Abstract
Extreme sample complexity is an inherent challenge in shotgun proteomics that positions quality of chromatographic separations as one of the key determinants of attainable proteome coverage. In search of better separations, macroscopic physical characteristics of capillary columns, i.e., length and properties of stationary phase particles, are typically considered and optimized, while significance of packing bed morphology is frequently underappreciated. Here, we describe a technology that enables packing of capillary columns at excess of 30,000 psi and demonstrate that such columns exhibit reduced backpressure and remarkably reproducible chromatographic performance, improved on average by 23%. These enhancements afford up to 35% increase in the depth of commonplace bottom-up proteomic analyses, owning to augmented sensitivity and resolution of peptide separations and improvements in spectral quality. Our findings strongly corroborate advantages of ultra-high pressure packing of capillary columns for diverse shotgun proteomic workflows.
Graphical Abstract
Shotgun proteomics is a versatile approach to global protein analysis that employs two sophisticated and equally imperative technologies: nano-ultra-high-performance liquid chromatography (nano-UHPLC) and tandem mass spectrometry (MS/MS).1,2 Typically examined peptide mixtures are extremely complex, so that thousands of peptides coelute even under the best capillary LC conditions.3,4 Recent improvements in sensitivity and acquisition speed of mass spectrometers have accentuated the significance of chromatography, as the most efficient separations are required to further extend dynamic range of analysis and saturate rapid MS/MS scan rates of modern instruments.5–7
An analytical column is a critical component of any chromatographic setup; its physical characteristics, i.e., length, diameter, stationary phase properties, and packing bed structure, dictate separation efficiency.8–10 When high quality separations are desired in proteomic analyses, long capillary columns (>20 cm) packed with small (sub-2 μm diameter) particles are typically employed.6,11–13 Upper pressure limits of commercial UHPLC systems and the need for relatively high loading capacity, however, confine further exploration of these column characteristics beyond the current state-of-the-art practices.6,12,14,15 The significance of packing bed morphology, on the other hand, has been under-evaluated. High pressure column packing promotes homogeneity of packing bed structure, reducing Eddy diffusion and enhancing flow uniformity,9,16,17 and its advantages for peak symmetry and separation resolution are universally recognized.18–20 That said, due to technical difficulties associated with retaining a glass capillary of a submillimeter diameter under ultra-high pressure (uHP; >20,000 psi), the practice has not found broad application among proteomics practitioners. Thus, commercial and in-house made capillary columns are typically packed under 100–3,000 psi with rarely employed and still insufficient19,20 maximum pressure of ~9,000 psi.
Jorgenson and co-workers pioneered the practice of uHP packing of capillary columns and demonstrated its value for separations of simple mixtures detected via ultraviolet visible spectroscopy.19–22 The setup also produced columns without integrated electrospray emitters that are critical for interfacing UHPLC and MS. Here, we built on their work and developed a straightforward approach for uHP packing of emitter-enabled capillary columns to assess their utility for LC-MS/MS analysis of complex peptide mixtures. Our findings revealed that the new columns offered lowered backpressure, remarkable column-to-column reproducibility, and up to 23% reduction in median base peak width. These enhancements of chromatographic performance translated into systematic increases (10–35%) in the depth of various commonplace proteomic analyses. The most pronounced improvements (19–35%) were observed in experiments on low amounts of material and post-translationally modified peptides, showcasing augmented sensitivity of the new methodology. Thus, our work strongly corroborated advantages of uHP packing of capillary columns and emphasized the general importance of chromatographic separations in modern shotgun proteomics.
EXPERIMENTAL SECTION
Design of the uHP Column Packing Station
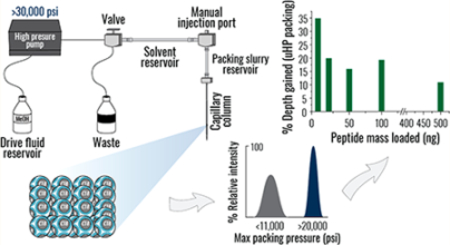
The capillary column packing system was manufactured in-house. To provide pressures up to 50,000 psi, a Haskel air driven liquid pump (model DSHF-300, Burbank, CA) was employed. All fluid connections from the pump to the slurry chamber were constructed using uHP valves, fittings, and tubing obtained from HiP High Pressure Equipment Co. (Erie, PA), rated for pressures up to 60,000 psi. Methanol from the pump was split off to a 0 to 50,000 psi gauge (HiP part number 6PG50), employed to monitor the applied pressure. The methanol not directed toward the pressure gauge flowed to a three-way uHP valve (HiP part number 60–13HF2), used to release pressure and provide an exit for excess solvent used in the back-flushing process (Figure S1A), as described in the Supplemental Experimental Section. To pack columns, pressure was gradually applied causing the pumped methanol to push on the slurry solvent that in turn pushed on the packing slurry. The rate of packing was monitored with a Dino-Lite digital microscope series AM4000 attached to a monitor (Dunwell Tech, Inc., Torrance, CA). The position of the column in the front of the microscope was fixed by a custom 3D printed structure, which maintained the column’s distance from the microscope while allowing the microscope to be traversed along the length of the column. Vertical movement of the microscope was enabled by a motorized camera slider (Robotshop, Mirabel, Canada). The setup was enclosed within a cabinet constructed from T-slot aluminum (80/20 Inc., Columbia City, IN) and polycarbonate panels.
Column Fabrication
A laser puller (Sutter Instruments Co., Novato, CA) was used to generate 75 × 360 μm inner–outer diameter bare-fused silica capillary columns with electrospray emitter tips (~10 × 25 μm inner–outer diameter). The tips were briefly etched with 100% hydrofluoric acid and plugged with 5 μm, 130 Å pore size Bridged Ethylene Hybrid (BEH) C18 particles (Waters, Milford, MA) using an in-house made pressure injection cell with maximum gas pressure grading of ~1500 psi. Then, using the same packing unit, three columns were filled with 1.7 μm diameter, 130 Å pore size BEH particles (Waters, Milford, MA). Two additional sets of three columns were packed with 1.7 μm diameter particles at the uHP column packing station, reaching maximum pressure of ~20,000 and ~30,000 psi. In all cases, 1.7 μm packing material was resuspended in chloroform at unspecified concentrations of 40–160 mg/mL, as varying slurry concentration across this range did not detectably impact the number of identified unique peptides (data not shown). On average, packing a column at 20,000 or 30,000 psi using the uHP station took ~2 h, in contrast to 3–7 h required to pack a column using the pressure injection cell.
Cell and Mice Information
K562 cells were purchased from the ACTT (Manassas, Virginia), cultured according to the ACTT guidelines in IMBM medium with the addition of 10% FBS, and collected at 70–80% confluence. Wild type Saccharomyces cerevisiae strain BY4741 (Open Biosystems, Lafayette, CO) was grown in YPD media (1% yeast extract, 2% peptone, 2% dextrose) to the optical density of ~0.6 at 600 nm. Both yeast and human cells were harvested by centrifugation, washed with ice-cold phosphate-buffered saline, pelleted, and frozen for storage at −80 °C. Brains were harvested from C57BL/6J adult female mice after euthanasia and immediately frozen in liquid nitrogen.
Sample Preparation
A cell pellet was resuspended in lysis buffer (100 mM Tris, 8 M urea, 10 mM TCEP, 40 mM 2-chloracetamide) and rigorously vortexed. Mouse brain samples were resuspended in the lysis buffer and homogenized with a probe sonicator (QSonica, Newtown, CT) at 4 °C. Proteins were precipitated by the addition of methanol to the final concentration of 90% and consequent 10 min centrifugation at 14,000g. Resulting protein pellets were resuspended in the lysis buffer, and Reducing Agent Compatible Protein BCA (Pierce, Rockford, IL) was used to measure protein concentration. Lysate was digested overnight with LysC in 1:50 ratio (enzyme/protein; Wako Chemicals, Richmond, VA) at ambient temperature, followed by additional digestion with trypsin in 1:50 ratio (enzyme/protein, Promega, Madison, WI) for 3 h. Peptides were desalted over a StrataX solid phase extraction column (Phenomenex, Torrance, CA) and lyophilized to dryness in a SpeedVac (Thermo Fisher, Waltham, MA). Samples were resuspended in 0.2% formic acid (Pierce, Rockford, IL), and final peptide concentration was determined using Quantitative Colorimetric Peptide Assay (Pierce, Rockford, IL).
Peptide Fractionation
For high pH reverse phase fractionation, ~200 μg of human peptides was separated across an XBridge Peptide BEH C18 Column, 130 Å, 3.5 μm, 4.6 mm × 150 mm column (Waters, Milford, MA), at the flow rate of 0.8 mL/min over a 25 min gradient into 8 fractions using a 1260 Infinity II High Pressure Liquid Chromatography system with configured Analytical-Scale Fraction Collector (Agilent, Santa Clara, CA). Mobile phase A consisted of 10 mM ammonium formate, pH 10; mobile phase B contained 10 mM ammonium formate in 80% UHPLC-MS grade methanol.
Phosphorylation and Acetylation Enrichments
~1 mg of desalted tryptic peptides obtained from the digest of mouse brain proteins was used to enrich for phosphopeptides with a 50 μL aliquot of MagResyn Ti-IMAC Ti4+ (ReSyn Biosciences, Edenvale, South Africa), according to the manufacturer’s instruction. Acetylated peptides were enriched from 2 mg of human tryptic peptides using one aliquot of pan-specific Acetylated-Lysine Antibodies (Cell Signaling, Danvers, MA), according to the manufacturer’s instruction.
Nano UHPLC-MS/MS
Columns were installed on a Dionex Ultimate 3000 nano UHPLC system (Thermo Fisher, Sunnyvale, CA) using a stainless steel uHP union (IDEX, Oak Harbor, WA). Upon the first installation, they were equilibrated and compressed by sequentially flowing mobile phases A (0.2% formic acid in water) and B (0.2% formic acid in 70% UHPLC-MS grade acetonitrile) at the rate adjusted to achieve backpressure of ~11,000 psi. Columns were heated to 55 °C inside an in-house made heater. Peptides were loaded onto a column and separated at a flow rate of 325 nL/min over 90, 120, and 180 min gradients, including injection time, column wash, and re-equilibration. Eluting peptides were analyzed on an Orbitrap Fusion Lumos (Thermo Scientific, San Jose, CA). Orbitrap survey scans were performed at a resolving power of 240,000 at 200 m/z with an AGC target of 1 × 106 ions. The instrument was operated in the Top Speed mode with 2 s cycles using the Advanced Precursor Determination algorithm.4 See Supplemental Experimental Section for additional details of instrumental methods.
Data Analysis
Raw data were processed using the COMPASS proteomics software suite and MaxQuant quantitative software package (version 1.5.2.8). Spectra were searched against UniProt databases containing protein isoforms. MaxQuant analyses were performed using default settings and the “Calculate peak properties” advanced option. MaxQuant reported full peak widths at the base (full width at 10% height, seconds) and full width at half-maximum height (FWHM, seconds). Peaks corresponding to peptides systematically observed in all experiments with the same charge and variable modifications were used to calculate median peak widths and intensities. MaxQuant was also used to identify and localize sites of phosphorylation and acetylation. See Supplemental Experimental Section for other search parameters.
Data Availability
All raw proteomics data files were deposited into the Chorus repository (Accession #1476).
RESULTS AND DISCUSSION
Design and Operation of the uHP Column Packing Station
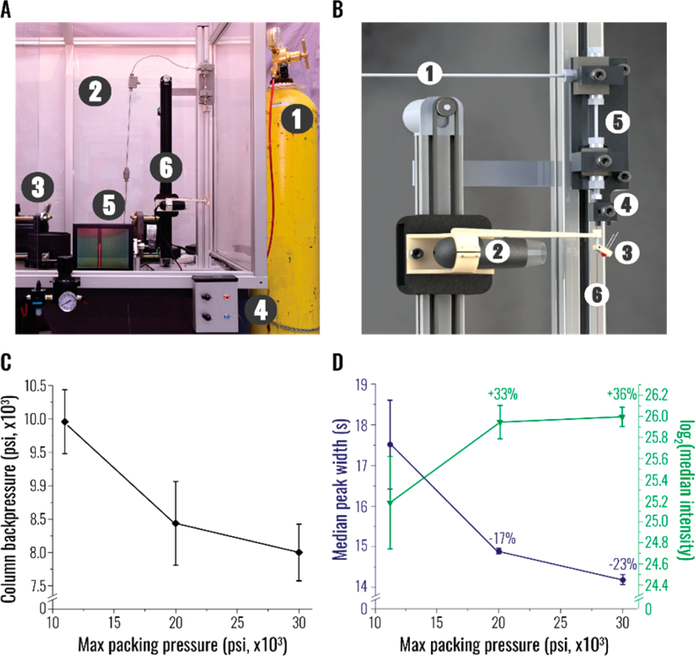
The newly designed station for downward constant-pressure packing combined elaborate safety features with the relative ease of operation (Figure 1A). To protect the user, the uHP pump and the capillary were housed inside a shatter-resistant glass case. Since maintaining the structural integrity of the fittings is exceedingly challenging under uHP, we intentionally minimized the overall number of connections and valves (Figure S1A). The capillary was restricted upsidedown via an uHP fitting with the internal support of a capillary ferrule and a peak tubing sleeve (Figure 1B). An additional safety clamp below increased the total surface area interacting with the capillary and prevented its complete detachment in the case of upper fitting failure. Suspended in chloroform,11 packing material slurry was placed inside a stainless steel tube, one end of which was machined to accommodate the uHP fitting restricting the column. Methanol was selected as the pump push solvent for its low compressibility and relative chemical inertness.19 Packing progress was visualized by a motorized microscopic camera connected to a small monitor (Figure 1A,B). As the level of packing material inside the column increased, the height of the camera could be adjusted accordingly. Due to similar refractive indexes of chloroform and C18 silica particles,19 monochromatic red light was supplied to illuminate the packing front.
Figure 1.
Design of the ultra-high pressure (uHP) packing station and the impact of uHP on column chromatographic performance. All comparisons in panels C and D were performed using three column replicates packed under the indicated maximum pressures; error bars represent standard error of the mean. (A) The outside view of the station: (1) gas tank with compressed air; (2) shatter resistant (bulletproof) glass casing; (3) uHP pump (up to 50,000 psi) using methanol as the push solvent; (4) regulator of light intensity and camera height; (5) microscopic camera monitor; (6) motorized camera track, adjacent to the column holding unit (more detail in B). (B) The detailed view of the column holding compartment: (1) line from the uHP pump; (2) microscopic camera; (3) red light-emitting diode (LED); (4) safety clamp; (5) packing material reservoir; (6) empty capillary. (C) The effect of maximum packing pressure (psi) on column backpressure (psi) upon installation on an UHPLC. Backpressure was measured on 30 cm columns flowing mobile phase A at 325 nL/min. (D) Relationship between maximum packing pressure (psi), median base peak width (blue, seconds), and median peak intensity (green, arbitrary units) of peptides detected in a 90 min LC-MS/MS analysis of the whole proteome tryptic digest of K562 cell line.
Characterizing Chromatographic Performance of uHP Packed Columns
To assess the effect of uHP packing on chromatographic performance, we fabricated sets of three columns reaching different maximum packing pressures. The first group of columns was packed with a commonly used pressure injection cell (i.e., a packing bomb) at ~1,000 psi. Note that upon installation these columns were equilibrated and further compressed at ~11,000 psi, the maximum pressure of the UHPLC system. Two additional sets of columns were packed on the uHP station at maximum pressures of 20,000 and 30,000 psi, respectively. After equilibration on the UHPLC, no further compression was detected in these columns, suggesting that stable beds of stationary phase particles were formed during packing.18,23
The first striking feature of the uHP packed columns was the reduced backpressure reported by the UHPLC system upon their installation (Figure 1C). This observation indicated that the anticipated changes in the structure of the packing bed had occurred, as a more morphologically homogeneous packing is characterized by augmented porosity and enhanced flow uniformity across the column.9,16 The decline in backpressure offered several benefits: (i) longer columns could be operated without the risk of exceeding upper pressure limit of the UHPLC system;6,7,14 (ii) higher flow rates could be maintained, optimizing linear velocity of the separation;8,9 (iii) lower temperature was required to mitigate the backpressure, potentially extending lifespan of the column.24,25
Another, even more valuable feature of the uHP packed columns was the reproducible improvement in chromatographic performance. The columns afforded a 17–23% decrease in the median base peak width of identified peptide precursors (Figure 1D in blue), as compared to the elution profiles generated by the low pressure packed columns. Only a modest reduction (~5%) in peak widths was detected between the columns packed at 20,000 and 30,000 psi, suggesting that further elevation of packing pressure would likely bring diminishing returns. The narrowing of chromatographic peaks predictably translated into proportional gains in achieved peak capacity (Figure S1B) and up to 36% increase in peak intensities (Figure 1D in green), pointing toward plausible improvements in sensitivity of the method. As evident by the error bars in Figure 1D, chromatographic performance achieved by the columns packed at higher pressure was not only better but also notably more reproducible.
Benefits of uHP Column Packing for Diverse Proteomic Analyses
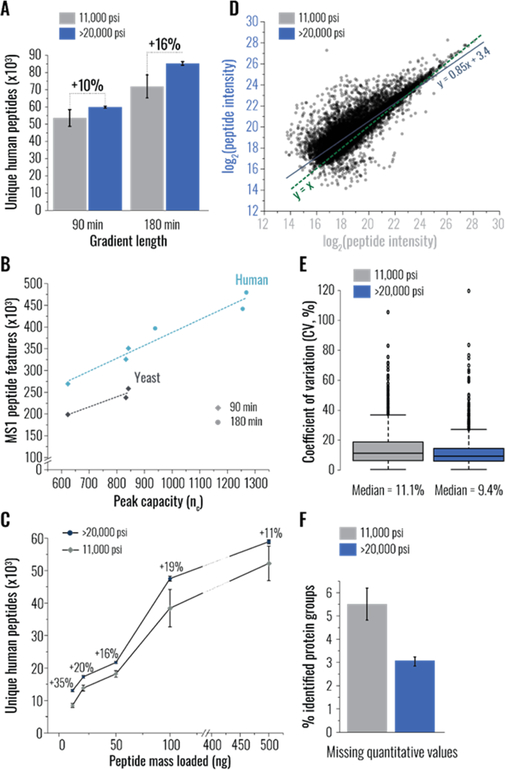
Having established that uHP packed columns consistently generated higher quality separations, we assessed the value of these improvements for analysis of commonplace proteomic samples. First, we examined tryptic digest of the whole yeast proteome over a 90 min gradient and observed an ~11% increase in the number of unique peptides detected using uHP packed columns (Figure S2A). A comparable improvement (+10%) was observed in the 90 min analysis of a complex human peptide mixture, generated by digesting proteins extracted from K562 cells (Figure 2A). To examine the effect of gradient length, we also separated and analyzed the human peptides over 180 min. The comparison revealed that better separations were especially valuable for longer analyses, as ~16% more peptides were detected in the 180 min experiments owing to the use of uHP packed columns. The number of peptide-like isotope patterns3 observed in survey (MS1) scans in analyses of both yeast and human samples increased linearly with the improvements in peak capacity, produced by narrowing of chromatographic peaks and/or extending the gradient (Figure 2B). This finding corroborates that one of the primary benefits of better separations is the increased resolution of closely eluting species that renders more precursors amenable to sequencing via MS/MS.26,27 Additionally, we evaluated the effect of uHP column packing on analyses of relatively less complex samples. We fractionated the human peptide mixture into four fractions and detected an ~11% increase in the number of identified human peptides using uHP packed columns (Figure S2B).
Figure 2.
Benefits of ultra-high pressure (uHP) column packing for diverse shotgun proteomics analyses. In all panels, data in gray and blue correspond to experiments conducted using columns packed at low (11,000 psi) and ultra-high (>20,000 psi) pressure, respectively; “>20,000 psi” and “11,000 psi” groups consist of six and three column replicates, respectively, each an average of two injection replicates. In panels A, C, and F, error bars represent standard error of the mean. (A) Unique human peptides identified in 90 and 180 min LC-MS/MS analyses of the whole proteome tryptic digest of K562 cells using capillary columns packed at the specified maximum pressure. (B) The effect of peak capacity (nc) on the total number of peptide-like features3 observed in MS1 (survey) scans of analyses performed using columns packed at the indicated maximum pressure. Results combined from 90 and 180 min analyses of human peptide mixtures are shown in light blue, and 90 min analyses of yeast samples are in dark gray. (C) Unique human peptides identified in indicated amounts of the whole proteome tryptic digest of K562 cells using columns packed at the specified maximum pressure. Percent increase achieved using uHP packed columns is indicated above each data point. (D) Scatter plot comparing log2-tranformed intensities of identical peptide precursors detected in analyses of 10 ng peptide injections using columns packed at different pressure. The line of best fit for the data set is shown in dark blue, and the y = x line is in dark green. (E) Box plots comparing coefficient of variation (CV, %) of label-free quantification (MaxQuant) observed in analyses of 10 ng peptide injections using columns packed at the specified maximum pressure. Data set medians are indicated below the boxes. (F) Missing label-free quantitative values (protein groups lacking MaxLFQ abundance measurements), reported as a percent of all identified protein groups, quantified in analyses of 10 ng peptide injections using columns packed at the specified pressures.
Next, we conducted 90 min analyses across a range of peptide masses and revealed that, regardless of the amount injected, experiments using uHP packed columns systematically generated a greater number of identified human peptides (Figure 2C). The most pronounced differences (16–35%) were observed in the lower (10–100 ng) portion of the range. This finding could be attributed to the improved sensitivity afforded by higher quality separations, as identical peptide precursors appeared more intense in analyses using uHP packed columns (Figure 2D). Note that in the 10–100 ng range increasing the number of detected peptides readily translated into up to a 22% boost in protein identifications, considerably enhancing proteome coverage (Figure S2C). Likewise, reproducibility and repeatability of label-free quantification (Max LFQ28) were also positively impacted (Figure 1E,F, respectively); the analyses performed using uHP packed columns exhibited lower median inter-replicate coefficient of variation (9.4% vs. 11.1%) and contained fewer missing quantitative values (3.0% vs. 5.5%).
Benefits of uHP Packing for Analyses of Post-Translationally Modified Peptides
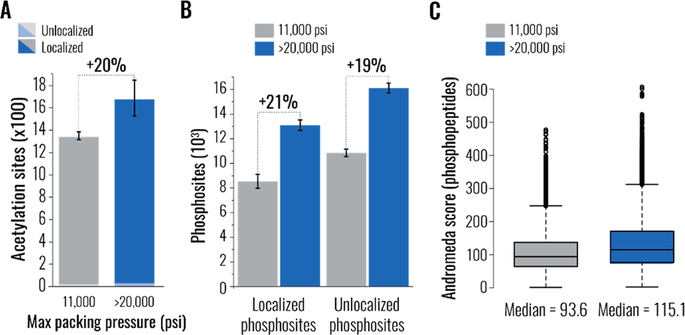
The persistent observation of enhanced method sensitivity prompted us to investigate how better separations impacted analyses of posttranslationally modified peptides, mixtures of which tend to contain numerous low abundance species.29 We enriched a whole human proteome digest for peptides containing acetylated lysine residues and analyzed them without prefractionation. Consistently with our expectations, analyses conducted using uHP columns reproducibly detected 20% more acetylated peptides (Figure 3A). Next, we enriched phosphopeptides from mouse brain tissue and analyzed them without prefractionation over a 180 min gradient. Similarly, 21% and 19% more localized and unlocalized phosphosites, respectively, were identified thanks to the use of uHP columns, affording detection of >11,000 unique phosphosites in a single experiment (Figure 3B). When repeated in injection triplicates, the analyses using uHP packed columns facilitated a 25% and 20% increase in the number of localized and unlocalized phosphosites, respectively, confidently identifying >1,000 unique phosphorylation events in a single unfractionated sample (Figure S3A). These results indicated that the use of uHP packed columns was particularly beneficial for phosphosite localization (i.e., 25% vs. 20% increase), implying that higher quality separations could positively affect spectral quality. In agreement with this suggestion, both median Andromeda peptide matching scores and delta scores30 (Figures 3C and S3B, respectively) were considerably elevated in analyses performed using uHP packed columns.
Figure 3.
Benefits of ultra-high pressure (uHP) column packing for analysis of post-translationally modified peptides. In all panels, data in gray and blue correspond to experiments conducted using columns packed at low (11,000 psi) and ultra-high (>20,000 psi) pressure, respectively. In panels A and B, error bars represent standard error of the mean. (A) Unique lysine acetylation sites detected in the antibody-assisted enrichment of modified tryptic peptides from K562 cells (120 min analysis). Each bar consists of two column replicates, each a sum of two injection replicates. (B) Phosphorylation sites detected in the enrichment of modified tryptic peptides from mouse brain lysates in 180 min analyses using capillary columns packed at the specified maximum pressure. Each bar consists of two column replicates, each a sum of three injection replicates. (C) Box plots comparing distributions of Andromeda scores (reported by MaxQuant) obtained in analyses of phosphopeptides (panel B) conducted using columns packed at the specified maximum pressure. Median of each population is indicated below the box. Higher scores signify greater confidence in the peptide match.
CONCLUSIONS
Frequently referred to as an art form,19,22,23 column packing is an exceptionally complex process. Numerous interdependent variables simultaneously influence morphology and stability of the generated packing bed,18,19,21,22 and such connectivity and complexity make systematic examination of the process extremely challenging, rendering it a specialty domain of chromatography experts. Nonetheless, our findings suggest that implementation of a single, rather straightforward practice of uHP packing could bring myriad benefits to all shotgun proteomics practitioners. Our findings repeatedly pointed toward augmented resolution and sensitivity of separations achieved using more efficiently packed columns. The value of these improvements was particularly evident in the analyses of small amounts of material and post-translationally modified peptides (up to 35% increase in depth), both of which constitute special challenges in bottom-up proteomics.29,31–33 Further, chromatographic performance of the uHP packed columns was also notably more reproducible than that of the conventionally packed columns (Figure 1D). Column-tocolumn reproducibility is generally sought-after by all proteomics methodologies and particularly appreciated in high-throughput large-scale studies that have become increasingly more commonplace.34–37 Lastly, the unexpected side product of the process—reduced column backpressure (Figure 1C)—may reinforce the ease of UHPLC system operation and potentially its long-term stability, alleviating the burden of instrument maintenance and daily use.10,14 In all, regardless of the sample identity, its relative complexity, and the amount examined, uHP column packing is an effective and reliable way of enhancing depth and sensitivity of shotgun proteomics analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Profs. James Jorgenson (Univ. of North Carolina at Chapel Hill) and Robert Kennedy (Univ. of Michigan–Ann Arbor) for helpful discussions. We gratefully acknowledge support from the NIH grants P41GM108538 and R35GM118110 (to J.J.C.).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.8b02766.
Flow path and operation of the ultra-high pressure (uHP) packing station and the impact of uHP packing on column peak capacity; benefits of uHP column packing for depth of bottom-up proteomics analyses; benefits of uHP column packing for depth of bottom-up proteomics analyses of phosphorylated peptides; Extended Experimental Methods section (PDF)
REFERENCES
- (1).Aebersold R; Mann M Nature 2016, 537, 347–355. [DOI] [PubMed] [Google Scholar]
- (2).Riley NM; Hebert AS; Coon JJ Cell Syst. 2016, 2, 142–143. [DOI] [PubMed] [Google Scholar]
- (3).Michalski A; Cox J; Mann M J. Proteome Res 2011, 10, 1785–1793. [DOI] [PubMed] [Google Scholar]
- (4).Hebert AS; et al. Anal. Chem 2018, 90, 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Scheltema RA; et al. Mol. Cell. Proteomics 2014, 13, 3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Shishkova E; Hebert AS; Coon JJ Cell Syst. 2016, 3, 321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bekker-Jensen DB; et al. Cell Syst. 2017, 4, 587–599.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Liu H; et al. J. Chromatogr. A 2007, 1147, 30–36. [DOI] [PubMed] [Google Scholar]
- (9).Gritti F; Guiochon G Anal. Chem 2013, 85, 3017–3035. [DOI] [PubMed] [Google Scholar]
- (10).Blue LE; et al. J. Chromatogr. A 2017, 1523, 17–39. [DOI] [PubMed] [Google Scholar]
- (11).Thakur SS; et al. Mol. Cell. Proteomics 2011, 10, M110.003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Pirmoradian M; et al. Mol. Cell. Proteomics 2013, 12, 3330–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hebert a. S.; et al. Mol. Cell. Proteomics 2014, 13, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Šesták J; Moravcová D; Kahle V J. Chromatogr. A 2015, 1421, 2–17. [DOI] [PubMed] [Google Scholar]
- (15).Grinias KM; Godinho JM; Franklin EG; Stobaugh JT; Jorgenson JW J. Chromatogr. A 2016, 1469, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Daneyko A; ltzel A; Khirevich S; Tallarek U Anal. Chem 2011, 83, 3903–3910. [DOI] [PubMed] [Google Scholar]
- (17).Reising AE; Godinho JM; Hormann K; Jorgenson JW; Tallarek U J. Chromatogr. A 2016, 1436, 118–132. [DOI] [PubMed] [Google Scholar]
- (18).Lancas FM; Rodrigues JC; de S Freitas S J. Sep. Sci 2004, 27, 1475–1482. [DOI] [PubMed] [Google Scholar]
- (19).Wahab MF; Patel DC; Wimalasinghe RM; Armstrong DW Anal. Chem 2017, 89, 8177–8191. [DOI] [PubMed] [Google Scholar]
- (20).Kennedy RT; Jorgenson JW Anal. Chem 1989, 61, 1128–1135. [DOI] [PubMed] [Google Scholar]
- (21).Bruns S; et al. J. Chromatogr. A 2013, 1318, 189–197. [DOI] [PubMed] [Google Scholar]
- (22).Blue LE; Jorgenson JW J. Chromatogr. A 2015, 1380, 71–80. [DOI] [PubMed] [Google Scholar]
- (23).Kirkland JJ; DeStefano JJ J. Chromatogr. A 2006, 1126, 50–57. [DOI] [PubMed] [Google Scholar]
- (24).Claessens HA; Van Straten MA J. Chromatogr. A 2004, 1060, 23–41. [PubMed] [Google Scholar]
- (25).Teutenberg T; Tuerk J; Holzháuser M; Giegold SJ Sep. Sci 2007, 30, 1101–1114. [DOI] [PubMed] [Google Scholar]
- (26).Shi Y; Xiang R; Horvath C; Wilkins JA J. Chromatogr. A 2004, 1053, 27–36. [PubMed] [Google Scholar]
- (27).Neverova I; Van Eyk JE J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2005, 815, 51–63. [DOI] [PubMed] [Google Scholar]
- (28).Cox J; et al. Mol. Cell. Proteomics 2014, 13, 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Olsen JV; Mann M Mol. Cell. Proteomics 2013, 12, 3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Cox J; et al. J. Proteome Res 2011, 10, 1794–1805. [DOI] [PubMed] [Google Scholar]
- (31).Angel TE; et al. Chem. Soc. Rev 2012, 41, 3912.22498958 [Google Scholar]
- (32).Specht H; Slavov N J. Proteome Res 2018, 17, 2565–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Shishkova E; Zeng.; et al. Nat. Commun 2017, 8, 15571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Chick JM; et al. Nature 2016, 534, 500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Floyd BJ; et al. Mol. Cell 2016, 63, 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Gillet LC; Leitner A; Aebersold R Annu. Rev. Anal. Chem 2016, 9, 449–472. [DOI] [PubMed] [Google Scholar]
- (37).Stefely JA; et al. Nat. Biotechnol 2016, 34, 1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw proteomics data files were deposited into the Chorus repository (Accession #1476).