Abstract
Background
Current treatment guidelines for limited‐stage small‐cell lung cancer (SCLC) recommend concomitant platinum‐based chemo‐radiotherapy plus prophylactic cranial irradiation, based on the premise that SCLC disseminates early, and is chemosensitive. However, although there is usually a favourable initial response, relapse is common and the cure rate for limited‐stage SCLC remains relatively poor. Some recent clinical practice guidelines have recommended surgery for stage 1 (limited) SCLC followed by adjuvant chemotherapy, but this recommendation is largely based on the findings of observational studies.
Objectives
To determine whether, in patients with limited‐stage SCLC, surgical resection of cancer improves overall survival and treatment‐related deaths compared with radiotherapy or chemotherapy, or a combination of radiotherapy and chemotherapy, or best supportive care.
Search methods
We performed searches on CENTRAL, MEDLINE, Embase, CINAHL, and Web of Science up to 11 January 2017. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria
We included randomised controlled trials (RCTs) with adults diagnosed with limited‐stage SCLC, confirmed by cytology or histology, and radiological assessment, considered medically suitable for resection and radical radiotherapy, which randomised participants to surgery versus any other intervention.
Data collection and analysis
We imported studies identified by the search into a reference manager database. We retrieved the full‐text version of relevant studies, and two review authors independently extracted data. The primary outcome measures were overall survival and treatment‐related deaths; and secondary outcome measures included loco‐regional progression, quality of life, and adverse events.
Main results
We included three trials with 330 participants. We judged the quality of the evidence as very low for all the outcomes. The quality of the data was limited by the lack of complete outcome reporting, unclear risk of bias in the methods in which the studies were conducted, and the age of the studies (> 20 years). The methods of cancer staging and types of surgical procedures, which do not reflect current practice, reduced our confidence in the estimation of the effect.
Two studies compared surgery to radiation therapy, and in one study chemotherapy was administered to both arms. One study administered initial chemotherapy, then responders were randomised to surgery versus control; following, both groups underwent chest and whole brain irradiation.
Due to the clinical heterogeneity of the trials, we were unable to pool results for meta‐analysis.
All three studies reported overall survival. One study reported a mean overall survival of 199 days in the surgical arm, compared to 300 days in the radiotherapy arm (P = 0.04). One study reported overall survival as 4% in the surgical arm, compared to 10% in the radiotherapy arm at two years. Conversely, one study reported overall survival at two years as 52% in the surgical arm, compared to 18% in the radiotherapy arm. However this difference was not statistically significant (P = 0.12).
One study reported early postoperative mortality as 7% for the surgical arm, compared to 0% mortality in the radiotherapy arm. One study reported the difference in mean degree of dyspnoea as −1.2 comparing surgical intervention to radiotherapy, indicating that participants undergoing radiotherapy are likely to experience more dyspnoea. This was measured using a non‐validated scale.
Authors' conclusions
Evidence from currently available RCTs does not support a role for surgical resection in the management of limited‐stage small‐cell lung cancer; however our conclusions are limited by the quality of the available evidence and the lack of contemporary data. The results of the trials included in this review may not be generalisable to patients with clinical stage 1 small‐cell lung cancer carefully staged using contemporary staging methods. Although some guidelines currently recommend surgical resection in clinical stage 1 small‐cell lung cancer, prospective randomised controlled trials are needed to determine if there is any benefit in terms of short‐ and long‐term mortality and quality of life compared with chemo‐radiotherapy alone.
Plain language summary
Surgery for limited‐stage small‐cell lung cancer
Background
There are different types of lung cancer. One type is called small‐cell lung cancer. Small‐cell lung cancer is considered limited‐stage if it is still within the chest or extensive‐stage if it has spread outside the chest. Currently, chemotherapy and radiation therapy is recommended for treatment of limited‐stage small‐cell lung cancer if it is localised and has not spread outside one side of the chest.
Review question
We wanted to know if people with small‐cell lung cancer that has not spread outside the chest live longer with an operation to remove the tumour, whether accompanied by chemotherapy, radiotherapy, or both or neither, compared to chemotherapy, with or without radiotherapy.
Study characteristics
We searched for clinical trials up to 11 January 2017, and we included three studies with 330 people who had been diagnosed with small‐cell lung cancer which had not spread outside the chest. Some were given surgery only, and some were not. Also, some were given chemotherapy and radiotherapy along with their surgery, and some were given chemotherapy and radiotherapy without surgery. We looked for a difference in how long people lived, and if their treatment caused any side effects.
Key findings
The data were all of very low quality. All three studies were quite different so could not be combined. One study reported that people lived longer without surgery (but with radiotherapy) than with surgery. One study reported 4% of people surviving at two years with surgery compared to 10% of people surviving with radiotherapy. One study reported 52% of people surviving with surgery compared to 18% of people surviving with radiotherapy. Our evidence does not support the use of surgery for people with small‐cell lung cancer, but the quality of data is low and from more than 20 years ago. Better trials are needed to properly compare surgery with no surgery in people with small‐cell lung cancer.
Quality of the evidence
We rated the quality of the evidence using one of the following grades: very low, low, moderate, or high. Very low quality evidence means we are uncertain about the results. High‐quality evidence means we are very certain about the results. For this Cochrane Review, we found that the evidence was of very low quality for all the outcomes studies. We could not combine the trials as they were all very different, and the trials were very old. Some trials did not give enough information about their quality.
Summary of findings
for the main comparison.
| Surgery compared with no surgery for limited‐stage small‐cell lung cancer | |||
|
Patient or population: People with limited‐stage small‐cell lung cancer Settings: ambulatory care Intervention: surgery Comparison: no surgery | |||
| Outcomes | Impact | No of Participants (studies) | Quality of the evidence (GRADE) |
| Survival | Survival is difficult to interpret in these studiesa | 3 studies (330 participants)b | ⊕⊖⊖⊖ very lowc |
| Treatment related mortality | Treatment‐related mortality is difficult to interpret in these studiesd | 2 studies (290 participants)e | ⊕⊖⊖⊖ very lowc |
| Loco‐regional progression | Loco‐regional progression is difficult to interpret in these studiesf | 2 studies (186 participants)g | ⊕⊖⊖⊖ very lowc |
| Quality of life | Quality of life is difficult to interpret in these studiesh | 1 study (144 participants)i | ⊕⊖⊖⊖ very lowj |
|
GRADE Working Group grades of evidence
High quality: We are very confident that the true effect lies close to the estimate of effect
Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different
Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect
Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect aTreatment across trials were heterogenous: Fox 1973 compared surgery to radiotherapy. Lad 1994a administered induction chemotherapy to all participants, and then compared surgery to no surgery; following, all participants had chest and brain irradiation. Liao 1995 administered induction chemotherapy to all participants and then compared surgery to radiotherapy. The effect is difficult to interpret as the types of surgical procedures used in these studies do not reflect current clinical practice. bThis includes Fox 1973: surgery ‐ 71 participants; and radiotherapy ‐ 73 participants. Lad 1994a: surgery ‐ 70 participants; and no surgery ‐ 76 participants. Liao 1995: surgery ‐ 20 participants; and radiotherapy ‐ 20 participants. cStudies contributing to this outcome were at an unclear risk of bias which reduced our confidence in the estimation of effect. Downgraded once. Staging and surgical techniques don't necessarily reflect best current practice. Downgraded twice. dThe effect is difficult to interpret as the type of surgical procedures used in these studies do not reflect current clinical practice. eThis includes Fox 1973: surgery ‐ 71 participants; and radiotherapy ‐ 73 participants. Lad 1994a: surgery ‐ 70 participants; and no surgery ‐ 76 participants. fTreatments across trials were heterogenous: Fox 1973 compared surgery to radiotherapy. Lad 1994a administered induction chemotherapy to all participants, and then compared surgery to no surgery; following, all participants had chest and brain irradiation. Liao 1995 administered induction chemotherapy to all participants and then compared surgery to radiotherapy. gThis includes Lad 1994a: surgery ‐ 70 participants; and no surgery ‐ 76 participants; Liao 1995: surgery ‐ 20 participants; and radiotherapy ‐ 20 participants. hFox 1973 reported quality of life and dyspnoea using non‐validated scales. iThis includes Fox 1973: surgery ‐ 71 participants; and radiotherapy ‐ 73 participants. jStudies contributing to this outcome were at an unclear risk of bias which reduced our confidence in the estimation of effect. Downgraded once. Staging and surgical techniques don't necessarily reflect best current practice. Downgraded twice. The scales utilised were not validated. | |||
Background
Description of the condition
Lung cancer is a significant health burden around the world. It is the fifth most common malignancy and accounts for approximately 20% of all cancer‐related deaths and 5% of overall deaths. Overall survival of lung cancer is poor. The five‐year survival rate is 15% in Australia and the USA (AIHW & AACR 2012; NHMRC 2004).
Lung cancer is classified according to its histopathological subtype (Travis 2011; Travis 2013). Two major classes are non‐small‐cell lung cancer (NSCLC) (including adenocarcinoma, squamous cell carcinoma, and NSCLC not otherwise specified), accounting for 85% of lung cancers; and small‐cell lung cancer (SCLC). Histopathological characteristics guide further diagnosis and management. The treatment of NSCLC has been examined in other reviews (Manser 2005).
Small‐cell lung cancer is a specific clinical and histological entity. It comprises approximately 15% of all newly diagnosed lung cancers worldwide or 180,000 cases per year. It is particularly associated with tobacco use: 90% of those with SCLC are or were heavy smokers. Clinically SCLC tends to present in current or ex‐smokers over 70 years of age with a rapid onset of symptoms, generally consists of central and bulky tumours on chest imaging, and tends to spread early (van Meerbeeck 2011; Watson 1962). SCLC may cause paraneoplastic syndromes including, but not limited to, syndrome of inappropriate antidiuretic hormone secretion (SIADH), Cushing syndrome, and Lambert–Eaton syndrome (van Meerbeeck 2011).
Staging for SCLC differs from NSCLC and other cancers. Because of its tendency for early dissemination, staging for SCLC was initially defined by the Veterans' Administration Lung Study Group (VALSG) in the 1950s as "limited" (within one radiation portal, defined as a single hemithorax and its corresponding ipsilateral, supraclavicular lymph nodes) or "extensive" (beyond one portal, and including distant metastases, and malignant pleural effusions) (Zelen 1973). Treatment is generally guided by this staging system. In 1989, the International Association for the Study of Lung Cancer (IASLC) recommended the expansion of the definition of "limited" to include all tumours limited to one hemithorax with regional lymph node metastases, including hilar, ipsilateral and contralateral mediastinal and ipsilateral and contralateral supraclavicular lymph nodes. The IASLC also recommended any patients with an ipsilateral pleural effusion, malignant or not, be included in the "limited stage" if no other metastases were present (Stahel 1989). Differentiating between these stages may mean the difference between offering chemotherapy plus radiotherapy, or chemotherapy alone; and given the advancement of radiation therapy since the 1950s, more accurate staging of nodal involvement may be of particular relevance for radiation treatment (Shepherd 2007). More recently clinicians recommend using the TNM staging system for SCLC (Appendix 1), to assist prognostication and guide future management. A large, retrospective analysis of the IASLC database demonstrated a significant difference in survival between T1 and other T categories, and between N0/N1 and N2/N3 categories, and between N1 and N2 categories (Shepherd 2007).
Description of the intervention
Current treatment guidelines recommend platinum‐based chemotherapy plus thoracic radiotherapy for the treatment of limited‐stage SCLC; and chemotherapy alone for extensive disease, with prophylactic cranial irradiation. These recommendations are based on the premise that SCLC disseminates early, and that it is very chemosensitive (Jett 2013; NCCN 2015; NHMRC 2015; NICE 2011). Consideration of surgery is currently recommended for those who have a solitary nodule, no hilar or mediastinal involvement based on adequate mediastinal staging, no distant metastases, and no contraindications to surgery (Jett 2013; NCCN 2015).
Although there is usually an initial good response to chemotherapy, the overall prognosis is poor. Median survival for patients with limited disease is 15 to 20 months, with 20% surviving to two years; and for those with extensive stage disease, median survival is 8 to 13 months with a two‐year survival at 5% (van Meerbeeck 2011).
Initially surgery was the treatment of choice for all types of lung cancer. However in 1969 a randomised controlled trial compared radiotherapy to surgery in patients with limited SCLC (Fox 1973). Although median survival rates were less than one year, it demonstrated a small but significant difference in survival favouring radiotherapy. After this trial, surgery was mostly abandoned in favour of radiotherapy. Shortly after, the first chemotherapeutic agents demonstrated benefit over radiotherapy and so chemo‐radiotherapy became the acceptable form of treatment (Anraku 2006). The dichotomous taxonomy of SCLC and NSCLC came about in the 1960s and 1970s following a trial showing the very poor outcomes of surgery for small‐cell or ‘oat cell’ cancer (Fox 1973). Criticisms of this trial are that it did not include many participants who would now be considered to have T1‐2 N0 disease; and that new diagnostic and surgical tools have been developed since this time (Brock 2005).
The principle of surgery in limited‐stage SCLC is an attempt to remove all viable tumour with curative intent. Surgical resection of presumed early SCLC may also be beneficial so as not to miss a mistakenly diagnosed SCLC which may indeed be a NSCLC or mixed neuroendocrine tumour, and may offer better local control than chemoradiation therapy (Anraku 2006).
Recently some large retrospective cohort studies have demonstrated a potential benefit from resection of limited‐stage SCLC. The International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project, involving 349 cases of early resection, demonstrated five‐year survival rates with pathologic stage I, II and III SCLC of 48%, 39% and 15% respectively (Vallieres 2009). Similar results were seen in the Surveillance, Epidemiology and End Results (SEER) retrospective study of 247 participants with stage I SCLC who underwent resection. This is compared to evidence from multiple clinical trials using non‐surgical chemo‐radiotherapy protocols with a five‐year survival rate of 10% to 15% (Yu 2010).
Numerous other retrospective cohort studies and case series have demonstrated a survival benefit for those undergoing surgery, including studies by Karrer 1995 which demonstrated a survival of 63% in the surgical arm compared to 37% with conventional therapy, Rostad 2004 which demonstrated a five‐year survival of 44.9% in the surgical arm compared to 11% with conventional therapy and Badzio 2004 which demonstrated a median survival of 22 months in the surgery arm compared to 11 months with conventional therapy.
These results conflict with some randomised controlled trials which demonstrate no significant benefit of surgery compared to conventional treatment (Lad 1994a), but these non‐randomised observational studies may be flawed by selection bias.
Why it is important to do this review
Given the poor prognosis of this condition and the current conflicting literature about the role of surgery in SCLC, this review sought to identify and appraise all randomised controlled trials available to determine if surgical resection of limited‐stage SCLC improves clinically important outcomes including survival.
Objectives
To determine whether, in patients with limited‐stage SCLC, surgical resection of cancer improves overall survival and treatment‐related deaths compared with radiotherapy or chemotherapy, or a combination of radiotherapy and chemotherapy, or best supportive care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, parallel‐group controlled trials. Randomised trials were defined as studies which are described by the author as ‘randomised’ anywhere in the manuscript. We included trials where randomisation is implied by the description of how participants were assigned to treatment and control groups. There were no language restrictions. All identified trials, published and unpublished, were potentially eligible for inclusion.
Types of participants
We included participants with a cytological or histopathological diagnosis of small‐cell lung carcinoma and limited‐stage disease, as defined by the authors in each paper. Methods used to qualify staging were recorded.
Types of interventions
We included surgical resection of lung cancer alone or in combination with any other therapy, compared to non‐surgical treatment or no treatment, and we excluded trials comparing surgery alone with surgery plus chemotherapy or radiotherapy.
Types of outcome measures
Primary outcomes
Overall survival, defined as the time of randomisation to the time of death.
Treatment‐related deaths.
Secondary outcomes
Progression‐free survival, defined as the time of randomisation to the time of progression of disease or death.
Loco‐regional progression.
Quality of life.
Adverse events, graded according the National Cancer Institute Common Toxicity Criteria (NCI CTCAE 2010).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases (using the search strategies listed in Appendix 2).
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library.
MEDLINE Ovid (1946 to 11 January 2017).
Embase Ovid (1974 to 11 January 2017).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 11 January 2015).
Web of Science (to 11 January 2017).
We included publications from any year, language, and type or article.
Searching other resources
We handsearched reference lists of included studies, relevant chapters and review articles. We also searched clinical trial registries including the World Health Organization's International Clinical Trials Registry (apps.who.int/trialsearch/); and the US National Institutes of Health's ClinicalTrials.gov. We translated any relevant article into English for potential inclusion. Where data were missing, we attempted to contact authors.
Data collection and analysis
Selection of studies
Two independent review authors (KS and HB) independently screened all abstracts to determine whether they met the inclusion criteria. Full‐text publications were sought for those which possibly or definitely met the inclusion criteria. Then two independent authors (KS and HB) reviewed full‐text articles to determine eligibility. They resolved disagreement through discussion or reached consensus by consulting a third author (RM) where necessary.
Data extraction and management
Two review authors (KS and HB) independently extracted data from the included studies. We used a data collection form for study characteristics and outcome data, which we piloted on one study included in the review.
We extracted the following data.
Methods: study design, duration of the study, study setting, and date of study.
Participants: number, mean age and age range, gender, inclusion and exclusion criteria.
Intervention: intervention, dose, mode of administration, concomitant treatments, and exclusions.
Outcomes: primary and secondary outcomes as specified, type of scale used, and time points collected.
Notes: funding for trial and any conflicts of interest for trial authors.
'Risk of bias' summary.
We aimed to present results for time‐to‐event outcomes (such as OS and PFS) as hazard ratios (HRs) with 95% confidence intervals (CIs); and to present the treatment effects of dichotomous outcomes (such as OR and serious adverse events) as risk ratios (RRs) with 95% CIs (Higgins 2011).
Assessment of risk of bias in included studies
Two independent authors (HB and KS) assessed the included studies for risk of bias using the Cochrane's 'Risk of bias' assessment tool (Higgins 2011). We assessed the following.
Allocation (random sequence generation and allocation concealment).
Blinding of participants and personnel (checking for possible performance bias).
Blinding of outcome assessors (checking for possible performance bias).
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations).
Selective reporting bias (checking for whether the prespecified outcomes were met).
Other bias (bias due to problems not covered elsewhere in the table).
We scored each of these domains separately as either low risk of bias, unclear risk of bias (insufficient information to make a judgement), or high risk of bias as outlined below.
Generation of allocation sequence
For each included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method used to generate the allocation sequence as either: low risk of bias (any truly random process such as random number table or computer random number generator); or unclear risk of bias (method used to generate sequence not clearly stated).
Allocation concealment
For each included study we described the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of (or during) recruitment, or changed after assignment. We assessed the methods as either: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); or unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
Blinding or masking
For this type of intervention, it was likely that the participant was aware of what group they were assigned to. For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed the methods as either: low risk of bias (study stated that it was blinded and described the method used to achieve blinding); or unclear risk of bias.
Incomplete outcome data
We assessed the methods used to deal with incomplete data as either: low risk of bias (information from all participants were included in the main results, any dropouts are reported, any systematic differences between the two treatment arms are reported); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
Selective reporting bias
We assessed the methods as either low risk of bias (when the study fully reported all prespecified outcomes); unclear risk of bias (when it appeared not all prespecified outcomes were fully reported); or high risk of bias (not all prespecified outcomes were reported).
Other bias
For each included study we described any important concerns we had about other possible sources of bias (e.g. baseline imbalance, bias of the presentation data, representation of gender, age of studies, and whether any other treatments were administered outside of the protocol).
We resolved any disagreement which arose whilst conducting the above procedures by discussion and consensus.
Measures of treatment effect
We planned to measure treatment effect using HRs for time‐to‐event variables. We planned to pool dichotomous data such as adverse events and present these using RRs and 95% CIs. We planned to pool continuous data such as quality of life scores, mean difference (MD) or the standardised mean difference (SMD) into Review Manager 2014. When data aggregation was not feasible, results were presented in a descriptive analysis.
Unit of analysis issues
Our unit of analysis is the participant. We did not identify any cluster randomised controlled trials.
Dealing with missing data
We attempted to contact the authors to obtain missing data, but at the time of the publication of the review there has been no response. We distinguished between intention‐to‐treat (ITT) and per protocol (PP) analyses when judging quality.
Assessment of heterogeneity
To assess statistical heterogeneity of pooled analyses we planned to use the I² statistic, which describes the percentage of the total variation across trials due to heterogeneity rather than sampling error. We considered significant statistical heterogeneity to be present if the I² was greater than 50% (Higgins 2011). Where significant heterogeneity was identified, we expected to further assess using pre‐determined subgroups.
Assessment of reporting biases
We planned to assess publication bias using funnel plot analysis, but given the small number of studies included this was not possible.
Data synthesis
We planned to pool measures of effect using a fixed‐effect model, unless there was significant heterogeneity (I² > 50%). In that case, we planned to analyse data using a random‐effects model. Based on the heterogeneity of the trials, we decided it was not possible to pool measures of effect. We reported trials as descriptive analyses.
We assessed the overall quality of the evidence using the GRADE system (GradePro 2015), according to the following parameters where applicable.
Limitations in the design and implementation.
Indirectness of evidence.
Unexplained heterogeneity or inconsistency of results.
Imprecision of results.
High probability of publication bias.
The GRADE system uses the following criteria for assigning the grade of evidence based on RCTs.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We decreased the grade of evidence if the following occurred.
Serious (−1) or very serious (−2) limitation to study quality.
Important inconsistency (−1).
Some (−1) or major (−2) uncertainty about directness.
Imprecise or sparse data (−1).
High probability of reporting bias (−1).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses to determine if the impact of the intervention varied across these groups, and if these groups identified a source of heterogeneity. However due to the small number and variability of trials we were unable to combine data and perform subgroup analyses.
By TNM stage (to determine if more contemporary staging had a different effect on outcomes).
Induction treatment (chemotherapy/radiotherapy/chemo‐radiotherapy) (to determine if different induction treatments had a different effect on outcomes).
Adjuvant treatment (chemotherapy/radiotherapy/chemo‐radiotherapy) (to determine if different adjuvant treatments had different effects on outcomes).
By publication date (to determine if this had an effect on heterogeneity).
Sensitivity analysis
Where applicable, we planned to perform sensitivity analyses by quality of studies as assessed by the risk of bias criteria; however, the small number of trials and variability between trials ruled this out.
Results
Description of studies
Results of the search
We identified 2954 citations using our search strategy, and selected 76 articles for full‐text review after screening the abstracts of the initial search results. Many trials were in languages other than English, and we sought Cochrane translators to assist in the screening of these studies. We found five papers eligible for inclusion. Fox 1973, Miller 1969 and Scadding 1966 described the same cohort of participants with different time points of outcome measurement and so we reported these as part of one trial. We included three trials with 330 participants. See Figure 1 for more details.
1.
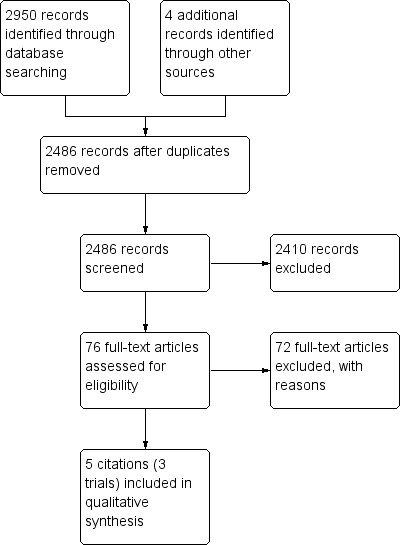
Study flow diagram.
Included studies
See the 'Characteristics of included studies' table.
We included three trials with 330 participants (Fox 1973; Lad 1994a; Liao 1995).
Study characteristics
All studies were described as randomised, parallel controlled trials. The three included studies were reported between 1973 and 1995, and their follow‐up ranged from 1 to 3 years (Liao 1995) to up to 10 years (Fox 1973).
Participants
Each study included participants diagnosed with histologically or cytologically confirmed SCLC, and were considered to have limited disease following radiological assessment. Bone marrow biopsy was performed in two trials (Lad 1994a; Liao 1995). Participants were only included if they were considered medically suitable for surgical resection and radical radiotherapy, except in Lad 1994a where participants were only included for randomisation after an initial response to chemotherapy.
Intervention
Surgical arm
Participants allocated to the surgical arm underwent thoracotomy with the intention of complete resection. Lad 1994a described complete resection in 77% of surgical participants, with 6% resulting in incomplete resection (positive margins), and 17% considered unresectable at time of thoracotomy. Fox 1973 described complete resection with pneumonectomy in 48%, a palliative pneumonectomy in 1%, thoracotomy only in 34%, and no resection in 18% of surgical participants. Liao 1995 did not describe any details of the methods or extent of the surgical resection, or the exact number of participants who ultimately underwent resection.
One trial performed nodal sampling from at least pretracheal, subcarinal, and intrapulmonary nodal stations during mediastinal nodal dissection, with the mean number of 3.6 N1 lymph nodes and mean 6.4 N2 nodes removed (Lad 1994a). Two trials did not provide details about nodal dissection/sampling (Fox 1973; Liao 1995).
Control arm
Lad 1994a compared surgery to no intervention. All participants underwent induction chemotherapy (cyclophosphamide, 1 g/m²; doxorubicin, 50 mg/m²; and vincristine 1.4 mg/m² every 3 weeks for five cycles), then objective responders (defined as “objective shrinking”, but specific criteria for this were not provided) were subsequently randomised to surgery with intent of complete resection followed by chest and whole brain irradiation, compared to chest and whole brain irradiation alone. Radiotherapy doses were 50 Gy in 25 fractions to the chest, and 30 Gy in 30 fractions in whole brain prophylaxis, delivered concurrently, in all participants.
Liao 1995 compared surgery to radiotherapy. All participants underwent two cycles of induction chemotherapy (IAO: Ifosfamide 1.2 g/m² intravenous (IV) for days 1 to 5, MESNA 400 mg IV TDS for days 1 to 5, Adriamycin 50 mg/m² IV for day 1, Vincristine 1 mg/m² for day 1). Subsequently, participants were randomised to surgical resection or radiotherapy (60 Gy). This was followed by an average of 2.8 cycles of chemotherapy in the surgical group and an average of 1.9 cycles of chemotherapy in the radiotherapy group.
Fox 1973 initially randomised participants to surgery or radiotherapy. Radical radiotherapy (3000 rads over 20 to 40 days), was applied in 85%, palliative radiotherapy in 11%, and no radiotherapy in 4% of control participants (due to refusal or deterioration).
Co‐interventions
Only one trial reported treatment outside the specified protocol (Fox 1973). In 55% of participants in the surgical arm, treatment other than surgery was given at some time during the two‐year period: chest radiotherapy was given to 34% in the first three months; radiotherapy for distant metastases was given in 11%; and chemotherapy was given to 18%. Twenty‐nine per cent of radiotherapy arm participants received further treatment over the two‐year period: 21% received radiotherapy for distant metastases; and chemotherapy was administered to 12% of participants (13/71 in the surgical arm and 9/73 in the radiotherapy arm).
Outcomes
For the primary outcome of survival, Lad 1994a reported median survival; Fox 1973 reported overall survival at 1, 2, 5, 6 and 10 years and mean survival; Liao 1995 reported overall survival at 1, 2 and 3 years.
Fox 1973 reported treatment‐related deaths and treatment‐related complications, and dyspnoea (but did not describe the scale on which this was measured).
Two studies reported loco‐regional progression (Lad 1994a; Liao 1995).
Excluded studies
See the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We assessed the risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011), and included the domains of allocation, blinding, incomplete outcome data, and other bias.
Please see Figure 2 and Figure 3 for the risk of bias findings.
2.
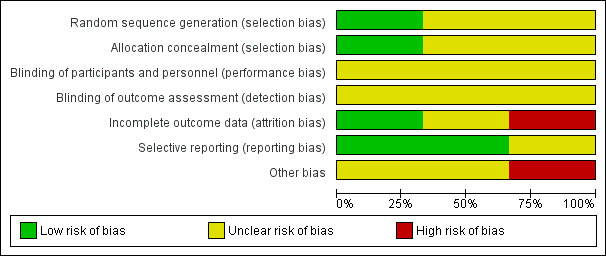
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
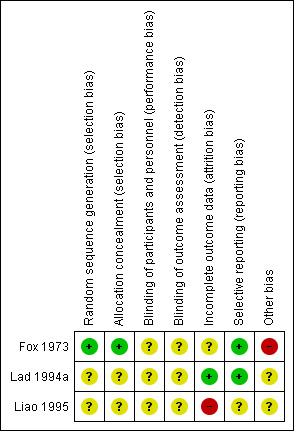
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We assessed random sequence generation as 'low risk' in one study (Fox 1973); and 'unclear risk' in two studies (Lad 1994a; Liao 1995).
We assessed allocation concealment as 'low risk' in one study (Fox 1973); and 'unclear risk' in two studies (Lad 1994a; Liao 1995), as methods of allocation concealment were not reported.
Blinding
We assessed blinding of participants and personnel and outcome assessors as 'unclear risk' in all three studies. It is likely that participants were aware of the intervention they were assigned to and it is likely the treating physicians were aware of the intervention received, and this may have affected the choice of co‐interventions. Lack of blinding is unlikely to have affected the results in the main outcome of survival but may have had an effect on the measurement of other outcomes.
Incomplete outcome data
Incomplete outcome data bias was assessed as low for one study (Lad 1994a), as reasons for participants not receiving allocated treatment were detailed, and all participants were accounted for at the time of follow‐up. We judged incomplete outcome data bias as high in one study (Liao 1995), as some participants were lost to follow‐up and further details were not provided. We judged incomplete outcome data bias as unclear in one study as although the reason for withdrawal was made clear, there were more withdrawals in the surgical group than the radiotherapy group (Fox 1973).
Only one study clearly used intention‐to‐treat analysis (Fox 1973).
Selective reporting
We judged selective reporting bias as low for two studies (Fox 1973; Lad 1994a), as all prespecified outcomes were detailed. We reported one study to be at high risk as prespecified outcomes (progression‐free survival and adverse events) were not reported and the reasons for this were unclear (Liao 1995).
Other potential sources of bias
We judged one study as 'high risk' as participants were eligible to receive further treatment following randomised intervention (Fox 1973). Intention‐to‐treat analysis was performed. We judged two studies as 'unclear risk' as it was not reported whether other participants received treatment outside the protocol (Lad 1994a; Liao 1995). Liao 1995 noted that the mean age of participants undergoing surgical intervention was lower (mean 50 years, range 33 to 74 years) compared to radiotherapy (mean age 54 years, range 31 to 66 years); however it is unclear how this may have affected the results.
All studies were published more than 20 years ago. Since this time, the requirements and reporting standards for conducting randomised controlled trials have become more rigorous. It is possible these trials did not adhere to current standards, leading to the introduction of bias.
Effects of interventions
See: Table 1
Primary outcomes
Survival
We intended to report overall survival using hazard ratios and 95% confidence intervals, but there were not enough appropriate extractable data to do this and so a descriptive analysis based on available evidence has been used.
Lad 1994a reported median survival as 15.4 months for the surgical arm and 18.6 months for the non‐surgical arm (146 participants, log‐rank P = 0.78).
Fox 1973 reported mean survival as 199 days in the surgical arm, and 300 days in the radiotherapy arm (P = 0.04; 144 participants). The number of participants alive in the surgical and radiotherapy arms was 21% and 22% respectively at 1 year, 4% and 10% respectively at 2 years, 1% and 4% respectively at 5 years, and 0% and 4% respectively at 10 years.
Liao 1995 reported overall survival at 1, 2 and 3 years. One‐year overall survival was: surgery group, 79%; radiotherapy group, 63%. Two‐year overall survival was: surgery group, 52%; radiotherapy group, 18%. Three‐year overall survival was: surgery group, 24%; radiotherapy group, 18% (P = 0.12, Chi² = 2.42).
Treatment‐related deaths
Fox 1973 reported treatment‐related deaths. Five deaths were directly attributable to surgical intervention (of 58 participants submitted to surgery), with an early postoperative mortality of 7%. Reasons included empyema, pulmonary embolus, myocardial infarction, chronic pleural space infection, and unknown cause. No radical radiotherapy participants died as a direct cause of treatment. Lad 1994a reported two deaths (3%) relating to surgery. No radiotherapy deaths were reported. Liao 1995 did not report treatment‐related deaths.
Secondary outcomes
Loco‐regional progression
Lad 1994a reported local progression in 25% and an additional 13% with distal relapse. They reported that patterns of treatment failure did not differ between the two groups; however individual group data were not available. Liao 1995 reported three out of 20 participants developed local recurrence in the surgical group compared to two out of 20 participants in the radiotherapy group. Distal metastases occurred in seven of 20 surgical participants, compared to ten out of 20 radiotherapy participants. Data was presented on the proportion of loco‐regional progression in each group at the end of the study but time‐to‐event data such as progression‐free survival were not reported.
Quality of life
Fox 1973 reported "well‐being" and "dyspnoea", using non‐validated scales (see Characteristics of included studies).
Well‐being was reported as "good" or "fair" in the majority of survivors in both arms. However at 3 months, 31% of surgical participants and 20% of radiotherapy participants were described as in "poor" condition; at 6 months, 43% of surgical participants and 29% of radiotherapy participants were similarly described; and at 12 months, 36% of surgical participants and 46% of radiotherapy participants were again described as in "poor" condition.
Mean degree of dyspnoea was recorded at 3, 6 and 12 months (adjusted for baseline assessment) (see Characteristics of included studies). Comparing surgery to radiotherapy, there was a difference of 0.1 at 3 months, −0.3 at 6 months, and −1.2 at 12 months, indicating participants in the radiotherapy group experienced more dyspnoea compared to the surgical group.
Discussion
Summary of main results
Conclusions about the role of surgery in the management of limited‐stage small‐cell lung cancer are constrained by the quality of the available evidence and the lack of contemporary randomised trial data. We identified three trials, all conducted more than 20 years ago.
The trial of Fox 1973 considerably changed the way SCLC was treated. It found survival was better in the non‐surgical compared to the surgical treatment group. Following this trial, surgery was largely abandoned in favour of radiotherapy. Chemotherapy was not included as part of the standard treatment protocol for this study; however subsequent studies supported the role of chemotherapy in addition to radiation in small‐cell carcinoma (Anraku 2006). The results of this study are not generalisable to current practice as most participants enrolled in this study had advanced disease, and staging was not comparable to current standards as chest imaging with computed tomography (CT) and positron emission tomography (PET) was not available at that time. Diagnosis was also made via rigid bronchoscopy, so peripheral nodules were not included. Treatment also did not include adjuvant chemotherapy at that time. In the surgical arm, only 34/71 participants underwent surgical resection; and during the 10‐year follow‐up other treatments were available — analysis was made as intention‐to‐treat, and subsequent interventions were not always completely recorded. There was also a considerably high rate of pneumonectomy, peri‐operative mortality, and R2 resections. Pneumonectomy is now rarely performed, and would not be advocated (Powell 2009). The high rate of mortality in the surgical arm may be due in part to the ongoing survival‐limiting effect of functioning with only one lung rather than being from a beneficial effect of radiotherapy on survival.
Lad 1994a included standard chemotherapy and radiotherapy for both arms, which is more comparable to current practice; and following this treatment, randomised participants to surgery or no surgery. They found no significant difference in survival between groups. Ten per cent of participants randomised to the surgical arm did not undergo resection; many participants had bulky nodal disease; and as tumours were diagnosed bronchoscopically, peripheral nodules were excluded. Liao 1995 treated all participants with chemotherapy, then randomised to surgery or radiotherapy. They found a higher survival rate in the surgical group compared to the radiotherapy group, but this was not statistically significant and because of the small number of participants and the risk of bias in this study it is difficult to interpret the significance of these findings.
Overall completeness and applicability of evidence
This review is limited by the paucity of good‐quality randomised controlled trials, and it is hard to compare participant characteristics and methods used 20 to 40 years ago to the diagnostic and therapeutic methods of our current practice. There were variations between the trials included in the review, particularly in the types of treatments delivered, and due to this clinical heterogeneity and the lack of appropriate quantitative data provided in trial reports it was not possible to draw firm conclusions or perform meta‐analyses. The review included trials which had examined the role of surgery in limited‐stage disease but none of the studies provided TNM staging for those included and it is likely the majority of the participants included in these studies had central and/or nodal disease. Therefore the findings of the review may not be generalisable to stage 1 small‐cell lung cancer, particularly stage 1A disease.
Quality of the evidence
Selection bias was judged as 'high' as the methods of randomisation and concealment allocation were often poorly described. Not all participants received their allocated treatments, and this was not always clearly reported; and some participants received other treatments during follow‐up which were incompletely reported and may have affected the overall outcome. There was also limited information provided regarding the extent of surgery, and the experience of the surgeons or the centre in which the treatment was conducted.
We judged the quality of evidence as 'very low' for all of the outcomes, according to the GRADE approach (GradePro 2015). There was an unclear risk of bias in the ways the studies were conducted, which reduced our confidence in the estimation of the effect. The way in which cancer was staged and methods of surgery which do not reflect current clinical practice led to concerns regarding indirectness of evidence. We were unable to combine studies as they were too heterogeneous.
Potential biases in the review process
We conducted this review in accordance with established Cochrane standards. Two review authors independently screened search results and resolved discrepancies by discussion and consensus. We did not restrict the literature search by language and we translated many studies into English to determine suitability for inclusion and for data extraction. We also contacted the study authors where it was unclear if a study met the inclusion criteria and to obtain further data, though none of the study authors responded.
Publication bias is possible, whereby a failure to identify unpublished trials could have led to an overestimation or underestimation of the effect of surgery on the treatment of SCLC.
Agreements and disagreements with other studies or reviews
To our knowledge this is the only systematic review available which examines the available evidence from randomised controlled trials for the use of surgery for limited‐stage small‐cell lung cancer. Our search only yielded a small number of older studies eligible for inclusion in the review, which demonstrated very little evidence for the role of surgery in SCLC. However there have been a number of recent, retrospective analyses performed that offer different findings.
The largest is a recent retrospective analysis of the Surveillance, Epidemiology and End Results (SEER) database which identified 3556 patients with stage I and II SCLC. Of these, 895 underwent resection. Median survival was 34.0 months (95% CI 29.0 to 39.0), compared to 16.0 months (95% CI 15.3 to 16.70, P < 0.001) in the non‐surgical patients. Median survival was significantly greater following lobectomy compared to wedge resection, which was still significantly better than no surgery. However the surgical cohort was younger, and likely a healthier cohort. These findings are similar to other retrospective studies, including the International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project which identified 349 cases of early stage resection which demonstrated five‐year survival rates with pathologic stage I, II and III SCLC of 48%, 39% and 15% respectively (Vallieres 2009), another retrospective study by Karrer 1995 which demonstrated a survival of 63% in the surgical arm compared to 37% with conventional therapy, Rostad 2004 which demonstrated a five‐year survival of 44.9% in the surgical arm compared to 11% with conventional therapy and Badzio 2004 which demonstrated a median survival of 22 months in the surgery arm compared to 11 months with conventional therapy. A more recent retrospective review from Lim 2008 analysed 59 patients who underwent surgical resection for known SCLC and demonstrated an excellent overall survival of 76% at one year (95% CI 65 to 88) and an overall five‐year survival of 52% (95% CI 40 to 68).
The findings of such observational studies have lent support to current clinical practice guidelines which recommend surgery for carefully staged stage 1 small‐cell lung cancer in those without contraindications for surgery; however these studies are likely affected by selection bias (Jett 2013; NCCN 2015). Whilst the results of this review may not be generalisable to stage 1 small‐cell lung cancers carefully staged in the modern era, the trend to increased treatment‐related deaths in the surgical arms of two of the studies included in this review emphasises the need for well‐conducted randomised controlled trials comparing surgery for stage 1 small‐cell cancer with non‐surgical treatment.
Authors' conclusions
Implications for practice.
Evidence from currently available randomised controlled trials does not support a role for surgical resection in the management of limited‐stage small‐cell lung cancer. However our conclusions are limited by the quality of the available evidence and the lack of contemporary data. The results of trials included in this review may not be generalisable to patients with clinical stage 1 small‐cell lung cancer carefully staged using contemporary staging methods.
Implications for research.
Although some guidelines currently recommend surgical resection in clinical stage 1 small‐cell lung cancer, prospective randomised controlled trials are needed to determine if there is any benefit in terms of short‐ and long‐term mortality and quality of life compared with chemo‐radiotherapy alone, as well as to assess the potential morbidity and mortality associated with surgery.
Acknowledgements
We would like to thank Ivan Sola, Information Specialist, Lung Cancer Group, for providing the search strategy. We would also like to thank the editors Fergus Macbeth, Ramon Rami‐Porta, Noelle O'Rourke, Jack Ruckdeschel and Tom Treasure, statistician Marta Roqué, Managing Editor of the Cochrane Lung Cancer Group Corynne Marchal, and editor Virginie Westeel for their comments on the review. We would also like to thank Darren Low, Ashley Tan and Nai Ming Lai for providing translations.
Appendices
Appendix 1. TNM staging system for lung cancer (7th edition)
| Primary tumor (T) | |||
| Tx | Primary tumour cannot be assessed, or tumour proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy | ||
| T0 | No evidence of primary tumour | ||
| Tis | Carcinoma in situ | ||
| T1 | Tumour ≤3 cm diameter, surrounded by lung or visceral pleura, without invasion more proximal than lobar bronchus | ||
| T1a | Tumour ≤2 cm in diameter | ||
| T1b | Tumour >2 cm but ≤3 cm in diameter | ||
| T2 | Tumour >3 cm but ≤7 cm, or tumour with any of the following features: | ||
| Involves main bronchus, ≥2 cm distal to carina | |||
| Invades visceral pleura | |||
| Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung | |||
| T2a | Tumour >3 cm but ≤5 cm | ||
| T2b | Tumour >5 cm but ≤7 cm | ||
| T3 | Tumour >7 cm or any of the following: | ||
| Directly invades any of the following: chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, main bronchus <2 cm from carina (without involvement of carina) | |||
| Atelectasis or obstructive pneumonitis of the entire lung | |||
| Separate tumour nodules in the same lobe | |||
| T4 | Tumour of any size that invades the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina, or with separate tumour nodules in a different ipsilateral lobe | ||
| Regional lymph nodes (N) | |||
| Nx | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastases | ||
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension | ||
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) | ||
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) | ||
| Distant metastasis (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| M1a | Separate tumour nodule(s) in a contralateral lobe; tumour with pleural nodules or malignant pleural or pericardial effusion | ||
| M1b | Distant metastasis (in extrathoracic organs) | ||
| Stage groupings | |||
| Stage IA | T1a‐T1b | N0 | M0 |
| Stage IB | T2a | N0 | M0 |
| Stage IIA | T1a,T1b,T2a | N1 | M0 |
| T2b | N0 | M0 | |
| Stage IIB | T2b | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T1a,T1b,T2a,T2b | N2 | M0 |
| T3 | N1,N2 | M0 | |
| T4 | N0,N1 | M0 | |
| Stage IIIB | T4 | N2 | M0 |
| Any T | N3 | M0 | |
| Stage IV | Any T | Any N | M1a or M1b |
Adapted from:Goldstraw 2007
Appendix 2. Search Strategy
Ovid MEDLINE, 1946 to 11 January 2017
1 exp Small Cell Lung Carcinoma/
2 exp Carcinoma, Small Cell/
3 exp Lung Neoplasms/
4 2 and 3
5 small cell lung.ti,ab.
6 (small cell carcinoma adj3 lung).ti,ab.
7 SCLC.ti,ab.
8 1 or 4 or 5 or 6 or 7
9 non small cell lung.ti.
10 8 not 9
11 exp Pneumonectomy/
12 surgery.fs.
13 surg*.ti,ab.
14 resect*.ti,ab.
15 lobectom*.ti,ab.
16 pneumonectom*.ti,ab.
17 11 or 12 or 13 or 14 or 15 or 16
18 10 and 17
19 randomised controlled trial.pt.
20 controlled clinical trial.pt.
21 randomized.ab.
22 placebo.ab.
23 drug therapy.fs.
24 randomly.ab.
25 trial.ab.
26 groups.ab.
27 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
28 exp animals/ not humans.sh.
29 27 not 28
30 18 and 29
Ovid Embase, 1974 to 11 January 2017
1 exp lung small cell cancer/
2 small cell lung.ti,ab.
3 (small cell carcinoma adj3 lung).ti,ab.
4 SCLC.ti,ab.
5 1 or 2 or 3 or 4
6 non small cell lung.ti.
7 5 not 6
8 exp lung resection/
9 su.fs.
10 surg*.ti,ab.
11 resect*.ti,ab.
12 lobectom*.ti,ab.
13 pneumonectom*.ti,ab.
14 8 or 9 or 10 or 11 or 12 or 13
15 7 and 14
16 random:.tw. or placebo:.mp. or double‐blind:.mp.
17 15 and 16
Cochrane Central Register of Controlled Trials (Cochrane Library, Issue 4, 2016)
#1 MeSH descriptor: [Small Cell Lung Carcinoma] explode all trees
#2 MeSH descriptor: [Carcinoma, Small Cell] explode all trees
#3 MeSH descriptor: [Lung Neoplasms] explode all trees
#4 #2 and #3
#5 small cell lung:ti,ab
#6 (small cell carcinoma near/3 lung):ti,ab
#7 SCLC:ti,ab
#8 #1 or #4 or #5 or #6 or #7
#9 non small cell lung:ti
#10 #8 not #9
#11 MeSH descriptor: [Pneumonectomy] explode all trees
#12 surg*:ti,ab
#13 resect*:ti,ab
#14 lobectom*:ti,ab
#15 pneumonectom*:ti,ab
#16 #11 or #12 or #13 or #14 or #15
#17 #10 and #16
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fox 1973.
| Methods | Parallel randomised controlled trial | |
| Participants | Adults with SCLC diagnosed on bronchial biopsy, with no radiologic evidence of extrathoracic metastases, and appropriate for operation/radiotherapy. | |
| Interventions | Surgery ‐ 71 participants Complete resection in 34 (48%), thoracotomy in 24 (34%), resection in 23 (33%), refused surgery in 2, and no surgery prior to deterioration in 13 (18%) of participants. In 55% of the surgical arm participants, treatment other than surgery was given at some time during the 2‐year period: chest radiotherapy was given to 34% in the first 3 months; radiotherapy for distant metastases was given in 11%; and chemotherapy was given to 18%. Compared to radiotherapy ‐ 73 participants. Radical (3000 rads over 20 to 40 days) in 62 (85%), palliative in 8 (11%), and refused or deteriorated in 3 (4%) of participants. 29% of radiotherapy arm participants received further treatment over the 2‐year period: 21% received radiotherapy for distant metastases; and chemotherapy was administered to 12% of participants. |
|
| Outcomes |
|
|
| Notes | This is the same cohort as the Scadding and Miller trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to treatment was made by reference to lists based on random sampling numbers. |
| Allocation concealment (selection bias) | Low risk | A separate list was used for each surgeon. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is likely that participants were aware of the intervention they were assigned to. It is likely the treating physicians were aware of the intervention received and this may have affected the choice of co‐interventions. Lack of blinding is unlikely to have affected the results in the main outcome of survival but may have an effect on the measurement of other outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear whether outcome assessors were truly blinded to the intervention. Lack of blinding is unlikely to have affected the results in the main outcome of survival, but it is unclear how it may have affected other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were not equal across groups, with 13/71 in the surgical group and only 3/73 in the radiotherapy group dropping out. For the surgical group 13/71 participants did not receive the intervention; 11 deteriorated in the interval between the allocation to treatment and the planned date of the operation, so that they were then judged unfit for surgery, and 2 refused surgery. In the radiotherapy group 3/73 participants did not receive any radiotherapy, in 2 because of deterioration before treatment could be commenced; the 3rd refused radiotherapy. There may have been a long delay between allocation of treatment and actual commencement, especially in the surgical group, which could have affected results. |
| Selective reporting (reporting bias) | Low risk | The study fully reported all prespecified outcomes. |
| Other bias | High risk | Participants were eligible to receive further treatment following randomised intervention. Intention to treat analysis was performed. |
Lad 1994a.
| Methods | Parallel randomised trial | |
| Participants | Adults with SCLC diagnosed on bronchial biopsy, with no radiologic evidence of extrathoracic metastases, and appropriate for operation/radiotherapy | |
| Interventions | All participants had induction chemotherapy for 5 cycles prior to randomisation (cyclophosphamide, 1 g/m²; doxorubicin, 50 mg/m²; and vincristine, 1.4 mg/m², every 3 weeks for five cycles). Surgery ‐ 70 participants. Complete resection in 77% of surgical participants, with 6% resulting in incomplete resection (positive margins), and 17% considered unresectable at time of thoracotomy. Compared to no intervention ‐ 76 participants. Following, all had chest and brain irradiation. (The radiation therapy doses were 50 Gy in 25 fractions to the chest, the target being the initial (pre‐chemotherapy) tumour volume and mediastinum, and 30 Gy in 15 fractions whole‐brain prophylaxis. Brain and chest irradiation were delivered concurrently). |
|
| Outcomes | Median survival, loco‐regional progression. Adverse events, including treatment related deaths, were not reported. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was reported as randomised, but no details for methods were given. |
| Allocation concealment (selection bias) | Unclear risk | No details for allocation concealment were given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is likely that participants were aware of the intervention they were assigned to. It is likely the treating physicians were aware of the intervention received and this may have affected the choice of co‐interventions. Lack of blinding is unlikely to have affected the results in the main outcome of survival but may have an effect on the measurement of other outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear whether outcome assessors were truly blinded to the intervention. Lack of blinding is unlikely to have affected the results in the main outcome of survival, but it is unclear how it may have affected other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | The study fully reported all prespecified outcomes. |
| Other bias | Unclear risk | It was not clear if any participants crossed over to other intervention arms. |
Liao 1995.
| Methods | Parallel randomised controlled trial. | |
| Participants | Adult participants SCLC diagnosed clinically, radiologically and histologically. | |
| Interventions | Surgery ‐ 20 participants Radiotherapy ‐ 20 participants All participants received up to 4 cycles of chemotherapy using IAO regimen (Ifosfamide 1.2 g/m² IV for days 1 to 5, MESNA 400 mg IV TDS for days 1 to 5, Adriamycin 50mg/m² IV for day 1, Vincristine 1 mg/m² for day 1) before and after intervention (surgical participants received an average of 2.1 cycles prior and 2.8 cycles post intervention, radiotherapy participants received an average of 2.2 cycles pre and 1.9 cycles post intervention). |
|
| Outcomes | Overall survival at 3 years. | |
| Notes | Article was in Chinese, translated into English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the method of random sequence generation was detailed. |
| Allocation concealment (selection bias) | Unclear risk | No information on sequence generation and allocation to enable assessment of the independence between the two stages of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is likely that participants were aware of the intervention they were assigned to. It is likely the treating physicians were aware of the intervention received and this may have affected the choice of co‐interventions. Lack of blinding is unlikely to have affected the results in the main outcome of survival but may have an effect on the measurement of other outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear whether outcome assessors were truly blinded to the intervention. Lack of blinding is unlikely to have affected the results in the main outcome of survival, but it is unclear how it may have affected other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 7 from 40 participants were lost to follow up (3 in surgery group and 4 in radiotherapy group, no reasons given). |
| Selective reporting (reporting bias) | Unclear risk | Progression‐free survival and adverse events not reported. |
| Other bias | Unclear risk | It was not clear if any participants crossed over to other intervention arms. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1991 | Not randomised |
| Anraku 2006 | Review (to be clarified) |
| Bychkov 2001 | Wrong intervention |
| Candel 2009 | Review |
| Clemente 2013 | Review |
| Coolen 1995 | Retrospective |
| Crisci 1989 | Not randomised |
| Cui 1994 | Retrospective |
| Davis 1993 | Not randomised |
| de Antonio 2006 | Not randomised |
| Dusmet 2004 | Review |
| Eguchi 1988 | Review |
| Eichhorn 1975 | Wrong intervention |
| Elias 1993 | Wrong intervention |
| Friedberg 2012 | Review |
| Fujimori 1997 | Wrong study design |
| Fujimura 2001 | Review |
| Gatzemeier 1986 | Not randomised |
| Graham 1993 | Review |
| Grants 1989 | Not randomised |
| Greschuchna 1978 | Retrospective |
| Gridelli 1994 | Not randomised |
| Gridelli 1994a | Not randomised |
| Hansen 1984 | Review |
| Hara 1988 | Retrospective |
| Hara 1991a | Not randomised |
| Hara 1991b | Retrospective |
| Ju 2012 | Retrospective |
| Karrer 1978 | Review |
| Karrer 1982a | Review |
| Karrer 1982b | Duplicate |
| Karrer 1986 | Review |
| Karrer 1987 | Wrong intervention |
| Karrer 1988 | Wrong intervention |
| Karrer 1989a | Wrong intervention |
| Karrer 1989b | Duplicate |
| Karrer 1990 | Wrong intervention |
| Karrer 1995 | Wrong intervention |
| Kobayashi 1988 | Not randomised |
| Kodama 1998 | Review |
| Krishnamurthy 2011 | Not randomised |
| Lad 1991 | Duplicate |
| Lad 1994b | Duplicate |
| Lagerwaard 2010 | Wrong participant population |
| Lassen 1999 | Review |
| Lewinski 2001 | Not randomised |
| Lexer 1988 | Not randomised |
| Li 2010 | Not randomised |
| Lucchi 1997 | Retrospective |
| Lukianski 1988 | Not randomised |
| Macchiarini 1989a | Retrospective |
| Macchiarini 1989b | Duplicate |
| Namikawa 1988 | Not randomised |
| Osterlind 1986a | Wrong intervention |
| Osterlind 1986b | Not randomised |
| Prager 1984 | Not randomised |
| Rea 1998 | Wrong intervention |
| Schamanek 1994 | Wrong intervention |
| Shepherd 1983 | Retrospective |
| Shepherd 1989 | Retrospective |
| Shepherd 1991 | Not randomised |
| Sorenson 1981 | Review |
| Szczesny 2003 | Review |
| Theuer 1992 | Review |
| Tsuchiya 2005 | Wrong intervention |
| Ulsperger 1990 | Duplicate |
| Ulsperger 1991 | Duplicate |
| Veronesi 2007 | Not randomised |
| Watanabe 1988 | Not randomised |
| Yamaguchi 1988 | Not randomised |
Differences between protocol and review
We revised the title to 'Surgery for limited‐stage small‐cell lung cancer' to more accurately reflect current guidelines on staging for small‐cell lung cancer. We further discussed the different definitions of 'limited' in the manuscript. We amended the primary outcomes to include overall survival and treatment‐related deaths, and made progression‐free survival a secondary outcome.
Contributions of authors
HB and RM drafted the protocol.
HB and KS screened and extracted studies.
HB, KS, SB, and RM drafted the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Hayley Barnes: none known
Katharine See: none known
Stephen Barnett: none known
Renée Manser: none known
New
References
References to studies included in this review
Fox 1973 {published data only}
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small‐celled or oat‐celled carcinoma of bronchus. Ten‐year follow‐up. Lancet 1973;2(7820):63‐5. [DOI] [PubMed] [Google Scholar]
- Miller AB, Fox W, Tall R. Five‐year follow‐up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oat‐celled carcinoma of the bronchus. Lancet 1969;294(7619):501. [DOI] [PubMed] [Google Scholar]
- Scadding JG, Bignall JR, Blair LG. Comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oatcelled carcinoma of the bronchus: first report to the medical research council by the working party on the evaluation of different methods of therapy in carcinoma of the bronchus. Lancet 1966;288(7471):979‐86. [PubMed] [Google Scholar]
Lad 1994a {published data only}
- Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit if surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106(6 Suppl):320S–3S. [DOI] [PubMed] [Google Scholar]
Liao 1995 {published data only}
- Liao M, Zhao J, Zhou Y. Multimodality therapy of late stage lung cancer. Chinese Journal of Oncology 1995;17(5):384‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1991 {published data only}
- Anonymous. Surgery of small cell carcinoma. Kyobu Geka [Japanese Journal of Thoracic Surgery] 1991;39(5):582‐97. [PubMed] [Google Scholar]
Anraku 2006 {published data only}
- Anraku M, Waddell TK. Surgery for Small‐Cell Lung Cancer. Seminars in Thoracic and Cardiovascular Surgery 2006;18(3):211‐6. [DOI] [PubMed] [Google Scholar]
Bychkov 2001 {published data only}
- Bychkov MB, Orel NF, Naskhletashvili DR. Present‐day possibilities of the treatment of small cell cancer of the lung. Voprosy Onkologii 2001;47(6):757‐61. [PubMed] [Google Scholar]
Candel 2009 {published data only}
- Candel VA, Blesa JMG, Vidal OJ. Treatment of small‐cell lung cancer. Cancer & Chemotherapy Reviews 2009;4(3):131‐40. [Google Scholar]
Clemente 2013 {published data only}
- Clemente M, Soccal PM, Karenovics W, Adler D, Rochat T, Mach N. The role of surgery in early stage small cell lung cancer: Should it be re‐evaluated?. Revue Medicale Suisse 2013;9(407):2172‐4. [PubMed] [Google Scholar]
Coolen 1995 {published data only}
- Coolen L, Eeckhout A, Deneffe G, Demedts M, Vansteenkiste J. Surgical treatment of small cell lung cancer. European Journal of Cardio‐thoracic Surgery 1995;9(2):59‐64. [DOI] [PubMed] [Google Scholar]
Crisci 1989 {published data only}
- Crisci C, Nutini S, Righi R, Zottola V, Giannini GM, Camassa M, et al. Role of surgery in undifferentiated small cell carcinoma of the lung. Preliminary results of an international multicenter study. Minerva Chirurgica 1989;44(4):589‐98. [PubMed] [Google Scholar]
Cui 1994 {published data only}
- Cui SY, Li HZ, Gu YT. The combined treatment with surgery and chemotherapy: primary approach to small cell lung carcinoma (SCLC). Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1994;16(6):432‐4. [PubMed] [Google Scholar]
Davis 1993 {published data only}
- Davis S, Crino L, Tonato M, Darwish S, Pelicci PG, Grignani F. A prospective analysis of chemotherapy following surgical resection of clinical stage I‐II small‐cell lung cancer. American Journal of Clinical Oncology 1993;16(2):93‐5. [DOI] [PubMed] [Google Scholar]
de Antonio 2006 {published data only}
- Antonio DG, Alfageme F, Gámez P, Córdoba M, Varela A, Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology. Results of surgery in small cell carcinoma of the lung. Lung Cancer (Amsterdam, Netherlands) 2006;52(3):299‐304. [DOI] [PubMed] [Google Scholar]
Dusmet 2004 {published data only}
- Dusmet M, Goldstraw P. Surgery for small cell lung cancer. Hematology/oncology Clinics of North America 2004;18(2):323‐41. [DOI] [PubMed] [Google Scholar]
Eguchi 1988 {published data only}
- Eguchi K. Overview of current studies on the role of surgery of small‐cell carcinoma of the lung. Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):205‐10. [PubMed] [Google Scholar]
Eichhorn 1975 {published data only}
- Eichhorn HJ, Eule H, Lessel A, Menne W. Results of a controlled clinical trial for evaluation of intensive preoperative irradiation in operable bronchial cancer. Archiv fur Geschwulstforschung 1975;45(4):376‐84. [PubMed] [Google Scholar]
Elias 1993 {published data only}
- Elias AD, Ayash L, Frei E 3rd, Skarin AT, Hunt M, Wheeler C, et al. Intensive combined modality therapy for limited‐stage small‐cell lung cancer. Journal of the National Cancer Institute 1993;85(7):559‐66. [DOI] [PubMed] [Google Scholar]
Friedberg 2012 {published data only}
- Friedberg J, Lotano VE. Role of surgery for small cell lung cancer. Current Problems in Cancer 2012;36(3):117‐30. [DOI] [PubMed] [Google Scholar]
Fujimori 1997 {published data only}
- Fujimori K, Yokoyama A, Kurita Y, Terashima M. A pilot phase 2 study of surgical treatment after induction chemotherapy for resectable stage I to IIIA small cell lung cancer. Chest 1997;111(4):1089‐93. [DOI] [PubMed] [Google Scholar]
Fujimura 2001 {published data only}
- Fujimura S. Limited surgery for bronchogenic carcinoma. Kyobu Geka [Japanese Journal of Thoracic Surgery] 2001;54(8 Suppl):707‐14. [PubMed] [Google Scholar]
Gatzemeier 1986 {published data only}
- Gatzemeier U, Radenbach D. Chemotherapy (CAV) and surgical resection as combined modality in the therapy of small‐cell lung cancer. Panminerva Medica 1986;28(2):63‐6. [PubMed] [Google Scholar]
Graham 1993 {published data only}
- Graham P. 5‐year actuarial survival results for an admittedly highly selected group of patients with small cell lung cancer treated with surgery and adjuvant chemotherapy. American Journal of Clinical Oncology 1993;16(2):181‐2. [PubMed] [Google Scholar]
Grants 1989 {published data only}
- Grants EA, Berzinia V, Bashko I, Zitare I, Berzin'sh I. The results of the adjuvant chemotherapy of patients with small‐cell lung cancer. Voprosy Onkologii 1989;35(2):172‐4. [PubMed] [Google Scholar]
Greschuchna 1978 {published data only}
- Greschuchna D. Results of operative therapy for small‐cell bronchogenic carcinoma. Thoraxchirurgie, Vaskulare Chirurgie 1978;26(4):300‐3. [DOI] [PubMed] [Google Scholar]
Gridelli 1994 {published data only}
- Gridelli C, D'Aprile M, Curcio C, Brancaccio L, Palmeri S, Comella G, et al. Carboplatin plus epirubicin plus VP‐16, concurrent 'split course' radiotherapy and adjuvant surgery for limited small cell lung cancer. Gruppo Oncologico Centro‐Sud‐Isole (GOCSI). Lung Cancer 1994;11(1‐2):83‐91. [DOI] [PubMed] [Google Scholar]
Gridelli 1994a {published data only}
- Gridelli C, Ianniello GP, Maiorino A, Curcio C, D'Aprile M, Brancaccio L, et al. Carboplatin, epirubicin, and VP‐16 chemotherapy in the treatment of small cell lung cancer. American Journal of Clinical Oncology 1994;17(2):160‐2. [DOI] [PubMed] [Google Scholar]
Hansen 1984 {published data only}
- Hansen HH. Treatment of small cell carcinoma of the lung. Gan to Kagaku Ryoho. Cancer & Chemotherapy 1984;11(2):171‐85. [PubMed] [Google Scholar]
Hara 1988 {published data only}
- Hara N, Motohiro A, Takeo S, Ohtsu Y, Tanaka K, Yamazaki S, et al. Multimodality therapy of small carcinoma of the lung‐‐the role of surgical treatment. Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):190‐4. [PubMed] [Google Scholar]
Hara 1991a {published data only}
- Hara N, Ichinose Y, Kuda T, Asoh H, Yano T, Kawasaki M, et al. Long‐term survivors in resected and nonresected small cell lung cancer. Oncology 1991;48(6):441‐7. [DOI] [PubMed] [Google Scholar]
Hara 1991b {published data only}
- Hara N, Ohta M, Ichinose Y, Motohiro A, Kuda T, Asoh H, et al. Influence of surgical resection before and after chemotherapy on survival in small cell lung cancer. Journal of Surgical Oncology 1991;47(1):53‐61. [DOI] [PubMed] [Google Scholar]
Ju 2012 {published data only}
- Ju MH, Kim HR, Kim JB, Kim YH, Kim DK, Park S‐II. Surgical outcomes in small cell lung cancer. The Korean Journal of Thoracic and Cardiovascular Surgery 2012;45(1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karrer 1978 {published data only}
Karrer 1982a {published data only}
- Karrer K, Denck H, Pridun N, Zwintz E. Prevention of recurrence of small‐cell lung cancer by adjuvant polychemotherapy. Wiener Klinische Wochenschrift 1982; Vol. 94, issue 7:159‐65. [PubMed]
Karrer 1982b {published data only}
- Karrer K, Denck H, Pridun N, Zwintz E. Prevention of recurrence of small‐cell lung cancer by adjuvant polychemotherapy. Wiener Klinische Wochenschrift 1982;94(7):159‐65. [PubMed] [Google Scholar]
Karrer 1986 {published data only}
- Karrer K, Denck H, Drings P, Orel J, Bruno MF. Combination of surgery and chemotherapy for small‐cell bronchial carcinoma. Drugs under Experimental and Clinical Research 1986;12(1‐3):191‐200. [PubMed] [Google Scholar]
Karrer 1987 {published data only}
- Karrer K, Denck H. Multimodality treatment with surgery in small‐cell lung cancer. International Journal of Clinical Pharmacology Research 1987; Vol. 7, issue 4:313‐28. [PubMed]
Karrer 1988 {published data only}
- Karrer K, Denck H, Karnicka‐Mlodkowska H, Drings P, Erzen J, Dell'Amore D, et al. Surgery for cure followed by combined modality treatment for small cell bronchial carcinoma. ISC Lung Cancer Study Group. International Journal of Clinical Pharmacology Research 1988; Vol. 8, issue 6:415‐21. [PubMed]
Karrer 1989a {published data only}
- Karrer K, Shields TW, Denck H, Hrabar B, Vogt‐Moykopf I, Salzer GM. The importance of surgical and multimodality treatment for small cell bronchial carcinoma. Journal of Thoracic and Cardiovascular Surgery 1989; Vol. 97, issue 2:168‐76. [PubMed]
Karrer 1989b {published data only}
- Karrer K, Denck H, Karnicka‐Mlodkowska H, Drings P, Erzen J, Salzer GM, et al. Surgery for cure of small cell bronchial carcinoma (SCLC) at early stages and postoperative combined modality treatment. International Journal of Clinical Pharmacology Research 1989;1(4 Suppl):1207‐8. [PubMed] [Google Scholar]
Karrer 1990 {published data only}
- Karrer K, Denck H, Karnicka‐Mlodkowska H, Drings P, Salzer G M, Hrabar B, et al. The importance of surgery as the first step in multimodality treatment of small cell bronchial carcinoma. The ISC Lung Cancer Study Group. International Journal of Clinical Pharmacology Research 1990; Vol. 10, issue 5:257‐63. [PubMed]
Karrer 1995 {published data only}
- Karrer K, Ulsperger E. Surgery for cure followed by chemotherapy in small cell carcinoma of the lung. For the ISC‐Lung Cancer Study Group. Acta Oncologica 1995;34(7):899‐906. [DOI] [PubMed] [Google Scholar]
Kobayashi 1988 {published data only}
- Kobayashi K, Hashizume N, Horinouchi H, Nakayama M, Kawamura M, Nomori H, et al. The role of surgery in the treatment for small cell lung cancer. Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):200‐4. [PubMed] [Google Scholar]
Kodama 1998 {published data only}
- Kodama K, Doi O, Higashiyama M, Yokouchi H, Takami K. Does induction therapy followed by surgery improve the survival rate in limited‐stage small‐cell lung cancer patients?. Nihon Geka Gakkai Zasshi. Journal of Japan Surgical Society 1998;99(5):291‐8. [PubMed] [Google Scholar]
Krishnamurthy 2011 {published data only}
- Krishnamurthy J, Tashi T, Gonsalves W, Ganti A K. Treatment of small cell lung cancer. International Journal of Cancer Research and Prevention 2011;4(2):151‐61. [Google Scholar]
Lad 1991 {published data only}
- Lad T, Thomas P, Piantadosi S. Surgical resection of small cell lung cancer ‐ a prospective randomized evaluation [abstract]. Proceedings of the American Society of Clinical Oncology 1991; Vol. 10, issue 244:A835.
Lad 1994b {published data only}
- Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest 1994;106(6 Suppl):320S‐3S. [DOI] [PubMed] [Google Scholar]
Lagerwaard 2010 {published data only}
- Lagerwaard FJ. New trials in early disease comparing surgery and radiation. Journal of Thoracic Oncology 2010;1:S24. [Google Scholar]
Lassen 1999 {published data only}
- Lassen U, Hansen HH. Surgery in limited stage small cell lung cancer. Cancer Treatment Reviews 1999;25(2):67‐72. [DOI] [PubMed] [Google Scholar]
Lewinski 2001 {published data only}
- Lewinski T, Zulawski M, Turski C, Pietraszek A. Small cell lung cancer I‐‐III A: cytoreductive chemotherapy followed by resection with continuation of chemotherapy. European Journal of Cardio‐thoracic Surgery 2001;20(2):391‐8. [DOI] [PubMed] [Google Scholar]
Lexer 1988 {published data only}
- Lexer G, Weitensfelder W, Haiderer O, Wieser O. Long‐term survival following pneumonectomy, chemotherapy and radiotherapy in central small cell bronchial cancer. Praxis und Klinik der Pneumologie 1988;42(7):588‐9. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li GH, Wu Y, Zhang XJ, Cui YS. A comparative study of survival time of surgery combined with chemotherapy and non‐surgical chemotherapy in SCLC. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2010; Vol. 90, issue 31:2212‐4. [PubMed]
Lucchi 1997 {published data only}
- Lucchi M, Mussi A, Chella A, Janni A, Ribechini A, Menconi GF, et al. Surgery in the management of small cell lung cancer. European Journal of Cardio‐thoracic Surgery 1997;12(5):689‐93. [DOI] [PubMed] [Google Scholar]
Lukianski 1988 {published data only}
- Lukianski M. [Surgical treatment of patients with small cell carcinoma of the lungs followed by chemotherapy]. Pneumonologia Polska 1988;56(2):63‐8. [PubMed] [Google Scholar]
Macchiarini 1989a {published data only}
- Macchiarini P, Mussi A, Basolo F, Bruno J, Angeletti CA. Optimal treatment for T1‐3NOMO small cell lung cancer: surgery plus adjuvant chemotherapy. Anticancer Research 1989;9(6):1623‐5. [PubMed] [Google Scholar]
Macchiarini 1989b {published data only}
- Macchiarini P, Mussi A, Basolo F, Bruno J, Angeletti CA. Optimal treatment for T1‐3N0M0 small cell lung cancer: Surgery plus adjuvant chemotherapy. Anticancer Research 1989;9(6):1623‐6. [PubMed] [Google Scholar]
Namikawa 1988 {published data only}
- Namikawa S, Kusagawa K, Tani K, Kanamori Y, Higashi K, Takeuchi Y, et al. The role of surgical resection and the effect of neo‐adjuvant therapy in the management of small cell lung cancer (SCLC). Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):195‐9. [PubMed] [Google Scholar]
Osterlind 1986a {published data only}
- Osterlind K, Hansen HH, Hansen M, Dombernowsky P. Mortality and morbidity in long‐term surviving patients treated with chemotherapy with or without irradiation for small‐cell lung cancer. Journal of Clinical Oncology 1986;4(7):1044‐52. [DOI] [PubMed] [Google Scholar]
Osterlind 1986b {published data only}
- Osterlind K, Hansen M, Hansen HH, Dombernowsky P. Influence of surgical resection prior to chemotherapy on the long‐term results in small cell lung cancer. A study of 150 operable patients. European Journal of Cancer & Clinical Oncology 1986;22(5):589‐93. [DOI] [PubMed] [Google Scholar]
Prager 1984 {published data only}
- Prager RL, Foster JM, Hainsworth JD, Hande KR, Johnson DH, Wolff SN, et al. The feasibility of adjuvant surgery in limited‐stage small cell carcinoma: a prospective evaluation. Annals of Thoracic Surgery 1984;38(6):622‐6. [DOI] [PubMed] [Google Scholar]
Rea 1998 {published data only}
- Rea F, Callegaro D, Favaretto A, Loy M, Paccagnella A, Fantoni U, et al. Long term results of surgery and chemotherapy in small cell lung cancer. European Journal of Cardio‐thoracic Surgery 1998;14(4):398‐402. [DOI] [PubMed] [Google Scholar]
Schamanek 1994 {published data only}
- Schamanek A, Karrer K. Importance of surgery in the multimodality treatment for small cell lung cancer (SCLC). Radiology and Oncology 1994;28(4):351‐8. [Google Scholar]
Shepherd 1983 {published data only}
- Shepherd FA, Ginsberg RJ, Evans WK. Reduction in local recurrence and improved survival in surgically treated patients with small cell lung cancer. Journal of Thoracic and Cardiovascular Surgery 1983;86(4):498‐506. [PubMed] [Google Scholar]
Shepherd 1989 {published data only}
- Shepherd FA, Ginsberg RJ, Patterson GA, Evans WK, Feld R. A prospective study of adjuvant surgical resection after chemotherapy for limited small cell lung cancer. A University of Toronto Lung Oncology Group study. Journal of Thoracic and Cardiovascular Surgery 1989;97(2):177‐86. [PubMed] [Google Scholar]
Shepherd 1991 {published data only}
- Shepherd FA, Ginsberg RJ, Feld R, Evans WK, Johansen E. Surgical treatment for limited small‐cell lung cancer. The University of Toronto Lung Oncology Group experience. Journal of Thoracic and Cardiovascular Surgery 1991;101(3):385‐93. [PubMed] [Google Scholar]
Sorenson 1981 {published data only}
- Sorenson S. Treatment of small cell carcinoma of the lung. European Journal of Respiratory Diseases 1981;62(5):315‐31. [PubMed] [Google Scholar]
Szczesny 2003 {published data only}
- Szczesny TJ, Szczesna A, Shepherd FA, Ginsberg RJ. Surgical treatment of small cell lung cancer. Seminars in Oncology 2003;30(1):47‐56. [DOI] [PubMed] [Google Scholar]
Theuer 1992 {published data only}
Tsuchiya 2005 {published data only}
- Tsuchiya R, Suzuki K, Ichinose Y, Watanabe Y, Yasumitsu T, Ishizuka N, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I‐IIIa small cell lung cancer: the Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101). Journal of Thoracic and Cardiovascular Surgery 2005;129(5):977‐83. [DOI] [PubMed] [Google Scholar]
Ulsperger 1990 {published data only}
- Ulsperger E, Karrer K, Shields TW. Surgery for cure followed by multimodality treatment for small cell bronchial carcinoma (SCLC). Annals of Oncology 1990; Vol. 1, issue Suppl:51.
Ulsperger 1991 {published data only}
- Ulsperger E, Karrer K, Denck H. Multimodality treatment for small cell bronchial carcinoma. Preliminary results of a prospective, multicenter trial. The ISC‐Lung Cancer Study Group. European Journal of Cardio‐thoracic Surgery 1991;5(6):306‐9; discussion 310. [DOI] [PubMed] [Google Scholar]
Veronesi 2007 {published data only}
- Veronesi G, Scanagatta P, Leo F, Pas T, Pelosi G, Catalano G, et al. Adjuvant surgery after carboplatin and VP16 in resectable small cell lung cancer. Journal of Thoracic Oncology 2007;2(2):131‐4. [PubMed] [Google Scholar]
Watanabe 1988 {published data only}
- Watanabe Y, Kimoto H, Shimizu J, Murakami N, Oda M, Yoshida M, et al. [The role of surgery in the management of small cell carcinoma of the lung]. Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):178‐83. [PubMed] [Google Scholar]
Yamaguchi 1988 {published data only}
- Yamaguchi Y, Momiki S, Kimura H, Ogawa T, Baba M. The role of surgical treatment in the combination therapy of small cell lung cancer (SCLC). Kyobu Geka [Japanese Journal of Thoracic Surgery] 1988;41(3):184‐9. [PubMed] [Google Scholar]
Additional references
AIHW & AACR 2012
- Australian Institute of Health and Welfare & Australasian Association of Cancer Registries. Cancer in Australia: an overview (Cancer series no. 74. Cat. no. CAN 70. Canberra: AIHW). www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129542353 (accessed 24 May 2014).
Badzio 2004
- Badzio A, Kurowski K, Karnicka‐Mlodkowska H, Jassem J. A retrospective study of surgery followed by chemotherapy vs. Non‐surgical management in limited‐disease small cell lung cancer. European Journal of Cardio‐thoracic Surgery 2004;26(1):183‐8. [DOI] [PubMed] [Google Scholar]
Brock 2005
- Brock M, Hooker C, Syphard J, Westra W, Xu L, Alberg AJ, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. Journal of Thoracic and Cardiovascular Surgery 2005;129(1):64‐72. [DOI] [PubMed] [Google Scholar]
Goldstraw 2007
- Goldstraw P, Crowley J, Chansky K, Diroux DJ, Groome PA, Rami‐Porta R, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groups in the forthcoming (seventh) edition of the TNM classification of malignant tumours. Journal of Thoracic Oncology 2007;2(8):706. [DOI] [PubMed] [Google Scholar]
GradePro 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) 2015. GRADEpro GuidelineDevelopment Tool [Software].. Available from www.gradepro.org. McMaster University (developed by Evidence Prime, Inc.), 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jett 2013
- Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd edition: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013;143(5 Suppl):e400S‐19S. [DOI] [PubMed] [Google Scholar]
Lim 2008
- Lim, E, Belcher E, Yap, Y, Nicholson A, Goldstraw P. The role of surgery in the treatment of limited disease small cell lung cancer: time to reevaluate. Journal of Thoracic Oncology 2008;3(11):1267–71. [DOI] [PubMed] [Google Scholar]
Manser 2005
- Manser R, Wright G, Hart D, Byrnes G, Campbell D, Wainer Z, al. Surgery for local and locally advanced non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004699.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1969
- Miller AB, Fox W, Tall R. Five‐year follow‐up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oat‐celled carcinoma of the bronchus. Lancet 1969;2(7619):501‐5. [DOI] [PubMed] [Google Scholar]
NCCN 2015
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer. Version 2. 2015. www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 24 May 2015).
NCI CTCAE 2010
- National Cancer Institute. Common Terminology Criteria for Adverse Events. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf (accessed 24 May 2015).
NHMRC 2004
- National Health and Medical Research Council (NHMRC). Clinical Practice Guidelines for the Prevention, Diagnosis and Management of Lung Cancer. www.nhmrc.gov.au/guidelines‐publications/cp97 (accessed 24 May 2015).
NHMRC 2015
- Cancer Council Australia Lung Cancer Guidelines Working Party. Clinical practice guidelines for the treatment of lung cancer. Sydney: Cancer Council Australia. [Version URL: wiki.cancer.org.au/australiawiki/index.php?oldid=99968. Available from: wiki.cancer.org.au/australia/Guidelines:Lung_cancer (accessed 24 May 2015).
NICE 2011
- National Institute for Health and Clinical Excellence (NICE). NICE clinical guideline 121 — Lung cancer: The diagnosis and treatment of lung cancer. www.nice.org.uk/guidance/cg121 (accessed 24 May 2015).
Powell 2009
- Powell ES, Pearce AC, Cook D, Davies P, Bishay E, Bowler GM, et al. UK pneumonectomy outcome study (UKPOS): a prospective observational study of pneumonectomy outcome. Journal of Cardiothoracic Surgery 2009;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rostad 2004
- Rostad H, Naalsund A, Jacobsen R, Strand T, Scott H, Strom E, et al. Small cell lung cancer in Norway. Should more patients have been offered surgical therapy?. European Journal of Cardiothoracic Surgery 2004;26(4):782‐6. [DOI] [PubMed] [Google Scholar]
Scadding 1966
- Scadding JG, Bignall JR, Blair LG. Comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oatcelled carcinoma of the bronchus: first report to the medical research council by the working party on the evaluation of different methods of therapy in carcinoma of the bronchus. Lancet 1966;288(7471):979‐86. [PubMed] [Google Scholar]
Shepherd 2007
- Shepherd F, Crowley J, Houtte P, Postmus P, Carney D, et al. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals Regarding the Clinical Staging of Small Cell Lung Cancer in the Forthcoming (Seventh) Edition of the Tumor, Node, Metastasis Classification for Lung Cancer. Journal of Thoracic Oncology 2007;2(12):1067–77. [DOI] [PubMed] [Google Scholar]
Stahel 1989
- Stahel R, Ginsberg R, Havemann K, Hirsch FR, Ihde DC, Jassem J, et al. Staging and prognostic factors in small cell lung cancer: a consensus report. Lung Cancer 1989;5:119–26. [Google Scholar]
Travis 2011
- Travis W, Brambilla E, Noguchi M, Nicholson A, Geisinger K, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma. Journal of Thoracic Oncology 2011;6(2):244‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Travis 2013
- Travis W, Brambilla E, Riely G. New Pathologic Classification of Lung Cancer: Relevance for Clinical Practice and Clinical Trials. Journal of Clinical Oncology 2013;31(8):992‐1001. [DOI] [PubMed] [Google Scholar]
Vallieres 2009
- Vallieres E, Shepherd FA, Crowley J, Houtte P, Postmus PE, Carney D, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. Journal of Thoracic Oncology 2009;4(9):1049‐59. [DOI] [PubMed] [Google Scholar]
van Meerbeeck 2011
- Meerbeeck JP, Fennell DA, Ruysscher DM. Small‐cell lung cancer. Lancet 2011;378(9804):1741–55. [DOI] [PubMed] [Google Scholar]
Watson 1962
- Watson WL, Berg JW. Oat cell lung cancer. Cancer 1962;15:759‐68. [DOI] [PubMed] [Google Scholar]
Yu 2010
- Yu J, Decker R, Detterbeck F, Wilson L. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. Journal of Thoracic Oncology 2010;5(2):215‐9. [DOI] [PubMed] [Google Scholar]
Zelen 1973
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemotherapy Reports. Part 3 1973;4(2):31–42. [PubMed] [Google Scholar]


