Abstract
Background
Darunavir 800 mg once (q24h) or 600 mg twice (q12h) daily combined with low-dose ritonavir is used to treat HIV-positive pregnant women. Decreased total darunavir exposure (17%–50%) has been reported during pregnancy, but limited data on unbound exposure are available.
Objectives
To evaluate total and unbound darunavir exposures following standard darunavir/ritonavir dosing and to explore the value of potential optimized darunavir/ritonavir dosing regimens for HIV-positive pregnant women.
Patients and methods
A population pharmacokinetic analysis was conducted based on data from 85 women. The final model was used to simulate total and unbound darunavir AUC0–τ and Ctrough during the third trimester of pregnancy, as well as to assess the probability of therapeutic exposure.
Results
Simulations predicted that total darunavir exposure (AUC0–τ) was 24% and 23% lower in pregnancy for standard q24h and q12h dosing, respectively. Unbound darunavir AUC0–τ was 5% and 8% lower compared with post-partum for standard q24h and q12h dosing, respectively. The probability of therapeutic exposure (unbound) during pregnancy was higher for standard q12h dosing (99%) than for q24h dosing (94%).
Conclusions
The standard q12h regimen resulted in maximal and higher rates of therapeutic exposure compared with standard q24h dosing. Darunavir/ritonavir 600/100 mg q12h should therefore be the preferred regimen during pregnancy unless (adherence) issues dictate q24h dosing. The value of alternative dosing regimens seems limited.
Introduction
Physiological changes during pregnancy can alter the pharmacokinetics of antiretroviral agents, mostly resulting in decreased antiretroviral exposure during pregnancy.1 This may lead to virological breakthrough and/or development of antiretroviral resistance, as well as an increased risk of perinatal HIV transmission.2,3 To ensure effective ART, the impact of pregnancy on the pharmacokinetic properties of antiretroviral drugs must be considered.
Darunavir in combination with low-dose cobicistat (150 mg) should be explicitly avoided during pregnancy because of subtherapeutic exposure.4 Darunavir in combination with low-dose ritonavir, however, can be used for treatment of HIV-positive pregnant women.5 Lowered total darunavir exposure has been reported during pregnancy with decreases of AUC0–τ ranging from 17% to 50%.6–10 To a lesser degree, lower unbound darunavir exposure has also been observed.11 To maintain sufficient exposure, it has been recommended to use the standard twice-daily (q12h) 600/100 mg darunavir/ritonavir dosing regimen during pregnancy and not the standard once-daily (q24h) 800/100 mg regimen.5,12 However, it has also been argued that the standard dosing regimens are inadequate during pregnancy and an increased dose is needed.13
The search for optimized darunavir/ritonavir dosing in HIV-positive pregnant women is complicated by several aspects of the darunavir/ritonavir pharmacokinetics. First, in plasma, darunavir is ∼94% protein bound, mainly to α-1-acid glycoprotein (AAG). During pregnancy, protein binding may be altered because of lower AAG plasma levels.14,15 This may result in decreased total darunavir exposure, but not necessarily in the unbound darunavir exposure (pharmacologically active), as observed previously for darunavir in pregnancy.9 Moreover, it has been reported that darunavir protein binding is non-linear in its therapeutic range.16
Secondly, darunavir biotransformation is almost exclusively mediated by cytochrome P450 (CYP) 3A4 and darunavir is a substrate for P-glycoprotein (P-gp).17 It has been suggested that both the CYP3A4 and P-gp activities are altered during pregnancy.15 This may lead to changes in darunavir pharmacokinetics.
Furthermore, in clinical practice, ritonavir co-administration is essential to reduce darunavir clearance, increase darunavir bioavailability, reduce pill burden and maintain adequate plasma concentrations throughout the dosing interval.18 This is commonly referred to as pharmacokinetic boosting. Ritonavir is a potent CYP3A4 inhibitor and reduces darunavir clearance, leading to higher plasma concentrations throughout the dosing interval.18 Ritonavir is also known to have potent inhibitory effects on the efflux drug transporter (P-gp), which, in the intestine, may also contribute to the increased darunavir bioavailability and hence exposure.17 Ritonavir biotransformation is also mediated by CYP3A4. Changes in ritonavir exposure, resulting from pregnancy-induced CYP3A4 activity, may result in altered darunavir CYP3A4/P-gp inhibition during pregnancy compared with non-pregnant women.9 Failure to take these pharmacokinetic aspects into account could result in (proposed) suboptimal dosing regimens in pregnant women with respect to exposure and hence treatment outcomes.
To investigate further the adequacy of standard darunavir/ritonavir dosing in pregnancy we performed a semi-mechanistic population pharmacokinetic analysis of the pharmacokinetics of darunavir and ritonavir in pregnant women. Ultimately, the developed model was used to evaluate total and unbound darunavir exposures following standard darunavir/ritonavir dosing, but also to explore the value of potential optimized dosing regimens for HIV-positive pregnant women.
Methods
Pharmacokinetic data
Data from two studies that included pregnant women taking darunavir/ritonavir were pooled. Study details have been reported previously.9,10 Both studies had a similar observational design. Women had intensive pharmacokinetic assessments during pregnancy (second and/or third trimester) and post-partum. The post-partum visit served as the control for the non-pregnant situation. In total, 2265 plasma concentrations were available from 85 women, of which 1431 plasma concentrations were during pregnancy. Corresponding unbound darunavir concentrations were determined in 74 plasma samples from 20 women, during pregnancy and post-partum. Further details and demographics are provided in Table 1.
Table 1.
Patient and study characteristics summarized by study
| Study 19 | Study 210 | |||
|---|---|---|---|---|
| Number of women | 24 | 64 | ||
| Number of patients included |
23a |
62a |
||
| Number of ritonavir samples | total | unbound | total | unbound |
| pregnant | 194 | NA | 519 | NA |
| post-partum | 132 | NA | 284 | NA |
| Number of darunavir samples | ||||
| pregnant | 194 | 44 | 524 | NA |
| post-partum |
132 |
30 |
286 |
NA |
| Gestational age at sampling times (weeks), median (range) | 34 (32–37) | 33 (20–39) | ||
| Post-partum visit (weeks after delivery), median (range) | 5 (3–14) | 7 (2–14) | ||
| Sampling design | 0 h (pre-dose) and 0.5, 1, 2, 3, 4, 6, 8, 12 (and 24) h post-dose | 0 h (pre-dose) and 1, 2, 4, 6, 8, 12 (and 24) h post-dose | ||
| Lower limit of quantification (mg/L) | ||||
| darunavir | 0.1 (n = 3, 1% BLQ) | 0.09 (n = 7, 1% BLQ) | ||
| ritonavir | 0.045 (n = 39, 12% BLQ) | 0.059 (n = 137, 17% BLQ) | ||
| Weight (kg), median (range) | ||||
| second trimester | NA | 88 (57–200) | ||
| third trimester | 80 (65–117) | 83 (56–204) | ||
| not pregnant | 76 (62–109) | 80 (51–194) | ||
| Dosing regimen | ||||
| 600/100 mg q12h | 5 | 30 | ||
| 800/100 mg q24h | 18 | 32 | ||
| Recruitment sites | Europe (Spain, the UK, Italy, Belgium, Germany, the Netherlands) | USA | ||
| Race, n (%) | ||||
| Caucasian | 11 (48) | 6 (10) | ||
| black or African American | 12 (52) | 28 (45) | ||
| other or mixed | 0 (0) | 28 (45) | ||
| HIV-1 RNA at delivery ≤50 copies/mL, % | 67 | 57 | ||
| Infant birth weight (g), median (range) | 3090 (2060–3718) | 3023 (1800–4560) | ||
NA, not applicable.
Women excluded due to non-adherence.
The number of darunavir plasma samples below the lower limit of quantification (BLQ) was small (<1%) and excluded. For ritonavir the number of plasma samples BLQ was substantial (∼15%; Table 1) and the M3 method19 was used to deal with BLQ data. With this method the likelihood of being BLQ is incorporated into the model. In both studies, validated (ultra) HPLC assays were used.9,10
Population pharmacokinetics
Several population pharmacokinetic models for darunavir/ritonavir in a non-pregnant population are available in the literature. These models are largely empirical and hence not able to deal with the complicating pharmacokinetic processes specified in the Introduction section and not based on data from pregnant women.14,20 Nevertheless, these models formed the background for further darunavir/ritonavir model development in pregnant women. The final model was developed in three steps. First, the darunavir protein (AAG)-binding dissociation constant (Kd) and the maximal protein binding capacity (Bmax) were estimated based on non-linear mixed-effects analysis of a subset of paired samples of total and unbound darunavir concentrations in pregnancy and post-partum. Then, separate population pharmacokinetic models were developed for ritonavir and darunavir. The darunavir Kd and Bmax estimated from the previous step were used and fixed in the darunavir model. Thereafter, the darunavir–ritonavir interaction was simultaneously modelled based on the darunavir and ritonavir data in pregnancy and post-partum. Pregnancy and gestational age were tested as covariate on all ritonavir and darunavir model parameters. Model development in each step is further detailed in Supplementary data S1 (available at JAC Online).
Model evaluation and qualification
Throughout the model building process, we evaluated precision in parameter estimates obtained by the covariance step ($COV) and standard goodness-of-fit plots. For the final models, parameter uncertainty was calculated with the sampling importance resampling (SIR) procedure.21 To qualify the final model for simulation we evaluated prediction-corrected visual predictive checks (pcVPCs) and goodness-of-fit plots.22
Simulation
For simulation we focused on the third trimester of pregnancy, rather than earlier phases of pregnancy, since the risks of mother-to-child transmission are highest during late pregnancy and labour and the potential changes in pharmacokinetics are presumed to be at their peak.5 The final darunavir/ritonavir model was used to simulate relevant secondary pharmacokinetic parameters (AUC0–τ and Ctrough) following standard and alternative q24h and q12h darunavir dosing in typical pregnant (at 38 weeks of gestation) and non-pregnant (i.e. post-partum) women. For exploration of higher ritonavir doses (i.e. increased boosting), linear pharmacokinetics were assumed for ritonavir up to 200 mg q12h, at steady-state.23,24
In addition, the probability of therapeutic exposure was assessed. The target for darunavir was set to the protein-adjusted EC50 for resistant virus: 0.55 mg/L.16 The SimCYP simulator was employed to create a dataset with demographics [fat free mass (FFM) and total bodyweight (TBW)] for 5000 virtual pregnant (38 weeks of gestation) and non-pregnant (0 weeks of gestation) women using correlated Monte Carlo sampling. This dataset was used to simulate Ctrough for the different dosing regimens and, subsequently, assess the probability of therapeutic exposure.
Additionally, we explored simulated unbound darunavir AUC0–τ and Ctrough. This was based on the predicted (concentration-dependent) fu predicated on pregnancy status and the model-simulated total darunavir plasma concentration (Ctot), using: Cfree = fu × Ctot.
The probability of therapeutic exposure was also assessed for unbound Ctrough. For this purpose, the target was set to 0.0275 mg/L; the unbound EC50 assuming 95% protein binding in typical non-pregnant adults.
Software
Data were analysed using NONMEM® 7.4.1 (ICON Development Solutions, Hanover, MD, USA). The first-order conditional estimation method was used. Pirana 2.9.1 (http://www.pirana-software.com) was used as an interface for NONMEM to structure and document model development.25 R version 3.4.2 (with Rstudio interface version 1.1.383) was used for data preparation, exploratory analyses, and graphical visualization and evaluation. Perl Speaks Nonmem 4.6.0 was used for automation of a diverse range of processes related to non-linear mixed-effects model development.26 SimCYP V15 Simulator was used to obtain demographic datasets for simulation.
Results
Darunavir protein binding
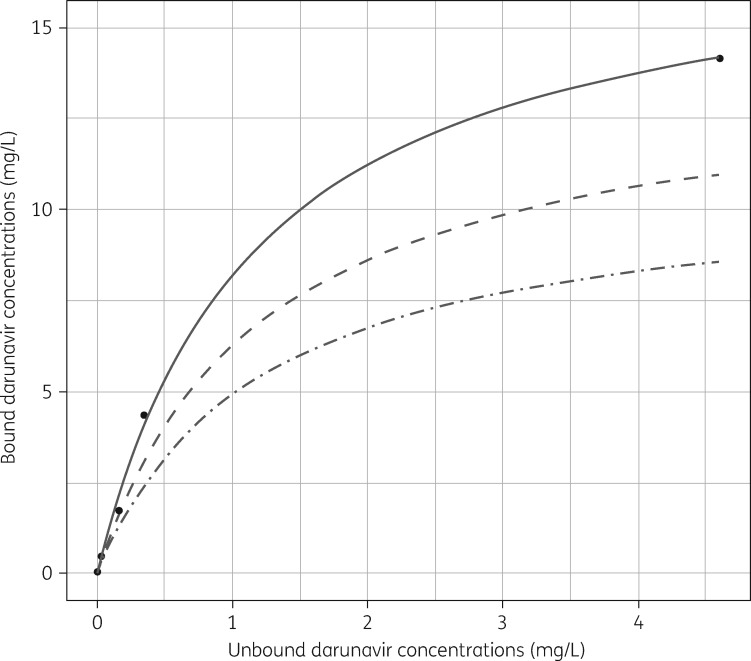
The unbound darunavir concentrations ranged from 0.04 to 1.35 mg/L and the paired total concentration ranged from 0.32 to 9.2 mg/L. Inclusion of pregnancy as a dichotomous covariate for Bmax significantly improved the model fit [change in objective function value (ΔOFV) −22.1; P < 0.01]. The parameter estimates for the darunavir protein binding model are listed in Table 2. These results are in line with data on darunavir protein binding in healthy volunteers presented in the registration package (Figure 1).16 The parameter uncertainties in Bmax and Kd were highly correlated (83%). Goodness-of-fit plots and pcVPCs are included in Supplementary data S2.
Table 2.
Final darunavir protein binding model
| Parameter | Parameter estimate | RSE (%) from SIR |
|---|---|---|
| B max, non-pregnant (mg/L) | 13.8 | 7 |
| B max, pregnant (mg/L)a | 10.8 | 5 |
| K d (mg/L) | 1.2 | 21 |
| IIV Bmax (%) | 15 | 14 |
| Proportional residual error (%) | 18 | 6 |
B max, maximal protein binding capacity; Kd, the darunavir protein (AAG)-binding dissociation constant; IIV, inter-individual variability; RSE, relative standard error of estimate.
According to Equation (12) (Supplementary data S1).
Figure 1.
Darunavir protein binding. Based on unpublished data from the registration package, bound and unbound darunavir plasma concentrations were plotted.16 Each dot represents the mean of two values. Using non-linear least-squares estimation based on Supplementary data S1, Equation (1), the Bmax and Kd (95% CI) were 18 (16–20) mg/L and 1.16 (0.83–1.7) mg/L, respectively. Dashed and dotted-dashed lines represent the typical binding kinetics in non-pregnant (i.e. post-partum) and pregnant (i.e. third trimester) women, respectively, based on the parameters listed in Table 2.
Darunavir and ritonavir population pharmacokinetic analyses
The observed ritonavir concentrations ranged from <0.045 (lower limit of quantification) to 0.9 mg/L. A one-compartment disposition model with sequential zero- and first-order absorption and first-order elimination best described the data. Inter-individual variability was included for clearance (CL/F; F was fixed to 1) and central volume of distribution (Vc/F) with the covariance term estimated for CL/F and Vc/F (ΔOFV −15.4). Inter-occasion variability was included for F (systemic bioavailability; ΔOFV −318.9). The residual error structure was proportional. Fixed allometric scaling with TBW for volume of distribution and clearance best described the data (total ΔOFV −13.8). Standard stepwise covariate modelling led to the inclusion of linear parameter–gestational age relationships for F (ΔOFV −25.4; P < 0.01). Population estimates are shown in Table 3. Goodness-of-fit plots and pcVPCs (continuous and censored for BLQ19) are included in Supplementary data S3.
Table 3.
Final darunavir/ritonavir model parameter estimates
| Parameter | Parameter estimate | RSE (%) from SIR |
|---|---|---|
| Ritonavir | ||
| CL/F (L/h)a | 25.1 | 8 |
| V c/F (L)a | 20.6 | 16 |
| ka (h−1) | 0.12 | 2 |
| duration of absorption (h) | 2.15 | 6 |
| θGA for Fb | −0.012 | 13 |
| IIV CL/F (%) | 37 (shrinkage 18%) | 17 |
| IIV Vc/F (%) | 162 (shrinkage 21%) | 23 |
| correlation ηCL/F–ηVc/F (%) | 24 | 32 |
| IOV F (%) | 51 (shrinkage 19%) | 7 |
| proportional residual error (%) | 36 | 2 |
| Darunavir | ||
| MAT (h) | 4.3 | 5 |
| CLint/F (L/h)a,c | 130 | 10 |
| V c/F (L)a | 8.3 | 15 |
| Q/F (L/h)a | 13 | 11 |
| Vp/F (L)a | 134 | 17 |
| B max, non-pregnant (mg/L) | 13.8 | FIX |
| B max, pregnant (mg/L) | 10.8 | FIX |
| K d (mg/L) | 1.2 | FIX |
| RTV Imax (%) | 35 | 28 |
| RTV IC50 (mg/L) | 0.015 | 16 |
| IIV Vc/F (%) | 146 (shrinkage 26%) | 19 |
| IOV CLint/F (%) | 49 (shrinkage 18%) | 8 |
| proportional residual error (%) | 29 | 2 |
CL/F, clearance; Vc/F, central volume of distribution; F, relative bioavailability; IIV, inter-individual variability; IOV, inter-occasion variability; MAT, mean absorption time; CLint/F, intrinsic clearance; Q/F, inter-compartmental clearance; Vp/F, peripheral volume of distribution; RTV, ritonavir; Imax, the maximum inhibitory effect on CLint/F; IC50, the inhibitor concentration producing 50% of the Imax; RSE, relative standard error of estimate.
Values refer to a typical individual of 70 kg (FFM 45 kg).
According to Equation (13) (Supplementary data S1).
According to Equation (5) (Supplementary data S1).
The observed total darunavir concentrations ranged from 0.1 to 18.5 mg/L. In addition to the well-stirred liver model, a two-compartment disposition model and absorption through one absorption transit compartment best described the data. Inclusion of the parameters Bmax (predicated on pregnancy status) and Kd to describe the relation between fu, and hence plasma clearance (CLhep/F), and total darunavir concentrations improved the model fit (ΔOFV of −61.0). Inter-individual variability was included for Vc/F (total ΔOFV −299.5). Inter-occasion variability was included for CLint/F (ΔOFV −195.1). The residual error structure was proportional. Body size scaling did not significantly improve the model fit. FFM was used for parameters related to the liver and central compartment, and TBW was used for scaling of the parameters related to the peripheral compartments. Stepwise covariate modelling did not lead to further inclusion of parameter–pregnancy relationships for darunavir pharmacokinetics in the simultaneous fit.
Darunavir–ritonavir interaction model
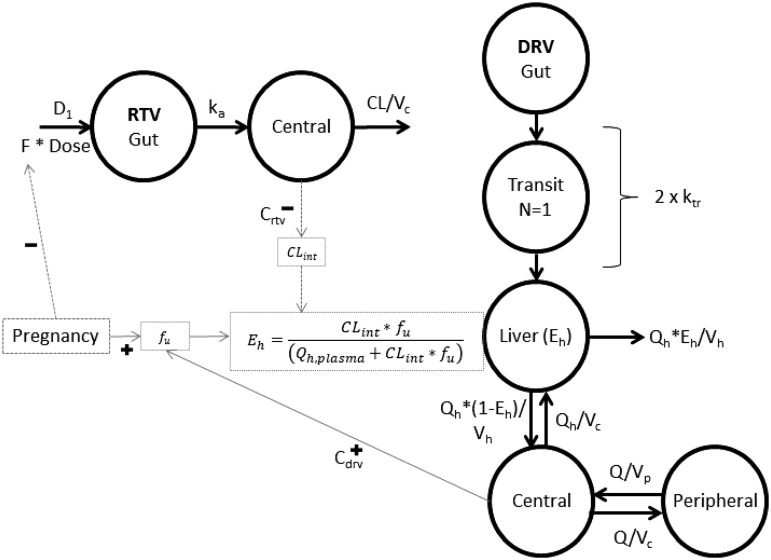
For darunavir, a direct proportional relationship between ritonavir and darunavir clearance improved the model fit as expected (ΔOFV −78.4). The maximum-inhibition model further improved the model fit (2df; ΔOFV −16.4; P < 0.01). Population estimates are shown in Table 3. Goodness-of-fit plots and pcVPCs are included in Supplementary data S4. Several stratifications of the pcVPCs were evaluated, including study (study 1 versus study 2), but no misspecifications were observed (data not shown). The final model used for simulation is graphically represented in Figure 2.
Figure 2.
Schematic of the final darunavir/ritonavir model. Left: final structural model for ritonavir. Right: final structural model for darunavir. A fraction of the ritonavir dose (F) enters the central compartment through sequential zero-order absorption (D1, duration of zero-order process) and first-order absorption (ka, absorption rate constant). Ritonavir is cleared from the central compartment. Darunavir is absorbed through one transit compartment into the liver compartment, based on two identical first-order rate constants (ktr). For the first pass through the liver a fraction of the darunavir amount is extracted (Eh) and cleared; the fraction remaining (1 − Eh) reaches the systemic circulation and becomes available for redistribution into the peripheral compartment. Darunavir recirculates from the central compartment to the liver with a flow equivalent to liver plasma flow (Qh) and at each pass the liver extracts a further fraction (Eh). Pregnancy impacts ritonavir bioavailability and darunavir Bmax. Total darunavir concentrations in the central compartment impact fu. Total ritonavir concentrations in the central compartment inhibit CLint. Relations indicated with dashed lines were estimated. Relations indicated with solid lines were fixed in the final darunavir/ritonavir model. DRV, darunavir; RTV, ritonavir.
Simulation
Relevant exposure parameters and probabilities of therapeutic exposure for standard darunavir/ritonavir dosing regimens are listed in Table 4. In the third trimester of pregnancy total darunavir exposure (AUC0–τ) was reduced by 24% and 23% compared with post-partum for darunavir/ritonavir 800/100 mg q24h and 600/100 mg q12h, respectively. Total darunavir Ctrough in the third trimester of pregnancy was reduced by 31% and 28% compared with post-partum for darunavir/ritonavir 800/100 mg q24h and 600/100 mg q12h, respectively. For darunavir, unbound pharmacokinetic parameters were also explored based on predicted fu. In the third trimester of pregnancy unbound darunavir AUC0–τ was reduced by 5% and 8% compared with post-partum for darunavir/ritonavir 800/100 mg q24h and 600/100 mg q12h, respectively. Unbound darunavir Ctrough in the third trimester of pregnancy was reduced by 14% and 11% compared with post-partum for darunavir/ritonavir 800/100 mg q24h and 600/100 mg q12h, respectively. The probability of therapeutic exposure for darunavir/ritonavir 600/100 mg q12h was >95% based on the total and unbound target concentrations, irrespective of pregnancy status. The probability of therapeutic exposure following darunavir/ritonavir 800/100 mg q24h in pregnancy was 78% based on the total target concentration. The probability of therapeutic exposure for darunavir/ritonavir 800/100 mg q24h in pregnancy based on the unbound target concentration was higher (94%; Table 4). Nevertheless, the latter indicates that still 1 of ∼17 pregnant women will have subtherapeutic unbound exposure, compared with 1 of ∼33 non-pregnant women.
Table 4.
Simulated typical darunavir pharmacokinetic parameters and probability of therapeutic exposure for standard and doubled ritonavir boosting
| AUCta (mg·h/L) | AUCua (mg·h/L) | C trough, t (mg/L) | C trough, u (mg/L) | Probability of TAt (%) | Probability of TAu (%) | |
|---|---|---|---|---|---|---|
| Pregnant (GA = 38 weeks) | ||||||
| 800/100 mg q24h | 58 | 7.6 | 1.1 | 0.12 | 78 | 94 |
| 600/100 mg q12h | 82 | 11 | 2.1 | 0.25 | 96 | 99 |
| 800/200 mg q24hb | 59 | 7.7 | 1.1 | 0.12 | 80 | 94 |
| 600/200 mg q12hb | 83 | 11 | 2.1 | 0.28 | 96 | 100 |
| 1200/100 mg q24hb | 78 | 11 | 1.4 | 0.16 | 87 | 97 |
| 800/100 mg q12hb | 102 | 15 | 2.6 | 0.31 | 99 | 100 |
| Non-pregnant | ||||||
| 800/100 mg q24h | 76 | 8.0 | 1.6 | 0.14 | 92 | 97 |
| 600/100 mg q12h | 107 | 12 | 2.9 | 0.28 | 99 | 100 |
| 800/200 mg q24hb | 77 | 8.1 | 1.6 | 0.14 | 93 | 97 |
| 600/200 mg q12hb | 107 | 12 | 2.9 | 0.28 | 99 | 100 |
| 1200/100 mg q24hb | 102 | 12 | 2.1 | 0.19 | 96 | 98 |
| 800/100 mg q12hb | 132 | 16 | 3.5 | 0.35 | 100 | 100 |
GA, gestational age; Ctrough, concentrations at the end of the dosing interval; TA, therapeutic exposure; t, total; u, unbound.
Values refer to the daily exposure (0–24 h for both q24h and q12h dosing).
Explored darunavir/ritonavir dosing regimens.
Several strategies were explored to increase darunavir exposure during pregnancy, including higher ritonavir dosing (i.e. more boosting) and/or higher darunavir dosing. The impact of higher ritonavir dosing on darunavir AUC0–τ and Ctrough was minor (Table 4). Additionally, the probability of therapeutic exposure following darunavir/ritonavir 800/200 mg q24h in the third trimester of pregnancy did not increase to the probability of therapeutic exposure for the standard 600/100 mg q12h dosing in pregnancy. For darunavir/ritonavir 600/200 mg q12h, increments in AUC0–τ, Ctrough and probability of therapeutic exposure were minor and insignificant when compared with darunavir/ritonavir 600/100 mg q12h.
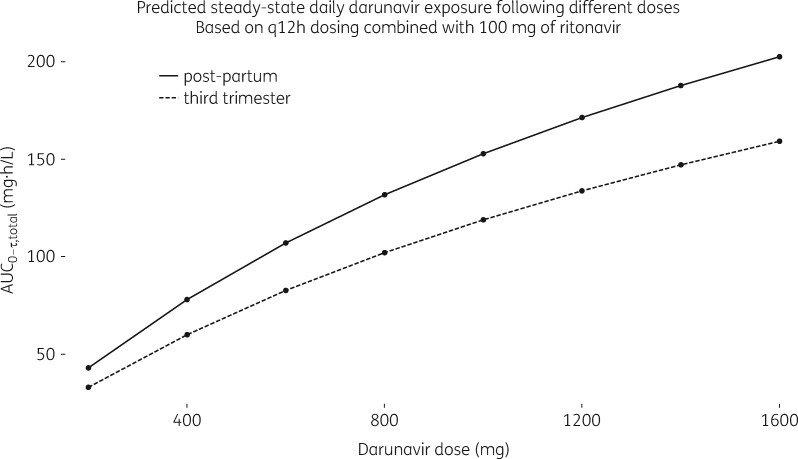
As highlighted in the Introduction section, higher darunavir q12h dosing has been proposed for pregnant women. In Figure 3 the simulated typical exposure for pregnant and non-pregnant women is plotted as a function of different darunavir q12h doses with standard 100 mg ritonavir boosting. As expected, a non-linear relationship between dose and total darunavir exposure was observed. This can be distinguished from a linear relationship between dose and unbound darunavir exposure (predictions not shown). The probability of therapeutic exposure for darunavir/ritonavir 1200/100 mg increased, but was still lower than with standard 600/100 mg q12h dosing (Table 4). Darunavir/ritonavir 800/100 mg q12h further increased darunavir AUC0–12 and Ctrough in pregnancy compared with the standard dosing regimens in pregnancy, yet the gains in probability of therapeutic exposure were minor and not relevant (Table 4).
Figure 3.
Exploration of darunavir dosing. Simulated typical daily darunavir exposure (AUC0–12) during the third trimester of pregnancy (at 38 weeks of gestation) and post-partum versus darunavir dose for q12h dosing; darunavir always co-administered with standard ritonavir (100 mg).
Discussion
In this semi-mechanistic population pharmacokinetic analysis, darunavir exposure following the standard darunavir/ritonavir dosing regimens was reduced in the third trimester of pregnancy compared with non-pregnant women. Total darunavir exposure (AUC0–τ) was reduced by 24% and 23% during the end of the third trimester compared with post-partum for darunavir/ritonavir 800/100 mg q24h and 600/100 mg q12h, respectively. This was in line with results from previous studies that showed reductions in total darunavir plasma concentrations of between 20% and 50% during the third trimester of pregnancy.6–10
The clinical relevance of these pregnancy-induced alterations in exposure is under debate. Often exposures are assessed by means of bioequivalence boundaries, often 80%–125%. For darunavir exposure in pregnant women, this criterion was not met in the present analysis. This was consistent with findings from previous studies. However, results from in vitro studies demonstrate alterations in antiviral activity resulting from changes in darunavir protein binding.27In vivo, non-linearities and pregnancy-induced changes in darunavir protein binding could therefore result in shifts in apparent antiviral activity. Under such conditions, unbound plasma concentrations are a better proxy for the pharmacologically active concentration at the site of action.28 Evaluating the simulated unbound darunavir AUC0–τ, reductions were much smaller (5%–8%) and the bioequivalence criterion was met for both standard q24h and q12h dosing. This was in line with the results from a previous study.11
Exposure in pregnancy can also be examined relative to therapeutic targets. Preferably, therapeutic targets are set based on exposure–response relationships from clinical data, but these were not available for darunavir (in pregnant women). The longstanding and widely used (in therapeutic drug monitoring) darunavir therapeutic target of 0.55 mg/L is translated from in vitro to in vivo potency (i.e. protein-binding adjusted) and 10 times higher than the EC50 for the least susceptible multi-PI-resistant HIV-1 strains isolated from patients.27 This target is therefore considered relatively conservative. The probability of therapeutic exposure following darunavir/ritonavir 800/100 mg q24h in pregnancy, however, was 78% based on the total target concentration. This indicates subtherapeutic exposure for 1 in 5 pregnant women. Nevertheless, for reasons outlined above, unbound therapeutic targets seem more appropriate to examine darunavir exposure in pregnancy. Assuming 95% protein binding in typical non-pregnant women the unbound target was set to 0.0275 mg/L (5% of the 0.55 mg/L target). The probability of therapeutic exposure for darunavir/ritonavir 800/100 mg q24h in pregnancy based on the unbound target was higher (94%), but indicated that still 1 of ∼17 pregnant women will have subtherapeutic unbound exposure, compared with 1 of ∼33 non-pregnant women. This could be explained by lower ritonavir exposures in pregnant women and thus less inhibition.
Alternative darunavir/ritonavir dosing regimens were also explored. Higher ritonavir (i.e. more boosting) or darunavir dosing increased the probability of therapeutic exposure for darunavir q24h dosing in pregnancy. These strategies may provide an occasional solution for pregnant women that should in fact be on a q12h regimen, but that are for some reason bound to q24h dosing. Such strategies can be considered combined with therapeutic drug monitoring. The results of the current study do not necessarily warrant higher ritonavir or darunavir dosing. It should be noted that increments in total exposure were not proportional to dose and thus less than expected based on linear darunavir pharmacokinetics. This was, in fact, observed in a preliminary analysis of data on darunavir/ritonavir 800/100 mg pharmacokinetics in pregnancy.13
The pharmacokinetic models developed in the current study were consistent with previously developed models for darunavir and ritonavir, in terms of compartmental structure and parameter estimates.14,20 In contrast to previous work, however, the present analysis used a well-stirred liver model for darunavir and focused on the impact of pregnancy on the pharmacokinetics. Gestational age (i.e. pregnancy) was found to reduce ritonavir relative bioavailability. This is most likely explained by pregnancy-induced alterations in CYP3A4 and/or P-gp activity.29 In this analysis the post-partum assessments served as the control for the non-pregnant situation (i.e. gestational age 0) and it can be questioned to what extent pregnancy-induced physiological processes (e.g. enzyme expression or protein levels) have normalized during the early post-partum period. Fortunately, in the current study, post-partum samples were mostly taken at least 4–6 weeks after delivery and previous work30 indicated that this time span is sufficient for relevant physiological processes to normalize, supporting our approach.
Another important aspect of our analysis was the interaction between ritonavir and darunavir. Separating ritonavir-mediated inhibition from the inherent correlation between pharmacokinetics of both compounds is cumbersome, though essential. Here, this was handled in a pragmatic way supported by mechanistic reasoning and information. In the absence of enzyme saturation (i.e. linear biotransformation), darunavir and ritonavir clearance are directly proportional to enzyme abundance. Consequently, a directly proportional relation between ritonavir clearance and darunavir clearance was assumed. Although this assumption is arguable, it is a reasonable approximation of the inherent relation between ritonavir and darunavir pharmacokinetics and particularly more reasonable than no relation at all. Subsequently, the ritonavir concentration-dependent inhibition of darunavir clearance was estimated and the identified IC50 was in line with those previously reported for ritonavir inhibition of darunavir,14 but also lopinavir.31 Maximum inhibition was estimated to be ∼35%. Fixing the maximum inhibition to 100% or testing complete competitive inhibition described the data less well. This was clearly reflected in the data where darunavir clearance did not further decrease with high versus higher ritonavir exposures. This indicates that ritonavir is not able to inhibit completely the darunavir clearance. This may be related to known un- or non-competitive elements to ritonavir inhibition, but also to other pathways for darunavir biotransformation or elimination.32,33 Notably, the ritonavir concentrations observed clinically are mostly well above the IC50 and inhibition is maximal. Only few subjects with concentrations at the lower end of the distributions (particularly in pregnancy) will have submaximal inhibition.
A strength of this study was its semi-mechanistic nature. The mechanistic aspects of the darunavir model allowed us to distinguish between changes in total darunavir exposure resulting from changes in protein binding from those resulting from pregnancy-induced intrinsic clearance or ritonavir-mediated inhibition of intrinsic clearance. This, in turn, allowed us to distinguish between pregnancy-related changes in total and unbound darunavir concentrations in the simulations, which provided relevant insight for exploring darunavir/ritonavir dosing regimens in HIV-positive pregnant women. Ritonavir was modelled in a more empirical manner and mostly relevant as input to the darunavir model. Although it could be argued that the darunavir–ritonavir interaction should be modelled based on unbound ritonavir concentrations, ritonavir protein binding is linear over the clinical concentration range and pregnancy-induced alterations in plasma binding were found to be minor.34 We therefore think total ritonavir concentrations were a good (enough) proxy to model the direct ritonavir-mediated inhibition of darunavir clearance.
A limitation to the current study was that only small numbers of matched total and unbound concentrations were available. Yet, estimating the binding parameters from the subset of matched total and unbound darunavir concentrations allowed inclusion of non-linear protein binding in the final model. The estimated Bmax was largely in line with the Bmax in plasma from healthy volunteers (Figure 1). The minor differences in Bmax observed are consistent with reported differences in fraction unbound for healthy volunteers16 and HIV-infected (pregnant) women.9 The effect of pregnancy on Bmax (22% decrease) was in line with the overall average change in AAG concentrations during (the third trimester of) pregnancy found in the literature (15% decrease35), which is expected to be directly proportional to Bmax. It should be noted that the Bmax estimate (but also other estimates) was based on data from the third trimester. Therefore, extrapolations to the first and second trimesters based on this model may not be valid. Additionally, simulation in the current study did not take parameter uncertainty into account.
Overall, the standard darunavir/ritonavir 600/100 mg q12h regimen resulted in maximal rates of therapeutic exposure in pregnancy and was quantitatively superior to 800/100 mg q24h in pregnancy, based on total and unbound darunavir exposure (AUC0–τ and Ctrough) and probability of therapeutic exposure. It has been argued to limit the use of darunavir/ritonavir 800/100 mg q24h to antiretroviral-naive pregnant women for whom PI mutations can be excluded. For these women, lower target concentrations have been proposed (0.2 mg/L).11 In addition, in case of good adherence and therapeutic drug monitoring, q24h dosing can be considered. In all other cases though, our analysis indeed suggests that darunavir/ritonavir 600/100 mg q12h should be the regimen of preference during pregnancy.
Supplementary Material
Acknowledgements
This work was presented at the Population Approach Meeting, Montreux, Switzerland, 2018 (Abstract IV-75).
We thank the patients for participating in the studies. We thank the staff from the centres participating in the PANNA network: M. E. van der Ende, Erasmus MC Rotterdam, The Netherlands; A. J. A. M. van der Ven, Radboud university medical center, Nijmegen, The Netherlands; A. Antinori, IRCSS, Roma, Italy; E. Nicastri, MD, National Institute for Infectious Diseases ‘L. Spallanzani’, Rome, Italy; C. Giaquinto, University of Padua, Italy; K. Weizsäcker, Klinik für Geburtsmedizin, Charité Universitätsmedizin, Berlin, Germany; A. Gingelmaier, Klinikum der Universität München, Frauenklinik Innenstadt, München, Germany; F. Lyons, St James’s Hospital Dublin, Ireland; J. Lambert, Mater Misericordiae University Hospital Dublin, Ireland; C. Wyen and G. Faetkenheuer, University of Cologne, Germany; J. K. Rockstroh and C. Schwarze-Zander, University of Bonn, Germany; S. Tariq Sadiq, Institution for Infection and Immunity, St George’s, University of London, London, UK; Y. Gilleece, Brighton and Sussex University Hospitals NHS Trust, Brighton, UK; C. Wood, North Middlesex University Hospital, London, UK. We thank T. P. Dorlo, PhD, NKI, The Netherlands, for valuable input to the manuscript.
Funding
Funding was received for some of the individual clinical trials from which data were used in this project (listed below). However, the current study did not receive any specific funding. The Pharmacokinetics of newly developed ANtiretroviral agents in HIV-infected pregNAnt women (PANNA) network is funded by European AIDS Treatment Network (NEAT)/Paediatric European Network for Treatment of AIDS (PENTA), Bristol-Myers Squibb (BMS), Merck, ViiV Healthcare and Janssen Pharmaceutica. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH).
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1. Gilbert EM, Darin KM, Scarsi KK. et al. Antiretroviral pharmacokinetics in pregnant women. Pharmacotherapy 2015; 35: 838–55. [DOI] [PubMed] [Google Scholar]
- 2. Harris NS, Fowler MG, Sansom SL. et al. Use of enhanced perinatal human immunodeficiency virus surveillance methods to assess antiretroviral use and perinatal human immunodeficiency virus transmission in the United States, 1999–2001. Am J Obstet Gynecol 2007; 197 Suppl: S33–41. [DOI] [PubMed] [Google Scholar]
- 3. Townsend CL, Byrne L, Cortina-Borja M. et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000–2011. AIDS 2014; 28: 1049–57. [DOI] [PubMed] [Google Scholar]
- 4.FDA. FDA: Label PREZCOBIX https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205395s000lbl.pdf.
- 5.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States November 2017. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 6. Zorrilla CD, Wright R, Osiyemi OO. et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100 mg administered twice daily. HIV Med 2014; 15: 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Courbon E, Matheron S, Mandelbrot L. et al. Safety, efficacy and pharmacokinetic of darunavir/ritonavir containing regimen in pregnant HIV-infected women. In: Abstracts of the Nineteenth Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 2012. Abstract 1011. Foundation for Retrovirology and Human Health, Alexandria, VA, USA.
- 8. Curran A, Ocana I, Deig E. et al. Darunavir/ritonavir once daily total and unbound plasmatic concentrations in HIV-infected pregnant women. In: Abstracts of the Fourteenth International Workshop on Clinical Pharmacology of HIV Therapy, Amsterdam, The Netherlands, 2013. Abstract P-19.
- 9. Colbers A, Moltó J, Ivanovic J. et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother 2015; 70: 534–42. [DOI] [PubMed] [Google Scholar]
- 10. Stek A, Best BM, Wang J. et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquir Immune Defic Syndr 2015; 70: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khoo S, Peytavin G, Burger D. et al. Pharmacokinetics and safety of darunavir/ritonavir in HIV-infected pregnant women. AIDS Rev 2017; 19: 16–23. [PubMed] [Google Scholar]
- 12. Taylor GP, Clayden P, Dhar J. et al. British HIV Association guidelines for the management of HIV infection in pregnant women 2012. HIV Med 2012; 13 Suppl 2: 87–157. [DOI] [PubMed] [Google Scholar]
- 13. Stek A, Best B, Caparelli E. et al. Pharmacokinetics of increased dose darunavir during late pregnancy and postpartum. In: Abstracts of the Twenty-third Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA, 2016. Abstract 775. Foundation for Retrovirology and Human Health, Alexandria, VA, USA.
- 14. Moltó J, Xinarianos G, Miranda C. et al. Simultaneous pharmacogenetics-based population pharmacokinetic analysis of darunavir and ritonavir in HIV-infected patients. Clin Pharmacokinet 2013; 52: 543–53. [DOI] [PubMed] [Google Scholar]
- 15. Abduljalil K, Furness P, Johnson TN. et al. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet 2012; 51: 365–96. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Prezista, Summary of Product Characteristics 2006.
- 17. Holmstock N, Annaert P, Augustijns P.. Boosting of HIV protease inhibitors by ritonavir in the intestine: the relative role of cytochrome P450 and P-glycoprotein inhibition based on Caco-2 monolayers versus in situ intestinal perfusion in mice. Drug Metab Dispos 2012; 40: 1473–7. [DOI] [PubMed] [Google Scholar]
- 18. Colbers A, Greupink R, Litjens C. et al. Physiologically based modelling of darunavir/ritonavir pharmacokinetics during pregnancy. Clin Pharmacokinet 2016; 55: 381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergstrand M, Karlsson MO.. Handling data below the limit of quantification in mixed effect models. AAPS J 2009; 11: 371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arab-Alameddine M, Lubomirov R, Fayet-Mello A. et al. Population pharmacokinetic modelling and evaluation of different dosage regimens for darunavir and ritonavir in HIV-infected individuals. J Antimicrob Chemother 2014; 69: 2489–98. [DOI] [PubMed] [Google Scholar]
- 21. Dosne AG, Bergstrand M, Harling K. et al. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn 2016; 43: 583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Svensson E, van der Walt JS, Barnes KI. et al. Integration of data from multiple sources for simultaneous modelling analysis: experience from nevirapine population pharmacokinetics. Br J Clin Pharmacol 2012; 74: 465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu A, Granneman GR, Cao G. et al. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob Agents Chemother 1998; 42: 2784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu A, Granneman GR, Witt G. et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother 1997; 41: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keizer RJ, van Benten M, Beijnen JH. et al. Piraña and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput Methods Programs Biomed 2011; 101: 72–9. [DOI] [PubMed] [Google Scholar]
- 26. Keizer RJ, Karlsson MO, Hooker A.. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Meyer S, Azijn H, Surleraux D. et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother 2005; 49: 2314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schalkwijk S, Greupink R, Burger D.. Free dug concentrations in pregnancy: bound to measure unbound? Br J Clin Pharmacol 2017; 83: 2595–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 2005; 44: 989–1008. [DOI] [PubMed] [Google Scholar]
- 30. Colbers A, Timing of the postpartum curve in pharmacokinetic studies in pregnancy should not be too early In: Abstracts of the Seventeenth International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, DC, USA, 2016. Abstract O3. [Google Scholar]
- 31. Moltó J, Barbanoj MJ, Miranda C. et al. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet 2008; 47: 681–92. [DOI] [PubMed] [Google Scholar]
- 32. Ernest CS 2nd, Hall SD, Jones DR.. Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther 2005; 312: 583–91. [DOI] [PubMed] [Google Scholar]
- 33. Vermeir M, Lachau-Durand S, Mannens G. et al. Absorption, metabolism, and excretion of darunavir, a new protease inhibitor, administered alone and with low-dose ritonavir in healthy subjects. Drug Metab Dispos 2009; 37: 809–20. [DOI] [PubMed] [Google Scholar]
- 34. Patterson KB, Dumond JB, Prince HA. et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr 2013; 63: 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dallmann A, Ince I, Meyer M. et al. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin Pharmacokinet 2017; 56: 1303–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.