Abstract
Background
The AIDS Clinical Trials Group study A5353 demonstrated the efficacy and safety of dolutegravir and lamivudine for initial treatment of HIV-1 infection at week 24 in individuals with HIV-1 RNA 1000–500 000 copies/mL. Optimal ART for treatment-naive individuals must be durable.
Objectives
The aim of this study was to estimate the efficacy and safety of dolutegravir plus lamivudine at week 48 and compare the efficacy in participants with baseline HIV-1 RNA ≤100 000 copies/mL versus >100 000 copies/mL.
Methods
Virological success was defined as HIV-1 RNA <50 copies/mL by FDA Snapshot criteria. Definition of virological failure included confirmed HIV-1 RNA >200 copies/mL at week 24 or later. The proportion of participants with virological success was estimated using two-sided exact Clopper–Pearson 95% CI. Comparison between screening HIV-1 RNA (≤100 000 versus >100 000 copies/mL) strata was carried out by Fisher’s exact test. The study was registered with ClinicalTrials.gov, number NCT02582684.
Results
A total of 120 enrolled eligible participants were included in the analysis. At week 48, 102 of the 120 participants (85%; 95% CI 77%–91%) had virological success. Virological success was similar between screening HIV-1 RNA groups. Six (5%) participants had virological non-success and one additional participant experienced virological failure while on study but off study treatment. No new drug resistance mutations were observed. Six (5%) participants had study-related grade 3 or higher adverse events and none discontinued study treatment.
Conclusions
These results add to the evidence that dolutegravir plus lamivudine is a safe and effective option for initial ART in individuals with HIV-1 RNA <500 000 copies/mL.
Introduction
Optimal ART for treatment-naive individuals must be safe, effective and durable. Although three-drug regimens are standard, recent preliminary data suggest that two-drug regimens may be safe and effective.1,2 In the pilot ACTG A5353 study, dolutegravir plus lamivudine was safe and efficacious through to week 24 in treatment-naive participants with a screening plasma HIV-1 RNA of 1000–500 000 copies/mL, with similar virological responses in those with a baseline HIV-1 RNA of ≤100 000 copies/mL or >100 000 copies/mL.2 The PADDLE study, which restricted baseline HIV-1 RNA to ≤100 000 copies/mL and CD4+ T cell (CD4) count to ≥200 cells/mm3, provided preliminary evidence of the durability of dolutegravir plus lamivudine treatment through week 96.3 Here we present the durability of dolutegravir plus lamivudine, through week 48, in the population investigated in A5353.
Patients and methods
The complete A5353 study procedures are described in the week 24 primary report.2 Briefly, A5353 was a single-arm, open-label, phase II study in which treatment-naive adults with HIV-1 RNA <500 000 copies/mL and no reverse transcriptase, integrase or major protease resistance mutations, as defined by the IAS-USA HIV Drug Resistance Mutation List,4 received oral dolutegravir 50 mg and lamivudine 300 mg as separate tablets (ViiV Healthcare, Brentford, UK) once daily. There was no CD4 count eligibility criterion. Enrolment of at least 25% of participants with baseline HIV-1 RNA >100 000 copies/mL was pre-specified. During 52 weeks of follow-up, participants underwent clinical evaluations and laboratory safety, lipid, HIV-1 RNA and CD4 count measurements, as well as adherence assessments by self-reported 4 day recall.5 Real-time HIV-1 drug resistance testing was performed in participants with confirmed virological failure along with retrospective testing of dolutegravir plasma levels if the viral failure occurred on study treatment.
This report focuses on the secondary study objectives of virological success, safety assessments, and changes in CD4 count and serum lipids at week 48. Virological success was defined as HIV-1 RNA <50 copies/mL by FDA Snapshot criteria. Protocol-defined virological failure was confirmed HIV-1 RNA >400 copies/mL at week 16 or 20, or >200 copies/mL at week 24 or later. The proportion of participants with virological success was estimated using two-sided exact Clopper–Pearson 95% CI. Comparison between baseline HIV-1 RNA strata (≤100 000 versus >100 000 copies/mL) was carried out by Fisher’s exact test. All P values were nominal, with no adjustment for multiple comparisons. All analyses were carried out using Statistical Analysis System (SAS), version 9.4 (SAS Institute, Cary, NC, USA).
Ethics
The local institutional review boards approved the study at each of the sites, and each participant provided written informed consent. The study was registered with ClinicalTrials.gov, number NCT02582684.
Results
The study enrolled 120 eligible participants, 87% were male with a median age of 30 years (IQR 24–41), and was racially diverse (40% black and 27% Latino). The median (IQR) baseline HIV-1 RNA was 4.61 (3.94–5.05) log10 copies/mL and 37 (31%) participants had baseline HIV-1 RNA >100 000 copies/mL. The median (IQR) CD4 count was 387 (288–596) cells/mm3. One hundred (83%) participants completed study treatment per protocol whereas 20 (17%) prematurely discontinued the study treatment owing to inability to attend the clinic visits (n = 8), loss to follow-up (n = 7), non-compliance with treatment (n = 3), pregnancy (n = 1) or virological failure (n = 1).
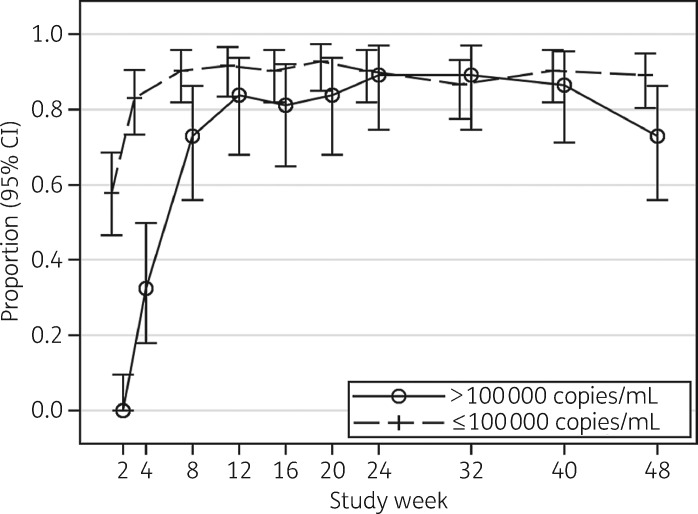
At week 48, 102 of the 120 participants (85%; 95% CI 77%–91%) had virological success by FDA Snapshot (Table 1). Virological success by baseline HIV-1 RNA stratum was similar with 29/37 participants (78%; 95% CI 62%–90%) in the >100 000 copies/mL stratum and 73/83 participants (88%; 95% CI 79%–94%) in the ≤100 000 copies/mL stratum achieving HIV-1 RNA <50 copies/mL (P = 0.18). Using an ITT missing/off treatment = failure approach (ITT-F), 101 of the 120 participants (84%; 95% CI 76%–90%) achieved HIV-1 RNA <50 copies/mL with no significant differences between the two baseline HIV-1 RNA strata (Figure 1). Using an as-treated approach, 101 of the 104 (97%; 95% CI 92%–99%) achieved HIV-1 RNA <50 copies/mL, similarly with no significant differences between the HIV-1 RNA strata. At every visit, 87% or more of participants reported no missed doses in the four preceding days.
Table 1.
Virological outcomes by US FDA Snapshot at week 48
| Baseline HIV-1 RNA (copies/mL) |
|||
|---|---|---|---|
| >100 000 (N = 37) | ≤100 000 (N = 83) | Total (N = 120) | |
| Virological successa, n (%) | 29 (78) | 73 (88) | 102 (85) |
| Virological successa, 95% CI | 62%–90% | 79%–94% | 77%–91% |
| Virological non-success, n (%) | 3 (8) | 3 (4) | 6 (5) |
| HIV-1 RNA ≥50 copies/mL | 1 | 1 | 2 |
| Discontinued for lack of efficacy; HIV-1 RNA ≥50 copies/mL | 1 | 0 | 1 |
| Discontinued study treatment for other reasonsb while HIV-1 RNA ≥50 copies/mL | 1 | 2 | 3 |
| No virological data in window, n (%) | 5 (14) | 7 (8) | 12 (10) |
| On study but missing data in window | 3 | 0 | 3 |
| Discontinued study treatment for other reasonsc | 2 | 7 | 9 |
The confidence intervals in the table are exact binomial 95% CIs.
HIV-1 RNA <50 copies/mL.
Lost to follow-up, poor adherence.
Lost to follow-up, pregnancy.
Figure 1.
Proportion (95% CI) of participants with HIV-1 RNA <50 copies/mL by week (ITT/missing = failure).
Six (5%) participants had virological non-success (HIV-1 RNA ≥50 copies/mL) at week 48. Two of these participants were on study treatment, one discontinued study treatment early and three were lost to follow-up/deemed non-adherent by site investigators. All of these participants had HIV-1 RNA <50 copies/mL at one or more timepoints in the first 20 weeks of treatment, prior to virological rebound. Twelve (10%) participants did not have virological data during the week 48 visit evaluation window. Three of these participants had missing data and nine had discontinued study treatment due to loss to follow-up or pregnancy, all with HIV-1 RNA <50 copies/mL at the last available timepoint. In addition to the three participants who experienced protocol-defined virological failure by week 24,2 one participant had virological failure after week 24. This participant achieved HIV-1 RNA <50 copies/mL by week 8 and was found to be pregnant at study week 20 (∼5 weeks post-conception). The participant’s HIV-1 RNA was <50 copies/mL and she was switched to raltegravir and tenofovir disoproxil fumarate/emtricitabine. The participant was non-adherent to the new regimen and virological failure occurred at study week 33 with an HIV-1 RNA of 5459 copies/mL. No reverse transcriptase or integrase region resistance mutations were detected. She remained viraemic through the remainder of her pregnancy despite further ART modification, and delivered at 37 weeks, via elective caesarean section, an HIV-negative girl with an ectopic kidney.
Sixteen (13.3%) participants experienced Grade 3 or greater adverse events which the site investigator categorized as related or possibly related to study treatment in 6 (5%) participants: change in creatinine clearance (n = 2), and one each of angio-oedema, paraesthesia, palpitations and suicidal ideation. There was no premature discontinuation of study treatment or study follow-up due to an adverse event.
Among all participants, the median CD4 count increased by 182 (IQR 104–284) cells/mm3 whereas the median calculated creatinine clearance decreased by 10 (IQR −18 to 2) mL/min. The median changes in lipids were total cholesterol +10 (IQR −6 to 24) mg/dL, LDL cholesterol +3.5 (IQR −11 to 6) mg/dL and HDL cholesterol +6 (IQR 1–11) mg/dL, whereas there was no change in triglyceride levels (0, IQR −27 to 26 mg/dL).
Discussion
Although dolutegravir plus lamivudine demonstrated promising safety and efficacy at week 24 in ART-naive participants with screening plasma HIV RNA <500 000 copies/mL,2 week 48 is the standard timepoint for comparative evaluation of the safety, efficacy and durability of initial ART. Thus at week 48, virological success (HIV-1 RNA <50 copies/mL by the FDA Snapshot criterion) was achieved in 85% of participants treated with dolutegravir plus lamivudine, with no significant differences between those whose pre-treatment HIV-1 RNA was above or below 100 000 copies/mL. These results were consistent in ITT, missing/off treatment = failure and as-treated analyses. These results were similar to the findings of larger, fully powered, randomized clinical trials. The week 48 efficacy of the US Department of Health and Human Services guideline-recommended bictegravir-, dolutegravir-, elvitegravir/cobicistat- or raltegravir-based three-drug regimens ranged from 86.1% to 92.4%.6–12
It is reassuring that only one participant had virological failure between weeks 24 and 48 on dolutegravir plus lamivudine in this study. The participant was off dolutegravir plus lamivudine due to pregnancy and was on raltegravir-based three-drug therapy at the time of virological failure. There were no failures with HIV-1 drug resistance between week 24 and week 48. At week 24, as previously reported, one participant developed resistance mutations to the study drugs (R263R/K and M184V),2 and additional virological analyses to evaluate minority variants and phenotyping are currently underway. Dolutegravir plus lamivudine was generally safe and well tolerated in this pilot study. No neural tube defects occurred in the baby that was exposed to dolutegravir in the first 5 weeks of pregnancy and, based on data from the Antiretroviral Pregnancy Registry, the renal defect was unlikely to be related to study treatment.13–15
Our results are limited by the relatively small sample size, the lack of a comparator arm, short follow-up period and enrolment of few women and few participants with a CD4 count <200 cells/mm3. Nevertheless, our results agree with the pooled analysis of the ongoing fully powered phase III clinical trials, GEMINI 1 and 2. In the GEMINI studies, dolutegravir plus lamivudine was non-inferior to dolutegravir plus tenofovir disoproxil fumarate/emtricitabine at week 48 by FDA Snapshot; virological success 91% versus 93%; treatment difference −1.7% (95% CI −4.4% to 1.1%).16 Similar to our study, women and individuals with CD4 counts <200 cells/mm3 were under-represented in the GEMINI studies. Among participants with CD4 counts <200 cells/mm3, viral suppression by FDA Snapshot was numerically lower with dolutegravir plus lamivudine, but was comparable between arms when failure was defined as treatment-related discontinuation. Follow-up through week 144 is planned. Like other tenofovir-free regimens, dolutegravir plus lamivudine is insufficient treatment in individuals co-infected with HBV. Additionally, the efficacy of dolutegravir plus lamivudine when administered with rifampicin in TB co-infection is unknown, and pre-treatment resistance testing is required, which will constrain its use in resource-limited settings and for same-day ART initiation.
In conclusion, the week 48 results of A5353 add to the evidence that dolutegravir plus lamivudine may be a safe and effective option for initial ART, with lower antiretroviral exposure than three-drug alternatives.
Acknowledgements
We thank the study participants. We acknowledge the contributions of the staff at the sites and grants supporting their work, including Mark Mall, RN, and Antoinette Lewis, MPA, at Rush University Medical Center Clinical Research Site (CRS) (site 2702; grant U01 AI06947; Cornelius N. Van Dam and Kimberly Epperson, RN, at Greensboro CRS (site 3203; grant 5UM1A069423-11); Johnny Perez and Nina Lambert at Northwestern University CRS (site 2701; grant 2UM1 AI069471); Roberto C. Arduino and Aristoteles Villamil at Houston AIDS Research Team (site 31473; grants 5UM1 AI069503-11 and 2UM1 AI068636-11); Cathi Basler and Christine Griesmer at University of Colorado Hospital CRS (site 6101; grants 2UM1AI069432 and UL1 TR001082); Margaret A. Fischl, MD, and Hector Bolivar, MD, at University of Miami AIDS Clinical Research Unit (site 901; grants AI069477 and 5UM1AI069477); Roger Bedimo, MD, and Lauren Rogers at Trinity Health and Wellness Center (site 31443; grant U01 AI069471); Becky Straub, BSN, MPH, and Chris Evans, MSN, at Chapel Hill CRS [site 3201; grants UM1 AI069423, Clinical and Translational Science Award (CTSA) 1UL1TR001111, Center for AIDS Research (CFAR) P30 AI50410]; Beverly Woodward, MSNRN, and Michael Leonard at Vanderbilt Therapeutics (site 3652; grants AI069439 and TR002243); Susan Koletar, MD, and Leah Kofmehl at Ohio State University (site 2301; grant UM1AI06949); Teresa Spitz and Lisa Kessels at Washington University Therapeutics CRS (site 2101; grant 5UM1AI069439-10); Helen Patterson, LPN, and Karen Tashima, MD, at the Miriam Hospital (site 2951; grant AI069412); Todd Stroberg and Tiina Ilmet at Weill Cornell Chelsea CRS [site 7804; grants UM1AI069419 and UL1TR000457 (Clinical and Translational Science Center)]; Phillip Dube, MD, and Frances Canchola, RN, at University of Southern California CRS (site 1201; grant 2UM1AI069432); Jorge L. Santana, MD, FIDSA, and Sigrid Perez, MD, at Puerto Rico AIDS Clinical Trails Unit CRS (site 5401; grant 5 UM1 AI069415-12); Paul Sax, MD, and Cheryl Keenan, RN, BC, at Brigham and Women’s Hospital (site 107; grant 5UM1AI068636); Eva Whitehead, RN, and Carl J. Fichtenbaum, MD, at Cincinnati CRS (site 2401; grant AI69439); Michael T. Yin, MD, MS, and Jolene Noel-Connor, RN, at Columbia Physicians and Surgeons CRS [site 30329; ACTG Clinical Trials Unit (CTU)], grants 2UM1-AI069470 and CTSA UL1RR024156]; Mary Adams, RN, MPH, and Elizabeth Keller, RN, at University of Rochester (site 31787; grant UM1 AI069511); Carlos Del Rio, MD, and Ericka R Patrick, RN, MSN, at Emory-Centers for Disease Control and Prevention Ponce de Leon (site 5802; grant 1U01AI069418-01 and Emory University CFAR P30AI050409); Eric Daar and Ruben Lopez at Harbor–University of California–Los Angeles (UCLA) Medical Center (site 603; grants A1069424 and UL1TR000124); Pablo Tebas, MD, and Yan Jiang, MSN, at Philadelphia HIV Therapeutics and Prevention CTU (site 6201; grants UM1AI068636 and UM1AI069534); Dee Dee Pacheco and Constance Benson, MD, at University of California–San Diego (site 701; grant AI069432); Valery Hughes and Brian Mangano at Weill Cornell Uptown CRS (site 7803; grants U01 AI69419 and UL1 TR000457); Teri Flynn, ANP, and Amy Sbrolla, RN, at Massachusetts General Hospital (site 101; grant AI069412); Raphael Landovitz, MD, and Sana Majid at UCLA Care Center CRS (site 601; grants AI069424, UCLA CTSI UL-1TR000124 and CFAR P30-AI028697).
Other members of the ACTG A5353 Study Team
Elizabeth Hawkins, Johnstone Kumwenda, Angel Hernandez, Belinda Ha, Bernadette Jarocki, Gerald Tegha and Tanisha Sullivan.
Funding
This work was supported by the Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants UM1 AI068634, UM1 AI068636 and UM1 AI106701).
Transparency declarations
C. L. W. received honoraria from Abbott Molecular and Mylan. E. P. A. reports non-financial support from ViiV. P. E. S. has served as a paid consultant to Gilead, Abbvie, Merck, Janssen, Glaxo-SmithKline/ViiV and Bristol-Myers Squibb. K. Y. S. is employed by ViiV Healthcare. C. N. V. D. served as a paid consultant/speaker to ViiV Healthcare, Gilead and Janssen. B. O. T. served as a paid consultant to GlaxoSmithKline/ViiV, Gilead and Janssen, and received research funding through Northwestern University from GlaxoSmithKline/ViiV. All remaining authors have none to declare.
Disclaimer
This paper was written by C. G. in her capacity as an NIH employee, but the views expressed in this paper do not necessarily represent those of the NIH.
Contributor Information
ACTG A5353 Study Team:
Elizabeth Hawkins, Johnstone Kumwenda, Angel Hernandez, Belinda Ha, Bernadette Jarocki, Gerald Tegha, and Tanisha Sullivan
References
- 1. Cahn P, Rolón MJ, Figueroa MI. et al. Dolutegravir–lamivudine as initial therapy in HIV-1 infected, ARV-naive patients, 48-week results of the PADDLE (Pilot Antiretroviral Design with Dolutegravir LamivudinE) study. J Int AIDS Soc 2017; 20: 21678.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taiwo BO, Zheng L, Stefanescu A. et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66: 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Figueroa MI, Rolón MJ, Patterson P. et al. Dolutegravir–lamivudine as initial therapy in HIV-infected, ARV naive patients: 96 week results of the PADDLE trial In: Abstracts of Ninth International AIDS Society Conference on HIV Science, Paris, France ,2017. Abstract MOPEB0287. International AIDS Society, Geneva, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wensing AM, Calvez V, Günthard HF. et al. Special contribution 2014 update of the drug resistance mutations in HIV-1. Top Antivir Med 2014; 22: 642–50. [PMC free article] [PubMed] [Google Scholar]
- 5. Chesney MA, Ickovics JR, Chambers DB. et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 2000; 12: 255–66. [DOI] [PubMed] [Google Scholar]
- 6. Gallant J, Lazzarin A, Mills A. et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017; 390: 2063–72. [DOI] [PubMed] [Google Scholar]
- 7. Sax PE, Pozniak A, Montes ML. et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390: 2073–82. [DOI] [PubMed] [Google Scholar]
- 8. Walmsley SL, Antela A, Clumeck N. et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369: 1807–18. [DOI] [PubMed] [Google Scholar]
- 9. DeJesus E, Rockstroh JK, Henry K. et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012; 379: 2429–38. [DOI] [PubMed] [Google Scholar]
- 10. Clotet B, Feinberg J, van Lunzen J. et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383: 2222–31. [DOI] [PubMed] [Google Scholar]
- 11. Lennox JL, DeJesus E, Lazzarin A. et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374: 796–806. [DOI] [PubMed] [Google Scholar]
- 12. Sax PE, DeJesus E, Mills A. et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012; 379: 2439–48. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA to Evaluate Potential Risk of Neural Tube Birth Defects with HIV Medicine Dolutegravir (Juluca, Tivicay, Triumeq) https://www.fda.gov/Drugs/DrugSafety/ucm608112.htm.
- 14. Zash R, Holmes L, Makhema J. et al. Surveillance for neural tube defects following antiretroviral exposure from conception, the Tsepamo study (Botswana) In: Symposium Session: Safety of Dolutegravir in Pregnancy. Twenty-second International AIDS Conference, Amsterdam, The Netherlands ,2018. Session TUSY15. International AIDS Society, Geneva, Switzerland. [Google Scholar]
- 15.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry Interim Report for 1 January 1989 through 31 January2018. www.APRegistry.com.
- 16. Cahn P, Madero JS, Arribas JR. et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2018; doi:10.1016/S0140-6736(18)32462-0. [DOI] [PubMed] [Google Scholar]