Abstract
Background
Uterine fibroids are smooth muscle tumours arising from the uterus. These tumours, although benign, are commonly associated with abnormal uterine bleeding, bulk symptoms and reproductive dysfunction. The importance of progesterone in fibroid pathogenesis supports selective progesterone receptor modulators (SPRMs) as effective treatment. Both biochemical and clinical evidence suggests that SPRMs may reduce fibroid growth and ameliorate symptoms. SPRMs can cause unique histological changes to the endometrium that are not related to cancer, are not precancerous and have been found to be benign and reversible. This review summarises randomised trials conducted to evaluate the effectiveness of SPRMs as a class of medication for treatment of individuals with fibroids.
Objectives
To evaluate the effectiveness and safety of SPRMs for treatment of premenopausal women with uterine fibroids.
Search methods
We searched the Specialised Register of the Cochrane Gynaecology and Fertility Group, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and clinical trials registries from database inception to May 2016. We handsearched the reference lists of relevant articles and contacted experts in the field to request additional data.
Selection criteria
Included studies were randomised controlled trials (RCTs) of premenopausal women with fibroids who were treated for at least three months with a SPRM.
Data collection and analysis
Two review authors independently reviewed all eligible studies identified by the search. We extracted data and assessed risk of bias independently using standard forms. We analysed data using mean differences (MDs) or standardised mean differences (SMDs) for continuous data and odds ratios (ORs) for dichotomous data. We performed meta‐analyses using the random‐effects model. Our primary outcome was change in fibroid‐related symptoms.
Main results
We included in the review 14 RCTs with a total of 1215 study participants. We could not extract complete data from three studies. We included in the meta‐analysis 11 studies involving 1021 study participants: 685 received SPRMs and 336 were given a control intervention (placebo or leuprolide). Investigators evaluated three SPRMs: mifepristone (five studies), ulipristal acetate (four studies) and asoprisnil (two studies). The primary outcome was change in fibroid‐related symptoms (symptom severity, health‐related quality of life, abnormal uterine bleeding, pelvic pain). Adverse event reporting in the included studies was limited to SPRM‐associated endometrial changes. More than half (8/14) of these studies were at low risk of bias in all domains. The most common limitation of the other studies was poor reporting of methods. The main limitation for the overall quality of evidence was potential publication bias.
SPRM versus placebo
SPRM treatment resulted in improvements in fibroid symptom severity (MD ‐20.04 points, 95% confidence interval (CI) ‐26.63 to ‐13.46; four RCTs, 171 women, I2 = 0%; moderate‐quality evidence) and health‐related quality of life (MD 22.52 points, 95% CI 12.87 to 32.17; four RCTs, 200 women, I2 = 63%; moderate‐quality evidence) on the Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL, scale 0 to 100). Women treated with an SPRM showed reduced menstrual blood loss on patient‐reported bleeding scales, although this effect was small (SMD ‐1.11, 95% CI ‐1.38 to ‐0.83; three RCTs, 310 women, I2 = 0%; moderate‐quality evidence), along with higher rates of amenorrhoea (29 per 1000 in the placebo group vs 237 to 961 per 1000 in the SPRM group; OR 82.50, 95% CI 37.01 to 183.90; seven RCTs, 590 women, I2 = 0%; moderate‐quality evidence), compared with those given placebo. We could draw no conclusions regarding changes in pelvic pain owing to variability in the estimates. With respect to adverse effects, SPRM‐associated endometrial changes were more common after SPRM therapy than after placebo (OR 15.12, 95% CI 6.45 to 35.47; five RCTs, 405 women, I2 = 0%; low‐quality evidence).
SPRM versus leuprolide acetate
In comparing SPRM versus other treatments, two RCTs evaluated SPRM versus leuprolide acetate. One RCT reported primary outcomes. No evidence suggested a difference between SPRM and leuprolide groups for improvement in quality of life, as measured by UFS‐QoL fibroid symptom severity scores (MD ‐3.70 points, 95% CI ‐9.85 to 2.45; one RCT, 281 women; moderate‐quality evidence) and health‐related quality of life scores (MD 1.06 points, 95% CI ‐5.73 to 7.85; one RCT, 281 women; moderate‐quality evidence). It was unclear whether results showed a difference between SPRM and leuprolide groups for reduction in menstrual blood loss based on the pictorial blood loss assessment chart (PBAC), as confidence intervals were wide (MD 6 points, 95% CI ‐40.95 to 50.95; one RCT, 281 women; low‐quality evidence), or for rates of amenorrhoea (804 per 1000 in the placebo group vs 732 to 933 per 1000 in the SPRM group; OR 1.14, 95% CI 0.60 to 2.16; one RCT, 280 women; moderate‐quality evidence). No evidence revealed differences between groups in pelvic pain scores based on the McGill Pain Questionnaire (scale 0 to 45) (MD ‐0.01 points, 95% CI ‐2.14 to 2.12; 281 women; moderate‐quality evidence). With respect to adverse effects, SPRM‐associated endometrial changes were more common after SPRM therapy than after leuprolide treatment (OR 10.45, 95% CI 5.38 to 20.33; 301 women; moderate‐quality evidence).
Authors' conclusions
Short‐term use of SPRMs resulted in improved quality of life, reduced menstrual bleeding and higher rates of amenorrhoea than were seen with placebo. Thus, SPRMs may provide effective treatment for women with symptomatic fibroids. Evidence derived from one RCT showed no difference between leuprolide acetate and SPRM with respect to improved quality of life and bleeding symptoms. Evidence was insufficient to show whether effectiveness was different between SPRMs and leuprolide. Investigators more frequently observed SPRM‐associated endometrial changes in women treated with SPRMs than in those treated with placebo or leuprolide acetate. As noted above, SPRM‐associated endometrial changes are benign, are not related to cancer and are not precancerous. Reporting bias may impact the conclusion of this meta‐analysis. Well‐designed RCTs comparing SPRMs versus other treatments are needed.
Plain language summary
Drugs to treat fibroids
Review question
We reviewed the evidence on effectiveness and safety of a new class of medications called selective progesterone receptor modulators (SPRMs) for treating premenopausal women with uterine fibroids.
Background
Fibroids (non‐cancerous masses within the muscle layer of the womb) are a common condition. Fibroids can negatively impact a woman's health by causing heavy periods, creating symptoms related to their size (such as pressing on the bladder or rectum) and/or making it difficult to conceive.
A new class of medication called SPRMs has shown promise for treatment of women with fibroids. The class of SPRMs includes various drugs such as mifepristone, ulipristal acetate and asoprisnil. SPRMs can cause benign changes to the endometrium that are not related to cancer and are not precancerous.
Search date
We searched the literature up to May 2016.
Study characteristics
Review authors included 14 randomised controlled trials (RCTs) (1215 women) but could not obtain data from three studies. In addition, several completed registered trials had not yet reported findings. This review evaluated results of 11 RCTs that included 1021 women with fibroids. Investigators treated women with mifepristone (five studies), ulipristal acetate (four studies) or asoprisnil (two studies) and compared SPRMs with either placebo or leuprolide acetate. More than half of these studies were at low risk of bias in all domains. The most common limitation of the other studies was poor reporting of methods.
Key results
The main outcomes studied were changes in symptoms (fibroid‐related symptom severity, quality of life, menstrual bleeding, pelvic pain). When compared with placebo (identical "dummy" tablet that contains no active medication), SPRMs improved fibroid‐related symptoms (by an average effect of 20 points on a 100‐point scale), improved women's quality of life (by an average effect of 22 points on a 100‐point scale) and resulted in a small decrease in menstrual bleeding. Between 24% and 96% of women treated with SPRMs had no period at all (compared with 3% taking placebo). Review authors could draw no conclusions about changes in pelvic pain, as this was not consistently evaluated. Two studies compared SPRMs versus a gonadotropin‐releasing hormone agonist (leuprolide) and found that both drugs (SPRMs and leuprolide) were effective in improving symptoms related to fibroids (improving quality of life, reducing menstrual bleeding, causing cessation of periods, decreasing pelvic pain). However, we are not sure if researchers noted a difference in effectiveness between SPRMs and leuprolide.
Women treated with SPRMs were more likely to develop changes to the lining of the womb (endometrium) than women treated with placebo or leuprolide. These changes are benign and reversible once SPRMs are discontinued.
In summary, the studies included in this review show that SPRMs improve fibroid‐related symptoms, quality of life and menstrual bleeding. However, we need larger, well‐designed studies comparing SPRMs against other treatments currently available for the management of fibroids.
Quality of the evidence
In comparisons with placebo, moderate‐quality evidence showed improvements in quality of life, reduction in menstrual bleeding and cessation of periods with SPRMs. Low‐quality evidence suggested a higher rate of changes to the endometrium with SPRM treatment than with placebo. Comparisons with leuprolide were based on moderate‐quality evidence for changes in quality of life, cessation of periods, pelvic pain and endometrial changes. The main limitation in the overall quality of evidence was potential publication bias.
Summary of findings
Background
Description of the condition
Uterine fibroids are common smooth muscle tumours arising from the uterus. They are also known as leiomyomata or myomas. The prevalence of these tumours depends on the population’s ethnicity and the method of detection. More than 80% of black women and nearly 70% of white women will develop fibroids before the age of 50 years (Baird 2003). These tumours, although benign, can cause significant distortion of the uterus and result in symptoms in up to 50% of women (Baird 2003). Fibroids are frequently associated with abnormal uterine bleeding, bulk symptoms (pelvic pressure, urinary dysfunction, constipation, pain) and reproductive dysfunction (subfertility, miscarriage, pregnancy complications) (Stovall 2001).
In the United States alone, the direct cost of treatment for women with fibroids is estimated to be over four billion dollars annually (Cardozo 2012). Fibroid‐related symptoms can be treated with surgery (hysterectomy, myomectomy, endometrial ablation, myolysis), minimally invasive procedures (uterine artery embolisation, magnetic resonance‐guided focused ultrasound) or medical therapies (Wallach 2004). Despite these treatment options, hysterectomy is the second most frequently performed surgical procedure in the United States, with fibroids the most common indication (Merrill 2008); this has contributed to significant surgical morbidity and escalating healthcare costs. Thus, focus on more conservative options is needed.
Description of the intervention
Currently, no pharmacological agents have received global approval specifically for long‐term treatment of individuals with uterine fibroids. The mainstay of medical management has comprised use of gonadotropin‐releasing hormone analogues (GnRHa) for preoperative optimisation seen as decreased blood loss, corrected anaemia and reduced fibroid volume (Sabry 2012). Leuprolide acetate is one of the most frequently used GnRHa treatments for fibroids and is approved in the United States and Europe for this indication. Challenges associated with GnRHa therapy include decreased bone mineral density, development of vasomotor symptoms and an initial oestrogen flare that may exacerbate symptoms. Although medical therapies such as combined hormonal contraceptives, progestins, progestin‐releasing intrauterine systems and danazol may be used to decrease menstrual blood flow, their specific effects on fibroids and bulk symptoms are limited, and they often cause side effects that lead to discontinuation (Ke 2009; Sangkomkamhang 2013; Van Voorhis 2009).
Traditionally, oestrogen has been considered the most important hormone for stimulating fibroid growth. Recently, progesterone was found to be essential for the maintenance and growth of fibroids (Bulun 2013). For this reason, selective progesterone receptor modulators (SPRMs) have shown promise for the treatment of women with uterine fibroids (Chwalisz 2005). These molecules bind to the progesterone receptor and show varying levels of antagonistic activity. SPRMs were first discovered in 1980, and mifepristone, a powerful progesterone antagonist, was the pioneer drug. It has been used mainly for pregnancy termination but has also been evaluated as a therapeutic agent for fibroids. Meta‐analysis of three randomised trials showed that mifepristone is effective in reducing bleeding symptoms and improving fibroid‐related quality of life, with no effect on fibroid volume (Tristan 2012).
Other SPRMs were subsequently developed, each with different affinity for the progesterone receptor and showing varying degrees of antagonistic activity. The clinical activity of each SPRM class member reflects the subtlety of its spectrum of agonist and antagonist activity, along with tissue‐specific expression of progesterone receptor (PR) subtypes.
How the intervention might work
The ‘progesterone hypothesis’ suggests that progesterone acts as a key hormone in the development of fibroids by increasing mitotic rates and reducing apoptosis of fibroid smooth muscle cells (Bulun 2013). Data also suggest that signalling occurs between oestrogen and progesterone receptors, whereby oestrogen induces increased expression of the progesterone receptor in fibroid cells (Ishikawa 2010). The importance of progesterone in fibroid pathogenesis supports SPRMs as effective treatment for women with fibroids. Fibroid cells cultured with SPRMs demonstrate inhibited proliferation and increased apoptosis, without affecting normal myometrium (Bouchard 2011). SPRMs can also downregulate the number of growth factors while reducing collagen synthesis in cultured fibroid cells (Bouchard 2011). SPRMs act upon the uterine endometrium to provide relief of bleeding symptoms in women with fibroids (Wagenfeld 2016). SPRMs are known to cause unique changes to the endometrium. Histological endometrial changes have been labelled as progesterone receptor modulator‐associated endometrial changes (PAECs) on the basis of international consensus (Mutter 2008). These changes are benign and reversible.
SPRMs may be used to treat women with fibroids in several clinical scenarios. Currently, the only SPRM approved for medical management of fibroids is ulipristal acetate (Esmya, Gedeon‐Richter, Europe, February 2012; Fibristal, Watson Laboratories Inc, Canada, July 2013). This drug was approved to treat bleeding symptoms while decreasing fibroid size for up to three months before surgery. Recently, it was approved in Europe and Canada for ongoing intermittent use. Long‐term use of SPRMs for fibroid‐related symptoms may decrease the need for surgical intervention and associated morbidity and costs. Long‐term medical therapy may be particularly beneficial for bridging perimenopausal women until menopause, when fibroids would then spontaneously decrease. Although pregnancy is contraindicated with SPRMs, evidence shows that the decrease in fibroid size is sustained after the medication has been discontinued (Donnez 2012). This may cause fibroid‐related subfertility, for which medical management may reduce fibroid volume and facilitate pregnancy after discontinuation of SPRM treatment.
Why it is important to do this review
Despite the prevalence of uterine fibroids, only a few high‐quality studies have examined the effectiveness of medical therapies. With increasing demand for less invasive fibroid therapies, the benefits and risks of medical treatments must be critically evaluated. Furthermore, women are delaying childbearing, hence fertility‐sparing therapeutic options are needed. Biochemical and clinical evidence shows that SPRMs may decrease fibroid growth and ameliorate symptoms (Chwalisz 2005). Although a Cochrane review on mifepristone has been completed (Tristan 2012), the newer SPRMs require systematic evaluation of their benefits and harms.
Objectives
To evaluate the effectiveness and safety of selective progesterone receptor modulators (SPRMs) for treatment of premenopausal women with uterine fibroids.
Methods
Criteria for considering studies for this review
Types of studies
We included data from all published and unpublished randomised controlled trials (RCTs). For cross‐over studies, we included for meta‐analysis only data from the first phase of the trial.
Types of participants
Premenopausal women with uterine fibroids, with or without symptoms. The presence of fibroids was confirmed surgically (laparoscopy, laparotomy or hysteroscopy) or through at least one of the following imaging modalities: ultrasonography, computed tomography or magnetic resonance imaging (MRI).
Types of interventions
Treatment with any SPRM for at least three months versus:
placebo;
no treatment;
another medical therapy (another SPRM, a GnRHa or another class of medication);
surgery (myomectomy or hysterectomy); or
uterine artery embolisation (UAE).
Commercially available SPRMs included, but were not limited to, mifepristone, asoprisnil, telapristone acetate and ulipristal acetate. Additional interventions were permitted as long as they were uniformly used in all study arms. Leuprolide acetate is a GnRHa that is commonly used to treat fibroids. We searched for comparisons of SPRM versus leuprolide acetate or other medications in the GnRHa class.
Types of outcome measures
Primary outcomes
-
Change in fibroid‐related symptoms
Quality of life assessed through standardised and validated measures. Examples of scales that measured health‐related quality of life for women with uterine fibroids (Williams 2006) included but were not limited to Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL) (Spies 2002), EuroQoL (Brooks 1996) and Short Form‐36 (SF‐36) (Ware 1992)
Abnormal uterine bleeding measured objectively (e.g. haemoglobin levels, haematocrit, ferritin levels, alkaline haematin technique) or subjectively (e.g. pictorIal blood loss assessment)
Pain and pelvic pressure measured subjectively (e.g. visual analogue scales, Likert scales)
Secondary outcomes
Change in fibroid or uterine size, or both, as measured by ultrasonography or MRI
SPRM‐related effects including, but not limited to, SPRM‐associated endometrial changes (Mutter 2008), endometrial hyperplasia, endometrial carcinoma, abnormal liver enzymes and prolactin levels, osteoporosis, breast discomfort, hot flushes, headache and nausea
Search methods for identification of studies
We searched for all published and unpublished RCTs of SPRMs used for treatment of uterine fibroids. We applied no language restrictions. We developed and executed the search strategy in consultation with the Cochrane Gynaecology and Fertility Group Information Specialist and a Mount Sinai Hospital librarian.
Electronic searches
We searched the following databases from inception until 15 May 2016.
Cochrane Gynaecology and Fertility Group Specialised Register (Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2).
MEDLINE (Appendix 3).
Embase (Appendix 4).
PsycINFO (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL)0 (Appendix 6).
Database of Abstracts of Reviews of Effects (DARE) (Appendix 7).
Other.
Trial registers for ongoing and registered trials: www.clinicaltrials.gov.
Web of Knowledge: http://wokinfo.com/.
Clinical study results for clinical trials of marketed pharmaceuticals: www.clinicalstudyresults.org.
World Health Organization (WHO) International Clinical Trials Registry Platform: www.who.int/trialsearch.
OpenGrey for unpublished literature from Europe: www.opengrey.eu.
Latin American Caribbean Health Sciences Literature (LILACS) for Portuguese and Spanish trials.
Searching other resources
We handsearched appropriate journals recommended by the Gynaecology and Fertility Group that were not captured in the above databases. We also handsearched reference lists of relevant articles and contacted experts in the field to obtain additional data.
Data collection and analysis
We performed statistical analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used Review Manager 5.3 (RevMan 2014) software for the analysis.
Selection of studies
Two review authors independently completed the initial title and abstract screening. We retrieved full texts when studies met the following criteria: used an SPRM as an intervention for treatment of uterine fibroids, and had a prospective design. If we had any doubts based on these screening criteria, we retrieved the full text. We excluded studies if full‐text articles did not mention randomisation. We resolved disagreements during the screening process by consulting a third review author. We documented the selection process in a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1).
1.
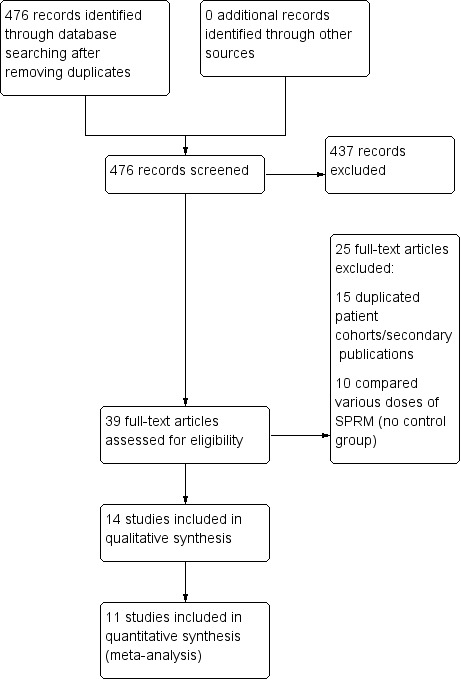
Study flow diagram.
Data extraction and management
Two review authors independently extracted data from all eligible studies to be included in the review. We extracted data using forms that we had pilot‐tested. These forms included specifics of study characteristics and outcomes data. When data from a trial had been published more than once, review authors extracted data that were additional and were not repeated. We contacted trial authors for clarification when required. We resolved disagreements between review authors by consensus after involving an additional review author.
Assessment of risk of bias in included studies
Two review authors independently assessed bias for included studies using the Cochrane risk of bias tool (www.cochrane‐handbook.org). We assessed the following elements: selection bias (random sequence generation, allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other biases. We paid special attention to within‐trial selective reporting when study authors failed to report obvious outcomes or reported them with insufficient detail. We compared studies against published protocols to determine whether planned outcome measures were indeed reported.
Measures of treatment effect
We analysed the various comparisons separately using RevMan 5.3. We reported dichotomous data as odds ratios (ORs) with 95% confidence intervals (CIs). We reported continuous data as mean differences (MDs) with 95% CIs. When outcomes were reported as continuous data on different scales, we reported standardised mean differences (SMDs) and 95% CIs.
We interpreted the SMD using the following rule‐of‐thumb guide: 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988).
Unit of analysis issues
We performed the primary analysis per woman randomised.
We prepared additional tables to briefly summarise data that did not allow valid analysis (e.g. 'per cycle' data) and did not meta‐analyse these data. This applied to "change in fibroid size data", which we analysed on the basis of number of fibroids tracked ‐ not number of participants (i.e. some participants contributed more fibroids to the analysis than others).
We included only first‐phase data from cross‐over trials.
Dealing with missing data
We contacted primary authors electronically to request missing data and clarification of any issues that arose. We analysed data on the basis of intention‐to‐treat analysis. When all randomised participants were not included in the analysis, we calculated and separately reported the percentage of participants lost to follow‐up. In these cases, we imputed values only for primary outcomes. For other outcomes, we analysed only available data.
When data were reported in a form unsuitable for analysis (e.g. did not report standard deviations or reported medians rather than means), we obtained statistical advice and imputed the data (see Appendix 12).
Assessment of heterogeneity
We evaluated included trials to determine whether studied participants, interventions and outcomes were similar enough that we could meta‐analyse them. If we determined that trials could be meta‐analysed to yield clinically relevant results, we assessed these trials for statistical heterogeneity. We performed tests for heterogeneity across studies by using the Q statistic and the I2 statistic. We used the following criteria for heterogeneity: I2 < 25% showed low, 25% to 50% moderate and > 50% high heterogeneity (Higgins 2011). When we found high heterogeneity across any of these criteria, we conducted subgroup and sensitivity analyses.
Assessment of reporting biases
We aimed to minimise reporting bias by completing a comprehensive search for eligible studies while staying conscious of data duplication. If we found a sufficient number of trials for inclusion (> 10), we used a funnel plot to assess for publication bias (under‐reporting of small negative studies).
Data synthesis
We pooled data for clinically similar studies using a random‐effects model for the meta‐analysis. When studies could not be pooled, we described outcomes in narrative form. We analysed different comparisons separately.
All SPRMs versus placebo or no treatment.
-
All SPRMs versus alternative active therapy, stratified by alternatives.
SPRMs versus medical therapy (stratified by class of medical therapy).
SPRMs versus surgical management (stratified by type of surgical management).
SPRMs versus UAE.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses if sufficient data were available (i.e. more than five studies).
Individual types of SPRMs versus placebo, no treatment or each alternative active therapy.
Duration of therapy (< 6 months, 6 to 12 months, > 12 months).
SPRM dose (low, medium, high).
Fibroid location (submucous, intramural, subserosal).
When high heterogeneity was present, we planned to explore possible explanations including individual study risk of bias, participant population (age, ethnicity, types and sizes of fibroids), dose of SPRM, duration of treatment and follow‐up.
Sensitivity analysis
We planned to conduct sensitivity analyses for primary outcomes when data were sufficient (more than five studies) to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These planned analyses included consideration of whether review conclusions would have differed if:
eligibility had been restricted to studies without high risk of bias;
a fixed‐effect model had been adopted;
alternative imputation strategies had been implemented; or
the summary effect measure had been risk ratio rather than odds ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro (GRADEpro GDT 2014) and Cochrane methods (Higgins 2011). These tables evaluated the overall quality of the body of evidence for main review outcomes (symptom severity, health‐related quality of life, menstrual blood loss, rate of amenorrhoea, pelvic pain/pressure, SPRM‐associated endometrial changes) for main review comparisons (SPRM vs placebo and SPRM vs leuprolide acetate). We assessed the quality of the evidence using the following GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors independently judged evidence quality (high, moderate, low or very low) and resolved disagreements by discussion. We justified, documented and incorporated these judgements into reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The electronic search of databases generated 476 records after we eliminated duplicates. We identified no additional records through handsearching and other sources. After screening titles and abstracts, we eliminated 437 records that did not meet review inclusion criteria. We retrieved 39 full‐text manuscripts and excluded 25 citations (15 were secondary publications/duplicated cohorts, 10 compared various doses of SPRM ‐ see Characteristics of excluded studies). We identified 10 ongoing trials (see Characteristics of ongoing studies) and included 14 studies in the review. Bigatti 2014 presented preliminary data on surgical outcomes after pretreatment with a variety of hormonal agents including ulipristal acetate. We could not extract data as no participants were treated with SPRM at the time of publication and investigators reported only surgery‐related outcomes. We did not include this study in further analyses in this review. Furthermore, we could not extract data from Liu 2015 and Prasad 2013, as the numbers of participants assigned to treatment and control groups were unclear. Our attempts to contact study authors were unsuccessful. Hence, we used 11 studies for meta‐analysis. Two publications reported on the same participant cohort but reported different clinical outcomes; Wilkens 2008 reported these details. We have outlined details of the study screening process in Figure 1. The search was current as of 15 May 2016.
Included studies
For more information on included studies, see Characteristics of included studies.
Methods and setting
All studies were RCTs. Four studies were multi‐centre trials: three from European centres (Donnez 2012; Donnez 2012a; Wilkens 2008) and one from a North American centre (Chwalisz 2007). Four single‐site studies were conducted in the United States (Fiscella 2006; Levens 2008; Nieman 2011; Reinsch 1994); two in India (Bagaria 2009; Prasad 2013) and the remainder in Cuba (Esteve 2013), China (Liu 2015), Sweden (Engman 2009) and Italy (Bigatti 2014).
Participants
Studies included a total of 1215 study participants: 685 received SPRM and 336 were given a control. For two studies, numbers of participants in SPRM and control groups were unclear (Liu 2015; Prasad 2013). All studies, with one exception, included only patients with symptomatic fibroids. In Chwalisz 2007, although participants were not expected to be symptomatic at baseline, most of them experienced symptoms (76% had abnormal uterine bleeding and 94% had bulk symptoms). Three studies scheduled participants for surgery for their symptomatic fibroids (Engman 2009; Esteve 2013; Wilkens 2008). Eight studies diagnosed uterine fibroids by ultrasonography, and three by MRI (Donnez 2012; Levens 2008; Nieman 2011).
Eight studies reported the following ethnicities for 931 participants (91% of participants were included in this analysis): 644 White/Caucasian (69%), 183 Black (20%), 31 Asian (3%), 29 Afro‐Cuban (3%), 4 Hispanic (< 1%) and 40 “other” (4%).
Interventions
The SPRM in seven studies was mifepristone (see Table 3). Five studies investigated ulipristal acetate (see Table 4), and two investigated asoprisnil. Chwalisz 2007 investigated three daily doses of asoprisnil (5 mg, 10 mg, 25 mg) and compared them with placebo over a three‐month period. Wilkens 2008 compared 10 mg and 25 mg daily of asoprisnil versus placebo over three months in a cohort of 33 participants (see Table 5).
1. Mifepristone studies.
| Study | Participants | Daily dose | Control | Follow‐up (months) |
| Esteve 2013 | 124 | 5 mg | Placebo | 3 |
| Fiscella 2006 | 42 | 5 mg | Placebo | 6 |
| Bagaria 2009 | 40 | 10 mg | Placebo | 3 |
| Liu 2015 | 62 | 10 mg | Placebo | 3 |
| Prasad 2013 | 132 | 10 mg | Placebo | 3 |
| Reinsch 1994 | 14 | 25 mg | Leuprolide acetate | 3 |
| Engman 2009 | 30 | 50 mg | Vitamin B | 3 |
2. Ulipristal acetate studies.
| Study | Participants | Daily dose | Control | Follow‐up (months) |
| Bigatti 2014 | Unknown | 5 mg | No treatment | Not stated |
| Donnez 2012 | 242 | 5 or 10 mg | Placebo | 3 |
| Donnez 2012a | 303 | 5 or 10 mg | Leuprolide acetate | 3 |
| Levens 2008 | 22 | 10 or 20 mg | Placebo | 3 |
| Nieman 2011 | 42 | 10 or 20 mg | Placebo | 3 |
3. Asoprisnil studies.
| Study | Participants | Daily dose | Control | Follow‐up (months) |
| Chwalisz 2007 | 129 | 5 or 10 or 25 mg | Placebo | 3 |
| Wilkens 2008 | 33 | 10 or 25 mg | Placebo | 3 |
Outcomes
All participant cohorts, with three exceptions (Liu 2015; Prasad 2013; Reinsch 1994), reported on the primary outcome: fibroid‐related symptoms. Researchers assessed fibroid symptoms by measuring quality of life, menstrual bleeding and pelvic pain/pressure.
Seven studies reported quality of life. Six studies used the Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL) (Donnez 2012a; Esteve 2013; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008). In addition to the UFS‐QoL, three studies (Fiscella 2006; Levens 2008; Nieman 2011) reported quality of life using Short Form‐36. Donnez 2012 assessed quality of life by using a unique questionnaire on discomfort associated with uterine fibroids.
Studies used both the symptom severity scale (SS‐QoL) and the Health‐Related Quality of Life scale (HR‐QoL) of the UFS‐QoL. Both measure aspects of fibroid‐related symptoms. They are mutually exclusive, and no overlap in scoring occurs between the two outcomes. The SS‐QoL (range 0 to 100) assesses bleeding, abdominal pressure, urinary frequency and fatigue. The HR‐QoL ranges from 0 to 100 points and is comprised of six domains: Concern, Activities, Energy/Mood, Control, Self‐Conscious and Sexual Function.
With the exception of Reinsch 1994 and Liu 2015, all participant cohorts reported on the change in menstrual bleeding. At a minimum, investigators reported attainment of amenorrhoea at the end of the follow‐up period as a proportion. They frequently reported haemoglobin at baseline and at follow‐up. Four studies used standardised outcome measures to better quantify menstrual blood loss. Three studies (Bagaria 2009; Donnez 2012; Donnez 2012a) used the pictorial blood loss assessment chart (PBAC) (Higham 1990). A PBAC score of 100 or higher correlates with menorrhagia, which is defined as menstrual blood loss greater than 80 mL (Higham 1990). Wilkens 2008 used a visual analogue menstrual pictogram (Wyatt 2001), and how Prasad 2013 assessed menstrual blood loss remains unclear.
Eight studies reported pelvic pain/pressure in a heterogeneous fashion. Outcome measures for pelvic pain included the McGill Pain Questionnaire, which has a score range of 0 to 45 (higher scores indicating greater pain) (Donnez 2012; Donnez 2012a; Fiscella 2006), a visual analogue scale (Bagaria 2009; Donnez 2012; Donnez 2012a; Esteve 2013), study‐specific Likert scales (Chwalisz 2007; Engman 2009; Fiscella 2006) or a daily calendar log assessing the number of days pain was present (Levens 2008). Five studies reported changes in pelvic pressure symptoms following treatment using Likert scales (Chwalisz 2007; Engman 2009; Fiscella 2006), a visual analogue scale (Bagaria 2009) or participant‐reported presence/absence of pressure symptoms (Esteve 2013).
Researchers assessed fibroid and uterine volume using ultrasonography in all patient cohorts, with the exception of Donnez 2012, which used serial MRI. Each study used different methods of calculation. See Characteristics of included studies.
Studies, with the exception of Reinsch 1994, reported endometrial histology for all participant cohorts. Studies differed in their criteria and timelines for performing endometrial biopsy and evaluated endometrial specimens using different pathological criteria. Four studies (Donnez 2012; Donnez 2012a; Esteve 2013; Nieman 2011) used the standard definition of SPRM‐associated endometrial changes (PAEC) provided by Mutter 2008. Three studies (Chwalisz 2007; Engman 2009; Wilkens 2008) evaluated specimens using their own semiquantitative assessment of glandular architecture as described in their respective manuscripts. Four studies (Bagaria 2009; Fiscella 2006; Levens 2008; Prasad 2013) evaluated endometrial histology but did not report on non‐physiological endometrial changes nor on PAEC.
Excluded studies
Of 39 full‐text articles assessed, we excluded 25 studies for various reasons. Duplication of participant cohorts and studies that compared various doses of the same SPRM were the major reasons for exclusion. For more information on excluded studies, see Characteristics of excluded studies.
Ongoing studies
We identified 10 ongoing studies through searches of trials registers: seven ulipristal acetate, two vilaprisan and one telapristone acetate. See Characteristics of ongoing studies. From trial registers, we identified an additional 10 studies that had been completed or prematurely terminated. For details of these studies, see Characteristics of studies awaiting classification. We attempted to contact study authors to obtain data but were unsuccessful.
Risk of bias in included studies
2.
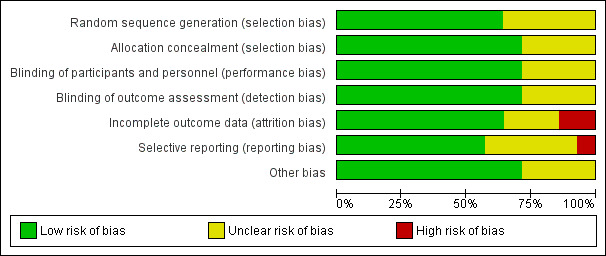
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
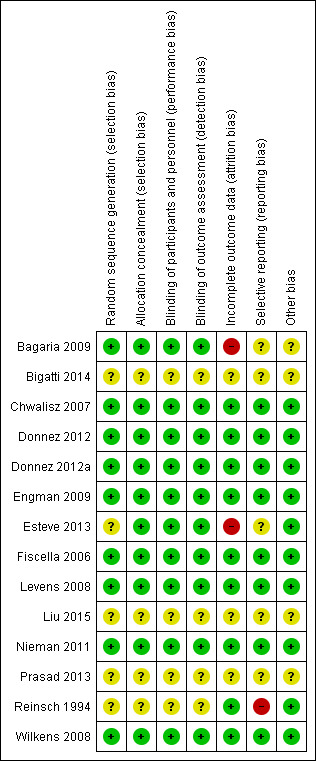
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Nine studies described true random sequence generation, and we graded them as having low risk (Bagaria 2009; Chwalisz 2007; Donnez 2012; Donnez 2012a; Engman 2009; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008). FIve studies provided insufficient details regarding the randomisation process; we therefore graded them as having unclear risk (Bigatti 2014; Esteve 2013; Liu 2015; Prasad 2013; Reinsch 1994). For concealment of allocation, we graded 10 studies as having low risk (Bagaria 2009; Chwalisz 2007; Donnez 2012; Donnez 2012a; Engman 2009; Esteve 2013; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008). Four studies provided no details of allocation concealment; we considered them as having unclear risk (Bigatti 2014; Liu 2015; Prasad 2013; Reinsch 1994). When risk of bias was unclear, we attempted to contact study authors to obtain clarification.
Blinding
Ten studies described in detail adequate blinding of both study participants/personnel (performance bias) and outcome assessors (detection bias), and we graded them as having low risk (Bagaria 2009; Chwalisz 2007; Donnez 2012; Donnez 2012a; Engman 2009; Esteve 2013; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008). We considered the remaining four included studies as having unclear risk for both performance and detection bias owing to inadequate details on blinding (Bigatti 2014; Liu 2015; Prasad 2013; Reinsch 1994).
We considered the same risk of bias criteria to be applicable to all outcomes.
Incomplete outcome data
We considered attrition bias to introduce low risk in nine included studies (Chwalisz 2007; Donnez 2012; Donnez 2012a; Engman 2009; Fiscella 2006; Levens 2008; Nieman 2011; Reinsch 1994; Wilkens 2008). We graded three studies as having unclear risk owing to insufficient details (Bigatti 2014; Liu 2015; Prasad 2013). We considered only two studies as having high risk (Bagaria 2009; Esteve 2013). The Bagaria study included a disproportionate number of participants lost to follow‐up from placebo versus mifepristone groups (1/20 vs 4/20), and this was magnified by small study size. Similarly, the Esteve study reported unbalanced loss to follow‐up, with significantly more drop‐outs among placebo versus mifepristone groups (15/62 vs 4/62).
Selective reporting
Eight studies were at low risk for reporting bias, with protocols available for each study and preselected outcome measures consistent with final reported outcomes in the published manuscripts (Chwalisz 2007; Donnez 2012; Donnez 2012a; Engman 2009; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008). We graded five studies as having unclear risk (Bagaria 2009; Bigatti 2014; Esteve 2013; Liu 2015; Prasad 2013). For the Bagaria and Esteve studies, we identified no study protocol. The Bigatti, Liu and Prasad studies provided insufficient details of outcome measures, and we found no protocols for these studies. We attempted to contact study authors for clarification without success. We considered only one study to have high risk (Reinsch 1994) as we could not identify a protocol and, although investigators described fibroid volume in the methods section, they did not report this measure as an outcome.
Other potential sources of bias
We identified four studies as having additional potential sources of bias (Bagaria 2009; Bigatti 2014; Liu 2015; Prasad 2013). In the Bagaria study, we noted potential for dose variation in the mifepristone group, as researchers provided no specific details regarding the capsule derivation method from 200 mg tablets and associated quality control. The Liu, Prasad and Bigatti studies were published as conference proceedings, and we graded them as having unclear risk owing to an overall lack of details.
Effects of interventions
Summary of findings for the main comparison. SPRM versus placebo.
| SPRM vs placebo | ||||||
| Patient or population: women with uterine fibroids Setting: outpatient clinic Intervention: SPRM Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with SPRM | |||||
| Quality of life: change in symptom severity score measured with Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL): scale 0 to 100 | Mean change in symptom severity score (QoL) in the intervention group was 20.04 points lower (26.63 lower to 13.46 lower), indicating improvement in symptom severity with SPRM treatment for 3 months | ‐ | 171 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ||
| Quality of life: change in health‐related quality of life score measured with UFS‐QoL: scale 0 to 100 | Mean change in health‐related quality of life score in the intervention group was 22.52 points higher (12.87 higher to 32.17 higher), indicating improvement in quality of life with SPRM treatment for 3 to 6 months | ‐ | 200 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | 1 RCT (Fiscella 2006) reported outcomes at 6 months. Remaining studies had a 3‐month follow‐up period | |
| Abnormal uterine bleeding: change in menstrual blood loss | Mean change in menstrual blood loss in the intervention group was 1.11 points lower (1.38 lower to 0.83 lower), indicating a decrease in menstrual blood loss with SPRM treatment for 3 months | ‐ | 310 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | Measured by PBAC score or similar menstrual pictorial score. PBAC score ≥ 100 correlates with menorrhagia, which is defined as > 80 mL menstrual blood loss | |
| Abnormal uterine bleeding: amenorrhoea | 29 per 1000 | 477 per 1000 (237 to 961) with 3 to 6 months of SPRM treatment | OR 82.50 (37.10 to 183.90) | 590 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa | 1 RCT (Fiscella 2006) reported outcomes at 6 months. Remaining studies had a 3‐month follow‐up period |
| Pelvic pain (measured subjectively) | No conclusions could be drawn owing to variability in estimates | 629 (7 RCTs) |
||||
| Adverse effects: SPRM‐associated endometrial changes | 77 per 1000 | 351 per 1000 (176 to 697) with 3 months of SPRM treatment | OR 15.12 (6.45 to 35.47) | 405 (5 RCTs) | ⊕⊕⊝⊝ LOWa,b | |
| *Risk in the intervention group (and its 95% confidence interval) is based on mean risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level as publication bias suspected because no small negative studies included. Also, many studies were conducted and not published
bDowngraded one level because of serious issues with indirectness of evidence when criteria for evaluating endometrial specimens differed between studies
Summary of findings 2. SPRM versus leuprolide acetate for uterine fibroids.
| SPRM versus leuprolide acetate for uterine fibroids | ||||||
| Patient or population: uterine fibroids Setting: outpatient clinic Intervention: SPRM Comparison: leuprolide acetate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with leuprolide acetate | Risk with SPRM | |||||
| Quality of life: change in symptom severity score measured with Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL): scale 0 to 100 | Mean change in symptom severity score (QoL) in the SPRM group was 3.7 points lower (9.85 lower to 2.45 higher) compared with the leuprolide group at 3 months | ‐ | 281 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| Quality of life: change in health‐related quality of life score measured with UFS‐QoL: scale 0 to 100 | Mean change in health‐related quality of life score in the SPRM group was 1.06 points higher (5.73 lower to 7.85 higher) compared with the leuprolide group at 3 months | ‐ | 281 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| Abnormal uterine bleeding: change in menstrual blood loss (measured using PBAC score) | Mean change in menstrual blood loss in the SPRM group was 6 points higher (40.95 lower to 52.95 higher) compared with the leuprolide group at 3 months | ‐ | 281 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | PBAC score ≥ 100 correlates with menorrhagia, which is defined as > 80 mL menstrual blood loss | |
| Abnormal uterine bleeding: amenorrhoea | 804 per 1000 | 828 per 1000 (732 to 933) at 3 months | OR 1.14 (0.60 to 2.16) | 280 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | |
| Pelvic pain (measured using McGill Pain Questionnaire: range 0 to 45) | Mean change in pelvic pain in the SPRM group was 0.01 points lower (2.14 lower to 2.12 higher) than in the leuprolide group at 3 months | ‐ | 281 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| Adverse effects: SPRM‐associated endometrial changes | 119 per 1000 | 585 per 1000 (340 to 1000) after 3 months of treatment | OR 10.45 (5.38 to 20.33) | 301 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | |
| *Risk in the intervention group (and its 95% confidence interval) is based on mean risk in the comparison group and relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level as publication bias strongly suspected
bDowngraded one level owing to serious issue with imprecision as point estimate has very wide confidence interval
Comparison of SPRM versus placebo
Nine studies compared SPRM with placebo (Bagaria 2009; Chwalisz 2007; Donnez 2012; Esteve 2013; Fiscella 2006; Levens 2008; Nieman 2011; Wilkens 2008) or vitamin B (Engman 2009). See Table 1.
PRIMARY OUTCOMES
Fibroid‐related symptoms
Quality of life
The UFS‐QoL is a validated measure of quality of life of patients with uterine fibroids (Spies 2002). It consists of a symptom severity scale (SS‐QoL) and a health‐related quality of life scale (HR‐QoL). Subscales of the UFS‐QoL evaluate different domains of fibroid‐related symptoms and are mutually exclusive of each other; therefore, we meta‐analysed these separately. The SS‐QoL (range 0 to 100) assesses bleeding, abdominal pressure, urinary frequency and fatigue. A high symptom severity score means more severe fibroid symptoms. Investigators noted greater improvement in symptom severity scores in the SPRM group than in the placebo group, from baseline to end of treatment. Four studies that investigated mifepristone (Esteve 2013), ulipristal acetate (Levens 2008; Nieman 2011) and asoprisnil (Wilkens 2008) demonstrated this over a three‐month treatment period. The mean difference in symptom severity score from baseline to end of treatment was ‐20.04 points (95% CI ‐26.63 to ‐13.46; 171 women, I2 = 0%; moderate‐quality evidence; Analysis 1.1; Figure 4).
1.1. Analysis.
Comparison 1 SPRM versus placebo, Outcome 1 Change in symptom severity score (QoL).
4.
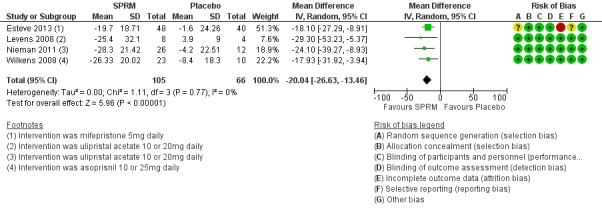
Forest plot of comparison: 1 SPRM versus placebo, outcome: 1.1 Change in symptom severity score (QoL).
The HR‐QoL subscale of the UFS‐QoL ranges from 0 to 100 points and comprises six domains: Concern, Activities, Energy/Mood, Control, Self‐Conscious and Sexual Function. A higher HR‐QoL score means better quality of life. Improvements in HR‐QoL were found during analysis of four studies investigating mifepristone (Esteve 2013; Fiscella 2006), ulipristal acetate (Nieman 2011) and asoprisnil (Wilkens 2008). The treatment course was three months in all studies except Fiscella 2006, which provided a six‐month treatment period. The mean difference in HR‐QoL scores from baseline to end of treatment was 22.52 points (95% CI 12.87 to 32.17; 200 women; I2 = 63%; moderate‐quality evidence; Analysis 1.2; Figure 5). This suggests greater improvement in HR‐QoL in the SPRM group than in the placebo group. Inclusion of few studies with each investigating different SPRMs may be contributing to heterogeneity.
1.2. Analysis.
Comparison 1 SPRM versus placebo, Outcome 2 Change in health‐related quality of life score.
5.
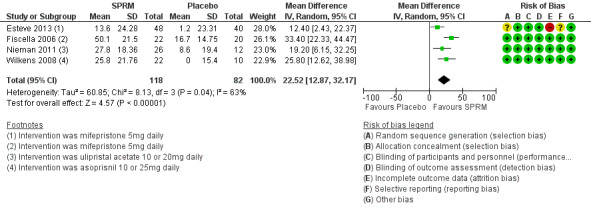
Forest plot of comparison: 1 SPRM versus placebo, outcome: 1.2 Change in health‐related quality of life score.
Abnormal uterine bleeding
Relief of bleeding
Fibroid‐related abnormal uterine bleeding symptoms were assessed in a heterogeneous fashion. We analysed various aspects separately: objective assessment of menstrual blood loss, proportion of amenorrhoeic participants at end of treatment and changes in haemoglobin (pretreatment and posttreatment).
Menstrual blood loss
Two studies investigating mifepristone (Bagaria 2009) and ulipristal acetate (Donnez 2012) used the pictorial blood loss assessment chart (PBAC). A third study evaluating asoprisnil (Wilkens 2008) used a similar menstrual pictorial score. This tool had the same directionality as PBAC, in which higher scores translated to greater menstrual blood loss. Meta‐analysis of these studies revealed improvement in menstrual bleeding at the end of three months of SPRM treatment compared with placebo (SMD ‐1.11 points, 95% CI ‐1.38 to ‐0.83; 310 women, I2 = 0%; moderate‐quality evidence; Analysis 1.3; Figure 6).
1.3. Analysis.
Comparison 1 SPRM versus placebo, Outcome 3 Change in menstrual blood loss.
6.
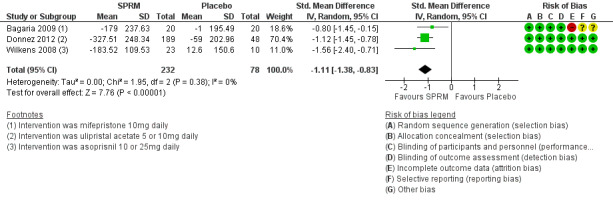
Forest plot of comparison: 1 SPRM versus placebo, outcome: 1.3 Change in menstrual blood loss.
Amenorrhoea
Seven studies investigating all three SPRMs consistently reported the proportion of participants who were amenorrhoeic at completion of treatment. Analysis demonstrates that participants treated with SPRMs for three to six months were more likely to be amenorrhoeic than those who received placebo (OR 82.50, 95% CI 37.10 to 183.90; 590 women, I2 = 0%; moderate‐quality evidence; Analysis 1.4). However, the definition of amenorrhoea was not standard between studies. A range of definitions of amenorrhoea included the following: no bleeding/spotting for the entire study period (Chwalisz 2007; Levens 2008; Nieman 2011), six or fewer days of spotting (Esteve 2013), PBAC score of 2 or less during weeks 9 to 12 (Donnez 2012), cessation of menstruation (Bagaria 2009) and not specifically defined (Fiscella 2006).
1.4. Analysis.
Comparison 1 SPRM versus placebo, Outcome 4 Amenorrhoea.
Haemoglobin
We could not meta‐analyse change in haemoglobin levels from baseline to end of treatment to yield meaningful conclusions for the following reasons: inconsistent reporting of values (means, medians, percentage point change, P value only, etc.), missing data such as measures of spread and some groups taking iron with study drug.
Eight studies reported on haemoglobin. Seven studies demonstrated an increase in haemoglobin at follow‐up in the SPRM group compared with the placebo group (Bagaria 2009; Chwalisz 2007; Donnez 2012; Engman 2009; Esteve 2013; Fiscella 2006; Nieman 2011). In Levens 2008, haemoglobin levels were unchanged over the study period in placebo and SPRM groups.
Pain and pelvic pressure
Investigators reported pelvic pain in a heterogenous manner, precluding meta‐analysis. Two studies (Donnez 2012; Fiscella 2006) used the McGill Pain Questionnaire (MPQ), two studies (Bagaria 2009; Esteve 2013) used a visual analogue scale (VAS) and the remaining studies used their own unique Likert scales (Chwalisz 2007; Engman 2009; Levens 2008).
Change in pain scores on the MPQ was less at completion of treatment compared with baseline only for the 10 mg ulipristal acetate dose compared with placebo (Donnez 2012). Fiscella 2006 reported a decrease in MPQ pain scores after treatment with 5 mg mifepristone, but this finding was not statistically significant.
Bagaria 2009 reported a non‐significant decrease in the proportion of participants experiencing complete resolution of pelvic pain, as measured by VAS, after treatment with 10 mg mifepristone. Esteve 2013 also used VAS and reported that participants treated with 5 mg mifepristone were more likely to be free of pelvic pain after completion of treatment.
Of the three studies that used their own Likert scales to evaluate pelvic pain, two did not report improvement in symptoms (Chwalisz 2007; Engman 2009). Levens 2008 presented data descriptively from daily participant journals, making it difficult for researchers to draw conclusions.
SECONDARY OUTCOMES
Change in fibroid or uterine size
Change in fibroid volume
We reported data for this outcome per fibroid tracked rather than per woman randomised and have presented this information in Table 6.
4. Change in fibroid volume: SPRM versus placebo.
| Study | SPRM type | SPRM | Placebo | |||||
| MD | SD | n | MD | SD | n | Finding | ||
| Bagaria 2009 | Mifepristone | ‐41.5 cc | 220.59 | 19 | 0.6 cc | 266.63 cc | 16 | No significant difference |
| Engman 2009 | Mifepristone | ‐10.0 cc | 107.39 | 12 | ‐16.0 cc | 98.54 cc | 15 | No significant difference |
| Esteve 2013 | Mifepristone | ‐37.0 cc | 96.24 | 58 | 4.0 cc | 99.1 cc | 47 | Favours SPRM |
| Donnez 2012 | Ulipristal acetate | ‐16.88% | 31.34 | 165 | 3.0% | 31.63 | 45 | Favours SPRM |
| Nieman 2011 | Ulipristal acetate | ‐20.5% | 20.6 | 26 | 7.0% | 25.0 | 12 | Favours SPRM |
cc: cubic centimetres
MD: mean difference
n: fibroids tracked
SD: standard deviation
Three mifepristone studies reported ‘mean difference’ in fibroid volume from baseline to end of treatment (Bagaria 2009; Engman 2009; Esteve 2013). Two of these studies reported a decrease in fibroid volume among participants who received mifepristone compared with placebo/vitamin B. Esteve 2013 found that fibroids treated with mifepristone showed a decrease in volume compared with those treated with placebo. Two ulipristal acetate studies reported a ‘percent change’ in fibroid volume over the study period (Donnez 2012; Nieman 2011). Both of these studies reported that participants treated with ulipristal acetate had a reduction in fibroid volume compared with those given placebo. Five additional studies (Chwalisz 2007; Levens 2008; Liu 2015; Prasad 2013; Wilkens 2008) reported change in fibroid volume, but the quality of data prohibited data extraction and data were not analysed. However, qualitatively, these five studies reported a decrease in fibroid volume with SPRM treatment.
Change in uterine volume
Three studies (Bagaria 2009; Esteve 2013; Fiscella 2006) reported change in uterine volume after mifepristone treatment. Investigators reported a mean uterine volume reduction of 153.25 cc after treatment (MD ‐53.25 cc, 95% CI ‐262.19 to ‐44.32; 182 women). Donnez 2012 also assessed uterine volume after ulipristal acetate therapy but reported data as ‘percent change’ in uterine volume. The treatment group had a non‐significant reduction in median uterine volume (5 mg dose, ‐12.1 percentage point change, 95% CI ‐28.3 to 2.9; 10 mg dose, ‐12.0 percentage point change, 95% CI ‐27.7 to 6.1) compared with the placebo group (5.9 percentage point change, 95% CI ‐3.8 to 18.4). Analysis of these four studies together revealed a reduction in uterine volume after SPRM treatment compared with placebo (SMD ‐0.63, 95% CI ‐0.91 to ‐0.36; 419 women, I2 = 0; Analysis 1.5).
1.5. Analysis.
Comparison 1 SPRM versus placebo, Outcome 5 Change in uterine volume.
SPRM‐related effects
Endometrial histology
Six studies comparing all three SPRMs versus placebo (Donnez 2012; Esteve 2013; Nieman 2011; Prasad 2013; Wilkens 2008) and vitamin B (Engman 2009) assessed endometrial histology. Pathology criteria for evaluation of endometrial specimens differed between studies. Three studies (Donnez 2012; Esteve 2013; Nieman 2011) used the standard definition of SPRM‐associated endometrial changes (PAEC) provided by Mutter 2008. Engman 2009 and Wilkens 2008 evaluated specimens using their own semiquantitative assessment of glandular architecture as described in their respective manuscripts. Meta‐analysis of five studies revealed that PAEC was more common after SPRM therapy than after placebo (OR 15.12, 95% CI 6.45 to 35.47; 405 women, I2 = 0%; low‐quality evidence; Analysis 1.6). These six studies reported three cases of endometrial hyperplasia, all of which occurred in the SPRM treatment group (3/488).
1.6. Analysis.
Comparison 1 SPRM versus placebo, Outcome 6 SPRM‐associated endometrial changes.
Chwalisz 2007 used a different approach to evaluate endometrial histology after asoprisnil treatment. Investigators developed a new classification system for endometrial biopsies that included two additional subcategories: non‐physiological secretory effect and secretory pattern mixed‐type. Among participants for which biopsies were available, 60/85 in the asoprisnil group were classified in these new histological categories compared with 4/30 participants in the placebo group.
Four studies evaluated endometrial histology but did not report on non‐physiological endometrial changes or PAEC. Fiscella 2006 included no participants with hyperplasia in the study group. Levens 2008 reported one case of hyperplasia in the ulipristal acetate group (1/12). Bagaria 2009 included 12/19 participants in the mifepristone group showing endometrial hyperplasia compared with none in the placebo group. The pathology definition used to classify endometrial hyperplasia in this study was not clearly specified nor was any mention made of a PAEC category. Prasad 2013 reported eight cases (25%) of 'cystic glandular hyperplasia' in the mifepristone group but provided unclear pathology criteria and an unclear rate of abnormal pathology in the placebo group.
Comparison of SPRMs versus alternative active therapy
Two studies compared SPRMs versus medical therapy (leuprolide acetate). No studies compared SPRMs versus surgical management or UAE.
SPRM versus medical therapy: leuprolide acetate
See Table 2.
We included two studies for this comparison. Reinsch 1994 evaluated 25 mg of mifepristone for three months, and we could extract only data for the uterine volume outcome. Donnez 2012a evaluated 5 mg and 10 mg of ulipristal acetate for three months and reported on other patient‐reported outcomes, in addition to uterine volume.
PRIMARY OUTCOMES
Fibroid‐related symptoms
Quality of life
Donnez 2012a found probably little or no difference in improvements in symptom severity score and HR‐QoL between ulipristal acetate and leuprolide groups using the UFS‐QoL. Results show no clear evidence of a difference in symptom severity score between SPRM and leuprolide groups (MD ‐3.70 points, 95% CI ‐9.85 to 2.45; 281 women; moderate‐quality evidence; Analysis 2.1;Figure 7), and probably little or no difference in HR‐QoL between SPRM and leuprolide groups (MD 1.06 points, 95% CI ‐5.73 to 7.85; 281 women; moderate‐quality evidence; Analysis 2.2;Figure 8).
2.1. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 1 Change in symptom severity score (QoL).
7.
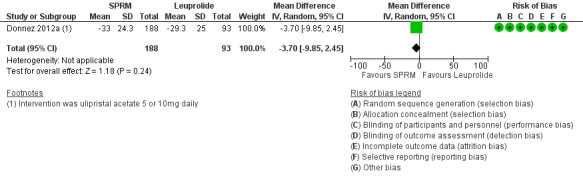
Forest plot of comparison: 2 SPRM versus leuprolide acetate, outcome: 2.1 Change in symptom severity score (QoL).
2.2. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 2 Change in health‐related quality of life score.
8.
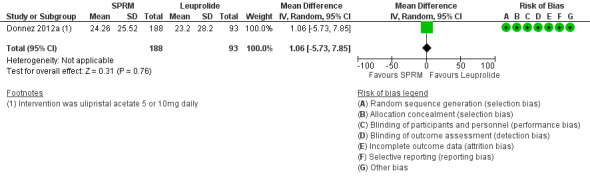
Forest plot of comparison: 2 SPRM versus leuprolide acetate, outcome: 2.2 Change in health‐related quality of life score.
Abnormal uterine bleeding
Menstrual blood loss
Donnez 2012a found little or no difference in bleeding scores on the PBAC between ulipristal acetate and leuprolide (mean difference 6 percentage point change, 95% CI ‐40.95 to 50.95; 281 women; low‐quality evidence; Analysis 2.3;Figure 9).
2.3. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 3 Change in menstrual blood loss.
9.
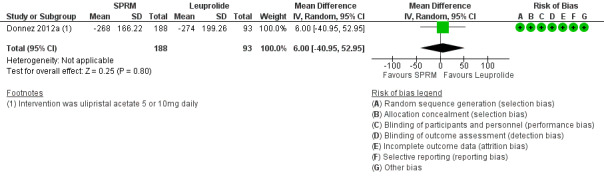
Forest plot of comparison: 2 SPRM versus leuprolide acetate, outcome: 2.3 Change in menstrual blood loss.
Amenorrhoea
Results showed probably little or no difference between groups in rates of amenorrhoea (5 mg dose vs leuprolide: ‐5.2 percentage point change difference, 95% CI ‐18.7 to 8.6; 10 mg dose vs leuprolide: 9.0 percentage point change difference, 95% CI ‐2.8 to 21.0). Overall, the odds ratio for amenorrhoea for the SPRM group compared with the leuprolide group was 1.14 (95% CI 0.60 to 2.16; 280 women; moderate‐quality evidence; Analysis 2.4).
2.4. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 4 Amenorrhoea.
Haemoglobin
Donnez 2012a found probably no difference in haemoglobin between groups (5 mg dose vs leuprolide: ‐0.02 percentage point change difference, 95% CI ‐0.3 to 0.3; 10 mg dose vs leuprolide: 0.03 percentage point change difference 95% CI ‐0.3 to 0.3).
Pain and pelvic pressure
Donnez 2012a used the McGill Pain Questionnaire and found clinically significant improvements in pelvic pain in all treatment groups. Pooled data showed probably little or no difference in improvement in pain scores between SPRM and leuprolide groups (MD ‐0.01 points on a 0 to 45 scale, 95% CI ‐2.14 to 2.12; 281 women; moderate‐quality evidence; Analysis 2.5).
2.5. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 5 Change in pelvic pain.
SECONDARY OUTCOMES
Change in fibroid or uterine size
Change in fibroid size
We reported data for this outcome per fibroid tracked rather than per woman randomised. We have provided this information in Table 7.
5. Change in fibroid volume (%): SPRM versus leuprolide.
| Study | SPRM | Leuprolide | |||||
| MD | SD | n | MD | SD | n | Finding | |
| Donnez 2012a | ‐39.03% | 37.92 | 188 | ‐53.0% | 24.44 | 93 | Favours leuprolide |
MD: mean difference
n: fibroids tracked
SD: standard deviation
Donnez 2012a evaluated change in total volume of the three largest myomas. Participants treated with leuprolide showed a greater reduction in fibroid volume than those treated with ulipristal acetate. The median fibroid volume reduction at end of treatment was 36 percentage point change in the 5 mg group, 42 percentage point change in the 10 mg group and 53 percentage point change in the leuprolide group. When we pooled data for the 5 mg and 10 mg doses, we found that the leuprolide group (93 participants) showed greater shrinkage in fibroid volume than the SPRM group (188 participants).
Change in uterine size
Donnez 2012a and Reinsch 1994 reported the percent change in uterine volume. Leuprolide was associated with decreased uterine volume at completion of treatment compared with SPRM. The reduction in uterine volume was 26% greater in the leuprolide group than in the SPRM group (MD 25.94%, 95% CI 20.49 to 31.39; 295 women, I2 = 0%; Analysis 2.6).
2.6. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 6 Percent change in uterine volume.
SPRM‐related effects
Donnez 2012a reported assessment of endometrial histology. This study used the definition of PAEC provided by Mutter 2008 and reported that PAEC was more common after SPRM therapy than after leuprolide treatment (OR 10.45, 95% CI 5.38 to 20.33; 301 women; moderate‐quality evidence; Analysis 2.7).
2.7. Analysis.
Comparison 2 SPRM versus leuprolide acetate, Outcome 7 SPRM‐associated endometrial changes.
Other analyses
Formal assessment of reporting bias
We identified too few studies to construct a funnel plot to investigate publication bias.
Subgroup analyses
Included studies were few and did not allow meaningful subgroup analyses with sufficient power.
Sensitivity analyses
We applied a fixed‐effect model to our comparisons and found no significant changes in findings. When ORs were reported, we performed sensitivity analysis using risk ratio and encountered no changes to our results. Owing to the limited number of studies for primary outcomes, other planned sensitivity analyses were not possible.
Discussion
Summary of main results
This review included 14 randomised controlled trials (RCTs) that evaluated three types of selective progesterone receptor modulators (SPRMs; mifepristone, ulipristal acetate, asoprisnil). Moderate‐quality evidence shows that compared with placebo, SPRMs may improve quality of life, decrease menstrual blood loss and induce amenorrhoea. Trial results support treatment of individuals with uterine fibroids with SPRMs to improve fibroid‐related symptoms. Only two studies provided evidence obtained by comparing SPRMs versus a gonadotropin‐releasing agonist (GnRHa), and only one of these studies used sound study methods (Donnez 2012a). These limited data of moderate quality show probably little or no difference between leuprolide and SPRM in improving quality of life, achieving amenorrhoea and improving pelvic pain. Data from only one study provided insufficient evidence to allow a recommendation regarding the efficacy of one class of drugs over another for treatment of individuals with fibroid‐related symptoms.
Investigators evaluated quality of life by using two components of the Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL): symptom severity (SS‐QoL) and health‐related quality of life (HR‐QoL). Moderate‐quality evidence for both quality of life outcomes showed probable improvement with SPRM treatment compared with placebo.
Moderate‐quality evidence shows that SPRMs are probably effective in achieving amenorrhoea. The odds of achieving amenorrhoea with SPRM treatment were calculated at 82.50 compared with placebo (or 29 per 1000 in the placebo group vs 477 per 1000 in the SPRM group). See Table 1. Furthermore, moderate‐quality evidence showed that menstrual blood loss is probably reduced with SPRM treatment. Measurement of menstrual blood loss differed between studies. Four studies used pictorial blood loss assessment charts, and the others used daily diaries, numerical rating scores or composite scores extracted from the UFS‐QoL. No studies used the alkaline‐hematin method ‐ a well‐validated quantitative assessment of menstrual bleeding.
There remains a relative paucity of data on comparison of SPRMs against other medical management options for fibroids. One RCT (Donnez 2012a) reported probably little or no difference between SPRM and leuprolide with respect to improved quality of life and menstrual bleeding scores. This same study showed that leuprolide was more effective than SPRMs in reducing fibroid volume (Analysis 2.6). Similarly, two RCTs showed that leuprolide was more effective than SPRM in reducing uterine volume (Analysis 2.6). This discrepancy between differential change in uterine/fibroid volume and similar improvements in bleeding symptoms suggests that SPRM mechanisms underpinning control of bleeding may be independent of fibroid shrinkage; this interaction is poorly understood (Wagenfeld 2016).
In keeping with known effects of SPRMs, low‐quality evidence suggests increased risk of selective progesterone receptor modulator‐associated endometrial changes (PAEC) in women treated with SPRMs versus placebo, and moderate‐quality evidence shows similar risk in comparisons with leuprolide. However, PAEC was not universally observed in all study participants (odds ratio (OR) 15.12, 95% confidence interval (CI) 4.65 to 35.47 in the comparison of SPRM vs placebo). Overall, endometrial hyperplasia was not increased after SPRM treatment. Increased rates of endometrial hyperplasia seen in one small study (Bagaria 2009) may be explained by misclassification of endometrial pathology. In light of published criteria for classifying SPRM endometrial effects (Mutter 2008), this study made no mention of a PAEC category and could have misclassified PAEC for hyperplasia.
Overall completeness and applicability of evidence
This review sought to assess the efficacy and safety of SPRMs. Researchers have reported improvements in key outcome measures when comparing SPRMs with placebo. However, additional studies comparing SPRMs versus other treatments for fibroids are needed. At present, an RCT fitting our inclusion criteria identified only leuprolide as a comparator. Other clinical trials evaluating SPRMs have not yet published results in peer‐reviewed journals nor on clinicaltrials.gov. Failure of investigators and industry to publish results of RCTs promptly has made our review vulnerable to publication bias.
With regard to safety outcomes, study authors have reported no increase in endometrial hyperplasia/malignancy after SPRM treatment. One study demonstrated an increase in endometrial hyperplasia (Bagaria 2009), but this study had serious limitations, as was previously discussed. Safety and efficacy data from this review are valid only for a treatment course of three months, as most included trials were of 12 weeks duration. The exception was Fiscella 2006, in which participants were treated for six months with no reports of hyperplasia in either group. Since the time of publication of these RCTs, more data have become available regarding endometrial safety and sustained efficacy over longer treatment periods (Donnez 2014; Donnez 2015).
Studies were conducted in Europe, North America, Cuba and India and included participants from diverse ethnic groups. However, it should be noted that participants from the PEARL (PGL4001 Efficacy Assessment in Reduction of Symptoms due to Uterine Leiomyomata) studies (Donnez 2012; Donnez 2012a) represented a significant number of those included in this meta‐analysis (549/1021). Inclusion criteria for the PEARL studies required that women have high pictorial blood loss assessment chart (PBAC) scores, anaemia and myomata larger than 3 cm. Participants were predominantly White Eastern European women. Thus, the results of this review may not be fully applicable to women with smaller, less symptomatic myomata or to those from other ethnic groups. Some evidence suggests that ulipristal acetate (UPA) may result in variable outcomes according to ethnicity (Murji 2016).
Quality of the evidence
More than half (8/14) of the included studies were at low risk of bias in all domains. The most common limitation in other studies was poor reporting of methods. We graded four studies in particular as having unclear risk across most domains primarily owing to insufficient detail in their description of study methods (Bigatti 2014; Liu 2015; Prasad 2013; Reinsch 1994). The Bigatti, Liu and Prasad papers were conference proceedings from which we could not extract reliable data, and we did not include these studies in the meta‐analyses.
Comparison of SPRM versus placebo yielded moderate‐quality evidence for most primary outcomes. We downgraded all evidence at least one level owing to strong suspicion of publication bias. Most of these studies included a small number of participants with positive results. We included no large negative studies in this review. Many unpublished industry‐sponsored studies further raise our concern regarding publication bias. Assessment of risk of SPRM‐associated endometrial changes yielded low‐quality evidence mainly owing to serious risk of measurement bias (due to inconsistencies in evaluation of endometrial histology).
Comparison of SPRM versus leuprolide generated mostly evidence of moderate quality. Again, we consistently downgraded the quality by one level owing to publication bias. For the outcome of change in menstrual blood loss, low‐quality evidence was due to very serious issues with imprecision.
One of the challenges for authors of this review was variable reporting of outcomes in these studies, particularly with regard to methods of assessing menstrual blood loss, fibroid/uterine size and quality of life parameters. Future trials assessing impact on fibroid size via imaging may wish to consider stereological assessment of the uterus rather than use of standard calliper methods (Thrippleton 2015). Consensus decisions regarding core reporting outcomes will facilitate future reviews and will permit greater ease of comparison in both future meta‐analyses and network analysis (Khan 2014; http://www.crown‐initiative.org).
Potential biases in the review process
Our literature search was comprehensive; in addition to conducting database searches, we handsearched appropriate journals, clinical trial databases and conference proceedings. Other SPRMs for which results have not yet been published are undergoing evaluation. Seven of ten studies awaiting classification were investigating telapristone acetate. Although these phase 2 and 3 studies have been completed, results are not yet published. Each of asoprisnil, vilaprisan or ulipristal acetate was the topic of one study with unpublished results.
Review authors made all important decisions regarding inclusion, bias and other aspects of analysis by discussion and consensus. When necessary, we sought additional evidence from trial authors that would help us accurately determine risk of bias. The main risk of bias involves the quality of some studies, small sample sizes and variable reporting of outcomes.
Agreements and disagreements with other studies or reviews
A previously published Cochrane review examined the use of mifepristone for uterine fibroids (Tristan 2012). Our review includes two additional studies investigating mifepristone (Esteve 2013; Reinsch 1994). Data from Esteve 2013 were published subsequent to the Tristan 2012 review. In the spirit of inclusiveness, we decided to include Reinsch 1994 data in our meta‐analysis but discussed its methodological issues both in the text and in risk of bias tables. However, owing to the small number of participants, the Reinsch study did not have a significant impact on the overall magnitude of effect size.
We also included in our analysis the three studies that were included in the Tristan 2012 mifepristone review (Bagaria 2009; Engman 2009; Fiscella 2006). Some minor differences in risk of bias assessments are evident. All review authors re‐evaluated these discrepancies and assigned final bias ratings by consensus in a manner that was consistent with ratings for other studies included in our review. Our findings are consistent with and extend those presented in the Tristan 2012 review. As a result of the larger number of participants in our study (1021 vs 112), we were able to demonstrate that SPRMs reduce fibroid volume and improve fibroid‐specific quality of life. Our review confirmed the finding that SPRMs reduce heavy menstrual bleeding.
Another Cochrane review investigated the role of pretreatment with GnRH analogues before a major surgical procedure ‐ hysterectomy or myomectomy ‐ for uterine fibroids (Lethaby 2001). An update of this review with an expanded scope including all medical therapies used before surgery for fibroids is under editorial review.
Our findings also build upon the results of other systematic reviews. For example, a meta‐analysis of ulipristal acetate for treatment of uterine fibroids found that this drug was associated with improved quality of life and reduced fibroid size when compared with placebo (Kalampokas 2016). This meta‐analysis was based on the same four ulipristal acetate studies included in our review.
Another systematic review of medical treatments for fibroids included 75 RCTs, 47 of which contributed to the network meta‐analysis (Gurusamy 2016). This meta‐analysis included the same 11 studies as were included in our review. We included three additional studies as well: Wilkens 2008 (Williams 2007 publication) reported on outcome measures of interest in our protocol, and Liu 2015 and Prasad 2013 were published subsequent to the Gurusamy 2016 review. The Gurusamy 2016 review rated risk of study bias as higher than we did, possibly because we solicited additional information/protocols from study authors that allowed us to downgrade some risk of bias assessments. The biggest difference between the two reviews involved the outcomes chosen for comparison. Our review focused on analysis of outcomes that directly impact and are clinically meaningful to patients. Our primary outcomes were changes in fibroid‐related symptoms (quality of life, amenorrhoea, reduction in bleeding, etc.). The Gurusamy review reported on the proportion of patients treated medically who underwent surgery, as well as haemoglobin levels and adverse events. On the basis of these limited comparisons, review authors concluded that evidence was insufficient to recommend medical treatment for patients with fibroids. On the basis of outcomes that we evaluated, we arrived at a different conclusion. We found that moderate‐quality evidence showed that SPRMs are more effective in improving patient‐reported outcomes when compared with placebo.
Several ongoing clinical trials are examining different drugs in the class of SPRMs. Results from these trials may alter subsequent updates of this review. Many of these ongoing trials are comparing SPRMs with other drugs rather than with placebo. These results will be of particular interest, as they will add to the literature and perhaps will inform clinical decision making.
Authors' conclusions
Implications for practice.
In comparison with placebo, evidence suggests that SPRMs may improve fibroid‐related symptoms and quality of life, while inducing amenorrhoea, controlling heavy menstrual bleeding and producing fibroid and uterine shrinkage. A three‐month treatment course with this class of drugs does not result in premalignant or malignant transformation of the endometrium. However, SPRM‐associated endometrial changes are frequently observed with administration of all SPRMs, and histopathologists must be apprised of these benign endometrial features. Moderate‐quality data based on one study show that differences between SPRM and leuprolide acetate (in the class of gonadotropin‐releasing hormone agonists) in treating fibroid‐related symptoms are probably few if they are noted at all. Both classes of medications are effective in improving quality of life and controlling menstrual bleeding. For secondary outcomes, limited evidence shows that leuprolide causes greater shrinkage of fibroid and uterine volume. However, although less fibroid shrinkage was reported after UPA administration, overall reduction in this group occurred as a change of 36 percentage points, which is clinically meaningful.For example, a reduction in fibroid size of this magnitude may facilitate a laparoscopic rather than an abdominal approach.
Given that both SPRM and leuprolide improve quality of life and that there is probably little or no difference between them, the choice of medication should be made based on patient/clinician preferences, taking into account issues such as route of administration, costs, presence of other disease processes and side‐effect profiles.
Overall, limited evidence is available on the use of SPRMs over other medical treatment options for fibroids.
Implications for research.
Well‐designed RCTs are needed to compare SPRMs versus other treatment options for fibroids. Study cohorts should reflect patient demographics, particularly with respect to ethnicity. Multiple potentially useful SPRMs are available, and evidence of efficacy compared with placebo, other medical treatments or surgical interventions is required. Outcome measures must assess quality of life, treatment adherence and patient satisfaction, as well as objective and standardised measures of uterine bleeding, fibroid and uterine size. Investigators should use consistent core reporting outcomes (Khan 2014) to facilitate meaningful comparisons.
Publication bias impacts meta‐analyses and potentially influenced this review. As such, transparency in clinical research should continue to be advocated at all levels. Cost‐effectiveness analysis should be considered if SPRMs are to be used as longer‐term medical treatment options.
Acknowledgements
We acknowledge the significant contributions of Drs Hilary OD Critchley and Andrew H Horne to the review.
Many thanks to Marian Showell, Information Specialist with the Cochrane Gynaecology and Fertility Group, and Vicky Lynham, at Sidney Liswood Library Mount Sinai Hospital, Toronto, Ontario, for help in devising and executing the literature search strategy.
Many thanks also to Professor Joseph Beyenne, Professor of Biostatistics, Faculty of Health Sciences, McMaster University, Hamilton, who assisted with statistical analysis and imputation of missing data.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register search strategy
From inception until 15.05.16
Procite platform
Keywords CONTAINS "Leiomyoma" or "leiomyomata" or "fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "uterine fibroids" or "myoma" or "myomas" or Title CONTAINS "Leiomyoma" or "leiomyomata" or "fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "uterine fibroids" or "myoma" or "myomas"
AND
Keywords CONTAINS "mifepristone" or "RU486" or "selective progesterone receptor modulator" or "CDB‐2914" or "asoprisnil" or "Ulipristal" or "Progesterone Receptor Modulator" or Title CONTAINS "mifepristone" or "RU486" or "selective progesterone receptor modulator" or "CDB‐2914" or "asoprisnil" or "Ulipristal" or "Progesterone Receptor Modulator" (42 hits)
Appendix 2. CENTRAL
From inception until 15.05.16
CRS online platform
#1 MESH DESCRIPTOR Leiomyoma EXPLODE ALL TREES (410) #2 fibroid*:TI,AB,KY (399) #3 (fibromyoma* or myoma*):TI,AB,KY (515) #4 (Leiomyom* or hysteromyoma*):TI,AB,KY (644) #5 (uter* adj3 fibroma*):TI,AB,KY (12) #6 #1 OR #2 OR #3 OR #4 OR #5 (1116) #7 MESH DESCRIPTOR Receptors, Progesterone EXPLODE ALL TREES (379) #8 (progesterone receptor*):TI,AB,KY (716) #9 (SPRM* or PRM*):TI,AB,KY (112) #10 MESH DESCRIPTOR Mifepristone EXPLODE ALL TREES (370) #11 Mifepristone:TI,AB,KY (703) #12 (r38486 or ru‐38486):TI,AB,KY (1) #13 (ru38486 or ru‐486 or ru486):TI,AB,KY (147) #14 (Asoprisnil or vilaprisan):TI,AB,KY (11) #15 J867:TI,AB,KY (1) #16 Ulipristal:TI,AB,KY (34) #17 Ella:TI,AB,KY (6) #18 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 (1681) #19 #6 AND #18 (152)
Appendix 3. MEDLINE
From 1946 until 15.05.16
Ovid platform
1 exp Leiomyoma/ (18861) 2 fibroid$.tw. (4992) 3 fibromyoma$.tw. (705) 4 myoma$.tw. (5083) 5 hysteromyoma$.tw. (58) 6 Leiomyom$.tw. (11885) 7 (uter$ adj3 fibroma$).tw. (347) 8 or/1‐7 (25960) 9 exp Receptors, Progesterone/ (17191) 10 progesterone receptor modulator$.tw. (274) 11 (SPRM$ or PRM$).tw. (3443) 12 exp Mifepristone/ (5528) 13 Mifepristone.tw. (2979) 14 mifegyne.tw. (12) 15 mifeprex.tw. (12) 16 r38486.tw. (1) 17 ru‐38486.tw. (442) 18 ru38486.tw. (374) 19 ru‐486.tw. (1666) 20 ru486.tw. (2091) 21 (Asoprisnil or vilaprisan).tw. (47) 22 J867.tw. (13) 23 Telapristone.tw. (9) 24 Progenta.tw. (1) 25 Ulipristal.tw. (216) 26 Ella.tw. (224) 27 Proellex.tw. (8) 28 CDB‐4124.tw. (17) 29 or/9‐28 (27704) 30 8 and 29 (534) 31 randomized controlled trial.pt. (428367) 32 controlled clinical trial.pt. (91556) 33 randomized.ab. (358910) 34 randomised.ab. (74152) 35 placebo.tw. (180116) 36 clinical trials as topic.sh. (178949) 37 randomly.ab. (256577) 38 trial.ti. (157027) 39 (crossover or cross‐over or cross over).tw. (69212) 40 or/31‐39 (1096048) 41 exp animals/ not humans.sh. (4299057) 42 40 not 41 (1009366) 43 30 and 42 (86)
Appendix 4. Embase
From 1974 until 15.05.16
Ovid platform
1 exp leiomyoma/ (15529) 2 fibroid$.tw. (7957) 3 fibromyoma$.tw. (608) 4 myoma$.tw. (6963) 5 hysteromyoma$.tw. (130) 6 Leiomyom$.tw. (14521) 7 (uter$ adj3 fibroma$).tw. (382) 8 or/1‐7 (32175) 9 exp progesterone receptor modulator/ (445) 10 progesterone receptor modulator$.tw. (427) 11 (SPRM$ or PRM$).tw. (4609) 12 exp mifepristone/ (11147) 13 Mifepristone.tw. (3773) 14 mifegyne.tw. (181) 15 mifeprex.tw. (106) 16 r38486.tw. (1) 17 ru38486.tw. (402) 18 ru‐38486.tw. (911) 19 ru‐486.tw. (4121) 20 ru486.tw. (2449) 21 (Asoprisnil or vilaprisan).tw. (64) 22 J867.tw. (14) 23 Telapristone.tw. (15) 24 Progenta.tw. (4) 25 Ulipristal.tw. (442) 26 Ella.tw. (383) 27 Proellex.tw. (22) 28 CDB‐4124.tw. (47) 29 or/9‐28 (17311) 30 8 and 29 (620) 31 Clinical Trial/ (862238) 32 Randomized Controlled Trial/ (413467) 33 exp randomization/ (71619) 34 Single Blind Procedure/ (22711) 35 Double Blind Procedure/ (130713) 36 Crossover Procedure/ (48263) 37 Placebo/ (279471) 38 Randomi?ed controlled trial$.tw. (141716) 39 Rct.tw. (21228) 40 random allocation.tw. (1552) 41 randomly allocated.tw. (25411) 42 allocated randomly.tw. (2146) 43 (allocated adj2 random).tw. (762) 44 Single blind$.tw. (17830) 45 Double blind$.tw. (164731) 46 ((treble or triple) adj blind$).tw. (580) 47 placebo$.tw. (237405) 48 prospective study/ (346790) 49 or/31‐48 (1604891) 50 case study/ (39627) 51 case report.tw. (312069) 52 abstract report/ or letter/ (969653) 53 or/50‐52 (1314128) 54 49 not 53 (1563365) 55 30 and 54 (221)
Appendix 5. PsycINFO
From 1806 until 15.05.16
Ovid platform
1 fibroid$.tw. (55) 2 myoma$.tw. (23) 3 fibromyoma$.tw. (1) 4 hysteromyoma$.tw. (2) 5 Leiomyom$.tw. (18) 6 (uter$ adj3 fibroma$).tw. (4) 7 or/1‐6 (97) 8 selective progesterone receptor modulator$.tw. (1) 9 SPRM$.tw. (3) 10 Mifepristone.tw. (216) 11 Mifegyne.tw. (0) 12 mifeprex.tw. (1) 13 r38486.tw. (0) 14 ru‐38486.tw. (42) 15 ru‐486.tw. (88) 16 Ulipristal.tw. (3) 17 CDB‐4124.tw. (2) 18 or/8‐17 (326) 19 7 and 18 (1)
Appendix 6. CINAHL (Cumulative Index to Nursing and Allied Health Literature)
From 1982 to 15.05.16
EBSCO Platfrom
| # | Query | Results |
| S35 | S22 AND S34 | 23 |
| S34 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 1,066,484 |
| S33 | TX allocat* random* | 5,196 |
| S32 | (MH "Quantitative Studies") | 14,755 |
| S31 | (MH "Placebos") | 9,774 |
| S30 | TX placebo* | 39,205 |
| S29 | TX random* allocat* | 5,196 |
| S28 | (MH "Random Assignment") | 41,421 |
| S27 | TX randomi* control* trial* | 108,575 |
| S26 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 845,197 |
| S25 | TX clinic* n1 trial* | 188,356 |
| S24 | PT Clinical trial | 79,704 |
| S23 | (MH "Clinical Trials+") | 201,384 |
| S22 | S7 AND S21 | 62 |
| S21 | S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 970 |
| S20 | TX CDB‐4124 | 2 |
| S19 | TX Ulipristal | 87 |
| S18 | TX Telapristone | 3 |
| S17 | TX J867 | 1 |
| S16 | TX Asoprisnil | 5 |
| S15 | TX ru‐486 | 163 |
| S14 | TX ru‐38486 | 7 |
| S13 | TX ru38486 | 7 |
| S12 | TX mifeprex | 20 |
| S11 | TX Mifepristone | 831 |
| S10 | (MM "Mifepristone") | 397 |
| S9 | TX SPRM* | 5 |
| S8 | TX selective progesterone receptor modulator* | 22 |
| S7 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 | 3,181 |
| S6 | TX fibromyoma | 2 |
| S5 | TX (uter* N3 fibroma*) | 5 |
| S4 | TX Leiomyoma* | 2,594 |
| S3 | TX myoma* | 404 |
| S2 | TX fibroid* | 1,096 |
| S1 | (MM "Leiomyoma") OR (MH "Myoma+") | 2,017 |
Appendix 7. DARE (Database of Abstracts of Reviews and Effect)
Cochrane Library
Searched 15.05.16
#1 fibroid* or myoma* or fibromyoma* or Leiomyom* (Word variations have been searched) (1202) #2 progesterone receptor* or SPRM* or PRM* or Mifepristone or Ulipristal (2250) #3 #1 and #2 (3 hits in DARE)
Appendix 8. Clinicaltrials.gov
Search Aug 25 2016
Field = Conditions
Myoma OR leiomyoma OR hysteromyoma OR “uterine fibroid” OR fibromyoma OR fibroma
AND
Field = Interventions
Mifepristone OR Mifegyne OR Mifeprex OR ru‐38486 OR ru‐486 OR Asoprisnil OR Telapristone OR Progenta OR Ulipristal OR Proellex OR j867 OR "bay 1002670" OR vilaprisan OR CDB4124 OR Sprm OR "selective progesterone receptor modulator"
No restriction by date or status of study
Results = 59 studies
Appendix 9. WHO trials register
Searched 25 August 2016
Field = Conditions
Myoma OR leiomyoma OR hysteromyoma OR uterine fibroid OR fibromyoma OR fibroma
AND
Field = Interventions
Mifepristone OR Mifegyne OR Mifeprex OR ru‐38486 OR ru‐486 OR Asoprisnil OR Telapristone OR Progenta OR Ulipristal OR Proellex OR j867 OR bay 1002670 OR vilaprisan OR CDB4124 OR Sprm OR selective progesterone receptor modulator
No restriction by date or status of trial
Results = 15 trials
Appendix 10. Web of Science
Searched 24 August 2016
Myoma* OR leiomyom* OR hysteromyoma* OR uterine fibroid* OR fibromyoma* OR fibroma*
Mifepristone OR Mifegyne OR Mifeprex OR ru‐38486 OR ru38486 OR ru‐486 OR ru486 OR Asoprisnil OR Telapristone OR Progenta OR Ulipristal OR Proellex OR j867 OR bay 1002670 OR vilaprisan OR Sprm* OR selective progesterone receptor modulator*
Results = 59
Appendix 11. LILACS
Searched 25 August 2016
Myoma* OR leiomyom* OR hysteromyoma* OR fibroid* OR fibromyoma* OR fibroma*
AND
Mifepristone OR Mifegyne OR Mifeprex OR ru38486 OR ru‐38486 OR ru486 OR ru‐486 OR Asoprisnil OR Telapristone OR Progenta OR Ulipristal OR Proellex OR j867 OR "bay 1002670" OR vilaprisan OR CDB4124 OR Sprm* OR "selective progesterone receptor modulator" OR "selective progesterone receptor modulators"
Results = 3
Appendix 12. Statistical methods used to impute missing data
The following summary presents selected portions of a report prepared by Joseph Beyenne, 10 March 2016
Selective progesterone receptor modulators (SPRMs) for uterine fibroids: a meta‐analysis
In this report, we summarise meta‐analysis results for the above study. The inverse variance method of weighting studies was used for all meta‐analyses.
1.1 Quality of life
1.1.1 Symptom severity
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 1: Esteve 2013
We needed to calculate change scores and the change score SD. To get the SD for each treatment group, we assumed a correlation of 0.4 and applied the following formula:
SD of change=sqrt((SD at baseline)^2 + (SD at final)^2 ‐ (2(Corr)(SD at baseline)(SD at final)))
For example, for the treatment group:
sqrt((16.4)^2 + (17.7)^2 ‐ (2(0.4)(16.4)*(17.7)))
and for the placebo group:
sqrt((23)^2 + (21.2)^2 ‐ (2(0.4)(23)*(21.2)))
Study 2: Levens 2008
We assumed +/‐ was SD.
Study 3: Nieman 2011
We converted SE=4.2 into SD=21.42. We converted SE=6.5 into SD=22.51
Study 4: Wilkens 2008
We combined treatment groups as follows:
MD=(12(‐21.6)+11(‐31.5))/(12+11)
SD=sqrt(((12‐1)(26.22)+(11‐1)(9.32))/(12+11‐2))
1.1.2 Health‐related quality of life
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 1: Esteve 2013
We needed to calculate change scores and change score SD. To get the SD for each treatment group, we assumed a correlation of 0.4 and applied the following formula:
Study 2: Levens 2008
We had to convert range to SD. So we assumed range is MD +/‐ 2SD, so 4SD=range, so SD=range/4.
Study 3: Nieman 2011
We converted SE into SD. Treatment group: SE=sqrt(26)3.6 = 18.36. Placebo: sqrt(12)5.6 = 19.4.
Study 4: Wilkens 2008
We combined treatment groups in the same way as for the previous outcome.
1.2 Abnormal uterine bleeding
1.2.1 Menstrual blood loss
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 1: Bagaria 2009
We imputed SDs from the other two studies in this meta‐analysis by taking their weighted average:
For treatment:
sqrt(((189‐1)(248.342)+(23‐1)(109.532))/(189+23‐2)) = 237.6312
For control:
sqrt(((48‐1)(202.962)+(10‐1)(150.62))/(48+10‐2)) = 195.4931
Study 2: Donnez 2012
We assumed medians to be means and imputed SD for the means as IQR/1.35.
For example, for placebo:
SD: (58‐(‐216))/1.35 =
Furthermore, we had to combine two treatment groups. So after completing this imputation for both treatment groups, we combined using the standard approach as shown previously.
SD (UPA 5mg): (‐205‐(‐571))/1.35 = 271.1111 SD (UPA 10mg): (‐226‐(‐527))/1.35 = 222.963
Pooling: sqrt(((95‐1)(271.11112)+(94‐1)(222.9632))/(95+94‐2))=248.3354
Study 3: Wilkens 2008
We combined treatment groups in the same way as for the previous outcome.
1.2.2 Amenorrhoea at the end of treatment
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 4: Donnez 2012
We combined events and sample sizes from UA 5 mg and UA 10 mg.
Study 5: Levens 2008
We combined events and sample sizes from UPA 10 mg and UPA 20 mg.
Study 6: Nieman 2011
We combined events and sample sizes from UA 10 mg and UA 20 mg.
Study 7: Chwalisz 2007
We combined events and sample sizes from 5 mg ASO, 10 mg ASO and 25 mg ASO.
2.2 Change in uterine volume
For this outcome, some studies reported mean differences and some reported percent changes. We therefore performed three separate meta‐analyses: a) mean differences only, b) percent changes only, c) both, combined via SMD.
2.2a Change in uterine volume; mean differences only
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 1: Bagaria 2009
We needed to calculate change scores and change score SD. To get the SD for each treatment group, we assumed a correlation of 0.4 and applied the same formula as before.
For treatment:
sqrt((235.6)^2 + (203.5)^2 ‐ (2(0.4)(235.6)(203.5))) = 241.9999
For placebo:
sqrt((417.5)^2 + (417.2)^2 ‐ (2(0.4)(417.5)(417.2))) = 457.1841
Study 2: Esteve 2013
For treatment:
sqrt((236)^2 + (202)^2 ‐ (2(0.4)(236)(202))) = 241.5831
For placebo:
sqrt((211)^2 + (210)^2 ‐ (2(0.4)(211)(210))) = 230.5927
Study 3: Fiscella 2009
We imputed SDs from the other two studies in this meta‐analysis by taking their weighted average:
For treatment:
sqrt(((19‐1)(2422)+(58‐1)(241.582))/(19+58‐2)) = 241.6809
For control:
sqrt(((16‐1)(457.182)+(47‐1)(230.592))/(16+47‐2)) = 302.4789
Study 4: Donnez 2012
We excluded this study for now because we cannot impute MDs from percent changes.
2.2b Change in uterine volume; percent changes only
Comparison: SPRM vs placebo
Study 4: Donnez 2012
Combined UPA 5 mg and UPA 10 mg.
For placebo:
(18.4‐(‐3.8))/1.35 = 16.44444
For treatment:
(95(‐12.1)+94(‐12))/189 = ‐12.05026
(2.9‐(‐28.3))/1.35 = 23.11111
(6.1‐(‐27.7))/1.35 = 25.03704
sqrt(((95‐1)23.111112+(94‐1)25.037042)/(95+94‐2)) = 24.08818
2.2c Change in uterine volume; both, combined via SMD
Comparison: SPRM vs placebo
2.3 SPRM‐associated endometrial changes (PAEC)
Comparison: SPRM vs placebo
Data management
We had to make some assumptions.
Study 3: Donnez 2012
We had to combine UPA 5 mg and UPA 10 mg.
We had to calculate number of events based on percentages and sample sizes. We rounded to the nearest integer for number of events.
Study 4: Donnez 2012a
We had to combine UPA 5 mg and UPA 10 mg.
We had to calculate number of events based on percentages and sample sizes. We rounded to the nearest integer for number of events.
Study 5: Nieman 2011
We assumed 9/9 normal meant number of events was 0, for placebo.
Study 6: William 2007
We combined events and sample sizes from 10 mg ASO, 25 mg ASO.
Data and analyses
Comparison 1. SPRM versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in symptom severity score (QoL) | 4 | 171 | Mean Difference (IV, Random, 95% CI) | ‐20.04 [‐26.63, ‐13.46] |
| 2 Change in health‐related quality of life score | 4 | 200 | Mean Difference (IV, Random, 95% CI) | 22.52 [12.87, 32.17] |
| 3 Change in menstrual blood loss | 3 | 310 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.38, ‐0.83] |
| 4 Amenorrhoea | 7 | 590 | Odds Ratio (IV, Random, 95% CI) | 82.50 [37.01, 183.90] |
| 5 Change in uterine volume | 4 | 419 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.91, ‐0.36] |
| 6 SPRM‐associated endometrial changes | 5 | 405 | Odds Ratio (IV, Random, 95% CI) | 15.12 [6.45, 35.47] |
Comparison 2. SPRM versus leuprolide acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in symptom severity score (QoL) | 1 | 281 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐9.85, 2.45] |
| 2 Change in health‐related quality of life score | 1 | 281 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐5.73, 7.85] |
| 3 Change in menstrual blood loss | 1 | 281 | Mean Difference (IV, Random, 95% CI) | 6.00 [‐40.95, 52.95] |
| 4 Amenorrhoea | 1 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.60, 2.16] |
| 5 Change in pelvic pain | 1 | 281 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐2.14, 2.12] |
| 6 Percent change in uterine volume | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 25.94 [20.49, 31.39] |
| 7 SPRM‐associated endometrial changes | 1 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 10.45 [5.38, 20.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bagaria 2009.
| Methods | Single‐center blinded placebo RCT | |
| Participants | Participants randomised to 1 of 2 groups in a 1:1 ratio (n = 40) ‐ 20 mifepristone 10 mg: 1 withdrawn, 19 analysed ‐ 20 placebo: 4 withdrawn, 16 analysed Baseline demographics similar in each group Inclusion criteria ‐ Premenopausal ‐ Symptomatic fibroids (confirmed on ultrasonography) Exclusion criteria ‐ Pregnancy/lactation ‐ Ovarian/cervical/uterine malignancy ‐ Histopathological evidence of endometrial hyperplasia ‐ Hormonal treatment previous 3 months ‐ Liver, respiratory, renal, cardiac disease ‐ Pelvic inflammatory disease ‐ Need for early surgical intervention for fibroids Setting: Delhi, India |
|
| Interventions | Mifepristone 10 mg orally daily vs placebo Duration: 3 months starting on cycle day 1 to 3 |
|
| Outcomes | Outcomes assessed monthly for 3 months in the post‐menstrual phase or on a fixed day if amenorrhoeic ‐ Uterine/fibroid ultrasound volumes ‣ Uterine (Viscomi formula) ‣ Average and largest fibroid (formula for sphere) ‐ Blood loss ‣ PBAC ‣ Mean Hgb level ‣ Amenorrhoea ‐ Fibroid symptoms (VAS) ‣ Dysmenorrhoea ‣ Pelvic pain ‣ Backache ‣ Urinary complaints ‣ Dyspareunia ‐ Serum haemoglobin, liver and renal function tests ‐ Side effects ‣ Nausea/vomiting ‣ Fatigue ‣ Diarrhoea ‣ Headache ‣ Weakness ‣ Hot flashes ‣ Loss of libido ‐ Endometrial biopsy for hyperplasia (only at end of study) but did not report on PAEC |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random tables were used to randomise packets to contain mifepristone or placebo |
| Allocation concealment (selection bias) | Low risk | As participants were enrolled, they were assigned numbers 1 to 40 and received the corresponding numbered drug packet prepared/dispensed by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Mifepristone and placebo capsules were identical in appearance and were prepared/dispensed by a third party |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not stated Main outcomes objective (e.g. fibroid volume, Hgb), so less influenced by bias if present |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 and 4 participants lost to follow‐up in mifepristone vs placebo group. This represents unequal loss of follow‐up between groups (5% vs 20%). No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not found. Study authors emailed for protocol |
| Other bias | Unclear risk | Potential for dose variation in mifepristone group, as no specific detail regarding capsule derivation method from 200 mg tablets and associated quality control |
Bigatti 2014.
| Methods | Randomised prospective comparative study | |
| Participants | Unclear but likely premenopausal women with submucosal fibroids undergoing hysteroscopic myomectomy | |
| Interventions | ‐ Group A: norethisterone acetate 10 mg 2 times per day ‐ Group B: micronised progesterone 200 mg 1 time per day ‐ Group C: dienogest 2 mg 1 time per day ‐ Group D: ulipristal acetate 5 mg 1 time per day ‐ Group E: control group with no treatment | |
| Outcomes | Fluid balance, cervical canal dilatation time, resection and total operation time, complications, second‐look procedures, conversion to bipolar resectoscopy | |
| Notes | Conference proceeding. Irrelevant clinical outcomes reported in this conference proceeding. This trial included 5 arms (ulipristal acetate, dienogest, micronised progesterone, norethindrone acetate, placebo). Investigators reported on surgical outcomes at hysteroscopic myomectomy, including fluid balance, cervical canal dilatation time, resection and total operation time, complications, second‐look procedures and conversion to bipolar resectoscopy. Total of 7 participants were recruited at time of publication. None of these participants were treated with an SPRM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | Data in form of conference proceedings. No published follow‐up data |
Chwalisz 2007.
| Methods | Multi‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 4 groups in a 1:1:1:1 ratio (n = 129) ‐ 33 asoprisnil 5 mg: 1 withdrawn, 32 analysed ‐ 29 asoprisnil 10 mg: 2 withdrawn, 27 analysed ‐ 36 asoprisnil 25 mg: 4 withdrawn, 32 analysed ‐ 31 placebo: 2 withdrawn, 29 analysed Baseline demographics similar in each group Inclusion criteria ‐ 18 to 49 years old ‐ Good general health ‐ No history of uterine artery embolisation, cryomyolysis or electrical myolysis ‐ At least 1 fibroid ≥ 3 cm diameter or uterine volume > 200 cm3 caused by multiple small fibroids (ultrasonography) Exclusion criteria ‐ Pelvic pathology (e.g. endometriosis, ovarian cysts ‐ simple > 3 cm or complex) ‐ Adenomyosis on ultrasonography (confirmed on MRI) ‐ Endometrial polyp (confirmed on saline infusion sonography) Setting: 28 sites in United States and 1 site in Canada |
|
| Interventions | Asoprisnil 5, 10 and 25 mg orally daily vs placebo Duration: 12 weeks starting on cycle day 1 to 4 Participants previously on hormone preparations underwent washout period (3 months to 1 year, depending on agent) |
|
| Outcomes | Outcomes assessed at baseline and at 2, 4, 8 and 12 weeks Efficacy ‐ Bleeding (daily diaries) ‣ Suppression of bleeding ‣ Amenorrhoea ‣ Haemoglobin concentrations and iron parameters ‐ Uterine/largest fibroid ultrasound volumes (formula for ellipsoid) ‐ Patient‐reported symptoms (unvalidated questionnaire) ‣ Bulk symptoms (bloating, pressure, urinary frequency) ‣ Heavy uterine bleeding ‣ Pelvic pain ‣ Dyspareunia ‣ Dysmenorrhoea ‐ Global efficacy question (only at 12 weeks) Safety ‐ Endometrial changes (only at 12 weeks). To report on non‐physiological changes, investigators developed a new classification system for endometrial biopsies that included 2 additional subcategories: non‐physiological secretory effect and secretory pattern mixed‐type ‣ Thickness (ultrasonography) ‣ Histology (pipelle endometrial biopsy) ‐ Hormone parameters ‣ Serum oestradiol, estrone, progesterone, cortisol, FHS, LH, sex hormone binding globulin, androstenedione, DHEAS, prolactin, TSH, T4 ‣ Serum and urine N‐telopeptide ‐ Lipids ‣ Total cholesterol, HDL, LDL ‐ Physical examination ‣ Weight, blood pressure, pulse, breast and pelvic exams ‐ Ultrasound assessment of ovarian cysts ‐ Papanicolaou test ‐ Electrocardiography ‐ Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer generated central randomization schedule was used to assign each patient in a 1:1:1:1 ratio to the four treatment groups” |
| Allocation concealment (selection bias) | Low risk | From study author‐provided protocol: "Fisher US packaged and labelled all study drug. The study drug was then shipped to Fisher UK who were responsible for supplying study drug to the clinical sites... When the randomization number was assigned for each subject, the duplicate label with the attached blinded label was removed from the subject kit and affixed to the appropriate CRF by study personnel. The identity of the contents of the blister card was not disclosed on the label" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “This study was…double‐blind…All asoprisnil and placebo tablets were identical in appearance” From study author‐provided protocol: "The investigator, clinical research coordinator, monitors, subject, and pathologist were blinded to each subject’s treatment group throughout the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patient‐reported outcomes were blinded: “Representatives of TAP collected the data, and statisticians at TAP conducted all statistical analyses” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for drop‐out given and plausible. Dropouts balanced between placebo (6.5%) and SPRM (7%) groups and unlikely “Nine of 129 randomized patients prematurely ... Among these nine patients, three terminated prematurely as a result of an adverse event" One drop‐out from AE in placebo group (3%) and 2 in SPRM group (2%). Drop‐outs for other reasons (less likely to affect outcomes) occurred in placebo group 1 (3%) and SPRM group 5 (5%) |
| Selective reporting (reporting bias) | Low risk | Protocol NCT00160459 reviewed |
| Other bias | Low risk | None identified |
Donnez 2012.
| Methods | Multi‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 3 groups in a 2:2:1 ratio (n = 242) ‐ 96 ulipristal acetate 5 mg: 5 withdrawn, 91 analysed ‐ 98 ulipristal acetate 10 mg: 6 withdrawn, 92 analysed ‐ 48 placebo: 1 withdrawn, 47 analysed Randomisation stratified according to ‐ Baseline haematocrit (≤ 28% or > 28%) ‐ Race (black or other) Baseline demographics similar in each group Inclusion criteria ‐ 18 to 50 years old ‐ PBAC score > 100 during day 1 to 8 of menstruation ‐ Fibroid‐related anaemia (Hgb ≤ 10.2 g per decilitre without macrocytosis) ‐ Fibroid uterus with size ≤ 16 weeks ‐ At least 1 fibroid ≥ 3 cm and no fibroid > 10 cm in diameter (ultrasonography) ‐ BMI 18 to 40 Exclusion criteria ‐ History of uterine surgery (except for cesarean section or cervical conisation) ‐ Endometrial ablation or uterine artery embolisation ‐ History of concurrent gynaecological cancer ‐ Current endometrial hyperplasia ‐ Hgb ≤ 6 g/L or any condition requiring immediate blood transfusion ‐ Known haemoglobinopathy ‐ Known severe coagulation disorder ‐ Large uterine polyp (> 2 cm) ‐ 1 or more ovarian cyst ≥ 4 cm in diameter (ultrasonography) ‐ Previous or current fibroid treatment with an SPRM or GnRH agonist ‐ Treatment with agents known to affect hepatic cytochrome CYP3A4 ‐ Progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments Setting: 38 centres in 6 European countries |
|
| Interventions | Ulipristal acetate 5 and 10 mg orally daily vs placebo Duration: 13 weeks starting on cycle day 1 to 4 ‐ After 13 weeks, participant could undergo surgery ‐ No further pharmacological treatment of fibroid administered All participants received 80 mg oral iron supplementation once daily |
|
| Outcomes | Outcomes assessed at baseline and at 13 weeks Co‐primary efficacy endpoints (baseline and 13 weeks) ‐ Reduction in bleeding (PBAC < 75) ‐ Total fibroid volume (MRI, sum of all fibroid volumes) Secondary endpoints ‐ Bleeding pattern (change in PBAC) ‐ Amenorrhoea ‐ Reduction in uterine and fibroid volume ‐ Change in Hgb, Hct and ferritin levels ‐ Pain (Short‐Form McGill Questionnaire and VAS) ‐ Discomfort questionnaire ‐ Adverse events (up to week 38) Safety endpoints ‐ Endometrial thickness (MRI at week 13, also at weeks 26 and 38 if no surgery) ‐ Endometrial biopsy (screening, week 13, week 38 if no hysterectomy or ablation). Used standard definition of SPRM‐associated endometrial changes (PAEC) described by Mutter 2008 ‐ Serum oestradiol, progesterone, corticotropin, thyrotropin, prolactin (screening and weeks 5, 9, 13 and 17) ‐ Haematological, coagulation, biochemical variables, lipids, glucose (all visits) ‐ FSH (baseline and 13 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned, in a 2:2:1 ratio, to receive 5 mg of ulipristal acetate per day, 10 mg of ulipristal acetate per day, or placebo. Randomization was stratified according to the hematocrit level at screening” From protocol: "a randomization list will be generated by a designated statistician from MDSL to be transmitted to the assigned clinical packaging organization for labelling" |
| Allocation concealment (selection bias) | Low risk | “The investigator assigned patients to a study group with the use of a Web‐integrated interactive voice‐response system” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Study materials and medication packaging were identical for all three groups” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant outcomes were blinded “Change in total fibroid volume as assessed by MRI…by a radiologist who was unaware of study group assignments” “Endometrial biopsy samples were assessed by three independent pathologists who were unaware of the study‐group assignments, the visit sequence” “Data were collected by an independent contract research organization and were handled and analyzed by an independent data management organization” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for drop‐out are given. See Figure 1. "Intention to treat" analysis performed. Missing data have been imputed using appropriate methods: "In general, missing values were imputed for the statistical analyses with the use of the last available post‐baseline value up to the time point of interest. We performed sensitivity analysis that included four patients (all in the 10mg UPA group), who did not have any efficacy data while receiving treatment, using baseline data carried forward" |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov NCT00755755 |
| Other bias | Low risk | None identified |
Donnez 2012a.
| Methods | Multi‐centre blinded non‐inferiority active‐comparator RCT | |
| Participants | Participants randomised to 1 of 3 groups in a 1:1:1 ratio (n = 307; 4 excluded): ‐ 98 ulipristal acetate (UA) 5 mg, 3 withdrawn, 95 analysed ‐ 104 ulipristal acetate 10 mg (UA), 4 withdrawn, 100 analysed ‐ 101 leuprolide acetate (LA) 3.75 IM, 6 withdrawn, 95 analysed Randomisation stratified according to ‐ Race (black or other) Baseline demographics similar in each group Inclusion criteria ‐ Premenopausal women 18 to 50 years old ‐ BMI 18 to 40 ‐ Heavy uterine bleeding caused by fibroids (PBAC > 100) ‐ At least 1 fibroid ≥ 3 cm and < 10 cm ‐ Uterine size ≤ 16 weeks ‐ Eligible for surgery Exclusion criteria ‐ History of uterine surgery (except for cesarean section or cervical conisation) ‐ Endometrial ablation or uterine artery embolisation ‐ History of concurrent gynaecological cancer ‐ Current endometrial hyperplasia ‐ Hgb ≤ 6 g/L or any condition requiring immediate blood transfusion ‐ Known haemoglobinopathy ‐ Known severe coagulation disorder ‐ Large uterine polyp (> 2 cm) ‐ 1 or more ovarian cyst ≥ 4 cm in diameter on ultrasound ‐ Previous or current fibroid treatment with an SPRM or GnRH agonist ‐ Treatment with agents known to affect hepatic cytochrome CYP3A4 ‐ Progestins, acetylsalicylic acid, mefenamic acid, anticoagulants, antifibrinolytic drugs or systemic glucocorticoid treatments Setting: 38 centres in 6 European countries |
|
| Interventions | Ulipristal acetate 5 mg or 10 mg orally daily vs leuprolide acetate 3.75 mg IM injection once monthly Duration: 13 weeks starting on cycle day 1 to 4 ‐ After 13 weeks, participants could undergo surgery ‐ No further pharmacological treatment of fibroids administered ‐ Iron supplementation left to the discretion of the treating physician |
|
| Outcomes | Outcomes assessed at baseline and at 13 weeks Primary efficacy endpoint ‐ Reduction in bleeding (PBAC < 75; non‐inferiority margin ‐20%) Secondary efficacy endpoints ‐ Bleeding pattern (PBAC scores) ‐ Amenorrhoea (PBAC score ≤ 2) ‐ Uterine/fibroid (3 largest) ultrasound volumes ‐ Global pain score (Short‐Form McGill Pain Questionnaire and VAS) ‐ Uterine Fibroid Symptom and Quality of Life (UFS‐QoL) questionnaire ‐ Haemoglobin, haematocrit and ferritin levels Co‐primary safety endpoints ‐ Serum oestradiol levels ‐ Moderate to severe hot flashes Secondary safety endpoints ‐ Adverse events ‐ Serum oestradiol, progesterone, corticotropin, thyrotropin, prolactin, lipids and glucose ‐ Endometrial thickness and ovaries on ultrasound ‐ Endometrial biopsy. Used the standard definition of SPRM‐associated endometrial changes (PAEC) provided by Mutter 2008 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomly assigned in a 1:1:1 ratio to receive either 5 mg or 10 mg of daily oral ulipristal acetate plus an intramuscular saline injection once monthly or a daily oral placebo plus an intramuscular injection of 3.75 mg of leuprolide acetate once monthly” From protocol: "a randomization list will be generated by a designated statistician from MDSL to be transmitted to the assigned clinical packaging organization for labelling" |
| Allocation concealment (selection bias) | Low risk | “A Web‐integrated voice‐response system transmitted the randomization to the packaging organization, which delivered the medications to the treatment centres” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study is described in the article as “double‐blind and double‐dummy” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant outcomes were blinded “Endometrial biopsy samples were assessed by three independent pathologists who were unaware of the study‐group assignments, the visit sequence” “Data were collected by an independent contract research organization and handled and analyzed by an independent data‐management organization” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups: "The modified intention‐to‐treat analyses did not include five patients: two patients (one in each ulipristal‐acetate group) who never received the study drug and were not followed and three patients (one who was assigned to receive 10 mg of ulipristal acetate and two in the leuprolide acetate group) with missing efficacy data after baseline" Appropriate imputation of missing data: "Missing data for week 13 were imputed with the use of data for the last available 28 days during treatment. A sensitivity analysis in the modified intention‐to‐treat population (including three patients without any on treatment efficacy data) was performed with the use of baseline data carried forward" |
| Selective reporting (reporting bias) | Low risk | Protocol available on clinicaltrials.gov NCT00740831 |
| Other bias | Low risk | None identified |
Engman 2009.
| Methods | Single‐center blinded placebo (vitamin B) RCT | |
| Participants | Participants randomised to 1 of 2 groups in a 1:1 ratio (n = 30) ‐ 14 mifepristone, 14 analysed ‐ 16 placebo (vitamin B), 2 withdrawn, 14 analysed Baseline demographics similar in each group (except for free testosterone greater in mifepristone group) Inclusion criteria ‐ Healthy, non‐pregnant women ‐ Fibroid‐related problems indicating surgical intervention ‐ No steroid hormones within 3 months of recruitment Exclusion criteria ‐ Breast cancer or other malignancy ‐ Bleeding not controlled by tranexamic acid and iron ‐ Abnormal mammogram and breast biopsy at baseline ‐ Adnexal abnormality ‐ Suspicion of leiomyosarcoma ‐ Abnormal FSH or LH or any other hormonal dysfunction of clinical significance ‐ Labs: suspicion of blood, liver or renal dysfunction ‐ Abnormal Papanicolaou test at screening ‐ Contraindication to mifepristone Setting: Stockholm, Sweden |
|
| Interventions | Mifepristone 50 mg vs vitamin B (placebo) orally every other day Duration: 3 months starting on cycle day 1 until surgery |
|
| Outcomes | Outcomes assessed at baseline and monthly for 3 months ‐ Uterine/fibroid (largest and total) ultrasound volumes (formula for an ellipsoid) ‐ Uterine/fibroid blood flow (Doppler ultrasound of PI and peak flow) ‐ Uterine bleeding ‣ Number of bleeding days ‣ Amenorrhoea Safety ‐ Blood work (every 4 weeks) ‣ Haematological, renal and liver ‣ Hormone profile ‐ Endometrial biopsy (baseline and study end during surgery). To describe non‐physiological changes, they devised their own semiquantitative assessment of glandular architecture ‐ Mammogram (baseline) ‐ Breast biopsy (baseline and study end) Fibroid symptoms (Likert scale 0 to 4) ‐ Bleeding ‐ Pelvic pain or pressure ‐ Bladder pressure ‐ Micturition problems ‐ Low back pain ‐ Proctodynia ‐ Dyspareunia ‐ Flushes ‐ Headache ‐ Nausea ‐ Vomiting ‐ Diarrhoea ‐ Mood fluctuations ‐ Libido ‐ Weakness ‐ Fatigue Medication side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation by third party |
| Allocation concealment (selection bias) | Low risk | Packed and coded by third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical pills prepared and distributed by third party |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ultrasound blinding not specifically mentioned, but "Patients and staff were blinded to treatment groups", as were assessors of endometrial pathology |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two in placebo arm excluded |
| Selective reporting (reporting bias) | Low risk | Protocol available and reviewed on clinicaltrials.gov NCT00579475 |
| Other bias | Low risk | None identified |
Esteve 2013.
| Methods | Single‐center blinded placebo RCT | |
| Participants | Participants randomised to 1 of 2 groups in a 1:1 ratio (n = 124) ‐ 62 mifepristone 5 mg, 4 withdrawn, 58 analysed ‐ 62 placebo, 15 withdrawn, 47 analysed Baseline demographics similar in each group except for haemoglobin (higher in placebo group) Inclusion criteria ‐ 18 years of age or older ‐ Symptomatic uterine fibroids (ultrasound) ‐ Indication for surgery (hysterectomy or myomectomy) ‐ Agreement to record on a monthly basis all vaginal bleeding episodes and mifepristone side effects and to have ultrasound examinations at every evaluation session Exclusion criteria ‐ Pregnancy or desire to get pregnant ‐ Breastfeeding ‐ Hormonal contraception or any hormonal therapy in the past 3 months ‐ Signs or symptoms of pelvic inflammation ‐ Adnexal tumours ‐ Suspicion or diagnosis of cervical–uterine or ovarian cancer ‐ Signs or symptoms of mental illness ‐ Unexplained genital bleeding ‐ Anaemia due to sickle cell disease ‐ Suffering from a serious illness ‐ Antiprogesterone contraindications Setting: Havana, Cuba |
|
| Interventions | Mifepristone 5 mg orally daily vs placebo Duration: 3 months |
|
| Outcomes | Outcomes assessed at baseline and at 3 months Efficacy outcomes ‐ Uterine/largest fibroid ultrasound volumes (formula for ellipsoid) ‐ Fibroid symptoms (self‐reported VAS) ‣ Pelvic pain ‣ Lumbar pain ‣ Rectal pain ‣ Pelvic pressure ‣ Urinary symptoms ‣ Dyspareunia ‣ Hypermenorrhoea ‣ Metrorrhagia ‐ Uterine Fibroid Symptom and Quality of Life (UFS‐QoL) Safety outcomes ‐ Endometrial thickness ‐ Mifepristone side effects (VAS) ‣ Amenorrhoea ‣ Hot flashes ‣ Nausea ‣ Vomiting ‣ Dizziness ‣ Fatigue/tiredness ‣ Headache ‐ Endometrial biopsy ‐ At baseline and 45 days during treatment (only if any of below criteria) and everyone post treatment. Used standard definition of PAEC provided by Mutter 2008 ‣ Endometrial thickness > 8 mm ‣ Vaginal bleeding > 10 days ‣ Vaginal bleeding during 3 weeks before menses ‣ Copious vaginal bleeding ‐ Serum biochemistry ‣ Haemoglobin ‣ Liver function tests (AST, ALT) ‣ Hormones (oestradiol, testosterone, LH, FHS, prolactin) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods lacked detail regarding randomisation process – unsure how randomisation was achieved |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes prepared by a third party "Staff not directly involved in the study prepared sealed opaque envelopes, each envelope containing a card… Once the subject [met] inclusion and exclusion criteria and had signed the informed consent, the envelope corresponding to the subject’s numbered incorporation into the study was opened and she was included in the treatment group indicated on the card contained in the envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “This [tablet] code was revealed once the initial data processing was completed” “Calibrations [from ultrasonography] taken at the end of each treatment visit were performed by sonographers who were ignorant of previous measurements, knowing only the localization of the myoma to be measured” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15 vs 4 drop‐outs in placebo vs mifepristone. Reasons for drop‐outs included, but a disproportionate number in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not found. Study authors emailed for protocol |
| Other bias | Low risk | None identified |
Fiscella 2006.
| Methods | Single‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 2 groups in a 1:1 ratio (n = 42) ‐ 22 mifepristone 5 mg, 2 withdrawn, 20 analysed ‐ 20 placebo, 3 withdrawn, 17 analysed Randomisation stratified according to ‐ Uterine Fibroid Symptom Quality of Life (UFS‐QoL) symptom severity (> 64 vs ≤ 63) Baseline demographics similar in each group except for ‐ BMI and baseline uterine volume (higher in treatment group) Inclusion criteria ‐ Age ≥ 18 years ‐ Premenopausal ‐ At least moderately severe fibroid‐related symptoms (> 39 on UFS‐QoL symptom severity subscale) ‐ Total uterine volume ≥ 160 mL ‐ At least 1 fibroid ≥ 2.5 cm ‐ Had not used short‐acting hormone analogues or other long‐acting hormone medications in past 6 months Exclusion criteria ‐ Pregnant or intending pregnancy during next 6 months ‐ Major medical morbidity ‐ Severe anaemia ‐ Active mental illness ‐ Elevated liver enzymes ‐ Substance abuse Setting: New York, USA |
|
| Interventions | Mifepristone 5 mg orally daily vs placebo Duration: 26 weeks ‐ Participants agreed to use barrier contraception and not to use hormonal or surgical treatments for fibroids during the study ‐ Participants were allowed to use analgesics and were asked to record use |
|
| Outcomes | Outcomes assessed at baseline and at 1, 3 and 6 months Primary outcome ‐ Fibroid‐specific overall QoL (UFS‐QoL) Secondary outcomes ‐ Global health status (Medical Outcomes 36‐item Short Form (SF‐36) survey) ‐ Global pain (McGill Pain Questionnaire) assessed monthly ‐ Bleeding (daily menstrual logs and pictorial charts) ‣ Amenorrhoea ‣ Mean monthly blood loss index ‣ Haemoglobin ‐ Uterine/fibroid (5 largest summed) ultrasound volumes (measured in 3 planes) ‐ Fibroid symptoms (5‐point Likert scale) assessed monthly ‣ Pelvic pain ‣ Pelvic pressure ‣ Bladder pressure ‣ Urinary frequency ‣ Low back pain ‣ Rectal pain ‣ Pain with intercourse ‐ Adverse effects (5‐point Likert scale) assessed monthly ‐ Liver function tests ‐ Endometrial biopsy assessed at baseline and at 6 months. Did not report on PAEC |
|
| Notes | 19/20 women in the treatment group correctly guessed that they had been receiving mifepristone because of cessation of bleeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Using random numbers generated with SAS 9, women were randomly assigned in blocks of four, stratified by Uterine Fibroid Symptom Quality of Life” |
| Allocation concealment (selection bias) | Low risk | “Study assignments were placed in opaque sealed envelopes that were opened by the study pharmacist once the participant was fully qualified” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The study pharmacist prepared mifepristone 5 mg and placebo in capsules that were identical in appearance and weight” “None of the study personnel, with the exception of the pharmacist, were aware of treatment assignments” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "None of the study personnel, with the exception of the pharmacist, were aware of treatment assignments" However, blinding of outcome assessors was not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data relatively balanced in numbers across intervention groups and unlikely to be related to the true outcome “3 [women] completed the intake measures and were randomised (two to treatment and one to placebo) but then declined to participate, and 39 began the trial and participated for at least one month” “Two other women (from placebo group) dropped out during the course of the study. Neither reported leaving due to adverse effects” |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. Protocol available at clinicaltrials.gov NCT00133705 |
| Other bias | Low risk | None identified |
Levens 2008.
| Methods | Single‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 3 groups in a 1:1:1 ratio (n = 22) ‐ 8 ulipristal acetate 10 mg, 2 withdrawn, 6 analysed ‐ 6 ulipristal acetate 20 mg, 0 withdrawn, 6 analysed ‐ 8 placebo, 2 withdrawn, 6 analysed Baseline demographics similar in each group Inclusion criteria ‐ Age 33 to 50 years ‐ Regular menses ‐ At least 1 fibroid > 2 cm diameter (MRI) ‐ Healthy ‐ Non‐pregnant ‐ Desired hysterectomy ‐ Hgb > 10 g/dL ‐ Ovulatory cycles every 24 to 35 days ‐ Current use of non‐hormonal contraception ‐ BMI < 33 kg/m2 Exclusion criteria ‐ Inability to complete study requirements ‐ Prior uterine artery embolisation ‐ Menopausal status (FSH > 20 milli‐international units/mL) ‐ Cervical dysplasia ‐ Adnexal mass ‐ Genetic cause or rapid growth of fibroid (doubled size within 6 months) ‐ Unexplained vaginal bleeding ‐ Use of glucocorticoids, progestins or agents that alter ovarian or hepatic function Setting: USA |
|
| Interventions | Ulipristal acetate 10 mg or 20 mg orally daily vs placebo Duration ‐ 3 menstrual cycles or 90 to 102 days if amenorrhoeic ‐ Initiated on cycle day 1 to 2 ‐ Participants admitted for hysterectomy after LH surge in third treatment cycle or follicular phase of fourth cycle; if anovulatory, then at 90 to 102 days of treatment ‐ Study drug discontinued immediately before surgery |
|
| Outcomes | Outcomes assessed at baseline and at end of treatment Primary outcome ‐ Total fibroid MRI volume (formula for ellipsoid) Secondary outcomes ‐ Alteration in menstrual function (PBAC scores) ‐ Change in Hgb and Hct ‐ Treatment‐dependent inhibition of ovulation ‐ Change in fibroid‐related symptoms ‣ General QoL (SF‐36 version 2) ‣ Fibroid‐specific QoL (UFS‐QoL) Safety ‣ Adverse events ‣ CBC, hepatic panel, electrolytes, BUN, creatinine and glucose (every month) ‣ Urine 24‐hour specimens for cortisol and creatinine (every month) ‣ Serum ACTH, cortisol, FSH, LH, P4 and E2 (every 2 to 4 weeks) ‣ Endometrial hyperplasia (biopsy at time of surgery plus surgical specimen). Did not report on PAEC |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Pharmaceutical Development Service at the National Institutes of Health Clinical Center randomly assigned subjects to computer‐generated blocks of six to receive CDB‐2914 at a dose of 10 or 20 or placebo” |
| Allocation concealment (selection bias) | Low risk | “Allocation concealment was assured by this Service” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “To blind both patients and health‐care providers, gelatin capsules containing either CDB‐2914 in doses of 10 mg and 20 mg or inert material (PLC) were prepared” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant outcomes were blinded “After both baseline and presurgical MR imaging was performed, a senior radiologist, unaware of treatment allocation, identified and mapped the leiomyomata seen at baseline assessment, noting the location and 3‐D dimensions” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few drop‐outs and reasons given Placebo: 1 unable to follow up, 1 had pain 10 mg CDB‐2914 UA: 2 declined surgery upon treatment completion 20 mg CDB‐2914: none withdrew |
| Selective reporting (reporting bias) | Low risk | Protocol available and reviewed on clinicaltrials.gov NCT00290251 |
| Other bias | Low risk | None identified |
Liu 2015.
| Methods | Single‐centre blinded RCT | |
| Participants | Participants randomised to 1 of 2 groups (n = 132) ‐ Mifepristone 10 mg ‐ Placebo Inclusion criteria ‐ Symptomatic fibroids No additional details available Setting: Beijing, China |
|
| Interventions | Mifepristone 10 mg orally daily vs placebo Duration: 3 months |
|
| Outcomes | Outcomes assessed at baseline and at 3 months ‐ Fibroid volume ‐ Hgb ‐ Liver function tests ‐ FSH |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" ‐ no details. Attempts to contact study authors unsuccessful |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Attempts to contact study authors unsuccessful |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" ‐ no further details. Attempts to contact study authors unsuccessful |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" ‐ no further details. Attempts to contact study authors unsuccessful |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. Attempts to contact study authors unsuccessful |
| Selective reporting (reporting bias) | Unclear risk | No details. Attempts to contact study authors unsuccessful |
| Other bias | Unclear risk | Data in form of conference proceedings. No published follow‐up data. Protocol not found on clinicaltrials.gov |
Nieman 2011.
| Methods | Single‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 3 groups in a 1:1:1 ratio (n = 42) ‐ 14 ulipristal acetate 10 mg, 1 withdrawn, 13 analysed ‐ 14 ulipristal acetate 20 mg, 1 withdrawn, 13 analysed ‐ 14 placebo, 2 withdrawn, 12 analysed Baseline demographics similar in each group Inclusion criteria ‐ Symptomatic fibroids > 2 cm in diameter (MRI) ‐ Age 25 to 50 years ‐ Ovulatory menstrual cycles of 24 to 35 days ‐ Haemoglobin > 10 g/dL ‐ Creatinine < 1.3 mg/dL ‐ Liver function tests within 130% of upper normal range ‐ BMI < 35 kg/m2 Exclusion criteria ‐ Use of glucocorticoids or megestrol within 1 year ‐ Cervical dysplasia ‐ Adnexal mass ‐ Previous malignancy ‐ Inability to complete study requirements ‐ Serum FSH > 20 U/L ‐ Anovulation ‐ Rapidly growing fibroid ‐ Unexplained vaginal bleeding ‐ Pregnancy ‐ Lactation ‐ Use of hormonal compounds within 8 weeks of study start ‐ Therapy affecting ovarian or hepatic function Setting: USA |
|
| Interventions | Ulipristal acetate 10 mg or 20 mg orally daily vs placebo Duration ‐ 3 menstrual cycles or 90 to 102 days if amenorrhoeic ‐ Initiated on cycle day 1 to 2 ‐ After initial treatment, women could elect hysterectomy, myomectomy or a second 3‐month treatment with ulipristal acetate 10 mg or 20 mg (part 2) ‐ Only part 1 of study included in this review |
|
| Outcomes | Outcomes assessed at baseline and at end of treatment Primary outcome ‐ Fibroid MRI volume (formula for an ellipsoid) Secondary outcomes ‐ Bleeding (logs of daily vaginal bleeding) ‣ Amenorrhoea ‣ Change in Hgb and Hct ‐ Treatment‐dependent inhibition of ovulation ‐ QoL outcomes ‣ General QoL (SF‐36 version 2) ‣ Fibroid‐specific QoL (UFS‐QoL) ‐ Safety ‣ Adverse events ‣ Serum FSH, ACTH, cortisol, prolactin, LH, P4 and E2 (every 2 weeks) ‣ CBC, LFTs and acute care panel (every 4 weeks) ‣ Urine cortisol and creatinine excretion (days 20 to 30, 50 to 60 and 80 to 90) ‣ Endometrial hyperplasia (biopsy at treatment end). Used standard definition of PAEC provided by Mutter 2008 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “randomised participants to receive CDB‐2914 10 mg (CDB10) or 20 mg (CDB20), or a placebo (PLC), using computer‐generated blocks of six” |
| Allocation concealment (selection bias) | Low risk | Central randomisation and concealment but details unclear “The Pharmaceutical Development Service assured allocation concealment” However, this study used the same treatment design and allocation concealment service as the Levens study; details from the Levens study have been extrapolated here to grade this as low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Laboratoire HRA‐Pharma provided 10‐mg CDB‐2914 tablets and a look alike inert placebo.Women received two bottles and were instructed to swallow one tablet from each bottle every morning before eating” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was MRI measurements “PDS assured allocation concealment”, and this body seems to be at arms length from study “Women with paired MRI results were included in this ITT analysis even if they did not take all study medications” Participants were definitely blinded, so patient‐reported outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced with plausible reasons. 1 drop‐out from each group after allocation “The baseline characteristics of these women were similar to study completers. Two women withdrew from treatment 2 because of inconvenience” Reasons for drop‐out were reported early in treatment (within 2 weeks of starting, 1 was on day 2) ‐ not lack of efficacy |
| Selective reporting (reporting bias) | Low risk | Study protocol available (NCT00290251) on clinical trials.gov; report includes all expected study outcomes |
| Other bias | Low risk | None identified |
Prasad 2013.
| Methods | Single‐centre placebo RCT | |
| Participants | Participants randomised to 1 of 2 groups (n = 62) ‐ Mifepristone 10 mg ‐ Placebo Inclusion criteria ‐ Symptomatic fibroids No additional details available Setting: Delhi, India |
|
| Interventions | Mifepristone 10 mg orally daily vs placebo Duration: 3 months |
|
| Outcomes | Outcomes assessed at baseline and at 3 months Primary outcome ‐ Fibroid volume Secondary outcomes ‐ Endometrial biopsy. Reported on 'cystic glandular hyperplasia' but pathology criteria not available ‐ Hormone profile ‐ Fibroid symptoms ‣ Bleeding/amenorrhoea ‣ Dysmenorrhoea |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized" ‐ no details. Attempts to contact study authors unsuccessful |
| Allocation concealment (selection bias) | Unclear risk | Not stated. Attempts to contact study authors unsuccessful |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Single blind" ‐ no further details. Attempts to contact study authors unsuccessful |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Single blind" ‐ no further details. Attempts to contact study authors unsuccessful |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details. Attempts to contact study authors unsuccessful |
| Selective reporting (reporting bias) | Unclear risk | No details. Attempts to contact study authors unsuccessful |
| Other bias | Unclear risk | Data in form of conference proceedings. No published follow‐up data. Protocol not found on clinicaltrials.gov |
Reinsch 1994.
| Methods | Single‐centre randomised prospective active‐comparator trial | |
| Participants | Participants randomised to 1 of 2 groups (n = 14) ‐ 8 mifepristone 25 mg, 8 analysed ‐ 6 leuprolide acetate 3.75 mg, 6 analysed Baseline demographics similar in each group Inclusion criteria ‐ Women with uterine fibroids on ultrasound ‐ Scheduled to have myomectomy or hysterectomy Setting: California, USA |
|
| Interventions | Mifepristone 25 mg orally daily vs leuprolide acetate 3.75 mg IM monthly Duration ‐ 3 months ‐ Initiated in early follicular phase ‐ Study drug discontinued immediately before surgery |
|
| Outcomes | Outcomes assessed at baseline and at 3 months Primary outcomes ‐ Uterine ultrasound volume (formula for a sphere) ‐ Uterine artery blood flow (RI on Doppler ultrasound) Secocondary outcomes (for mifepristone group only) ‐ Side effects ‣ Nausea ‣ Hot flushes ‣ Night sweats ‣ Fatigue ‣ Vaginal dryness ‐ 24‐Hour urine‐free cortisol, creatinine, BUN, AST, ALT and LDH (baseline and monthly) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation are not provided: “They were randomly assigned to group A or group B” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | High risk | No protocol provided. Chose not to measure/report fibroid volume |
| Other bias | Low risk | None identified |
Wilkens 2008.
| Methods | Multi‐centre blinded placebo RCT | |
| Participants | Participants randomised to 1 of 3 groups in a 1:1:1 ratio (n = 33) ‐ 12 asoprisnil 10 mg, 12 analysed ‐ 11 asoprisnil 25 mg, 11 analysed ‐ 10 placebo, 10 analysed Baseline demographics similar in each group Inclusion criteria ‐ Premenopausal women over 18 years of age ‐ Good general health ‐ Menstrual cycle 17 to 42 days ‐ Symptoms related to overall fibroid size, pressure and/or heavy uterine bleeding ‐ Scheduled for hysterectomy ‐ At least 1 intramural non‐pedunculated, submucosal or subserosal fibroid (diameter ≥ 2 cm) or multiple smaller fibroids (volume ≥ 200 cm3) confirmed by ultrasound ‐ Washout period of 2 to 12 months for hormonal medications ‐ Agreement to use double barrier contraception method ‐ Normal endometrial biopsy within 3 months of study commencement Exclusion criteria ‐ Pregnancy ‐ Abnormal Papanicolaou test NSAIDs and tranexamic acid were allowed during screening and treatment Setting: 4 centres in the UK |
|
| Interventions | Asinoprisnil 10 mg or 25 mg orally daily vs placebo Duration ‐ 12 weeks until hysterectomy ‐ Initiated before cycle day 5 ‐ Hysterectomy performed within 24 hours of final dose |
|
| Outcomes | Outcomes assessed at baseline and at 12 months Primary outcome ‐ Uterine artery blood flow (Doppler ultrasound) ‣ Resistance index (RI) ‣ Pulsatility index (PI) Secondary outcomes ‐ Uterine/fibroid ultrasound volumes (formula for ellipsoid) ‣ Uterine ‣ Largest fibroid ‐ Uterine bleeding using menstrual pictogram (MP) ‣ Blood loss ‣ Days with bleeding ‣ Amenorrhoea ‐ Fibroid symptoms ‐ Uterine fibroid symptom quality of life (USF‐QoL) questionnaire ‐ Ovarian activity (urinary pregnanediol glucuronide (PdG) and estrone glucuronide (E1G) twice weekly) ‣ Evidence of luteal activity (PdG) ‣ Ovarian follicular activity (E1G) ‐ Adverse events Endometrial biopsy at baseline and assessment of endometrium from pathology specimens after treatment. To describe non‐physiological endometrial changes, investigators used their own semiquantitative assessment of glandular architecture |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From Williams 2007: "women were sequentially assigned subject numbers in ascending numerical order that encoded the assignment of the woman, via a randomization schedule, to one of the three treatment arms of the study. Subjects were randomised to one of three parallel dose groups in a 1:1:1 ratio to receive daily doses of asoprisnil 10, 25 mg or placebo" |
| Allocation concealment (selection bias) | Low risk | From Williams 2007: “Asoprisnil or placebo tablets were supplied in blister cards of identical appearance, supplied to the site packaged in sealed kits” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “double‐blind” “Subjects…and all study personnel were blinded to treatment groups” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | From study author‐provided protocol: "The investigator, clinical research coordinator (CRC), monitor, subject, and TAP will remain blinded to each subject’s treatment assignment throughout the course of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Protocol obtained by emailing study authors |
| Other bias | Low risk | None identified |
Abbreviations
ACTH: adrenocorticotropic hormone
AE: adverse event
ALT: aspartate aminotransferase
AST: alanine aminotransferase
BMI: body mass index
BUN: blood urea nitrogen
CBC: complete blood count
CDB: an SPRM
cm: centimetres
CRF: case report form
DHEAS: dehydroepiandrosterone
E1G: estrone glucuronide
E2: estradiol
FSH: follicle‐stimulating hormone
g/dL: grams per decilitre
g/L: grams per litre
GnRH: gonadotropin‐releasing hormone
Hct: haematocrit
HDL: high‐density lipoprotein
HgB: haemoglobin
IM: intramuscular
LA: leuprolide acetate
LDH: lactate dehydrogenase
LDL: low‐density lipoprotein
LFT: liver function test
LH: luteinising hormone
MDSL: Market Data Management Services
mg: milligrams
MP: menstrual pictogram
MRI: magnetic resonance imaging
NSAIDs: non‐steroidal anti‐inflammatory drugs
P4: progesterone
PAEC: progesterone receptor modulator‐associated endometrial changes
PBAC: pictorial blood loss assessment chart
PdG: pregnanediol glucuronide
PI: pulsatility index
QoL: quality of life
RCT: randomised controlled trial
RI: resistance index
SF‐36: Short Form‐36
SPRM: selective progesterone receptor modulator
T4: thyroxine
TAP: TAP pharmaceutical products
TSH: thyroid‐stimulating hormone
UA: ulipristal acetate
UFS‐QoL: Uterine Fibroid Symptom and Quality of Life questionnaire
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carbonell 2008 | Compared different doses, no placebo group |
| Carbonell 2012 | Compared different doses, no placebo group |
| Carbonell 2013 | Compared different doses, no placebo group |
| Carbonell 2013a | Compared different doses, no placebo group |
| Chwalisz 2003 | Compared different doses, no placebo group |
| Donnez 2014 | Compared different doses, no placebo group |
| Eisinger 2003 | Compared different doses, no placebo group |
| Eisinger 2005 | Compared different doses, no placebo group |
| Esteve 2012 | Compared different doses, no placebo group |
| Kulshrestha 2013 | Compared different doses, no placebo group |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00044876.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids |
| Interventions | Ulipristal acetate 10 mg vs 25 mg vs placebo. |
| Outcomes | Unclear |
| Notes | Protocol NCT00044876 in clinicaltrials.gov of ulipristal acetate 10 mg vs 25 mg vs placebo. No results published in database nor in published literature. States trial was completed |
NCT00152269.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with uterine fibroids |
| Interventions | Asoprisnil 10 mg, 25 mg vs placebo |
| Outcomes | Primary outcome: Percent of participants who demonstrate a clinically meaningful improvement in bleeding and do not have surgical/invasive intervention |
| Notes | Protocol NCT00152269 in clinicaltrials.gov of asoprisnil 10 mg, 25 mg vs placebo. No results published in database nor in published literature. States trial was completed. Principal study authors communicated to us that presentation of results is expected in 2016 |
NCT00683917.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with symptomatic fibroids |
| Interventions | Telapristone acetate 25 mg vs 50 mg vs Lupron Depot |
| Outcomes | Primary outcome measure is pharmacokinetic characteristics of 25 mg and 50 mg Proellex. Transformed Uterine Fibroid Symptom Quality of Life Scale (UFS‐QoL) severity score, uterine fibroid size as measured by magnetic resonance imaging (MRI), relapse of symptoms as recorded on participant diary cards and persistence of effect as measured by UFS‐QoL |
| Notes | Protocol NCT00683917 in clinicaltrials.gov of telapristone acetate 25 mg vs 50 mg vs Lupron Depot. No results published in database nor in published literature. States trial was terminated |
NCT00702702.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids and anaemia |
| Interventions | Telapristone acetate 25 mg vs 50 mg vs placebo |
| Outcomes | Change in haemoglobin vs placebo |
| Notes | Protocol NCT00702702 in clinicaltrials.gov of telapristone acetate 25 mg vs 50 mg vs placebo. No results published in database nor in published literature. States trial was terminated |
NCT00735553.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with uterine fibroids and heavy menstrual bleeding |
| Interventions | Telapristone acetate 25 mg vs 50 mg vs placebo |
| Outcomes | To determine the efficacy of 50 mg Proellex vs placebo in the treatment of participants with symptomatic uterine fibroids from baseline to month 4 as determined by scoring changes in the pictorial blood loss assessment chart (PBAC) |
| Notes | Protocol NCT00735553 in clinicaltrials.gov of telapristone acetate 25 mg vs 50 mg vs placebo. No results in database nor in published literature. States trial was terminated |
NCT00785356.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids and anaemia |
| Interventions | Telapristone acetate 25 mg vs 50 mg vs placebo |
| Outcomes | Comparison between 50 mg Proellex dose level and placebo in the change in haemoglobin from baseline to 3 months |
| Notes | Protocol NCT00785356 in clinicaltrials.gov of telapristone acetate 25 mg vs 50 mg vs placebo. No results in database nor in published literature. States trial was terminated |
NCT00853567.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids and heavy menstrual bleeding |
| Interventions | Telapristone acetate 25 mg vs 50 mg vs placebo |
| Outcomes | To determine the efficacy of 50 mg Proellex vs placebo in the treatment of participants with symptomatic uterine fibroids from baseline to month 4 as determined by scoring changes in the pictorial blood loss assessment chart (PBAC) |
| Notes | Protocol NCT00853567 in clinicaltrials.gov of telapristone acetate 25 mg vs 50 mg vs placebo. No results in database nor in published literature. States trial was terminated |
NCT00882258.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids |
| Interventions | Telapristone acetate 12.5 mg vs 25 mg vs placebo |
| Outcomes | Unclear |
| Notes | Protocol NCT00882258 in clinicaltrials.gov of telapristone acetate 12.5 mg vs 25 mg vs placebo. No results in database nor in published literature. States trial was completed |
NCT01069094.
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with fibroids |
| Interventions | Telapristone acetate (12.5 mg, 25 mg and 50 mg) vs Lupron depot vs placebo |
| Outcomes | Assess the safety of Progenta when administered in premenopausal women with symptomatic leiomyomata |
| Notes | Protocol NCT01069094 in clinicaltrials.gov of telapristone acetate vs Lupron depot vs placebo. No results in database nor in published literature. States trial was completed |
NCT01816815.
| Methods | Randomised phase 1 study |
| Participants | Healthy females |
| Interventions | Vilaprisan (0.1 mg, 0.5 mg, 1 mg, 2 mg, 5 mg) vs placebo |
| Outcomes | Non‐bleeding rate (i.e. women without bleeding from treatment day 9 until the end of treatment) |
| Notes | Protocol NCT01816815 in clinicaltrials.gov of Vilaprisan vs placebo. No results in database nor in published literature. States trial was completed |
Characteristics of ongoing studies [ordered by study ID]
NCT02131662.
| Trial name or title | Bay1002670, Fibroids, Safety and Efficacy EU, US, Can, Jap (ASTEROID 1) |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with heavy menstrual bleeding and fibroid ≥ than 3 cm |
| Interventions | Vilaprisan 0.5 mg, 1 mg, 2 mg, 4 mg vs placebo |
| Outcomes | Amenorrhoea at 3 months |
| Starting date | May 2014 |
| Contact information | Bayer |
| Notes |
NCT02147158.
| Trial name or title | A Study of the Safety and Efficacy of Intermittent Ulipristal Treatment of Abnormal Uterine Bleeding Associated With Leiomyomas |
| Methods | Randomised controlled trial |
| Participants | Cyclic abnormal uterine bleeding (heavy or prolonged) Menstrual blood loss (MBL) ≥ 80 mL in the first 8 days of menses Minimum of 1 discrete leiomyoma observable by transvaginal ultrasound |
| Interventions | Various permutations of ulipristal acetate 5 mg or 10 mg or placebo |
| Outcomes | Absence of bleeding (time frame: 12 weeks) Time to absence of bleeding (time frame: 12 weeks) |
| Starting date | January 2014 |
| Contact information | Watson Pharmaceuticals |
| Notes |
NCT02147197.
| Trial name or title | A Study of the Efficacy and Safety of a Single Ulipristal Treatment Course for the Treatment of Abnormal Uterine Bleeding Associated With Leiomyomas |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women, 18 to 50 years of age, inclusive Experienced cyclic abnormal uterine bleeding (heavy or prolonged) Menstrual blood loss (MBL) ≥ 80 mL as measured once by the alkaline hematin method during screening over first 8 days of menses Minimum of 1 discrete leiomyoma observable by transvaginal ultrasound at screening assessment |
| Interventions | Ulipristal acetate 5 mg vs 10 mg vs placebo |
| Outcomes | Absence of bleeding (time frame: 12 weeks) Time to absence of bleeding (time frame: 12 weeks) |
| Starting date | April 2014 |
| Contact information | Watson Pharmaceuticals |
| Notes | Expected completion date March 2016 |
NCT02288130.
| Trial name or title | Ulipristal vs. GnRHa Prior to Laparoscopic Myomectomy (MYOMEX) |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women eligible for laparoscopic myomectomy with fibroid size 5 to 12 cm |
| Interventions | Leuprolide 11.25 mg IM × 1 vs ulipristal acetate 5 mg daily × 3 months |
| Outcomes | Blood loss during surgery |
| Starting date | December 2014 |
| Contact information | I. de Milliano, MD, VU University Medical Center |
| Notes |
NCT02323646.
| Trial name or title | A Phase 2 Study to Evaluate the Safety and Efficacy of Proellex (Telapristone Acetate) Administered Vaginally in the Treatment of Uterine Fibroids |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with symptomatic uterine fibroids |
| Interventions | Two vaginal doses of Proellex (6 mg vs 12 mg vs placebo) administered for up to 2 courses of treatment (18 weeks each), each separated by an off‐drug interval (ODI) |
| Outcomes | Percentage of participants who become amenorrhoeic after 1 course of treatment (6 months) |
| Starting date | December 2014 |
| Contact information | Repros Therapeutics Inc |
| Notes |
NCT02357563.
| Trial name or title | Ulipristal Acetate Versus GnRH Analogue and Myometrial Preservation |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with G2 submucosal leiomyoma < 3 cm, symptoms of menometrorrhagia, menstrual disorder, infertility, pelvic pain |
| Interventions | Ulipristal acetate 5 mg/d for 2 courses of 3 months each vs IM on leuprolide acetate 11.25 in luteal phase repeated 3 months later |
| Outcomes | Proportion of restored uterine cavity 1 year after enrolment |
| Starting date | February 2015 |
| Contact information | Fulvio Zullo, University Magna Graecia |
| Notes |
NCT02361879.
| Trial name or title | Ulipristal Acetate Versus GnRH Analogue Treatment Before Hysteroscopic Resection of Uterine Leiomyoma |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with submucosal leiomyoma, symptoms of menometrorrhagia, menstrual disorder, infertility, pelvic pain |
| Interventions | 5 mg/d of oral ulipristal acetate for 3 months vs IM injection of leuprolide acetate 11.25 mg in luteal phase |
| Outcomes | Uterine bleeding assessed by pictorial blood loss assessment chart (PBAC) |
| Starting date | February 2015 |
| Contact information | Fulvio Zullo, University Magna Graecia |
| Notes |
NCT02361905.
| Trial name or title | Ulipristal Acetate for the Preoperative Management of Hypoechoic Cellular Leiomyomas |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with hypoechoic uterine leiomyoma (echogenicity < 3), intramural leiomyomas with ultrasonographic size < 20 cm but > 4 cm, indication to surgery (symptoms of menometrorrhagia, menstrual disorder, infertility, pelvic pain or pelvic pressure) |
| Interventions | 5 mg/d of oral ulipristal acetate for 3 months vs IM injection of leuprolide acetate 11.25 mg in luteal phase |
| Outcomes | Operative time (minutes) (time frame: at time of skin closure at the end of the myomectomy) |
| Starting date | February 2015 |
| Contact information | Fulvio Zullo, University Magna Graecia |
| Notes |
NCT02425878.
| Trial name or title | Ulipristal Acetate 10 mg and Assisted Reproduction |
| Methods | Randomised controlled trial |
| Participants | Patients > 18 and < 50 years of age Patients who undergo first/second cycle OVD Patients who present with 1 to 3 intramural myomata > 2 cm and < 5 cm that do not distort the cavity, type 3 and 4 FIGO classification |
| Interventions | Ulipristal acetate 10 mg vs placebo |
| Outcomes | Increase in rate of clinical pregnancy at 12 weeks |
| Starting date | May 2015 |
| Contact information | Instituto Valenciano de Infertilidad, IVI VALENCIA: Davinia Oltra, PhD ‐ Davinia.Oltra@ivi.es |
| Notes |
NCT02465814.
| Trial name or title | Assess Safety and Efficacy of Vilaprisan in Patients With Uterine Fibroids (ASTEROID 2) |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women with heavy menstrual bleeding and fibroid ≥ 3 cm |
| Interventions | Vilaprisan 2 mg vs ulipristal 5 mg in varying regimens |
| Outcomes | Amenorrhoea at 3 months |
| Starting date | June 2015 |
| Contact information | Bayer |
| Notes |
Abbreviations
cm: centimetres
IM: intramuscular
mg: milligrams
ml: millilitres
Differences between protocol and review
Secondary outcomes
We removed the outcome that looked at recurrence rate over time. When conducting the review, we found this outcome to be not workable as it applied only to the post‐randomisation subgroup.
Measures of treatment effect
We added a paragraph to explain how SMDs would be interpreted because we wished to ensure that they would be interpreted in a consistent manner in the review.
Unit of analysis issues
In the protocol, we stated, "Change in fibroid size data will be analysed based on the number of fibroids tracked and not the number of patients". We decided that we would not pool any data not analysed per woman but would include them in an additional table, because we wished to avoid a unit of analysis error.
Dealing with missing data
We added a sentence to make it clear that when data manipulation was undertaken (such as imputing standard deviations or calculating medians from means), we obtained statistical advice and imputed the data using methods included in an appendix. Our rationale was that we wished to be transparent about our methods.
Subgroup analyses
The protocol stated that subgroup analyses would be undertaken "if sufficient data were available". Because "sufficient data" was poorly defined, we specified that this would mean more than five studies.
Sensitivity analyses
The protocol stated that we would conduct the following sensitivity analyses.
Exclusion of studies with high risk of bias.
Application of a fixed‐effect model.
Exclusion of unpublished studies.
Exclusion of trials for which data were imputed for primary outcomes.
Exclusion of studies that used unpublished rating scales or scales that had not been validated to assess for symptom relief.
We decided not to conduct the third and fifth sensitivity analyses in the list above because we considered that these factors would be reflected in an assessment of study risk of bias, and we changed the wording of the fourth subgroup analysis to "if alternative imputation strategies had been implemented" because it allows consideration of a wider range of scenarios for imputation of data.
Overall quality of the body of evidence: 'Summary of findings' tables
We added a paragraph explaining the methods we would use to compile 'Summary of findings' tables, and we added details of a second comparison because this information was not included in the protocol.
Contributions of authors
All review authors contributed equally in drafting and reviewing the protocol and review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
AM participated in a speakers bureau or served on the advisory board for Allergan (manufacturer of ulipristal acetate in Canada), Abbvie (manufacturer of leuprolide), Bayer, Hologic and Medtronic.
MS, TC and LW have no conflicts of interest to declare.
New
References
References to studies included in this review
Bagaria 2009 {published data only}
- Bagaria M, Suneja A, Vaid NB, Guleria K, Mishra K. Low‐dose mifepristone in treatment of uterine leiomyoma: a randomised double‐blind placebo‐controlled clinical trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009;49:77‐83. [DOI] [PubMed] [Google Scholar]
Bigatti 2014 {published data only}
- Bigatti G, Lemmello R, Desgro M, Pollino S, Santirocco M. Progesterone preoperative treatment for myomectomy with the intrauterine bigatti shaver (IBS). Gynecological Surgery 2014;1:119‐20. [Google Scholar]
Chwalisz 2007 {published data only}
- Chwalisz K, Larsen L, Edmonds A, Winkel C. Effectiveness of asoprisnil, a selective progesterone receptor modulator (SPRM) in treating uterine leiomyomata. Advances in Uterine Leiomyoma Research: 2nd NIH International Congress. 2005.
- Chwalisz K, Larsen L, Mattia‐Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertility and Sterility 2007;87:1399‐412. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, McCrary K, Edmonds A. Effects of the novel selective progesterone receptor modulator (SPRM) asoprisnil on bleeding patterns in subjects with leiomyomata. Journal of the Society for Gynecologic Investigation 2004;11:320A‐1A. [Google Scholar]
- Larsen L, Coyne K, Chwalisz K. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reproductive Sciences (Thousand Oaks, Calif.) 2013;20:680‐7. [DOI] [PubMed] [Google Scholar]
Donnez 2012 {published data only}
- Barlow DH, Lumsden MA, Fauser BCJM, Terrill P, Bestel E. Individualized vaginal bleeding experience of women with uterine fibroids in the PEARL I randomized controlled trial comparing the effects of ulipristal acetate or placebo. Human Reproduction 2014;29:480‐9. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. New England Journal of Medicine 2012;366:409‐20. [DOI] [PubMed] [Google Scholar]
- Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. International Journal of Gynecological Pathology 2012;31:556‐69. [DOI] [PubMed] [Google Scholar]
Donnez 2012a {published data only}
- Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. New England Journal of Medicine 2012;366:421‐32. [DOI] [PubMed] [Google Scholar]
Engman 2009 {published data only}
- Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell‐Danielsson K. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Human Reproduction 2009;24:1870‐9. [DOI] [PubMed] [Google Scholar]
Esteve 2013 {published data only}
- Esteve JL, Acosta R, Perez Y, Rodriguez B, Seigler I, Sanchez C, et al. Mifepristone versus placebo to treat uterine myoma: a double‐blind, randomized clinical trial. International Journal of Womens Health 2013;5:361‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiscella 2006 {published data only}
- Eisinger SH, Fiscella K, Meldrum S, Feng C, Fisher S, Guzick DS. Effect of mifepristone on quality of life for women with symptomatic fibroids. Fertility and Sterility 2006;86:S41‐2. [Google Scholar]
- Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: a randomized controlled trial. Obstetrics and Gynecology 2006;108:1381‐7. [DOI] [PubMed] [Google Scholar]
Levens 2008 {published data only}
- Levens ED, Potlog‐Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, et al. CDB‐2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstetrics and Gynecology 2008;111:1129‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G, Avila N, Armstrong AY, Nieman L. Does the selective progesterone receptor modulator ulipristal normalize the uterine cavity in women with leiomyoma. Reproductive Sciences 2011;1:95A. [Google Scholar]
- Segal TR, Zarek SM, Mumford SL, Plowden TC, Nieman LK, Segars JH, et al. Radiographic and histopathologic endometrial characteristics of women undergoing treatment with ulipristal acetate (UPA). Fertility and Sterility 2014;102:e286. [Google Scholar]
Liu 2015 {published data only}
- Liu C. Low‐dose mifepristone versus placebo to treat uterine myoma: a double‐blind, randomized clinical trial. Journal of Minimally Invasive Gynecology 2015;22(6):S91. [DOI] [PubMed] [Google Scholar]
Nieman 2011 {published data only}
- Cox J, Malik M, Britten‐Webb J, Patel A, Malik S, Segars J, et al. Ulipristal acetate inhibits extracellular matrix production in human leiomyomas in vivo: a laboratory analysis of a randomized controlled trial. Reproductive Sciences 2015;22:88A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fru KN, Levy G, Wesley R, Neiman L, Venkatesan A, Armstrong A. Prolactin as a biomarker for tumor burden and response to UPA therapy in African American women with symptomatic leiomyoma. Reproductive Sciences 2013;20:150A. [Google Scholar]
- Green L J, Levy G, Wesley R, Nieman L, Armstrong A. Efficacy of ulipristal acetate for the treatment of symptomatic uterine leiomyomas in African Americans. Fertility and Sterility 2013;98:S96. [Google Scholar]
- Levy G, Avila N, Armstrong AY, Nieman L. Does the selective progesterone receptor modulator ulipristal normalize the uterine cavity in women with leiomyoma. Reproductive Sciences 2011;1:95A. [Google Scholar]
- Nieman L, Chabbert‐Buffet N, Bouchard P. Endocrine safety of ulipristal acetate, a selective progesterone receptor modulator (SPRM): results from two phase II randomised, placebo‐controlled studies. Endocrine Abstracts 2010;22:P480. [Google Scholar]
- Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, et al. Efficacy and tolerability of CDB‐2914 treatment for symptomatic uterine fibroids: a randomized, double‐blind, placebo‐controlled, phase IIb study. Fertility and Sterility 2011;95:767‐72 e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal TR, Zarek SM, Mumford SL, Plowden TC, Nieman LK, Segars JH, et al. Radiographic and histopathologic endometrial characteristics of women undergoing treatment with ulipristal acetate (UPA). Fertility and Sterility 2014;102:e286. [Google Scholar]
Prasad 2013 {published data only}
- Prasad S, Varun N, Kumar A. Effect of low dose mifepristone on uterine leiomyoma in reproductive age group. Fertility and Sterility 2013;3:S78. [Google Scholar]
Reinsch 1994 {published data only}
- Reinsch RC, Murphy AA, Morales AJ, Yen SS. The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: a prospective, randomized study. American Journal of Obstetrics and Gynecology 1994;170:1623‐7; discussion 1627‐8. [PubMed] [Google Scholar]
Wilkens 2008 {published data only}
- Larsen L, Coyne K, Chwalisz K. Validation of the menstrual pictogram in women with leiomyomata associated with heavy menstrual bleeding. Reproductive Sciences 2013;20:680‐7. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. Journal of Clinical Endocrinology and Metabolism 2008;93:4664‐71. [DOI] [PubMed] [Google Scholar]
- Wilkens J, Male V, Ghazal P, Forster T, Gibson DA, Williams AR, et al. Uterine NK cells regulate endometrial bleeding in women and are suppressed by the progesterone receptor modulator asoprisnil. Journal of Immunology 2013;191(5):2226‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Human Reproduction 2009;24:1036‐44. [DOI] [PubMed] [Google Scholar]
- Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Human Reproduction 2007;22:1696‐704. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carbonell 2008 {published data only}
- Carbonell Esteve JL, Acosta R, Heredia B, Perez Y, Castaneda MC, Hernandez AV. Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstetrics and Gynecology 2008;112:1029‐36. [DOI] [PubMed] [Google Scholar]
Carbonell 2012 {published data only}
- Carbonell Esteve JL, Riveron AM, Cano M, Ortiz AI, Valle A, Texido CS, et al. Mifepristone 2.5 mg versus 5 mg daily in the treatment of leiomyoma before surgery. International Journal of Womens Health 2012;4:75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbonell 2013 {published data only}
- Carbonell JL, Acosta R, Perez Y, Garces R, Sanchez C, Tomasi G. Treatment of uterine myoma with 2.5 or 5 mg mifepristone daily during 3 months with 9 months posttreatment followup: randomized clinical trial. ISRN Obstetrics and Gynecology 2013;Article ID 649030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbonell 2013a {published data only}
- Carbonell JLL, Acosta R, Perez Y, Marrero AG, Trellez E, Sanchez C, et al. Safety and effectiveness of different dosage of mifepristone for the treatment of uterine fibroids: a double‐blind randomized clinical trial. International Journal of Womens Health 2013;5:115‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chwalisz 2003 {published data only}
- Chwalisz K, Parker RL, Williamson S, Larsen L, McCrary K, Elger W. Treatment of uterine leiomyomas with the novel selective progesterone receptor modulator (SPRM). Journal of the Society for Gynecologic Investigation 2003;10:301A. [Google Scholar]
Donnez 2014 {published data only}
- Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BCJM, et al. Long‐term treatment of uterine fibroids with ulipristal acetate. Fertility and Sterility 2014;101:1565‐73.e18. [DOI] [PubMed] [Google Scholar]
Eisinger 2003 {published data only}
- Eisinger SH, Meldrum S, Fiscella K, Roux HD, Guzick DS. Low‐dose mifepristone for uterine leiomyomata. Obstetrics & Gynecology 2003;101:243‐50. [DOI] [PubMed] [Google Scholar]
Eisinger 2005 {published data only}
- Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve‐month safety and efficacy of low‐dose mifepristone for uterine myomas. Journal of Minimally Invasive Gynecology 2005;12:227‐33. [DOI] [PubMed] [Google Scholar]
Esteve 2012 {published data only}
- Esteve JL, Acosta R, Perez Y, Campos R, Hernandez AV, Texido CS. Treatment of uterine myoma with 5 or 10mg mifepristone daily during 6 months, post‐treatment evolution over 12 months: double‐blind randomised clinical trial. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2012;161:202‐8. [DOI] [PubMed] [Google Scholar]
Kulshrestha 2013 {published data only}
- Kulshrestha V, Kriplani A, Agarwal N, Sareen N, Garg P, Hari S, et al. Low dose mifepristone in medical management of uterine leiomyoma ‐ an experience from a tertiary care hospital from North India. Indian Journal of Medical Research 2013;137:1154‐62. [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00044876 {published data only}
- NCT00044876. Treatment of uterine fibroids with CDB‐2914, an experimental selective progesterone receptor antagonist. https://clinicaltrials.gov/ct2/show/NCT00044876 (first received 5 September 2002).
NCT00152269 {published data only}
- NCT00152269. Treatment of uterine fibroids with asoprisnil (J867). https://clinicaltrials.gov/ct2/show/NCT00152269 (first received 7 September 2005).
NCT00683917 {published data only}
- NCT00683917. Pharmacokinetics, safety and efficacy study of Proellex in pre‐menopausal women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00683917 (first received 22 May 2008).
NCT00702702 {published data only}
- NCT00702702. Safety and efficacy of Proellex in pre‐menopausal anemic women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00702702 (first received 18 June 2008).
NCT00735553 {published data only}
- NCT00735553. Evaluating the safety and efficacy of Proellex (CDB‐4124) in premenopausal women with asymptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00735553 (first received 13 August 2008).
NCT00785356 {published data only}
- NCT00785356. Safety and efficacy of Proellex in pre‐menopausal anemic women with symptomatic uterine fibroids. https://clinicaltrialbase.com/study/NCT00785356 (first received 2 November 2008).
NCT00853567 {published data only}
- NCT00853567. Evaluating the safety and efficacy of Proellex in premenopausal women with symptomatic uterine fibroids. https://clinicaltrialbase.com/study/NCT00853567 (first received 26 February 2009).
NCT00882258 {published data only}
- NCT00882258. Study of Proellex in pre‐menopausal women with symptomatic uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT00882258 (first received 14 April 2009).
NCT01069094 {published data only}
- NCT01069094. A Study of Progenta (CDB‐4124) in pre‐menopausal women with symptomatic leiomyomata. https://clinicaltrials.gov/ct2/show/NCT01069094 (first received 12 February 2010).
NCT01816815 {published data only}
- NCT01816815. Study in healthy tubal ligated women to evaluate pharmacodynamics, safety and pharmacokinetics of BAY1002670. https://clinicaltrials.gov/ct2/show/NCT01816815 (first received 20 March 2013).
References to ongoing studies
NCT02131662 {published data only}
- NCT02131662. Bay1002670, fibroids, safety and efficacy EU, US, Can, Jap (ASTEROID 1). https://clinicaltrials.gov/ct2/show/NCT02131662 (first received 5 May 2014).
NCT02147158 {published data only}
- NCT02147158. A study of the safety and efficacy of intermittent ulipristal treatment of abnormal uterine bleeding associated with leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02147158 (first received 10 May 2014) 2016.
NCT02147197 {published data only}
- NCT02147197. A study of the efficacy and safety of a single ulipristal treatment course for the treatment of abnormal uterine bleeding associated with leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02147197 (first received 21 May 2014).
NCT02288130 {published data only}
- NCT02288130. Ulipristal vs. GnRHa prior to laparoscopic myomectomy. https://clinicaltrials.gov/ct2/show/NCT02288130 (first received 6 November 2014).
NCT02323646 {published data only}
- NCT02323646. A phase 2 study to evaluate the safety and efficacy of Proellex (telapristone acetate) administered vaginally in the treatment of uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT02323646 (first received 18 December 2014).
NCT02357563 {published data only}
- NCT02357563. Ulipristal acetate versus GnRH analogue and myometrial preservation. https://clinicaltrials.gov/ct2/show/NCT02357563 (first received 2 February 2015).
NCT02361879 {published data only}
- NCT02361879. Ulipristal acetate versus GnRH analogue treatment before hysteroscopic resection of uterine leiomyoma. https://clinicaltrials.gov/ct2/show/NCT02361879 (first received 2 February 2015).
NCT02361905 {published data only}
- NCT02361905. Ulipristal acetate for the preoperative management of hypoechoic cellular leiomyomas. https://clinicaltrials.gov/ct2/show/NCT02361905 (first received 2 February 2015).
NCT02425878 {published data only}
- NCT02425878. Ulipristal acetate 10 mg and assisted reproduction. https://clinicaltrials.gov/ct2/show/NCT02425878 (first received 21 April 2015).
NCT02465814 {published data only}
- NCT02465814. Assess safety and efficacy of vilaprisan in patients with uterine fibroids. https://clinicaltrials.gov/ct2/show/NCT02465814 (first received 22 May 2015).
Additional references
Baird 2003
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American Journal of Obstetrics and Gynecology 2003;188(1):100‐7. [PUBMED: 12548202] [DOI] [PubMed] [Google Scholar]
Bouchard 2011
- Bouchard P, Chabbert‐Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertility and Sterility 2011;96(5):1175‐89. [PUBMED: 21944187] [DOI] [PubMed] [Google Scholar]
Brooks 1996
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37(1):53‐72. [PUBMED: 10158943] [DOI] [PubMed] [Google Scholar]
Bulun 2013
- Bulun SE. Uterine fibroids. New England Journal of Medicine 2013;369(14):1344‐55. [PUBMED: 24088094] [DOI] [PubMed] [Google Scholar]
Cardozo 2012
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. American Journal of Obstetrics and Gynecology 2012;206(3):211.e1‐9. [PUBMED: 22244472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chwalisz 2005
- Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocrine Reviews 2005;26(3):423‐38. [PUBMED: 15857972] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Donnez 2015
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertility and Sterility 2015;103(2):519‐27.e3. [PUBMED: 25542821] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed before November 2016. Hamilton, ON: GRADE Working Group, McMaster University, 2014.
Gurusamy 2016
- Gurusamy KS, Vaughan J, Fraser IS, Best LM, Richards T. Medical therapies for uterine fibroids ‐ a systematic review and network meta‐analysis of randomised controlled trials. PloS one 2016;11(2):e0149631. [PUBMED: 26919185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Higham 1990
- Higham JM, O'Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. BJOG 1990;97:734‐9. [DOI] [PubMed] [Google Scholar]
Ishikawa 2010
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010;151(6):2433‐42. [PUBMED: 20375184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalampokas 2016
- Kalampokas T, Kamath M, Boutas I, Kalampokas E. Ulipristal acetate for uterine fibroids: a systematic review and meta‐analysis. Gynecological Endocrinology 2016;32(2):91‐6. [PUBMED: 26572056] [DOI] [PubMed] [Google Scholar]
Ke 2009
- Ke LQ, Yang K, Li J, Li CM. Danazol for uterine fibroids. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD007692.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2014
Lethaby 2001
- Lethaby A, Vollenhoven B, Sowter MC. Pre‐operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000547] [DOI] [PubMed] [Google Scholar]
Merrill 2008
- Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Medical Science Monitor 2008;14(1):CR24‐31. [PUBMED: 18160941] [PubMed] [Google Scholar]
Murji 2016
- Murji A, Crosier R, Chow T, Ye XY, Shirreff L. Role of ethnicity in treating uterine fibroids with ulipristal acetate. Fertility and Sterility 2016;106(5):1165‐9. [PUBMED: 27336213] [DOI] [PubMed] [Google Scholar]
Mutter 2008
- Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Modern Pathology 2008;21(5):591‐8. [PUBMED: 18246050] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sabry 2012
- Sabry M, Al‐Hendy A. Medical treatment of uterine leiomyoma. Reproductive Sciences 2012;19(4):339‐53. [PUBMED: 22378865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sangkomkamhang 2013
- Sangkomkamhang US, Lumbiganon P, Laopaiboon M, Mol BW. Progestogens or progestogen‐releasing intrauterine systems for uterine fibroids. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD008994.pub2] [DOI] [PubMed] [Google Scholar]
Spies 2002
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz‐Murphy K, Gonzalves SM. The UFS‐QOL, a new disease‐specific symptom and health‐related quality of life questionnaire for leiomyomata. Obstetrics and Gynecology 2002;99(2):290‐300. [PUBMED: 11814511] [DOI] [PubMed] [Google Scholar]
Stovall 2001
- Stovall DW. Clinical symptomatology of uterine leiomyomas. Clinical Obstetrics and Gynecology 2001;44(2):364‐71. [PUBMED: 11344999] [DOI] [PubMed] [Google Scholar]
Thrippleton 2015
- Thrippleton MJ, Munro KI, McKillop G, Newby DE, Marshall I, Roberts N, et al. Unbiased and efficient estimation of the volume of the fibroid uterus using the Cavalieri method and magnetic resonance imaging. Reproductive Sciences 2015;22(1):15‐22. [PUBMED: 25332217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tristan 2012
- Tristan M, Orozco LJ, Steed A, Ramirez‐Morera A, Stone P. Mifepristone for uterine fibroids. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD007687.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Voorhis 2009
- Voorhis B. A 41‐year‐old woman with menorrhagia, anemia, and fibroids: review of treatment of uterine fibroids. JAMA 2009;301(1):82‐93. [PUBMED: 19050179] [DOI] [PubMed] [Google Scholar]
Wagenfeld 2016
- Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opinion on Therapeutic Targets 2016;20(9):1‐10. [PUBMED: 27138351] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallach 2004
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstetrics and Gynecology 2004;104(2):393‐406. [PUBMED: 15292018] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
Williams 2006
- Williams VS, Jones G, Mauskopf J, Spalding J, DuChane J. Uterine fibroids: a review of health‐related quality of life assessment. Journal of Womens Health 2006;15(7):818‐29. [PUBMED: 16999637] [DOI] [PubMed] [Google Scholar]
Wyatt 2001
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PMS. Determination of total menstrual blood loss. Fertility and Sterility 2001;76:125‐31. [DOI] [PubMed] [Google Scholar]


