Abstract
Background
The treatment of people with pancreatic necrosis differs from that of people with oedematous pancreatitis. It is important to know the diagnostic accuracy of serum C‐reactive protein (CRP), serum procalcitonin, and serum lactate dehydrogenase (LDH) as a triage test for the detection of pancreatic necrosis in people with acute pancreatitis, so that an informed decision can be made as to whether the person with pancreatic necrosis needs further investigations such as computed tomography (CT) scan or magnetic resonance imaging (MRI) scan and treatment for pancreatic necrosis started. There is currently no standard clinical practice, although CRP, particularly an increasing trend of CRP, is often used as a triage test to determine whether the person requires further imaging. There is also currently no systematic review of the diagnostic test accuracy of CRP, procalcitonin, and LDH for the diagnosis of pancreatic necrosis in people with acute pancreatitis.
Objectives
To compare the diagnostic accuracy of CRP, procalcitonin, or LDH (index test), either alone or in combination, in the diagnosis of necrotising pancreatitis in people with acute pancreatitis and without organ failure.
Search methods
We searched MEDLINE, Embase, Science Citation Index Expanded, National Institute for Health Research (NIHR HTA and DARE), and other databases until March 2017. We searched the references of the included studies to identify additional studies. We did not restrict studies based on language or publication status, or whether data were collected prospectively or retrospectively. We also performed a 'related search' and 'citing reference' search in MEDLINE and Embase.
Selection criteria
We included all studies that evaluated the diagnostic test accuracy of CRP, procalcitonin, and LDH for the diagnosis of pancreatic necrosis in people with acute pancreatitis using the following reference standards, either alone or in combination: radiological features of pancreatic necrosis (contrast‐enhanced CT or MRI), surgeon's judgement of pancreatic necrosis during surgery, or histological confirmation of pancreatic necrosis. Had we found case‐control studies, we planned to exclude them because they are prone to bias; however, we did not locate any. Two review authors independently identified the relevant studies from the retrieved references.
Data collection and analysis
Two review authors independently extracted data, including methodological quality assessment, from the included studies. As the included studies reported CRP, procalcitonin, and LDH on different days of admission and measured at different cut‐off levels, it was not possible to perform a meta‐analysis using the bivariate model as planned. We have reported the sensitivity, specificity, post‐test probability of a positive and negative index test along with 95% confidence interval (CI) on each of the different days of admission and measured at different cut‐off levels.
Main results
A total of three studies including 242 participants met the inclusion criteria for this review. One study reported the diagnostic performance of CRP for two threshold levels (> 200 mg/L and > 279 mg/L) without stating the day on which the CRP was measured. One study reported the diagnostic performance of procalcitonin on day 1 (1 day after admission) using a threshold level of 0.5 ng/mL. One study reported the diagnostic performance of CRP on day 3 (3 days after admission) using a threshold level of 140 mg/L and LDH on day 5 (5 days after admission) using a threshold level of 290 U/L. The sensitivities and specificities varied: the point estimate of the sensitivities ranged from 0.72 to 0.88, while the point estimate of the specificities ranged from 0.75 to 1.00 for the different index tests on different days of hospital admission. However, the confidence intervals were wide: confidence intervals of sensitivities ranged from 0.51 to 0.97, while those of specificities ranged from 0.18 to 1.00 for the different tests on different days of hospital admission. Overall, none of the tests assessed in this review were sufficiently accurate to suggest that they could be useful in clinical practice.
Authors' conclusions
The paucity of data and methodological deficiencies in the studies meant that it was not possible to arrive at any conclusions regarding the diagnostic test accuracy of the index test because of the uncertainty of the results. Further well‐designed diagnostic test accuracy studies with prespecified index test thresholds of CRP, procalcitonin, LDH; appropriate follow‐up (for at least two weeks to ensure that the person does not have pancreatic necrosis, as early scans may not indicate pancreatic necrosis); and clearly defined reference standards (of surgical or radiological confirmation of pancreatic necrosis) are important to reliably determine the diagnostic accuracy of CRP, procalcitonin, and LDH.
Plain language summary
Blood tests for the diagnosis of pancreatic necrosis (pancreatic destruction due to inflammation of pancreas)
Background
The pancreas is an organ in the abdomen (tummy) that secretes several digestive enzymes (substances that break down the food that we eat) into the pancreatic ductal system, which empties into the small bowel. The pancreas also contains the islets of Langerhans, which secrete several hormones including insulin (which helps regulate blood sugar). Acute pancreatitis is a sudden inflammation of the pancreas that can lead to destruction of the pancreas (pancreatic necrosis). The treatment of people with pancreatic necrosis differs from that of people without pancreatic necrosis. Blood tests such as C‐reactive protein (CRP), procalcitonin, and lactate dehydrogenase (LDH) may be used to find out whether a person with acute pancreatitis has pancreatic necrosis. This is usually followed by CT scan to confirm that the person has pancreatic necrosis. If the person is found to have pancreatic necrosis, the intensity of care is increased and additional treatments are performed as required. At present it is unclear whether measuring the levels of CRP, procalcitonin, or LDH is useful in identifying pancreatic necrosis.
Study characteristics
We performed a thorough literature search for studies reporting the accuracy of CRP, procalcitonin, or LDH in identifying pancreatic necrosis. We included studies reported until 20 March 2017. We identified three studies reporting information on 242 people with pancreatitis. The studies included pancreatitis due to all causes.
Key results
Variations in when the studies carried out the blood tests and what level was considered abnormal meant that we were unable to combine the data to provide the overall results. It was not possible to arrive at any firm conclusions about how accurate the tests are for the following reasons.
• The studies included few participants. As a result, there was significant uncertainty in the results.
• The studies were of poor methodological quality, which introduced additional uncertainty in the results.
• For the results to be trusted, they must be reproduced in another group of participants. Since this was not done, there was uncertainty in the results.
Quality of evidence
All of the studies were of unclear or low methodological quality, which may result in arriving at false conclusions.
Summary of findings
Summary of findings'. 'Serum C‐reactive protein, procalcitonin and lactate dehydrogenase for the diagnosis of pancreatic necrosis.
| Population | People with acute pancreatitis | |||||||||
| Setting | Secondary care in various countries | |||||||||
| Target condition | Acute pancreatic (or peripancreatic) necrosis | |||||||||
| Reference standard | Radiology (contrast enhanced computed tomography scan) or surgery | |||||||||
| Median prevalence of pancreatic leak | 53.3% | |||||||||
| Index test1 | Sensitivity | Specificity | Study specific pre‐test probability | Post‐test probability of a positive test2 | Post‐test probability of a negative test2 | Number of studies | Number of participants | Risk of bias | Applicability concerns | Plain language interpretation |
| C‐reactive protein (day 3) > 140 mg/L | 0.82 (95% CI 0.66 to 0.92) | 0.84 (95% CI 0.66 to 0.95) | 55.7% | 85.3% (95% CI 72.0% to 92.9%) | 19.7% (95% CI 10.9% to 32.7%) | 1 | 70 | High | Unclear | At the pre‐test probability of 56%, out of 100 people with positive test, 85 people (95% CI 72 to 93) have pancreatic necrosis. At the same pre‐test probability, out of 100 people with negative test, 20 people (95% CI 11 to 33) have pancreatic necrosis. |
| C‐reactive protein (day not stated) > 200 mg/L | 0.88 (95% CI 0.69 to 0.97) | 0.75 (95% CI 0.67 to 0.82) | 15.9% | 80.1% (95% CI 74.3% to 84.8%) | 15.5% (95% CI 5.9% to 34.7%) | 1 | 157 | High | Unclear | At the pre‐test probability of 16%, out of 100 people with positive test, 80 people (95% CI 74 to 85) have pancreatic necrosis. At the same pre‐test probability, out of 100 people with negative test, 16 people (95% CI 6 to 35) have pancreatic necrosis. |
| C‐reactive protein (day not stated) > 279 mg/L | 0.72 (95% CI 0.51 to 0.88) | 0.89 (95% CI 0.82 to 0.93) | 15.9% | 87.9% (95% CI 80.9% to 92.5%) | 26.5% (95% CI 16.1% to 40.4%) | 1 | 157 | High | Unclear | At the pre‐test probability of 16%, out of 100 people with positive test, 88 people (95% CI 81 to 93) have pancreatic necrosis. At the same pre‐test probability, out of 100 people with negative test, 27 people (95% CI 16 to 40) have pancreatic necrosis. |
| Procalcitonin (day 1) > 0.5 ng/mL | 0.75 (95% CI 0.35 to 0.97) | 0.57 (95% CI 0.18 to 0.90) | 53.3% | 66.7% (95% CI 43.8% to 83.7%) | 33.3% (95% CI 11.4% to 66.1%) | 1 | 15 | High | Unclear | At the pre‐test probability of 56%, out of 100 people with positive test, 67 people (95% CI 44 to 84) have pancreatic necrosis. At the same pre‐test probability, out of 100 people with negative test, 33 people (95% CI 11 to 66) have pancreatic necrosis. |
| Lactate dehydrogenase (day 5) > 290 U/L | 0.87 (95% CI 0.73 to 0.96) | 1.00 (95% CI 0.89 to 1.00) | 55.7% | 98.5% (95% CI 80.6% to 99.9%) | 12.8% (95% CI 6.1% to 24.9%) | 1 | 70 | High | Unclear | At the median pre‐test probability of 56%, out of 100 people with positive test, 99 people (95% CI 81 to 100) have pancreatic necrosis. At the same pre‐test probability, out of 100 people with negative test, 13 people (95% CI 6 to 25) have pancreatic necrosis. |
| Intepretation: Lactate dehydrogenase (day 5) > 290 U/L appears to perform best, missing the diagnosis in 13 (95% CI 4 to 27) out of 100 people with acute pancreatic necrosis and overdiagnosing in 0 (95% CI 0 to 11) out of 100 people without acute pancreatic necrosis. However, the study is at high risk of bias, and neither the day on which the measurement was made nor the threshold for positive diagnosis was determined in advance, which is likely to increase the test performance incorrectly. Consequently, the results are highly unreliable. | ||||||||||
1The number following 'day' indicates the number of days after admission that the index test was performed. The information that follows this indicates the threshold.
2 The post‐test probabilities were calculated at the median pre‐test probability.
CI: confidence interval
Background
(See Appendix 1 for a glossary of terms.)
The pancreas is an abdominal organ that secretes several digestive enzymes into the pancreatic ductal system, which empties into the small bowel. It also contains the islets of Langerhans, which secrete several hormones, including insulin (NCBI 2014a). Acute pancreatitis is a sudden inflammatory process in the pancreas, with variable involvement of adjacent organs or other organ systems (Bradley 1993). The annual incidence of acute pancreatitis ranges from 5 to 30 per 100,000 population (Roberts 2013; Yadav 2006). In the last one to two decades there has been an increase in the incidence of acute pancreatitis in the UK and the USA (Roberts 2013; Yang 2008). Acute pancreatitis is the most common gastrointestinal (digestive tract) cause of hospital admission in the USA (Peery 2012). Gallstones and alcohol are the two main causes of acute pancreatitis. Approximately 50% to 70% of acute pancreatitis cases are caused by gallstones (Roberts 2013; Yadav 2006). Increasing age, male gender, and lower socioeconomic class are associated with a higher incidence of acute pancreatitis (Roberts 2013).
According to a consensus conference on the classification of acute pancreatitis, the diagnosis of acute pancreatitis is generally made when at least two of the following three features are present (Banks 2013).
Acute onset of a persistent, severe epigastric pain often radiating to the back.
Serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal.
Characteristic findings of acute pancreatitis on contrast‐enhanced computed tomography (CECT) and, less commonly, magnetic resonance imaging (MRI) or transabdominal ultrasonography.
Acute pancreatitis can be classified into interstitial oedematous pancreatitis (diffuse or occasionally localised enlargement of the pancreas due to inflammatory oedema as seen on CECT) or necrotising pancreatitis (necrosis involving either the pancreas or peripancreatic tissues or both) (Banks 2013). Approximately 90% to 95% of people with acute pancreatitis have interstitial oedematous pancreatitis, while the remainder have necrotising pancreatitis (Banks 2013). Necrotising pancreatitis may be sterile or infected (Banks 2013). Various theories exist as to how pancreatic and peripancreatic tissues become infected. These include spread from blood circulation, lymphatics, bile, from the small bowel (duodenum) through the pancreatic duct, and migration through the large bowel wall (translocation) (Schmid 1999).
Local complications of acute pancreatitis include acute peripancreatic fluid collection, pancreatic pseudocyst, acute necrotic collection, and walled‐off necrosis (Banks 2013). Systemic complications of acute pancreatitis include worsening of pre‐existing illnesses, such as heart or chronic lung disease (Banks 2013). The mortality rate following an attack of acute pancreatitis is between 6% and 20% (Roberts 2013; Yadav 2006), and depends upon the severity of the acute pancreatitis and the presence of infection. Acute pancreatitis can be classified as mild, moderate, or severe depending upon the presence of local or systemic complications, transient organ failure involving one of more of lungs, kidneys, and cardiovascular system (heart and blood vessels) lasting up to 48 hours, or persistent failure of the same organs mentioned above, lasting beyond 48 hours. In mild pancreatitis, there are no local or systemic complications, or organ failure. In moderately severe acute pancreatitis, there may be local or systemic complications, or transient organ failure. In severe acute pancreatitis, there is persistent organ failure (Banks 2013). (See summary in Table 2.) Acute severe pancreatitis carries the worst prognosis in terms of mortality, while mild pancreatitis has the best prognosis (Banks 2013). Infected necrotising pancreatitis carries a significantly worse prognosis than sterile necrotising pancreatitis, with an average in‐hospital mortality of more than 30% for people with infected necrotising pancreatitis, which increases to more than 40% in the subgroup of people with organ failure in addition to infection (Petrov 2010).
1. Acute pancreatitis classification.
| Mild acute pancreatitis | Moderate acute pancreatitis | Severe acute pancreatitis |
|
|
|
Target condition being diagnosed
Acute necrotising pancreatitis in people with an established diagnosis of acute pancreatitis.
Index test(s)
All of the index tests evaluated in this review are performed by the laboratory technician and interpreted by the clinician.
Diagnosis of necrotising pancreatitis in people with an established diagnosis of acute pancreatitis
Serum C‐reactive protein (CRP)
C‐reactive protein is a plasma protein that increases during inflammation and after tissue damage (NCBI 2014b). Inflammation and tissue damage occur in people with pancreatic necrosis. However, activation of inflammatory pathways is considered to be one of the reasons for the clinical manifestation of acute pancreatitis (Banks 2013), and hence serum CRP can be elevated even in oedematous pancreatitis. One of the thresholds proposed for distinguishing oedematous pancreatitis and necrotising pancreatitis is 140 mg/L (Rau 1998). An increasing trend in the values of the test may also be used for the triage of people who require radiological examination.
Serum procalcitonin
Procalcitonin is the precursor of the hormone calcitonin found in the thyroid C cells and the pulmonary endocrine cells. However, all tissues have the potential to produce procalcitonin. In people with sepsis and severe inflammation, procalcitonin is elevated (Becker 2010). Since pancreatic necrosis is associated with severe inflammation, serum procalcitonin may distinguish between oedematous pancreatitis and necrotising pancreatitis. Procalcitonin levels are undetectable in healthy adults. Hence, any detectable levels of serum procalcitonin can be considered to be abnormal. An increasing trend in the values of the test may also be used for the triage of people who require radiological examination.
Serum lactate dehydrogenase (LDH)
Lactate dehydrogenase is an indicator of cell death. Since pancreatic necrosis is associated with cell death, LDH may distinguish between oedematous pancreatitis and pancreatic necrosis. Normal LDH levels range from 140 units/L to 280 units/L. One of the thresholds proposed for distinguishing oedematous pancreatitis and necrotising pancreatitis is 290 units/L (Rau 1998). An increasing trend in the values of the test may also be used for the triage of people who require radiological examination.
Clinical pathway
For people with acute onset of a persistent, severe, epigastric pain or people with diffuse abdominal pain that started in the epigastric region (or if the person is unsure about the region in which diffuse abdominal pain began), clinical examination including recording of blood pressure, pulse rate, and oxygen saturations (when available) are performed. Routine blood tests such as full blood count, urea, creatinine, and electrolytes are also performed. Blood tests such as amylase and lipase are performed to confirm (or rule out) the diagnosis of acute pancreatitis. Radiological findings of acute pancreatitis evolve over a few days, and the radiological features may not be apparent in the early stages, or may even be normal (Banks 2013; Vissers 1999); thus, one cannot rely on radiological tests to diagnose acute pancreatitis, at least in the early stages. Radiological examination with computed tomography (CT scan) or MRI scan is not routinely performed if a diagnosis of acute pancreatitis is suspected. If acute pancreatitis can be ruled out, other causes of acute epigastric pain should be considered. Peptic ulcer, functional dyspepsia, and gallstones can present with acute epigastric pain (Gurusamy 2014; Moayyedi 2006). All of these alternative causes of epigastric pain are generally investigated and treated after discharge of the person unless there is a strong suspicion of perforated peptic ulcer, usually because of features of peritonitis or because pain control could not be achieved. In such instances, either a plain X‐ray of the abdomen or emergency CT scan, or both may be performed to identify the presence of free‐intraperitoneal gas (Ghekiere 2007; Grassi 2004). The usual treatment for perforated peptic ulcer is emergency surgical closure, which can be performed by open or laparoscopic surgery (Sanabria 2013).
If a diagnosis of acute pancreatitis can be established, usually based on the consensus criteria, the person is admitted to hospital and the severity of pancreatitis assessed. The treatment of acute pancreatitis is generally supportive treatment, that is maintenance of fluid and electrolyte imbalance. Despite various pharmacological interventions being evaluated in acute pancreatitis, none is currently recommended as treatment. Abdominal ultrasound and magnetic resonance cholangiopancreatography or endoscopic ultrasound may be performed to investigate the aetiology of acute pancreatitis. In the presence of gallstones, cholecystectomy is performed. The timing of cholecystectomy in acute pancreatitis is controversial, and different factors must be considered depending upon the severity of the acute pancreatitis (Gurusamy 2013). Endoscopic sphincterotomy or common bile duct exploration may need to be performed in the presence of common bile duct stones (Ayub 2004; Larson 2006). In the absence of gallstones, investigation of other causes of acute pancreatitis is required. People are generally monitored clinically. If the person improves clinically with supportive treatment, the person with gallstone pancreatitis is discharged after cholecystectomy or after scheduling a cholecystectomy or on a planned list, within the two weeks. For those people with severe acute pancreatitis, cholecystectomy is undertaken when clinically appropriate after resolution of pancreatitis (NCEPOD 2016). If the person deteriorates clinically, the person undergoes a CT scan and may require high‐dependency or intensive care unit care in the presence of organ failure or in the presence of infected pancreatic necrosis.
In the presence of organ failure, the person undergoes a CT scan or MRI to identify any local complications. C‐reactive protein, procalcitonin, and LDH might distinguish between oedematous and necrotising pancreatitis (Alfonso 2003; Khanna 2013; Rau 1998), and could potentially be used as a triage test to identify who among those without organ failure needs further radiological tests (Alfonso 2003). Some centres use CRP routinely to determine whether people require radiological investigations to diagnose necrotising pancreatitis. Frequently, a rising trend in CRP, procalcitonin, or LDH rather than a single test may be used to determine whether people require radiological investigations to diagnose necrotising pancreatitis. It should be noted that CT scan or MRI is not routinely performed during the initial stages of acute pancreatitis but usually in the presence of organ failure or due to the results of the serum CRP. The various treatment strategies in acute necrotising pancreatitis include non‐surgical (conservative) treatment, percutaneous drainage, endoscopic transluminal drainage, early surgical debridement (necrosectomy, which can be performed by open surgery or by minimally invasive retroperitoneal debridement), delayed necrosectomy (delaying the surgery by about four weeks), or a step‐up approach that consists of endoscopic or percutaneous drainage followed by laparoscopic necrosectomy if required, and non‐surgical (conservative) treatment (Bakker 2012; Mouli 2013; Tenner 2013; van Brunschot 2014; van Santvoort 2010; van Santvoort 2011). A recent Cochrane systematic review found that a step‐up approach may be preferable to direct surgery in people with acute necrotising pancreatitis (Gurusamy 2016). All of these treatments are supported by appropriate fluid therapy and nutritional support. This is in comparison with severe acute oedematous pancreatitis, where the main treatment is supportive treatment for systemic complications including organ failure and treatment of local complications such as pseudocyst if symptomatic (Cannon 2009; Cheruvu 2003; Johnson 2009; Varadarajulu 2008; Varadarajulu 2013). In the case of infected pancreatic necrosis, appropriate antibiotics are administered in addition to the treatment outlined above for non‐infected pancreatic necrosis. In the case of acute peripancreatic collections or pseudocysts on the radiological tests, the person requires clinical and radiological follow‐up to ensure resolution of these collections.
If the diagnosis of acute pancreatitis cannot be ruled out on the basis of the clinical presentation and serum amylase or lipase, the person is admitted to hospital and the evolution of signs and symptoms is noted. Serum amylase and lipase may be repeated, or radiological examinations may be performed to establish or rule out acute pancreatitis with a reasonable amount of certainty. Tests for organ failure (e.g. urea and creatinine for identifying renal failure, blood pressure, pulse rate, respiratory rate, urine output, and arterial blood gases) may also be performed to ensure that moderately severe or severe pancreatitis is not present irrespective of the results of serum amylase and lipase. The possible clinical pathway in the diagnosis and management of acute pancreatitis is shown in Figure 1.
1.
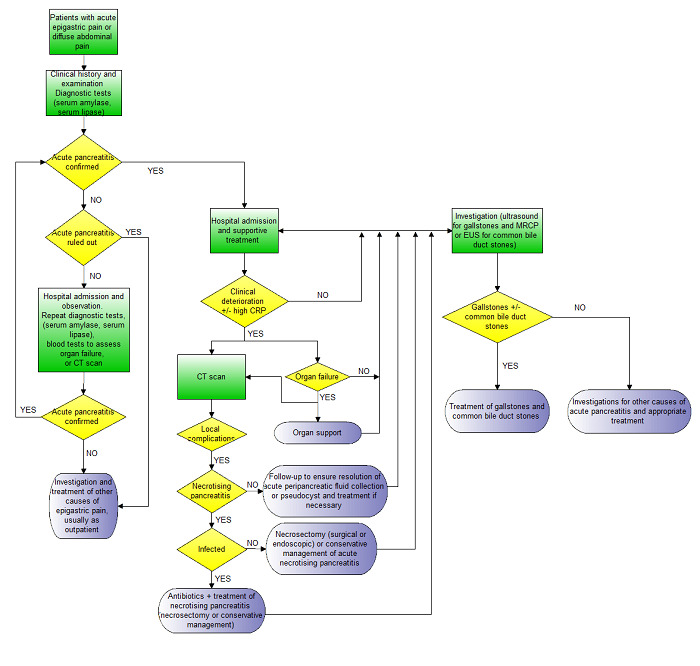
Clinical pathway.
Footnotes:
- Acute pancreatitis is usually confirmed by consensus criteria (Banks 2013).
- Irrespective of the CT scan findings and presence or absence of necrosis, people with organ failure will require organ support and will receive a CT scan.
- CT scan may also be performed in people without organ failure if there is clinical deterioration (not amounting to organ failure) or in some centres based on an elevated CRP.
- Necrotising pancreatitis is usually confirmed by the findings on the CT scan and by histopathological examination of the biopsy obtained during necrosectomy if early necrosectomy is performed.
- Infected necrotising pancreatitis is usually confirmed by the findings on the CT scan and by microbiological examination of fluid aspirated under radiological guidance or from the tissue biopsy obtained during necrosectomy if early necrosectomy is performed.
- Organ failure is diagnosed on the basis of clinical examination and blood tests (urea, creatinine, blood pressure, pulse rate, respiratory rate, arterial blood gas analysis).
Abbreviations:
CRP: C‐reactive protein CT: computed tomography EUS: endoscopic ultrasound MRCP: magnetic resonance cholangiopancreatography
Prior test(s)
The tests that are performed before the index tests, such as serum lipase or amylase, are used to establish the diagnosis of acute pancreatitis. If necessary, these are supported with radiological tests such as CECT, MRI, or transabdominal ultrasonography, and clinical examination and blood tests to rule out organ failure (e.g. urea and creatinine for identifying renal failure, blood pressure, pulse rate, respiratory rate, urine output, and arterial blood gases). Of these tests, serum tests for the diagnosis of acute pancreatitis, clinical examination, and blood tests to rule out organ failure are routinely performed, while CT scan is performed if there is uncertainty in the diagnosis of acute pancreatitis. The minimum prior tests are thus serum lipase, serum amylase, clinical examination, and blood tests to rule out organ failure.
Role of index test(s)
Currently, if necrotising pancreatitis is suspected in people without organ failure, radiological investigations are performed directly, although some units may use CRP (in particular an increasing trend in CRP values) to identify those who require radiological investigations. In people where the diagnosis of acute pancreatitis was based on CT scan, it is quite possible that the radiological features of necrosis are not manifest initially, as there may be a delay in their appearance (Banks 2013). In such cases, CRP may be used to identify people who require additional radiological investigations. We evaluated the index tests (CRP, procalcitonin, and LDH) as triage tests for detecting pancreatic necrosis in people with acute pancreatitis in whom the diagnosis of pancreatic necrosis has not been made. Further radiological tests such as CECT will be necessary for confirming pancreatic necrosis, and the location and extent of pancreatic necrosis, before treatment can be planned. We did not evaluate the role of these tests in monitoring necrotising pancreatitis once the diagnosis of necrotising pancreatitis is made.
Alternative test(s)
Other tests used in the diagnosis of pancreatic necrosis include CECT, MRI, or transabdominal ultrasonography (Banks 2013). Various other blood tests such as blood haematocrit, blood urea, serum creatinine, and procarboxypeptidase B have been evaluated as diagnostic tests for pancreatic necrosis, but these are not in routine use for the diagnosis of pancreatic necrosis (Muddana 2009; Rau 1998).
Rationale
The treatment of people with acute pancreatitis differs between people with and those without pancreatic necrosis, as mentioned in the clinical pathway (Figure 1). People with organ failure routinely undergo radiological investigations, while those without organ failure do not routinely undergo CT scans. Some units already use CRP as a triage test to identify people without organ failure who require radiological investigations and admission to high dependency unit or intensive therapy unit, while others do not. The role of CRP, procalcitonin, and LDH as triage tests is thus unclear. There is no current systematic review of the diagnostic test accuracy of CRP, procalcitonin, or LDH in the diagnosis of pancreatic necrosis. A Cochrane systematic review of the diagnostic test accuracy of CRP, procalcitonin, or LDH in the diagnosis of pancreatic necrosis was needed to understand the value of these tests as triage tests to identify people who require radiological investigation.
Objectives
To compare the diagnostic accuracy of CRP, procalcitonin, or LDH, either alone or in combination, in the diagnosis of necrotising pancreatitis in people with acute pancreatitis and without organ failure.
Secondary objectives
We planned to explore the following sources of heterogeneity.
Studies at low risk of bias versus those at unclear or high risk of bias (as assessed by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool, recommended by the Cochrane Diagnostic Test Accuracy Group) (Whiting 2006; Whiting 2011).
Prospective studies versus retrospective studies (to determine whether there is a difference in diagnostic accuracy between prospective and retrospective studies).
Full‐text publications versus abstracts (this can be indicative of publication bias, since there may be an association between the results of the study and the study reaching full publication status) (Eloubeidi 2001).
Previous history of acute pancreatitis.
Different aetiology for acute pancreatitis (gallstone versus alcohol versus other aetiology). The accuracy of the test may depend upon the aetiology of the acute pancreatitis.
Presence or absence of infection. The accuracy of the test may depend upon the presence or absence of infection.
Pancreatic versus peripancreatic necrosis.
Average time to performance of the test. The accuracy of the test may depend upon the interval between the onset of clinical symptoms and the performance of the test.
Different test manufacturers.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that evaluated the accuracy of the index tests mentioned above in the appropriate population (see below). We included relevant studies irrespective of language or publication status; whether the data were collected prospectively or retrospectively; and whether there was a comparison between the tests. However, we excluded case reports (which describe how the diagnosis of acute pancreatitis or acute necrotising pancreatitis was made on an individual participant or a group of participants and which do not provide sufficient diagnostic test accuracy data, i.e. true positive, false positive, false negative, and true negative). We also planned to exclude case‐control studies because they are prone to bias (Whiting 2011); however, we did not identify any case‐control studies.
Participants
Adult participants with acute pancreatitis within 14 days of the onset of symptoms (irrespective of the interval between the onset of symptoms and the time at which the test was performed). The diagnosis of acute pancreatitis should have been made on the basis of the consensus conference definition (Banks 2013). Participants who had already developed organ failure at the time of performing these tests were excluded, since all such participants undergo radiological investigations. Although we had planned to exclude participants in whom pancreatic necrosis was present on the CT scan used to diagnose acute pancreatitis, this information was not available from the studies.
Index tests
Serum CRP, procalcitonin, and LDH either alone or in combination immediately prior to radiological investigation. A variety of kits are available for measuring these tests. We included kits from all manufacturers, and included studies irrespective of the threshold used. We included studies that reported a single test and sequential tests of serum CRP, procalcitonin, and LDH. If the study reported sequential testing, we planned to consider a progressive increase as a positive index test irrespective of the degree of increase, and stationary or decrease in the levels as a negative test; however, none of the studies reported this information despite measuring the levels on different days.
Target conditions
Pancreatic necrosis (i.e. infected or sterile pancreatic or peripancreatic necrosis)
Reference standards
While considered to be the gold standard for confirming necrosis, biopsy may not have been performed in all participants due to ethical concerns over performing an invasive treatment (during which biopsy is taken) in those without a diagnosis of pancreatic necrosis. As a result, study authors may use radiological features of pancreatic necrosis (an area of reduced enhancement or non‐enhancing area of pancreatic parenchyma on CECT or contrast‐enhanced MRI). However, this reference standard may miss some cases of pancreatic necrosis, resulting in underestimation of diagnostic test accuracy of the index tests. In addition, using radiological features of pancreatitis might introduce an intrinsic threshold effect because of interobserver variation between radiologists. As per protocol, we accepted any of the following reference standards, used alone or in combination: radiological features of pancreatic necrosis (CECT or contrast‐enhanced MRI) or histological confirmation of pancreatic necrosis. We also included a combination of radiological features of pancreatic necrosis (CECT or contrast‐enhanced MRI) and surgeon's judgement of pancreatic necrosis during surgery, as we considered this equivalent to radiologist judgement of the presence of pancreatic necrosis on CECT or contrast‐enhanced MRI. In terms of ranking the reference standards, we considered biopsy in all participants as the best reference standard (although it is unlikely to be performed in participants with a negative test for pancreatic necrosis) followed by biopsy in participants with positive test and radiological or surgical features of pancreatic necrosis in participants with negative test, and radiological tests or surgery alone as the reference standard, in that order.
Search methods for identification of studies
We included all studies irrespective of the language of publication and publication status. We translated non‐English language articles.
Electronic searches
We searched the following databases.
MEDLINE (In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R)) via OvidSP (January 1946 to 20 March 2017) (Appendix 2).
Embase via OvidSP (January 1947 to 20 March 2017) (Appendix 3).
Science Citation Index Expanded via Web of Knowledge (January 1980 to 20 March 2017) (Appendix 4).
Conference Proceedings Citation Index‐Science (CPCI‐S) via Web of Knowledge (January 1990 to 20 March 2017) (Appendix 4)
National Insitute for Health Research (NIHR HTA and DARE) via Centre for Reviews and Dissemination (20 March 2017) (Appendix 5).
Zetoc via British Library (20 March 2017) (Appendix 6).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) (20 March 2017) (Appendix 7).
ClinicalTrials.gov (clinicaltrials.gov/) (20 March 2017) (Appendix 8).
We used this same strategy in another review on diagnosis of acute pancreatitis in people with acute epigastric or diffuse abdominal pain (Gurusamy 2015).
Searching other resources
We searched the references of the included studies to identify additional studies. We also searched for articles related to the included studies by performing the 'related search' function in MEDLINE (OvidSP) and Embase (OvidSP) and a 'citing reference' search (by searching the articles that cite the included articles) in these databases (Sampson 2008).
Data collection and analysis
Selection of studies
Two review authors (OK and KSG) independently identified relevant studies from the retrieved references. We obtained the full texts of references considered to be relevant by at least one of the review authors. Two review authors independently screened the full‐text papers against the inclusion criteria and resolved any differences through discussion. We planned to contact the study authors if there were any doubts about study eligibility.
Data extraction and management
Two review authors (OK and KSG) independently extracted the following data from each included study using a data extraction form designed and piloted by KSG, resolving any differences by discussion.
First author.
Year of publication.
Study design (prospective or retrospective cohort studies; cross‐sectional studies or randomised comparisons of index tests).
Inclusion and exclusion criteria for individual studies.
Total number of participants.
Number of females.
Average age of the participants.
Average time between onset of symptoms and index test.
Aetiology of acute pancreatitis.
Proportion of participants with infected pancreatic necrosis.
Description of the index test.
Threshold used for the index test.
Reference standard.
Information to complete the QUADAS‐2 assessment (please see below).
Number of true positives, false positives, false negatives, and true negatives.
If the same study reported multiple index tests, we extracted the number of true positives, false positives, false negatives, and true negatives for each index test. If the same study reported the number of true positives, false positives, false negatives, and true negatives for each index test at different thresholds, we extracted this information for each threshold. If the study reported the results for a combination of tests, we planned to extract the number of true positives, false positives, false negatives, and true negatives for each different combination of tests; however, we did not find any such studies.
A common way that the diagnostic accuracy of a combination of tests is assessed is at least one test positive versus all tests positive. We planned to extract the number of true positives, false positives, false negatives, and true negatives for both the scenarios (at least one test positive and all tests positive). If the study reported the test at multiple time points, we planned to obtain the trend in sequential testing of CRP, procalcitonin, or LDH if the author used a progressively increasing trend in index test values for distinguishing acute necrotising pancreatitis and oedematous pancreatitis. For this purpose, we planned to consider an increasing trend as a positive index test irrespective of the degree of increase, and consider stationary levels or a decrease in the levels as a negative test in order to calculate the number of true positives, false positives, false negatives, and true negatives. If the authors provided the final values of these index tests prior to radiological examination, we planned to obtain these values for calculating the true positives, false positives, false negatives, and true negatives. We did this because we wanted to evaluate the role of these index tests used as a test with a prespecified threshold, and the role of an increasing trend in the values of these index tests for distinguishing acute necrotising pancreatitis and oedematous pancreatitis. The rationale for using the final values to calculate the diagnostic test accuracy is as follows. Participants may receive treatment for organ failure if they developed organ failure between the index test and reference standard. We anticipated that a radiological investigation would have been performed within 24 hours of diagnosis of organ failure. Pancreatic necrosis does not resolve in 24 hours, and there will be no alteration of the final diagnosis by the treatment in participants with pancreatic necrosis. People with oedematous pancreatitis and organ failure may develop pancreatic necrosis in the absence of appropriate treatment. Consequently, there is a possible interaction between inadequate treatment and the final diagnosis. The final values, which have the shortest time interval between the index test and reference standard, are the least likely to be affected by inappropriate treatment and are likely to provide the best estimates of diagnostic test accuracy. Although studies measured the index tests at several time points, the diagnostic test accuracy results were provided only at a specific time point, therefore we did not use trend in values as a threshold in this review.
We excluded participants with uninterpretable index test results (irrespective of the reason given for lack of interpretation) from the diagnostic test accuracy data since in clinical practice, uninterpretable index test results would result in additional tests for the diagnosis of acute pancreatitis. However, we recorded the number of uninterpretable index test results in a separate data column, as this would provide information on the applicability of the test in clinical practice (i.e. the number of individuals in whom the test provides interpretable results) and may affect the cost‐effectiveness of a test. Although cost‐effectiveness is outside the scope of this review, cost‐effectiveness studies may use data from this review. If there was an overlap of participants between multiple reports, as suggested by common authors and centres, we planned to contact the study authors to seek clarification about the overlap. If we were unable to contact the authors, we planned to extract the maximum possible information from all of the reports. However, we did not find any such reports. We attempted to contact the study authors for further information where necessary.
Assessment of methodological quality
Two review authors (OK and KSG) independently assessed study quality using the QUADAS‐2 assessment tool (Whiting 2006; Whiting 2011). Any differences were resolved by discussion using the QUADAS‐2 table from the protocol shown in Table 3. We considered studies classified as 'low risk of bias' and 'low concern' in all of the domains (except for the reference standard domain, where we accepted a 'No' for the signalling question 'Is the reference standard likely to correctly classify the target condition?') as studies with high methodological quality, that is we accepted a study to be of high methodological quality despite not using histological confirmation of pancreatic necrosis (as it is unethical to perform a biopsy in a person with a low likelihood of not having pancreatic necrosis), provided that it was classified as at low risk of bias for all other domains and low concern in all domains. We have presented the results in a 'Risk of bias' summary and graphs in addition to a narrative summary.
2. QUADAS‐2 classification (acute necrotising pancreatitis).
| Domain 1: Participant selection | Patient sampling | Adult participants with acute pancreatitis and without organ failure. |
| Was a consecutive or random sample of patients enrolled? | Yes: If a consecutive sample or a random sample of participants with acute pancreatitis and without organ failure was included in the study. No: If a consecutive sample or a random sample of participants with acute pancreatitis and without organ failure was not included in the study. Unclear: If this information was not available. | |
| Did the study avoid inappropriate exclusions? | Yes: If all participants with acute pancreatitis and without organ failure were included. No: If the study excluded participants based on high or low probability of pancreatic necrosis (e.g. those with normal white cell count were excluded). Unclear: If this information was not available. | |
| Could the selection of participants have introduced bias? | Low risk of bias: If 'yes' classification for both of the above two questions. High risk of bias: If 'no' classification for either of the above two questions. Unclear risk of bias: If 'unclear' classification for either of the above two questions, but without a 'no' classification for either of the above two questions. |
|
| Participant characteristics and setting | We recorded the following characteristics: sample size, females, age, presentation (inclusion and exclusion criteria), and setting (primary or secondary care and country). | |
| Are there concerns that the included participants and setting do not match the review question? | Low concern: If the participant characteristics and setting is classified as 'yes'. Unclear concern: If the participant characteristics and setting is classified as 'unclear'. High concern: If the participant characteristics and setting is classified as 'no'. |
|
| Domain 2: Index test | Index test(s) | Serum C‐reactive protein, procalcitonin, lactate dehydrogenase |
| Were the index test results interpreted without knowledge of the results of the reference standard? | The index test would always be conducted, though not interpreted before the reference standard. Yes: If the index test is conducted and interpreted without knowledge of the results of the reference standard. No: If the index test is interpreted with knowledge of the results of the reference standard. Unclear: If it is not clear whether the index test was interpreted without knowledge of the results of the reference standard. |
|
| If a threshold was used, was it prespecified? | Yes: If a prespecified threshold was used. No: If a prespecified threshold was not used. Unclear: If it was not clear whether the threshold used was prespecified. |
|
| Could the conduct or interpretation of the index test have introduced bias? | Low risk of bias: If 'yes' classification for both of the above two questions. High risk of bias: If 'no' classification for either of the above two questions. Unclear risk of bias: If 'unclear' classification for either of the above two questions, but without a 'no' classification for either of the above two questions. |
|
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: If the criteria for positive index test were clearly stated. High concern: If the criteria for positive index test were not stated. |
|
| Domain 3: Target condition and reference standard | Target condition and reference standard(s) | Target condition: pancreatic or peripancreatic necrosis (infected or sterile). While considered to be the gold standard for confirming necrosis, biopsy may not have been performed in all participants due to ethical concerns over performing an invasive treatment (during which biopsy is taken) in those without a diagnosis of pancreatic necrosis. As a result, study authors may use radiological features of pancreatic necrosis (an area of impairment enhancement or non‐enhancing area of pancreatic parenchyma on CECT) or surgical features of pancreatic necrosis during surgery (presence of necrotic tissue). However, this reference standard may miss some cases of pancreatic necrosis. In terms of ranking the reference standards, we considered biopsy in all participants as the best reference standard (although it is unlikely to be performed in participants with negative test for pancreatic necrosis) followed by biopsy in participants with positive test and radiological or surgical features of pancreatic necrosis in participants with negative test, and radiological tests or surgical tests alone as the reference standard, in that order. |
| Is the reference standard likely to correctly classify the target condition? | Yes: If histological confirmation of pancreatic necrosis was obtained in all participants or at least all participants with positive test.
No: If the reference standard was CECT (or contrast enhanced MRI) in all participants. Unclear: If the reference standard was not described adequately. |
|
| Were the reference standard results interpreted without knowledge of the results of the index test? | Yes: If the reference standard was interpreted without knowledge of the results of the index test. No: If the reference standard was interpreted with knowledge of the results of the index test. Unclear: If it was not clear if the reference standard was interpreted without knowledge of the results of the index test. | |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk of bias: If 'yes' classification for both of the above two questions. High risk of bias: If 'no' classification for either of the above two questions. Unclear risk of bias: If 'unclear' classification for either of the above two questions, but without a 'no' classification for either of the above two questions. As anticipated, we assessed all studies as at high risk of bias as they all used CECT or surgery as the reference standard and were therefore classified as 'no' for the question "Is the reference standard likely to correctly classify the target condition?". |
|
| Are there concerns that the target condition as defined by the reference standard does not match the question? | As anticipated, considering the inclusion criteria for this review, we classified all of the included studies as 'low concern', as they all reported on pancreatic necrosis. | |
| Domain 4: Flow and timing | Flow and timing | Participants may have progression or regression of pancreatic necrosis if there is a long delay between index test and reference standard. In addition, participants may receive treatment for organ failure if they develop organ failure between the index test and reference standard. We anticipated that a radiological investigation would have been performed within 24 hours of diagnosis of organ failure. Pancreatic necrosis does not resolve in 24 hours, and there will be no alteration of the final diagnosis by the treatment in participants with pancreatic necrosis. People with oedematous pancreatitis and organ failure may develop pancreatic necrosis in the absence of appropriate treatment. Consequently there is a possible interaction between inadequate treatment and the final diagnosis. We have minimised this misclassification error due to the final diagnosis being altered by inappropriate treatment by choosing 24 hours as an acceptable delay between index test and reference standard. |
| Was there an appropriate interval between index test and reference standard? | Yes: If the time interval between index test and reference standard was less than 24 hours. No: If the time interval between index test and reference standard was more than 24 hours. Unclear: If the time interval between index test and reference standard was unclear. | |
| Did all participants receive a reference standard? | Yes: If all participants received a reference standard.
No: If some participants did not receive a reference standard. Such studies were excluded.
Unclear: If it was not clear whether all participants received a reference standard. Such studies were excluded. As anticipated, we classified all studies included in the review as 'yes' for this item. |
|
| Did all participants receive the same reference standard? | Yes: If all participants received the same reference standard.
No: If the reference standard participants received varied. Unclear: If this information was not clear. |
|
| Were all participants included in the analysis? | Yes: If all participants were included in the analysis irrespective of whether the results were interpretable. No: If some participants were excluded from the analysis because of uninterpretable results. Unclear: If this information was not clear. | |
| Could the patient flow have introduced bias? | Low risk of bias: If 'yes' classification for all of the above four questions. High risk of bias: If 'no' classification for any of the above four questions. Unclear risk of bias: If 'unclear' classification for any of the above four questions, but without a 'no' classification for any of the above four questions. |
CECT: contrast enhanced computed tomography MRI: magnetic resonance imaging
Statistical analysis and data synthesis
We stratified the analysis by the test thresholds (i.e. tests at different thresholds were considered as different index tests) and planned to stratify the analysis by different reference standards (if the same test was assessed in different studies using different reference standards, it was considered as different index tests). If the study used increasing trend in the values of CRP, procalcitonin, or LDH as the diagnostic criteria for distinguishing necrotising pancreatitis from oedematous pancreatitis, we planned to consider this as the 'threshold' for the purpose of this review. We plotted study estimates of sensitivity and specificity on forest plots and in receiver operating characteristic (ROC) space to explore between‐study variation in the performance of each test stratified by the threshold and reference standard. To estimate the summary sensitivity and specificity of each test at each threshold level and each reference standard, we planned to perform the meta‐analysis by fitting the bivariate model (Chu 2006; Reitsma 2005). This model accounts for between‐study variability in estimates of sensitivity and specificity through the inclusion of random effects for the logit sensitivity and logit specificity parameters of the bivariate model. If sparse data resulted in unreliable estimation of the covariance matrix of the random effects as indicated by very large variance of logit sensitivity and specificity, we planned to perform the analysis using simpler models suggested by Takwoingi 2015 and colleagues using the distribution of sensitivities and specificities as noted in the forest plots or ROC space and ‐2 log likelihood to choose the model.
We planned to compare the diagnostic accuracy of the different tests by including a single covariate term for test type in the bivariate model to estimate differences in the sensitivity and specificity of the tests. We planned to consider a combination of tests for each of the scenarios (any test positive or all tests positive) as different index tests. We planned to allow the variances of the random effects and their covariance to also depend on test type, thus allowing the variances to differ between tests. We planned to use the hierarchical summary receiver operating characteristics curve (HSROC) to test hypotheses about whether one test is superior to another and to investigate heterogeneity (Rutter 2001). For this purpose, we planned to combine tests irrespective of the thresholds and reference standards, as we expected few studies at each threshold level and reference standard. In case the study reported results at multiple thresholds, we planned to employ the threshold used for primary analysis by the authors for inclusion in the HSROC model. We planned to use likelihood ratio tests to compare the model with and without covariate (test type). A P value of less than 0.05 for the likelihood ratio test would have indicated differences in diagnostic accuracy between the tests. We also planned to compare the estimates of sensitivity and specificity between models to check the robustness of our assumptions about the variances of the random effects. If at least four studies that evaluated different tests in the same study population were available (e.g. in studies that performed more than one index test in all participants, individual index tests and combination of index tests in all participants, or randomised controlled trials in which participants were randomised to the different index tests), we planned to perform a direct head‐to‐head comparison by limiting the test comparison to such studies. We also planned to present the relative sensitivities and relative specificities of the index tests from the direct comparisons in a table.
We planned to perform the meta‐analysis using the NLMIXED command in SAS version 9.3 (SAS Institute Inc, Cary, North Carolina, USA). We planned to create a graph of pre‐test probabilities (using the observed median and range of prevalence from the included studies) against post‐test probabilities for each test stratified by different thresholds and reference standards. The post‐test probabilities would have been calculated using these pre‐test probabilities and the summary positive and negative likelihood ratios. The summary likelihood ratios and their confidence intervals would have been calculated from the functions of the parameter estimates from the bivariate model that we planned to fit to estimate the summary sensitivities and specificities. Post‐test probability associated with positive test is the probability of having the target condition (acute pancreatitis or acute necrotising pancreatitis) on the basis of a positive test result, and is the same as the term 'positive predictive value' used in a single diagnostic accuracy study. Post‐test probability associated with a negative test is the probability of having the target condition (acute pancreatitis or acute necrotising pancreatitis) on the basis of a negative test result and is 1 ‐ 'negative predictive value'. Negative predictive value is the term used in a single diagnostic accuracy study to indicate the chance that the participant has no target condition when the test is negative. We planned to report the summary sensitivity, specificity, positive and negative likelihood ratios, and post‐test probabilities for the median, lower quartile, and upper quartile of the pre‐test probabilities.
However, because of paucity of data, we did not perform any meta‐analysis. We calculated the sensitivity and specificity of each test and have reported these with their 95% confidence intervals (95% CI), along with the post‐test probability of positive and negative test at the pre‐test probability in the studies.
Investigations of heterogeneity
Of the nine sources of heterogeneity mentioned in the Secondary objectives, we planned to use risk of bias, prospective or retrospective studies, publication status, presence or absence of infection, and different test manufacturers as categorical covariates, and the proportion of participants with a previous history of acute pancreatitis, the proportion of participants with different aetiologies, the proportion of participants with pancreatic necrosis and peripancreatic necrosis, and the average time to performance of the test as continuous covariates in the regression model. As before, we planned to include one covariate at a time in the regression model and use the likelihood ratio test to determine whether the covariate is statistically significant. We did not investigate heterogeneity because of the paucity of data.
Sensitivity analyses
We did not plan any sensitivity analyses except when the data available from the studies were ambiguous (e.g. the numbers in the text differed from the numbers in the figures), in which case we planned to assess the impact of different data used by a sensitivity analysis. We did not find any ambiguous data in the studies.
Assessment of reporting bias
We planned to investigate whether the summary sensitivity and specificity were different between studies published as full text and those that were available only as abstracts (at least two years prior to the search date) using the methods described in the Investigations of heterogeneity section. We did not investigate reporting bias because of the paucity of data.
Results
Results of the search
We identified a total of 23,360 references through the electronic searches of MEDLINE (n = 7326), Embase (n = 11,502), Science Citation Index Expanded (n = 4293), National Institute for Health Research (NIHR HTA and DARE) (n = 142), Zetoc (n = 360), WHO ICTRP (n = 1), and ClinicalTrials.gov (n = 36). We excluded 10,657 duplicates and 12,790 clearly irrelevant references through reading the titles or abstracts, or both. We sought full‐text articles for 213 references, but were unable to obtain the full text for four references (Djurasinovic 2013; Grenier 1968; Issekutz 2003; Pindak 2003). We retrieved the full‐text articles of 209 references for further assessment against our review protocol inclusion criteria. We excluded 204 of these 209 references for the reasons provided in the Characteristics of excluded studies section. Three studies (five references) fulfilled the inclusion criteria and provided the diagnostic accuracy data for the review (Alfonso 2003; Bertsch 1997; Rau 1998). We have shown the reference flow in Figure 2.
2.
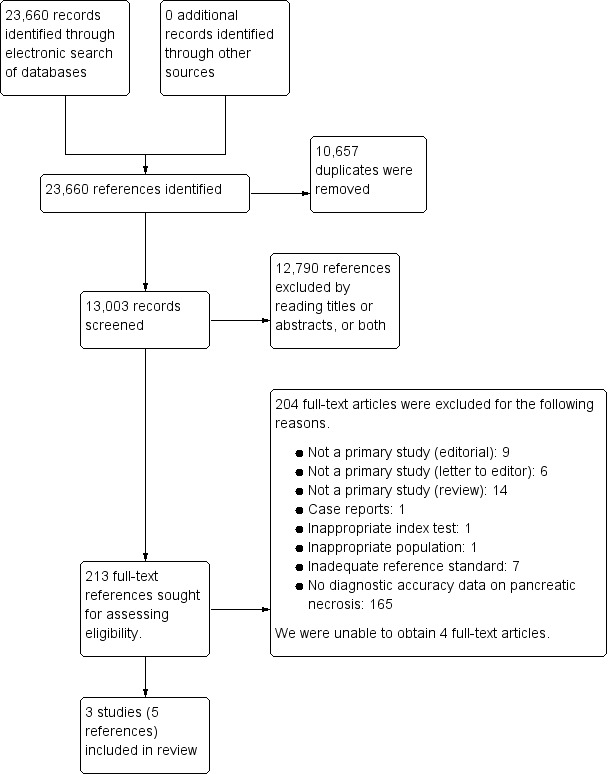
Study flow diagram.
Included studies
Three studies including 242 participants met the inclusion criteria for this review and assessed the diagnostic accuracy of the index tests in participants with established acute pancreatitis. The average age of participants in the studies was 49 years (Rau 1998), 53 years (Bertsch 1997), and 67 years (Alfonso 2003). About two‐fifths of participants (41%) were females in these three studies (Alfonso 2003; Bertsch 1997; Rau 1998). One study was a prospective study (Rau 1998), and one was a retrospective study (Alfonso 2003). It was unclear whether the third study was prospective or retrospective (Bertsch 1997). All of the studies were full‐text publications. The studies did not report whether they included participants with a previous history of acute pancreatitis. Two studies reported that they included acute pancreatitis of varied aetiology (Alfonso 2003; Bertsch 1997); information on aetiology was not available in the third study (Rau 1998). None of the studies reported data separately for different aetiologies. None of the studies reported the presence or absence of infection in participants. One study clearly indicated that the presence of pancreatic and peripancreatic necrosis was considered as the target condition (Rau 1998); the remaining studies did not provide this information. None of the studies reported data separately for pancreatic and peripancreatic necrosis.
One study reported the diagnostic performance of CRP for two threshold levels (> 200 mg/L and > 279 mg/L) without stating the day on which the CRP was measured (Alfonso 2003). One study reported the diagnostic performance of procalcitonin on day 1 using a threshold level of 0.5 ng/mL (Bertsch 1997). One study reported the diagnostic performance of CRP on day 3 using a threshold level of 140 mg/L and LDH on day 5 using a threshold level of 290 U/L (Rau 1998).
Excluded studies
We excluded a total of 204 studies at the full‐text stage for the following reasons.
Not a primary study (editorial): 9 (Chen 2004; Fan 1994; Folch‐Puy 2007; Gosling 1992; Lipsett 2001; Lott 1991; Manabe 2004; Petrov 2011; Samso 2002).
Not a primary study (letter to editor): 6 (Beger 1989; Bihari 2004; Choudhary 2012; Economou 1997; Neoptolemos 2001; Wilson 1989b).
Not a primary study (review): 14 (Bassi 1994; Brailski 1975; Buchler 1991; Frossard 2001; Geng 2014; Johnson 2003; Korczowski 2006; Lempinen 2005; Liu 2008; Malfertheiner 1993; Millat 1999; Mulholland 1996; Purkayastha 2006; Rau 2004).
Case reports: 1 (Wong 1993).
Inappropriate index test: 1 (Pezzilli 1998b).
Inappropriate population: 1 (Machiedo 1974).
Inadequate reference standard: 7 (Barauskas 2004; Cardoso 2013; Gluskina 1967; Khanna 2013; Pallisera 2014; Puolakkainen 1987; Schaffler 2010).
No diagnostic accuracy data on pancreatic necrosis: 165 (Abishek 2014; Aggelopoulos 1996; Ammori 2003; Appasani 2011a; Appasani 2011b; Appasani 2012; Bajec 2010; Bapat 1986; Berry 1982; Bezmarevic 2012a; Bezmarevic 2012b; Bezmarevic 2012c; Blum 2001; Boskovic 2014; Brand 2014; Brisinda 1999; Buchler 1986a; Buchler 1986b; Buchler 1986c; Buchler 1987; Bulbuller 2006; Cai 2014; Cardoso 2011; Cardoso 2015; Chen 1992; Chen 2012; Choi 2012; Choi 2013; Chooklin 2010; Cooper 1981; Cravo 1988; d'Eril 2000; Dambrauskas 2010; Dammann 1979; Daniel 2010; de Beaux 1996; De la Pena 1991; Del Prete 2001; Digalakis 2009; Duarte‐Rojo 2009; Ferguson 1990; Fisic 2013; Frasquet 2003; Gao 2014; Garcia‐Cantu 2004; Garcia Lozano 1992; Gelfand 2005; Gross 1990; Guenther 2010; Gurda‐Duda 2008; Gurleyik 2004; Gurleyik 2005; Gvozdenovic 2001; Hamalainen 2002; Han 2011; Hjalmarsson 2009; Huang 2013; Huang 2015; Imamura 2002a; Imamura 2002b; Inagaki 1997; Isenmann 1993; Isogai 1998; Jia 2015; Jiang 2004; Jimenez 2015; Jimin 2015; Kaiyasah 2013; Kaya 2007; Kazda 2002; Khvatova 1973; Khvatova 1977; Kibar 2016; Kim 2013a; Kim 2013b; Kitsanou 2004; Kusnierz‐Cabala 2004; Kylanpaa‐Back 2001a; Kylanpaa‐Back 2001b; Kylanpaa‐Back 2001c; Leese 1987; Leese 1988; Lempinen 1999; Lewandowski 2007; Li 2013; Liang 2014; Lindner 1995; Lobo 1999; Ma 2013; Makay 2003; Makela 2007; Mandi 2000a; Mandi 2000b; Manes 1994; Mantke 2002; Marek 1996; Mayer 1984; Mayer 2002; Melzi D'Eril 2000; Modrau 2005; Modzelewski 2005; Muller 1997; Muller 2000; Nunes 2009; Oezcueruemez‐Porsch 1998; Olah 2005; Omoto 2015; Ostrovskii 2012; Paajanen 1995; Palani 1977; Park 2012; Park 2013; Pezzilli 1994; Pezzilli 1995a; Pezzilli 1995b; Pezzilli 1997; Pezzilli 1998a; Pezzilli 2000; Pongprasobchai 2010; Qiu 2014; Raraty 2002; Rau 1997; Rau 2000; Rau 2007; Ricardo 2011; Riche 2003; Ruzafa 1991; Sanchez‐Lozada 2005; Santotoribio 2015; Sato 2004; Savel'ev 2002; Schaffler 2011; Sharma 2011; Stimac 2010; Stimac 2012; Stimac 2013; Stoelben 1996; Sugumar 2011; Tao 2013; Teerenhovi 1988; Tesinsky 2008; Trunin 1985; Uhl 1991; Uomo 1995; Vaz 2013; Vesentini 1993; Viedma 1992; Viedma 1994; Vlachos 2014; Wei 2013; Wetherill 2012; Wetherill 2013a; Wetherill 2013b; Wilson 1987; Wilson 1988; Wilson 1989a; Woo 2011; Xu 2015; Yadav 2015a; Yadav 2015b; Yasuda 2011; Yin 2014; Yu 2011; Zhu 2013; Zrnic 2007).
Methodological quality of included studies
We have summarised the methodological quality of included studies in Figure 3 and Figure 4.
3.
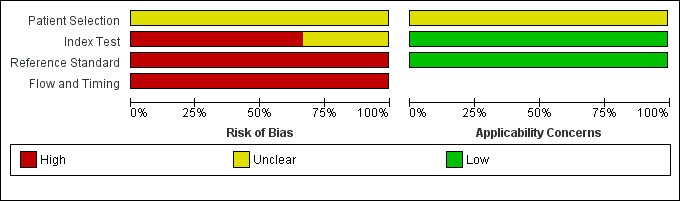
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.
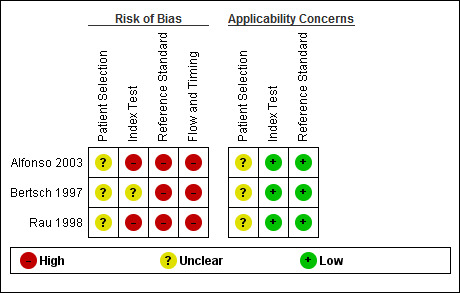
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Participant selection
All studies were at unclear risk of bias in the participant selection domain and were also of unclear concern about applicability in this domain, because none of the included studies mentioned if participants were excluded inappropriately or whether a consecutive or random selection of participants was included.
Index test
Two studies were at high risk of bias in the index test domain, because the thresholds used were not prespecified; it was also unclear whether the index tests were interpreted without knowledge of the reference standard (Alfonso 2003; Rau 1998). One study had an unclear risk of bias, because it was unclear whether the index tests were interpreted without knowledge of the reference standard (Bertsch 1997). However, all studies were of low concern with regards to applicability since all studies reported the threshold at which the diagnosis was made.
Reference standard
All studies were at high risk of bias in the reference standard domain; in two studies the reference standard was CECT alone (Alfonso 2003; Bertsch 1997), and in the third study the reference standard was a combination of CT scan and laparotomy findings (Rau 1998). We considered all of the studies to be low concern with regards to applicability since they all used pancreatic or peripancreatic necrosis, or both as the target condition.
Flow and timing
All studies were at high risk of bias in this domain because the interval between the measurement of the index test and the reference standard in all studies was longer than 24 hours.
Findings
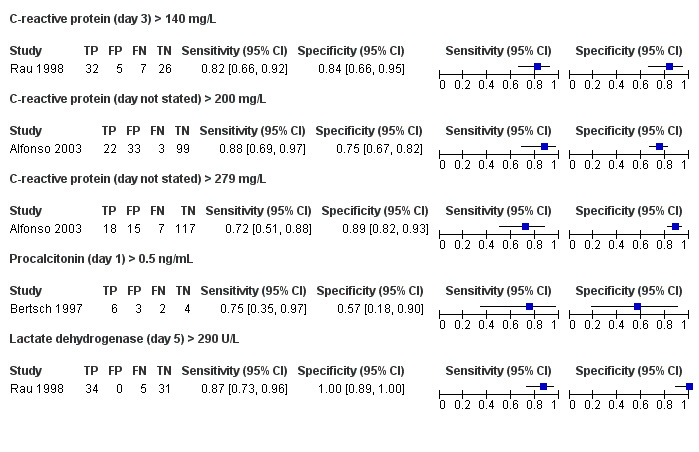
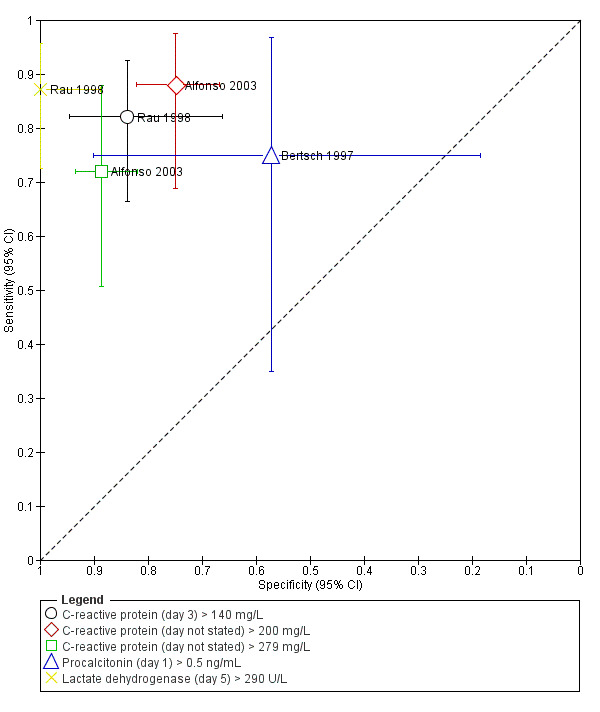
Since the studies reported the tests at different thresholds, we did not perform meta‐analysis. The sensitivities and specificities and their 95% confidence intervals (CI) are visually represented in the forest plots and ROC space in Figure 5 and Figure 6. The sensitivities and specificities are summarised in Table 1. The median pre‐test probability in the studies was 53.3%. The days indicate the number of days after admission that the measurements were made.
5.
Forest plot of tests: 1 C‐reactive protein (day 3) > 140 mg/L; 2 C‐reactive protein (day not stated) > 200 mg/L; 3 C‐reactive protein (day not stated) > 279 mg/L; 4 Procalcitonin (day 1) > 0.5 ng/mL; 5 Lactate dehydrogenase (day 5) > 290 U/L.
6.
Summary ROC plot of tests: 1 C‐reactive protein (day 3) > 140 mg/L; 2 C‐reactive protein (day not stated) > 200 mg/L; 3 C‐reactive protein (day not stated) > 279 mg/L; 4 Procalcitonin (day 1) > 0.5 ng/mL; 5 Lactate dehydrogenase (day 5) > 290 U/L.
C‐reactive protein
Day 3: > 140 mg/L
One study including 70 participants reported the diagnostic accuracy of day 3 CRP at threshold > 140 mg/L (Rau 1998). The sensitivity and specificity of CRP at this threshold was 0.82 (95% CI 0.66 to 0.92) and 0.84 (95% CI 0.66 to 0.95), respectively. The positive and negative likelihood ratios were 5.08 (95% CI 2.25 to 11.50) and 0.21 (95% CI 0.11 to 0.43), respectively. At the pre‐test probability of 53.3%, the post‐test probabilities of pancreatic necrosis of positive and negative tests were 85.3% (95% CI 72.0% to 92.9%) and 19.7% (95% CI 10.9% to 32.7%), respectively.
Day not stated: > 200 mg/L
One study including 157 participants reported the diagnostic accuracy of CRP (day not stated) at threshold > 200 mg/L (Alfonso 2003). The sensitivity and specificity of CRP at this threshold was 0.88 (95% CI 0.69 to 0.97) and 0.75 (95% CI 0.67 to 0.82), respectively. The positive and negative likelihood ratios were 3.52 (95% CI 2.53 to 4.89) and 0.16 (95% CI 0.06 to 0.46), respectively. At the pre‐test probability of 53.3%, the post‐test probabilities of pancreatic necrosis of positive and negative tests were 80.1% (95% CI 74.3% to 84.8%) and 15.5% (95% CI 5.9% to 34.7%), respectively.
Day not stated: > 290 mg/L
One study including 157 participants reported the diagnostic accuracy of CRP (day not stated) at threshold > 290 mg/L (Alfonso 2003). The sensitivity and specificity of CRP at this threshold was 0.72 (95% CI 0.51 to 0.88) and 0.89 (95% CI 0.82 to 0.93), respectively. The positive and negative likelihood ratios were 6.34 (95% CI 3.71 to 10.82) and 0.32 (95% CI 0.17 to 0.59), respectively. At the pre‐test probability of 53.3%, the post‐test probabilities of pancreatic necrosis of positive and negative tests were 87.9% (95% CI 80.9% to 92.5%) and 26.5% (95% CI 16.1% to 40.4%), respectively.
Procalcitonin (day 1 > 0.5 ng/mL)
One study including 15 participants reported the diagnostic accuracy of day 1 procalcitonin at threshold > 0.5 ng/mL (Bertsch 1997). The sensitivity and specificity of procalcitonin at this threshold was 0.75 (95% CI 0.35 to 0.97) and 0.57 (95% CI 0.18 to 0.90), respectively. The positive and negative likelihood ratios were 55.79 (95% CI 3.64 to 856.23) and 0.13 (95% CI 0.06 to 0.29), respectively. At the pre‐test probability of 53.3%, the post‐test probabilities of pancreatic necrosis of positive and negative tests were 66.7% (95% CI 43.8% to 83.7%) and 33.3% (95% CI 11.4% to 66.1%), respectively.
Lactate dehydrogenase (day 5 > 290 U/L)
One study including 70 participants reported the diagnostic accuracy of day 5 LDH at threshold > 0.5 ng/mL (Rau 1998). The sensitivity and specificity of LDH at this threshold was 0.87 (95% CI 0.73 to 0.96) and 1.00 (95% CI 0.89 to 1.00), respectively. The positive and negative likelihood ratios were 1.75 (95% CI 0.68 to 4.50) and 0.44 (95% CI 0.11 to 1.71), respectively. At the pre‐test probability of 53.3%, the post‐test probabilities of pancreatic necrosis of positive and negative tests were 98.5% (95% CI 80.6% to 99.9%) and 12.8% (95% CI 6.1% to 24.9%), respectively.
Investigation of heterogeneity and reporting bias
We did not investigate heterogeneity because of the paucity of data. We did not assess reporting bias since all of the studies were full‐text publications.
Discussion
Summary of main results
Three studies including 242 participants met the inclusion criteria for this review and assessed the diagnostic accuracy of the index tests in participants with established acute pancreatitis (Alfonso 2003; Bertsch 1997; Rau 1998). These three studies reported the diagnostic test accuracy of the index tests at different thresholds and different time points. C‐reactive protein was assessed at three different time points (day 3 and not known for two thresholds), and the point estimate of the sensitivities ranged from 0.72 to 0.88, while the point estimate of the specificities ranged from 0.75 to 0.89. The confidence intervals of the sensitivities ranged from 0.51 to 0.97, and those of the specificities ranged from 0.66 to 0.93. Procalcitonin was assessed on day 1 using a threshold of 0.5 ng/mL, and LDH was assessed on day 5 using a threshold of 290 U/L. The sensitivity and specificity of procalcitonin were 0.75 (95% CI 0.35 to 0.97) and 0.57 (95% CI 0.18 to 0.90), respectively, while the sensitivity and specificity of LDH were 0.87 (95% CI 0.73 to 0.96) and 1.00 (95% CI 0.89 to 1.00), respectively.
Avoiding CECT may be beneficial to the patient, as it avoids unnecessary radiation exposure, particularly if the patient has undergone CECT for the diagnosis of acute pancreatitis. It also benefits the healthcare funder, as it can decrease costs thereby allowing limited resources to be used more appropriately. In addition, patients with acute gallstone pancreatitis may be able to undergo early laparoscopic cholecystectomy, if the patient is stable and acute necrotising pancreatitis can be ruled out early. A triage test to avoid CECT is thus useful. However, such a triage test should have high sensitivity with at least a reasonable specificity. If it has a low specificity, it is not a useful triage test even if it has a very high sensitivity, since one might skip the test altogether and perform CECT directly. The sensitivity of the tests varied and was moderate, with mean sensitivities between 0.75 and 0.89 for all of the tests. This means that these tests can miss about 11% to 25% of people with pancreatic necrosis. To miss 11% to 25% of people with pancreatic necrosis is unacceptable clinically, as patients can be discharged or denied further investigations or intensive treatment. Overall, none of the tests assessed in this review was sufficiently accurate to suggest that they may be useful in clinical practice. In clinical practice, a rising trend is usually considered important rather than a single value, although very high values of CRP or LDH along with organ failure will raise the suspicion of necrotising pancreatitis. However, we were unable to determine the accuracy of a rising trend in CRP or LDH, as none of the studies reported this information.
Strengths and weaknesses of the review
Strengths
One of the main strengths of this review was that the literature was searched thoroughly, without any publication or language restrictions. We did not use any diagnostic test accuracy filters in our literature search because such filters could have led to the exclusion of some relevant studies (Doust 2005). Inclusion of abstracts and non‐English articles may decrease the impact of publication bias to a certain extent, although the determinants and extent of publication bias and selective reporting are not well known for diagnostic accuracy studies. We also planned to exclude case‐control studies because these studies are prone to bias (Whiting 2011). Two review authors (OK and KSG) independently searched the references located by the search to identify relevant studies, screened the full‐text papers against the inclusion criteria, and extracted data. Data extractions by two review authors potentially reduced the chance of errors related to data extraction by a single review author (Buscemi 2006). Another strength of this review was that we used the recommended methodological quality methods to assess the risk of bias and applicability concerns in the included studies and took these into consideration while interpreting the evidence.
Weaknesses
There were several shortcomings in our review. Firstly, the studies included in the review had several methodological deficiencies. The major methodological deficiency was that the two studies that contributed the most participants to this review did not use a prespecified threshold (Alfonso 2003; Rau 1998). In one of these studies it was unclear how the day on which the measurement was performed was determined (Alfonso 2003), while in the other study, the day of measurement was determined by selecting the day (along with the threshold) on which the test had maximum accuracy (Rau 1998). This is likely to overestimate the diagnostic accuracy. In addition, none of the studies reported whether the index tests and reference standards were interpreted independently of each other. If they were not interpreted independently of each other, the accuracy of the tests would have been overestimated. None of the studies reported whether all the participants were included in the study. Exclusion of participants with borderline values close to the threshold used or participants with other causes of elevation of these tests will overestimate the diagnostic test accuracy of these tests.
Secondly, the sample sizes in the studies were small, resulting in wide confidence intervals. It was not possible to perform a meta‐analysis to improve the precision since the studies reported the tests on different days of admission using different thresholds. Additionally, the measurement of CRP on different days using different thresholds for diagnosis of pancreatic necrosis made it impossible for us to explore whether the results could be replicated in another group of participants.
Comparison with other reviews
We did not identify any other systematic reviews on the topic.
Applicability of findings to the review question
Generalisability of the results
The studies did not restrict the participants to specific aetiologies of acute pancreatitis, therefore the findings of this review are applicable to all aetiologies of acute pancreatitis. Although the studies did not specify the restriction of participants to acute severe pancreatitis, it is likely that two studies used these tests in participants with acute severe pancreatitis, since more than 50% of participants in these studies had pancreatic necrosis (Bertsch 1997; Rau 1998). These two studies reported procalcitonin and LDH along with CRP > 140 mg/L (Bertsch 1997; Rau 1998). Consequently, the results of these two studies are applicable to people with severe pancreatitis, while the results from the third study, which reported CRP > 200 mg/L and CRP > 290 mg/L (Alfonso 2003), are applicable to all people with acute pancreatitis.
Use of the test in clinical setting
The main role of the index test is as a triage test to identify people who require further scanning such as CT. Such a test needs to be highly sensitive with at least reasonable specificity, so that it is possible to rule out pancreatic necrosis, which will avoid further testing. The confidence intervals of post‐test probability of a negative test ranged from 1% to 66.1% when the pre‐test probability was 15.9%, and from 5.9% to 66.1% when the pre‐test probability was 53.3%. Adding to the uncertainty due to random errors resulting from small sample sizes, there were many systematic errors, resulting in further uncertainty. Given these uncertainties, the role of these tests in people with acute pancreatitis is not clear.
Authors' conclusions
Implications for practice.
Because of the paucity of data and methodological deficiencies in the studies, it was not possible to arrive at any conclusions regarding the diagnostic test accuracy of C‐reactive protein, procalcitonin, and lactate dehydrogenase.
Implications for research.
Further well‐designed diagnostic test accuracy studies with a prespecified index test threshold of C‐reactive protein, procalcitonin, and lactate dehydrogenase, as well as appropriate follow‐up (for at least two weeks to ensure that the person does not have pancreatic necrosis; early scans may not indicate pancreatic necrosis) and a clearly defined reference standard (of surgical or radiological confirmation of pancreatic necrosis) are important to determine the diagnostic accuracy of C‐reactive protein, procalcitonin, and lactate dehydrogenase reliably.
Acknowledgements
We thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, the United Kingdom Support Unit for Diagnostic Test Accuracy (DTA) Reviews, and the DTA editorial team for their advice in the preparation of this review.
Appendices
Appendix 1. Glossary of terms
Adipose: fat.
Aetiology: cause.
Autodigestion: breaking down of the same organ that secretes the substance.
Debridement: surgical removal of damaged, dead, or infected tissue; in this context, identical with necrosectomy.
Endoscopic: using an endoscope, a flexible tube with a light and camera attached to it to view the inner aspects of the food pipe, stomach, and upper small intestine.
Epigastric: upper central abdomen.
Heterogeneity: differences between studies.
Histological: by examination of the tissue under a microscope.
Hyperamylasaemia: excess amylase in circulation.
Inflammation: localised physical condition in which part of the body becomes reddened, swollen, hot, and often painful, especially as a reaction to injury or infection.
Interstitial: small, narrow spaces between tissues or parts of an organ.
Intraperitoneal: inside the abdominal cavity.
Laparoscopic: key‐hole surgery.
Magnetic resonance cholangiopancreatography: medical imaging technique that uses magnetic resonance imaging (use of magnetic field to differentiate between different structures) to visualise the biliary and pancreatic ducts in a non‐invasive manner.
Necrosectomy: removal of dead tissue.
Necrosis: death and decomposition of living tissue usually caused by lack of blood supply, but can be the result of other pathological insult.
Necrotising: presence of necrosis.
Oedema: swelling.
Oedematous: tissue with an excess of interstitial fluid.
Pancreatic ductal system: tubular system that transports the pancreatic juice secreted by the pancreatic cells to the small intestine.
Pancreatic pseudocysts: fluid collections in the pancreas or the tissues surrounding the pancreas, enclosed by a well‐defined wall and containing only fluid with little or no solid material.
Pancreatitis: inflammation of the pancreas.
Parenchyma: functional parts of an organ.
Paucity: insufficient.
Percutaneous: through the skin.
Percutaneous drainage: drainage carried out by insertion of drain from the external surface of the body, usually guided by an ultrasound or computed tomography (CT) scan.
Peripancreatic tissues: tissues surrounding the pancreas.
Radiating to the back: pain in front going to the back (in this context).
Retroperitoneal: behind the abdominal cavity.
Sphincterotomy: partial division of the sphincter of Oddi, a circular band of muscle at the junction of the biliary tree (tubes that conduct bile from the liver to the small intestine) and pancreatic duct (tubes that conduct pancreatic juice into the second part of the duodenum).
Transabdominal: through the abdominal cavity.
Transluminal: through the lumen (inner cavity of a tubular structure).
Transperitoneal: through the abdominal cavity.
Ultrasonography: using high‐frequency sound to view internal structures of the body (in this context).
Appendix 2. MEDLINE search strategy
1. Pancreatitis, Acute Necrotizing/
2. Pancreatitis/et
3. Pancreas/ab, pa, pp
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea*).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp Amylases/ or exp Lipase/ or exp Trypsinogen/
10. (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia).mp.
11. exp C‐Reactive Protein/
12. ("c‐reactive protein" or "c reactive protein" or CRP).mp.
13. procalcitonin.mp.
14. exp L‐Lactate Dehydrogenase/
15. ("lactate dehydrogenase" or LDH).mp.
16. 9 or 10 or 11 or 12 or 13 or 14 or 15
17. 8 and 16
Appendix 3. Embase search strategy
1. acute hemorrhagic pancreatitis/
2. Pancreatitis/et
3. acute pancreatitis/
4. (acute adj3 pancrea*).mp.
5. (necro* adj3 pancrea*).mp.
6. (inflam* adj3 pancrea*).mp.
7. ((interstitial or edema* or oedema*) adj2 pancrea*).mp.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp amylase/
10. exp triacylglycerol lipase/
11. exp trypsinogen/
12. (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia).mp.
13. exp C reactive protein/
14. ("c‐reactive protein" or "c reactive protein" or CRP).mp.
15. exp procalcitonin/
16. procalcitonin.mp.
17. exp lactate dehydrogenase/
18. ("lactate dehydrogenase" or LDH).mp.
19. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. 8 and 19
Appendix 4. Science Citation Index and Conference Proceedings Citation Index‐Science search strategy
# 1 TS=((acute or necro* or inflam* or interstitial or edema* or oedema*) near/3 pancrea*)
# 2 TS=(amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia or "c‐reactive protein" or "c reactive protein" or CRP or procalcitonin or "lactate dehydrogenase" or LDH)
# 3 #2 AND #1
Appendix 5. National Institute for Health Research ‐ HTA and DARE search strategy
acute pancreatitis
Appendix 6. Zetoc search strategy
Each of the following lines will be searched separately. since the Boolean operator 'or' is not available for searching Zetoc database.
1. acute pancreatitis amylase
2. acute pancreatitis lipase
3. acute pancreatitis trypsinogen
4. acute pancreatitis hyperamylasaemia
5. acute pancreatitis hyperamylasemia
6. acute pancreatitis "c‐reactive protein"
7. acute pancreatitis "c reactive protein"
8. acute pancreatitis CRP
9. acute pancreatitis procalcitonin
10. acute pancreatitis "lactate dehydrogenase"
11. acute pancreatitis LDH
Appendix 7. WHO ICTRP search strategy
Title: (amylase or lipase or trypsinogen or hyperamylasaemia or hyperamylasemia or "c‐reactive protein" or "c reactive protein" or CRP or procalcitonin or "lactate dehydrogenase" or LDH)
Condition: acute pancreatitis
Appendix 8. ClinicalTrials.gov search strategy
amylase OR lipase OR trypsinogen OR hyperamylasaemia OR hyperamylasemia OR "c‐reactive protein" OR "c reactive protein" OR CRP OR procalcitonin OR "lactate dehydrogenase" OR LDH | acute pancreatitis
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 C‐reactive protein (day 3) > 140 mg/L | 1 | 70 |
| 2 C‐reactive protein (day not stated) > 200 mg/L | 1 | 157 |
| 3 C‐reactive protein (day not stated) > 279 mg/L | 1 | 157 |
| 4 Procalcitonin (day 1) > 0.5 ng/mL | 1 | 15 |
| 5 Lactate dehydrogenase (day 5) > 290 U/L | 1 | 70 |
1. Test.

C‐reactive protein (day 3) > 140 mg/L.
2. Test.

C‐reactive protein (day not stated) > 200 mg/L.
3. Test.

C‐reactive protein (day not stated) > 279 mg/L.
4. Test.

Procalcitonin (day 1) > 0.5 ng/mL.
5. Test.

Lactate dehydrogenase (day 5) > 290 U/L.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfonso 2003.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 157. Females: 63 (40.1%). Age: 67 years. Presentation: Participants with acute pancreatitis. Setting: secondary setting in Spain. |
||
| Index tests | Index test: C‐reactive protein (day not stated). Further details: Technical specifications: Nephelometry (Dade Behring Marburg GmbH, Marburg, Germany). Performed by: not stated. Criteria for positive diagnosis: > 200 mg/L and > 279 mg/L. | ||
| Target condition and reference standard(s) | Target condition: pancreatic necrosis. Reference standard: CT scan. |
||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | This study reported the diagnostic test accuracy at 2 threshold levels. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Bertsch 1997.
| Study characteristics | |||
| Patient sampling | Type of study: unclear whether prospective or retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 15. Females: 5 (33.3%). Age: 53 years. Presentation: Participants with acute pancreatitis. Setting: secondary care setting, Germany. | ||
| Index tests | Index test: procalcitonin (day 1). Further details: Technical specifications: a luminometric immunoassay (Fa.Brahms, Berlin). Performed by: not stated. Criteria for positive diagnosis: > 0.5 ng/mL. | ||
| Target condition and reference standard(s) | Target condition: pancreatic necrosis. Reference standard: CT scan. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
Rau 1998.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 70. Females: 31 (44.3%). Age: 49 years. Presentation: Participants with acute pancreatitis (within fewer than 4 days of onset of symptoms). Setting: secondary care setting, Germany. | ||
| Index tests | Index test: C‐reactive protein (day 3).
Further details:
Technical specifications: laser nephelometry.
Performed by: not stated.
Criteria for positive diagnosis: > 140 mg/L. Index test: lactate dehydrogenase (day 5). Further details: Technical specifications: enzyme kinetic method. Performed by: not stated. Criteria for positive diagnosis: > 290 U/L. |
||
| Target condition and reference standard(s) | Target condition: pancreatic necrosis. Reference standard: CT scan or intra‐operative findings, or both. Further details: Technical specifications: CT scan: CT 9800 (General Electric) and CT Twin Flash (Elscint); Surgery: not applicable. Performed by: not stated. Criteria for positive diagnosis: not stated. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard were available: 0 (0%). Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | This study reported 2 index tests. Authors provided additional information that the index tests were interpreted without knowledge of reference standards. The authors also stated that the interval between the index tests and CT scan was 2 to 6 days, and the interval between index tests and laparotomy was 18 days. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Unclear | ||
| High | |||
CT: computed tomography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abishek 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Aggelopoulos 1996 | No diagnostic accuracy data on pancreatic necrosis |
| Ammori 2003 | No diagnostic accuracy data on pancreatic necrosis |
| Appasani 2011a | No diagnostic accuracy data on pancreatic necrosis |
| Appasani 2011b | No diagnostic accuracy data on pancreatic necrosis |
| Appasani 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Bajec 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Bapat 1986 | No diagnostic accuracy data on pancreatic necrosis |
| Barauskas 2004 | Inadequate reference standard |
| Bassi 1994 | Not a primary study (review) |
| Beger 1989 | Not a primary study (letter to editor) |
| Berry 1982 | No diagnostic accuracy data on pancreatic necrosis |
| Bezmarevic 2012a | No diagnostic accuracy data on pancreatic necrosis |
| Bezmarevic 2012b | No diagnostic accuracy data on pancreatic necrosis |
| Bezmarevic 2012c | No diagnostic accuracy data on pancreatic necrosis |
| Bihari 2004 | Not a primary study (letter to editor) |
| Blum 2001 | No diagnostic accuracy data on pancreatic necrosis |
| Boskovic 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Brailski 1975 | Not a primary study (review) |
| Brand 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Brisinda 1999 | No diagnostic accuracy data on pancreatic necrosis |
| Buchler 1986a | No diagnostic accuracy data on pancreatic necrosis |
| Buchler 1986b | No diagnostic accuracy data on pancreatic necrosis |
| Buchler 1986c | No diagnostic accuracy data on pancreatic necrosis |
| Buchler 1987 | No diagnostic accuracy data on pancreatic necrosis |
| Buchler 1991 | Not a primary study (review) |
| Bulbuller 2006 | No diagnostic accuracy data on pancreatic necrosis |
| Cai 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Cardoso 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Cardoso 2013 | Inadequate reference standard |
| Cardoso 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Chen 1992 | No diagnostic accuracy data on pancreatic necrosis |
| Chen 2004 | Not a primary study (editorial) |
| Chen 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Choi 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Choi 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Chooklin 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Choudhary 2012 | Not a primary study (letter to editor) |
| Cooper 1981 | No diagnostic accuracy data on pancreatic necrosis |
| Cravo 1988 | No diagnostic accuracy data on pancreatic necrosis |
| d'Eril 2000 | No diagnostic accuracy data on pancreatic necrosis |
| Dambrauskas 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Dammann 1979 | No diagnostic accuracy data on pancreatic necrosis |
| Daniel 2010 | No diagnostic accuracy data on pancreatic necrosis |
| de Beaux 1996 | No diagnostic accuracy data on pancreatic necrosis |
| De la Pena 1991 | No diagnostic accuracy data on pancreatic necrosis |
| Del Prete 2001 | No diagnostic accuracy data on pancreatic necrosis |
| Digalakis 2009 | No diagnostic accuracy data on pancreatic necrosis |
| Duarte‐Rojo 2009 | No diagnostic accuracy data on pancreatic necrosis |
| Economou 1997 | Not a primary study (letter to editor) |
| Fan 1994 | Not a primary study (editorial) |
| Ferguson 1990 | No diagnostic accuracy data on pancreatic necrosis |
| Fisic 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Folch‐Puy 2007 | Not a primary study (editorial) |
| Frasquet 2003 | No diagnostic accuracy data on pancreatic necrosis |
| Frossard 2001 | Not a primary study (review) |
| Gao 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Garcia Lozano 1992 | No diagnostic accuracy data on pancreatic necrosis |
| Garcia‐Cantu 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Gelfand 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Geng 2014 | Not a primary study (review) |
| Gluskina 1967 | Inadequate reference standard |
| Gosling 1992 | Not a primary study (editorial) |
| Gross 1990 | No diagnostic accuracy data on pancreatic necrosis |
| Guenther 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Gurda‐Duda 2008 | No diagnostic accuracy data on pancreatic necrosis |
| Gurleyik 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Gurleyik 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Gvozdenovic 2001 | No diagnostic accuracy data on pancreatic necrosis |
| Hamalainen 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Han 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Hjalmarsson 2009 | No diagnostic accuracy data on pancreatic necrosis |
| Huang 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Huang 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Imamura 2002a | No diagnostic accuracy data on pancreatic necrosis |
| Imamura 2002b | No diagnostic accuracy data on pancreatic necrosis |
| Inagaki 1997 | No diagnostic accuracy data on pancreatic necrosis |
| Isenmann 1993 | No diagnostic accuracy data on pancreatic necrosis |
| Isogai 1998 | No diagnostic accuracy data on pancreatic necrosis |
| Jia 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Jiang 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Jimenez 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Jimin 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Johnson 2003 | Not a primary study (review) |
| Kaiyasah 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Kaya 2007 | No diagnostic accuracy data on pancreatic necrosis |
| Kazda 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Khanna 2013 | Inadequate reference standard |
| Khvatova 1973 | No diagnostic accuracy data on pancreatic necrosis |
| Khvatova 1977 | No diagnostic accuracy data on pancreatic necrosis |
| Kibar 2016 | No diagnostic accuracy data on pancreatic necrosis |
| Kim 2013a | No diagnostic accuracy data on pancreatic necrosis |
| Kim 2013b | No diagnostic accuracy data on pancreatic necrosis |
| Kitsanou 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Korczowski 2006 | Not a primary study (review) |
| Kusnierz‐Cabala 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Kylanpaa‐Back 2001a | No diagnostic accuracy data on pancreatic necrosis |
| Kylanpaa‐Back 2001b | No diagnostic accuracy data on pancreatic necrosis |
| Kylanpaa‐Back 2001c | No diagnostic accuracy data on pancreatic necrosis |
| Leese 1987 | No diagnostic accuracy data on pancreatic necrosis |
| Leese 1988 | No diagnostic accuracy data on pancreatic necrosis |
| Lempinen 1999 | No diagnostic accuracy data on pancreatic necrosis |
| Lempinen 2005 | Not a primary study (review) |
| Lewandowski 2007 | No diagnostic accuracy data on pancreatic necrosis |
| Li 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Liang 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Lindner 1995 | No diagnostic accuracy data on pancreatic necrosis |
| Lipsett 2001 | Not a primary study (editorial) |
| Liu 2008 | Not a primary study (review) |
| Lobo 1999 | No diagnostic accuracy data on pancreatic necrosis |
| Lott 1991 | Not a primary study (editorial) |
| Ma 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Machiedo 1974 | Inappropriate population |
| Makay 2003 | No diagnostic accuracy data on pancreatic necrosis |
| Makela 2007 | No diagnostic accuracy data on pancreatic necrosis |
| Malfertheiner 1993 | Not a primary study (review) |
| Manabe 2004 | Not a primary study (editorial) |
| Mandi 2000a | No diagnostic accuracy data on pancreatic necrosis |
| Mandi 2000b | No diagnostic accuracy data on pancreatic necrosis |
| Manes 1994 | No diagnostic accuracy data on pancreatic necrosis |
| Mantke 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Marek 1996 | No diagnostic accuracy data on pancreatic necrosis |
| Mayer 1984 | No diagnostic accuracy data on pancreatic necrosis |
| Mayer 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Melzi D'Eril 2000 | No diagnostic accuracy data on pancreatic necrosis |
| Millat 1999 | Not a primary study (review) |
| Modrau 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Modzelewski 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Mulholland 1996 | Not a primary study (review) |
| Muller 1997 | No diagnostic accuracy data on pancreatic necrosis |
| Muller 2000 | No diagnostic accuracy data on pancreatic necrosis |
| Neoptolemos 2001 | Not a primary study (letter to editor) |
| Nunes 2009 | No diagnostic accuracy data on pancreatic necrosis |
| Oezcueruemez‐Porsch 1998 | No diagnostic accuracy data on pancreatic necrosis |
| Olah 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Omoto 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Ostrovskii 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Paajanen 1995 | No diagnostic accuracy data on pancreatic necrosis |
| Palani 1977 | No diagnostic accuracy data on pancreatic necrosis |
| Pallisera 2014 | Inadequate reference standard |
| Park 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Park 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Petrov 2011 | Not a primary study (editorial) |
| Pezzilli 1994 | No diagnostic accuracy data on pancreatic necrosis |
| Pezzilli 1995a | No diagnostic accuracy data on pancreatic necrosis |
| Pezzilli 1995b | No diagnostic accuracy data on pancreatic necrosis |
| Pezzilli 1997 | No diagnostic accuracy data on pancreatic necrosis |
| Pezzilli 1998a | No diagnostic accuracy data on pancreatic necrosis |
| Pezzilli 1998b | Inappropriate index test |
| Pezzilli 2000 | No diagnostic accuracy data on pancreatic necrosis |
| Pongprasobchai 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Puolakkainen 1987 | Inappropriate reference standards |
| Purkayastha 2006 | Not a primary study (review) |
| Qiu 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Raraty 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Rau 1997 | No diagnostic accuracy data on pancreatic necrosis |
| Rau 2000 | No diagnostic accuracy data on pancreatic necrosis |
| Rau 2004 | Not a primary study (review) |
| Rau 2007 | No diagnostic accuracy data on pancreatic necrosis |
| Ricardo 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Riche 2003 | No diagnostic accuracy data on pancreatic necrosis |
| Ruzafa 1991 | No diagnostic accuracy data on pancreatic necrosis |
| Samso 2002 | Not a primary study (editorial) |
| Sanchez‐Lozada 2005 | No diagnostic accuracy data on pancreatic necrosis |
| Santotoribio 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Sato 2004 | No diagnostic accuracy data on pancreatic necrosis |
| Savel'ev 2002 | No diagnostic accuracy data on pancreatic necrosis |
| Schaffler 2010 | inadequate reference standard |
| Schaffler 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Sharma 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Stimac 2010 | No diagnostic accuracy data on pancreatic necrosis |
| Stimac 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Stimac 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Stoelben 1996 | No diagnostic accuracy data on pancreatic necrosis |
| Sugumar 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Tao 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Teerenhovi 1988 | No diagnostic accuracy data on pancreatic necrosis |
| Tesinsky 2008 | No diagnostic accuracy data on pancreatic necrosis |
| Trunin 1985 | No diagnostic accuracy data on pancreatic necrosis |
| Uhl 1991 | No diagnostic accuracy data on pancreatic necrosis |
| Uomo 1995 | No diagnostic accuracy data on pancreatic necrosis |
| Vaz 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Vesentini 1993 | No diagnostic accuracy data on pancreatic necrosis |
| Viedma 1992 | No diagnostic accuracy data on pancreatic necrosis |
| Viedma 1994 | No diagnostic accuracy data on pancreatic necrosis |
| Vlachos 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Wei 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Wetherill 2012 | No diagnostic accuracy data on pancreatic necrosis |
| Wetherill 2013a | No diagnostic accuracy data on pancreatic necrosis |
| Wetherill 2013b | No diagnostic accuracy data on pancreatic necrosis |
| Wilson 1987 | No diagnostic accuracy data on pancreatic necrosis |
| Wilson 1988 | No diagnostic accuracy data on pancreatic necrosis |
| Wilson 1989a | No diagnostic accuracy data on pancreatic necrosis |
| Wilson 1989b | Not a primary study (letter to editor) |
| Wong 1993 | Case reports |
| Woo 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Xu 2015 | No diagnostic accuracy data on pancreatic necrosis |
| Yadav 2015a | No diagnostic accuracy data on pancreatic necrosis |
| Yadav 2015b | No diagnostic accuracy data on pancreatic necrosis |
| Yasuda 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Yin 2014 | No diagnostic accuracy data on pancreatic necrosis |
| Yu 2011 | No diagnostic accuracy data on pancreatic necrosis |
| Zhu 2013 | No diagnostic accuracy data on pancreatic necrosis |
| Zrnic 2007 | No diagnostic accuracy data on pancreatic necrosis |
Characteristics of studies awaiting classification [ordered by study ID]
Djurasinovic 2013.
| Study characteristics | |||
| Patient sampling | Unable to obtain full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Grenier 1968.
| Study characteristics | |||
| Patient sampling | Unable to obtain full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Issekutz 2003.
| Study characteristics | |||
| Patient sampling | Unable to obtain full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Pindak 2003.
| Study characteristics | |||
| Patient sampling | Unable to obtain full text | ||
| Patient characteristics and setting | |||
| Index tests | |||
| Target condition and reference standard(s) | |||
| Flow and timing | |||
| Comparative | |||
| Notes | |||
Differences between protocol and review
As per protocol, we accepted any of the following reference standards, used alone or in combination: radiological features of pancreatic necrosis (contrast‐enhanced computed tomography or magnetic resonance imaging) or histological confirmation of pancreatic necrosis. In addition, we also included a combination of radiological features of pancreatic necrosis (contrast‐enhanced computed tomography or magnetic resonance imaging) and surgeon's judgement of pancreatic necrosis during surgery, as we considered this equivalent to radiologist judgement of the presence of pancreatic necrosis on radiology.
Contributions of authors
Oluyemi Komolafe wrote the first draft of the review.
Kurinchi Selvan Gurusamy wrote the protocol and revised the review.
Stephen P Pereira and Brian R Davidson critically commented on the review.
Sources of support
Internal sources
University College London, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
OK: none known.
SPP: none known.
BRD: none known.
KSG: none known.
New
References
References to studies included in this review
Alfonso 2003 {published and unpublished data}
- Alfonso V, Gomez F, Lopez A, Moreno‐Osset E, Valle R, Anton MD, et al. C‐reactive protein level in the detection of necrosis in acute pancreatitis. Gastroenterologia y Hepatologia 2003;26(5):288‐93. [DOI] [PubMed] [Google Scholar]
Bertsch 1997 {published data only}
- Bertsch T, Richter A, Hofheinz H, Bohm C, Hartel M, Aufenanger J. Procalcitonin ‐ a new marker for the acute‐phase reaction in acute pancreatitis. Langenbecks Archiv fur Chirurgie 1997;382(6):367‐72. [DOI] [PubMed] [Google Scholar]
Rau 1998 {published data only}
- Rau B, Cebulla M, Uhl W, Schoenberg MH, Beger HG. The clinical value of human pancreas‐specific protein procarboxypeptidase B as an indicator of necrosis in acute pancreatitis : Comparison to CRP and LDH. Pancreas 1998;17(2):134‐9. [DOI] [PubMed] [Google Scholar]
- Rau B, Steinbach G, Baumgart K, Gansauge F, Grunert A, Beger HG. Serum amyloid A versus C‐reactive protein in acute pancreatitis: Clinical value of an alternative acute‐phase reactant. Critical Care Medicine 2000;28(3):736‐42. [DOI] [PubMed] [Google Scholar]
- Rau B, Steinbach G, Gansauge F, Mayer JM, Grunert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut 1997;41(6):832‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abishek 2014 {published data only}
- Abishek V, Babu V, Muthukumaran, Ramkumar, Balamurali, Murali A, et al. Role of C‐reactive protein in predicting severity of acute pancreatitis. Indian Journal of Gastroenterology 2014;33(Suppl 1):A92. [Google Scholar]
Aggelopoulos 1996 {published data only}
- Aggelopoulos C, Aloizos S, Touliatou A, Papanastasopoulos C, Tsaglis B, Papanastasopoulous B. APACHE III and CRP as early predictive indexes in acute pancreatitis. HPB Surgery 1996;9(Supp 2):121. [P200] [Google Scholar]
Ammori 2003 {published data only}
- Ammori BJ, Becker KL, Kite P, Snider RH, Nylen ES, White JC, et al. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. British Journal of Surgery 2003;90(2):197‐204. [DOI] [PubMed] [Google Scholar]
Appasani 2011a {published data only}
- Appasani S, Thandassery RB, Dixit P, Yadav TD, Attri SV, Rana SV, et al. Serum ADA levels in predicting the severity in acute pancreatitis. Indian Journal of Gastroenterology 2011;1:A87. [Google Scholar]
Appasani 2011b {published data only}
- Appasani S, Thandassery RB, Varma N, Yadav TD, Attri SV, Rana SV, et al. D‐dimer as a single marker for early prediction of severity, necrosis, organ failure and mortality in acute pancreatitis. Indian Journal of Gastroenterology 2011;1:A12. [Google Scholar]
Appasani 2012 {published data only}
- Appasani S, Rana S, Babu Thandassery R, Basha J, Yadav TD, Attri SV, et al. Serum ADA levels in predicting the severity in acute pancreatitis. Journal of Gastroenterology and Hepatology 2012;27:374‐5. [Google Scholar]
Bajec 2010 {published data only}
- Bajec D, Gunjic D, Radenkovic D, Gregoric P, Karadzic B, Pejovic I. Evaluation of Glasgow and APACHE II scores and CRP in prediction of severity of acute pancreatitis. Pancreatology 2010;10(2‐3):349. [Google Scholar]
Bapat 1986 {published data only}
- Bapat RD, Nazareth HM, Kulkarni AG, Shah AB. Prognostic markers in acute pancreatitis ‐ a prospective study. Indian Journal of Gastroenterology 1986;5(2):113‐5. [PubMed] [Google Scholar]
Barauskas 2004 {published data only}
- Barauskas G, Svagzdys S, Maleckas A. C‐reactive protein in early prediction of pancreatic necrosis. Medicina 2004;40(2):135‐40. [PubMed] [Google Scholar]
Bassi 1994 {published data only}
- Bassi C. Infected pancreatic necrosis. International Journal of Pancreatology 1994;16(1):1‐10. [DOI] [PubMed] [Google Scholar]
Beger 1989 {published data only}
- Beger HG, Buchler M. C‐reactive protein and acute pancreatitis. British Journal of Surgery 1989;76(8):877. [DOI] [PubMed] [Google Scholar]
Berry 1982 {published data only}
- Berry AR, Taylor TV, Davies GC. Diagnostic tests and prognostic indicators in acute pancreatitis. Journal of the Royal College of Surgeons of Edinburgh 1982;27(6):345‐52. [PubMed] [Google Scholar]
Bezmarevic 2012a {published data only}
- Bezmarevic M, Kostic Z, Jovanovic M, Mickovic S, Mirkovic D, Soldatovic I, et al. Procalcitonin and BISAP score versus C‐reactive protein and APACHE II score in early assessment of severity and outcome of acute pancreatitis. Vojnosanitetski Pregled 2012;69(5):425‐31. [PubMed] [Google Scholar]
Bezmarevic 2012b {published data only}
- Bezmarevic M, Mirkovic D, Mickovic S, Perisic N. BISAP score and procalcitonin versus APACHE II score and C‐reactive protein in early assessment of the severity and outcome of acute pancreatitis. Pancreatology 2012;12(6):534. [PubMed] [Google Scholar]
Bezmarevic 2012c {published data only}
- Bezmarevic M, Mirkovic D, Soldatovic I, Stamenkovic D, Mitrovic N, Perisic N, et al. Correlation between procalcitonin and intra‐abdominal pressure and their role in prediction of the severity of acute pancreatitis. Pancreatology 2012;12(4):337‐43. [DOI] [PubMed] [Google Scholar]
Bihari 2004 {published data only}
- Bihari D. Monitoring procalcitonin is of value in acute pancreatitis. BMJ 2004;329(7459):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blum 2001 {published data only}
- Blum T, Maisonneuve P, Lowenfels AB, Lankisch PG. Fatal outcome in acute pancreatitis: Its occurrence and early prediction. Pancreatology 2001;1(3):237‐41. [DOI] [PubMed] [Google Scholar]
Boskovic 2014 {published data only}
- Boskovic A, Pasic S, Soldatovic I, Milinic N, Stankovic I. The role of D‐dimer in prediction of the course and outcome in paediatric acute pancreatitis. Pancreatology 2014;14(5):330‐4. [DOI] [PubMed] [Google Scholar]
Brailski 1975 {published data only}
- Brailski KH. Acute pancreatitis (etiology, pathogenesis and pathobiochemistry). Vutreshni Bolesti 1975;14(4):1‐11. [PubMed] [Google Scholar]
Brand 2014 {published data only}
- Brand M, Gotz A, Zeman F, Behrens G, Leitzmann M, Brunnler T, et al. Acute necrotizing pancreatitis: Laboratory, clinical, and imaging findings as predictors of patient outcome. American Journal of Roentgenology 2014;202(6):1215‐31. [DOI] [PubMed] [Google Scholar]
Brisinda 1999 {published data only}
- Brisinda G, Maria G, Ferrante A, Civello IM. Evaluation of prognostic factors in patients with acute pancreatitis. Hepato‐Gastroenterology 1999;46(27):1990‐7. [PubMed] [Google Scholar]
Buchler 1986a {published data only}
- Buchler M, Malfertheiner P, Schoetensack C, Uhl W, Beger HG. Sensitivity of antiproteases, complement factors and C‐reactive protein in detecting pancreatic necrosis ‐ results of a prospective clinical‐study. International Journal of Pancreatology 1986;1(3‐4):227‐35. [DOI] [PubMed] [Google Scholar]
Buchler 1986b {published data only}
- Buchler M, Malfertheiner P, Schoetensack C, Uhl W, Scherbaum W, Beger HG. Value of biochemical and imaging procedures for the diagnosis and prognosis of acute pancreatitis ‐ results of a prospective clinical study. Zeitschrift Fur Gastroenterologie 1986;24(2):100‐9. [PubMed] [Google Scholar]
Buchler 1986c {published data only}
- Buchler M, Malfertheiner P, Uhl W, Beger HG. A new staging system in patients with acute‐pancreatitis using serum alpha‐2‐macroglobulin and C‐reactive protein. Gastroenterology 1986;90(5):1361. [Google Scholar]
Buchler 1987 {published data only}
- Buchler M, Malfertheiner P, Uhl W, Beger HG. C‐reactive protein as a marker of inflammation and necrosis in gastroenterology. Medizinische Klinik 1987;82(5):180‐5. [PubMed] [Google Scholar]
Buchler 1991 {published data only}
- Buchler M. Objectification of the severity of acute‐pancreatitis. Hepato‐Gastroenterology 1991;38(2):101‐8. [PubMed] [Google Scholar]
Bulbuller 2006 {published data only}
- Bulbuller N, Dogru O, Ayten R, Akbulut H, Ilhan YS, Cetinkaya Z. Procalcitonin is a predictive marker for severe acute pancreatitis. Ulusal Travma ve Acil Cerrahi Dergisi [Turkish Journal of Trauma & Emergency Surgery: TJTES] 2006;12(2):115‐20. [PubMed] [Google Scholar]
Cai 2014 {published data only}
- Cai YY, Ee J, Liew YX, Lee WN, Chlebicki MP, Goh YC, et al. A procalcitonin‐based guideline promotes shorter duration of antibiotic use safely in acute pancreatitis. Journal of Infection 2014;69(4):412‐5. [DOI] [PubMed] [Google Scholar]
Cardoso 2011 {published data only}
- Cardoso F, Ricardo L, Oliveira A, Rodrigues C, Horta D, Figueiredo A, et al. Value of C‐reactive protein in predicting organ failure, complications and mortality in acute pancreatitis. Pancreas 2011;40(8):1315. [Google Scholar]
Cardoso 2013 {published data only}
- Cardoso FS, Ricardo LB, Oliveira AM, Canena JM, Horta DV, Papoila AL, et al. C‐reactive protein prognostic accuracy in acute pancreatitis: Timing of measurement and cutoff points. European Journal of Gastroenterology & Hepatology 2013;25(7):784‐9. [DOI] [PubMed] [Google Scholar]
Cardoso 2015 {published data only}
- Cardoso FS, Ricardo L, Gondar P, Deus JR, Horta D. C‐reactive protein may influence decisively the prescription of prophylactic antibiotics in acute pancreatitis: a population‐based cohort study. Pancreas 2015;44(3):404‐8. [DOI] [PubMed] [Google Scholar]
Chen 1992 {published data only}
- Chen CC, Wang SS, Chao Y, Lu CW, Lee SD, Tsal YT, et al. C‐reactive protein and lactate‐dehydrogenase isoenzymes in the assessment of the prognosis of acute‐pancreatitis. Journal of Gastroenterology and Hepatology 1992;7(4):363‐6. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen CC. Serum markers in the early assessment of severity of acute pancreatitis: Which is the most useful?. Journal of the Chinese Medical Association: JCMA 2004;67(9):439‐41. [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen D, Lu S, Xu F. Study for the value of serum lactate dehydrogenase in predicting multiple organ dysfunction syndrome caused by acute pancreatitis. Chinese Journal of Critical Care Medicine 2012;32(4):349‐51. [Google Scholar]
Choi 2012 {published data only}
- Choi JS, Cho JH, Jeon TJ, Kim HM, Kim YJ, Han KJ, et al. Prognostic value of procalcitonin, CRP, BISAP, RANSON'S, APACHE II and CTSI scores according to the various etiologies of acute pancreatitis. Pancreas 2012;41(8):1353. [Google Scholar]
Choi 2013 {published data only}
- Choi JS, Cho JH, Jeon TJ, Kim HM, Kim YJ, Han KJ, et al. Prognostic value of procalcitonin, CRP, BISAP RANSON'S, APACHE II and CTSI scores according to the various etiologies of acute pancreatitis. Pancreatology 2013;13(2):e20. [Google Scholar]
Chooklin 2010 {published data only}
- Chooklin S, Lyba M, Bihalskyy I. Pancreatic necrosis markers in severe acute pancreatitis. European Journal of Clinical Investigation 2010;40:14. [Google Scholar]
Choudhary 2012 {published data only}
- Choudhary RK, Choudhary M. A useful diagnostic and prognostic tool for acute appendicitis and acute pancreatitis. BMJ 2012;344:e1404; author reply e9. [DOI] [PubMed] [Google Scholar]
Cooper 1981 {published data only}
- Cooper MJ, Whicher JT, Walters G, Williamson RCN. Predictive value of C‐reactive protein and complement in acute‐pancreatitis. Gastroenterologie Clinique Et Biologique 1981;5(10):920‐1. [Google Scholar]
Cravo 1988 {published data only}
- Cravo M, Santos P, Marques A, Deus J, Santos ML, Geada H, et al. Antiproteases and C‐reactive protein in the early assessment of severity of acute‐pancreatitis. Digestion 1988;40(2):74. [Google Scholar]
d'Eril 2000 {published data only}
- d'Eril GM, Merlini G, Finazzi S, Bosoni T, Barakat B, Pezzilli R. Procalcitonin is not a reliable marker for the assessment of severity in acute pancreatitis without infectious complications. Clinical Chemistry 2000;46(3):428‐30. [PubMed] [Google Scholar]
Dambrauskas 2010 {published data only}
- Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scandinavian Journal of Gastroenterology 2010;45(7‐8):959‐70. [DOI] [PubMed] [Google Scholar]
Dammann 1979 {published data only}
- Dammann HG, Wichert P, Schreiber HW. Prognostic indices in acute pancreatitis. Zentralblatt Fur Chirurgie 1979;104(6):397‐404. [PubMed] [Google Scholar]
Daniel 2010 {published data only}
- Daniel P, Lesniowski B, Mokrowiecka A, Jasinska A, Pietruczuk M, Malecka‐Panas E. Circulating levels of Visfatin, Resistin and pro‐inflammatory cytokine Interleukin‐8 in acute pancreatitis. Pancreatology 2010;10(4):477‐82. [DOI] [PubMed] [Google Scholar]
de Beaux 1996 {published data only}
- Beaux AC, Goldie AS, Ross JA, Carter DC, Fearon KC. Serum concentrations of inflammatory mediators related to organ failure in patients with acute pancreatitis. British Journal of Surgery 1996;83(3):349‐53. [DOI] [PubMed] [Google Scholar]
De la Pena 1991 {published data only}
- Pena J, las Heras G, Galo Peralta F, Casafont F, Pons Romero F. Prospective study of the prognostic value of C reactive protein, alpha 1‐antitrypsin and alpha 1‐acid glycoprotein in acute pancreatitis. Revista Espanola de Enfermedades Digestivas 1991;79(5):337‐40. [PubMed] [Google Scholar]
Del Prete 2001 {published data only}
- Prete M, Castiglia D, Meli M, Perri S, Nicita A, Dalla Torre A, et al. Prognostic value of C reactive protein in acute pancreatitis. Chirurgia Italiana 2001;53(1):33‐8. [PubMed] [Google Scholar]
Digalakis 2009 {published data only}
- Digalakis MK, Katsoulis IE, Biliri K, Themeli‐Digalaki K. Serum profiles of C‐reactive protein, interleukin‐8, and tumor necrosis factor‐alpha in patients with acute pancreatitis. HPB Surgery 2009;2009:878490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duarte‐Rojo 2009 {published data only}
- Duarte‐Rojo A, Suazo‐Barahona J, Ramirez‐Iglesias MT, Uscanga LF, Robles‐Diaz G. Time frames for analysis of inflammatory mediators in acute pancreatitis: Improving admission triage. Digestive Diseases and Sciences 2009;54(10):2282‐7. [DOI] [PubMed] [Google Scholar]
Economou 1997 {published data only}
- Economou MS. The potential role of procalcitonin and interleukin‐8 in the prediction of infected necrosis in acute pancreatitis. Hellenic Journal of Gastroenterology 1997;10(3):248‐9. [Google Scholar]
Fan 1994 {published data only}
- Fan ST. Early recognition of severe acute‐pancreatitis. Journal of Gastroenterology and Hepatology 1994;9(3):289‐90. [DOI] [PubMed] [Google Scholar]
Ferguson 1990 {published data only}
- Ferguson CM, Bradley EL. Can markers for pancreatic necrosis be used as indicators for surgery?. American Journal of Surgery 1990;160(5):459‐61. [DOI] [PubMed] [Google Scholar]
Fisic 2013 {published data only}
- Fisic E, Poropat G, Bilic‐Zulle L, Licul V, Milic S, Stimac D. The role of IL‐6, 8, and 10, sTNFr, CRP, and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Gastroenterology Research and Practice 2013;2013:282645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Folch‐Puy 2007 {published data only}
- Folch‐Puy E. Markers of severity in acute pancreatitis. Medicina Clinica 2007;128(11):417‐8. [DOI] [PubMed] [Google Scholar]
Frasquet 2003 {published data only}
- Frasquet J, Saez J, Trigo C, Martinez J, Such J, Perez‐Mateo M. Early measurement of procalcitonin does not predict severity in patients with acute pancreatitis. British Journal of Surgery 2003;90(9):1129‐30. [DOI] [PubMed] [Google Scholar]
Frossard 2001 {published data only}
- Frossard JL, Hadengue A, Pastor CM. New serum markers for the detection of severe acute pancreatitis in humans. American Journal of Respiratory and Critical Care Medicine 2001;164(1):162‐70. [DOI] [PubMed] [Google Scholar]
Gao 2014 {published data only}
- Gao K, Zhang LJ, Wang GY, Li J, Zhang H. Role of serum IL‐6, IL‐8 and procalcitonin in diagnosis of secondary infection in severe acute pancreatitis. World Chinese Journal of Digestology 2014;22(16):2343‐6. [Google Scholar]
Garcia‐Cantu 2004 {published data only}
- Garcia‐Cantu DA, Gonzalez‐Gonzalez JA, Dominguez NR, Perez DOR, Maldonado‐Garza HJJ. A simple score plus serum C‐reactive protein at admission increase the positive predictive value for organ failure in severe acute pancreatitis. A prospective trial. Gastroenterology 2004;126(4):A227. [Google Scholar]
Garcia Lozano 1992 {published data only}
- Garcia Lozano A, Garcia‐Villanova J, Martin L, Martinez D, Mansilla D, Rodenas V, et al. The prediction of the severity of acute pancreatitis by C‐reactive protein. Revista Espanola de Enfermedades Digestivas 1992;82(4):231‐3. [PubMed] [Google Scholar]
Gelfand 2005 {published data only}
- Gelfand B, Filimonov M, Burnevich S, Brazhnik T, Sergeyeva N. The procalcitonin test in the evaluation of clinical severity in patients with acute pancreatitis. Critical Care 2005;9(Supp 1):P171. [Google Scholar]
Geng 2014 {published data only}
- Geng P, Ding Y. Advances in studies on procalcitonin and acute pancreatitis. Chinese Journal of Gastroenterology 2014;19(7):439‐41. [Google Scholar]
Gluskina 1967 {published data only}
- Gluskina VM. On the differential diagnosis of edema and hemorrhagic necrosis of the pancreas. Vestnik Khirurgii Imeni I. I. Grekova 1967;99(9):69‐72. [PubMed] [Google Scholar]
Gosling 1992 {published data only}
- Gosling P, Shearman CP. Can we predict disease severity in acute‐pancreatitis. Annals of Clinical Biochemistry 1992;29:9‐10. [DOI] [PubMed] [Google Scholar]
Gross 1990 {published data only}
- Gross V, Scholmerich J, Leser HG, Salm R, Lausen M, Ruckauer K, et al. Granulocyte elastase in assessment of severity of acute‐pancreatitis ‐ comparison with acute‐phase proteins C‐reactive protein, alpha‐1‐antitrypsin, and protease inhibitor alpha‐2‐macroglobulin. Digestive Diseases and Sciences 1990;35(1):97‐105. [DOI] [PubMed] [Google Scholar]
Guenther 2010 {published data only}
- Guenther A, Aghdassi AA, Nitsche CJ, Schulte J, Struck J, Lerch MM, et al. Comparison of peroxiredoxin‐4 with CRP as a severity marker for acute pancreatitis. Pancreatology 2010;10(2‐3):345. [Google Scholar]
Gurda‐Duda 2008 {published data only}
- Gurda‐Duda A, Kusnierz‐Cabala B, Nowak W, Naskalski JW, Kulig J. Assessment of the prognostic value of certain acute‐phase proteins and procalcitonin in the prognosis of acute pancreatitis. Pancreas 2008;37(4):449‐53. [DOI] [PubMed] [Google Scholar]
Gurleyik 2004 {published data only}
- Gurleyik G, Cirpici OZ, Aktekin A, Saglam A. The value of Ranson and APACHE II scoring systems, and serum levels of interleukin‐6 and C‐reactive protein in the early diagnosis of the severity of acute pancreatitis. Ulusal Travma Ve Acil Cerrahi Dergisi 2004;10(2):83‐8. [PubMed] [Google Scholar]
Gurleyik 2005 {published data only}
- Gurleyik G, Emir S, Kilicoglu G, Arman A, Saglam A. Computed tomography severity index, APACHE II score, and serum CRP concentration for predicting the severity of acute pancreatitis. Journal of the Pancreas 2005;6(6):562‐7. [PubMed] [Google Scholar]
Gvozdenovic 2001 {published data only}
- Gvozdenovic M, Lausevic Z, Ignjatovic S, Bukumirovic V. Significance of C‐reactive protein in the treatment of acute necrotizing pancreatitis. Acta Chirurgica Iugoslavica 2001;48(3):29‐34. [PubMed] [Google Scholar]
Hamalainen 2002 {published data only}
- Hamalainen MT, Gronroos P, Gronroos JM. Do normal leucocyte count and C‐reactive protein on admission to hospital exclude a life‐threatening attack of acute pancreatitis?. Scandinavian Journal of Surgery 2002;91(Part 4):353‐6. [DOI] [PubMed] [Google Scholar]
Han 2011 {published data only}
- Han L, Lu S. Early predictive value of lactate dehydrogenase, serum creatinine and C‐reactive protein in severe acute pancreatitis. Chinese Journal of Critical Care Medicine 2011;31(Part 12):1058‐61. [Google Scholar]
Hjalmarsson 2009 {published data only}
- Hjalmarsson C, Stenflo J, Borgstrom A. Activated protein C‐protein C inhibitor complex, activation peptide of carboxypeptidase B and C‐reactive protein as predictors of severe acute pancreatitis. Pancreatology 2009;9(5):700‐7. [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang HL, Nie X, Cai B, Tang JT, He Y, Miao Q, et al. Procalcitonin levels predict acute kidney injury and prognosis in acute pancreatitis: a prospective study. PLoS ONE 2013;8(12):e82250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang J, Wu ZW, Lu SQ, Shen JQ, Kong XM, Shen YP. Soluble B7‐H2 as a novel marker in early evaluation of the severity of acute pancreatitis. Labmedicine 2015;46(2):109‐17. [DOI] [PubMed] [Google Scholar]
Imamura 2002a {published data only}
- Imamura T, Tanaka S, Yoshida H. Significance of measurement of high‐sensitivity C‐reactive protein in acute pancreatitis. Journal of Gastroenterology 2002;37(Part 11):935‐8. [DOI] [PubMed] [Google Scholar]
Imamura 2002b {published data only}
- Imamura T, Tanaka S, Yoshida H, Kitamura K, Takahashi A, Ikegami A, et al. Clinical significance of measurement of high‐sensitivity C‐reactive protein in acute pancreatitis. Gastroenterology 2002;122(4):A368. [DOI] [PubMed] [Google Scholar]
Inagaki 1997 {published data only}
- Inagaki T, Hoshino M, Hayakawa T, Ohara H, Yamada T, Yamada H, et al. Interleukin‐6 is a useful marker for early prediction of the severity of acute pancreatitis. Pancreas 1997;14(1):1‐8. [DOI] [PubMed] [Google Scholar]
Isenmann 1993 {published data only}
- Isenmann R, Buchler M, Uhl W, Malfertheiner P, Martini M, Beger HG. Pancreatic necrosis: An early finding in severe acute pancreatitis. Pancreas 1993;8(3):358‐61. [PubMed] [Google Scholar]
Isogai 1998 {published data only}
- Isogai M, Yamaguchi A, Hori A, Kaneoka Y. LDH to AST ratio in biliary pancreatitis ‐ a possible indicator of pancreatic necrosis: preliminary results. American Journal of Gastroenterology 1998;93(3):363‐7. [DOI] [PubMed] [Google Scholar]
Jia 2015 {published data only}
- Jia R, Tang M, Qiu L, Sun R, Cheng L, Ma X, et al. Increased interleukin‐23/17 axis and C‐reactive protein are associated with severity of acute pancreatitis in patients. Pancreas 2015;44(2):321‐5. [DOI] [PubMed] [Google Scholar]
Jiang 2004 {published data only}
- Jiang CF, Shiau YC, Ng KW, Tan SW. Serum interleukin‐6, tumor necrosis factor alpha and C‐reactive protein in early prediction of severity of acute pancreatitis. Journal of the Chinese Medical Association 2004;67(9):442‐6. [PubMed] [Google Scholar]
Jimenez 2015 {published data only}
- Jimenez E, Moreno S, Guadiana LG, Pedregosa J, Jimenez R, Rebollo S, et al. Procalcitonin for predicting severity of acute pancreatitis. Clinical Chemistry and Laboratory Medicine 2015;53:S941. [Google Scholar]
Jimin 2015 {published data only}
- Jimin Z, Jian Z, Juncha G. Early evaluations of BISAP plus C‐reactive protein in predicting the severity of acute pancreatitis. Zhonghua Yi Xue za Zhi 2015;95(12):925‐8. [PubMed] [Google Scholar]
Johnson 2003 {published data only}
- Johnson CD. Early severity indexes in acute pancreatitis. Acta Gastro‐Enterologica Belgica 2003;66(2):174‐6. [PubMed] [Google Scholar]
Kaiyasah 2013 {published data only}
- Kaiyasah H, Alozaibi L, Anwar S, Al‐Jufairi F, Naim R. Can serum procalcitonin be a reliable single bio‐marker in predicting the severity of acute pancreatitis?. Journal of Gastroenterology and Hepatology 2013;28:681. [Google Scholar]
Kaya 2007 {published data only}
- Kaya E, Dervisoglu A, Polat C. Evaluation of diagnostic findings and scoring systems in outcome prediction in acute pancreatitis. World Journal of Gastroenterology 2007;13(22):3090‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kazda 2002 {published data only}
- Kazda A, Brodska H, Valenta J, Uhrova I, Hendl J, Belohlavek J, et al. Procalcitonin in acute pancreatitis and in septic states. Klinicka Biochemie a Metabolismus 2002;10(1):32‐7. [Google Scholar]
Khanna 2013 {published data only}
- Khanna AK, Meher S, Prakash S, Tiwary SK, Singh U, Srivastava A, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE II, CTSI scores, IL‐6, CRP, and procalcitonin in predicting severity, organ failure, pancreatic necrosis, and mortality in acute pancreatitis. HPB Surgery 2013 Sep 24 [Epub ahead of print]:32‐7. [DOI: 10.1155/2013/367581] [DOI] [PMC free article] [PubMed]
Khvatova 1973 {published data only}
- Khvatova EA. Role of certain enzymatic systems in different forms of acute pancreatitis. Klinicheskaia Khirurgiia 1973;2:48‐53. [PubMed] [Google Scholar]
Khvatova 1977 {published data only}
- Khvatova EA. Value of thermal fractionation of lactate dehydrogenase and the protein composition of the blood in the differential diagnosis of the phases of acute pancreatitis. Vestnik Khirurgii Imeni I. I. Grekova 1977;118(1):34‐7. [PubMed] [Google Scholar]
Kibar 2016 {published data only}
- Kibar YI, Albayrak F, Arabul M, Dursun H, Albayrak Y, Ozturk Y. Resistin: New serum marker for predicting severity of acute pancreatitis. Journal of International Medical Research 2016;44(2):328‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013a {published data only}
- Kim BG, Noh MH, Ryu CH, Nam HS, Woo SM, Ryu SH, et al. A comparison of the BISAP score and serum procalcitonin for predicting the severity of acute pancreatitis. Korean Journal of Internal Medicine 2013;28(3):322‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim KH, Kim BG, Ryu CH, Nam HS, Woo SM, Ryu SH, et al. Comparative analysis of BISAP system and serum procalcitonin for predicting severity of acute pancreatitis. Pancreatology 2013;13(Supp):S25‐7. [Google Scholar]
Kitsanou 2004 {published data only}
- Kitsanou M, Katsanos KH, Christodoulou D, Kalabokis G, Katsaraki A, Kontodimou N, et al. The role of PMN elastase and CRP on the diagnosis and assessment of severity of acute pancreatitis. Gastroenterology 2004;126(4):A228. [Google Scholar]
Korczowski 2006 {published data only}
- Korczowski B. Diagnostic usefulness of procalcitonin in acute pancreatitis. Gastroenterologia Polska 2006;13(1):63‐5. [Google Scholar]
Kusnierz‐Cabala 2004 {published data only}
- Kusnierz‐Cabala B, Naskalski JW, Kedra B, Panek J. Comparison of sensitivity and specificity of serum poly‐C avid ribonuclease activity and C‐reactive protein concentration in detection of mild and severe acute pancreatitis. Clinical Chemistry and Laboratory Medicine 2004;42(5):549‐55. [DOI] [PubMed] [Google Scholar]
Kylanpaa‐Back 2001a {published data only}
- Kylanpaa‐Back ML, Takala A, Kemppainen E, Puolakkainen P, Haapiainen R, Repo H. Procalcitonin strip test in the early detection of severe acute pancreatitis. British Journal of Surgery 2001;88(2):222‐7. [DOI] [PubMed] [Google Scholar]
Kylanpaa‐Back 2001b {published data only}
- Kylanpaa‐Back ML, Takala A, Kemppainen EA, Puolakkainen PA, Haapiainen RK, Repo H. The strip test of procalcitonin in early detection of severe acute pancreatitis. Gastroenterology 2001;120(5):A469. [DOI] [PubMed] [Google Scholar]
Kylanpaa‐Back 2001c {published data only}
- Kylanpaa‐Back ML, Takala A, Kemppainen EA, Puolakkainen PA, Leppaniemi AK, Karonen SL, et al. Procalcitonin, soluble interleukin‐2 receptor, and soluble E‐selectin in predicting the severity of acute pancreatitis. Critical Care Medicine 2001;29(1):63‐9. [DOI] [PubMed] [Google Scholar]
Leese 1987 {published data only}
- Leese T, Fuller M, Holliday M. C‐reactive protein and alpha‐2 macroglobulin in the assessment and monitoring of acute‐pancreatitis. Digestion 1987;38(1):35‐6. [Google Scholar]
Leese 1988 {published data only}
- Leese T, Shaw D, Holliday M. Prognostic markers in acute pancreatitis: Can pancreatic necrosis be predicted?. Annals of the Royal College of Surgeons of England 1988;70(4):227‐32. [PMC free article] [PubMed] [Google Scholar]
Lempinen 1999 {published data only}
- Lempinen M, Kylanpaa‐Back ML, Stenman UH, Puolakkainen P, Haapiainen R, Finne P, et al. Predicting the severity of acute pancreatitis by rapid measurement of trypsinogen‐2 in urine. Clinical Chemistry 1999;47(12):2103‐7. [PubMed] [Google Scholar]
Lempinen 2005 {published data only}
- Lempinen M, Puolakkainen P, Kemppainen E. Clinical value of severity markers in acute pancreatitis. Scandinavian Journal of Surgery: SJS 2005;94(2):118‐23. [DOI] [PubMed] [Google Scholar]
Lewandowski 2007 {published data only}
- Lewandowski A, Kopec W, Diakowska D, Garbien M. Proinflammatory cytokines IL‐6, IL‐8 and C‐reactive protein in acute pancreatitis ‐ the role of IL‐8 in prognosing complications. Gastroenterologia Polska 2007;14(3):165‐72. [Google Scholar]
Li 2013 {published data only}
- Li Y, Li Y, Lu S. The value of lactate dehydrogenase combined with BISAP scoring in predicting the prognosis of acute pancreatitis. Chinese Journal of Critical Care Medicine 2013;33(Part 6):523‐6. [Google Scholar]
Liang 2014 {published data only}
- Liang K. Early predictive value of serum calcium, C‐reactive protein and serum amylase like protein A on severe acute pancreatitis. Chinese Journal of Critical Care Medicine 2014;34(Part 8):720‐3. [Google Scholar]
Lindner 1995 {published data only}
- Lindner J, Skokanova V. Acute phase proteins in acute pancreatitis. Rozhledy V Chirurgii 1995;74(1):21‐4. [PubMed] [Google Scholar]
Lipsett 2001 {published data only}
- Lipsett PA. Serum cytokines, proteins, and receptors in acute pancreatitis: Mediators, markers, or more of the same?. Critical Care Medicine 2001;29(8):1642‐4. [DOI] [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu C, Haldene C. C‐reactive protein or lactate dehydrogenase to assess the severity of pancreatic necrosis. Emergency Medicine Journal 2008;25(10):687‐8. [DOI] [PubMed] [Google Scholar]
Lobo 1999 {published data only}
- Lobo SM, Meirelles RF, Lupino P, Pires MD, Kuga ML, Beolchi G. Increased procalcitonin serum levels as predictive parameter of multiple organ failure and outcome in acute pancreatitis patients. Critical Care 1999;3(Supp 1):P93. [Google Scholar]
Lott 1991 {published data only}
- Lott JA. The value of clinical laboratory studies in acute‐pancreatitis. Archives of Pathology & Laboratory Medicine 1991;115(4):325‐6. [PubMed] [Google Scholar]
Ma 2013 {published data only}
- Ma J, Fan H, Kou JT, Li LX, He Q. Clinical significance for monitoring of serum ghrelin in acute pancreatitis severity assessment. National Medical Journal of China 2013;93(48):3864‐6. [PubMed] [Google Scholar]
Machiedo 1974 {published data only}
- Machiedo GW, Brown CS, Rush BF Jr. Comparison of biochemical changes in blood and abdominal fluid during acute hemorrhagic pancreatitis. Review of Surgery 1974;31(1):60‐2. [PubMed] [Google Scholar]
Makay 2003 {published data only}
- Makay R, Issekutz A, Banga P, Belagyi T, Olah A. Role of procalcitonin rapid test in the differential diagnosis of uninfected and infected forms of acute pancreatitis. Magyar Sebeszet 2003;56(1):31‐3. [PubMed] [Google Scholar]
Makela 2007 {published data only}
- Makela JT, Eila H, Kiviniemi H, Laurila J, Laitinen S. Computed tomography severity index and C‐reactive protein values predicting mortality in emergency and intensive care units for patients with severe acute pancreatitis. American Journal of Surgery 2007;194(1):30‐4. [DOI] [PubMed] [Google Scholar]
Malfertheiner 1993 {published data only}
- Malfertheiner P, Dominguez‐Munoz JE. Prognostic factors in acute‐pancreatitis. International Journal of Pancreatology 1993;14(1):1‐8. [DOI] [PubMed] [Google Scholar]
Manabe 2004 {published data only}
- Manabe T, Okada Y. Serum markers for predicting the severity of acute pancreatitis. Journal of Gastroenterology 2004;39(5):504‐6. [DOI] [PubMed] [Google Scholar]
Mandi 2000a {published data only}
- Mandi Y, Farkas G, Takacs T, Boda K, Lonovics J. Diagnostic relevance of procalcitonin, IL‐6, and sICAM‐1 in the prediction of infected necrosis in acute pancreatitis. International Journal of Pancreatology 2000;28(1):41‐9. [DOI] [PubMed] [Google Scholar]
Mandi 2000b {published data only}
- Mandi Y, Farkas G, Skolak E, Takacs T. Relevance of procalcitonin in infected pancreatic necrosis. 5th World Congress on Trauma, Shock, Inflammation and Sepsis: Pathophysiology, Immune Consequences and Therapy; 2000 Feb 29‐Mar 4; Munich, Germany. Augusta (GA): BioMedical, 2000:419‐24.
Manes 1994 {published data only}
- Manes G, Rabitti PG, Laccetti M, Perrotti F, Cavallera A, Uomo G. Early prognostic assessment of acute pancreatitis with sterile necrosis. A perspective clinical study. Recenti Progressi in Medicina 1994;85(10):490‐3. [PubMed] [Google Scholar]
Mantke 2002 {published data only}
- Mantke R, Pross M, Kunz D, Ebert M, Kahl S, Peters B, et al. Soluble thrombomodulin plasma levels are an early indication of a lethal course in human acute pancreatitis. Surgery 2002;131(4):424‐32. [DOI] [PubMed] [Google Scholar]
Marek 1996 {published data only}
- Marek TA, Nowak A, Dziurkowska‐Marek A, Nowakowska‐Dulawa E, Cholewka A, Kaczor R, et al. C‐reactive protein (CRP) in the early assessment of severity in acute biliary pancreatitis (ABP). Gastroenterology 1996;110(4):A415. [Google Scholar]
Mayer 1984 {published data only}
- Mayer AD, McMahon MJ, Bowen M, Cooper EH. C‐reactive protein ‐ an aid to assessment and monitoring of acute‐pancreatitis. Journal of Clinical Pathology 1984;37(2):207‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayer 2002 {published data only}
- Mayer JM, Raraty M, Slavin J, Kemppainen E, Fitzpatrick J, Hietaranta A, et al. Serum amyloid A is a better early predictor of severity than C‐reactive protein in acute pancreatitis. British Journal of Surgery 2002;89(2):163‐71. [DOI] [PubMed] [Google Scholar]
Melzi D'Eril 2000 {published data only}
- Melzi D'Eril GV, Merlini G, Finazzi S, Bosoni T, Barakat B, Pezzilli R. Procalcitonin is not a reliable marker for the assessment of severity in acute pancreatitis without infectious complications. Clinical Chemistry 2000;46(3):428‐30. [PubMed] [Google Scholar]
Millat 1999 {published data only}
- Millat B. Acute pancreatitis. Etiology, diagnosis, prognosis. Revue du Praticien 1999;49(3):311‐9. [PubMed] [Google Scholar]
Modrau 2005 {published data only}
- Modrau IS, Floyd AK, Thorlacius‐Ussing O. The clinical value of procalcitonin in early assessment of acute pancreatitis. American Journal of Gastroenterology 2005;100(7):1593‐7. [DOI] [PubMed] [Google Scholar]
Modzelewski 2005 {published data only}
- Modzelewski B, Holynski J. The role of selected enzymes and acute phase proteins in necrotizing pancreatitis. Polski Merkuriusz Lekarski 2005;18(106):412‐4. [PubMed] [Google Scholar]
Mulholland 1996 {published data only}
- Mulholland MW, Eckhauser FE. Prognostic criteria in necrotizing pancreatitis. Problems in General Surgery 1996;13(4):29‐36. [Google Scholar]
Muller 1997 {published data only}
- Muller C, Uhl W, Printzen G, Friess K, Vogel R, Reber P, et al. Value of procalcitonin (PCT) and granulocyte colony‐stimulating factor (G‐CSF) in predicting infected pancreatic necrosis. Gut 1997;41:A134. [Google Scholar]
Muller 2000 {published data only}
- Muller CA, Uhl W, Printzen G, Gloor B, Bischofberger H, Tcholakov O, et al. Role of procalcitonin and granulocyte colony stimulating factor in the early prediction of infected necrosis in severe acute pancreatitis. Gut 2000;46(2):233‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neoptolemos 2001 {published data only}
- Neoptolemos JP. Procalcitonin strip test in the early detection of severe acute pancreatitis. British Journal of Surgery 2001;88(10):1418. [DOI] [PubMed] [Google Scholar]
Nunes 2009 {published data only}
- Nunes QM, Gardner‐Thorpe J, Dajani K, Raraty M, Ghaneh P, Sutton R, et al. CRP as an indicator for using CT‐guided fine needle aspiration in the diagnosis of infected pancreatic necrosis. Pancreatology 2009;9(4):454. [Google Scholar]
Oezcueruemez‐Porsch 1998 {published data only}
- Oezcueruemez‐Porsch M, Kunz D, Hardt PD, Fadgyas T, Kress O, Schulz HU, et al. Diagnostic relevance of interleukin pattern, acute‐phase proteins, and procalcitonin in early phase of post‐ERCP pancreatitis. Digestive Diseases and Sciences 1998;43(8):1763‐9. [DOI] [PubMed] [Google Scholar]
Olah 2005 {published data only}
- Olah A, Belagyi T, Issekutz A, Makay R, Zaborszky A. Value of procalcitonin quick test in the differentiation between sterile and infected forms of acute pancreatitis. Hepato‐Gastroenterology 2005;52(61):243‐5. [PubMed] [Google Scholar]
Omoto 2015 {published data only}
- Omoto S, Kitano M, Sakamoto H, Imai H, Yamao K, Kamata K, et al. Usefulness of serum procalcitonin for diagnosis of acute pancreatitis. Journal of Gastroenterology and Hepatology 2015;30:141. [Google Scholar]
Ostrovskii 2012 {published data only}
- Ostrovskii VK, Rodionov PN, Makarov SV. Some of criteria in the evaluation of severity and prognosis with different forms of acute pancreatitis. Anesteziologiia i Reanimatologiia 2012, (3):56‐9. [PubMed] [Google Scholar]
Paajanen 1995 {published data only}
- Paajanen H, Laato M, Jaakkola M, Pulkki K, Niinikoski J, Nordback I. Serum tumour necrosis factor compared with C‐reactive protein in the early assessment of severity of acute pancreatitis. British Journal of Surgery 1995;82(2):271‐3. [DOI] [PubMed] [Google Scholar]
Palani 1977 {published data only}
- Palani CK, Sehgal LR, Kraft AR, Duarte B, Dulski R, Levine H, et al. Predictive value of biochemical indexes in acute alcoholic pancreatitis. Surgical Forum 1977;28:441‐3. [PubMed] [Google Scholar]
Pallisera 2014 {published data only}
- Pallisera A, Jorba R, Ramia JM, Rodriguez JA, Subirana H, Zarate LO, et al. Biological markers of severity in acute pancreatitis. Central European Journal of Medicine 2014;9(4):550‐5. [Google Scholar]
Park 2012 {published data only}
- Park JY, Jeon TJ, Hwang JT, Sinn DH, Oh TH, Shin WC, et al. Bedside index for severity in acute pancreatitis: an early marker of severity. Journal of Gastroenterology and Hepatology 2012;27:355. [Google Scholar]
Park 2013 {published data only}
- Park JY, Jeon TJ, Ha TH, Hwang JT, Sinn DH, Oh TH, et al. Bedside index for severity in acute pancreatitis: Comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary & Pancreatic Diseases International 2013;12(6):645‐50. [DOI] [PubMed] [Google Scholar]
Petrov 2011 {published data only}
- Petrov MS. Predicting the severity of acute pancreatitis: Choose the right horse before hitching the cart. Digestive Diseases and Sciences 2011;56(12):3402‐4. [DOI] [PubMed] [Google Scholar]
Pezzilli 1994 {published data only}
- Pezzilli R, Billi P, Plate L, Barakat B, Bongiovanni F, Miglioli M. Human pancreatic secretory trypsin inhibitor in the assessment of the severity of acute pancreatitis. A comparison with C‐reactive protein. Journal of Clinical Gastroenterology 1994;19(2):112‐7. [DOI] [PubMed] [Google Scholar]
Pezzilli 1995a {published data only}
- Pezzilli R, Billi P, Miniero R, Fiocchi M, Cappelletti O, Sprovieri G, et al. Serum‐beta‐2 microglobulin in the early assessment of the severity of acute pancreatitis ‐ a comparative study with Interleukin‐6, Interleukin‐8 and C‐reactive protein. Gastroenterology 1995;108(4 Suppl):A384. [Google Scholar]
Pezzilli 1995b {published data only}
- Pezzilli R, Billi P, Miniero R, Fiocchi M, Cappelletti O, Morselli‐Labate AM, et al. Serum interleukin‐6, interleukin‐8, and beta 2‐microglobulin in early assessment of severity of acute pancreatitis. Comparison with serum C‐reactive protein. Digestive Diseases and Sciences 1995;40(11):2341‐8. [DOI] [PubMed] [Google Scholar]
Pezzilli 1997 {published data only}
- Pezzilli R, Billi P, Cappelletti O, Barakat B. Serum C‐positive protein in acute biliary pancreatitis. Is it a reliable marker for the early assessment of severity of the disease?. Italian Journal of Gastroenterology and Hepatology 1997;29(6):554‐7. [PubMed] [Google Scholar]
Pezzilli 1998a {published data only}
- Pezzilli R, Labate AMM, Barakat B, Fiocchi M, Cappelletti O, Miglio F. Is the association of serum lipase with beta 2‐microglobulin or with C‐reactive protein useful in simultaneously establishing the diagnosis and prognosis of patients with acute pancreatitis?. Gastroenterology 1998;114(4):A490‐1. [Google Scholar]
Pezzilli 1998b {published data only}
- Pezzilli R, Morselli‐Labate AM, Barakat B, Fiocchi M, Cappelletti O. Is the association of serum lipase with alpha‐2‐microglobulin or C‐reactive protein useful for establishing the diagnosis and prognosis of patients with acute pancreatitis?. Clinical Chemistry and Laboratory Medicine 1998;36(12):963‐8. [DOI] [PubMed] [Google Scholar]
Pezzilli 2000 {published data only}
- Pezzilli R, D'Eril GVM, Morselli‐Labate AM, Merlini G, Barakat B, Bosoni T. Serum amyloid A, procalcitonin, and C‐reactive protein in early assessment of severity of acute pancreatitis. Digestive Diseases and Sciences 2000;45(6):1072‐8. [DOI] [PubMed] [Google Scholar]
Pongprasobchai 2010 {published data only}
- Pongprasobchai S, Jianjaroonwong V, Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S, et al. Erythrocyte sedimentation rate and C‐reactive protein for the prediction of severity of acute pancreatitis. Pancreas 2010;39(8):1226‐30. [DOI] [PubMed] [Google Scholar]
Puolakkainen 1987 {published data only}
- Puolakkainen P, Valtonen V, Paananen A, Schroder T. C‐reactive protein (CRP) and serum phospholipase‐A2 in the assessment of the severity of acute pancreatitis. Gut 1987;28(6):764‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Purkayastha 2006 {published data only}
- Purkayastha S, Chow A, Athanasiou T, Cambaroudis A, Panesar S, Kinross J, et al. Does serum procalcitonin have a role in evaluating the severity of acute pancreatitis? A question revisited. World Journal of Surgery 2006;30(9):1713‐21. [DOI] [PubMed] [Google Scholar]
Qiu 2014 {published data only}
- Qiu L, Yu Q, Yu J, Wang H, Zhou Y, Li Q, et al. The prognostic value of CD14+HLA‐DRlow/‐ for evaluating the severity of acute pancreatitis. Chinese Journal of Microbiology and Immunology 2014;34(8):620‐3. [Google Scholar]
Raraty 2002 {published data only}
- Raraty MGT, Mayer JM, Slavin M, Kemppainen E, Fitzpatrick J, Hietaranta A, et al. Serum amyloid A is a better early predictor of severity than C‐reactive protein in acute pancreatitis. British Journal of Surgery 2002;89(3):380. [DOI] [PubMed] [Google Scholar]
Rau 1997 {published data only}
- Rau B, Steinbach G, Gansauge F, Mayer J, Gruenert A, Beger HG. The role of procalcitonin and interleukin‐8 in the prediction of infected necrosis in acute pancreatitis. Gastroenterology 1997;112(4):A476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rau 2000 {published data only}
- Rau B, Steinbach G, Baumgart K, Gansauge F, Grunert A, Beger HG. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Medicine 2000;26:S159‐64. [DOI] [PubMed] [Google Scholar]
Rau 2004 {published data only}
- Rau B, Kruger CM, Schilling MK. Procalcitonin: Improved biochemical severity stratification and postoperative monitoring in severe abdominal inflammation and sepsis. Langenbecks Archives of Surgery 2004;389(2):134‐44. [DOI] [PubMed] [Google Scholar]
Rau 2007 {published data only}
- Rau BM, Kemppainen EA, Gumbs AA, Buchler MW, Wegscheider K, Bassi C, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Annals of Surgery 2007;245(5):745‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricardo 2011 {published data only}
- Ricardo L, Cardoso F, Oliveira A, Rodrigues C, Horta D, Figueiredo A, et al. Comparison of BISAP, Ranson's, and CTSI scores, and C‐reactive protein in predicting organ failure, complications and mortality in acute pancreatitis. Pancreas 2011;40(8):1351. [Google Scholar]
Riche 2003 {published data only}
- Riche FC, Cholley BP, Laisne MJ, Vicaut E, Panis YH, Lajeunie EJ, et al. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery 2003;133(3):257‐62. [DOI] [PubMed] [Google Scholar]
Ruzafa 1991 {published data only}
- Ruzafa A, Granada ML, Gener J, Manterola JM, Oller B, Mesalles E, et al. C reactive protein concentrations, measured by a latex slide test, as a prognostic factor in acute pancreatitis. Revista de la Sociedad Espanola de Quimica Clinica 1991;10(5):372‐6. [Google Scholar]
Samso 2002 {published data only}
- Samso CT. Early assessment of severity in acute pancreatitis. Revista Espanola De Enfermedades Digestivas 2002;94(9):519‐22. [PubMed] [Google Scholar]
Sanchez‐Lozada 2005 {published data only}
- Sanchez‐Lozada R, Camacho‐Hernandez MI, Vega‐Chavaje RG, Garza‐Flores JH, Campos‐Castillo C, Gutierrez‐Vega R. Acute pancreatitis: Five year experience at the hospital general de Mexico. Gaceta Medica de Mexico 2005;141(2):123‐7. [PubMed] [Google Scholar]
Santotoribio 2015 {published data only}
- Santotoribio JD, Canavate‐Solano C, Garcia‐De La Torre A, Arce‐Matute F, Perez‐Ramos S. C‐reactive protein for diagnosis of severe acute pancreatitis. Clinical Chemistry and Laboratory Medicine 2015;53:S1250. [Google Scholar]
Sato 2004 {published data only}
- Sato N, Endo S, Kasai T, Inoue Y, Fujino Y, Onodera M, et al. Relationship of the serum procalcitonin level with the severity of acute pancreatitis. Research Communications in Molecular Pathology & Pharmacology 2004;115‐116:243‐9. [PubMed] [Google Scholar]
Savel'ev 2002 {published data only}
- Savel'ev VS, Gel'fand BR, Filimonov MI, Burnevich SZ, Sergeeva NA, Brazhnik TB, et al. Clinical significance of the procalcitonin test in differential diagnosis of systemic inflammatory reaction in pancreatonecrosis. Anesteziologiia i Reanimatologiia 2002, (1):25‐9. [PubMed] [Google Scholar]
Schaffler 2010 {published data only}
- Schaffler A, Hamer O, Dickopf J, Goetz A, Landfried K, Voelk M, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. American Journal of Gastroenterology 2010;105(11):2474‐84. [DOI] [PubMed] [Google Scholar]
Schaffler 2011 {published data only}
- Schaffler A, Hamer OW, Dickopf J, Goetz A, Landfried K, Voelk M, et al. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. American Journal of Gastroenterology 2011;106(5):957‐67. [DOI] [PubMed] [Google Scholar]
Sharma 2011 {published data only}
- Sharma M, Goswami B. C reactive protein, PCV and CT scan in assessing severity of acute pancreatitis. Journal of Gastroenterology and Hepatology 2011;26(Supp 5):232. [Google Scholar]
Stimac 2010 {published data only}
- Stimac D, Fisic E, Zulle LB, Zrnic IK, Milic S. Clinical value of C‐reactive protein and elastase in assessment of acute pancreatitis. Pancreatology 2010;10(2‐3):347. [Google Scholar]
Stimac 2012 {published data only}
- Stimac D, Fisic E, Poropat G, Bilic Zulle L, Licul V, Milic S. The role of IL‐6, IL‐8, IL‐10, sTNFr CRP and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Pancreas 2012;41(8):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stimac 2013 {published data only}
- Stimac D, Fisic E, Poropat G, Bilic Zulle L, Licul V, Milic S. The role of IL‐6, IL‐8, IL‐10, sTNFr, CRP and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Pancreatology 2013;13(2):e75‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoelben 1996 {published data only}
- Stoelben E, Nagel M, Ockert D, Quintel M, Scheibenbogen C, Klein B, et al. Clinical relevance of cytokines IL‐6, IL‐8, and C‐reactive protein in the blood of patients with acute pancreatitis. Chirurg 1996;67(12):1231‐6. [DOI] [PubMed] [Google Scholar]
Sugumar 2011 {published data only}
- Sugumar A, Alkubeyyer M, Erickson B, Vege SS. Can machine learning predict the severity of acute pancreatitis. Gastroenterology 2011;1:S853. [Google Scholar]
Tao 2013 {published data only}
- Tao J, Li L, Wu B. The value of combining earlier period C reactive protein and hematocrit level in acute pancreatitis. Journal of Gastroenterology and Hepatology 2013;28:455. [Google Scholar]
Teerenhovi 1988 {published data only}
- Teerenhovi O, Nordback I. C‐reactive protein (CRP) and pancreatic necrosis in acute necrotizing pancreatitis. Annales Chirurgiae Et Gynaecologiae 1988;77(2):61‐3. [PubMed] [Google Scholar]
Tesinsky 2008 {published data only}
- Tesinsky P, Holubec L. P016 predictive value of procalcitonin and cytokines in ERCP induced acute pancreatitis. Clinical Nutrition Supplements 2008;3(Supp 1):35. [Google Scholar]
Trunin 1985 {published data only}
- Trunin MA, Khvatova EA. Lactate dehydrogenase enzymes and isoenzymes in the diagnosis of forms of acute and postoperative pancreatitis. Khirurgiia 1985, (1):50‐5. [PubMed] [Google Scholar]
Uhl 1991 {published data only}
- Uhl W, Buchler M, Malfertheiner P, Martini M, Beger HG. PMN‐elastase in comparison with CRP, antiproteases, and LDH as indicators of necrosis in human acute pancreatitis. Pancreas 1991;6(3):253‐9. [DOI] [PubMed] [Google Scholar]
Uomo 1995 {published data only}
- Uomo G, Rabitti PG, Laccetti M, Manes G, Carraturo I, Esposito P, et al. Predictive evaluation of acute necrotizing pancreatitis: Results of a prospective study. Presse Medicale 1995;24(5):263‐6. [PubMed] [Google Scholar]
Vaz 2013 {published data only}
- Vaz PS, Caldeira A, Sousa R, Gouveia A, Banhudo A, Loureiro A. The value of procalcitonin, antithrombin III and BISAP score at predicting the severity of acute pancreatitis. Pancreatology: Abstracts presented at the 45th Annual Meeting of the European Pancreatic Club, 2013 June 26‐29, Zurich, Switzerland 2013;13(Supp):S34‐5. [Google Scholar]
Vesentini 1993 {published data only}
- Vesentini S, Bassi C, Talamini G, Cavallini G. Prospective comparison of C‐reactive protein level, Ranson score and contrast‐enhanced computed tomography in the prediction of septic complications of acute pancreatitis. British Journal of Surgery 1993;80(6):755. [DOI] [PubMed] [Google Scholar]
Viedma 1992 {published data only}
- Viedma JA, Perez‐Mateo M, Dominguez JE, Carballo F. Role of interleukin‐6 in acute pancreatitis. Comparison with C‐reactive protein and phospholipase A. Gut 1992;33(9):1264‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viedma 1994 {published data only}
- Viedma JA, Perez‐Mateo M, Agullo J, Dominguez JE, Carballo F. Inflammatory response in the early prediction of severity in human acute pancreatitis. Gut 1994;35(6):822‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlachos 2014 {published data only}
- Vlachos S, Tsaroucha AK, Konstantoudakis G, Papachristou F, Trypsianis G, Schizas D, et al. Serum profiles of M30, M65 and Interleukin‐17 compared with C‐reactive protein in patients with mild and severe acute pancreatitis. Journal of Hepato‐Biliary‐Pancreatic Sciences 2014;21(12):911‐8. [DOI] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei H, Wang YX. Significance of C‐reactive protein to prediction and severity evaluation of acute pancreatitis. Journal of Gastroenterology and Hepatology 2013;28(Suppl 3):885‐6. [Google Scholar]
Wetherill 2012 {published data only}
- Wetherill C, Melling J, Jones M. C‐reactive protein accurately predicts severity of acute pancreatitis in children. Pancreas 2012;41(8):1412‐3. [DOI] [PubMed] [Google Scholar]
Wetherill 2013a {published data only}
- Wetherill C, Melling J, Jones M. 48 hour C‐reactive protein accurately predicts severity of acute pancreatitis in children. Pancreatology 2013;13(1):e6. [DOI] [PubMed] [Google Scholar]
Wetherill 2013b {published data only}
- Wetherill C, Melling J, Jones M. C‐reactive protein accurately predicts severity of acute pancreatitis in children. Pancreatology 2013;13(2):e84. [DOI] [PubMed] [Google Scholar]
Wilson 1987 {published data only}
- Wilson C, Fraser WD, Gardner MD, Imrie CW. Source of lactate‐dehydrogenase elevation in acute‐pancreatitis. Digestion 1987;38(1):64. [Google Scholar]
Wilson 1988 {published data only}
- Wilson C, Shenkin A, Imrie CW. Value of C‐reactive protein and antiproteases in the objective monitoring of acute pancreatitis. Gut 1988;29(2):A269. [Google Scholar]
Wilson 1989a {published data only}
- Wilson C, Heads A, Shenkin A, Imrie CW. C‐reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. British Journal of Surgery 1989;76(2):177‐81. [DOI] [PubMed] [Google Scholar]
Wilson 1989b {published data only}
- Wilson C, Shenkin A, Imrie C. C‐reactive protein and acute pancreatitis ‐ reply. British Journal of Surgery 1989;76(8):877. [DOI] [PubMed] [Google Scholar]
Wong 1993 {published data only}
- Wong ECC, Butch AW, Rosenblum JL, Ladenson JH, Scott MG. (Washington University Case Conference) The clinical chemistry laboratory and acute pancreatitis. Clinical Chemistry 1993;39(2):234‐43. [PubMed] [Google Scholar]
Woo 2011 {published data only}
- Woo SM, Noh MH, Kim BG, Hsing CT, Han JS, Ryu SH, et al. Comparison of serum procalcitonin with Ranson, APACHE II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean Journal of Gastroenterology 2011;58(1):31‐7. [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu YH, Yan L, Bian C, Tian ZB, Jing X. Value of procalcitonin in assessing severity of acute pancreatitis. World Chinese Journal of Digestology 2015;23(30):4898‐904. [Google Scholar]
Yadav 2015a {published data only}
- Yadav A, Rafferty S, Rasool J, Keohane J, Sengupta S. C reactive protein ‐ a rapid and cost effective marker of severity of acute pancreatitis. Gastroenterology 2015;148(4):S684. [Google Scholar]
Yadav 2015b {published data only}
- Yadav A, Rafferty S, Rasool J, Keohane J, Sengupta S. C‐reactive protein: A rapid and cost effective marker of acute pancreatitis severity. Irish Journal of Medical Science 2015;184:S217. [Google Scholar]
Yasuda 2011 {published data only}
- Yasuda H, Suzaki S. Is procalcitonin useful to the diagnosis of infected pancreatic necrosis and pancreatic abscess?. Critical Care Medicine 2011;39:104. [Google Scholar]
Yin 2014 {published data only}
- Yin G, Hu G, Cang X, Yu G, Hu Y, Xing M, et al. C‐reactive protein: Rethinking its role in evaluating the severity of hyperlipidemic acute pancreatitis. Pancreas 2014;43(8):1323‐8. [DOI] [PubMed] [Google Scholar]
Yu 2011 {published data only}
- Yu YH, Han DS, Kim EK, Park HS, Kim TY, Eun CS, et al. Prevalence, mortality rate and predictive factors for severe acute pancreatitis according to the revised Atlanta classification. Journal of Gastroenterology and Hepatology 2011;26:235. [Google Scholar]
Zhu 2013 {published data only}
- Zhu GF. Early predictive values of the combined detection of serum calcium and CRP in the severity of acute pancreatitis. Journal of Gastroenterology and Hepatology 2013;28:891‐2. [Google Scholar]
Zrnic 2007 {published data only}
- Zrnic IK, Milic S, Fisic E, Radic M, Stimac D. C‐reactive protein and lactate dehydrogenase as single prognostic factors of severity in acute pancreatitis. Lijecnicki Vjesnik 2007;129(1):1‐3. [PubMed] [Google Scholar]
References to studies awaiting assessment
Djurasinovic 2013 {published data only}
- Djurasinovic T, Subota V, Vujanic S, Andjelic T, Pejovic J, Bezmarevic M. Prognostic values of procalcitonin and CRP in acute pancreatitis. Biochimica Clinica 2013;37:S663. [Google Scholar]
Grenier 1968 {published data only}
- Grenier JF, Gillet M, Sava G, Crevoisier R, Weiss AG. Acute pancreatitis. Anatomo‐clinical and biological confrontations; diagnostic, prognostic and therapeutic deductions. Apropos of a statistic of 37 cases. Presse Medicale 1968;76(29):1481‐4. [PubMed] [Google Scholar]
Issekutz 2003 {published data only}
- Issekutz A, Belagy T, Olah A. Clinical value of procalcitonin test in severe acute pancreatitis. Hpb 2003;5, SUPP 1:140. [Google Scholar]
Pindak 2003 {published data only}
- Pindak D, Parrak V, Pechan J, Vavrecka A, Kuzela L, Fuchs D, et al. The clinical value of the procalcitonin in prediction of severity and outcome in acute pancreatitis. Hepato‐Gastroenterology 2003;50(Suppl 2):ccviii‐ccix. [PubMed] [Google Scholar]
Additional references
Ayub 2004
- Ayub K, Slavin J, Imada R. Endoscopic retrograde cholangiopancreatography in gallstone‐associated acute pancreatitis. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003630.pub2] [DOI] [PubMed] [Google Scholar]
Bakker 2012
- Bakker OJ, Santvoort HC, Brunschot S, Geskus RB, Besselink MG, Bollen TL, et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA 2012;307(10):1053‐61. [DOI] [PubMed] [Google Scholar]
Banks 2013
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis ‐ 2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102‐11. [DOI] [PubMed] [Google Scholar]
Becker 2010
- Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. British Journal of Pharmacology 2010;159(2):253‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1993
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis; 1992 September 11‐13; Atlanta, GA. Archives of Surgery 1993;128(5):586‐90. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of Clinical Epidemiology 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Cannon 2009
- Cannon JW, Callery MP, Vollmer CM Jr. Diagnosis and management of pancreatic pseudocysts: What is the evidence?. Journal of the American College of Surgeons 2009;209(3):385‐93. [DOI] [PubMed] [Google Scholar]
Cheruvu 2003
- Cheruvu CV, Clarke MG, Prentice M, Eyre‐Brook IA. Conservative treatment as an option in the management of pancreatic pseudocyst. Annals of the Royal College of Surgeons of England 2003;85(5):313‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Doust 2005
- Doust JA, Pietrzak E, Sanders S, Glasziou PP. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. Journal of Clinical Epidemiology 2005;58(5):444‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2001
- Eloubeidi MA, Wade SB, Provenzale D. Factors associated with acceptance and full publication of GI endoscopic research originally published in abstract form. Gastrointestinal Endoscopy 2001;53(3):275‐82. [DOI] [PubMed] [Google Scholar]
Ghekiere 2007
- Ghekiere O, Lesnik A, Hoa D, Laffargue G, Uriot C, Taourel P. Value of computed tomography in the diagnosis of the cause of nontraumatic gastrointestinal tract perforation. Journal of Computer Assisted Tomography 2007;31(2):169‐76. [DOI] [PubMed] [Google Scholar]
Grassi 2004
- Grassi R, Romano S, Pinto A, Romano L. Gastro‐duodenal perforations: conventional plain film, US and CT findings in 166 consecutive patients. European Journal of Radiology 2004;50(1):30‐6. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Nagendran M, Davidson BR. Early versus delayed laparoscopic cholecystectomy for acute gallstone pancreatitis. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010326.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Davidson BR. Gallstones. BMJ 2014;348:g2669. [DOI] [PubMed] [Google Scholar]
Gurusamy 2016
- Gurusamy KS, Belgaumkar AP, Haswell A, Pereira SP, Davidson BR. Interventions for necrotising pancreatitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011383.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2009
- Johnson MD, Walsh RM, Henderson JM, Brown N, Ponsky J, Dumot J, et al. Surgical versus nonsurgical management of pancreatic pseudocysts. Journal of Clinical Gastroenterology 2009;43(6):586‐90. [DOI] [PubMed] [Google Scholar]
Larson 2006
- Larson SD, Nealon WH, Evers BM. Management of gallstone pancreatitis. Advances in Surgery 2006;40:265‐84. [DOI] [PubMed] [Google Scholar]
Moayyedi 2006
- Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia?. JAMA 2006;295(13):1566‐76. [DOI] [PubMed] [Google Scholar]
Mouli 2013
- Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta‐analysis. Gastroenterology 2013;144(2):333‐40.e2. [DOI] [PubMed] [Google Scholar]
Muddana 2009
- Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. American Journal of Gastroenterology 2009;104(1):164‐70. [DOI] [PubMed] [Google Scholar]
NCBI 2014a
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. www.ncbi.nlm.nih.gov/mesh/68010179 2014 (accessed 4 July 2014).
NCBI 2014b
- NCBI. MeSH. NLM Controlled Vocabulary. C‐reactive protein. www.ncbi.nlm.nih.gov/mesh/68010179 2014 (accessed 2 August 2014).
NCEPOD 2016
- NCEPOD. National Confidential Enquiry Into Patient Outcome and Death. Acute pancreatitis: Treat the cause (2016). www.ncepod.org.uk/2016ap.html 2016 (accessed 19 September 2016).
Peery 2012
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrov 2010
- Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139(3):813‐20. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
Roberts 2013
- Roberts SE, Akbari A, Thorne K, Atkinson M, Evans PA. The incidence of acute pancreatitis: impact of social deprivation, alcohol consumption, seasonal and demographic factors. Alimentary Pharmacology and Therapeutics 2013;38(5):539‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Sampson 2008
- Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. Journal of Clinical Epidemiology 2008;61(8):755‐62. [DOI] [PubMed] [Google Scholar]
Sanabria 2013
- Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004778.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmid 1999
- Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut 1999;45(2):311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2015
- Takwoingi Y, Guo B, Riley RD, Deeks JJ. Performance of methods for meta‐analysis of diagnostic test accuracy with few studies or sparse data. Statistical Methods in Medical Research 2015 Jun 26 [Epub ahead of print]. [DOI: 10.1177/0962280215592269] [DOI] [PMC free article] [PubMed]
Tenner 2013
- Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. American Journal of Gastroenterology 2013;108(9):1400‐15. [DOI] [PubMed] [Google Scholar]
van Brunschot 2014
- Brunschot S, Fockens P, Bakker OJ, Besselink MG, Voermans RP, Poley JW, et al. Endoscopic transluminal necrosectomy in necrotising pancreatitis: a systematic review. Surgical Endoscopy 2014;28(5):1425‐38. [DOI] [PubMed] [Google Scholar]
van Santvoort 2010
- Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, et al. A step‐up approach or open necrosectomy for necrotizing pancreatitis. New England Journal of Medicine 2010;362(16):1491‐502. [DOI] [PubMed] [Google Scholar]
van Santvoort 2011
- Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology 2011;141(4):1254‐63. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2008
- Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointestinal Endoscopy 2008;68(6):1102‐11. [DOI] [PubMed] [Google Scholar]
Varadarajulu 2013
- Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145(3):583‐90. [DOI] [PubMed] [Google Scholar]
Vissers 1999
- Vissers RJ, Abu‐Laban RB, McHugh DF. Amylase and lipase in the emergency department evaluation of acute pancreatitis. Journal of Emergency Medicine 1999;17(6):1027‐37. [DOI] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology. 2006/03/08 2006; Vol. 6:9. [DOI] [PMC free article] [PubMed]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Yadav 2006
- Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33(4):323‐30. [DOI] [PubMed] [Google Scholar]
Yang 2008
- Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol‐related liver and pancreatic disease in the United States. Archives of Internal Medicine 2008;168(6):649‐56. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2015
- Gurusamy KS, Davidson BR. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis and serum C‐reactive protein, procalcitonin and lactate dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD012010] [DOI] [Google Scholar]


