Abstract
Background
Patients with newly diagnosed high‐risk (HR) neuroblastoma (NBL) still have a poor outcome, despite multi‐modality intensive therapy. This poor outcome necessitates the search for new therapies, such as treatment with 131I‐meta‐iodobenzylguanidine (131I‐MIBG).
Objectives
To assess the efficacy and adverse effects of 131I‐MIBG therapy in patients with newly diagnosed HR NBL.
Search methods
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 2016, Issue 3), MEDLINE (PubMed) (1945 to 25 April 2016) and Embase (Ovid) (1980 to 25 April 2016). In addition, we handsearched reference lists of relevant articles and reviews. We also assessed the conference proceedings of the International Society for Paediatric Oncology, Advances in Neuroblastoma Research and the American Society of Clinical Oncology; all from 2010 up to and including 2015. We scanned the International Standard Randomized Controlled Trial Number (ISRCTN) Register (www.isrctn.com) and the National Institutes of Health Register for ongoing trials (www.clinicaltrials.gov) on 13 April 2016.
Selection criteria
Randomised controlled trials (RCTs), controlled clinical trials (CCTs), non‐randomised single‐arm trials with historical controls and cohort studies examining the efficacy of 131I‐MIBG therapy in 10 or more patients with newly diagnosed HR NBL.
Data collection and analysis
Two review authors independently performed the study selection, risk of bias assessment and data extraction.
Main results
We identified two eligible cohort studies including 60 children with newly diagnosed HR NBL. All studies had methodological limitations, with regard to both internal (risk of bias) and external validity. As the studies were not comparable with regard to prognostic factors and treatment (and often used different outcome definitions), pooling of results was not possible. In one study, the objective response rate (ORR) was 73% after surgery; the median overall survival was 15 months (95% confidence interval (CI) 7 to 23); five‐year overall survival was 14.6%; median event‐free survival was 10 months (95% CI 7 to 13); and five‐year event‐free survival was 12.2%. In the other study, the ORR was 56% after myeloablative therapy and autologous stem cell transplantation; 10‐year overall survival was 6.25%; and event‐free survival was not reported. With regard to short‐term adverse effects, one study showed a prevalence of 2% (95% CI 0% to 13%; best‐case scenario) for death due to myelosuppression. After the first cycle of 131I‐MIBG therapy in one study, platelet toxicity occurred in 38% (95% CI 18% to 61%), neutrophil toxicity in 50% (95% CI 28% to 72%) and haemoglobin toxicity in 69% (95% CI 44% to 86%); after the second cycle this was 60% (95% CI 36% to 80%) for platelets and neutrophils and 53% (95% CI 30% to 75%) for haemoglobin. In one study, the prevalence of hepatic toxicity during or within four weeks after last the MIBG treatment was 0% (95% CI 0% to 9%; best‐case scenario). Neither study reported cardiovascular toxicity and sialoadenitis. One study assessed long‐term adverse events in some of the children: there was elevated plasma thyroid‐stimulating hormone in 45% (95% CI 27% to 65%) of children; in all children, free T4 was within the age‐related normal range (0%, 95% CI 0% to 15%). There were no secondary malignancies observed (0%, 95% CI 0% to 9%), but only five children survived more than four years.
Authors' conclusions
We identified no RCTs or CCTs comparing the effectiveness of treatment including 131I‐MIBG therapy versus treatment not including 131I‐MIBG therapy in patients with newly diagnosed HR NBL. We found two small observational studies including chilren. They had high risk of bias, and not all relevant outcome results were available. Based on the currently available evidence, we cannot make recommendations for the use of 131I‐MIBG therapy in patients with newly diagnosed HR NBL in clinical practice. More high‐quality research is needed.
Plain language summary
Iodine‐131‐meta‐iodobenzylguanidine therapy for patients with newly diagnosed high‐risk neuroblastoma
Review question
We reviewed the evidence of the effectiveness and side effects of 131I‐meta‐iodobenzylguanidine (131I‐MIBG) therapy in patients with newly diagnosed high‐risk (HR) neuroblastoma (NBL).
Background
NBL is a rare solid cancer that develops from special nerves cells. Patients with newly diagnosed HR NBL have a poor outcome, despite intensive treatments such as high‐dose chemotherapy to kill the cancer and surgery. This poor outcome needs research to look for new therapies, such as treatment with 131I‐MIBG, which is a type of targeted radiotherapy (radiation directed at the cancer without causing too much damage to surrounding area).
Study characteristics
The evidence is current to April 2016.
We found two cohort studies (where a group of people (the cohort) is followed over time, to examine different treatments received and subsequent outcomes) looking at 131I‐MIBG treatment in 60 children with newly diagnosed HR NBL.
Key results
The studies were not comparable with regard to the children, the ways they were treated and the ways the different outcomes were defined and so it was impossible to combine the results in an analysis. Not all relevant outcome results were available.
The percentages of children whose cancer reduced or disappeared after treatment (response rate) were 56% and 73% in the two studies, but survival was still poor: overall survival (length of time that the child remained alive) was about 15 months, event‐free survival (time during which there were no objective signs of tumor recurrence) was about 10 months. Overall survival five years after treatment was 14.6%, and after 10 years was 12.2%.
With regard to short‐term side effects, there were some low blood cell counts. There was no liver toxicity. The studies did not report on heart problems and infections of the salivary glands. One study assessed long‐term side effects in some of the children: there was some evidence of thyroid (a gland in the neck) problems, which was brief in three children, but remained high in seven children, of whom five were prescribed medicine. There were no secondary cancers (where a different type of cancer has returned following the original cancer).
Based on the currently available evidence, we cannot make recommendations for the use of 131I‐MIBG therapy in patients with newly diagnosed HR NBL in clinical practice. More high‐quality research is needed before definite conclusions can be made.
Quality of the evidence
All studies had problems relating to quality of the evidence.
Background
Description of the condition
Neuroblastoma (NBL) is the most common extracranial solid tumour of childhood, derived from the sympathetic nervous system (Gurney 1996). The median age of diagnosis is 18 months. According to the International Neuroblastoma Staging System (INSS), NBL is classified into stages 1 to 4, with a special stage termed 4S. Children with stage 4 NBL present with metastatic disease at diagnosis, mainly involving lymph nodes and bone marrow. Traditionally, the defining characteristics of high‐risk NBL included an age of more than one year, metastatic disease, unfavourable Shimada histology and MYCN amplification (Bernstein 1992; Shimada 1995; Shimada 1999). In recent years, an age cut‐off of 18 months was discovered and implemented for pretreatment risk stratification, as opposed to the traditional cut‐off of one year (Cohn 2009). However, most currently published studies use the old definition. Current high‐risk treatment consists of intensive multi‐agent chemotherapy induction (Peinemann 2015a), extensive surgical resection of the primary tumour, external beam irradiation of residual primary tumour, myeloablative chemotherapy (Yalçin 2015), and maintenance with differentiation and immunotherapy (Peinemann 2015b).
Despite this very intensive treatment, children with advanced‐stage high‐risk NBL still have a poor prognosis; the five‐year overall survival rate of HR NBL in the Children's Oncology Group in the era between 2005 and 2010 is now 50% + 0.02 standard error (Pinto 2015). This poor outcome necessitates the search for new therapies (Matthay 1999; Simon 2011).
Description of the intervention
The majority of NBL tumours accumulate meta‐iodobenzylguanidine (MIBG). When radiolabelled with 123iodine, MIBG can be used for imaging and when labelled with 131iodine it can be used as a form of targeted radiotherapy (Bleeker 2015; Hattner 1984; Suc 1996). In various studies around the world, the radiopharmaceutical 131I‐MIBG has been shown to have a significant antitumour effect against NBL (Hutchinson 1991; Klingebiel 1991a; Klingebiel 1991b; Lashford 1992; Lumbroso 1991; Matthay 1998; Matthay 2007; Simon 2011; Voute 1991). More than 95% of NBL tumours are able to accumulate 131I‐MIBG actively (Leung 1997). Given the unsatisfactory results of high‐intensity induction chemotherapy it is rational to add 131I‐MIBG, as a 'targeted radiotherapy' to the treatment of HR NBL.
Extensive experience exists with 131I‐MIBG treatment of children with NBL. Hoefnagel and colleagues reported the value of 131I‐MIBG in the detection of NBL (Hoefnagel 1985). In the following years, it became clear that there was also a role for therapeutic use of 131I‐MIBG. Initially 131I‐MIBG therapy was given to children with recurrent NBL (Matthay 1998; Matthay 2001). After some time, a second group was included, that is, children with residual disease after chemotherapy and surgery. From these studies, it became clear that the most prominent response was obtained in children with a large tumour burden at the time of 131I‐MIBG treatment (Matthay 1998; Matthay 2001). This finding has served as the basis for a study performed in Amsterdam, with the objectives to document response in untreated children with stage 4 or non‐operable stage 3 disease, and to further characterise the adverse effects of 131I‐MIBG treatment. The study was closed in 1999. In summary, 131I‐MIBG therapy has a very high response rate at induction of children with HR NBL and can be combined with induction chemotherapy followed by mega‐therapy and autologous stem cell transplantation (ASCT). In this study, the chemotherapy was not dose intense and since then we have learned that dose intense chemotherapy results in better outcomes (De Kraker 2008). The current Dutch Children's Oncology Group high‐risk NBL 2009 treatment protocol combines upfront 131I‐MIBG with induction chemotherapy followed by mega‐therapy and ASCT. Matthay and colleagues reported in 2009 the results of a phase I study in children with refractory or relapsed HR NBL. It showed that closely spaced infusions of 131I‐MIBG can be administered safely using ASCT without dose‐limiting non‐haematological toxicity and with rapid and reliable reconstitution of haematopoiesis (Matthay 2009a).
How the intervention might work
131I‐MIBG is a radiopharmaceutical; it is a radio‐labelled molecule similar to noradrenaline which can be taken up by NBL tissue (Hattner 1984). When NBL has taken up the 131I‐MIBG (gamma and beta emitting isotope), its beta particles will irradiate neighbouring tissue with a median range of 1 cm causing cell death by double‐strand DNA breaks and damage to lipid bilayers (Hutchinson 1991; Matthay 2007).
Why it is important to do this review
At the moment, the prognosis for patients with HR NBL is still very poor. Relapses remain common, despite the achievement of a complete clinical remission after induction therapy. The place of 131I‐MIBG in newly diagnosed HR NBL treatment is not yet well established.
Objectives
To assess the efficacy and adverse effects of 131I‐MIBG therapy in patients with newly diagnosed HR NBL.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs), controlled clinical trials (CCTs), non‐randomised single‐arm trials with historical controls and cohort studies examining the efficacy of 131I‐MIBG therapy in patients with newly diagnosed HR NBL. We excluded cross‐sectional studies, case‐reports and case series (i.e. a series of non‐consecutive participants). We defined a cohort study as one in which a group of consecutive participants treated for HR NBL was followed from the time of diagnosis. The described study could be the original cohort or a subgroup of the original cohort based on well‐defined inclusion criteria.
We excluded studies including fewer than 10 patients.
Types of participants
Patients with newly diagnosed HR NBL. The defining characteristics of HR NBL include an age of more than one year, regional or metastatic disease, unfavourable Shimada histology or MYCN amplification. We excluded patients with esthesioneuroblastoma or newly diagnosed patients who received prior chemotherapy (or both). If studies included both eligible and non‐eligible participants, the results of eligible patients should have been separately available in order to be included in the review.
Types of interventions
131I‐MIBG therapy.
Types of outcome measures
Primary outcomes
Response as measured by the International Neuroblastoma Response Criteria (INRC) (Brodeur 1993).
Overall survival (as defined in the original study).
Event‐free survival, defined as the time span that follows therapy during which there are no objective signs of recurrence or other events (as defined in the original study).
Adverse events, as defined in the original studies. We particularly looked at haematological, cardiovascular and hepatic problems, and sialoadenitis as short‐term events. Long‐term events were thyroid dysfunction and secondary malignancies.
Secondary outcomes
Dose intensity after 131I‐MIBG treatment: the actual administered amount of 131I‐MIBG in mega Becquerel/milliCurie (MBq/mCi).
Time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy.
Yield of peripheral stem cell collection during harvest sessions (defined as the mean number of collected autologous haematopoietic stem cells).
Number of successful peripheral stem cell harvests (defined as more than 2 × 106/kg autologous haematopoietic stem cells).
Number of peripheral stem cell harvest sessions necessary to obtain a sufficient amount of autologous haematopoietic stem cells (i.e. more than 2 × 106/kg autologous haematopoietic stem cells).
Percentage of patients in which bone marrow harvest was performed
Stem cell engraftment defined as the time to haematopoietic recovery after myeloablative chemotherapy and ASCT were measured for each of the haematopoietic cell lineages.
Stem cell harvest could have taken place at any stage of the treatment protocols (i.e. before or after 131I‐MIBG therapy).
Search methods for identification of studies
We imposed no language restrictions.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 3), MEDLINE in PubMed (from 1945 to 25 April 2016) and Embase in Ovid (from 1980 to 25 April 2016). The appendices show the search strategies for the different electronic databases (using a combination of controlled vocabulary and text words; Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or Embase, either published or unpublished, by searching the reference lists of relevant articles and review articles. We handsearched the conference proceedings of the International Society for Paediatric Oncology, Advances in Neuroblastoma Research and the American Society of Clinical Oncology published from 2010 up to and including 2015. We scanned the International Standard Randomized Controlled Trial Number (ISRCTN) Register (www.isrctn.com) and the National Institutes of Health Register for ongoing trials (www.clinicaltrials.gov); both registers were searched on 13 April 2016 using this search strategy: (131I‐MIBG OR 131I‐meta‐iodobenzylguanidine) AND neuroblastoma.
Data collection and analysis
Selection of studies
Two authors independently selected studies meeting the inclusion criteria. We resolved discrepancies by consensus. If this was not possible, a third‐party arbitrator resolved the issue. We obtained in full‐text any study which seemed to meet the inclusion criteria based on the title or abstract (or both) for closer inspection. We clearly stated details of reasons for exclusion of any study considered for this review (see Characteristics of excluded studies table). We included a flow chart of the selection of studies in the review (Figure 1).
Data extraction and management
Two authors independently performed data extraction using standardised data extraction forms. We resolved discrepancies between authors by discussion. We extracted the following data.
Study characteristics, including:
design;
number of patients enrolled in the study;
number of patients fulfilling the predefined inclusion criteria.
Participants characteristics, including:
gender;
age at time of diagnosis (range, mean, median, or a combination of these);
stage of disease according to the INSS;
tumour biology and genetic aberrations (MYCN amplified and loss of heterozygosity chromosome 1P);
tumour localisation (primary and metastasis).
Interventions, including:
schedule of 131I‐MIBG treatment and total amount of 131I‐MIBG administered (in MBq/mCi);
additive (radio‐sensitising) therapeutics (dose and schedule) during 131I‐MIBG treatment and chemotherapy schedules thereafter.
Outcome measures (as described above).
Length of follow‐up.
Assessment of risk of bias in included studies
Two authors independently performed the assessment of risk of bias of the included studies. If we had identified RCTs and CCTs we would have used the risk of bias items as described in the module of Cochrane Childhood Cancer (Kremer 2012), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The assessment of risk of bias in observational studies was based on previously described checklists according to Evidence‐Based Medicine Criteria (Grimes 2002; Laupacis 1994). See Table 1 for the definitions of the different risk of bias criteria. For attrition bias, detection bias and outcome reporting bias (i.e. biases that can be assessed for each outcome separately), we only assessed the risk of bias for the primary outcomes. The risk of bias in included studies was taken into account in the interpretation of the review's results. We resolved discrepancies between authors by consensus.
1. Risk of bias criteria for observational studies.
| Internal validity | External validity | |
| Study group |
Selection bias (representative: yes/no): If the described study group consisted of > 90% of patients with HR NBL treated with 131I‐MIBG included in the original cohort Or If it was a random sample of these patients with respect to important prognostic factors (i.e. age, stage according to INSS (bone marrow involvement), MYCN amplification and loss of chromosome 1p), type of disease (i.e. newly diagnosed, relapsed, refractory) and cancer treatment. |
Reporting bias (well‐defined: yes/no): If the mean, median or range of the cumulative 131I‐MIBG dose was mentioned And When it was described what other treatment (including the received doses) was given. |
| Follow‐up |
Attrition bias (adequate: yes/no): If the outcome was assessed for > 90% of the study group of interest (++) Or If the outcome was assessed for 60‐90% of the study group of interest (+). |
Reporting bias (well‐defined: yes/no): If the length of follow‐up was mentioned. |
| Outcome |
Detection bias (blind: yes/no): If the outcome assessors were blinded to the investigated determinant. |
Reporting bias (well‐defined: yes/no): If the outcome definition was provided. |
HR: high risk; INSS: International Neuroblastoma Staging System; MIBG: meta‐iodobenzylguanidine; NBL: neuroblastoma.
Measures of treatment effect
If a control group had been available, we would have analysed dichotomous variables using risk ratios (RRs); continuous outcomes using mean differences (MDs) and survival using hazard ratios (HRs). We would have used the Parmar's method if HRs had not been explicitly presented in the study (Parmar 1998). However, as all included studies did not have a control group, we used the prevalence (and corresponding 95% confidence intervals (CI)) to analyse tumour response and adverse effects; we summarised other outcomes descriptively.
Dealing with missing data
When relevant data regarding study selection were missing, we contacted the study authors to retrieve the missing data. If RCTs or CCTs had been included, we would have extracted data by the allocated intervention, irrespective of compliance with the allocated intervention, in order to allow an intention‐to‐treat analysis. If this was not possible, we would have stated this and we would have performed an 'as treated' analysis.
Assessment of heterogeneity
As pooling of results was not possible, assessment of heterogeneity was not applicable. Otherwise, we would have assessed heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is, the I2 statistic. In the absence of significant heterogeneity (I2 less than 50%) (Higgins 2011), we would have used a fixed‐effect model for the estimation of treatment effects. Otherwise, we would have explored possible reasons for the occurrence of heterogeneity and we would have taken appropriate measures.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we would have assessed reporting bias by constructing a funnel plot when there would have been a sufficient number of included studies (that is at least 10 studies included in a meta‐analysis). When there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry (Higgins 2011). As pooling of results was not possible, this was not applicable.
Data synthesis
We entered data into Cochrane's statistical software, Review Manager 5 (RevMan 2014), and undertook analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included outcome measures only if it was the intention of the study to perform the necessary assessments in all participants (i.e. not only optional or only performed in some centres). We performed pooling of results only if studies were comparable with regard to important prognostic factors (i.e. age, stage according to INSS (bone marrow involvement), MYCN amplification and loss of chromosome 1P), treatment and used outcome definitions. Different study designs were taken into account in the analyses. Studies for which pooling of results was not possible were summarised descriptively. We used the Wilson method to calculate the corresponding 95% CIs of the prevalences. As this was not possible in Review Manager 5, we used a different tool (EpiTools epidemiological calculators).
Sensitivity analysis
For all outcomes for which pooling was possible, we would have performed sensitivity analyses for all risk of bias criteria separately. We would have excluded studies with a high risk of bias and studies for which the risk of bias was unclear and compared the results of studies with a low risk of bias with the results of all available studies. As pooling of results was not possible, this was not applicable.
Results
Description of studies
Results of the search
Running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and EMBASE (Ovid) yielded 3366 references. Following initial screening of the titles, abstracts, or both, we excluded 3304 references which clearly did not meet all criteria for this review. We assessed the full‐text of the 62 remaining references, of which two fulfilled all the criteria and were eligible for inclusion. Three articles are awaiting classification (see Characteristics of studies awaiting classification table) and the 57 excluded references are described in the Characteristics of excluded studies table.
Scanning the reference lists of the included articles and reviews did not identify any additional eligible studies. By scanning the conference proceedings we identified three additional studies (see Characteristics of studies awaiting classification table) and by scanning the ongoing trials databases we identified one possible ongoing study (see Characteristics of ongoing studies table).
In summary, two studies were eligible for inclusion in the review; six studies were awaiting classification, 57 were excluded and one was ongoing. See Figure 1 for a flow diagram of the selection of studies for this systematic review.
1.
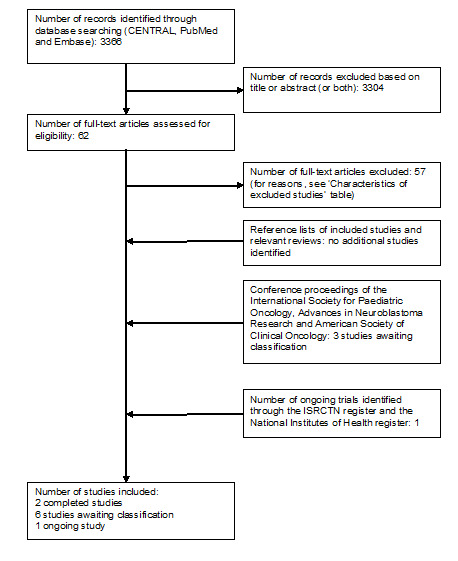
Study flow diagram
Included studies
The characteristics of the included studies are summarised below. For more detailed information, see the Characteristics of included studies table.
We identified one single‐centre prospective cohort study (De Kraker 2008), and one multi‐centre prospective phase II windows study (Kraal 2015), both conducted in the Netherlands. The total number of children with newly diagnosed HR NBL included in these studies was 60. In both studies children had metastatic disease (i.e. INSS stage 4) (De Kraker 2008, Kraal 2015). Children were aged between 1 and 15.4 years at diagnosis. In one study, children received 131I‐MIBG single agent therapy (De Kraker 2008), while in the other study, children received 131I‐MIBG in combination with topotecan (Kraal 2015). For complete detailed treatment information, see the Characteristics of included studies table. None of the studies reported the length of follow‐up.
Risk of bias in included studies
See the risk of bias section of the Characteristics of included studies table and Figure 2 for the exact scores per study and the support for the judgements made. We have looked both at internal and external validity.
2.
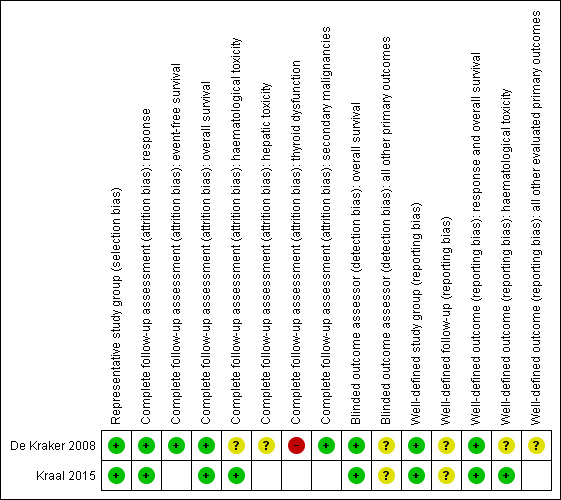
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Internal validity
Selection bias
For evaluating selection bias, we assessed if there was a representative study group. In both studies, the risk of selection bias was low.
Attrition bias
For evaluating attrition bias, we assessed the completeness of follow‐up for the different primary outcomes. Both studies assessed response, overall survival and haematological toxicity; for the first two outcomes, the risk of attrition bias was low in both studies, for haematological toxicity, the risk of bias was low in one study (Kraal 2015), and unclear in the other study (De Kraker 2008). De Kraker 2008 also assessed event‐free survival (low risk of attrition bias), hepatic toxicity (unclear risk of attrition bias), thyroid dysfunction (high risk of attrition bias) and secondary malignancies (low risk of attrition bias).
Detection bias
For evaluating detection bias, we assessed if the outcome assessors were blinded to the investigated determinant for the different primary outcomes. For overall survival, this was not applicable and therefore the risk of detection bias was low in both studies. For all other outcomes that were assessed in both studies, the risk of detection bias was unclear.
External validity
Reporting bias
In both studies, the study group was well‐defined and the risk of reporting bias was low.
In both studies, the length of follow‐up was not mentioned, so the risk of reporting bias was unclear.
For overall survival, the outcome is well‐defined by default, so the risk of reporting bias was low in both studies. Both studies also assessed response (low risk of reporting bias in both studies) and haematological toxicity (low risk of reporting bias in Kraal 2015, unclear risk of reporting bias in De Kraker 2008). For all other primary outcomes that were assessed in both studies, the risk of reporting bias was unclear.
Overall, neither of the included studies scored good on all applicable reporting bias items.
Effects of interventions
Not all articles allowed data extraction for all endpoints (see the Characteristics of included studies table for a more detailed description of the extractable endpoints from each article).
As the studies were not comparable with regard to prognostic factors and treatment (and often used different outcome definitions), pooling of results was not possible. For response and adverse effects (both short‐term and long‐term), we used the Wilson method to calculate the prevalence and 95% CI; for all other outcomes, we presented results as described in the publications.
Response
De Kraker 2008 used the INRC to define response (Brodeur 1993). The objective response rate (ORR, i.e. complete response (CR), very good partial response (VGPR) or partial response (PR)) was evaluated at two different time points. After two cycles of 131I‐MIBG therapy, the ORR was 66% (95% CI 51% to 78%); 1/41 children had a CR and 26/41 had a PR. After surgery, the ORR was 73% (95% CI 58% to 84%); 16/41 children had a CR, 1/41 a VGPR and 13/41 a PR. For the ORRs in 131I‐MIBG‐treated children and 131I‐MIBG plus chemotherapy‐treated children, see Table 2.
2. Objective response rate for children treated with 131I‐MIBG only and 131I‐MIBG plus chemotherapy.
| Treatment | ORR (95% CI) before surgery | ORR (95% CI) after surgery |
| 131I‐MIBG only (24 children) | 79% (60% to 91%) | 92% (74% to 98%) |
| 131I‐MIBG and chemotherapy (17 children) | 35% (17% to 59%) | 47% (26% to 69%) |
CI: confidence interval; MIBG: meta‐iodobenzylguanidine; ORR: objective response rate.
Kraal 2015 also used the INRC to define response (Brodeur 1993). The ORR was evaluated at two different time points. After two cycles of 131I‐MIBG therapy plus topotecan the ORR was 56% (95% CI 33% to 77%); 1/16 children had a VGPR and 8/16 children had a PR. After myeloablative therapy and ASCT, the ORR was also 56% (95% CI 33% to 77%); 5/16 children had a CR and 4/16 children a PR.
Overall survival
De Kraker 2008 provided no definition for overall survival. The median overall survival for all 41 children was 15 months (95% CI 7 to 23) and five‐year overall survival was 14.6%.
Kraal 2015 provided no definition for overall survival. The 10‐year overall survival was 6.25%.
Event‐free survival
De Kraker 2008 provided no definition for event‐free survival. The median event‐free survival for all 41 children was 10 months (95% CI 7 to 13) and five‐year event‐free survival was 12.2%.
Kraal 2015 did not report event‐free survival.
Short‐term adverse events
Haematological adverse events
De Kraker 2008 defined haematological adverse events as death due to myelosuppression. One child died 43 days after stem cell reinfusion of a cerebral bleeding while still thrombocytopenic (no definition provided); the child had received a cumulative dose of 800 mCi of MIBG. A toxic myelosuppression is highly suspective for the death. It was unclear if all children were assessed for this outcome, but the best‐case scenario showed a prevalence of 2% (95% CI 0% to 13%).
Kraal 2015 defined haematological adverse events as grade 3 or 4 according to the Common Terminology Criteria Adverse Event version 3 (CTCAEv3) criteria. They assessed platelets, neutrophils and haemoglobin (Hb) at two different time points. After the first cycle of 131I‐MIBG therapy with topotecan, 6/16 children had grade 3 or 4 platelets (38%, 95% CI 18% to 61%), 8/16 had grade 3 or 4 neutrophils (50%, 95% CI 28% to 72%) and 11/16 had grade 3 or 4 Hb (69%, 95% CI 44% to 86%). After the second cycle of 131I‐MIBG therapy with topotecan 9/15 children had grade 3 or 4 platelets and grade 3 or 4 neutrophils (60%, 95% CI 36 to 80%), and 8/15 children had grade 3 or 4 Hb (53%, 95% CI 30 to 75%).
Cardiovascular adverse events
Neither De Kraker 2008 nor Kraal 2015 reported cardiovascular adverse events.
Hepatic adverse events
De Kraker 2008 defined hepatic adverse events as higher than grade 1, but no definition was provided. It was assessed during or within four weeks after last the 131I‐MIBG treatment. It was not clear if all participants were assessed for this outcome, but the best‐case scenario showed a prevalence of 0% (95% CI 0% to 9%).
Kraal 2015 did not report hepatic adverse events.
Sialoadenitis
Neither De Kraker 2008 nor Kraal 2015 reported sialoadenitis.
Long‐term adverse events
Thyroid dysfunction
De Kraker 2008 tested thyroid function in 22/41 children (54%) before or after 131I‐MIBG treatment. They assessed thyroid‐stimulating hormone (TSH) (greater than 4.5 mU/L) and T4 (cut‐off point not provided). Children were measured at a median follow‐up of 19 months (range 0.7 to 129). Ten out of 22 children had an elevated plasma TSH (45%, 95% CI 27% to 65%), which was transient in three children. It remained elevated in seven children of which five were prescribed thyroxine. In all 22 children, free T4 was within the age‐related normal range (0%, 95% CI 0% to 15%).
Kraal 2015 did not report thyroid dysfunction.
Secondary malignancies
De Kraker 2008 provided no definition for secondary malignancies. None were observed (0%, 95% CI 0% to 9%), but only five children survived more than four years.
Kraal 2015 did not report secondary malignancies.
Dose intensity after 131I‐MIBG treatment: the actual administered amount of 131I‐MIBG
In De Kraker 2008, children received a cumulative dose of 350 mCi to 950 mCi (13 GBq to 35 GBq) 131I‐MIBG (median 500 mCi) as induction therapy.
In Kraal 2015, the administered activity was median 0.5 GBq/kg (range 0.4 to 0.6) and 14.5 mCi/kg (range 10.1 to 16.8) in the first cycle and median 0.4 GBq/kg (range 0.3 to 0.5) and 10.6 mCi/kg (range 6.9 to 12.6) in the second cycle. The cumulative administered activity was median 0.9 GBq/kg (range 0.5 to 1.1) and median 25 mCi/kg (range 14.3 to 29.4).
Time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy
De Kraker 2008 did not report time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy.
In Kraal 2015, the researchers aimed to give treatment at four‐week intervals. The actual mean interval time between the second MIBG plus topotecan and the first VECI (vincristine, etoposide, carboplatin and ifosfamide) chemotherapy course was 30 days (range 20 to 47). The mean interval time for the subsequent VECI chemotherapy courses was 30 days (range 18 to 40) for the first course, 31 days (range 28 to 38) for the second course and 29 days (range 21 to 46) between the third and fourth course.
Stem cells
Yield of peripheral stem cell collection during harvest sessions
De Kraker 2008 did not report the mean number of collected autologous haematopoietic stem cells, but they did report the median value for the 17 children who underwent a stem cell harvest. In the first eight children, it was 11.6 × 104/kg colony‐forming unit in culture (CFU‐C) colonies (range 2.9 to 31.6), while in the subsequent nine children, the median was 4.9 × 106/kg CD34+ cells (range 1.2 to 17).
Kraal 2015 did report the mean number of collected stem cells for the 13 children who underwent a stem cell harvest: the mean yield of peripheral blood stem cells harvest was 2.2 × 106/kg (0 to 6.9) CD34+ stem cells. For the bone marrow harvest, this was 3.4 × 106/kg (1.0 to 9.1) CD34+ stem cells.
Number of successful peripheral stem cell harvests
De Kraker 2008 did not report the number of successful peripheral stem cell harvests.
Kraal 2015 did not provide a definition for a successful peripheral stem cell harvest, so we could not be certain it was according our prespecified definition of 2 × 106/kg autologous haematopoietic stem cells. They reported that peripheral blood stem cell apheresis was successful in 6/13 children (46%).
Number of peripheral stem cell harvest sessions necessary to obtain a sufficient amount of autologous haematopoietic stem cells
Neither De Kraker 2008 nor Kraal 2015 reported the number of peripheral stem cell harvest sessions necessary to obtain a sufficient amount of autologous haematopoietic stem cells.
Percentage of children in which bone marrow harvest was performed
De Kraker 2008 did not report the percentage of children in which bone marrow harvest was performed.
In Kraal 2015, 6/13 children (46%) underwent further bone marrow harvesting.
Stem cell engraftment defined as the time to haematopoietic recovery after myeloablative chemotherapy and autologous stem cell transplantation for each of the haematopoietic cell lineages
De Kraker 2008 assessed stem cell engraftment for platelets and neutrophils, but not for Hb. The median time to platelet engraftment (greater than 50 × 103/μL) was 47 days. The median time to neutrophil engraftment (greater than 1 × 103/μL) was 23 days.
Kraal 2015 also assessed stem cell engraftment for platelets and neutrophils, but not for Hb. The median time to platelet engraftment (greater than 25 × 109/L) was 37 days (range 14 to 74). For neutrophil engraftment (greater than 0.5 × 109/L), this was 21 days (range 12 to 62).
Discussion
Summary of main results
Patients with newly diagnosed HR NBL still have a poor outcome, despite multi‐modality intensive therapy. This poor outcome necessitates the search for new therapies, such as treatment with 131I‐MIBG. In this systematic review, we assessed the efficacy and adverse effects of 131I‐MIBG therapy in patients with newly diagnosed HR NBL.
We identified two prospective (cohort) studies investigating efficacy and toxicity of upfront 131I‐MIBG therapy in children with newly diagnosed HR NBL. The first study included 44 children (De Kraker 2008), the second study included 16 children (Kraal 2015). As the studies were not comparable with regard to prognostic factors and treatment (and often used different outcome definitions), pooling of results was not possible.
Both studies assessed response according to the INRC. In De Kraker 2008, the ORR was 73% after surgery. In Kraal 2015 the ORR was 56% after myeloablative therapy and ASCT.
In De Kraker 2008, median overall survival was 15 months (95% CI 7 to 23) and five‐year overall survival was 14.6%. Median event‐free survival was 10 months (95% CI 7 to 13) and five‐year event‐free survival was 12.2%. In Kraal 2015, 10‐year overall survival was 6.25% and event‐free survival was not reported.
With regard to short‐term adverse effects, De Kraker 2008 assessed death due to myelosuppression. It was unclear if all children were assessed for this outcome, but the best‐case scenario showed a prevalence of 2% (95% CI 0% to 13%). Kraal 2015 assessed platelets, neutrophils and Hb grade 3 or 4 according to the CTCAEv3 criteria. After the first cycle of 131I‐MIBG therapy plus topotecan there was platelet toxicity in 38% (95% CI 18% to 61%), neutrophil toxicity in 50% (95% CI 28% to 72%) and Hb toxicity in 69% (95% CI 44% to 86%). After the second cycle, this was 60% (95% CI 36% to 80%) for platelets and neutrophils and 53% (95% CI 30% to 75%) for Hb. Only De Kraker 2008 reported hepatic toxicity: it was assessed during or within four weeks after last the MIBG treatment (higher than grade 1). It was unclear if all children were assessed for this outcome, but the best‐case scenario showed a prevalence of 0% (95% CI 0% to 9%). Neither study reported cardiovascular toxicity and sialoadenitis. Kraal 2015 did not look at long‐term adverse events, but De Kraker 2008 reported elevated plasma TSH in 45% of the 22 assessed children (95% CI 27% to 65%); in all 22 children, the free T4 was within the age‐related normal range (0%, 95% CI 0% to 15%). There were no secondary malignancies observed (0%, 95% CI 0% to 9%), but only five children survived more than four years.
With regard to dose intensity after 131I‐MIBG treatment, in De Kraker 2008, children received a cumulative dose of 350 mCi to 950 mCi (13 GBq to 35 GBq) 131I‐MIBG (median 500 miC) as induction therapy, while in Kraal 2015 the administered activity was median 0.5 GBq/kg (range 0.4 to 0.6) and 14.5 mCi/kg (range 10.1 to 16.8) in the first cycle and median 0.4 GBq/kg (range 0.3 to 0.5) and 10.6 mCi/kg (range 6.9 to 12.6) in the second cycle. The cumulative administered activity was median 0.9 GBq/kg (range 0.5 to 1.1) and median 25 mCi/kg (range 14.3 to 29.4).
Only Kraal 2015 reported time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy, who aimed to give treatment at four‐week intervals. The actual mean interval time between the second MIBG plus topotecan and the first VECI chemotherapy course was 30 days (range 20 to 47). The mean interval time for the subsequent VECI chemotherapy courses was 30 days (range 18 to 40) for the first course, 31 days (range 28 to 38) for the second course and 29 days (range 21 to 46) between the third and fourth course.
We also collected information on stem cells. De Kraker 2008 reported the median number of collected autologous haematopoietic stem cells during harvest sessions for the 17 children who underwent a stem cell harvest. In the first eight children, it was 11.6 × 104/kg CFU‐C colonies (range 2.9 to 31.6), while in the subsequent nine children, the median was 4.9 × 106/kg CD34+ cells (range 1.2 to 17). Kraal 2015 reported the mean number of collected stem cells for the 13 children who underwent a stem cell harvest: the mean yield of peripheral blood stem cells harvest was 2.2 × 106/kg CD34+ stem cells (range 0 to 6.9). For the bone marrow harvest, this was 3.4 × 106/kg CD34+ stem cells (range 1.0 to 9.1). De Kraker 2008 provided no information on the number of successful stem cell harvests; Kraal 2015 reported that peripheral blood stem cell apheresis was successful in 46% of the children, but did not provide a definition. Neither study provided information on the number of peripheral stem cell harvest sessions necessary to obtain a sufficient amount of autologous haematopoietic stem cells. In Kraal 2015, 46% of children underwent bone marrow harvesting; De Kraker 2008 provided no information. Both studies reported on stem cell engraftment for platelets and neutrophils, but not for Hb. In De Kraker 2008, the median time to platelet engraftment (greater than 50 × 103/μL) was 47 days; the median time to neutrophil engraftment (greater than 1 × 103/μL) was 23 days. In Kraal 2015, the median time to platelet engraftment (greater than 25 × 109/L) was 37 days (range 14 to 74); for neutrophil engraftment (greater than 0.5 × 109/L) this was 21 days (range 12 to 62).
Overall completeness and applicability of evidence
The external validity of a study indicates how well its results can be extrapolated to individual children with newly‐diagnosed HR NBL treated with 131I‐MIBG therapy. It includes the following items: well‐defined study group, well‐defined follow‐up and well‐defined outcome. If important information is missing, it is difficult to correctly interpret the results and extrapolate them to individual children. In both included studies, the study group was well‐defined and the risk of reporting bias was low. In both studies, the length of follow‐up was not mentioned, so the risk of reporting bias was unclear. Follow‐up could have been too short for children to develop, for example, long‐term adverse effects. For overall survival, the outcome is well‐defined by default, so the risk of reporting bias was low in both studies. Both studies assessed response (low risk of reporting bias in both studies) and haematological toxicity (low risk of reporting bias in Kraal 2015; unclear risk of reporting bias in De Kraker 2008). For all other primary outcomes assessed by the included studies, the risk of reporting bias was unclear. Overall, neither of the included studies scored good on all applicable reporting bias items, on many occasions due to a lack of reporting.
In addition, the studies were performed in a different treatment era (1989 to 1999 (De Kraker 2008) and 2000 to 2003 (Kraal 2015)). Supportive care, like antibiotic use, anticancer treatments, diagnostic techniques and reporting standards have changed substantially since then, so consequently, the results may not be applicable to children who are treated in the 2010s. The overall survival reported in both included studies (five‐year overall survival in De Kraker 2008 of 14.6% and 10‐year overall survival in Kraal 2015 of 6.25%) is very much less than the current norm in other studies of treatment in children with newly diagnosed HR NBL. For example, Pinto 2015 found a five‐year overall survival rate of 50% in the treatment era between 2005 and 2010 (Pinto 2015). This can be explained by the fact that the two included studies were carried out in different treatment era (1989 to 2003). In both studies, the 131I‐MIBG therapy was followed by VECI chemotherapy, which later was shown to be less effective chemotherapy than other regimens (Pearson 2008).
Supportive care, such as antibiotic use, and anticancer treatments have changed substantially within this period, so consequently, the results may not all be applicable to children who are treated in the 2010s.
It should be kept in mind that the time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy does not only depend on the effects of 131I‐MIBG therapy, but, for example, also on adverse effects after chemotherapy.
The absence of reporting of either whole‐body dose (which correlates with toxicity (Trieu 2016)) or tumour dose (which may correlate with response and survival (Trieu 2016)), or both, in the included studies is a major methodological flaw.
Despite all children having newly diagnosed HR NBL, in this review we used the old definition of HR NBL. Recently, the age cut‐off has been changed from one year to 18 months (Cohn 2009). As a result, some included children might now be classified as having intermediate‐risk disease. However, the number of children that will switch from high‐risk to intermediate‐risk treatment is low.
Even though RCTs provide the highest level of evidence, it should be recognised that data from non‐randomised studies on the efficacy of 131I‐MIBG therapy in children with newly diagnosed NBL are available (Kraal 2015; De Kraker 2008). The overall response rate after two courses of 131I‐MIBG therapy was 66% (De Kraker 2008) and 56% (Kraal 2015). Although the results of these studies are promising, they should be interpreted with caution due to the biases associated with non‐randomised study designs, in these cases, two retrospective cohort studies. A study by Park and colleagues described a lower response rate to chemotherapy of 12/31 (39%) children after two cycles of topotecan/cyclophosphamide in children with HR NBL (Park 2011).
Finally, data were not available for all outcomes of interest. As a result, we cannot draw conclusions regarding those outcomes, which are important for clinical practice.
Quality of the evidence
To adequately assess the efficacy and adverse effects of 131I‐MIBG therapy in patients with newly diagnosed HR NBL, the best study design ‐ provided that the design and execution are correct ‐ is an RCT in which the only difference between the intervention and control group is use of 131I‐MIBG therapy. CCTs can also provide reliable information, keeping in mind their limitations, but we found none of these. This review included other study designs, but they were associated with a high risk of bias.
The internal validity gives an indication of the bias present in a study and thus how valid the results of a certain study are. It includes the following issues: selection bias, attrition bias and detection bias. In both studies, selection bias could be ruled out. The risk of attrition bias was low for response, event‐free survival, overall survival, secondary malignant disease, stem cells and, in one study (Kraal 2015), for haematological toxicity. In the other study, the risk of attrition bias was unclear for haematological toxicity and hepatic toxicity, and high for thyroid dysfunction (De Kraker 2008). Attrition bias can lead to an overestimation or underestimation of the risk of these adverse effects. The risk of detection bias was low for overall survival, but it could not be ruled out for all other assessed primary outcomes. Knowledge of prognostic factors can increase the possibility of classifying a person as having a certain outcome.
Potential biases in the review process
This systematic review used a very broad search strategy for identifying eligible studies. However, although it is unlikely that eligible studies were missed, it is never possible to completely rule out reporting bias.
Authors' conclusions
Implications for practice.
We found no randomised controlled trials or controlled clinical trials comparing the effectiveness of treatment including 131I‐meta‐iodobenzylguanidine (131I‐MIBG) therapy versus treatment not including 131I‐MIBG therapy in patients with newly diagnosed high‐risk (HR) neuroblastoma (NBL). We found two observational studies in children, but, besides the associated high risk of bias, results were not available for all relevant outcomes. In addition, it should be kept in mind that recently the age cut‐off for high‐risk disease was changed from one year to 18 months. As a result, it is possible that children with what is now classified as intermediate‐risk disease were included in the high‐risk groups. Based on the currently available evidence, we cannot make recommendations for the use of 131I‐MIBG therapy in patients with newly diagnosed HR NBL in clinical practice. At the moment, 131I MIBG treatment should only be given in the context of a well‐designed randomised controlled trials.
Implications for research.
Prospective randomised controlled trials are essential to strengthen the evidence base on the use of 131I‐MIBG therapy in patients with newly diagnosed HR NBL. The studies should be performed in homogeneous study populations (e.g. stage of disease; using the most recent definitions for high‐risk disease) and have a long‐term follow‐up. Dosimetry for 131I‐MIBG therapy should be incorporated into the treatment protocol. The number of included participants should be sufficient to obtain the power needed for the results to be reliable. Accurate and transparent reporting of findings will make it possible for readers to critically appraise the results of these studies.
Acknowledgements
Huib Caron was a coauthor of the protocol for this systematic review and we thank him for his valuable input. We would like to acknowledge the Editorial Base of Cochrane Childhood Cancer for their advice and support. The Editorial Base of Cochrane Childhood Cancer is funded by 'Stichting Kinderen Kankervrij' (KiKa), the Netherlands.
We would like to thank the peer reviewers for their comments.
Appendices
Appendix 1. Search strategy for CENTRAL (the Cochrane Library)
1. For 'Neuroblastoma' the following text words were used:
neuroblastoma OR neuroblastomas OR neuroblast* OR ganglioneuroblastoma OR ganglioneuroblastomas OR neuroepithelioma OR neuroepitheliomas OR Peripheral Primitive Neuroectodermal Tumors OR Peripheral Primitive Neuroectodermal Neoplasm OR Primitive Neuroectodermal Tumor, Extracranial OR Neuroectodermal Tumor, Peripheral OR Neuroectodermal Tumors, Peripheral OR Peripheral Neuroectodermal Tumor OR Peripheral Neuroectodermal Tumors OR Tumor, Peripheral Neuroectodermal OR Tumors, Peripheral Neuroectodermal OR pPNET OR PNET OR PNET* OR Peripheral Primitive Neuroectodermal Tumor OR Extracranial Primitive Neuroectodermal Tumor OR Extracranial Primitive Neuroectodermal Tumors OR Neuroectodermal Neoplasm, Peripheral Primitive OR Neuroectodermal Tumor, Peripheral Primitive
2. For '131I‐meta‐iodobenzylguanidine' the following text words were used:
131I‐Meta‐iodobenzylguanidine or 131I‐MIBG or 131I‐metaiodobenzylguanidine or Iodine‐131 Metaiodobenzylguanidine or Iobenguane (131I) or (3‐Iodo‐ (131I) benzyl) guanidine OR iodine‐131‐metaiodobenzylguanidine or 131I‐MIBG therapy or I‐metaiodobenzylguanidine or I‐131‐MIBG or I‐131‐Metaiodobenzylguanidine or (131) I‐MIBG or (131) I‐metaiodobenzylguanidine or (MIBG and (treatment or therapy)) OR 3‐Iodobenzylguanidine
Final search 1 AND 2
The search was performed in title, abstract or keywords
[* = zero or more characters]
Appendix 2. Search strategy for MEDLINE (PubMed)
1. For 'Neuroblastoma' the following MeSH headings and text words were used:
neuroblastoma OR neuroblastomas OR neuroblast* OR ganglioneuroblastoma OR ganglioneuroblastomas OR neuroepithelioma OR neuroepitheliomas OR (Peripheral Primitive Neuroectodermal Tumors OR Peripheral Primitive Neuroectodermal Neoplasm OR Primitive Neuroectodermal Tumor, Extracranial OR Neuroectodermal Tumor, Peripheral OR Neuroectodermal Tumors, Peripheral OR Peripheral Neuroectodermal Tumor OR Peripheral Neuroectodermal Tumors OR Tumor, Peripheral Neuroectodermal OR Tumors, Peripheral Neuroectodermal) OR (pPNET OR PNET OR PNET*) OR Peripheral Primitive Neuroectodermal Tumor OR Extracranial Primitive Neuroectodermal Tumor OR Extracranial Primitive Neuroectodermal Tumors OR Neuroectodermal Neoplasm, Peripheral Primitive OR Neuroectodermal Tumor, Peripheral Primitive
2. For '131I‐meta‐iodobenzylguanidine' the following MeSH headings and text words were used:
(131I‐Meta‐iodobenzylguanidine OR 131I‐MIBG OR 131I‐metaiodobenzylguanidine OR Iodine‐131 Metaiodobenzylguanidine OR Iobenguane (131I) OR (3‐Iodo‐(131I)benzyl)guanidine OR Iodine Radioisotopes/therapeutic use OR 3‐Iodobenzylguanidine/therapeutic use) OR (iodine‐131‐metaiodobenzylguanidine OR 131I‐MIBG therapy OR I‐metaiodobenzylguanidine OR I‐131‐MIBG OR I‐131‐Metaiodobenzylguanidine OR (131) I‐MIBG OR 3‐Iodobenzylguanidine[mh] OR (131) I‐metaiodobenzylguanidine OR (MIBG AND (treatment OR therapy)))
Final search 1 AND 2
[* = zero or more characters]
Appendix 3. Search strategy for Embase (Ovid)
1. For 'Neuroblastoma' the following Emtree terms and text words were used:
exp neuroblastoma/
(neuroblastoma or neuroblastomas or neuroblast$).mp.
(ganglioneuroblastoma or ganglioneuroblastomas or ganglioneuroblast$).mp.
(neuroepithelioma or neuroepitheliomas or neuroepitheliom$).mp.
exp neuroectoderm tumor/ or (peripheral primitive neuroectodermal tumors or peripheral primitive neuroectodermal tumours).mp.
(peripheral primitive neuroectodermal neoplasm or peripheral primitive neuroectodermal neoplasms).mp.
(peripheral neuroectodermal tumor or peripheral neuroectodermal tumors or peripheral neuroectodermal tumour or peripheral neuroectodermal tumours).mp.
(pPNET or PNET or PNET$).mp.
(peripheral primitive neuroectodermal tumor or peripheral primitive neuroectodermal tumour).mp.
(extracranial primitive neuroectodermal tumor or extracranial primitive neuroectodermal tumors or extracranial primitive neuroectodermal tumour or extracranial primitive neuroectodermal tumours).mp.
or/1‐10
2. For '131I‐meta‐iodobenzylguanidine' the following Emtree terms and text words were used:
exp "(3 iodobenzyl)guanidine i 131"/ or 131I‐Meta‐iodobenzylguanidine.mp.
131I‐MIBG.mp.
131I‐metaiodobenzylguanidine.mp.
Iodine‐131 Metaiodobenzylguanidine.mp.
Iobenguane 131I.mp.
"(3 iodobenzyl)guanidine"/
Iodine Radioisotopes.mp. or exp radioactive iodine/
radiopharmaceutical agent/ad, dt
131I‐MIBG therapy.mp.
I‐metaiodobenzylguanidine.mp.
(I‐131‐MIBG or I‐131‐Metaiodobenzylguanidine or 131‐I‐MIBG).mp.
3‐Iodobenzylguanidine.mp.
131‐I‐metaiodobenzylguanidine.mp.
(MIBG and (treatment or therapy)).mp.
or/1‐14
Final search 1 AND 2
[mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; / = Emtree term; $=zero or more characters; ad=drug administration; dt=drug therapy]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
De Kraker 2008.
| Methods | Prospective cohort study. Single‐centre study performed in the Netherlands. Start and end date: April 1989 and October 1999. |
|
| Participants | 44 children (median age at diagnosis 2.6 years (range 1‐15.4); 21 girls and 23 boys) with newly diagnosed high‐risk NBL (INSS stage not explicitly mentioned, but based on the metastatic disease, deduced as stage 4). Only 41 children evaluable (age range at start treatment 1.2‐15.5 years; 20 girls and 21 boys; 24 received MIBG, 17 received MIBG + chemotherapy): in 2 children, it was not feasible to start 131I‐MIBG therapy (1 early progression requiring immediate treatment; 1 sepsis and too unstable for MIBG treatment); in 1 child with progressive disease, parents refused further therapy after 1 MIBG infusion. 2 other children with only 1 MIBG infusion remained in the study. Tumour biology: NBL. Genetics: 10/44 (23%) MYCN amplification (4/24 (16%) MIBG and 6/17 (35%) MIBG + chemotherapy), 20/43 (47%; value for 1 child not available) loss of heterozygosity chromosome 1p (LOH1p) (12 (50%) MIBG and 8 (47%) MIBG + chemotherapy). Primary tumour location: not mentioned. Tumour metastasis: BM all children. Previous treatment received: not mentioned (but see exclusion criteria for further information). Inclusion criteria: children aged 1‐18 years at diagnosis with high‐risk, MIBG‐positive NBL. Exclusion criteria: previous chemotherapy. Initial surgery as the only treatment modality was allowed. |
|
| Interventions |
Induction therapy: 2 cycles of 131I‐MIBG single‐agent therapy; if objective response occurred then third MIBG treatment (24 children); if stable disease or progression preoperative VECI chemotherapy (17 children) then surgery (as soon as the surgeon felt that > 95% resection was feasible) then 4 courses of VECI chemotherapy at 4‐weekly intervals then consolidation therapy 4 weeks after last VECI: HDCT/ASCT then retinoic acid. VECI: vincristine (1.5 mg/m2 IV on day 1), ifosfamide (3000 mg/m2 IV over 1 hour on days 1 and 2), carboplatin (400 mg/m2 IV in a 24‐hour infusion on day 3), etoposide (150 mg/m2 IV over 4 hours on day 4). Consolidation therapy: carboplatin (800 mg/m2 IV over 6 hours on day 1), melphalan (180 mg/m2 IV on day 3), reinfusion of BM or PBCS on day 5. Harvest of stem cells: during postoperative VECI courses as soon as the child was in VGPR or CR with a clear BM. After stem cell reinfusion, 13‐cis‐retinoic acid 160 mg/m2 per day administered orally in 2 divided doses for 14 consecutive days in a 28‐day cycle, given for 6 months, beginning 4 weeks after stem‐cell reinfusion, in 14‐day cycles. 131I‐MIBG therapy: first infusion fixed dose 200 mCi (7.4 GBq), 2nd and all subsequent infusions 100‐150 mCi (3.7‐5.6 GBq) by IV 4‐hour infusion. Interval between MIBG infusions 4 weeks. MIBG dosimetry not performed. Thyroid blocking: potassium iodide 100 mg for 14 days, beginning on day before MIBG infusion. 14 children had no surgery; 2 had only 1 MIBG infusion; 17 had stem cell transplant. No control participants. |
|
| Outcomes |
Response (according to INRC (Brodeur 1993)). Overall survival (no definition provided). Event‐free survival (no definition provided). Toxicity:
Dose intensity after 131I‐MIBG treatment (the actual administered amount of 131I‐MIBG MBq/mCi). Stem cells:
|
|
| Notes |
Follow‐up: not mentioned, but the maximal follow‐up according to the survival curves was approximately 175 months; thyroid dysfunction was assessed after a mean follow‐up of 19 months (range 0.7‐129). Influence of funders: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | > 90% of the original cohort. |
| Complete follow‐up assessment (attrition bias): response | Low risk | Response assessed for > 90% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): event‐free survival | Low risk | Event‐free survival assessed for > 90% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): overall survival | Low risk | Overall survival assessed for > 90% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): haematological toxicity | Unclear risk | Not mentioned. |
| Complete follow‐up assessment (attrition bias): hepatic toxicity | Unclear risk | Not mentioned. |
| Complete follow‐up assessment (attrition bias): thyroid dysfunction | High risk | Thyroid dysfunction only assessed in 50% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): secondary malignancies | Low risk | Secondary malignancies were assessed in > 90% of the study group of interest. |
| Blinded outcome assessor (detection bias): overall survival | Low risk | Blinding of outcome assessor not applicable for overall survival. |
| Blinded outcome assessor (detection bias): all other primary outcomes | Unclear risk | Not mentioned. |
| Well‐defined study group (reporting bias) | Low risk | All necessary items (see Table 1) provided. |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow‐up not mentioned. |
| Well‐defined outcome (reporting bias): response and overall survival | Low risk | Outcome definition provided for response, not applicable for overall survival. |
| Well‐defined outcome (reporting bias): haematological toxicity | Unclear risk | No outcome definition provided. |
| Well‐defined outcome (reporting bias): all other evaluated primary outcomes | Unclear risk | No outcome definition provided. |
Kraal 2015.
| Methods | Prospective phase II windows study. Multi‐centre study performed in the Netherlands (2 centres). Start and end date: March 2000 and August 2003. |
|
| Participants | 16 children (median age at diagnosis 2.8 years (range 1.6‐8.3); age at start treatment not mentioned; 10 boys and 6 girls) with newly diagnosed HR NBL (INSS stage not explicitly mentioned, but based on the metastatic disease, deduced as stage 4). Tumour biology: NBL. Genetics: 8/14 (57%; value for 2 children not available) MYCN amplification, 6/15 (40%; value for 1 child not available) loss of heterozygosity chromosome 1p (LOH1p), MYCN amplification and LOH1p 6/14 (43%; value for 2 children not available). Primary tumour location: 15/16 (94%) abdominal and 1/16 (6%) thoraco‐abdominal. Tumour metastasis: 16/16 (100%) BM, 4/16 (25%) lymph node and 1/16 (6%) pleura. Previous treatment received: none (no prior cancer treatment was allowed). Inclusion criteria: histologically confirmed HR NBL, no prior cancer treatment, a non‐complicated clinical condition which allowed for a 5‐day radioprotective isolation for 131I‐MIBG treatment, aged 0‐16 years at diagnosis, signed and dated informed consent. Exclusion criteria: not conform inclusion criteria stated above. |
|
| Interventions |
Upfront treatment: 2 cycles of 131I‐MIBG therapy + topotecan (16 children) then 4 courses of VECI chemotherapy at 4 week intervals (15 children) then surgery (12 children; optimal timing of surgery was discussed when the distant metastases were inactive and the tumour operable) then myeloablative therapy/ASCT (9 children) then 13 cis retinoic acid (13 children). Topotecan: daily 1‐hour infusion of topotecan 0.7 mg/m2 IV for 5 days after MIBG therapy. VECI: vincristine (1.5 mg/m2 IV on day 1), ifosfamide (3000 mg/m2 IV on days 1 and 2), carboplatin (400 mg/m2 IV on day 3), etoposide (150 mg/m2 IV on day 4). Harvest of stem cells: PBSC harvesting took place after the first VECI course if the BM was clear; if not successful this was repeated after the next course. Myeloablative therapy: carboplatin (800 mg/m2 on day ‐3) and melphalan (180 mg/m2 IV on day ‐1), ASCT on day 0. 13‐cis‐retinoic acid 160 mg/m2 in 2 doses for 2 weeks followed by 2 weeks' rest (6 cycles). 131I‐MIBG therapy: first dose 7.4 GBq/200 mCi and second dose 5.6 GBq/150 mCi. Interval between MIBG infusions 4 weeks. MIBG dosimetry not performed. Thyroid blocking: not reported. 4 children had no surgery; 1 had only 1 MIBG infusion; 9 had stem cell transplant. No control participants. |
|
| Outcomes |
Response (according to INRC (Brodeur 1993)). Overall survival (no definition provided). Toxicity:
Dose intensity after 131I‐MIBG treatment (defined as the actual administered amount of 131I‐MIBG MBq/mCi). Time span of delivering chemotherapy induction treatment after 131I‐MIBG therapy. Stem cells:
|
|
| Notes |
Follow‐up: not mentioned, but maximal length of follow‐up 10 years. Influence of funders: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | All eligible consecutive participants. |
| Complete follow‐up assessment (attrition bias): response | Low risk | Response assessed for > 90% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): overall survival | Low risk | Overall survival assessed for > 90% of the study group of interest. |
| Complete follow‐up assessment (attrition bias): haematological toxicity | Low risk | Haematological toxicity assessed for > 90% of the study group of interest. |
| Blinded outcome assessor (detection bias): overall survival | Low risk | Blinding of outcome assessor not applicable for overall survival. |
| Blinded outcome assessor (detection bias): all other primary outcomes | Unclear risk | Not mentioned. |
| Well‐defined study group (reporting bias) | Low risk | All necessary items (see Table 1) provided. |
| Well‐defined follow‐up (reporting bias) | Unclear risk | Length of follow‐up not mentioned. |
| Well‐defined outcome (reporting bias): response and overall survival | Low risk | Outcome definition provided for response, not applicable for overall survival. |
| Well‐defined outcome (reporting bias): haematological toxicity | Low risk | Outcome definition provided. |
ASCT: autologous stem cell transplantation; BM: bone marrow; CFU‐C: colony‐forming unit culture; CR: complete response; CTCAEv3: Common Terminology Criteria for Adverse Events version 3 (publication date: 9 August 2006); Hb: haemoglobin; HDCT: high‐dose chemotherapy; HR: high risk; INRC: International Neuroblastoma Response Criteria; INSS: International Neuroblastoma Staging System; IV: intravenous; LOH1p: loss of heterozygosity chromosome 1p; MBq/mCi: megaBecquerel/milliCurie; MIBG: meta‐iodobenzylguanidine; NBL: neuroblastoma; PBSC: peripheral blood stem cell; TSH: thyroid‐stimulating hormone; VECI: vincristine, etoposide, carboplatin and ifosfamide; VGPR: very good partial response.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bleeker 2009 | Unclear number of patients with HR NBL. |
| Bleeker 2013 | Unclear number of patients with HR NBL. |
| Brans 2002 | < 10 patients with HR NBL. |
| Brendel 1989 | Case reports. |
| Claudiani 1988 | < 10 patients with MIBG therapy. |
| Clement 2013 | Unclear if they are newly diagnosed or patients with HR NBL, or both. |
| Clement 2015 | Unclear if they are newly diagnosed or patients with HR NBL, or both. |
| Corbett 1991 | < 10 patients with HR NBL. |
| De Kraker 1995 | Included all stages, no separate analysis for patients with HR NBL. |
| Edeling 1986 | < 10 patients with HR NBL. |
| El‐Sabban 2013 | Unclear number of patients with HR NBL. |
| Garaventa 1995 | Unclear number of patients. |
| Garaventa 2003 | Unclear if they were patients with HR NBL. |
| Gerrard 1987 | Case report. |
| Hartmann 1988 | Not MIBG therapy. |
| Hoefnagel 1987 | Unclear number of patients with HR NB. |
| Hoefnagel 1988 | Unclear number of patients with HR NBL. |
| Hoefnagel 1991 | < 10 patients with HR NBL. |
| Hoefnagel 1994 | Unclear if they were patienst with HR NBL. |
| Hoefnagel 1995 | Included all stages, no separate analysis for patients with HR NBL. |
| Klingebiel 1991 | Unclear number of patients with HR NBL. |
| Klingebiel 1994 | < 10 patients with HR NBL. |
| Klingebiel 1998 | Prior chemotherapy before MIBG therapy. |
| Kosmin 2012 | Unclear if they were patients with HR NBL. |
| Lashford 1990 | Unclear if they were patients with HR NBL. |
| Mastrangelo 1987 | Editorial, no primary data. |
| Mastrangelo 1991 | Case report. |
| Mastrangelo 2001 | Unclear if they were patients with HR NBL. |
| Mastrangelo 2011 | No separate analysis for patients with HR NBL; prior chemotherapy before MIBG therapy. |
| Matthay 2009b | Relapsed/refractory patients. |
| McEwan 1986 | No participant data, biochemical analysis. |
| Mitjavila 1989 | < 10 patients with HR NBL. |
| Monsieurs 2001 | Unclear if they were patients with HR NBL. |
| Mussa 1987 | Diagnostic MIBG scan. |
| O'Donoghue 1991 | < 10 patients with HR NBL. |
| Picco 1995 | Unclear if they were patients with HR NBL. |
| Rachh 2011 | < 10 patients with HR NBL. |
| Schoot 2009a | < 10 patients with HR NBL. |
| Schoot 2009b | < 10 patients with HR NBL. |
| Shapiro 1991 | Unclear number of patients with HR NBL. |
| Sudbrock 2010 | Unclear number of patients with HR NBL. |
| Sutton 1982 | Unclear number of patients with HR NBL. |
| Sze 2013 | < 10 patients with HR NBL. |
| Teszler 2013 | Editorial. |
| Van Hasselt 1996 | Includes all stages, no separate analysis for patients with HR NBL. |
| Van Santen 2002 | No outcome parameters mentioned as included in the eligibility criteria of this review. |
| Van Santen 2005 | Includes all stages, no separate analysis for patients with HR NBL. |
| Van Santen 2012 | Unclear if they were patients with newly diagnosed or HR NBL, or both. |
| Van Santen 2013 | Includes all stages, no separate analysis for patients with HR NBL. |
| Vitale 2012 | Includes all stages, no separate analysis for patients with HR NBL. |
| Voute 1985 | Unclear number of patients with HR NBL. |
| Voute 1987a | Unclear number of patients with HR NBL. |
| Voute 1987b | Unclear number of patients with HR NBL. |
| Voute 1988a | Unclear number of patients with HR NBL. |
| Voute 1988b | Unclear number of patients with HR NBL. |
| Voute 1991 | Includes all stages, no separate analysis for patients with HR NBL. |
| Wong 2013 | Patients with refractory NBL. |
HR: high risk; NBL: neuroblastoma; MIBG: meta‐iodobenzylguanidine.
It should be noted that the list of excluded studies is not exhaustive, and that other valid reasons for exclusion may also exist.
Characteristics of studies awaiting assessment [ordered by study ID]
Hamidieh 2014.
| Methods | Single‐centre study. |
| Participants | 13 children (mean age at diagnosis 42.5 months (range 17‐65) and mean (± SD) age at transplantation 60.2 ± 21.3 months (range 34‐92)) with HR NBL and MIBG avid lesions. |
| Interventions | Therapeutic 131I‐ MIBG therapy (12 mCi/kg) followed by myeloablative therapy (etoposide 1200 mg/m2, carboplatin 1500 mg/m2 and melphalan 210 mg/m2) and ASCT. After ASCT, all children received cis‐retinoic acid. |
| Outcomes | Median time to neutrophil engraftment after ASCT was 10 days (range 9‐13) and median platelet engraftment was 13 days (range 10‐20). None of the children failed to engraft after ASCT. In studied children, 3‐year overall survival (mean ± SD) was 66 ± 21% while 3‐year EFS was 53 ± 20%. |
| Notes | This study has not been published in full text (as of 16 May 2016), but has been published as an abstract for Bone Marrow Transplantation meeting. |
Kraal 2012.
| Methods | Multi‐centre pilot study. |
| Participants | Children with HR NBL. |
| Interventions | 2 cycles of upfront 131I‐MIBG therapy. Of the 33 evaluable children, 16 (48%) received 2 cycles of upfront 131I‐MIBG therapy (group A), 17 (51%) children were treated without upfront 131I‐MIBG therapy (group B; insufficient MIBG uptake, weak clinical condition and hypertension) and 2 children were excluded (they received prior chemotherapy). |
| Outcomes | 131I‐MIBG therapy within 2 weeks from diagnosis was feasible in all children eligible for 131I‐MIBG therapy. Interval between subsequent N5/N6 chemotherapy courses was similar in both groups (22‐30 days). There was no serious haematological toxicity. Stem cell harvest after 131I‐MIBG therapy was undisturbed and did not compromise high‐dose chemotherapy with ASCT treatment. Response analysis (follow‐up 1 January 2005 to 1 January 2012) showed an effect of 2 131I‐MIBG courses in 10/15 (67%) children (1 missing) after 3 times N5/N6 of 15/16 (94%) and at follow‐up of 13/16 (81%). |
| Notes | This study has not been published in full text (as of 16 May 2016), but was presented at the Advances in Neuroblastoma Research conference 2012. |
Leung 2011.
| Methods | Retrospective chart review. |
| Participants | 15 children (median age 3.6 years) with stage 4 (HR) disease with 131I‐MIBG‐avid lesions at initial diagnosis. |
| Interventions | All children received 131I‐MIBG therapy (14 had 1 infusion; 1 had 2 infusions). 14 131I‐MIBG‐therapies were administered as part of the conditioning regimen for HSCT. Lugol's solution was given for thyroid protection. For 131I‐MIBG therapy administered as curative treatment, the dose range was 9‐12.9 mCi/kg, with median dose of 12 mCi/kg. Carboplatin, etoposide and melphalan was given 7‐10 days after MIBG treatment as conditioning for HSCT. |
| Outcomes | At a median follow‐up of 1.2 years (range 0 to 7), 5/14 children receiving therapeutic 131I‐MIBG treatment relapsed (36%) and 4 of them died (7%). The estimated 2‐year overall survival was 12.8% and EFS was 15.3%. 6 children (43%) developed primary hypothyroidism with elevation of TSH at 5 months to 1 year after treatment and 3 required thyroxine replacement. No child experienced liver derangement immediately after 131I‐MIBG treatment. |
| Notes | Overlap in children with Leung 2013 is very likely. This study has not been published in full text (as of 16 May 2016), but was presented at the Societé International Oncologie et Pediatrie (International Society of Paediatric Oncology) conference 2011. |
Leung 2013.
| Methods | Retrospective chart review. |
| Participants | 22 children (median age 2.9 years) with stage 4 (HR) NBL with MIBG avid lesions at diagnosis. |
| Interventions | All children received 131I‐MIBG therapy. 21 131I‐MIBG therapies were administered as part of the conditioning regimen for HSCT. The median dose of 131I‐MIBG administered for curative treatment was 12 mCi/kg (range 5.8 to 12.9). Lugol's solution was given for thyroid protection. |
| Outcomes | At a median follow‐up of 1.2 years (range 0 to 9), 12/21 (57%) children receiving therapeutic 131I‐MIBG treatment relapsed and 8 of them died (38%). The estimated 2‐years overall survival (mean ± SD) was 66.1 ± 12.5%. Among 15 children who had evaluable thyroid function result, 9 (60%) developed primary hypothyroidism with elevation of TSH at 0.3‐2.9 years' post‐treatment and 4 required thyroxine replacement. No children experienced liver derangement. |
| Notes | Overlap in participants with Leung 2011 was very likely. This study has not been published in full text (as of 16 May 2016), but has been presented at the Societé International Oncologie et Pediatrie (International Society of Paediatric Oncology) conference 2013. |
López‐Aguilar 2003.
| Methods | Cohort study. |
| Participants | Children with newly diagnosed NBL stage III and IV; not mentioned if they all had HR disease. |
| Interventions | Chemotherapy (cisplatin, epirubicin, etoposide, ifosfamide), massive doses of 131I‐MIBG and surgical ablation of the remaining tumour were possible. |
| Outcomes | Not reported for the children treated with MIBG. |
| Notes | We are currently awaiting the translation of this article from Spanish. Based on the currently available information it is unclear whether this study fulfils the inclusion criteria for this review. |
Nakajo 1987.
| Methods | Unclear. |
| Participants | Participants with pheochromocytoma and NBL; no further information available. |
| Interventions | 131I‐MIBG; no further information available. |
| Outcomes | Unclear. |
| Notes | Article in Japanese. We were unable to obtain a copy of this article. Based on the currently available information it is unclear whether this study fulfils the inclusion criteria for this review. |
ASCT: autologous stem cell transplantation; EFS: event‐free survival; HR: high risk; HSCT: haematopoietic stem cell transplantation; MIBG: meta‐iodobenzylguanidine; NBL: neuroblastoma; SD: standard deviation; TSH: thyroid‐stimulating hormone.
Characteristics of ongoing studies [ordered by study ID]
NCT01175356.
| Trial name or title | Induction Therapy Including 131I‐MIBG and Chemotherapy in Treating Patients with Newly Diagnosed High‐risk Neuroblastoma Undergoing Stem Cell Transplant, Radiation Therapy, and Maintenance Therapy with Isotretinoin. |
| Methods | Interventional study. |
| Participants | Patients with newly diagnosed neuroblastoma or ganglioneuroblastoma stage 4 disease according to International Neuroblastoma Staging System. |
| Interventions | 131I‐MIBG therapy followed by myeloablative busulfan/melphalan. |
| Outcomes | Response, event‐free survival and adverse effects. |
| Starting date | October 2010. |
| Contact information | Children's Oncology Group; principal investigator: Brian Weiss, MD. |
| Notes | No full‐text publication as of 22 May 2016. |
MIBG: meta‐iodobenzylguanidine.
Differences between protocol and review
The protocol was written for newly diagnosed, relapsed and refractory HR NBL (Kraal 2013). Since then, we decided that it would be preferable to prepare separate reviews for newly diagnosed and relapsed/refractory disease. This review focused on newly diagnosed disease.
As recommended by Cochrane Childhood Cancer, we classified the outcome "Toxicity and adverse effects" as a primary outcome instead of a secondary outcome to comply with the MECIR standards.
The protocol stated that when relevant data regarding data extraction and risk of bias assessment were missing, we would attempt to contact the study authors to retrieve the missing data. This was not done. In addition, in consultation with Cochrane Childhood Cancer, we restricted the risk of bias assessment of observational studies to the primary outcomes for attrition bias, detection bias and outcome reporting bias (i.e. biases that can be assessed for each outcome separately).
Data synthesis: after the publication of our protocol, Cochrane Childhood Cancer changed its policy regarding the calculation of prevalences and the corresponding 95% confidence intervals. So instead of using the generic inverse variance function of Review Manager 5 to calculate the 95% confidence intervals we were advised to use the Wilson method. As this was not possible in Review Manager 5 we used the EpiTools epidemiological calculators.
Contributions of authors
KK designed the study and wrote the protocol; identified the studies meeting the inclusion criteria (both by initial screening and thereafter); searched for unpublished and ongoing studies; performed the data extraction and risk of bias assessment of the included studies; interpreted the results; wrote and revised the manuscript.
ED designed the study and critically reviewed the protocol; identified studies meeting the inclusion criteria and searched for ongoing studies; acted as a third‐party arbitrator for study selection; performed the data extraction and risk of bias assessment of the included studies; analysed the data and contributed to the interpretation of the results; wrote the manuscript and critically reviewed the manuscript.
GT critically reviewed the protocol; identified studies meeting the inclusion criteria; contributed to the interpretation of the results and critically reviewed the manuscript.
BES critically reviewed the protocol; identified studies meeting the inclusion criteria; contributed to the interpretation of the results and critically reviewed the manuscript.
Sources of support
Internal sources
-
Cochrane Netherlands, Netherlands.
Training
External sources
Stichting Kinderen Kankervrij (KiKa), Netherlands.
Koningin Wilhelmina Fonds (KWF), Netherlands.
Declarations of interest
Some of the authors of this systematic review were also authors of the included studies: KK, BES and GT are authors of Kraal 2015, and BES is also an author of De Kraker 2008.
New
References
References to studies included in this review
De Kraker 2008 {published data only}
- Kraker J, Hoefnagel KA, Verschuur AC, Eck BLF, Santen HM, Caron HN. Iodine‐131‐metaiodobenzylguanidine as initial induction therapy in stage 4 neuroblastoma patients over 1 year of age. European Journal of Cancer 2008;44:551‐6. [DOI] [PubMed] [Google Scholar]
Kraal 2015 {published data only}
- Kraal KC, Tytgat GAM, Eck‐Smit BLF, Kam B, Caron HN, Noesel MM. Upfront treatment of high‐risk neuroblastoma with a combination of 131I‐MIBG and topotecan. Pediatric Blood and Cancer 2015;62(11):1886‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bleeker 2009 {published data only}
- Bleeker G, Schoot R, Caron H, Eck B, Kraker J, Tytgat G. 20 years of 131iodine‐metaiodobenzylguanidine (131I‐MIBG) therapy upfront: a retrospective analysis of acute toxicity. Pediatric Blood and Cancer. 2009; Vol. 53:736.
Bleeker 2013 {published data only}
- Bleeker G, Schoot RA, Caron HN, Kraker J, Hoefnagel CA, Eck‐Smit BL, et al. Toxicity of upfront 131I‐meta‐iodobenzylguanidine (131I‐MIBG) therapy in newly diagnosed neuroblastoma patients: a retrospective analysis. European Journal of Nuclear Medical Molecular Imaging 2013;40:1711‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brans 2002 {published data only}
- Brans B, Monsieurs M, Laureys G, Kaufman JM, Thierens H, Dierckx RA. Thyroidal uptake and radiation dose after repetitive I‐131‐MIBG treatments: influence of potassium iodide for thyroid blocking. Medical and Pediatric Oncology 2002;38(1):41‐6. [DOI] [PubMed] [Google Scholar]
Brendel 1989 {published data only}
- Brendel AJ, Jeandot R, Guyot M, Lambert B, Drouillard J. Radionuclide therapy of pheochromocytomas and neuroblastomas using iodine‐131 metaiodobenzylguanidine (MIBG). Clinical Nuclear Medicine 1989;14(1):19‐21. [DOI] [PubMed] [Google Scholar]
Claudiani 1988 {published data only}
- Claudiani F, Garaventa A, Scopinaro G, Bertolazzi L, Villavecchia GP, Dini G, et al. Diagnostic and therapeutic use of 131I‐metaiodobenzylguanidine in children with neuroblastoma. Journal of Nuclear Medicine and Allied Sciences 1988;32(1):1‐6. [PubMed] [Google Scholar]
Clement 2013 {published data only}
- Clement SC, Eck‐Smit BLF, Trotsenburg ASP, Kremer LCM, Tytgat GAM, Santen HM. Long term follow‐up of the thyroid gland after treatment with 131I‐metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatric Blood and Cancer 2013;60:1833‐8. [DOI] [PubMed] [Google Scholar]
Clement 2015 {published data only}
- Clement SC, Rijn RR, Eck‐Smit BLF, Trotsenburg ASP, Caron HN, Tytgat GAM, et al. Long term efficacy of current thyroid prophylaxis and future perspectives on thyroid protection during 131I‐metaiodobenzylguanidine treatment in children with neuroblastoma. European Journal of Medical Molecular Imaging 2015;42:706‐15. [DOI] [PubMed] [Google Scholar]
Corbett 1991 {published data only}
- Corbett R, Pinkerton R, Tait D, Meller S. [131I]metaiodobenzylguanidine and high‐dose chemotherapy with bone marrow rescue in advanced neuroblastoma. Journal of Nuclear Biology and Medicine 1991;35:228‐31. [PubMed] [Google Scholar]
De Kraker 1995 {published data only}
- Kraker J, Hoefnagel CA, Caron H, Valdes Olmos RA, Zsiros J, Heij HA, et al. First line targeted radiotherapy, a new concept in the treatment of advanced stage neuroblastoma. European Journal of Cancer 1995;31A(4):600‐2. [DOI] [PubMed] [Google Scholar]
Edeling 1986 {published data only}
- Edeling CJ, Buchler FP, Kamper J, Jeppesen P. Diagnosis and treatment of neuroblastoma using 131I‐meta‐iodobenzylguanidine. Nuklearmedizin 1986;25:172‐5. [PubMed] [Google Scholar]
El‐Sabban 2013 {published data only}
- El‐Sabban K, Al Sakhri H, Alsulaimani AA, Al Malky T. Management of neuroblastoma. Comparative study between 1st and 2nd line radioionated MIBG therapy and chemotherapy alone. Clinical Cancer Investigation Journal 2013;2(1):41‐7. [Google Scholar]
Garaventa 1995 {published data only}
- Garaventa A, Pianca C, Conte M, Nigro M, De BB, Claudiani F, et al. Place of meta‐[131I]iodobenzylguanidine in the treatment of neuroblastoma: the Genoa experience. Quarterly Journal of Nuclear Medicine 1995;39(4, Suppl 1):58‐60. [PubMed] [Google Scholar]
Garaventa 2003 {published data only}
- Garaventa A, Gambini C, Villavecchia G, Cataldo A, Bertolazzi L, Pizzitola MR, et al. Second malignancies in children with neuroblastoma after combined treatment with 131I‐metaiodobenzylguanidine. Cancer 2003;97(5):1332‐8. [DOI] [PubMed] [Google Scholar]
Gerrard 1987 {published data only}
- Gerrard M, Eden OB, Merrick MV. Imaging and treatment of disseminated neuroblastoma with 131I‐metaiodobenzylguanidine. British Journal of Radiology 1987;60:393‐5. [DOI] [PubMed] [Google Scholar]
Hartmann 1988 {published data only}
- Hartmann O, Lumbroso JD, Lemerle M, Schlumberger M, Ricard M, Aubert B. The therapeutic use of I‐131 meta‐iodobenzylguanidine (mIBG) in neuroblastoma: a phase II study in 12 patients. Progressive Clinical Biological Research 1988;271:655‐67. [PubMed] [Google Scholar]
Hoefnagel 1987 {published data only}
- Hoefnagel CA, Voute PA, Kraker J, Marcuse HR. Radionuclide diagnosis and therapy of neural crest tumors using iodine‐131 metaiodobenzylguanidine. Journal of Nuclear Medicine 1987;28(3):308‐14. [PubMed] [Google Scholar]
Hoefnagel 1988 {published data only}
- Hoefnagel CA, Kraker J, Voute PA, Hartog Jager FCA, Taal BG, Engelsman E. Diagnosis and therapy of neural crest tumors with 131I‐meta‐iodobenzylguanidine [Diagnostiek en therapie van tumoren uitgaande van de neurale lijst met behulp van joodbenzylguanidine I 131]. Nederlands Tijdschrift voor Geneeskunde 1988;132:1367‐72. [PubMed] [Google Scholar]
Hoefnagel 1991 {published data only}
- Hoefnagel CA, Kraker J, Voute PA, Valdes Olmos RA. Preoperative [131I]metaiodobenzylguanidine therapy of neuroblastoma at diagnosis ("MIBG de novo"). Journal of Nuclear Biology and Medicine 1991;35(4):248‐51. [PubMed] [Google Scholar]
Hoefnagel 1994 {published data only}
- Hoefnagel CA, Kraker J, Valdes Olmos RA, Voute PA. 131I‐MIBG as a first‐line treatment in high‐risk neuroblastoma patients. Nuclear Medicine Communications 1994;15(9):712‐7. [DOI] [PubMed] [Google Scholar]
Hoefnagel 1995 {published data only}
- Hoefnagel CA, Kraker J, Valdes Olmos RA, Voute PA. [131I]MIBG as a first line treatment in advanced neuroblastoma. Quarterly Journal of Nuclear Medicine 1995;39(4 (Suppl 1)):61‐4. [PubMed] [Google Scholar]
Klingebiel 1991 {published data only}
- Klingebiel T, Feine U, Treuner J, Reuland P, Handgretinger R, Niethammer D. Treatment of neuroblastoma with [131I]metaiodobenzylguanidine: long‐term results in 25 patients. Journal of Nuclear Biology and Medicine 1991;35(4):216‐9. [PubMed] [Google Scholar]
Klingebiel 1994 {published data only}
- Klingebiel T, Handgretinger R, Herter M, Lang P, Dopfer R, Scheel‐Walter H, et al. Peripheral stem cell transplantation in neuroblastoma stage 4 with the use of [131I‐m]IBG. Progress in Clinical and Biological Research 1994;385:309‐17. [PubMed] [Google Scholar]
Klingebiel 1998 {published data only}
- Klingebiel T, Bader P, Bares R, Beck J, Hero B, Jurgens H, et al. Treatment of neuroblastoma stage 4 with 131I‐meta‐iodo‐benzylguanidine, high‐dose chemotherapy and immunotherapy. A pilot study. European Journal of Cancer 1998;34(9):1398‐402. [DOI] [PubMed] [Google Scholar]
Kosmin 2012 {published data only}
- Kosmin MA, Bomanji JB, Cork NJ, Shankar A, Gaze MN. Hypertension complicating 131I‐metaiodobenzylguanidine therapy for neuroblastoma. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(4):597‐601. [DOI] [PubMed] [Google Scholar]
Lashford 1990 {published data only}
- Lashford LS, Clarke J, Kemshead JT. Systemic administration of radionuclides in neuroblastoma as planned radiotherapeutic intervention. Medical and Pediatric Oncology 1990;18(1):30‐6. [DOI] [PubMed] [Google Scholar]
Mastrangelo 1987 {published data only}
- Mastrangelo R. The treatment of neuroblastoma with 131I‐MIBG. Medical and Pediatric Oncology 1987;15(4):157‐8. [DOI] [PubMed] [Google Scholar]
Mastrangelo 1991 {published data only}
- Mastrangelo R, Lasorella A, Troncone L, Rufini V, Iavarone A, Riccardi R. [131I]metaiodobenzylguanidine in neuroblastoma patients at diagnosis. Journal of Nuclear Biology and Medicine 1991;35:252‐4. [PubMed] [Google Scholar]
Mastrangelo 2001 {published data only}
- Mastrangelo S, Tornesello A, Diociaiuti L, Pession A, Prete A, Rufini V, et al. Treatment of advanced neuroblastoma: feasibility and therapeutic potential of a novel approach combining 131‐I‐MIBG and multiple drug chemotherapy. British Journal of Cancer 2001;84(4):460‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mastrangelo 2011 {published data only}
- Mastrangelo S, Rufini V, Ruggiero A, Giannatale A, Riccardi R. Treatment of advanced neuroblastoma in children over 1 year of age: the critical role of 131I‐metaiodobenzylguanidine therapy combined with chemotherapy in a rapid induction regimen. Pediatric Blood & Cancer 2011;56(7):1032‐40. [DOI] [PubMed] [Google Scholar]
Matthay 2009b {published data only}
- Matthay KK, Quach A, Huberty J, Franc BL, Hawkins RA, Jackson H. Iodine‐131‐metaiodobenzylguanidine double infusion with autologous stem‐cell rescue for neuroblastoma: a new approaches to neuroblastoma therapy phase I study. Journal Clinical Oncology 2009;27(7):1020‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEwan 1986 {published data only}
- McEwan AJ, Wyeth P, Ackery D. Radioiodinated iodobenzylguanidines for diagnosis and therapy. Applied Radiation and Isotopes, Part A 1986;37:765‐75. [DOI] [PubMed] [Google Scholar]
Mitjavila 1989 {published data only}
- Mitjavila M, Gonzalez A, Munoz A, Crespo A, Diez L. Therapeutic possibilities of (m‐iodo(131I)benzyl)guanidine. Revista Espanola de Medicina Nuclear 1989;8(1):7‐17. [Google Scholar]
Monsieurs 2001 {published data only}
- Monsieurs MA, Thierens HM, Vral A, Brans B, Ridder L, Dierckx RA. Patient dosimetry after 131I‐MIBG therapy for neuroblastoma and carcinoid tumours. Nuclear Medicine Communications 2001;22(4):367‐74. [DOI] [PubMed] [Google Scholar]
Mussa 1987 {published data only}
- Mussa GC, Silvestro L. Radiometabolic diagnosis and treatment of neuroblastoma using 131I‐MIBG: personal research. Rivista Italiana di Pediatria 1987;13:510‐4. [Google Scholar]
O'Donoghue 1991 {published data only}
- O'Donoghue JA, Wheldon TE, Babich JW, Moyes JS, Barrett A, Meller ST. Therapeutic implications of the uptake of radiolabelled mIBG for the treatment of neuroblastoma. Progress in Clinical and Biological Research 1991;366:455‐61. [PubMed] [Google Scholar]
Picco 1995 {published data only}
- Picco P, Garaventa A, Claudiani F, Gattorno M, Bernardi B, Borrone C. Primary hypothyroidism as a consequence of 131‐I‐metaiodobenzylguanidine treatment for children with neuroblastoma. Cancer 1995;76(9):1662‐4. [DOI] [PubMed] [Google Scholar]
Rachh 2011 {published data only}
- Rachh SH, Abhyankar S, Basu S. 131I Metaiodobenzylguanidine therapy in neural crest tumors: varying outcome in different histopathologies. Nuclear Medicine Communication 2011;32(12):1201‐10. [DOI] [PubMed] [Google Scholar]
Schoot 2009a {published data only}
- Schoot R, Bleeker G, Heij H, Eck B, Caron H, Kraker J. The role of 131I‐metaiodobenzylguanidine (131i‐MIBG) therapy in unresectable and compromising localized neuroblastoma. Pediatric Blood and Cancer. 2009; Vol. 53:807‐8.
Schoot 2009b {published data only}
- Schoot RA, Bleeker G, Heij HA, Eck BL, Caron HN, Kraker J. The role of 131‐I‐metaiodobenzylguanidine (13II‐MIBG) therapy in irresectable and compromising localized neuroblastoma. Journal of Clinical Oncology. 2009; Vol. 15 Suppl:10053.
Shapiro 1991 {published data only}
- Shapiro B. Summary, conclusions, and future directions of [131I]metaiodobenzylguanidine therapy in the treatment of neural crest tumors. Journal of Nuclear Biology and Medicine 1991;35:357‐63. [PubMed] [Google Scholar]
Sudbrock 2010 {published data only}
- Sudbrock F, Schmidt M, Simon T, Eschner W, Berthold F, Schicha H. Dosimetry for 131I‐MIBG therapies in metastatic neuroblastoma, phaeochromocytoma and paraganglioma. European Journal of Nuclear Medicine and Molecular Imaging 2010;37:1279‐90. [DOI] [PubMed] [Google Scholar]
Sutton 1982 {published data only}
- Sutton H, Green C, Wyeth P, Ackery D. Iodine labelled meta‐iodobenzyl guanidine (mIBG) in the localisation and treatment of malignant phaeochromocytoma and neuroblastoma. Nuclear Medicine Communications 1982;3:101. [Google Scholar]
Sze 2013 {published data only}
- Sze WC, Grossman AB, Goddard I, Amendra D, Shieh SCC, Plowman PN, et al. Sequalae and survivorship in patients treated with 131I‐MIBG therapy. British Journal of Cancer 2013;109:565‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teszler 2013 {published data only}
- Teszler CB. Differentiated thyroid carcinoma after iodine‐131 metaiodobenzylguanidine treatment for neuroblastoma: assessment of causality. Thyroid 2013;23(2):248‐9. [DOI] [PubMed] [Google Scholar]
Van Hasselt 1996 {published data only}
- Hasselt EJ, Heij HA, Kraker J, Vos A, Voute PA. Pretreatment with [131I] metaiodobenzylguanidine and surgical resection of advanced neuroblastoma. European Journal of Pediatric Surgery 1996;6(3):155‐8. [DOI] [PubMed] [Google Scholar]
Van Santen 2002 {published data only}
- Santen HM, Kraker J, Eck BL, Vijlder JJ, Vulsma T. High in despite prophylaxis with potassium iodide during 131I‐metaiodobenzylguanide treatment in children with neuroblastoma. Cancer 2002;94(7):2081‐9. [DOI] [PubMed] [Google Scholar]
Van Santen 2005 {published data only}
- Santen HM, Kraker KJ, Vulsma T. Endocrine late effects from multi‐modality treatment of neuroblastoma. European Journal of Cancer 2005;41(12):1767‐74. [DOI] [PubMed] [Google Scholar]
Van Santen 2012 {published data only}
- Santen HM, Clement S, Eck BLF, Tytgat LA, Trotsenburg PASP. Increasing incidence and severity of thyroid damage 15 years after 131I‐metaiodobenzylguanidine treatment in children with neuroblastoma. 94th Annual Meeting and Expo of the Endocrine Society; Jun 23‐26; Houston (TX). 2012.
Van Santen 2013 {published data only}
- Clement SC, Eck‐Smit BLF, Trotsenburg ASP, Kremer LCM, Tytgat GAM, Santen HM. Long‐term follow‐up of the thyroid gland after treatment with131I‐metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatric Blood and Cancer 2013;60:1833‐8. [DOI] [PubMed] [Google Scholar]
Vitale 2012 {published data only}
- Vitale V, Hanau G, Caruso S, Cabria M, Piccardo A, Altrinetti V, et al. Late toxicity after 131 I‐MIBG therapy in children with neuroblastoma. European Journal of Nuclear Medicine and Molecular Imaging. 2012; Vol. 2:S317.
Voute 1985 {published data only}
- Voute PA, Hoefnagel CA, Kraker J, Marcuse HR. Treatment of neuroblastoma (NBL) with 131I‐metaiodobenzylguanidine (131I‐MIBG). Proceedings of the American Association for Cancer Research 1985;26. [Google Scholar]
Voute 1987a {published data only}
- Voute PA, Hoefnagel CA, Kraker J, Evans AE, Hayes A, Green A. Radionuclide therapy of neural crest tumors. Medical and Pediatric Oncology 1987;15(4):192‐5. [DOI] [PubMed] [Google Scholar]
Voute 1987b {published data only}
- Voute PA, Hoefnagel CA, Kraker J. 131I‐meta‐iodobenzylguanidine in diagnosis and treatment of neuroblastoma [131I‐meta‐iodobenzylguanidine dans le diagnostic et le traitement des neuroblastomes]. Gazette Medicale 1987;94:107‐11. [Google Scholar]
Voute 1988a {published data only}
- Voute PA, Hoefnagel CA, Kraker J. 131I‐meta‐iodobenzylguanidine in diagnosis and treatment of neuroblastoma. Bulletin du Cancer 1988;75(1):107‐11. [PubMed] [Google Scholar]
Voute 1988b {published data only}
- Voute PA, Hoefnagel CA, Kraker J, Majoor M. Side effects of treatment with I‐131‐meta‐iodobenzylguanidine (I‐131‐MIBG) in neuroblastoma patients. Progress in Clinical and Biological Research 1988;271:679‐87. [PubMed] [Google Scholar]
Voute 1991 {published data only}
- Voute PA, Hoefnagel CA, Kraker J, Valdes OR, Bakker DJ, Kleij AJ. Results of treatment with 131 I‐metaiodobenzylguanidine (131 I‐MIBG) in patients with neuroblastoma. Future prospects of zetotherapy. Progress in Clinical and Biological Research 1991;366:439‐45. [PubMed] [Google Scholar]
Wong 2013 {published data only}
- Wong T, Matthay KK, Bscardin WJ, Hawkins RA, Brakeman PR, Dubois SG. Acute changes in blood pressure in patients with neuroblastoma treated with 131I‐metaiodobenzylguanidine (MIBG). Pediatric Blood and Cancer 2013;60:1424‐30. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hamidieh 2014 {published data only}
- Hamidieh AA, Hosseini AS, Shamshiri A, Eftekhari M, Ghavamzadeh A. A mean to optimize transplant regimen for neuroblastoma: the role of pre‐transplant MIBG scintigraphy in targeted administration of 131I‐MIBG accompanied by autologous stem cell transplantation. Bone Marrow Transplantation 2014;49:S156‐7. [Google Scholar]
Kraal 2012 {published data only}
- Kraal KCJM, Bleeker GM, Kwaasteniet M, Eck‐Smit BLF, Noesel MM. Pilot feasibility and toxicity of 2 cycles of upfront 131I‐MIBG. Advances in Neuroblastoma Research 2012 Conference; Jun 18‐21 2012; Toronto, Canada. 2012.
Leung 2011 {published data only}
- Leung WK, Lee V, Kam M, Wai Tsoi Cheng F, Shing MK, Kong C. Treatment outcome of high dose 131I‐MIBG treatment in stage 4 neuroblastoma in Hong Kong. 26th Annual SIOP Conference; 2011 Apr 14‐16; Auckland, New Zealand. 2011.
Leung 2013 {published data only}
- Leung WK, Lee V, Kam MKM, Cheung JSS, Cheng FWT, Shing MK, et al. High dose 131I‐MIBG treatment in stage 4 neuroblastoma patients in a tertiary centre in Hong Kong. 28th Annual SIOP Conference; 2011 Apr 11‐13; Houston (TX). 2013.
López‐Aguilar 2003 {published data only}
- López‐Aguilar E, Cerecedo‐Díaz F, Rivera‐Márquez H, Valdéz‐Sánchez M, Sepúlveda‐Vildósola AC, Delgado Huerta S, et al. Neuroblastoma: prognostic factors and survival. Experience in Hospital de Pediatria del Centro Medico Nacional del Siglo XXI and review of the literature. Gaceta Medico de Mexico 2003;139(3):209‐14. [PubMed] [Google Scholar]
Nakajo 1987 {published data only}
- Nakajo M, Shinohara S. 131I‐meta‐iodobenzylguanidine (MIBG) for the detection and therapy of pheochromocytoma and neuroblastoma. Rinsho Hoshasen 1987;32(4):461‐70. [PubMed] [Google Scholar]
References to ongoing studies
NCT01175356 {published data only}
- NCT01175356. Induction therapy including 131 I‐MIBG and chemotherapy in treating patients with newly diagnosed high‐risk neuroblastoma undergoing stem cell transplant, radiation therapy, and maintenance therapy with isotretinoin. clinicaltrials.gov/ct2/show/NCT01175356 Date first received: 3 August 2010.
Additional references
Bernstein 1992
- Bernstein ML, Leclerc JM, Bunin G, Brisson L, Robison L, Shuster J, et al. A population‐based study of neuroblastoma incidence, survival, and mortality in North America. Journal of Clinical Oncology 1992;10(2):323‐9. [DOI] [PubMed] [Google Scholar]
Bleeker 2015
- Bleeker G, Tytgat GAM, Adam JA, Kremer LCM, Hooft L, Dalen EC. 123I‐MIBG scintigraphy and 18F‐FDG‐PET imaging for diagnosing neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD009263.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodeur 1993
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of Clinical Oncology 1993;11(8):1466‐77. [DOI] [PubMed] [Google Scholar]
Cohn 2009
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of Clinical Oncology 2009;27(2):289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2002
- Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. Lancet 2002;359(9303):341‐5. [DOI] [PubMed] [Google Scholar]
Gurney 1996
- Gurney JG, Davis S, Severson RK, Fang JY, Ross JA, Robison LL. Trends in cancer incidence among children in the US. Cancer 1996;78(3):532‐41. [DOI] [PubMed] [Google Scholar]
Hattner 1984
- Hattner RS, Huberty JP, Engelstad BL, Gooding CA, Ablin AR. Localization of m‐iodo(131I)benzylguanidine in neuroblastoma. American Journal of Roentgenology 1984;143(2):373‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoefnagel 1985
- Hoefnagel CA, Voute PA, Kraker J, Marcuse HR. Total‐body scintigraphy with 131I‐meta‐iodobenzylguanidine for detection of neuroblastoma. Diagnostic Imaging in Clinical Medicine 1985;54(1):21‐7. [PubMed] [Google Scholar]
Hutchinson 1991
- Hutchinson RJ, Sisson JC, Miser JS, Zasadny KR, Normolle DP, Shulkin BL, et al. Long‐term results of [131I]metaiodobenzylguanidine treatment of refractory advanced neuroblastoma. Journal of Nuclear Biology and Medicine 1991;35(4):237‐40. [PubMed] [Google Scholar]
Klingebiel 1991a
- Klingebiel T, Berthold F, Treuner J, Schwabe D, Fischer M. Metaiodobenzylguanidine (mIBG) in treatment of 47 patients with neuroblastoma: results of the German Neuroblastoma Trial. Medical and Pediatric Oncology 1991;19(2):84‐8. [DOI] [PubMed] [Google Scholar]
Klingebiel 1991b
- Klingebiel T, Feine U, Treuner J, Reuland P, Handgretinger R, Niethammer D. Treatment of neuroblastoma with [131I]metaiodobenzylguanidine: long‐term results in 25 patients. Journal of Nuclear Biology and Medicine 1991;35(4):216‐9. [PubMed] [Google Scholar]
Kremer 2012
- Kremer LCM, Dalen EC, Moher D, Caron HN. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGSs)) 2012, Issue 4. Art. No.: CHILDCA.
Lashford 1992
- Lashford LS, Lewis IJ, Fielding SL, Flower MA, Meller S, Kemshead JT, et al. Phase I/II study of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: a United Kingdom Children's Cancer Study Group investigation. Journal of Clinical Oncology 1992;10(12):1889‐96. [DOI] [PubMed] [Google Scholar]
Laupacis 1994
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. JAMA 1994;272(3):234‐7. [DOI] [PubMed] [Google Scholar]
Leung 1997
- Leung A, Shapiro B, Hattner R, Kim E, Ghazzar N. Specificity of radioiodinated MIBG for neural crest tumors in childhood. Journal of Nuclear Medicine 1997;38(9):1352‐7. [PubMed] [Google Scholar]
Lumbroso 1991
- Lumbroso J, Hartmann O, Schlumberger M. Therapeutic use of [131I]metaiodobenzylguanidine in neuroblastoma: a phase II study in 26 patients. "Societe Francaise d'Oncologie Pediatrique" and Nuclear Medicine Co‐investigators. Journal of Nuclear Biology and Medicine 1991;35(4):220‐3. [PubMed] [Google Scholar]
Matthay 1998
- Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A. Phase I dose escalation of 131I‐metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. Journal of Clinical Oncology 1998;16(1):229‐36. [DOI] [PubMed] [Google Scholar]
Matthay 1999
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high‐risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13‐cis‐retinoic acid. Children's Cancer Group. New England Journal of Medicine 1999;341(16):1165‐73. [DOI] [PubMed] [Google Scholar]
Matthay 2001
- Matthay KK, Panina C, Huberty J, Price D, Glidden DV, Tang HR, et al. Correlation of tumor and whole‐body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I‐MIBG. Journal of Nuclear Medicine 2001;42(11):1713‐21. [PubMed] [Google Scholar]
Matthay 2007
- Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine‐131‐metaiodobenzylguanidine therapy in refractory neuroblastoma. Journal of Clinical Oncology 2007;25(9):1054‐60. [DOI] [PubMed] [Google Scholar]
Matthay 2009a
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas‐Kogan D, et al. Long‐term results for children with high‐risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13‐cis‐retinoic Acid: a Children's Oncology Group Study. Journal of Clinical Oncology 2009;27(7):1007‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2011
- Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high‐risk neuroblastoma: a Children’s Oncology Group study. Journal Clinical Oncology 2011;29(33):4351‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics Medical 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pearson 2008
- Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. High‐dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncology 2008;3:247‐56. [DOI] [PubMed] [Google Scholar]
Peinemann 2015a
- Peinemann F, Kahangire DA, Dalen EC, Berthold F. Rapid COJEC versus standard induction therapies for high‐risk neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD010774.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peinemann 2015b
- Peinemann F, Dalen EC, Kahangire DA, Berthold F. Retinoic acid post consolidation therapy for high‐risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010685.pub2] [DOI] [PubMed] [Google Scholar]
Pinto 2015
- Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, et al. Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology 2015;33:3008‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shimada 1995
- Shimada H, Stram DO, Chatten J, Joshi VV, Hachitanda Y, Brodeur GM, et al. Identification of subsets of neuroblastomas by combined histopathologic and N‐myc analysis. Journal National Cancer Institute 1995;87(19):1470‐6. [DOI] [PubMed] [Google Scholar]
Shimada 1999
- Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 1999;86(2):364‐72. [PubMed] [Google Scholar]
Simon 2011
- Simon T, Berthold F, Borkhardt A, Kremens B, Carolis B, Hero B. Treatment and outcomes of patients with relapsed, high‐risk neuroblastoma: results of German trials. Pediatric Blood and Cancer 2011;56(4):578‐83. [DOI] [PubMed] [Google Scholar]
Suc 1996
- Suc A, Lumbroso J, Rubie H, Hattchouel JM, Boneu A. Metastatic neuroblastoma in children older than one year: prognostic significance of the initial metaiodobenzylguanidine scan and proposal for a scoring system. Cancer 1996;77(4):805‐11. [DOI] [PubMed] [Google Scholar]
Trieu 2016
- Trieu M, DuBois SG, Pon E, Nardo L, Hawkins RA, Marachelian A, et al. Impact of whole‐body radiation dose on response and toxicity in patients with neuroblastoma after therapy with 131 I‐metaiodobenzylguanidine (MIBG). Pediatric Blood & Cancer 2016;63(3):436‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yalçin 2015
- Yalçin B, Kremer LC, Dalen EC. High‐dose chemotherapy and autologous haematopoietic stem cell rescue for children with high‐risk neuroblastoma. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD006301.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kraal 2013
- Kraal KCJM, Dalen EC, Tytgat GAM, Eck‐Smit BL, Caron HN. Iodine‐131‐meta‐iodobenzylguanidine therapy for patients with high‐risk neuroblastoma. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010349] [DOI] [PMC free article] [PubMed] [Google Scholar]


