Abstract
Objective
Although, accumulating evidence is delineating a neuroprotective and neurotrophic role for lithium (Li), inconsistent findings have also been reported in human studies especially. Moreover, the effects of Li infusion into the hippocampus are still unknown. The aims of this work were (a) to assess whether basal synaptic activity and long-term potentiation (LTP) in the hippocampus are different in regard to intrahippocampal Li infusion; (b) to assess spatial learning and memory in rats chronically treated with LiCO3 in the Morris water maze.
Methods
Field potentials were recorded form the dentate gyrus, stimulating perforant pathways, in rats chronically (20 mg/kg for 40 days) or acutely treated with LiCO3 and their corresponding control rats. In addition, performance of rats in a Morris water maze was measured to link behaviour of rats to electrophysiological findings.
Results
LiCO3 infusion into the hippocampus resulted in enhanced LTP, especially in the late phases, but attenuated LTP was observed in rats chronically treated with Li as compared to controls. Li-treated rats equally performed a spatial learning task, but did spend less time in target quadrant than saline-treated rats in Morris water maze.
Conclusion
Despite most data suggest that Li always yields neuroprotective effects against neuropathological conditions; we concluded that a 40-day treatment of Li disrupts hippocampal synaptic plasticity underlying memory processes, and that these effects of prolonged treatment are not associated with its direct chemical effect, but are likely to be associated with the molecular actions of Li at genetic levels, because its short-term effect preserves synaptic plasticity.
Keywords: Lithium, Hippocampus, Learning, Memory, Long-term potentiation
INTRODUCTION
The hippocampal formation is involved in a circuit that is required for several types of memory in both humans1) and rodents.2) At the physiological/cellular level, memory processes generally are believed to involve long-term potentiation (LTP) of synaptic function.3) Lithium (Li) salts like lithium carbonate (Li2CO3) is a neuroprotector that improves memory and learning in preclinical models of aging,4) traumatic brain injury,5) drug addiction,6) glutamate neurotoxicity,7) and Alzheimer’s disease.8) However, inconsistent findings have been reported in human studies. Li treatment inhibited learning, memory, and speed of information processing in patients with bipolar disorder and to some extent in control subjects9–12) and many patients administered Li2CO3 complained of memory impairment.9,13) So far, only a limited attempt has been made to study the effect of Li treatment on the LTP, which is believed to underlie learning and memory.
Although it has been previously demonstrated that the LTP in the dentate gyrus (DG) of hippocampal formation is enhanced in rats treated intraperitoneally with Li for 2 to 28 days, compared to control rats,4,14,15) neither effect of more prolonged exposure nor acute effect have not been studied up to date. The objective of this study was to determine, in normal adult rats, whether Li treatment for 40 days produces deficits in spatial learning in the Morris water maze and synaptic plasticity in the DG of the hippocampal formation in vivo. To further investigate the contribution of direct chemical effect of Li to its prolonged effect on synaptic plasticity, we also present the results of Li infusion into the DG.
METHODS
Animals and Treatment with Lithium
The experiments were performed on adult male Wistar rats between the ages of 4 and 6 months in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) regarding the protection of animals used for experimental purposes and with the guiding principles for the care and use of laboratory animals approved by the Erciyes University.
In experiments investigating the outcome of long-term administration, Li2CO3 (Sigma Chemical Co., St. Louis, MO, USA) was administered intraperitoneally (1 mEq/kg/day) for 40 days. This treatment protocol resulted in serum Li concentrations of 1.18±0.54 mM/L (mean±standard error of mean) which are also close to the range of 0.30–1.30 mM/L, concentrations that are achieved and maintained during Li therapy in human subjects.16) Saline was provided ad libitum to the Li-treated rats to reduce potential toxicity. In experiments investigating the outcome of short-term Li administration, Li2CO3 (10 mM) was infused at the rate of 4.0 μl/min for 20 μl before LTP induction.
Morris Water Maze
Performance in the Morris water maze of 22 saline-treated and 19 Li-treated rats was evaluated for measurement of spatial memory in a circular, galvanized steel maze (2 m in diameter and 75 cm in depth), which was filled with water to a depth of 50 cm and kept at 22°C. The water was made dark blue with non-toxic dye. The maze was located in a large quiet test room, surrounded by many visual cues external to the maze, which were visible from within the pool and could be used by the rats for spatial orientation. Locations of the cues were unchanged throughout the period of testing. There were four equally divided quadrants in the pool. In one of the quadrants, a hidden platform (10 cm in diameter, 2 cm below the surface) was located centrally and fixed in a position and was kept constant throughout the acquisition trials. The platform was removed on probe phase. The rats performed four trials per day for four consecutive days (16 trials). In the swimming trials, each individual rat was released gently into the water in a randomly chosen quadrant other than the target one that contained the platform for facing an extra maze cue. During an acquisition period, rats learned how to find the platform within 120 seconds. After reaching it, the rat was allowed to stay on the platform for 15 seconds and was then put back into its cage. The rats were placed on the platform by hand for 15 seconds if they could not escape to the platform within 120 seconds by themselves. During the inter-trial intervals, animals were kept in a dry home cage for 60 seconds. The probe trial was performed 24 hours after the training period. Latency of finding the platform (escape latency, EL), distance moved (DM), swimming speed, and the time in seconds spent in the quadrant occupied by the platform was measured for individual animals in each trial by the EthoVision video tracking system (Noldus, Wageningen, the Netherlands). The EL of rats who could not escape to the platform within 120 seconds was accepted as 120 seconds. The experimenter always stood at the same position. All the trials were completed between 10.00 to 12.00 hour.
Electrophysiology
Details of the protocols used for electrophysiological experiments are described elsewhere.17) Briefly, after rats were anesthetized with intraperitoneally injected urethane (1.2 g/kg), a bipolar tungsten electrode (stainless steel, Teflon-coated, 127 μm in diameter, insulated except at its tips) was used to stimulate the medial perforant path (PP; from bregma; anteroposterior: −8.0 mm, mediolateral: 4.2 mm, dorsoventral: 2–2.5 mm below the dura) of the right hemisphere. A two-barrel micropipette (Borosilicate, outer diameter 1.5 mm, length 10 cm length; World Precision Instruments, Sarasota, FL, USA) was inserted into the granule cell layer of the DG in the right hemisphere (from bregma; anteroposterior: −3.5 mm, mediolateral: 2.15 mm, dorsoventral: 2.5–3 mm below the dura) to record the field potential. The depth of recording and stimulating electrodes (dorsoventral coordinate) was adjusted to obtain a large positive excitatory postsynaptic potential (EPSP) followed by a negative-going population spike (PS) in response to PP stimulation. These positions of both electrodes were previously verified to be in the granule cell layer of the DG and in the PP.18,19) One of the barrels was filled with 3 M NaCl (tip resistance: 2–10 MΩ) for recording of field potentials. The other was filled with Li2CO3 in experiments investigating the outcome of short-term administration, or saline in control rats and rats chronically treated with Li2CO3, and connected to a Hamilton syringe (25 μl) driven by a syringe pump (Stoelting Co., Wood Dale, IL, USA). Five minutes prior to LTP induction, a 20 μl volume was infused into the DG at a rate of 4.0 μl/minute.
After a stable EPSP was obtained, the PP was stimulated by pulses at an intensity that ranged from 0.1 to 1.5 mA at 0.05Hz three times and by increasing the intensities from a 0.1 mA to a 1.5 mA step by 0.2 mA per step to create an input-output (I/O) curve, which was stored for off-line analysis. The stimulus intensity produced by half of the maximum PS amplitude was determined (test stimulus) and then used throughout the experiment. LTP was induced using high-frequency stimulation (HFS; 100 Hz, 1 second, 4 times). Test stimulus intensities were 0.81±0.17 and 0.90±0.16 mA in control and Li groups, respectively. Following the delivery of HFS, the test stimulus was repeated every 30 seconds for up to the end of recording.
Data Analysis and Statistics
Two components of each field potential, the EPSP and PS, were used for statistical purposes. The slope of the EPSP was calculated as the amplitude change at 20% to 80% of the voltage difference between the start and the peak of the waveform. The PS amplitude was calculated as the average of the two potential differences of the negative spike peak to the preceding and following positive peaks. Raw values for the EPSP slopes and PS amplitudes obtained during the I/O experiment were analyzed separately using repeated measures ANOVA, with the drug (saline vs. Li) used as between-subjects variables and stimulus intensity (8 levels of intensity) used as a within-subjects factor. The mean value of the EPSP slope and the PS amplitude during baseline recording was chosen to represent 100%, and each EPSP and PS was expressed as a percentage of this value. The average slope or amplitude was calculated from ten sweeps at the end of recording as a measure of LTP. Unpaired Student’s t test was used to compare groups’ means. Significance was set at p<0.05 (two-tailed). All statistical analyses were performed using IBM SPSS Statistics (ver. 22.0; IBM Corp., Armonk, NY, USA).
RESULTS
Learning Behavior of Rats Chronically Treated with Lithium Carbonate
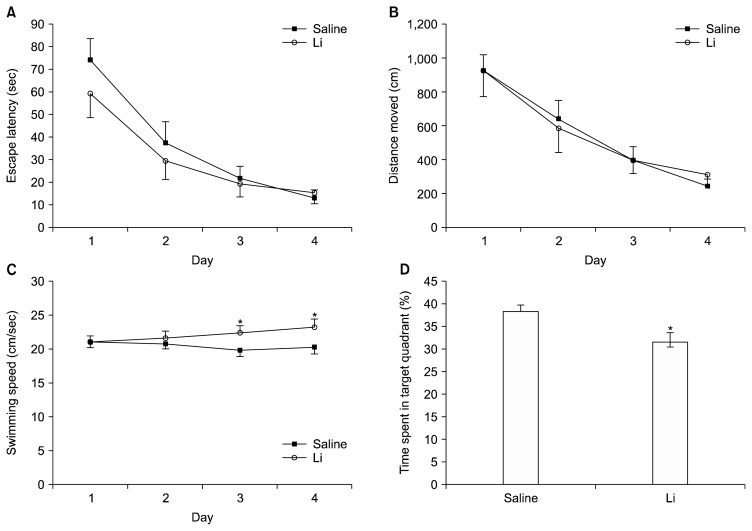
To address the question of whether Li treatment results in altered learning capabilities, we performed the Morris water maze test. During acquisition trials, Li-treated rats as well as saline-treated rats improved their ability to find the hidden platform as showed by decreasing EL (F3,510= 85.356, p<0.001) and DM (F3,510=71.263, p<0.001) as a result of training (Fig. 1). Between-subjects effect was not significant for EL (F1,170=3.833, p=0.052) and DM (F1,170=0.192, p>0.05). Interaction effects group×EL and group×DM were not significant (Fs3,519=1.955 and 0.405, p>0.05). Repeated measures ANOVA showed that speed did not change as a function of day (F3,510=1.974, p> 0.05) in control group, however was affected by Li treatment (group effect: F1,170=14.084, p<0.001; interaction: F3,510=4.245, p=0.006). Li-treated rats swam faster than control group at the 3rd day (F1,170=19.165, p<0.010) and at the 4th day (F1,170=9.733, p=0.002).
Fig. 1.
Effects of lithium (Li) treatment on acquisition and memory performance in the water maze. All rats improved their ability to find the hidden platform as showed by decreasing escape latency (A) and distance moved (B) over training days. Swimming speed (C) did change as a function of day and was affected by Li treatment. Li-treated rats (n=19) spent less time in target quadrant than control rats (n=22) (D). Data are expressed as mean±standard error of mean. *vs. control rats. Note that Li-treated rats performed spatial learning task as control rats however underperformed retrieval task.
Twenty-four hours after the last trial session a probe trial, in which the platform had been removed, was given in order to test the rats’ spatial accuracy and their actual ability to remember the position of the hidden platform. Under normal conditions in such a probe trial, it is expected that animals spend more time than the chance level (25%) in the quadrant where the platform had been located. The time spent in target quadrant was significantly above the 25% chance level for control (38.22± 1.62%; df=21; t=8.182, p<0.001; one-sample t test) and Li-treated rats (31.44±2.15%; degree of freedom [df]=18; t=3.046; p=0.007). However, Li-treated rats spent significantly less time in the target quadrant than did control rats in probe trial (df=39; t=2.584; p=0.014) (Fig. 1D). These results showed that Li-treated rats underperformed retrieval task in spite of similar spatial learning performance to control.
Electrophysiology
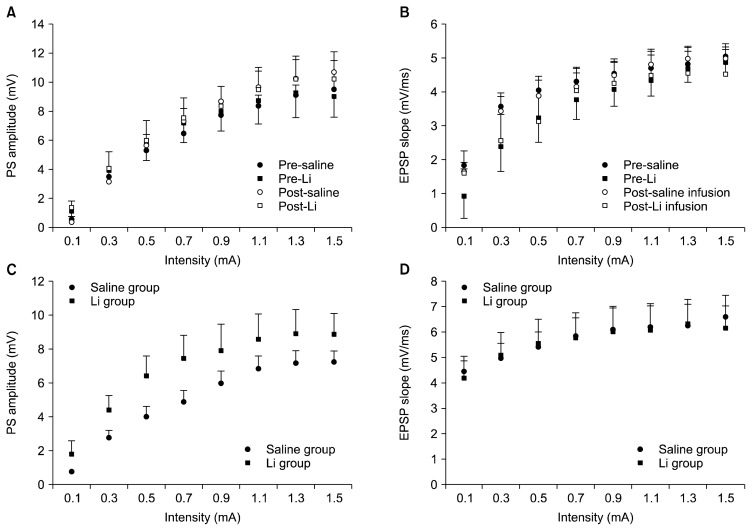
To establish that transmission at PP-DG synapses was altered by Li2CO3 administration, I/O curves were constructed using eight stimulus levels. The PS amplitude and EPSP slope were not different, neither between prior to and after infusions in cases where I/O recordings were taken prior to and after Li2CO3 (or saline) infusion from the same animal nor between Li group and control group in case where I/O recordings were taken prior to LTP induction across the eight stimulus intensities as indicated by no significant interactions between intensity and drug (Fig. 2). These data indicate no effectiveness of Li2CO3 on transmission at PP-DG synapses, and no gross differences in baseline function between the control and Li groups that complicate with measure of synaptic plasticity.
Fig. 2.
Effect of Li2CO3 on baseline synaptic transmission in the dentate gyrus of hippocampal formation. The input-output curves were generated by plotting population spike (PS) amplitude and excitatory postsynaptic potential (EPSP) slope across a range of stimulation intensities (0.1–1.5 mA). Input-output (I/O) curves in A and B were taken prior to and after Li2CO3 (or saline) infusion from the same animal (n=5/group). I/O curves in C and D were prior to long-term potentiation induction in the lithium (Li) group and control group (n=7/group). PS and EPSP at each intensity level are mean of three consecutive responses in 5 rats for each group. Data are expressed as mean±standard error of mean. Note that the PS amplitude and EPSP slope were not different across the eight stimulus intensities as indicated by no significant interactions between intensity and drug.
Effects of Intrahippocampal Li2CO3 on the LTP in the Dentate Gyrus
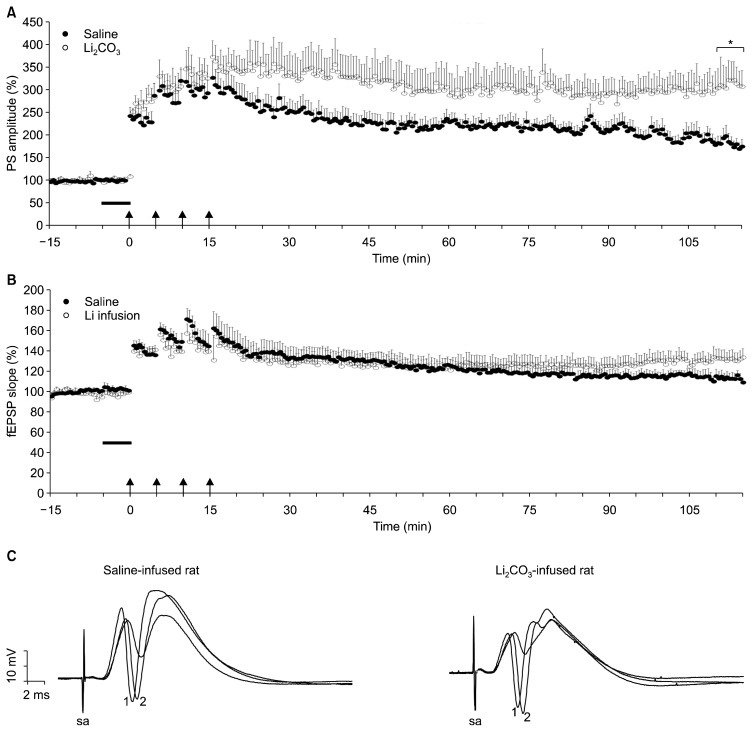
The potentiation of the field EPSP (fEPSP) is associated with the formation and enlargement of dendritic spines, whereas the PS potentiation reflects the somatic response, the amplitude of which is dependent on the number of granule cells that discharge in synchrony. The product of the synaptic component of LTP (fEPSP) together with the non-synaptic component (PS) has a much stronger effect on the neuronal output in comparison to cases when only one of these two components is potentiated. As shown in Figure 3, four tetanus trains of 100 pulses at 100 Hz induced LTP of both PS (Fig. 3A) and fEPSP (Fig. 3B) in the DG neurons of both groups. Independent samples t test failed to show a significant difference between saline- and Li-infused rats in the magnitude of the short-term potentiation measured at 1 to 5 minutes after tetanus as the PS amplitude (304±32% vs. 352±34% of baseline, respectively; p>0.10) and the EPSP slope (153±15% vs. 144±9% of baseline; p>0.10). Nevertheless, induced LTP of the PS, but not of fEPSP, measured at 100 minutes after the last HFS was greatly enhanced by pre-HFS application of Li2CO3 (10 mM, 5 minutes; 312±42% vs. 182±16% of baseline, p=0.012). The difference between LTP magnitudes of fEPSP remained a trend level for significance (132±8% vs. 113±5% of baseline, p=0.055). These data indicates that induction of LTP at the DG synapses was enhanced by infusion of Li2CO3, by a mechanism so-called non-synaptic plasticity such as increased somatic and dendritic excitability or increases in the membrane potential.20)
Fig. 3.
A 5-minute infusion intrahippocampal Li2CO3 infusion enhances the long-term potentiation (LTP) of population spike (PS) amplitude in the dentate gyrus of hippocampal formation. After a 10-minute baseline recording, saline or Li2CO3 was infused into the dentate gyrus (horizontal black bar) and LTP was induced by means of high-frequency stimulation (HFS) (arrows; 100 Hz, 1 second, 4 times), which was applied beginning at time 0. This protocol induced LTP of both PS (A) and field excitatory postsynaptic potential (fEPSP, B) in the dentate gyrus neurons of both group. The magnitudes of the short-term potentiation of both fEPSP and PS measured at 1 to 5 minutes after tetanus are comparable between saline-infused rats and Li2CO3-infused rats. PS amplitude was more potentiated between 110 and 115 minutes in lithium-infused (Li) group compared to saline-infused group (*). Error bars denote the standard errors of the means. n=7 for each group. (C) Traces are representative of field potential recordings made immediately before (time point, −1 minute; not depicted) and after HFS (time point, 15 minutes; depicted with “1”) and at the end of the experiment (time point, 75 minutes; depicted with “2”). Note the higher ratio of PS (the down-going deflection) amplitude in trace depicted with “2” to that in trace not depicted (the magnitude of LTP) in Li2CO3-infused rat.
sa, stimulus artifact.
Effects of Chronic Li2CO3 Treatment on the LTP in the Dentate Gyrus
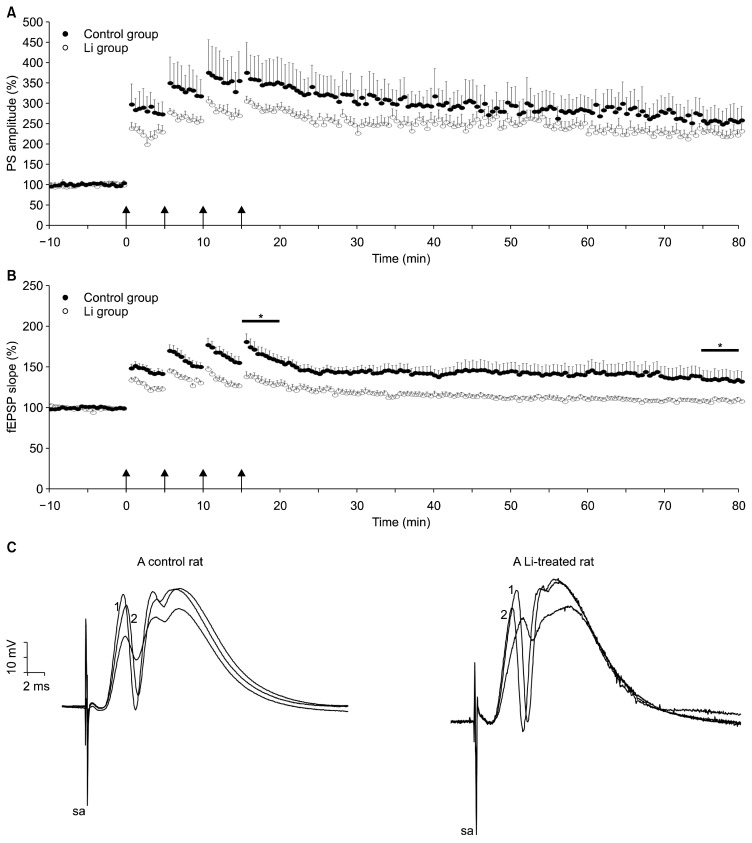
Independent samples t test failed to show a significant difference between control and Li groups in the magnitude of the short-term potentiation measured at 1 to 5 minutes after tetanus (349±36% vs. 285±10% of baseline, respectively; p>0.10) and the LTP measured at 75 to 80 minutes after tetanus (256±20% vs. 227±16% of baseline, respectively; p>0.10). Nevertheless, induced LTP of fEPSP, measured at 75 to 80 minutes after the last HFS was significantly attenuated by chronic treatment of Li2CO3 (1 mEq/kg/day, 40 day; 107±1.1% vs. 135±11% of baseline, p=0.038). The difference between short-term potentiation of fEPSP also reached significance (144±4% vs. 165±8% of baseline, p=0.004). These data indicates that induction of LTP at the DG synapses was attenuated by Li2CO3 treatment.
DISCUSSION
Although it has been previously reported that prolonged Li treatment promptly increased the LTP,14,15) to our best knowledge, no study has addressed the Morris water maze performance of Li-treated rats, as well as in-vivo effects of Li infusion on the DG-LTP. The major result of this study is that infusion of Li magnifies the potentiation of PS amplitude (Fig. 3A) whereas chronic treatment for 40 days impairs hippocampus-dependent spatial memory without affecting platform learning task and attenuates the potentiation of EPSP slope (Fig. 4B) in the DG of hippocampal formation compared with control rats, without enhancing baseline synaptic transmission (Fig. 2). The differences in the LTP between Li-infused and Li-treated rats suggest that effect of prolonged Li treatment may not be associated with its direct chemical effect, but is likely to be associated with the molecular actions of Li at genetic levels. Moreover, two components of LTP, which is previously well defined,21) can be differentially affected by Li so that synaptic component is attenuated when Li administrated chronically, whereas magnification of non-synaptic component is observed when given acutely.
Fig. 4.
Time course of popultion spike (PS)-long-term potentiation (LTP) (A) and excitatory postsynaptic potential (EPSP)-LTP (B) in the dentate gyrus from the control group (filled) and the lithium group (empty circle, a 40-day treatment). After a 10-minute baseline recording, LTP was induced by means of high-frequency stimulation (arrows; 100 Hz, 1 second, 4 times), which was applied beginning at time 0 (arrows). This protocol induced LTP of both PS (A) and field EPSP (fEPSP) (B) in the dentate gyrus neurons of both group. The fEPSP slope was less potentiated between 15–20 minutes (short-term potentiation) and between 75–80 minutes (LTP) in lithium-infused (Li) group compared to saline-infused group (*). (C) Traces are representative of field potential recordings made immediately before (time point, −1 minute; not depicted) and after high-frequency stimulation (time point, 15 minutes; depicted with “1”) and at the end of the experiment (time point, 75 minutes; depicted with “2”). Note the higher ratio of fEPSP slope in trace depicted with “2” to that in trace not depicted (the magnitude of LTP) in a control rat.
sa, stimulus artifact.
Non-synaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and affects synaptic integration, sub-threshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level.20,22) At doses used in this study, Li infusion led to a marked increase in PS-LTP without affecting on EPSP-LTP, indicating an effect on a non-synaptic rather than synaptic plasticity. Previously reported effects of injection of Li confirm EPSP-spike (E-S) potentiation, namely a larger PS amplitude for a given EPSP size. A microdialysis study in rat prefrontal cortex showed that acute application of Li increased the concentration of γ-aminobutyric acid (GABA) at doses of 4 meq/kg,23) and the activation of GABAA receptors increased the E-S potentiation.24)
Our findings suggest that long-term Li treatment attenuates synaptic plasticity in the hippocampus. Nevertheless, it is generally reported that enhanced synaptic plasticity in the DG is a functional consequence of long-term treatment with Li. Previous electrophysiological works showed that sub-chronic (1 mEq/kg/day for 2 weeks) and chronic treatments (4 mEq/kg/day for 4 weeks) of Li increased the LTP in the granule cells in the DG of the hippocampus.14,15) There are discrepancies in the methodology of studies. For instance, we obtained the field potentials from the principle cell layer which is made up largely of densely packed granule cells, whereas Son et al.14) from the molecular layer which is occupied by, among other things, the dendrites of the dentate granule cells. Rats were exposed to Li chloride for shorter time (14 days) relative to our study in Shim et al.’s study.15) Yu et al.4) studied the 20-month-old aged rats following chronic Li treatment.
The enhanced LTP, which has been reported by other authors, is best explained by the inhibitory action of Li on glycogen synthase kinase 3 (GSK-3β).25,26) Unlike most protein kinases involved in signaling, GSK-3β is active in unstimulated, resting cells and degrades β-catenin, blocking reach to nucleus. Therefore, inhibition of GSK-3β activates Wnt signaling pathway, promoting expression of neuroplastic factors involved in synaptic plasticity27) and this leads to favoring LTP. It was shown that induction of LTP resulted in deactivation of GSK-3β through phosphor-ylation28,29) and after 15 minutes’ HFS, through one hour, the inhibitory phosphorylation of GSK-3β was increased in the DG where LTP had been potentiated relative to uninduced regions in the ipsilateral hippocampus, to the contralateral hippocampus or to native control animals.29) Deficient LTP in GSK-3β overexpressing mice could be rescued by Li.29,30) The other possible effect such as upregulation of brain-derived neurotrophic factor,31) Bcl-2 and CREB32) may underline increase in LTP by Li. However, contradictory data concerning an attenuation of LTP can be expected because Li has effects on multiple cellular processes in regard to synaptic proteins, depending on duration and dose of Li. Some effects of Li can negatively affect the cell signaling involved in synaptic trafficking, such as inhibition of N-methyl-D-aspartate receptor activity,7,33,34) down regulation of the gene for the glutamate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionate (AMPA) receptor,35) reduction in the expression of the protein kinase C and myristoylated alanine rich C kinase substrate.36) Although molecular studies are not covered in the current study, we have thought that forty days of Li treatment is long enough to produce inhibitor actions through the gene expression of proteins involved in synaptic plasticity.
The Morris water maze is a test of hippocampus-dependent spatial memory,37) the closest parallel to episodic memory in humans.38) The testing procedure used during the 4 days of locating the hidden platform provides a measure of spatial reference memory, while the probe trial is a measure of the strength of spatial learning.38) In the present experiments, lower efficacy of LTP, which is thought to be the major neurophysiological basis for learning and memory,39) was correlated with impaired spatial memory behavior in Li-treated rats. In line with the present study, intra-CA1 administration of Li after training on a one-trial step-down inhibitory avoidance task impaired memory when retrieval was tested 24 hours later.40) In humans, higher Li levels were found to be correlated with impairment of memory performance in psychotic patients treated with Li.41) Some investigators have suggested that Li treatment inhibited learning, memory, and speed of information processing in patients with bipolar disorder and to some extent in control subjects.9–12) Li therapy did not improve deficit in episodic memory in bipolar patients.42) Some investigators, however, have revealed inconsistent findings with those obtained from studies mentioned above. Chronic Li treatment enhanced spatial working memory in rats43) and improved spatial learning and memory ability after transient global cerebral ischemia44) and cranial irradiation45) in Morris water maze. Li treated rats outperformed control rats in the training trails at dose higher than used in this study.46) In addition, as distinct from Morris water maze, Li increased learning in a hole-board arena, a conditioned place-preference box and a T-maze in rats fed with Li carbonate.47) Nevertheless, Li-treated rats shown upper motor ability in terms of swimming velocity (23.66±0.56 cm/sec vs. 21.47±0.43 cm/sec; F1,40=9.430, p=0.004) and DM (2,830±68 cm vs. 2,575±51 cm; F1,40=8.825; p=0.005) in the probe trial. Therefore this anxiety-like behavior in Li-treated rats can also be responsible from worse performance in the probe trial.48)
As a result, although explanation for the present results apparently needs further investigations, we concluded that a 40-day treatment of Li disrupts hippocampal synaptic plasticity underlying memory processes, and that these effects of prolonged treatment are not associated with its direct chemical effect, but are likely to be associated with the molecular actions of Li at genetic levels, because its short-term effect preserves synaptic plasticity. However, it may be suspected that the data of these articles were contaminated by the neuronal toxicity of Li, because of contradictory data concerning an improvement of hippocampal plasticity that has been previously reported.
Acknowledgments
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK, 113S345) and was partly supported by the Erciyes University Research Fund (TOA-2013-4555).
REFERENCES
- 1.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Moser E, Moser MB, Trommald M. Cellular correlates to spatial learning in the rat hippocampus. J Physiol Paris. 1996;90:349. doi: 10.1016/S0928-4257(97)87917-X. [DOI] [PubMed] [Google Scholar]
- 3.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 4.Yu IT, Kim JS, Lee SH, Lee YS, Son H. Chronic lithium enhances hippocampal long-term potentiation, but not neurogenesis, in the aged rat dentate gyrus. Biochem Biophys Res Commun. 2003;303:1193–1198. doi: 10.1016/S0006-291X(03)00494-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhu ZF, Wang QG, Han BJ, William CP. Neuroprotective effect and cognitive outcome of chronic lithium on traumatic brain injury in mice. Brain Res Bull. 2010;83:272–277. doi: 10.1016/j.brainresbull.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Zarrindast MR, Fazli-Tabaei S, Ahmadi S, Yahyavi SH. Effect of lithium on morphine state-dependent memory of passive avoidance in mice. Physiol Behav. 2006;87:409–415. doi: 10.1016/j.physbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Heng X, Li T, Li L, Yang D, Zhang X, et al. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-β production in an aged Alzheimer’s disease transgenic mouse model. J Alzheimers Dis. 2011;24:739–749. doi: 10.3233/JAD-2011-101875. [DOI] [PubMed] [Google Scholar]
- 9.Pachet AK, Wisniewski AM. The effects of lithium on cognition: an updated review. Psychopharmacology (Berl) 2003;170:225–234. doi: 10.1007/s00213-003-1592-x. [DOI] [PubMed] [Google Scholar]
- 10.Honig A, Arts BM, Ponds RW, Riedel WJ. Lithium induced cognitive side-effects in bipolar disorder: a qualitative analysis and implications for daily practice. Int Clin Psychopharmacol. 1999;14:167–171. [PubMed] [Google Scholar]
- 11.Kocsis JH, Shaw ED, Stokes PE, Wilner P, Elliot AS, Sikes C, et al. Neuropsychologic effects of lithium discontinuation. J Clin Psychopharmacol. 1993;13:268–275. doi: 10.1097/00004714-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Stip E, Dufresne J, Lussier I, Yatham L. A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord. 2000;60:147–157. doi: 10.1016/S0165-0327(99)00178-0. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard P, Poulstrup I, Schou M. Prospective studies on a lithium cohort. 3. Tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand. 1988;78:434–441. doi: 10.1111/j.1600-0447.1988.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 14.Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, et al. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003;85:872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- 15.Shim SS, Hammonds MD, Ganocy SJ, Calabrese JR. Effects of subchronic lithium treatment on synaptic plasticity in the dentate gyrus of rat hippocampal slices. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:343–347. doi: 10.1016/j.pnpbp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Amdisen A. Clinical and serum-level monitoring in lithium therapy and lithium intoxication. J Anal Toxicol. 1978;2:193–202. doi: 10.1093/jat/2.5.193. [DOI] [Google Scholar]
- 17.Bitiktaş S, Tan B, Kavraal Ş, Yousef M, Bayar Y, Dursun N, et al. The effects of intra-hippocampal L-thyroxine infusion on long-term potentiation and long-term depression: a possible role for the αvβ3 integrin receptor. J Neurosci Res. 2017;95:1621–1632. doi: 10.1002/jnr.23985. [DOI] [PubMed] [Google Scholar]
- 18.Artis AS, Bitiktas S, Taşkın E, Dolu N, Liman N, Suer C. Experimental hypothyroidism delays field excitatory post-synaptic potentials and disrupts hippocampal long-term potentiation in the dentate gyrus of hippocampal formation and Y-maze performance in adult rats. J Neuroendocrinol. 2012;24:422–433. doi: 10.1111/j.1365-2826.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 19.Suer C, Dolu N, Artis AS, Sahin L, Aydogan S. Electrophysiological evidence of biphasic action of carnosine on long-term potentiation in urethane-anesthetized rats. Neuropeptides. 2011;45:77–81. doi: 10.1016/j.npep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Nikitin ES, Balaban PM. Compartmentalization of non-synaptic plasticity in neurons at the subcellular level. Neurosci Behav Physiol. 2014;44:725–730. doi: 10.1007/s11055-014-9975-5. [DOI] [Google Scholar]
- 21.Chavez-Noriega LE, Halliwell JV, Bliss TV. A decrease in firing threshold observed after induction of the EPSP-spike (E-S) component of long-term potentiation in rat hippocampal slices. Exp Brain Res. 1990;79:633–641. doi: 10.1007/BF00229331. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli T, Ferioli V, Lo Gallo G, Tomasini MC, Fernandez M, O’Connor WT, et al. Differential effects of acute and short-term lithium administration on dialysate glutamate and GABA levels in the frontal cortex of the conscious rat. Synapse. 2000;38:355–362. doi: 10.1002/1098-2396(20001201)38:3<355::AID-SYN15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Chavez-Noriega LE, Bliss TV, Halliwell JV. The EPSP-spike (ES) component of long-term potentiation in the rat hippo-campal slice is modulated by GABAergic but not cholinergic mechanisms. Neurosci Lett. 1989;104:58–64. doi: 10.1016/0304-3940(89)90329-7. [DOI] [PubMed] [Google Scholar]
- 25.Hernández F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- 26.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 28.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci. 2007;25:81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harwood AJ, Agam G. Search for a common mechanism of mood stabilizers. Biochem Pharmacol. 2003;66:179–189. doi: 10.1016/S0006-2952(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 32.Shim SS. Lithium enhances synaptic plasticity: implication for treatment of bipolar disorder. In: Barnhill J, editor. Bipolar disorder - a portrait of a complex mood disorder. London: InTech; 2012. pp. 41–54. [Google Scholar]
- 33.Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 35.Seelan RS, Khalyfa A, Lakshmanan J, Casanova MF, Parthasarathy RN. Deciphering the lithium transcriptome: microarray profiling of lithium-modulated gene expression in human neuronal cells. Neuroscience. 2008;151:1184–1197. doi: 10.1016/j.neuroscience.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Lenox RH, McNamara RK, Watterson JM, Watson DG. Myristoylated alanine-rich C kinase substrate (MARCKS): a molecular target for the therapeutic action of mood stabilizers in the brain? J Clin Psychiatry. 1996;57(Suppl 13):23–31. discussion 32–33. [PubMed] [Google Scholar]
- 37.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 38.Jeltsch H, Bertrand F, Lazarus C, Cassel JC. Cognitive performances and locomotor activity following dentate granule cell damage in rats: role of lesion extent and type of memory tested. Neurobiol Learn Mem. 2001;76:81–105. doi: 10.1006/nlme.2000.3986. [DOI] [PubMed] [Google Scholar]
- 39.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 40.Ghorbanalizadeh-Khalifeh-Mahaleh B, Taheri S, Sahebgharani M, Rezayof A, Haeri-Rohani A, Zarrindast MR. Intra-dorsal hippocampal microinjections of lithium and scopolamine induce a cross state-dependent learning in mice. Arch Iran Med. 2008;11:629–638. [PubMed] [Google Scholar]
- 41.Bora E, Vahip S, Akdeniz F, Gonul AS, Eryavuz A, Ogut M, et al. The effect of previous psychotic mood episodes on cognitive impairment in euthymic bipolar patients. Bipolar Disord. 2007;9:468–477. doi: 10.1111/j.1399-5618.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 42.López-Jaramillo C, Lopera-Vásquez J, Ospina-Duque J, García J, Gallo A, Cortez V, et al. Lithium treatment effects on the neuropsychological functioning of patients with bipolar I disorder. J Clin Psychiatry. 2010;71:1055–1060. doi: 10.4088/JCP.08m04673yel. [DOI] [PubMed] [Google Scholar]
- 43.Tsaltas E, Kontis D, Boulougouris V, Papakosta VM, Giannou H, Poulopoulou C, et al. Enhancing effects of chronic lithium on memory in the rat. Behav Brain Res. 2007;177:51–60. doi: 10.1016/j.bbr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 46.Sharifzadeh M, Aghsami M, Gholizadeh S, Tabrizian K, Soodi M, Khalaj S, et al. Protective effects of chronic lithium treatment against spatial memory retention deficits induced by the protein kinase AII inhibitor H-89 in rats. Pharmacology. 2007;80:158–165. doi: 10.1159/000103265. [DOI] [PubMed] [Google Scholar]
- 47.Nocjar C, Hammonds MD, Shim SS. Chronic lithium treatment magnifies learning in rats. Neuroscience. 2007;150:774–788. doi: 10.1016/j.neuroscience.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 48.Youngs RM, Chu MS, Meloni EG, Naydenov A, Carlezon WA, Jr, Konradi C. Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J Neurosci. 2006;26:6031–6039. doi: 10.1523/JNEUROSCI.0580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]