Abstract
BACKGROUND
Massive burns induce a hypermetabolic response that leads to total body wasting and impaired physical and psychosocial recovery. The administration of propranolol or oxandrolone positively affects postburn metabolism and growth. The combined administration of oxandrolone and propranolol (OxProp) for one year restores growth in children with large burns. Here we investigated whether the combined administration of oxandrolone and propranolol for one year would reduce scarring and improve quality of life compared to control.
STUDY DESIGN
Children with large burns (n=480) were enrolled into this IRB-approved study; patients were randomized to control (n=226) or administration of OxProp (n=126) for 1 year postburn. Assessments were conducted at discharge and 6, 12, and 24 months postburn. Scar biopsies were obtained for histology. Physical scar assessments and patient reported outcome measures of physical and psychosocial function were obtained.
RESULTS
Reductions in cellularity, vascular structures, inflammation, and abnormal collagen (p<0.05) occurred in OxProp-treated scars. With OxProp, scar severity was attenuated and pliability increased (both p<0.05). Analyses of patient reported outcomes showed improved general and emotional health within the OxProp-treated group (p<0.05).
CONCLUSIONS
Here we have shown improvements in objective and subjective measures of scarring and an increase in overall patient-reported physical function. The combined administration of oxandrolone and propranolol for up to a year after burn injury should be considered for the reduction of postburn scarring and improvement of long-term psychosocial outcomes in children with massive burns.
MINI-ABSTRACT
Severe burn injury induces a hypermetabolic, hypercatabolic state, leading to inflammation, body wasting, scarring, and related morbidity that decrease quality of life. Here, we show that modulation of postburn metabolic dysfunction with the yearlong combined administration of propranolol and oxandrolone reduces scarring and improves related long-term outcomes.
INTRODUCTION
Severe burn injuries covering ≥ 30% of the total body surface area (TBSA) significantly disrupt metabolism by inducing a hypermetabolic and catabolic state that leads to body mass wasting.1 Mechanistically, this process is driven by hypercatecholaminemia,2 an increase in cortisol and pro- and anti- inflammatory mediators, and futile cycling of substrates.3 Significant loss of lean body mass results, leading to debility and loss of physical function.4 Despite significant progress in delineating the causes and consequences of the hypermetabolic response, this state remains incompletely understood.5 However, studies suggest that modulation of postburn hypermetabolism both acutely and over the longer term improves outcomes.6 In addition to numerous non-pharmacologic strategies that have been developed to support the burn-induced hypermetabolic response, the current leading experimental pharmacological interventions for blunting postburn hypermetabolism and catabolism include the administration of propranolol or oxandrolone.
Propranolol is a non-selective β1 and β2 adrenoreceptor antagonist that decreases heart rate and reduces hypertension in the general population. Based on the hypothesis that elevated catecholamines are responsible for the catabolic phenotype associated with burn injury,7 research began into the use of propranolol to attenuate postburn hypermetabolism.8 Since then, the benefits of postburn propranolol has included9 decreasing myocardial oxygen demand10, cardiac work11–13, and peripheral lipolysis12 without increasing the risk of sepsis14 or exacerbating altered immunity.15 Similarly, long-term propranolol administration decreased resting energy expenditure, heart rate, and central fat accretion while preventing bone loss and improving lean mass.16 When combined with participation in an exercise program, propranolol increased lean mass and aerobic capacity.17
Oxandrolone is a synthetic anabolic steroid hormone that is a less virilizing testosterone analog 18 and is used to modulate postburn loss of lean mass.19 While oxandrolone had been used for its anabolic effects in other wasting diseases20,21, use of this anabolic agent to curb the catabolism associated with burns began in the late 1990s.22 Early results suggested that twice daily oral dosing of 10 mg of oxandrolone, in combination with a high protein diet, significantly accelerated weight gain in the recovery phase following severe burns.22 Subsequent research showed that both younger and older groups of adult burn patients experienced a significant effect of oxandrolone treatment.23 Next, the salutary effects of oxandrolone on body composition following burn injury were found to persist for at least 6 months after drug discontinuation, suggesting an extended benefit of the therapy.24 In the acute phase following burn, oxandrolone decreases length of stay for adults 25 and may enhance erythropoiesis in children.26 Although the duration of oxandrolone administration in adults was short, in children, the long-term administration of oxandrolone for up to one year postburn improved lean mass, lung function, and both bone mineral density and bone mineral content. 27–29,30 The benefits of long-term oxandrolone administration persisted well beyond the duration of therapy in severely burned children as well.31,32
Patients with large burns are at risk for developing pathologic scarring that is pruritic and inflamed and that limits movement and function; postburn hypertrophic scars affects 70-90% of these patients.33 These scars continue to be a source of pain and discomfort and negatively impact quality of life for up to 2 years postburn.34 Therapies such as laser or surgical revision can decrease the severity of burn scar.33 However, there is a dearth of pharmacologic approaches that successfully reduce the effects of burn scars on function and quality of life after massive burns. One of the greatest risk factors for hypertrophic scarring following burn injury is delayed wound healing.33 We have hypothesized that therapies that reduce wound healing time following a large burn, such as propranolol35 or oxandrolone,36 may also decrease hypertrophic scarring.
With declining mortality from burns, attention has naturally shifted to improving long-term quality of life.37 To improve quality of life, we now focus on reducing the impact of scar and increasing physical activity.38,39 The chronic stress, inflammation, and catabolism in the first year following burn injury are likely to underlie negative outcomes.40 Therefore, modulation of these chronic stressors would be reasonably expected to improve long-term outcomes in burn survivors. Previously we demonstrated that the co-administration of oxandrolone and propranolol (OxProp) for one year attenuated the burn-induced growth arrest in children with large burns.41 Based on the promising results of singly and co-administered oxandrolone and propranolol, we hypothesized that the yearlong OxProp administration to severely burned children would attenuate the development of hypertrophic scar, objectively measured in vivo scar characteristics, and overall patient-reported physical function.
METHODS
One thousand one hundred eighty-six eligible patients were admitted to Shriners Hospitals for Children®—Galveston between 2003 and 2015 (Fig. 1). Patients were between the ages of 6 months and 18 years at the time of burn, with burns over ≥30% of TBSA, and required surgery for skin grafting after scald, flame, or electrical burn. This study, which is part of an ongoing evaluation of the long-term administration of anti-adrenergic, anti-catabolic, or anabolic agents, 8,11–14,17,19,28–32,35,41–60 was approved by the University of Texas Medical Branch Institutional Review Board (ClinicalTrials.gov number, NCT00675714). Patients, or their legal caregiver if younger than 18 years of age, consented to participate in this trial; children between the ages of 7 and 18 assented to participate in the protocol as well. Patients were randomized to control (n=226) or OxProp for one year following injury (n=126); patients were assessed for up to 2 years postburn.
Fig 1.
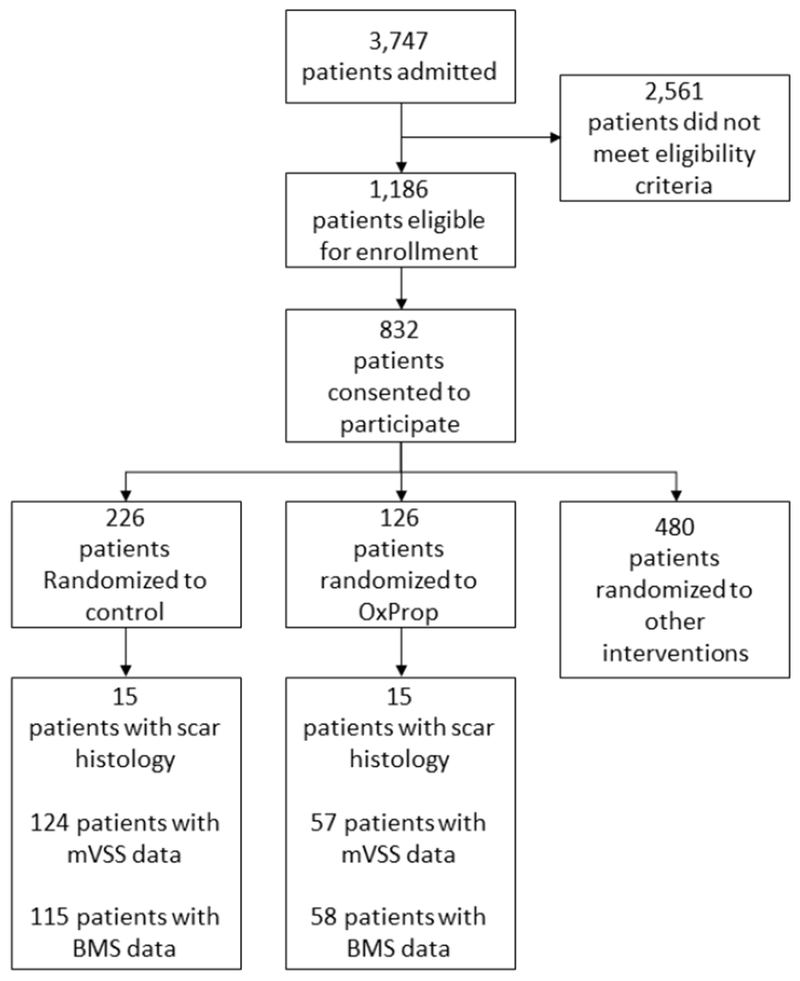
CONSORT diagram
In the case of anoxic brain injury and/or when the decision was made to not treat because of injury severity, patients were excluded. Demographics, including age, sex, ethnicity, and burn size, were recorded at the time of admission. All patients received standard of care for inhalation injury, wound treatment, and nutrition as described previously.41
Patients allocated to Control were enrolled continuously from 2003 to 2017. Because of the balanced design of the randomization schedule for all patients participating in studies at Shriners Hospitals for Children®—Galveston (Fig 1), more patients were allocated to the Control group. Beginning in 2003, patients were randomized to OxProp; breaks in OxProp randomization were taken from 2005 to 2007 and 2008 to 2009 in order to randomize patients to the Ox or Prop groups. OxProp administration began within 4 ± 0.5 days following admission. Treatment group assignment was not revealed to the patients. Oxandrolone (BTG Pharmaceuticals, West Conshohocken, PA) was administered at a dose of 0.1 mg/kg every 12 hours for a minimum of 1 year; propranolol (Roxane Laboratories, Columbus, OH) was administered at a dose of 4.0 ± 0.2 mg/kg/day for a minimum of 1 year. The propranolol dose was titrated to decrease heart rate by 15%. Propranolol dosage was determined by a physician blinded to the patient’s randomization.
Bradycardia was treated by holding a single dose of propranolol and recommencing administration 16 hours later with one half of the original dose. Over the next 24 hours, the propranolol dose was re-escalated to the original dose.
Tissue processing.
Scar samples were obtained during surgical revisions from a total of 30 pediatric patients (15 control, 15 OxProp) between 6 and 12 months following injury. Tissues were fixed in 10% neutral-buffered formalin, processed through absolute alcohol, and embedded in paraffin prior to sectioning at 4 μm. Sections were then stained in hematoxylin & eosin (H&E) for histological scar assessments or processed for subsequent immunohistochemical (IHC) stains. Immunohistochemistry included staining for the beta-adrenergic and androgen receptors, through which propranolol and oxandrolone act, respectively. As previous studies have demonstrated that oxandrolone also inhibits the signaling downstream from the glucocorticoid receptor, staining was also performed for this receptor.61
Histological scar assessment and scoring.
Histology of scar H&E sections were evaluated using a scoring scale developed by a board-certified pediatric pathologist. Scarring parameters of circumscription, loss of rete ridges, epidermal thickening, inflammation, abnormal collagen distribution, and vascularity were assigned ordinal scores from 0 (none) to 4 (most severe). Tissue nodularity was assessed by percent of scar area for each section. Three trained observers in scar histopathology then scored each section under microscopic evaluation (Olympus BX41) of the tissue. H&E sections were randomized and blinded to histopathology scorers prior to analysis. Subsequently, each section was digitized at 400× absolute magnification and gridded with a reticle to yield a 0.1 mm2 area (version 1.12, Olympus DP22, cellSens software, Olympus Corp of the Americas, Waltham Massachusetts, USA). Tissue cellularity was quantified by counting positively stained nuclei in the papillary and mid dermis in 15 separate fields. For the scar assessment analysis, 6- and 12-month groups were combined.
Immunohistochemical analyses.
Tissues were processed as described above. Following sectioning, tissues were then deparaffinized and rehydrated. Antigen retrieval was performed in modified citrate buffer at pH 6.1 (DAKO target retrieval solution, Agilent, Santa Clara California, USA) and heated to 95°C for 15 minutes. To quench endogenous peroxidase activity, sections were exposed to 2% hydrogen peroxide diluted in methanol for 30 minutes and then washed in tris-buffered saline with 0.1% tween (TBS-T). Sections were blocked in 3% horse serum in TBS-T for one hour then incubated in primary antibody diluted in background-reducing antibody diluent (DAKO) at 4°C overnight. Negative controls received no primary antibody. Tissues were rinsed 3 times in TBS-T and then incubated in biotinylated secondary antibody for one hour at room temperature. After being washed 3 times in TBS-T, sections were further incubated in an avidin-biotinylated horseradish peroxidase (HRP) complex for 30 minutes and then exposed to hydrogen peroxide and diaminobenzidine (DAB) substrate (SK4105, Vector Labs, Burlingame California, USA) for 3 minutes.
The following primary antibodies were used for IHC: anti-CD31 (Abcam, ab199012, 1:250), anti-Ki67 (Abcam, ab15580, 1:1000), anti-glucocorticoid receptor (GCR) (Abcam, ab2768, 1:150), anti-alpha smooth muscle actin (α-SMA) (SigmaAldrich, A5691, 1:250), anti-beta2-adrenergic receptor (β2-AR) (Abcam, ab182136, 1:100), anti-androgen receptor (Abcam, ab108341, 1:250), anti-collagen 1 (Abcam, ab138492, 1:250), anti-collagen 3 (Abcam, ab6310, 1:250). Secondary antibodies corresponded to the primary antibody host: biotinylated anti-rabbit IgG (PK6101, 1:150, Vector Labs) and biotinylated anti-mouse IgG (PK6102, 1:150, Vector Labs). IHC analyses for each tissue section included quantification by blinded observers of microvascular structures • mm−2 by endothelial cell IHC for CD31, and α-SMA+ or Ki67+ fibroblasts • mm−2, and scaled scores for tissue DAB intensity for β2-AR, Col-1, Col-3, GCR, and androgen receptor staining from 0 (none to lightly intense DAB) to 4 (highly intense DAB).
Tissue scores for H&E scar assessments and immunohistochemical sections were averaged for all blinded observers. After unblinding, scores for control and OxProp groups were compared by a two-tailed student’s t test using GraphPad Prism (version 7.03, GraphPad Software, La Jolla California, USA). Unless otherwise indicated, data is presented as mean ± SEM; significant differences between groups were accepted at p ≤ 0.05.
Modified Vancouver Scar Scale.
A trained clinician completed the modified Vancouver Scar Scale (mVSS)62. The mVSS is a validated tool that examines six parameters: pigmentation, vascularity, pliability, scar height, itch, and pain.63 A total of 3,267 individual records were gathered; matching the data for time postburn resulted in 124 control and 57 OxProp-treated patients from 6-months to 9-years postburn. mVSS records with null scores, non-matched wounds, or unverified time points were excluded, resulting in 2,792 records for analyses. Normally distributed data differences in total mVSS score and individual mVSS parameters between treatment groups were assessed and compared for each time point by one-way analysis of variance (ANOVA). Data is represented as mean ± SEM.
Patient and Caregiver Reported Outcome Measures.
The Burn Model Systems (BMS) National Longitudinal Database is a National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR) funded, multi-center, longitudinal research project that includes patient reported outcome measures. These surveys assess emotional, social, and physical outcomes following burn injury, including measures of satisfaction with life, appearance, and scarring.64–77 The patients completed self-reported outcome measures, including Community Integration Questionnaire, the Satisfaction with Appearance Scale, the Burn Specific Health Scale, and in children 14+ years of age, the Short Form 12 (SF-12), at discharge and 6, 12, and 24 months postburn. Statistical significance was assessed with the Wilcoxon Rank Sum test using R version 2.3.2.78
RESULTS
OxProp decreases cellularity, vascular structures, inflammation, and deposition of abnormal collagen in burn scars.
No significant differences were detected between the groups for age, burn size, sex, or length of stay. Histologically, scars from patients in the control group were more cellular (12.65 ±0.45 cells • 0.1mm−2) than those in OxProp-treated patients (9.27 ± 0.33 cells • 0.1mm−2) (p<0.001), suggesting a more physiologically active scar (Fig. 2a). Significantly reduced Ki67 expression in OxProp-treated scars (20.33 ± 3.13 Ki67+ fibroblasts • mm−2) compared to control scars (38.89 ± 8.43 Ki67+ fibroblasts • mm−2, p=0.039, Fig. 2b) confirmed that cellular proliferation was greater in the untreated scars. The number of αSMA+ fibroblasts did not vary between treatment groups (data not shown).
Fig 2.
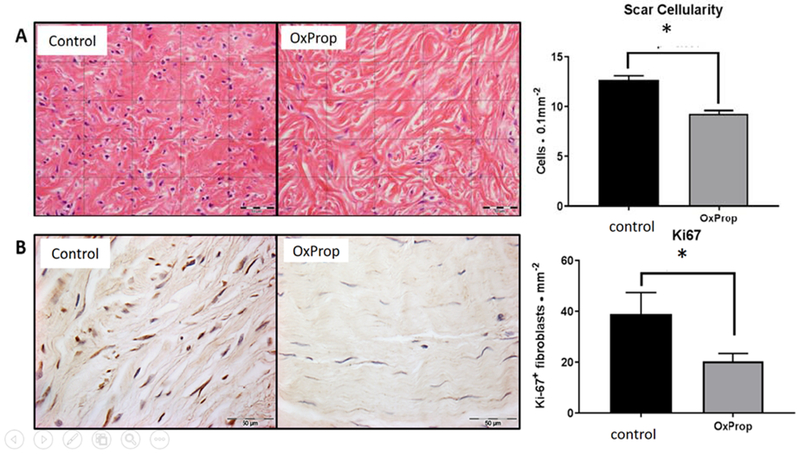
OxProp treatment reduces scar cellularity and dermal fibroblast proliferation. (A) Representative images of H&E stained pediatric scar tissue after 12 months of control or OxProp treatment with associated quantification. When compared to control, scars from OxProp treated patient scars show significantly decreased dermal cellularity (*p<0.001). (B) Ki67 immuno-stained sections of pediatric scar tissue 12 months postburn and accompanying quantification per mm2. OxProp treatment significantly lessens the proliferative capacity of dermal fibroblasts (*p = 0.0392) compared to control patients. Scale bars = 50 μm.
Expression of CD31, a marker of vascular structures, was reduced (p<0.0001) with OxProp treatment, indicating significantly fewer microvascular structures in OxProp scars (10.56 ± 0.79 • mm−2) than in control scars (16.58 ± 1.301 • mm−2) (Fig. 3b). This finding may correlate with a lower vascularity score in H&E sections (1.45 ± 0.13, control; 1.14 ± 0.12, OxProp, p = 0.087, Fig. 3a); however, examination of more samples is necessary to determine whether a difference exists.
Fig. 3.
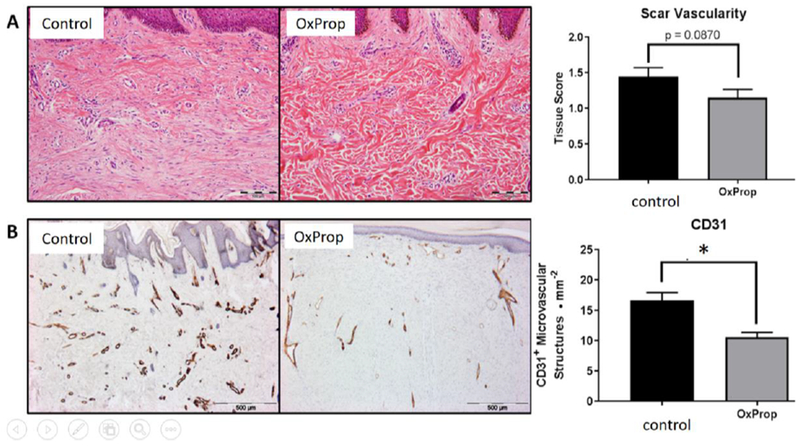
OxProp mitigates angiogenic potential in post burn scars. (A) Representative H&E images of 12-month postburn treated scars with associated quantification. Scale bar = 100 μm. Tissue score show decreased vascularization in OxProp treated patients’ tissues compared to control (*p = 0.087). (B) DAB immunostained scar sections after 12 months of injury and treatment with accompanying quantification Scale bar = 500 μm. CD31 expression is significantly decreased (*p<0.0001) in scars of patients treated with OxProp compared to control after 12 months indicating significant reduction in neo-vascularization during the postburn wound healing process.
Dermal inflammation was reduced with OxProp treatment (scar score: 1.02 ± 0.09; p = 0.0019) compared to controls (scar score: 1.60 ± 0.16) in the papillary and mid-dermal regions (Fig. 4a). Additionally, percent nodularity decreased (p < 0.0001) with OxProp treatment (control: 36.22% ± 4.41; OxProp: 12.22% ± 3.33) (Fig. 4b). Here we show that OxProp substantially diminishes (p < 0.0001) tissue histopathology scores for abnormal collagen deposition (1.28 ± 0.14) when compared to controls (2.25 ± 0.19, Fig. 4c). Expression of collagen I and collagen III, however, was similar between treatment groups (data not shown).
Fig. 4.
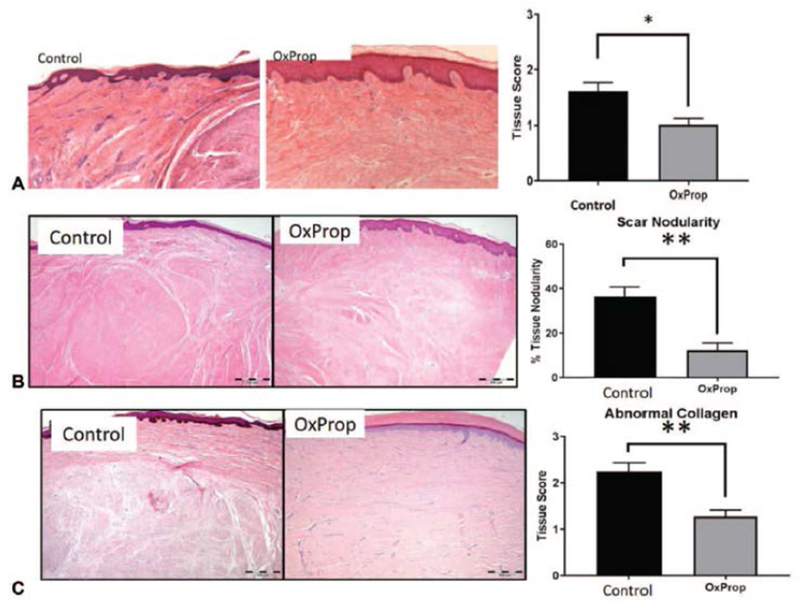
OxProp significantly reduces dermal inflammation, nodularity and abnormal collagen deposition in postburn scars. (A) Dermal inflammation, (B) nodularity, and (C) abnormal collagen deposition in postburn scars are significantly reduced following OxProp year-long administration compared to control (*p<0.05; **p<0.0001).
OxProp treatment significantly increased glucocorticoid receptor expression in scar (Fig. 6). Scars from control patients expressed less glucocorticoid receptors in dermal fibroblasts (tissue score: 1.43 ± 0.20) than those from OxProp-treated patients (tissue score: 2.20 ± 0.22) (p = 0.043). Differences were not found in expression of the β2-AR or the androgen receptor (data not shown).
Fig. 6.
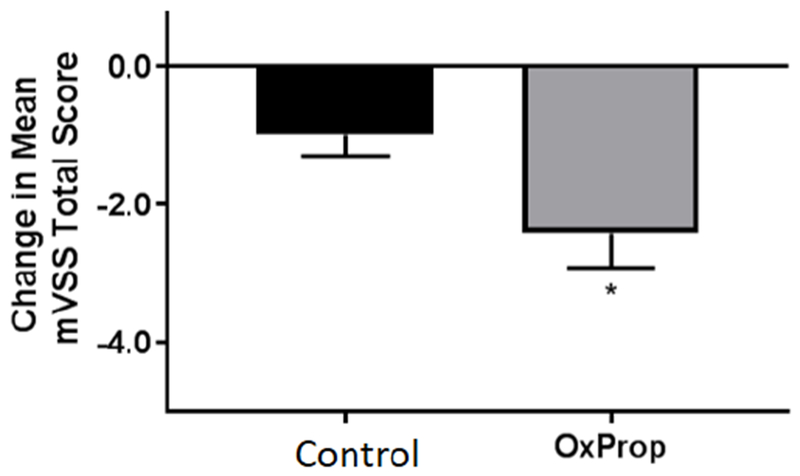
Severity of postburn hypertrophic scarring is reduced with OxProp following one year treatment. Total mVSS is significantly reduced in OxProp-treated patients between 6 and 12 months following burn injury. *p<0.05.
OxProp reduces severity of hypertrophic scarring in severely burned patients
Mean mVSS total scores were reduced in OxProp-treated patients over time, most significantly at between 6 and 12 months postburn (p<0.05) compared to the control patients. In OxProp-treated patients, mVSS scores were reduced from 8.8±0.2 to 6.4±1.0 compared to control patients, 8.5±0.3 to 7.5±0.5. OxProp showed the most significant reduction in total mean mVSS from 6 to 12 months (−2.4 ± 0.5, p=0.02) compared to control (−0.99 ± 0.3) (Fig. 5). One individual mVSS parameter, pliability, was significantly greater with OxProp (p<0.0001, Fig. 7). No other individual mVSS scar parameters changed significantly.
Fig. 5.
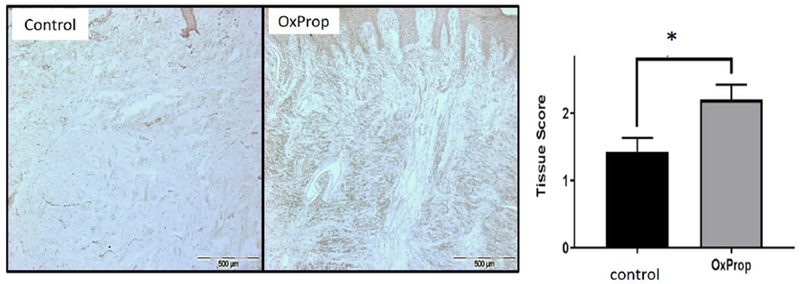
OxProp increases glucocorticoid receptor expression compared to control. (*p<0.0426).
Fig. 7.
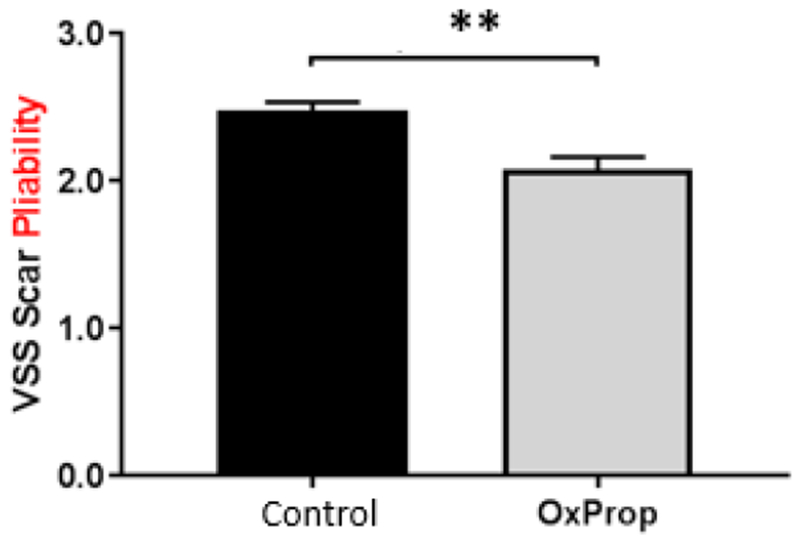
OxProp makes scar more pliable. Data shown is combined analyses means for all patients over all time points ± SEM. **p<0.0001
Patient-reported physical function is improved with OxProp
We evaluated answers to questions on the questionnaires that addressed activity, overall health, and extent of physical or emotional limitations (TABLE 2). The control group contained 115 patients; the OxProp group contained 58 patients. There were no significant differences with respect to age, percent TBSA burned, percent third degree TBSA burned, or length of stay. When asked “How often do you perform leisure activities (movies, sports, restaurants)?” patients treated with OxProp reported significantly greater participation in activities at 2 years (p=0.007). Patients completing the SF-12 reported better general health at 2 years (p=0.049) compared to controls. When asked how often participants accomplished less than they would like because of emotional problems, the OxProp group reported that the frequency of emotional limitations was reduced significantly (p=0.018) two years post burn. Finally, OxProp patients reported significantly better overall mental health as measured by the SF-12 Mental Health composite score at two years when compared to control (p=0.0012).
Table 2.
Quality of Life Assessments
| Assessment | P | Interpretation | Time Point |
|---|---|---|---|
| How often do you perform leisure activities (movies, sports, restaurants)? | 0.007 | More activities performed with OxProp | 2 years |
| How often do you accomplish less than you would like because of emotional problems? | 0.018 | Accomplishing more with OxProp | 2 years |
| In general, would you say your health is excellent, very good, good, fair, or poor? | 0.049 | Perceived overall health is better with OxProp | 2 years |
| Mental health composite score (based on results of SF12) | 0.0012 | Perceived mental health is better with OxProp | 2 years |
DISCUSSION
In this prospective, randomized, double-blind, controlled study of the yearlong co-administration of OxProp following severe burn injury in children, we have shown improvements in both objective and subjective measures of scarring and an increase in overall patient-reported physical function. These results demonstrate benefits of the long-term, combined administration of propranolol and oxandrolone. The present findings add to the already established benefits of the individual administration of oxandrolone and propranolol, in addition to the improved growth with the combined therapies8,10,12–15,17,19,26,28–32,35,41,43–47,49,50,52,79–81.
From our histological analysis, we hypothesize that OxProp reduces postburn scarring via several potential mechanisms. First, dermal fibroproliferative capacity is reduced with OxProp treatment, as shown by the decrease in overall cellularity of the scar tissue treated with OxProp. The persistent decrease in Ki-67–positive cells at 12 months indicates that the proliferative capacity of deep dermal fibroblasts is reduced longitudinally with OxProp treatment. We previously demonstrated that propranolol changes β-AR expression and trafficking in scar dermal fibroblasts, providing precedence supporting direct action of propranolol on this cell population.82 Taken together, these findings may indicate that the scar matures faster with OxProp such that the scar is less physiologically active at the time of assessment. Next, we identified an overall reduction in blood vessels as detected via CD31 expression, suggesting that OxProp decreases angiogenic potential in postburn scars. This data also supports the contention that OxProp results in a less physiologically active scar, as fewer blood vessels correlate with reduced metabolic activity. Currently, there is a dearth of investigations into oxandrolone’s effect on angiogenic potential; however, several studies show that propranolol suppresses neovascularization.83–85 Therefore, the anti-angiogenic effect shown in this study may be primarily induced by propranolol, although more investigations into the mechanism of action of oxandrolone are needed. The histological findings from our study have suggested mechanisms that may be responsible for the reduction in hypertrophic scarring with OxProp. In light of the changes in angiogenesis as detected by histology, we are now using laser speckle to assess blood flow through the scar to reduce the subjective nature of our assessments of blood flow / vascularity in burn scar.
We have also found that OxProp reduces dermal inflammation, nodularity, and abnormal collagen deposition. Twelve months following burn injury, OxProp diminished the severity of inflammatory cell infiltration within scars. Previous studies have concluded that both oxandrolone and propranolol alone can reduce collagen production through androgen receptor signaling perturbation81 and β-AR antagonism, reducing VEGF expression. 86,87 This further supports the hypothesis that OxProp treatment may improve wound healing and reduce scarring following burns. Collagen I or III presence was not different as assessed by immunohistochemistry. Future analyses will be focused on the expression of other extracellular matrix proteins or activity of proteins that modify or breakdown collagen. Additionally, we found that OxProp increases glucocorticoid receptor expression, which may be a compensatory response to glucocorticoid antagonism through an androgen receptor-dependent manner.61 Further study into the relationship among adrenergic blockade, androgen supplementation, and glucocorticoids is warranted.
The decrease in mean mVSS scores showed an overall reduction in scarring with OxProp administration. The change in pliability with OxProp indicated reduced stiffness of the tissue so that the scar was more flexible and moveable. This finding correlates well with our histological findings that abnormal collagen deposition was reduced.
From our analyses of patient-reported outcome measures, we conclude that the long-term co-administration of OxProp improves recovery from burn injury. The data show that patients treated with OxProp believed that their health was better and engaged in leisure activities more frequently than their counterparts in the control group. By two years post injury, significantly more patients treated with OxProp were participating in activities. Patients on OxProp self-reported less frequent emotional limitations at 2 years postinjury than did the controls. The patients in the treatment group were also found to have greater improvement in mental health. These findings may result from improved healing with OxProp—these patients have resumed growing41 and have more lean mass and muscle strength28; this would translate directly into an easier ability to function, allowing the patient to resume more daily activity and improving mental outlook.
A limitation of the study is the younger patient group who participated only in the quality of life questionnaires; these patients also had smaller burns. Even though these burns were smaller with a shorter length of stay, the outcomes were still better for patients treated with oxandrolone.
While we have suggested several hypotheses as to the mechanisms underlying the ability of co-administration of propranolol and oxandrolone to improve hypertrophic scarring and physical function, several alternative possibilities deserve mention. First, amelioration of the long-term adrenergic stress associated with burns improves anthropometric measures, specifically lean mass,16 as does treatment with oxandrolone.32 This increase in lean mass, when contrasted with the muscle catabolism of untreated patients, contributes to an overall increase in functional strength,28 thereby increasing physical function.88 Since patients treated with the combination of oxandrolone and propranolol reported improved general health, perhaps the increase in activity level creates a beneficial effect on scar tissue, such as the scar being stretched more because of increased activity. Alternatively, given that dermal fibroblasts proliferate in response to catecholamines, the excessive adrenergic stimulation after burn injury could lead to hyperproliferation within the developing scar.89 Therefore, modulation of the persistent adrenergic stress associated with burn could decrease the activation of cutaneous fibroblasts, leading to a decrease in scar tissue formation.
CONCLUSION
Given the increasing evidence for the salutary effects of combined beta-blockade of burn-related hypermetabolism and the use of anabolic agents to blunt catabolism and increase anabolism, we conclude that the long-term administration of oxandrolone with propranolol should be considered for the treatment of pediatric burn survivors. The benefits of this combined treatment strategy, including resumed growth, increased muscle mass, greater strength, and now reduction in scar and greater activity, suggest that OxProp may be the most efficacious modulator of the hypermetabolic response that we have tested in burned children. Although long-term treatment of burned adults with oxandrolone has not been trialed, our reports of beneficial effects of OxProp suggest that this combination should be trialed in adults as well.
Table 1.
Demographics
| Characteristic | Control (n=226) | OxProp (n=126) |
|---|---|---|
| Age (years) | 7.1 ± 0.4 | 7.4 ± 0.5 |
| Sex (% Male) | 63 | 72 |
| Hispanic (%) | 94 | 90 |
| Type of Burn (Scald : Flame : Electrical) | 73 : 134 : 19 | 41 : 70 : 15 |
| TBSA (%) | 52 ± 1 | 50 ± 1 |
| 3rd Degree TBSA (%) | 37 ± 2 | 35 ± 2 |
| Delay to Admit (Days, IQR) | 3 (2.25) | 3 (7.75) |
| Hospital Length of Stay (Days, IQR) | 25 (20.2) | 25 (14.75) |
Data presented as mean ± standard deviation. IQR, Interquartile Range.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Hal Hawkins and Sam Jacobs of the SHC-G Histology Core for their support in preparation of the histological samples, and Dr. Ted T. Huang for sharing his insight into burn scar behavior. We thank the clinical and research staff for their assistance.
GRANT SUPPORT:
This work was supported by the National Institutes of Health (P50 GM060338, R01 GM056687, R01 HD049471, R01 GM112936, and T32 GM008256), the National Institute on Disability, Independent Living, and Rehabilitation Research (H133A120091, 90DP00430100), the Department of Defense (W81XWH-15-1-0143), and Shriners Hospitals for Children (84080, 79141, 79135, 71009, 80100, 71008, 84060, 87300, 71001, and 71000). The project was also supported by the University of Texas Medical Branch’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences.
REFERENCES
- 1.Porter C, Tompkins RG, Finnerty CC, et al. The metabolic stress response to burn trauma: current understanding and therapies. Lancet. 2016;388:1417–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of Severe Burn Injury on Substrate Cycling by Glucose and Fatty Acids. New England Journal of Medicine. 1987;317:403–408. [DOI] [PubMed] [Google Scholar]
- 4.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. [DOI] [PubMed] [Google Scholar]
- 5.Sidossis L, Porter C, Saraf M, et al. Browning of Subcutaneous White Adipose Tissue in Humans after Severe Adrenergic Stress. Cell Metab. 2015;22:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. The Lancet. 2004;363:1895–1902. [DOI] [PubMed] [Google Scholar]
- 7.Wilmore DW, Long JM, Mason ADJ, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Annals of surgery. 1988;208:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnerty CC, Herndon DN. Is propranolol of benefit in pediatric burn patients? Adv Surg. 2013;47:177–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minifee PK, Barrow RE, Abston S, et al. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. Journal of Pediatric Surgery. 1989;24:806–811. [DOI] [PubMed] [Google Scholar]
- 11.Williams FN, Herndon DN, Kulp GA, et al. PROPRANOLOL DECREASES CARDIAC WORK IN A DOSE-DEPENDENT MANNER IN SEVERELY BURNED CHILDREN. Surgery. 2011;149:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron PW, Barrow RE, Pierre EJ, et al. Prolonged Use of Propranolol Safely Decreases Cardiac Work in Burned Children. The Journal of Burn Care & Rehabilitation. 1997;18:223–227. [DOI] [PubMed] [Google Scholar]
- 13.Wurzer P, Branski LK, Clayton RP, et al. Propranolol Reduces Cardiac Index But does not Adversely Affect Peripheral Perfusion in Severely Burned Children. Shock. 2016;46:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeschke MG, Norbury WB, Finnerty CC, et al. Propranolol does not increase inflammation, sepsis, or infectious episodes in severely burned children. Journal of Trauma and Acute Care Surgery. 2007;62:676–681. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Jeschke MG, Asai A, et al. Propranolol as a modulator of M2b monocytes in severely burned patients. J Leukoc Biol. 2011;89:797–803. [DOI] [PubMed] [Google Scholar]
- 16.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-Term Propranolol Use in Severely Burned Pediatric Patients: A Randomized Controlled Study. Annals of surgery. 2012;256:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porro LJ, Al-Mousawi AM, Williams F, et al. Effects of propranolol and exercise training in children with severe burns. J Pediatr. 2013;162:799–803 e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kicman AT. Pharmacology of anabolic steroids. British Journal of Pharmacology. 2008;154:502–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy KD, Thomas S, Mlcak RP, et al. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136:219–224. [DOI] [PubMed] [Google Scholar]
- 20.Jekot WF, Purdy DW. Treating HIV/AIDS patients with anabolic steroids: a retrospective study. AIDS Patient Care. 1993;7:68–74. [Google Scholar]
- 21.Mendenhall CL, Anderson S, Garcia-Pont P, et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine. 1984;311:1464–1470. [DOI] [PubMed] [Google Scholar]
- 22.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. Journal of trauma and acute care surgery. 1997;43:47–51. [DOI] [PubMed] [Google Scholar]
- 23.Demling RH, DeSanti L. The rate of restoration of body weight after burn injury, using the anabolic agent oxandrolone, is not age dependent. Burns. 2001;27:46–51. [DOI] [PubMed] [Google Scholar]
- 24.Demling RH, DeSanti L. Oxandrolone induced lean mass gain during recovery from severe burns is maintained after discontinuation of the anabolic steroid. Burns. 2003;29:793–797. [DOI] [PubMed] [Google Scholar]
- 25.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. Journal of burn care & research. 2006;27:131–139. [DOI] [PubMed] [Google Scholar]
- 26.Capek KV CD; Andersen CR; Watson KJ; Bohanon FJ; Finnerty CC; Sousse LE; Porter CR; Guillory AN; Meyer WJ; Suman OE; Herndon DN. OXANDROLONE ENHANCES ERYTHROPOIESIS IN PEDIATRIC BURNS. SHOCK. 2017;47:66–67. [Google Scholar]
- 27.Murphy KD, Thomas S, Mlcak RP, et al. Effects of long-term oxandrolone administration in severely burned children. Surgery. 2004;136:219–224. [DOI] [PubMed] [Google Scholar]
- 28.Chao T, Porter C, Herndon DN, et al. Propranolol and Oxandrolone Therapy Accelerated Muscle Recovery in Burned Children. Med Sci Sports Exerc. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuvdendorj D, Chinkes DL, Zhang XJ, et al. Long-term oxandrolone treatment increases muscle protein net deposition via improving amino acid utilization in pediatric patients 6 months after burn injury. Surgery. 2011;149:645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousse LE, Herndon DN, Mlcak RP, et al. Long-Term Administration of Oxandrolone Improves Lung Function in Pediatric Burned Patients. J Burn Care Res. 2016;37:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reeves PT, Herndon DN, Tanksley JD, et al. Five-Year Outcomes after Long-Term Oxandrolone Administration in Severely Burned Children: A Randomized Clinical Trial. Shock. 2016;45:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porro LJ, Herndon DN, Rodriguez NA, et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214:489–502; discussion 502-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurzer P, Forbes AA, Hundeshagen G, et al. Two-year follow-up of outcomes related to scarring and distress in children with severe burns. Disabil Rehabil. 2017;39:1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali A, Herndon DN, Mamachen A, et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit Care. 2015;19:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000;15:12–17. [DOI] [PubMed] [Google Scholar]
- 37.Pereira C, Murphy K, Herndon D. Outcome measures in burn care: is mortality dead? Burns. 2004;30:761–771. [DOI] [PubMed] [Google Scholar]
- 38.Pallua N, Künsebeck HW, Noah EM. Psychosocial adjustments 5 years after burn injury. Burns.29:143–152. [DOI] [PubMed] [Google Scholar]
- 39.Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. The Lancet. 2016;388:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PloS one. 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herndon DN, Voigt CD, Capek KD, et al. Reversal of Growth Arrest With the Combined Administration of Oxandrolone and Propranolol in Severely Burned Children. Ann Surg. 2016;264:421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guillory AN, Herndon DN, Silva MB 3rd, et al. Oxandrolone Coadministration Does Not Alter Plasma Propranolol Concentrations in Severely Burned Pediatric Patients. J Burn Care Res. 2017;38:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001;233:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeschke MG, Finnerty CC, Suman OE, et al. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg. 2007;246:351–360; discussion 360-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aarsland A, Chinkes D, Wolfe RR, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Rate of hepatic very low density lipoprotein triglyceride secretion remains unchanged. Ann Surg. 1996;223:777–787; discussion 787-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrow RE, Wolfe RR, Dasu MR, et al. The use of beta-adrenergic blockade in preventing trauma-induced hepatomegaly. Ann Surg. 2006;243:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillory AN, Herndon DN, Silva MB 3rd, et al. Propranolol kinetics in plasma from severely burned adults. Burns. 2017;43:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart DW, Wolf SE, Chinkes DL, et al. Beta-blockade and growth hormone after burn. Ann Surg. 2002;236:450–456; discussion 456-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herndon D, Rodriguez N, Diaz E, et al. Long-term propranolol use in severely burned pediatric patients: a randomized controlled study. Ann Surg. 2012;256:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. [DOI] [PubMed] [Google Scholar]
- 51.Jeschke MG, Finnerty CC, Kulp GA, et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med. 2008;9:209–216. [DOI] [PubMed] [Google Scholar]
- 52.Olah G, Finnerty CC, Sbrana E, et al. Increased poly(ADP-ribosyl)ation in skeletal muscle tissue of pediatric patients with severe burn injury: prevention by propranolol treatment. Shock. 2011;36:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Branski LK, Herndon DN, Barrow RE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg. 2009;250:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cree MG, Zwetsloot JJ, Herndon DN, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007. 245:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finnerty CC, Ali A, McLean J, et al. Impact of stress-induced diabetes on outcomes in severely burned children. J Am Coll Surg. 2014;218:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gauglitz GG, Herndon DN, Kulp GA, et al. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herndon DN, Ramzy PI, DebRoy MA, et al. Muscle protein catabolism after severe burn: effects of IGF-1/IGFBP-3 treatment. Ann Surg. 1999;229:713–720; discussion 720-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeschke MG, Finnerty CC, Herndon DN, et al. Severe injury is associated with insulin resistance, endoplasmic reticulum stress response, and unfolded protein response. Ann Surg. 2012;255:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeschke MG, Kulp GA, Kraft R, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuvdendorj D, Zhang XJ, Chinkes DL, et al. Intensive insulin treatment increases donor site wound protein synthesis in burn patients. Surgery. 2011;149:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Bauman WA, Huang R, et al. Oxandrolone blocks glucocorticoid signaling in an androgen receptor-dependent manner. Steroids. 2004;69:357–366. [DOI] [PubMed] [Google Scholar]
- 62.Nedelec B, Shankowsky HA, Tredget EE. Rating the resolving hypertrophic scar: comparison of the Vancouver Scar Scale and scar volume. J Burn Care Rehabil. 2000;21:205–212. [DOI] [PubMed] [Google Scholar]
- 63.Ta Sullivan, Smith J, Kermode J, et al. Rating the burn scar. The Journal of burn care & rehabilitation. 1990;11:256–260. [DOI] [PubMed] [Google Scholar]
- 64.Amtmann D, Gibran NS, Herndon DN, et al. Letter to the Editor #2: Description of the Burn Model System National Database sample. Burns. 2016;42:704–705. [DOI] [PubMed] [Google Scholar]
- 65.Amtmann D, McMullen K, Bamer A, et al. National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model System: Review of Program and Database. Arch Phys Med Rehabil. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amtmann D, Project Directors of the National Institute on Disability IL, Rehabilitation Research Burn Model System Centers P, et al. BMS Letter to the Editor #1: Introduction to the Burn Model System Centers Program. Burns. 2016;42:944–946. [DOI] [PubMed] [Google Scholar]
- 67.Gerrard P, Kazis LE, Ryan CM, et al. Validation of the Community Integration Questionnaire in the adult burn injury population. Qual Life Res. 2015;24:2651–2655. [DOI] [PubMed] [Google Scholar]
- 68.Goverman J, Mathews K, Goldstein R, et al. Adult Contractures in Burn Injury: A Burn Model System National Database Study. J Burn Care Res. 2017;38:e328–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goverman J, Mathews K, Goldstein R, et al. Pediatric Contractures in Burn Injury: A Burn Model System National Database Study. J Burn Care Res. 2017;38:e192–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goverman J, Mathews K, Holavanahalli RK, et al. The National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model System: Twenty Years of Contributions to Clinical Service and Research. J Burn Care Res. 2017;38:e240–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goverman J, Mathews K, Nadler D, et al. Satisfaction with life after burn: A Burn Model System National Database Study. Burns. 2016;42:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kazis LE, Lee AF, Rose M, et al. Recovery Curves for Pediatric Burn Survivors: Advances in Patient-Oriented Outcomes. JAMA Pediatr. 2016;170:534–542. [DOI] [PubMed] [Google Scholar]
- 73.Kazis LE, Sheridan RL, Shapiro GD, et al. Development of clinical process measures for pediatric burn care: Understanding variation in practice patterns. J Trauma Acute Care Surg. 2018;84:620–627. [DOI] [PubMed] [Google Scholar]
- 74.Levi B, Jayakumar P, Giladi A, et al. Risk factors for the development of heterotopic ossification in seriously burned adults: A National Institute on Disability, Independent Living and Rehabilitation Research burn model system database analysis. J Trauma Acute Care Surg. 2015;79:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider JC, Nadler DL, Herndon DN, et al. Pruritus in pediatric burn survivors: defining the clinical course. J Burn Care Res. 2015;36:151–158. [DOI] [PubMed] [Google Scholar]
- 76.Schneider JC, Simko LC, Goldstein R, et al. Predicting Heterotopic Ossification Early After Burn Injuries: A Risk Scoring System. Ann Surg. 2017;266:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simko LC, Espinoza LF, McMullen K, et al. Fatigue Following Burn Injury: A Burn Model System National Database Study. J Burn Care Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.(2015). RCT, . R: A language and environment for statistical computing. 2015; https://www.R-project.org/.
- 79.Williams FN, Herndon DN, Kulp GA, et al. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pham TN, Klein MB, Gibran NS, et al. Impact of oxandrolone treatment on acute outcomes after severe burn injury. J Burn Care Res. 2008;29:902–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiang L, Wiren K, Xiao-Wei Z, et al. The Effect of Oxandrolone Treatment on Human Osteoblastic Cells. Journal of Burns and Wounds. 2007;6:53–64. [PMC free article] [PubMed] [Google Scholar]
- 82.El Ayadi A, Prasai A, Wang Y, et al. beta-Adrenergic receptor trafficking, degradation, and cell-surface expression are altered in dermal fibroblasts from hypertrophic scars. J Invest Dermatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamy S, Lachambre M, Lord-Dufour S, et al. Propranolol suppresses angiogenesis in vitro: Inhibition of proliferation, migration, and differentiation of endothelial cell. Vascular Pharmacology. 2010;53:200–208. [DOI] [PubMed] [Google Scholar]
- 84.Pasquier E, Ciccolini J, Carre M, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Mai HM, Zheng J, et al. Propranolol inhibits angiogenesis via down-regulating the expression of vascular endothelial growth factor in hemangioma derived stem cell. Int J Clin Exp Pathol. 2014;7:48–55. [PMC free article] [PubMed] [Google Scholar]
- 86.Hajighasemi F, Mirshafiey A. Propranolol effect on proliferation and vascular endothelial growth factor secretion in human immunocompetent cells. Journal of Clinical Immunology and Immunopathololgy Research. 2010;2:22–27. [Google Scholar]
- 87.Romana-Souza B, Nascimento AP, Monte-Alto-Costa A. Low-dose propranolol improves cutaneous wound healing of burn-injured rats. Plast Reconstr Surg. 2008;122:1690–1699. [DOI] [PubMed] [Google Scholar]
- 88.Lee JO, Herndon DN, Andersen C, et al. Effect of Exercise Training on the Frequency of Contracture-Release Surgeries in Burned Children. Ann Plast Surg. 2017;79:346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pullar CE, Isseroff RR. The β2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. Journal of cell science. 2006;119:592–602. [DOI] [PubMed] [Google Scholar]


