Abstract
Background
Nitric oxide (NO) has multiple effects that may be beneficial in acute stroke, including lowering blood pressure, and promoting reperfusion and cytoprotection. Some forms of nitric oxide synthase inhibition (NOS‐I) may also be beneficial. However, high concentrations of NO are likely to be toxic to brain tissue. This is an update of a Cochrane review first published in 1998, and last updated in 2002.
Objectives
To assess the safety and efficacy of NO donors, L‐arginine, and NOS‐I in people with acute stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched 6 February 2017), MEDLINE (1966 to June 2016), Embase (1980 to June 2016), ISI Science Citation Indexes (1981 to June 2016), Stroke Trials Registry (searched June 2016), International Standard Randomised Controlled Trial Number (ISRCTN) (searched June 2016), Clinical Trials registry (searched June 2016), and International Clinical Trials Registry Platform (ICTRP) (searched June 2016). Previously, we had contacted drug companies and researchers in the field.
Selection criteria
Randomised controlled trials comparing nitric oxide donors, L‐arginine, or NOS‐I versus placebo or open control in people within one week of onset of confirmed stroke.
Data collection and analysis
Two review authors independently applied the inclusion criteria, assessed trial quality and risk of bias, and extracted data. The review authors cross‐checked data and resolved issues through discussion. We obtained published and unpublished data, as available. Data were reported as mean difference (MD) or odds ratio (OR) with 95% confidence intervals (CI).
Main results
We included five completed trials, involving 4197 participants; all tested transdermal glyceryl trinitrate (GTN), an NO donor. The assessed risk of bias was low across the included studies; one study was double‐blind, one open‐label and three were single‐blind. All included studies had blinded outcome assessment. Overall, GTN did not improve the primary outcome of death or dependency at the end of trial (modified Rankin Scale (mRS) > 2, OR 0.97, 95% CI 0.86 to 1.10, 4195 participants, high‐quality evidence). GTN did not improve secondary outcomes, including death (OR 0.78, 95% CI 0.40 to 1.50) and quality of life (MD ‐0.01, 95% CI ‐0.17 to 0.15) at the end of trial overall (high‐quality evidence). Systolic/diastolic blood pressure (BP) was lower in people treated with GTN (MD ‐7.2 mmHg (95% CI ‐8.6 to ‐5.9) and MD ‐3.3 (95% CI ‐4.2 to ‐2.5) respectively) and heart rate was higher (MD 2.0 beats per minute (95% CI 1.1 to 2.9)). Headache was more common in those randomised to GTN (OR 2.37, 95% CI 1.55 to 3.62). We did not find any trials assessing other nitrates, L‐arginine, or NOS‐I.
Authors' conclusions
There is currently insufficient evidence to recommend the use of NO donors, L‐arginine or NOS‐I in acute stroke, and only one drug (GTN) has been assessed. In people with acute stroke, GTN reduces blood pressure, increases heart rate and headache, but does not alter clinical outcome (all based on high‐quality evidence).
Keywords: Humans, Acute Disease, Arginine, Arginine/therapeutic use, Brain Ischemia, Brain Ischemia/drug therapy, Enzyme Inhibitors, Enzyme Inhibitors/therapeutic use, Headache, Headache/chemically induced, Nitric Oxide Donors, Nitric Oxide Donors/adverse effects, Nitric Oxide Donors/therapeutic use, Nitric Oxide Synthase, Nitric Oxide Synthase/antagonists & inhibitors, Nitroglycerin, Nitroglycerin/adverse effects, Nitroglycerin/therapeutic use, Quality of Life, Randomized Controlled Trials as Topic, Stroke, Stroke/drug therapy, Stroke/mortality
Plain language summary
Nitric oxide donors (nitrates), L‐arginine, or nitric oxide synthase inhibitors for acute stroke
Question: Are nitric oxide donors, L‐arginine, or nitric oxide synthase inhibitors safe and effective drugs for use in people soon after they have suffered a stroke?
Background: Nitric oxide is a key molecule involved in the control of blood pressure, blood flow, and brain activity, both before and during a brain attack (stroke, either due to a blockage or rupture of an artery in the brain). Drugs that produce nitric oxide or control its production may be beneficial in acute stroke. This review looked at trials that tested such drugs in people with a stroke that came on within the last few days.
Study characteristics: This review is up‐to‐date as of September 2016. We included five trials involving 4197 people; all trials assessed glyceryl trinitrate, a drug that is given as a skin patch and which releases nitric oxide. One study was international, whilst the remainder were studies performed at single centres. Not all trials contributed data to all outcomes. We used both unpublished and published information, where available. We did not find any trials assessing other nitrate drugs, L‐arginine, or nitric oxide synthase inhibitors.
Key results: Overall, glyceryl trinitrate did not improve the rate of death or dependency compared with those who did not receive glyceryl trinitrate after acute stroke. Glyceryl trinitrate did not improve other outcomes including death and quality of life. Glyceryl trinitrate lowers blood pressure, and increases heart rate and headache in people with acute stroke.
Quality of the evidence: The key results are based on high‐quality evidence.
Conclusions: There is currently insufficient evidence to recommend the use of drugs affecting nitric oxide production in acute stroke. Overall, glyceryl trinitrate is inexpensive, lowers blood pressure, increases heart rate and headache, but does not change clinical outcomes in people who have suffered a stroke.
Summary of findings
Summary of findings for the main comparison. Glyceryl trinitrate (GTN) compared with no GTN for acute stroke.
| Glyceryl trinitrate (GTN) compared with no GTN for acute stroke | ||||||
|
Patient or population: people with acute stroke Settings: pre‐hospital / hospital Intervention: GTN Comparison: no GTN | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no GTN / placebo | Risk with GTN compared with no GTN for acute stroke | |||||
| Death or dependency (mRS>2), end of trial (90 days) | 609 per 1,000 | 601 per 1,000 (573 to 631) |
OR 0.97 (0.86 to 1.10) |
4195 (5 studies) | ⊕⊕⊕⊕ high | |
| Death, end of trial (90 days) | 131 per 1,000 | 115 per 1,000 (90 to 184) | OR 0.78 (0.40 to 1.50) | 4197 (5 studies) | ⊕⊕⊕⊕ high | |
| Quality of life, end of trial (90 days) | The mean quality of life (EQ‐5D3L), end of trial ranged across control groups from 0.25 to 0.9. | The mean quality of life (EQ‐5D3L), end of trial in the intervention group was 0.01 lower (0.17 lower to 0.15 higher) | ‐ | 4088 (4 studies) | ⊕⊕⊕⊕ high | Health utility status derived from European Quality of life‐5 dimensions‐3 levels (EQ‐5D3L). Significant heterogeneity between trials (I2 = 78%). |
| Systolic BP, first treatment measurement (<24 hours after randomisation) | The mean systolic BP, first treatment measurement ranged across control groups from 151.13 to 185.17 mmHg. | The mean systolic BP, first treatment measurement in the intervention group was 7.21 mmHg lower (8.58 lower to 5.85 lower) | ‐ | 4197 (5 studies) | ⊕⊕⊕⊕ high | |
| Diastolic BP, first treatment measurement (<24 hours after randomisation) | The mean diastolic BP, first treatment measurement ranged across control groups from 82.34 to 91.0 mmHg. | The mean diastolic BP, first treatment measurement in the intervention group was 3.31 mmHg lower (4.18 lower to 2.45 lower) | ‐ | 4197 (5 studies) | ⊕⊕⊕⊕ high | |
| Heart rate, first treatment measurement (<24 hours after randomisation) | The mean heart rate, first treatment measurement ranged across control groups from 65.5 to 77.27 beats per minute. | The mean heart rate, first treatment measurement in the intervention group was 2.02 beats per minute higher (1.13 higher to 2.91 higher) | ‐ | 4197 (5 studies) | ⊕⊕⊕⊕ high | Significant heterogeneity between trials (I2 = 61%). |
| Adverse event: Headache, on treatment (7‐12 days) | 84 per 1,000 |
180 per 1,000 (125 to 250) |
OR 2.37 (1.55 to 3.62) | 4186 (5 studies) | ⊕⊕⊕⊕ high | |
| BP: blood pressure; CI: confidence interval; GTN: glyceryl trinitrate; MD: mean difference; OR: odds ratio GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Stroke is the third most common cause of death and the most common cause of disability in the western world (Mackay 2004). Blood pressure (BP) is elevated in 75%, or more, of people with acute stroke (whether due to blockage (ischaemic stroke) or rupture (intracerebral haemorrhage (ICH)) of an artery), and half of those have an existing diagnosis of hypertension (Britton 1986; Oppenheimer 1992). In two‐thirds of people with stroke, BP spontaneously falls in the first week after stroke, whilst the remainder have high BP, which is associated with a poor outcome (Willmot 2004). Systolic BP is related to outcome in a 'U‐shaped' relationship with both high and low systolic BP associated with increased death, and death or dependency (Leonardi‐Bee 2002). Elevated systolic BP is also associated with early stroke recurrence (Sprigg 2006).
Cerebral autoregulation maintains cerebral blood flow (CBF) despite changes in cerebral perfusion pressure (CPP: blood pressure supplying the brain). In acute stroke, autoregulation is lost so that cerebral perfusion becomes related to CPP, and therefore systemic BP (Tikhonoff 2009). Thus, large and precipitous reductions in BP reduce CBF, leading to further cerebral infarction (brain cell death from lack of blood flow; Owens 2011) or perihaematomal ischaemia (reduced blood flow to brain tissue surrounding the ICH; Qureshi 2008). Similarly, high BP increases the risk of haematoma (bleed) growth in ICH, haemorrhagic transformation in ischaemic stroke, and cerebral oedema (brain swelling) in both stroke types (Fagan 1998; Willmot 2004).
Description of the intervention
Nitric oxide (NO) (endothelium‐derived relaxing factor) is an endogenous (present within humans) inorganic soluble gas formed from the guanidino nitrogen of L‐arginine and molecular oxygen by the enzyme (catalyst) nitric oxide synthase (NOS) (Palmer 1987; Palmer 1988). This enzyme is present within endothelial cells (endothelial NOS, eNOS, type III NOS) and some neurones (brain cells; neuronal NOS, nNOS, type I NOS) in a calcium‐dependent constitutive form (Knowles 1989), and in macrophages (white blood cells), astrocytes, microglia (brain cells), and vascular smooth muscle cells (inducible NOS, iNOS, type II NOS) in a calcium‐independent inducible form (Fleming 1991; Cho 1992). NO is one of the most versatile molecules in animal and human biology with diverse roles in both physiology and pathophysiology: it is a vasodilator (dilates blood vessels) with anti‐smooth muscle cell activity (Murad 1987); protects endothelium; inhibits platelet (circulating cell fragments involved in clotting) adhesion and aggregation (Radomski 1987a; Radomski 1987b) and leucocyte (white blood cells) adhesion and chemotaxis (movement of cells to an attractant) (Bath 1991), and has other anti‐inflammatory properties. Further, it is a neurotransmitter and neuromodulator (chemical messenger) (Garthwaite 1988; Manzoni 1992; Dawson 1994), and is involved in cerebral blood flow auto‐ and chemo‐regulation (control of brain blood flow) (Tanaka 1996; White 2000; Lavi 2003).
In experimental pre‐clinical studies of ischaemic stroke, significant changes relating to NO occur in a time‐dependent fashion. In models of focal ischaemic stroke, there is increased NO production for up to 30 minutes following middle cerebral artery occlusion or blockage (Kader 1993; Malinski 1993), likely due to increased calcium availability and nNOS activation (Huang 1994). eNOS and nNOS activity increases in line with NO within the first minutes following middle cerebral artery occlusion and significantly reduces thereafter (Kader 1993), whilst iNOS is upregulated (increased activity) from 12 hours after the event, this lasting for up to seven days (Niwa 2001). Within brain tissue, NO is undetectable for up to seven days following ictus (stroke onset) (Malinski 1993).
Intravenous (injected into a vein) L‐arginine given after middle cerebral artery occlusion improved cerebral blood flow within the ischaemic penumbra (area of brain tissue surrounding the infarct that is at risk of infarction) and reduced infarct size and volume (Morikawa 1992a; Morikawa 1994). However, this observation was not seen in eNOS‐deficient mice, who also have lower post‐ischaemic cerebral blood flow, larger infarcts, smaller penumbral areas (Huang 1996; Lo 1996), and absent angiogenesis (development of new blood vessels), potentially leading to increased post‐ischaemic damage (Cui 2009). The advantageous effects of eNOS activity are supported by evidence that eNOS phosphorylation‐deficient mice (mice with inactive eNOS) (Atochin 2007) and mice deficient in the alpha‐1 subunit of soluble guanylate cyclase have increased infarct size (Atochin 2010). Whilst eNOS and eNOS‐derived NO are neuroprotective (protecting brain cells against further damage) following cerebral infarction, nNOS‐derived NO (Huang 1994; Hara 1996; Zaharchuk 1997) and iNOS‐derived NO (Iadecola 1995; Zhang 1996; Iadecola 1997; Zhao 2003) are detrimental to tissue survival resulting in poorer neurological outcome. Despite being neurotoxic in the acute phase following stroke, iNOS and nNOS seem to be important in neurogenesis (formation of new brain cells) post‐stroke, with iNOS promoting (Sehara 2006; Corsani 2008) and nNOS attenuating this process (Sun 2005).
With the weight of evidence for NO‐mediated neuroprotection post‐stroke, several therapeutic strategies have been proposed: replacement or supplementation of NO deficiency through administration of NO, NO donors or precursors; enhancement of eNOS activity to modulate or increase endogenous NO production (Endres 2004); and direct or indirect inhibition of NOS.
How the intervention might work
Vascular NO levels are low in acute stroke and so replacement might be beneficial (Ferlito 1998; Rashid 2003). NO, however delivered, may have multiple effects that could improve outcome after stroke.
BP lowering: NO donors (intravenous sodium nitroprusside (SNP) and transdermal glyceryl trinitrate (GTN)) lower BP, pulse pressure, and peak systolic BP; improve arterial compliance; and maintain CBF in acute stroke (Butterworth 1998; Bath 2001; Rashid 2002; Willmot 2006). Whilst SNP inhibits platelet function, thereby negating its use in ICH (Butterworth 1998), GTN has no effect on platelets and can therefore be used in people with stroke, regardless of stroke type (Bath 2001).
Reperfusion (improving blood supply): NO dilates cerebral arteries and could then increase perilesional perfusion (blood flow around the stroke lesion via the 'front door') without inducing cerebral steal (Willmot 2006). Pial artery (brain surface artery) blood flow and, therefore, collateral flow may provide 'back‐door' reperfusion to the ischaemic penumbra in ischaemic stroke (Morikawa 1992b) and the perihaematomal region in ICH.
Cytoprotection (cell protection): NO exhibits neuroprotective effects, as found in experimental studies (see above). Whether these effects involve cellular protection per se or just reflect reperfusion remains unclear.
NO donors could also have adjunctive effects, enhancing the effects of existing reperfusion therapies by:
Lowering BP: NO donors may prepare people with hyper‐acute (within a few hours) ischaemic stroke for thrombolysis (clot‐dissolving therapy; non‐significant tendencies to more and earlier intravenous thrombolysis were seen in the pilot RIGHT trial (Ankolekar 2013);
Dilating cerebral arteries around occluding clot, NO donors may enhance access to the clot by both endogenous and exogenous fibrinolysis (clot‐dissolving compounds found within the body and administered as treatment).
Why it is important to do this review
Evidence‐based treatments for acute stroke are limited. In hyper‐acute ischaemic stroke, there are interventions with high efficacy but limited utility (intravenous thrombolysis (Emberson 2014); thrombectomy (removal of clot causing large vessel occlusion) (Goyal 2016); and decompressive hemicraniectomy (surgery to remove one side of the skull to relieve intracranial pressure) (Vahedi 2007)), and those with low efficacy but high utility (aspirin (Sandercock 2014)). In comparison, there are no definitive treatments for ICH other than early BP lowering (Anderson 2013). All patients with stroke should have access to stroke unit care (Stroke Unit Trialists' Collaboration 2013).
NO donors are inexpensive (e.g. GTN costs approximately £1 per patch) and, if efficacious, could be used in low, middle, and high income economies.
Objectives
To assess the safety and efficacy of NO donors, L‐arginine, or NOS‐I in people with acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomised controlled trials (RCTs) of NO donors, L‐arginine, or NOS‐I in people with stroke randomised within one week of ictus with either acute ischaemic stroke or ICH. We excluded studies of people with subarachnoid haemorrhage, uncontrolled studies, and confounded controlled studies (i.e. where two or more active interventions were compared).
Types of participants
Men and women of any age with recent ischaemic stroke or intracerebral haemorrhage (ICH) eligible for randomisation to active treatment or control.
Types of interventions
NO donors, L‐arginine, and/or NOS‐I given by any route of administration versus placebo or open control.
Types of outcome measures
We assessed haemodynamic measures after first treatment, and clinical measures at end of treatment and end of follow‐up.
Primary outcomes
End‐of‐trial death or dependency, defined as modified Rankin Scale (mRS) > 2. The mRS is a 7‐level ordinal hierarchical scale ascribing a score to grades of disability from 0: no symptoms; 1: symptoms but independent; 2: mostly independent but needs some help; 3: moderate dependency but can walk; 4: dependency needing help for walking and bodily needs; 5: severe dependency and bedridden; and 6: death.
Secondary outcomes
First blood pressure and heart rate measurements after randomisation
Early case fatality (end of treatment)
Late case fatality (end of trial)
Early neurological deterioration by end of treatment defined as a decrease in the Scandinavian Stroke Scale (SSS) by > 5 points or a decrease in the consciousness part of the SSS by > 2 points
Headache on treatment
Treatment stopped early
National Institutes of Health Stroke Scale (NIHSS) at end of treatment derived from SSS (Gray 2009)
Late dependency or disability defined as end‐of‐trial Barthel Index (BI)
Mood at end‐of‐trial using the short‐form Zung Depression Scale (Zung 1965)
Quality of life at end‐of‐trial using health utility status derived from European Quality of life‐5 dimensions‐3 levels (EQ‐5D3L) and EQ‐visual analogue scale (EQ‐VAS)
Cognition at end‐of‐trial using telephone Mini‐Mental State Examination (t‐MMSE, subscore derived from MMSE), telephone interview cognition scale (TICS) and verbal fluency (animal naming)
Physiotherapy during hospital stay
Occupational therapy during hospital stay
Speech and language therapy during hospital stay
Feeding route (non‐oral feeding at day seven)
Length of hospital stay (days)
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We did not apply any language restrictions and sought translations of articles where required.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched 6 February 2017), MEDLINE (Ovid) (1966 to June 2016, Appendix 1), Embase (Ovid) (1980 to June 2016, Appendix 2), Science Citation Index (ISI, Web of Science, 1900 to June 2016, Appendix 3), Stroke Trials Registry (www.strokecenter.org/trials, searched June 2016), International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.controlled‐trials.com, searched June 2016), Clinical Trials registry (www.clinicaltrials.gov, searched June 2016), and the International Clinical Trials Registry Platform (ICTRP) (www.apps.who.int/trialsearch/, searched June 2016).
Searching other resources
For the 2002 version of this review we contacted pharmaceutical companies who made nitric oxide donors (Schwarz Pharma, Schering‐Plough) or nitric oxide synthase inhibitors (GlaxoWellcome) and researchers in the field to help identify relevant trials (Bath 2002). For this update, we also searched references lists of included studies and systematic reviews.
Data collection and analysis
We extracted data using a standard proforma, which one review author (JA) entered into Review Manager 5 (RevMan 2014) and another review author (KK) checked.
Selection of studies
One author (JA) screened the outputs from the electronic searches, and excluded irrelevant studies. We obtained full paper copies of the remaining studies and both review authors (JA and PB) selected studies based on the aforementioned inclusion criteria. We resolved any disagreements through discussion.
Data extraction and management
We extracted data from published and unpublished material. We recorded information on the following parameters: randomisation method; allocation concealment; blinding of treatment; analysis method; stroke type; treatment type, dose, route of administration and timing; blood pressure and heart rate during treatment; case fatality; functional status; quality of life; mood; cognition; length of hospital stay; rates of physiotherapy, occupational therapy and speech and language therapy; and feeding status. Individual patient data were available for all included trials and this allowed subgroup analyses and calculation of means and standard deviations to facilitate parametric analyses of ordinal outcomes such as mRS. The search criteria did not require that individual patient data were present in the included studies.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using Cochrane's 'Risk of bias' tool, which is based on the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other sources of bias.
Each domain includes one or more specific entries in a 'Risk of bias' table. Within each entry, the first part of the tool describes what was reported to have happened in the study in sufficient detail to support a judgement on the risk of bias. The second part assigns a judgement relating to the risk of bias: 'Low risk' of bias, 'High risk' of bias, or 'Unclear risk' of bias, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We calculated the weighted estimate of the typical treatment effect across trials using RevMan 5.3 (RevMan 2014). We calculated odds ratios (OR) using the Mantel‐Haenszel random‐effects model for binary data, and mean difference (MD) using the inverse variance method for continuous data, all with 95% confidence intervals (CIs).
Unit of analysis issues
Where stroke severity was measured by the Scaninavian Stroke Scale (SSS), the National Institutes of Health Stroke Scale (NIHSS) score was calculated using a published conversion algorithm (Gray 2009). Since many scales include a value for people who have died (e.g. modified Rankin Scale = 6, Health Utility Status = 0, Barthel Index = ‐5), extreme worst values were assigned for death for other outcomes including mood (short‐form Zung Depression Scale (Zung 1965) = 102.5; EQ‐VAS = ‐1; t‐MMSE = ‐1; TICS = ‐1; and animal naming = ‐1). Where secondary outcomes were not assessed, trials were excluded from analysis of that particular outcome.
Dealing with missing data
We made extensive attempts to find missing data, including utilising unpublished data from study authors.
Assessment of heterogeneity
We calculated heterogeneity between RCT results using the I2 statistic based upon the DerSimonian‐Laird formula (DerSimonian 1986).
Assessment of reporting biases
We demonstrated reporting bias using funnel plots.
Data synthesis
We performed statistical analysis using RevMan 5.3 (RevMan 2014). We reported outcomes for dichotomous data as OR with 95% CIs, and continuous data as MD with 95% CIs. We used random‐effects models to analyse individual results, a conservative approach appropriate for the heterogenous designs and populations of the trials involved. We used fixed‐effect models to analyse haemodynamic variables, given the size and precision of ENOS 2015 in relation to the other included studies.
Summary of findings table
We presented the primary outcome and important secondary outcomes (including quality of life) of the review in Table 1. We used the GRADE approach to assess the quality of the supporting evidence for each analysis. We downgraded quality depending upon: limitations in study design and implementation suggesting a high potential of bias; unexplained heterogeneity or inconsistency of results; imprecision of results (wide CIs); indirectness of evidence; or publication bias.
Subgroup analysis and investigation of heterogeneity
We reviewed the primary and secondary outcomes in the following prespecified subgroups.
Intervention type
Type of stroke: ischaemic stroke or ICH
Time from stroke onset to randomisation: ≤ 6 hours; 6.1 to 12 hours; 12.1 to 24 hours; 24.1 to 36 hours; and > 36 hours
Baseline systolic blood pressure (systolic BP): ≤ 160 mmHg; 160.1 to 180 mmHg; 180.1 to 200 mmHg; > 200 mmHg
We considered heterogeneity to be significant if I2 was greater than 50%; if present, we sought the potential reasons, e.g. different trial designs, trial populations, time to treatment. If individual subgroups had zero participants in either the intervention or no intervention arms, then we excluded the trial concerned from that particular individual subgroup analysis. As a result, the denominators for subgroups analyses vary.
Sensitivity analysis
Since ordinal or continuous analyses are more sensitive to treatment effects than dichotomous measures (OAST 2007), we assessed the comparison of the primary outcome, mRS, as MD in addition to OR.
Results
Description of studies
We included five randomised trials of participants with ultra‐acute, hyper‐acute, acute and sub‐acute stroke.
Results of the search
Figure 1 shows the PRISMA study flow diagram. Of the 4772 records identified and screened, we excluded 4750. After full‐text review of the remaining 22 studies, we excluded 16 studies including four ongoing studies that did not meet eligibility criteria. One study met inclusion criteria but is currently ongoing. Therefore, we included five trials, which are completed and summarised in Characteristics of included studies.
1.
Results of Database Search
Included studies
We identified five trials that fulfilled the inclusion criteria (Ankolekar 2013; Bath 2001; ENOS 2015; Rashid 2002; Willmot 2006). All five trials involved administration of transdermal glyceryl trinitrate (GTN) 5 to 10 mg daily via a patch. Individual patient data were provided for all five studies, totaling 4197 participants. All studies recruited participants with mixed stroke (ischaemic stroke and ICH). One of the trials was multicentre and international (ENOS 2015), whilst the remainder were UK‐based, single‐centre studies.
Trial recruitment occurred at different time intervals following stroke:
within four hours of onset (Ankolekar 2013);
within 48 hours of onset (ENOS 2015);
within 72 hours of onset (Rashid 2002);
within 168 hours of onset (Bath 2001; Willmot 2006).
The duration of the intervention period varied:
seven days (Ankolekar 2013; ENOS 2015; Willmot 2006);
10 days (Rashid 2002);
12 days (Bath 2001).
Four studies used transdermal GTN patch 5 mg once daily (Ankolekar 2013; Bath 2001; ENOS 2015; Willmot 2006), whilst Rashid 2002 adopted a different strategy with three treatment groups: transdermal GTN patch 5 mg once daily for 10 days; 5 mg for four days then 10 mg for six days; and 10 mg for 10 days. For this review, as with a previous version (2002), we combined the three treatment arms into one group. All studies detailed the equipment used and patient posture when blood pressure recordings were performed.
Multiple outcomes were reported:
mRS end‐of‐trial in all studies (Ankolekar 2013; Bath 2001; ENOS 2015; Rashid 2002; Willmot 2006);
BI end‐of‐trial in all studies (Ankolekar 2013; Bath 2001; ENOS 2015; Rashid 2002; Willmot 2006);
case fatality at end‐of‐treatment and end‐of‐trial in all studies (Ankolekar 2013; Bath 2001; ENOS 2015; Rashid 2002; Willmot 2006);
early neurological deterioration by end‐of‐treatment in four studies (Ankolekar 2013; ENOS 2015; Rashid 2002; Willmot 2006);
mood (Zung Depression Scale) end‐of‐trial in three studies (Ankolekar 2013; ENOS 2015; Willmot 2006);
quality of life (EQ‐5D and EQ‐VAS) end‐of‐trial in four studies (Ankolekar 2013; ENOS 2015; Rashid 2002; Willmot 2006);
cognition at end‐of‐trial in three studies: MMSE (Ankolekar 2013; Willmot 2006), t‐MMSE (ENOS 2015), TICS (ENOS 2015), animal naming (ENOS 2015);
physiotherapy, occupational therapy and speech and language therapy rates in three studies (Ankolekar 2013; ENOS 2015; Willmot 2006);
feeding route (non‐oral feeding at day seven) in three studies (Ankolekar 2013; ENOS 2015; Willmot 2006);
length of stay in four studies (Ankolekar 2013; Bath 2001; ENOS 2015; Willmot 2006).
Excluded studies
We excluded 16 studies that either lacked randomisation, were uncontrolled, were confounded, or were not relevant to this review (Characteristics of excluded studies); four of these are ongoing studies. One other study met the inclusion criteria and is currently ongoing (Characteristics of ongoing studies).
Risk of bias in included studies
For full details see the corresponding 'Risk of bias' tables in Characteristics of included studies, and Figure 2 and Figure 3.
2.
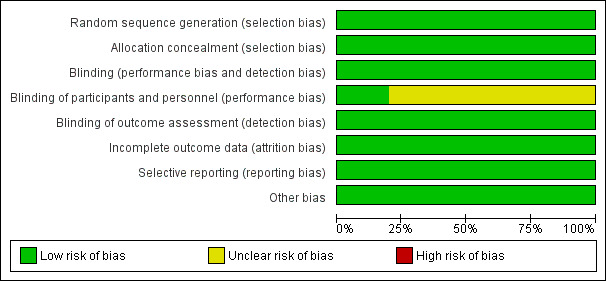
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation can be summarised as follows (Characteristics of included studies).
Simple randomisation 1:1 (Ankolekar 2013) was deemed at low risk of selection bias and ensured allocation concealment.
Computer minimisation 1:1 (Bath 2001; ENOS 2015; Rashid 2002) were deemed at low risk of selection bias and ensured allocation concealment.
Computer minimisation 2:1 (Willmot 2006) was deemed at low risk of selection bias and ensured allocation concealment.
Blinding
Participants and investigators were blinded to treatment as follows.
Double‐blind (participant and investigator): one trial (Bath 2001).
Single‐blind (participant): three trials (Ankolekar 2013; ENOS 2015; Willmot 2006).
Open‐label: one trial (Rashid 2002).
Blinding of participants and personnel (performance bias) were deemed unclear risk for the three single‐blind trials (Ankolekar 2013; ENOS 2015; Willmot 2006) and the open‐label trial (Rashid 2002). Blinding of outcome assessment (detection bias) was deemed low risk for all included trials as outcomes were assessed centrally blinded to treatment group.
Incomplete outcome data
All five included trials were analysed by intention‐to‐treat with no differences in follow‐up rates between treatment groups.
Selective reporting
The likelihood of reporting bias was demonstrated using funnel plots (Figure 4; Figure 5) and was not evident in any of the included trials. Figure 4 assessed the primary outcome (mRS end‐of‐trial) across the included studies, whilst Figure 5 assessed the first BP measurement after treatment; this was included because it was the primary outcome in the four phase II trials.
4.
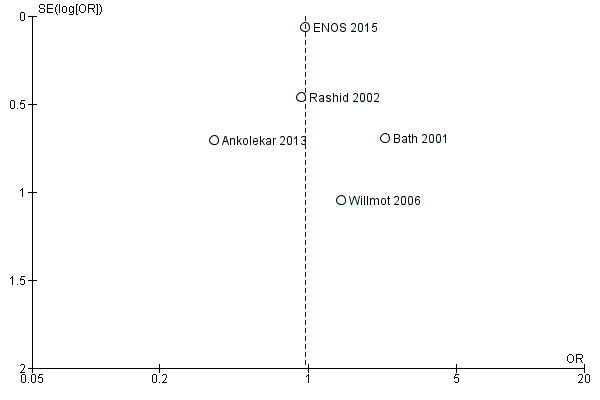
Funnel plot of comparison: Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome: 1.1 Death or dependency (mRS>2), end of trial.
5.
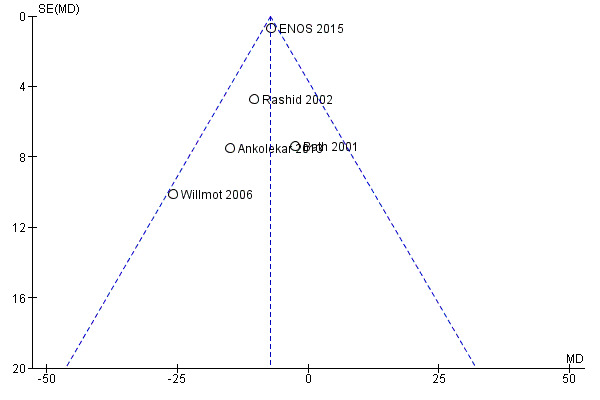
Funnel plot of comparison: Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, outcome: 1.81 Systolic BP, first treatment measurement.
Other potential sources of bias
We did not identify any other potential sources of bias that might have impacted upon the validity of the included studies.
Effects of interventions
See: Table 1
Death or dependency, end of trial
Death or dependency was assessed as mRS > 2 at day 90 in all five included trials totaling 4195 participants (Table 1; Analysis 1.1). There was no significant difference between GTN and control (OR 0.97, 95% CI 0.86 to 1.10), and no evidence of heterogeneity (I2 = 0%). When assessed as continuous data to compare mean mRS, there was no significant difference between GTN and control (MD ‐0.08, 95% CI ‐0.52 to 0.36, I2 = 47%, Analysis 1.5).
1.1. Analysis.
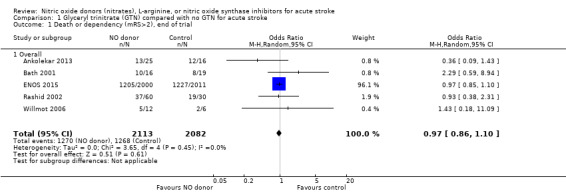
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 1 Death or dependency (mRS>2), end of trial.
1.5. Analysis.
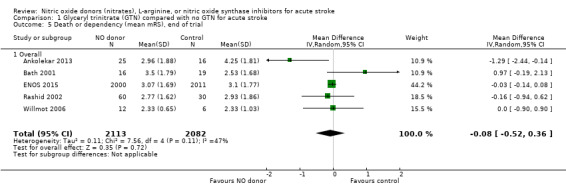
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 5 Death or dependency (mean mRS), end of trial.
Stroke type
There was no differential effect by stroke type (Analysis 1.2; Analysis 1.6).
1.2. Analysis.
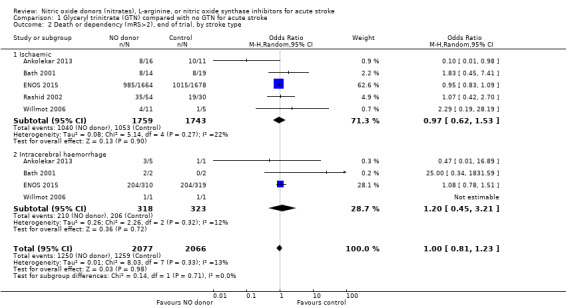
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 2 Death or dependency (mRS>2), end of trial, by stroke type.
1.6. Analysis.
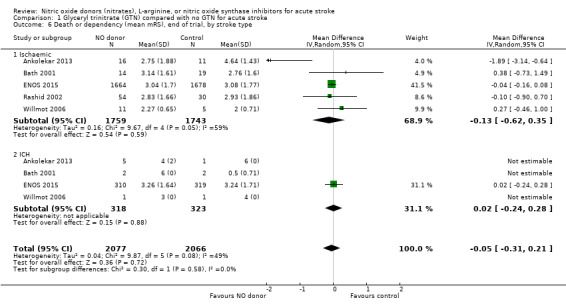
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 6 Death or dependency (mean mRS), end of trial, by stroke type.
Time to randomisation
Those participants randomised to GTN within six hours of stroke onset (two trials, 312 participants) had a tendency toward less death and dependency (OR 0.65, 95% CI 0.41 to 1.02; P = 0.06; I2 = 0%). Randomisation at more than six hours after ictus did not influence the primary outcome and overall subgroup differences were nonsignificant (P = 0.28, Analysis 1.3). In the sensitivity analysis, mean mRS was lower in those randomised to GTN within six hours of onset as compared with control participants (MD ‐0.79, 95% CI ‐1.35 to ‐0.23; P = 0.005); an effect not seen in any other time period from randomisation although the interaction between time and mRS was significant (P = 0.05, Analysis 1.7).
1.3. Analysis.
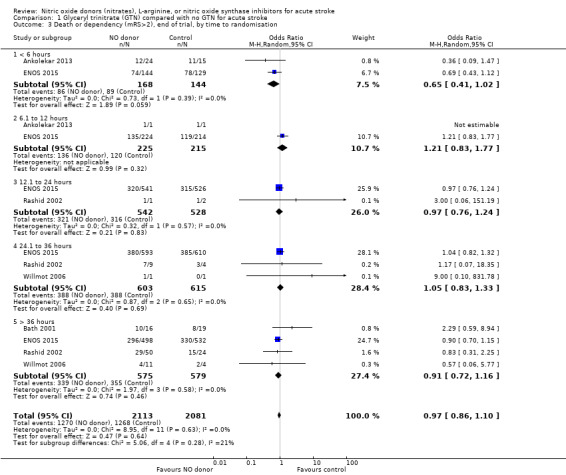
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 3 Death or dependency (mRS>2), end of trial, by time to randomisation.
1.7. Analysis.
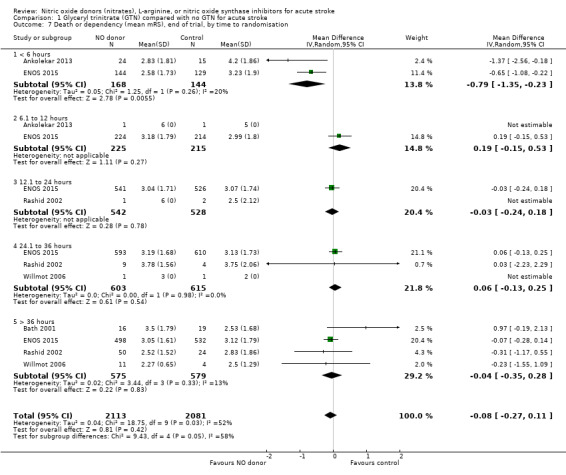
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 7 Death or dependency (mean mRS), end of trial, by time to randomisation.
Baseline systolic BP
There was no differential effect by baseline systolic BP (Analysis 1.4; Analysis 1.8).
1.4. Analysis.
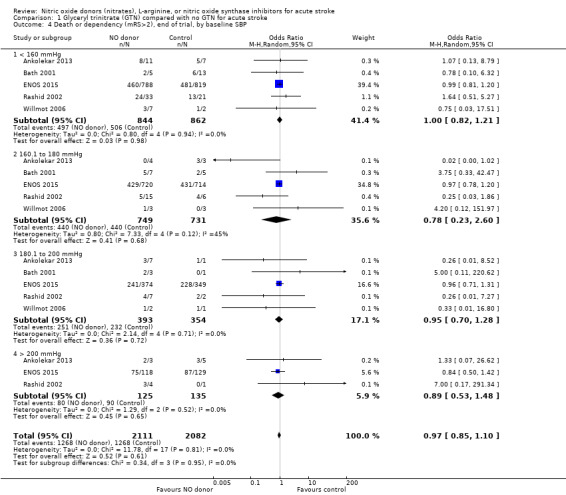
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 4 Death or dependency (mRS>2), end of trial, by baseline SBP.
1.8. Analysis.
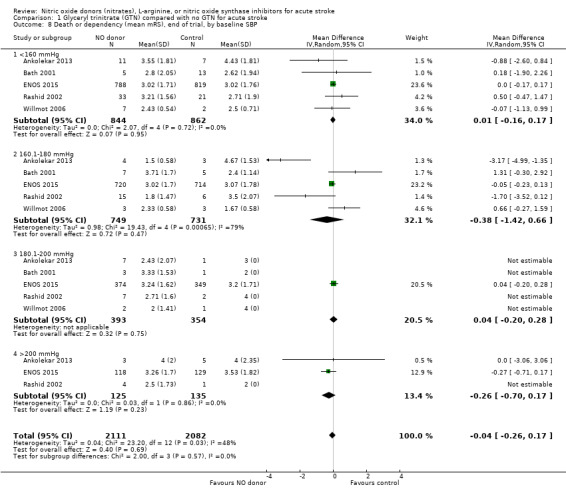
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 8 Death or dependency (mean mRS), end of trial, by baseline SBP.
Death, end of treatment and end of trial
There was no significant treatment effect of GTN compared with control on death, whether measured early (Analysis 1.9) or end of trial (Analysis 1.13; 4197 participants; Table 1). No significant differences were noted in any subgroups for death at end of treatment (Analysis 1.10; Analysis 1.11; Analysis 1.12), whilst death at end of trial was reduced in those randomised to GTN within six hours of stroke onset (OR 0.30, 95% CI 0.15 to 0.60; P = 0.0006; I2 = 0%; Analysis 1.15), with significant interaction between time and death (P = 0.01).
1.9. Analysis.
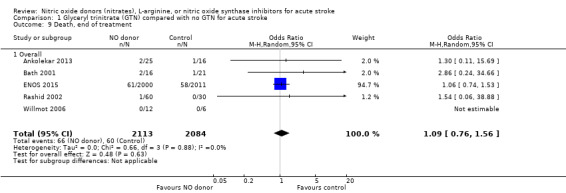
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 9 Death, end of treatment.
1.13. Analysis.
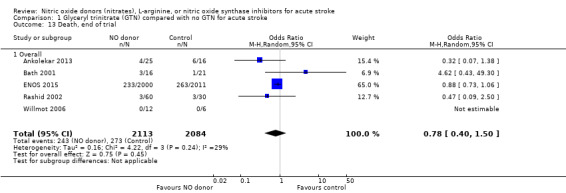
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 13 Death, end of trial.
1.10. Analysis.
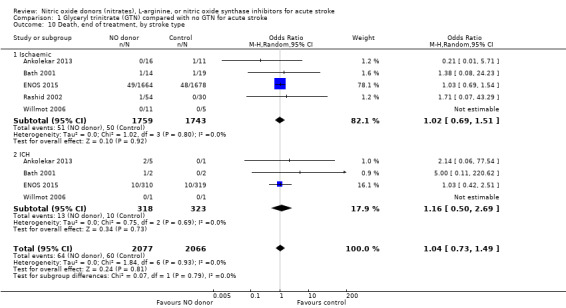
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 10 Death, end of treatment, by stroke type.
1.11. Analysis.
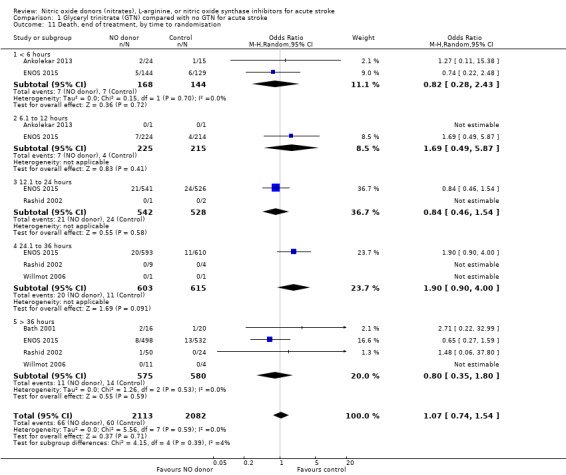
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 11 Death, end of treatment, by time to randomisation.
1.12. Analysis.
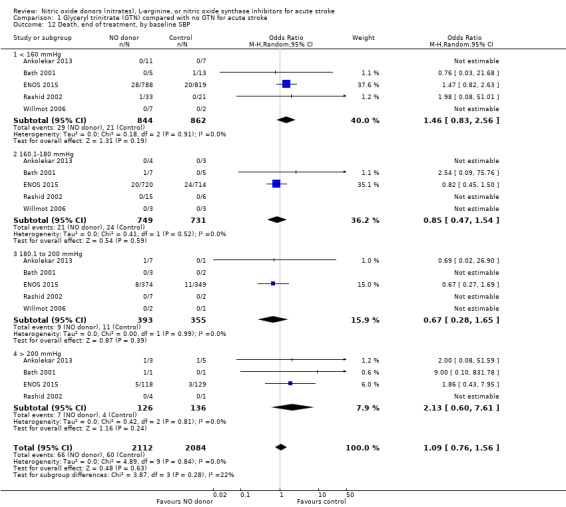
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 12 Death, end of treatment, by baseline SBP.
1.15. Analysis.
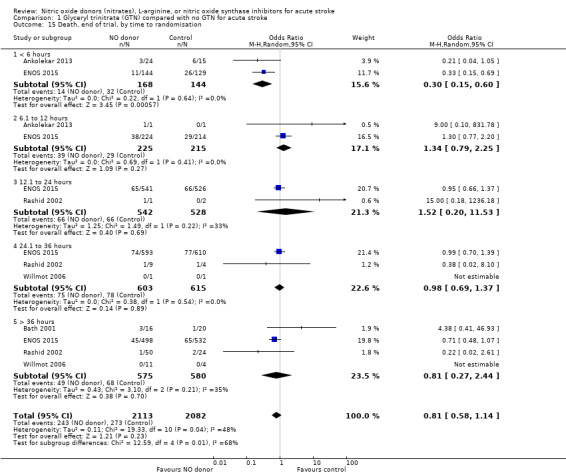
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 15 Death, end of trial, by time to randomisation.
Neurological deterioration, end of treatment
No significant difference was noted in early neurological deterioration between treatment and control (Analysis 1.17) in the four studies with data (4158 participants). Stroke type did not change the neutral effect seen overall (Analysis 1.18). Those randomised between 24 and 36 hours to GTN in one study (1218 participants; two studies were not estimable due to no events) had a higher likelihood of neurological deterioration than control participants (OR 2.00, 95% CI 1.19 to 3.35; P = 0.009). The other time groups revealed no difference between GTN and control (Analysis 1.19). Participants randomised to GTN with a baseline systolic BP of < 160 mmHg (1688 participants) or 180 to 200 mmHg in two studies (743 participants; two studies were not estimable due to no events) were more likely to have neurological deterioration at end of treatment than controls (OR 1.62, 95% CI 1.05 to 2.50, P = 0.03 and OR 1.81, 95% CI 1.05 to 3.12, P = 0.03, respectively; Analysis 1.20).
1.17. Analysis.
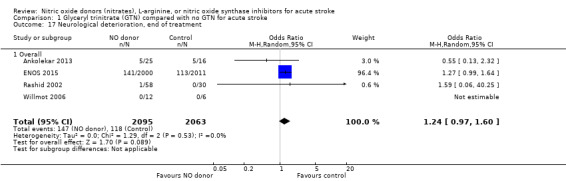
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 17 Neurological deterioration, end of treatment.
1.18. Analysis.
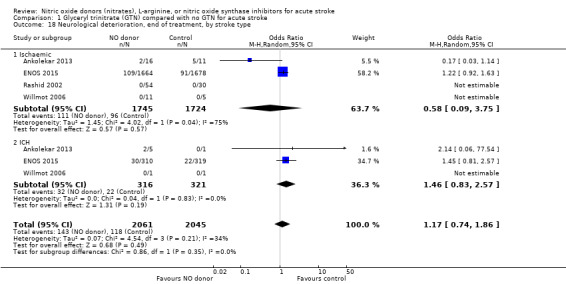
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 18 Neurological deterioration, end of treatment, by stroke type.
1.19. Analysis.
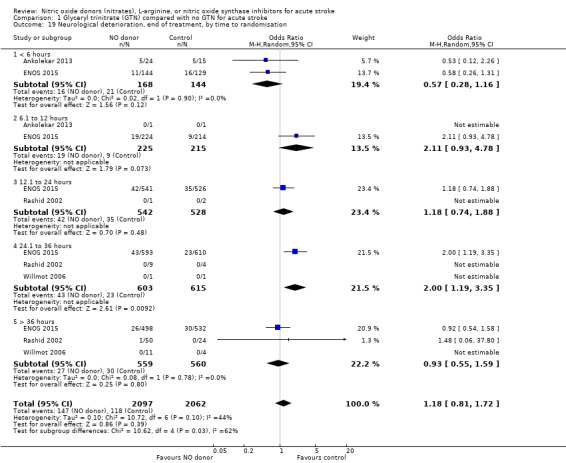
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 19 Neurological deterioration, end of treatment, by time to randomisation.
1.20. Analysis.
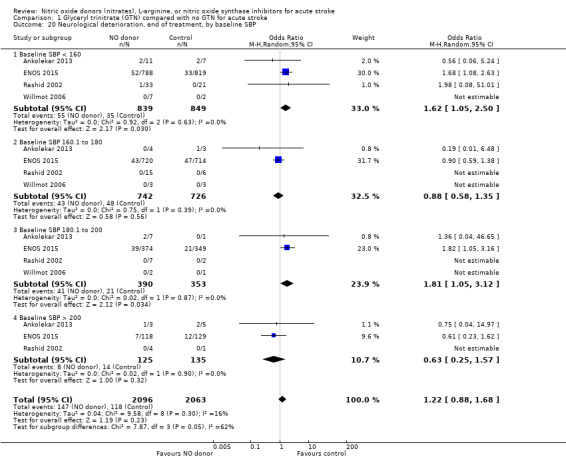
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 20 Neurological deterioration, end of treatment, by baseline SBP.
NIHSS, end of treatment
NIHSS was calculated using the SSS available from four trials (4137 participants). There was no significant difference between treatment and control (Analysis 1.21). Stroke type and baseline systolic BP had no influence on the neutral effect seen overall (Analysis 1.22; Analysis 1.24). Participants randomised to GTN within six hours of stroke onset had lower NIHSS scores at end of treatment compared with control (MD ‐2.07, 95% CI ‐3.81 to ‐0.34, P = 0.02), with no heterogeneity seen between the two trials, and significant interaction between time and NIHSS (P = 0.03, Analysis 1.23).
1.21. Analysis.
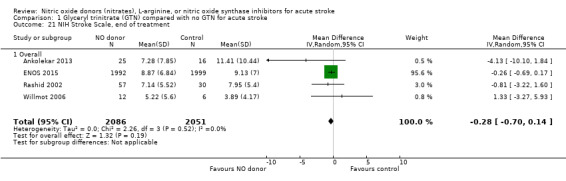
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 21 NIH Stroke Scale, end of treatment.
1.22. Analysis.
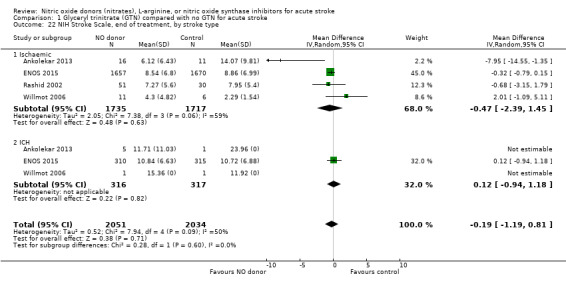
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 22 NIH Stroke Scale, end of treatment, by stroke type.
1.24. Analysis.
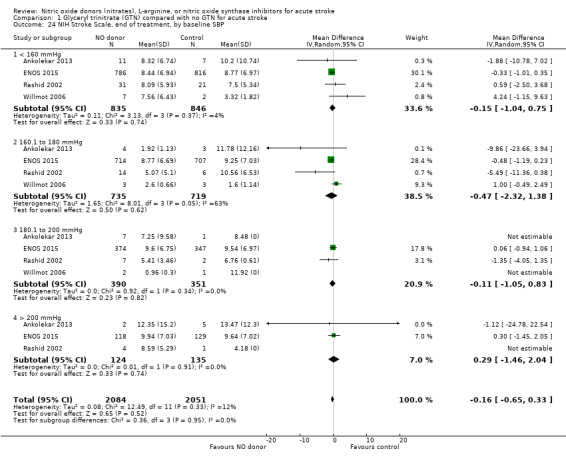
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 24 NIH Stroke Scale, end of treatment, by baseline SBP.
1.23. Analysis.
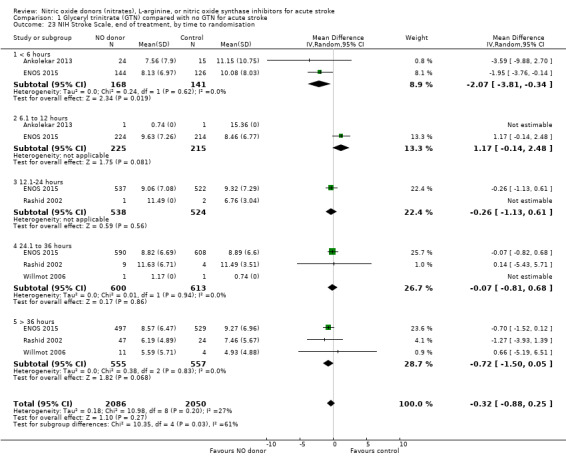
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 23 NIH Stroke Scale, end of treatment, by time to randomisation.
Disability (Barthel Index), end of trial
Disability data in the form of BI was available for all five studies (4153 participants) with no significant difference detected between GTN and control (Analysis 1.25). Those randomised to GTN within six hours of stroke ictus (2 trials, 312 participants) had improved disability scores compared with control participants (MD 14.57, 95% CI 5.95 to 23.19, P = 0.0009), with no heterogeneity seen between the studies, and significant interaction between time and BI (P = 0.03, Analysis 1.27). Baseline systolic BP and stroke type subgroups had no associations of note for this outcome (Analysis 1.26; Analysis 1.28).
1.25. Analysis.
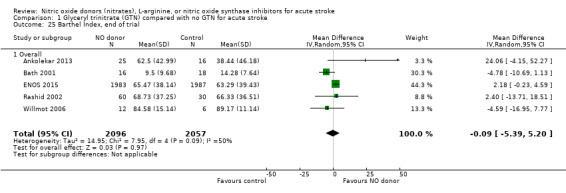
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 25 Barthel Index, end of trial.
1.27. Analysis.
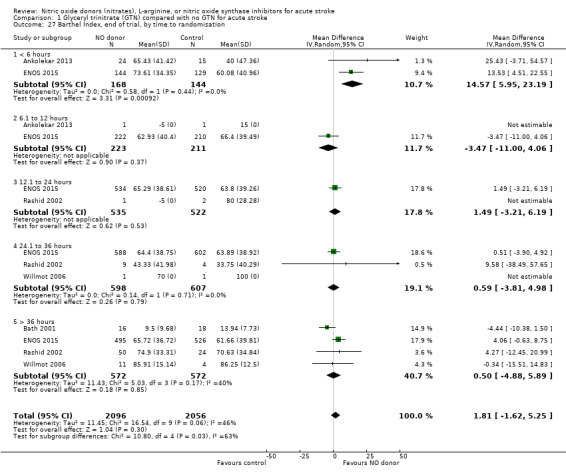
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 27 Barthel Index, end of trial, by time to randomisation.
1.26. Analysis.
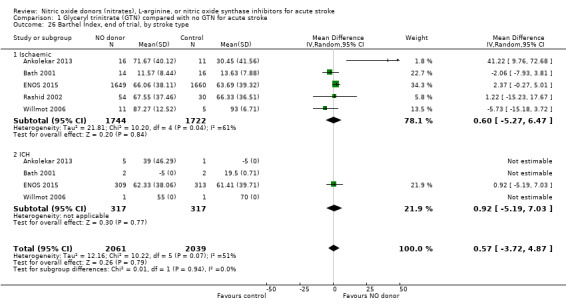
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 26 Barthel Index, end of trial, by stroke type.
1.28. Analysis.
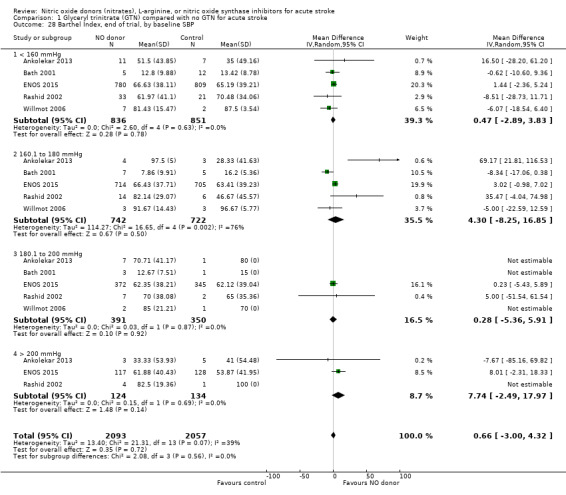
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 28 Barthel Index, end of trial, by baseline SBP.
Mood (Zung), end of trial
Mood was assessed in three studies (3312 participants) using the Zung depression scale (ZDS). No significant difference was seen between GTN and control (Analysis 1.29). Improved mood scores were seen in those randomised to GTN within six hours of the index event compared with control participants (MD ‐11.12, 95% CI ‐17.35 to ‐4.90, P = 0.0005) with no heterogeneity seen between the two studies (268 participants), and significant interaction between time and ZDS (P = 0.007, Analysis 1.31). Stroke type and baseline systolic BP had no associations of note for this outcome (Analysis 1.30; Analysis 1.32).
1.29. Analysis.
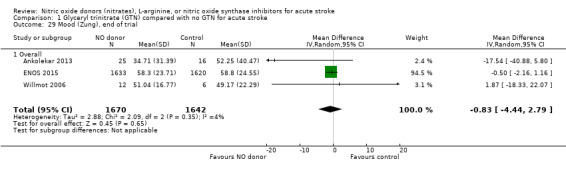
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 29 Mood (Zung), end of trial.
1.31. Analysis.
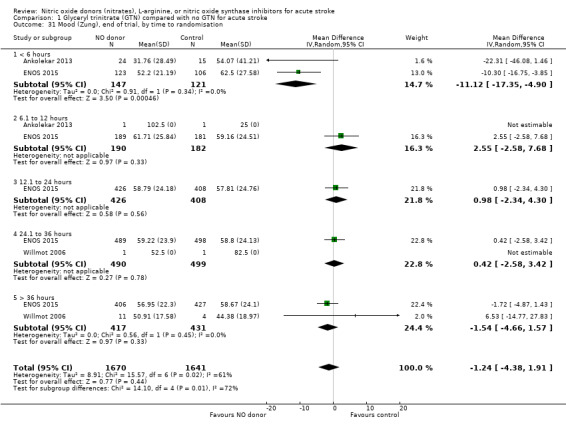
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 31 Mood (Zung), end of trial, by time to randomisation.
1.30. Analysis.
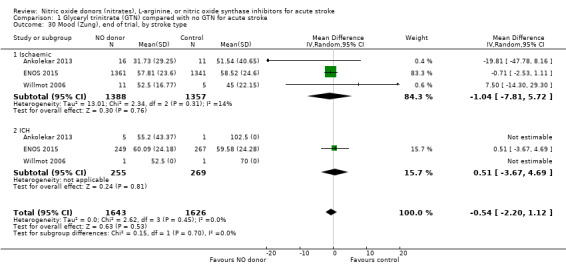
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 30 Mood (Zung), end of trial, by stroke type.
1.32. Analysis.
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 32 Mood (Zung), end of trial, by baseline SBP.
Quality of life, end of trial
Two measures of quality of life were assessed: the EQ‐5D was transformed into a health utility status from data available in four studies (4088 participants); and the EQ‐VAS from four studies (3575 participants). There was no significant difference between treatment and control for either measure (Analysis 1.33; Analysis 1.37). Whilst the subgroups stroke type and baseline systolic BP had no impact on the neutral effect seen overall, early treatment with GTN within six hours of onset was associated with improved quality of life at day 90 compared with those randomised to control (EQ‐5D (2 trials, 312 participants) MD 0.11, 95% CI 0.02 to 0.20, P = 0.02, with minimal heterogeneity (I2 = 3%; Analysis 1.35); and EQ‐VAS (2 trials, 295 participants) MD 9.96, 95% CI 2.49 to 17.43, P = 0.009 with no heterogeneity (Analysis 1.39)). Across time to randomisation, there was a significant interaction with EQ‐VAS (P = 0.04), but not EQ‐5D (P = 0.10).
1.33. Analysis.
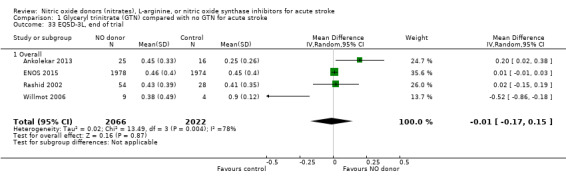
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 33 EQ5D‐3L, end of trial.
1.37. Analysis.
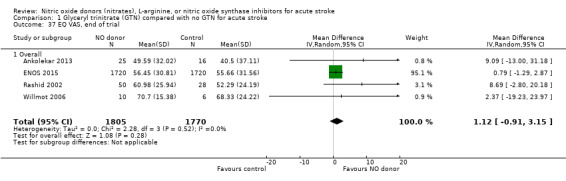
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 37 EQ VAS, end of trial.
1.35. Analysis.
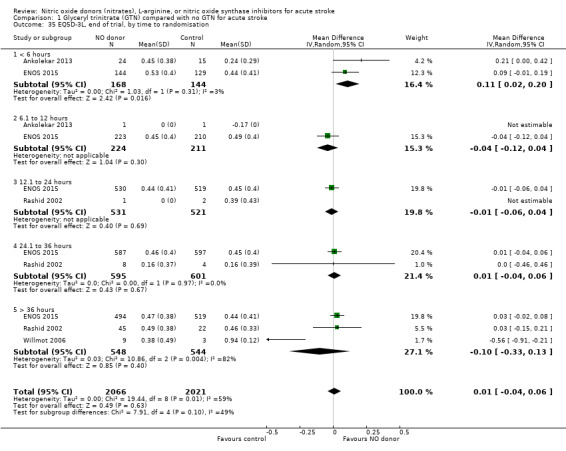
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 35 EQ5D‐3L, end of trial, by time to randomisation.
1.39. Analysis.
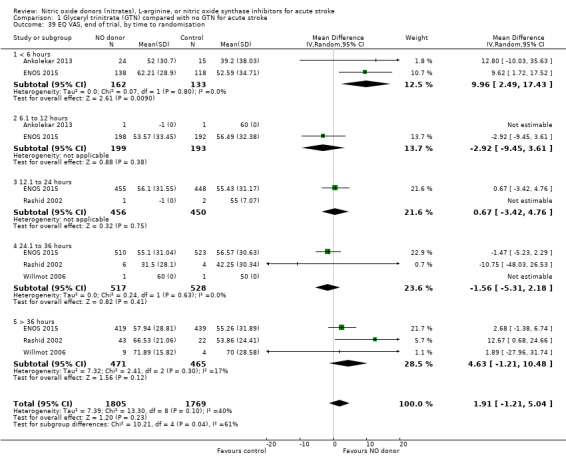
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 39 EQ VAS, end of trial, by time to randomisation.
Cognition, end of trial
Three measures of cognition were assessed: three studies had t‐MMSE data (2078 participants, in two studies t‐MMSE was derived from MMSE); one study also used TICS and animal naming (ENOS 2015). Overall, there was no significant difference between GTN and control (Analysis 1.41; Analysis 1.45; Analysis 1.49). Stroke type and baseline systolic BP did not influence this finding (Analysis 1.42; Analysis 1.44; Analysis 1.46; Analysis 1.48; Analysis 1.50; Analysis 1.52). Improved cognitive scores were seen across all three measures in those randomised to GTN within six hours of onset (t‐MMSE: MD 3.61, 95% CI 1.68 to 5.55, P = 0.0002 (2 trials, 219 participants, interaction P = 0.003; Analysis 1.43); TICS: MD 5.59, 95% CI 2.63 to 8.55, P = 0.0002 (1 trial, 182 participants, interaction P = 0.002; Analysis 1.47); and animal naming: MD 2.94, 95% CI 0.88 to 5.00, P = 0.005 (1 trial, 192 participants, interaction P = 0.04; Analysis 1.51)).
1.41. Analysis.
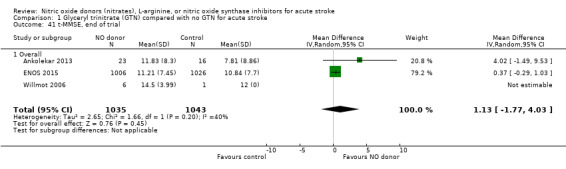
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 41 t‐MMSE, end of trial.
1.45. Analysis.
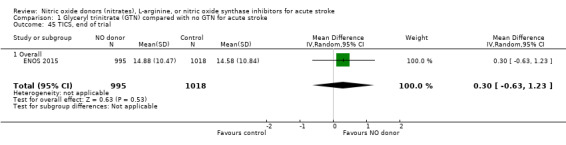
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 45 TICS, end of trial.
1.49. Analysis.
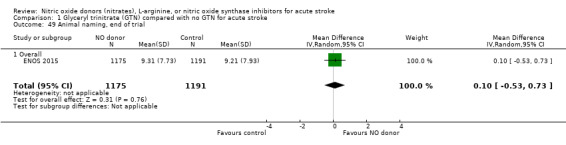
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 49 Animal naming, end of trial.
1.42. Analysis.
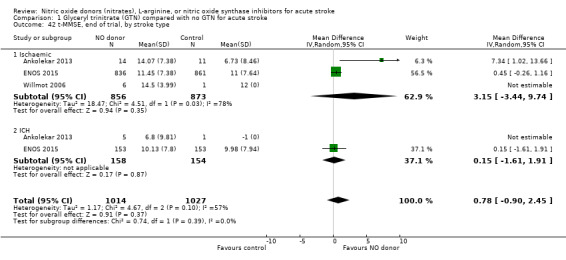
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 42 t‐MMSE, end of trial, by stroke type.
1.44. Analysis.
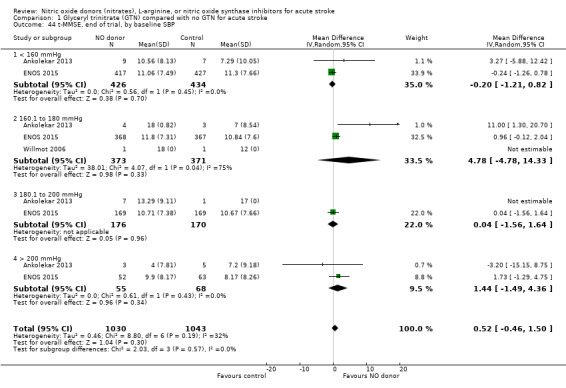
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 44 t‐MMSE, end of trial, by baseline SBP.
1.46. Analysis.
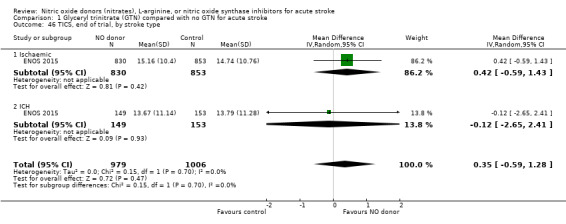
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 46 TICS, end of trial, by stroke type.
1.48. Analysis.
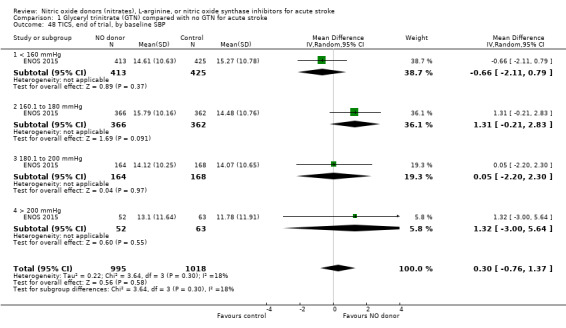
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 48 TICS, end of trial, by baseline SBP.
1.50. Analysis.
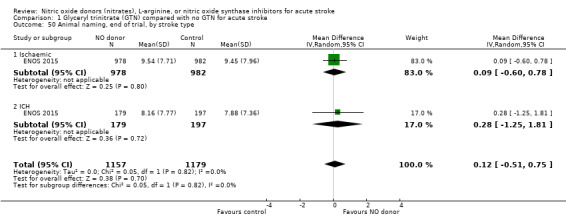
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 50 Animal naming, end of trial, by stroke type.
1.52. Analysis.
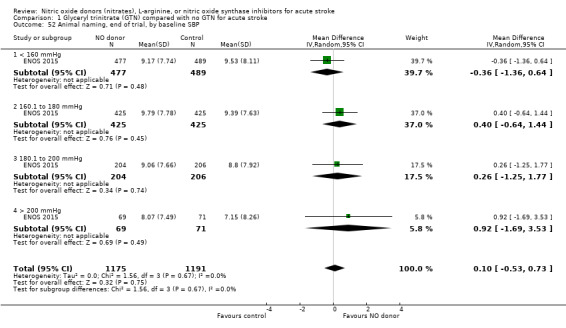
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 52 Animal naming, end of trial, by baseline SBP.
1.43. Analysis.
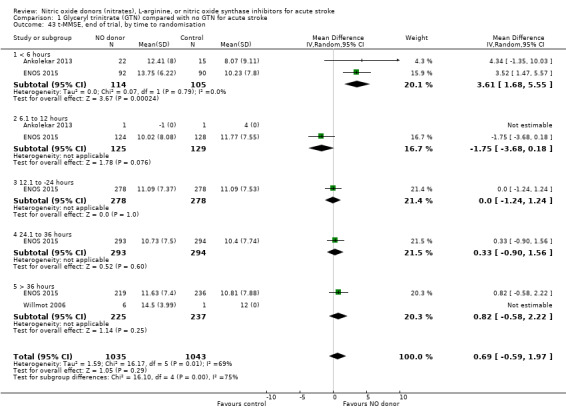
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 43 t‐MMSE, end of trial, by time to randomisation.
1.47. Analysis.
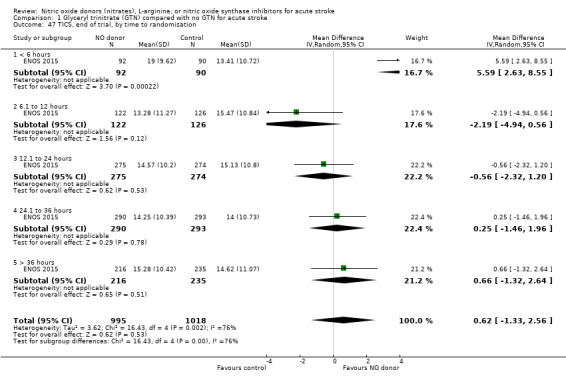
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 47 TICS, end of trial, by time to randomisation.
1.51. Analysis.
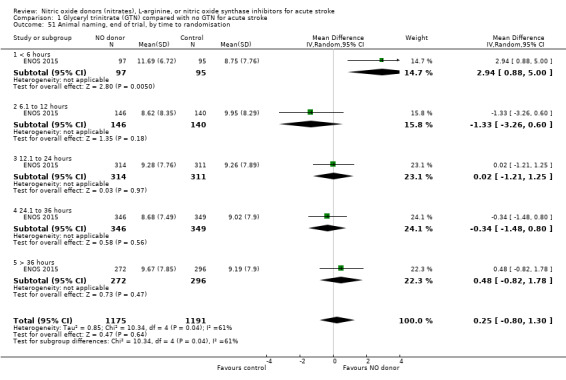
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 51 Animal naming, end of trial, by time to randomisation.
Physiotherapy, occupational therapy, and speech and language therapy
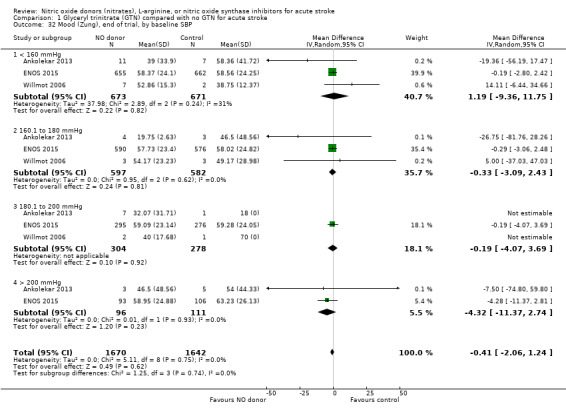
Data on whether individuals received physiotherapy, occupational therapy or speech and language therapy during their admission were available from three studies (4042 participants). There was no difference between treatment groups in the rates of therapy required (Analysis 1.53; Analysis 1.57; Analysis 1.61). Rates of occupational therapy were lower, with borderline significance, in those randomised to GTN with baseline systolic BP level of 180.1 to 200 mmHg compared with control participants (OR 0.74, 95% CI 0.54 to 1.00, P = 0.05), with no heterogeneity seen between the three studies (730 participants), and higher in those randomised to GTN with baseline systolic BP > 200 mmHg compared with controls (OR 2.11, 95% CI 1.25 to 3.54, P = 0.005), with no heterogeneity between the two studies (255 participants; Analysis 1.60). No other associations regarding baseline systolic BP were noted for rates of either physiotherapy (Analysis 1.56) or speech and language therapy (Analysis 1.64). Neither time to randomisation nor stroke type had any effect on therapy rates between treatment and control.
1.53. Analysis.
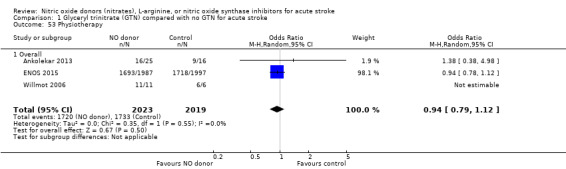
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 53 Physiotherapy.
1.57. Analysis.
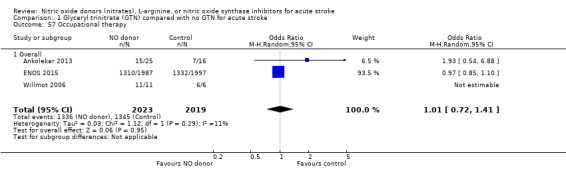
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 57 Occupational therapy.
1.61. Analysis.
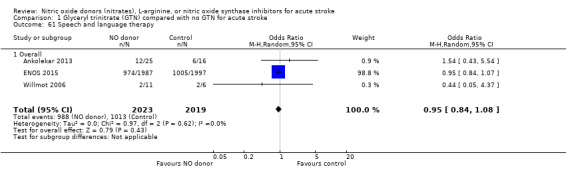
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 61 Speech and language therapy.
1.60. Analysis.
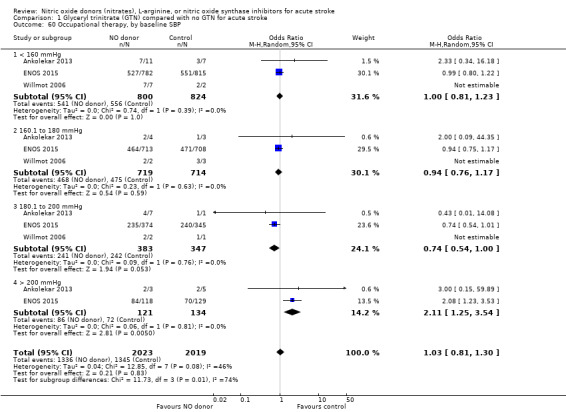
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 60 Occupational therapy, by baseline SBP.
1.56. Analysis.
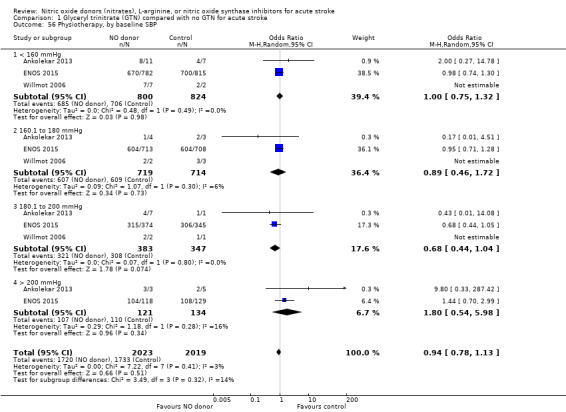
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 56 Physiotherapy, by baseline SBP.
1.64. Analysis.
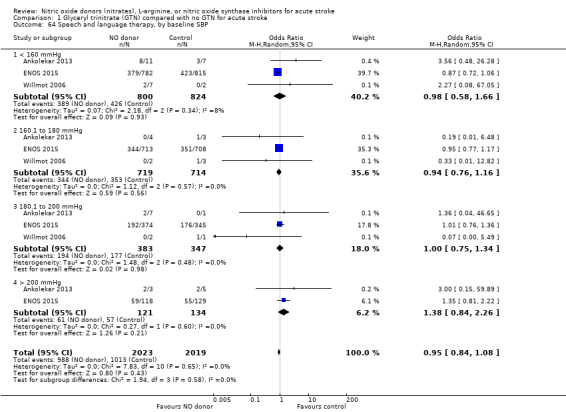
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 64 Speech and language therapy, by baseline SBP.
Feeding route (non‐oral feeding at day 7)
Feeding route was recorded in three trials (4009 participants) and assessed as non‐oral feeding at day seven. Overall, there was no difference between GTN versus no GTN regarding rates of non‐oral feeding at day seven (Analysis 1.65). Early treatment with GTN within six hours of onset was associated with a nonsignificant tendency towards less non‐oral feeding (OR 0.59, 95% CI 0.32 to 1.08, P = 0.09), with no heterogeneity seen between the two studies (301 participants; Analysis 1.67). No associations of note were seen regarding other factors (time groups from randomisation, stroke type or baseline systolic BP (Analysis 1.66; Analysis 1.68)).
1.65. Analysis.
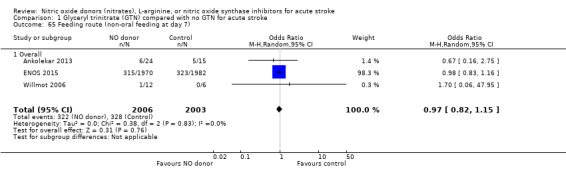
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 65 Feeding route (non‐oral feeding at day 7).
1.67. Analysis.
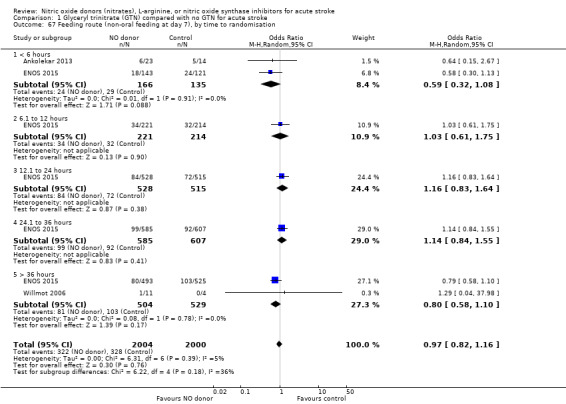
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 67 Feeding route (non‐oral feeding at day 7), by time to randomisation.
1.66. Analysis.
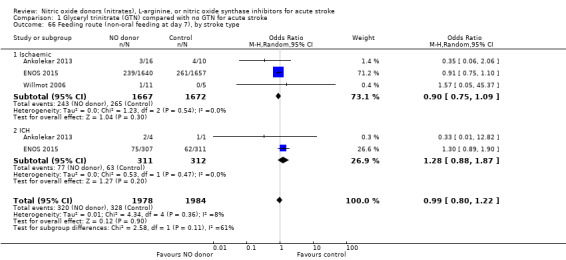
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 66 Feeding route (non‐oral feeding at day 7), by stroke type.
1.68. Analysis.
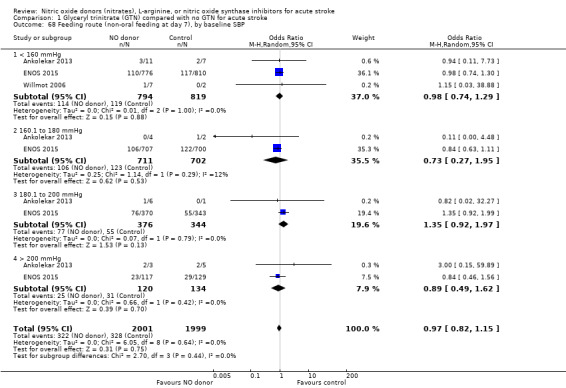
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 68 Feeding route (non‐oral feeding at day 7), by baseline SBP.
Length of stay
The length of hospital stay relating to the index randomising event was assessed in four studies (4078 participants), with no significant treatment effect noted (Analysis 1.69). No subgroup effects were noted (Analysis 1.70; Analysis 1.71; Analysis 1.72).
1.69. Analysis.
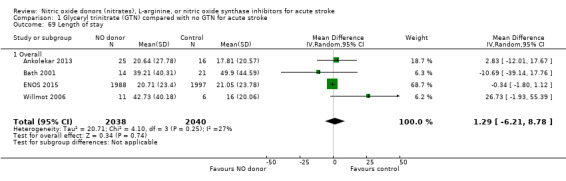
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 69 Length of stay.
1.70. Analysis.
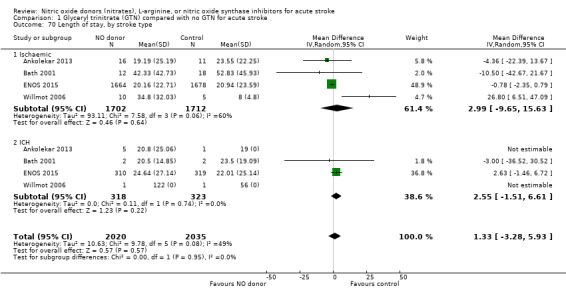
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 70 Length of stay, by stroke type.
1.71. Analysis.
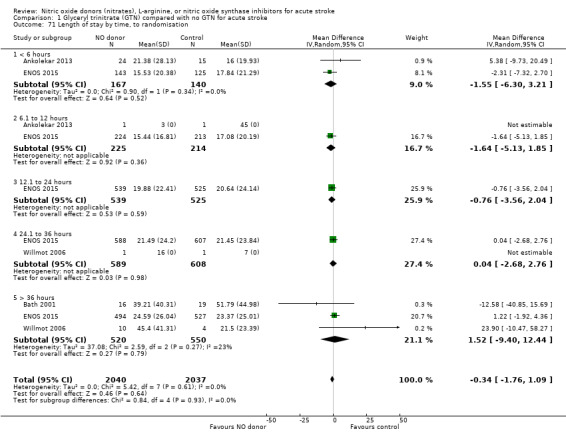
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 71 Length of stay by time, to randomisation.
1.72. Analysis.
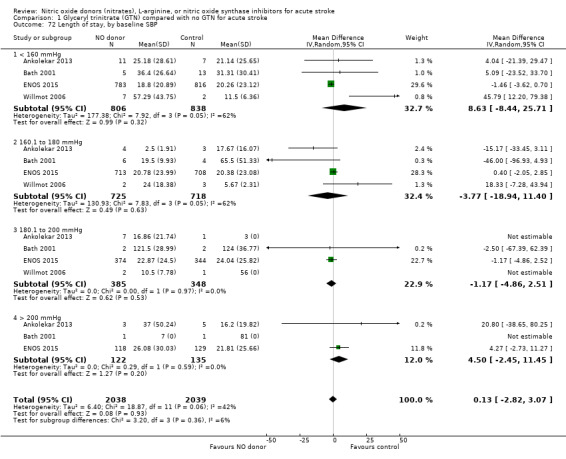
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 72 Length of stay, by baseline SBP.
Headache on treatment
Headache on treatment was more common in people randomised to GTN than to control (OR 2.37, 95% CI 1.55 to 3.62; 4186 participants), with no significant heterogeneity seen (Analysis 1.73; Table 1). GTN led to increased reporting of headache on treatment in both ischaemic stroke (OR 2.39, 95% CI 1.59 to 3.61, P < 0.0001; 3409 participants; I2 = 4%) and ICH (OR 1.91, 95% CI 1.24 to 2.93, P = 0.003; 639 participants; I2 = 0%) compared with control (Analysis 1.74). Headache was more common in participants randomised to GTN than controls for all time periods of randomisation (Analysis 1.75) and all baseline systolic BP subgroups except > 200 mmHg, which was neutral (Analysis 1.76).
1.73. Analysis.
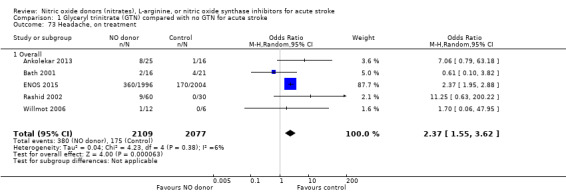
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 73 Headache, on treatment.
1.74. Analysis.
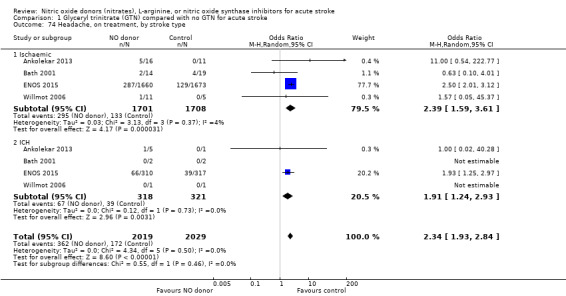
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 74 Headache, on treatment, by stroke type.
1.75. Analysis.
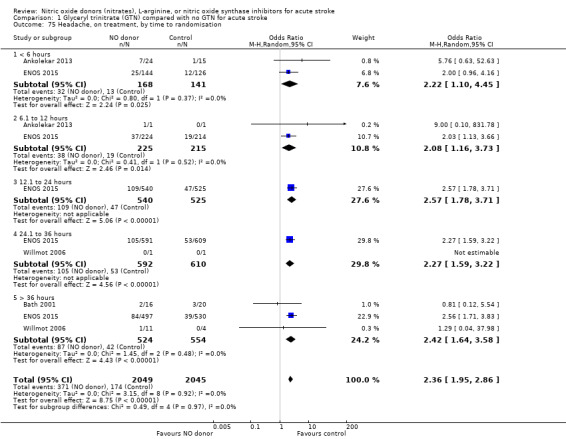
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 75 Headache, on treatment, by time to randomisation.
1.76. Analysis.
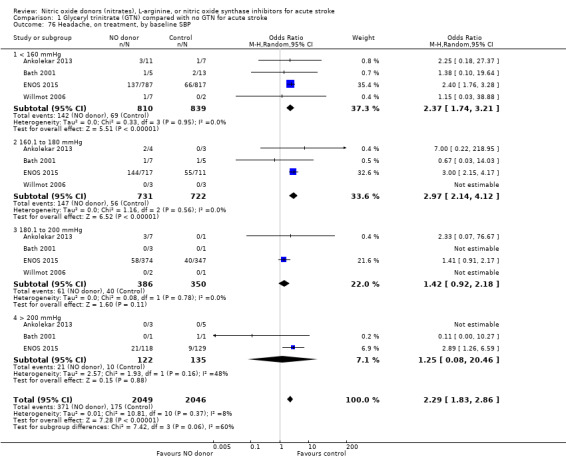
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 76 Headache, on treatment, by baseline SBP.
Treatment stopped early
There was no significant difference in the rate of treatment being stopped early between those randomised to GTN or control (4193 participants), although there was significant heterogeneity between trials (I2 = 88%; Analysis 1.77). Treatment was more likely to be stopped in those randomised to GTN after six, and before 36, hours but these groups were dominated by ENOS 2015 (Analysis 1.79). Stroke type and baseline systolic BP did not alter the overall neutral effect seen.
1.77. Analysis.
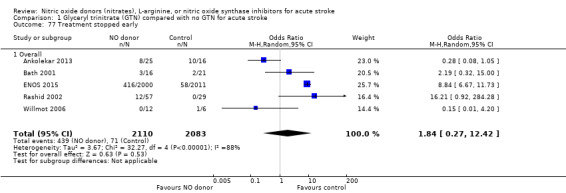
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 77 Treatment stopped early.
1.79. Analysis.
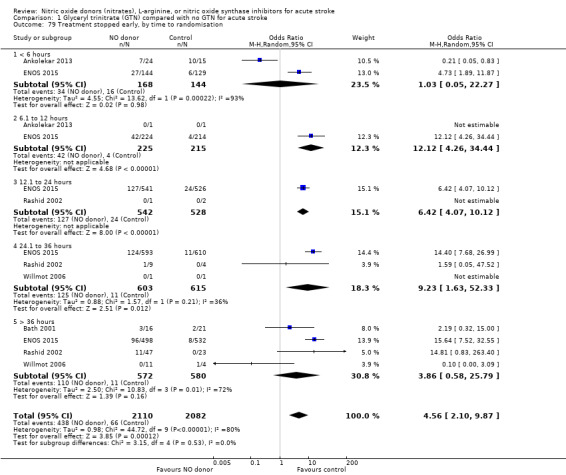
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 79 Treatment stopped early, by time to randomisation.
Haemodynamics
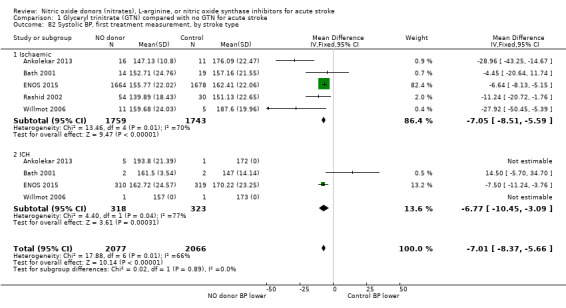
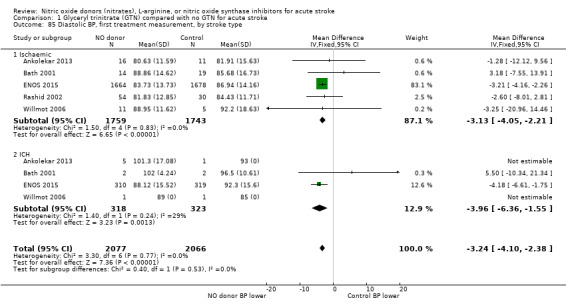
Haemodynamics are the first measurements on treatment (4197 participants; Table 1; high‐quality evidence). BP (mmHg) was significantly lowered in those randomised to GTN compared with control (systolic BP: MD ‐7.21, 95% CI ‐8.58 to ‐5.85, Analysis 1.81; diastolic BP: MD ‐3.31, 95% CI ‐4.18 to ‐2.45, Analysis 1.84), whilst heart rate (beats per minute) was significantly increased (MD 2.02, 95% CI 1.13 to 2.91, Analysis 1.87). Of note, for heart rate there was significant heterogeneity (I2 = 61%) between trials. Regardless of stroke type, BP was significantly lowered by GTN compared with control, and heart rate was increased (Analysis 1.82; Analysis 1.85; Analysis 1.88). BP was significantly lowered in those randomised to GTN compared with control in all time to randomisation groups (Analysis 1.83; Analysis 1.86), whilst heart rate was significantly increased in those randomised to GTN after 24, and before 36, hours (MD 3.44, 95% CI 1.79 to 5.09; 1218 participants; I2 = 32%), with nonsignificant results seen in other time groups (Analysis 1.89).
1.81. Analysis.
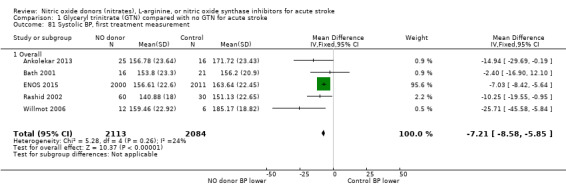
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 81 Systolic BP, first treatment measurement.
1.84. Analysis.
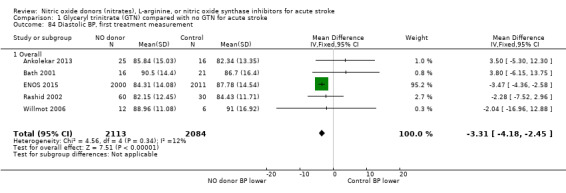
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 84 Diastolic BP, first treatment measurement.
1.87. Analysis.
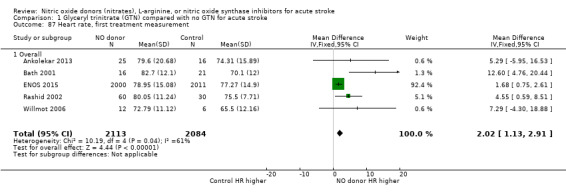
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 87 Heart rate, first treatment measurement.
1.82. Analysis.
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 82 Systolic BP, first treatment measurement, by stroke type.
1.85. Analysis.
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 85 Diastolic BP, first treatment measurement, by stroke type.
1.88. Analysis.
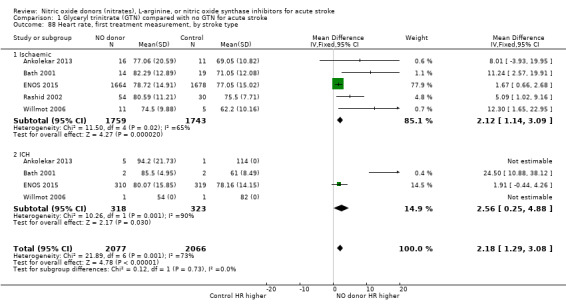
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 88 Heart rate, first treatment measurement, by stroke type.
1.83. Analysis.
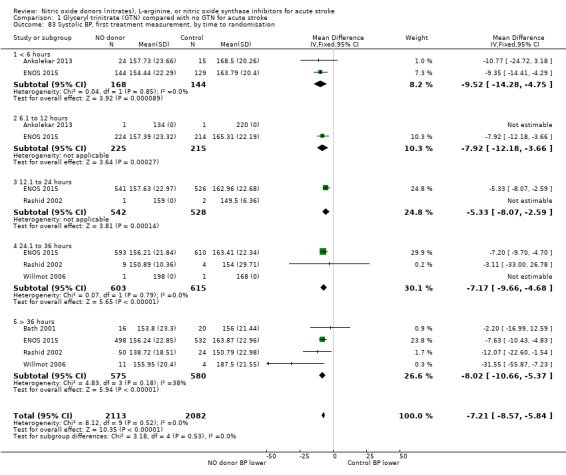
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 83 Systolic BP, first treatment measurement, by time to randomisation.
1.86. Analysis.
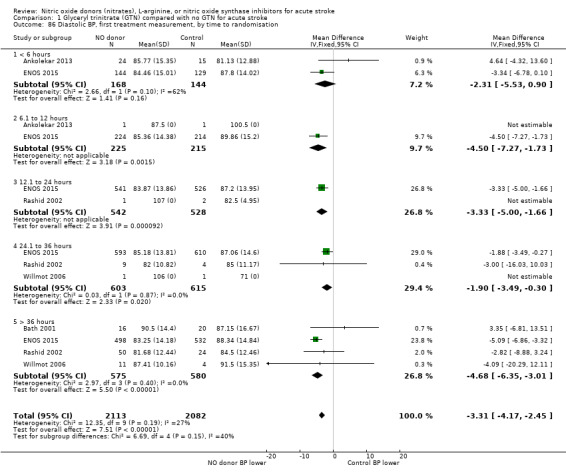
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 86 Diastolic BP, first treatment measurement, by time to randomisation.
1.89. Analysis.
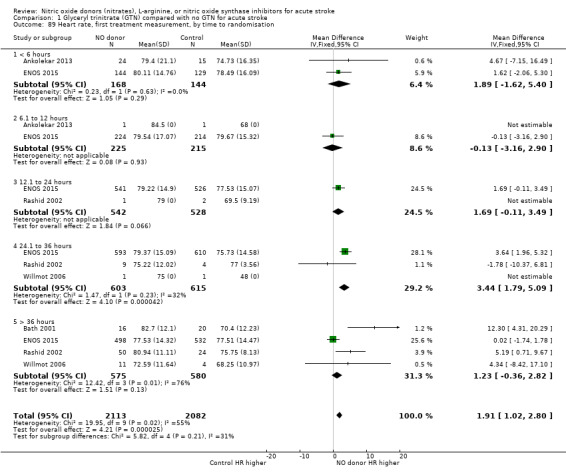
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 89 Heart rate, first treatment measurement, by time to randomisation.
Discussion
Summary of main results
Five trials involving 4197 participants assessed transdermal GTN in acute stroke. Overall, GTN did not significantly influence clinical outcomes including death or disability, death, neurological deterioration, severity, mood, quality of life, cognition, length of hospital stay, therapy requirements, or feeding route.
Subgroup analyses showed that participants randomised to GTN with baseline systolic BP <160 or 180 to 200 mmHg had increased rates of neurological deterioration compared with controls. Conversely, receiving GTN with a baseline systolic BP 180 to 200 mmHg was associated with lower rates of occupational therapy but higher rates were seen with a baseline systolic BP > 200 mmHg. There were no consistent effects across outcomes regarding baseline systolic BP. Participants randomised to GTN within six hours of symptom onset had nonsignificant reductions in the rates of death or dependency (mRS > 2) and non‐oral feeding, and significant improvements in NIHSS at end of treatment, and death or dependency (mean mRS), death, disability, mood, quality of life, and cognition at end of trial. Although participants randomised to GTN between 24 and 36 hours after stroke onset had an increased chance of neurological deterioration, no significant findings were noted in any other clinical outcomes for this time period. Some of the heterogeneity seen between the trials for several outcomes may relate to the differing times from stroke onset to randomisation, which ranged from within four hours of onset up to 168 hours from onset.
In respect of haemodynamics, BP was significantly lowered by GTN as compared with control regardless of stroke type and time to randomisation, whilst heart rate was significantly increased overall, regardless of stroke type, and in those randomised between 24 and 36 hours after stroke onset. Headache, a recognised side effect of GTN, was more commonly seen in those randomised to GTN overall, in both stroke types, at all times of randomisation and in all but those with baseline systolic BP > 200 mmHg.
Of note, there was no significant difference in the rate of treatment being stopped early between GTN and control but there was significant heterogeneity between the trials. Those who received GTN between six and 36 hours of stroke onset were more likely to stop treatment early; a finding driven by ENOS (ENOS 2015).
Overall completeness and applicability of evidence
This review includes all identified trials of NO donors in people with recent stroke. All five trials studied transdermal GTN and no RCTs assessing other NO donors, L‐arginine or nitric oxide synthase inhibitors have been completed. The results from trials assessing GTN should not be extrapolated to other NO donors, L‐arginine or nitric oxide synthase inhibitors. With 4197 participants across five trials, this review has substantial external validity and is able to comment on the safety and efficacy of GTN in acute stroke. Overall, GTN was safe, lowered BP and increased heart rate, but had no beneficial effect on primary and secondary clinical outcomes.
The potential benefit of GTN seen in participants randomised within six hours of onset may represent chance, but there are several explanations to suggest that the association could be real.
The beneficial effects seen were across multiple outcomes including death, dependency (mRS), disability (BI), mood (ZDS), NIHSS, quality of life (EQ‐5D health utility status and EQ‐VAS) and cognition (t‐MMSE, TICS, animal naming).
The effects were seen in two separate trials (Ankolekar 2013; ENOS 2015).
The effects were seen in a population of over 300 participants. This group is equivalent in size to each of the parts of the positive NINDS trials of intravenous alteplase (NINDS 1995) and recent trials of mechanical thrombectomy (Goyal 2016).
The time‐dependent effect of NO donors has been demonstrated in a meta‐analysis of pre‐clinical studies in which early treatment within 60 minutes of ischaemia was associated with positive outcomes as compared with neutral outcomes in those studies assessing treatment up to 48 hours following induction of ischaemic stroke (Willmot 2005).
Hyperacute administration of NO donors, such as GTN, has several potential mechanisms of action alluded to in the background of this review.
NO levels are low in acute stroke and replacing this deficiency may be beneficial.
NO donors lower BP, pulse pressure and peak systolic BP, and improve arterial compliance. Lowering BP acutely may reduce early recurrence following ischaemic stroke, and haematoma expansion in ICH (Anderson 2013).
NO dilates cerebral arteries thereby increasing perilesional perfusion through the 'front door' without resultant cerebral steal (Willmot 2006).
NO is a potent vasodilator of pial arteries leading to 'back‐door' collateral reperfusion (Morikawa 1992b).
NO donors are neuroprotective when administered early after preclinical ischaemic stroke (Willmot 2005).
NO donors might augment the effects of established interventions such as intravenous alteplase, both through preparing patients for treatment by lowering BP, and potentially by increasing access of lytics to occluding clot.
Quality of the evidence
This review has several strengths.
The study cohort was large, involving more than 4000 participants.
Individual patient data were used from all identified controlled trials of NO donors, facilitating subgroup analyses.
Differing participant characteristics between trials broadened external validity.
The trials examined different time‐windows after stroke onset including ultra‐acute/pre‐hospital (Ankolekar 2013), hyper‐acute (< 6 hours) (ENOS 2015), acute (< 48 hours) (ENOS 2015), and sub‐acute (< 168 hours) (Bath 2001; Rashid 2002; Willmot 2006).
Safety and efficacy were assessed across a multitude of outcomes encompassing multiple clinical domains; similar results across outcome domains and trials suggest intra‐ and inter‐trial consistency.
There are also several limitations of this review.
GTN was the only NO donor assessed in the included RCTs and therefore the results cannot be extrapolated to other NO donors, for which there is no RCT‐based evidence.
ENOS 2015 accounted for 95.6% of all participants included in this review, and therefore dominated the data and subsequent analyses.
All these data were reported by the same research group and therefore the results need to be validated and extended by other research groups.
Only one of the included studies was double‐blind (Bath 2001), with the remainder being single‐blind (Ankolekar 2013; ENOS 2015; Willmot 2006) or open‐label (Rashid 2002). Further, headache, a common side effect of GTN, may have unblinded some participants. In addition, despite all end‐of‐trial outcome measures being performed by assessors blinded to treatment in the included studies, observer bias cannot be excluded.
The results relating to ultra‐acute and hyper‐acute treatment involved a relatively small number of participants (Ankolekar 2013; ENOS 2015); these findings should be considered provisional and require formal testing.
This review contains analyses involving a variety of outcomes assessed in several subgroups. Such multiple testing can lead to spurious results and it is possible that some findings may simply reflect chance, e.g. increased neurological deterioration in certain BP subgroups (in the absence of a trend across BP, or a negative effect on the NIHSS), or variation in occupational therapy utilisation in BP subgroups.
In summary, the overall quality of the evidence as assessed by GRADE criteria was high, due to the aforementioned strengths of the included studies.
Potential biases in the review process
This review was compiled following an extensive literature search, without language restrictions, by both the review authors and Cochrane Stroke Group. The resultant risk for publication bias is therefore low. However, all the included trials were from the same research group ‐ of which the authors are members ‐ which may have potentially introduced bias in the review process, e.g. in choice of outcomes and subgroups. Nevertheless, all outcomes reported in the included trials were summarised here (including mRS, BI, EQ‐5D, EQ‐VAS, t‐MMSE, TICS‐M, animal naming, ZDS, NIHSS, death), and subgroups (time, stroke type, blood pressure) were used as prespecified in the ENOS trial (ENOS 2015).
Agreements and disagreements with other studies or reviews
A recently published systematic review and individual patient data meta‐analysis assessed the same five trials of transdermal GTN under the auspices of the Blood pressure in Acute Stroke Collaboration (BASC 2016). Both unadjusted and adjusted analyses were performed including a predefined subgroup analysis by time to randomisation. In this regard, the results and conclusions of both reviews were similar showing that GTN is safe though ineffective overall, but may improve outcome in a time‐dependent manner if given within six hours of stroke onset.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to determine the efficacy of NO donors, L‐arginine, or nitric oxide synthase inhibitors in acute stroke, with only RCT evidence available for the NO donor, GTN. In people with acute stroke, transdermal GTN reduces blood pressure, increases heart rate and headache, but does not alter outcome. When administered within six hours, GTN may improve clinical outcomes, a finding that requires confirmation in an independent trial (e.g. RIGHT‐2 2015).
Implications for research.
Large phase III randomised trials are required to assess the administration of NO donors in ultra‐acute and hyper‐acute strokes.
The safety and efficacy of NO donors other than GTN.
The safety and efficacy of GTN administered in the ultra‐acute phase/prehospital environment (e.g. RIGHT‐2 2015).
The effect of GTN on long‐term functional outcome and death, i.e. beyond 90 days (e.g. RIGHT‐2 2015).
Mechanisms by which GTN might work, e.g. potentially through reducing haematoma expansion in ICH, and improving collateral supply in ischaemic stroke (e.g. RIGHT‐2 2015).
The health economics of GTN (although the low cost of treatment, i.e. < £5 per patient, means that GTN will dominate if efficacy is seen for mRS).
Whether there are particular subgroups who benefit, or do not benefit, from GTN.
Whether GTN acts as adjunctive therapy to intravenous thrombolysis in ischaemic stroke.
Whether GTN acts as adjunctive therapy to mechanical thrombectomy in ischaemic stroke.
Whether GTN reduces the need for hospital‐based therapies such as mechanical thrombectomy, hemicraniectomy, need for rehabilitation therapy.
Whether low and middle income countries can utilise GTN in the hyperacute period.
What's new
| Date | Event | Description |
|---|---|---|
| 7 September 2016 | New search has been performed | Addition of three new trials with 4070 participants. In total, five trials with 4197 participants are now included in the review. We have added subgroup analyses and new outcomes, and updated the Background. |
| 7 September 2016 | New citation required and conclusions have changed | With the addition of new data we conclude that glyceryl trinitrate (GTN) does not alter outcome overall in acute stroke. When administered within six hours, GTN may improve clinical outcomes and therefore warrants further study. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 4 September 2008 | Amended | Converted to new review format. |
| 13 August 2002 | New search has been performed | Data from two trials have been added. The review has been expanded to include patients with primary intracerebral haemorrhage (as well as ischaemic stroke). |
Acknowledgements
We are grateful to the Editorial Board of the Cochrane Stroke Group, external peer reviewer, and consumer reviewer (Katarina Paunovic) for making constructive comments on this review. No pharmaceutical company was involved in this review.
Appendices
Appendix 1. MEDLINE search strategy
1. Cerebrovascular disorders/
2. exp Brain ischemia/
3. Carotid artery diseases/ or Carotid artery thrombosis/
4. exp stroke/
5. exp Hypoxia‐ischemia, brain/
6. Cerebral arterial diseases/ or Intracranial arterial diseases/
7. exp "Intracranial embolism and thrombosis"/
8. exp basal ganglia cerebrovascular disease/
9. exp intracranial hemorrhages/
10. (stroke$ or cerebral vasc$ or cerebrovasc$ or cva or transient isch?emic attack$ or tia$).tw.
11. (brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation).tw.
12. (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$).tw.
13. 11 and 12
14. (brain or cerebral or intracranial).tw.
15. (h?emorrhage or h?ematoma or bleed$).tw.
16. 14 and 15
17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 13 or 16
18. exp nitric oxide donors/
19. nitric oxide/
20. nitroglycerin/
21. Nitric‐Oxide Synthase/ai [Antagonists & Inhibitors]
22. arginine/
23. nitroprusside/
24. (nitric$ or nitro$ or glyceryl trinitrat$ or GTN or arginine).tw.
25. or/18‐24
26. 17 and 25
27. limit 26 to human
Appendix 2. Embase search strategy
1. Cerebrovascular disorders.mp. or cerebrovascular disease/
2. exp Brain ischemia/
3. Carotid artery diseases/ or Carotid artery thrombosis/
4. exp cerebrovascular accident/
5. exp brain hypoxia/ or exp brain ischemia
6. Cerebral artery disease/
7. exp occlusive cerebrovascular disease/
8. exp basal ganglion hemorrhage/
9. exp brain hemorrhage/
10. (stroke$ or cerebral vasc$ or cerebrovasc$ or cva or transient isch?emic attack$ or tia$).tw.
11. (brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation).tw.
12. (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$).tw.
13. 11 and 12
14. (brain or cerebral or intracranial).tw.
15. (h?emorrhage or h?ematoma or bleed$).tw.
16. 14 and 15
17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 13 or 16
18. exp nitric oxide donor/
19. nitric oxide/
20. glyceryl trinitrate/
21. Nitric‐Oxide Synthase/
22. arginine/
23. nitroprusside sodium/
24. (nitric$ or nitro$ or glyceryl trinitrat$ or GTN or arginine).tw.
25. or/18‐24
26. 17 and 25
27. limit 26 to human
28. limit 27 to (clinical trial or randomized controlled trial or controlled clinical trial or multicentre study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial)
Appendix 3. Web of science / Science Citation Index search strategy
1. Cerebrovascular disorders.TI/TS
2. Brain ischemia.TI/TS
3. Carotid artery diseases or Carotid artery thrombosis.TI/TS
4. Stroke.TI/TS
5. Brain hypoxia.TI/TS
6. Cerebral artery disease.TI/TS
7. Occlusive cerebrovascular disease.TI/TS
8. Basal ganglia cerebrovascular disease.TI/TS
9. Intracranial hemorrhages.TI/TS
10. (stroke$ or cerebral vasc$ or cerebrovasc$ or cva or transient isch?emic attack$ or tia$).TI/TS
11. (brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation).TI/TS
12. (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$).TI/TS
13. 11 and 12
14. (brain or cerebral or intracranial).TI/TS
15. (h?emorrhage or h?ematoma or bleed$).TI/TS
16. 14 and 15
17. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 13 or 16
18. nitric oxide donor.TI/TS
19. nitric oxide.TI/TS
20. glyceryl trinitrate.TI/TS
21. Nitric‐Oxide Synthase.TI/TS
22. arginine.TI/TS
23. nitroprusside sodium.TI/TS
24. (nitric$ or nitro$ or glyceryl trinitrat$ or GTN or arginine).TI/TS
25. or/18‐24
26. 17 and 25
27. 26 Refined by: DOCUMENT TYPES: (CLINICAL TRIAL)
Data and analyses
Comparison 1. Glyceryl trinitrate (GTN) compared with no GTN for acute stroke.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency (mRS>2), end of trial | 5 | 4195 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 1.1 Overall | 5 | 4195 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 2 Death or dependency (mRS>2), end of trial, by stroke type | 5 | 4143 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
| 2.1 Ischaemic | 5 | 3502 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.62, 1.53] |
| 2.2 Intracerebral haemorrhage | 4 | 641 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.45, 3.21] |
| 3 Death or dependency (mRS>2), end of trial, by time to randomisation | 5 | 4194 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.86, 1.10] |
| 3.1 < 6 hours | 2 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.41, 1.02] |
| 3.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.83, 1.77] |
| 3.3 12.1 to 24 hours | 2 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 3.4 24.1 to 36 hours | 3 | 1218 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.83, 1.33] |
| 3.5 > 36 hours | 4 | 1154 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.16] |
| 4 Death or dependency (mRS>2), end of trial, by baseline SBP | 5 | 4193 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.10] |
| 4.1 < 160 mmHg | 5 | 1706 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.21] |
| 4.2 160.1 to 180 mmHg | 5 | 1480 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.23, 2.60] |
| 4.3 180.1 to 200 mmHg | 5 | 747 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 4.4 > 200 mmHg | 3 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.53, 1.48] |
| 5 Death or dependency (mean mRS), end of trial | 5 | 4195 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.52, 0.36] |
| 5.1 Overall | 5 | 4195 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.52, 0.36] |
| 6 Death or dependency (mean mRS), end of trial, by stroke type | 5 | 4143 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 6.1 Ischaemic | 5 | 3502 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.62, 0.35] |
| 6.2 ICH | 4 | 641 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.24, 0.28] |
| 7 Death or dependency (mean mRS), end of trial, by time to randomisation | 5 | 4194 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 7.1 < 6 hours | 2 | 312 | Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.35, ‐0.23] |
| 7.2 6.1 to 12 hours | 2 | 440 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.15, 0.53] |
| 7.3 12.1 to 24 hours | 2 | 1070 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 7.4 24.1 to 36 hours | 3 | 1218 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.13, 0.25] |
| 7.5 > 36 hours | 4 | 1154 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.28] |
| 8 Death or dependency (mean mRS), end of trial, by baseline SBP | 5 | 4193 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.17] |
| 8.1 <160 mmHg | 5 | 1706 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.16, 0.17] |
| 8.2 160.1‐180 mmHg | 5 | 1480 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐1.42, 0.66] |
| 8.3 180.1‐200 mmHg | 5 | 747 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.20, 0.28] |
| 8.4 >200 mmHg | 3 | 260 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.70, 0.17] |
| 9 Death, end of treatment | 5 | 4197 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.76, 1.56] |
| 9.1 Overall | 5 | 4197 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.76, 1.56] |
| 10 Death, end of treatment, by stroke type | 5 | 4143 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.49] |
| 10.1 Ischaemic | 5 | 3502 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.69, 1.51] |
| 10.2 ICH | 4 | 641 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.50, 2.69] |
| 11 Death, end of treatment, by time to randomisation | 5 | 4195 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.74, 1.54] |
| 11.1 < 6 hours | 2 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.28, 2.43] |
| 11.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.49, 5.87] |
| 11.3 12.1 to 24 hours | 2 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.46, 1.54] |
| 11.4 24.1 to 36 hours | 3 | 1218 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [0.90, 4.00] |
| 11.5 > 36 hours | 4 | 1155 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.35, 1.80] |
| 12 Death, end of treatment, by baseline SBP | 5 | 4196 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.76, 1.56] |
| 12.1 < 160 mmHg | 5 | 1706 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.83, 2.56] |
| 12.2 160.1‐180 mmHg | 5 | 1480 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.47, 1.54] |
| 12.3 180.1 to 200 mmHg | 5 | 748 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.28, 1.65] |
| 12.4 > 200 mmHg | 4 | 262 | Odds Ratio (M‐H, Random, 95% CI) | 2.13 [0.60, 7.61] |
| 13 Death, end of trial | 5 | 4197 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.40, 1.50] |
| 13.1 Overall | 5 | 4197 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.40, 1.50] |
| 14 Death, end of trial, by stroke type | 5 | 4143 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.72, 1.04] |
| 14.1 Ischaemic | 5 | 3502 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 14.2 ICH | 4 | 641 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.22, 5.21] |
| 15 Death, end of trial, by time to randomisation | 5 | 4195 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.14] |
| 15.1 < 6 hours | 2 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.60] |
| 15.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.79, 2.25] |
| 15.3 12.1 to 24 hours | 2 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 1.52 [0.20, 11.53] |
| 15.4 24.1 to 36 hours | 3 | 1218 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.37] |
| 15.5 > 36 hours | 4 | 1155 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.27, 2.44] |
| 16 Death, end of trial, by baseline SBP | 5 | 4196 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.05] |
| 16.1 < 160 mmHg | 5 | 1706 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.71, 1.27] |
| 16.2 160.1 to 180 mmHg | 5 | 1480 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.44, 1.41] |
| 16.3 180.1 to 200 mmHg | 5 | 748 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.33] |
| 16.4 > 200 mmHg | 4 | 262 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.40, 1.50] |
| 17 Neurological deterioration, end of treatment | 4 | 4158 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.97, 1.60] |
| 17.1 Overall | 4 | 4158 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.97, 1.60] |
| 18 Neurological deterioration, end of treatment, by stroke type | 4 | 4106 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.74, 1.86] |
| 18.1 Ischaemic | 4 | 3469 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.09, 3.75] |
| 18.2 ICH | 3 | 637 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.83, 2.57] |
| 19 Neurological deterioration, end of treatment, by time to randomisation | 4 | 4159 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.81, 1.72] |
| 19.1 < 6 hours | 2 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.28, 1.16] |
| 19.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [0.93, 4.78] |
| 19.3 12.1 to 24 hours | 2 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.74, 1.88] |
| 19.4 24.1 to 36 hours | 3 | 1218 | Odds Ratio (M‐H, Random, 95% CI) | 2.00 [1.19, 3.35] |
| 19.5 > 36 hours | 3 | 1119 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.55, 1.59] |
| 20 Neurological deterioration, end of treatment, by baseline SBP | 4 | 4159 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.88, 1.68] |
| 20.1 Baseline SBP < 160 | 4 | 1688 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [1.05, 2.50] |
| 20.2 Baseline SBP 160.1 to 180 | 4 | 1468 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.58, 1.35] |
| 20.3 Baseline SBP 180.1 to 200 | 4 | 743 | Odds Ratio (M‐H, Random, 95% CI) | 1.81 [1.05, 3.12] |
| 20.4 Baseline SBP > 200 | 3 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.25, 1.57] |
| 21 NIH Stroke Scale, end of treatment | 4 | 4137 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.70, 0.14] |
| 21.1 Overall | 4 | 4137 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.70, 0.14] |
| 22 NIH Stroke Scale, end of treatment, by stroke type | 4 | 4085 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.19, 0.81] |
| 22.1 Ischaemic | 4 | 3452 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.39, 1.45] |
| 22.2 ICH | 3 | 633 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.94, 1.18] |
| 23 NIH Stroke Scale, end of treatment, by time to randomisation | 4 | 4136 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.88, 0.25] |
| 23.1 < 6 hours | 2 | 309 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐3.81, ‐0.34] |
| 23.2 6.1 to 12 hours | 2 | 440 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐0.14, 2.48] |
| 23.3 12.1‐24 hours | 2 | 1062 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.13, 0.61] |
| 23.4 24.1 to 36 hours | 3 | 1213 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.81, 0.68] |
| 23.5 > 36 hours | 3 | 1112 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.50, 0.05] |
| 24 NIH Stroke Scale, end of treatment, by baseline SBP | 4 | 4135 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.65, 0.33] |
| 24.1 < 160 mmHg | 4 | 1681 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐1.04, 0.75] |
| 24.2 160.1 to 180 mmHg | 4 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐2.32, 1.38] |
| 24.3 180.1 to 200 mmHg | 4 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐1.05, 0.83] |
| 24.4 > 200 mmHg | 3 | 259 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐1.46, 2.04] |
| 25 Barthel Index, end of trial | 5 | 4153 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐5.39, 5.20] |
| 25.1 Overall | 5 | 4153 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐5.39, 5.20] |
| 26 Barthel Index, end of trial, by stroke type | 5 | 4100 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐3.72, 4.87] |
| 26.1 Ischaemic | 5 | 3466 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐5.27, 6.47] |
| 26.2 ICH | 4 | 634 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐5.19, 7.03] |
| 27 Barthel Index, end of trial, by time to randomisation | 5 | 4152 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐1.62, 5.25] |
| 27.1 < 6 hours | 2 | 312 | Mean Difference (IV, Random, 95% CI) | 14.57 [5.95, 23.19] |
| 27.2 6.1 to 12 hours | 2 | 434 | Mean Difference (IV, Random, 95% CI) | ‐3.47 [‐11.00, 4.06] |
| 27.3 12.1 to 24 hours | 2 | 1057 | Mean Difference (IV, Random, 95% CI) | 1.49 [‐3.21, 6.19] |
| 27.4 24.1 to 36 hours | 3 | 1205 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐3.81, 4.98] |
| 27.5 > 36 hours | 4 | 1144 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐4.88, 5.89] |
| 28 Barthel Index, end of trial, by baseline SBP | 5 | 4150 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐3.00, 4.32] |
| 28.1 < 160 mmHg | 5 | 1687 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐2.89, 3.83] |
| 28.2 160.1 to 180 mmHg | 5 | 1464 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐8.25, 16.85] |
| 28.3 180.1 to 200 mmHg | 5 | 741 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐5.36, 5.91] |
| 28.4 > 200 mmHg | 3 | 258 | Mean Difference (IV, Random, 95% CI) | 7.74 [‐2.49, 17.97] |
| 29 Mood (Zung), end of trial | 3 | 3312 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐4.44, 2.79] |
| 29.1 Overall | 3 | 3312 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐4.44, 2.79] |
| 30 Mood (Zung), end of trial, by stroke type | 3 | 3269 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐2.20, 1.12] |
| 30.1 Ischaemic | 3 | 2745 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐7.81, 5.72] |
| 30.2 ICH | 3 | 524 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐3.67, 4.69] |
| 31 Mood (Zung), end of trial, by time to randomisation | 3 | 3311 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐4.38, 1.91] |
| 31.1 < 6 hours | 2 | 268 | Mean Difference (IV, Random, 95% CI) | ‐11.12 [‐17.35, ‐4.90] |
| 31.2 6.1 to 12 hours | 2 | 372 | Mean Difference (IV, Random, 95% CI) | 2.55 [‐2.58, 7.68] |
| 31.3 12.1 to 24 hours | 1 | 834 | Mean Difference (IV, Random, 95% CI) | 0.98 [‐2.34, 4.30] |
| 31.4 24.1 to 36 hours | 2 | 989 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐2.58, 3.42] |
| 31.5 > 36 hours | 2 | 848 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐4.66, 1.57] |
| 32 Mood (Zung), end of trial, by baseline SBP | 3 | 3312 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐2.06, 1.24] |
| 32.1 < 160 mmHg | 3 | 1344 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐9.36, 11.75] |
| 32.2 160.1 to 180 mmHg | 3 | 1179 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐3.09, 2.43] |
| 32.3 180.1 to 200 mmHg | 3 | 582 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐4.07, 3.69] |
| 32.4 > 200 mmHg | 2 | 207 | Mean Difference (IV, Random, 95% CI) | ‐4.32 [‐11.37, 2.74] |
| 33 EQ5D‐3L, end of trial | 4 | 4088 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 33.1 Overall | 4 | 4088 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 34 EQ5D‐3L, end of trial, by stroke type | 4 | 4084 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.08, 0.09] |
| 34.1 Ischaemic | 4 | 3457 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.17, 0.18] |
| 34.2 ICH | 2 | 627 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 35 EQ5D‐3L, end of trial, by time to randomisation | 4 | 4087 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 35.1 < 6 hours | 2 | 312 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.02, 0.20] |
| 35.2 6.1 to 12 hours | 2 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 35.3 12.1 to 24 hours | 2 | 1052 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 35.4 24.1 to 36 hours | 2 | 1196 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 35.5 > 36 hours | 3 | 1092 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.33, 0.13] |
| 36 EQ5D‐3L, end of trial, by baseline SBP | 4 | 4086 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.05, 0.08] |
| 36.1 < 160 mmHg | 4 | 1664 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.34, 0.10] |
| 36.2 160.1 to 180 mmHg | 4 | 1438 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.08, 0.51] |
| 36.3 180.1 to 200 mmHg | 3 | 726 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 36.4 > 200 mmHg | 3 | 258 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.01, 0.18] |
| 37 EQ VAS, end of trial | 4 | 3575 | Mean Difference (IV, Random, 95% CI) | 1.12 [‐0.91, 3.15] |
| 37.1 Overall | 4 | 3575 | Mean Difference (IV, Random, 95% CI) | 1.12 [‐0.91, 3.15] |
| 38 EQ VAS, end of trial, by stroke type | 4 | 3523 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐1.13, 2.96] |
| 38.1 Ischaemic | 4 | 2974 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐1.06, 3.37] |
| 38.2 ICH | 2 | 549 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐5.72, 4.86] |
| 39 EQ VAS, end of trial, by time to randomisation | 4 | 3574 | Mean Difference (IV, Random, 95% CI) | 1.91 [‐1.21, 5.04] |
| 39.1 < 6 hours | 2 | 295 | Mean Difference (IV, Random, 95% CI) | 9.96 [2.49, 17.43] |
| 39.2 6.1 to 12 hours | 2 | 392 | Mean Difference (IV, Random, 95% CI) | ‐2.92 [‐9.45, 3.61] |
| 39.3 12.1 to 24 hours | 2 | 906 | Mean Difference (IV, Random, 95% CI) | 0.67 [‐3.42, 4.76] |
| 39.4 24.1 to 36 hours | 3 | 1045 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐5.31, 2.18] |
| 39.5 > 36 hours | 3 | 936 | Mean Difference (IV, Random, 95% CI) | 4.63 [‐1.21, 10.48] |
| 40 EQ VAS, end of trial, by baseline SBP | 4 | 3574 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐1.16, 2.92] |
| 40.1 < 160 mmHg | 4 | 1480 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐2.74, 3.57] |
| 40.2 160.1 to 180 mmHg | 4 | 1258 | Mean Difference (IV, Random, 95% CI) | 2.02 [‐6.59, 10.64] |
| 40.3 180.1 to 200 mmHg | 4 | 617 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐4.21, 5.75] |
| 40.4 > 200 mmHg | 3 | 219 | Mean Difference (IV, Random, 95% CI) | 5.22 [‐3.55, 14.00] |
| 41 t‐MMSE, end of trial | 3 | 2078 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐1.77, 4.03] |
| 41.1 Overall | 3 | 2078 | Mean Difference (IV, Random, 95% CI) | 1.13 [‐1.77, 4.03] |
| 42 t‐MMSE, end of trial, by stroke type | 3 | 2041 | Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.90, 2.45] |
| 42.1 Ischaemic | 3 | 1729 | Mean Difference (IV, Random, 95% CI) | 3.15 [‐3.44, 9.74] |
| 42.2 ICH | 2 | 312 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐1.61, 1.91] |
| 43 t‐MMSE, end of trial, by time to randomisation | 3 | 2078 | Mean Difference (IV, Random, 95% CI) | 0.69 [‐0.59, 1.97] |
| 43.1 < 6 hours | 2 | 219 | Mean Difference (IV, Random, 95% CI) | 3.61 [1.68, 5.55] |
| 43.2 6.1 to 12 hours | 2 | 254 | Mean Difference (IV, Random, 95% CI) | ‐1.75 [‐3.68, 0.18] |
| 43.3 12.1 to ‐24 hours | 1 | 556 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.24, 1.24] |
| 43.4 24.1 to 36 hours | 1 | 587 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.90, 1.56] |
| 43.5 > 36 hours | 2 | 462 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐0.58, 2.22] |
| 44 t‐MMSE, end of trial, by baseline SBP | 3 | 2073 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.46, 1.50] |
| 44.1 < 160 mmHg | 2 | 860 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.21, 0.82] |
| 44.2 160.1 to 180 mmHg | 3 | 744 | Mean Difference (IV, Random, 95% CI) | 4.78 [‐4.78, 14.33] |
| 44.3 180.1 to 200 mmHg | 2 | 346 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.56, 1.64] |
| 44.4 > 200 mmHg | 2 | 123 | Mean Difference (IV, Random, 95% CI) | 1.44 [‐1.49, 4.36] |
| 45 TICS, end of trial | 1 | 2013 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.63, 1.23] |
| 45.1 Overall | 1 | 2013 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.63, 1.23] |
| 46 TICS, end of trial, by stroke type | 1 | 1985 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.59, 1.28] |
| 46.1 Ischaemic | 1 | 1683 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.59, 1.43] |
| 46.2 ICH | 1 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐2.65, 2.41] |
| 47 TICS, end of trial, by time to randomisation | 1 | 2013 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐1.33, 2.56] |
| 47.1 < 6 hours | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 5.59 [2.63, 8.55] |
| 47.2 6.1 to 12 hours | 1 | 248 | Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐4.94, 0.56] |
| 47.3 12.1 to 24 hours | 1 | 549 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐2.32, 1.20] |
| 47.4 24.1 to 36 hours | 1 | 583 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.46, 1.96] |
| 47.5 > 36 hours | 1 | 451 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐1.32, 2.64] |
| 48 TICS, end of trial, by baseline SBP | 1 | 2013 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.76, 1.37] |
| 48.1 < 160 mmHg | 1 | 838 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.11, 0.79] |
| 48.2 160.1 to 180 mmHg | 1 | 728 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐0.21, 2.83] |
| 48.3 180.1 to 200 mmHg | 1 | 332 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐2.20, 2.30] |
| 48.4 > 200 mmHg | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 1.32 [‐1.00, 5.64] |
| 49 Animal naming, end of trial | 1 | 2366 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.53, 0.73] |
| 49.1 Overall | 1 | 2366 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.53, 0.73] |
| 50 Animal naming, end of trial, by stroke type | 1 | 2336 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.51, 0.75] |
| 50.1 Ischaemic | 1 | 1960 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.60, 0.78] |
| 50.2 ICH | 1 | 376 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.25, 1.81] |
| 51 Animal naming, end of trial, by time to randomisation | 1 | 2366 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.80, 1.30] |
| 51.1 < 6 hours | 1 | 192 | Mean Difference (IV, Random, 95% CI) | 2.94 [0.88, 5.00] |
| 51.2 6.1 to 12 hours | 1 | 286 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐3.26, 0.60] |
| 51.3 12.1 to 24 hours | 1 | 625 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐1.21, 1.25] |
| 51.4 24.1 to 36 hours | 1 | 695 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.48, 0.80] |
| 51.5 > 36 hours | 1 | 568 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.82, 1.78] |
| 52 Animal naming, end of trial, by baseline SBP | 1 | 2366 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.53, 0.73] |
| 52.1 < 160 mmHg | 1 | 966 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.36, 0.64] |
| 52.2 160.1 to 180 mmHg | 1 | 850 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.64, 1.44] |
| 52.3 180.1 to 200 mmHg | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐1.25, 1.77] |
| 52.4 > 200 mmHg | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 0.92 [‐1.69, 3.53] |
| 53 Physiotherapy | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 53.1 Overall | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.79, 1.12] |
| 54 Physiotherapy, by stroke type | 3 | 3994 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 54.1 Ischaemic | 3 | 3363 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.74, 1.11] |
| 54.2 ICH | 3 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.70] |
| 55 Physiotherapy, by time to randomisation | 3 | 4041 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.80, 1.15] |
| 55.1 < 6 hours | 2 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.40, 2.05] |
| 55.2 6.1 to 12 hours | 2 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.36] |
| 55.3 12.1 to 24 hours | 1 | 1064 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.77, 1.51] |
| 55.4 24.1 to 36 hours | 2 | 1198 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.49] |
| 55.5 > 36 hours | 2 | 1033 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.54, 1.27] |
| 56 Physiotherapy, by baseline SBP | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 56.1 < 160 mmHg | 3 | 1624 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.32] |
| 56.2 160.1 to 180 mmHg | 3 | 1433 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.46, 1.72] |
| 56.3 180.1 to 200 mmHg | 3 | 730 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.04] |
| 56.4 > 200 mmHg | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 1.80 [0.54, 5.98] |
| 57 Occupational therapy | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.72, 1.41] |
| 57.1 Overall | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.72, 1.41] |
| 58 Occupational therapy, by stroke type | 3 | 3994 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.84, 1.24] |
| 58.1 Ischaemic | 3 | 3363 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 58.2 ICH | 3 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.92, 1.73] |
| 59 Occupational therapy, by time to randomisation | 3 | 4041 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.13] |
| 59.1 < 6 hours | 2 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.36, 3.68] |
| 59.2 6.1 to 12 hours | 2 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.37] |
| 59.3 12.1 to 24 hours | 1 | 1064 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.82, 1.36] |
| 59.4 24.1 to 36 hours | 2 | 1198 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.79, 1.28] |
| 59.5 > 36 hours | 2 | 1033 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.72, 1.28] |
| 60 Occupational therapy, by baseline SBP | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.30] |
| 60.1 < 160 mmHg | 3 | 1624 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
| 60.2 160.1 to 180 mmHg | 3 | 1433 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.17] |
| 60.3 180.1 to 200 mmHg | 3 | 730 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.00] |
| 60.4 > 200 mmHg | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 2.11 [1.25, 3.54] |
| 61 Speech and language therapy | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 61.1 Overall | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 62 Speech and language therapy, by stroke type | 3 | 3994 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.09] |
| 62.1 Ischaemic | 3 | 3363 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 62.2 ICH | 3 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.14, 4.19] |
| 63 Speech and language therapy, by time to randomisation | 3 | 4041 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.09] |
| 63.1 < 6 hours | 2 | 307 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.44, 2.29] |
| 63.2 6.1 to 12 hours | 2 | 439 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.64, 1.40] |
| 63.3 12.1 to 24 hours | 1 | 1064 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.21] |
| 63.4 24.1 to 36 hours | 2 | 1198 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.20] |
| 63.5 > 36 hours | 2 | 1033 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.79, 1.29] |
| 64 Speech and language therapy, by baseline SBP | 3 | 4042 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.08] |
| 64.1 < 160 mmHg | 3 | 1624 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.58, 1.66] |
| 64.2 160.1 to 180 mmHg | 3 | 1433 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.16] |
| 64.3 180.1 to 200 mmHg | 3 | 730 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.75, 1.34] |
| 64.4 > 200 mmHg | 2 | 255 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.84, 2.26] |
| 65 Feeding route (non‐oral feeding at day 7) | 3 | 4009 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.15] |
| 65.1 Overall | 3 | 4009 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.15] |
| 66 Feeding route (non‐oral feeding at day 7), by stroke type | 3 | 3962 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.22] |
| 66.1 Ischaemic | 3 | 3339 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.75, 1.09] |
| 66.2 ICH | 2 | 623 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.88, 1.87] |
| 67 Feeding route (non‐oral feeding at day 7), by time to randomisation | 3 | 4004 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.16] |
| 67.1 < 6 hours | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.08] |
| 67.2 6.1 to 12 hours | 1 | 435 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.61, 1.75] |
| 67.3 12.1 to 24 hours | 1 | 1043 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.83, 1.64] |
| 67.4 24.1 to 36 hours | 1 | 1192 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.84, 1.55] |
| 67.5 > 36 hours | 2 | 1033 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.10] |
| 68 Feeding route (non‐oral feeding at day 7), by baseline SBP | 3 | 4000 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.82, 1.15] |
| 68.1 < 160 mmHg | 3 | 1613 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.29] |
| 68.2 160.1 to 180 mmHg | 2 | 1413 | Odds Ratio (M‐H, Random, 95% CI) | 0.73 [0.27, 1.95] |
| 68.3 180.1 to 200 mmHg | 2 | 720 | Odds Ratio (M‐H, Random, 95% CI) | 1.35 [0.92, 1.97] |
| 68.4 > 200 mmHg | 2 | 254 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.49, 1.62] |
| 69 Length of stay | 4 | 4078 | Mean Difference (IV, Random, 95% CI) | 1.29 [‐6.21, 8.78] |
| 69.1 Overall | 4 | 4078 | Mean Difference (IV, Random, 95% CI) | 1.29 [‐6.21, 8.78] |
| 70 Length of stay, by stroke type | 4 | 4055 | Mean Difference (IV, Random, 95% CI) | 1.33 [‐3.28, 5.93] |
| 70.1 Ischaemic | 4 | 3414 | Mean Difference (IV, Random, 95% CI) | 2.99 [‐9.65, 15.63] |
| 70.2 ICH | 4 | 641 | Mean Difference (IV, Random, 95% CI) | 2.55 [‐1.51, 6.61] |
| 71 Length of stay by time, to randomisation | 4 | 4077 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.76, 1.09] |
| 71.1 < 6 hours | 2 | 307 | Mean Difference (IV, Random, 95% CI) | ‐1.55 [‐6.30, 3.21] |
| 71.2 6.1 to 12 hours | 2 | 439 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐5.13, 1.85] |
| 71.3 12.1 to 24 hours | 1 | 1064 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐3.56, 2.04] |
| 71.4 24.1 to 36 hours | 2 | 1197 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐2.68, 2.76] |
| 71.5 > 36 hours | 3 | 1070 | Mean Difference (IV, Random, 95% CI) | 1.52 [‐9.40, 12.44] |
| 72 Length of stay, by baseline SBP | 4 | 4077 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐2.82, 3.07] |
| 72.1 < 160 mmHg | 4 | 1644 | Mean Difference (IV, Random, 95% CI) | 8.63 [‐8.44, 25.71] |
| 72.2 160.1 to 180 mmHg | 4 | 1443 | Mean Difference (IV, Random, 95% CI) | ‐3.77 [‐18.94, 11.40] |
| 72.3 180.1 to 200 mmHg | 4 | 733 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐4.86, 2.51] |
| 72.4 > 200 mmHg | 3 | 257 | Mean Difference (IV, Random, 95% CI) | 4.50 [‐2.45, 11.45] |
| 73 Headache, on treatment | 5 | 4186 | Odds Ratio (M‐H, Random, 95% CI) | 2.37 [1.55, 3.62] |
| 73.1 Overall | 5 | 4186 | Odds Ratio (M‐H, Random, 95% CI) | 2.37 [1.55, 3.62] |
| 74 Headache, on treatment, by stroke type | 4 | 4048 | Odds Ratio (M‐H, Random, 95% CI) | 2.34 [1.93, 2.84] |
| 74.1 Ischaemic | 4 | 3409 | Odds Ratio (M‐H, Random, 95% CI) | 2.39 [1.59, 3.61] |
| 74.2 ICH | 4 | 639 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [1.24, 2.93] |
| 75 Headache, on treatment, by time to randomisation | 4 | 4094 | Odds Ratio (M‐H, Random, 95% CI) | 2.36 [1.95, 2.86] |
| 75.1 < 6 hours | 2 | 309 | Odds Ratio (M‐H, Random, 95% CI) | 2.22 [1.10, 4.45] |
| 75.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 2.08 [1.16, 3.73] |
| 75.3 12.1 to 24 hours | 1 | 1065 | Odds Ratio (M‐H, Random, 95% CI) | 2.57 [1.78, 3.71] |
| 75.4 24.1 to 36 hours | 2 | 1202 | Odds Ratio (M‐H, Random, 95% CI) | 2.27 [1.59, 3.22] |
| 75.5 > 36 hours | 3 | 1078 | Odds Ratio (M‐H, Random, 95% CI) | 2.42 [1.64, 3.58] |
| 76 Headache, on treatment, by baseline SBP | 4 | 4095 | Odds Ratio (M‐H, Random, 95% CI) | 2.29 [1.83, 2.86] |
| 76.1 < 160 mmHg | 4 | 1649 | Odds Ratio (M‐H, Random, 95% CI) | 2.37 [1.74, 3.21] |
| 76.2 160.1 to 180 mmHg | 4 | 1453 | Odds Ratio (M‐H, Random, 95% CI) | 2.97 [2.14, 4.12] |
| 76.3 180.1 to 200 mmHg | 4 | 736 | Odds Ratio (M‐H, Random, 95% CI) | 1.42 [0.92, 2.18] |
| 76.4 > 200 mmHg | 3 | 257 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.08, 20.46] |
| 77 Treatment stopped early | 5 | 4193 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.27, 12.42] |
| 77.1 Overall | 5 | 4193 | Odds Ratio (M‐H, Random, 95% CI) | 1.84 [0.27, 12.42] |
| 78 Treatment stopped early, by stroke type | 4 | 4102 | Odds Ratio (M‐H, Random, 95% CI) | 2.49 [0.72, 8.59] |
| 78.1 Ischaemic | 4 | 3465 | Odds Ratio (M‐H, Random, 95% CI) | 1.26 [0.07, 21.87] |
| 78.2 ICH | 3 | 637 | Odds Ratio (M‐H, Random, 95% CI) | 6.81 [3.46, 13.43] |
| 79 Treatment stopped early, by time to randomisation | 5 | 4192 | Odds Ratio (M‐H, Random, 95% CI) | 4.56 [2.10, 9.87] |
| 79.1 < 6 hours | 2 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.05, 22.27] |
| 79.2 6.1 to 12 hours | 2 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 12.12 [4.26, 34.44] |
| 79.3 12.1 to 24 hours | 2 | 1070 | Odds Ratio (M‐H, Random, 95% CI) | 6.42 [4.07, 10.12] |
| 79.4 24.1 to 36 hours | 3 | 1218 | Odds Ratio (M‐H, Random, 95% CI) | 9.23 [1.63, 52.33] |
| 79.5 > 36 hours | 4 | 1152 | Odds Ratio (M‐H, Random, 95% CI) | 3.86 [0.58, 25.79] |
| 80 Treatment stopped early, by baseline SBP | 4 | 4159 | Odds Ratio (M‐H, Random, 95% CI) | 2.84 [1.37, 5.88] |
| 80.1 < 160 mmHg | 4 | 1687 | Odds Ratio (M‐H, Random, 95% CI) | 1.33 [0.09, 20.26] |
| 80.2 160.1 to 180 mmHg | 4 | 1467 | Odds Ratio (M‐H, Random, 95% CI) | 2.17 [0.17, 27.08] |
| 80.3 180.1 to 200 mmHg | 4 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.17, 11.13] |
| 80.4 > 200 mmHg | 3 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 2.16 [0.21, 22.44] |
| 81 Systolic BP, first treatment measurement | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | ‐7.21 [‐8.58, ‐5.85] |
| 81.1 Overall | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | ‐7.21 [‐8.58, ‐5.85] |
| 82 Systolic BP, first treatment measurement, by stroke type | 5 | 4143 | Mean Difference (IV, Fixed, 95% CI) | ‐7.01 [‐8.37, ‐5.66] |
| 82.1 Ischaemic | 5 | 3502 | Mean Difference (IV, Fixed, 95% CI) | ‐7.05 [‐8.51, ‐5.59] |
| 82.2 ICH | 4 | 641 | Mean Difference (IV, Fixed, 95% CI) | ‐6.77 [‐10.45, ‐3.09] |
| 83 Systolic BP, first treatment measurement, by time to randomisation | 5 | 4195 | Mean Difference (IV, Fixed, 95% CI) | ‐7.21 [‐8.57, ‐5.84] |
| 83.1 < 6 hours | 2 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐9.52 [‐14.28, ‐4.75] |
| 83.2 6.1 to 12 hours | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐12.18, ‐3.66] |
| 83.3 12.1 to 24 hours | 2 | 1070 | Mean Difference (IV, Fixed, 95% CI) | ‐5.33 [‐8.07, ‐2.59] |
| 83.4 24.1 to 36 hours | 3 | 1218 | Mean Difference (IV, Fixed, 95% CI) | ‐7.17 [‐9.66, ‐4.68] |
| 83.5 > 36 hours | 4 | 1155 | Mean Difference (IV, Fixed, 95% CI) | ‐8.02 [‐10.66, ‐5.37] |
| 84 Diastolic BP, first treatment measurement | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐4.18, ‐2.45] |
| 84.1 Overall | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐4.18, ‐2.45] |
| 85 Diastolic BP, first treatment measurement, by stroke type | 5 | 4143 | Mean Difference (IV, Fixed, 95% CI) | ‐3.24 [‐4.10, ‐2.38] |
| 85.1 Ischaemic | 5 | 3502 | Mean Difference (IV, Fixed, 95% CI) | ‐3.13 [‐4.05, ‐2.21] |
| 85.2 ICH | 4 | 641 | Mean Difference (IV, Fixed, 95% CI) | ‐3.96 [‐6.36, ‐1.55] |
| 86 Diastolic BP, first treatment measurement, by time to randomisation | 5 | 4195 | Mean Difference (IV, Fixed, 95% CI) | ‐3.31 [‐4.17, ‐2.45] |
| 86.1 < 6 hours | 2 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐2.31 [‐5.53, 0.90] |
| 86.2 6.1 to 12 hours | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐7.27, ‐1.73] |
| 86.3 12.1 to 24 hours | 2 | 1070 | Mean Difference (IV, Fixed, 95% CI) | ‐3.33 [‐3.00, ‐1.66] |
| 86.4 24.1 to 36 hours | 3 | 1218 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.49, ‐0.30] |
| 86.5 > 36 hours | 4 | 1155 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐6.35, ‐3.01] |
| 87 Heart rate, first treatment measurement | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | 2.02 [1.13, 2.91] |
| 87.1 Overall | 5 | 4197 | Mean Difference (IV, Fixed, 95% CI) | 2.02 [1.13, 2.91] |
| 88 Heart rate, first treatment measurement, by stroke type | 5 | 4143 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [1.29, 3.08] |
| 88.1 Ischaemic | 5 | 3502 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [1.14, 3.09] |
| 88.2 ICH | 4 | 641 | Mean Difference (IV, Fixed, 95% CI) | 2.56 [0.25, 4.88] |
| 89 Heart rate, first treatment measurement, by time to randomisation | 5 | 4195 | Mean Difference (IV, Fixed, 95% CI) | 1.91 [1.02, 2.80] |
| 89.1 < 6 hours | 2 | 312 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [‐1.62, 5.40] |
| 89.2 6.1 to 12 hours | 2 | 440 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐3.16, 2.90] |
| 89.3 12.1 to 24 hours | 2 | 1070 | Mean Difference (IV, Fixed, 95% CI) | 1.69 [‐0.11, 3.49] |
| 89.4 24.1 to 36 hours | 3 | 1218 | Mean Difference (IV, Fixed, 95% CI) | 3.44 [1.79, 5.09] |
| 89.5 > 36 hours | 4 | 1155 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐0.36, 2.82] |
1.14. Analysis.
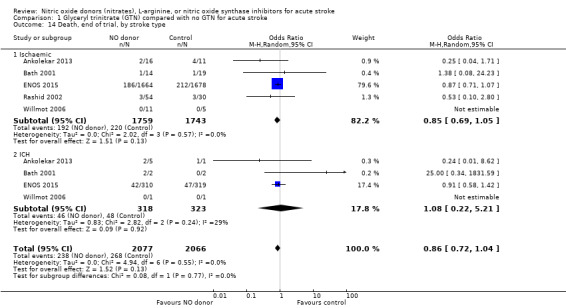
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 14 Death, end of trial, by stroke type.
1.16. Analysis.
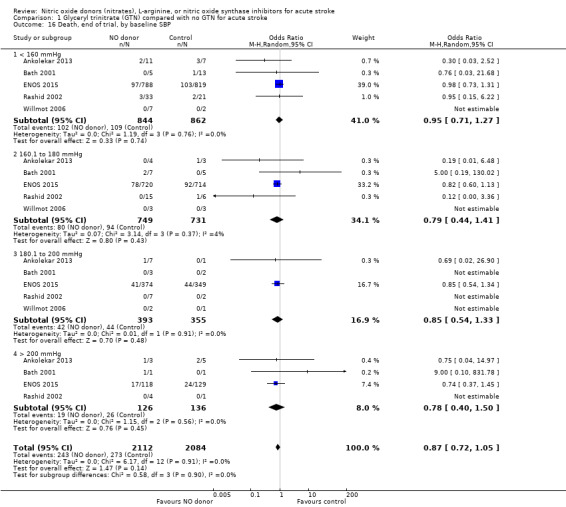
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 16 Death, end of trial, by baseline SBP.
1.34. Analysis.
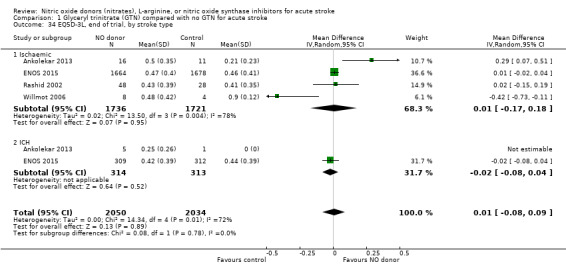
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 34 EQ5D‐3L, end of trial, by stroke type.
1.36. Analysis.
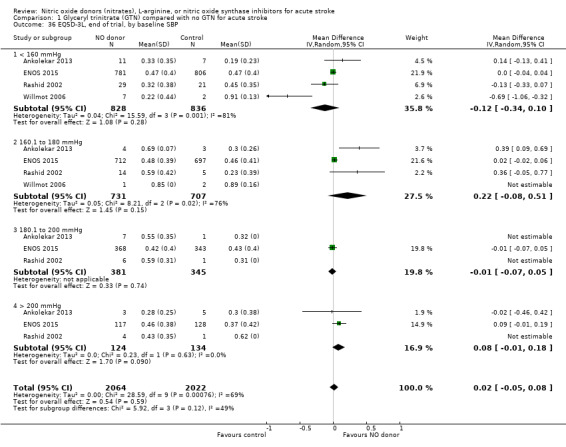
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 36 EQ5D‐3L, end of trial, by baseline SBP.
1.38. Analysis.
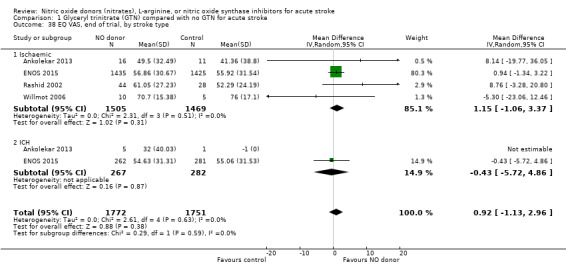
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 38 EQ VAS, end of trial, by stroke type.
1.40. Analysis.
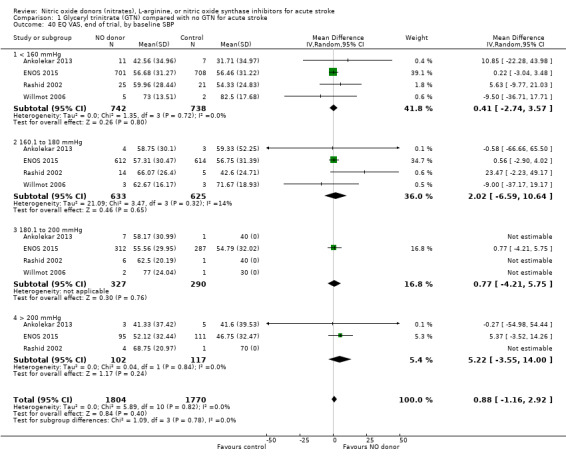
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 40 EQ VAS, end of trial, by baseline SBP.
1.54. Analysis.
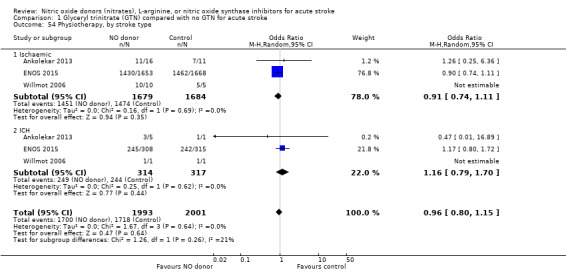
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 54 Physiotherapy, by stroke type.
1.55. Analysis.
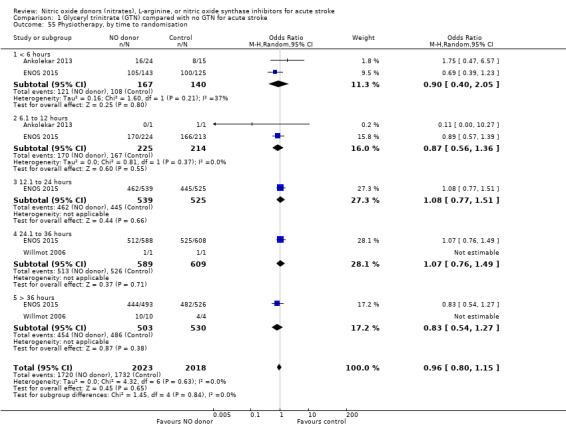
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 55 Physiotherapy, by time to randomisation.
1.58. Analysis.
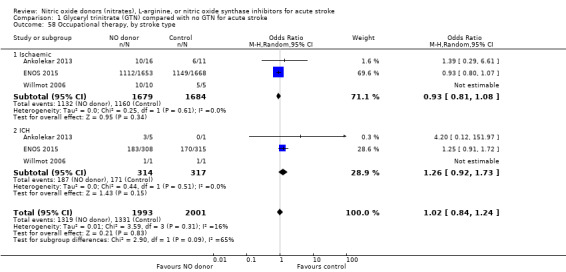
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 58 Occupational therapy, by stroke type.
1.59. Analysis.
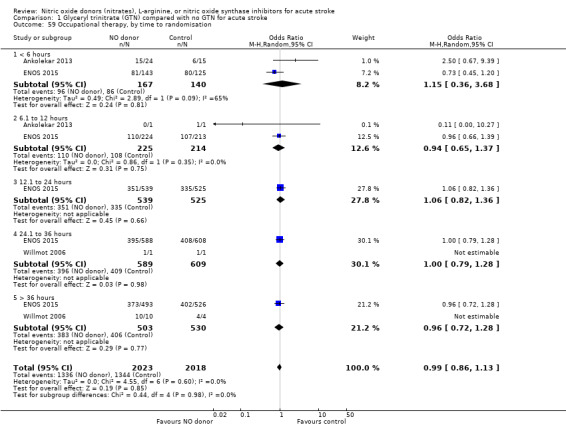
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 59 Occupational therapy, by time to randomisation.
1.62. Analysis.
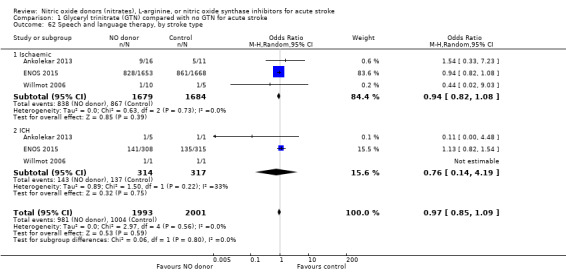
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 62 Speech and language therapy, by stroke type.
1.63. Analysis.
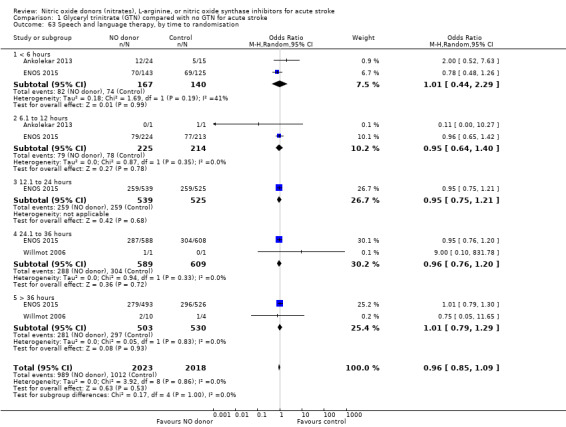
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 63 Speech and language therapy, by time to randomisation.
1.78. Analysis.
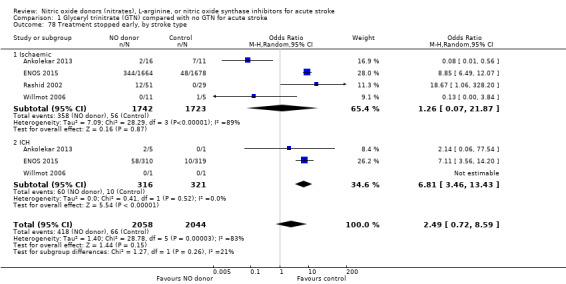
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 78 Treatment stopped early, by stroke type.
1.80. Analysis.
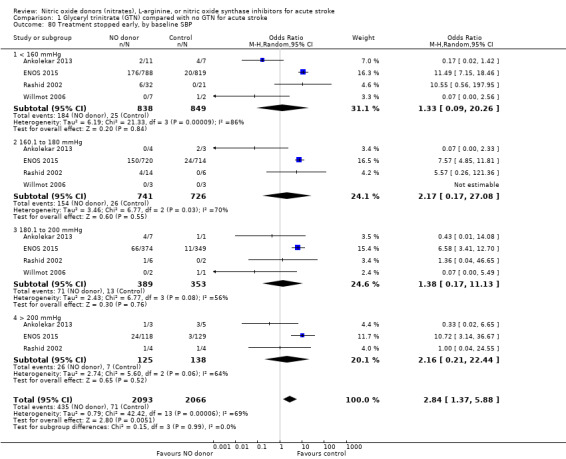
Comparison 1 Glyceryl trinitrate (GTN) compared with no GTN for acute stroke, Outcome 80 Treatment stopped early, by baseline SBP.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ankolekar 2013.
| Methods | Single‐blind, blinded endpoint Randomisation (1:1) |
|
| Participants | UK, single centre 41 participants: treatment 25; control 16 Mean age: treatment 79 years; control 81 years Male: treatment 15 (65%); control 7 (43.8%) Inclusion: FAST positive test; systolic BP ≥ 140 mmHg; age > 40 years for men and > 55 years for women Time to randomisation: within 4 hours of onset |
|
| Interventions | Treatment: transdermal GTN patch 5 mg once daily Control: no patch (Blinding with gauze dressing over patch/equivalent area of skin) Duration: 7 days |
|
| Outcomes | Primary: systolic BP at 2 hours Secondary:
|
|
| Notes | Exclusion: requirement for or contraindication to nitrate therapy; GCS ≤ 8; blood glucose < 2.5 mmol/L; non‐ambulatory prior to symptom onset | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation. Equal distribution between trial groups |
| Allocation concealment (selection bias) | Low risk | Opaque envelope containing a gauze dressing +/‐ GTN patch was only opened after consent was obtained |
| Blinding (performance bias and detection bias) All outcomes | Low risk | GTN was given single‐blinded (participant), whilst outcomes were assessed blinded to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | GTN was given single‐blinded as no placebo patches were available. A gauze dressing was placed over the GTN patch or equivalent area of skin out of sight. As a result, participants were blinded whilst the treating clinician was unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. No difference between trial groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | None found |
Bath 2001.
| Methods | Double‐blind, placebo‐controlled Randomisation by computer (minimisation by age, mean arterial BP, baseline SSS, hours from onset, presence of stroke lesion on CT) |
|
| Participants | UK, single centre 37 participants: treatment 16; control 21 Age: treatment 76 years; control 72 years Male: treatment 6 (38%); control; 12 (57%) Inclusion: IS or ICH Time to randomisation: within five days of onset |
|
| Interventions | Treatment: transdermal GTN patch 5 mg once daily
Control: matching placebo Duration: 12 days |
|
| Outcomes | Primary: BP on day 1 (24 hour ambulatory BP measured 3 times per hour during the day and hourly at night at days 0, 1, 8) Secondary: death at end of treatment; death or dependency (mRS > 2), death, and BI at 3 months | |
| Notes | Exclusion: taking part in another trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation with minimisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching treatment and placebo patches |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching treatment and placebo patches |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | None found |
ENOS 2015.
| Methods | Single‐blind, parallel‐group, partial factorial study Randomisation via password‐protected, data‐encrypted website (minimisation by age, sex, stroke severity, time to treatment and total anterior circulation syndrome) |
|
| Participants | International (23 countries), multicentre (173 sites) 4011 participants: treatment 2000; control 2011 Age: treatment 70 years; control 70 years Male: treatment 1147 (57%); control 1150 (57%) Inclusion: IS or ICH; motor deficit in arm or leg, or both; systolic BP 140 to 220 mmHg Time to randomisation: within 48 hours of onset |
|
| Interventions |
In appropriate participants:
Duration: 7 days |
|
| Outcomes | Primary: mRS at day 90 Secondary:
|
|
| Notes | Exclusions: GCS < 8; pure sensory stroke; preceding dependency (mRS 3 to 5); confounding neurological or psychiatric illness; stroke mimic; severe liver or renal dysfunction; severe comorbidity; pregnant or breastfeeding; planned surgical intervention; previous participation in ENOS 2015; contraindication to or definite need for nitrates and/or prestroke antihypertensive medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Central website‐based |
| Blinding (performance bias and detection bias) All outcomes | Low risk | GTN was given single‐blinded (participant), whilst outcomes were assessed centrally and blinded to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | GTN was given single‐blinded as no placebo patches were available. A gauze dressing was placed over the GTN patch or equivalent area of skin out of sight. As a result, participants were blinded whilst the treating clinician was unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed centrally, blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No difference between trial groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | None found |
Rashid 2002.
| Methods | Open‐label, blinded endpoint dose comparison controlled trial Randomisation by computer (minimisation by age, gender, SSS, mean arterial pressure) |
|
| Participants | UK, single centre 90 participants: treatment 60; control 30 Mean age: treatment 70.8 years; control 73.9 years Male: treatment 28 (46.7%); control 13 (43.3%) Inclusion: IS or ICH Time to randomisation: within 72 hours of onset |
|
| Interventions | Treatment: transdermal GTN patch 5 mg for 10 days; 5 mg for 4 days, then 10 mg for 6 days; 10 mg for 10 days
Control: no patch Duration: 10 days |
|
| Outcomes | Primary: BP on day 1 (24 hour ambulatory BP measured 3 times per hour during the day and hourly at night at days 0, 1, 4, 5, 10) Secondary: death at end of treatment; death or dependency (mRS > 2), death, BI and QoL at 3 months | |
| Notes | Exclusion: systolic BP > 230 or < 100 mmHg; diastolic BP > 130 or < 60 mmHg; HR > 130 or < 50 bpm; mild stroke; coma; premorbid dependence (mRS ≥ 3); requirement for or contraindication to nitrate therapy; presence of illness that could confound neurological or functional evaluation Any antihypertensive medication was stopped on admission and recommenced after 10 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation with minimisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | GTN was given single‐blinded (participant), whilst outcomes were assessed blinded to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label as no matching treatment and placebo patches were available |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differences between trial groups |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | None found |
Willmot 2006.
| Methods | Participant‐ and measurement‐blinded RCT Randomisation by computer (2:1, minimisation by age, sex, baseline systolic BP, baseline SSS, time from onset, presence of stroke lesion on CT) |
|
| Participants | UK, single centre 18 participants: treatment 12; control 6 Age: treatment 69 years; control 70 years Male: treatment 2 (16.7%); control 3 (50%) Inclusion: IS or ICH; previously independent; clinical stroke syndrome and limb weakness Time to randomisation: within 5 days of onset |
|
| Interventions | Treatment: transdermal GTN patch 5 mg once daily Control: no patch (Blinding with gauze dressing over patch/equivalent area of skin) Duration: 7 days |
|
| Outcomes | Primary: BP (immediately before the baseline Xenon‐CT and immediately after the post‐treatment scan), cerebral blood flow (Xenon‐CT and transcranial doppler) and cerebral perfusion pressure (transcranial doppler) Secondary:
|
|
| Notes | Exclusion: requirement for or contraindication to nitrate therapy; definite need for previous antihypertensive or vasoactive drugs; unable to cooperate with scanning Prior antihypertensive medication was stopped on admission |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation with minimisation |
| Allocation concealment (selection bias) | Low risk | Computer randomisation with minimisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | GTN was given single‐blinded (participant), whilst outcomes were assessed blinded to treatment group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | GTN was given single‐blinded as no placebo patches were available. A gauze dressing was placed over the GTN patch or equivalent area of skin out of sight. As a result, participants were blinded whilst the treating clinician was unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | None found |
BI: Barthel Index bpm: beats per minute BP: blood pressure CT: computed tomography DBP: diastolic blood pressure EQ‐5D: European Quality of life ‐ 5 Dimensions EQ‐VAS: European Quality of life ‐ Visual Analogue Scale GCS: Glasgow Coma Scale GTN: glyceryl trinitrate HR: heart rate ICH: intracerebral haemorrhage IS: ischaemic stroke MMSE: Mini Mental State Examination mRS: modified Rankin Scale QoL: quality of life SBP: systolic blood pressure SSS: Scandinavian Stroke Scale TICS‐M: Modified Telephone Interview Cognition Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Butterworth 1998 | Nonrandomised study assessing sodium nitroprusside in acute IS |
| El Mously 2014 | Case‐control study |
| El‐Zammer 2012 | Not randomised or controlled |
| FAST‐BP 2013 | Not randomised or controlled (ongoing) |
| Flinders 2014 | Nonrandomised study as part of Field Administration of Stroke Therapy‐Magnesium (FAST‐MAG) clinical trial |
| Kate 2015 | Nonrandomised, controlled study assessing treatment with sublingual nitroglycerin +/‐ intravenous labetalol depending on baseline systolic BP on CBF |
| Kerr 2000 | Unable to obtain published or unpublished data |
| MAPAS 2009 | Non‐controlled, target‐based study (ongoing) |
| Nakamura 2010 | Head‐to‐head comparison study comparing perindopril vs candesartan vs conventional therapy (including nitrates) |
| PATICH 2014 | Target‐based study (ongoing) |
| PATIS 2009 | Non‐randomised, target‐based study (ongoing) |
| Roitberg 2008 | Confounded randomised controlled trial of nicardipine versus sodium nitroprusside in ICH and subarachnoid haemorrhage |
| Sakamoto 2015 | Not randomised or controlled |
| Sanossian 2012 | Nonrandomised study as part of FAST‐MAG clinical trial |
| Suri 2009 | Retrospective study on the use of nicardipine or sodium nitroprusside in ICH |
| Xu 2007 | Assessing vertebrobasilar insufficiency, not acute stroke |
CBF: cerebral blood flow ICH: intracerebral haemorrhage IS: ischaemic stroke SBP: systolic blood pressure vs: versus
Characteristics of ongoing studies [ordered by study ID]
RIGHT‐2 2015.
| Trial name or title | Rapid Intervention with Glyceryl trinitrate in Hypertensive stroke Trial‐2 (RIGHT‐2) |
| Methods | Prospective multicentre single‐blind randomised controlled trial |
| Participants | 850 participants Inclusion: FAST positive test, BP ≥ 120 mmHg, presenting to ambulance service following a 999 emergency call Time to randomisation: within 4 hours of onset |
| Interventions | Treatment: transdermal GTN patch 5 mg once daily Control: sham patch (Blinding with gauze dressing over patch) Duration: 4 days |
| Outcomes | Primary: mRS at 90 days Secondary:
|
| Starting date | October 2015 |
| Contact information | Philip Bath, Stroke Association Professor of Stroke Medicine, Stroke, Division of Clinical Neuroscience, B52, Clinical Sciences Building, University of Nottingham, City Hospital Campus, Hucknall Road, Nottingham, NG5 1PB (philip.bath@nottingham.ac.uk) |
| Notes |
BI: Barthel index BP: Blood pressure CT: Computed tomography DBP: Diastolic blood pressure EQ‐5D: European quality of life‐5 dimensions EQ‐VAS: European quality of life‐visual analogue scale GTN: Glyceryl trinitrate HR: Heart rate ICH: Intracerebral haemorrhage IS: Ischaemic stroke MMSE: Mini‐mental state examination MRI: Magnetic resonance imaging mRS: Modified Rankin scale NIHSS: National Institutes of Health Stroke Scale QoL: Quality of life SBP: Systolic blood pressure SSS: Scandanavian stroke scale TICS‐M: modified Telephone Interview for Cognitive Status
Differences between protocol and review
The methods of the present review have been substantially updated since the initial publication of the protocol in 1996 to include multiple secondary clinical outcomes and utilisation of individual patient data where available.
Contributions of authors
PM Bath: conceived, designed and coordinated the review; developed the search strategy for the previous reviews; interpreted data; wrote the review; and is guarantor of the review.
JP Appleton: updated the search strategy; performed data collection, collation and analysis; interpreted data; co‐wrote the review.
K Krishnan: checked and interpreted data; co‐wrote the review.
Previous versions of the review involved:
Richard Butterworth: undertook searches; entered data;
Mark Willmot: provided advice on the review; co‐wrote the review;
Jo Leonardi‐Bee: analysed individual patient data from two trials;
Fiona Bath‐Hextall (supported by South Thames & Trent NHS R&D Executive): coordinated the review; undertook searches; was responsible for data management; entered data.
Sources of support
Internal sources
No sources of support supplied
External sources
NHS South Thames Region Research and Development Programme, UK.
Stroke Association, UK.
Wolfson Foundation, UK.
Declarations of interest
PM Bath was chief investigator for the five included trials (Ankolekar 2013; Bath 2001; ENOS 2015; Rashid 2002; Willmot 2006) and is chief investigator of the ongoing RIGHT‐2 2015 trial; he was Wolfson Foundation Senior Lecturer in Stroke Medicine, and is Stroke Association Professor of Stroke Medicine, and a NIHR Senior Investigator.
JA was funded by the British Heart Foundation (RIGHT‐2 2015 trial).
KK was funded by the Medical Research Council (ENOS 2015 trial).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ankolekar 2013 {published and unpublished data}
- Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, et al. Feasibility of an ambulance‐based stroke trial, and safety of glyceryl trinitrate in ultra‐acute stroke: the Rapid Intervention with Glyceryl Trinitrate in Hypertensive Stroke Trial (RIGHT). Stroke 2013;44:3120‐8. [ISRCTN66434824] [DOI] [PubMed] [Google Scholar]
- Ankolekar S, Parry R, Sprigg N, Siriwardena AN, Bath PMW. Views of paramedics on their role in an out‐of‐hospital ambulance‐based trial in ultra‐acute stroke: qualitative data from the Rapid Intervention with Glyceryl trinitrate in Hypertensive stroke Trial (RIGHT). Annals of Emergency Medicine 2014;64(6):640‐8. [DOI] [PubMed] [Google Scholar]
- Ankolekar S, Sare G, Geeganage C, Fuller M, Stokes L, Sprigg N, et al. Determining the feasibility of ambulance‐based randomised controlled trials in patients with ultra‐acute stroke: study protocol for the "Rapid Intervention with GTN in Hypertensive Stroke Trial" (RIGHT). Stroke Research and Treatment 2012;2012:385753. [ISRCTN66434824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 2001 {published and unpublished data}
- Bath PMW, Pathansali R, Iddenden R, Bath FJ. The effect of nitric oxide, given as transdermal glyceryl trinitrate, on blood pressure in acute stroke. Cerebrovascular Diseases 1999;9 Suppl 1:101. [DOI] [PubMed] [Google Scholar]
- Bath PMW, Pathansali R, Iddenden R, Bath FJ. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and platelet function in acute stroke. Cerebrovascular Diseases 2001;11:265‐72. [DOI] [PubMed] [Google Scholar]
ENOS 2015 {published and unpublished data}
- Bath PMW, Houlton A, Woodhouse L, Sprigg N, Wardlaw J, Pocock S, the ENOS Trialists. Statistical analysis plan for the 'Efficacy of Nitric Oxide in Stroke' (ENOS) trial. International Journal of Stroke 2014;9:372‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOS Investigators. Baseline characteristics of the 4011 patients recruited into the 'Efficacy of Nitric Oxide in Stroke' (ENOS) trial. International Journal of Stroke 2014;9:711‐20. [DOI] [PubMed] [Google Scholar]
- The ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial‐factorial randomised controlled trial. Lancet 2015;385:617‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ENOS Trial Investigators. Glyceryl trinitrate vs control, and continuing vs stopping temporarily prior antihypertensive therapy, in acute stroke: rationale and design of the efficacy of nitric oxide in stroke (ENOS) trial. International Journal of Stroke 2006;1:245‐9. [ISRCTN99414122] [DOI] [PubMed] [Google Scholar]
Rashid 2002 {published and unpublished data}
- Rashid PA, Leonardi‐Bee JA, Weaver CS, Bath FJ, Bath PM. The effect of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure and middle cerebral artery blood velocity in acute stroke. Stroke 2002;33:383. [Google Scholar]
- Rashid PA, Weaver C, Leonardi‐Bee JA, Bath FJ, Fletcher S, Bath PMW. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac hemodynamics, and plasma nitric oxide levels in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2003;12(3):143‐51. [DOI] [PubMed] [Google Scholar]
Willmot 2006 {published and unpublished data}
- Willmot M, Ghadami A, Whysall B, Clarke W, Wardlaw J, Bath PMW. Transdermal glyceryl trinitrate lowers blood pressure and maintains cerebral blood flow in recent stroke. Hypertension 2006;47:1209‐15. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Butterworth 1998 {published data only}
- Butterworth RJ, Cluckie A, Jackson SHD, Buxton‐Thomas M, Bath PMW. Sodium nitroprusside, a spontaneous nitric oxide donor in acute ischaemic stroke. Cerebrovascular Diseases 1998;8:158‐65. [DOI] [PubMed] [Google Scholar]
El Mously 2014 {published data only}
- Mously SM, Bialy RB, Khayat ZK, Masoud HM, Mously LM. Assessment of S100B biomarker in hypertensive patients with acute ischemic stroke treated with glyceryl trinitrate. Cerebrovascular Diseases 2014;37 Suppl 1:418. [Google Scholar]
El‐Zammer 2012 {published data only}
- El‐Zammer ZM, Latorre JG, Wang D, Satyan S, Elnour E, Kamel A, et al. Intra‐arterial vasodilator use during endovascular therapy for acute ischaemic stroke might improve reperfusion rate. Annals of the New York Academy of Sciences 2012;1268:134‐40. [DOI] [PubMed] [Google Scholar]
FAST‐BP 2013 {published data only}
- NCT01811693. Field Administration of Stroke Therapy ‐ Blood Pressure lowering (FAST‐BP). clinicaltrials.gov/ct2/show/NCT01811693?term=NCT01811693&rank=1 (accessed prior to 6 April 2017). [NCT01811693]
Flinders 2014 {published data only}
- Flinders A, Sanossian N, Kim MA, Liebeskind D, Eckstein M, Stratton S, et al. Antihypertensive utilization in hyperacute intracerebral haemorrhage presenting to the emergency room. Stroke 2014;45 Suppl 1:A199. [Google Scholar]
Kate 2015 {published data only}
- Kate M, Asdaghi N, Gioia L, Buck B, Jeerakathil T, Shuaib A, et al. Blood pressure reduction in acute ischaemic stroke does not affect ischemic core tissue perfusion.. International Journal of Stroke 2015;10 Suppl S2:226. [Google Scholar]
Kerr 2000 {published data only}
- Kerr S. The effect of nitric oxide donation on regional cerebral blood flow and metabolism in acute ischaemic stroke. National Research Register: sites.google.com/a/york.ac.uk/yhectrialsregisters/home/researchregisters/uk‐national‐research‐register‐1 (accessed prior to 6 April 2017).
MAPAS 2009 {published data only}
- NCT00848770. Manipulation of Arterial Pressure in Acute ischemic Stroke (MAPAS). clinicaltrials.gov/ct2/show/NCT00848770?term=NCT00848770&rank=1 (accessed prior to 6 April 2017). [NCT00848770]
Nakamura 2010 {published data only}
- Nakamura T, Tsutsumi Y, Shimizu Y, Uchiyama S. Renin‐angiotension system blockade safely reduces blood pressure in patients with minor ischemic stroke during the acute phase. Journal of Stroke and Cerebrovascular Diseases 2010;19(6):435‐40. [DOI] [PubMed] [Google Scholar]
PATICH 2014 {published data only}
- Zheng J, Lin S, Li H, Ma J, Fang Y, Ma L, et al. Perioperative Antihypertensive Treatment in patients of spontaneous IntraCerebral Haemorrhage (PATICH): a clinical trial protocol. Contemporary Clinical Trials 2014;39(1):9‐13. [DOI] [PubMed] [Google Scholar]
PATIS 2009 {published data only}
- NCT02327793. Perfusion and Antihypertensive Therapy in Acute Ischemic Stroke (PATIS). clinicaltrials.gov/ct2/show/NCT02327793?term=NCT02327793&rank=1 (accessed prior to 6 Apri 2017). [NCT02327793]
Roitberg 2008 {published data only}
- Roitberg BZ, Hardman J, Urbaniak K, Merchant A, Mangubat EZ, Alaraj A, et al. Prospective randomised comparison of safety and efficacy of nicardipine and nitroprusside drip for control of hypertension in the neurosurgical intensive care unit. Neurosurgery 2008;63(1):115‐20. [DOI] [PubMed] [Google Scholar]
Sakamoto 2015 {published data only}
- Sakamoto Y, Koga M, Todo K, Okuda S, Okada Y, Kimura K, et al. Relative systolic blood pressure reduction and clinical outcomes in hyperacute intracerebral haemorrhage: the SAMURAI‐ICH observational study. Journal of Hypertension 2015;33(5):1069‐73. [DOI] [PubMed] [Google Scholar]
Sanossian 2012 {published data only}
- Sanossian N, Flinders A, Olivas E, Starkman S, Liebeskind D, Eckstein M, et al. Door to blood pressure goal achievement in community management of hyperacute intracerebral haemorrhage. Annals of Emergency Medicine 2012;60:S56. [Google Scholar]
Suri 2009 {published data only}
- Suri MF, Vazquez G, Ezzeddine MA, Qureshi AI. A multicenter comparison of outcomes associated with intravenous nitroprusside and nicardipine treatment among patients with intracerebral haemorrhage. Neurocritical Care 2009;11(1):50‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2007 {published data only}
- Xu D, Liu J, Wang A, Zhao C. Nitrogen monoxide vector of ultrasonic atomizing inhalation improves vertebro‐basilar artery insufficiency: hemodynamic changes are detected by transcranial Doppler test. Neural Regeneration Research 2007;2(8):506‐9. [Google Scholar]
References to ongoing studies
RIGHT‐2 2015 {published data only}
- ISRCTN26986053. Rapid Intervention with Glyceryl trinitrate in Hypertensive stroke Trial‐2. www.isrctn.com/ISRCTN26986053 (accessed prior to 6 April 2017). [ISRCTN26986053]
Additional references
Anderson 2013
- Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, INTERACT2 Investigators. Rapid blood‐pressure lowering in patients with acute intracerebral hemorrhage. New England Journal of Medicine 2013;368(25):2355‐65. [DOI] [PubMed] [Google Scholar]
Atochin 2007
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft‐Wilson R, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. Journal of Clinical Investigation 2007;117:1961‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atochin 2010
- Atochin DN, Yuzawa I, Li Q, Rauwerdink KM, Malhotra R, Chang J, et al. Soluble guanylate cyclase alpha1beta1 limits stroke size and attenuates neurological injury. Stroke 2010;41:1815‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
BASC 2016
- Bath PM, Woodhouse L, Krishnan K, Anderson C, Berge E, Ford GA, Blood pressure in Acute Stroke Collaboration (BASC). Effect of treatment delay, stroke type, and thrombolysis on the effect of glyceryl trinitrate, a nitric oxide donor, on outcome after acute stroke: a systematic review and meta‐analysis of individual patient from randomised trials. Stroke Research and Treatment 2016;2016:1‐12. [DOI: 10.1155/2016/9706720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bath 1991
- Bath PMW, Hassall DG, Gladwin AM, Plamer RMJ, Martin JF. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterisclerosis & Thrombosis 1991;11:254‐60. [DOI] [PubMed] [Google Scholar]
Britton 1986
- Britton M, Carlsson A, Faire U. Blood pressure course in patients with acute stroke and matched controls. Stroke 1986;17(5):861‐4. [DOI] [PubMed] [Google Scholar]
Cho 1992
- Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, et al. Calmodulin is a subunit of nitric oxide synthase from macrophages. Journal of Experimental Medicine 1992;176:599‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corsani 2008
- Corsani L, Bizzoco E, Pedata F, Gianfriddo M, Faussone‐Pellegrini MS, Vannucchi MG. Inducible nitric oxide synthase appears and is co‐expressed with the neuronal isoform in interneurons of the rat hippocampus after transient ischemia induced by middle cerebral artery occlusion. Experimental Neurology 2008;211:433‐40. [DOI] [PubMed] [Google Scholar]
Cui 2009
- Cui X, Chopp M, Zacharek A, Zhang C, Roberts C, Chen J. Role of endothelial nitric oxide synthase in arteriogenesis after stroke in mice. Neuroscience 2009;159:744‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 1994
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. Journal of Neuroscience 1994;14:5147‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Emberson 2014
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet 2014;384(9958):1929‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endres 2004
- Endres M, Laufs U, Liao JK, Moskowitz MA. Targeting eNOS for stroke protection. Trends in Neurosciences 2004;27:283‐9. [DOI] [PubMed] [Google Scholar]
Fagan 1998
- Fagan SC, Bowes MP, Lyden PD, Zivin JA. Acute hypertension promotes hemorrhagic transformation in a rabbit embolic stroke model: effect of labetalol. Experimental Neurology 1998;150(1):153‐8. [DOI] [PubMed] [Google Scholar]
Ferlito 1998
- Ferlito S, Gallina M, Pitari GM, Bianchi A. Nitric oxide plasma levels in patients with chronic and acute cerebrovascular disorders. Panminerva Medicine 1998;40:51‐4. [PubMed] [Google Scholar]
Fleming 1991
- Fleming I, Gray GA, Schott C, Stoclet JC. Inducible but not constitutive production of nitric oxide by vascular smooth muscle cells. European Journal of Pharmacology 1991;200:375‐6. [DOI] [PubMed] [Google Scholar]
Garthwaite 1988
- Garthwaite J, Charles SL, Chess‐Williams R. Endothelium derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988;336:385‐8. [DOI] [PubMed] [Google Scholar]
Goyal 2016
- Goyal M, Menon BK, Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, the HERMES Collaborators. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet 2016;387(10029):1723‐31. [DOI] [PubMed] [Google Scholar]
Gray 2009
- Gray LJ, Ali M, Lyden PD, Bath PM, Virtual International Stroke Trials Archive Collaboration. Interconversion of the National Institutes of Health Stroke Scale and Scandinavian Stroke Scale in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2009;18:466‐8. [DOI] [PubMed] [Google Scholar]
Hara 1996
- Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. Journal of Cerebral Blood Flow & Metabolism 1996;16:605‐11. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Huang 1994
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 1994;265:1883‐5. [DOI] [PubMed] [Google Scholar]
Huang 1996
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro‐L‐arginine. Journal of Cerebral Blood Flow & Metabolism 1996;16:981‐7. [DOI] [PubMed] [Google Scholar]
Iadecola 1995
- Iadecola C, Zhang F, Xu X. Inhibition of inducible nitric oxide synthase ameliorates cerebral ischemic damage. American Journal of Physiology 1995;268:R286‐92. [DOI] [PubMed] [Google Scholar]
Iadecola 1997
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. Journal of Neuroscience 1997;17:9157‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kader 1993
- Kader A, Frazzini VI, Solomon RA, Trifiletti RR. Nitric oxide production during focal cerebral ischemia in rats. Stroke 1993;24:1709‐16. [DOI] [PubMed] [Google Scholar]
Knowles 1989
- Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L‐arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proceedings of the National Academy of Sciences of the United States of America 1989;86:5159‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavi 2003
- Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation 2003;107:1901‐5. [DOI] [PubMed] [Google Scholar]
Leonardi‐Bee 2002
- Leonardi‐Bee J, Bath PM, Phillips SJ, Sandercock PA, IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33(5):1315‐20. [DOI] [PubMed] [Google Scholar]
Lo 1996
- Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, et al. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke 1996;27:1381‐5. [DOI] [PubMed] [Google Scholar]
Mackay 2004
- Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. http://www.who.int/cardiovascular_diseases/resources/atlas/en/ (accessed prior to 6 April 2017).
Malinski 1993
- Malinski T, Bailey F, Zhang ZG, Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. Journal of Cerebral Blood Flow & Metabolism 1993;13:355‐8. [DOI] [PubMed] [Google Scholar]
Manzoni 1992
- Manzoni O, Prezeau L, Marin P, Deshager S, Bockaert J, Fagni L. Nitric oxide induced blockade of NMDA receptors. Neuron 1992;8:653‐62. [DOI] [PubMed] [Google Scholar]
Morikawa 1992a
- Morikawa E, Huang Z, Moskowitz MA. L‐arginine decreases infarct size caused by middle cerebral arterial occlusion in SHR. American Journal of Physiology 1992;263:H1632‐5. [DOI] [PubMed] [Google Scholar]
Morikawa 1992b
- Morikawa E, Rosenblatt S, Moskowitz MA. L‐arginine dilates rat pial arterioles by nitric oxide‐dependent mechanisms and increases blood flow during focal cerebral ischaemia. British Journal of Pharmacology 1992;107(4):905‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morikawa 1994
- Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. L‐arginine infusion promotes nitric oxide‐dependent vasodilation, increases regional cerebral blood flow, and reduces infarction volume in the rat. Stroke 1994;25:429‐35. [DOI] [PubMed] [Google Scholar]
Murad 1987
- Murad F, Waldman S, Molina C, Bennett B, Leitman D. Regulation and role of guanylate cyclase‐cyclic GMP in vascular relaxation. Progress in Clinical and Biological Research 1987;249:65‐76. [PubMed] [Google Scholar]
NINDS 1995
- National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. New England Journal of Medicine 1995;333(24):1581‐7. [DOI] [PubMed] [Google Scholar]
Niwa 2001
- Niwa M, Inao S, Takayasu M, Kawai T, Kahita Y, Nihashi T, et al. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurologia Medico‐Chirurgica 2001;41:63‐72. [DOI] [PubMed] [Google Scholar]
OAST 2007
- Optimising Analysis of Stroke Trials (OAST) Collaboration. Can we improve the statistical analysis of stroke trials? Statistical re‐analysis of functional outcomes in stroke trials. Stroke 2007;38:1911‐5. [DOI] [PubMed] [Google Scholar]
Oppenheimer 1992
- Oppenheimer S, Hachinski V. Complications of acute stroke. Lancet 1992;339(8795):721‐4. [DOI] [PubMed] [Google Scholar]
Owens 2011
- Owens WB. Blood pressure control in acute cerebrovascular disease. Journal of Clinical Hypertension 2011;13(3):205‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 1987
- Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium derived relaxing factor. Nature 1987;327:524‐6. [DOI] [PubMed] [Google Scholar]
Palmer 1988
- Palmer RMJ, Ashton DS, Moncada S. Vascular endothelial cells synthesise nitric oxide from L‐arginine. Nature 1988;333:664‐6. [DOI] [PubMed] [Google Scholar]
Qureshi 2008
- Qureshi AI. Acute hypertensive response in patients with stroke ‐ pathophysiology and management. Circulation 2008;118(2):176‐87. [DOI] [PubMed] [Google Scholar]
Radomski 1987a
- Radomski MW, Palmer RMJ, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;ii:1057‐8. [DOI] [PubMed] [Google Scholar]
Radomski 1987b
- Radomski MW, Palmer RMJ, Moncada S. The anti‐aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. British Journal of Pharmacology 1987;92:639‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rashid 2003
- Rashid PA, Whitehurst A, Lawson N, Bath PMW. Plasma nitric oxide (nitrate/nitrite) levels in acute stroke and their relationship with severity and outcome. Journal of Stroke and Cerebrovascular Diseases 2003;12:82‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, the Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Sandercock 2014
- Sandercock PAG, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD000029.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sehara 2006
- Sehara Y, Hayashi T, Deguchi K, Nagotani S, Zhang H, Shoji M, et al. Distribution of inducible nitric oxide synthase and cell proliferation in rat brain after transient middle cerebral artery occlusion. Brain Research 2006;1093:190‐7. [DOI] [PubMed] [Google Scholar]
Sprigg 2006
- Sprigg N, Gray LJ, Bath PM, Boysen G, Deyn PP, Friis P, TAIST Investigators. Relationship between outcome and baseline blood pressure and other haemodynamics in ischaemic stroke: data from the TAIST trial. Journal of Hypertension 2006;24(7):1413‐7. [DOI] [PubMed] [Google Scholar]
Stroke Unit Trialists' Collaboration 2013
- Stroke Unit Triallists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000197.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2005
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Neuronal nitric oxide synthase and ischemia‐induced neurogenesis. Journal of Cerebral Blood Flow & Metabolism 2005;25:485‐92. [DOI] [PubMed] [Google Scholar]
Tanaka 1996
- Tanaka K. Is nitric oxide really important for regulation of the cerebral circulation? Yes or no?. Keio Journal of Medicine 1996;45:14‐27. [DOI] [PubMed] [Google Scholar]
Tikhonoff 2009
- Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurology 2009;8(10):938‐48. [DOI] [PubMed] [Google Scholar]
Vahedi 2007
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, DECIMAL, DESTINY, and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurology 2007;6(3):215‐22. [DOI] [PubMed] [Google Scholar]
White 2000
- White RP, Vallance P, Markus HS. Effect of inhibition of nitric oxide synthase on dynamic cerebral autoregulation in humans. Clinical Science 2000;99:555‐60. [PubMed] [Google Scholar]
Willmot 2004
- Willmot M, Leonardi‐Bee J, Bath PMW. High blood pressure in acute stroke and subsequent outcome ‐ a systematic review. Hypertension 2004;43(1):18‐24. [DOI] [PubMed] [Google Scholar]
Willmot 2005
- Willmot M, Gray L, Gibson C, Murphy S, Bath PMW. A systematic review of nitric oxide donors and L‐arginine in experimental stroke; effects on infarct size and cerebral blood flow. Nitric Oxide 2005;12(3):141‐9. [DOI] [PubMed] [Google Scholar]
Zaharchuk 1997
- Zaharchuk G, Hara H, Huang PL, Fishman MC, Moskowitz MA, Jenkins BG, et al. Neuronal nitric oxide synthase mutant mice show smaller infarcts and attenuated diffusion coefficient changes in the peri‐infarct zone during focal cerebral ischemia. Magnetic Resonance in Medicine 1997;37:170‐5. [DOI] [PubMed] [Google Scholar]
Zhang 1996
- Zhang F, Casey RM, Ross ME, Iadecola C. Aminoguanidine ameliorates and L‐arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke 1996;27:317‐23. [DOI] [PubMed] [Google Scholar]
Zhao 2003
- Zhao X, Ross ME, Iadecola C. L‐arginine increases ischemic injury in wild‐type mice but not in iNOS‐deficient mice. Brain Research 2003;966:308‐11. [DOI] [PubMed] [Google Scholar]
Zung 1965
- Zung WW. A self‐rating depression scale. Archives of General Psychiatry 1965;12:63‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bath 2002
- Bath PMW, Willmot MM, Leonardi‐Bee J, Bath‐Hextall FJ. Nitric oxide donors (nitrates), L‐arginine, or nitric oxide synthase inhibitors for acute stroke. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000398] [DOI] [PubMed] [Google Scholar]


