Abstract
Cognitive impairments are amongst the most debilitating deficits of schizophrenia and the best predictor of functional outcome. Schizophrenia is hypothesized to have a neurodevelopmental origin, making animal models of neurodevelopmental insult important for testing predictions that early insults will impair cognitive function. Rats exposed to methylazoxymethanol acetate (MAM) at gestational day 17 display morphological, physiological and behavioral abnormalities relevant to schizophrenia. Here we investigate the cognitive abilities of adult MAM rats. We examined brain activity in MAM rats by histochemically assessing cytochrome oxidase enzyme activity, a metabolic marker of neuronal activity. To assess cognition, we used a hippocampus-dependent two-frame active place avoidance paradigm to examine learning and spatial memory, as well as cognitive control and flexibility using the same environment and evaluating the same set of behaviors. We confirmed that adult MAM rats have altered hippocampal morphology and brain function, and that they are hyperactive in an open field. The latter likely indicates MAM rats have a sensorimotor gating deficit that is common to many animal models used for schizophrenia research. On first inspection, cognitive control seems impaired in MAM rats, indicated by more errors during the two-frame active place avoidance task. Because MAM rats are hyperactive throughout place avoidance training, we considered the possibility that the hyperlocomotion may account for the apparent cognitive deficits. These deficits were reduced on the basis of measures of cognitive performance that account for motor activity differences. However, though other aspects of memory are intact, the ability of MAM rats to express trial-to-trial memory is delayed compared to control rats. These findings suggest that spatial learning and cognitive abilities are largely intact, that the most prominent cognitive deficit is specific to acquiring memory in the MAM neurodevelopmental model, and that hyperactivity can confound assessments of cognition in animal models of mental dysfunction.
Keywords: MAM, Methylazoxymethanol acetate, Neurodevelopmental insult, Cognition, Spatial memory, Hyperactivity, Schizophrenia
1. Introduction
Cognitive deficits are the most debilitating of schizophrenia and the best predictor of functional outcome (Nuechterlein et al., 2008). Antipsychotics primarily target the dopaminergic system and do little to improve cognitive deficits (Weinberger, 2007). As such, animal models with well-characterized schizophrenia-related cognitive deficits are important for developing procognitive treatments. Efforts to study cognition in rodents have focused on place learning and other navigation behaviors that require spatial computations (Buzsaki & Moser, 2013). In the present study, we examine learning, memory, cognitive control, and cognitive flexibility within a single behavioral paradigm, the active place avoidance task, which facilitates comparisons across the cognitive domains.
Schizophrenia is increasingly hypothesized to be a neurodevelopmental disorder (Insel, 2010) in an attempt to explain the relationships between genetic susceptibilities, altered development, and the clinical symptoms. Understanding the link between these factors is a major challenge for schizophrenia research that may be best investigated using controlled manipulations in animal models that make it possible to identify the relationships between specific genes, developmental phases, neural circuit function, and cognitive behaviors. The gestational day 17 methylazoxymethanol acetate (MAM) exposure model of neurodevelopmental insult has emerged as an important model for schizophrenia research (Lodge & Grace, 2009). The MAM model results in multi-level deficits including structural, physiological, and behavioral abnormalities, many of which have been described in schizophrenia. Timing MAM administration at gestational day 17 specifically disrupts the development of paralimbic, frontal and temporal cortices, regions also altered in schizophrenia (Lodge & Grace, 2009). Disruption of cell proliferation with the MAM methylating agent increases cell density in the prefrontal cortex and alters hippocampal size and architecture (Le Pen et al., 2006; Matricon et al., 2010; Moore, Jentsch, Ghajarnia, Geyer, & Grace, 2006). Exposure to MAM also results in physiological deficits, such as enhanced baseline dopamine activity, thought to be due to abnormal interactions between the ventral hippocampus and medial prefrontal cortex (Lodge & Grace, 2009).
Not only has the MAM model had success in producing subclinical aspects of schizophrenia, MAM exposure also produces dysfunctional dopaminergic responses and disrupted sensorimotor gating measured as impaired prepulse inhibition of startle and hyperlocomotion (Moore, Jentsch, Ghajarnia, Geyer, & Grace, 2006). Although sensorimotor gating deficits are common to almost every animal model used in schizophrenia research, and are related to the positive symptoms, they have no established relationship to the cognitive symptoms that are the contemporary target of schizophrenia treatment research. Building on the success of the MAM model, we were motivated to ask whether this standard neurodevelopmental insult could result in cognitive abnormalities in adulthood.
We are not the first to examine the cognitive abilities of MAM rats. Deficits in working memory have been observed in Y-maze alternation (Gastambide et al., 2015; Moore et al., 2006) and the radial eight-arm maze (Gourevitch, Rocher, Le Pen, Krebs, & Jay, 2004). Deficits in spatial memory have been reported in water maze tasks (Gastambide et al., 2015; Ratajczak et al., 2015) and selective cognitive impairments in the two-frame active place avoidance task (Jenks et al., 2013) that we use in the present work. However, all of these studies, including the measures of pre-pulse inhibition of startle, have relied on assessing motor activity and the interpretations from observed performance deficits are potentially confounded by hyperactivity and other potential locomotor abnormalities that are also a feature of this and other models used to investigate the origins of schizophrenia symptoms.
Our goals were to evaluate a variety of the components of cognitive ability in MAM rats: learning, and spatial memory, as well as cognitive control and flexibility, and to determine if apparent deficits could be the result of the concomitant hyperlocomotion. In addition to locomotion, the use of sensory information, motivation and motor behavior, such as conditioned responses, are important baseline components of tests that evaluate cognition. Consequently, we examined learning, memory, cognitive control and cognitive flexibility using a single behavioral paradigm with distinct task variants that keep the physical, locomotor and motivational requirements the same across the variants. In the basic two-frame place avoidance task variant, a rat on a slowly rotating arena must learn to avoid the location of shock that is defined by stationary room coordinates. In these tasks, it is necessary for rats to use cognitive control in order to use the relevant room cues to remember the shock zone, while ignoring irrelevant arena cues (such as location on the arena surface when shock was delivered), as we have demonstrated using brain manipulations and place cell physiology (Kelemen & Fenton, 2010; Wesierska, Dockery, & Fenton, 2005). This task is sensitive to dysfunction of multiple brain areas including dorsal hippocampus (Cimadevilla, Wesierska, Fenton, & Bures, 2001), basolateral amygdala (Serrano et al., 2008), retrosplenial cortex (Wesierska, Adamska, & Malinowska, 2009) and detects selective adult cognitive control deficits after neonatal ventral hippocampal lesion (NVHL), another schizophrenia-relevant rat model of a neurodevelopmental insult (Lee et al., 2012; O’Reilly, Kao, Lee, and Fenton, 2014). Task performance is also sensitive to psychotomimetics such as the NMDA receptor antagonist MK-801 (Stuchlik & Vales, 2005) and the hallucinogen psilocin (Rambousek, Palenicek, Vales, & Stuchlik, 2014).
Cognitive flexibility can be assessed with the conflict variant of the task, in which the rat is challenged to learn the location of a new shock zone that is opposite the initial location. This variant reveals cognitive flexibility deficits in the Fmr1 knockout mouse model of Fragile X Syndrome (Radwan, Dvorak, & Fenton, 2016) as well as the schizophrenia-relevant NVHL model (Lee et al., 2012; O’Reilly et al., 2014).
We find that MAM rats have altered prefrontal-ventral hippocampal functional connectivity and that MAM rats are hyperactive in a novel environment, consistent with prior reports. Although MAM rats appear to have cognitive deficits in the two frame-active place avoidance task as has been reported, they are hyperactive throughout the training paradigm. However, when this hyperactivity is accounted for, the cognitive deficits become marginal. We also find that MAM rats have a deficit in developing trial-to-trial memory during a training session, but development of memory is largely intact across days between the daily training sessions.
2. Materials and methods
All methods complied with Public Health and Service Policy on Humane Care and Use of Laboratory Animals and were approved by New York University Animal Welfare Committee.
2.1. Animals
Timed pregnant Long Evans rats arrived at the New York University animal facilities on embryonic day 10 (E10) and were housed individually. On E17, the female rats were administered 26 mg/kg MAM (in 500 μL saline), or saline intraperitoneally (i.p.). Male pups were weaned at P24 and group-housed (2–4 rats) until P42–56, at which time they were single housed. The rats had free access to food and water and were studied at 59–72 days old.
2.2. Neuroarchitectural examination
MAM rats were deeply anesthetized with pentobarbital (100 mg/kg) and transcardially perfused with 0.1 M phosphate buffer (PB, pH = 7.6) followed by 4% paraformaldehyde in PB. The brains were post fixed overnight in 4% paraformaldehyde, cryoprotected (0.1 M PB containing 2% dimethyl sulfoxide and 20% glycerol), cut on a freezing microtome (40 μm) and Nissl stained. Slides were visualized with an Olympus VS 120 microscope (2× and 10×).
2.3. Cytochrome oxidase activity and Nissl stain
Naïve MAM and control rats aged 70 days were anesthetized with isoflurane, immediately decapitated and the brains quickly extracted. The brains were hemisected, rapidly frozen in isopentane (−70 °C) and stored at −80 °C. Sets of brains (left hemisphere), consisting of two animals per group were cut simultaneously on the cryostat (40 μm), and sorted into three series, one for Nissl stain used in measuring hippocampal volume and two for cytochrome oxidase staining. The slides were stored at −80 °C until processed using quantitative cytochrome oxidase histochemistry.
Histochemical staining was performed according to (O’Reilly, Shumake, Bailey, Gonzalez-Lima, and Lane, 2009). To control for variability across batches of histochemical staining, 20, 40, 60 and 80 μm cryosections of fresh rat brain tissue homogenate (prepared as in (Shumake, Poremba, Edwards, & Gonzalez-Lima, 2000)) were included. Considering 40 μm as baseline (1.0 arbitrary unit), the other sections were assigned relative values (0.5, 1.5, and 2.0 arbitrary units for 20, 60, and 80 μm respectively). The optical density (OD) was correlated with the arbitrary units and the resulting linear regression equations (r > 0.97) were used to normalize OD readings from brain regions into relative cytochrome oxidase activity. To control for cutting thickness for each set of brains, the thickness of five random sections per set were averaged. The relative activity was normalized to the thickness (relative cytochrome oxidase activity/μm). The resulting values were then used in statistical analysis.
Optical densities were measured using ImageJ while blind to the group identity. The slides were scanned on an Olympus VS 120 microscope (2×) and OD subsequently recorded from the captured images. ImageJ was calibrated using a gray scale (5% resolution), the Rodbard function, and setting OD as the unit. All OD readings were taken from the blue component of images that were converted to RGB stacks. Four to six OD readings were taken for each region, two readings from each of two to three sections (~120 μm apart), and averaged. The OD reading size was set for each brain region and kept the same for all groups.
2.4. Hippocampal volume measurements
Areas were measured from every other slide from the Nissl stained series of fresh frozen tissue using ImageJ. Hippocampal area was measured as dentate gyrus, CA3, CA1 and subiculum from the first coronal section containing anterior CA3 until the last section containing posterior subiculum. Volume was calculated as area × thickness between sections × number of sections.
2.5. Two-frame active place avoidance
Rats were handled ~5 min/day, for five days before active place avoidance training (Fig. 1A). One day prior to training, rats were given two 10-min pretraining trials to habituate on the stationary arena. On days one and two, the rats were given eight 10-min trials per day on the rotating arena (one rpm) with a 60° shock zone stationary within the room. On day three, retention of the initial avoidance was tested. Cognitive flexibility was then assessed using eight 10-min conflict trials with the shock zone relocated 180° (Fig. 1B). The time between trials was ~10 min.
Fig. 1.
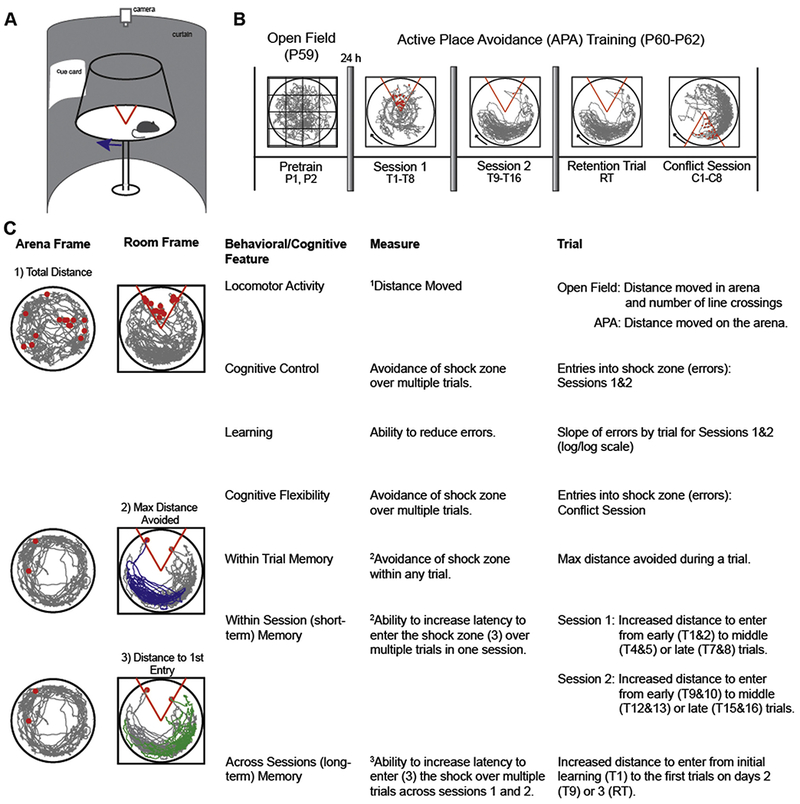
The two-frame active place avoidance task is used to assess locomotor activity and cognitive abilities. (A) A schematic of the two-frame active place avoidance task shows the rat on a metal disk that rotates at 1 rpm. The movement of the animal is tracked by a computer and an overhead camera. An LED that rotates with the arena allows us to track the animal with respect to the both the stationary room and rotating arena spatial frames. (B) Pretraining, conducted the day before training starts (P1 and P2), consists of two trials in which the animal is exposed to the stationary arena. The pretraining sessions is used to measure spontaneous locomotor activity and habituation to a novel environment. Training in the active place avoidance task occurs over two sessions consisting of eight trials per session, with 10 min between trials. Each session is conducted approximately 24 h apart. On the third day, a retention trial (RT) is conducted to test memory for the shock zone, followed by a Conflict Session, during which the shock zone is relocated 180” from the initial location. The Conflict Session also consists of eight trials. (C) Locomotor activity is assessed with respect to the arena frame while cognitive ability is assessed with respect to the room frame.
Active place avoidance allows a multidimensional analysis of cognitive behavior to assess locomotor activity (as in an open field test), place learning, within- and between-session memory (as in a water maze test), and cognitive control (Fig. 1C, (Abdel Baki, Kao, Kelemen, Fenton, & Bergold, 2009; Wesierska, Dockery, & Fenton, 2005)). Cognitive control describes the processes necessary to make judicious use of information from multiple sources, typically for optimal perceptual judgments, cognitive discriminations and action selection (Posner & Snyder, 1975; Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004). Because the rotating arena dissociates spatial information into two distinct spatial frames, the rotating arena and stationary room, the locations of shock are potentially defined in two ways. As such, animals are required to make cognitive discriminations to selectively associate shock reinforcement with the locations defined by room cues and not by arena cues (Wesierska et al., 2005). Alternating activations of distinct room and arena representations of location are explicitly reflected in hippocampal place cell ensemble discharge during place avoidance (Kelemen & Fenton, 2010). Optimal place avoidance requires moving to avoid room locations rather than arena locations (O’Reilly et al., 2014). The conflict task variant evaluates cognitive flexibility as a particular form of cognitive control, similar to the cognitive challenge in reversal learning paradigms (Burghardt, Park, Hen, & Fenton, 2012; Park, Burghardt, Dvorak, Hen, & Fenton, in press) and intradimensional shift tests (Rogers, Andrews, Grasby, Brooks, & Robbins, 2000). Because the behavioral contingencies do not reverse when the shock zone is relocated, we do not refer to the conflict variant as reversal learning.
2.6. Statistical analysis
Cytochrome oxidase activity
The group average cytochrome oxidase activity was calculated for each region and expressed as mean ± SEM Relative Cytochrome Oxidase Activity/μm of tissue. A two-tailed Student’s t test was performed to compare groups.
Functional connectivity, represented by coordinated changes in cytochrome oxidase activity between regions, was examined by calculating Pearson Correlations. Between groups comparisons were made using Fisher’s z test on the z-transformed correlations.
Behavior
Group and trial comparisons were made using multi-variate analysis of variance (MANOVA). Each session was evaluated separately with trials as a repeated measure. A two-tailed t test evaluated retention. Statistical significance was set at 0.05 for all comparisons. The statistics are reported only in the figure legends when possible for optimal readability.
3. Results
3.1. MAM rats have abnormal hippocampal architecture and functional connectivity, and are hyperactive
We began by assessing the MAM model for the characteristic non-cognitive abnormalities. Similar to what has been previously reported (Matricon et al., 2010), MAM rats have thinned hippocampal CA1 and CA3 pyramidal layers with discontinuities (Fig. 2A). We observed enlarged ventricles in some, but not all MAM rats. However, hippocampal volumes do not differ between groups (control = 50.7 ± 6.3 mm3, MAM = 46.0 ± 5.4 mm3, t14 = 0.56, p = 0.59).
Fig. 2.
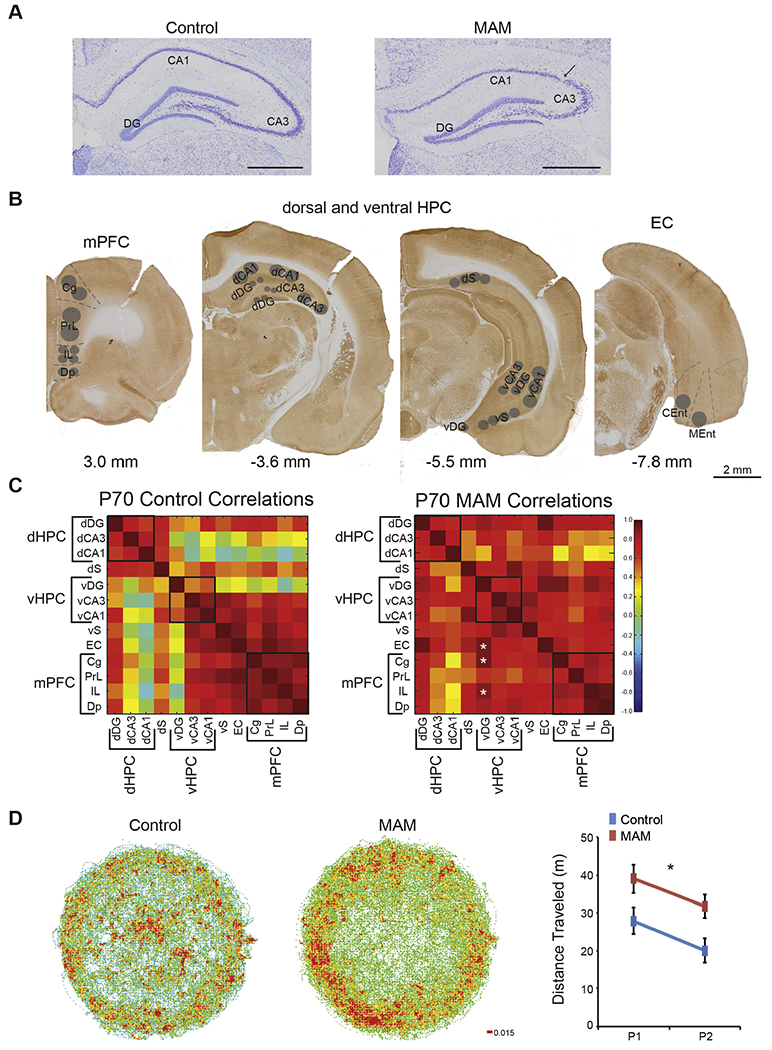
MAM rats are hyperactive and have altered functional connectivity between hippocampus and prefrontal cortex. (A) Nissl stained tissues show that MAM rats have a thinned and disrupted pyramidal cell layer in the hippocampus. (B) Cytochrome oxidase activity was measured in the prefrontal cortex, hippocampus, and entorhinal cortex. Subregions are as follows: Cg = cingulate cortex, PrL = prelimbic cortex, IL = infralimbic cortex, Dp = dorsal peduncular nucleus, dDG = dorsal dentate gyrus, dCA3 = dorsal Cornu Ammonis 3 of the hippocampus, dCA1 = dorsal Cornu Ammonis 1 of the hippocampus, dS = dorsal subiculum, vDG = ventral dentate gyrus, vCA3 = ventral Cornu Ammonis 3 of the hippocampus, vCA1 = ventral Cornu Ammonis 1 of the hippocampus, vS = ventral subiculum, EC = entorhinal cortex, CEnt = caudal entorhinal cortex, and MEnt = medial entorhinal cortex. (C) MAM rats have altered functional connectivity between the ventral dentate gyrus and the prefrontal cortex as well as between the ventral dentate gyrus and the entorhinal cortex. Black boxes represent groupings of subregions into functional domains: dHPC = dorsal hippocampus, vHPC = ventral hippocampus, and mPFC = medial prefrontal cortex. All other abbreviations are the same as in B. Values are Pearson Product Correlations. MAM, n = 8. Control, n = 8. * Group differences, p < 0.05. (D) MAM rats display spontaneous hyperactivity in the open field and habituate to a new environment. (Group: F1,14 = 7.15, p = 0.02; Trial: F1,14 = 5.76, p = 0.03; Interaction: F1,14 = 0.00, p = 0.97). P1 and P2 are pretraining trial 1 and 2 respectively. Values for the locomotor activity are average ± SEM. MAM, n = 8. Control n = 8. * p < 0.05.
We next assessed functional connectivity using cytochrome oxidase activity as a marker of neuronal activity in the prefrontal, hippocampal, and entorhinal cortices (Fig. 2B). While there are no group differences in regional cytochrome oxidase activity (Table 1), functional connectivity is different between MAM and control rats (Fig. 2C). Interregional coupling of neuronal activity with the ventral dentate gyrus is higher for several brain regions in MAM rats, including the cingulate cortex (control R = 0.08, MAM R = 0.92, p = 0.02), infralimbic cortex (control R= −0.13, MAM R = 0.88, p< 0.001), and the entorhinal cortex (control R = 0.23, MAM R = 0.92, p = 0.03). Other interregional changes between dorsal hippocampus and other cortical regions did not reach significance.
Table 1.
Average CO activity by brain region between MAM and control rats.
| Relative CO activity/μm tissue (×102) |
||||
|---|---|---|---|---|
| Brain region | P70Control | P70MAM | p-value | t14 |
| dDG | 12.42 ± 0.84 | 12.82 ± 1.19 | 0.79 | 0.28 |
| dCA3 | 6.95 ± 0.64 | 7.27 ± 0.52 | 0.71 | 0.39 |
| dCA1 | 7.35 ± 0.56 | 8.21 ± 0.85 | 0.41 | 0.85 |
| dS | 7.79 ± 0.62 | 8.31 ± 0.88 | 0.63 | 0.49 |
| vDG | 10.47 ± 0.39 | 10.08 ±0.94 | 0.72 | 0.37 |
| vCA3 | 12.02 ± 0.79 | 13.80 ± 0.89 | 0.16 | 1.49 |
| vCA1 | 10.18 ± 0.79 | 10.03 ± 0.74 | 0.89 | 0.14 |
| vS | 12.43 ± 0.98 | 13.50 ± 1.33 | 0.53 | 0.64 |
| EC | 9.89 ± 1.01 | 8.80 ± 0.84 | 0.42 | 0.82 |
| Cg | 11.23 ± 0.79 | 10.39 ± 1.13 | 0.55 | 0.61 |
| PrL | 10.65 ± 0.88 | 9.78 ± 0.64 | 0.44 | 0.80 |
| IL | 10.11 ± 0.77 | 9.21 ± 0.65 | 0.39 | 0.89 |
| Dp | 8.70 ± 0.63 | 9.29 ± 0.82 | 0.57 | 0.57 |
dDG = dorsal dentate gyrus, dCA3 = dorsal Cornu Ammonis 3, dCA1 = dorsal Cornu Ammonis 1, dS = dorsal subiculum, vDG = ventral dentate gyrus, vCA3 = ventral Cornu Ammonis 3, vCA1 = ventral Cornu Ammonis1, vS = ventral subiculum, EC = entorhinal cortex, Cg = cingulate cortex, PrL = prelimbic cortex, IL = infralimbic cortex, Dp = dorsal peduncular nucleus. Control n = 8, MAM n = 8.
Finally, we assessed spontaneous locomotor activity in a novel open field environment in which all rats are thigmotaxic. Adult MAM rats are hyperactive (Fig. 2D). Like controls, MAM rats habituate (Fig. 2D), but nonetheless are hyperactive, even throughout the place avoidance training that assesses cognitive functions, confirming the sensorimotor integration deficit in MAM rats (Le Pen et al., 2006; Moore et al., 2006).
3.2. Assessment of cognitive control and flexibility
Cognitive control and flexibility are key therapeutic targets of contemporary psychiatry so we first assessed these two cognitive functions. MAM rats make more errors (entries into the shock zone) than control rats during Session 1, and training improves their performance during the following sessions (Fig. 3A,B), similar to previous reports using the active place avoidance task (Jenks et al., 2013; O’Reilly et al., 2014).
Fig. 3.
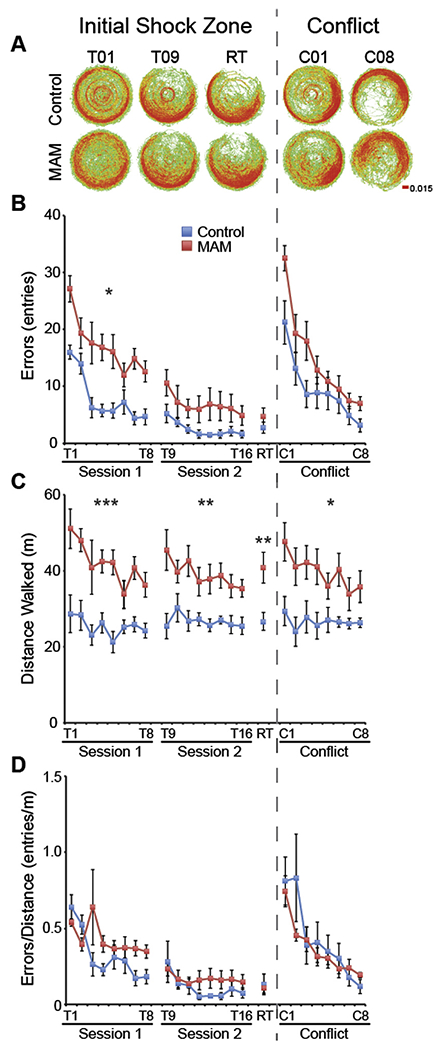
Hyperactivity accounts for increased errors of MAM rats in the two-frame active place avoidance task. (A) Dwell maps of average time spent in each location of the arena. (B) MAM rats make more errors (entries into the shock zone) on the first training day (Session 1: Group: F1,14 = 18.07, p < 0.001; Trial: F7,8 = 20.67, p < 0.001; Interaction: F7,8 = 1.17, p = 0.41), but perform similarly to control rats on the second training day (Session 2: Group: F1,14 = 3.52, p = 0.08; Trial: F7,8 = 3.16, p = 0.06; Interaction: F7,8 = 0.88, p = 0.56). By the end of training, MAM rats perform as well as control rats (RT: t14 = 1.03, p = 0.32). Cognitive flexibility appears intact in MAM rats as performance in the two frame active place avoidance is not different between MAM and control rats during the conflict trials when the shock zone is shifted opposite to its initial location (Conflict Session: Group: F1,13 = 4.15, p = 0.06; Trial: F7,7 = 31.61, p < 0.0001; Interaction: F7,7 = 0.63, p = 0.72). However, (C) MAM rats are hyperactive during all trials (Session 1: Group: F1,14 = 22.14, p < 0.001; Trial: F7,8 = 4.69, p = 0.02; Interaction: F7,8 = 1.69, p = 0.24. Session 2: Group: F1,14 = 11.46, p < 0.01; Trial: F7,8 = 1.88, p = 0.20; Interaction: F7,8 = 1.01, p = 0.49. RT: t14 = 2.99, p = 0.01. Conflict Session: Group: F1,13 = 11.99, p < 0.01; Trial: F7,7 = 3.47, p = 0.06; Interaction: F7,7 = 1.85, p = 0.22), which may account for the increased number of errors. (D) When the number of errors is normalized to locomotor activity, cognitive control, measured as place avoidance, in MAM rats is not different from control rats (Session 1: Group: F1,14 = 2.71, p = 0.12; Trial: F7,8 = 4.30, p = 0.03; Interaction: F7,8 = 1.94, p = 0.19. Session 2: Group F1,14 = 0.84, p = 0.37; Trial: F7,8 = 1.10, p = 0.44; Interaction: F7,8 = 0.36, p = 0.90. RT: t14 = 0.10, p = 0.92. Conflict Session: Group: F1,13 = 0.47, p = 0.51; Trial: F7,7=4.84, p = 0.03; Interaction: F7,7 = 0.74, p = 0.65). Values are presented as average ± SEM. MAM n = 8, Control n = 8. * p < 0.05. ** p < 0.01. *** p < 0.001.
MAM rats are hyperactive throughout place avoidance training (Fig. 3C), leading us to hypothesize that this hyperactivity may account for the increased number of errors because opportunity to enter the shock zone increases with the distance animals walk. The ANCOVA on number of errors made during Session 1, where deficits seem to be the greatest, with average distance walked on day 1 as a covariate, indicated that hyperactivity accounts for the errors made by MAM rats during Session 1 (Group: F1,13 = 0.84, p = 0.38; Trial: F7,7 = 5.33, p = 0.02; Interaction: F7,7 = 3.70, p = 0.05). Because the contribution of hyperactivity to entrances may be complex and the ANCOVA assumes the contribution is linear, we also considered an approach with fewer assumptions. To understand the influence of hyperactivity on place avoidance performance, we normalized the number of errors to the distance walked during each of the trials. Although at the end of Session 1 it appeared that MAM rats still make more errors (Fig. 3D), the ANOVA indicated that MAM rats perform as well as controls, suggesting again that they do not have a robust cognitive control impairment. Consequently, we examined the details of the learning curves to conclusively assess whether or not there is a deficit in place avoidance learning, independent of the hyperactivity. MAM and control rats learn at the same rate, assessed as an exponential fit of the learning curves (coefficients of decay: Initial Shock Zone: MAM = −0.67 ±0.12, control = −0.77 ±0.10, t14 = 0.61, p = 0.55. Conflict Shock Zone: MAM = −0.80 ±0.05, control = −1.00 ±0.31, t14 = 0.69, p = 0.50), again suggesting that MAM rats do not have an impairment in place avoidance learning in general and in cognitive control in particular.
Cognitive flexibility is normal in MAM rats, determined by performance when the shock zone is relocated 180° (Fig. 3, right side).
Taken together, these data indicate that any cognitive deficit that we observed in MAM rats disappears with training and hyperactivity can account for the apparent differences.
3.3. Assessment of within- and across-session memory
Along with cognitive control, memory deficits are an important therapeutic target for improving outcomes in schizophrenia (Ragland et al., 2009). We assessed spatial memory within sessions as well as across days using latency to enter the shock zone (Pastalkova et al., 2006). The latency to enter the shock zone on Trial 1, when the animals experienced shock for the first time, estimates chance. The latency on Trial 9, the first trial 24-h after day 1 training, and the 24-h retention test on day 3 estimate long-term memory without confounds of within-session learning or extinction. Because MAM rats are hyperlocomotive, we examined the distance walked before entering the shock zone for the first time in a trial; MAM rats do not differ from control rats (Fig. 4A). In addition to memory, path to first entry may be influenced by a variety of behaviors. For example, animals might enter because they are patrolling or evaluating whether shock is still present. In this case, we expect that once they receive confirmation of shock they will delay entering the shock zone a second time. The path to second entry is also not different between MAM or control rats across days (Fig. 4B).
Fig. 4.
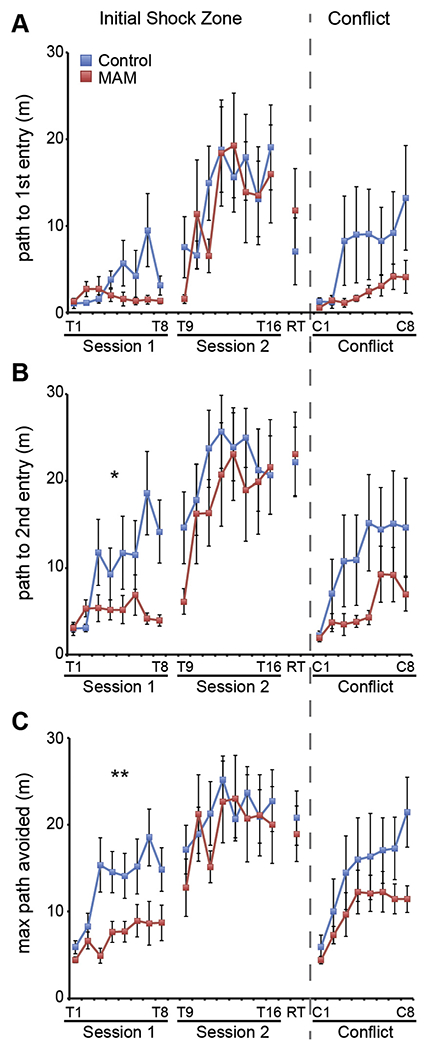
Within and between session memory in MAM rats. Across session memory was measured as the ability to increase the path to first (A) or second (B) entrance across sessions 1, 2, and 3 (Trial 1, Trial 9, and RT respectively). MAM rats have normal long-term memory (Path to first entry: Group: F1,14 = 0.02, p = 0.90; Trial F2,13 = 4.09, p = 0.05; Interaction: F2,13 = 1.71, p = 0.14, Path to second entry: Group: F1,14 = 0.72, p = 0.41; Trial: F2,13 = 18.98, p < 0.001; Interaction: F2,13 = 2.33, p = 0.14). Within session memory was measured as the ability to increase the path to enter the shock zone from trial-to-trial within a session. When examining the path to first entry, MAM rats are not different from control rats (Session 1: Group: F1,14 = 3.58, p = 0.08; Trial: F7,8 = 2.82, p = 0.08; Interaction: F7,8 = 1.45, p = 0.30; Session 2: Group: F1,14 = 0.11, p = 0.75; Trial: F7,8 = 3.53, p = 0.05; Interaction: F7,8 = 1.17, p = 0.41. RT: t14 = 0.77 p = 0.45; Conflict Session: Group: F1,13 = 1.88, p = 0.19; Trial: F7,7 = 1.92, p = 0.20; Interaction: F7,7 = 1.04, p = 0.48). When considering the path to second entry, MAM rats have impaired within session memory during the first session (Session 1: Group: F1,14 = 7.89, p = 0.01; Trial: F7,8 = 4.61, p = 0.02; Interaction: F7,8 = 2.74, p = 0.07). Short-term memory is not different after the first session (Session 2: Group: F1,14 = 0.53, p = 0.48; Trial: F7,8 = 2.81, p = 0.80; Interaction: F7,8 = 0.51, p = 0.81. RT: t14 = 0.15, p = 0.88. Conflict Session: Group: F1,13 = 1.84, p = 0.20; Trial: F7,7 = 1.50, p = 0.30; Interaction: F2,13 = 0.46, p = 0.83). (C) Within trial memory was also assessed as the maximum path the rat could walk without entering the shock zone. MAM rats are impaired on the first day of training (Session 1: Group: F1,14 = 9.06, p = 0.001; Trial: F7,8 = 3.01, p = 0.07; Interaction: F7,8 = 2.75, p = 0.09) but express normal memory on days 2 and 3 (Session 2: Group F1,14 = 0.16, p = 0.69; Trial: F7,8 = 2.96, p = 0.08; Interaction: F7,8 = 0.87, p = 0.56. RT: t14 = 0.41, p = 0.69. Conflict Session: Group: F1,13 = 1.44, p = 0.25; Trial: F7,7 = 1.73, p = 0.24; Interaction: F7,7 = 0.65, p = 0.71). Values are presented as average ± SEM. MAM, n = 8. Control, n = 8. *p < 0.05. **p < 0.01. *** p < 0.001.
We next examined memory within each session by measuring entrance latency on a trial-by-trial basis. MAM rats do not differ from control rats in their ability to delay entering the shock zone at the beginning of each trial within a session (Fig 4A), but while control rats increase latency to enter a second time, MAM rats do not during Session 1 (Fig. 4B). The following day, both MAM and control rats improve similarly across the Session 2 trials. Although by inspection MAM rats appear impaired, performance is statistically indistinguishable between the two groups during the Conflict Session.
Memory performance within a session can also be assessed by the maximum time the rat is able to stay away from the shock zone. Because the MAM group is hyperactive, we estimated the optimal avoidance by the maximum path the rats could walk without getting a shock. According to this measure, MAM rats are impaired during the first session of training (Fig. 4C) and like with the other estimate of within session memory (Fig. 4B) they are no longer impaired after the 24-h break from training.
Together these data indicate that across day memory is intact in MAM rats but they are slower to express place avoidance memory between the trials within a daily session of training.
4. Discussion
4.1. Summary
Cognitive impairments are amongst the most debilitating of schizophrenia, which is why animal models that can drive development of novel procognitive treatments are invaluable. We therefore investigated cognitive abilities in a model of neurodevelopmental insult that has provided insight into schizophrenia-related pathophysiology, gestational day 17 MAM exposure (Lodge & Grace, 2009). Adult MAM rats have neuroarchitectural abnormalities, such as thinned and disrupted pyramidal cell layers in the hippocampus, enlarged ventricles, and excessive functional connectivity to the ventral hippocampus (Fig. 2). MAM rats are hyperlocomotive and this is sufficient to account for most of the apparent cognitive deficits in the two-frame active place avoidance task (Fig. 3). As reported in previous studies [12], these cognitive deficits are transient because with training, cognitive performance improves to the level of controls (Figs. 3 and 4).
4.2. Neuroarchitecture and functional changes
Others have reported reduced hippocampus weight (Featherstone, Rizos, Nobrega, Kapur, & Fletcher, 2007), area (Matricon et al., 2010; Moore et al., 2006), and volume (Chin et al., 2011) in rats treated with MAM at gestational day 17. We found that the total volume of the dorsal and ventral hippocampal formation (including the subiculum) was not different between groups. The differences between our results and area reductions in MAM rats (Matricon et al., 2010; Moore et al., 2006) could be due to 2D versus 3D measurements. Indeed, if we consider only the area, we find that, on average, the hippocampal area in MAM rats is 84.5 ± 2.5% of control rats. Previously reported volume reduction in MAM rats measured by MRI (Chin et al., 2011) yielded similar volumes for MAM rats to those reported here, but volumes of control rats in that study were slightly higher than we report.
We confirmed that the MAM neurodevelopmental insult has a measurable impact on functional connectivity. The excessive functional connectivity that we observed between the ventral hippocampus and neocortical areas is consistent with reports of reduced parvalbumin expression in ventral hippocampus (Lodge, Behrens, & Grace, 2009; Penschuck, Flagstad, Didriksen, Leist, & Michael-Titus, 2006), reduced anxiety (Gastambide et al., 2015) that is associated with abnormal ventral hippocampal function (Bannerman et al., 2003; Kheirbek et al., 2013), and abnormal prefrontal-hippocampal function (Lodge & Grace, 2009) in rats exposed to MAM at gestational day 17. It is difficult to directly compare the electrophysiology studies of prefrontal-hippocampal function with the cytochrome oxidase studies presented here because cytochrome oxidase activity is assessed over multiple cell layers and measures global activity within a region, including activity of neurons, interneurons, and astrocytes, as well as dendritic activity from local networks and aberrant fibers from distal locations (Wong-Riley, 1989). These observations confirm abnormal global brain function in adult MAM rats, although cognition itself was largely intact, as we discuss next.
4.3. Hyperactivity can explain some cognitive deficits in MAM rats
It is difficult to dissociate relative contributions of sensorimotor gating dysfunction, such as hyperactivity, from abnormal mechanisms underlying deficits in cognitive control – the ability to use relevant information and ignore irrelevant information. Agents such as NMDA receptor antagonists that induce hyperactivity, do so by altering brain mechanisms that may disrupt other aspects of cognitive abilities. We would like to be clear that MAM rats make more errors in the active place avoidance task, however, we do not interpret this as a fundamental cognitive deficit because the difference is not due to difficulties separating relevant room information from irrelevant arena information. MAM rats reduce their errors at the same rate as control rats, and plateau in performance with the same amount of training, though the hyperactivity of MAM rats persists.
Active exploration is essential for rodents to make the spatial computations required to perform most cognitive tests (McHugh & Tonegawa, 2007; Whishaw & Brooks, 1999), such as avoiding shock in the active place avoidance task used here. Locomotor activity is an important component of cognitive behavior, and abnormal locomotor activity should be taken into consideration when assessing cognitive ability. For example, both competitive and noncompetitive NMDA-receptor antagonists were thought to impair learning and memory by inhibiting LTP induction, but antagonism of NMDA receptors also induces sensorimotor deficits that include hyperactivity. This hyperactivity was hypothesized to confound the cognitive impairments and indeed, pretraining to water maze test conditions prevented the learning deficits caused by NMDA-receptor antagonists despite the drugs continuing to impair LTP, leaving the authors to conclude that NMDA receptor activation and NMDA receptor dependent LTP are not necessary for acquiring place memory itself (Bannerman, Good, Butcher, Ramsay, & Morris, 1995; Saucier & Cain, 1995).
The effects of hyperactivity and locomotor ability on spatial learning and memory have garnered little attention in studies of mental illness, in spite of the use of hyperactivity to vet animal models as useful for schizophrenia research (Abbott, 2010). As demonstrated here, hyperactivity appears to confound cognitive performance in MAM rats, and after accounting for differences in locomotor activity, cognitive control and flexibility appear intact. Similar to the NMDA-receptor antagonist memory experiments discussed above, two active place avoidance training sessions are sufficient for MAM rats to overcome difficulties accounted for by hyperlocomotion. When cognitive flexibility was tested in the Conflict Session, MAM rats perform as well as controls, in spite of maintained hyperactivity. Like MAM rats, NVHL rats are hyperactive in adulthood, but unlike MAM rats, hyperactivity does not account for learning impairments using the same task and protocol (Lee et al., 2012). Thus, hyperactivity does not always account for cognitive deficits (Lee et al., 2012), but it is often the most parsimonious interpretation of an apparent deficit and needs to be ruled out as an explanation before concluding the deficit is in cognitive ability. Hyperactivity has been reported in MAM rats (Ratajczak et al., 2015) and water maze learning and memory deficits have been described in MAM rats without accounting for the potential impact of hyperactivity on the interpretation of cognitive deficits (Gastambide et al., 2015; Gourevitch et al., 2004). In light of our findings and those of Saucier et al. (Saucier & Cain, 1995; Saucier, Hargreaves, Boon, Vanderwolf, & Cain, 1996), it is important to consider whether hyperactivity could have contributed to these deficits. For example, pretraining the rats to swim prior to learning the paradigm may have eliminated the learning deficits, similar to the effect of pretraining on water maze performance during NMDA antagonism. Given MAM rats have sensorimotor deficits, it would be prudent to measure performance details of abnormal swim behavior (Saucier, Hargreaves, Boon, Vanderwolf, & Cain, 1996; Wolfer, Stagljar-Bozicevic, Errington, & Lipp, 1998). In addition, it would also be prudent to use measures of behavioral performance that are less sensitive to hyperactivity, such as distance swum and swim linearity, as well as other measures (Wolfer & Lipp, 1992, 2000). For example, the sensorimotor abnormalities in GD15 MAM exposed rats precluded assessment of reversal learning behavior, whereas locomotor function was effectively normal in GD17 MAM exposed rats, allowing detection of both sensorimotor prepulse inhibition deficits, as well as reversal deficits in a Y-maze in spite of better-than-normal Y-maze learning (Moore et al., 2006). The reversal deficit contrasts with normal conflict learning observed in the present study. It is unclear whether the reversal deficit is due to cognitive inflexibility itself, or due to better-than-normal learning of the initial arm in the Y-maze, which may have established a stronger than normal memory to overcome in the reversal test. Notably, normal win-shift learning was reported in the radial 8 arm maze at short delays, the assessment of which may not be sensitive to hyperlocomotion (Gourevitch et al., 2004). Similar to the present findings of a between-trial memory deficit, the MAM rats were impaired at 30 min delays. Thus, because locomotor activity can account for some cognitive deficits, but not all, as will be discussed with respect to memory in MAM rats, the present results indicate it is appropriate to perform control studies and analyses to dissociate cognitive and sensorimotor components of behavior.
4.4. Memory in MAM rats
While hyperactivity accounted for cognitive control deficits, we found that MAM rats have spatial memory deficits similar to those reported by others (Gastambide et al., 2015; Gourevitch et al., 2004; Ratajczak et al., 2015). MAM rats are slower to express between-trial memory, measured as the ability to increase path to enter the shock zone at the beginning of the trial (‘‘entrance latency”), and do not express between-trial memory until the second day of training. On the second day of training, memory appears normal after the rat experiences the first reminder trial and memory performance remains normal on the day 3, 24-h retention trial. This suggests MAM rats require a longer-than-normal consolidation period estimated to be between 3 and 24 h before longterm memory is unambiguously expressed. A similar phenomenon for memory to strengthen with the passage of time is known as the incubation effect (Houston, Stevenson, McNaughton, & Barnes, 1999). Note however, that MAM rats require a reminder (Trial 9 of Session 2) in addition to the overnight incubation before they express long-term avoidance memory at the same level as controls. Further work is required to elucidate the nature of long-term memory in MAM rats, which like Fmr1 KO mice may express memory-related abnormalities at the level of electrophysiological network mechanisms although they do not express overt memory deficits in behavior (Brennan, Albeck, & Paylor, 2006; D’Hooge et al., 1997; Radwan et al., 2016; Till et al., 2015).
4.5. Conclusion
While neurodevelopmental insult due to MAM treatment produces some of the dopaminergic and basal ganglia circuit abnormalities observed in schizophrenia and epilepsy (Jenks et al., 2013; Lodge & Grace, 2009; Lucas, Lenck-Santini, Holmes, & Scott, 2011), gestational MAM exposure does not sufficiently reproduce deficits in cognitive control - the ability to dissociate relevant and irrelevant streams of information - which are amongst the most debilitating deficits in schizophrenia. Because MAM rats lack a cognitive control deficit, it may be relevant to consider the two-hit hypotheses that posit a neurodevelopmental and/or genetic abnormality predisposes the subject to the illness, but that clinical symptoms only emerge after a second, environmental hit such as stress or infection (Bayer, Falkai, & Maier, 1999; Maynard, Sikich, Lieberman, & LaMantia, 2001). The MAM model may be well suited to test two-hit hypotheses that predict cognitive control deficits in MAM rats following a manipulation that constitutes a second hit.
Acknowledgements
This work was supported by NIMH R01MH08438. The authors would like to thank Claudia Franke for help with the cytochrome oxidase assays, Dr. Hsin-Yi Kao for help generating the MAM rats, and Dr. Fraser Sparks for his comments on the manuscript.
Footnotes
Conflicts of interest
The authors have no conflicts to report.
References
- Abbott A (2010). Schizophrenia: The drug deadlock. Nature, 468, 158–159 [DOI] [PubMed] [Google Scholar]
- Abdel Baki SG, Kao HY, Kelemen E, Fenton AA, & Bergold PJ (2009). A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Research, 1280, 98–106. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, & Morris RG (1995). Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature, 378, 182–186. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, & Rawlins JN (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research, 139, 197–213. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, & Maier W (1999). Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the ‘‘two hit hypothesis”. Journal of Psychiatric Research, 33, 543–548. [DOI] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, & Paylor R (2006). Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes, Brain and Behavior, 5, 467–471. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, & Fenton AA (2012). Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus, 22, 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, & Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience, 16, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CL, Curzon P, Schwartz AJ, O’Connor EM, Rueter LE, Fox GB, … Basso AM (2011). Structural abnormalities revealed by magnetic resonance imaging in rats prenatally exposed to methylazoxymethanol acetate parallel cerebral pathology in schizophrenia. Synapse, 65, 393–403. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, & Bures J (2001). Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proceedings of the National Academy of Sciences of the United States of America, 98, 3531–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, … De Deyn PP (1997). Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience, 76, 367–376. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Rizos Z, Nobrega JN, Kapur S, & Fletcher PJ (2007). Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: Parallels to schizophrenia. Neuropsychopharmacology, 32, 483–492. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Taylor AM, Palmer C, Svard H, Karjalainen M, Janhunen SK, … Bannerman DM (2015). Alterations in spatial memory and anxiety in the MAM E17 rat model of hippocampal pathology in schizophrenia. Psychopharmacology (Berlin), 232, 4099–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourevitch R, Rocher C, Le Pen G, Krebs MO, & Jay TM (2004). Working memory deficits in adult rats after prenatal disruption of neurogenesis. Behavioural Pharmacology, 15, 287–292. [DOI] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, & Barnes CA (1999). Effects of age on the generalization and incubation of memory in the F344 rat. Learning & Memory, 6, 111–119. [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2010). Rethinking schizophrenia. Nature, 468, 187–193. [DOI] [PubMed] [Google Scholar]
- Jenks KR, Lucas MM, Duffy BA, Robbins AA, Gimi B, Barry JM, & Scott RC (2013). Enrichment and training improve cognition in rats with cortical malformations. PLoS One, 8, e84492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen E, & Fenton AA (2010). Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS Biology, 8, e1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, … Hen R (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron, 77, 955–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, & Krebs MO (2006). Peri-pubertal maturation after developmental disturbance: A model for psychosis onset in the rat. Neuroscience, 143, 395–405. [DOI] [PubMed] [Google Scholar]
- Lee H, Dvorak D, Kao HY, Duffy AM, Scharfman HE, & Fenton AA (2012). Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron, 75, 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, & Grace AA (2009). A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. Journal of Neuroscience, 29, 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, & Grace AA (2009). Gestational methylazoxymethanol acetate administration: A developmental disruption model of schizophrenia. Behavioural Brain Research, 204, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas MM, Lenck-Santini PP, Holmes GL, & Scott RC (2011). Impaired cognition in rats with cortical dysplasia: Additional impact ofearly-life seizures. Brain, 134, 1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricon J, Bellon A, Frieling H, Kebir O, Le Pen G, Beuvon F, … Krebs MO (2010). Neuropathological and Reelin deficiencies in the hippocampal formation of rats exposed to MAM; differences and similarities with schizophrenia. PLoS One, 5, e10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard TM, Sikich L, Lieberman JA, & LaMantia AS (2001). Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophrenia Bulletin, 27, 457–476. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, & Tonegawa S (2007). Spatial exploration is required for the formation of contextual fear memory. Behavioral Neuroscience, 121, 335–339. [DOI] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, & Grace AA (2006). A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biological Psychiatry, 60, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, … Marder SR (2008). The MATRICS consensus cognitive battery, Part 1: Test selection, reliability, and validity. American Journal of Psychiatry, 165, 203–213. [DOI] [PubMed] [Google Scholar]
- O’Reilly KC, Kao HY, Lee H, & Fenton AA (2014). Converging on a core cognitive deficit: The impact of various neurodevelopmental insults on cognitive control. Frontiers in Neuroscience, 8, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KC, Shumake J, Bailey SJ, Gonzalez-Lima F, & Lane MA (2009). Chronic 13-cis-retinoic acid administration disrupts network interactions between the raphe nuclei and the hippocampal system in young adult mice. European Journal of Pharmacology, 605, 68–77. [DOI] [PubMed] [Google Scholar]
- Park EH, Burghardt NS, Dvorak D, Hen R, & Fenton AA (in press). Experience-dependent regulation of dentate gyrus excitability by adult-born granule cells. Journal of Neuroscience (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, & Sacktor TC (2006). Storage of spatial information by the maintenance mechanism of LTP. Science, 313, 1141–1144. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, & Michael-Titus AT (2006). Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. European Journal of Neuroscience, 23, 279–284. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Snyder CRR (1975). Attention and cognitive control In Solso RL (Ed.), Information processing and cognition: The Loyola symposium (pp. 55–85). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Radwan B, Dvorak D, & Fenton AA (2016). Impaired cognitive discrimination and discoordination of coupled theta-gamma oscillations in Fmrl knockout mice. Neurobiology of Diseases, 88, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Cools R, Frank M, Pizzagalli DA, Preston A, Ranganath C, & Wagner AD (2009). CNTRICS final task selection: Long-term memory. Schizophrenia Bulletin, 35, 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambousek L, Palenicek T, Vales K, & Stuchlik A (2014). The effect of psilocin on memory acquisition, retrieval, and consolidation in the rat. Frontiers in Behavioral Neuroscience, 8, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak P, Kus K, Murawiecka P, Slodzinska I, Giermaziak W, & Nowakowska E (2015). Biochemical and cognitive impairments observed in animal models of schizophrenia induced by prenatal stress paradigm or methylazoxymethanol acetate administration. Acta Neurobiologiae Experimentalis (Wars), 75, 314–325. [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, & Carter CS (2004). Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56, 129–140. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, & Robbins TW (2000). Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. Journal of Cognitive Neuroscience, 12, 142–162. [DOI] [PubMed] [Google Scholar]
- Saucier D, & Cain DP (1995). Spatial learning without NMDA receptor-dependent long-term potentiation. Nature, 378, 186–189. [DOI] [PubMed] [Google Scholar]
- Saucier D, Hargreaves EL, Boon F, Vanderwolf CH, & Cain DP (1996). Detailed behavioral analysis of water maze acquisition under systemic NMDA or muscarinic antagonism: Nonspatial pretraining eliminates spatial learning deficits. Behavioral Neuroscience, 110, 103–116. [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, … Fenton AA (2008). PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biology, 6, 2698–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Poremba A, Edwards E, & Gonzalez-Lima F (2000). Congenital helpless rats as a genetic model for cortex metabolism in depression. NeuroReport, 11, 3793–3798. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, & Vales K (2005). Systemic administration of MK-801, a noncompetitive NMDA-receptor antagonist, elicits a behavioural deficit of rats in the Active Allothetic Place Avoidance (AAPA) task irrespectively of their intact spatial pretraining. Behavioural Brain Research, 159, 163–171. [DOI] [PubMed] [Google Scholar]
- Till SM, Asiminas A, Jackson AD, Katsanevaki D, Barnes SA, Osterweil EK, … Kind PC (2015). Conserved hippocampal cellular pathophysiology but distinct behavioural deficits in a new rat model of FXS. Human Molecular Genetics, 24, 5977–5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR (2007). Schizophrenia drug says goodbye to dopamine. Nature Medicine, 13, 1018–1019. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Adamska I, & Malinowska M (2009). Retrosplenial cortex lesion affected segregation of spatial information in place avoidance task in the rat. Neurobiology of Learning and Memory, 91, 41–49. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Dockery C, & Fenton AA (2005). Beyond memory, navigation, and inhibition:Behavioral evidence for hippocampus-dependent cognitive coordination in the rat. Journal of Neuroscience, 25, 2413–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, & Brooks BL (1999). Calibrating space: Exploration is important for allothetic and idiothetic navigation. Hippocampus, 9, 659–667. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, & Lipp HP (1992). A new computer program for detailed off-line analysis of swimming navigation in the Morris water maze. Journal of Neuroscience Methods, 41, 65–74. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, & Lipp HP (2000). Dissecting the behaviour of transgenic mice: Is it the mutation, the genetic background, or the environment? Experimental Physiology, 85, 627–634. [PubMed] [Google Scholar]
- Wolfer DP, Stagljar-Bozicevic M, Errington ML, & Lipp HP (1998). Spatial memory and learning in transgenic mice: Fact or artifact? News in Physiological Sciences, 13, 118–123. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT (1989). Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends in Neurosciences, 12, 94–101. [DOI] [PubMed] [Google Scholar]


