Abstract
Objective
This study investigated changes in urotensin-II (U-II) and endocan levels which can be used as an early biological marker of endothelial injury in the episode and remission phases of bipolar affective disorder (BAD).
Methods
We compared endocan and U-II levels, which has been shown to be closely associated with neurotransmitter systems in addition to continuity of endothelial structure and inflammatory response, in patients with BAD in remission for at least one year (n=42) and in patients still in manic or depressive episodes (n=16) with healthy controls (n=30).
Results
Both endocan and U-II levels were significantly higher in the bipolar patients than in the controls. Endocan and U-II levels were also significantly correlated with one another (p=0.000, r=0.833). Both endocan (p=0.000) and U-II levels (p=0.000) were significantly higher in the bipolar attack group compared to the subjects in remission, and in the remission group compared to the controls.
Conclusion
In this study we determined significantly higher endocan and U-II levels in BAD compared to the controls, while serum endocan and U-II levels of patients undergoing attacks were also significantly higher than those of the controls and also those of patients in remission.
Keywords: Endocan, Urotensins, Bipolar disorder, Endothelial injury
INTRODUCTION
OBipolar affective disorder (BAD) is a chronic disease exhibiting early onset and variation in terms of response to treatment, in addition to combinations of heterogeneous clinical symptoms with an episodic course and a high level of genetic characteristics.1–3) It also constitutes a severe psychiatric disease of unclear etiology and course and a serious medical problem representing the 6th largest cause of disability.4)
Oxidative and immunological mechanisms are thought to be involved in many chronic psychiatric and neurodegenerative processes. Tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are proinflammatory cytokines most associated with BAD. TNF-α has been shown to remain high after episodes and in intervening periods, while IL-6 is high in both depressive and manic episodes. Studies have reported that less effective mono-amine signaling is associated with increased IL-6 levels in cerebrospinal fluid (CSF). Increased IL-6 in CSF in association with recent manic attack in BAD has recently been confirmed. Elevated IL-4, which is thought to be involved in maintaining the balance between cellular and antibody-related immune response, is thought to be more associated with adaptive compensatory mechanisms.5–9)
There is considerable evidence pointing to a relation between BAD and vascular diseases. Adult bipolar patients have an earlier and 5-fold higher risk of cardiovascular disease compared to non-bipolar subjects.10) Studies of schizophrenia and BAD (and as in one study showing a linear relation between Von Willebrand factor levels and basal ganglion volume) provide evidence for the involvement of inflammation-relayed endothelial injury.11,12)
Endocan, also known as endothelial cell-specific molecule 1, is an endothelium-derived proteoglycan capable of binding to numerous bioactive molecules associated with cell signaling, adhesion, proliferation, differentiation and migration, soluble intermediate molecules and the cell surface.13) It has angiogenic and mitogenic properties and prevents leukocyte migration when inflammation is not present. It is closely associated with immunological response related to inflammation and infection. Its release is increased by vascular endothelial growth factor A (VEGF-A), VEGF-C, IL-1, TNF-α, transforming growth factor (TGF) β1 and fibroblast growth factor 2 (FGF-2) and reduced by phosphatidylinositol 3-kinase (P13K) and interferon gamma (INF-γ). It has been reported to be capable of forming as a result of endothelial function with impaired endocan levels.14) There is increasing suggestion in the literature that serum endocan levels can be used as an early biological marker of endothelial injury, as well as of its probable significance in determining arteriosclerosis and asymptomatic cardiovascular changes in the early stages.15–17) In addition, higher endocan levels have been linked to insulin resistance and hypothyroidism.18,19)
Urotensin-II (U-II) is a cyclic 11Aa peptide first identified in the caudal neurosecretory system of the teleost fish in 1969.20,21) U-II receptors have been determined in the vascular endothelium, myocardium, smooth muscle and skeletal muscles, adrenal glands, thyroid and renal cortex.22) Although U-II is known as a potent vasoconstrictor, it can exhibit different effects depending on its source, endothelial integrity, vascular size and presence or absence of disease.23) Elevation has been reported in conditions whose etiology is thought to involve oxidative injury and immunological systems, such as atherosclerosis, endothelial injury, hypertension, diabetes mellitus, obesity, heart failure, kidney failure, and liver diseases.24,25) U-II is thought to have diffuse receptor distribution in the central nervous system (CNS), particularly in the cerebral cortex, the olfactory bulb, the pineal gland, and the hypothalamus26,27) and to have behavioral and emotional effects in association with neurotransmitter systems, and particularly the dopaminergic, serotonergic, cholinergic and adrenergic systems.28–32)
This study investigated changes in U-II and endocan levels in the episode and remission phases of BAD, which can cause endothelial injury despite being a psychiatric disease involving a chronic course and episodes. This will support the previous literature on the subjects of their place in the etiology and monitoring of endothelial injury associated with this disease.
METHODS
We compared endocan levels, a marker of endothelial injury and part of the inflammatory process, and levels of U-II, which like endocan has been shown to be closely associated with neurotransmitter systems in addition to continuity of endothelial structure and inflammatory response, in patients with BAD in remission for at least one year (n=42) and in patients still in manic or depressive episodes (n=16) with healthy controls (n=30). We hypothesized that U-II and endocan, as markers of probable endothelial injury also thought to continue at the cellular level in the euthymic phase, in patients with BAD would differ from levels in the controls.
Patients presenting to our clinics on an in or outpatient basis, aged 18 to 65 years and diagnosed with BAD-I on the basis of diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision and in remission or attack phases and a control group were included in the study after providing written consent. Remission periods were defined as at least one year. Remission was identified on the basis of Hamilton Depression Scale and Young Mania Rating Scale scores of 7 or less. Patients or controls with a history of head trauma, severe metabolic, neurological or systemic disease or with intellectual insufficiency were excluded. Patients’ treatments were not managed by the authors, and no intervention took place into their treatment. Treatment and observation were continued as regulated on our ward if the patient was hospitalized or in the clinic if they were treated as outpatients. Outpatients receiving parenteral medication due to excitation were not included in the study until they were able to comply with treatment and progressed to oral drug treatment. If subjects’ compliance with treatment was sufficiently impaired as to refuse oral medication, then that consent was not considered valid. However, once they agreed to take medications by the oral route, consent was considered valid.
Our study was completed with 88 subjects, 58 patients with BAD-I and 30 controls. Forty-two patients were in remission and were enrolled from the psychiatric clinic, and 16 were enrolled from patients hospitalized on our ward. The age- and sex-matched control group was selected at random.
Ethical committee approval for the study was granted by the Atatürk University Faculty of Medicine Clinical Research Ethical Committee (No. B30.2.ATA.0.01.00/8 dated 28.22-2014).
Evaluation Tools
Sociodemographic and clinical evaluation form
Prepared by the author, this form was used to assess not only the participant’s sociodemographic characteristics, but also clinical characteristics such as age at onset of disease, duration of treatment, attack type and number and drugs used.
Biochemical analysis
Endocan ELISA kit (Catalogue No. SK00318-06) was purchased from the Aviscera Bioscience Company (Santa Clara, CA, USA). Urotensin-II ELISA kit (Catalogue No. E14913h) was purchased from the EIAab Company (Whuan, China). Biochemistry tubes containing blood specimens collected using the vacuum method from the patient and control groups were left to stand for 20 to 30 minutes at room temperature and away from the light. This allowed fiber networks to form. Specimens were then centrifuges at 3,500 rpm for 10 minutes at +4°C. Each serum specimen obtained was divided into two Eppendorf tubes (1.5 ml) and labeled. We checked that the specimen code on the blood collection tubes matched the number on the Eppendorf tubes. Specimens divided into portions were stored at −80°C until analysis. On the day of study, the specimens in the deep freeze were gradually thawed and investigated once thawing was complete.
Analysis of Serum Endocan Levels
Six different concentrations were obtained from the main solution: 100, 50, 25, 12.5; 6.25 and 3.125 ng/ml. Blind and serum specimens were each pipetted into 100-μl wells. Next, 100 mL of detection antibody was added to each well. After being stored at room temperature for 120 minutes, these were aspirated five times. One hundred microliters of horseradish peroxidase conjugate were pipetted into each well and incubated in the dark for 45 minutes. All wells were washed five times. One hundred microliters of substrate solution were added, and the mixture was shaken in a microplate mixer in the dark for 10 minutes. Finally, 100-ml stop solution was added to each well, and the reaction was halted. Blue color proportional to the level of U-II formed as a result of the reaction, and the color change was measured at 450 nm.
Evaluation of Serum Urotensin-II Levels
Concentrations of 10, 5, 2.5, 1.25, 0.62, 0.31, 0.15, and 0 ng/ml were prepared from the main stock. Blind and serum specimens were each pipetted into 100-μl wells and stored for 120 minutes at 37°C. The fluids were emptied from each well, and 100 μl of detection A reactive was placed into the wells. These were then incubated for 1 hour at 37°C. All wells were washed three times. Next, 100 μl of detection B reactive was placed into each well and incubated for 1 hour at 37°C. These were then again washed three times. In the following step, 100 μl of substrate solution was added and incubated in the dark for 15 minutes. Finally, the reaction was halted by the addition of 100-μl of stop solution.
The end reaction was measured spectrophotometrically on an ELISA reader at a wavelength of 450 nm.
Statistical Analysis
Our data were analyzed on IBM SPSS Statistics software (ver. 20; IBM Corp., Armonk, NY, USA). Data were expressed as mean, standard deviation and percentage and evaluated using descriptive analysis. Relation analysis on numerical data was performed using the correlation test, and using the chi-square test for categorical data. Relations among numerical and categorical data were assessed using linear regression analysis. Parametric tests or non-parametric comparisons were selected depending on the sample numbers of the groups to be compared and on homogeneous distribution. Differences between the groups were subjected to post-hoc Tukey test analysis.
RESULTS
Participants’ Demographic Characteristics
The mean age of the participants was 36.0±9 years. The mean age of the patient group was 36.9±10 years and the mean age of the control group was 34.3±8 years. The ages of the participants ranged between 18 and 56 years. Women constituted 52.3% (n=46) of the participants and men 47.7% (n=42). Women comprised 55.2% (n=32) of the bipolar group and men comprised 44.8% (n=26). No significant difference was determined in terms of sex between the controls and the bipolar patients. No significant difference was also determined in terms of other demographic characteristics. Smoking levels were 50% (n=15) among the controls and 43.9% (n=25) in bipolar disorder, and no significant difference was determined between the groups. No alcohol and substance use disorder was determined in the control group, but alcohol use disorder was present in three patients with bipolar disorder and substance use disorder in one.
Data concerning psychiatric disease in families were obtained on the basis of information provided by participants. Accordingly, a family history of psychiatric disease was present in 51.7% (n=45) of all subjects, in 57.9% (n=33) of the bipolar disorder group and in 40% (n=12) of the controls. The difference was not statistically significant.
The level of comorbidity with other physical diseases was 16.7% (n=5). Figure for the bipolar group was 42.1% (n=24), and the difference between the groups was statistically significant (χ2=5.724, p=0.017). Degree of functionality, assessed by means of clinical global observation scores, varied significantly between the groups. Although mean values for the control and remission groups were close to one another (100 and 94.8±8.4, respectively), the mean functionality evaluation score in the attack group, 36.0±12.4, was significantly lower compared to the other two groups (p=0.000, f=387.976).
Evaluation of Clinical Characteristics in Bipolar Disorder
Of the 58 patients with BAD enrolled in this study, 27.6% were still in the depressive (n=4) or manic episode (n=12), while 72.4% (n=42) were in remission persisting for at least one year. Mean age at onset of disease was 25.4±7.8 and mean duration of disease was 11.9±8.9 years. Mean number of attacks was 4.6±2.2, total number of hospitalizations was 3.4±3.1, mean number of manic episodes was 3.4±2.7, mean number of hypomanic episodes was 3.6±2.5, and mean number of depressive episodes was 2.3±1.7.
The first attack type was manic in 70.7% (n=41) of the bipolar patients and depressive in the remaining 29.3% (n=17). Time between diagnosis and treatment ranged between 1 and 7,200 days. Excluding two patients for whom the available data were inadequate, none of the remaining 56 patients started treatment on the same day that they were diagnosed. In terms of degree of delay, 44.8% (n=26) of patients started treatments within one week, 32.8% (n=19) started treatment after longer than one week but within 100 days, while first treatment took place after more than 100 days in 19% (n=11).
All patients were using medication; 3.4% (n=2) were using lithium bicarbonate alone, 5.2% (n=3) valproic acid alone, 27.6% lithium+antipsychotics (n=16), 37.9% (n=22) valproic acid+antipsychotics, 8.6% (n=5) lithium+valproic acid+antipsychotics, and 17.2% (n=10) antipsychotics alone. Electroconvulsive therapy (ECT) had been applied in the treatment history of 10 (17.2%) of the 58 patients with bipolar disorder, but none were currently receiving ECT.
In terms of the course characteristics of bipolar disorder, seasonality data for eight patients were excluded due to inadequacy of record data and since interviews were not sufficiently reliable. Analysis then revealed seasonality of attacks in 24.1% of patients (n=14), while no seasonality was determined in 58.6% (n=34). Attempted suicide was determined in 20.7% of patients. Since all the female bipolar patients (n=32) had children, postpartum episode characteristics were evaluated in all these subjects, and attacks were determined to be postpartum in onset in 43.8% (n=14) of patients. Attacks triggered by stressors not matching a definition of trauma were present in 44.8% (n=26) of patients, while 31 (53.4%) patients experienced attacks without these being triggered by stressor factors.
Serum Endocan and U-II Values
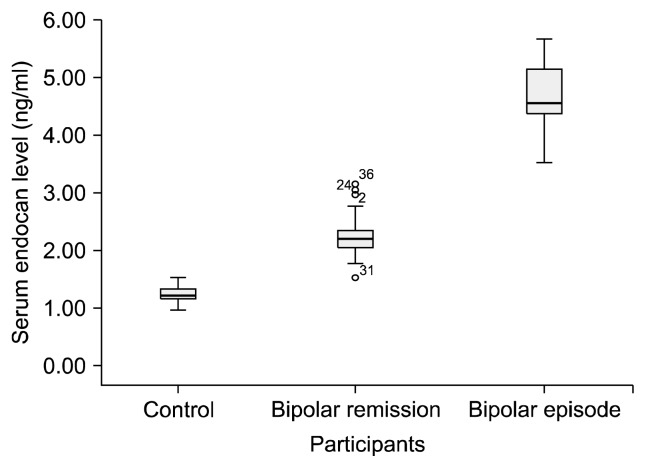
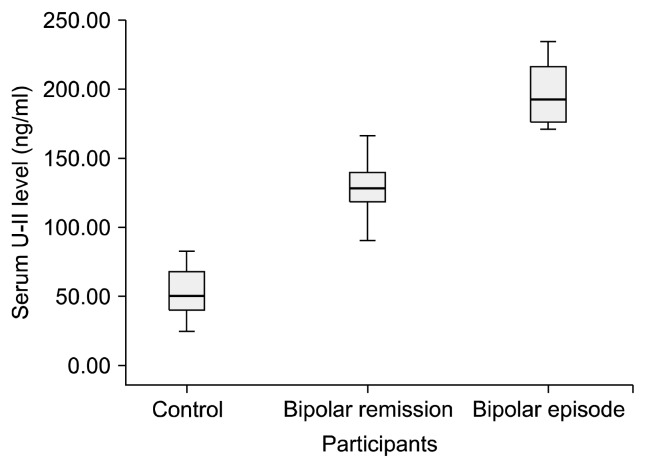
The mean serum endocan level of the participants was 2.34±1.23 and the mean U-II level was 115.72±54.14. Both endocan and U-II levels were significantly higher in the bipolar patients than in the controls. Endocan and U-II levels were also significantly correlated with one another (p=0.000 and r=0.833). Both endocan and U-II levels were significantly higher in the bipolar attack group compared to the subjects in remission, and in the remission group compared to the controls (Figs. 1, 2).
Fig. 1.
Serum endocan levels of the participants.
Fig. 2.
Serum urotensin-II (U-II) levels of the participants.
When the significant difference between the groups (Table 1) was subjected to post-hoc Tukey test analysis, the significant difference between endocan and U-II values persisted in all combinations when the three groups were subjected to two-way comparison. In addition, correlation analysis performed when the groups were classified as control=0, remission=1, and attack=2 and were analyzed in the form of numerical data, both endocan levels and U-II levels exhibited significant positive correlation with presence of disease and attack status; r values were 0.920 for endocan and 0.876 for U-II.
Table 1.
Serum endocan and urotensin-II (U-II) levels
| Participant | Endocan | U-II |
|---|---|---|
| BAD episode (n=16) | 4.65±0.56** | 196.12±21.48** |
| BAD remission (n=42) | 2.23±0.33** | 127.22±16.48** |
| Control (n=30) | 1.22±0.14** | 53.40±17.19** |
| F | 529.97 | 359.57 |
Values are presented as mean±standard deviation.
BAD, bipolar affective disorder.
p=0.000.
Seasonal characteristics, presence of postpartum episode, presence of attempted suicide, presence of stressor-triggered attacks and comorbidity with psychiatric and other physical diseases were not significantly correlated with endocan and U-II levels. Serum endocan and U-II levels were significantly correlated with time between diagnosis of BAD and treatment.
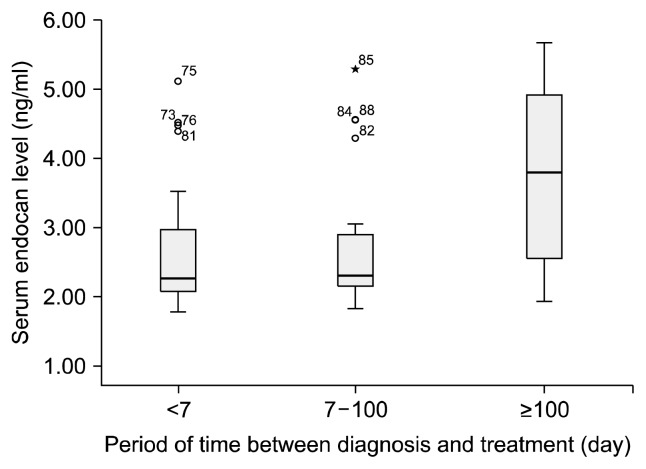
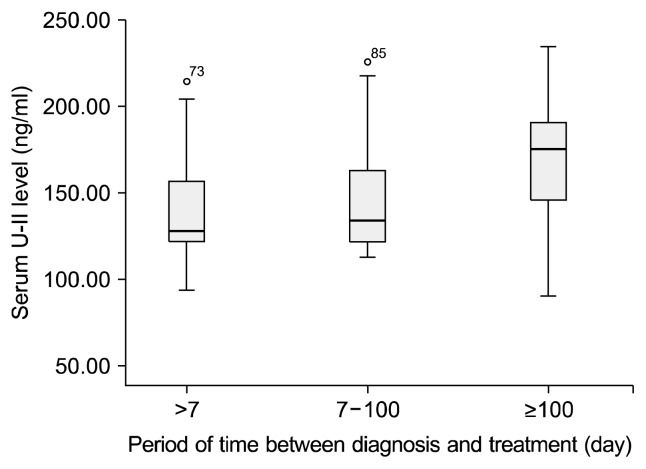
When the time between onset of disease and treatment was calculated in the form of days elapsed, serum endocan and U-II exhibited significant correlation with length of delay. This significance was determined as r=0.470 and p=0.000 for U-II and r=0.440 and p=0.001 for endocan. However, since the extreme values were highly pronounced the present correlation may be disregarded. When the time between onset of disease and initial treatment was subdivided as one week or less, 8 to 100 days and more than 100 days, significant differences were observed between the groups in terms of both endocan (p=0.031 and f=3.552) and U-II (p=0.040 and f=3.151) levels (Figs. 3, 4). When assessed using Tukey post-hoc analysis in order to determine the origin of the difference between the groups, the principal difference in terms of both endocan and U-II levels was identified between the group with less than one week between diagnosis and treatment (endocan level, 2.67±0.93) and the group with an intervening period exceeding 100 days (endocan level, 3.68±1.41).
Fig. 3.
Relationship between serum endocan levels and treatment period after diagnosis.
Fig. 4.
Relationship between serum urotensin-II (U-II) levels and treatment period after diagnosis.
Endocan levels were significantly correlated with number of depressive episodes (p=0.026, r=0.463), total number of episodes (p=0.044, r=0.270) and total number of hospitalizations (p=0.035, r=0.285).
U-II levels were significantly correlated with total episode numbers (p=0.039, r=0.277). U-II levels were correlated with number of depressive episodes (p=0.056, r=0.405) and total number of hospitalizations (p=0.051, r=0.265) at a level approaching statistical significance.
No significant correlation was determined between serum endocan or U-II levels and seasonality, comorbidity of psychiatric and other physical diseases or medication use.
DISCUSSION
Inflammation in an organism, whether in the CNS or not, may be regarded as both positive and negative. It is thought to initially appear with a healing code, but its long-lasting nature, the fact that it involves numerous inter-related systems and sometimes emerges in an unnecessary or inadequate form can make it difficult to observe its traces and outcomes. However, the immune inflammatory response resulting from adaptation, either initially or during the process involved, is known to lead to injury in the CNS and the periphery. Various consequences, such as neurodegenerative and psychiatric disorders, atherosclerosis, dyslipidemia, insulin resistance and increased mortality may all be observed as a result of this response.33,34)
Proinflammatory cytokines (such as ILs, TNFs, IFNs, TGFs, and chemokines) released from T lymphocytes, macrophages and endothelial cells during inflammation activate neutrophils, cause B cell proliferation, increase vascular permeability and lead to the release of acute phase reactants.35,36) Peripheral immune response can extend to the CNS. This can occur as a result of endothelial cells and pericytes in the blood brain barrier (BBB) stimulating inflammatory signals or peripheral immune cells such as T lymphocytes or macrophages passing into the CNS, since cytokines can pass the BBB through active transport, through structures not surrounded by the BBB or by means of afferent vagal fibers such as the nucleus tractus solitarius.5,6) The BBB protects the brain against endothelial injury. Recent studies have shown that the brain is not as free from peripheral immunological response as had once been thought and that cytokines can enter the CNS.37) Capillary endothelial injury has been observed on the first day of stress in the frontal cortex and hippocampus, and a thinner and irregular capillary membrane structure has been observed in the hippocampus after the 21st day.38) Microglia in the CNS also contribute to the inflammatory response by behaving like macrophages. Although all these can serve to prevent neuronal damage in an acute infection, in chronic or recurring conditions, such as in mood episodes, they can cause neuronal damage through the impairment of homeostasis.7) In conclusion, neuronal functions are compromised, synaptogenesis is affected, dendritic losses may occur and apoptotic stages are initiated, resulting in neuron death.8) Activation of microglia in the brain by inflammatory signals strengthens the inflammatory response through the release of oxygen and nitrogen species, chemokines and cytokines.5–9) The role of inflammation and the immune system in BAD has also been supported by a large number of studies.5–9,39,40) Immune dysregulation is associated with changes in monoamine glutamate signaling and affected neurotrophic neuroplastic support in BAD, and all these can explain the rich metabolic and vascular co-morbidity as well as the etiopathogenesis of the disease and contributing to the variation in its course.41,42)
There is also evidence that inflammation-related endothelial injury plays a role in schizophrenia and BAD.11,12) There are studies associating depressive mood with endothelial injury in major depressive disorder and BAD, as well as studies reporting endothelial injury in euthymic BAD patients or even suggesting an association between endothelial injury and hypomanicmanic symptoms, but not depressive symptoms.43) There are studies showing an increase in endothelial cell activity in BAD44) and studies implicating endothelium-related inflammation.45)
Endocan and U-II may be involved in endothelial injury associated with inflammatory mechanisms in BAD. Endocan binds to leukocyte ligands that acquire high affinity for E and P secretins during inflammation, and thus becomes significantly involved in inflammation response. Its involvement in this process that supports leukocyte migration is interpreted as preventing leukocyte migration and clustering.46,47) Although it appears to prevent migration in conditions in which cell stabilization is not impaired, its activity is rather different after onset of inflammation. While IL-1 and TNF-α increase the release of endocan, INF-γ and PI3K reduce it. INF-γ inhibits the release of endocan, and when combined with TNF-α it causes even more powerful endocan mRNA inhibition.48,49) U-II has also been shown to be involved in the process in inflammation. It contributes to the release of proangiogenic cytokines from endothelial cells.50) It activates monocytes and macrophages and increases the release of IL-1 and IL-6,51) and these effects have been shown to be antagonized with U-II receptor blockers.52) Due to the anti-inflammatory effect of U-II receptor blockage, it is regarded as a novel therapeutic target for several diseases thought to be based on inflammation.53)
Endocan stimulated by proangiogenic factors such as FGF-2 and VEGF becomes involved in vascularization by stimulating types of cells that determine the direction of the vessel and the extent to which it will grow.
Endocan also plays an important role in angiogenesis in stem cells during endothelial-mesenchymal transition.54,55) Endocan is although thought to play an important role in arterial medial thickening, plaque formation, and determining the activity zone of endothelial nitric oxide synthase (eNOS).56) Thirty-five percent greater nitric oxide (NO) cyclic guanosine monophosphate (cGMP) activity has been determined in tip cells compared to stalk cells that are not associated with vascular growth. Tip cells are known to have a greater endocan release capacity than stalk cells. eNOS/NO/cGMP signaling in tip cells also initiates blood vessel formation.57)
Although U-II is regarded as a potent vasoconstrictor, depending on its origin, endothelial integrity, vessel size and presence of disease, it is also capable of performing vasodilation in association with prostaglandin (PG) synthesis, although PGs are not thought to be involved in U-II-mediated vasoconstriction. Vascular tone has a more pronounced vasoconstriction effect in small arteries regulated in an endothelium-dependent manner, although this effect decreases as the vessel diameter increases and the effect in vasodilation associated with smooth muscle functions is almost non-existent. High U-II levels have been determined in patients with atherosclerosis, and U-II predicts a 1.6-fold increased risk for carotid plaque formation.58) Intervention in U-II can improve metabolic and atherosclerotic sequelae, and there is also evidence that the use of antagonists or gene deletion can treat atherosclerosis. Inflammatory cells appear to play an important role in U-II-dependent atherosclerosis.
U-II has a proangiogenic effect in endothelial cells in humans and rats and an angiogenic cytokine fibroblast F-like effect and increases mRNA expression of proangiogenic factors. U-II is also thought to activate endothelial proliferation by the P38 P44/42 MAPK pathway.58,59) Depending on the binding site, it can also cause an increase in intracellular Ca++ and vasoconstriction, or hyperpolarization and vasodilation by causing the release of NO.23,58,60,61) U-II is thought to play a role in both systemic and arterial hypertension, and there may be a positive correlation between CSF U-II concentrations and mean arterial blood pressure.60) When U-II binds to its receptor, phospholipase c, activation occurs and inositol trisphosphate (IP3) and diacylglycerol are formed from PIP2. It subsequently binds to the IP3 receptor. The Ca++ channels on ER are activated and Ca++ increase. To summarize, U-II increases intracellular Ca++ mobilization. Calcium-related cellular injury is one of the mechanisms discussed in the pathogenesis of BAD. In addition to all the other mechanisms, endocan and U-II are associated with the endocrine system, insulin resistance, obesity and diabetes mellitus. The high comorbidity of metabolic disease and BAD may indicate a common effect.18,19,62–64)
The distribution of U-II and its receptors in the CNS and their close relation with areas of the brain that regulate neurotransmitters implicated in psychiatric diseases, the locomotor system, the neuroendocrine system and sleep is also worthy of note.
In terms of U-II receptor distribution in the central nervous system, these are found in numerous regions, including the cerebral cortex, olfactory bulb, hippocampus, tegmentum, brain stem, cerebellum and spinal canal.26,27)
The fact that U-II is present in significant amounts in the locus coeruleus shows that it may have an effect on both mood and the sleep wakefulness cycle, because U-II increases noradrenaline (NA) release, while its agonists prevent this. U-II affects not only NA, but also the release of neurotransmitters such as dopamine, serotonin and histamine that are known to be involved in several events such as emotional control, sleep, attention, and memory and pain transmission.28)
Few studies have investigated the relation between serum endocan and U-II levels in psychiatric disorders. One study of subjects with depression in Alzheimer’s disease reported significantly lower endocan levels in subjects with depression and that depressive symptoms decreased as endocan levels increased. This was attributed to the probable effect of cortisol levels that rise in depression on serum endocan levels.65) Cortisol inhibits endocan gene expression. However, comorbidity of Alzheimer’s and depression is not the same thing as the depressive condition that emerges without Alzheimer’s. For one thing, it must be remembered that they differ in terms of cholinergic reserves. Additionally, the unknown process that initiates plaque formation appears long before clinical forgetfulness appears, and numerous adaptive mechanisms that restore balances for the continuation of connectivity in the nervous system go into operation during that process. Endocan levels may also be affected by inflammatory cytokines associated with depression.
In this study we determined significantly higher endocan levels in BAD compared to the controls, while serum endocan levels of patients undergoing attacks were also significantly higher than those of the controls and also those of patients in remission. In this study, based on the idea that high endocan may be a marker of endothelial injury, such injury was greater in patients with BAD than in the controls, and endothelial injury was also higher in patients currently experiencing manic or depressive attacks compared to patients in long-term remission.
Serum endocan and U-II levels being higher in patients in the attack period compared to those in remission may indicate that endothelial and inflammatory factors underlying the disease persist despite clinical improvement.
The association between time from diagnosis to first treatment and serum endocan and U-II levels, and the significant difference particularly between duration of less than one week and one exceeding 100 days, may be related to endothelial injury persisting throughout the period in the absence of any therapeutic intervention. The length of time in which treatment is delayed, which was significantly correlated with a higher number of depressive episodes, may be associated with a more destructive course of disease.
The significant association in this study between serum endocan elevation and number of depressive attacks, total number of attacks and number of hospitalizations suggests that endothelial injury may be associated with a worse disease course.
Subjects with severe metabolic disease being excluded from the study may be regarded as a limitation. Although, in one respect, this was an advantage since potentially confusing factors were excluded, a potential common biochemical mechanism may perhaps exist between metabolic diseases and bipolar disease, and this could not therefore be identified.
Since the investigation of other comorbid physical conditions in BAD lay outside the scope of this study, our participants did not receive cardiovascular examination, although further studies with larger numbers of subjects may make a significant contribution to the subject. Our subject numbers were not sufficient to reveal the effects of drugs used on serum endocan and U-II levels. In addition, since the drug load calculated on the basis of the levels of drugs used by patients and in the community by years and doses were unknown, we were unable to include the effect of treatment in the analysis.
Further studies with higher sample numbers and supported by blood drug levels are now needed to clarify the effects on the endothelial and inflammatory process of mood regulators and antipsychotics such as lithium and valproic acid.
Although the objectives set out in the methodology were scrupulously adhered to, and the findings were interpreted with the inclusion of confusing factors in the calculations, it is not possible to generalize the results of this study to this disease population.
One of the most powerful aspects of the study is the inclusion of a group with remission for at least one year and an attack group, which is important in revealing biochemical variation in the period in which symptoms do not persist compared to the controls. In addition, this is the first study to evaluate serum endocan and U-II levels in BAD. We therefore think that our study will make a significant contribution to the growing medical literature concerning the role of inflammatory processes and endothelial injury in the etiology and course of BAD.
REFERENCES
- 1.Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell’Osso MC, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Houenou J, Perlini C, Brambilla P. Epidemiological and clinical aspects will guide the neuroimaging research in bipolar disorder. Epidemiol Psychiatr Sci. 2015;24:117–120. doi: 10.1017/S2045796014000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni J, Filia S, Berk L, Filia K, Dodd S, de Castella A, et al. Treatment and outcomes of an Australian cohort of out-patients with bipolar I or schizoaffective disorder over twenty-four months: implications for clinical practice. BMC Psychiatry. 2012;12:228. doi: 10.1186/1471-244X-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martino DJ, Marengo E, Igoa A, Scápola M, Ais ED, Perinot L, et al. Neurocognitive and symptomatic predictors of functional outcome in bipolar disorders: a prospective 1 year follow-up study. J Affect Disord. 2009;116:37–42. doi: 10.1016/j.jad.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Söderlund J, Olsson SK, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, et al. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J Psychiatry Neurosci. 2011;36:114–118. doi: 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins CC, Sawa A, Pomper MG. Glia and immune cell signaling in bipolar disorder: insights from neuropharmacology and molecular imaging to clinical application. Transl Psychiatry. 2014;4:e350. doi: 10.1038/tp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stertz L, Magalhães PV, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry. 2013;26:19–26. doi: 10.1097/YCO.0b013e32835aa4b4. [DOI] [PubMed] [Google Scholar]
- 8.Fakhoury M. Role of Immunity and Inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis. 2015;15:63–69. doi: 10.1159/000369933. [DOI] [PubMed] [Google Scholar]
- 9.Muneer A. Bipolar disorder: role of inflammation and the development of disease biomarkers. Psychiatry Investig. 2016;13:18–33. doi: 10.4306/pi.2016.13.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dieset I, Haukvik UK, Melle I, Røssberg JI, Ueland T, Hope S, et al. Association between altered brain morphology and elevated peripheral endothelial markers--implications for psychotic disorders. Schizophr Res. 2015;161:222–228. doi: 10.1016/j.schres.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein BI, Young LT. Toward clinically applicable bio-markers in bipolar disorder: focus on BDNF, inflammatory markers, and endothelial function. Curr Psychiatry Rep. 2013;15:425. doi: 10.1007/s11920-013-0425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kali A, Shetty KS. Endocan: a novel circulating proteoglycan. Indian J Pharmacol. 2014;46:579–583. doi: 10.4103/0253-7613.144891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canpolat U, Kocyigit D, Yildirim A. Role of endothelial dysfunction and endocan in atherosclerosis: point of origin or end point? Angiology. 2016 doi: 10.1177/0003319716654627. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Qiu CR, Fu Q, Sui J, Zhang Q, Wei P, Wu Y, et al. Serum endothelial cell-specific molecule 1 (endocan) levels in patients with acute myocardial infarction and its clinical significance. Angiology. 2017;68:354–359. doi: 10.1177/0003319716651349. [DOI] [PubMed] [Google Scholar]
- 16.Kanbay A, Ceylan E, Köseoğlu Hİ, Çalışkan M, Takir M, Tulu S, et al. Endocan: a novel predictor of endothelial dysfunction in obstructive sleep apnea syndrome. Clin Respir J. 2018;12:84–90. doi: 10.1111/crj.12487. [DOI] [PubMed] [Google Scholar]
- 17.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Celik T, Iyisoy A. Endocan: a novel inflammatory indicator in cardiovascular disease? Atherosclerosis. 2015;243:339–343. doi: 10.1016/j.atherosclerosis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Westergren HU, Svedlund S, Momo RA, Blomster JI, Wåhlander K, Rehnström E, et al. Insulin resistance, endothelial function, angiogenic factors and clinical outcome in non-diabetic patients with chest pain without myocardial perfusion defects. Cardiovasc Diabetol. 2016;15:36. doi: 10.1186/s12933-016-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gungor A, Palabiyik SS, Bayraktutan Z, Dursun H, Gokkaya N, Bilen A, et al. Levels of endothelial cell-specific molecule-1 (ESM-1) in overt hypothyroidisim. Endocr Res. 2016;41:275–280. doi: 10.3109/07435800.2015.1135443. [DOI] [PubMed] [Google Scholar]
- 20.Onan D, Hannan RD, Thomas WG. Urotensin II: the old kid in town. Trends Endocrinol Metab. 2004;15:175–182. doi: 10.1016/j.tem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Coulouarn Y, Jégou S, Tostivint H, Vaudry H, Lihrmann I. Cloning, sequence analysis and tissue distribution of the mouse and rat urotensin II precursors. FEBS Lett. 1999;457:28–32. doi: 10.1016/S0014-5793(99)01003-0. [DOI] [PubMed] [Google Scholar]
- 22.Ong KL, Lam KS, Cheung BM. Urotensin II: its function in health and its role in disease. Cardiovasc Drugs Ther. 2005;19:65–75. doi: 10.1007/s10557-005-6899-x. [DOI] [PubMed] [Google Scholar]
- 23.Vaudry H, Leprince J, Chatenet D, Fournier A, Lambert DG, Le Mével JC, et al. International Union of Basic and Clinical Pharmacology. XCII. Urotensin II, urotensin II-related peptide, and their receptor: from structure to function. Pharmacol Rev. 2015;67:214–258. doi: 10.1124/pr.114.009480. [DOI] [PubMed] [Google Scholar]
- 24.McDonald J, Batuwangala M, Lambert DG. Role of urotensin II and its receptor in health and disease. J Anesth. 2007;21:378–389. doi: 10.1007/s00540-007-0524-z. [DOI] [PubMed] [Google Scholar]
- 25.Watson AM, May CN. Urotensin II, a novel peptide in central and peripheral cardiovascular control. Peptides. 2004;25:1759–1766. doi: 10.1016/j.peptides.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire JJ, Kuc RE, Kleinz MJ, Davenport AP. Immunocytochemical localization of the urotensin-II receptor, UT, to rat and human tissues: relevance to function. Peptides. 2008;29:735–742. doi: 10.1016/j.peptides.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Ono T, Kawaguchi Y, Kudo M, Kushikata T, Hashiba E, Yoshida H, et al. Urotensin II evokes neurotransmitter release from rat cerebrocortical slices. Neurosci Lett. 2008;440:275–279. doi: 10.1016/j.neulet.2008.05.096. [DOI] [PubMed] [Google Scholar]
- 29.Bucharles C, Bizet P, Arthaud S, Arabo A, Leprince J, Lefranc B, et al. Concordant localization of functional urotensin II and urotensin II-related peptide binding sites in the rat brain: Atypical occurrence close to the fourth ventricle. J Comp Neurol. 2014;522:2634–2649. doi: 10.1002/cne.23553. [DOI] [PubMed] [Google Scholar]
- 30.Dubessy C, Cartier D, Lectez B, Bucharles C, Chartrel N, Montero-Hadjadje M, et al. Characterization of urotensin II, distribution of urotensin II, urotensin II-related peptide and UT receptor mRNAs in mouse: evidence of urotensin II at the neuromuscular junction. J Neurochem. 2008;107:361–374. doi: 10.1111/j.1471-4159.2008.05624.x. [DOI] [PubMed] [Google Scholar]
- 31.Bruzzone F, Cervetto C, Mazzotta MC, Bianchini P, Ronzitti E, Leprince J, et al. Urotensin II receptor and acetylcholine release from mouse cervical spinal cord nerve terminals. Neuroscience. 2010;170:67–77. doi: 10.1016/j.neuroscience.2010.06.070. [DOI] [PubMed] [Google Scholar]
- 32.Clark SD, Nothacker HP, Blaha CD, Tyler CJ, Duangdao DM, Grupke SL, et al. Urotensin II acts as a modulator of mesopontine cholinergic neurons. Brain Res. 2005;1059:139–148. doi: 10.1016/j.brainres.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Hamdani N, Doukhan R, Kurtlucan O, Tamouza R, Leboyer M. Immunity, inflammation, and bipolar disorder: diagnostic and therapeutic implications. Curr Psychiatry Rep. 2013;15:387. doi: 10.1007/s11920-013-0387-y. [DOI] [PubMed] [Google Scholar]
- 34.Muneer A. Staging models in bipolar disorder: a systematic review of the literature. Clin Psychopharmacol Neurosci. 2016;14:117–130. doi: 10.9758/cpn.2016.14.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, et al. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord. 2015;17:269–277. doi: 10.1111/bdi.12259. [DOI] [PubMed] [Google Scholar]
- 36.Wang TY, Lee SY, Chen SL, Chang YH, Wang LJ, Chen PS, et al. Comparing clinical responses and the biomarkers of BDNF and cytokines between subthreshold bipolar disorder and bipolar II disorder. Sci Rep. 2016;6:27431. doi: 10.1038/srep27431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 38.Sántha P, Veszelka S, Hoyk Z, Mészáros M, Walter FR, Tóth AE, et al. Restraint stress-induced morphological changes at the blood-brain barrier in adult rats. Front Mol Neurosci. 2016;8:88. doi: 10.3389/fnmol.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari P, Parisi MM, Colombo R, Becker M, Fries G, Ascoli BM, et al. Depression and mania induce pro-inflammatory activation of macrophages following application of serum from individuals with bipolar disorder. Clin Psychopharmacol Neurosci. 2018;16:103–108. doi: 10.9758/cpn.2018.16.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa IG, Vaz GN, Rocha NP, Machado-Vieira R, Ventura MRD, Huguet RB, et al. Plasma levels of tumor necrosis factor superfamily molecules are increased in bipolar disorder. Clin Psychopharmacol Neurosci. 2017;15:269–275. doi: 10.9758/cpn.2017.15.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: A review. J Clin Psychiatry. 2006;67:1034–1041. doi: 10.4088/JCP.v67n0704. [DOI] [PubMed] [Google Scholar]
- 42.Laursen TM, Wahlbeck K, Hällgren J, Westman J, Ösby U, Alinaghizadeh H, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8:e67133. doi: 10.1371/journal.pone.0067133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiedorowicz JG, Coryell WH, Rice JP, Warren LL, Haynes WG. Vasculopathy related to manic/hypomanic symptom burden and first-generation antipsychotics in a sub-sample from the collaborative depression study. Psychother Psychosom. 2012;81:235–243. doi: 10.1159/000334779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willebrand factor. Bipolar Disord. 2009;11:726–734. doi: 10.1111/j.1399-5618.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 45.Dieset I, Djurovic S, Tesli M, Hope S, Mattingsdal M, Michelsen A, et al. Up-regulation of NOTCH4 gene expression in bipolar disorder. Am J Psychiatry. 2012;169:1292–1300. doi: 10.1176/appi.ajp.2012.11091431. [DOI] [PubMed] [Google Scholar]
- 46.Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–537. doi: 10.1097/01.CCM.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 47.Béchard D, Scherpereel A, Hammad H, Gentina T, Tsicopoulos A, Aumercier M, et al. Human endothelial-cell specific molecule-1 binds directly to the integrin CD11a/CD18 (LFA-1) and blocks binding to intercellular adhesion molecule-1. J Immunol. 2001;167:3099–3106. doi: 10.4049/jimmunol.167.6.3099. [DOI] [PubMed] [Google Scholar]
- 48.Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, et al. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458–20464. doi: 10.1074/jbc.271.34.20458. [DOI] [PubMed] [Google Scholar]
- 49.Rennel E, Mellberg S, Dimberg A, Petersson L, Botling J, Ameur A, et al. Endocan is a VEGF-A and PI3K regulated gene with increased expression in human renal cancer. Exp Cell Res. 2007;313:1285–1294. doi: 10.1016/j.yexcr.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Albertin G, Guidolin D, Sorato E, Oselladore B, Tortorella C, Ribatti D. Urotensin-II-stimulated expression of pro-angiogenic factors in human vascular endothelial cells. Regul Pept. 2011;172:16–22. doi: 10.1016/j.regpep.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, et al. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:238–250. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- 52.Park SL, Lee BK, Kim YA, Lee BH, Jung YS. Inhibitory effect of an urotensin II receptor antagonist on proinflammatory activation induced by urotensin II in human vascular endothelial cells. Biomol Ther (Seoul) 2013;21:277–283. doi: 10.4062/biomolther.2013.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cadirci E, Halici Z, Yayla M, Toktay E, Bayir Y, Karakus E, et al. Blocking of urotensin receptors as new target for treatment of carrageenan induced inflammation in rats. Peptides. 2016;82:35–43. doi: 10.1016/j.peptides.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 54.del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maurage CA, Adam E, Minéo JF, Sarrazin S, Debunne M, Siminski RM, et al. Endocan expression and localization in human glioblastomas. J Neuropathol Exp Neurol. 2009;68:633–641. doi: 10.1097/NEN.0b013e3181a52a7f. [DOI] [PubMed] [Google Scholar]
- 56.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Kurtoglu E, Demir M, et al. Endocan--a novel inflammatory indicator in newly diagnosed patients with hypertension: a pilot study. Angiology. 2014;65:773–777. doi: 10.1177/0003319713513492. [DOI] [PubMed] [Google Scholar]
- 57.Priya MK, Sahu G, Soto-Pantoja DR, Goldy N, Sundaresan AM, Jadhav V, et al. Tipping off endothelial tubes: nitric oxide drives tip cells. Angiogenesis. 2015;18:175–189. doi: 10.1007/s10456-014-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You Z, Genest J, Jr, Barrette PO, Hafiane A, Behm DJ, D’Orleans-Juste P, et al. Genetic and pharmacological manipulation of urotensin II ameliorate the metabolic and atherosclerosis sequalae in mice. Arterioscler Thromb Vasc Biol. 2012;32:1809–1816. doi: 10.1161/ATVBAHA.112.252973. [DOI] [PubMed] [Google Scholar]
- 59.Hassan GS, Douglas SA, Ohlstein EH, Giaid A. Expression of urotensin-II in human coronary atherosclerosis. Peptides. 2005;26:2464–2472. doi: 10.1016/j.peptides.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 60.Jani PP, Narayan H, Ng LL. The differential extraction and immunoluminometric assay of Urotensin II and Urotensin-related peptide in heart failure. Peptides. 2013;40:72–76. doi: 10.1016/j.peptides.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Bennett RT, Jones RD, Morice AH, Smith CF, Cowen ME. Vasoconstrictive effects of endothelin-, endothelin-, and urotensin II in isolated perfused human lungs and isolated human pulmonary arteries. Thorax. 2004;59:401–407. doi: 10.1136/thx.2003.011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson JP, Watt P, Sanghavi S, Strupish JW, Lambert DG. A comparison of cerebrospinal fluid and plasma urotensin II concentrations in normotensive and hypertensive patients undergoing urological surgery during spinal anesthesia: a pilot study. Anesth Analg. 2003;97:1501–1503. doi: 10.1213/01.ANE.0000086723.97421.BC. [DOI] [PubMed] [Google Scholar]
- 63.Romanova EV, Sasaki K, Alexeeva V, Vilim FS, Jing J, Richmond TA, et al. Urotensin II in invertebrates: from structure to function in Aplysia californica. PLoS One. 2012;7:e48764. doi: 10.1371/journal.pone.0048764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yasuda T, Masaki T, Gotoh K, Chiba S, Kakuma T, Yoshimatsu H. Intracerebroventricular administration of urotensin II regulates food intake and sympathetic nerve activity in brown adipose tissue. Peptides. 2012;35:131–135. doi: 10.1016/j.peptides.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Yoon KH, Kim SY, Moon YS, Roh D, Lee SK, Kim DH. The relationship between serum endocan levels and depression in Alzheimer’s disease. Dis Markers. 2016;2016 doi: 10.1155/2016/8254675. 8254675. [DOI] [PMC free article] [PubMed] [Google Scholar]