Abstract
In this third paper the 1962 He3 Scale of Temperatures is evaluated both as to its precision and its deviations from the thermodynamic Kelvin Scale. Various thermodynamic quantities of He3 consistent with the 1962 He3 Scale are derived and listed. The correction to an observed vapor pressure for small amounts of He4 is discussed and tabulated. A description is given of the method of multiple variable least squares analysis used for deriving the final scale equation and for re-analysis of isotherm data. Finally the present status of the 1962 He3 Scale is discussed along with some considerations for the future.
1. An Evaluation of the 1962 He3 Scale
1.1. Fit of the Input 1961 L.A.S.L. Vapor-Pressure Data
One factor in evaluating the 1962 He3 Scale is the fit of the input vapor-pressure data of Part I [46] to the Scale. Figure 1 and table 1 of Part II [6] show the deviations of the observed data from the final scale as T62(P3) — T58(P4). The symbol T62 is the temperature on the 1962 He3 Scale corresponding to a He3 vapor pressure, P3, while T58 is the temperature on the 1958 He4 Scale [2] corresponding to the experimentally determined isothermal He4 vapor pressure, P4. The standard deviation of the data from the scale is 0.25 mdeg and the maximum deviation over the full range is 0.6 mdeg. The data may not scatter completely randomly. For example, the data points just below 2 °K are all below the 1958 He4 Scale and the points just above 2 °K are all above the 1958 He4 Scale. Hence, if one wished to obtain the vapor pressure of He3 which is most probably isothermal with a given He4 vapor pressure, a slightly better value (in terms of consistency with the observed data) may be obtained by drawing a smooth curve through these data points or by using direct interpolation equations as discussed in Part I. However, the overall fit of the Scale to the input data is very satisfactory in comparison with the errors of measurement of the two vapor pressures.
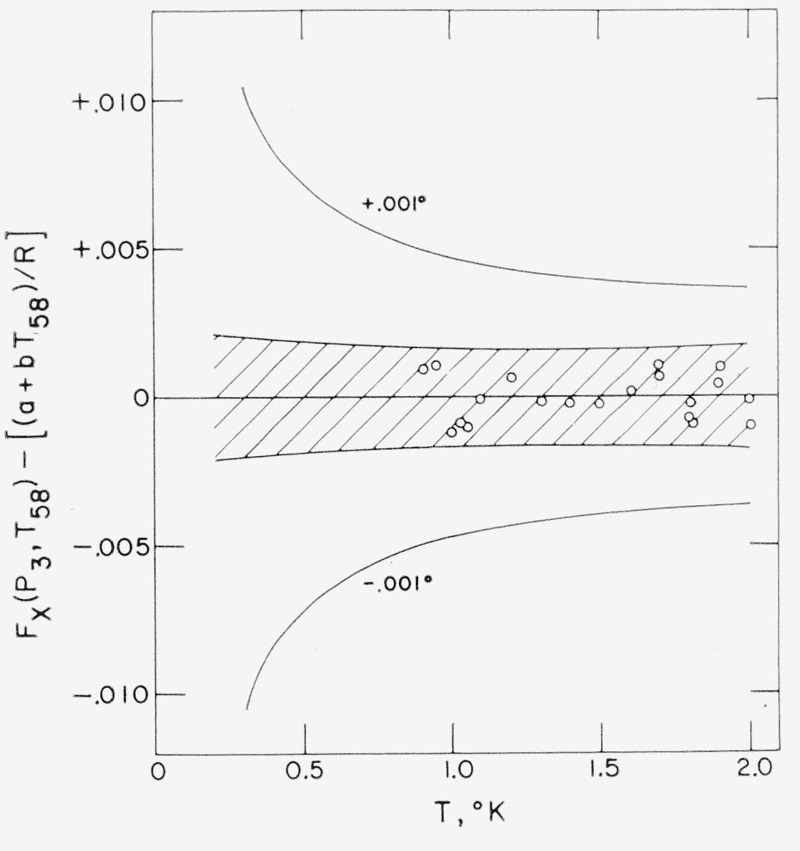
Figure 1.— Deviations from the ETE Scale, eq (1).
The circles are deviations from the fitted equation of the function Fx(P3, T58) calculated from eq (2) for each input (P3, T58) data point. The central cross-hatched area is the 95 percent prediction interval [53] for a prediction of Fx(P3, T) from eq (1) as calculated from the ± values for a and b given in eqs (3) and (4). The outermost solid curves represent a change in Fx(P3, T) corresponding to a 1 mdeg change in temperature and are calculated as 0.001 T(dln P3/dT)sat.
Table 1. Effect of possible systematic or random errors on the ETE scale, eq (1).
Systematic or random changes in the constants a and b of eqs (3) and (4) and of temperatures on the ETE scale below 1 °K are listed for the following arbitrary, but plausible, cases:
Case 1. the fit of the input (P3, T58) data expressed as the 95 percent confidence limit [53] for the prediction of a value of ln P at any single temperature (see fig. 1);
Case 2. random errors of ±3 percent in fx (Csat);
Case 3. random errors of ±3 percent in ϵ, eq (4a) of Part II;
Case 4. a systematic error of −3 percent in all Csat values, and hence in fx(Csat);
Case 5. a systematic error of +3 percent in all values of ϵ;
Case 6. the difference between the empirical function, fx(VL), and the numerically integrated values of the thermodynamic term, ; and
Case 7. the increase of all values of input temperatures between 0.9 and 2.0 °K by adding 0.002 deg to each T58.
| Case | Δa | Δb | ΔT |
||||
|---|---|---|---|---|---|---|---|
| 1.0 °K | 0.8 °K | 0.6 °K | 0.4 °K | 0.2 °K | |||
| J/mole | J/mole deg | mdeg | mdeq | mdeq | mdeg | mdeq | |
| 1- | ±0.006 | ±0.004 | ±0.4 | ±0.3 | ±0.3 | ±0.2 | ±0.1 |
| 2- | - | - | 0. 0 | ±0.1 | ±0.2 | ±0.4 | ±0.4 |
| 3- | - | - | ±0. 2 | ±0.1 | 0.0 | 0.0 | 0.0 |
| 4- | 0. 069 | −0.060 | 0.2 | 0.5 | 0.8 | 1.0 | 1.0 |
| 5- | 0. 073 | −0.076 | 0.2 | 0.4 | 0.5 | 0.6 | 0.5 |
| 6- | - | - | −0.1 | −0.1 | −0.1 | 0.0 | 0.0 |
| 7- | 0.094 | −0.026 | - | 1.7 | 1.5 | 1.2 | 0.7 |
1.2. Fit of the Experimental Thermodynamic Equation (ETE) Scale
Below 0.9 °K the 1962 He3 Scale was evaluated by examining its fit to the ETE Scale described in Part II. The ETE Scale is defined by a linear equation fitted to an empirical function, Fx(P3, T), of He3 vapor pressure and temperature,
| (1) |
As shown by the full line curve in figure 1 of Part II, temperatures calculated from eq (1) are in good agreement with the 1962 He3 Scale; nowhere below 2 °K do the scales differ by more than 0.4 mdeg. the 1962 He3 Scale, defined by eq (9b) of Part II, is therefore in effect an ETE scale from 0.2 to 2° and an empirical scale above 2 °K.
The effect of possible errors in the various terms of the equation for the ETE scale, over the temperature range from 0.2 to 1.0 °K, should also be considered in evaluating the 1962 He3 Scale. The ETE scale was obtained from a least squares fit of the He3-He4 vapor-pressure intercomparisons between 0.9 and 2.0 °K to eq (1). The function Fx(P3, T) is calculated for each experimental P3,T58-observation; deviations of this “observed” value of Fx(P3,T) from the fitted value, [(a+bT58)/R], from eq (1) are shown as circles in figure 1. The function Fx (Ρ3, Τ) is derived in Part II and in ref. [10] as
| (2) |
where i0 is the chemical constant including the nuclear spin degeneracy; fx(VL) is an empirical power series representing the theoretical and exact integral term,
involving the molar liquid volume, VL; fx(Csat) is an equation for the theoretical and exact double integral term of eq (3a) of Part II, , based on an empirical power series fitted between 0.2 and 2.0 °K to data for (Csat, the specific heat of the saturated liquid; and ϵ is the gas imperfection term, eq (4a) of Part II.
The least squares values for the coefficients a and b of eq (1), and their equivalent expressions from eqs (6) and (7) of Part II are
| (3) |
and
| (4) |
The plus-or-minus values for a and b were found from the fit of the data points to the straight line, a+bT. Mood [53] has given an equation for the prediction interval for a single prediction of Fx(P3, T) from eq (1) for any value of T. The ETE Scale is just the set of P3 values obtained by solving eq (2) for In P3 at any given temperature using the predicted value of Fx(P3,T). Hence a prediction interval for Fx(P3, T) can be expressed as an equivalent prediction interval in P3 by neglecting the contribution of possible errors in the other terms on the right hand side of eq (2).
The 95 percent prediction interval for a single prediction of Fx(P3, T) [with a 95 percent probability of containing the statistically “true” value of Fx(P3,T)] is shown as the central cross-hatched area in figure 1. The outermost curves in this figure show the change in Fx(P3, T) corresponding to a one millidegree change in temperature. The same prediction intervals have been converted to equivalent temperature scale errors as listed in table 1.
The effects of random errors of ±3 percent in fx (Csat) and ϵ on individual temperatures below 1° are also shown in table 1.
Systematic errors in fx(Csat), 𝜖 and in fx(VL) would be compensated between 0.9° and 2.0° by the least squares process of fitting the (P3, T58) data to eq (1). The scale below 1° would be skewed by such errors as is shown in table 1 for assumed 3 percent systematic errors in all values of Csat or ϵ. Table 1 also shows the systematic errors below 1° resulting from use of the approximate empirical function, fx(VL), instead of the graphically integrated values of the true thermodynamic function, f(VL) [see table 4 of Part II].
Table 4. Thermodynamic properties of He3 and vapor pressures of He4 consistent with the 1962 He3 Scale.
| T | P3 | P4(T58) | at X = 1 (see note†) | Csat | SL | Virial coeff |
VG | VL | L | ||
| B | C | ||||||||||
| °K | microns Hg at 0 °C and std. g | deg−1 | J/deg mole | J/deg mole | cm3/mole | cm6/mole2 | cm3/mole | cm3/mole | J/mole | ||
| 0 | 0 | 0 | 36.8346 | 20.56 | |||||||
| 0.2 | 0.0121 | 73.32 | 0. 23 | 2.728 | 3.703 | −1350 | 6409 | 103×107 | 36.7809 | 24.39 | |
| 0.4 | 28.115 | 21.04 | 0.47 | 3.146 | 5.731 | −672.5 | 4532 | 887×103 | 36.7203 | 27.97 | |
| 0.6 | 544.49 | 0.2812 | 10.57 | 0. 70 | 3.477 | 7.071 | −446.7 | 3700 | 68274 | 36.7197 | 31.42 |
| 0.8 | 2892.5 | 11.445 | 6.650 | 0. 88 | 3.812 | 8.117 | −333.8 | 3204 | 16909 | 36.7849 | 34.61 |
| 1.0 | 8842.4 | 120.00 | 4.721 | 0. 92 | 4.222 | 9.010 | −266.0 | 2866 | 6776.6 | 36.9073 | 37.51 |
| 1.2 | 20163 | 625.02 | 3.613 | 0. 92 | 4.753 | 9.824 | −220.9 | 2616 | 3476. 7 | 37.0897 | 40.08 |
| 1.4 | 38516 | 2155.4 | 2.908 | 0.91 | 5.414 | 10.605 | −188.6 | 2422 | 2060. 7 | 37.3517 | 42.29 |
| 1.6 | 65467 | 5689.9 | 2.425 | 0.89 | 6.201 | 11.378 | −164.4 | 2266 | 1338.9 | 37.7151 | 44.07 |
| 1.8 | 102516 | 12466 | 2.077 | 0. 88 | 7.079 | 12.159 | −145.6 | 2136 | 925.5 | 38.201 | 45.34 |
| 2.0 | 151112 | 23767 | 1.815 | 0. 86 | 7.983 | 12.951 | −130.6 | 2027 | 667.8 | 38.854 | 45.99 |
| 2.2 | 212673 | 40466 | 1.611 | 0. 810 | −118.2 | 1932 | 496.6 | 39.680 | 45.91 | ||
| 2.4 | 288613 | 63304 | 1.448 | 0. 781 | —108.0 | 1850 | 376. 7 | 40. 734 | 44.94 | ||
| 2.6 | 380383 | 93733 | 1.317 | 0. 754 | −99.3 | 1777 | 288.8 | 42.124 | 42.84 | ||
| 2.8 | 489549 | 132952 | 1.210 | 0. 728 | −91.8 | 1713 | 221. 0 | 44.049 | 39.11 | ||
| 3.0 | 617907 | 182073 | 1.122 | 0. 705 | −85.4 | 1655 | 163.1 | 46.818 | 32.25 | ||
Here × is the He3 mole fmcLioll.
The He4 IlIole fraction, (1–×), is used to make the impurity correction.
Another possible source of systematic error is a smooth deviation of the 1958 He4 Scale from the thermodynamic Kelvin Scale. The effect on the ETE scale of adding 2 mdeg to each scale input temperature is listed in table 1.
The overall effect on the 1962 He3 Scale of random errors in the specific heat of liquid He3, the virial coefficient equations, and the 1958 He4 Scale might amount to as much as three millidegrees. Durieux [54] has analyzed the 1962 He3 Scale for the effects of possible errors and has reached similar conclusions.
1.3. Fit of the Argonne Laboratory Vapor-Pressure Data
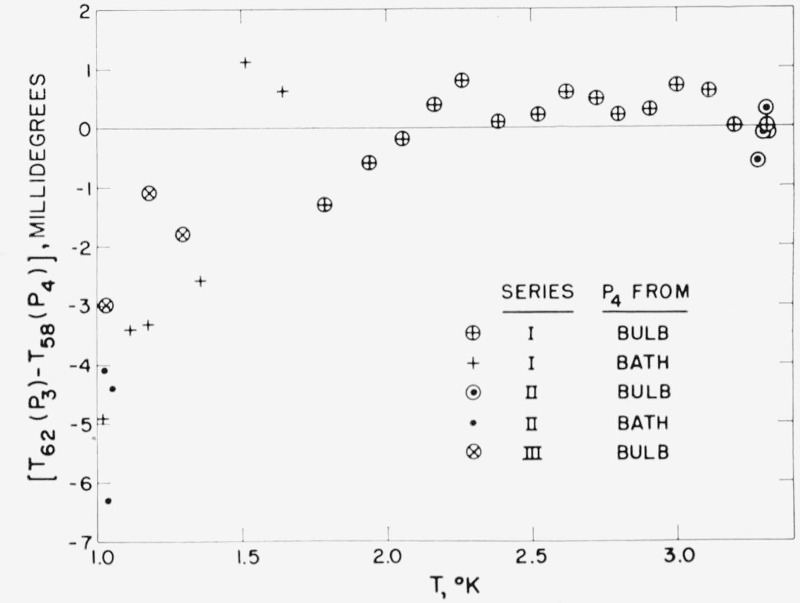
The He3-He4 vapor-pressure intercomparisons of Abraham, Osborne, and Weinstock [5] have been used in deriving all previous He3 temperature scales, but these were rejected for the 1962 He3 Scale because of apparent thermodynamic inconsistency (see refs. [10 and 45]) with recent precise measurements of the latent heat of vaporization of He3 by the same workers [4, 55]. The fit of the AOW vapor-pressure data to the 1962 He3 Scale is shown in figure 2. Above 1.9° the agreement is within 0.5 mdeg. Below 1.5° the AOW data deviate systematically from the 1962 Scale indicating either that their He3 vapor pressures were lower or that their He4 pressures were higher than the data of Sydoriak and Sherman (Part I). For encircled points in figure 2, a He4 vapor pressure bulb was fastened to the He3 bulb. The He4 pressure may have been high because of insufficient correction for effects due to the refluxing superfluid film. For the uncircled crosses and dots, the He4 bath pressure was measured in the pumping line in a warm part of the apparatus. The corrected He4 pressures as published may have been high because the effluent gas was significantly colder than the tip of the pressure-sensing tube, thus increasing the actual thermomolecular pressure difference.
Figure 2.— The deviation in millidegrees of the 1950 Abraham, Osborne, and Weinstock [5] (P3, P4) data from the 1962 He3 Scale: T62(P3) − T58(P4).
The uncircled crosses and small dots are data points below the λ-point for which the He4 cryostat bath pressure was published as P4. For the encircled points a He4 vapor pressure bulb was fastened to the He3 bulb.
1.4. Fit of Heat-of-Vaporization Data
Heat-of-vaporization, L, data may be used to test the thermodynamic consistency of the temperature scale. The equations used may be either
| (5) |
with the vapor entropy, SG, calculated from the Sackur-Tetrode equation, or
| (6) |
from the Clausius-Clapeyron equation. The use of these equations for testing He4 scales of temperature has been discussed extensively; see van Dijk and Durieux [25], Durieux [15], Berman and Mate [56], and Keller [43].
The liquid entropy at 1.0 °K (or any other temperature) may be calculated from eq (5) and data for Csat as
| (7) |
The Argonne (WAO) heat-of-vaporization measurements [4] were undertaken in order to determine the entropy of liquid He3 in this way.
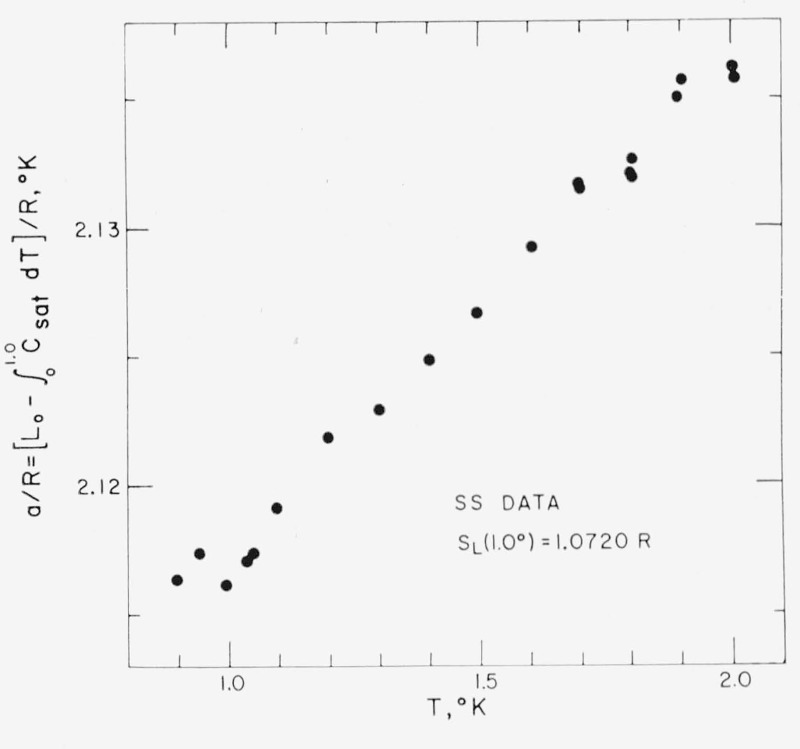
At one stage of the derivation of the 1962 He3 Scale, it was proposed [10] to use all the WAO heat-of-vaporization data and eq (7) to get an average value for SL(1.0°), which is just the coefficient, b, in eqs (1) and (4). Then, values of (a/R) = Fx(P3, T) − (bT/R) from eq (1) were computed for every vapor-pressure datum point between 0.9 and 2.0 °K. This two-step method failed to converge on a stable, consistent pair of constants a and b, and led to the decision to undertake the new L.A.S.L. intercomparisons of the vapor pressures of He3 and He4. Analysis of the new intercomparisons [45] by this same two-step method also failed to yield a consistent set of a and b values, although these data showed much less scatter, as shown in figure 3. The heat-of-vaporization data were not used to determine the 1962 He3 Scale; instead a and b values were obtained by a least squares fit of the He3 vapor-pressure data to eq (1) using for T the T58 values that corresponded to the He4 vapor pressures observed concurrently with the He3 vapor pressures.
Figure 3.— Demonstration of the remaining thermodynamic inconsistency between eqs (1) and (7) involving the He3 latent-heat data, (L, P3), of Weinstock, Abraham, and Osborne [4], [55]; the (P3, P4) intercomparisons of Sydoriak and Sherman [46]; and the 1958 He4 Scale [2], T58(P4).
The indicated value of SL(1.0°) is the average of nine values calculated from eq (7) for the WAO (L,P3) data and is about 1 percent lower than the 1962 He3 Scale value for that entropy, eq (4). The solid circles are the individual values of the theoretical constant, (a/R), in eq (1) calculated for each (P3, T58) data point as Fx(P3, T58) — [SL(1.0°) T58/R] using the indicated average value of SL(1.0°).
The 1958 WAO heat-of-vaporization data may be used to test the 1962 He3 Scale in the manner explained by Durieux [15]. In terms of an apparent heat of vaporization, La (defined as the heat necessary to evolve a mole of gas outside of a calorimeter), eq (6) becomes
| (8) |
where the compressibility coefficient, from the density virial form of the equation of state.
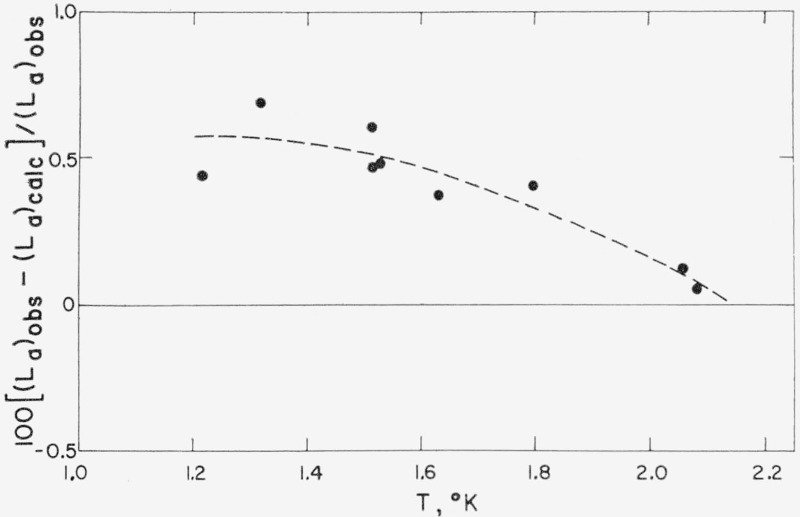
As shown in figure 4, the observed values of La average about 0.5 percent higher than the values calculated from the 1962 He3 Scale using eq (8), a deviation considerably in excess of the estimated 0.1 percent accuracy [55] of the La measurements. Durieux [15] calculates that the La values of Berman and Mate [56] for He4 average 0.76 percent higher than the values calculated from the 1958 He4 Scale between 2.2 and 3.0 °K. These differences may be due in part to a deviation of the 1962 He3 and 1958 He4 Scales from the true thermodynamic temperature scale. An estimate of such a difference may be obtained by neglecting the variation of (d ln P/dT)sat and Z in eq (8). In this case δT/T ≈ (1/2)(δLa)/La. From the dashed curve drawn through the He3 data of figure 4 this estimate of δΤ varies from 3 mdeg at 1.2 °K to 1 mdeg at 2.0 °K, a not unreasonable range of deviations. For the He4 data, this approximation yields values from 8 mdeg at 2.2 °K up to 11 mdeg at 3.0 °K which are much larger than appears reasonable for the departure of the 1958 He4 Scale from the thermodynamic scale. An error in the temperature scale also would cause both (d ln P/dT)sat and Z to change.
Figure 4.—
Deviations of the values of La, the apparent latent heat of vaporization (see sec. 1.4), observed by Weinstock, Abraham, and Osborne [4, 55] from values of La calculated for the 1962 He3 Scale from eq (8) using eqs (10) and (11).
1.5. Fit of Gas Thermometer, Isotherm, and Acoustic Interferometer Measurements
In principle, gas thermometer, isotherm and acoustic interferometer measurements of the absolute temperature associated with a He4 vapor pressure may be used to check the 1962 He3 Scale by directly interpolating the He4 vapor pressure to an isothermal He3 vapor pressure. The direct interpolation equations and table described in Part I are usable for this interpolation of He4 vapor pressures. Since the 1962 He3 Scale has been made to agree so closely with the 1958 He4 Scale, at least in terms of the (P3, P4) data of Part I, little really significant new information would be expected from this kind of comparison.
Van Dijk [57] has critically reanalyzed all published gas thermometer and isotherm measurements and compared them with the 1958 He4 Scale. The majority of these measurements between 1.5 and 3.3 °K seem to indicate that the thermodynamic temperature may be several millidegrees higher than T58, although the data scatter over a range of about ±10 mdeg from T58.
Two preliminary acoustic interferometer results of Cataland and Plumb [58] indicate thermodynamic temperatures that are 3 ± 2 mdeg higher than T58 at 2.0 and 2.2 °K.
The isotherm measurements of Keller [11, 12] are generally conceded to be the most accurate in the liquid helium temperature range, and are the only data for gaseous He3. During the derivation of the 1962 He3 Scale, the isotherm data of Keller were reanalyzed [10, 48] by Deming’s method [47] of least squares adjustment with errors in more than one measured variable (see appendix A). For the isotherm measurements the observed pressures for each data point were corrected or normalized to a calculated value which would have been observed if the cell volume were at the normalized temperature for the set of data points. This normalized temperature, TN,58, was taken as the temperature on the 1958 He4 Scale corresponding to , the average He4 vapor pressure for the set of data. The values of and TN, 58 are listed in tables 2 and 3. The method of normalization was essentially the same as that described in detail by Keller [11].
Table 2. Summary of reanalysis of Keller’s gaseous He3 isotherms [12].
The isotherm designations, the data points, and the method of normalization for each isotherm are the same as those used by Keller. is the average He4 vapor pressure for each isotherm and is_the average He3 vapor pressure for the α isotherm. is the normalized temperature for each isotherm and is the 1958 He4 Scale temperature corresponding to . is the directly interpolated He3 vapor pressure corresponding to , using the direct interpolation equations described in Part I, and is the 1962 He3 Scale temperature corresponding to . The values of Tiso, B, and C listed under the heading “Three-constant fit” were obtained by solving eq (9) using Deming’s method of least squares adjustment with errors in two measured variables (see appendix A). The standard errors and the weighted variance are calculated in accordance with the statistical formulas given by Deming [47]. The two constant fit values of Tiso and B were obtained by solving eq (9) assuming C=0. For the For the γ, δ, and ϵ isotherms the variance of the three-constant fit is lower and the values of C are greater than the magnitude of their statistically assigned errors. Therefore the three constant fit solutions were used for fitting He3 virial coefficient equations, eqs (10) and (11), and for the temperature differences shown in figure 2 of Part II.
| Isotherm | Units | γ | δ | α | ϵ | β |
|---|---|---|---|---|---|---|
| No.of points | 18 | 18 | 11 | 10 | 8 | |
| mm Hg | 489.52 | 178. 09 | 36.16 | 13.070 | 3.782 | |
| T58() | mdeg | 3779.1 | 2985.3 | 2154.2 | 1813.4 | 1510.3 |
| P3() | mm Hg | 607. 70 | 197.43 | 105.33 | 52.21 | |
| mm Hg | 197.62 | |||||
| T62[P3()] | mdeg | 2985. 2±0.2 | 2154.4±0.2 | 1813.1±0.2 | 1510.2±0.2 | |
| T62() | mdeg | 2.1550 | ||||
| THREE-CONSTANT FIT | ||||||
| Tiso- | mdeg | 3782.3 ±2.2 | 2992.4±1.6 | 2153.7±1.0 | 1816.4±0.8 | 1510.4±2.5 |
| B- | cm3/mole | −65.50±0.86 | −86.31 ±0.52 | −118.81 ±1.35 | −145. 08±1.61 | −162.15±10. 7 |
| C- | cm6/mole2 | 1192 ±306 | 1746±136 | 548±822 | 2611 ±1289 | −11,069±16, 390 |
| Variance | 7.0 | 13.8 | 6.1 | 0. 86 | 4.8 | |
| (T58–Tiso) | mdeg | −3.3±2.2 | −7.1±1.6 | 0.5±1.0 | −3. 0±0.8 | −0.1 ±2. 5 |
| (T62–Tiso)- | mdeg | −7.2±1.8 | 0.7±1.2 | −3.2±1.0 | −0.2±2.7 | |
| (T62()–Tiso) | mdeg | 1.3±1. 0 | ||||
| TWO-CONSTANT FIT | ||||||
| Tiso- | mdeg | 3774.4±1.3 | 2974.4±2.8 | 2153.2±0.6 | 1814. 9±0.4 | 1512.0±0. 7 |
| B- | cm3/mole | −62.18±0.19 | −79.66±0.33 | −117.92 ±0.26 | −141.87 ±0.29 | −169.30±1.15 |
| Variance- | 13.2 | 156 | 5.7 | 1.20 | 4.4 | |
Table 3. Summary of reanalysis of Keller’s gaseous He4 isotherms [11].
The various Quantities have the same meaning as in table 2. The three constant fits gave a statistically better fit for only one isotherm, so the results of the two constant fits have been accepted as being more meaningful and have been used for figure 2 of Part II.
| Isotherm | Units | A | B | C | D | E |
| No. of points | 12 | 14 | 12 | 17 | 9 | |
| mm Hg | 588.55 | 291.31 | 147.55 | 52.758 | 35.541 | |
| T58() | mdeg | 3954.2 | 3339.2 | 2864.0 | 2315.1 | 2147.3 |
| P3() | mm Hg | 528.42 | 254.50 | 195.21 | ||
| T62[P3()] | mdeg | 2864.0 ± 0.2 | 2315.0 ± 0.2 | 2147.6 ± 0.2 | ||
| TWO-CONSTANT FIT | ||||||
| Tiso | mdeg | 3953.7 ± 2.1 | 3338.4 ± 2.1 | 2863.5 ± 0.9 | 2316.7 ± 2.3 | 2148.9 ± 0.9 |
| B | cm3/mole | −83.97±0.66 | −103.60±0.78 | −124.16±0.63 | − 153.38±4.21 | −176.04±2.04 |
| Variance | 8.7 | 17.8 | 3.6 | 23.4 | 1.69 | |
| (T58 – Tiso) | mdeg | −1. 5±2.1 | 0.8±2.1 | 0.5±0.9 | −1.6±2.3 | −1.6±0.9 |
| (T62 – Tiso) | mdeg | 0.5±1.1 | −1.7±2. 5 | −1.3±1.1 | ||
| THREE-CONSTANT FIT | ||||||
| Tiso | mdeg | 3953.8±4.3 | 3335.3±3. 7 | 2860.9±1.5 | 2311.9±6.1 | 2146. 5±2.8 |
| B | cm3/mole | −82.16±3.52 | −99.80±3.78 | −118.47±2.86 | −132.1 ±25.7 | −162.6±15.8 |
| C | cm6/mole2 | −1452±2773 | −3133 ±3042 | −6741 ±3331 | − 50,490±60,020 | −38, 900±45,160 |
| Variance | 9.4 | 17.7 | 2.8 | 23.9 | 1.75 | |
For the isotherm measurements the quantity minimized by the least squares adjustment was Σ[Wp(P0−Pc)2+Wn(N0−Nc)2] where the independently observed variables are P0, the normalized helium gas pressure, and N0, the molar density. The fitted function was
| (9) |
where B and C are the second and third virial coefficients, T is the isotherm temperature for a normalized set of data, and Pc and Nc are the calculated or adjusted values of P and N. The individual weights, Wp and Wn, were calculated from Keller’s assignment of errors in the various observed quantities.
The results of the reanalysis of Keller’s gaseous He3 data [12] were fitted to empirical equations for the He3 second and third virial coefficients. These equations are
| (10) |
and
| (11) |
The deviations of both He3 and He4 isotherm temperatures from the 1962 He3 and 1958 He4 Scales are shown in figure 2 of Part II. Keller used a He4 vapor-pressure thermometer for all but one of his isotherms, but his observed P4’s have been interpolated to P3’s by use of the direct interpolation equations of Part I. The weighted average of the isotherm temperatures is 1.50 ± 1.0 mdeg above the corresponding T58 values and 1.52 ± 1.2 mdeg above the T62 values. For one isotherm He3 containing about 0.25 percent He4, was condensed in the vapor-pressure bulb. The corrected average pressure for pure He3 was 197.62 ± 0.02 mm Hg (T62 = 2.1550 °K) at an isotherm temperature of 2.1537 ± 0.0010 °K. It was concluded that nothing would be gained by trying to base a He3 scale more directly on these isotherm temperatures since the 1958 He4 Scale is based on these data. Moreover, the 1962 He3 Scale, as it has been set up to agree with the 1958 He4 Scale, adequately expresses the experimental He3—He4 vapor-pressure relation for most practical purposes.
2. Thermodynamic Properties of He3 Consistent with the 1962 He3 Scale
Two temperatures which are frequently given in discussing helium vapor pressure scales are the boiling point and the critical point. The boiling point of He3 on the 1962 He3 Scale, at a vapor pressure of 760 mm Hg at 0 °C and standard gravity, is 3.1905 °K. The temperature at the critical point for He3 was measured by Sydoriak and Sherman [46] by observing the He4 vapor pressure. This measured critical temperature on the 1958 He4 Scale was 3.3240 ± 0.0018 °K at a He3 critical pressure, Pc, of 873.0± 1.5 mm Hg. The calculated critical temperature on the 1962 He3 Scale is the temperature given by eq (9b) of Part II [6] corresponding to Pc and is 3.3246 ± 0.0017 °K. This close agreement with the directly measured temperature is quite gratifying and somewhat unexpected since the scale-defining equation was fitted over the full range of the vapor-pressure intercomparisons.
Table 4 gives a number of quantities which are consistent with the 1962 He3 Scale, including, for comparison, values of He4 vapor pressures from the 1958 He4 Scale. The concentration derivative of In ΡX, (d ln PX/dX)T, X = 1, where X is the mole fraction of He3 and PX is the corresponding mixture vapor pressure, is useful for correcting for the He4 impurity in a He3 vapor-pressure thermometer. This correction is discussed in section 3.
The table values for Csat, the specific heat of the saturated liquid, were calculated from the empirical equation, eq (3b) of Part II, fitted to specific-heat data for the derivation of the 1962 He3 Scale. The calculated values agree with the experimental data to ±1 percent; the deviations are given in table 2 of Part II. Values for SL, the entropy of the saturated liquid, were calculated from the relation
| (12) |
using the above-mentioned Csat equation and the 1962 He3 Scale value of SL( 1.0°), given in eq (4).
Values for the second and third virial coefficient were calculated from eqs (10) and (11) while VG, the molar volume of the saturated vapor, was calculated from the equation given for Z in eq (8). These calculated values of VG agree to within 1 percent with the smoothed fit to the experimental data of Kerr [59] up to 2.8 °K. The values of VL, the molar volume of the saturated liquid, are taken from the table of Kerr and Taylor [60]. The values of L, the heat of vaporization, are calculated from eq (6).
The heat of vaporization at absolute zero, L0, can be obtained from the fitted constant, a, of eq (3) if the specific heat integral is evaluated. The specific heat of liquid He3 under a pressure of a few centimeters of mercury has been measured by Anderson, Wheatley, and coworkers [61] down to a temperature of 0.015 °K. Their empirical equation for Cp, the specific heat at a constant pressure of 0.12 atm, is (Cp/R) = 2.89T−7.80T2+7.09T3 for 0<T<0.3°. Integration of this equation yields . This value is in excellent agreement with the value 0.333 ± 0.010 J/mole obtained by graphical integration of the smooth curve through recent specific heat data given by Strongin, Zimmerman, and Fairbank [62]. From the empirical equation for Csat used for the T62 scale, . Our corresponding value of L0 is 20.56 ± 0.07 J/mole.
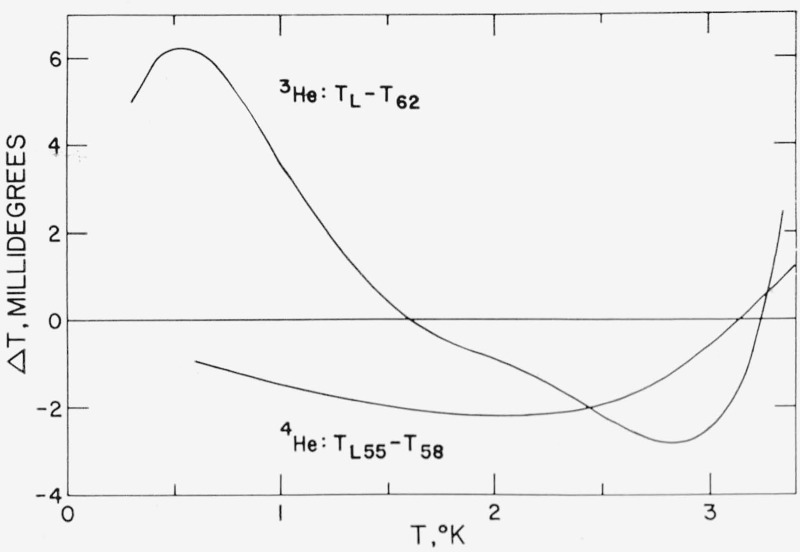
The entropy of the saturated liquid can be assumed to be approximately equal to from the 0.12 atm Cp equation of Anderson et al. [61] given above. From this approximation SL(0.23 °K) is 4.05 ± 0.17 J/mole deg. Strongin et al. [62] also have computed the entropy from their extrapolation of specific heat data to 0 °K. Their value for the entropy at 0.23 °K is 3.97 ± 0.17 J/mole deg. These values are in good agreement with the value 4.02 ± 0.25 J/mole deg obtained by Weinstock, Abraham and Osborne [4], who used eq (5) to calculate SL(1.5°) from their three separate latent heat measurements at that temperature. The equivalent of eq (12) was used by WAO to compute other entropies in the range of the then available specific heat data. The value of SL(0.23°) consistent with the 1962 He3 Scale, calculated from eq (12), is SL,(0.23°) = 4.09 ± 0.10 J/mole deg, estimating a ±2 percent limit of error for the specific heat integral term. The good agreement among these values is gratifying but it may be fortuitous. For example, the He3 temperature scale used by Weinstock et al. [4] to assign temperatures to observed He3 vapor pressures was the TL Scale, eq (9) of ref. [9]. The deviations of the TL Scale from the 1962 He3 Scale are shown in figure 5 and range from +6 to −3 mdeg. At 1.5 °K, however, the two scales are in good agreement as to the temperature. Thus the value of T used in computing both SG and L/T in eq (7) would give essentially the same value for liquid entropy at T ≈ 1.5 °K on the TL and 1962 He3 Scales. If, however, a value of L calculated from eq (6) is used in eq (7) instead of a WAO experimental value of L, significantly different values are obtained for SL depending upon which of the two scales, TL or 1962 He3, is used for the calculation of (dP3/dT)sat since there is a difference of about 0.4 percent in these derivatives on the two scales as inferred from the slope of the ∆T curve in figure 5 (see, for example, ref. [63]).
Figure 5.— Graphs of differences between old and new He3 and He4 temperature scales.
The He3 curve, TL(P3)—T62(P3), is a graph of the difference between the TL Scale [9] and the 1962 He3 Scale. The He4 curve is the difference between the TL55 Scale [25] and the 1958 He4 Scale given in table 6 of ref [2], It was intended in their derivations that the TL Scale would reproduce the TL55 Scale, and that the 1962 He3 Scale would reproduce the 1958 He4 Scale. The difference between the two curves is explained principally by the new (P3,P4) intercomparison data of Sydoriak and Sherman [46] used in deriving the 1962 He3 Scale.
3. Corrections to the Measured Pressure of a He3 Vapor-Pressure Thermometer for the He4 Impurity
Sydoriak and Sherman have discussed in Part I corrections to be applied to an observed pressure as measured in the laboratory in order to obtain the vapor pressure at the surface of liquid He3. The correction for a small He4 impurity will be discussed in more detail here.
The presence of He4 in liquid He3 thermometers lowers the vapor pressure below that of pure He3. Much of the He3 available for purchase up to the present has contained significant quantities of He4; even the He3 used for the 1962 He3 Scale input data measurements [46] contained about 0.04 percent He4. Specially purified He3 containing less than 0.01 percent is being made available for purchase through the Division of Research of the United States Atomic Energy Commission [64].
The correction for small amounts of He4 may be calculated from the approximate relation
| (13) |
where Px is the observed vapor pressure for mole fraction, X, of He3. Although very little data has been published for the vapor pressures of dilute solutions of He4 in He3, the liquid phase diagrams of Sydoriak and Roberts [32] and Esel’son and Berezniak [38] indicate that (dPx/dX)T is probably a constant for X≥0.9 and hence eq (13) may be valid for concentrations of up to 10 percent He4.
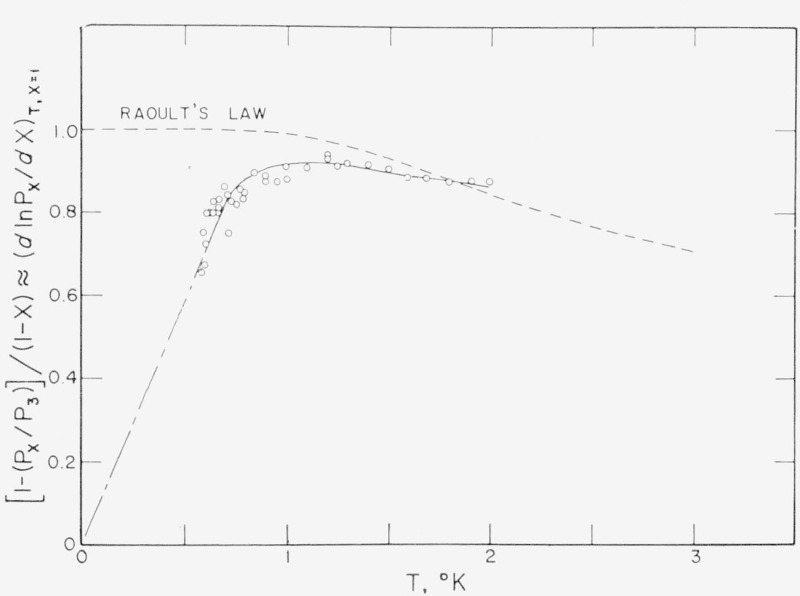
Smooth values of this derivative in reduced form,
| (14) |
are listed in table 4 and are compared with the raw data of Sydoriak and Roberts [32] in figure 6. Between 0.6 and 2.0 °K the table 4 entries and the solid curve in figure 6 were calculated from eq (14) using the X = 0.9 values from the smoothed table of ref. [32]. Below 0.6 °K the derivative has been assumed to go linearly to zero at 0 °K mainly because such an extrapolation is consistent with the data below 1 °K, as shown in figure 6. Actually for all practical purposes this derivative may be zero below about 0.2 °K since at these temperatures liquid He3—He4 mixtures with very small amounts of He4 undergo phase separation [65] and, when this happens, the vapor pressure becomes independent of further decreases in X. Above 2.0 °K, the table 4 values were calculated from Raoult’s law,
| (15) |
shown as the dashed curve in figure 6. That law is in fairly good agreement with all existing vapor-pressure data for X ≥ 0.89 and T ≥ 1.7 °K.
Figure 6.— The correction factor for He4 impurity.
The circles are the data points of Sydoriak and Roberts [32] for He3 mole fraction, X, of 0.897. The solid curve from 0.6 to 2.0 °K is drawn through the smoothed table values of ref. [32] for X=0.9. The long and short dashed curve is an arbitrary extrapolation to 0 ° K. The dashed curve is Raoult’s Law, eq (15).
The concentration of He4 in the saturated vapor will be much less than that of the liquid. This fact can cause fractionation and may require further correction in precise thermometry if much of the He3 in the thermometer system is not condensed.
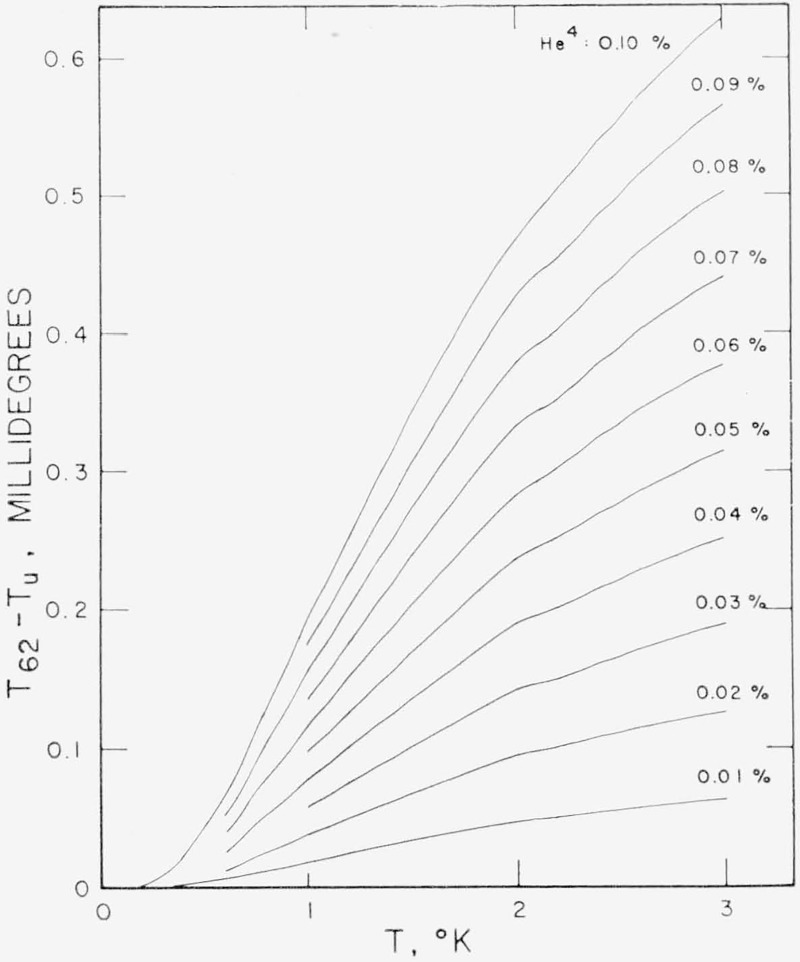
The correction to a temperature measurement made with a He3 sample containing 0.1 percent He4 in the liquid phase ranges from 0.02 mdeg at 0.4 °K to 0.71 mdeg at 3.2 °K. For most purposes the correction may be calculated from the approximate relation
| (16) |
The derivative below 2 °K may be interpolated from a curve through the values listed in table 4 or from figure 6. Above 2 °K the derivative can be easily calculated from eq (15). A careful experimental determination of the correction above 2 °K would improve the accuracy of this correction. Figure 7 may be used to read the temperature correction directly for known He4 concentrations in the liquid.
Figure 7.— Correction for He4 impurity, (T62 —Tu), to be added to the uncorrected temperatures, Tu, calculated from the observed vapor pressure and the 1962 He3 Scale.
The correction is calculated as T62—Tu=(1—X) (dlnPx/dX)T,X=1/(dlnP3/dT) based on eqs (13) and (14) and using the derivative values listed in table 4. The curves can be used for ten times the indicated percent of He4 in the liquid if the ordinate is multiplied by ten.
4. Status of the 1962 He3 Scale of Temperatures
The 1962 He3 Scale was presented [66] at the Eighth International Conference on Low Temperature Physics in London, September 17–21, 1962. During the next week the new scale was discussed in detail by the Advisory Committee on Thermometry of the International Committee on Weights and Measures. A scale proposal along with ref. [54] has been published along with the minutes of the meeting of the Advisory Committee in Sèvres, France, September 26 and 27, 1962. Quoting from the draft of those minutes [67]:
“Il a estimé que l’Échelle 3He 1962 doit également être recommandée pour l’usage général, avec la désignation T62.
“Les deux échelles T58 (l’Échelle 4He 1958) et T62 peuvent être utilisées concurremment dans le domaine où elles sont valables. Cependant, quand il s’agit de l’adoption de cette nouvelle échelle 3He comme partie de l’E.Ι.Ρ.Τ., on doit prendre soin d’éviter toute ambiguité dans le domaine de recouvrement avec l’échelle 4He.” 3
The recommendation by the Advisory Committee of the 1962 He3 Scale was approved in October 1962, by the International Committee on Weights and Measures meeting in Sevres. The International Committee requested the United States and Russian Governments to take steps to make high-purity He3 available internationally for vapor-pressure thermometry, and to prepare and distribute known mixtures of He3 and He4 for the calibration of or the checking of the calibration of apparatus for measuring the isotopic purity of He3.
The status of the 1962 He3 Scale and proposed changes in the International Practical Temperature Scale have been discussed by Stimson [68] and Brickwedde [69].
5. Practical Lower Limits for He3 and He4 Vapor-Pressure Thermometry
Practical lower limits for helium vapor-pressure thermometry are determined by the desired precision of temperature determination, the accuracy with which the vapor pressure can be measured, and by how small or how well known the temperature difference is between the thermometric fluid and the object of the measurement.
Below about 2.2 °K, He3 can much more easily be made isothermal with an object the temperature of which is being measured than can He4, for the reasons discussed in Part I. A major factor in fixing a practical lower limit for He3 thermometry is the time necessary to achieve an equilibrium thermomolecular ratio, Pc/Pw, of cold pressure, Pc, to warm pressure, Pw, and more importantly, of sufficient data to permit accurate calculation of that ratio.
To measure temperatures from 0.3 to 0.25 °K to a millidegree precision using He3, vapor pressures from 1.9 to 0.24 μ would have to be measured to 4 or 5 percent accuracy. This accuracy in the pressure system is relatively easy to achieve. However, the error in Pc/Pw may amount to several percent because of marked quantum effects in the gas viscosity and because no He3 measurements have been made of the empirical thermal transpiration coefficients postulated by Weber [70]. The He3 thermomolecular pressure measurements made by Roberts and Sydoriak [37] showed that for a cold temperature of 2 °K observed He3 Pc/Pw ratios from 1.0 to 0.5 agreed within 1 percent with ratios calculated for He4 using the Weber-Schmidt equation [71].
Roberts and Sydoriak also discuss the possible effect of the low-temperature viscosity on pressure ratios in the tubing at liquid helium temperatures. For warm and cold temperatures, Tw and Tc, below 5 °K and pressure ratios close to 1, eq (5) of ref. [37] is (Pc/Pw)2= 1 — 0.005642 where R is the tubing radius in centimeters and pw is in microns of mercury. In order to be reasonably sure that the calculated thermomolecular ratio is within 4 or 5 percent of the true ratio for He3 it is suggested that the calculated (pc/pw) be kept greater than 0.5 in the rest of the pressure sensing tube. For the stepped pressure sensing tube used by Sydoriak and Sherman with dimensions shown in figure 4 of Part I [46], this suggests a practical lower limit of microns or a vapor pressure of 1.16 μ (T62 = 0.287°).
The practical lower limit for He4 vapor-pressure thermometry is strongly dependent on numerous details of the particular technique and apparatus used. In many cases experimental temperatures are determined from the vapor pressure of the bath in which the experimental apparatus is immersed. For bath temperatures and with proper corrections for pressure drops associated with film reflux and/or motion of effluent vapor from the bath, temperatures may be determined down to about 1 °K. Figure 1 of Part I shows errors due to film reflux pressure drops for typical sizes of pressure sensing tubing. However, for temperatures determined by thermal contact with a closed-bulb He4 vapor-pressure thermometer the practical lower limit is raised to just below the λ point, because of the large and variable temperature discontinuity at the bulb wall associated with recondensation of the refluxing film. Figure 1 of Part I also shows this “correction” for a variety of conditions.,
If the bulb wall is a poor thermal conductor, a still higher limit is imposed due to the effects of the density maximum at about 2.18 °K, discussed by Chase, Maxwell, and coworkers [72].
In the case of precision measurements using He3 thermometry, the publication of only a few pertinent details, such as the diameters and temperatures of any stepped junctions in the pressure sensing tube, will enable future correction of data as improved thermal transpiration corrections become possible.
6. Conclusion and Considerations for the Future
These papers on the development of the 1962 He3 Temperature Scale have emphasized marked differences between liquid He3 and He4. One is in the low-temperature variation of the liquid entropy. The quantum mechanical exchange forces in liquid He3 lead to appreciable correlation of the nuclear spins in the liquid at temperatures as high as 1 °K. The difficulty in fitting the entropy curve with a simple analytical expression has led to difficulty in attaching theoretical significance to certain terms in empirical vapor-pressure equations at temperatures below the temperature of fitted data.
The superfluid transition of liquid He4 makes accurate isothermal intercomparisons of He3 and He4 vapor pressures difficult below the He4 λ-point. The 1961 intercomparison data of Sydoriak and Sherman [46], however, are considered to be valid to a few tenths of a millidegree. The 1962 He3 Scale, which is based on these intercomparisons, is believed to be in agreement with the 1958 He4 Scale to within two or three tenths of a millidegree over the full range of the intercomparison data, from 0.9 °K to the critical point, 3.324 °K. A lower limit for practical He3 vapor-pressure thermometry is 0.25 °K corresponding to a He3 vapor pressure of 0.24 μ Hg.
A number of independent measurements, including measurements of the latent heats of vaporization of He3 and He4, precise isotherm measurements, and preliminary acoustical interferometer measurements, indicate that both the 1962 He3 Scale and the 1958 He4 Scale may be 2 or 3 mdeg lower than true thermodynamic temperatures between 1 and 3.3 °K When more accurate data on the thermodynamic properties of He3 and He4 are available, together with better determinations of absolute thermodynamic temperatures, the precise intercomparison data will allow both the He3 and the He4 scales to be adjusted consistently and simultaneously. He3 and He4 scales, thermodynamically consistent between 1 and 3 °K, may be extended by precision para magnetic salt thermometry above 3 °K using He4 and below 1 °K using He3. Also, the scales may be extended downward by thermodynamic calculations using coefficients evaluated between 1 and 3 °K as in the development of the 1962 He3 Scale and the TL55 He4 Scale [25]. Further measurements on He3 that would be helpful in improving the Scale are measurements of the vapor pressure and thermo-molecular pressure ratio down to temperatures of 0.25 °K, more accurate specific heat measurements on the liquid below 2 °K, and specific heat, latem heat, and high-precision vapor-density measurements above 2 °K.
7. Appendix A. Method of Multiple Variable Least Squares Analysis
In the usual least squares adjustment of experimental data the implicit assumption is made that only one measured quantity, often called the dependent variable, is in error. This dependent variable is measured as a function of one or more independent variables. The usual procedures for the adjusting of sets of observations to an empirical or theoretical equation assume no error in the independent variables. Frequently, however, the “independent variable” is also determined by a physical measurement and is just as subject to error as the dependent variable.
Deming [47] has discussed a method of adjustment of sets of observations of two or more variables when all variables are subject to error. His method is essentially equivalent to statistical weighting of the data. The method has been coded by P. McWilliams of the Los Alamos laboratory for use with the Gaussian method of iterative least squares analysis adapted by Moore and Zeigler [73] for high-speed computers. We will give a simple example of the method of analysis with exaggerated scatter and uncertainty.
7.1. Simple Example
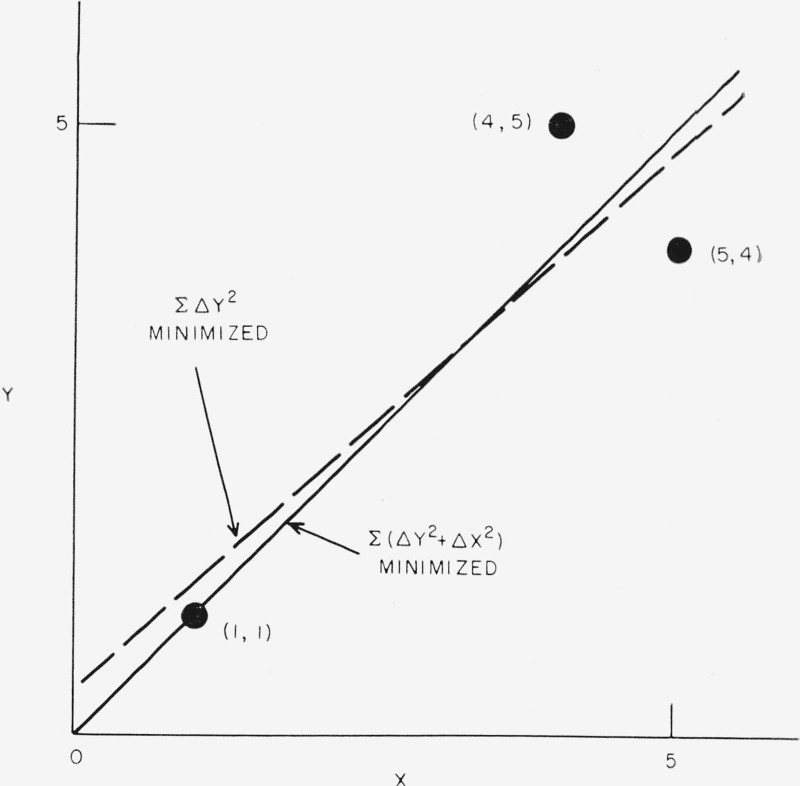
As a simple illustration consider fitting the best straight line to the following three points:
The dashed line in figure 8 shows the fit of the usual least squares solution of Y=a+bX assuming that only Y is in error and equally weighting the points. The solid line, Y=X, is the solution assuming that the X and Y values are all equally subject to equal absolute errors. The general case of fitting to a straight line is discussed in detail by Deming. For our special case of equal weighting, the two solutions go through the centroid but have different slopes.
Figure 8.— Simple example of the least squares fit of a straight line to the three observed points shown as dots.
The dashed line is the usual least squares solution of Y=a+bX which minimizes Σ(Y0—Yc)2. The solid line is the solution assuming both X and Y are subject to equal absolute errors.
Actually the quantity which is minimized by the least squares solution is
| (17) |
where the subscript o indicates the observed value; subscript c indicates the calculated or adjusted value; and wy, i and wx, i are the weights of the ith of M sets of observations. Thus in figure 8 the solid line is identified as minimizing
since all the individual weights are equal. ∆Y is defined as (Y0—Yc) and ∆X = (X0—Xc).
The geometrical relation between ∆Y and ∆X that is assumed in Deming’s method of solution is illustrated in figure 18 of ref. [47]. The relation for each point depends on the individual weights for that point.
If wx=wy, this condition requires that the line from the observed point, (X,Y0), to the calculated point, (Xc, Yc), be perpendicular to the calculated curve.
7.2. General Discussion of Method
In many cases the least squares problem can be expressed as minimizing eq (17) subject to M equations:
| (18) |
As an example consider a case in which three coefficients, a, b, and c, are to be fitted by least squares to the relation Y = f(X, a, b, c). We take
| (19) |
Using his geometrical relation between ΔY and ΔX, and using the Gaussian method of linearization of the problem by a truncated Taylor’s Series expansion of the function, Deming derives a typical least squares solution “normal” equation as
| (20) |
In this equation, the subscripts a,b, and c designate the partial derivatives of F with respect to the respective coefficients; Δα=α1—ac, the difference between the initial value, α1, and the calculated or adjusted value, ac; F0, i=F(X0, i, Y0, i, α1, b1, c1); and the weights are
| (21) |
Fx,i and Fy,i are the partial derivatives of F with respect to X and F, respectively. Where a set of observations, Χ0, i and Y0,i, can be assigned respective standard deviations 𝜎χ,i and 𝜎y,i, we take the weights as:
| (22) |
If variations in X and Y are independent, substitution of eq (22) in eq (21) yields
| (23) |
and hence the method is equivalent to statistical weighting of the individual sets of observations.
Using the Gaussian method, several iterations using the successively improved values of the coefficients may be used. The partial derivatives are usually evaluated using the observed values of X and Y in each iteration.
As pointed out by Deming, an advantage of the method is that the solution is unequivocal; i.e., the coefficients are independent of variations in the way of writing the equation. Thus a straight line can be written as either Y=a+bX or X=−a/b+Y/b and correspondingly F would be (Y—a—bX) or (X+a/b—Y/b). The values of a and b and the adjusted points will be the same to within higher powers of the residuals, ∆X and ∆Y.
The least squares code used computes standard deviations of the fitted parameters based on the fit of the observed points to the calculated points. The code also permits computation of standard deviations of points calculated at interpolated or extrapolated points as well as at data points.
Acknowledgments
We are indebted to R. K. Zeigler and P. McWilliams for many discussions and for the development of the computer code.
Footnotes
This list of references applies to papers I, II, III, and IV of this series. For completeness and convenience, it is reproduced in its entirety at the end of each paper.
Much of the material in this paper III has been included in a review chapter [52] on the development of the scale.
Work performed under the auspices of the U.S. Atomic Energy Commission.
“It (the Advisory Committee) has deemed that the 1962 He3 Scale ought equally to be recommended for general usage with designation T62.
“The two scales T58 and T62 can be used concurrently in the range where they are valid. However, when it is a question of adopting this new He3 scale as a part of the I.P.T.S. (International Practical Temperature Scale), care should be taken to avoid any ambiguity in the region of overlap with the He4 scale.
References*
- [1].Sydoriak S. G., Roberts T. R., and Sherman R. H., Proc. 7th Intern. Conf. Low Temperature Physics, ed. Graham G. M. and Hollis-Hallett A. C. (Univ. of Toronto Press, Toronto, 1961), ch. 30, p. 717. [Google Scholar]
- [2].Brickwedde F. G., van Dijk H., Durieux M., Clement J. R., and Logan J. K., J. Res. NBS 64A (Phys. & Chem.), 1 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brewer D. F., Sreedhar A. K., Kramers H. C., and Daunt J. G., Phys. Rev. 115, 836 (1959). [Google Scholar]
- [4].Weinstock B., Abraham B. M., and Osborne D. W., Nuovo Cimento Suppi. 9, 310 (1958). [Google Scholar]
- [5].Abraham B. M., Osborne D. W., and Weinstock B., Phys. Rev. 80, 366 (1950). [Google Scholar]
- [6].Sydoriak S. G., Roberts T. R., and Sherman R. H., Paper II of this series.
- [7].Roberts T. R., Sherman R. H., and Sydoriak S. G., Paper III of this series.
- [8].Sherman R. H., Sydoriak S. G., and Roberts T. R., Paper IV of this series. See also Los Alamos Scientific Lab. Rept. LAMS 2701 (July 1962).
- [9].Sydoriak S. G. and Roberts T. R., Phys. Rev. 106, 175 (1957). [Google Scholar]
- [10].Roberts T. R., Sydoriak S. G., and Sherman R. H., Proc. 4th Symp. Temperature, Its Measurement and Control in Science and Industry, ed. Herzfeld C. M. (Reinhold Publ. Corp., New York, 1962), Vol. 3, pt. 1, p. 75. [Google Scholar]
- [11].Keller W. E., Phys. Rev. 97, 1 (1955); 100, 1790 (1955). [Google Scholar]
- [12].Keller W. E., Phys. Rev. 98, 1571 (1955). [Google Scholar]
- [13].Hoare F. E. and Zimmerman J. E., Rev. Sei. Instr. 30, 184 (1959); [Google Scholar]; Swim R. T., Advances in Cryogenic Engineering, ed. Timmerhaus K. D. (Plenum Press, Inc., New York, 1960), Vol. 5, pp. 498–504. [Google Scholar]
- [14].Plumb H. H., Proc. 10th Intern. Congr. Refrigeration, Copenhagen, 1959, ed. Jul M. and Jul A. M. S. (Pergamon Press, Paris, 1960;, Vol. 1, pp. 184–187. [Google Scholar]
- [15].Durieux M., Thermometry at Liquid Helium and Liquid Hydrogen Temperatures, Thesis, Leiden Univ; (16 March 1960). [Google Scholar]
- [16].Brickwedde F. G., Report on the Conference Agreement on the Helium Vapor-Pressure Scale of Temperatures, Conf. Physique des Basses Temperatures, Paris, 2–8 Septembre 1955, pp. 608–610. [Google Scholar]
- [17].Bleaney B. and Simon F., Trans. Faraday Soc. 35, 1205 (1939). [Google Scholar]
- [18].Bleaney B. and Hull R. A., Proc. Roy. Soc. (London) 178A, 74 (1941). [Google Scholar]
- [19].de Boer J. and Cohen E. G. D., Physica 17, 993 (1951). [Google Scholar]
- [20].Becker E. W. and Misenta R., Phys. Rev. 93, 244 (1954). [Google Scholar]
- [21].Becker E. W., Misenta R., and Schmeissner F., Z Physik 137, 126 (1954). [Google Scholar]
- [22].Mendelssohn K. and White G. K., Proc. Phys. Soc. (London) A63, 1328 (1950). [Google Scholar]
- [23].Bowers R. and Mendelssohn K., Nature 163, 870 (1949); Proc. Phys. Soc. (London) A63, 1318 (1950). [DOI] [PubMed] [Google Scholar]
- [24].Taconis K. W., Progress in Low Temperature Physics, ed. Gorter C. J. (North Holland Publ. Co, Amsterdam, The Netherlands, 1961), Vol. 3, p. 154. [Google Scholar]
- [25].van Dijk H. and Durieux M., Physica 24, 1 (1958). [Google Scholar]
- [26].Sydoriak S. G. and Sommers H. S. Jr., Rev. Sei. Instr. 22, 915 (1951). [Google Scholar]
- [27].Kapitza P., Phys J.. (U.S.S.R.) 4, 181 (1941). [Google Scholar]
- [28].Fairbank H. A. and Wilks J., Proc. Roy. Soc. (London) A231, 545 (1955). [Google Scholar]
- [29].Challis L. J., Dransfeld K., and Wilks J., Proc. Roy. Soc. (London) A260, 31 (1961) [Google Scholar]
- [30].Kuang Wey-Yen Zh. Experim. i Teor. Fiz. (U.S.S.R.) 42, 921 (1962); Soviet Phys. JETP (English Transí.) 15, 635 (1962). [Google Scholar]
- [31].Khalatnikov I.M., Zh. Experim. i Teor. Fiz. (U.S.S.R.) 22, 687 (1952). [Google Scholar]
- [32].Sydoriak S. G. and Roberts T. R., Phys. Rev. 118, 901 (1960). [Google Scholar]
- [33].Mills R. L., Grilly E.R., and Sydoriak S. G., Ann. Phys. 12, 41 (1961). [Google Scholar]
- [34].Cawood W. and Patterson H. S., Trans. Faraday Soc. 29, 522 (1933). [Google Scholar]
- [35].Kistemaker J., Leiden Commun. 268(1 (1944); Physica 11, 277 (1944–1946); B. E. Blaisdell, J. Math. Phys. 19, 186 (1940). [Google Scholar]
- [36].Brombacher W. G., Johnson D. P., and Cross J. L., Mercury Barometers and Manometers, N BS Mono. 8 (U.S. Govt. Printing Office, Washington, 1960). [Google Scholar]
- [37].Roberts T. R. and Sydoriak S. G., Phys. Rev. 102, 304 (1956). [Google Scholar]
- [38].Esel’son B. N. and Berezniak N. G., Zh. Experim. i Teor. Fiz. (U.S.S.R.) 30, 628 (1956); Soviet Phys. JETP (English Transí.) 3, 568 (1956). [Google Scholar]
- [39].Sydoriak S. G., Grilly E. R., and Plammel E. F., Phys. Rev. 75, 303 (1949). [Google Scholar]
- [40].Peshkov V. P., Zh. Experim. i Teor. Fiz. (U.S.S.R.) 33, 833 (1957); Soviet Phys. JETP (English Transí.) 4, 645 (1958). [Google Scholar]
- [41].van Dijk H. and Shoenberg D., Nature 164, 151 (1949). [Google Scholar]
- [42].Chen T. C. and London F., Phys. Rev. 89, 1038 (1953). [Google Scholar]
- [43].Keller W. E., Nature 178, 883 (1956); [Google Scholar]; Clement J. R., Low Temperature Physics and Chemistry (Univ. of Wisconsin Press, Madison, 1958), p. 187 (Proc. 5th Intern. Conf. Low Temperature Physics and Chemistry, Madison, Wis., 1957). [Google Scholar]
- [44].Goldstein L., Phys. Rev. 96, 1455 (1954); Phys. Rev. 102, 1205 (1956). [Google Scholar]
- [45].Sherman R. H., Roberts T. R., and Sydoriak S. G., Supplement au Bulletin de lTnstitut International du Froid, Annexe 1961–5, p. 125 (Proc. Meeting of Commission I of the International Institute of Refrigeration, London, 1961). [Google Scholar]
- [46].Sydoriak S. G. and Sherman R. H., Paper I of this series.
- [47].Deming W. E., Statistical Adjustment of Data (John Wiley & Sons, New York, 1943). [Google Scholar]
- [48].Roberts T. R., Sydoriak S. G., and Sherman R. H., see ref. [45], p. 115.
- [49].Grilly E. R. and Hammel E. F., Progress in Low Temperature Physics III (1961), pp. 113–152.
- [50].Kerr E. C. and Taylor R. D., Proc. 7th Intern. Conf. Low Temperature Physics, ch. 24, pp. 605–608, ed, Graham G. M. and Hollis-Hallett A. C. (Univ. of Toronto Press, Toronto, 1961). [Google Scholar]
- [51].Sherman R. H. and Edeskuty F. J., Ann. Phys. 9, 522 (1960). [Google Scholar]
- [52].Roberts T. R., Sherman R. H., Sydoriak S. G., and Brickwedde F. G., Progress in Low Temperature Physics IV, ch. 10 (North Holland Publ. Co., Amsterdam, The Netherlands, 1964), p. 480. [Google Scholar]
- [53].Mood A. M., Introduction to the Theory of Statistics, p. 299 (McGraw-Hill, New York, N.Y., 1950). [Google Scholar]
- [54].Durieux M., Procès-Verbaux du Comité Consultatif de Thermométrie auprès du Comité International des Poids et Mesures, 6e Session, 1962 (Gauthier-Villars, Paris, France, 1964), p. 190. [Google Scholar]
- [55].Osborne D. W., private communication.
- [56].Berman R. and Mate C. F., Phil. Mag. 3, 461 (1958). [Google Scholar]
- [57].van Dijk H., Progr. Cryog. 2, p. 125 (Academic Press, Inc., New York, N.Y., 1962). [Google Scholar]
- [58].Cataland G. and Plumb Η. H., Proc. 8th Intern. Conf. Low Temperature Physics, London, Sept. 16–22, 1962, ed. Davies R. O. (Butterworths, Washington, D.C., 1963), pp. 439–40 [Google Scholar]
- [59].Kerr E. C., Phys. Rev. 96, 551 (1954). [Google Scholar]
- [60].Kerr E. C. and Taylor R. D., Ann. Phys. 20, 450 (1962). [Google Scholar]
- [61].Anderson A. C., Salinger G. L., Steyert W. A., and Wheatley J. C., Phys. Rev. Letters 6, 331 ( 1961); [Google Scholar]; Anderson A. C., Reese W., and Wheatley J. C., Phys. Rev. 130, 495 (1963). [Google Scholar]
- [62].Strongin M., Zimmerman G. O., and Fairbank H. A., Phys. Rev. 128, 1983 (1962). [Google Scholar]
- [63].Abraham B. M., Osborne D. W., and Weinstock B., Phys. Rev. 98, 551 (1955). [Google Scholar]
- [64].Grove G. R. and Haubach W. J. Jr.; see ref. [58], p. 441 For further information, write Gaseous Isotope Sales, Montsanto Research Corporation, Mound Laboratory, Miamisburg, Ohio. [Google Scholar]
- [65].Edwards D. O. and Daunt J. G., Phys. Rev. 124, 640 (1961). [Google Scholar]
- [66].Sydoriak S. G., Roberts T. R., and Sherman R. H.; see ref. [58], pp. 437–8.
- [67].Hall J. A., Sixième Rapport du Comité Consultatif de Thermométrie au Comité International des Poids et Mesures; see ref. [54].
- [68].Stimson H. F., NBS Tech. News Bull. 47, No. 2, 30 (February 1963); Nature 197, 1055 (1963). [Google Scholar]
- [69].Brickwedde F. G., Phys. Today 16, 24 (1963). [Google Scholar]
- [70].Weber S., Commun. Kamerlingh Onnes Lab. Univ. Leiden Suppi; 71b (1932), 264b and 264d (1936). [Google Scholar]
- [71].Weber S. and Schmidt G., Commun. Kamerlingh Onnes Lab. Univ. Leiden; 264c (1936). [Google Scholar]
- [72].Chase C. E., Maxwell E., and Whitney W. M., Physica 26, 160 (1960); [Google Scholar]; Chase C. E., Maxwell E., and Millett W. E., Physica 27, 1129 (1961). [Google Scholar]
- [73].Moore R. H. and Zeigler R. K., Trans. Am. Nuclear Soc. 1, 128–9 (1958); The Solution of the General Least Squares Problem with Special Reference to High-Speed Computers, Los Alamos Scientific Lab. Rept. LA-2367 (Mar. 4, 1960). [Google Scholar]
- [74].Roberts T. R. and Swartz B. K., Proc. 2d Symp. Liquid and Solid Helium Three, ed. Daunt J. G. (Ohio State Univ. Press, Columbus, 1960), p. 163. [Google Scholar]