Abstract
Background
The treatment of people with clinically significant postoperative pancreatic leaks is different from those without clinically significant pancreatic leaks. It is important to know the diagnostic accuracy of drain fluid amylase as a triage test for the detection of clinically significant pancreatic leaks, so that an informed decision can be made as to whether the patient with a suspected pancreatic leak needs further investigations and treatment. There is currently no systematic review of the diagnostic test accuracy of drain fluid amylase for the diagnosis of clinically relevant pancreatic leak.
Objectives
To determine the diagnostic accuracy of amylase in drain fluid at 48 hours or more for the diagnosis of pancreatic leak in people who had undergone pancreatic resection.
Search methods
We searched MEDLINE, Embase, the Science Citation Index Expanded, and the National Institute for Health Research Health Technology Assessment (NIHR HTA) websites up to 20 February 2017. We searched the references of the included studies to identify additional studies. We did not restrict studies based on language or publication status, or whether data were collected prospectively or retrospectively. We also performed a 'related search' and 'citing reference' search in MEDLINE and Embase.
Selection criteria
We included all studies that evaluated the diagnostic test accuracy of amylase in the drain fluid at 48 hours or more for the diagnosis of pancreatic leak in people who had undergone pancreatic resection excluding total pancreatectomy. We planned to exclude case‐control studies because these studies are prone to bias, but did not find any. At least two authors independently searched and screened the references produced by the search to identify relevant studies.
Data collection and analysis
Two review authors independently extracted data from the included studies. The included studies reported drain fluid amylase on different postoperative days and measured at different cut‐off levels, so it was not possible to perform a meta‐analysis using the bivariate model as planned. We have reported the sensitivity, specificity, post‐test probability of a positive and negative drain fluid amylase along with 95% confidence interval (CI) on each of the different postoperative days and measured at different cut‐off levels.
Main results
A total of five studies including 868 participants met the inclusion criteria for this review. The five studies included in this review reported the value of drain fluid amylase at different thresholds and different postoperative days. The sensitivities and specificities were variable; the sensitivities ranged between 0.72 and 1.00 while the specificities ranged between 0.73 and 0.99 for different thresholds on different postoperative days. At the median prevalence (pre‐test probability) of 15.9%, the post‐test probabilities for pancreatic leak ranged between 35.9% and 95.4% for a positive drain fluid amylase test and ranged between 0% and 5.5% for a negative drain fluid amylase test.
None of the studies used the reference standard of confirmation by surgery or by a combination of surgery and clinical follow‐up, but used the International Study Group on Pancreatic Fistula (ISGPF) grade B and C as the reference standard. The overall methodological quality was unclear or high in all the studies.
Authors' conclusions
Because of the paucity of data and methodological deficiencies in the studies, we are uncertain whether drain fluid amylase should be used as a method for testing for pancreatic leak in an unselected population after pancreatic resection; and we judge that the optimal cut‐off of drain fluid amylase for making the diagnosis of pancreatic leak is also not clear. Further well‐designed diagnostic test accuracy studies with pre‐specified index test threshold of drain fluid amylase (at three times more on postoperative day 5 or another suitable pre‐specified threshold), appropriate follow‐up (for at least six to eight weeks to ensure that there are no pancreatic leaks), and clearly defined reference standards (of surgical, clinical, and radiological confirmation of pancreatic leak) are important to reliably determine the diagnostic accuracy of drain fluid amylase in the diagnosis of pancreatic leak.
Keywords: Aged, Female, Humans, Male, Middle Aged, Pancreatectomy, Pancreaticoduodenectomy, Amylases, Amylases/analysis, Anastomotic Leak, Anastomotic Leak/diagnosis, Biomarkers, Biomarkers/analysis, Clinical Enzyme Tests, Clinical Enzyme Tests/standards, Drainage, Pancreas, Pancreas/surgery, Prospective Studies, Retrospective Studies, Sensitivity and Specificity
Plain language summary
Amylase in drain fluid for the diagnosis of pancreatic leak after partial removal of the pancreas
Background
The pancreas is an organ in the abdomen that secretes pancreatic juice that aids digestion; and it contains cells that produce important hormones such as insulin. Partial removal of the pancreas (pancreatic resection) is performed to remove cancerous and non‐cancerous growths in the pancreas. During this process, new connections (anastomoses) are made between the pancreas and intestines and bile duct (a tube that transports bile from the liver to the intestines). These connections may break down and result in leakage of pancreatic content into the abdomen; this can lead to severe infections within the abdomen and in the blood stream, which can even lead to the death of the patient.
At the end of the operation, a drainage tube is inserted into the abdomen for two purposes: firstly, the detection of any fluid collections within the abdomen (intra‐abdominal collections), usually resulting from the pancreatic leaks; and secondly, as the treatment of intra‐abdominal collections, so that fluid collection decreases or, at least, does not worsen within the abdomen. The fluids from the drain can be tested for amylase (one of the contents of the pancreatic juice which digests carbohydrates) to find out whether the fluid in the drain is because of a pancreatic leak. If there is a high suspicion of a pancreatic leak, further scans are performed to confirm it or to rule it out. If the leak is major and the patient is unwell, urgent reoperation may be required. Moderate leaks can lead to intra‐abdominal infections: patients may need antibiotics, drugs that decrease pancreatic secretion, insertion of a new drainage tube or repositioning of the existing drainage tube to drain the infected collection, and supportive care to recover. Currently, it is unclear whether measuring the amylase content in the fluid from the drain inserted after pancreatic resection is useful in identifying pancreatic leaks.
Study characteristics
We performed a thorough literature search for studies reporting the accuracy of drain fluid amylase in identifying pancreatic leaks. We included studies reported up to 20 February 2017. We identified five studies reporting information on 868 people who underwent pancreatic resections for cancer and non‐cancerous growths. Most studies included only people in whom the head of the pancreas (right side of the pancreas) was removed.
Key results
Variations in when the studies measured the amylase content in the drain and what level was considered abnormal meant that we were not able to combine the data to provide the overall results. We are uncertain whether drain fluid amylase is useful in identifying pancreatic leaks because of the following reasons.
1. The way that study authors confirmed that a participant had or did not have pancreatic leak was itself subject to error (i.e. there was no true 'gold standard').
2. The studies included few participants. As a result, there was significant uncertainty in the results.
3. The studies were of poor methodological quality. This introduced additional uncertainty in the results.
Quality of evidence
All of the studies were of unclear or low methodological quality, which may result in arriving at false conclusions.
Summary of findings
Summary of findings'. 'Summary of findings table.
| Population | People undergoing pancreatic resection | ||||||||
| Setting | Secondary care in various countries | ||||||||
| Target condition | Clinically significant pancreatic leak | ||||||||
| Reference standard | International Study Group on Pancreatic Fistula (ISGPF) grade B or C | ||||||||
| Median prevalence of pancreatic leak | 15.9% | ||||||||
| Index test1 | Sensitivity | Specificity | Post‐test probability of a positive test2 | Post‐test probability of a negative test2 | Number of studies | Number of participants | Risk of bias | Applicability concerns | Plain language interpretation |
| POD:3 DFA > 600 IU/L | 0.86 (95% CI 0.68 to 0.96) | 0.73 (95% CI 0.65 to 0.80) | 37.9% (95% CI 31.1% to 45.1%) | 3.4% (95% CI 1.4% to 8.2%) | 1 | 182 | Unclear | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 38 people (95% CI 31 to 45) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 3 people (95% CI 1 to 8) have clinically significant pancreatic leak. |
| POD:3 to 5 DFA > 3 times serum amylase | 0.79 (95% CI 0.49 to 0.95) | 0.78 (95% CI 0.65 to 0.89) | 40.8% (95% CI 27.7% to 55.5%) | 4.9% (1.8% to 12.5%) | 1 | 65 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 41 people (95% CI 28 to 56) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 5 people (95% CI 2 to 13) have clinically significant pancreatic leak. |
| POD:4 DFA > 647 U/L | 0.72 (95% CI 0.53 to 0.86) | 0.91 (95% CI 0.82 to 0.97) | 60.7% (95% CI 41.1% to 77.4%) | 5.5% (95% CI 3.2% to 9.3%) | 1 | 100 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 61 people (95% CI 41 to 77) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 6 people (95% CI 3 to 9) have clinically significant pancreatic leak. |
| POD:5 DFA > 3 times serum amylase | 1.00 (95% CI 0.29 to 1.00) | 0.94 (95% CI 0.82 to 0.99) | 74.8% (95% CI 49.8% to 89.9%) | 0% (95% CI not estimable) | 1 | 50 | Unclear | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 75 people (95% CI 50 to 90) have clinically significant pancreatic leak. It was not possible to estimate the number of people with clinically significant pancreatic leak when the test was negative. |
| POD:5 DFA > 4000 U/L | 0.75 (95% CI 0.58 to 0.88) | 0.99 (95% CI 0.98 to 1.00) | 95.4% (95% CI 86.8% to 98.5%) | 4.6% (95% CI 2.6% to 7.8%) | 1 | 471 | High | High | At the median pre‐test probability of 16%, out of 100 people with positive test, 95 people (95% CI 87 to 99) have clinically significant pancreatic leak. At the same pre‐test probability, out of 100 people with negative test, 5 people (95% CI 3 to 8) have clinically significant pancreatic leak. |
| Interpretation | The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. A negative test more or less rules out pancreatic leak. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings. | ||||||||
1The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold.
2All post‐test probabilities were calculated at the median prevalence (pre‐test probability) of pancreatic leak in the studies. At the lower quartile of the prevalence of 7.6%, the post‐test probabilities of pancreatic leak of positive POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 21.0% (95% CI 16.5% to 26.4%), 23.2% (95% CI 14.3% to 35.2%), 40.3% (95% CI 23.4% to 59.9%), 56.5% (95% CI 30.3% to 79.5%), and 90.0% (95% CI 74.2% to 96.6%) respectively. At the same pre‐test probability, the post‐test probabilities of pancreatic leak of negative POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 1.5% (95% CI 0.6% to 3.7%), 2.2% (95% CI 0.8% to 5.9%), 2.5% (95% CI 1.4% to 4.3%), 0% (95% CI not estimable), and 2.0% (95% CI 1.2% to 3.5%) respectively. At the upper quartile of the prevalence of 21.5%, the post‐test probabilities of pancreatic leak of positive POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 46.9% (95% CI 39.6% to 54.4%), 50.0% (95% CI 35.7% to 64.3%), 69.1% (95% CI 50.3% to 83.2%), 81.1% (95% CI 59.0% to 92.8%), and 96.8% (95% CI 90.5% to 98.9%) respectively. At the same pre‐test probability, the post‐test probabilities of pancreatic leak of negative POD:3 DFA > 600 IU/L, POD:3 to 5 DFA > 3 times serum amylase, POD:4 DFA > 647 U/L, POD:5 DFA > 3 times serum amylase, and POD:5 DFA > 4000 U/L were 4.9% (95% CI 2.0% to 11.4%), 7.0% (95% CI 2.7% to 17.1%), 7.8% (95% CI 4.6% to 12.9%), 0% (95% CI not estimable), and 6.5% (95% CI 3.8% to 10.8%) respectively.
CI = confidence intervals
Background
Please see glossary of terms in Appendix 1.
The pancreas is an abdominal organ that secretes several digestive enzymes into the pancreatic ductal system that empties into the small bowel. It also houses the Islets of Langerhans, which secrete several hormones including insulin (NCBI 2014). Pancreatic resection is performed to treat pancreatic diseases, including pancreatic cancer, pre‐cancerous pancreatic lesions, and chronic pancreatitis. Pancreatic resection is in the form of pancreaticoduodenectomy for lesions and disease of the head of the pancreas, and distal pancreatectomy for lesions in the body and tail of the pancreas (Park 2013). After pancreaticoduodenectomy, pancreato‐enteric anastomosis is performed to allow the drainage of pancreatic fluid into the small bowel. After distal pancreatectomy, the cut surface of the pancreatic remnant (pancreatic stump) is closed using staples or sutures (Diener 2011). Generally, an abdominal drain is placed after pancreatic resection, although this practice has been questioned (van der Wilt 2013).
Pancreatic resection is a surgical procedure with high morbidity. It carries a postoperative mortality of around 4.5% (Gurusamy 2013). Approximately 30% of patients develop one or more postoperative complications (Gurusamy 2013). Approximately 18% of patients develop postoperative pancreatic leak or postoperative pancreatic fistula (POPF) making it one of the common complications of pancreatic resection (Gurusamy 2013). POPF is an abnormal communication containing enzyme‐rich pancreatic fluid between the pancreatic ductal epithelium and another epithelial surface. It represents a failure of healing or sealing of the pancreato‐enteric anastomosis or it may represent a parenchymal leak not directly related to an anastomosis, such as a leak from the raw pancreatic surface after distal pancreatectomy (Bassi 2005). Pancreatic leak includes leak of pancreatic fluid or intestinal contents into the general abdominal cavity. The leak may be self‐contained and minimal or may lead to peritonitis or life‐threatening general sepsis.
Clinically, POPF can be defined as an output via an operatively placed drain (or a subsequently placed, percutaneous drain) of any measurable volume of drain fluid on or after postoperative day 3, with an amylase content greater than three times the upper normal serum value according to the definition by the International Study Group on Pancreatic Fistula (ISGPF) (Bassi 2005). Various other definitions exist (Bassi 2005). ISGPF has graded postoperative fistulas as Grade A, Grade B, and Grade C based on their respective clinical impact, as shown below and Table 2 (Bassi 2005).
1. International study group postoperative pancreatic fistula.
| Grade | A | B | C |
| Clinical conditions | Well | Often well | Usually ill |
| Ultrasound/CT (computed tomogram) (if obtained) | Negative | Negative/positive | Positive |
| Persistent drainage (after 3 weeks) | No | Usually yes | Yes |
| Reoperation | No | No | Yes |
| Death related to postoperative pancreatic fistula | No | No | Possibly yes |
| Signs of infections | No | Yes | Yes |
| Sepsis | No | No | Yes |
| Readmission | No | Yes/no | Yes/no |
Modified from Bassi 2005.
Grade A: This grade of fistula has no clinical impact and requires little change in management or deviation from the normal clinical pathway.
Grade B: This grade of fistula requires a change in management or adjustment in the clinical pathway. Many people with this grade of fistula can be discharged with drains in situ and observed in the outpatient setting. However, there is no requirement for an invasive procedure.
Grade C: This grade of fistula requires a major change in clinical management or deviation from the normal clinical pathway. People with this grade of fistula typically require an extended hospital stay with a major delay in hospital discharge; and they often undergo reoperation.
Various interventions to decrease postoperative leaks include pancreaticogastrostomy rather than pancreaticojejunostomy after pancreatic resections (McKay 2006), somatostatin analogues to decrease pancreatic fluid secretion (Gurusamy 2013), and fibrin sealants (in the form of glue (Suzuki 1995) or patches (Montorsi 2012)) to seal the pancreatic stump. Despite one or more of these measures, approximately 14% of patients develop a pancreatic fistula (Gurusamy 2013).
Target condition being diagnosed
Clinically significant postoperative pancreatic leak (clinically significant pancreatic fistula or other leaks requiring intervention).
Index test(s)
Amylase in drain fluid
Amylase is an enzyme secreted by the pancreas. Various other tissues including salivary glands, small intestines, ovaries, adipose tissue and skeletal muscles secrete amylase. There are two major isoforms of amylase — pancreatic amylase and salivary amylase (Vissers 1999). High amylase in the drain fluid indicates pancreatic leak since the pancreas is the source of pancreatic amylase and without a leak, the pancreatic fluid drains into the small intestine. Amylase can be measured by immunochemical assays, usually with monoclonal antibodies (Maeda 2008; Mifflin 1985). The test is conducted by the laboratory technicians and interpreted by the clinicians managing the patient. Drain fluid amylase content greater than three times the upper normal serum value is considered to be abnormal (Bassi 2005). Normal serum amylase can vary between laboratories but is usually between 100 IU/L and 300 IU/L (Vissers 1999).
Clinical pathway
When there is a high suspicion of pancreatic fistula, usually based on high amylase content of drain fluid, further radiological investigations such as a computed tomography (CT) scan are performed to identify and subsequently deal with identified pancreatic leaks. Grade A POPF is not associated with any peripancreatic fluid collections and the patient is clinically well. The major difference in management of people with Grade A POPF and those without pancreatic fistula is the delayed removal of drains. Grade B POPF may be associated with peripancreatic collections on CT scan. The patient may require repositioning of the drain if there is a peripancreatic collection, and usually requires enteral or parenteral nutritional support. Depending upon the clinical signs and symptoms such as abdominal pain, fever, and elevated white cell count, antibiotics and somatostatin analogues may be required. Grade C POPF is usually associated with peripancreatic fluid collections on CT scan and these often require reoperation. Patients with grade B and C POPF usually require enteral or parenteral nutritional support, intravenous antibiotics, and somatostatin analogues, and are usually managed in an intensive therapy unit setting. If there is clinical deterioration and development of sepsis and organ dysfunction, reoperation with a view to repair the site of leakage, conversion to an alternative means of pancreato‐enteric anastomosis (e.g. conversion of pancreaticojejunostomy to pancreaticogastrostomy), or a complete pancreatectomy may be necessary. Thus, the presence and grade of pancreatic leak alters the treatment pathway. This is shown in Figure 1. If there is a high suspicion of pancreatic leak because of the presence of peritonitis or sepsis, patients may undergo further radiological investigations directly, without waiting for the drain fluid amylase measurement.
1.
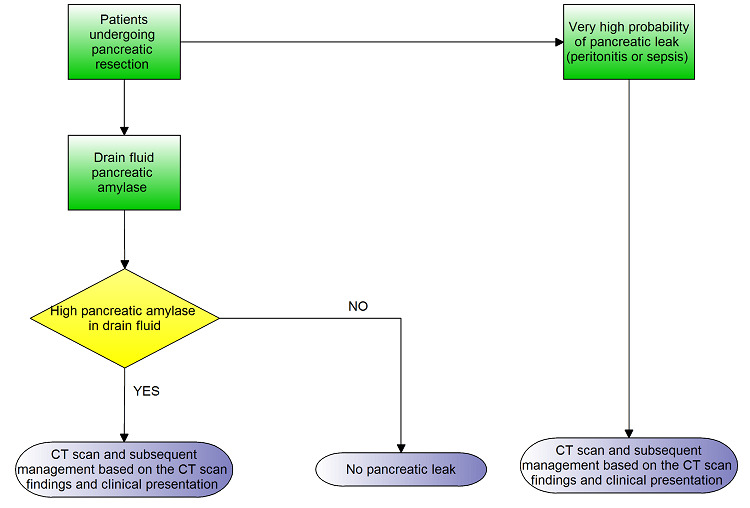
Clinical pathway
Prior test(s)
Amylase in the drain fluid (the index test) is usually the first investigation performed in people with suspected pancreatic leak.
Role of index test(s)
The index test is used to test for pancreatic leak in an unselected population after pancreatic resection. It is usually followed by CT scan or magnetic resonance cholangiopancreatography (MRCP) to confirm the presence or absence of peripancreatic collection and pancreatic leak. Thus, drain fluid amylase can be considered as a triage test prior to CT scan or MRCP in the diagnosis of pancreatic leak.
Rationale
The treatment of patients with clinically significant pancreatic leaks is different from those without clinically significant pancreatic leaks as mentioned in the clinical pathway. It is important to know the true diagnostic accuracy of drain fluid amylase as a method for testing for clinically significant pancreatic leak in an unselected population after pancreatic resection, so that an informed decision can be made as to whether the person with a suspected pancreatic leak needs further investigations. There is currently no systematic review of the diagnostic test accuracy of amylase in the drain fluid for the diagnosis of pancreatic leak. Hence, a Cochrane Review of this subject is necessary.
Objectives
To determine the diagnostic accuracy of amylase in drain fluid at 48 hours or more for the diagnosis of pancreatic leak in people who had undergone pancreatic resection.
Secondary objectives
If we identified heterogeneity, we planned to explore heterogeneity by using the following sources of heterogeneity as covariate(s) in the regression model.
Studies at low risk of bias in all the domains versus those at unclear or high risk of bias (as assessed by the QUADAS‐2 tool, recommended by the Cochrane Diagnostic Test Accuracy Group) (Whiting 2006; Whiting 2011).
Full‐text publications versus abstracts (this can give an idea about publication bias since there may be an association between the results of the study and the study reaching full publication status) (Eloubeidi 2001).
Prospective studies versus retrospective studies.
Pancreatoduodenectomies versus distal pancreatic resection.
Participants with cancers versus those with benign diseases.
Different reference standards (confirmation by surgical resection in all participants versus a combination of surgical resection and clinical follow‐up).
Methods
Criteria for considering studies for this review
Types of studies
We included all studies that evaluated the diagnostic test accuracy of amylase in drain fluid for the diagnosis of clinically significant pancreatic leak in people who had undergone pancreatic resection excluding total pancreatectomy. We included studies that provided information on the index test and reference standards irrespective of language or publication status, or whether the data was collected prospectively or retrospectively. However, we excluded case reports that describe how the diagnosis of pancreatic leak was made on an individual participant or a group of participants and which did not provide sufficient diagnostic test accuracy data, i.e. true positive, false positive, false negative, and true negative. We also excluded case‐control studies because these studies are prone to bias (Whiting 2011).
Participants
People who have undergone pancreatic resection with drain fluid at least 48 hours after pancreatic resection irrespective of the volume of the drain fluid.
Index tests
Drain fluid amylase.
Target conditions
Clinically significant pancreatic leak (pancreatic leaks that require radiological or surgical intervention)
Reference standards
We planned to accept one of the following reference standards as per our review protocol. However, according to these reference standards we could not have included any studies as none of the studies reported used one of the two reference standards below.
Pancreatic leak confirmed at surgery. This is confirmation of pancreatic leak at surgery usually on the basis of the presence of partial or complete separation of the anastomosis allowing leakage of contents, abdominal collections, or fistula (a tract between the anastomosis and exterior), and is a subjective decision made by the surgeon. Nevertheless, this is the best reference standard available.
Pancreatic leak confirmed at surgery for participants with elevated amylase and clinical follow‐up for a minimum period of six weeks (to ensure that they do not have complications due to pancreatic leak such as abdominal collections requiring drainage, intra‐abdominal sepsis, generalised sepsis resulting from intra‐abdominal sepsis, or mortality due to intra‐abdominal sepsis) in people with negative amylase. The clinical follow‐up should have included clinical examination of the patient, and may or may not have included radiological follow‐up done as follow‐up of suspected pancreatic leak or routine radiological follow‐up to detect the recurrence of cancer. In retrospective studies, we accepted hospital records of physical examination of the patient after a minimum follow‐up period of six weeks as an acceptable reference standard. The presence of one or more complications due to pancreatic leak such as abdominal collections requiring drainage, intra‐abdominal sepsis, generalised sepsis resulting from intra‐abdominal sepsis, or mortality due to intra‐abdominal sepsis was considered as a positive reference standard.
Because of the lack of any studies using one of the two reference standards mentioned above, we accepted ISGPF grades B and C POPF as reference standards. People with grade C POPF require surgery while those with grade B POPF usually do not undergo surgery but may require additional radiological drainage (Bassi 2005). These people with grade B POPF do not have systemic sepsis but have localised intra‐abdominal infection. Although the intra‐abdominal infections are usually because of pancreatic leaks in people undergoing pancreatic resection (these leaks are usually at least partially 'contained' (i.e. the effects limited) by the body's defence mechanism), one cannot be sure that the intra‐abdominal infection was because of pancreatic leak. So, the reference standards used in this review might misclassify the target condition of pancreatic leak.
Search methods for identification of studies
We included all studies irrespective of the language of publication and publication status. We obtained translations for articles found in non‐English language.
Electronic searches
We searched the following databases up to 20 February 2017.
MEDLINE OvidSP (January 1946 to 20 February 2017) (Appendix 2).
Embase OvidSP (January 1947 to 20 February 2017) (Appendix 3).
Science Citation Index Expanded via Web of Knowledge (January 1980 to 20 February 2017) (Appendix 4).
National Institute for Health Research (NIHR HTA) via Centre for Reviews and Dissemination (up to 20 February 2017) (Appendix 5).
Searching other resources
We searched the references of the included studies to identify additional studies. We also searched for articles related to the included studies by performing the 'related search' function in MEDLINE (OvidSP) and Embase (OvidSP) and a 'citing reference' search (by searching the articles which cite the included articles; Sampson 2008) in these two databases.
Data collection and analysis
Selection of studies
Two review authors (TD and KG) independently searched the references produced by the search to identify relevant studies. We obtained the full texts of the references that were considered relevant by at least one of the review authors. Two authors (TD and KG) independently screened the full‐text papers against the inclusion criteria. We resolved any differences in study selection by discussion. We selected studies that met the inclusion criteria for data extraction irrespective of the publication status.
Data extraction and management
Two review authors (TD and KG) independently extracted the following data from each included study using a pre‐piloted data extraction form; the two authors settled any differences by discussion.
First author and contact details.
Year of publication.
Publication status (abstract or full‐text).
Study design (prospective or retrospective cohort studies; cross‐sectional studies or randomised controlled trials).
Inclusion and exclusion criteria for individual studies.
Total number of participants.
Number of females.
Average age of the participants.
Proportion of pancreatoduodenectomies.
Proportion of participants with cancers.
Description of the index test.
Threshold used for index test.
Reference standard.
Number of true positives, false positives, false negatives, and true negatives (diagnostic test accuracy data).
If the same study reported the index test at different thresholds, we planned to calculate true positives, false positives, false negatives, and true negatives for the index test at different thresholds, and extract this information for each threshold. We excluded participants with uninterpretable index test results (no matter the reason given for lack of interpretation, for example low volume of drain fluid) since in clinical practice, uninterpretable index test results will result in additional tests such as CT scan for diagnosis of pancreatic leak. However, we recorded the number of uninterpretable index test results as this provides information on the applicability of the test in clinical practice, and may affect the cost‐effectiveness of a test (the cost‐effectiveness is outside the scope of this review; cost‐effectiveness studies may use data from this review). Further information was sought from study authors if necessary.
Assessment of methodological quality
Two authors (TD and KG) independently assessed study quality using the QUADAS‐2 assessment tool (Whiting 2006; Whiting 2011). We resolved any differences in the methodological quality assessment by discussion between us until we reached a consensus. The criteria used for this is shown in Table 3; we decided these a priori and published them in the protocol (except for the reference standard, which was revised to include the new reference standard that we accepted). We considered studies which were classified as 'low risk of bias' and 'low concern' in all the domains as studies of high methodological quality.
2. QUADAS‐2 classification.
| Domain 1: Patient selection | Patient sampling | Patients who have undergone pancreatic resection with drain fluid at least 48 hours after pancreatic resection irrespective of the volume of the drain fluid. |
| Was a consecutive or random sample of patients enrolled? | Yes: if a consecutive sample or a random sample of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was included in the study. No: if a consecutive sample or a random sample of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was not included in the study. Unclear: if this information was not available. | |
| Was a case‐control design avoided? | Yes: if a cohort of patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection was studied. No: if patients with pancreatic leak were compared with patients without pancreatic leak (controls). We planned to exclude such studies. Unclear: as anticipated, we were able to determine whether the design was case‐control. There were no case‐control studies. Hence, as anticipated, all studies included in the review were classified as 'yes' for this item. | |
| Did the study avoid inappropriate exclusions? | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included. No: if the study excluded patients based on high or low probability of pancreatic leak (for example, those with high volume in the drain). Unclear: if this information was not available. | |
| Could the selection of patients have introduced bias? | Low risk of bias: if 'yes' classification for all of the above 3 questions. High risk of bias: if 'no' classification for any of the above 3 questions. Unclear risk of bias: if 'unclear' classification for any of the above 3 questions but without a 'no' classification for any of the above 3 questions. |
|
| Patient characteristics and setting | Yes: if all patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were included. No: if some patients with pancreatic resection with drain fluid at least 48 hours after pancreatic resection were excluded on the basis of the results of drain fluid volume. Unclear: if it was not clear whether the patients had been included on the basis of the results of drain fluid volume. | |
| Are there concerns that the included patients and setting do not match the review question? | Low concern: if the patient characteristics and setting were classified as 'yes'. Unclear concern: if the patient characteristics and setting were classified as 'unclear'. High concern: if the patient characteristics and setting were classified as 'no'. |
|
| Domain 2: Index test | Index test(s) | Amylase in drain fluid. |
| Were the index test results interpreted without knowledge of the results of the reference standard? | The index test would always be conducted though not interpreted before the reference standard. Yes: if the index test was conducted and interpreted without the knowledge of the results of the reference standard. No: if the index test was interpreted with the knowledge of the results of the reference standard. Unclear: if it was not clear whether the index test was interpreted without the knowledge of the results of the reference standard. |
|
| If a threshold was used, was it pre‐specified? | Yes: if a pre‐specified threshold was used. No: if a pre‐specified threshold was not used. Unclear: if it was not clear whether the threshold used was pre‐specified. |
|
| Could the conduct or interpretation of the index test have introduced bias? | Low risk of bias: if 'yes' classification for both of the above questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. |
|
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: if the criteria for positive index test was clearly stated. High concern: if the criteria for positive index test was not stated. |
|
| Domain 3: Target condition and reference standard | Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak (requiring clinical intervention). Planned reference standards (see below).
|
| Is the reference standard(s) likely to correctly classify the target condition? | Yes: if pancreatic leak was confirmed at reoperation.
No: if the reference standard was a combination of pancreatic leak and clinical follow‐up for a minimum period of 6 weeks (to ensure that they do not have complications due to pancreatic leak such as abdominal collections requiring drainage, intra‐abdominal sepsis, or generalised sepsis) in people with negative amylase. Unclear: although we planned to exclude studies if the reference standard was not described adequately or was not one of the above planned reference standards, this would have meant that there would have been no studies included in the review. So, we accepted the ISGPF grades B and C as an appropriate references standard and classified the answer to this signalling question as unclear. |
|
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes: if the reference standard was interpreted without the knowledge of the results of the index test. No: if the reference standard was interpreted with the knowledge of the results of the index test. Unclear: it is not clear if the reference standard was interpreted without the knowledge of the results of the index test. | |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk of bias: if 'yes' classification for both of the above 2 questions. High risk of bias: if 'no' classification for any of the above 2 questions. Unclear risk of bias: if 'unclear' classification for any of the above 2 questions but without a 'no' classification for any of the above 2 questions. |
|
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Although we anticipated that all of the included studies would be classified as 'low concern' because of the reference standards we planned to use, we have classified all the studies as 'high concern' because of the reference standards that we accepted. | |
| Domain 4: Flow and timing | Flow and timing | Patients may have progression or resolution of pancreatic leak if there is a long delay between index test and reference standard. An arbitrary 2 weeks was chosen as an acceptable delay between index test and reference standard. |
| Was there an appropriate interval between index test and reference standard? | Yes: if the time interval between index test and reference standard was less than 2 weeks. No: if the time interval between index test and reference standard was more than 2 weeks. Unclear: if the time interval between index test and reference standard was unclear. | |
| Did all patients receive a reference standard? | Yes: if all patients received a reference standard. No: if some of the patients did not receive a reference standard. Such studies were excluded. Unclear: if it was not clear whether all patients received a reference standard. Such studies were excluded. As anticipated, all studies included in the review were classified as 'yes' for this item. | |
| Did all patients receive the same reference standard? | Yes: if all the patients received the same reference standard.
No: if different patients received different reference standards. Unclear: if this information was not clear. Because of the inclusion criteria, all the studies in this review were classified as 'yes' for this signalling question. |
|
| Were all patients included in the analysis? | Yes: if all the patients are included in the analysis irrespective of whether the results were interpretable. No: if some patients are excluded from the analysis because of uninterpretable results. Unclear: if this information is not clear. | |
| Could the patient flow have introduced bias? | Low risk of bias: if 'yes' classification for all the above 4 questions. High risk of bias: if 'no' classification for any of the above 4 questions. Unclear risk of bias: if 'unclear' classification for any of the above 4 questions but without a 'no' classification for any of the above 4 questions. |
ISGPF = International Study Group on Pancreatic Fistula
Statistical analysis and data synthesis
We plotted study estimates of sensitivity and specificity on forest plots and in receiver operating characteristics (ROC) space to explore variation in the performance of drain fluid amylase due to differences in threshold. When a study evaluated an increasing trend in drain fluid amylase values (by repeated testing) in the same cohort study group, we considered this as the 'threshold' for the purpose of this review.
To estimate the summary sensitivity and specificity of drain fluid amylase at each threshold, we had planned to perform meta‐analyses using the bivariate model (Chu 2006; Reitsma 2005). However, because there were few studies and the studies were performed on different postoperative days using different thresholds, meta‐analysis was not possible. To summarise the findings from individual studies, we estimated median, and lower and upper quartiles of pre‐test probabilities across the included studies. Post‐test probabilities were then calculated for each study using these pre‐test probabilities and the positive and negative likelihood ratios from the study. The post‐test probability associated with a positive test is the probability of having pancreatic leak following a positive amylase test result. The post‐test probability associated with a negative test is the probability of having pancreatic leak following a negative test result.
Investigations of heterogeneity
We had planned to use bivariate meta‐regression (adding a covariate to a bivariate model) to investigate the following potential sources of heterogeneity: risk of bias, publication status, type of recruitment (prospective versus retrospective), type of pancreatic resection (pancreatoduodenectomies versus distal pancreatic resection), different aetiologies, and different reference standards. We were unable to formally explore heterogeneity as we did not perform a meta‐analysis.
Sensitivity analyses
We did not plan to conduct any sensitivity analyses except when the data available from the studies were ambiguous (for example, the numbers in the text are different from the numbers in the figures); we did not find any such ambiguity in our review.
Assessment of reporting bias
We did not explore any of the planned investigation to see whether the summary sensitivity and specificity were different between studies that are published as full texts and those that are available only as abstracts (at least two years prior to the search date). This is because only one of the included studies was published as an abstract and the thresholds of index tests were different between the included studies (Araki 2012).
Results
Results of the search
We identified a total number of 2701 references through the electronic searches of MEDLINE (n = 594), Embase (n = 1695), Science Citation Index Expanded (n = 389), and National Institute for Health Research (NIHR HTA) (n = 23). We excluded 897 duplicates and 1724 clearly irrelevant references through reading the titles or abstracts, or both. We retrieved full‐text articles of 80 references for further assessment against our review protocol inclusion criteria. Of the 80 references (68 studies), we excluded 73 references (63 studies) for the reasons listed in Characteristics of excluded studies. Five studies (seven references) fulfilled the inclusion criteria and provided the diagnostic accuracy data for the review (Araki 2012; El Nakeeb 2013; Facy 2012; Kong 2008; Kosaka 2014). We have shown the reference flow in Figure 2.
2.
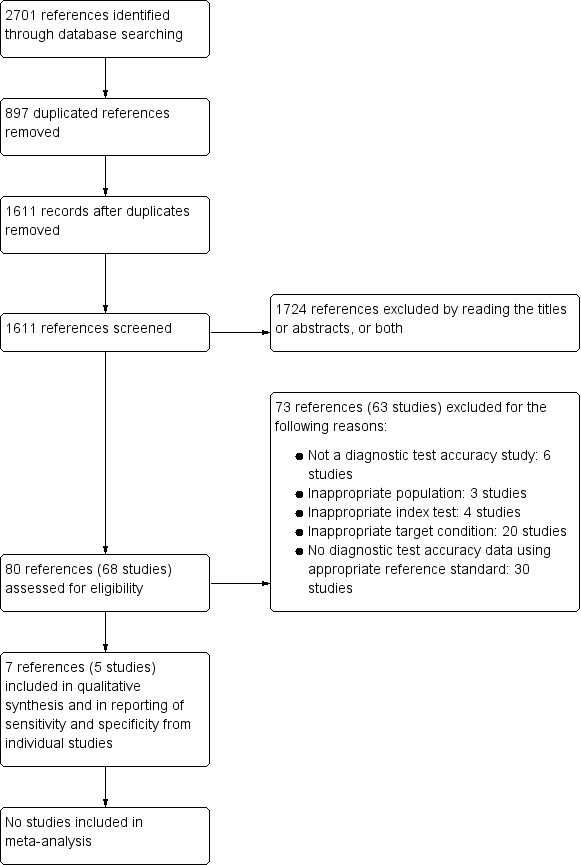
Study flow diagram.
Included studies
All the included studies assessed pancreatic leak following pancreatic resection excluding total pancreatectomy. A total of 868 participants were analysed in the five included studies. All the studies except Facy 2012 included only participants who underwent pancreaticoduodenectomy. The average age of participants in the studies that reported this information ranged between 53 years and 67 years; and about a third of participants (32%) were females in these four studies (El Nakeeb 2013; Facy 2012; Kong 2008; Kosaka 2014). Except for Araki 2012, which was an abstract, the remaining four studies were full‐text publications. Only two studies were prospective (Facy 2012; Kong 2008); the remaining three studies were retrospective studies (Araki 2012; El Nakeeb 2013; Kosaka 2014).
Excluded studies
A total of 64 studies were excluded at the full‐text stage for the following reasons.
Not a diagnostic test accuracy study: six studies (Fong 2016; Palani Velu 2015; Ramesh 2006; Sutcliffe 2015; Teixeira 2016; Yang 2015).
Inappropriate population: three studies (Kanda 2014; Kobayashi 2015; Mcmillan 2015).
Inappropriate index test: four studies (Kawai 2011; Kosaka 2013; Kosaka 2014a; Prakash 2011).
Inappropriate target condition: 20 studies (Cherian 2010; Cirocchi 2015; Israel 2014; Kumar 2013; Lee 2014; Malleo 2014; Menon 2012; Molinari 2007; Nissen 2012; Partelli 2014; Raja 2015; Sanchez Acedo 2013; Saxena 2014; Shi 2009; Shinchi 2006; Shyr 2003; Srivastava 2016; Sutcliffe 2012; Sutcliffe 2014; Zelga 2015).
No diagnostic test accuracy data using appropriate reference standard: 30 studies (Ansorge 2014; Burdy 1999; Ceroni 2014; Chen 2015; Chhabra 2011; Dugalic 2014; Furukawa 2015; Gebauer 2012; Graham 2013; Hashimoto 2003; Hashimoto 2014; Hiyoshi 2013; Ho 2014; Kim 2014; Kurahara 2011; Mimura 2012; Moskovic 2010; Musiewicz 2010; Noji 2012; Okano 2011; Robinson 2010; Shimizu 2015; Sugimoto 2013; Tang 2015; Tsujie 2012; Uemura 2011; Uemura 2014; Veillette 2010; Ven Fong 2015; Yamaguchi 2003).
Methodological quality of included studies
The methodological quality of the included studies is shown in Characteristics of included studies table and a summary of the methodological quality is shown in Figure 3.
3.
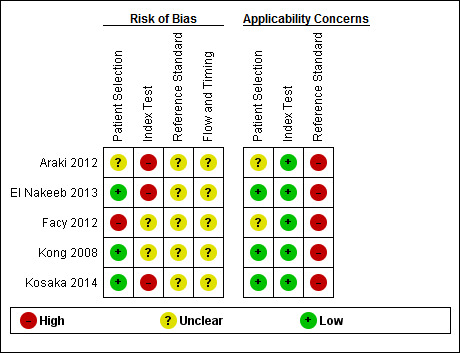
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Participant selection
Three studies had low risk of bias and low applicability concerns regarding the selection of participants, as these studies included consecutive patients (El Nakeeb 2013; Kong 2008; Kosaka 2014). One study had high risk of bias regarding the selection of participants and unclear applicability concerns because the participants without lipase tests were excluded (Facy 2012). The remaining one study was at unclear risk of bias with unclear concerns about applicability because this study did not mention whether a consecutive or random sample of patients was included, and whether there were any exclusions that we considered inappropriate (Araki 2012).
Index test
Regarding the index test, Facy 2012 and Kong 2008 were at unclear risk of bias; and Araki 2012, El Nakeeb 2013 and Kosaka 2014 were at high risk of bias. Three studies, in which it was clear that the thresholds were based on the ROC curve, were classified as high risk of bias (Araki 2012; El Nakeeb 2013; Kosaka 2014). It was not clear whether the index test results were interpreted without knowledge of the reference standard results in any of the studies. So, all studies have been classified as unclear or high risk of bias. All included studies had low concerns about applicability in the 'index test' domain because the criteria for positive index tests were clearly stated in the included studies.
Reference standard
None of the studies were at low risk of bias in this domain. All included studies were at unclear risk of bias because the reference standards used were the ISGPF definition. It was also not clear whether the reference standards were interpreted without knowledge of the index test results. All studies were at high concern about applicability. This is because the reference standards used in all the studies were the ISGPF definitions (grade B or C). There is a possibility of misclassification of target condition by this reference standard as detailed in the discussion.
Flow and timing
All the studies were at unclear risk of bias since the studies did not report the period that they followed up participants to determine whether they had grade B or C POPF. In addition, it was not clear whether some participants were excluded prior to analysis in two studies (Araki 2012; Facy 2012).
Findings
The included studies reported the value of drain fluid amylase measured on different days and at different thresholds, so a meta‐analysis was not performed. Three studies reported the participant flow clearly; in these studies, none of the participants has uninterpretable results (El Nakeeb 2013; Kong 2008; Kosaka 2014). The remaining two studies did not report any participants with uninterpretable results; however, one cannot rule out uninterpretable results in these studies since the participant flow was not reported (Araki 2012; Facy 2012).
The median pre‐test probability of clinically significant pancreatic leak (proportion with pancreatic leak out of total number of included participants) was 15.9%, with minimum of 6% and maximum of 32%. The lower and upper quartiles were 7.6% and 21.5% respectively. The sensitivity and specificity along with the 95% CI for each different threshold on different postoperative days are shown in forest plot (Figure 4), ROC space (Figure 5), and in the Table 1.
4.
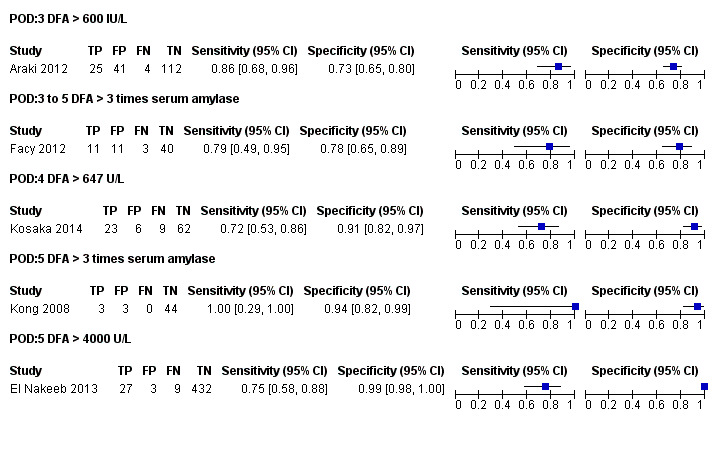
Forest plot of tests: The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold. The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings.
5.
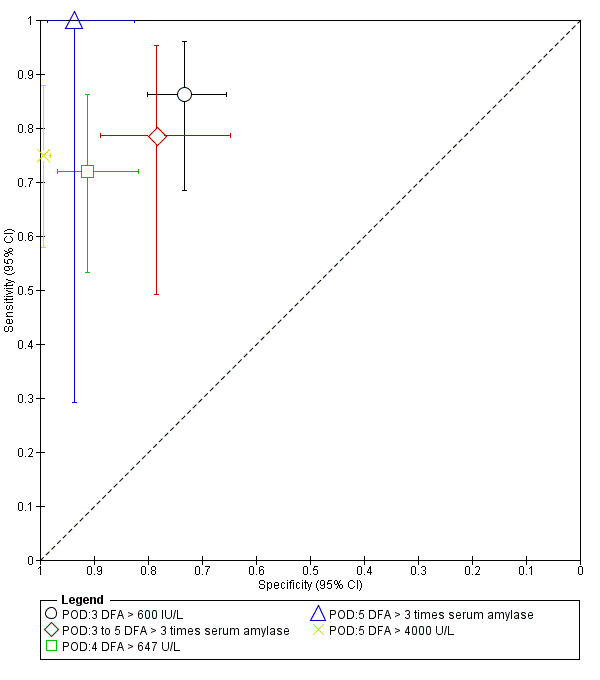
Plot of sensitivity and specificity in the ROC (receiver operating characteristics) space: The numbers following POD (postoperative day) indicate the number of the postoperative day. The numbers or text following DFA (drain fluid amylase) indicate the threshold. The drain fluid amylase measured on 5th postoperative day using a threshold of more than three times serum amylase provides the best sensitivity with high specificity. However, this is based on a single study with small sample size. Another study which used the same threshold between 3 days and 5 days has much less diagnostic test accuracy introducing significant uncertainty in the findings.
Drain fluid amylase at a cut‐off of greater than 600 IU/L on postoperative day 3
There were 182 participants in the only study with drain fluid amylase measured on postoperative day 3 using a cut‐off level of more than 600 IU/L (Araki 2012). At this threshold level, the sensitivity of diagnosing clinically relevant pancreatic leak was 0.86 (95% CI 0.68 to 0.96) and the specificity was 0.73 (95% CI 0.65 to 0.80).
Drain fluid amylase at a cut‐off of greater than three times serum amylase on postoperative days 3 to 5
There were 65 participants in the only study with drain fluid amylase measured on postoperative days 3 to 5 using a cut‐off value of more than three times serum amylase level (Facy 2012). At this cut‐off value, the sensitivity of diagnosing clinically significant pancreatic leak was 0.79 (95% CI 0.49 to 0.95) and its specificity was 0.78 (95% CI 0.65 to 0.89).
Drain fluid amylase at a cut‐off of greater than 647 IU/L on postoperative day 4
There were 100 participants in the only study with a drain fluid amylase measured on postoperative day 4 using a cut‐off value of more than 647 IU/L (Kosaka 2014). At this cut‐off value, the sensitivity of diagnosing clinically relevant pancreatic leak was 0.72 (95% CI 0.53 to 0.86) and its specificity was 0.91 (95% CI 0.82 to 0.97).
Drain fluid amylase at a cut‐off of greater than three times serum amylase on postoperative day 5
There were 50 participants in the only study with a drain fluid amylase measured on postoperative day 5 using a cut‐off value of more than three times serum amylase level (Kong 2008). At this cut‐off value, the sensitivity of diagnosing clinically relevant pancreatic leak was 1.00 (95% CI 0.29 to 1.00) and the specificity of this index test was 0.94 (95% CI 0.82 to 0.99).
Drain fluid amylase at a cut‐off of greater than 4000 IU/L on postoperative day 5
There were 471 participants in the only study with drain fluid amylase measured on postoperative day 5 using a cut‐off value of more than 4000 IU/L (El Nakeeb 2013). At this cut‐off level, the sensitivity of clinically significant pancreatic leak diagnostic accuracy was 0.75 (95% CI 0.58 to 0.88) and the specificity was 0.99 (95% CI 0.98 to 1.00).
Discussion
Summary of main results
A total of five studies including 868 participants met the inclusion criteria for this review (Araki 2012; El Nakeeb 2013; Facy 2012; Kong 2008; Kosaka 2014). The median prevalence of clinically significant POPF (grades B and C) was 15.9%.
The five studies included in this review reported the value of drain fluid amylase at different thresholds and different postoperative days. We were unable to perform any meta‐analysis or exploration of heterogeneity because of paucity of data. The sensitivities and specificities were variable: the sensitivities ranged between 0.72 and 1.00 while the specificities ranged between 0.73 and 0.99 for different thresholds on different postoperative days. At the median prevalence (pre‐test probability) of 15.9%, the mean post‐test probabilities for pancreatic leak ranged between 35.9% and 95.4% for a positive drain fluid amylase test and ranged between 0% and 5.5% for a negative drain fluid amylase test.
Strengths and weaknesses of the review
Strengths
One of the main strengths of this review was that we searched the literature thoroughly, without any publication or language restrictions. We did not use any diagnostic test accuracy filters in our literature search because filters of this kind could lead to exclusion of some relevant studies (Doust 2005). Inclusion of abstracts and non‐English articles may decrease the impact of publication bias to a certain extent although the determinants and extent of publication bias and selective reporting are not well known for diagnostic accuracy studies. We also planned to exclude case‐control studies because these studies are prone to bias (Whiting 2011). Two review authors (TD and KG) independently searched the references produced by the search to identify relevant studies, screened the full‐text papers against the inclusion criteria and extracted data. Data extractions by two authors potentially reduce the errors related to a single author data extraction (Buscemi 2006). Another strength of this review is that we used the recommended methodological quality methods to assess the risk of bias and applicability concerns in the included studies and took these into consideration while interpreting the evidence.
Weaknesses
There were several shortcomings in our review. First and foremost is the change of reference standard. In the protocol, we stated that we would use either surgical confirmation of presence or absence of pancreatic leak in all participants or at least surgical confirmation of presence or absence of pancreatic leak in participants with positive drain fluid amylase and clinical follow‐up in those with negative drain fluid amylase. We did not identify any studies that used either of these reference standards. So, we have accepted ISGPF grades B and C POPF as reference standards. While grade C POPF equates to surgical confirmation of presence of pancreatic leak, grade B POPF includes only POPF that do not require reoperation. However, grade B POPF includes POPF with intra‐abdominal infection and drainage usually persists for more than three weeks. Although persistent drainage of POPF can be managed by discharging the patient home with a drain with intermittent follow‐up visits to the hospital, it is likely to have a significant impact on return to work. So, there is no controversy on whether grade B POPF is clinically significant; the controversy is whether these are caused by pancreatic leaks. Pancreatic leak is the major cause of intra‐abdominal infection in people who undergo pancreatic resections. However, one cannot be absolutely sure that there was a clinically significant pancreatic leak in people with grade B POPF. While major aspects of treatment in people with grade B POPF is the same as that of confirmed pancreatic leaks not requiring reoperation but causing intra‐abdominal collections (minimally invasive drainage, antibiotics, and supportive treatment), knowing whether the fistula is due to pancreatic leak may help in deciding whether patients require interventions such as somatostatin analogues to decrease the pancreatic secretion. So, it is useful to distinguish the cause of grade B POPF. By using grade B or C POPF, there is a possibility of misclassification of the target condition (pancreatic leak) by the reference standards used (grade B or C POPF), i.e. some people without pancreatic leak may have been classified as having pancreatic leak by the reference standards used. The use of grade C POPF alone as reference standards would have resulted in an error in the opposite direction, i.e. some people with clinically significant pancreatic leak may have been misclassified as not having pancreatic leak. However, we were not able to test the diagnostic accuracy of the index test using grade C POPF as the reference standards since none of the studies reported diagnostic test accuracy data using grade C POPF alone as reference standards. While we agree that surgical confirmation cannot be performed in everyone with suspected pancreatic leak, one can expect that surgical confirmation can be performed in people with high suspicion of pancreatic leak based on the index test (drain pancreatic amylase) and clinical follow‐up for at least six weeks in those with low suspicion of pancreatic leak based on the index test (drain pancreatic amylase). Because of lack of these appropriate reference standards (and use of grade B or C as reference standards), there is a possibility of underestimation or overestimation of diagnostic accuracy of the index test.
Second, there were other methodological deficiencies besides the bias and concern related to reference standards. For example, three studies did not pre‐specify the drain fluid amylase threshold (Araki 2012; El Nakeeb 2013; Kosaka 2014); this would have resulted in overestimation of the diagnostic test accuracy. In addition, none of the studies reported whether the index tests and references standards were interpreted independent of each other. If they were not interpreted independent of each other, the accuracy of the tests would have been overestimated. None of the studies reported whether the participants were followed up for sufficient period of time to rule out grade B or C POPF. This could cause underestimation or overestimation of the diagnostic accuracy.
Third, the sample sizes in most of the studies were small, resulting in wide confidence intervals. It was not possible to perform a meta‐analysis since the studies reported drain fluid amylase on different postoperative days using different thresholds. Additionally, the measurement of drain fluid amylase on different postoperative days using different thresholds for diagnosis of pancreatic leak made it impossible for us to explore whether the results could be replicated in another group of people.
Comparison with other reviews
We identified two systematic reviews which evaluated the ability of drain fluid amylase on postoperative day 1 to predict the development of pancreatic fistula (Giglio 2016; Lu 2016); and one systematic review which included drain fluid amylase measured at any time in the diagnosis of any POPF (Yang 2015). All these studies concluded that drain fluid amylase on the first postoperative day is a good predictor of development of pancreatic fistula. However, the objectives of this review were different; we wanted to evaluate the role of drain fluid amylase in the diagnosis of clinically significant pancreatic leak. We were unable to find any systematic reviews addressing this question.
Applicability of findings to the review question
Generalisability of the results
Most of the participants included in this review were people who underwent pancreaticoduodenectomy surgery for benign and malignant conditions involving the pancreas. So, the findings of this review are applicable only for those undergoing pancreaticoduodenectomy.
Use of the test in clinical setting
The main role of the index test is as a triage test to identify people who require further scanning such as CT or MRI. Such a test needs to be a highly sensitive test, so that it is possible to rule out pancreatic leak, which will result in avoidance of further testing and allow the drain to be removed. The median prevalence of grade B or C POPF in the studies included in the review was 15.9%. The mean post‐test probabilities of pancreatic leak when the drain fluid amylase was negative ranged between 0% and 5.5%. However, the confidence intervals in these studies were higher and these ranged between 0% and 12.5%. Adding to this uncertainty were random errors resulting from small sample sizes: these generated a lot of systematic errors, resulting in further uncertainty. Because of these uncertainties, the role of drain fluid amylase in the diagnosis of pancreatic leak in people who undergo pancreatic resection is not clear.
Authors' conclusions
Implications for practice.
Because of the paucity of data and methodological deficiencies in the studies, it is not possible to arrive at any definitive conclusions i.e. there is no clear evidence whether clinicians should continue to use drain fluid amylase as a method for testing for clinically significant pancreatic leak in an unselected population after pancreatic resection; the optimal cut‐off of drain fluid amylase for making the diagnosis of pancreatic leak is also not clear.
Implications for research.
Further well‐designed diagnostic test accuracy studies with a pre‐specified index test threshold of drain fluid amylase (at three times more on postoperative day 5 or another suitable pre‐specified threshold), appropriate follow‐up (for at least six to eight weeks to ensure that there are no pancreatic leaks), and clearly defined reference standard (of surgical, clinical, and radiological confirmation of pancreatic leak) are important to determine the diagnostic accuracy of drain fluid amylase reliably.
Acknowledgements
We thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group, the United Kingdom Support Unit for Diagnostic Test Accuracy (DTA) Reviews, and the DTA editorial team for their advice in the preparation of this review. We also thank the librarians at Knowledge Centre, Bodleian Health Care Libraries, University of Oxford for supporting the author/student TD with a laptop and obtaining full texts of articles.
Appendices
Appendix 1. Glossary
Analogues: a substance that is similar to another substance.
Anastomoses: to be linked by anastomosis.
Anastomosis: connection of two structures (in this context, connection between pancreas and small bowel).
Antibodies: a blood protein produced in response to and counteracting a specific antigen.
Covariate: variable that is possibly predictive of the outcome under study.
Enteral: intestinal.
Epithelial surface: a surface lined by epithelium.
Epithelium: membranous tissue composed of one or more layers of cells. It forms the covering of most internal and external surfaces of the body and its organs.
Fistula: an abnormal duct or passage connecting a cavity or hollow organ to the body surface or to another hollow organ.
Heterogeneity: differences in results between studies.
Immunochemical: using antibodies (blood proteins produced in response to and counteracting a specific antigen such as bacteria, virus, or part of tissue) to find the presence of a substance or to measure the amount of a substance.
In situ: in its original position in the body.
Intra‐abdominal: situated within the abdomen.
Isoforms: two or more functionally similar proteins that have a similar but not identical composition.
Magnetic resonance cholangio pancreatography: medical imaging technique that uses magnetic resonance imaging (use of magnetic field to differentiate between different structures) to visualize the biliary and pancreatic ducts in a non‐invasive manner.
Monoclonal: forming a clone from a single individual or cell.
Morbidity: illness (in this context, it means complications).
Mortality: death.
Pancreatectomy: removal of part of pancreas.
Pancreatic ductal system: tubular system that transports the pancreatic juice secreted by the pancreatic cells to the small intestine.
Pancreatic leak: leakage of pancreatic section or intestinal contents into the abdomen, resulting in localised or blood stream infection.
Pancreaticoduodenectomy: removal of part of pancreas and duodenum (first part of the small intestine).
Pancreaticogastrostomy: connecting the pancreatic duct to the stomach.
Pancreaticojejunostomy: connecting the pancreatic duct to the jejunum (second part of the small intestine).
Pancreato‐enteric: connecting the pancreatic duct to the intestine.
Parenchymal: functional parts of an organ.
Parenteral: administered into the body in a manner other than through the gut (in this context by a drip).
Paucity: presence of something in a small amount.
Percutaneous: through the skin.
Peripancreatic: adjacent to the pancreas.
Peritonitis: inflammation of the lining of the abdomen, usually due to chemical irritation or infection.
Resection: the surgical removal of a body part.
Sepsis: life‐threatening illness due to blood infection with bacteria, fungus, or virus.
Thresholds: limits.
Appendix 2. MEDLINE search strategy
1. (ampulla vateri or ampullovateric or papilla vateri or vater papilla or vater ampulla or periampull* or peri‐ampull* or choledoch* or alcholedoch* or bile duct* or biliary or cholangio* or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*).ti,ab.
2. exp "Ampulla of Vater"/ or exp Pancreas/ or exp Bile Ducts/ or exp Duodenum/
3. 1 or 2
4. (surger* or surgical* or operat* or resection*).ti,ab.
5. 3 and 4
6. (pancreatect* or pancreaticojejunost* or pancreaticogastros* or pancreaticoduodenect* or duodenopancreatectom*).ti,ab.
7. exp Pancreatectomy/
8. exp Pancreaticojejunostomy/
9. exp Pancreaticoduodenectomy/
10. 5 or 6 or 7 or 8 or 9
11. (amylase or amylases).ti,ab.
12. exp Amylases/
13. 11 or 12
14. (drain* or leak or fistula).ti,ab.
15. exp Drainage/
16. exp Anastomotic Leak/
17. exp Pancreatic Fistula/
18. 14 or 15 or 16 or 17
19. 10 and 13 and 18
Appendix 3. Embase search strategy
1. (ampulla vateri or ampullovateric or papilla vateri or vater papilla or vater ampulla or periampull* or peri‐ampull* or choledoch* or alcholedoch* or bile duct* or biliary or cholangio* or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*).ti,ab.
2. exp duodenum cancer/ or Vater papilla tumor/ or exp pancreas cancer/ or exp bile duct tumor/
3. 1 or 2
4. (surger* or surgical* or operat* or resection*).ti,ab.
5. exp Surgery/
6. 4 or 5
7. 3 and 6
8. (pancreatect* or pancreaticojejunost* or pancreaticogastros* or pancreaticoduodenect* or duodenopancreatectom*).ti,ab.
9. exp pancreas surgery/
10. 7 or 8 or 9
11. amylase.ti,ab.
12. exp amylase/
13. 11 or 12
14. (drain* or leak or fistula).ti,ab.
15. exp drain/
16. exp anastomosis leakage/
17. exp pancreas fistula/
18. 14 or 15 or 16 or 17
19. 10 and 13 and 18
Appendix 4. Science Citation Index search strategy
#1 TS=(ampulla vateri or ampullovateric or papilla vateri or vater papilla or vater ampulla or periampull* or peri‐ampull* or choledoch* or alcholedoch* or bile duct* or biliary or cholangio* or gall duct or duoden* or small bowel or small intestin* or enter* or pancrea*) #2 TS=(operat* OR surger* OR surgical* OR resection*) #3 #1 AND #2 #4 TS=(pancreatect* OR pancreaticojejunost* OR pancreaticogastros* OR pancreaticoduodenect* OR duodenopancreatectom*) #5 #3 OR #4 #6 TS=(amylase) #7 TS=(drain* or leak or fistula) #8 #5 AND #6 AND #7
Appendix 5. National Institute for Health Research ‐ Health Technology Assessment search strategy
pancrea* AND amylase
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 POD:3 DFA > 600 IU/L | 1 | 182 |
| 2 POD:3 to 5 DFA > 3 times serum amylase | 1 | 65 |
| 3 POD:4 DFA > 647 U/L | 1 | 100 |
| 4 POD:5 DFA > 3 times serum amylase | 1 | 50 |
| 5 POD:5 DFA > 4000 U/L | 1 | 471 |
1. Test.

POD:3 DFA > 600 IU/L.
2. Test.

POD:3 to 5 DFA > 3 times serum amylase.
3. Test.

POD:4 DFA > 647 U/L.
4. Test.

POD:5 DFA > 3 times serum amylase.
5. Test.

POD:5 DFA > 4000 U/L.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Araki 2012.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: unclear. | ||
| Patient characteristics and setting | Sample size: 182. Females: not stated. Age: not stated. Presentation: people who underwent pancreaticoduodenectomy in a single center in Japan between April 2003 and May 2012 were included. Setting: secondary care, Japan. | ||
| Index tests | Index test: postoperative day 3 drain fluid amylase. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: > 600 IU/L. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. Reference standard: ISGPF grade B or C. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: ISGPF definitions. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). Number of patients who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
El Nakeeb 2013.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive sample. | ||
| Patient characteristics and setting | Sample size: 471. Females: 193 (41.0%). Age: 53 years. Presentation: people who underwent pancreaticoduodenectomy from January 2001 to June 2012 were included. Setting: secondary care, Egypt. | ||
| Index tests | Index test: postoperative day 5 drain fluid amylase. Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: > 4000 U/L. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. Reference standard: ISGPF grade B or C. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: ISGPF definitions. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). Number of patients who were excluded from the analysis: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Facy 2012.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study. Consecutive or random sample: neither. | ||
| Patient characteristics and setting | Sample size: 65. Females: 31 (47.69%). Age: 62 years. Presentation: people who underwent pancreatic resection between 2008 and 2010 and had the concentration of amylase and lipase measured in abdominal drains were included. People who underwent total pancreatectomy were not included. People in whom the lipase concentration was not measured were excluded from analysis. Setting: tertiary care, France. | ||
| Index tests | Index test: post operative day 3 to 5 drain fluid amylase. Further details: Technical specifications: Dimension Vista Colorimetric Analyser. Performed by: Dr David Masson. Criteria for positive diagnosis: 3 times normal limit. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. Reference standard: ISGPF grade B or C. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: ISGPF definitions. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). Number of participants who were excluded from the analysis: not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Kong 2008.
| Study characteristics | |||
| Patient sampling | Type of study: prospective study.. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 50. Females: 15 (30%). Age: 67 years. Presentation: people who underwent modified extended pancreaticoduodenectomy for a periampullary tumour between April 2004 and August 2006 at two hospitals in Australia were included. Setting: secondary and tertiary care, Australia. | ||
| Index tests | Index test: postoperative day 5 drain fluid amylase. Further details: Technical specifications: Roche Modular System and Roche Reagent Assays. Performed by: not stated. Criteria for positive diagnosis: > 125 u/ml (3 times serum amylase and 50 mls/24 h on D5). | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. Reference standard: ISGPF grade B or C. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: ISGPF definitions. | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). Number of patients who were excluded from the analysis: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Kosaka 2014.
| Study characteristics | |||
| Patient sampling | Type of study: retrospective study. Consecutive or random sample: consecutive patients. | ||
| Patient characteristics and setting | Sample size: 100. Females: 36 (36%). Age: 67 years. Presentation: people who underwent pancreaticoduodenectomy between January 2009 and October 2012 were included. Setting: secondary care, Japan. | ||
| Index tests | Index test: postoperative day 4 drain fluid amylase Further details: Technical specifications: not stated. Performed by: not stated. Criteria for positive diagnosis: 647 U/L. | ||
| Target condition and reference standard(s) | Target condition: clinically significant pancreatic leak. Reference standard: ISGPF grade B or C. Further details: Technical specifications: not applicable. Performed by: clinicians. Criteria for positive diagnosis: ISGPF definitions | ||
| Flow and timing | Number of indeterminates for whom the results of reference standard was available: 0 (0%). Number of patients who were excluded from the analysis: 0 (0%). | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
ISGPF = International Study Group on Pancreatic Fistula
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ansorge 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Burdy 1999 | No diagnostic test accuracy data using appropriate reference standard |
| Ceroni 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Chen 2015 | No diagnostic test accuracy data using appropriate reference standard |
| Cherian 2010 | Inappropriate target condition (target condition not defined adequately) |
| Chhabra 2011 | No diagnostic test accuracy data using appropriate reference standard |
| Cirocchi 2015 | Inappropriate target condition |
| Dugalic 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Fong 2016 | Not a diagnostic test accuracy study. |
| Furukawa 2015 | No diagnostic test accuracy data using appropriate reference standard |
| Gebauer 2012 | No diagnostic test accuracy data using appropriate reference standard |
| Graham 2013 | No diagnostic test accuracy data using appropriate reference standard |
| Hashimoto 2003 | No diagnostic test accuracy data using appropriate reference standard |
| Hashimoto 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Hiyoshi 2013 | No diagnostic test accuracy data using appropriate reference standard |
| Ho 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Israel 2014 | Inappropriate target condition |
| Kanda 2014 | Inappropriate population (patients without pancreatic fistula were excluded from the study) |
| Kawai 2011 | Inappropriate index test (drain fluid amylase was measured on post‐operative day 1 only) |
| Kim 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Kobayashi 2015 | Inappropriate population (not in patients undergoing pancreatic resection) |
| Kosaka 2013 | Inappropriate index test (not on drain fluid amylase) |
| Kosaka 2014a | Inappropriate index test |
| Kumar 2013 | Inappropriate target condition |
| Kurahara 2011 | No diagnostic test accuracy data using appropriate reference standard |
| Lee 2014 | Inappropriate target condition |
| Malleo 2014 | Inappropriate target condition |
| Mcmillan 2015 | Inappropriate population (people with amylase > 5000 IU were initially excluded; in addition, people with low unvalidated fistula risk score were excluded from the analysis) |
| Menon 2012 | Inappropriate target condition |
| Mimura 2012 | No diagnostic test accuracy data using appropriate reference standard |
| Molinari 2007 | Inappropriate target condition |
| Moskovic 2010 | No diagnostic test accuracy data using appropriate reference standard |
| Musiewicz 2010 | No diagnostic test accuracy data using appropriate reference standard |
| Nissen 2012 | Inappropriate target condition |
| Noji 2012 | No diagnostic test accuracy data using appropriate reference standard |
| Okano 2011 | No diagnostic test accuracy data using appropriate reference standard |
| Palani Velu 2015 | Not a diagnostic test accuracy study |
| Partelli 2014 | Inappropriate target condition |
| Prakash 2011 | Inappropriate index test |
| Raja 2015 | Inappropriate target condition |
| Ramesh 2006 | Not a diagnostic test accuracy study |
| Robinson 2010 | No diagnostic test accuracy data using appropriate reference standard |
| Sanchez Acedo 2013 | Inappropriate target condition |
| Saxena 2014 | Inappropriate target condition |
| Shi 2009 | Inappropriate target condition |
| Shimizu 2015 | No diagnostic test accuracy data using appropriate reference standard |
| Shinchi 2006 | Inappropriate target condition |
| Shyr 2003 | Inappropriate target condition |
| Srivastava 2016 | Inappropriate target condition. |
| Sugimoto 2013 | No diagnostic test accuracy data using appropriate reference standard |
| Sutcliffe 2012 | Inappropriate target condition |
| Sutcliffe 2014 | Inappropriate target condition |
| Sutcliffe 2015 | Not a diagnostic test accuracy study |
| Tang 2015 | No diagnostic test accuracy data using appropriate reference standard |
| Teixeira 2016 | Not a diagnostic test accuracy study |
| Tsujie 2012 | No diagnostic test accuracy data using appropriate reference standard |
| Uemura 2011 | No diagnostic test accuracy data using appropriate reference standard |
| Uemura 2014 | No diagnostic test accuracy data using appropriate reference standard |
| Veillette 2010 | No diagnostic test accuracy data using appropriate reference standard |
| Ven Fong 2015 | No diagnostic test accuracy data using appropriate reference standard |
| Yamaguchi 2003 | No diagnostic test accuracy data using appropriate reference standard |
| Yang 2015 | Not a diagnostic test accuracy study (systematic review) |
| Zelga 2015 | Inappropriate target condition |
Differences between protocol and review
Because of the lack of any studies using the reference standards described in the protocol, we accepted ISGPF grades B and C POPF as reference standards. People with grade C POPF require surgery while those with grade B POPF usually do not undergo surgery and may require minimally invasive drainage (Bassi 2005). These people with grade B POPF do not have sepsis but have localised intra‐abdominal infection. Although the intra‐abdominal infections are usually because of pancreatic leaks in people undergoing pancreatic resection (these leaks are usually at least partially 'contained' (i.e. the effects limited) by the body's defence mechanism), one cannot be sure that the intra‐abdominal infection was because of pancreatic leak. So, the reference standards used in this review might misclassify the target condition of pancreatic leak.
We searched MEDLINE through OvidSP platform rather than the PubMed platform. This was to avoid limitations of the search strategy in truncating the words searched to 600 variations when truncation terms were used.
Contributions of authors
TD independently screened the literature, extracted and analysed data. TD also wrote the first draft of the review based on a previously published protocol as per part of a MSc project in Evidence‐based Healthcare.
KS Gurusamy wrote the protocol, independently screened the literature, extracted data, and guided and supervised TD. He also revised the review draft significantly after submission of the MSc project.
M Yaghoobi and BR Davidson critically commented on the review.
Sources of support
Internal sources
-
University College London, UK.
Support salary, consumables (including printing, photocopying), and equipment (including laptop, scanner, printer, and any other equipment) necessary to complete the review in addition to payment for editorial support.
External sources
-
National Institute for Health Research, UK.
Support salary, consumables (including printing, photocopying), and equipment (including laptop, scanner, printer, and any other equipment) necessary to complete the review in addition to payment for editorial support.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR or the Department of Health.
TBUD: none known.
MY: none known.
BD: none known.
KSG: none known.
New
References
References to studies included in this review
Araki 2012 {published data only}
- Araki M, Yasuda T, Yoshioka Y, Nakata Y, Ishikawa H, Yamazaki M, et al. Utility of drain fluid amylase measurement on the third postoperative day after pancreaticoduodenectomy. Pancreas 2012;41(8):1347. [Google Scholar]
- Araki M, Yasuda T, Yoshioka Y, Nakata Y, Ishikawa H, Yamazaki M, et al. Utility of drain fluid amylase measurement on the third postoperative day after pancreaticoduodenectomy. Pancreatology 2013;13(2):e14. [Google Scholar]
El Nakeeb 2013 {published data only}
- Nakeeb A, Salah T, Sultan A, Hemaly M, Askr W, Ezzat H, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World Journal of Surgery 2013;37(6):1405‐18. [DOI] [PubMed] [Google Scholar]
- El‐Hanafy E, Askr W. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors and patients outcomes. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:294. [Google Scholar]
Facy 2012 {published data only}
- Facy O, Chalumeau C, Poussier M, Binquet C, Rat P, Ortega‐Deballon P. Diagnosis of postoperative pancreatic fistula. British Journal of Surgery 2012;99(8):1072‐5. [DOI] [PubMed] [Google Scholar]
Kong 2008 {published data only}
- Kong J, Gananadha S, Hugh TJ, Samra JS. Pancreatoduodenectomy: Role of drain fluid analysis in the management of pancreatic fistula. ANZ Journal of Surgery 2008;78(4):240‐4. [DOI] [PubMed] [Google Scholar]
Kosaka 2014 {published data only}
- Kosaka H, Kuroda N, Suzumura K, Asano Y, Okada T, Fujimoto J. Multivariate logistic regression analysis for prediction of clinically relevant pancreatic fistula in the early phase after pancreaticoduodenectomy. Journal of Hepato‐biliary‐pancreatic Sciences 2014;21(2):128‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ansorge 2014 {published data only}
- Ansorge C, Nordin J, Strommer L, Lundell L, Rangelova E, Blomberg J, et al. The diagnostic value of pancreatic amylase analyses from prophylactic abdominal drainage in identifying pancreatic fistula following pancreaticoduodenectomy. Pancreatology 2013;13(3 Suppl):S82. [Google Scholar]
- Ansorge C, Nordin JZ, Lundell L, Strommer L, Rangelova E, Blomberg J, et al. Diagnostic value of abdominal drainage in individual risk assessment of pancreatic fistula following pancreaticoduodenectomy. British Journal of Surgery 2014;101(2):100‐8. [DOI] [PubMed] [Google Scholar]
- Segersvard R, Blomberg J, Chiaro M, Rangelova E, Ansorge C. The diagnostic value of abdominal drainage in the individual risk assessment for pancreatic fistula following pancreaticoduodenectomy. Pancreatology 2013;13(4 Suppl):S8‐9. [DOI] [PubMed] [Google Scholar]
Burdy 1999 {published data only}
- Burdy G, Attal E, Frileux P, Dalban‐Sillas B, Safar MH, Voinchet O, et al. Analysis of the drainage fluid after cephalic duodenopancreatectomy: A reliable clinical criterion. Annales de Chirurgie 1999;53(3):191‐200. [PubMed] [Google Scholar]
Ceroni 2014 {published data only}
- Ceroni M, Galindo J, Guerra JF, Salinas J, Martinez J, Jarufe N. Amylase level in drains after pancreatoduodenectomy as a predictor of clinically significant pancreatic fistula. Pancreas 2014;43(3):462‐4. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen JY, Feng J, Wang XQ, Cai SW, Dong JH, Chen YL. Risk scoring system and predictor for clinically relevant pancreatic fistula after pancreaticoduodenectomy. World Journal of Gastroenterology 2015;21(19):5926‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherian 2010 {published data only}
- Cherian PT, Coldham C, Bramhall SR, Mirza DF, Buckels J, Mayer D. Drain fluid analysis post pancreaticoduodenectomy‐are we any wiser? A 10‐year, retrospective analysis of 558 patients. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2010;12:22. [Google Scholar]
Chhabra 2011 {published data only}
- Chhabra DG, Sutariya K, Shah RC, Jagannath P. Clinical validation of ISGPF for postoperative pancreatic fistula. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2011;13:102. [Google Scholar]
- Chhabra DG, Sutariya KG, Shah RG, Jagannath P. Clinical validation of postoperative pancreatic fistula. Pancreatology 2011;11:69. [Google Scholar]
Cirocchi 2015 {published data only}
- Cirocchi R, Graziosi L, Sanguinetti A, Boselli C, Polistena A, Renzi C, et al. Can the measurement of amylase in drain after distal pancreatectomy predict post‐operative pancreatic fistula?. International Journal of Surgery (London, England) 2015;21 Suppl 1:S30‐3. [DOI] [PubMed] [Google Scholar]
Dugalic 2014 {published data only}
- Dugalic VD, Knezevic DM, Obradovic VN, Gojnic‐Dugalic MG, Matic SV, Pavlovic‐Markovic AR, et al. Drain amylase value as an early predictor of pancreatic fistula after cephalic duodenopancreatectomy. World Journal of Gastroenterology 2014;20(26):8691‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fong 2016 {published data only}
Furukawa 2015 {published data only}
- Furukawa K, Gocho T, Horiuchi T, Shirai Y, Iwase R, Haruki K, et al. Amylase level of pancreatic juice after pancreaticoduodenectomy predicts postoperative pancreatic fistula. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2015;17:50‐1. [Google Scholar]
- Furukawa K, Gocho T, Shirai Y, Iwase R, Haruki K, Fujiwara Y, et al. The decline of amylase level of pancreatic juice after pancreaticoduodenectomy predicts postoperative pancreatic fistula. Pancreas 2016;45(10):1474‐7. [DOI] [PubMed] [Google Scholar]
Gebauer 2012 {published data only}
- Gebauer F, Kloth K, Tachezy M, Vashist YK, Cataldegirmen G, Izbicki JR, et al. Options and limitations in applying the fistula classification by the international study group for pancreatic fistula. Annals of Surgery 2012;256(1):130‐8. [DOI] [PubMed] [Google Scholar]
Graham 2013 {published data only}
- Graham JA, Kayser R, Smirniotopoulos J, Nusbaum JA, Johnson LB. Early predictor of outcome after pancreaticoduodenectomy with postoperative pancreatic fistula risk calculator. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2013;15:27. [Google Scholar]
Hashimoto 2003 {published data only}
- Hashimoto N, Yasuda C, Ohyanagi H. Pancreatic fistula after pancreatic head resection; incidence, significance and management. Hepato‐Gastroenterology 2003;50(53):1658‐60. [PubMed] [Google Scholar]
Hashimoto 2014 {published data only}
- Hashimoto N. Pancreatic juice output and amylase level in the drainage fluid after pancreatoduodenectomy in relation to leakage. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:652‐3. [Google Scholar]
- Hashimoto N, Ohyanagi H. Pancreatic juice output and amylase level in the drainage fluid after pancreatoduodenectomy in relation to leakage. Hepato‐gastroenterology 2002;49(44):553‐5. [PubMed] [Google Scholar]
Hiyoshi 2013 {published data only}
- Hiyoshi M, Chijiiwa K, Fujii Y, Imamura N, Nagano M, Ohuchida J. Usefulness of drain amylase, serum c‐reactive protein levels and body temperature to predict postoperative pancreatic fistula after pancreaticoduodenectomy. World Journal of Surgery 2013;37(10):2436‐42. [DOI] [PubMed] [Google Scholar]
Ho 2014 {published data only}
- Ho IG, Kim JK, Hwang HK, Kim JY, Park JS, Yoon DS. Does international study group on pancreatic fistula (ISGPF) classification need modification after distal pancreatectomy?. Korean Journal of Hepatobiliarypancreatic Surgery 2014;18(3):90‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Israel 2014 {published data only}
- Israel JS, Hanks LR, Rettammel RJ, Cho CS, Winslow ER, Weber SM. Does postoperative drain amylase predict pancreatic fistula following pancreatectomy?. Journal of Surgical Research 2013;179(2):193. [Google Scholar]
- Israel JS, Rettammel RJ, Leverson GE, Hanks LR, Cho CS, Winslow ER, et al. Does postoperative drain amylase predict pancreatic fistula after pancreatectomy?. Journal of the American College of Surgeons 2014;218(5):978‐87. [DOI] [PubMed] [Google Scholar]
Kanda 2014 {published data only}
- Kanda M, Fujii T, Takami H, Suenaga M, Inokawa Y, Yamada S, et al. Novel diagnostics for aggravating pancreatic fistulas at the acute phase after pancreatectomy. World Journal of Gastroenterology 2014;20(26):8535‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawai 2011 {published data only}
- Kawai M, Kondo S, Yamaue H, Wada K, Sano K, Motoi F, et al. Predictive risk factors for clinically relevant pancreatic fistula analyzed in 1,239 patients with pancreaticoduodenectomy: Multicenter data collection as a project study of pancreatic surgery by the japanese society of hepato‐biliary‐pancreatic surgery. Journal of Hepato‐biliary‐pancreatic Sciences 2011;18(4):601‐8. [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim JK, Yoon DS, Park JS. Which one is better for predicting pancreatic fistula after pancreaticoduodenectomy; drain amylase or lipase?. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:142‐3. [Google Scholar]
Kobayashi 2015 {published data only}
- Kobayashi D, Iwata N, Tanaka C, Kanda M, Yamada S, Nakayama G, et al. Factors related to occurrence and aggravation of pancreatic fistula after radical gastrectomy for gastric cancer. Journal of Surgical Oncology 2015;112(4):381‐6. [DOI] [PubMed] [Google Scholar]
Kosaka 2013 {published data only}
- Kosaka H, Kuroda N, Suzumura K, Uda Y, Kondo Y, Asano Y, et al. The prediction of clinically relevant pancreatic fistula after distal pancreatectomy by a multivariate logistic regression model. Pancreatology 2013;13(4 SUPPL.):S60‐S1. [Google Scholar]
Kosaka 2014a {published data only}
- Kosaka H, Asano Y, Suzumura K, Sueoka H, Uyama N, Okada T, et al. The validation analysis of our prediction method for postoperative pancreatic fistula after pancreas head resection. Pancreas 2014;43(8):1382. [Google Scholar]
Kumar 2013 {published data only}
- Kumar S, Bramhall SR, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, et al. Exclusion of pancreatic fistula after pancreatico‐duodenectomy in patients with a low drain fluid amylase on the first postoperative day. Pancreatology 2013;13 (1):e7. [Google Scholar]
Kurahara 2011 {published data only}
- Kurahara H, Shinchi H, Maemura K, Mataki Y, Iino S, Sakoda M, et al. Indicators of complications and drain removal after pancreatoduodenectomy. Journal of Surgical Research 2011;170(2):e211‐6. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee CW, Pitt H, Riall TS, Ronnekleiv‐Kelly S, Israel JS, Leverson G, et al. Does drain fluid amylase accurately predict pancreatic fistula?. Gastroenterology 2014;146(5 SUPPL):S1027‐S8. [Google Scholar]
- Lee CW, Pitt HA, Riall TS, Ronnekleiv‐Kelly SS, Israel JS, Leverson GE, et al. Low drain fluid amylase predicts absence of pancreatic fistula following pancreatectomy. Journal of Gastrointestinal Surgery 2014;18(11):1902‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malleo 2014 {published data only}
- Malleo G, Salvia R, Butturini G, D'Onofrio M, Martone E, Marchegiani G, et al. Is routine imaging necessary after pancreatic resection? An appraisal of postoperative ultrasonography for the detection of pancreatic fistula. Pancreas 2014;43(2):319‐23. [DOI] [PubMed] [Google Scholar]
Mcmillan 2015 {published data only}
- McMillan MT, Malleo G, Bassi C, Butturini G, Salvia R, Roses RE, et al. Drain management after pancreatoduodenectomy: Reappraisal of a prospective randomized trial using risk stratification. Journal of the American College of Surgeons 2015;221(4):798‐809. [DOI] [PubMed] [Google Scholar]
Menon 2012 {published data only}
- Menon V, Annamalai A, Puri V, Kotler H, Nissen NN. A simple algorithm of early drain removal after pancreaticoduodenectomy. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:53‐4. [Google Scholar]
Mimura 2012 {published data only}
- Mimura T, Niguma T, Kojima T. Pancreas texture and postoperative amylase value in the drainage fluid as better predictive factors for popf after pd. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2012;14:257. [Google Scholar]
Molinari 2007 {published data only}
- Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: Results of a prospective study in 137 patients. Annals of Surgery 2007;246(2):281‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moskovic 2010 {published data only}
- Moskovic D, Hodges S, Wu MF, Hilsenbeck S, Brunicardi FC, Fisher W. Drain data to predict clinically significant pancreatic leak. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2010;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musiewicz 2010 {published data only}
- Musiewicz M, Lampe P, Mrowiec S, Ciosek J, Badora A, Goyszny R, et al. Usefulness of indicating the value of the amylase in the drain after operations of the pancreas. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2010;12:326‐7. [Google Scholar]
Nissen 2012 {published data only}
- Nissen NN, Menon VG, Puri V, Annamalai A, Boland B. A simple algorithm for drain management after pancreaticoduodenectomy. American Surgeon 2012;78(10):1143‐6. [PubMed] [Google Scholar]
Noji 2012 {published data only}
- Noji T, Nakamura T, Ambo Y, Suzuki O, Nakamura F, Kishida A, et al. Clinically relevant pancreas‐related infectious complication after pancreaticoenteral anastomosis could be predicted by the parameters obtained on postoperative day 3. Pancreas 2012;41(6):916‐21. [DOI] [PubMed] [Google Scholar]
Okano 2011 {published data only}
- Okano K, Kakinoki K, Suto H, Oshima M, Kashiwagi H, Yamamoto N, et al. Persisting ratio of total amylase output in drain fluid can predict postoperative clinical pancreatic fistula. Journal of Hepato‐biliary‐pancreatic Sciences 2011;18(6):815‐20. [DOI] [PubMed] [Google Scholar]
Palani Velu 2015 {published data only}
- Palani Velu LK, Chandrabalan V, McMillan DC, McKay CJ, Carter CR, Jamieson NB, et al. Routine drainage after pancreaticoduodenectomy: Serum amylase can guide early, selective drain removal. Annals of Surgery 2015;262(6):e107. [DOI] [PubMed] [Google Scholar]
Partelli 2014 {published data only}
- Partelli S, Tamburrino D, Crippa S, Facci E, Zardini C, Falconi M. Evaluation of a predictive model for pancreatic fistula based on amylase value in drains after pancreatic resection. American Journal of Surgery 2014;208(4):634‐9. [DOI] [PubMed] [Google Scholar]
Prakash 2011 {published data only}
- Prakash K. Drain amylase value alone on day‐3 in diagnosis of pancreatic fistula: Is there no relevance for the drain output amount?. Pancreatology 2011;11:70. [Google Scholar]
Raja 2015 {published data only}
- Raja K, Pottakkat B, Gaurav K, Kate V. Can early estimation of drain fluid amylase predict postoperative pancreatic fistula in patients with chronic pancreatitis? A pilot study. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2015;17:185. [Google Scholar]
Ramesh 2006 {published data only}
- Ramesh H, Sikora SS. Drain amylase levels following pancreaticoduodenectomy for cancer; correlation with outcomes and proposal for a uniform grading system. Gastroenterology 2006;130(4):A887‐A. [Google Scholar]
Robinson 2010 {published data only}
- Robinson S, Rahman A, Lochan R, Sen G, Jacob M, French JJ, et al. Drain amylase after a pancreaticoduodenectomy (PPD) does it predict a pancreatic leak?. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2010;12:320. [Google Scholar]
Sanchez Acedo 2013 {published data only}
- Sanchez Acedo P, Zazpe Ripa C, Herrera Cabezen J, Tarifa Castilla A, Marin G, Lera Tricas JM. Cephalic duodenopancreatectomy: When to remove the drainages?. Pancreatology 2013;13(4 Suppl):e2‐3. [Google Scholar]
Saxena 2014 {published data only}
- Saxena R, Parthasarthy G, Prakash A, Singh R, Behari A, Kumar A, et al. Postoperative laboratory parameters after pancreaticoduodenectomy‐can they predict complications? A prospective study. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2014;16:647. [Google Scholar]
Shi 2009 {published data only}
- Shi CY, Jin DY, Xu B, Lou WH. Value of monitoring postoperative intra‐abdominal drainage fluid for the diagnosis of postoperative pancreatic fistula: Results of a prospective study in 134 patients. Surgical Practice 2009;13(4):102‐7. [Google Scholar]
Shimizu 2015 {published data only}
- Shimizu T, Ito M, Horiguchi A. Parameters and risk factors for appropriate drain management after distal pancreatectomy. European Surgical Research 2015;55:46‐7. [Google Scholar]
Shinchi 2006 {published data only}
- Shinchi H, Wada K, Traverso LW. The usefulness of drain data to identify a clinically relevant pancreatic anastomotic leak after pancreaticoduodenectomy?. Journal of Gastrointestinal Surgery 2006;10(4):490‐8. [DOI] [PubMed] [Google Scholar]
Shyr 2003 {published data only}
- Shyr YM, Su CH, Wu CW, Lui WY. Does drainage fluid amylase reflect pancreatic leakage after pancreaticoduodenectomy?. World Journal of Surgery 2003;27(5):606‐10. [DOI] [PubMed] [Google Scholar]
Srivastava 2016 {published data only}
- Srivastava M, Kumaran V, Nundy S. Does drain amylase < 666 IU/L on the third post‐operative day effectively predicts the absence of a high‐impact postoperative pancreatic fistula following pancreaticoduodenectomy?. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2016;18(Supplement 1):e111. [DOI: 10.1016/j.hpb.2016.02.260] [DOI] [Google Scholar]
Sugimoto 2013 {published data only}
- Sugimoto M, Takahashi S, Gotohda N, Kato Y, Kinoshita T, Shibasaki H, et al. Schematic pancreatic configuration: A risk assessment for postoperative pancreatic fistula after pancreaticoduodenectomy. Journal of Gastrointestinal Surgery 2013;17(10):1744‐51. [DOI] [PubMed] [Google Scholar]
Sutcliffe 2012 {published data only}
- Sutcliffe RP, Battula N, Haque A, Ali A, Srinivasan P, Atkinson SW, et al. Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World Journal of Surgery 2012;36(4):879‐83. [DOI] [PubMed] [Google Scholar]
Sutcliffe 2014 {published data only}
- Sutcliffe R, Hamoui M, Pitchaimuthu M, Isaac J, Marudanayagam R, Mirza D, et al. First postoperative day drain fluid amylase greater than 2000 IU/l predicts grade C pancreatic fistula after pancreaticoduodenectomy. British Journal of Surgery 2014;101:7. [Google Scholar]
Sutcliffe 2015 {published data only}
- Sutcliffe RP, Hamoui M, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, et al. Implementation of an enhanced recovery pathway after pancreaticoduodenectomy in patients with low drain fluid amylase. World Journal of Surgery 2015;39(8):2023‐30. [DOI] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang ELS, Huey CWT, Junnarkar SP, Low JK, Woon WLW. Most accurate post operative day drain amylase level in predicting post operative pancreatic fistulas. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2015;17:209. [Google Scholar]
Teixeira 2016 {published data only}
- Teixeira UF, Goldoni MB, Waechter FL. Early drain amylase value predicts the occurrence of pancreatic fistula after pancreaticoduodenectomy. Annals of Surgery 2016;4 March:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Tsujie 2012 {published data only}
- Tsujie M, Nakamori S, Miyamoto A, Yasui M, Ikenaga M, Hirao M, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy ‐ patients with low drain amylase level on postoperative day 1 are safe from developing pancreatic fistula. Hepato‐Gastroenterology 2012;59(120):2657‐60. [DOI] [PubMed] [Google Scholar]
Uemura 2011 {published data only}
- Uemura K, Murakami Y, Sudo T, Hashimoto Y, Nakashima A, Sueda T. Predictive clinical factor for clinically relevant postoperative pancreatic fistula after pancreaticoduodenectomy. Gastroenterology 2011;140(5 Suppl):S1040‐S1. [Google Scholar]
Uemura 2014 {published data only}
- Uemura K, Murakami Y, Sudo T, Hashimoto Y, Kondo N, Nakagawa N, et al. Indicators for proper management of surgical drains following pancreaticoduodenectomy. Journal of Surgical Oncology 2014;109(7):702‐7. [DOI] [PubMed] [Google Scholar]
- Uemura K, Murakami Y, Sudo T, Hashimoto Y, Nakashima A, Ohge H, et al. Indicator for proper management of surgical drains following pancreaticoduodenectomy. Gastroenterology 2012;142(5 Suppl):S1061. [DOI] [PubMed] [Google Scholar]
Veillette 2010 {published data only}
- Veillette GR, Correa‐Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, et al. A drain amylase < 1000 U/l on the first post‐operative day effectively predicts the absence of a high‐impact fistula following pancreatic resection. Gastroenterology 2010;138(Suppl 5):S855. [Google Scholar]
Ven Fong 2015 {published data only}
- Ven Fong Z, Correa‐Gallego C, Ferrone CR, Veillette GR, Warshaw AL, Lillemoe KD, et al. Early drain removal‐‐the middle ground between the drain versus no drain debate in patients undergoing pancreaticoduodenectomy: A prospective validation study. Annals of Surgery 2015;262(2):378‐83. [DOI] [PubMed] [Google Scholar]
Yamaguchi 2003 {published data only}
- Yamaguchi M, Nakano H, Midorikawa T, Yoshizawa Y, Sanada Y, Kumada K. Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepato‐Gastroenterology 2003;50(52):1155‐8. [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang J, Huang Q, Wang C. Postoperative drain amylase predicts pancreatic fistula in pancreatic surgery: A systematic review and meta‐analysis. International Journal Of Surgery 2015;22:38‐45. [DOI] [PubMed] [Google Scholar]
Zelga 2015 {published data only}
- Zelga P, Ali J, Brais R, Harper S, Liau SS, Huguet E, et al. Negative predictive value of drain amylase concentration for development of pancreatic fistula after pancreaticoduodenectomy. Pancreatology 2015;1:S98‐S9. [DOI] [PubMed] [Google Scholar]
- Zelga P, Ali JM, Brais R, Harper SJ, Liau SS, Huguet EL, et al. Negative predictive value of drain amylase concentration for development of pancreatic fistula after pancreaticoduodenectomy. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2016;18:e851. [DOI] [PubMed] [Google Scholar]
- Zelga P, Ali JM, Brais R, Harper SJ, Liau SS, Huguet EL, et al. Negative predictive value of drain amylase concentration for development of pancreatic fistula after pancreaticoduodenectomy. Pancreatology 2016;15(2):179‐84. [DOI] [PubMed] [Google Scholar]
Additional references
Bassi 2005
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138(1):8‐13. [DOI] [PubMed] [Google Scholar]
Buscemi 2006
- Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. Journal of clinical epidemiology 2006;59(7):697‐703. [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Diener 2011
- Diener MK, Seiler CM, Rossion I, Kleeff J, Glanemann M, Butturini G, et al. Efficacy of stapler versus hand‐sewn closure after distal pancreatectomy (DISPACT): a randomised, controlled multicentre trial. Lancet 2011;377(9776):1514‐22. [DOI] [PubMed] [Google Scholar]
Doust 2005
- Doust JA, Pietrzak E, Sanders S, Glasziou PP. Identifying studies for systematic reviews of diagnostic tests was difficult due to the poor sensitivity and precision of methodologic filters and the lack of information in the abstract. Journal of clinical epidemiology 2005;58(5):444‐9. [DOI] [PubMed] [Google Scholar]
Eloubeidi 2001
- Eloubeidi MA, Wade SB, Provenzale D. Factors associated with acceptance and full publication of GI endoscopic research originally published in abstract form. Gastrointestinal Endoscopy 2001;53(3):275‐82. [DOI] [PubMed] [Google Scholar]
Giglio 2016
- Giglio MC, Spalding DR, Giakoustidis A, Zarzavadjian Le Bian A, Jiao LR, Habib NA, et al. Meta‐analysis of drain amylase content on postoperative day 1 as a predictor of pancreatic fistula following pancreatic resection. The British journal of Surgery 2016;103(4):328‐36. [DOI] [PubMed] [Google Scholar]
Gurusamy 2013
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008370.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016
- Lu X, Wang X, Fang Y, Chen H, Peng C, Li H, et al. Systematic review and meta‐analysis of pancreatic amylase value on postoperative day 1 after pancreatic resection to predict postoperative pancreatic fistula. Medicine 2016;95(5):e2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeda 2008
- Maeda E, Kataoka M, Yatsushiro S, Kajimoto K, Hino M, Kaji N, et al. Accurate quantitation of salivary and pancreatic amylase activities in human plasma by microchip electrophoretic separation of the substrates and hydrolysates coupled with immunoinhibition. Electrophoresis 2008;29(9):1902‐9. [DOI] [PubMed] [Google Scholar]
McKay 2006
- McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, et al. Meta‐analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. British Journal of Surgery 2006;93(8):929‐36. [DOI] [PubMed] [Google Scholar]
Mifflin 1985
- Mifflin TE, Benjamin DC, Bruns DE. Rapid quantitative, specific measurement of pancreatic amylase in serum with use of a monoclonal antibody. Clinical Chemistry 1985;31(8):1283‐8. [PubMed] [Google Scholar]
Montorsi 2012
- Montorsi M, Zerbi A, Bassi C, Capussotti L, Coppola R, Sacchi M, et al. Efficacy of an absorbable fibrin sealant patch (TachoSil) after distal pancreatectomy: a multicenter, randomized, controlled trial. Annals of Surgery 2012;256(5):853‐9. [DOI] [PubMed] [Google Scholar]
NCBI 2014
- NCBI. MeSH. NLM Controlled Vocabulary. Pancreas. http://www.ncbi.nlm.nih.gov/mesh/68010179 (accessed on 4 July 2014) 2014.
Park 2013
- Park JW, Jang JY, Kim EJ, Kang MJ, Kwon W, Chang YR, et al. Effects of pancreatectomy on nutritional state, pancreatic function and quality of life. British Journal of Surgery 2013;100(8):1064‐70. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
Sampson 2008
- Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. Journal of Clinical Epidemiology 2008;61(8):755‐62. [DOI] [PubMed] [Google Scholar]
Suzuki 1995
- Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Archives of Surgery 1995;130(9):952‐5. [DOI] [PubMed] [Google Scholar]
van der Wilt 2013
- Wilt AA, Coolsen MM, Hingh IH, Wilt GJ, Groenewoud H, Dejong CH, et al. To drain or not to drain: a cumulative meta‐analysis of the use of routine abdominal drains after pancreatic resection. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2013;15(5):337‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vissers 1999
- Vissers RJ, Abu‐Laban RB, McHugh DF. Amylase and lipase in the emergency department evaluation of acute pancreatitis. The Journal of Emergency Medicine 1999;17(6):1027‐37. [DOI] [PubMed] [Google Scholar]
Whiting 2006
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology. 2006/03/08 2006; Vol. 6:9. [DOI] [PMC free article] [PubMed]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]


