Abstract
Context:
The worldwide prevalence of rheumatoid arthritis (RA) is about 1%, whereas in India, it is approximately 0.75%. The current therapy for RA includes nonsteroidal anti-inflammatory drugs corticosteroids, disease-modifying anti-rheumatic drugs and some recently developed biologic agents, but all of these are associated with adverse effects. Some herbal drugs, such as Boswellia serrata, have been reported to possess anti-inflammatory activity.
Aims:
The aim of this study is to evaluate the anti-arthritic activity of Boswellia serrata extract (BSE) in complete Freund's adjuvant (CFA)-induced arthritis in rats.
Materials and Methods:
Thirty-six Wistar rats were divided into six equal groups. RA was induced by intradermal injection of 0.1 ml CFA in hind paw. Body weight, ankle diameter, paw volume, arthritic index, tumor necrosis factor-α (TNF-α), and histopathological examination were assessed. The experimental data were statistically assessed by one-way analysis of variance (ANOVA).
Statistical Analysis Used:
The recorded data were analyzed using paired t-test and ANOVA test using SPSS. The data were analyzed and represented as mean difference. Value of P < 0.05 was considered statistically significant.
Results:
BSE at dose 180 mg/kg showed statistically significant improvement in body weight and decrease in ankle diameter and arthritic index (P < 0.05); however, there was insignificant change in paw volume (P = 0.056). This improvement was comparable with Indomethacin. The level of TNF-α did not show any statistically significant change (P = 0.076). Histopathological results also exhibited a reduction in inflammatory parameters.
Conclusions:
BSE might have usefulness as an adjunct to conventional therapy of RA.
Keywords: Boswellia serrata extract, complete Freund's adjuvant, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is an inflammatory condition of synovial tissue leading to the destruction of joints.[1] When this tissue remains constantly inflamed, it leads to deformity by loosening joint ligaments and joint destruction by eroding nearby cartilage and bones.[2] The prevalence of RA varies between 0.3% and 1% worldwide and approximately 0.75% in India.[3] The current treatment of RA only provides symptomatic relief but not totally prevent joint damage. The commonly used drugs for the treatment of RA such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease-modifying antirheumatic drugs (DMARDs), and biological response modifiers are associated with many adverse effects.[4] The gastrointestinal adverse drug effects of NSAIDs range from minor uneasiness to life-threatening peptic ulcers. The common adverse effects such as nausea, anorexia, gastritis, epigastric pain, peptic ulcers, flatulence, or diarrhea may occur in 15%–60% of patients.[5] Renal and cardiovascular complications such as acute kidney failure, hypertension, and electrolyte abnormalities can also be attributed to NSAIDs. Prolong use of steroids is associated with impaired wound healing, osteoporosis, muscular weakness, cataract, and peptic ulcer.[6] Side effects of DMARDs include dysfunction of gastrointestinal organ, liver, kidney coupled with stomatitis, and bone marrow suppression.[7] Secondary infections and malignancies such as lymphoma and nonmelanoma skin cancers may develop with the use of biological response modifiers like infliximab.[8]
As a result, there is a need for alternative well-tolerated anti-inflammatory remedies. Gum resin of Boswellia serrata extract (BSE) has been found to possess anti-inflammatory potential with better tolerability.[9] In India, extract of BSE has been used for centuries as traditional Ayurvedic medicine for treatment of the inflammatory conditions.[10] Boswellia serrata has also shown the ability to inhibit pro-inflammatory cytokines. It suppresses interleukin-1 β (IL-1 β), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and enhances production IL-10 in collagen-induced arthritis rats. These cytokines have a crucial role in chronic inflammation and tissue damage during the progression of RA.[11,12]
Several animal models were developed for RA and complete Freund's adjuvant (CFA)-induced arthritis is commonly used model which shares many features of RA in humans. Hence, we planned this study to evaluate the anti-inflammatory effect of BSE in the acute condition of CFA-induced animal model of RA in Wistar rats.
Materials and Methods
After being approved by the Institutional Animal Ethics Committee (Project No-72/IAEC/2016 dated 24/09/2016), this study was carried out in 36 healthy adult male Wistar rats having an almost similar physical constitution (in terms of age and body weight), weighing between 150 and 200 g, 6 in each group as-Group 1-Normal Control, Group 2-Arthritic Control, Group 3-Arthritic rats treated with indomethacin (3 mg/kg), Group 4-Arthritic rats treated with Boswellia serrata (45 mg/kg), Group 5-Arthritic rats treated with Boswellia serrata (90 mg/kg), and Group 6-Arthritic rats treated with Boswellia serrata (180 mg/kg). They were kept under standard laboratory conditions of temperature (25°C ± 2°C), humidity (55% ±5%) and 12 h light-dark cycle controlled environment. The animals were provided pellet food diet and water ad libitum.
For induction of arthritis, 0.1 ml CFA was injected intradermally in the footpad of left hind paw in rats barring control group.[13] All rats studied for an acute inflammatory lesion of arthritis. The treatment was scheduled from day 5 to 15. During the entire course of the study, the standard protocols were followed. Baseline measurement for parameters body weight, paw thickness, ankle diameter, and paw volume was done on day 0. Subsequently, above-mentioned parameters along with an arthritic index and TNF-α was measured on day 5 and 15. On day 15, all rats were sacrificed by using a high dose of pentobarbitone (50 mg/kg i. p).[14] The inflamed limbs were excised above the ankle joints and examined for a histopathological finding of rheumatic arthritis.
CFA was purchased from Sigma Aldrich Chemical Co, USA. Boswellia serrata tablets (containing Boswellia serrata ole-gum resin extract; Oleo-gum-resin is tapped from the incision made on the trunk of the tree) were purchased from Himalaya Healthcare Co., India. Indomethacin 25 mg tablet was purchased from Jagsonpal Pharmaceuticals. An enzyme-linked immunosorbent assay (ELISA) kit for TNF-α was obtained from Diaclone, France. BSE in the dose of 45 mg/kg, 90 mg/kg, 180 mg/kg, and indomethacin 3 mg/kg was administered in distilled water for oral administration to each rat.[15,16]
The body weight (g) of all rats was recorded on day 0, 5, and 15 in the experiment. The percentage change of body weight was calculated using the formula: Percentage change = (Wc − Wt) × 100/Wc where Wc is mean change in body weight of arthritic control group and Wt is mean change in body weight of the treated group.
Ankle diameter was measured on day 0, 5, and 15 to assess inflammation as acute lesion on an injected limb. The percentage inhibition of ankle diameter was calculated using the formula: Percentage inhibition = (Dc − Dt) × 100/Dc where Dc is mean change in ankle diameter of the arthritic control group and Dt is mean change in ankle diameter of the treated group.
Paw volume of the injected limb was measured on day 0, day 5, and day 15 by immersed vertically to the level of the lateral malleolus in the plethymometer. Mean change in paw volume was calculated and percentage inhibition of paw edema was calculated using the formula: Percentage inhibition = (Vc − Vt) × 100/Vc: where Vc is mean change in paw volume of the arthritic control group and Vt is mean change in paw volume of the treated group.
Rats were kept under observation and periodic parametric evaluation for arthritis was done on day 0, 5, and 15. The severity of arthritis was evaluated by grading system: Normal Paw = 0, Mild swelling and erythema of digits = 1, Swelling and erythema of digits = 2, Severe swelling and erythema = 3, Gross deformity and inability to bend the limb = 4. Arthritic index for each rat was calculated by adding the four scores of individual paws.[17] If adjuvant injected limb (left hind) was inflamed, the experimental animal was considered to have developed acute arthritis.
The blood sample was withdrawn from the retro-orbital route and serum was separated by centrifugation. Serum level of TNF-α was determined by using commercially available ELISA kits.
For histopathological examinations, rats were sacrificed on day 15 by using a high dose of pentobarbitone (50 mg/kg i. p) and the inflamed limbs were excised above the ankle joints and examined for histopathological findings of rheumatic arthritis. Excised left hind limb tissue fixed in 10% buffer formalin solution from control and treated rats sent to the pathologist for evaluation who was blind to the specimen.
The recorded data were analyzed using paired t-test and analysis of variance test using SPSS (IBM SPSS Statistics Version 24.0. Armonk, NY). The data were analyzed and represented as mean difference. Value of P < 0.05 was considered statistically significant.
Results
Effects of Boswellia serrata extract on body weight as compared to indomethacin
On day 0, body weight was measured of each rat from every group and taken as baseline values. During the course of the study, the mean % increase in weight in control group was 2.21 (P = 0.07), from day 0 to 5 and mean % increase in weight was 0.43 (P = 0.78) from day 5 to 15. On day 5, the body weight was found to be decreased in all CFA induced arthritic groups (Groups 2–6). On day 15, weight was increased in indomethacin and BSE treated groups as compared to day 5. Body weight was gained significantly in indomethacin (P < 0.05) while insignificant in all BSE treated groups [Table 1a]. On intergroup comparison, body weight was significantly increased in indomethacin-treated group (P < 0.01) and BSE 180 mg/kg treated group (P < 0.05) as compared to the arthritic control group [Table 1b].
Table 1a.
Change in body weight (recorded in grams) of different groups of animals on day 0, 5, and 15 (all values represented here are in mean±standard deviation; values on day 0 and 5 represent the value before treatment and values on day 15 are after treatment; n=6/group; all treatment administered orally)
| Groups | Days of study (mean±SD) |
% change |
|||
|---|---|---|---|---|---|
| Day 0 | Day 5 | Day 15 | Days 0-5 | Days 5-15 | |
| I | 188.5±5.577 | 192.67±4.63 | 193.5±5.32 | −2.21 | −0.43 |
| II | 186.83±6.113 | 176.83±10.64 | 169.83±10.304 | 5.35 | 3.96 |
| III | 185.83±8.085 | 176.33±9.688 | 189.5±6.74 | 5.11# | −7.47# |
| IV | 185±8.438 | 177.17±9.02 | 180±8.877 | 4.23 | −1.60 |
| V | 184.17±7.548 | 177.67±9.50 | 183.33±7.005 | 3.53 | −3.19 |
| VI | 183.17±6.338 | 175.83±6.79 | 186.33±7.11 | 4.01 | −5.97 |
#P<0.05. SD: Standard deviation
Table 1b.
Change in body weight of treated groups as compared with arthritic control group
| Groups | Mean difference |
||
|---|---|---|---|
| Days 0 | Day 5 | Day 15 | |
| II versus | |||
| III | 1.00 | 0.50 | −19.67* |
| IV | 1.83 | −0.33 | −10.17 |
| V | 2.67 | −0.83 | −13.50 |
| VI | 3.67 | 1.00 | −16.50# |
*P<0.01, #P<0.05
Effects of Boswellia serrata extract on ankle diameter as compared to indomethacin
Before CFA injection ankle diameter of both hind limbs of each rat from every group was measured on day 0 and taken as baseline values. During the course of the study, ankle diameter remained almost unchanged in normal control. In CFA-induced arthritic groups (2–6), ankle diameter values were significantly increased on day 5 as compared to day 0 (P < 0.01). In Group 2 (arthritic control), ankle diameter was increased significantly on day 15 as compared to day 5. Following the drug treatment ankle diameter values on day 15 were found to have decreased from day 5 values which were significant in Groups 3, 5, and 6 [Table 2a]. On intergroup comparison, ankle diameter was significantly reduced in indomethacin 3 mg/kg treated group (P < 0.05) and BSE 180 mg/kg treated group (P < 0.05) as compared to that of arthritic control group [Table 2b].
Table 2a.
Change in ankle diameter (measured in cm) of different groups of animals on day 0, 5, and 15 (all values represented here are in mean±standard deviation; values on day 0 and 5 represent the value before treatment and values on day 15 are after treatment; n=6/group; all treatment administered orally)
| Groups | Day 0 | Day 5 | Day 15 | % change |
|
|---|---|---|---|---|---|
| Days 0-5 | Days 5-15 | ||||
| I | 0.32±0.03 | 0.32±0.06 | 0.33±0.01 | 0.01 | −1.03 |
| II | 0.33±0.02 | 0.60±0.03 | 0.64±0.04 | −86.15* | −6.89# |
| III | 0.32±0.02 | 0.62±0.04 | 0.40±0.03 | −93.32* | 35.81* |
| IV | 0.31±0.22 | 0.56±0.55 | 0.58±0.03 | −81.64* | −4.77 |
| V | 0.30±0.13 | 0.66±0.06 | 0.55±0.04 | −116.82* | 16.05# |
| VI | 0.32±0.03 | 0.65±0.03 | 0.51±0.03 | −105.72* | 22.53* |
*P<0.01, #P<0.05
Table 2b.
Change in ankle diameter of treated groups as compared with arthritic control group
| Variables | Groups | Mean difference |
||
|---|---|---|---|---|
| Days 0 | Day 5 | Day 15 | ||
| Ankle diameter | II versus | |||
| III | −0.005 | −0.023 | 0.243# | |
| IV | 0.0116 | 0.045 | 0.060 | |
| V | 0.0133 | −0.060 | 0.088 | |
| VI | 0.001 | −0.053 | 0.136# | |
#P<0.05
Effects of Boswellia serrata extract on paw volume as compared to indomethacin
Before CFA injection paw volume of both hind limbs of each rat from every group was measured on day 0 and taken as baseline values. Slight increase in paw volume was observed in the normal control group on day 15 as compared to day 0. Paw volume values were significantly (P < 0.01) increased in all CFA-induced arthritic groups (2-6), on day 5 as compared to day 0. Following the drug treatment, paw volume values on day 15 were found to have decreased from day 5 values, but it was significant only in indomethacin-treated group [Table 3a]. Intergroup comparative analysis showed that there was reduction in paw volume on day 15 in all the groups when compared to the arthritic control group, but it was significantly reduced in Group 3 and 6 [Table 3b].
Table 3a.
Change in paw volume (measured in ml) of different groups of animals on day 0, 5, and 15 (all values represented here are in mean±standard deviation values on day 0 and 5 represent the value before treatment and values on day 15 are after treatment; n=6/group; all treatment administered orally)
| Groups | Day 0 | Day 5 | Day 15 | % change |
|
|---|---|---|---|---|---|
| Days 0-5 | Days 0-5 | ||||
| I | 0.64±0.01 | 0.65±0.013 | 0.68±0.01 | 0.01 | −0.26 |
| II | 0.63±0.02 | 0.99±0.29 | 1.21±0.14 | −52.1# | −21.73 |
| III | 0.64±0.01 | 1.12±0.25 | 0.70±0.03 | −73.27* | 37.39# |
| IV | 0.66±0.02 | 1.23±0.41 | 0.89±0.08 | −84.99# | 27.16 |
| V | 0.64±0.02 | 0.99±0.20 | 0.86±0.08 | −54.95* | 12.44 |
| VI | 0.63±0.02 | 0.98±0.11 | 0.80±0.07 | −56.08* | 17.80# |
*P<0.01, #P<0.05
Table 3b.
Effects of Boswellia serrata extract on paw volume in control and treatment group-on day 0, 5, and 15
| Variables | Groups | Mean difference |
||
|---|---|---|---|---|
| Day 0 | Day 5 | Day 15 | ||
| Paw volume | II versus | |||
| III | −0.018 | −0.126 | 0.510# | |
| IV | −0.036 | −0.236 | 0.315 | |
| V | −0.010 | 0.005 | 0.345 | |
| VI | 0.001 | 0.013 | 0.405# | |
#P < 0.05
Effects of Boswellia serrata extract on arthritis index as compared to indomethacin
Complete Freund's Adjuvant-induced arthritic rats produced statistically significant (P < 0.01) increase in the arthritic index in all arthritic group. Following the drug treatment, arthritic index values on day 15 were found to have decreased from day 5 values, but it was significant only in indomethacin-treated group [Table 4a]. Arthritis index kept on increasing from day 5 to 15 in all the groups, whether control or treated with indomethacin/BSE [Table 4a]. Intergroup comparative analysis showed that there was reduction in the arthritic index on day 15 in all the groups when compared to the arthritic control group, but it was significantly reduced in Group 3, 5, and 6 [Table 4b].
Table 4a.
Arthritis index of different groups of animals on day 0, 5 and 15 (all values represented here are in mean±standard deviation; values on day 0 and 5 represent the value before treatment and values on day 15 are after treatment; n=6/group; all treatment administered orally)
| Groups | Day 0 | Day 5 | Day 15 | % change (days 5-15) |
|---|---|---|---|---|
| I | 0 | 0±0 | 0±0 | 0.00 |
| II | 0 | 5.66±0.51 | 19.5±1.22 | −244.12* |
| III | 0 | 5.66±0.51 | 10.16±0.98 | −79.41* |
| IV | 0 | 5.33±0.51 | 16.16±2.04 | −203.13* |
| V | 0 | 5.5±0.54 | 15.5±2.66 | −181.82* |
| VI | 0 | 5.66±0.51 | 12.66±2.94 | −123.53* |
*P<0.01
Table 4b.
Effects of Boswellia serrata extract on Arthritis index in control and treatment group-on day 5 and 15
| Variables | Groups | Mean difference |
|
|---|---|---|---|
| Days 5 | Days 15 | ||
| Arthritic index | II versus | ||
| III | 0.001 | 9.33* | |
| IV | 0.333 | 3.33 | |
| V | 0.167 | 4.00# | |
| VI | 0.001 | 6.83* | |
*P<0.01, #P<0.05
Effects of Boswellia serrata extract on serum tumor necrosis factor-α as compared to indomethacin
In CFA-induced arthritic rats, TNF-α was increased as compared to the normal control group on day 5. TNF-α is an inflammatory marker so its increased level promotes inflammation and joint tissue destruction [Table 5a]. When compared day 15 values from day 5, TNF-α values kept on increasing significantly in the arthritic control group. Only indomethacin-treated group showed a significant decrease in TNF-α levels while treatment with BSE (in any dose) did not show any significant change. Intergroup comparative analysis also showed that there was reduction only in TNF-α on day 15 in indomethacin-treated groups when compared to the arthritic control group [Table 5b].
Table 5a.
Tumor necrosis factor-α of different groups of animals on day 5 and 15 (all values represented here are in mean±standard deviation; values on day 5 represent the value before treatment and values on day 15 are after treatment; n=6/group; all treatment administered orally)
| Groups | Day 5 | Day 15 | % change (days 5-15) |
|---|---|---|---|
| I | 24.47±0.83 | 24.35±0.63 | 0.49 |
| II | 24.08±1.04 | 32.72±1.03 | −16.52* |
| III | 27.93±1.25 | 25.25±0.81 | 9.60# |
| IV | 28.8±0.17 | 28.08±2.81 | 2.50 |
| V | 27.43±1.16 | 26.32±1.75 | 4.05 |
| VI | 28.47±1.01 | 26.3±1.22 | 7.62 |
*P<0.01, #P<0.05
Table 5b.
Effects of Boswellia serrata extract on tumor necrosis factor-α in control and treatment group on day 5, and 15
| Variables | Groups | Day 5 |
Day 15 |
||
|---|---|---|---|---|---|
| Mean difference | P | Mean difference | P | ||
| TNF-α | II versus | ||||
| III | 0.15 | 0.94 | 7.46# | 0.01 | |
| IV | −0.71 | 0.82 | 3.63 | 0.08 | |
| V | 0.65 | 0.87 | 5.40 | 0.07 | |
| VI | −0.38 | 0.98 | 5.41 | 0.07 | |
#P<0.05. TNF-α: Tumor necrosis factor-α
Histopathological changes
The histopathological results of inflamed hind limb of arthritic control were compared with that of the normal control group and found that in arthritic control the synovial membrane was thickened, synovial space was reduced and edematous; the articular cartilage found to be damaged with edematous background along with dilated blood vessels; dense inflammatory cells were also present [Figures 1 and 2]. Indomethacin-(3 mg/kg) treated Group-III showed a significant low influx of inflammatory cells and revealed a reduction in pannus formation with reduced neutrophil infiltration and also showing bony spicules along with regenerating marrow tissue [Figures 3 and 4]. BSE treated Group-VI (180 mg/kg) showed reduction in inflammation, cartilage disruption, fibro-osseous proliferation, pannus formation, vascular proliferation, synovial hyperplasia, vasculitis, and fibrinoid necrosis [Figures 5 and 6].
Figure 1.
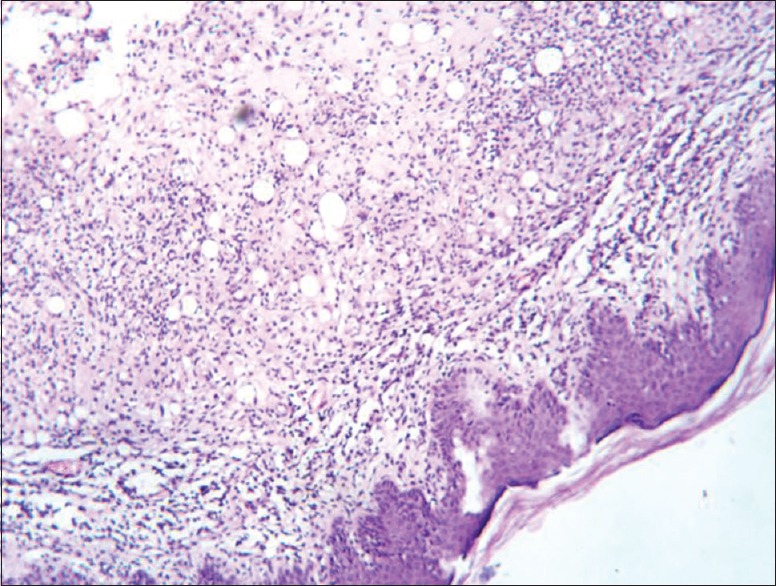
Histopathology of ankle joint cartilage in arthritis control group showing keratinized epidermis with underlying loose subepidermal zone infiltrated by chronic inflammatory cells entrapping fat cells
Figure 2.
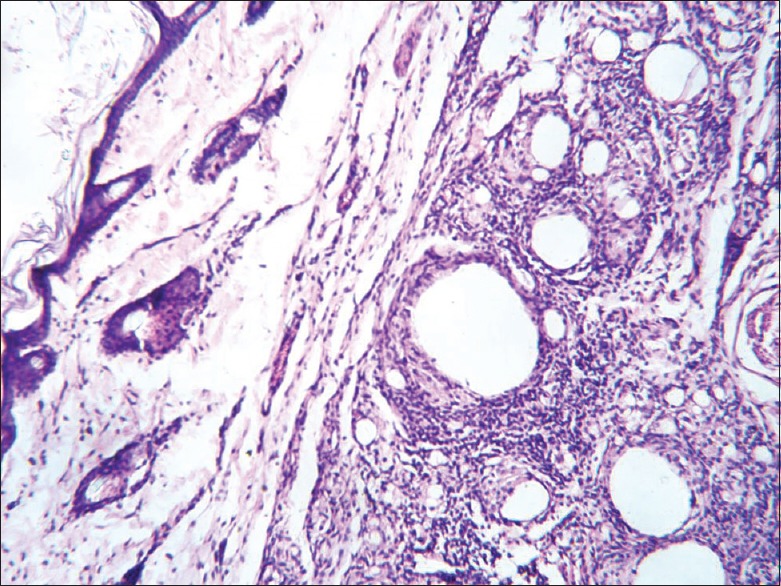
Histopathology of ankle joint bone in arthritis control group showing dense inflammation and pannus
Figure 3.
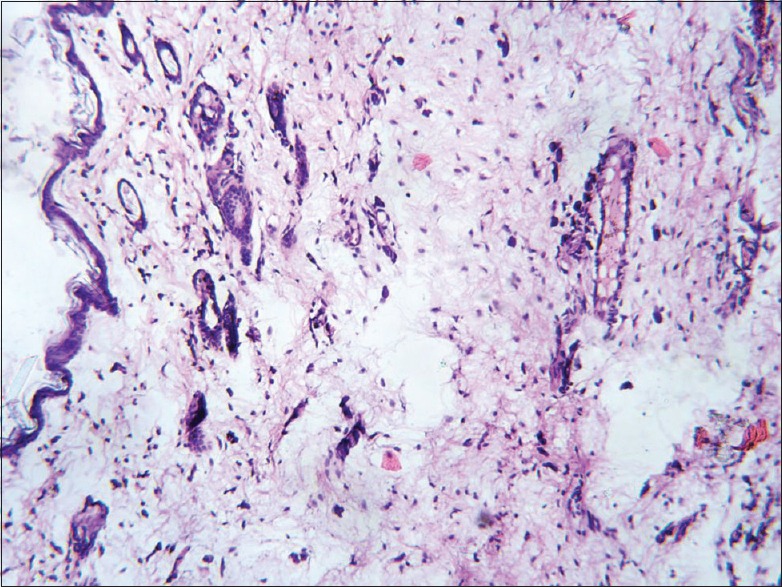
Histopathology of ankle joint in Indomethacin treated group. Response to drug seen in the form of normal thin epidermis and skin appendages
Figure 4.
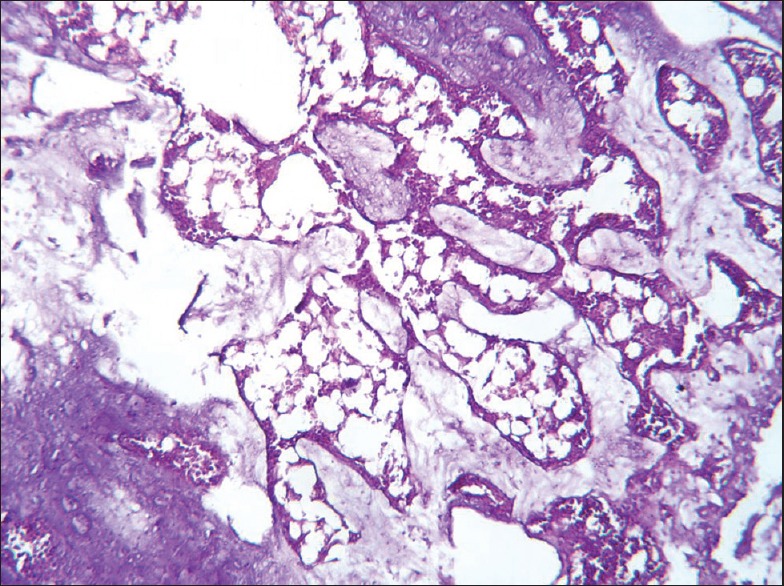
Histopathology of ankle joint bone in Indomethacin treated group showing bony spicules with regenerating marrow tissue
Figure 5.
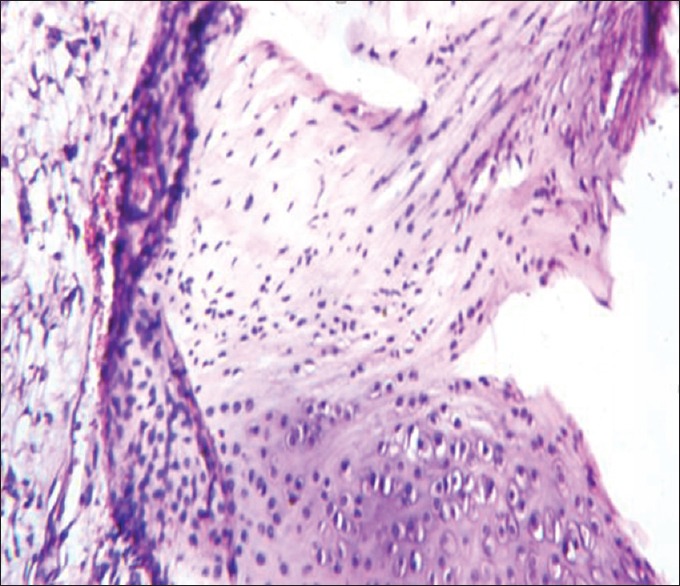
Histopathology of ankle joint cartilage in Boswellia serrata extract treated group
Figure 6.
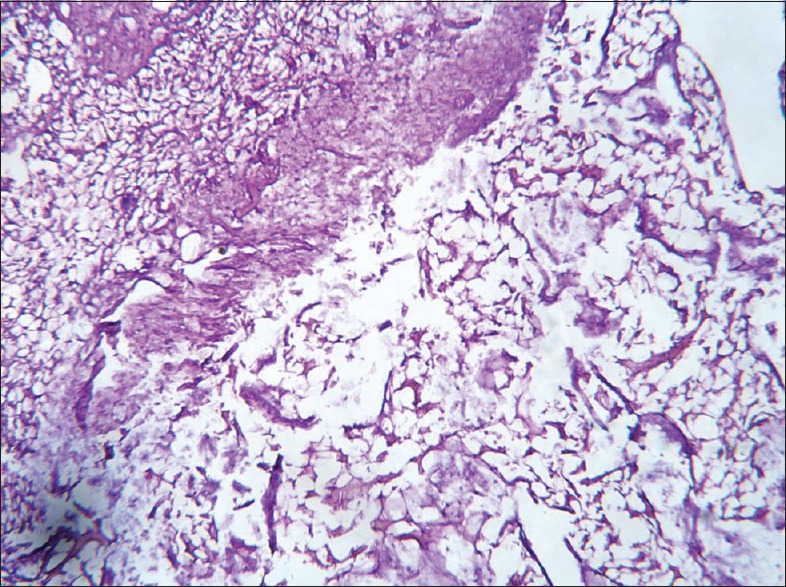
Histopathology of ankle joint bone in Boswellia serrata extract treated group. Partial effect of drug appears as bone, marrow, and connective tissue are seen regenerating
Discussion
Oleo-gum-resin is prepared from the bark of Boswellia tree found in dry deciduous forests of India, Pakistan, and Arabia.[18] BSEs consist of Boswellic acids such as 11-keto-beta-boswellic acid (KBA) and acetyl-KBA which affect the cellular defense system by alteration in the levels of different cytokines.[19] Anti-inflammatory, antioxidant, antipyretic, antiatherosclerotic, and analgesic activity of Boswellia serrata has been described in earlier studies.[7,9,20]
The symptoms of RA such as pain, stiffness, swelling, redness, and weight loss are mainly due to inflammation and immunological injury. The present study showed that BSE lessened the inflammation observed by changes in inflammatory parameters of joint-like reduction in ankle diameter, paw volume, and arthritis index. Singh and Atal also showed the anti-inflammatory effects of Boswellia serrata in carrageenan-induced rat model of inflammation.[9] Anti-inflammatory effects of BSE were further supported by our histopathological findings of the joints. BSE showed a marked reduction in cartilage disruption, fibro-osseous proliferation, pannus formation, vascular proliferation, synovial hyperplasia, vasculitis, and fibrinoid necrosis which are the hallmark of RA. Our histopathological observations are also supported by a study in which Boswellia serrata reduces cellular infiltrates in carrageenan-induced inflammation model of RA.[21] Along with other symptoms, decrease in weight also has been observed in RA. This condition has been referred to as rheumatoid cachexia (RC). RC is attributed to the increased levels of pro-inflammatory cytokines as IL-1 beta and TNF-alpha.[22,23] The present study showed that body weight improves after treatment with different doses of BSE.
However, no significant change was found on serum TNF-α levels by any dose of BSE. Only indomethacin treated group showed a significant decrease in TNF-α levels. While in other studies, BSE treatment showed a significant reduction in levels of several inflammatory mediators including TNF-α.[24,25] We were unable to find the reason for this no significant change on serum TNF-α levels by BSE in our study.
Thus, this study suggests that the antiarthritic effect of BSE on joints, bone, and cartilage in CFA-induced arthritic rats was probably mediated by anti-inflammatory action. Therefore, BSE has significant potential as a phytomedicine and might represent an alternative for classical medicine treatments for chronic inflammatory diseases like RA. We believe that our results will contribute to the clinical applications in the treatment of RA. Keeping in view the results obtained in the present study, the following conclusions may be drawn regarding the potential effectiveness of BSE against RA. An effective and dose-dependent anti-arthritic effect of BSE in three doses, i.e., 45, 90, and 180 mg/kg was clearly evident with a more pronounced effect at 180 mg/kg.
A higher dose of BSE was more effective than its lower dose by showing improvement in the body weight, reduction of the paw thickness, reduction of the ankle diameter, reduction of the paw volume, reduction of the arthritic index for acute inflammation in RA. However, it was less effective than the standard drug (Indomethacin) for acute inflammation in RA that shows good anti-inflammatory effect.
Conclusions
The present animal study has revealed that the BSE has anti-inflammatory potential and can be used as an antiarthritic drug. BSE at the dose of 180 mg/kg is more effective as compared to other BSE doses (45, 90 mg/kg), but less than standard drug (indomethacin 3 mg/kg) in RA parameters. The results of the present study are encouraging and reveal the importance of BSE as a potential anti-arthritic agent. In light of the above evidence, its efficacy should be evaluated in human subjects and the combination of BSE might have potential usefulness as an adjunct to conventional modern therapy in the further management of RA as well as in other inflammatory diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–11. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, et al. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3:360–5. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O'Fallon WM, et al. Survival in rheumatoid arthritis: A population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–8. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 4.Schaffer D, Florin T, Eagle C, Marschner I, Singh G, Grobler M, et al. Risk of serious NSAID-related gastrointestinal events during long-term exposure: A systematic review. Med J Aust. 2006;185:501–6. doi: 10.5694/j.1326-5377.2006.tb00665.x. [DOI] [PubMed] [Google Scholar]
- 5.Laine L. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin Arthritis Rheum. 2002;32:25–32. doi: 10.1053/sarh.2002.37217. [DOI] [PubMed] [Google Scholar]
- 6.Ravindran V, Rachapalli S, Choy EH. Safety of medium- to long-term glucocorticoid therapy in rheumatoid arthritis: A meta-analysis. Rheumatology (Oxford) 2009;48:807–11. doi: 10.1093/rheumatology/kep096. [DOI] [PubMed] [Google Scholar]
- 7.Pirotta M. Arthritis disease – The use of complementary therapies. Aust Fam Physician. 2010;39:638–40. [PubMed] [Google Scholar]
- 8.Elalouf O, Elkayam O. Long-term safety and efficacy of infliximab for the treatment of ankylosing spondylitis. Ther Clin Risk Manag. 2015;11:1719–26. doi: 10.2147/TCRM.S55928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh GB, Atal CK. Pharmacology of an extract of Salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18:407–12. doi: 10.1007/BF01965005. [DOI] [PubMed] [Google Scholar]
- 10.Henderson CJ, Panush RS. Diets, dietary supplements, and nutritional therapies in rheumatic diseases. Rheum Dis Clin North Am. 1999;25:937–68, i ×. doi: 10.1016/s0889-857x(05)70112-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh D, Nainwani R, Sharma T, Gautam RK. In vitro anti-inflammatory and anti-arthritic activity of hydroalcoholic extract of Pongamia pinnata (L.) Pierre seed. Int J Pharm Res Rev. 2013;2:20–5. [Google Scholar]
- 13.Augustine B, Dash S, Lahkar M, Lihite R, Samudrala PK, Pitta S. Effect of Mirabilis jalapa linn flowers in experimentally induced arthritis and consecutive oxidative stress. Int J Pharm Pharm Sci. 2013;5:190–3. [Google Scholar]
- 14.Pal R, Chaudhary MJ, Tiwari PC, Nath R, Babu S, Pant KK, et al. Pharmacological studies on the anti-inflammatory and immunomodulatory role of pentoxifylline and its interaction with nitric oxide (NO) in experimental arthritis in rats. Inflammopharmacology. 2016;24:221–31. doi: 10.1007/s10787-016-0281-4. [DOI] [PubMed] [Google Scholar]
- 15.Mishra NK, Bstia S, Mishra G, Chowdary KA, Patra S. Anti-arthritic activity of Glycyrrhiza glabra Boswellia serrata and their synergistic activity in combined formulation studied in Freund's adjuvant induced arthritic rats. J Pharm Educ Res. 2011;2:92–8. [Google Scholar]
- 16.Mico JA, Berrocoso E, Vitton O, Ladure P, Newman-Tancredi A, Bardin L, et al. Effects of milnacipran, duloxetine and indomethacin, in polyarthritic rats using the Randall-Selitto model. Behav Pharmacol. 2011;22:599–606. doi: 10.1097/FBP.0b013e328345ca4e. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Sanmiguel AB, Nieto-Bona MP, Fernandez-Galaz C, Priego T, Martin AI, Lopez-Calderon A, et al. Melanocortin-4 receptor agonist (RO27-3225) ameliorates soleus but not gastrocnemius atrophy in arthritic rats. J Physiol Pharmacol. 2017;68:191–9. [PubMed] [Google Scholar]
- 18.Ghorpade RP, Chopra A, Nikam TD. In vitro zygotic embryo germination and propagation of an endangered Boswellia serrata roxb. a source of boswellic acid. Physiol Mol Biol Plants. 2010;16:159–65. doi: 10.1007/s12298-010-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ammon HP. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine. 2010;17:862–7. doi: 10.1016/j.phymed.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J Pharm Sci. 2011;73:255–61. doi: 10.4103/0250-474X.93507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismail SM, Rao SK, Bhaskar M. Evaluation of anti-inflammatory activity of Boswellia serrata on carrageenan induced paw edema in albino wistar rats. Int J Res Med Sci. 2016;4:2980–6. [Google Scholar]
- 22.Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: Cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. 1994;93:2379–86. doi: 10.1172/JCI117244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 24.Umar S, Umar K, Sarwar AH, Khan A, Ahmad N, Ahmad S, et al. Boswellia serrata extract attenuates inflammatory mediators and oxidative stress in collagen induced arthritis. Phytomedicine. 2014;21:847–56. doi: 10.1016/j.phymed.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Gayathri B, Manjula N, Vinaykumar KS, Lakshmi BS, Balakrishnan A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int Immunopharmacol. 2007;7:473–82. doi: 10.1016/j.intimp.2006.12.003. [DOI] [PubMed] [Google Scholar]


