Abstract
Background:
Increased life expectancy in sickle cell disease (SCD) has resulted in increased recognition of the consequences of repeated intravascular vaso-occlusion and chronic hemolysis to multiple organ systems.
Aim:
To report the long-term consequences of liver dysfunction in SCD.
Methods:
A cohort of SCD patients was prospectively evaluated at the NIH Clinical Center. The association of mortality with liver enzymes, parameters of liver synthetic function and iron overload was evaluated using Cox regression.
Results:
247 SCD patients were followed for 30 months of whom 22 (9%) died. After controlling for predictors, increased direct bilirubin, ferritin, alkaline phosphatase and decreased albumin were independently associated with mortality. In a multivariable model, only high direct bilirubin and ferritin remained significant. Ferritin correlated with hepatic iron content and total blood transfusions but not hemolysis markers. 40 patients underwent liver biopsies and 11 (28%) had fibrosis. 12 of 26 patients (48%) had portal hypertension by hepatic venous pressure gradient (HVPG) measurements. All patients with advanced liver fibrosis had iron overload; however most patients (69%) with iron overload were without significant hepatic fibrosis. Ferritin did not correlate with left ventricular dysfunction by echocardiography. Direct bilirubin correlated with bile acid levels suggesting liver pathology. Platelet count and soluble CD14 correlated with HVPG indicating portal hypertension.
Conclusions:
Ferritin and direct bilirubin are independently associated with mortality in SCD. Ferritin likely relates to transfusional iron overload, while direct bilirubin suggests impairment of hepatic function, possibly impairing patients’ ability to tolerate systemic insults.
Keywords: sickle cell disease, portal hypertension, direct bilirubin, ferritin, iron overload, cirrhosis, nodular regenerative hyperplasia
Introduction
Sickle cell disease (SCD) is associated with significantly reduced long-term survival. Patients with SCD typically die of septic and/or vascular complications1, however management improvements have led to greater life expectancy2–4. With prolonged survival, there has been increasing recognition of the consequences of repeated intravascular sickling and chronic hemolysis to multiple organ systems2.
Known liver complications of SCD include acute intrahepatic vaso-occlusion, hepatic sequestration and chronic or acute intrahepatic cholestasis. Additionally, therapy of SCD can cause liver injury from transfusional iron overload and transfusion-related viral hepatitis. The frequency of cirrhosis from one or several of these complications has been reported at autopsy as high as 11% to 14%5, 6. Less recognized is the complication of non-cirrhotic portal hypertension due to chronic portal venopathy and nodular regenerative hyperplasia, which has been increasingly reported in patients with chronic illnesses7–10. Despite this multiplicity of hepatic complications, liver failure and end-stage liver disease are rarely identified as the immediate cause of death in patients with SCD1; however chronic liver disease is often silent, may require decades to manifest and affect morbidity and mortality and is often listed a contributory rather than the primary cause of death.
To assess the impact of hepatic injury in SCD, predictors of mortality in a large cohort of prospectively followed patients with SCD were evaluated, with a particular focus on liver-related factors.
Methods
Patients
A cohort of SCD patients followed prospectively in a natural history protocol at the National Institutes of Health (NIH) was evaluated (NCT00081523 & NCT00001971). All co-authors had access to the data and reviewed and approved the final manuscript. Baseline demographic, clinical and laboratory data were collected. Patients with suspected liver-related complications of SCD were referred for complete hepatologic evaluation by the hepatology consult service between January 2005 through July 2008. Reasons for referral included persistent elevation of alanine aminotransferase (ALT), alkaline phosphatase (ALP) or direct bilirubin (DB), elevation of ferritin or other iron parameters, and evidence of viral hepatitis. Because the focus was on chronic liver injury, all laboratory analyses were performed at least 2 weeks after or before any acute illness. If clinically indicated, patients were offered liver biopsy. Liver biopsies were preferentially performed by the transjugular route which allowed for estimation of the hepatic venous pressure gradient (HVPG). Hepatic iron load was evaluated (see supplemental methods) and soluble CD14 was measured according to the manufacturer’s protocol (R&D systems, Minneapolis, MN, USA).
Statistical Analysis
To assess the importance of liver parameters on mortality, Cox proportional hazards regression was performed. Liver variables included liver-associated enzymes (aspartate aminotransferase [AST], ALT, ALP, lactate dehydrogenase [LDH]), measures of hepatic synthetic function (total and DB, prothrombin time [PT] and serum albumin) and serum iron indices (serum iron, transferrin and ferritin). Time to event was calculated using date of first laboratory data at the NIH and date of final follow-up or death. For details please see supplementary methods section.
Results
Patients
A total of 247 patients were followed prospectively in a natural history protocol on sickle cell disease for a median of 30 months (13–49). The average age was 36.2 years (range 18–74) and 40% were males. All were anemic and had symptoms of SCD. Patients underwent standard therapies for sickle cell disease. 85 of 247 were treated with hydroxyurea and 186 of 247 with folic acid. Of those referred for evaluation, 17 of 80 (22%) were on chelation therapy. In those with Fe overload (Deugnier>20), only 11 of 24 (46%) were on chelation at the time of referral, including only 2 of 4 with iron overload and advanced fibrosis. No patients without Fe overload were on chelation therapy. Only 16% had elevated ALT and 33% elevated ALP values. Viral hepatitis testing was performed in a subpopulation of the cohort, including all that were referred for hepatologic evaluation. Chronic hepatitis B was diagnosed in 1 out of 123 patients tested and chronic hepatitis C was diagnosed in 9 out of 118 patients tested (12 were anti-HCV antibody positive). No other chronic liver diseases, including alcoholic liver disease, were identified in all patients referred for hepatologic evaluation.
Upon reviewing mortality for this cohort, it became apparent that those with markers of liver disease died more frequently. Therefore, patients with abnormal liver associated tests were evaluated. Comparing individuals referred for hepatologic evaluation with those who were not, only mean AST was significantly higher in the referred group (AST 48 vs 40 IU/mL, p=0.01) where as no significant differences were seen in ALT, ALP, ALB, direct bilirubin and ferritin. 80 patients underwent hepatology evaluation of which 40 were offered and agreed to liver biopsy. Ishak fibrosis scores ranged from 0–6 (median 0) and HAI inflammatory scores ranged from 1–9 (median 3). Those who underwent liver biopsy had higher baseline ALT (40 vs 25 U/L, p=0.004), AST (60 vs 38 U/L, p=0.02) and ferritin (2365 vs 874 μg/L, p=0.005) levels than those who did not have a biopsy. Portal venopathy was seen in 23% of patients and central venopathy in none. Portal venopathy scores correlated with increasing HVPG (ρ=0.45, p=0.016). Of particular interest, regenerative changes were noted in more than half of patients and nodular regenerative hyperplasia (defined as a score of 2 or 3 on a scale of 0–3) was seen in 36%. The regeneration score also correlated with HVPG (ρ=0.40, p=0.036).
Mortality
Twenty-two (9%) patients died during follow-up; 18 of complications possibly related to SCD (8-sudden death, 4-sepsis, 2-stroke, 4-multiorgan failure including one with liver failure and 4-unknown). Baseline characteristics comparing the 225 patients who were alive at final follow up to the 22 who died are shown in Table 1.
Table 1.
Baseline Characteristics of Patients Who Died and Those Alive at the end of Follow-up
| Patients Alive at End of Follow-up (n=225) |
Patients Who Died (n=22) |
P-Value♦ |
|
|---|---|---|---|
| Age (Years)* | 35.7 (18–70) |
41.2 (18–74) |
0.03 |
| Follow-up (Months)* | 27 (1.3–4.9) | 20 (0.1–45) | 0.012 |
| Male | 87 (39%) | 12 (55%) | 0.15 |
|
SS SC Other |
181 (80%) 38 (17%) 6 (3%) |
17 (77%) 4 (18%) 1 (5%) |
0.86 |
|
SCD Complications >12 Pain Crises/Year Stroke Leg Ulcers Priapism >10 Lifetime Transfusions Severe Pulmonary Hypertension** |
94 (48%) 38 (17%) 48 (22%) 34 (40%) 75 (43%) 23 (10%) |
13 (59%) 1 (5%) 4 (21%) 6 (60%) 4 (50%) 6 (21%) |
0.14 0.15 0.91 0.24 0.70 0.02 |
| Body Mass Index [kg/m2]* | 25.4 (4.0) | 25.1 (8.7) | 0.89 |
|
Baseline Lab Values
Hematologic (SD)* Hemoglobin (g/dL) White Blood Count (1,000/μL) Platelets (1,000/μL) Renal* Creatinine (mg/dL) Blood Urea Nitrogen (mg/dL) Iron Indices* Serum Iron (μg/dL) Ferritin (μg/L) Transferrin (mg/dL) Liver* ALT (U/L) >ULN n(%) AST (U/L) >ULN n(%) ALP (U/L) >ULN n(%) LDH (U/L) Albumin (g/L) >ULN n(%) Bilirubin - Direct (mg/dL) >ULN n(%) Total (mg/dL) >ULN n(%) |
9.5 (1.8) 10.0 (3.5) 353 (133) 0.84 (1.0) 10.3 (10) 99 (53) 754 (1137) 209 (52) 26 (14) 30 (14) 40 (21) 107 (50) 109 (84) 71 (33) 352 (168) 4.1 (0.4) 44 (20) 0.44 (0.3) 115 (53) 2.6 (1.8) 179 (83) |
9.1 (2.0) 12.3 (5.0) 384 (149) 1.51 (2.9) 14.6 (11) 125 (56) 2125 (2096) 175 (51) 32 (17) 6 (29) 48 (25) 14 (67) 173 (86) 15 (17) 383 (136) 3.8 (0.4) 10 (48) 0.83 (0.7) 18 (86) 2.6 (1.5) 19 (90) |
0.38 0.006 0.32 0.023 0.07 0.053 <0.0001 0.005 0.10 0.10 0.11 0.17 0.001 0.001 0.44 0.0004 0.011 <0.0001 0.005 0.95 0.54 |
| Anti-HCV +ve HBsAg +ve |
10/112 (9%) 1/115 (0.9%) |
2/6 (33%) 0/8 (0%) |
0.054 0.79 |
Pulmonary hypertension defined as tricuspid regurgitant jet > 2.5 m/s
Hepatitis B testing available in 123 and hepatitis C testing in 118 patients.
Mean (standard deviation), normal ranges of laboratory values are available at http://cclnprod.cc.nih.gov/dlm/testguide.nsf/HRRAll?OpenForm&Count=1183
p-value calculated using student’s t-test for continuous variables or Fisher’s exact test for proportions.
By Cox proportional hazards regression, iron indices (ferritin, transferrin and iron) and liver parameters (ALP, DB and albumin) were associated with increased mortality (Table 2). Although baseline creatinine was higher in patients who died, this was not significant by univariate Cox regression and did not affect the multivariable models. By stepwise multivariable Cox regression, only ferritin (log-transformed), DB, and age remained independently associated with mortality after controlling for factors that have been previously shown to associated with mortality in sickle cell disease (age, sex, WBC, DB, log ferritin and pulmonary hypertension) (Table 3).11 The proportional hazards assumptions were valid in all models.
Table 2.
Cox Regression Analysis for Association with Mortality
| Parameter§ | Unadjusted HR (95% CI) |
Unadjusted P-value* |
HR (95% CI)^ Adjusted for PH and WBC | P-Value* Adjusted for PH and WBC |
|---|---|---|---|---|
|
Log Ferritin Transferrin > 165 mg/dL ≤ 165 mg/dL Iron < 90 μg/dL ≥ 90 μg/dL |
1.9 (1.4–2.7) 1.0 3.9 (1.6–9.2) 1.0 3.6 (1.3–10.4) |
<0.0001 0.002 0.01 |
1.6 (1.2–2.3) 1.0 2.4 (0.91–6.1) 1.0 2.7 (0.93–8.0) |
0.02 0.08 0.05 |
|
ALT
AST < 37 U/L ≥ 37 U/L Log ALP LDH <324 U/L ≥ 324 U/L |
1.2 (1.0–1.5) 1.0 2.2 (0.89–5.5) 3.8 (2.0–7.1) 1.0 2.1 (0.78–5.4) |
0.14 0.086 <0.0001 0.13 |
1.1 (0.98–1.5) 1.0 1.1 (0.40–3.0) 2.2 (1.0–4.5) 1.0 1.1 (0.40–3.0) |
0.42 0.85 0.05 0.87 |
|
Direct Bilirubin Total Bilirubin Albumin |
2.8 (1.8–4.4) 1.0 (0.77–1.3) 0.16 (0.07–0.39) |
<0.0001 1.0 <0.0001 |
2.4 (1.4–4.2) 0.90 (0.68–1.2) 0.29 (0.11–0.76) |
0.02 0.47 0.02 |
| Anti-HCV+ | 3.3 (0.59–17.9) | 0.18 | 4.0 (0.65–25.1) | 0.13 |
Parameters were evaluated in regression models as continuous variables, by quartile and as dichotomous variables (dichotomized at median, or at the 25th or 75th percentile), as appropriate for the relationship with mortality
P-values calculated using likelihood ratio test
Hazard ratios adjusted for presence of pulmonary hypertension and white blood cell count
Table 3.
Stepwise Multivariable Cox Regression for Association with Mortality
| Adjusted HR (95% CI)* | Adjusted P-Value* | |
|---|---|---|
| Age | 1.04 (1.00–1.09) | 0.047 |
| Log Ferritin | 1.90 (1.34–2.71) | <0.001 |
| Direct Bilirubin | 2.68 (1.54–4.68) | 0.001 |
Hazard ratio and p-value adjusted for other factors significant by univariate analysis as well as age, sex, WBC, direct bilirubin, log ferritin and pulmonary hypertension. P-value determined by likelihood ratio test.
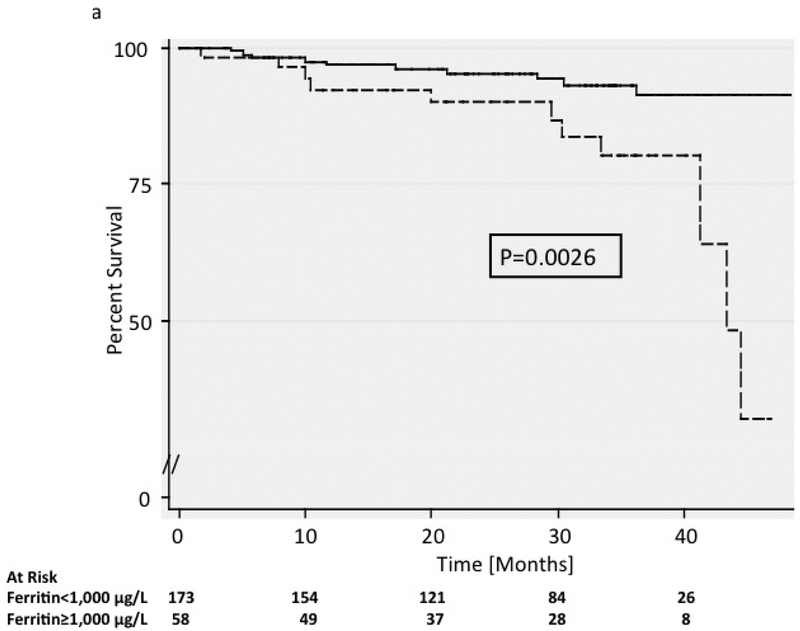
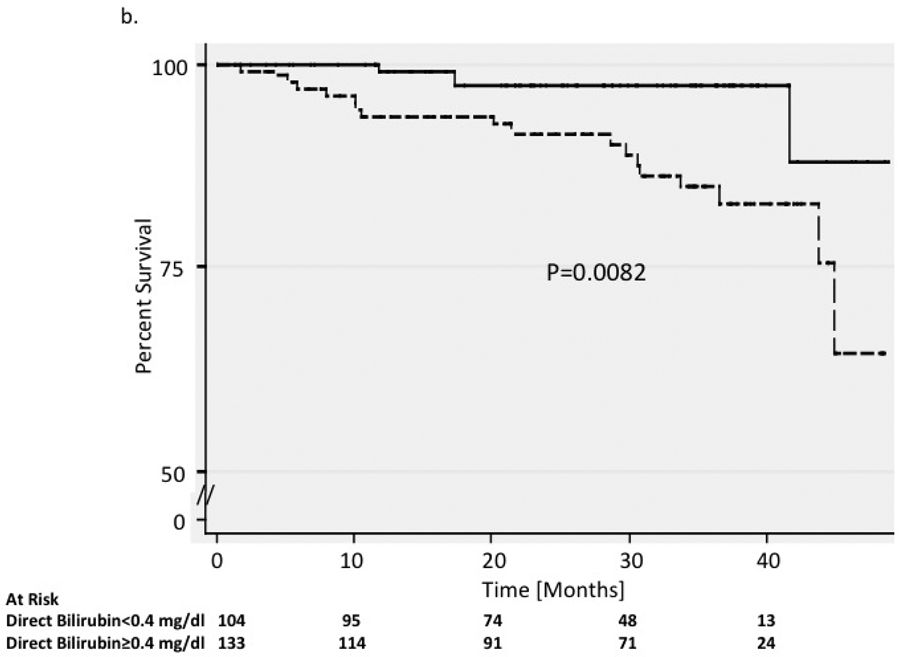
Mean ferritin level at the initial visit was 2,125 mg/dL (range 31 to 6,865 mg/dL) in the 22 who died during follow-up compared to 754 mg/dL (range 11 to 6,429 mg/dL) in survivors (p<0.0001). The 3-year mortality was 19.9% (95% CI 0.11–0.37) in those with ferritin greater than 1,000 mg/dL, compared to 7.0% (95% CI 0.036–0.13) in those with lower ferritin levels (p=0.003) (Figure 1a). Serum iron and transferrin levels were not significantly different between groups. DB levels were also associated with mortality, ranging from 0.2 to 3.2 (mean = 0.83) mg/dL in the 22 patients who died, compared to 0.1 to 3.6 (mean = 0.44) mg/dL in survivors (p<0.0001). The 3-year mortality rate was 17.1% (95% CI 0.12–0.27) in patients with DB ≥0.4 mg/dL at initial visit compared to 2.4% (95% CI 0.01–0.094) in those with lower bilirubin levels (Figure 1b). There was no difference in total bilirubin or liver enzyme levels between groups.
Figure 1. Survival.
Kaplan-Meier survival curves are shown for patients with a) ferritin levels above and below 1,000 μg/L and b) patients with direct bilirubin levels above and below 0.4 mg/dL. P-values were calculated using the log-rank test. In figure 1a, patients with elevated ferritin greater than 1000 μg/L had a lower rate of survival compared with those who had ferritin levels less than 1000 μg/L. In figure 1b, patients with elevated direct bilirubin levels above 0.4 mg/dL had a lower rate of survival compared with those who had direct bilirubin levels below 0.4 mg/dL.
Ferritin
By multivariable logistic regression, ferritin levels ≥1,000 μg/L were independently associated with >10 lifetime blood transfusions, higher WBC and elevated ALT levels (Table 4). LIC from 32 liver biopsy specimens ranged from 181 to 49,642 mg/g dry weight, above the accepted safety level of 4,000 mg/g dry weight in 23 (72%). Only 9 of 23 patients were on chelation therapy before the liver biopsy and the remaining patients were started after liver biopsy. The different methods of assessing hepatic iron load (LIC, Deugnier and Rowe iron staining scores) showed high concordance (see Supplementary Figure 1). Serum ferritin correlated well with liver biopsy assessments of hepatic iron (LIC: ρ=0.87, p<0.0001, Deugnier score: ρ=0.90, p<0.0001) (see Supplementary Figure 2a, b). For every increase of 1,000 μg/L of ferritin, the LIC increased by 3,825 (95% CI 2,036–5,614) mg/g dry weight and the iron score increased by 3.6 on the Deugnier scale.
Table 4.
Logistic Regression for Associations with Ferritin > 1,000 μg/Land Direct Bilirubin > 0.4 mg/dL
| Parameter | OR (95% CI) |
P-value | Adjusted OR (95% CI)* | Adjusted P-Value* |
|---|---|---|---|---|
| Ferritin >1,000 μg/L | ||||
| > 10 Lifetime Transfusions | 20.1 (7.3–55.2) |
<0.0001 | 17.1 (5.9–49.6) |
<0.0001 |
| WBC | 1.2 (1.1–1.3) |
<0.0001 | 1.3 (1.1–1.4) |
0.002 |
|
ALT† AST† ALP Protein-Albumin |
1.3 (1.1–1.5) 1.1 (1.0–1.3) 3.6 (2.0–6.7) 1.9 (1.2–2.9) |
0.002 0.052 <0.0001 0.005 |
1.4 (1.1–1.8) |
0.023 |
|
Log BUN Log BNP |
2.5 (1.6–4.1) 1.3 (1.1–1.6) |
<0.0001 0.014 |
||
| Direct Bilirubin (>0.4 g/L) | ||||
|
LDH† AST† Arginine Iron† WBC |
1.1 (1.1–1.1) 1.8 (1.6–11.1) 0.98 (0.96–0.99) 1.1 (1.1–1.2) 1.3 (1.2–1.4) |
<0.0001 <0.0001 0.009 <0.0001 <0.0001 |
(1.0–1.1) (1.0–1.2) 1.2 (1.1–1.4) |
<0.0001 0.003 0.008 |
|
IVC Diameter Mean Arterial Pressure† |
1.2 (1.1–1.3) 0.97 (0.95–0.99) |
<0.0001 0.009 |
1.2 (1.1–1.4) 0.96 (0.93–0.98) |
0.003 0.002 |
|
ALT† ALP Protein-Albumin |
1.6 (1.3–1.8) 3.0 (1.7–5.4) 1.7 (1.1–2.5) |
<0.0001 <0.0001 0.015 |
||
Odds ratio and p-value adjusted for other factors significant by univariate analysis.
Odds ratio reflects change of 10 units for AST, iron, mean arterial blood pressure and ALT and 100 units for LDH.
BNP – Brain Natriuretic Peptide; BUN – Blood Urea Nitrogen
Although ferritin levels may be elevated from causes other than iron overload, in patients with ferritin >1,000 μg/L who underwent liver biopsy, all 20 had an LIC of at least 4,000 mg/g dry weight. Ferritin was unlikely to be related to infection or inflammation, as all patients were evaluated at least 2 weeks from resolution of any acute illness, including veno-occlusive crisis. Ferritin and hepatic iron measurements correlated well with self-reported estimates of lifetime blood transfusions (see Supplementary Figure 3a, b). Increasing ferritin and hepatic iron measurements were associated with increasing liver fibrosis but not inflammation. Patients with advanced fibrosis (Ishak≥3) had higher average LIC values (25,070±10,546 vs. 9,270±1,791 mg/g dry weight, p=0.013), Deugnier scores (39.8±5.2 vs. 23.0±2.1, p=0.007) and ferritin (7,663±2,539 vs. 1,677±274 μg/L, p=0.008) than those with milder liver disease. All with advanced fibrosis had iron overload (ferritin range 1,473–15,780 μg/L, Deugnier score range 22–53 and LIC range 7,487–49,652 mg/g dry weight). However, in contrast, many with severe iron overload had minimal or no fibrosis. Of patients with Deugnier scores >20, 22 of 32 (69%) patients had Ishak fibrosis scores of 0 or 1. Patients with iron overload and advanced fibrosis had higher ALT (90 vs 21 U/L, p=0.0001), AST (163 vs 39 U/L, p=0.0001), and ALP (231 vs 108 U/L, p=0.002) levels, more inflammation on liver biopsy (HAI 7 vs 2, p=0.006) and more significant iron overload (ferritin 7663 vs 2390 μg/L, p=0.0007, Deugnier Score 42 vs 32, p=0.02, LIC 30,932 vs 14,167 mg/g, p=0.03) than patients with high iron scores but minimal fibrosis.
Echocardiographic estimation of ejection fraction (EF) was available in 123 (54%) from the main survival cohort, of whom 50 (41%) had impaired left ventricular (LV) function (EF<60%). EF was similar in patients with ferritin levels above and below 1,000 μg/L and ferritin levels were similar in patients with normal and depressed LV function. Declining EF was associated with increased mortality (HR 0.92 95% CI 0.85–0.99, p=0.039), but this was no longer significant after controlling for ferritin (HR 0.94 95% CI 0.86–1.0, p=0.073).
Bilirubin
Factors associated with increased DB included diameter of the inferior vena cava (IVC), mean arterial blood pressure, LDH, iron and WBC (Table 4). The presence of HCV antibody was marginally associated with DB elevation (OR 7.2, 95% CI 0.90–58.1, p=0.063).
To determine if DB levels suggested hepatic pathology rather than hemolysis, serum levels of total and individual bile acids were measured. Primary bile acids, cholic and chenodeoxycholic acid, which are synthesized in the liver, and secondary bile acids, deoxycholic and ursodeoxycholic acid, which depend upon conjugation by gut bacteria, were measured. Of patients with elevated DB levels, 81% had total bile acids above normal (7 μmol/L). DB levels correlated with levels of total bile acids and primary bile acids but not with levels of secondary bile acids (see Supplementary Table 1). The correlation between bile acid levels and DB was strongest in patients with sinusoidal portal hypertension (HVPG≥5 mmHg) (see Supplementary Table 1). As expected, total bilirubin levels correlated with DB levels, however total bilirubin did not correlate with bile acid levels (see Supplementary Table 1). ALP, GGT, ALT and AST correlated with total and primary bile acid levels, in a similar pattern to that seen with DB. DB was negatively correlated with albumin (r=−0.27, p<0.00001). Collectively these data suggest that DB elevations in SCD reflect liver pathology to a greater extent than hemolysis.
The relationship between hepatic biomarkers and portal hypertension was assessed. Although platelet count has not been considered a reliable marker of portal hypertension in SCD due to autosplenectomy, there was a significant negative correlation between platelet count and HVPG (r=−0.65, p=0.0003) (Supplementary Figure 4). In the cohort as a whole, among patients with an elevated DB, there was a negative correlation between DB and platelet count. For every increase in 1 mg/dL of DB, platelets decreased by 49,900/μL(95% CI −96,000 to −3,740/μL, p=0.034).
Microbial Translocation:
Portal hypertension has been postulated to increase intestinal microbial translocation12. To assess microbial translocation, levels of soluble CD14 (sCD14), which is released upon monocyte or macrophage stimulation by lipopolysaccharide (LPS)13, were measured. sCD14 levels are considered a more consistent assessment of serum LPS concentration14. sCD14 correlated with HVPG, but only in patients with elevated portal pressures (Supplementary Figure 5a, b). sCD14 levels correlated with TRV (r=0.39, p=0.003), which is an indicator of the post-hepatic component to portal pressure. Similarly, IgG levels, which also increase in portal hypertension, correlated with sCD14 (r=0.30, p=0.02). Collectively, this suggests that even though patients did not have overt clinical manifestations of portal hypertension, in keeping with the relatively low HVPG values, those with HVPG measurements above 5 mmHg still had physiologically relevant sinusoidal portal hypertension.
Discussion
As patients are living longer with SCD, the causes of mortality in this disease are becoming more complex. Improvements in survival have been achieved through understanding and improved management of acute complications as well as the use of strategies to reduce their frequency2. One consequence of this success has been the increasing significance of the chronic complications that develop in multiple organs after decades of disease. One such organ includes the liver, and in fact, liver disease is under-diagnosed as a cause of death in general15, 16. This has been shown using careful analyses of death records; usually focusing on hepatitis C, but it most likely applies to all liver disease. This may be an important reason that the contribution of liver disease to mortality in SCD has been underappreciated. In this study, we focused on the importance of hepatic involvement to survival in SCD. In a large cohort of well-characterized patients with SCD, elevated ferritin and DB were independently associated with mortality.
In an effort to gain understanding of the mechanisms underlying the observed associations, factors associated with ferritin and bilirubin elevation were determined in the cohort as a whole and in a smaller cohort with more detailed evaluation. Ferritin elevation appeared to reflect predominantly iron overload.
Previous studies have suggested that ferritin does not correlate well with transfusion history or total body iron stores in patients with SCD. In contrast, we found that there was a very good correlation between ferritin levels, transfusion history and measurements of hepatic iron. As expected, ferritin and LIC levels varied widely and did not follow a normal distribution. The major implication of this observation is that for a given individual, it may be difficult to accurately predict hepatic iron based on ferritin17. However, the major utility of ferritin is to identify patients who are likely to be iron overloaded; not necessarily to define the precise degree of iron overload. This discrimination will not be affected by the lack of a truly linear relationship; effective thresholds of ferritin that require further investigation and/or therapy can still be defined. This is borne out in this cohort, in which all 20 patients with ferritin values over 1,000 μg/L who underwent liver biopsy had LIC values of at least 4,000 mg/g dry weight, above the threshold proposed as a conservative goal to prevent complications18. A recent study reported that regularly transfused SCD patients were more likely to require hospitalization and to die, suggesting more severe disease, but of concern, those that died started chelation therapy much later in life than those who survived19, suggesting that iron overload itself may contribute to mortality. These findings suggest that the relationship between ferritin levels and mortality likely relates directly to the degree of transfusional iron overload, and thus emphasizes the need for early and aggressive chelation therapy.
Although greater degrees of iron overload were on average associated with more hepatic fibrosis, many patients had severe iron overload with little or no fibrosis17, 20, 21. However, all patients with advanced fibrosis were iron overloaded, suggesting that iron is necessary but insufficient to cause hepatic fibrosis in SCD. It is likely that genetic factors, duration of iron overload and the presence of other insults, influence the outcome of iron overload in patients with SCD.
Markers of liver disease can be challenging to evaluate in patients with SCD22, 23. Mild elevations of bilirubin are often assumed related to hemolysis and although DB should not be affected by hemolysis, the accuracy of measurements may be poor in the presence of high total bilirubin. To clarify this, bile acids were measured. Direct, but not total bilirubin correlated with bile acid levels and specifically, DB correlated only with levels of primary bile acids, which are made in the liver, but not with secondary bile acids, which rely on conjugation by gut bacteria. Higher DB was also associated with portal hypertension and evidence of impaired liver synthetic function. The increased bilirubin load resulting from hemolysis may act as ‘stress-test’ to the liver, thus helping to identify otherwise subclinical impairment of hepatic function.
If the association of elevated DB with mortality is related to intrinsic liver disease, the question arises as to the type of liver injury that is occurring. Patients with SCD are at risk for numerous hepatic insults. Pulmonary hypertension is a well-recognized complication and significant predictor of mortality in SCD.3 The association between DB and IVC diameter suggests that chronic hepatic congestion due to pulmonary hypertension may contribute to the elevation of DB. However bilirubin elevation was associated with mortality even after controlling for pulmonary hypertension. In fact, pulmonary hypertension was no longer associated with mortality after inclusion of bilirubin and ferritin in the model, making it unlikely that bilirubin elevation represents only congestion from increased pulmonary pressure.
Patients with SCD also develop non-cirrhotic portal hypertension, likely as a consequence of injury to the hepatic microvasculature24. In fact, both portal venopathy and regenerative changes, including nodular regenerative hyperplasia, were relatively common in this cohort and were associated with increasing HVPG. In the subset of patients with measurement of HVPG, there was a negative correlation between platelet count and HVPG, and in the whole cohort, there was a negative correlation between bilirubin and platelet count in those with elevated DB. In addition, patients with portal hypertension had elevated levels of sCD14, suggesting increased microbial translocation, potentially increasing the risk of infection, a major cause of morbidity and mortality in SCD in general1, and in this cohort specifically. While the presence of asplenia may affect sCD14 levels, all patients in this cohort were asplenic. Therefore, the differences seen between patients are more likely from causes other than asplenia. The risk of infection may be further compounded in patients with SCD due to secondary iron overload by providing bacteria with increased access to iron25. Taken together, these data suggest that elevation of DB is a marker of intrinsic liver disease and possibly portal hypertension, likely due to multiple etiologies, and furthermore that this hepatic impairment is associated with mortality.
However the question remains: why is this degree of hepatic dysfunction associated with mortality? Although not generally severe enough to cause end-stage liver disease with its usual complications, mild degrees of liver dysfunction, particularly if compounded by portal hypertension may be sufficient to impair the response to systemic insults such as infection26. This is compounded by a predisposition to infection in SCD, which is increased further once portal hypertension, and potentially iron overload, develop27, 28. The observation that subtle degrees of hepatic impairment and relatively modest portal hypertension can increase the risk of mortality, particularly in patients with chronic systemic illnesses, is an important emerging concept_ENREF_4029.
Conclusions
Elevated ferritin and DB are associated with an increased risk of mortality in patients with SCD. Ferritin levels are associated with transfusional iron overload supporting close monitoring and early and aggressive chelation therapy once ferritin levels increase. Direct hyperbilirubinemia suggests subtle hepatic dysfunction and possibly portal hypertension, which, although not severe enough to cause overt symptoms, may impair the ability to respond to systemic insults and thus cause significant morbidity and contribute to mortality.
Supplementary Material
Supplementary Figure 1. Correlation between Liver Iron Concentration and Deugnier Iron Staining Score. The p-value was calculated using Spearman’s correlation coefficient. The Deugnier iron score significantly correlated with liver iron concentration.
Supplementary Figure 2. Correlation of ferritin and liver biopsy assessment of iron. The correlation between a) the log serum ferritin and liver iron concentration and b) the log serum ferritin and the Deugnier iron staining score. P-values were calculated using Spearman’s correlation coefficient. In figure 2a, log serum ferritin significantly correlated with liver iron concentration. In figure 2b, log serum ferritin significantly correlated with the Deugnier iron staining score.
Supplementary Figure 3. Correlation between Ferritin and Deugnier Iron Staining Score with Estimated Lifetime Blood Transfusions. The correlation between a) log serum ferritin and estimated lifetime blood transfusions and b) between Deugnier iron staining score with estimated lifetime blood transfusions. Estimated lifetime blood transfusions were categorized as: 0, 1 to 5, 6 to 10, 11–25, 26–50, 51–100 and >100. P-values were calculated using Spearman’s correlation coefficient. In figure 3a, serum log ferritin significantly correlated with estimated lifetime blood transfusions. In figure 3b, the Deugnier iron staining score significantly correlated with the estimated lifetime blood transfusions.
Supplementary Figure 4. Correlation of HVPG with platelet count. Increasing HVPG significantly correlated with decreasing platelet counts. P-values were calculated using Spearman’s correlation coefficient.
Supplementary Figure 5. Correlation of HVPG and sCD14 levels. The correlation between HVPG and sCD14 levels, a marker of microbial translocation, is shown in a) all patients and b) those with HVPG≥5 mmHg. P-values were calculated using Spearman’s correlation coefficient. Patients with elevated HVPG had significantly higher sCD14 levels.
Funding:
This research was supported by the Intramural Research Programs of the NIDDK (DK075008), NHLBI, NIAID, CC, and NCI, NIH.
Role of the Sponsor: None of the sponsors had any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations:
- SCD
sickle cell disease
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- HVPG
hepatic-venous pressure gradient
- TRV
tricuspid regurgitant velocity
- NIH
National Institutes of Health
- LIC
liver iron concentration
- LDH
lactic dehydrogenase
- PT
prothrombin time
- WBC
white blood cell count
- DB
direct bilirubin
Footnotes
Financial Disclosures: Disclosures: None of the authors has any financial interest or conflict of interest related to this research.
Writing Assistance: None.
References
- 1.Manci EA, Culberson DE, Yang YM, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol 2003;123:359–65. [DOI] [PubMed] [Google Scholar]
- 2.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood 2004;103:4023–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehari A, Gladwin MT, Tian X, et al. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA 2012;307:1254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TW, Moore GW, Hutchins GM. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med 1980;69:833–7. [DOI] [PubMed] [Google Scholar]
- 6.Darbari DS, Kple-Faget P, Kwagyan J, et al. Circumstances of death in adult sickle cell disease patients. Am J Hematol 2006;81:858–63. [DOI] [PubMed] [Google Scholar]
- 7.Fuss IJ, Friend J, Yang Z, et al. Nodular regenerative hyperplasia in common variable immunodeficiency. J Clin Immunol 2013;33:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain N, Feld JJ, Kleiner DE, et al. Hepatic abnormalities in patients with chronic granulomatous disease. Hepatology 2007;45:675–83. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mukhaizeem KA, Lamoureux E, Rosenberg A, et al. Nodular regenerative hyperplasia of the liver and focal global glomerulosclerosis associated with sickle cell anemia. Dig Dis Sci 2002;47:443–7. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien K, Hussain N, Warady BA, et al. Nodular regenerative hyperplasia and severe portal hypertension in cystinosis. Clin Gastroenterol Hepatol 2006;4:387–94. [DOI] [PubMed] [Google Scholar]
- 11.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004;350:886–95. [DOI] [PubMed] [Google Scholar]
- 12.Such J, Frances R, Munoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology 2002;36:135–41. [DOI] [PubMed] [Google Scholar]
- 13.Hiki N, Berger D, Prigl C, et al. Endotoxin binding and elimination by monocytes: secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun 1998;66:1135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichbaum EB, Harris HW, Kane JP, et al. Chylomicrons can inhibit endotoxin activity in vitro. J Surg Res 1991;51:413–6. [DOI] [PubMed] [Google Scholar]
- 15.Vong S, Bell BP. Chronic liver disease mortality in the United States, 1990–1998. Hepatology 2004;39:476–83. [DOI] [PubMed] [Google Scholar]
- 16.Thomas AR, Zaman A, Bell BP. Deaths from chronic liver disease and viral hepatitis, Multnomah County, Oregon, 2000. J Clin Gastroenterol 2007;41:859–62. [DOI] [PubMed] [Google Scholar]
- 17.Karam LB, Disco D, Jackson SM, et al. Liver biopsy results in patients with sickle cell disease on chronic transfusions: poor correlation with ferritin levels. Pediatr Blood Cancer 2008;50:62–5. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood 1997;89:739–61. [PubMed] [Google Scholar]
- 19.Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol 2007;82:255–65. [DOI] [PubMed] [Google Scholar]
- 20.Vichinsky E, Butensky E, Fung E, et al. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol 2005;80:70–4. [DOI] [PubMed] [Google Scholar]
- 21.Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol 2001;38:57–62. [DOI] [PubMed] [Google Scholar]
- 22.Berry PA, Cross TJ, Thein SL, et al. Hepatic dysfunction in sickle cell disease: a new system of classification based on global assessment. Clin Gastroenterol Hepatol 2007;5:1469–76; quiz 1369. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Owen C, Chopra S. Sickle cell hepatopathy. Hepatology 2001;33:1021–8. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mukhaizeem KA, Rosenberg A, Sherker AH. Nodular regenerative hyperplasia of the liver: an under-recognized cause of portal hypertension in hematological disorders. Am J Hematol 2004;75:225–30. [DOI] [PubMed] [Google Scholar]
- 25.Rahav G, Volach V, Shapiro M, et al. Severe infections in thalassaemic patients: prevalence and predisposing factors. Br J Haematol 2006;133:667–74. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002;35:140–8. [DOI] [PubMed] [Google Scholar]
- 27.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol 1995;22:165–72. [DOI] [PubMed] [Google Scholar]
- 28.Deschenes M, Villeneuve JP. Risk factors for the development of bacterial infections in hospitalized patients with cirrhosis. Am J Gastroenterol 1999;94:2193–7. [DOI] [PubMed] [Google Scholar]
- 29.Feld JJ, Hussain N, Wright EC, et al. Hepatic involvement and portal hypertension predict mortality in chronic granulomatous disease. Gastroenterology 2008;134:1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Correlation between Liver Iron Concentration and Deugnier Iron Staining Score. The p-value was calculated using Spearman’s correlation coefficient. The Deugnier iron score significantly correlated with liver iron concentration.
Supplementary Figure 2. Correlation of ferritin and liver biopsy assessment of iron. The correlation between a) the log serum ferritin and liver iron concentration and b) the log serum ferritin and the Deugnier iron staining score. P-values were calculated using Spearman’s correlation coefficient. In figure 2a, log serum ferritin significantly correlated with liver iron concentration. In figure 2b, log serum ferritin significantly correlated with the Deugnier iron staining score.
Supplementary Figure 3. Correlation between Ferritin and Deugnier Iron Staining Score with Estimated Lifetime Blood Transfusions. The correlation between a) log serum ferritin and estimated lifetime blood transfusions and b) between Deugnier iron staining score with estimated lifetime blood transfusions. Estimated lifetime blood transfusions were categorized as: 0, 1 to 5, 6 to 10, 11–25, 26–50, 51–100 and >100. P-values were calculated using Spearman’s correlation coefficient. In figure 3a, serum log ferritin significantly correlated with estimated lifetime blood transfusions. In figure 3b, the Deugnier iron staining score significantly correlated with the estimated lifetime blood transfusions.
Supplementary Figure 4. Correlation of HVPG with platelet count. Increasing HVPG significantly correlated with decreasing platelet counts. P-values were calculated using Spearman’s correlation coefficient.
Supplementary Figure 5. Correlation of HVPG and sCD14 levels. The correlation between HVPG and sCD14 levels, a marker of microbial translocation, is shown in a) all patients and b) those with HVPG≥5 mmHg. P-values were calculated using Spearman’s correlation coefficient. Patients with elevated HVPG had significantly higher sCD14 levels.