Abstract
Background
Chronic bacterial prostatitis (CBP) is a difficult-to-treat infection as only a few antibiotics achieve therapeutic concentrations in the prostate. Data on the efficacy and safety of oral fosfomycin for the treatment of CBP are limited.
Objectives
To analyse the efficacy and safety of fosfomycin in CBP due to MDR pathogens.
Methods
In a prospective observational study, an oral regimen of 3 g of fosfomycin q24h for 1 week followed by 3 g q48h for a treatment duration of 6–12 weeks was administered. The outcome was clinical and microbiological cure rate at the end of treatment (EOT) and rate of relapse at 3 and 6 months.
Results
The study included 44 patients. The most common pathogen was Escherichia coli (66%), followed by Klebsiella spp. (14%) and Enterococcus faecalis (14%). Most strains were MDR (59%) and 23% had an ESBL phenotype; 33 of 44 strains were resistant to fluoroquinolones, but all were susceptible to fosfomycin (median MIC for Gram-negative pathogens 1.5 mg/L). In 25 patients, treatment was administered for 6 weeks, whereas in the remaining 19 patients it was prolonged to 12 weeks based on the presence of calcifications in the prostate. Cure rate was 82% at EOT and 80% and 73% at 3 and 6 months accordingly. Microbiological eradication was achieved in 86% and 77% at EOT and at 6 months, respectively. Failure was observed in 12 patients. The most common adverse event was diarrhoea (18%).
Conclusions
Oral fosfomycin, particularly in the era of MDR prevalence, represents an attractive, safe and effective alternative to fluoroquinolones for the treatment of CBP.
Introduction
Chronic bacterial prostatitis (CBP) is a difficult-to-treat infection as only few oral antibiotics are able to distribute to the prostatic tissue and achieve sufficient concentrations at the site of infection.1 According to the US NIH consensus definition, category II CBP occurs when patients experience recurrent symptomatic episodes of urinary tract infection caused by the same organism, with the most common pathogen being Escherichia coli followed by other Gram-negative organisms (i.e. Klebsiella spp., Proteus spp., Pseudomonas spp.) or Enterococcus faecalis.2
Fluoroquinolones are considered the cornerstone of treatment of CBP due to their in vitro activity and advantageous pharmacokinetics in prostatic tissue.3,4 International recommendations and guidelines indicate a 6–12 week course.5 However, urinary and prostatic infections due to MDR Gram-negative Enterobacteriaceae are steeply increasing worldwide, even in the community, with the prevalence of resistance of community uropathogens to fluoroquinolones >10% in many countries,6–8 as well as the presence of ESBL-producing bacteria,6 rendering therapeutic options even more limited.9
The evolving changes in resistance have a serious impact on treatment options and alternative antimicrobial treatment is needed.9 Trimethoprim, combined or not with sulfamethoxazole, has been one of the most prescribed alternative drugs for the treatment of CBP caused by traditional pathogens, but, due to high resistance rates in the community and subsequently low eradication rates achieved, this drug is now indicated as a last-line agent.10
Recently, researchers have shown an increased interest in fosfomycin tromethamine, an oral phosphonic acid derivative that was first identified and reported from various strains of Streptomyces spp. in 1969. Fosfomycin is an antimicrobial class of its own and is structurally unrelated to any other agent currently approved for clinical use.11 Formally, it is an antibiotic traditionally utilized for urinary tract infections in women.12 However, fosfomycin is a broad-spectrum antibiotic with bactericidal activity against Gram-negative and -positive bacteria that has been gaining considerable attention due to its effectiveness as a treatment for MDR pathogens13 and has been reported to achieve acceptable intraprostatic concentrations in the uninflamed prostate.14
The purpose of the present study was to evaluate the effectiveness and safety of oral fosfomycin in the treatment of CBP. The current study presents a novel and alternative therapeutic approach for the treatment of CBP in the era of MDR prevalence evaluating a 6 week as well as prolonged 12 week treatment.
Patients and methods
Subjects
This was a prospective observational study that included patients with CBP referred to the Outpatient ID Clinic of Hygeia General Hospital, Athens, Greece from September 2014 to March 2018. Every patient was evaluated by a member of the Infectious Diseases Unit at the beginning of treatment and during the follow-up. Patients were characterized as having CBP, if they satisfied the following criteria: (i) voiding symptoms (irritative or obstructive) and/or pain (genitourinary, pelvic or rectal) for >3 months to coordinate with the NIH classification of prostatitis syndromes;2 (ii) positive urine culture (colony count of ≥104 cfu/mL was considered positive)15 or a positive Meares–Stamey 4-glass procedure conducted by the urologist [Meares–Stamey procedure was considered positive when pathogens from expressed prostatic secretions or post-prostatic massage voided urine (VB3) grew exclusively or at a level 10-fold higher than in urethral and bladder samples (VB1 and VB2)];16,17 (iii) negative multiplex-PCR for sexually transmitted diseases on a urine or urethral swab;1,5 (iv) transrectal ultrasound (TRUS) and/or an MRI of the prostate indicating signs of inflammation;5,18,19 and (v) no active in vitro antimicrobials with intraprostatic penetration (i.e. fluoroquinolones, minocycline or trimethoprim/sulfamethoxazole) were available according to susceptibility testing, or treatment failure or recurrence or adverse events with other potentially active antimicrobials had occurred. Patients with a prostatic abscess were excluded. For each patient, the following data were recorded: demographic characteristics, comorbidities, clinical symptoms, previous therapeutic treatments for CBP, the isolated pathogens and their susceptibility profiles as well as toxicity. Follow-up clinical evaluation and urine cultures were performed at the end of treatment or in case of relapse and at 3 and 6 months, whereas TRUS and/or MRI of the prostate were repeated at 6 weeks of therapy.
Ethics
The study protocol was approved by the Ethics Committee of Hygeia General Hospital (registration no. 590/18-06-2104). All included patients gave their written consent for their participation.
Fosfomycin administration
Oral fosfomycin (as fosfomycin trometamol; Vocate Pharmaceutical SA, Athens, Greece) was administered once daily at a dosage of 3 g for the first week of treatment to be followed by 3 g q48h for the remaining duration of treatment. The patient was advised to dissolve the contents of the sachet in a glass of water and take this immediately on an empty stomach, before bedtime after emptying the bladder.20 The duration of treatment was 6 weeks, whereas treatment was prolonged to 12 weeks in patients with the presence of calcifications in the prostate.
Microbiological methods
Strains were identified and antimicrobial susceptibilities were determined using an automated system (Vitek 2; bioMérieux, Marcy-l’Étoile, France). Detection of ESBL was achieved using CHROMagar™ ESBL chromogenic medium (bioMérieux) and was confirmed based on the double-disc synergy test and disc diffusion with third-generation cephalosporins alone and in combination with clavulanic acid.21 MICs of fosfomycin were also evaluated with the Etest (bioMérieux), in accordance with the manufacturer’s instructions. Results were interpreted in accordance with CLSI (regarding lower urinary tract infections, as breakpoints for other body sites have not been reported) as follows: MIC ≤64 mg/L, susceptible (S); MIC 128 mg/L, intermediate (I); and MIC ≥256 mg/L, resistant (R).21 MDR was defined as non-susceptibility to at least one agent in three or more antimicrobial categories.22 In patients presenting with diarrhoea, a two-step algorithm utilizing an enzyme immunoassay in stool samples detecting glutamate dehydrogenase and toxin A/B (R-Biopharm AG, Darmstadt, Germany) was performed.23
Outcome
The primary endpoint was cure at the end of therapy and rate of relapse at 3 and 6 months of follow-up. Cure was defined as meeting the criteria for both clinical and microbiological cure. Clinical cure was determined when all signs of infections (clinical and imaging) resolved or improved during therapy, at the end of treatment and at follow-up. Clinical failure was defined whenever the clinical symptoms persisted or relapsed during therapy or follow-up and signs of inflammation required TRUS or MRI. Microbiological cure was defined as eradication of the original causative organism from the Meares–Stamey procedure or from urine culture at the end of treatment and at follow-up, whereas microbiological failure was determined whenever positive urine cultures were observed during treatment or follow-up. Relapse was defined when the same causative pathogen was identified during treatment or follow-up, whereas reinfection was defined as when a different bacterium was isolated. Discontinuation, defined as termination of treatment due to adverse events, was categorized as clinical failure.
Results
A total of 44 patients were included during the study period. Clinical and demographic characteristics of the patients are shown in Table 1. The median age was 54 years (range: 28–82 years) and diabetes mellitus and benign prostatic hyperplasia were present in 7% and 34% of patients, respectively. The majority of patients (86%) had experienced previous episodes of CBP (median: 2 episodes, range: 0–5).
Table 1.
Characteristics of patients with CBP treated with fosfomycina
| Fosfomycin treatment duration |
|||
|---|---|---|---|
| Characteristics | All patients (N = 44) | 6 weeks (N = 25) | 12 weeks (N = 19) |
| Comorbidities | |||
| age, years, mean ± SD | 53±14.7 | 53±14.4 | 53±15 |
| diabetes | 3 (7) | 2 (8) | 1 (5) |
| benign prostatic hyperplasia | 15 (34) | 8 (32) | 7 (37) |
| prior manipulation of urinary tact | 6 (14) | 3 (12) | 3 (16) |
| episodes of prostatitis prior to current treatment, median (range) | 2 (0–5) | 3 (0–4) | 2 (0–5) |
| prior use of fluoroquinolones | 24 (55) | 17 (68) | 7 (37) |
| Symptoms | |||
| pain (in the perineum, lower abdomen, testicles, penis) | 35 (80) | 19 (76) | 16 (84) |
| dysuria | 21 (48) | 14 (56) | 7 (37) |
| frequency | 16 (36) | 5 (20) | 11 (58) |
| bladder outlet obstruction | 7 (16) | 4 (16) | 3 (16) |
| Diagnosis | |||
| TRUS of prostate | 31 (70) | 18 (72) | 13 (68) |
| MRI of prostate | 26 (59) | 15 (60) | 11 (58) |
| both | 13 (30) | 8 (32) | 5 (26) |
| Treatment | |||
| α-adrenergic antagonists | 18 (41) | 11 (44) | 7 (37) |
| duration, days, median (range) | 45 (15–120) | 45 (15–46) | 90 (90–120) |
Values shown are n (%) unless specified otherwise.
According to the microbiological profile, the most common pathogen was E. coli (66%), followed by Klebsiella spp. (14%) and E. faecalis (14%), whereas a minority of isolates were Proteus mirabilis and Pseudomonas aeruginosa (Table 2). Most strains were MDR (59%) and 23% had an ESBL phenotype. Resistance to fluoroquinolones was found in 33 of 44 strains, whereas 65% of strains were resistant to trimethoprim/sulfamethoxazole, but all were susceptible to fosfomycin. The median MIC of fosfomycin was 1.5 mg/L (range: 0.125–32 mg/L) for Gram-negative pathogens and 8 mg/L (range: 4–24 mg/L) for E. faecalis.
Table 2.
Pathogens isolated from urine cultures and expressed prostate secretions and resistance rates to antimicrobials that penetrate the prostate
| Fosfomycin treatment duration |
||||
|---|---|---|---|---|
| Pathogens | All patients (N = 44) | 6 weeks (N = 25) | 12 weeks (N = 19) | Fosfomycin MICa, mg/L, median (range) |
| Prevalence, n (%) | ||||
| E. coli | 29 (66) | 17 (68) | 12 (63) | 1 (0.125–16) |
| Klebsiella oxytoca | 3 (7) | 3 (12) | 0 (0) | 10 (4–32) |
| K. pneumoniae | 3 (7) | 0 (0) | 3 (16) | 10 (4–32) |
| P. mirabilis | 2 (5) | 2 (8) | 0 (0) | 6 (4–8) |
| P. aeruginosa | 1 (2) | 0 (0) | 1 (5) | 32 (32) |
| E. faecalis | 6 (14) | 3 (12) | 3 (16) | 8 (4–24) |
| Resistance rates, n/N (%) | ||||
| fluoroquinolone resistant | 33/44 (75) | 17/25 (68) | 16/19 (84) | |
| SXT resistant | 24/37 (65) | 13/22 (59) | 11/15 (73) | |
| MDR | 26/44 (59) | 15/25 (60) | 11/19 (58) | |
| ESBL positive | 10/44 (23) | 5/25 (20) | 5/19 (26) | |
SXT, trimethoprim/sulfamethoxazole.
Fosfomycin MIC determined by Etest.
Regarding the diagnostic procedure, the causative bacteria of CBP was identified with the gold-standard Meares–Stamey examination in 19 cases and in the remaining 25 cases with urine cultures. During follow-up, all patients were re-evaluated with urine cultures at 6 and 12 weeks (with the exception of five patients who underwent a second Meares–Stamey procedure) as well as at 3 and 6 months.
As far as imaging findings are concerned, TRUS was conducted in 31 cases, MRI of the prostate in 26 and both in 13 at initiation of treatment. The most common imaging findings were high-density and mid-range echoes, echolucent zones, capsular irregularity and thickening, ejaculatory duct echoes and periurethral zone irregularity as well as signs of calcifications, which were identified in 22 cases.
In all patients oral fosfomycin was administered as monotherapy at a dose scheme of 3 g once daily for the first week followed by 3 g every 48 h for the rest of therapy. In four patients, the dosage interval was extended to q72h due to the adverse effect of diarrhoea, whereas in one patient treatment discontinuation was obligatory due to severe diarrhoea. In 25 patients, treatment was administered for a median of 42 days, whereas in the remaining patients, therapy was extended for a median of 90 days. Fosfomycin was also administered in 11 cases in which fluoroquinolone susceptibility was documented, due to previous treatment failure or recurrence with fluoroquinolone treatment (four cases) or reported adverse events with previous administration of fluoroquinolones, mainly tendinopathy (seven cases).
Cure at the end of therapy was achieved in 36 of 44 (82%) of the study population, whereas cure at the 6 and 12 week duration of therapy was accomplished in 21 of 25 (84%) and in 15 of 19 (79%), respectively. Clinical and microbiological cure were achieved in 84% (37 of 44) and 86% (38 of 44) of patients at the end of treatment accordingly. It must be pointed out that patients treated with prolongation of the dosage intervals to q72h were all cured. It is interesting to note that failure at the end of treatment was observed in eight cases in this study (four in each treatment group). Regarding the 6 week treatment group, in two patients there was persistence of clinical symptoms and isolation of a fosfomycin-resistant strain and they were finally treated with a fluoroquinolone; in one patient there was reappearance of E. coli susceptible to fosfomycin and due to clinical symptoms the patient was retreated with fosfomycin with no relapse after 3 or 6 months; one patient discontinued treatment due to adverse effects and was categorized as failure. In the 12 week treatment group, in three patients a fosfomycin-resistant strain was detected during therapy in urine culture or at the end of treatment and therapy was reconsidered, whereas in one patient, clinical symptoms reappeared with no microbiological documentation at the end of therapy. Cure at 3 and 6 months follow-up was achieved in 35 of 44 (80%) and 32 of 44 (73%) accordingly. Clinical and microbiological cure were achieved in 80% (35 of 44) for both parameters at 3 months and 80% (35 of 44) and 77% (34 of 44) at 6 months follow-up, respectively. One patient was considered as a failure at follow-up at 3 months with a fosfomycin-susceptible strain, whereas recurrence of infection was observed in three patients after 6 months follow-up. In one patient, clinical symptoms reappeared with no microbiological evidence of infection, whereas in two patients a fosfomycin-susceptible strain was re-isolated in urine cultures. True or possible relapse was observed in seven cases, whereas in the remaining five cases, reinfection was detected (Table 3).
Table 3.
Cases of clinical and/or microbiological failure of patients with CBP treated with fosfomycin
| Susceptibility |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no. | Age (years) | Comorbidities | No. of previous episodes | Previous treatment | Pathogen from EPS or urine (FOF MIC)a | CIP | SXT | Duration of FOF (days) | Clinical failure | Microbiological failure | Pathogen at time of relapse (S/R, FOF MIC)a | Comment/ treatment of relapse |
| During therapy or end of treatment | ||||||||||||
| 1 | 67 | DM, BPH | 1 | ETP | E. coli (0.75) | R | R | 45 | yes | yes | K. pneumoniae (R, >256) | CIP for 6 weeks |
| 2 | 52 | none | 3 | CIP | E. coli (0.5) | R | R | 21 | yes | no | sterile | discontinuation of FOF due to adverse effect |
| 3 | 70 | liver cancer | 1 | CIP | E. coli (16) | S | R | 45 | yes | yes | E. coli (R, >256) | LVX for 6 weeks |
| 4 | 55 | none | 0 | none | E. coli (0.75) | R | R | 45 | yes | yes | E. coli (S, 0.75) | FOF for 6 weeks; no relapse at 3 months and 6 months |
| 5 | 50 | BPH | 0 | none | P. aeruginosa (32) | R | R | 21 | yes | yes | K. pneumoniae (R, >256) | LVX for 6 weeks |
| 6 | 50 | BPH | 4 | CIP | E. coli (1.5) | S | R | 90 | yes | no | sterile | NSAIDs |
| 7 | 59 | none | 1 | CIP | K. pneumoniae (16) | R | R | 60 | yes | yes | K. pneumoniae (R, >256) | MIN for 6 weeks |
| 8 | 52 | none | 2 | CIP | E. coli (0.75) | R | R | 90 | no | yes | K. pneumoniae (R, >256) | LVX + FOF for 3 months |
| 3 months follow-up | ||||||||||||
| 9 | 37 | none | 0 | none | E. coli (1) | R | S | 90 | yes | yes | E. coli (S, 1) | LVX for 6 weeks |
| K. pneumoniae (S, 16) | ||||||||||||
| 6 months follow-up | ||||||||||||
| 10 | 36 | none | 3 | CIP | E. faecalis (32) | R | NA | 42 | yes | no | none | NSAIDs |
| 11 | 66 | BPH | 1 | CIP | P. mirabilis (8) | R | R | 45 | no | yes | E. coli (S, 16) | MIN for 6 weeks |
| 12 | 66 | BPH | 4 | CIP | E. coli (4) | R | R | 90 | no | yes | E. coli (S, 4) | FOF for 3 months; no relapse at 3 months and 6 months |
BPH, benign prostatic hyperplasia; CIP, ciprofloxacin; DM, diabetes mellitus; EPS, expressed prostatic secretions; ETP, ertapenem; FOF, fosfomycin; LVX, levofloxacin; MIN, minocycline; NA, not applicable; NSAIDs, non-steroidal anti-inflammatory drugs; R, resistant; S, susceptible; SXT, trimethoprim/sulfamethoxazole.
Fosfomycin MIC (mg/L) determined by Etest.
The administration of α-adrenergic antagonists (mainly alfuzosin and tamsulosin), which is recommended as adjuvant treatment in CBP with significant voiding or obstructive symptoms,5 was commenced at initiation of treatment in 18 patients. The mean age of patients on α-adrenergic antagonists was 56 years and five patients on adjuvant therapy presented with failure at the end of treatment and two at 6 months follow-up.
With regard to treatment safety, oral fosfomycin was well tolerated with minor side effects even when treatment was prolonged to 90 days. The only adverse effect was diarrhoea in 18% (8 of 44) that subsided with prolongation of dose intervals (in four cases) and/or dietary modification on day of fosfomycin administration, with the exception of one patient who discontinued treatment. In two patients, diarrhoea appeared in the first week of treatment, whereas in the remaining cases diarrhoea appeared later during therapy. All patients who presented with diarrhoea were tested for Clostridium difficile toxin production and were found to be negative.
Discussion
This is the largest cohort study to make an important contribution to the field of treatment of CBP caused by MDR pathogens administering oral fosfomycin as a new therapeutic approach and as an alternative option for at least 6 weeks up to 12 weeks.
It is well known that fluoroquinolones are established as first-line treatment of CBP based on longitudinal studies.1,10,24,25 However, the issue of alternatives has grown in importance in light of the alarming increase in resistance rates for fluoroquinolones. In Greece, resistance rates to ciprofloxacin in uropathogens gradually increased from 2.2% in 200526 to 17% in 2017,27 whereas resistance rates for Enterobacteriaceae isolated from male patients (period 2005–10) were found to be 15%.28 Similar increases have been observed worldwide.6,7 In the current study, 75% of the isolates were found to be resistant to fluoroquinolones. The recommendation of a 10% fluoroquinolone resistance prevalence of community uropathogens as the threshold for using an alternative agent has been proposed by the IDSA.8
Intravenous therapy with ertapenem has been utilized as a treatment for CBP in cases where ESBL-producing bacteria have been isolated and resistance to oral agents with penetration in the prostate tissue has been documented.29 However, the necessity of intravenous access as outpatient treatment and the limited data on efficacy29 have not established intravenous treatment as standard of care in guidelines.4,5
This study aimed to contribute to this growing area of research for novel treatments of CBP in the era of MDR prevalence by exploring alternative options. First, oral fosfomycin, administered as fosfomycin trometamol exhibits excellent activity against uropathogens, including MDR and ESBL-positive pathogens.11,13 In Greece, susceptibility rates are ∼97%–98%26,30 as well as globally,31 whereas in a recent systemic review on fosfomycin susceptibility regarding ESBL-producing E. coli, ESBL-producing Klebsiella pneumoniae and E. faecalis, susceptibility rates were found to be 95%, 83.8% and 96.8%, respectively.32 Secondly, significant information on fosfomycin penetration in the prostate tissue has been reported. The effect of fosfomycin in a bacterial prostatitis rat model induced by E. coli revealed reduction of bacterial growth, inhibition of inflammation (with significant lower IL-6, IL-8, anti-TNF-α and prostate-specific antigen levels in the prostate tissue) and improvement of prostatic tissue injury, whereas the concentration of fosfomycin in infected prostate was higher than that in the normal prostate.33 Moreover, fosfomycin distribution into human prostatic tissues was analysed by Gardiner et al.14 and indicated an overall prostate fosfomycin level of 6.5 ± 4.9 μg/g (in the peripheral prostate region: 4.4 ± 4.1 μg/g) after administration of a single dose of 3 g preoperatively in patients undergoing transurethral resection of the prostate. Interestingly, the majority of patients in that study achieved prostate fosfomycin levels ≥4 mg/L, whereas following a single dose, potential therapeutic concentrations in uninflamed prostate were observed up to 17 h.14
This study set out with the aim of assessing the efficacy of oral fosfomycin in CBP. A daily dose for the first week was implemented to rapidly reduce the bacterial burden in the prostate tissue. As previously shown, fosfomycin mean prostate levels were found to be ∼4 μg/g,14 whereas the median fosfomycin MIC for Gram-negative pathogens in the current study was 1.5 mg/L, indicating effective fosfomycin concentrations in the prostate to minimize the infectious load. A maintenance dose interval every 48 h was continued based on limited experience in humans34,35 and to minimize gastrointestinal adverse effects, as fosfomycin’s most common adverse event is diarrhoea.11,13 Previous studies of fosfomycin treatment in CBP have emphasized the relationship between once or twice daily doses of oral fosfomycin and frequency of diarrhoea36 as well as higher probability of failure with a 72 h dose interval.36,37 The evidence presented thus far supports the idea that a 48 h dose interval is the most appropriate.
An introductory analysis of the first 20 patients provided a brief overview of the effectiveness and safety of a 6 week duration of fosfomycin (the minimum treatment for CBP)1,3–5,10 with a clinical success of 85% and minimal toxicity. Furthermore, in patients with diffuse inflammation on imaging and calcifications, prolongation of treatment for a median period of 12 weeks as has been suggested38,39 was applied and was very well tolerated. The presence of prostate stones contributes to the persistence of infection and patients with prostate calcifications and CBP are considered to have a biofilm infection and are more likely to experience relapse following antimicrobial therapy; therefore, prolongation of therapy is indicated in these patients.38,39
Clinical and microbiological success at the end of treatment was achieved in 82% of the study population, whereas success after a 6 month follow-up was achieved in 73%. To the best of our knowledge, clinical data on oral fosfomycin for the treatment of CBP is mainly found in the work undertaken by Los-Arcos et al.34 who reported 15 difficult-to-treat cases of CBP caused by E. coli in the majority of the study population, and administered a dose of 3 g every 48–72 h for 6 weeks, with a clinical cure rate of 42% and a 6 month microbiological eradication rate of 53%. There are three likely causes for the differences between the results of that study and ours: (i) isolates were determined by semen culture of the ejaculate alone, which is not reliable for diagnosis;1,3–5 (ii) an exact MIC of fosfomycin was not determined and it is possible some cases with an MIC >4 mg/L were treated with oral fosfomycin, with a high probability of failure; and (iii) more than half of patients had prostatic calcifications precluding failure with a 6 week treatment duration. In addition, four case studies have also been reported (three with monotherapy administering a dose of 3 g q24h in two cases for 12–16 weeks36 and 3 g q48h for 12 weeks in the third,35 and one with a combination of 3 g of fosfomycin q72h and 100 mg of minocycline q12h for 2 weeks37) with clinical cure and microbiological eradication. On the other hand, a considerable amount of literature has been published on first-line treatment with fluoroquinolones with a similar overall clinical and microbiological response of 70%–90% at the end of therapy, but only ∼60% after 6 months.1,10,24,25
Failure of fosfomycin treatment, shown in Table 3, was observed in 12 patients. True or possible relapse was noted in seven cases, whereas reinfection was illustrated in five cases. As expected, the majority of the patients (five) that relapsed or presented with reinfection during therapy or at the end of therapy had a fosfomycin-resistant pathogen isolated in urine and were treated with other antimicrobials to which the isolates were susceptible in vitro. It should be pointed out that in the majority of cases that failed fosfomycin treatment, a pathogen with MIC ≥16 mg/L was isolated, raising great concern that fosfomycin administration q48h may not be adequate to achieve therapeutic intraprostatic levels to treat an organism with a fosfomycin MIC >8 mg/L. However, the most surprising aspect of the data was that patients that appeared with recurrence during the 3 and 6 month follow-up (three patients) revealed fosfomycin-susceptible bacteria, indicating a reinfection or possibly a minor reservoir of bacterial load in the prostate tissue at the end of therapy and the necessity for prolongation of treatment in this specific group of patients.
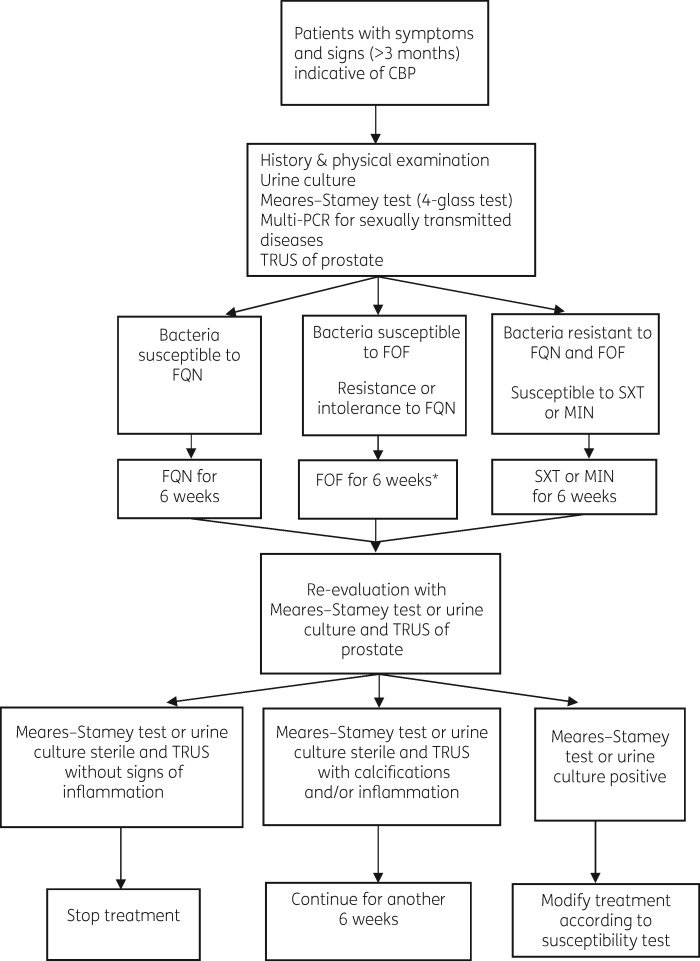
An important area where the current study makes an original contribution is the utilization of imaging and particularly TRUS of the prostate at initiation of treatment and at 6 weeks. MRI of the prostate did not provide adequate information for the monitoring of prostatitis treatment, except for the exclusion of prostatic abscesses. In contrast, TRUS revealed calcifications in the prostate, dilatation of the seminal vesicles as well as mid-range echoes and echolucent zones, consistent with inflammation. However, the low sensitivity of the ultrasound features of prostatitis preclude identification of any one feature as being diagnostic of CBP and thus are not included in guidelines as a diagnostic tool.4,5,18,40 However, according to our experience, TRUS could be included in a multifaceted approach as shown in Figure 1. Our suggestion regarding the diagnostic approach to patients with CBP includes for the initial evaluation, a history and medical examination with a microbiological documentation of infection (with a urine culture or Meares–Stamey procedure, and excluding sexually transmitted diseases with a molecular test)1,5 in combination with TRUS to determine the extent of inflammation, the presence of calcifications or the formation of an abscess. Based on microbiological results, fluoroquinolones have been considered first-line treatment,1,3,4,10,24,25 followed by oral fosfomycin in case of bacterial resistance to fluoroquinolones or in cases of intolerance (for bacteria susceptible to fosfomycin with an MIC <16 mg/L). A 6 week re-evaluation is suggested with a urine culture or a Meares–Stamey procedure (however, a second Meares–Stamey procedure is difficult to obtain and causes discomfort to patients) and a TRUS to determine the length of treatment.
Figure 1.
Suggested diagnostic algorithm of evaluation of patients with CBP. *Indicates that this is applicable for bacteria susceptible to fosfomycin with an MIC <16 mg/L. FQN, fluoroquinolone; FOF, fosfomycin; SXT, trimethoprim/sulfamethoxazole; MIN, minocycline.
This study has some limitations. The reader should bear in mind that the study is based on a single centre and small sample size with the lack of a control group receiving the best available therapy. However, our patients had either limited or no available antimicrobial agents achieving sufficient concentrations at the site of infection, highlighting oral fosfomycin as the most appropriate choice. It must be pointed out that among the limitations of the current study was the fact that microbiological evaluation on follow-up was based mostly on urine cultures, owing to obstacles to the repetition of a Meares–Stamey procedure, thus cure rates could be overestimated. In contrast, the advantages of our study were the uniform dose of fosfomycin implemented in all our patients with a multifaceted approach and a Meares–Stamey test that is considered the diagnostic reference standard1,3–5,16,17 as well as an exact fosfomycin MIC determination by Etest. Lastly, it is important to point out that, to the best of our knowledge, the case series in the present paper is the largest so far of CBP treated with oral fosfomycin.
In conclusion, this study sought to obtain data that will help to address the research gap of alternative treatments for CBP in the era of MDR prevalence. Oral fosfomycin at a dose of 3 g once daily for the first week of treatment followed by 3 g q48h for the remaining duration of treatment (a total of 6 weeks up to 12 weeks based on the presence of prostate calcifications) was proven as a safe and effective therapy with minimal toxicity and could be recommended in case of bacterial resistance to fluoroquinolones or in case of poor tolerability of the first-line agent and whenever there is laboratory confirmation of susceptibility to fosfomycin.
Acknowledgements
Part of the work has been presented at the Fifty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, CA, USA, 2015 (Poster L-1253) and the Twenty-eighth European Congress of Clinical Microbiology and Infectious Diseases, Madrid, Spain, 2018 (Oral Poster O0737).
We thank Vasiliki Papoutsaki and Rania Karantani for outstanding technical work.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
- 1. Lipsky BA, Byren I, Hoey CT.. Treatment of bacterial prostatitis. Clin Infect Dis 2010; 50: 1641–52. [DOI] [PubMed] [Google Scholar]
- 2. Krieger JN, Nyberg L Jr, Nickel JC.. NIH consensus definition and classification of prostatitis. JAMA 1999; 282: 236–7. [DOI] [PubMed] [Google Scholar]
- 3. Wagenlehner FM, Pilatz A, Bschleipfer T. et al. Bacterial prostatitis. World J Urol 2013; 31: 711–6. [DOI] [PubMed] [Google Scholar]
- 4. Bonkat G, Pickard R, Bartoletti R. et al. EAU Guidelines on Urological Infections European Association of Urology, 2017. http://uroweb.org/wp-content/uploads/19-Urological-infections_2017_web.pdf.
- 5. Rees J, Abrahams M, Doble A. et al. Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: a consensus guideline. BJU Int 2015; 116: 509–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zowawi HM, Harris PN, Roberts MJ. et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol 2015; 12: 570–84. [DOI] [PubMed] [Google Scholar]
- 7. Sanchez GV, Master RN, Karlowsky JA. et al. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012; 56: 2181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta K, Hooton TM, Naber KG. et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52: e103–20. [DOI] [PubMed] [Google Scholar]
- 9. Zhanel GG, Zhanel MA, Karlowsky JA.. Oral fosfomycin for the treatment of acute and chronic bacterial prostatitis caused by multidrug-resistant Escherichia coli. Can J Infect Dis Med Microbiol 2018; 2018: 1404813.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perletti G, Marras E, Wagenlehner FM. et al. Antimicrobial therapy for chronic bacterial prostatitis. Cochrane Database Syst Rev 2013; issue 8: CD009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sastry S, Doi Y.. Fosfomycin: resurgence of an old companion. J Infect Chemother 2016; 22: 273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keating GM. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 2013; 73: 1951–66. [DOI] [PubMed] [Google Scholar]
- 13. Karaiskos I, Giamarellou H.. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother 2014; 15: 1351–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardiner BJ, Mahony AA, Ellis AG. et al. Is fosfomycin a potential treatment alternative for multidrug-resistant gram-negative prostatitis? Clin Infect Dis 2014; 58: e101–5. [DOI] [PubMed] [Google Scholar]
- 15. Rubin RH, Shapiro ED, Andriole VT. et al. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis 1992; 15 Suppl 1: S216–27. [DOI] [PubMed] [Google Scholar]
- 16. Meares EM, Stamey TA.. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol 1968; 5: 492–518. [PubMed] [Google Scholar]
- 17. Schaeffer AJ. Clinical practice. Chronic prostatitis and the chronic pelvic pain syndrome. N Engl J Med 2006; 355: 1690–8. [DOI] [PubMed] [Google Scholar]
- 18. Weidner W, Anderson RU.. Evaluation of acute and chronic bacterial prostatitis and diagnostic management of chronic prostatitis/chronic pelvic pain syndrome with special reference to infection/inflammation. Int J Antimicrob Agents 2008; 31 Suppl 1: S91–5. [DOI] [PubMed] [Google Scholar]
- 19. Schull A, Monzani Q, Bour L. et al. Imaging in lower urinary tract infections. Diagn Interv Imaging 2012; 93: 500–8. [DOI] [PubMed] [Google Scholar]
- 20.FDA. Fosfomycin Tromethamine https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050717s005lbl.pdf.
- 21.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Eighth Edition: M100. CLSI, Wayne, PA, USA, 2018. [Google Scholar]
- 22. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]
- 23. McDonald LC, Gerding DN, Johnson S. et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: 987–94. [DOI] [PubMed] [Google Scholar]
- 24. Naber KG, Roscher K, Botto H. et al. Oral levofloxacin 500 mg once daily in the treatment of chronic bacterial prostatitis. Oral levofloxacin 500 mg once daily in the treatment of chronic bacterial prostatitis. Int J Antimicrob Agents 2008; 32: 145–53. [DOI] [PubMed] [Google Scholar]
- 25. Bundrick W, Heron SP, Ray P. et al. Levofloxacin versus ciprofloxacin in the treatment of chronic bacterial prostatitis: a randomized double-blind multicenter study. Urology 2003; 62: 537–41. [DOI] [PubMed] [Google Scholar]
- 26. Katsarolis I, Poulakou G, Athanasia S. et al. Acute uncomplicated cystitis: from surveillance data to a rationale for empirical treatment. Int J Antimicrob Agents 2010; 35: 62–7. [DOI] [PubMed] [Google Scholar]
- 27.Greek System for the Surveillance of Antimicrobial Resistance. WHONET—Greece http://www.mednet.gr/whonet/.
- 28. Maraki S, Mantadakis E, Michailidis L. et al. Changing antibiotic susceptibilities of community-acquired uropathogens in Greece, 2005-2010. J Microbiol Immunol Infect 2013; 46: 202–9. [DOI] [PubMed] [Google Scholar]
- 29. Trad MA, Zhong LH, Llorin RM. et al. Ertapenem in outpatient parenteral antimicrobial therapy for complicated urinary tract infections. J Chemother 2017; 29: 25–9. [DOI] [PubMed] [Google Scholar]
- 30. Kahlmeter G, Poulsen HO.. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO·SENS study revisited. Int J Antimicrob Agents 2012; 39: 45–51. [DOI] [PubMed] [Google Scholar]
- 31. Giske CG. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clin Microbiol Infect 2015; 21: 899–905. [DOI] [PubMed] [Google Scholar]
- 32. Vardakas KZ, Legakis NJ, Triarides N. et al. Susceptibility of contemporary isolates to fosfomycin: a systematic review of the literature. Int J Antimicrob Agents 2016; 47: 269–85. [DOI] [PubMed] [Google Scholar]
- 33. Fan L, Shang X, Zhu J. et al. Pharmacodynamic and pharmacokinetic studies and prostatic tissue distribution of fosfomycin tromethamine in bacterial prostatitis or normal rats. Andrologia 2018; 50: e13021. [DOI] [PubMed] [Google Scholar]
- 34. Los-Arcos I, Pigrau C, Rodríguez-Pardo D. et al. Long-term fosfomycin-tromethamine oral therapy for difficult-to-treat chronic bacterial prostatitis. Antimicrob Agents Chemother 2015; 60: 1854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gian J, Cunha BA.. Raoultella planticola chronic bacterial prostatitis with prostatic calcifications: successful treatment with prolonged fosfomycin therapy. Int J Antimicrob Agents 2016; 47: 414.. [DOI] [PubMed] [Google Scholar]
- 36. Grayson ML, Macesic N, Trevillyan J. et al. Fosfomycin for treatment of prostatitis: new tricks for old dogs. Clin Infect Dis 2015; 61: 1141–3. [DOI] [PubMed] [Google Scholar]
- 37. Cunha BA, Gran A, Raza M.. Persistent extended-spectrum β-lactamase-positive Escherichia coli chronic prostatitis successfully treated with a combination of fosfomycin and doxycycline. Int J Antimicrob Agents 2015; 45: 427–9. [DOI] [PubMed] [Google Scholar]
- 38. Bartoletti R, Cai T, Nesi G. et al. The impact of biofilm-producing bacteria on chronic bacterial prostatitis treatment: results from a longitudinal cohort study. World J Urol 2014; 32: 737–42. [DOI] [PubMed] [Google Scholar]
- 39. Zhao WP, Li YT, Chen J. et al. Prostatic calculi influence the antimicrobial efficacy in men with chronic bacterial prostatitis. Asian J Androl 2012; 14: 715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doble A, Carter SS.. Ultrasonographic findings in prostatitis. Urol Clin North Am 1989; 16: 763–72. [PubMed] [Google Scholar]