Abstract
Background
Delirium is a common and distressing mental disorder. It is often caused by a combination of stressor events in susceptible people, particularly older people living with frailty and dementia. Adults living in institutional long‐term care (LTC) are at particularly high risk of delirium. An episode of delirium increases risks of admission to hospital, development or worsening of dementia and death. Multicomponent interventions can reduce the incidence of delirium by a third in the hospital setting. However, it is currently unclear whether interventions to prevent delirium in LTC are effective. This is an update of a Cochrane Review first published in 2014.
Objectives
To assess the effectiveness of interventions for preventing delirium in older people in institutional long‐term care settings.
Search methods
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group (CDCIG) ’s Specialised Register of dementia trials (dementia.cochrane.org/our‐trials‐register), to 27 February 2019. The search was sufficiently sensitive to identify all studies relating to delirium. We ran additional separate searches in the Cochrane Central Register of Controlled Trials (CENTRAL), major healthcare databases, trial registers and grey literature sources to ensure that the search was comprehensive.
Selection criteria
We included randomised controlled trials (RCTs) and cluster‐randomised controlled trials (cluster‐RCTs) of single and multicomponent, non‐pharmacological and pharmacological interventions for preventing delirium in older people (aged 65 years and over) in permanent LTC residence.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Primary outcomes were prevalence, incidence and severity of delirium; and mortality. Secondary outcomes included falls, hospital admissions and other adverse events; cognitive function; new diagnoses of dementia; activities of daily living; quality of life; and cost‐related outcomes. We used risk ratios (RRs) as measures of treatment effect for dichotomous outcomes, hazard ratios (HR) for time‐to‐event outcomes and mean difference (MD) for continuous outcomes. For each outcome, we assessed the overall certainty of the evidence using GRADE methods.
Main results
We included three trials with 3851 participants. All three were cluster‐RCTs. Two of the trials were of complex, single‐component, non‐pharmacological interventions and one trial was a feasibility trial of a complex, multicomponent, non‐pharmacological intervention. Risk of bias ratings were mixed across the three trials. Due to the heterogeneous nature of the interventions, we did not combine the results statistically, but produced a narrative summary.
It was not possible to determine the effect of a hydration‐based intervention on delirium incidence (RR 0.85, 95% confidence interval (CI) 0.18 to 4.00; 1 study, 98 participants; very low‐certainty evidence downgraded for risk of bias and very serious imprecision). This study did not assess delirium prevalence, severity or mortality.
The introduction of a computerised system to identify medications that may contribute to delirium risk and trigger a medication review was probably associated with a reduction in delirium incidence (12‐month HR 0.42, CI 0.34 to 0.51; 1 study, 7311 participant‐months; moderate‐certainty evidence downgraded for risk of bias) but probably had little or no effect on mortality (HR 0.88, CI 0.66 to 1.17; 1 study, 9412 participant‐months; moderate‐certainty evidence downgraded for imprecision), hospital admissions (HR 0.89, CI 0.72 to 1.10; 1 study, 7599 participant‐months; moderate‐certainty evidence downgraded for imprecision) or falls (HR 1.03, CI 0.92 to 1.15; 1 study, 2275 participant‐months; low‐certainty evidence downgraded for imprecision and risk of bias). Delirium prevalence and severity were not assessed.
In the enhanced educational intervention study, aimed at changing practice to address key delirium risk factors, it was not possible to determine the effect of the intervention on delirium incidence (RR 0.62, 95% CI 0.16 to 2.39; 1 study, 137 resident months; very low‐certainty evidence downgraded for risk of bias and serious imprecision) or delirium prevalence (RR 0.57, 95% CI 0.15 to 2.19; 1 study, 160 participants; very low‐certainty evidence downgraded for risk of bias and serious imprecision). There was probably little or no effect on mortality (RR 0.82, CI 0.50 to 1.34; 1 study, 215 participants; moderate‐certainty evidence downgraded for imprecision). The intervention was probably associated with a reduction in hospital admissions (RR 0.67, CI 0.57 to 0.79; 1 study, 494 participants; moderate‐certainty evidence downgraded due to indirectness).
Authors' conclusions
Our review identified limited evidence on interventions for preventing delirium in older people in LTC. A software‐based intervention to identify medications that could contribute to delirium risk and trigger a pharmacist‐led medication review, probably reduces incidence of delirium in older people in institutional LTC. This is based on one large RCT in the US and may not be practical in other countries or settings which do not have comparable information technology services available in care homes. In the educational intervention aimed at identifying risk factors for delirium and developing bespoke solutions within care homes, it was not possible to determine the effect of the intervention on delirium incidence, prevalence or mortality. This evidence is based on a small feasibility trial. Our review identified three ongoing trials of multicomponent delirium prevention interventions. We identified no trials of pharmacological agents. Future trials of multicomponent non‐pharmacological delirium prevention interventions for older people in LTC are needed to help inform the provision of evidence‐based care for this vulnerable group.
Plain language summary
Interventions for preventing delirium in older people in institutional long‐term care (LTC)
Review question
How effective are treatments to prevent delirium in older people living in long‐term care (LTC)?
Background
LTC is the name used for residential homes, which provide personal care, supervision with medications and help with day‐to‐day activities, and nursing homes, which provide 24‐hour nursing care. Delirium is a common and serious illness for older people living in LTC. Delirium is a condition that causes confusion, usually over a few hours or days. Some people with delirium become quiet and sleepy while others become agitated and disorientated, so it can be a very distressing condition. Delirium can increase the chances of being admitted to hospital, developing dementia and can increase the risk of death.
Importantly, studies of people in hospital have shown that it is possible to prevent around a third of cases of delirium by providing an environment and care plan that target the main delirium risk factors, including, providing better lighting and signs to avoid disorientation; avoiding unnecessary use of catheters to help prevent infection; and avoiding certain medications which increase the risk of delirium.
This review has searched for and assessed research on preventing delirium in older people living in LTC.
Study characteristics
The evidence is current to February 2019. We found three studies that included 3851 participants. Two studies took place in the US and one study in the UK.
One study tested whether delirium could be prevented by calculating how much fluid an older person in a care home needs each day and ensuring hydration was maintained. There were 98 people in the study, which lasted four weeks.
One study tested the effect of a computer program which searched for prescriptions of medications that might increase the chance of developing delirium, to enable a pharmacist to adjust or stop them. There were 3538 people in the study, which lasted 12 months.
One study tested an enhanced educational intervention which included learning sessions on delirium with care home staff and group meetings to identity targets for preventing delirium. There were 215 people in the study, which lasted 16 months.
Key findings
It was not possible to determine if the hydration intervention reduced the occurrence of delirium. This was a small study of short duration with serious design problems.
The study of a computerised medication search programme probably reduced delirium, but there was no clear reduction in hospital admissions, deaths or falls. A potential problem is that it might not be possible to use this computer program in different countries that do not have similar computer systems available.
It was not possible to determine if the enhanced education intervention reduced the occurrence of delirium and there was no clear reduction in the number of deaths. The intervention was probably associated with a reduction in hospital admissions. This is based on findings from a small study.
Quality of the evidence
There is very low‐quality evidence on the effectiveness of hydration interventions for reducing the incidence of delirium. Therefore, it was not possible to draw firm conclusions.
There is moderate‐quality evidence that a computerised medication search programme may reduce the incidence of delirium. There is no clear evidence for reducing hospitalisations, mortality or falls.
There is very low‐quality evidence of the effectiveness of an enhanced educational intervention for reducing delirium. Therefore, it was not possible to draw firm conclusions. There is moderate‐quality evidence for reducing hospital admissions.
As this review only found a small number of research studies, we recommend that further research be conducted, testing different ways of preventing delirium for older people in LTC.
Summary of findings
Background
Long‐term care (LTC) facilities are institutions which are considered to be an individual's 'usual place of residence'. This distinguishes them from other more temporary facilities, such as respite care, intermediate care and postacute care settings. There is significant variation internationally in the terminology used to describe LTC settings (Burton 2017). Broadly, LTC is an umbrella term for facilities including: residential homes, which provide personal care, supervision with medications; and some help with activities of daily living; and nursing homes, which provide 24‐hour nursing care by staff with specialist skills in management of physical and mental health conditions (Sanford 2015).
Delirium is a common and serious acute change in mental status, which develops rapidly, normally over several hours to a couple of days. It consists of a sudden confusion which is generally associated with serious illness, undergoing surgery, a change in physical condition or receiving pharmacological treatment (Inouye 2014). Delirium can present with hyperactive features (restlessness and agitated behaviour) and hypoactive features (where the individual is withdrawn and sleepy), and the clinical picture is often mixed (Yang 2009). All subtypes are associated with increased risk of mortality, but for people with dementia, the hypoactive form is more serious (Yang 2009). Older adults are particularly vulnerable to delirium, with age a significant risk factor in hospital settings (Inouye 2014). Although a single event can precipitate delirium, it is more common for several factors to interact and a multifactorial model of delirium has been established to help illustrate how delirium is precipitated in people at risk (Inouye 1996). Using this model, a seemingly small insult, such as a minor infection or new medication in people at high risk, can lead to delirium.
Delirium during hospital admission is associated with increased risk of mortality, prolonged length of stay, functional decline and new admission to LTC (Siddiqi 2006; Witlox 2010). Delirium is more likely to occur in people with an established diagnosis of dementia and is also associated with an increased likelihood of subsequent cognitive impairment and development of dementia (Fong 2015).
Delirium is common throughout the health and social care system and has substantial health and socioeconomic costs (Inouye 2006; Leslie 2008). In hospitalised people with delirium, mean costs per day have been estimated as two and a half times those of a person without delirium (Leslie 2008). The majority of delirium research has focused on hospitalised people, but LTC residents are also at high risk, with a point prevalence of delirium of around 14% in these settings (Siddiqi 2009). The multifactorial model of delirium susceptibility has been validated in this setting (Voyer 2010), and residents with moderate‐to‐severe cognitive impairment are at particularly high risk (McCusker 2011). Level of education, malnutrition, antipsychotic medication use and physical restraint use are also associated with delirium risk in LTC settings (Morichi 2018). The development of delirium in older people in LTC is associated with increases in risk of admission to hospital, rates of readmission and mortality (Siddiqi 2009). Notably, delirium in LTC residents is typically of longer duration than in hospitalised people (Cole 2012). Similarly, LTC residents with delirium have been shown to have less frequent improvement patterns and more frequent worsening patterns compared to people in acute care settings (Ciampi 2017). Although it is possible to prevent delirium in the hospital setting by providing multicomponent delirium prevention interventions (Siddiqi 2016), it is currently unclear whether interventions to prevent delirium in LTC are effective.
About 2% to 5% of older adults worldwide live in nursing home settings (Ribbe 1997). Considering the combined effects of population ageing, multimorbidity and dementia prevalence, it is likely that LTC facility provision will need to expand to provide care for increasing numbers of dependent older adults (Kingston 2018). The environment and systems of care in LTC facilities share features with hospitals that are likely to increase the risk of delirium. As being older age and having cognitive impairment or dementia are important risk factors for delirium, the high point prevalence of delirium is likely to reflect clustering of these risk factors in LTC.
Description of the condition
The fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) defines delirium as a disturbance of attention (e.g. reduced ability to focus or shift attention), awareness (e.g. reduced orientation of surrounding environment) and cognition (e.g. memory impairment, disorientation); developing rapidly and usually fluctuating in severity over the day (APA 2013).
Key indicators in the presentation of delirium are change and fluctuation in a range of key symptoms and behaviours including:
cognitive function (e.g. worsened concentration, slow responses, confusion);
perception (e.g. visual or auditory hallucinations);
physical function (e.g. reduced mobility, reduced movement, restlessness, agitation, changes in appetite, sleep disturbance);
social behaviour (e.g. lack of co‐operation, withdrawal, or alterations in communication, mood or attitude or both (NICE 2010)).
Delirium is triggered when a susceptible person is exposed to often multiple precipitating factors, including infection, medications, pain and dehydration (Inouye 1998). These multiple factors are considered to interact in a cumulative manner; the greater the number of factors, the greater the risk of delirium. The pathophysiology of delirium is incompletely understood, but a complex interaction between acetylcholine and multiple other neurotransmitters, including dopamine, noradrenaline, glutamate and gamma‐amino hydroxybutyric acid (GABA), is thought to be important (Alagiakrishnan 2004; Clegg 2011; Hshieh 2008).
Description of the intervention
This review examined the effectiveness of single‐ and multicomponent, pharmacological and non‐pharmacological interventions for preventing delirium in older people in LTC.
Non‐pharmacological interventions target the important precipitating factors for delirium and usually incorporate a multicomponent approach to address the multiple potential factors, including: actively looking for and treating infection; avoiding unnecessary urinary catheterisation; undertaking a medication review to identify medications associated with increased risk of delirium; assessing for pain and initiating treatment where appropriate; addressing sensory impairment by providing visual and hearing aids; assessing and encouraging physical capabilities; and addressing and maintaining nutrition and hydration (Boockvar 2016; NICE 2010). Multicomponent delirium prevention interventions incorporating such strategies have been demonstrated to be effective at reducing delirium incidence in hospitalised adults by one third (Siddiqi 2016). Introduction of protocols, staff education or systems redesign are methods that have been used to introduce these interventions (Inouye 1999; Rockwood 1999). As many of the reported risk factors for delirium are similar in both hospitalised people and LTC residents (Siddiqi 2009), non‐pharmacological interventions that have been shown to be effective in hospitals by targeting these risk factors may have a role in reducing the incidence of delirium in LTC, with appropriate modification to account for differences in environmental factors and care processes (McCusker 2013).
Although it is biologically plausible that pharmacological agents could prevent delirium by acting on neurotransmitter pathways, a small number of trials of pharmacological interventions for preventing delirium in hospitalised people have demonstrated limited effectiveness (Siddiqi 2016; Kalisvaart 2005; Tabet 2009).
How the intervention might work
Delirium is associated with various risk factors: predisposing (including, dementia, cognitive impairment, history of delirium, functional impairment, visual impairment, hearing impairment, comorbidity, depression, alcohol abuse and older age) and precipitating factors (including, medications, use of physical restraints, urinary catheterisation, metabolic abnormalities, surgery and infections) (Inouye 2014). Non‐pharmacological interventions target the multiple potential precipitating factors of delirium to reduce their cumulative effect. Pharmacological interventions target neurotransmitter pathways that have been implicated in the complex pathophysiology of delirium.
Why it is important to do this review
This is an update of a Cochrane Review last updated in 2014, which examined the clinical and cost‐effectiveness of pharmacological and non‐pharmacological interventions for preventing delirium in LTC settings. The 2014 review found limited evidence for delirium prevention interventions due to the small number of trials conducted in this setting. We collated evidence from randomised controlled trials and cluster‐randomised controlled trials to provide an up‐to‐date evaluation. Many residents in LTC will experience at least one episode of delirium. As the population ages, the number of residents in LTC is on the rise. With the numerous significant adverse outcomes associated with delirium (including increased risk of mortality and functional decline), and the growing economic costs that are attributable to delirium, it is important to identify which interventions are effective in preventing delirium in this setting. This evidence will help inform the development and commissioning of evidence‐based services to improve the health and well‐being of this vulnerable group. It will also help improve knowledge about delirium in LTC, inform the development of LTC staff education programmes and help stimulate future research into prevention of delirium in LTC residents.
Objectives
To assess the effectiveness of interventions for preventing delirium in older people in institutional long‐term care settings.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster‐RCTs in this review.
Eligible trials investigated interventions for preventing delirium in older people in LTC. It is possible that any general health intervention for older people in LTC will have the effect of reducing delirium. Therefore, we only considered trials that used a validated method of delirium diagnosis, such as DSM‐5 and International Classification of Diseases 10th Edition (ICD‐10) (APA 2013; WHO 1992), or a diagnostic tool validated against these, for example, Confusion Assessment Method (CAM) (Inouye 1990), and Delirium Rating Scale (DRS) (Trzepacz 1988).
Types of participants
We included trials in which participants were residents of LTC facilities and in which the mean participant age was 65 years or older. In this review, LTC was defined as an institution that was the permanent residence of an individual, providing accommodation together with personal or nursing care. We excluded trials taking place in other settings, such as hospitals, palliative care settings and settings that were not the permanent residence of study participants (e.g. postacute care, intermediate care, continuing care).
Types of interventions
Experimental interventions were any interventions designed to prevent delirium in LTC settings. These could have been single‐ or multicomponent, pharmacological or non‐pharmacological. Comparator interventions were standard care for non‐pharmacological interventions (defined as the usual care provided on that unit), or placebo for pharmacological interventions. These interventions are described as complex interventions. Complex interventions contain characteristics of different complexities, including; number of interacting components, number and variability of outcomes, the degree of flexibility in delivering the intervention and the number of organisational levels targeted by the intervention (Campbell 2000).
Types of outcome measures
We identified the primary, secondary and adverse outcome measures that are important both for older people in LTC and for health and social care systems.
Primary outcomes
Prevalence and incidence of delirium, using a validated diagnostic method (see Types of studies).
Severity of delirium, using a validated diagnostic method (e.g. DRS; Trzepacz 1988)).
Mortality.
Secondary outcomes
Duration of delirium episode.
Proportion of time spent with delirium (total number of days of delirium/length of follow‐up).
Total number of delirium episodes.
Cognitive function, using any validated continuous scale.
New diagnosis of dementia.
Worsening severity of dementia, using a validated diagnostic method (e.g. Clinical Dementia Rating (CDR) scale; Morris 1993; Dementia Severity Rating Scale (DSRS); Clark 1996)).
Quality of life.
Direct costs of intervention.
Health utility change and cost effectiveness of intervention.
Activities of daily living.
Adverse events (adverse medication outcomes, falls, new pressure ulcers, hospital admissions).
Where data allowed, we included the following outcomes in the 'Summary of findings' tables.
Prevalence of delirium.
Incidence of delirium.
Severity of delirium.
Mortality.
Cognitive function, using any validated continuous scale.
Falls.
Hospital admissions.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the CDCIG’s Specialised Register of dementia trials (dementia.cochrane.org/our‐trials‐register), on 27 February 2019. The search was sufficiently sensitive to identify all studies relating to delirium.
The Information Specialists of the CDCIG maintain ALOIS, which contains dementia and cognitive improvement studies identified from:
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) on the Cochrane Library;
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, PsycINFO, CINAHL, and LILACS;
Monthly searches of a number of trial registers: meta Register of Controlled Trials; Umin Japan Trial Register; World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical trials Register; German Clinical trials register; Iranian Registry of Clinical trials; Netherlands National Trials Register, plus others);
Monthly searches of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS (see About ALOIS on the ALOIS website).
We ran additional separate searches in CENTRAL (the Cochrane Library), MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), Web of Science and conference proceedings (Web of Knowledge), LILACS (BIREME), Clinicaltrials.gov (www.clinicaltrials.gov), and ICTRP Search Portal (apps.who.int/trialsearch) to ensure that the search was as comprehensive as possible. All search strategies and the number of hits retrieved can be viewed in Appendix 1.
Searching other resources
We checked the reference lists of all papers of included studies for further potentially eligible studies.
Data collection and analysis
Selection of studies
CDCIG information specialists conducted a first assessment on all search results. Two review authors independently examined the titles and abstracts of citations identified by the search for eligibility. We retrieved full‐text copies of potentially relevant studies and two review authors independently assessed them for inclusion, based on the stated eligibility criteria. We settled any disagreements by consensus. We collated studies represented by more than one publication under one study reference. Review authors were not blind to author names and affiliations when assessing studies for inclusion.
Data extraction and management
Two review authors independently extracted data using a piloted data extraction form, and settled any disagreements by consensus. We created Characteristics of included studies tables and 'Summary of findings' tables using GRADEpro (GRADEpro 2015) and Review Manager 5 (Review Manager 2012). Review authors were not blind to author names and affiliations of studies when extracting data. Review authors who had been investigators on an included study were not involved in extracting data from that study.
We contacted study authors via email to resolve any data queries and to obtain relevant data where required.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed included trials for adequacy of sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential sources of bias. For the other potential sources of bias domain, we assessed for contamination, retention of clusters, recruitment bias, and any other bias that may have been caused by the design or conduct of the trial. For each domain, we made a judgement of low risk, high risk or unclear risk of bias. We settled any disagreements by discussion to reach consensus. We generated 'risk of bias' summary figures using Review Manager 5 for each study (Review Manager 2012). Review authors who had been investigators on an included study were not involved in assessing risk of bias of that study.
Measures of treatment effect
We used risk ratios (RR) with 95% confidence interval (CI) as measures of treatment effect for dichotomous outcomes. We used hazard ratios (HR) with 95% CI for time to event data.
Unit of analysis issues
For cluster‐RCTs, we extracted the effect measures (RR, HR) and their 95% CIs that were adjusted for clustering, where available. If unadjusted analyses had been performed, we sought to calculate approximately correct analyses, by extracting data on number of clusters, mean size of each cluster, primary outcome data and estimates of the intracluster correlation coefficient (ICC). If an approximately correct analysis was not possible, then we extracted primary data and calculated RRs with 95% CIs.
Dealing with missing data
We recorded missing data due to loss of participants or clusters from follow‐up, with reasons where possible. We reported the number of participants included in the final analysis as a proportion of all randomised participants. We preferred Intention‐to‐treat data. If these were not available, we recorded per‐protocol data.
Assessment of heterogeneity
We anticipated that different models of LTC in different countries may lead to clinical heterogeneity. In the UK, residential homes and nursing homes comprise residents who have different levels of dependence and associated care needs. Furthermore, different interventions for preventing delirium in older people in LTC were likely to lead to methodological and statistical heterogeneity. For example, there may be heterogeneity between strategies targeting LTC residents or LTC facilities, or heterogeneity due to timing of the delirium prevention intervention.
We planned separate categorisation and analysis of non‐pharmacological and pharmacological, single and multicomponent interventions to help address trial heterogeneity. Due to clear clinical heterogeneity (see Included studies), we did not conduct any meta‐analysis of the included trials.
Assessment of reporting biases
We sought clinical trial registration data and trial protocols to assess potential reporting biases, and documented the funding source for all trials to assist the assessment.
Data synthesis
Where adjusted HRs were presented, we analysed data using generic inverse variance methods, deploying natural logarithms of HRs and associated standard errors.
We did not perform a meta‐analysis because of clinical and methodological differences between the trials.
Subgroup analysis and investigation of heterogeneity
Sensitivity analysis
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The results of the search are outlined in a PRISMA diagram (Figure 1). In this update, the search identified 238 records following deduplication and assessment of titles and abstracts by Cochrane Dementia Group information specialists and review authors. Of these, 227 did not meet inclusion criteria and we excluded them. We retrieved the full‐text of the 11 remaining studies, six of which we excluded (see Excluded studies) and three are ongoing (see Ongoing studies), leaving one study (represented by two papers) eligible for inclusion. This study was added to the two studies from the previous review (see Included studies), totalling three studies for inclusion. We identified three potentially eligible trials that are ongoing (see Ongoing studies).
1.
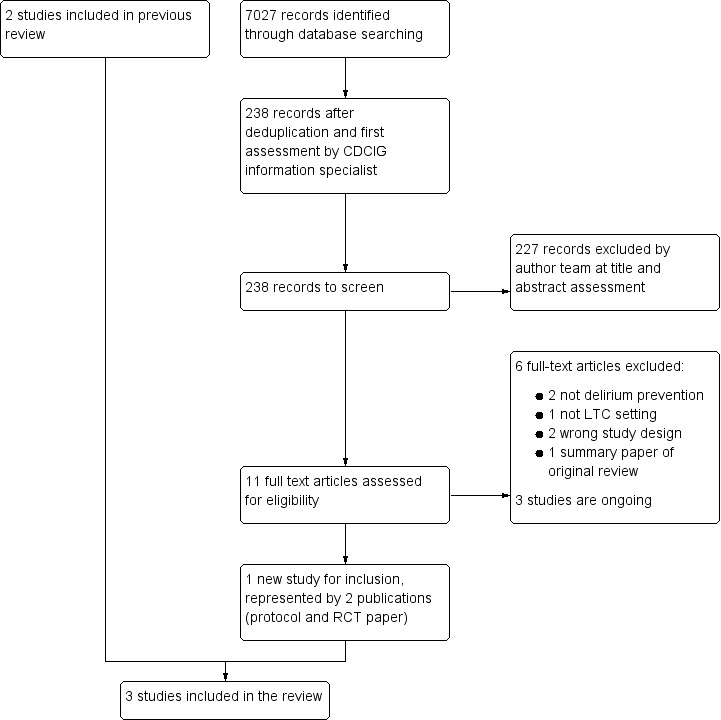
Study flow diagram. CDCIG: Cochrane Dementia and Cognitive Improvement Group; LTC: long‐term care; RCT: randomised controlled trial.
Included studies
We included three trials representing 3851 participants (Culp 2003; Lapane 2011; Siddiqi 2016). Two trials were complex single‐component, non‐pharmacological, delirium prevention interventions (Culp 2003; Lapane 2011), and one trial was a complex multicomponent non‐pharmacological, delirium prevention intervention (Siddiqi 2016).
One study was a cluster‐RCT of a four‐week hydration management intervention (Culp 2003). It recruited 98 residents across seven nursing homes in the US. All residents were considered eligible for inclusion; however, those with acute confusion at baseline, terminal illness, uncontrolled diabetes, nasogastric or gastrostomy tube, severe renal failure, severe congestive heart failure, current urinary tract infection or serum sodium less than 135 mEq/L were excluded. The intervention was a hydration management programme whereby an individual fluid intake goal was calculated according to participant's bodyweight. Seventy‐five per cent of the fluid intake goal was delivered with meals, and the remaining 25% during non‐meal times. Nursing staff were instructed on the treatment regimen. A research assistant calculated the fluid goal and measured fluid intake randomly to ensure protocol compliance. Control arm participants had no individual fluid intake goal. Follow‐up was at four weeks postrandomisation. The trial was funded by the National Institute for Nursing Research.
One study was a cluster‐RCT of the Geriatric Risk Assessment MedGuide (GRAM) software program (Lapane 2011). This trial included 3538 residents across 25 care homes in the US. Medicare‐ and Medicaid‐certified nursing homes with contracts with Omnicare pharmacies, 50 or more geriatric beds and few short‐stay residents were considered for inclusion. All residents were considered eligible; individual resident consent was assessed as not required on the basis that the intervention involved a wholesale change in clinical and administrative practices at the nursing home. The GRAM was used to identify medications that may contribute to delirium and falls risk for individual residents. Pharmacy automatically generated a GRAM report within 24 hours of nursing home admission. For those identified as being on medication contributing to risk of delirium or falls, an automatic report was sent to the pharmacist to coincide with a monthly visit to the nursing home. A medication review was then undertaken at the visit and a proactive monitoring plan was initiated by the care‐home staff to assess for medication side effects. Control nursing homes did not receive the triggered pharmacist visit or proactive monitoring plan. All outcomes were recorded electronically by participating care‐home staff over a 12‐month period. The trial used resident months rather than individuals as its unit of outcome measurement. Results applied only to new admissions during 2004. The trial was funded by the Agency for Healthcare Research and Quality and the National Institutes of Health Center for Research Resources.
One study was a cluster randomised, controlled feasibility trial of a 16‐month educational package delivered to 14 independent sector care homes in one Metropolitan district in the UK (Siddiqi 2016). All residents at the care homes were eligible to take part unless they had severe communication difficulties, were unable to communicate in English or were receiving end‐of‐life care. The trial included 215 care home residents. The intervention called 'Stop delirium!' was an enhanced educational package which incorporated multiple strategies to change practice (Siddiqi 2011). A specialist delirium practitioner delivered three 20‐minute interactive educational sessions to care‐home staff and facilitated monthly staff working groups to identify targets for delirium prevention and to develop bespoke solutions for each home. A delirium champion was also trained at each home to deliver the educational sessions and facilitate the working groups. Control care homes continued with care as usual and were offered the Stop delirium! intervention package at the end of the trial. Delirium assessments were conducted by researchers 16‐months postrandomisation, over a one‐month period. Other outcomes were collected electronically from care home records in a six‐month period starting 10‐months postrandomisation, and hospitalisations were obtained from routinely collected hospital data (hospital episode statistics). The hospital admissions data and delirium incidence data were obtained directly from correspondence with the author. The trial was funded by the National Institute of Health Research.
Excluded studies
In this update, we excluded six studies after assessing full‐texts: two were not specifically delirium prevention trials (García‐Gollarte 2014; Snider 2012); one was not conducted in a LTC setting (Faustino 2016); two studies were not RCTs (Alagiakrishnan 2016; NCT03066232); and one study was a summary paper of the original review (González‐Gil 2016).
Ongoing studies
We found three ongoing studies (Mestres Gonzalvo 2017; NCT02994979; NCT03718156).
Risk of bias in included studies
Our assessment of risk of bias for the three included trials is presented in the Characteristics of included studies table and in Figure 2. Risk of bias was mixed across the three trials; no trial was at low risk of bias across all domains. There was no evidence of blinding of trial participants and personnel in any of the three studies and no evidence of blinding of assessors in two studies (Culp 2003; Lapane 2011). Risk of bias for some domains was rated unclear, due to insufficient information.
2.
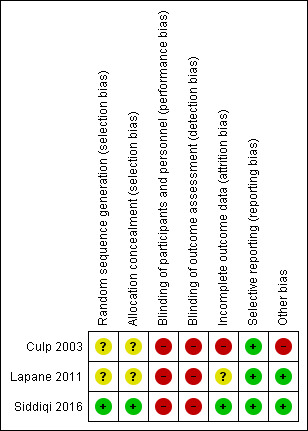
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial reported computer‐generated randomisation and was at low risk of selection bias (Siddiqi 2016). Two trials reported insufficient information on sequence generation or allocation concealment, and risk of selection bias was unclear (Culp 2003; Lapane 2011).
Blinding
Performance bias
Three trials did not report blinding for participants and personnel. Two studies reported that it was not feasible to blind due to the nature of the intervention (Culp 2003; Siddiqi 2016). All three trials were rated at high risk of performance bias.
Detection bias
All three studies reported that outcome assessments were performed by staff or researchers with knowledge of intervention allocation, resulting in a high risk of detection bias (Culp 2003; Lapane 2011: Siddiqi 2016).
Incomplete outcome data
Attrition bias was mixed across the three studies. Culp 2003 was at high risk due to lack of reporting of information on losses to follow‐up and intention‐to‐treat analysis. Lapane 2011 was at unclear risk due to not reporting intention‐to‐treat analysis. Siddiqi 2016 was at low risk due to clear reporting of attrition and intention‐to‐treat analysis.
Selective reporting
There was no evidence of selective outcome reporting in any of the three trials, and all were at low risk of reporting bias.
Other potential sources of bias
Culp 2003 reported that staff alerted researchers to change in cognition; therefore, identification of delirium was partly dependent on staff knowledge. The nursing facility director recommended which unit should be used in the study, introducing further potential for bias. There was a significantly higher baseline urea:creatinine ratio in the intervention group, indicating that this group was more dehydrated at baseline and analyses were not adjusted to account for this. No adjustments were made for the potential effects of clustering. There may have been potential for between‐cluster contamination of the relatively simple hydration‐based intervention, and the investigators reported no measures to prevent this. On the basis of these additional considerations, Culp 2003 was at high risk of other bias.
Lapane 2011 reported that only one trial cluster was lost and they used Poisson regression to account for the cluster design. Therefore, this trial was at low risk of other bias.
Siddiqi 2016 had no evidence of other risk of bias and was classified at low risk.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Single‐component hydration intervention versus control for preventing delirium in older people in institutional long‐term care.
| Single‐component hydration intervention versus control for preventing delirium in older people in institutional long‐term care | ||||||
| Patient or population: people at risk of delirium in institutional long‐term care Settings: long‐term care institutions Intervention: single‐component hydration intervention Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with single‐component hydration intervention versus control | |||||
|
Prevalence of delirium Not measured |
— | — | — | — | — | — |
|
Incidence of delirium NEECHAM Confusion Scale Follow‐up: mean 4 weeks |
Study population | RR 0.85 (0.18 to 4.0) | 98 (1 study) | ⊕⊝⊝⊝ Very lowa,b,c | ||
| 67 per 1000 | 57 per 1000 (12 to 268) | |||||
| Severity of delirium | — | — | — | — | — | Not measured |
| Mortality | — | — | — | — | — | Not measured |
| Cognitive function | — | — | — | — | — | Not measured |
| Falls | — | — | — | — | — | Not measured |
| Hospital admissions | — | — | — | — | — | Not measured |
| *Therisk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NEECHAM: Neelon and Champagne; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aAssessed at high risk of methodological bias for blinding, outcome data and other bias. bOne trial only so not possible to assess for consistency. cVery low rate of delirium events. Wide confidence limits indicate uncertainty; downgraded two levels for imprecision.
Summary of findings 2. Multicomponent educational intervention compared to control for preventing delirium in older people in institutional long‐term care.
| Multicomponent educational intervention compared to control for preventing delirium in older people in institutional long‐term care | ||||||
| Patient or population: preventing delirium in older people in institutional long‐term care Setting: long‐term care institutions Intervention: multicomponent educational intervention Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with multicomponent educational intervention versus control | |||||
|
Prevalence of delirium Assessed with: short‐CAM Follow‐up: period prevalence at 16 months postrandomisation (assessed over a 1‐month period) |
Study population | RR 0.57 (0.15 to 2.19) | 160a (1 RCT) | ⊕⊝⊝⊝ Very lowb,c,d | — | |
| 71 per 1000 | 40 per 1000 (11 to 155) | |||||
|
Incidence of delirium Assessed with: short‐CAM Follow‐up:16 months postrandomisation (assessed over a 1‐month period) |
Study population |
RR 0.62 (0.16 to 2.39) |
137e (1 RCT) |
⊕⊝⊝⊝ Very lowb,c,d | Rate data reported in paper: 4.9 (95% CI 0.7 to 15) per 100 resident‐months at risk in intervention homes and 7.9 (95% CI 1.4 to 22.0) per 100 resident‐months at risk in control homes. |
|
| 100 per 1000 |
62 per 1000 (16 to 239) |
|||||
|
Severity of delirium Assessed with: DRS‐R‐98 Follow‐up: 16 months postrandomisation (assessed over a 1‐month period) |
N/A | N/A | — | N/A | N/A | DRS‐R‐98 completed for 12/13 short CAM positive residents. All rated as high severity (score >15.25) |
|
Mortality Assessed with: care home records Follow‐up: 10 months postrandomisation (recorded over a 6‐month period) |
Study population | RR 0.82 (0.50 to 1.34) | 215 (1 RCT) | ⊕⊕⊕⊝ Moderatec,d | — | |
| 250 per 1000 | 205 per 1000 (125 to 335) | |||||
|
Cognitive function Assessed with: 6‐CIT |
N/A | — | N/A | N/A | Baseline assessment only. | |
|
Falls Assessed with: care home records |
N/A | N/A | — | N/A | N/A | Due to fall recording issues, falls were not analysed further following baseline. |
|
Hospital admissions Assessed with: hospital episode statistics Follow‐up: 10 months postrandomisation (assessed over a 6‐month period) |
Study population | RR 0.67 (0.57 to 0.79) | 494f (1 RCT) | ⊕⊕⊕⊝ Moderated,f | — | |
| 642 per 1000 | 430 per 1000 (366 to 507) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6‐CIT: 6‐item Cognitive Impairment Test; CAM: Confusion Assessment Method; CI: confidence interval; DRS‐R‐98: Delirium Rating Scale, Revised; N/A: not applicable; RCT: randomised controlled trial; RR: risk ratio; Short‐CAM: Short Confusional Assessment Method. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aTotal number included in the analysis. bAssessed as high risk of methodological bias for blinding of participants and personnel. cDowngraded due to imprecision. dOne trial only so not possible to assess for consistency. eNumber of participants was number of resident‐months. Residents were assessed over a 1‐month period, not all residents completed assessments for the full month. fDowngraded due to indirectness. The hospital admissions data were based on a national methodology to quantify admissions from care homes, incorporating care‐home postcode combined with an age cut‐off. This meant older adults living in the same postcode area as a care home may have been included in the results.
Summary of findings 3. Single‐component medication monitoring and adjustment intervention versus control for preventing delirium in older people in institutional long‐term care.
| Single‐component medication monitoring and adjustment intervention versus control for preventing delirium in older people in institutional long‐term care | ||||||
| Patient or population: people at risk of delirium in institutional long‐term care Settings: long‐term care institutions Intervention: single‐component medication monitoring and adjustment intervention Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participant‐months (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with single‐component medication monitoring and adjustment intervention versus control | |||||
| Prevalence of delirium | — | — | — | — | — | Not measured |
|
Incidence of delirium NH‐CAM Follow‐up: mean 12 months |
Study population | HR 0.42 (0.34 to 0.51) | 7311 (1 study)a | ⊕⊕⊕⊝ Moderateb,c,d | — | |
| 104 per 1000 | 45 per 1000 (37 to 54) | |||||
| Severity of delirium | — | — | — | — | — | Not measured |
|
Mortality Follow‐up: mean 12 months |
Study population | HR 0.88 (0.66 to 1.17) | 9412 (1 study)a | ⊕⊕⊕⊝ Moderatec,d,e | — | |
| 25 per 1000 | 22 per 1000 (17 to 29) | |||||
| Cognitive function | — | — | — | — | — | Not measured |
|
Falls Fall events Follow‐up: mean 12 months |
Study population | RR 1.03 (0.92 to 1.15) | 2275 (1 study)a | ⊕⊕⊝⊝ Lowb,c,d | — | |
| 523 per 1000 | 539 per 1000 (481 to 601) | |||||
|
Hospital admissions Follow‐up: mean 12 months |
Study population | HR 0.89 (0.72 to 1.10) | 7599 (1 study)a | ⊕⊕⊕⊝ Moderatec,d,e | — | |
| 55 per 1000 | 49 per 1000 (40 to 60) | |||||
| *Therisk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; NH‐CAM: Nursing Home Confusional Assessment Method; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aNumber of participant months is defined as the number of days from first assessment to the first outcome occurrence, the last date in the nursing home, death date or 31 December 2004. bAssessed as high risk of methodological bias for blinding of participants and personnel. cOnly one trial, therefore, unable to assess consistency. dLarge effect size observed but only one trial, therefore, not eligible for upgrade. eDowngraded due to imprecision.
We did not pool data from the included studies because we considered the interventions to be too diverse.
Primary outcomes
Prevalence of delirium
One trial reported data on prevalence of delirium.
It was not possible to determine an effect on delirium prevalence of the 'Stop delirium! intervention in Siddiqi 2016. Although the RR favoured the intervention group, the result was very imprecise (RR 0.57, 95% CI 0.15 to 2.19; 1 study, 160 participants; very low‐certainty evidence downgraded due to risk of bias and serious imprecision; Table 2).
Incidence of delirium
All three trials reported data on incidence of delirium.
It was not possible to determine an effect on delirium incidence of the hydration‐based intervention in Culp 2003, because of the very low‐certainty of evidence (RR 0.85, 95% CI 0.18 to 4.00; 1 study, 98 participants; very low‐certainty evidence downgraded due to risk of bias and very serious imprecision; Table 1).
The intervention (GRAM report, pharmacist‐led medication review and subsequent proactive monitoring plan) in Lapane 2011 was probably associated with a reduction in delirium incidence compared to control (12‐month HR 0.42, CI 0.34 to 0.51; 1 study, 7311 participant‐months; moderate‐certainty evidence downgraded due to risk of bias; Table 3).
It was not possible to determine an effect on delirium incidence of the 'Stop delirium! intervention in Siddiqi 2016 due to the very low‐certainty evidence (RR 0.62, 95% CI 0.16 to 2.39; 1 study, 137 resident months; downgraded due to risk of bias and very serious imprecision). The study reported delirium incidence rates for both groups. The intervention group had a delirium incidence rate of 4.9 (95% CI 0.7 to 15) and the control group of 7.9 (95% CI 1.4, 22) per 100‐resident months (Table 2).
Severity of delirium
None of the included trials reported data on the severity of delirium.
Mortality
Two trials reported data on mortality.
In the Lapane 2011 study, there was probably little or no effect of the system for reviewing medication on mortality (HR 0.88, CI 0.66 to 1.17; 1 study, 9412 participant‐months; moderate‐certainty evidence downgraded due to imprecision; Table 3).
In the Siddiqi 2016 study, there was probably little of no effect of the 'Stop delirium! intervention on mortality (RR 0.82, 95% CI 0.50 to 1.34; 1 study, 215 participants; moderate‐certainty evidence downgraded for imprecision; Table 2).
Secondary outcomes
Culp 2003 did not report data for any of our secondary outcomes.
Lapane 2011 reported data on hospital admissions and falls. There was probably little or no effect of the intervention on hospital admissions (HR 0.89, CI 0.72 to 1.10; 1 study, 7599 participant‐months; moderate‐certainty evidence downgraded due to imprecision) or falls (HR 1.03, CI 0.92 to 1.15; 1 study, 2275 participant‐months; low‐certainty evidence downgraded for risk of bias and imprecision) (Table 3). The hospitalisation data was not separated into planned and unplanned admissions. Therefore, the data reported were for all hospital admissions. The study reported a 3% absolute reduction in use of opiates and use of miscellaneous anticonvulsant medication and an approximate 4% reduction in tranquillisers, in the intervention homes but not the control homes.
Siddiqi 2016 reported data on hospital admissions, quality of life, direct costs, hospital resource use and monthly costs. The 'Stop delirium! intervention was probably associated with a reduction in hospital admissions compared to the control (RR 0.67, 95% CI 0.57 to 0.79; 1 study, 494 participants; moderate‐certainty evidence downgraded due to indirectness; Table 2). The indirectness in this study was because the hospital admissions data were based on a national methodology to quantify admissions from care homes, based on care‐home postcode combined with an age cut‐off. This meant older adults living in the same postcode area as a care home may have been included in the results. Therefore, the data may have included people who were not part of the trial. The study authors reported difficulty in obtaining accurate care home‐level and individual resident data. The hospitalisation data were not separated by planned and unplanned admissions. Therefore, the data reported were for all hospital admissions. The intervention probably led to similar follow‐up scores as the control group on the quality of life measure, EQ‐5D (MD 0.04, 95% CI ‐0.09 to 0.17; 1 study, 160 participants: moderate‐certainty evidence downgraded due to risk of bias). The total cost of delivering the intervention was GBP 138 per resident. This included the costs for care home staff and for the delirium practitioner. Overall, the hospital resource use for the intervention homes was lower (estimated costs GBP 3281) than control homes (estimated costs GBP 7210). These figures were estimated using national sources, including the National Health Service reference cost databases. In terms of monthly costs, the intervention homes cost per resident was lower at GBP 219.72 compared with GBP 253.01 in control. This included the cost of the intervention and the healthcare resource use.
Subgroup analyses
Limitations of data reporting precluded subgroup analysis for participants with and without dementia.
Discussion
Summary of main results
Our review identified three RCTs of delirium prevention interventions for older people in institutional LTC, recruiting 3851 participants.
One small cluster‐RCT (98 participants) of a hydration‐based intervention was not able to show any reduction in delirium incidence in the intervention group compared to control because of very serious imprecision in the result. Additionally, the analysis was not adjusted for the effects of clustering and there were serious limitations in trial design, so there is a high level of uncertainty associated with the effect estimate. Importantly, the investigators reported that both intervention and control groups were consuming approximately the same volume of fluids over the follow‐up period, and only 51% of intervention participants had 90% or greater compliance with the fluid goal. Previous research has identified that many LTC residents do not consume adequate fluid (Armstrong‐Esther 1996), and this result may indicate that achieving target fluid intake in care‐home residents is challenging, even in the context of a clinical trial.
One large cluster‐RCT (3538 participants) of a computerised system to identify medications that may contribute to delirium risk and trigger a pharmacist‐led medication review found moderate‐certainty evidence of a large reduction in delirium incidence but of little or no effect on hospital admissions, mortality or falls.
One feasibility cluster‐RCT (215 participants) of an enhanced educational package to identify delirium risk targets and develop bespoke solutions specific to individual care homes, was not able to show any reduction in delirium incidence or prevalence due to the serious imprecision in the results. There was moderate‐certainty evidence of a reduction in hospital admissions. The hospital admissions data are based on a national methodology to quantify admissions from care homes, incorporating care‐home postcode combined with an age cut‐off. This means older adults living in the same postcode area as a care home may have been included in the results. Therefore, the data may have included people who were not part of the trial and did not receive the intervention or control.
Overall completeness and applicability of evidence
The small number of included trials represented a limited body of evidence on the effectiveness of interventions for preventing delirium in older people in institutional LTC. We identified only two single‐component non‐pharmacological interventions with methodological limitations and one multicomponent non‐pharmacological intervention. We did not find any pharmacological delirium prevention interventions for this population. Two of the trials were conducted in the US and one in the UK. International differences in the organisation of LTC may mean that the results are not directly applicable to other settings.
Quality of the evidence
We used GRADEpro software to inform the generation of evidence certainty statements.
On the basis of a single RCT with serious limitations in trial design and very imprecise results, there was very low‐certainty evidence on the effectiveness of hydration‐based interventions for reducing the incidence of delirium in older people in institutional LTC. The evidence was downgraded two levels due to serious imprecision. Therefore, it was not possible to draw firm conclusions about this intervention (Table 1).
On the basis of one large RCT, there was moderate‐certainty evidence that a single‐component medication monitoring and adjustment intervention may have reduced the incidence of delirium in older people in institutional LTC (Table 3).
On the basis of one large RCT, there was moderate‐certainty evidence that a single‐component medication monitoring and adjustment intervention did not appear to be associated with reduced hospitalisation or mortality for older people in institutional LTC. There was low‐certainty evidence that the intervention did not appear to be associated with falls (Table 3).
On the basis of a single RCT, there was very low‐certainty evidence on the effectiveness of an enhanced educational intervention for reducing delirium incidence or prevalence in older people in LTC. The evidence was downgraded two levels due to serious imprecision. Therefore, it was not possible to draw firm conclusions about this intervention (Table 2).
On the basis of a single RCT, there was moderate‐certainty evidence that an enhanced educational intervention may have reduced hospitalisations in older people in LTC. The evidence was downgraded due to indirectness. There was moderate‐certainty evidence that the intervention did not appear to be associated with reduced mortality (Table 2).
Potential biases in the review process
This review has followed Cochrane procedures and there were only minor amendments to the review protocol following initial publication. The very small number of included trials precluded an accurate assessment of consistency of results or a statistical assessment of reporting bias.
Agreements and disagreements with other studies or reviews
To our knowledge there are no previous systematic reviews on the effectiveness of delirium prevention interventions for older people in institutional LTC settings.
Authors' conclusions
Implications for practice.
Introduction of a software‐based intervention to identify medications that could contribute to delirium risk, so that a pharmacist‐led medication review and monitoring plan can be initiated, was probably associated with a reduction in delirium incidence for older people in institutional LTC without affecting hospital admissions, falls or mortality. This is based on one large randomised controlled trial (RCT) in the US and the intervention may not be practical in other countries which do not have comparable information technology services available in care homes. One small RCT of a weight‐based hydration intervention for older people in nursing homes had serious methodological limitations and poor concordance with the intervention; it is not possible to determine the usefulness of this approach. The enhanced educational intervention delivering training sessions to staff and developing targets for delirium prevention bespoke to each care home, was not able to provide any clear evidence for the reduction of delirium episodes or mortality. The intervention may be able to reduce hospital admissions, although, due to the possible inclusion of hospital admissions data from non‐trial participants, further research is required.
Implications for research.
There is very limited evidence on the effectiveness of interventions for preventing delirium in older people in institutional LTC. Further large trials of computerised medication management interventions and of enhanced educational interventions are justified. These trials should be supported by research investigating implementation across different care systems.
Delirium is a common and very distressing condition with devastating outcomes. Interventions that are effective in preventing delirium are of high importance. Future studies should pay particular attention to accurate recording of delirium incidence and prevalence. The challenges of conducting research in LTC settings are well described in the international literature (Lam 2018). In the UK, the lack of a systematic recording of care home residency in health and care data systems makes it difficult to use these methods to reliably ascertain outcomes such as hospital admission (Burton 2018). Researchers need to be aware of the limitations of the methods they select to collect outcome data and consider how these may be overcome when designing trials.
What's new
| Date | Event | Description |
|---|---|---|
| 27 February 2019 | New search has been performed | The most recent search for this review was performed on 27 February 2019 |
| 27 February 2018 | New citation required and conclusions have changed | Review updated and conclusions changed. One study added to the review. Review authors have changed. One existing author and four new authors completed this update. |
Acknowledgements
We thank the authors (Andrew Clegg, Anne Heaven, John Young, and Rachel Holt) of the original version of the review, for their substantial contribution to the previous research, which has been updated in the current review.
We would like to acknowledge the contribution of Kenneth Boockvar and Sarah Ward who peer reviewed this update.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) (Date of most recent search: 27 February 2019) |
delirium | Jul 2012: 96 Apr 2013: 9 Nov 2016: 8 Feb 2018: 3 Feb 2019: 3 |
| 2. MEDLINE In‐process Nov 2016:and other non‐indexed citations and MEDLINE 1950 ‐ present (OvidSP) (Date of most recent search: 27 February 2019) |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. Primary Prevention/ 17. prevent*.mp. 18. reduc*.ti,ab. 19. stop*.ti,ab. 20. taper*.ti,ab. 21. avoid*.ti,ab. 22. "cut* down".ti,ab. 23. or/16‐22 24. 15 and 23 25. randomized controlled trial.pt. 26. controlled clinical trial.pt. 27. randomi?ed.ab. 28. placebo.ab. 29. drug therapy.fs. 30. randomly.ab. 31. trial.ab. 32. groups.ab. 33. or/25‐32 34. (animals not (humans and animals)).sh. 35. 33 not 34 36. 24 and 35 |
Jul 2012: 821 Apr 2013: 118 Nov 2016: 120 Feb 2018: 263 Feb 2019: 192 |
| 3. Embase 1980 ‐ 2019 February 26 (OvidSP) (Date of most recent search: 27 February 2019) |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. Delirium, Dementia, Amnestic, Cognitive Disorders/su [Surgery] 14. obnubilat*.ti,ab. 15. or/1‐14 16. primary prevention/ 17. prevent*.mp. 18. reduc*.ti,ab. 19. stop*.ti,ab. 20. taper*.ti,ab. 21. avoid*.ti,ab. 22. "cut* down".ti,ab. 23. or/16‐22 24. 15 and 23 25. randomized controlled trial/ 26. random*.ti,ab. 27. placebo.ti,ab. 28. trial.mp. 29. controlled clinical trial/ 30. or/25‐29 31. 24 and 30 |
Jul 2012: 835 Apr 2013: 161 Nov 2016: 191 Feb 2018: 562 Feb 2019: 366 |
| 4. PsycINFO 1806 ‐ February week 4 2019 (OvidSP) (Date of most recent search: 27 February 2019) |
1. Delirium/ 2. deliri*.mp. 3. "acute confusion*".ti,ab. 4. "acute organic psychosyndrome".ti,ab. 5. "acute brain syndrome".ti,ab. 6. "metabolic encephalopathy".ti,ab. 7. "acute psycho‐organic syndrome".ti,ab. 8. "clouded state".ti,ab. 9. "clouding of consciousness".ti,ab. 10. "exogenous psychosis".ti,ab. 11. "toxic psychosis".ti,ab. 12. "toxic confusion".ti,ab. 13. obnubilat*.ti,ab. 14. or/1‐13 15. Prevention/ 16. prevent*.mp. 17. reduc*.ti,ab. 18. stop*.ti,ab. 19. taper*.ti,ab. 20. avoid*.ti,ab. 21. "cut* down".ti,ab. 22. or/15‐21 23. 14 and 22 24. random*.mp. 25. trial.mp. 26. placebo*.mp. 27. group.ab. 28. or/24‐27 29. 23 and 28 |
Jul 2012: 163 Apr 2013: 19 Nov 2016: 16 Feb 2018: 45 Feb 2019: 17 |
| 5. CINAHL (EBSCOhost) (Date of most recent search: 27 February 2019) |
S1 (MH "Delirium") OR (MH "Delirium Management (Iowa NIC)") OR (MH "Delirium, Dementia, Amnestic, Cognitive Disorders/SU") S2 TX deliri* S3 TX "acute confusion*" S4 TX "acute organic psychosyndrome" S5 TX "acute brain syndrome" S6 TX "metabolic encephalopathy" S7 TX "acute psycho‐organic syndrome" S8 TX "clouded state" S9 TX "clouding of consciousness" S10 TX "exogenous psychosis" S11 TX "toxic psychosis" S12 TX "toxic confusion" S13 TX obnubilat* S14 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S15 (MH "Preventive Trials") OR (MH "Preventive Health Care") S16 TX prevent* S17 TX reduc* S18 TX stop* S19 TX taper* S20 TX avoid* S21 TX "cut* down" S22 S15 or S16 or S17 or S18 or S19 or S20 or S21 S23 S14 and S22 S24 TX random* S25 TX placebo S26 TX trial S27 (MH "Clinical Trials") OR (MH "Intervention Trials") S28 S24 or S25 or S26 or S27 S29 S23 and S28 |
Jul 2012: 189 Apr 2013: 0 Nov 2016: 2 Feb 2018: 86 Feb 2019: 115 |
| 6. Web of Science Core Collection (ISI Web of Science) (Date of most recent search: 27 February 2019) |
Topic=(deliri* OR "acute confusion*" OR "acute organic psychosyndrome" OR "acute brain syndrome" OR "metabolic encephalopathy" OR "acute psycho‐organic syndrome" OR "clouded state" OR "clouding of consciousness" OR "exogenous psychosis" OR "toxic psychosis" OR "toxic confusion" OR obnubilat*) AND Topic=(prevent* OR reduc* OR stop* OR taper* OR avoid* OR "cut* down") AND Topic=(random* or placebo or "double‐blind" or trial OR groups OR "controlled study" OR "time series" OR "Comparative Study" OR "Pretest‐Posttest Design") Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH. Lemmatization=On |
Jul 2012: 654 Apr 2013: 163 Nov 2016: 176 Feb 2018: 620 Feb 2019: 325 |
| 7. LILACS (BIREME) (Date of most recent search: 27 February 2019) |
randomly OR randomised OR randomized OR trial OR ensaio clínico OR control OR controlled [Words] and delirium OR delious OR deliria OR delirio OR loucura [Words] | Jul 2012: 47 Apr 2013: 1 Nov 2016: 5 Feb 2018: 8 Feb 2019: 6 |
| 8. CENTRAL (the Cochrane Library) (Issue 2 of 12, 2019) (Date of most recent search: 27 February 2019) |
#1 MeSH descriptor Delirium, this term only #2 deliri* #3 "acute confusion*" #4 "acute organic psychosyndrome" #5 "acute brain syndrome" #6 "metabolic encephalopathy" #7 "acute psycho‐organic syndrome" #8 "clouded state" #9 "clouding of consciousness" #10 "exogenous psychosis" #11 "toxic psychosis" #12 "toxic confusion" #13 obnubilat* #14 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 MeSH descriptor Primary Prevention, this term only #16 prevent* #17 reduc* #18 stop* #19 taper* #20 avoid* #21 "cut* down" #22 (#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#14 AND #22), trials |
Jul 2012: 230 Apr 2013: 7 Nov 2016: 42 Feb 2018: 80 Feb 2019: 365 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) (Date of most recent search: 27 February 2019) |
care home OR institutionalised OR institutionalized OR long term care OR home | Interventional Studies | delirium OR toxic psychosis OR toxic confusion OR metabolic encephalopathy OR clouded state OR exogenous psychosis | Senior | Jul 2012: 156 Apr 2013: 23 Nov 2016: 11 Feb 2018: 27 Feb 2019: 12 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) (includes: Australian New Zealand Clinical Trials Registry; ClinicalTrials.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register) (Date of most recent search: 27 February 2019) |
care home OR institutionalised OR institutionalized OR long term care OR home | Interventional Studies | delirium OR toxic psychosis OR toxic confusion OR metabolic encephalopathy OR clouded state OR exogenous psychosis | Jul 2012: 72 Apr 2013: 0 Nov 2016: 1 Feb 2018: 5 Feb 2019: 1 |
| TOTAL before deduplication | July 2012: 3263 April 2013: 501 Nov 2016: 572 Feb 2018: 1699 Feb 2019: 1037 TOTAL: 7027 |
|
| TOTAL after deduplication and first assessment by CDCIG Information Specialists | July 2012: 120 April 2013: 15 Nov 2016: 31 Feb 2018: 70 Feb 2019: 2 TOTAL: 238 |
|
Data and analyses
Comparison 1. Single‐component hydration intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of delirium | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
1.1. Analysis.
Comparison 1 Single‐component hydration intervention versus control, Outcome 1 Incidence of delirium.
Comparison 2. Single‐component medication monitoring and adjustment intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of delirium | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Mortality | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 Falls | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4 Hospital admissions | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
2.1. Analysis.
Comparison 2 Single‐component medication monitoring and adjustment intervention versus control, Outcome 1 Incidence of delirium.
2.2. Analysis.
Comparison 2 Single‐component medication monitoring and adjustment intervention versus control, Outcome 2 Mortality.
2.3. Analysis.
Comparison 2 Single‐component medication monitoring and adjustment intervention versus control, Outcome 3 Falls.
2.4. Analysis.
Comparison 2 Single‐component medication monitoring and adjustment intervention versus control, Outcome 4 Hospital admissions.
Comparison 3. Multicomponent delirium prevention intervention (MCI) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prevalence of delirium | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Incidence of delirium | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4 Quality of Life EQ‐5D | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Hospital admissions | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only |
3.1. Analysis.
Comparison 3 Multicomponent delirium prevention intervention (MCI) versus control, Outcome 1 Prevalence of delirium.
3.2. Analysis.
Comparison 3 Multicomponent delirium prevention intervention (MCI) versus control, Outcome 2 Incidence of delirium.
3.3. Analysis.
Comparison 3 Multicomponent delirium prevention intervention (MCI) versus control, Outcome 3 Mortality.
3.4. Analysis.
Comparison 3 Multicomponent delirium prevention intervention (MCI) versus control, Outcome 4 Quality of Life EQ‐5D.
3.5. Analysis.
Comparison 3 Multicomponent delirium prevention intervention (MCI) versus control, Outcome 5 Hospital admissions.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Culp 2003.
| Methods | Cluster‐randomised controlled trial with nursing home as the unit of randomisation | |
| Participants | 98 residents of 7 care homes in Iowa, USA Mean age: 84.5 (SD 9.3) years in intervention group; 83.8 (SD 8.1) years in control group 54.7% women in intervention group; 53.3% women in control group |
|
| Interventions | Intervention group: 4‐week weight‐based hydration management intervention for nursing‐home residents. Individual fluid intake goal was calculated according to bodyweight. 75% of the fluid intake goal was delivered with meals, the remaining 25% during non‐meal times. Nursing staff were instructed on the treatment regimen. A research assistant calculated the fluid goal and measured fluid intake randomly to ensure protocol compliance. Control group: no individual fluid intake goal. |
|
| Outcomes | Incidence of delirium, measured using the NEECHAM Confusion Scale (Neelon 1996). Outcomes recorded at 4 weeks postrandomisation. |
|
| Notes | Funding source: National Institute for Nursing Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on generation of allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Cluster randomised trial. Unclear if all care homes recruited prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants or personnel (or both) aware of allocation to intervention or control group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessments made by the research team who were not blind to intervention allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on loss to follow‐up. No intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | High risk | Staff alerted researchers to change in cognition so dependent on staff knowledge. Nursing facility director recommended which unit should be used in the study. A higher urea:creatinine ratio in the intervention group, indicating that this group were more dehydrated at baseline. No adjustment made for effects of clustering. Potential for between‐cluster contamination of the relatively simple hydration‐based intervention, and measures to prevent this were not reported by the investigators. |
Lapane 2011.
| Methods | Cluster‐randomised controlled trial with nursing home as the unit of randomisation | |
| Participants | 3538 residents of 25 nursing homes in Virginia, USA, recruited between 2003 and 2004 Medicare‐ and Medicaid‐certified nursing homes with contracts with Omnicare pharmacies, ≥ 50 geriatric beds and few short‐stay residents were considered for inclusion. 73.9% women 39.0% aged ≥ 85 years |
|
| Interventions | Intervention group: GRAM software used to identify resident‐specific medications that may contribute to delirium and falls risk. Pharmacy automatically generated GRAM report within 24 hours of nursing‐home admission. For those who triggered GRAM resident assessment protocols for delirium or falls risk, an automatic report was sent to the pharmacist to coincide with a monthly visit to the nursing home. A medication review was then undertaken at the visit and a proactive monitoring plan was initiated by the care home staff to assess for medication adverse effects. Control group: nursing homes did not receive the triggered pharmacist visit or proactive monitoring plan. |
|
| Outcomes | Incidence of delirium, measured using the NH‐CAM (Dosa 2007) Fall events, measured using MDS records Hospital admissions, measured using MDS records. Mortality, measured using MDS records. The trial used resident months (defined as the number of days from date of first assessment to the first outcome occurrence, the last date in the nursing home, the death date or 31 December 2004), rather than individuals as its unit of outcome measurement. Results applied only to new admissions during 2004. All outcomes were recorded electronically by participating care‐home staff over a 12‐month period. |
|
| Notes | Funding source: Agency for Healthcare Research and Quality and the National Institutes of Health Center for Research Resources. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of allocation sequence generation not provided. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if all care homes recruited prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants or personnel (or both) aware of allocation to intervention or routine care. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessed using data from the minimum dataset and assessments were made by staff aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 cluster lost. No information on intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | Only 1 cluster was lost. Poisson regression accounting for the cluster design was used. |
Siddiqi 2016.
| Methods | Cluster‐randomised controlled trial with care home as the unit of randomisation | |
| Participants | 215 participants from 14 independent sector care homes in 1 metropolitan district in the UK (residential and nursing care) Mean age: 83.9 (SD 8.1) years 69.3% women 96.7% white British ethnicity |
|
| Interventions | Intervention group: Stop Delirium! Multicomponent educational package, multiple strategies to change practice. Delivered to care homes over 16‐months. Specialist delirium practitioner delivered 3 × 20‐minute interactive educational sessions and facilitated working groups with care home staff – to identify delirium prevention targets and develop bespoke solutions for each home. A 'delirium champion' was also trained at each home. It aims to modify key resident and environmental delirium risk factors (pain, infections, dehydration, poor nutrition, constipation, polypharmacy, sensory impairment, limited mobility and sleep disturbance) by improving the quality of care. Control group: care as usual. Stop delirium package offered at the end of the trial. Delirium assessments: 16‐months postrandomisation, over a 1‐month period. Other outcomes: collected electronically from care home records in a 6‐month period starting 10 months postrandomisation, and hospitalisations were obtained from routinely collected hospital data (hospital episode statistics). |
|
| Outcomes | Delirium point prevalence (at baseline) Delirium period prevalence assessed by CAM Delirium incidence assessed by CAM and case note review Delirium severity assessed using the DRS‐R‐98 Proportion of residents with ≥ 1 CAM‐positive assessment during follow‐up Hospital admissions (6 months and 16 months) Number of medications Mortality Feasibility of baseline and outcome assessments Health and social care resource use Quality of life using EQ‐5D, SCRQoL, and DEMQoL‐5D Intervention delivery |
|
| Notes | National Institute of Health Research for Patient Benefit Programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Homes were randomised on a 1:1 basis using a computer‐generated minimisation programme which stratified homes based on care home size and percentage of residents with dementia. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated minimisation programme by Leeds Clinical Trials Research Unit. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants or personnel (or both) were aware of allocated intervention due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors reported that it was not possible to blind researchers collecting outcome measures to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 215 residents recruited, 160 included in analysis. Attrition of participants between recruitment and follow‐up was 27.2% for the intervention group and 24.1% for the control group. Similar reasons for dropout across both groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol was available and changes to the original protocol were outlined, which included the introduction of a second phase of resident recruitment 12 months after randomisation because of a high attrition rate, and conducting structured case note reviews in order to explore the possibility that reliance on face‐to‐face assessments alone might be underestimating delirium. |
| Other bias | Low risk | No evidence of other bias. |
CAM: Confusional Assessment Method; DEMQoL‐5D: Dementia Quality of Life – 5 Dimension; DRS‐R‐98: Delirium Rating Scale Revised‐98; EQ‐5D: EuroQol‐5‐Dimensions; GRAM: Geriatric Risk Assessment MedGuide; MDS: minimum data set; NEECHAM: Neelon and Champagne; NH‐CAM: Nursing Home Confusion Assessment Method; SCRQoL: Social Care Related Quality of Life; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alagiakrishnan 2016 | Not an RCT. |
| Faustino 2016 | Trial not conducted in a long‐term care setting. |
| García‐Gollarte 2014 | Not a delirium prevention trial. |
| González‐Gil 2016 | Summary paper of original review. |
| Greendyke 1986 | Not a delirium prevention trial. |
| Grover 2011 | Not a delirium prevention trial. |
| Hofferberth 1989 | Not a delirium prevention trial. |
| Isaia 2009 | Trial not conducted in a long‐term care setting. |
| Kim 2010 | Not a delirium prevention trial. |
| Marcantonio 2010 | Trial not conducted in a long‐term care setting. |
| Mittal 2004 | Not a delirium prevention trial. |
| Moretti 2004 | Not a delirium prevention trial. |
| NCT03066232 | Not an RCT. |
| Overshott 2010 | Not a delirium prevention trial. |
| Pellfolk 2010 | Not a delirium prevention trial. |
| Snider 2012 | Not a delirium prevention trial. |
| Tahir 2010 | Not a delirium prevention trial. |
| Ushijima 2008 | Not a delirium prevention trial. |
| Yoon 2011 | Not a delirium prevention trial. |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Mestres Gonzalvo 2017.
| Trial name or title | Supporting Clinical Rules Engine in the Adjustment of Medication (SCREAM) |
| Methods | Cluster‐RCT of nursing homes in the Netherlands |
| Participants | Nursing home residents |
| Interventions | Intervention group: clinical decision support system will be used to screen medication lists, laboratory values and medical history in order to obtain potential clinically relevant remarks. The remarks will be sent to the main physician and feedback will be provided whether the advice was followed or not. Control group: regular care. |
| Outcomes | Hospital referrals, delirium, falls, and deaths |
| Starting date | June 2013 |
| Contact information | Carlota Mestres Gonzalvo; c.mestresgonzalvo@zuyderland.nl |
| Notes | The complete SCREEN project (Supporting clinical rules in the evaluation of elderly patients with neuropsychiatric disorders), which includes the SCREAM study, is supported by a grant from the ZonMw (the Netherlands Organisation for Health Research and Development) (Grant number: 113101001). |
NCT02994979.
| Trial name or title | Nursing assistant intervention to prevent delirium in nursing homes |
| Methods | People on 17 long‐term care units at a large, urban nursing home who experience onset of an acute change in condition according to established criteria, will be screened. Delirium will be assessed 5 days a week by a research assistant blinded to study hypotheses and group assignment. Cognitive and physical function decline and hospital transfer will be ascertained during 1‐month follow‐up. |
| Participants | Nursing home residents |
| Interventions | Intervention group: multicomponent intervention targeting delirium risk factors (immobility, cognitive impairment, dehydration, undernutrition, sleep and medication use). Daily visits from an Elder Life Specialist, a mobile Certified Nursing Assistant trained to provide services to counter risks for delirium, for the duration of the acute illness and for 1 week following, in collaboration with the patient's primary medical and nursing team. Control group: usual care from the unit‐based nurses and the patient's primary care team |
| Outcomes | Delirium incidence measured by Confusional Assessment Method Physical function, cognitive function, hospital admission |
| Starting date | November 2016 |
| Contact information | Kimberly Judon, kjudon@jewishhome.org |
| Notes |
NCT03718156.
| Trial name or title | The Prevention Program for Alzheimer's Related Delirium (PREPARED) trial |
| Methods | 4‐year, cluster RCT of long‐term care facilities in Canada. Clusters will be assigned to either the PREPARED trial intervention group or the control (usual care) group. 40–50 long‐term care facilities will be recruited. Residents will be assessed weekly for a follow‐up of 18 weeks. |
| Participants | Long‐term care residents |
| Interventions | Intervention group: multicomponent intervention provided to nursing staff working in long‐term care facilities. The intervention consists of 4 components: a decision tree, an instruction manual, a training package and a tool kit. Nursing staff will be trained to adjust the therapeutic nursing plans for residents in the intervention group, by providing optimal stimulation (including, surveying the use of glasses and hearing aids and room lighting and space organisation), and by assessing the presence of modifiable delirium risk factors (antipsychotic use, sensory impairment, restraint use and dehydration), then taking specific action when a risk factor is identified. Control group: care as usual. Staff in this group will be provided with the PREPARED trial training programme at the end of follow‐up. |
| Outcomes | Incidence of delirium measured by Confusional Assessment Method Delirium severity measured by Delirium Index Delirium episode duration and number of delirium episodes Falls, cognitive functioning, change in functional autonomy, change in level of social engagement |
| Starting date | June 2018 |
| Contact information | Machelle Wilchesky; Machelle.Wilchesky@mcgill.ca |
| Notes |
RCT: randomised controlled trial.
Differences between protocol and review
2019 update of the review
We rated the blinding section of the risk of bias in two parts (blinding of participants and personnel; blinding of outcome assessment) for all included studies for consistency with the delirium prevention in hospitalised adults review.
We changed the outcome previously listed as 'unplanned hospitalisations', to 'hospital admissions' to better represent the data, which included both planned and unplanned hospital admissions.
Previous review version (2014)
Following publication of the protocol, amendments were made to Measures of treatment effect and Data synthesis to incorporate the analysis of adjusted data from cluster‐randomised trials using generic inverse variance methods. A post hoc decision was made to include the adverse outcome of falls in the 'Summary of findings' tables. We planned participant‐level subgroup analyses for those with and without dementia, but we were unable to conduct these analyses because of limitations in reporting. We planned sensitivity analyses for trials at low risk of methodological bias, but these were not possible because of the very small number of included trials.
Contributions of authors
For this 2018 update contributions were as follows.
RW and NS screened all titles and abstracts.
RW and JKB assessed full texts for inclusion.
RW, JKB and JL extracted data for included studies and assessed risk of bias.
RW completed 'Summary of findings' tables and generated GRADE Evidence Profiles.
All authors contributed to the drafting and editing of this update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
RW: none.
JKB is an author on one of the included studies. She had no part in data extraction or assessing risk of bias for this study.
NR: none.
YLP: none.
JL: none.
NS was chief investigator for a National Institute for Health Research (NIHR) Research for Patient Benefit (RfPB) grant to investigate the effects of a delirium prevention intervention for older people in long‐term care and is an author on one of the included studies. She had no part in decisions about inclusion, data extraction, risk of bias or interpretation of findings from this study. She provided additional unpublished data on hospital admissions, at the request of RW.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Culp 2003 {published data only}
- Culp K, Mentes J, Wakefield B. Hydration and acute confusion in long‐term care residents. Western Journal of Nursing Research 2003;25(3):251‐66. [DOI] [PubMed] [Google Scholar]
Lapane 2011 {published data only}
- Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist‐led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. Journal of the American Geriatrics Society 2011;59(7):1238‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siddiqi 2016 {published data only}
- Heaven A, Cheater F, Clegg A, Collinson M, Farrin A, Forster A, et al. Pilot trial of stop delirium! (PiTStop) – a complex intervention to prevent delirium in care homes for older people: study protocol for a cluster randomised controlled trial. Trials 2014;15(47):15‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi N, Cheater F, Collinson M, Farrin A, Forster A, George D, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age and Ageing 2016;45:651‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alagiakrishnan 2016 {published data only}
- Alagiakrishnan, K. Melatonin based therapies for delirium and dementia. Discovery Medicine 2016;21(117):363‐71. [ISSN: 1539‐6509] [PubMed] [Google Scholar]
Faustino 2016 {published data only}
- Faustino TN, Pedreira LC, Freitas YS, Silva RM, Amaral JB. Prevention and monitoring of delirium in older adults: an educational intervention. Brazilian Journal of Nursing 2016;69(4):678‐85. [DOI] [PubMed] [Google Scholar]
García‐Gollarte 2014 {published data only}
- García‐Gollarte F, Baleriola‐Júlvez J, Ferrero‐López I, Cuenllas‐Díaz A, Cruz‐Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. Journal of American Medical Directors Association 2014;15(12):885‐91. [DOI] [PubMed] [Google Scholar]
González‐Gil 2016 {published data only}
- González‐Gil T. Interventions for preventing delirium in older people in institutional long‐term care. International Journal of Nursing Studies 2015;55:133‐4. [DOI] [PubMed] [Google Scholar]
Greendyke 1986 {published data only}
- Greendyke RM, Kanter DR, Schuster DB, Verstreate S, Wootton J. Propranolol treatment of assaultive patients with organic brain disease. a double‐blind crossover, placebo‐controlled study. Journal of Nervous and Mental Disease 1986;174(5):290‐4. [DOI] [PubMed] [Google Scholar]
Grover 2011 {published data only}
- Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. Journal of Psychosomatic Research 2011;71(4):277‐81. [DOI] [PubMed] [Google Scholar]
Hofferberth 1989 {published data only}
- Hofferberth B. The effect of Ginkgo biloba extract on neurophysiological and psychometric measurement results in patients with psychotic organic brain syndrome. A double‐blind study against placebo. Arzneimittel‐Forschung 1989;39(8):918‐22. [PubMed] [Google Scholar]
Isaia 2009 {published data only}
- Isaia G, Astengo MA, Tibaldi V, Zanocchi M, Bardelli B, Obialero R, et al. Delirium in elderly home‐treated patients: a prospective study with 6‐month follow‐up. Age 2009;31(2):109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim SW, Yoo JA, Lee SY, Kim SY, Bae KY, Yang SJ, et al. Risperidone versus olanzapine for the treatment of delirium. Human Psychopharmacology 2010;25(4):298‐302. [DOI] [PubMed] [Google Scholar]
Marcantonio 2010 {published data only}
- Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. Journal of the American Geriatrics Society 2010;58(6):1019‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittal 2004 {published data only}
- Mittal D, Jimerson NA, Neely EP, Johnson WD, Kennedy RE, Torres RA, et al. Risperidone in the treatment of delirium: results from a prospective open‐label trial. Journal of Clinical Psychiatry 2004;65(5):662‐7. [DOI] [PubMed] [Google Scholar]
Moretti 2004 {published data only}
- Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G. Cholinesterase inhibition as a possible therapy for delirium in vascular dementia: a controlled, open 24‐month study of 246 patients. American Journal of Alzheimer's Disease and Other Dementias 2004;19(6):333‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT03066232 {published data only}
- NCT03066232. Delirium in home‐dwelling old people receiving home nursing care. clinicaltrials.gov/ct2/show/record/NCT03066232 (first received 28 February 2017).
Overshott 2010 {published data only}
- Overshott R, Vernon M, Morris J, Burns A. Rivastigmine in the treatment of delirium in older people: a pilot study. International Psychogeriatrics 2010;22(5):812‐8. [DOI] [PubMed] [Google Scholar]
Pellfolk 2010 {published data only}
- Pellfolk TE, Gustafson Y, Bucht G, Karlsson S. Effects of a restraint minimization program on staff knowledge, attitudes, and practice: a cluster randomized trial. Journal of the American Geriatrics Society 2010;58(1):62‐9. [DOI] [PubMed] [Google Scholar]
Snider 2012 {published data only}
- Snider KT, Snider EJ, Johnson JC, Hagan C, Schoenwald C. Preventative osteopathic manipulative treatment and the elderly nursing home resident: a pilot study. Journal of the American Osteopathic Association 2012;112(8):489‐501. [PubMed] [Google Scholar]
Tahir 2010 {published data only}
- Tahir TA, Eeles E, Karapareddy V, Muthuvelu P, Chapple S, Phillips B, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. Journal of Psychosomatic Research 2010;69(5):485‐90. [DOI] [PubMed] [Google Scholar]
Ushijima 2008 {published data only}
- Ushijima M, Yokoyama S, Sugiyama E, Amano N. Contribution of perospirone and risperidone to reduce delirium in senile patients. Psychogeriatrics 2008;8(1):4‐7. [Google Scholar]
Yoon 2011 {published data only}
- Yoon HK, Kim YK, Han C, Ko YH, Lee HJ, Kwon DY, et al. Paliperidone in the treatment of delirium: results of a prospective open‐label pilot trial. Acta Neuropsychiatrica 2011;23(4):179‐83. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Mestres Gonzalvo 2017 {published data only}
- Mestres Gonzalvo C, Wit H, Oijen B, Hurkens K, Janknegt R, Schols J, et al. Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study. Biomed Central Geriatrics 2017;17(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02994979 {published data only}
- NCT02994979. Nursing assistant intervention to prevent delirium in nursing homes. clinicaltrials.gov/ct2/show/NCT02994979 (first received 16 December 2016).
NCT03718156 {published data only}
- NCT03718156. The prevention program for Alzheimer's related delirium trial (PREPARED). clinicaltrials.gov/ct2/show/NCT03718156 (first received 24 October 2018).
Additional references
Alagiakrishnan 2004
- Alagiakrishnan K, Wiens CA. An approach to drug‐induced delirium in the elderly. Postgraduate Medical Journal 2004;80(945):388‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Vol. 5, Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Armstrong‐Esther 1996
- Armstrong‐Esther CA, Browne KD, Armstrong‐Esther DC, Sander L. The institutionalized elderly: dry to the bone. International Journal of Nursing Studies 1996;33(6):619‐28. [DOI] [PubMed] [Google Scholar]
Boockvar 2016
- Boockvar K, Teresi J, Inouye S. Preliminary data: an adapted hospital elder life program to prevent delirium and reduce complications of acute illness in long‐term care delivered by certified nursing assistants. Journal of the American Geriatrics Society 2016;64(5):1108‐13. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 2017
- Burton JK, Quinn TJ, Gordon AL, MacLullich AM, Reynish E, Shenkin SD. Identifying published studies of care home research: an international survey of researchers. Journal of Nursing Home Research Sciences 2017;3:99‐102. [Google Scholar]
Burton 2018
- Burton JK, Guthrie B. Identifying who lives in a care home – a challenge to be conquered. Age and Ageing 2018;47(3):322‐3. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ciampi 2017
- Ciampi A, Bai C, Dyachenko A, McCusker J, Cole MG, Belzile E. Longitudinal patterns of delirium severity scores in long‐term care settings. International Psychogeriatrics 2017;29(1):11‐7. [DOI] [PubMed] [Google Scholar]
Clark 1996
- Clark CM, Ewbank DC. Performance of the Dementia Severity Rating Scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Disease and Associated Disorders 1996;10(1):31‐9. [PubMed] [Google Scholar]
Clegg 2011
- Clegg A, Young J. Which medications to avoid in people at risk of delirium: a systematic review. Age and Ageing 2011;40(1):23‐9. [DOI] [PubMed] [Google Scholar]
Cole 2012
- Cole MG, McCusker J, Voyer P, Monette J, Champoux N, Ciampi A, et al. The course of delirium in older long‐term care residents. International Journal of Geriatric Psychiatry 2012;27(12):1291‐7. [DOI] [PubMed] [Google Scholar]
Dosa 2007
- Dosa D, Intrator O, McNicoll L, Cang Y, Teno J. Preliminary derivation of a Nursing Home Confusion Assessment Method based on data from the Minimum Data Set. Journal of the American Geriatrics Society 2007;55(7):1099‐105. [DOI] [PubMed] [Google Scholar]
Fong 2015
- Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface of delirium and dementia in older persons. Lancet Neurology 2015;14(8):823‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015.
Higgins 2011
- Higgins JP, Altman DG. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hshieh 2008
- Hshieh TT, Fong TJ, Marcantonio ER, Inouye SK. Cholinergic deficiency hypothesis in delirium: synthesis of current evidence. Journal of Gerontology: Medical Sciences 2008;63A(7):764‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941‐8. [DOI] [PubMed] [Google Scholar]
Inouye 1996
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. Journal of the American Medical Association 1996;275(11):852‐7. [PubMed] [Google Scholar]
Inouye 1998
- Inouye SK. Delirium in hospitalized older patients: recognition and risk factors. Journal of Geriatric Psychiatry and Neurology 1998;11(3):118‐25. [DOI] [PubMed] [Google Scholar]
Inouye 1999
- Inouye SK, Bogardus ST Jr, Charpentier PA, Leo‐Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine 1999;340(9):669‐76. [DOI] [PubMed] [Google Scholar]
Inouye 2006
- Inouye SK. Delirium in older persons. New England Journal of Medicine 2006;354:1157‐65. [DOI] [PubMed] [Google Scholar]
Inouye 2014
- Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalisvaart 2005
- Kalisvaart KJ, Jonghe JF, Bogaards MJ, Vreeswijk R, Egberts TC, Burger BJ, et al. Haloperidol prophylaxis for elderly hip‐surgery patients at risk for delirium: a randomized placebo‐controlled study. Journal of the American Geriatrics Society 2005;53(10):1658‐66. [DOI] [PubMed] [Google Scholar]
Kingston 2018
- Kingston A, Comas‐Herrera A, Jagger C, for the MODEM project. Forecasting the care needs of the older population in England over the next 20 years: estimates from the Population Ageing and Care Simulation (PACSim) modelling study. Lancet Public Health 2018;3(9):447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2018
- Lam HR, Chow S, Taylor K, Chow R, Lam H, Bonin K, et al. Challenges of conducting research in long‐term care facilities: a systematic review. Biomed Central Geriatrics 2018;18(242):934‐9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leslie 2008
- Leslie DL, Marcantonio ER, Zhang Y, Leo‐Summers L, Inouye SK. One year health‐care costs associated with delirium in the elderly population. Archives of Internal Medicine 2008;168(1):27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCusker 2011
- McCusker J, Cole MG, Voyer P, Monette J, Champoux N, Ciampi A, et al. Prevalence and incidence of delirium in long‐term care. International Journal of Geriatric Psychiatry 2011;26(11):1152‐61. [DOI] [PubMed] [Google Scholar]
McCusker 2013
- McCusker J, Cole MG, Voyer P, Vu M, Ciampi A, Monette J, et al. Environmental factors predict the severity of delirium symptoms in long‐term care residents with and without delirium. Journal of the American Geriatrics Society 2013;61(4):502‐11. [DOI] [PubMed] [Google Scholar]
Morichi 2018
- Morichi V, Fedecostante M, Morandi A, Santo SG, Mazzone A, Mossello E, et al. A point prevalence study of delirium in Italian nursing homes. Dementia and Geriatric Cognitive Disorders 2018;46(1‐2):27‐41. [DOI] [PubMed] [Google Scholar]
Morris 1993
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412‐4. [DOI] [PubMed] [Google Scholar]
Neelon 1996
- Neelon VJ, Champagne MT, Carlson JR, Funk SG. The NEECHAM Confusion Scale: construction, validation, and clinical testing. Nursing Research 1996;45(6):324‐30. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence (NICE). Delirium: diagnosis, prevention and management. NICE Clinical Guideline 103, 2010. www.nice.org.uk/nicemedia/live/13060/49909/49909.pdf (accessed 16 January 2014). [PubMed]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribbe 1997
- Ribbe MW, Ljunggren G, Steel K, Topinková E, Hawes C, Ikegami N, et al. Nursing homes in 10 nations: a comparison between countries and settings. Age and Ageing 1997;Suppl 2:3‐12. [DOI] [PubMed] [Google Scholar]
Rockwood 1999
- Rockwood K. Educational interventions in delirium. Dementia and Geriatric Cognitive Disorders 1999;10(5):426‐9. [DOI] [PubMed] [Google Scholar]
Sanford 2015
- Sanford AM, Orrell M, Tolson D, Abbatecola AM, Arai H, Bauer JM, et al. An international definition for "nursing home". Journal of the American Medical Directors Association 2015;16(3):181‐4. [DOI] [PubMed] [Google Scholar]
Siddiqi 2006
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in‐patients: a systematic literature review. Age and Ageing 2006;35(4):350‐64. [DOI] [PubMed] [Google Scholar]
Siddiqi 2009
- Siddiqi N, Clegg A, Young J. Delirium in care homes. Reviews in Clinical Gerontology 2009;19:309‐16. [Google Scholar]
Siddiqi 2011
- Siddiqi N, Young J, House AO, Featherstone I, Hopton A, Martin C, et al. Stop delirium! A complex intervention to prevent delirium in care homes: a mixed methods feasibility study. Age and Ageing 2011;40(1):90‐8. [DOI] [PubMed] [Google Scholar]
Tabet 2009
- Tabet N, Howard R. Pharmacological treatment for the prevention of delirium: review of current evidence. International Journal of Geriatric Psychiatry 2009;24(10):1037‐44. [DOI] [PubMed] [Google Scholar]
Trzepacz 1988
- Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Research 1988;23(1):89‐97. [DOI] [PubMed] [Google Scholar]
Voyer 2010
- Voyer P, Richard S, Doucet L, Cyr N, Carmichael PH. Examination of the multifactorial model of delirium among long‐term care residents with dementia. Geriatric Nursing 2010;31(2):105‐14. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Vol. 10, Geneva: World Health Organization, 1992. [Google Scholar]
Witlox 2010
- Witlox J, Eurelings LS, Jonghe JF, Kalisvaart KJ, Gool WA. Delirium in elderly patients and the risk of post‐discharge mortality, institutionalization, and dementia. Journal of the American Medical Association 2010;304(4):443‐51. [DOI] [PubMed] [Google Scholar]
Yang 2009
- Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009;50(3):248‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clegg 2012
- Clegg A, Siddiqi N, Holt R, Young J. Interventions for preventing delirium in older people in institutional long‐term care (protocol). Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009537] [DOI] [PubMed] [Google Scholar]
Clegg 2014
- Clegg A, Siddiqi N, Heaven, A, Young J, Holt, R. Interventions for preventing delirium in older people in institutional long‐term care. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009537.pub2] [DOI] [PubMed] [Google Scholar]


