Abstract
Background
Perioperative hypertension requires careful management. Angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II type 1 receptor blockers (ARBs) have shown efficacy in treating hypertension associated with surgery. However, there is lack of consensus about whether they can prevent mortality and morbidity.
Objectives
To systematically assess the benefits and harms of administration of ACEIs or ARBs perioperatively for the prevention of mortality and morbidity in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia.
Search methods
We searched the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12), Ovid MEDLINE (1966 to 8 December 2014), EMBASE (1980 to 8 December 2014), and references of the retrieved randomized trials, meta‐analyses, and systematic reviews. We reran the search on February 3, 2017. Three potential new studies of interest were added to a list of 'Studies awaiting Classification' and will be incorporated into the formal review findings during the review update.
Selection criteria
We included randomized controlled trials (RCTs) comparing perioperative administration of ACEIs or ARBs with placebo in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia. We excluded studies in which participants underwent procedures that required local anaesthesia only, or participants who had already been on ACEIs or ARBs.
Data collection and analysis
Two review authors independently performed study selection, assessed the risk of bias, and extracted data. We used standard methodological procedures expected by Cochrane.
Main results
We included seven RCTs with a total of 571 participants in the review. Two of the seven trials involved 36 participants undergoing non‐cardiac vascular surgery (infrarenal aortic surgery), and five involved 535 participants undergoing cardiac surgery, including valvular surgery, coronary artery bypass surgery, and cardiopulmonary bypass surgery. The intervention was started from 11 days to 25 minutes before surgery in six trials and during surgery in one trial. We considered all seven RCTs to carry a high risk of bias. The effects of ACEIs or ARBs on perioperative mortality and acute myocardial infarction were uncertain because the quality of the evidence was very low. The risk of death was 2.7% in the ACEIs or ARBs group and 1.6% in the placebo group (risk ratio (RR) 1.61; 95% confidence interval (CI) 0.44 to 5.85). The risk of acute myocardial infarction was 1.7% in the ACEIs or ARBs group and 3.0% in the placebo group (RR 0.55; 95% CI 0.14 to 2.26). ACEIs or ARBs may improve congestive heart failure (cardiac index) perioperatively (mean difference (MD) ‐0.60; 95% CI ‐0.70 to ‐0.50, very low‐quality evidence). In terms of rate of complications, there was no difference in perioperative cerebrovascular complications (RR 0.48; 95% CI 0.18 to 1.28, very low‐quality evidence) and hypotension (RR 1.95; 95% CI 0.86 to 4.41, very low‐quality evidence). Cardiac surgery‐related renal failure was not reported. ACEIs or ARBs were associated with shortened length of hospital stay (MD ‐0.54; 95% CI ‐0.93 to ‐0.16, P value = 0.005, very low‐quality evidence). These findings should be interpreted cautiously due to likely confounding by the clinical backgrounds of the participants. ACEIs or ARBs may shorten the length of hospital stay, (MD ‐0.54; 95% CI ‐0.93 to ‐0.16, very low‐quality evidence) Two studies reported adverse events, and there was no evidence of a difference between the ACEIs or ARBs and control groups.
Authors' conclusions
Overall, this review did not find evidence to support that perioperative ACEIs or ARBs can prevent mortality, morbidity, and complications (hypotension, perioperative cerebrovascular complications, and cardiac surgery‐related renal failure). We found no evidence showing that the use of these drugs may reduce the rate of acute myocardial infarction. However, ACEIs or ARBs may increase cardiac output perioperatively. Due to the low and very low methodology quality, high risk of bias, and lack of power of the included studies, the true effect may be substantially different from the observed estimates. Perioperative (mainly elective cardiac surgery, according to included studies) initiation of ACEIs or ARBs therapy should be individualized.
Plain language summary
Giving blood pressure‐lowering drugs around the time of surgery to reduce the risk of death and serious illness in adults
Review question
We reviewed the evidence on two drugs that are used to lower blood pressure (angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin II type 1 receptor blockers (ARBs)) around the time of surgery for reducing the risk of death and serious illness in adults undergoing surgery using a general anaesthetic.
Background
People with high blood pressure around the time of surgery are carefully treated as they have a higher risk of complications such as reduced blood flow to the heart muscle (myocardial ischaemia), heart attack, and even death. ACEIs or ARBs relax the blood vessels and are effective in treating high blood pressure associated with surgery, but the outcome is uncertain when these are used for the prevention of surgery‐related complications.
Study characteristics
We searched the databases to 8 December 2014. We found seven randomized controlled trials (from 1992 to 2014) with 571 participants that met our inclusion criteria. Two of the seven trials involved 36 participants undergoing non‐cardiac vascular surgery (infrarenal aortic surgery), and five involved 535 participants undergoing cardiac surgery, including valvular surgery, coronary artery bypass surgery, and cardiopulmonary bypass surgery. The interventions started from 11 days to 25 minutes before surgery in six trials and during surgery in one. All of the seven studies were conducted in Europe and the United States. One of the seven studies was funded by a drug company.
Key results
Three trials involving 419 participants reported on deaths, but the results were imprecise with no evidence of a difference between the intervention and placebo groups (perioperative mortality). Two trials with 345 participants reported a similar number of participants in the two groups with changes in their electrocardiogram that indicated a heart attack (acute myocardial infarction). The output of the heart (cardiac index) appeared to be increased in one trial only.
The two trials that reported the risk of low blood pressure as a potential complication of the intervention found no apparent difference; and the risk of stroke was similar with and without the intervention in three trials.
The results from three studies showed that ACEIs or ARBs may reduce length of hospital stay, but these findings should be interpreted cautiously because of the possible influence of the clinical backgrounds of the participants studied. Two trials that assessed adverse events found no evidence of a difference between ACEIs or ARBs and placebo (no treatment).
Quality of the evidence
The quality of evidence for the outcomes was low or very low. The overall number of participants was small. Most participants were undergoing cardiac surgery, which meant the findings cannot be generalized to other types of surgery. We reran the search on February 3, 2017. We will deal with the three studies of interest when we update the review.
Summary of findings
Summary of findings for the main comparison. ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults.
| ACEIs or ARBs compared to placebo for preventing surgery‐related mortality and morbidity in adults | ||||||
| Patient or population: Patients undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively Settings: All settings Intervention: ACEIs or ARBs Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | ACEIs or ARBs | |||||
| All‐cause mortality | Study population | RR 1.61 (0.44 to 5.85) | 419 (3 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital |
|
| 16 per 1000 | 25 per 1000 (7 to 90) | |||||
| Moderate | ||||||
| 7 per 1000 | 11 per 1000 (3 to 41) | |||||
| Risk of acute myocardial ischaemia | Study population | RR 0.55 (0.14 to 2.26) | 345 (2 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total sample size is lower than the calculated. Duration of follow‐up: until discharge from hospital |
|
| 30 per 1000 | 16 per 1000 (4 to 67) | |||||
| Moderate | ||||||
| 56 per 1000 | 31 per 1000 (8 to 127) | |||||
| Congestive heart failure | The mean cardiac index in the intervention groups was 0.6 higher (0.7 to 0.5 higher) | ‐ | 34 (2 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified |
|
| Hypotension | ‐ | RR 1.95 (0.86 to 4.41) | 298 (1 study) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: not specified |
|
| Rate of perioperative cerebrovascular complications | Study population | RR 0.48 (0.18 to 1.28) | 459 (3 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Duration of follow‐up: until discharge from hospital (Billings 2012; Pretorius 2012); 90 days after surgery (Flesch 2009) |
|
| 50 per 1000 | 24 per 1000 (9 to 65) | |||||
| Moderate | ||||||
| 71 per 1000 | 34 per 1000 (13 to 92) | |||||
| Length of hospital stay | The mean length of hospital stay in the intervention groups was 0.54 lower (0.93 lower to 0.16 lower) | ‐ | 372 (2 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total population size is less than 400. Duration of follow‐up: until discharge from hospital |
|
| Treatment related adverse events | ‐ | ‐ | ‐ | 385 (2 studies) | ⊕⊝⊝⊝ very low1 | All the included trials were at high risk of bias. Total population size is less than 400. Authors did not provided detailed information on adverse events, which made the synthesis of the results less clinically relevant. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACEIs: angiotensin‐converting enzyme inhibitors; ARBs: angiotensin II type 1 receptor blockers; CI: confidence interval; ECG: electrocardiograph; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded by three levels due to very serious study limitations (all the trials included were at high risk of bias) and serious imprecision (total population size is less than 400).
Background
Hypertension is closely related to cardiac diseases such as myocardial infarction, congestive heart failure, and even sudden death (Lloyd‐Jones 2010). An increasing number of patients scheduled to undergo surgery suffer from hypertension, which is an important contributing factor to perioperative cardiac complications. Patients who undergo non‐cardiac surgery run the risk of myocardial infarction or cardiac death, which also leads to considerably increased costs (Freeman 2009). Perioperative myocardial infarction occurs in 6% of patients, with a mortality rate of 3% in patients with acquired cardiac diseases (Wiesbauer 2007). Due to the high risk of perioperative cardiac complications, strategies for prevention are worth examining (Fleisher 2007).
Description of the condition
As cardiovascular events remain a major threat perioperatively in hypertensive patients undergoing cardiac or non‐cardiac operative procedures, considerable effort has been expended to lower the extent of myocardial ischaemia in these patients. There is also a high economic cost associated with cardiovascular morbidity and mortality. Perioperative medical therapy is one of three categories of interventions intended to reduce the rate of perioperative cardiac complications. Pharmacological therapies include beta‐blockers, alpha 2‐adrenergic agonists, nitrates, diuretics, calcium‐channel blockers, angiotensin‐converting enzyme inhibitors (ACEIs), and angiotensin II receptor blockers (ARBs). Pharmacologic attenuation of sympathetic nervous system activity is thought to improve participant outcomes, and beta‐blockers have been found to reduce perioperative arrhythmias and myocardial ischaemia (Wiesbauer 2007). However, they also seem to be associated with increased mortality and a higher risk of cerebrovascular complications (Devereaux 2008). Several randomized studies showed that alpha 2‐adrenergic agonists did not reduce rates of fatal cardiac events and cardiac death (Ellis 1994; Stuhmeier 1996). Nitrates and calcium‐channel blockers have not shown benefits in reducing the rate of cardiac events (Dodds 1993). Despite the variety of therapeutic interventions available, cardiac complications remain a threat to the safety of patients undergoing surgery.
Description of the intervention
ACEIs and ARBs are two types of effective and widely used antihypertensive drugs targeting the renin‐angiotensin system (RAS). They can be used perioperatively to control hypertension via similar mechanisms. ACEIs prevent the production of angiotensin II from angiotensin I and interfere with the regulation of blood pressure by impairing degradation of bradykinin and inhibit the receptor binding of angiotensin II (Tschope 2002). ARBs exert their vasodilation effect at the receptor level by inhibiting the binding of angiotensin II to the type 1 receptors (AT1R), irrespective of whether angiotensin II is generated by renin‐angiotensin cascade or in local tissues by other means (Zou 2009).
How the intervention might work
Perioperative usage of ACEIs and ARBs is thought to be helpful in controlling high blood pressure. Perioperatively, aggressive and early treatment of hypertensive reactions is suggested to reduce cardio‐cerebral complications. In hypertensive emergencies, intravenous infusion of ACEI enalaprilat lowered blood pressure in more than 60% of participants (Strauss 1984). The use of ACEIs and ARBs may work on multiple foci. Heightened sympathetic nervous system activity contributes greatly to perioperative cardiac complications, and pharmacologic intervention of that pathway improves participant outcomes (Warltier 2000). Research has showed that ACEIs can reduce sympathetic drive and augment vagal tone (Fariello 1989). However, conflicting opinions have been proposed indicating that sympathetic nervous inhibition is not a major component of the blood pressure‐lowering action (Krum 2006). Can RAS inhibitors, apart from decreasing blood pressure, lower hard endpoints like myocardial ischaemia, perioperative mortality, and length of hospitalization?
The RAS are activated during cardiac surgery with cardiopulmonary bypass (CPB), which disturbs the balance of pro‐ and anti‐inflammatory cytokines and modifies regional blood flow, contributing to morbidity (Kwapisz 2004). The systemic vascular resistance index of participants treated with ACEI quinapril was significantly lower compared with those treated with isotonic saline solution, without an increased risk of deleterious haemodynamic episodes (Kwapisz 2004). Thus, administration of RAS inhibitors during cardiac surgery with CPB may lower cardiac complications through modifying regional blood flow during CPB.
In people routinely treated with ACEIs, adrenergic receptor hyporesponsiveness and regression of cardiovascular hypertrophy are thought to be implicated (Licker 1996). Long‐term administration of ACEIs and ARBs has been demonstrated to be beneficial to participants with cardiovascular and renal diseases (Garg 1995; Lee 2004). When taken properly in adequate dosages, ACEIs/ARBs slowed the progression of heart failure and greatly reduced morbidity and mortality for participants with heart failure (Cohn 1991; Hunt 2001). Thus, an increasing number of patients scheduled to undergo cardiac surgery are now chronically treated with ACEIs (Licker 2000). However, some of these patients develop perioperative hypotensive episodes (Coriat 1994; Tuman 1995), which are due to impaired adrenergic vasoconstrictive response in people chronically treated with ACEIs (Licker 2000). In these cases, can perioperative administration of RAS inhibitors for the treatment of hypertensive reactions improve outcomes?
In both diabetic and non‐diabetic proteinuria renal disease, blockade of the RAS is regarded as reno‐protective (Brenner 2003; Nakao 2003). During cardiac surgery with CPB, effective renal plasma flow and glomerular filtration rate decreased in the control group whereas they remained unchanged in the captopril group (Colson 1990). The reno‐protective effect of RAS inhibitors may be beneficial to patients undergoing cardiopulmonary bypass or those with renal diseases.
Why it is important to do this review
The beneficial effects of chronic administration of ACEIs and ARBs have been demonstrated, but the value of the administration of these drugs in the operative setting remains controversial. Different investigators have different opinions (Boeken 1999; Colson 1992; Coriat 1994; Deakin 1998; Di Pasquale 1993; Licker 1996; Pigott 1999; Ryckwaert 2001; Webb 1998). In light of the uncertain evidence for perioperative administration of ACEIs and ARBs, a systematic review of randomized trials appraising the perioperative usage of ACEIs and ARBs is necessary to test whether these two types of RAS inhibitors are suitable for controlling haemodynamic condition and then preventing mortality and morbidity in patients undergoing surgery. Blessberger 2014 conducted a systematic review on perioperative beta‐blockers for preventing surgery‐related mortality and morbidity, but due to the low‐ to moderate‐quality evidence, the authors were uncertain as to whether the intervention had an important effect on these outcomes, which made the present review more interesting.
Objectives
To systematically assess the benefits and harms of administration of ACEIs or ARBs perioperatively for the prevention of mortality and morbidity in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) comparing perioperative administration of ACEIs or ARBs with placebo.
Types of participants
We included adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia receiving ACEIs or ARBs perioperatively, including those participants with pre‐existing hypertension, heart failure, or ventricular dysfunction.
We excluded:
participants undergoing procedures that require local anaesthesia only;
participants who are already on chronic treatment with ACEIs or ARBs.
Types of interventions
We included trials that compared perioperative administration of any kind of ACEI or ARB via any route versus placebo. ACEIs and ARBs are started preoperatively (after hospital admission), during operation, or up to one day after surgery.
Types of outcome measures
Primary outcomes
All‐cause mortality and cardiac mortality (occurring 30 days postoperatively or before hospital discharge).
Risk of acute myocardial infarction, defined as the presence of characteristic chest pain, an ST elevation, or increase in myocardial isoenzymes, occurring up to 30 days postoperatively or before discharge from hospital.
Risk of myocardial ischaemia (defined as the presence of clinical symptoms and significant ST segment depression, occurring up to 30 days postoperatively or before hospital discharge).
Secondary outcomes
Congestive heart failure (occurring 30 days postoperatively or before hospital discharge).
Hypotension (defined as hypotension in the individual studies selected for the review).
Cerebrovascular complications (diagnosed by clinical symptoms and computed tomography or magnetic resonance imaging).
Renal insufficiency (diagnosed by clinical symptoms and laboratory examination, including serum creatinine and urinary diagnostic indices, occurring 30 days postoperatively or before hospital discharge).
Length of hospital stay.
Treatment related adverse events
Search methods for identification of studies
Electronic searches
We searched the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12), Ovid MEDLINE (1966 to 8 December 2014), and EMBASE (1980 to 8 December 2014).
We reran the search on February 3, 2017. We will deal with the three studies of interest when we update the review.
We retrieved relevant RCTs without language or date restrictions. Please see Appendix 1 for our search terms.
We searched for ongoing clinical trials and unpublished studies via:
Searching other resources
We screened the references of the retrieved randomized trials, meta‐analyses, and systematic reviews for additional trials. We contacted the main authors of studies to ask for missing or unreported data.
Data collection and analysis
Selection of studies
After searching the literature, we reviewed the titles and abstracts of all studies identified, and determined which publications were suitable for further consideration. We then obtained the full records of these publications. Two review authors (ZZ, XYC) independently assessed the eligibility of each trial for inclusion in the review. We conferred with a third review author (XYS) to resolve disagreements. In order to avoid duplication, we only included the data from the latest study if the same group of participants were involved in the different reports.
Data extraction and management
Two review authors (ZZ, XYC) independently extracted data from each identified trial and recorded them on a standardized data extraction form (see Appendix 2). We resolved disagreements by consensus. When additional information was required, we contacted the first author of the relevant trial.
Assessment of risk of bias in included studies
Two review authors (ZZ, XYS) independently appraised the methodological quality of the eligible trials. We resolved any disagreements by discussion. A third review author (HBY) arbitrated when necessary. We assessed each trial according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential threats to validity (Higgins 2011; Kjaergard 2001; Moher 1998; Schulz 1995). We considered a trial to be at low risk of bias if all domains were assessed as adequate. We considered a trial to be at high risk of bias if one or more domains were assessed as inadequate or unclear. We reported the 'Risk of bias' table as part of the Characteristics of included studies table and present a 'Risk of bias' summary figure that details all of the judgements made for all included studies in the review (Higgins 2011). We resolved disagreements in a consensus meeting. To avoid selection bias, we did not exclude trials because of low quality or any methodological characteristics. We used study quality assessment to assess the stability of the meta‐analytic results by features of study design such as randomization. We carried out the analysis before and after we excluded certain studies of lower methodological quality. If the effect measures differed significantly between the analyses, with or without the lower methodological quality studies, we reported stratified results according to study quality.
Measures of treatment effect
We summarized dichotomous data as risk ratio and continuous data as mean difference or standardized mean difference. We calculated the number of participants who suffered from treatment‐related adverse effects. In the original studies, "adverse events considered as related with the study medication" or other similar expressions were recognized as the outcomes of interest. We calculated risk ratio and number needed to treat for an additional beneficial outcome with 95% confidence intervals (Cook 1995) if the data allowed.
Unit of analysis issues
We combined different ACEIs or ARBs when trials compared ACEIs or ARBs with placebo. We analysed cardiac surgery and non‐cardiac surgery separately. We ensured that cluster randomized trials were treated appropriately.
Dealing with missing data
We tried to contact the first author of included trials to obtain missing data necessary for the meta‐analysis. We calculated missing standard deviations from the standard errors or confidence intervals (Higgins 2011). When we were unable to calculate standard deviations, we planned to impute these data using the mean value of reported standard deviations of other trials. We addressed the influence of missing data in the Discussion section of the review.
Assessment of heterogeneity
We assessed clinical heterogeneity and statistical heterogeneity. We solved clinical heterogeneity by subgroup analysis and sensitivity analysis. We also examined heterogeneity using the Chi2 statistic with significance set at P value < 0.1. We used the I2 statistic to describe the proportion of any variability due to heterogeneity (Higgins 2002). When P value > 0.1, we carried out the meta‐analysis in a fixed‐effect model; otherwise, we used a random‐effects model. We assumed the corresponding outcomes between groups to be statistically significant when the 95% confidence interval of risk ratio did not include 1.
Assessment of reporting biases
We planned to use a funnel plot to assess publication bias if we included more than 10 trials in the review. We planned to use weighted linear regression to test for funnel plot asymmetry (Egger 1997). However, due to the limited number of trials for each outcome, we did not produce a funnel plot.
Data synthesis
We undertook a meta‐analysis to measure the effect size if the degree of clinical and statistical heterogeneity was not excessive. We performed the meta‐analysis using RevMan 5.3. We used the fixed‐effect and random‐effects model according to the value of P and the I2 statistic. When the data extracted from the original reports did not warrant a quantitative summary measure, we carried out a qualitative description of the outcomes. For trials where continuous data were not given as means, no standard deviations (SDs) or standard errors were presented, or data were difficult to decipher (for instance the results were shown in figures and difficult to quantify accurately), we tried to contact the first authors and corresponding authors as stated in Dealing with missing data. When the attempt failed, we moved these studies to the studies awaiting classification. Only one study, Boldt 1996, should have been classified as awaiting classification since the data reporting in this study was incomplete. However, the lead author of that study has been accused of fraud. Consequently the reliability of the results of that study is questionable, and we have excluded the study.
According to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), when SDchange were unavailable in the original report, we imputed standard deviations for changes from baseline with the following technique: SDchange = (SDbaseline2 + SDfinal2 ‐ 2*Corr*SDbaseline*SDfinal)0.5. Default value (0.8) imputed for the correlation value.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses according to participants and interventions.
Subgroups of participants
We planned to conduct subgroup analyses according to types of surgery, anaesthesia, and other potentially influential factors.
Type of surgery: cardiac surgery or non‐cardiac surgery.
Type of anaesthesia: general anaesthesia only, or general anaesthesia combined with local anaesthesia. However, since none of the trials contained any information about combination of general anaesthesia and local anaesthesia, we could not perform subgroup analysis to assess the impact of anaesthesia method.
Type of potentially influential factors: factors that may increase the perioperative risk (presence of heart failure, recent acute myocardial infarction, diabetes, cerebrovascular insufficiency, lung disease, etc.). However, since individual raw data were not available, we could not perform subgroup analyses to assess the impact of perioperative risk of participants.
Subgroups of interventions
Perioperative AECIs versus placebo.
Perioperative ARBs versus placebo.
Sensitivity analysis
We performed sensitivity analyses to exclude trials with a high risk of bias. We performed 'trim and fill' sensitivity analysis of the primary outcomes if publication bias existed. To assess the influence of each trial on the result, we excluded them one by one using Stata. When we came across studies where the standard deviation of change from baseline was missing, we imputed the missing standard deviation using an imputed value, Corr, for the correlation coefficient. Besides the default value (0.8), we used different hypothesized values of Corr based on reasoned argument to determine whether the overall result of the analysis was robust to the use of imputed correlation coefficients.
Summary of findings tables and GRADE
We assessed the quality of the body of evidence associated with specific outcomes (all‐cause mortality, risk of acute myocardial ischaemia, risk of myocardial ischaemia, hypotension, cerebrovascular complications, and cardiac mortality) using the principles of the GRADE system (Guyatt 2008). We constructed a 'Summary of findings' table using the GRADE software (GRADEpro). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident an estimate of effect or association reflects the item being assessed. The assessment of the quality of the body of evidence was considered within study risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our initial electronic search yielded 2258 publications (last searched December 2014). We scanned these publications and identified 46 studies that we could not exclude by scrutiny of titles and abstracts alone. After reading the full texts, we found seven randomized controlled trials (RCTs) that met the inclusion criteria (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Ryckwaert 2001; Walter 2002):
We included seven trials in this review and excluded 39 studies for the reasons stated in the Characteristics of excluded studies table. One trial, Billings 2012, used more than one eligible drug (candesartan and ramipril), and we combined the two intervention groups numerically using the statistical methods in Chapters 7 and 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), so in effect we compared one intervention group of 46 participants with the placebo group of 28 participants (Figure 1)
1.
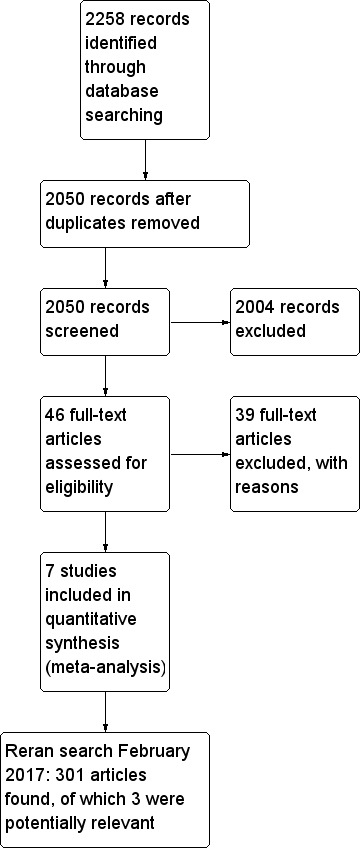
Flow diagram of study selection process.
We reran the search on February 3, 2017. The 301 studies yielded were scanned and three studies were found of interest. The three potential new studies of interest were added to a list of 'Studies awaiting Classification' and will be incorporated into the formal review findings during the review update.
Included studies
All the eligible trials were conducted in Europe and the United States. The RCTs were parallel‐group and single‐centre designed. Three trials had more than one ACEIs or ARBs group (Billings 2012; Colson 1992; Pretorius 2012); we discarded the unrelated groups. The seven included studies included 571 enrolled participants, with sample size varying from 14 to 305 participants. The participants in the Colson 1992 and Licker 1996 studies were undergoing non‐cardiac surgery (infrarenal aortic surgery) (n = 36), and the participants in the other RCTs were undergoing cardiac surgery (n = 535), including valvular surgery, coronary artery bypass surgery, and cardiopulmonary bypass surgery. Demographic data in each RCT were specified in a data extraction form. Flesch 2009 and Walter 2002 detailed the disease status of participants preoperatively. Four trials specified prior drug therapies (Billings 2012; Flesch 2009; Licker 1996; Pretorius 2012). We contacted the investigators of the trials for missing data and added this information to the Characteristics of included studies table.
The agents used in the ACEIs or ARBs groups included enalapril (Colson 1992; Licker 1996; Ryckwaert 2001; Walter 2002), ramipril (Billings 2012; Pretorius 2012), and candesartan (Billings 2012; Flesch 2009). In six RCTs pharmaceutical therapy was started preoperatively (11 days to 25 minutes prior to surgery) (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002), and in one RCT it was started intraoperatively (Ryckwaert 2001). Drugs were administered orally or intravenously in the seven included trials.
Three RCTs reported the primary outcomes (Billings 2012; Pretorius 2012; Walter 2002). Of these, acute myocardial infarction was measured in dichotomous data (ST elevation or new Q wave in electrocardiogram test). One trial reported glomerular filtration rate as a measurement of renal function (Colson 1992). Two RCTs reported the rate of hypotension (Pretorius 2012; Walter 2002), but the definitions were different. Pretorius et al defined hypotension as systolic blood pressure less than 90 mmHg or prolonged need for vasopressors, while Walter et al defined hypotension as blood pressure below 80/50 mmHg.
We considered the included studies to be underpowered because only two studies, Flesch 2009 and Pretorius 2012, reported the methods of sample size calculations. Compared with the sample size of these two studies, the other five studies contained fewer participants, which made them underpowered. Besides, in Flesch 2009 and Pretorius 2012, mortality was not included as primary outcome, which means the sample size calculations in these two trials might not be correct for testing statistical significance of mortality.
Excluded studies
We excluded 39 studies for the reasons detailed in Characteristics of excluded studies.
Ongoing studies
No ongoing studies were identified.
Studies awaiting classification
There are three studies awaiting classification (Fan 2016; Fuentes‐Reyes 2016;Tian 2015). For further details see Characteristics of studies awaiting classification.
Risk of bias in included studies
See: Figure 2; Figure 3Characteristics of included studies.
2.
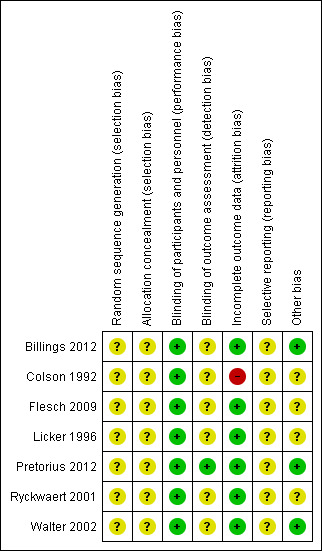
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
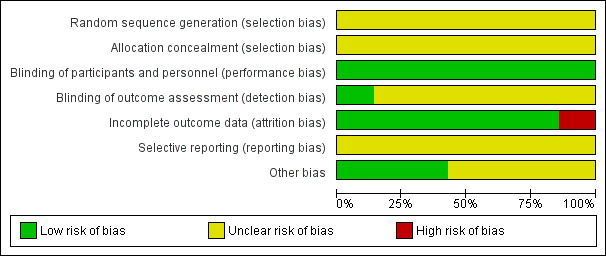
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The seven included RCTs did not provide an adequate description of the methods used for generating the allocation sequence; all were described as randomized. We considered that all of the included studies carried an unclear risk of bias. None of the trials specified allocation concealment.
Blinding
One trial had no relevant description (Billings 2012), while the remaining six studies claimed to be double‐blind designs. After contacting the author, we recognized the Billings 2012 study as a double‐blind design. In addition, in Pretorius 2012, the study personnel who assessed the outcomes were blinded.
Incomplete outcome data
Five studies detailed information of dropouts (Billings 2012; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002). Among these five studies, Flesch 2009, and Pretorius 2012 carried out intention‐to‐treat analysis. In one study all data were included in the analysis, although no information about dropouts was provided (Ryckwaert 2001). Another study provided no relevant description, so we considered this study to be at high risk of bias (Colson 1992).
Selective reporting
We found no protocols for any of the studies, and were therefore unable to assess reporting bias.
Other potential sources of bias
We did not recognize any other potential sources of bias.
Effects of interventions
See: Table 1
See: Table 1.
Primary outcomes
All‐cause mortality
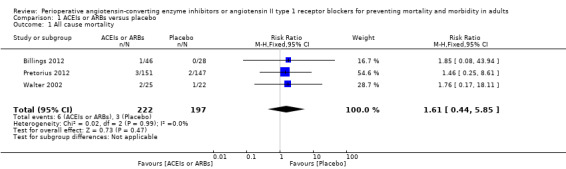
Three RCTs involving 419 participants reported perioperative mortality (Billings 2012; Pretorius 2012; Walter 2002). There was no significant difference between the ACEIs or ARBs and control groups (risk ratio (RR) 1.61; 95% confidence interval (CI) 0.44 to 5.85, P value = 0.48) with no statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 4). All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded this outcome to very low quality.
4.
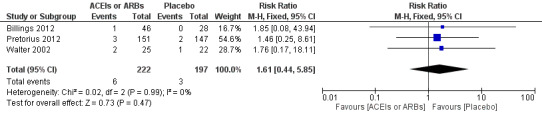
Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality.
Risk of acute myocardial infarction
ST elevation or new Q wave in electrocardiogram test
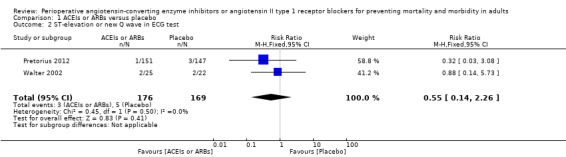
Two RCTs involving 345 participants reported the outcome of perioperative electrocardiogram test (Pretorius 2012; Walter 2002). All of the participants underwent elective cardiac surgery. Positive findings included ST segment elevation or new Q wave, which indicated acute myocardial infarction. The rates of myocardial infarction were similar in both groups without significant difference during the study period (RR 0.55; 95% CI 0.14 to 2.26, P value = 0.41), with no statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 5). However, the direction of the analysis favoured administration of ACEIs or ARBs. All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.
5.

Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.2 ST‐elevation or new Q wave in ECG test.
Myocardial ischaemia
None of the trials reported myocardial ischaemia (defined as the presence of clinical symptoms and significant ST segment depression, occurring up to 30 days postoperatively or before hospital discharge).
Secondary outcomes
Congestive heart failure
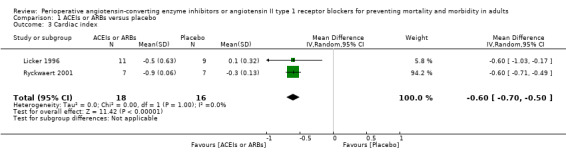
None of the studies reported the rate of congestive heart failure. Two trials involving 34 participants compared enalapril with placebo on cardiac index (Licker 1996; Ryckwaert 2001). Cardiac index favoured ACEIs (mean difference (MD) ‐0.60; 95% CI ‐0.70 to ‐0.50, P value < 0.00001) (Figure 6), with no significant statistical heterogeneity across the studies (I2 statistic = 0%). When the study containing participants undergoing cardiac surgery, Licker 1996, was removed, we found similar results as follows: cardiac index (MD ‐0.60; 95% CI ‐0.71 to ‐0.49, P value < 0.00001). All of the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.
6.
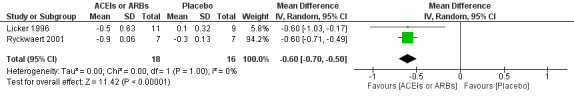
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.3 Cardiac index.
Hypotension
Two studies reported the occurrence of hypotension (Pretorius 2012; Walter 2002). As Walter 2002 did not describe the results clearly, we discarded this study when we synthesized the rate of hypotension. There was no statistical difference in the rate of hypotension (RR 1.95; 95% CI 0.86 to 4.41, P value = 0.11) (Table 2).
1. Rate of hypotension.
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Rate of hypotension | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.86, 4.41] |
Risk ratio < 1 favours angiotensin‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers group. Risk ratio > 1 favours control group.
Rate of perioperative cerebrovascular complications
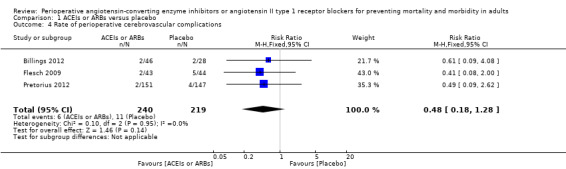
Three trials involving 459 participants compared ACEIs or ARBs with placebo (Billings 2012; Flesch 2009; Pretorius 2012). In total, 19 cerebrovascular events occurred; the rate was similar in both groups without significant difference during the study period (RR 0.48; 95% CI 0.18 to 1.28, P = 0.14), and there was no significant statistical heterogeneity across the studies (I2 statistic = 0%) (Figure 7). The ACEIs or ARBs arm in two studies used ARB (candesartan) as the experimental drug (Billings 2012; Flesch 2009). We conducted subgroup analyses according to the experimental drugs used. We found that when the ACEIs or ARBs group contained ACEIs only (ramipril arm in Billings 2012; Pretorius 2012), the rate of perioperative cerebrovascular complications was similar in both groups (RR 0.51; 95% CI 0.12 to 2.13, P value = 0.35), with no heterogeneity across the studies (I2 statistic = 0%). When the ACEIs or ARBs group contained ARBs only, the rate of perioperative cerebrovascular complications was also similar in both groups (RR 0.45; 95% CI 0.12 to 1.77, P value = 0.26), and there was no heterogeneity across studies (I2 statistic = 0%). All the included trials were at high risk of bias. For these reasons, we downgraded the outcome to very low quality. Subgroup difference was insignificant (P value = 0.80, I2 statistic = 0%).
7.
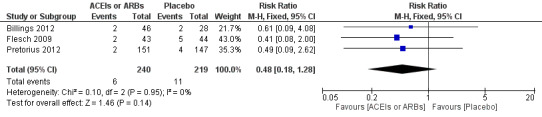
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.4 Rate of perioperative cerebrovascular complications.
Renal insufficiency
No trial reported the rate of perioperative renal insufficiency, but one trial reported glomerular filtration rate as a measurement of renal function (Colson 1992). There was no significant difference between the ACEIs or ARBs group and control group (MD ‐1.40; 95% CI ‐10.30 to 7.50, P value = 0.76) (Table 3).
2. Glomerular filtration rate.
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
| Glomerular filtration rate | 1 | 16 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐10.30, 7.50] |
IV ‐ inverse variance
IV: intravenous
Length of hospital stay
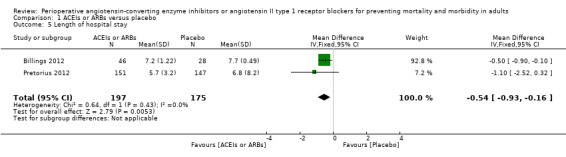
Two trials involving 372 participants reported the length of hospital stay (Billings 2012; Pretorius 2012). There was a statistical difference between the two groups (MD ‐0.54; 95% CI ‐0.93 to ‐0.16, P value = 0.005), with no significant statistical heterogeneity across the studies (I2 statistic = 0%). However, we found that the clinical backgrounds of the participants varied between trials, which might give rise to confounding factors (Figure 8). All the included trials were at high risk of bias, and the sample sizes were small. For these reasons, we downgraded the outcome to very low quality.
8.
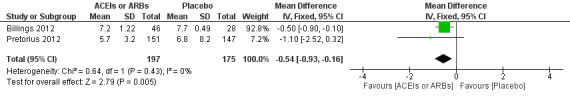
Forest plot of comparison: 1 ACEIs or ARBs versus placebo, outcome: 1.5 Length of hospital stay.
Treatment related adverse events
Two studies reported adverse events, and there was no evidence of a difference between the ACEIs or ARBs and control groups (Flesch 2009; Pretorius 2012). There were no serious adverse events considered as related to the study drug in Flesch 2009, while Pretorius 2012 reported similar incidence of serious adverse events between the two groups (P value = 0.7) but the authors did not describe what the adverse events were.
Sensitivity analysis
Since all of the included studies were at high risk of bias, we did not perform sensitivity analysis based on the risk of bias. Sensitivity analyses using different hypothesized values of Corr (0.4 to 0.9) yielded stable result. (Corr = 0.4, cardiac index favoured ACEIs (MD ‐0.60; 95% CI ‐0.77 to ‐0.43, P value < 0.00001), with no significant statistical heterogeneity across studies (I2 statistic = 0%); Corr = 0.9, cardiac index favoured ACEIs (MD ‐0.60; 95% CI ‐0.69 to ‐0.51, P value < 0.00001), with no significant statistical heterogeneity across studies (I2 statistic = 0%)).
Discussion
Summary of main results
Seven RCTs with a total of 571 participants, comparing perioperative administration of ACEIs or ARBs with placebo and reporting mortality and morbidity, met the inclusion criteria of this review. As all seven RCTs were underpowered and considered to carry a high risk of bias, no firm conclusion could be drawn. We found insufficient evidence to determine the effects of perioperative administration of ACEIs or ARBs on perioperative mortality and acute myocardial infarction. However, ACEIs or ARBs might improve cardiac output perioperatively. In terms of hypotension, perioperative cerebrovascular complications, and cardiac surgery related renal failure, ACEIs or ARBs might not be helpful in reducing the rate of these complications. Due to the potential confounding factors, the estimation of length of hospital stay should be interpreted cautiously, though the results showed that ACEIs or ARBs might result in an earlier discharge.
Overall completeness and applicability of evidence
The present review suggests that the existing evidence on effectiveness of perioperative ACEIs or ARBs administration is far from sufficient. The generalization of the findings is limited.
All the participants in the eligible RCTs underwent cardiac or vascular surgery, which seriously limited the generalization of the findings in this review. As the necessary raw data such as details of anaesthesia/surgery, disease status, comorbidities, and prior drug therapies were not available in every RCT, we could not perform some of the planned subgroup analyses. Moreover, the male‐dominated population of the studies might also limit the generalization. In summary, we could draw no robust conclusion.
All seven studies provided sufficient information on their interventions. However, it is worth noting that experimental drugs were different among the RCTs. The use of different kinds of ACEIs or ARBs could introduce clinical heterogeneity. In six RCTs pharmacological therapy started preoperatively (11 days to 25 minutes prior to surgery) (Billings 2012; Colson 1992; Flesch 2009; Licker 1996; Pretorius 2012; Walter 2002), and in one RCT it started intraoperatively (Ryckwaert 2001); this difference might also introduce clinical heterogeneity.
Although we included seven studies, there were a limited number of trials for each outcome. None of the seven included studies reported cardiac mortality. Regarding all‐cause mortality, sample sizes were small and total events were few; hence, we were unable to make a firm conclusion. Most of the included RCTs presented their results in table form but failed to detail the diagnostic methods used, which might introduce heterogeneity. For instance, theoretically, cerebrovascular complication should be confirmed by computed tomography or magnetic resonance imaging, but none of the three trials reported this (Billings 2012; Flesch 2009; Pretorius 2012). In addition, none of these RCTs reported the rate of congestive heart failure.
Quality of the evidence
The major weakness of all seven included studies was the high risk of bias. None of the studies strictly abided by the reporting criteria laid down in the Consolidated Standards of Reporting Trials (CONSORT) statement. None of the seven trials reported randomization sequence generation as well as allocation concealment, which might indicate potential selection bias. Only one of the trials attempted to blind the study personnel who assessed the outcome, and Colson 1992 did not report any information of dropouts when not all the participants completed the research. All of these were sources of information bias. Not thoroughly describing the type of potentially influential factors that might increase the perioperative risk of participants (presence of heart failure, recent acute myocardial infarction, diabetes, cerebrovascular insufficiency, and lung disease) could bring about confounding bias. The other weakness for all seven included studies was that the sample sizes were small, which made them susceptible to being underpowered to detect clinically significant differences. None reported a sample size calculation based on mortality, which indicated faulty methodology in these RCTs (Figure 2). Poor methodology was the most common reason for the downgrading of quality of evidence. Since the quality of evidence in each outcome was low or very low, further research is very likely to have an important impact on our confidence in the estimation of effect and is likely to change the estimate.
Potential biases in the review process
Our process for searching for studies was thorough. We followed the review protocol strictly in the process of study selection, data extraction, and analysis. However, there is always the possibility that we missed unreported trials or those that only appeared in unpublished conference abstracts. We also excluded studies whose authors could not provide us required information, which could also introduce bias. Challenges in optimizing search terms/poor indexing of studies were a potential source of bias.
Agreements and disagreements with other studies or reviews
A recently published, well‐designed prospective observational study (Drenger 2012) involving 4224 participants on patterns of perioperative ACEIs usage in coronary artery bypass surgery (CABG) with cardiopulmonary bypass effects suggested that regardless what pattern was adopted (continuation, withdrawal, addition, or no ACEI), no differences in in‐hospital mortality and cerebral events were noted, which is identical with our results. However, another retrospective observational cohort study reported that preoperative therapy with ACEI was associated with an increased risk of mortality, use of inotropic support, postoperative renal dysfunction, and new onset of postoperative atrial fibrillation (Miceli 2009). The IMAGINE trial drew the same conclusion: In participants at low risk of cardiovascular events after CABG, routine early initiation (less than seven days) of ACEI therapy did not appear to improve clinical outcome up to three years after CABG but increased the rate of adverse events, particularly early after CABG (Rouleau 2008). These studies did not meet the criteria of the present review, but our objectives were similar. Their conclusions should be tested with well‐designed RCTs.
A retrospective cohort study with large sample size (n = 1358) suggested that preoperative use of ACEI/ARB was associated with a 27.6% higher risk of acute kidney injury postoperatively (Arora 2008). Stopping ACEIs or ARBs before cardiac surgery might reduce the rate of acute kidney injury. On the other hand, a propensity score‐based analysis of 536 participants undergoing CABG on cardiopulmonary bypass suggested that preoperative ACEIs were associated with a reduced rate of acute kidney injury after on‐pump CABG surgery (Benedetto 2008). Our comprehensive and systematic search did not find any RCT supporting either of these conclusions.
Authors' conclusions
Implications for practice.
Overall, this review did not find evidence in preventing mortality, morbidity, and complications (hypotension, perioperative cerebrovascular complications, and cardiac surgery‐related renal failure) of perioperative ACEIs or ARBs. There was also no evidence that the use of these drugs may reduce the risk of acute myocardial infarction. However, ACEIs or ARBs may increase cardiac output perioperatively. Due to the low and very low methodology quality, high risk of bias, and lack of power of the included studies, the true effect may be substantially different from the observed estimates. Perioperative (mainly elective cardiac surgery, according to included studies) initiation of ACEIs or ARBs therapy should be individualized.
Implications for research.
Poor methodology was the most common reason for the downgrading of quality of evidence. Since the evidence this review provides is still weak, further clinical trials should control the risk of bias through rigorous study design and adopt a relatively large sample size. Moreover, the efficacy of perioperative administration of ACEIs or ARBs on patients undergoing non‐cardiac surgery should be studied. As the quality of evidence for each outcome was low or very low, we are unable to determine the effects of perioperative ACEIs or ARBs. Further research is very likely to have an important impact on the results of this review.
What's new
| Date | Event | Description |
|---|---|---|
| 3 April 2017 | Amended | We rearn the searches on 3rd February 2017. Three potential studies of interest were added to Studies awaiting classification and will be incorporated into the formal review findings during the review update. |
Acknowledgements
We would like to thank Javier Eslava‐Schmalbach (content editor), Nathan Pac (statistical editor), Pierre Foex, Claudio Bravo (peer reviewers), and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid SP)
1. exp angiotensin‐converting‐enzyme‐inhibitors/ or (alacepril or benazepril* or captopril or ceranapril or cilazapril*or delapril or enalapril* or fosinopril* or imidapril or libenzapril or quinaprilat or ramipril* or rentiapril or saralasin or spirapril or temocapril hydrochloride or teprotide or trandolapril or zofenopril or cozaar or valsartan or diovan or telmisartan or micardis or candesartan or tasosartan or verdia or eprosartan or irbesartan).mp. or exp ramipril/ or exp receptors, angiotensin/ or exp losartan/ 2. (surg* or perioperative or preoperative or intraoperative or postoperative).mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
EMBASE (Ovid SP)
1. angiotensin‐converting‐enzyme‐inhibitors/ or alacepril/ or alacepril.mp. or benazepril/ or benazepril*.mp. or captopril/ or captopril.mp. or ceranapril/ or ceranapril*.mp. or cilazapril/ or cilazapril*.mp. or cilazaprilat/ or delapril/ or delapril.mp. or enalapril/ or enalapril*.mp. or enalaprilat/ or fosinopril/ or fosinopril*.mp. or fosinoprilic acid/ or fosinoprilic acid.mp. or imidapril/ or imidapril.mp. or libenzapril/ or libenzapril.mp. or quinaprilat/ or quinaprilat.mp. or ramipril/ or ramipril*.mp. or ramiprilat/ or rentiapril/ or rentiapril*.mp. or saralasin/ or saralasin.mp. or spirapril/ or spirapril.mp. or temocapril hydrochloride/ or temocapril hydrochloride.mp. or teprotide/ or teprotide.mp. or trandolapril/ or trandolapril.mp. or zofenopril/ or zofenopril.mp. or angiotensin II receptor blocker/ or losartan/ or losartan.mp. or cozaar/ or cozaar.mp. or valsartan/ or valsartan.mp. or diovan/ or diovan.mp. or telmisartan/ or telmisartan.mp. or micardis/ or micardis.mp. or candesartan/ or candesartan.mp. or tasosartan/ or tasosartan.mp. or verdia/ or verdia.mp. or eprosartan/ or eprosartan.mp. or irbesartan/ or irbesartan.mp. 2. (surg* or perioperative or preoperative or intraoperative or postoperative).ti,ab. 3. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
The Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library)
#1 MeSH descriptor: [Angiotensin‐Converting Enzyme Inhibitors] explode all trees #2 alacepril or benazepril* or captopril or ceranapril or cilazapril*or delapril or enalapril* or fosinopril* or imidapril or libenzapril or quinaprilat or ramipril* or rentiapril or saralasin or spirapril or temocapril hydrochloride or teprotide or trandolapril or zofenopril or cozaar or valsartan or diovan or telmisartan or micardis or candesartan or tasosartan or verdia or eprosartan or irbesartan #3 MeSH descriptor: [Ramipril] explode all trees #4 MeSH descriptor: [Receptors, Angiotensin] explode all trees #5 MeSH descriptor: [Losartan] explode all trees #6 #1 or #2 or #3 or #4 #7 surg* or perioperative or preoperative or intraoperative or postoperative #8 #6 and #7
Appendix 2. Data extraction form
Study Selection, Quality Assessment & Data Extraction Form
| First author | Journal/Conference Proceedings etc | Year |
| |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Issue relates to selective reporting, when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in Studies awaiting assessment until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are 'No'. If study to be included in 'Excluded studies' section of the review, record below the information to be inserted into 'Table of excluded studies' |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Disease status / type, etc 02(if applicable) | |
| Comorbidities (diabetes, hypertension, etc) | |
| Prior drug therapy (beta‐blockers, statins, ACEIs/ ARBs and other antihypertensive drugs) | |
| Other | |
Trial characteristics
seeAppendix 1, usually just completed by one reviewer
Methodological quality
| State here method used to generate allocation and reasons for grading | Grade (circle) | |
| |
Low risk of bias | |
| High risk of bias | ||
| Unclear | ||
| Blinding | ||
| Person responsible for participants care | Low/high/unclear | |
| Participant | Low/high/unclear | |
| Outcome assessor | Low/high/unclear | |
| Other (please specify) | Low/high/unclear | |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. |
||
| All participants entering trial | ||
| 15% or fewer excluded | ||
| More than 15% excluded | ||
| Not analysed as 'intention‐to‐treat' | ||
| Unclear | ||
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| All cause mortality (up to 30 days postoperatively) | Yes / No |
| Long term all cause mortality | Yes / No |
| Rate of acute myocardial infarction (AMI) | Yes / No |
| Myocardial ischaemia | Yes / No |
| Cerebrovascular complications | Yes / No |
| Congestive heart failure | Yes / No |
| Length of hospital stay | Yes / No |
| For Continuous data | |||||||
| Code of paper | Outcomes (rename) |
Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc | Length of hospital stay | ||||||
| For Dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| A | All cause mortality (up to 30 days postoperatively) | ||
| Long term all cause mortality | |||
| Rate of acute myocardial infarction (AMI) | |||
| Myocardial ischaemia | |||
| Cerebrovascular complications | |||
| Congestive heart failure | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| Trial characteristics | |
| Further details | |
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? |
|
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose / frequency of administration | |
| Duration of treatment (State weeks / months, etc, if cross‐over trial give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Data and analyses
Comparison 1. ACEIs or ARBs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality | 3 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.44, 5.85] |
| 2 ST‐elevation or new Q wave in ECG test | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.14, 2.26] |
| 3 Cardiac index | 2 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.70, ‐0.50] |
| 4 Rate of perioperative cerebrovascular complications | 3 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.18, 1.28] |
| 5 Length of hospital stay | 2 | 372 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.93, ‐0.16] |
1.1. Analysis.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 1 All cause mortality.
1.2. Analysis.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 2 ST‐elevation or new Q wave in ECG test.
1.3. Analysis.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 3 Cardiac index.
1.4. Analysis.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 4 Rate of perioperative cerebrovascular complications.
1.5. Analysis.
Comparison 1 ACEIs or ARBs versus placebo, Outcome 5 Length of hospital stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Billings 2012.
| Methods | Prospective randomized controlled trial | |
| Participants | Adults scheduled for cardiac surgery involving cardiopulmonary bypass were eligible for the study Mean age (years): 1. 66.1 ± 2.1; 2. 64.4 ± 2.1; 3. 67.0 ± 1.7 Sample size (male): 1. 28(9); 2. 24(12); 3. 22(10) |
|
| Interventions | 1. placebo; 2. ramipril (2.5 mg on the first 3 days followed by 5 mg/day, with the dose reduced to 2.5 mg/day on the first postoperative day only); 3. candesartan (16 mg/day) | |
| Outcomes | All‐cause mortality; rate of perioperative stroke; length of hospital stay | |
| Notes | Intervention started 5 to 7 days before surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
Colson 1992.
| Methods | Prospective randomized controlled trial | |
| Participants | Scheduled for infrarenal aortic surgery because of aortic aneurysm (n = 8) or aorta occlusive disease (n = 16) Mean age (years): 1. 63 ± 1; 2. 63 ± 3; 3. 58 ± 4 Sample size (male): 24(23) |
|
| Interventions | 2 days before surgery, participants were allocated to 1 of 3 groups in randomized, double‐blind fashion: 1. control group; 2. nicardipine group; 3. enalapril group. The enalapril group received enalapril (10 mg twice daily), whereas the control and nicardipine groups received a placebo (1 tablet twice daily). The last dose of either enalapril or placebo was given at the time of preanaesthetic medication, approximately 2 h before surgery. Both treatments were well tolerated. At skin incision, nicardipine was administered to the nicardipine group (2 mg IV bolus injection, then 2 mg/h), and placebo (5% glucose solution) was infused in participants in the other groups | |
| Outcomes | Glomerular filtration rate | |
| Notes | Intervention started 2 days before surgery and continued until 2 h before surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No relevant description |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
Flesch 2009.
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective coronary artery bypass grafting Mean age (years): 1. 71.0 ± 4.6; 2. 69.9 ± 4.6 Sample size (male): 1. 43(36); 2. 44(33) Duration: 1. 84.1 ± 42.7 days; 2. 93.1 ± 41.5 days |
|
| Interventions | Eligible and consenting patients were enrolled 6 to 11 days before coronary artery bypass surgery. At this day, called visit 1. ACEIs or ARBs were to be discontinued if part of the previous medication. All other antihypertensive medications including long‐acting calcium channel blockers and beta blockers were allowed to be continued. Participants were randomized to receive either 8 mg of candesartan cilexetil or placebo. Treatment with the study drug was continued for 6 to 11 days until the day of operation. 1 day prior to CABG (visit 2) renal clearance was determined. 8 ± 3 days after CABG (visit 3) endpoint cognitive function tests were performed and renal clearance was determined again | |
| Outcomes | Rate of perioperative stroke; incidence of treatment related adverse events | |
| Notes | Drugs were given between 8 and 11 days prior to surgery The 8th page of the study reported the results of adverse events.There were no significant differences in the number of patients with adverse events with candesartan and placebo. The majority of adverse events were non‐serious. And most adverse events were considered as unlikely or not related with the study medication. A possible causal relationship was indicated in two adverse events in 52 patients in the candesartan and three adverse events in 53 patients in the placebo group. The authors did not provide further information on adverse events including what the exact adverse events were. We have tried to contact the corresponding author via email but did not obtain any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
Licker 1996.
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective infrarenal aortic surgery because of aortic aneurysm or atherosclerotic occlusive disease Mean age (years): 1. 68; 2. 69 Sample size (male): 1. 9 (7); 2. 11(10) |
|
| Interventions | 1. enalapril 50 μg/kg diluted in 20 ml of normal saline and injected IV over 5 min. 2. same volume of saline solution | |
| Outcomes | Cardiac index | |
| Notes | The drugs were given 25 min before induction of anaesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
Pretorius 2012.
| Methods | Prospective randomized controlled trial | |
| Participants | Undergoing elective cardiac surgery including CABG or valvular surgery Mean age (years): 1. 60.0 ± 12.0; 2. 58.7 ± 12.3; 3. 59.2 ± 12.3 Sample size (male): 1. 147(94); 2. 151(106); 3. 147(96) |
|
| Interventions | 1 week to 4 days prior to surgery, participants were randomized to treatment with placebo, ramipril (2.5 mg the first 3 days followed by 5 mg/day, with the dose reduced to 2.5 mg/day on the first postoperative day only), or spironolactone (25 mg/day) | |
| Outcomes | All‐cause mortality; ST segment change or new Q wave in ECG test; rate of perioperative stroke; length of hospital stay; hypotension; incidence of treatment related adverse events | |
| Notes | Ramipril group: 2.5 mg the first 3 days followed by 5 mg/d, with the dose reduced to 2.5 mg/d on the first postoperative day only The authors listed adverse events and serious adverse events in the table 3 but no further information in the main text. We have tried to contact the corresponding author via email but did not obtain any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All electrocardiograms and rhythm strips were reviewed in a blinded fashion by a single cardiac electrophysiologist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
Ryckwaert 2001.
| Methods | Prospective randomized controlled trial | |
| Participants | Patients scheduled for elective CABG Mean age (years): 1. 66.3 ± 4.2; 2. 60.1 ± 3.6 Sample size (male): 14(13) |
|
| Interventions | 1. IV enalapril 1 mg at intervals of 6 h for 2 days, starting at the time of surgical incision 2. placebo | |
| Outcomes | Cardiac index | |
| Notes | Enalapril started at the time of surgical incision and lasted for 2 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Unclear risk | Funding source not reported |
Walter 2002.
| Methods | Prospective randomized controlled trial | |
| Participants | Patients undergoing elective cardiopulmonary bypass surgery Mean age (years): 1. 64.8 ± 1.7 2. 61.9 ± 2.0 Sample size (male): 1. 22(17); 2. 21(16) |
|
| Interventions | 1. oral 7.5 mg enalapril on the first day and oral 20 mg/d enalapril on the following days 2. placebo |
|
| Outcomes | All‐cause mortality; concentration of creatine kinase, MB form ; ST segment change or new Q wave in ECG test; hypotension | |
| Notes | Participants in enalapril group were given 7.5 mg on the first day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, but no details available |
| Allocation concealment (selection bias) | Unclear risk | No relevant description, and no further details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, but no details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No relevant description |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information on dropouts specified |
| Selective reporting (reporting bias) | Unclear risk | Protocol inaccessible |
| Other bias | Low risk | No other source of bias found |
ACEIs: angiotensin‐converting enzyme inhibitors ARBs: angiotensin II type 1 receptor blockers CABG: coronary artery bypass graft surgery ECG: electrocardiograph IV: intravenous ST: the ST segment in electrocardiography
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andres 2006 | Drugs were not given perioperatively |
| Aronson 2011 | Review |
| Arora 2012 | Review |
| Bader 2012 | Review |
| Benedetto 2008 | Retrospective study |
| Boldt 1995a | The lead author has been accused of fraud, therefore the reliability of the results is questionable |
| Boldt 1995b | The lead author has been accused of fraud, therefore the reliability of the results is questionable |
| Boldt 1996 | The lead author has been accused of fraud, therefore the reliability of the results is questionable. We tried to contact the other authors of the research Boldt 1996 but received no response. We have decided that it is not helpful to include primary studies in a review when the results of the studies are likely to be biased |
| Briot 1988 | Did not measure our interested outcomes |
| Cieciura 2000 | Not perioperative drug administration |
| Coca 2013 | Not perioperative drug administration |
| Colson 1990 | Did not measure our interested outcomes |
| Dag 2013 | Retrospective study |
| Dahl 2013 | Not perioperative drug administration |
| Di Pasquale 1993 | Did not measure our interested outcomes |
| Fontes 2012 | Review |
| Friedrich 2009 | Review |
| Heck 2012 | Participants receiving adjuvant breast cancer therapy |
| Heropoulos 1995 | Did not measure our interested outcomes |
| Ibrahim 2013 | Not perioperative drug administration |
| Issa 2014 | Not perioperative drug administration |
| Kerut 2006 | Review |
| Kortekaas 2014 | The study design was not randomized. The data in the control group were retrieved from a biobank |
| Kottenberg‐Assenmacher 2008 | Did not measure our interested outcomes |
| Lazar 2008 | Review |
| Magnusson 1993 | Increase of the arterial pressure during the application of a tourniquet |
| Manche 1999 | Did not measure our interested outcomes |
| McCarthy 1990 | Did not measure our interested outcomes |
| Muller 2000 | Did not measure our interested outcomes |
| Pigott 1999 | Participants were already on ACEI |
| Poulsen 2014 | Participants in 1 group were already on ACEI 3 months prior to surgery |
| Proshchaev 2003 | No relevant compression |
| Schuetz 1998 | Did not measure our interested outcomes |
| Sharaf 2013 | Retrospective study |
| Taniguchi 2008 | Did not measure our interested outcomes |
| Tohmo 1993 | Did not measure our interested outcomes |
| Twersky 2014 | Not perioperative drug administration |
| Webb 1998 | Did not measure our interested outcomes |
| Zentner 2012 | Surgery under local anaesthesia only |
ACEI: angiotensin‐converting enzyme inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
Fan 2016.
| Methods | Parallel randomized controlled trial |
| Participants | Patients diagnosed with rheumatic valve diseases undergoing heart valve replacement operations |
| Interventions | Telmisartan, captopril, and placebo |
| Outcomes | Pulmonary vascular resistance, A‐aDO2, pulmonary neutrophil count, SOD malondialdehyde, NO, angiotensin II; Complications and hospital time. |
| Notes |
Fuentes‐Reyes 2016.
| Methods | Prospective, randomized, double‐blind study |
| Participants | Undergoing laparoscopic surgery |
| Interventions | Telmisartan and placebo |
| Outcomes | Plasma creatinine, creatinine clearance, etc. |
| Notes |
Tian 2015.
| Methods | Randomized trial |
| Participants | Patients selected for heart valve replacement surgery |
| Interventions | Untreated control, captopril pretreatment, single dose captopril |
| Outcomes | ICU stay, hospital stay, death; Postischemic Myocardial Cellular Injury and Proinflammatory Cytokines. |
| Notes |
Differences between protocol and review
Two new authors joined the team: Bo Yang and Fengying Xu.
We changed the original title of the protocol, 'Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing surgery‐related mortality and morbidity in adults', to 'Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults', as suggested by referee Pierre Foex.
We changed the original objectives of the protocol, 'to systematically assess the benefits and harms of administration (prophylaxis or treatment or both) of angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II type I receptors blockers (ARBs) in the short‐term perioperative period for the prevention of surgery related mortality and morbidity', to 'to systematically assess the benefits and harms of administration of ACEIs or ARBs perioperatively for the prevention of mortality and morbidity in adults (aged 18 years and above) undergoing any type of surgery under general anaesthesia' to keep coincident with the revised title and ensure the precision of the objectives description.
We rearranged the criteria for considering studies for this review and refined the presentation but did not change the exact meaning.
We added length of hospital stay as a secondary outcome to enrich the results.
We also added treatment related adverse effects to the secondary outcomes to enrich the results
We added description in the Data synthesis section 'SDchange were unavailable in the original report, we imputed standard deviations for changes from baseline with the following technique: SDchange = (SDbaseline2 + SDfinal2 ‐ 2*Corr*SDbaseline*SDfinal)0.5. Default value (0.8) imputed for the correlation value' to provide more detailed methodology.
We added the following to the Sensitivity analysis section: 'when we came across studies where the standard deviation of changes from baseline was missing, we imputed the missing standard deviation using an imputed value, Corr, for the correlation coefficient. Besides the default value (0.8), we used different hypothesized values of Corr based on reasoned argument to determine whether the overall result of the analysis was robust to the use of imputed correlation coefficients', to provide more detailed methodology.
We did not perform funnel plots because there were no more than three trials in each analysis.
Since all the included studies had high risk of bias, we did not perform sensitivity analysis. It was only possible to perform subgroup analysis according to the types of surgery and interventions because of the sparse data in other groups.
Contributions of authors
Zui Zou (ZZ), Hong B Yuan (HBY), Bo Yang (BY), Fengying Xu (FYX), Xiao Y Chen (XYC), Guan J Liu (GJL), Xue Y Shi (XYS)
Joint first authors: Zui Zou, Hong B Yuan, Bo Yang
Conceiving the review: ZZ, XYS
Co‐ordinating the review: ZZ, XYS
Undertaking manual searches: XYC
Screening search results: ZZ, XYC
Organizing retrieval of papers: ZZ, HBY
Screening retrieved papers against inclusion criteria: ZZ
Appraising quality of papers: ZZ, XYS
Abstracting data from papers: ZZ, XYC
Writing to authors of papers for additional information: XYS
Providing additional data about papers: HBY, BY
Obtaining and screening data on unpublished studies: HBY
Data management for the review: ZZ, XYS
Entering data into Review Manager (RevMan 5.3): BY
RevMan statistical data: ZZ, XYS
Other statistical analysis not using RevMan: ZZ, GJL
Double entry of data: ZZ, HBY
Interpretation of data: XYS
Statistical inferences: GJL
Writing the review: ZZ, BY, XYS
Revising the review: FYX, BY
Securing funding for the review: XYS
Performing previous work that was the foundation of the present study: ZZ, XYS
Guarantor for the review (one author): XYS
Person responsible for reading and checking review before submission: ZZ, XYS
Sources of support
Internal sources
No sources of support supplied
External sources
National Nature Science Foundation of China (81372103), China.
Key Program of Medical Science Development of PLA (BWS12J027), China.
Program of Shanghai Municipal Health Planning Commission (2013SY025), China.
Shanghai Rising‐Star Program (15QA1405000), China.
Natural Science Foundation of Shanghai (14ZR1413700), China.
Declarations of interest
See: Sources of support.
Zui Zou: none known
Hong B Yuan: none known
Bo Yang: none known
Fengying Xu: none known
Xiao Y Chen: none known
Guan J Liu: none known
Xue Y Shi: none known
Joint first author
Joint first author
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Billings 2012 {published data only}
- Billings FT 4th, Balaguer JM, C‐Y, Wright P, Petracek MR, Byrne JG, et al. Comparative effects of angiotensin receptor blockade and ACE inhibition on the fibrinolytic and inflammatory responses to cardiopulmonary bypass. Clinical Pharmacology and Therapeutics 2012;91(6):1065‐73. [PUBMED: 22549281] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colson 1992 {published data only}
- Colson P, Ribstein J, Seguin JR, Marty‐Ane C, Roquefeuil B. Mechanisms of renal hemodynamic impairment during infrarenal aortic cross‐clamping. Anesthesia and Analgesia 1992;75(1):18‐23. [PUBMED: 1616156] [DOI] [PubMed] [Google Scholar]
Flesch 2009 {published data only}
- Flesch M, Knipp S, Kessler G, Geissler HJ, Massoudy P, Wilhelm H, et al. ARTA: AT1‐receptor blocker therapy in patients undergoing coronary artery bypass grafting. Clinical Research in Cardiology: Official Journal of the German Cardiac Society 2009;98(1):33‐43. [PUBMED: 18853093] [DOI] [PubMed] [Google Scholar]
Licker 1996 {published data only}
- Licker M, Bednarkiewicz M, Neidhart P, Pretre R, Montessuit M, Favre H, et al. Preoperative inhibition of angiotensin‐converting enzyme improves systemic and renal haemodynamic changes during aortic abdominal surgery. British Journal of Anaesthesia 1996;76(5):632‐9. [PUBMED: 8688261] [DOI] [PubMed] [Google Scholar]
Pretorius 2012 {published data only}
- Pretorius M, Murray KT, Yu C, Byrne JG, Billings FT 4th, Petracek MR, et al. Angiotensin‐converting enzyme inhibition or mineralocorticoid receptor blockade do not affect prevalence of atrial fibrillation in patients undergoing cardiac surgery. Critical Care Medicine 2012;40(10):2805‐12. [PUBMED: 22824930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryckwaert 2001 {published data only}
- Ryckwaert F, Colson P, Ribstein J, Boccara G, Guillon G. Haemodynamic and renal effects of intravenous enalaprilat during coronary artery bypass graft surgery in patients with ischaemic heart dysfunction. British Journal of Anaesthesia 2001;86(2):169‐75. [PUBMED: 11573655] [DOI] [PubMed] [Google Scholar]
Walter 2002 {published data only}
- Walter T, Helber U, Bail D, Heller W, Hoffmeister HM. Influence of ACE inhibition on myocardial damage, the Kallikrein‐Kinin system and hemostasis during cardiopulmonary bypass surgery. The Thoracic and Cardiovascular Surgeon 2002;50(3):150‐4. [PUBMED: 12077687] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andres 2006 {published data only}
- Andres A, Morales E, Morales JM, Bosch I, Campo C, Ruilope LM. Efficacy and safety of valsartan, an angiotensin II receptor antagonist, in hypertension after renal transplantation: a randomized multicenter study. Transplantation Proceedings 2006;38(8):2419‐23. [PUBMED: 17097955] [DOI] [PubMed] [Google Scholar]
Aronson 2011 {published data only}
- Aronson S, Varon J. Hemodynamic control and clinical outcomes in the perioperative setting. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):509‐25. [PUBMED: 21493093] [DOI] [PubMed] [Google Scholar]
Arora 2012 {published data only}
- Arora P, Kolli H, Nainani N, Nader N, Lohr J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2012;26(4):687‐97. [PUBMED: 22516466] [DOI] [PubMed] [Google Scholar]
Bader 2012 {published data only}
- Bader AM. Advances in preoperative risk assessment and management. Current Problems in Surgery 2012;49(1):11‐40. [PUBMED: 22137353] [DOI] [PubMed] [Google Scholar]
Benedetto 2008 {published data only}
- Benedetto U, Melina G, Capuano F, Comito C, Bianchini R, Simon C, et al. Preoperative angiotensin‐converting enzyme inhibitors protect myocardium from ischemia during coronary artery bypass graft surgery. Journal of Cardiovascular Medicine (Hagerstown, Md.) 2008;9(11):1098‐103. [PUBMED: 18852580] [DOI] [PubMed] [Google Scholar]
Boldt 1995a {published data only}
- Boldt J, Schindler E, Harter K, Gorlach G, Hempelmann G. Influence of intravenous administration of angiotensin‐converting enzyme inhibitor enalaprilat on cardiovascular mediators in cardiac surgery patients. Anesthesia and Analgesia 1995;80(3):480‐5. [PUBMED: 7864411] [DOI] [PubMed] [Google Scholar]
Boldt 1995b {published data only}
- Boldt J, Schindler E, Wollbruck M, Gorlach G, Hempelmann G. Cardiorespiratory response of intravenous angiotensin‐converting enzyme inhibitor enalaprilat in hypertensive cardiac surgery patients. Journal of Cardiothoracic and Vascular Anesthesia 1995;9(1):44‐9. [PUBMED: 7718754] [DOI] [PubMed] [Google Scholar]
Boldt 1996 {published data only}
- Boldt J, Rothe G, Schindler E, Doll C, Gorlach G, Hempelmann G. Can clonidine, enoximone, and enalaprilat help to protect the myocardium against ischaemia in cardiac surgery?. Heart (British Cardiac Society) 1996;76(3):207‐13. [PUBMED: 8868976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Briot 1988 {published data only}
- Briot D, Schmitt JP, Coumaros G, Conraux C, Dupeyron JP. Captopril potentiation of the hypotension induced by halothane [Potentialisation par le captopril de l'hypotension induite par l'halothane]. Annales Francaises d'Anesthesie et de Reanimation 1988;7(1):22‐5. [PUBMED: 2831758] [DOI] [PubMed] [Google Scholar]
Cieciura 2000 {published data only}
- Cieciura T, Senatorski G, Rell K, Baczkowska T, Paczek L, Gradowska L, et al. Influence of angiotensin‐converting enzyme inhibitor treatment of the carotid artery intima‐media complex in renal allograft recipients. Transplantation Proceedings 2000;32(6):1335‐6. [PUBMED: 10995971] [DOI] [PubMed] [Google Scholar]
Coca 2013 {published data only}
- Coca SG, Garg AX, Swaminathan M, Garwood S, Hong K, Thiessen‐Philbrook H, et al. Preoperative angiotensin‐converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association ‐ European Renal Association 2013;28(11):2787‐99. [PUBMED: 24081864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colson 1990 {published data only}
- Colson P, Ribstein J, Mimran A, Grolleau D, Chaptal PA, Roquefeuil B. Effect of angiotensin converting enzyme inhibition on blood pressure and renal function during open heart surgery. Anesthesiology 1990;72(1):23‐7. [PUBMED: 2404429] [DOI] [PubMed] [Google Scholar]
Dag 2013 {published data only}
- Dag O, Kaygin MA, Aydin A, Limandal HK, Arslan U, Kiymaz A, et al. Is administration of preoperative angiotensin‐converting enzyme inhibitors important for renal protection after cardiac surgery?. Renal Failure 2013;35(5):754‐60. [PUBMED: 23521631] [DOI] [PubMed] [Google Scholar]
Dahl 2013 {published data only}
- Dahl JS, Videbaek L, Poulsen MK, Pellikka PA, Veien K, Andersen LI, et al. Prevention of atrial fibrillation in patients with aortic valve stenosis with candesartan treatment after aortic valve replacement. International Journal of Cardiology 2013;165(2):242‐6. [PUBMED: 21907422] [DOI] [PubMed] [Google Scholar]
Di Pasquale 1993 {published data only}
- Pasquale P, Paterna S, Valenza M, Valdes L, Albano V, Trombino G, et al. Effects of captopril on myocardial protection during cardioplegia. International Journal of Cardiology 1993;42(3):225‐30. [PUBMED: 8138330] [DOI] [PubMed] [Google Scholar]
Fontes 2012 {published data only}
- Fontes ML, Varon J. Perioperative hypertensive crisis: newer concepts. International Anesthesiology Clinics 2012;50(2):40‐58. [PUBMED: 22481555] [DOI] [PubMed] [Google Scholar]
Friedrich 2009 {published data only}
- Friedrich A, Helmy T. Prevention of perioperative myocardial infarction. International Anesthesiology Clinics 2009;47(4):13‐36. [PUBMED: 19820476] [DOI] [PubMed] [Google Scholar]
Heck 2012 {published data only}
- Heck SL, Gulati G, Ree AH, Schulz‐Menger J, Gravdehaug B, Rosjo H, et al. Rationale and design of the prevention of cardiac dysfunction during an Adjuvant Breast Cancer Therapy (PRADA) Trial. Cardiology 2012;123(4):240‐7. [PUBMED: 23207160] [DOI] [PubMed] [Google Scholar]
Heropoulos 1995 {published data only}
- Heropoulos M, Schieren H, Seltzer JL, Bartkowski RR, Lessin J, Torjman M, et al. Intraoperative hemodynamic, renin, and catecholamine responses after prophylactic and intraoperative administration of intravenous enalaprilat. Anesthesia and Analgesia 1995;80(3):583‐90. [PUBMED: 7864430] [DOI] [PubMed] [Google Scholar]
Ibrahim 2013 {published data only}
- Ibrahim HN, Jackson S, Connaire J, Matas A, Ney A, Najafian B, et al. Angiotensin II blockade in kidney transplant recipients. Journal of the American Society of Nephrology 2013;24(2):320‐7. [PUBMED: 23308016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Issa 2014 {published data only}
- Issa N, Ortiz F, Reule SA, Kukla A, Kasiske BL, Mauer M, et al. The renin‐aldosterone axis in kidney transplant recipients and its association with allograft function and structure. Kidney International 2014;85(2):404‐15. [PUBMED: 23965522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerut 2006 {published data only}
- Kerut EK, Geraci SA, Falterman C, Hunter D, Hanawalt C, Giles TD. Atherosclerotic renal artery stenosis and renovascular hypertension: clinical diagnosis and indications for revascularization. Journal of Clinical Hypertension (Greenwich, Conn.) 2006;8(7):502‐9. [PUBMED: 16849904] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kortekaas 2014 {published data only}
- Kortekaas KE, Meijer CA, Hinnen JW, Dalman RL, Xu B, Hamming JF, et al. ACE inhibitors potently reduce vascular inflammation, results of an open proof‐of‐concept study in the abdominal aortic aneurysm. PLoS One 2014;9(12):e111952. [PUBMED: 25474105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kottenberg‐Assenmacher 2008 {published data only}
- Kottenberg‐Assenmacher E, Massoudy P, Jakob H, Philipp T, Peters J. Chronic AT1‐receptor blockade does not alter cerebral oxygen supply/demand ratio during cardiopulmonary bypass in hypertensive patients. Acta Anaesthesiologica Scandinavica 2008;52(1):73‐80. [PUBMED: 17976222] [DOI] [PubMed] [Google Scholar]
Lazar 2008 {published data only}
Magnusson 1993 {published data only}
- Magnusson L, Rebagliati M, Buchser E. Elevation of arterial pressure during surgery with a pneumatic tourniquet: an independent action of renin? [Elevation de la tension arterielle lors de chirurgie sous garrot pneumatique: un mecanisme independant de la renine?]. Canadian Journal of Anaesthesia/Journal canadien d'anesthesie 1993;40(7):596‐600. [PUBMED: 8403133] [DOI] [PubMed] [Google Scholar]
Manche 1999 {published data only}
- Manche A, Galea J, Busuttil W. Tolerance to ACE inhibitors after cardiac surgery. European Journal of Cardio‐Thoracic Surgery: Official Journal of the European Association for Cardio‐Thoracic Surgery 1999;15(1):55‐60. [PUBMED: 10077374] [DOI] [PubMed] [Google Scholar]
McCarthy 1990 {published data only}
- McCarthy GJ, Hainsworth M, Lindsay K, Wright JM, Brown TA. Pressor responses to tracheal intubation after sublingual captopril. A pilot study. Anaesthesia 1990;45(3):243‐5. [PUBMED: 2185673] [DOI] [PubMed] [Google Scholar]
Muller 2000 {published data only}
- Muller M, Schindler E, Kwapisz M, Klemm S, Akinturk H, Heidt M, et al. Effect of intraoperative angiotensin‐converting enzyme inhibition by quinaprilat on hypertension after coronary artery surgery. British Journal of Anaesthesia 2000;84(3):396‐8. [PUBMED: 10793603] [DOI] [PubMed] [Google Scholar]
Pigott 1999 {published data only}
- Pigott DW, Nagle C, Allman K, Westaby S, Evans RD. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. British Journal of Anaesthesia 1999;83(5):715‐20. [PUBMED: 10690132] [DOI] [PubMed] [Google Scholar]
Poulsen 2014 {published data only}
- Poulsen FR, Munthe S, Soe M, Halle B. Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: a randomized trial. Clinical Neurology and Neurosurgery 2014;123:4‐8. [PUBMED: 25012003] [DOI] [PubMed] [Google Scholar]
Proshchaev 2003 {published data only}
- Proshchaev KI. Use of enalapril in the preparation of patients with arterial hypertension to the surgical procedures [Ispol'zovanie enalaprila glia podgotovki bol'nykh arterial'noi hypertension k khirurgicheskim vmeshatel'stvam]. Terapevticheskii arkhiv 2003;75(12):43‐7. [PUBMED: 14959469] [PubMed] [Google Scholar]
Schuetz 1998 {published data only}
- Schuetz WH, Lindner KH, Georgieff M, Mueller S, Oertel F, Radermacher P, et al. The effect of i.v. enalaprilat in chronically treated hypertensive patients during cardiac surgery. Acta Anaesthesiologica Scandinavica 1998;42(8):929‐35. [PUBMED: 9773137] [DOI] [PubMed] [Google Scholar]
Sharaf 2013 {published data only}
- Sharaf A, Davoodi S, Karimi AA, Ahmadi H, Abbasi K, Fathollahi MS, et al. Impact of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on mortality of coronary artery bypass grafting. Journal of Tehran University Heart Center 2013;8(4):177‐81. [PMC free article] [PubMed] [Google Scholar]
Taniguchi 2008 {published data only}
Tohmo 1993 {published data only}
- Tohmo H, Karanko M, Scheinin M, Viinamaki O, Salonen M, Nieminen V. Enalapril premedication attenuates the blood pressure response to tracheal intubation and stabilizes postoperative blood pressure after controlled hypotension with sodium nitroprusside in neurovascular patients. Journal of Neurosurgical Anesthesiology 1993;5(1):13‐21. [PUBMED: 8431665] [DOI] [PubMed] [Google Scholar]
Twersky 2014 {published data only}
- Twersky RS, Goel V, Narayan P, Weedon J. The risk of hypertension after preoperative discontinuation of angiotensin‐converting enzyme inhibitors or angiotensin receptor antagonists in ambulatory and same‐day admission patients. Anesthesia and Analgesia 2014;118(5):938‐44. [PUBMED: 24681657] [DOI] [PubMed] [Google Scholar]
Webb 1998 {published data only}
- Webb CM, Underwood R, Anagnostopoulos C, Bennett JG, Pepper J, Lincoln C, et al. The effect of angiotensin converting enzyme inhibition on myocardial function and blood pressure after coronary artery bypass surgery‐‐a randomised study. European Journal of Cardio‐Thoracic Surgery: Official Journal of the European Association for Cardio‐Thoracic Surgery 1998;13(1):42‐8. [PUBMED: 9504729] [DOI] [PubMed] [Google Scholar]
Zentner 2012 {published data only}
- Zentner D, Pedagogos E, Yapanis A, Karapanagiotidis S, Kinghorn A, Alexiou A, et al. Can losartan and blood pressure control peri arteriovenous fistula creation ameliorate the early associated left ventricular hypertrophic response a randomised placebo controlled trial. BMC Research Notes 2012;5:260. [PUBMED: 22642740] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fan 2016 {published data only}
- Fan Y, Zhang D, Xiang D. Delayed protective effect of telmisartan on lung ischemia/reperfusion injury in valve replacement operations. Experimental and Therapeutic Medicine 2016;12(4):2577‐81. [PUBMED: 27698759 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuentes‐Reyes 2016 {published data only}
- Fuentes‐Reyes RA, Pacheco‐Patiño MF, Ponce‐Escobedo AN, Muñoz‐Maldonado GE, Hernandez‐Guedea MA. [Impact of telmisartan on glomerular filtration in laparoscopic surgery. A double blinded randomised controlled study]. [Article in Spanish]. Cirugía y Cirujanos 2017;85(1):34‐40. [PUBMED: 27417705] [DOI] [PubMed] [Google Scholar]
Tian 2015 {published data only}
- Tian Y, Li H, Liu P, Xu JM, Irwin MG, Xia Z, et al. Captopril pretreatment produces an additive cardioprotection to isoflurane preconditioning in attenuating myocardial ischemia reperfusion injury in rabbits and in humans. Mediators of Inflammation 2015:819232. [PUBMED: 26273143 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Arora 2008
- Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, et al. Preoperative use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(5):1266‐73. [PUBMED: 18667735] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blessberger 2014
- Blessberger H, Kammler J, Domanovits H, Schlager O, Wildner B, Azar D, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD004476.pub2; PUBMED: 25233038] [DOI] [PubMed] [Google Scholar]
Boeken 1999
- Boeken U, Feindt P, Mohan E, Zimmermann N, Micek M, Kalweit G, et al. Post‐perfusion syndrome and disturbed microcirculation after cardiac surgery: the role of angiotensin‐converting‐enzyme inhibitors. The Thoracic and Cardiovascular Surgeon 1999;47(6):347‐51. [PUBMED: 10670790] [DOI] [PubMed] [Google Scholar]
Brenner 2003
- Brenner BM, Zagrobelny J. Clinical renoprotection trials involving angiotensin II‐receptor antagonists and angiotensin‐converting‐enzyme inhibitors. Kidney International 2003;83:S77‐85. [PUBMED: 12864880] [DOI] [PubMed] [Google Scholar]
Cohn 1991
- Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine‐isosorbide dinitrate in the treatment of chronic congestive heart failure. The New England Journal of Medicine 1991;325(5):303‐10. [PUBMED: 2057035] [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [PUBMED: 7873954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coriat 1994
- Coriat P, Richer C, Douraki T, Gomez C, Hendricks K, Giudicelli JF, et al. Influence of chronic angiotensin‐converting enzyme inhibition on anesthetic induction. Anesthesiology 1994;81(2):299‐307. [PUBMED: 8053578] [DOI] [PubMed] [Google Scholar]
Deakin 1998
- Deakin CD, Dalrymple‐Hay MJ, Jones P, Monro JL. Effects of angiotensin converting enzyme inhibition on systemic vascular resistance and vasoconstrictor requirements during hypothermic cardiopulmonary bypass. European Journal of Cardio‐Thoracic Surgery 1998;13(5):546‐50. [PUBMED: 9663536] [DOI] [PubMed] [Google Scholar]
Devereaux 2008
- Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. The Lancet 2008;371(9627):1839‐47. [PUBMED: 18479744] [DOI] [PubMed] [Google Scholar]
Dodds 1993
- Dodds TM, Stone JG, Coromilas J, Weinberger M, Levy DG. Prophylactic nitroglycerin infusion during noncardiac surgery does not reduce perioperative ischemia. Anesthesia and Analgesia 1993;76(4):705‐13. [PUBMED: 8466005] [DOI] [PubMed] [Google Scholar]
Drenger 2012
- Drenger B, Fontes ML, Miao Y, Mathew JP, Gozal Y, Aronson S, et al. Patterns of use of perioperative angiotensin‐converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in‐hospital morbidity and mortality. Circulation 2012;126(3):261‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 1994
- Ellis JE, Drijvers G, Pedlow S, Laff SP, Sorrentino MJ, Foss JF, et al. Premedication with oral and transdermal clonidine provides safe and efficacious postoperative sympatholysis. Anesthesia and Analgesia 1994;79(6):1133‐40. [PUBMED: 7978438] [DOI] [PubMed] [Google Scholar]
Fariello 1989
- Fariello R, Alicandri C, Boni E, Zaninelli A, Cantalamessa A, Corda L, et al. Angiotensin converting enzyme inhibition, parasympathetic activity and exercise in essential hypertension. Drugs Under Experimental and Clinical Research 1989;15(11‐12):571‐6. [PUBMED: 2561594] [PubMed] [Google Scholar]
Fleisher 2007
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta‐blocker therapy‐‐a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesthesia and Analgesia 2007;104(1):15‐26. [PUBMED: 17179239] [DOI] [PubMed] [Google Scholar]
Freeman 2009
- Freeman WK, Gibbons RJ. Perioperative cardiovascular assessment of patients undergoing noncardiac surgery. Mayo Clinic Proceedings 2009;84(1):79‐90. [PUBMED: 19121258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 1995
- Garg R, Yusuf S. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995;18:1450‐6. [PUBMED: 7654275] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 2001
- Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). Journal of the American College of Cardiology 2001;38(7):2101‐13. [PUBMED: 11738322] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Krum 2006
- Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. American Journal of Physiology. Heart and Circulatory Physiology 2006;290(4):H1706‐12. [PUBMED: 16284232] [DOI] [PubMed] [Google Scholar]
Kwapisz 2004
- Kwapisz MM, Muller M, Schindler E, Demir S, Veit M, Roth P, et al. The effect of intravenous quinaprilat on plasma cytokines and hemodynamic variables during cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(1):53‐8. [PUBMED: 14973800] [DOI] [PubMed] [Google Scholar]
Lee 2004
- Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta‐analysis: angiotensin‐receptor blockers in chronic heart failure and high‐risk acute myocardial infarction. Annals of Internal Medicine 2004;141(9):693‐704. [PUBMED: 15520426] [DOI] [PubMed] [Google Scholar]
Licker 2000
- Licker M, Schweizer A, Hohn L, Farinelli C, Morel DR. Cardiovascular responses to anesthetic induction in patients chronically treated with angiotensin‐converting enzyme inhibitors. Canadian Journal of Anaesthesia 2000;47(5):433‐40. [PUBMED: 10831200] [DOI] [PubMed] [Google Scholar]
Lloyd‐Jones 2010
- Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, Simone G, et al. Heart disease and stroke statistics‐‐2010 update: a report from the American Heart Association. Circulation 2010;121(7):e46‐215. [PUBMED: 20019324] [DOI] [PubMed] [Google Scholar]
Miceli 2009
- Miceli A, Capoun R, Fino C, Narayan P, Bryan AJ, Angelini GD, et al. Effects of angiotensin‐converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. Journal of the American College of Cardiology 2009;54(19):1778‐84. [PUBMED: 19682819] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. The Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Nakao 2003
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin‐II receptor blocker and angiotensin‐converting‐enzyme inhibitor in non‐diabetic renal disease (COOPERATE): a randomised controlled trial. The Lancet 2003;361(9352):117‐24. [PUBMED: 12531578] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rouleau 2008
- Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S, Johnstone D, et al. Effects of angiotensin‐converting enzyme inhibition in low‐risk patients early after coronary artery bypass surgery. Circulation 2008;117(1):24‐31. [PUBMED: 18071079] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Stata
- StataCorp, College Station, TX, USA. Stata. version 11.
Strauss 1984
- Strauss R, Gavras I, Vlahakos D, Gavras H. Enalaprilat in hypertensive emergencies. Journal of Clinical Pharmacology 1986;26(1):39‐43. [PUBMED: 3005376] [DOI] [PubMed] [Google Scholar]
Stuhmeier 1996
- Stuhmeier KD, Mainzer B, Cierpka J, Sandmann W, Tarnow J. Small, oral dose of clonidine reduces the incidence of intraoperative myocardial ischemia in patients having vascular surgery. Anesthesiology 1996;85(4):706‐12. [PUBMED: 8873539] [DOI] [PubMed] [Google Scholar]
Tschope 2002
- Tschope C, Schultheiss HP, Walther T. Multiple interactions between the renin‐angiotensin and the kallikrein‐kinin systems: role of ACE inhibition and AT1 receptor blockade. Journal of Cardiovascular Pharmacology 2002;39(4):478‐87. [PUBMED: 11904521] [DOI] [PubMed] [Google Scholar]
Tuman 1995
- Tuman KJ, McCarthy RJ, O'Connor CJ, Holm WE, Ivankovich AD. Angiotensin‐converting enzyme inhibitors increase vasoconstrictor requirements after cardiopulmonary bypass. Anesthesia and Analgesia 1995;80(3):473‐9. [PUBMED: 7864410] [DOI] [PubMed] [Google Scholar]
Warltier 2000
- Warltier DC, Pagel PS, Kersten JR. Approaches to the prevention of perioperative myocardial ischemia. Anesthesiology 2000;92(1):253‐9. [PUBMED: 10638923] [DOI] [PubMed] [Google Scholar]
Wiesbauer 2007
- Wiesbauer F, Schlager O, Domanovits H, Wildner B, Maurer G, Muellner M, et al. Perioperative beta‐blockers for preventing surgery‐related mortality and morbidity: a systematic review and meta‐analysis. Anesthesia and Analgesia 2007;104(1):27‐41. [PUBMED: 17179240] [DOI] [PubMed] [Google Scholar]
Zou 2009
- Zou Z, Xi GL, Yuan HB, Zhu QF, Shi XY. Telmisartan versus angiotension‐converting enzyme inhibitors in the treatment of hypertension: A meta‐analysis of randomized controlled trials. Journal of Human Hypertension 2009;23(5):339‐49. [PUBMED: 18987649] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zou 2011
- Zou Z, Yuan HB, Chen XY, Liu GJ, Shi XY. Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing surgery‐related mortality and morbidity. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009210] [DOI] [PMC free article] [PubMed] [Google Scholar]


