Abstract
Background
Post‐dural puncture headache (PDPH) is one of the most common complications of diagnostic and therapeutic lumbar punctures. PDPH is defined as any headache occurring after a lumbar puncture that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of the patient lying down. Researchers have suggested many types of interventions to help prevent PDPH. It has been suggested that aspects such as needle tip and gauge can be modified to decrease the incidence of PDPH.
Objectives
To assess the effects of needle tip design (traumatic versus atraumatic) and diameter (gauge) on the prevention of PDPH in participants who have undergone dural puncture for diagnostic or therapeutic causes.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL and LILACS, as well as trial registries via the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal in September 2016. We adopted the MEDLINE strategy for searching the other databases. The search terms we used were a combination of thesaurus‐based and free‐text terms for both interventions (lumbar puncture in neurological, anaesthesia or myelography settings) and headache.
Selection criteria
We included randomized controlled trials (RCTs) conducted in any clinical/research setting where dural puncture had been used in participants of all ages and both genders, which compared different tip designs or diameters for prevention of PDPH
Data collection and analysis
We used the standard methodological procedures expected by Cochrane.
Main results
We included 70 studies in the review; 66 studies with 17,067 participants were included in the quantitative analysis. An additional 18 studies are awaiting classification and 12 are ongoing. Fifteen of the 18 studies awaiting classification mainly correspond to congress summaries published before 2010, in which the available information does not allow the complete evaluation of all their risks of bias and characteristics. Our main outcome was prevention of PDPH, but we also assessed the onset of severe PDPH, headache in general and adverse events. The quality of evidence was moderate for most of the outcomes mainly due to risk of bias issues. For the analysis, we undertook three main comparisons: 1) traumatic needles versus atraumatic needles; 2) larger gauge traumatic needles versus smaller gauge traumatic needles; and 3) larger gauge atraumatic needles versus smaller gauge atraumatic needles. For each main comparison, if data were available, we performed a subgroup analysis evaluating lumbar puncture indication, age and posture.
For the first comparison, the use of traumatic needles showed a higher risk of onset of PDPH compared to atraumatic needles (36 studies, 9378 participants, risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67, I2 = 9%).
In the second comparison of traumatic needles, studies comparing various sizes of large and small gauges showed no significant difference in effects in terms of risk of PDPH, with the exception of one study comparing 26 and 27 gauge needles (one study, 658 participants, RR 6.47, 95% CI 2.55 to 16.43).
In the third comparison of atraumatic needles, studies comparing various sizes of large and small gauges showed no significant difference in effects in terms of risk of PDPH.
We observed no significant difference in the risk of paraesthesia, backache, severe PDPH and any headache between traumatic and atraumatic needles. Sensitivity analyses of PDPH results between traumatic and atraumatic needles omitting high risk of bias studies showed similar results regarding the benefit of atraumatic needles in the prevention of PDPH (three studies, RR 2.78, 95% CI 1.26 to 6.15; I2 = 51%).
Authors' conclusions
There is moderate‐quality evidence that atraumatic needles reduce the risk of post‐dural puncture headache (PDPH) without increasing adverse events such as paraesthesia or backache. The studies did not report very clearly on aspects related to randomization, such as random sequence generation and allocation concealment, making it difficult to interpret the risk of bias in the included studies. The moderate quality of the evidence for traumatic versus atraumatic needles suggests that further research is likely to have an important impact on our confidence in the estimate of effect.
Plain language summary
Needle characteristics that reduce the occurrence of post‐dural puncture headache (PDPH)
Background
A lumbar puncture is a needle inserted into the lower part of the spine to draw fluid, to test for conditions affecting the brain and spinal cord. It can also be used for treatment (for instance, for the management of pain in caesarean section).
In general, lumbar punctures are considered safe; however, a number of adverse effects such as backache, tickling sensations (paraesthesia) or even post‐dural puncture headache (PDPH) have been reported. These conditions are not life‐threatening, but can impair the person's physical activity and can be very painful. Several different needle tips (classified as traumatic or atraumatic) and gauges (size/diameter) are used to perform a lumbar puncture. We compared different types of needles to assess the effects of the needle tip and its thickness on the prevention of post‐dural puncture headache.
Study characteristics
We searched the medical literature for studies carried out in any setting comparing needles of different characteristics (i.e. different tip designs and sizes) for the prevention of PDPH. The evidence is current to September 2016. We included 70 studies and were able to include information from 66 of those studies (17,067 participants) in the numerical analysis. An additional 18 studies are awaiting classification and 12 are ongoing.
Key findings
We found that the use of needles with a traumatic tip resulted in a higher risk of PDPH when compared to needles with atraumatic tips. When we compared the different studies comparing various sizes of large and small traumatic gauges, we did not find any difference in effects in terms of the risk of PDPH. Finally, when we compared atraumatic needles with a higher gauge to those with a smaller gauge, we observed no significant differences in terms of the development of PDPH in any of the scenarios analysed. We also found no significant differences in the use of traumatic versus atraumatic needles in the development of adverse effects such as paraesthesia, backache and severe PDPH.
Quality of the evidence
The studies did not report clearly on aspects of their design related to randomization. (This is a method that uses the play of chance to assign participants to comparison groups in a trial). This made it difficult for us to interpret the risk of bias in the included studies. We therefore considered the quality of the evidence for most of the outcomes assessed in this review to be moderate.
Summary of findings
Background
Description of the condition
Post‐dural (post‐lumbar or post‐spinal) puncture headache (PDPH) is one of the most common complications of diagnostic, therapeutic or inadvertent lumbar punctures (Bezov 2010; Davignon 2002; Raskin 1990; Sadashivaiah 2009). PDPH is defined as any headache after a lumbar puncture that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of the patient lying down (González‐Martínez 2005; Headache Classification Subcommittee IHS 2004). Most PDPHs occur within three days of the procedure and more than 50% start within the first 48 hours (Turnbull 2003).
The pathophysiology of PDPH has not been fully established. It is well known that puncture of the dura allows cerebrospinal fluid (CSF) to leak from the subarachnoid space, which results in decreased CSF volume and pressure (Grande 2005). This CSF volume loss may cause a downward pull on pain‐sensitive structures, which could explain the occurrence of PDPH (Ahmed 2006; Baumgarten 1987; Davignon 2002; Denny 1987; Harrington 2004). In addition, loss of CSF may cause an increase in blood flow, leading to arterial and venous vasodilatation, which could result in PDPH. A third PDPH mechanism may involve the role of substance P (neurotransmitter/neuromodulator involved in pain perception) and the regulation of neurokinin 1 receptors (NK1Rs) (Clark 1996). Defects in manufactured needles have also been described as a possible source of PDPH (Parker 1997). Laboratory studies have shown significant alteration of the tips of traumatic needles when their introducer needle protrudes through the inner hole of the needle. These altered tips can produce holes in the dura mater of increased diameter, which may require longer healing times and consequently increase the time allowed for leakage of CSF (Bezov 2010; Calthorpe 2004; Parker 1997).
Studies about the incidence of PDPH have reported a wide range of estimates, depending on target populations, types of needles and lumbar puncture techniques (Alstadhaug 2012; Arendt 2009; Lavi 2006; Shaikh 2008; Vallejo 2000). For example, during anaesthetic procedures such as epidural anaesthesia, PDPH is most commonly caused by an unintentional dural puncture (Thew 2008; Turnbull 2003). However, in diagnostic or therapeutic lumbar punctures, the need for adequate CSF flow requires an intentional lesion that may trigger the PDPH phenomenon (Kuczkowski 2006). Estimated frequencies of this event vary from less than 10% after spinal anaesthesia (Vallejo 2000) to 36% after diagnostic lumbar puncture (Lavi 2006; Vallejo 2000), and up to 81% in obstetric patients with inadvertent dural puncture during active labour (Berger 1998; Choi 2003).
The characteristics of PDPH are often variable. It may be accompanied by neck stiffness, tinnitus, hearing loss, photophobia and nausea, among other symptoms. Other characteristics such as the location and duration of the headache are also unpredictable (Grande 2005). Although PDPH is not a life‐threatening condition, physical activity is often restricted. Patients are usually required to stay in bed for the entire day, and length of hospital stay and use of medical services are increased (Angle 2005). The variability in symptom profiles makes PDPH a diagnosis of exclusion. Alternative diagnoses (e.g. viral meningitis, sinus headache, intracranial haemorrhage) should be ruled out first (Turnbull 2003).
Once PDPH is diagnosed, initial treatment involves conservative measures such as bed rest and analgesics. If PDPH continues for longer than 72 hours, more specific treatment is indicated (Ahmed 2006). Severe PDPH may respond to some therapeutic drugs and to an epidural blood patch (Boonmak 2010; Lavi 2006).
Description of the intervention
Many interventions have been suggested for the prevention of PDPH (e.g. body postures and fluid intake after lumbar puncture). One of the most relevant strategies involves the features of the needles (Arendt 2009). Although the choice of the needle depends mostly on the purpose of the lumbar puncture, several experts have remarked that facets such as the tip and the gauge could be modified to decrease the incidence of PDPH (American Society of Anesthesiologists 2007; Armon 2005).
According to tip design, needles can be divided into traumatic and atraumatic types. Atraumatic needles include Whitacre, Atraucan, Sprotte, Cappe and Deutsch, among others. Traumatic needles include Quincke, Greene, Hingson Ferguson, Lutz, Brace and Rovenstine, among others. Traumatic needles are characterized by a bevelled tip that cuts the dura mater. In contrast, atraumatic needles are characterized by a pencil‐point design. It has been stated that noncutting or atraumatic needles produce a separation of the tissue fibres that heals easily after removal of the needle. Cutting or traumatic needles, on the other hand, favour loss of tissue and trigger a large inflammatory reaction that requires a long time to heal (Calthorpe 2004; Lynch 1992; Wu 1991).
The external diameter of the needle is another factor that may be involved in the mechanisms of PDPH. The external diameter is determined by the cross‐sectional area of the needle; larger diameters are expected to produce larger orifices in the dura mater, thereby allowing increased CSF leakage. Larger gauges are represented by smaller numbers (e.g. 16 gauge, 17 gauge), and smaller gauges are represented by larger numbers (e.g. 29 gauge, 32 gauge) (Calthorpe 2004).
How the intervention might work
Studies that have compared needle internal diameters have found that needles of larger diameter produce larger holes in the dura mater and this could lead to a greater risk of post‐dural puncture headache (Bezov 2010; Lavi 2006; Shaikh 2008; Santanen 2004). However, evidence also suggests that the use of thinner needles increases the difficulty of the procedure and hence the number of bone punctures, causing needle tip deformities (Angle 2003). Some authors advocate the use of needles with cutting/traumatic tips based on the theory that these needles can cause larger lesions than are produced by pencil‐point/atraumatic needles (Calthorpe 2004; Lynch 1992a; Srivastava 2010a). Pencil‐point needles were thought to penetrate and then separate dura mater fibres, resulting in less trauma and subsequently less loss of CSF and a lower incidence of PDPH (Arendt 2009). A large inflammatory reaction caused by larger lesions can lead to faster closing of the injury through rapid migration of the cells involved in scar formation. Microscopic analyses of corpses have revealed that injuries produced by pencil‐point needles are more complex than those produced by cutting needles (Arendt 2009).
Why it is important to do this review
Lumbar puncture is part of everyday clinical practice and is associated with potential adverse effects (Evans 2009; Grande 2005). Prevention strategies should be preferred over treatment of adverse effects (Turnbull 2003). Morbidities associated with CSF loss, besides PDPH, include peripartum seizures, cranial subdural haematomas and subdural fluid collections (Arendt 2009; Janssens 2003). Even though most cases of PDPH are resolved within a few days, a significant number of patients experience at least one week of disability, and others require prolonged or recurrent hospitalizations (van Kooten 2008). Prevention strategies, such as the use of a prophylactic epidural blood patch, caffeine or different postures after lumbar puncture, have not proved effective for the prevention of PDPH in several Cochrane Reviews (Arevalo‐Rodriguez 2013; Basurto 2013; Boonmak 2010).
Objectives
To assess the effects of needle tip design (traumatic versus atraumatic) and diameter (gauge) on the prevention of post‐dural puncture headache (PDPH) in participants who have undergone dural puncture for diagnostic or therapeutic causes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) conducted in any clinical/research setting where dural puncture has been used.
Types of participants
We included participants of all ages and both genders who have undergone lumbar puncture for medical reasons.
Types of interventions
We included studies in participants undergoing lumbar puncture that assessed one of the following interventions.
A needle tip design/bevel used for lumbar puncture (i.e. traumatic or atraumatic) versus another needle tip design/bevel.
A specified needle gauge (i.e. from 16 gauge to 32 gauge) versus another needle gauge for the same type of tip design (i.e. traumatic or atraumatic).
Any combination of the above.
Types of outcome measures
Primary outcomes
Onset of PDPH, defined as each headache that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of lying down after a lumbar puncture. We used the valid PDPH diagnostic criteria specified by the International Headache Society (Headache Classification Subcommittee IHS 2004).
Adverse events related to lumbar puncture: total adverse events and total serious adverse events. We defined an adverse event as "any untoward medical occurrence that may present during treatment with a pharmaceutical product but that does not necessarily have a causal relationship with this treatment". Due to heterogeneity in the report of adverse events, we choose paraesthesia and backache as the most important adverse events, additional to PDPH, related to needle gauge and tip. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and it is explained in the Differences between protocol and review section.
Secondary outcomes
Severe PDPH, according to the definition used in each study, which could be based on specific features (e.g. duration of PDPH), a visual analogue scale (VAS) or other criteria such as the need for specialized treatments to manage the episode of headache (e.g. epidural blood patch).
Any headache subsequent to a lumbar puncture, to incorporate any possible data that had not been catalogued as PDPH, according to the definition used in each study.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 9) (see Appendix 2 for details of the search strategy), PubMed, MEDLINE (1966 to September 2016, see Appendix 3), EMBASE via Ovid SP (1982 to September 2016, see Appendix 4), CINAHL (EBSCOhost, 1982 to September 2016, see Appendix 5) and LILACS (1982 to September 2016 see Appendix 6).
We adopted the MEDLINE search strategy in searching the other databases. The search terms are a combination of thesaurus‐based and free‐text terms for both the intervention (lumbar puncture in neurological, anaesthesia or myelography settings) and the headache. We did not impose any language restriction.
Searching other resources
We searched trial registries via the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal up to September 2016. In addition, we searched the reference lists from retrieved studies, information from clinical trial registration websites and conference proceedings.
Data collection and analysis
Selection of studies
Two review authors (JJA and LM) independently selected studies for eligibility using Early Review Organizing Software (EROS) (Ciapponi 2011; Ciapponi 2011a; Glujovsky 2010). We reviewed the titles and abstracts of all identified studies to determine whether they fulfilled the inclusion criteria. We assessed the full texts of selected studies to confirm their relevance for inclusion. We resolved any disagreement by consulting with a third review author (AC). We were not blinded to the authors' names and institutions, the journal of publication or the study results at any stage of the review.
Data extraction and management
Three review authors (NG‐C, SB and LM) independently used pre‐designed data forms to extract information from the original study reports about participants, methods of randomization, blinding, comparisons of interest, numbers of participants originally randomly assigned by arm, follow‐up losses and outcomes (double data entry) (Appendix 7). We recorded the reasons for exclusion of potential studies in the Characteristics of excluded studies table. We resolved any disagreement by discussion with a fourth review author (IA‐R). We entered the extracted data into Review Manager 5 for the analyses (RevMan 5.3).
Assessment of risk of bias in included studies
Two review authors (NG‐C and IA‐R) independently assessed the risk of bias of included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered seven domains (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias). We did not consider blinding of personnel because of the nature of the intervention (lumbar puncture). We resolved any disagreements by discussion with a third review author (MRF).
Measures of treatment effect
We presented results as summary risk ratios (RRs) for incidence of PDPH, adverse events, severe PDPH and any headache along with 95% confidence intervals (CIs). We calculated the number needed to treat for an additional beneficial outcome (NNTB) as the reciprocal of risk differences (RDs) (McQuay 1998).
Unit of analysis issues
We did not expect to encounter any unit of analysis issues, as we did not expect to find cross‐over studies or cluster‐randomized trials. However, we identified four such studies with our search strategies and excluded them from quantitative analysis. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
Dealing with missing data
For all outcomes we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis (i.e. we attempted to include in the analyses all randomized patients in the denominator of the assessed groups).
Assessment of heterogeneity
We assessed statistical heterogeneity of effect sizes by means of the I2 statistic. The I2 statistic describes the percentage of total variation across trials that is due to heterogeneity rather than to sampling error (Higgins 2003; Higgins 2011). If we identified at least moderate heterogeneity (i.e. I2 > 30%), we explored it by performing prespecified subgroup analyses. If we identified substantial heterogeneity (I2 > 80%), we did not present the pooled result.
Assessment of reporting biases
We assessed reporting bias through careful attention to assessment of quality, particularly the quality of study methodology. We also used funnel plot analysis to assess publication bias.
Data synthesis
We summarized the findings using random‐effects models with the DerSimonian‐Laird method. We carried out statistical analyses using Review Manager 5 (RevMan 5.3).
Subgroup analysis and investigation of heterogeneity
For the primary outcomes, we considered subgroup analyses for the following factors, as appropriate.
Participants undergoing dural puncture for anaesthesia only, diagnosis only or myelography only.
Pregnant women only.
Gender: it has been reported that women are at twice the risk of men (Alstadhaug 2012; Bezov 2010; Evans 2009).
Age (younger than 18 years of age, older than 65 years of age and 18 to 65 years of age). Due to heterogeneity in the reporting of age, we classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section
Posture during the lumbar puncture (e.g. lateral, sitting).
Type of surgery: in participants receiving anaesthesia, we analysed the primary outcome by type of surgical procedure if data were available. As we mentioned in the Background, some patients such as obstetric women have an increased risk of PDPH. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
Sensitivity analysis
We performed a sensitivity analysis to compare the results from using only those RCTs classified as having a 'low risk of bias' in three core domains: allocation concealment, incomplete outcome data and blinding of outcome assessment (Higgins 2011). In addition, we performed a sensitivity analysis to measure the risk difference (RD) in those analysis that presented zero events in both treatment arms. This is a difference from our protocol (Arevalo‐Rodriguez 2013a) and is explained in the Differences between protocol and review section.
'Summary of findings' tables
We used the principles of the GRADE system (Guyatt 2008) to assess the quality of the body of evidence associated with all outcomes (onset of PDPH and adverse events), and we constructed a 'Summary of findings' table using the GRADE profiler software. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the quality of a body of evidence considers within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias (Balshem 2011; Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g). For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations. We included the following outcomes in the 'Summary of findings' tables: onset of PDPH, adverse events (i.e. paraesthesia, backache), severe PDPH and any headache.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We searched the databases in February 2015, identifying a total of 1201 references. We found four additional references using other research strategies. After reviewing the references by title and abstract, we selected 138 of them to review as full texts (see Figure 1). After reading the articles, we included 70 studies (distributed in 75 references). We excluded 35 studies. We classified 12 as ongoing studies and 15 as studies awaiting assessment. We reran the search in September 2016, identifying a total of 96 new references. We selected a further three studies for in‐depth review (Castrillo 2015; Fama 2015; Hong 2015). We added these three potential new studies of interest to a list of ‘Characteristics of studies awaiting classification' and we will incorporate them into the formal review findings during the review update.
1.
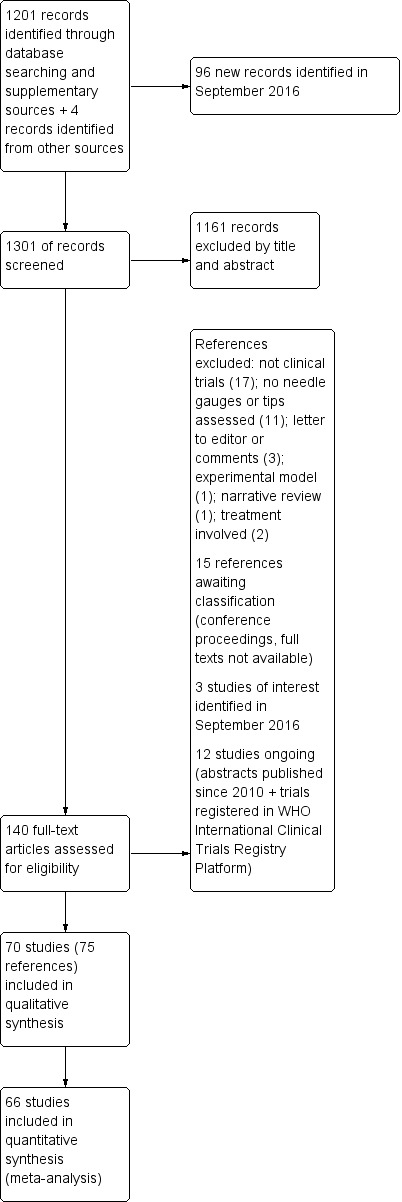
Study flow diagram
Included studies
We included 70 studies in the qualitative synthesis of the review, accounting for 75 references (see Figure 1 and Characteristics of included studies). However, we excluded four of these from the quantitative data analysis as their results were obtained using a different unit of analysis to the one planned for this review (procedures instead of participants: four studies). One of these studies was a study with a cross‐over design (Crock 2014), and it included children receiving treatment for leukaemia. The remaining three were parallel‐group trials that included all the lumbar punctures undertaken on the participants during the lifespan of the study (Hafer 1997; Kokki 1999; Lavi 2006); however, in some cases it was not clear if the procedures or the participants were randomized (Kokki 1999; Lavi 2006).
The quantitative analysis included 66 studies with a total of 17,067 participants (mean 258.6 participants; standard deviation (SD) 236.7; interquartile range (IQR) 100 to 311), published between 1972 and 2013. The sample sizes of the studies included ranged from 40 to 1522 participants (see Characteristics of included studies).
We classified the studies according to the needle tip design used, as follows: traumatic needles = Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon; atraumatic needles = Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor. Thirty‐nine studies (10,715 participants) compared traumatic needles versus atraumatic needles. Eleven studies compared traumatic needles of different gauges (2896 participants) and 15 studies compared atraumatic needles of different gauges (4095 participants). Four studies provided information for two different comparisons (Kokki 1998; Shah 2010; Shaikh 2008; Shutt 1992). The type of needle tip used could not be determined in seven of the studies (Geurts 1990; Harrison 1993; McGann 1992; Rasmussen 1989a; Rasmussen 1989b; Tourtellotte 1972; Wilkinson 1991). In one case, a hybrid point needle (a combination of diamond and pencil points) was compared to an atraumatic needle (Standl 2004). Two references provided information on two studies in the same publication and we analysed these as two independent groups of data (Rasmussen 1989a; Rasmussen 1989b; Srivastava 2010a; Srivastava 2010b).
Most of the studies included both genders, however 20 only included women and one only included men (Saenghirunvattana 2008). Similarly, most of the studies included patients in all age ranges; three only included under 18 year‐olds (Kokki 1996; Kokki 1998; Kokki 2000), and one study only included over 60 year‐olds (Kim 2011). The 25 gauge needle was the most frequently assessed (414 groups), followed by the 22 gauge (20 groups). In one study, it was not possible to identify the gauge of the needle used or its brand (Kokki 2000). A Quincke needle was used in 57 groups, followed by Whitacre needles (31 groups) and Sprotte (21 groups).
Among the indications for lumbar puncture, 57 studies undertook this procedure to administer anaesthesia. The most common reasons for the administration of anaesthesia were caesarean section (15 studies), followed by orthopaedic interventions (eight studies). The remaining studies combined different types of subumbilical surgery such as urologic surgery, outpatient surgery and tubal ligation among others. Five studies used lumbar puncture as a diagnosis method, including for the detection of infections, while a further seven studies used lumbar puncture for myelography. The most common site for puncture was between lumbar vertebrae (L) 2‐3 and 3‐4 (12 studies), followed by L3 to 4 (nine studies). Nineteen studies did not report puncture site and 25 studies reported that the puncture was undertaken by trained and experienced professionals, whereas 35 studies did not provide such information. The most common body position during the procedure was a lateral position (23 studies) and a seated position (21 studies).
Excluded studies
We excluded a total of 35 studies from the review as most of them were not clinical trials. In 11 cases, the studies were not designed to evaluate needles, their gauge or tip for the prevention of PDPH. Readers can find more information in the Characteristics of excluded studies table.
Studies awaiting classification
In total we classified 18 studies as awaiting classification. We found 15 of these during the February 2015 search (Bano 2004; Buttner 1990; De Andres 1994; Fyneface‐Ogan 2006; Harrison 1994; Jager 1995; Jensen 1999; Kaul 1996; Knudsen 1998; Lim 1992; Maclean 1994; Mignonsin 1991; Palmieri 1993; Puolakka 1997; Vandana 2004). These 15 studies mainly correspond to congress summaries published before 2010, in which the available information does not allow the complete evaluation of all their risks of bias and other characteristics. Also, the fact that they were written so long ago makes the likelihood of them being published as complete articles very low. We also classified articles that could not be obtained as full texts from the authors, the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group and the Iberoamerican Cochrane Centre as awaiting assessment.
We reran the search in September 2016 and selected a further three studies for in‐depth review (Castrillo 2015; Fama 2015; Hong 2015).
Ongoing studies
We classified 12 studies as ongoing (Ahmed 2012; Akdemir 2011; Bertolotto 2014; Bertolotto 2014a; Bham 2010; IRCT201009292080N4; Lorthe 2014; NCT00370604; NCT01821807; NCT02384031; Shah 2011; Shaikh 2013), given that we were only able to find summaries of their results. However, we considered that they could be subject to publication in a short time given the year of reference (after 2010). See Characteristics of ongoing studies.
Risk of bias in included studies
We assessed the risk of bias of the studies in seven categories. We provide a summary of our assessment of the risk of bias of the included studies in Figure 2 and Figure 3.
2.
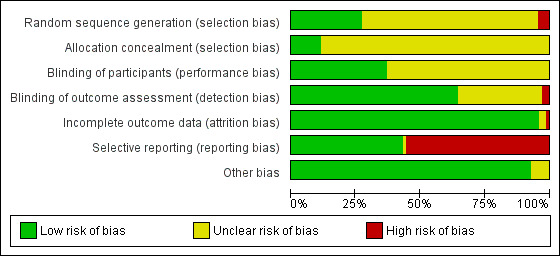
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
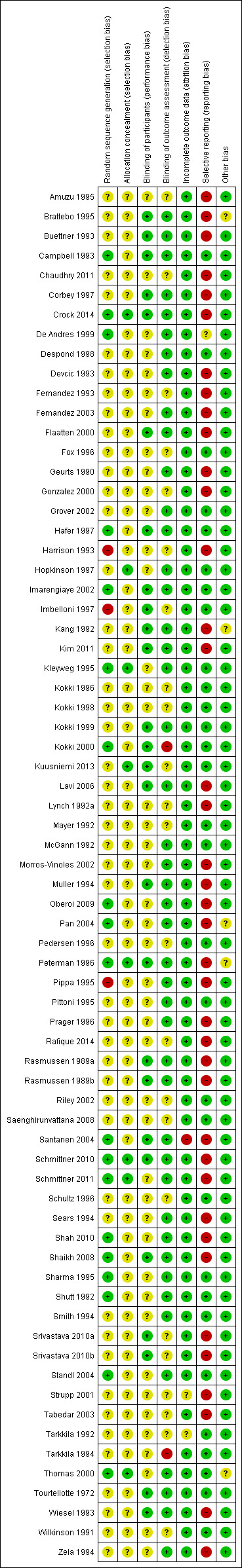
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 19 studies, the authors reported a valid method of randomization (Campbell 1993; Crock 2014; De Andres 1999; Hafer 1997; Imarengiaye 2002; Kleyweg 1995; Kokki 2000; Oberoi 2009; Pan 2004; Peterman 1996; Santanen 2004; Schmittner 2010; Schmittner 2011; Shah 2010; Shaikh 2008; Sharma 1995; Shutt 1992; Standl 2004; Thomas 2000), whereas this information was not clearly reported in the remaining 48 studies. As mentioned above, in three studies the authors reported an invalid method of randomization (Harrison 1993; Imbelloni 1997; Pippa 1995), and we rated them as at high risk of selection bias.
Eight studies undertook and reported adequate random allocation concealment (Crock 2014; Hopkinson 1997; Kleyweg 1995; Kuusniemi 2013; Peterman 1996; Schmittner 2010; Schmittner 2011; Thomas 2000), whereas this information was absent in the rest of the included studies.
Blinding
Twenty‐six studies reported blinding of participants (Brattebo 1995; Buettner 1993; Campbell 1993; Corbey 1997; Crock 2014; Flaatten 2000; Hafer 1997; Imarengiaye 2002; Imbelloni 1997; Kang 1992; Kim 2011; Kokki 1999; Kokki 2000; Kuusniemi 2013; Lavi 2006; Muller 1994; Peterman 1996; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Tourtellotte 1972; Wiesel 1993), and we assessed them as at low risk of bias. However, the remaining 44 studies did not report this information clearly. Two studies reported an open assessment process to the researchers and assessors, and we considered them to have a high risk of bias for blinding of outcome assessment (Kokki 2000; Tarkkila 1994). Twenty‐one studies did not provide enough information to assess the blinding of outcome assessment, and in the remaining 47 studies we classified the risk of bias as low. In 21 studies we classified the risk of bias as low for both blinding of participants and blinding of outcome assessment (Brattebo 1995; Buettner 1993; Campbell 1993; Corbey 1997; Crock 2014; Flaatten 2000; Hafer 1997; Imarengiaye 2002; Kang 1992; Kim 2011; Kokki 1999; Lavi 2006; Muller 1994; Peterman 1996; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Shaikh 2008; Tourtellotte 1972; Wiesel 1993).
Incomplete outcome data
Significant numbers of patients were lost or excluded from the final analysis of one study (Santanen 2004), and two further studies presented unclear data (Strupp 2001; Tarkkila 1992). In the studies with minimal attrition bias, we often found that the data analyses were undertaken by protocol and we took this into account for data gathering, including all the randomized patients in the denominators of the assessed groups.
Selective reporting
A full report of adverse events associated with the different types of needle is fundamental for the complete assessment of their usefulness in the assessed clinical scenarios. We found that 39 studies did not report other adverse events associated with the needles (such as paraesthesia and backache) (Amuzu 1995; Brattebo 1995; Buettner 1993; Chaudhry 2011; Corbey 1997; Crock 2014; Devcic 1993; Fernandez 1993; Fernandez 2003; Flaatten 2000; Geurts 1990; Gonzalez 2000; Harrison 1993; Kang 1992; Kim 2011; Lavi 2006; Lynch 1992a; Morros‐Vinoles 2002; Muller 1994; Oberoi 2009; Pan 2004; Peterman 1996; Pippa 1995; Prager 1996; Rafique 2014; Rasmussen 1989a; Rasmussen 1989b; Santanen 2004; Schmittner 2010; Schmittner 2011; Sears 1994; Shah 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003; Wiesel 1993; Zela 1994), whereas the remaining studies reported at least one additional adverse event to PDPH.
Other potential sources of bias
We found other sources of bias in five studies, mainly related to the unclear role of the sponsors in the development of the research (Brattebo 1995; Kang 1992; Pan 2004; Schmittner 2010; Thomas 2000). We identified no additional sources of bias in the remaining studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Traumatic needles compared to atraumatic needles for prevention of post‐dural puncture headache (PDPH).
| Traumatic needles compared to atraumatic needles for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures Settings: all settings (countries: Argentina, Austria, Brazil, Canada, Denmark, Finland, France, Germany, India, Israel, Italy, Korea, Mexico, Nepal, Netherlands, Nigeria, Norway, Pakistan, Spain, Thailand, UK and USA) Intervention: traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) Comparison: atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needles | Traumatic needles | |||||
| Onset of PDPH | 30 per 1000 | 64 per 1000 (52 to 80) | RR 2.14 (1.72 to 2.67) | 9378 (36 studies) | ⊕⊕⊕⊝ moderate1 | — |
| Adverse events: paraesthesia | 52 per 1000 | 50 per 1000 (25 to 102) | RR 0.96 (0.47 to 1.96) | 573 (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
| Adverse events: backache | 155 per 1000 | 147 per 1000 (118 to 183) | RR 0.94 (0.78 to 1.13) | 3027 (12 studies) | ⊕⊕⊕⊝ moderate1 | — |
| Severe PDPH | 0 per 1000 | 10 per 1000 | RD 0 (0.00 to 0.01) | 6420 (24 studies) | ⊕⊕⊝⊝ low1,2 | — |
| Any headache | 221 per 1000 | 290 per 1000 (228 to 367) | RR 1.35 (1.17 to 1.57) | 4104 (18 studies) | ⊕⊕⊕⊝ moderate1 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PDPH: post‐dural puncture headache; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Inconsistency downgraded by one level due to presence of considerable heterogeneity (I2 = 42%), caused by one study focused on diagnostic lumbar punctures (Muller 1994).
Summary of findings 2. Larger traumatic needles compared to smaller traumatic needles for prevention of post‐dural puncture headache (PDPH).
| Traumatic needle(major gauge) compared to traumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with traumatic needles (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) Settings: all settings (countries: Finland, Germany, India, Italy, Korea, Pakistan and USA) Intervention: traumatic needle ‐ larger gauge (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) Comparison: traumatic needle ‐ smaller gauge (Quincke, Greene, Hingson Ferguson, Lutz, Brace, Rovenstine, Lemmon) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Traumatic needle ‐ smaller gauge | Traumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — |
RR ranged from 0.86 to 6.47 |
2288 (10 studies) | ⊕⊕⊝⊝ low1,3 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Adverse events: paraesthesia ‐ not reported | See comment | See comment | Not estimable | — | See comment | We did not identify any studies reporting this outcome. |
| Adverse event: backache | — | — | RR ranged from 0.81 to 2.00 | 948 (3 studies) | ⊕⊕⊕⊝ moderate1 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Severe PDPH | — | — | RD ranged from 0.00 to 0.00 | 1128 (6 studies) | ⊕⊕⊝⊝ low1,2 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| Any headache | — | — | RR ranged from 0.75 to 1.56 | 771 (3 studies) | ⊕⊕⊕⊝ moderate1 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in another. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PDPH: post‐dural puncture headache; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues).
2Imprecision downgraded by one level due to few events reported in each arm.
3Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit.
Summary of findings 3. Larger atraumatic needles compared to smaller atraumatic needles for prevention of post‐dural puncture headache (PDPH).
| Atraumatic needle (major gauge) compared to atraumatic needle (minor gauge) for prevention of PDPH | ||||||
| Patient or population: patients undergoing lumbar punctures with atraumatic needles (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) Settings: all settings (countries: Canada, France, India, Italy, Spain, UK and USA) Intervention: atraumatic needle ‐ larger gauge (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) Comparison: atraumatic needle ‐ smaller gauge (Whitacre, Atraucan, Sprotte, Cappe‐Deutsh, Pajunk, Gertie Marx, Durasafe, Cappe, Deutsch and Eldor) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Atraumatic needle ‐ smaller gauge | Atraumatic needle ‐ larger gauge | |||||
| Onset of PDPH | — | — | RR ranged from 0.38 to 9.3 | 3134 (13 studies) | ⊕⊕⊝⊝ low1,2 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: paraesthesia | — | — | RR ranged from 1.03 to 7.61 | 439 (2 studies) | ⊕⊕⊕⊝ moderate1 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Adverse events: backache | — | — | RR ranged from 0.95 to 5.00 | 526 (4 studies) | ⊕⊕⊕⊝ moderate1 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Severe PDPH | — | — | RD ranged from 0 to 0.01 | 1983 (8 studies) | ⊕⊕⊝⊝ low1,2 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| Any headache | — | — | RR ranged from 1.13 to 2.17 | 1791 (7 studies) | ⊕⊕⊕⊝ moderate1 | We decided against overall pooling of results because the gauge of a needle could be considered small in one comparison but large in other. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PDPH: post‐dural puncture headache; RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Risk of bias downgraded by one level due to unclear reporting (especially related to allocation concealment and random sequence generation issues). 2Imprecision downgraded by one level due unclear clinical decisions indicated by each confidence interval limit.
See: Table 1; Table 2; Table 3.
Comparison between traumatic and atraumatic needles
Primary outcome: Onset of post‐dural puncture headache (PDPH)
This comparison included information from 36 studies with a total of 9378 participants and 448 events (incidence of PDPH = 4.77%). The traumatic needles showed a greater risk of PDPH compared with the atraumatic ones (risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67), with low heterogeneity among the studies (I2 = 9%) (Analysis 1.1; Figure 4). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 1).
1.1. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 1 PDPH by indication.
4.
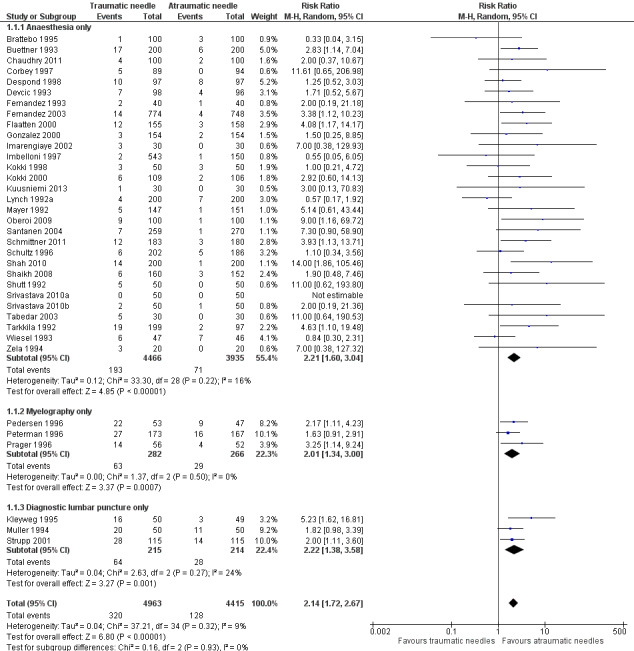
Forest plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
In the subgroup analysis of needle gauge size, 20 studies (6213 participants) compared 22, 25 or 27 gauge traumatic and atraumatic needles (Buettner 1993; Chaudhry 2011; Corbey 1997; Despond 1998; Fernandez 1993; Flaatten 2000; Kleyweg 1995; Kuusniemi 2013; Oberoi 2009; Pedersen 1996; Peterman 1996; Prager 1996; Santanen 2004; Schmittner 2010; Shah 2010; Shaikh 2008; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003). We observed no significant heterogeneity between the three subgroups (I2 subgroup test = 0%). The estimated RR for each of these subgroups is similar to the overall estimate reported above (22 gauge RR 2.15, 95% CI 1.56 to 2.97; 25 gauge RR 2.48, 95% CI 1.56 to 3.95; 27 gauge RR 2.87, 95% CI 1.81 to 4.53) (Analysis 1.2), with no evidence of significant heterogeneity in any of the subgroups (I2 = 0%).
1.2. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 2 PDPH by gauge.
In the subgroup analysis performed for indication of lumbar puncture, we observed no differences in the results (I2 subgroup test = 0%). Most of the studies involved anaesthesia procedures (30 studies, 8401 participants, incidence of PDPH = 3.14%). In this subgroup, the atraumatic needles presented significantly less risk of PDPH in comparison with the use of traumatic needles, similar to the analysis using the whole sample (RR 2.21, 95% CI 1.60 to 3.04; I2 = 16%) (Analysis 1.1). The results were similar for the myelography by lumbar puncture subgroup (three studies: Pedersen 1996; Peterman 1996; Prager 1996) (RR 2.01, 95% CI 1.34 to 3; I2 = 0%), and the diagnostic lumbar puncture subgroup (three studies: Kleyweg 1995; Muller 1994; Strupp 2001) (RR 2.22, 95% CI 1.38 to 3.58; I2 = 24%). The funnel plot figure indicates slight asymmetry related to the studies with small sample sizes and null or favourable outcomes when using traumatic needles (Figure 5).
5.
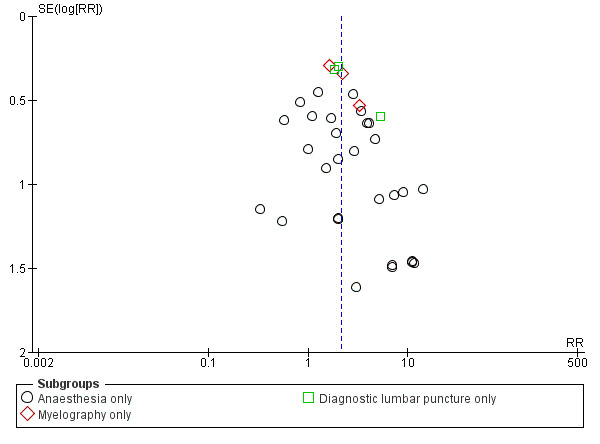
Funnel plot of comparison: 1 Traumatic needle versus atraumatic needle, outcome: 1.1 PDPH by indication.
In addition, we identified nine studies that only included women (mostly in labour) (Devcic 1993; Imarengiaye 2002; Mayer 1992; Oberoi 2009; Pedersen 1996; Shaikh 2008; Shutt 1992; Srivastava 2010b; Tabedar 2003) and we found no studies that only included men (Analysis 1.3).
1.3. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 3 PDPH by gender.
In the subgroup analysis for the type of surgery used in the anaesthesia studies, there were no significant subgroup differences between caesarean section, orthopaedic interventions and subumbilical or lower limb surgeries (test of subgroup differences: I2 = 12%). Orthopaedic surgical studies presented moderate heterogeneity (I2 = 55%), but there was no significant difference in risk between traumatic and atraumatic needles (RR 1.35, 95% CI 0.58 to 3.19). In contrast, the risk of PDPH for caesarian and other surgeries was lower in the atraumatic needle group, with no or minimal heterogeneity (I2 = 0% and 18%, respectively) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 4 PDPH/anaesthesia: type of surgery.
In addition, in the subgroup analysis performed for body position during the lumbar puncture there was heterogeneity (I2 subgroup test = 76.9%) (Analysis 1.5). These differences may be due to the results observed in the subgroup of punctures administered to patients in a lateral position, in which the risk associated with traumatic needles increased significantly when compared to the global result (nine studies, RR 4.70, 95% CI 2.39 to 9.24; I2 = 0%). In the subgroup of punctures administered to sitting participants, with traumatic needles the risk ratio was similar to the analysis including the whole sample (11 studies, RR 2.11, 95% CI 1.52 to 2.94; I2 = 0%).
1.5. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 5 PDPH by position.
Finally, in the subgroup analysis performed for age range, we observed no differences (I2 subgroup test = 0%). In this comparison, only two studies focused on children under 18 (Kokki 1998; Kokki 2000), and the estimate in this subgroup was not precise (RR 1.69, 95% CI 0.56 to 5.12; I2 = 0%), due to the low number of events (14 in total) (Analysis 1.6).
1.6. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 6 PDPH by age.
Primary outcome: adverse events/paraesthesia
Paraesthesia was reported in three studies, which included a total of 573 participants and 29 paraesthesias (incidence of paraesthesia = 5.06%) (Imarengiaye 2002; Kuusniemi 2013; Mayer 1992). We found no differences between the use of traumatic needles versus atraumatic needles for this adverse event (RR 0.96, 95% CI 0.47 to 1.96; I2 = 0%) (Analysis 1.7). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 1).
1.7. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 7 AE: paraesthesia.
Primary outcome: adverse events/backache
Backache was reported in 12 studies (Brattebo 1995; Chaudhry 2011; Flaatten 2000; Imarengiaye 2002; Imbelloni 1997; Kokki 1998; Kokki 2000; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Schultz 1996; Thomas 2000), including a total of 3027 participants and 454 backache events (incidence of backache = 14.9%). We found no differences between the use of traumatic needles versus atraumatic needles for this adverse event (RR 0.94, 95% CI 0.78 to 1.13; I2 = 14%) (Analysis 1.8). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 1).
1.8. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 8 AE: backache.
Secondary outcome: severe PDPH
For this comparison, we analysed the information taken from 24 studies with a total of 6420 participants and 87 events (incidence of severe PDPH = 1.35%) (Brattebo 1995; Chaudhry 2011; Corbey 1997; Despond 1998; Devcic 1993; Fernandez 1993; Fernandez 2003; Imbelloni 1997; Kokki 1998; Lynch 1992a; Mayer 1992; Muller 1994; Pedersen 1996; Peterman 1996; Prager 1996; Shah 2010; Shaikh 2008; Shutt 1992; Srivastava 2010a; Srivastava 2010b; Strupp 2001; Tabedar 2003; Tarkkila 1992; Wiesel 1993). Nine studies presented zero events in both arms and they do not count for the RR analysis (Brattebo 1995; Fernandez 1993; Imbelloni 1997; Kokki 1998; Lynch 1992a; Mayer 1992; Shah 2010; Srivastava 2010a; Srivastava 2010b). A sensitivity analysis measuring the risk difference (RD) allowed us to include all the studies and presents a similar risk between traumatic and atraumatic needles, with considerable heterogeneity (RD 0.00, 95% CI 0.00 to 0.01; I2 = 42%). The heterogeneity observed in this analysis is due to the study focused on diagnostic lumbar punctures (Muller 1994). Excluding this study eliminates the heterogeneity completely and maintains the non‐significant results (Analysis 1.9). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well the presence of the aforementioned considerable heterogeneity. (See Table 1).
1.9. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 9 Severe PDPH by indication.
Secondary outcome: any headache
For this comparison, we analysed the information taken from 18 studies with a total of 4104 participants and 636 events (general incidence of any headache = 15.4%) (Brattebo 1995; Buettner 1993; Chaudhry 2011; Corbey 1997; Despond 1998; Flaatten 2000; Fox 1996; Imarengiaye 2002; Kokki 1998; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Peterman 1996; Prager 1996; Saenghirunvattana 2008; Santanen 2004; Shutt 1992; Thomas 2000). The estimated RR for this outcome was 1.35 (95% CI 1.17 to 1.57) (Analysis 1.10), with minimal heterogeneity (I2 = 5%). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 1).
1.10. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 10 Any headache by indication.
Comparison between larger gauge traumatic needles versus smaller gauge traumatic needles
Primary outcome: Onset of PDPH
For this comparison, we analysed the information taken from 10 studies with a total of 2288 participants and 185 events (incidence of PDPH = 8.09%) (Grover 2002; Kang 1992; Kim 2011; Kokki 1996; Pippa 1995; Rafique 2014; Schmittner 2010; Shah 2010; Shaikh 2008; Tarkkila 1994). We decided against overall pooling of results because a needle gauge could be considered small in one comparison but large in another (for example, a 25 gauge needle could be considered as smaller in a 23 versus 25 gauge comparison, but larger in a 25 versus 27 gauge comparison). Instead, we grouped and analysed studies according to the gauges evaluated (23 versus 25 gauge, 25 versus 27 gauge, 25 versus 29 gauge, 26 versus 27 gauge and 21 versus 25 gauge). The RRs for these comparisons ranged from 0.86 to 6.47 and they were not were not statistically significant except for a single study in the 26 versus 27 gauge subgroup (23 versus 25 gauge RR 2.08, 95% CI 0.20 to 21.55; 25 versus 27 gauge RR 1.82, 95% CI 0.98 to 3.39; 25 versus 29 gauge RR 2.13, 95% CI 0.46 to 9.78; 26 versus 27 gauge RR 6.47, 95% CI 2.55 to 16.43; 21 versus 25 gauge RR 0.86, 95% CI 0.30 to 2.44) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.
The results obtained when comparing 29 with 25 gauge needles present the greatest heterogeneity (I2 = 69%; Analysis 2.1). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See Table 2).
All the studies included in this comparison were undertaken using anaesthesia and included a mixed population, which is the reason why we did not carry out a subgroup analysis for indication for lumbar puncture or gender. Analysis by type of surgery showed no subgroup differences. The estimates presented in the caesarean section subgroup and the orthopaedic surgeries subgroup showed no differences in the risk of PDPH with the use of traumatic needles of any gauge (Analysis 2.2). In the analyses performed for age subgroups, we found no differences by subgroup. In the studies in children and the over 60 years age group there were no significant differences in the risk of PDPH between the use of larger or smaller gauges; however, this information was derived from only one study for each of the subgroups mentioned (Analysis 2.3). In studies in the no age distinction group, we found a significantly higher risk of PDPH for larger gauge needles (RR 2.09, 95% CI 1.11 to 3.95), but with significant heterogeneity (I2 = 69%, P = 0.002). There were no significant differences in the risk of PDPH between the use of larger or smaller gauges in the subgroup analyses by body position (Analysis 2.4).
2.2. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 2 PDPH by type of surgery.
2.3. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 3 PDPH by age.
2.4. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 4 PDPH by position.
Primary outcome: adverse events/paraesthesia
No studies in this comparison reported this outcome.
Primary outcome: adverse events/backache
Backache was reported in three studies that included a total of 948 participants and 188 events (backaches) (incidence of backache = 19.8%) (Grover 2002; Kang 1992; Tarkkila 1994). The RRs for these comparisons ranged from 0.81 to 2.00 and were not statistically significant (25 versus 29 gauge RR 2.00, 95% CI 1.00 to 4.02; 26 versus 27 gauge RR 0.91, 95% CI 0.66 to 1.24; 25 versus 27 gauge RR 0.81, 95% CI 0.44 to 1.49) (Analysis 2.5). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 2).
2.5. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 5 AE: backache.
Secondary outcome: severe PDPH
For this outcome, we analysed the information from six studies with a total of 1128 participants and three events (incidence of severe PDPH = 0.2%) (Grover 2002; Kim 2011; Pippa 1995; Rafique 2014; Shah 2010; Shaikh 2008). We grouped and analysed studies according to the gauges evaluated (23 versus 25 gauge, 25 versus 27 gauge, 25 versus 29 gauge and 21 versus 25 gauge). We conducted analyses with risk differences, which allowed us to incorporate all studies in the estimate. The RDs for these comparisons were 0.00 in all cases and were not statistically significant (23 versus 25 gauge RD 0.00, 95% CI ‐0.07 to 0.07; 25 versus 27 gauge RD 0.00, 95% CI ‐0.01 to 0.01; 25 versus 29 gauge RD 0.00, 95% CI ‐0.04 to 0.04; 21 versus 25 gauge RD 0.00, 95% CI ‐0.02 to 0.02) (Analysis 2.6). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as the few events reported. (See Table 2).
2.6. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 6 Severe PDPH by gauge.
Secondary outcome: any headache
For this comparison, we analysed the information taken from three studies with a total of 771 participants and 195 events (incidence of any headache = 25.2%) (Kang 1992; Kim 2011; Kokki 1996). The RRs for these comparisons ranged from 0.75 to 1.56 and were not statistically significant (23 versus 25 gauge RR 1.29, 95% CI 0.98 to 1.68; 25 versus 29 gauge RR 1.56, 95% CI 0.86 to 2.82; 26 versus 27 gauge RR 0.75, 95% CI 0.18 to 3.07) (Analysis 2.7). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 2).
2.7. Analysis.
Comparison 2 Larger gauge traumatic needles versus smaller gauge traumatic needles, Outcome 7 Any headache.
Comparison between larger gauge atraumatic needles versus smaller gauge atraumatic needles
This comparison involved all studies that compared different gauges of atraumatic needles. From each study we selected only comparisons between larger gauge versus smaller gauge needles for this analysis.
Primary outcome: Onset of PDPH
For this comparison, we analysed the information taken from 13 studies with a total of 3134 participants and 75 events (incidence PDPH = 2.33%) (Amuzu 1995; Campbell 1993; De Andres 1999; Hopkinson 1997; Kokki 1998; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sears 1994; Shah 2010; Sharma 1995; Shutt 1992; Smith 1994). As we mentioned above, we decided against overall pooling of results because a needle gauge could be considered small in one comparison but large in other (for example, a 25 gauge needle could be considered as smaller in a 23 versus 25 gauge comparison, but larger in a 25 versus 27 gauge comparison). We found no significant differences in the analyses comparing gauges (22 versus 24 gauge RR 0.98, 95% CI 0.20 to 4.81; 22 versus 25 gauge RR 3.00, 95% CI 0.32 to 28.50; 24 versus 25 gauge RR 5.62, 95% CI 1.00 to 31.67; 25 versus 26 gauge RR 0.76, 95% CI 0.30 to 1.90; 25 versus 27 gauge RR 3.72, 95% CI 0.59 to 23.64; 26 versus 27 gauge RR 1.79, 95% CI 0.30 to 10.73; 27 versus 29 gauge RR 1.59, 95% CI 0.58 to 4.37) (Analysis 3.1). We found few incidence data for each of the gauge subgroups mentioned and we did not find benefits derived from the use of smaller atraumatic needles compared to larger ones. The funnel plot figure does not show any asymmetry in relation to the data classified by gauge (Figure 6). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See Table 3).
3.1. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 1 PDPH larger gauge vs smaller gauge.
6.
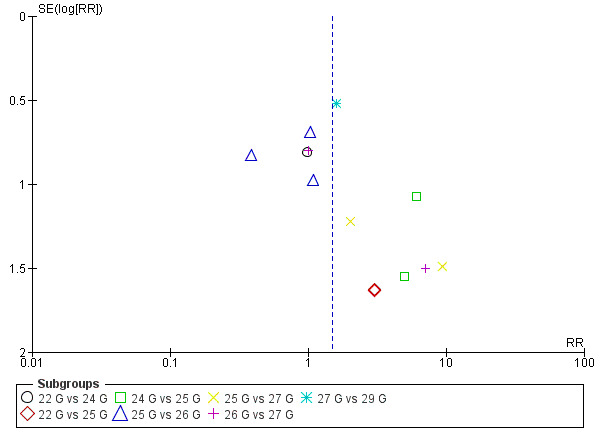
Funnel plot of comparison: 3 Atraumatic needles: different gauges, outcome: 3.1 PDPH major gauge versus minor gauge by number.
The studies included in this comparison had participants with indication for anaesthesia. Analyses by type of surgery showed no effect derived from the type of needles with respect to the presentation of PDPH (Analysis 3.2). The subgroup analyses performed for gender and body position also showed no differences in the results for PDPH.
3.2. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 2 PDPH by type of surgery.
Primary outcome: adverse events/paraesthesia
Two studies that included a total of 439 participants reported 51 paraesthesias (incidence of paraesthesia = 11.6%) (Hopkinson 1997; Sharma 1995). We found no statistically significant difference in paraesthesia related to the size of gauge used; the pooled estimate presented considerable heterogeneity (RR 2.19, 95% CI 0.31 to 15.30; I2 = 72%) (Analysis 3.5). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 3).
3.5. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 5 AE: paraesthesia.
Primary outcome: adverse events/backache
Four studies including a total of 526 participants reported 105 incidences of backache (incidence = 19.9%) (De Andres 1999; Kokki 1998; Sharma 1995; Smith 1994). The RRs for these comparisons ranged from 0.95 to 5.00 and they were not statistically significant (25 versus 29 gauge RR 5.00, 95% CI 0.62 to 40.28; 26 versus 27 gauge RR 1.29, 95% CI 0.69 to 2.40; 25 versus 27 gauge RR 0.95, 95% CI 0.56 to 1.61; 25 versus 26 gauge RR 1.19, 95% CI 0.58 to 2.42) (Analysis 3.6). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 3).
3.6. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 6 AE: backache.
Secondary outcome: severe PDPH
For this outcome, we analysed the information taken from eight studies with a total of 1983 participants and five events (incidence of severe PDPH = 0.25%) (Campbell 1993; De Andres 1999; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sears 1994; Sharma 1995; Smith 1994). We grouped and analysed studies according to the gauges evaluated (22 versus 24 gauge, 22 versus 25 gauge, 24 versus 25 gauge, 25 versus 26 gauge, 25 versus 27 gauge, 26 versus 27 gauge and 27 versus 29 gauge). We conducted analyses with RDs, which allowed us to incorporate all studies in the estimate. The RDs for these comparisons ranged from 0.00 to 0.01 and they were not statistically significant (22 versus 24 gauge RD 0.00, 95% CI ‐0.01 to 0.01; 22 versus 25 gauge RD 0.00, 95% CI ‐0.02 to 0.02; 24 versus 25 gauge RD 0.01, 95% CI ‐0.02 to 0.03; 25 versus 26 gauge RD 0.01, 95% CI ‐0.01 to 0.03; 25 versus 27 gauge RD 0.01, 95% CI ‐0.02 to 0.04; 26 versus 27 gauge RD 0.00, 95% CI ‐0.02 to 0.02; 27 versus 29 gauge RD 0.00, 95% CI ‐0.01 to 0.01) (Analysis 3.7). We downgraded the quality of evidence from high to low due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation, as well as imprecision. (See Table 3).
3.7. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 7 Severe PDPH by gauge.
Secondary outcome: any headache
For this outcome, we analysed the information taken from seven studies with a total of 1791 participants and 206 events (incidence of any headache = 11.5%) (Campbell 1993; Hopkinson 1997; Morros‐Vinoles 2002; Pan 2004; Pittoni 1995; Sharma 1995; Smith 1994). We grouped and analysed studies according to the gauges evaluated (22 versus 25 gauge, 24 versus 25 gauge, 25 versus 26 gauge, 25 versus 27 gauge and 27 versus 29 gauge). The RRs for these comparisons ranged from 1.13 to 2.17 and they were not statistically significant (22 versus 25 gauge RR 2.17, 95% CI 0.85 to 5.51; 24 versus 25 gauge RR 1.17, 95% CI 0.49 to 2.77; 25 versus 26 gauge 1.13, 95% CI 0.65 to 1.99; 25 versus 27 gauge RR 1.87, 95% CI 0.65 to 5.39; 27 versus 29 gauge RR 1.80, 95% CI 0.85 to 3.83) (Analysis 3.8). We downgraded the quality of evidence from high to moderate due to risk of bias issues such as unclear reporting of allocation concealment and random sequence generation. (See Table 3).
3.8. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 8 Any headache by gauge.
Sensitivity analysis
In accordance with our protocol, we selected studies with a low risk of bias for allocation concealment, blinding of outcome assessment and presence of incomplete data (attrition bias). Six studies fulfilled these requirements for the main outcome of onset of PDPH (Hopkinson 1997; Kleyweg 1995; Peterman 1996; Schmittner 2010; Schmittner 2011; Thomas 2000). Only three of them could be analysed together as they made similar comparisons (traumatic needles versus atraumatic needles) and possessed data regarding the main outcome (PDPH) (Kleyweg 1995; Peterman 1996; Schmittner 2011). The analysis of these three studies showed significant risk of PDPH when using traumatic needles (RR 2.78, 95% CI 1.26 to 6.15), but with moderate heterogeneity (I2 = 51%) (Analysis 1.11).
1.11. Analysis.
Comparison 1 Traumatic needle versus atraumatic needle, Outcome 11 PDPH sensitivity analysis.
Discussion
Summary of main results
We assessed the evidence from 66 studies in 17,067 participants, which showed several important aspects for each comparison analysed.
Firstly, in the comparison between traumatic versus atraumatic needles, after analysing information from 9378 participants, we found that the risk of post‐dural puncture headache (PDPH) is almost doubled when a traumatic needle is used (risk ratio (RR) 2.14, 95% confidence interval (CI) 1.72 to 2.67). The number of participants required to be treated with atraumatic needles to prevent an additional new episode of PDPH (NNTB) is 24 (95% CI 20 to 30 participants undergoing lumbar punctures). We observed these results regardless of lumbar puncture indication, gender, age or risk of bias issues. Likewise, we found that only three of the studies included in this review reported paraesthesia as a possible primary outcome after lumbar puncture, with an incidence of 5.06% (Imarengiaye 2002; Kuusniemi 2013; Mayer 1992). We identified no difference in the occurrence of paraesthesia between traumatic and atraumatic needles. This may be due to the low number of events. Twelve studies reported backache, with an incidence of 14.9% (Brattebo 1995; Chaudhry 2011; Flaatten 2000; Imarengiaye 2002; Imbelloni 1997; Kokki 1998; Kokki 2000; Kuusniemi 2013; Lynch 1992a; Mayer 1992; Schultz 1996; Thomas 2000). Despite the higher number of events, we found no important differences between the two needle types. Finally, we found significant differences in the risk of any headache in this comparison (RR 1.35, 95% CI 1.17 to 1.57), but not in the risk of severe PDPH or backache.
Secondly, with respect to the comparison of different gauges of traumatic needles, after analysing the information from 2288 participants of both genders, we found heterogeneous results about the risk associated with larger gauges versus smaller gauges. Overall, studies comparing various sizes of large and small gauges showed no significant differences in the effects on risk of PDPH. We analysed this information by factors such as type of surgery, age and body position, but these factors did not explain the heterogeneity. In addition, we found a scarcity of data related to adverse events: only three studies reported backache and found no differences in risk according to gauge (Grover 2002; Kang 1992; Tarkkila 1994).
Finally, in the comparison of gauges for atraumatic needles, after analysing the information from 3134 participants, we found a large number of gauge comparisons, all with few data. Studies comparing various gauge sizes (large and small) showed no significant differences in the effects on risk of PDPH. Similarly, we did not find significant differences in adverse events, severe PDPH or any headache.
Overall completeness and applicability of evidence
We carried out a thorough search and identified a reasonable number of studies evaluating the effectiveness and safety of different gauges and needle types for the prevention of PDPH. The 66 studies included in the numerical analysis enrolled 17,067 participants. Needle tips, gauges, indications for lumbar puncture and operators all varied and participants were from different age groups and genders. The studies were also conducted over a long period of time. The included studies represent the characteristics of the population usually undergoing lumbar puncture procedures either for diagnostic or therapeutic reasons, which is important for the external validity of this review and should increase the applicability of the results.
The systematic search for study selection and data extraction that we undertook should have minimized the likelihood of missing relevant studies. Also, the funnel plots we produced were highly symmetric, suggesting that a minimal chance of having missed relevant studies and that there is no evidence of publication bias.
The evidence presented consistently showed benefits derived from the use of atraumatic needles and is sufficient to address the main objectives of this review. However, new studies (including those that are ongoing) could help to increase the precision of the different measures of effect, as well as to clarify the actual risk in some selected subgroups (for instance, the comparison between traumatic needles by gauge). Similarly, we think that new studies could also help to provide additional data on adverse events related to the use of needles, or even information about technical difficulties related to the use of smaller gauge needles.
Finally, we did not find any information related to gauge differences in diagnostic and myelography settings. New studies might help to identify any benefits related to greater gauge versus finer gauge needles in these specific scenarios.
Quality of the evidence
We considered the quality of the evidence for the first comparison (traumatic versus atraumatic needles) to be moderate for most of the outcomes assessed. We downgraded the quality of the evidence in these cases due to lack of reporting of aspects related to randomization, such as random sequence generation and allocation concealment, which made it difficult for us to interpret the risk of bias for the included studies. Given that it is not possible to blind the personnel to the needle used, we only assessed the blinding of participants. However, we found that participant blinding was only reported in 50% of included studies. Likewise, we found that a considerable number of studies did not report other adverse events associated with the use of needles, for example paraesthesia and backache. The quality of the evidence for the secondary outcome of severe PDPH was also downgraded from high to low due to both the presence of risk of bias and inconsistency (42%), which was caused by one study focusing on diagnostic lumbar punctures. The secondary outcome 'any headache' was affected by similar reporting problems to those previously mentioned for the primary outcomes and we therefore reduced the quality of this evidence to moderate from high.
The primary outcomes for the second comparison (larger gauge versus smaller gauge traumatic needles) were also affected by concerns about risk of bias and we downgraded the quality of the evidence from high to moderate. The secondary outcomes were not affected by heterogeneity but we considered the quality of the evidence to be moderate due to concerns about risk of bias, similar to those related to the primary outcomes.
Finally, we considered the quality of evidence for the outcomes in the third comparison (larger gauge versus smaller gauge atraumatic needles) to be moderate for most of the outcomes, due to imprecision and risk of bias issues.
Potential biases in the review process
We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
This review was comprehensive in identifying clinical trials addressing the issue of the effectiveness and safety of needle gauges and tips in the prevention of post‐dural puncture headache. However, 18 studies did not provide enough information to be able to classify them as included or excluded, because they were published only as conference proceedings, or because we did not have access to the full texts when we were completing this review. Also, we considered 12 of the studies to be 'ongoing' due to their date of publication as abstracts. We may be able to decide whether or not to include these studies once they have been published as full texts.
A potential source of bias in the review process was that we made some decisions about the analysis after seeing the data from the included studies. First, in order to assess adverse events, we had to define those events (except PDPH) related to the use of needles in anaesthesia, myelography and diagnostic lumbar punctures after the publication of the protocol. Most of the events usually reported in studies, such as nausea and vomiting, were already included in the definition of PDPH; we therefore selected paraesthesia and backache as the two most important adverse events related to the intervention assessed. In this review, we did not consider other events related to technical difficulties with the use of smaller needles; for example, the number of attempts before a successful puncture or the anaesthesiologist’s satisfaction regarding the use of these needles. Secondly, we did not expect to encounter any unit of analysis issues, as we do not expect to find cross‐over studies or cluster‐randomized trials. However, we did identify one cross‐over study with our search strategies. In order to avoid bias in the development of our review, we did not include numerical results related to this study in our analyses because we consider that the patients' history of PDPH could be an important factor to take into account when analysing the possibility of a new episode of PDPH. In a future update we can examine other analysis options in order to try to deal with this information. Finally, we modified the subgroup analysis for age due to heterogeneity in the reporting of this outcome. We classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more, and we analysed the numerical information in these three new categories. Although we planned to present risk ratios, in cases where there were no events in one of the arms we presented risk differences. However, we also presented risk ratios in these cases as a sensitivity analysis.
It is also important to mention as a potential source of bias in the review process the fact that we reran the search strategy in September 2016 and found three studies of interest. We added these studies to the list of Studies awaiting classification and we will incorporate them into the review during a review update.
Agreements and disagreements with other studies or reviews
The literature includes a number of examples of reviews that have evaluated several issues related to the use of needles for different purposes. One of our identified studies included a meta‐analysis of other trials using 27 gauge atraumatic versus 27 gauge traumatic needles , which found a RR of developing PDPH of 0.38 (95% CI 0.19 to 0.75) in the atraumatic group compared to the traumatic group (Flaatten 2000). In our review, we found an effect in all the gauges assessed (22, 25 and 27 gauge), confirming the conclusions presented by these authors. Likewise, Halpern 1994 compared noncutting spinal needles (Sprotte or Whitacre) with cutting needles and larger spinal needles with smaller needles. They found a reduction in the incidence of severe PDPH when noncutting spinal needles were used rather than cutting needles (odds ratio (OR) 0.26, 95% CI 0.11 to 0.62) and no important difference in back pain. They also found a reduction in severe PDPH when a small spinal needle was used compared with a large needle of the same type (OR 0.18, 95% CI 0.09 to 0.36). There was no important difference in the incidence of back pain. The direction of the effect is consistent with our findings.
Bradbury et al assessed different methods to decrease accidental dural punctures and interventions to reduce PDPH following these punctures in parturients (Bradbury 2013). They identified 14 randomized controlled trials with 11,536 epidural insertions, finding that prophylactic epidural blood patch, lateral positioning of the epidural needle bevel upon insertion, use of Sprotte needles, epidural morphine and administration of cosyntropin reduce PDPH. In the same subgroup of participants, Choi et al found that the use of atraumatic spinal needles with a smaller gauge decreased the risk of PDPH in the obstetric population (Choi 2003). However, the authors remarked that the incidence of this complication in labour is considerable, with an estimate of 52.1% accidental dural punctures (95% CI 51.4% to 52.8%). We found similar benefits in the subgroup of obstetric participants when atraumatic needles were used.
Other reviews included other factors related to needles but these are not assessed in the present review. In 2006, Richman assessed the effect of lumbar puncture needle bevel direction on the incidence of post‐dural puncture headache in adult participants when cutting needles were used (Richman 2006). The authors also evaluated the use of a parallel versus a perpendicular orientation during needle insertion. The results derived from five trials suggested that a parallel/longitudinal insertion resulted in a lower incidence of PDPH (OR 0.29, 95% CI 0.17 to 0.50). Our review did not include information about bevel orientation but we noticed that the needles used in spinal anaesthesia are larger than those usually used. Likewise, Tung et al in 2012 developed a decision‐analytic model to determine the cost of diagnostic lumbar punctures using atraumatic versus traumatic needles (Tung 2012). The authors assumed a healthcare system perspective and determined that the difference in estimated costs between the two needles was the economic outcome measure selected. They found that lumbar punctures performed with an atraumatic needle are associated with an average cost saving of USD 26.07 per patient. Average total healthcare costs with traumatic needles are USD 192.15 versus 166.08 using atraumatic needles in diagnostic lumbar punctures.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that atraumatic needles reduce the risk of post‐dural puncture headache (PDPH) without increasing adverse events such as paraesthesia or backache. The moderate quality of the evidence suggests that further research is likely to have an important impact on our confidence in this estimate. Health professionals in charge of lumbar punctures in daily clinical practice (anaesthesiologists, neurologists or radiologists, among others) could choose to use atraumatic needles in order to avoid the onset of PDPH.
We found variable results when we assessed the risk associated with larger versus smaller needle gauges, which precludes conclusions about needle size. However, our results for anaesthesia procedures found benefits in terms of the prevention of 'any headache' with the use of fine‐gauge needles. It is important to point out, however, that practitioners would need to be well trained in the use of such needles in order to avoid additional complications such as an increased number of attempts.
Implications for research.
The relative benefit of using atraumatic needles is modest and their widespread use should be determined by additional economic evaluations, which assess the costs of newer needles against the excess cases of post‐dural puncture headache from traumatic needles. Likewise, because we only found moderate‐quality evidence for two adverse events (paraesthesia and backache), we think that large, well‐designed cohort studies are necessary to evaluate the occurrence of other neurological complications from the use of atraumatic needles. Due to the low quality of the evidence related to severe PDPH, additional studies are needed to determine which factors are associated with its occurrence and the interaction of these factors with needle tip designs. This is important because while non‐severe case of PDPH will continue to occur, it is the cases of severe PDPH that are the largest burden to patients and account for the extra healthcare costs.
What's new
| Date | Event | Description |
|---|---|---|
| 21 December 2017 | Amended | We have corrected an error in the data from Smith 1994 regarding the incidence of PDPH. The RR point estimates, confidence intervals and I‐square values were modified for the following comparisons Analysis 3.1. 5; 5, Analysis 3.2.1; and Analysis 3.4.1. However, these changes did not change the interpretations and conclusions of the published version. |
Acknowledgements
We would like to thank Bronagh Blackwood (content editor), Vibeke E Horstmann (statistical editor), Stephen Halpern and James D Griffiths (peer reviewers), and Patricia Tong (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Mathew Zacharias (content editor), Marialena Trivella (statistical editor) and Andrew Moore, Stephen Halpern and Polpun Boonmak (peer reviewers) for their help and editorial advice during the preparation of the protocol for this systematic review. The review authors would also like to thank Arturo Marti‐Carvajal for his support and assistance in the design of this review's search strategies and for giving feedback on this protocol (Arevalo‐Rodriguez 2013a).
Appendices
Appendix 1. Glossary of terms
| Term | Definition | Source |
| Analgesia, epidural | Relief of pain without loss of consciousness through the introduction of an analgesic agent into the epidural space of the vertebral canal. | http://www.ncbi.nlm.nih.gov/mesh |
| Analgesia, obstetric | Elimination of pain, without loss of consciousness, during obstetrical labour; obstetrical delivery; or the postpartum period, usually through the administration of analgesics. | http://www.ncbi.nlm.nih.gov/mesh |
| Blood patch, epidural | Injection of autologous blood into the epidural space either as a prophylactic treatment immediately after an epidural puncture or for treatment of headache resulting from an epidural puncture. | http://www.ncbi.nlm.nih.gov/mesh |
| Cerebrospinal fluid pressure | Manometric pressure of the cerebrospinal fluid as measured by lumbar, cerebroventricular or cisternal puncture. Within the cranial cavity, it is called intracranial pressure. | http://www.ncbi.nlm.nih.gov/mesh |
| Dura mater | The outermost of the three meninges, a fibrous membrane of connective tissue that covers the brain and the spinal cord. | http://www.ncbi.nlm.nih.gov/mesh |
| Myelography | X‐ray visualization of the spinal cord after injection of contrast medium into the spinal arachnoid space. | http://www.ncbi.nlm.nih.gov/mesh |
| Needle | Sharp instruments used for puncturing or suturing. | http://www.ncbi.nlm.nih.gov/mesh |
| Primary prevention | Specific practices for the prevention of disease or mental disorders in susceptible individuals or populations. These include health promotion, including mental health; protective procedures, such as communicable disease control; and monitoring and regulation of environmental pollutants. | http://www.ncbi.nlm.nih.gov/mesh |
| Post‐dural puncture headache | A secondary headache disorder attributed to low cerebrospinal fluid pressure caused by spinal puncture, usually after dural or lumbar puncture. | http://www.ncbi.nlm.nih.gov/mesh |
| Spinal puncture | Tapping fluid from the subarachnoid space in the lumbar region, usually between the third and fourth lumbar vertebrae. | http://www.ncbi.nlm.nih.gov/mesh |
Appendix 2. CENTRAL, the Cochrane Library search strategy
#1 MeSH descriptor: [Post‐Dural Puncture Headache] explode all trees #2 (pdph or plph or pph or post dural or postdural or headach* or cephalea* or cephalalgi*):ti,ab #3 MeSH descriptor: [Anesthesia, Epidural] explode all trees #4 MeSH descriptor: [Anesthesia, Spinal] explode all trees #5 MeSH descriptor: [Injections, Spinal] explode all trees #6 MeSH descriptor: [Myelography] explode all trees #7 MeSH descriptor: [Spinal Puncture] explode all trees #8 (#1 or #2) and (#3 or #4 or #5 or #6 or #7) #9 ((spinal or intraspinal or dural or intradural or epidural or lumbar* or thecal* or intrathecal or sub?arachnoid*) near (puncture* or inject* or anesth* or anaesth* or needle* or tap)):ti,ab #10 #8 or #9 #11 (caliber or needle gauge* or needle tip* or needle size* or traumatic tap* or traumatic needle* or atraumatic needle* or pencil point* or diamond tip* or spinal needle* or ((quincke or greene or hingson or lutz or brace or rovenstine or lemmon or whitacre or atraucan or sprotte or cappe or gertie marx or deutsch) and (needle*))):ti,ab #12 #10 and #11
Appendix 3. MEDLINE (PubMed) search strategy
(Post‐Dural Puncture Headache[Mesh] OR PDPH[tiab] OR PLPH[tiab] OR PPH[tiab] OR Post dural[tiab] OR Postdural[tiab] OR Headache[Mesh] OR Headach*[tiab] OR cephalea*[tiab] OR cephalalgi*[tiab]) AND (Anesthesia, Epidural[Mesh] OR Anesthesia, Spinal[Mesh] OR Injections, Spinal[Mesh] OR Myelography[Mesh] OR Spinal Puncture[Mesh] OR ((spinal[tiab] OR intraspinal[tiab] OR dural[tiab] OR intradural[tiab] OR epidural[tiab] OR lumbar*[tiab] OR thecal*[tiab] OR intrathecal[tiab] OR subarachnoid*[tiab] OR sub arachnoid*[tiab]) AND (Spinal Puncture[Mesh] OR puncture*[tiab] OR inject*[tiab] OR anesth*[tiab] OR anaesth*[tiab] OR needle*[tiab] OR Tap[tiab]))) AND (caliber[tiab] OR Needle Gauge*[tiab] OR Needle Tip*[tiab] OR Needle size*[tiab] OR Traumatic Tap*[tiab] OR Traumatic Needle*[tiab] OR Atraumatic Needle*[tiab] OR Pencil Point*[tiab] OR Diamond Tip*[tiab] OR Spinal Needle*[tiab] OR ((Quincke[tiab] OR Greene[tiab] OR Hingson[tiab] OR Lutz[tiab] OR Brace[tiab] OR Rovenstine[tiab] OR Lemmon[tiab] OR Whitacre[tiab] OR Atraucan[tiab] OR Sprotte[tiab] OR Cappe[tiab] OR Gertie Marx[tiab] OR Deutsch[tiab]) AND (Needle*[tiab])))
Appendix 4. EMBASE (Ovid SP) search strategy
1. (postdural puncture headache/ or pdph.ti,ab. or plph.ti,ab. or pph.ti,ab. or post dural.ti,ab. or postdural.ti,ab. or headache/ or headach*.ti,ab. or cephalea*.ti,ab. or cephalalgi*.ti,ab.) and (epidural anesthesia/ or spinal anesthesia/ or intraspinal drug administration/ or myelography/ or puncture/ or ((spinal or intraspinal or dural or intradural or epidural or lumbar* or thecal* or intrathecal or sub?arachnoid*) and (puncture* or inject* or anesth* or anaesth* or needle* or tap)).ti,ab.) and (caliber or needle gauge* or needle tip* or needle size* or traumatic tap* or traumatic needle* or atraumatic needle* or pencil point* or diamond tip* or spinal needle* or ((quincke or greene or hingson or lutz or brace or rovenstine or lemmon or whitacre or atraucan or sprotte or cappe or gertie marx or deutsch) and needle*)).ti,ab.
Appendix 5. CINAHL (EBSCOhost) search strategy
S1. ( (MM "Anesthesia, Epidural") OR (MM "Analgesia, Epidural") OR (MM "Anesthesia, Spinal") OR (MM "Injections, Intraspinal+") OR (MM "Myelography") OR (MH "Spinal Puncture") ) AND ( (MH "Headache") OR TI ( pdph or plph or pph or post dural or postdural or headach* or cephalea* or cephalalgi* ) OR AB ( pdph or plph or pph or post dural or postdural or headach* or cephalea* or cephalalgi* ) ) S2. TI ( (spinal or intraspinal or dural or intradural or epidural or lumbar* or thecal* or intrathecal or subarachnoid* or sub arachnoid*) and (puncture* or inject* or anesth* or anaesth* or needle* or tap) ) OR AB ( (spinal or intraspinal or dural or intradural or epidural or lumbar* or thecal* or intrathecal or subarachnoid* or sub arachnoid*) and (puncture* or inject* or anesth* or anaesth* or needle* or tap) ) S3. S1 OR S2 S4. TI ( (caliber or needle gauge* or needle tip* or needle size* or traumatic tap* or traumatic needle* or atraumatic needle* or pencil point* or diamond tip* or spinal needle* or ((quincke or greene or hingson or lutz or brace or rovenstine or lemmon or whitacre or atraucan or sprotte or cappe or gertie marx or deutsch) and (needle*))) ) OR AB ( (caliber or needle gauge* or needle tip* or needle size* or traumatic tap* or traumatic needle* or atraumatic needle* or pencil point* or diamond tip* or spinal needle* or ((quincke or greene or hingson or lutz or brace or rovenstine or lemmon or whitacre or atraucan or sprotte or cappe or gertie marx or deutsch) and (needle*))) ) S5. S3 AND S4
Appendix 6. LILACS (BIREME) search strategy
((pdph or plph or pph or post dural or postdural or headach$ or cephalea$ or cephalalgi$) and (epidural or spinal anesthesia or spinal injections or myelography or spinal puncture)) or ((spinal or intraspinal or dural or intradural or epidural or lumbar$ or thecal$ or intrathecal or subarachnoid$ or sub arachnoid$) and (puncture$ or inject$ or anesth$ or anaesth$ or needle$ or tap)) [Words] and (caliber or needle gauge$ or needle tip$ or needle size$ or traumatic tap$ or traumatic needle$ or atraumatic needle$ or pencil point$ or diamond tip$ or spinal needle$ or ((quincke or greene or hingson or lutz or brace or rovenstine or lemmon or whitacre or atraucan or sprotte or cappe or gertie marx or deutsch) and (needle$)))
Appendix 7. Study eligibility screening and data extraction form
Needle Gauge and Tip designs for Preventing PDPH— Intervention Cochrane Review
Study Selection, Quality Assessment & Data Extraction Form
| First Author | Journal/Conference proceedings, etc | Year |
1. Study Eligibility
| RCT/CCT | Relevant participants | Relevant interventions | Relevant outcomes* | |
| Yes | ||||
| No | ||||
| Unclear |
aIssues related to selective reporting when authors may have taken measurements for particular outcomes but did not report these within the paper(s). Review authors should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in 'Studies awaiting assessment' until clarified. If no clarification is received after three attempts, study should then be excluded.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Do not proceed if any of the above answers is "No".
2. References to Trial
Check other references identified in searches. If further references to this trial are identified, link the papers and list below. All references to a trial should be linked under one Study ID in RevMan.
| Author | Journal/Conference proceedings, etc | Year |
3. Participant and Trial Characteristics
| Further details | |
| Age, years (mean, median, range, etc.) | |
| Gender of participants | |
| Country | |
| Reason for puncture (dx, anaesthesia, radiology) | |
| Surgical procedure (obstetrical, orthopaedic, etc.) | |
| Type of anaesthesia used | |
| Trial design (parallel, etc.) | |
| Single centre/multi‐centre | |
| Eligibility criteria | |
| Exclusion criteria | |
| Follow‐up, years (mean, median, range, etc.) | |
| Time points reported in the study | |
| Other |
4. Intervention Characteristics
| Further details | |
| Needle tip | |
| Needle gauge | |
| Number of attempts |
5. Number of Participants
| Enrolled participants | Randomly assigned participants | Participants included in analysis | Lost to follow‐up | Reasons | |
| At beginning | |||||
| A Group | |||||
| B Group | |||||
| C Group | |||||
| D Group |
6. Methodological Quality
| Low risk of bias | Unclear risk of bias | High risk of bias | Details | |
| Random sequence generation | ||||
| Allocation concealment | ||||
| Blinding of participants and personnel | ||||
| Blinding of outcome assessment | ||||
| Selective reporting bias | ||||
| Incomplete outcome data | ||||
| Other bias | ||||
| Withdrawals | ||||
| Other (describe) |
7. Results
| A Group (define) | B Group (define) | |||
| # Participants with outcome | # Participants analysed | # Participants with outcome | # Participants analysed | |
| PDPH | ||||
| Severe PDPH (define) |
||||
| Any headache after spinal anaesthesia | ||||
| Other (define) |
Data and analyses
Comparison 1. Traumatic needle versus atraumatic needle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PDPH by indication | 36 | 9378 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.72, 2.67] |
| 1.1 Anaesthesia only | 30 | 8401 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.60, 3.04] |
| 1.2 Myelography only | 3 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 2.01 [1.34, 3.00] |
| 1.3 Diagnostic lumbar puncture only | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.38, 3.58] |
| 2 PDPH by gauge | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 22 gauge | 5 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.56, 2.97] |
| 2.2 25 gauge | 5 | 1260 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.56, 3.95] |
| 2.3 27 gauge | 11 | 4076 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [1.81, 4.53] |
| 3 PDPH by gender | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 9 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 2.60 [1.62, 4.17] |
| 4 PDPH/anaesthesia: type of surgery | 30 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Caesarean section | 8 | 1324 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.60, 6.10] |
| 4.2 Orthopaedic procedures | 3 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.58, 3.19] |
| 4.3 Other surgeries | 19 | 6083 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.50, 3.51] |
| 5 PDPH by position | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Lateral position | 9 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [2.39, 9.24] |
| 5.2 Sitting position | 11 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.52, 2.94] |
| 6 PDPH by age | 36 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 No distinctions by age | 34 | 9063 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.73, 2.73] |
| 6.2 Only < 18 years | 2 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.56, 5.12] |
| 7 AE: paraesthesia | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.47, 1.96] |
| 8 AE: backache | 12 | 3027 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 9 Severe PDPH by indication | 24 | 6420 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.20, 2.94] |
| 9.1 Anesthesia | 19 | 5542 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.88, 3.53] |
| 9.2 Myelography | 4 | 778 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.68, 4.28] |
| 9.3 Diagnostic lumbar puncture | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.18, 7.63] |
| 10 Any headache by indication | 18 | 4104 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.17, 1.57] |
| 10.1 Anaesthesia | 16 | 3656 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.17, 1.63] |
| 10.2 Myelography | 2 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.81, 2.21] |
| 11 PDPH sensitivity analysis | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [1.26, 6.15] |
Comparison 2. Larger gauge traumatic needles versus smaller gauge traumatic needles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PDPH larger gauge vs smaller gauge | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 23 G vs 25 G | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 1.2 25 G vs 27 G | 4 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.98, 3.39] |
| 1.3 25 G vs 29 G | 3 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [0.46, 9.78] |
| 1.4 26 G vs 27 G | 1 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.55, 16.43] |
| 1.5 21 G vs 25 G | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.30, 2.44] |
| 2 PDPH by type of surgery | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 2 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.64, 2.57] |
| 2.2 Orthopaedic surgeries | 2 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.38, 2.58] |
| 2.3 Other surgeries | 6 | 1620 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.23, 7.03] |
| 3 PDPH by age | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 No distinctions about age | 8 | 2175 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [1.11, 3.95] |
| 3.2 Only children | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.83] |
| 3.3 Only > 60 years | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [0.20, 21.55] |
| 4 PDPH by position | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Lateral position | 5 | 859 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.98, 3.16] |
| 4.2 Sitting position | 2 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.64, 1.56] |
| 5 AE: backache | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Severe PDPH by gauge | 6 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 23 G vs 25 G | 1 | 53 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.07, 0.07] |
| 6.2 25 G vs 27 G | 3 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 6.3 25 G vs 29 G | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6.4 21 G vs 25 G | 1 | 160 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7 Any headache | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Larger gauge atraumatic needles versus smaller gauge atraumatic needles.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PDPH larger gauge vs smaller gauge | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 22 G vs 24 G | 1 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.81] |
| 1.2 22 G vs 25 G | 2 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.32, 28.50] |
| 1.3 24 G vs 25 G | 2 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [1.00, 31.67] |
| 1.4 25 G vs 26 G | 3 | 519 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.30, 1.90] |
| 1.5 25 G vs 27 G | 2 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.59, 23.64] |
| 1.6 26 G vs 27 G | 2 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.30, 10.73] |
| 1.7 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.58, 4.37] |
| 2 PDPH by type of surgery | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Caesarean section | 6 | 1263 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.64, 5.79] |
| 2.2 Orthopaedic procedures | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.30, 5.07] |
| 2.3 Other surgeries | 5 | 1479 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.73, 2.83] |
| 3 PDPH by gender | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Only women | 8 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.51, 2.20] |
| 4 PDPH by position | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Sitting position | 5 | 1106 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.45, 2.06] |
| 4.2 Lateral position | 5 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.65, 5.41] |
| 5 AE: paraesthesia | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.31, 15.30] |
| 6 AE: backache | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7 Severe PDPH by gauge | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 22 G vs 24 G | 1 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 7.2 22 G vs 25 G | 1 | 234 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.3 24 G vs 25 G | 1 | 304 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.4 25 G vs 26 G | 2 | 311 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.5 25 G vs 27 G | 1 | 212 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.6 26 G vs 27 G | 1 | 158 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.02, 0.02] |
| 7.7 27 G vs 29 G | 1 | 389 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 Any headache by gauge | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 22 G vs 25 G | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.85, 5.51] |
| 8.2 24 G vs 25 G | 2 | 645 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.49, 2.77] |
| 8.3 25 G vs 26 G | 2 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.65, 1.99] |
| 8.4 25 G vs 27 G | 1 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.65, 5.39] |
| 8.5 27 G vs 29 G | 1 | 389 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.85, 3.83] |
3.3. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 3 PDPH by gender.
3.4. Analysis.
Comparison 3 Larger gauge atraumatic needles versus smaller gauge atraumatic needles, Outcome 4 PDPH by position.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amuzu 1995.
| Methods |
|
|
| Participants | 1. 208 patients enrolled (obstetric patients undergoing elective caesarean section) Patients randomized to:
2. No randomized patients were excluded 3. No patients lost to follow‐up 4. Main characteristics of patients: unclear. "There were no significant differences between the patients in the two groups in terms of age, weight, height, previous history of c‐section and past history of headache" |
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned to…" (page 150) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "were randomly assigned to…" (page 150) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Brattebo 1995.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (patients scheduled for surgery in the lower part of the body)
2. 2 patients randomized to Quincke group were excluded due to failures in identification of subarachnoidal space 3. No patients lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "… were randomised into two groups after written informed consent was obtained" (page 535) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients did not know which needle was used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After 72 hours all patient were contacted personally or by telephone, and questioned in a structured interview about problems or symptoms which could have been a result of the spinal anaesthetic. This interview was done by a nurse anaesthetist who was unaware of which needle that had been used, and whether any problems had occurred during the anaesthetic." (page 536) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of the funder during the study was unclear (Medisinsk forskning I Finnmark) |
Buettner 1993.
| Methods |
|
|
| Participants | 1. 400 women enrolled (consecutive patients receiving spinal anaesthesia for orthopaedic operations of the lower extremities) Patients randomized to:
2. No randomized patients lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to (…)" (page 166) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Neither the interviewer nor the patient were aware of the kind of needle that had been used". (page 167) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the interviewer nor the patient were aware of the kind of needle that had been used" (page 167) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Campbell 1993.
| Methods |
|
|
| Participants | 1. 354 women enrolled (ASA 1 and 2 undergoing spinal anaesthesia for elective caesarean section) Patients randomized to:
2. 4 patients (2 for each group) with failure to identify the subarachnoid space were excluded Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized, using a randomization table, into two groups (…)". (page 1132) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients were blinded to the needle utilized". (page 1132) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were assessed after operation by an investigator blinded to the needle and not involved in their perioperative care". (page 1132) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.31% patients enrolled were not analysed |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Chaudhry 2011.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (patients from different surgical departments of Nawaz Sharif Social Security Hospital Lahore having different surgical procedures on the lower abdomen and lower limbs such as hernias, amputations, debridements, vesicolithotomy, total hip replacements, tibial nailing or plating, external fixators, caesarian sections and hysterectomies) Excluded patients: patients with systemic disease such as uncontrolled diabetes mellitus and hypertension, congestive cardiac failure, severe anaemia, pulmonary oedema, coagulopathies and vertebral column deformities Patients randomized to:
2. No patients were excluded 3. Main characteristics of patients:
|
|
| Interventions |
Co‐intervention: needle directed cephalic slightly upwards towards umbilicus |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Two groups consisting 100 patients each were randomly chosen". (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Several outcomes unreported in results section: speed of CSF back flow, aspiration of CSF, ease of injection |
| Other bias | Low risk | No other biases were identified |
Corbey 1997.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (less than 45 years of age, presenting for daycare surgery on the lower half of the body to be performed under spinal anaesthesia) Excluded patients: unclear
2. 9 patients failed to return their questionnaires and 2 patients were recorded as failures 3. Main characteristics of patients:
|
|
| Interventions | Spinal anaesthesia with either a 27 G Quincke needle or 27 G Whitacre
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated to receive spinal anaesthesia" (page 780) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients were not aware of which needle had been used to perform the anaesthesia". (page 780) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The replies to the questionnaires were assessed by one of the authors who was not aware of which needle had been used to perform the anaesthesia". (page 780) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Crock 2014.
| Methods |
|
|
| Participants | 1. 133 children having LP as part of their standard treatment protocol for leukaemia, recruited during visits to the Day Surgery Unit of the Royal Children’s Hospital. Aged 4 to 15 years at the time of first procedure. Exclusion criteria: excluded if they had insufficient LPs remaining in their planned treatment, had significant coexisting medical problems causing headache or were routinely using 25 G needles at parental request, or if there were significant social or communication problems. 3. 40 were excluded for meeting exclusion criteria
4. 93 were allocated to a random sequence of 4 LPs, 2 with 22 G (A) and 2 with 25 G (B), and completed 341 LPs
2. No randomized patients were excluded 3. 18 patients lost to follow‐up: 2 children had their last LP after their 16th birthday (excluded). 16 did not complete all 4 procedures for reasons such as moving interstate or finishing their treatment protocol, giving a total of 341 procedures (167 with the 22 G and 174 with the 25 G needle) 4. Main characteristics of patients (not specifying groups):
|
|
| Interventions |
|
|
| Outcomes | Primary outcomes:
Secondary outcomes: assessed by a 1‐page questionnaire and telephone interview on days 1, 3 and 7 following the procedure
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The treatment allocation was computer‐generated by an independent statistician." (page 204) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated a sequential study number which corresponded to a large envelope containing four smaller sealed envelopes, labelled a, b, c and d, containing details of the needle sizes to be used for four procedures" (page 204) |
| Blinding of participants (performance bias) | Low risk | Quote: "All LPs were performed under general anaesthesia by the same experienced doctor (CC) who was given the relevant sealed envelope immediately before the procedure. This doctor was not involved in data collection after the procedure. All other staff and study participants were blinded to the needle gauge." (page 204) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A study researcher blinded to the needle size recorded the time from first needle insertion to successful commencement of CSF collection and the time required for collection of 22 drops of CSF (approximately 1 mL) (…) Following each procedure, parents were given a one‐page questionnaire to take home which asked them to record details of any headache in the child on days 1, 3 and 7 following the procedure (…) A researcher also phoned families on days 1, 3 and 7 after each procedure to ensure the data were recorded, and confirm the nature of any headache." (page 204) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.3% (31 out of 372 procedures) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
De Andres 1999.
| Methods |
|
|
| Participants | 1. 158 patients enrolled during a 12‐month period (ASA I and II, aged from 20 to 40 years, undergoing lower limb orthopaedic surgery) Exclusion criteria: presence of hypovolaemia, coagulation disorders, infection at the puncture site, use of general anaesthesia, history of headaches, chronic back pain or pregnancy Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On arrival at the operating room, the patients were assigned to one of two groups using a randomization table: (…)." (page 548) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After surgery, close follow‐up of patients was performed by an investigator blinded to the study protocol". (page 549) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Information about non‐specific headaches is unclear |
| Other bias | Low risk | No other biases were identified |
Despond 1998.
| Methods |
|
|
| Participants | 1. 200 patients ASA I and II, aged 18 to 45 years and scheduled for knee arthroscopy were randomly assigned to 2 groups Unclear if patients were enrolled but not randomized Exclusion criteria: history of migraine headaches, previous PDPH Patients randomized to:
2. 6 patients were excluded from analysis because exclusion criteria had been missed or they could not be contacted Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias Quote: "…were randomly assigned to two groups." (page 1107) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The interview was conducted by an anaesthetist unaware of the anaesthetic technique used..." (page 1107) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients (3%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Devcic 1993.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (healthy obstetric patients requiring caesarean section) Exclusion criteria: patients in whom labour epidural analgesia had been attempted or performed previously, or in whom a spinal anaesthetic had been attempted with other needles 4 patients in the Sprotte group (3 Sprotte with fentanyl and 1 Sprotte with plain local anaesthetic) and 2 in the Quincke group (1 randomized to receive fentanyl and 1 Sprotte with plain local bupivacaine) were not available for follow‐up
2. 6 (6%) patients randomized were excluded because exclusion criteria had been missed or because they could not be contacted
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "This randomized, blinded study (…)." (page 222) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all patients were evaluated daily during the first 4 postoperative days by the designated nurse, who was blinded to the type of needle and medication used (...) Investigators conducting telephone follow‐up were blinded to the type of needle and anesthetic solution used". (page 223) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients (8%) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Fernandez 1993.
| Methods |
|
|
| Participants | 1. 80 patients undergoing different surgical procedures and receiving regional anaesthesia were randomized. Unclear if patients were enrolled but not randomized. Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Individuals were randomly assigned to receive…" (page 241) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Fernandez 2003.
| Methods |
|
|
| Participants | 1. 1555 patients enrolled (ASA I‐II patients undergoing lower abdominal surgery and hospitalization no more than 24 hours) Exclusion criteria: history of PDPH in previous surgeries Number of patients randomized per group: unclear 2. 33 patients randomized were excluded due to (unclear numbers by group):
3. 1522 patients were analysed in 2 groups:
4. No patients were lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Distribution of patients in each group were randomly using the last two digits of their medical history" (page 183) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Once the patient had started mobilisation was visited by a team member who did not know the type of needle used and asked specifically for headache occurrence." (page 183) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Flaatten 2000.
| Methods |
|
|
| Participants | 1. 313 patients aged 18 to 55 years were enrolled (scheduled for non‐obstetric outpatient surgery below the umbilicus to be performed during spinal anaesthesia) Exclusion criteria: unclear Patients randomized to:
2. 12 patients were excluded from analysis
3. 301 patients were analysed (lost to follow‐up: 3.83%)
3. Main characteristics of patients:
|
|
| Interventions | 1. 27 G Pencan group: 0.40 mm O.D. B Braun, Germany 2. 27 G Quincke group: Spinocan 27 G, B. Braun, Germany. The bevel of the Quincke‐type spinal needle was kept parallel to the longitudinal direction of the dural sac. |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was performed using the sealed envelope technique.…" (page 643) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was performed using the sealed envelope technique.…" (page 643) |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients were blinded to the choice of spinal needle, and only the needle size of the spinal needle was documented in the anaesthetic record." (page 644) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients were followed up by a single anaesthesiologist (HF) also blinded to the choice of spinal needle." (page 644) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.83% patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Fox 1996.
| Methods |
|
|
| Participants | 1. 412 patients undergoing thoracic/cervical or lumbar myelographies were enrolled Exclusion criteria: unclear Patients randomized to:
Also patients inside each group were randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "In a prospective randomized trial the incidence of complaints after lumbar puncture…" (page 922) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Geurts 1990.
| Methods |
|
|
| Participants | 1. 40 patients healthy ASA I patients under 40 years of age were enrolled. Indications for surgery varied, but all operations were subumbilical. Exclusion criteria: patients complaining of pre‐existing headache or backache Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "a restricted randomised double‐blind study to ensure equal numbers in each group was initiated…" (page 350). |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The use of a 0.90 mm introducer needle ensured that the patients were unable to differentiate between the two spinal needles." (page 350) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively, patients were visited by two of the authors (MCH and RMW), who had no knowledge of which needle size had been used for spinal anaesthesia." (page 350) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Gonzalez 2000.
| Methods |
|
|
| Participants | 1. 308 patients aged 18 to 45, ASA I, undergoing surgery in lower limbs Exclusion criteria: column injuries, cognitive or coagulation comorbidities, infection in site of lumbar puncture Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to one of two groups…" (page 162) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Grover 2002.
| Methods |
|
|
| Participants | 1. 100 ASA Grade I and II of either sex in the age group between 25 to 45 years, who were to receive spinal anaesthesia to undergo subumbilical surgery Exclusion criteria: obstetric patients, patients with abnormalities of spine, soft tissue infection at the site of needle insertion, acute ear infection and respiratory tract infection, coagulation disorders and neurological symptoms Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
Co‐intervention: patients were premedicated with tablet diazepam 5 mg a night before and 5 mg on the morning of surgery. Morphine sulphate 0.15 mg/kg and promethazine 0.5 mg/kg was also administered intramuscularly to all patients 45 minutes before anaesthesia. |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly divided into two groups…" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All the patients were visited at the end of 24 hours and then on the third and fourth post‐operative day by an anaesthetist who was not present during the performance of spinal anaesthesia." (page 2) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Hafer 1997.
| Methods |
|
|
| Participants | 1. 493 ASA Grade I to III patients undergoing orthopaedic surgery of the lower limbs were included 19 patients receive 2 surgeries, therefore the authors included 512 procedures in this study. However, 12 patients were excluded due to anatomical factors (1 procedure). 500 procedures randomized to:
Also patients were assigned to different regimes of mobilization (not analysed in the present review) 2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According with a random list patients were assigned to one of four groups" (page 861) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Examiners and patients had no knowledge of the needle type used (double‐blind)." (page 861) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examiners and patients had no knowledge of the needle type used (double‐blind)." (page 861) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Harrison 1993.
| Methods |
|
|
| Participants | 1. 128 patients referred to lumbar, thoracic, cervical or total column myelography were included 128 patients assigned to:
2. 15 patients were lost to follow‐up and excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were numbered sequentially: in even‐numbered patients a 22 gauge needle was used and for odd‐numbered patients, a 25 gauge needle " (page 487) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% of patients (15) were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Hopkinson 1997.
| Methods |
|
|
| Participants | 1. 688 women undergoing caesarean section in whom spinal anaesthesia was clinically indicated Exclusion criteria: anticoagulation therapy, aortic valve disease, NYHA class 3 or 4 cardiac symptomology, sepsis at the site of injection, severe pre‐eclampsia and systemic hypotension Patients randomized to:
2. 7 patients were not studied because 1 withdrew and 5 were entered twice A further 16 were excluded from the analysis of headache due to protocol deviations Patients analysed (3.34% lost to follow‐up):
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Randomisation was achieved using sealed envelopes, which contained the needle to be used as well as the documentation" (page 1006) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was achieved using sealed envelopes, which contained the needle to be used as well as the documentation" (page 1006) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all patients were seen within 48 h of surgery by a member of the study team who had not been involved with the performance of the block" (page 1007) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.34% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Imarengiaye 2002.
| Methods |
|
|
| Participants | 1. 60 women ASA I or II scheduled for elective caesarean section were enrolled Exclusion criteria: abnormal lumbar spaces, coagulopathy, infection, pre‐eclampsia or obesity Patients randomized to:
2. No patients were excluded at follow‐up 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized, pulling out of a hat method (…)". (page 379) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All patients and the assessor of postoperative complications but not the attending anesthetists were blinded to the needle used" (page 380) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively, the patients were visited daily for five days by an anaesthetist not involved in the perioperative care (..)" (page 1007) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Imbelloni 1997.
| Methods |
|
|
| Participants | 1. 693 patients under 50 years undergoing spinal anaesthesia were included Exclusion criteria: patients with diseases that could affect CSF pressure were excluded from the study Patients divided into 2 groups:
2. No patients reported as lost to follow‐up 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "693 patients younger than 50 years, submitted to spinal anesthesia, were divided into two groups corresponding to each type of disposable needle used" (page 3) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients did not know which type of needle to use" (page 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were reported as lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kang 1992.
| Methods |
|
|
| Participants | 1. 730 ambulatory surgery patients, 18 years or older, ASA I or II, and electing to receive spinal anaesthesia, were enrolled Exclusion criteria: patients with history of migraine headache or chronic back pain Number of patients randomized to each group: unclear 2. 72 patients (9.86%) were excluded at follow‐up Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomly assigned (…)". (page 734) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "In operating room, while patients were blinded to the needle size used (…)". (page 380) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One of the nurse investigators (..), who had no knowledge of the patient´s needle assignment, made (…)" (page 1007) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9.86% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Severity of headache and back pain are mentioned, but results are not reported. Adverse events, additional to PDPH, were not reported. |
| Other bias | Unclear risk | The role of the funder in this research is unclear |
Kim 2011.
| Methods |
|
|
| Participants | 1. 53 patients who underwent elective orthopaedic knee or hip surgery under spinal anaesthesia were enrolled (age > 60 years, ASA classes I–II, recumbent in bed for the first 24 hours postoperatively, and administration of intravenous patient‐controlled analgesia for the first 48 hours postoperatively) Exclusion criteria: history of migraine headache, previous history of PDPH, cardiovascular or central nervous disease, and coagulation abnormality Patients were randomized to:
2. 3 patients (5.66%) were excluded due to severe hypotension, heart problems after operation or refusal to participate in follow‐up Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The 53 patients were randomly allocated to either the experimental group (…)" (page 1316) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.66% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Kleyweg 1995.
| Methods |
|
|
| Participants | 1. 100 patients enrolled (Dutch patients, both sexes, > 18 years of age) Patients randomized to:
2. 1 randomized patient was excluded due to:
3. 0 patients lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At random allocation a few minutes before lumbar puncture, through telephone via the trial bureau |
| Allocation concealment (selection bias) | Low risk | Allocation was controlled by a central and independent randomization unit. The allocation sequence was unknown to the investigators. |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was assessed by a medical doctor not involved in the lumbar puncture and blinded to the intervention type |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient excluded from the study |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kokki 1996.
| Methods |
|
|
| Participants | 1. 60 ASA physical status 1 and 2 children aged one to 13 years, scheduled for day case operations of the lower abdomen, genital area or lower extremities, were enrolled Patients were randomized to:
2. No patients were excluded at follow‐up 3. Main characteristics of patients:
|
|
| Interventions |
Co‐intervention: at the end of the operation the children were given ibuprofen 10 mg/kg‐1 as a suppository for pre‐emptive pain therapy |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: " The children were randomly allocated (…)" (page 116) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kokki 1998.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (ASA I‐II children, aged 2 to 128 months, and scheduled for day care surgery) Exclusion criteria: known contraindication to spinal puncture, such as an increased intracranial pressure, haemorrhagic diathesis or infection at the puncture site. Children with neurologic disorders or allergy to bupivacaine or other local anaesthetics were also excluded. Patients randomized to:
2. 5 patients randomized were excluded due to:
3. 1 patients lost to follow‐up:
4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated to receive spinal anaesthesia with either (…)" (page 1077) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The parents were blinded to the needle used." (page 1077) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kokki 1999.
| Methods |
|
|
| Participants | 1. 57 patients enrolled (ASA I‐II children, aged 8 months to 15 years, with cancer or neurological symptoms having a diagnostic and/or therapeutic LP) Exclusion criteria: unclear Patients randomized to:
2. No exclusions 3. No losses to follow‐up: 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The 53 patients were randomly allocated to either the experimental group (…)Those children having repeated LPs remained in the same needle group throughout the study" (page 1316) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were blinded to the intervention allocations. In addition, research assistants who were working as a nurse on the orthopedic nursing unit and measured postdural puncture headache and post‐operative back pain, were blinded to the intervention allocations (…)" (page 1316) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.66% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kokki 2000.
| Methods |
|
|
| Participants | 1. 215 patients enrolled (ASA I‐II children, aged 1 to 18 years, undergoing surgery below the umbilicus)
2. 1 patient randomized was excluded from the complication analysis 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation schedule was generated by a computer and concealed until the patient arrived in the operating theatre (…)" (page 211) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess this item as low or high risk |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients, parents and post‐anaesthesia care unit (PACU) nurses were unaware of the type of needle used. (…)" (page 211) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "using an open‐randomised, parallel‐groups, and prospective design (…)" (page 211) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Kuusniemi 2013.
| Methods |
|
|
| Participants | 1. 60 consecutive outpatients (ASA) physical status I–III, ages ranging between 18 and 60 years, scheduled for unilateral lower limb surgery, with spinal block being used as the sole anaesthetic without any intraoperative sedation were enrolled Exclusion criteria: previous history of intolerance to the study drug or related compounds and existing contraindications for spinal anaesthesia, patients with a body mass index (BMI) of 30 kg/m2, those with a history of alcoholism, drug abuse, or psychological or other emotional problems, patients who were pregnant or lactating Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
In both groups a 20 G introducer was applied |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "using a sealed envelope technique, the patients were randomized to two groups". (page 225) |
| Allocation concealment (selection bias) | Low risk | Quote: "using a sealed envelope technique, the patients were randomized to two groups" (page 225) |
| Blinding of participants (performance bias) | Low risk | Quote: "patients, nurses, and the anesthetist performing the motor and sensory block assessments were blinded for the spinal needle type used". (page 225) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Lavi 2006.
| Methods |
|
|
| Participants | 1. 63 patients enrolled (consecutive patients older than 18 years scheduled for a diagnostic or therapeutic lumbar puncture as a part of their routine clinical management)
2. 5 patients randomized were excluded due to:
3. 0 patients lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary A. Incidence of PDPH B. Adverse events: not reported C. Severity PDPH D. Any headache subsequent to a lumbar puncture: not reported |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to undergo LP with a standard (...).Patients were randomized only once. Therefore, those who required repeated LPs had them done with the same needle type." (page 1492) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The study was blinded to the patient. However, because the different needles have different structures, the physician knew which needle was used and could not be blinded to the needle." (page 1492) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Follow‐up was performed by a physician, blinded to the randomization, on days 2 (...)". (page 1492) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Lynch 1992a.
| Methods |
|
|
| Participants | 1. 300 patients (ASA I or II) aged 15 to 40 years (196 male, 104 female) undergoing elective orthopaedic procedures were enrolled Exclusion criteria: migraine or chronic severe headache, infection, local anaesthetic allergy or a preference for general anaesthesia Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
All punctures were done with a 20 G introducer (Braun, Germany) |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to have (…)" (page 58) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported. Presence of associated symptoms is mentioned in methods, but not reported. |
| Other bias | Low risk | No other biases were identified |
Mayer 1992.
| Methods |
|
|
| Participants | 1. 298 patients enrolled (patients consenting to spinal anaesthesia for elective and emergency caesarean section)
3. Losses to follow‐up or exclusions: not reported 2. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The needle to be used was assigned in a random manner: (…)". (page 58) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
McGann 1992.
| Methods |
|
|
| Participants | 1. 160 patients attending for myelography were included. Further details were not provided. Exclusion criteria: patients with marked obstruction of CSF flow Number of patients randomized by arm: unknown 2. 14 patients (8.75%) were excluded from analysis due to incomplete follow‐up or death Patients analysed:
3. Main characteristics of patients (in general):
|
|
| Interventions |
After the study, patients rested in bed with head elevated for 24 hours and were encouraged to consume fluids |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomized to undergo (..)". (page 1102) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients were questioned by an independent observer at 24 hours (…)". (page 1102) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Morros‐Vinoles 2002.
| Methods |
|
|
| Participants | 1. 389 patients undergoing orthopaedic surgery or general surgery were enrolled Exclusion criteria: refusal of technique, allergy to anaesthesia, neurological comorbidities or coagulation conditions Patients randomized to:
2. 12 patients (3%) were excluded from analysis, due to protocol deviations Patients analysed:
Telephone interview was complete in (lost to follow‐up: 10%):
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized into two groups (..)" (page 449) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "patients were interviewed by telephone to the second and seventh day after surgery, by an anesthesiologist unaware of who and who and how the puncture was made, and following a specific and as a template for all patients (…)" (page 450) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Muller 1994.
| Methods |
|
|
| Participants | 1. 100 consecutive patients undergoing diagnostic LP were enrolled Exclusion criteria: contraindications against any type of LP Patients randomized to:
2. 10 patients (10%) were excluded from analysis, due to protocol deviations Patients analysed:
3. Main characteristics of patients (only analysed patients):
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The study was carried out as a prospective randomized blind study on a general neurological ward (..)" (page 376) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The LP was carried out by a resident who was asked not to disclose the type of needle to the patient or to the masked examiner." (page 377) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All examinations were carried out by an examiner who was unaware of the puncture technique and observations were recorded on standardised check‐lists (…)" (page 377) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Oberoi 2009.
| Methods |
|
|
| Participants | 1. 200 patients enrolled (obstetric female patients aged 20 to 35 belonging to ASA I undergoing elective or emergency lower segment caesarean section) Patients randomized to:
2. Losses to follow‐up and exclusions were not reported 2. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly allocated to one of the two groups Q or W according to computer generated numbers". (page 420) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Post‐operatively, follow up was done up to 7 days after the surgery or till the time of discharge by an anaesthesiologist who had no knowledge of the spinal needle." (page 421) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Severity of post‐dural puncture headache was assessed according to Corbey severity grading and visual analogue scale (VAS). This information is not reported. |
| Other bias | Low risk | No other biases were identified |
Pan 2004.
| Methods |
|
|
| Participants | 1. 215 American Society of Anesthesiology Class I to II postpartum patients presenting for elective postpartum bilateral tubal ligations under spinal anaesthesia were enrolled Patients randomized to:
2. 11 patients (5.1%) were excluded from analysis, because of loss to follow‐up, cancellation of surgery or inability to identify the sub‐arachnoid space Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomized by means of a computer‐generated random number table into either" (page 360) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively, an investigator who was blinded to the group assignment interviewed the patients daily while in the hospital" (page 360) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of funder is unclear |
Pedersen 1996.
| Methods |
|
|
| Participants | 1. 107 consecutive patients (inpatient and outpatient) referred to the Department of Radiology for lumbar myelography were enrolled Number of patients randomized per arm: unclear 2. 7 patients (6.5%) were excluded from analysis because they were operated within the first 7 days or they did not returned the questionnaire 146 patients were analysed:
3. Main characteristics of patients: 58 men, 42 women, age range: 20 to 82 years, mean: 50.5 years) |
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "were randomized into two groups (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Peterman 1996.
| Methods |
|
|
| Participants | 1. 778 patients undergoing myelography from 26 April 1993 to 7 September 1994, at a large, tertiary care, academic hospital were eligible for this study Of the 778 eligible patients, 340 consented to participate in the study 340 patients were randomized to:
2. 26 patients received another needle than the randomized needle; additionally, 49 patients were not followed up. Authors include all data (as randomized) in the final analysis. 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sequential order of Whitacre and Quincke needle assignments was randomly assigned by computer in blocks of 10 to ensure an equal number of patients in each needle group." (page 772) |
| Allocation concealment (selection bias) | Low risk | Quote: "When a patient consented to enter the study, the fluoroscopy technologist received the needle assignment from the chief radiologic technologist, who kept the randomization list." (page 772) |
| Blinding of participants (performance bias) | Low risk | Quote: "There was no formal masking of the research nurses or patients; however, there was probable masking in effect. At follow‐up, most patients did not seem to know their needle group assignment." (page 772) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The principal investigator (S.B.P.), who was masked to the needle group assignment, coded the patient diagnosis by chart review." (page 772) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14.4% of patients were lost to follow‐up. However, all randomized patients were included in the analysis. |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Unclear risk | The role of the funder in this research is unclear |
Pippa 1995.
| Methods |
|
|
| Participants | 1. 160 ASA grade I or II patients undergoing orthopaedic surgery or manipulations to reduce lower limb fractures were included Exclusion criteria: patients with a history of migraine or frequent headaches and neurological problems Patients divided into 2 groups:
2. No patients reported as lost to follow‐up 3. Main characteristics of patients: not fully reported |
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly allocated, on the basis of date (but not the year) of their birth, to receive spinal anaesthesia with either (...)" (page 560) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two of the authors, who were unaware of the needle gauge employed, examined each patient on the first, second and third postoperative days and inquired about the occurrence of headache" (page 561) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were reported as lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Pittoni 1995.
| Methods |
|
|
| Participants | 1. 234 ASA I‐II outpatients undergoing elective arthroscopy of the knee joint Exclusion criteria: contraindication to regional anaesthesia Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to receive (…)" (page 73) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "and were interviewed by one of the authors (blind with respect to needle size(…)" (page 74) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Prager 1996.
| Methods |
|
|
| Participants | 1. 108 patients enrolled (patients referred for myelograms) Exclusion criteria: inability to sit or stand, inability to reliably communicate, a situation that would tend to decrease the presence and reporting of spinal headache 108 patients randomized to:
2. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "108 were randomized to a 22‐gauge" (page 1290) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An observer contacted each subject by telephone 5‐14 days after the myelogram. The observer did not know which type of needle had been used on the subjects." (page 1290) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Rafique 2014.
| Methods |
|
|
| Participants | 1. 90 patients enrolled (female patients of 20 to 38 years old, undergoing caesarian sections) Exclusion criteria: ASA above III Number of patients randomized per arm: unclear 2.Number of patients excluded (who required more than one prick): unclear. 3 patients were excluded from analysis for unknown reasons. Number of analysed patients:
3. No patients lost to follow‐up 4. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "ninety female patients of 20 to 38 years of age, undergoing caesarian sections were randomly distributed to either 25 or 27 gauge Quincke needle groups" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were interviewed first through third post‐operative days about the occurrence of headache and their satisfaction regarding spinal anesthesia." (page 1) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Rasmussen 1989a.
| Methods |
|
|
| Participants | 1. 200 admitted for elective total unilateral hip replacement were enrolled Number of patients randomized per arm: unclear 2. 17 patients (8.5%) were excluded from analysis, in the pre and postoperative period. It was impossible to perform the spinal procedure with the prescribed 25 G needle in 4 patients; 7 had an incomplete block, of whom 5 had a supplementary general anaesthetic and 2 another spinal injection; 4 had major cardiovascular complications, 1 had a classical migraine first noticed postoperatively and another needed a further spinal anaesthetic on the second postoperative day because the artificial hip dislocated. 183 patients were analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated in a double blind manner (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "Spinal anaesthesia was performed by the department anaesthetists, but did not include the authors." (page 571) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "The patients in study 1 were interviewed by one of the authors on the fourth day after surgery (...)" (page 571) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Rasmussen 1989b.
| Methods |
|
|
| Participants | 1. 200 patients aged between 20 and 40 years and admitted for either elective or acute orthopaedic, lower abdominal or urogenital surgery were enrolled Number of patients randomized per arm: unclear 2. 7 patients (3.5%) were excluded. It was impossible to perform the spinal procedure with a 25 G needle in 1 patient; 2 needed a supplementary general anaesthetic, 1 another spinal anaesthetic, while 3 had a history of migraine first noticed postoperatively 193 patients were analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated in a double blind manner (..)" (page 184) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "Spinal anaesthesia was performed by the department anaesthetists, but did not include the authors." (page 571) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The authors as well as the patients were blinded with respect to needle size" (page 571) "The patients in study 1 were interviewed by one of the authors on the fourth day after surgery (..)" (page 571) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.5% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Riley 2002.
| Methods |
|
|
| Participants | 1. 73 patients enrolled (women in active labour who requested labour analgesia and accepted a combined spinal‐epidural technique) Patients randomized to:
2. 6 (8.21%) patients lost to follow‐up because no cerebrospinal fluid was obtained with the Sprotte needle Patients analysed:
3. Main characteristics of patients were not provided. Quote: "The two groups were similar with regard to cervical dilation, parity, height, weight, and initial pain score." (page 575) |
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized to have the spinal component (..)" (page 574) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.21% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Saenghirunvattana 2008.
| Methods |
|
|
| Participants | 1. 91 patients undergoing spinal anaesthesia for operations in the departments of orthopaedics, general surgery and urology from August 2006 to October 2007 were enrolled Patients randomized to:
2. No patients were excluded from analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly allocated into (…)" (page S157) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Santanen 2004.
| Methods |
|
|
| Participants | 1. 676 outpatients (ASA physical status I‐II, aged 18 to 60 years) given spinal anaesthesia for elective day‐case surgery were enrolled Exclusion criteria: use of oral opioids or regular use of nonsteroidal anti‐inflammatory drugs, history of allergy to any study medication, patient refusal, contraindication for spinal anaesthesia, abuse of drugs or alcohol, headache preoperatively on the morning of surgery and body mass index not within normal limits (17 to 28) Number of patients randomized per arm: unclear 2. 54 patients (33 in the Quincke group and 21 in the Whitacre group) were excluded from the study for various reasons such as if they received midazolam for sedation (15/10), general anaesthesia was required because of insufficient spinal block (12/6), pethidine was required for postoperative shivering (2/2), or pain medication different from the study protocol had been given to the patient in the ward or at home (2/1) Of the remaining 622 patients, 529 patients returned the questionnaire (85.1%) and were available for the final analysis Total of exclusions: 147 (21.74%) 3. Patients analysed:
4. Main characteristics of patients:
|
|
| Interventions |
The choice of whether to use an introducer needle (22 G (0.7 mm) 30 mm long, Yale1 needle, Becton Dickinson Ltd) was left to the individual anaesthesiologist performing the spinal block. |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was computer‐generated and double‐blind except for the anaesthetist performing (…)" (page 475) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The patients, surgeons, as well as the postoperative ward personnel did not know which spinal needle had been used." (page 475) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The anaesthesiologist who analyzed patient outcome was unaware of the spinal needle type used." (page 475) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Schmittner 2010.
| Methods |
|
|
| Participants | 1. 216 patients ASA I to III, undergoing in‐house and ambulatory anorectal surgery, performed in lithotomy position, were enrolled Exclusion criteria: contraindications against spinal anaesthesia, patients considered to be ASA status IV–I, operation techniques other than in lithotomy position and prior participation in the study. After inclusion of 216 patients, the study was terminated when interim analysis showed unexpected high rates of PDPH in both study groups. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Upon arrival in the operating theatre the patients were randomly allocated 1:1 using sealed envelopes in blocks of 20 to receive a spinal saddle block with either a 25‐G or a 29‐G Quincke type spinal needle" (page 776) |
| Allocation concealment (selection bias) | Low risk | Quote: "Upon arrival in the operating theatre the patients were randomly allocated 1:1 using sealed envelopes in blocks of 20 to receive a spinal saddle block with either a 25‐G or a 29‐G Quincke type spinal needle" (page 776) |
| Blinding of participants (performance bias) | Low risk | Quote: "Study participants were blinded to the type of needle used." (page 776) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A consultant anaesthesiologist who was blinded towards the needles used and who was not involved in the study assessed the incidence of PDPH" (page 776) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Schmittner 2011.
| Methods |
|
|
| Participants | 1. 363 patients (male/female, 18 to 80 years; American Society of Anesthesiologists (ASA) physical grade I–III) undergoing in‐house and ambulatory anorectal surgery, performed in lithotomy position, were enrolled and randomized Exclusion criteria: general contraindications against spinal anaesthesia, patients' history of recurrent headaches or a previous PDPH, patients considered to be ASA grade IV–VI, operation techniques other than in lithotomy position and prior participation in the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients (in general):
Quote: "The groups did not differ in their demographic data" (page 99) |
|
| Interventions | 1. Group A: 27 G PP needle, 10 minutes pre‐operative time in upright sitting position. 27 G PP needle with introducer (Pencan ® 0.42 × 88 mm– G27 × 3½, B. Braun, Melsungen, Germany) 2. Group B: 27 G Q needle, 10 minutes pre‐operative time in upright sitting position. 27 G Q needle with introducer (Spinocan ® 0.42 × 88 mm–G27 × 3½, B‐Braun, Melsungen, Germany) 1. Group C: 27 G PP needle, 30 minutes pre‐operative time in upright sitting position. 27 G PP needle with introducer (Pencan ® 0.42 × 88 mm– G27 × 3½, B. Braun, Melsungen, Germany) 1. Group D: 27 G Q needle, 30 minutes pre‐operative time in upright sitting position. 27 G Q needle with introducer (Spinocan ® 0.42 × 88 mm–G27 × 3½, B‐Braun, Melsungen, Germany) A vertical bevel direction was used. |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "upon arrival in the operating theatre, the patients were randomised via sealed envelopes in order to assign each patient into one of four study groups" (page 98) |
| Allocation concealment (selection bias) | Low risk | Quote: "upon arrival in the operating theatre, the patients were randomised via sealed envelopes in order to assign each patient into one of four study groups" (page 98) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A consultant anaesthesiologist who was blinded towards the needles used and who was not involved in the study assessed the incidence of PDPH" (page 99) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Schultz 1996.
| Methods |
|
|
| Participants | 1. 388 ASA I‐III patients, aged 15 to 80 years, who were scheduled for subumbilical surgery, were enrolled Exclusion criteria: obstetric patients Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
Both needles were used with a 20 G introducer to facilitate puncture |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "the patients were randomly assigned" (page 462) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Sears 1994.
| Methods |
|
|
| Participants | 1. 375 ASA physical status I and II caesarean section and postpartum tubal ligation patients at 4 hospitals participated in the study Exclusion criteria: unclear Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions | 1. 22 G Sprotte needle 2. 24 G Sprotte needle All patients received an infusion of at least 1000 mL of lactated Ringer's solution over 30 minutes prior to the block |
|
| Outcomes | Outcomes were not classified as primary or secondary 1. Complication: headache 2. PDPH 3. Severity of PDPH |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly assigned to receive" (page 43) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were visited at least once by the anesthesiologist during the postoperative period, and nurses on the obstetrics floor were instructed to notify the anesthesiologist of any complication, including headache. In addition, patients were contacted by telephone 1 week or more after discharge by an investigator who was blinded to the type of needle used." (page 43) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Shah 2010.
| Methods |
|
|
| Participants | 1. 800 young patients (16 to 40 years old) with ASA risk I/II scheduled for endoscopic urological procedures under spinal anaesthesia between January 2008 and December 2009 were enrolled in this study Exclusion criteria: history of headache, use of oral opioids or non‐steroidal anti‐inflammatory drugs, or contraindications to spinal anaesthesia Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided by computer‐generated random numbers into four groups of 200 patients each." (page 25) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively, all patients were visited successively for three days by a staff member, who was unaware of the type of needle used, to inquire about headache." (page 25) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Shaikh 2008.
| Methods |
|
|
| Participants | 1. 480 American Society of Anesthesiologists physical status classification (ASA) I‐II women, aged 18 to 45 years, undergoing elective caesarean section, were enrolled Exclusion criteria: patient refusal, contraindication to spinal anaesthesia for infectious haemodynamic, haemostatic or neurological reasons, emergency caesarean section, severe pre‐eclampsia or failure of the spinal anaesthesia. Patients with more than one attempt were excluded from the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary.
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were selected randomly by balloting." (page 10) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patient, surgeon and the assessor in the ward did not know which spinal needle was used." (page 10) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively, all patients were assessed daily for 4‐days by an investigator, blinded to the type and size of the needle used.." (page 11) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Sharma 1995.
| Methods |
|
|
| Participants | 1. 96 women (ASA I and II) scheduled for elective post‐partum tubal ligation under spinal anaesthesia were enrolled Exclusion criteria: abnormal lumbar spaces due to deformities of the spine or obesity Exclusion criteria: patient refusal, contraindication to spinal anaesthesia for infectious haemodynamic, haemostatic or neurological reasons, emergency caesarean section, severe pre‐eclampsia or failure of the spinal anaesthesia. Patients with more than one attempt were excluded from the study. Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned randomly, using computer generated numbers." (page 707) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All patients were evaluated daily throughout their hospital course by an observer blinded to group assignment and then interviewed by telephone one week after discharge from hospital for the presence of headache, backache, or any other complication". (page 707) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Shutt 1992.
| Methods |
|
|
| Participants | 1. 150 women of ASA grade I undergoing spinal anaesthesia for elective caesarean section were enrolled Patients randomized to:
2. 6 patients (4%) were excluded from further analysis because of a failure to identify the subarachnoid space with the trial needle 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each woman was allocated by random number selection to one of." (page 589) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At 24 h also, the second "blind " anaesthetist visited the patient. His duty was to check that the questionnaire had been completed and to record the patient's temperature. If a headache had been reported, he completed a second questionnaire ascertaining the onset and distribution of the headache, the effect of posture and if there was any visual or auditory disturbance." (page 590) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% of patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Smith 1994.
| Methods |
|
|
| Participants | 1. 212 women of ASA grade I undergoing spinal anaesthesia for elective caesarean section were enrolled Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated to receive a subarachnoid block using either a 25G or a 27G Whitacre (...)" (page 859) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were interviewed daily on the 1st to 5th postoperative days, by an anaesthetist unaware of the needle size used (..)". (page 860) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 patients (3.7%) were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Srivastava 2010a.
| Methods |
|
|
| Participants | 1. 100 patients enrolled (either sex, age group 14 to 75, ASA I and II, admitted for elective or emergency lower segment caesarian section and other surgical procedures) Losses at follow‐up and reasons for exclusions not reported 2. Patients randomized to:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated into four groups" (page 711) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All the patients were blinded to the needle utilized. The anaesthetist conducting the procedure was not blinded as the two needles have different appearance making blinding impossible". (page 711) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Srivastava 2010b.
| Methods |
|
|
| Participants | 1. 100 patients enrolled (either sex, age group 14 to 75, ASA I and II, admitted for elective or emergency lower segment caesarian section and other surgical procedures) Losses at follow‐up and reasons for exclusions not reported 2. Patients randomized to:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomly allocated into four groups" (page 711) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "All the patients were blinded to the needle utilized. The anaesthetist conducting the procedure was not blinded as the two needles have different appearance making blinding impossible". (page 711) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Standl 2004.
| Methods |
|
|
| Participants | 1. 700 patients enrolled (ASA I/II/III patients were scheduled for lower abdominal or extremity surgery (orthopaedic, trauma, urology, visceral, gynaecology) and underwent the same protocol) Patients randomized to:
23 randomized patients (15 group B, 18 group S) were excluded due to:
2. 0 patients lost to follow‐up 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "according to a randomization protocol that was created by a computerized program for each study site". (page 513) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "During postoperative Day 2 and 4, all patients were visited by an anesthesiologist who was blinded to the type of spinal needle" (page 514) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 patients (3.2%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Strupp 2001.
| Methods |
|
|
| Participants | 1. 230 patients enrolled (who had a neurologic indication for an LP (e.g. MS, neuroborreliosis or other CNS infections), between 18 and 59 years, no recent headache (at least up to 1 week before LP, years; 2) no recent headache, i.e. at least up to 1 week), no evidence of increased intracranial pressure, no LP in the last 4 weeks, ability to be mobilized and no previous headache or other pain medication. Patients randomized to:
2. No exclusions or loses to follow‐up were reported 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were allocated randomly to one or the other group according to Efron." (page 2311) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Tabedar 2003.
| Methods |
|
|
| Participants | 1. 60 ASA I and II primi and multipara parturient undergoing elective caesarean section aged 19 to 40 years. Exclusion criteria: parturient refusal, weight more than 75 kg, eclampsia/pre‐eclampsia, bleeding disorders Patients randomized to:
2. 6 (8.21%) patients lost to follow‐up because no cerebrospinal fluid was obtained with the Sprotte needle 3. Main characteristics:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "60 ASA I and II primi and multipara parturient undergoing elective caesarean section aged 19‐40 years were randomly divided (...)" (page 264) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Tarkkila 1992.
| Methods |
|
|
| Participants | 1. 300 co‐operative ASA I and II who had spinal anaesthesia for minor orthopaedic or urologic operations, and who were not expected to need a blood transfusion Patients randomized to:
2. 256 patients (86.5%) returned the second questionnaire and were included in the study
1. Main characteristics:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomized into three groups of equal size" (page 284) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13.5% of patients lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Tarkkila 1994.
| Methods |
|
|
| Participants | 1. 300 patients undergoing surgery under spinal anaesthesia Patients randomized to:
2. Patients analysed: 2% failure rate, thus 6 patients were excluded from analysis. 94% were interviewed postoperatively. 3. Main characteristics:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Three hundred patients undergoing surgery under spinal anaesthesia were randomly allocated (...)". (page 723) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All the spinal anaesthetics were performed by the authors"... "The patients were contacted by one of the authors one week after the surgery." (page 723) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 patients (6%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Thomas 2000.
| Methods |
|
|
| Participants | 1. 116 patients enrolled (patients attending the investigation ward on a regional neurology unit for elective diagnostic lumbar puncture) Not randomized (n = 15) Consent refused (n = 8) Incomplete training for senior house officers (n = 7) 99 patients randomized to:
2. 2 patients (2%) did not receive the allocated intervention in each arm and they were excluded. 97 patients randomized to: standard needle (48, 46.56%); atraumatic needle (49, 47.53%) 6. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done by a computer generated code stored in opaque envelopes that were serially numbered and sealed" (page 987) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done by a computer generated code stored in opaque envelopes that were serially numbered and sealed" (page 987) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "One week after lumbar puncture, the patients were telephoned by a single observer who was blinded to needle allocation" (page 987) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients were lost to follow‐up (2%) |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Unclear risk | The role of funder is unclear |
Tourtellotte 1972.
| Methods |
|
|
| Participants | 1. 100 patients enrolled (healthy volunteers rated normal on physical and neurological examinations)
2. No randomized patients were excluded from the study
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Members of each successive pair of incoming volunteers were randomly" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "The subjects were blinded with respect to size of needle used. They were all interviewed by the same neurologist (W.W.T.), who was also blinded as to needle size" (page 2) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "They were all interviewed by the same neurologist (W.W.T.), who was also blinded as to needle size" (page 2) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Wiesel 1993.
| Methods |
|
|
| Participants | 1. 96 patients less than 45 years of age undergoing elective or emergency surgery were enrolled Exclusion criteria: obstetric patients Number of patients randomized to each group: unclear 2. 3 patients were excluded from further analysis due to incomplete interviews Patients analysed:
3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were randomized to receive spinal anesthesia with either the 24 gauge Sprotte needle or the 27 G Quincke needle." (page 608) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients were interviewed in person or by telephone (if discharged from the hospital) by an anesthetist not involved with the case or by a research nurse. Both were blinded to the spinal needle used" (page 608) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients were interviewed in person or by telephone (if discharged from the hospital) by an anesthetist not involved with the case or by a research nurse. Both were blinded to the spinal needle used" (page 608) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Wilkinson 1991.
| Methods |
|
|
| Participants | 1. 284 patients enrolled (patients referred for myelography)
2. 6 patients were excluded following failed lumbar puncture with 26 G needles 3. Main characteristics of patients:
|
|
| Interventions |
All lumbar punctures were performed with the patient in the left lateral decubitus position Patients were routinely ambulatory following the examination |
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "The patients were randomly assigned to the 22 G or the 26 G needle group." (page 338) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "Patients were given a questionnaire to complete on discharge from hospital 24h after the myelogram. Late complications were obtained by telephone" (page 338) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All patient‐important outcomes were reported |
| Other bias | Low risk | No other biases were identified |
Zela 1994.
| Methods |
|
|
| Participants | 1. 40 patients ASA I‐II, aged from 18 to 50 years, undergoing subumbilical surgery were enrolled Exclusion criteria: history of headache, refusal of method, hypertension Patients randomized to:
2. No patients were excluded from further analysis 3. Main characteristics of patients:
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias. Quote: "(we) developed a clinical trial with 40 patients" (page 1) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information to score this item as low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Follow‐up to detect patients who developed PDPH was realized by an anesthesiologist different from the one who performed the procedure" (page 67) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse events, additional to PDPH, were not reported |
| Other bias | Low risk | No other biases were identified |
Acronyms and abbreviations used in this table
ASA: American Society of Anesthesiologists; ASN: Atraucan spinal needle; BMI: body mass index; CI: confidence interval; c‐section: caesarean section; CSF: cerebrospinal fluid; G: gauge; IQR: interquartile range; L2‐3 to L3‐4: lumbar vertebrae 2‐3 to 3‐4; LP: lumbar puncture; NRS: numerical rating scale; NYHA: New York Heart Association; PDPH: post‐dural puncture headache; RCT: randomized controlled trial; SD: standard deviation; SEM: standard error of the mean; VAS: visual analogue scale; vs: versus; WSN: Whitacre spinal needle
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ansaloni 2000 | In this study, the authors did not evaluate the use of a specific type of needle or its gauge for the evaluation of PDPH. |
| Benedetti 1992 | This study was excluded because it was performed without any random allocation process. |
| Braune 1992 | This study is not a randomized controlled trial. |
| Browne 2005 | This study was excluded because an intervention to treat PDPH is included. |
| Carrada 1997 | This study was excluded because it was performed without any random allocation process. |
| Charuluxananan 2005 | In this study, the units of randomization were anaesthesiologists in training (learning curve) and not a specific type of needle. |
| Das‐Neves 2001 | This study was excluded because it was performed without any random allocation process. |
| Eldor 2003 | This study was excluded because it was a letter to the editor. |
| Eshuis 1995 | This study was excluded because it was a comment. |
| Flaatten 1998 | This study was excluded because the unit of randomization was the procedure and not the patient. |
| Ginosar 2012 | In this study, the authors used a pulsatile cerebrospinal fluid model to test a spinal needle. |
| Guclu 2006 | This study was excluded because it was a letter to the editor. |
| Herbstman 1998 | This study was excluded because an intervention to treat PDPH is included. |
| Huffnagle 1998 | This study was excluded because the intervention assessed in this review was not addressed. |
| Jones 1994 | This study was excluded because it did not use an adequate method of randomization (odd and even hospital record numbers). |
| Landau 2001 | This study was excluded because it was performed without any random allocation process. |
| Lynch 1992 | This study was excluded because it was performed without any random allocation process. |
| Malhotra 2007 | This study was excluded because it was performed without any random allocation process. |
| Mardirosoff 2001 | This study was excluded because it evaluated the duration of time sitting and spinal needle type on the maximal spread of local anaesthetics and not the presence of PDPH. |
| Mazze 1993 | This study was excluded because the unit of randomization was the procedure and not the patient. |
| Merlo 1989 | This study was excluded because it was performed without any random allocation process. |
| Nunes 1999 | This study was excluded because it was performed without any random allocation process. |
| Pjevic 1993 | This study is not a randomized controlled trial. |
| Quinn 2013 | This study was excluded because it was a narrative review. |
| Russell 2002 | This study was excluded because it evaluated different positions (oxford position, lateral and sitting positions) during spinal‐epidural anaesthesia and not the use of different types of needles. |
| Samayoa 2004 | This study was excluded because it was performed without any random allocation process. |
| Shah 2002 | This study is not a randomized controlled trial. |
| Sinikoglu 2013a | This study was excluded because it evaluated the effects of reinsertion of the stylet after a spinal anaesthesia procedure on PDPH and not the type or size of the needle. |
| Strupp 1998 | This study was excluded because it evaluated the effects of reinsertion of the stylet after a spinal anaesthesia procedure on PDPH and not the type or size of the needle. |
| Strupp 2009 | This study was excluded because it was a narrative review. |
| Thoren 1994 | This study was excluded because it evaluated different types of anaesthesia techniques (sequential combined spinal epidural block versus spinal block) and not different types of needles. |
| Vallejo 2000 | This study was excluded because the authors randomized the days on which each different needle would be used and not the patients. |
| Van Den Berg 2011 | This study was excluded because it was performed without any random allocation process. |
| Vilming 2001 | This study was excluded because it was performed without any random allocation process. |
| Wilhelm 1997 | This study was excluded because it was not focused on the prevention of PDPH. |
PDPH: post‐dural puncture headache
Characteristics of studies awaiting assessment [ordered by study ID]
Bano 2004.
| Methods | Single blinded, interventional, experimental study |
| Participants | A total of 100 females, aged 18 to 35 years, ASA physical status I and II, with singleton pregnancy undergoing elective or emergency caesarean section under spinal anaesthesia |
| Interventions | Participants were randomly allocated to receive spinal anaesthesia either by using 25 G Quincke or 25 G Whitacre needles. Patients were followed for 3 days postoperatively |
| Outcomes | The primary outcome was the assessment of headache and the relation to posture. Secondary outcomes were onset of headache, its duration, severity and response to the treatment |
| Notes | — |
Buttner 1990.
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 400 patients who received spinal anaesthesia for operation of the lower extremities |
| Interventions | Patients were randomly assigned to 2 groups (25 G Whitacre and a 25 G Quincke needles) and were interviewed postoperatively on days 1, 3, 5 and 7 to assess PDPH |
| Outcomes | The primary outcome was PDPH. Secondary outcomes were: duration of PDPH, non‐postural headache and the duration of non‐postural headache. |
| Notes | — |
Castrillo 2015.
| Methods | Prospective, randomized and single‐blinded clinical trial |
| Participants | Patients older than 14 years were scheduled for a diagnostic or therapeutic lumbar puncture |
| Interventions | 2 kinds of spinal needle: atraumatic or S‐type or traumatic or Q‐type |
| Outcomes | Development of PDPH according to the International Headache Association criteria |
| Notes | — |
De Andres 1994.
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 158 patients, ASA I and II, ranging in age from 20 to 40 years undergoing lower limb orthopaedic surgery |
| Interventions | Patients were randomly assigned to 2 groups (26 G Atraucan and 27 G Whitacre needles) for the realization of spinal anaesthesia |
| Outcomes | The primary outcome was: frequency and degree of PDPH. Secondary outcomes were: performance of the subarachnoid technique and intraoperative side effects. |
| Notes | — |
Fama 2015.
| Methods | Prospective, randomized, experimental study in healthy participants |
| Participants | 330 parturients scheduled for caesarean section |
| Interventions | 25, 26 or 27 G pencil point, Whitacre type (with introducer) needles |
| Outcomes | Puncture failure rates, post‐dural puncture headache |
| Notes | — |
Fyneface‐Ogan 2006.
| Methods | Prospective, single‐blind, randomized study |
| Participants | A total of 100 women undergoing elective and emergency caesarean delivery under spinal anaesthesia were recruited |
| Interventions | Patients were randomly allocated to receive spinal anaesthesia either by using 2 spinal needles (Becton Dickinson Whitacre sizes 25 G and 26 G needles) |
| Outcomes | Incidence of PDPH |
| Notes | — |
Harrison 1994.
| Methods | Randomized, prospective study |
| Participants | A total of 113 patients referred for lumbar, thoracic, cervical or total column myelography |
| Interventions | Participants were numbered sequentially; in even‐numbered patients a 22 G needle was used and for odd‐numbered patients, a 25 G needle |
| Outcomes | The primary outcome was the incidence of headache following myelography. Secondary outcomes were: the influence of needle type, sex, myelogram type and operators. |
| Notes | — |
Hong 2015.
| Methods | Prospective, randomized trial |
| Participants | 149 patients undergoing lumbar transforaminal epidural steroid injection for radicular leg pain |
| Interventions | Whitacre and Quincke type needles |
| Outcomes | After final confirmation of intravascular injection with digital subtraction angiography, total procedure time and amount of radiation exposure during the procedure were measured |
| Notes | — |
Jager 1995.
| Methods | Only title is available |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | — |
Jensen 1999.
| Methods | Prospective, randomized study |
| Participants | A total of 197 patients aged below 40 years were included in this study |
| Interventions | Participants were randomized to receive spinal analgesia using one of the following needles: Sprotte G24, Spinocan G27 or Atraucan G26 |
| Outcomes | The primary outcome of this study was the incidence of postoperative complications including post‐dural puncture headache (PDPH) |
| Notes | — |
Kaul 1996.
| Methods | Prospective, randomized study |
| Participants | A total of 90 adult patients who underwent elective surgical operations under spinal anaesthesia were evaluated |
| Interventions | Patients were randomly allocated to 3 groups of 30 each to receive spinal anaesthesia using 20‐ G, 22 G or 24‐gague spinal needles |
| Outcomes | The primary outcome was: incidence of headache and frequency of hearing loss |
| Notes | — |
Knudsen 1998.
| Methods | Prospective, randomized study |
| Participants | A total of 106 patients, aged below 40 years, scheduled for surgery in the lower part of the body were chosen for this study |
| Interventions | Patients were allocated randomly to have spinal analgesia with either a Sprotte 24 G or an Atraucan 26 G spinal needle |
| Outcomes | The primary outcome was: incidence of PDPH. Secondary outcomes were: ease of needle insertion and number of puncture attempts |
| Notes | — |
Lim 1992.
| Methods | Prospective, randomized study |
| Participants | A total of 56 patients were recruited in this study |
| Interventions | Patients underwent spinal anaesthesia for extra‐corporeal shockwave lithotripsy using either a Sprotte 24 G (n = 28) or Vygon 29 G or Quincke type needle (n = 28) |
| Outcomes | Frequency of PDPH |
| Notes | — |
Maclean 1994.
| Methods | Prospective, randomized, double‐blind study |
| Participants | A total of 60 nulliparous women |
| Interventions | Participants were randomized to receive an epidural infusion of either 0.125% plain bupivacaine or 0.0625% bupivacaine with 2.5µg/ml fentanyl |
| Outcomes | The primary outcome was pain and motor block. Secondary outcomes were maternal side effects and cardio‐tocograph abnormalities |
| Notes | — |
Mignonsin 1991.
| Methods | Prospective, controlled study |
| Participants | 30 ASA I or II patients |
| Interventions | Lumbar puncture was carried out with 26 G in group I and 18 G in group II |
| Outcomes | Complications during spinal anaesthesia included: vomiting, nausea, allergia and low blood pressure. Postspinal headache. |
| Notes | — |
Palmieri 1993.
| Methods | Prospective, randomized study |
| Participants | A total of 92 pregnant patients undergoing elective caesarean section |
| Interventions | Patients undergoing lumbar puncture were randomized to 2 groups (Group I: 22 G Quincke disposable needle and group II: 22 G Quincke reusable needle) |
| Outcomes | The primary outcome was the assessment of PDPH. There were no secondary outcomes. |
| Notes | — |
Puolakka 1997.
| Methods | Prospective follow‐up study |
| Participants | A total of 400 patients were included in this study |
| Interventions | Patients were randomly selected to have a spinal anaesthesia using either a 27 G Quincke‐type needle or a 27 G pencil point needle |
| Outcomes | The primary outcome was the severity of needle damage according to the type and number of attempts |
| Notes | — |
Vandana 2004.
| Methods | Prospective study |
| Participants | 200 patients between 18 and 45 years of age belonging to ASA grade I and II of either sex |
| Interventions | Spinal anaesthesia with 25 G or 29 G Quincke type spinal needle |
| Outcomes | Incidence, type, severity, duration, day of onset and site of post‐dural puncture headache were recorded for the first 5 postoperative days |
| Notes | — |
ASA: American Society of Anesthesiologists; G: gauge; PDPH: post‐dural puncture headache
Characteristics of ongoing studies [ordered by study ID]
Ahmed 2012.
| Trial name or title | 'Incidence and severity of post dural puncture headache after spinal anaesthesia for caesarean section; a comparison between 25G Quincke cutting and 25G Pencan pencil point spinal needles' |
| Methods | Study design: double‐blind randomized controlled trial |
| Participants | Patients and methods: 200 adult female patients aged 20 to 40 years, ASA I and II, presenting for elective or emergency caesarean deliveries under spinal anaesthesia were randomly divided into 2 groups of 100 patients each |
| Interventions | In group P, spinal anaesthesia was performed by Pencan needle while in group Q spinal anaesthesia was performed by Quincke cutting needle using a standardized technique |
| Outcomes | Level of block (sympathetic, sensory, motor) was assessed intraoperatively. Patients were followed for 3 consecutive days postoperatively for headache, its onset, severity and associated symptoms |
| Starting date | August 2009 to August 2010 |
| Contact information | Ahmed J |
| Notes | — |
Akdemir 2011.
| Trial name or title | 'The association between needle types and headache' |
| Methods | Not known |
| Participants | 664 ASA I‐II group elective caesarean patients who had no contraindications for spinal anaesthesia were included to this study. The thickness of the needle and the shape of tip of the spinal needle was recorded after anaesthesia. The education period of the anaesthesia performer, number of attempts, the space used for anaesthesia (L3‐4, LL4‐5) and movement of patient during anaesthesia were recorded |
| Interventions | Patients were randomly divided into 2 groups: group I (Atraucan 26G n = 323) and group II (Quincke 26G n = 342) |
| Outcomes | Patients were questioned about headache for 72 hours. Chi2 and comparison of proportions were used for statistical evaluations |
| Starting date | Not known |
| Contact information | AkdemirMS |
| Notes | — |
Bertolotto 2014.
| Trial name or title | 'Post‐dural puncture headache is markedly reduced when 25 Sprotte needles are used' |
| Methods | To evaluate the frequency of post‐dural puncture headache (PDPH) using 4 types of needles with a prospective, rater‐blind study |
| Participants | 365 lumbar punctures were performed using 4 different types of needles as follows: 39 with 20 G Quincke traumatic needle, 62 with 22G Sprotte needle, 133 with 25G Whitacre needle, 131 with 25G Sprotte needle |
| Interventions | 25 G Whitacre needle, 25 G Sprotte needle |
| Outcomes | The patient was blinded to the needle used; a neurologist, blinded to the type of the needle, interviewed the patient for PDPH. Safety and time consumption were evaluated. |
| Starting date | Not known |
| Contact information | Bertolotto A |
| Notes | — |
Bertolotto 2014a.
| Trial name or title | '25G Sprotte needle strongly reduces the risk of post‐lumbar puncture headache in clinical practice' |
| Methods | To evaluate the frequency of post‐lumbar puncture headache (PLPH) using 5 types of needles |
| Participants | 363 lumbar punctures were performed using 5 different types of needles as follows: 39 with 20 G Quincke traumatic needle, 11 with 22 G Quincke needle, 53 with 22 G Whitacre needle, 134 with 25 G Whitacre needle, 126 with 25 G Sprotte needle |
| Interventions | 25 G Whitacre needle, 25 G Sprotte needle |
| Outcomes | The patient was blinded to the needle used; a neurologist, blinded to the type of the needle, interviewed the patient for PLPH. Safety and time consumption were evaluated. |
| Starting date | Unclear |
| Contact information | Bertolotto A |
| Notes | — |
Bham 2010.
| Trial name or title | 'Comparison of 22/27g Microtip vs 25g Pencan spinal needle; insertion characteristic and complications' |
| Methods | Single‐blind, randomized study |
| Participants | A total of 101 parturients admitted for elective lower segment caesarian sections under spinal anaesthesia were admitted in the study |
| Interventions | Patients were randomly assigned to have Pencan (n = 50) or Microtip (n = 51) needle |
| Outcomes | The outcomes of this study were: ease of needle insertion, first attempt success rate and CSF flow rate as well as incidence of paraesthesia, post‐dural puncture headache (PDPH) and backache (PDPB), transient neurological symptoms (TNS) |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
IRCT201009292080N4.
| Trial name or title | 'Comparison of Sprotte and Quincke needles with respect to post dural puncture headache' |
| Methods | Randomization: randomized Blinding: double‐blind Placebo: not used Assignment: parallel Purpose: others. |
| Participants | Inclusion criteria: age 16 to 65, patients with major surgery of the lower limb or lower abdominal segment under spinal anaesthesia
Exclusion criteria: patients with chronic headache and drug‐induced headache Age minimum: 16 Age maximum: 65 Gender: both male and female |
| Interventions | Intervention 1: Sprotte spinal needle. Intervention 2: Quincke spinal needle |
| Outcomes | Headache Hypotension Nausea & vomiting Nuchal rigidity Time point (all outcomes): every 4 hours until 24 hours after surgery. Method of measurement (all outcomes): checklist and physical exam. |
| Starting date | 21 April 2010 |
| Contact information | Afsane Norouzi |
| Notes | — |
Lorthe 2014.
| Trial name or title | 'CSE for caesarean section: Gertie Marx versus Pencan spinal needles' |
| Methods | Compared Gertie Marx spinal needle with PENCAN needle to determine which one is preferred by obstetric patients |
| Participants | Following IRB approval and informed consent, 124 ASA I‐II parturients, who requested neuraxial block for C/S, were included. The epidural space was located with ESPOCAN 18 gauge epidural 'Braun' needle (B. Braun Medical Inc.) at L3‐4 or L4‐5 interspace with loss of resistance to air technique using a midline approach in the lateral or sitting flexed position |
| Interventions | Patients were then randomized to 1 of 2 groups. Group I: 59 patients had a 25 G PENCAN spinal needle placed in the subarachnoid space. Group II: 65 had a 26 G Gertie Marx spinal needle (IMD Inc. USA) placed in the subarachnoid space. |
| Outcomes | An investigator recorded patients' height, weight, parity, position, the distance of the epidural space from the skin, technical problems, paraesthesia and pain upon insertion of the spinal needle, time to incision, difficulty with catheter insertion, post‐dural puncture headache, transient radicular irritability, duration of procedure and overall satisfaction with the technique use |
| Starting date | Not known |
| Contact information | Lorthe J |
| Notes | — |
NCT00370604.
| Trial name or title | 'Effect of small versus large epidural needles on postdural puncture headache study' |
| Methods | Allocation: randomized Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: single‐blind (outcomes assessor) Primary purpose: prevention |
| Participants | Inclusion criteria:
‐ American Society of Anesthesiologists status 1 to 2
‐ Must have provided written informed consent = or < 6 cm cervical dilation
‐ Fetus 37 to 42 weeks gestation
‐ Must be able to read and write English well enough to provide written informed consent
Exclusion criteria:
‐ BMI = or > 40
‐ Multiple gestation pregnancy
‐ Known contraindications to use of epidural analgesia
‐ Pregnancy‐induced hypertension
‐ Investigator concern for maternal or neonatal welfare
‐ Receipt of spinal or epidural anaesthesia within 14 days of labour epidural request
‐ Women with chronic headaches (defined as headaches that occur 15 or more days per month for more than 3 months)
‐ Already participated in study
‐ History of narcotic abuse Age minimum: 18 years Age maximum: N/A Gender: female |
| Interventions | Device: => 18 G Tuohy‐type needle Device: 19 G Tuohy‐type epidural needle, 23 G catheter |
| Outcomes | Incidence of post‐dural puncture headache (time frame: within the first 14 days of epidural placement) Anaesthesiologist satisfaction with the 19 G Tuohy epidural needle and 23 G catheter compared with traditional Tuohy‐type epidural needles and traditional catheters (time frame: during labour and delivery) Degree of dysfunction and disability related to PDPH symptoms (time frame: within first 14 days post‐epidural placement and, if necessary, up to 1 year post‐epidural placement) |
| Starting date | June 2007 |
| Contact information | Pamela J Angle |
| Notes | — |
NCT01821807.
| Trial name or title | 'Comparison of two spinal needles regarding postdural puncture headache' |
| Methods | Time perspective: prospective |
| Participants | Inclusion Criteria:
‐ Pregnant female patients between 18‐40 years old undergoing caesarean section
‐ Patient accepting spinal anaesthesia
Exclusion Criteria:
‐ Infection at the spinal needle insertion cite
‐ Coagulability disorder
‐ Patient not accepting the procedure Age minimum: 18 Years Age maximum: 40 Years Gender: Female |
| Interventions | Two kind of spinal anaesthesia needles will be used:
|
| Outcomes | Post‐dural puncture headache in patients receiving spinal anaesthesia for caesarean section (time frame: 1 week) Backache in patients receiving spinal anaesthesia for caesarean section (time frame: 1 week) |
| Starting date | June 2013 |
| Contact information | Ruslan Abdullayev |
| Notes | — |
NCT02384031.
| Trial name or title | 'Post‐dural puncture headache ‐ needles and biomarkers in CSF' |
| Methods | Allocation: randomized Intervention model: parallel assignment Masking: double‐blind (subject, investigator) Primary purpose: prevention |
| Participants | Inclusion criteria:
1. Patients at Department of Neurology, Nordland Hospital Trust in Bodø, scheduled for diagnostic LP
Exclusion criteria:
1. Dementia
2. Non‐compliance or coma
3. Local skin infections over proposed puncture site
4. Suspicion of raised intracranial pressure due to neurological or radiological findings
5. Bleeding diathesis (thrombocytopenia < 50 x 109/L) or ongoing anticoagulant therapy
6. Major spinal column deformities
7. Procedural complications whereby needle type or size change is a requisite
8. Recent LP (< 7 days) Age minimum: 18 years Age maximum: 60 years Gender: both |
| Interventions | Device: atraumatic needle Device: traumatic needle |
| Outcomes | Post‐dural puncture headache (PDPH) (time frame: at day 7 post LP) Levels of inflammatory mediators in CSF (time frame: during lumbar puncture) Levels of metabolites in CSF (time frame: during lumbar puncture) Levels of neuropeptides in CSF (time frame: during lumbar puncture) |
| Starting date | February 2012 |
| Contact information | Francis Odeh, MD, PhD |
| Notes | — |
Shah 2011.
| Trial name or title | 'Combined spinal epidural (CSE) for cesarean section: Gertie Marx versus Pencan spinal needles' |
| Methods | Prospective, randomized study |
| Participants | A total of 124 ASA I‐II parturients who requested neuraxial block for caesarean section were included in this study |
| Interventions | Patients were randomized into 2 groups (Group I: n = 59 has a 25 G PENCAN spinal needle placed in the subarachnoid space and Group II: n = 65 had a 26 G Gertie Marx spinal needle in the subarachnoid space |
| Outcomes | Need to rotate or reinsert the epidural needle, the efficacy of the block, side effects from the block, difficulty with catheter insertion and the sensory level overall satisfaction |
| Starting date | Not known |
| Contact information | Not known |
| Notes | — |
Shaikh 2013.
| Trial name or title | 'Post dural puncture headache after spinal anaesthesia for caesarean section: a comparison of 25G Quincke, 27G Quincke and 27G Whitacre spinal needles' |
| Methods | Comparative, randomized, double‐blind, interventional study |
| Participants | A total of 480 ASA I‐II full‐term pregnant women, 18 to 45 years of age, scheduled for elective caesarean section, under spinal anaesthesia |
| Interventions | Participants were randomized into 3 groups: Group I (25 G Quincke spinal needle: n = 168), Group II (27 G Quincke spinal needle: n = 160) and Group III (27 G Whitacre spinal needle: n = 152) |
| Outcomes | The primary outcome was the frequency of PDPH. Secondary outcomes were the severity and onset of PDPH. |
| Starting date | From October 2005 to December 2006 |
| Contact information | Not known |
| Notes | — |
Acronyms and abbreviations used in this table
ASA: American Society of Anesthesiologists; ASN: Atraucan spinal needle; BMI: body mass index; CI: confidence interval; c‐section: caesarean section; CSF: cerebrospinal fluid; G: gauge; IQR: interquartile range; L2‐3 to L3‐4: lumbar vertebrae 2‐3 to 3‐4; LP: lumbar puncture; NRS: numerical rating scale; NYHA: New York Heart Association; PDPH: post‐dural puncture headache; RCT: randomized controlled trial; SD: standard deviation; SEM: standard error of the mean; VAS: visual analogue scale; WSN: Whitacre spinal needle
Differences between protocol and review
We made the following changes to the published protocol (Arevalo‐Rodriguez 2013a).
Due to heterogeneity in the reporting of adverse events, we chose paraesthesia and backache as the most important adverse events(additional to PDPH) related to needle gauge and tip designs. We extracted all numerical information related to these two events and we reported the results in the corresponding sections.
In order to make a comprehensive 'Risk of bias' assessment, we considered seven domains (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias) instead of the six domains planned in our protocol (Arevalo‐Rodriguez 2013a). However, we did not consider blinding of personnel because of the nature of the intervention (lumbar puncture).
We did not expect to encounter any unit of analysis issues, as we do not expect to find cross‐over studies or cluster‐randomized trials. However, we did identify four such studies (one cross‐over trial and three parallel‐group studies with punctures instead of patients as the unit of analysis) with our search strategies. We included these trials in our review in the qualitative report, but we did not include their results in our main analyses.
Subgroup analysis for age (younger than 18 years of age, older than 65 years of age and 18 to 65 years of age). Due to heterogeneity in the reporting of age, we classified studies into three groups: a) only children; b) no distinctions about age; c) 60 years or more. We analysed the numerical information into these three categories.
Subgroup analysis by type of surgery: in participants receiving anaesthesia, we analysed the primary outcome by type of surgical procedure in order to explain all sources of heterogeneity. We identified at least three groups: caesarean section, orthopaedic surgeries and other surgeries. It has been reported that some subgroups of patients, such as obstetric women, have an increased risk of PDPH.
We did not use number needed to treat to harm (NNTH) figures to illustrate the harms or benefits of interventions, taking into account the quality of evidence and its limitations.
In order to consider all possible studies, we performed a sensitivity analysis to measure the risk difference (RD) in those analyses that presented zero events in both treatment arms; they were then not included in the risk ratio analysis.
Contributions of authors
Ingrid Arevalo‐Rodriguez: (IA‐R), Luis Muñoz (LM), Natalia Godoy‐Casasbuenas (NG‐C), Jimmy J Arevalo (JJA), Sabine Boogaard (SB), Agustín Ciapponi (AC), Marta Roqué i Figul (MRF)
Conceiving the review: IA‐R, LM
Designing the review: IA‐R, LM, JJA, AC, MRF
Co‐ordinating the review: IA‐R
Undertaking manual searches: IA‐R, NG‐C
Screening search results: IA‐R, LM, JJA, NG‐C
Organizing retrieval of papers: LM, NG‐C
Screening retrieved papers against inclusion criteria: IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Appraising quality of papers: IA‐R, LM, SB, AC, MRF, NG‐C
Abstracting data from papers: LM, JJA, NG‐C, IAR
Writing to authors of papers for additional information: IAR
Providing additional data about papers: IA‐R, AC
Obtaining and screening data on unpublished studies: IA‐R, LM
Providing data management for the review: RM, IAR
Entering data into Review Manager (RevMan 5.3): IA‐R, LM, NG‐C
Managing RevMan statistical data: MRF, IAR
Performing other statistical analyses not using RevMan: MR
Ensuring double entry of data (data entered by person one: MRF; data entered by person two: IA‐R)
Interpreting data: IA‐R, LM, AC, MRF, NG‐C
Making statistical inferences: IA‐R, LM, AC, MRF
Writing the review: IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Providing guidance on the review: AC, MRF
Securing funding for the review: IA‐R
Performing previous work that served as the foundation of the present study: IA‐R, LM, AC, MRF
Serving as guarantor for the review (one author): IA‐R
Taking responsibility for reading and checking the review before submission:IA‐R, LM, JJA, AC, MRF, NG‐C, SB
Sources of support
Internal sources
Fundación Universitaria de Ciencias de la Salud, Bogotá D.C., Colombia.
Institute for Clinical Effectiveness and Health Policy IECS, Buenos Aires, Argentina.
Iberoamerican Cochrane Centre, Barcelona, Spain.
-
Universidad El Bosque, Bogotá, Colombia.
Luis Muñoz is a Master of Science degree student at the Department of Clinical Epidemiology of El Bosque University, Bogotá, Colombia.
External sources
Agencia de Calidad del Sistema Nacional de Salud, Ministry of Health, Spain.
Declarations of interest
Ingrid Arevalo‐Rodriguez: none known.
Luis Muñoz: none known.
Natalia Godoy‐Casasbuenas: none known.
Jimmy J Arevalo: none known.
Sabine Boogaard: none known
Agustín Ciapponi: none known.
Marta Roqué i Figuls: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Amuzu 1995 {published data only}
- Amuzu J, Patel S, Maitra‐D'Cruze A. Incidence of postdural puncture headache after Cesarean section: comparison of 26g Atraucan and 25g Whitacre spinal needles. Regional Anesthesia 1995;20(2S):150. [Google Scholar]
Brattebo 1995 {published data only}
- Brattebo G, Wisborg T, Rodt S A, Roste I. Is the pencil point spinal needle a better choice in younger patients? A comparison of 24G Sprotte with 27G Quincke needles in an unselected group of general surgical patients below 46 years of age. Acta Anaesthesiologica Scandinavica 1995;39(4):535‐8. [PUBMED: 7676793] [DOI] [PubMed] [Google Scholar]
Buettner 1993 {published data only}
- Buettner J, Wresch K P, Klose R. Postdural puncture headache: comparison of 25‐gauge Whitacre and Quincke needles. Regional Anesthesia 1993;18(3):166‐9. [PUBMED: 8323889 ] [PubMed] [Google Scholar]
Campbell 1993 {published data only}
- Campbell DC, Douglas MJ, Pavy TJ, Merrick P, Flanagan ML, McMorland GH. Comparison of the 25‐gauge Whitacre with the 24‐gauge Sprotte spinal needle for elective caesarean section: cost implications. Canadian Journal of Anaesthesia 1993;40(12):1131‐5. [PUBMED: 8281588 ] [DOI] [PubMed] [Google Scholar]
Chaudhry 2011 {published data only}
- Chaudhry MA, Ahmad RZ, Qureshi ZA. Postdural puncture headache comparative study between 25 guage pencil point needle and 25 gauge quincky needle. Pakistan Journal of Medical and Health Sciences 2011;5(1):50‐4. [EMBASE: 2012548141] [Google Scholar]
Corbey 1997 {published data only}
- Corbey MP, Bach AB, Lech K, Frorup AM. Grading of severity of postdural puncture headache after 27‐gauge Quincke and Whitacre needles. Acta Anaesthesiologica Scandinavica 1997;41:779‐84. [PUBMED: 9241342] [DOI] [PubMed] [Google Scholar]
Crock 2014 {published data only}
- Crock C, Orsini F, Lee KJ, Phillips RJ. Headache after lumbar puncture: randomised crossover trial of 22‐gauge versus 25‐gauge needles. Archives of Disease in Childhood 2014;99(3):203‐7. [PUBMED: 24233069] [DOI] [PubMed] [Google Scholar]
De Andres 1999 {published data only}
- Andres J, Valia JC, Errando C, Rico G, Lopez‐Alarcon MD. Subarachnoid anesthesia in young patients: a comparative analysis of two needle bevels. Regional Anesthesia and Pain Medicine 1999;24:547‐52. [PUBMED: 10588560] [DOI] [PubMed] [Google Scholar]
Despond 1998 {published data only}
- Despond O, Meuret P, Hemmings G. Postdural puncture headache after spinal anaesthesia in young orthopaedic outpatients using 27‐g needles. Canadian Journal of Anaesthesia 1998;45:1106‐9. [PUBMED: 10021962] [DOI] [PubMed] [Google Scholar]
Devcic 1993 {published data only}
- Devcic A, Maitra‐D'Cruze A, Sprung J, Patel S, Kettler R. PDPH in obstetric anesthesia: comparison of 24‐gauge Sprotte and 25‐gauge Quincke needles and effect of subarachnoid administration of fentanyl. Regional Anesthesia 1993;18:222‐5. [PUBMED: 8398955 ] [PubMed] [Google Scholar]
Fernandez 1993 {published data only}
- Fernandez Lasalde FD. Post dural punction migrane: a comparative study of its effects using needles of the Quincke and Whitacre type [Cefalea post punción dural: estudio comparativo de su incidencia utilzando agujas tipo Quincke y Whitacre]. Revista Argentina de Anestesiologia 1993;51(4):241‐4. [172411] [Google Scholar]
Fernandez 2003 {published data only}
- Diego‐Fernandez R, Tisner Madrid ML, Cabrerizo Torrente P, Sanjoaquin Mur T. Comparison of two 27‐G‐caliber needles for spinal anesthesia. Study of 1,555 patients [Comparación de dos agujas de calibre 27G para anestesia raquídea.Estudio sobre 1.555 pacientes]. Revista Española de Anestesiología y Reanimación 2003;50(4):182‐7. [ibc‐28291] [PubMed] [Google Scholar]
Flaatten 2000 {published data only}
- Flaatten H, Felthaus J, Kuwelker M, Wisborg T. Postural post‐dural puncture headache. A prospective randomised study and a meta‐analysis comparing two different 0.40 mm O.D. (27 g) spinal needles. Acta Anaesthesiologica Scandinavica 2000;44(6):643‐7. [PUBMED: 10903010] [DOI] [PubMed] [Google Scholar]
Fox 1996 {published data only}
- Fox RGT, Reiche W, Kiefer M, Hagen T, Huber G. The influence of an atraumatic needle with a Sprotte bevel and a needle with a Quincke bevel on the incidence of complaints following myelography after lumbar puncture [Inzidenz des Postmyelographiesyndroms (PMS) und postmyelographischer Beschwerden nach lumbaler Punktion mit der bleistiftformigen Nadel nach Sprotte im Vergleich zur Nadel nac Quincke]. Radiologe 1996;36(11):921‐7. [PUBMED: 9036434] [DOI] [PubMed] [Google Scholar]
Geurts 1990 {published data only}
- Geurts JW, Haanschoten MC, Wijk RM, Kraak H, Besse T C. Post‐dural puncture headache in young patients. A comparative study between the use of 0.52 mm (25‐gauge) and 0.33 mm (29‐gauge) spinal needles. Acta Anaesthesiologica Scandinavica 1990;34(5):350‐3. [PUBMED: 2143882] [DOI] [PubMed] [Google Scholar]
Gonzalez 2000 {published data only}
- González Santillán JM, Cedillo Maguey A, Cárdenas Jurado J, Gómez Ortiz I, Cortés Rosas NE. Post puncture headache in young ambulatory patients, comparing two different kinds of spinal anesthesia needles for lower limb surgery [Cefalea postpunción en pacientes jóvenes y "ambulatorios", comparando dos tipos de agujas para anestesia espinal en cirugía de extremidad inferior]. Revista Mexicana de Anestesiología 2000;23(4):161‐6. [304288] [Google Scholar]
Grover 2002 {published data only}
- Grover VK, Bala I, Mahajan R, Sharma S. Post‐dural puncture headache following spinal anaesthesia: comparison of 25g vs 29g spinal needles. Bahrain Medical Bulletin 2002;24(4):131‐4. [EMBASE: 2003022034] [Google Scholar]
Hafer 1997 {published data only}
- Hafer J, Rupp D, Wollbrück M, Engel J, Hempelmann G. The effect of needle type and immobilization on postspinal headache [Die Bedeutung von Nadeltypund Immobilisation für denpostspinalen Kopfschmerz]. Der Anaesthesist 1997;46(10):860‐6. [PUBMED: 9424969 ] [DOI] [PubMed] [Google Scholar]
Harrison 1993 {published data only}
- Harrison PB. The contribution of needle size and other factors to headache following myelography. Neuroradiology 1993;35(7):487‐9. [PUBMED: 8232869] [DOI] [PubMed] [Google Scholar]
Hopkinson 1997 {published data only}
- Hopkinson JM, Samaan AK, Russell IF, Birks RJS, Patrick MR. A comparative multicentre trial of spinal needles for Caesarean section. Anaesthesia 1997;52(10):1005‐11. [PUBMED: 9370847] [DOI] [PubMed] [Google Scholar]
Imarengiaye 2002 {published data only}
- Imarengiaye CO, Edomwonyi NP. Evaluation of 25‐gauge Quincke and 24‐gauge Gertie Marx needles for spinal anaesthesia for caesarean section. East African Medical Journal 2002;79(7):379‐81. [PUBMED: 12638834] [DOI] [PubMed] [Google Scholar]
Imbelloni 1997 {published data only}
- Imbelloni LE. Whitacre and 26G Atraucan needles in patients under 50 years [Comparaçäo entre agulha 27G Whitacre com 26G Atraucan para cirurgias eletivas em pacientes abaixo de 50 anos]. Revista Brasileira de Anestesiologia 1997;47(4):288‐96. [198071] [Google Scholar]
- Imbelloni LE, Carneiro AN. Is the Huber point needle a better choice for young patients? a comparison of 26G Atraucan with 27G Quincke needles in general surgical patients under 50 years [É a agulha ponta de huber a melhor escolha em pacientes jovens? comparaçäo entre agulha 26G atraucan com 27G quincke para cirurgias em pacientes abaixo de 50 anos]. Revista Brasileira de Anestesiologia 1997;47(5):408‐16. [238812] [Google Scholar]
Kang 1992 {published data only}
- Kang SB, Goodnough DE, Lee YK, Olson RA, Borshoff JA, Furlano MM, et al. Comparison of 26‐ and 27‐G needles for spinal anesthesia for ambulatory surgery patients. Anesthesiology 1992;76(5):734‐8. [PUBMED: 1575341] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim M, Yoon H. Comparison of post‐dural puncture headache and low back pain between 23 and 25 gauge Quincke spinal needles in patients over 60 years: randomized, double‐blind controlled trial. International Journal of Nursing Studies 2011;48(11):1315‐22. [PUBMED: 21561619] [DOI] [PubMed] [Google Scholar]
Kleyweg 1995 {published data only}
- Kleyweg RP, Hertzberger LI, Carbaat PA. Less headache following lumbar puncture with the use of an atraumatic needle; double‐blind randomized study. Nederlands Tijdschrift voor Geneeskunde 1995;139(5):232‐4. [PUBMED: 7854485 ] [PubMed] [Google Scholar]
- Kleyweg RP, Hertzberger LI, Carbaat PAT. Significant reduction in post lumbar puncture headache using an atraumatic needle: a double‐blind controlled clinical trial. Journal of the Neurological Sciences 1997;18(9):S304. [DOI] [PubMed] [Google Scholar]
- Kleyweg RP, Hertzberger LI, Carbaat PAT. Significant reduction in post‐lumbar puncture headache using an atraumatic needle. A double‐blind, controlled clinical trial. Cephalalgia 1998;18(9):635‐7. [PUBMED: 9876888] [DOI] [PubMed] [Google Scholar]
Kokki 1996 {published data only}
- Kokki H, Hendolin H. Comparison of 25 G and 29 G Quincke spinal needles in paediatric day case surgery. A prospective randomized study of the puncture characteristics, success rate and postoperative complaints. Paediatric Anaesthesia 1996;6(2):115‐9. [PUBMED: 8846276] [DOI] [PubMed] [Google Scholar]
Kokki 1998 {published data only}
- Kokki H, Hendolin H, Turunen M. Postdural puncture headache and transient neurologic symptoms in children after spinal anaesthesia using cutting and pencil point paediatric spinal needles. Acta Anaesthesiologica Scandinavica 1998;42(9):1076‐82. [PUBMED: 9809091] [DOI] [PubMed] [Google Scholar]
Kokki 1999 {published data only}
- Kokki H, Salonvaara M, Herrgard E, Riikonen P. Postdural puncture headache is not an age‐related symptom in children: a prospective, open‐randomized, parallel group study comparing a 22‐gauge Quincke with a 22‐gauge Whitacre needle. Paediatric Anaesthesia 1999;9(5):429‐34. [PUBMED: 10447907 ] [DOI] [PubMed] [Google Scholar]
Kokki 2000 {published data only}
- Kokki H, Heikkinen M, Turunen M, Vanamo K, Hendolin H. Needle design does not affect the success rate of spinal anaesthesia or the incidence of postpuncture complications in children. Acta Anaesthesiologica Scandinavica 2000;44(2):210‐3. [PUBMED: 10695916] [DOI] [PubMed] [Google Scholar]
Kuusniemi 2013 {published data only}
Lavi 2006 {published data only}
- Lavi R, Yernitzky D, Rowe JM, Weissman A, Segal D, Avivi I. Standard vs atraumatic Whitacre needle for diagnostic lumbar puncture: a randomized trial. Neurology 2006;67(8):1492‐4. [PUBMED: 17060584] [DOI] [PubMed] [Google Scholar]
Lynch 1992a {published data only}
- Lynch J, Arhelger S, Krings‐Ernst I. Post‐dural puncture headache in young orthopaedic in‐patients: comparison of a 0.33 mm (29‐gauge) Quincke‐type with a 0.7 mm (22‐gauge) Whitacre spinal needle in 200 patients. Acta Anaesthesiologica Scandinavica 1992;36(1):58‐61. [PUBMED: 1539481 ] [DOI] [PubMed] [Google Scholar]
Mayer 1992 {published data only}
- Mayer DC, Quance D, Weeks SK. Headache after spinal anesthesia for cesarean section: a comparison of the 27‐gauge Quincke and 24‐gauge Sprotte needles. Anesthesia and Analgesia 1992;75(3):377‐80. [PUBMED: 1510258] [DOI] [PubMed] [Google Scholar]
McGann 1992 {published data only}
- McGann GM, Gleeson FV, Kelly I, Valentine AR, Platts A, Butler P, et al. The influence of needle size on post‐myelography headache: a controlled trial. British Journal of Radiology 1992;65(780):1102‐4. [PUBMED: 1286418] [DOI] [PubMed] [Google Scholar]
Morros‐Vinoles 2002 {published data only}
- Morros‐Vinoles C, Perez‐Cuenca MD, Cedo‐Lluis E, Colls C, Bueno J, Cedo‐Valloba F. Comparison of efficacy and complications of 27G and 29G Sprotte needles for subarachnoid anesthesia. Revista Española de Anestesiologia y Reanimacion 2002;49(9):448‐54. [PUBMED: 12516488 ] [PubMed] [Google Scholar]
Muller 1994 {published data only}
- Muller B, Adelt K, Reichmann H, Toyka K. Atraumatic needle reduces the incidence of post‐lumbar puncture syndrome. Journal of Neurology 1994;241(6):376‐80. [PUBMED: 7931432 ] [DOI] [PubMed] [Google Scholar]
Oberoi 2009 {published data only}
- Oberoi R, Kaul TK, Singh MR, Grewal A, Dhir R. Incidence of post dural puncture headache: 25 gauge Quincke vs 25 gauge Whitacre needles. Journal of Anaesthesiology Clinical Pharmacology 2009;25(4):420‐2. [EMBASE: 2009564360] [Google Scholar]
Pan 2004 {published data only}
- Pan PH, Fragneto R, Moore C, Ross V. Incidence of postdural puncture headache and backache, and success rate of dural puncture: comparison of two spinal needle designs. Southern Medical Journal 2004;97(4):359‐63. [PUBMED: 15108829 ] [DOI] [PubMed] [Google Scholar]
- Pan PH, Fragneto R, Moore C, Ross V, Justis G. The incidence of failed spinal anesthesia, postdural puncture headache and backache is similar with Atraucan and Whitacre spinal needles. Canadian Journal of Anesthesia 2002;49(6):636‐7. [PUBMED: 12067883 ] [DOI] [PubMed] [Google Scholar]
Pedersen 1996 {published data only}
- Pedersen ON. Use of a 22‐gauge Whitacre needle to reduce the incidence of side effects after lumbar myelography: a prospective randomised study comparing Whitacre and Quincke spinal needles. European Radiology 1996;6(2):184‐7. [PUBMED: 8797976 ] [DOI] [PubMed] [Google Scholar]
Peterman 1996 {published data only}
- Peterman SB. Postmyelography headache rates with Whitacre versus Quincke 22‐gauge spinal needles. Radiology 1996;200(3):771‐8. [PUBMED: 8756930] [DOI] [PubMed] [Google Scholar]
Pippa 1995 {published data only}
- Pippa P, Barbagli R, Rabassini M, Doni L, Rucci FS. Postspinal headache in Taylor's approach: a comparison between 21‐ and 25‐gauge needles in orthopaedic patients. Anaesthesia and Intensive Care 1995;23(5):560‐3. [PUBMED: 8787254 ] [DOI] [PubMed] [Google Scholar]
Pittoni 1995 {published data only}
- Pittoni G, Toffoletto F, Calcarella G, Zanette G, Giron G P. Spinal anesthesia in outpatient knee surgery: 22‐gauge versus 25‐gauge Sprotte needle. Anesthesia and Analgesia 1995;81(1):73‐9. [PUBMED: 7598286 ] [DOI] [PubMed] [Google Scholar]
Prager 1996 {published data only}
- Prager JM, Roychowdhury S, Gorey MT, Lowe GM, Diamond CW, Ragin A. Spinal headaches after myelograms: comparison of needle types. American Journal of Roentgenology 1996;167(5):1289‐92. [PUBMED: 8911197] [DOI] [PubMed] [Google Scholar]
Rafique 2014 {published data only}
- Rafique K, Saeed M, Chaudhry IA, Mahmood S, Tamur M, Sadiq T, et al. Post spinal headache (PDPH), spinal headache, spinal needle. Pakistan Journal of Medical and Health Sciences 2014; Vol. 8, issue 3:774‐7. [EMBASE: 2014808829]
Rasmussen 1989a {published data only}
- Rasmussen BS, Blom L, Hansen P, Mikkelsen SS. Postspinal headache in young and elderly patients. Two randomised, double‐blind studies that compare 20‐ and 25‐gauge needles. Anaesthesia 1989;44:571‐3. [DOI] [PubMed] [Google Scholar]
Rasmussen 1989b {published data only}
- Rasmussen BS, Blom L, Hansen P, Mikkelsen SS. Postspinal headache in young and elderly patients. Two randomised, double‐blind studies that compare 20‐ and 25‐gauge needles. Anaesthesia 1989;44:571‐3. [DOI] [PubMed] [Google Scholar]
Riley 2002 {published data only}
- Riley ET, Hamilton CL, Ratner EF, Cohen SE. A comparison of the 24‐gauge Sprotte and Gertie Marx spinal needles for combined spinal‐epidural analgesia during labor. Anesthesiology 2002;97(3):574‐7. [PUBMED: 12218522] [DOI] [PubMed] [Google Scholar]
Saenghirunvattana 2008 {published data only}
- Saenghirunvattana R, Tantivitayatan K, Chumnanvech W, Tangsukkasemsun S, Siritongtaworn P. A comparison study between newly‐designed pencil‐point and cutting needles in spinal anesthesia. Journal of the Medical Association of Thailand 2008;91(Suppl 1):S156‐61. [PUBMED: 18672608 ] [PubMed] [Google Scholar]
Santanen 2004 {published data only}
- Santanen U, Rautoma P, Luurila H, Erkola O, Pere P. Comparison of 27‐gauge (0.41‐mm) Whitacre and Quincke spinal needles with respect to post‐dural puncture headache and non‐dural puncture headache. Acta Anaesthesiologica Scandinavica 2004;48(4):474‐9. [PUBMED: 15025611] [DOI] [PubMed] [Google Scholar]
Schmittner 2010 {published data only}
- Schmittner MD, Terboven T, Dluzak M, Janke A, Limmer ME, Weiss C, et al. High incidence of post‐dural puncture headache in patients with spinal saddle block induced with Quincke needles for anorectal surgery: a randomised clinical trial. International Journal of Colorectal Disease 2010;25(6):775‐81. [PUBMED: 20148254 ] [DOI] [PubMed] [Google Scholar]
Schmittner 2011 {published data only}
- Schmittner M, Dluzak M, Janke A, Bussen D, Beck G. Comparison of 29‐ and 25‐gauge Quincke spinal needles for patients undergoing anorectal surgery in saddle block technique. European Journal of Anaesthesiology 2009;26(Suppl 45):21. [Google Scholar]
- Schmittner MD, Urban N, Janke A, Weiss C, Bussen DG, Burmeister MA, et al. Influence of the pre‐operative time in upright sitting position and the needle type on the incidence of post‐dural puncture headache (PDPH) in patients receiving a spinal saddle block for anorectal surgery. International Journal of Colorectal Disease 2011;26(1):97‐102. [PUBMED: 20652572 ] [DOI] [PubMed] [Google Scholar]
Schultz 1996 {published data only}
- Schultz AM, Ulbing S, Kaider A, Lehofer F. Postdural puncture headache and back pain after spinal anesthesia with 27‐gauge Quincke and 26‐gauge Atraucan needles. Regional Anesthesia 1996;21(5):461‐4. [PUBMED: 8896009 ] [PubMed] [Google Scholar]
Sears 1994 {published data only}
- Sears DH, Leeman MI, Jassy LJ, O'Donnell LA, Allen SG, Reisner LS. The frequency of postdural puncture headache in obstetric patients: a prospective study comparing the 24‐gauge versus the 22‐gauge Sprotte needle. Journal of Clinical Anesthesia 1994;6(1):42‐6. [PUBMED: 8142098 ] [DOI] [PubMed] [Google Scholar]
Shah 2010 {published data only}
- Shah VR, Bhosale GP. Spinal anaesthesia in young patients: evaluation of needle gauge and design on technical problems and postdural puncture headache. Southern African Journal of Anaesthesia and Analgesia 2010;16(3):24‐8. [EMBASE: 2010571960] [Google Scholar]
Shaikh 2008 {published data only}
- Shaikh JM, Memon A, Memon MA, Khan M. Post dural puncture headache after spinal anaesthesia for caesarean section: a comparison of 25 g Quincke, 27 g Quincke and 27 g Whitacre spinal needles. Journal of Ayub Medical College 2008;20(3):10‐3. [PUBMED: 19610505] [PubMed] [Google Scholar]
Sharma 1995 {published data only}
- Sharma SK, Gambling DR, Joshi GP, Sidawi JE, Herrera ER. Comparison of 26‐gauge Atraucan and 25‐gauge Whitacre needles: insertion characteristics and complications. Canadian Journal of Anaesthesia 1995;42(8):706‐10. [PUBMED: 7586110 ] [DOI] [PubMed] [Google Scholar]
Shutt 1992 {published data only}
- Shutt LE, Valentine SJ, Wee MYK, Page RJ, Prosser A, Thomas TA. Spinal anaesthesia for Caesarean section: comparison of 22‐gauge and 25‐gauge Whitacre needles with 26‐gauge Quincke needles. British Journal of Anaesthesia 1992;69(6):589‐94. [PUBMED: 1467102] [DOI] [PubMed] [Google Scholar]
Smith 1994 {published data only}
- Smith EA, Thorburn J, Duckworth RA, Reid JA. A comparison of 25 g and 27 g Whitacre needles for Caesarean section. Anaesthesia 1994;49(10):859‐62. [PUBMED: 7802179 ] [DOI] [PubMed] [Google Scholar]
Srivastava 2010a {published data only}
- Srivastava V, Jindal P, Sharma J P. Study of post dural puncture headache with 27g Quincke & Whitacre needles in obstetrics/non obstetrics patients. Middle East Journal of Anesthesiology 2010;20(5):709‐18. [PUBMED: 20803861] [PubMed] [Google Scholar]
Srivastava 2010b {published data only}
- Srivastava V, Jindal P, Sharma J P. Study of post dural puncture headache with 27g Quincke & Whitacre needles in obstetrics/non obstetrics patients. Middle East Journal of Anesthesiology 2010;20(5):709‐18. [PUBMED: 20803861] [PubMed] [Google Scholar]
Standl 2004 {published data only}
- Standl T, Stanek A, Burmeister MA, Gruschow S, Wahlen B, Muller K, et al. Spinal anesthesia performance conditions and side effects are comparable between the newly designed Ballpen and the Sprotte needle: results of a prospective comparative randomized multicenter study. Anesthesia and Analgesia 2004;98(2):512‐7. [PUBMED: 14742396] [DOI] [PubMed] [Google Scholar]
Strupp 2001 {published data only}
- Strupp M, Schueler O, Straube A, Stuckrad‐Barre S, Brandt T. "Atraumatic" Sprotte needle reduces the incidence of post‐lumbar puncture headaches. Neurology 2001;57(12):2310‐2. [PUBMED: 11756618 ] [DOI] [PubMed] [Google Scholar]
Tabedar 2003 {published data only}
- Tabedar S, Maharjan SK, Shrestha BR, Shrestha BM. A comparison of 25 gauge Quincke spinal needle with 26 gauge Eldor spinal needle for the elective Caesarian sections: insertion characteristics and complications. Kathmandu University Medical Journal 2003;1(4):263‐6. [PUBMED: 16388267 ] [PubMed] [Google Scholar]
Tarkkila 1992 {published data only}
- Tarkkila PJ, Heine H, Tervo RR. Comparison of Sprotte and Quincke needles with respect to post dural puncture headache and backache. Regional Anesthesia 1992;17(5):283‐7. [PUBMED: 1419942 ] [PubMed] [Google Scholar]
Tarkkila 1994 {published data only}
- Tarkkila P, Huhtala J, Salminen U. Difficulties in spinal needle use. Insertion characteristics and failure rates associated with 25‐, 27‐ and 29‐gauge Quincke‐type spinal needles. Anaesthesia 1994;49(8):723‐5. [PUBMED: 7943709 ] [DOI] [PubMed] [Google Scholar]
Thomas 2000 {published data only}
- Thomas SR, Jamieson DRS, Muir KW. Randomised controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture. BMJ 2000;321(7267):986‐90. [PUBMED: 11039963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tourtellotte 1972 {published data only}
- Tourtellotte WW, Henderson WG, Tucker RP, Gilland O, Walker JE, Kokman E. A randomized, double‐blind clinical trial comparing the 22 versus 26 gauge needle in the production of the post‐lumbar puncture syndrome in normal individuals. Headache 1972;12(2):73‐8. [PUBMED: 4262477 ] [DOI] [PubMed] [Google Scholar]
Wiesel 1993 {published data only}
- Wiesel S, Tessler MJ, Easdown LJ. Postdural puncture headache: a randomized prospective comparison of the 24 gauge Sprotte and the 27 gauge Quincke needles in young patients. Canadian Journal of Anaesthesia 1993;40(7):607‐11. [PUBMED: 8403134] [DOI] [PubMed] [Google Scholar]
Wilkinson 1991 {published data only}
- Wilkinson AG, Sellar RJ. The influence of needle size and other factors on the incidence of adverse effects caused by myelography. Clinical Radiology 1991;44(5):338‐41. [PUBMED: 1836989 ] [DOI] [PubMed] [Google Scholar]
Zela 1994 {published data only}
- Zela JR, Espinoza R, Ulibarri A, Hernandez DM. Post dural headache: the use of Whitacre BD No. 25 vs. Quincke No. 25 needle [Cefalea post bloqueo subaracnoideo con aguja Whitacre B‐D No. 25 vs Quincke No. 25]. Revista Mexicana de Anestesiologia 1994;17(2):66‐9. [138928] [Google Scholar]
References to studies excluded from this review
Ansaloni 2000 {published data only}
- Ansaloni L, Balzani C, Falaschi F, Pazè E. Post‐spinal headache after dural puncture with perpendicular or horizontal needle bevel direction: a randomized controlled trial in an African rural hospital. Tropical Doctor 2000;30(3):167‐9. [PUBMED: 10902480 ] [DOI] [PubMed] [Google Scholar]
Benedetti 1992 {published data only}
- Benedetti A, Calliari G, Leli G, Welber D. Sub‐arachnoid anesthesia with Sprotte and Whitacre types atraumatic needles in cesarean section. Minerva Anestesiologica 1992;58(7‐8):437‐9. [PUBMED: 1508356 ] [PubMed] [Google Scholar]
Braune 1992 {published data only}
- Braune HJ, Huffmann G. A prospective double‐blind clinical trial, comparing the sharp Quincke needle (22G) with an 'atraumatic' needle (22G) in the induction of post‐lumbar puncture headache. Acta Neurologica Scandinavica 1992;86(1):50‐4. [PUBMED: 1519474 ] [DOI] [PubMed] [Google Scholar]
Browne 2005 {published data only}
- Browne IM, Birnbach DJ, Stein DJ, O'Gorman DA, Kuroda M. A comparison of Espocan and Tuohy needles for the combined spinal‐epidural technique for labor analgesia. Anesthesia and Analgesia 2005;101(2):535‐40, table of contents. [PUBMED: 16037172 ] [DOI] [PubMed] [Google Scholar]
- Browne IM, Birnbach DJ, Stein DJ, O'Gorman DA, Santos AC, Kelly‐Francis SB, et al. Comparison of Espocan and Tuohy needles for combined spinal‐epidural (CSE) analgesia [abstract]. Anesthesiology 2001; Vol. 94, issue 1A:Abstract no: A18. [CN‐00363793]
Carrada 1997 {published data only}
- Carrada S, Whizar V, Pérez A, Cabrera N. Postdural puncture headache incidence in young patients. A double blind comparative study with Atraucan 26, Quincke 26 and Whitacre 27 [Incidencia de cefalea postraquia en pacientes jóvenes. Estudio doble ciego, comparativo con Atraucan 26, Quincke 26 y Whitacre 2]. Revista Mexicana de Anestesiologia 1997;20(1):3‐10. [225059] [Google Scholar]
Charuluxananan 2005 {published data only}
- Charuluxananan S, Kyokong O, Premsamran P. Comparison of 25 and 27 gauge needle in spinal anesthesia learning curve for anesthesia residency training. Journal of the Medical Association of Thailand 2005;88(11):1569‐73. [PUBMED: 16471104 ] [PubMed] [Google Scholar]
Das‐Neves 2001 {published data only}
- Das‐Neves JFNP, Monteiro GA, De‐Almeida JR, Brun A, Sant'Anna RS, Soldate‐Duarte E. Spinal anesthesia with 27G and 29G Quincke and 27G Whitacre needles. Technical difficulties, failures and headache [Raquianestesia com agulha de Quincke 27G, 29G eWhitacre 27G. Análise da dificuldade técnica, incidência defalhas e cefaléia]. Revista Brasileira de Anestesiología 2001;51(3):196‐201. [DOI: ] [Google Scholar]
Eldor 2003 {published data only}
- Eldor J. Whitacre spinal needle vs. Eldor spinal needle regarding the incidence of transient neurologic symptoms. Acta Anaesthesiologica Scandinavica 2003;47(5):635‐6. [PUBMED: 12699529 ] [DOI] [PubMed] [Google Scholar]
Eshuis 1995 {published data only}
- Eshuis JH. Fewer headaches following lumbar puncture when using an atraumatic needle; double‐blind randomized study. Nederlands Tijdschrift voor Geneeskunde 1995;139(13):693‐4. [PUBMED: 7723873 ] [PubMed] [Google Scholar]
Flaatten 1998 {published data only}
- Flaatten H, Krakenes J, Vedeler C. Post‐dural puncture related complications after diagnostic lumbar puncture, myelography and spinal anaesthesia. Acta Neurologica Scandinavica 1998;98(6):445‐51. [PUBMED: 9875625 ] [DOI] [PubMed] [Google Scholar]
Ginosar 2012 {published data only}
- Ginosar Y, Smith Y, Ben‐Hur T, Lovett JM, Clements T, Ginosar YD, et al. Novel pulsatile cerebrospinal fluid model to assess pressure manometry and fluid sampling through spinal needles of different gauge: support for the use of a 22 G spinal needle with a tapered 27 G pencil‐point tip. British Journal of Anaesthesia 2012;108(2):308‐15. [PUBMED: 22157954 ] [DOI] [PubMed] [Google Scholar]
Guclu 2006 {published data only}
- Guclu E, Demiraran Y, Sezen G. Hearing loss after spinal anaesthesia: comparison of 22 and 25 G Quincke needles in a non‐elderly population. Clinical Otolaryngology 2006;31(4):344‐6. [PUBMED: 16911667] [DOI] [PubMed] [Google Scholar]
Herbstman 1998 {published data only}
- Herbstman CH, Jaffee JB, Tuman KJ, Newman LM. An in vivo evaluation of four spinal needles used for the combined spinal‐epidural technique. Anesthesia and Analgesia 1998;86(3):520‐2. [PUBMED: 9495405] [DOI] [PubMed] [Google Scholar]
Huffnagle 1998 {published data only}
- Huffnagle SL, Norris MC, Arkoosh VA, Huffnagle HJ, Ferouz F, Boxer L, et al. The influence of epidural needle bevel orientation on spread of sensory blockade in the laboring parturient. Anesthesia and Analgesia 1998;87(2):326‐30. [PUBMED: 9706925 ] [DOI] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones MJ, Selby IR, Gwinnutt CL, Hughes DG. Technical note: the influence of using an atraumatic needle on the incidence of post‐myelography headache. British Journal of Radiology 1994;67(796):396‐8. [PUBMED: 8173883 ] [DOI] [PubMed] [Google Scholar]
Landau 2001 {published data only}
- Landau R, Ciliberto CF, Goodman SR, Kim‐Lo SH, Smiley RM. Complications with 25‐gauge and 27‐gauge Whitacre needles during combined spinal‐epidural analgesia in labor. International Journal of Obstetric Anesthesia 2001;10(3):168‐71. [PUBMED: 15321605 ] [DOI] [PubMed] [Google Scholar]
Lynch 1992 {published data only}
- Lynch J, Arhelger S, Krings‐Ernst I, Grond S, Zech D. Whitacre 22‐gauge pencil‐point needle for spinal anaesthesia. A controlled trial in 300 young orthopaedic patients. Anaesthesia and Intensive Care 1992;20(3):322‐5. [PUBMED: 1524172 ] [DOI] [PubMed] [Google Scholar]
Malhotra 2007 {published data only}
- Malhotra SK, Iyer BA, Gupta AK, Raghunathan M, Nakra D. Spinal analgesia and auditory functions: a comparison of two sizes of Quincke needle. Minerva Anestesiologica 2007;73(7‐8):395‐9. [PUBMED: 17159767 ] [PubMed] [Google Scholar]
Mardirosoff 2001 {published data only}
- Mardirosoff C, Dumont L, Deyaert M, Leconte M. Posture‐related distribution of hyperbaric bupivacaine in cerebro‐spinal fluid is influenced by spinal needle characteristics. Acta Anaesthesiologica Scandinavica 2001;45(6):772‐5. [PUBMED: 11421839 ] [DOI] [PubMed] [Google Scholar]
Mazze 1993 {published data only}
- Mazze RI, Fujinaga M. Postdural puncture headache after continuous spinal anesthesia with 18‐gauge and 20‐gauge needles. Regional Anesthesia 1993;18(1):47‐51. [PUBMED: 8448099 ] [PubMed] [Google Scholar]
Merlo 1989 {published data only}
- Merlo A, Morant R, Ketz E, Gerig HJ, Senn HJ. Does post‐puncture syndrome following lumbar puncture depend on needle diameter?. Schweizerische Medizinische Wochenschrift 1989;119(49):1781‐6. [PUBMED: 2694368 ] [PubMed] [Google Scholar]
Nunes 1999 {published data only}
- Nunes JF, Alves G, Almeida JR, Silva R, Pedrosa G, Baptista A. Spinal anesthesia for cesarean section: headache evaluation with 25 G and 27 G Quincke and Whitacre needles [Raquianestesia para cesariana: avaliaçäo da cefaléia com agulhas de Quincke e Whitacre 25G e 27G]. Revista Brasileira de Anestesiologia 1999;49(3):173‐5. [277484] [Google Scholar]
Pjevic 1993 {published data only}
- Pjevic M, Gvozdenovic L. Postspinal headache‐‐incidence and prognosis. Medicinski Pregled 1993;46(5‐6):201‐4. [PUBMED: 7869977 ] [PubMed] [Google Scholar]
Quinn 2013 {published data only}
- Quinn C, MacKlin EA, Atassi N, Bowser R, Boylan K, Cudkowicz M, et al. Post‐lumbar puncture headache is reduced with use of atraumatic needles in ALS. Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration 2013;14(7‐8):632‐4. [PUBMED: 23834161 ] [DOI] [PubMed] [Google Scholar]
Russell 2002 {published data only}
- Russell R, Popat M, Richards E, Burry J. Combined spinal epidural anaesthesia for caesarean section: a randomised comparison of Oxford, lateral and sitting positions. International Journal of Obstetric Anesthesia 2002;11(3):190‐5. [PUBMED: 15321547 ] [DOI] [PubMed] [Google Scholar]
Samayoa 2004 {published data only}
- Samayoa F, Ramos N, Sánchez A. Cefalea post punción dural al utilizar agujas de Quincke vrs. agujas de Whitacre en pacientes obstétricas. Revista Colombiana de Anestesiología 2004;32(4):253‐60. [195118230003] [Google Scholar]
Shah 2002 {published data only}
- Shah A, Bhatia PK, Tulsiani KL. Post dural puncture headache in caesarean section ‐ a comparative study using 25 G Quincke, 27 G Quincke and 27 G Whitacre needle. Indian Journal of Anaesthesia 2002;46(5):373‐7. [Google Scholar]
Sinikoglu 2013a {published data only}
- Sinikoglu NS, Yeter H, Gumus F, Belli E, Alagol A, Turan N. Reinsertion of the stylet does not affect incidence of post dural puncture headaches (PDPH) after spinal anesthesia [A reinserção do estilete não afeta a incidência de cefaleia pós‐punção dural (CPPD) após raquianestesia]. Revista Brasileira de Anestesiologia 2013;63(2):188‐92. [DOI: 10.1590/S0034-70942013000200005] [DOI] [Google Scholar]
Strupp 1998 {published data only}
- Strupp M, Brandt T, Müller A. Incidence of post‐lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. Journal of Neurology 1998;245(9):589‐92. [PUBMED: 9758296 ] [DOI] [PubMed] [Google Scholar]
Strupp 2009 {published data only}
- Strupp M, Katsarava Z. Post‐lumbar puncture syndrome and spontaneous low CSF pressure syndrome. Der Nervenarzt 2009;80(12):1509‐19. [PUBMED: 19921503 ] [DOI] [PubMed] [Google Scholar]
Thoren 1994 {published data only}
- Thoren T, Holmstrom B, Rawal N, Schollin J, Lindeberg S, Skeppner G. Sequential combined spinal epidural block versus spinal block for cesarean section: effects on maternal hypotension and neurobehavioral function of the newborn. Anesthesia and Analgesia 1994;78(6):1087‐92. [PUBMED: 8198262 ] [PubMed] [Google Scholar]
Vallejo 2000 {published data only}
- Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesthesia and Analgesia 2000;91(4):916‐20. [PUBMED: 11004048] [DOI] [PubMed] [Google Scholar]
Van Den Berg 2011 {published data only}
- Berg AA, Ghatge S, Armendariz G, Cornelius D, Wang S. Responses to dural puncture during institution of combined spinal‐epidural analgesia: a comparison of 27 gauge pencilpoint and 27 gauge cutting‐edge needles. Anaesthesia and Intensive Care 2011;39(2):247‐51. [PUBMED: 21485674 ] [DOI] [PubMed] [Google Scholar]
Vilming 2001 {published data only}
- Vilming ST, Kloster R, Sandvik L. The importance of sex, age, needle size, height and body mass index in post‐lumbar puncture headache. Cephalalgia 2001;21(7):738‐43. [PUBMED: 11595002 ] [DOI] [PubMed] [Google Scholar]
Wilhelm 1997 {published data only}
- Wilhelm S, Standl T. Continuous spinal anesthesia vs. combined spinal‐epidural anesthesia in emergency surgery. The combined spinal‐epidural anesthesia technique does not offer an advantage of spinal anesthesia with a microcatheter. Der Anaesthesist 1997;46(11):938‐42. [PUBMED: 9490580 ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bano 2004 {published data only}
- Bano Farooq F, Haider S, Aftab S, Sultan ST. Comparison of 25‐gauge, Quincke and Whitacre needles for postdural puncture headache in obstetric patients. Journal of the College of Physicians and Surgeons Pakistan 2004;14(11):647‐50. [PUBMED: 15530271 ] [PubMed] [Google Scholar]
Buttner 1990 {published data only}
- Buttner J, Wresch KP, Klose R. Does a cone‐shaped cannula needle offer an advantage in spinal anesthesia?. Regional‐Anaesthesie 1990;13(5):124‐8. [PUBMED: 2202024 ] [PubMed] [Google Scholar]
Castrillo 2015 {published data only}
- Castrillo A, Tabernero C, Garcia‐Olmos LM, Gil C, Gutierrez R, Zamora MI, et al. Postdural puncture headache: impact of needle type, a randomized trial. Spine 2015;15:1571‐6. [DOI] [PubMed] [Google Scholar]
De Andres 1994 {published data only}
- Andres J, Valia JC, Errando C, Rico G, Lopez‐Alarcon MD. Subarachnoid anesthesia in young patients: a comparative analysis of two needle bevels. Regional Anesthesia and Pain Medicine 1999;24(6):547‐52. [PUBMED: 10588560 ] [DOI] [PubMed] [Google Scholar]
Fama 2015 {published data only}
- Fama F, Linard C, Bierlaire D, Gioffre'‐Florio M, Fusciardi J, Laffon M. Influence of needle diameter on spinal anaesthesia puncture failures for caesarean section: a prospective, randomised, experimental study. Anaesthesia, Critical Care and Pain Medicine 2015;34:277‐80. [DOI] [PubMed] [Google Scholar]
Fyneface‐Ogan 2006 {published data only}
- Fyneface‐Ogan S, Mato CN, Odagme MT. Post‐dural puncture headache following caesarean section in Nigerian parturients: a comparison of two spinal needles. Nigerian Postgraduate Medical Journal 2006;13(3):200‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Harrison 1994 {published data only}
- Harrison DA, Langham BT. Post‐dural puncture headache: a comparison of the Sprotte and Yale needles in urological surgery. European Journal of Anaesthesiology 1994;11(4):325‐7. [PUBMED: 7925339 ] [PubMed] [Google Scholar]
Hong 2015 {published data only}
- Hong J, Jung S, Chang H. Whitacre needle reduces the incidence of intravascular uptake in lumbar transforaminal epidural steroid injections. Pain Physician 2015;18:325‐31. [PubMed] [Google Scholar]
Jager 1995 {published data only}
- Jager H, Fenzl G, Schleifer J, Galli C. The postmyelography syndrome‐‐very rare in use of an "atraumatic needle". A controlled double‐blind study. Röntgenpraxis; Zeitschrift für radiologische Technik 1995;48(8):226‐8. [PUBMED: 7482038 ] [PubMed] [Google Scholar]
Jensen 1999 {published data only}
- Jensen KM, Jensen LB, Felding M, Golbaekdal K I, Nielsen JA. Complications after spinal analgesia using three different spinal needles: Sprotee, Spinocan and Atraucan. Ugeskrift for Laeger 1999;161(49):6775‐8. [PUBMED: 10643362 ] [PubMed] [Google Scholar]
Kaul 1996 {published data only}
- Kaul TK, Chopra H, Gautam PL, Anjali H. Hearing loss after spinal anaesthesia: relation to needle size. Journal of Anaesthesiology Clinical Pharmacology 1996;12(2):113‐6. [NI000672] [Google Scholar]
Knudsen 1998 {published data only}
- Knudsen L, Dich‐Nielsen JO, Molgaard O, Staach LJ, Rasmussen A, Rajan RM. Atraucan, a new needle for spinal analgesia. Ugeskrift for Laeger 1998;160(32):4636‐9. [PUBMED: 9719744 ] [PubMed] [Google Scholar]
Lim 1992 {published data only}
- Lim M, Cross GD, Sold M. Postdural puncture headache: a comparison between the 29 G Vygon and 24 G Sprotte needles. Der Anaesthesist 1992;41(9):539‐43. [PUBMED: 1416009 ] [PubMed] [Google Scholar]
Maclean 1994 {published data only}
- Maclean AR, Lyons G, Dresner M. Post dural puncture headache; a comparison of the 27 gauge Quincke, 25 gauge Whitacre and the 24 gauge Sprotte needles. International Journal of Obstetric Anesthesia 1994;3(3):175‐6. [EMBASE: 1994224228] [Google Scholar]
Mignonsin 1991 {published data only}
- Mignonsin D, Adande P, Bouabre E, Bondurand A. Isobar 0.5% bupivacaine spinal anesthesia: effects of 18 and 26 G spinal needles. Urgences Medicales 1991;10(2):58‐61. [EMBASE: 1991168386] [Google Scholar]
Palmieri 1993 {published data only}
- Palmieri JT, Muniz M, Silva MC, Menezes P do T, Vianna A. Post dural puncture headache. A comparison between disposable and reusable Quincke needles. Regional Anesthesia 1993;18S1(15):17. [Google Scholar]
Puolakka 1997 {published data only}
- Puolakka R, Jokinen M, Pitkänen MT, Rosenberg P. Comparison of postanaesthetic sequelae of clinical use of 27‐gauge cutting and noncutting spinal needles. Acta Anaesthesiologica Scandinavica 1997;41(6):164. [PUBMED: 9425967 ] [PubMed] [Google Scholar]
Vandana 2004 {published data only}
- Vandana, Katyal S, Kaul Tej K, Sood D, Narula N, Singh A. Post dural puncture headache in spinal anaesthesia using 25 gauge versus 29 gauge Quincke needles. Journal of Anaesthesiology Clinical Pharmacology 2004;20(4):407‐9. [EMBASE: 2005134893] [Google Scholar]
References to ongoing studies
Ahmed 2012 {published data only}
- Ahmed J, Siddiqui SZ, Haider S, Siddiqui AS. Incidence and severity of post dural puncture headache after spinal anaesthesia for cesarean section; a comparison between 25G Quincke cutting and 25G Pencan pencil point spinal needles. Anaesthesia, Pain and Intensive Care 2012;16(1):106‐7. [Google Scholar]
Akdemir 2011 {published data only}
- Akdemir MS, Karaman H, Olmez Kavak G, Tufek A, Celik F, Tokgoz O, et al. The association between needle types and headache. Regional Anesthesia and Pain Medicine 2011;36(5 Suppl 2):E223. [Google Scholar]
Bertolotto 2014 {published data only}
- Bertolotto A, Motuzova Y, Sperli F, Capobianco M, Malentacchi M, Pulizzi A, et al. Post‐dural puncture headache is markedly reduced when 25 Sprotte needles are used. Multiple Sclerosis 2014;20(Suppl 1):160‐1. [Google Scholar]
Bertolotto 2014a {published data only}
- Bertolotto A, Sperli F, Capobianco M, Malentacchi M, Pulizzi A, Malucchi S. 25G Sprotte needle strongly reduces the risk of post‐lumbare puncture headache in clinical practice. Neurology. 2014; Vol. 82 (10 Suppl 1).
Bham 2010 {published data only}
- Bham E, Ung LK, Chan L. Comparison of 22/27g microtip vs 25g Pencan spinal needle; insertion characteristic and complications. Regional Anesthesia and Pain Medicine 2010;35(5):E68‐9. [Google Scholar]
IRCT201009292080N4 {unpublished data only}
- IRCT201009292080N4. Comparison of Sprotte and Quincke needles with respect to post dural puncture headache. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201009292080N4. Iranian Registry of Clinical Trials, (accessed 15 April 2015).
Lorthe 2014 {published data only}
- Lorthe J, Shah S, Cohen S, Ramos D, Rah K, Kapadia A, et al. CSE for cesarean section: Gertie Marx versus Pencan spinal needles. Anesthesia and Analgesia 2014;118(5 Suppl 1):S188. [Google Scholar]
NCT00370604 {unpublished data only}
- NCT00370604. Effect of small versus large epidural needles on postdural puncture headache study. https://clinicaltrials.gov/show/NCT00370604 (accessed 15 April 2015). [NCT00370604]
NCT01821807 {unpublished data only}
- NCT01821807. Comparison of two spinal needles regarding postdural puncture headache. https://clinicaltrials.gov/show/NCT01821807 (Accessed 15 April 2015).
NCT02384031 {unpublished data only}
- NCT02384031. Post‐dural puncture headache ‐ needles and biomarkers in CSF. https://clinicaltrials.gov/ct2/show/NCT02384031 (accessed 15 April 2015).
Shah 2011 {published data only}
- Shah S, Cohen S, Negron‐Gonzalez M, Kiss G, Hunter CW. Combined spinal epidural (CSE) for cesarean section: Gertie Marx versus Pencan spinal needles. Anesthesia and Analgesia. 2011; Vol. 112 (5 Suppl 1).
Shaikh 2013 {published data only}
- Shaikh H, Cohen S, Shah S, Shkolnikova T, Mohiuddin A, Shapiro P, et al. A comparison of gravity flow epidural block with combined spinal epidural (CSE) for cesarean section. Anesthesia and Analgesia. 2013; Vol. 116 Suppl 1.
Additional references
Ahmed 2006
- Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgraduate Medicine 2006;82(973):713‐6. [PUBMED: 7099089] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alstadhaug 2012
- Alstadhaug KB, Odeg F, Baloch FK, Berg DH, Salvesen R. Post‐lumbar puncture headache. Tidsskrift for Den Norske Laegeforening 2012;132(7):818‐21. [PUBMED: 22511093] [DOI] [PubMed] [Google Scholar]
American Society of Anesthesiologists 2007
- American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology 2007;106(4):843‐63. [PUBMED: 17413923] [DOI] [PubMed] [Google Scholar]
Angle 2003
- Angle PJ, Kronberg JE, Thompson DE, Ackerley C, Szalai JP, Duffin J, et al. Dural tissue trauma and cerebrospinal fluid leak after epidural needle puncture: effect of needle design, angle, and bevel orientation. Anesthesiology 2003;99(6):1376‐82. [PUBMED: 14639152] [DOI] [PubMed] [Google Scholar]
Angle 2005
- Angle P, Tang SL, Thompson D, Szalai JP. Expectant management of postdural puncture headache increases hospital length of stay and emergency room visits. Canadian Journal of Anaesthesia 2005;52(4):397‐402. [PUBMED: 15814755] [DOI] [PubMed] [Google Scholar]
Arendt 2009
- Arendt K, Demaerschalk BM, Wingerchuk DM, Camann W. Atraumatic lumbar puncture needles: after all these years, are we still missing the point?. Neurologist 2009;15(1):17‐20. [PUBMED: 9131853] [DOI] [PubMed] [Google Scholar]
Arevalo‐Rodriguez 2013
- Arevalo‐Rodriguez I, Ciapponi A, Munoz L, Roqué I Figuls M, Bonfill Cosp X. Posture and fluids for preventing post‐dural puncture headache. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009199.pub2] [DOI] [PubMed] [Google Scholar]
Armon 2005
- Armon C, Evans RW. Addendum to assessment. Prevention of post‐lumbar puncture headaches: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005;65(4):510‐2. [PUBMED: 16116106] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Basurto 2013
- Basurto OX, Uriona TSM, Martínez GL, Solà I, Bonfill CX. Drug therapy for preventing post‐dural puncture headache. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD001792.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumgarten 1987
- Baumgarten RK. Should caffeine become the first‐line treatment for postdural puncture headache?. Anesthesia and Analgesia 1987;66(9):913‐4. [PUBMED: 3619102] [PubMed] [Google Scholar]
Berger 1998
- Berger CW, Crosby ET, Grodecki W. North American survey of the management of dural puncture occurring during labour epidural analgesia. Canadian Journal of Anaesthesia 1998;45(2):110‐4. [PUBMED: 9512843] [DOI] [PubMed] [Google Scholar]
Bezov 2010
- Bezov D, Lipton RB, Ashina S. Post‐dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache 2010;50(7):1144‐52. [PUBMED: 20533959] [DOI] [PubMed] [Google Scholar]
Boonmak 2010
- Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post‐dural puncture headache. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001791.pub2] [DOI] [PubMed] [Google Scholar]
Bradbury 2013
- Bradbury CL, Singh SI, Badder SR, Wakely LJ, Jones PM. Prevention of post‐dural puncture headache in parturients: a systematic review and meta‐analysis. Acta Anaesthesiologica Scandinavica 2013;57(4):417‐30. [PUBMED: 23278515] [DOI] [PubMed] [Google Scholar]
Calthorpe 2004
- Calthorpe N. The history of spinal needles: getting to the point. Anaesthesia 2004;59(12):1231‐41. [PUBMED: 15549987] [DOI] [PubMed] [Google Scholar]
Choi 2003
- Choi PT, Galinski SE, Takeuchi L, Lucas S, Tamayo C, Jadad AR. PDPH is a common complication of neuraxial blockade in parturients: a meta‐analysis of obstetrical studies. Canadian Journal of Anaesthesia 2003;50(5):460‐9. [PUBMED: 12734154] [DOI] [PubMed] [Google Scholar]
Ciapponi 2011
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. HTAi 2011 Conference. Rio de Janeiro, Brazil, 2011.
Ciapponi 2011a
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. ISPOR 3rd Latin America Conference. Hilton Mexico City Reforma in Mexico City, Mexico, 2011.
Clark 1996
- Clark JW, Solomon GD, Senanayake PD, Gallagher C. Substance P concentration and history of headache in relation to postlumbar puncture headache: towards prevention. Journal of Neurology, Neurosurgery, and Psychiatry 1996;60(6):681‐3. [PUBMED: 8648338 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davignon 2002
- Davignon KR, Dennehy KC. Update on postdural puncture headache. International Anesthesiology Clinics 2002;40(4):89‐102. [PUBMED: 12409935] [DOI] [PubMed] [Google Scholar]
Denny 1987
- Denny N, Masters R, Pearson D, Read J, Sihota M, Selander D. Postdural puncture headache after continuous spinal anesthesia. Anesthesia and Analgesia 1987;66(8):791‐4. [PUBMED: 3605700] [PubMed] [Google Scholar]
Evans 2009
- Evans RW. Diagnostic testing for migraine and other primary headaches. Clinical Neurology 2009;27(2):393‐415. [PUBMED: 19289222] [DOI] [PubMed] [Google Scholar]
Glujovsky 2010
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. XVIII Cochrane Colloquium. The Joint Colloquium of the Cochrane & Campbell Collaborations. Keystone Resort, Colorado, USA, 2010.
González‐Martínez 2005
- González‐Martínez F, León‐Belmar J, Navarro‐Gutierrez S, Herráiz‐de Castro C, Montero‐López L, Liaño‐Martínez H, et al. Lowered incidence of post‐lumbar puncture headache following the application of the second edition of the International Headache Society classification [Disminución en la incidencia de la cefalea pospunción lumbar tras la aplicación de la segunda edición de la Sociedad Internacional de Cefaleas]. Revista de Neurologia 2005;41(10):582‐6. [PUBMED: 16288419] [PubMed] [Google Scholar]
Grande 2005
- Grande PO. Mechanisms behind postspinal headache and brain stem compression following lumbar dural puncture—a physiological approach. Acta Anaesthesiologica Scandinavica 2005;49(5):619‐26. [PUBMED: 15836674] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Halpern 1994
- Halpern S, Preston R. Postdural puncture headache and spinal needle design. Metaanalyses. Anesthesiology 1994;81:1376‐83. [DOI] [PubMed] [Google Scholar]
Harrington 2004
- Harrington BE. Postdural puncture headache and the development of the epidural blood patch. Regional Anesthesia and Pain Medicine 2004;29(2):136‐63; discussion 135. [PUBMED: 15029551] [DOI] [PubMed] [Google Scholar]
Headache Classification Subcommittee IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia 2004;24(Suppl 1):9‐160. [PUBMED: 14979299] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICTRP
- WHO. International Clinical Trials Registry Platform Search Portal. http://apps.who.int/trialsearch/Default.aspx.
Janssens 2003
- Janssens E, Aerssens P, Alliet P, Gillis P, Raes M. Post‐dural puncture headaches in children. A literature review. European Journal of Pediatrics 2003;162(3):117‐21. [PUBMED: 12655411] [DOI] [PubMed] [Google Scholar]
Kuczkowski 2006
- Kuczkowski KM. The treatment and prevention of post‐dural puncture headache. Acta Anaesthesiologica Belgica 2006;57(1):55‐6. [PUBMED: 16617759] [PubMed] [Google Scholar]
McQuay 1998
- McQuay HJ, Moore RA. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [Google Scholar]
Parker 1997
- Parker RK, White PF. A microscopic analysis of cut‐bevel versus pencil‐point spinal needles. Anesthesia and Analgesia 1997;85(5):1101‐4. [PUBMED: 9356107 ] [DOI] [PubMed] [Google Scholar]
Raskin 1990
- Raskin NH. Lumbar puncture headache: a review. Headache 1990;30(4):197‐200. [PUBMED: 2186014] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richman 2006
- Richman JM, Joe EM, Cohen SR, Rowlingson AJ, Michaels RK, Jeffries MA, et al. Bevel direction and postdural puncture headache: a meta‐analysis. The Neurologist 2006;12(4):224‐8. [PUBMED: 16832241] [DOI] [PubMed] [Google Scholar]
Sadashivaiah 2009
- Sadashivaiah J, McLure H. 18‐G Tuohy needle can reduce the incidence of severe post dural puncture headache. Anaesthesia 2009;64(12):1379‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thew 2008
- Thew M, Paech MJ. Management of postdural puncture headache in the obstetric patient. Current Opinion in Anaesthesiology 2008;21(3):288‐92. [PUBMED: 18458543] [DOI] [PubMed] [Google Scholar]
Tung 2012
- Tung CE, So YT, Lansberg MG. Cost comparison between the atraumatic and cutting lumbar puncture needles. Neurology 2012;78(2):109‐13. [PUBMED: 22205758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull DK, Shepherd DB. Post‐dural puncture headache: pathogenesis, prevention and treatment. British Journal of Anaesthesia 2003;91(5):718‐29. [PUBMED: 14570796] [DOI] [PubMed] [Google Scholar]
van Kooten 2008
- Kooten F, Oedit R, Bakker SL, Dippel DW. Epidural blood patch in post dural puncture headache: a randomised, observer‐blind, controlled clinical trial. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(5):553‐8. [PUBMED: 17635971] [DOI] [PubMed] [Google Scholar]
Wu 1991
- Wu YW, Hui YL, Tan PP. Incidence of post‐dural puncture headache with 25‐gauge Quincke spinal needle. Anaesthesiologica Sinica 1991;29(1):538‐41. [PUBMED: 1758245] [PubMed] [Google Scholar]
References to other published versions of this review
Arevalo‐Rodriguez 2013a
- Arevalo‐Rodriguez I, Muñoz L, Arevalo Jimmy J, Ciapponi A, Roqué iFM. Needle gauge and tip designs for preventing post‐dural puncture headache (PDPH). Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010807; CD010807] [DOI] [PMC free article] [PubMed] [Google Scholar]


