Abstract
Background
Staphylococcus aureus causes pulmonary infection in young children with cystic fibrosis. Prophylactic antibiotics are prescribed hoping to prevent such infection and lung damage. Antibiotics have adverse effects and long‐term use might lead to infection with Pseudomonas aeruginosa. This is an update of a previously published review.
Objectives
To assess continuous oral antibiotic prophylaxis to prevent the acquisition of Staphylococcus aureus versus no prophylaxis in people with cystic fibrosis, we tested these hypotheses. Prophylaxis: 1. improves clinical status, lung function and survival; 2. causes adverse effects (e.g. diarrhoea, skin rash, candidiasis); 3. leads to fewer isolates of common pathogens from respiratory secretions; 4. leads to the emergence of antibiotic resistance and colonisation of the respiratory tract with Pseudomonas aeruginosa.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register, comprising references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings. Companies manufacturing anti‐staphylococcal antibiotics were contacted.
Most recent search of the Group's Register: 29 September 2016.
Selection criteria
Randomised trials of continuous oral prophylactic antibiotics (given for at least one year) compared to intermittent antibiotics given 'as required', in people with cystic fibrosis of any disease severity.
Data collection and analysis
The authors assessed studies for eligibility and methodological quality and extracted data.
Main results
We included four studies, with a total of 401 randomised participants aged zero to seven years on enrolment; one study is ongoing. The two older included studies generally had a higher risk of bias across all domains, but in particular due to a lack of blinding and incomplete outcome data, than the two more recent studies. We only regarded the most recent study as being generally free of bias, although even here we were not certain of the effect of the per protocol analysis on the study results. Evidence was downgraded based on GRADE assessments and outcome results ranged from moderate to low quality. Downgrading decisions were due to limitations in study design (all outcomes); for imprecision (number of people needing additional antibiotics); and for inconsistency (weight z score).
Fewer children receiving anti‐staphylococcal antibiotic prophylaxis had one or more isolates of Staphylococcus aureus (low quality evidence). There was no significant difference between groups in infant or conventional lung function (moderate quality evidence). We found no significant effect on nutrition (low quality evidence), hospital admissions, additional courses of antibiotics (low quality evidence) or adverse effects (moderate quality evidence). There was no significant difference in the number of isolates of Pseudomonas aeruginosa between groups (low quality evidence), though there was a trend towards a lower cumulative isolation rate of Pseudomonas aeruginosa in the prophylaxis group at two and three years and towards a higher rate from four to six years. As the studies reviewed lasted six years or less, conclusions cannot be drawn about the long‐term effects of prophylaxis.
Authors' conclusions
Anti‐staphylococcal antibiotic prophylaxis leads to fewer children having isolates of Staphylococcus aureus, when commenced early in infancy and continued up to six years of age. The clinical importance of this finding is uncertain. Further research may establish whether the trend towards more children with CF with Pseudomonas aeruginosa, after four to six years of prophylaxis, is a chance finding and whether choice of antibiotic or duration of treatment might influence this.
Plain language summary
Giving antibiotics regularly to people with cystic fibrosis to prevent infection with a germ called Staphylococcus aureus
Review question
We reviewed the evidence about the benefits and adverse effects of giving regular antibiotics to people with cystic fibrosis to prevent infection with a germ called Staphylococcus aureus.
Background
Cystic fibrosis blocks the airways with mucus and causes frequent airway infections. These can lead to death from breathing failure. People with cystic fibrosis are sometimes given regular antibiotics to prevent infections from a germ called Staphylococcus aureus. However, antibiotics can also have adverse effects such as oral thrush or diarrhoea. This is an update of a previously published review.
Search date
The evidence is current to: 29 September 2016.
Study characteristics
The review includes four studies with 401 children; there were no adult studies. The children were put into groups at random and received either an oral antibiotic continuously as a prevention for at least one year or no antibiotic treatment to prevent infection with Staphylococcus aureus. All children could be given additional antibiotics if their doctor thought they needed them, based on symptoms and germs grown in their respiratory secretions. Studies lasted for a maximum of six years.
Key results
The review found some evidence that giving regular antibiotics to young children (continued up to six years of age) leads to fewer infections with Staphylococcus aureus. For other outcomes in the review, there was no difference between giving regular antibiotics or not. Since none of the studies lasted longer than six years, we can't draw any conclusions about long‐term use. Also, since all studies were in children, we can not comment on the use of these drugs in adults. Future research should look at patterns of antibiotic resistance and survival.
Quality of the evidence
All the studies were of variable quality and the quality of the evidence for different outcomes ranged from low to moderate. We judged that the two older studies had a higher risk of bias overall compared to the two newer studies. In particular this was because those taking part in the studies (or their parents or caregivers) would be able to guess which treatment they were receiving, and also one study did not state if anyone had dropped out and if so what the reasons were. Only the newest study seemed to be free of bias, although even here we were not certain if the study results were affected by the way the data were analysed. Further research might change the estimate of the size of the treatment effect and would certainly affect our confidence in the estimated effect.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ Prophylactic compared with 'as required' anti‐staphylococcal antibiotics for cystic fibrosis.
| Prophylactic compared with 'as required' anti‐staphylococcal antibiotics for cystic fibrosis | ||||||
|
Patient or population: children with cystic fibrosis Settings: outpatients Intervention: prophylactic anti‐staphylococcal antibiotics (prophylaxis) Comparison: anti‐staphylococcal antibiotics 'as required' | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 'As required' | Prophylaxis | |||||
|
FEV₁ (% predicted (Knudson 1983)) Follow up: 6 years |
The mean FEV₁ was 1.1 % predicted in the 'as required' group. | The mean FEV₁ was 0% predicted higher (0.08 % lower to 0.08 % higher) in the prophylaxis group. | NA | 119 (1 study) |
⊕⊕⊕⊝ moderate1 | |
|
Number of people with one or more isolates of S aureus (sensitive strains) Follow up: 2 years |
541 per 1000. | 114 per 1000 (70 to 189). | OR 0.21 (95% CI 0.13 to 0.35). | 315 (3 studies) | ⊕⊕⊝⊝ low2,3 | Significant advantages to prophylaxis antibiotics were also shown at the following time points. 1 year: OR 0.27 (95% CI 0.15 to 0.48). 3 years: OR 0.22 (95% CI 0.13 to 0.38). 4 years: OR 0.10 (95% CI 0.04 to 0.25). 5 years: OR 0.09 (95% CI 0.03 to 0.26). 6 years: OR 0.11 (95% CI 0.03 to 0.46). |
|
Number of people with one or more isolates of P aeruginosa Follow up: 2 years |
346 per 1000. | 256 per 1000 (156 to 426). |
OR 0.74 (95% CI 0.45 to 1.23). | 312 (3 studies) |
⊕⊕⊝⊝ low2,3 | Trend towards more P aeruginosa in the intervention group at 4, 5 and 6 years. |
|
Number of people needing additional antibiotics Follow up: up to 7 years |
1000 per 1000. | 180 per 1000 (10 to 1000). |
OR 0.18 (95% CI 0.01 to 3.60). | 119 (1 study) |
⊕⊕⊝⊝ low1,4 | |
|
Weight (z score) Follow up: 2 years |
The mean z score for weight ranged from ‐0.25 to ‐0.69 in the 'as required' group. | The mean z score for weight was 0.06 higher (0.03 lower to 0.45 higher) in the prophylaxis group. | NA | 140 (2 studies) |
⊕⊕⊝⊝ low2,5 | There was also no significant difference between treatment groups at the following time points. 6 months: MD 0.30 (95% CI ‐0.54 to 1.14). 1 year: MD ‐0.12 (95% CI ‐0.50 to 0.26). 3 years: MD ‐0.14 (95% CI ‐0.58 to 0.30). |
|
Acquisition of multiply‐resistant S aureus Follow up: NA |
Outcome not reported | NA | ||||
|
Adverse events of treatment Follow up: up to 7 years |
See comment. | See comment. | NA | 119 (1 study) |
⊕⊕⊕⊝ moderate1 | There were no significant differences between treatment groups in terms of generalised rash, nappy rash and increased stool frequency. |
| *The basis for the assumed risk is the control group risk (mean risk or event rate depending on type of outcome data) across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval;FEV₁: forced expiratory volume in one second;FVC: forced vital capacity;MD: mean difference;NA: not applicable; OR: risk ratio; P aeruginosa: Pseudomonas aeruginosa; S aureus: Staphylococcus aureus. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded once due to incomplete outcome data: outcomes were measured only in the children completing treatment per protocol (Stutman 2002). 2. Downgraded once due to applicability: nose and throat swabs were used to assess for infection, rather than sputum samples, which have been shown to poorly predict lower respiratory infection (Armstrong 1996). 3. Downgraded once due to risk of bias: studies contributing evidence were not blinded or had incomplete outcome data (or both) (Chatfield 1991; Stutman 2002; Weaver 1994). 4. Downgraded once due to imprecision: only a small number of children did not require additional antibiotics; this small number has results in a wide confidence interval around the relative effect. 5. Downgraded once due to inconsistency: high levels of heterogeneity present in analysis (I² = 81%).
Background
Description of the condition
Cystic fibrosis (CF) is a genetic disorder characterised by abnormal mucociliary clearance, primarily affecting the respiratory tract and the gut. Poor clearance of respiratory secretions and an increased susceptibility to respiratory infection lead to chronic inflammation and structural airway damage. Most deaths from CF are due to end‐stage respiratory failure (Kerem 1992).
Bacterial infection due to Staphylococcus aureus (S aureus) may be found in CF infants as early as three months of age (Armstrong 1995) and can be accompanied by evidence of inflammation and abnormal lung function (Pillarisetti 2011).
Description of the intervention
Most CF centres treat people with CF with antibiotics when they are symptomatic, guided by the results of recent specimens of respiratory secretions. Many will also collect sputum or 'cough swab' specimens routinely and prescribe antibiotics if a pathogen, such as Pseudomonas aeruginosa (P aeruginosa), is found even if the person is asymptomatic. It is the practice in some CF centres to give continuous anti‐staphylococcal antibiotic prophylaxis to people with CF from diagnosis through early childhood.
How the intervention might work
The aim of prophylactic antibiotic use in this population is to reduce infection with S aureus and inflammation in the developing lung and to slow the onset of bronchiectasis. However, prophylactic antibiotics may be associated with earlier age at acquisition of P aeruginosa as well as adverse effects such as diarrhoea or oral candidiasis.
Why it is important to do this review
There is significant debate globally regarding the role of anti‐staphylococcal antibiotic prophylaxis in young children with CF, with guidelines varying widely by country based on differing views of the risks versus benefits. This is an update of a previously published review (Smyth 2003; Smyth 2014).
Objectives
To assess the effect on the outcome in young children with CF, of continuous oral antibiotic prophylaxis compared to no prophylaxis to prevent the acquisition of S aureus. The following hypotheses were tested to investigate whether antibiotic prophylaxis:
improves clinical status;
improves lung function;
improves survival;
causes adverse effects (diarrhoea, skin rash, candidiasis);
leads to fewer isolates of common pathogens from respiratory secretions;
leads to the emergence of antibiotic resistance and earlier isolation of P aeruginosa from respiratory cultures
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). Cross‐over studies were not considered because we felt this study design would not allow evaluation of the effects of prophylaxis on long‐term outcome measures such as lung function, nutrition and the acquisition of resistant organisms.
Types of participants
People with CF, of any age, diagnosed on the basis of clinical criteria and sweat testing or genotype analysis.
Types of interventions
Any oral prophylactic antibiotic, used continuously for a period of at least one year, compared with controls who do not receive prophylactic antibiotics to prevent the acquisition of S aureus. Both groups could receive intermittent courses of antibiotics 'as required', on the basis of symptoms and organisms found in respiratory secretions.
Types of outcome measures
Primary outcomes
-
Lung function (spirometry)
forced expiratory volume in one second (FEV₁) % predicted (Knudson 1983)
forced vital capacity (FVC) % predicted (Knudson 1983)
Number of people with one or more isolates of S aureus (sensitive strains)
NB: in only one study were the children old enough to perform the spirometry. We also included a study measuring infant lung function.
Secondary outcomes
Growth as measured by weight for age and height for age standard deviation (SD) scores*
Survival on a yearly basis commencing at one year
Number of people admitted to hospital and days spent as an inpatient
Number of people receiving additional antibiotics and number of days received
Number of people with one or more isolates of Haemophilus influenzae (H influenzae)
Number of people with one or more isolates of P aeruginosa
Number of people acquiring of multiply‐resistant S aureus
Frequency of adverse effects including: diarrhoea; skin rash; and oral, nappy or vulval candidiasis
Quality of life (if well‐validated measures are used)
* standard deviation (SD) score = observed weight or height ‐ mean/SD
Search methods for identification of studies
Electronic searches
We identified relevant trials from the Group's Cystic Fibrosis Trials Register using the terms: antibiotics AND (staphylococcus aureus OR mixed infections) AND (preventative treatment OR unknown) AND (oral OR not stated).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of theCochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the most recent search of the Group's CF Trials Register: 29 September 2016.
Search of ongoing trials registers
Clinicaltrials.gov Advanced search form Search terms: cystic fibrosis AND staphylococcus aureus Study type: interventional studies WHO ICTRP Cystic fibrosis AND staphylococcus aureus
Date of the most recent search of the ongoing trials registers: 16 February 2017.
Searching other resources
We have checked the reference lists of the trials on the Cochrane CF and Genetic Disorders Group relevant to this review to find any studies not previously identified.
We have contacted the authors of published trials to obtain any unpublished observations or long‐term follow‐up data. We also wrote to the manufacturers of antibiotics commonly used as prophylaxis to establish if unpublished data are held on file. Ten manufacturers were approached: Smith Kline Beecham; Ashbourne Pharmaceuticals; Approved Prescription Services; Galen; Trinity Pharmaceuticals; Yamanouchi Pharma; Bristol Myers Squibb Pharmaceuticals; Glaxo Wellcome; Eli Lilley; and Kent Pharmaceuticals. Five of these replied, but no new data were uncovered.
Data collection and analysis
Selection of studies
Two authors independently selected studies for inclusion in the review. The authors resolved disagreements as to which studies should be included by negotiation.
Data extraction and management
Each author recorded the following: concealment of treatment allocation; generation of allocation sequence; blinding; and whether intention‐to‐treat analysis had been used or was possible from the available data. Each author extracted data independently. The authors collected data for the outcome events listed above.
Where possible, the authors reported all outcome measures at yearly intervals or calculated annualised rates.
Assessment of risk of bias in included studies
In the current version of the review, we assessed the risk of bias to each included study relative to six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias) as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the generation of allocation sequence or concealment of that sequence was deemed to be adequate, then we judged the study to have a low risk of bias. If these were deemed to be inadequate, then we judged the study to have a potential risk of bias. If these were unclear, then the risk of bias for the study was also unclear. For blinding, the risk of bias was judged to increase as the number of people blinded to the intervention decreased. We also deemed there to be a risk of bias if there were any withdrawals or drop outs from the study which were not accounted for and explained, or if there were an unequal number of drop outs from a particular intervention group. We planned to examine the protocol for each included study where possible to establish whether results from any outcomes measured were selectively reported. Where protocols were not available, we compared the 'Methods' sections of the published papers to the 'Results' sections and also used clinical experience to judge whether they would expect outcomes to be measured as 'standard'. If we identified any outcomes that had been clearly measured but not reported, we judged there to be a high risk of bias; if all outcomes measured were clearly reported, we judged there to be a low risk of bias; if it was not clear if outcomes may have been measured and not reported, we judged there to be an unclear risk of bias. Finally, we assessed the studies for any other potential sources of bias, again judging there to be a high risk of bias if any sources were identified, a low risk of bias if it was clear that there were no other sources of bias and an unclear risk of bias if we were not able to judge this without any doubts.
Measures of treatment effect
We calculated a pooled estimate of treatment effect across all studies. For dichotomous outcomes, we presented the the odds ratio (OR) with corresponding 95% confidence intervals (CIs). Dichotomous outcomes include: acquisition of S aureus, survival, hospitalisation, requirement for additional antibiotics, acquisition of additional pathogens (including resistant S aureus), and presence of any adverse events. For continuous outcome data, we calculated the mean difference between treatment groups with corresponding 95% CIs. Continuous outcomes include: lung function tests, growth, number of days in hospital, number of days of additional antibiotics and quality of life. For one study, adverse events were presented as the number of days with a range of adverse events and we analysed these as continuous data. For longitudinal data, we undertook analysis at six months and thereafter at yearly intervals from diagnosis.
Unit of analysis issues
We did not consider cross‐over studies because we felt this study design would not allow evaluation of the effects of prophylaxis on long‐term outcome measures such as lung function, nutrition and the acquisition of resistant organisms.
Dealing with missing data
Where insufficient data were available from published work, we requested additional data from the trial investigators.
Assessment of heterogeneity
We planned to test for heterogeneity between study results using the I² statistic. We aimed to use the following grading.
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity
Assessment of reporting biases
In future updates of this review, if we are able to include sufficient studies (at least 10), we plan to assess publication bias by constructing a funnel plot. If the funnel plot is not symmetrical, publication bias may be present. However, there are other reasons for funnel plot asymmetry (i.e. heterogeneity), so we will interpret any results with caution. To minimise publication bias, we planned to search trial registries for any unpublished trials and contact experts in the field.
We aimed to compare original study protocols with final published papers to identify any selective reporting. If the original study protocols were not available, we examined the final published papers to identify any outcomes stated as being measured, but not reported in the study results.
Data synthesis
We analysed the data in the review using a fixed‐effect model. If in future, we identify a moderate to high degree of heterogeneity (see above) we will analyse the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We did not plan or undertake any subgroup analyses.
Summary of findings and quality of the evidence (GRADE)
In a post hoc change from protocol, we have presented a summary of findings tables for the comparison of prophylactic compared with 'as required' anti‐staphylococcal antibiotics for CF (Table 1). We report the following outcomes in the tables (chosen based on relevance to clinicians and consumers): lung function (FEV₁); number of people with at least one isolate of S aureus (sensitive strains); number of people having one or more isolates of P aeruginosa; number of people needing additional antibiotics; weight (z score); acquisition of multiply‐resistant S aureus; adverse effects of treatment. We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results and a high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
Of a total of 19 studies identified by the searches, four studies were included and 14 studies were excluded and one study is ongoing.
Included studies
Four studies met the inclusion criteria (Chatfield 1991; Schlesinger 1984; Stutman 2002; Weaver 1994). The Chatfield study is the subject of three conference abstracts and a full paper; individual patient data have been obtained from the authors (Chatfield 1991). The Schlesinger study is reported in a single conference abstract (Schlesinger 1984) and the Weaver study is reported in one conference abstract and two full papers (Weaver 1994). The most recently published study is by Stutman and colleagues, which is reported in a conference abstract and a full paper; the investigators have also supplied data to us directly (Stutman 2002).
Trial design
All four included studies were described as randomised, but only one was placebo‐controlled (Stutman 2002). Study duration ranged from one year (Schlesinger 1984) to a total of seven years (Stutman 2002). In the seven‐year study, each participant was followed for between five and seven years depending on the date of enrolment. Three studies were multicentre (Chatfield 1991; Stutman 2002; Weaver 1994) ‐ one stated 27 individual centres (Stutman 2002), but two did not explicitly state the number of centres. The fourth study did not indicate if it was single‐ or multicentre (Schlesinger 1984), One study was run in Germany (Schlesinger 1984), one in the USA (Stutman 2002) and two in the UK (Chatfield 1991; Weaver 1994).
Participant characteristics
The four included studies enrolled a total of 401 children and provided data from a total of 305 children (144 boys) who completed treatment per protocol (randomised to receive either prophylactic antibiotics or no prophylaxis). The number of participants enrolled in each study ranged from 28 (Schlesinger 1984) to 209 (Stutman 2002). Dropout rates were generally low, except in the Stutman study where 90 out of 209 participants withdrew (Stutman 2002). In one study of neonatal screening (Chatfield 1991), many clinicians declined to randomise infants presenting with meconium ileus (27 infants).
All studies recruited paediatric participants, but the ages at enrolment differed. Two studies were linked to newborn screening programmes (Chatfield 1991; Weaver 1994). The youngest participants were in the Weaver study; the mean age at enrolment was seven weeks in the prophylaxis group and five weeks in the 'as required' group (Weaver 1994). In the Chatfield study, the mean age was 122 days in the prophylaxis group and 260 in the 'as required' group (Chatfield 1991). Participants in the Stutman study were older; 15.6 months in the antibiotic group and 14.1 months in the placebo group at enrolment (Stutman 2002). The fourth study did not give details of age at enrolment (Schlesinger 1984).
One study did not give any information on the gender of participants (Chatfield 1991); in the remaining three studies, there were approximately equal numbers of males and females (Schlesinger 1984; Stutman 2002; Weaver 1994).
Interventions
Three studies compared continuous prophylactic antibiotics to 'as required' antibiotics (Chatfield 1991; Schlesinger 1984; Weaver 1994). Two studies used flucloxacillin in both the prophylactic and the 'as required' treatment arms (Chatfield 1991; Weaver 1994); Weaver further stated that the dose given prophylactically was 125 mg twice daily (Weaver 1994). Schlesinger used a cycle of antibiotics (cotrimoxazole, cefadroxil and dicloxacillin), with changes being made every three months, versus the same drugs being used intermittently (Schlesinger 1984). Stutman compared continuous daily cephalexin (80 mg/kg/day to 100 mg/kg/day in three equally divided doses) to placebo (Stutman 2002).
In each of these studies additional antibiotics could be prescribed to children in both arms of the study. In the Stutman study, children who received an additional antibiotic stopped their prophylaxis temporarily and, if additional treatment was required for more than six weeks, the participant was withdrawn (Stutman 2002).
Outcomes
Two of the included studies reported on our primary outcome of lung function (Stutman 2002; Weaver 1994). All four studies reported on the second primary outcome of isolation of S aureus and Weaver additionally reported on isolation of flucloxacillin‐resistant S aureus (Weaver 1994). All four studies also reported on the secondary outcome of growth (Chatfield 1991; Schlesinger 1984; Stutman 2002; Weaver 1994). Three studies reported on inpatient days and isolation of P aeruginosa (Chatfield 1991; Stutman 2002; Weaver 1994) and two studies reported on courses of additional oral antibiotics (Stutman 2002; Weaver 1994).
One study reported the first data at the six‐month time point (Weaver 1994) and all studies reported at one year and yearly intervals after this for as long as the studies lasted (Chatfield 1991; Schlesinger 1984; Stutman 2002; Weaver 1994).
Excluded studies
A total of 14 studies were excluded from the review. Cross‐over studies were not considered (see 'Types of studies'). This resulted in the exclusion of one study (Loening‐Baucke 1979). A study comparing two prophylactic antibiotics and which did not include a placebo group was also excluded (Harrison 1985). For similar reasons, we excluded two studies looking at a group of participants receiving oral prophylaxis where an additional antibiotic was given by aerosol (Nolan 1982) or parenterally (Shapera 1981). One study was a pharmacokinetic study of linezolid (Keel 2011) and another study was of a new formulation of tobramycin (Keller 2010). Finally, non‐randomised studies were excluded (Ballestero 1992; Brown 1980; Denning 1977; Feigelson 1993; Jensen 1990; Kerrebijn 1984; Szaff 1982; Wright 1970).
Ongoing studies
One ongoing study has been identified which is a randomised registry study to assess the safety and efficacy of long‐term anti‐staphylococcal antibiotic prophylaxis with flucloxacillin (CF START 2016). It is an open study of parallel design comparing prophylaxis to antibiotics given in a targeted manner as per national guidelines and being run at 130 centres in the UK. It is expected that 480 participants with diagnosed CF will be randomised. Age at enrolment is up to 70 days and each participant will be followed up to four years of age. The primary outcome is the age at first growth of P aeruginosa which will be assessed at all routine clinical encounters to study completion. Secondary outcomes include the need for extra antibiotic treatment, the number and type of respiratory cultures taken, the number and proportion of respiratory cultures positive for S aureus or for P aeruginosa or for other significant CF pathogens, chronic airway infection, frequency of hospital admissions, adverse events, nutritional status and cost. All secondary outcomes will be assessed at all encounters to study completion.
Risk of bias in included studies
We assessed the risk of bias to each included study relative to six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other potential sources of bias) as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (see table Characteristics of included studies).
Allocation
Two studies described the method of generating the allocation sequence and were judged to have a low risk of bias (Stutman 2002; Weaver 1994). The Stutman study randomised participants in blocks of six, stratified by initial respiratory culture status (Stutman 2002). The Weaver study employed block randomisation (Weaver 1994). Two studies were described as 'randomised' but do not discuss the generation of allocation sequence in their publications (Chatfield 1991; Schlesinger 1984); although Chatfield describes a neonatal screening programme which operated on alternate weeks (so infants were identified both clinically and by screening) and randomisation to either continuous prophylactic flucloxacillin or 'as required' antibiotic treatment took place at diagnosis (Chatfield 1991). These two studies were judged to have an unclear risk of bias (Chatfield 1991; Schlesinger 1984).
The two studies which described the method of randomisation, also discussed allocation concealment and the methods were judged to be adequate leading to a low risk of bias (Stutman 2002; Weaver 1994). In the Stutman study, all investigators apart from the study pharmacist were blind to treatment allocation. The pharmacist was responsible for increasing the dose of the prophylactic antibiotic as the children grew (Stutman 2002). In the Weaver study allocation was given by telephone from the co‐ordinating centre and concealed from the local investigator until the participant was enrolled (Weaver 1994). The remaining two studies did not discuss concealment of allocation and were judged to have an unclear risk of bias (Chatfield 1991; Schlesinger 1984).
Blinding
Only one of the included studies was double‐blinded and placebo‐controlled (Stutman 2002). We judged this study to have a low risk of bias.
The other three studies were not blinded and did not use a placebo (Chatfield 1991; Schlesinger 1984; Weaver 1994). These studies were judged to have a high risk of bias.
Incomplete outcome data
One study did not report any participants withdrawn from the study (Schlesinger 1984). Two studies performed analysis on those participants who completed the study and did not provide any data on participants withdrawn from the studies (Chatfield 1991; Weaver 1994). Chatfield reported data on varying numbers of participants at one, two and three year time points (Chatfield 1991). Weaver stated that analysis was per protocol (Weaver 1994). In the Stutman study, analysis was per protocol (outcome variables measured yearly up to six years of age) (Stutman 2002). Of 209 children recruited, 90 children withdrew; 119 completed the study, of which 68 were in the prophylaxis group and 51 in the placebo group. When children were withdrawn, this was most commonly at the parents' request, due to "the rigors of the study". They also analysed data on those completing at least one year of the study, although this does not constitute a formal intention‐to‐treat analysis (Stutman 2002). The use of per protocol analysis in the Stutman study will tend to favour the intervention. Two studies were felt to have a low risk of bias (Chatfield 1991; Weaver 1994) and one study, with a large number of withdrawals, was judged to have a high risk of bias (Stutman 2002). The risk of bias in the final study was unclear (Schlesinger 1984).
Selective reporting
We judged two studies to have a low risk of bias; one study reported outcome variables at one and two years following study entry (Weaver 1994); another study measured and reported those stated outcomes yearly up to six years of age (Stutman 2002).
We judged two studies to have an unclear risk of bias. One study reported outcome variables at only one year after enrolment; furthermore, the protocol was not available and since this study has only been published as a abstract, we were unable to compare any detailed methods with results (Schlesinger 1984). The final study reported outcomes up to three years of age. However, only summary statistics were presented in the three published abstracts and the only full paper describes the methodology but does not present results by antibiotic groups (Chatfield 1991).
Other potential sources of bias
For two studies there is a high risk of bias. In the first, the original data were published only in abstract form and the authors cannot be traced (Schlesinger 1984). In the second many clinicians declined to randomise infants presenting with meconium ileus, and these infants were therefore excluded from the analysis (27 infants) (Chatfield 1991).
In each of these studies additional antibiotics could be prescribed to children in both arms of the study and the use of additional antibiotics is a potential confounding factor. However, we still judge there to be is a low risk of bias for this domain for the other two studies, as we have not identified any other potential sources of bias (Stutman 2002; Weaver 1994). One study stated that the investigators performed a sample‐size calculation when designing the trial (Stutman 2002).
Effects of interventions
See: Table 1
Data from three studies could be combined (Chatfield 1991; Stutman 2002; Weaver 1994). In each case data on participants completing the study per protocol were used. The Stutman study is the only one giving data beyond three years and hence graphical data for years four, five and six refer to the Stutman study alone (Stutman 2002). In these three studies, additional antibiotics could be given to children receiving prophylaxis, when they were unwell, and so prophylaxis is evaluated as an adjunct to 'as required' treatment.
Primary outcomes
1. Lung function
Stutman used conventional tests of lung function, measured at the end of follow‐up (six years) in 119 participants (Stutman 2002). They found no significant difference between prophylaxis and placebo for FEV₁ (% predicted) or FVC (% predicted) (Analysis 1.1).
1.1. Analysis.
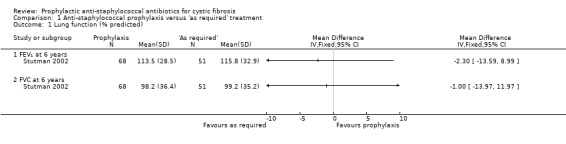
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 1 Lung function (% predicted).
Infant lung function was measured by Beardsmore in the Weaver study, which looked at infants enrolled in the newborn period (Beardsmore 1994). They aimed to measure lung function shortly after diagnosis (achieved in 19 infants receiving prophylaxis and 18 on 'as required' treatment) and again at one year (achieved in 18 infants receiving prophylaxis and 17 on 'as required' treatment). Specialised tests of lung function (not in routine clinical practice) were used, namely: thoracic gas volume (TGV); airway conductance (Gaw); maximum flow at functional residual capacity (Vmax FRC). The study authors reported no significant difference in infant lung function between the two regimens at either age. The results were expressed as scores (the number of standard errors by which the participant's value differed from a predicted value). For the mean values of scores, please refer to the additional table (Table 2).
1. Results of infant lung function testing (Beardsmore 1994).
| Measurement* | Baseline | At 1 year | ||
| Prophylaxis | As required | Prophylaxis | As required | |
| TGV (thoracic gas volume) | 0.05 | 0.98 | ‐0.22 | 0.09 |
| Gaw (airway conductance) | 1.16 | 0.00 | ‐1.79 | ‐1.13 |
| Vmax FRC (maximum flow at functional residual capacity) | ‐0.69 | ‐0.75 | ‐0.61 | ‐0.85 |
*All lung function values expressed as standard error scores
2. Number of people with one or more isolates of S aureus (sensitive strains)
The Weaver study reported the number of children in whom an organism was found and the number of months when there were positive isolates (Weaver 1994). However, the methods section does not specify how often routine samples were taken. For the sake of clarity and comparability, the data presented in 'Data and analyses' show the number of children with at least one isolate of S aureus. Pooled data from the Chatfield, Stutman and Weaver studies were used and are presented by years in the study from one to six years (Chatfield 1991; Stutman 2002; Weaver 1994). These data show significantly fewer children with one or more isolates of S aureus (at any time from the start of the study) in the group receiving prophylaxis for every year of follow up: at one year (n = 315), OR 0.27 (95% CI 0.15 to 0.48); at two years (n = 260), OR 0.21 (95% 0.13 to 0.35); at three years (n = 127), OR 0.22 (95% 0.13 to 0.38); at four years (n = 127), OR 0.10 (95% CI 0.04 to 0.25); at five years (n = 98), OR 0.09 (95% CI 0.03 to 0.26); and at six years (n = 43), OR 0.11 (95% CI 0.03 to 0.46) (Analysis 1.2). At the three‐year time point we note the substantial level of heterogeneity (I² = 70%) which might be linked to the age at diagnosis; the Chatfield study was linked to a neonatal screening programme and the Stutman study enrolled infants and young children diagnosed up to two years of age.
1.2. Analysis.
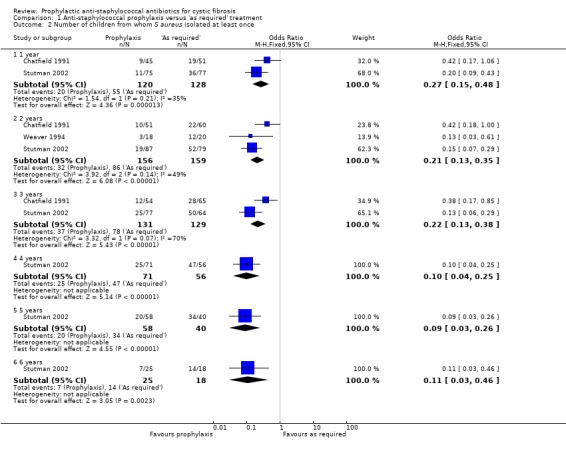
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 2 Number of children from whom S aureus isolated at least once.
Schlesinger reported only the results of throat swabs at the beginning and end of the one‐year prophylaxis period for S aureus only (Schlesinger 1984):
| Prophylaxis | 'As required' | |
| Start of study | 2/14 | 7/14 |
| End of study | 0/14 | 5/14 |
Given the lower prevalence of S aureus at the start of this study in participants who were subsequently randomised to prophylaxis, the finding that S aureus was isolated from none of the participants receiving prophylaxis at the end of the study must be interpreted with caution. The results do not give cumulative isolation rates for the one‐year study period and so are not included in 'Data and analyses'.
Secondary outcomes
1. Growth
Four studies measured children's growth (Chatfield 1991; Schlesinger 1984; Stutman 2002; Weaver 1994). Schlesinger reported the weight for age SD score (z score) after 12 months and found a statistically significant difference in favour of the children receiving prophylaxis; the statistical test used and the level of significance were not stated (Schlesinger 1984). The results from the Schlesinger study could not be presented graphically as the SDs were not given. Stutman and colleagues gave mean weight and height in each group, at the end of six years follow up (no significant difference) (Stutman 2002). However, SD scores were not given.
Weaver recorded weight for age and length for age SD scores at six months, one year and two years (Weaver 1994). Chatfield recorded the same SD scores at one, two and three years (Chatfield 1991). The results for the one‐ and two‐year assessment have therefore been combined. The combined data from the two studies did not find a statistically significant difference in either the weight for age or the length for age SD scores, in favour of either regimen, at either the one‐ or two‐year time points (Analysis 1.3; Analysis 1.4). The SD scores at six months and three years, for the individual studies, are also presented in 'Data and analyses'. Again, there was no significant difference in favour of either regimen. Length is difficult to measure accurately in this age group.
1.3. Analysis.
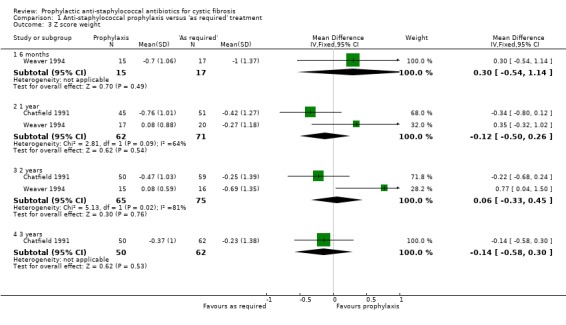
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 3 Z score weight.
1.4. Analysis.
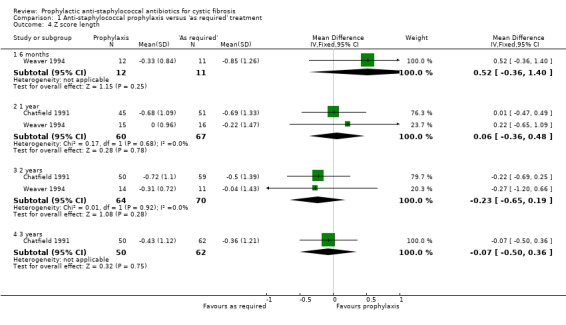
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 4 Z score length.
We note the substantial heterogeneity present when data from the Chatfield and Weaver studies for weight for age z score are combined at both one year (I² = 64%) and two years (I² = 81%) (Analysis 1.3). We have considered possible reasons for this heterogeneity and are unable to explain it, although we judge the Weaver study to be more methodologically robust.
2. Survival
This could not be included in the graphs as an outcome. No deaths were reported in three studies (Schlesinger 1984; Stutman 2002; Weaver 1994). One death was reported in the prophylaxis group in the other study, but no details were published (Chatfield 1991).
3. Number of people admitted to hospital and days spent as an inpatient
Frequency of hospital admissions was reported in three studies (n = 243) (Chatfield 1991; Stutman 2002; Weaver 1994). There was no significant difference between the two regimens in the number of participants having at least one hospital admission (Analysis 1.5).
1.5. Analysis.
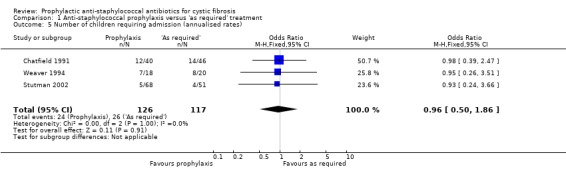
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 5 Number of children requiring admission (annualised rates).
The mean number of days spent in hospital per child per year of the study was calculated for three studies (n = 242) (Chatfield 1991; Stutman 2002; Weaver 1994). There was no significant difference between the two regimens (Analysis 1.6).
1.6. Analysis.
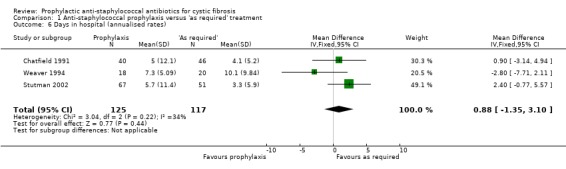
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 6 Days in hospital (annualised rates).
4. Number of people receiving additional antibiotics and number of days received
Weaver described number of additional 'courses' of antibiotics given but did not define the length of a course (Weaver 1994). These data have therefore not been presented in graphical form. Additional antibiotic treatment was not reported in three studies (Chatfield 1991; Schlesinger 1984; Stutman 2002). There was no significant difference between groups for either the number of children receiving additional antibiotics (n = 119) (Analysis 1.7) or for the mean number of days received per child (n = 119) (Analysis 1.8).
1.7. Analysis.
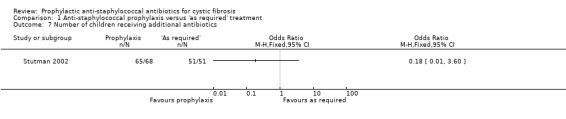
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 7 Number of children receiving additional antibiotics.
1.8. Analysis.
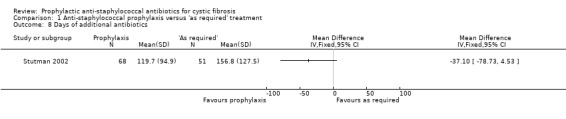
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 8 Days of additional antibiotics.
5. Number of people with one or more isolates H influenzae
Only one study reported isolates of H influenzae (n = 38) (Weaver 1994). This study found no significant difference between the two regimens in the number of children from whom H influenzae was isolated (Analysis 1.9).
1.9. Analysis.
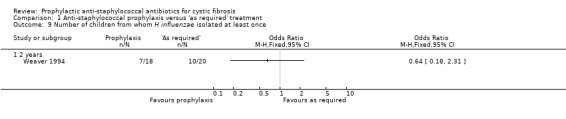
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 9 Number of children from whom H influenzae isolated at least once.
6. Number of people with one or more isolates of P aeruginosa
This was not reported by Schlesinger (Schlesinger 1984). When the results of the Chatfield, Stutman and Weaver studies were combined, there were no significant differences between groups for this outcome (n = 315) (Chatfield 1991; Stutman 2002; Weaver 1994). However, the results suggest that this outcome may depend on the duration of treatment. As shown in the graph, after two and three years of treatment there was a trend towards fewer isolates of P aeruginosa in the treatment group, but at years four, five and six the trend was towards fewer isolates of P aeruginosa in the control group (Analysis 1.10). However, only one study followed children up for four years or more (Stutman 2002).
1.10. Analysis.
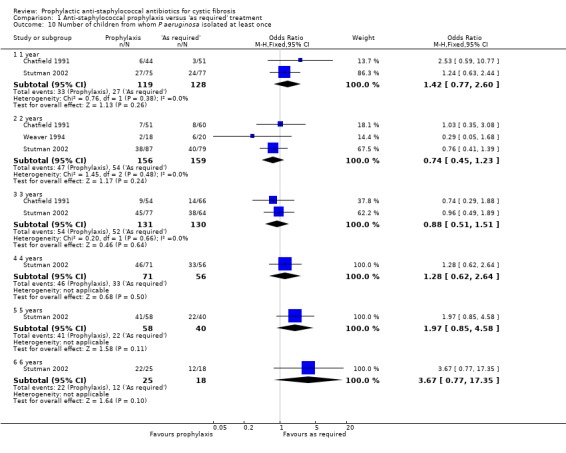
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 10 Number of children from whom P aeruginosa isolated at least once.
Weaver reported isolates of P aeruginosa in both upper respiratory and stool specimens (Weaver 1994). There was no significant difference in the isolation rate from stools in children (n = 38) on the two regimens.
7. Acquisition of multiply resistant S aureus
Three studies did not report isolation of resistant organisms such as MRSA or Burkholderia cepacia (Chatfield 1991; Schlesinger 1984; Stutman 2002). Weaver specifically stated that no flucloxacillin resistant strains of S aureus were isolated from study participants in either arm (Weaver 1994).
8. Adverse effects of prophylactic antibiotics
These data were presented in one study (n = 119) as the mean number of days experiencing each adverse event (Stutman 2002). There was no significant difference between the groups in the mean number of days with generalised rash, nappy rash or increased stool frequency (Analysis 1.11).
1.11. Analysis.
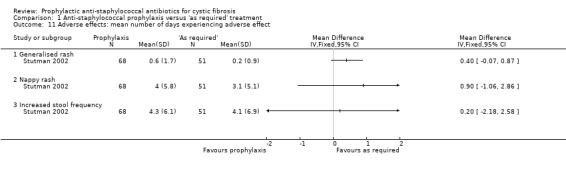
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 11 Adverse effects: mean number of days experiencing adverse effect.
9. Quality of life
Quality of life for parent or child was not reported in any of the studies.
Additional outcomes which have arisen from the review
1. Clinical and radiological scoring
Although this was not an a priori hypothesis of this review, data from the Chatfield study are available for Shwachman and Chrispin‐Norman scores in the 109 children in whom data were available at three years (Chatfield 1991). The Shwachman score is a clinical score which includes symptoms, clinical examination findings, nutrition and radiology (Shwachman 1958). The Chrispin‐Norman score is an objective chest radiograph score (Chrispin 1974). There was no significant difference in either the Shwachman score (Analysis 1.12), or the Chrispin‐Norman score (Analysis 1.13).
1.12. Analysis.
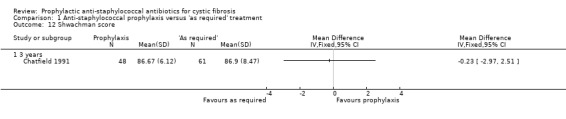
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 12 Shwachman score.
1.13. Analysis.
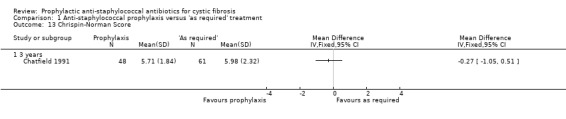
Comparison 1 Anti‐staphylococcal prophylaxis versus 'as required' treatment, Outcome 13 Chrispin‐Norman Score.
Discussion
Summary of main results
We present data on 305 participants with cystic fibrosis (CF) from four studies, three of which compared a prophylactic antibiotic regimen to an 'as required' regimen (Chatfield 1991; Schlesinger 1984; Weaver 1994) and one which compared a continuous antibiotic regimen to placebo (Stutman 2002).
With regards to the review's first primary outcome, lung function, the Stutman study was the only one to measure conventional spirometry and found no difference in forced expiratory volume in one second (FEV₁) % predicted or forced vital capacity (FVC) % predicted between the active and placebo arms after six years of follow‐up (Stutman 2002). Similarly, the Beardsmore report from the Weaver study, using infant pulmonary function tests, found antibiotic prophylaxis has no significant effect on infant lung function over a one‐year period (Beardsmore 1994). In terms of the second primary outcome, isolation of Staphylococcus aureus (S aureus) from respiratory cultures, data from all four included studies indicate that fewer children in the group receiving continuous anti‐staphylococcal prophylaxis had one or more isolates of S aureus (Chatfield 1991; Schlesinger 1984; Stutman 2002; Weaver 1994).
In terms of the review's secondary outcome measures, data are presented for a number of clinical outcome measures: nutrition; Shwachman score; and Chrispin‐Norman chest radiograph score. Nutritional data are available from three eligible studies (Chatfield 1991; Schlesinger 1984; Weaver 1994). One study suggested an improvement in weight for age standard deviation score in the prophylaxis group after one year of treatment (Schlesinger 1984). This study could not be combined with the other two studies reporting standard deviation scores (Chatfield 1991; Weaver 1994). The pooled data from the studies of Chatfield and Weaver show no significant difference in weight for age or height for age standard deviation scores between regimens (Chatfield 1991; Weaver 1994). The Chatfield study provides data up to three years, again with no significant difference seen. This may be because this study looked at young children who are still showing rapid 'catch up' growth (Morison 1997), whereas the participants studied by Schlesinger may have already achieved this 'catch up' in weight. Alternatively, this negative result may be because there is no therapeutic effect. The data from the Chatfield study showed no significant difference in Shwachman or Chrispin‐Norman scores between the two regimens (Chatfield 1991).
Only one death was reported in one of the eligible studies, so no conclusions can be drawn about the likely effects of prophylaxis on survival. Mortality is likely to be very low in the young children studied and during such a short follow‐up period.
The requirement for additional antibiotics and hospital admission may be thought of as indirect measures of clinical status, although both these measures will be influenced by local treatment protocols and clinicians' preferences. The use of additional antibiotics is also a potential confounding factor. Admissions to hospital may also have a negative effect on the quality of life of the child and their family. The pooled data show no significant difference in additional antibiotics or hospital admissions between groups.
We found no significant difference in the isolation rate of other common organisms (such as Haemophilus influenzae and Streptococcus pneumoniae) between the prophylaxis and 'as required' groups. Although there was no significant difference between groups in the number of children having one or more isolates of Pseudomonas aeruginosa (P aeruginosa), there was a trend towards fewer affected children in years two and three of the study and a similar trend to more children having at least one isolate of P aeruginosa in years four to six. The data from the years four to six all come from one study (Stutman 2002), as none of the other studies had more than three years of follow up. These trends may be a chance finding. However, if the trend to more children having P aeruginosa with a longer duration of prophylaxis is a genuine finding, then this is a cause for concern. There are two possible explanations: firstly, a period of prophylaxis of more than three years' duration predisposes to pseudomonas infection; or secondly, the use of a broad‐spectrum antibiotic (cephalexin) rather than a narrow spectrum anti‐staphylococcal antibiotic (flucloxacillin) promotes pseudomonas infection.
Overall completeness and applicability of evidence
Reporting of the presence of organisms in the respiratory secretions is difficult to standardise, since prevalence is dependent on the frequency with which samples are taken. The eligible studies involved young children and so nose and throat swabs were used, rather than sputum samples. Armstrong showed that oropharyngeal specimens predict lower respiratory infection poorly (positive predictive value 41%) (Armstrong 1996).
The eligible studies had a maximum follow‐up period of six years and all the participants studied were under seven years of age. It is therefore important not to extrapolate these results to longer periods of prophylaxis or to older individuals.
Quality of the evidence
In common with other Cochrane authors (Walters 1999), we have found it difficult to establish whether the randomisation method used in many studies allows true concealment of allocation and therefore prevents bias. It is to be hoped that medical journals will increasingly follow the recommendations of the CONSORT statement on reporting the results of randomised controlled trials (Moher 2001). One of the eligible studies included in this review by Schlesinger did not report withdrawals, making it impossible to determine whether intention‐to‐treat or per protocol analysis had been used (Schlesinger 1984). The two citations of the Weaver study contained discrepancies in the number of participants in prophylaxis and 'as required' groups (Weaver 1994). This suggests that data on lung function have been presented on some (but not all) of the participants who withdrew from the study. The Chatfield study has a number of methodological weaknesses (Chatfield 1991). There was a lack of proper randomisation of infants with meconium ileus, leading to their exclusion from our analysis. Neonatal screening was undertaken only on alternate weeks leading to a heterogeneous population, containing screened and unscreened infants, being randomised to prophylaxis or intermittent treatment. Data were not available on every child in the study at each time point of follow‐up. This has led to data on different numbers of children being reported at different time points, e.g. data for weight and length standard deviation score at one, two and three years (Analysis 1.3; Analysis 1.4). This is a potential source of bias. The Stutman study was methodologically superior in having a clear description of concealment of allocation, allocation sequence generation, and double‐blinded placebo‐controlled design (Stutman 2002). However, even in this study, a formal intention‐to‐treat analysis was not possible due to a lack of outcome data on those children who were withdrawn.
Overall the number of studies is small, and those studies which have been undertaken are of poor quality, with small numbers of participants. The effect on S aureus is likely to be genuine, as it is seen in all three studies with data, when taken individually and when they are combined in the meta‐analysis. It is also consistent at all time points up to six years. However, the lack of a beneficial effect of prophylaxis on any outcome measure other than S aureus may be genuine or due to insufficient statistical power, bias or the 'lumping' together of different regimens. The data on Paeruginosa must be interpreted with caution, as there was no statistically significant difference between regimens. Data for this outcome measure for years four to six came from the Stutman study alone, where attrition may have led to bias (Stutman 2002). The Stutman study was the only study to report on adverse effects and uncommon adverse effects may be missed in randomised trials because of the small numbers involved (Stutman 2002). Based on GRADE criteria, the overall strength of the evidence is moderate to low. Downgrading decisions were due to limitations in study design (all outcomes); for imprecision (number of people needing additional antibiotics); and for inconsistency (weight z score).
Potential biases in the review process
The authors of this review were not involved in any of the included studies. However, both authors are involved with an ongoing study of prophylactic antibiotics in infants with CF identified by newborn screening (AS as a co‐investigator and MR as chair of the data safety monitoring committee) (CF START 2016).
Agreements and disagreements with other studies or reviews
This update agrees with the prior Cochrane review on this topic as there have been no new studies. A previous systematic review (McCaffery 1999), did not include data from the Stutman study (Stutman 2002) and did not perform meta‐analysis.
Authors' conclusions
Implications for practice.
Our review includes four studies of anti‐staphylococcal antibiotic prophylaxis in children with CF, with data from 305 participants. The quality of studies is concerning, with important deficiencies in each. Significantly fewer children with CF will have one or more isolates of S aureus in upper respiratory secretions when anti‐staphylococcal antibiotic prophylaxis is prescribed for the first six years of life. However, the importance of this finding is uncertain, as this review has not shown that this is associated with an improvement in clinical outcome measures. The currently available evidence does not allow conclusions to be drawn regarding the effect of prophylaxis on acquisition of P aeruginosa. There is no significant difference in the rate of common adverse effects.
There is insufficient evidence in this review to say whether the use of anti‐staphylococcal antibiotic prophylaxis in older children or adults is beneficial or harmful. Hence, clinicians should exercise caution, if prophylactic anti‐staphylococcal antibiotics are used in older individuals or for longer periods.
Implications for research.
The four studies included in this review had important methodologic limitations, including small sample size and high risk of bias. Thus, important questions regarding the benefits and risks of staphylococcal prophylaxis remain unanswered, including long‐term clinical benefit and risk of earlier acquisition of P aeruginosa. A prospective, multicentre randomized clinical trial comparing continuous flucloxacillin and "as required" antibiotic therapy in infants identified by newborn screening is underway in the UK (CF START 2016) (for further details see Characteristics of ongoing studies). Additional or future clinical trials, particularly with a placebo arm, would greatly strengthen the evidence base for or against anti‐staphylococcal prophylaxis in people with CF.
What's new
| Date | Event | Description |
|---|---|---|
| 19 February 2020 | Amended | Clarification statement added from Alan Smyth, Co‐ordinating Editor on 19 February 2020: This review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. It will be updated by February 2021. The update will have a majority of authors and lead author free of conflicts. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 11 April 2017 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group Cystic Fibrosis Trials Register did not identify any potentially relevant new references. A summary of findings table has been added to the review and changes made to bring the format of the review into line with current Cochrane standards. |
| 11 April 2017 | New citation required but conclusions have not changed | Co‐author Dr Sarah Walters has stepped down from the author team. A new co‐author, Professor Margaret Rosenfeld, has joined the review team. |
| 20 November 2014 | New citation required but conclusions have not changed | No new information has been added to this review, therefore our conclusions remain the same. |
| 20 November 2014 | New search has been performed | An updated search of the Cystic Fibrosis & Genetic Disorders Review Group's Cystic Fibsrosis Trials Register did not identify any new references which were potentially eligible for inclusion in this review. The Plain Language Summary has been updated in line with new guidance. |
| 30 October 2012 | New citation required but conclusions have not changed | No new data were included at this update and so the conclusions of the review remain the same. |
| 30 October 2012 | New search has been performed | A new search of the Group's Cystic Fibrosis Trials Register identified two new references potentially eligible for inclusion in this review both of which were excluded (Keel 2011; Keller 2010). |
| 13 September 2010 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified no studies which were potentially relevant for inclusion in the review. |
| 12 August 2009 | Amended | Contact details updated. |
| 9 September 2008 | Amended | Converted to new review format. |
| 2 September 2008 | New search has been performed | A new search of the Group's trials register was run but no new references were identified. |
| 23 May 2007 | New search has been performed | A new search of the Group's trials register was run but no new references were identified. |
| 24 May 2006 | New search has been performed | A new search of the Group's trials register was run but no new references were identified. |
| 23 February 2005 | New search has been performed | A new search was run but no new references were identified. |
| 23 February 2005 | Amended | To more accurately reflect the content and scope of the review, the title was changed from 'Prophylactic antibiotics for cystic fibrosis'. |
| 29 April 2004 | New search has been performed | A new search was run but no new references were identified. |
| 20 May 2003 | New citation required and conclusions have changed | Substantive amendment. Since the previous version of this review, the results of a large North American trial of cephalexin versus placebo have been published (Stutman 2002). The authors have made further data available, allowing us to evaluate the effect of prophylaxis on conventional lung function tests, number of people receiving additional antibiotics, days of additional treatment and adverse effects. This further data also allows pooling of data on the following outcome measures: number of people admitted to hospital, duration of admission, isolates S. aureus, and isolates of P. aeruginosa. Our earlier findings of a beneficial effect on the number of children with one or more isolates of S. aureus, are confirmed. There is no significant difference in the number of isolates of P. aeruginosa between groups, though there is a trend towards fewer children with one or more isolates of P. aeruginosa infection, with prophylaxis, in years two and three and a similar trend towards more children with P. aeruginosa from years four to six. |
| 21 March 2001 | New search has been performed | We have received individual patient data on 109 children at two‐year follow up who were enrolled in the Wales and West Midlands neonatal screening study (reported by Chatfield 1991). The published report did not include analysis by allocation to the prophylaxis or intermittent treatment group. We have now gone back to the original data and undertaken this analysis. This has allowed pooling of data on the following outcome measures: nutrition, isolates of common pathogens, isolates of P. aeruginosa, number of children admitted to hospital, and duration of admission. Our earlier findings of a beneficial effect of prophylaxis on the frequency of isolation of S. aureus from upper respiratory secretions are confirmed. Pooled data demonstrate no effect on nutrition. There is a non‐significant trend towards a lower prevalence of P. aeruginosa infection with prophylaxis. |
Acknowledgements
We acknowledge the help of Dr Harris Stutman, who provided data from the cephalexin study (Stutman 2002). Dr Henry Ryley supplied individual patient data from the Chatfield study (Chatfield 1991). Prof Lawrence Weaver and Dr Michael Green, provided data from the Weaver study (Weaver 1994).
The current author team would also like to acknowledge the previous contributions of Dr Sarah Walters who stepped down from the review in 2016.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Anti‐staphylococcal prophylaxis versus 'as required' treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung function (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 FEV₁ at 6 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 FVC at 6 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of children from whom S aureus isolated at least once | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 1 year | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.15, 0.48] |
| 2.2 2 years | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.13, 0.35] |
| 2.3 3 years | 2 | 260 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.13, 0.38] |
| 2.4 4 years | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.04, 0.25] |
| 2.5 5 years | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.26] |
| 2.6 6 years | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.46] |
| 3 Z score weight | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.54, 1.14] |
| 3.2 1 year | 2 | 133 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.50, 0.26] |
| 3.3 2 years | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.33, 0.45] |
| 3.4 3 years | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.58, 0.30] |
| 4 Z score length | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.36, 1.40] |
| 4.2 1 year | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.36, 0.48] |
| 4.3 2 years | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.65, 0.19] |
| 4.4 3 years | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.50, 0.36] |
| 5 Number of children requiring admission (annualised rates) | 3 | 243 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.86] |
| 6 Days in hospital (annualised rates) | 3 | 242 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.35, 3.10] |
| 7 Number of children receiving additional antibiotics | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Days of additional antibiotics | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Number of children from whom H influenzae isolated at least once | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 2 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of children from whom P aeruginosa isolated at least once | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 1 year | 2 | 247 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.77, 2.60] |
| 10.2 2 years | 3 | 315 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.23] |
| 10.3 3 years | 2 | 261 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.51] |
| 10.4 4 years | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.62, 2.64] |
| 10.5 5 years | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.85, 4.58] |
| 10.6 6 years | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.77, 17.35] |
| 11 Adverse effects: mean number of days experiencing adverse effect | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Generalised rash | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Nappy rash | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Increased stool frequency | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Shwachman score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Chrispin‐Norman Score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chatfield 1991.
| Methods | RCT. Duration: follow up to each child's third birthday. Multicentre in Wales and West Midlands (UK) (number of centres not stated). |
|
| Participants | Infants with CF diagnosed by neonatal screening or clinically (alternate weeks).
Followed up to age 3 years. n = 132: 57 prophylaxis; 75 'as required'. Mean (SD) age at diagnosis: 122 (255) days prophylaxis; 260 (379) 'as required'. Data available at 1, 2 & 3 years. |
|
| Interventions | Continuous oral flucloxacillin versus intermittent antibiotics 'as required'. | |
| Outcomes | Growth; inpatient days; participants with isolates of common pathogens; P aeruginosa. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described, unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded. No placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis: not possible (infants with meconium ileus not randomised and therefore excluded from analysis). 1 participant lost to follow up and 1 infant died. |
| Selective reporting (reporting bias) | Unclear risk | Study reported outcomes up to 3 years of age. Only summary statistics have been presented in abstracts, and full paper describes the methodology, but does not present results by antibiotic groups. |
| Other bias | High risk | Infants with meconium ileus not randomised. Additional information from the authors. |
Schlesinger 1984.
| Methods | RCT. Duration: 1 year. |
|
| Participants | Children aged 1 to 7 years with mild CF lung disease. 28 participants enrolled, no withdrawals documented. Prophylaxis = 14 (8 males), 'as required' = 14 (8 males). Mean (range) age at enrolment: prophylaxis group 42 (10 ‐ 81) months; 'as required' group 53 (16 ‐ 81) months. Similar z scores for weight and height on enrolment. Important differences in prevalence of S aureus in prophylaxis (2/14) and 'as required' groups (7/14) on enrolment. | |
| Interventions | Co‐trimoxazole, or cefadroxil, or dicloxacillin in 3‐monthly cycles for 1 year. | |
| Outcomes | Growth, participants with isolates of common pathogens. Data collected at enrolment and 1 year. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described, unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded. No placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis: not possible, (numbers assessed for eligibility and participants withdrawn not described). |
| Selective reporting (reporting bias) | Unclear risk | Study reported outcome variables at only one year after enrolment. Published as abstract only, so not able to compare Methods and Results sections. |
| Other bias | High risk | The original data were published only in abstract form and the authors cannot be traced. |
Stutman 2002.
| Methods | RCT. Duration: 7 years (each participant had a 5 ‐ 7 year course of therapy). Multicentre (27 centres) in the USA. |
|
| Participants | Children under 2 years diagnosed with CF by conventional criteria.
209 enrolled, 90 withdrew and 119 completed the study (68 prophylaxis, 51 'as required').
Mean (SD) age at enrolment: overall 14.6 (6.8) months; cephalexin 15.6 months; placebo 14.1 months. 54% of those enrolled were female. Followed up for between 5 and 7 years. |
|
| Interventions | Continuous daily cephalexin (80 ‐ 100 mg/kg/day in 3 equally divided doses) versus placebo. | |
| Outcomes | Lung function; growth; inpatient days; courses of 'as required' oral antibiotics; participants with isolates of common pathogens; P aeruginosa. Data collected at yearly intervals from year 1. |
|
| Notes | Statistical power calculations undertaken. Additional information from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participant stratified by respiratory culture status. Permuted block design (blocks of 6, with 3 participants in each block randomised to cephalexin or placebo). |
| Allocation concealment (selection bias) | Low risk | Treatment allocation known only to the study pharmacist. The pharmacist was responsible for increasing the dose of the prophylactic antibiotic as the children grew. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded and placebo‐controlled. Placebo was identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Formal intention‐to‐treat analysis: not possible. However, analysis performed of children completing treatment per protocol (n = 119) and those completing at least 1 year of the trial (n = 165). |
| Selective reporting (reporting bias) | Low risk | Measured and reported stated outcome variables yearly up to 6 years of age. |
| Other bias | Low risk | No other potential source of bias identified. |
Weaver 1994.
| Methods | RCT. Duration: 2 years. Multicentre in East Anglia, UK (does not state exact number of centres). |
|
| Participants | Infants with CF diagnosed by neonatal screening.
42 participants enrolled, 4 withdrew, n = 38 (prophylaxis = 18, 'as required' = 20).
Similar mean ages at enrolment: prophylaxis 7 weeks; 'as required' 5 weeks. Gender split: 20 males (10 in each group). Followed up to age 2 years. |
|
| Interventions | Continuous oral flucloxacillin (250 mg/day in 2 divided doses) versus intermittent antibiotics 'as required'. | |
| Outcomes | Lung function; growth; inpatient days; courses of 'as required' oral antibiotics; participants with isolates of common pathogens (P aeruginosa); flucloxacillin resistant S. aureus. Data collected at 6 months, 1 and 2 years. |
|
| Notes | Additional information from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised in the published paper. Authors confirmed treatment was allocated on the basis of block randomisation and allocation was given by telephone from the co‐ordinating centre. |
| Allocation concealment (selection bias) | Low risk | Allocation was given by telephone from the co‐ordinating centre and concealed from the local investigator until the participant was enrolled. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded. No placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis: not possible. |
| Selective reporting (reporting bias) | Low risk | Study reported outcome variables at 1 and 2 years following study entry. |
| Other bias | Low risk | No other potential source of bias identified. |
CF: cystic fibrosis P aeruginosa: Pseudomonas aeruginosa RCT: randomised controlled trial S aureus: Staphylococcus aureus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ballestero 1992 | Non‐randomised. |
| Brown 1980 | Non‐randomised. |
| Denning 1977 | Non‐randomised. |
| Feigelson 1993 | Non‐randomised. |
| Harrison 1985 | Both participant groups received prophylaxis. Comparison was not made with a group receiving intermittent antibiotics 'as required'. |
| Jensen 1990 | Non‐randomised. |
| Keel 2011 | Pharmacokinetic study of linezolid. |
| Keller 2010 | Study of new formulation of tobramycin for use in treating Pseudomonas aeruginosa. |
| Kerrebijn 1984 | Non‐randomised. |
| Loening‐Baucke 1979 | Cross‐over study design. |
| Nolan 1982 | All participants on oral prophylaxis (cloxacillin). Nebulised cephaloridine given to alternate participants in a quasi‐randomised design. |
| Shapera 1981 | Parenteral and oral clindamycin versus oral clindamycin alone. |
| Szaff 1982 | Non‐randomised. |
| Wright 1970 | Non‐randomised. |
Characteristics of ongoing studies [ordered by study ID]
CF START 2016.
| Trial name or title | The cystic fibrosis (CF) anti‐staphylococcal antibiotic prophylaxis trial (CF START); a randomised registry trial to assess the safety and efficacy of flucloxacillin as a long‐term prophylaxis agent. |
| Methods | Randomised controlled open trial. Parallel design. Location: multicentre (130 centres) in UK. Initial estimate of the duration of the trial: 8 years (follow‐up of each participant to 4 years of age). |
| Participants | Expected enrolment: 480 participants up to 70 days of age at enrolment. Expected age range: preterm newborn infants (up to gestational age < 37 weeks) n = 10; newborns (0‐27 days) n = 100; infants and toddlers (28 days‐23 months) n = 370. Diagnosis of CF through either identification of 2 CF‐causing mutations OR 1 or no CF‐ causing mutations identified and a sweat chloride test result greater than 59 mmol/L OR 2 CFTR mutations (not known CF‐causing mutations) and a sweat chloride test result greater than 29 mmol/L. Exclusion criteria: 1. An inconclusive diagnosis after newborn screening (NBS). 2. A condition (non‐CF) that, in the opinion of the recruiting investigator will impact on the long‐term management and outcome of a participant with CF. 3. Previous growth of Pseudomonas aeruginosa from respiratory culture. 4. Infants with a history of hypersensitivity to β‐lactam antibiotics (e.g. penicillins) or excipients. 5. Infants with a history of flucloxacillin associated jaundice/hepatic dysfunction. |
| Interventions | Intervention: 2x daily oral prophylactic flucloxacillin ("prevent and treat"). Comparator: antibiotics given in a targeted manner as per national guidelines ("detect and treat"). |
| Outcomes |
Primary outcome (assessed at all routine clinical encounters to trial completion (age, 48 months)) Age at first growth of Pseudomonas aeruginosa from respiratory culture collected as part of routine care Secondary outcomes Need for extra antibiotic treatment Number and type of respiratory culture taken during the trial period Number and proportion of respiratory cultures positive for Staphylococcus aureus Number and proportion of respiratory cultures positive for Pseudomonas aeruginosa Number and proportion of respiratory cultures positive for other significant CF pathogens Chronic airway infection Frequency of hospital admission Adverse events Nutritional status Costs to the NHS All secondary outcomes will be assessed at all encounters to trial completion (age, 48 months). Apart from determining if anti‐staphylococcal prophylaxis improves respiratory function in pre‐school children with CF which will be evaluated at trial visits between the age of 40 ‐ 48 months. As well as determining nutritional status which will be evaluated at encounters between 40 ‐ 48 months. |
| Starting date | 16 September 2016. |
| Contact information | Prof Kevin Southern, Alder Hey Children's NHS Foundation Trust, Liverpool, UK. |
| Notes | EudraCT Number: 2016‐002578‐11. |
CF: cystic fibrosis CFTR: cystic fibrosis transmembrane regulator NHS: National Health Service
Differences between protocol and review
We included a further outcome after the protocol was published: Clinical and radiological scoring. Although this was not an a priori hypothesis of this review, data from the Chatfield study are available for Shwachman and Chrispin‐Norman scores at three years (Chatfield 1991). The Shwachman score is a clinical score which includes symptoms, clinical examination findings, nutrition and radiology (Shwachman 1958). The Chrispin‐Norman score is an objective chest radiograph score (Chrispin 1974).
Contributions of authors
Original review and updates up to and including 2014
Both AS and SW evaluated which studies should be included in the review. AS analysed the data. AS and SW both interpreted the results. AS liaised with the authors of the studies included in this review to obtain additional data.
AS completed the updates with additional comments from SW and he acts as guarantor for this review.
From 2016
Both AS and MR evaluated studies identified as potentially eligible for inclusion in the review. AS completed the updates with MR. Both AS and MR wrote the manuscript. AS acts as guarantor for this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
ARS declares relevant activities of membership of a MPEX steering committee, advisory board member (Vertex, Gilead and MPEX), lectures paid for by Gilead and Novartis.
MR declares no known conflict of interest.
Clarification statement added from Alan Smyth, Co‐ordinating Editor on 19 February 2020: This review was found by the Cochrane Funding Arbiters, post‐publication, to be noncompliant with the Cochrane conflict of interest policy, which includes the relevant parts of the Cochrane Commercial Sponsorship Policy. It will be updated by February 2021. The update will have a majority of authors and lead author free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Chatfield 1991 {published and unpublished data}
- Chatfield S, Owen G, Ryley MC, Williams J, Alfaham M, Goodchild MC, et al. Neonatal screening for cystic fibrosis in Wales and the West Midlands: clinical assessment after five years of screening. Archives of Disease in Childhood 1991;66(1 Spec No):29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen G, West J, Maguire S, Ryley H, Goodchild M, Weller PH. Continuous and intermittent antibiotic therapy in cystic fibrosis patients to age 4 years [abstract]. Proceedings of the 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:95.
- West J, Smith AW, Brown MRW, Weller PH. Longitudinal relationship between clinical status, lung infection and immune responses in young cystic fibrosis patients [abstract]. Pediatric Pulmonology 1990;Suppl 5:222. [Google Scholar]
- Williams J, Alfaham M, Ryley HC, Goodchild MC, Weller PH, Dodge JA. Screening for cystic fibrosis in Wales and the West Midlands 2: Clinical evaluation [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:G(b)3. [Google Scholar]
Schlesinger 1984 {unpublished data only}
- Schlesinger E, Muller W, Hardt H, Schirg E, Rieger CHL. Effect of long‐term continuous anti‐staphylococcal antibiotic treatment in young children with cystic fibrosis [abstract]. Proceedings of the 9th International Cystic Fibrosis Congress. 1984:4.14.
Stutman 2002 {published and unpublished data}
- Stutman HR, Lieberman JM, Nussbaum E, Marks MI. Antibiotic prophylaxis in infants and young children with cystic fibrosis: A randomised controlled trial. Journal of Pediatrics 2002;140(3):299‐305. [DOI] [PubMed] [Google Scholar]
- Stutman HR, Marks MI. Cephalexin prophylaxis in newly diagnosed infants with cystic fibrosis [abstract]. Proceedings of the 6th Annual North American Cystic Fibrosis Conference; 1992. 1992:147‐8.
Weaver 1994 {published and unpublished data}
- Beardsmore CS, Thompson JR, Williams A, McArdle EK, Gregory GA, Weaver LT, et al. Pulmonary function in infants with cystic fibrosis: the effect of antibiotic treatment. Archives of Disease in Childhood 1994;71(2):133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LT, Green MR, Nicholson K, Heeley AF, Mills J, Kuzemko JA. Continuous prophylactic flucloxacillin improves outcome of infants with cystic fibrosis (CF) detected soon after birth [abstract]. Proceedings of 63rd Annual Meeting of the British Paediatric Association; 1991; Warwick. 1991:24.
- Weaver LT, Green MR, Nicholson K, Mills J, Heeley ME, Kuzemko JA, et al. Prognosis in cystic fibrosis treated with continuous flucloxacillin from the neonatal period. Archives of Disease in Childhood 1994;70(2):84‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ballestero 1992 {published data only}
- Ballestero S, Villaverde R, Escobar H, Baquero F. Susceptibility to various anti‐microbial agents of Staphylococcus aureus isolates from cystic fibrosis patients. European Journal of Clinical Microbiology & Infectious Diseases 1992;11(12):1193‐4. [DOI] [PubMed] [Google Scholar]
Brown 1980 {published data only}
- Brown J. Efficacy of antimicrobial drugs against staphylococci in cystic fibrosis. Australian Paediatric Journal 1980;16(3):207‐9. [DOI] [PubMed] [Google Scholar]
Denning 1977 {unpublished data only}
- Denning CR, Park S, Grece CA, Mellin GW. Continuous versus intermittent oral antibiotics in the management of patients with cystic fibrosis [abstract]. 18th Annual Meeting Cystic Fibrosis Club Abstracts. 1977:23.
Feigelson 1993 {published data only}
- Feigelson J, Pecau Y. Fusidic acid in cystic fibrosis [Action therapeutique de l'acide fusidique dans la mucoviscidose]. Pediatre 1993;29(138):111‐3. [Google Scholar]
Harrison 1985 {published data only}
- Harrison CJ, Marks MI, Welch DF, Sharma BB, Baker D, Dice J. A multicentric comparison of related pharmacologic features of cephalexin and dicloxacillin given for two months to young children with cystic fibrosis. Pediatric Pharmacology 1985;5(1):7‐16. [PubMed] [Google Scholar]
Jensen 1990 {published data only}
- Jensen T, Lanng S, Faber M, Rosdahl VT, Hoiby N, Koch C. Clinical experiences with fusidic acid in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1990;25(Suppl B):45‐52. [DOI] [PubMed] [Google Scholar]
Keel 2011 {published data only}
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2011;55(7):3393‐8. [CFGD Register: PI251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keller 2010 {published data only}
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS® [abstract]. Journal of Cystic Fibrosis 2010;9(Suppl 1):S22, Abstract no: 84. [CFGD Register: PI241] [Google Scholar]
Kerrebijn 1984 {published data only}
- Kerrebijn KF. Prospective study on the effect of daily and intermittent antibiotic treatment in cystic fibrosis. In: Lawson D editor(s). CF: horizons. Chichester: John Wiley and Son, 1984:273. [Google Scholar]
Loening‐Baucke 1979 {published data only}
- Loening‐Baucke V, Mischler E, Myers MG. A placebo controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. [DOI] [PubMed] [Google Scholar]
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis [abstract]. 19th Annual Meeting Cystic Fibrosis Club Abstracts. 1978:69.
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study [abstract]. Pediatric Research 1978;12(4 Pt 2):495. [Google Scholar]
Nolan 1982 {published data only}
- Nolan G, McIvor P, Levinson H, Fleming PC, Corey M, Gold R. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. Journal of Pediatrics 1982;101(4):626‐30. [DOI] [PubMed] [Google Scholar]
Shapera 1981 {published data only}
- Shapera RM, Warwick WJ, Matsen JM. Clindamycin therapy of staphylococcal pulmonary infections in patients with cystic fibrosis. Journal of Pediatrics 1981;99(4):647‐50. [DOI] [PubMed] [Google Scholar]
Szaff 1982 {published data only}
- Szaff M, Hoiby N. Antibiotic treatment of staphylococcus aureus infection in cystic fibrosis. Acta Paediatrica Scandinavica 1982;71(5):821‐6. [DOI] [PubMed] [Google Scholar]
Wright 1970 {published data only}
- Wright GL, Harper J. Fusidic acid and lincomycin therapy in staphylococcal infections in cystic fibrosis. Lancet 1970;1(7636):9‐14. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CF START 2016 {published data only}
- 2016‐002578‐11. CF START [The cystic fibrosis (CF) anti‐staphylococcal antibiotic prophylaxis trial (CF START); a randomised registry trial to assess the safety and efficacy of flucloxacillin as a long‐term prophylaxis agent.]. www.clinicaltrialsregister.eu/ctr‐search/search?query=2016‐002578‐11 2016.
Additional references
Armstrong 1995
- Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995;310(6994):1571‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 1996
- Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatric Pulmonology 1996;21(5):267‐75. [DOI] [PubMed] [Google Scholar]
Beardsmore 1994
- Beardsmore CS, Thompson JR, Williams A, McArdle EK, Gregory GA, Weaver LT, et al. Pulmonary function in infants with cystic fibrosis: the effect of antibiotic treatment. Archives of Disease in Childhood 1994;71(2):133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chrispin 1974
- Chrispin AR, Norman AP. The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatric Radiology 1974;2:101‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higggins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kerem 1992
- Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. New England Journal of Medicine 1992;326(18):1187‐91. [DOI] [PubMed] [Google Scholar]
Knudson 1983
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow‐volume curve with growth and aging. American Review of Respiratory Disease 1983;127(6):725–34. [DOI] [PubMed] [Google Scholar]
McCaffery 1999
- McCaffery K, Olver RE, Franklin M, Mukhopadhyay S. Systematic review of antistaphyloccal antibiotic therapy in cystic fibrosis.. Thorax 1999;54:380‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG, for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Morison 1997
- Morison S, Dodge JA, Cole TJ, Lewis PA, Coles EC, Geddes D, et al. Height and weight in cystic fibrosis: a cross sectional survey. Archives of Disease in Childhood 1997;77(6):497‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillarisetti 2011
- Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, Ranganathan S. Infection, inflammation, and lung function decline in infants with cystic fibrosis. American Journal of Respiratory & Critical Care Medicine 2011;184(1):75‐81. [DOI] [PubMed] [Google Scholar]
Shwachman 1958
- Shwachman H, Kulczycki LL. Long term study of one hundred five patients with cystic fibrosis. American Journal of Diseases of Children 1958;96:6‐15. [DOI] [PubMed] [Google Scholar]
Walters 1999
- Walters E, Walters JAE. Many reports of RCTs give insufficient data for Cochrane reviewers. BMJ 1999;319(7204):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Smyth 2003
- Smyth AR, Walters S. Prophylactic anti‐staphylococcal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001912] [DOI] [PubMed] [Google Scholar]
Smyth 2014
- Smyth AR, Walters S. Prophylactic anti‐staphylococcal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD001912.pub3] [DOI] [PubMed] [Google Scholar]


