Abstract
Background
Decision aids are interventions that support patients by making their decisions explicit, providing information about options and associated benefits/harms, and helping clarify congruence between decisions and personal values.
Objectives
To assess the effects of decision aids in people facing treatment or screening decisions.
Search methods
Updated search (2012 to April 2015) in CENTRAL; MEDLINE; Embase; PsycINFO; and grey literature; includes CINAHL to September 2008.
Selection criteria
We included published randomized controlled trials comparing decision aids to usual care and/or alternative interventions. For this update, we excluded studies comparing detailed versus simple decision aids.
Data collection and analysis
Two reviewers independently screened citations for inclusion, extracted data, and assessed risk of bias. Primary outcomes, based on the International Patient Decision Aid Standards (IPDAS), were attributes related to the choice made and the decision‐making process.
Secondary outcomes were behavioural, health, and health system effects.
We pooled results using mean differences (MDs) and risk ratios (RRs), applying a random‐effects model. We conducted a subgroup analysis of studies that used the patient decision aid to prepare for the consultation and of those that used it in the consultation. We used GRADE to assess the strength of the evidence.
Main results
We included 105 studies involving 31,043 participants. This update added 18 studies and removed 28 previously included studies comparing detailed versus simple decision aids. During the 'Risk of bias' assessment, we rated two items (selective reporting and blinding of participants/personnel) as mostly unclear due to inadequate reporting. Twelve of 105 studies were at high risk of bias.
With regard to the attributes of the choice made, decision aids increased participants' knowledge (MD 13.27/100; 95% confidence interval (CI) 11.32 to 15.23; 52 studies; N = 13,316; high‐quality evidence), accuracy of risk perceptions (RR 2.10; 95% CI 1.66 to 2.66; 17 studies; N = 5096; moderate‐quality evidence), and congruency between informed values and care choices (RR 2.06; 95% CI 1.46 to 2.91; 10 studies; N = 4626; low‐quality evidence) compared to usual care.
Regarding attributes related to the decision‐making process and compared to usual care, decision aids decreased decisional conflict related to feeling uninformed (MD −9.28/100; 95% CI −12.20 to −6.36; 27 studies; N = 5707; high‐quality evidence), indecision about personal values (MD −8.81/100; 95% CI −11.99 to −5.63; 23 studies; N = 5068; high‐quality evidence), and the proportion of people who were passive in decision making (RR 0.68; 95% CI 0.55 to 0.83; 16 studies; N = 3180; moderate‐quality evidence).
Decision aids reduced the proportion of undecided participants and appeared to have a positive effect on patient‐clinician communication. Moreover, those exposed to a decision aid were either equally or more satisfied with their decision, the decision‐making process, and/or the preparation for decision making compared to usual care.
Decision aids also reduced the number of people choosing major elective invasive surgery in favour of more conservative options (RR 0.86; 95% CI 0.75 to 1.00; 18 studies; N = 3844), but this reduction reached statistical significance only after removing the study on prophylactic mastectomy for breast cancer gene carriers (RR 0.84; 95% CI 0.73 to 0.97; 17 studies; N = 3108). Compared to usual care, decision aids reduced the number of people choosing prostate‐specific antigen screening (RR 0.88; 95% CI 0.80 to 0.98; 10 studies; N = 3996) and increased those choosing to start new medications for diabetes (RR 1.65; 95% CI 1.06 to 2.56; 4 studies; N = 447). For other testing and screening choices, mostly there were no differences between decision aids and usual care.
The median effect of decision aids on length of consultation was 2.6 minutes longer (24 versus 21; 7.5% increase). The costs of the decision aid group were lower in two studies and similar to usual care in four studies. People receiving decision aids do not appear to differ from those receiving usual care in terms of anxiety, general health outcomes, and condition‐specific health outcomes. Studies did not report adverse events associated with the use of decision aids.
In subgroup analysis, we compared results for decision aids used in preparation for the consultation versus during the consultation, finding similar improvements in pooled analysis for knowledge and accurate risk perception. For other outcomes, we could not conduct formal subgroup analyses because there were too few studies in each subgroup.
Authors' conclusions
Compared to usual care across a wide variety of decision contexts, people exposed to decision aids feel more knowledgeable, better informed, and clearer about their values, and they probably have a more active role in decision making and more accurate risk perceptions. There is growing evidence that decision aids may improve values‐congruent choices. There are no adverse effects on health outcomes or satisfaction. New for this updated is evidence indicating improved knowledge and accurate risk perceptions when decision aids are used either within or in preparation for the consultation. Further research is needed on the effects on adherence with the chosen option, cost‐effectiveness, and use with lower literacy populations.
Plain language summary
Decision aids to help people who are facing health treatment or screening decisions
Review question
We reviewed the effects of decision aids on people facing health treatment or screening decisions. In this update, we added 18 new studies for a total of 105.
Background
Making a decision about the best treatment or screening option can be hard. People can use decision aids when there is more than one option and neither is clearly better, or when options have benefits and harms that people value differently. Decision aids may be pamphlets, videos, or web‐based tools. They state the decision, describe the options, and help people think about the options from a personal view (e.g. how important are possible benefits and harms).
Study characteristics
For research published up to April 2015, there were 105 studies involving 31,043 people. The decision aids focused on 50 different decisions. The common decisions were about: surgery, screening (e.g. prostate cancer, colon cancer, prenatal), genetic testing, and medication treatments (e.g. diabetes, atrial fibrillation).The decision aids were compared to usual care that may have included general information or no intervention. In the 105 studies, 89 evaluated a patient decision aid used by people in preparation for the visit with the clinician, and 16 evaluated its use during the visit with the clinician.
Key results with quality of the evidence
When people use decision aids, they improve their knowledge of the options (high‐quality evidence) and feel better informed and more clear about what matters most to them (high‐quality evidence). They probably have more accurate expectations of benefits and harms of options (moderate‐quality evidence) and probably participate more in decision making (moderate‐quality evidence). People who use decision aids may achieve decisions that are consistent with their informed values (evidence is not as strong; more research could change results). People and their clinicians were more likely to talk about the decision when using a decision aid. Decision aids have a variable effect on the option chosen, depending on the choice being considered. Decision aids do not worsen health outcomes, and people using them are not less satisfied. More research is needed to assess if people continue with the option they chose and also to assess what impact decision aids have on healthcare systems.
Summary of findings
for the main comparison.
| Patient decision aids compared with usual care for adults considering treatment or screening decisions | ||||||
|
Patient or population: adults considering treatment or screening decisions Settings: all settings Intervention: patient decision aid Comparison: usual care | ||||||
| Outcomes | Illustrative comparative benefits* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed benefit | Corresponding benefit | |||||
| Usual care | Patient decision aid | |||||
|
Knowledge ‐ all studies Standardized on score from 0 (no knowledge) to 100 (perfect knowledge), soon after exposure to the decision aid |
The mean knowledge score was 56.9% across control groups, ranging from 27.0% to 85.2% | The mean knowledge score in the intervention groups was 13.27 higher (11.32 to 15.23 higher) | — | 13,316 (52 studies) | ⊕⊕⊕⊕ Higha,b | Higher scores indicate better knowledge. 46 out of 52 studies showed a statistically significant improvement in knowledge |
|
Accurate risk perceptions ‐ all studies Assessed soon after exposure to the decision aid |
269 per 1000c | 565 per 1000 (447 to 716 per 1000) | RR 2.10 (1.66 to 2.66) | 5096 (17 studies) | ⊕⊕⊕⊝ Moderatea,d | — |
|
Congruence between the chosen option and informed values ‐ all studies Assessed soon after exposure to the decision aid |
289 per 1000c | 595 per 1000 (422 to 841 per 1000) | RR 2.06 (1.46 to 2.91) | 4626 (10 studies) |
⊕⊕⊝⊝ Lowa,d,e,f | — |
|
Decisional conflict: uninformed subscale ‐ all studies Standardized on score from 0 (not uninformed) to 100 (uninformed) Assessed soon after exposure to the decision aid |
The mean for outcome 'feeling uninformed' ranged across control groups from 11.1 to 61.1. Scores ≤ 25 associated with following through on decisions. Scores > 38 associated with delay in decision making |
The mean feeling uninformed in the intervention groups was 9.28 lower (12.20 to 6.36 lower) | — | 5707 (27 studies) |
⊕⊕⊕⊕ Higha,b | Lower scores indicate feeling more informed |
|
Decisional conflict: unclear about personal values subscale ‐ all studies Standardized on score from 0 (not unclear) to 100 (unclear) Assessed soon after exposure to the decision aid |
The mean for outcome 'feeling unclear about personal values' ranged across control groups from 15.5 to 53.2. Scores ≤ 25 associated with follow‐through with decisions. Scores > 38 associated with delay in decision making |
The mean feeling unclear values in the intervention groups was 8.81 lower (11.99 to 5.63 lower) | — | 5068 (23 studies) |
⊕⊕⊕⊕ Higha,b | Lower scores indicate feeling clearer about values |
|
Participation in decision making: clinician‐controlled decision making ‐ all studies Assessed soon after consultation with clinician |
228 per 1000c | 155 per 1000 (125 to 189 per 1000) | RR 0.68 (0.55 to 0.83) | 3180 (16 studies) | ⊕⊕⊕⊝ Moderatea,e | Patient decision aids aim to increase patient involvement in making decisions; lower proportion of clinician‐controlled decision making is better |
| Adverse events | There were no adverse effects on health outcomes or satisfaction, and no other adverse effects reported. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aThe vast majority of studies measuring this outcome were not at high risk of bias. bThe GRADE ratings for these outcomes were not downgraded for heterogeneity given the generally consistent direction of effects across studies for the decision aid compared to usual care groups. cThe data source for the assumed risk was the mean control event rate. dThe GRADE rating was downgraded given the lack of precision. eThe GRADE rating was downgraded given the lack of consistency. fThe GRADE rating was downgraded given the lack of directness. As well, the outcome was measured using various approaches with no gold standard approach.
Background
Many health treatment and screening decisions have no single 'best' choice. These types of decisions are considered 'preference‐sensitive' because there is insufficient evidence about outcomes or there is a need to trade off known benefits and harms. Clinical Evidence analyzed 3000 treatments, classifying 50% as having insufficient evidence, 24% as likely to be beneficial, 7% as requiring trade‐offs between benefits and harms, 5% as unlikely to be beneficial, 3% as likely to be ineffective or harmful, and only 11% as being clearly beneficial (Clinical Evidence 2013). Not only does one have to take into account the strength of the evidence, but even for the 11% of treatments that show beneficial effects for populations, physicians need to translate the probabilistic nature of the evidence for individual patients to help them reach a decision based on informed values. Patient decision aids are an intervention that can be used to present such evidence (Brouwers 2010). This review is an update of the review last published in 2014 of the comparisons between patient decision aids and usual care (Stacey 2014b). To provide a more focused review, we removed 28 studies that compared detailed versus simple decision aids.
Description of the intervention
According to the International Patient Decision Aids Standards (IPDAS) Collaboration (Elwyn 2006; IPDAS 2005a; Joseph‐Williams 2013), decision aids are evidence‐based tools designed to help patients make specific and deliberated choices among healthcare options. Patient decision aids supplement (rather than replace) clinicians' counselling about options. The specific aims of decision aids and the type of decision support they provide may vary slightly, but in general they:
explicitly state the decision that needs to be considered;
provide evidence‐based information about a health condition, the options, associated benefits, harms, probabilities, and scientific uncertainties;
help patients to recognize the values‐sensitive nature of the decision and to clarify, either implicitly or explicitly, the value they place on the benefits and harms. (To accomplish this, patient decision aids may describe the options in enough detail that clients can imagine what it is like to experience the physical, emotional, and social effects, or they may guide clients to consider which benefits and harms are most important to them.)
Decision aids differ from usual health education materials. Decision aids make the decision being considered explicit, providing a detailed, specific, and personalized focus on options and outcomes for the purpose of preparing people for decision making. In contrast, health education materials help people to understand their diagnosis, treatment, and management in general terms, but given their broader perspective, these materials are not focused on decision points and thus do not necessarily help them to participate in decision making. Many decision aids are based on a conceptual model or theoretical framework (Durand 2008; Mulley 1995; O'Connor 1998b; Rothert 1987).
In response to concerns about variability in the quality of patient decision aids, the IPDAS Collaboration reached agreement on criteria for judging their quality (Elwyn 2006). More than 100 researchers, clinicians, patients, and policymakers from 14 countries participated. Participants addressed three domains of quality: clinical content, development process, and evaluation of a patient decision aid's effectiveness. A series of background papers informing the original IPDAS criteria were updated in 2013 (IPDAS 2013). Subsequently, an international team of researchers reached consensus on a shorter set of qualifying and certifying criteria (Joseph‐Williams 2013). Informed by IPDAS, the Washington State Health Authority launched the first programme for certifying patient decision aids in 2016 (Washington State 2016).
How the intervention might work
Decision aids can be used before, during, or after a clinical encounter to enable patients to become active, informed participants. Providing the patient decision aid in preparation for the consultation allows people more time to digest the information and be ready to discuss the decision, but this may not be feasible in some health decisions (e.g. antibiotics for upper respiratory infections). Decision aids can also facilitate shared decision making. Shared decision making is defined as a process through which clinicians and patients make healthcare choices together (Charles 1997; Makoul 2006), representing the crux of people‐centred care (Weston 2001). However, the way the clinician provides information may strongly affect people's preferences (Hibbard 1997), prompting the need for standardized information such as patient decision aids. Patients who are more active in making decisions about their health have better health outcomes and healthcare experiences (Hibbard 2013; Kiesler 2006). In summary, patient decision aids may help clinicians and patients come to quality decisions, grounded in patients' values and taking into account the potential trade‐offs in benefits and risks of different options.
Why it is important to do this review
Given the broad range of stakeholders interested in patient decision aids and the rapidly expanding field of research, there was a need to update this review to identify studies on new decisions or conducted in new countries and to strengthen the synthesized evidence supporting use of patient decision aids for outcomes that do not yet have high‐quality evidence. In fact, the 2014 publication was the most cited Cochrane Review in 2015 based on 1888 reviews published in 2013 and 2014. With growing development of patient decision aids for use in the consultation, we wanted to conduct a subgroup analysis of patient decision aids used in preparation for versus within the consultation.
Results from previous reviews were used to inform clinical practice guidelines such as Patient Experience in Adult NHS Services (NCGC/NICE 2012) and Decision Support for Adults Living with Chronic Kidney Disease (RNAO 2009). Subgroup analyses of included studies have focused on anxiety (Bekker 2003), adherence (Trenaman 2016), values congruence (Munro 2016), participant trial identity (Brown 2015), and heterogeneity (Gentles 2013).
Other systematic reviews have been conducted on the use of patient decision aids as one type of intervention to facilitate shared decision making in clinical practice (Coyne 2013; Duncan 2010; Elwyn 2013; Legare 2010; Legare 2014).
Objectives
To assess the effects of decision aids in people facing treatment or screening decisions.
Methods
Criteria for considering studies for this review
Types of studies
We included all published studies that used a randomized controlled trial (RCT) design evaluating patient decision aids.
Types of participants
We included studies involving adults aged 18 years or older who were making decisions about screening or treatment options for themselves, a child, or an incapacitated significant other. We excluded studies in which participants were making hypothetical choices.
Types of interventions
We included studies that evaluated a patient decision aid as part of the intervention. Decision aids were defined as interventions designed to help people make specific and deliberated choices among options (including the status quo), by making the decision explicit and by providing (at the minimum) information on the options and outcomes relevant to a person's health status as well as implicit methods to clarify values. The aid also may have included: information on the disease/condition; costs associated with options; probabilities of outcomes tailored to personal health risk factors; an explicit values clarification exercise; information on others' opinions; a personalized recommendation on the basis of clinical characteristics and expressed preferences; and guidance or coaching in the steps of making and communicating decisions with others.
We excluded studies if interventions focused on: decisions about lifestyle changes, clinical trial entry, or general advance directives (e.g. do not resuscitate); education programmes not geared to a specific decision; and interventions designed to promote adherence or elicit informed consent regarding a recommended option. We also excluded studies when the relevant decision aid(s) were not available to us and not adequately described in the article(s), because we could not determine the aids’ characteristics and whether or not they met the minimum criteria to qualify as patient decision aids.
Types of comparisons
We included studies that compared patients exposed to a patient decision aid to patients in comparison groups that were exposed to usual care, general information, clinical practice guideline, placebo intervention, or no intervention. For the purposes of this review, we refer to all such control comparisons as 'usual care'.
We excluded studies that compared two different types of patient decision aids.
Types of outcome measures
To ascertain whether the decision aids achieved their objectives, we examined a broad range of outcomes. Although the decision aids focused on diverse clinical decisions, many had similar objectives such as improving knowledge scores, the accuracy of risk perceptions, and participation in decision making. Many of these evaluation criteria mapped onto the International Patient Decision Aids Standards (IPDAS) criteria for evaluating the effectiveness of decision aids (Elwyn 2006; IPDAS 2005b; Sepucha 2013). The IPDAS criteria were attributes related to the choice (e.g. match between the chosen option and the features that matter most to the informed patient) and to the decision‐making process (e.g. helps patients to recognize that a decision needs to be made; know the options and their features; understand that values affect the decision; be clear about the features that matter most; discuss values with their clinician; and become involved in their preferred ways). A complete list of outcomes, specified in advance of the review, included primary and secondary outcomes.
Primary outcomes
Evaluation criteria that map onto the IPDAS criteria
Attributes of the choice made: does the patient decision aid improve the match between the chosen option and the features that matter most to the informed patient (demonstrated by outcomes such as knowledge, accurate risk perceptions, values‐choice congruence)?
Attributes of the decision‐making process: does the patient decision aid help patients to recognize that a decision needs to be made, feel informed about the options and their features, be clear about the option features that matter most, discuss values with their clinician, and become involved in decision making?
Other decision‐making process variables
Decisional conflict
Patient‐clinician communication
Participation in decision making
Proportion undecided
Satisfaction with the choice, with the process of decision making, and with the preparation for decision making
Secondary outcomes
Behaviour
Choice (the actual choice implemented; if not reported, the participants’ preferred option was used as a surrogate measure)
Adherence to chosen option
Health outcomes
Health status and quality of life (generic and condition‐specific)
Anxiety, depression, emotional distress, regret, confidence
Healthcare system
Costs, cost‐effectiveness
Consultation length
Litigation rates
Search methods for identification of studies
Our search strategy for the review included:
searching electronic medical and social science databases; and
searching other resources.
Electronic searches
For this update, we used the same search strategy that was revised by the Trials Search Coordinator at the Cochrane Consumers and Communication Group in the last update (Stacey 2014b).
Therefore, the cumulative search of electronic databases is as follows.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 6) in the Cochrane Library (searched to 24 April 2015).
MEDLINE Ovid (1966 to 24 April 2015).
Embase Ovid (1980 to 24 April 2015).
PsycINFO Ovid (1806 to 24 April 2015).
CINAHL Ovid (1982 to September 2008), then in Ebsco (to 24 April 2015).
We present the search strategies in Appendix 1 and Appendix 2.
Searching other resources
On 18 December 2015 we also searched trial registries (World Health Organization, ClinicalTrials.gov), the Internet using Google and Google Scholar, and the Decision Aid Library Inventory (decisionaid.ohri.ca). Finally, reference lists of all newly included trials were searched.
Data collection and analysis
For this current update, we focused only on new publications that had appeared since the previous publication (Stacey 2014b), and we limited the inclusion to patient decision aids versus usual care. As such, we removed studies from the previous reviews that compared detailed versus simple patient decision aids to provide a more focused review.
Selection of studies
Pairs of eight review authors (CB, DS, RT, MB, MHR, KE, NC, DR) screened all identified citations. We retrieved the full text of any papers identified as potentially relevant by at least one author, listing all papers excluded from the review at this stage, with reasons, in the 'Characteristics of excluded studies' table. We also provided citation details and any available information about ongoing studies, and we collated and reported details of additional publications, so that each study (rather than each report) was the unit of interest. We report the screening and selection process in Figure 1.
1.
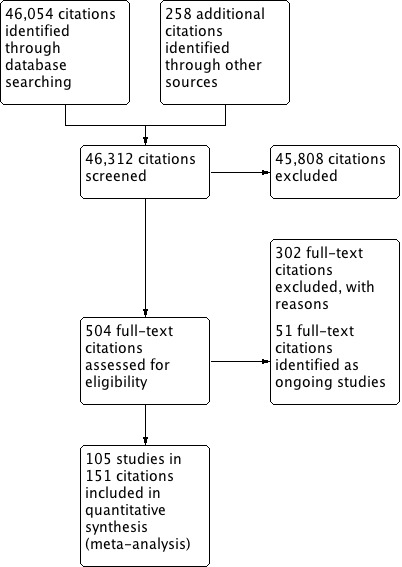
Study flow diagram.
Data extraction and management
Two research assistants extracted data independently (KL, IS). We compared findings and resolved inconsistencies through discussion with the principal investigator (DS) and, when necessary, with a co‐author (CB). No review authors extracted data for their own studies in this update nor in any other versions of this review.
One review author entered all extracted data into Review Manager 5 (RevMan 5), and a second one worked independently to check for accuracy against the data extraction sheets (RevMan 2014).
Assessment of risk of bias in included studies
Two research assistants independently appraised studies using the Cochrane 'Risk of bias' tool (current update: KL, IS) (Higgins 2011, Chapter 8). We judged each item as conferring high, low, or unclear risk of bias as set out in the criteria provided by Higgins 2011, and we provided a quote from the study report and a justification for our judgement for each item in the 'Risk of bias' table. For the item on 'other' potential sources of bias, the assessment included: whether the same clinician provided consultation to both the intervention and usual care groups with measures taken postconsultation, whether clustering was accounted for in the analysis; and potential sources of bias reported by the authors in the study limitations.
We resolved inconsistencies by discussion with the principal investigator (DS) and, when necessary, with a co‐author (CB). No review authors appraised risk of bias for their own studies in this update nor in any other versions of this review.
Studies were deemed to be at the highest risk of bias if they were scored as at high risk on any of the items of the risk of bias tool (Higgins 2011).
Measures of treatment effect
For dichotomous outcomes, we analyzed data based on the number of events and the number of people assessed in the intervention and comparison groups. We will use these to calculate the risk ratio (RR) and 95% confidence interval (CI). For continuous measures, we analyzed data based on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI.
First, we described study characteristics individually. The a priori comparison was usual care versus decision aids. For studies in which there were more than one intervention group, we extracted data from the groups that provided the strongest contrast between the intervention and control groups. We pooled results across studies in cases where investigators used similar outcome measures and the effects were expected to be independent of the type of decision studied. For example, we expected decision aids to improve knowledge and create accurate percetions of options, benefits, and harms; to reduce decisional conflict; and to enhance active participation in decision making. Therefore, we pooled data from included RCTs for these outcomes if trials used comparable measures. To facilitate pooling of data for some outcomes (e.g. knowledge, decisional conflict), we standardized the scores to range from 0 to 100 points. When analysing the effects of decision aids on choices, we pooled outcomes on more homogeneous subgroups of decisions (choice of major surgery versus conservative options; screening test or not, etc.).
Unit of analysis issues
We checked for unit‐of‐analysis errors. Where we found errors and sufficient information was available, we re‐analyzed the data using the appropriate unit of analysis by taking account of the intracluster correlation (ICC). We obtained estimates of the ICC by contacting authors of included studies, or we imputed them using estimates from external sources. For two studies (Kupke 2013; Lewis 2010), it was not possible to obtain sufficient information to re‐analyze the data, and we reported these studies as being at high risk for 'other' bias based on these unit‐of‐analysis errors. We made no adjustments to the data based on these two studies that were included in meta‐analysis for knowledge only.
Dealing with missing data
We contacted authors to obtain missing data. Where possible, we conducted analysis on an intention‐to‐treat basis; otherwise, we analyzed data as reported. We reported on the levels of loss to follow‐up and assessed this as a source of potential bias.
Assessment of heterogeneity
For this update and in previous versions of the review, we grouped studies together across populations and settings. The aim was to enable an assessment of the effectiveness of decision aids across conditions, rather than to focus on disease‐specific contexts. Given that decision aids are a well‐defined and clearly delineated type of intervention, we decided that this approach was defensible. On the basis of grouping studies across populations and decision aid elements, we anticipated that there would be a substantial degree of heterogeneity in our pooled effect estimates. However, we decided that we would consider the direction of effects and variability in these rather than variability in the size of effects, as the major basis for our interpretation of heterogeneity. This meant that for those pooled effect estimates where the direction of effect was consistent across studies, we did not downgrade for inconsistency, despite some variability in the size of effects across individual studies. We did downgrade for inconsistency for one outcome: congruence between the chosen option and informed values. This was because there is no accepted gold standard measure for assessing this outcome, and we considered that variability in measurement by the included studies added further uncertainty about the effects of decision aids for this outcome.
Where heterogeneity was present in pooled effect estimates, we explored possible reasons for variability by conducting subgroup analysis in the 2009 update (O'Connor 2009b). The post hoc analysis included the IPDAS effectiveness criteria to explore heterogeneity according to the following factors: the type of decision (treatment versus screening), the type of media of the decision aid (video/computer versus audio booklet/pamphlet), and the possibility of a ceiling effect based on usual‐care scores (resulting in the removal of studies with lower scores for knowledge and accurate risk perception and higher scores for decisional conflict using the subscales measuring levels of informedness and clarity of values). We analyzed the effect of removing the biggest outlier(s) (defined by visual inspection of forest plots). Given that the post hoc analysis did not alter the findings from the 2009 update, we did not re‐conduct the post hoc analysis for the IPDAS effectiveness criteria.
Assessment of reporting biases
We used funnel plots to assess publication bias.
Data synthesis
We used RevMan 5 software to estimate a weighted intervention effect with 95% confidence intervals (RevMan 2014). For continuous measures, we used mean differences (MD); for dichotomous outcomes, we calculated pooled relative risks (RR). We analyzed all data with a random‐effects model because of the diverse nature of the studies being combined and then anticipated variability in the populations and interventions of the included studies. We summarized all of the results for the primary outcomes and rated the strength of evidence using GRADE (Andrews 2013), presenting these in a 'Summary of findings' table (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
For this update, we conducted a subgroup analysis to compare the effects of the intervention when used in preparation for the consultation with the effects of those used during the consultation to usual care.
Sensitivity analysis
We performed post hoc sensitivity analyses to examine the effect of excluding studies of lower methodological quality. The analysis excluded studies that were at high risk of bias for any of the categories in the 'Risk of bias' assessment (Higgins 2011).
'Summary of findings' table
We prepared a 'Summary of findings' table to present the results of meta‐analysis, based on the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We presented the results of meta‐analysis for the major comparison of the review for each of the key outcomes. We provided a source and rationale for each assumed risk cited in the table and used the GRADE criteria to rank the quality of the evidence for each outcome on each of the following domains: risk of bias, inconsistency, imprecision, indirectness, and publication bias. Two authors independently assessed the quality of the evidence using the GRADEprofiler (GRADEpro) software (GRADEpro GDT).
Results
Description of studies
The current version of our review updates our 2014 version, Stacey 2014b, with 18 new studies (Bozic 2013; Brazell 2014; Chabrera 2015; Fraenkel 2012; Knops 2014; Köpke 2014; Kuppermann 2014; Lam 2013; LeBlanc 2015; Legare 2012; Lepore 2012; Mathers 2012; Mott 2014; Sawka 2012; Shourie 2013; Stacey 2014a; Taylor 2006; Williams 2013). For this update, we excluded 28 previously included studies due to the comparisons being limited to detailed versus simple patient decision aids (Deschamps 2004; Deyo 2000; Dodin 2001; Goel 2001; Green 2004; Hunter 2005; Kuppermann 2009; Labrecque 2010; Lalonde 2006; Legare 2003; Leung 2004; Myers 2005a; Myers 2011; O'Connor 1998a; O'Connor 1999a; Raynes‐Greenow 2010; Rostom 2002; Rothert 1997; Schapira 2000; Schapira 2007; Solberg 2010; Street 1995; Tiller 2006; Van Roosmalen 2004; Volk 2008; Wakefield 2008a; Wakefield 2008b; Wakefield 2008c).
Results of the search
In total, we identified 46,054 citations from the electronic database searches and 258 citations from other sources. Of these, we assessed 504 citations for eligibility using the full text (see Figure 1).
Included studies
The remaining 151 citations provided data on 105 studies that met our inclusion criteria, 18 of which are new for this update. The 105 RCTs, involving 31,043 participants, presented results from 10 countries: Australia (10 studies), Canada (15 studies), China (1 study), Finland (2 studies), Germany (6 studies), Netherlands (2 studies), Spain (1 study), Sweden (1 study), the UK (16 studies), the USA (50 studies), and Australia plus Canada (1 study). We present study details below and in the Characteristics of included studies table.
Unit of randomization
Ninety studies randomized individual patients, and 15 randomized clusters. For cluster trials, Allen 2010 randomized 12 company worksites; Fraenkel 2012, 2 groups of primary care physicians; Hamann 2006, 12 inpatient psychiatric units; Kupke 2013, 49 dental students; Legare 2011, 4 family medicine group practices; Legare 2012, 12 family medicine group practices; Lewis 2010, 32 family medicine group practices; Loh 2007, 30 general practitioners; Mathers 2012, 49 general medicine practices; McAlister 2005, 102 primary care practices; Mullan 2009, 40 clinicians; Nagle 2008, 60 general practitioners; Shourie 2013, 50 general medicine practices; Weymiller 2007, 21 endocrinologists; and Whelan 2004, 27 surgeons.
For 10 studies (Allen 2010; Legare 2011; Legare 2012; Loh 2007; Mathers 2012; Mullan 2009; Nagle 2008; Shourie 2013; Weymiller 2007; Whelan 2004), the cluster effect was taken into account in the published outcome data, and the meta‐analysis used published results. Although Hamann 2006 did not account for the cluster effect in the published outcome data, the way this study was reported did not allow us to include it in the meta‐analysis, so we did not re‐analyze the data and report the study separately. For McAlister 2005, meta‐analysis was done applying the design effect (based on the published intracluster correlation coefficient (ICC)). For Fraenkel 2012, the authors stated that adding a random effect for physician clusters did not contribute to better‐fitting regression models, and we removed it from the analysis. The analysis by Kupke 2013 and Lewis 2010 did not account for clustering.
Decision aids and comparisons
The 105 included studies evaluated decision aids that focused on 50 different decisions (Table 2). The most common decisions were about prostate cancer screening (14 studies), colon cancer screening (10 studies), medication for diabetes (4 studies), breast cancer genetic testing (4 studies), prenatal screening (4 studies), medication for atrial fibrillation (4 studies), and surgery (mastectomy for breast cancer, 4 studies; hysterectomy, 3 studies; prostate cancer treatment, 4 studies). New decision topics added in this update included surgery for prolapsed pelvic organs (1 study) and asymptomatic aortic abdominal aneurysm (1 study); restoration for tooth decay (1 study); measles, mumps, and rubella vaccine for infants (1 study); treatment of post‐traumatic stress disorder (1 study); and radioactive iodine treatment for thyroid cancer (1 study).
1. Decision aids evaluated in the trials.
| Study | Topic | Availability | Source | Contact Information |
| Allen 2010 | Prostate cancer screening | No | Allen, Center for Community‐Based Research, Dana‐Farber Cancer Institute, Boston, MA, USA, 2010 | Requested access |
| Arterburn 2011 | Bariatric surgery | Yes | Informed Medical Decisions Foundation, MA,USA, 2010 | informedmedicaldecisions.org/imdf_decision_aid/making‐decisions‐about‐weight‐loss‐surgery/ |
| Auvinen 2004 | Prostate cancer treatment | Yes | Auvinen, Helsinki, Finland, 1993 | Included in publication |
| Barry 1997 | Benign prostate disease treatment | Yes | Informed Medical Decisions Foundation, MA, USA, 2001 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐options‐for‐benign‐prostatic‐hyperplasia/ |
| Bekker 2004 | Prenatal screening | Yes | Bekker, Leeds, UK, 2003 | Included in publication |
| Bernstein 1998 | Ischaemic heart disease treatment | Yes | Informed Medical Decisions Foundation, MA,USA, 2002 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐carotid‐artery‐disease/ |
| Berry 2013 | Prostate cancer treatment | No | Berry, Phyllis F. Cantor Center, MA, USA, 2011 | donna_berry@dfci.harvard.edu |
| Bjorklund 2012 | Antenatal Down syndrome screening | Yes | Södersjukhuset, Department of Obstetrics and Gynecology, Stockholm, Sweden | vimeo.com/34600615/ |
| Bozic 2013 | Osteoarthritis of the knee or hip | No | Informed Medical Decisions Foundation and Health Dialog; USA | www.healthdialog.com |
| Brazell 2014 | Pelvic Organ Prolapse | Yes | Healthwise, USA | decisionaid.ohri.ca |
| Chabrera 2015 | Prostate cancer treatment | No | C Chabrera. School of Health Sciences, Department of Nursing. Mataro, Spain | cchabrera@tecnocampus.cat |
| Chambers 2012 | Healthcare personnel’s influenza immunization | Yes | A McCarthy. Ottawa Influenza Decision Aid Planning Group, CA, 2008 | decisionaid.ohri.ca/decaids.html#oida |
| Clancy 1988 | Hepatitis B Vaccine | No | Clancy, Richmond VA, USA, 1983 | — |
| Davison 1997 | Prostate cancer treatment | No | Davison, Manitoba CA, 1992‐1996 | — |
| De Achaval 2012 | Total knee arthroplasty treatment | Yes | Informed Medical Decisions Foundation, MA,USA | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐knee‐osteoarthritis/ |
| Dolan 2002 | Colon cancer screening | No | Dolan, Rochester NY, USA, 1999 | — |
| Evans 2010 | Prostate cancer screening | Yes | Elwyn, Cardiff, UK | www.prosdex.com |
| Fagerlin 2011 | Breast cancer prevention | Yes | Fagerlin, Ann Arbor, MI, USA | — |
| Fraenkel 2007 | Osteoarthritis knee treatment | No | Fraenkel, New Haven CT, USA | Author said DA never fully developed, all info in paper |
| Fraenkel 2012 | Atrial fibrillation | No | Veterans Affairs Connecticut Healthcare System, USA | Obtained from author terri.fried@yale.edu |
| Frosch 2008a | Prostate cancer screening | No | Frosch, Los Angeles, USA | Screenshots from author |
| Gattellari 2003 | Prostate cancer screening | Yes | Gatellari, Sydney, AU, 2003 | included in publication |
| Gattellari 2005 | Prostate cancer screening | Yes | Gatellari, Sydney, AU, 2003 | Included in publication |
| Green 2001 | Breast cancer genetic testing | Yes | Green, Hershey PA, USA, 2000 | 1‐800‐757‐4868 dwc@mavc.com |
| Hamann 2006 | Schizophrenia treatment | Yes | Hamann, Munich, GER | Emailed by author (in German) |
| Hanson 2011 | Feeding options in advanced dementia | Yes | Mitchell, Tetroe, O'Connor; 2001 (updated 2008) | decisionaid.ohri.ca/decaids.html#feedingtube |
| Heller 2008 | Breast reconstruction | Yes | University of Texas MD Anderson Cancer Center, Houston TX, USA, 2003 | Disc mailed |
| Hess 2012 | Stress testing for chest pain | Yes | Hess, Rochester, MN, USA, 2012 | Included in publication |
| Jibaja‐Weiss 2011 | Breast cancer treatment | Yes | Jibaja‐Weiss, Baylor College of Medicine, 2010 | www.bcm.edu/patchworkoflife |
| Johnson 2006 | Endodontic treatment | Yes | Johnson, Chicago, USA, 2004 | Included in publication |
| Kasper 2008 | Multiple sclerosis | No | Jürgen Kasper | — |
| Kennedy 2002 | Abnormal uterine bleeding treatment | No | Kennedy/Coulter, London UK, 1996 | — |
| Knops 2014 | Asymptomatic Abdominal Aortic Aneurysm treatment | Yes | Amsterdam, The Netherlands | www.keuzehulp.info/amc/AAA/landing‐page |
| Krist 2007 | Prostate cancer screening | Yes | Krist, Fairfax VA, USA | www.familymedicine.vcu.edu/research/misc/psa/index.html |
| Kupke 2013 | Dental ‐ posterior tooth decay | Yes | University of Cologne, Cologne, Germany | jana.kupke@uk‐koeln.de |
| Kuppermann 2014 | Prenatal screening | No | Kuppermann, San Francisco CA, USA | Interactive web‐based decision aid |
| Lam 2013 | Breast cancer treatment | Yes | Kwong Wah Hospital, Hong Kong, China | Obtained from author. wwtlam@hku.hk |
| Langston 2010 | Contraceptive method choice | Yes | World Health Organization, 2005 | www.who.int/reproductivehealth/publications/family_planning/9241593229index/en/index.html |
| Laupacis 2006 | Pre‐operative autologous blood donation | No | Laupacis, Ottawa, CA, 2001 | Decisionaid.ohri.ca/decaids‐archive.html |
| LeBlanc 2015 | Treatment for osteoporosis | Yes | Mayo Clinic | — |
| Legare 2008a | Natural health products | No | Legare, Quebec City, CA, 2006 | — |
| Legare 2011 | Use of antibiotics for acute respiratory infections | Yes | Legare, Quebec City, CA, 2007 | www.decision.chaire.fmed.ulaval.ca/index.php?id=192&L=2 |
| Legare 2012 | Antibiotics for acute respiratory infections | Yes | Legare, Quebec City, CA | www.decision.chaire.fmed.ulaval.ca/index.php? |
| Leighl 2011 | Advanced colorectal cancer chemotherapy | Yes | Princess Margaret Hospital, Toronto, 2011 | Natasha.Leighl@uhn.on.ca |
| Lepore 2012 | Prostate cancer screening | Yes | Sally Weinrich University of Louisville, USA | Obtained from author slepore@temple.edu |
| Lerman 1997 | Breast cancer genetic testing | No | Lerman/Schwartz, Washington DC, USA, 1997 | — |
| Lewis 2010 | Colorectal cancer screening | Yes | Lewis, University of North Carolina, Chapel Hill, NC, USA, 2010 | decisionsupport.unc.edu/CHOICE6/ |
| Loh 2007 | Depression treatment | Yes | Loh, Freiburg, GER | Emailed to us by author ‐ in German |
| Man‐Son‐Hing 1999 | Atrial fibrillation treatment | No | McAlister/Laupacis, Ottawa CA, 2000 | decisionaid.ohri.ca/decaids‐archive.html |
| Mann D 2010 | Diabetes treatment ‐ statins | Yes | Montori, Rochester MN, USA | mayoresearch.mayo.edu/mayo/research/ker_unit/form.cfm |
| Mann E 2010 | Diabetes screening | Yes | Marteau, King's College London, London, England, 2010 | Additional file 2 of publication |
| Marteau 2010 | Diabetes screening | Yes | Marteau, King's College London, London, England, 2010 | Provided by author, same DA as Mann E 2010 |
| Mathieu 2007 | Mammography | Yes | Mathieu, Sydney, AU | DA emailed by author |
| Mathers 2012 | Diabetes treatment | Yes | The University of Sheffield, Sheffield, UK, 2008 | Obtained from author C.Ng@sheffield.ac.uk |
| Mathieu 2010 | Mammography | Yes | Mathieu, University of Sydney, AUS, 2010 | www.psych.usyd.edu.au/cemped/com_decision_aids.shtml |
| McAlister 2005 | Atrial fibrillation treatment | No | McAlister/Laupacis, Ottawa CAN, 2000 | decisionaid.ohri.ca/decaids‐archive.html |
| McBride 2002 | Hormone replacement therapy | Yes, update in progress | Sigler/Bastien, Durham NC, USA, 1998 | basti001@mc.duke.edu |
| McCaffery 2010 | Screening after mildly abnormal pap smear | Yes | Screening & test evaluation program, School of public health, University of Sydney 2007 | kirstenm@health.usyd.edu.au |
| Miller 2005 | BRCA1/BRCA2 gene testing | No | Miller, Fox Chase PA, USA | — |
| Miller 2011 | Colorectal cancer screening | Yes | University of North Carolina, Chapel Hill, NC, USA, 2007 | intmedweb.wakehealth.edu/choice/choice.html (no longer available) |
| Montgomery 2003 | Hypertension treatment | No | Montgomery, UK, 2000 | — |
| Montgomery 2007 | Birthing options after caesarean | Yes | Montgomery, Bristol, UK, last update 2004 | www.computing.dundee.ac.uk/acstaff/cjones/diamond/Information.html |
| Montori 2011 | Osteoporosis treatment | Yes | Montori, Mayo Foundation for Medical Education and Research, 2007 | shareddecisions.mayoclinic.org/decision‐aids‐for‐diabetes/other‐decision‐aids/ |
| Morgan 2000 | Ischaemic heart disease treatment | Yes | Informed Medical Decisions Foundation, MA, USA, 2002 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐carotid‐artery‐disease/ |
| Mott 2014 | PTSD treatment | Yes | Michael E DeBakey Veterans Affairs Medical Center, Houston, USA | Obtained from author juliette.mott@va.gov |
| Mullan 2009 | Diabetes treatment | Yes | Montori or Mayo Foundation(?) Rochester MN, USA, | Included in publication |
| Murray 2001a | Benign prostate disease treatment | Yes | Informed Medical Decisions Foundation, MA, USA, 2001 | informedmedicaldecisions.org/imdf_decision_aid/treatment‐options‐for‐benign‐prostatic‐hyperplasia/ |
| Murray 2001b | Hormone replacement therapy | No, update in progress | Informed Medical Decisions Foundation, MA, USA | informedmedicaldecisions.org/imdf_decision_aid/treatment‐choices‐for‐managing‐menopause/ |
| Nagle 2008 | Prenatal screening | Yes | Nagle, Victoria, AU | www.mcri.edu.au/Downloads/PrenatalTestingDecisionAid.pdf |
| Nassar 2007 | Birth breech presentation | Yes | Nassar, West Perth WA, AU | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php |
| Oakley 2006 | Osteoporosis treatment | No | Cranney, Ottawa CA, 2002 | decisionaid.ohri.ca/decaids‐archive.html |
| Ozanne 2007 | Breast cancer prevention | No | Ozanne, Boston MA, USA | — |
| Partin 2004 | Prostate cancer screening | Yes | Informed Medical Decisions Foundation, MA,USA, 2001 | informedmedicaldecisions.org/imdf_decision_aid/deciding‐if‐the‐psa‐test‐is‐right‐for‐you/ |
| Pignone 2000 | Colon cancer screening | Yes | Pignone, Chapel Hill NC, USA, 1999 | www.med.unc.edu/medicine/edusrc/colon.htm |
| Protheroe 2007 | Menorrhagia treatment | No | Protheroe, Manchester, UK | Computerized decision aid, Clinical Guidance Tree ‐ no longer in existence, author sent chapter in thesis |
| Rubel 2010 | Prostate cancer screening | No | Centers for Disease Control and Prevention (CDC), USA, 2010 | No longer available |
| Ruffin 2007 | Colorectal cancer screening | Yes | Regents of the University of Michigan (copyright info), Ann Arbor MI, USA, 2006 | colorectalweb.org |
| Sawka 2012 | Adjuvant radioactive iodine treatment for patients with early‐stage papillary thyroid cancer | No | University Health Network, Toronto, Canada, 2009 | — |
| Schroy 2011 | Colorectal cancer screening | Yes | Schroy III, Boston, USA | Paul.schroy@bmc.org |
| Schwalm 2012 | Coronary angiogram access site | Yes | Schwalm, Hamilton, ON, Canada, 2009 | www.phri.ca/workfiles/studies/presentations/PtDA%20Vascular%20Access%2023‐May−2012.pdf |
| Schwartz 2001 | Breast cancer genetic testing | No | Schwartz/Lerman, Washington DC, USA, 1997 | — |
| Schwartz 2009a | BRCA mutation prophylactic surgery | No | Schwartz, Washington DC, USA | — |
| Sheridan 2006 | Cardiovascular prevention | Yes | Sheridan, Chapel Hill, NC, USA | www.med‐decisions.com/cvtool/ |
| Sheridan 2011 | Coronary heart disease prevention | Yes | Sheridan, University of North Carolina at Chapel Hill, Division of General Internal Medicine, North Carolina, USA, 2011 | www.med‐decisions.com/h2hv3/ |
| Shorten 2005 | Birthing options after previous caesarean | Yes (updated 2006) | Shorten, Wollongong, AU, 2000 | ashorten@uow.edu.au or www.capersbookstore.com.au/product.asp?id=301 |
| Shourie 2013 | Measles mumps and rubella vaccination | Yes | University of Leeds, UK & NSIRS Australia | www.leedsmmr.co.uk |
| Smith 2010 | Bowel cancer screening | Yes | Smith, Sydney, AU 2008 | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php |
| Stacey 2014a | Osteoarthritis of the hip and knee | No | Informed Medical Decisions Foundation and Health Dialog; USA | www.healthdialog.com |
| Steckelberg 2011 | Colorectal cancer screening | Yes | Steckelberg, Hamburg, Germany | — |
| Taylor 2006 | Prostate cancer screening | Yes | Georgetown University Medical Center, Washington DC, USA, 2000 | Obtained from author taylorkl@georgetown.edu |
| Thomson 2007 | Atrial fibrillation treatment | Yes | Thomson, Newcastle Upon Thyne, UK | Disc sent by mail |
| Trevena 2008 | Colorectal cancer screen | Yes | Trevena, Sydney, AU | sydney.edu.au/medicine/public‐health/shdg/resources/decision_aids.php |
| Van Peperstraten 2010 | Embryos transplant | Yes | Radboud University Nijmegen Medical Centre; 2006 | www.umcn.nl/ivfda‐en |
| Vandemheen 2009 | Cystic Fibrosis referral transplant | Yes | Aaron, Ottawa ON, CA, 2009 (last update 2011) | decisionaid.ohri.ca/decaids.html#cfda |
| Vodermaier 2009 | Breast cancer surgery | Yes | Vodermaier, Vancouver BC, CA | Received by email (in German) |
| Volk 1999 | Prostate cancer screening | Yes | Informed Medical Decisions Foundation, MA, USA, 1999 | informedmedicaldecisions.org/imdf_decision_aid/deciding‐if‐the‐psa‐test‐is‐right‐for‐you/ |
| Vuorma 2003 | Menorrhagia treatment | No | Vuorma, Helsinki Finland, 1996 | — |
| Watson 2006 | Prostate cancer screening | Yes | Oxford, UK | Included in publication |
| Weymiller 2007 | Diabetes mellitus type 2 treatment | Yes | Montori, Rochester MN, USA | mayoresearch.mayo.edu/mayo/research/ker_unit/form.cfm |
| Williams 2013 | Prostate cancer screening | Yes | Georgetown University, Washington, DC, USA | Obtained from author taylorkl@georgetown.edu |
| Whelan 2003 | Breast cancer chemotherapy | Yes | Whelan, Hamilton CA, 1995 | Included in publication |
| Whelan 2004 | Breast cancer surgery | Yes | Whelan, Hamilton CA, 1997 | Included in publication |
| Wolf 1996 | Prostate cancer screening | Yes | Wolf, Charlottesville VA, USA, 1996 | Script in publication |
| Wolf 2000 | Colon cancer screening | Yes | Wolf, Charlottesville VA, USA, 2000 | Script in publication |
| Wong 2006 | Pregnancy termination | No | Bekker, Leeds, UK, 2002 | — |
The decision aids used different formats and were compared to a variety of control interventions (e.g. usual care, general information, no intervention, guideline, placebo intervention). We noted the nature of usual care when reported (see Characteristics of included studies table). For this review, we have grouped control interventions and refer to them as 'usual care'.
According to the definition of a patient decision aid, all of the studies evaluated patient decision aids that included information about the options and outcomes and provided at least implicit clarification of values. Most patient decision aids included information on the clinical problem (90.5%) as well as outcome probabilities (89.5%). Fewer patient decision aids provided guidance in the steps of decision making (65.7%), explicit methods to clarify values (57.1%), and/or examples of others' experiences (41.0%) (see table Characteristics of included studies).
Excluded studies
We excluded 302 studies upon close perusal of the relevant papers (see Characteristics of excluded studies). The reasons for exclusion were: the study was not a randomized controlled trial; the decision was hypothetical, with participants not actually at a point of decision making; the intervention was not focused on making a choice; the intervention offered no decision support in the form of a decision aid or did not provide enough information about the decision aid; no comparison outcome data were provided; the study did not evaluate the decision aid; the study was a protocol; the decision aid was about clinical trial entry, lifestyle choice, or advanced care planning; the study involved testing the presentation of the decision aid, but with no difference in the content of the decision aid between study groups; or the study compared a detailed versus simple decision aid.
We also identified 61 ongoing RCTs through trial registration databases, personal contact, and published protocols in the electronic database searches (see references to Ongoing studies and table Characteristics of ongoing studies).
Risk of bias in included studies
Details on the ratings and rationale for risk of bias are in the Characteristics of included studies table and displayed in Figure 2 and Figure 3.
2.
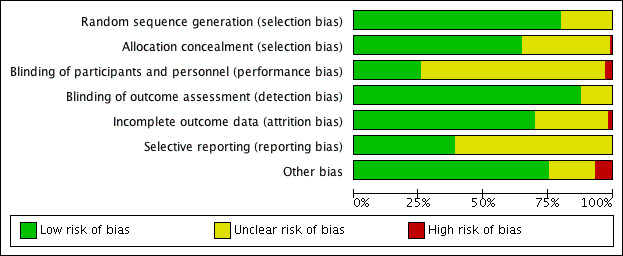
Risk of bias summary as percentages across all included studies.
3.
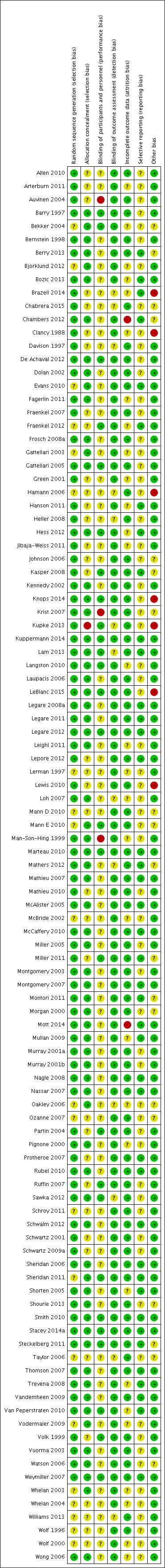
Risk of bias summary for each included study.
Allocation
When assessing risk of selection bias, we rated all 105 studies as being at low or unclear risk of bias. Allocation concealment methods prompted a rating of low or unclear risk of bias in 104 studies and high risk of bias in 1 study (Kupke 2013).
Blinding
We judged 102 studies to be at low or unclear risk of performance and detection bias for the blinding of participants and personnel, while 3 (2.9%) studies were at high risk of bias. High risk of bias was due to lack of blinding of physicians to the status of patients randomized to the patient decision aid and alternative interventions (Auvinen 2004; Krist 2007; Man‐Son‐Hing 1999).
We rated the blinding of outcome assessment as leading to low or unclear risk of bias in all 105 studies.
Incomplete outcome data
For 103 studies, aspects related to incomplete outcome data conferred low or unclear risk of bias. In two (1.9%) studies (Chambers 2012; Mott 2014), there was high risk of bias due to high attrition rates.
Selective reporting
We rated all 105 studies as being at either low risk of bias because the protocol was registered publicly or at unclear risk of bias because we could not assess the extent or the impact of any reporting bias.
Other potential sources of bias
Of 105 studies, we rated 98 as being at low or unclear risk of other potential sources of bias. The other seven (6.7%) discussed other potential risks of bias (Brazell 2014; Clancy 1988; Hamann 2006; Knops 2014; Kupke 2013; LeBlanc 2015; Lewis 2010). We rated Brazell 2014 and LeBlanc 2015 as being at high risk of bias given that the same physicians provided consultation to both intervention and control groups, and measures were taken after physician consultation. Clancy 1988 describes a potential for selection bias because non‐randomized medical residents were added to the decision aid group, and there was a low response rate among those offered decision aid. We rated Knops 2014 as being at high risk of bias given that a large number of potential participants did not participate in the study. Hamann 2006, Kupke 2013, and Lewis 2010 did not account for clustering in their analyses.
Effects of interventions
See: Table 1
In addition to Table 1, see the Data and analyses figures for pooled data and Additional tables for outcome data that we did not pool. This section presents the attributes of the choice made, the attributes of the decision process, and secondary outcomes.
Primary outcomes
Attributes of the choice made: does the patient decision aid improve the match between the chosen option and the features that matter most to the informed patient?
The randomized controlled trials used three measures that correspond to this outcome: knowledge scores, accuracy of risk perceptions, and congruence between the chosen option and the patient's values.
Knowledge
Seventy‐one of the 105 studies (67.6%) assessed the effects of decision aids on knowledge. The studies' knowledge tests were based on information contained in the decision aid. The proportion of accurate responses was transformed to a percentage scale ranging from 0% (no correct responses) to 100% (fully correct responses).
There is high‐quality evidence that patient decision aids were more effective than usual care (52 studies) on knowledge scores (MD 13.27, 95% CI 11.32 to 15.23; Analysis 1.1). In absolute terms the group receiving usual care had, on average, 57 of 100 answers correct. Those in the decision aid group scored better, with 70 of 100 answers correct on average (from 68 to 72 correct).
1.1. Analysis.
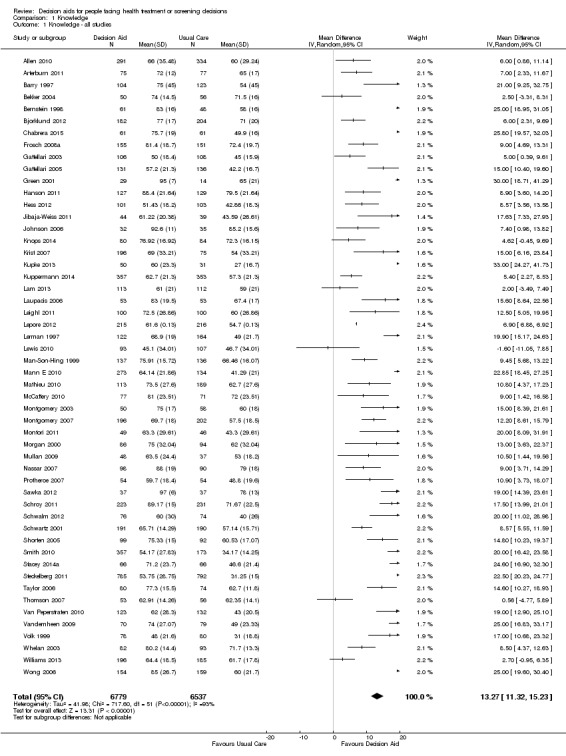
Comparison 1 Knowledge, Outcome 1 Knowledge ‐ all studies.
Nineteen additional studies presented knowledge scores that could not be included in the pooled outcome (see Table 3). Most of these other studies reported statistically‐significantly higher knowledge scores for those exposed to the decision aid compared to usual care. The funnel plot for knowledge as an outcome in studies comparing decision aid to usual care shows that these studies are at low risk for publication bias (Figure 4).
2. Knowledge.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Bozic 2013 | Decision quality instrument, 19 items re knowledge (> 50%) | After 1st consultation with surgeon | 60 | 58.3% | 60 | 33.3% | P = 0.01 |
| Evans 2010 | 12 true or false questions; scores ranging from −12 to 12 | Immediately post | 89 | 4.9 | 103 | 2.17 | P < 0.001 |
| Fagerlin 2011 | Insufficient (≤ 50% correct) | Immediately post | 383 | 31.8% | 102 | 93.1% | P < 0.001 |
| Sufficient | Immediately post | 383 | 61.9% | 102 | 6.9% | — | |
| Fraenkel 2012 | Open‐ended questions about medication options to reduce stroke ‐ knows medications | Postintervention | 66 | 61% | 62 | 31% | OR 3.5 (95% CI: 1.6 to 7.7, P = 0.001) |
| Open‐ended questions about side effects of medications ‐ knows side effects | Postintervention | 53 | 49% | 46 | 37% | OR 1.9 (95%CI: 0.9 to 4.0; P = 0.07) | |
| Hamann 2006 | 7‐item multiple choice knowledge test (unable to standardize results) | On discharge (˜ 1 month) | 49 | 15 (4.4 SD) | 58 | 10.9 (5.4 SD) | P = 0.01 |
| Heller 2008 | 12‐item multiple choice | Pre‐operatively | 66 | 14%* | 67 | 8%* | *mean increase from baseline P = 0.02 |
|
LeBlanc 2015 (in consultation) |
13‐item questionnaire (median, IQR) total score | Immediately post | 32 | 7 (4.5 to 9.0) | 45 | 5.5 (2.5 to 8.0) | P = 0.11 |
| 9‐items knowledge based on decision aid | Immediately post | 32 | 6 (3.5 to 6.5) | 45 | 4 (2.0 to 8.0) | P = 0.01 | |
| Legare 2008a | 10‐item yes/no/unsure general knowledge test about natural health products (not specific to outcomes of options) | Change scores from baseline to 2 weeks | 43 | 0.86 ± 1.77 P = 0.002 |
41 | 0.51 ± 1.47 P = 0.031 | No difference between groups (P = 0.162) |
|
Mann D 2010 (in consultation) |
14‐item survey | Immediately post | — | — | — | — | No difference in level of knowledge between groups |
| Mathers 2012 | Correctly answers question about best option to lower blood sugar | 6 months postintervention | 95 | 51.6% | 80 | 28.8% | P < 0.001 |
| Correctly answers question about best option to lower complications | 6 months postintervention | 95 | 31.0% | 80 | 29% | P = 0.90 | |
| Mathieu 2007 | 9‐item ‐ 4 concept questions and 5 numeric questions | — | 351 | — | 357 | — | Significantly higher mean increase for the intervention group (2.62 ) compared to control group (0.68) from baseline, P < 0.001 |
| Miller 2005 | 8‐item survey | 2‐week, 2‐month, and 6‐month follow‐ups | — | — | — | — | Intervention type had no impact on general or specific knowledge |
| Nagle 2008 | Good level knowledge was scored higher than the mid point of the knowledge scale (greater than 4) | — | — | — | — | — | 88% (147/167) in DA group compared to 72% (123/171) pamphlet group. OR 3.43 (95% CI 1.79 to 6.58) |
| Ozanne 2007 (in consultation) | Change in knowledge from baseline | Post‐test | 15 | 48% to 64% | 15 | 45% to 57% | change in knowledge score was significant for decision aid (P = 0.01) but not control (P = 0.13) |
| Partin 2004 | 10‐item knowledge index score | 2 weeks | 308 | 7.44 | 290 | 6.9 | P = 0.001 |
| Rubel 2010 | 24‐items adapted from existing prostate cancer knowledge measures | Immediately post | 100 | — | 100 | — | The total mean standardized knowledge score was 84.38 (SD 12.38) |
| Trevena 2008 | Adequate knowledge (positive score: understanding benefits/harms) | 1 month | 134 | 28/134 | 137 | 8/137 | P = 0.0001 |
| Watson 2006 | 12‐item true/false/don't know | Post‐test | 468 | 75% (range 0 to 100) | 522 | 25% (range 0 to 100) | P < 0.0001 |
| Weymiller 2007 (in consultation) | 14‐item ‐ 9 addressed by decision aid; 5 were not | Immediately post | 52 | 46 | — | Mean difference between groups 2.4 (95% CI 1.5 to 3.3) P < 0.05 (when decision aid administered during the consultation only ‐ not if prior to the consultation) |
CI: confidence interval; DA: decision aid; OR: odds ratio; SD: standard deviation.
4.
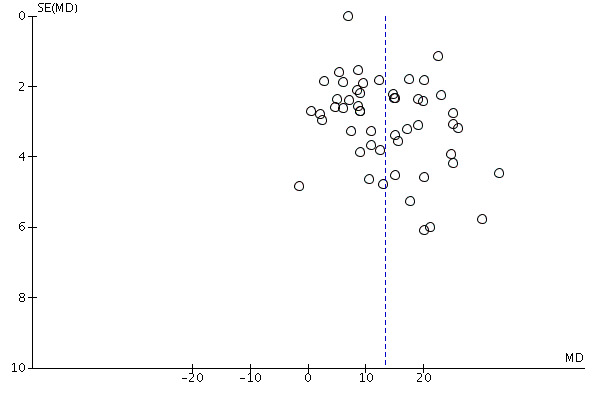
Funnel plot of comparison: 1 Knowledge, outcome: 1.1 Knowledge ‐ all studies.
Accurate risk perceptions (i.e. perceived probabilities of outcomes)
Of 105 studies, 25 (23.8%) examined the effects of patient decision aids on the accuracy of patients' perceived probabilities of outcomes (see Analysis 2.1; Table 4). We classified the accuracy of perceived outcome probabilities according to the percentage of individuals whose judgments corresponded to the scientific evidence about the chances of an outcome for similar people. For studies that elicited risk perceptions using multiple items, we averaged the proportion of accurate risk perceptions.
2.1. Analysis.
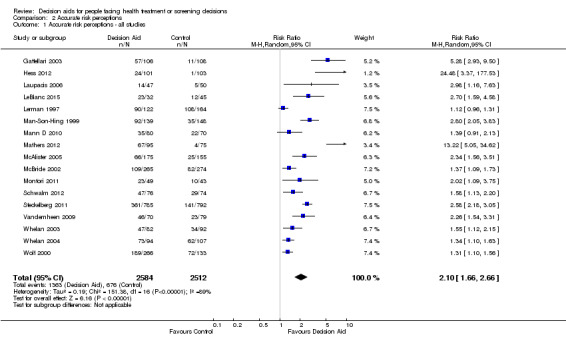
Comparison 2 Accurate risk perceptions, Outcome 1 Accurate risk perceptions ‐ all studies.
3. Accurate risk perceptions.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Fraenkel 2012 | Accuracy of stroke risk (reported by taking the absolute value of the difference between the participant's risk as estimated by the DA and the estimate provided by the participant ‐ out of 100; lower score indicates more accurate estimation of risk) |
Postintervention | 69 | 9.1 (SD 13.3) | 66 | 14.2 (SD 13) | P = 0.002 |
| Accuracy of bleeding risk (reported same as above) |
Postintervention | 69 | 8.7 (SD 12.5) | 66 | 13.1 (SD 12.2) | P = 0.004 | |
| Hanson 2011 | Expectation of benefit index 11 items score from 1 to 4 with lower score indicating better knowledge | Post (after reviewing DA) | 127 | 2.3 | 129 | 2.6 | P = 0.001 |
| Kuppermann 2014 | Correct estimate of amniocentesis miscarriage risk | 3‐6 months postintervention | 357 | 263 (73.8%) | 353 | 208 (59.0%) | P < 0.001 |
| Correct estimate of Down syndrome risk | 3‐6 months postintervention | 357 | 210 (58.7%) | 353 | 163 (46.1%) | P = 0.001 | |
| Mann E 2010 | 3 of 8 multiple choice items in the knowledge test (question 4, 5, 7) | 2 weeks post | — | — | — | — | Total knowledge reported only |
| Mathieu 2010 | 5 item numerical questions (max = 5) | Post | 113 | 3.02 | 189 | 2.45 | P < 0.001 |
| Miller 2005 | — | 2‐week, 2‐month, and 6‐month follow‐ups | — | — | — | — | Intervention type had no impact on risk perceptions |
| Smith 2010 | 8 numerical questions (max = 8) | — | 357 | 2.93 (SD 2.91) | 173 | 0.58 (SD 1.28) | P < 0.001 |
| Weymiller 2007 (in consultation) | — | Immediately | 52 | — | 46 | — | Difference between group OR 22.4 (95% CI 5.9 to 85.8) when decision aid administered during the consultation only (not if prior to) OR 6.7 (95% CI 2.2 to 19.7) when the decision aid administered prior to or during the consultation |
CI: confidence interval; DA: decision aid; OR: odds ratio; SD: standard deviation.
There is moderate‐quality evidence that patient decision aids were more effective than usual care for transmitting accurate risk perceptions (risk ratio (RR) 2.10, 95% CI 1.66 to 2.66, 17 studies; Analysis 2.1). This means that for every 1000 people receiving usual care, 269 were likely to accurately interpret risk, whereas far more people (565 people per 1000; from 447 to 716) accurately interpreted risk after using a decision aid.
Eight studies reported results that were not amenable to pooling (see Table 4). Fraenkel 2012; Hanson 2011; Kuppermann 2014; Mathieu 2010; and Smith 2010 reported a statistically significant improvement in accurate perceptions of outcomes for the decision aid group compared to usual care, and Miller 2005 reported no effect on risk perception. In another study, Weymiller 2007 reported participants allocated to the decision aid had a significantly more accurate perception of their estimated cardiovascular risk without statin therapy compared to the usual care group; this effect was greater when the clinician used the decision aid during the consultation rather than when the researcher used the decision aid in preparation for the consultation (Pinteraction= 0.03). For the final study by Mann E 2010, three of eight knowledge test items measured accurate risk perceptions, but results were presented for total knowledge and not individual items.The funnel plot for accurate risk perception as an outcome in studies comparing decision aid to usual care shows low risk for publication bias.
Congruence between chosen option and values
Of 105 studies, 16 (15.3%) measured congruence between the chosen options and the patients' values. Six measured values‐choice congruence without considering knowledge (Arterburn 2011; Berry 2013; Frosch 2008a; Legare 2008a; Lerman 1997; Vandemheen 2009). Of 10 studies that measured informed values‐choice congruence, eight used the Multi‐Dimensional Measure of Informed Choice (Bjorklund 2012; Fagerlin 2011; Mathieu 2007; Mathieu 2010; Nagle 2008; Smith 2010; Steckelberg 2011; Trevena 2008), which assesses the extent to which the choice is based on relevant knowledge, is consistent with a person's values/attitudes, and is behaviourally implemented (Michie 2002). These studies operationalized the measure in terms of knowledge scores higher than the mid‐point of the scale, attitude scale scores higher than the mid‐point, and choice being congruent with attitude.Two other studies measured informed values‐based choice: Schwalm 2012 assessed the extent to which the choice was based on knowledge score ≥ 60% and a score for three values‐importance ratings that matched the choice; and Stacey 2014a assessed the extent to which the choice was based on knowledge score ≥ 66% and measured values‐choice congruence using a logistic regression model. For the 10 studies that measured informed values‐choice congruence, two used preferred choice (Mathieu 2010; Trevena 2008), and the other eight used actual choice.
There is low quality evidence that patient decision aids were more effective than usual care for selecting an option that was congruent with their informed values (RR 2.06, 95% CI 1.46 to 2.91, 10 studies; Analysis 3.1). Of the 10 studies, 8 individually showed statistically higher congruence scores for the patient decision aid compared to usual care, and 2 showed no difference (Bjorklund 2012; Mathieu 2010). Repeating this analysis using the studies that measured actual choice and not preferred choice revealed a pooled RR of 2.13 (95% CI 1.44 to 3.14; 8 studies). A sub‐analysis of studies using the Multi‐Dimensional Measure of Informed Choice revealed a pooled RR of 2.08 (95% CI 1.40 to 3.08, 8 studies; Analysis 3.3).
3.1. Analysis.
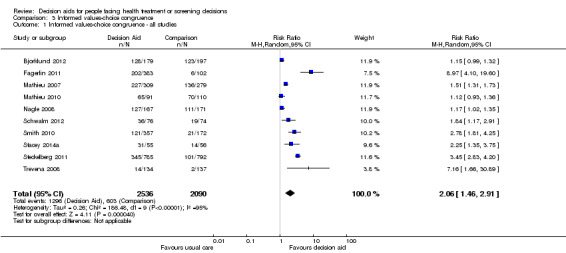
Comparison 3 Informed values‐choice congruence, Outcome 1 Informed values‐choice congruence ‐ all studies.
3.3. Analysis.
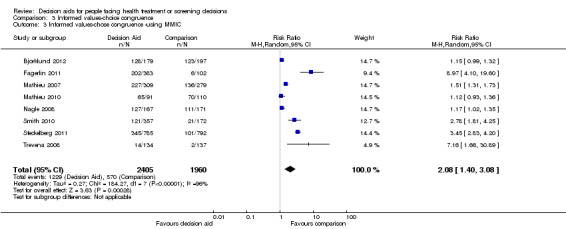
Comparison 3 Informed values‐choice congruence, Outcome 3 Informed values‐chose congruence ‐using MMIC.
There was no difference between patient decision aid and usual care for the six studies that measured values‐choice congruence without considering knowledge scores (Arterburn 2011; Berry 2013; Frosch 2008a; Legare 2008a; Lerman 1997; Vandemheen 2009; see Table 5). We did not pool these studies because of how they reported results. Arterburn 2011 reported that, compared to the control group, those exposed to the decision aid experienced a more rapid early improvement of value‐choice concordance immediately after exposure. Legare 2008a reported that women's valuing of the non‐chemical aspect of natural health products was positively associated with their choice of natural health products in managing menopausal symptoms (P = 0.006). The other four studies reported no differences between groups. However, Frosch 2008a observed that men exposed to the decision aid who chose not to have a prostate‐specific antigen (PSA) test rated their concern about prostate cancer lower than men who requested a PSA test, while men assigned to the usual care group provided similar ratings of concern regardless of their PSA choice.
4. Values congruent with chosen option.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Arterburn 2011 | Percent match procedures described by Sepucha et al (2007; 2008). For values items were most predictive and used to specify logistic models to estimate predicted probability of selecting surgery > 0.5. | Postintervention | 75 | — | 77 | — | The intervention group experienced a more rapid early improvement in value concordance immediately after the intervention compared to control |
| Berry 2013 | Concordant when men reported:a) sexual function influenced decision and they had radiation therapy; b) bowel function influenced decision and they had surgery; c) all effects influenced decision and they had surveillance | 6 months postintervention | 239 | — | 209 | — | No difference OR = 0.82; 95% CI 0.56 to 1.2 |
| Frosch 2008a | Concordance between participant's preferences and values for potential outcomes related to the decision and the choice made | within weeks | 155 | — | 151 | — | Men assigned to the decision aid who chose not to have a PSA test rated their concern about prostate cancer lower than did men who requested a PSA test. Men assigned to usual care provided similar ratings of concern about prostate cancer regardless of their PSA decision. There was no statistically significant difference between groups. |
| Legare 2008a | — | — | — | — | — | — | Women valuing of non‐chemical aspect of natural health products was positively associated with their choice of nature health products, P = 0.006. No difference between groups |
| Lerman 1997 | Association between values and choice | — | — | — | — | — | No difference; between‐group differences were not reported |
| Vandemheen 2009 | Congruence between personal values and decision | 3 weeks | 70 | — | 70 | — | Patient choices were consistent with their values across both randomized groups |
DA: decision aid; SD: standard deviation.
Attributes of the decision process: does the decision aid help patients to recognize that a decision needs to be made, know the options and their features, understand that values affect the decision, be clear about the features that matter most to them, discuss values with their clinician, and become involved in their preferred ways?
In relation to the International Patient Decision Aids Standards (IPDAS) decision process criteria, no studies evaluated the extent to which patient decision aids helped participants to recognize that a decision needed to be made or understand that values affect the decision. Some studies measured participants' self‐reports about feeling informed and clear about personal values. The measures used to evaluate these criteria were two subscales of the previously validated Decisional Conflict Scale (DCS) (O'Connor 1995).
Decisional conflict
Of 105 studies, 63 (60.0%) evaluated decisional conflict using the DCS (O'Connor 1995). The DCS is reliable, discriminates between those who make or delay decisions, is sensitive to change, and discriminates between different decision support interventions (Morgan 2000; O'Connor 1995; O'Connor 1998b). The scale measures the constructs of overall decisional conflict and the particular factors contributing to uncertainty (e.g. feeling uncertain, uninformed, unclear about values, and unsupported in decision making). A final subscale measures perceived effective decision making. The scores were standardized to range from 0 (no decisional conflict) to 100 points (extreme decisional conflict). Scores of 25 or lower are associated with follow‐through with decisions, whereas scores that exceed 38 are associated with delay in decision making (O'Connor 1998b). When decision aids are compared to usual care, a negative score indicates a reduction in decisional conflict, favouring the decision aid.
Analysis 4.1.1 summarizes the decisional conflict results for the 42 studies that compared decision aids to usual care. We report on 21 studies that were not amenable to pooling in Table 6 (original DCS), Table 7 (low literacy version), and Table 8 (SURE test version).
4.1. Analysis.
Comparison 4 Decisional conflict, Outcome 1 Decisional conflict ‐ all studies.
5. Decisional Conflict Score.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Arterburn 2011 | Total decisional conflict‐ change from baseline (standardised values) | Immediately post | 75 | Mean −20 SD 19.44 | 77 | Mean −11.8 SD 22.83 | P = 0.03 |
| Berry 2013 | Decisional conflict scale | Uncertainty | — | −3.61 units | — | — | P = 0.04 |
| Uninformed | — | — | — | — | No significant difference | ||
| Unclear values | — | −3.57 units | — | — | P = 0.002 | ||
| Unsupported | — | — | — | — | No significant difference | ||
| Ineffective decision | — | — | — | — | No significant difference | ||
| Total | — | −1.75 units | — | — | P = 0.07 | ||
| Fagerlin 2011 | Decisional conflict scale | Immediately post | — | — | — | — | DCS was higher in the intervention group compared to control, P < 0.001. |
| Frosch 2008a | Decisional conflict ‐ subscales only | Feeling uninformed | 155 | 23.37 | 151 | 29.68 | P < 0.05 |
| Feeling unclear values | 155 | 32.25 | 151 | 37.93 | P < 0.05 | ||
| Feeling supported | 155 | 30.51 | 151 | 35.21 | P < 0.05 | ||
| Feeling uncertain | 155 | — | 151 | — | No difference | ||
| Effective decisions | 155 | — | 151 | — | No difference | ||
| Knops 2014 | Decisional conflict (total score) | 4 months | 73 | 19 SD 14 | 81 | 22 SD 17 | No difference |
| 10 months | 73 | 21 SD 17 | 81 | 18 SD 17 | No difference | ||
| Krist 2007 | Decisional conflict | Immediately after office visit | 196 | 1.54 | 75 | 1.58 | No difference |
| LeBlanc 2015 (in consult) | Decision conflict (overall) median, IQR | Immediately post | 28 | 10.9 (95% CI 1.6 to 26.6) | 36 | 22.7 (95% CI 7.8 to 28.5) | P = 0.18 |
| Informed subscale | Immediately post | 28 | 4.2 (95% CI 0 to 25) | 36 | 20.8 (95% CI 0 to 33.3) | P = 0.14 | |
| Values subscale | Immediately post | 28 | 16.7 (95% CI 0 to 25) | 36 | 25.0 (95% CI 8.3 to 33.3) | P = 0.25 | |
| Support subscale | Immediately post | 28 | 8.3 (95% CI 0 to 25) | 36 | 16.7 (95% CI 0 to 25) | P = 0.35 | |
| Certainty subscale | Immediately post | 28 | 8.3 (95% CI 0 to 25) | 36 | 25 (95% CI 0 to 25) | P = 0.3 | |
| Effectiveness subscale | Immediately post | 28 | 12.5 (95% CI 0 to 25) | 36 | 18.8 (95% CI 0 to 25) | P = 0.15 | |
| Legare 2012 (in consult) | Decisional conflict ‐ proportion who had a value of 2.5 or more on the 1−5 DCS. (n,%) | Immediately post | 163 | 4.6% (95% CI 2.6 to 7.4) | 165 | 6.3% (95% CI 0 to 12.8) | Absolute difference 1.7; RR 0.8 (95% CI 0.2 to 2.4) |
| Leighl 2011 | Decisional conflict scale median (range) |
1‐2 weeks postintervention | 107 | 26 (range 0‐79) | 100 | 26 (range 0‐67) | No difference |
| Mathieu 2010 | Based on approaches suggested by Marteau et al. (informed choice) | Immediately after intervention | 91 | 71% | 110 | 64% | P = 0.24 |
| Ozanne 2007 (in consult) | Decisional conflict | Postconsultation | 15 | — | 15 | — | Both groups showed lower decisional conflict postconsultation (P < 0.001) but no difference between groups |
| Rubel 2010 | Decisional conflict | Immediately post | — | — | — | — | The total mean score was 24.5 with a SD of 15.25 (N = 200) |
| Schwartz 2009a | Decisional conflict | 12 of 16 items of the original scale | — | — | — | — | Significant longitudinal impact of the decision aid was moderated by baseline decision status; decision aid led to significant decreases in decisional conflict for those who were undecided at the time of randomisation |
| Thomson 2007 (in consult) | Decisional conflict | Postconsultation | 53 | — | 56 | — | Difference between decision aid and control group were −0.18 (95% CI −0.34 to −0.01). P = 0.036 |
| 3‐months post | 51 | — | 55 | — | Difference between decision aid and control group were −0.15 (95% CI −0.37 to 0.06), no significant difference | ||
| Van Peperstraten 2010 | 15 item questionnaire (1‐5) ‐ satisfaction‐uncertainty | Postintervention, pre IVF | 124 | 72.5 | 128 | 75 | P = 0.76 |
| 15 item questionnaire (1‐5) ‐ informed (includes some items from DCS) | Postintervention, pre IVF | 124 | 77.5 | 128 | 87.5 | P = 0.001 | |
| Weymiller 2007 (in consult) | Decisional conflict | Immediately post | 52 | — | 46 | — | Mean difference indicates statistically significantly lower decisional conflict for decision aid compared to usual care. Total DCS −10.6 (95% CI −15.4 to −5.9) Uncertain −12.8 (95% CI −18.4 to −7.3) Informed −17.3 (95% CI −22.6 to −12.0) if administered during consult −6.6 (95% CI −14.3 to −1.1) if administered prior to consult Values clarity −8.5 (95% CI−15.7 to −1.3) Support −9.4 (95% CI −14.8 to −3.9) Effective decision −10.0 (95% CI −15.0 to −5.0) |
CI: confidence interval; DA: decision aid; DCS: decisional conflict scale; IVF: in vitro fertilisation; SD: standard deviation.
6. Decisional Conflict Score ‐ low literacy version.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Fraenkel 2012 | Informed | Immediately post | 69 | 13.0 | 66 | 24.8 | P = 0.01 |
| Values | Immediately post | 69 | 6.4 | 66 | 21.0 | P <.001 | |
| Smith 2010 | Total DCS | 2 week follow‐up | 357 | 13.63 (SD 20.55) | 173 | 14.91 (SD 18.34) | P = 0.02 |
| Taylor 2006 | Total DCS | Used 8 of 10 items only 1 month post |
80 | 24.1% high | 74 | 41.9% high | Results were dichotomized (items removed choosing without pressure from others; know what options are available to you) |
| Williams 2013 | Total DCS | 2 months post | 153 | 27.5% | 136 | 38.2% | Significant decrease for DA group compared to usual care in the home condition site |
| 13 months post | 153 | 38.6% | 136 | 31.6% | No difference |
DA: decision aid; DCS: decisional conflict scale; SD: standard deviation.
7. Decisional Conflict Score ‐ SURE test.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Stacey 2014a | SURE tool Item: 'Feels sure about the best choice' |
Postintervention; prior to surgical consult | 65 | 72.3% | 66 | 80.3% | No difference |
| 'Knows the benefits and harms . . .' | Postintervention; prior to surgical consult | 65 | 92.3% | 66 | 66.7% | No difference | |
| 'Clear about which benefits and harms . . .' | Postintervention; prior to surgical consult | 65 | 87.7% | 66 | 74.2% | No difference | |
| 'Has enough support and advice . . .' | Postintervention; prior to surgical consult | 65 | 76.9% | 66 | 77.3% | No difference | |
| Total SURE score | Postintervention; prior to surgical consult | 65 | 69.2% | 66 | 57.6% | No difference |
The mean difference (MD) for total DCS scores was −7.22 points out of 100, favouring the patient decision aid over usual care groups (95% CI −9.12 to −5.31; see Analysis 4.1.1). Sixteen studies that could not be pooled (Table 6) reported mixed results on the original DCS. Of four studies that used the low literacy version (Fraenkel 2012; Smith 2010; Taylor 2006; Williams 2013), all reported statistically significant improvement (i.e. reduced) in total (or subscale) decisional conflict scores in the decision aid group, compared to usual care (Table 7). Stacey 2014a reported no difference between groups using the SURE test version.
The 'feeling uninformed' subscale of the DCS measures self‐reported comfort with knowledge, not actual knowledge. We elected to consider this as a process measure and to reserve the gold standard of objective knowledge tests for assessing decision quality. There was high‐quality evidence that patient decision aids were more effective than usual care in reducing patients' 'feeling uninformed' about options, benefits, and harms (MD −9.28, 95% CI −12.20 to −6.36; 27 studies; Analysis 4.1.2). The funnel plot for 'feeling uninformed' as an outcome in studies comparing decision aid to usual care shows low risk for publication bias (Figure 5).
5.
Funnel plot of comparison: 4.1 Decisional conflict: DA vs usual care ‐ all studies, outcome: 4.1.2 Uninformed subscale
There was high‐quality evidence that patient decision aids were more effective than usual care for reducing patients' 'feeling unclear about values' subscale of the DCS (MD −8.81; 95% CI −11.99 to −5.63; 23 studies; Analysis 4.1.3). The funnel plot for using 'feeling unclear about values' as an outcome in studies comparing decision aid to usual care shows low risk for publication bias (Figure 6).
6.
Funnel plot of comparison: 4.1 Decisional conflict: DA vs usual care ‐ all studies, outcome: 4.1.3 Unclear subscale
Patient‐clinician communication
Of 105 studies, 10 (9.5%) measured the effect of decision aids on patient‐clinician communication. Of these 10 studies, 5 evaluated a patient decision aid used primarily within the consultation with the clinician, and 5 evaluated a patient decision aid used in preparation for the consultation.
Five studies compared the effect of usual care versus a decision aid used within the clinical encounter (or, in Weymiller 2007, half the decision aid participants were exposed just prior to the encounter), evaluating the extent of shared decision making communication by analyzing the audio recordings using the OPTION scale (Hess 2012; LeBlanc 2015; Montori 2011; Mullan 2009; Weymiller 2007). The OPTION scale measures the extent to which healthcare providers use behaviours that involve patients in decision making (Elwyn 2005). All five studies reported statistically higher mean OPTION scores in the patient decision aid group compared to usual care (see Table 9).
8. Patient‐clinician communication.
| Study | Scale used | Timing | N decision aid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Fraenkel 2012 | Discussed risk of stroke | Immediately post | 69 | 71% | 66 | 12% | P < 0.001 |
| Discussed risk of major bleeding | Immediately post | 69 | 69% | 66 | 20% | P < 0.001 | |
| Hanson 2011 | Discussed feeding with physician, nurse clinician, or physician's assistant | 3 months | 126 | 46% | 127 | 33% | P = 0.04 |
| Discussed feeding with other nursing home staff | 3 months | 126 | 64% | 127 | 71% | P = 0.42 | |
| Hess 2012 (in consult) | OPTION scale | Analysis of the consultation using video‐recordings | 101 | Mean 26.6% (95% CI 24.9 to 8.2) | 103 | Mean 7% (95% CI 5.9 to 8.1) | Significantly greater in the intervention arm |
| LeBlanc 2015 (in consult) | OPTION scale | Analysis of the consultation using video‐recordings | 25 | Mean 57% (95% CI 50 to 64) | 13 | Mean 43% (95% CI 37 to 48) | P = 0.001 |
| Lepore 2012 | Discussed PSA testing with physician postintervention | 8 months postintervention | 215 | 15.8% | 216 | 8.3% | P < 0.001 |
| Montori 2011 (in consult) | OPTION 100‐point scale | Analysis of the consultation using video‐recorded consultations | 38 | 49.8 | 32 | 27.3 | P < 0.001 |
| Mullan 2009 (in consult) | OPTION scale | Analysis of the consultation using video‐recorded consultations | 48 used decision aid within consultation | Mean 49.7% (SD 17.74) | 37 usual care | Mean 27.7% (SD 11.75) | MD 21.8 (95% CI 13.0 to 30.5) for decision aid vs usual care. All but 2 of the 12 items significantly favoured the decision aid |
| Sheridan 2006 | Discussed CHD with doctor | Patient reported Immediately post | 16/41 decision aid pre‐consult with summary report to bring to consult | — | 8/34 usual care | — | Absolute difference 16% (95% CI −4 to 37) |
| Plan to reduce CHD risk and discussed with doctor | Patient reported Immediately post | 15/41 decision aid pre‐consult with summary report to bring to consult | — | 8/34 usual care | — | Absolute difference 13% (95% CI −7 to 34). | |
| Plan to reduce CHD risk and not discussed with doctor | Patient reported Immediately post | 37/41 decision aid pre‐consult with summary report to bring to consult | — | 25/34 usual care | — | Absolute difference 16% (95% CI −1 to 33) | |
| Sheridan 2011 | Had CHD discussion with provider | Patient reported Immediately post |
79 | 89% | 78 | 58% | Absolute difference 31% (95% CI 15 to 45; P < 0.001) |
| Patient‐raised discussion | Patient reported Immediately post |
79 | 63% | 78 | 35% | Absolute difference 28% (95% CI 9 to 45; P = 0.02) | |
| Modified Healthcare Climate Questionnaire: 1. "My provider provided me with choices and options about lowering my chances of heart disease" | Patient reported Immediately post |
79 | 91% | 78 | 76% | Absolute difference 15% (95% CI −0.1 to 31; P = 0.02) | |
| 2. "My provider understands how I see things with respect to lowering my chances of heart disease." | Patient reported Immediately post |
79 | 95% | 78 | 86% | Absolute difference 9% (95% CI −7 to 25; P = 0.21) | |
| 3. "My provider conveyed confidence in my ability to make changes regarding lowering my chances of heart disease" | patient reported Immediately post |
79 | 88% | 78 | 77% | Absolute difference 11% (95% CI −5 to 27; P = 0.15) | |
| 4. "My provider encouraged me to ask questions" | Patient reported Immediately post |
79 | 78% | 78 | 67% | Absolute difference 11% (95% CI −4% to 27%; P = 0.13) | |
| 5. "My provider listened to how I would like to do things" | Patient reported Immediately post |
79 | 92% | 78 | 71% | Absolute difference 21% (CI 95% 6 to 37; P < 0.01) | |
| 6. "My provider tried to understanding how I see things before suggesting new ways to lower my chances of heart disease." | Patient reported Immediately post |
79 | 84% | 78 | 69% | Absolute difference 15% (CI 95% −0.3 to 31; P = 0.05) | |
| Weymiller 2007 (in consult) | OPTION Scale | Analysis of the consultation using video‐recorded consultations | 1/2 used decision aid prior to consult and 1/2 used it during consult | — | Usual care | — | Greater patient participation MD 4.4 (95% CI 2.9 to 6.0) in decision aid compared to usual care |
CHD: coronary heart disease;CI: confidence interval; DA: decision aid; DCS: decisional conflict scale; ICC: intraclass correlation coefficient;MD: mean difference; OPTION scale: observing patient involvement scale; RR: risk ratio; SD: standard deviation
Four of five studies reported that compared to those in the usual care group, significantly higher proportions of participants exposed to the patient decision aid in preparation for the consultation reported that they discussed the decision with their clinician (Fraenkel 2012; Hanson 2011; Lepore 2012; Sheridan 2011; see Table 9). The fifth study showed no between‐group difference in discussion of cardiovascular disease with the clinician (Sheridan 2006; see Table 9).
Participation in decision making
Of 105 studies, 24 (22.9%) measured the effect of decision aids on patients' perceived participation in decision making (Analysis 5.1; Table 10). Davison 1997 used the Control Preferences Scale (Degner 1992). This scale uses five response statements to measure the role in decision making: two represent an active or patient‐controlled role; one a shared or collaborative role; and two response statements represent a passive or clinician‐controlled role. Most other studies used comparable response statements that could be classified within each of the three groupings of the Control Preferences Scale, except for Hamann 2006, which used the COMRADE instrument to measure patient perception of involvement, and two others that used other measures of perceived involvement (Hanson 2011; Loh 2007; see Table 10).
5.1. Analysis.
Comparison 5 Participation in decision making, Outcome 1 Participation in decision making ‐ all studies.
9. Participation in decision making.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Allen 2010 | Control preferences ‐ patients choosing active/collaborative decision making | Postintervention | 291 | 95% | 334 | 92% | No difference |
| Control preferences did not change | Postintervention | 291 | 92% | 334 | 87% | No difference | |
| Control preferences changed to passive | Postintervention | 291 | 3% | 334 | 5% | No difference | |
| Control preferences changed to active/ collaborative | Postintervention | 291 | 3% | 334 | 7% | No difference | |
| Hamann 2006 | COMRADE used to measure patients' perceived involvement in decisions | Postconsultation | 49 | 79.5 (SD 18.6) 76.8 (SD 20.9) |
58 | 69.7 (SD 20.0) 73.5 (SD 19.3) |
Increased patient involvement in decision aid group postintervention compared to usual care at baseline. At discharge there was no difference between groups. |
| Hanson 2011 | Surrogates feeling somewhat or very involved in decision making | Postintervention | — | 83% | — | 77% | P = 0.18 |
| Leighl 2011 | Achieved decision involvement | Postintervention | — | 32% | — | 35% | No difference |
| Loh 2007 (in consult) | Patients' perceived involvement in decision making | Postconsultation | 191 | 26.3 pre 28.0 post | 96 | 24.5 pre 25.5 post |
Improved patient participation from baseline to post exposure to the decision aid (P = 0.010) and in comparison to the usual care group (P = 0.003) but there was no change in the control group for the pre‐post comparison |
| Rubel 2010 | Adapted from the Control Preferences Scale | Postintervention | — | — | — | — | The total mean scores were: 2.74 (SD 1.25) (N = 99) pre and 2.83 (SD 1.16) (N = 199) post, no statistically significant difference |
| Sheridan 2011 | Patient participation: 'Any' |
Immediately post | 79 | 79% | 78 | 51% | Absolute difference 28% (95% CI 9 to 45; P = 0.01) |
| 'None' | Immediately post | 79 | 21% | 78 | 49% | Absolute difference −28% (95% CI −45 to −9) | |
| Van Peperstraten 2010 | Decision Evaluation scale (15 item questionnaire) Decision control subscale | Postconsultation | 124 | 85 | 128 | 87.5 | P = 0.33 |
DA: decision aid; SD: standard deviation.
Using the groupings of the Control Preferences Scale, 16 of 24 studies reported on clinician‐controlled decision making. Consistent with the hypothesis that patient decision aids increase patient participation in decision making, there was moderate‐quality evidence that patient decision aids were more effective than usual care for reducing clinician‐controlled decision making (RR 0.68; 95% CI 0.55 to 0.83; Analysis 5.1.1). In this field, there is no consensus on the hypothesized effects of decision aids on measures of patient‐controlled decision making or shared decision making. Of 24 studies, 15 reported on participants assuming an active (patient‐controlled) role in decision making and were pooled for analysis. Compared to usual care, decision aid use increased patient‐controlled decision making (RR 1.28, 95% CI 1.05 to 1.55; Analysis 5.1.2). The 15 studies that reported on a shared decision‐making role showed no difference between decision aid and usual care (RR 0.95; 95% CI 0.83 to 1.10; Analysis 5.1.3).
Of eight studies that could not be pooled, Allen 2010, Leighl 2011,Rubel 2010, and Van Peperstraten 2010 reported no between‐group differences in these roles (Table 10). Three studies reported that a statistically significant proportion of patients exposed to the decision aid either participated (Sheridan 2011) – or at least felt involved – in decision making (Hamann 2006; Loh 2007). However, Hamann 2006 did not analyze results accounting for the use of design clusters. Hanson 2011 reported that a higher proportion described feeling involved (83% vs. 77%), but the difference between groups was not statistically significant.
Proportion undecided
Of 105 studies, 24 (22.9%) measured the proportion of participants remaining undecided: of these, 22 studies could be pooled. A significantly lower proportion of people remained undecided after exposure to a decision aid (RR 0.64; 95% CI 0.52 to 0.79; Analysis 6.1).
6.1. Analysis.
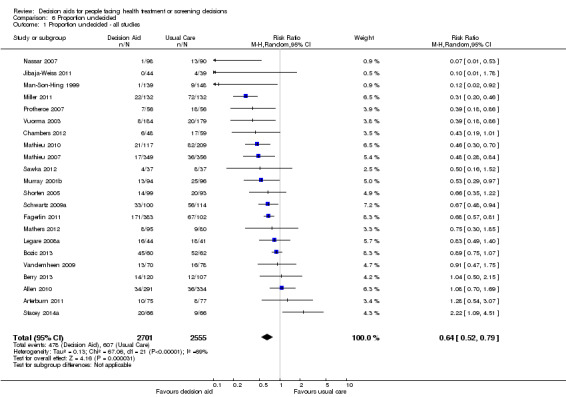
Comparison 6 Proportion undecided, Outcome 1 Proportion undecided ‐ all studies.
Kasper 2008 measured progress in decision making using a single item ranging from '0 = completely undecided' to '100 = made my decision'. Given the difference in the measure Kasper used, these results were not included in the meta‐analysis. In this study, both the patients exposed to a decision aid and the usual care group progressed in their decision making, with no difference between the groups (Table 11). Sawka 2012 reported that 10.8% in the patient decision aid group versus 21.6% in the usual care group reported not knowing if they preferred taking adjuvant radioactive iodine.
10. Proportion undecided.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Kasper 2008 | Single item ‐ ranging from '0 = completely undecided' to '100 = made my decision' | — | — | — | — | — | No difference |
| Sawka 2012 | Answer "I don't know" to question "I favor taking adjuvant radioactive iodine" | Immediately post ‐ treatment preference | 37 | 10.8% | 37 | 21.6% | — |
| 6.3 months (mean) post ‐ actual decision | 37 | 13.5% | 37 | 8.1% | — | ||
| Answer "I don't know" to question "I favor not taking adjuvant radioactive iodine" | Immediately post ‐ treatment preference | 37 | 43.2% | 37 | 37.8% | — | |
| 6.3 months (mean) post ‐ actual decision | 37 | 40.5% | 37 | 51.4% | — |
DA: decision aid
Satisfaction
Nineteen included studies (18.1%) measured satisfaction as it relates to the choice and the preparation for and the process of decision making. When possible, we standardized the scores to a 0 to 100 point scale, with higher scores reflecting greater satisfaction.
Nineteen studies (18.1%) measured satisfaction with the choice. Of these 19 studies, 4 reported that people exposed to the decision aid had higher satisfaction with their choice compared to usual care, and the other 15 reported no statistically significant differences (Chabrera 2015; Heller 2008; Laupacis 2006; Montgomery 2007; see Analysis 7.1 and Table 12). For results that used a similar measure (Analysis 7.1), there was high satisfaction for all participants, with a median score of 82.5% for the decision aid and 80.0% for the usual care groups.
7.1. Analysis.
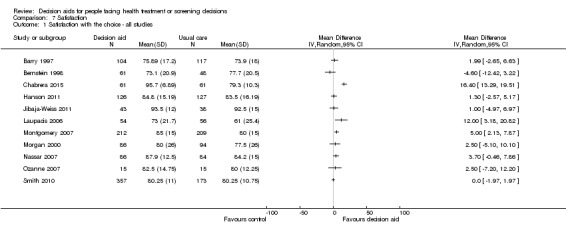
Comparison 7 Satisfaction, Outcome 1 Satisfaction with the choice ‐ all studies.
11. Satisfaction with the choice.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Heller 2008 | 1‐item; pleased with treatment choice | 1 month postsurgery | 62/66 | — | 55/67 | — | P = 0.03 |
| Legare 2012 (in consult) | Single question Likert scale to assess the quality of the decision made (0 = very low quality; 10 = very high quality) | Immediately post | 162 | 8.54 (SD 1.56) | 159 | 8.53 (SD 1.51) | No difference; MD 0.0 (95% CI −0.4 to 0.4) |
| Leighl 2011 | Satisfaction with decision scale: median (range) |
1 month postintervention | 107 | 22 (13‐25) | 100 | 21(15‐25) | No difference |
| Marteau 2010 | Scale: ranging from 1−7 and standardized out 100 | 4 weeks | — | 91.17 (SD 14) | — | 91.33 (SD 14.50) | No difference |
| Schwartz 2009b | 6‐item | 1, 6, 12 months | 100 | — | 114 | — | Overall, no difference between groups; decision aid led to significantly increased satisfaction compared to usual care among those who were undecided at randomization but not among those who had made a decision before randomization; (only graph in paper with no raw data) |
| Taylor 2006 | Single item ‐ "Are you satisfied with your decision about prostate cancer testing? | 1 month | 80 | 79.7% | 74 | 75.7% | — |
| Trevena 2008 | Satisfaction with the decision | Immediately post | 134 | — | 137 | — | No difference (P = 0.56) |
| Williams 2013 | 6‐item Satisfaction with Decision Scale | Baseline | — | > 95% | — | > 95% | — |
DA: decision aid.
Of 105 total studies, 11 (10.5%) measured satisfaction with the decision, 11 (10.5%) measured satisfaction with the decision‐making process (see Analysis 7.6; plus Hess 2012 and Vodermaier 2009 in Table 13), 4 measured satisfaction with information provided (LeBlanc 2015; Laupacis 2006; Montori 2011; Oakley 2006), 3 measured satisfaction with the clinician (Laupacis 2006; Miller 2005; Vodermaier 2009), and 1 measured satisfaction with participating in decision making (Kennedy 2002). There were mixed results, but no studies reported that those exposed to patient decision aids were significantly less satisfied compared to usual care. For results that used a similar measure of satisfaction with the decision‐making process (Analysis 7.4), there was high satisfaction for all participants, with median scores of 83.8% for the decision aid and 77.8% for the usual care groups. Although there were no differences between participant groups in satisfaction with the information in the Montori 2011, clinicians using the decision aid had higher satisfaction.
7.6. Analysis.
Comparison 7 Satisfaction, Outcome 6 Satisfaction with the decision making process ‐ in preparation for consultation.
12. Satisfaction with the decision‐making process.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Satisfaction with the decision‐making process | |||||||
| Hess 2012 (in consult) | Satisfaction with decision process (0 for strongly agree to 5 for strongly disagree) | — | 101 | — | 103 | — | Patients in DA group reported greater satisfaction with the DM process (strongly agree, 61% DA vs 40% usual care) |
| Vodermaier 2009 | Satisfied with process | 1 week follow‐up | 53 | 42 | 56 | 50 | High satisfaction with no difference by group |
| Satisfaction with participating in decision making | |||||||
| Kennedy 2002 | Measured satisfaction with opportunities to participate in decision making using a single item | — | — | — | — | — | Compared to usual care, women who received the decision aid followed by nurse coaching were significantly more satisfied with the opportunities to participate in decision making (OR 1.5, 95% CI 1.1 to 2.0). |
| Satisfaction with the information provided | |||||||
| LeBlanc 2015 (in consult) | Amount of information was just right | Postconsultation | 29 | 25 (86%) | 37 | 34 (92%) | P = 0.69 |
| Information received was clear | Postconsultation | 27 | 17 (63%) | 36 | 26 (72%) | P = 0.43 | |
| Information received was helpful | Postconsultation | 28 | 21 (75%) | 34 | 23 (68%) | P = 0.53 | |
| Would recommend method to others | Postconsultation | 28 | 24 (86%) | 35 | 27 (77%) | P = 0.52 | |
| Laupacis 2006 | Satisfaction with information received subscale 4‐item (0 to 100; low to high) | Average 10 days | 54 | 76 (15.5 SD) | 56 | 59 (23.3 SD) | P = 0.001 |
| Montori 2011 (in consult) | (7 point scales) Participants' satisfaction with knowledge transfer
|
Postintervention | 49 | 6.6 6 6 6.1 6.4 |
46 | 6.3 6 5.8 5.8 6.2 |
P = 0.798 P = 0.296 P = 0.624 P = 0.248 P = 0.435 |
Clinicians' satisfaction with knowledge transfer
|
Postintervention | 39 | 5.8 6.1 5.9 |
33 | 5.2 4.9 4.8 |
P = 0.006 P < 0.001 P < 0.001 |
|
| Oakley 2006 | Satisfaction with information about medicines | 4 months post | 16 | 10.4 (SD 2.9) | 17 | 10.1 (SD 2.2) | No difference |
| Satisfaction with the clinician | |||||||
| Laupacis 2006 | Satisfaction with practitioner treatment during decision process subscale 4‐item (0 to 100; low to high) | Average 10 days | 54 | 69 (25.3 SD) | 56 | 54 (26.7 SD) | P = 0.004 |
| Miller 2005 | Satisfaction with cancer information service 1‐item (1 to 5; low to high) | 2 weeks | — | 4.37 (0.84 SD) | — | 4.38 (0.86 SD) | No difference |
| 6 months | — | 4.51 (0.75 SD) | — | 4.51 (0.64 SD) | No difference | ||
| Vodermaier 2009 |
|
1 week follow‐up | 53 | 49 (92.5%) 47 47 44 36 |
56 | 53 (94.6%) 50 51 45 36 |
High satisfaction with no difference by group |
DA: decision aid; SD: standard deviation.
7.4. Analysis.
Comparison 7 Satisfaction, Outcome 4 Satisfaction with the decision making process ‐ all studies.
Three studies (2.9%) measured satisfaction with preparation for decision making using the Preparation for Decision Making Scale (Bennett 2010) (Table 14). Compared to usual care, two studies reported significant improvements in people's satisfaction with their preparation for making decisions: in Fraenkel 2007 after using decision aids about management of knee osteoarthritis, and in Vandemheen 2009 regarding referral to a lung transplant centre. The third study found no statistically significant difference on this subscale's four items (Stacey 2014a).
13. Preparation for decision making.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Fraenkel 2007 | Preparation for Decision Making Scale | Pre‐consultation | 43 | 35 (median) | 40 | 20.5 (median) | P < 0.001 |
| Stacey 2014a | Preparation for Decision Making Scale item (5‐point scale from: 1 not at all to 5 a great deal) 'Help recognize decision to be made' |
Postintervention; pre‐consultation | 66 | 4.12 (SD 1.21) | 64 | 3.78 (SD 1.25) | No difference |
| Preparation for Decision Making Scale item 'Help know decision depends on what matters most' |
Postintervention; pre‐consultation | 66 | 4.48 (SD 0.85) | 64 | 4.14 (SD 1.10) | No difference | |
| Preparation for Decision Making Scale item 'Help think about how involved you want to be in decision' |
Postintervention; pre‐consultation | 66 | 4.48 (SD 0.81) | 64 | 4.25 (SD 1.05) | No difference | |
| Preparation for Decision Making Scale item 'Prepare you to talk to your doctor about what matters most' |
Postintervention; pre‐consultation | 66 | 4.36 (SD 0.91) | 64 | 4.23 (SD 1.04) | No difference | |
| Vandemheen 2009 | Preparation for Decision Making Scale | 3 weeks | 70 | 65.1 (SD 24.9) | 79 | 53.9 (SD 27.1) | P = 0.009 |
DA: decision aid; SD: standard deviation.
Secondary outcomes
Behaviour
Choice
Choice was defined as the actual choice implemented. However, when studies did not report the actual choice, we used the patients' preferred option as a surrogate measure. Actual choices or preferences were reported as the percentage of individuals actually implementing or stating a preference for the most intensive or most invasive option.
In summary, patient decision aids decreased the number of patients choosing elective surgical procedures (excluding prophylactic mastectomy) and PSA testing in multiple studies. Single studies showed that decision aids increased the number of people choosing hepatitis B vaccination, psycho‐educational therapies for mental health conditions, and medication for cardiovascular disease prevention. In contrast, decision aids decreased the rate of cardiac stress testing, the number of embryos being transplanted, and the rate of antibiotic use for upper respiratory infections. The effect on patients' choice in other situations was more variable. There were mixed results for the choice of colon cancer screening, genetic testing, prenatal testing, anti‐thrombosis therapy, breast screening, and diabetes medications. There was no difference between groups for choices about natural health products, hypertension therapy, breast cancer chemotherapy, schizophraenia medication, immunotherapy for multiple sclerosis, vaccines (for flu or measles, mumps, rubella), diabetes screening, birth control, osteoporosis treatment, chemotherapy for advanced cancer, chemopreventive medications, use of blood transfusions, childbirth procedures, treatment of prolapsed pelvic organs, or radioactive iodine treatment for thyroid cancer.
Choice for major elective surgery
Eighteen studies (17.1%) focused on choices regarding major elective surgery (Analysis 8.1).
8.1. Analysis.
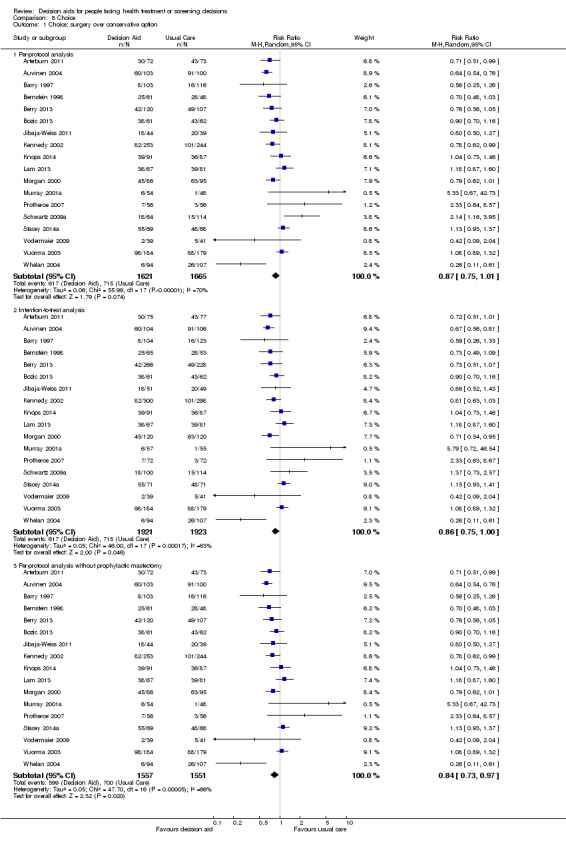
Comparison 8 Choice, Outcome 1 Choice: surgery over conservative option.
Using intention‐to‐treat analysis, there was a non‐significant reduction in the number of patients choosing major elective surgery in the group receiving the decision aid compared to usual care (RR 0.86; 95% CI 0.75 to 1.00, 18 studies; Analysis 8.1.2). Schwartz 2009a reported a statistically significant uptake of prophylactic mastectomy for women who are BRCA1/2 gene carriers (114%). And after removing this study from the pooled results, there was a statistically significant reduction in the number of patients choosing major elective surgery (RR 0.84 95% CI 0.73 to 0.97; 17 studies; Analysis 8.1.3).
Four other studies showed statistically significant reductions in surgery rates: −29% for cardiac revascularization and bariatric surgery (Arterburn 2011; Morgan 2000), −33% for orchiectomy (Auvinen 2004), and −74% for mastectomy (Whelan 2004). The other 15 studies showed no difference between the decision aid or usual care groups.
Choice for other elective surgery
Two studies evaluated the effect of decision aids versus usual care on other elective surgical decisions. Decision aids did not significantly influence surgical abortion rates in Wong 2006 or feeding tube insertions in Hanson 2011 (Table 15).
14. Choice.
| Study | Type of comparison | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Surgery ‐ elective more minor surgery | ||||||
| Hanson 2011 | Actual choice (feeding tube) | 127 | 1 | 129 | 3 | No difference |
| Wong 2006 | Actual choice (abortion) | — | — | — | — | No difference |
| Screening ‐ breast cancer genetic testing | ||||||
| Miller 2005 | Preference | — | — | — | — | Intervention decreased intention for genetic testing in women at average risk; increased in women at high risk |
| Screening ‐ breast screening | ||||||
| Mathieu 2007 | Actual choice | — | — | — | — | No difference in women who participated in screening within 1 month |
| Mathieu 2010 | Preference of women who were decided | 96 | 52% | 127 | 65% | P = 0.05 |
| Screening ‐ cardiac stress testing | ||||||
| Hess 2012 (in consult) | Actual choice | 101 | 58% | 100 | 77% | P < 0.001 |
| Screening ‐ diabetes | ||||||
| Marteau 2010 | Actual choice | 633 | 353 | 639 | 368 | P = 0.51 |
| Mann E 2010 | Preference | 273 | — | 134 | — | No difference |
| Screening ‐ prenatal | ||||||
| Bekker 2004 (in consult) | Actual choice | — | — | — | — | No difference |
| Nagle 2008 | Actual choice | — | — | — | — | No difference |
| Screening ‐ prostate cancer testing | ||||||
| Frosch 2008a | Actual choice | — | — | — | — | The experimental interventions led to significant reductions in requests for prostate‐specific antigen tests ( ˜2 times greater decline). |
| Lepore 2012 | Actual choice 2 years postintervention |
215 | 62.7% | 216 | 66.7% | No difference Exp (B) = 0.829 CI 95% 0.564 to 1.218 |
| Williams 2013 | Actual choice | — | — | — | — | No difference (P > 0.3) |
| Lepore 2012 | Preference | 215 | 80.9% | 216 | 80.1% | No difference Exp (B) = 0.994 95% CI 0.614 to 1.610 |
| Diagnostic testing ‐ prenatal genetic testing | ||||||
| Kuppermann 2014 | Invasive diagnostic testing without screening test | 357 | 11 (3.0%) | 353 | 16 (4.6%) | P = 0.37 |
| Screening test followed by invasive diagnostic test | 357 | 10 (2.9%) | 353 | 27 (7.7%) | Not reported | |
| Medication ‐ antibiotics for upper respiratory infections | ||||||
| Legare 2011 (in consult) | Actual choice | 81 | 33 | 70 | 49 | P = 0.08 |
| Legare 2012 (in consult) | Actual choice | — | 27.2% | — | 52.2% | Absolute difference 25.0; RR 0.5 (95% CI 0.3 to 0.7) |
| Medication ‐ atrial fibrillation anti‐thrombosis ‐ uptake | ||||||
| Man‐Son‐Hing 1999 | Actual choice | — | — | — | — | 25% decrease in DA group, not statistically significant |
| McAlister 2005 | Actual choice | — | — | — | — | No difference |
| Thomson 2007 (in consult) | Actual choice | — | 93.8% | — | 25% | RR 0.27 (95% CI 0.11 to 0.63) |
| Medication ‐ breast cancer prevention | ||||||
| Fagerlin 2011 | Actual choice | 383 | 0.5% | 102 | 0% | No difference |
| Medication ‐ cardiovascular disease prevention | ||||||
| Sheridan 2011 | DA versus usual care. Any effective CHD risk reducing strategy | 79 | 63% | 78 | 42% | Absolute difference 21%, 95% CI 5 to 37 |
| Blood pressure medication, if hypertensive (n = 55) | — | 26% | — | 29% | Absolute difference −3%, 95% CI −30 to 25 | |
| Cholesterol medication, if abnormal cholesterol (n = 69) | — | 39% | — | 9% | Absolute difference 30%, 95% CI 14 to 46 | |
| Smoking cessation, if smoking (n = 21) | — | 80% | — | 50% | Absolute difference 30%, 95% CI −16 to 76 | |
| Aspirin, if CHD risk > 6% (n = 140) | — | 43% | — | 24% | Absolute difference 19%, 95% CI −1 to 39 | |
| Diet low in saturated fat | 79 | 29% | 78 | 40% | Absolute difference −11%, 95% CI −27 to 6 | |
| Regular exercise | 79 | 53% | 78 | 54% | Absolute difference −1%, 95% CI −17 to 16 | |
| Medication ‐ chemotherapy | ||||||
| Leighl 2011 | For advanced cancer | 107 | 77% | 100 | 71% | No difference |
| Whelan 2003 (in consult) | For early breast cancer | — | — | — | — | No difference |
| Medication ‐ diabetes management insulin | ||||||
| Mathers 2012 | Preference for insulin | 92 | 18.5% | 78 | 11.5% | P = 0.41 |
| Medication ‐ hypertension | ||||||
| Montgomery 2003 | Uptake | — | — | — | — | No difference |
| Medication ‐ menopausal symptom treatment | ||||||
| Murray 2001b | Uptake hormone therapy | — | — | — | — | 8% decrease in DA group, not statistically significant |
| Legare 2008a | preference for natural health products | 41% | 41% | No difference | ||
| Medication ‐ multiple sclerosis immunotherapy | ||||||
| Kasper 2008 | Uptake | — | — | — | — | No difference |
| Medication ‐ osteoporosis | ||||||
| LeBlanc 2015 (in consult) | Preference | 29 | 12 (41%) | 38 | 11 (29%) | P = 0.57 |
| Prescription during encounter | 29 | 13 (41%) | 38 | 12 (27%) | P = 0.2 | |
| Montori 2011 (in consult) | Uptake | 52 | 44% | 48 | 40% | No difference |
| Mental health treatment | ||||||
| Hamann 2006 | Uptake prescribed medication | — | — | — | — | No difference |
| Hamann 2006 | Uptake psychoeducation | — | — | — | — | Higher uptake in DA group (P = 0.003) |
| Mott 2014 | Uptake of 9 psychoeducation sessions | 9 | 44% | 11 | 9% | All 4 decision aid participants received 9 or more sessions. 1 of 5 usual care received 9 or more sessions. |
| Obstetrics ‐ birth control method | ||||||
| Langston 2010 | Preference | 114 | — | 108 | — | No difference in the methods chosen between groups, participants in the intervention group were not more likely to initiate the requested method immediately compared to those in the usual care group (OR 0.65, 95% CI 0.31 to 1.34) |
| Obstetric ‐ childbirth procedure | ||||||
| Montgomery 2007 | Uptake | — | — | — | — | No difference |
| Nassar 2007 | Uptake | — | — | — | — | No difference |
| Shorten 2005 | preference | — | — | — | — | No difference |
| Obstetric ‐ embryo transplant | ||||||
| Van Peperstraten 2010 ‐ single embryo transfer | Uptake | 152 | 43% | 156 | 32% | P = 0.05 |
| Other‐ lung transplant referral | ||||||
| Vandemheen 2009 | — | — | — | — | No difference | |
| Other ‐ pre‐operative blood transfusion | ||||||
| Laupacis 2006 | Uptake | — | — | — | — | No difference |
| Other ‐ pelvic organ prolapse treatment | ||||||
| Brazell 2014 | Uptake | — | — | — | — | No difference; P = 0.835 |
| Other ‐ thyroid cancer adjuvant radioactive iodine treatment | ||||||
| Sawka 2012 | Preferred treatment Immediately post | 37 | 35.1% | 37 | 32.4% | — |
| Uptake at follow‐up (˜ 6.3 months post) | 37 | 29.7% | 37 | 18.9% | No difference. (Chi2=1.18; df = 1; P = 0.28) |
|
| Vaccines | ||||||
| Chambers 2012 | Uptake flu shot | 48 | 46% | 59 | 27% | No difference |
| Clancy 1988 | Uptake hepatitis B | — | — | — | — | Significant increase of 76% in the DA group |
| Shourie 2013 | Measles, mumps, rubella in infant | 48 | 48 (100%) | 71 | 70 (99%) | No difference |
CHD: congenital heart disease;DA: decision aid; OR: odds ratio; RR: risk ratio.
Choice for prostate‐specific antigen screening
The effects of decision aids on prostate‐specific antigen (PSA) screening decisions were variable in 13 studies (12.4%) that compared decision aids to usual care. The pooled RR for 10 studies was 0.88 (95% CI 0.80 to 0.98; Analysis 8.2.1); Frosch 2008a, Lepore 2012, and Williams 2013 could not be included in the pooled data (Table 15). Frosch reported a reduction in screening rates and the other two reported no difference.
8.2. Analysis.
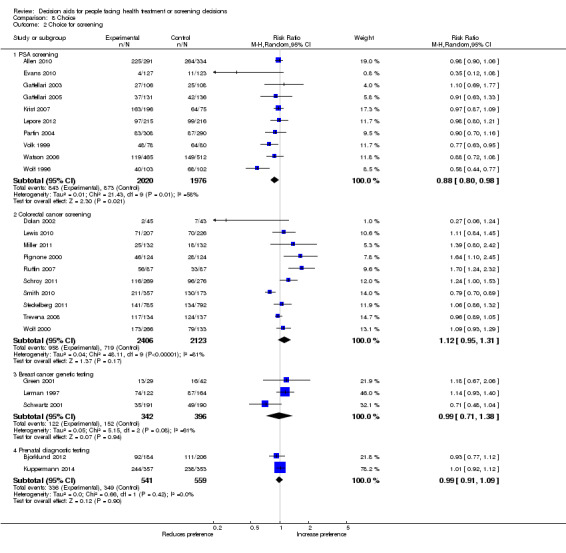
Comparison 8 Choice, Outcome 2 Choice for screening.
Choice for colon cancer screening
Of 10 studies (9.5%) on colon cancer screening, 3 reported statistically significant differences in choices, and 7 showed no difference. Two studies reported that compared to usual care, the decision aid significantly increased the screening rates by 64% and 70% (Pignone 2000; Ruffin 2007). The other study reported a statistically significant reduction of 21% for screening (Smith 2010). There was an increase in screening rates in five studies, by 6% to 39%, but the difference was not statistically significant (Lewis 2010; Miller 2011; Schroy 2011; Steckelberg 2011; Wolf 2000). In two studies (Dolan 2002; Trevena 2008), there was a 73% and 4% decrease in screening rates that was not statistically significant. The pooled RR was 1.12 (95% CI 0.95 to 1.31, 10 studies; Analysis 8.2.2).
Choice for cancer genetic screening
Four studies reported preferences or uptake rates for breast cancer genetic screening (3.8%). The decision aid did not significantly affect preferences for breast cancer genetic screening when compared to usual care. The pooled RR was 0.99 (95% CI 0.71 to 1.38, 3 studies; Analysis 8.2.3). One study reported an increase in screening rates by 14% (Lerman 1997), a second study reported an increase of 18% (Green 2001), and a third study reported a decrease of 29% (Schwartz 2001). Miller 2005 reported that women exposed to the decision aid who were at higher risk of breast cancer increased their intention to obtain genetic testing, while those at average risk decreased their intention (Table 15).
Choice for breast screening
There were lower mammography screening rates among women aged 38 to 45 years of age (Mathieu 2010), but no between‐group difference in women aged 70 or older who were exposed to a decision aid versus usual care (Mathieu 2007; Table 15).
Choice for prenatal screening
In all four studies focusing on decisions around prenatal screening, prenatal testing rates were not affected by a decision aid compared to usual care (Bekker 2004; Bjorklund 2012; Kuppermann 2014; Nagle 2008). Meta‐analysis included two studies, showing no effect (RR 0.99, 95% CI 0.91 to 1.09, 2 studies; Bjorklund 2012; Kuppermann 2014; Analysis 8.2.4).
Choice for stress test for chest pain
Compared to usual care, adults presenting with chest pain in the emergency department who received the decision aid had significantly lower rates of stress testing (58% versus 77%) (Hess 2012; Table 15).
Choice for screening for diabetes
Compared to usual care, there was no difference in diabetes screening rates in Marteau 2010 or preferences for screening in Mann E 2010 in adults exposed to a decision aid (Table 15).
Choice to take antibiotics for upper respiratory infection
Compared to usual care, using a decision aid in the consultation decreased prescriptions for antibiotics for upper respiratory infections in Legare 2012, although this difference was not statistically significant in Legare 2011 (Table 15).
Choice for atrial fibrillation treatment
Three studies evaluated the effect of a decision aid on the use of anti‐thrombotic therapy for atrial fibrillation versus usual care (Table 15). One study demonstrated a non‐significant reduction in warfarin use of 25% (Man‐Son‐Hing 1999). The second study evaluated the proportions of patients choosing the option that was appropriate relative to their level of risk, and found no significant difference between the groups (McAlister 2005). Thomson 2007 reported that patients in the usual care group (guided by practice recommendations) were much more likely to start warfarin (15/16; 93.8%) compared to the decision aid group (4/16; 25%; RR 0.27; 95% CI: 011 to 0.63).
Choice to take breast cancer prevention medication
There was no difference in medication use among women at risk of breast cancer who were exposed to the decision aid versus usual care (Fagerlin 2011; Table 15).
Choice for cardiovascular disease prevention
There was an increase in patient preferences for any effective cardiovascular disease risk‐reducing strategy (including medication) when using a decision aid versus usual care (63% versus 42%) (Sheridan 2011; Table 15).
Choice for chemotherapy for cancer
There was no statistically significant difference in the rates of chemotherapy for adults with advanced colorectal cancer (77% versus 71%) (Leighl 2011; Table 15). Whelan 2003 also found no significant effect on preferences for adjuvant chemotherapy versus no chemotherapy for early stage breast cancer.
Choice for diabetes treatment with new medications
Four studies evaluated patient decision aids compared to usual care on decisions about starting new medications for diabetes (Mann D 2010; Mathers 2012; Mullan 2009; Weymiller 2007). Although there was no statistically significant difference between groups for individual studies, pooled results indicated a significant increase in starting new medications (RR 1.65, 95% CI 1.06 to 2.56; Analysis 8.3).
8.3. Analysis.
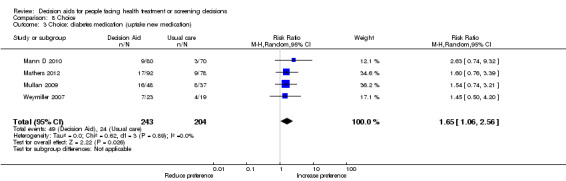
Comparison 8 Choice, Outcome 3 Choice: diabetes medication (uptake new medication).
Choice to take hypertension medication
Montgomery 2003 found no significant effect for decision aids over usual care on the initiation of medication for hypertension (Table 15).
Choice for menopausal symptom treatment
In a study comparing a decision aid to usual care (Murray 2001b), there was a non‐significant decrease of 8% in hormone therapy (Table 15). Preferences for natural health products in women experiencing menopausal symptoms were no different for women exposed to the decision aid compared to women exposed to the usual education materials (Legare 2008a).
Choice for multiple sclerosis immunotherapy
Kasper 2008 reported no difference in the uptake of immunotherapy in people with multiple sclerosis who were exposed to a decision aid compared to usual care based on practice guidelines (Table 15).
Choice to take osteoporosis treatment
There was no difference in prescriptions for bisphosphonates for osteoporosis treatment (LeBlanc 2015;Table 15). Montori 2011 found no significant effect of decision aids over usual care on the uptake of medication for osteoporosis treatment.
Mental health
Hamann 2006 found no difference in prescription rates for antipsychotic medications but reported a statistically significant increase in the uptake in psycho‐education (P = 0.003) in people with schizophraenia exposed to the decision aid compared to usual care (Table 15). Mott 2014 reported that a higher proportion of participants in the decision aid group with post‐traumatic stress disorder completed psychotherapy sessions (4 of 9) compared to usual care (1 of 11).
Obstetrical choices
Childbirth procedures
Three studies focused on childbirth issues, using a decision aid compared to usual care. There was no difference in preference for vaginal birth in Shorten 2005 or actual vaginal mode of delivery in Montgomery 2007 following a previous cesarean section. Another study found no difference in actual choice to undergo external cephalic version for women with breech presentation (Nassar 2007).
Birth control approaches
There was no difference in the birth control methods chosen for those in the decision aid versus usual care groups (Langston 2010).
Embryo transplantation
Compared to usual care, those in the decision aid group were significantly more likely to choose a single embryo transplant (43% versus 32%) (Van Peperstraten 2010).
Vaccines
Compared to usual care, there was a non‐significant increase in intentions to get the flu vaccine in those exposed to the decision aid (46% versus 27%) (Chambers 2012), a statistically significant increase in uptake of hepatitis B vaccination with decision aids (Clancy 1988), and no difference in uptake of measles, mumps, rubella vaccine in infants (Shourie 2013).
Other choices
Blood transfusions
There was no difference in the uptake of preoperative autologous blood donation when a decision aid was compared to usual care (Laupacis 2006).
Lung transplant referral
There was no difference in referral rates for consideration of lung transplant in people with advanced cystic fibrosis exposed to a decision aid versus usual care (Vandemheen 2009).
Pelvic organ prolapse treatment
There was no difference in treatment rates for prolapsed pelvic organs (Brazell 2014).
Thyroid cancer radioactive iodine treatment
There was no difference in the rates of adjuvant radioactive iodine treatment for thyroid cancer (Sawka 2012).
Adherence (continuance/compliance) with chosen option
Of 105 studies, 16 (15.2%) measured adherence using various approaches (Table 16).
15. Adherence with chosen option.
| Reference | Scale used | N decision aid | Mean (SD) Decision aid | N comparison | Mean (SD) Comparison | Notes |
| Langston 2010 | 3 months ‐ using a contraceptive method that was in the same effectiveness group as the method requested at enrolment, 'very effective', as chosen option ‐ e.g. if chose sterilization and ended up using an IUD counted as adhering | 48 | 85% | 52 | 77% | P = 0.28 No difference in adherence to baseline choice |
| 3 months ‐ using a contraceptive method that was in the same effectiveness group, 'effective', as chosen option | 41 | 68% | 31 | 68% | P = 0.96 No difference in adherence to baseline choice |
|
| LeBlanc 2015 (in consult) | Filled prescription (of those who were given prescriptions), n/N (%) | 29 | 10/13 (83%) (1 missing) | 38 | 4/12 (40%) (2 missing) | P = 0.07 No difference in adherence to baseline choice |
| % of days covered out of 180 (median, 95% CI) | 29 | 46.7% (95% CI 39.2 to 46.7) | 38 | 85% (95% CI 55.3 to 92.6) | P = 0.08 No difference in adherence to treatment |
|
| Legare 2012 (in consult) | 2 weeks post ‐ single question asking if the patient maintained the decision made, n (%) | 163 | 143 (87.7%) | 165 | 150 (91.5%) | Absolute difference 3.8; RR 1.0 (95% CI 0.9 to 1.0) No difference in adherence to baseline choice |
| Lepore 2012 | Congruence between intention to test and verified PSA test ‐ 1 year | 244 | 55.3% | 246 | 58.1% | No difference in adherence to baseline choice. 95% CI 0.62 to 1.28 |
| Congruence between intention to test and verified PSA test ‐ 2 year | 244 | 59.0% | 246 | 59.3% | No difference in adherence to baseline choice. 95% CI 0.69 to 1.42 | |
| Loh 2007 (in consult) | 6‐8 weeks ‐ patient reported ‐ 5‐point Likert scale on steadiness of following the treatment plan: 1 = very bad to 5 = very good | 191 | 4.3 (0.9) | 96 | 3.9 (1.0) | No difference in adherence to treatment P = 0.073 |
| 6‐8 weeks ‐ physician reported ‐ 5‐point Likert scale steadiness of following the treatment plan: 1 = very bad to 5 = very good | 191 | 4.8 (0.6) | 96 | 4.3 (1.1) | No difference in adherence to treatment P = 0.56 |
|
| Mann D 2010 (in consult) | 3 months ‐ telephone administration of the 8‐item Morisky adherence (7 yes/no items and 1 item with 5‐point Likert scale to elicit behaviours such as skipping medicines when they have no symptoms) | — | — | — | — | No difference in adherence to treatment 70% reported good adherence to statins; no difference between groups |
| 6 months ‐ telephone administration of the 8‐item Morisky adherence (7 yes/no items and 1 item with 5‐point Likert scale to elicit behaviours such as skipping medicines when they have no symptoms) | — | — | — | — | No difference in adherence to treatment 80% reported good adherence to statins; no difference between groups |
|
| Man‐Son‐Hing 1999 | 6 months ‐ self‐reported – measured % of participants taking therapy initially chosen | 129 | 95.35% | 134 | 93.28% | No difference in adherence to baseline choice P = 0.44 |
| Mathers 2012 | 6 months ‐ Self‐reported. Measured % of patients who did not change their initially chosen treatment. | 95 | 68.1% | 80 | 56.3% | PtDA higher aderence to baseline choice P = 0.041 |
| Montgomery 2003 | ˜ 3 years ‐ self‐reported – 6‐item adherence questionnaire: from 'I take all my tablets at the same time of day' to 'I take hardly any of my tablets' | — | — | — | — | No difference to adherence to baseline choice or adherence to treatment |
| Montori 2011 (in consult) | 6 months ‐ percentage of participants that self‐reported currently taking medication who have not missed 1 dose within last week | 17 | 65% | 19 | 63% | No difference in adherence to treatment P = 0.92 |
| 6 months ‐ percentage of participants who opted to take bisphosphonates who took their medication on more than 80% of the days for which it was prescribed, based on pharmacy records | 23 | 100% | 19 | 74% | No difference in adherence to baseline choice P = 0.009 |
|
| Mott 2014 | 4 months ‐ percentage of participants who engaged in psychotherapy sessions | 9 | 44% | 11 | 45% | — |
| 4 months ‐ number of participants who engaged in 9 or more psychotherapy sessions | 4 | 100% | 5 | 20% | Adherence to treatment | |
| Mullan 2009 (in consult) | 6 months ‐ pharmacy records ‐ days covered (range) | 48 | 97.5% (range 0 to 100) | 37 | 100 (range 73.9 to 100) | Higher adherence to treatment for usual care AMD −8.88 (−13.6% to −4.14%) Statistically significant |
| 6 months ‐ self‐reported by telephone call – did not miss a dose in last week | 41 | 76% | 31 | 81% | No difference in adherence to treatment OR 0.74 (95% CI 0.24 to 2.32) |
|
| Oakley 2006 | 4 months ‐ extent to which the participants' behaviour in taking medications coincides with the clinical prescription | 16 | 10.4% (32) (improvement from baseline) | 17 | 2% (26) (improvement from baseline) | No difference in adherence to treatment |
| Sheridan 2011 | 3 month ‐ adherence to treatment | |||||
| Any therapy promoted in decision aid | 76 | 45 (59%) | 73 | 25 (34%) | P < 0.01 DA group showed higher adherence to treatment |
|
| Any therapy promoted in decision aid + others (e.g. diet or physical activity) | 77 | 64 (83%) | 77 | 52 (68%) | P = 0.02 | |
| Aspirin | 32 | 30 (94%) | 19 | 11 (58%) | P < 0.01 | |
| Cholesterol medicine | 14 | 12 (86%) | 6 | 5 (83%) | The intervention had little effect blood pressure or cholesterol medication, however, the sample sizes for these estimates were small and underpowered | |
| Blood pressure medicine | 9 | 9 (100%) | 12 | 11 (92%) | ||
| Stop smoking | 8 | 25% | 5 | 20% | No effect on smoking, although subgroups were small and underpowered | |
| Trevena 2008 | 1 month ‐ faecal occult blood test uptake | 134 | 5.2% | 137 | 6.6% | No difference in adherence to baseline choice P = 0.64 |
| Weymiller 2007 (in consult) | 3 months ‐ self‐reported – mailed surveys and telephone call to non‐respondents On adherence to statin use: missed 1 dose or more within the last week |
33 | 93.94% | 29 | 79.31% | No difference in adherence to baseline choice or treatment when analysis adjusted by sex, cardiovascular disease, and number of medications |
AMD: absolute mean difference; DA: decision aid; OR: odds ratio
Based on the measurement framework by Trenaman 2016, we grouped adherence according to adherence to the baseline choice and adherence to the treatment. Six studies measured only adherence to the baseline choice (Langston 2010; Legare 2012; Lepore 2012; Man‐Son‐Hing 1999; Mathers 2012; Trevena 2008), 6 studies measured only adherence to treatment (Loh 2007; Mann D 2010; Mott 2014; Mullan 2009; Oakley 2006; Sheridan 2011), and 4 studies measured both (LeBlanc 2015; Montgomery 2003; Montori 2011; Weymiller 2007).
For the 10 studies that measured adherence to choice, two studies reported that patients exposed to decision aids had higher adherence compared to usual care (Mathers 2012; Montori 2011), and 8 reported no difference between groups. For example, Mathers 2012 asked participants, 6 months after their decision, whether or not they had changed their initial choice about starting insulin for type II diabetes (decision aid 68.1% versus 56.3% usual care; P = 0.041). Montori used pharmacy records to determine if participants who chose bisphosphonates actually took their medication on more than 80% of the days for which it was prescribed (100% decision aid versus 74% usual care; P = 0.009).
For the 10 studies that measured adherence to treatment, 2 studies reported that patients exposed to decision aids had higher adherence compared to usual care (Mott 2014; Sheridan 2011), 1 study reported that patients exposed to decision aids had lower adherence (Mullan 2009), and 7 reported no difference. Mott reported the percentage of participants at four months who engaged in nine or more psychotherapy sessions (4 of 4 decision aid group participants versus 1 of 5 usual care). Sheridan measured the percentage of participants who, 3 months after initiating therapy, were continuing (59% decision aid versus 34% usual care; P < 0.01). Mullan used pharmacy records to determine the days covered by medication use (97.5% decision aid versus 100% usual care).
Health outcomes
General health outcomes
Eleven studies (10.5%) compared a decision aid to usual care in terms of general health outcomes (Table 17). Ten of these used either the previously validated Medical Outcomes Study 36‐item Short‐Form Health Survey (SF‐36) or the 12‐item Short‐Form Health Survey (SF‐12) (Stewart 1992), while Vuorma 2003 used the RAND‐36 (Hays 1993). As shown in Table 17, there were no significant differences for mental health function or social function in any of the seven studies. In one study (Barry 1997), general health and physical function outcome scores were significantly better in the decision aid group compared to usual care for men considering treatments for benign prostatic disease. Of the two studies evaluating the effect of a decision aid for women considering treatment for abnormal uterine bleeding, Kennedy 2002 found a statistically significant improvement in role physical function, and Vuorma 2003 found a statistically significant improvement in emotional role functioning for women.
16. General quality of life.
| Reference | Timing | N decision aid | Mean Decision aid (SD) | Change from baseline | N comparison | Mean comparison (SD) | Change from Baseline | Notes |
| General health | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 67.2 (19.0) | — | 123 | 71.1 (17.6) | — | P = 0.02 |
| 3 months | — | — | −0.96 (1.41) | — | — | −3.59 (1.57) | ||
| 6 months | — | — | −1.46 (1.41) | — | — | −4.93 (1.45) | ||
| 12 months | — | — | 0.61 (1.58) | — | — | −4.99 (1.44) | ||
| Legare 2011 (percentage of people who felt they had a stable and better health, (SF‐12)) | 2 weeks post | Not reported | 94 | +7 | Not reported | 85 | −6 | P = 0.08 |
| Morgan 2000 (SF‐36) | 6 months post | 72 | 62 (23) | + 4.0 | 88 | 65 (20) | + 7.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 2.2 | 159 | — | 2.8 | No difference |
| Physical function | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 81.9 (20.0) | — | 123 | 83.0 (18.9) | — | P = 0.02 |
| 3 months | — | — | −0.34 (1.61) | — | — | −1.81 (1.07) | ||
| 6 months | — | — | 0.10 (1.28) | — | — | −3.26 (1.37) | ||
| 12 months | — | — | 0.15 (1.40) | — | — | −3.74 (1.18) | ||
| Knops 2014 (SF‐12) | Baseline | 91 | 45 | — | 87 | 44 | — | — |
| 1 month | 80 | 44 | — | 84 | 43 | — | — | |
| 4 months | 80 | 43 | — | 84 | 43 | — | — | |
| 10 months | 80 | 44 | — | 84 | 42 | — | — | |
| Legare 2012 (SF‐12) | 2 weeks post | 160 | 49.4 (SD 7.5) | + 0.08 | 162 | 48.16 (7.80) | + 0.43 | Absolute difference 1.2; MD 0.4 (95% CI−2.6 to 3.3) |
| Morgan 2000 (SF‐36) | 6 months post | 72 | 67 (29) | + 7.0 | 88 | 71 (24) | + 10.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 2.4 | 159 | — | 2.2 | No difference |
| Bernstein 1998 (SF‐12) | 3 months post | 61 | 38 (12.1) | + 0.6 | 48 | 37.6 (10.6) | + 3.8 | No difference |
| Social function | ||||||||
| Barry 1997 (SF‐36) | Baseline | 104 | 90.6 (15.5) | 123 | 91.7 (15.7) | P = 0.17 | ||
| 3 months | — | — | 0.34 (1.58) | — | — | −2.26 (1.36) | ||
| 6 months | — | — | −0.05 (1.92) | — | — | −2.46 (1.45) | ||
| 12 months | — | — | −1.46 (1.85) | — | — | −3.52 (1.71) | ||
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 84.7 | — | 71 | 82.1 | — | P = 0.39 |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 5.2 | 159 | — | 7.1 | No difference |
| Mental function | ||||||||
| Legare 2012 (SF‐12) | 2 weeks post | 160 | 50.79 (SD 9.28) | −0.38 | 162 | 51.21 (8.36) | + 2.7 | Absolute difference 0.4; MD −1.9 (95% CI−4.9 to 1.1) |
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 71.3 | — | 71 | 71.6 | — | P = 0.46 |
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 4.7 | 159 | — | 5.3 | No difference |
| Bernstein 1998 (SF‐12) | 3 months post | 61 | 49.1 (11.4) | 0.0 | 48 | 48.9 (10.8) | + 0.9 | No difference |
| Role function | ||||||||
| Morgan 2000 (SF‐36) | 6 months post | 72 | 62 (44) | + 20.0 | 88 | 58 (43) | + 15.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | P = 0.04 |
| Vuorma 2003 (RAND‐36) | 1 year | — | 9.2 | — | — | 6.3 | No difference | |
| Bodily pain | ||||||||
| Morgan 2000 (SF‐36) | 6 months post | 72 | 81 (22) | + 6.0 | 88 | 77 (24) | + 5.0 | No difference |
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | 157 | — | No difference | ||
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 6.5 | 159 | — | 6.2 | No difference |
| Role emotional | ||||||||
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 80.3 | — | 71 | 77.4 | — | P = 0.61 |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 12.6 | 159 | — | 1.9 | P = 0.01 |
| Energy/vitality | ||||||||
| Kennedy 2002 (SF‐36) | 2 years | 176 | — | — | 157 | — | — | No difference |
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 55.2 | — | 71 | 54.1 | — | P = 0.09 |
| Vuorma 2003 (RAND‐36) | 1 year | 156 | — | 8.9 | 159 | — | 8.8 | No difference |
| SF‐36 all dimensions | ||||||||
| McCaffery 2010 (SF‐36) | 2 weeks | 77 | 47 | — | 71 | 46.3 | — | P = 0.35 |
| Murray 2001b (SF‐36) | 9 months | 93 | — | — | 94 | — | — | No difference |
| Murray 2001a (SP−36) | 9 months | 54 | — | — | 48 | — | — | No difference |
| Health utilities | ||||||||
| Murray 2001a (Euroqol EQ‐5D) | — | — | — | — | — | — | — | No difference |
| Murray 2001b (Euroqol EQ‐5D) | — | — | — | — | — | — | — | No difference |
| Euroqol 5D ‐ Health Thermometer (scale of 0 to 100) | ||||||||
| LeBlanc 2015 | Postconsultation | 29 | 85 (IQR 80, 95) | — | 85 (IQR 73, 90) | — | — | P = 0.19 |
DA: decision aid; SF‐36: Medical Outcomes Study 36‐item Short‐Form Health Survey; SF‐12: 12‐item Short‐Form Health Survey;
RAND‐36: the 36‐item short form survey from the RAND Medical Outcomes Study
In two studies measuring health utilities using the Euroqol EQ‐5D (Murray 2001a; Murray 2001b), there was no difference between the decision aid and usual care groups. There was also no between‐group difference in the LeBlanc 2015 study, which used the Euroqol 5D health thermometer.
Condition‐specific health outcomes
Seven studies (6.7%) used various measures to assess condition‐specific health outcomes (Table 18). Outcomes included urinary symptoms (Barry 1997; Murray 2001a), angina (Bernstein 1998), functional assessment of cancer therapy (Leighl 2011), menopausal symptoms (Murray 2001b), and menstrual symptoms (Protheroe 2007; Vuorma 2003). Five studies found no significant effects on condition‐specific health outcomes (Bernstein 1998; Leighl 2011; Murray 2001a; Murray 2001b; Vuorma 2003). Protheroe 2007 reported significantly higher menorrhagia‐related quality of life scores in women exposed to the decision aid compared to usual care. Barry 1997 showed an improvement in urinary symptoms in favour of the decision aid group, but it was not statistically significant.
17. Condition‐specific quality of life.
| Study | Outcome | Scale used | Timing | N decision aid | Decision aid mean change (SD) | N comparison | Comparison mean change (SD) | Notes |
| Barry 1997 | Urinary symptoms | AUA Symptom Index (0 to 100) | 3 months | 104 | −4.80% (1.74) | 117 | −1.40% (1.37) | No difference; trend toward DA |
| Urinary symptoms | AUA | 6 months | 104 | −3.66% (2.06) | 117 | −3.17% (1.77) | No difference | |
| Urinary symptoms | AUA | 12 months | 104 | −2.51% (2.11) | 117 | −4.14% (1.66) | No difference; trend toward control | |
| Impact of symptoms | BPH Impact Index (0 to 100) | 3 months | 104 | −6.58% (1.10) | 117 | −3.00% (1.05) | No difference; trend toward DA | |
| Impact of symptoms | BPH | 6 months | 104 | −4.37% (1.32) | 117 | −3.89% (1.16) | No difference; trend toward DA | |
| Impact of symptoms | BPH | 12 months | 104 | −5.53% (1.32) | 117 | −2.63% (1.32) | No difference; trend toward DA | |
| Bernstein 1998 | Satisfaction | SAQ (0 to 100) | 3 months | 61 | + 6.2% | 48 | + 10.5% | Control significantly more satisfied |
| Angina stability | SAQ | 3 months | 61 | + 17.2% | 48 | + 28.3% | No difference | |
| Angina frequency | SAQ | 3 months | 61 | + 5.5% | 48 | + 15.3% | No difference | |
| Disease Perception | SAQ | 3 months | 61 | + 14.1% | 48 | + 18.8% | No difference | |
| Physical Capacity | SAQ | 3 months | 61 | −0.5% | 48 | + 7.1% | No difference | |
|
Leighl 2011 (FACT‐G) median (range) |
Functional status at 1 month post | 74 | 17 (6‐28) | — | 68 | 17.5 (7‐28) | — | P = 0.02 |
| Physical function at 1 month post | 74 | 21 (0‐28) | — | 68 | 20 (4‐28) | — | No difference | |
| Role emotional at 1 month post | 74 | 17 (0‐20) | — | 68 | 17(7‐20) | — | No difference | |
| Murray 2001a | Urinary symptoms | AUA symptom Index (0 to100) | — | — | — | — | — | No difference |
| Murray 2001b | Menopausal symptoms | MenQol | — | — | — | — | — | No difference |
| Protheroe 2007 | Menorrhagia specific utility scale | (0 to 100) | 6 months | 60 | 59.3 (30.0) | 56 | 50.9 (25.1) | P = 0.03 higher menorrhagia quality of life favouring DA group |
| Vuorma 2003 | Inconvenience due to menstrual bleeding | (5 to 25) | 1 year | 156 | 10.4 | 159 | 10.5 | No difference |
| Menstrual pain | (0 to 12) | 1 year | 156 | 4.7 | 159 | 4.6 | No difference |
AUA: American Urological Association; BPH: benign prostatic hyperplasia; DA: decision aid; SAQ: Seattle Angina Questionnaire; FACT‐G: Functional Assessment of Cancer Therapy‐General.
Other health outcomes
Seven studies (6.7%) reported on other health outcomes (Table 19), including death (Auvinen 2004; Knops 2014), glycated haemoglobin (Mathers 2012), angina (Morgan 2000), stroke (Thomson 2007), successful pregnancy (Van Peperstraten 2010), and pain (Vuorma 2003). There were no statistically significant differences between groups.
18. Other condition‐specific health outcomes.
| Study | Outcome | Scale used | Timing | N decision aid | Decision aid outcome | N comparison | Comparison outcome | Notes |
| Auvinen 2004 | Death | — | 5 years | 104 | 41 (39%) | 106 | 33 (31%) | No difference |
| Disease‐free survival | — | 10 years | 104 | 74 (70.8%) | 106 | 66 (62.5%) | P = 0.14 | |
| Biochemical failure (rising serum PSA) | — | 5 years | 100 | 42 (42%) | 96 | 34 (35%) | P = 0.57 | |
| Disease progression | — | 5 years | 97 | 31 (32%) | 92 | 28 (30%) | P = 0.94 | |
| Knops 2014 | Postoperative mortality | — | 10 months | 91 | 0 (0%) | 87 | 0 (0%) | |
| Postoperative major morbidity | — | 10 months | 91 | 0 (0%) | 87 | 2 (6%) | P = .23 | |
| Aneurysm rupture during watchful waiting | — | 10 months | 91 | 0 (0%) | 87 | 3 (8%) | P = 0.12 | |
| Mathers 2012 | HbA1c (change from baseline) | — | 6 months | 95 | −0.37% | 80 | −0.24% | P = 0.12 |
| Morgan 2000 | No angina | CCVA | 6 months | 72 | + 49% | 88 | + 48% | No difference |
| Class I angina | CCVA | 6 months | 72 | −1% | 88 | + 6% | No difference | |
| Class II angina | CCVA | 6 months | 72 | −23% | 88 | −26% | No difference | |
| Class III angina | CCVA | 6 months | 72 | −26% | 88 | −28% | No difference | |
| Class IV angina | CCVA | 6 months | 72 | 0% | 88 | 0% | No difference | |
| Thomson 2007 | Strokes or bleeds requiring admission | — | 3 months | 51 | — | 55 | — | No strokes and no bleeds requiring admission. 1 bleed and 1 transient stroke both in control group that required GP consultation |
| Van Peperstraten 2010 | Ongoing pregnancies (> 12 weeks gestation) | — | After 1st IVF cycle | 152 | — | 156 | — | 32% of participants in the intervention group and 38% of participants in the control group had ongoing pregnancies, P = 0.25 |
| Twin pregnancies (> 12 weeks gestation) | — | After 1st IVF cycle | 152 | — | 156 | — | 4% of participants in intervention group and 6% of participants in control group had twin pregnancies, P = 0.33 | |
| Vuorma 2003 | Inconvenience due to menstrual bleeding | (5 to 25) | 1 year | 156 | 10.4 | 159 | 10.5 | No difference |
| Menstrual pain | (0 to 12) | 1 year | 156 | 4.7 | 159 | 4.6 | No difference |
AUA: American Urological Association; CCVA: Canadian Cardiovascular Angina; BPH: benign prostatic hyperplasia; DA: decision aid; SAQ: Seattle Angina Questionnaire.
Preference‐linked health outcomes
None of the 105 studies measured preference‐linked health outcomes – that is, whether the patients experienced the outcomes they preferred and avoided the outcomes they wanted to avoid.
Anxiety
Of 105 studies, 31 (29.5%) measured anxiety, with 24 using the previously validated State Trait Anxiety Inventory (Spielberger 1970), 2 using the anxiety subscale of the Hospital Anxiety and Depression Scale (Knops 2014; Lam 2013), 2 using questions about worry (Fraenkel 2012; Smith 2010), 2 measuring intrusive thoughts (Lewis 2010; McCaffery 2010), and 1 using a single question on a seven‐point Likert scale (Johnson 2006; see Table 20). Of 18 studies that used the State Trait Anxiety inventory within 1 month postintervention, 2 (11.1%) reported that the decision aid group had significantly lower anxiety scores for people considering birthing options after a previous caesarean (Montgomery 2007) and for women considering options for the treatment of menorrhagia (Protheroe 2007). None of the studies demonstrated significant differences in effects on people's state anxiety at one month (2 studies), three months (6 studies), six months (4 studies), or one year (2 studies). There was no significant difference between groups for the other instruments that measured anxiety.
19. Anxiety.
| Study | Timing |
N decision aid |
Mean decision aid (SD) | Change from baseline |
N comparison |
Mean comparison(SD) | Change from baseline | Notes |
| State Anxiety Inventory: < 30 days postintervention (standardized scores) | ||||||||
| Bekker 2004; prenatal screening | Immediately post | 50 | 58.9 (16.6) | — | 56 | 61.2 (13.7) | — | No difference |
| Evans 2010; PSA screening | Immediately post‐DA | 89 | 4.98 | — | 103 | 4.88 | — | No difference P = 0.98 |
| Fraenkel 2012; atrial fibrillation | Immediately post‐DA | 69 | 13.0 | — | 66 | 13.4 | — | No difference P =0.48 |
| Leighl 2011 | Post consult, 1‐2 weeks and 4 weeks post | — | — | — | — | — | — | No difference |
| Mathieu 2007; mammography screening | Immediately after | 321 | 29.61 | — | 315 | 29.34 | — | No difference |
| McCaffery 2010; HPV screening (state trait anxiety inventory) | 2 weeks | 77 | 10.5 | — | 71 | 10.6 | — | No difference P = 0.25 |
| Montgomery 2003; hypertension | Immediately post‐DA | 44 | 35.45 (10.52) | — | 50 | 37.67 (13.92) | — | No difference |
| Montgomery 2007; previous cesarean section | 37 weeks gestation | 196 | 38.7 (12.2) | — | 195 | 42.1 (12.2) | — | P = 0.016 |
| Nassar 2007; breech presentation | 1 week | 98 | 41.4 (12.5) | — | 90 | 44.4 (13.9) | — | No difference |
| Protheroe 2007; menorrhagia | 2 weeks | 59 | 11.6 (3.7) |
— | 61 | 12.2 (3.7) | — | P = 0.016 |
| Rubel 2010; PSA screening | Immediately after 20 items adapted from state portion of State‐Trait Anxiety Inventory Scale STAI ‐ Form Y; |
— | — | — | — | — | — | No difference Mean score = 1.66 (SD 0.59) (N = 200) for both groups |
| Smith 2010; bowel cancer screening | 2‐week follow‐up | 357 | 13.67 | — | 173 | 14.05 | — | No difference P = 0.80 |
| Thomson 2007; anti‐thrombotic treatment for atrial fibrillation | Immediately after | 53 | — | — | 56 | — | — | Significant fall in anxiety (−4.57) but no difference between groups (P = 0.98) |
| Trevena 2008 colorectal cancer screening | Immediately after | 134 | — | — | 137 | — | — | No difference (P = 0.59) |
| Van Peperstraten 2010; number of embryos transferred | Immediately after | 152 | 27.33% | — | 156 | 24.5% | — | No difference P = 0.14 |
| Whelan 2004; breast cancer surgery | 7 days post‐DA | 94 | 42.3 (1.3) | — | 107 | 41.9 (1.3) | — | No difference |
| Whelan 2003; breast chemotherapy | 7 days post‐DA | 82 | 45.6 | + 2.2 | 93 | 47.4 | + 0.8 | No difference |
| Wong 2006; pregnancy termination | Immediately post | 154 | 54 (15.8) | — | 159 | 54 (16.1) | — | No difference |
| State Anxiety Inventory: 1 month postintervention (standardized scores) | ||||||||
| Bekker 2004; prenatal screening | 1 month post‐DA | 29 | 35.3 (12.5) | — | 39 | 34.7 (14.8) | — | No difference |
| Davison 1997; prostate cancer treatment | 5‐6 weeks post‐DA | 30 | 35.5 | −9.0 | 30 | 34.5 | −2.5 | No difference |
| State Anxiety Inventory: 3 months postintervention (standardized scores) | ||||||||
| Murray 2001a; benign prostatic hypertrophy | 3 months post‐DA | 55 | 36.36 (14.99) | +2.4 | 48 | 32.08 (9.836) | +0.7 | No difference |
| Murray 2001b; hormone replacement therapy | 3 months post‐DA | 93 | 38.42 (10.83) | −0.5 | 95 | 40.53 (12.96) | +1.8 | No difference |
| Nagle 2008; prenatal screening | ˜1 to 12 weeks post‐DA | 167 | 37.2 (12.1) | — | 171 | 37.36 (12.6) | — | No difference |
| Nassar 2007; breech presentation | 3 months post‐DA | 86 | 29.2 (9.9) | — | 84 | 30.8 (10.5) | — | No difference |
| Vuorma 2003; menorrhagia treatment | 3 months post‐DA | 184 | 37.1 | +1.0 | 179 | 35.9 | −1.0 | No difference |
| Whelan 2003; breast chemotherapy | 3 months post‐DA | 82 | 36.0 | — | 93 | 37.8 | — | No difference |
| State Anxiety Inventory: 6 months postintervention (standardized scores) | ||||||||
| Lepore 2012; prostate screening | 8 months post‐DA | 215 | 9.6 (10.3) | — | 216 | 10.3 (10.2) | — | No difference No condition by time interaction on anxiety. Low in both groups. |
| Protheroe 2007; menorrhagia | 6 months post‐DA | 47 | 11.2 (4.2) | — | 52 | 13.3 (4.9) | — | No difference P = 0.067 |
| Whelan 2004; breast cancer surgery | 6 months post‐DA | 94 | 39.3 (1.3) | — | 107 | 38.9 (1.6) | — | No difference |
| Whelan 2003; breast chemotherapy | 6 months post‐DA | 82 | 38.2 | — | 93 | 38.2 | — | No difference |
| State Anxiety Inventory: 12 months postintervention (standardized scores) | ||||||||
| Whelan 2004; breast cancer surgery | 12 months post‐DA | 94 | 37.5 (1.4) | — | 107 | 36.6 (1.5) | — | No difference |
| Whelan 2003; breast chemotherapy | 12 months post‐DA | 82 | 39.2 | — | 93 | 40.2 | — | No difference |
| Anxiety subscale of the Hospital Anxiety and Depression Scale (HADS) | ||||||||
| Knops 2014; asymptomatic abdominal aortic aneurysm | 1 month post‐DA ‐ (HADS standardized) | 81 | 21.0 (17.1) | — | 85 | 23.8 (19.1) | — | No difference P = 0.73 |
| 4 months post‐DA (HADS) | 81 | 20.0 (19.1) | — | 85 | 21.9 (17.6) | — | — | |
| 10 months post‐DA (HADS) | 81 | 20.5 (20.0) | — | 85 | 21.4 (20.5) | — | — | |
| Lam 2013; breast cancer surgery | 1 week post‐DA Hospital Anxiety and Depression Scale (HADS standardized | 101 | 25.2 (22.4) | — | 97 | 24.8 (23.3) | — | No difference P = 0.655 |
| 1 month postsurgery | 101 | 11.9 (15.2) | — | 97 | 12.4 (15.7) | — | No difference P = 0.859 |
|
| 4 months postsurgery | 91 | 10.5 (15.2) | — | 88 | 10.0 (14.8) | — | No difference P = 0.908 |
|
| 10 months postsurgery | 88 | 12.9 (16.8) | — | 90 | 13.3 (17.1) | — | No difference P = 0.553 |
|
| Other measures indicating anxiety | ||||||||
| Chabrera 2015; prostate cancer | Seeking and using social support | 61 | 22.3 (5.20) | + 7.8 | 61 | 16.2 (5.44) | + 1.8 | P < 0.001 |
| Focusing on the positive | 61 | 15.1 (6.93) | + 0.3 | 61 | 16.2 (9.47) | + 0.9 | P < 0.001 | |
| Behavioural escape‐avoidance | 61 | 23.7 (5.53) | + 4.5 | 61 | 22.0 (4.22) | + 1.2 | P < 0.001 | |
| Cognitive escape avoidance | 61 | 11.7 (5.37) | + 4.47 | 61 | 10.5 (4.65) | + 1.84 | P < 0.001 | |
| Distancing | 61 | 8.75 (3.90) | + 1.85 | 61 | 8.54 (4.28) | + 0.47 | P < 0.001 | |
| Fraenkel 2012; atrial fibrillation | Worry about having a stroke over next 5 years (10 point scale ‐ lower scores=less worry) | 69 | 1.8 (SD 1.7) | — | 66 | 1.6 (SD 1.6) | — | P = 0.47 |
| Worry about having a bleed over next 5 years (10 point scale ‐ lower scores = less worry) |
69 | 1.5 (SD 3.3) | — | 66 | 1.9 (SD 3.2) | — | P = 0.24 | |
| Johnson 2006; endodontic treatment | Immediately post ‐ single question 7‐point Likert scale | 32 | 3.2 (1.7) | — | 35 | 3.8 (2.1) | — | P = 0.27 |
| Lewis 2010; colorectal cancer screening | Intrusive thoughts ‐ 3 items; 4 point scale ‐ not at all | 139 | 66.2% | — | 157 | 68.0% | — | P = 0.92 |
| Intrusive thoughts ‐ 3 items; 4 point scale ‐ sometimes | 66 | 31.4% | — | 69 | 29.9% | — | ||
| intrusive thoughts ‐ 3 items; 4 point scale ‐ often | 5 | 2.4% | — | 5 | 2.2% | — | ||
| McCaffery 2010 | Intrusive thoughts ‐ measured using 1 item from the impact of events scale | 77 | 43% | — | 71 | 32% | — | No difference |
| Smith 2010 | Worry about developing bowel cancer ‐ quite or very | 357 | 6% | — | 173 | 8% | — | P = 0.78 |
| Worry about developing bowel cancer ‐ none or a bit | 357 | 94% | — | 173 | 92% | — | ||
DA: decision aid; HPV: human papilloma virus; PSA: prostate‐specific antigen.
Depression
Of 105 studies, 6 (5.7%) measured the effect of decision aids on depression using various instruments (Table 21). None of the studies reported a statistically significant difference between groups for decisions about cancer treatment (Davison 1997; Whelan 2004), depression (Loh 2007), prenatal genetic testing (Nagle 2008), or for women considering the number of embryos to transplant (Van Peperstraten 2010). At 10 months' postintervention, there were lower levels of depression in women deciding about breast cancer surgery who were exposed to the patient decision aid versus the usual care, but no differences at 1 week, 1 month, or 4 months postintervention (Lam 2013).
20. Depression.
| Study | Timing |
N decision aid |
Mean decision aid (SD) | Change from baseline |
N comparison |
Mean comparison (SD) | Change from Baseline | Notes |
| Davison 1997 (20‐item CES‐D) | 5‐6 weeks | 30 | 29.8 | −0.6 | 30 | 29.5 | + 1.3 | No difference |
| Lam 2013 (Hospital and Anxiety Depression Scale) | 1 week post‐DA | 101 | 16.7 (17.1) | — | 97 | 16.7 (19.5) | — | No difference P = 0.849 |
| 1 month postsurgery | 101 | 11.0 (12.9) | — | 97 | 11.0 (12.9) | — | No difference P = 0.649 |
|
| 4 months postsurgery | 91 | 10.0 (15.7) | — | 88 | 9.0 (11.4) | — | No difference P = 0.637 |
|
| 10 months postsurgery | 88 | 6.7 (9.0) | — | 90 | 11.9 (16.2) | — | P = 0.001 | |
| Loh 2007 (Brief Patient Health Questionnaire‐D) | 6 to 8 weeks | 191 | 29.8 (2.7) | — | 96 | 27.0 (3.6) | — | No difference P = 0.236 |
| Nagle 2008 (Edinburgh Postnatal Depression Scale) | ˜1‐12 weeks post‐DA | 167 | 19 (11.6) | — | 171 | 19 (11.2) | — | No difference |
| Van Peperstraten 2010 (Beck Depression Inventory) | After multifaceted intervention/ before IVF | 126 | 16 (13%) | — | 136 | 5 (4%) | — | P = 0.01 |
| At uptake of IVF | 147 | 16 (11%) | — | 151 | 113 (9%) | — | No difference | |
| Whelan 2004 (20‐item CES‐D) | 1 week post‐DA | 94 | 13.8 (1.0) | — | 107 | 13.4 (1.1) | — | No difference |
| 6 months post‐DA | 94 | 15.1 (1.1) | — | 107 | 14.2 (1.2) | — | No difference | |
| 12 months post‐DA | 94 | 13.2 (1.3) | — | 107 | 12.8 (1.2) | — | No difference |
CES‐D: Centre for Epidemiology Studies Depresion Scale; DA: decision aid; IVF: in vitro fertilization.
Regret
Of 105 studies, 7 (6.7%) measured the effect of decision aids on decision regret, using the five‐item Decisional Regret scale (Brehaut 2003; see Table 22). At 4 and 10 months postintervention, women with breast cancer who were considering surgery and used a decision aid reported lower regret scores compared to women receiving usual care (Lam 2013). There was no statistically significant between‐group difference in the other six studies.
21. Decisional regret.
| Author | Item |
N decision aid |
Proportion or mean (SD) |
N control |
Proportion or mean (SD) | Notes |
| Brazell 2014 | Decision Regret Scale at 3 months postchoice |
28 | 12.1 (18.5) | 26 | 10 (20.1) | No difference P = 0.969 |
| Hanson 2011 | 5‐item Decisional Regret Index | 126 | 11.9 | 127 | 14.3 | No difference P = 0.14 |
| Kuppermann 2014 | Decision Regret Scale (out of 100) at 3‐6 months postintervention |
357 | 8.29 (12.5) | 353 | 6.83(10.8) | No difference P = 0.12; 95% CI 1.46 (−0.36 to 3.29) |
| Lam 2013 | Decision Regret Scale at 1 month postsurgery |
101 | 21.4 (17.2) | 97 | 23.1 (18.3) | No difference Adjusted P = 1.0 |
| Decision Regret Scale at 4 months postsurgery |
91 | 18.8 (15.8) | 88 | 24.4 (18.9) | P = 0.026 | |
| Decision Regret Scale at 10 months postsurgery |
88 | 20.1 (14.5) | 90 | 24.6 (18.8) | P = 0.014 | |
| Legare 2011 | Proportion of patients with decisional regret | — | 7% | — | 9% | No difference P = 0.91 |
| Legare 2012 | Decision Regret Scale 2 weeks postconsultation | 162 | 12.38(19.08) | 164 | 7.59 (13.67) | No clinically significant difference; Absolute difference 4.8; MD 4.8 (95% CI 0.9 to 8.7) |
| Mathers 2012 | Decision Regret Scale at 6 months postintervention |
95 | 44.63 | 80 | 44.57 | No difference P = 0.872 |
DA: decision aid.
Confidence
Of 105 studies, 8 (7.8%) measured the effect of decision aids on confidence levels (see Table 23). Four of these studies used the Decisional Self‐efficacy Scale (Allen 2010; Arterburn 2011; Fraenkel 2007; Smith 2010). Four studies reported a statistically significant improvement in confidence or self‐efficacy with decision making in the decision aid compared to the usual care groups (Chambers 2012; Fraenkel 2007; Gattellari 2003; McBride 2002), and the other studies reported no difference between groups.
22. Confidence.
| Study | Scale used | Timing | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Notes |
| Allen 2010 | 11‐item self‐efficacy scale | Postintervention | 291 | 83% (SD 40.26) |
334 | 79% (SD 33.08) |
No difference |
| Arterburn 2011 | Decisional self‐efficacy | Changes from baseline | 75 | + 3.0 (95% CI 0.6 to 5.4) | 77 | + 2.8 (95% CI 0.9 to 4.8) | No difference P = 0.78 |
| Chambers 2012 | Mean confidence with decision: scale from 1 (low confidence) to 5 (high confidence) | Postintervention | 48 | 4 | 59 | 3.6 | P = 0.02 |
| Fraenkel 2007 | Decisional self‐efficacy scale | Pre‐consultation | 43 | 32 (median) | 40 | 27 (median) | P = 0.001 |
| Gattellari 2003 | Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | 3 days post | 106 | — | 108 | — | P = 0.008; DA group more likely to agree that they could make an informed choice about PSA screening |
| Gattellari 2005 | Perceived ability to make an informed choice 1‐item; 5‐point Likert scale | Immediately post | 131 | — | 136 | — | No difference |
| McBride 2002 | Confidence with ability to understand outcomes of hormone therapy, make a decision, engage in discussion with practitioner, 3 items (0 to 10; low to high confidence) | 1 month post | 273 | 78% (18% SD) | 284 | 70% (19% SD) | P < 0.001 |
| 9 months post | 261 | 80% (17%SD) | 278 | 75% (20% SD) | P = 0.0004 | ||
| Smith 2010 | 3 items adapted from the Decisional self‐efficacy scale | 2‐week follow‐up | 357 | 4.67 (0.54 SD) | 173 | 4.61 (0.62 SD) | No difference P = 0.26 |
CI: confidence interval; DA: decision aid; SD: standard deviation.
Healthcare system effects
Cost and resource use
Of eight studies (7.6%) examining cost and resource use, one conducted a cost‐effectiveness analysis (Kennedy 2002), five evaluated the effect of decision aids compared to usual care on total costs (Montgomery 2007; Murray 2001a; Murray 2001b; Van Peperstraten 2010; Vuorma 2003), and two measured resource use (Legare 2012; Thomson 2007) (see Table 24).
23. Healthcare system effects.
| Study | Scale used | N decisionaid | Decision aid ‐ mean | N comparison | Comparison ‐ mean | Difference between groups | Notes |
| Consultation length | |||||||
| Bekker 2004 (in consultation) | Consultation length using DA in the consultation (minutes) | 50 | 32.2 (SD 13.0) | 56 | 26.3 (SD 11.5) | + 5.9 minutes | P = 0.01 (longer with decision aid) |
| Bozic 2013 | Consultation length with practitioner post‐DA (minutes) | 61 | 20.9 (SD 6.8) | 62 | 21.0 (SD 7.2) | −0.1 minutes | No difference; P = 0.91 |
| Krist 2007 | Time spent discussing prostate cancer with practitioner post‐DA (minutes) ‐ patient reported | 196 | 5.3 | 75 | 5.2 | + 0.1 minutes | No difference between groups |
| Time spent discussing prostate cancer with practitioner post‐DA (minutes) ‐ physician reported | 196 | 3.8 | 75 | 4.2 | −0.4 minutes | No difference between groups but physicians thought they spent less time than patients (P < 0.001) | |
| LeBlanc 2015 (in consultation) | Consultation length with practitioner using DA in consultation (median, range in minutes) | 29 | 11.5 (5.4 to 21.4) | 37 | 10.7 (2.5 to 54.9) | + 0.8 minutes (−33.6 to 3.0) | — |
| Loh 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 191 | 29.2 (10.7) | 96 | 26.7 (12.5) | +2.5 minutes | P = 0.681 |
| Ozanne 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 15 | 24 | 15 | 21 | +3 minutes | P = 0.42 |
| Thomson 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 8 | 44 (39 to 55) | 10 | 21 (19 to 26) | +23 minutes | P = 0.001 Compared computerized decision aid with standard gamble within the consultation to guideline driven consultation |
| Vodermaier 2009 | Consultation length with practitioner post‐DA | ||||||
| 5 to 10 min | 53 | 6 (11.3%) | 54 | 5 (9.3%) | — | P = 0.91 | |
| 10 to 15 min | 17 (32.1%) | 19 (35.2%) | — | ||||
| 15 to 25 min | 15 (28.3%) | 14 (25.9%) | — | ||||
| 25 to 35 min | 7 (13.2%) | 5 (9.3%) | — | ||||
| Above 35 min | 8 (15.1%) | 11 (20.4%) | — | ||||
| Whelan 2003 (in consultation) | Consultation length using DA in consultation (minutes) | 50 | 68.3 | 50 | 65.7 | + 2.6 minutes | P = 0.53 |
| Weymiller 2007 (in consultation) | Consultation length using DA in consultation (minutes) | 52 | — | 46 | — | + 3.8 minutes in DA group | Not statistically significant 3.8 min (95% CI −2.9 to 10.5) |
| Cost and resource use | |||||||
| Hollinghurst 2010; Montgomery 2007 | Total costs in the UK for decision about mode of delivery post previous cesarean | 235 | GBP 2019 (SD 741) | 238 | GBP 2033 (SD 677) | — | No difference |
| Kennedy 2002 | Cost‐effectiveness in the UK for decision about benign heavy menstruation | 296 300 |
USD 2026 (DA alone) USD 1556 (DA plus nurse coaching |
298 | USD 2751 | — | Mean differences: DA versus usual care USD 461 (95% CI 236 to 696) DA plus coaching versus usual care USD 1184 (95% CI 684 to 2110) |
| Murray 2001a | Total costs excluding intervention in the UK for decision about treatment of benign enlarged prostate | 57 | GBP 310.3 (SD 602.0) | 48 | GBP 188.8 (SD 300.4) | — | Mean difference GBP 121.5 (95% CI −58.9 to 302.0) |
| Total costs including intervention (interactive video disk equipment) in the UK for decision about treatment of benign enlarged prostate | 57 | GBP 594.10 (SD 602) | 48 | GBP 188.8 (SD 300.4) | — | Mean difference GBP 405.4 (95% CI GBP 224.9 to GBP 585.8) P < 0.001 |
|
| Murray 2001b | Total costs excluding intervention in the UK for decision about hormone replacement therapy | 85 | GBP 90.5 | 84 | GBP 90.9 (SD 39.2) | — | No difference |
| Total costs including intervention (interactive video disk equipment) in the UK for decision about hormone replacement therapy | 85 | GBP 306.5 (SD 42.8) | 84 | GBP 90.9 (SD 39.2) | — | Mean difference GBP 215.5 (95% CI 203.1 to 228.0) P < 0.001 | |
| Van Peperstraten 2010 | Mean total savings per couple in the Netherlands for decision about embryo transfer for invitro fertilization | — | — | — | — | — | Mean total saving per couple in the intervention group were EUR 169.75 (USD 219.12) |
| Vuorma 2003 | Total estimated costs in Finland for treatment decision about heavy benign menstruation | 184 | EUR 2760 | 179 | EUR 3094 | — | P = 0.1 No difference between intervention and control |
| Resource use | |||||||
| Legare 2012 (in consultation) | Repeat consultation for the same reason, n (%) | 163 | 37 (22.7%) | 165 | 25 (15.2%) | Absolute difference 7.5 | RR 1.3 (95% CI 0.7 to 2.3) |
| Thomson 2007 (in consultation) | GP consultations postintervention | 51 | 39 (76.5%) | 54 | 32 (59.3%) | — | P = 0.35 |
| Hospital appointments postintervention | 51 | 29 (56.9%) | 54 | 10 (18.5%) | — | P = 0.06 | |
| Wait time from screening of eligibility to decision | |||||||
| Stacey 2014a | Wait time in weeks | 69 | 33.4 weeks | 71 | 33 weeks | — | No difference |
CI: confidence interval; DA: decision aid; RR: risk ratio; SD: standard deviation.
The cost‐effectiveness analysis (Kennedy 2002) was conducted from the healthcare system perspective, using USD values from 1999 to 2000 and calculating costs over two years. The decision aid with nurse coaching demonstrated the lowest mean cost (USD 1566) compared to decision aid alone (USD 2026) or usual care (USD 2751).
Of the five studies that evaluated total costs, two reported no statistically significant difference in the patient decision aid compared to usual care (Montgomery 2007; Vuorma 2003). Two studies reported higher costs for the patient decision aid group when including the cost of the interactive video disc equipment (USD 216 at 1999 prices) and no statistically significant difference between groups when removing this cost (Murray 2001a; Murray 2001b). The fifth study reported that the mean total savings in the decision aid group versus usual care was EUR 169.75 per couple (Van Peperstraten 2010).
For healthcare resource use in upper respiratory infection, Legare 2012 reported no difference in the rates of repeat consultations for the same reason, and Thomson 2007 reported no difference in the rates of general clinician consultations in the three months following the intervention. Both studies used the patient decision aid in the consultation.
Consultation length
Of 105 studies, 10 (9.5%) evaluated the effect of a decision aid compared to usual care on consultation length (see Table 24). The median consultation length was 24 minutes (range 3.8 to 68.3) for patient decision aid compared to 21 minutes (range 4.2 to 65.7) for usual care. The difference was 2.6 minutes longer (7.5% increase) than usual care consultations (range 0.4 minutes shorter to 23 minutes longer). The length of consultation was significantly longer for the patient decision aid group in two studies (Bekker 2004; Thomson 2007), and eight studies reported no difference. Bekker 2004 reported that consultations about prenatal diagnostic testing were 5.9 minutes longer, and Thomson 2007 reported consultations about treatment for atrial fibrillation were 23 minutes longer when using a computerized decision aid with standard gamble method within the consultation.
Litigation rates
None of the 105 studies examined the effect of decision aids on litigation.
Adverse events
There were no adverse effects on health outcomes or satisfaction, and no other adverse events reported.
Subgroup analysis ‐ in preparation for versus during the consultation
Of 105 studies, 89 (84.8%) primarily evaluated the patient decision aid when used by the patient in preparation for the consultation, and 16 (15.2%) primarily evaluated the patient decision aid when used within the consultation. The patient decision aids used during the consultation focused on prenatal screening (Bekker 2004); cardiac stress testing (Hess 2012); dental surgery (Johnson 2006); restoration of tooth decay (Kupke 2013); antibiotics for upper respiratory infection (Legare 2011; Legare 2012); medication use for depression (Loh 2007), diabetes (Mann D 2010; Mullan 2009; Weymiller 2007), osteoporosis (LeBlanc 2015; Montori 2011), prevention of breast cancer (Ozanne 2007), and atrial fibrillation (Thomson 2007); surgery for breast cancer (Whelan 2004); and chemotherapy for breast cancer (Whelan 2003).
Knowledge
When considered separately by subgroups, there was no difference between knowledge scores for those exposed to the decision aid in preparation for the consultation compared to those used in the consultation itself (Analysis 1.2: MD 13.77% versus 10.57%, test for subgroup difference P = 0.31, I2: 3%). Weymiller 2007 reported a higher mean difference when the decision aid was administered during the consultation but not if it was administered by research staff in preparation for the consultation. For the studies evaluating decision aids used in the consultation not included in the pooled outcome, two showed a statistically significant improvement in knowledge (LeBlanc 2015; Ozanne 2007), and two showed no difference (Mann D 2010; Thomson 2007).
1.2. Analysis.
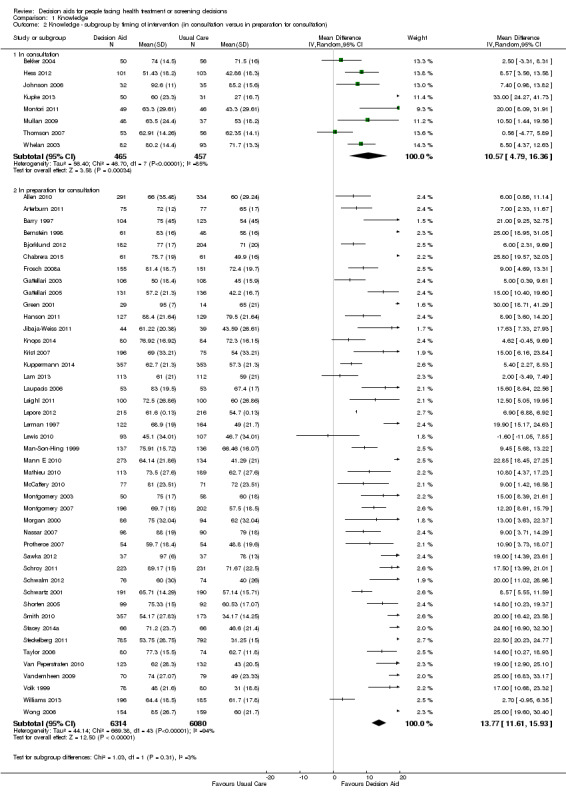
Comparison 1 Knowledge, Outcome 2 Knowledge ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation).
Accurate risk perceptions
When analyzing pre‐consultation and in‐consultation decision aids further, accurate risk perceptions were not different between studies that used the decision aid in preparation for the consultation and those where the intervention occurred during the consultation (Analysis 2.2: RR 2.25 versus RR 1.79, test for subgroup differences: P = 0.33, I2: 0%). The only study evaluating a decision aid within the consultation that was not included in the meta‐analysis, Weymiller 2007, reported a higher proportion with accurate risk perception when the decision aid was administered during the consultation, but found no difference between groups when administered by research staff in preparation for the consultation.
2.2. Analysis.
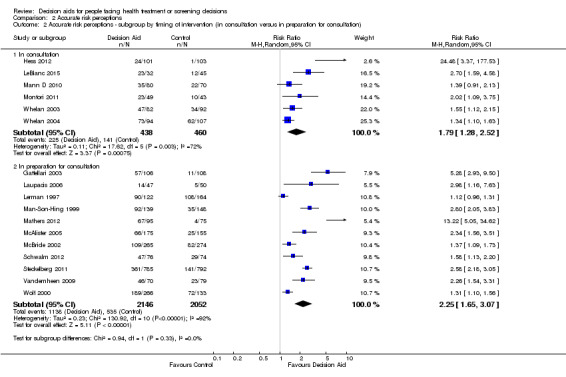
Comparison 2 Accurate risk perceptions, Outcome 2 Accurate risk perceptions ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation).
Decisional conflict uninformed subscale
Too few studies measured the uninformed subscale in those exposed to decision aid within the consultation to be able to compare with those who used decision aids in preparation for the consultation. Weymiller 2007 reported that participants felt less uninformed when the decision aid was administered during the consultation, but not if it was administered by research staff in preparation for the consultation.
Decisional conflict unclear values subscale
Too few studies measured the unclear values subscale in those exposed to decision aid within the consultation to be able to compare with those who used decision aids in preparation for the consultation. Weymiller 2007 reported that participants felt less unclear about values when the decision aid was administered during the consultation, but not if it was administered by research staff in preparation for the consultation.
Patient‐clinician communication
Due to variation in the reporting of data for this outcome, we were unable to investigate the effect of intervention timing on the variation in the effect on communication. Five studies evaluated a patient decision aid primarily used within the consultation with the clinician, and five evaluated a patient decision aid used in preparation for the consultation (see Table 9). All five studies that used the decision aid during consultations reported statistically higher mean OPTION scores in the patient decision aid group compared to usual care (Hess 2012; LeBlanc 2015; Montori 2011; Mullan 2009; Weymiller 2007). Four of five studies assessing the effects of pre‐consultation decision aid delivery (Fraenkel 2012; Hanson 2011; Lepore 2012; Sheridan 2011) reported that, compared to those in the usual care group, significantly higher proportions of participants exposed to the patient decision aid in preparation for the consultation reported that they discussed the decision with their clinician, and the fifth study showed no between‐group difference (Sheridan 2006).
Participation in decision making
There were too few studies on decision aids used during the consultation to interpret findings from the subgroup analysis (Analysis 5.2; Analysis 5.3).
5.2. Analysis.
Comparison 5 Participation in decision making, Outcome 2 Participation in decision making ‐ in consultation.
5.3. Analysis.
Comparison 5 Participation in decision making, Outcome 3 Participation in decision making ‐ in preparation for consultation.
Length of the consultation
Due to variation in the reporting of data for this outcome, we were unable to investigate the effects of intervention timing on the length of consultation. Of seven studies that evaluated decision aids used within the consultation (Bekker 2004; LeBlanc 2015; Loh 2007; Ozanne 2007; Thomson 2007; Weymiller 2007; Whelan 2003), two reported that the length of the consultation was significantly longer for the patient decision aid group (Bekker 2004; Thomson 2007). There was no difference for the other studies. The three studies that evaluated decision aids used in preparation for the consultation reported no between group difference in the length of the consultation (Bozic 2013; Krist 2007; Vodermaier 2009).
Other outcomes
For values‐choice congruence and proportion undecided, none of the studies of patient decision aids used during the consultation measured these outcomes. For satisfaction, there were a range of different approaches to measuring this outcome with mixed results and too few studies to make any descriptive comparisons. For choice, there were too few studies to conduct a subgroup analysis of pooled comparisons.
Post hoc analysis
Effects of study quality
To examine the potential bias arising from including studies of low methodological quality, we excluded 12 studies with a high risk of bias for any of the seven risk of bias criteria from the analysis (Auvinen 2004; Brazell 2014; Chambers 2012; Clancy 1988; Hamann 2006; Knops 2014; Krist 2007; Kupke 2013; LeBlanc 2015; Lewis 2010; Man‐Son‐Hing 1999; Mott 2014; see Figure 3). Overall, the results remained the same (Table 25; Analysis 1.3; Analysis 2.3; Analysis 3.5; Analysis 4.4).
24. Subanalysis using higher quality trials.
| Outcome | Overall mean effect (95% CI), 105 total studies | Without trials having high risk of bias on at least 1 of 7 criteria (N = 16) |
| Knowledge | 13.27 (95% CI 11.32 to 15.25) 52 studies | 13.43 (95% CI 11.37 to 15.49) 47 studies |
| Accurate risk perceptions ‐ with probabilities versus no probabilities | 2.10 (95% CI 1.66 to 2.66) 17 studies | 2.02 (95% CI 1.57 to 2.59) 15 studies |
| Values congruent with chosen option | 2.06 (95% CI 1.46 to 2.91) 10 studies | 2.06 (95% CI 1.46 to 2.91) 10 studies |
| Uninformed subscale of Decisional Conflict Scale | −9.28 (95% CI −12.20 to −6.36) 27 studies | −9.96 (95% CI −13.13 to −6.78) 25 studies |
| Unclear values subscale of Decisional Conflict Scale | ‐8.81 (95% Ci −11.99 to −5.63) 23 studies | −9.55 (95% CI −13.08 to −6.02) 21 studies |
CI: confidence interval.
1.3. Analysis.
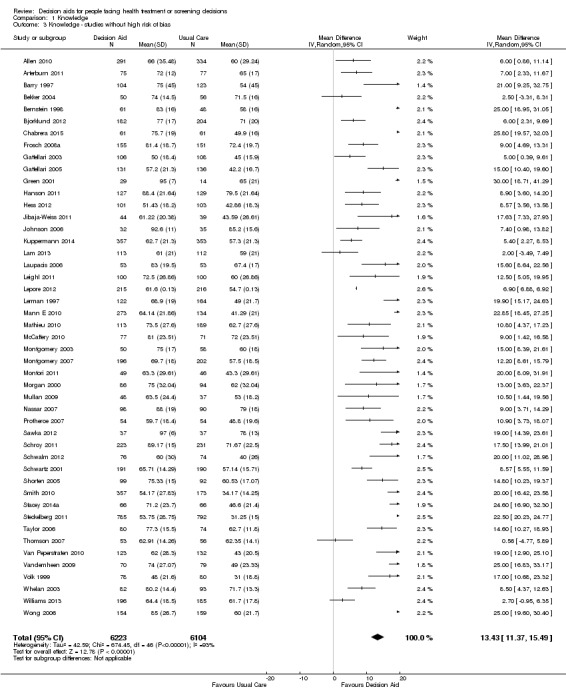
Comparison 1 Knowledge, Outcome 3 Knowledge ‐ studies without high risk of bias.
2.3. Analysis.
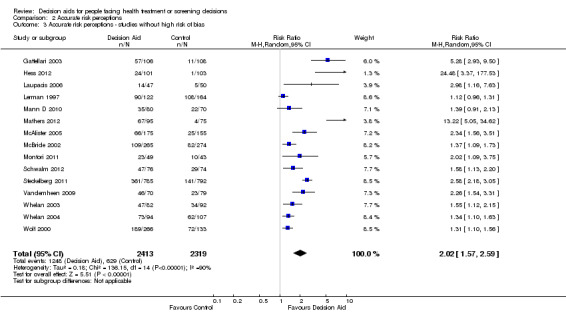
Comparison 2 Accurate risk perceptions, Outcome 3 Accurate risk perceptions ‐ studies without high risk of bias.
3.5. Analysis.
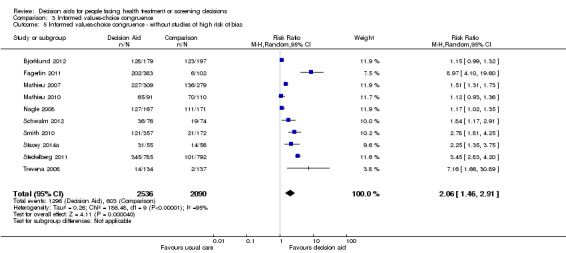
Comparison 3 Informed values‐choice congruence, Outcome 5 Informed values‐choice congruence ‐ without studies of high risk of bias.
4.4. Analysis.
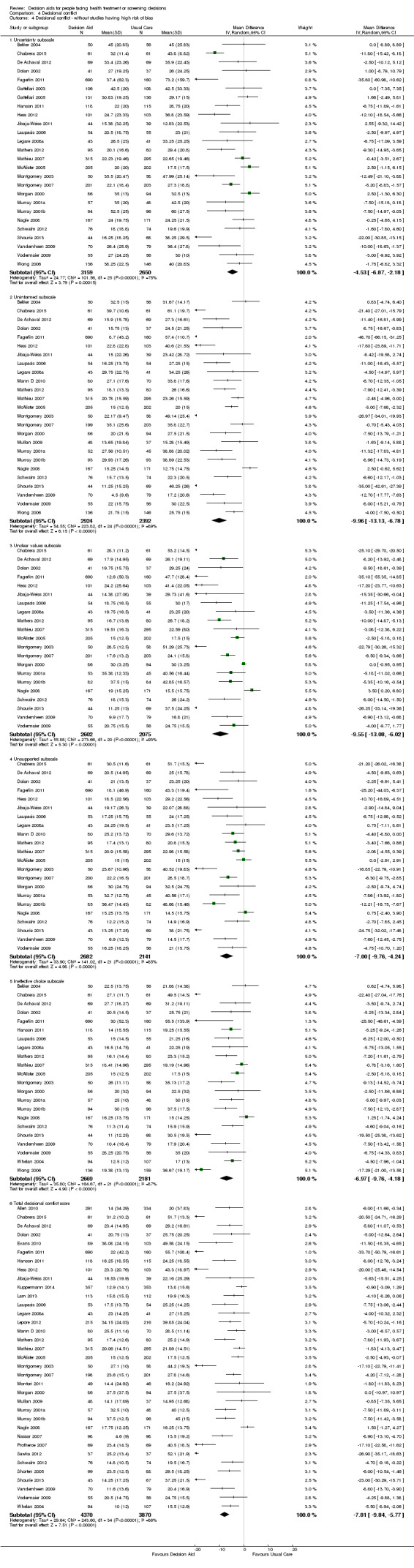
Comparison 4 Decisional conflict, Outcome 4 Decisional conflict ‐ without studies having high risk of bias.
Heterogeneity
When comparing patient decision aids to usual care, there was statistically significant heterogeneity in five of six of the IPDAS effectiveness criteria: knowledge scores, accurate risk perceptions, congruence between values and choice; feeling uninformed, and feeling unclear regarding personal values. There was no statistically significant heterogeneity for participation in decision making. It should be noted that the heterogeneity of the effect was not manifested in its direction but only in its size. For the 2009 update (O'Connor 2009b), we explored the potential factors contributing to heterogeneity (Table 26). Overall, regardless of the subgroup analyses conducted, scores for outcomes were similar to the overall effect, as indicated by overlapping confidence intervals.
25. Heterogeneity (based on 55 trials in search to 2006).
| Outcome | Overall effect | Treatment decision | Screening decision | Video/computer decision aid | Audio/pamphlet Decision aid | Base risk control | Removal of outliers* |
| Knowledge ‐ decision aid versus usual care | 15.2 (11.7 to 18.7) | 16.5 (11.9 to 21.2) | 13.1 (7.7 to 18.5) | 21.3 (16.3 to 26.2) | 11.9 (8.3 to 15.6) | 15.5 (11.3 to 19.8) | 17.3 (13.6 to 20.9) (*Bekker 2004, Gattellari 2003, Johnson 2006) |
| Accurate risk perceptions ‐ probabilities versus no probabilities | 1.6 (1.4 to 1.9) | 1.6 (1.4 to 1.9) | 1.6 (1.1 to 2.3) | No data | 1.6 (1.4 to 1.9) | 1.3 (1.2 to 1.5) (P = 0.3) | 1.5 (1.3 to 1.7) (*Gattellari 2003) |
| Uninformed subscale of the Decisional Conflict Scale ‐ decision aid versus usual care | −8.4 (−11.9 to −4.8) | −9.4 (−13.3 to −5.5) | −3.5 (−12.9 to 5.8) | −12.6 (−19.5 to −5.8) | −4.9 (−7.6 to −2.3) (P = 0.06) | −5.4 (−7.7 to −3.2) (P = 0.11) | −6.2 (−8.4 to −4.1) (P = 0.06) (*Montgomery 2003) |
| Unclear values subscale of the Decisional Conflict Scale ‐ decision aid versus usual care | −6.3 (−10.0 to −2.7) | −6.0 (−9.8 to −2.3) | Insufficient data | −8.0 (−15.1 to −1.0) | −4.5 (−8.4 to −0.6) | −3.6 (−6.8 to −0.5) | −4.0 (−6.7 to −1.3) (*Montgomery 2003) |
Discussion
Summary of main results
In this updated review, we added 18 new studies for a total of 105 studies comparing patient decision aids to usual care. This update also removed 28 studies that compared detailed versus simple patient decision aids that were included in the previous update. Based on the GRADE assessment (Table 1), there is high‐quality evidence that compared to usual care, decision aids improve people's knowledge regarding options and reduce the decisional conflict stemming from feeling uninformed and unclear about their personal values. There is moderate‐quality evidence that decision aids stimulate people to take a more active role in decision making and increase the accuracy of their risk perceptions. There is lower‐quality evidence that decision aids improve congruence between the chosen option and personal values. This outcome is measured using a variety of different approaches, and the evidence could be strengthened by more standardized measurement. Moreover, decision aids decreased the proportion of people remaining undecided.
Although not a primary outcome of the review, the effect of decision aids on patients' choosing particular options continues to be variable. The numbers of patients choosing to have major elective surgery continues to decrease in favour of more conservative options, except when the baseline rates are low (e.g. surgery for benign prostate hyperplasia, prophylactic mastectomy for women who are carriers of the BRCA gene). The numbers of men choosing prostate‐specific antigen (PSA) testing were fewer after exposure to decision aids.
Decision aids do no better than usual care in terms of their effects on people's satisfaction with decision making or health outcomes such as general quality of life or condition‐specific quality of life. However, no studies measured preference‐linked health outcomes, nor were adverse events reported. There was also no difference in anxiety. For length of consultation, eight studies found no difference, while two studies found a median increase of 2.6 minutes (7.5%) in the decision aid group compared to usual care consultations.There continue to be too few studies to determine the effects of decision aids on costs/resource use (Trenaman 2014). Although there may be additional costs involved in delivering decision aids, an independent review of decision aid studies with economic outcomes concluded that "this was likely to be small relative to the benefit to patients in terms of improved decision quality when effective decision aids are used" (NCGC/NICE 2012). Given the variability in measurement strategies, it difficult to determine the effect of patient decision aids on adherence to the chosen option or treatment.
New for this update, we analyzed the pooled data for decision aids used in preparation for the consultation separately from decision aids used in the consultation, and we found that there were similar improvements in knowledge, accurate risk perceptions, and patient‐clinician communication.
Overall completeness and applicability of evidence
Main effects of decision aids
The largest and most consistent benefits of decision aids, relative to usual care, are better knowledge of options and outcomes, and more accurate perceptions of outcome probabilities. These observations are clinically important because the usual care groups' scores for knowledge and perception of outcome probabilities were lower than the intervention groups'; both knowledge and perception of outcome probabilities are important for ensuring informed decision making. These effects suggest that current 'usual care' may not be good enough when informing people about these complex, values‐sensitive decisions. People need to comprehend the options and outcome probabilities in order to consider and communicate to their clinicians the personal value they place on the benefits versus the harms. Likewise, pooling results from additional studies in this update shows a significant increase in informed values‐based choice when decision aids were compared to usual care, and the results appear to be similar across subgroup analyses of studies that used the same composite measure.
Decision aids also help people feel more comfortable with their choices than usual care. This is revealed by the reduced scores for overall decisional conflict and for the decisional conflict subscales. People who use decision aids generally feel more informed about options and clearer regarding their personal values.
Compared to usual care strategies, decision aids improve individuals' perception of involvement in decision making. This observation suggests that the International Patient Decision Aids Standards criterion of helping patients participate 'in ways that they prefer' needs to be assessed after a patient has adequate information about what involvement means using interventions such as patient decision aids. People may have a mistaken preference for passivity because they believe that the best choice relies on the expertise of the clinician (which option is medically reasonable?) rather than understand the importance of their own preferences for outcomes of options (which outcomes matter most to me?).
Evidence continues to build that decision aids have a positive effect on the patient‐clinician consultation (in 9 of the 10 studies that assessed this effect). Of the studies that measured patient‐clinician communication, five involved using decision aids within the consultation and five in preparation for the consultation. At the same time, evidence on length of consultation indicates either no difference (8 studies) or slightly longer (2 studies) consultations in the decision aid group compared to usual care consultations.
However, few studies have reported on the impact of the context in which the patient decision aids are used. A previous subgroup analysis of 29 studies evaluating patient decision aids for treatment decisions reported greater improvement in knowledge scores (P = 0.03) when the patient decision aid was evaluated within the clinical pathway of care, compared to when patients volunteered to participate in the study independent of their clinician (Brown 2015).
Variable effects of decision aids
There may be several reasons for the variable effect of decision aids on the outcome of choices. First, most studies were under‐powered to detect important differences in the outcome of choices. Second, not enough is known about baseline rates for optimal use of specific options. Third, in the studies reporting the outcome 'choices' at baseline and postdecision aid, some options may have been under‐used and others over‐used, relative to the choices individuals would make if they were more fully informed. Under these circumstances, one could expect to observe directional effects on choices once people become better informed and more involved in decision making.
Relatively under‐used options at baseline were prostate surgery for benign prostatic hyperplasia and prophylactic mastectomy for breast cancer gene carriers. In this prostate‐related example, there was a shortage of urologists and low referral rates for benign prostatic hyperplasia, whereas the breast‐related example reflects the growing number of women who test gene positive and become aware of their options for preventing breast cancer. Hence, under‐use of an option may be corrected with exposure to a decision aid.
In the other surgical decision aid studies, there were higher numbers of people choosing surgery in the control group (e.g. cardiac revascularization, back surgery, hysterectomy, orchiectomy, mastectomy). The procedure may have been chosen due to people's inflated perceptions of the probabilities of benefits, lack of appreciation of the probabilities of harms, and lack of awareness of alternatives (Hoffman 2015). Exposure to the decision aid reduced the number of people choosing elective surgery in favour of more conservative alternatives.
Limited effects of decision aids
The limited effects of decision aids on reported satisfaction with the decision‐making process and with the actual choice made may indicate that decision aids have a limited effect on satisfaction. The null effects may also be due to measurement insensitivity. This is especially likely when satisfaction with usual care is already quite high (e.g. ceiling effects) and when choices are inherently difficult to make because of competing benefits and harms. Furthermore, once the decision is made, people may find it psychologically more comforting to say that they are satisfied rather than entertain doubts about what they have chosen (Gruppen 1994).
There is a need to establish the 'essential ingredients' in decision aids and to identify the people who are most likely to benefit from them. As the body of available research grows, it will become easier and more important to assess the usefulness of different components of decision support for different clinical contexts, decision problems, and groups of people. For example, an analysis of decision aids used in higher versus lower socioeconomic groups indicated greater improvements for those of lower socioeconomic status (Durand 2014). Recently, the IPDAS Collaboration completed a set of evidence reviews underlying the IPDAS checklist (IPDAS 2013), proposing criteria for defining the intervention as a patient decision aid and minimal certifying criteria (Joseph‐Williams 2013). These are being used to inform the certification of patient decision aids in the USA, England, and Norway.
It is not surprising that decision aids had limited effects on health outcomes. One reason for using a decision aid is that there is often no option with a clear health outcome advantage. For example, when men with localized prostate cancer consider active treatment options, their health outcomes can be different, depending on whether they choose surgery with higher risks of impotence or radiation therapy with higher risks of longer term bowel irritation. Therefore, if health outcomes are used in future investigations of decision aids in situations in which there is clearly no health outcome advantage, the key question to pose is: do patients experience the health outcomes they prefer and avoid the outcomes to which they are averse?
More recently, decision aids are being used in situations in which there may be a longer‐term health advantage, for example, in preventive decisions about the management of type II diabetes and/or hypertension, when the longer‐term health outcome may be to avoid stroke (Mann D 2010; Mathers 2012; Montgomery 2003; Mullan 2009; Weymiller 2007). Interestingly, the pooled results showed a statistically significant increase in medication initiation when participants were exposed to the decision aid compared to usual care.
Unknown effects of decision aids
The effect of patient decision aids on adherence to the chosen option is an area of uncertainty. The adherence results are difficult to interpret due to incomplete data, primarily self‐reported data, varying length of follow‐ups, and small sample sizes. Moreover, studies reporting this outcome such as Man‐Son‐Hing 1999 had very little variation in choice (over 90% of long‐term aspirin users decided to stay on aspirin). When examining adherence, it would be important to do so in the early phase, when presumably the issue is actually decisional in nature (e.g. filling the prescription, picking up the prescription, refilling the prescription) rather than involving the management of side effects and in a manner that separates those choosing to change versus those remaining with the status quo.
Despite the positive effects of decision aids on patient‐clinician communication, some authors are concerned about the potential negative influence that decision aids may have on the relational aspects of the decision‐making process; this concern highlights the need for further evaluation when decision aids are implemented as part of the routine process of care (Charles 2010; LeBlanc 2010).
In the context of decision aid use, cost‐effectiveness and health utilities are other secondary outcome measures about which little is known and further evaluation is required (Trenaman 2014). We also need to establish ways of measuring preference‐linked health outcomes to better determine the effect on quality of life. It is unlikely that we will observe the effect of decision aids on litigation rates in studies of decision aids, given the time delay to litigation and the rarity of this type of event. There do not appear to be any adverse events from using decision aids, but this could be more clearly examined in future studies. In fact, a mock trial that used a patient decision aid for prostate‐specific antigen testing found that the majority of jurors (94%) would indicate that the standard of care had been met (Barry 2008). A recent systematic review concluded that there was insufficient evidence to determine if patient decision aids could reduce medical malpractice litigation (Durand 2014).
Quality of the evidence
Risk of bias ratings reveal between‐study variability. We rated few studies as being at low risk of bias for blinding of participants and personnel and most studies as being at unclear risk of bias. Likewise, the majority of studies were rated as being at unclear risk of bias for selective reporting. When we conducted a post hoc analysis that involved removing studies at high risk of bias from the meta‐analysis, there was no effect on the results. The conclusions of this review are limited by inadequate power to detect important between‐subgroup differences in effectiveness and by the wide variability in the decision contexts, the elements within the patient decision aids, the type of comparison delivered (collectively referred to as usual care here), the targeted outcomes, and the evaluation procedures. The small number of studies for most outcomes did not allow for analysis of publication bias due to failure to publish negative studies. Moreover, most studies were at unclear risk of selective outcome reporting, indicating that there may have been bias arising from a failure to report all negative findings.
We rated the six primary outcomes in the 'Summary of findings' table using GRADE and assessed outcomes as high quality (knowledge, feeling uninformed, feeling unclear values), moderate quality (accurate risk perception, clinician‐controlled role in decision making), and low quality (values‐choice congruence). For values‐choice congruence, the GRADE rating was downgraded for lack of consistency, directness, and precision. More specifically, congruence was measured using various approaches, as there is no gold standard measurement approach (Munro 2016). Several of the outcomes demonstrated statistically significant levels of heterogeneity. For the outcome of knowledge, for example, heterogeneity would be expected, given that the knowledge tests themselves were not standardized. However, we did not downgrade the ratings for knowledge, feeling uninformed, and feeling unclear values based on heterogeneity given the consistent direction of findings across studies. Moreover, the heterogeneity found in the various outcomes reflects differences across clinically diverse studies; therefore, the pooled effect size and confidence intervals should be interpreted as a range across conditions, which may not be applicable to a specific condition.
Potential biases in the review process
The strength of this systematic review is that patient decision aids improve several key primary outcomes across a wide variety of populations and decision contexts. The potential biases in the review process are due to limitations associated with having inadequate power to detect potentially important differences in effectiveness between subgroups, to differentiate between the most effective elements within the patient decision aid, and to investigate any differences associated with the type of comparison interventions used in studies. Several of the outcomes demonstrated statistically significant heterogeneity. This reflects differences across clinically diverse studies; therefore, the pooled effect size and confidence intervals should be interpreted as a range across conditions, which may not be applicable to a specific condition. In the Gentles 2013 subgroup analysis exploring three potential sources of heterogeneity (e.g. type of control intervention, decision aid IPDAS quality score, participants' baseline accurate risk perception), participants' baseline accurate risk perception was an important variable for explaining heterogeneity. Authors reported that when participants' baseline scores for accurate risk perception were lower, decision aids led to great improvement. Furthermore, we limited the extracted study data to only two comparison groups (e.g. most intensive intervention including a patient decision aid and usual care); therefore, we did not investigate the possibility of intermediate effects with less intensive decision aid interventions.
Agreements and disagreements with other studies or reviews
Our results confirm many of the observations reported in the previous versions of our review and in a comparative effectiveness review that focused on studies evaluating oncology‐specific patient decision aids (Trikalinos 2014). We published the first systematic review of 17 randomized trials of decision aids in 1999 (O'Connor 1999b; O'Connor 2001), followed by updates in 2003 with a total of 35 studies (O'Connor 2003), in 2009 with a total of 55 studies (O'Connor 2009b), in 2011 with a total of 86 studies (Stacey 2011), and 2014 with a total of 115 studies (Stacey 2014b).
Authors' conclusions
Implications for practice.
The positive effects of decision aids on improving people's knowledge of risks and benefits, feeling informed, and feeling clear about their values across a wide variety of decision contexts provides sufficient evidence for using them in clinical practice. They probably also facilitate accurate risk perception and active participation in decision making. However, several conditions may be necessary for successful implementation, including: good quality decision aids that meet the needs of the population; clinicians who are willing to use decision aids in their practice; effective systems for delivering decision support; and clinicians and healthcare consumers who are skilled in shared decision making. Although there have been some strides in achieving these conditions (Elwyn 2013; O'Connor 2007), the use of patient decision aids will not occur without adequate attention to implementation barriers to implementation and careful design of effective strategies for introducing and maintaining their use in routine clinical practice (Elwyn 2013; Gravel 2006; Legare 2008b; Legare 2010;Legare 2014).
New in this update was a subgroup analysis of the findings based on timing of decision aid used either before or during a consultation. Although knowledge scores and accurate risk perceptions were significantly higher in the decision aid group compared to the usual care, there was no difference in these outcomes when comparing decision aids used in preparation for versus during the consultation.
Implications for research.
Studies are needed to deepen our understanding of interactions between patient decision aid use and the patterns of patient‐clinician communication; format issues such as the web‐based delivery of patient decision aids; and downstream effects on cost, resource use, and adherence. Although this update shows new studies conducted in Spain and China, most studies have taken place in North America, the UK, Europe, and Australia. There were far fewer studies of patient decision aids used within the consultation than those delivered pre‐consultation, and this is an area of further research given the important issue of implementation.
With the addition of more studies in the systematic review, it may be possible to tease out the reasons for heterogeneity of results, including variability in: study quality; comparison intervention; elements within patient decision aids; decision type; setting where it was used; and format of decision aid (e.g. video, Internet, booklet). Research should also explore the degree of detail in patient decision aids that is required for positive effects according to the IPDAS criteria. In particular, evaluation is needed to compare the effect of those decision aids that meet the minimal IPDAS criteria for certification versus those that meet the full roster of IPDAS quality criteria (Joseph‐Williams 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2017 | New search has been performed | We updated the search in April 2015 and added 18 new studies comparing decision aids to usual care. For this update, we removed 28 studies that were focused on detailed versus simple decision aids. We also conducted a subanalysis of decision aids used within the consultation and those used in preparation for the consultation. |
| 6 April 2017 | New citation required and conclusions have changed | New for this update is growing evidence that decision aids may improve informed values‐congruence choices and the sub‐analysis indicated improved knowledge and accurate risk perceptions when decision aids are used either within or in preparation for the consultation. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 5 December 2013 | New citation required and conclusions have changed | This update added 33 new studies for a total of 115 studies involving 34,444 participants. GRADE was used to summarize the quality of the evidence, and findings were reported using a 'Summary of findings' table. We excluded three previously‐included trials on the basis of their quasi‐randomized controlled trial (q‐RCT) design identified using the more rigorous 'Risk of bias' assessment tool, as well as one other study that used the same decision aid content for both groups but varied the format used. Overall, the results are similar to the previous update, but this update indicates the quality of the evidence to support the reported outcomes (high‐quality evidence that decision aids compared to usual care improve people’s knowledge and reduce their decisional conflict related to feeling uninformed and unclear about their personal values; moderate‐quality evidence that decision aids compared to usual care stimulate people to take a more active role in decision making and improve accurate risk perceptions when probabilities are included; and low‐quality evidence that decision aids improve the congruence between the chosen option and their values). We added two new authors to the review, LT in Sydney and JW in Ottawa who helped coordinate this update. |
| 30 June 2012 | New search has been performed | Search strategies were updated and new searches run in June 2012. |
| 18 January 2012 | Amended | Minor change to wording, Plain Language Summary. |
| 5 September 2011 | New search has been performed | An update of this review was conducted in 2010 and published on issue 10 2011 of The Cochrane Library. Citations were searched from 2006 to December 2009. |
| 5 September 2011 | New citation required but conclusions have not changed | This update added 31 new studies, and all 86 included studies were assessed for risk of bias. Overall the results were consistent with the previous update. New in this update is the meta‐analysis of informed values‐based choices for decision aids including explicit values‐clarification compared to those with no explicit values‐clarification. We have also conducted a post‐hoc analysis to evaluate the effect of risk of bias assessment ratings on outcomes. |
| 29 April 2009 | New search has been performed | See the 'History' items dated 29 April 2009 and 28 July 2006. |
| 29 April 2009 | New citation required and conclusions have changed | A substantially updated version of this review was published on issue 1 2009 of The Cochrane Library. The changes are outlined in the 'History' (date 28 July 2006). The updated review ought to have had a new citation to reflect the new authorship and substantial changes to the review and its conclusions; however because of a technical error this new citation was not given to the updated review. The new citation for this review for issue 3 2009 (O'Connor 2009b) reflects the updated review contents as actually published from issue 1 2009 onwards. |
| 28 April 2009 | Amended | Corrected mislabelled table 'Summary of pooled outcomes'. |
| 17 July 2008 | Amended | Converted to new review format. |
| 28 July 2006 | New search has been performed | Changes for the 2006 update (first published on issue 1 2009 of The Cochrane Library):
Findings from the 2006 update (*new to this update):
|
| 21 February 2003 | New search has been performed | For the 2002 update (O'Connor 2003), the following changes were made:
|
Acknowledgements
The Cochrane Consumers and Communication Group (editors, academic and consumer referees) provided peer review and advice regarding the review and checked extracted data for newly included studies. We thank John Kis‐Rigo at La Trobe University who revised the search strategy used since the 2014 update. David Rovner and Nananda Col helped with screening studies for inclusion, and Intissar Souli assisted with data extraction and 'Risk of bias' assessment. Anton Saarimaki set up and managed a web‐based title and abstract screening application that facilitated independent screening of citations by the review authors, and he also verified references for patient decision aids in Table 2. Alain Mayhew provided guidance in the interpretation of the 'Summary of findings' table. Dean Fergusson provided consultation on the statistical analysis.
Appendices
Appendix 1. Revised Search Strategies January 2009 to April 2015
CENTRAL via the Cochrane Library
(decision‐support or decision‐aid):kw in Trials
decision‐tree:kw in Trials
patient‐decision‐making:kw
(decision‐making or choice‐behavior):ti,ab,kw and (informed‐consent:kw,ti or (patient or parent* or carer or caregiver or care‐giver):ti,ab,kw) in Trials
((decision or decid*) near/4 (support* or aid* or tool or instrument or technolog* or technique or system or program* or algorithm or process or method or intervention or material)):ti,ab,kw
(decision next (board or guide or counseling)):ti,ab,kw
((risk‐communication or risk‐assessment or risk‐information) near/4 (tool or method)):ti,ab,kw
(computer* near/2 decision‐making):ti,ab,kw
(interactive‐health‐communication or (interacti* near/4 tool)):ti,ab,kw
(interactive next (internet or online or graphic* or booklet)):ti,ab,kw
((interactiv* or evidence‐based) near/3 (risk‐information or risk‐communication or risk‐presentation or risk‐graphic*)):ti,ab,kw
shared‐decision‐making:ti,ab,kw
(informed next (choice or decision)):ti,ab,kw
adaptive‐conjoint‐analysis:ti,ab,kw
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14), from 2009 to 2015
(Last line restricted to “Trials”, and to date range 2009 to 2015)
MEDLINE Ovid
1. decision support techniques/
2. decision support systems clinical/
3. decision trees/
4. (decision making or choice behavior).mp. and informed consent.sh.
5. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).tw.
6. (decision adj (board* or guide* or counseling)).tw.
7. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw.
8. decision‐making computer assisted/
9. (computer* adj2 decision making).tw.
10. interactive health communication*.tw.
11. (interactive adj (internet or online or graphic* or booklet*)).tw.
12. (interacti* adj4 tool*).tw.
13. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw.
14. shared decision making.tw.
15. (informed adj (choice* or decision*)).tw.
16. adaptive conjoint analys#s.tw.
17. or/1‐16
18. randomized controlled trial.pt.
19. controlled clinical trial.pt.
20. randomized.ab.
21. placebo.ab.
22. clinical trials as topic.sh.
23. randomly.ab.
24. trial.ti.
25. or/18‐24
26. exp animals/ not humans.sh.
27. 25 not 26
28. 17 and 27
29. limit 28 to yr="2009 ‐Current"
Embase Ovid
1. decision support system/
2. patient decision making/
3. decision aid/
4. "decision tree"/
5. decision making.hw,kw,tw. and informed consent.hw,kw.
6. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).tw,kw.
7. (decision adj (board* or guide* or counseling)).tw,kw.
8. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).tw,kw.
9. (computer* adj2 decision making).tw,kw.
10. interactive health communication*.tw,kw.
11. (interactive adj (internet or online or graphic* or booklet*)).tw,kw.
12. (interacti* adj4 tool*).tw,kw.
13. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).tw,kw.
14. shared decision making.tw,kw.
15. (informed adj (choice* or decision*)).tw,kw.
16. adaptive conjoint analys#s.tw,kw.
17. or/1‐16
18. randomized controlled trial/
19. controlled clinical trial/
20. single blind procedure/ or double blind procedure/
21. crossover procedure/
22. random*.tw.
23. placebo*.tw.
24. ((singl* or doubl*) adj (blind* or mask*)).tw.
25. (crossover or cross over or factorial* or latin square).tw.
26. (assign* or allocat* or volunteer*).tw.
27. or/18‐26
28. nonhuman/ not (human/ and nonhuman/)
29. 27 not 28
30. 17 and 29
31. 30 and 20012:2015.(sa_year).
32. limit 31 to exclude medline journals
PsycINFO Ovid
1. decision support systems/
2. (decision making or choice behavior).mp. and (informed consent.sh. or (patient* or parent* or carer* or caregiver* or care giver*).mp.)
3. ((decision* or decid*) adj4 (support* or aid* or tool* or instrument* or technolog* or technique* or system* or program* or algorithm* or process* or method* or intervention* or material*)).ti,ab,id.
4. (decision adj (board* or guide* or counseling)).ti,ab,id.
5. ((risk communication or risk assessment or risk information) adj4 (tool* or method*)).ti,ab,id.
6. computer assisted therapy/
7. (computer* adj2 decision making).ti,ab,id.
8. interactive health communication*.ti,ab,id.
9. (interactive adj (internet or online or graphic* or booklet*)).ti,ab,id.
10. (interacti* adj4 tool*).ti,ab,id.
11. ((interactiv* or evidence based) adj3 (risk information or risk communication or risk presentation or risk graphic*)).ti,ab,id.
12. shared decision making.ti,ab,id.
13. (informed adj (choice* or decision*)).ti,ab,id.
14. adaptive conjoint analys#s.ti,ab,id.
15. or/1‐14
16. random*.ti,ab,hw,id.
17. intervention.ti,ab,hw,id.
18. trial.ti,ab,hw,id.
19. placebo*.ti,ab,hw,id.
20. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
21. (cross over or crossover).ti,ab,hw,id.
22. latin square.ti,ab,hw,id.
23. (assign* or allocat* or volunteer*).ti,ab,hw,id.
24. treatment effectiveness evaluation/
25. mental health program evaluation/
26. exp experimental design/
27. or/16‐26
28. 15 and 27
29. limit 28 to yr="2009 ‐Current"
CINAHL (EBSCO)
| # | Query | Limiters/Expanders |
| S31 | S30 | Limiters ‐ Exclude MEDLINE records Search modes ‐ Boolean/Phrase |
| S30 | S28 and S29 | Search modes ‐ Boolean/Phrase |
| S29 | EM 2009‐ | Search modes ‐ Boolean/Phrase |
| S28 | S17 and S27 | Search modes ‐ Boolean/Phrase |
| S27 | S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 | Search modes ‐ Boolean/Phrase |
| S26 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S25 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) | Search modes ‐ Boolean/Phrase |
| S24 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) | Search modes ‐ Boolean/Phrase |
| S23 | MH Quantitative Studies | Search modes ‐ Boolean/Phrase |
| S22 | MH Placebos | Search modes ‐ Boolean/Phrase |
| S21 | MH Random Assignment | Search modes ‐ Boolean/Phrase |
| S20 | MH Clinical Trials+ | Search modes ‐ Boolean/Phrase |
| S19 | PT Clinical Trial | Search modes ‐ Boolean/Phrase |
| S18 | PT "randomi?ed controlled trial" | Search modes ‐ Boolean/Phrase |
| S17 | S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 | Search modes ‐ Boolean/Phrase |
| S16 | "informed choice*" or "informed decision*" | Search modes ‐ Boolean/Phrase |
| S15 | "shared decision making" | Search modes ‐ Boolean/Phrase |
| S14 | "adaptive conjoint analys?s" | Search modes ‐ Boolean/Phrase |
| S13 | (interactive N2 "risk information") or (interactive N2 "risk communication") or (interactive N2 "risk presentation") or (interactive N2 "risk graphic*") | Search modes ‐ Boolean/Phrase |
| S12 | "interactive internet" or "interactive online" or "interactive graphic*" or "interactive booklet*" or (interacti* N3 tool*) | Search modes ‐ Boolean/Phrase |
| S11 | "interactive health communication*" | Search modes ‐ Boolean/Phrase |
| S10 | computer* N1 "decision making" | Search modes ‐ Boolean/Phrase |
| S9 | ("risk communication" N3 tool*) or ("risk communication" N3 method*) or ("risk information" N3 tool*) or ("risk information" N3 method*) or ("risk assessment" N3 tool*) or ("risk assessment" N3 method*) | Search modes ‐ Boolean/Phrase |
| S8 | "evidence based risk communication" or "evidence based risk information" | Search modes ‐ Boolean/Phrase |
| S7 | "decision board*" or "decision guide*" or "decision counseling" | Search modes ‐ Boolean/Phrase |
| S6 | (decision* N3 support*) or (decision* N3 aid*) or (decision* N3 tool*) or (decision* N3 instrument*) or (decision* N3 technolog*) or (decision* N3 technique*) or (decision* N3 system*) or (decision* N3 program*) or (decision* N3 algorithm*) or (decision* N3 process*) or (decision* N3 method*) or (decision* N3 intervention*) or (decision* N3 material*) | Search modes ‐ Boolean/Phrase |
| S5 | ("decision making" or "choice behavior") and MH consent | Search modes ‐ Boolean/Phrase |
| S4 | MH decision making, computer assisted | Search modes ‐ Boolean/Phrase |
| S3 | MH decision making, patient | Search modes ‐ Boolean/Phrase |
| S2 | MH decision support systems, clinical | Search modes ‐ Boolean/Phrase |
| S1 | MH decision support techniques+ | Search modes ‐ Boolean/Phrase |
Appendix 2. Search strategies to 2009
CENTRAL
CENTRAL in the Cochrane Library was searched using the MEDLINE search above in Ovid to the end of 2006; for the 2011 update, the CENTRAL search was conducted at www.thecochranelibrary.com to the end of 2009 using the following search strategy:
1. decision.tw,hw.
2. patient.tw,hw.
3. consumer.tw,sh.
4. 1 and (2 or 3)
5. shared decision making.tw.
6. decision aid$.tw.
7. informed choice.tw.
8. or/4‐7
9. clinical trial.pt.
10. randomized controlled trial.pt.
11. random$.tw.
12. or/9‐11
13. 8 and 12
MEDLINE Ovid (1966 to December 2009)
1. choice behavior/
2. decision making/
3. exp decision support techniques/
4. Educational Technology/
5. decision$.tw.
6. (choic$ or preference$).tw.
7. communication package.tw.
8. or/1‐7
9. exp health education/
10. Health Knowledge, Attitudes, Practice/
11. informed consent.tw,hw.
12. patient.tw,hw.
13. consumer.tw,hw.
14. or/9‐13
15. 8 and 14
16. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
17. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
18. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
19. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
20. shared decision making.tw.
21. decision aid$.tw.
22. informed choice.tw.
23. or/16‐22
24. 15 or 23
25. clinical trial.pt.
26. randomized controlled trial.pt.
27. random$.tw.
28. (double adj blind$).tw.
29. double‐blind method/
30. or/25‐29
31. 24 and 30
CINAHL Ovid (1982 to September 2008)
1. exp Decision Making/
2. information seeking behavior/
3. Help Seeking Behavior/
4. (choic$ or preference$).tw.
5. decision$.tw.
6. Educational Technology/
7. or/1‐6
8. exp Health Behavior/
9. consumer participation/
10. exp Health Education/
11. health knowledge/ or exp professional knowledge/
12. exp Consent/
13. informed consent.tw.
14. patient.tw,hw.
15. consumer.tw,sh.
16. or/8‐15
17. 7 and 16
18. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
19. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
20. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
21. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
22. shared decision making.tw.
23. decision aid$.tw.
24. informed choice.tw.
25. or/18‐24
26. 17 or 25
27. exp clinical trials/
28. Clinical trial.pt.
29. (clinic$ adj trial$1).tw.
30. random$.tw.
31. Random assignment/
32. placebo$.tw,sh.
33. Quantitative studies/
34. Allocat$ random$.tw.
35. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$3 or mask$3)).tw.
36. or/27‐35
37. 26 and 36
Embase Ovid (1980 to December 2009)
1. decision making/
2. decision theory/
3. decision$.tw.
4. Educational Technology/
5. or/1‐4
6. exp health behavior/
7. exp Patient Attitude/
8. exp health education/
9. informed consent.tw,sh.
10. patient.tw,sh.
11. consumer.tw,sh.
12. or/6‐11
13. 5 and 12
14. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
15. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
16. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
17. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
18. shared decision making.tw.
19. decision aid$.tw.
20. informed choice.tw.
21. or/14‐20
22. 13 or 21
23. Controlled Study/
24. Randomized Controlled Trial/
25. Clinical Study/
26. Clinical Trial/
27. Major Clinical Study/
28. Prospective Study/
29. Multicenter Study/
30. Randomization/
31. Double Blind Procedure/
32. Single Blind Procedure/
33. Crossover Procedure/
34. Placebo.tw,sh.
35. random$.tw.
36. (double adj blind$).tw.
37. or/23‐36
38. 22 and 37
PsycINFO Ovid (1806 to December 2009)
1. decision$.tw.
2. (choic$ or preference$).tw.
3. exp decision making/
4. computer assisted instruction/
5. or/1‐4
6. exp health education/
7. exp health personnel attitudes/
8. informed consent.tw,sh.
9. patient.tw,hw.
10. consumer.tw,hw.
11. exp health behavior/
12. or/6‐11
13. 5 and 12
14. ((patient$ or consumer$) adj1 (decision$ or choice or preference or participation)).tw.
15. ((women or men) adj1 (decision$ or choice or preference or participation)).tw.
16. (parent$ adj1 (decision$ or choice or preferenc$ or participat$)).tw.
17. ((personal or interpersonal or individual) adj (decision$ or choice or preference$ or participat$)).tw.
18. shared decision making.tw.
19. decision aid$.tw.
20. informed choice.tw.
21. or/14‐20
22. 13 or 21
23. random$.tw.
24. (double adj blind$).tw.
25. placebo$.tw,hw.
26. or/23‐25
27. 22 and 26
Data and analyses
Comparison 1. Knowledge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Knowledge ‐ all studies | 52 | 13316 | Mean Difference (IV, Random, 95% CI) | 13.27 [11.32, 15.23] |
| 2 Knowledge ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation) | 52 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 In consultation | 8 | 922 | Mean Difference (IV, Random, 95% CI) | 10.57 [4.79, 16.36] |
| 2.2 In preparation for consultation | 44 | 12394 | Mean Difference (IV, Random, 95% CI) | 13.77 [11.61, 15.93] |
| 3 Knowledge ‐ studies without high risk of bias | 47 | 12327 | Mean Difference (IV, Random, 95% CI) | 13.43 [11.37, 15.49] |
Comparison 2. Accurate risk perceptions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Accurate risk perceptions ‐ all studies | 17 | 5096 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [1.66, 2.66] |
| 2 Accurate risk perceptions ‐ subgroup by timing of intervention (in consultation versus in preparation for consultation) | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 In consultation | 6 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.28, 2.52] |
| 2.2 In preparation for consultation | 11 | 4198 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.65, 3.07] |
| 3 Accurate risk perceptions ‐ studies without high risk of bias | 15 | 4732 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.57, 2.59] |
Comparison 3. Informed values‐choice congruence.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Informed values‐choice congruence ‐ all studies | 10 | 4626 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.46, 2.91] |
| 2 Informed values‐choice congruence ‐ actual choice only | 8 | 4154 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.44, 3.14] |
| 3 Informed values‐chose congruence ‐using MMIC | 8 | 4365 | Risk Ratio (M‐H, Random, 95% CI) | 2.08 [1.40, 3.08] |
| 4 Informed values‐chose congruence ‐ heterogeneous measures | 2 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.44, 2.83] |
| 5 Informed values‐choice congruence ‐ without studies of high risk of bias | 10 | 4626 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.46, 2.91] |
3.2. Analysis.
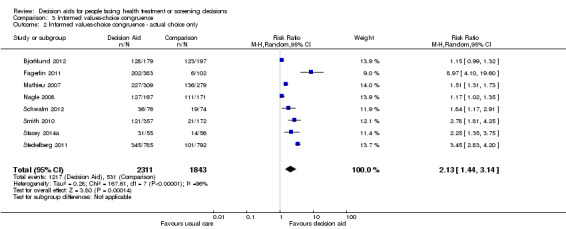
Comparison 3 Informed values‐choice congruence, Outcome 2 Informed values‐choice congruence ‐ actual choice only.
3.4. Analysis.
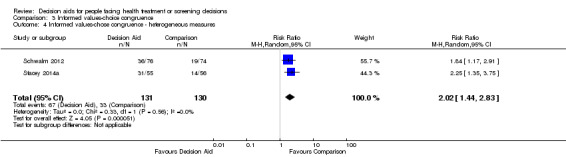
Comparison 3 Informed values‐choice congruence, Outcome 4 Informed values‐chose congruence ‐ heterogeneous measures.
Comparison 4. Decisional conflict.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decisional conflict ‐ all studies | 42 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Total decisional conflict score | 38 | 8785 | Mean Difference (IV, Random, 95% CI) | ‐7.22 [‐9.12, ‐5.31] |
| 1.2 Uninformed subscale | 27 | 5707 | Mean Difference (IV, Random, 95% CI) | ‐9.28 [‐12.20, ‐6.36] |
| 1.3 Unclear values subscale | 23 | 5068 | Mean Difference (IV, Random, 95% CI) | ‐8.81 [‐11.99, ‐5.63] |
| 1.4 Uncertainty subscale | 28 | 6200 | Mean Difference (IV, Random, 95% CI) | ‐4.04 [‐6.27, ‐1.81] |
| 1.5 Unsupported subscale | 24 | 5214 | Mean Difference (IV, Random, 95% CI) | ‐6.27 [‐8.86, ‐3.68] |
| 1.6 Ineffective choice subscale | 24 | 5241 | Mean Difference (IV, Random, 95% CI) | ‐6.31 [‐8.93, ‐3.70] |
| 2 Decisional conflict ‐ in consultation | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Uncertainty subscale | 2 | 310 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐18.29, 5.38] |
| 2.2 Uninformed subscale | 4 | 545 | Mean Difference (IV, Random, 95% CI) | ‐6.37 [‐14.58, 1.85] |
| 2.3 Unclear values subscale | 1 | 204 | Mean Difference (IV, Random, 95% CI) | ‐17.2 [‐23.77, ‐10.63] |
| 2.4 Unsupported subscale | 2 | 354 | Mean Difference (IV, Random, 95% CI) | ‐7.16 [‐13.28, ‐1.03] |
| 2.5 Ineffective choice subscale | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐7.31, 2.58] |
| 2.6 Total decisional conflict score | 5 | 735 | Mean Difference (IV, Random, 95% CI) | ‐6.46 [‐12.78, ‐0.14] |
| 3 Decisional conflict ‐ in preparation for consultation | 36 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Uncertainty subscale | 26 | 5890 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐6.12, ‐1.55] |
| 3.2 Uninformed subscale | 23 | 5162 | Mean Difference (IV, Random, 95% CI) | ‐9.81 [‐13.00, ‐6.61] |
| 3.3 Unclear values subscale | 22 | 4864 | Mean Difference (IV, Random, 95% CI) | ‐8.40 [‐11.59, ‐5.21] |
| 3.4 Unsupported subscale | 22 | 4860 | Mean Difference (IV, Random, 95% CI) | ‐6.18 [‐8.96, ‐3.40] |
| 3.5 Ineffective choice subscale | 22 | 4934 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐9.59, ‐3.90] |
| 3.6 Total decisional conflict score | 33 | 8050 | Mean Difference (IV, Random, 95% CI) | ‐7.32 [‐9.35, ‐5.28] |
| 4 Decisional conflict ‐ without studies having high risk of bias | 39 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Uncertainty subscale | 26 | 5809 | Mean Difference (IV, Random, 95% CI) | ‐4.53 [‐6.87, ‐2.18] |
| 4.2 Uninformed subscale | 25 | 5316 | Mean Difference (IV, Random, 95% CI) | ‐9.96 [‐13.13, ‐6.78] |
| 4.3 Unclear values subscale | 21 | 4677 | Mean Difference (IV, Random, 95% CI) | ‐9.55 [‐13.08, ‐6.02] |
| 4.4 Unsupported subscale | 22 | 4823 | Mean Difference (IV, Random, 95% CI) | ‐7.00 [‐9.76, ‐4.24] |
| 4.5 Ineffective choice subscale | 22 | 4850 | Mean Difference (IV, Random, 95% CI) | ‐6.97 [‐9.76, ‐4.18] |
| 4.6 Total decisional conflict score | 35 | 8240 | Mean Difference (IV, Random, 95% CI) | ‐7.81 [‐9.84, ‐5.77] |
4.2. Analysis.
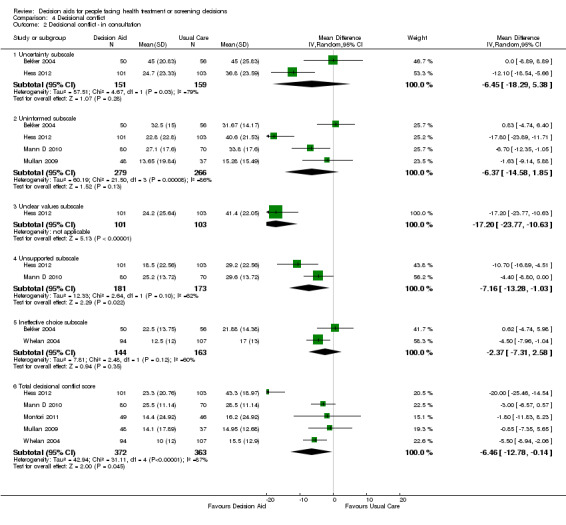
Comparison 4 Decisional conflict, Outcome 2 Decisional conflict ‐ in consultation.
4.3. Analysis.
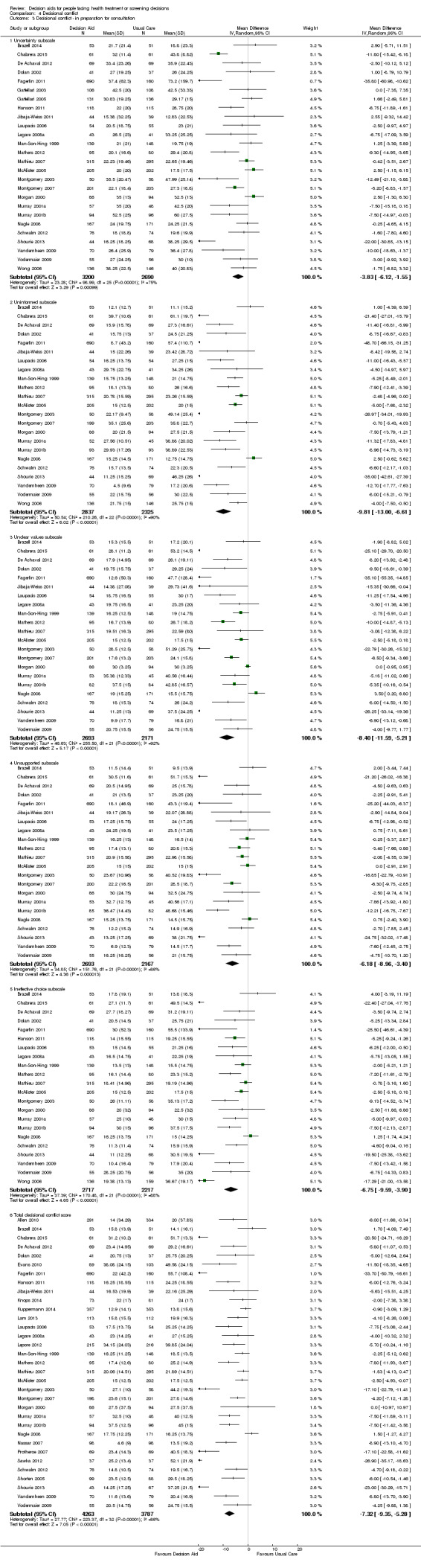
Comparison 4 Decisional conflict, Outcome 3 Decisional conflict ‐ in preparation for consultation.
Comparison 5. Participation in decision making.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participation in decision making ‐ all studies | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Clinician‐controlled decision making | 16 | 3180 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.55, 0.83] |
| 1.2 Patient‐controlled decision making | 15 | 3009 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.05, 1.55] |
| 1.3 Shared decision making | 15 | 2973 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.10] |
| 2 Participation in decision making ‐ in consultation | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Clinician‐controlled decision making ‐ in consultation | 3 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.70, 1.12] |
| 2.2 Patient‐controlled decision making ‐ in consultation | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.27] |
| 2.3 Shared decision making ‐ in consultation | 2 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.84, 1.55] |
| 3 Participation in decision making ‐ in preparation for consultation | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Clinician‐controlled decision making | 13 | 2530 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.75] |
| 3.2 Patient‐controlled decision making | 13 | 2530 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.08, 1.73] |
| 3.3 Shared decision making | 13 | 2494 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.09] |
Comparison 6. Proportion undecided.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion undecided ‐ all studies | 22 | 5256 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.52, 0.79] |
Comparison 7. Satisfaction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with the choice ‐ all studies | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Satisfaction with the choice ‐ in consultation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Satisfaction with the choice ‐ in preparation for consultation | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Satisfaction with the decision making process ‐ all studies | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Satisfaction with the decision making process ‐ in consultation | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Satisfaction with the decision making process ‐ in preparation for consultation | 8 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
7.2. Analysis.
Comparison 7 Satisfaction, Outcome 2 Satisfaction with the choice ‐ in consultation.
7.3. Analysis.
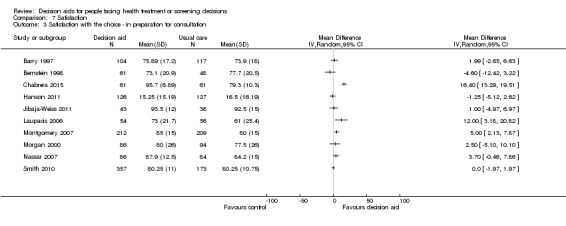
Comparison 7 Satisfaction, Outcome 3 Satisfaction with the choice ‐ in preparation for consultation.
7.5. Analysis.
Comparison 7 Satisfaction, Outcome 5 Satisfaction with the decision making process ‐ in consultation.
Comparison 8. Choice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Choice: surgery over conservative option | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Per‐protocol analysis | 18 | 3286 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.01] |
| 1.2 Intention‐to‐treat analysis | 18 | 3844 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 1.00] |
| 1.3 Per‐protocol analysis without prophylactic mastectomy | 17 | 3108 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.73, 0.97] |
| 2 Choice for screening | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 PSA screening | 10 | 3996 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.80, 0.98] |
| 2.2 Colorectal cancer screening | 10 | 4529 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.95, 1.31] |
| 2.3 Breast cancer genetic testing | 3 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.71, 1.38] |
| 2.4 Prenatal diagnostic testing | 2 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.09] |
| 3 Choice: diabetes medication (uptake new medication) | 4 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [1.06, 2.56] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2010.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 398 + 414 men considering prostate cancer screening in the USA | |
| Interventions | DA: computer tailored programme on clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step‐by‐step process for making the decision; interactive computer programme: inherently guided the patient through the decision aid and decision making process), tailored printout given to patients to promote discussion with others (practitioner, significant others) Comparator: no intervention |
|
| Outcomes | Primary outcomes: decisional status, knowledge, decision self‐efficacy, decisional consistency Secondary outcomes: desire for involvement in decision making, decisional conflict, preferred options Outcomes assessed pre‐ and postintervention |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sites were blocked on size and percent of male employees and randomly assigned by computer‐generated random numbers to condition within blocks" (p 2173, Setting) |
| Allocation concealment (selection bias) | Unclear risk | The study does not address this criterion. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study does not address this criterion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes measured were not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and low rate of attrition that was consistent between groups |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Intervention delivery: mention of money incentive to complete paperwork, but was judged to have no effect on outcomes measured (p 2175) |
Arterburn 2011.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 75 + 77 participants considering bariatric surgery in the USA | |
| Interventions | DA: booklet + video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to discuss with clinician) Comparator: usual care (general information pamphlets on clinical problem) |
|
| Outcomes | Primary outcomes: knowledge, values, values concordance Secondary outcomes: treatment preference, decisional conflict, decisional self‐efficacy, proportion undecided Primary outcomes assessed at baseline, postintervention and 3 months follow‐up; secondary outcomes assessed at baseline and postintervention |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[U]sed computer‐assisted, block randomisation process to ensure balanced allocation of participants" (p 1670, Participants and randomization) |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment and no mention of impact on study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[S]tudy was not blinded" (p 1670, Participants and randomization); no mention of impact on study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Measures: mentioned 4 choices for treatment preference (surgery, drug therapy, diet and/or exercise programme and unsure) but only reported on surgery and unsure options (p 1671); minimal attrition that was consistent between groups |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol or trial registration; all pre‐specified outcomes included |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Auvinen 2004.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 100 men newly diagnosed with prostate cancer in Finland | |
| Interventions | DA: pamphlet patient decision aid created for study on options' outcomes, outcome probability, guidance Comparator: usual care by clinical guideline | |
| Outcomes | Primary outcome: uptake of options Secondary outcome: participation in decision making Other outcomes (from Huang 2014): death (5 years), disease‐free survival (10‐years), biochemical failure (serum PSA elevation) (5 years), biochemical failure‐free survival (5 years), disease progression (5 years), disease progression‐free survival (5 years) (data from 104 + 106 men) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Auvinen 2001, p 2: "randomized centrally, using software based on a random number generator"; no blocking used Auvinen 2004, (primary study), p 1: "randomized using a computer algorithm based on random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Auvinen 2001,p 2, Patients and Methods: randomized centrally at the Finnish Cancer Registry Auvinen 2004, (primary study), p 1: randomized centrally Comment: central allocation confers low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Auvinen 2001, p 3: "recognized carry‐over effect because same physician in charge for intervention and control groups, diminish contrast between groups, as these physicians were more motivated to inform patients than those physicians not participating" Auvinen 2004 (primary study): no blinding but primary outcome is choice of treatment for prostate, objectively recorded. But unsure how physicians may have influenced decisions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but primary outcome is choice of treatment for prostate, objectively recorded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Auvinen 2001, p 3: flow‐chart "Imbalance in the numbers of patients between the arms within two hospitals. Not expected to affect the results in any way"; "some participants refused to give informed consent, health deterioration, not seen by urologist" (p 4) Auvinen 2004 (primary study), p 2: flow diagram and results; low attrition and consistent between groups |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry. Auvinen 2001, p 2: "The study protocol was approved by an ethical committee in each participating hospital" Auvinen 2004 (primary study), p 1: "The study protocol was approved by the institutional review board at each participating hospital" |
| Other bias | Low risk | Appears to be free of other potential biases |
Barry 1997.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 104 + 123 patients considering benign prostatic hyperplasia treatment in the USA | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinion Comparator: usual care using general information on the clinical problem | |
| Outcomes | Primary outcome: knowledge Secondary outcomes: uptake of option, satisfaction with DM process, satisfaction with decision, interest in DM, general health outcomes, condition specific health outcomes |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified by study site in concealed blocks of 10" (p 2) |
| Allocation concealment (selection bias) | Low risk | Study coordinator opening serially numbered, opaque, sealed envelopes (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but phase 1 eliminated risk of contamination |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but phase 1 eliminated risk of outcome assessor interfering with decision |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient accrual and follow‐up reported; post‐randomization withdrawals could have biased the results (more in intervention group) ‐ however they reported no evidence of a differential effect of the study group (p 3) |
| Selective reporting (reporting bias) | Unclear risk | No indication that trial registered in central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
Bekker 2004.
| Methods | Randomized to detailed vs routine consultation | |
| Participants | 59 + 58 pregnant women who have received a maternal serum screening positive test result for Down syndrome in the UK | |
| Interventions | DA (in consult): decision analysis plus routine consultation on options' outcomes, clinical problem, outcome probability, values clarification, guidance/coaching Comparator: routine consultation on options' outcomes, outcome probability | |
| Outcomes | Primary outcome: anxiety Secondary outcomes: uptake of option, knowledge, decisional conflict, informed decision making, satisfaction with consultation, consultation length |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Bekker 2003, p 2 ‐ section 2.3 Sample and Procedure: "randomly allocated... using previously numbered... envelopes" Bekker 2004 (primary study), p 3: "Participants were randomly allocated by previously numbered envelopes"; does not mention how sequence was generated |
| Allocation concealment (selection bias) | Low risk | Bekker 2003, p 2 ‐ section 2.3 Sample and Procedure: "Using previously numbered, sealed, opaque envelopes" Bekker 2004 (primary study), p 3: previously numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded, personnel not blinded. Same personnel did control & intervention. Tape recorded sessions to ensure no bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Bekker 2003 flow diagram indicates postrandomization attrition with more attrition in decision aid group; no discussion on implications of attrition Bekker 2004 (primary study), p 4: results/flow diagram; baseline characteristics not included |
| Selective reporting (reporting bias) | Unclear risk | Bekker 2003: the coding frame was developed from literature. Does not mention protocol Bekker 2004 (primary study): no information provided about central trials registry |
| Other bias | Unclear risk | Bekker 2003: does not directly address baseline characteristics of participants Bekker 2004 (primary study): appears to be free of other potential biases |
Bernstein 1998.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 65 + 53 patients with coronary artery disease considering revascularization surgery in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinion Comparator: usual care (no information provided) | |
| Outcomes | Primary outcome: satisfaction with decision and decision making process Secondary outcomes: uptake of option, knowledge, satisfaction with care, general health outcomes, condition specific health outcomes |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by study site in blocks of 10" (p 3) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization performed by a study coordinator opening opaque, sealed envelopes at study headquarters" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither subjects nor study staff were blinded to treatment assignment ‐ could lead to different satisfaction ratings based on knowing the treatment received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3); low attrition of eligible participants randomized and consistent between group |
| Selective reporting (reporting bias) | Unclear risk | No information provided indicating trial was included in central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
Berry 2013.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 266 + 228 men considering prostate cancer treatment in the USA | |
| Interventions | DA: interactive web based video on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (list of questions to ask doctor and automated summary) Comparator: usual care |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcome: preferred/actual treatment choice (pre‐ and post‐DA), proportion undecided Other outcomes (Bosco 2012): choice concordance (6 months post‐DA). (Data from 239 + 209 men) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods section‐ second paragraph, p 3: "Participants were randomized automatically by the P3P application to study groups (1:1 using a simple randomization scheme with no blocking)" |
| Allocation concealment (selection bias) | Low risk | Methods section, p 3: "Participants were randomized automatically by the P3P application to study groups" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded and study does not address the effect on the results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear whether outcome assessors are blinded, but outcomes are not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis and low dropout (p 4) |
| Selective reporting (reporting bias) | Low risk | Protocol made available |
| Other bias | Unclear risk | Was a multicentre trial which could have lead to contamination, protocol violation and biased questionnaire completion |
Bjorklund 2012.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 236 + 247 women less than 11 weeks pregnant considering Down syndrome screening in Sweden | |
| Interventions | DA: linear video on options' outcomes, clinical problem, outcome probabilities, others' opinion, and guidance (step‐by‐step process for making the decision) Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), attitude (post‐DA), uptake of combined ultrasound and biochemical screening (post‐DA) Secondary outcomes: values congruent with chosen option (post‐DA) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The midwife allocated the participants randomly by sealed envelopes" (p 391) but does not state the actual sequence generation method |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes, "prepared, sequentially coded and distributed to the maternity units by the research group" (p 391) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was not possible to blind neither [sic] the midwives nor the participants due to the characteristics of the intervention" (p 395). The study does not address the effects of this on the results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of why some participants' data were excluded in Tables 2, 3 and 4 |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
Bozic 2013.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 95 + 103 participants with hip and/or knee osteoarthritis considering hip/knee surgery | |
| Interventions | DA: DVD and booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, and guidance/coaching with health coach Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: informed decision/knowledge (pre, immediately post, and 6 weeks follow‐up) Secondary outcomes: preferred treatment choice (pre and immediately post), patient and provider satisfaction (immediately post), length of consultation time |
|
| Notes | Trial registration: NCT01492257 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was blocked with use of random permuted blocks in groups of four, six, or eight to help ensure that the groups were balanced" (p 1634) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to either the intervention group or the control group with use of the sealed envelop method" (p 1634) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[S]urgeons were not blinded to the intervention" (p 1635). Knowing the allocation of participants, surgeons' favourable scoring could be due to greater investment in decision‐making. Insufficient information to make a judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 62% (123/198) retention rate therefore high attrition rate ‐ however the attrition was balanced between groups |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Appears to be free of other sources of bias |
Brazell 2014.
| Methods | Randomized to DA + standard counselling vs usual care + standard counselling | |
| Participants | 53 + 51 women presenting for the management and treatment of pelvic organ prolapse | |
| Interventions | DA: paper‐based or web‐based DA on clinical problem, options' outcomes, outcome probabilities, patient stories and standard counselling Comparator: standard counselling alone |
|
| Outcomes | Primary outcomes: decisional conflict (immediately postconsultation) Secondary outcomes: choice (3 months after making decision), decisional regret (3 months after making decision) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized 1:1 using a random numbers table in blocks of 6" (p 231) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to make judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to make judgment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition but balanced between groups: "39 randomized subjects were either missed by the research assistant at their new patient visit and thus did not receive a DCS questionnaire to complete or they canceled their appointments and did not reschedule a new one" (p 233). There was a 48% (50/104) attrition rate for Decisional Regret measures. |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | High risk | Risk of contamination due to same physicians in both groups. Also, outcomes measured after the PtDA and physician consult |
Chabrera 2015.
| Methods | Randomized to DA vs usual care | |
| Participants | 73 + 74 men recently diagnosed with prostate cancer considering treatment options | |
| Interventions | DA: 2‐part decision support booklet with clinical problem, options' outcomes, outcome probabilities, patient stories, explicit values clarification, and guidance Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, satisfaction with decision‐making process Secondary outcome: coping Outcomes assessed at 3 months postintervention |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[S]tudy participants were randomized into 1 of 2 arms using a computer‐generated random list with unequal blocks" (p E44) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided to make judgment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided to make judgment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced attrition in both groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided; trial not registered |
| Other bias | Unclear risk | Prostate cancer in Catalonia is common; however, only 147 were recruited for this trial (p E44) |
Chambers 2012.
| Methods | Randomized to DA vs usual care | |
| Participants | 74 + 77 healthcare workers who did not receive the influenza vaccine considering receiving the vaccine in Canada | |
| Interventions | DA: web‐based DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: confidence in decision (post‐DA) Secondary outcomes: impact on immunization intent (post‐DA), proportion undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated using the randomization function in Excel 2002 (version 10.6856.6856 SP3)" (p 199) |
| Allocation concealment (selection bias) | Low risk | "The list was imported from Excel into a Microsoft SQL Server database. The online application would sequentially assign a random identification number and their decision aid status (seeing the decision aid or not) from the randomization list when users logged into the survey." (p 199) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported whether or not they were blinded during the course of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Questionnaire scores are objective and not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65% completion rate in intervention arm and 77% completion rate in control arm: attrition could be different where the respondents and non‐respondents are different |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Unclear risk | Figure 1 numbers for exclusion are not logical |
Clancy 1988.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 753 + 263 health physicians considering Hep B vaccine in the USA | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification (personal decision analysis), guidance/coaching Comparator: usual care (no information provided) | |
| Outcomes | Uptake of option | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table; all incoming residents were assigned to Group 2 (non‐randomized residents identified as subgroup) (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or personnel. Did not report on how this may affect their findings |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but decisions for screening were retrieved from health records (objective data) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow chart not included. Insufficient information to make a judgment |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Potential selection bias ‐ non‐randomized residents were added to group 2 and therefore potential unbalanced distribution (p 287) Low response rate among those offered decision analysis |
Davison 1997.
| Methods | Randomized to decision aid + audio‐taped consultation vs usual care | |
| Participants | 30 + 30 men with prostate cancer considering treatment in Canada | |
| Interventions | DA: written + audiotape consultation of options' outcomes, clinical problem, outcome probability, others' opinion Comparator: usual care (general information pamphlets on clinical problem) | |
| Outcomes | Primary outcomes: role in decision making Secondary outcomes: anxiety, depression |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The group to which subjects were assigned was predetermined by a block randomization procedure. This ensured there were an equal number of subjects in both groups for each physician." (p 5, Data collection) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned; group assignment predetermined by block randomization procedure (p 5) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding; study does not report on how the results could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding and whether outcomes could be affected by unblinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No flow diagram; p 12 explains why certain men did not listen to audiotape. All men approached by study investigator agreed to participate; only 1 man refused to complete the second set of questionnaires. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other sources of bias; similar baseline characteristics |
De Achaval 2012.
| Methods | Randomized to detailed vs simple vs usual care | |
| Participants | 70 + 70 + 71 patients diagnosed with knee osteoarthritis considering treatment in the USA | |
| Interventions | Complex DA: video booklet + interactive joint analysis on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (list of questions) Comparator DA: video booklet on options' outcomes, clinical problem, outcome probabilities, others' opinion and guidance (list of questions) Comparator: usual care receiving generic booklet |
|
| Outcomes | Decisional conflict (baseline and postintervention) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list with uneven blocks (p 231) |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed and opaque envelopes (p 231) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Likely not blinded, but low threat of bias in study (p 231) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were not blinded but outcome was objectively measured (p 231) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts; missing data effect size unlikely to have significant impact on study outcome |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Appears to be free of other sources of bias |
Dolan 2002.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 50 + 47 average risk for colorectal cancer considering screening in the USA | |
| Interventions | DA: computer with analytic hierarchy process on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance/coaching Comparator: usual care with information on options, clinical problem | |
| Outcomes | Primary outcomes: uptake of option, decisional conflict Secondary outcomes: role in decision making |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomization schedules were created using a computer random number generator" (p 2, Study interventions) |
| Allocation concealment (selection bias) | Low risk | Computer‐based (p 2, Study interventions) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding of participants. All patient interviews in both the experimental and control groups were done by the same investigator, unclear on how this could contribute to risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram ‐ low attrition |
| Selective reporting (reporting bias) | Unclear risk | Nothing specifically mentioned re study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
Evans 2010.
| Methods | Randomized to online decision aid vs paper decision aid vs questionnaire vs usual care | |
| Participants | 129 + 126 + 127 + 132 men considering PSA screening in Wales | |
| Interventions | DA: online programme on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer programme; summary) Comparator: paper version of online DA on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (interactive computer programme; summary) Comparator: received a questionnaire Comparator: received nothing |
|
| Outcomes | Primary outcomes: knowledge (post‐DA) Secondary outcomes: attitude (post‐DA), intention to undergo PSA testing (post‐DA), anxiety (post‐DA), uptake of PSA test (post‐DA), total decisional conflict |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[A] random sample of 100 men was selected from the list." "The process ensured individual level randomization" (p 4, Recruitment process) |
| Allocation concealment (selection bias) | Low risk | "[A]ffirmative consent forms from each practice were transferred to the research officer who allocated each participant with a number provided remotely by the trial statistician to ensure concealment" (p 4, Recruitment process) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study does not address this outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram indicating high attrition consistently across groups |
| Selective reporting (reporting bias) | Low risk | Registered as a trial |
| Other bias | Low risk | The study appears free of other sources of bias |
Fagerlin 2011.
| Methods | Decision aid vs delayed intervention vs control | |
| Participants | 382 + 159 + 100 women with an elevated 5‐year risk of breast cancer considering breast cancer prevention medication in the USA | |
| Interventions | DA: tailored DA on options' outcomes, clinical problem, outcome probabilities, and explicit values clarification Comparator 1: given DA after 3‐month follow‐up Comparator 2: given DA after all outcome measures were taken |
|
| Outcomes | Decisional conflict (post‐DA), behavioural intent (post‐DA), actual behaviour (post‐DA), proportion undecided, perception of benefits (post‐DA), perception of risk (post‐DA) Other outcomes:
|
|
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was provided by the author |
| Allocation concealment (selection bias) | Low risk | Central and web‐based allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding ‐ using an online decision aid would have avoided control participants accessing the decision aid |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not report exclusions; inadequate reporting on participant flow through the study to determine risk for attrition bias or incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
Fraenkel 2007.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 47 + 40 patients with knee pain considering treatment options in the USA | |
| Interventions | DA: interactive computer tool options' outcomes, outcome probability, explicit values clarification Comparator: usual care using the Arthritis Foundation information pamphlet |
|
| Outcomes | Decisional self‐efficacy, preparation for decision making | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided; computer generated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but study does not report if it had an impact on the outcomes measured |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low risk of attrition bias ‐ outcome data for all 40 controls and 44 of 47 intervention (p 3, Results) |
| Selective reporting (reporting bias) | Unclear risk | No information provided; no indication of trial was registered centrally |
| Other bias | Low risk | Appears to be free of other potential biases |
Fraenkel 2012.
| Methods | Cluster‐randomized control trial of clinics to decision aid versus usual care | |
| Participants | 69 + 66 patients with nonvalvular atrial fibrillation considering anticoagulation with aspirin or warfarin | |
| Interventions | DA: computer‐based tool on options' outcomes, clinical problem, options' probabilities, guidance, explicit values clarification Comparator: control arm (no further information provided) |
|
| Outcomes | Primary outcomes: feeling informed and having clear values (baseline, immediately post) Secondary outcomes: knowledge (baseline, immediately post), accuracy of risk (baseline, immediately post), anxiety (baseline, immediately post), worry (baseline, immediately post), rationale for preferred treatment (during the encounter ‐ DA group only), discussion of related outcomes (during the encounter as captured on audiotape), change in treatment plan (post intervention), anxiety, accurate risk expectations (stroke, bleeding) |
|
| Notes | Trial registration NCT00829478 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Inadequate information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | inadequate information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To avoid contamination, participants were randomized at the level of the firm so that all participants in one firm received the intervention, and all participants in the second firm were included in the control arm" (p 1435) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An interviewer blinded to the participant's group assignment reassessed the primary and secondary outcomes after participant's primary care visit" (p 1436) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not appear to be incomplete outcome data; flow diagram does not report participation beyond randomization |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Does not appear to be any other potential sources of bias |
Frosch 2008a.
| Methods | Randomized to decision aid vs. decision aid + chronic disease trajectory vs chronic disease trajectory vs usual care (Internet information) | |
| Participants | 155 + 152 + 153 + 151 men considering prostate cancer screening | |
| Interventions | DA: information on options' outcomes, clinical problem, outcome probabilities, others' opinions Comparator 1: information on options' outcomes, clinical problem, outcome probabilities, others' opinions, explicit values clarification (utilities for outcomes associated with prostate cancer) Comparator 2: explicit values clarification (utilities for outcomes associated with prostate cancer) Comparator 3: usual care using public information on prostate cancer screening on American Cancer Society and Centers for Disease Control and Prevention websites 2005‐2006 |
|
| Outcomes | Primary outcomes: knowledge, actual option, decisional conflict Secondary outcomes: concern about prostate cancer, treatment preference if prostate cancer diagnosed |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm randomly assigned participants to the 4 study groups |
| Allocation concealment (selection bias) | Low risk | Revealed after signed consent and completed baseline measures |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Accessed a secure Internet site that hosted all study materials; participants had unlimited access to assigned intervention, unclear blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were measured via questionnaires and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis; imputed missing data for participants who did not complete follow‐up assessments; minimal attrition |
| Selective reporting (reporting bias) | Unclear risk | No indication of published protocol |
| Other bias | Low risk | Appears to be free of other potential biases |
Gattellari 2003.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 126 + 122 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification Comparator: usual care using brief information on screening test and chances of false‐positive results | |
| Outcomes | Preferred option, knowledge, decisional conflict, accurate risk perceptions, perceived ability to make an informed choice | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pre‐randomized code ‐ no further information (p 1) |
| Allocation concealment (selection bias) | Low risk | Pre‐randomized code unobtrusively marked on envelopes (p 1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Consenting men were blinded to allocation, but unclear if personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pre‐test characteristics included. Flow chart not included and reasons for attrition not mentioned; some attrition but balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Gattellari 2005.
| Methods | Randomized to decision aid booklet vs decision aid video vs usual care | |
| Participants | 140 + 141 + 140 men considering PSA testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probability, explicit values clarification Comparator 1: video on clinical problem, outcome probability, others' opinion Comparator 2: usual care using brief information on screening test and chances of false‐positive results | |
| Outcomes | Preferred option, knowledge, decisional conflict, perceived ability to make an informed choice | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unique identification codes assigned to participants according to date and time enrolled into the interventional component of the study. Block randomization of identification codes then performed via computer software (p 2 ‐ 2.3.1) |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured as the interviewers, responsible for enrolling participants onto the trial, were blinded to the randomized study design while one of the authors (MG) was responsible for randomisation. Hence, it was not possible for either participants or interviewers to be aware of the randomisation sequence." (p 2 ‐ 2.3.1) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and interviewers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At post‐test, it was not possible to blind the interviewers but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal attrition that is consistent across groups (figure 1) |
| Selective reporting (reporting bias) | Unclear risk | "[S]uccess of study protocol" limitation to protocol: men not confronted with actual decision to undergo PSA screening; no indication that trial registered in central trials registry (p 13, paragraph 5) |
| Other bias | Low risk | "[H]igh follow‐up rate and allocation concealment; study not subjected to selection bias" (p 13, paragraph 5). Appears to be free of other sources of bias |
Green 2001.
| Methods | Randomized to decision aid + counselling vs counselling alone vs usual care | |
| Participants | 29 + 14 women with a first degree relative with breast cancer interested in learning about genetic testing in the USA | |
| Interventions | DA: CD‐ROM plus counselling on options' outcomes, clinical problem, others' opinions, guidance/coaching Comparator: counselling Comparator: usual care |
|
| Outcomes | Primary outcome: preferred options Secondary outcome: knowledge |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[B]lock randomization schedule to one of three groups in a 2:2:1 ratio" (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[G]enetic counsellor blinded to randomization until just prior to the session" (p 2), unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Values do not always add up to the number of participants due to missing data"; reasons not mentioned (p 4). "Participants' baseline knowledge was reflected in the control group's answers"; participants balanced in study groups |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other sources of bias |
Hamann 2006.
| Methods | Cluster‐randomized trial of decision aid vs usual care | |
| Participants | 54 + 59 patients with schizophraenia considering treatment options (cluster‐RCT with 12 wards paired and randomized) in Germany | |
| Interventions | DA: 16‐page booklet on options' outcomes, outcome probabilities, explicit values clarification, coaching/guidance Comparator: usual care | |
| Outcomes | Knowledge, participation in decision making (COMRADE ‐ doctor gave me a chance to decided which treatment I thought was best for me), uptake of psycho‐education, rehospitalization, adherence, satisfaction with care, severity of illness (baseline only), attitudes about drug use, decision making preference | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[O]ne member of each pair being randomly assigned to the control or to the interventional condition" (p 266). Sequence generation method was not stated |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Clustering was not accounted for in the analysis |
Hanson 2011.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 127 + 129 patients diagnosed with advanced dementia and eating problems considering long‐term feeding tube placement in the USA | |
| Interventions | DA: booklet or audio recording on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (steps in decision making, worksheet, summary) Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (3 months post‐DA) Secondary outcomes: surrogate knowledge, risk perceptions, frequency of communication with providers (3 months post‐DA), feeding treatment use (3, 6 and 9 months post‐DA), participation in decision making, satisfaction with the decision, decisional regret |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number generation (p 2010, Randomization) |
| Allocation concealment (selection bias) | Unclear risk | No description of method used to conceal allocation (p 2010, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Cluster randomization prevented double blinding and may have introduced bias due to site effects" (p 2014, Discussion); study authors unsure of effect on study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[B]ecause of cluster randomization, data collectors were not blinded to group assignment" (p 2010, Randomization); authors believe has little impact on study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention group missing data for 1 participant, reason for omission not reported (table 1) No explanation for number of participants in each group (n = 127) given numbers vary from those in 'recruitment and retention' figure (table 4) |
| Selective reporting (reporting bias) | Low risk | Registered with clinicaltrials.gov, protocol on website |
| Other bias | Low risk | Appears to be free of other potential biases |
Heller 2008.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 66 + 67 breast cancer patients eligible for breast reconstruction in the USA | |
| Interventions | DA: interactive software programme on options' outcomes, others' opinions Comparator: standard patient education | |
| Outcomes | Knowledge, anxiety, satisfaction with treatment choice, satisfaction with decision‐making ability | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "upon study entry, the participants were randomized (computer generated) to one of two groups" (p 2) |
| Allocation concealment (selection bias) | Unclear risk | Not enough information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline anxiety and knowledge included in graphs. Participant numbers between study groups balanced (p 3). Reasons for incomplete questionnaires and study withdrawals mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No information provided re protocol |
| Other bias | Low risk | Appears to be free of other potential biases |
Hess 2012.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 105 patients in the the emergency department with primary symptoms of nontraumatic chest pain and were being considered of admission to the emergency department observation unit for monitoring and cardiac stress testing within 24 hours | |
| Interventions | DA (in consultation): 1‐page printout on options' outcomes, clinical problem, and outcome probabilities Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge Secondary outcomes: risk perceptions, decisional conflict, actual choice, satisfaction with decision making process, patient‐practitioner communication |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" (p 253) |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized to either usual care or shared decision making through a Web‐based, computer‐generated allocation sequence in a 1:1 concealed fashion" (p 253) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel were blinded, but unclear if patients were blinded (p 253, Outcome measures). However, the primary outcome is unlikely to be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators assessing outcomes were blinded (p 253, Outcome measures). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some of the numbers of patients reported in the results did not match the flow chart |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | Appears to be free of other biases |
Jibaja‐Weiss 2011.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 51 + 49 women diagnosed with breast cancer considering surgical treatment in the USA | |
| Interventions | DA: computer programme on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (step‐by‐step process for making the decision) Comparator: usual care + breast cancer treatment educational materials normally provided to patients |
|
| Outcomes | Surgical treatment preference (post‐DA), breast cancer knowledge (pre, post‐DA, post‐DA and consult), satisfaction with surgical decision (post‐DA), satisfaction with decision‐making process (post‐DA), decisional conflict (pre, post‐DA, post‐DA and consult), proportion undecided | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients at each hospital were randomized using permuted blocks" (p 42, Methods section) |
| Allocation concealment (selection bias) | Unclear risk | Not addressed in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not addressed in the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no way to know if the plots include all of the participants' data since they do not specify what was the number of patients used to obtain these mean scores |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Appears to be free of other potential biases |
Johnson 2006.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 32 + 35 patients considering endodontic treatment options in the USA | |
| Interventions | DA (in consultation): decision board on options' outcomes, clinical problem, outcome probability, guidance Comparator: usual care | |
| Outcomes | Primary outcomes: knowledge, satisfaction with decision making process, anxiety | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[F]our computerized random generation lists to assign to one of two groups" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | Not for residents: computer‐generated randomization lists (1 for each resident) were prepared by the PI (p 3‐4); therefore residents would have had pre‐generated lists; Unclear for patients: "allocation was concealed from patients" (p 3) but does not explain how |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not mentioned. Allocation was concealed from patients only (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 6); all 40 patients agreed to participate in the study, but only 32 questionnaires were useable several residents did not understand need for entering data on the envelope and placing matched questionnaire in it (p 5) |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Unclear risk | "[B]aseline data obtained because possible that clinicians training in the EndoDB would alter usual care discussions" (p 5). Mentions taking baseline characteristics, but not included in article |
Kasper 2008.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 150 + 147 multiple sclerosis patients considering immunotherapy in Germany | |
| Interventions | DA: booklet and worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification (based on IPDAS) Comparator: information material on immunotherapy (80 pages) |
|
| Outcomes | Primary outcomes: role in decision making Secondary outcomes: choice, feeling undecided, helpfulness with making a decision, attitudes toward immunotherapy, expectations of side effects realized at 6 months |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[A]llocation using computer generated random numbers" (p 5) |
| Allocation concealment (selection bias) | Unclear risk | Randomization was carried out by concealed allocation, but method of concealment was not described (p 2, Assignment) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not told whether the information they received was standard information or the newly developed DA (p 3, Masking) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were not told whether the information they received was standard information or the newly developed DA (p 3, Masking) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow of participants (p 2, Fig 1); baseline data/characteristics included |
| Selective reporting (reporting bias) | Low risk | "The protocol of this study has been published with the trial registration at http://controlled‐trials.com/ ISRCTN25267500" (p 2) |
| Other bias | Unclear risk | Difference in preferred interaction style between groups at baseline (P value 0.04) (p 5) |
Kennedy 2002.
| Methods | Randomized to decision aid + coaching vs decision aid only vs usual care | |
| Participants | 215 + 206 + 204 women considering treatment for menorrhagia in the UK | |
| Interventions | DA: video + booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance/coaching Coaching: ˜ 20 minute coaching with explicit values clarification by a registered nurse prior to seeing physician Comparator: usual care | |
| Outcomes | Primary outcomes: general quality of life Secondary outcomes: uptake of option, satisfaction, menorrhagia severity, cost‐effectiveness |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated by computer and stratified by consultant and the age at which the woman left full‐time education (p 3) |
| Allocation concealment (selection bias) | Low risk | "Secure randomization ensured by using a central telephone randomization system" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Possibility of contamination bias; clinicians could have applied the experience gained from consultations with the interventions groups in their consultations with the control group (p 6) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if blinding used but most outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 1 and Figure 1 flow diagram (p 4‐5) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free from other risks of bias |
Knops 2014.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 91 + 87 patients with asymptomatic abdominal aortic aneurysm considering elective surgery vs watchful waiting | |
| Interventions | DA: interactive CD‐ROM on options' outcomes, clinical problem, outcome probabilities, explicit values clarification Comparator: usual care with regular information |
|
| Outcomes | Primary outcomes: decisional conflict (baseline, 1, 4, and 10 months) Secondary outcomes: patient knowledge (baseline and 1 month), anxiety (baseline, 1, 4, and 10 months), satisfaction with conversation with the surgeon (baseline and 1 month), final treatment choice (10 months), aneurysm rupture (10 months), possible date of surgery (10 months), postoperative morbidity and mortality (10 months), physical quality of life (baseline, 1, 4, and 10 months) |
|
| Notes | Trial registration: NTR1524 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation ALEA v.2.2, NKI‐AVL, the Netherlands) was performed by the investigators" (p 2) |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomisation ALEA v.2.2, NKI‐AVL, the Netherlands) was performed by the investigators" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and investigators could not be blinded after group assignment, a factor which is inherent to the decision aid and the design of the study. Surgeons and nurses involved in the outpatient care of the participants were blinded to the patient's allocation group, although patients were not prohibited from sharing their allocation with them." (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measurement is not likely to be influenced by lack of blinding as all outcomes were measured objectively using validated scales and data retrieved from medial records. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears to have similar attrition between groups. The proportion of values missing varied from 2% to 9% per outcome measure. Missing values were completed by multiple imputation analysis. If one of the outcome measures had more than 25% missing values, that outcome measure for that patient was excluded from analysis. Therefore, missing data have been handled appropriately (p 3). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make judgment |
| Other bias | High risk | "Considerable number of patients could not be included, were not asked to participation, or declined to participate. Selection bias may have occured in patients that were not included" (p 6) "Both patients and surgeons were aware of the aim and subject of the study and could not be blinded to the allocation. It is possible that surgeons in the contributing centres offered more than average information to their patients" (p 6). Performance bias may have been introduced in terms of altered communication style. |
Krist 2007.
| Methods | Randomized to decision aid booklet vs decision aid web‐based vs usual care | |
| Participants | 196 + 226 + 75 patients considering prostate cancer screening in the USA | |
| Interventions | DA: 4 page pamphlet with options' outcomes, clinical problem, outcome probability Comparator: web‐site with same information as paper based DA Comparator: usual care |
|
| Outcomes | Primary outcomes: role in decision making Secondary outcomes: knowledge, decisional conflict, time spent discussing screening, choice (PSA test ordered) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]oordinator referred to pre‐generated randomisation tables to inform the participant to which arm he was randomised" (p 2) |
| Allocation concealment (selection bias) | Low risk | At the time of enrolment, the allocation was concealed from the coordinator (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Physicians were not blinded ‐ could affect decision making process and uptake of screening |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p 3, Results; p 4, Flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Uneven groups but done intentionally, ration of 1:3:3 but appears to be free of other potential biases |
Kupke 2013.
| Methods | Cluster‐randomized trial of 2 groups of dental students to decision board group and non‐decision board group. Patients randomized to students in either group. | |
| Participants | 57 + 36 patients with defect in posterior tooth (Class II defect) considering 6 treatment options, including no therapy | |
| Interventions | DA (in consultation): options' outcomes, outcome probabilities Comparator: usual care with discussion of the treatment options |
|
| Outcomes | Knowledge (costs/self‐payment, survival rate, characteristics and treatment time) (postintervention); overall satisfaction with consultation (postintervention) | |
| Notes | Primary outcome not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned by a dice (selection of students and patient allocation) (p 20) |
| Allocation concealment (selection bias) | High risk | "The patients were assigned to the students according to common standards of the university independently and without knowing which group the student belonged to." (p 20) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were assigned to the students independently and without knowing which group the students belonged to" (p 20) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge if blinding of outcome assessment occurred |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attribution in both groups; "missing answers were treated as incorrect answers, while illegible answers were treated as missing values" (p 22) |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol or trial registration. No way to ensure the outcomes they intended to measure are fully reported |
| Other bias | High risk | Did not adjust for clustering in analysis |
Kuppermann 2014.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 375 + 369 11‐week pregnant women who had not yet undergone prenatal screening or diagnostic testing | |
| Interventions | DA: describes clinical condition, options, outcome probabilities, values clarification Comparator: usual care |
|
| Outcomes | Primary outcomes: invasive prenatal diagnostic testing (3 to 6 months) Secondary outcomes: testing strategy undergone (3 to 6 months), knowledge (3 to 6 months), accurate risk perception (procedure related miscarriage, DS affected fetus) (3 to 6 months), decisional conflict (3 to 6 months), decisional regret (3 to 6 months) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated random allocation sequence assigned participants to experimental groups within permuted blocks of random size, with a 1:1 allocation ratio, stratified by age, clinical site, parity, and interviewer" (p 1211) |
| Allocation concealment (selection bias) | Low risk | "The randomization code was not available to any study‐related personnel until data analysis was complete" (p 1211) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Different research associates facilitated baseline and follow‐up interviews and medical record review to ensure blinding to the randomization assignment" (p 1211) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Different research associates facilitated baseline and follow‐up interviews and medical record review to ensure blinding to the randomization assignment" (p 1211) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups. "[A]ll reported analyses were based on a modified intention‐to‐treat sample" (p 1211) |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | Low risk | Appears to be free of other sources of bias |
Lam 2013.
| Methods | Randomized to decision aid or standard information booklet after initial consultation | |
| Participants | 138 + 138 women considering breast cancer surgery for early‐stage breast cancer | |
| Interventions | DA: take‐home booklet on clinical problem, options' outcomes, outcome probabilities, guidance, explicit values clarification Comparator: standard information booklet |
|
| Outcomes | Primary outcomes: treatment decision making difficulties and decisional conflict scale at 1 week post consultation, knowledge at 1‐week postconsultation, decision regret at 1 month after surgery Secondary outcomes: postoperative psychological distress (anxiety and depression) at 1, 4, and 10 months after surgery, decision regret at 4 and 10 months after surgery, treatment decision |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient assignment to treatment and control arms was performed using a prior computer‐generated random‐number sequence" (p 2880) |
| Allocation concealment (selection bias) | Low risk | "A serially labeled, opaque, sealed‐envelope method was used for block randomization" (p 2880) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Two research staff members ‐ one responsible for preintervention assessment and block allocation and the other for postintervention assessments ‐ ensured that the researcher performing follow‐up assessments was blinded regarding women's allocation status." "Blinding surgeons to allocation status proved impractical." (p 2880) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 1 research staff member was responsible for postintervention assessments to ensure that the researcher performing follow‐up assessments was blinded regarding women's allocation status (p 2880). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data; similar attrition in both groups |
| Selective reporting (reporting bias) | Low risk | Study protocol available online with published study |
| Other bias | Low risk | Does not appear to be subject to other sources of bias |
Langston 2010.
| Methods | Randomized to decision aid + coaching vs usual care | |
| Participants | 114 + 108 women pregnant women in their first trimester considering use of contraceptives in the USA | |
| Interventions | DA: double‐sided flip chart on clinical problem, outcome probabilities, guidance (administered by a research assistant), coaching (structured, standardized, non‐directive contraceptive counselling) + usual care Comparator: usual care |
|
| Outcomes | Primary outcomes: proportion of participants choosing very effective contraceptive method (post‐DA and consult) Secondary outcomes: actual choice on day of procedure (post‐DA and consult), adherence of very effective and/or effective methods at 3 months and at 6 months (post‐DA and consult) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random‐number table, we determined the sequence for 1:1 allocation constrained by blocks of 10" (p 363, Methods‐study procedures) |
| Allocation concealment (selection bias) | Low risk | "Randomization assignments were sealed inside numbered, opaque envelopes" (p 363, Methods‐study procedures) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "No blinding of participants or coordinators was feasible due to the nature of the intervention. Physician‐providers did not know the participant's allocation group, did not discuss the study with patients, and were asked not to change their counselling" (p 363, Methods‐study procedures) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | For "method initiation on the day of the procedure" it is only said that the "[p]articipants in the intervention group were not more likely to initiate the requested method immediately compared to those in the usual care group"; possible that the results contradicted the hypothesis and were excluded for this reason |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol; not enough information to permit judgement |
| Other bias | Low risk | Appears to be free of other potential biases |
Laupacis 2006.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 60 + 60 patients undergoing elective open heart surgery considering pre‐operative autologous blood donation in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework) Comparator: usual care | |
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: uptake of option, satisfaction with decision making process, satisfaction with decision, accurate risk perceptions |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization envelopes were prepared centrally by a statistician" (p 2) |
| Allocation concealment (selection bias) | Low risk | "The envelopes were labeled with identification numbers and contained a card specifying the patient's group assignment. The envelopes were opened by the interviewer after completion of the baseline interview." (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results, p 4; fig 1, flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
LeBlanc 2015.
| Methods | Randomized to decision aid vs individualized score only vs usual care | |
| Participants | 32 + 33 + 14 women over 50 years diagnosed with osteopenia or osteoporosis not taking biphosphonates or other prescription medication | |
| Interventions | DA (in consultation): clinical problem, individualized risk of condition, options' outcomes, guidance Comparator 1: individualized risk Comparator 2: usual care |
|
| Outcomes | Primary outcomes: knowledge (immediately post), decisional conflict (immediately post), participation in decision‐making process (immediately post), decision to start (immediately post), adherence (6 months), acceptability (timing not specified), satisfaction with the decision‐making process (not specified), quality of life (not specified), time (review of video consultation) Secondary outcome: decision quality (not reported) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion" (p 5) |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated using a computer‐generated sequence that randomized them 1:1:1 in a concealed fashion" (p 5) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and clinicians were aware of the overall objective, presented as improvement in communication between patients and clinicians during the clinical encounter, but remained blinded to the specific aims" (p 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After randomization, only data analysts remained blind to allocation" (p 5) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used intention‐to‐treat analysis; similar attrition in both groups |
| Selective reporting (reporting bias) | Unclear risk | Trial registered; Checklists available for CONSORT and protocol. Sample size originally calculated based on adherence but re‐calculated for decisional conflict given inability to reach original target |
| Other bias | High risk | "Possible contamination at the clinician level (i.e. clinician who, having used the decision aid with a prior patient, recreates elements of the decision aid with a subsequent patient allocated to receive FRAX alone or usual care) was monitored by a detailed review of the available video recorded encounters" (p 5) |
Legare 2008a.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 45 + 45 women considering use of natural health products for managing menopausal symptoms | |
| Interventions | DA: booklet with worksheet on options' outcomes, clinical problem, explicit values clarification, guidance/coaching (Ottawa Decision Support Framework) Comparator: general information brochure on the clinical problem (did not address risks and benefits) | |
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge of natural health products in general (not specific option outcomes), preferred choice, values‐choice agreement, proportion undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization scheme was carried out by a biostatistician using computer‐generated unequal blocks. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes containing 1 or the other documents (a PDA in the intervention group and a general information brochure in the control group) were prepared by another individual, external to the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigators were blinded but no mention of blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Figure 1 for flow diagram, reason for loss to follow‐up was described. |
| Selective reporting (reporting bias) | Low risk | Trial registration identifier is NCT00325923 |
| Other bias | Low risk | No statistically significant difference in women's characteristics between groups (Table 1) |
Legare 2011.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 245 + 214 patients with non‐emergent acute respiratory infections considering using antibiotics in Canada | |
| Interventions | DA (in consultation): pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance and coaching Comparator: delayed intervention |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A biostatistician simultaneously randomised all FMGs and allocated them to groups using Internet‐based software" (p 99) |
| Allocation concealment (selection bias) | Low risk | "Using Internet‐based software" (p 99) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding of participants and personnel: only biostatistician was blinded (p 99) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biostatistician who assesses the outcomes is blinded, outcomes were objectively measured (p 99) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no missing data |
| Selective reporting (reporting bias) | Low risk | No missing pre‐specified outcomes |
| Other bias | Low risk | Appears to be free of other sources of bias |
Legare 2012.
| Methods | Cluster‐randomized controlled trial to decision aid vs usual care | |
| Participants | 239+210 adults and children with with a diagnosis of acute respiratory infection (e.g., bronchitis, otitis media, pharyngitis, rhinosinusitis) | |
| Interventions | DA (in consultation): pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance and coaching (participating physicians also received training in the form of a 2‐hour online tutorial and a 2‐hour on‐site interactive workshop). Comparator: usual care |
|
| Outcomes | Primary outcome: use of antibiotics (immediately post consultation) Secondary outcomes: decisional conflict (immediately post), control preference scale (immediately post), quality of decision (immediately post), adherence to the decision (2 weeks post), repeat consultation (2 weeks post), decisional regret (2 weeks post), quality of life (2 weeks post) and intention to engage in SDM in future consultations regarding antibiotics for acute respiratory infections (2 weeks post) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A biostatistician used internet‐based software to simultaneously randomize all 12 family practice teaching units to either the intervention group or control group. The teaching units were stratified according to rural or urban location" (p E728) |
| Allocation concealment (selection bias) | Low risk | "A biostatistician used internet‐based software to simultaneously randomize all 12 family practice teaching units to either the intervention group or control group. The teaching units were stratified according to rural or urban location" (p E728) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients with symptoms suggestive of an acute respiratory infection were initially recruited by a RA in the waiting room before consultation with a physician" (p E728) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The biostatistician was unaware of group allocation, the researchers and research assistants who recruited patients and collected data were not" and "Statistical analysis was performed by a statistician who was unaware of the teaching unit allocations" (p E729) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol registered and published |
| Other bias | Low risk | "To avoid contamination bias, access to the online tutorial was denied to providers in the control group during the trial" (p E728) |
Leighl 2011.
| Methods | Randomized to DA + usual care vs usual care | |
| Participants | 107 + 100 patients diagnosed with metastatic CRC considering advanced chemotherapy in Australia and Canada | |
| Interventions | DA: booklet and audiotape on option' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance (steps in decision making + worksheet) Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), satisfaction with decision (post‐DA) Secondary outcomes: anxiety (pre and post‐DA), satisfaction with consultation (post‐DA), choice leaning (post‐DA), decisional conflict (post‐DA). achievement of their information preference (post‐DA), participation in decision making (post‐DA), acceptability (post‐DA), quality of life (post‐DA) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomized lists (p 2078, Study design) |
| Allocation concealment (selection bias) | Low risk | Code concealed in sealed envelopes until time of random assignment (p 2078, Study design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients not blinded and subjective outcomes may be affected by them knowing their assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes are not subjected to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31% dropout rate, but similar losses across all groups |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Appears to be free of other sources of bias |
Lepore 2012.
| Methods | Randomized to decision support intervention (decision coaching by telephone + educational pamphlet) vs control | |
| Participants | 244 + 246 African American men aged 45‐70 in the USA | |
| Interventions | DA: condition‐specific educational pamphlet on prostate cancer screening and tailored telephone education on options' outcomes, explicit values clarification, others' opinions, and guidance (decision coaching) Comparator: attention control (education on fruit and vegetable consumption) |
|
| Outcomes | Primary outcomes: knowledge (pretest and post‐test at 8 months postrandomization), decisional conflict (posttest), physician visit to discuss testing (post‐test), adherence as congruence between testing intentions and behaviors (post‐test) Secondary outcomes: testing intention (post‐test), benefit‐to‐risk ratio of testing (post‐test), PSA screening (post‐test), anxiety (pretest and post‐test) |
|
| Notes | Trial registration NCT01415375 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The principal investigator used a computer‐generated randomization schedule to randomize the participant." (p 322) |
| Allocation concealment (selection bias) | Unclear risk | "The principal investigator used a computer‐generated randomization schedule to randomize the participant and emailed the randomization assignment to the interventionist." (p 322) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Interventionists were not blind to condition. We can assume that patients were blinded as the study design was a telephone call for both intervention and control groups (p 322) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collectors were blind to condition but the interventionists were not" (p 322). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data |
| Selective reporting (reporting bias) | Low risk | Appears to have reported on all pre‐specified outcomes (protocol). |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Lerman 1997.
| Methods | Randomized to decision aid vs waiting list control | |
| Participants | 122 + 114 + 164 women considering BRCA1 gene testing in the USA | |
| Interventions | DA: education and counselling on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching Comparator: no intervention | |
| Outcomes | Primary outcome: preferred option Secondary outcomes: knowledge, accurate risk perceptions, perceived personal risk/benefits/limitations, agreement between values and choice |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 440 women, 400 completed 1‐month follow‐up interviews; no reasons provided; baseline data/characteristics included (p 2) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Lewis 2010.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 211 + 232 patients considering colorectal cancer screening in the USA | |
| Interventions | DA: web‐based, DVD and VHS videotape formats + stage targeted brochures (and booster kit if patients had not been screened) on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encouraged patients to communicate with their practitioners by asking questions and sharing preferences; summary) Comparator: usual care using Aetna annual reminders to obtain CRC screening |
|
| Outcomes | Knowledge of the age at which screening should begin (post‐DA), completion of colorectal cancer screening (pre, post‐DA), intrusive thoughts (pre, post‐DA), interest in CRC screening (pre, post‐DA), intent to ask provider about screening (pre, post‐DA), readiness to be screened (pre, post‐DA), perceived risk of colon cancer (pre, post‐DA), general beliefs about colon cancer (pre, post‐DA), fears about colorectal cancer screening (pre, post‐DA), perceptions about whether participants had enough information (post‐DA), whether participants had enough information about specific screening tests (post‐DA), willingness to pay for screening tests (post), desire to participate in medical decision (post) Practice level measures: assess CRC screening practices (pre, post‐DA), referrals (pre, post‐DA), quality improvement initiatives |
|
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done using matched pairs and a blocking procedure." (p 2, Practice recruitment and randomization section) |
| Allocation concealment (selection bias) | Unclear risk | "Thus, purposive assignment to treatment group was used, resulting in a hybrid randomisation" (p 3, Practice recruitment and randomization section). There is no mention of the effect of this purposive assignment on the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | As mentioned above, staff used purposive assignment and were therefore not blinded, but there is no mention of the effect on the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study did not address this outcome, but outcomes were objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No mention of study protocol |
| Other bias | High risk | Unadjusted cluster analysis |
Loh 2007.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 263 + 142 patients with physician diagnosed depression (cluster RCT with 30 general practitioners randomized) in Germany | |
| Interventions | DA (in consultation): options' outcomes, clinical problem, explicit values clarification, guidance/coaching Comparator: usual care |
|
| Outcomes | Participation in decision making, adherence, satisfaction with clinical care, depression severity, consultation length | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[T]wo‐thirds of the general practitioners were randomly assigned to the intervention group by drawing blinded lots under the supervision of the principal investigator and two researchers" (p 3) |
| Allocation concealment (selection bias) | Low risk | Drawing blinded lots (p 3 ‐ 2.1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding, not enough information provided to assess whether this contributes to bias on outcomes not measured by using a scale (e.g. consultation time was documented in minutes by the physicians following each consultation) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Further results resting on the baseline phase of this trial were already presented elsewhere" (p 5, fig); "unequal distribution of physicians was due to possibility of higher dropout rate in intervention group because of additional time and effort" (p 3). |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases (p 5‐6, details pt and physician baseline characteristics). Statistically significant differences were controlled for in outcome analyses |
Man‐Son‐Hing 1999.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 139 + 148 patients on atrial fibrillation trial considering continuing on aspirin vs change to Warfarin in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) Comparator: usual care | |
| Outcomes | Primary outcomes: uptake of options, adherence Secondary outcomes: help with making a decision, knowledge, accurate risk perceptions, decisional conflict, satisfaction with decision making process, role in decision making |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme (p 2) |
| Allocation concealment (selection bias) | Low risk | Administered from a central location (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear blinding however, "contamination, physicians may have provided DA information to patients receiving usual care" (p 7) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | P 4, fig 2 flow chart. Reasons for attrition not mentioned. Baseline data not included. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential risks of bias |
Mann D 2010.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 80 + 70 participants diagnosed with diabetes considering the use of statins to reduce coronary risk | |
| Interventions | DA (in consultation): healthcare provider led discussion using developed tool (Statin Choice) on options' outcomes,outcome probabilities, guidance (step‐by‐step process for making the decision; administered by the physician in the consultation) Comparator: usual primary care visit + pamphlet |
|
| Outcomes | Knowledge (postconsult and post‐DA), decisional conflict (postconsult and post‐DA), risk estimation (postconsult and post‐DA), beliefs (postconsult and post‐DA), adherence (3 and 6 months postconsult and post‐DA) | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized but there is no mention of method used (p 138, Methods section) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline data was provided |
| Selective reporting (reporting bias) | Unclear risk | Only reports on improvement (i.e. decisional conflict scale); does not present outcome data to fullest (no numerical data on knowledge results between groups, only describes in words) |
| Other bias | Unclear risk | "We did not adjust the clustering of effects given that few participants received care by the same clinicians" (p 139, Analysis section). No mention of magnitude in change of data due to this choice |
Mann E 2010.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 278 + 139 participants considering diabetes screening in the UK | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities and explicit values clarification Comparator: usual care using screening invitation on clinical problem |
|
| Outcomes | Primary outcomes: preferred option (post‐DA) Secondary outcomes: whether invitation type impacts on intention (post‐DA), impact on knowledge (post‐DA), impact on attitude (post‐DA), risk perception |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Invitation taken from the top of a randomly ordered pile (either standard or one of two versions of an informed decision choice invitation). The materials were ordered in a way that the invitation type was hidden until the recruitment process was completed" (p 2‐3, Methods, Participants section). Unclear how invitation type was hidden |
| Allocation concealment (selection bias) | Low risk | "Invitation taken from the top of a randomly ordered pile; materials were ordered in a way that the invitation type was hidden until the recruitment process was completed" (p 2‐3, Methods, Participants section). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Interviewers were not aware of the direction of anticipated effect of materials, and materials were dummy‐coded so that no sense of intervention or control would have been communicated to interviewers or participants (p 3, Methods, Participants section). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study did not address this outcome, but outcomes were objectively measured and not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol; insufficient information to permit judgment |
| Other bias | Unclear risk | "Present sample was … not necessarily representative of the highest risk individuals in this age group"; "£5 incentive might have also added a selection bias"; "Lack of anonymity with verbally delivered questionnaire might encourage socially desirable responding" (p 6, Discussion section) |
Marteau 2010.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 633 + 639 patients considering diabetes screening in England | |
| Interventions | DA: screening invitation on clinical problem, outcome probabilities and explicit values clarification Comparator: usual care using screening invitation on clinical problem |
|
| Outcomes | Primary outcome: attendance for screening (post‐DA and consult) Secondary outcomes: intention to make changes to lifestyle (post‐DA and consult), satisfaction with decisions made among attenders (post‐DA and consult) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[G]enerated simultaneously in a batch by random numbers using Excel spreadsheet software, stratifying by number of participants in household" (p 2, Randomization section) |
| Allocation concealment (selection bias) | Low risk | "Randomisation … was undertaken by the study statistician from a central site" (p 2, Randomization section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Personnel were blinded and appears that patients were unaware which arm they were in (members of the same household received the same intervention) (p 2, Randomization section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and trial staff taking measurements and entering data were unaware of the study arm to which participants had been assigned (p 2, Randomization section) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published protocol (p 2, Methods) |
| Other bias | Low risk | Appears free of other potential biases |
Mathers 2012.
| Methods | Cluster‐randomized controlled trial of 49 general practices in the UK to decision aid, healthcare professional training workshop and use of PDA in consultation, or usual care. | |
| Participants | 95 + 80 participants with type 2 diabetes considering adding or changing to insulin therapy | |
| Interventions | DA: booklet about clinical problem, treatment options, options' outcomes, outcome probabilities, explicit values clarification, structured guidance Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (immediately postintervention), glycaemic control (glycosolated haemoglobin, HbA1c) at 6 months Secondary outcomes: knowledge (immediately post), realistic expectations (immediately post), preference option (immediately post), proportion undecided (immediately post), participation in decision‐making (immediately post), regret (6 months), adherence with chosen option (6 months) |
|
| Notes | Trial registration: ISRCTN14842077 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All eligible and willing practices were randomly allocated by a computer" (p 3) |
| Allocation concealment (selection bias) | Low risk | "A statistician generated the random allocation sequence while a secretary who was not involved in the research study assigned participants to either the intervention or control groups" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Blinding of the intervention and assessment of the process measures were not feasible in view of the nature of the intervention studied" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Blinding of the intervention and assessment of the process measures were not feasible in view of the nature of the intervention studied" (p 3) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | Unclear risk | Cannot make a judgment with information provided regarding cessation of recruitment at 175 (yet 320 required to allow detection of 0.5% difference in HbA1c) |
Mathieu 2007.
| Methods | Randomized to decision aid versus usual care | |
| Participants | 367 + 367 women aged 70 to 71 years and considering a subsequent screening mammography in Australia | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance with worksheet (Ottawa Decision Support Framework) Comparator: BreastScreen NSW brochure ‐ includes information for women 70 + but no numeric information about the outcomes of screening |
|
| Outcomes | Primary outcomes: actual decision, informed choice Secondary outcomes: knowledge (includes 5 questions about risk perceptions), anxiety, decisional conflict, breast cancer worry, preference/intension, attitudes about screening, relationship between objective and perceived risk of breast cancer |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer programme, which assigned allocations in accordance with a simple randomization schedule (p 2, Methods) |
| Allocation concealment (selection bias) | Low risk | Randomized by interview staff who accessed a previously concealed computer programme (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers [at follow‐up] were blinded, outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 1 flow diagram (p 2) |
| Selective reporting (reporting bias) | Low risk | "The trial was registered with the Australian Clinical Trials Registry and the Clinical Trials Registration System" (p 5) |
| Other bias | Low risk | Appears to be free of other potential biases |
Mathieu 2010.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 189 + 223 women considering mammography screening | |
| Interventions | DA: Internet programme + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (worksheet with questions relevant to decision making process; one or more questions that asked patients to clarify their preferences; summary) Comparator: delayed intervention |
|
| Outcomes | Primary outcomes: knowledge (post‐DA), risk perception Secondary outcomes: intention (post‐DA), values (post‐DA), informed choice (post‐DA), proportion undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer generated simple randomization schedule" (p 66, Randomization and baseline questions section) |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomization was conducted in a concealed manner" (p 66). Method of allocation concealment not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes mentioned in Outcome measures section were reported in the results section (p 68, Table 2; information for intention as well as anxiety and acceptability can be found in text format in the secondary outcomes section on pg.67‐68) |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
McAlister 2005.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 219 + 215 patients considering antithrombotic therapy for nonvalvular atrial fibrillation (cluster‐RCT with 102 primary care practices randomized) in Canada | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) Comparator: usual care | |
| Outcomes | Primary outcomes: uptake of (appropriate) option Secondary outcomes: knowledge, decisional conflict, accurate risk perceptions |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]luster randomization at level of primary care practice to minimize contamination; randomization was done centrally to preserve allocation concealment using a computer generated sequence" (p 2) |
| Allocation concealment (selection bias) | Low risk | Randomization was done centrally to preserve allocation concealment (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded, but not sure whether the lack of blinding would affect the outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results and Fig 1 ‐ flow diagram (p 3) |
| Selective reporting (reporting bias) | Low risk | DAAFI trial protocol, including copies of the various questionnaires we employed, has been published (p 1, Methods) |
| Other bias | Low risk | Appears to be free of other potential biases |
McBride 2002.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 289 + 292 perimenopausal women considering hormone replacement therapy in the USA | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, values clarification, others' opinions, guidance/coaching Comparator: delayed intervention | |
| Outcomes | Primary outcome: accurate risk perceptions Secondary outcomes: satisfaction with decision, confidence with knowledge and making/discussing decision |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided; Bastian 2002, no information provided ‐ Study design is described elsewhere (p 4) |
| Allocation concealment (selection bias) | Unclear risk | No information provided; Bastian 2002, no information provided ‐ Study design is described elsewhere (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data are available for 520 (90%) of the women (p 2). Reasons why not mentioned (Bastian 2002, p 5, Results; p 6, Baseline characteristics/data included) |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases; Bastian 2002, p 8 ‐ Eligible participants were willing to consider HRT and this may have favoured recruitment of women with higher SES and those who had prior experience with HRT |
McCaffery 2010.
| Methods | Randomized to decision aid + informed choice vs HPV testing vs repeat smear | |
| Participants | 104 + 104 + 106 women screened as HPV indeterminate considering HPV testing in Australia | |
| Interventions | DA: pamphlet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion and guidance (worksheet) Comparator 1: no decision support, received immediate HPV testing Comparator 2: no decision support, received a repeat cervical smear at 6 months |
|
| Outcomes | Primary outcomes: quality of life (post‐DA) Secondary outcomes: waiting time anxiety (post‐DA), , perceived risk (post‐DA), perceived seriousness of cancer (post‐DA), worriedness (post‐DA), intrusive thoughts (post‐DA), satisfaction with care (post‐DA), anxiety (post‐DA), distress and concerns (post‐DA), self‐esteem (post‐DA), effect on sexual behaviour (post‐DA), help seeking behaviour (post‐DA), knowledge (post‐DA) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomised centrally by the research team within each clinic in blocks of three" (p 2, Design) |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised centrally by the research team within each clinic in blocks of three" (p 2, Design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and staff were unblinded, but objective outcomes were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All outcomes are on questionnaires; not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 3: sensitivity analysis was done to include most of the patients |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Low risk | Appears to be free of other sources of bias |
Miller 2005.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 279 women considering BRCA1‐BRCA2 gene testing in the USA | |
| Interventions | DA: educational intervention on options' outcomes, personal family cancer history; clinical problem, outcome probability, explicit values clarification, others' opinions, guidance/coaching Comparator: provision of general information about cancer risk | |
| Outcomes | Preferred option, knowledge, perceived risk, satisfaction | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomized by the CATI system" (p 4) after self‐initiated telephone contact |
| Allocation concealment (selection bias) | Low risk | "[C]omputerized assisted telephone interview system (CATI)" (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not addressed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons stated for initial drop‐out of study participants (p 8). Patients contacted offered reasons for dropping out. Study protocol allowed patients to be reached up to 13 times at follow‐up; but still not able to be reached |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other sources of bias |
Miller 2011.
| Methods | Decision aid vs attention placebo | |
| Participants | 132 + 132 participants considering colon cancer screening in the USA | |
| Interventions | DA: computer‐based web programme on options' outcomes, clinical problem, outcome probabilities, others' opinion, guidance (encourages patient‐practitioner communication, summary) Comparator: computer‐based web programme on prescription drug refills and safety |
|
| Outcomes | Primary outcomes: receipt of CRC screening (post‐DA) Secondary outcomes: ability to state a preference, change in readiness to receive screening (pre and post‐DA), CRC test ordering (post‐DA), proportion undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐randomized, stratified by literacy level (p 609, Methods) |
| Allocation concealment (selection bias) | Unclear risk | Study does not address this domain |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Health care providers were not notified of patients' enrolment in the study at any time (p 609, Methods) RAs that administered post‐DA questionnaire were not blinded but believed to be a low risk of bias (p 613, Discussion) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[C]linical outcome assessors were [blinded]" (p 613, Discussion) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol on ClinicalTrials.gov |
| Other bias | Unclear risk | USD 10 gift card for participation could affect participant pool |
Montgomery 2003.
| Methods | Randomized to decision aid + decision analysis vs decision analysis vs decision aid vs usual care | |
| Participants | 51 + 52 + 55 + 59 newly diagnosed hypertensive patients considering drug therapy for blood pressure in the UK | |
| Interventions | DA: decision analysis plus information video and leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification Comparator: decision analysis on options' outcomes, outcome probability, explicit values clarification Comparator: video and leaflet on options' outcomes, clinical problem Comparator: usual care | |
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: uptake of option, knowledge, anxiety |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation schedule was computer‐generated by an individual not involved in the study (p 2) |
| Allocation concealment (selection bias) | Low risk | "[A]llocation was concealed to the author in advance by the nature of the minimization procedure" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 5) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Montgomery 2007.
| Methods | Randomized to decision aid with values clarification vs decision aid without values clarification vs usual care | |
| Participants | 245 + 250 + 247 women with previous caesarean section in the UK | |
| Interventions | DA: options' outcomes, clinical problem, outcome probability, explicit values clarification Comparator: options' outcomes, clinical problem, outcome probability Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: choice, anxiety, knowledge, satisfaction with decision |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked by using randomly permuted and selected blocks of sizes 6, 9, 12, and 15 generated by computer (p 2 Methods, Randomization) |
| Allocation concealment (selection bias) | Low risk | 1 member of the study team generated the randomization sequence by computer, and another member of staff with no other involvement in the trial performed the allocation (p 2 Methods, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow of women through the study |
| Selective reporting (reporting bias) | Low risk | Trials registry ISRCTN84367722 |
| Other bias | Low risk | Recruited more than planned to account for lost data (p 4, Sample size); baseline characteristics were balanced |
Montori 2011.
| Methods | Randomized to decision aid vs usual care + booklet | |
| Participants | 52 + 48 women with low bone mass or osteoporosis considering taking bisphosphonates in the USA | |
| Interventions | DA (in consultation): worksheet on options' outcomes, clinical problem, outcome probabilities, guidance (administered by physician) Comparator: usual care + general information booklet on osteoporosis |
|
| Outcomes | Patient knowledge (post‐DA), satisfaction with knowledge transfer (post‐DA), decisional conflict (post‐DA), patient‐clinician communication (OPTION), trust with physician (during intervention), clinician's perception of decision quality (post‐DA), clinician's satisfaction with knowledge transfer (post‐DA), uptake (post‐DA), adherence (post‐DA), fidelity (post‐DA), contamination (post‐DA), risk perception | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated allocation" (p 551, Randomization) |
| Allocation concealment (selection bias) | Low risk | Patients randomized "in a concealed fashion (using a secure study website)" (p 551, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of participants being blinded to their allocation; only mention of data collectors and analysts blinding (p 551, Randomization) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "After randomization, data collectors and data analysts were blind to allocation" (p 551, Randomization); Outcomes were not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | "The protocol for this trial has been reported in full" (p 550, Design) |
| Other bias | Unclear risk | Appears to be free of other potential biases |
Morgan 2000.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 120 + 120 patients with ischaemic heart disease considering revascularization surgery in Canada | |
| Interventions | DA: Health Dialog interactive videodisc on options' outcomes, clinical problem, outcome probability, others' opinions Comparator: usual care | |
| Outcomes | Primary outcome: satisfaction with the decision making process Secondary outcomes: uptake of option, knowledge |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Morgan 1997, p 29: all randomization enrolment was performed by telephone at which time the participant was assigned Morgan 2000 (primary study), p 2, Methods, Patient Population: "Only the statistician was privy to the two randomisation schedules and blocking factor used" |
| Allocation concealment (selection bias) | Low risk | Morgan 1997, p 29: only the statistician was privy to the two randomization schedules and blocking factor; Morgan 2000, (primary study), p 2, Methods, Patient Population: "only the statistician was privy to the two randomisation schedules and blocking factor used. All randomization enrolment was performed by telephone" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[D]ue to nature of trial, neither patients or investigators were blinded to the study" ‐ may introduce bias to subjective outcomes such as satisfaction |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Morgan 1997, p 39, Patient accrual and follow‐up: baseline characteristics included Morgan 2000 (primary study): 78% completed follow‐up (90 of 120 in the intervention; 97 of 120 in the control). reasons for attrition were provided |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Unclear risk | Morgan 1997, p 56: significant number of patients were lost to follow‐up (25%); Morgan 2000 (primary study): baseline data imbalance (high school grad, income, no. of diseased arteries). Dropout group reported lower incomes, may have affected results. (discussion par. 6) "Selection bias was minimized by enrolling available consecutive patients" |
Mott 2014.
| Methods | Randomized to shared decision‐making process with DA versus usual care | |
| Participants | 13 +14 military veterans in USA diagnosed with PTSD and had served in Iraq or Afghanistan | |
| Interventions | DA: booklet on clinical problem, options' outcomes, structured guidance Comparator: usual care |
|
| Outcomes | Satisfaction with SDM qualitatively (postintervention), perceived advantages and disadvantages of SDM qualitative (postintervention), treatment preferences (4 months), adherence using treatment engagement (4 months) | |
| Notes | Not reported as registered in trials database; no primary outcome reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized to SDM or UC using a computer‐generated randomization sequence" (p 146) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization envelopes were prepared by the study statistician to ensure that study staff remained masked to randomization sequence" (p 146) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information provided to make judgment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff not blinded but because outcomes were taken from medical records. "At 4‐month follow‐up, study staff reviewed participants' medical records to extract information on treatment preferences and engagement. Medical‐record reviews were conducted by a single rater trained in use of the dataextraction form. A second rater, masked to initial ratings, reextracted data from 20% of patients" (p 146). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27 participants were consented and enrolled , yet only 20 (UC = 11; SMD = 9) completed the study (p 146‐147). Only 5 participants in the SDM arm completed the exit interview. No mention of missing data. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all expected outcomes reported on |
| Other bias | Low risk | Does not appear to be any other sources of bias |
Mullan 2009.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 48 + 37 patients with type 2 diabetes considering treatment options (cluster RCT with 40 clinicians randomized) in the USA | |
| Interventions | DA (in consultation): decision cards with information on options, outcomes, outcome probability, explicit values clarification Compare: 12‐page pamphlet on oral antihyperglycaemic medications |
|
| Outcomes | Knowledge, decisional conflict, participation in decision making, acceptability of the information, change in medication, adherence, HbA1C levels, trust in physician, OPTION to analyse audio‐taped encounters | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were blinded, the clinicians were not, but each session was recorded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for attrition not included |
| Selective reporting (reporting bias) | Low risk | Trial registration no. at clinicaltrials.gov reported |
| Other bias | Low risk | Appears to be free of other sources of bias |
Murray 2001a.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 57 + 55 men considering treatment for benign prostatic hypertrophy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options, outcomes, clinical problem, outcome probability, others' opinions Comparator: usual care | |
| Outcomes | Primary outcomes: uptake of option, prostate symptoms, costs, anxiety Secondary outcomes: decisional conflict, role in decision making, general health status, utility |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomisation schedule, stratified according to recruitment centre, was generated by computer" (p 4) |
| Allocation concealment (selection bias) | Low risk | "Allocation were sealed in opaque numbered envelopes, opened by the study nurse" (p 4) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded but not sure how this would introduce bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 5); baseline data/characteristics included and balanced |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other sources of bias |
Murray 2001b.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 102 + 102 women considering hormone replacement therapy in the UK | |
| Interventions | DA: Health Dialog interactive videodisc on options outcomes, clinical problem, outcome probability, other's opinion Comparator: usual care | |
| Outcomes | Primary outcomes: preferred option Secondary outcomes: help with making a decision, decisional conflict, role in decision making anxiety, menopausal symptoms, costs, utility, general health status |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomisation schedule, stratified according to recruitment centre, was generated by computer" (p 3 Methods, Randomization) |
| Allocation concealment (selection bias) | Low risk | "Allocations were sealed in opaque numbered envelopes, opened by the study nurse after collection of the baseline data" (p 3 Methods, Randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See page 3 figure for Progress of patients through trial |
| Selective reporting (reporting bias) | Unclear risk | Protocol is not mentioned |
| Other bias | Low risk | Similar baseline characteristics, appears to be free of other potential biases. Educational achievement was higher in control group. Quote "Subsequent analysis showed that educational level not related to use of HRT nor was there an interaction between educational attainment and the intervention" |
Nagle 2008.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 167 + 172 women in early pregnancy considering genetic testing (26 + 29 general physicians) (cluster RCT with 60 general practitioners randomized) in Australia | |
| Interventions | DA: 24‐page booklet and worksheet on options, benefits and risks, test limitations, outcomes; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework) Comparator: standard pamphlet on prenatal testing |
|
| Outcomes | Primary outcomes: informed choice, decisional conflict Secondary outcomes: anxiety, depression, attitudes toward pregnancy, acceptability of the intervention, choice |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (p 3) |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers by an independent statistician; allocation concealment was achieved (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Due to the nature of the intervention, it was not possible to blind women, GP's or researchers" (p 3); unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results, p 4; Fig 1 ‐ flow diagram, p 5 |
| Selective reporting (reporting bias) | Low risk | Trial Registration ‐ The ADEPT trial was registered in the UK with Current Controlled Trials [ISRCTN22532458] and with the Australian Clinical Trials Registry (No: 012606000234516) (p 4) |
| Other bias | Low risk | Appears to be free of other potential biases (p 8); selection bias but was adjusted for in analysis |
Nassar 2007.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 102 + 98 women diagnosed with a breech presentation from 34 weeks gestation considering external cephalic version in Australia | |
| Interventions | DA: 24‐page booklet, 30‐minute audio‐CD and worksheet; clinical problem, outcome probability, explicit values clarification, opinions of others', guidance (Ottawa Decision Support Framework) Comparator: usual care counselling and information on the management of breech presentation |
|
| Outcomes | Primary outcomes: knowledge, decisional conflict, anxiety, satisfaction with the decision, Secondary outcomes: preferred role in decision making, preferred choice |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomly generated using computer and stratified by parity and center using random variable block sizes" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[P]articipants were randomized by telephoning a remote, central location" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Womens were not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up because of onset of labour or incomplete data forms (p 3). Baseline characteristics are included and equal. Minimum of 84 participants in each study group achieved; p 4 ‐ flow diagram |
| Selective reporting (reporting bias) | Low risk | ISRCTN14570598 |
| Other bias | Low risk | "Maternal characteristics and baseline measures of cognitive and affective outcomes were comparable between groups" (p 3 Results, Table 1) "Blinding clinicians and employment of a research midwife to interact with women" (p 6) |
Oakley 2006.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 16 + 17 postmenopausal women with osteoporosis considering treatment options to prevent further bone loss in the UK | |
| Interventions | DA: audiotape booklet on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinions, guidance (Ottawa Decision Support Framework) Comparator: usual care | |
| Outcomes | Satisfaction with information, decisional conflict (intervention group only), improvement in adherence | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Group allocation was done by a third party, unconnected to the study and blinded to the identity of the patients (p 1) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear blinding, some outcomes were assessed by open‐ended questions, do not know whether this contributes to risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample characteristics not included; baseline satisfaction score included. "No evaluation was carried out to determine the reasons for non‐participation" (p 2) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No baseline characteristics (p 2). Only 16 patients in intervention group and 17 in control group; small sample size. |
Ozanne 2007.
| Methods | Randomized to decision aid + standard counselling vs usual care (standard counselling) | |
| Participants | 15 + 15 women considering breast cancer prevention in the USA | |
| Interventions | DA (in consultation): interactive computer decision aid on options outcomes, outcome probability Comparator: standard counselling | |
| Outcomes | Primary outcomes: consultation length Secondary outcomes: knowledge, decisional conflict, satisfaction with the decision, acceptability of the decision aid, physician satisfaction with the consultation |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomized evenly between groups; no information provided about generation (p 149) |
| Allocation concealment (selection bias) | Unclear risk | No information provided (p 149) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Demographic data included; reasons for attrition mentioned |
| Selective reporting (reporting bias) | Unclear risk | No reference to study protocol |
| Other bias | Unclear risk | Small sample size, does not say how many physicians participated in study, mentions that there were observed changes in physician behaviour (based on doing both intervention and control) |
Partin 2004.
| Methods | Randomized to decision aid with others' opinions vs decision aid without others' opinions vs usual care | |
| Participants | 384 + 384 + 384 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog video on options' outcomes, clinical problem, outcome probability, others' opinions Comparator 1: pamphlet on options' outcomes, clinical problem, outcome probability Comparator 2: usual care | |
| Outcomes | Primary outcomes: knowledge Secondary outcomes: preferred option, help with making a decision, decisional conflict |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated algorithm (p 2) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "[P]roviders were blinded to the fact that their patients were participating in a trial" "coordinator did not have direct contact with subjects" (p 5) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[F]ollow‐up interviewers blinded, statisticians were not". Outcomes were objectively measured and not subjective to to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 2); reasons for attrition mentioned and participants balanced across study groups. Sample characteristics included |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
Pignone 2000.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 125 + 124 adults considering colon cancer screening in the USA | |
| Interventions | DA: video of options' outcomes, clinical problem, others' opinion Comparator: video on car safety | |
| Outcomes | Primary outcome: uptake of options | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputerized random number generator" (p 2, Methods, Group assignment) |
| Allocation concealment (selection bias) | Low risk | "[R]andomization was performed centrally and was not balanced among centers. Assignments were placed in sealed, opaque, sequentially numbered envelopes and were distributed to the three sites" (p 2, Methods, Group assignment) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The providers and staff were not blinded to intervention status" "3 to 6 months after, different RA blinded to participant intervention examined clinic records" (p 2) Does not mention whether patients were blinded; unclear if lack of blinding contributed to potential risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A different research assistant who was blinded to participants' intervention status examined participants' clinic records in a standardized and validated manner to determine whether colon cancer screening tests were actually completed within 3 months of the index visit. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Because of an administrative error, 18 controls did not complete the second and third questionnaires (p 4). |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not mentioned |
| Other bias | Low risk | Baseline characteristics similar, appear to be no other potential sources of biases. Minimized bias from repeated measurements by administering the same questionnaires to the intervention and control participants |
Protheroe 2007.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 60 + 56 women considering treatment options for menorrhagia in the UK | |
| Interventions | DA: interactive computerized DA on options' outcomes, clinical problem, outcome probability, explicit values clarification, guidance Comparator: information leaflet | |
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge, anxiety, condition specific health outcomes, treatment preference, undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization, stratified by practice and minimized according to age (p 2, Methods) |
| Allocation concealment (selection bias) | Unclear risk | Random allocation was concealed from the individual who was making judgments of eligibility, but the method of concealment was not stated (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig 6 flow diagram (p 5); baseline data/characteristics included and balanced (p 4) |
| Selective reporting (reporting bias) | Low risk | ISRCTN72253427 |
| Other bias | Low risk | Appears to be free of other potential biases |
Rubel 2010.
| Methods | Randomized to pretest + decision aid + post‐test vs decision aid + post‐test vs pretest + posttest vs posttest | |
| Participants | 50 + 50 + 50 + 50 men considering prostate cancer screening in the USA | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + pretest and post‐test Comparator : booklet on options' outcomes, clinical problem, outcome probabilities, others' opinions + post‐test Comparator: pretest + post‐test Comparator: post‐test |
|
| Outcomes | Knowledge (pre, post‐DA), decisional anxiety (post‐DA), decisional conflict (post‐DA), participation in decision making (pre, post‐DA), schema for PSA testing (pre, post‐DA), perception of quality and interpretation of recommendation (post‐DA) | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronically generated random number sequence (p 309, Study design section) |
| Allocation concealment (selection bias) | Low risk | They were given sealed, sequentially numbered packets (p 309, Study design section) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding, but the outcomes were objectively measured and not subject to interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol followed CONSORT checklist (p 310, Study design section) |
| Other bias | Low risk | Appears to be free of other potential biases |
Ruffin 2007.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 87 + 87 community dwelling adults not previously screened for CRC in the USA | |
| Interventions | DA: interactive website with information on options' outcomes, clinical problem, outcome probability, explicit values clarification, others' opinion, guidance Comparator: non‐interactive website with information on clinical problem |
|
| Outcomes | Primary outcome: uptake of option | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A block randomisation process programmed by the study computer support staff and verified by a statistician was used including two strata, race and gender" (p 3) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators, data collectors, data entry, and data analyst were all blinded to study arm assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Sawka 2012.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 37 + 37 individuals with early‐stage papillary thyroid cancer | |
| Interventions | DA: web‐based decision aid with clinical problem, options' outcomes, outcome probabilities, guidance, printout summary Comparator: usual care (consultation with a specialized head and neck surgeon, and with 1 or more medical specialist). |
|
| Outcomes | Primary outcomes: knowledge (baseline and immediately post intervention) Secondary outcomes: decisional conflict, undecided, treatment decision (baseline, immediately post intervention, 6 to12 months), individual primarily responsible for the treatment decision (6 to 12 months) |
|
| Notes | Trial registration: NCT01083550 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central computerized randomization in a 1:1 ratio was performed at a patient level by using variable block sizes of 2 and 4 (allocation designed by a study statistician)" (p 2908) |
| Allocation concealment (selection bias) | Low risk | "Before the random assignment/testing visit, neither the participant, study staff, investigators, nor treating physicians were aware of the allocation, because it had not yet been assigned" (p 2908) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "There was no blinding of participants, study staff, or treating physicians after random assignment was completed" (p 2908), yet it is unlikely that the outcomes are affected by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "There was no blinding of participants, study staff, or treating physicians after random assignment was completed. However, the statistician was blinded to the allocation of groups at the time of data analysis." (p 2908) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Authors state the trial is registered, but no link to trial number |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Schroy 2011.
| Methods | Randomized to detailed vs simple decision aid vs control | |
| Participants | 223 + 212 + 231 average‐risk patients considering CRC screening in the USA | |
| Interventions | Detailed DA: CRC risk assessment + web‐based interactive audio‐visual DA on options' outcomes, clinical problem, outcome probabilities, others' opinion and guidance Comparator 1: web‐based decision aid only Comparator 2: usual care using pamphlet |
|
| Outcomes | Knowledge (pre and post‐DA), satisfaction with decision making process (pre and post‐DA), preferred choice (pre and post‐DA) | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of randomization process |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Providers were not blinded, subjective outcomes such as satisfaction with decision‐making process could have been affected, unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors not blinded but outcome measures not believed to be influenced by it |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No data appears to be missing |
| Selective reporting (reporting bias) | Unclear risk | No mention of examination of selective outcome reporting or study protocol |
| Other bias | Low risk | Appears to be free of other sources of bias |
Schwalm 2012.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 76 + 74 patients undergoing coronary angiography | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict Secondary outcomes: knowledge, risk perception, value congruent with chosen option |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized random number generator (p 261, Study design) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes (p 261, Study design) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients and physicians were not blinded to the allocation (p 261, Study design) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear if DCS score assessed by unblinded individuals, but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Did not seem to have incomplete data |
| Selective reporting (reporting bias) | Low risk | Protocol is available |
| Other bias | Low risk | Appeared to be free of other biases |
Schwartz 2001.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 181 + 190 Ashkenazi Jewish women considering genetic testing in the USA | |
| Interventions | DA: 16‐page booklet on genetic testing with options' outcomes, clinical problem Comparator: general information on breast cancer, Understanding Breast Changes: A Health Guide for all Women, published by the National Cancer Institute | |
| Outcomes | Primary outcome: preferred option Secondary outcomes: knowledge, accurate risk perceptions |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (p 3) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High retention rate, baseline data and reasons for lost to follow‐up were provided (p 2, Participants section) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Schwartz 2009a.
| Methods | Randomized to decision aid + genetic counselling vs genetic counselling alone | |
| Participants | 100 + 114 women considering prophylactic mastectomy for being BRCA1/2 mutation carriers in the USA | |
| Interventions | DA: CD‐Rom on options' outcomes, clinical problem, risk communication with individually tailored risk graphs, explicit values clarification, others' opinion; guidance/counselling ‐ genetic counselling as usual care (Ottawa Decision Support Framework) Comparator: genetic counselling on benefits and risks of testing, clinical problem (risk assessment, cancer risks associated with mutations, process of testing and interpretation of results) plus written letter outlining all guidelines and recommendations |
|
| Outcomes | Primary outcomes: decisional conflict, satisfaction with decision, actual choice (risk reduction mastectomy) Secondary outcomes: remaining undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized via computer‐generated random number in a 1:1 ratio (p 3, Procedure) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fig. 1 ‐ flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other sources of bias (p 8) "when variable for not watching DA cd was considered in multivariate models, the results did not change substantively (data not shown)" |
Sheridan 2006.
| Methods | Randomized to decision aid vs usual care (list of risk factors) | |
| Participants | 49 + 38 adults with no history of cardiovascular disease in the USA | |
| Interventions | DA: computerized decision aid on options' outcomes, outcome probabilities Comparator: list of CHD risk factors to present to doctor | |
| Outcomes | Patient‐practitioner communication (e.g. discussion with doctor, specific plan to reduce risk discussed with doctor) | |
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputerized random number generator" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[S]ealed in security envelopes" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded but the doctors who saw both groups were not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcome was patient reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results (p 5); Flow diagram (p 10); Baseline characteristics/data included |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov NCT00315978 |
| Other bias | Low risk | Appears to have no other potential risk of bias |
Sheridan 2011.
| Methods | Randomized to decision aid + tailored messages vs usual care | |
| Participants | 81 + 79 patients with moderate or high risk for CHD considering CHD prevention strategies in the USA | |
| Interventions | DA: web‐based decision aid on options' outcomes, clinical problem, outcome probabilities, explicit values clarification and guidance Comparator: usual care using computer programme |
|
| Outcomes | Preferred choice (post‐DA), adherence Other outcomes (Sheridan 2014): patient‐provider communication (post‐DA), patient participation (post‐DA), patients perceptions of discussions and the health care visit (post‐DA), preferred choice (baseline and post‐DA) (data from 81 +79 patients). |
|
| Notes | Primary outcome was not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised by study staff who accessed an online randomised schedule" (p 2). Sequence generation method not stated |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised by study staff who accessed an online randomised schedule" (p 2). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients blinded and physicians unblinded but objective outcomes are not likely affected by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes deemed objective therefore lack of blinding did not influence assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be no missing data |
| Selective reporting (reporting bias) | Low risk | Protocol made available |
| Other bias | Low risk | Appears to be free of other sources of bias |
Shorten 2005.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 85 + 84 pregnant women who have experienced previous cesarean section considering birthing options in Australia | |
| Interventions | DA: decision aid booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (Ottawa Decision Support Framework) Comparator: usual care | |
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: preferred option, help with making a decision |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomized generation (p 3, Procedure) |
| Allocation concealment (selection bias) | Low risk | "[O]paque envelopes containing a random allocation for each participant code number" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants/midwives/doctors were blinded to patients' allocation. However, women who used the decision aid as specified and in a process of consultation with their midwife or doctor would have negated the blinding of their clinicians, and perhaps of the women themselves. For the intervention group, this may have affected the level and type of information exchanged between them and their caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 women were lost to follow‐up from the intervention group and 18 from the control group (no reasons listed) (p 4, Results) |
| Selective reporting (reporting bias) | Low risk | Reference to published protocol |
| Other bias | Low risk | Appears to be free of other potential biases |
Shourie 2013.
| Methods | Cluster‐randomized controlled trial of GP practices to web‐based MMR DA + usual care, MMR leaflet + usual care, versus usual care | |
| Participants | 50 + 93 + 77 parents' of children facing their first dose MMR vaccination | |
| Interventions | Web‐based DA: clinical problem, options' outcomes, explicit values clarification, guidance MMR leaflet: Health Scotland leaflet, 'MMR: your questions answered' Comparator: usual care |
|
| Outcomes | Primary outcomes: decisional conflict (baseline and 2 weeks postintervention) Secondary outcomes: choice uptake of first dose MMR (when child was 15 months), knowledge (baseline and 2 weeks; results not provided), MMR immunization cognitions (baseline and 2 weeks post; results not provided), immunization trade‐off beliefs (baseline and 2 weeks post; results not provided), anxiety (baseline and 2 weeks post; results not provided), use of the intervention (baseline and 2 weeks post) |
|
| Notes | Trial registration: UK Clinical Research Network ‐ UKCRN ID 4811 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation using a computer‐generated random list allocated GP practices on a 1:1:1 basis" (p 3) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher who had no contact with participants generated the allocation sequence and assigned the GP practices to their allocated arm" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "On receipt of the completed baseline questionnaire and consent form, the appropriate intervention was delivered. At this point the researchers and participants were no longer blind to allocation" (p 3). We don't know if receiving the intervention had an effect on the ultimate decision that was made. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data assessment does not depend on the assessor. It is an objective questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing primary outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol registered. Primary outcome reported as stated. Secondary outcomes are not reported (p 3). |
| Other bias | Unclear risk | Difference in allocation to groups (50 + 93 + 77). Unclear what effect this difference had on the results. |
Smith 2010.
| Methods | Randomized to detailed vs simple decision aid vs usual care | |
| Participants | 196 + 188 + 188 socioeconomically disadvantaged participants diagnosed with average or slightly above average risk of bowel cancer considering bowel cancer screening in Australia | |
| Interventions | DA: booklet + DVD + worksheet + question prompt list on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary) Comparator: booklet + DVD + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making the decision; worksheet; encourages patients to communicate with practitioners by asking questions; summary) Comparator: usual care using standard information booklet |
|
| Outcomes | Primary outcomes: values congruent with chosen option (post‐DA), participation in decision making (pre, post‐DA) Secondary outcomes: knowledge (pre, post‐DA), attitude, actual choice (post‐DA), decisional conflict (post‐DA), decision satisfaction (post‐DA), confidence in decision making (post‐DA), general anxiety (post‐DA), worry about developing bowel cancer (pre, post‐DA), risk perception Other outcomes (Smith 2014): screening participation (357 + 173 participants) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants who verbally consented to take part were then randomised to one of the three groups using random permutated blocks of size 6 and 9 for each sex stratum" (p 3, Participants and recruitment section) |
| Allocation concealment (selection bias) | Low risk | Central allocation; "interviewers responsible for recruiting participants were not aware of the randomization sequence or allocation and therefore did not know which intervention respondents would receive" (p 3, Participants and recruitment section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "It was not possible for the reviewers to be blinded to the group allocation. However, all questions used standardised wording with pre‐coded responses and were asked within a supervised environment, where interviewer performances were regularly monitored to ensure scripts were read as written" (p 3, Outcome measures section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "[A]nalyses were by intention to treat and carried out blinded to intervention" (p 5, Statistical analysis section); outcomes measured were not subject to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Explanation for the missing data reported at base of tables |
| Selective reporting (reporting bias) | Low risk | Study protocol available (ClinicalTrials.gov NCT00765869 and Australian New Zealand Clinical Trials Registry 12608000011381) |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Stacey 2014a.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 71 + 71 adults diagnosed with knee osteoarthritis considering joint replacement in Canada | |
| Interventions | DA: DVD + booklet + worksheet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, others' opinion, guidance (1 page summary for the surgeon) Comparator: usual care |
|
| Outcomes | Primary outcomes: feasibility (including recruitment, data collection), preliminary effectiveness Secondary outcomes: knowledge (post‐DA, pre‐surgeon consult), informed values‐congruent with chosen option (post‐DA, pre‐surgeon consult), uptake of chosen option at 1 year; decisional conflict (SURE test), preparation for decision making (4 items), wait times |
|
| Notes | Trial registration: NCT00743951 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation schedule was computer‐generated centrally by a statistician using a permuted block design with randomly varying block lengths of 4, 6, or 8." (p 3) |
| Allocation concealment (selection bias) | Low risk | "Allocations were concealed in numbered opaque sealed envelopes" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients were not informed of the intervention characteristics" (p 3) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Although the research assistant was not blinded to group allocation, study outcomes for effectiveness were objective and obtained from clinic data (e.g. date of surgery or wait list status)" (p 3). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Protocol registered on ClinicalTrials.gov |
| Other bias | Low risk | Appears to be free of other potential sources of bias |
Steckelberg 2011.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 785 + 792 patients with no CRC history considering CRC screening in Germany | |
| Interventions | DA: brochure on options' outcomes, clinical problem, and outcome probabilities Comparator: usual care using pamphlet |
|
| Outcomes | Primary outcomes: values congruent with chosen option (post‐DA) Secondary outcomes: knowledge (post‐DA), combination of actual and planned uptake (post‐DA), risk perception |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence (p 2, Randomization and blinding) |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed. Identity numbers were independent of allocation, and study members did not have access to the data. (p 2, Randomization and blinding) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial staff who sent out questionnaires and reminders were not aware of study arm, unclear if participants were blinded (p 2, Randomization and blinding) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial staff and statistician who entered data were blinded (p 2, Randomization and blinding) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% missing one or both questionnaires in intervention group vs 9.2% in control; judged to have low impact on study outcome (p 2) |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Unclear risk | Participants who completed the trial do not add up |
Taylor 2006.
| Methods | Randomized to print DA versus video DA versus wait list control | |
| Participants | 98 + 95 + 92 African American men with no history of prostate cancer to consider prostate cancer screening | |
| Interventions | Print DA: clinical problem; outcome probabilities; guidance (list of questions to ask at next appointment); others' opinions Video DA: clinical problem; others' opinions Wait list comparator: no information provided until 1 month postrandomization (baseline assessment for this group coincided with 1‐month assessment of print and video arms) |
|
| Outcomes | Prostate cancer screening intention (baseline and 1 month; not reported), prostate screening uptake (1 year; not included because wait list received intervention before 1 year) process variables including use and perception of the intervention materials (1 month), prostate cancer knowledge (baseline and 1 month post), decisional conflict (baseline and 1 month post), satisfaction with screening decision (baseline and 1 month post) | |
| Notes | No primary outcome reported; not found in trials registry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information related to random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to judge blinding; however, participants were requested to not share intervention materials with others to prevent contamination between groups (p 2180) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Does not appear to be missing any outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol registered or published |
| Other bias | Unclear risk | "All participants were mailed $25 for their participation following completion of the 1‐month interview" (p 2181) "Men who reported that they had not yet had a chance to read/watch the materials were given an additional week to do so and called again to complete the follow‐up assessment" (p 2181) |
Thomson 2007.
| Methods | Randomized to decision aid vs usual care by clinical guidelines | |
| Participants | 69 + 67 patients with atrial fibrillation considering treatment options in the UK | |
| Interventions | DA (in consultation): computerized decision on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance/coaching by physician Comparator: guidelines applied as direct advice |
|
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: anxiety, knowledge, resource use, choice, health outcomes (stroke, transient ischaemic attack, bleeding events) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[E]lectronically‐generated random permuted blocks via a web‐based randomisation service" (p 2, Recruitment and randomization) |
| Allocation concealment (selection bias) | Low risk | "[E]lectronically‐generated random permuted blocks via a web‐based randomisation service" (p 2, Recruitment and randomization) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Physicians were blinded. Unclear if patients are blinded and how that may affect the outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See flow diagram |
| Selective reporting (reporting bias) | Low risk | ISRCTN24808514 |
| Other bias | Low risk | Baseline characteristics similar, sample size similar, not stopped early |
Trevena 2008.
| Methods | Randomized to decision aid vs usual care by consumer guidelines | |
| Participants | 157 + 157 patients not previously screened for colorectal cancer in Australia | |
| Interventions | DA: age‐gender‐family history specific DA booklet with information on options, outcome probabilities, explicit values clarification, guidance (personal worksheet with steps in decision making) (Theory of planned behaviour) Comparator: consumer guidelines recommending faecal occult blood testing |
|
| Outcomes | Primary outcome: informed choice Secondary outcomes: knowledge, values, screening intention (choice); test uptake, anxiety, acceptability of the intervention, satisfaction with the decision |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequential ID numbers were randomly assigned by computer program to DA or Guidelines (G) in blocks of four" (p 3) |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed via the password‐protected program" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded to the intervention type ‐ not sure about GPs |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers were blinded to allocation for all telephone interviews, outcomes were objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics included (p 3). Fig 2 flow chart (p 5). Reasons for loss to follow‐up not mentioned |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov ‐ NCT00148226 |
| Other bias | Low risk | Appears to be free of other potential biases |
Van Peperstraten 2010.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 152 + 156 infertile women on wait list for in vitro fertilization in the Netherlands | |
| Interventions | DA: self‐administered booklet on options' outcomes, clinical problem, outcome probabilities, explicit values clarification, guidance (step‐by‐step process for making decision, worksheet with questions relevant to decision‐making process; 1 or more questions that asked patients to clarify their preferences; summary to be shared with practitioner), coaching (by trained in vitro fertilization nurse) + standard in vitro fertilization care Comparator: standard in vitro fertilization care, including a session in which the number of embryos transferred was discussed |
|
| Outcomes | Primary outcomes: actual choice (postintervention and consult) Secondary outcomes: knowledge (pre, post‐DA and consult), empowerment (pre, post‐DA and consult), participation in decision making, decisional conflict (post‐DA and consult), levels of anxiety (pre, post‐DA and consult), depression (pre, post‐DA and consult), cost evaluation of empowerment strategy (post‐DA and consult), condition‐specific health outcomes (pregnancies) (post‐DA and consult) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list (p 2, Methods section) |
| Allocation concealment (selection bias) | Low risk | Central allocation (p 2, Methods section) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Because of the nature of the intervention it was not possible to blind the participants or in vitro fertilisation doctors to the allocation. Participation in our trial did not change the normal in vitro routine." (p 2, Methods section) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes assessed were not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There are categories in each column of table 1 (p 3) where the denominators do not match the number of people in the group and no reason was given to explain why this would be or if this affects the study |
| Selective reporting (reporting bias) | Low risk | Outcomes same as those registered with ClinicalTrials.gov |
| Other bias | Low risk | The study appear to be free of other sources of bias |
Vandemheen 2009.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 70 + 79 patients with cystic fibrosis considering referral for lung transplantation in Canada | |
| Interventions | DA: self‐administered booklet with clinical problem, outcome probability, explicit values clarification, guidance (Ottawa Decision Support Framework) Comparator: blank pages |
|
| Outcomes | Primary outcomes: knowledge, accurate risk perceptions, decisional conflict Secondary outcomes: preparation for decision making, choice, durability of decision, undecided |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[C]omputer‐generated random listing of two treatment allocations blocked in blocks of 2 or 4, stratified by site and infection status of Burkholderia cepacia" (p 2) |
| Allocation concealment (selection bias) | Low risk | Central allocation (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blinded RCT; patients and researchers were blinded but physicians were not because they were involved with patients before being randomized. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff, who were blinded to treatment allocation, telephoned each patient and had them complete a follow‐up questionnaire; other outcomes reported are objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline characteristics included (Flow diagram, p 2) |
| Selective reporting (reporting bias) | Low risk | Clinical trial registered with www.clinicaltrials.gov (NCT00345449) |
| Other bias | Low risk | Appears to be free of other potential biases |
Vodermaier 2009.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 74 + 78 women with breast cancer considering treatment options in Germany | |
| Interventions | DA: Decision board administered by research psychologists and booklet on options' outcomes, clinical problem, outcome probability Comparator: booklet on clinical problem | |
| Outcomes | Primary outcome: decisional conflict Secondary outcomes: choice, length of consultation, satisfaction with decision making, participation in decision making |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation after the patient gave written informed consent" "Random assignment was performed by means of numbered cards in envelopes" "stratified by age group" (p 2) |
| Allocation concealment (selection bias) | Low risk | "[N]umbered cards in envelopes" (p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded ‐ unclear if this would introduce bias to outcome assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram, p 5; baseline characteristics not included |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
Volk 1999.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 80 + 80 men considering PSA testing in the USA | |
| Interventions | DA: Health Dialog videotape and brochure on options' outcomes, clinical problem, outcome probability, others' opinion Comparator: usual care | |
| Outcomes | Primary outcomes: knowledge, preferred/uptake of option | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volk 1999 (primary study), p 3: "[r]andomization by permuted blocks" "Each block included the numbers 1 through 4"; Volk 2003, p 2, Methods: Randomization by permuted blocks was used to balance the number of subjects in each arm of the study. |
| Allocation concealment (selection bias) | Unclear risk | Volk 1999 (primary study): no information provided Volk 2003, p 2: "[d]etails of the study procedures, subjects, and 2‐week follow‐up results can be found elsewhere" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded to the treatment assignment, but the physicians were; therefore outcomes were unlikely to be biased. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Volk 1999 (primary study), p 2, Procedures: baseline values included. Volk 2003, p 4 Fig 1 ‐ flow diagram; baseline data not included |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Volk 1999 (primary study): appears to be free of other potential biases Volk 2003: appears to be free of other sources of bias |
Vuorma 2003.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 184 + 179 women considering treatment for menorrhagia in Finland | |
| Interventions | DA: booklet on options' outcomes, clinical problem, outcome probability Comparator: usual care | |
| Outcomes | Primary outcomes: uptake of option Secondary outcomes: knowledge, proportion remaining undecided, anxiety, satisfaction, health outcomes, use and cost of healthcare services |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Vuorma 2003 (primary study), p 2, Randomization: computer‐generated; done by a researcher who did not participate in the planning or concealment procedures "[D]one in STAKES, by researcher separately for each hospital in computer‐generated varying clusters"(p 2) Vuorma 2004: no information provided |
| Allocation concealment (selection bias) | Low risk | Vuorma 2003 (primary study), p 2 "sequentially numbered, opaque and sealed envelopes" Vuorma 2004, p 2 "sequentially numbered, opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, unclear if measurements could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff were not blinded but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vuorma 2003 (primary study): flow chart balanced. Reasons for non‐eligibility. "One women on HRT was randomized by mistake and included in analyses." Baseline characteristics included and balanced across groups (p 4‐5) Vuorma 2004, flow diagram (p 3) |
| Selective reporting (reporting bias) | Unclear risk | Vuorma 2003 (primary study): no mention of study protocol Vuorma 2004: no information provided |
| Other bias | Low risk | Vuorma 2003 (primary study), p 7: "increase in knowledge in both study groups, carry‐over effect; change in decision‐making process of intervention group may have altered physician's negotiation with patients" appears to be free of other potential biases Vuorma 2004, p 5: "comparison of the baseline characteristics presented elsewhere" In the pre‐trial group compared with the control group, there was a greater increase in the dimensions of physical role functioning and emotional role functioning of the RAND‐36 |
Watson 2006.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 475 + 522 men considering prostate cancer screening in the UK | |
| Interventions | DA: leaflet on options' outcomes, clinical problem, outcome probability Comparator: usual care |
|
| Outcomes | Primary outcomes: knowledge, screening intention, attitudes Secondary outcomes: preferred role in decision making |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andom numbers generated centrally by Stata v8.2" (p 3) |
| Allocation concealment (selection bias) | Low risk | "[R]andom numbers generated centrally by Stata v8.2" (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 2); reason for exclusion from analysis mentioned. Sample characteristics of risk included |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | "Adjustment for multiple testing was not accounted for and hence a degree of caution with interpretation is required, particularly in relation to findings with a P‐value close to 0.05" (p 3) |
Weymiller 2007.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 51 + 46 patients with type 2 diabetes in the USA | |
| Interventions | DA (in consultation): 1‐page decision aid options' outcomes, clinical problem, tailored outcome probability, guidance/coaching Comparator: booklet on cholesterol management | |
| Outcomes | Primary outcomes: knowledge, decisional conflict Secondary outcomes: consultation length, acceptability of the intervention, adherence, estimated personal risk, trust, patient participation (OPTION), choice |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence (p 2) Nannenga 2009: no information provided |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation sequence, unavailable to personnel enrolling patients. "[W]ith concealed allocation" (Abstract); "maintained allocation concealment" (p 5); randomized by concealed central allocation (Nannenga 2009, p 2) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded to the study objectives, providers and patients were naive to this study objective |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data analysts and statisticians blinded to allocation; intervention and outcomes; adequate blinding wherever possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram (p 3); reasons for attrition mentioned (p 4); baseline characteristics included; flow diagram Nannenga 2009, p 3: reasons for attrition mentioned and study groups balanced; baseline characteristics included |
| Selective reporting (reporting bias) | Low risk | ClinicalTrials.gov identifier: NCT00217061 |
| Other bias | Low risk | Enrollment of patients already receiving statin therapy and limited statin uptake decreased the precision of our results; results should best be interpreted as preliminary and requiring verification Nannenga 2009: appears to be free of other potential biases |
Whelan 2003.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 82 + 93 women with node negative breast cancer considering adjuvant chemotherapy in Canada | |
| Interventions | DA: decision board and booklet on options' outcomes, clinical problem, outcome probability, guidance/coaching Comparator: booklet on clinical problem | |
| Outcomes | Primary outcomes: knowledge, satisfaction of participant Secondary outcomes: preferred option, anxiety, accurate risk perceptions, participation in decision making |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Randomization, which was performed at a central location (p 3) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unable to blind participants in our trial for practical reasons, measures were taken to minimize bias in the design of the study and the assessment of outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram not included. "[O]ne patient excluded from analysis, determined by physician not to be candidate for chemotherapy" (p 4). Baseline data/characteristics included. |
| Selective reporting (reporting bias) | Unclear risk | Unclear if lack of blinding contributed to potential risk of bias |
| Other bias | Low risk | Appears to be free of other potential biases |
Whelan 2004.
| Methods | Cluster‐randomized to decision aid vs usual care | |
| Participants | 94 + 107 women with Stage 1 or 2 breast cancer considering surgery (cluster‐RCT with 27 surgeons randomized) in Canada | |
| Interventions | DA: decision board on options' outcomes, outcome probability, guidance/coaching Comparator: usual care | |
| Outcomes | Primary outcomes: preferred option, knowledge, decisional conflict, satisfaction Secondary outcomes: accurate risk perceptions, anxiety |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not specify how the sequence was generated; a paired cluster randomization process was used (p 2, Study design and procedures). |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned in a concealed fashion, but method of concealment was not stated (p 2, Study design and procedures) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "[C]hose cluster randomization method to avoid contamination that might have occurred if surgeons used decision board for some patients and not others" (p 6); unclear if this would introduce bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics not included; reasons given for loss of participants |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Appears to be free of other potential biases |
Williams 2013.
| Methods | Randomized to decision aid at home or in clinic versus usual care at home or in clinic | |
| Participants | 134 + 138 + 134 +137 men aged 40‐70 years with no history of prostate cancer who had pre‐registered for screening | |
| Interventions | DA: content adapted from the Centers for Disease Control and Prevention's PCS educational tool. Includes clinical problem, treatment options, outcome probabilities, explicit values clarification, others' stories, summary worksheet Comparator: information booklet. A 3‐page fact sheet requiring 5 minutes to read. Information presented in a Q&A format on who is recommended for testing, how to interpret results, and the limitations of testing |
|
| Outcomes | Knowledge, decisional conflict, screening outcomes, satisfaction with decision Outcomes assessed at baseline, 2 months, 13 months, except satisfaction with decision (2 months and 13 months) |
|
| Notes | No primary outcome reported; trial registration not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to judge random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to judge allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to judge blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to judge blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There does not appear to be any outcome data missing |
| Selective reporting (reporting bias) | Unclear risk | No registered or published protocol |
| Other bias | Low risk | Appears to be free of other potential biases |
Wolf 1996.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 103 + 102 men considering PSA testing in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probability, others' opinions Comparator: usual care (single sentence) | |
| Outcomes | Preferred option | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Wolf 1996 (primary study): no information provided Wolf 1998, p 2: "the methodology of the randomized trial has been reported previously" |
| Allocation concealment (selection bias) | Unclear risk | Wolf 1996 (primary study): no information provided Wolf 1998, p 2: "The methodology of the randomized trial has been reported previously" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Wolf 1996 (primary study), p 2: needed a minimum sample size of 150 participants, and was achieved with total sample size of 205. Reasons for attrition mentioned; baseline characteristics included Wolf 1998: no information provided except that methodology of the randomized trial and the content of the informational intervention reported previously (p 2). Baseline characteristics included; flow of participants not included |
| Selective reporting (reporting bias) | Unclear risk | No indication that the trial was registered in a central trials registry |
| Other bias | Low risk | Wolf 1996 (primary study): participant population had lower SES therefore external validity of the findings limited, but overall appears to be free of other potential biases Wolf 1998: appears to be free of other potential biases |
Wolf 2000.
| Methods | Randomized to decision aid vs usual care | |
| Participants | 266 + 133 elderly (≥ 65 years) considering CRC screening in the USA | |
| Interventions | DA: script of options' outcomes, clinical problem, outcome probabilities Comparator: usual care (5 sentences) | |
| Outcomes | Primary outcome: preferred option Secondary outcomes: accurate risk perceptions |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[P]atients were randomised" (p 2); does not indicate how |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline data not included (p 2, Results) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not mentioned |
| Other bias | Low risk | Appears to be free of other potential biases |
Wong 2006.
| Methods | Randomized to decision aid vs placebo control leaflet | |
| Participants | 162 + 164 women referred for pregnancy termination in the UK | |
| Interventions | DA: decision aid leaflet on options' outcomes, clinical problem, outcome probability, explicit values clarification Comparator: placebo leaflet on contraception use post pregnancy termination | |
| Outcomes | Primary outcomes: uptake of option, knowledge, decisional conflict, anxiety | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1 ratio, balanced block of 10"; "envelope preparation by drawing slips of paper labelled either control or intervention"; "the slip determined leaflet placed into envelope" (p 2) |
| Allocation concealment (selection bias) | Low risk | Consecutive numbered, opaque trial envelope (p 2, Methods) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear blinding but outcomes were objectively measured and not subjective to interpretation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Baseline characteristics not included (p 3); reasons for attrition and incompletion mentioned. |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | Appears to be free of other potential biases |
CHD: coronary heart disease; CRC: colorectal cancer; DA: decision aid; HPV: human papilloma virus; HRT: hormone replacement therapy; NSW: New South Wales; OA: osteoarthritis; PSA: prostate‐specific antigen; PTSD: post‐traumatic stress disorder; RCT: randomized controlled trial; SES: socioeconomic status.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadie 2009 | Study did not evaluate the decision aid (evaluated clinician use of the decision aid in one arm of a study only) |
| Adab 2003 | Hypothetical choice, not at a point of decision making |
| Al Saffar 2008 | Study not focused on making a choice; adhering to medications only |
| Alegría 2014 | Not a patient decision aid |
| Altiner 2007 | Not a patient decision aid |
| Anderson 2011 | Not a randomized controlled trial |
| Arimori 2006 | Not a patient decision aid (not including benefits and harms) |
| Armstrong 2005 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Arterburn 2013 | Not evaluating a patient decision aid |
| Au 2011 | Not a randomized controlled trial |
| Bakken 2014 | Not a patient decision aid; related to lifestyle choices |
| Becker 2009 | Hypothetical choice; not at the point of decision making |
| Belkora 2012 | Not a patient decision aid |
| Bellmunt 2010 | Not a patient decision aid |
| Bennett 2011 | Compares 3 versions of the same patient decision aid |
| Bieber 2006 | Study did not evaluate the patient decision aid (evaluated shared decision‐making process); not a patient decision aid |
| Branda 2013 | 2 patient decision aids with findings aggregated |
| Brenner 2014 | Not a patient decision aid |
| Breslin 2008 | Not a randomized controlled trial |
| Brown 2004 | Not focused on making a choice (no specific decision to be made) |
| Brundage 2001 | Not a randomized controlled trial |
| Burton 2007 | Not a patient decision aid (general patient education only) |
| Buzhardt 2011 | Not evaluating patient decision making |
| Campbell 2014 | Not evaluating a patient decision aid |
| Carling 2008 | Hypothetical choice, not at point of decision making |
| Causarano 2015 | Not a patient decision aid |
| Chadwick 1991 | Not a randomized controlled trial |
| Chan 2011 | Not a patient decision aid |
| Chewning 1999 | Not a randomized controlled trial |
| Chiew 2008 | Not a randomized controlled trial |
| Clouston 2014 | Not a patient decision aid |
| Col 2007 | Unable to ascertain characteristics of the patient decision aid. Additional information requested from author but not provided (e.g. values clarification) |
| Colella 2004 | Not a patient decision aid (describes model of care) |
| Costanza 2011 | Not a randomized controlled trial |
| Coulter 2003 | Not a randomized controlled trial (editorial) |
| Cox 2012 | Not a randomized controlled trial |
| Crang‐Svalenius 1996 | Not a randomized controlled trial |
| Davison 1999 | Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid |
| Davison 2007 | Not a patient decision aid |
| De Boer 2012 | Not a randomized controlled trial |
| De Haan 2013 | Not a randomized controlled trial of a patient decision aid |
| Deen 2012 | Not a patient decision aid |
| Deinzer 2009 | Not a patient decision aid |
| Denig 2014 | not a patient decision aid |
| Deschamps 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Deyo 2000 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Diefenbach 2012 | Not a patient decision aid |
| Dobke 2008 | Not focused on making a choice |
| Dodin 2001 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Donovan 2012 | Does not report results of the randomized controlled trial; descriptive article offering techniques of provision of information. |
| Driscoll 2008 | Not a patient decision aid |
| Dunn 1998 | Quasi‐RCT: randomization was by days of the week |
| Eaton 2011 | Not a decision aid (no decision support) |
| Eden 2009 | Hypothetical choice, not at point of decision making |
| Eden 2014 | The educational brochure (control group) provided information about the options, benefits, and harms making it a simple patient decision aid |
| Eden 2015 | Not a treatment or screening decision |
| Edwards 2012 | Hypothetical choice, not a randomized controlled trial |
| El‐Jawahri 2010 | End of life decision |
| Ellison 2008 | Not a randomized controlled trial (Quasi‐experimental design); unclear whether at point of decision making |
| Elwyn 2004 | No difference in intervention between arms; risk communication did not have values clarification |
| Emery 2007 | Not a patient decision aid |
| Emmett 2007 | Not a randomized controlled trial |
| Feldman‐Stewart 2006 | Hypothetical choice, not at point of decision making |
| Feldman‐Stewart 2012 | Same patient decision aid with vs without values clarification |
| Fiks 2013a | Not patient decision making (uptake of vaccine) |
| Flood 1996 | Non‐randomized allocation; wait list control |
| Francis 2009 | Not a patient decision aid |
| Fraval 2015 | Not a patient decision aid; general education material to obtain informed consent for surgery |
| Frosch 2001 | Not a randomized controlled trial |
| Frosch 2003 | Same decision aid delivered on the Internet versus on DVD plus booklet |
| Frosch 2008b | Not a randomized controlled trial |
| Frosch 2011 | Not a patient decision aid |
| Frost 2009 | Qualitative study for an included RCT |
| Fujiwara 2015 | Not a patient decision aid and aims to increase screening rates |
| Garvelink 2013 | Hypothetical decision |
| Genz 2012 | Not a patient decision aid |
| Giordano 2014 | Not a patient decision aid |
| Goel 2001 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Graham 2000 | Not a patient decision aid (general information) |
| Gray 2009 | Hypothetical choice, not at the point of decision making |
| Green 2001b | Not a patient decision aid (educational intervention) |
| Green 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Greenfield 1985 | Not focused on making a choice (intervention to increase patient involvement in care) |
| Griffith 2008a | Hypothetical choice, not at the point of decision making |
| Griffith 2008b | Not a randomized controlled trial |
| Gruppen 1994 | Not a patient decision aid |
| Gummersbach 2015 | Not a patient decision aid and a hypothetical decision |
| Hacking 2013 | Not a patient decision aid |
| Hall 2007 | Not about evaluating a patient decision aid |
| Hall 2011 | Not a patient decision aid |
| Hamann 2014 | not a patient decision aid |
| Harmsen 2014 | Not a patient decision aid |
| Harwood 2011 | Not a randomized controlled trial |
| Healton 1999 | Not a patient decision aid (education to promote compliance) |
| Henderson 2013 | Not a treatment or screening decision |
| Herrera 1983 | Quasi‐RCT: assigned to 1 of 2 alternating groups |
| Hess 2015 | Conjoint analysis for values clarification without information on options, pros and cons |
| Hewison 2001 | Not a patient decision aid; no values clarification |
| Heyn 2013 | Not a randomized controlled trial |
| Hickish 1995 | Not a randomized controlled trial (letter) |
| Hochlehnert 2006 | Not a patient decision aid (general information; no values clarification) |
| Hofbauer 2008 | Not a randomized controlled trial |
| Hoffman 2009 | Not a patient decision aid |
| Holbrook 2007 | Hypothetical choice, not at the point of decision making |
| Hollen 2013 | Not a treatment or screening decision |
| Holloway 2003 | Not focused on making a choice (promotes complying with a recommended option) |
| Holmes‐Rovner 2011 | Not a randomized controlled trial |
| Holt 2009 | Study does not evaluate a decision aid; evaluation of spiritual versus non‐spiritual framework |
| Hope 2010 | Same content |
| Huijbregts 2013 | Not a patient decision aid |
| Hunt 2005 | Not focused on making a choice (promotes complying with a recommended option) |
| Hunter 1999 | Not focused on making a choice (no specific decision) |
| Hunter 2005 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Huyghe 2009 | Hypothetical choice, not at point of decision making for all participants |
| Ilic 2008 | No difference in content of interventions ‐ testing mode of delivery |
| Isebaert 2007 | Not a randomized controlled trial (English paper published in 2008 Urologia Internationalis) |
| Jackson 2011 | Not a patient decision aid |
| Jerant 2007 | Not focused on making a choice ‐ adherence to screening |
| Jibaja‐Weiss 2006 | No comparison outcome data provided (only presents data for intervention group) |
| Joosten 2009 | Not a patient decision aid |
| Joosten 2011 | Not a patient decision aid |
| Jorm 2003 | Hypothetical choice, not at point of decision making ‐ community sample asked to evaluate information booklet on depression |
| Kakkilaya 2011 | Hypothetical choice, not at point of decision making |
| Kaplan 2014a | Not a patient decision aid |
| Kaplan 2014b | Not randomized controlled trial results; cross‐sectional analysis of baseline data |
| Kassan 2012 | Web arm only, not a randomized controlled trial |
| Kellar 2008 | Hypothetical choice, not at point of decision making |
| Kiatpongsan 2014 | No specific decision to be made and not a true randomized controlled trial |
| Kobelka 2009 | Not a randomized controlled trial; not a patient decision aid |
| Koelewijn‐van Loon 2009 | Lifestyle only |
| Krawczyk 2012 | Uptake of a recommended option |
| Kripalani 2007 | Not a patient decision aid |
| Krones 2008 | Not a patient decision aid ‐ no benefits and harms |
| Kuppermann 2009 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Kurian 2009 | Not a randomized controlled trial; not a patient decision aid |
| Köpke 2009 | Not a patient decision aid |
| Köpke 2014 | Not a patient decision aid |
| Labrecque 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| LaCroix 1999 | Inadequate comparison outcome data provided, secondary report of pilot study |
| Lairson 2011 | Not a patient decision aid (to increase uptake of screening) |
| Lalonde 2006 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Lancaster 2009 | Not a patient decision aid |
| Landrey 2013 | Not a patient decision aid |
| Lazcano Ponce 2000 | Not a patient decision aid (no values clarification) |
| Legare 2003 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Leung 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Levin 2011 | Not a patient decision aid |
| Lewis 2003 | Hypothetical choice, not at the point of decision making |
| Lewis 2012 | Uptake of a recommended option |
| Lopez‐Jornet 2012 | Not a patient decision aid/not at point of decision‐making |
| Lukens 2013 | Not a patient decision aid. Results in response to clinical vignettes (hypothetical scenarios) |
| Lurie 2011 | Not a randomized controlled trial (all patients received DA) |
| Maisels 1983 | Not a patient decision aid (no values clarification) |
| Mancini 2006 | Not about evaluating a patient decision aid |
| Manne 2009 | Not focused on making a choice (about adherence not decision making) |
| Manns 2005 | Not focused on making a choice (Promotes complying with a recommended option) |
| Markham 2003 | Not a patient decision aid (review of patient information pamphlets on pre‐operative fasting) |
| Martin 2012 | Hypothetical choice, not at the point of decision making |
| Maslin 1998 | Insufficient outcome data provided in publication; requested from author but not provided |
| Matlock 2014 | End of life |
| Matloff 2006 | Not a patient decision aid ‐ genetic counselling only |
| Mazur 1994 | Hypothetical choice, not at the point of decision making |
| McCaffery 2007 | Not a patient decision aid |
| McGinley 2002 | Not a patient decision aid (no values clarification) |
| McGowan 2008 | Not a patient decision aid |
| McInerney‐Leo 2004 | Not a patient decision aid (no risk/benefit information; no values clarification) |
| Mclaren 2012 | Not a patient decision aid; hypothetical choice, not at point of decision making |
| Meropol 2013 | Not a patient decision aid |
| Michie 1997 | Unable to ascertain whether intervention meets criteria (values clarification) to qualify as a patient decision aid; additional information requested but author was unable to provide the intervention. |
| Miller 2014a | No specific decision; related to increasing visits to healthcare provider |
| Miller 2014b | Aims to increase visits to healthcare providers; intervention targeted to partners |
| Mishel 2009 | Not a patient decision aid (information only) |
| Mohammad 2012 | Not a patient decision aid; presents only benefits, not harms |
| Molenaar 2001 | Not a randomized controlled trial |
| Mulley 2006 | Not a randomized controlled trial (editorial) |
| Myers 2005a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Myers 2005b | Not a randomized controlled trial (editorial) |
| Myers 2007 | Not a patient decision aid |
| Myers 2011 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Myers 2013 | Uptake of screening |
| Neubeck 2008 | Study protocol, does not appear to be patient decision aid |
| Newton 2001 | Not a randomized controlled trial |
| O'Cathain 2002 | Suite of 8 decision aids (not an efficacy trial) |
| O'Connor 1999a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| O'Connor 1996 | No patient decision aid ‐ framing effects |
| O'Connor 1998a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| O'Connor 2009a | Not a patient decision aid |
| O'Connor 2011 | Not a patient decision aid |
| Owens 2014A | Not an RCT; doctoral dissertation |
| Patanwala 2011 | Not a patient decision aid |
| Patel 2014 | Not an RCT |
| Pearson 2005 | Not a patient decision aid (focus on provision of information) |
| Peele 2005 | Not a patient decision aid (decision aid only supplies mortality risk information; no risk info; no values clarification) |
| Petty 2014 | Not a randomized controlled trial and not a patient decision aid |
| Philip 2010 | Not a randomized controlled trial, not a patient decision aid (promotes complying with a recommended option) |
| Phillips 1995 | Quasi‐RCT: alternating order based on patients' initial appointment sequence |
| Pignone 2013 | Not a patient decision aid; compared the effect of 3 different values clarification methods |
| Pinto 2008 | About clinical trial entry |
| Powers 2011 | Not a patient decision aid |
| Proctor 2006 | Not a patient decision aid (general patient education resource) |
| Prunty 2008 | About a lifestyle choice ‐ whether or not to have a child or have another child if I have multiple sclerosis |
| Ranta 2015 | Not a patient decision aid; intended to increase guideline adherence for transient ischaemic attack/stroke |
| Rapley 2006 | Not a randomized controlled trial |
| Raynes‐Greenow 2009 | No difference in intervention content; comparison of presentation formats; audio‐guided decision aid versus booklet only |
| Raynes‐Greenow 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Rimer 2001 | Not focused on making a choice (promotes complying with a recommended option) |
| Rimer 2002 | Not focused on making a choice (promotes complying with a recommended option) |
| Robinson 2013 | Not a patient decision aid |
| Ronda 2014 | Benefits or harms of self‐testing are not provided as information on the website; values clarification exercise asks users to qualify value statements as benefits or harms |
| Rostom 2002 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Roter 2012 | Not a patient decision aid |
| Rothert 1997 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Rovner 2004 | Not a randomized controlled trial |
| Rubinstein 2011 | Not a patient decision aid |
| Ruddy 2009 | Not a patient decision aid |
| Ruehlman 2012 | Not a patient decision aid |
| Ruland 2013 | No specific decision to be made |
| Ryser 2004 | Not focused on making a choice (promotes complying with a recommended option) |
| Sassen 2014 | Not a patient decision aid evaluation study; healthcare professionals were recruited, not patients |
| Saver 2007 | Not a patient decision aid ‐ general information; not a specific decision |
| Sawka 2011 | Not a randomized controlled trial |
| Scaffidi 2014 | Not an RCT |
| Schapira 2000 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Schapira 2007 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Schwartz 2009b | Hypothetical choice, not at the point of decision making |
| Sears 2007 | About do not resuscitate versus initiating cardiopulmonary resuscitation decision |
| Sequist 2011 | Not a patient decision aid (promotes complying with a recommended option) |
| Shah 2012 | Not a patient decision aid, lifestyle choices |
| Sheppard 2012 | Not a randomized controlled trial |
| Sheridan 2004 | Not a randomized controlled trial |
| Sheridan 2010 | Hypothetical choice, not at point of decision making |
| Sheridan 2012 | Not a patient decision aid ‐ no benefits and harms |
| Sherman 2014 | Not a randomized controlled trial |
| Shirai 2012 | Not a patient decision aid |
| Silver 2012 | Hypothetical choice, not at point of decision making |
| Siminoff 2006 | Not a patient decision aid (no discussion of harms) |
| Simon 2012a | Not a patient decision aid |
| Simon 2012b | Not a patient decision aid |
| Smith 2011a | No decision regarding treatment or screening to be made (decision regarding full disclosure) |
| Smith 2011b | Not a patient decision aid, not an RCT |
| Solberg 2010 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Sorenson 2004 | Not a randomized controlled trial |
| Sparano 2006 | Not a patient decision aid |
| Stalmeier 2009 | Not a randomized controlled trial (about instrument development) |
| Starosta 2015 | Not a patient decision aid ‐ benefits and harms of screening are missing. |
| Stein 2013 | End of life |
| Steiner 2003 | Not a patient decision aid (only effectiveness not cons of options; not at point of decision making) |
| Stephens 2008 | Not a randomized controlled trial |
| Stiggelbout 2008 | Not a patient decision aid |
| Stirling 2012 | Not a treatment or screening decision |
| Street 1995 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Street 1998 | Not focused on making a choice (promotes complying with a recommended option) |
| Sundaresan 2011 | Hypothetical choice, not at the point of decision making, not a randomized controlled trial |
| Tabak 1995 | Not a randomized controlled trial |
| Taylor 2013 | Not a patient decision aid ‐ benefits and harms of screening not included |
| Ten 2008 | Not a patient decision aid; about stopping medication use |
| Thomas 2013 | Not a patient decision aid |
| Thomson 2006 | Not a randomized controlled trial; not at point of decision making |
| Thornton 1995 | Unable to ascertain whether intervention meets criteria to qualify as a patient decision aid; additional information requested from author but not provided |
| Tiller 2006 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Tinsel 2013 | Not a patient decision aid |
| Tomko 2015 | Not a patient decision aid ‐ benefits and harms of screening are missing |
| Ukoli 2013 | Not an RCT |
| Valdez 2001 | Not a randomized controlled trial; not focused on making a choice (complying with a recommended option) |
| Van der Krieke 2013 | Not a patient decision aid, no benefits/harms |
| Van Roosmalen 2004 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Van Steenkiste 2008 | Not a randomized controlled trial |
| Van Til 2009 | Hypothetical choice, not at the point of decision making |
| Van Tol‐Geerdink 2013 | Not a randomized controlled trial; insufficient information to judge random sequence generation, allocation concealment, and blinding |
| Veroff 2012 | Not a patient decision aid |
| Volandes 2009 | Advanced care planning options |
| Volandes 2011 | Hypothetical choice, end‐of‐life decision |
| Volandes 2013 | Advanced care planning |
| Volk 2008 | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Von Wagner 2011 | Not a randomized controlled trial (commentary) |
| Wagner 1995 | Not a randomized controlled trial |
| Wakefield 2008a | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wakefield 2008b | Simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wakefield 2008c | simple versus detailed patient decision aid (excluded in update after 2014 publication) |
| Wallston 1991 | Not a patient decision aid ‐ patient preference study |
| Wang 2004 | Not a patient decision aid ‐ intent of intervention to facilitate genetic counselling process, no focused decision |
| Warner 2015 | Not a treatment or screening decision |
| Watts 2014 | Simple versus detailed patient decision aid |
| Welschen 2012 | Not a patient decision aid |
| Wennberg 2010 | Same decision aid in both groups |
| Westermann 2013 | Not a patient decision aid |
| Weymann 2015 | Patients not at the point of decision making |
| Wilhelm 2009 | Not a patient decision aid |
| Wilkes 2013 | Unable to ascertain characteristics of the patient decision aid. Additional information requested from author but not provided (e.g. values clarification) |
| Wilkie 2013 | Not treatment or screening decision |
| Wilkins 2006 | Not a randomized controlled trial |
| Willemsen 2006 | Lifestyle change |
| Williams‐Piehota 2008 | Not a randomized controlled trial |
| Williamson 2014 | Lifestyle decision ‐ not treatment or screening |
| Woltmann 2011 | Not a patient decision aid |
| Wroe 2005 | Not focused on making a choice ‐ promotes complying with a recommended option |
| Yee 2014 | Not a patient decision aid |
| Yun 2011 | End‐of‐life decision |
| Zajac 2012 | Hypothetical |
| Zapka 2004 | Not focused on making a choice ‐ promotes complying with a recommendation |
| Zikmund‐Fisher 2008 | No difference in intervention content ‐ comparison of presentation of probabilities |
| Zoffman 2012 | Not a randomized controlled trial, not a patient decision aid |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12615000523505.
| Trial name or title | The motherhood choices decision aid for women with rheumatoid arthritis increases knowledge and reduces decisional conflict: a randomized controlled study |
| Methods | RCT |
| Participants | 130 women diagnosed with rheumatoid arthritis and currently under the care of a rheumatologist |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, knowledge, anxiety, depression, self‐efficacy |
| Starting date | May 2015 |
| Contact information | Tanya Meade; School of Social Science and Psychology University of Western Sydney; Sydney, Australia |
| Notes | Trial #: ACTRN12615000523505 |
ACTRN12615000843550.
| Trial name or title | Evaluation of decision aids for parents about the benefits and harms of antibiotic use for coughs and colds in children |
| Methods | Pilot RCT |
| Participants | 108 adult parents or primary caregivers of a child |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Informed choice, knowledge, attitudes towards antibiotic use, intention to use antibiotic, decisional conflict, confidence in decision‐making, usability and accessibility of the written materials |
| Starting date | August 2015 |
| Contact information | Mr Peter D Coxeter; pcoxeter@bond.edu.au; Bond University, Queensland, Australia |
| Notes | ACTRN12615000843550 |
Al‐Itejawi 2015.
| Trial name or title | (Cost‐)effectiveness and implementation of a decision aid for patients with prostate cancer |
| Methods | Stepped wedge cluster RCT |
| Participants | Newly diagnosed adult participants with localized prostate cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Decisional conflict, quality of life, treatment preferences, participation in decision making, knowledge, patient‐provider communication |
| Starting date | May 2015 |
| Contact information | Hoda Al‐Itejawi; Afdeling Urologie, Amsterdam, the Netherlands |
| Notes | Trial #: NTR5177 |
Anderson 2014.
| Trial name or title | Shared decision making in the emergency department: Chest Pain Choice Trial (CPC) |
| Methods | RCT |
| Participants | Presenting to the emergency department with chest pain |
| Interventions | Chest Pain Choice decision aid vs usual care |
| Outcomes | Knowledge, patient engagement, decisional conflict, satisfaction, adverse events, admissions, healthcare utilization |
| Starting date | October 2013 |
| Contact information | Erik P Hess, Mayo Clinic |
| Notes | NCT01969240; verified September 2014, estimated study completion March 2016 |
Aslani 2014.
| Trial name or title | Computerized decision aid on mode of delivery |
| Methods | Cluster RCT |
| Participants | Pregnant Iranian women |
| Interventions | Computerized decision aid |
| Outcomes | Decisional conflict, knowledge |
| Starting date | Not reported |
| Contact information | Azam Aslani, Mashhad University, Iran |
| Notes | — |
Buhse 2013.
| Trial name or title | Efficacy of an evidence‐based informed shared decision making program for prevention of myocardial infarction in type 2 diabetes |
| Methods | RCT |
| Participants | 154 patients with type 2 diabetes |
| Interventions | Shared decision‐making programme consisting of a decision aid booklet and a curriculum for group counselling vs placebo counselling |
| Outcomes | Knowledge, sustainability of knowledge, achievement of individual treatment goals, achievement of treatment goals prioritized by individual patients, medication uptake |
| Starting date | March 2013 |
| Contact information | Matthias Lenz, University of Hamburg |
| Notes | ISRCTN84636255 |
Carroll 2012.
| Trial name or title | Development of and feasibility testing of decision support for patients who are candidates for an implantable defibrillator |
| Methods | RCT |
| Participants | Referred for consideration of an implantable cardioverter‐defibrillators (non‐cardiac resynchronization therapy) for a primary prevention indication |
| Interventions | Patient decision aid provided prior to the consultation with the physician, which provides a lay summary that outlines the facts, risks, benefits (including probabilities), specific to the option of an implantable defibrillator or the option of medical management vs usual care |
| Outcomes | Decision aid development and evaluation, decisional conflict and decision quality, sure test, reparation for decision‐making scale, medical outcomes trust short form (SF‐36v2) |
| Starting date | June 2012 |
| Contact information | Sandra Carroll, McMaster University |
| Notes | Trial #: NCT01876173 |
Chambers 2008.
| Trial name or title | ProsCan for Men: randomized controlled trial of a decision support intervention for men with localised prostate cancer |
| Methods | RCT |
| Participants | 700 men newly diagnosed with localized prostate cancer |
| Interventions | A tele‐based nurse delivered 5‐session decision support/psychosocial intervention vs usual care |
| Outcomes | Cancer threat appraisal; decision‐related distress and bother from treatment side effects; involvement in decision making; satisfaction with health care; heathcare utilization; use of healthcare resources; and a return to previous activities |
| Starting date | Not yet assessed |
| Contact information | Suzanne K Chambers, Griffith University |
| Notes | Trials #: ACTRN012607000233426 |
Coylewright 2012.
| Trial name or title | Shared decision making in patients with stable coronary artery disease: PCI Choice |
| Methods | RCT |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Starting date | — |
| Contact information | Megan Coylewright, Mayo Clinic |
| Notes | Upcoming RCT |
Cuypers 2015.
| Trial name or title | Prostate cancer patient‐centered care: impact of a treatment decision aid in a pragmatic, cluster randomized controlled trial |
| Methods | Pragmatic RCT |
| Participants | 400 men newly diagnosed with early stage prostate cancer |
| Interventions | Decision aid (online) vs usual care |
| Outcomes | Decisional conflict, decisional regret, treatment satisfaction, decision making role, knowledge, satisfaction with decision‐making process, preparation for decision‐making, health‐related quality of life, personality (anxiety, depression, optimism), skills measures (self‐efficacy, health literacy, numeracy) |
| Starting date | May 2014 |
| Contact information | M Cuypers; M.Cuypers@uvt.nl; Tilburg University Social and Behavioral Sciences Tilburg, the Netherlands |
| Notes | NTR4554 |
Den Ouden 2015.
| Trial name or title | Shared decision‐making in type 2 diabetes with a support decision tool that takes into account clinical factors, the intensity of treatment and patient preferences |
| Methods | Cluster RCT |
| Participants | 150 adults with type 2 diabetes mellitus for 8‐15 years |
| Interventions | Patient decision aid with training vs usual care |
| Outcomes | Achievement of diabetes‐specific health goals, satisfaction with treatment, quality of life, well‐being, coping, evidence of shared decision‐making |
| Starting date | March 2012 |
| Contact information | h.denouden@umcutrecht.nl; Henk den Ouden; Julius Cntre for Health Sciences and Primary Care, University Medical Centre, Utrecht, the Netherlands |
| Notes | Trial #: NCT02285881 |
Dirmaier 2013.
| Trial name or title | Tailored, dialogue‐based health communication application for patients with chronic low back pain |
| Methods | RCT |
| Participants | 414 patients with self‐reported chronic low back pain |
| Interventions | Web‐based interactive health communication application (IHCA) vs control (standard info) |
| Outcomes | Knowledge, patient empowerment, website usage, preparation for decision making, decisional conflict |
| Starting date | 2012 |
| Contact information | Martin Härter, University Medical Center Hamburg‐Eppendorf |
| Notes | International Clinical Trials Registry DRKS00003322 |
Geiger 2011.
| Trial name or title | Investigating a training supporting Shared Decision Making (IT'S SDM 2011): study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | 40 physicians that contribute a sequence of 4 medical consultations including a diagnostic or treatment decision |
| Interventions | A training curriculum for the doctors ‐ intend to stimulate efforts to involve their patients in the decision‐making process. |
| Outcomes | Physician‐patient communication, effect of SDM on perceived quality of the decision process and on the elaboration of the decision, decisional conflict |
| Starting date | Not yet assessed |
| Contact information | Friedemann Geiger, University Medical Center Schleswig ‐ Holstein |
| Notes | Trials #: ISRCTN78716079 |
Hersch 2014.
| Trial name or title | Effect of information about over detection of breast cancer on women's decision‐making about mammography screening |
| Methods | RCT |
| Participants | 970 women aged 48‐50 |
| Interventions | Intervention (evidence‐based information booklet including over detection, breast cancer mortality reduction and false positives) vs control information booklet (including mortality reduction and false positives only) |
| Outcomes | Knowledge, consistency between attitudes and intentions, decision conflict, confidence, regret, anxiety, perceived risk, quality of life |
| Starting date | June 2014 |
| Contact information | Kirsten McCaffery, University of Sydney |
| Notes | Australian New Zealand Clinical Trials Registry ACTRN12613001035718 |
Hess 2014.
| Trial name or title | Shared decision making in parents of children with head trauma: head CT choice |
| Methods | RCT |
| Participants | 1004 parent‐child dyad, seeking care for a child who had blunt trauma above the eyebrows and is positive for at least 1 PECARN clinical prediction rules |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, engagement in decision‐making process, decisional conflict, trust in the physician, satisfaction with the decision‐making process, choice, healthcare utilization 7‐days post ER visit, rate of clinically important traumatic brain injury |
| Starting date | April 2014 |
| Contact information | Erik Hess; Mayo Clinic; Rochester, MN |
| Notes | Trial #: NCT02063087 |
Jimbo 2012.
| Trial name or title | Decision aid to technologically enhance shared decision making |
| Methods | RCT |
| Participants | Patients who are not current with colorectal cancer screening |
| Interventions | Web based decision aid + interactive component (preferences and risk assessment) vs web based decision aid only |
| Outcomes | Uptake of screening on patient determinants/preference/intention before the patient‐physician encounter, and on shared decision making, concordance and patient intention during/after the patient‐physician encounter |
| Starting date | May 2012 |
| Contact information | Masahito Jimbo, University of Michigan |
| Notes | Trial # :NCT01514786; last updated December 2013, estimated study completion October 2014 |
Layton 2012.
| Trial name or title | Effects of a web‐based decision aid on African American men's prostate screening knowledge and behavior |
| Methods | — |
| Participants | 128 African American men |
| Interventions | — |
| Outcomes | — |
| Starting date | — |
| Contact information | Beverly Layton, Walden University |
| Notes | Unpublished thesis |
LeBlanc 2013.
| Trial name or title | Translating comparative effectiveness of depression medications into practice by comparing the depression medication choice decision aid to usual care: study protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | 300 patients |
| Interventions | Use of the Depression Medication Choice decision aid by patients and their primary care clinician during the clinical encounter vs usual care |
| Outcomes | Decisional conflict, knowledge, satisfaction, preference in decision making style, patient involvement in decision making, depression outcomes, medication adherence |
| Starting date | December 2011 |
| Contact information | Victor Montori, Mayo Clinic, USA |
| Notes | NCT01502891 |
Mann 2012.
| Trial name or title | Increasing efficacy of primary care‐based counselling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial |
| Methods | RCT |
| Participants | Primary care providers |
| Interventions | Using the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) system to enhance providers' effectiveness to counsel about lifestyle behaviour changes |
| Outcomes | Outcome measurements are designed to detect changes in patient behaviours that are most likely to result from the use of ADAPT tool: difference between intervention and control patients in the change in mean steps per day at baseline and after 6 months, and 6 month difference of differences in haemoglobin A1C and self‐reported diet between the 2 groups |
| Starting date | Not yet assessed |
| Contact information | Devin Mann, Boston University School of Medicine |
| Notes | Trial #: NCT01473654 |
NCT00813033.
| Trial name or title | Use of a patient decision aid for gastrologic endoscopy in a paediatric setting |
| Methods | Interventional efficacy study |
| Participants | 80 parents considering gastro‐endoscopy for child |
| Interventions | Not yet assessed |
| Outcomes | Knowledge, expectations of outcomes, clarity of values, decision, decision conflict |
| Starting date | December 2008 |
| Contact information | Nancy Neilan, Children's Mercy Hospital, Kansas City |
| Notes | Trials #: NCT00813033; completed March 2011 |
NCT01077037.
| Trial name or title | Shared decision making in the emergency department: the Chest Pain Choice Trial |
| Methods | RCT |
| Participants | 1500 adults admitted to the emergency department for chest pain, being considered by the treating clinical for admission for cardiac testing |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, healthcare utilization (rate of hospital admission, rate of cardiac testing, etc), patient engagement in decision‐making process, decisional conflict, trust in the physician, satisfaction with decision, safety (major adverse cardiac events within 30 days) |
| Starting date | October 2013 |
| Contact information | hess.erik@mayo.edu; Mayo Clinic, Rochester, Minnesota, USA |
| Notes | Trial #: NCT01969240 |
NCT01152294.
| Trial name or title | Measuring quality of decisions about treatment of menopausal symptoms |
| Methods | RCT |
| Participants | Patients talked with healthcare provider about ways to manage menopause or seriously considered taking medicine or supplement to manage menopause |
| Interventions | Decision aid (DVD/booklet) vs usual care |
| Outcomes | Knowledge, value concordance |
| Starting date | June 2010 |
| Contact information | Karen R Sepucha, Massachusetts General Hospital |
| Notes | NCT01152294; completed, study results on clinicaltrials.gov |
NCT01152307.
| Trial name or title | Measuring quality of decisions about treatment of depression |
| Methods | RCT |
| Participants | Patients that talked to a healthcare provider about starting or stopping a treatment (prescription medicine for depression or counselling) |
| Interventions | Decision aid (DVD/booklet) vs usual care |
| Outcomes | Knowledge, value concordance |
| Starting date | June 2010 |
| Contact information | Karen R Sepucha, Massachusetts General Hospital |
| Notes | NCT01152307; completed, study results on clinicaltrials.gov |
NCT01447186.
| Trial name or title | Informed decisions about lung cancer screening |
| Methods | RCT |
| Participants | 500 adults between 55 and 77 years olds who are currently smoking or quit within the past 15 years |
| Interventions | Patient decision aid vs standard educational information |
| Outcomes | Decisional conflict: value subscale and informed subscale |
| Starting date | March 2015 |
| Contact information | MD Anderson Cancer Center; USA |
| Notes | Trial #: NCT02286713 |
NCT01618097.
| Trial name or title | Evaluation of DVD and Internet decision aids for hip and knee osteoarthritis: focus on health literacy |
| Methods | RCT |
| Participants | Osteoarthritis patients |
| Interventions | DVD decision aid vs Internet‐based decision aid |
| Outcomes | Decisional conflict, decision self‐efficacy, knowledge |
| Starting date | January 2012 |
| Contact information | Kelli D Allen, Duke University |
| Notes | Trial #: NCT01618097; last updated March 2014, study completion date January 2014 |
NCT01713894.
| Trial name or title | Utility of a clinically relevant decision aid, for parents facing extremely premature delivery |
| Methods | RCT |
| Participants | 300 women who are receiving counselling at the limits of viability |
| Interventions | Decision aid vs usual care |
| Outcomes | Decisional conflict, knowledge |
| Starting date | May 2013 |
| Contact information | uguillen@christianacare.org; Ursula Guillen, Christiana Care Health Systems; University of Michigan |
| Notes | Trial # NCT01713894 |
NCT01771536.
| Trial name or title | Study to test use of a decision aid in a clinical visit to help patients choose a diabetes medication. Translating Information on Comparative Effectiveness Into Practice (TRICEP) |
| Methods | RCT |
| Participants | Type 2 diabetes mellitus patients |
| Interventions | Diabetes medication decision aid vs usual care |
| Outcomes | Patient satisfaction and knowledge. Physician adoption and satisfaction with the decision aid |
| Starting date | January 2011 |
| Contact information | Nilay D Shah, Mayo Clinic |
| Notes | NCT01293578; estimated completion date December 2014 |
NCT01851785.
| Trial name or title | Behavioral and social science research on understanding and reducing health disparities: African American preference for knee replacement: a patient‐centred intervention (ACTION) |
| Methods | RCT |
| Participants | African‐American participants referred to orthopaedic doctor with presence of knee OA |
| Interventions | Decision aid video + communication, skill‐building intervention vs educational programme (an NIH‐developed booklet) that summarizes how to live with knee OA but does not mention joint replacement |
| Outcomes | Recommendation and receipt of knee joint replacement |
| Starting date | July 2010 |
| Contact information | Said A Ibrahim, University of Pennsylvania |
| Notes | Trial #: NCT01851785; last verified May 2013, estimated completion date June 2015 |
NCT01941186.
| Trial name or title | A family centered intervention to promote optimal child development |
| Methods | RCT |
| Participants | 64 parent‐child dyad in which the child is aged 0‐36 months screening positive for developmental concern |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Evaluation by early intervention specialist, attitudes, knowledge, uncertainty, intervention acceptability, intervention feasibility |
| Starting date | December 2013 |
| Contact information | Children's Hospital of Philadelphia Philadelphia, PN, USA, 19104 |
| Notes | Trial #: NCT01941186 |
NCT01976325.
| Trial name or title | Incorporation of the 'Ottawa Malaria Decision Aid' into the pre‐travel consultation process |
| Methods | RCT |
| Participants | 100 adults attending a travel clinic before travelling to an area with known chloroquine‐resistant malaria |
| Interventions | Decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict, preparation for decision‐making, medication adherence |
| Starting date | January 2014 |
| Contact information | amccarthy@toh.on.ca; Anne E McCarthy; Ottawa Hospital Research Institute |
| Notes | Trial # NCT01976325 |
NCT02026102.
| Trial name or title | A pilot trial of patient decision aids for implantable cardioverter‐defibrillators (ICDs) |
| Methods | RCT |
| Participants | 60 patients with heart failure referred for primary prevention implantable cardioverter‐defibrillators |
| Interventions | Decision aid toolkit vs usual care |
| Outcomes | Intervention acceptability, decision quality (knowledge and values concordance), quality of life, depressive symptoms, health status, spiritual well‐being |
| Starting date | September 2014 |
| Contact information | amy.jenkins@ucdenver.edu; University of Colorado Hospital (UCH) |
| Notes | Trial #: NCT02026102 |
NCT02084290.
| Trial name or title | Evaluating a prediction tool and decision aid for patients with Crohn's disease |
| Methods | RCT |
| Participants | 300 adults with Crohn's disease |
| Interventions | Patient decision aid and SDM programme vs usual care |
| Outcomes | Preferred choice, actual choice, adherence, cost of care, remission, patient on steroids, surgeries, Crohn's disease related hospitalizations |
| Starting date | March 2014 |
| Contact information | corey.a.siegel@hitchcock.org; Corey A Siegel; Dartmouth‐Hitchcock Medical Center |
| Notes | Trial #: NCT02084290 |
NCT02110979.
| Trial name or title | Validation of a patient decision aid for type 2 diabetes |
| Methods | RCT |
| Participants | 200 type 2 diabetes patients |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict |
| Starting date | April 2014 |
| Contact information | EPI‐Q Inc, Oak Brook, IL, USA, 60523 www.epi‐q.com/our‐approach |
| Notes | Trial #: NCT02110979 |
NCT02145481.
| Trial name or title | Decisional quality for patients with stable coronary artery disease |
| Methods | RCT |
| Participants | 846 adults with stable coronary artery disease |
| Interventions | Patient decision aid vs standard education |
| Outcomes | Quality of the decision‐making process, knowledge, communication, involvement, treatment preferences |
| Starting date | May 2014 |
| Contact information | R. Adams Dudley; University of California, San Francisco |
| Notes | Trial # NCT02145481 |
NCT02198690.
| Trial name or title | Randomized trial of a mammography decision aid for women aged 75 and older |
| Methods | RCT |
| Participants | 550 women aged 75‐89 years |
| Interventions | Decision aid vs usual care |
| Outcomes | Receipt of mammography screening, acceptability, anxiety, decision‐making role, decisional conflict, home safety, home safety discussions, knowledge, preparation for decision‐making, screening discussions, screening intentions |
| Starting date | September 2014 |
| Contact information | Mara A Schonberg, MD, MPH; mschonbe@bidmc.harvard.edu; Beth Israel Deaconess Medical Center; Boston, MA, USA |
| Notes | NCT02198690 |
NCT02235571.
| Trial name or title | iChoose kidney decision aid for treatment options among end‐stage renal disease (ESRD) patients |
| Methods | RCT |
| Participants | 450 adults with end‐stage renal disease on dialysis for < 1 year and being evaluated for kidney transplant |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, evidence of shared decision‐making, access to transplant, treatment preferences |
| Starting date | September 2014 |
| Contact information | Rachel Patzer; Emory Transplant Center; Atlanta, GA, USA |
| Notes | Trial # NCT02235571 |
NCT02248974.
| Trial name or title | Development and user testing of a decision aid for left ventricular assist device (LVAD) placement |
| Methods | RCT |
| Participants | 144 adults who are candidates for a left ventricular assist device |
| Interventions | Patient decision aid vs. standard education |
| Outcomes | Knowledge, decisional conflict, control preferences scale, CollaboRATE score, perceived quality of care, satisfaction with decision‐making process, decisional regret, satisfaction with life, preparation for decision‐making, usability and acceptability of the intervention |
| Starting date | February 2014 |
| Contact information | Jennifer Blumenthal‐Barby; Baylor College of Medicine; Houston, TX |
| Notes | Trial #: NCT02248974 |
NCT02259699.
| Trial name or title | Ovarian cancer patient‐centered decision aid |
| Methods | RCT |
| Participants | 221 women with stage III optimally debulked advanced ovarian cancer |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Satisfaction with decision, evidence of shared decision‐making, quality of life, satisfaction with care and satisfaction with cancer treatment |
| Starting date | December 2014 |
| Contact information | lwenzel@uci.edu; Lari Wenzel; University of California, Irvine, USA |
| Notes | Trial #: NCT02259699 |
NCT02308592.
| Trial name or title | Patient decision aid for antidepressant use in pregnancy |
| Methods | RCT |
| Participants | 50 women aged 18 years or older planning a pregnancy or <30 weeks pregnant |
| Interventions | Patient decision aid vs standard resource sheet |
| Outcomes | depression, anxiety, decisional conflict, knowledge, intervention acceptability, choice, satisfaction with DA |
| Starting date | January 2015 |
| Contact information | simone.vigod@wchospital.ca Women's College Hospital, Toronto, Ontario, Canada |
| Notes | Trial #: NCT02308592 |
NCT02319525.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
NCT02326597.
| Trial name or title | Decision aid for therapeutic options in sickle cell disease |
| Methods | RCT |
| Participants | 120 individuals with sickle cell disease ages 8 to 80 years |
| Interventions | Decision aid vs usual care |
| Outcomes | Knowledge, self‐efficacy, decisional conflict, values, realistic expectations, preparation for decision‐making, choice predisposition, stage of decision‐making, decisional regret |
| Starting date | September 2014 |
| Contact information | diana.ross@emory.edu; principal investigator Lakshmanan Krishnamurti; Emory University, Atlanta, GA, USA |
| Notes | Trial # NCT02326597 |
NCT02344576.
| Trial name or title | A multicenter trial of a shared decision support intervention for patients and their caregivers offered destination therapy for end‐stage heart failure |
| Methods | RCT |
| Participants | 400 adults advanced heart failure and are being evaluated for destination left ventricular assist device |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, values, decisional conflict, decisional regret, stress, anxiety, depression, quality of life, control preferences scale, illness acceptance, health status |
| Starting date | May 2015 |
| Contact information | jocelyn.thompson@ucdenver.edu; University of Colorado, Denver |
| Notes | Trial #: NCT02344576 |
NCT02488317.
| Trial name or title | Empowering patients on choices for renal replacement therapy |
| Methods | RCT |
| Participants | 150 adults with kidney disease |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Preference for shared decision‐making (CPS), decisional conflict, decision self‐efficacy, knowledge, preparation for decision making |
| Starting date | May 2015 |
| Contact information | Francesca Tentori; Arbor Research Collaborative for Health; Ann Arbor, MI |
| Notes | Trial #: NCT02488317 |
NCT02488603.
| Trial name or title | Utilization of decision aids for tamoxifen treatment in breast cancer patients |
| Methods | RCT |
| Participants | 360 breast cancer patients referred for tamoxifen treatment |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict scale, satisfaction with decision, quality of life |
| Starting date | August 2015 |
| Contact information | Eun Sook Lee; National Cancer Center, Korea |
| Notes | Trial # NCT02488603 |
NCT02492009.
| Trial name or title | Patient decision aid for antidepressant use in pregnancy |
| Methods | RCT |
| Participants | 50 women aged 18 years or older planning a pregnancy or < 30 weeks pregnant |
| Interventions | Patient decision aid vs standard resource sheet |
| Outcomes | Depression, anxiety, decisional conflict, knowledge, intervention acceptability, choice |
| Starting date | June 2015 |
| Contact information | hind.khalifeh@kcl.ac.uk or ruth.brauer@kcl.ac.uk Section of Women's Mental Health, King's College London |
| Notes | Trial #: NCT02492009 |
NCT02503553.
| Trial name or title | Decision aids in cerebral aneurysm treatment |
| Methods | RCT |
| Participants | 60 patients undergoing treatment for cerebral aneurysm |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Participation in the shared‐decision making process; stress levels, patient satisfaction level |
| Starting date | August 2015 |
| Contact information | Kimon Bekelis; Dartmouth‐Hitchcock Medical Center; New Hampshire, USA |
| Notes | Trial #: NCT02503553 |
NCT02516449.
| Trial name or title | Assessment of shared decision making aids in asthma |
| Methods | RCT |
| Participants | 51 adults with mild to severe asthma |
| Interventions | Patient decision aid vs usual care |
| Outcomes | Knowledge, decisional conflict, treatment adherence, asthma control |
| Starting date | March 2013 |
| Contact information | Centre de recherche de l'Institut universitaire de cardiologie et de pneumologie de Québec, Québec, Canada, G1V 4G5 |
| Notes | Trial # NCT02516449 |
NCT02540044.
| Trial name or title | Supporting patient care with electronic resource (SuPER): efficacy of an online decision aid for patients considering biologic therapy for rheumatoid arthritis |
| Methods | RCT |
| Participants | 144 adults with rheumatoid arthritis whose rheumatologists have recommended initiating a biologic/subsequent entry biologic or switching to another biologic agent |
| Interventions | Online patient decision aid vs online standard information |
| Outcomes | Decisional conflict, knowledge, self‐efficacy, self‐management behaviours, health resource utilization, choice, evidence of shared decision‐making |
| Starting date | January 2016 |
| Contact information | Linda Li; University of British Columbia; Vancouver, Canada |
| Notes | Trial #: NCT02540044 |
NCT02611050.
| Trial name or title | Treatment decisions for multi‐vessel CAD |
| Methods | RCT |
| Participants | 160 adults with stable multi‐vessel CAD at relative equipoise for at least 2 potential treatment options |
| Interventions | Option grid decision aid vs usual care |
| Outcomes | Decisional conflict, CollaboRATE score, knowledge, patient experience, treatment received |
| Starting date | December 2015 |
| Contact information | Elizabeth L Nichols; the Dartmouth Institute |
| Notes | Trial #: NCT02611050 |
Oostendorp 2011.
| Trial name or title | Assessing the information desire of patients with advanced cancer by providing information with a decision aid, which is evaluated in a randomized trial: a study protocol |
| Methods | RCT |
| Participants | Patients with advanced colorectal, breast, or ovarian cancer and have started treatment with first‐line palliative chemotherapy |
| Interventions | Patients are randomized to receive either usual care or usual care + decision aid |
| Outcomes | Not yet assessed |
| Starting date | Not yet assessed |
| Contact information | Linda JM Oostendorp, Radbound University |
| Notes | Netherlands Trial Register (NTR): NTR1113 |
Yu 2015.
| Trial name or title | Impact of an interprofessional shared decision‐making and goal setting decision aid for patients with diabetes |
| Methods | Cluster‐randomized controlled trial |
| Participants | 112 patients with diabetes |
| Interventions | Multicomponent patient decision aid toolkit vs patient education pamphlet |
| Outcomes | Decisional conflict, diabetes distress, health‐related quality of life, chronic illness care, intention to engage in SDM |
| Starting date | April 2015 |
| Contact information | yuca@smh.ca |
| Notes | Trial # NCT02379078 |
CA‐125: cancer antigen 125; CAD: coronary artery disease; CT: computerized tomography; NIH: National Institutes of Health; NSW: New South Wales; OA: osteoarthritis; RCT: randomized controlled trial; SDM: shared decision making.
Differences between protocol and review
There are three main differences between the original protocol and the review. We re‐structured the 2009 update, O'Connor 2009b, to organize the long list of outcomes into primary and secondary outcomes based on the new effectiveness criteria of the International Patient Decision Aid (IPDAS) Collaboration (Elwyn 2006). For the 2011 update, Stacey 2011, we changed the study quality assessment to the 'Risk of bias' assessment (Higgins 2011). For the 2014 update, Stacey 2014b, we used GRADE to summarize the quality of the evidence and reported the results using Table 1.
For the 2016 (current) update, we removed 28 studies that compared detailed versus simple decision aids. This update is limited to comparisons of patient decision aids versus usual care to provide a more focused review. This change resulted in removal of these comparisons for pooled results including knowledge scores, decisional conflict, perceived participation in decision making, proportion undecided, choice, and satisfaction. For other outcomes including congruence between chosen option‐values and accurate risk perception, the new pooled comparisons only focus on patient decision aid versus usual care, rather than previous comparisons that reported on patient decision aids with explicit values clarification and probabilities of outcomes versus any comparisons without these features.
Contributions of authors
1999 Review (O'Connor 1999b): AO, AR, VF, JT, VE, HLT, MHR, VF, MB, and JJ contributed to the design of the protocol, the interpretation of results, and the revision and approved the final paper. AO led the team, and JT coordinated the project. AO, MH‐R, AR, VF, and JT pilot tested the data extraction forms. AR, VF, and JT screened studies and extracted data. AR, JT, and AO analyzed the results.
2001 Review (O'Connor 2001): AO, DS, DR, MHR, HLT, VE, MB, JT, VF, and AR contributed to the interpretation of results and the revision and approved the final paper. AO led the team, and DS coordinated the update. AO, DR, MHR, HLT, JT, DS, and JP screened studies and extracted data. DS and JP evaluated decision aids using the CREDIBLE criteria. AO and DS analyzed the results.
2002 Review (O'Connor 2003): AO, DS, DR, MHR, HLT, VE, MB, JT, and VF contributed to the interpretation of results and the revision and approved the final paper. AO led the team, and DS coordinated the update. DS, JP, VT, and JT screened studies and extracted data. DS, JP, VT, and SK evaluated decision aids using the CREDIBLE criteria. AO and DS analyzed the results.
2006 Review (O'Connor 2009b): AO, CB, DS, MB, NC, KE, VE, VF, MHR, SK, HLT, DR, contributed to the interpretation of results, and the revision and final approval of the paper. AO led the team and CB coordinated the update. CB, SK, DS, AO, VF screened studies and extracted data. AO and CB analyzed the results.
2009 Review (Stacey 2011):
DS, CB, MB, NC, KE, FL, AL, MHR, HLT, and RT contributed to the interpretation of results, and the revision and approved the final paper. DS led the team, and CB coordinated the update. CB and DS screened studies; SM and AD extracted data; CB entered the data; DS verified the data entered. DS and CB analyzed the results.
2013 Review (Stacey 2014b):
DS, CB, MB, NC, KE, FL, AL, MHR, HLT, RT, and LT contributed to the interpretation of results and the revision and approved the final paper. DS led the team with help coordinating the update from SB and JW. CB, DS, RT, MB, MHR, NC, KE, BV, DR, and AS screened studies; SB, RW, JW, and CC extracted data; SB and JW entered the data; DS verified the data entered. DS and JW analyzed the results.
2016 (current) Review:
DS, CB, MB, KE, FL, AL, MHR, HLT, RT, LT, and KL contributed to the interpretation of results and the revision and approved the final paper. DS led the team with help coordinating the update from KL. CB, DS, RT, MB, MHR, KE, DR, and AS screened studies; KL and IS extracted data; KL entered the data; DS verified the data entered. DS analyzed the results.
Sources of support
Internal sources
-
University of Ottawa, Canada.
University Research Chair in Knowledge Translation to Patients
-
Ottawa Hospital Research Institute, Canada.
Scientific Director, Patient Decision Aids Research Group
External sources
No sources of support supplied
Declarations of interest
Several of the investigators have developed patient decision aids (DS, FL, HL, MHR, MB, KE, RT, LT, KL), but none reviewed their own studies.
Within the last five years, two investigators (HL, MB) have received financial support from the not‐for‐profit Informed Medical Decisions Foundation (IMDF). MB serves on the Board of and received salary and grant support as President of the Foundation. In 2014, the Foundation merged with another not‐for‐profit, Healthwise. MB continues to receive salary and grant support as Chief Science Officer at Healthwise. Healthwise develops, licenses, and distributes patient decision aids. Several investigators (DS, FL, HL, MHR, MB, KE, RT, LT) who were involved in a special issue in BMC Medical Informatics and Decision Making that included a series of 14 papers focused on the theoretical and empirical evidence underlying the International Patient Decision Aid Standards (IPDAS), received partial funding from the Foundation to cover publishing costs.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Allen 2010 {published data only}
- Allen JD, Othus MK, Hart A Jr, Tom L, Li Y, Berry D, et al. A randomized trial of a computer‐tailored decision aid to improve prostate cancer screening decisions: results from the take the wheel trial. Cancer Epidemiology, Biomarkers and Prevention 2010;19(9):2172‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Othus MKD, Hart A Jr, Mohllajee AP, Bowen D. Do men make informed decisions about prostate cancer screening? Baseline results from the "Take the Wheel" Trial. Medical Decision Making 2011;31:108‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arterburn 2011 {published data only}
- Arterburn D, Westbrook E, Bogart T, Sepucha K, Bock S, Weppner W. Randomized trial of a video‐based patient decision aid for bariatric surgery. Obesity 2011;19(8):1669‐75. [DOI] [PubMed] [Google Scholar]
Auvinen 2004 {published data only}
- Auvinen A, Hakama M, Ala‐Opas M, Vornanen T, Leppilahti M, Salminen P, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU International 2004;93(1):52‐6. [DOI] [PubMed] [Google Scholar]
- Auvinen A, Vornanen T, Tammela TL, Ala‐Opas M, Leppilahti M, Salminen P, et al. A randomized trial of the choice of treatment in prostate cancer: design and baseline characteristics. BJU International 2001;88(7):708‐15. [DOI] [PubMed] [Google Scholar]
- Huang RC, Auvinen A, Hakama M, Tammela TLJ, Ala‐Opas M, Leppilahti M, et al. Effect of intervention on decision making of treatment for disease progression, prostate‐specific antigen biochemical failure and prostate cancer death. Health Expectations 2014;17(6):776‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 1997 {published and unpublished data}
- Barry MJ, Cherkin DC, Chang Y, Fowler FJ, Skates S. A randomized trial of a multimedia shared decision‐making program for men facing a treatment decision for benign prostatic hyperplasia. Disease Management and Clinical Outcomes 1997;1(1):5‐14. [Google Scholar]
- Rovner DR, Wills CE, Bonham V, Williams G, Lillie J, Kelly‐Blake K, et al. Decision aids for benign prostatic hyperplasia: applicability across race and education. Medical Decision Making 2004;24(4):359‐66. [DOI] [PubMed] [Google Scholar]
Bekker 2004 {published data only}
- Bekker HL, Hewison J, Thornton JG. Applying decision analysis to facilitate informed decision making about prenatal diagnosis for Down syndrome: a randomised controlled trial. Prenatal Diagnosis 2004;24(4):265‐75. [DOI] [PubMed] [Google Scholar]
- Bekker HL, Hewison J, Thornton JG. Understanding why decision aids work: linking process with outcome. Patient Education and Counseling 2003;50(3):323‐9. [DOI] [PubMed] [Google Scholar]
Bernstein 1998 {published and unpublished data}
- Bernstein SJ, Skarupski KA, Grayson CE, Starling MR, Bates ER, Eagle KA. A randomized controlled trial of information‐giving to patients referred for coronary angiography: effects on outcomes of care. Health Expectations 1998;1(1):50‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2013 {published data only}
- Berry DL, Halpenny B, Hong F, Wolpin S, Lober WB, Russell KJ, et al. The personal patient profile‐prostate decision support for men with localized prostate cancer: a multi‐center randomized trial. Urologic Oncology 2013;31(7):1012‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Wang Q, Halpenny B, Hong F. Decision preparation, satisfaction and regret in a multi‐center sample of men with newly diagnosed localized prostate cancer. Patient Education and Counseling 2012;88(2):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco JLF, Halpenny B, Berry DL. Personal preferences and discordant prostate cancer treatment choice in an intervention trial of men newly diagnosed with localized prostate cancer. Health and Quality of Life Outcomes 2012;10(123):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill ML, Hong F, Berry DL. When study site contributes to outcomes in a multi‐center randomized trial: a secondary analysis of decisional conflict in men with localized prostate cancer. Health and Quality of Life Outcomes 2014;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjorklund 2012 {published data only}
- Bjorklund U, Marsk A, Levin C, Ohman SG. Audiovisual information affects informed choice and experience of information in antenatal Down syndrome screening‐a randomized controlled trial. Patient Education and Counseling 2012;86(3):390‐5. [DOI] [PubMed] [Google Scholar]
- Öhman SG, Björklund U, Marsk A. Does an informational film increase women's possibility to make an informed choice about second trimester ultrasound?. Prenatal Diagnosis 2012;32(9):833‐9. [DOI] [PubMed] [Google Scholar]
Bozic 2013 {published data only}
- Bozic KJ, Belkora J, Chan V, Youm J, Zhou T, Dupaix J, et al. Shared decision making in patients with osteoarthritis of the hip and knee: results of a randomized controlled trial. Journal of Bone and Joint Surgery: American Volume 2013;95(18):1633‐9. [DOI] [PubMed] [Google Scholar]
- Bozic KJ, Chenok KE, Schindel J, Chan V, Huddleston JI, Braddock C, Belkora J. Patient, surgeon, and healthcare purchaser views on the use of decision and communication aids in orthopaedic surgery: a mixed methods study. BMC Health Services Research 2014;14(366):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm J, Chan V, Belkora J, Bozic KJ. Impact of socioeconomic factors on informed decision making and treatment choice in patients with hip and knee OA. The Journal of Arthroplasty 2015;30(2):171‐5. [DOI] [PubMed] [Google Scholar]
Brazell 2014 {published data only}
- Brazell HD, O'Sullivan DM, Forrest A, Greene JF. Effect of a decision aid on decision making for the treatment of pelvic organ prolapse. Female Pelvic Medicine & Reconstructive Surgery 2014;21(4):231‐5. [DOI] [PubMed] [Google Scholar]
Chabrera 2015 {published data only}
- Chabrera C, Zabalegui A, Bonet M, Caro M, Areal J, González JR, Font A. A decision aid to support informed choices for patients recently diagnosed with prostate cancer. Cancer Nursing 2015;38(3):E42‐E50. [DOI] [PubMed] [Google Scholar]
Chambers 2012 {published data only}
- Chambers LW, Wilson K, Hawken S, Puxty J, Crowe L, Lam PP, et al. Impact of the Ottawa influenza decision aid on healthcare personnel's influenza immunization decision: a randomized trial. Journal of Hospital Infection 2012;82(3):194‐202. [DOI] [PubMed] [Google Scholar]
Clancy 1988 {published data only}
- Clancy CM, Cebul RD, Williams SV. Guiding individual decisions: a randomized, controlled trial of decision analysis. American Journal of Medicine 1988;84(2):283‐8. [DOI] [PubMed] [Google Scholar]
Davison 1997 {published data only}
- Davison BJ, Degner LF. Empowerment of men newly diagnosed with prostate cancer. Cancer Nursing 1997;20(3):187‐96. [DOI] [PubMed] [Google Scholar]
De Achaval 2012 {published data only}
- Achaval S, Fraenkel L, Volk R, Cox V, Suarez‐Almazor M. Impact of educational and patient decision aids on decisional conflict associated with total knee arthroplasty. Arthritis Care & Research 2012;64(2):229‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2002 {published data only}
- Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Medical Decision Making 2002;22(2):125‐39. [DOI] [PubMed] [Google Scholar]
Evans 2010 {published data only}
- Evans R, Joseph‐Williams N, Edwards A, Newcombe R, Wright P, Kinnersley P, et al. Supporting informed decision making for prostate specific antigen (PSA) testing on the web: an online randomized controlled trial. Journal of Medical Internet Research 2010;12(3):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fagerlin 2011 {published data only}
- Banegas MP, McClure JB, Barlow WE, Ubel PA, Smith DM, Zikmund‐Fisher BJ, et al. Results from a randomized trial of a web‐based, tailored decision aid for women at high risk for breast cancer. Patient Education and Counseling 2013;91:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlin A. Randomization for Guide to Decide phase II. Word document provided by the authors.
- Fagerlin A, Dillard AJ, Smith DM, Zikmund‐Fisher BJ, Pitsch R, McClure JB, et al. Women's interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Research and Treatment 2011;127(3):681‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfage IJ, Fuhrel‐Forbis A, Ubel PA, Zikmund‐Fisher BJ, Greene SM, McClure JB, et al. Informed choice about breast cancer prevention: randomized controlled trial of an online decision aid intervention. Breast Cancer Research 2013;15(R74):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraenkel 2007 {published data only}
- Fraenkel L, Rabidou N, Wittink D, Fried T. Improving informed decision‐making for patients with knee pain. Journal of Rheumatology 2007;34(9):1894‐8. [PubMed] [Google Scholar]
Fraenkel 2012 {published data only}
- Fraenkel L, Street RL Jr, Towle V, O'Leary JR, Iannone L, Ness PH, Fried TR. A pilot randomized controlled trial of a decision support tool to improve the quality of communication and decision‐making in individuals with atrial fibrillation. Journal of the American Geriatrics Society 2012;60(8):1434‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2008a {published data only}
- Frosch DL, Bhatnagar V, Tally S, Hamori CJ, Kaplan RM. Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Archives of Internal Medicine 2008;168(4):363‐9. [DOI] [PubMed] [Google Scholar]
Gattellari 2003 {published data only}
- Gattellari M, Ward JE. Does evidence‐based information about screening for prostate cancer enhance consumer decision‐making? A randomised controlled trial. Journal of Medical Screening 2003;10(1):27‐39. [DOI] [PubMed] [Google Scholar]
Gattellari 2005 {published data only}
- Gattellari M, Ward JE. A community‐based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Education and Counseling 2005;57(2):168‐82. [DOI] [PubMed] [Google Scholar]
Green 2001 {published data only}
- Green MJ, Biesecker BB, McInerney AM, Mauger D, Fost N. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. American Journal of Medical Genetics 2001;103(1):16‐23. [DOI] [PubMed] [Google Scholar]
Hamann 2006 {published data only}
- Hamann J, Cohen R, Leucht S, Busch R, Kissling W. Shared decision making and long‐term outcome in schizophrenia treatment. Journal of Clinical Psychiatry 2007;68(7):992‐7. [DOI] [PubMed] [Google Scholar]
- Hamann J, Langer B, Winkler V, Busch R, Cohen R, Leucht S, et al. Shared decision making for in‐patients with schizophrenia. Acta Psychiatrica Scandinavica 2006;114(4):265‐73. [DOI] [PubMed] [Google Scholar]
Hanson 2011 {published data only}
- Ersek M, Sefcik JS, Feng‐Chang L, Lee TJ, Gilliam R, Hanson LC. Provider staffing effect on a decision aid intervention. Clinical Nursing Research 2014;23:36‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L, Carey T, Caprio A, Joon Lee T, Ersek M, Garrett J, et al. Improving decision making for feeding options in advanced dementia: a randomized, controlled trial. Journal of the American Geriatrics Society 2011;59(11):2009‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EA, Caprio AJ, Wessell K, Lin FC, Hanson LC. Impact of a decision aid on surrogate decision‐makers’ perceptions of feeding options for patients with dementia. American Medical Directors Association 2013;14(2):114‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heller 2008 {published data only}
- Heller L, Parker PA, Youssef A, Miller MJ. Interactive digital education aid in breast reconstruction. Plastic & Reconstructive Surgery 2008;122(3):717‐24. [DOI] [PubMed] [Google Scholar]
Hess 2012 {published data only}
- Hess EP, Knoedler MA, Shah ND, Kline JA, Breslin M, Branda ME, et al. The chest pain choice decision aid: a randomized trial. Circulation: Cardiovascular Quality and Outcomes 2012;5(3):251‐9. [DOI] [PubMed] [Google Scholar]
Jibaja‐Weiss 2011 {published data only}
- Jibaja‐Weiss M, Volk R, Granchi T, Neff N, Robinson E, Spann S, et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Education and Counseling 2011;84(1):41‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson BR, Schwartz A, Goldberg J, Koerber A. A chairside aid for shared decision making in dentistry: a randomized controlled trial. Journal of Dental Education 2006;70(2):133‐41. [PubMed] [Google Scholar]
Kasper 2008 {published data only}
- Kasper J, Kopke S, Muhlhauser I, Nubling M, Heesen C. Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): A randomized controlled trial. European Journal of Neurology 2008;15(12):1345‐52. [DOI] [PubMed] [Google Scholar]
Kennedy 2002 {published data only}
- Kennedy AD, Sculpher MJ, Coulter A, Dwyer N, Rees M, Abrams KR, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA 2002;288(21):2701‐8. [DOI] [PubMed] [Google Scholar]
Knops 2014 {published data only}
- Knops AM, Goossens A, Ubbink DT, Balm R, Koelemay MJ, Vahl AC, et al. DECAID Trial Group. A decision aid regarding treatment options for patients with an asymptomatic abdominal aortic aneurysm: a randomised clinical trial. European Journal of Vascular and Endovascular Surgery 2014;48(3):276‐283. [DOI] [PubMed] [Google Scholar]
Krist 2007 {published data only}
- Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Annals of Family Medicine 2007;5(2):112‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kupke 2013 {published data only (unpublished sought but not used)}
- Kupke J, Wicht MJ, Stützer H, Derman SH, Lichtenstein NV, Noack MJ. Does the use of a visualised decision board by undergraduate students during shared decision‐making enhance patients' knowledge and satisfaction? A randomised controlled trial. European Journal of Dental Education 2013;17(1):19‐25. [DOI] [PubMed] [Google Scholar]
Kuppermann 2014 {published data only}
- Kuppermann M, Pena S, Bishop JT, Nakagawa S, Gregorich SE, Sit A, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. Journal of the American Medical Association 2014;312(12):1210‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2013 {published data only}
- Lam WW, Chan M, Or A, Kwong A, Suen D, Fielding R. Reducing treatment decision conflict difficulties in breast cancer surgery: a randomized controlled trial. Journal of Clinical Oncology 2013;31(23):2879‐85. [DOI] [PubMed] [Google Scholar]
Langston 2010 {published data only}
- Langston A, Rosario L, Westhoff C. Structured contraceptive counseling: a randomised controlled trial. Patient Education and Counseling 2010;81(3):362‐7. [DOI] [PubMed] [Google Scholar]
Laupacis 2006 {published data only}
- Laupacis A, O'Connor AM, Drake ER, Rubens FD, Robblee JA, Grant FC, et al. A decision aid for autologous pre‐donation in cardiac surgery ‐ a randomized trial. Patient Education and Counseling 2006;61(3):458‐66. [DOI] [PubMed] [Google Scholar]
LeBlanc 2015 {published data only}
- LeBlanc A, Wang AT, Wyatt K, Branda ME, Shah ND, Houten H, et al. Encounter decision aid vs. clinical decision support or usual care to support patient‐centered treatment decisions in osteoporosis: the osteoporosis choice randomized trial II. PLOS ONE 2015;10(5):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2008a {published data only}
- Legare F, Dodin S, Stacey D, Leblanc A, Tapp S. Patient decision aid on natural health products for menopausal symptoms: randomized controlled trial. Menopause International 2008;14(3):105‐10. [DOI] [PubMed] [Google Scholar]
Legare 2011 {published data only}
- Legare F, Labrecque M, LeBlanc A, Njoya M, Laurier C, Cote L, et al. Training family physicians in shared decision making for the use of antibiotics for acute respiratory infections: a pilot clustered randomized controlled trial. Health Expectations 2011;14:96‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2012 {published and unpublished data}
- Legare F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision‐making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. Canadian Medical Association Journal 2012;184(13):E726‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leighl 2011 {published data only}
- Leighl NB, Shepherd HL, Butow PN, Clarke SJ, McJannett M, Beale PJ, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. Journal of Clinical Oncology 2011;29(15):2077‐84. [DOI] [PubMed] [Google Scholar]
Lepore 2012 {published data only}
- Lepore SJ, Wolf RL, Basch CE, Godfrey M, McGinty E, Shmukler C, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Annals of Behavioral Medicine 2012;44(3):320‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 1997 {published data only}
- Lerman C, Biesecker B, Benkendorf JL, Kerner J, Gomez‐Caminero A, Hughes C, et al. Controlled trial of pretest education approaches to enhance informed decision‐making for BRCA1 gene testing. Journal of the National Cancer Institute 1997;89(2):148‐57. [DOI] [PubMed] [Google Scholar]
Lewis 2010 {published data only}
- Lewis C, Pignone M, Schild L, Scott T, Winquist A, Rimer B, et al. Effectiveness of a patient and practice‐level colorectal cancer screening intervention in health plan members: design and baseline findings of the CHOICE trial. Cancer 2010;116(7):1664‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignone M, Winquist A, Schild L, Lewis C, Scott T, Hawley J, et al. Effectiveness of a patient and practice‐level colorectal cancer screening intervention in health plan members. Cancer 2011;117(15):3252‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignone MP, Brenner AT, Hawley S, Sheridan SL, Lewis CL, Jonas DE, et al. Conjoint analysis versus rating and ranking for values elicitation and clarification in colorectal cancer screening. Journal of General Internal Medicine 2011;27(1):45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loh 2007 {published data only}
- Loh A, Simon D, Harter M. Effects of shared decision making in primary care of depressive patients ‐ better compliance and treatment effects. Klinikarzt 2007;36(1):38‐41. [Google Scholar]
- Loh A, Simon D, Wills CE, Kriston L, Niebling W, Harter M. The effects of a shared decision‐making intervention in primary care of depression: a cluster‐randomized controlled trial. Patient Education and Counseling 2007;67(3):324‐32. [DOI] [PubMed] [Google Scholar]
Mann D 2010 {published data only}
- Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The statin choice decision aid in primary care: a randomized trial. Patient Education and Counseling 2010;80(1):138‐40. [DOI] [PubMed] [Google Scholar]
Mann E 2010 {published data only}
- Mann E, Kellar I, Sutton S, Kinmonth AL, Hankins M, Griffin S, et al. Impact of informed‐choice invitations on diabetes screening knowledge, attitude and intentions: an analogue study. BMC Public Health 2010;10:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Man‐Son‐Hing 1999 {published and unpublished data}
- Man‐Son‐Hing M, Laupacis A, O'Connor AM, Biggs J, Drake E, Yetisir E, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999;282(8):737‐43. [DOI] [PubMed] [Google Scholar]
Marteau 2010 {published data only}
- Kellar I, Mann E, Kinmonth AL, Prevost AT, Sutton S, Marteau TM. Can informed choice invitations lead to inequities in intentions to make lifestyle changes among participants in a primary care diabetes screening programme? Evidence from a randomized trial. Public Health 2011;125(9):645‐52. [DOI] [PubMed] [Google Scholar]
- Marteau TM, Mann E, Prevost AT, Vasconcelos JC, Kellar I, Sanderson S, et al. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial. BMJ 2010;340:c2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathers 2012 {published data only}
- Brown I, Bradley A, Ng CJ, Colwell B, Mathers N. Investigating active ingredients in a complex intervention: a nested study within the Patient and Decision Aids (PANDAs) randomised controlled trial for people with type 2 diabetes. BMC Research Notes 2014;7:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open 2012;2(6):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2007 {published data only}
- Mathieu E, Barratt A, Davey HM, McGeechan K, Howard K, Houssami N. Informed choice in mammography screening: a randomized trial of a decision aid for 70‐year‐old women. Archives of Internal Medicine 2007;167(19):2039‐46. [DOI] [PubMed] [Google Scholar]
Mathieu 2010 {published data only}
- Mathieu E, Barratt AL, McGeechan K, Davey HM, Howard K, Houssami N. Helping women make choices about mammography screening: an online randomized trial of a decision aid for 40‐year‐old women. Patient Education and Counseling 2010;81(1):63‐72. [DOI] [PubMed] [Google Scholar]
McAlister 2005 {published data only}
- McAlister FA, Man‐Son‐Hing M, Straus SE, Ghali WA, Anderson D, Majumdar SR, et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ 2005;173(5):496‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBride 2002 {published data only}
- Bastian LA, McBride CM, Fish L, Lyna P, Farrell D, Lipkus IM, et al. Evaluating participants' use of a hormone replacement therapy decision‐making intervention. Patient Education and Counseling 2002;48(3):283‐91. [DOI] [PubMed] [Google Scholar]
- McBride CM, Bastian LA, Halabi S, Fish L, Lipkus IM, Bosworth HB, et al. A tailored intervention to aid decision making about hormone replacement therapy. American Journal of Public Health 2002;92(7):1112‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCaffery 2010 {published data only}
- McCaffery KJ, Irwig L, Turner R, Chan SF, Macaskill P, Lewicka M, et al. Psychosocial outcomes of three triage methods for the management of borderline abnormal cervical smears: an open randomised trial. BMJ 2010;340:b4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller SM, Fleisher L, Roussi P, Buzaglo JS, Schnoll R, Slater E, et al. Facilitating informed decision making about breast cancer risk and genetic counseling among women calling the NCI's Cancer Information Service. Journal of Health Communication 2005;10(Suppl 1):119‐36. [DOI] [PubMed] [Google Scholar]
Miller 2011 {published data only}
- Duren‐Winfield V, Onsomu EO, Case DL, Pignone M, Miller D. Health literacy and computer‐assisted instruction: usability and patient preference. Journal of Health Communication 2015;20:491‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D, Spangler J, Case D, Goff D, Singh S, Pignone M. Effectiveness of a web‐based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed‐literacy population. American Journal of Preventive Medicine 2011;40(6):608‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2003 {published and unpublished data}
- Emmett CL, Montgomery AA, Peters TJ, Fahey T. Three‐year follow‐up of a factorial randomised controlled trial of two decision aids for newly diagnosed hypertensive patients. British Journal of General Practice 2005;55(516):551‐3. [PMC free article] [PubMed] [Google Scholar]
- Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. British Journal of General Practice 2003;53(491):446‐53. [PMC free article] [PubMed] [Google Scholar]
Montgomery 2007 {published data only}
- Frost J, Shaw. Women's views on the use of decision aids for decision making about the method of delivery following a previous caesarean section: qualitative interview study. British Journal of Obstetrics and Gynecology 2009;116(7):896‐905. [DOI] [PubMed] [Google Scholar]
- Hollinghurst S, Emmett C, Peters TJ, Watson H, Fahey T, Murphy DJ, et al. Economic evaluation of the DIAMOND randomized trial: cost and outcomes of 2 decision aids for mode of delivery among women with previous caesarian section. BMJ 2010;30:453‐63. [DOI] [PubMed] [Google Scholar]
- Montgomery AA, Emmett CL, Fahey T, Jones C, Ricketts I, Patel RR, et al. Two decision aids for mode of delivery among women with previous caesarean section: randomised controlled trial. BMJ 2007;334(7607):1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montori 2011 {published data only}
- Montori VM, Shah ND, Pencille LJ, Branda ME, Houten HK, Swiglo BA. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. American Journal of Medicine 2011;124(6):549‐56. [DOI] [PubMed] [Google Scholar]
- Pencille LJ, Campbell ME, Houten HK, Shah ND, Mullan RJ, Swiglo BA, et al. Protocol for the Osteoporosis Choice trial. A pilot randomized trial of a decision aid in primary care practice. Trials 2009;10:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2000 {published and unpublished data}
- Morgan MW. A Randomized Trial of the Ischemic Heart Disease Shared Decision Making Program: An Evaluation of a Decision Aid [Masters Thesis]. Toronto: University of Toronto, 1997. [Google Scholar]
- Morgan MW, Deber RB, Llewellyn‐Thomas HA, Gladstone P, Cusimano RJ, O'Rourke K, et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease. Journal of General Internal Medicine 2000;15(10):685‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mott 2014 {published data only}
- Mott JM, Stanley MA, Street RL Jr, Grady RH, Teng EJ. Increasing engagement in evidence‐based PTSD treatment through shared decision‐making: a pilot study. Military Medicine 2014;179(2):143‐9. [DOI] [PubMed] [Google Scholar]
Mullan 2009 {published data only}
- Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Archives of Internal Medicine 2009;169(17):1560‐8. [DOI] [PubMed] [Google Scholar]
Murray 2001a {published and unpublished data}
- Murray E, Davis H, Tai SS, Coulter A, Gray A, Haines A. Randomised controlled trial of an interactive multimedia decision aid on benign prostatic hypertrophy in primary care. BMJ 2001;323(7311):493‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2001b {published and unpublished data}
- Murray E, Davis H, Tai SS, Coulter A, Gray A, Haines A. Randomized controlled trial of an interactive multimedia decision aid on hormone replacement therapy in primary care. BMJ 2001;323(7311):490‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagle 2008 {published data only}
- Nagle C, Gunn J, Bell R, Lewis S, Meiser B, Metcalfe S, et al. Use of a decision aid for prenatal testing of fetal abnormalities to improve women's informed decision making: a cluster randomised controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology 2008;115(3):339‐47. [DOI] [PubMed] [Google Scholar]
- Nagle C, Lewis S, Meiser B, Metcalfe S, Carlin JB, Bell R, et al. Evaluation of a decision aid for prenatal testing of fetal abnormalities: a cluster randomised trial [ISRCTN22532458]. BMC Public Health 2006;6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nassar 2007 {published data only}
- Nassar N, Roberts CL, Raynes‐Greenow CH, Barratt A, Peat B, Decision Aid for Breech Presentation Trial Collaborators. Evaluation of a decision aid for women with breech presentation at term: a randomised controlled trial [ISRCTN14570598]. BJOG: An International Journal of Obstetrics & Gynaecology 2007;114(3):325‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oakley 2006 {published data only}
- Oakley S, Walley T. A pilot study assessing the effectiveness of a decision aid on patient adherence with oral bisphosphonate medication. Pharmaceutical Journal 2006;276(7399):536‐8. [Google Scholar]
Ozanne 2007 {published data only}
- Ozanne EM, Annis C, Adduci K, Showstack J, Esserman L. Pilot trial of a computerized decision aid for breast cancer prevention. Breast Journal 2007;13(2):147‐54. [DOI] [PubMed] [Google Scholar]
Partin 2004 {published and unpublished data}
- Partin MR, Nelson D, Flood AB, Friedemann‐Sanchez G, Wilt TJ. Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions. Health Expectations 2006;9(3):285‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin MR, Nelson D, Radosevich D, Nugent S, Flood AB, Dillon N, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. Journal of General Internal Medicine 2004;19(8):835‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pignone 2000 {published data only}
- Pignone M, Harris R, Kinsinger L. Videotape‐based decision aid for colon cancer screening. A randomized, controlled trial. Annals of Internal Medicine 2000;133(10):761‐9. [DOI] [PubMed] [Google Scholar]
Protheroe 2007 {published data only}
- Patel S, Ngunjiri A, Hee SW, Yang Y, Brown S, Friede T, et al. Primum non nocere: shared informed decision making in low back pain ‐ a pilot cluster randomised trial. BMC Musculoskeletal Disorders 2014;15:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protheroe J, Bower P, Chew‐Graham C. The use of mixed methodology in evaluating complex interventions: identifying patient factors that moderate the effects of a decision aid. Family Practice 2008;24(6):594‐600. [DOI] [PubMed] [Google Scholar]
- Protheroe J, Bower P, Chew‐Graham C, Peters TJ, Fahey T. Effectiveness of a computerized decision aid in primary care on decision making and quality of life in menorrhagia: results of the MENTIP randomized controlled trial. Medical Decision Making 2007;27(5):575‐84. [DOI] [PubMed] [Google Scholar]
Rubel 2010 {published data only}
- Rubel SK, Miller JW, Stephens RL, Xu Y, Scholl LE, Holden EW, et al. Testing the effects of a decision aid for prostate cancer screening. Journal of Health Communication 2010;15(3):307‐21. [DOI] [PubMed] [Google Scholar]
Ruffin 2007 {published data only}
- Ruffin MT, Fetters MD, Jimbo M. Preference‐based electronic decision aid to promote colorectal cancer screening: results of a randomized controlled trial. Preventive Medicine 2007;45(4):267‐73. [DOI] [PubMed] [Google Scholar]
Sawka 2012 {published and unpublished data}
- Sawka AM, Straus S, Rotstein L, Brierley JD, Tsang RW, Asa S, et al. Randomized controlled trial of a computerized decision aid on adjuvant radioactive iodine treatment for patients with early‐stage papillary thyroid cancer. Journal of Clinical Oncology 2012;30(23):2906‐11. [DOI] [PubMed] [Google Scholar]
Schroy 2011 {published data only}
- Schroy PC 3rd, Emmons K, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. The impact of a novel computer‐based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Medical Decision Making 2011;31(1):93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroy PC 3rd, Emmons KM, Peters E, Glick JT, Robinson PA, Lydotes MA, et al. Aid‐assisted decision making and colorectal cancer screening: a randomized controlled trial. American Journal of Preventive Medicine 2012;43(6):573‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwalm 2012 {published data only}
- Schwalm JD, Stacey D, Pericak D, Natarajan MK. Radial artery versus femoral artery access options in coronary angiogram procedures: randomized controlled trial of a patient‐decision aid. Circulation: Cardiovascular Quality and Outcomes 2012;5(3):260‐6. [DOI] [PubMed] [Google Scholar]
Schwartz 2001 {published data only}
- Schwartz MD, Benkendorf J, Lerman C, Isaacs C, Ryan‐Robertson A, Johnson L. Impact of educational print materials on knowledge, attitudes, and interest in BRCA1/BRCA2: testing among Ashkenazi Jewish women. Cancer 2001;92(4):932‐40. [DOI] [PubMed] [Google Scholar]
Schwartz 2009a {published data only}
- Hooker GW, Leventhal KG, DeMarco T, Peshkin BN, Finch C, Wahl E, et al. Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial. Medical Decision Making 2011;31(3):412‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Valdimarsdottir HB, DeMarco TA, Peshkin BN, Lawrence W, Rispoli J, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychology 2009;28(1):11‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2006 {published data only}
- Sheridan SL, Shadle J, Simpson RJ Jr, Pignone MP. The impact of a decision aid about heart disease prevention on patients' discussions with their doctor and their plans for prevention: a pilot randomized trial. BMC Health Services Research 2006;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2011 {published data only}
- Sheridan SL, Draeger LB, Pignone MP, Keyserling TC, Simpson RJ Jr, Rimer B, et al. A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies. BMC Health Services Research 2011;11:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan SL, Draeger LB, Pignone MP, Rimer B, Bangdiwala SI, Cai J, Gizlice Z, Keyserling TC, Simpson RJ. The effect of a decision aid intervention on decision making about coronary heart disease risk reduction: secondary analyses of a randomized trial. BMC Medical Informatics and Decision Making 2014;14(14):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shorten 2005 {published and unpublished data}
- Shorten A, Shorten B, Keogh J, West S, Morris J. Making choices for childbirth: a randomized controlled trial of a decision‐aid for informed birth after cesarean. Birth 2005;32(4):252‐61. [DOI] [PubMed] [Google Scholar]
Shourie 2013 {published data only}
- Shourie S, Jackson C, Cheater FM, Bekker HL, Edlin R, Tubeuf S, et al. A cluster randomised controlled trial of a web based decision aid to support parents' decisions about their child's Measles Mumps and Rubella (MMR) vaccination. Vaccine 2013;31(50):6003‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2010 {published data only}
- Smith SK, Barratt A, Trevana L, Simpson JM, Jansen J, McCaffery KJ. A theoretical framework for measuring knowledge in screening decision aid trials. Patient Education and Counseling 2012;89:330‐6. [DOI] [PubMed] [Google Scholar]
- Smith SK, Kearney P, Trevena L, Barratt A, Nutbeam D, McCaffery KJ. Informed choice in bowel cancer screening:a qualitative study to explore how adults with lower education use decision aids. Health Expectations 2012;17:511‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SK, Simpson JM, Trevena LJ, McCaffery KJ. Factors associated with informed decisions and participation in bowel cancer screening among adults with lower education and literacy. Medical Decision Making 2014;34(6):756‐72. [DOI] [PubMed] [Google Scholar]
- Smith SK, Trevena L, Simpson JM, Barratt A, Nutbeam D, McCaffery KJ. A decision aid to support informed choices about bowel cancer screening among adults with low education: randomised controlled trial. BMJ 2010;341:c5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2014a {published and unpublished data}
- Stacey D, Hawker G, Dervin G, Tugwell P, Boland L, Pomey MP, et al. Decision aid for patients considering total knee arthroplasty with preference report for surgeons: A pilot randomized controlled trial. BMC Musculoskeletal Disorders 2014;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steckelberg 2011 {published data only}
- Steckelberg A, Hulfenhaus C, Haastert B, Muhlhauser I. Effect of evidence based risk information on "informed choice" in colorectal cancer screening: randomised controlled trial. BMJ 2011;342:d3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor KL, Davis JL 3rd, Turner RO, Johnson L, Schwartz MD, Kerner JF, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiology, Biomarkers & Prevention 2006;15(11):2179‐88. [DOI] [PubMed] [Google Scholar]
Thomson 2007 {published data only}
- Kaner E, Heaven B, Rapley T, Murtagh M, Graham R, Thomson R, et al. Medical communication and technology: a video‐based process study of the use of decision aids in primary care consultations. BMC Medical Informatics and Decision Making 2007;7(2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, et al. A patient decision aid to support shared decision‐making on anti‐thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Quality & Safety in Health Care 2007;16(3):216‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trevena 2008 {published data only}
- Trevena LJ, Irwig L, Barratt A. Randomized trial of a self‐administered decision aid for colorectal cancer screening. Journal of Medical Screening 2008;15(2):76‐82. [DOI] [PubMed] [Google Scholar]
Vandemheen 2009 {published data only}
- Vandemheen KL, O'Connor A, Bell SC, Freitag A, Bye P, Jeanneret A, et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. American Journal of Respiratory & Critical Care Medicine 2009;180(8):761‐8. [DOI] [PubMed] [Google Scholar]
Van Peperstraten 2010 {published data only}
- Kreuwel I, Peperstraten A, Hulscher M, Kremer J, Grol R, Nelen W, Hermens R. Evaluation of an effective multifaceted implementation strategy for elective single‐embryo transfer after in vitro fertilization. Human Reproduction 2013;28(2):336‐42. [DOI] [PubMed] [Google Scholar]
- Peperstraten A, Nelen W, Grol R, Zielhuis G, Adang E, Stalmeier P, et al. The effect of a multifaceted empowerment strategy on decision making about the number of embryos transferred in in vitro fertilisation: randomised controlled trial. BMJ 2010;341:c2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vodermaier 2009 {published data only}
- Vodermaier A, Caspari C, Koehm J, Kahlert S, Ditsch N, Untch M. Contextual factors in shared decision making: a randomised controlled trial in women with a strong suspicion of breast cancer. British Journal of Cancer 2009;100(4):590‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volk 1999 {published and unpublished data}
- Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Archives of Family Medicine 1999;8(4):333‐40. [DOI] [PubMed] [Google Scholar]
- Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1‐year follow‐up. Annals of Family Medicine 2003;1(1):22‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vuorma 2003 {published data only}
- Vuorma S, Rissanen P, Aalto AM, Hurskainen R, Kujansuu E, Teperi J. Impact of patient information booklet on treatment decision ‐ a randomized trial among women with heavy menstruation. Health Expectations 2003;6(4):290‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorma S, Teperi J, Aalto AM, Hurskainen R, Kujansuu E, Rissanen P. A randomized trial among women with heavy menstruation ‐ impact of a decision aid on treatment outcomes and costs. Health Expectations 2004;7(4):327‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2006 {published data only}
- Watson E, Hewitson P, Brett J, Bukach C, Evans R, Edwards A, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men's knowledge, attitudes and intention to be tested. Patient Education and Counseling 2006;63(3):367‐79. [DOI] [PubMed] [Google Scholar]
Weymiller 2007 {published data only}
- Jones LA, Weymiller AJ, Shah N, Bryant SC, Christianson TJH, Guyatt GH, et al. Should clinicians deliver decision aids? further exploration of the statin choice randomized trial results. Medical Decision Making 2009;29(4):468‐74. [DOI] [PubMed] [Google Scholar]
- Nannenga MR, Montori VM, Weymiller AJ, Smith SA, Christianson TJ, Bryant SC, et al. A treatment decision aid may increase patient trust in the diabetes specialist. The Statin Choice randomized trial. Health Expectations 2009;12(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymiller AJ, Montori VM, Jones LA, Gafni A, Guyatt GH, Bryant SC, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Archives of Internal Medicine 2007;167(10):1076‐82. [DOI] [PubMed] [Google Scholar]
Whelan 2003 {published and unpublished data}
- Whelan T, Sawka C, Levine M, Gafni A, Reyno L, Willan A, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node‐negative breast cancer. Journal of the National Cancer Institute 2003;95(8):581‐7. [DOI] [PubMed] [Google Scholar]
Whelan 2004 {published and unpublished data}
- Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA 2004;292(4):435‐41. [DOI] [PubMed] [Google Scholar]
Williams 2013 {published and unpublished data}
- Williams RM, Davis KM, Luta G, Edmond SN, Dorfman CS, Schwartz MD, et al. Fostering informed decisions: A randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Education and Counseling 2013;91:329‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 1996 {published data only}
- Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate‐specific antigen screening. Archives of Internal Medicine 1996;156(12):1333‐6. [PubMed] [Google Scholar]
- Wolf AM, Schorling JB. Preferences of elderly men for prostate‐specific antigen screening and the impact of informed consent. Journals of Gerontology Series A‐Biological Sciences & Medical Sciences 1998;53(3):M195‐200. [DOI] [PubMed] [Google Scholar]
Wolf 2000 {published and unpublished data}
- Wolf AM, Schorling JB. Does informed consent alter elderly patients' preferences for colorectal cancer screening? Results of a randomized trial. Journal of General Internal Medicine 2000;15(1):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong SS, Thornton JG, Gbolade B, Bekker HL. A randomised controlled trial of a decision‐aid leaflet to facilitate women's choice between pregnancy termination methods. BJOG: An International Journal of Obstetrics & Gynaecology 2006;113(6):688‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abadie 2009 {published data only}
- Abadie R, Weymiller AJ, Tilburt J, Shah ND, Charles C, Gafni A, et al. Clinician's use of the Statin Choice decision aid in patients with diabetes: a videographic study nested in a randomized trial. Journal of Evaluation in Clinical Practice 2009;15(3):492‐7. [DOI] [PubMed] [Google Scholar]
Adab 2003 {published data only}
- Adab P, Marshall T, Rouse A, Randhawa B, Sangha H, Bhangoo N. Randomised controlled trial of the effect of evidence based information on women's willingness to participate in cervical cancer screening. Journal of Epidemiology & Community Health 2003;57(8):589‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alegría 2014 {published data only}
- Alegría M, Carson N, Flores M, Li X, Shi P, Lessios AS, et al. Activation, self‐management, engagement, and retention in behavioral health care: a randomized clinical trial of the DECIDE intervention. JAMA Psychiatry 2014;71(5):557‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al Saffar 2008 {published data only}
- Al Saffar N, Abdulkareem A, Abdulhakeem A, Salah AQ, Heba M. Depressed patients' preferences for education about medications by pharmacists in Kuwait. Patient Education and Counseling 2008;72(1):94‐101. [DOI] [PubMed] [Google Scholar]
Altiner 2007 {published data only}
- Altiner A, Brockmann S, Sielk M, Wilm S, Wegscheider K, Abholz HH. Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: a cluster‐randomized intervention study. Journal of Antimicrobial Chemotherapy 2007;60(3):638‐44. [DOI] [PubMed] [Google Scholar]
Anderson 2011 {published data only}
- Anderson C, Carter J, Nattress K, Beale P, Philp S, Harrison J, et al. "The booklet helped me not to panic": a pilot of a decision aid for asymptomatic women with ovarian cancer and with rising CA‐125 levels. International Journal of Gynecological Cancer 2011;21(4):737‐43. [DOI] [PubMed] [Google Scholar]
Arimori 2006 {published data only}
- Arimori N. Randomized controlled trial of decision aids for women considering prenatal testing: the effect of the Ottawa Personal Decision Guide on decisional conflict. Japan Journal of Nursing Science 2006;3(2):119‐30. [Google Scholar]
Armstrong 2005 {published data only}
- Armstrong K, Weber B, Ubel PA, Peters N, Holmes J, Schwartz JS. Individualized survival curves improve satisfaction with cancer risk management decisions in women with BRCA1/2 mutations. Journal of Clinical Oncology 2005;23(36):9319‐28. [DOI] [PubMed] [Google Scholar]
Arterburn 2013 {published data only}
- Arterburn D, Flum DR, Westbrook EO, Fuller S, Shea M, Bock SN, Landers J, Kowalski K, Turnbull E, Cummings DE, CROSSROADS Study Team. A population‐based, shared decision‐making approach to recruit for a randomized trial of bariatric surgery versus lifestyle for type 2 diabetes. Surgery for Obesity and Related Diseases 2013;9(6):837‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Au 2011 {published data only}
- Au AH, Lam WW, Chan MC, Or AY, Kwong A, Suen D, et al. Development and pilot‐testing of a decision aid for use among Chinese women facing breast cancer surgery. Health Expectations 2011;14(4):405‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakken 2014 {published data only}
- Bakken S, Jia H, Chen ES, Choi J, John RM, Lee NJ, et al. The effect of a mobile health decision support system on diagnosis and management of obesity, tobacco use, and depression in adults and children. The Journal for Nurse Practitioners 2014;10(10):774‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2009 {published data only}
- Becker H, Stuifbergen AK, Dormire SL. The effects of hormone therapy decision support for women with mobility impairments. Health Care for Women International 2009;30(9):845‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belkora 2012 {published data only}
- Belkora J, Stupar L, O'Donnell S, Loucks A, Moore D, Jupiter C, et al. Decision support by telephone: randomized controlled trial in a rural community setting. Patient Education and Counseling 2012;89(1):134‐42. [DOI] [PubMed] [Google Scholar]
Bellmunt 2010 {published data only}
- Bellmunt J, Eisen T, Szczylik C, Mulders P, Porta C. A new patient‐focused approach to the treatment of metastatic renal cell carcinoma: establishing customized treatment options. BJU International 2010;107(8):1190‐9. [DOI] [PubMed] [Google Scholar]
Bennett 2011 {published data only}
- Bennett PA. Making the choice: cesarean delivery by maternal request versus planned vaginal birth [PhD thesis]. University of Colorado at Denver. ProQuest. Ann Arbor: University of Colorado at Denver, 2011.
Bieber 2006 {published data only}
- Bieber C, Muller KG, Blumenstiel K, Eich W. Participative decision‐making as a measure to improve the doctor‐patient interaction with fibromyalgia patients [Partizipative Entscheidungsfindung als Maßnahme zur Verbesserung der Arzt‐Patient‐Interaktion mit Fibromyalgie‐Patientinnen]. Zeitschrift fur Medizinische Psychologie 2006;15(2):53‐60. [Google Scholar]
- Bieber C, Muller KG, Blumenstiel K, Hochlehnert A, Wilke S, Hartmann M, et al. A shared decision‐making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. Journal of Psychosomatic Research 2008;64(1):13‐20. [DOI] [PubMed] [Google Scholar]
Branda 2013 {published data only}
- Branda ME, LeBlanc A, Shah ND, Tiedje K, Ruud K, Houten H, et al. Shared decision making for patients with type 2 diabetes: a randomized trial in primary care. BMC Health Services Research 2013;13(301):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenner 2014 {published data only}
- Brenner A, Howard K, Lewis C, Sheridan S, Crutchfield T, Hawley S, et al. Comparing 3 values clarification methods for colorectal cancer screening decision‐making: a randomized trial in the US and Australia. Journal of General Internal Medicine 2014;29(3):507‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breslin 2008 {published data only}
- Breslin M, Mullan RJ, Montori VM. The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Education and Counseling 2008;73(3):465‐72. [DOI] [PubMed] [Google Scholar]
Brown 2004 {published data only}
- Brown RF, Butow PN, Sharrock MA, Henman M, Boyle F, Goldstein D, et al. Education and role modelling for clinical decisions with female cancer patients. Health Expectations 2004;7(4):303‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brundage 2001 {published data only}
- Brundage MD, Feldman‐Stewart D, Cosby R, Gregg R, Dixon P, Youssef Y, et al. Phase I study of a decision aid for patients with locally advanced non‐small‐cell lung cancer. Journal of Clinical Oncology 2001;19(5):1326‐35. [DOI] [PubMed] [Google Scholar]
Burton 2007 {published data only}
- Burton MJ. Booklet‐based education in vestibular rehabilitation or symptom control improved subjective health in Meniere disease. Evidence‐Based Medicine 2007;12(4):111. [DOI] [PubMed] [Google Scholar]
Buzhardt 2011 {published data only}
- Buzhardt J, Greenwood CR, Walker D, Anderson R, Howard W, Carta JJ. Effects of web‐based support on early head start home visitors' use of evidence‐based intervention decision making and growth in children's expressive communication. NHSA Dialog 2011;14(3):121‐46. [Google Scholar]
Campbell 2014 {published data only}
- Campbell SR, Holter MC, Manthey TJ, Rapp CA. The effect of CommonGround Software and Decision Support Center. American Journal of Psychiatric Rehabilitation 2014;17(2):166‐80. [Google Scholar]
Carling 2008 {published data only}
- Carling C, Kristoffersen DT, Herrin J, Treweek S, Oxman AD, Schunemann H, et al. How should the impact of different presentations of treatment effects on patient choice be evaluated? A pilot randomized trial. PLOS ONE 2008;3(11):e3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Causarano 2015 {published data only}
- Causarano N, Platt J, Baxter NN, Bagher S, Jones JM, Metcalfe KA, Hofer SOP, O'Neill AC, Cheng T, Starenkyj E, Zhong T. Pre‐consultation educational group intervention to improve shared decision‐making for postmastectomy breast reconstruction: a pilot randomized controlled trial. Support Cancer Care 2015;23:1365‐1375. [DOI] [PubMed] [Google Scholar]
Chadwick 1991 {published data only}
- Chadwick DJ, Gillatt DA, Gingell JC. Medical or surgical orchidectomy: the patients' choice. BMJ 1991;302(6776):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan EC, McFall SL, Byrd TL, Mullen PD, Volk RJ, Ureda J, et al. A community‐based intervention to promote informed decision making for prostate cancer screening among Hispanic American men changed knowledge and role preferences: a cluster RCT. Patient Education and Counseling 2011;84(2):e44‐51. [DOI] [PubMed] [Google Scholar]
Chewning 1999 {published data only}
- Chewning B, Mosena P, Wilson D, Erdman H, Potthoff S, Murphy A, et al. Evaluation of a computerized contraceptive decision aid for adolescent patients. Patient Education and Counseling 1999;38(3):227‐39. [DOI] [PubMed] [Google Scholar]
Chiew 2008 {published data only}
- Chiew KS, Shepherd H, Vardy J, Tattersall MHN, Butow PN, Leighl NB. Development and evaluation of a decision aid for patients considering first‐line chemotherapy for metastatic breast cancer. Health Expectations 2008;11(1):35‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clouston 2014 {published data only}
- Clouston K, Katz A, Martens PJ, Sisler J, Turner D, Lobchuk M, et al. CIHR/CCMB Team in Primary Care Oncology (PCO‐NET). Does access to a colorectal cancer screening website and/or a nurse‐managed telephone help line provided to patients by their family physician increase fecal occult blood test uptake? Results from a pragmatic cluster randomized controlled trial. BMC Cancer 2014;14:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Col 2007 {published data only}
- Col NF, Ngo L, Fortin JM, Goldberg RJ, O'Connor AM. Can computerized decision support help patients make complex treatment decisions? A randomized controlled trial of an individualized menopause decision aid. Medical Decision Making 2007;27(5):585‐98. [DOI] [PubMed] [Google Scholar]
Colella 2004 {published data only}
- Colella KM, DeLuca G. Shared decision making in patients with newly diagnosed prostate cancer: a model for treatment education and support. Urologic Nursing 2004;24(3):187‐91, 195‐6. [PubMed] [Google Scholar]
Costanza 2011 {published data only}
- Costanza ME, Luckmann RS, Rosal M, White MJ, LaPelle N, Partin M, et al. Helping men make an informed decision about prostate cancer screening: a pilot study of telephone counseling. Patient Education and Counseling 2011;82(2):193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulter 2003 {published data only}
- Coulter A. Patient information and shared decision‐making in cancer care. British Journal of Cancer 2003;89(Suppl 1):S15‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2012 {published data only}
- Cox CE, Lewis CL, Hanson LC, Hough CL, Kahn JM, White DB, et al. Development and pilot testing of a decision aid for surrogates of patients with prolonged mechanical ventilation. Critical Care Medicine 2012;40(8):2327‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crang‐Svalenius 1996 {published data only}
- Crang‐Svalenius E, Dykes AK, Jorgensen C. Women's informed choice of prenatal diagnosis: early ultrasound examination‐routine ultrasound examination‐age‐independent amniocentesis. Fetal Diagnosis & Therapy 1996;11(1):20‐5. [DOI] [PubMed] [Google Scholar]
Davison 1999 {published data only}
- Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Education and Counseling 1999;37(3):255‐63. [DOI] [PubMed] [Google Scholar]
Davison 2007 {published data only}
- Davison BJ, Goldenberg SL, Wiens KP, Gleave ME. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nursing 2007;30(5):E7‐15. [DOI] [PubMed] [Google Scholar]
De Boer 2012 {published data only}
- Boer JC, Blijderveen G, Dijk G, Duivenvoorden HJ, Williams M. Implementing structured, multiprofessional medical ethical decision‐making in a neonatal intensive care unit. Journal of Medical Ethics 2012;38(10):596‐601. [DOI] [PubMed] [Google Scholar]
Deen 2012 {published data only}
- Deen D, Lu WH, Weintraub MR, Maranda MJ, Elshafey S, Gold MR. The impact of different modalities for activating patients in a community health center setting. Patient Education and Counseling 2012;89(1):178‐83. [DOI] [PubMed] [Google Scholar]
De Haan 2013 {published data only}
- Haan MC, Wijkerslooth TR, Stoop E, Bossuyt P, Fockens P, Thomeer M, et al. Informed decision‐making in colorectal cancer screening using colonoscopy or CT‐colonography. Patient Education and Counseling 2013;91(3):318‐25. [DOI] [PubMed] [Google Scholar]
Deinzer 2009 {published data only}
- Deinzer A, Veelken R, Kohnen R, Schmieder RE. Is a shared decision‐making approach effective in improving hypertension management?. Journal of Clinical Hypertension 2009;11(5):266‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Denig 2014 {published data only}
- Denig P, Schuling J, Haaijer‐Ruskamp F, Voorham J. Effects of a patient oriented decision aid for prioritising treatment goals in diabetes: pragmatic randomised controlled trial. BMJ 2014;349:g5651. [DOI] [PubMed] [Google Scholar]
Deschamps 2004 {published and unpublished data}
- Deschamps MA, Taylor JG, Neubauer SL, Whiting S, Green K. Impact of pharmacist consultation versus a decision aid on decision making regarding hormone replacement therapy. International Journal of Pharmacy Practice 2004;12(1):21‐8. [Google Scholar]
Deyo 2000 {published and unpublished data}
- Deyo RA, Cherkin DC, Weinstein J, Howe J, Ciol M, Mulley AG. Involving patients in clinical decisions: impact of an interactive video program on use of back surgery. Medical Care 2000;38(9):959‐69. [DOI] [PubMed] [Google Scholar]
- Phelan EA, Deyo RA, Cherkin DC, Weinstein JN, Ciol MA, Kreuter W, et al. Helping patients decide about back surgery: a randomized trial of an interactive video program. Spine 2001;26(2):206‐12. [DOI] [PubMed] [Google Scholar]
Diefenbach 2012 {published data only}
- Diefenbach MA, Mohamed NE, Butz BP, Bar‐Chama N, Stock R, Cesaretti J, et al. Acceptability and preliminary feasibility of an internet/CD‐ROM‐based education and decision program for early‐stage prostate cancer patients: randomized pilot study. Journal of Medical Internet Research 2012;14(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobke 2008 {published data only}
- Dobke MK, Bhavsar D. Pilot trial of telemedicine as a decision aid for patients with chronic wounds. Telemedicine Journal and e‐health 2008;14(3):245‐9. [DOI] [PubMed] [Google Scholar]
Dodin 2001 {published and unpublished data}
- Dodin S, Legare F, Daudelin G, Tetroe J, O'Connor A. Making a decision about hormone replacement therapy. A randomized controlled trial [Prise de decision en matière d'hormonothérapie de remplacement]. Canadian Family Physician 2001;47:1586‐93. [PMC free article] [PubMed] [Google Scholar]
Donovan 2012 {published data only}
- Donovan JL. Presenting treatment options to men with clinically localized prostate cancer: the acceptability of active surveillance/monitoring. Journal of the National Cancer Institute. Monographs 2012;45:191‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 2008 {published data only}
- Driscoll DL, Rupert DJ, Golin CE, McCormack LA, Sheridan SL, Welch BM. Promoting prostate‐specific antigen informed decision‐making. Evaluating two community‐level interventions. American Journal of Preventive Medicine 2008;35(2):87‐94. [DOI] [PubMed] [Google Scholar]
Dunn 1998 {published and unpublished data}
- Dunn RA, Shenouda PE, Martin DR, Schultz AJ. Videotape increases parent knowledge about poliovirus vaccines and choices of polio vaccination schedules. Pediatrics 1998;102(2):e26. [DOI] [PubMed] [Google Scholar]
Eaton 2011 {published data only}
- Eaton L, Cherry C, Cain D, Pope H. A novel approach to prevention for at‐risk HIV negative men who have sex with men: creating a teachable moment to promote informed sexual decision making. American Journal of Public Health 2011;101(3):539‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eden 2009 {published data only}
- Eden KB, Dolan JG, Perrin NA, Kocaoglu D, Anderson N, Case J, et al. Patients were more consistent in randomized trial at prioritizing childbirth preferences using graphic‐numeric than verbal formats. Journal of Clinical Epidemiology 2009;62(4):415‐24. [DOI] [PubMed] [Google Scholar]
Eden 2014 {published data only}
- Eden KB, Perrin NA, Vesco KK, Guise JM. A randomized comparative trial of two decision tools for pregnant women with prior cesareans. Journal of Obstetric, Gynecologic, & Neonatal Nursing 2014;43:568‐79. [DOI] [PubMed] [Google Scholar]
Eden 2015 {published data only}
- Eden KB, Perrin NA, Hanson GC, Messing JT, Bloom TL, Campbell JC, et al. Use of online safety decision aid by abused women. American Journal of Preventive Medicine 2015;48(4):372‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2012 {published data only}
- Edwards JA, Snyder FJ, Allen PM, Makinson KA, Hamby DM. Decision making for risk management: a comparison of graphical methods for presenting quantitative uncertainty. Risk Analysis 2012;32(12):2055‐70. [DOI] [PubMed] [Google Scholar]
El‐Jawahri 2010 {published data only}
- El‐Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. Journal of Clinical Oncology 2010;28(2):305‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellison 2008 {published data only}
- Ellison GL, Weinrich SP. A randomized trial comparing web‐based decision aids on prostate cancer knowledge for African‐American men. Journal of the National Medical Association 2008;100(10):1139‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2004 {published data only}
- Elwyn G, Edwards A, Hood K, Robling M, Atwell C, Russell I, et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice 2004;21(4):337‐46. [DOI] [PubMed] [Google Scholar]
Emery 2007 {published data only}
- Emery J, Morris H, Goodchild R, Fanshawe T, Prevost AT, Bobrow M, et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. British Journal of Cancer 2007;97(4):486‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmett 2007 {published data only}
- Emmett CL, Murphy DJ, Patel RR, Fahey T, Jones C, Ricketts IW, et al. Decision‐making about mode of delivery after previous caesarean section: development and piloting of two computer‐based decision aids. Health Expectations 2007;10(2):161‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman‐Stewart 2006 {published data only}
- Feldman‐Stewart D, Brennenstuhl S, Brundage MD, Roques T. An explicit values clarification task: development and validation. Patient Education and Counseling 2006;63(3):350‐6. [DOI] [PubMed] [Google Scholar]
Feldman‐Stewart 2012 {published data only}
- Feldman‐Stewart D, Tong C, Siemens R, Alibhai S, Pickles T, Robinson J, Brundage MD. The impact of explicit values clarification exercises in a patient decision aid emerges after the decision is actually made: evidence from a randomized controlled trial. Medical Decision Making 2012;32(4):616‐26. [DOI] [PubMed] [Google Scholar]
Fiks 2013a {published data only}
- Fiks AG, Grundmeier RW, Mayne S, Song L, Feemster K, Karavite D, et al. Effectiveness of decision support for families, clinicians, or both on HPV vaccine receipt. Pediatrics 2013;131(6):1114‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flood 1996 {published data only}
- Flood AB, Wennberg JE, Nease RF Jr, Fowler FJ Jr, Ding J, Hynes LM. The importance of patient preference in the decision to screen for prostate cancer. Prostate Patient Outcomes Research Team. Journal of General Internal Medicine 1996;11(6):342‐9. [DOI] [PubMed] [Google Scholar]
Francis 2009 {published data only}
- Francis NA, Butler CC, Hood K, Simpson S, Wood F, Nuttall J. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ 2009;339:b2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraval 2015 {published data only}
- Fraval A, Chandrananth J, Chong YM, Tran P, Coventry LS. Internet based patient education improves informed consent for elective orthopaedic surgery: a randomized controlled trial. BMC Musculoskeletal Disorders 2015;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2001 {published data only}
- Frosch DL, Kaplan RM, Felitti V. Evaluation of two methods to facilitate shared decision making for men considering the prostate‐specific antigen test. Journal of General Internal Medicine 2001;16(6):391‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2003 {published data only}
- Frosch DL, Kaplan RM, Felitti VJ. A randomized controlled trial comparing internet and video to facilitate patient education for men considering the prostate specific antigen test. Journal of General Internal Medicine 2003;18(10):781‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2008b {published data only}
- Frosch DL, Legare F, Mangione CM. Using decision aids in community‐based primary care: a theory‐driven evaluation with ethnically diverse patients. Patient Education and Counseling 2008;73(3):490‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2011 {published data only}
- Frosch DL, Uy V, Ochoa S, Mangione CM. Evaluation of a behavior support intervention for patients with poorly controlled diabetes. Archives of Internal Medicine 2011;171(22):2011‐7. [DOI] [PubMed] [Google Scholar]
Frost 2009 {published data only}
- Frost J, Shaw A, Montgomery A, Murphy DJ. Women's views on the use of decision aids for decision making about the method of delivery following a previous caesarean section: qualitative interview study. BJOG: An International Journal of Obstetrics & Gynaecology 2009;116(7):896‐905. [DOI] [PubMed] [Google Scholar]
Fujiwara 2015 {published data only}
- Fujiwara H, Shimoda A, Ishikawa Y, Taneichi A, Ohashi M, Takahashi Y, et al. Effect of providing risk information on undergoing cervical cancer screening: a randomized controlled trial. Archives of Public Health 2015;73:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garvelink 2013 {published data only}
- Garvelink MM, ter Kuile MM, Fischer MJ, Louwé LA, Hilders CG, Kroep JR, et al. Development of a decision aid about fertility preservation for women with breast cancer in the Netherlands. Journal of Psychosomatic Obstetrics and Gynecology 2013;34(4):170‐8. [DOI] [PubMed] [Google Scholar]
Genz 2012 {published data only}
- Genz J, Haastert B, Müller H, Verheyen F, Cole D, Rathmann W, et al. Blood glucose testing and primary prevention of Type 2 diabetes ‐ evaluation of the effect of evidence‐based patient information: a randomized controlled trial. Diabetic Medicine 2012;29(8):1011‐20. [DOI] [PubMed] [Google Scholar]
Giordano 2014 {published data only}
- Giordano A, Lugaresi A, Confalonieri P, Granella F, Radice D, Trojano M, et al. Implementation of the "Sapere Migliora" information aid for newly diagnosed people with multiple sclerosis in routine clinical practice: a late‐phase controlled trial. Multiple Sclerosis Journal 2014;20(9):1234‐43. [DOI] [PubMed] [Google Scholar]
Goel 2001 {published and unpublished data}
- Goel V, Sawka CA, Thiel EC, Gort EH, O'Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Medical Decision Making 2001;21(1):1‐6. [DOI] [PubMed] [Google Scholar]
Graham 2000 {published data only}
- Graham W, Smith P, Kamal A, Fitzmaurice A, Smith N, Hamilton N. Randomised controlled trial comparing effectiveness of touch screen system with leaflet for providing women with information on prenatal tests. BMJ 2000;320(7228):155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2009 {published data only}
- Gray SW, O'Grady C, Karp L, Smith D, Schwartz JS, Hornik RC, et al. Risk information exposure and direct‐to‐consumer genetic testing for BRCA mutations among women with a personal or family history of breast or ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention 2009;18(4):1303‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2001b {published data only}
- Green MJ, McInerney AM, Biesecker BB, Fost N. Education about genetic testing for breast cancer susceptibility: patient preferences for a computer program or genetic counselor. American Journal of Medical Genetics 2001;103(1):24‐31. [DOI] [PubMed] [Google Scholar]
Green 2004 {published data only}
- Green MJ, Peterson SK, Baker MW, Friedman LC, Harper GR, Rubinstein WS, et al. Use of an educational computer program before genetic counseling for breast cancer susceptibility: effects on duration and content of counseling sessions. Genetics in Medicine 2005;7(4):221‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, et al. Effect of a computer‐based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA 2004;292(4):442‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenfield 1985 {published data only}
- Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Annals of Internal Medicine 1985;102(4):520‐8. [DOI] [PubMed] [Google Scholar]
Griffith 2008a {published data only}
- Griffith JM, Lewis CL, Brenner AR, Pignone MP. The effect of offering different numbers of colorectal cancer screening test options in a decision aid: a pilot randomized trial. BMC Medical Informatics and Decision Making 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffith 2008b {published data only}
- Griffith JM, Fichter M, Fowler FJ, Lewis C, Pignone MP. Should a colon cancer screening decision aid include the option of no testing? A comparative trial of two decision aids. BMC Medical Informatics and Decision Making 2008;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gruppen 1994 {published data only}
- Gruppen LD, Margolin J, Wisdom K, Grum CM. Outcome bias and cognitive dissonance in evaluating treatment decisions. Academic Medicine 1994;69(10 Suppl):S57‐9. [DOI] [PubMed] [Google Scholar]
Gummersbach 2015 {published data only}
- Gummersbach E, in der Schmitten J, Mortsiefer A, Abholz HH, Wegscheider K, Pentzek M. Willingness to participate in mammography screening ‐ a randomized controlled questionnaire study of responses to two patient information leaflets with different factual content. Deutsches Ärzteblatt International 2015;112(5):61‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hacking 2013 {published data only}
- Hacking B, Wallace L, Scott S, Kosmala‐Anderson J, Belkora J, McNeill A. Testing the feasibility, acceptability and effectiveness of a 'decision navigation' intervention for early stage prostate cancer patients in Scotland ‐ a randomised controlled trial. Psycho‐Oncology 2013;22(5):1017‐1024. [DOI] [PubMed] [Google Scholar]
Hall 2007 {published data only}
- Hall S, Chitty L, Dormandy E, Hollywood A, Wildschut HIJ, Fortuny A, et al. Undergoing prenatal screening for Down's syndrome: presentation of choice and information in Europe and Asia. European Journal of Human Genetics 2007;15(5):563‐9. [DOI] [PubMed] [Google Scholar]
Hall 2011 {published data only}
- Hall MJ, Manne SL, Winkel G, Chung DS, Weinberg DS, Meropol NJ. Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing. Cancer Epidemiology, Biomarkers and Prevention 2011;20(2):249‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamann 2014 {published data only}
- Hamann J, Maris N, Iosifidou P, Mendel R, Cohen R, Wolf P, Kissling W. Effects of a question prompt sheet on active patient behaviour: a randomized controlled trial with depressed outpatients. International Journal of Social Psychiatry 2014;60(3):227‐35. [DOI] [PubMed] [Google Scholar]
Harmsen 2014 {published data only}
- Harmsen CG, Kristiansen IS, Larsen PV, Nexøe J, Støvring H, Gyrd‐Hansen D, et al. Communicating risk using absolute risk reduction or prolongation of life formats: cluster‐randomised trial in general practice. British Journal of General Practice 2014;64(621):e199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harwood 2011 {published data only}
- Harwood R, Douglas C, Clark D. Decision aids for breast and nodal surgery in patients with early breast cancer: development and a pilot study. Asia‐Pacific Journal of Clinical Oncology 2011;7:114‐22. [DOI] [PubMed] [Google Scholar]
Healton 1999 {published data only}
- Healton C, Taylor S, Messeri P, Weinberg G, Bamji M. Effects of ZDV‐based patient education on intentions toward ZDV use, HIV testing and reproduction among a US cohort of women. AIDS Care 1999;11(6):675‐86. [DOI] [PubMed] [Google Scholar]
Henderson 2013 {published data only}
- Henderson C, Brohan E, Clement S, Williams P, Lassman F, Schauman O, et al. Decision aid on disclosure of mental health status to an employer: feasibility and outcomes of a randomised controlled trial. British Journal of Psychiatry 2013;203(5):350‐7. [DOI] [PubMed] [Google Scholar]
Herrera 1983 {published data only}
- Herrera AJ, Cochran B, Herrera A, Wallace B. Parental information and circumcision in highly motivated couples with higher education. Pediatrics 1983;71(2):233‐4. [PubMed] [Google Scholar]
Hess 2015 {published data only}
- Hess LM, Litwiller A, Byron J, Stutsman J, Kasper K, Learman LA. Preference elicitation tool for abnormal uterine bleeding treatment: a randomized controlled trial. The Patient: Patient Centered Outcomes Research 2015;8(2):217‐27. [DOI] [PubMed] [Google Scholar]
Hewison 2001 {published data only}
- Hewison J, Cuckle H, Baillie C, Sehmi I, Lindow S, Jackson F, et al. Use of videotapes for viewing at home to inform choice in Down syndrome screening: a randomised controlled trial. Prenatal Diagnosis 2001;21(2):146‐9. [DOI] [PubMed] [Google Scholar]
Heyn 2013 {published data only}
- Heyn L, Finset A, Eide H, Ruland CM. Effects of an interactive tailored patient assessment on patient‐clinician communication in cancer care. Psycho‐Oncology 2013;22(1):89‐96. [DOI] [PubMed] [Google Scholar]
Hickish 1995 {published data only}
- Hickish TF, Smith IE, Middleton G, Nicolson M. Patient preference for extended palliative chemotherapy for non‐small cell lung cancer. Lancet 1995;345(8953):857‐8. [DOI] [PubMed] [Google Scholar]
Hochlehnert 2006 {published data only}
- Hochlehnert A, Richter A, Bludau HB, Bieber C, Blumenstiel K, Mueller K, et al. A computer‐based information‐tool for chronic pain patients: computerized information to support the process of shared decision‐making. Patient Education and Counseling 2006;61(1):92‐8. [DOI] [PubMed] [Google Scholar]
Hofbauer 2008 {published data only}
- Hofbauer GFL, Buhler RPN, French LE, Brockes M, Scheuer E. Patient‐centered care in dermatology: an online system that provides accessible and appropriate information to guide patients' decision making. Archives of Dermatology 2008;144(9):1225‐7. [DOI] [PubMed] [Google Scholar]
Hoffman 2009 {published data only}
- Hoffman RM, Walter LC. Colorectal cancer screening in the elderly: the need for informed decision making. Journal of General Internal Medicine 2009;24(12):1336‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holbrook 2007 {published data only}
- Holbrook A, Labiris R, Goldsmith CH, Ota K, Harb S, Sebaldt RJ. Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial. CMAJ 2007;176(11):1583‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollen 2013 {published data only}
- Hollen PJ, Tyc VL, Donnangelo SF, Shannon SV, O'Laughlen MC, Hinton I, et al. A substance use decision aid for medically at‐risk adolescents: results of a randomized controlled trial for cancer‐surviving adolescents. Cancer Nursing 2013;36(5):355‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holloway 2003 {published data only}
- Holloway RM, Wilkinson C, Peters TJ, Russell I, Cohen D, Hale J, et al. Cluster‐randomised trial of risk communication to enhance informed uptake of cervical screening. British Journal of General Practice 2003;53(493):620‐5. [PMC free article] [PubMed] [Google Scholar]
Holmes‐Rovner 2011 {published data only}
- Holmes‐Rovner M, Kelly‐Blake K, Dwamena F, Dontje K, Henry R, Olomu A, et al. Shared decision making guidance reminders in practice (SDM‐GRIP). Patient Education and Counseling 2011;85(2):219‐24. [DOI] [PubMed] [Google Scholar]
Holt 2009 {published data only}
- Holt CL, Wynn TA, Litaker MS, Southward P, Jeames S, Schulz E. A comparison of a spiritually based and non‐spiritually based educational intervention for informed decision making for prostate cancer screening among church‐attending African‐American men. Urologic Nursing 2009;29(4):249‐58. [PMC free article] [PubMed] [Google Scholar]
Hope 2010 {published data only}
- Hope N, Rombauts L. Can an educational DVD improve the acceptability of elective single embryo transfer? A randomized controlled study. Fertility and Sterility 2010;94(2):489‐95. [DOI] [PubMed] [Google Scholar]
Huijbregts 2013 {published data only}
- Huijbregts KML, Jong FJ, Marwijk HWJ, Beekman ATF, Adèr HJ, Hakkaart‐van Roijen L, et al. A target‐driven collaborative care model for major depressive disorder is effective in primary care in the Netherlands. A randomized clinical trial from the depression initiative. Journal of Affective Disorders 2013;146:328‐37. [DOI] [PubMed] [Google Scholar]
Hunt 2005 {published data only}
- Hunt LM, Voogd KB, Castaneda H. The routine and the traumatic in prenatal genetic diagnosis: does clinical information inform patient decision‐making?. Patient Education and Counseling 2005;56(3):302‐12. [DOI] [PubMed] [Google Scholar]
Hunter 1999 {published data only}
- Hunter M, O'Dea I. An evaluation of a health education intervention for mid‐aged women: five year follow‐up of effects upon knowledge, impact of menopause and health. Patient Education and Counseling 1999;38(3):249‐55. [DOI] [PubMed] [Google Scholar]
Hunter 2005 {published data only}
- Hunter AG, Cappelli M, Humphreys L, Allanson JE, Chiu TT, Peeters C, et al. A randomized trial comparing alternative approaches to prenatal diagnosis counseling in advanced maternal age patients. Clinical Genetics 2005;67(4):303‐13. [DOI] [PubMed] [Google Scholar]
Huyghe 2009 {published data only}
- Huyghe E, Martinetti P, Sui D, Schover LR. Banking on Fatherhood: pilot studies of a computerized educational tool on sperm banking before cancer treatment. Psycho‐Oncology 2009;18(9):1011‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilic 2008 {published data only}
- Ilic D, Egberts K, McKenzie JE, Risgridger G, Green S. Informing men about prostate cancer screening: a randomized controlled trial of patient education materials. Journal of General Internal Medicine 2008;23(4):466‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Isebaert 2007 {published data only}
- Isebaert S, Audenhove C, Haustermans K, DeRidder K, Junius S, Joniau S, et al. A decision aid for patients with localized prostate cancer: first results [Een beslissingshulp voor patienten met gelokaliseerde prostaatkanker: eerste resultaten]. Tijdschrift voor Geneeskunde 2007;63(1):15‐21. [Google Scholar]
Jackson 2011 {published data only}
- Jackson C, Cheater FM, Harrison W, Peacock R, Bekker H, West R, et al. Randomised cluster trial to support informed parental decision‐making for the MMR vaccine. BMC Public Health 2011;11:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2007 {published data only}
- Jerant A, Kravitz RL, Rooney M, Amerson S, Kreuter M, Franks P. Effects of a tailored interactive multimedia computer program on determinants of colorectal cancer screening: a randomized controlled pilot study in physician offices. Patient Education and Counseling 2007;66(1):67‐74. [DOI] [PubMed] [Google Scholar]
Jibaja‐Weiss 2006 {published data only}
- Jibaja‐Weiss ML, Volk RJ, Granchi TS, Neff NE, Spann SJ, Aoki N, et al. Entertainment education for informed breast cancer treatment decisions in low‐literate women: development and initial evaluation of a patient decision aid. Journal of Cancer Education 2006;21(3):133‐9. [DOI] [PubMed] [Google Scholar]
Joosten 2009 {published data only}
- Joosten EA, Jong CA, Weert‐van Oene GH, Sensky T, Staak CP. Shared decision‐making reduces drug use and psychiatric severity in substance‐dependent patients. Psychotherapy and Psychosomatics 2009;78:245‐53. [DOI] [PubMed] [Google Scholar]
Joosten 2011 {published data only}
- Joosten EA, Jong CA, Weert‐van Oene GH, Sensky T, Staak CP. Shared decision‐making: increases autonomy in substance‐dependent patients. Substance Use and Misuse 2011;48:1037‐48. [DOI] [PubMed] [Google Scholar]
Jorm 2003 {published data only}
- Jorm AF, Griffiths KM, Christensen H, Korten AE, Parslow RA, Rodgers B. Providing information about the effectiveness of treatment options to depressed people in the community: a randomized controlled trial of effects on mental health literacy, help‐seeking and symptoms. Psychological Medicine 2003;33(6):1071‐9. [DOI] [PubMed] [Google Scholar]
Kakkilaya 2011 {published data only}
- Kakkilaya V, Groome L, Platt D, Kurepa D, Pramanik A, Caldito G, et al. Use of a visual aid to improve counseling at the threshold of viability. Pediatrics 2011;128(6):e1511‐9. [DOI] [PubMed] [Google Scholar]
Kaplan 2014a {published data only}
- Kaplan CP, Livaudais‐Toman J, Tice JA, Kerlikowske K, Gregorich SE, Pérez‐Stable EJ, et al. A randomized, controlled trial to increase discussion of breast cancer in primary care. Cancer Epidemiology, Biomarkers & Prevention 2014;23(7):1245‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaplan 2014b {published data only}
- Kaplan AL, Crespi CM, Saucedo JD, Connor SE, Litwin MS, Saigal CS. Decisional conflict in economically disadvantaged men with newly diagnosed prostate cancer. Cancer 2014;120(17):2721‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassan 2012 {published data only}
- Kassan EC, Williams RM, Kelly SP, Barry SA, Penek S, Fishman MB, Cole CA, et al. Men's use of an internet‐based decision aid for prostate cancer screening. Journal of Health Communication 2012;17(6):677‐97. [DOI] [PubMed] [Google Scholar]
Kellar 2008 {published data only}
- Kellar I, Sutton S, Griffin S, Prevost AT, Kinmonth AL, Marteau TM. Evaluation of an informed choice invitation for type 2 diabetes screening. Patient Education and Counseling 2008;72(2):232‐8. [DOI] [PubMed] [Google Scholar]
Kiatpongsan 2014 {published data only}
- Kiatpongsan S, Carlson K, Feibelmann S, Sepucha K. Decision aid reduces misperceptions about hormone therapy: a randomized controlled trial. Menopause: The Journal of The North American Menopause Society 2014;21(1):33‐38. [DOI] [PubMed] [Google Scholar]
Kobelka 2009 {published data only}
- Kobelka C, Mattman A, Langlois S. An evaluation of the decision‐making process regarding amniocentesis following a screen‐positive maternal serum screen result. Prenatal Diagnosis 2009;29(5):514‐9. [DOI] [PubMed] [Google Scholar]
Koelewijn‐van Loon 2009 {published data only}
- Koelewijn‐van Loon MS, Weijden T, Steenkiste B, Ronda G, Winkens B, Severens JL, et al. Involving patients in cardiovascular risk management with nurse‐led clinics: a cluster randomized controlled trial. CMAJ 2009;181(12):E267‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Köpke 2009 {published data only}
- Köpke S, Kasper J, Mühlhauser I, Nübling M, Heesen C. Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized‐controlled trial. Multiple Sclerosis 2009;15(1):96‐104. [DOI] [PubMed] [Google Scholar]
Köpke 2014 {published data only}
- Köpke S, Kern S, Ziemssen T, Berghoff M, Kleiter I, Marziniak M, et al. Evidence‐based patient information programme in early multiple sclerosis: a randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(4):411‐18. [DOI] [PubMed] [Google Scholar]
Krawczyk 2012 {published data only}
- Krawczyk A. Cancer Prevention and the Human Papillomavirus Vaccine: Psychosocial and Behavioural Factors Involved in Vaccination Decision‐making [PhD thesis]. Montreal: McGill Library, 2012. [Google Scholar]
Kripalani 2007 {published data only}
- Kripalani S, Sharma J, Justice E, Justice J, Spiker C, Laufman LE, et al. Low‐literacy interventions to promote discussion of prostate cancer: a randomized controlled trial. American Journal of Preventive Medicine 2007;33(2):83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krones 2008 {published data only}
- Krones T, Keller H, Becker A, Sonnichsen A, Baum E, Donner‐Banzhoff N. The theory of planned behaviour in a randomized trial of a decision aid on cardiovascular risk prevention. Patient Education and Counseling 2009;78(2):169‐76. [DOI] [PubMed] [Google Scholar]
- Krones T, Keller H, Sönnichsen A, Sadowski EM, Baum E, Wegscheider K, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Annals of Family Medicine 2008;6(3):218‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuppermann 2009 {published data only}
- Kuppermann M, Norton ME, Gates E, Gregorich SE, Learman LA, Nakagawa S, et al. Computerized prenatal genetic testing decision‐assisting tool: a randomized controlled trial. Obstetrics & Gynecology 2009;113(1):53‐63. [DOI] [PubMed] [Google Scholar]
Kurian 2009 {published data only}
- Kurian B, Trivedi M, Grannemann B, Claassen C, Daly E, Sunderajan P. A computerized decision support system for depression in primary care. Primary Care Companion to the Journal of Clinical Psychiatry 2009;11(4):140‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Labrecque 2010 {published data only}
- Labrecque M, Paunescu C, Plesu I, Stacey D, Legare F. Evaluation of the effect of a patient decision aid about vasectomy on the decision‐making process: a randomized trial. Contraception 2010;82(6):556‐62. [DOI] [PubMed] [Google Scholar]
LaCroix 1999 {published data only}
- LaCroix AZ, Newton KM, Buist DSM, Curry SJ, Scholes D, Anderson LA, et al. Population‐based strategy for improving informed decision making about hormone replacement therapy in managed care settings. Women's Health Issues 1999;9(6):306‐18. [Google Scholar]
Lairson 2011 {published data only}
- Lairson DR, Chan W, Chang YC, Junco DJ, Vernon SW. Cost‐effectiveness of targeted versus tailored interventions to promote mammography screening among women military veterans in the United States. Evaluation and Program Planning 2011;34(2):97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalonde 2006 {published data only}
- Lalonde L, O'Connor AM, Duguay P, Brassard J, Drake E, Grover SA. Evaluation of a decision aid and a personal risk profile in community pharmacy for patients considering options to improve cardiovascular health: the OPTIONS pilot study. International Journal of Pharmacy Practice 2006;14(1):51‐62. [Google Scholar]
Lancaster 2009 {published data only}
- Lancaster T. Physician training in the use of a decision aid increased patient participation in decision making for CVD prevention. Evidence‐Based Medicine 2009;14(1):24. [DOI] [PubMed] [Google Scholar]
Landrey 2013 {published data only}
- Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate‐specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. Journal of Primary Care & Community Health 2013;4(1):67‐74. [DOI] [PubMed] [Google Scholar]
Lazcano Ponce 2000 {published data only}
- Lazcano Ponce EC, Sloan NL, Winikoff B, Langer A, Coggins C, Heimburger A, et al. The power of information and contraceptive choice in a family planning setting in Mexico. Sexually Transmitted Infections 2000;76(4):277‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legare 2003 {published data only}
- Legare F, O'Connor AM, Graham ID, Wells GA, Jacobsen MJ, Elmslie T, et al. The effect of decision aids on the agreement between women's and physicians' decisional conflict about hormone replacement therapy. Patient Education and Counseling 2003;50(2):211‐21. [DOI] [PubMed] [Google Scholar]
Leung 2004 {published data only}
- Leung KY, Lee CP, Chan HY, Tang MH, Lam YH, Lee A. Randomised trial comparing an interactive multimedia decision aid with a leaflet and a video to give information about prenatal screening for Down syndrome. Prenatal Diagnosis 2004;24(8):613‐8. [DOI] [PubMed] [Google Scholar]
Levin 2011 {published data only}
- Levin W, Campbell D, McGovern K, Gau J, Kosty D, Seeley J, Lewinsohn P. A computer‐assisted depression intervention in primary care. Psychological Medicine 2011;41(7):1373‐83. [DOI] [PubMed] [Google Scholar]
Lewis 2003 {published data only}
- Lewis CL, Pignone MP, Sheridan SL, Downs SM, Kinsinger LS. A randomized trial of three videos that differ in the framing of information about mammography in women 40 to 49 years old. Journal of General Internal Medicine 2003;18(11):875‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
- Lewis CL, Brenner AT, Griffith JM, Moore CG, Pignone MP. Two controlled trials to determine the effectiveness of a mailed intervention to increase colon cancer screening. North Carolina Medical Journal 2012;73(2):93‐8. [PMC free article] [PubMed] [Google Scholar]
Lopez‐Jornet 2012 {published data only}
- López‐Jornet P, Camacho‐Alonso F, Sanchez‐Siles M. Patient information preferences and behaviour in relation to oral biopsies. British Journal of Oral & Maxillofacial Surgery 2012;50(8):e115‐8. [DOI] [PubMed] [Google Scholar]
Lukens 2013 {published data only}
- Lukens JM, Solomon P, Sorenson SB. Shared decision‐making for clients with mental illness: a randomized factorial survey. Research on Social Work Practice 2013;23(6):694‐705. [Google Scholar]
Lurie 2011 {published data only}
- Lurie J, Spratt K, Blood E, Tosteson T, Tosteson A, Weinstein J. Effects of viewing an evidence based video decision aid on patients' treatment preferences for spine surgery. Spine 2011;36(18):1501‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maisels 1983 {published data only}
- Maisels MJ, Hayes B, Conrad S, Chez RA. Circumcision: the effect of information on parental decision making. Pediatrics 1983;71(3):453‐5. [PubMed] [Google Scholar]
Mancini 2006 {published data only}
- Mancini J, Santin G, Chabal F, Julian‐Reynier C. Cross‐cultural validation of the Decisional Conflict Scale in a sample of French patients. Quality of Life Research 2006;15(6):1063‐8. [DOI] [PubMed] [Google Scholar]
Manne 2009 {published data only}
- Manne SL, Coups EJ, Markowitz A, Meropol NJ, Haller D, Jacobsen PB, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Annals of Behavioral Medicine 2009;37(2):207‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manns 2005 {published data only}
- Manns B J, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, et al. The impact of education on chronic kidney disease patients'' plans to initiate dialysis with self‐care dialysis: a randomized trial. Kidney International 2005;68(4):1777‐83. [DOI] [PubMed] [Google Scholar]
Markham 2003 {published data only}
- Markham R, Smith A. Limits to patient choice: example from anaesthesia. BMJ 2003;326(7394):863‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2012 {published data only}
- Martin R, Brower M, Geralds A, Gallagher P, Tellinghuisen D. An experimental evaluation of patient decision aid design to communicate the effects of medications on the rate of progression of structural joint damage in rheumatoid arthritis. Patient Education and Counseling 2012;86(3):329‐34. [DOI] [PubMed] [Google Scholar]
Maslin 1998 {published data only}
- Maslin AM, Baum M, Walker JS, A'Hern R, Prouse A. Shared decision‐making using an interactive video disk system for women with early breast cancer. NT Research 1998;3(6):444‐55. [Google Scholar]
- Maslin AM, Baum M, Walker JS, A'Hern R, Prouse A. Using an interactive video disk in breast cancer patient support. Nursing Times 1998;94(44):4‐10. [PubMed] [Google Scholar]
Matlock 2014 {published data only}
- Matlock DD, Keech TA, McKenzie MB, Bronsert MR, Nowels CT, Kutner JS. Feasibility and acceptability of a decision aid designed for people facing advanced or terminal illness: a pilot randomized trial. Health Expectations 2014;17(1):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matloff 2006 {published data only}
- Matloff ET, Moyer A, Shannon KM, Niendorf KB, Col NF. Healthy women with a family history of breast cancer: impact of a tailored genetic counseling intervention on risk perception, knowledge, and menopausal therapy decision making. Journal of Women's Health 2006;15(7):843‐56. [DOI] [PubMed] [Google Scholar]
Mazur 1994 {published data only}
- Mazur DJ, Hickam DH. The effect of physician's explanations on patients' treatment preferences: five‐year survival data. Medical Decision Making 1994;14(3):255‐8. [DOI] [PubMed] [Google Scholar]
McCaffery 2007 {published data only}
- McCaffery K, Irwig L, Bossuyt P. Patient decision aids to support clinical decision making: evaluating the decision or the outcomes of the decision. Medical Decision Making 2007;27(5):619‐25. [DOI] [PubMed] [Google Scholar]
McGinley 2002 {published data only}
- McGinley AM. Effect of Web‐based Computer‐tailoring on Women's Intention to Continue or Begin to Use Hormone Replacement Therapy to Lower their Risk for Osteoporosis [PhD thesis]. Philadelphia: University of Pennsylvania, 2002. [Google Scholar]
McGowan 2008 {published data only}
- McGowan J, Hogg W, Campbell C, Rowan M. Just‐in‐time information improved decision‐making in primary care: a randomized controlled trial. PLOS ONE 2008;3(11):e3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
McInerney‐Leo 2004 {published data only}
- McInerney‐Leo A, Biesecker BB, Hadley DW, Kase RG, Giambarresi TR, Johnson E, et al. BRCA1/2 testing in hereditary breast and ovarian cancer families: effectiveness of problem‐solving training as a counseling intervention. American Journal of Medical Genetics. Part A 2004;130(3):221‐7. [DOI] [PubMed] [Google Scholar]
Mclaren 2012 {published data only}
- Mclaren PJ, Hyde MK, White KM. Exploring the role of gender and risk perceptions in people's decisions to register as a bone marrow donor. Health Education Research 2011;27(3):513‐22. [DOI] [PubMed] [Google Scholar]
Meropol 2013 {published data only}
- Meropol NJ, Egleston BL, Buzaglo JS, Balshem A, Benson AB 3rd, Cegala DJ, et al. A web‐based communication aid for patients with cancer: the CONNECT study. Cancer 2013;119(7):1437‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 1997 {published data only}
- Michie S, Smith D, McClennan A, Marteau TM. Patient decision making: An evaluation of two different methods of presenting information about a screening test. British Journal of Health Psychology 1997;2(4):317‐26. [Google Scholar]
Miller 2014a {published data only}
- Miller MJ, Allison JJ, Cobaugh DJ, Ray MN, Saag KG. A group‐randomized trial of shared decision making for non‐steroidal anti‐inflammatory drug risk awareness: primary results and lessons learned. Journal of Evaluation in Clinical Practice 2014;20:638‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2014b {published data only}
- Miller SM, Roussi P, Scarpato J, Wen KY, Zhu F, Roy G. Randomized trial of print messaging: the role of the partner and monitoring style in promoting provider discussions about prostate cancer screening among African American men. Psycho‐Oncology 2014;23:404‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishel 2009 {published data only}
- Mishel MH, Germino BB, Lin L, Pruthi RS, Wallen EM, Crandell J, et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial. Patient Education and Counseling 2009;77(3):349‐59. [DOI] [PubMed] [Google Scholar]
Mohammad 2012 {published data only}
- Mohammad‐Alizadeh‐Charandabi S, Shahnazi M, Jahanbakhsh. Communicating contraceptive effectiveness: a randomized controlled trial. Journal of Caring Sciences 2012;1(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molenaar 2001 {published data only}
- Molenaar S, Sprangers MA, Rutgers EJ, Luiten EJ, Mulder J, Bossuyt PM, et al. Decision support for patients with early‐stage breast cancer: effects of an interactive breast cancer CDROM on treatment decision, satisfaction, and quality of life. Journal of Clinical Oncology 2001;19(6):1676‐87. [DOI] [PubMed] [Google Scholar]
Mulley 2006 {published data only}
- Mulley AG Jr. Developing skills for evidence‐based surgery: ensuring that patients make informed decisions. Surgical Clinics of North America 2006;86(1):181‐92. [DOI] [PubMed] [Google Scholar]
Myers 2005a {published data only}
- Myers RE, Daskalakis C, Cocroft J, Kunkel EJ, Delmoor E, Liberatore M, et al. Preparing African‐American men in community primary care practices to decide whether or not to have prostate cancer screening. Journal of the National Medical Association 2005;97(8):1143‐54. [PMC free article] [PubMed] [Google Scholar]
Myers 2005b {published data only}
- Myers RE. Decision counseling in cancer prevention and control. Health Psychology 2005;24(4 Suppl):S71‐7. [DOI] [PubMed] [Google Scholar]
Myers 2007 {published data only}
- Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer 2007;110(9):2083‐91. [DOI] [PubMed] [Google Scholar]
Myers 2011 {published data only}
- Myers RE, Daskalakis C, Kunkel EJ, Cocroft JR, Riggio JM, Capkin M, et al. Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counseling. Patient Education and Counseling 2011;83(2):240‐6. [DOI] [PubMed] [Google Scholar]
Myers 2013 {published data only}
- Myers RE, Bittner‐Fagan H, Daskalakis C, Sifri R, Vernon SW, Cocroft J, et al. A randomized controlled trial of a tailored navigation and a standard intervention in colorectal cancer screening. Cancer Epidemiology, Biomarkers & Prevention 2013;22(1):109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neubeck 2008 {published data only}
- Neubeck L, Redfern J, Briffa T, Bauman A, Hare D, Freedman SB. The CHOICE (Choice of Health Options In prevention of Cardiovascular Events) replication trial: study protocol. BMC Cardiovascular Disorders 2008;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2001 {published data only}
- Newton KM, LaCroix AZ, Buist DS, Delaney KM, Anderson LA. Women's responses to a mailed hormone replacement therapy workbook. Menopause 2001;8(5):361‐7. [DOI] [PubMed] [Google Scholar]
O'Cathain 2002 {published data only}
- O'Cathain A, Walters SJ, Nicholl JP, Thomas KJ, Kirkham M. Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice. BMJ 2002;324(7338):643‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 1996 {published data only}
- O'Connor AM, Pennie RA, Dales RE. Framing effects on expectations, decisions, and side effects experienced: the case of influenza immunization. Journal of Clinical Epidemiology 1996;49(11):1721‐6. [DOI] [PubMed] [Google Scholar]
O'Connor 1998a {published and unpublished data}
- O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. Randomized trial of a portable, self‐administered decision aid for postmenopausal women considering long‐term preventive hormone therapy. Medical Decision Making 1998;18:295‐303. [DOI] [PubMed] [Google Scholar]
O'Connor 1999a {published data only}
- O'Connor AM, Wells GA, Tugwell P, Laupacis A, Elmslie T, Drake E. The effects of an 'explicit' values clarification exercise in a women's decision aid regarding postmenopausal hormone therapy. Health Expectations 1999;2:21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2009a {published data only}
- O'Connor PJ, Sperl‐Hillen J, Johnson PE, Rush WA, Crain AL. Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial. Diabetes Care 2009;32(7):1158‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2011 {published data only}
- Connor PJ, Sperl‐Hillen JM, Rush WA, Johnson PE, Amundson GH, Asche SE, et al. Impact of electronic health record clinical decision support on diabetes care: a randomized trial. Annals of Family Medicine 2011;9(1):12‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owens 2014A {published data only}
- Owens OL. A Community‐driven Approach to the Development of a Digital Decision Aid to Facilitate Informed Decision Making for Prostate Cancer Screening among African‐American men in Communities of Faith [PhD thesis]. Columbia: University of South Carolina, 2014. [Google Scholar]
Patanwala 2011 {published data only}
- Patanwala IM, Brocklebank V, Inglis J, Trewby PN. A randomized questionnaire‐based study on the impact of providing numerical information on colorectal cancer screening. Journal of the Royal Society of Medicine Short Reports 2011;2(6):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2014 {published data only}
- Patel S, Ngunjiri A, Wan Hee S, Yang Y, Brown S, Friede T, et al. Primum non nocere: shared informed decision making in low back pain ‐ a pilot cluster randomised trial. BMC Musculoskeletal Disorders 2014;15:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearson 2005 {published data only}
- Pearson S, Maddern GJ, Hewett P. Interacting effects of preoperative information and patient choice in adaptation to colonoscopy. Diseases of the Colon & Rectum 2005;48(11):2047‐54. [DOI] [PubMed] [Google Scholar]
Peele 2005 {published data only}
- Peele PB, Siminoff LA, Xu Y, Ravdin PM. Decreased use of adjuvant breast cancer therapy in a randomized controlled trial of a decision aid with individualized risk information. Medical Decision Making 2005;25(3):301‐7. [DOI] [PubMed] [Google Scholar]
Petty 2014 {published data only}
- Petty J. Exploring the effectiveness of an interactive, technology‐enabled learning tool to enhance knowledge for neonatal nurses. Neonatal, Paediatric and Child Health Nursing 2014;17(1):2‐10. [Google Scholar]
Philip 2010 {published data only}
- Philip E, DuHamel K, Jandorf L. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: a preliminary study. Cancer Causes Control 2010;21(10):1685‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 1995 {published data only}
- Phillips C, Hill BJ, Cannac C. The influence of video imaging on patients' perceptions and expectations. Angle Orthodontist 1995;65(4):263‐70. [DOI] [PubMed] [Google Scholar]
Pignone 2013 {published data only}
- Pignone MP, Howard K, Brenner AT, Crutchfield TM, Hawley ST, Lewis CL, et al. Comparing 3 techniques for eliciting patient values for decision making about prostate‐specific antigen screening: a randomized controlled trial. JAMA Internal Medicine 2013;173(5):362‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2008 {published data only}
- Pinto H, Rumball D, Maskrey V, Holland R. A pilot study for a randomized controlled and patient preference trial of buprenorphine versus methadone maintenance treatment in the management of opiate dependent patients. Journal of Substance Use 2008;13(2):73‐82. [Google Scholar]
Powers 2011 {published data only}
- Powers B, Danus S, Grubber J, Olsen M, Oddone E, Bosworth H. The effectiveness of personalized coronary heart disease and stroke risk communication. American Heart Journal 2011;161(4):673‐80. [DOI] [PubMed] [Google Scholar]
Proctor 2006 {published data only}
- Proctor A, Jenkins TR, Loeb T, Elliot M, Ryan A. Patient satisfaction with 3 methods of postpartum contraceptive counseling: a randomized, prospective trial. Journal of Reproductive Medicine 2006;51(5):377‐82. [PubMed] [Google Scholar]
Prunty 2008 {published data only}
- Prunty MC, Sharpe L, Butow P, Fulcher G. The motherhood choice: a decision aid for women with multiple sclerosis. Patient Education and Counseling 2008;71(1):108‐15. [DOI] [PubMed] [Google Scholar]
Ranta 2015 {published data only}
- Ranta A, Dovey S, Weatherall M, O'Dea D, Gommans J, Tilyard M. Cluster randomized controlled trial of TIA electronic decision support in primary care. American Academy of Neurology 2015;84(15):1545‐51. [DOI] [PubMed] [Google Scholar]
Rapley 2006 {published data only}
- Rapley T, May C, Heaven B, Murtagh M, Graham R, Kaner EF, et al. Doctor‐patient interaction in a randomised controlled trial of decision‐support tools. Social Science & Medicine 2006;62(9):2267‐78. [DOI] [PubMed] [Google Scholar]
Raynes‐Greenow 2009 {published data only}
- Raynes‐Greenow CH, Roberts CL, Nassar N, Trevena L. Do audio‐guided decision aids improve outcomes? A randomized controlled trial of an audio‐guided decision aid compared with a booklet decision aid for Australian women considering labour analgesia. Health Expectations 2009;12(4):407‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raynes‐Greenow 2010 {published data only}
- Raynes‐Greenow CH, Nassar N, Torvaldsen S, Trevena L, Roberts CL. Assisting informed decision making for labour analgesia: a randomised controlled trial of a decision aid for labour analgesia versus a pamphlet. BMC Pregnancy and Childbirth 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rimer 2001 {published data only}
- Rimer BK, Halabi S, Sugg Skinner C, Kaplan EB, Crawford Y, Samsa GP, et al. The short‐term impact of tailored mammography decision‐making interventions. Patient Education and Counseling 2001;43(3):269‐85. [DOI] [PubMed] [Google Scholar]
Rimer 2002 {published data only}
- Rimer BK, Halabi S, Sugg Skinner C, Lipkus IM, Strigo TS, Kaplan EB, et al. Effects of a mammography decision‐making intervention at 12 and 24 months. American Journal of Preventive Medicine 2002;22(4):247‐57. [DOI] [PubMed] [Google Scholar]
Robinson 2013 {published data only}
- Robinson JK, Gaber R, Hultgren B, Eilers S, Blatt H, Stapleton J, et al. Skin self‐examination education for early detection of melanoma: a randomized controlled trial of internet, workbook and in‐person interventions. Journal of Medical Internet Research 2013;16(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronda 2014 {published and unpublished data}
- Ronda G, Grispen JEJ, Ickenroth M, Dinant GJ, Vries NK, Weijden T. The effects of a web‐based decision aid on the intention to diagnostic self‐testing for cholesterol and diabetes: a randomized controlled trial. BMC Public Health 2014;14:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rostom 2002 {published data only}
- Rostom A, O'Connor A, Tugwell P, Wells G. A randomized trial of a computerized versus an audio‐booklet decision aid for women considering post‐menopausal hormone replacement therapy. Patient Education and Counseling 2002;46(1):67‐74. [DOI] [PubMed] [Google Scholar]
Roter 2012 {published data only}
- Roter DL, Wexler R, Naragon P, Forrest B, Dees J, Almodovar A, Wood J. The impact of patient and physician computer mediated communication skill training on reported communication and patient satisfaction. Patient Education and Counseling 2012;88(3):406‐13. [DOI] [PubMed] [Google Scholar]
Rothert 1997 {published and unpublished data}
- Holmes‐Rovner M, Kroll J, Rovner DR, Schmitt N, Rothert M, Padonu G, et al. Patient decision support intervention: increased consistency with decision analytic models. Medical Care 1999;37(3):270‐84. [DOI] [PubMed] [Google Scholar]
- Rothert ML, Holmes‐Rovner M, Rovner D, Kroll J, Breer L, Talarczyk G, et al. An educational intervention as decision support for menopausal women. Research in Nursing & Health 1997;20(5):377‐87. [DOI] [PubMed] [Google Scholar]
Rovner 2004 {published data only}
- Rovner DR, Wills CE, Bonham V, Williams G, Lillie J, Kelly‐Blake K, et al. Decision aids for benign prostatic hyperplasia: applicability across race and education. Medical Decision Making 2004;24(4):359‐66. [DOI] [PubMed] [Google Scholar]
Rubinstein 2011 {published data only}
- Rubinstein W, Acheson L, O'Neill S, Ruffin M, Wang C, Beaumont J, et al. Clinical utility of family history for cancer screening and referral in primary care: a report from the Family Healthware Impact Trial. Genetics in Medicine 2011;13(11):956‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruddy 2009 {published data only}
- Ruddy KJ, Partridge AH. Breast cancer in young women: clinical decision‐making in the face of uncertainty. Oncology 2009;23(6):474‐7. [PubMed] [Google Scholar]
Ruehlman 2012 {published data only}
- Ruehlman LS, Karoly P, Enders C. A randomized controlled evaluation of an online chronic pain self management program. Pain 2012;153(2):319‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruland 2013 {published data only}
- Ruland CM, Andersen T, Jeneson A, Moore S, Grimsbø GH, Børøsund E, Ellison MC. Effects of an internet support system to assist cancer patients in reducing symptom distress: a randomized controlled trial. Cancer Nursing 2013;36(1):6‐17. [DOI] [PubMed] [Google Scholar]
Ryser 2004 {published data only}
- Ryser FG. Breastfeeding attitudes, intention, and initiation in low‐income women: the effect of the best start program. Journal of Human Lactation 2004;20(3):300‐5. [DOI] [PubMed] [Google Scholar]
Sassen 2014 {published data only}
- Sassen B, Kok G, Schepers J, Vanhees L. Supporting health care professionals to improve the processes of shared decision making and self‐management in a web‐based intervention: randomized controlled trial. Journal of Medical Internet Research 2014;16(10):e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saver 2007 {published data only}
- Saver BG, Gustafson D, Taylor TR, Hawkins RP, Woods NF, Dinauer S, et al. A tale of two studies: the importance of setting, subjects and context in two randomized, controlled trials of a web‐based decision support for perimenopausal and postmenopausal health decisions. Patient Education and Counseling 2007;66(2):211‐22. [DOI] [PubMed] [Google Scholar]
Sawka 2011 {published data only}
- Sawka AM, Straus S, Gafni A, Brierley JD, Tsang RW, Rotstein L, et al. How can we meet the information needs of patients with early stage papillary thyroid cancer considering radioactive iodine remnant ablation?. Clinical Endocrinology 2011;74:419‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scaffidi 2014 {published data only}
- Scaffidi RM, Posmontier B, Bloch JR, Wittmann‐Price R. The relationship between personal knowledge and decision self‐efficacy in choosing trial of labor after cesarean. Journal of Midwifery & Women's Health 2014;59(3):246‐53. [DOI] [PubMed] [Google Scholar]
Schapira 2000 {published data only}
- Schapira MM, VanRuiswyk J. The effect of an illustrated pamphlet decision‐aid on the use of prostate cancer screening tests. Journal of Family Practice 2000;49(5):418‐24. [PubMed] [Google Scholar]
Schapira 2007 {published data only}
- Schapira MM, Gilligan MA, McAuliffe T, Garmon G, Carnes M, Nattinger AB. Decision‐making at menopause: a randomized controlled trial of a computer‐based hormone therapy decision‐aid. Patient Education and Counseling 2007;67(1‐2):100‐7. [DOI] [PubMed] [Google Scholar]
Schwartz 2009b {published data only}
- Schwartz LM, Woloshin S, Welch HG. Using a drug facts box to communicate drug benefits and harms: two randomized trials. Annals of Internal Medicine 2009;150(8):516‐27. [DOI] [PubMed] [Google Scholar]
Sears 2007 {published data only}
- Sears SR, Woodward JT, Twillman RK. What do I have to lose? effects of a psycho‐educational intervention on cancer patient preference for resuscitation. Journal of Behavioral Medicine 2007;30(6):533‐44. [DOI] [PubMed] [Google Scholar]
Sequist 2011 {published data only}
- Sequist T, Zaslavsky A, Colditz G, Ayanian J. Electronic patient message to promote colorectal cancer screening. Archives of Internal Medicine 2011;171(7):636‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG, et al. CARRS Trial Writing Group. Improving diabetes care: multi‐component cardiovascular disease risk reduction strategies for people with diabetes in South Asia ‐ the CARRS multi‐center translation trial. Diabetes Research and Clinical Practice 2012;98(2):285‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheppard 2012 {published data only}
- Sheppard VB, Wallington SF, Williams KP, Lucas W. A decision‐support intervention for black women eligible for adjuvant systematic therapy: Sisters informing sisters about breast cancer treatment ‐ An intervention to reduce treatment disparities. In: Elk R, Landrine H editor(s). Cancer Disparities: Causes and Evidence‐Based Solutions. American Cancer Society, 2012. [Google Scholar]
Sheridan 2004 {published data only}
- Sheridan SL, Felix K, Pignone MP, Lewis CL. Information needs of men regarding prostate cancer screening and the effect of a brief decision aid. Patient Education and Counseling 2004;54(3):345‐51. [DOI] [PubMed] [Google Scholar]
Sheridan 2010 {published data only}
- Sheridan SL, Griffith JM, Behrend L, Gizlice Z, Jianwen C, Pignone MP. Effect of adding a values clarification exercise to a decision aid on heart disease prevention: a randomized trial. Medical Decision Making 2010;30(4):E28‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheridan 2012 {published data only}
- Sheridan SL, Golin C, Bunton A, Lykes JB, Schwartz B, McCormack L, Driscoll D, Bangdiwala SI, Harris RP. Shared decision making for prostate cancer screening: the results of a combined analysis of two practice‐based randomized controlled trials. BMC Medical Informatics & Decision Making 2012;12:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2014 {published data only}
- Sherman KA, Harcourt DM, Lam TC, Shaw LK, Boyages J. BRECONDA: Development and acceptability of an interactive decisional support tool for women considering breast reconstruction. Psycho‐Oncology 2014;23:835‐8. [DOI] [PubMed] [Google Scholar]
Shirai 2012 {published data only}
- Shirai Y, Fujimori M, Ogawa A, Yamada Y, Nishiwaki Y, Ohtsu A, Uchitomi Y. Patients' perception of the usefulness of a question prompt sheet for advanced cancer patients when deciding the initial treatment: a randomized, controlled trial. Psycho‐Oncology 2012;21(7):706‐713. [DOI] [PubMed] [Google Scholar]
Silver 2012 {published data only}
- Silver B, Zaman IF, Ashraf K, Majed Y, Norwood EM, Schuh LA, et al. A randomized trial of decision‐making in asymptomatic carotid stenosis. Neurology 2012;78(5):315‐21. [DOI] [PubMed] [Google Scholar]
Siminoff 2006 {published data only}
- Siminoff LA, Gordon NH, Silverman P, Budd T, Ravdin PM. A decision aid to assist in adjuvant therapy choices for breast cancer. Psycho‐Oncology 2006;15(11):1001‐13. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Elkin EB, Peele PB, Dickler M, Siminoff LA. Long‐term health outcomes of a decision aid: data from a randomized trial of adjuvant! in women with localized breast cancer. Medical Decision Making 2009;29(4):461‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2012a {published data only}
- Simon D, Kriston L, Wolff A, Buchholz A, Vietor C, Hecke T, et al. Effectiveness of a web‐based, individually tailored decision aid for depression or acute low back pain: a randomized controlled trial. Patient Education and Counseling 2012;87(3):360‐8. [DOI] [PubMed] [Google Scholar]
Simon 2012b {published data only}
- Simon W, Lambert MJ, Harris MW, Busath G, Vazquez A. Providing patient progress information and clinical support tools to therapists: Effects on patients at risk of treament failure. Psychotherapy Research 2012;22(6):638‐47. [DOI] [PubMed] [Google Scholar]
Smith 2011a {published data only}
- Smith T, Dow L, Virago E, Khatcheressian J, Matsuyama R, Lyckholm L. A pilot trial of decision aids to give truthful prognostic and treatment information to chemotherapy patients with advanced cancer. Journal of Supportive Oncology 2011;9(2):79‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2011b {published data only}
- Smith SW, Nazione S, LaPlante C, Clark‐Hitt R, Park HS, Sung R, Leichtman A. Living kidney donor decision making and communication. Journal of Health Communication: International Perspectives 2011;16(8):870‐88. [DOI] [PubMed] [Google Scholar]
Solberg 2010 {published data only}
- Solberg LI, Asche SE, Sepucha K, Thygeson NM, Madden JE, Morrissey L, et al. Informed choice assistance for women making uterine fibroid treatment decisions: a practical clinical trial. Medical Decision Making 2010;30(4):444‐52. [DOI] [PubMed] [Google Scholar]
Sorenson 2004 {published data only}
- Sorenson JR, Lakon C, Spinney T, Jennings‐Grant T. Assessment of a decision aid to assist genetic testing research participants in the informed consent process. Genetic Testing 2004;8(3):336‐46. [DOI] [PubMed] [Google Scholar]
Sparano 2006 {published data only}
- Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx). Clinical Breast Cancer 2006;7(4):347‐50. [DOI] [PubMed] [Google Scholar]
Stalmeier 2009 {published data only}
- Stalmeier PF, Roosmalen MS. Concise evaluation of decision aids. Patient Education and Counseling 2009;74(1):104‐9. [DOI] [PubMed] [Google Scholar]
Starosta 2015 {published data only}
- Starosta AJ, Luta G, Tomko CA, Schwartz MD, Taylor KL. Baseline attitudes about prostate cancer screening moderate the impact of decision aids on screening rates. Annals of Behavioral Medicine 2015;49:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2013 {published data only}
- Stein RA, Sharpe L, Bell ML, Boyle FM, Dunn SM, Clarke SJ. Randomized controlled trial of a structured intervention to facilitate end‐of‐life decision making in patients with advanced cancer. Journal of Clinical Oncology 2013;31(27):3403‐10. [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner MJ, Dalebout S, Condon S, Dominik R, Trussell J. Understanding risk: a randomized controlled trial of communicating contraceptive effectiveness. Obstetrics & Gynecology 2003;102(4):709‐17. [DOI] [PubMed] [Google Scholar]
Stephens 2008 {published data only}
- Stephens RL, Xu Y, Volk RJ, Scholl LE, Kamin SL, Holden EW. Influence of a patient decision aid on decisional conflict related to PSA testing: a structural equation model. Health Psychology 2008;27(6):711‐21. [DOI] [PubMed] [Google Scholar]
Stiggelbout 2008 {published data only}
- Stiggelbout AM, Molewijk AC, Otten W, Bockel JH, Bruijninckx CM, Salm I, et al. The impact of individualized evidence‐based decision support on aneurysm patients' decision making, ideals of autonomy, and quality of life. Medical Decision Making 2008;28(5):751‐62. [DOI] [PubMed] [Google Scholar]
Stirling 2012 {published data only}
- Stirling C, Leggett S, Lloyd B, Scott J, Blizzard L, Quinn S, Robinson A. Decision aids for respite service choices by carers of people with dementia: development and pilot RCT. BMC Medical Informatics and Decision Making 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Street 1995 {published data only}
- Street RLJ, Voigt B, Geyer CJ, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer 1995;76(11):2275‐85. [DOI] [PubMed] [Google Scholar]
Street 1998 {published data only}
- Street RL Jr, Order A, Bramson R, Manning T. Preconsultation education promoting breast cancer screening: does the choice of media make a difference?. Journal of Cancer Education 1998;13(3):152‐61. [DOI] [PubMed] [Google Scholar]
Sundaresan 2011 {published data only}
- Sundaresan P, Turner S, Kneebone A, Pearse M, Butow P. Evaluating the utility of a patient decision aid for potential participants of a prostate cancer trial (RAVES‐TROG 08.03). Radiotherapy and Oncology 2011;101(3):521‐4. [DOI] [PubMed] [Google Scholar]
Tabak 1995 {published data only}
- Tabak N. Decision making in consenting to experimental cancer therapy. Cancer Nursing 1995;18(2):89‐96. [PubMed] [Google Scholar]
Taylor 2013 {published data only}
- Taylor KL, Williams RM, Davis K, Luta G, Penek S, Barry S, et al. Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Internal Medicine 2013;173(18):1704‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ten 2008 {published data only}
- Wolde GB, Dijkstra A, Empelen P, Hout W, Neven AK, Zitman F. Long‐term effectiveness of computer‐generated tailored patient education on benzodiazepines: a randomized controlled trial. Addiction 2008;103(4):662‐70. [DOI] [PubMed] [Google Scholar]
Thomas 2013 {published data only}
- Thomas KL, Zimmer LO, Dai D, Al‐Khatib SM, Allen LaPointe NM, Peterson ED. Educational videos to reduce racial disparities in ICD therapy via innovative designs (VIVID): a randomized clinical trial. American Heart Journal 2013;166(1):157‐63. [DOI] [PubMed] [Google Scholar]
Thomson 2006 {published data only}
- Thomson P, Dowding D, Swanson V, Bland R, Mair C, Morrison A, et al. A computerised guidance tree (decision aid) for hypertension, based on decision analysis: development and preliminary evaluation. European Journal of Cardiovascular Nursing 2006;5(2):146‐9. [DOI] [PubMed] [Google Scholar]
Thornton 1995 {published data only}
- Thornton JG, Hewison J, Lilford RJ, Vail A. A randomised trial of three methods of giving information about prenatal testing. BMJ 1995;311(7013):1127‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tiller 2006 {published data only}
- Tiller K, Meiser B, Gaff C, Kirk J, Dudding T, Phillips KA, et al. A randomized controlled trial of a decision aid for women at increased risk of ovarian cancer. Medical Decision Making 2006;26(4):360‐72. [DOI] [PubMed] [Google Scholar]
Tinsel 2013 {published data only}
- Tinsel I, Buchholz A, Vach W, Siegel A, Dürk T, Buchholz A, et al. Shared decision‐making in antihypertensive therapy: a cluster randomised controlled trial. BMC Family Practice 2013;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomko 2015 {published data only}
- Tomko C, Davis K, Ludin S, Kelly S, Stern A, Luta G, et al. Decisional outcomes following use of an interactive web‐based decision aid for prostate cancer screening. Translational Behavioral Medicine: Practice, Policy, Research 2015;5(2):189‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoli 2013 {published data only}
- Ukoli FA, Patel K, Hargreaves M, Beard K, Moton PJ, Bragg R, et al. A tailored prostate cancer education intervention for low‐income African Americans: impact on knowledge and screening. Journal of Health Care for the Poor and Underserved 2013;24(1):311‐331. [DOI] [PubMed] [Google Scholar]
Valdez 2001 {published data only}
- Valdez A, Banerjee K, Fernandez M, Ackerson L. Impact of a multimedia breast cancer education intervention on use of mammography by low‐income Latinas. Journal of Cancer Education 2001;16(4):221‐4. [DOI] [PubMed] [Google Scholar]
Van der Krieke 2013 {published data only}
- Krieke L, Emerencia AC, Boonstra N, Wunderink L, Jonge P, Sytema S. A web‐based tool to support shared decision making for people with a psychotic disorder: randomized controlled trial and process evaluation. Journal of Medical Internet Research 2013;15(10):e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Roosmalen 2004 {published and unpublished data}
- Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra‐Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation. British Journal of Cancer 2004;90(2):333‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosmalen MS, Stalmeier PF, Verhoef LC, Hoekstra‐Weebers JE, Oosterwijk JC, Hoogerbrugge N, et al. Randomized trial of a shared decision‐making intervention consisting of trade‐offs and individualized treatment information for BRCA1/2 mutation carriers. Journal of Clinical Oncology 2004;22(16):3293‐301. [DOI] [PubMed] [Google Scholar]
Van Steenkiste 2008 {published data only}
- Steenkiste B, Weijden TM, Stoffers JHEH, Grol RPTM. Patients' responsiveness to a decision support tool for primary prevention of cardiovascular diseases in primary care. Patient Education and Counseling 2008;72(1):63‐70. [DOI] [PubMed] [Google Scholar]
Van Til 2009 {published data only}
- Til JA, Stiggelbout AM, IJzerman MJ. The effect of information on preferences stated in a choice‐based conjoint analysis. Patient Education and Counseling 2009;74(2):264‐71. [DOI] [PubMed] [Google Scholar]
Van Tol‐Geerdink 2013 {published data only}
- Tol‐Geerdink JJ, Leer JW, Weijerman PC, Oort IM, Vergunst H, Lin EN, et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU International 2013;111(4):564‐73. [DOI] [PubMed] [Google Scholar]
Veroff 2012 {published data only}
- Veroff D, Sullivan L, Shoptaw EJ, Venator B, Ochoa‐Arvelo T, Baxter J, et al. Improving self‐care for heart failure for seniors: Impact of video and written education and decision aids. Population Health Management 2012;15(1):37‐45. [DOI] [PubMed] [Google Scholar]
Volandes 2009 {published data only}
- Volandes AE, Paasche‐Orlow MK, Barry MJ, Gillick MR, Minaker KL, Chang Y, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ 2009;338:b2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volandes 2011 {published data only}
- Volandes A, Ferguson L, Davis A, Hull N, Green M, Chang Y, et al. Assessing end‐of‐life preferences for advanced dementia in rural patients using an educational video: a randomised controlled trial. Journal of Palliative Medicine 2011;14(2):169‐77. [DOI] [PubMed] [Google Scholar]
Volandes 2013 {published data only}
- Volandes AE, Paasche‐Orlow MK, Mitchell SL, El‐Jawahri A, Davis AD, Barry MJ, et al. Randomized controlled trial of a video decision support tool for cardiopulmonary resuscitation decision making in advanced care. Journal of Clinical Oncology 2013;31(3):380‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volk 2008 {published data only}
- Volk RJ, Jibaja‐Weiss ML, Hawley ST, Kneuper S, Spann SJ, Miles BJ, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Education and Counseling 2008;73(3):482‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Wagner 2011 {published data only}
- Wagner C. A decision aid to support informed choice about bowel cancer screening in people with low educational level improves knowledge but reduces screening uptake. Evidence‐Based Nursing 2011;14(2):36‐7. [DOI] [PubMed] [Google Scholar]
Wagner 1995 {published data only}
- Wagner EH, Barrett P, Barry MJ, Barlow W, Fowler FJ Jr. The effect of a shared decision making program on rates of surgery for benign prostatic hyperplasia. Pilot results. Medical Care 1995;33(8):765‐70. [DOI] [PubMed] [Google Scholar]
Wakefield 2008a {published data only}
- Wakefield CE, Meiser B, Homewood J, Ward R, O'Donnell S, Kirk J, et al. Randomized trial of a decision aid for individuals considering genetic testing for hereditary nonpolyposis colorectal cancer risk. Cancer 2008;113(5):956‐65. [DOI] [PubMed] [Google Scholar]
Wakefield 2008b {published data only}
- Wakefield CE, Meiser B, Homewood J, Peate M, Taylor A, Lobb E, et al. A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Research and Treatment 2008;107(2):289‐301. [DOI] [PubMed] [Google Scholar]
Wakefield 2008c {published data only}
- Wakefield CE, Meiser B, Homewood J, Taylor A, Gleeson M, Williams R. A randomized trial of a breast/ovarian cancer genetic testing decision aid used as a communication aid during genetic counseling. Psycho‐Oncology 2008;17(8):844‐54. [DOI] [PubMed] [Google Scholar]
Wallston 1991 {published data only}
- Wallston KA, Smith RA, King JE, Smith MS, Rye P, Burish TG. Desire for control and choice of antiemetic treatment for cancer chemotherapy. Western Journal of Nursing Research 1991;13(1):12‐23. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang C, Gonzalez R, Milliron KJ, Strecher VJ, Merajver SD. Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process. American Journal of Medical Genetics. Part A 2005;134(1):66‐73. [DOI] [PubMed] [Google Scholar]
Warner 2015 {published data only}
- Warner DO, LeBlanc A, Kadimpati S, Vickers KS, Shi Y, Montori V. Decision aid for cigarette smokers scheduled for elective surgery. Anesthesiology 2015;123(1):18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watts 2014 {published data only}
- Watts KJ, Meiser B, Wakefield CE, Barratt AL, Howard K, Cheah BC, et al. Online prostate cancer screening decision aid for at‐risk men: a randomized trial. Health Psychology 2014;33(9):986‐97. [DOI] [PubMed] [Google Scholar]
Welschen 2012 {published data only}
- Welschen LM, Bot SD, Kostense PJ, Dekker JM, Timmermans DR, Weijden T, et al. Effects of cardiovascular disease risk communication for patients with type 2 diabetes on risk perception in a randomized controlled trial: the @RISK study. Diabetes Care 2012;35:2485‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wennberg 2010 {published data only}
- Wennberg DE, Marr A, Lang L, O'Malley S, Bennett G. A randomized trial of a telephone care‐management strategy. New England Journal of Medicine 2010;363(13):1245‐55. [DOI] [PubMed] [Google Scholar]
Westermann 2013 {published data only}
- Westermann GM, Verheij F, Winkens B, Verhulst FC, Oort FV. Structured shared decision‐making using dialogue and visualization: a randomized controlled trial. Patient Education and Counseling 2013;90(1):74‐81. [DOI] [PubMed] [Google Scholar]
Weymann 2015 {published data only}
- Weymann N, Dirmaier J, Wolff A, Kriston L, Härter M. Effectiveness of a web‐based tailored interactive health communication application for patients with type 2 diabetes or chronic low back pain: randomized controlled trial. Journal of Medical Internet Research 2015;17(3):e53 1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilhelm 2009 {published data only}
- Wilhelm D, Gillen S, Wirnhier H, Kranzfelder M, Schneider A, Scmidt A, et al. Extended preoperative patient education using a multimedia DVD: impact on patients receiving a laparoscopic cholecystectomy: a randomised controlled trial. Langenbeck's Archives of Surgery 2009;394(2):227‐33. [DOI] [PubMed] [Google Scholar]
Wilkes 2013 {published data only}
- Wilkes MS, Day FC, Srinivasan M, Griffin E, Tancredi DJ, Rainwater JA, et al. Pairing physician education with patient activation to improve shared decisions in prostate cancer screening: a cluster randomized controlled trial. Annals of Family Medicine 2013;11(4):324‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkie 2013 {published data only}
- Wilkie DJ, Gallo AM, Yao Y, Molokie RE, Stahl C, Hershberger PE, et al. Reproductive health choices for young adults with sickle cell disease or trait: randomized controlled trial immediate posttest effects. Nursing Research 2013;62(5):352‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 2006 {published data only}
- Wilkins EG, Lowery JC, Copeland LA, Goldfarb SL, Wren PA, Janz NK. Impact of an educational video on patient decision making in early breast cancer treatment. Medical Decision Making 2006;26(6):589‐98. [DOI] [PubMed] [Google Scholar]
Willemsen 2006 {published data only}
- Willemsen MC, Wiebing M, Emst A, Zeeman G. Helping smokers to decide on the use of efficacious smoking cessation methods: a randomized controlled trial of a decision aid. Addiction 2006;101(3):441‐9. [DOI] [PubMed] [Google Scholar]
Williamson 2014 {published data only}
- Williamson LEA, Lawson KL, Downe PJ, Pierson RA. Informed reproductive decision‐making: The impact of providing fertility information on fertility knowledge and intentions to delay childbearing. Journals of Obstetrics and Gynaecology Canada 2014;36(5):400‐5. [DOI] [PubMed] [Google Scholar]
Williams‐Piehota 2008 {published data only}
- Williams‐Piehota PA, McCormack LA, Treiman K, Bann CM. Health information styles among participants in a prostate cancer screening informed decision‐making intervention. Health Education Research 2008;23(3):440‐53. [DOI] [PubMed] [Google Scholar]
Woltmann 2011 {published data only}
- Woltmann EM, Wilkniss SM, Teachout A, McHugo GJ, Drake RE. Trial of an electronic decision support system to facilitate shared decision making in community mental health. Psychiatric Services 2011;62(1):54‐60. [DOI] [PubMed] [Google Scholar]
Wroe 2005 {published data only}
- Wroe AL, Turner N, Owens RG. Evaluation of a decision‐making aid for parents regarding childhood immunizations. Health Psychology 2005;24(6):539‐47. [DOI] [PubMed] [Google Scholar]
Yee 2014 {published data only}
- Yee LM, Wolf M, Mullen R, Bergeron AR, Cooper Bailey S, Levine R, Grobman WA. A randomized trial of a prenatal genetic testing interactive computerized information aid. Prenatal Diagnosis 2014;34(6):552‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2011 {published data only}
- Yun YH, Lee MK, Park S, Lee JL, Park J, Choi YS, et al. Use of a decision aid to help caregivers discuss terminal disease status with a family member with cancer: a randomized controlled trial. Journal of Clinical Oncology 2011;29(36):4811‐9. [DOI] [PubMed] [Google Scholar]
Zajac 2012 {published data only}
- Zajac LE. Making Difficult Health Decisions: A Motivated Decision Processing Model [PhD thesis]. Pittsburgh: University of Pittsburgh, 2012. [Google Scholar]
Zapka 2004 {published data only}
- Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Annals of Internal Medicine 2004;141(9):683‐92. [DOI] [PubMed] [Google Scholar]
Zikmund‐Fisher 2008 {published data only}
- Zikmund‐Fisher BJ, Ubel PA, Smith DM, Derry HA, McClure JB, Stark A, et al. Communicating side effect risks in a tamoxifen prophylaxis decision aid: the debiasing influence of pictographs. Patient Education and Counseling 2008;73(2):209‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoffman 2012 {published data only}
- Zoffman V, Kirkevold M. Realizing empowerment in difficult diabetes care: a guided self‐determination intervention. Qualitative Health Research 2012;22(1):103‐18. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12615000523505 {published data only}
- ACTRN12615000523505. The Motherhood Choices Decision Aid for Women with Rheumatoid Arthritis Increases Knowledge and Reduces Decisional Conflict: A Randomized Controlled Study. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12615000523505 (first received May 25, 2015). [DOI] [PMC free article] [PubMed]
ACTRN12615000843550 {published data only}
- ACTRN12615000843550. Evaluation of decision aids for parents about the benefits and harms of antibiotic use for coughs and colds in children [Pilot randomised controlled trial of decision aids for parents about the benefit and harm of antibiotics for common acute respiratory infections in children to aid informed decision making]. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12615000843550 (first received August 13, 2015).
Al‐Itejawi 2015 {published data only}
- Al‐Itejawi HH, Uden‐Kraan CF, Vis AN, Nieuwenhuijzen JA, Hofstee MJ, Moorselaar RJ, Verdonck‐de Leeuw IM. Development of a patient decision aid for the treatment of localised prostate cancer: a participatory design approach. J Clin Nurs 2016;25(7‐8):1131‐1144. [DOI] [PubMed] [Google Scholar]
Anderson 2014 {published data only}
- Anderson RT, Montori VM, Shah ND, Ting HH, Pencille LJ, Demers M, et al. Effectiveness of the Chest Pain Choice decision aid in emergency department patients with low‐risk chest pain: study protocol for a multicenter randomized trial. Trials 2014;15(166):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aslani 2014 {published data only}
- Aslani A, Tara F, Ghalichi L, Eslami S. The impact of computerized decision aid on mode of delivery ‐ a study protocol. Studies in Health Technology and Informatics 2014;200:170‐2. [PubMed] [Google Scholar]
Buhse 2013 {published data only}
- Buhse S, Heller T, Kasper J, Mühlhauser I, Müller UA, Lehmann T, Lenz M. An evidence‐based shared decision making programme on the prevention of myocardial infarction in type 2 diabetes: protocol of a randomised‐controlled trial. BMC Family Practice 2013;14(155):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2012 {published data only}
- Carroll SL, McGillion M, Stacey D, Healey JS, Browne G, Arthur HM, Thabane L. Development and feasibility testing of decision support for patients who are candidates for a prophylactic implantable defibrillator: a study protocol for a pilot randomized controlled trial. Trials 2013;14(346):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chambers 2008 {published data only}
- Chambers SK, Ferguson M, Gardiner RA, Nicol D, Gordon L, Occhipinti S, et al. ProsCan for men: randomised controlled trial of a decision support intervention for men with localised prostate cancer. BMC Cancer 2008;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coylewright 2012 {published data only}
- Coylewright M, Shepel K, LeBlanc A, Pencille L, Hess E, Shah N, Montori VM, Ting HH. Shared decision making in patients with stable coronary artery disease: PCI choice. PLOS One 2012;7(11):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuypers 2015 {published data only}
- Cuypers M, Lamers RE, Kil PJ, Poll‐Franse LV, Vries M. Impact of a web‐based treatment decision aid for early‐stage prostate cancer on shared decision‐making and health outcomes: study protocol for a randomized controlled trial.. Trials 2015;16:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Den Ouden 2015 {published data only}
- Ouden H, Vos RC, Reidsma C, Rutten GEHM. Shared decision making in type 2 diabetes with a support decision tool that takes into account clinical factors, the intensity of treatment and patient preferences: design of a cluster randomised (OPTIMAL) trial. BMC Family Practice 2015;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dirmaier 2013 {published data only}
- Dirmaier J, Härter M, Weymann N. A tailored, dialogue‐based health communication application for patients with chronic low back pain: study protocol of a randomised controlled trial. BMC Medical Informatics & Decision Making 2013;13(66):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geiger 2011 {published data only}
- Geiger F, Liethmann K, Hoffmann F, Paschedag J, Kasper J. Investigating a training supporting Shared Decision Making (IT'S SDM 2011): study protocol for a randomized controlled trial. Trials 2011;12:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hersch 2014 {published data only}
- Hersch J, Barratt A, Jansen J, Houssami N, Irwig L, Jacklyn G, et al. The effect of information about overdetectection of breast cancer on women's decision‐making about mammography screening: study protocol for a randomised controlled trial. BMJ Open 2014;4(5):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2014 {published data only}
- Hess EP, Wyatt KD, Kharbanda AB, Louie JP, Dayan PS, Tzimenatos L, et al. Effectiveness of the head CT choice decision aid in parents of children with minor head trauma:study protocol for a multicenter randomized trial. Trials 2014;14(253):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimbo 2012 {published data only}
- Jimbo M, Kelly‐Blake K, Sen A, Hawley ST, Ruffin MT 4th. Decision Aid to Technologically Enhance Shared decision making (DATES): study protocol for a randomized controlled trial. Trials 2013;14(381):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Layton 2012 {published data only}
- Effects of a web‐based decision aid on African American men's prostate screening knowledge and behavior. Ongoing study —.
LeBlanc 2013 {published data only}
- LeBlanc A, Bodde AE, Branda ME, Yost KJ, Herrin J, Williams MD, et al. Translating comparative effectiveness of depression medications into practice by comparing the depression medication choice decision aid to usual care: study protocol for a randomized controlled trial. Trials 2013;14(127):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 2012 {published data only}
- Mann DM, Lin JJ. Increasing efficacy of primary care‐based counseling for diabetes prevention: rationale and design of the ADAPT (Avoiding Diabetes Thru Action Plan Targeting) trial. Implementation Science 2012;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00813033 {published data only}
- NCT00813033. Use of a Patient Decision Aid for Gastrologic Endoscopy in a Pediatric Setting [Creation and Pilot Evaluation of a Patient Decision Aid as an Adjunct to the Consenting Process for Gastrointestinal Endoscopy in a Pediatric Setting]. https://clinicaltrials.gov/show/NCT00813033 (first received December 19, 2008).
NCT01077037 {published data only}
- NCT01077037. Impact of a Decision Aid on Patient Decision Making in Emergency Department Chest Pain Patients [Impact of a Decision Aid on Patient Participation in Decision Making and Resource Use in Low Risk Chest Pain Patients: A Randomized Trial]. clinicaltrials.gov/show/NCT01077037 (first received February 24, 2010).
NCT01152294 {published data only}
- NCT01152294. Measuring Quality of Decisions About Treatment of Menopausal Symptoms [Measuring Quality of Decisions About Treatment of Menopausal Symptoms]. clinicaltrials.gov/show/NCT01152294 (first received June 22, 2010).
NCT01152307 {published data only}
- NCT01152307. Measuring Quality of Decisions About Treatment of Depression [Measuring Quality of Decisions About Treatment of Depression]. clinicaltrials.gov/show/NCT01152307 (first received June 22, 2010).
NCT01447186 {published data only}
- NCT01447186. Adaptation of the American Cancer Society (ACS) Early Detection of Prostate Cancer Patient Decision Aid for Spanish Speaking Men [Adaptation of the American Cancer Society (ACS) Early Detection of Prostate Cancer Patient Decision Aid for Spanish Speaking Men]. clinicaltrials.gov/show/NCT01447186 (first received October 3, 2011).
NCT01618097 {published data only}
- NCT01618097. Evaluation of DVD and Internet Decision Aids for Hip and Knee Osteoarthritis: Focus on Health Literacy. clinicaltrials.gov/show/NCT01618097 (first received May 29, 2012).
NCT01713894 {published data only}
- NCT01713894. Decision Aid ‐ Extreme Prematurity [Utility of a Clinically Relevant Decision Aid, for Parents Facing Extremely Premature Delivery]. clinicaltrials.gov/show/NCT01713894 (first received October 22, 2012).
NCT01771536 {published data only}
- NCT01771536. The PCI Choice Trial: a Pilot Randomized Trial of a Decision Aid for Patients With Stable Coronary Artery Disease. clinicaltrials.gov/show/NCT01771536 (first received December 15, 2012).
NCT01851785 {published data only}
- NCT01851785. African American Preference for Knee Replacement: A Patient‐Centered Intervention (ACTION) [Behavioral & Social Science Research on Understanding and Reducing Health Disparities]. clinicaltrials.gov/show/NCT01851785 (first received May 8, 2013).
NCT01941186 {published data only}
- NCT01941186. A Family Centered Intervention to Promote Optimal Child Development [A Family Centered Intervention to Promote Optimal Child Development at the Interface of the Health System and Community]. clinicaltrials.gov/show/NCT01941186 (first received September 9, 2013).
NCT01976325 {published data only}
- NCT01976325. Evaluating the Ottawa Malaria Decision Aid (OMDA) [Incorporation of the 'Ottawa Malaria Decision Aid' Into the Pre‐travel Consultation Process: Assessment of Travelers' Knowledge, Decisional Conflict, Preparation for Decision‐making and Medication Adherence Compared to Standard Care]. clinicaltrials.gov/show/NCT01976325 (first received October 29, 2013).
NCT02026102 {published data only}
- NCT02026102. A Pilot Trial of Patient Decision Aids for Implantable Cardioverter‐Defibrillators (ICDs) [A Pilot Trial of Patient Decision Aids for Implantable Cardioverter‐Defibrillators (ICDs)]. clinicaltrials.gov/show/NCT02026102 (first received December 12, 2013).
NCT02084290 {published data only}
- NCT02084290. Evaluating a Shared Decision Making Program for Crohn's Disease [Evaluating a Prediction Tool and Decision Aid for Patients With Crohn's Disease]. clinicaltrials.gov/show/NCT02084290 (first received January 29, 2014).
NCT02110979 {published data only}
- NCT02110979. Validation of a Patient Decision Aid for Type 2 Diabetes [Validation of a Patient Decision Aid for Type 2 Diabetes]. clinicaltrials.gov/show/NCT02110979 (first received April 4, 2014).
NCT02145481 {published data only}
- NCT02145481. Decisional Quality for Patients With Coronary Artery Disease (DeQCAD). clinicaltrials.gov/show/NCT02145481 (first received May 15, 2014).
NCT02198690 {published data only}
- NCT02198690. Trial of a Mammography Decision Aid for Women Aged 75 and Older [Randomized Trial of a Mammography Decision Aid for Women Aged 75 and Older]. clinicaltrials.gov/show/NCT02198690 (first received July 16, 2014).
NCT02235571 {published data only}
- NCT02235571. iChoose Decision Kidney Aid for End‐Stage Renal Disease Patients [iChoose Kidney Decision Aid for Treatment Options Among End‐Stage Renal Disease (ESRD) Patients]. clinicaltrials.gov/show/NCT02235571 (first received September 6, 2014).
NCT02248974 {published data only}
- NCT02248974. Development & Testing of a Decision Aid for LVAD Placement (VADDA) [Development and User Testing of a Decision Aid for Left Ventricular Assist Device (LVAD) Placement]. clinicaltrials.gov/show/NCT02248974 (first received September 17, 2014).
NCT02259699 {published data only}
- NCT02259699. Ovarian Cancer Patient‐Centered Decision Aid (PCOA) [Ovarian Cancer Patient‐Centered Decision Aid]. clinicaltrials.gov/show/NCT02259699 (first received May 15, 2014).
NCT02308592 {published data only}
- NCT02308592. Patient Decision Aid for Antidepressant Use in Pregnancy [Patient Decision Aid (PDA) for Antidepressant Use In Pregnancy]. clinicaltrials.gov/show/NCT02308592 (first received December 2, 2014).
NCT02319525 {published data only}
- NCT02319525. Individualized Patient Decision Making for Treatment Choices Among Minorities With Lupus [Individualized Patient Decision Making for Treatment Choices Among Minorities With Lupus]. clinicaltrials.gov/show/NCT02319525 (first received November 5, 2014). [Google Scholar]
NCT02326597 {published data only}
- NCT02326597. Decision Aid for Therapeutic Options In Sickle Cell Disease [Comparative Effectiveness of a Decision Aid for Therapeutic Options in Sickle Cell Disease]. clinicaltrials.gov/show/NCT02326597 (first received December 18, 2014).
NCT02344576 {published data only}
- NCT02344576. Trial of a Decision Support Intervention for Patients and Caregivers Offered Destination Therapy Heart Assist Device (DECIDE‐LVAD) [A Multicenter Trial of a Shared Decision Support Intervention for Patients and Their Caregivers Offered Destination Therapy for End‐Stage Heart Failure]. clinicaltrials.gov/show/NCT02344576 (first received January 16, 2015).
NCT02488317 {published data only}
- NCT02488317. Empowering Patients On Choices for Renal Replacement Therapy (Aim 3) (EPOCH‐RRT) (EPOCH‐RRT) [Empowering Patients On Choices for Renal Replacement Therapy (Aim 3)]. clinicaltrials.gov/show/NCT02488317 (first received June 15, 2015).
NCT02488603 {published data only}
- NCT02488603. Decision Aids for Tamoxifen Treatment in Breast Cancer Patients [Utilization of Decision Aids for Tamoxifen Treatment in Breast Cancer Patients: A Randomized Controlled Trial.]. clinicaltrials.gov/show/NCT02488603 (first received June 25, 2015).
NCT02492009 {published data only}
- NCT02492009. Patient Decision Aid for Antidepressant Use in Pregnancy [Patient Decision Aid (PDA) for Antidepressant Use In Pregnancy: a Pilot RCT]. clinicaltrials.gov/show/NCT02492009 (first received June 22, 2015).
NCT02503553 {published data only}
- NCT02503553. Decision Aids in Cerebral Aneurysm Treatment. clinicaltrials.gov/show/NCT02503553 (first received July 17, 2015).
NCT02516449 {published data only}
- NCT02516449. Assessment of Shared Decision Making Aids in Asthma [Utility of Two Patients Decision Aids About Asthma Inhaled Controller Medication Use in Adult Patients With Asthma]. clinicaltrials.gov/show/NCT02516449 (first received July 2, 2015).
NCT02540044 {published data only}
- NCT02540044. Supporting Patient Care With Electronic Resource (SuPER) (SuPER) [Supporting Patient Care With Electronic Resource (SuPER): Efficacy of an Online Decision Aid for Patients Considering Biologic Therapy for Rheumatoid Arthritis]. clinicaltrials.gov/show/NCT02540044 (first received September 1, 2015).
NCT02611050 {published data only}
- NCT02611050. Treatment Decisions for Multi‐vessel CAD [Treatment Decisions for Multi‐vessel Coronary Artery Disease Patients]. clinicaltrials.gov/show/NCT02611050 (first received Noovember 9, 2015).
Oostendorp 2011 {published data only}
- Oostendorp L, Ottevanger P, Graaf W, Stalmeier P. Assessing the information desire of patients with advanced cancer by providing information with a decision aid, which is evaluated in a randomized trial: a study protocol. BMC Medical Informatics & Decision Making 2011;11(9):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2015 {published data only}
- Yu CH, Ivers NM, Stacey D, Rezmovitz J, Telner D, Thorpe K, et al. Impact of an interprofessional shared decision‐making and goal‐setting decision aid for patients with diabetes on decisional conflict: study protocol for a randomized controlled trial. Trials 2015;16(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Andrews 2013
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines 15: Going from evidence to recommendation ‐ determinants of a recommendation's direction and strength. Journal of Clinical Epidemiology 2013;66(7):726‐35. [DOI: 10.1016/j.jclinepi.2013.02.003] [DOI] [PubMed] [Google Scholar]
Barry 2008
- Barry MJ, Wescott PH, Reifler EJ, Chang Y, Moulton BW. Reactions of potential jurors to a hypothetical malpractice suit. Alleging failing to perform a prostate‐specific‐antigen test. Journal of Law, Medicine & Ethics 2008;Summer:396‐402. [DOI] [PubMed] [Google Scholar]
Bekker 2003
- Bekker HL, Legare F, Stacey D, O'Connor A, Lemyre L. Is anxiety a suitable measure of decision aid effectiveness: a systematic review. Patient Education and Counselling 2003;50(3):255‐62. [DOI] [PubMed] [Google Scholar]
Bennett 2010
- Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O'Connor AM. Validation of a preparation for decision making scale. Patient Education and Counseling 2010;78(1):130‐33. [DOI] [PubMed] [Google Scholar]
Brehaut 2003
- Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Medical Decision Making 2003;23(4):281‐92. [DOI] [PubMed] [Google Scholar]
Brouwers 2010
- Brouwers M, Stacey D, O’Connor A. Knowledge creation: synthesis, tools and products. CMAJ 2010;182(2):E68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015
- Brown JG, Joyce KE, Stacey D, Thomson MD. Patients or volunteers? The impact of motivation for trial participation on the efficacy of patient decision aids: a secondary analysis of a Cochrane Systematic Review. Medical Decision Making 2015;35(4):419‐35. [DOI] [PubMed] [Google Scholar]
Charles 1997
- Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean?. Social Science and Medicine 1997;44(5):681‐92. [DOI] [PubMed] [Google Scholar]
Charles 2010
- Charles C, Gafni A, Freeman E. Implementing shared treatment decision making and treatment decision aids: a cautionary tale. Psicooncologia 2010;7(2‐3):243‐55. [Google Scholar]
Clinical Evidence 2013
- Clinical Evidence. How much do we know?. Available from: clinicalevidence.bmj.com/x/set/static/cms/efficacy‐categorisations.html (accessed 29 October 2013).
Coyne 2013
- Coyne I, O'Mathuna DP, Gibson F, Shields L, Sheaf G. Interventions for promoting participation in shared decision‐making for children with cancer. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008970.pub2] [DOI] [PubMed] [Google Scholar]
Degner 1992
- Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play. Journal of Clinical Epidemiology 1992;45(9):941‐50. [DOI] [PubMed] [Google Scholar]
Duncan 2010
- Duncan E, Best C, Hagen S. Shared decision making interventions for people with mental health conditions. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007297.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durand 2008
- Durand MA, Stiel M, Boivin J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Education and Counseling 2008;71(1):125‐35. [DOI] [PubMed] [Google Scholar]
Durand 2014
- Durand MA, Carpenter L, Dolan H, Bravo P, Mann M, Bunn F, et al. Do interventions designed to support shared decision‐making reduce health inequalities? A systematic review and meta‐analysis. PLOS One 2014;9(4):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2005
- Elwyn G, Hutchings H, Edwards A, Rapport F, Wensing M, Cheung WY, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision‐making tasks. Health Expectations 2005;8(1):34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2006
- Elwyn G, O’Connor A, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006;333(7565):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elwyn 2013
- Elwyn G, I Scholl, Tietbohl C, Mann M, Edwards AGK, Clay C, et al. "Many miles to go...": A systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC: Medical Informatics and Decision Making 2013;13(Suppl 2):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentles 2013
- Gentles SJ, Stacey D, Bennett C, Alshurafa M, Walter SD. Factors explaining the heterogeneity of effects of patient decision aids on knowledge of outcome probabilities: a systematic review sub‐analysis. Systematic Reviews 2013;2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 21 March 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gravel 2006
- Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: a systematic review of health professionals' perceptions. Implementation Science 2006;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hays 1993
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36‐item Health Survey 1.0. Health Economics 1993;2(3):217‐27. [DOI] [PubMed] [Google Scholar]
Hibbard 1997
- HIbbard JH, Slovic P, Jewett JJ. Informing consumer decisions in health care: Implications from decision‐making research. Milbank Quarterly 1997;75(3):395‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hibbard 2013
- Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Affairs 2013;32(2):207‐14. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffman 2015
- Hoffmann TC, Mar C. Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. Journal of the American Medical Association 2015;175(2):274‐86. [DOI] [PubMed] [Google Scholar]
Hollinghurst 2010
- Hollinghurst S, Emmett C, Peters TJ, Watson H, Fahey T, Murphy DJ, et al. Economic evaluation of the DIAMOND randomized trial: cost and outcomes of 2 decision aids for mode of delivery among women with previous caesarian section. BMJ 2010;30:453‐63. [DOI] [PubMed] [Google Scholar]
IPDAS 2005a
- International Patient Decision Aid Standards Collaboration. Background Document. 2005. ipdas.ohri.ca/IPDAS_Background.pdf (accessed 29 Oct 2013).
IPDAS 2005b
- International Patient Decision Aid Standards Collaboration. IPDAS Voting Document ‐ 2nd Round. 2005. ipdas.ohri.ca/IPDAS_Second_Round.pdf (accessed 29 Oct 2013).
IPDAS 2013
- Volk RJ, Llewellyn‐Thomas H, Stacey D, Elwyn G. Ten years of the International Patient Decision Aid Standards collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC: Medical Informatics and Decision Making 2013;13(Suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph‐Williams 2013
- Joseph‐Williams N, Newcombe R, Politi M, Durand MA, Sivell S, Stacey D, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Medical Decision Making 2014;34(6):699‐710. [DOI: 10.1177/0272989X13501721] [DOI] [PubMed] [Google Scholar]
Kiesler 2006
- Kiesler DJ, Auerbach SM. Optimal matches of patient preferences for information, decision‐making and interpersonal behavior: evidence, models and interventions. Patient Education and Counseling 2006;61(3):319‐41. [DOI] [PubMed] [Google Scholar]
LeBlanc 2010
- LeBlanc A, Kenny DA, O'Connor AM, Legare F. Decisional conflict in patients and their physicians: a dyadic approach to shared decision making. Medical Decision Making 2010;29(1):61‐8. [DOI] [PubMed] [Google Scholar]
Legare 2008b
- Legare F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision‐making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Education and Counseling 2008;73(3):526‐35. [DOI] [PubMed] [Google Scholar]
Legare 2010
- Legare F, Ratte S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD006732.pub2] [DOI] [PubMed] [Google Scholar]
Legare 2014
- Legare F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]
Makoul 2006
- Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Education and Counseling 2006;60(3):301‐12. [DOI] [PubMed] [Google Scholar]
Michie 2002
- Michie S, Dormandy E, Marteau TM. The multi‐dimensional measure of informed choice: a validation study. Patient Education and Counseling 2002;48(1):87‐91. [DOI] [PubMed] [Google Scholar]
Mulley 1995
- Mulley A. Outcomes research: implications for policy and practice. In: Smith R, Delamother T editor(s). Outcomes in Clinical Practice. London: BMJ Publishing Group, 1995. [Google Scholar]
Munro 2016
- Munro S, Stacey D, Lewis KB, Bansback N. Choosing treatment and screening options congruent with values: do decision aids help? Sub‐analysis of a systematic review. Patient Education & Counseling 2016;99(4):491‐500. [DOI] [PubMed] [Google Scholar]
NCGC/NICE 2012
- National Clinical Guideline Centre. Patient experience in adult NHS services: improving the experience of care for people using adult NHS services. 2012. www.nice.org.uk/nicemedia/live/13668/58283/58283.pdf. London, UK: The Author, (accessed prior to 27 March 2017).
O'Connor 1995
- O'Connor AM. Validation of a decisional conflict scale. Medical Decision Making 1995;15(1):25‐30. [DOI] [PubMed] [Google Scholar]
O'Connor 1998b
- O'Connor AM, Tugwell P, Wells GA, Elmslie T, Jolly E, Hollingworth G, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Education and Counselling 1998;33(3):267‐79. [DOI] [PubMed] [Google Scholar]
O'Connor 2007
- O'Connor AM, Wennberg JE, Legare F, Llewellyn‐Thomas HA, Moulton BW, Sepucha KR, et al. Toward the 'tipping point': decision aids and informed patient choice. Health Affairs 2007;26(3):716‐25. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RNAO 2009
- Registered Nurses’ Association of Ontario. Decision support for adults living with chronic kidney disease. 2009. rnao.ca/bpg/guidelines/decision‐support‐adults‐living‐chronic‐kidney‐disease. Toronto, Ontario: The Author, (accessed prior to 27 March 2017).
Rothert 1987
- Rothert M, Talarcyzk GJ. Patient compliance and the decision making process of clinicians and patients. Journal of Compliance in Health Care 1987;2(1):55‐71. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Sepucha 2013
- Sepucha KR, Borkhoff CM, Lally J, Levin CA, Matlock DD, Ng CJ, et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC: Medical Informatics and Decision Making 2013;13(Suppl 2):S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State‐Trait Anxiety Inventory (Self‐evaluations questionnaire). Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
Stewart 1992
- Stewart AL, Ware JE Jr (editors). Measuring Functioning and Well‐being: The Medical Outcomes Study Approach. Durham NC: Duke University Press, 1992. [Google Scholar]
Trenaman 2014
- Trenaman L, Stirling B, Bansback N. The cost‐effectiveness of patient decision aids: a systematic review. Healthcare 2014;2(4):2510257. [DOI] [PubMed] [Google Scholar]
Trenaman 2016
- Trenaman L, Selva A, Desroches S, Singh K, Bissonnette J, Bansback N, et al. A measurement framework for adherence in patient decision aid trials applied in a systematic review subanalysis. Journal of Clinical Epidemiology 2016;77:15‐23. [DOI] [PubMed] [Google Scholar]
Trikalinos 2014
- Trikalinos TA, Wieland LS, Adam GP, Zgodic A, Ntzani EE. Decision Aids for Cancer Screening and Treatment. Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
Washington State 2016
- Washington State Health Authority. Patient decision aid certification criteria. 2016. www.hca.wa.gov/hw/Documents/sdm_cert_criteria.pdf (accessed prior to 27 March 2017).
Weston 2001
- Weston WW. Informed and shared decision‐making: the crux of patient‐centered care. CMAJ 2001;165(4):438‐9. [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
O'Connor 1999b
- O'Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn‐Thomas H, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ 1999;319(7212):731‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2001
- O'Connor AM, Stacey D, Rovner D, Holmes‐Rovner M, Tetroe J, Llewellyn‐Thomas H, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001431] [DOI] [PubMed] [Google Scholar]
O'Connor 2003
- O'Connor AM, Stacey D, Rovner D, Holmes‐Rovner M, Tetroe J, Llewellyn‐Thomas H, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001431] [DOI] [PubMed] [Google Scholar]
O'Connor 2009b
- O'Connor AM, Bennett C, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001431.pub2] [DOI] [PubMed] [Google Scholar]
Stacey 2011
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes‐Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD001431.pub3] [DOI] [PubMed] [Google Scholar]
Stacey 2014b
- Stacey D, Legare F, Col NF, Bennett CL, Barry MJ, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD001431.pub4] [DOI] [PubMed] [Google Scholar]


