Abstract
Purpose:
The objectives of this study were to test the acceptability and feasibility of a survivorship needs assessment planning (SNAP) tool for head and neck cancer (HNC) survivors and caregivers, evaluate short-term changes in psychosocial outcomes after completing the SNAP session and develop strategies for system refinement.
Methods:
We used a prospective one-group design and mixed methods with HNC survivors and caregivers (N=25 dyads). Participants completed baseline and 6-week surveys before and after completing a SNAP clinic visit to assess psychosocial outcomes and acceptability. Intervention sessions included tablet-based needs assessments driving tailored care plans. Dyads’ open-ended feedback and clinician interviews (N=12) evaluated acceptability and feasibility.
Results:
SNAP data collection time burden and technology challenges were minimal and care plans included messages (M=19), educational materials (M=13) and referrals (M=4.5; 86% Behavioral Medicine, 77% Nutrition, 65% Physical Therapy). Participants reported high satisfaction with the session and care plan, highlighting the key strengths of pulling complex medical information together and the focus on caregiver well-being, with multiple suggestions to facilitate clinic workflow. Depression and unmet needs decreased and survivorship knowledge increased significantly in survivors and caregivers (p <.05) over the 6-week period.
Conclusions:
The SNAP tool is an innovative technology-based survivor-centered strategy to assess and manage needs in HNC survivors and caregivers. Results support its acceptability and ability to address dyads’ needs; the tool merits further testing in a clinical trial.
Implications for Cancer Survivors:
Technology-enabled care planning may be a productive way to assess and address HNC dyads’ dynamic needs after treatment.
Keywords: head and neck cancer, survivorship, patient-reported outcomes, dyads
Introduction
Head and neck cancers (HNCs) in the oral cavity, larynx and pharynx lead to unique and dynamic survivorship challenges with intense late and long-term effects critically impacting activities of daily living [1, 2]. Patients’ informal, unpaid caregivers also face demanding roles in the recovery process and often suffer adverse effects on their own well-being [3–5]. As national guidelines advance regarding the delivery of survivorship care to improve transitions from treatment to the post-treatment period [6–9], a specialized approach is needed in HNC. In addition to providing survivors with information about their diagnosis, treatment, follow-up care and health promotion [8, 10], quality HNC survivorship care must address a wide array of physical, emotional and social concerns in survivor-caregiver dyads [1, 11–14] . Therefore, it may be ideal to incorporate the systematic assessment of patient-reported outcomes (PROs) and consider caregiver needs in HNC survivorship care.
Research capitalizing on technology to systematically assess and address PROs in cancer patients is growing at a fast pace and has demonstrated improved symptom management and survival outcomes [15–17]. Research has highlighted that tablet computers are a feasible way to collect data for HNC patients [18, 19] and may be particularly beneficial to address speech barriers and facilitate identification of current, critical needs in survivors facing multiple potential challenges [2, 12, 20]. Technology-focused HNC interventions have shown benefits in promoting adherence to swallowing exercises [21, 22], recovery from surgery [23], self-care after total laryngectomy [24] and self-management outcomes [25, 26]. However, research is lacking in the use of technology to assess and address survivors’ needs at the end of treatment and there are few cancer caregiver-focused interventions capitalizing on technology [27].
Given the need for a specialized and dyad-focused approach to survivorship care in HNC, we developed a survivorship needs assessment planning (SNAP) tool to systematically assess needs in post-treatment HNC survivors and their primary caregivers and generate tailored care plans [19]. The objectives of the current study were to test the acceptability and feasibility of the SNAP system, evaluate short-term changes in psychosocial outcomes after completing the SNAP session and refine the system for future use.
Methods
Study Design and Data Collection Overview
We used a prospective one-group design and mixed methods to carry out the study. Participants completed a baseline survey by telephone or mail, an in-person clinic session concluding with a brief evaluation survey by tablet computer, and a 6-week follow-up survey by telephone or mail (N=25 dyads). Intervention delivery details were monitored using an implementation tracking log and a nurse rating form. Finally, we included open-ended questions in survivor and caregiver follow-up surveys and conducted a set of key informant interviews with HNC healthcare providers (N=12) after completing the intervention period to gather feedback about the SNAP program.
Setting and Study Participants
Following study protocol approval by the Institutional Review Board at the Medical University of South Carolina (MUSC), a convenience sample of survivors was identified using the electronic medical record. We screened survivors ≥ 21 years old who had completed all treatment for stage I-IVA cancer of the upper aerodigestive tract (lip/oral cavity, pharynx, larynx, salivary gland, paranasal sinus) at the MUSC Hollings Cancer Center from November 2015 to May 2016. We recruited a sample diverse by sociodemographic and clinical variables to assure representation of the clinic population but narrowed the focus to survivors who had received at least two treatment types (surgery, radiation, chemotherapy). Participants were excluded when they did not speak English, had cognitive challenges interfering with questionnaire completion, did not have a caregiver or had recurrent disease. Potential participants were sent a study introduction letter and called to screen for eligibility and nominate their primary caregiver, the person they reported relying on most for cancer-related support. All participants signed informed consent forms in person, survivors and caregivers completed separate questionnaires and received a gift card after completing each survey.
SNAP Intervention
Details regarding the development of the SNAP tool were previously published [19]; key informant interviews and focus groups with HNC patients, caregivers and healthcare providers guided SNAP item development and session delivery processes. The SNAP tool is an Enterprise Data Management System with an administrator planning interface, a clinic data collection application and a personalized algorithm-driven care report delivery component (Figure 1). The web-based interfaces feed into REDCap (Research Electronic Data Capture) [28], a secure application supporting SNAP needs assessment data entry with branching logic and import/export procedures. The SNAP administrator interface includes menus to design survey formatting (change font, add colors, add images), develop libraries of messages, referrals and educational materials for care plans and build logic algorithms based on both survivor and caregiver responses to set targets to generate resources for care plans.
Figure 1.
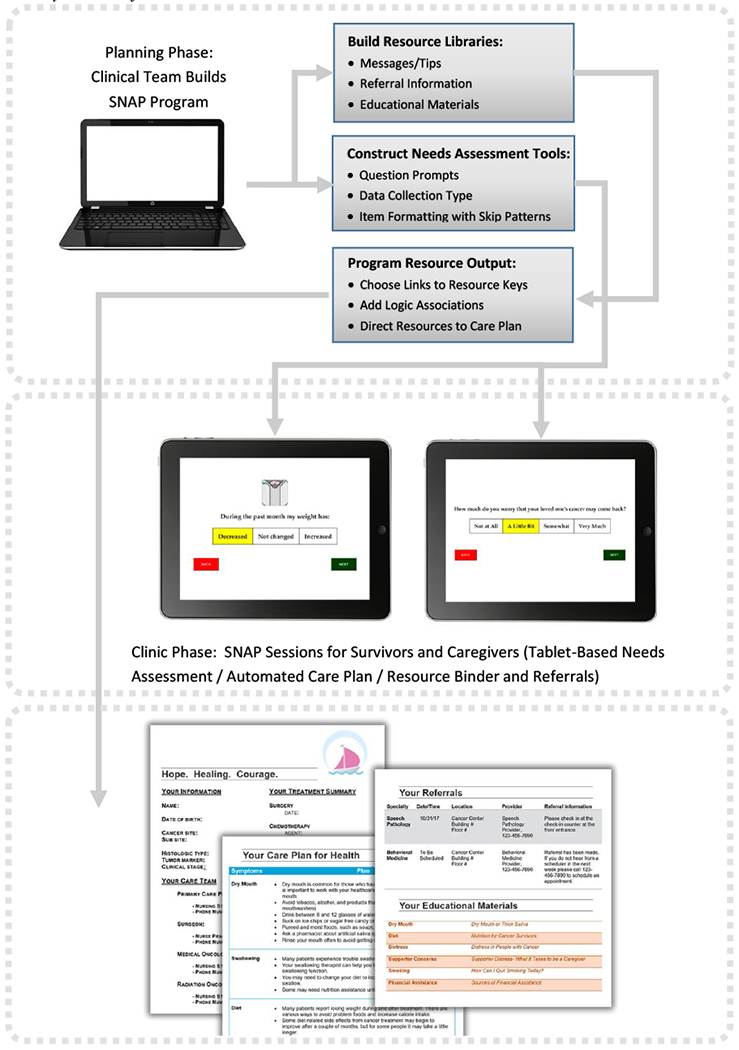
SNAP System Workflow
In the SNAP clinic visit, a wireless touchscreen tablet device renders a web-based interface designed for ease of data collection with one question per page, color displays, large font and images. The system authenticates to a data tracking system, registers assessments, records data and, based on administrator-designated logic considering both survivor and caregiver responses, generates a draft tailored care plan. Care plans include a care summary cover page with diagnosis, treatment and care team information followed by details about follow-up care and a set of algorithm-driven tailored messages, referrals and listing of educational materials mapped to reported concerns, symptoms and behaviors of survivors and caregivers (Figure 1). A Nurse Practitioner reviewed draft care plans with survivor-caregiver dyads, discussed questions and finalized referrals, offering a final printed care plan and tailored resource binder.
Guided by PRO assessment principles [29], we adopted a screening approach for SNAP assessments with single screener items [17, 19] to reduce respondent burden. This process yielded a set of 63 survivor and 28 caregiver items previously published [19] with item selection guided by local resource availability and algorithms driving care plan resources developed by Clinical Advisory Board consensus [19]. We assessed 1) unmet needs [30, 31], satisfaction with nutritional status, fear of recurrence, dyadic efficacy, tobacco and alcohol use and self-efficacy in both survivors and caregivers, 2) symptom severity and interference [32, 33], nutritional intake and weight change status in survivors only, and 3) caregiver distress and burden in caregivers only.
Measures
We assessed a comprehensive set of survivor, caregiver and dyadic primary and secondary outcomes guided by emerging measurement [34] and conceptual frameworks [35] in survivorship care planning. As described below, we also monitored program delivery elements to examine feasibility (factors critical to clinic uptake) and acceptability (factors relevant to survivor and caregiver system use). Feasibility factors included ability to complete SNAP protocol steps, time burden to complete assessments and nurse visits and technology challenges encountered. Acceptability factors included survivor and caregiver satisfaction with session elements and timing and comfort with data collection and nurse ratings of session length and survivor/caregiver engagement and understanding.
Primary Outcome Variables (assessed by survey at baseline and 6-week follow-up)
Depression was assessed in survivors and caregivers using the Patient-Reported Outcomes Measure Information System (PROMIS) short-form instrument [36, 37]. Participants rated the extent to which they were feeling depressed, worthless, hopeless and helpless in the past 7 days on a 5-point response scale from never (1) to always (5). Total raw scores for each participant were translated into standardized t-scores ranging from 41 to 79.9 with higher scores reflecting higher depression. Cronbach’s alphas in the current study sample were 0.50 for survivors and 0.88 for caregivers at baseline. Further analyses of the survivor data showed the 95% confidence intervals for patients’ baseline scores was large (0.18 to 0.83) and 9/26 (35%) had zero variance across the 4 items, potentially impacting the alpha score with the modest sample size.
Unmet needs were assessed in survivors and caregivers using an adapted version of the Cancer Survivors/Partners Unmet Needs instruments [30, 31]. After pilot testing the instrument with staff and volunteers, we modified the wording of several items and the response options. Respondents rated 30 items concerning whether they currently needed help with comprehensive care, information, quality of life, existential survivorship and relationship issues (yes or no). Total number of unmet needs was calculated.
Survivorship knowledge was assessed using the 11-item Preparing for Life As a New Survivor (PLANS) measure developed at the University of Michigan and used in prior research [38–40] evaluating survivor and caregiver knowledge about follow-up care, who to call with questions and the extent to which they felt prepared for the next phase of their/their loved one’s cancer experience and believed the health care team had prepared them for what to expect during recovery (1=Strongly Disagree to 6=Strongly Agree). Items were averaged and Cronbach’s alphas in the current study sample were 0.71 for survivors and 0.90 for caregivers at baseline.
Dyadic coping was assessed using a modified cancer-specific version of the 5-item dyadic coping subscale of the Dyadic Coping Inventory [41]. Participants reported the frequency (1=Neverto 5=Always) with which they engaged in dyadic coping efforts (e.g., tried to cope with problems related to cancer together, engaged in serious discussions about cancer, were satisfied with the way they dealt together with cancer-related stress). Cronbach’s alphas in the current study sample were 0.87 for survivors and 0.81 for caregivers at baseline.
Caregiver burden was evaluated using a 4-item screening version of the Zarit Burden Inventory, which has demonstrated strong properties [42]. Scores range from 0 to 16; higher scores represent greater burden. Cronbach’s alpha in the current study sample was 0.74 for caregivers at baseline.
Secondary Outcome Variables (assessed by survey at baseline and 6-week follow-up)
Symptom distress was assessed in survivors and caregivers with participant report of current distress concerning the survivors’ symptoms on a scale from 0 (Not at All Distressing) to 10 (Extremely Distressing). Symptom management abilities was assessed with 1 item about how well survivors and caregivers believed survivors were able to manage current symptoms on a scale from 0 (Cannot Manage at All) to 10 (Can Manage Extremely Well).
Self-efficacy was assessed using two single item questions from the National Cancer Institute Follow-up Care Use and Health Outcomes of Cancer Survivors (FOCUS) survey [43] to assess survivor and caregiver confidence in one’s ability to get advice and information about cancer if needed and to keep to the follow-up care schedule recommended by their doctors (0=Not at all Confident to 4=Completely Confident). Follow-up care satisfaction was assessed with one item from the FOCUS survey examining the quality of cancer-related follow-up care from 1 (Poor) to 5 (Excellent).
Dyadic efficacy was assessed by asking survivors and caregivers to report their level of confidence in abilities to work together as a team to address daily cancer-related problems on a scale from 0 (Not at all Confident) to 10 (Extremely Confident) [44].
Sociodemographic and Clinical Variables
We assessed age, gender, race/ethnicity, education, and marital and employment status at baseline. Using a standardized abstraction form, data were collected from the electronic medical record, including cancer site, cancer stage, date of diagnosis, human papilloma virus HPV status (positive, negative, unknown assessed by surrogate marker pi6), treatment types and dates (surgery, radiation therapy, chemotherapy) and end of treatment date.
Feasibility and Acceptability
Using detailed implementation tracking logs, study staff recorded details about the delivery of the SNAP intervention, including length of time to complete needs assessments, technology challenges encountered, length of time with nurse, SNAP protocol steps completed, number of messages and educational materials generated on care plan, and number and types of referrals flagged with outcomes (accepted or declined and reasons).
At the end of the clinic session, participants completed brief surveys by tablet computer to evaluate session acceptability. We assessed survivor and caregiver satisfaction with the session (1=Poor to 5=Excellent) and comfort completing questions on the tablet, moving from question to question, and completing questions with one’s loved one in the room (1=Not at all Satisfied to 5=Extremely Satisfied). After completing each session, Nurse Practitioners reported their perceptions (1=Not at all to 4=Very Much) about the session length, survivor/caregiver level of engagement and understanding of the care plan.
Acceptability was also assessed in the 6-week follow-up surveys as survivors and caregivers reported their beliefs about the extent to which 1) the session made them feel prepared for the post-treatment period and 2) the information provided was practical and helpful to them emotionally (1=Slightly Disagree to 6=Strongly Agree). In addition, participants rated the appropriateness of the timing of the session (preferred earlier, just right or preferred later). Survivors and caregivers also reported whether they reviewed the care plan and binder materials again or shared with others and whether they discussed any of the intervention materials with their health care providers. We also used open-ended questions to assess survivor and caregiver acceptability and suggestions to improve the SNAP care plan and session. Finally, we conducted 20-40-minute interviews with HNC healthcare providers (N=12) using a structured interview guide to obtain feedback about the timing, content and delivery of the SNAP system and suggestions to improve feasibility.
Data Analysis
Participant characteristics and implementation tracking log data (i.e., length of time to complete needs assessment, technology challenges encountered, length of time with nurse, protocol steps completed, number of resources generated on care plan, referral outcomes) were summarized using descriptive statistics. To examine whether the change in outcome variables over time from baseline to 6-week follow-up was different from zero on our primary (depression, unmet needs, survivorship knowledge, dyadic coping, caregiver burden) and secondary (symptom distress, symptom management abilities, self-efficacy, dyadic efficacy and follow-up care satisfaction) outcome variables, we calculated average scores and 95% confidence intervals (CI) at each time point and used paired t-tests or Wilcoxon signed-rank tests to test the differences. Finally, we used descriptive statistics and content analysis to explore participant use of and satisfaction with the intervention and suggestions to improve the intervention.
Results
Participant Characteristics
Eighty-two potential participants were mailed study introduction letters and 69 (77%) were reached for telephone screening. Forty-nine of those screened (49/69; 71%) were eligible and 20 (29%) did not meet study criteria. Twenty-nine of eligible dyads (29/49; 59%) enrolled. Main reasons for ineligibility included lack of a caregiver and main reasons for declining included lack of interest or being too busy, ill or overwhelmed. Twenty-six dyads completed the baseline survey and clinic session and 25 completed the 6-week follow-up survey.
As shown in Table 1, the majority of patients were male while most caregivers (77% partners) were female. While the majority of participants were white, age and education level varied widely. The most common cancer sites were oral cavity (42%) and oropharynx (46%; 10/12 human papilloma virus positive) and the majority (65%) had stage IVA cancer. Time since treatment completion also varied with 42% completing treatment within the past 6 months. HNC healthcare providers completing interviews included 6 nurses, 2 physicians and 4 other providers (Maxillofacial Prosthodontist, Dietitian, Clinical Psychologist and Speech Pathologist).
Table 1.
Participant Characteristics
| Survivors (N=26) | Caregivers (N=26) | ||
|---|---|---|---|
| Age, median (range) | 63 (32 – 77) | 56 (33 −75) | |
| ≤ 50, n (%) | 9 (35) | 6 (23) | |
| >50, n (%) | 17 (65) | 20 (77) | |
| Gender, n (%) | |||
| Female | 9 (35) | 19 (73) | |
| Male | 17 (65) | 7 (27) | |
| Race, n (%) | |||
| African American | 2 (8) | 3 (12) | |
| White | 23 (88) | 22 (85) | |
| Asian | 1 (4) | 1 (4) | |
| Caregiver Type | |||
| Partner | 20 (77) | ||
| Sibling | 1 (4) | ||
| Child | 4 (15) | ||
| Parent | 1 (4) | ||
| Education, n (%) | |||
| ≤High school | 4 (15) | 7 (27) | |
| Some college or technical school | 9 (35) | 8 (31) | |
| ≥College degree | 13 (50) | 11 (42) | |
| Employment status | |||
| Employed | 12 (46) | ||
| Retired | 11 (42) | ||
| Disability | 3 (12) | ||
| Other | 2 (8) | ||
| Cancer Site | |||
| Oral Cavity | 11 (42) | ||
| Oropharynx | 12 (46) | ||
| Larynx | 2 (8) | ||
| Other | 1 (4) | ||
| Stage, n (%) | |||
| I-III | 8 (35) | ||
| IVA | 15 (65) | ||
| Unknown | 3 | ||
| Surgery, n (%) | 23 (88) | ||
| Chemotherapy, n (%) | 14 (54) | ||
| Radiation Therapy, n (%) | 24 (92) | ||
| Time Since Treatment Completed | |||
| 0-6 months | 11 (42) | ||
| >6-12 months | 6 (23) | ||
| >12 months | 9 (35) | ||
| Number of Follow-up Care Doctors, median (range) | 3(1 – 5) |
Intervention Delivery Factors and Session Acceptability
SNAP needs assessments by tablet in the clinic took an average of 11 and 6 minutes to complete for survivors and caregivers, respectively, and the nurse discussion of care plans took approximately 13 minutes (Table 2). Few technology challenges were experienced, including server errors when opening the system (n=2) and issues with final care plan generation when the care plan draft was edited (n=2). All issues were resolved by refreshing the system. SNAP session protocol steps were completed in full for all 26 sessions. Care plans included an average of 19 messages and 13 educational materials and multiple referrals (M=4.5) were flagged for each dyad (Table 2). Figure 2 illustrates the frequency of referrals by type and referral outcomes. The highest referral rates were observed for Behavioral Medicine (86%), Nutrition (77%) and Physical Therapy (65%) and the highest referral acceptance rates were for the Chaplain (1/2; 50%), Maxillofacial Prosthodontics (4/12; 40%) and Smoking Cessation Pharmacist (1/3; 33%). Referral decline reasons varied by type with one common reason being that the survivor was already seeing a local provider (Maxillofacial Prosthodontics, n=6, 75% of declines; Physical Therapy, n=9, 53% of declines). Other common decline reasons included the survivor indicating no need for the referral (Drug Addiction Counseling, n=4, 80% of declines; Behavioral Medicine, n=10, 59% of declines; Smoking Cessation Pharmacist, n=1, 50% of declines) or lack of interest (Smoking Cessation Pharmacist, n=1, 50% of declines).
Table 2.
SNAP Session Tracking and Participant Satisfaction Ratings
| Survivors (N=26) | Caregivers (N=26) | |
|---|---|---|
| Session Elements (mean, range unless otherwise specified)a | ||
| Time to complete tablet surveys (minutes) | 11.4 (5 – 26) | 5.9 (3 – 11) |
| Preferred using stylus to answer questions on tablet (%) | 77 | 89 |
| Length of nurse visit (minutes) | 13.0 (2 – 25) | |
| Number of educational messages generated | 19.1 (8-38) | |
| Number of educational materials provided | 12.6 (4 – 28) | |
| Referrals flagged | 4.5 (1-9) | |
| Session Satisfaction (% Extremely Satisfied)b | ||
| Using tablets to answer questions | 83 | 88 |
| Following instructions | 77 | 85 |
| Moving from question to question | 85 | 88 |
| Completing survey with loved one in the room | 77 | 77 |
Assessed by staff using implementation tracking log
assessed by tablet computer after completing session
Figure 2.
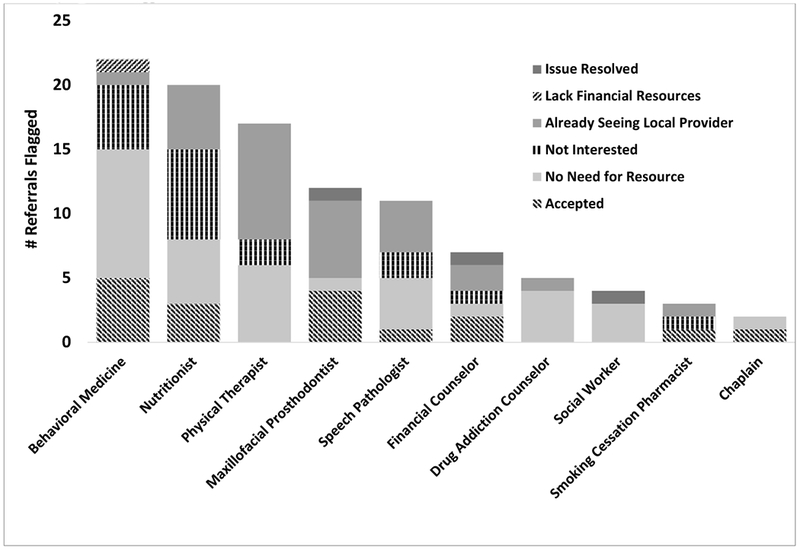
Number of Referrals Flagged and Outcomes
Participants reported high satisfaction with the session (96.1% of survivors and 92.3% of caregivers rated the overall quality as Excellent or Very Good). The majority of participants also reported high satisfaction with completing questions by tablet, following instructions, moving from question to question and completing needs assessments with one’s survivor/caregiver in the room (Table 2). Nurses conducting study sessions perceived that the majority of sessions were appropriate in length (80%), survivors and caregivers were engaged (80% and 88.5%, respectively) and dyads had a good understanding of the care plan (92.3%) and were well-prepared after the session (84.6%).
Outcomes
For primary outcomes, as highlighted in Table 3 and illustrated in the Online Resource, depression decreased significantly from baseline to 6-week follow-up in survivors (p=.01) and caregivers (p=.001) as did the number of unmet needs in survivors (p=.001) and caregivers (p=.02). Also, survivorship knowledge increased significantly in both survivors and caregivers (p=.02 and.03, respectively) over the 6-week study period. Dyadic coping increased slightly over time in both survivors and caregivers but these findings were not significant (Table 3). Finally, caregiver burden decreased marginally over time (p=.08).
Table 3.
Survivor and Caregiver Baseline and Follow-up Outcome Variables and Difference Scores Over Time by Group
| Dependent Variable | Survivors (N=25) | Caregivers (N=25) | ||||||
|---|---|---|---|---|---|---|---|---|
| (scale range) | Baseline | Follow-upa | Change | p-valueb | Baseline | Follow-upa | Change | p-valueb |
| Depression (41-79) | 49.1 (46.7 – 51.5) | 45.4 (42.5 – 48.2) | −3.4 (−5.9 – −0.9) | 0.01 | 52.1 (48.9 – 55.4) | 46.4 (43.0 – 49.8) | −5.4 (−8.4 – −2.3) | 0.001 |
| Unmet needs (0-30) | 7.7 (4.7 – 10.6) | 2.9 (1.2 – 4.6) | −4.4 (−6.8 – −1.9) | 0.001 | 7.0 (4.3 – 9.7) | 4.1 (2.1 – 6.1) | −2.4 (−4.5 – −0.3) | 0.02 |
| Survivorship knowledge (1-6) | 4.9 (4.6 – 5.2) | 5.2 (4.9 – 5.5) | 0.4 (0.1 – 0.6) | 0.02 | 4.9 (4.5 – 5.3) | 5.2 (4.8 – 5.6) | 0.3 (0.03 – 0.6) | 0.03 |
| Dyadic coping (1-5) | 4.5 (4.1 – 4.9) | 4.6 (4.4 – 4.9) | 0.2 (−0.2 – 0.6) | 0.43 | 3.5 (3.1 – 3.9) | 3.7 (3.4 – 3.9) | 0.1 (−0.2 – 0.5) | 0.48 |
| Caregiver burden (0-16) | -- | -- | -- | -- | 3.9 (2.7 – 5.2) | 2.9 (1.6 – 4.3) | −0.8 (−1.6 – 0.1) | 0.08 |
| Symptom distress (0-10) | 2.7 (1.8 – 3.5) | 2.5 (1.6 – 3.4) | −0.2 (−1.3 – 0.7) | 0.66 | 4.3 (3.4 – 5.3) | 3.0 (1.9 – 4.0) | −1.5 (−2.8 – −0.2) | 0.03 |
| Symptom management abilities (0-10) | 8.5 (7.8 – 9.2) | 8.6 (8.0 – 9.2) | 0.2 (−0.5 – 0.9) | 0.64 | 6.0 (4.8 – 7.2) | 8.2 (7.3 – 9.2) | 2.3 (0.8 – 3.8) | 0.004 |
| Dyadic efficacy (0-10) | 9.3 (8.8 – 9.8) | 9.5 (9.1 – 10.0) | 0.2 (−0.3 – 0.8) | 0.40 | 9.0 (8.3 – 9.7) | 9.3 (8.8 – 9.7) | 0.2 (−0.6 – 1.1) | 0.55 |
| Follow-up care satisfaction (1-5) | 4.5 (4.2 – 4.8) | 4.5 (4.2 – 4.8) | 0 (−0.2 – 0.2) | 1 | 4.4 (4.1 – 4.8) | 4.5 (4.1 – 4.9) | 0 (−0.4 – 0.4) | 1 |
| Follow-up care adherence self-efficacy (0-4) | 3.6 (3.4 – 3.8) | 3.6 (3.4 – 3.8) | 0 (−0.2 – 0.2) | 1 | 3.7 (3.4 – 3.9) | 3.8 (3.6 – 3.9) | 0.1 (−0.2 – 0.4) | 0.45 |
| Self-efficacy to obtain cancer-related advice (0-4) | 3.2 (2.8 – 3.6) | 3.6 (3.3 – 3.9) | 0.4 (−0.1 – 0.9) | 0.10 | 3.2 (2.9 – 3.6) | 3.5 (3.1 – 3.8) | 0.2 (−0.1 – 0.5) | 0.11 |
Note: All results are presented as means and 95% confidence intervals for each outcome.
Six week follow-up;
paired t test or Wilcoxon signed rank test
For secondary outcomes (Table 3), while symptom distress and symptom management abilities remained stable over time in survivors (p=.66 and .64, respectively), these outcomes improved in caregivers (p=.03 and .004, respectively; Table 3 and Online Resource). Dyadic efficacy increased slightly in both survivors and caregivers but these findings were not statistically significant. Follow-up care satisfaction remained stable over time as did follow-up care adherence self-efficacy in both survivors and caregivers. Finally, self-efficacy to obtain cancer related advice increased over time in survivors but these findings were not statistically significant.
SNAP Program Evaluation
At 6 week follow-up, participants rated the impact of the SNAP session favorably with the majority in moderate to strong agreement that the session made them feel prepared for the post-treatment period (84% survivors, 80% caregivers) and that the care plan had the right amount of information (100% survivors, 84% caregivers), provided practical information (92% survivors, 88% caregivers), and was helpful emotionally (80% survivors and caregivers). When considering the timing of the session in cancer care, 32% of survivors and 40% of caregivers rated the timing as “just right” while 68% of survivors and 56% caregivers preferred the session earlier in care. Finally, the majority of survivors and caregivers reported using the care plan document (64% and 56%, respectively) and educational materials (72%and 52%, respectively) after the session by sharing them with family or other healthcare providers.
Open-ended feedback concerning the SNAP session to complement session acceptability ratings revealed three main parallel themes from dyad and healthcare provider perspectives (Table 4). Key themes included that the session 1) pulled together complex clinical information and facilitated care coordination, 2) offered continued support and addressed post-treatment needs and 3) confirmed importance of caregiver well-being and dyad communication. While similar themes were supported by dyads and healthcare providers, survivors and caregivers emphasized appreciation for the support and perception of extended care whereas providers uniquely focused on the value of SNAP promoting adherence to follow-up care and improving clinic efficiency.
Table 4.
Interview Findings: Impact of SNAP Program
| Theme | Illustrative Quotes | |
|---|---|---|
| Dyads (N=25 survivors and caregivers) | Providers (N=12) | |
| Pulled together complex clinical information and facilitated care coordination | • A wealth of information and makes me realize all this is normal what I have gone through… (Tongue cancer survivor, age 66) • Gives you a lot of information: aftercare, treatment, knowing who to contact if you need additional help. (Caregiver, age 62) • Everything you could ever need was all in one place: the symptoms we should be looking for, the educational materials that you could go through and come up with questions to ask. (Caregiver, age 49) |
• Important to stay in touch with patients post-treatment…many drop off after completing follow-up visits during the first year. (Oncology Dietitian) • Many come in unnecessarily because they have difficulty deciphering between emergent issues and normal changes after treatment. (Radiation Oncology Nurse) • Tool is a time-saving mechanism that will improve efficiency by streamlining concerns and triaging appropriate referrals. (Clinical Psychologist) |
| Offers continued support and addressed post-treatment needs | • Gave us a sense of security and extended care. (Caregiver, age 48) • Overall knowledge that there are people there if we need it. Knowing who to call could be difficult through this process, so it’s nice to have that information. (HPV+ tonsil/oropharyngeal cancer survivor, age 60) • Knowing that there’s an additional support system in place. (Caregiver, age 63) • Seemed to care for us personally. Made us feel like we could sit down and talk about things. (Caregiver, age 59) |
• Social and emotional needs of patients and supporters may not always be identified or addressed under current practice. (Head and Neck Cancer Nurse) |
| Confirmed importance of caregiver well-being and dyad communication | • Knowing there’s support for me was helpful…usually the caretaker is left out and I felt selfish for thinking about myself. Now I know I should be thinking about myself. (Caregiver, age 56) • We don’t often talk about how it makes me feel. We now do this more. (Caregiver, age 54) • Forced us to sit together and discuss things out in the open because it had been a while since we talked about his cancer. (Caregiver, age 55) |
• Cancer experience can be just as tough on family members as it is on the patient as patients may take out their frustrations on their caregiver. (Oncology Dietitian) • Important to ask about the caregiver’s perception of patients symptoms/issues (especially tobacco and alcohol use). (Head and Neck Cancer Nurse Practitioner) • Caregiving support is one issue that arises during treatment and persists during the post-treatment period. (Radiation Oncology Nurse) |
With respect to suggestions for the program, both survivors and caregivers recommended including additional supportive care information (e.g., in the areas of cancer staging, care of teeth, financial concerns and general caregiving resources). Some caregivers also highlighted interest in receiving a separate, private visit to address their concerns. Healthcare providers valued the program but highlighted time, space, coordination with other clinic visits and literacy concerns as barriers, suggesting the option of remote SNAP assessments before clinic visits. While both survivors and caregivers preferred to have the session earlier in their follow-up care, providers identified the 6-month follow-up visit as ideal timing when survivors’ initial post-treatment symptoms had improved; both highlighted the potential benefits of implementing multiple SNAP assessments over the course of HNC follow-up care.
Discussion
To build on growing research capitalizing on technology to promote better cancer outcomes [15–17, 45–47], the current research evaluated a survivorship needs assessment planning (SNAP) tool for HNC survivors and caregivers. We included both survivors and their nominated primary caregivers aiming to address their unique and complex needs at the end of HNC treatment, facilitate positive transitions in care and enhance teamwork [41, 49] around caregiving and recovery. Results highlighted promising acceptability and feasibility of the SNAP system and improved short-term changes in psychosocial outcomes after completing the session, and also provided helpful information to guide program improvements for the future.
The SNAP tool adds to previously tested technology-supported HNC interventions such as home-based PRO self-management programs [50, 51], with an explicit focus to develop a feasible, minimal intervention with consideration of limited clinic resources [52] and to address the limitation that few eHealth interventions have been designed and tested for caregivers [27]. A PRO monitoring system may be especially beneficial in HNC because survivors and caregivers face a variety of potential physical, emotional and social challenges after treatment [2, 3, 5, 20, 53–55] and desire information in multiple formats [56]; tailored resource algorithms can be used to drive rapid identification of top concerns and more focused follow-up care discussions about these concerns. Key design features and barriers to PRO system implementation have been outlined for optimal uptake of systems and the SNAP system was designed with these criteria in mind (e.g., response automation, tailoring of item selection and resource algorithms, flexibility of data collection, brief assessments) [57, 58]. The SNAP system’s web-based technology was designed for dynamic needs assessment, logic builder and resource library interfaces so that clinic staff can make changes in planning stages and in real time. SNAP needs assessments took approximately 11 and 6 minutes to complete by survivors and caregivers, respectively, in varied clinic locations (e.g., waiting room, exam room) and data collection methods were rated as acceptable by users and staff.
Preliminary testing of the SNAP system demonstrated that high rates of messages, educational materials and referrals were generated yet we also observed high rates of declines for referrals for a variety of reasons that should be addressed in future work. First, when participants indicated they were not in need of a flagged referral, results can drive updated algorithms to more accurately identify those in need of such resources. For example, our targets for Drug Addiction Counseling, Behavioral Medicine and the Smoking Cessation Pharmacist had high referral decline rates (≥ 50%) so the algorithms for these referrals should be adjusted. Also, when participants declined referrals due to lack of interest, we learned that this was commonly due to being too busy with other appointments or feeling overwhelmed. These findings open the opportunity to re-visit referral interest at future visits or implement patient engagement strategies such as messaging or reminder techniques to activate possible referral uptake later. We also identified that multiple survivors declined referrals when they were already seeing or scheduled to see a provider for the concern flagged. Productive discussions about care received by other healthcare providers offers the opportunity to improve care coordination with documentation and shared communication across providers [59, 60].
Patterns observed in participant outcomes showed improvements in psychosocial factors after completing the intervention. In particular, depression, unmet needs, and survivorship knowledge improved significantly in both survivors and caregivers, and symptom distress and perceptions about survivors’ symptom management abilities improved significantly for caregivers. In addition, dyadic coping and dyadic efficacy scores improved slightly in both survivors and caregivers but these changes over time were not significant. A similar pattern was observed for caregiver burden and self-efficacy in survivors and caregivers to obtain cancer-related information and adhere to recommended follow-up care. Future larger-scale studies should follow to optimize selection of key outcome variables in the growing survivorship field [34], monitor outcomes over a longer time period in a randomized controlled study design and examine whether the intervention impact is clinically significant. Findings can then guide whether additional teamwork building, caregiver-focused or skill-building intervention strategies are needed to impact dyadic, caregiver and self-efficacy outcomes, respectively.
High ratings of satisfaction were reported concerning the SNAP clinic session and care plan content by survivors, caregivers and nurses. However, the majority of survivors and caregivers (> 55%) recommended providing the session earlier in one’s cancer care. Of note, over half of survivors were greater than six months beyond treatment conclusion and this likely impacted timing preferences. Qualitative results highlighted that dyads and especially caregivers may benefit from resources provided at the end of treatment and during the initial post-treatment recovery, with a focus on caregiving tools for caregivers, who may benefit from an individual visit. A primary theme emphasized by survivors and caregivers was that the SNAP session provided a sense of extended care, and this may help alleviate the common isolation reported by survivors at the end of treatment [61]. In contrast, providers recommended the session ideally be provided 6-months following treatment completion when survivors’ symptoms have improved; it may be that visit timing should vary by preference, treatment type and other factors.
Feasible implementation of the SNAP system was demonstrated with a limited time burden placed on dyads and clinic staff for data collection and care plan discussion, minimal technology challenges, low dropout rates and our ability to complete all SNAP visits according to the protocol. However, qualitative findings highlighted that while clinical staff valued the efficiency of the SNAP protocol, they still had concerns about resources to support the SNAP system and suggested alternative data collection techniques, such as remote needs assessments at home, to streamline clinic processes. Finally, we found that most survivors and about half of caregivers used care plans and educational materials after the session and shared them with other family members and healthcare providers. Qualitative findings confirmed that having “everything in one place” was helpful and the visit offered a setting in which to ask questions and understand next steps in care. Importantly, the SNAP sessions also directed attention to the caregiver’s well-being in line with growing recent attention on the key roles played and burdens experienced by cancer caregivers [62].
Results from this study support the acceptability and feasibility of a new system to assess symptoms, unmet needs, concerns and health behaviors in HNC survivors and their caregivers with the generation of tailored care plans in a survivorship clinic visit. Strengths of this study include its mixed methods approach and reliance on key stakeholders (survivors, caregivers, healthcare providers) to evaluate the system. Preliminary testing of the system provides evidence of the potential benefits of a PRO system for use in HNC survivorship care on survivor and caregiver psychosocial outcomes and key data to guide system improvements. However, the single-group design and small sample size were limitations. In addition, while a study strength was inclusion of caregivers, results from this study do not provide evidence of the acceptability of the SNAP system in survivors lacking a caregiver and this will require future study to facilitate wide system scalability. Also, one critical SNAP system limitation is that it is not directly integrated in the electronic medical record, a common workflow barrier in PRO systems [57]. Essential next steps to advance the SNAP system include modifying the system to include additional resources and new intervention modules to support caregivers and promote survivor activation. It is also critical to determine optimal delivery timing and system acceptability in survivors without a caregiver. Finally, the SNAP system should be tested in a larger-scale study to monitor optimal long-term clinical, psychosocial and cost outcomes using a randomized controlled trial study design.
Supplementary Material
Acknowledgements
Funding: This research was funded by the National Cancer Institute (grant # 1R21CA173271) with support from the South Carolina Clinical and Translational Research Institute (UL1TR000062) and the Biostatistics Shared Resource of the Hollings Cancer Center (P30 CA138313) and technology development by the Technology Applications Center for Healthful Lifestyles at the Medical University of South Carolina. Katherine Sterba’s work on this manuscript was also supported by a Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-12-221-01-CPPB) from the American Cancer Society.
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Miller MC, Shuman AG, American H, Survivorship NSsCo. Survivorship in head and neck cancer: A primer. JAMA Otolaryngol Head Neck Surg. 2016;142(10):1002–1008. [DOI] [PubMed] [Google Scholar]
- 2.Survivorship Ringash J. and quality of life in head and neck cancer. J Clin Oncol. 2015;33(29):3322–3327. [DOI] [PubMed] [Google Scholar]
- 3.Longacre ML, Ridge JA, Burtness BA, Galloway TJ, Fang CY. Psychological functioning of caregivers for head and neck cancer patients. Oral Oncol. 2012;48(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penner JL, McClement S, Lobchuk M, Daeninck P. Family members’ experiences caring for patients with advanced head and neck cancer receiving tube feeding: a descriptive phenomenological study. J Pain Symptom Manage. 2012;44(4):563–571. [DOI] [PubMed] [Google Scholar]
- 5.Sterba KR, Zapka, J., Cranos, C., Laursen, A., Day, T. Quality-of-life in head and neck cancer patient-caregiver dyads: A systematic review. Cancer Nursing. 2016;39(3):238–250. [DOI] [PubMed] [Google Scholar]
- 6.Birken SA, Ellis SD, Walker JS, et al. Guidelines for the use of survivorship care plans: a systematic quality appraisal using the AGREE II instrument. Implementation Sci. 2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: an integrative review of studies of cancer survivorship care plans (2006-2013). Cancer. 2015;121(7):978–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Implementing cancer survivorship care planning workshop summary. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 9.Stricker CT, Jacobs LA, Palmer SC. Survivorship care plans: An argument for evidence over common sense. J Clin Oncol. 2012. [DOI] [PubMed] [Google Scholar]
- 10.Ganz PA, Casillas J, Hahn EE. Ensuring quality care for cancer survivors: implementing the survivorship care plan. Semin Oncol Nurs. 2008;24(3):208–217. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network practice guidelines in oncology: head and neck cancers V.1.2018. 2018; http://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf. Accessed 1/02/2018.
- 12.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203–239. [DOI] [PubMed] [Google Scholar]
- 13.Nekhlyudov L, Lacchetti C, Davis NB, et al. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement of the American Cancer Society guideline. J Clin Oncol. 2017;35(14):1606–1621. [DOI] [PubMed] [Google Scholar]
- 14.Chen SC, Lai YH, Liao CT, et al. Unmet supportive care needs and characteristics of family caregivers of patients with oral cancer after surgery. Psychooncology. 2014;23(5):569–577. [DOI] [PubMed] [Google Scholar]
- 15.Basch E Patient-reported outcomes - harnessing patients’ voices to improve clinical care. N Engl J Med. 2017;376(2):105–108. [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder CF, Aaronson NK, Choucair AK, et al. Implementing patient-reported outcomes assessment in clinical practice: a review of the options and considerations. Qua Life Res. 2012;21(8):1305–1314. [DOI] [PubMed] [Google Scholar]
- 18.de Bree R, Verdonck-de Leeuw IM, Keizer AL, Houffelaar A, Leemans CR. Touch screen computer-assisted health-related quality of life and distress data collection in head and neck cancer patients. Clin Otolaryngol. 2008;33(2):138–142. [DOI] [PubMed] [Google Scholar]
- 19.Sterba KR, Zapka J, LaPelle N, et al. Development of a survivorship needs assessment planning tool for head and neck cancer survivors and their caregivers: a preliminary study. J Cancer Surviv. 2017;11(6):822–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314–3321. [DOI] [PubMed] [Google Scholar]
- 21.Constantinescu G, Kuffel K, King B, Hodgetts W, Rieger J. Usability testing of an mHealth device for swallowing therapy in head and neck cancer survivors. Health Informatics J. 2018:1460458218766574. [DOI] [PubMed] [Google Scholar]
- 22.Constantinescu G, Loewen I, King B, Brodt C, Hodgetts W, Rieger J. Designing a mobile health app for patients with dysphagia following head and neck cancer: A qualitative study. JMIR Rehabil Assist Technol. 2017;4(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosa A, Heineman N, Thomas K, et al. Improving patient health engagement with mobile texting: A pilot study in the head and neck postoperative setting. Head Neck. 2017;39(5):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnossen IC, van Uden-Kraan CF, Eerenstein SE, et al. An online self-care education program to support patients after total laryngectomy: feasibility and satisfaction. Support Care Cancer. 2016;24(3):1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Brink JL, Moorman PW, de Boer MF, et al. Impact on quality of life of a telemedicine system supporting head and neck cancer patients: a controlled trial during the postoperative period at home. J Am Med Inform Assoc. 2007;14(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Brink JL, Moorman PW, de Boer MF, Pruyn JF, Verwoerd CD, van Bemmel JH. Involving the patient: a prospective study on use, appreciation and effectiveness of an information system in head and neck cancer care. Int J Med Inform. 2005;74(10):839–849. [DOI] [PubMed] [Google Scholar]
- 27.Slev VN, Mistiaen P, Pasman HR, Verdonck-de Leeuw IM, van Uden-Kraan CF, Francke AL. Effects of eHealth for patients and informal caregivers confronted with cancer: A meta-review. Int J Med Inform. 2016;87:54–67. [DOI] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International Society for Quality of Life Research (prepared by Aaronson N, Elliott T, Greenhalgh J, Halyard M, Hess R, Mille D, Reeve B, Santana M, Snyder C). User’s guide to implementing patient reported outcomes assessment in clinical practice version 2. 2015. 2015; http://www.isoqol.org/researchpublications/isoqol-publications. Accessed 1/10/2018. [DOI] [PubMed]
- 30.Hodgkinson K, Butow P, Hobbs KM, Hunt GE, Lo SK, Wain G. Assessing unmet supportive care needs in partners of cancer survivors: the development and evaluation of the Cancer Survivors’ Partners Unmet Needs measure (CaSPUN). Psychooncology. 2007;16(9):805–813. [DOI] [PubMed] [Google Scholar]
- 31.Hodgkinson K, Butow P, Hunt GE, et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the CaSUN (Cancer Survivors’ Unmet Needs measure). Psychooncology. 2007;16(9):796–804. [DOI] [PubMed] [Google Scholar]
- 32.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 33.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007;29(10):923–931. [DOI] [PubMed] [Google Scholar]
- 34.Parry C, Beckjord E, Moser RP, Vieux SN, Padgett LS, Hesse BW. It takes a (virtual) village: crowdsourcing measurement consensus to advance survivorship care planning. Transl Behav Med. 2014;5(1):53–9. PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parry C, Kent EE, Forsythe LP, Alfano CM, Rowland JH. Can’t see the forest for the care plan: a call to revisit the context of care planning. J Clin Oncol. 2013;31(21):2651–2653. Epub 2013/06/26. doi: 10.1200/JCO.2012.48.4618. PubMed PMID: ; PubMed Central PMCID: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J Psychiatr Res. 2014;56:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer SC, Stricker CT, DeMichele AM, et al. The use of a patient-reported outcome questionnaire to assess cancer survivorship concerns and psychosocial outcomes among recent survivors. Support Care Cancer. 2017;25(8):2405–2412. [DOI] [PubMed] [Google Scholar]
- 39.Rocque GB, Wisinski KB, Buhr KA, et al. Development and evaluation of a survey to assess survivor knowledge change after survivorship care plans: WiSDOM-B (Wisconsin Survey of cancer DiagnOsis and Management in Breast cancer). J Cancer Educa. 2014;29(2):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olagunju TO, Liu Y, Liang LJ, et al. Disparities in the survivorship experience among Latina survivors of breast cancer. Cancer. 2018;124(11):2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodenmann G Dyadic coping - a systemic-transactional conceptualization of stress and coping among couples: theory and empirical findings. European Review of Applied Psychology. 1997;47:137–140. [Google Scholar]
- 42.Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652–657. [DOI] [PubMed] [Google Scholar]
- 43.Office of Cancer Survivorship, National Cancer Institute. Follow-up care use among survivors (FOCUS) survey. http://cancercontrol.cancer.gov/ocs/focus.html. Accessed 09/01/17.
- 44.Sterba KR, DeVellis RF, Lewis MA, Baucom DH, Jordan JM, DeVellis B. Developing and testing a measure of dyadic efficacy for married women with rheumatoid arthritis and their spouses. Arthritis Rheum. 2007;57(2):294–302. [DOI] [PubMed] [Google Scholar]
- 45.Abernethy AP, Ahmad A, Zafar SY, Wheeler JL, Reese JB, Lyerly HK. Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. 2010;48(6 Suppl):S32–38. [DOI] [PubMed] [Google Scholar]
- 46.Bantum EO, Albright CL, White KK, et al. Surviving and thriving with cancer using a Web-based health behavior change intervention: randomized controlled trial. J Med Internet Res. 2014;16(2):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vickers AJ, Salz T, Basch E, et al. Electronic patient self-assessment and management (SAM): a novel framework for cancer survivorship. BMC Med Inform Decis Mak. 2010;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsini N, Fish J, Ramsey I, et al. Cancer survivorship monitoring systems for the collection of patient-reported outcomes: a systematic narrative review of international approaches. J Cancer Surv. 2017;11(4):486–497. [DOI] [PubMed] [Google Scholar]
- 49.Revenson TA, Kayser K, Bodenmann G. Couples coping with stress: Emerging perspectives on dyadic coping. Washington DC: American Psychological Association; 2005. [Google Scholar]
- 50.Duman-Lubberding S, van Uden-Kraan CF, Jansen F, et al. Feasibility of an eHealth application “OncoKompas” to improve personalized survivorship cancer care. Support Care Cancer. 2016;24(5):2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Hout A, van Uden-Kraan CF, Witte BI, et al. Efficacy, cost-utility and reach of an eHealth self-management application ‘Oncokompas’ that helps cancer survivors to obtain optimal supportive care: study protocol for a randomised controlled trial. Trials. 2017;18(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glasgow RE, Fisher L, Strycker LA, et al. Minimal intervention needed for change: definition, use, and value for improving health and health research. Transl Behav Med. 2014;4(1):26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen NA, Ringash J. Head and neck cancer survivorship care: A review of the current guidelines and remaining unmet needs. Curr Treat Options Oncol. 2018;19(8):44. [DOI] [PubMed] [Google Scholar]
- 54.Ringash J, Bernstein LJ, Devins G, et al. Head and neck cancer survivorship: Learning the needs, meeting the needs. Semin Radiat Oncology. 2018;28(1):64–74. [DOI] [PubMed] [Google Scholar]
- 55.So WK, Chan RJ, Chan DN, et al. Quality-of-life among head and neck cancer survivors at one year after treatment--a systematic review. Eur J Cancer. 2012;48(15):2391–2408. [DOI] [PubMed] [Google Scholar]
- 56.Jabbour J, Milross C, Sundaresan P, et al. Education and support needs in patients with head and neck cancer: A multi-institutional survey. Cancer. 2017;123(11):1949–1957. [DOI] [PubMed] [Google Scholar]
- 57.Jensen RE, Rothrock NE, DeWitt EM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care. 2015;53(2):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu AW, White SM, Blackford AL, et al. Improving an electronic system for measuring PROs in routine oncology practice. J Cancer Surviv. 2016;10(3):573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010(40):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. Journal of the National Cancer Institute. Monographs. 2010;2010(40):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewitt ME, Greenfield S, Stovall E. From cancer patient to cancer survivor: lost in translation. Washington DC: The National Academies Press; 2006. [Google Scholar]
- 62.National Alliance of Caregiving, in partnershop with the National Cancer Institute and the Cancer Support Community. Cancer caregiving in the United States: An intense, episodic, and challenging care experience. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


