Abstract
Maternal immunization directed to control RSV infection in newborns and infants is an appealing vaccination strategy currently under development. In this work we have modeled maternal vaccination against RSV in cotton rats (CR) to answer two fundamental questions on maternal vaccine safety. We tested (i), whether a known, unsafe RSV vaccine (i.e., FI-RSV Lot 100 vaccine) induces vaccine enhanced disease in the presence of passively transferred, RSV maternal immunity, and (ii) whether the same FI-RSV vaccine could induce vaccine enhanced disease in CR litters when used to immunize to their RSV-primed mothers. Our data show that FI-RSV immunization of pups with subsequent RSV infection results in vaccine-enhanced disease independent of whether the pups were born to RSV-seropositive or RSV-seronegative mothers, and that FI-RSV immunization of RSV-seropositive mothers does not present a health risk to either the mother or the infant. Our study also raises a novel concern regarding infant immunization, namely that “safe” RSV vaccines (e.g., live RSV administered intramuscularly) may induce vaccine-enhanced disease in RSV-infected pups born to seropositive mothers. Finally, we describe for the first time a sharp decrease in RSV neutralizing antibody titers in immunized seropositive CR at the time of delivery. This decline may reflect maternal immune suppression, potentially pinpointing a window of increased vulnerability to RSV infection that could be alleviated by effective immunization of expectant mothers.
Keywords: Cotton rat, RSV, maternal immunization
1. Introduction
Since there is no safe and effective RSV vaccine for infants, maternal vaccination has been considered as an alternative for controlling early RSV-induced severe disease early in life [1–3]. It is during this time that infants are unlikely to benefit from active immunization, due to the fact that passively transferred maternal immunity strongly inhibits the efficacy of many vaccination protocols [4–6].
Although the reduction in the incidence of RSV acute lower respiratory infection (ALRI) during the first months of life has been correlated with higher levels of RSV-specific maternal antibodies [7, 8], the peak incidence of RSV disease is observed between 2 and 8 months [8–12], when maternal IgG levels are waning [1–3]. This suggests that increasing levels of maternal antibodies against RSV could potentially benefit infants during this critical window of susceptibility to severe RSV infection.
Approval for maternal vaccination requires vaccination trials in one group (mothers), but further testing for the ultimate outcome in another group (infants). These studies tend to take long periods of time and significant resources. Moreover, efficacy surrogates are still under discussion. Formalin-inactivated RSV (FI-RSV Lot 100) vaccine used in early clinical trials led to “vaccine-enhanced disease” after infants and young children contracted naturally occurring RSV infection [13–16]. This effect has been studied extensively in preclinical animal models, but the majority of these studies have utilized naïve animals that were not exposed to RSV prior to FI-RSV vaccination [17–20].
We demonstrated that the cotton rat (CR) model of RSV infection can be used to test the efficacy of maternal vaccination against RSV [21]. However, many issues regarding the immunology of RSV maternal vaccination still remain unknown. Among the most important is the fact that maternal RSV vaccine studies in preclinical models have been performed using naïve animals, rather than the target vaccine population, i.e., RSV-seropositive adult females [11, 22–24]. In addition, the safety of FI-RSV vaccine has never been tested in pregnant females or infants. Yet, in the context of regulatory process towards the approval of maternal vaccines, it is important to demonstrate that both the mother and the child benefit from vaccination and that no adverse effects are seen in either population.
Our study shows that vaccine-enhanced disease develops in pups when they were vaccinated with an unsafe RSV vaccine (i.e., FI-RSV) at the time of waning maternal immunity. Importantly, the studies reported here raise a novel concern regarding infant immunization, namely that “safe” RSV vaccines (e.g., live RSV administered intramuscularly) may facilitate vaccine-enhanced disease in RSV-infected pups of seropositive mothers. Additionally, we investigated whether a FI-RSV vaccine, when used for maternal vaccination of RSV-seropositive females, can trigger RSV-induced vaccine-enhanced disease in the litter. Our data suggest that FI-RSV immunization of RSV-seropositive mothers does not present a health risk to either mother or infant. We also describe for the first time a sharp decrease in RSV neutralizing antibody titers in immunized, seropositive pregnant CR at the time of delivery. This decline may reflect a state of maternal immune suppression, potentially indicating a window of increased vulnerability to RSV infection that could be alleviated by effective immunization of expectant mothers.
2. Materials and Methods
2.1. Animals
Inbred Sigmodon hispidus cotton rats (CR) were obtained from an inbred colony maintained at Sigmovir Biosystems, Inc. (Rockville, MD). Three to 5-week-old female CR were used. At ≥9 weeks, females were paired with males for mating. Animals were pre-bled before inclusion in the study to rule out the possibility of pre-existent anti-RSV antibodies. Animals were housed in large polycarbonate cages and fed a standard diet of rodent chow and water ad libitum. The colony was monitored for antibodies to paramyxoviruses and rodent viruses, and none were detected. All studies were conducted after approval from the Institutional Animal Care and Use Committee. All CR born as a result of breeding during these studies are referred to as “pups,” irrespective of whether they were used for RSV infection at 1 or 4 weeks of age.
2.2. Viruses and viral assays
The prototype Long strain of RSV was obtained from American Type Culture Collection (ATCC VR-26, Manassas, VA). Virus was propagated in HEp-2 cells and serially plaque-purified to reduce defective-interfering particles. A single virus pool (107.6 PFU/ml) was used for all experiments. Stock aliquots were diluted with PBS for intranasal (i.n.) infections. Viral titers in the lungs and in the noses of RSV-infected pups were determined as described [21] and normalized for the weight of the lung portion or expressed per nose.
2.3. RSV neutralizing antibody (NA) assay
RSV NA titers were measured by 60% plaque reduction assay as described [25]. Plaques were quantified and reciprocal NA titers were determined as previously described (the limit of detection of this assay is 4.32 Log2) [21].
2.4 Cytokine gene expression by real-time RT-PCR
Total RNA was extracted from lung tissue using the RNeasy purification kit (QIAGEN). qRT-PCR was carried out as previously described [26]. Reactions were set up in 96-well plates and amplifications performed on a Bio-Rad iCycler (MyiQ Single Color). Delta-delta Ct method was used to calculate relative gene expression after normalization to β-actin as a housekeeping gene [26].
2.5. Lung Histopathology
Lungs were dissected and inflated with 10% neutral buffered formalin to their normal volume, and then immersed in the same fixative solution. After fixation, lungs were paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). An average pathology score was determined based on four parameters of pulmonary inflammation: peribronchiolitis (inflammatory cell infiltration around the bronchioles), perivasculitis (inflammatory cell infiltration around the small blood vessels), interstitial pneumonia (inflammatory cell infiltration and thickening of alveolar walls), and alveolitis (cells within the alveolar spaces). Slides were scored blindly from 0–4 (severity scale) as previously described [27].
2.6. Experimental design
Experiment 1
In the experiment of FI-RSV vaccination in the presence of maternal immunity (outlined in Figure 1A), one group of female CR was primed by RSV infection with 105 PFU RSV/animal (100 µl per animal intranasally (i.n.), whereas another group was left unprimed. Five weeks later, females were mated with RSV-seronegative males. Females started delivering pups at week 9. Pups born to primed or unprimed mothers were subsequently segregated at 4 weeks into 3 groups of balanced sexes: One group was vaccinated with FI-RSV Lot 100 vaccine (produced in the mid 1950s by Pfizer, Inc. for the National Institutes of Health under contract PH43-63-582, 1:125 dilution in PBS) [13], a second was vaccinated with live RSV i.m. (105 PFU RSV A/Long), and a third remained unvaccinated. At week 8, all sera from pups were analyzed for RSV NA, the pups challenged with RSV i.n (105 PFU RSV/animal), and sacrificed on day 4 p.i. for lung and nose viral titers, lung histopathology, and lung cytokine mRNA expression.
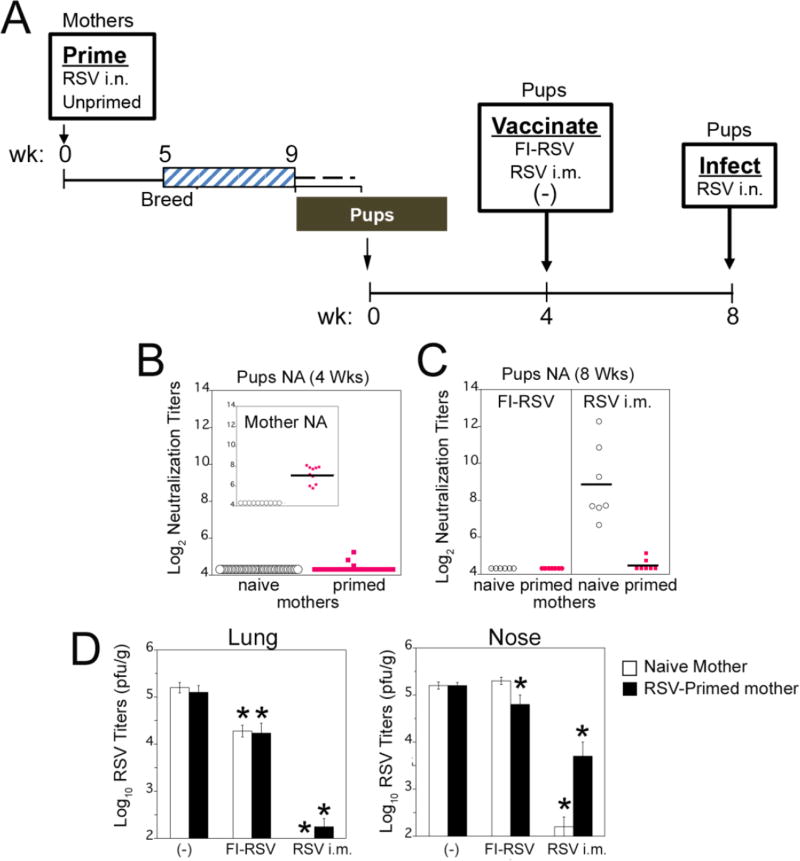
Figure 1. FI-RSV vaccination of animals with passively transferred maternal RSV immunity.
(A) Diagram of the experiment. Female CR were primed by infection with RSV A/Long (105 PFU i.n.) or left unprimed. All females were set in breeding pairs at 5 weeks after RSV infection and on week 9 females began delivering pups. At the age of 4 weeks, pups were vaccinated with FI-RSV, live RSV i.m., or left unvaccinated (−). Four weeks later, serum was collected from each pup and animals were challenged with RSV i.n. Four days after infection pups were sacrificed for determination of lung and nose viral titers, analysis of the mRNA expression of lung cytokines, and lung histopathology. (B) NA titers of 4-week-old pups born to unprimed or primed mothers. Insert shows mothers’ NA titers in sera collected before delivery, indicating that all primed mothers produced NA. (C) NA titers in sera obtained from pups born to naïve or RSV-primed mothers after FI-RSV or live RSV i.m. vaccination (samples collected at week 8 prior to RSV challenge). (D) Lung and nose viral titers measured in samples obtained from pups born to naïve or RSV-primed mothers after vaccination of pups with FI-RSV, live RSV i.m, or left unvaccinated (−). All animals were challenged i.n. with RSV/A Long (105 PFU) and sacrificed 4 days later. Bars represent mean ± SEM. Significant differences between mock vaccinated groups and the respective treated groups were assessed by Student t Test. * p<0.01. (E and F) Lung pathology scores in RSV-infected pups born to naïve or RSV-primed mothers and vaccinated FI-RSV, live RSV i.m., or left unvaccinated (−). (F) Representative pictures of H&E-stained lung tissue obtained from animals in the indicated groups after challenge with RSV (magnification is 100X). (G) Expression of mRNA for IFN-γ and IL-4 in the lungs of RSV-infected pups born to naïve or RSV-primed mothers vaccinated with FIRSV, RSV i.m., or left unvaccinated. Bars represent mean ± SEM. Significant differences between mock vaccinated groups and the respective treated groups were assessed by Student t Test. * p<0.01.
Experiment 2
For maternal vaccine safety experiment, female CR were first primed by RSV infection (105 PFU RSV/animal i.n.) to induce anti-RSV antibodies (outlined in Figure 2A). Eight weeks later animals were separated into two groups (5 per group), with one group vaccinated with the original FI-RSV Lot 100 vaccine. The other group remained unvaccinated. Two weeks later (week 10), all females were mated with RSV-seronegative males. Two weeks later (the time generally corresponding to the middle of pregnancy), females were re-immunized with FI-RSV. Females started delivering pups after week 14. Pups of both sexes were challenged with RSV i.n. at either 1 or 4 weeks of age (105 PFU RSV/animal). On day 4 post-infection (p.i), all pups were sacrificed for analysis of nose and lung viral titers, lung histopathology, and expression of mRNA corresponding to select Th1 and Th2 cytokines as described [26]. An additional group of 5 females remained unprimed and unvaccinated. This group was used as a source of pups that remained uninfected, and provided basal levels of lung gene expression for comparison. Serum samples were obtained from pups before RSV challenge. Mothers were kept on the study for additional 2 months after delivery of pups. At week 22 from the start of the experiment, pups were bled and challenged with RSV (105 PFU RSV/animal) to evaluate pulmonary response to infection.
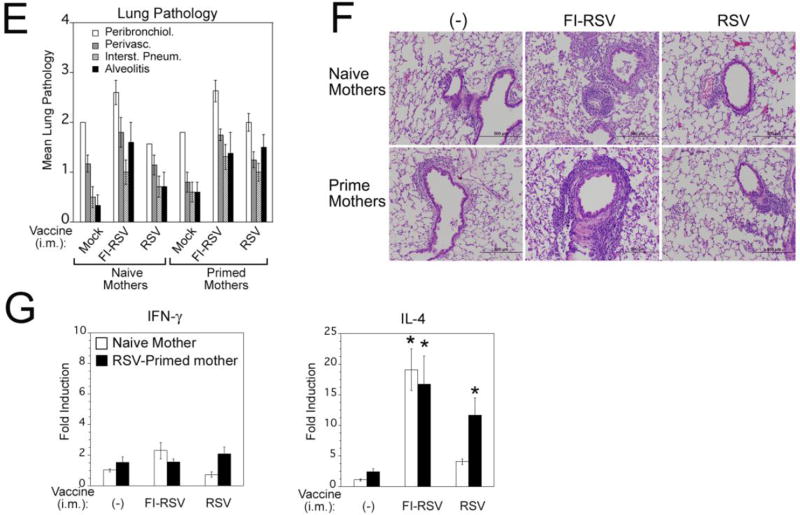
Figure 2. FI-RSV immunization of RSV-primed mothers and its effect on RSV infection of pups.
(A) Diagram of the experiment. Female CR were primed by infection with RSV A/Long (105 PFU i.n.). Eight weeks later primed females were divided into two groups and immunized with FI-RSV Lot 100 (100 µl i.m. of a 1:125 dilution of the original Lot 100 vaccine) or left unvaccinated (−). All females were set in breeding pairs at 10 weeks after RSV-priming, females previously vaccinated with FI-RSV were boosted during pregnancy (12 weeks after RSV priming), and began delivering pups after week 14. Two sex-balanced groups were formed from the pups: pups challenged with RSV at 1 week of age and pups challenged with RSV at 4 weeks of age (both groups were sacrificed 4 days p.i.). Mothers remaining on the experiment were bled at week 22 and challenged with RSV A/Long (105 PFU i.n.) for sacrifice 4 days later. (B) Pups delivered by RSV-primed mothers vaccinated with FI-RSV or unvaccinated (−) were bled at 1 or 4 weeks of age and serum NA titers were determined. Bars represent mean ± SEM. (C) Lung and nose mean viral titers ± SEM in pups (1-week old) born to RSV-primed mothers that were vaccinated with FI-RSV or unvaccinated (−). A slight but significant increase in protection of the lung and the nose was detected in animals born to mothers vaccinated with FI-RSV (* p<0.01 by Student t Test) in 1-week old pups. Neither lung nor nose of animals challenged at 4-weeks of age showed protection in the group born to FI-RSV-vaccinated mothers. (D) Lung pathology scored in animals (1- and 4-weeks old) born to RSV primed-mothers vaccinated with FI-RSV or unvaccinated. Numbers in parentheses above each bar represent the number of animals used for analysis. (E-F) Expression of mRNA for IFN-γ and IL-4 in RSV-infected (+) or uninfected (−) animals born to RSV-primed mothers that were vaccinated with FI-RSV or unvaccinated (−) during pregnancy. Unchallenged pups from naïve mothers were used as control for base-level gene expression (c). Bars represent mean ± SEM. (G) NA titers of RSV-primed mothers that were vaccinated with FI-RSV- or unvaccinated (−) before challenge on week 20 post-priming (left panel) and lung histopathology scores in the same animals determined at day 6 post-challenge (right panel). Transversal lines represent mean values in each group.
Experiment 3
For experiments evaluating dynamics of RSV NA in pregnant mothers (Figure 3, Table 1), groups of female CR (3 weeks of age, 5 rats/group) were primed by infection with live RSV (105 PFU/animal i.n.). At different times post post-priming, females were immunized i.m. with various vaccines described in previous studies: 105 PFU of RSV A/Long [21] (“RSV i.m.”); a formulation with RSV Fusion (F) protein [28] with MPL (Avanti Polar Lipids) (10 µg of F and 15 µg of MPL) (“RSV-F + MPL”); or one with RSV F protein alone (“RSV-F”) [29], and were subsequently bred to naïve, male CR. Blood samples were obtained during early pregnancy (6 weeks after priming in for animals vaccinated with RSV and 12 weeks after priming for animals vaccinated with preparations containing RSV F), and again before the time of delivery (≤7 days), and serum samples were tested for RSV NA. As control, RSV NA was measured in serum samples from RSV-primed (105 PFU RSV/animal) female CR that were never pregnant (Control).
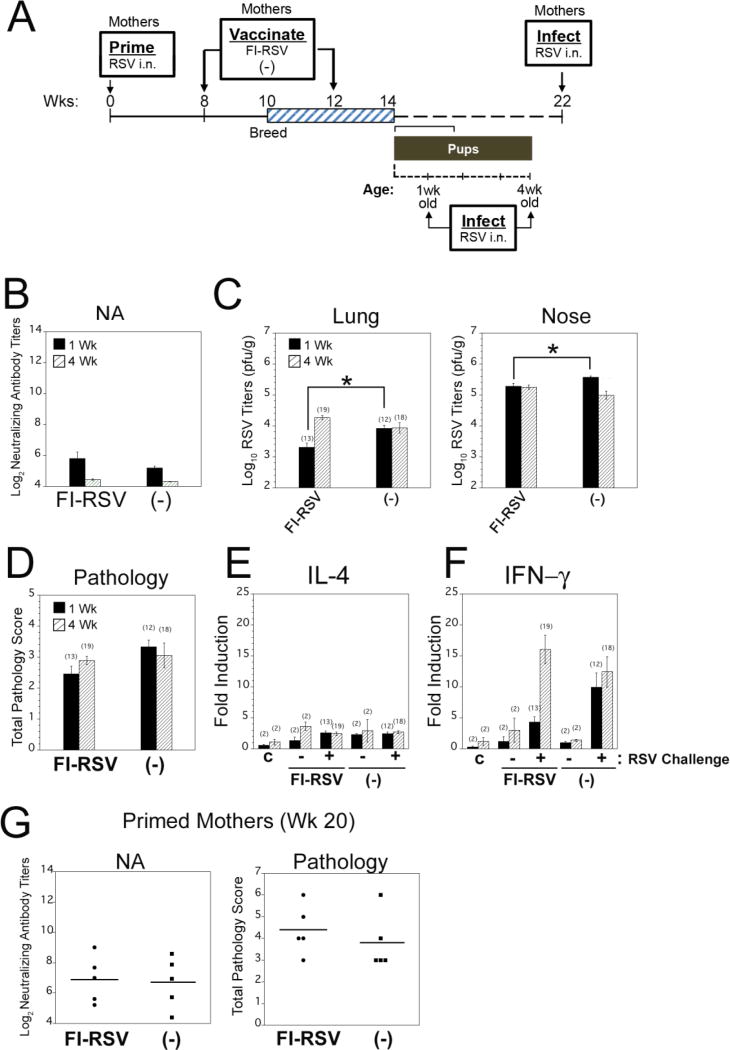
Figure 3. Reduction of RSV NA activity in pregnant female cotton rats just before delivery.
Female (3 week old) CR were primed i.n. by infection with 105 PFU of RSV/Long. After priming, females were vaccinated with either RSV Long i.m. (105 PFU, Group A), with a vaccine formulated with the RSV F protein in the presence of MPL (10 µg of F + 15 µg/rat of MPL"RSV-F + MPL”), or with the RSV F alone (“RSV-F) and set in breeding pairs at different time from priming (“RSV i.m.” at d35, RSV-F preparations at day 70). Animals were bled once during early pregnancy (first measurement, open arrowhead) and the second time just before the time of delivery (second measurement, filled arrowhead). Percentage of total NA was defined as 100% for all females after infection during early pregnancy. Black dots and line represent natural decay of RSV-induced NA in females that were not pregnant. Dots represent mean ± SEM.
TABLE 1.
Three groups of female CR (3 weeks old) were primed by i.n. infection with 105 PFU of RSV A/Long and subsequently immunized with live RSV i.m. (105 PFU of RSV A/Long [21]) (“RSV i.m.), or with a vaccine formulated with RSV F protein in the presence or absence or MPL (10 µg of F and 15 µg of MPL [29]) (“RSVF+ MPL” and “RSV-F”, respectively). Animals were set in breeding pairs at the indicated times post-priming (Breeding Setup) and serum sample were obtained during early pregnancy and just before delivery. Titers are shown as the reciprocal dilution that resulted in 60% RSV plaque reduction in HEp-2 cells ±SE.
| Group | Breeding Setup (wk post priming) |
Serum Sample Early Pregnancy |
Serum Sample Before Delivery |
Females (n) |
||
|---|---|---|---|---|---|---|
| wk | Titer ± SE | wk | Titer ± SE | |||
| RSV i.m. | 5 | 6 | 1720±832 | 8 | 342±49 | 5 |
| RSV-F + MPL | 10 | 12 | 1090±100 | 14–16 | 205±28 | 5 |
| RSV-F | 10 | 12 | 839±123 | 14–16 | 158±30 | 5 |
3. Results
3.1 FI-RSV and RSV immunization of pups born to RSV-primed or naïve mothers: effect of pups’ immunization on pups’ response to RSV. Experiment 1 (Figure 1)
Infants and older babies were vaccinated in 1965–67 with the FI-RSV Lot 100 in four different clinical trials [13–16]. The most profound adverse effects of FI-RSV vaccination were described in the trial that took place in the Washington, D.C. [13]. What differentiated this study from the others was that the population in the Kim et al. trial was composed exclusively of infants between 2 and 7 months [13], the youngest group used for the overall FI-RSV Lot 100 trials. This study showed the highest rates of hospitalizations: 80% of FI-RSV Lot 100 vaccinees were hospitalized vs. only 5% of the infants vaccinated with the control vaccine. In addition, it was reported that all infant vaccinees possessed detectable levels of pre-vaccination anti-RSV antibodies, most likely reflecting maternally transferred immunity [13].
The enhanced pathology associated with FI-RSV Lot 100 vaccination has been recapitulated in many animal models [19, 20, 31], including CR [17, 26]. However, no studies used young animals born to RSV-seropositive mothers. To recapitulate the conditions of Kim’s study and to validate this model of infant vaccination, CR born to RSV-seropositive or naïve mothers were subsequently vaccinated with FI-RSV Lot 100. This experiment was carried out as follows (Figure 1A) as described in the Experimental Design section.
RSV NA were measured in serum samples of all pups before vaccination (Figure 1B). Four-week-old pups born to RSV-primed mothers had low to undetectable levels of maternally-derived anti-RSV NA, consistent with our previous results that show that by four weeks of age, only trace maternal NA remain in the pups [21], confirming maternal transfer (maternal NA levels shown in Figure 1B, inset panel). After vaccination, but before challenge (8 wks), pups vaccinated with FI-RSV showed no detectable NA, independent of the maternal immunological status (Fig. 1C, “FI-RSV”). In pups vaccinated at 4 weeks with live RSV i.m., only those born to immunologically naive mothers showed an increased RSV NA at 8 weeks (Fig. 1C “RSV i.m.”). Thus, in spite of almost undetectable maternal RSV NA in 4-week-old pups born to RSV-primed mothers (Fig. 1B), we observed a strong inhibitory effect of the maternally transferred immunity on the efficacy of RSV i.m. vaccination of the pups.
As expected, vaccination of pups with live RSV i.m. induced the strongest protection of the lung and nose (Fig. 1D). Only weak protection (~ 1 log10 PFU/g tissue reduction) was detected in the lungs of animals vaccinated with FI-RSV (Fig. 1D). In support of the inhibitory effect of passively transferred maternal immunity to RSV, pups derived from primed mothers and vaccinated with live RSV i.m. showed strong, but incomplete, protection against virus challenge in the lung, in contrast to pups derived from RSV-naïve mothers (Fig. 1D, Lungs) where full protection was achieved. The same trend was seen when the nose was examined (Fig. 1D, Nose). Despite the differences in protection between pups from primed vs. unprimed mothers, it is clear that measurement of vaccine-induced NA in the pups was not a good correlate of protection since protection in these animals was significant (Figure 1D, Lung and Nose, p<0.01), despite the lack of NA in pups from primed mothers at 4 weeks post-vaccination (Fig. 1C, RSV i.m., red dots).
Pups vaccinated with FI-RSV Lot 100 showed the strongest lung histopathology (Fig. 1E and F), including high scores for alveolitis, the hallmark of vaccine-enhanced disease in the CR model, regardless of whether their mothers were RSV-primed or not. Furthermore, animals born to RSV-primed mothers and vaccinated with live RSV i.m. at 4 weeks of age also showed enhanced lung pathology upon RSV challenge. The level of alveolitis in RSV i.m.-immunized animals born to RSV-primed mothers was comparable to that detected in FI-RSV-immunized animals born to RSV-primed mothers (Figure 1E and F).
The enhanced expression of lung mRNA for the cytokines IL-4 and IFN-γ has been correlated with the level of vaccine enhanced disease found after infection in CR [26, 29]. Increased expression of mRNA for IL-4 was detected in FI-RSV-immunized pups born to either naïve or RSV-primed mothers. Significant increase IL-4 expression was also detected in RSV i.m.-immunized pups born to RSV-primed mothers (Fig. 1G). No changes in the expression of IFN-γ was seen in pups vaccinated with FI-RSV or RSV i.m. irrespective of their maternal priming history (Fig. 1G). Interestingly, while alveolitis was increased in RSV i.m.-immunized animals born to RSV-primed mother, peribronchiolitis was not (Figure 1E and F), potentially suggesting different mechanisms of disease enhancement in the presence of passive immunity.
3.2 Effect of FI-RSV immunization of RSV-primed mothers on pups’ response to RSV. Experiment 2 (Figure 2)
Vaccine safety in offspring should be explored for all RSV vaccine candidates used during pregnancy. Thus, we tested the effects of maternal FI-RSV vaccination of RSV-primed mothers in the response of their pups to RSV challenge. One-week old pups born to RSV-primed mothers that had been vaccinated/boosted with FI-RSV (scheme on Figure 2A) showed slightly higher mean NA titers than pups born to RSV-primed, unvaccinated mothers (Figure 2B), although this increase was not statistically significant. The minor increase in RSV NA titer detected in 1-week old pups born to RSV-primed, FI-RSV-immunized mothers correlated with significantly increased protection of the lungs in this group (Fig. 2C, Lung). In the nose, there was a similar trend for improved protection in pups born to primed, FI-RSV-immunized mothers; however, statistical significance was not achieved (Figure 2C, Nose). No RSV NA titers (Fig 2B) and no protection were detected in pups born to either group of mothers and challenged at 4 weeks of age (data not shown). This result was expected since we previously reported that by four weeks of age, only trace maternal NA remain in the pups [21].
Lung histopathology was evaluated in pups born to RSV-primed mothers vaccinated with FI-RSV or unvaccinated and challenged with RSV at 1 or 4 weeks of age (Figure 2D). No increase in lung pathology and no evidence of alveolitis, the histopathologic hallmark of vaccine-enhanced disease in CR [26, 27], were detected in RSV-infected pups born to FI-RSV-vaccinated mothers. The lack of enhanced pathology in RSV-challenged pups born to RSV-primed FI-RSV-vaccinated mothers correlated with low levels of the expression of IL-4 mRNA and moderate expression of IFN-γ mRNA in both 1- and 4-week-old animals (Fig. 2,E and F, respectively). Higher expression of IFN-γ was noted in all RSV-infected pups born to RSV-primed mothers, independent of whether the mother was FI-RSV-immunized or not (Fig. 2E). Expression of IL-4 mRNA was low and comparable between pups born to FI-RSV-immunized or unvaccinated mothers (Fig. 2F), further ruling out the possibility of vaccine enhancement of disease in animals born to FI-RSV-immunized mothers.
FI-RSV vaccination of RSV-primed mothers failed to increase RSV NA titers in mothers (Figure 2G, left panel). As previously shown in the mouse [30], vaccination of RSV-seropositive CR with FI-RSV did not induce enhance lung pathology after challenge with RSV (Figure 2H, right panel). These data reinforce the concept that pre-existent immunity to RSV as a consequence of prior infection prevents vaccine-enhanced disease.
In the experiment just presented, RSV-primed females were vaccinated with FI-RSV twice: the first time before pregnancy and the second time 2 weeks after mating. However, similar results were obtained in parallel studies where only one FI-RSV-vaccination was performed during pregnancy (data not shown).
3.3 The level of RSV neutralizing antibodies in mothers drops shortly before delivery. Experiments 3, (Figure 3, Table 1)
Groups of naïve female CR were infected i.n. with RSV A/Long (105 PFU/animal) to elicit circulating anti-RSV NA (Table I). At different times p.i. (2 weeks for the “RSV i.m.” and 8 weeks for animals vaccinated with preparations of “RSV-F+MPL” or “RSV-F alone”), all females were vaccinated [29]. Females were mated to seronegative male CR. Serum samples were obtained from each female early during pregnancy and then close to the time of delivery (≤7 days “Before Delivery” samples) (Table 1, Figure 3)). Mean serum NA were quantified. All animals in both vaccination experiments showed a strong reduction in serum NA at the bleeding taken closest to the time of delivery. Figure 4 shows the percent change in RSV NA as the time of delivery approaches. Control infected, non-pregnant or vaccinated females CR exhibited a gradual decrease in the total amount of NA (solid black line) over time, with mean decreases of 10, 25, and 30% at 49, 63, and 91 days p.i., respectively. However, a much more precipitous decrease in NA titers against RSV was measured in pregnant females during the time preceding delivery, a result that was observed in two independent experiments (blue and red dashed lines). This set of data supports the conclusion that during the last week of pregnancy in CR, a strong reduction in RSV NA takes place that might deleteriously impact maternal defenses against RSV.
4. Discussion
Maternal immunity is a major form of protection against many infectious diseases early in life. IgG antibodies can be transferred from pregnant women to their fetuses through placental transfer, colostrum, and milk [4]. Thus, maternal immunization has emerged as an important strategy to protect both pregnant women and their newborn infants against several infectious diseases, including influenza, tetanus, and pertussis [1, 32]. In the case of RSV, it has been assumed that maternal vaccination could provide strong benefit for full-term newborn babies by increasing their maternally-transferred NA titers during the early period of life associated with the greatest vulnerability [1, 3].
We showed that maternal vaccination against RSV in the CR can be used to define vaccine preparations that could improve preexistent immunity and induce subsequent transfer of efficient immunity to infants [21]. However, a set of premises related to safety of maternal vaccination against RSV have not been tested rigorously using preclinical RSV models of vaccination. In the late 1960s, the results of a clinical trial in infants and very young children using FI-RSV Lot 100 raised strong safety concerns that seriously affected development of RSV subunit vaccines [33]. Concerns over vaccination with non-replicative vaccines also extends to expectant mothers. FI-RSV vaccination in naïve female mice and the impact in their offspring was previously evaluated [22]. In our study, we investigated whether RSV-seropositive CR mothers vaccinated with FI-RSV Lot 100 prior to pregnancy and boosted during their pregnancy, would predispose their litters to the enhanced vaccine disease (Figure 1A). Our data suggest that this unsafe RSV vaccine administered to RSV-seropositive expectant mothers will not predispose offspring to a deleterious outcome upon RSV challenge. Our study extends previous findings to maternal immunity model by showing that FI17 RSV immunization of seropositive animals does not result in potentiation of disease in offspring [30].
Most humans are seropositive for RSV. Infants born prematurely (32 to 35 wks) and infants born during the spring, who are unlikely to encounter the virus prior to the following winter when they no longer have maternal antibodies, are at the highest risk of RSV ALRI and hospitalization. In the case of full-term infants, extension of their passive immunity through maternal vaccination has been postulated as a possible solution to reduce disease and hospitalization [1–3, 12]. Thus, the model presented here reproduces the immunological status of this population. Passive immunity from the mother to the baby could potentially delay onset of and decrease the severity of infection during early life, but even at low or undetectable levels, passive immunity can interfere with the active immune responses of the offspring [6]. Interestingly, we have shown that in addition to low or even undetectable levels of maternal NA in 4-week-old pups born to RSV-primed mothers, a strong inhibitory effect is detected in the pups immunized with live RSV i.m. (Figure 2C). We consider this observation to be particularly important since it implies that clinical trials in infants with low to undetectable RSV NA would likely fail to mount a robust NA response upon immunization. Furthermore, we show that despite the low induction of NA in these animals, vaccine-induced protection is induced, indicating that RSV NA and RSV protection do not tightly correlate in this population. An understanding of the mechanism underlying this phenomenon is strongly needed before a successful RSV vaccine for the infant population can be produced.
Our data also confirm that the deleterious effects of FI-RSV vaccination seen in the Kim trial can be modeled in the CR [6]. The strong pathology seen in FI-RSV-vaccinated animals was comparable between the groups, whether or not the vaccinees had passive antibody titers (Figure 3B). This indicates that (1) FI-RSV vaccine’s deleterious effect on pups is independent of the presence or absence of passive maternal NA; (2) parameters associated with RSV vaccine enhancement of lung pathology remain the same, i.e., enhanced alveolitis and increased expression of the Th2 cytokine IL-4; and (3) vaccine-enhanced disease could be related to immunization of pups during the time when maternal immunity is waning and NA levels are low or undetectable. We consider that these studies generate important parameters with which to evaluate programs directed at the delineation of mechanisms involved in maternal transfer of immunity, with the goal of improving maternal and infant RSV vaccination. It is important to mention that studies using a model of bovine RSV (bRSV) infection and bovine FI-RSV vaccination has also demonstrated that the effects of FI-RSV are upheld in the presence of maternal antibodies against bRSV [34].
Our studies have revealed a consistent decrease in important mediators of immunity specific for RSV in pregnant CR, i.e., an abrupt reduction in RSV serum NA when delivery is imminent. An early study has suggested certain degree in immunosuppression for RSV during late pregnancy [35]. Although we did not evaluate the consequences that this reduction in NA has on the health of females during the perinatal period, this data would certainly support the argument that an RSV vaccine has to benefit vaccine recipients directly. Recently, three cases of maternal RSV infection and hospitalization have been reported, highlighting the under-recognized effects of maternal RSV infection in expecting mothers [36, 37]. Cases of severe RSV in pregnant women have also recently been presented (Dr. Pedro Piedra’s lab, 10th Internationals RSV Symposium, 2016, Argentina). They showed reduced RSV NA during the last trimester of pregnancy and a subsequent increase after birth. Reduction in total IgG(2) has been associated with increased frequency of influenza H1N1 infection during pregnancy [36]. Taken together, this data supports the value of RSV vaccination during pregnancy to overcome late-pregnancy reduction of RSV neutralizing antibodies and thereby increase resistance to RSV infection in the mothers.
Acknowledgments
The authors would like to thank Ms. Martha Malache, Mr. Charles Smith, and Mr. Fredy Rivera, and Ms. Ana Rivera for their technical support with the animals.
Funding Statement: This work was supported by Sigmovir Biosystems, Inc. corporate funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chu HY, Englund JA. Maternal immunization. Clin Infect Dis. 2014;59:560–8. doi: 10.1093/cid/ciu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development - A global agenda. Vaccine. 2016;34:2870–5. doi: 10.1016/j.vaccine.2016.03.109. [DOI] [PubMed] [Google Scholar]
- 3.Munoz FM. Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy? Current opinion in infectious diseases. 2015;28:221–4. doi: 10.1097/QCO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 4.Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine. 1998;16:1456–63. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 5.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–84. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewien KE, Barbosa V, de Lima OS, Osiro K. The influence of maternally derived antibody on the efficacy of further attenuated measles vaccine. Infection. 1978;6:207–10. doi: 10.1007/BF01642310. [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–15. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, et al. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176:794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143:S118–26. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharma A, Wendland R, Sung B, Wu W, Grunwald T, Worgall S. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine. 2014;32:5761–8. doi: 10.1016/j.vaccine.2014.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Human vaccines & immunotherapeutics. 2013;9:1263–7. doi: 10.4161/hv.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 14.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–48. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 15.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–21. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 16.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 17.Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, Horswood RL, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986;57:721–8. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–40. [PubMed] [Google Scholar]
- 19.Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC, 3rd, Sotnikov AV, et al. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–51. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Waal L, Power UF, Yuksel S, van Amerongen G, Nguyen TN, Niesters HG, et al. Evaluation of BBG2Na in infant macaques: specific immune responses after vaccination and RSV challenge. Vaccine. 2004;22:915–22. doi: 10.1016/j.vaccine.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Blanco JC, Pletneva LM, Oue RO, Patel MC, Boukhvalova MS. Maternal transfer of RSV immunity in cotton rats vaccinated during pregnancy. Vaccine. 2015;33:5371–9. doi: 10.1016/j.vaccine.2015.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon YM, Hwang HS, Lee JS, Ko EJ, Yoo SE, Kim MC, et al. Maternal antibodies by passive immunization with formalin inactivated respiratory syncytial virus confer protection without vaccine-enhanced disease. Antiviral Res. 2014;104:1–6. doi: 10.1016/j.antiviral.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buraphacheep W, Sullender WM. The guinea pig as a model for the study of maternal immunization against respiratory syncytial virus infections in infancy. J Infect Dis. 1997;175:935–8. doi: 10.1086/513994. [DOI] [PubMed] [Google Scholar]
- 24.Glenn GM, Fries LF, Smith G, Kpamegan E, Lu H, Guebre-Xabier M, et al. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine. 2015;33:6488–92. doi: 10.1016/j.vaccine.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 26.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027–35. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 27.Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385–92. [PubMed] [Google Scholar]
- 28.Blanco J, Boukhvalova M, Pletneva L, Shirey K, Vogel SN. A Recombinant Anchorless Respiratory Syncytial Virus (RSV) Fusion (F) Protein/Monophosphoryl Lipid A (MPL) Vaccine Protects against RSV-induced Replication and Lung Pathology. Vaccine. 2014 doi: 10.1016/j.vaccine.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine. 2014;32:1495–500. doi: 10.1016/j.vaccine.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waris ME, Tsou C, Erdman DD, Day DB, Anderson LJ. Priming with live respiratory syncytial virus (RSV) prevents the enhanced pulmonary inflammatory response seen after RSV challenge in BALB/c mice immunized with formalin-inactivated RSV. J Virol. 1997;71:6935–9. doi: 10.1128/jvi.71.9.6935-6939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–60. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsey B, Kampmann B, Jones C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Current opinion in infectious diseases. 2013;26:248–53. doi: 10.1097/QCO.0b013e3283607a58. [DOI] [PubMed] [Google Scholar]
- 33.Anderson LJ. Respiratory syncytial virus vaccine development. Seminars in immunology. 2013;25:160–71. doi: 10.1016/j.smim.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 34.Gerswin LJ, Schelegle ES, Gunther RA, Anderson ML, Woolums AR, Larochelle DR, et al. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine. 1998;11–12:1225–36. doi: 10.1016/s0264-410x(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 35.Nandapalan N, Taylor CE, Greenwell J, Scott M, Scott R, Hey EN, et al. Seasonal variations in maternal serum and mammary immunity to RS virus. J Med Virol. 1986;20:79–87. doi: 10.1002/jmv.1890200110. [DOI] [PubMed] [Google Scholar]
- 35.Wheeler SM, Dotters-Katz S, Heine RP, Grotegut CA, Swamy GK. Maternal Effects of Respiratory Syncytial Virus Infection during Pregnancy. Emerg Infect Dis. 2015;21:1951–5. doi: 10.3201/eid2111.150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon CL, Johnson PD, Permezel M, Holmes NE, Gutteridge G, McDonald CF, et al. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin Infect Dis. 2010;50:672–8. doi: 10.1086/650462. [DOI] [PubMed] [Google Scholar]
- 37.Chu HY, Katz J, Tielsch J, Khatry SK, Shrestha L, LeClerq SC, et al. Clinical presentation and birth outcome associated with respiratory syncytial virus in pregnancy. PLOS One. 2016;11(3):e0152015. doi: 10.1371/journal.pone.0152015. [DOI] [PMC free article] [PubMed] [Google Scholar]