Abstract
Background
Decreased concentration of nitric oxide has been proposed as one of the possible cellular mechanisms of necrotising enterocolitis (NEC). Arginine can act as a substrate for production of nitric oxide in the tissues, and arginine supplementation may help to prevent NEC.
Objectives
To examine the effect of arginine supplementation (administered by any route) on the incidence of NEC in preterm neonates. To conduct subgroup analyses based on the dose of arginine and the gestational age of participants (≤ 32 weeks, > 32 weeks).
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4), MEDLINE via PubMed (from 1966 to 12 May 2016), Embase (from 1980 to 12 May 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; from 1982 to 12 May 2016). We also searched clinical trials databases, conference proceedings and reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised and quasi‐randomised controlled trials of arginine supplementation (administered orally or parenterally for at least seven days, in addition to what an infant may be receiving from an enteral or parenteral source) compared with placebo or no treatment.
Data collection and analysis
We assessed the methodological quality of trials by using information obtained from study reports and through personal communication with study authors. We extracted data on relevant outcomes and estimated and reported the effect size as risk ratio (RR), risk difference (RD) and mean difference (MD), as appropriate. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Main results
We identified three eligible studies that included a total of 285 neonates (140 received arginine) from three countries. We assessed the overall methodological quality of the included studies as good. We noted a statistically significant reduction in risk of development of NEC (any stage) among preterm neonates in the arginine group compared with the placebo group (RR 0.38, 95% confidence interval (CI) 0.23 to 0.64; I2 = 27%) (RD ‐0.19, 95% CI ‐0.28 to ‐0.10; I2 = 0%) and rated the quality of evidence as moderate. The number needed to treat for an additional beneficial outcome (NNTB) as required to prevent the development of NEC (any stage) was 6 (95% CI 4 to 10). Study results showed a statistically significant reduction in risk of development of NEC stage 1 (RR 0.37, 95% CI 0.15 to 0.90; I2 = 52%) (RD ‐0.07, 95% CI ‐0.14 to ‐0.01; I2 = 0%) and NEC stage 3 (RR 0.13, 95% CI 0.02 to 1.03; I2 = 0%) (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; I2 = 89%) in the arginine group compared with the control group; the quality of evidence was moderate.
Arginine supplementation was associated with a significant reduction in death related to NEC (RR 0.18, 95% CI 0.03 to 1.00; I2 = 0%) (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; I2 = 87%). Results showed clinical heterogeneity in mortality rates. Mortality due to any cause was not significantly different between arginine and control or no treatment groups (RR 0.77, 95% CI 0.41 to 1.45; I2 = 42%) (RD ‐0.03, 95% CI ‐0.10 to 0.04; I2 = 79%). Investigators noted no significant side effects directly attributable to arginine, including hypotension or alterations in glucose homeostasis. Follow‐up data from one trial revealed no statistically significant differences in adverse outcomes (cerebral palsy, cognitive delay, bilateral blindness or hearing loss requiring hearing aids) at 36 months. Limitations of the present findings include a relatively small overall sample size.
Authors' conclusions
Administration of arginine to preterm infants may prevent development of NEC. Because information was provided by three small trials that included 285 participants, the data are insufficient at present to support a practice recommendation. A multi‐centre randomised controlled study that is focused on the incidence of NEC, particularly at more severe stages (2 and 3), is needed.
Keywords: Humans; Infant, Newborn; Arginine; Arginine/adverse effects; Arginine/therapeutic use; Cause of Death; Enterocolitis, Necrotizing; Enterocolitis, Necrotizing/epidemiology; Enterocolitis, Necrotizing/prevention & control; Glutamine; Glutamine/therapeutic use; Hypotension; Hypotension/chemically induced; Infant, Premature; Infant, Premature, Diseases; Infant, Premature, Diseases/epidemiology; Infant, Premature, Diseases/prevention & control; Numbers Needed To Treat; Randomized Controlled Trials as Topic
Plain language summary
Adding arginine to prevent necrotising enterocolitis in preterm infants
What is the issue? Necrotising enterocolitis (NEC) is a condition in which inflammation damages an infant's gastrointestinal (GI) tract. The rate of NEC ranges from 4% to 22% in very low birth weight infants. Necrotising enterocolitis may be caused by an infant's immaturity, lack of blood flow to the GI tract and surface (mucosa) breakdown resulting from infection or feeding with formula. To protect the GI tract, the body makes a natural substance ‐ nitric oxide ‐ from the amino acid arginine. Plasma arginine concentrations are reported to be low in very low birth weight infants and preterm infants who develop NEC. Adding extra arginine to the feeding solution may prevent NEC.
Why is this important? NEC can result in permanent damage to the intestine, the need for multiple surgeries, prolonged hospital stay, death and increased cost to the healthcare system.
What evidence did we find? Review authors searched the literature for controlled studies evaluating the efficacy and safety of arginine supplementation. Adding extra arginine to a preterm infant's feed reduced the risk of NEC in three good quality studies that included 285 infants born at less than 34 weeks' gestation. Six infants had to be treated, for one to benefit from treatment. Researchers reported no significant side effects directly attributable to too much arginine in the first 28 days, and one study reported no long‐term (36 months) developmental delays. Possible effects of supplementing arginine include lower blood pressure and changes in blood glucose control.
What does this mean? Arginine supplementation may reduce the incidence and severity of NEC in preterm infants. Results are limited, as studies included only a few patients. A large study that includes infants from multiple centres is needed to verify these findings.
Summary of findings
Summary of findings for the main comparison. Arginine compared with placebo for prevention of necrotising enterocolitis.
| Arginine compared with placebo for prevention of necrotising enterocolitis | ||||||
| Patient or population: prevention of necrotising enterocolitis in preterm infants Settings: neonatal intensive care units Intervention: arginine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Arginine | |||||
| NEC any stage Bell's criteria | 303 per 1000 | 115 per 1000 (70 to 194) |
RR 0.38 (0.23 to 0.64) |
285 (3 studies) | ⊕⊕⊕⊝ moderatea,b | |
| Stage 1 NEC Bell's criteria | 110 per 1000 | 41 per 1000 (17 to 99) | RR 0.37 (0.15 to 0.9) | 285 (3 studies) | ⊕⊕⊕⊝ moderatea,b | |
| Stage 2 NEC Bell's criteria | 138 per 1000 | 70 per 1000 (34 to 146) | RR 0.51 (0.25 to 1.06) | 285 (3 studies) | ⊕⊕⊕⊝ moderatea,b | |
| Stage 3 NEC Bell's criteria | 55 per 1000 | 7 per 1000 (1 to 57) | RR 0.13 (0.02 to 1.03) | 285 (3 studies) | ⊕⊕⊕⊝ moderatea,b | |
| Mortality due to any cause | 124 per 1000 | 94 per 1000 (24 to 164) |
RR 0.77 (0.41 to 1.45) |
285 (3 studies) | ⊕⊕⊝⊝ lowa,b,c | |
| Side effects ‐ hypotension | 104 per 1000 | 107 per 1000 (104 to 104) |
RR 1.03 (0.41 to 2.59) |
192 (2 studies) | ⊕⊕⊕⊝ moderatea,b | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effects of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aMinor sources of indirectness included (1) age and weight of included neonates (< 32 weeks and < 1250 grams vs < 34 weeks and < 1500 grams); (2) placebo composition (saline vs 5% glucose vs no treatment). These were not deemed by review authors to warrant downgrading of recommendations. bSmall sample size. cResults showed clinical heterogeneity in the studied population, as Amin 2002 reported overall mortality of 2%, El‐Shimi 2015 16% and Polycarpou 2013a 25%.
Background
Description of the condition
Bell 1978 reported that necrotising enterocolitis (NEC) occurs in three different stages; this report was later modified (Walsh 1986). Clinical features of NEC vary from constitutional symptoms such as feeding intolerance to severe systemic symptoms including cardiorespiratory decompensation, coagulopathy, peritonitis and ascites with or without pneumoperitoneum. As the result of marked variability in clinical manifestations, the reported incidence of NEC varies from 4% to 22% among very low birth weight infants at various centres (Stoll 1994).
The exact cause of NEC is unknown. Suspected pathophysiological mechanisms include immaturity, ischaemia, disruption of mucosal integrity, formula feeding, hyperosmolar load to intestine, infection and bacterial translocation (Caplan 2001). Animal experiments have shown that subsequent to ischaemia, intestinal permeability is increased (Langer 1993), and this may lead to passage of bacteria and endotoxins into the intestinal wall, affecting mucosal integrity. Intestinal dysmotility and disruption in mucosal integrity play important roles in the pathogenesis of NEC (Di Lorenzo 1995).
Nitric oxide is important for normal gastrointestinal (GI) function from several perspectives. First, nitric oxide is an important regulator of vasomotor function (Stark 1992). An inadequate concentration of nitric oxide leads to vasoconstriction of the intestinal vessels, which might result in ischaemia and a predisposition to NEC. Second, nitric oxide acts as a neurotransmitter for enteric non‐adrenergic non‐cholinergic neurons that regulate peristalsis (Boeckxstaens 1991). Lack or inadequacy of nitric oxide can alter intestinal motility. Third, nitric oxide inhibits leucocyte adherence and modulates inflammatory responses to various insults within the intestine (Akisu 2002). Therefore, normal concentrations of nitric oxide must be maintained within the GI tract. Owing to its volatile nature, nitric oxide cannot be delivered to the GI tract in its original form. An indirect method of achieving adequate concentration involves supplementing substrates such as arginine to promote production.
Description of the intervention
Arginine is an amino acid that is a precursor for proteins (Wu 1998) and nitric oxide synthase. The availability of plasma arginine is important for formation of nitric oxide (Castillo 1995; Zamora 1998). Plasma arginine concentration was found to be 95 ± 25 μmol/L in term breast‐fed (N = 16) infants (Wu 1986). In extremely low birth weight infants (birth weight < 1000 grams) who were given formula feeds (N = 2), the mean arginine concentration was found to be 37 μmol/L (range 13 to 60 μmol/L), and the mean arginine concentration was 53 μmol/L (range 3 to 116 μmol/L) in breast‐fed (N = 9) infants (Ventura 1987). In a case control study (Becker 2000), researchers found that arginine and glutamine levels were similar at day 3, but were significantly lower at days 7 and 14 in infants who developed NEC. Zamora 1997 reported statistically significantly reduced plasma arginine concentrations in preterm infants who developed NEC compared with control infants, even after adjustments were made for intake of arginine and day of life. Recommended minimum and maximum concentrations of arginine in preterm infant formula are 72 mg/100 kcal and 104 mg/100 kcal, respectively (Klein 2002). These requirements are greater in infants who are stressed owing to higher utilisation in conditions such as NEC or pulmonary hypertension. The concentration of arginine found in formula today is 47 to 51 mg/100 kcal (according to manufacturer specifications). Therefore, preterm infants have low levels of arginine and low intake. Supplementation of arginine may help prevent NEC by promoting nitric oxide synthesis.
How the intervention might work
In an experimental model of hypoxaemia/reoxygenation‐induced NEC in mice, dietary supplementation of arginine had a protective effect, as evidenced by lower injury scores on histopathological examination (Akisu 2002). In a piglet model of NEC, continuous infusion of arginine reduced the severity of intestinal injury (Di Lorenzo 1995). However, in a rat model of ischaemia‐reperfusion injury, investigators found that nitric oxide did not play a protective role in the immature or newborn intestine (Chan 2002). Therefore, conflicting results have been reported when animal experiments were conducted to determine the role of nitric oxide in the pathogenesis of NEC.
Arginine supplementation can cause alterations in glucose homeostasis (both hypoglycaemia and hyperglycaemia) and histaminergic side effects (Vosatka 1994). Researchers found that inhibition of nitric oxide synthesis reduced hypotension in rats with endotoxin‐mediated septic shock; thus promotion of nitric oxide synthesis in infants with sepsis may lead to irreversible septic shock (Thiemermann 1990). This makes it imperative that any human studies of arginine supplementation to prevent NEC should be rigorously evaluated for both benefit and risk.
Why it is important to do this review
The objective of this review was to evaluate the efficacy and safety of arginine supplementation in decreasing the incidence of all stages of NEC among preterm neonates. As the rise in plasma levels of arginine depends on the dose of arginine used, we planned a subgroup analysis based on the dose of arginine administered. In addition, as the baseline incidence of NEC is low among infants after 32 weeks' gestation, we planned a subgroup analysis based on this cut‐off for the purpose of examining the efficacy of arginine in infants of lower gestational age.
Objectives
To examine the effect of arginine supplementation (administered by any route) on the incidence of NEC in preterm neonates. To conduct subgroup analyses based on the dose of arginine and the gestational age of participants (≤ 32 weeks, > 32 weeks).
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials.
Types of participants
Preterm infants before 37 weeks' gestation at birth.
Types of interventions
Arginine supplementation (in addition to what an infant may be receiving from an enteral or parenteral source) versus placebo or no treatment administered orally or parenterally for at least seven days to achieve plasma arginine levels at or above the upper end of the normal range (145 μmol/L in term breast‐fed infants).
Types of outcome measures
Primary outcomes
Incidence of NEC (any stage and specific stage (1, 2 or 3)) based on Bell's criteria (Bell 1978; Walsh 1986) and diagnosed before discharge.
Secondary outcomes
Death before discharge
Death attributed to NEC at any time
Surgery for NEC (including placement of peritoneal drains)
Duration of total parenteral nutrition administration (days)
Plasma concentrations of arginine and glutamine (μmol/L) measured at least every seven days after the start of supplementation
-
Side effects of arginine supplementation
Systemic hypotension, defined as mean blood pressure below the mean for corrected gestational age during the period of intervention
Alteration in glucose homeostasis (number of infants with blood glucose levels < 2.6 mmol/L or > 8 mmol/L during the period of intervention)
Post hoc secondary outcomes
Neurodevelopmental outcomes among survivors (including incidences of cerebral palsy, cognitive delay, blindness and deafness), patent ductus arteriosus (PDA), intraventricular haemorrhage (IVH) grades III and IV, faecal calprotectin levels and median age at NEC diagnosis were included on the basis of outcomes reported in the included studies.
Search methods for identification of studies
For the May 2016 update, we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library; MEDLINE via PubMed (from 1996 to 12 May 2016); Embase (from 1980 to 12 May 2016); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; from 1982 to 12 May 2016) and used the following search terms: (L‐Arginine OR Arginine, L‐Isomer OR Arginine, L Isomer OR L‐Isomer Arginine OR DL‐Arginine Acetate, Monohydrate OR DL Arginine Acetate, Monohydrate OR Monohydrate DL‐Arginine Acetate), plus database‐specific limiters for randomised controlled trials (RCTs) and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions. We searched clinical trials registries (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform (www.whoint/ictrp/search/en/); and the ISRCTN Registry) for ongoing and recently completed trials.
For previous publications of this review, review authors searched MEDLINE (from 1966 to May 2016) using the following medical subject heading (MeSH) terms: enterocolitis necrotizing; enterocolitis; enteritis; colitis; enterocolitis, pseudomembranous; enterocolitis, acute; infant, premature; infant, newborn; infant, premature, disease; clinical trials; randomised controlled trials; random allocation; prospective studies; and arginine.
Review authors searched other databases as well, including Embase (from 1980 to May 2016); CINAHL (from 1982 to May 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 4) in the Cochrane Library; the reference lists of identified trials; and abstracts from annual meetings of the Society for Pediatric Research, the American Pediatric Society and Pediatric Academic Societies, which were published in Pediatric Research (1991 to 2016). Review authors applied no language restrictions.
Review authors excluded the following types of articles: letters, editorials/commentaries, reviews and lectures.
We updated the first search in August 2010, and again in May 2016. See Appendix 2.
We searched clinical trials registries (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp) for ongoing and recently completed trials.
Data collection and analysis
We employed standard methods and followed Guidelines of the Cochrane Neonatal Review Group in creating this update.
Selection of studies
We included all randomised and quasi‐randomised controlled trials that fulfilled the selection criteria described in the previous section. Two review authors (LK, PS) reviewed search results and separately selected studies for inclusion. Review authors resolved disagreements by discussion or with involvement of the third review author (VS).
Data extraction and management
We assessed retrieved articles and independently abstracted data. We resolved discrepancies between review authors by reaching consensus.
Assessment of risk of bias in included studies
Two review authors used the Cochrane ‘Risk of bias’ tool (Higgins 2011) to independently assess risk of bias (low, high or unclear) of all included trials according to the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessors (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
We resolved disagreements by discussion or by consultation with a third assessor. See Appendix 3 for a detailed description of risk of bias for each domain.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Measures of treatment effect
We performed all statistical analyses using Review Manager software. We analysed categorical data according to risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB). We analysed continuous data using weighted mean difference (WMD) and reported the 95% confidence interval (CI) for all estimates.
Assessment of heterogeneity
We planned to estimate treatment effects in individual trials and to examine heterogeneity between trials by inspecting forest plots and quantified the impact of heterogeneity by using the I2 statistic. If we detected statistical heterogeneity, we planned to explore possible causes (e.g. differences in study quality, participants, intervention regimens or outcome assessments) by performing post hoc subgroup analyses.
Data synthesis
If appropriate, we planned to perform meta‐analysis by using Review Manager software (RevMan 5) supplied by Cochrane. For estimates of typical risk ratio and risk difference, we planned to use the Mantel‐Haenszel method. For measured quantities, we planned to use the inverse variance method. We planned to perform meta‐analyses by using the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We performed unplanned subgroup analyses based on stage of NEC (stage 1, 2 or 3) because of the subjective nature of the diagnosis of stage 1 NEC. We planned to perform subgroup analysis to investigate arginine concentrations based on gestational age. We have reported heterogeneity scores (I2 values) for all analyses.
Results
Description of studies
We identified three eligible studies ‐ Amin 2002, Polycarpou 2013a and El‐Shimi 2015 (Figure 1). We have provided clinical details of participants, interventions and outcomes in the Characteristics of included studies table.
1.
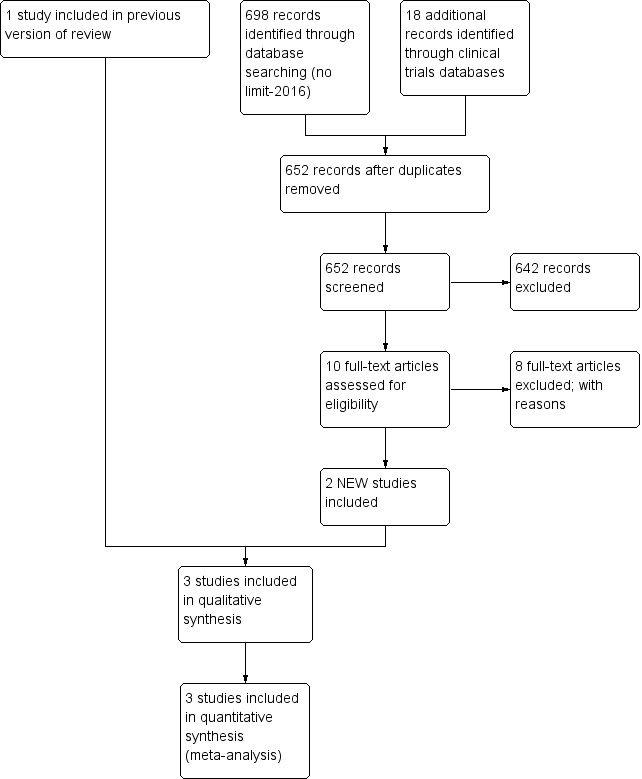
Study flow diagram: review update.
In Amin 2002, investigators randomised preterm neonates at ≤ 32 weeks and at ≤ 1250 grams to receive L‐arginine (1.5 mmol/kg/d) or placebo (normal saline) in equivalent volume. Researchers added L‐arginine to the parenteral nutrition solution until infants were able to tolerate more than 40% of feeds, after which investigators provided L‐arginine or placebo supplementation via the enteral route. Researchers administered study medication at between two and five days of age and continued the medication until 28 days of age. The goal was to increase plasma arginine concentrations from a baseline level of 99 μmol/L to 164 μmol/L (upper limit of normal in healthy full‐term breast‐fed infants was 148 μmol/L). Other clinical management decisions were left to the clinical care team responsible for the participants. Investigators enrolled a total of 152 participants (75 in the L‐arginine group and 77 in the placebo group).
The primary outcome assessed in Amin 2002 was the incidence of NEC (stage 1, 2 and 3, according to Bell 1978). Secondary outcomes assessed included incidences of various stages of NEC, death due to NEC, death due to any cause and concentrations of arginine, glutamine and ammonia at baseline and at 14 and 28 days of age. Both groups recorded incidences of IVH, sepsis, hypotension and PDA and followed up all survivors until completion of the study (28 days of age). They reported long‐term outcome data in abstract format and published a report of the follow‐up study in 2009 (Amin 2002).
In Polycarpou 2013a, researchers randomised preterm neonates at ≤ 34 weeks and at ≤ 1500 grams to receive L‐arginine (1.5 mmol/kg/d) or placebo (5% glucose in equivalent volume). They provided L‐arginine in liquid form in two equal doses, with nasogastric tube enteral feeds from three to 28 days of age. Investigators commenced enteral feeds at 10 to 25 mL/kg/d on the first day of life and increased these feeds on day 4 up to a maximum tolerance of 150 to 180 mL/kg/d. This study enrolled a total of 83 participants (40 in the L‐arginine group and 43 in the placebo group).
The primary outcome in Polycarpou 2013a was the incidence of NEC (all stages according to Bell 1978) in the first three months of life. Secondary outcomes assessed included age at NEC diagnosis and age at death due to NEC. Both groups evaluated the incidences of IVH, respiratory distress and PDA. Researchers monitored the intervention group for arginine‐related side effects (diarrhoea, vomiting, low blood pressure and hypo/hyperglycaemia). A previous report revealed levels of faecal calprotectin in these 83 neonates (Polycarpou 2013b).
In El‐Shimi 2015, researchers randomised 75 neonates born at ≤ 34 weeks to three study arms: arginine (N = 25), glutamine (N = 25) and no treatment (control; N = 25). They administered L‐arginine orally or via nasogastric tube, starting with the introduction of enteral feeds until day 30 of postnatal life. The primary outcome assessed in El‐Shimi 2015 was the incidence of any stage of NEC from the time of enrolment to discharge, death or completion of 30 days of life.
These studies were conducted in three different settings: Canada (Amin 2002), Egypt (El‐Shimi 2015) and Greece (Polycarpou 2013a). The baseline population was ≤ 32 weeks in Amin 2002 and ≤ 34 weeks in the remaining two studies. However, baseline rates of any NEC were 17% (Amin 2002), 18% (El‐Shimi 2015) and 30% (Polycarpou 2013a). Thus, we anticipated a degree of clinical heterogeneity in the results. However, we found little methodological heterogeneity between studies (all of which were relatively well‐conducted RCTs) and lower risk of bias. Thus, we decided to pool results statistically and to explore heterogeneity if identified by using a random‐effects model.
Risk of bias in included studies
For studies included in the review, we have provided assessments of methodological quality in the Characteristics of included studies table and have summarised findings in Figure 2 and Figure 3. We extracted methodological details of each study by reviewing published information and by contacting the primary author.
2.
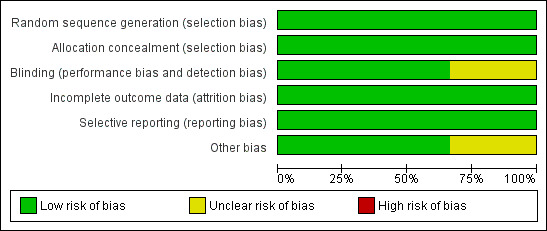
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
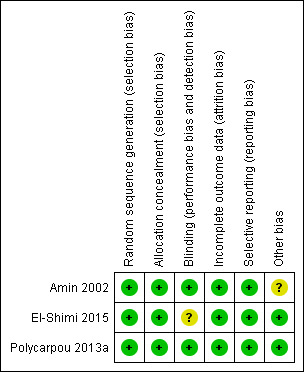
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In Amin 2002, the pharmacist performed randomisation centrally (information provided by study author ‐ Dr Amin). In this study, allocation was concealed and the intervention was masked, so that investigators, parents and caretakers were unaware of treatment allocation. Cross‐over was not allowed (information provided by study author ‐ Dr Amin) and contamination was not possible because the study drug was not available outside the trial. Outcome assessors (at various stages of NEC before study completion) were blinded and were not aware of treatment allocation. All randomised patients were accounted for in the analysis of outcomes.
Amin 2002 reported outcomes among survivors at 36 months' postmenstrual age. Individuals who assessed outcomes at follow‐up were blinded to the intervention. Among 145 infants eligible for follow‐up, 135 (93%) were followed.
In Polycarpou 2013a, the randomisation sequence was computer generated, as described in Polycarpou 2013b. A pharmacist prepared intervention and control solutions in non‐transparent bottles at equal volumes, meaning that nurses who administered the dilutions were blinded to the allocated intervention. Neonatalogists and radiologists evaluating x‐rays of the abdomen were also blinded to the treatment arm. All participants were accounted for in the analysis of outcomes. Investigators included an additional evaluation of neonates whose parents declined consent.
Researchers in El‐Shimi 2015 used a simple, computer‐generated randomisation approach and concealed allocation (information obtained from study author ‐ Dr Khafagy). Participants, attending physicians, nurses and data analysts were blinded to the group to which babies were assigned (information obtained from study author ‐ Dr Khafagy). Investigators reported all outcomes and accounted for all randomised participants in the analysis of outcomes. It is difficult to understand blinding of nurses and doctors involved in the study to interventions, as the control group received no intervention. We contacted study authors to request clarification but received no response.
Effects of interventions
See: Table 1
Arginine supplementation versus placebo
Primary outcomes
Incidence of NEC (at any stage according to Bell 1978) among all randomised participants (Outcome 1.1)
Results showed a statistically significant reduction in risk of development of NEC (any stage) in the arginine group as compared with the placebo group (RR 0.38, 95% CI 0.23 to 0.64; N = 285; three studies; I2 = 27%) (RD ‐0.19, 95% CI ‐0.28 to ‐0.10; N = 285; three studies; I2 = 0%). The number of participants needed to treat with arginine for an additional beneficial outcome of preventing development of NEC among preterm infants was 6 (95% CI 4 to 10). It should be noted that investigators in Amin 2002 followed neonates for 28 days, and El‐Shimi 2015 for 30 days; Polycarpou 2013a followed neonates for three months (Analysis 1.1).
1.1. Analysis.
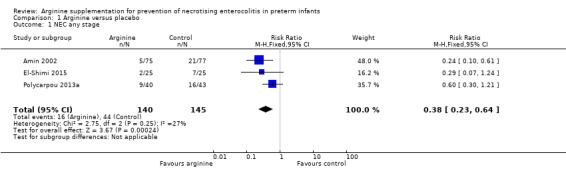
Comparison 1 Arginine versus placebo, Outcome 1 NEC any stage.
We have reported below unplanned subgroup analyses according to various stages of NEC.
NEC stage 1 (according to Bell 1978 staging criteria) (Outcome 1.2)
Results showed a statistically significant reduction in risk of developing NEC stage 1 (RR 0.37, 95% CI 0.15 to 0.90; N = 285; three studies; I2 = 52%) (RD ‐0.07, 95% CI ‐0.14 to ‐0.01; N = 285; three studies; I2 = 0%). The number of participants needed to treat with arginine to prevent development of stage 1 NEC among preterm infants was 15 (95% CI 8 to 100) (Analysis 1.2).
1.2. Analysis.
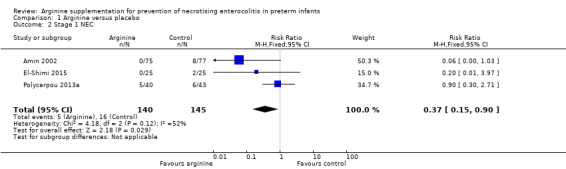
Comparison 1 Arginine versus placebo, Outcome 2 Stage 1 NEC.
NEC stage 2 (according to Bell 1978 staging criteria) (Outcome 1.3)
Results showed no reduction in risk of developing NEC stage 2 (RR 0.51, 95% CI 0.25 to 1.06; N = 285; three studies; I2 = 2%) (RD ‐0.07, 95% CI ‐0.14 to 0.00; N = 285; three studies; I2 = 51%) (Analysis 1.3).
1.3. Analysis.
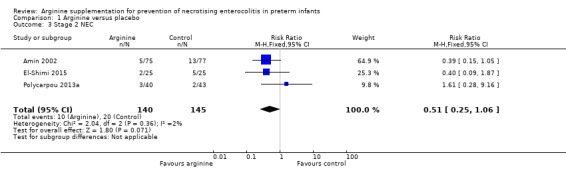
Comparison 1 Arginine versus placebo, Outcome 3 Stage 2 NEC.
NEC stage 3 (according to Bell 1978 staging criteria) (Outcome 1.4)
Results for stage 3 NEC were different depending upon the type of estimate used for analysis because none of the participants in two studies (Amin 2002 and El‐Shimi 2015) developed NEC stage 3. Thus the RR for developing NEC stage 3 (RR 0.13, 95% CI 0.02 to 1.03; N = 285; three studies; I2 = 0%) was not significantly different but the risk difference was statistically significantly different (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; N = 285; three studies; I2 = 89%). The risk difference revealed that the number of patients needed to treat with arginine to prevent development of stage 3 NEC among preterm infants was 20 (95% CI 12 to 100) (Analysis 1.4).
1.4. Analysis.
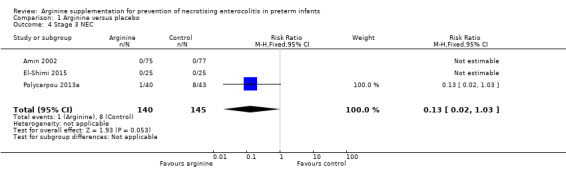
Comparison 1 Arginine versus placebo, Outcome 4 Stage 3 NEC.
Secondary outcomes
Mortality (Outcome 1.5)
Death due to any cause
In Amin 2002, three deaths were reported in the arginine group and none in the placebo group. Polycarpou 2013a reported eight deaths in the arginine group and 12 in the placebo group. In El‐Shimi 2015, two deaths occurred in the arginine group and six in the control group. Combined results from all three studies showed no differences in risk of mortality between the two groups (RR 0.77, 95% CI 0.41 to 1.45; N = 285; three studies; I2 = 42%) (RD ‐0.03, 95% CI ‐0.10 to 0.04; N = 285; three studies; I2 = 79%). Clinical heterogeneity was noted in the populations included in these three studies, with Amin 2002 reporting overall mortality of 2%, El‐Shimi 2015 16% and Polycarpou 2013a 25% in the entire cohort (Analysis 1.5).
1.5. Analysis.
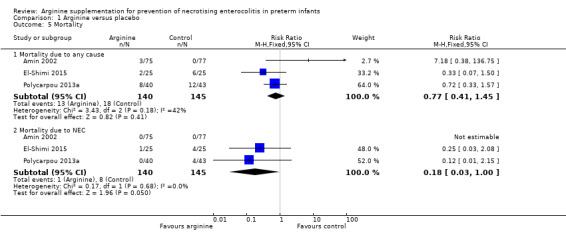
Comparison 1 Arginine versus placebo, Outcome 5 Mortality.
Death due to NEC
Results showed a statistically significant reduction in risk of death due to NEC (RR 0.18, 95% CI 0.03 to 1.00; N = 285; three studies; I2 = 0%) (RD ‐0.05, 95% CI ‐0.09 to ‐0.01; N = 285; three studies; I2 = 87%). The number of patients needing treatment with arginine to prevent NEC‐related mortality was 20 (95% CI 12 to 100) (Analysis 1.5).
Surgery for NEC
In all three included studies, none of the participants in either group required surgical intervention for NEC.
Duration of total parenteral nutrition administration
Data on this outcome are not available.
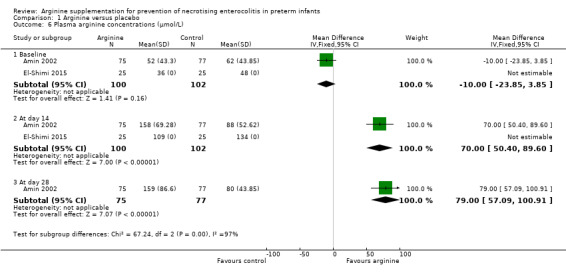
Plasma concentrations of arginine and glutamine at baseline and after the start of supplementation (Outcomes 1.6, 1.7)
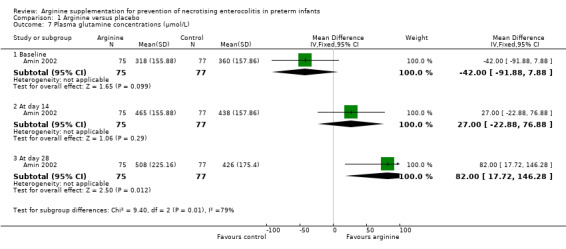
In Amin 2002, plasma arginine concentrations at baseline before supplementation were not significantly different between the two groups (MD ‐10 μmol/L, 95% CI ‐23.85 to 3.85). However, plasma arginine concentrations were statistically significantly higher in the group supplemented with arginine at 14 days (MD 70.00 μmol/L, 95% CI 50.40 to 89.60) and at 28 days of age (MD 79.00 μmol/L, 95% CI 57.09 to 100.91). Plasma glutamine concentrations at baseline before supplementation (MD ‐42 μmol/L, 95% CI ‐91.88 to 7.88) and at 14 days after supplementation (MD 27.00 μmol/L, 95% CI ‐22.88 to 76.88) were not statistically significantly different between the two groups; however, results showed a statistically significant difference (higher in the arginine‐supplemented group) in plasma glutamine concentrations at 28 days of age (MD 82.00 μmol/L, 95% CI 17.72 to 146.28).
El‐Shimi 2015 evaluated serum arginine concentrations at baseline, at day 14 and at time of NEC diagnosis. Baseline arginine levels were significantly lower in the arginine group (6.1 ng/mL vs 8.4 ng/mL; P = 0.009). In both groups, plasma arginine levels significantly increased over the 14‐day period. Baseline and day 14 arginine levels were reported in El‐Shimi 2015 as median and interquartile range (IQR). Study authors clarified that the single value reported for IQR was the difference between the 25th and 75th centiles, but without values for the IQR, we were unable to pool these data points.
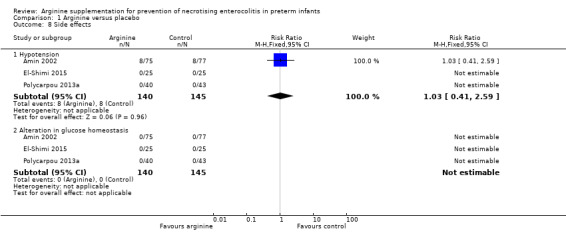
Side effects of arginine supplementation (Outcome 1.8)
Outcome 1.8.1 ‐ Hypotension
In Amin 2002, results showed no statistically significant difference in risk of developing hypotension after 24 hours of age between treatment and placebo groups (RR 1.03, 95% CI 0.41 to 2.59; RD 0.00, 95% CI ‐0.05 to 0.06; N = 285; three studies; I2 = 0%). Polycarpou 2013a and El‐Shimi 2015 reported that hypotension was not observed in neonates receiving arginine (Analysis 1.8).
1.8. Analysis.
Comparison 1 Arginine versus placebo, Outcome 8 Side effects.
Outcome 1.8.2 ‐ Alteration in glucose homeostasis
In Amin 2002, Polycarpou 2013a and El‐Shimi 2015, no participants in either group developed hypoglycaemia during the study period (RD 0.00, 95% CI ‐0.02 to 0.02; N = 285; three studies; I2 = 0%) (Analysis 1.8).
Review authors did not undertake planned subgroup analyses based on gestational age and dose of arginine owing to lack of available data.
Post hoc secondary outcomes (Outcomes 1.9 to 1.18)
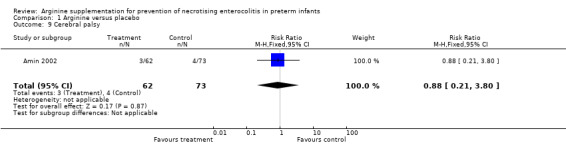
Cerebral palsy (Outcome 1.9)
At 36‐month follow‐up, results showed no statistically significant differences in risk of cerebral palsy between the two groups (RR 0.88, 95% CI 0.21 to 3.80; N = 135; one study; I2 = 0%).
Cognitive delay (cognitive index < 70) (Outcome 1.10)
At 36‐month follow‐up, results showed no statistically significant differences in risk of cerebral palsy between the two groups (RR 0.78, 95% CI 0.14 to 4.55; N = 135; one study; I2 = 0%).
Visual impairment (Outcome 1.11)
None of the infants in either group developed visual impairment.
Deafness (Outcome 1.12)
At 36‐month follow‐up, results showed no statistically significant differences in risk of deafness requiring a hearing aid between the two groups (RR 3.52, 95% CI 0.15 to 84.98; N = 135; one study; I2 = 0%).
Major neurodevelopmental disability (Outcome 1.13)
Results showed no statistically significant differences in risk of major neurodevelopmental disability between the two groups (RR 0.65, 95% CI 0.23 to 1.83; N = 132; one study; I2 = 0%) at 36 months.
Patent ductus arteriosis (Outcome 1.14)
Results showed no statistically significant differences in the incidence of PDA (RR 0.96, 95% CI 0.75 to 1.24; N = 285; three studies; I2 = 0%).
Intraventricular haemorrhage (Outcome 1.16)
Results showed no statistically significant differences in risk of grade II or III IVH (RR 0.85, 95% CI 0.43 to 1.68; N = 235; two studies; I2 = 0%).
Faecal calprotectin (Outcome 1.17)
Polycarpou 2013a measured effects of arginine on intestinal inflammation, as estimated by faecal calprotectin levels and reported no statistically significant differences between groups in calprotectin levels at day 3 (MD 32.10, 95% CI ‐114.31 to 178.51), at day 14 (MD 55.80, 95% CI ‐46.13 to 157.73) and at day 28 (MD ‐13.40, 95% CI ‐105.05 to 78.25).
Age at NEC diagnosis (Outcome 1.18)
Only median values for age at NEC diagnosis were reported. Amin 2002 reported a borderline significantly higher median age at diagnosis of 20 days in the arginine group (vs 10 days in the control group; P = 0.049). El‐Shimi 2015 reported a mean age at diagnosis of 10.7 ± 2.5 days in the arginine group (9.8 ± 5.2 days in the control group), which was not significantly different. Polycarpou 2013a reported a median age at diagnosis of 17 days in the arginine group and 15 days in the control group, which was not significantly different. Without variance measures for two of the studies (Amin 2002; Polycarpou 2013a), we were unable to pool these outcomes.
Discussion
We conducted an extensive literature search to identify trials that might qualify for inclusion in this review. However, we identified only three trials (Amin 2002; El‐Shimi 2015; Polycarpou 2013a). The number of neonates included in this analysis was 285. It appears from these studies that arginine may be effective in preventing necrotising enterocolitis (NEC).
The overall quality of the included studies was good, suggesting that extracted data were valid. However, review authors noted some issues of concern. In Amin 2002, the incidence of NEC in the control group was 27.3% ‐ a rate well above that (5% to 10%) reported by studies including similar populations. Study authors included stage 1 NEC as an outcome, but diagnosis of this disorder is highly subjective and non‐specific. Use of stage 1 NEC as an outcome in a prophylactic study is questionable owing to the inherent possibility of over‐diagnosis (i.e. labelling cases as NEC when patients do not have NEC). Because of masking in this study, it is unlikely that any such over‐diagnosis was biased. Factors responsible for the high incidence of NEC at this centre are unknown. When only participants with stage 2 NEC were analysed, a trend towards reduction that was not statistically significant was noted. Lack of significance of these post hoc analyses may be due to reduced sample size at each stage. Follow‐up reports reveal no increase in risk of neurodevelopmental impairment among survivors.
In El‐Shimi 2015, neonates who developed NEC were of significantly lower gestational age and weight. Owing to the small sample size, study authors were unable to apply regression modelling, which presented a limitation in interpretation, as the effects of arginine cannot be isolated. Results of this study showed a significant baseline imbalance in the control group, which included smaller neonates (based on birth weight) with significantly higher baseline arginine levels. A larger sample size may have resulted in groups that were more comparable.
Based on data provided by the three included studies, we have downgraded recommendations for the use of arginine to prevent NEC to moderate. The included studies are of good methodological design, are consistent in their findings regarding the overall incidence of NEC and appear free of publication bias. We noted minor sources of indirectness related to populations examined in the included studies (< 32 weeks vs < 34 weeks) and to composition of the comparator (placebo‐glucose vs placebo‐saline vs no treatment); however, we believe this would not affect the overall strength of the recommendation. We downgraded evidence as the result of imprecision resulting from the relatively small sample size. We graded evidence on the use of arginine supplementation to reduce mortality to low owing to clinical heterogeneity in baseline mortality rates, likely reflecting variation in population demographics and perinatal health services across regions.
Arginine supplementation for prevention of NEC could be an important avenue for further research. However, the sample size required for prevention of stage 2 or 3 NEC will be significantly larger than the sample previously studied (for an absolute risk reduction of 50% from a baseline incidence of 6%, with an alpha of 0.05 and power of 0.8, each arm would have to include 750 participants), requiring a multi‐centre design. The fact that researchers in Amin 2002 were able to demonstrate reduced concentrations of arginine among patients who develop NEC compared with those who do not develop NEC points towards the possibility of increased utilisation or reduced absorption of the substrate, thereby supporting the rationale for undertaking such a study. Conversely, El‐Shimi 2015 reported similar arginine levels at the time of enrolment among neonates who developed NEC compared with those who did not. The findings of El‐Shimi 2015 may be confounded by baseline imbalances and significantly higher arginine concentrations in the control group at the time of enrolment.
Results showed no significant side effects with supplementation of arginine at a dose of 1.5 mmol/kg, despite arginine levels that were marginally higher than those of normal breast‐fed term newborn infants. This finding further supports the rationale for future research.
Authors' conclusions
Implications for practice.
Findings of three small randomised controlled studies of prophylactic administration of arginine for prevention of NEC among preterm neonates show that arginine supplementation may be effective. However, at present, data are insufficient to support a practice recommendation.
Implications for research.
A multi‐centre randomised controlled study of arginine supplementation for preterm neonates must focus on the incidence of NEC stage 2 or 3.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2016 | New citation required but conclusions have not changed | We made no changes to conclusions. |
| 29 June 2016 | New search has been performed | This review updates the existing review, "Arginine supplementation for prevention of necrotising enterocolitis in preterm infants," published in the Cochrane Database of Systematic Reviews (Shah 2010). The search strategy, updated from 2010, revealed 2 new studies (Polycarpou 2013a and El‐Shimi 2015). We updated data tables accordingly. We also added PRISMA flow diagrams and GRADE tables. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 1 November 2010 | New search has been performed | This review updates the existing review, "Arginine supplementation for prevention of necrotising enterocolitis in preterm infants," published in the Cochrane Database of Systematic Reviews (Shah 2007). The updated search revealed 1 new report of follow‐up data from a previously included trial. We made no changes to conclusions. |
| 21 May 2008 | Amended | We converted this review to the new review format. |
| 1 May 2007 | New citation required but conclusions have not changed | New citation: We made no changes to conclusions. |
| 1 May 2007 | New search has been performed | This review updates the review, "Arginine supplementation for prevention of necrotising enterocolitis in preterm infants," published in the Cochrane Library, 2004, Issue 4 (Shah 2004). Follow‐up data from 1 included study (Amin 2002) were available in abstract format. In this version of the review, we included the data as post hoc analysis. |
Acknowledgements
Review authors would like to acknowledge Prof Stanley Zlotkin for reviewing the protocol and providing expert advice. We would also like to thank Dr Harish Amin and Dr Soha Khafagy for providing details of their studies.
Editorial support of the Cochrane Neonatal Review Group has been provided by Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Appendices
Appendix 1. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomised controlled trial or controlled clinical trial or randomised or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomised controlled trial OR controlled clinical trial OR randomised OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Search strategy, August 2010
MEDLINE, CCTR and Embase were searched according to the following strategies.
Ovid MEDLINE(R), 1950 to 27 August 2010
Search strategy:
1 Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab.
2 ("clinical trial, all" or clinical trial).pt. or clinical trials as topic/ or clinical trial, phase i.pt. or clinical trials, phase i as topic/ or clinical trial, phase iii.pt. or clinical trials, phase iii as topic/ or clinical trial, phase iv.pt. or clinical trials, phase iv as topic/ or controlled clinical trial.pt. or controlled clinical trials as topic/ or meta‐analysis.pt. or meta‐analysis as topic/ or multicentre study.pt. or multicentre studies as topic/ or randomised controlled trial.pt. or randomised controlled trials as topic/ or evaluation studies as topic/ or validation studies as topic/ or evaluation study.pt. or validation study.pt. or case‐control studies/ or retrospective studies/ or cohort studies/ or longitudinal studies/ or follow‐up studies/ or prospective studies/ or cross‐sectional studies/ or double‐blind method/ or random allocation/ or single‐blind method/ or ((singl* or doubl* or tripl* or trebl*) adj5 (blin or mask or blinded or masked)).ti,ab.
3 L‐Arginine/ or (Arginine, L‐Isomer or Arginine, L Isomer or L‐Isomer Arginine or DL‐Arginine Acetate, Monohydrate or DL Arginine Acetate, Monohydrate or Monohydrate DL‐Arginine Acetate). mp
4 1 and 2 and 3
5 1 and 3
EBM reviews ‐ Cochrane Central Register of Controlled Trials <1st quarter 2010>
Search strategy:
1 Infant, newborn/ or infant, low birth weight/ or infant, small for gestational age/ or infant, very low birth weight/ or infant, premature/ or exp Infant, Newborn, Diseases/ or pregnancy, high‐risk/ or quadruplets/ or quintuplets/ or superfetation/ or triplets/ or twins/ or twins, dizygotic/ or twins, monozygotic/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw).ti,ab. or ((intrauterine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab.
2 L‐Arginine/ or (Arginine, L‐Isomer or Arginine, L Isomer or L‐Isomer Arginine or DL‐Arginine Acetate, Monohydrate or DL Arginine Acetate, Monohydrate or Monohydrate DL‐Arginine Acetate). mp
3 1 and 2
Embase <1980 to Week 30 2010>
Search strategy:
1 newborn/ or newborn period/ or low birth weight/ or extremely low birth weight/ or small for date infant/ or very low birth weight/ or Prematurity/ or exp newborn disease/ or multiple pregnancy/ or twin pregnancy/ or twins/ or dizygotic twins/ or monozygotic twins/ or human triplets/ or intrauterine growth retardation/ or small for date infant/ or (infan: or neonat: or newborn: or prematur: or iugr or sga or vlbw or lbw or elbw or (intrautrine adj2 growth adj2 restrict:) or (intrauterine adj2 growth adj2 retard:)).ti,ab. (774681)
2 ct.fs. or clinical trial/ or controlled clinical trial/ or multicentre study/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or cohort analysis/ or double blind procedure/ or single blind procedure/ or triple blind procedure/ or meta analysis/ or randomised controlled trial/ or "systematic review"/ or case control study/ or longitudinal study/ or prospective study/ or retrospective study/ or multicentre study/ or validation study/ or (((evaluation or validation) adj2 study) or ((evaluation or validation) adj2 studies)).ti,ab. (882179)
3 L‐Arginine/ or (Arginine, L‐Isomer or Arginine, L Isomer or L‐Isomer Arginine or DL‐Arginine Acetate, Monohydrate or DL Arginine Acetate, Monohydrate or Monohydrate DL‐Arginine Acetate). mp
4 1 and 3
5 2 and 4
We also searched reference lists of identified trials and abstracts from annual meetings of the Society for Pediatric Research, the American Pediatric Society and Pediatric Academic Societies, published in Pediatric Research (2002‐2009). We applied no language restrictions.
Clinicaltrials.gov
Search strategy:
Arginine AND (neon* OR infant OR newborn)
Limited: Child (birth ‐ 17 years)
Controlled‐trials.com
Search strategy:
Arginine AND (neon* OR infant OR newborn)
No limits
Appendix 3. Risk of bias tool
The following issues were evaluated and entered into the risk of bias table: 1. Sequence generation (checking for possible selection bias): Was the allocation sequence adequately generated? For each included study, we categorised the method used to generate the allocation sequence as: a. low risk (any truly random process, e.g. random number table; computer random number generator); b. high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or c. unclear risk. 2. Allocation concealment (checking for possible selection bias): Was allocation adequately concealed? For each included study, we categorised the method used to conceal the allocation sequence as: a. low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); b. high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or c. unclear risk. 3. Blinding of participants and personnel (checking for possible performance bias): Was knowledge of the allocated intervention adequately prevented during the study? For each included study, we categorised the method used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised methods as: a. low risk for participants or personnel; b. high risk for participants or personnel; or c. unclear risk for participants or personnel. 4. Blinding of outcome assessment (checking for possible detection bias): Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment? For each included study, we categorised the method used to blind outcome assessors from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorised methods as: a. low risk for outcome assessors; b. high risk for outcome assessors; or c. unclear risk for outcome assessors. 5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described completeness of data, including attrition and exclusions from analysis. We noted whether attrition and exclusions were reported, numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes. When sufficient information was reported or supplied by trial authors, we re‐included missing data in the analyses. We categorised methods as: a. low risk (< 20% missing data); b. high risk (≥ 20% missing data); or c. unclear risk. 6. Selective reporting bias. Are reports of the study free of the suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as: a. low risk (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported); b. high risk (when not all of the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or c. unclear risk. 7. Other sources of bias. Was the study apparently free of other problems that could put it at high risk of bias? For each included study, we described any important concerns that we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design, whether the trial was stopped early owing to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: a. low risk; b. high risk; or c. unclear risk.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
Data and analyses
Comparison 1. Arginine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NEC any stage | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.64] |
| 2 Stage 1 NEC | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| 3 Stage 2 NEC | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.25, 1.06] |
| 4 Stage 3 NEC | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 1.03] |
| 5 Mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mortality due to any cause | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 5.2 Mortality due to NEC | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.03, 1.00] |
| 6 Plasma arginine concentrations (μmol/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Baseline | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐23.85, 3.85] |
| 6.2 At day 14 | 2 | 202 | Mean Difference (IV, Fixed, 95% CI) | 70.0 [50.40, 89.60] |
| 6.3 At day 28 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 79.0 [57.09, 100.91] |
| 7 Plasma glutamine concentrations (μmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Baseline | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐40.00 [‐91.88, 7.88] |
| 7.2 At day 14 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 27.0 [‐22.88, 76.88] |
| 7.3 At day 28 | 1 | 152 | Mean Difference (IV, Fixed, 95% CI) | 82.0 [17.72, 146.28] |
| 8 Side effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Hypotension | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.41, 2.59] |
| 8.2 Alteration in glucose homeostasis | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Cerebral palsy | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.21, 3.80] |
| 10 Cognitive delay (cognitive index < 70) | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.14, 4.55] |
| 11 Visual impairment | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Deafness | 1 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [0.15, 84.98] |
| 13 Major neurodevelopmental disability | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.23, 1.83] |
| 14 PDA (%) | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.24] |
| 15 IVH grade III or IV | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.43, 1.68] |
| 16 Faecal calprotectin (μg/g) | 1 | 249 | Mean Difference (IV, Random, 95% CI) | 20.13 [‐41.66, 81.92] |
| 16.1 At day 3 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 32.10 [‐114.31, 178.51] |
| 16.2 At day 14 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 55.80 [‐46.13, 157.73] |
| 16.3 At day 28 | 1 | 83 | Mean Difference (IV, Random, 95% CI) | ‐13.40 [‐105.05, 78.25] |
| 17 Median age at NEC diagnosis | Other data | No numeric data |
1.6. Analysis.
Comparison 1 Arginine versus placebo, Outcome 6 Plasma arginine concentrations (μmol/L).
1.7. Analysis.
Comparison 1 Arginine versus placebo, Outcome 7 Plasma glutamine concentrations (μmol/L).
1.9. Analysis.
Comparison 1 Arginine versus placebo, Outcome 9 Cerebral palsy.
1.10. Analysis.
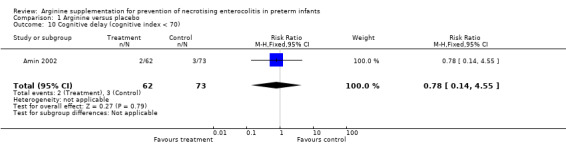
Comparison 1 Arginine versus placebo, Outcome 10 Cognitive delay (cognitive index < 70).
1.11. Analysis.
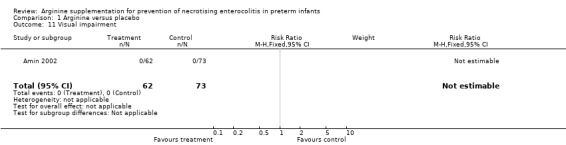
Comparison 1 Arginine versus placebo, Outcome 11 Visual impairment.
1.12. Analysis.
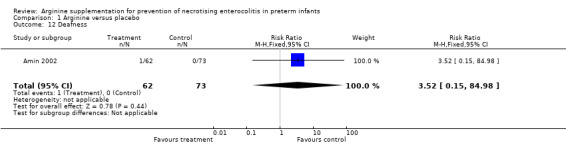
Comparison 1 Arginine versus placebo, Outcome 12 Deafness.
1.13. Analysis.
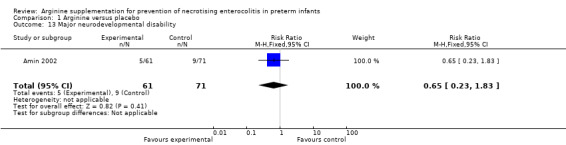
Comparison 1 Arginine versus placebo, Outcome 13 Major neurodevelopmental disability.
1.14. Analysis.
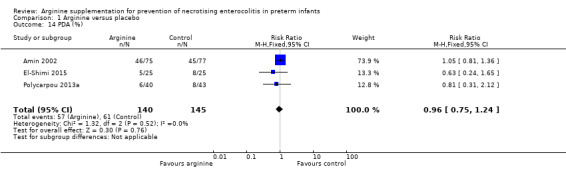
Comparison 1 Arginine versus placebo, Outcome 14 PDA (%).
1.15. Analysis.
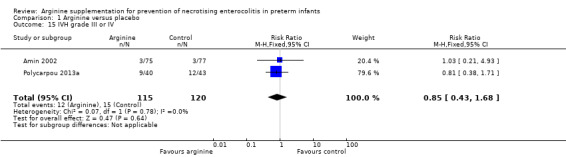
Comparison 1 Arginine versus placebo, Outcome 15 IVH grade III or IV.
1.16. Analysis.
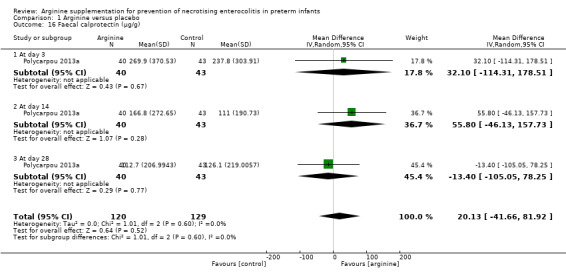
Comparison 1 Arginine versus placebo, Outcome 16 Faecal calprotectin (μg/g).
1.17. Analysis.
Comparison 1 Arginine versus placebo, Outcome 17 Median age at NEC diagnosis.
| Median age at NEC diagnosis | |||
|---|---|---|---|
| Study | Age of NEC diagnosis, median (SD if available) (Arginine group) | Age of NEC diagnosis, median (SD if available) (Control group) | Reported p‐value |
| Amin 2002 | 20 days | 10 days | 0.049 |
| El‐Shimi 2015 | 10.67 (2.5) days | 9.83 (5.2) days | NS |
| Polycarpou 2013a | 17 days | 15 days | |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amin 2002.
| Methods | Double‐blind, placebo‐controlled, randomised study 1. Masking of randomisation: yes 2. Masking of intervention: yes 3. Completeness of follow‐up: yes 4. Masking of outcome assessment: yes | |
| Participants | 152 infants ≤ 32 weeks' gestation at birth and birth weight ≤ 1250 grams were enrolled after parental consent. Arginine group (75 neonates): Mean (SD) birth weight was 952 (216) grams and gestational age was 27.4 (2.6) weeks. Placebo group (77 neonates): Mean (SD) birth weight was 955 (175) grams and gestational age was 27.6 (1.8) weeks. Exclusion criteria: severe congenital anomalies, congenital non‐bacterial infection, evidence of intraventricular haemorrhage grade ≥ II, conjugated hyperbilirubinaemia, inborn error of metabolism, exchange transfusion during the study period, preexisting renal failure defined as urine output < 0.5 mL/kg/h for > 8 hours) | |
| Interventions | Arginine group (N = 75): 1.5 mmol/kg/d of L‐arginine supplemented with parenteral nutrient solution or given enterally when the infant was able to tolerate > 40% of total feeds from randomisation (days 2‐5) until 28 days of age Placebo group (N = 77): Equal volumes of placebo solution (normal saline) and arginine supplementation were provided in identical vials from randomisation (days 2‐5) until 28 days of age | |
| Outcomes | NEC, any stage NEC, stage 1 NEC, stage 2 Mortality due to NEC Mortality due to any cause Plasma arginine; glutamine concentrations at baseline, at 14 days and at 28 days of age | |
| Notes | Follow‐up published in 2009 (Amin HJ, Soraisham AS, Sauve RS. Neurodevelopmental outcomes of premature infants treated with l‐arginine for prevention of necrotizing enterocolitis. J Paediatr Child Health 2009 Apr;45(4):219‐23. Epub 2009 Mar 23) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence was used. |
| Allocation concealment (selection bias) | Low risk | Masking of randomisation: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Masking of intervention: yes Masking of outcome assessment: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completeness of follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | We do not have access to the protocol, but it appears that investigators reported all important outcomes. |
| Other bias | Unclear risk | The rate of NEC in the control population was high compared with that reported for similar populations in other studies. |
El‐Shimi 2015.
| Methods | Three‐arm, comparative, prospective randomised study 1. Masking of randomisation: yes 2. Masking of intervention: unclear; yes according to study authors, but one group received no intervention. Thus it is unclear how this could have been achieved. 3. Completeness of follow‐up: yes 4. Masking of outcome assessment: yes | |
| Participants | 75 infants ≤ 34 weeks' gestation at birth were enrolled after parental consent.
Arginine group (N = 25): Mean (SD) birth weight was 1450 (260) grams and gestational age was 31.84 (2.3) weeks Glutamine group (N = 25): Mean (SD) birth weight was 1310 (250) grams and gestational age was 30.64 (2.3) weeks Control (no treatment) group (N = 25): Mean (SD) birth weight was 1450 (210) grams and gestational age was 31.86 (1.4) weeks Exclusion criteria: newborns with congenital malformations, chromosomal abnormalities, suspected inborn error of metabolism, sepsis, IVH grade ≥ II, undergoing intestinal surgery or having any contraindication to enteral feeding |
|
| Interventions | Arginine group: Infants received L‐arginine (0.75 mmol/kg/d) with the start of enteral feedings, doubled to 1.5 mmol/kg/d (261 mg/kg/d) when 40% of enteral feeding (60 cc/kg) was reached. Arginine was supplemented until 30 days' postnatal age. The dose was administered orally or via nasogastric tube every 12 hours Glutamine group: Infants received glutamine (156 mg/kg/d) with the start of enteral feedings, doubled to 312 mg/kg/d when 40% of enteral feeding (60 cc/kg) was reached. Glutamine was supplemented until 30 days' postnatal age. The dose was divided every 12 hours and was taken orally or via nasogastric tube. Control group: Infants started enteral feeding within first week without arginine or glutamine supplementation |
|
| Outcomes | NEC, all stages NEC, stage 2 NEC, stage 3 Mortality due to NEC Mortality due to any cause Arginine concentrations at baseline, at time of NEC diagnosis and at day 14 of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation method was based on computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed (information provided by study authors). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants, doctors and nurses evaluating outcomes were blinded to intervention allocation according to study authors; however, it is unclear how this may have been achieved when the control group received no intervention (information provided by study authors). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were accounted for in the analysis. |
| Selective reporting (reporting bias) | Low risk | The registered primary outcome and the reported primary outcome were aligned. No prespecified secondary outcomes were stated. |
| Other bias | Low risk | |
Polycarpou 2013a.
| Methods | Double‐blind, parallel‐group, placebo‐controlled randomised study
|
|
| Participants | After parental consent, 83 neonates with birth weight ≤ 1500 grams and gestational age ≤ 34 weeks underwent randomisation. Arginine group (40 neonates): Mean (IQR) birth weight was 1168 (1095.1 to 1242.2) grams and gestational age was 29.2 (28.9 to 29.4) weeks Placebo group (43 neonates) Mean (IQR) birth weight was 1127 (1047.1 to 1 207.6) grams and gestational age was 28.8 (28.5 to 29.1) weeks Exclusion criteria: lethal congenital or chromosomal abnormalities, inborn errors of metabolism, lack of parental consent |
|
| Interventions | Neonates in the arginine group received daily enteral L‐arginine supplementation (Nutricia, Amsterdam, The Netherlands) of 1.5 mmol/kg/d (261 mg/kg) in liquid form at 2 equal doses (bid), with nasogastric tube enteral feeds from the 3rd to the 28th day after birth, whereas neonates in the placebo group received 5% glucose at equivalent volumes | |
| Outcomes | Primary: incidence of NEC (2 and 3) in the first 3 months of life Secondary: age at NEC diagnosis and incidence of death due to NEC |
|
| Notes | Previously published study conducted to determine potential effect of oral L‐arginine supplementation on intestinal inflammation (faecal calprotectin) in very low birth weight neonates (N = 83). Polycarpou E, Zachaki S, Papaevangelou V, Tsolia M, Kyriacou A, Kostalos C, Kafetzis D. Oral L‐arginine supplementation and faecal calprotectin levels in very low birth weight neonates. J Perinatol 2013b;33:141‐6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators randomised 84 neonates to 2 groups using a computer‐generated programme. |
| Allocation concealment (selection bias) | Low risk | The pharmacy prepared dilutions (central allocation) of the same volumes of arginine supplement and placebo. Nurses providing the medication were not aware of the diluent constitute (transparent bottles) and were blinded to allocation of participants. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neonatologists who classified episodes of NEC, as well as the radiologist who evaluated abdominal x‐rays, were blinded to participant allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing. Outcomes of neonates whose parents declined consent were not different from those of participants. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes match those registered at ClinicalTrials.gov (NCT01336998). |
| Other bias | Low risk | This study appears to be free of other sources of bias. |
IQR: interquartile range. IVH: intraventricular haemorrhage. NEC: necrotising enterocolitis. SD: standard deviation.
Differences between protocol and review
We have added post hoc outcomes reported in subsequent trials.
Contributions of authors
Dr LE Kelly Literature search and identification of trials Evaluation of methodological quality of trials Data collection Verification of data and entry into RevMan Writing of review text
Dr V Shah Literature search and identification of trials Evaluation of methodological quality of trials Data collection Verification of data Revision of the review
Dr PS Shah Literature search and identification of trials Evaluation of methodological quality of trials Data collection Verification of data and entry into RevMan Interpretation of results Writing of review text
Dr PS Shah and Dr V Shah conducted the August 2010 search. Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton and Roger Soll) centrally conducted the November 2010 update, which was reviewed and approved by Dr PS Shaw.
Dr PS Shah and Dr LE Kelly conducted the May 2016 search, which was updated centrally by Cochrane Neonatal Review Group staff (Yolanda Brosseau, Colleen Ovelman and Roger Soll). Dr PS Shah reviewed and approved this update.
Sources of support
Internal sources
Mount Sinai Hospital, University of Toronto, Toronto, Canada.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been provided by Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Amin 2002 {published data only}
- Amin H, Soraisham AS, Sauve R. Does L‐arginine supplementation used in the prevention of NEC in ≤ 1250gm birth weight infants adversely impact neurodevelopment?. E‐PAS 2006;59:2862.228. [Google Scholar]
- Amin HJ, Soraisham AS, Sauve RS. Neurodevelopmental outcomes of premature infants treated with l‐arginine for prevention of necrotising enterocolitis. Journal of Paediatrics and Child Health 2009;45:219‐23. [DOI] [PubMed] [Google Scholar]
- Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. Journal of Pediatrics 2002;140:425‐31. [DOI] [PubMed] [Google Scholar]
El‐Shimi 2015 {published and unpublished data}
- El‐Shimi MS, Awad HA, Abdelwahed MA, Mohamed MH, Khafagy SM, Saleh G. Enteral l‐arginine and glutamine supplementation for prevention of NEC in preterm neonates. International Journal of Pediatrics 2015;2015:856091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polycarpou 2013a {published data only}
- Polycarpou E, Zachaki S, Papaevangelou V, Tsolia M, Kyriacou A, Kostalos C, et al. Oral L‐arginine supplementation and faecal calprotectin levels in very low birth weight neonates. Journal of Perinatology 2013;33(2):141‐6. [DOI] [PubMed] [Google Scholar]
- Polycarpou E, Zachaki S, Tsolia M, Papaevangelou V, Polycarpou N, Briana DD, et al. Enteral L‐arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: a double‐blind randomized pilot study of efficacy and safety. JPEN. Journal of Parenteral and Enteral Nutrition 2013;37(5):617‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Akisu 2002
- Akisu M, Ozmen D, Baka M, Habif S, Yalaz M, Arslanoglu S, et al. Protective effect of dietary supplementation with L‐arginine and L‐carnitine on hypoxia/reoxygenation‐induced necrotizing enterocolitis in young mice. Biology of the Neonate 2002;81:260‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Becker 2000
- Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, et al. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. Journal of Pediatrics 2000;137:785‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187:1‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boeckxstaens 1991
- Boeckxstaens GE, Pelckmans PA, Bult H, Man JG, Herman AG, Maercke YM. Evidence for nitric oxide as mediator of non‐adrenergic non‐cholinergic relaxations induced by ATP and GABA in the canine gut. British Journal of Pharmacology 1991;102:434‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caplan 2001
- Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Current Opinions in Pediatrics 2001;13:111‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castillo 1995
- Castillo L, DeRojas‐Walker T, Yu YM, Sanchez M, Chapman TE, Shannon D, et al. Whole body arginine metabolism and nitric oxide synthesis in newborns with persistent pulmonary hypertension. Pediatric Research 1995;38:17‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chan 2002
- Chan KL, Hui CW, Chan KW, Fung PC, Wo JY, Tipoe G, et al. Revisiting ischemia and reperfusion injury as a possible cause of necrotizing enterocolitis: role of nitric oxide and superoxide dismutase. Journal of Pediatric Surgery 2002;37:828‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Di Lorenzo 1995
- Lorenzo M, Bass J, Krantis A. Use of L‐arginine in the treatment of experimental necrotizing enterocolitis. Journal of Pediatric Surgery 1995;30:235‐41. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Klein 2002
- Klein CJ. Nutrient requirements for preterm infant formulas. Journal of Nutrition 2002;132:1395S‐577S. [DOI] [PubMed] [Google Scholar]
Langer 1993
- Langer JC, Sohal SS, Riddell RH. Mucosal permeability to 51Cr EDTA following subclinical intestinal ischemia‐reperfusion injury in the weanling rat. Journal of Pediatric Surgery 1993;28:601‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Polycarpou 2013b
- Polycarpou E, Zachaki S, Papaevangelou V, Tsolia M, Kyriacou A, Kostalos C, et al. Oral L‐arginine supplementation and faecal calprotectin levels in very low birth weight neonates. Journal of Perinatology 2013;33(2):141‐6. [DOI] [PubMed] [Google Scholar]
Stark 1992
- Stark ME, Szurszewski JH. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology 1992;103:1928‐49. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stoll 1994
- Stoll BJ. Epidemiology of necrotizing enterocolitis. Clinics in Perinatology 1994;21:205‐18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiemermann 1990
- Thiemermann C, Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. European Journal of Pharmacology 1990;182:591‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ventura 1987
- Ventura V, Brooke OG. Plasma amino acids in small preterm infants fed on human milk or formula. Archives of Disease in Childhood 1987;62:1257‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vosatka 1994
- Vosatka RJ, Kashyap S, Trifiletti RR. Arginine deficiency accompanies persistent pulmonary hypertension of the newborn. Biology of the Neonate 1994;66:65‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 1986
- Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric Clinics of North America 1986;33:179‐201. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 1986
- Wu PYK, Edwards N, Storm MC. Plasma amino acid pattern in normal term breast‐fed infants. Journal of Pediatrics 1986;109:347‐9. [DOI] [PubMed] [Google Scholar]
Wu 1998
- Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. The Biochemical Journal 1998;336:1‐17. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zamora 1997
- Zamora SA, Amin HJ, McMillan DD, Kubes P, Fick GH, Butzner JD, et al. Plasma L‐arginine concentrations in premature infants with necrotizing enterocolitis. Journal of Pediatrics 1997;131:226‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zamora 1998
- Zamora SA, Amin HJ, McMillan DD, Fick GH, Butzner JD, Parsons HG, et al. Plasma L‐arginine concentration, oxygenation index, and systemic blood pressure in premature infants. Critical Care Medicine 1998;26:1271‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shah 2004
- Shah P, Shah V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004339.pub2] [DOI] [PubMed] [Google Scholar]
Shah 2007
- Shah P, Shah V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004339.pub3] [DOI] [PubMed] [Google Scholar]
Shah 2010
- Shah P, Shah V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD004339] [DOI] [Google Scholar]


