or protection to females. Other mechanisms discussed are sex chromosome and sex hormone involvement. Specifically, fetal testosterone is involved in many aspects of development and may interact with neurotransmitter, neuropeptide, or immune pathways to contribute to male vulnerability. Finally, the possibilities of female underdiagnosis and a multi-hit hypothesis are discussed.
Summary:
This review highlights current theories of male bias in developmental disorders. Topics include environmental, genetic, and epigenetic mechanisms; theories of sex chromosomes, hormones, neuroendocrine and immune function; underdiagnosis of females; and a multi-hit hypothesis.
Keywords: Neurodevelopmental disorders, autism, sex differences, female protective effect, extreme male brain theory, fetal testosterone
Introduction
Autism spectrum disorder (ASD) comprises a set of neurodevelopmental disorders that affect 1 in 68 children [1]. ASD is defined by the early developmental onset of persistent, usually lifelong symptoms, primarily social communication deficits, and a pattern of restricted and repetitive behaviors. Heritability estimates of ASD have ranged from 0.5–0.9%. Eight hundred eighty one genes have been implicated in autism, at least one from every chromosome (see [2,3]), however the majority of cases of autism are due to unknown genetic etiology. A few prenatal or perinatal environmental contributors have been identified, but mechanisms of environmental factors are also largely unknown [4].
Despite striking heterogeneity in manifestation and severity of ASD, one of the most highly replicated findings is the male preponderance of the disorder. The male: female ratio of ASD has been reported as 4.5:1 [5]. However, the ratio of males to females with ASD without intellectual disability is 6–16:1, and the male: female ratio for those with moderate to severe intellectual disability is approximately 1–2:1 [6]. In fact, most neurodevelopmental disorders (ND) have a male bias, including attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and intellectual disability [6].
Various studies have attributed the male preponderance of ASD to sex-specific single nucleotide polymorphisms (SNPs), single-nucleotide variants (SNVs), microdeletions, copy number variants (CNVs) and proteins [6–11]. However, as has been common in studies of the highly heterogeneous ASD, these findings have not been consistently replicated [12]. A fairly new idea is that risk genes--rather than themselves being sex-specific--may interact with sex-specific pathways, possibly those associated with hormones or immune function [13,14]. Another hypothesis is that females may be more sensitive to genetic disruptions and therefore less likely to survive to term, but this remains to be demonstrated [14]. A recent report described a sex-specific transcriptome based on RNA sequencing data from lymphoblastoid cell lines from a small group of siblings discordant for ASD [15]. This is a fairly new, but promising avenue of study that could shed light on sex bias in ND. In this review, we will summarize recent research on possible mechanisms of the male bias in ASD and promising directions for future study.
Are Females Protected or are Males Vulnerable or Both?
Female Protective Effect (FPE) theory attempts to explain the differences in preponderance and severity of ASD between males and females. The FPE includes the Greater Variability Model, which states that males exhibit greater genetic variability, allowing for an increased incidence but decreased severity of ASD [16,17]. The FPE also incorporates the liability-threshold model, which states that females who meet diagnostic threshold for ASD will carry a higher mutational load than males, and that relatives of females with ASD are more likely to be affected than relatives of males with ASD [18]. These predictions are supported by several samples of individuals with ASD in which there are more genes within de novo CNVs and higher rates of de novo deletions in females than in males [19,20]. Larger and more numerous CNVs and SNVs in female versus male individuals with ND and ASD have been reported by several groups [21,22]. The median number of genes per CNV identified in autistic females is 10–15, compared to 2–3 in males with ASD [23,24].
A network-based analysis of genetic associations determined that CNVs affecting females with ASD were significantly more likely to involve genes that are central to the functionality of the gene network, suggesting that a more profound disruption of integral biological pathways is necessary to lead to an ASD phenotype in females [23]. Relatedly, a recent publication reported sex-specific sets of small noncoding RNAs (sncRNAs) in the temporal cortex of individuals with ASD. Specifically, there was more sncRNA dysregulation in brains of females with ASD than in males with ASD, compared to neurotypical controls of the same sex, indicating a necessity for a higher genetic load in ASD females [25]. Therefore, in agreement with the FPE, females seem to require a higher genetic burden to reach diagnostic threshold for ASD, and are more severely symptomatic, on average, than males with ASD [26].
Several studies fulfill the FPE’s prediction of increased risk in families of females with mutations as well. Recent reports including very large samples from multiple databases concluded that siblings of females with ASD have higher autistic trait scores and higher recurrence risk than siblings of males with ASD [6,27–29]. Evidence from large datasets of multiplex families with more than one child affected by autism also supports the FPE. The risk for subsequent children developing autism was higher when at least one of the affected siblings was female versus when the child had only brothers with ASD [30]. Another group similarly hypothesized that female-enriched multiplex families with two or more severely affected females represent a group with an extremely high recurrence risk [31]. These data indicate that affected females had larger or higher penetrance genetic disruptions that are more likely to result in siblings having an autism diagnosis. Similarly, CNV enrichment was identified in mothers of individuals with autism by several groups [21,22]. The Autism Science Foundation is currently dedicating substantial research effort to investigating the FPE through the Autism Sisters Project [32].
Other research evaluating groups of individuals with ASD and siblings of individuals with ASD did not find increased genetic burden in females with the disorder or increased incidence in relatives of females [12,33,34]. These differences may be attributable to the heterogeneity of samples and differences in methodology used. Replication with larger groups will be important in the future.
Another theory put forth to explain sex differences in the prevalence of ASD is the idea that males are more susceptible to ASD. This concept is not necessarily exclusive of a FPE, and may be explained by a proposed increase in genetic variability amongst males, as mentioned earlier, or other biological factors that confer vulnerability to males in particular [16,27]. Studies of gene networks found a sex-specific pattern of expression in a typically developing population, and the genes over-represented in male versus female brains were those often associated with ASD, including genes involved in cytoskeletal and extracellular matrix proteins, immune response, and chromatin [35,36]. It is possible, therefore, that perturbations in genes that are typically expressed at higher levels in males would have a bigger impact on male brain development.
Animal studies of epigenetic changes point to male vulnerability as well. Prenatal valproic acid (VPA) treatment in rats results in an ASD-like phenotype, as well as a male-specific decrease in the methyl-CpG-binding protein 2 (MeCP2), which binds methylated DNA and can repress transcription via histone acetylation, in the prefrontal cortex. In addition, knockdown of MeCP2 with small interfering RNA (siRNA) resulted in an increase in PSD95, an important postsynaptic scaffolding protein, in neural progenitor cells derived from males but not females [37]. In conclusion, it is not clear what factors contribute to female protection from and/or male vulnerability to ASD, but they may be environmental, genetic, or epigenetic in nature.
Chromosome and Hormone Mechanisms of Sex Differences and their Effects on Development, Behavior, Brain Function
The developmental origin of the many behavioral and anatomical differences between males and females is in the sex chromosomes. In addition to a maternally inherited X chromosome, there is a paternally inherited sex chromosome complement: a Y in the case of males, and an X in the case of females. In order to correct for gene dosage, whereas females have twice as many X chromosomes as males, one of the X chromosomes in each cell is silenced, known as X-inactivation. However, some genes (~10–15%) escape X-inactivation (escape genes) [38]. This earliest source of differences between males and females is discussed below as a possible mechanism for male bias in ND.
Secondary to sex chromosomes is the other main factor that differentiates males and females: sex hormones, primarily testosterone and estradiol. These steroids are produced by the gonads and have important developmental effects, particularly during two critical periods. First, an organizational period during development in utero results in the body and brain being permanently formed into male or female anatomy. In the case of males, the Y chromosome contains the SRY gene, which encodes the testis-determining factor. The testis-determining factor is a DNA-binding protein that upregulates transcription factors to initiate the formation of testes, which then produce testosterone. This fetal testosterone is responsible for masculinization, whereas the process of feminization mainly requires the absence of hormones. The second critical period is the activational, and it comprises the sex hormone surge during puberty: the cyclical levels of estradiol and progesterone in females until menopause (menstrual or estrous cycle) and the steady surge of testosterone in males until senescence. Sex hormones at that time have more transient effects [39,40] (Fig. 1). As ND, by definition, manifest early, and are male-biased, fetal testosterone is a strong candidate for male bias in ASD (discussed below).
Figure 1.
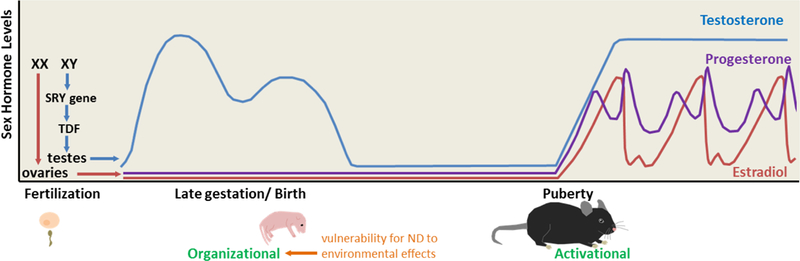
Sex chromosomes (XX=female; XY=male) determine which gonads will form and which sex hormones (mainly testosterone, estradiol, and progesterone) they will produce. Fetal testosterone is important for permanent masculinization of the male brain and body during the organizational period (late gestation/birth, in rodents). This is the proposed period for vulnerability to ND. Sex hormones then surge during the activational period (puberty) for more transient effects; males have relatively stable testosterone levels, and females have variable hormone levels over the 4–5 day estrous cycle. Modified from [54].
Testosterone in utero is critical for the development of many observed sex differences, from physical appearance to brain region size, neurotransmitter and receptor levels, neurogenesis, cell death, migration, differentiation, immune function, neuropeptide signaling, and many other factors, some of which will be addressed here. Many of the genes associated with autism encode proteins involved in synapse formation or maintenance, cell adhesion, and scaffolding. These molecules may be targets of hormones during the organizational period of development, resulting in the male preponderance observed in ND [41]. In addition, dendritic spines are mediated by sex hormones in many areas of the brain and are abnormal in several mouse models of ASD and individuals with ASD [42–50]. Finally, animal studies have indicated that DNA methyltransferase (DNMT) and histone deacetylase (HDAC) have sex-specific effects on behavior and can be influenced by sex hormones [51,52]. These epigenetic mechanisms, along with chromatin modifications, and microRNA expression, are new areas of interest in the ASD field and may be influenced by sex hormones to contribute to susceptibility [53].
Sex Chromosome Theory: Is XX protective or is XY a risk factor?
It has been proposed that possessing a Y chromosome, in the case of males, is a risk factor for neurodevelopmental disorders, and/or having a second X chromosome is protective in the case of females. There is some evidence for this in animal models with altered numbers of sex chromosomes, as well as from aneuploid individuals with increased risk for ASD (XYY, XXY, XXYY, XXY) [6,55–57]. However, aneuploid individuals are relatively rare and often also exhibit sex hormone dysregulation, so these studies can be difficult to interpret, i.e. do not necessarily distinguish the effects of sex chromosomes vs. hormones. Although most genetic mutations associated with ASD susceptibility are autosomal, some ASD-linked genes reside on the X chromosome, including FMRP, MECP2, NLGN3, and NLGN4X [6,58]. Often, X-linked phenotypes in males are much more severe than in females. For example, loss of function of the MECP2 gene, located on the X chromosome, is associated with Rett syndrome, which causes an ASD-like phenotype. The syndrome is seen almost exclusively in girls, because affected males –with only one copy of MeCP2 on one X chromosome-- are more severely affected, and usually die before or shortly after birth [59]. This led to the idea that escape genes (which survive X-inactivation, discussed above) may be protective to females. Several have been identified [58,60,61], but other studies found no differences in X inactivation in females without ASD compared to those with the disorder [62,63]. Importantly, many of these findings were based on relatively small sample sizes, and have not been replicated. Overall, it seems that the X chromosome theory cannot account for most cases of ASD and may not significantly contribute to the male bias, although a recent report suggested that in addition to rare X-linked loci and Mendelian diseases, sex-specific SNPs on the X chromosome may play a role in ASD risk [12]. Similarly, the Y chromosome contains few genes and the possibility of a role for these genes in ASD remains understudied. One group found no differences in Y haplotype distribution between individuals with ASD and controls [64]. Another group found some Y haplotypes that were more or less common in ASD individuals compared to a nonaffected population [65]. Thus a significant contribution of sex chromosomes in the etiology of the majority of cases of ASD seems unlikely but warrants further research.
Sex Hormone Theory: Are fetal testosterone levels linked to autism?
Fetal testosterone (fT) affects a number of downstream targets, as discussed earlier, and has been identified as a prime candidate for male-biased risk [41]. Levels of fetal testosterone have been directly measured through amniocentesis. fT is also indirectly approximated by digit ratio (higher levels of fT are associated with lower second to fourth digit length ratio (2D:4D), although this is slightly controversial). Evidence for the effects of fT also come from studies of girls with congenital adrenal hyperplasia (CAH), children of women with polycystic ovarian syndrome (PCOS), and females with male co-twins, all of whom are exposed to higher than normal levels of fT. Correlation of increased fT in these populations with autistic trait scores and ASD diagnosis was demonstrated in many studies but not all [41,53,58,66–74]. A recent study found that at 12 and 36 months, there was no relationship between levels of fT or other androgens in umbilical cord blood of children with older siblings diagnosed with ASD, and autistic traits, except in a small subset of children whose older sibling was a female with ASD [75]. This is an interesting finding that may relate to the liability-threshold model and FPE models (discussed earlier), but the sample sizes were small and the implications should be considered carefully. Finally, maternal blood levels of an endocrine disruptor, which has been shown to decrease testosterone levels, were negatively correlated with social responsiveness in boys and girls at 9 and 10 years of age [76].
A report from the Baron-Cohen group has directly compared fT levels of boys who later received a diagnosis of ASD or Asperger syndrome to typically developing controls. Individuals with ASD had higher amniotic levels of progesterone, 17α-hydroxy-progesterone, androstenedione, testosterone, and cortisol [53]. There were moderate sample sizes in this study, but it will be important to replicate these findings, especially including female subjects, and investigate the source of the increased levels, which could be environmental or genetic in nature, and could be either maternally or fetally derived. Additionally, although fT levels do not appear to be related to postnatal testosterone levels in young children or adults, some hormone dysfunction appears to be present in both males and females with ASD, including higher rates of precocious puberty and an increased incidence of PCOS and dysmenorrhea [8,68,77–83].
A number of studies in the past several years have implicated sex hormones, receptors, and related enzymes in ASD. Human studies are somewhat lacking, as manipulation of fetal hormone levels in humans is of course not possible, but one study looking at postmortem brains of a small group of adolescents demonstrated an interesting pattern of decreased estrogen receptor beta (ERβ), aromatase (an enzyme that converts androgens to into estrogens), and several ER coactivators in the frontal gyrus of individuals with ASD compared to controls (n=13 per group) [84]. Another group has demonstrated decreased aromatase levels in the frontal cortex of individuals diagnosed with ASD and a positive correlation between aromatase levels and the protein product of the ASD-associated gene RORA (retinoic acid-related orphan receptor-alpha) [85–88]. Then, using a neuronal cell line, they showed that aromatase is a transcriptional target of RORA and that RORA is sex hormone responsive--upregulated by estradiol and downregulated by dihydrotestosterone (DHT) [85]. Finally, using mice, they indicate that males seem to be more susceptible to disruptions in RORA due to reductions in aromatase, resulting in an increase in testosterone and decrease in estradiol [89]. Therefore, genes associated with ASD may be modulated by sex hormones and vice versa, and the enzyme aromatase may be a critical player in the male overrepresentation in autism.
Other animal studies have also pointed to a role of hormones in the development of ASD-like brain or behavioral traits. In a zebrafish model of ASD, animals with a mutation in contactin associated protein-like 2 (CNTNAP2) exhibited hyperactivity and sensitivity to seizures, which were rescued by treatment with a phytoestrogen [90]. A report of heterozygous reeler mice, which exhibit ASD-like social and cognitive deficits and amygdalar abnormalities, found that neonatal administration of estradiol rescued those phenotypes [91]. However, although estradiol is discussed as a “feminizing hormone”, a potential shortcoming of this study is that, in fact, estradiol has important masculinizing effects on the developing brain, particularly in rodents, so this may actually result in a “hypermasculinized” state. Treatment of pregnant rats with an aromatase inhibitor, which blocks the conversion of testosterone to estradiol, resulted in pups that emitted decreased numbers of maternal separation- induced ultrasonic vocalizations compared to controls, and a female-specific decrease in social interactions, both of which are considered ASD-relevant traits [92]. Although the authors claim that administration of an aromatase inhibitor will increase masculinization by preventing testosterone conversion to estradiol, this may, in fact, result in “dysmasculinization” instead, as many brain masculinizing effects are due to estradiol. Another recent study, which does not address autism directly, nevertheless has interesting implications for the disorder. The authors reported that prenatal testosterone administration to mice caused a significant increase in spine density [93], which has been associated with ASD [45–50].
In summary, fetal testosterone levels may be predictors of future ASD-related behaviors, but the exact effects of sex hormones during development are quite complex and warrant more study. Perturbations of fT in either direction may increase likelihood for later diagnosis of the disorder.
Extreme Male Brain (EMB) Theory: Is the autistic brain a hypermasculinized brain?
The Extreme Male Brain theory of autism (EMB) states that there are morphological and functional differences between male and female brains, but the “autistic brain” is a more extreme, or hypermasculinized, version of the male brain, possibly due to elevated fT [41,58]. Various reports indicate that males are more adept at construction and analysis of rule-based systems, attention to detail, and collecting (Systemizing quotient, SQ), whereas females score higher on tests of empathy, language abilities, and social cognition (Empathy quotient, EQ) [41,58,94]. Based on these innate differences, the EMB theory predicts that individuals with ASD will score higher on the SQ than typical males, who will in turn score higher than typical females. Likewise, autistic individuals should score lower on the EQ than typical males, who do not perform as well as typical females [58]. This has been supported by a number of studies [95,96]. EMB theory also predicts that sex differences found in the general population would be absent or reduced in individuals with ASD, which has also been demonstrated recently with SQ and EQ traits in adults and toddlers with ASD [97,98].
Some aspects of brain morphology follow the EMB theory as well and demonstrate sex differences in size or laterality, with individuals on the autism spectrum having an exaggerated male pattern [58,99–101]. Measurements of cortical thickness with MRI indicated that females with a male-typical organization were significantly more likely to have been diagnosed with ASD [102]. Another recent report indicated an exaggeration of a “male brain” phenotype in individuals with ASD, specifically, lower connectivity in the default mode network (DMN), a group of interacting brain areas with correlated activity associated with social processing and dysfunction in ASD [103].
Other findings are counter to what is predicted by the EMB theory or report complex relationships between ASD, sex, and brain morphometry [104–106]. For example, another study of the DMN found sex differences in a control population, but reduced connectivity in the anterior medial prefrontal cortex correlated with autistic traits in males only [107]. A more recent analysis of DMN and whole-brain connectivity in resting state fMRI, ASD males had a feminized profile of hypo-connectivity compared to control males, and females with ASD had a masculinized expression of increased connectivity. The authors suggest that ASD may not involve hypermasculinization but a general dysfunction of sex differentiation [108]. Finally, an investigation of neuroanatomical features of grey and white matter, some areas of the brains of females with ASD appeared to be masculinized, but those regions did not appear hypermasculinized in males with ASD [109]. Future work is needed to identify underlying factors that drive sex-specific neural expressions of ASD and determine whether they are a cause or effect of ASD.
Neurotransmitters and signaling molecules: Do sex differences in synaptic transmission contribute to ND?
Sex differences in neurotransmitter signaling may be involved in ASD risk, particularly GABA and glutamate signaling, which regulate the central excitatory/inhibitory balance, an important factor in ASD. Sex hormones have been shown to influence GABA receptor expression and GABA synthesis, and both suppress and facilitate GABA inhibitory action via age-, sex-, and brain region-specific mechanisms. Likewise, estradiol can cause an increase in glutamate release, affect metabotropic glutamate receptor signaling depending on subtype, and increase NMDA receptor expression. Progesterone can suppress glutamate response and glutamatergic neurons are sexually dimorphic in some brain regions [40,53,110]. Plasma glutamate levels were recently shown to be lower in individuals with ASD [111]. Animal studies have found related results. Juvenile male rats have higher extracellular glutamate levels in the lateral septum at baseline and during social play, and blockade of ionotropic glutamate receptors resulted in a female-specific decrease in social play [112]. Glutamatergic deficits were reported in a VPA-induced model of ASD. Male, but not female, rats exhibited disruptions in NMDAR, AMPAR, and mGluR5 pathways in the prefrontal cortex [37]. In fact, NMDAR deficits have been linked to a number of mouse models of ASD [45,113–117]. Finally, brain-derived neurotrophic factor (BDNF) and serotonin levels are both influenced by sex hormones and are higher in individuals with autism [41,118–121].
Can Immune Activation lead to sex-biased diagnoses?
The role of immune function is becoming more prominent in the ASD field, and immune function is modulated by sex hormones. ASD has been linked to a number of abnormalities in the immune system, including maternal infection, cytokine and chemokine activity [14,53,122], and many studies have demonstrated the effects of sex hormones on immune responses, including cytokine and microglia responses [123,124]. In humans, a sex-specific biomarker signature of inflammatory molecules and cytokines identified in blood was significantly different between individuals with Asperger syndrome and controls [125]. PET scans revealed an increase in microglia activation in males with ASD relative to control males [126]. Subsequent studies should include larger group sizes as well as female subjects. Finally, evaluation of the ASD transcriptome revealed a number of genes associated with neuroimmune function [127].
In animal research, lipopolysaccharides (LPS) or polyinosinic: polycytidylic acid (Poly I:C) are administered to pregnant dams to mimic the immune activation of bacterial and viral infections, respectively. Following prenatal LPS or Poly I:C administration, some groups have shown sex-specific ASD-related behavioral deficits in offspring. Only male offspring of mouse dams treated with LPS or Poly I:C exhibit repetitive behavior [128]. Male Swiss mouse offspring of LPS-challenged mothers exhibited repetitive behavior, anxiety, cognitive deficits, and decreased parvalbumin. Some cytokines and immune factors were increased in both sexes, and some in males only [129]. Similarly, IL-6 cytokine was increased in microglia cultures from male more than female rats perinatally exposed to LPS, although other immune responses were not sex-specific [130]. On the other hand, some groups have found immune-related phenotypes in females only. For example, the BTBR mouse model of ASD exhibited female-specific increases in self-grooming behavior, and levels of IL-6 and CD11c [131]. At 6 weeks, wildtype mice prenatally treated with Poly I:C had decreased DNA methylation globally, particularly in females, and decreased methylation at the promoter region of MeCP2, a gene associated with ND [132]. Finally, whole transcriptome profiling in purified microglia from wild-type mice uncovered a delay in gene expression pattern over the course of development in males compared to females. Administration of LPS in adult male mice caused an increase in developmental rate of microglia, indicating that the sex difference may involve immune function. Acceleration of microglial development was also observed in brain transcriptomes of individuals with ASD when compared to controls. Therefore, individuals with ASD appear to exhibit increased immune activation, but the results are contrary to predictions of the EMB theory. [133]. Overall, these data indicate that immune activation may be involved in male ND bias, but the exact mechanisms remain unclear, and continued research will be important.
Neuroendocrine Hypothesis: Neuropeptides are responsive to sex hormones
Another possible explanation for the male bias in autism falls under the Neuroendocrine hypothesis, which posits a role of neuropeptides in sex differences, especially oxytocin (OT), vasopressin, and corticotropin-releasing hormone (CRH). These hormones are synthesized in the hypothalamus, secreted centrally and peripherally, and have expression and response patterns that are sex-specific [134].
OT is known for increasing trust, facilitating bonding between partners as well as mothers and children, and augmenting social interaction, social memory, and social cognition [135–142]. Blood levels of OT are higher in females than males, and its activity, release and receptor expression are regulated by estradiol and progesterone [143–149]. Estrogen receptors are often expressed on oxytocin-containing cells and neurons expressing OT receptors [150,151].
Blood levels of OT are reduced in individuals with ASD (although this finding has not always been replicated) and OT has been used as a treatment in preclinical trials [149,152–157]. Animal studies generally support a relationship between sex hormones, OT, and ASD-related phenotypes. Neonatal testosterone administration to rats decreased early social behaviors as well as OT-expressing cells in the PVN, to a greater extent in males than females [158]. In addition, OT administration may affect males and females differently. OT improved working memory and social gaze only in male, not female, infant macaques [159]. Similarly, intranasal OT increased caudate response in males and decreased it in females participating in a cooperation task during an fMRI, indicating sex-specific alterations in social reward [160]. In contrast, levels of the related neuropeptide, arginine vasopressin (AVP), are higher in males than females. AVP is androgen-dependent; and the gene encoding its receptor has been linked to ASD and social behavior [149,161–163].
CRH is involved in stress response, cells that produce it are more abundant in males than females, and its receptors exhibit sex-specific signaling [164,165]. CRH stimulates the release of cortisol, which is increased in children with ASD under baseline conditions and in response to a nonsocial environmental stressors, unpleasant stimuli, and neutral social situations, but they have decreased cortisol responses to social stressors compared to controls [53,166,167]. Increases in CRH receptor gene are associated with deficits in rodent social exploration as well [168]. Therefore, some evidence suggests that interactions between neurotransmitters or neuropeptides and sex hormones may contribute to sex differences in ASD.
Are sex differences in autism overstated?: Possible underdiagnosis of females
An additional theory growing in popularity purports that the male preponderance of ASD is overstated because of underreporting or under-diagnosis of females with ASD. As mentioned previously, ASD females are more likely to present with comorbid intellectual disability, but they are also more likely to be comorbid for sensory issues, seizures, sleep disturbances, anxiety, and depression [169–173]. Diagnosis with a co-occurring disorder can lead to under-diagnosis of ASD in females (see [17] for review). Not only are fewer girls diagnosed with autism, but they are diagnosed later than males on average [174,175]. The reason for this is contentious.
Differences in brain structure, connectivity, or function in ASD indicate that the sexes may manifest the disorder distinctively. There is evidence of this in MRI and fractional anisotropy studies in individuals with ASD [176–182]. fMRI showed that males, but not females, with ASD had decreased activity in the posterior superior temporal sulcus in a social information processing task during fMRI [183]. Likewise, some potential treatments work better in one versus the other sex (see OT section above), including a ketogenic diet in a mouse model of ASD, which rescues social and repetitive abnormalities only in females [184].
Studies have been trying to parse out sex differences in individuals with ASD in order to determine whether the disorder “looks different” in females, which can make diagnosis more difficult. There is disagreement on sex-specific presentations of nearly every behavioral measure, including social aspects, communication, stereotyped and repetitive behaviors, cognition, motor scores, hyperactivity, externalizing and aggressive behaviors, executive function, processing speed, visuospatial skills, and theory of mind (see [17,185] for review), with some finding more severe deficits for males [186–190] and others for females [191,192]. Some report sex differences that include similar overall scores with subtle but specific variations in presentation [193–195]. Most studies conclude there are no, or very minor, sex differences in ASD manifestation, or only an increase in restrictive and repetitive behaviors in males [106,172,196–201]. A recent meta-analysis found sex differences in social and cognitive domains in opposite directions depending on the database of participants used [202]. These inconsistencies highlight the high level of heterogeneity in the autism spectrum as a whole, and warrant further research with increased attention to sampling, testing, and analysis to better define patterns of sex differences.
One study found that adult women with ASD self-reported more autistic traits than men with ASD, but clinician observation found no sex differences in social or communication skills [169]. This discrepancy between self-report and clinician observation has important implications for both diagnostic and self-report measures, and also indicates that societal expectations may lead to more self-criticism in females than in males. Besides discrepancies with self-reports, clinicians often find less sex differences in ASD presentation than represented by parent reports, on which much of the literature is based [203–206]. Hiller et al. [201] interestingly point out that parent and teacher reports of behavior often conflict with each other as well, with the parent reports often describing more impairments than teacher reports, particularly for females. This could be because parents are able to pay more attention and can report more accurately. On the other hand, parents may be more worried about their children, resulting in hypersensitivity to any perceived impairments. Results may even differ depending on which parent completes the questionnaire [207]. Other study design and methodology issues may have confounded estimates of sex differences in ASD as well. Some reports are under-sampled and do not control for age and IQ. Many are underpowered to separate males and females with ASD and not enough are longitudinal. In addition, different measures have varying numbers of questions per category, resulting in unintended emphasis on certain symptoms, or alternatively, increased sensitivity to differences. Some assays determine the presence or absence of symptoms, while others assess severity of traits. However, some researchers have argued that current diagnostic tools, when used correctly, are effective and do not exhibit sex bias [208].
Recently, some research has begun to focus on the role of sociocultural norms on the representation of females with ASD [17,201]. Males are more likely to act out in classrooms, which makes them more likely to receive attention, diagnoses, and treatment [17,172,209]. In addition, females with ASD are more likely to camouflage their symptoms and perceive them differently [188,194,210–216]. Most accepted knowledge of ASD phenotypes and diagnostic criteria is based on research on primarily male subjects, which can make diagnosis in females more difficult. Indeed, a recent report indicates that there were more discrepancies in females than males in meeting diagnostic criteria in the DSM V after previous diagnosis using the DSM IV [201]. In conclusion, there is still not a consensus on whether males and females with ASD present differently and whether the 4.5:1 male to female ratio is accurate. Assessing the influence of social and gender norms will be difficult, but will be an important factor for future treatment strategies.
Multi-hit hypothesis: (Epi)gene x environment x sex interactions
A recently proposed theory, the three-hit hypothesis, combines some of the previously discussed ideas and states that interactions between sex, genes, and environment lead to the male bias in ASD. In the first and only test of this three-hit hypothesis to date, mice showed social deficits and histone modifications most robustly when they were male, deficient for the contactin-associated protein-like 2 gene (Cntnap2), and exposed to LPS in utero, which activates maternal immune response. mRNA gene expression assays in the hippocampus revealed that the promoter area of the Crhr1 gene (a CRH receptor type) may bind SRY (the testis determining factor) and NF-κB, a nuclear factor involved in inflammation, which would confer risk to animals that were male and stressed [217]. As discussed earlier, maternal immune activation may affect males to a greater extent than females, and especially with regard to social behaviors [218]. However, it is worth noting that mice in this study with two hits performed better in some instances than controls with no hits. Also, other models of autism exhibit deficits with just one or two hits, discussed below.
Many studies indicate that two hits may be sufficient to confer ASD vulnerability, including some combination of a number of possible environmental factors; chromosomal or gonadal/hormonal sex; and/or genetic and epigenetic alterations. Therefore, the combinations of factors potentially resulting in an autism spectrum phenotype under this model are abundant.
Some recent mouse studies indicate that sex is one hit and the second is environmental. Male offspring of obese dams were more severely affected than females, with smaller embryo size and a greater number of dysregulated genes [219]. Additionally, female-specific behavioral deficits and male-specific changes in neuron and glia number in layers 2/3 and layers 5/6 of the mPFC occur after prenatal and early postnatal exposure to propionic acid and bisphenol A, respectively [220,221]. Other recent reports using mouse models of ASD indicate that two hits--male sex and particular genetic mutations--may be sufficient to result in autism-relevant phenotypes. Findings include sleep disturbances in 16p11.2 del/+ male mice, social and morphological deficits in male Pcdh10+/− mice, and increased social motivation in male Pten mutant mice [45,222,223]. Purkinje cell abnormalities were male-specific in the Reeler mouse model of ASD, and the Adnp mouse model of ASD shows sex-specific hippocampal gene expression, behavior alterations, and response to treatment [224,225]. The VPA mouse model shows complex patterns of sex specificity in androgen receptor expression and deep cerebellar nuclei morphology [226,227].
Therefore, many factors or combinations of factors may lead to sex differences in ASD or ND, and so there is a need for increased research focus on the complex interactions among such factors. As previously mentioned, most studies with human subjects have not included females and have not considered sex as a variable, although several mentioned previously have found sex differences in populations of people with ASD.
Conclusions
The striking sex bias in ASD and other neurodevelopmental disorders is an important phenomenon to examine in order to better understand the underlying biology of ASD and to work toward new and better treatment strategies. There have been many contradictory findings that complicate the question, perhaps due to a combination of the heterogeneity of ASD and the prevalence of under-powered studies. It seems likely that parts of many of the hypotheses and models discussed here interact to confer greater risk to males. In addition, different biological subtypes of ASD almost certainly exist, with different combinations of underlying biological factors involved. See Figure 2 for a summary.
Figure 2.
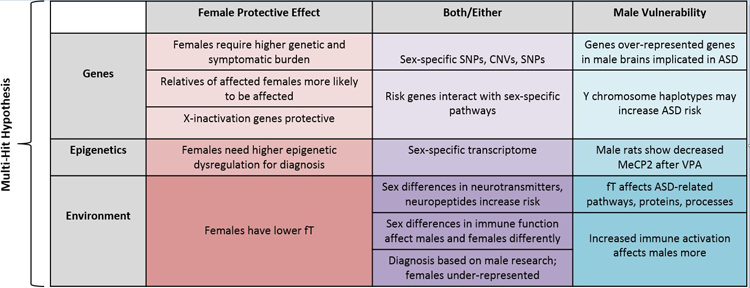
Summary of theories and phenomena that contribute to the male bias of ASD and ND. fT = fetal testosterone, SNP = single nucleotide polymorphism, SNV = single nucleotide variant, CNV = copy number variant, MeCP2 = methyl-CpG-binding protein 2, VPA = valproic acid.
Moving forward, more inclusive research is necessary, with increased numbers of human subjects, particularly females, better matched for age and IQ, and with an augmented effort to study different time points in development or utilize longitudinal designs. Using genomic data to parse out more specific sex differences and risk estimates will also be an important avenue for future studies. Replication of the genetic and epigenetic studies discussed here, again, with larger subject pools, will be vital to determine whether there are sex-specific genes and transcriptomes that confer male bias, or whether ND-associated genes interact with sex hormone pathways. Animal studies should include female as well as male subjects, and should be utilized as a tool to investigate manipulation of sex hormones and related enzymes during development.
Finally, care should be taken to try to separate cause and effect–or at least not make unwarranted assumptions about cause and effect—when it comes to ND phenotypes. For example, do ASD-associated gene mutations make children more susceptible to environmental risk factors or are they caused by them? Those dynamics can be difficult to disentangle. We should strive for better diagnostic tools and animal research that includes multidisciplinary components. Specifically, genetic, hormonal, environmental, and societal factors should be considered, with an emphasis on multiple “hits,” which is a promising future direction.
Acknowledgements
This work was supported by NIMH grant R34MH104407 (Brodkin, PI), the Asperger Syndrome Program of Excellence at University of Pennsylvania (Brodkin, co-Director), and Simons Foundation (SFARI) grant 345034 (Abel, PI). We would like to thank Joseph Lynch for his help editing the manuscript.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Sarah L. Ferri and Ted Abel declare no conflict of interest.
Edward S. Brodkin reports grants from National Institute of Mental Health and a gift to the University of Pennsylvania (Asperger Syndrome Program of Excellence).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
- 1. https://www.cdc.gov/ncbddd/autism/data.html.
- 2.Butler M, Rafi S, Hossain W, Stephan D, Manzardo A. Whole Exome Sequencing in Females with Autism Implicates Novel and Candidate Genes. Int. J. Mol. Sci 2015;16:1312–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://gene.sfari.org/.
- 4.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn. Sci. NIH Public Access; 2011;15:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR. Surveill. Summ 2016;65:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr. Opin. Neurol 2013;26:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carayol J, Schellenberg GD, Dombroski B, Genin E, Rousseau F, Dawson G. Autism risk assessment in siblings of affected children using sex-specific genetic scores. Mol. Autism 2011;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steeb H, Ramsey JM, Guest PC, Stocki P, Cooper JD, Rahmoune H, et al. Serum proteomic analysis identifies sex-specific differences in lipid metabolism and inflammation profiles in adults diagnosed with Asperger syndrome. Mol. Autism 2014;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato D, Lionel AC, Leblond CS, Prasad A, Pinto D, Walker S, et al. SHANK1 Deletions in Males with Autism Spectrum Disorder. Am. J. Hum. Genet 2012;90:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tropeano M, Ahn JW, Dobson RJB, Breen G, Rucker J, Dixit A, et al. Male-Biased Autosomal Effect of 16p13.11 Copy Number Variation in Neurodevelopmental Disorders. Liu C, editor. PLoS One 2013;8:e61365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tropeano M, Howley D, Gazzellone MJ, Wilson CE, Ahn JW, Stavropoulos DJ, et al. Microduplications at the pseudoautosomal SHOX locus in autism spectrum disorders and related neurodevelopmental conditions. J. Med. Genet 2016;53:536–47. [DOI] [PubMed] [Google Scholar]
- 12.Mitra I, Tsang K, Ladd-Acosta C, Croen LA, Aldinger KA, Hendren RL, et al. Pleiotropic Mechanisms Indicated for Sex Differences in Autism. Flint J, editor. PLOS Genet 2016;12:e1006425.• A study of single nucleotide polymorphisms uncovered no excess genetic load in females, contradicting much previous data, but pointed to sex-specific mutations, specifically on the X chromosome, that may contribute to male prevalence in autism.
- 13.Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nat. Commun 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy MM, Wright CL. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017;81:402–10.• An important developmental difference between males and females involves immune function. A number of immune mechanims are discussed that could confer male risk to neurodevelopmental disorders.
- 15.Tylee DS, Espinoza AJ, Hess JL, Tahir MA, McCoy SY, Rim JK, et al. RNA sequencing of transformed lymphoblastoid cells from siblings discordant for autism spectrum disorders reveals transcriptomic and functional alterations: Evidence for sex-specific effects. Autism Res 2017;10:439–55. [DOI] [PubMed] [Google Scholar]
- 16.Wing L Sex ratios in early childhood autism and related conditions. Psychiatry Res 1981;5:129–37. [DOI] [PubMed] [Google Scholar]
- 17.Kreiser NL, White SW. ASD in Females: Are We Overstating the Gender Difference in Diagnosis? Clin. Child Fam. Psychol. Rev. Springer US; 2014;17:67–84.• This review explores sociocultural factors and sex differences in manifestation that may affect females with neurodevelopmental disorders and how they may contribute to their underdiagnosis
- 18.Tsai L, Stewart MA, August G. Implication of sex differences in the familial transmission of infantile autism. J. Autism Dev. Disord Kluwer Academic Publishers-Plenum Publishers; 1981;11:165–73. [DOI] [PubMed] [Google Scholar]
- 19.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron, 87(6), 1215–1233. https://doi.org/10.1. Neuron. 2015;87:1215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am. J. Hum. Genet 2014;94:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desachy G, Croen LA, Torres AR, Kharrazi M, Delorenze GN, Windham GC, et al. Increased female autosomal burden of rare copy number variants in human populations and in autism families. Mol. Psychiatry 2015;20:170–5.• In support of a Female Protective Effect, a meta-analysis found increased large, rare autosomal copy number variant mutations in female family members of autistic individuals compared to those in control families.
- 23.Gilman SR, Iossifov I, Levy D, Ronemus M, Wigler M, Vitkup D. Rare De Novo Variants Associated with Autism Implicate a Large Functional Network of Genes Involved in Formation and Function of Synapses. Neuron 2011;70:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy D, Ronemus M, Yamrom B, Lee Y, Leotta A, Kendall J, et al. Rare De Novo and Transmitted Copy-Number Variation in Autistic Spectrum Disorders. Neuron 2011;70:886–97. [DOI] [PubMed] [Google Scholar]
- 25.Schumann CM, Sharp FR, Ander BP, Stamova B. Possible sexually dimorphic role of miRNA and other sncRNA in ASD brain. Mol. Autism. BioMed Central; 2017;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 2012;51. [DOI] [PubMed] [Google Scholar]
- 27.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl. Acad. Sci. U. S. A 2013;110:5258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Werling DM, Geschwind DH. Understanding sex bias in autism spectrum disorder. Proc. Natl. Acad. Sci. U. S. A 2013;110:4868–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer N, Beam A, Agniel D, Eran A, Manrai A, Spettell C, et al. Association of Sex With Recurrence of Autism Spectrum Disorder Among Siblings. JAMA Pediatr 2017; [DOI] [PMC free article] [PubMed]
- 30.Werling DM, Geschwind DH. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol. Autism 2015;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner TN, Sharma K, Oh EC, Liu YP, Collins RL, Sosa MX, et al. Loss of δ-catenin function in severe autism. Nature 2015;520:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. http://autismsciencefoundation.org/about-asf/media-center/press-releases/autism-sisters-project/.
- 33.Goin-Kochel RP, Abbacchi A, Constantino JN, Autism Genetic Resource Exchange Co. Lack of evidence for increased genetic loading for autism among families of affected females. Autism 2007;11:279–86. [DOI] [PubMed] [Google Scholar]
- 34.Messinger DS, Young GS, Webb SJ, Ozonoff S, Bryson SE, Carter A, et al. Early sex differences are not autism-specific: A Baby Siblings Research Consortium (BSRC) study. Mol. Autism 2015;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziats MN, Rennert OM. Sex-biased gene expression in the developing brain: implications for autism spectrum disorders. Mol. Autism 2013;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Zhang Z, Su B. Sex Biased Gene Expression Profiling of Human Brains at Major Developmental Stages. Sci. Rep 2016;6:21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KC, Choi CS, Kim J-W, Han S-H, Cheong JH, Ryu JH, et al. MeCP2 Modulates Sex Differences in the Postsynaptic Development of the Valproate Animal Model of Autism. Mol. Neurobiol 2016;53:40–56. [DOI] [PubMed] [Google Scholar]
- 38.Bhatnagar S, Zhu X, Ou J, Lin L, Chamberlain L, Zhu LJ, et al. Genetic and pharmacological reactivation of the mammalian inactive X chromosome. Proc. Natl. Acad. Sci. U. S. A 2014;111:12591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viveros M-P, Mendrek A, Paus T, López-Rodríguez AB, Marco EM, Yehuda R, et al. A comparative, developmental, and clinical perspective of neurobehavioral sexual dimorphisms. Front. Neurosci. Frontiers Media SA; 2012;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz JM, McCarthy MM. Cellular mechanisms of estradiol-mediated masculinization of the brain. J. Steroid Biochem. Mol. Biol 2008;109:300–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science 2005;310:819–23. [DOI] [PubMed] [Google Scholar]
- 42.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci 1992;12:2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J. Neurosci 2000;20:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J. Neurosci 2002;22:8586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoch H, Kreibich AS, Ferri SL, White RS, Bohorquez D, Banerjee A, et al. Sociability Deficits and Altered Amygdala Circuits in Mice Lacking Pcdh10, an Autism Associated Gene. Biol. Psychiatry 2017;81:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res 2010;1309:83–94. [DOI] [PubMed] [Google Scholar]
- 47.Tang G, Gudsnuk K, Kuo S-H, Cotrina ML, Rosoklija G, Sosunov A, et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron 2014;83:1131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet 2001;98:161–7. [DOI] [PubMed] [Google Scholar]
- 49.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. U. S. A 1997;94:5401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron 2006;50:377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright EC, Johnson SA, Hao R, Kowalczyk AS, Greenberg GD, Ordoñes Sanchez E, et al. Exposure to extrinsic stressors, social defeat or bisphenol A, eliminates sex differences in DNA methyltransferase expression in the amygdala. J. Neuroendocrinol 2017;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gámez-Del-Estal MM, Contreras I, Prieto-Pérez R, Ruiz-Rubio M. Epigenetic effect of testosterone in the behavior of C. elegans. A clue to explain androgen-dependent autistic traits? Front. Cell. Neurosci 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, et al. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015;20:369–76.• Males with autism spectrum disorders had elevated fetal levels of progesterone, 17α-hydroxy-progesterone, androstenedione and testosterone in amniotic samples compared to those of typically developing controls, providing a possible mechanism for the male bias in autism.
- 54.McCarthy MM. How it’s made: organisational effects of hormones on the developing brain. J. Neuroendocrinol 2010;22:736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox KH, Quinnies KM, Eschendroeder A, Didrick PM, Eugster EA, Rissman EF. Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice. Psychoneuroendocrinology 2015;51:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Printzlau F, Wolstencroft J, Skuse DH. Cognitive, behavioral, and neural consequences of sex chromosome aneuploidy. J. Neurosci. Res 2017;95:311–9. [DOI] [PubMed] [Google Scholar]
- 57.Tartaglia NR, Wilson R, Miller JS, Rafalko J, Cordeiro L, Davis S, et al. Autism Spectrum Disorder in Males with Sex Chromosome Aneuploidy. J. Dev. Behav. Pediatr 2017;38:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biol 2011;9:e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lombardi LM, Baker SA, Zoghbi HY. MECP2 disorders: from the clinic to mice and back. J. Clin. Invest 2015;125:2914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skuse DH. Imprinting, the X-chromosome, and the male brain: explaining sex differences in the liability to autism. Pediatr. Res 2000;47:9–16. [DOI] [PubMed] [Google Scholar]
- 61.Schaafsma SM, Pfaff DW. Etiologies underlying sex differences in Autism Spectrum Disorders. Front. Neuroendocrinol 2014;35:255–71. [DOI] [PubMed] [Google Scholar]
- 62.Gockley J, Willsey AJ, Dong S, Dougherty JD, Constantino JN, Sanders SJ. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol. Autism 2015;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong X, Bacchelli E, Blasi F, Toma C, Betancur C, Chaste P, et al. Analysis of X chromosome inactivation in autism spectrum disorders. Am. J. Med. Genet. Part B Neuropsychiatr. Genet 2008;147B:830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jamain S, Quach H, Quintana-Murci L, Betancur C, Philippe A, Gillberg C, et al. Y chromosome haplogroups in autistic subjects. Mol. Psychiatry 2002;7:217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serajee FJ, Mahbubul Huq AHM. Association of Y Chromosome Haplotypes With Autism. J. Child Neurol 2009;24:1258–61. [DOI] [PubMed] [Google Scholar]
- 66.Barona M, Kothari R, Skuse D, Micali N. Social communication and emotion difficulties and second to fourth digit ratio in a large community-based sample. Mol. Autism 2015;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eriksson JM, Lundström S, Lichtenstein P, Bejerot S, Eriksson E. Effect of co-twin gender on neurodevelopmental symptoms: a twin register study. Mol. Autism 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kung KTF, Spencer D, Pasterski V, Neufeld S, Glover V, O’Connor TG, et al. No relationship between prenatal androgen exposure and autistic traits: convergent evidence from studies of children with congenital adrenal hyperplasia and of amniotic testosterone concentrations in typically developing children. J. Child Psychol. Psychiatry 2016; [DOI] [PMC free article] [PubMed]
- 69.Falter CM, Plaisted KC, Davis G. Visuo-spatial processing in autism--testing the predictions of extreme male brain theory. J. Autism Dev. Disord 2008;38:507–15. [DOI] [PubMed] [Google Scholar]
- 70.van Honk J, Schutter DJ, Bos PA, Kruijt A-W, Lentjes EG, Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc. Natl. Acad. Sci 2011;108:3448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whitehouse AJO, Maybery MT, Hart R, Mattes E, Newnham JP, Sloboda DM, et al. Fetal androgen exposure and pragmatic language ability of girls in middle childhood: implications for the extreme male-brain theory of autism. Psychoneuroendocrinology 2010;35:1259–64. [DOI] [PubMed] [Google Scholar]
- 72.Voracek M, Dressler SG. High (feminized) digit ratio (2D : 4D) in Danish men: a question of measurement method? Hum. Reprod 2006;21:1329–31. [DOI] [PubMed] [Google Scholar]
- 73.Guyatt AL, Heron J, Knight BLC, Golding J, Rai D. Digit ratio and autism spectrum disorders in the Avon Longitudinal Study of Parents and Children: a birth cohort study. BMJ Open 2015;5:e007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Zaid FS, Alhader AA, AL-Ayadhi LY. The second to fourth digit ratio (2D:4D) in Saudi boys with autism: A potential screening tool. Early Hum. Dev 2015;91:413–5. [DOI] [PubMed] [Google Scholar]
- 75.Park BY, Lee BK, Burstyn I, Tabb LP, Keelan JA, Whitehouse AJO, et al. Umbilical cord blood androgen levels and ASD-related phenotypes at 12 and 36 months in an enriched risk cohort study. Mol. Autism 2017;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nowack N, Wittsiepe J, Kasper-Sonnenberg M, Wilhelm M, Schölmerich A. Influence of Low-Level Prenatal Exposure to PCDD/Fs and PCBs on Empathizing, Systemizing and Autistic Traits: Results from the Duisburg Birth Cohort Study. Carpenter DO, editor. PLoS One 2015;10:e0129906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Auyeung B, Ahluwalia J, Thomson L, Taylor K, Hackett G, O’Donnell KJ, et al. Prenatal versus postnatal sex steroid hormone effects on autistic traits in children at 18 to 24 months of age. Mol. Autism 2012;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruta L, Ingudomnukul E, Taylor K, Chakrabarti B, Baron-Cohen S. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology 2011;36:1154–63. [DOI] [PubMed] [Google Scholar]
- 79.Takagishi H, Takahashi T, Yamagishi T, Shinada M, Inukai K, Tanida S, et al. Salivary testosterone levels and autism-spectrum quotient in adults. Neuro Endocrinol. Lett 2010;31:837–41. [PubMed] [Google Scholar]
- 80.Geier DA, Geier MR. A prospective assessment of androgen levels in patients with autistic spectrum disorders: biochemical underpinnings and suggested therapies. Neuro Endocrinol. Lett 2007;28:565–73. [PubMed] [Google Scholar]
- 81.Pohl A, Cassidy S, Auyeung B, Baron-Cohen S. Uncovering steroidopathy in women with autism: a latent class analysis. Mol. Autism 2014;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ingudomnukul E, Baron-Cohen S, Wheelwright S, Knickmeyer R. Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Horm. Behav 2007;51:597–604. [DOI] [PubMed] [Google Scholar]
- 83.Tordjman S, Ferrari P, Sulmont V, Duyme M, Roubertoux P. Androgenic activity in autism. Am. J. Psychiatry 1997;154:1626–7. [DOI] [PubMed] [Google Scholar]
- 84.Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol. Autism 2014;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarachana T, Xu M, Wu R-C, Hu VW. Sex hormones in autism: androgens and estrogens differentially and reciprocally regulate RORA, a novel candidate gene for autism. PLoS One 2011;6:e17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu VW, Sarachana T, Kim KS, Nguyen A, Kulkarni S, Steinberg ME, et al. Gene expression profiling differentiates autism case-controls and phenotypic variants of autism spectrum disorders: evidence for circadian rhythm dysfunction in severe autism. Autism Res 2009;2:78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics 2006;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen A, Rauch TA, Pfeifer GP, Hu VW. Global methylation profiling of lymphoblastoid cell lines reveals epigenetic contributions to autism spectrum disorders and a novel autism candidate gene, RORA, whose protein product is reduced in autistic brain. FASEB J 2010;24:3036–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu VW, Sarachana T, Sherrard RM, Kocher KM. Investigation of sex differences in the expression of RORA and its transcriptional targets in the brain as a potential contributor to the sex bias in autism. Mol. Autism 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman EJ, Turner KJ, Fernandez JM, Cifuentes D, Ghosh M, Ijaz S, et al. Estrogens Suppress a Behavioral Phenotype in Zebrafish Mutants of the Autism Risk Gene, CNTNAP2. Neuron 2016;89:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Macrì S, Biamonte F, Romano E, Marino R, Keller F, Laviola G. Perseverative responding and neuroanatomical alterations in adult heterozygous reeler mice are mitigated by neonatal estrogen administration. Psychoneuroendocrinology 2010;35:1374–87. [DOI] [PubMed] [Google Scholar]
- 92.Xu XJ, Zhang HF, Shou XJ, Li J, Jing WL, Zhou Y, et al. Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring. Physiol. Behav 2015;138. [DOI] [PubMed] [Google Scholar]
- 93.Hatanaka Y, Wada K, Kabuta T. Abnormal instability, excess density, and aberrant morphology of dendritic spines in prenatally testosterone-exposed mice. Neurochem. Int 2015;85–86:53–8. [DOI] [PubMed]
- 94.Gur RC, Gur RE. Complementarity of sex differences in brain and behavior: From laterality to multimodal neuroimaging. J. Neurosci. Res 2017;95:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ashwin C, Ricciardelli P, Baron-Cohen S. Positive and negative gaze perception in autism spectrum conditions. Soc. Neurosci 2009;4:153–64. [DOI] [PubMed] [Google Scholar]
- 96.Tan DW, Russell-Smith SN, Simons JM, Maybery MT, Leung D, Ng HLH, et al. Perceived Gender Ratings for High and Low Scorers on the Autism-Spectrum Quotient Consistent with the Extreme Male Brain Account of Autism. McCormick CM, editor. PLoS One 2015;10:e0131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baron-Cohen S, Cassidy S, Auyeung B, Allison C, Achoukhi M, Robertson S, et al. Attenuation of Typical Sex Differences in 800 Adults with Autism vs. 3,900 Controls. Hu VW, editor. PLoS One 2014;9:e102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Øien RA, Hart L, Schjølberg S, Wall CA, Kim ES, Nordahl-Hansen A, et al. Parent-Endorsed Sex Differences in Toddlers with and Without ASD: Utilizing the M-CHAT. J. Autism Dev. Disord 2017;47:126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci 2007;27:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res 2011;1380:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beacher FD, Minati L, Baron-Cohen S, Lombardo MV., Lai M-C, Gray MA, et al. Autism Attenuates Sex Differences in Brain Structure: A Combined Voxel-Based Morphometry and Diffusion Tensor Imaging Study. Am. J. Neuroradiol 2012;33:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ecker C, Andrews DS, Gudbrandsen CM, Marquand AF, Ginestet CE, Daly EM, et al. Association Between the Probability of Autism Spectrum Disorder and Normative Sex-Related Phenotypic Diversity in Brain Structure. JAMA Psychiatry 2017;74:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ypma RJF, Moseley RL, Holt RJ, Rughooputh N, Floris DL, Chura LR, et al. Default Mode Hypoconnectivity Underlies a Sex-Related Autism Spectrum. Biol. psychiatry Cogn. Neurosci. neuroimaging 2016;1:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bejerot S, Eriksson JM, Bonde S, Carlström K, Humble MB, Eriksson E. The extreme male brain revisited: gender coherence in adults with autism spectrum disorder. Br. J. Psychiatry 2012;201:116–23. [DOI] [PubMed] [Google Scholar]
- 105.Beacher FDCC, Radulescu E, Minati L, Baron-Cohen S, Lombardo MV, Lai M-C, et al. Sex differences and autism: brain function during verbal fluency and mental rotation. Tsakiris M, editor. PLoS One. Public Library of Science; 2012;7:e38355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cauda F, Geda E, Sacco K, D’Agata F, Duca S, Geminiani G, et al. Grey matter abnormality in autism spectrum disorder: an activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry BioMed Central; 2011;82:1304–13. [DOI] [PubMed] [Google Scholar]
- 107.Jung M, Mody M, Saito DN, Tomoda A, Okazawa H, Wada Y, et al. Sex Differences in the Default Mode Network with Regard to Autism Spectrum Traits: A Resting State fMRI Study. Stamatakis EA, editor. PLoS One 2015;10:e0143126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alaerts K, Swinnen SP, Wenderoth N. Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females. Soc. Cogn. Affect. Neurosci Oxford University Press; 2016;11:1002–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai M-C, Lombardo MV, Suckling J, Ruigrok ANV, Chakrabarti B, Ecker C, et al. Biological sex affects the neurobiology of autism. Brain 2013;136:2799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci 2015;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai J, Ding L, Zhang J-S, Xue J, Wang L-Z. Elevated plasma levels of glutamate in children with autism spectrum disorders. Neuroreport 2016;27:272–6. [DOI] [PubMed] [Google Scholar]
- 112.Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema AH. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Neuroscience 2015;307:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Won H, Lee H-R, Gee HY, Mah W, Kim J-I, Lee J, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 2012;486:261–5. [DOI] [PubMed] [Google Scholar]
- 114.Toft AKH, Lundbye CJ, Banke TG. Dysregulated NMDA-Receptor Signaling Inhibits Long-Term Depression in a Mouse Model of Fragile X Syndrome. J. Neurosci 2016;36:9817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burket JA, Benson AD, Tang AH, Deutsch SI. NMDA receptor activation regulates sociability by its effect on mTOR signaling activity. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2015;60:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee E-J, Lee H, Huang T-N, Chung C, Shin W, Kim K, et al. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat. Commun 2015;6:7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duffney LJ, Zhong P, Wei J, Matas E, Cheng J, Qin L, et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep 2015;11:1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Belmonte MK, Cook EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy1. Mol. Psychiatry 2004;9:646–63. [DOI] [PubMed] [Google Scholar]
- 119.Shuffrey LC, Guter SJ, Delaney S, Jacob S, Anderson GM, Sutcliffe JS, et al. Is there sexual dimorphism of hyperserotonemia in autism spectrum disorder? Autism Res 2017; [DOI] [PMC free article] [PubMed]
- 120.Imwalle DB, Gustafsson J-Å, Rissman EF. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol. Behav 2005;84:157–63. [DOI] [PubMed] [Google Scholar]
- 121.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav. Brain Res 1999;105:53–68. [DOI] [PubMed] [Google Scholar]
- 122.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci 2015;16:469–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: Hormonal effects, antagonistic selection, and evolutionary consequences. Horm. Behav 2017;88:95–105. [DOI] [PubMed] [Google Scholar]
- 124.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J. Neurosci NIH Public Access; 2013;33:2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schwarz E, Guest PC, Rahmoune H, Wang L, Levin Y, Ingudomnukul E, et al. Sex-specific serum biomarker patterns in adults with Asperger’s syndrome. Mol. Psychiatry 2011;16:1213–20. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial Activation in Young Adults With Autism Spectrum Disorder. JAMA Psychiatry 2013;70:49. [DOI] [PubMed] [Google Scholar]
- 127.Gupta S, Ellis SE, Ashar FN, Moes A, Bader JS, Zhan J, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun 2014;5:5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xuan ICY, Hampson DR. Gender-Dependent Effects of Maternal Immune Activation on the Behavior of Mouse Offspring. Baudry M, editor. PLoS One 2014;9:e104433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Custódio CS, Mello BSF, Filho AJMC, de Carvalho Lima CN, Cordeiro RC, Miyajima F, et al. Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-lasting Sex- and Age-related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol. Neurobiol 2017; [DOI] [PubMed]
- 130.Turano A, Lawrence JH, Schwarz JM. Activation of neonatal microglia can be influenced by other neural cells. Neurosci. Lett 2017;657:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coretti L, Cristiano C, Florio E, Scala G, Lama A, Keller S, et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep 2017;7:45356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Basil P, Li Q, Dempster EL, Mill J, Sham P-C, Wong CCY, et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl. Psychiatry 2014;4:e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hanamsagar R, Alter MD, Block CS, Sullivan H, Bolton JL, Bilbo SD. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017;65:1504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schulkin J Autism and the amygdala: an endocrine hypothesis. Brain Cogn 2007;65:87–99. [DOI] [PubMed] [Google Scholar]
- 135.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature 2005;435:673–6. [DOI] [PubMed] [Google Scholar]
- 136.ZAK P, KURZBAN R, MATZNER W. Oxytocin is associated with human trustworthiness. Horm. Behav 2005;48:522–7. [DOI] [PubMed] [Google Scholar]
- 137.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron 2008;58:639–50. [DOI] [PubMed] [Google Scholar]
- 138.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin Attenuates Affective Evaluations of Conditioned Faces and Amygdala Activity. J. Neurosci 2008;28:6607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buchheim A, Heinrichs M, George C, Pokorny D, Koops E, Henningsen P, et al. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology 2009;34:1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 2008;33:368–74. [DOI] [PubMed] [Google Scholar]
- 141.White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev. Psychobiol 2009;51:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin Improves “Mind-Reading” in Humans. Biol. Psychiatry 2007;61:731–3. [DOI] [PubMed] [Google Scholar]
- 143.Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res 2009;169:249–52. [DOI] [PubMed] [Google Scholar]
- 144.Bale TL, Dorsa DM. Regulation of oxytocin receptor messenger ribonucleic acid in the ventromedial hypothalamus by testosterone and its metabolites. Endocrinology 1995;136:5135–8. [DOI] [PubMed] [Google Scholar]
- 145.Bale TL, Dorsa DM, Johnston CA. Oxytocin receptor mRNA expression in the ventromedial hypothalamus during the estrous cycle. J. Neurosci 1995;15:5058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Kloet ER, Voorhuis DA, Boschma Y, Elands J. Estradiol modulates density of putative “oxytocin receptors” in discrete rat brain regions. Neuroendocrinology 1986;44:415–21. [DOI] [PubMed] [Google Scholar]
- 147.Quiñones-Jenab V, Jenab S, Ogawa S, Adan RA, Burbach JP, Pfaff DW. Effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the uterus, pituitary, and forebrain of the female rat. Neuroendocrinology 1997;65:9–17. [DOI] [PubMed] [Google Scholar]
- 148.Schumacher M, Coirini H, Pfaff DW, McEwen BS. Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science 1990;250:691–4. [DOI] [PubMed] [Google Scholar]
- 149.Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, et al. Oxytocin and Vasopressin in Children and Adolescents With Autism Spectrum Disorders: Sex Differences and Associations With Symptoms. Autism Res 2013;6:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front. Neuroendocrinol 2006;27:217–32. [DOI] [PubMed] [Google Scholar]
- 151.Devidze N, Mong JA, Jasnow AM, Kow L-M, Pfaff DW. Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc. Natl. Acad. Sci 2005;102:14446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guastella AJ, Hickie IB. Oxytocin Treatment, Circuitry, and Autism: A Critical Review of the Literature Placing Oxytocin Into the Autism Context. Biol. Psychiatry 2016;79:234–42. [DOI] [PubMed] [Google Scholar]
- 153.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, et al. Plasma oxytocin levels in autistic children. Biol. Psychiatry 1998;43:270–7. [DOI] [PubMed] [Google Scholar]
- 154.Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol. Psychiatry 2001;50:609–13. [DOI] [PubMed] [Google Scholar]
- 155.Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biol. Psychiatry 2010;67:692–4. [DOI] [PubMed] [Google Scholar]
- 156.Guastella AJ, Gray KM, Rinehart NJ, Alvares GA, Tonge BJ, Hickie IB, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J. Child Psychol. Psychiatry 2015;56:444–52. [DOI] [PubMed] [Google Scholar]
- 157.Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A 2010;107:4389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Aiello TP, Whitaker-Azmitia PM. Sexual differentiation and the neuroendocrine hypothesis of autism. Anat. Rec. (Hoboken) 2011;294:1663–70. [DOI] [PubMed] [Google Scholar]
- 159.Simpson EA, Paukner A, Sclafani V, Kaburu SSK, Suomi SJ, Ferrari PF. Acute oxytocin improves memory and gaze following in male but not female nursery-reared infant macaques. Psychopharmacology (Berl) 2017;234:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, et al. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav 2015;9:754–64. [DOI] [PubMed] [Google Scholar]
- 161.CARTER C Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res 2007;176:170–86. [DOI] [PubMed] [Google Scholar]
- 162.Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev 2017;76:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.ISRAEL S, LERER E, SHALEV I, UZEFOVSKY F, REIBOLD M, BACHNERMELMAN R, et al. Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between. Adv. Vasopressin Oxytocin — From Genes to Behav. to Dis. Elsevier; 2008. p. 435–49. [DOI] [PubMed]
- 164.Bangasser DA, Curtis A, Reyes BAS, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 2010;15:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bao A-M, Swaab DF. Gender Difference in Age-Related Number of Corticotropin-Releasing Hormone-Expressing Neurons in the Human Hypothalamic Paraventricular Nucleus and the Role of Sex Hormones. Neuroendocrinology 2007;85:27–36. [DOI] [PubMed] [Google Scholar]
- 166.Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology 2006;31:59–68. [DOI] [PubMed] [Google Scholar]
- 167.Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014;49:207–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Veenit V, Riccio O, Sandi C. CRHR1 links peripuberty stress with deficits in social and stress-coping behaviors. J. Psychiatr. Res 2014;53:1–7. [DOI] [PubMed] [Google Scholar]
- 169.Lai M-C, Lombardo MV, Pasco G, Ruigrok AN V, Wheelwright SJ, Sadek SA, et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One 2011;6:e20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, et al. Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil. Health J 2010;3:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism Symptoms and Internalizing Psychopathology in Girls and Boys with Autism Spectrum Disorders. J. Autism Dev. Disord 2012;42:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.May T, Cornish K, Rinehart N. Does Gender Matter? A One Year Follow-up of Autistic, Attention and Anxiety Symptoms in High-Functioning Children with Autism Spectrum Disorder. J. Autism Dev. Disord. Springer US; 2014;44:1077–86. [DOI] [PubMed] [Google Scholar]
- 173.Hartley SL, Sikora DM. Sex Differences in Autism Spectrum Disorder: An Examination of Developmental Functioning, Autistic Symptoms, and Coexisting Behavior Problems in Toddlers. J. Autism Dev. Disord 2009;39:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, et al. Sex differences in the timing of identification among children and adults with autism spectrum disorders. J. Autism Dev. Disord 2013;43:1151–6. [DOI] [PubMed] [Google Scholar]
- 175.Rutherford M, McKenzie K, Johnson T, Catchpole C, O’Hare A, McClure I, et al. Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism 2016;20:628–34. [DOI] [PubMed] [Google Scholar]
- 176.Irimia A, Torgerson CM, Jacokes ZJ, Van Horn JD. The connectomes of males and females with autism spectrum disorder have significantly different white matter connectivity densities. Sci. Rep 2017;7:46401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kirkovski M, Enticott PG, Maller JJ, Rossell SL, Fitzgerald PB. Diffusion tensor imaging reveals no white matter impairments among adults with autism spectrum disorder. Psychiatry Res. Neuroimaging 2015;233:64–72. [DOI] [PubMed] [Google Scholar]
- 178.Retico A, Giuliano A, Tancredi R, Cosenza A, Apicella F, Narzisi A, et al. The effect of gender on the neuroanatomy of children with autism spectrum disorders: a support vector machine case-control study. Mol. Autism 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Supekar K, Menon V. Sex differences in structural organization of motor systems and their dissociable links with repetitive/restricted behaviors in children with autism. Mol Autism 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeestraten EA, Gudbrandsen MC, Daly E, de Schotten MT, Catani M, Dell’Acqua F, et al. Sex differences in frontal lobe connectivity in adults with autism spectrum conditions. Transl. Psychiatry 2017;7:e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V. Sex differences in cortical volume and gyrification in autism. Mol. Autism 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nordahl CW, Iosif A-M, Young GS, Perry LM, Dougherty R, Lee A, et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol. Autism 2015;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kirkovski M, Enticott PG, Hughes ME, Rossell SL, Fitzgerald PB. Atypical Neural Activity in Males But Not Females with Autism Spectrum Disorder. J. Autism Dev. Disord 2016;46:954–63. [DOI] [PubMed] [Google Scholar]
- 184.Ruskin DN, Fortin JA, Bisnauth SN, Masino SA. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav 2017;168:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Van Wijngaarden-Cremers PJM, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and Age Differences in the Core Triad of Impairments in Autism Spectrum Disorders: A Systematic Review and Meta-analysis. J. Autism Dev. Disord 2014;44:627–35. [DOI] [PubMed] [Google Scholar]
- 186.Head AM, McGillivray JA, Stokes MA. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol. Autism 2014;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Backer van Ommeren T, Koot HM, Scheeren AM, Begeer S. Sex differences in the reciprocal behaviour of children with autism. Autism 2017;21:795–803. [DOI] [PubMed] [Google Scholar]
- 188.Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, et al. An investigation of the “female camouflage effect” in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol. Autism 2016;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lai M-C, Lombardo MV., Ruigrok ANV, Chakrabarti B, Wheelwright SJ, Auyeung B, et al. Cognition in Males and Females with Autism: Similarities and Differences. Botbol M, editor. PLoS One 2012;7:e47198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, et al. Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res 2017;10:680–9. [DOI] [PubMed] [Google Scholar]
- 191.Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and Cognitive Characteristics of Females and Males With Autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 2014;53:329–340.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koyama T, Kamio Y, Inada N, Kurita H. Sex Differences in WISC-III Profiles of Children with High-functioning Pervasive Developmental Disorders. J. Autism Dev. Disord 2009;39:135–41. [DOI] [PubMed] [Google Scholar]
- 193.Sedgewick F, Hill V, Yates R, Pickering L, Pellicano E. Gender Differences in the Social Motivation and Friendship Experiences of Autistic and Non-autistic Adolescents. J. Autism Dev. Disord. Springer US; 2016;46:1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lehnhardt F-G, Falter CM, Gawronski A, Pfeiffer K, Tepest R, Franklin J, et al. Sex-Related Cognitive Profile in Autism Spectrum Disorders Diagnosed Late in Life: Implications for the Female Autistic Phenotype. J. Autism Dev. Disord 2016;46:139–54. [DOI] [PubMed] [Google Scholar]
- 195.Kauschke C, van der Beek B, Kamp-Becker I. Narratives of Girls and Boys with Autism Spectrum Disorders: Gender Differences in Narrative Competence and Internal State Language. J. Autism Dev. Disord 2016;46:840–52. [DOI] [PubMed] [Google Scholar]
- 196.Reinhardt VP, Wetherby AM, Schatschneider C, Lord C. Examination of Sex Differences in a Large Sample of Young Children with Autism Spectrum Disorder and Typical Development. J. Autism Dev. Disord Springer US; 2015;45:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Frazier TW, Hardan AY. Equivalence of symptom dimensions in females and males with autism. Autism 2017;21:749–59. [DOI] [PubMed] [Google Scholar]
- 198.Harrop C, Gulsrud A, Kasari C. Does Gender Moderate Core Deficits in ASD? An Investigation into Restricted and Repetitive Behaviors in Girls and Boys with ASD. J. Autism Dev. Disord Springer US; 2015;45:3644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pisula E, Pudło M, Słowińska M, Kawa R, Strząska M, Banasiak A, et al. Behavioral and emotional problems in high-functioning girls and boys with autism spectrum disorders: Parents’ reports and adolescents’ self-reports. Autism 2017;21:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Grove R, Hoekstra RA, Wierda M, Begeer S. Exploring sex differences in autistic traits: A factor analytic study of adults with autism. Autism 2017;21:760–8. [DOI] [PubMed] [Google Scholar]
- 201.Hiller RM, Young RL, Weber N. Sex Differences in Autism Spectrum Disorder based on DSM-5 Criteria: Evidence from Clinician and Teacher Reporting. J. Abnorm. Child Psychol Springer US; 2014;42:1381–93. [DOI] [PubMed] [Google Scholar]
- 202.Howe YJ, O’Rourke JA, Yatchmink Y, Viscidi EW, Jones RN, Morrow EM. Female Autism Phenotypes Investigated at Different Levels of Language and Developmental Abilities. J. Autism Dev. Disord. Springer US; 2015;45:3537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 2015;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jamison R, Bishop SL, Huerta M, Halladay AK. The clinician perspective on sex differences in autism spectrum disorders. Autism 2017;21:772–84. [DOI] [PubMed] [Google Scholar]
- 205.Hiller RM, Young RL, Weber N. Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism 2016;20:75–84. [DOI] [PubMed] [Google Scholar]
- 206.Charman T, Loth E, Tillmann J, Crawley D, Wooldridge C, Goyard D, et al. The EU-AIMS Longitudinal European Autism Project (LEAP): clinical characterisation. Mol. Autism 2017;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tsai P-C, Harrington RA, Lung F-W, Lee L-C. Disparity in report of autism-related behaviors by social demographic characteristics: Findings from a community-based study in Taiwan. Autism 2017;21:540–51. [DOI] [PubMed] [Google Scholar]
- 208.Murray AL, Allison C, Smith PL, Baron-Cohen S, Booth T, Auyeung B. Investigating diagnostic bias in autism spectrum conditions: An item response theory analysis of sex bias in the AQ-10. Autism Res 2017;10:790–800. [DOI] [PubMed] [Google Scholar]
- 209.Little LM, Wallisch A, Salley B, Jamison R. Do early caregiver concerns differ for girls with autism spectrum disorders? Autism 2017;21:728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cridland EK, Jones SC, Caputi P, Magee CA. Being a girl in a boys’ world: Investigating the experiences of girls with autism spectrum disorders during adolescence. J. Autism Dev. Disord 2014;44. [DOI] [PubMed] [Google Scholar]
- 211.Mandy W, Tchanturia K. Do women with eating disorders who have social and flexibility difficulties really have autism? A case series. Mol. Autism 2015;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bargiela S, Steward R, Mandy W. The Experiences of Late-diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord 2016;46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hull L, Petrides KV., Allison C, Smith P, Baron-Cohen S, Lai M-C, et al. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord 2017;47:2519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lai M-C, Lombardo MV, Ruigrok AN, Chakrabarti B, Auyeung B, Szatmari P, et al. Quantifying and exploring camouflaging in men and women with autism. Autism 2017;21:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Baldwin S, Costley D. The experiences and needs of female adults with high-functioning autism spectrum disorder. Autism 2016;20:483–95. [DOI] [PubMed] [Google Scholar]
- 216.Dean M, Harwood R, Kasari C. The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism 2017;21:678–89. [DOI] [PubMed] [Google Scholar]
- 217.Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Månsson K, Francis K, et al. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc. Natl. Acad. Sci. U. S. A 2017;114:1383–8.• This paper discusses risk for autism as a three-hit hypothesis, in which the factors are environmental, genetic, and male sex, as a possible explanation for male preponderance.
- 218.Connors EJ, Shaik AN, Migliore MM, Kentner AC. Environmental enrichment mitigates the sex-specific effects of gestational inflammation on social engagement and the hypothalamic pituitary adrenal axis-feedback system. Brain. Behav. Immun 2014;42:178–90. [DOI] [PubMed] [Google Scholar]
- 219.Edlow AG, Guedj F, Pennings JLA, Sverdlov D, Neri C, Bianchi DW. Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol 2016;214:623.e1–623.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Foley KA, MacFabe DF, Kavaliers M, Ossenkopp K-P. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: Relevance to autism spectrum disorders. Behav. Brain Res 2015;278:244–56. [DOI] [PubMed] [Google Scholar]
- 221.Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience 2014;279:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Angelakos CC, Watson AJ, O’Brien WT, Krainock KS, Nickl-Jockschat T, Abel T. Hyperactivity and male-specific sleep deficits in the 16p11.2 deletion mouse model of autism. Autism Res 2017;10:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tilot AK, Gaugler MK, Yu Q, Romigh T, Yu W, Miller RH, et al. Germline disruption of Pten localization causes enhanced sex-dependent social motivation and increased glial production. Hum. Mol. Genet 2014;23:3212–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Amram N, Hacohen-Kleiman G, Sragovich S, Malishkevich A, Katz J, Touloumi O, et al. Sexual divergence in microtubule function: the novel intranasal microtubule targeting SKIP normalizes axonal transport and enhances memory. Mol. Psychiatry 2016;21:1467–76. [DOI] [PubMed] [Google Scholar]
- 225.Magliaro C, Cocito C, Bagatella S, Merighi A, Ahluwalia A, Lossi L. The number of Purkinje neurons and their topology in the cerebellar vermis of normal and reln haplodeficient mouse. Ann. Anat. - Anat. Anzeiger 2016;207:68–75. [DOI] [PubMed] [Google Scholar]
- 226.Perez-Pouchoulen M, Miquel M, Saft P, Brug B, Toledo R, Hernandez ME, et al. Prenatal exposure to sodium valproate alters androgen receptor expression in the developing cerebellum in a region and age specific manner in male and female rats. Int. J. Dev. Neurosci 2016;53:46–52. [DOI] [PubMed] [Google Scholar]
- 227.Mowery TM, Wilson SM, Kostylev PV., Dina B, Buchholz JB, Prieto AL, et al. Embryological exposure to valproic acid disrupts morphology of the deep cerebellar nuclei in a sexually dimorphic way. Int. J. Dev. Neurosci 2015;40:15–23. [DOI] [PubMed] [Google Scholar]


