Abstract
Background:
Short-term exposure to air pollution has been associated with cardiovascular events, potentially by promoting endothelial cell activation and inflammation. A few large-scale studies have examined the associations and have had mixed results.
Methods:
We included 3820 non-current smoking participants (mean age 56 years, 54% women) from the Framingham Offspring cohort examinations 7 (1998-2001) and 8 (2005-2008), and Third Generation cohort examination 1 (2002-2005), who lived within 50 km of a central monitoring station. We calculated the 1- to 7-day moving averages of fine particulate matter (PM2.5), black carbon (BC), sulfate (SO42−), nitrogen oxides (NOx), and ozone before examination visits. We used linear mixed effect models for P-selectin, monocyte chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1, lipoprotein-associated phospholipase A2 activity and mass, and osteoprotegerin that were measured up to twice, and linear regression models for CD40 ligand and interleukin-18 that were measured once, adjusting for demographics, life style and clinical factors, socioeconomic position, time, and meteorology.
Results:
We found negative associations of PM2.5 and BC with P-selectin, of ozone with MCP-1, and of SO42− and NOx with osteoprotegerin. At the 5-day moving average, a 5 μg/m3 higher PM2.5 was associated with 1.6% (95% CI: −2.8, −0.3) lower levels of P-selectin; a 10 ppb higher ozone was associated with 1.7% (95% CI: −3.2, −0.1) lower levels of MCP-1; and a 20 ppb higher NOx was associated with 2.0% (95% CI: −3.6, −0.4) lower levels of osteoprotegerin.
Conclusions:
We did not find evidence of positive associations between short-term air pollution exposure and endothelial cell activation. On the contrary, short-term exposure to higher levels of ambient pollutants were associated with lower levels of P-selectin, MCP-1, and osteoprotegerin in the Framingham Heart Study.
Keywords: Air pollution, Endothelial dysfunction, Inflammation, Biomarker, Environment, Epidemiology
1. Introduction
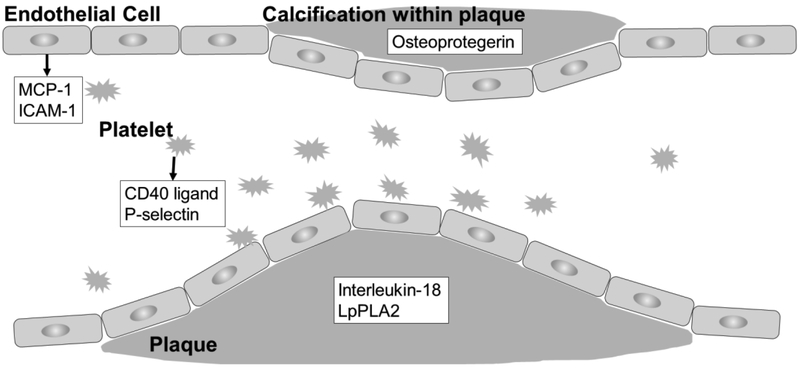
Short-term exposure to ambient air pollution has been associated with acute cardiovascular events 1, 2 One of the hypothesized underlying mechanisms is air pollution-induced oxidative stress and inflammation. Higher levels of oxidative stress and inflammation in the vascular system promote platelet and endothelial cell activation, leading to increased tendency towards thrombosis and erosion and/or disruption of atherosclerotic plaque 3-7 For example, CD40 ligand and P-selectin are released upon platelet activation and may enhance endothelial inflammation and upregulate expression of chemokines (such as monocyte chemoattractant protein 1 (MCP-1)) and adhesion molecules (such as intercellular adhesion molecule 1 (ICAM-1)), altering the properties of the endothelium and contributing to the formation of atherosclerotic plaque 3-7 Increased vascular inflammation also stimulates secretion of biomarkers such as interleukin-18, lipoprotein-associated phospholipase A2 (LpPLA2), and osteoprotegerin. Higher levels of interleukin-18 and LpPLA2 are associated with plaque disruption and thrombosis 8, 9, and higher levels of osteoprotegerin are associated with vascular calcification in human studies 10-12. Figure 1 illustrates the involvement of these biomarkers.
Fig. 1.
Involvement of CD40 ligand, P-selectin, chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), interleukin-18, lipoprotein-associated phospholipase A2 (LpPLA2), and osteoprotegerin in endothelial cell activation.
Several prior studies have examined the associations between short-term exposure to air pollutants and the aforementioned biomarkers that are involved in endothelial cell activation 13-33. While some of these studies found positive associations between some air pollutants and CD40 ligand 13-16, P-selectin 14-18, MCP-1 14, 31, and ICAM-1 29, 30, the findings were generally inconsistent across different air pollutants or studies, possibly because most of these previous studies were conducted with small sample sizes, limited age range, or among participants with conditions that may predispose them to the adverse health effects of air pollution. Some other studies have found null associations with interleukin-18 31, positive associations with LpPLA2 mass 32, and negative associations with osteoprotegerin 33.
Among participants from the Framingham Heart Study, we have found associations between short-term exposure to ambient air pollution and biomarkers of oxidative stress 34 and systemic inflammation 35. However, the associations for biomarkers of endothelial cell activation and atherosclerotic plaque have not been examined.
We therefore hypothesized that short-term exposure to higher levels of ambient air pollution is associated with higher levels of biomarkers of endothelial cell activation. We investigated the associations among participants from the Framingham Heart Study.
2. Methods
2.1. Study sample
We included participants from the Framingham Offspring cohort examinations 7 (1998-2001) and 8 (2005-2008), and Third Generation cohort examination 1 (2002-2005). The study design and selection criteria of the two cohorts have been described elsewhere 36, 37 We restricted our analyses to 4540 participants (6192 observations) who lived within 50 km from the Harvard Supersite air pollution monitor in Boston, Massachusetts. Interleukin-18 levels that were under the minimum detection limit (128 pg/ml) were treated as missing (N=162). We then excluded 879 observations contributed by current smokers because levels of inflammatory biomarkers may be influenced by smoking and may interfere with our ability to assess the relatively small variation in these biomarkers that can be attributed to ambient air pollution 38. Last, we excluded 97 observations where data on body mass index, alcohol intake, or pack years of smoking were missing, leaving 3820 participants (5216 observations) in the final dataset. At each examination visit, physical examinations were performed following standardized protocols, and data on demographics, medication history, smoking history, and alcohol intake were collected using standardized questionnaires. All participants provided written informed consent at each examination, and the Institutional Review Boards at Beth Israel Deaconess Medical Center, Harvard T.H. Chan School of Public Health, and Boston University Medical Center approved the study.
2.2. Air pollution and meteorology assessment
We calculated 1-, 2-, 3-, 5-, and 7-day moving averages of the air pollutants prior to the date of examination visit, based on measures of fine particulate matter (particles with diameters ≤2.5 μm; PM2.5), black carbon (BC), and sulfate (SO42−) from the Boston Harvard Supersite air pollution monitoring station, and measures of nitrogen oxides (NOx) and ozone (O3) from local state monitors within the Greater Boston area (three for NOx and two for O3) 39, 40. The 1-day moving average (lag 0) was calculated as the average from 9:00 am on the day before examination date to 9:00 am on the day of examination visit. The 2-day moving average was calculated as the mean of lag 0 and lag 1. If more than 25% of the days for a moving average were missing, we assigned “missing” to that moving average.
The Supersite monitor is located on the rooftop of the Francis A. Countway Library of Medicine (5 stories above ground level) and 50 m from the nearest street. PM2.5 was measured using a tapered element oscillating microbalance (Model 1400A, Rupprecht & Patashnick Co., Inc.); BC was measured using an aethalometer (Model AE16, Magee Scientific Corp.); and SO42− was measured from elemental sulfur that was measured by X-Ray Fluorescence analysis of the PM2.5 filter samples. On days when SO42− X-Ray Fluorescence data were not available, we used an SO42− analyzer (Model 5020, Thermo Electron Corp.). Hourly temperature and relative humidity were assessed at the Boston Logan International Airport Weather Station, located 12 km from the Supersite 41.
2.3. Biomarker assessment
All blood samples were obtained after an overnight fast, typically between 7 and 9 am. Blood specimens were stored at −80°C until assay. Commercially available enzyme-linked immunoassay kits were used to assess P-selectin, MCP-1, ICAM-1 (R&D Systems, Minneapolis, MN); CD40 ligand (Bender MedSystems, Vienna, Austria); interleukin-18 (MBL, Woburn, MA); and osteoprotegerin (BioMedica Incorporate, San Diego, CA). LpPLA2 activity was measured using a colorimetric activity method (diaDexus CAM Kit, Inc., San Francisco, CA).
LpPLA2 mass was measured using a commercially available sandwich enzyme immunoassay (diaDexus PLAC Test, Inc., San Francisco, CA). Details regarding biomarker measurements have been reported elsewhere 42-44 and can also be found at the Framingham Heart Study website (https://www.framinghamheartstudy.org).
2.4. Statistical methods
Because P-selectin, MCP-1, ICAM-1, LpPLA2 activity, LpPLA2 mass, and osteoprotegerin were measured in both Offspring cohort examination 7 (1998-2001) and 8 (2005-2008), we used multivariable linear mixed effects models with participant-specific random intercepts. We used multivariable linear regression models for CD40 ligand and interleukin-18 that were measured only once in the Offspring cohort examination 7. Levels of the biomarkers were loge transformed to meet the model assumptions, assessed by residual plots.
For each moving average, the model was adjusted for demographic variables, individual-level socioeconomic position, census tract-level socioeconomic position, lifestyle factors, clinical factors, time, and meteorology. The covariates were selected because of their role as potential confounders or predictors of the outcome. The demographic variables included centered age at the time of examination visit, (centered age)2, and sex. For individual-level socioeconomic position, we adjusted for educational attainment (high school or less, some college, and college graduate) and usual occupation (laborer; sales/homemaker/clerical; professional/executive/supervisory/technical; and unspecified) 35, 45. For census tract-level socioeconomic position, we adjusted for median household income (continuous), median value of owner-occupied housing units (continuous), and population density (people/km2, continuous), from U.S. Census 2000 data. For lifestyle factors, we included body mass index, alcohol intake (drinks/week; standardized to 15 ml alcohol/drink, continuous), pack years of smoking (continuous), smoking status (never or former smoker), and tertiles of physical activity index 46. For clinical factors, we adjusted for cardiovascular disease status, use of antihypertensive medications, and use of statins, all as binary variables. We adjusted for time by including a linear term of examination date, for seasonality by including sine and cosine terms of the examination date, and for meteorology by adding temperature and relative humidity. Last, we added an examination cycle identifier for biomarkers that were measured in multiple examinations to account for potential batch effects. We created missing indicators for participants with missing data on educational attainment (34 observations) or physical activity (88 observations).
We conducted a sensitivity analysis restricting analyses to participants who lived within 40 km (4456 observations, 85%) from the central site. We also examined whether associations differed by age (≤/> 65 years old), sex, and diabetes status. Due to the moderate negative correlation between NOx and O3 (r=−0.55), we conducted a post-hoc sensitivity analysis and included both pollutants in the same model. Last, we examined the associations between moving averages of daily maximum 8-hour O3 and the levels of measured biomarkers.
Parameter estimates (βS) were scaled by a factor close to the interquartile range of the 1-day moving average: 5 μg/m3 for PM2.5, 0.5 μg/m3 for BC, 2 μg/m3 for SO42−, 20 ppb for NOx, and 10 ppb for O3.
We reported percent difference ((e(scaled β)−1)×100%) with 95% confidence intervals (CIs). In interpreting the results, we focused on describing and highlighting association patterns that were consistently observed across multiple moving averages of the pollutants. Analyses were performed using PROC GLM and PROC MIXED in SAS 9.4 (SAS Institute, Inc., Cary, NC). Figures were plotted using Stata 13 (StataCorp LP, College Station, TX).
3. Results
Of the 5216 observations, the mean age was 56 years old (standard deviation (SD): 15) and 2794 (54%) were from women (Table 1). The mean level of the 1-day moving average PM2.5 was 10.2 μg/m3 (SD: 6.1). As expected, the correlations between PM2.5, BC, and SO42− were moderate to high, and NOx was negatively correlated with O3 (Table 2). The distribution of measured pollutants are shown in Supplemental Figure 1.
Table 1.
Characteristics of the 3820 participants (5216 observations) from the Framingham Offspring and Third Generation cohorts.
| Characteristics | Offspring Cohort Mean(SD) or N[%] |
Third Generation Cohort Mean(SD) or N[%] |
Overall Mean(SD) or N[%] |
|
|---|---|---|---|---|
| Cycle 7 | Cycle 8 | Cycle 1 | ||
| Number of observations | 1846 | 1549 | 1821 | 5216 |
| Age, years | 61.7 (9.5) | 67.3 (9.1) | 40.1 (8.9) | 55.8 (14.9) |
| Women | 987 [53.5] | 836 [54.0] | 971 [53.3] | 2794 [53.6] |
| Body mass index, kg/m2 | 28.4 (5.3) | 28.6 (5.4) | 27.0 (5.6) | 28.0 (5.5) |
| Alcohol, drinks/week | 4.5 (7.0) | 4.0 (6.7) | 4.3 (6.2) | 4.3 (6.6) |
| Smoking status | ||||
| Non-smoker | 792 [42.9] | 665 [42.9] | 1255 [68.9] | 2712 [52.0] |
| Former smoker | 1054 [57.1] | 884 [57.1] | 566 [31.1] | 2504 [48.0] |
| Pack year | 14.1 (20.6) | 14.1 (20.6) | 3.5 (9.0) | 10.4 (18.2) |
| Educational attainmenta | ||||
| High school or less | 667 [36.1] | 549 [35.4] | 266 [14.6] | 1482 [28.4] |
| Some college | 563 [30.5] | 483 [31.2] | 575 [31.6] | 1621 [31.1] |
| College graduate | 586 [31.7] | 517 [33.4] | 976 [53.6] | 2079 [39.9] |
| Diabetes | 245 [13.3] | 288 [18.6] | 55 [3.0] | 588 [11.3] |
| Cardiovascular disease | 240 [13.0] | 263 [17.0] | 28 [1.5] | 531 [10.2] |
| Antihypertensive use | 681 [36.9] | 920 [59.4] | 186 [10.2] | 1787 [34.3] |
| Statins use | 422 [22.9] | 661 [42.7] | 124 [6.8] | 1207 [23.1] |
| CD40 ligandb, ng/ml | 1.6 (2.0) | - | - | 1.6 (2.0) |
| ICAM-1b,c, ng/ml | 244.8 (57.9) | 291.3 (91.4) | 240.1 (57.8) | 255.8 (70.5) |
| Interleukin-18b, pg/ml | 250.8 (94.6) | - | - | 250.8 (94.6) |
| LpPLA2 activityb,c, nmol/min/ml | 138.2 (34.4) | 134.2 (34.8) | 160.8 (36.6) | 144.5 (37.2) |
| LpPLA2 massb,c, ng/ml | 282.5 (89.8) | 192.5 (51.4) | 225.8 (46.2) | 233.2 (72.2) |
| MCP-1b,c, pg/ml | 312.4 (104.4) | 374.4 (116.2) | 331.1 (109.9) | 336.3 (112.6) |
| P-selectinb,c, ng/ml | 35.3 (13.3) | 39.1 (12.6) | 45.4 (16.8) | 39.7 (14.9) |
| Osteoprotegerinb,c, pmol/l | 5.4 (1.7) | 4.8 (1.6) | - | 5.1 (1.7) |
Abbreviation: SD, standard deviation; ICAM-1, intercellular adhesion molecule 1; LpPLA2, lipoprotein-associated phospholipase A2; MCP-1, monocyte chemoattractant protein 1.
30 (1.6%) and 4 (0.2%) participants in examination cycle 7 and 1, respectively, were missing educational attainment data and were assigned missing indicators.
Geometric mean and standard deviation.
1352 participants had two measurements of ICAM-1, 1376 participants had two measurements of LpPLA2 activity and LpPLA2 mass, 1328 participants had two measurements of MCP-1, 1395 participants had two measurements of P-selectin, and 1392 participants had two measurements of osteoprotegerin.
Table 2.
Characteristics and the Spearman correlation coefficients between the 1-day moving averages of measured ambient air pollutants.
| Pollutant | Number of observations |
Mean (SD) | Interquartile Range |
Spearman Correlation Coefficients |
|||
|---|---|---|---|---|---|---|---|
| BC | SO42− | NOx | O3 | ||||
| PM2.5, μg/m3 | 5206 | 10.2 (6.1) | 6.4 | 0.73 | 0.82 | 0.41 | 0.00 |
| BC, μg/m3 | 5205 | 0.8 (0.5) | 0.5 | 0.53 | 0.57 | −0.25 | |
| SO42−, μg/m3 | 4461 | 3.2 (2.5) | 2.4 | 0.28 | 0.11 | ||
| NOx, ppb | 4912 | 39.1 (21.0) | 19.7 | −0.55 | |||
| O3, ppb | 5207 | 23.2 (11.2) | 15.4 | ||||
Abbreviation: PM2.5, fine particulate matter; BC, black carbon; SO42−, sulfate; NOx, nitrogen oxides; O3, ozone.
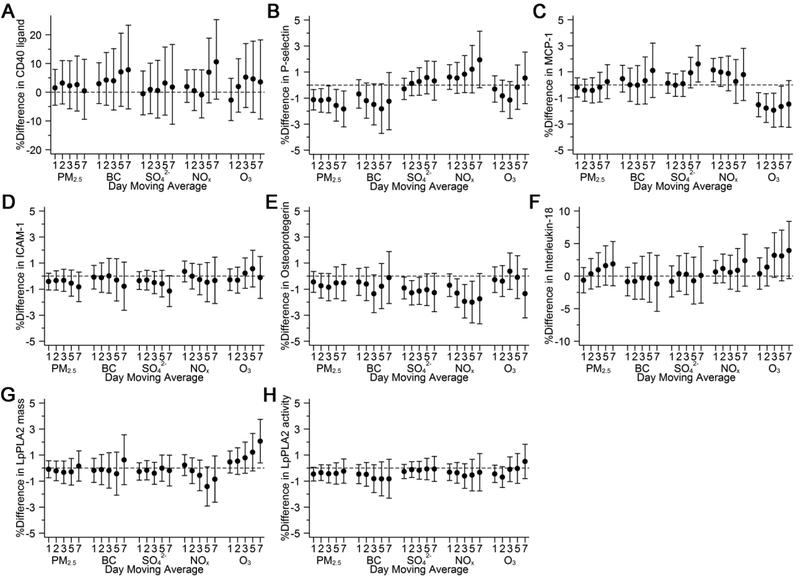
Higher levels of exposure to PM2.5 and BC were associated with lower levels of P-selectin: a 5 μg/m3 higher 5-day moving average of PM2.5 and a 0.5 μg/m3 higher 5-day moving average of BC were associated with 1.6% (−2.8, −0.3) and 1.8% (−3.7, 0.1) lower levels of P-selectin, respectively (Figure 2B). Exposure to higher levels of 1- to 5-day moving averages of O3 before exam visit was associated with lower levels of MCP-1: a 10 ppb higher 5-day moving average of O3 was associated with 1.7% (−3.2, −0.1) lower levels of MCP-1 (Figure 2C). We also found that higher levels of exposure to SO42− and NOx were associated with lower levels of osteoprotegerin: for the 5-day moving average, a 2 μg/m3 higher SO42− and a 20 ppb higher NOx were associated with 1.1% (−2.3, 0.2) and 2.0% (−3.6, −0.4) lower levels of osteoprotegerin, respectively (Figure 2E). Higher exposure to O3 at longer moving averages were associated with higher levels of interleukin-18 (Figure 2F) and LpPLA2 mass (Figure 2G). The associations otherwise appeared to be generally null.
Fig. 2.
Associations of 1- to 7-day moving averages of air pollutants with A) CD40 ligand; B) P-selectin; C) monocyte chemoattractant protein 1 (MCP-1); D) intercellular adhesion molecule 1 (ICAM-1); E) osteoprotegerin; F) interleukin-18; G) lipoprotein-associated phospholipase A2 (LpPLA2) mass; and H) LpPLA2 activity. Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, usual occupations, tertiles of physical activity index, census tract median household income, census tract population density, census tract median value of owner-occupied housing unit, cardiovascular disease, use of antihypertensives, use of statins, date of examination visit, sine and cosine season, day of week, temperature, and relative humidity. An exam identifier was added for P-selectin, MCP-1, ICAM-1, osteoprotegerin, LpPLA2 mass, and LpPLA2 activity. Results were scaled to 5 μg/m3 for PM2.5, 0.5 μg/m3 for BC, 2 μg/m3 for SO4 2−, 20 ppb for NOx, and 10 ppb for O3. Error bars indicate the 95% confidence intervals.
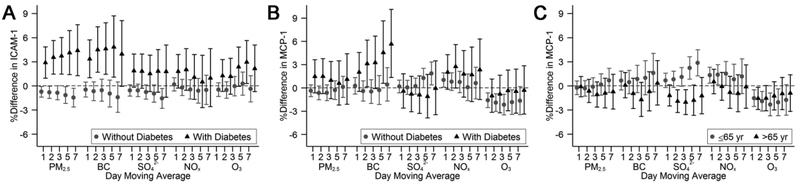
Restricting the study sample to participants who lived within 40 km from the Supersite, or replacing daily 24-hour average O3 with daily maximum 8-hour O3 did not alter our results materially (Supplemental Figures 2 and 3). In analyses where we included both NOx and O3 in the same model, both NOx and O3 appeared to be positively associated with interleukin-18 across multiple moving averages (Supplemental Figure 4). Among participants with diabetes, we observed consistent and positive associations of PM2.5, BC, and SO42− with ICAM-1 over multiple moving averages (Figures 3A). We also observed positive associations of BC with MCP-1 among participants with diabetes, and of SO42− with MCP-1 among participants younger than 65 years old (Figures 3B and 3C). However, the differing association pattern was not observed between other pollutants and MCP-1. The associations otherwise did not differ by age of 65 years old, sex, or diabetes status (Supplemental Figures 5-7).
Fig. 3.
Associations of 1- to 7-day moving averages of air pollutants with A) ICAM-1 and B) MCP-1 by diabetes status, and with C) MCP-1 by age of 65 years old. Models were adjusted for centered age, (centered age)2, sex, body mass index, smoking status, pack years, alcohol intake, educational attainment, usual occupations, tertiles of physical activity index, census tract median household income, census tract population density, census tract median value of owner-occupied housing unit, cardiovascular disease, use of antihypertensives, use of statins, date of examination visit, sine and cosine season, day of week, temperature, relative humidity, and an examination identifier. Results were scaled to 5 μg/m3 for PM2.5, 0.5 μg/m3 for BC, 2 μg/m3 for SO4 2−, 20 ppb for NOx, and 10 ppb for O3. Error bars indicate the 95% confidence intervals.
4. Discussion
Among participants from the Framingham Offspring and Third Generation cohorts, we found negative associations of PM2.5 and BC with P-selectin, of O3 with MCP-1, and of SO42− and NOx with osteoprotegerin; the associations otherwise appeared to be null. There was no consistent association for ICAM-1 in the overall study sample, however, higher levels of exposure to PM2.5, BC, and SO42− were associated with higher levels of ICAM-1 among participants with diabetes.
The current study adds to our previous work in the Framingham Heart Study 34, 35, in which we found short-term exposure to higher levels of ambient air pollution was associated with higher levels of myeloperoxidase, indexed 8-epi-PGF2α 34, C-reactive protein (CRP), interleukin-6, and tumor necrosis factor receptor 2 (TNFR2), but not with fibrinogen or TNFα35. Together we have provided a comprehensive coverage of the associations between short-term exposure to ambient air pollution and biomarkers of oxidative stress, systemic inflammation, and endothelial cell activation among participants from the community-based Framingham Heart Study. Because the magnitude of observed negative associations were rather small and the associations observed were likely transient, the interpretation of our findings may not be suitable for clinical interpretation.
Both CD40 ligand and P-selectin are released upon platelet and endothelial cell activation, and induce the production of reactive oxygen species, adhesion molecules, chemokines, and other inflammatory biomarkers 4, 5, 7 Some studies have found positive associations of short-term exposure to air pollution with CD40 ligand 13-16 and P-selectin 14-18, but not all 13, 19-21, 31. Moreover, one of the studies found a negative association for CD40 ligand 31. However, these studies were all conducted among a relatively small number of participants 13, 31, and many were conducted in regions with higher levels of air pollution than the current study 14-17, 21.
In the current study, we found generally null associations between PM2.5 and MCP-1 and ICAM-1, except that among potentially susceptible participants who had type 2 diabetes, we found positive associations between PM2.5 and ICAM-1. Expression of both MCP-1 and ICAM-1 by endothelial cells are upregulated upon stimulation by pro-inflammatory cytokines 4, 5. Prior studies of the associations between PM2.5 and MCP-1 have had inconsistent results; two studies reported positive associations 14, 31, but the association appeared to be null in another study 15.
The association between PM2.5 and ICAM-1 has been examined in a larger number of studies 14, 15, 22-31 and several of them had a relatively large sample size 27-30; however, the findings were not consistent across studies. For example, in the Multi-Ethnic Study of Atherosclerosis (MESA), Hajat etal. found negative associations between the 3- to 5-day moving averages of PM2.5 and ICAM-1 among 2865 participants (4007 observations in total), and reasons for this negative association were unclear 27. Moreover, PM2.5 was positively associated with ICAM-1 in two reports from the Normative Aging Study 29, 30.
Sustained baseline inflammation among participants with diabetes may upregulate the inflammatory response to air pollutants 47, and in our previous work we found a larger magnitude of the associations of short-term air pollution with C-reactive protein and interleukin-6 among participants with diabetes than those without 35. In the current study, we found positive associations of PM2.5, BC, and SO42− with ICAM-1 across multiple moving averages among participants with diabetes, suggesting potentially higher susceptibility to air pollution among individuals with diabetes.
Both interleukin-18 and LpPLA2 are highly expressed in atherosclerotic plaque, and may result in increased risk of plaque disruption and thrombosis 7, 8. LpPLA2 is an enzyme that hydrolyses oxidized phospholipids and generates oxidized free fatty acids and lysophosphatidylcholine, promoting atherogenesis and plaque formation 8, 9 Previous small-scale studies have found null associations of PM2.5 with interleukin-18 among 72 healthy young participants 31, but positive associations with LpPLA2 mass among 200 myocardial infarction survivors 32. The associations were generally null in the current study. It is possible that the different study sample characteristics contributed to the discrepancies between the current study and previous studies; the study participants in the current study were middle-aged and older, and were generally healthy with relatively low prevalence of cardiovascular disease and diabetes.
The exact role of osteoprotegerin in vascular biology is not clear. In controlled animal studies, osteoprotegerin was found to be a protective factor against atherosclerotic calcification 10-12 However, in human studies, osteoprotegerin was positively associated with vascular calcification and coronary heart disease 48-54. It has been proposed that the elevated osteoprotegerin level was the result of a compensatory mechanism 11. We found negative associations of SO42− and NOx with osteoprotegerin, similar to the previous study where negative associations were observed between indoor PM2.5 from burning of biomass and osteoprotegerin 33.
In summary, in the current study we examined the associations between short-term exposure to ambient air pollution and several biomarkers of endothelial cell activation, and found that the associations were generally null or negative, except for longer moving averages of O3 with interleukin-18 and LpPLA2 mass. This divergent associations may represent different underlying biological pathways: while P-selectin and MCP-1 are related to platelet activation and stimulation from pro-inflammatory cytokines, and osteoprotegerin is potentially related to vascular calcification, interleukin-18 and LpPLA2 are potentially related to existing plaques. However, the exact biological mechanism remains unclear. As we mentioned before, not many large-scale studies have examined the associations between short-term exposure to air pollution and the biomarkers that were measured in the current study. Thus, future large-scale studies are needed to examine these associations.
There are several limitations in the current study. First, we measured levels of air pollution at a central air pollution monitor. Previous studies in the Boston region have found moderate correlations between central site measured PM2.5 and SO42− and personal exposure levels (regression slope was 0.3 in winter and 0.8-0.9 in summer for PM2.5; and was 0.4-0.6 in winter and 0.7 in summer for SO42−) 55, 56, which supports the rationale of exposure assignment. Moreover, this potential exposure measurement error was likely non-differential. Second, participants from the Framingham Heart Study are mostly middle-aged and older adults of European ancestry; our findings may not be generalizable to different age groups or races/ethnicities. Last, although we adjusted for many potential confounders, we cannot rule out residual or unmeasured confounding, and cannot ascertain temporality in the current study. Thus, the observed associations should not be used to infer causality.
There are also several strengths of the current study. The current study is among a few large-scale studies that included participants from a community-based cohort and examined the associations between ambient air pollution and biomarkers of endothelial cell activation. The Framingham Heart Study utilized standardized protocols for physical examinations and biomarkers assessments. We adjusted for a robust set of potential confounders, including demographic characteristics, lifestyle, individual- and area-level socioeconomic position, meteorology, and time in our analyses. Additionally, levels of air pollutants and biomarkers were measured separately so that any measurement error was likely non-differential. Last, because the differences in air pollution levels between participants were mostly determined by the dates of participants’ examination visits and the participants could schedule their examination visit in advance and spread over the year, the possibility of strong residual confounding by examination date is unlikely.
5. Conclusions
Our findings do not support the hypothesis that short-term exposure to ambient air pollution is associated with higher levels of circulating biomarkers of endothelial cell activation among generally healthy adults. Rather, in this region with relatively low levels of ambient air pollution, we found negative associations of several air pollutants with these biomarkers, including P-selectin, MCP-1, and osteoprotegerin. Furthermore, among participants with diabetes, the associations for ICAM-1 appeared to be positive. Future longitudinal studies with large sample sizes and larger variations in air pollution levels are needed to confirm or refute our findings.
Supplementary Material
Highlights.
Higher PM2.5 and BC were associated with lower P-selectin levels
Higher ozone was associated with lower MCP-1 levels
Higher SO42− and NOx were associated with lower osteoprotegerin levels
Participants with type 2 diabetes may be more susceptible
Acknowledgements:
We thank the participants of the Framingham Offspring and Third Generation cohorts.
Funding sources:
This publication was made possible by USEPA grant (RD-835872-01) through the Harvard University USEPA sponsored Air, Climate & Environment (ACE) Centre. The contents of the study are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. Funding of the inflammation markers was through 1RO1 HL64753, R01 HL076784, 1 R01 AG028321, 1R01HL128914, 2R01 HL092577, and 1P50HL120163. Funding of the Framingham Heart Study was via HHSN268201500001I; N01-HC 25195. This work was also supported by the NIEHS grants P01 ES09825 and P30 ES000002, and the National Institute of General Medical Sciences grant 1P20GM109036-01A1.
Abbreviations:
- BC
Black carbon
- ICAM-1
Intercellular adhesion molecule 1
- LpPLA2
Lipoprotein-associated phospholipase A2
- MCP-1
Monocyte chemoattractant protein 1
- NOx
Nitrogen oxides
- O3
Ozone
- PM2.5
Fine particulate matter
- SO42−
Sulfate
Footnotes
Declarations of interest:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr., Tager I, Expert Panel on P, Prevention Science of the American Heart A. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the american heart association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD, on behalf of the American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Wagner DD, Burger PC. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 4.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 6.Stokes KY, Granger DN. Platelets: A critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part i. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 8.Szmitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S. Biomarkers of vascular disease linking inflammation to endothelial activation: Part ii. Circulation. 2003;108:2041–2048. doi: 10.1161/01.CIR.0000089093.75585.98. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MK, Bertoia ML, Cahill LE, Agarwal I, Rimm EB, Mukamal KJ. Novel metabolic biomarkers of cardiovascular disease. Nat Rev Endocrinol. 2014;10:659–672. doi: 10.1038/nrendo.2014.155. [DOI] [PubMed] [Google Scholar]
- 10.Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009;204:321–329. doi: 10.1016/j.atherosclerosis.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Vassalle C, Mazzone A. Bone loss and vascular calcification: A bi-directional interplay? Vascul Pharmacol. 2016;86:77–86. doi: 10.1016/j.vph.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Frampton MW, Bausch J, Chalupa D, Hopke PK, Little EL, Oakes D, Stewart JC, Utell MJ. Effects of outdoor air pollutants on platelet activation in people with type 2 diabetes. Inhal Toxicol. 2012;24:831–838. doi: 10.3109/08958378.2012.724117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu C, Cai J, Qiao L, Wang H, Xu W, Li H, Zhao Z, Chen R, Kan H. The acute effects of fine particulate matter constituents on blood inflammation and coagulation. Environ Sci Technol. 2017;51:8128–8137. doi: 10.1021/acs.est.7b00312. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhou L, Wang C, Chen R, Ma X, Xu B, Xiong L, Ding Z, Chen X, Zhou Y, Xu Y, Kan H. Associations between air quality changes and biomarkers of systemic inflammation during the 2014 nanjing youth olympics: A quasi-experimental study. Am J Epidemiol. 2017;185:1290–1296. doi: 10.1093/aje/kww209. [DOI] [PubMed] [Google Scholar]
- 16.Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang JJ. Association between changes in air pollution levels during the beijing olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307:2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, Weschler CJ, Ohman-Strickland PA, Sundell J, Weng W, Zhang Y, Zhang JJ. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med. 2017;177:1344–1353. doi: 10.1001/jamainternmed.2017.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, Dailey L, Devlin RB, Diaz-Sanchez D, Koenig W, Phipps R, Silbajoris R, Soentgen J, Soukup J, Peters A, Schneider A. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Ruckerl R, Phipps RP, Schneider A, Frampton M, Cyrys J, Oberdorster G, Wichmann HE, Peters A. Ultrafine particles and platelet activation in patients with coronary heart disease--results from a prospective panel study. Part Fibre Toxicol. 2007;4:1. doi: 10.1186/1743-8977-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu S, Deng F, Wei H, Huang J, Wang H, Shima M, Wang X, Qin Y, Zheng C, Hao Y, Guo X. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: A prospective panel study. Part Fibre Toxicol. 2012;9:49. doi: 10.1186/1743-8977-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan RM, Sullivan JH, Carlsten C, Wilkerson HW, Beyer RP, Bammler T, Farin F, Peretz A, Kaufman JD. A randomized cross-over study of inhalation of diesel exhaust, hematological indices, and endothelial markers in humans. Part Fibre Toxicol. 2013;10:7. doi: 10.1186/1743-8977-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: A mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S, Yang D, Pan L, Shan J, Li H, Wei H, Wang B, Huang J, Baccarelli AA, Shima M, Deng F, Guo X. Chemical constituents and sources of ambient particulate air pollution and biomarkers of endothelial function in a panel of healthy adults in beijing, china. Sci Total Environ. 2016;560-561:141–149. doi: 10.1016/j.scitotenv.2016.03.228. [DOI] [PubMed] [Google Scholar]
- 25.Hildebrandt K, Ruckerl R, Koenig W, Schneider A, Pitz M, Heinrich J, Marder V, Frampton M, Oberdorster G, Wichmann HE, Peters A. Short-term effects of air pollution: A panel study of blood markers in patients with chronic pulmonary disease. Part Fibre Toxicol. 2009;6:25. doi: 10.1186/1743-8977-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gong H Jr., Sioutas C, Linn WS. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient particles in metropolitan los angeles. Res Rep Health Eff Inst. 2003:1–36; discussion 37-47. [PubMed] [Google Scholar]
- 27.Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: A repeat-measures analysis in the multiethnic study of atherosclerosis (mesa). Epidemiology. 2015;26:310–320. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, Schwartz J. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2010;67:312–317. doi: 10.1136/oem.2009.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilker EH, Alexeeff SE, Suh H, Vokonas PS, Baccarelli A, Schwartz J. Ambient pollutants, polymorphisms associated with microrna processing and adhesion molecules: The normative aging study. Environ Health. 2011;10:45. doi: 10.1186/1476-069X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bind MA, Baccarelli A, Zanobetti A, Tarantini L, Suh H, Vokonas P, Schwartz J. Air pollution and markers of coagulation, inflammation, and endothelial function: Associations and epigene-environment interactions in an elderly cohort. Epidemiology. 2012;23:332–340. doi: 10.1097/EDE.0b013e31824523f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O'Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119:1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruske I, Hampel R, Baumgartner Z, Ruckerl R, Greven S, Koenig W, Peters A, Schneider A. Ambient air pollution and lipoprotein-associated phospholipase a(2) in survivors of myocardial infarction. Environ Health Perspect. 2011;119:921–926. doi: 10.1289/ehp.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha H, Mukherjee B, Bindhani B, Ray MR. Changes in rankl and osteoprotegerin expression after chronic exposure to indoor air pollution as a result of cooking with biomass fuel. J Appl Toxicol. 2016;36:969–976. doi: 10.1002/jat.3275. [DOI] [PubMed] [Google Scholar]
- 34.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr., Lin H, Vasan RS, Benjamin EJ, Mittleman MA. Short-term exposure to air pollution and biomarkers of oxidative stress: The framingham heart study. J Am Heart Assoc. 2016. doi: 10.1161/JAHA.115.002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Dorans KS, Wilker EH, Rice MB, Ljungman PL, Schwartz JD, Coull BA, Koutrakis P, Gold DR, Keaney JF Jr., Vasan RS, Benjamin EJ, Mittleman MA. Short-term exposure to ambient air pollution and biomarkers of systemic inflammation: The framingham heart study. Arterioscler Thromb Vasc Biol. 2017;37:1793–1800. doi: 10.1161/ATVBAHA.117.309799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 37.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB Sr., Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the national heart, lung, and blood institute's framingham heart study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 38.Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF Jr., Sutherland PA, Vasan A, Lipinska I, Evans JC, Benjamin EJ. Relation of smoking status to a panel of inflammatory markers: The framingham offspring. Atherosclerosis. 2008;201:217–224. doi: 10.1016/j.atherosclerosis.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta AJ, Zanobetti A, Koutrakis P, Mittleman MA, Sparrow D, Vokonas P, Schwartz J. Associations between short-term changes in air pollution and correlates of arterial stiffness: The veterans affairs normative aging study, 2007-2011. Am J Epidemiol. 2014;179:192–199. doi: 10.1093/aje/kwt271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang CM, Koutrakis P, Suh HH. Hourly measurements of fine particulate sulfate and carbon aerosols at the harvard-u.S. Environmental protection agency supersite in boston. J Air Waste Manag Assoc. 2010;60:1327–1334. doi: 10.3155/1047-3289.60.11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ljungman PL, Wilker EH, Rice MB, Schwartz J, Gold DR, Koutrakis P, Vita JA, Mitchell GF, Vasan RS, Benjamin EJ, Mittleman MA, Hamburg NM. Short-term exposure to air pollution and digital vascular function. Am J Epidemiol. 2014;180:482–489. doi: 10.1093/aje/kwu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murabito JM, Keyes MJ, Guo CY, Keaney JF Jr., Vasan RS, D'Agostino RB Sr., Benjamin EJ. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The framingham offspring study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham heart study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong SN, Gona P, Fontes JD, Oyama N, Chan RH, Kenchaiah S, Tsao CW, Yeon SB, Schnabel RB, Keaney JF, O'Donnell CJ, Benjamin EJ, Manning WJ. Atherosclerotic biomarkers and aortic atherosclerosis by cardiovascular magnetic resonance imaging in the framingham heart study. J Am Heart Assoc. 2013;2:e000307. doi: 10.1161/JAHA.113.000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loucks EB, Lynch JW, Pilote L, Fuhrer R, Almeida ND, Richard H, Agha G, Murabito JM, Benjamin EJ. Life-course socioeconomic position and incidence of coronary heart disease: The framingham offspring study. Am J Epidemiol. 2009;169:829–836. doi: 10.1093/aje/kwn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannel WB, Sorlie P. Some health benefits of physical activity. The framingham study. Arch Intern Med. 1979;139:857–861. doi: 10.1001/archinte.1979.03630450011006. [DOI] [PubMed] [Google Scholar]
- 47.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 49.Semb AG, Ueland T, Aukrust P, Wareham NJ, Luben R, Gullestad L, Kastelein JJ, Khaw KT, Boekholdt SM. Osteoprotegerin and soluble receptor activator of nuclear factor-kappab ligand and risk for coronary events: A nested case-control approach in the prospective epic-norfolk population study 1993-2003. Arterioscler Thromb Vasc Biol. 2009;29:975–980. doi: 10.1161/ATVBAHA.109.184101. [DOI] [PubMed] [Google Scholar]
- 50.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J. Soluble receptor activator of nuclear factor-kappa b ligand and risk for cardiovascular disease. Circulation. 2007;116:385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 51.Stenemo M, Nowak C, Byberg L, Sundstrom J, Giedraitis V, Lind L, Ingelsson E, Fall T, Arnlov J. Circulating proteins as predictors of incident heart failure in the elderly. Eur J Heart Fail. 2018;20:55–62. doi: 10.1002/ejhf.980. [DOI] [PubMed] [Google Scholar]
- 52.Nyrnes A, Njolstad I, Mathiesen EB, Wilsgaard T, Hansen JB, Skjelbakken T, Jorgensen L, Lochen ML. Inflammatory biomarkers as risk factors for future atrial fibrillation. An eleven-year follow-up of 6315 men and women: The tromso study. Gend Med. 2012;9:536–547 e532. doi: 10.1016/j.genm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Szulc P, Chapurlat R, Hofbauer LC. Prediction of fractures and major cardiovascular events in men using serum osteoprotegerin levels: The prospective strambo study. J Bone Miner Res. 2017;32:2288–2296. doi: 10.1002/jbmr.3213. [DOI] [PubMed] [Google Scholar]
- 54.di Giuseppe R, Biemann R, Wirth J, Menzel J, Isermann B, Stangl GI, Fritsche A, Boeing H, Schulze MB, Weikert C. Plasma osteoprotegerin, its correlates, and risk of heart failure: A prospective cohort study. Eur J Epidemiol. 2017;32:113–123. doi: 10.1007/s10654-016-0172-4. [DOI] [PubMed] [Google Scholar]
- 55.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: Implications for studying the health effects of particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 56.Brown KW, Sarnat JA, Suh HH, Coull BA, Spengler JD, Koutrakis P. Ambient site, home outdoor and home indoor particulate concentrations as proxies of personal exposures. J Environ Monit. 2008;10:1041–1051. doi: 10.1039/b805991h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.