Abstract
Systemic arterial hypertension is the most important modifiable risk factor for all-cause morbidity and mortality worldwide and is associated with increased risk of cardiovascular disease (CVD). Fewer than half of those with hypertension are aware of their condition, and many others are aware but not treated or inadequately treated, although successful treatment of hypertension reduces the global burden of disease and mortality. The aetiology of hypertension involves the complex interplay of environmental and pathophysiological factors that affect multiple systems, as well as genetic predisposition. Evaluation of patients with hypertension includes accurate standardized blood pressure (BP) measurement, assessing patients’ predicted risk of atherosclerotic CVD, evidence of target organ damage, detection of secondary causes of hypertension and presence of comorbidities, including CVD and kidney disease. Lifestyle changes, including dietary modifications and increased physical activity, are effective in lowering BP and preventing hypertension and its CVD sequelae. Pharmacological therapy is very effective in lowering BP and preventing CVD outcomes in most patients; first line antihypertensive medications include angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, dihydropyridine calcium channel blockers and thiazide diuretics.
INTRODUCTION
Systemic arterial hypertension (hereafter referred to as hypertension) is characterized by persistently high blood pressure (BP) in the systemic arteries. BP is commonly expressed as the ratio of the systolic BP (that is, the pressure that the blood exerts on the arterial walls when the heart contracts) and the diastolic BP (the pressure when the heart relaxes). The BP thresholds that define hypertension depend on the measurement method (Table 1). Several aetiologies can underlie hypertension. The majority (90–95%) of patients have a highly heterogeneous ‘essential’ or primary hypertension with a multifactorial gene-environment aetiology. A positive family history is a frequent occurrence in patients with hypertension, with the heritability (a measure of how much of the variation in a trait is due to variation in genetic factors) estimated between 35% and 50% in the majority of studies1,2. Genome-wide association studies (GWAS) have identified ~120 loci that are associated with BP regulation and together explain 3.5% of the trait variance3,4,5. These findings are becoming increasingly important as we search for new pathways and new biomarkers to develop more-modern ‘omics’-driven diagnostic and therapeutic modalities for hypertension in the era of precision medicine6.
Table 1 -.
Definitions of hypertension based on the 2013 ESH/ESC guidelines
| Category | Subtype | Systolic BP (mmHg) | Diastolic BP (mmHg) |
|---|---|---|---|
| Office BP | NA | ≥ 140 | ≥ 90 |
| Ambulatory BP | Daytime (awake) | ≥ 135 | ≥ 85 |
| Night time (asleep) | ≥ 120 | ≥ 70 | |
| 24hr | ≥ 130 | ≥ 80 | |
| Home BP | NA | ≥ 135 | ≥ 85 |
For the diagnosis of hypertension, systolic BP, diastolic BP or both have to exceed the reported values.
NA, not applicable. Modified from Ref77.
Several rare, monogenic forms of hypertension have been described (for example, the Liddle syndrome, glucocorticoid-remediable aldosteronism (a mineralocorticoid excess state) and mutations in PDE3A (which encodes cGMP-inhibited 3’,5’-cyclic phosphodiesterase A)), in which a single gene mutation fully explains the pathogenesis of hypertension and indicates the best treatment modality7,8,9. If hypertension is caused by another condition (for example, primary aldosteronism, pheochromocytoma (a neuroendocrine tumour of the adrenal glands or other neuroendocrine tissues) or renal artery stenosis), it is referred to as secondary hypertension.
Hypertension is the most common preventable risk factor for cardiovascular disease (CVD; including coronary heart disease, heart failure, stroke, myocardial infarction, atrial fibrillation and peripheral artery disease), chronic kidney disease (CKD) and cognitive impairment, and is the leading single contributor to all-cause death and disability worldwide10. The relationship between BP and the increased risk of CVD is graded and continuous, starting as low as 115/75 mmHg, well within what is considered to be the normotensive range. Successful prevention and treatment of hypertension are key in reducing disease burden and promoting longevity in the world’s population. In treating hyperteinsion, it is important to consider a person’s predicted atherosclerotic CVD (ASCVD) risk more than the level of BP alone, as persons with high CVD risk derive the greatest benefit from BP lowering treatment11.
This Primer will discuss the epidemiology and pathophysiology of primary hypertension, prevention strategies for slowing the progression of BP elevation, management strategies (including optimal BP targets) for lowering BP and preventing CVD outcomes in patients with established hypertension and the effects of antihypertensive treatment on quality of life; finally, we will explore knowledge gaps, future trends and the outlook for hypertension research and treatment over the next decade.
EPIDEMIOLOGY
In pre-industrial societies, BP levels had narrow distributions with mean values that changed little with age and averaged around 115/75 mmHg12, a value that probably represents the normal (or ideal) BP for humans. However, in most contemporary societies, systolic BP levels rise steadily and continuously with age in both men and women. This ubiquitous finding could be explained because age is a proxy for the probability and duration of exposure to the numerous environmental factors that increase BP gradually over time, such as excessive sodium consumption, insufficient intake of dietary potassium, overweight and obesity, alcohol intake and physical inactivity. Other factors, such as genetic predisposition or adverse intrauterine environment (such as gestational hypertension or pre-eclampsia), have small but definite associations with high BP levels in adulthood13. Even modest rises in mean population BP lead to large increases in the absolute number of people with hypertension14.
As economic development progresses, hypertension initially affects those with a high socioeconomic status, but at later stages of economic development, the prevalence of hypertension and its consequences are greatest in those with lower socioeconomic status; this phenomenon is seen both within and between countries. Further, the speed of change prevalence of hypertension since 2000 to 2010 has been much more rapid than in previous epidemiological transitions15.
Disease burden
Globally, 3.5 billion adults now have non-optimal systolic BP levels (that is, >110–115 mmHg) and 874 million adults have systolic BP ≥140 mmHg. Thus, approximately one in four adults has hypertension16. Between 1990 and 2015 there was a 43% increase in the total global number of healthy life years lost to non-optimal BP, driven by population increase, population aging and a 10% increase in the age-standardized prevalence of hypertension16. The Global Burden of Disease study has shown that non-optimal BP continues to be the biggest single risk factor contributing to the global burden of disease and to global all-cause mortality, leading to 9.4 million deaths and 212 million lost healthy life years (8.5% of the global total) each year10.
CVD risk
Prospective observational studies have repeatedly demonstrated a strong, continuous positive relationship between BP and CVD, with no evidence of a threshold for risk throughout the usual range of BP observed in clinical practice17,18,19. The relationship between BP and CVD applies to both systolic BP and diastolic BP, but is somewhat more robust for systolic BP in adults19. It is noted in both sexes, at all ages throughout adulthood and for all major manifestations of CVD, including stroke (ischaemic and haemorroagic), coronary artery disease, heart failure, peripheral vascular disease and end stage renal disease (although there are variations in the strength of the associations and the slopes of the curves)17,18,19,20 (Figure 1). The relationship is independent of other CVD risk factors, and level of BP has proven to be a major component of CVD risk in all prediction models21. Approximately two-thirds of all adults who have hypertension or receive treatment with BP lowering medication at 30 years of age have a ~40 % higher risk of experiencing a CVD event than their age-matched and sex-matched counterparts with a lower level of BP18. In addition, CVD events in individuals with hypertension tend to manifest about five years earlier than in individuals with a lower level of BP18.
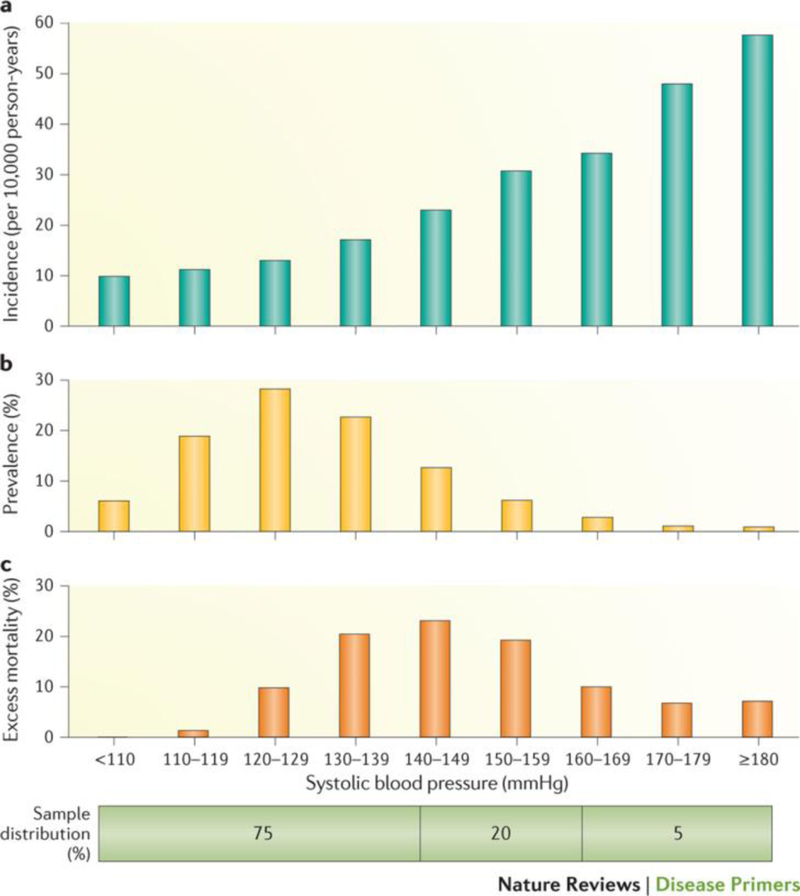
Figure 1. Association between systolic blood pressure and coronary heart disease mortality.
Relationship of systolic BP to subsequent risk of coronary heart disease mortality in >340,000 US men 35–57 years of age at the beginning of the study followed-up for an average 11.6 years. A | Distribution of the incidence of coronary heart disease mortality, adjusted for age, race, total serum cholesterol level, cigarettes smoked per day, use of medication for diabetes, and income. Individuals with the highest BPs were at greatest risk for CVD mortality. B | Prevalence of coronary heart disease mortality; only a minority of the sample was exposed to the high risk associated with hypertension (≥140 mmHg for systolic BP, as per office BP measurement). However, a much larger number of them, who had BP in the non-hypertensive range, were exposed to the more modest but still important increases in CVD risk. C | Estimation of the percent of excess coronary heart disease deaths occurring in each category of systolic BP, using those with a systolic BP <110 mm Hg as the reference group. About two-thirds of the overall burden of BP-related CHD mortality occurred in men who had a systolic BP ≥140 mmHg (25% of the sample). However, about two-thirds of the remaining disease burden could be attributed to the approximately 20% of adults who had a systolic BP in the high-normal range (systolic BP 130–139 mmHg)204. Data from Ref.19.
In individuals of 40–69 years of age, a 20 mmHg rise of systolic BP or a 10 mmHg rise of diastolic BP regardless of baseline values is associated with more than a doubling of the risk for stroke or ischaemic heart disease mortality17, whereas a systolic BP reduction of 5 mmHg can decrease stroke mortality by 14% and CVD mortality by 9%. At older ages (≥80 years), the corresponding relative risk is slightly lower, but the absolute risk is far greater than earlier in life17. For example, a 20 mm Hg difference in systolic BP between 120 and 140 mmHg is associated with an annual difference in absolute risk that is nearly ten times larger at ages 80–89 years than that at ages 50–59 years17.
MECHANISMS/PATHOPHYSIOLOGY
BP regulation
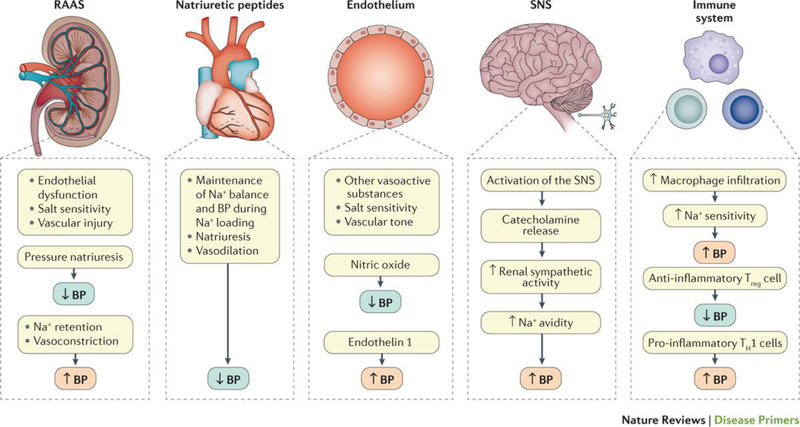
BP is determined by several parameters of the cardiovascular system, including blood volume and cardiac output (the amount of blood pumped by the heart per minute) as well as the balance of arterial tone that is affected by both intravascular volume and neurohumoral systems (discussed in the following sections). The maintenance of physiological BP levels involves a complex interplay of various elements of an integrated neurohumoral system that includes the renin-angiotensin-aldosterone system (RAAS), the role of natriuretic peptides and the endothelium, the sympathetic nervous system (SNS) and the immune system (Figure 2). Malfunction or disruption of factors involved in BP control in any of these systems can directly or indirectly lead to increases in mean BP, BP variability or both, over time resulting in target organ damage (for example, left ventricular hypertrophy and CKD) and CVD outcomes22.
Figure 2. The main neuroendocrine systems involved in the regulation of blood pressure.
Neurohumoral, immune and organ systems involved in the maintenance of blood pressure. BP: Blood pressure, RAAS: renin-angiotensin-aldosterone system.
The pathophysiological mechanisms responsible for hypertension are complex and act on a genetic background. Primary hypertension involves multiple types of genes; some allelic variants of several genes are associated with an increased risk of developing primary hypertension and are linked in almost all cases to a positive family history (Box 1) This genetic predisposition, along with a host of environmental factors, such as high Na+ intake, poor sleep quality or sleep apnoea, excess alcohol intake and high mental stress, contribute to the development of hypertension22,23,24. Finally, the probability of developing hypertension increases with aging, owing to progressive stiffening of the arterial vasculature caused by, among other factors, slowly developing changes in vascular collagen and increases in atherosclerosis25,26,27. Immunological factors can also play a major part, especially on the background of infectious or rheumatological diseases such as rheumatoid arthritis. The mosaic theory of hypertension describes its multifaceted pathophysiology28,29.
Box 1. Genetic predisposition to hypertension.
A large GWAS of 2.5 million genotyped single nucleotide polymorphisms (SNPs) in >69,000 individuals of European ancestry from 29 studies demonstrated that most SNPs related to BP regulation and CVD risk involved natriuretic peptides198. SNPs in genes that encode precursors for ANP and BNP had been previously identified199, and two other loci were identified in this study, containing genes involved in natriuretic peptide and NO signalling pathways; both these pathways regulate cyclic guanosine monophosphate, which promotes vasodilation. A 2016 study identified 66 BP–associated loci, which were enriched for cis-regulatory elements in vascular endothelial cells, consistent with a role in BP control through modulation of vascular tone. This information prompted development of a genetic risk score to predict target organ damage4.
Gene deletion studies in rodent models have evaluated cardiac ANP and BNP as paracrine regulators of vascular regeneration. Deletion of the genes encoding ANP and BNP exaggerates cardiac fibrosis and increase adverse left ventricular (LV) remodelling38, and natriuretic peptide receptor A (NPRA) deficiency leads to increased BP, severe fibrosis and LV dysfunction. Further, deletion of the gene encoding the endothelial guanylyl cyclase-A (GC-A) receptor, a cell surface receptor for natriuretic peptides, leads to diminished vascular regeneration and angiogenesis in response to critical hind limb ischemia, as well as cardiac fibrosis and diastolic dysfunction.
Further, clinical studies have observed an association between certain corin gene polymorphisms and risk of pre-eclampsia and hypertension, particularly among African-American but not Chinese populations200.
Sodium homeostasis regulation
Sodium (Na+) is a crucial regulator of blood volume: high serum Na+ concentration promotes fluid (water) retention, thereby increasing blood volume and BP. When dietary Na+ increases in normotensive individuals, compensatory haemodynamic changes occur to maintain constant BP. These changes include reduction in renal and peripheral vascular resistance and increased production of nitric oxide (a vasodilator) from the endothelium. However, if the effect of nitric oxide is impaired or absent, an increase in BP occurs. Endothelial dysfunction is a risk factor for the development of salt sensitivity and subsequent hypertension. Salt sensitivity is defined as a marked elevation in BP following a Na+ load of ≥5 g and is characterized by an elevation of systolic BP of at least 10 mmHg within a few hours of ingestion. Salt sensitive individuals have underlying endothelial dysfunction due to genetic or environmental influences. In response to a high salt load these individuals generally manifest overproduction of transforming growth factor β (TGF-β), which increases the risk of fibrosis, and oxidative stress, and have limited bioavailable nitric oxide. Chronic high salt ingestion can result in endothelial dysfunction, even in salt-resistant individuals30, and also affects the gut microbiota, with resultant changes that contribute to increased salt sensitivity and the development of hypertension31. High salt intake also appears to drive autoimmunity by inducing T helper 17 (TH17) cells31. High salt intake in mice has been shown to deplete Lactobacillus murinus in the gut microbiota. Treatment of mice with L. murinus prevented salt-induced exacerbation of salt-sensitive hypertension by modulating TH17 cells31. In line with these findings, a moderate high-salt challenge in a pilot study in humans reduced intestinal survival of Lactobacillus spp., increased the activity of TH17 cells and increased BP31. Thus, the gut microbiota appears to contribute to salt sensitivity of BP and the pathogenesis of hypertension.
Renin-Angiotensin-Aldosterone System
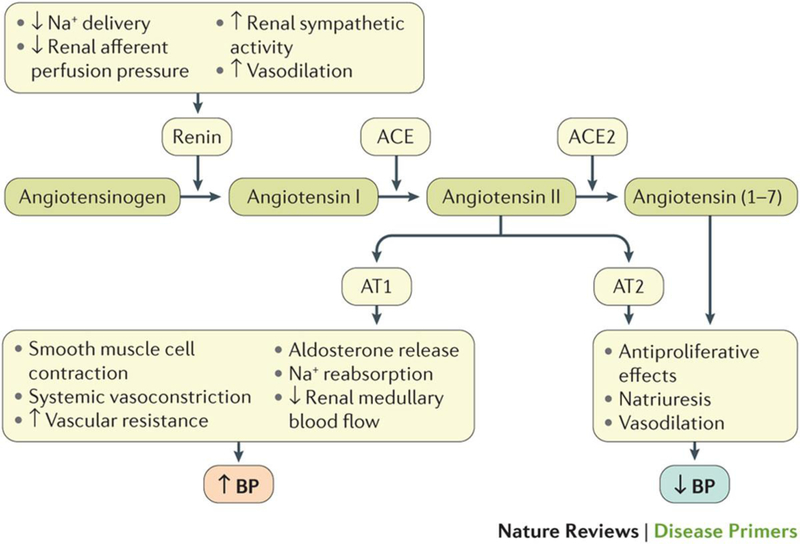
The RAAS has wide-ranging effects on BP regulation, mediating Na+ retention, pressure natriuresis (that is, the mechanism whereby increases in renal perfusion pressure (the gradient between renal arterial and venous blood pressure) lead to decreased Na+ reabsorption and increased Na+ excretion), salt sensitivity, vasoconstriction, endothelial dysfunction and vascular injury, and plays an important part in the pathogenesis of hypertension22. The RAAS is present at the cellular level in many organs, but its most crucial role is to help regulate pressure-volume homeostasis in the kidney, where it maintains perfusion in volume depleted states (that is, when there is a reduction in extracellular fluid volume as a result of sodium and fluid loss) and is suppressed in volume expanded (fluid overload) conditions. Renin and its precursor pro-renin are synthesized and stored in the juxtaglomerular cells of the kidney and are released in response to various stimuli (Figure 3). The main function of renin is to cleave angiotensinogen to form angiotensin I. Angiotensin-converting enzyme (ACE) cleaves angiotensin I to form angiotensin II, which is at the center of the pathogenetic role of the RAAS in hypertension (Figure 3)32..
Figure 3. Role of the renin-angiotensin-aldosterone system in the regulation of blood pressure.
Decreased renal afferent perfusion pressure, reduced Na+ delivery to the macula densa (an area lining the wall of the distal convoluted tubule in correspondence of the glomerulus), activation of renal sympathetic nerves (via β1 adrenergic receptor stimulation) and a variety of vasodilators, including prostaglandin E2, stimulate the release of renin. Angiotensin II activates the AT1 receptor, triggering smooth muscle cell contraction, systemic vasoconstriction, increased renovascular resistance and decreased renal medullary blood flow, a mediator of salt sensitivity. Stimulation of the AT2 receptor has opposite effects, resulting in vasodilation, natriuresis and anti-proliferative actions. Cross-transplantation studies using wild-type mice and mice lacking the AT1 receptor have shown that both systemic and renal actions of angiotensin II are relevant to physiologic BP regulation, but that the detrimental effects of angiotensin II in hypertension are mediated mainly via the kidney205,206. ACE inhibitors and AT1 receptor antagonists have been shown to increase Ang-(1–7) levels in plasma and urine of normotensive animals and enhance renal ACE2 activity33.. Studies in rodents and humans with non-diabetic kidney disease suggest that upregulation of ACE2 may delay progression of kidney disease207.
Angiotensin II enhances Na+ reabsorption in the proximal tubule by increasing the activity of the sodium-hydrogen exchanger (NHE3), sodium-bicarbonate exchanger and sodium-potassium ATPase, and by inducing aldosterone synthesis and release from the adrenal glomerulosa22. Angiotensin II is also associated with endothelial dysfunction and has pro-fibrotic and pro-inflammatory effects, mediated in large part by increased oxidative stress, resulting in renal, cardiac and vascular injury. Angiotensin II is tightly linked to target organ damage in hypertension via these mechanisms22..
Angiotensin-converting enzyme 2 (ACE2) has emerged as an important modulator in the pathophysiology of hypertension, CVD and renal disease, owing to its role in metabolizing angiotensin II into angiotensin-(1–7)33. Ang-(1–7) induces systemic and regional vasodilation, diuresis and natriuresis, and exerts antiproliferative and antigrowth effects on vascular smooth muscle cells, cardiac myocytes and fibroblasts as well as glomerular and proximal tubular cells33. Ang-(1–7) also has cardiorenal protective effects that are mediated by the proto-oncogene Mas receptor through signalling pathways that include mitogen-activated protein kinases (MAPK), PI3K-AKT, NADPH oxidase, TGF-β1, the EGF receptor, and NF-κB activity33,34,35.
Aldosterone plays a crucial part in hypertension: by binding to the mineralocorticoid receptor, it induces non-genomic effects (that is, without directly modifying gene expression) that include activation of the amiloride-sensitive sodium channel, commonly known as the epithelial sodium channel (ENaC) and result in the stimulation of renal Na+ reabsorption in the cortical collecting duct36. Aldosterone also has many non-epithelial effects that contribute to endothelial dysfunction, vasoconstriction and hypertension36,37. These include vascular smooth muscle cell proliferation, vascular extracellular matrix deposition, vascular remodeling, fibrosis, and increased oxidative stress36,37.
Natriuretic Peptides
Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) play an important part in salt sensitivity and hypertension. They have important natriuretic and vasodilator properties that allow maintenance of Na+ balance and BP during Na+ loading38,39. Upon administration of a Na+ load, atrial and ventricular stretch leads to release of ANP and BNP, respectively, which leads to systemic vasodilation and decreased plasma volume (owing to fluid shifts from the intravascular to the interstitial compartment) and results in BP lowering40. Natriuretic peptides increase glomerular filtration rate via an increase in efferent arteriolar tone in volume-expanded states and inhibit renal Na+ reabsorption through both direct and indirect effects. Direct effects include decreased activity of Na+-K+-ATPase and the sodium-glucose co-transporter in the proximal tubule and inhibition of the epithelial sodium channel in the distal nephron. Indirect effects include inhibition of renin and aldosterone release39.
Natriuretic peptide deficiency promotes hypertension. Corin is a serine protease that is largely expressed in the heart and converts the ANP and BNP precursors pro-ANP and pro-BNP to their active forms. Corin deficiency has been associated with volume overload, heart failure and salt-sensitive hypertension41. Natriuretic peptide deficiency also predisposes to insulin resistance and type 2 diabetes mellitus. Obesity is associated with natriuretic peptide deficiency, probably through upregulation of the natriuretic peptide scavenger receptor NPR-C in adipose tissue42. Natriuretic peptides have therapeutic potential for the metabolic syndrome; the metabolic syndrome is a cluster of conditions (including high BP, high fasting glucose levels, abdominal obesity, high triglycerides and microalbuminuria) that occur together, increasing the risk of CVD and diabetes mellitus42.
The Endothelium
The endothelium is a major regulator of vascular tone and major contributor to salt sensitivity through NO. Endothelial cells produce a host of vasoactive substances, of which NO is the most important in BP regulation43,44. NO is continuously released by endothelial cells in response to flow-induced shear stress, leading to vascular smooth muscle relaxation through activation of guanylate cyclase and generation of intracellular cyclic guanosine monophosphate45. Interruption of NO production via inhibition of constitutively expressed endothelial NO synthase (eNOS) causes BP elevation and development of hypertension in animals and humans46. Studies to evaluate NO activity in humans have demonstrated decreased whole-body production of NO in patients with hypertension compared with normotensive controls46,47.
Endothelial cells also secrete a variety of other vasoregulatory substances, including vasodilators such as prostacyclin and endothelium-derived hyperpolarizing factors, and vasoconstrictors such as endothelin 1 (ET-1), locally generated angiotensin II and the prostanoids thromboxane A2 and prostaglandin A2. ET-1 is a potent vasoconstrictor that activates ET-A receptors in vascular smooth muscle48. Other vasodilating substances, secreted by a variety of cells types, such as calcitonin gene related peptide, adrenomedullin and substance P act primarily through increases in NO release from endothelial cells49,50. The glucose-regulating gut hormone glucagon-like peptide-1 (GLP-1) also has vasodilating properties51. The balance between these factors, along with NO and ET-1, determines the final effect of the endothelium on vascular tone52,48,53,51. Circulating ET-1 levels are not consistently increased in hypertension, but there is a trend toward increased sensitivity to the vasoconstrictor and hypertensive effects of ET-1 in individuals with hypertension53. ET-A receptor antagonists attenuate or abolish hypertension in a variety of experimental models and are effective in lowering BP in humans48,53.
Endothelial dysfunction plays a seminal part in the pathogenesis of hypertension. Normotensive offspring of parents with hypertension often have impaired endothelium-dependent vasodilation, which implies a genetic component in the development of endothelial dysfunction47. Endothelial dysfunction in the setting of chronic hypertension is related to a combination of direct pressure-induced injury and increased oxidative stress. Several enzyme systems, including NADPH oxidase, xanthine oxidase and cyclooxygenase, as well as decreased activity of superoxide dismutase generate reactive oxygen species47,54. Excess superoxide anions bind to NO, decreasing NO bioavailability and generating the pro-inflammatory oxidant, peroxynitrite. Decreased NO bioavailability is the central factor that links oxidative stress to endothelial dysfunction and hypertension47. Salt-sensitive individuals may be very sensitive to the hemodynamic stress of increased blood volume, leading to overproduction of TGF-beta, oxidative stress, and limiting bioavailable NO30. Angiotensin II, along with other factors, including cyclic vascular stretch as a result of BP changes, endothelin-1 (ET-1), uric acid, systemic inflammation, norepinephrine, free fatty acids, and tobacco smoking, enhances NADPH oxidase activity and plays a central part in the generation of oxidative stress in hypertension52.
Sympathetic Nervous System
Baroreceptors, mechanoreceptors that sense pressure changes of the circulatory system, are housed in various locations in the arterial tree, a key place being the carotid sinus, a dilated area at the base of the internal carotid artery just superior to bifurcation of the common carotid artery. When this artery is stretched by elevated BP, nerve bundles projecting from the baroreceptors in the carotid sinus send messages to the brain to reduce sympathetic outflow of nerve impulses or nerve traffic and, thereby, BP55,56,57. The SNS is generally more activated in persons with hypertension than in normotensive individuals.58,59 SNS activity is also greater in individual with obesity, in men than in women, in younger than in older persons, and in those with advanced kidney disease.60,61 Many patients with hypertension are in a state of autonomic imbalance with increased sympathetic and decreased parasympathetic activity59,62. SNS hyperactivity is relevant to both the generation and maintenance of hypertension. Studies in humans have also identified markers (such as increased catecholamine spillover and sural nerve activity assessed by microneurography) of sympathetic overactivity in normotensive individuals with a family history of hypertension63. Among patients with hypertension, increasing severity of hypertension is associated with increasing levels of sympathetic activity measured by microneurography64,65. Plasma catecholamine levels, microneurographic recordings and systemic catecholamine spillover (the amount of catecholamines released from sympathetic nerves innervating blood vessels that enter the bloodstream) studies have given evidence of increased sympathetic activity in patients with hypertension who are obese, in those with the metabolic syndrome, and in those whose hypertension is complicated by heart failure or kidney disease65.
The importance of the SNS in the pathogenesis of hypertension has been defined in a variety of experimental models. Models of obesity-related hypertension demonstrate that increased renal sympathetic nerve activity and its attendant increase in renal sodium reabsorption are key factors in the maintenance of sustained hypertension62. In another animal model, rats that received daily infusions of phenylephrine for 8 weeks developed hypertension during the infusions; their BP normalized under a low salt diet after discontinuation of phenylephrine, but once re-challenged with a high salt diet, the animals became hypertensive again30. The degree of BP elevation on the high salt diet was directly related to the degree of renal tubulo-interstitial fibrosis and decrease in glomerular filtration rate, suggesting that catecholamine-induced hypertension causes renal interstitial injury and a salt-sensitive phenotype that persists even after sympathetic overactivity is no longer present. In addition, enhanced SNS activity results in alpha-1 adrenergic receptor mediated endothelial dysfunction, vasoconstriction, vascular smooth muscle proliferation and increased arterial stiffness, which contribute to the development and maintenance of hypertension66. Finally, there is evidence that sympathetic overactivity enhances salt-sensitivity owing to a reduction in activity of the WNK lysine deficient protein kinase 4 (WNK4) gene, which encodes a serine/threonine kinase that inhibits the thiazide-sensitive-Na-Cl co-transporter, resulting in increased distal tubular Na+ retention67. These mechanisms have been reviewed recently66.
Inflammation and the immune system
Inflammation makes an important contribution to the genesis of hypertension and related target organ damage. Inflammation is associated with increased vascular permeability and release of potent mediators, such as reactive oxygen species, NO, cytokines and metalloproteinases. Cytokines mediate the formation of neo-intima (a new or thickened layer of arterial intima), thereby decreasing the lumen diameter of resistance vessels (small arteries and arterioles highly innervated by autonomic nerves and the primary vessels involved in the regulation of BP), and promoting vascular fibrosis, leading to increased vascular resistance and stiffness. Cytokines also affect renal tubular function by increasing local synthesis of angiotensinogen and angiotensin II, as well as promoting sodium and volume retention in hypertension68. Matrix metalloproteinases stimulate the degradation of the extracellular matrix, allowing infiltration of immune cells through the vessel wall into the interstitium of the affected organs, promoting apoptosis and enhancing collagen synthesis and matrix deposition, leading to target organ damage68.
While animal data are clear about the relationship between inflammation and hypertension, the data in humans are limited. There are associations between C-reactive protein, TNF-alpha and various interleukins and hypertension, but no direct link68. GWASs have identified a single nucleotide polymorphism of SH2B3 (SNP rs3184504), which results in an amino acid substitution in SH2B adapter protein 3 (a protein involved in T cell receptor activation and signalling), that is associated with many autoimmune and cardiovascular disorders, including hypertension69. Further, drugs that are used to treat inflammation, such as non-steroidal anti-inflammatory drugs and cyclosporine, raise rather than lower BP in hypertensive individuals, highlighting the complex nature of the relationship between inflammation and hypertension69.
Both innate and adaptive immune responses participate in the generation of reactive oxygen species and inflammatory changes in the kidneys, blood vessels and brain in hypertension68,70. Innate immune responses, especially those mediated by macrophages, have been linked to hypertension induced by angiotensin II, aldosterone and NO antagonism68,70. Reductions in macrophage infiltration of the kidney or the peri-adventitial space of the aorta and medium sized arteries lead to reductions in BP and salt-sensitivity68. Adaptive immune responses via T cells have also been linked to the genesis of hypertension and its target organ damage. T cells express AT1 receptors and mediate angiotensin II-dependent hypertension,70 and it has been shown that depletion of mature lymphocytes ameliorated hypertension and kidney injury resulting from a high-salt diet in the Dahl SS rat71. Thus, a balance between proinflammatory T cell reactivity and inflammatory suppression induced by T regulatory cells determines the development of hypertension, as demonstrated by the amelioration of hypertension with the adoptive transfer of T regulatory cells in several animal models of hypertension68–70. Abnormalities in both pro-inflammatory T cells and regulatory T cells are implicated in hypertension-induced target organ damage, as they regulate the inflammatory processes in the kidney and vasculature that underlie hypertension-induced kidney disease68,70,71.
DIAGNOSIS, SCREENING AND PREVENTION
Diagnosis and screening
Essential or primary hypertension is usually asymptomatic; thus, in clinical practice all adults should have their BP measured at regular office visits. Hypertension is most commonly diagnosed based on repeated BP measurements in a clinical office setting. Accurate measurement and recording of BP is essential to categorize the level of BP, ascertain BP-related CVD risk and guide management. Since 2010, methods to measure out-of-office BP have been increasingly introduced to guide diagnosis and treatment of hypertension72,73. Table 1 These include home BP monitoring (HBPM) and ambulatory BP monitoring (ABPM). HBPM refers to the measurement of BP at regular intervals by an individual at their home or elsewhere outside the clinic setting. ABPM consists of measuring and recording the BP at regular intervals (usually every 20–30 minutes), typically for the 24-hour period and while individuals go about their daily activities. The ability to measure out-of-office BP has enabled the identification of distinct BP phenotypes, including white coat or isolated clinic hypertension and masked or isolated ambulatory hypertension74,75. White coat hypertension is characterised by elevated office BP but normal ABPM or HBPM readings. By contrast, masked hypertension is characterised by normal office readings but elevated out –of-office readings ( ABPM and HBPM )74,75.
Diagnosis
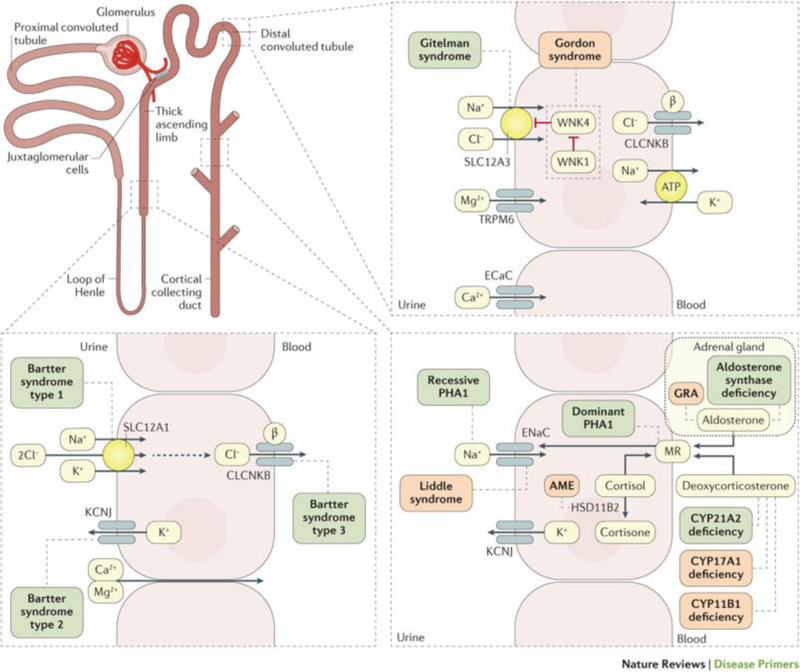
The evaluation of a patient with hypertension requires more than the diagnosis of elevated BP. It should also include assessment of the CVD risk, target organ damage, and concomitant clinical conditions that may affect the BP or related target organ damage as well as recognition of features suggestive of secondary hypertension. Some of these investigations are routine tests necessary in all patients, but others only in specific patient groups identified by history, clinical examination, and routine tests. In rare inherited forms of hypertension, a single gene mutation explains the pathogenesis of hypertension7,8,9. (Figure 4) A small proportion of patients have a potentially reversible cause of hypertension, and a correct diagnosis might lead to a cure or a substantial improvement in BP control with a reduction of CVD risk. It is therefore appropriate to implement a simple screening for secondary hypertension in all patients. The screening is based on clinical history, physical examination and routine laboratory investigations (Box 2 and 3). Secondary hypertension should also be considered in cases of a sudden worsening of hypertension, poor BP response to drug treatment or severe target organ damage, which is out of proportion to the duration and severity of hypertension. In these cases, specific diagnostic tests are indicated (Table 2).
Figure 4. Pathways affected in single gene, Mendelian hypertension and hypotension syndromes.
Some inherited diseases can affect the renal-angiotensin-aldosterone system pathways and, therefore, the blood pressure; hypertensive disorders are listed in red boxes and hypotensive disorders in green boxes. MR, mineralocorticoid receptor; GRA, glucocorticoid-remediable aldosteronism; PHA1, pseudohypoaldosteronism, type-1; AME, apparent mineralocorticoid excess; SLC12A1, solute carrier family 12 member 1; SLC12A3, solute carrier family 12 member 3; CLCNKB, chloride channel protein ClC-Kb; KCNJ, inward rectifier potassium channel; ECaC, epithelial calcium channel; ENaC; epithelial Na channel; WNK1, Serine/threonine-protein kinase WNK1; HSD11B1, corticosteroid 11-beta-dehydrogenase isozyme 1; CYP21A2, steroid 21-hydroxylase; CYP17A1, steroid 17-alpha-hydroxylase/17,20 lyase; CYP11B1, cytochrome P450 11B1, mitochondrial. Modified from Ref7
Box 2 – Physical examination for secondary hypertension, organ damage and obesity.
| Signs suggestive of secondary hypertension |
| ● Features of Cushing syndrome ● Neurofibromatosis (pheochromocytoma) ● Enlarged kidneys (polycystic kidney) ● Abdominal murmurs (renovascular hypertension) ● Precordial murmurs (aortic coarctation, aortic disease) |
|
Signs of target organ damage |
| ● Brain: motor or sensory deficit ● Retina: hypertensive retinopathy ● Heart: atrial fibrillation, arrhythmias, pulmonary congestion and peripheral oedema ● Peripheral arteries: absent, reduced or asymmetrical pulses and ischaemic skin lesions ● Carotid arteries: murmurs |
|
Evidence of obesity |
| ● BMI (body weight/height2) > 30 ● Waist circumference* >102 cm in man and >88 cm in women |
values might need to be adjusted based on ethnicity or other factors.
BMI, body mass index
Box 3 – Laboratory investigations in the diagnosis of hypertension.
|
Routine tests
● Haemoglobin and haematocrit ● Fasting plasma glucose ● Serum total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol ● Fasting serum triglycerides ● Serum potassium and sodium ● Serum uric acid ● Serum creatinine ● Estimated glomerular filtration rate (eGFR) ● Urine analysis including a test for microalbuminuria ● 12-lead EKG |
|
Additional tests based on history, clinical examination and routine tests ● Haemoglobin A1c ● Quantitative proteinuria ● Out-of-office BP measurements* ● Echocardiogram ● Holter monitoring ● Carotid ultrasound ● Abdominal ultrasound ● Pulse wave velocity ● Ankle-brachial index ● Further specialist tests for secondary hypertension (renin, aldosterone, catecholamines and their metabolites etc) |
Ambulatory BP monitoring (ABPM) is recommended as the preferred method for measurement of “out-of-office” BPs to confirm high BP and to diagnose masked hypertension. Careful measurement of home BPs is acceptable when ABPM is not feasible.
Table 2 –
Diagnostics of secondary hypertension
| Possible causes | Clinical indications | Diagnostics | |||
|---|---|---|---|---|---|
| Clinical history | Physical examination | Laboratory investigations | First-line test(s) | Additional confirmatory test(s) | |
| Common causes | |||||
| Renal parenchymal disease | ● History of urinary tract infection or obstruction, haematuria (blood in the urine), analgesic abuse ● family history of polycystic kidney disease |
Abdominal masses (in case of polycystic kidney disease) | ● Presence of protein, erythrocytes, or leucocytes in the urine ● decreased GFR |
Renal ultrasound | Detailed work-up for kidney disease |
| Renal artery stenosis | ● Fibromuscular dysplasia: early onset hypertension (especially in women). ● Atherosclerotic stenosis: hypertension of abrupt onset, worsening or increasingly difficult to treat; flash pulmonary oedema |
Abdominal bruit (abnormal sound) | ● Difference of >1.5 cm in length between the two kidneys (renal ultrasound), ● rapid deterioration in renal function (spontaneous or in response to RAA blockers) |
Renal Duplex Doppler ultrasonography | ● Magnetic resonance angiography, ● spiral computed tomography ● intra-arterial digital subtraction angiography |
| Primary aldosteronism | ● Muscle weakness ● family history of early onset hypertension and cerebrovascular events at <40 years of age |
Arrhythmias (in case of severe hypokalaemia) | ● Hypokalaemia (spontaneous or diuretic-induced) ● incidental discovery of adrenal masses |
Aldosterone–renin ratio under standardized conditions (corrected hypokalaemia and withdrawal of drugs affecting RAA system) | ● Confirmatory tests (oral sodium loading, saline infusion, fludrocortisone suppression, or captopril test) ● adrenal CT scan ● adrenal vein sampling |
| Uncommon causes | |||||
| Pheochromocytoma | ● Paroxysmal hypertension or a crisis superimposed to sustained hypertension; ● headache, sweating, palpitations and pallor; ● positive family history of pheochromocytoma |
Skin stigmata of neurofibromatosis (café-au-lait spots, neurofibromas) | Incidental discovery of adrenal (or in some cases, extra-adrenal) masses | Measurement of urinary fractionated metanephrines or plasma-free metanephrines | ● CT or MRI of the abdomen and pelvis; ● 123I-labelled meta-iodobenzyl-guanidine scanning; ● genetic screening for pathogenic mutations |
| Cushing syndrome | ● Rapid weight gain, ● polyuria (excessive production of urine), ● polydipsia (excessive thirst), ● psychological disturbances |
Typical body habitus (central obesity, moon-face, buffalo hump, red striae (stretch marks), hirsutism) | Hyperglycaemia | 24-h urinary cortisol excretion | Dexamethasone-suppression test |
CT, computed tomography; GFR, glomerular filtration rate; MRI, magnetic resonance imaging; RAA, renin–angiotensin–aldosterone
Modified from Ref77.
The medical history has to address the time of the first diagnosis of hypertension, current and past BP measurements and antihypertensive medications. A history of pregnancy-related hypertension is an important factor in the assessment of women with hypertension. Hypertension results in an increased risk of CVD complications, and chronic kidney disease (CKD). Thus, a careful medical history should be taken in all patients to allow for assessment of global CVD risk, with special emphasis on current and past smoking habits and evidence of dyslipidaemia and diabetes mellitus. CVD risk should be estimated using an established calculator (e.g. http://ASCVD-Risk-Estimator/). Adults at high risk for CVD have a high probability to benefit from antihypertensive drug therapy in addition to lifestyle change76.
The physical examination aims to establish the diagnosis of hypertension and screen for target organ damage and secondary causes (box 2). The patient should sit quietly for 5 minutes before a BP reading is taken and BP cuff should be at heart level. An average of 2 to 3 BP measurements obtained at 2 to 3 separate occasions provides an accurate basis for estimation of BP77,78. At least once, BP should be measured on both arms, and differences in SBP > 20 mmHg and/or in DBP >10mmHg should initiate investigations of vascular abnormalities77. Careful attention should be paid to choosing appropriately sized cuff, particularly for the increasing number of patients with obesity. Further, BP should be measured in both sitting and standing positions to rule out orthostatic hypotension (a sudden drop of the BP when a person stands up from a lying or sitting position). This is particularly important in elderly individuals.
All patients should undergo auscultation of the carotid arteries, heart and renal arteries. Detection of murmurs (sounds audible via the stethoscope) should lead to further investigations: carotid ultrasound, echocardiography and renal ultrasound, respectively. An irregular pulse frequently indicates atrial fibrillation, which should be confirmed by an electrocardiogram (EKG). Laboratory investigations are used to detect additional risk factors, to confirm or exclude secondary hypertension, to detect clinical or subclinical target organ damage and to estimate global CVD risk (box 3).
Screening
Despite overwhelming evidence that hypertension is a major treatable CVD risk factor, studies across the world show that a large proportion of individuals with hypertension are either unaware of their high BP or aware but not treated or inadequately treated79,15. Thus, there is a strong indication to screen middle-aged or younger persons in order to detect and treat more patients with hypertension. The most serious attempt by a healthcare system to improve the diagnostic aspects of hypertension has been done in the UK, based on pay-for-performance principle, that is, to give incentives to general practitioners (primary care physicians) for the appropriate diagnosis and treatment of chronic diseases, including hypertension. Early reports80,81 showed that this initiative was associated with an increased rate of BP monitoring and better BP control, but a later report suggested that this was not a sustained improvement82. It is possible that the initiative championed by the International Society of Hypertension and many national societies, which targeted entire populations by screening for hypertension in public places over the entire month of May 2017, might have better and more sustained results83.
Prevention
The association between BP and risk of CVD highlights the importance of treating hypertension, especially when severe. Further, it also underscores the importance of strategies to reduce BP-related CVD risk in those who have a higher than normal level of BP (average systolic BP 120–129 mmHg) but below the hypertension threshold. Reducing BP in adults with a high normal BP (referred to as elevated BP in the 2017 US guidelines) provides the potential to directly reduce CVD risk and to prevent or at least slow the age-related tendency for individuals to develop hypertension.
In most countries there is a strong tendency for BP, especially systolic BP, and the prevalence of hypertension to increase progressively from childhood until late in life79. However, studies in isolated societies that have limited contact with the outside world84,85 indicate that high BP is not an inevitable consequence of aging and that the rise in BP associated with local migration by members of isolated societies is related to changes in diet, decreased physical activity and consumption of alcohol84,86,87. These reports underscore the logic of efforts to prevent high BP in settings where an age-related increase in BP is common.
Lifestyle changes
A variety of nonpharmacological interventions have been shown to be effective in lowering BP and preventing hypertension. The most effective interventions are weight loss88,89,90, reduced Na+ intake88,89,90,91, increased potassium intake92,93, increased physical activity94, reduced consumption of alcohol95,96 and diets like the Dietary Approaches to Stop Hypertension (DASH) diet97 that combine several elements which favorably affect BP98,99 (table 3). The DASH diet is especially successful when combined with other effective BP lowering interventions such as a reduced intake of dietary sodium91. Lifestyle change is the best way for the individual to implement these interventions. Even small improvements in an individual’s lifestyle can be valuable. Government agency and professional society websites provide helpful tips for lifestyle change and monitoring of BP. Careful monitoring of BP is essential because the beneficial effects of lifestyle change are predicated on maintenance of the intervention100.
Table 3.
Dietary Approaches to Stop Hypertension (DASH) eating plan
| Food group | Servings* | Examples of a serving |
|---|---|---|
| Whole grains | 6–8 per day | 1 slice whole grain bread |
| Vegetables | 4–5 per day | 1 cup of raw leafy vegetables |
| Fruits | 4–5 per day | 1 medium sized fruit |
| Dairy products (low-fat or fat-free) | 2–3 per day | 1 cup of milk or yogurt |
| Fats and oils | 2–3 per day | 1 teaspoon of margarine or vegetable oil or 1 tablespoon of mayonnaise or 2 tablespoons of salad dressing |
| Lean meat, poultry, fish | 2–3 per day | 2 ounces of cooked meats, chicken or fish |
| Nuts, seeds and legumes | 4–5 per week | 1/3 cup (1.5 ounces) of nuts or 2 tablespoons of peanut butter or 2 tablespoons (0.5 ounce) of seeds or ½ cup of cooked peas or beans |
| Candy and added sugars | 5 or less per week | 1 tablespoon of sugar, jelly or jam or 1 cup of lemonade |
Recommended frequency of servings for a 2,000 calorie per day diet.
Two complementary strategies aimed at achieving a small population-wide reduction in BP or a larger reduction in those who are at higher risk to develop hypertension can be employed to implement hypertension prevention interventions98,99,101. Modeling studies suggest that a downward shift of as little as 2 mmHg in the population distribution of diastolic BP would result in a 17% reduction in the incidence of hypertension, a 14% reduction in the risk of stroke and transient ischemic attacks, and a 6% reduction in the risk of coronary heart disease102. Public health interventions focused on dietary improvements and increases in physical activity that are known to lower BP provide the basis for the population-wide strategy. Diet in the general population can be favorably influenced by means of public health education campaigns, food product labeling, and collaborations with food manufacturers to reduce the calorie and sodium content of their products, as well as with fast food companies and restaurants to reduce portion size and to promote healthier food preparation and promotion practices. Physical activity can be enhanced by making it easier for members of the community to engage in exercise on a routine basis.
Pharmacological interventions
Low-dose pharmacological therapy has also been shown to be effective in lowering BP and preventing hypertension in three randomized controlled trials conducted in adults with high normal BP103,104,105. The Brazilian multi-center PREVER-Prevention Trial compared treatment with the low-dose long-acting thiazide-like diuretic chlorthalidone in combination with the potassium sparing agent amiloride with treatment with placebo105. Treatment with the low-dose chlorthalidone and amiloride combination resulted in both a decrement in BP and prevention of hypertension and a reduction in left ventricular mass. A drug intervention is easier to implement and maintain than a lifestyle change intervention but there is a natural reluctance to recommend a lifetime of pharmaceutical therapy for prevention of hypertension. Consideration of low-dose pharmacotherapy should be restricted to those who are at high risk of developing hypertension despite energetic efforts to lower BP by means of one or more nonpharmacological interventions105.
MANAGEMENT
BP treatment thresholds and targets
Until 2015, most guidelines recommended a target BP < 140/90 mmHg for most patients and < 150/90 mmHg for elderly patients over 60 or 80 years of age (Table 4)77,106. However, after the publication of the Systolic blood PRessure Intervention Trial (SPRINT)107, target systolic BP values have been frequently debated. SPRINT was a randomized, open-label controlled trial that enrolled 9361 participants without diabetes mellitus but with increased CVD risk. Patients with a history of stroke were excluded. Participants were randomized to a standard systolic BP target < 140 mmHg or intensive systolic BP target < 120 mmHg. Intensive BP treatment in SPRINT resulted in a significantly greater (25%) reduction in the primary endpoint (first occurrence of myocardial infarction, acute coronary syndrome, stroke, heart failure or death from cardiovascular causes), compared with standard treatment. Office BP measurement in SPRINT was performed with an automated device timed to start measurement after 5 minutes of rest in an effort to standardize measurements in the various clinics and minimize the white coat effect. Because large differences had been observed between automated office BP measurement and conventional auscultatory measurements (with the automated technique showing lower values)108, some groups have questioned the applicability of the SPRINT intensive systolic BP target of < 120 mmHg to ordinary office practice109. Both the appropriate method(s) of measuring office BP (automated versus manual; unattended versus attended) and the appropriate BP targets for antihypertensive treatment are currently topics of vigorous debate. In summary, newer guidelines published after the SPRINT trial generally have more aggressive goals, at least for individuals < 65 years of age (Table 4).
Table 4.
Blood pressure targets recommended by various guidelines
| Guideline | Population | Goal BP (mmHg) |
|---|---|---|
| 2010 Chinese Guidelines208 | Adults < 65 years | < 140/90 |
| Adults 65 years and older | <150/90 (<140/90 if tolerated) | |
| Adults with diabetes, CHD or renal disease | <130/80 | |
| 2013 ESH/ESC77 | Non frail adults < 80 years | < 140/90 |
| Adults > 80 years | < 150/90 | |
| Adults with diabetes | < 140/85 | |
| Adults with CKD without proteinuria | < 140/90 | |
| Adults with CKD with overt proteinuria | < 130/90 | |
| Adults with CHD | < 140/90 | |
| 2013 ASH/ISH209 | Adults 55–80 years | < 140/90 |
| Young adults | < 130/80 | |
| Elderly > 80 years | < 150/90 | |
| 2014 Hypertension guideline106 (formerly known as JNC 8) | Adults < 60 years | < 140/90 |
| Adults ≥ 60 years | < 150/90 | |
| Adults with diabetes | < 140/90 | |
| Adults with CKD | < 140/90 | |
| 2014 South African Guidelines210 | Most adults | < 140/90 |
| Adults > 80 years | SBP 140–150 | |
| 2014 Japanese Guidelines211 | Most adults | < 140/90 |
| Late phase elderly patients | <150/90 (<140/90 if tolerated) | |
| Adults with diabetes or CKD | < 130/80 | |
| Adults with CHD or CVD | < 140/90 | |
| CHEP 2016212 | Adults < 80 years | < 140/90 |
| Adults ≥ 80 years | < 150 | |
| High-risk adults ≥ 50 years | ≤ 120* | |
| 2016 Australian guidelines213 | Adults at high CV risk without diabetes mellitus, including CKD patients and those >75 years | < 120 |
| Adults with diabetes in whom prevention of stroke is priority | < 120 | |
| ADA214 | Adults with diabetes | < 140/90 |
| Adults with diabetes and high risk for CVD | < 130/80 | |
| 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA110 | Adults with known CVD or 10-year ASCVD event risk ≥ 10% | < 130/80 |
| Adults without additional markers of increased CVD risk | < 130/80 | |
| Older adults ≥ 65 years of age, noninstitutionalized, ambulatory |
< 130/80 | |
| Older adults ≥ 65 years of age, with comorbidities and limited life expectancy | Individualized goal based on clinical judgement and patient preference |
The 2013 ASH/ISH guidelines were written to provide information for practitioners in low-income and middle-income countries as well as in developed countries.
should be guided by automated office BP measurement. BP, blood pressure; ESH, European Society of Hypertension; ESC, European Society of Cardiology; CKD; chronic kidney disease; CHD, coronary heart disease; CHEP, Canadian Hypertension Education Program; ADA, American Diabetes Association; CVD, cardiovascular disease. ACC, American College Cardiology; AHA, American Heart Association; AAPA, American Academy of Physician Assistants; ABC,; ACPM, American College of Preventive Medicine; AGE; AGS, American Geriatric Society; APhA, American Public Health Association; ASCVD, atherosclerotic cardiovascular disease; ASH, American Society of Hypertension; ASPC, American Society of Preventive Cardiology; NMA, National Medical Association; PCNA, Preventive Cardiovascular Nurses Association.
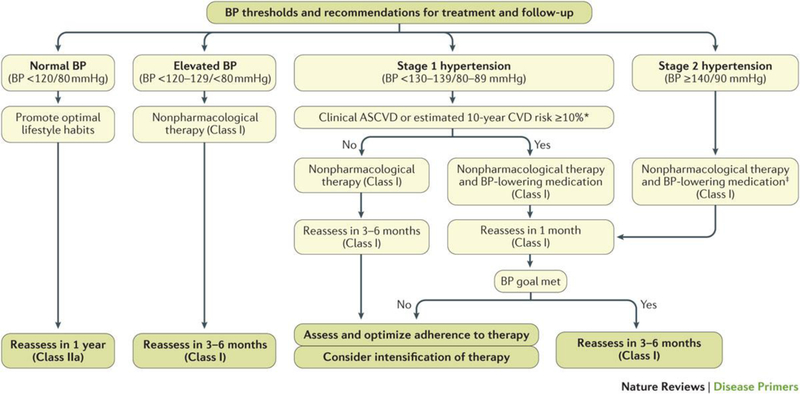
Patient’s global CVD risk and comorbidities should be considered in determining the need for pharmacologic antihypertensive treatment. The 2017 US ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults110 recommend the use of antihypertensive medication in patients with pre-existing CVD and those without a CVD event but an estimated 10-year atherosclerotic cardiovascular disease (ASCVD) risk of 10% or higher at BP levels ≥ 130/80 mmHg. In individuals without CVD and with 10-year ASCVD risk < 10%, antihypertensive medication should be initiated at BP ≥ 140/90 mmHg. (Figure 5).
Figure 5. Algorithm for the management of hypertension.
Reproduced from ref110.
Non-Pharmacological Management
Lifestyle advice is recommended for all patients with hypertension. The most effective interventions are the same as for prevention of hypertension. Targeted dietary approaches can reduce the systolic BP in individuals with hypertension. For example, reducing sodium intake (ideally to <2.3 g per day, or <1.5 g per day in those most susceptible to the effects of sodium on BP, but reduction by at least 1.0 g per day is desirable) can lower the systolic BP by 2–4 mmHg (Ref110,111,112). A similar reduction can be expected with increases in potassium intake to 3.5– 5.0 g per day92.
Reduced salt intake
For metabolic balance, the amount of salt consumed must be equal to that lost. Thus, under normal living conditions and physical activity levels, an intake of 5 g salt/day is considered sufficient, in line with the WHO recommendation (< 5 g per day)113. By contrast, the currently estimated dietary intake of salt is about 9–12 g per day in most countries. The current recommendations of the American Heart Association114 and American Society of Hypertension115 are stricter than the European guidelines, recommending lowering salt intake to 3.8 g per day, whereas the 2013 ESH/ESC guidelines recommend 5–6 g of salt per day77.
Randomized controlled trials carried out in persons with hypertension have consistently shown that reduced sodium intake is associated with reduction of BP116. The most convincing evidence is provided by the Dietary Approaches to Stop Hypertension (DASH-sodium) trial91, in which the effects of three different sodium intakes were tested separately in combination with two diets: the DASH diet, rich in fruit, vegetables, low-fat dairy products and reduced in saturated fat and cholesterol, and a control diet consisting of what many people in the United States typically eat. Reduction of sodium intake by ~0.9 g per day induced a greater BP reduction when the starting sodium intake was <2.3 g per day, which corresponds to about 6 g of salt per day; thus it is slightly more than the currently recommended < 1,500 mg/day of sodium by the 2017 US hypertension guidelines. Of note, sodium reduction reduced BP in non-hypertensive individuals on both diets. Reduced sodium intake can also prevent hypertension (relative risk reduction of about 20% with or without concomitant weight loss)90, improve hypertension control117 and thus, possibly, reduce need for antihypertensive medication100. In the Intersalt study118, lower sodium intake was associated with a blunted age-related rise in systolic BP.
There is strong evidence to support population-wide recommendations to lower salt intake119,120. As more than 75% of dietary salt comes from processed foods (in western countries), any population strategy to reduce salt intake must involve food manufacturers and restaurants, in order to progressively reduce salt added to foods. So far, only three countries (Japan, Finland and the United Kingdom) have successfully reduced population salt intake121.
Increased potassium intake
Healthy individuals with normal kidney function usually have a potassium intake of 4.7 g/day; a higher intake is not associated with increased risk because potassium is readily excreted in persons who do not have CKD. Increased potassium intake is associated with reduced BP in individuals with low as well as high baseline potassium intake91,92. Of note, potassium reduces BP to a greater extent in blacks than in whites122. The effect of potassium on BP is dependent on salt intake. There is a greater BP reduction with increased potassium intake in the context of lower salt intake123. Thus, the best strategy is to increase potassium intake and reduce sodium intake at the same time. The preferred strategy to increase potassium intake is to increase consumption of fruits and vegetables that are rich in potassium rather than using supplements115. In individuals with impaired urinary potassium excretion, a potassium intake <4.7 g per day is recommended124.
Moderate alcohol consumption
Keeping alcohol intake ≤2 standard drinks (~3.5 alcohol units) per day for men and ≤1 standard drink (~1.75 alcohol units) per day for women can also contribute to a 2–4 mmHg BP reduction. 95,96
Physical activity
Regular physical activity reduces BP in individuals with hypertension. Endurance training reduces BP more in persons with hypertension than in individuals with normal BP. A narrative review of 27 randomized clinical trials in individuals with hypertension showed that regular medium-intensity to high-intensity aerobic activity reduced BP by a mean of 11/5 mmHg125. Sessions lasting 40–60 minutes performed at least three times a week had the greatest effect on BP. Three randomized controlled trials of isometric exercise (strength training) showed a BP reduction of similar magnitude to that induced by aerobic exercise in individuals with hypertension125. A meta-analysis of 64 controlled studies of the efficacy of dynamic resistance training as stand-alone antihypertensive therapy showed BP reductions comparable with or greater than those with aerobic exercise training126. Greater BP reductions occurred in individuals with higher resting BP (approx. 6/5 mmHg for individuals with hypertension and 3/3 mmHg for individuals with pre-hypertension) and in non-white individuals126.
Weight Loss
Excess adiposity generally raises BP in susceptible individuals, and patients with hypertension who also have obesity require more antihypertensive medications to control their BP and are more likely to be treatment resistant127. In a recent meta-analysis, any reduction in body weight lowered systolic BP by on average 2.69 mmHg and in diastolic BP by on average 1.34 mmHg (Ref128). However, the response varies substantially between individuals. Lifestyle interventions, including hypocaloric diets and physical exercise, are commonly recommended for patients with obesity and hypertension, yet average weight loss is modest and most patients regain weight129 (box 4).
Box 4. Hypertension and obesity.
Weight loss is recommended for individuals with obesity, and may be particularly important if these patients also have hypertension. Medications have been developed for the treatment of obesity, but their approval status differs between the United States and Europe: some drugs are only approved in the United States (for example, lorcaserin and topiramate/phentermine), whereas others are approved in Europe only. BP reductions in patients with hypertension have been reported for some weight loss medications201, but their specific pharmacological actions may attenuate the positive influences of weight loss on BP and CVD outcomes133. Bariatric surgery is very effective in reducing body weight, and the risk for arterial hypertension is substantially reduced up to five years following bariatric surgery202. However, large and sustained body weight reductions are needed to substantially reduce BP following bariatric surgery203 and there are no large clinical trials specifically testing the effects of weight loss medications or bariatric surgery on hypertension control.
Antihypertensive Pharmacotherapy
Antihypertensive pharmacotherapy has evolved over several decades driven by development of various antihypertensive medication classes and large-scale outcomes trials proving their benefits on CVD morbidity and mortality130. Clinicians are now faced with a plethora of antihypertensive medications of different drug classes and a variety of fixed dose combinations. Typically, antihypertensive pharmacotherapy begins with first-line antihypertensive medications either in monotherapy or in combination131. Combination therapy may be preferable in patients with higher levels of pretreatment BP. First-line antihypertensive medications include ACE inhibitors, angiotensin II receptor blockers (also known as sartans), dihydropyridine calcium channel blockers, and thiazide diuretics106. Beta-blockers are also indicated in patients with heart failure and reduced left ventricular ejection fraction or post myocardial infarction, and some guidelines recommend beta-blockers as first line antihypertensive medications 77,132. The choice should be based on individual efficacy and tolerability. Ethnicity affects the response to antihypertensive medications, and it has been suggested that calcium channel blockers and diuretics may be the first choice in blacks106,133,134. Further, in specific clinical situations, for example hypertension in pregnant women, other medications such as alpha-methyldopa (an agonist of alpha adrenoreceptors in the central nervous system that inhibits the sympathetic nervous system) or labetalol (a beta adrenoreceptor blocker) are preferable, whereas some first line antihypertensives, for example ACE inhibitors and angiotensin II receptor blockers, are contraindicated because of increased risk for renal teratogenicity. Divided dosing of antihypertensive drugs tends to decrease adherence and should be avoided when possible135.
BP cannot be controlled with monotherapy in many patients, particularly those with severe hypertension. When combining antihypertensive medications, it is important to consider whether the drugs have additive effects on BP or adverse effects, and whether the patient has comorbidities that mandate particular drug choices77. ACE inhibitors or angiotensin II receptor blockers, thiazide diuretics and dihydropyridine calcium channel blockers are additive in lowering BP and can be combined as double or triple combination therapies. By contrast, combining ACE inhibitors and angiotensin II receptor blockers adds little BP lowering while increasing the risk for renal dysfunction and hyperkalemia (high blood potassium levels, which can lead to cardiac arrhythmias). Similarly, combining RAAS inhibitors with beta-adrenoreceptor blockers adds little BP reduction, but this combination is indicated in patients following acute myocardial infarction or heart failure with reduced left ventricular ejection fraction for reasons beyond BP reduction.
Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
Among medications that inhibit components of the RAAS, ACE inhibitors and angiotensin II receptor blockers are considered first line antihypertensives, whereas other antihypertensive medications targeting RAAS, including direct renin inhibitors and mineralocorticoid receptor antagonists, are usually considered reserve medications because there is less clinical trial evidence supporting their use as first line antihypertensive therapy. ACE inhibitors and angiotensin II receptor blockers have been tested extensively in large-scale hypertension trials136. In patients with heart failure with reduced left ventricular ejection fraction or with diabetic nephropathy, both drug classes improved outcomes, making them particularly good choices in these populations. Both classes appear to be comparable in reducing CVD risk137. and also tend to improve glucose metabolism and, therefore, could be preferable in younger patients and in patients with conditions predisposing to type 2 diabetes mellitus, including obesity and the metabolic syndrome138. ACE inhibitors are generally well tolerated, but reductions in kidney function, hyperkalemia, cough, and – less commonly – angioedema (swelling caused by fluid accumulation) could occur with their use. The risk for angioedema, which can be life threatening, is substantially increased in blacks139 and modestly increased in patients treated with dipeptidyl peptidase-IV inhibitors (used in the treatment of diabetes, examples of which include sitagliptin, vildagliptin, saxagliptin, and linagliptin)140. ACE inhibitors that can be dosed once daily are preferred. Angiotensin II receptor blockers can also elicit hyperkalemia and worsening of kidney function, but are not likely to cause cough or angioedema137.
Dihydropyridine calcium channel blockers.
Dihydropyridine calcium channel blockers elicit vasodilation by blocking vascular smooth muscle L-type calcium channels. They are effective antihypertensive drugs with extensive experience in large clinical trials136. A practical advantage of this drug class is that it can be combined with all other first-line antihypertensives. Peripheral edema, which is explained by peripheral arterial vasodilation rather than worsening heart failure or kidney dysfunction, is a common side effect, particularly in individuals with obesity. Non-dihydropyridine calcium channel blockers, especially verapamil, also inhibit cardiac calcium channels, which can reduce heart rate and cardiac contractility141. Calcium channel blockers can induce or worsen constipation, especially in institutionalized older persons142. All calcium channel blockers modestly inhibit the drug metabolizing enzyme cytochrome P450 3A4, and, therefore, could elicit important drug-interactions143.
Thiazide-type and thiazide-like diuretics.
Thiazide-type diuretics (for example, hydrochlorothiazide) have a benzothiadiazine ring, whereas thiazide-like diuretics (for example, chlorthalidone, metolazone and indapamide) lack the benzothiadiazine structure. Both subclasses of thiazide diuretics inhibit Na+ and CI- co-transporters in renal tubules, thereby promoting natriuresis, and have been an important component of pharmacological hypertension management ever since the first trials showing morbidity benefits of antihypertensive therapy144. Over the years, diuretic doses have been substantially reduced to attain better risk-benefit profiles. Thiazide-type and thiazide-like diuretics can worsen glucose metabolism increasing the risk for new onset diabetes mellitus, but whether or not this metabolic action translates into long-term increases in CVD risk has been called into question145. Hydrochlorothiazide, the most commonly prescribed thiazide-type diuretic worldwide, may be less effective in mitigating CVD risk compared to chlorthalidone or indapamide146,147. Drug-related electrolyte disturbances, including hypokalemia and hyponatremia (low blood potassium and sodium levels, respectively), are particularly important adverse effects; hypokalemia can lead to cardiac arrhythmias and muscle weakness, and hyponatremia can cause confusion, seizures and coma. The risk for hypokalemia is reduced when thiazide-type and thiazide-like diuretics are combined with potassium supplements or potassium-sparing agents, such as ACE inhibitors, angiotensin receptor blockers, or potassium-sparing diuretics. Hyponatremia is a potentially life threatening adverse effect, particularly in elderly persons.
Beta-adrenoreceptor blockers.
Beta-adrenoreceptor blockers lower BP reducing cardiac output, heart rate, renin release and adrenergic control nervous system effects148. They improve outcomes following acute myocardial infarction and in patients with heart failure with reduced left ventricular ejection fraction, but, in the absence of these comorbidities, beta-adrenoreceptor blockers are inferior to other first line antihypertensives in reducing CVD morbidity and mortality149. This effect has been attributed to lesser reductions in aortic BP150 and adverse effects on body weight151 and glucose metabolism with beta-adrenoreceptor blockade. Some of these disadvantages might be mitigated with newer vasodilator beta-adrenoreceptor blockers, such as nebivolol and carvedilol152. However, there are no large-scale antihypertensive trials demonstrating that this difference translates into better clinical outcomes. Beta-adrenoreceptor blockers may promote bronchial obstruction is patients with asthma and should not be combined with non-dihydropyridine calcium channel blockers such as verapamil that lower sinus node rate or atrioventricular conduction.
Newer pharmacological agents
Overall, the interest of the pharmaceutical industry in developing new antihypertensive medications has been limited in recent years. Moreover, most antihypertensive drugs are out of patent and, therefore, available as relatively inexpensive generics. Further, some of the currently approved drugs, such as angiotensin II receptor blockers, have placebo-like tolerability. Newer pharmacological agents approved for other indications, including combined angiotensin II receptor and neprilysin inhibitors153 (for heart failure), soluble guanylyl cyclase modulating drugs154 (for erectile dysfunction), and sodium-glucose cotransporter 2 (SGLT2) inhibitors155 (for type 2 diabetes mellitus) may also be useful in treating hypertension. Other pharmacological agents, such as newer mineralocorticoid receptor antagonists, aldosterone synthase inhibitors, activators of the angiotensin-converting enzyme 2/ angiotensin (1–7)/ MAS receptor axis, and natriuretic peptide receptor agonists, are in various stages of preclinical or clinical development156, often for indications other than hypertension. Drugs addressing novel pressor mechanisms could be useful in patients with treatment resistant hypertension, particularly those not responding to or not tolerating mineralocorticoid receptor antagonists. Moreover, drugs with actions in addition to BP reduction could prove clinically useful. For example, combined angiotensin II receptor blockade and neprilysin inhibition has been shown to ameliorate insulin resistance in patients with obesity and hypertension157 and decrease the progression to type 2 diabetes mellitus in patients with heart failure158.
Treatment Resistant Hypertension
Treatment resistant hypertension is commonly diagnosed when office BP is >140/90 mmHg despite treatment with three or more properly dosed antihypertensive drugs including a diuretic and secondary hypertension has been ruled out159. Poor treatment adherence is a common cause of apparent treatment resistant hypertension. The true prevalence of treatment resistant hypertension is unknown, but an estimated 12.8% of all individuals with hypertension in the United States and 15.3 % of those treated with antihypertensives fulfill the criteria for treatment resistant hypertension160. Adding a fourth or fifth drug could lead to satisfactory BP control in these patients. The PATHWAY trial rotated patients with treatment resistant hypertension through different add on drugs or placebo in a randomized fashion161. All patients received a standardized antihypertensive regimen comprising three drugs, including a diuretic. Compared with alpha-adrenoreceptor or beta-adrenoreceptor blockade, the mineralocorticoid receptor antagonist spironolactone was the most effective fourth antihypertensive drug. In another study in patients whose BP was uncontrolled despite receiving three drugs, sequential addition of a mineralocorticoid receptor antagonist followed by a loop diuretic (which acts at the ascending limb of the loop of Henle in the kidney) was more effective than adding an ACE inhibitor followed by a beta-adrenoreceptor blocker162. Overall, mineralocorticoid receptor antagonism is a reasonable choice in patients with difficult to control hypertension. Given the risk of inducing hyperkalemia163, serum potassium concentrations should be monitored.
Device-based Treatments
Device-based treatments have been primarily developed for patients with severe resistant hypertension whose BP cannot be controlled with antihypertensive drugs156. Catheter-based renal nerve ablation164,165, electrical carotid sinus stimulation166,167, modulation of baroreflex transduction with a dedicated carotid stent168, carotid body denervation169, and deep brain stimulation170 are thought to lower BP through SNS inhibition. Creation of a defined arteriovenous stent with a coupler device lowers BP by reducing peripheral vascular resistance171. These treatments are in various stages of clinical development, with the most extensive data available on renal nerve ablation and electrical carotid sinus stimulation. None has yet been proven efficacious in lowering BP in randomized sham-controlled clinical trials164,161,167, because either the primary endpoint was not achieved or no trials have been conducted. Finally, trials with hard clinical endpoints do not exist.
QUALITY OF LIFE
Health-related quality of life (HRQoL) is a multi-dimensional concept that includes domains related to physical, mental, emotional, and social functioning; studies demonstrate that each additional disease, as well as the severity of these diseases, is associated with declines in HRQOL 172. Population-based studies have consistently shown that being diagnosed with hypertension is associated with worsening of HRQoL even after adjusting for other comorbidities173,174. Altered HRQoL in persons with hypertension has been attributed to a variety of factors, including the diagnosis, treatment, and effects of alterations (both elevations and reductions) in BP173.
Labeling someone as having hypertension can result in worsening of self-perceived health status175. This was well-demonstrated in a classic study of otherwise healthy Canadian steelworkers identified as having hypertension as part of a screening program. In the year following diagnosis, absenteeism from work owing to illness more than tripled in those made newly aware of their hypertension, whereas it increased only slightly in those previously aware of their hypertension176. This finding could not be explained by hypertension treatment or BP level and was believed to be a direct consequence of people adopting a “sick role.” These findings have been replicated in studies carried out in diverse settings and using different measures of physical and mental health175.
Antihypertensive medication use is associated with a variety of symptoms that could lower HRQoL177. Observational studies showed an association between the number of antihypertensive medications prescribed and worsening of the HRQoL178. Some classes of antihypertensive medications, (for example, ACE inhibitors) are better tolerated than others (for example, beta blockers and centrally acting agents, such as alpha-methyldopa) and result in significantly better scores on measures of general well-being179. Further, small differences in HRQoL have even been reported among medications of the same class, e.g., enalapril vs. captopril180. However, clinical trials with newer antihypertensive agents have generally indicated that they are extremely well-tolerated and can enhance the effects of non-pharmacological treatment on HRQoL177,181. In the Treatment of Mild Hypertension Study (TOMHS), combining lifestyle modifications with any of five different antihypertensive medication classes resulted in greater improvements in HRQoL than lifestyle modifications plus placebo181.
Treatment-related reductions in BP could have a negative effect on HRQoL, particularly in older and more frail patients at high risk of hypotension. Clinical trials performed in the 1990s that evaluated patients with very high baseline BP, for example, the Systolic Hypertension in the Elderly Program Trial (SHEP) and the Systolic Hypertension in Europe Trial (Syst-Eur), generally found minimal effects of BP reductions on HRQoL182,183. Two more recent clinical trials have targeted lower BPs (intensive systolic BP target < 120 mmHg versus standard systolic BP target < 140 mmHg ), it had been postulated that this lower BP might be expected to cause cerebral hypoperfusion, resulting in falls, dizziness, and cognitive impairment184,185,107. In a substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, HRQoL was evaluated in 1,028 participants randomized to either intensive or standard therapy. No differences in mental function were noted between treatment groups, but intensive therapy was associated with a small, not clinically significant, decrease in physical function185. In the Systolic Blood Pressure Intervention Trial (SPRINT), targeting systolic BP <120 mmHg required 1 additional antihypertensive medication compared to standard treatment to target systolic BP < 140 mmHg and was generally safe and well tolerated107,186. Compared to standard treatment, intensive treatment did not affect the perceived HRQOL of SPRINT participants, measured by patient reported outcomes of physical and mental health, self-reported satisfaction with care and medication adherence, even when stratifying on age and comorbidities186. Almost 90% of participants in both treatment groups reported satisfaction with their BP care, and more than 1/3 described improvement in satisfaction over baseline levels.
Quality of life concerns remain an important aspect of hypertension management. SPRINT has demonstrated that with careful clinical management, lower BP can be targeted without concern of worsening physical and mental function. Clinicians must seek the optimal balance of reducing CVD morbidity and mortality while maximizing well-being for each individual patient.
OUTLOOK
Although there is regional variability in the outlook for hypertension over the next 5 to 10 years, It is clear overall that the prevalence of hypertension and, therefore, the associated global burden attributable to hypertension, will increase187. Global population growth and aging will largely contribute to this increase – 1.5 billion people are expected to be affected by 2025 (Ref. 188) – which will be focused in low and middle income countries187. However, these adverse trends in disease burden will be variably offset by improvements in prevention, awareness and treatment. The size of improvements in each of these 3 areas will vary from non-existent (-hypertension prevention could even worsen in some parts of the world, as exposure to factors that promote raised BP increases) to substantially large and important elsewhere in the world.
Overall, prevention will probably contribute least to any improvement in BP-associated disease burden. This is because 80% of the world is in the process of developing, which hitherto has inevitably been associated with increased exposure to the main environmental determinants of raised BP such as excess intake of calories, alcohol and salt. Food and drink industries, governments and education systems would be required to cooperate in order to reverse this pattern
Implementation of preventive strategies has largely been limited to high income countries. Despite reasonably compelling evidence to the contrary111, recommendations that the general population should restrict salt intake have been questioned on the basis of largely suboptimal observational data189. Such confusion worsens an already very difficult public health challenge. Data show that only approximately half of people with hypertension are aware of their condition190, and the Lancet Commission on hypertension identified improving awareness of hypertension is a critical action needed to improve the current disease burden191,192. The global BP awareness campaign promoted by the International Society of Hypertension whereby World Hypertension Day was extended to become May Measurement Month (MMM) in 2017 could contribute substantially to improving rates of routine BP screening around the world83. Over 1.20 million adults (≥18 years) from >100 countries were screened as part of MMM and the ensuing data allied to health-economic analyses will be used to persuade policy makers in each country that enhanced local BP screening and treatment facilities are wise financial investments.
Improving the efficacy of drug treatment also holds great promise for reducing hypertension-associated disease burden. Rather than focusing on rare secondary causes of hypertension or the optimal management of treatment resistant hypertension193, the greatest effect could be achieved by the delivery and distribution of affordable, effective single-pill combinations of 2 or 3 drugs to low-income and middle income countries where the burden of hypertension is considerable and where any such therapies are currently either largely unavailable or unaffordable.194. Unfortunately, optimal combinations of 2 antihypertensive agents have not been identified for the majority of the world’s hypertensive population: no such data are available for black, south Asian or east Asian patients195. However, the first in a series of trials in these ethnic groups is underway in Sub-Saharan Africa.
Meanwhile, single-pill formulations of the drug combinations most commonly recommended in current guidelines (calcium channel blocker plus a diuretic, calcium channel blocker plus a RAAS-blocker or diuretic plus a RAAS-blocker) are readily available and have low production costs. In addition, a 3-drug combination of a calcium channel blocker, a diuretic and a RAAS-blocker132 should also be produced for more severe hypertension, with low dose spironolactone available as a fourth-line agent161. Hence, 1 or 2 tablets will be able to control BPs of all but a small proportion of patients with hypertension.
These formulations should be made available and affordable in all countries of the world.191 Additional local obstacles to the distribution and delivery of these agents to patients with hypertension within each country will also have to be overcome – among which the lack of effective screening programmes is crucial191.
Antihypertensive medications are prescribed by different health professionals in different countries. However, even in high income countries, much of the “routine” uncomplicated hypertension management could, and probably should be carried out by nurse practitioners or other non-physician health workers. In more remote parts of the world, the use of e-healthcare techniques196 should be increasingly used to facilitate task-shifting or task sharing by non-physician health-workers where doctors are unavailable197.
In summary, although there are many interesting unanswered scientific research questions in the field of hypertension (Box 5), perhaps the most pressing need to reduce the diseas burden is to evaluate the best way(s), at a local level, to screen routinely for raised BP and then to deliver the best, most affordable, evidence-based combination of antihypertensive agents. Meanwhile, efforts to drive public health policy towards encouraging more healthy diets and lifestyles from a BP and CVD viewpoint should be encouraged and basic scientific research that might allow precision medicine to be applied to patients with hypertension must also continue.
Box 5. Outstanding Research Questions:
Measurement Issues
Is hypertension management improved by basing treatment strategies on serial unattended office BP measurements, out of office (home or ambulatory) BP measurements or central BP measurements?
How should BP be measured in patients with atrial fibrillation?
Treatment Issues
Should salt restriction at the population level continue to be recommended at current targets?
To what extent should age, estimated CVD risk and concomitant conditions influence treatment thresholds?
Should white-coat hypertension be treated?
If management strategy is to be influenced by central or out of office BP levels, what treatment thresholds and targets should be used?
Should reducing 24-hour and longer-term BP variability be a consideration in the selection of drug treatment for optimal CVD protection?
What combinations of antihypertensive agents give optimal CVD protection, stratified by age and ethnicity?
What is the optimal BP treatment target stratified by age, CVD risk and concomitant disease status?
What is the optimal management of treatment resistant hypertension that is resistant to 4 agents including spironolactone?
If treatment thresholds are to be driven by estimated CVD risk, at what level should antihypertensive drug treatment be initiated and what other CVD protective agents should be considered?
Is initiating drug therapy with 2 hypertensive agents more effective than initiating with monotherapy for optimal CVD prevention?
Acknowledgments
P.K.W. was supported by P20GM109036 (Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases) from the National Institute of General Medical Sciences.
Footnotes
Competing interests
G.B. served as Consultant for Janssen, Bayer, AbbVie, Vascular Dynamics, Relypsa and Merck; served/serves as Principal Investigator for FIDELIO trial (Bayer), Steering Committee member (CREDENCE)-Janssen, SONAR-AbbVie, and CALM-2-Vascular Dynamics. J.J. served as consultant for Novartis, Novo-Nordisc, Boehringer-Ingelheim, Sanofi, Orexigen, Riemser, Theravance, Vivus; and is cofounder of Eternygen GmbH. S.O. (in the previous 24 months) has received research grant support or reimbursement for travel to meetings or other, non-financial support from Actelion Clinical Research/George Clinical; AstraZeneca AB; Bayer; Lundbeck; Novartis; Novo Nordisk; Rox Medical; has consulted for Actelion/George Clinical, Lundbeck, Novo Nordisk and ROX Medical; served as Director/Principal Investigator, SPRINT University of Alabama at Birmingham (UAB) Clinical Center Network (CCN); and sub-investigator UAB CCN clinical site; for which Takeda and Arbor Pharmaceuticals donated 5% of medication used. N.R.P. served as advisory board member (ad hoc) for Pfizer, Takeda, MSD, Servier, and Medtronic (companies producing blood pressure lowering agents/devices); received speaker honoraria from Servier, AstraZeneca, Napi Labs, and Menarini; received research funding from Servier, Pfizer and Menarini; and is the President of the International Society of Hypertension. George Health Enterprises, the social enterprise arm of The George Institute for Global Health, has applied for a patent in the area of low-dose combinations on which A.R. is listed as an inventor; and has received investment to develop fixed-dose combinations containing aspirin, statin and BP lowering drugs. AR is an investigator on grants for several trials of blood pressure lowering interventions. G.G. has received lectures fees from Merck and Astra Zeneca. M.C.A., R.C., A.F.D., D.R.B. and P.K.W. declare no competing interests.
How to cite this Primer
Publisher’s note
References
- 1.Luft FC Twins in Cardiovascular Genetic Research. Hypertension 37, 350–356 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Fagard R et al. Heritability of Conventional and Ambulatory Blood Pressures : A Study in Twins. Hypertension 26, 919–924 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Surendran P et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet 48, 1151–1161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehret GB et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet 48, 1171–1184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu C et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet 48, 1162–1170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominiczak A, Delles C & Padmanabhan S Genomics and Precision Medicine for Clinicians and Scientists in Hypertension. Hypertension 69, e10–e13 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Lifton RP, Gharavi AG & Geller DS Molecular Mechanisms of Human Hypertension. Cell 104, 545–556 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Ehret GB & Caulfield MJ Genes for blood pressure: an opportunity to understand hypertension. Eur. Heart J 34, 951–961 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maass PG et al. PDE3A mutations cause autosomal dominant hypertension with brachydactyly. Nat. Genet 47, 647–653 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Forouzanfar MH et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1659–1724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blood Pressure Lowering Treatment Trialists’ Collaboration. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet 384, 591–598 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Page LB, Damon A & Moellering RC Antecedents of cardiovascular disease in six Solomon Islands societies. Circulation 49, 1132–46 (1974). [DOI] [PubMed] [Google Scholar]
- 13.Poulter NR, Prabhakaran D & Caulfield M Hypertension. Lancet 386, 801–812 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Rose G & Day S The population mean predicts the number of deviant individuals. BMJ 301, 1031–4 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills KT et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-based Studies from 90 Countries. Circulation 134, 441–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forouzanfar MH et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 317, 165 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Lewington S et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England) 360, 1903–13 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Rapsomaniki E et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 383, 1899–1911 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamler J, Stamler R & Neaton JD Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch. Intern. Med 153, 598–615 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Klag MJ et al. Blood pressure and end-stage renal disease in men. N. Engl. J. Med 334, 13–8 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Goff DC et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. J. Am. Coll. Cardiol 63, 2935–2959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall ME & Hall JE Pathogenesis of Hypertension. Hypertension: A Companion to Braunwald’s Heart Disease 33–51 (2018). doi: 10.1016/b978-0-323-42973-3.00005-6 [DOI] [Google Scholar]
- 23.Gangwisch JE A review of evidence for the link between sleep duration and hypertension. Am. J. Hypertens 27, 1235–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palagini L et al. Sleep loss and hypertension: a systematic review. Curr. Pharm. Des 19, 2409–19 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Mikael L. de R. et al. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol 109, 253–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sindler AL et al. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10, 429–37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steppan J, Barodka V, Berkowitz DE & Nyhan D Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract 2011, 263585 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PAGE IH PATHOGENESIS OF ARTERIAL HYPERTENSION. JAMA J. Am. Med. Assoc 140, 451 (1949). [DOI] [PubMed] [Google Scholar]
- 29.Harrison DG The Mosaic Theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J. Am. Soc. Hypertens 7, 68–74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W, Dell’Italia LJ & Sanders PW Novel Paradigms of Salt and Hypertension. J. Am. Soc. Nephrol 28, 1362–1369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilck N et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A & Williams GH Textbook of nephro-endocrinology
- 33.Varagic J, Ahmad S, Nagata S & Ferrario CM ACE2: angiotensin II/angiotensin-(1–7) balance in cardiac and renal injury. Curr. Hypertens. Rep 16, 420 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrario CM ACE2: more of Ang-(1–7) or less Ang II? Curr. Opin. Nephrol. Hypertens 20, 1–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmerman D & Burns KD Angiotensin-(1–7) in kidney disease: a review of the controversies. Clin. Sci 123, 333–346 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Zhou ZH & Bubien JK Nongenomic regulation of ENaC by aldosterone. Am. J. Physiol. Cell Physiol 281, C1118–30 (2001). [DOI] [PubMed] [Google Scholar]
- 37.McCurley A & Jaffe IZ Mineralocorticoid receptors in vascular function and disease. Mol. Cell. Endocrinol 350, 256–265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerkelä R, Ulvila J & Magga J Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events. J. Am. Heart Assoc 4, e002423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodard GE & Rosado JA Chapter 3 Natriuretic Peptides in Vascular Physiology and Pathology. in International review of cell and molecular biology 268, 59–93 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Curry F-RE Atrial natriuretic peptide: an essential physiological regulator of transvascular fluid, protein transport, and plasma volume. J. Clin. Invest 115, 1458–1461 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armaly Z, Assady S & Abassi Z Corin: a new player in the regulation of salt-water balance and blood pressure. Curr. Opin. Nephrol. Hypertens 22, 713–722 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Schlueter N et al. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol. Ther 144, 12–27 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Khaddaj Mallat R, Mathew John C, Kendrick DJ & Braun AP The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci 54, 458–470 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Sandoo A, van Zanten JJCSV, Metsios GS, Carroll D & Kitas GD The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J 4, 302–12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spieker LE, Flammer AJ & Lüscher TF The Vascular Endothelium in Hypertension. The Vascular Endothelium II 249–283 doi: 10.1007/3-540-36028-x_8 [DOI] [PubMed] [Google Scholar]
- 46.Ayub T, Khan SN, Ayub SG, Dar R & Andrabi KI Reduced nitrate level in individuals with hypertension and diabetes. J. Cardiovasc. Dis. Res 2, 172–176 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panza JA, Casino PR, Badar DM & Quyyumi AA Effect of increased availability of endothelium-derived nitric oxide precursor on endothelium-dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation 87, 1475–1481 (1993). [DOI] [PubMed] [Google Scholar]
- 48.Kohan DE & Barton M Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86, 896–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serrano-Ponz M et al. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Mol. Med. Rep 13, 3724–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vendégh Z et al. Calcitonin gene-related peptide, substance P, nitric oxide and epinephrine modulate bone marrow micro circulation of the rabbit tibia and femur. Clin. Hemorheol. Microcirc 45, 9–17 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Yu M et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J. Hypertens 21, 1125–1135 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Popolo A, Autore G, Pinto A & Marzocco S Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res 47, 346–356 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Lazich I & Bakris GL Endothelin Antagonism in Patients with Resistant Hypertension and Hypertension Nephropathy. Contributions to Nephrology 223–234 (2011). doi: 10.1159/000328988 [DOI] [PubMed] [Google Scholar]
- 54.Dharmashankar K & Widlansky ME Vascular Endothelial Function and Hypertension: Insights and Directions. Curr. Hypertens. Rep 12, 448–455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.HEYMANS C & DELAUNOIS AL Fundamental role of the tone and resistance to stretch of the carotid sinus arteries in the reflex regulation of blood pressure. Science 114, 546–7 (1951). [DOI] [PubMed] [Google Scholar]
- 56.Pijacka W et al. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J. Physiol 594, 6255–6266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Leeuw PW et al. Sustained Reduction of Blood Pressure With Baroreceptor Activation TherapyNovelty and Significance. Hypertension 69, 836–843 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Grassi G et al. Excessive Sympathetic Activation in Heart Failure With Obesity and Metabolic Syndrome: Characteristics and Mechanisms. Hypertension 49, 535–541 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Mancia G & Grassi G The Autonomic Nervous System and Hypertension. Circ. Res 114, 1804–1814 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Augustyniak RA et al. Sympathetic nerves and the progression of chronic kidney disease during 5/6 nephrectomy: Studies in sympathectomized rats. Clin. Exp. Pharmacol. Physiol 37, 12–18 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Augustyniak RA, Tuncel M, Zhang W, Toto RD & Victor RG Sympathetic overactivity as a cause of hypertension in chronic renal failure. J. Hypertens 20, 3–9 (2002). [DOI] [PubMed] [Google Scholar]
- 62.DiBona GF Sympathetic Nervous System and Hypertension. Hypertension 61, 556–560 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Grassi G, Mark A & Esler M The Sympathetic Nervous System Alterations in Human Hypertension. Circ. Res 116, 976–990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A & Mancia G Baroreflex Control of Sympathetic Nerve Activity in Essential and Secondary Hypertension. Hypertension 31, 68–72 (1998). [DOI] [PubMed] [Google Scholar]
- 65.Smith PA; Graham LN; Mackintosh AF; Stoker JB; Mary D Relationship between central sympathetic activity and stages of human hypertension. Am. J. Hypertens 17, 217–222 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Fujita T Mechanism of Salt-Sensitive Hypertension: Focus on Adrenal and Sympathetic Nervous Systems. J. Am. Soc. Nephrol 25, 1148–1155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mu S et al. Epigenetic modulation of the renal β-adrenergic–WNK4 pathway in salt-sensitive hypertension. Nat. Med 17, 573–580 (2011). [DOI] [PubMed] [Google Scholar]
- 68.Harrison DG & Bernstein KE Inflammation and Immunity in Hypertension. Hypertension: A Companion to Braunwald’s Heart Disease 60–69 (2018). doi: 10.1016/b978-0-323-42973-3.00007-x [DOI] [Google Scholar]
- 69.Devallière J & Charreau B The adaptor Lnk (SH2B3): An emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem. Pharmacol 82, 1391–1402 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Iturbe B Autoimmunity in the Pathogenesis of Hypertension. Hypertens. (Dallas, Tex. 1979) 67, 477–83 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Mattson DL et al. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. AJP Regul. Integr. Comp. Physiol 304, R407–R414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roush GC et al. Prognostic impact from clinic, daytime, and night-time systolic blood pressure in nine cohorts of 13 844 patients with hypertension. J. Hypertens 32, 2332–2340 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Stergiou GS et al. Methodology and technology for peripheral and central blood pressure and blood pressure variability measurement. J. Hypertens 34, 1665–1677 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Parati G et al. European Society of Hypertension Practice Guidelines for home blood pressure monitoring. J. Hum. Hypertens 24, 779–785 (2010). [DOI] [PubMed] [Google Scholar]
- 75.O’Brien E et al. European Society of Hypertension Position Paper on Ambulatory Blood Pressure Monitoring. J. Hypertens 31, 1731–1768 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Muntner P & Whelton PK Using Predicted Cardiovascular Disease Risk in Conjunction With Blood Pressure to Guide Antihypertensive Medication Treatment. J. Am. Coll. Cardiol 69, 2446–2456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mancia G et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. J. Hypertens 31, 1281–1357 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Pickering TG Recommendations for Blood Pressure Measurement in Humans and Experimental Animals: Part 1: Blood Pressure Measurement in Humans: A Statement for Professionals From the Subcommittee of Professional and Public Education of the American Heart Association Cou. Circulation 111, 697–716 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Whelton PK The elusiveness of population-wide high blood pressure control. Annu. Rev. Public Health 36, 109–30 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Primatesta P & Poulter NR Improvement in hypertension management in England: results from the Health Survey for England 2003. J. Hypertens 24, 1187–1192 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Ashworth M, Medina J & Morgan M Effect of social deprivation on blood pressure monitoring and control in England: a survey of data from the quality and outcomes framework. BMJ 337, a2030–a2030 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serumaga B et al. Effect of pay for performance on the management and outcomes of hypertension in the United Kingdom: interrupted time series study. BMJ 342, d108–d108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poulter NR & Lackland DT May Measurement Month: a global blood pressure screening campaign. Lancet 389, 1678–1680 (2017). [DOI] [PubMed] [Google Scholar]
- 84.He J et al. Migration, blood pressure pattern, and hypertension: the Yi Migrant Study. Am. J. Epidemiol 134, 1085–101 (1991). [DOI] [PubMed] [Google Scholar]
- 85.Poulter NR et al. The Kenyan Luo migration study: observations on the initiation of a rise in blood pressure. BMJ 300, 967–72 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenthal T The effect of migration on hypertension and other cardiovascular risk factors: A review. J. Am. Soc. Hypertens 8, 171–191 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Klag MJ et al. The contribution of urinary cations to the blood pressure differences associated with migration. Am. J. Epidemiol 142, 295–303 (1995). [DOI] [PubMed] [Google Scholar]
- 88.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 267, 1213–20 (1992). [DOI] [PubMed] [Google Scholar]
- 89.Whelton PK et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. Am. J. Clin. Nutr 65, 652S–660S (1997). [DOI] [PubMed] [Google Scholar]
- 90.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch. Intern. Med 157, 657–67 (1997). [PubMed] [Google Scholar]
- 91.Sacks FM et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med 344, 3–10 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Whelton PK et al. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 277, 1624–32 (1997). [DOI] [PubMed] [Google Scholar]
- 93.Aburto NJ et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 346, f1378–f1378 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whelton SP, Chin A, Xin X & He J Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann. Intern. Med 136, 493–503 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Xin X et al. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertens. (Dallas, Tex. 1979) 38, 1112–7 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Roerecke M et al. The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Heal 2, e108–e120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Appel LJ et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med 336, 1117–24 (1997). [DOI] [PubMed] [Google Scholar]
- 98.Whelton PK Hypertension curriculum review: epidemiology and the prevention of hypertension. J. Clin. Hypertens. (Greenwich) 6, 636–42 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whelton PK et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 288, 1882–8 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Whelton PK et al. Sodium Reduction and Weight Loss in the Treatment of Hypertension in Older Persons. JAMA 279, 839 (1998). [DOI] [PubMed] [Google Scholar]
- 101.National High Blood Pressure Education Program Working Group report on primary prevention of hypertension. Arch. Intern. Med 153, 186–208 (1993). [PubMed] [Google Scholar]
- 102.Cook NR, Cohen J, Hebert PR, Taylor JO & Hennekens CH Implications of small reductions in diastolic blood pressure for primary prevention. Arch. Intern. Med 155, 701–9 (1995). [PubMed] [Google Scholar]
- 103.Julius S et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N. Engl. J. Med 354, 1685–97 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Lüders S et al. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J. Hypertens 26, 1487–96 (2008). [DOI] [PubMed] [Google Scholar]
- 105.Fuchs SC et al. Effectiveness of Chlorthalidone Plus Amiloride for the Prevention of Hypertension: The PREVER‐Prevention Randomized Clinical Trial. J. Am. Heart Assoc 5, e004248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.James PA et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults. JAMA 311, 507 (2014). [DOI] [PubMed] [Google Scholar]
- 107.SPRINT Research Group et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med 373, 2103–2116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filipovský J et al. Automated compared to manual office blood pressure and to home blood pressure in hypertensive patients. Blood Press 25, 228–234 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Kjeldsen SE, Lund-Johansen P, Nilsson PM & Mancia G Unattended Blood Pressure Measurements in the Systolic Blood Pressure Intervention Trial. Hypertension 67, 808–812 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Whelton PK et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary. Hypertension HYP.0000000000000066 (2017). doi: 10.1161/HYP.0000000000000066 [DOI] [Google Scholar]
- 111.Aburto NJ et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 346, f1326 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He FJ, Li J & Macgregor GA Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346, f1325 (2013). [DOI] [PubMed] [Google Scholar]
- 113.WHO| Guideline Sodium intake for adults and children Sodium intake for adults and children [PubMed]
- 114.Appel LJ et al. Dietary Approaches to Prevent and Treat Hypertension: A Scientific Statement From the American Heart Association. Hypertension 47, 296–308 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Appel LJ ASH Position Paper: Dietary Approaches to Lower Blood Pressure. J. Clin. Hypertens 11, 358–368 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He FJ & MacGregor GA Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J. Hum. Hypertens 16, 761–770 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Langford HG Dietary therapy slows the return of hypertension after stopping prolonged medication. JAMA J. Am. Med. Assoc 253, 657–664 (1985). [PubMed] [Google Scholar]
- 118.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ 297, 319–328 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bibbins-Domingo K et al. Projected Effect of Dietary Salt Reductions on Future Cardiovascular Disease. N. Engl. J. Med 362, 590–599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graudal NA, Hubeck-Graudal T & Jurgens G Effects of Low-Sodium Diet vs. High-Sodium Diet on Blood Pressure, Renin, Aldosterone, Catecholamines, Cholesterol, and Triglyceride (Cochrane Review). Am. J. Hypertens 25, 1–15 (2012). [DOI] [PubMed] [Google Scholar]
- 121.He FJ & MacGregor GA Reducing Population Salt Intake-Time for Global Action. J. Clin. Hypertens 17, 10–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cappuccio FP & MacGregor GA Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J. Hypertens 9, 465–473 (1991). [DOI] [PubMed] [Google Scholar]
- 123.Chalmers J et al. Australian National Health and Medical Research Council dietary salt study in mild hypertension. J. Hypertens. Suppl 4, S629–37 (1986). [PubMed] [Google Scholar]
- 124.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis 43, S1–290 (2004). [PubMed] [Google Scholar]
- 125.Börjesson M, Onerup A, Lundqvist S & Dahlöf B Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. Br. J. Sports Med 50, 356–361 (2016). [DOI] [PubMed] [Google Scholar]
- 126.MacDonald HV et al. Dynamic Resistance Training as Stand‐Alone Antihypertensive Lifestyle Therapy: A Meta‐Analysis. J. Am. Heart Assoc 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Egan BM, Zhao Y, Axon RN, Brzezinski WA & Ferdinand KC Uncontrolled and Apparent Treatment Resistant Hypertension in the United States, 1988 to 2008. Circulation 124, 1046–1058 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zomer E et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes. Rev 17, 1001–1011 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Stevens VJ Long-Term Weight Loss and Changes in Blood Pressure: Results of the Trials of Hypertension Prevention, Phase II. Ann. Intern. Med 134, 1 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Ettehad D et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387, 957–967 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Garjón J et al. First-line combination therapy versus first-line monotherapy for primary hypertension. in Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd, 2017). doi: 10.1002/14651858.cd010316.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hypertension in adults: diagnosis and management | Guidance and guidelines | NICE.
- 133.James WPT et al. Effect of Sibutramine on Cardiovascular Outcomes in Overweight and Obese Subjects. N. Engl. J. Med 363, 905–917 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Flack JM et al. Management of High Blood Pressure in Blacks: An Update of the International Society on Hypertension in Blacks Consensus Statement. Hypertension 56, 780–800 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Iskedjian M et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: Evidence from a meta-analysis. Clin. Ther 24, 302–316 (2002). [DOI] [PubMed] [Google Scholar]
- 136.Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 362, 1527–1535 (2003). [DOI] [PubMed] [Google Scholar]
- 137.Yusuf S, et al. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med 358, 1547–1559 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Bosch J et al. Effect of Ramipril on the Incidence of Diabetes. N. Engl. J. Med 355, 1551–1562 (2006). [DOI] [PubMed] [Google Scholar]
- 139.Brown NJ, Ray WA, Snowden M & Griffin MR Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema*. Clin. Pharmacol. Ther 60, 8–13 (1996). [DOI] [PubMed] [Google Scholar]
- 140.Brown NJ, Byiers S, Carr D, Maldonado M & Warner BA Dipeptidyl Peptidase-IV Inhibitor Use Associated With Increased Risk of ACE Inhibitor-Associated Angioedema. Hypertension 54, 516–523 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guazzi MD et al. Disparate unloading efficacy of the calcium channel blockers, verapamil and nifedipine, on the failing hypertensive left ventricle. Am. Heart J 108, 116–23 (1984). [DOI] [PubMed] [Google Scholar]
- 142.Harari D, Gurwitz JH, Avorn J, Choodnovskiy I & Minaker KL Correlates of regular laxative use by frail elderly persons. Am. J. Med 99, 513–8 (1995). [DOI] [PubMed] [Google Scholar]
- 143.Bernard E, Goutelle S, Bertrand Y & Bleyzac N Pharmacokinetic Drug-Drug Interaction of Calcium Channel Blockers With Cyclosporine in Hematopoietic Stem Cell Transplant Children. Ann. Pharmacother 48, 1580–1584 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA J. Am. Med. Assoc 202, 1028–1034 (1967). [PubMed] [Google Scholar]
- 145.Barzilay JI, Long-Term Effects of Incident Diabetes Mellitus on Cardiovascular Outcomes in People Treated for Hypertension The ALLHAT Diabetes Extension Study. [DOI] [PMC free article] [PubMed]
- 146.Roush GC, Holford TR & Guddati AK Chlorthalidone Compared With Hydrochlorothiazide in Reducing Cardiovascular Events: Systematic Review and Network Meta-Analyses. Hypertension 59, 1110–1117 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Roush GC, Ernst ME, Kostis JB, Tandon S & Sica DA Head-to-Head Comparisons of Hydrochlorothiazide With Indapamide and Chlorthalidone: Antihypertensive and Metabolic Effects. Hypertension 65, 1041–1046 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Antman EM & Sabatine MS Cardiovascular therapeutics : a companion to Braunwald’s heart disease (Elsevier/Saunders, 2013). [Google Scholar]
- 149.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM & Opie LH Beta-blockers for hypertension. in Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd, 2017). doi: 10.1002/14651858.cd002003.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boutouyrie P, Achouba A, Trunet P & Laurent S Amlodipine-Valsartan Combination Decreases Central Systolic Blood Pressure More Effectively Than the Amlodipine-Atenolol Combination: The EXPLOR Study. Hypertension 55, 1314–1322 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Sharma AM, Pischon T, Hardt S, Kunz I & Luft FC Hypothesis: -Adrenergic Receptor Blockers and Weight Gain : A Systematic Analysis. Hypertension 37, 250–254 (2001). [DOI] [PubMed] [Google Scholar]
- 152.Bakris GL et al. Metabolic Effects of Carvedilol vs Metoprolol in Patients With Type 2 Diabetes Mellitus and Hypertension. JAMA 292, 2227 (2004). [DOI] [PubMed] [Google Scholar]
- 153.Ruilope LM et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 375, 1255–1266 (2010). [DOI] [PubMed] [Google Scholar]
- 154.Ghofrani H-A et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med 369, 330–340 (2013). [DOI] [PubMed] [Google Scholar]
- 155.Zinman B et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med 373, 2117–2128 (2015). [DOI] [PubMed] [Google Scholar]
- 156.Oparil S & Schmieder RE New Approaches in the Treatment of Hypertension. Circ. Res 116, 1074–1095 (2015). [DOI] [PubMed] [Google Scholar]
- 157.Jordan J et al. Improved Insulin Sensitivity With Angiotensin Receptor Neprilysin Inhibition in Individuals With Obesity and Hypertension. Clin. Pharmacol. Ther 101, 254–263 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Seferovic JP et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 5, 333–340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Calhoun DA et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific Statement From the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51, 1403–1419 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Sim JJ et al. Characteristics of Resistant Hypertension in a Large, Ethnically Diverse Hypertension Population of an Integrated Health System. Mayo Clin. Proc 88, 1099–1107 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams B et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet (London, England) 386, 2059–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bobrie G et al. Sequential nephron blockade versus sequential renin–angiotensin system blockade in resistant hypertension. J. Hypertens 30, 1656–1664 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Juurlink DN et al. Rates of Hyperkalemia after Publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med 351, 543–551 (2004). [DOI] [PubMed] [Google Scholar]
- 164.Krum H et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009). [DOI] [PubMed] [Google Scholar]
- 165.Bhatt DL et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med 370, 1393–1401 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Heusser K et al. Carotid Baroreceptor Stimulation, Sympathetic Activity, Baroreflex Function, and Blood Pressure in Hypertensive Patients. Hypertension 55, 619–626 (2010). [DOI] [PubMed] [Google Scholar]
- 167.Bisognano JD et al. Baroreflex Activation Therapy Lowers Blood Pressure in Patients With Resistant Hypertension. J. Am. Coll. Cardiol 58, 765–773 (2011). [DOI] [PubMed] [Google Scholar]
- 168.Spiering W et al. LB02.05: Controlling and lowering blood pressure with the Mobiushd device: first in-man results (CALM-FIM study). J. Hypertens 33, e86 (2015). [Google Scholar]
- 169.Narkiewicz K et al. Unilateral Carotid Body Resection in Resistant Hypertension. JACC Basic to Transl. Sci 1, 313–324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.O’Callaghan EL et al. Chronic Deep Brain Stimulation Decreases Blood Pressure and Sympathetic Nerve Activity in a Drug- and Device-Resistant Hypertensive Patient. Hypertension 69, 522–528 (2017). [DOI] [PubMed] [Google Scholar]
- 171.Lobo MD et al. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet 385, 1634–1641 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Testa MA & Simonson DC Assessment of Quality-of-Life Outcomes. N. Engl. J. Med 334, 835–840 (1996). [DOI] [PubMed] [Google Scholar]
- 173.Trevisol DJ, Moreira LB, Kerkhoff A, Fuchs SC & Fuchs FD Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J. Hypertens 29, 179–188 (2011). [DOI] [PubMed] [Google Scholar]
- 174.Bardage C & Isacson DG Hypertension and health-related quality of life. an epidemiological study in Sweden. J. Clin. Epidemiol 54, 172–81 (2001). [DOI] [PubMed] [Google Scholar]
- 175.Pickering TG Now we are sick: labeling and hypertension. J. Clin. Hypertens. (Greenwich) 8, 57–60 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Haynes RB, Sackett DL, Taylor DW, Gibson ES & Johnson AL Increased Absenteeism from Work after Detection and Labeling of Hypertensive Patients. N. Engl. J. Med 299, 741–744 (1978). [DOI] [PubMed] [Google Scholar]
- 177.Fogari R & Zoppi A Effect of antihypertensive agents on quality of life in the elderly. Drugs Aging 21, 377–93 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Carris NW & Smith SM Quality of Life in Treatment-Resistant Hypertension. Curr. Hypertens. Rep 17, 61 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Croog SH et al. The Effects of Antihypertensive Therapy on the Quality of Life. N. Engl. J. Med 314, 1657–1664 (1986). [DOI] [PubMed] [Google Scholar]
- 180.Testa MA, Anderson RB, Nackley JF & Hollenberg NK Quality of Life and Antihypertensive Therapy in Men -- A Comparison of Captopril with Enalapril. N. Engl. J. Med 328, 907–913 (1993). [DOI] [PubMed] [Google Scholar]
- 181.Grimm RH et al. Relationships of quality-of-life measures to long-term lifestyle and drug treatment in the Treatment of Mild Hypertension Study. Arch. Intern. Med 157, 638–48 (1997). [PubMed] [Google Scholar]
- 182.Applegate WB Quality of life during antihypertensive treatment. Lessons from the Systolic Hypertension in the Elderly Program. Am. J. Hypertens 11, 57S–61S (1998). [DOI] [PubMed] [Google Scholar]
- 183.Fletcher AE et al. Quality of life on randomized treatment for isolated systolic hypertension: results from the Syst-Eur Trial. J. Hypertens 20, 2069–79 (2002). [DOI] [PubMed] [Google Scholar]
- 184.Saper CB How low can you go? Ann. Neurol 78, 665–666 (2015). [DOI] [PubMed] [Google Scholar]
- 185.O’Connor PJ et al. Effect of intensive versus standard blood pressure control on depression and health-related quality of life in type 2 diabetes: the ACCORD trial. Diabetes Care 35, 1479–81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Berlowitz D; Foy C; Kazis L; Bolin L; Conroy M et al. Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes. N. Engl. J. Med 377, 733–744 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 389, 37–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kearney PM et al. Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223 (2005). [DOI] [PubMed] [Google Scholar]
- 189.O’Donnell MJ, Mente A, Smyth A & Yusuf S Salt intake and cardiovascular disease: why are the data inconsistent? Eur. Heart J 34, 1034–1040 (2012). [DOI] [PubMed] [Google Scholar]
- 190.Chow CK et al. Prevalence, Awareness, Treatment, and Control of Hypertension in Rural and Urban Communities in High-, Middle-, and Low-Income Countries. JAMA 310, 959 (2013). [DOI] [PubMed] [Google Scholar]
- 191.Adler AJ et al. Reducing Cardiovascular Mortality Through Prevention and Management of Raised Blood Pressure. Glob. Heart 10, 111–122 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Olsen MH et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388, 2665–2712 (2016). [DOI] [PubMed] [Google Scholar]
- 193.Bhatt DL et al. A Controlled Trial of Renal Denervation for Resistant Hypertension. N. Engl. J. Med 370, 1393–1401 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Mendis S et al. The availability and affordability of selected essential medicines for chronic diseases in six low- and middle-income countries. Bull. World Health Organ 85, 279–288 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park IU & Taylor AL Race and ethnicity in trials of antihypertensive therapy to prevent cardiovascular outcomes: a systematic review. Ann. Fam. Med 5, 444–52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Anchala R et al. The Role of Decision Support System (DSS) in Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS One 7, e47064 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.WHO, PEPFAR U Task Shifting Global Recommendations and Guidelines
- 198.Ehret GB et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478, 103–109 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Potter LR, Yoder AR, Flora DR, Antos LK & Dickey DM Natriuretic Peptides: Their Structures, Receptors, Physiologic Functions and Therapeutic Applications. in cGMP: Generators, Effectors and Therapeutic Implications 341–366 (Springer Berlin Heidelberg, 2009). doi: 10.1007/978-3-540-68964-5_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dries DL Corin Gene Minor Allele Defined by 2 Missense Mutations Is Common in Blacks and Associated With High Blood Pressure and Hypertension. Circulation 112, 2403–2410 (2005). [DOI] [PubMed] [Google Scholar]
- 201.Siebenhofer A et al. Long-term effects of weight-reducing drugs in people with hypertension. in Cochrane Database of Systematic Reviews (John Wiley & Sons, Ltd, 2016). doi: 10.1002/14651858.cd007654.pub4 [DOI] [PubMed] [Google Scholar]
- 202.Ricci C et al. Long-Term Effects of Bariatric Surgery on Type II Diabetes, Hypertension and Hyperlipidemia: A Meta-Analysis and Meta-Regression Study with 5-Year Follow-Up. Obes. Surg 25, 397–405 (2014). [DOI] [PubMed] [Google Scholar]
- 203.Sjöström CD, Lystig T & Lindroos AK Impact of weight change, secular trends and ageing on cardiovascular risk factors: 10-year experiences from the SOS study. Int. J. Obes 35, 1413–1420 (2011). [DOI] [PubMed] [Google Scholar]
- 204.Whelton PK Epidemiology and the Prevention of Hypertension. J. Clin. Hypertens 6, 636–642 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crowley SD et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci 103, 17985–17990 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Crowley SD et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J. Clin. Invest 115, 1092–1099 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Reich HN, Oudit GY, Penninger JM, Scholey JW & Herzenberg AM Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74, 1610–6 (2008). [DOI] [PubMed] [Google Scholar]
- 208.Liu L-S & Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. [2010 Chinese guidelines for the management of hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi 39, 579–615 (2011). [PubMed] [Google Scholar]
- 209.Weber MA et al. Clinical Practice Guidelines for the Management of Hypertension in the Community. J. Clin. Hypertens 16, 14–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Seedat YK, Rayner BL & Veriava Y South African hypertension practice guideline 2014 : review article. Cardiovasc. J. Afr 25, 288–294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shimamoto K et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens. Res 37, 253–253 (2014). [DOI] [PubMed] [Google Scholar]
- 212.Leung AA et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can. J. Cardiol 32, 569–588 (2016). [DOI] [PubMed] [Google Scholar]
- 213.National Heart Foundation. Australia. Guideline for the diagnosis and management of hypertension in adults (2016).
- 214.American Diabetes Association. Standards of medical care in diabetes - 2017. Diabetes Care 40, 1–142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]