Abstract
Background
Worldwide at least 100 million people are thought to have prevalent cardiovascular disease (CVD). This population has a five times greater chance of suffering a recurrent cardiovascular event than people without known CVD. Secondary CVD prevention is defined as action aimed to reduce the probability of recurrence of such events. Drug interventions have been shown to be cost‐effective in reducing this risk and are recommended in international guidelines. However, adherence to recommended treatments remains sub‐optimal. In order to influence non‐adherence, there is a need to develop scalable and cost‐effective behaviour‐change interventions.
Objectives
To assess the effects of mobile phone text messaging in patients with established arterial occlusive events on adherence to treatment, fatal and non‐fatal cardiovascular events, and adverse effects.
Search methods
We searched CENTRAL, MEDLINE, Embase, the Conference Proceedings Citation Index ‐ Science on Web of Science on 7 November 2016, and two clinical trial registers on 12 November 2016. We contacted authors of included studies for missing information and searched reference lists of relevant papers. We applied no language or date restrictions.
Selection criteria
We included randomised trials with at least 50% of the participants with established arterial occlusive events. We included trials investigating interventions using short message service (SMS) or multimedia messaging service (MMS) with the aim to improve adherence to medication for the secondary prevention of cardiovascular events. Eligible comparators were no intervention or other modes of communication.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. In addition, we attempted to contact all authors on how the SMS were developed.
Main results
We included seven trials (reported in 13 reports) with 1310 participants randomised. Follow‐up ranged from one month to 12 months. Due to heterogeneity in the methods, population and outcome measures, we were unable to conduct meta‐analysis on these studies. All seven studies reported on adherence, but using different methods and scales. Six out of seven trials showed a beneficial effect of mobile phone text messaging for medication adherence. Dale 2015a, reported significantly greater medication adherence score in the intervention group (Mean Difference (MD) 0.58, 95% confidence interval (CI) 0.19 to 0.97; 123 participants randomised) at six months. Khonsari 2015 reported less adherence in the control group (Relative Risk (RR) 4.09, 95% CI 1.82 to 9.18; 62 participants randomised) at eight weeks. Pandey 2014 (34 participants randomised) assessed medication adherence through self‐reported logs with 90% adherence in the intervention group compared to 70% in the control group at 12 months. Park 2014a (90 participants randomised) reported a greater increase of the medication adherence score in the control group, but also measured adherence with an event monitoring system for a number of medications with adherence levels ranging from 84.1% adherence to 86.2% in the intervention group and 79.7% to 85.7% in the control group at 30 days. Quilici 2013, reported reduced odds of non‐adherence in the intervention group (Odds Ratio (OR) 0.43, 95% CI 0.22 to 0.86, 521 participants randomised) at 30 days. Fang 2016, reported that participants given SMS alone had reduced odds of being non‐adherent compared to telephone reminders (OR 0.40 95% CI 0.18 to 0.63; 280 patients randomised). Kamal 2015 reported higher levels of adherence in the intervention arm (adjusted MD 0.54, 95% CI 0.22 to 0.85; 200 participants randomised).
Khonsari 2015 was the only study to report fatal cardiovascular events and only reported two events, both in the control arm. No study reported on the other primary outcomes. No study reported repetitive thumb injury or road traffic crashes or other adverse events that were related to the intervention.
Four authors replied to our questionnaire on SMS development. No study reported examining causes of non‐adherence or provided SMS tailored to individual patient characteristics.
The included studies were small, heterogeneous and included participants recruited directly after acute events. All studies were assessed as having high risk of bias across at least one domain. Most of the studies came from high‐income countries, with two studies conducted in an upper middle‐income country (China, Malaysia), and one study from a lower middle‐income country (Pakistan). The quality of the evidence was found to be very low. There was no obvious conflicts of interest from authors, although only two declared their funding.
Authors' conclusions
While the results of this systematic review are promising, there is insufficient evidence to draw conclusions on the effectiveness of text message‐based interventions for adherence to medications for secondary prevention of CVD. Sufficiently powered, high‐quality randomised trials are needed, particularly in low‐ and middle‐income countries.
Plain language summary
Text messaging to help people suffering from heart disease adhere to medications
Review question
We reviewed the evidence about the effect of text messaging on medication adherence in people with heart disease. We found seven studies including 1310 participants.
Background
Worldwide, at least 100 million people suffer from heart disease. While there are numerous cost‐effective treatments, the majority of these individuals are not taking the medications that they need to keep themselves from suffering more heart problems. One possible method of helping people with heart disease to take their medications is through the use of text message‐based reminders.
Study characteristics
The evidence is current to November 2016. We found seven studies that compared using text messages to not using text messages, with follow‐up ranging from one month to 12 months.
Key results
While the results of these studies appear promising that text messages can help people take their medicines, the studies were small and utilised very different methods and definitions. For that reason, we were not able to compile the findings of the studies. Most of the studies came from high‐income countries, and were primarily conducted on men. No studies reported any bad side effects from using text messages. There was no obvious conflicts of interest from authors, although only two declared their funding.
Quality of the evidence
The quality of evidence from these studies was very low. Additional high‐quality studies on the use of text messages for encouraging people suffering from heart disease to take their medication regularly are needed, particularly in low‐ and middle‐income countries.
Summary of findings
Summary of findings for the main comparison. Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease.
| Mobile phone text messaging to improve medication adherence in secondary prevention of cardiovascular disease | |||
| Patient or population: patients with established arterial occlusive events Setting: hospital/cardiac rehabilitation facility Intervention: mobile phone text messaging Comparison: no intervention or other modes of communication | |||
| Outcomes | Impact | № of participants (studies) | Quality of the evidence (GRADE) |
| Adherence to treatment | Six out of seven trials showed a beneficial effect of mobile phone text messaging for medication adherence. One trial showed an improved adherence score for the control group compared to the intervention group (smallest and shortest trial). | 1310 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||
1 Downgraded one level of evidence due to unclear or high risk of bias for all studies in at least one domain.
2 Downgraded one level of evidence due to inconsistent reporting of outcome.
3 Downgraded one level of evidence due to imprecision of the results in one study and different direction of effect in another study.
Background
Description of the condition
Worldwide, there are an estimated 13 million deaths due to coronary heart disease or stroke each year, and 80% of these deaths occur in low‐ and middle‐income countries (Lozano 2012). It is estimated that approximately three times as many people will suffer non‐fatal cardiovascular events and that each year 35 million people have an acute coronary or cerebrovascular event. Worldwide, at least 100 million people are thought to have prevalent cardiovascular disease (CVD) (Chambless 1997; WHO 2002; Yusuf 2011). This population has a five times greater chance of suffering a new cardiovascular event than people without known CVD (Kerr 2009).
Secondary CVD prevention is defined as action aimed to reduce the probability of recurrence of a cardiovascular event in patients with known atherosclerotic CVD. There are two main aspects to secondary CVD prevention: risk factor management and medications. Drug interventions (such as antiplatelet therapy, angiotensin‐converting enzyme (ACE) inhibitors/ angiotensin receptor blockers (ARBs), beta‐blockers and statins) have been shown to be cost‐effective in reducing the risk of subsequent fatal and non‐fatal cardiovascular events in patients with established atherosclerotic cardiovascular diseases and are recommended in international guidelines (ESC 2012; Smith 2011; WHO 2003a).
Unfortunately there is a well‐documented knowledge–practice gap in the implementation of these proven cost‐effective interventions. For example, the Prospective Urban Rural Epidemiology (PURE) study reported that in low‐ and middle‐income countries up to 75% of patients with known CVD are not using even one recommended medication (Yusuf 2011). Even in high‐income countries, adherence to recommended treatments remains sub‐optimal. A cross‐sectional survey of 12 European countries showed only 26% of patients on antihypertensives achieving control of hypertension and less than 31% of patients on lipid‐lowering medication achieving cholesterol control (Kotseva 2010). It has been shown that a considerable proportion of cardiovascular events could be attributed to poor adherence, with 9% of cardiovascular events in Europe attributed to poor adherence(Chowdhury 2013). It is estimated that good adherence may be associated with a 20% lower risk of CVD and a 35% reduction in all‐cause mortality (Chowdhury 2013). This evidence–practice gap might be influenced by different factors, including health system issues such as lack of accessibility and affordability; treatment complexity; or patients' non‐compliance with recommendations (Nieuwlaat 2013). In order to influence non‐compliance there is a need to develop scalable and cost‐effective behaviour‐change interventions.
Description of the intervention
The global number of mobile phone subscribers is estimated at nearly seven billion (ICT 2014). Even in low‐ and middle‐income countries the penetration rate of mobile phones is estimated to be 90% (ICT 2014). The widespread ownership of mobile phones and the possibility of automation leads to a potential to deliver behaviour‐change interventions to large numbers of people at low cost. Mobile phone interventions are a potentially promising means to deliver messages to increase medication adherence. The use of mobile devices such as phones to support the delivery of medical care is commonly referred to as mHealth.
How the intervention might work
Mobile phone text messages have been shown to improve medication adherence for a variety of conditions including HIV (Sharma 2012). The development of messages should follow some theoretical framework, and text messages should be developed specifically for the target population and intervention (Abroms 2015). Text messages as an intervention are relatively cost‐effective and quick, and do not require that the intended audience need to search for information as it is delivered to them (Douglas 2013). Two recent systematic reviews addressed the question of using mobile phones for all types of medication adherence (Anglada‐Martinez 2015; Park 2014b). The majority of studies found significant improvement in medication adherence through the use of text messages. Overall, few adverse events have been reported with mobile phone text messaging; however, potential rare adverse effects such as road traffic crashes may occur.
Why it is important to do this review
While there is a great deal of enthusiasm for mHealth interventions among researchers and policy makers, there is still limited evidence for its effectiveness (Free 2013). Systematic reviews have recently been conducted on adherence to medications and reported promising results (Anglada‐Martinez 2015; Park 2014b; Thakkar 2016); however, to date no systematic review has been conducted evaluating specifically the effect of mobile phone text messaging on secondary CVD prevention. Furthermore, no review has examined how text messages are created, and if short message service (SMS) are tailored based on individual patient characteristics, and if some patients benefit more than others from interventions. Mobile phone text messaging is of particular interest in low‐ and middle‐income countries because of wider accessibility of mobile phones with text‐messaging capabilities than smart phones.
Objectives
To determine whether mobile phone text messaging is effective in enhancing adherence to recommended medication in patients with established arterial occlusive events.
Methods
Criteria for considering studies for this review
Types of studies
We only included randomised controlled trials (RCTs). We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included participants with established arterial occlusive events, including coronary artery disease, cerebrovascular artery disease, peripheral artery disease, and atherosclerotic aortic disease, for whom antiplatelet, blood pressure lowering medications and lipid‐lowering medications are recommended. We included all studies regardless of where the patients were enrolled (community or clinic). We only included studies where at least 50% of participants had established cardiovascular disease (CVD).
Types of interventions
We included trials comparing interventions using short message service (SMS) or multimedia messaging service (MMS) to improve adherence to secondary cardiovascular prevention interventions. We compared mobile phone messaging with no intervention, and also with other modes of communication (for example, face‐to‐face, postal letters, or phone calls). We did not exclude studies based on how the text messages were developed, or if they were one way versus two ways. We only included trials that included adherence, but we also included trials that included both adherence and lifestyle modifications.
Types of outcome measures
Primary outcomes
Adherence to treatment (any definition used in trials)
Fatal cardiovascular events
Non‐fatal cardiovascular events (coronary heart disease (CHD), revascularisation, stroke)
Combined CVD event (fatal or non‐fatal events)
Secondary outcomes
Surrogate outcomes according to the different interventions recommended for secondary prevention including low‐density lipoprotein (LDL)‐cholesterol for the effect of statins, blood pressure for antihypertensive drugs, heart rate for the effect of beta blockers, urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin.
Adverse effects including self‐reported road traffic crashes and repetitive thumb strain
Search methods for identification of studies
Electronic searches
We identified relevant studies through systematic searches of the following bibliographic databases on 7 November 2016.
Cochrane Central Register of Controlled Trials (CENTRAL, Issue 10 of 12, 2016) in the Cochrane Library
MEDLINE in‐Process & Other Non‐Indexed Citations and MEDLINE (OVID, 1946 to 7 November 2016)
EmbaseE Classic and Embase (OVID, 1947 to 4 November 2016)
Conference Proceedings Citation Index – Science (CPCI‐S) (1990 to 4 November 2016) on Web of Science (Thomson Reuters)
The Cochrane sensitivity‐maximising RCT filter was applied to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL (Lefebvre 2011). The search strategies are shown in Appendix 1. We searched all databases from their inception to the present, and imposed no restriction on language of publication.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization's International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) on 12 November 2016. The search terms used are shown in Appendix 1.
We additionally searched for the clinical trial registry numbers of identified ongoing studies on 14 January 2017 to see if their status had changed and results had been published.
Searching other resources
We checked reference lists of all included primary studies and reviewed relevant systematic reviews and meta‐analyses (Anglada‐Martinez 2015; Chow 2016; de Jongh 2012; Ershad Sarabi 2016; Hamine 2015; Misono 2010; Sahu 2014b; Thakkar 2016; Vodopivec‐Jamsek 2012) for additional references.
Data collection and analysis
Selection of studies
Two of four review authors (AJA, LF, NM, NS) independently screened titles and abstracts for inclusion of all identified potential studies and decided to retrieve the full‐text copies or to discard them. If there were any disagreements, a third author arbitrated (PP or JPC). We retrieved full‐text study reports/publications and two of three review authors (AJA, NM, NS) independently screened the full text and identified studies for inclusion. We resolved any disagreement through discussion. If necessary, a third person (PP or JPC) arbitrated. We identified and excluded duplicates and collated multiple reports of the same study so that each study, instead of the report, is the unit of interest in the review. We completed a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form to extract study characteristics and outcome data previously piloted on at least one study in the review. Two of three review authors (NM, OO, AJA) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, gender, condition, diagnostic criteria, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications, how text messages were developed, behaviour‐change technique, time from arterial occlusive event, if SMS was personalised.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
We resolved disagreements by consensus or by involving a third person (PP or JPC). One review author (AJA) transferred data into the Review Manager 5 (RevMan 2014) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
Two of four review authors (CT, JM, AJA, NM) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other biases including industry funding.
We graded each potential source of bias as high, low or unclear and provided evidence from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
In addition to routine risk of bias, we also undertook to understand bias in the creation of the SMS. To obtain more information about how the text messages were written, we contacted all authors to request the following information.
Is the SMS intervention a reminder?
Did the authors describe the process to construct the content of the text messages?
Did they evaluate causes for non‐adherence in the target population?
Were psychological theories used to develop the messages to target the identified behavioural determinants of non‐adherence?
Were behaviour‐change techniques employed to develop the messages?
Were different text messages developed according to participants' characteristics?
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We planned to analyse dichotomous data as odds ratios or risk ratios with 95% confidence intervals and continuous data as mean difference or standardised mean difference with 95% confidence intervals. If it had been applicable, we would have entered data presented as a scale with a consistent direction of effect.
We would have narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
We did not include any cluster‐randomised trials. We did not carry out meta‐analysis because of the heterogeneity of the included studies with respect to their methods, population and outcome measures. Therefore, we did not have any unit of analysis issues.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only).
Assessment of heterogeneity
The included trials were too heterogeneous in methods, population and outcome measures to pool the data in a meta‐analysis. We therefore described the studies narratively.
Assessment of reporting biases
We did not assess reporting bias with a funnel plot as we included only seven studies which were too heterogenous to pool in a meta‐analysis.
Data synthesis
We did not undertake meta‐analyses as the included studies were too heterogeneous in their methods, population and outcome measures. Should more studies become available in future updates of this review which enable meta‐analyses, we will use a random‐effects model as we would still expect some degree of heterogeneity.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcome: adherence to treatment. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies for the prespecified outcome. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software. We justified all decisions to downgrade the quality of studies using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We had planed to carry out the following subgroup analyses for the primary outcome.
The baseline arteriosclerotic cardiovascular’ (ASCV) condition (i.e. coronary artery disease, cerebrovascular artery disease, peripheral artery disease, and atherosclerotic aortic disease).
Age (non‐elderly versus elderly, i.e. 64 or more years old).
According to the health system in the population background (universal health systems versus others).
Income region (by World Bank income group).
Type of setting (private versus public, and rural versus urban).
Time of duration of the intervention (less than one year versus one year or more).
Time since cardiovascular event (less than one year versus one year to two years versus two years or more).
Frequency of text messages (daily versus other).
How text messages are developed (theory‐based, validated, etc.).
If trials are text message only or text message plus phone calls.
By different measurements of adherence reported in the articles (for example MARS questionnaire, self‐reported, pill recounts, etc.).
However, we were unable to undertake meta‐analyses and therefore unable to conduct subgroup analyses. In future updates of this review, when more trials are available, we will re‐examine the subgroup analysis.
Sensitivity analysis
We planned to conduct a sensitivity analysis for studies with a low risk of bias. As we were unable to undertake meta‐analyses, no sensitivity analysis was done.
Reaching conclusions
We based our conclusions only on findings from the narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our implications for research suggested priorities for future research and outlines the remaining uncertainties.
Results
Description of studies
Results of the search
The search of the databases retrieved 3987 records. The search of the clinical trial registers retrieved an additional 103 records. After de‐duplication, 2567 records remained for title and abstract screening which led to the exclusion of 2425 records. Therefore 142 records were assessed as full text. This lead to the exclusion of 113 records. Eight studies (16 references) were identified as ongoing and seven studies (13 references) were eligible for inclusion.
A search for the clinical trial registry numbers of the eight ongoing studies revealed that three studies are completed but study results are not published yet (NCT01642355; NCT02354040; NCT02783287). One completed study is still classed as ongoing (Chow 2015) as contact with the author revealed that a sub‐analysis for medication adherence is planned. We also identified a published study protocol for one of the remaining ongoing studies (NCT01642355) and added this report.
The flow of studies through the process is shown in Figure 1.
1.
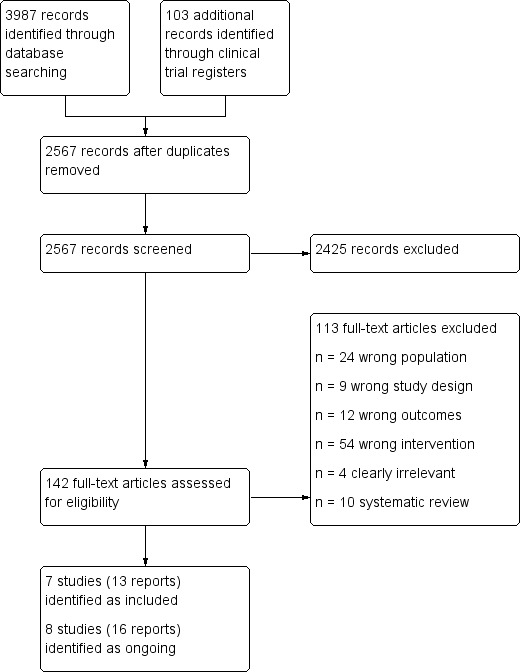
Study flow diagram.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies table.
We included seven studies (Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Pandey 2014; Park 2014a; Quilici 2013), which are heterogeneous in their methods, population and outcome measures. One study (Pandey 2014) was only available as abstracts, and despite attempts to contact the authors we were unable to obtain further information.
Participants
The sample size of included studies ranged from 34 (Pandey 2014) to 521 (Quilici 2013) with a total across all seven studies of 1310, of which 1225 completed follow‐up.
All studies included participants with coronary heart disease except Kamal 2015, that reported on stroke. In detail, Dale 2015a included 80% participants with myocardial infarction, 7% with unstable angina and 13% with angina. Two studies included participants with acute coronary syndrome (Khonsari 2015; Quilici 2013), and two studies included participants with myocardial infarction (Pandey 2014; Park 2014a). One study included participants with chronic stable angina (Fang 2016). Participants in one study had undergone coronary stenting for acute coronary syndrome (Quilici 2013).
The mean age ranged from 53.6 years (Fang 2016) to 64 years (Quilici 2013).
All studies had over 70% males, with the exception of (Pandey 2014) that had 59% males, and Kamal 2015 that had 67.5% males.
Settings
Five studies recruited from hospitals (large metropolitan hospitals (Dale 2015a; Fang 2016), tertiary teaching hospital (Kamal 2015; Khonsari 2015), non‐profit community hospital (Park 2014a). One study (Pandey 2014) was set in a cardiac rehabilitation facility. The setting of one study is unknown (Quilici 2013).
Five studies reported the country in which they took place (China (Fang 2016), New Zealand (Dale 2015a), Malaysia (Khonsari 2015), Pakistan (Kamal 2015), USA (Park 2014a)). The countries are not reported for two studies (Pandey 2014; Quilici 2013) but the affiliations of the authors suggest that one took place in France (Quilici 2013) and one in USA (Pandey 2014).
Development of SMS
Authors were emailed about how SMS were created. We were able to obtain responses from four studies (Dale 2015a; Khonsari 2015; Park 2014a; Kamal 2015) the responses are summarised in Table 2.
1. SMS development.
| Dale 2015a1 | Khonsari 20151 | Pandey 2014 | Park 2014a1 | Quilici 2013 | Fang 2016 | Kamal 2015 | |
| SMS = reminder | No | Yes | no contact details available | Yes | emailed 18/4/2016 | emailed 18/01/2017) |
yes |
| Description of process to design SMS | “We created and refined the Text4Heart intervention through formative and pretesting studies following the mHealth Development and Evaluation Framework.” (Dale 2014a) Also another study that helped inform the physical activity component (Dale 2015c). |
The text‐messages content was based on the World Health Organization (WHO) multidimensional adherence model (WHO 2003b). In constructing the content of the text messages, we focused on the most common reasons for medication non‐adherence based on the WHO model that are unintentional on the patient’s part (forgetfulness and carelessness with medication usage), and include a therapy‐related dimension (misunderstanding of treatment instructions: meds name, dosage and timing) (Gadkari 2012). | No information | ‐ | No information | ‐ | |
| Evaluation of causes for non‐adherence | No | According to the study method, patients were recruited during an admission for ACS prior to discharge from the cardiology ward. It means that all patients were primarily diagnosed with ACS without any experience of taking cardiac medications. Therefore, evaluating causes for non‐adherence in the target population was not applicable. | No information | ‐ | No information | ‐ | |
| Used psychological theories to develop SMS | Messages were based on social cognitive theory and the common sense model (Dale 2014b) |
The WHO multidimensional adherence model (WHO 2003b) that guided this study included many different aspects to describe medication non‐adherence behaviour including psychological factors. It is emphasised that no single determinant is responsible for non‐adherence to treatment because the adherence phenomenon is multidimensional and results from the interplay of five sets of factors (dimensions) including: A. Social and economic factors, B. Therapy‐related factors, C. Condition‐related factors, D. Healthcare team and system‐related factors and E. Patient‐related factors. |
No Information | ‐ | No information | ‐ | |
| Used behaviour‐change techniques to develop SMS | Yes – All messages were coded according to their theoretical construct and corresponding BCT | Development of the automated SMS reminder system in this study was useful for deploying spaced repetition strategies via text messaging. Basically, spaced repetition strategy posits that instruction which is repeated at intervals have a great impact on improving a behaviour (Ebbinghaus 1885) | No information | ‐ | No information | ‐ | |
| SMS designed according to participants characteristics | No, but participants could pick messages on the health behaviour they were most interested in changing (Physical activity, healthy eating, smoking cessation, or stress management). Messages were also personalised with participant’s preferred name. | No | No information | ‐ | No information | ‐ | |
| Pilot phase to evaluate clarity, grammar of SMS | Yes, we pilot tested the healthy eating messages. Feedback from participants was used to refine the messages (Dale 2014a) | We piloted the intervention with a sample size of ten cardiac patients during the first stage of the study. During this phase, a variety of test scenarios and clarity of SMS content were analysed and, consequently, the required changes and fixes were applied in order to achieve the desired functions. | No information | ‐ | No information | ‐ |
1Text in italics = communication from authors
Two studies reported that the SMS was developed as a reminder to take their medications (Khonsari 2015; Park 2014a), and as a result no work was put into their development.
One study specified that the automated computer program from which the messages were sent was developed particularly for this study (Pandey 2014). Four other studies stated that an automated system was used (Dale 2015a; Kamal 2015; Park 2014a; Khonsari 2015), which can also be assumed for the remaining study but was not explicitly stated (Quilici 2013). In detail, Dale 2015a specified that "we created and refined the Text4Heart intervention through formative and pre‐testing studies following the mHealth Development an Evaluation Framework" and "a SMS library of 503 messages has been developed. It is written in English at an appropriate reading level (RMS 800 Lexile: approximately age 13 years) tested using the Lexile Analyzer 2013 software program (MetaMetrics, Durham, NC, USA)." Park 2014a specified that "The primary intervention for this research study was based on Self‐Efficacy Theory by Bandura. Briefly, this theory postulates that in one's capability to successfully perform certain behaviours influences level of motivation, affective states, and action (Bandura 1997)."
No study reported evaluating causes for non‐adherence in the study. Dale 2015a and Khonsari 2015 reported on the psychological and behaviour‐change techniques used in the development of their text messages (Table 2).
Three studies tailored the text messages to the participants' name (Dale 2015a; Khonsari 2015; Park 2014a). One study stated that the messages were personalised without providing further details (Quilici 2013). One study (Pandey 2014) did not provide information on whether or not the messages were tailored. No study detailed that text messages were tailored to individual patient characteristics. Two studies stated that bi‐directional text messaging was required (Dale 2015a; Park 2014a). Participants were required to respond back to confirm receipt (Park 2014a) or send their step count, questions and feedback (Dale 2015a). One study stated that the formulation of the text messages were different every day (Quilici 2013).
Four studies provide details on the template texts used for the text messages (Dale 2015a; Pandey 2014; Park 2014a; Khonsari 2015). Dale 2015a, Kamal 2015, and Khonsari 2015 reported piloting the questionnaires before conducting the study; Fang 2016 did not discuss the method or timing of the SMS at all in the paper.
Interventions
Duration of the intervention ranged from 30 days (Park 2014a; Quilici 2013) to 12 months (Pandey 2014).
Daily text messages were sent in most studies (Khonsari 2015; Pandey 2014; Park 2014a; Quilici 2013). One study (Dale 2015a) sent daily text messages from week zero to 12 weeks, which were reduced in week 13 to week 24 to five messages a week. One study (Fang 2016) did not report on message frequency. Kamal 2015 stated that they were sent on "preset days of the week", and at particular times before each medication intake.
The control group was usual care in five studies (Dale 2015a; Kamal 2015; Khonsari 2015; Pandey 2014; Quilici 2013), text messaging for health education in one study (Park 2014a), and monthly phone calls in one study (Fang 2016). Usual care in Dale 2015a consisted of the standard outpatient cardiac rehabilitation program, involving education classes and supervised exercise.
Outcomes
All included studies measured adherence to medication. Quilici 2013 looked at aspirin adherence using self‐reported adherence. Two studies (Pandey 2014; Khonsari 2015) measured the overall adherence to several prescribed medications. Pandey 2014 included participants on a once‐daily regimen of aspirin, a beta‐blocker, an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and a statin using self‐reported logs. Most participants in Khonsari 2015 were on five or more daily medications, adherence was measured using the Morisky Meidication Adherence Scale. One study measured adherence to antiplatelet and statin medications separately using both electronic pill bottles and self‐reported adherence (Park 2014a). One study only looked at adherence to statins (Fang 2016). One study did not specify which medications the participants were taking and adherence was measured, but specified that it used self‐reported medication adherence (Dale 2015a).
One study reported on fatal cardiovascular events (Khonsari 2015).
Two studies provided outcome data for our secondary outcome of blood pressure (Dale 2015a; Kamal 2015) and one study reported on LDL cholesterol (Dale 2015a). Two studies reported on adverse events (Dale 2015a; Quilici 2013).
Four studies did not report on any of our secondary outcomes (Khonsari 2015; Pandey 2014; Park 2014a; Quilici 2013).
Funding
The source of funding was reported on in three studies ‐ government body (Dale 2015a), no funding received (Khonsari 2015), and research materials from not‐for‐profit organisation but for‐profit organisation provided use of the mobile Health manager platform (Park 2014a).
Excluded studies
Details of excluded studies which most closely missed the inclusion criteria can be found in the Characteristics of excluded studies table.
Ongoing studies
We have identified eight ongoing studies (ACTRN12616000422426; Chow 2015; NCT01642355; NCT02336919; NCT02354040; NCT02783287; NCT02883842; NCT02888769). Five are from high‐income countries (New Zealand, 330 participants, ACTRN12616000422426; Australia, 710 participants Chow 2015; USA, 400 participants NCT01642355; Canada, 75 participants NCT02336919, and 84 participants NCT02783287), one from a lower middle‐income country (Pakistan, 200 participants NCT02354040), and two from an upper middle‐income country (China, NCT02883842; NCT02888769). Details can be found in the Characteristics of ongoing studies table.
Risk of bias in included studies
Details are provided for each of the included studies in the 'Risk of bias' tables in Characteristics of included studies and in Figure 2 and Figure 3. Overall, studies were assessed as having high or unclear bias across multiple domains, and the quality was deemed to be very low (Table 1).
2.
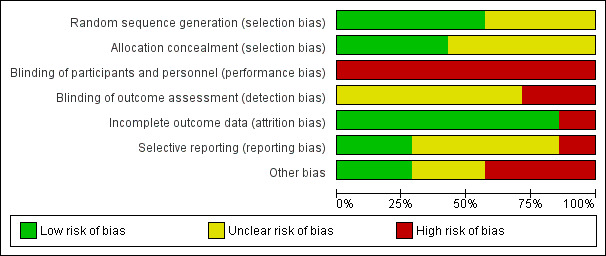
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
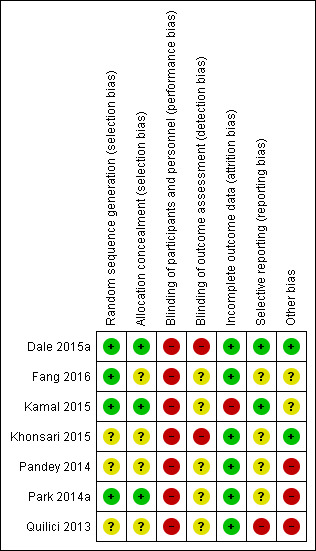
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies reported adequately on the random sequence generation and were therefore judged to be of low risk of bias in this domain (Dale 2015a; Fang 2016; Kamal 2015; Park 2014a). Three studies did not provide enough information and are therefore judged to be unclear risk of bias (Khonsari 2015; Pandey 2014; Quilici 2013).
Three studies reported adequately on allocation concealment and were judged to be of low risk of bias in this domain (Dale 2015a; Kamal 2015; Park 2014a). Four studies did not provide enough information and are therefore judged to be unclear risk of bias (Fang 2016; Khonsari 2015; Pandey 2014; Quilici 2013).
Blinding
While blinding of the participants is not possible with this intervention, blinding of outcome assessors could have been done. Two studies (Dale 2015a; Khonsari 2015) clearly state that no blinding occurred and are therefore at high risk of bias. Five studies (Fang 2016; Kamal 2015; Pandey 2014; Park 2014a; Quilici 2013) did not report on this domain and are therefore judged to be of unclear risk of bias.
Incomplete outcome data
Six studies had less than 8% loss to follow‐up, comparable in intervention and control group, and were judged to be at low risk of incomplete outcome data. One study had 20% loss to follow‐up and was considered to be at high risk of bias (Kamal 2015).
Selective reporting
For two studies we were able to access the trial protocol and all outcomes planned were also reported on (Dale 2015a; Kamal 2015). We therefore judged these studies to be of low risk of reporting bias. One study (Quilici 2013) was judged to be of high risk of bias in this domain as the data were minimal (published as a letter to the editor), and details within the report differed. The other four studies (Fang 2016; Khonsari 2015; Pandey 2014; Park 2014a) are of unclear risk of bias as we did not identify a protocol or trial registry entry to judge reporting bias.
Other potential sources of bias
Two studies were assessed as low risk of bias in this domain as they were funded by a government body (Dale 2015a) and clearly stated that no grant from any type of funding body has supported this trial (Khonsari 2015). Five studies have been judged to be at high (Pandey 2014; Park 2014a; Quilici 2013) or unclear (Fang 2016; Kamal 2015) risk of bias. Pandey 2014 is an abstract publication only and no contact with authors was possible to clarify missing information, the funding source is unclear and differing details are provided in two abstracts. Similarly with Quilici 2013, for which the only source of information is a published letter to an editor in which the outcome data for self‐reported non‐adherence differs between the text and Figure 2. A for‐profit organisation provided use of the mobile Health manager platform in Park 2014a.
Effects of interventions
See: Table 1
Primary outcomes
Adherence to treatment
All seven included studies (1310 randomised participants) reported on medication adherence. Due to the heterogeneity between the studies with respect to participants, methods and outcome measures, we did not pool the results in a meta‐analysis but describe the results in narrative form.
Validated Survey measures
Five studies measured medication adherence with the Morisky Medication Adherence Scale (MMAS‐8) (Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Park 2014a). MMAS‐8 is a patient‐reported metric and validated tool that is widely used in adherence research. Unfortunatetly the studies presented the results in different ways, making it difficult to pool the studies. Dale 2015a, with a follow‐up of six months and 116 participants analysed reports for the intervention group a "significantly greater medication adherence score (Mean Difference (MD) 0.58, 95% CI 0.19, 0.97; P=.004)". In particular, this was a MMAS‐8 score of 7.3 (SD 0.9) for the intervention group and 6.8 (SD 1.2) for the control group at the six months follow‐up. Fang 2016, had a three‐arm design with SMS, SMS + micro letter, or telephone calls (follow‐up of six months and 271 patients analysed) reported that participants given SMS alone had reduced odds of being non‐adherent compared to telephone reminders (Odds Ratio (OR) 0.40 95% CI 0.18 to 0.63) and patients that had SMS + micro letter had the lowest odds compared to telephone reminders (OR 0.07, 95% CI 0.03 to 0.15). Kamal 2015 (200 participants, two‐month follow‐up) reported higher levels of adherence in the intervention arm (adjusted MD 0.54, 95% CI 0.22 to 0.85). Khonsari 2015 (62 participants) reported that "the risk of being low adherent [(score 3‐8 according to Morisky 1986)] among the control group is 4.09 times greater than the intervention group (Relative Risk (RR) 4.09, 95% CI 1.82 to 9.18)" at eight weeks follow‐up. The same study also reported end of follow‐up at two months the low adherence was 16.1% in the intervention group and 58.1% in the control group. Park 2014a, with the shortest follow‐up of 30 days and 28 participants analysed in each group, reported a baseline MMAS‐8 score of 6.20 (SD 1.66) for the intervention group and 5.85 (SD 2.10) for the control group. At follow‐up, the score had risen for both groups, but was higher for the control group at 6.73 (SD 1.49) than for the intervention group at 6.43 (SD 1.22) (no P value reported).
Objective Measures
In addition to the MMAS‐8 score, Park 2014a used another measure to test for medication adherence. A Medication Event Monitoring System (opening of the two electronic pill bottles provided a time‐stamp corresponding with medication self‐administration) resulted in the following. Antiplatelet doses taken on schedule were 86.2% (SD 15.4) in the intervention group and 85.7% (SD 18.2) in the control group. For statins, 84.1% (SD 19.4) of doses were taken on schedule by the intervention group and 79.7% (SD 19.3) in the control group. The correct number of antiplatelet doses taken were 88.0% (SD 14.0) in the intervention group and 87.2% (SD 16.5) in the control group. For statins, 85.4% (SD 16.6) correct number of doses were taken in the intervention group and 81.3% (SD 16.4) in the control group.
Self‐Reported Measures
Pandey 2014 assessed medication adherence in 33 participants with self‐reported logs at 12 months. This resulted in 90% adherence in the intervention group compared to 70% in the control group (P < 0.0001).
At 30 days follow‐up, data from Quilici 2013 self‐reports differed between the text and Figure 2, but showed a higher adherence in the intervention group (96.4% (text)/97.2% (Figure 2)) than in the control group (93.6% (text)/92.8 (Figure 2).The OR for self‐reported aspirin non‐adherence as provided in the paper is 0.37, 95% CI 0.15 to 0.90, P = 0.02. The platelet testing confirmed this by showing a 94.8% adherence in the intervention group and 88.8% in the control group. The paper reported the OR for non‐adherence as 0.43, 95% CI 0.22 to 0.86, P = 0.01.
Fatal cardiovascular events
One study reported two deaths due to acute coronary syndrome (ACS) complications, both in the control group (Khonsari 2015).
Non‐fatal cardiovascular events (coronary heart disease (CHD), revascularisation, stroke)
No study reported this outcome.
Combined cardiovascular disease (CVD) event (fatal or non‐fatal events)
No study reported this outcome.
Secondary outcomes
Low‐density lipoprotein (LDL)‐cholesterol for the effect of statins
One study (Dale 2015a) reported some evidence of a reduction of LDL cholesterol (mmol/L) in the intervention arm (adjusted MD at six months: ‐0.25, 95% CI ‐0.49 to 0.01, P = 0.053).
Blood pressure for antihypertensive drugs
Two studies reported on blood pressure. Dale 2015a reported no difference between arms for change in blood pressure (mmHg) (systolic blood pressure adjusted MD at six months 0.09, 95% CI ‐6.43 to 6.61, P = 0.98; diastolic blood pressure ‐0.24, 95% CI ‐3.86 to 3.38, P = 0.90). Kamal 2015 reported a slightly lower mean diastolic blood pressure in intervention group (MD 2.6 mmHg 95% CI ‐5.5 to 0.15).
Heart rate for the effect of beta blockers
No study reported this outcome.
Urinary 11‐dehydrothromboxane B2 for the antiplatelet effects of aspirin
No study reported this outcome.
Adverse effects
No study reported repetitive thumb injury or road traffic crashes or other adverse effects as related to the intervention.
Discussion
Summary of main results
In this review, we were only able to include seven trials with a small number of participants. The seven included trials were small, of variable length (one to 12 months), and heterogenous so we were unable to pool them for meta‐analysis. Six out of the seven trials showed a beneficial effect of interventions of mobile phone text messaging for medication adherence (Dale 2015a; Fang 2016; Kamal 2015; Khonsari 2015; Pandey 2014; Quilici 2013). One trial (Park 2014a), using Morisky Medication Adherence Scale (MMAS‐8) showed an improved adherence score for the control group compared to the intervention group. However, this was only one outcome measure in the smallest of the included trials with a short follow‐up of 30 days. The other measures used to assess adherence in Park 2014a also showed a beneficial effect of text message reminders. Park 2014a also showed a beneficial effect of reminders compared with education. Only one study reported on fatal cardiovascular events (Khonsari 2015), and they reported two deaths due to acute coronary syndrome (ACS) complications, both of which were reported in the control group. No study reported on any of the other primary outcomes.
Only one study (Dale 2015a) reported on the secondary outcomes of low‐density lipoprotein (LDL)‐cholesterol, finding a small positive effect on lowering cholesterol. Two studies (Dale 2015a; Kamal 2015) reported no strong evidence of an effect on lowering blood pressure. Four authors replied to our questionnaire on SMS development. No study reported examining causes of non‐adherence or provided SMS tailored to individual patient characteristics. No study reported adverse effects that related to the intervention.
Overall completeness and applicability of evidence
The evidence of this review is applicable to a predominantly male population aged between 50 to 65 years, with coronary heart (CHD) disease recruited soon after the index event and with a short follow‐up, usually less than six months. The studies were mainly on CHD, with only one study examining stroke so the results are not applicable to other cardiovascular diseases.
Two trials for which information on the country is available took place in high‐income countries (New Zealand and USA) and two took place in upper middle‐income countries (China and Malaysia), and only one was conducted in a lower middle‐income country (Pakistan) (Worldbank 2015). It is uncertain where the other two studies were conducted, but they were likely from high‐income countries (France and USA). It is therefore unclear whether the results would apply to low‐ and middle‐income countries. Access to mobile phone technology does not seem to be a problem in low‐ and middle‐income countries (Worldbank 2012). We identified eight ongoing studies, that ranged from 75 individuals (NCT02336919) to 710 individuals (Chow 2015). Five of these studies are being conducted in high‐income countries (ACTRN12616000422426; Chow 2015; NCT01642355; NCT02336919; NCT02783287).
Most of the studies examined medications and diseases singly, this has implications for the generalisability of results, given that most people may have co‐morbidities, or be on multiple medications.
Quality of the evidence
Overall the 'Summary of findings' table shows that the evidence is of very low quality. The studies were small, heterogenous and underpowered for the following reasons: Dale 2015a provided a sample size calculation in the trial protocol but the primary outcome was not medication adherence; Quilici 2013 was a 'pilot study'; Park 2014a was reported as 'a convenience sample'. The studies were generally of short duration, with two trials only lasting 30 days.
Each study has at least one risk of bias domain judged as high risk. All studies were either at high or unclear risk of bias for blinding; both performance and detection bias, and only three studies (Dale 2015a; Kamal 2015; Park 2014a) were at low risk for allocation concealment and random sequence generation. Although all studies used mobile phones as the way to deliver the intervention, we identified substantial differences in the actual content of the SMS. Only one study (Dale 2015a) used behaviour‐change models to develop the content of the intervention, while two other studies used just "reminders", and for the other two there was lack of information for review authors to judge what type of content was used in the SMS. This heterogeneity not only has implications to the applicability of the evidence, but also raised the questions that quality of reporting for trials evaluating mobile phone interventions is very poor.
Potential biases in the review process
We acknowledge that, although systematic searches across a number of resources were conducted, any search has limitations for pragmatic reasons. Publication bias is a known problem for trials with negative results (Hopewell 2009). We tried to overcome this potential limitation by searching clinical trial registries for data on prospectively registered trials.
One of the studies was only reported in abstract form (Pandey 2014), and while we attempted to contact the authors on multiple occasions we were unable to obtain further information on this study.
Due to the heterogeneity of the identified trials we did not perform a meta‐analysis and therefore this review cannot benefit from pooled estimates based on a larger sample size than the individual trials.
Agreements and disagreements with other studies or reviews
While there is not a great deal of evidence on mobile text messaging for adherence in secondary prevention, It can be useful to look into research into what has been successful in tackling other chronic conditions (for example Viswanathan 2012). Our results are broadly in line with studies in other disciplines that have showed some promising results; particularly in the field of HIV research Anglada‐Martinez 2015, a systematic review of 20 generally low‐quality studies (7402 participants) of mhealth for adherence to HIV mediations found a great deal of heterogeneity, but reported 65% of studies reported a positive effect of mhealth on adherence. Another systematic review (Devi 2015) that included mHealth on adherence to medications found 70% (33 of 47) of studies reported positive effects. Al‐Ganmi and colleagues conducted a systematic review looking at cardiovascular medication adherence in cardiac patients, and also found too much heterogeneity of results to conduct meta‐analysis (Al‐Ganmi 2016). In a large systematic review of mhealth for behaviour‐change and disease management, Free 2013 found that text message‐based interventions increased adherence to antiretroviral (ART) and smoking cessation. One study on mobile text messaging for adherence on all chronic diseases found that mobile text messaging nearly doubled the odds of medication adherence (Thakkar 2016). Our study is the first review to include an assessment of SMS development.
Authors' conclusions
Implications for practice.
While the studies suggested positive effects of mobile text messaging for adherence to medications, the findings are of very low quality, and we were unable to conduct meta‐analysis. As a result we have very little confidence in the findings, and cannot make recommendations for practice.
Implications for research.
Mobile text messaging appears to have positive effects on adherence to medications for secondary prevention of cardiovascular disease (CVD), with very little evidence of adverse events. However, there is a lack of high quality evidence. Although we were able to identify eight ongoing studies, most of these are from high‐income countries. As a result we call for more, adequately powered, good quality, randomised studies to be conducted, particularly in low‐resource settings. Because in most cases there is a need for lifelong adherence to medications longer‐term trials are also needed. Future studies should also examine the frequency and timings that the messages should be sent; message content; optimal development processes, and process evaluations to assess the mechanisms by which messages have effect. It is of particular importance that standardised approaches to measure adherence (development of free and validated scores) are used so that outcomes can be pooled across studies.
Acknowledgements
We are grateful to Deirdre Beecher for her help with the assessment of one Italian paper (Capomolla 2005) and to Marina Karanikolos with the assessment of one Russian paper (Kiselev 2011).
Appendices
Appendix 1. Search strategies
CENTRAL
#1MeSH descriptor: [Reminder Systems] this term only #2MeSH descriptor: [Telemedicine] this term only #3MeSH descriptor: [Cell Phones] explode all trees #4sms #5mms #6short near/6 messag* #7text near/6 messag* #8texting #9telemedicine* #10reminder next/6 (text* or system* or messag*) #11telehealth #12mobile near/6 (health* or phone*) #13mhealth #14telemonitor* #15#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16MeSH descriptor: [Cardiovascular Diseases] explode all trees #17cardio* #18cardia* #19heart* #20coronary* #21angina* #22ventric* #23myocard* #24pericard* #25isch?em* #26emboli* #27arrhythmi* #28thrombo* #29atrial next fibrillat* #30tachycardi* #31endocardi* #32(sick near/2 sinus) #33MeSH descriptor: [Stroke] explode all trees #34stroke or strokes #35cerebrovasc* #36cerebral next vascular #37apoplexy #38brain near/2 accident* #39(brain* or cerebral or lacunar) near/2 infarct* #40peripheral next arter* next disease* #41aortic* #42arterial near/2 occlus* #43infarct* #44#16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 #45#15 and #44
MEDLINE OVID
1. Reminder Systems/ 2. Telemedicine/ 3. exp Cell Phones/ 4. sms.tw. 5. mms.tw. 6. (short adj messag*).tw. 7. (text adj messag*).tw. 8. texting.tw. 9. telemedicine*.tw. 10. (reminder adj (text* or system* or messag*)).tw. 11. telehealth.tw. 12. (mobile adj (health* or phone*)).tw. 13. mhealth.tw. 14. telemonitor*.tw. 15. or/1‐14 16. exp Cardiovascular Diseases/ 17. cardio*.tw. 18. cardia*.tw. 19. heart*.tw. 20. coronary*.tw. 21. angina*.tw. 22. ventric*.tw. 23. myocard*.tw. 24. pericard*.tw. 25. isch?em*.tw. 26. emboli*.tw. 27. arrhythmi*.tw. 28. thrombo*.tw. 29. atrial fibrillat*.tw. 30. tachycardi*.tw. 31. endocardi*.tw. 32. (sick adj sinus).tw. 33. exp Stroke/ 34. (stroke or strokes).tw. 35. cerebrovasc*.tw. 36. cerebral vascular.tw. 37. apoplexy.tw. 38. (brain adj2 accident*).tw. 39. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 40. peripheral arter* disease*.tw. 41. aortic*.tw. 42. (arterial adj occlus*).tw. 43. infarct*.tw. 44. or/16‐43 45. 15 and 44 46. randomized controlled trial.pt. 47. controlled clinical trial.pt. 48. randomized.ab. 49. placebo.ab. 50. drug therapy.fs. 51. randomly.ab. 52. trial.ab. 53. groups.ab. 54. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 55. exp animals/ not humans.sh. 56. 54 not 55 57. 45 and 56
Embase OVID
1. reminder system/ 2. telemonitoring/ 3. mobile phone/ 4. sms.tw. 5. mms.tw. 6. (short adj messag*).tw. 7. (text adj messag*).tw. 8. texting.tw. 9. telemedicine*.tw. 10. (reminder adj (text* or system* or messag*)).tw. 11. telehealth.tw. 12. (mobile adj (health* or phone*)).tw. 13. mhealth.tw. 14. telemonitor*.tw. 15. or/1‐14 16. exp cardiovascular disease/ 17. cardio*.tw. 18. cardia*.tw. 19. heart*.tw. 20. coronary*.tw. 21. angina*.tw. 22. ventric*.tw. 23. myocard*.tw. 24. pericard*.tw. 25. isch?em*.tw. 26. emboli*.tw. 27. arrhythmi*.tw. 28. thrombo*.tw. 29. atrial fibrillat*.tw. 30. tachycardi*.tw. 31. endocardi*.tw. 32. (sick adj sinus).tw. 33. cerebrovascular accident/ 34. (stroke or strokes).tw. 35. cerebrovasc*.tw. 36. cerebral vascular.tw. 37. apoplexy.tw. 38. (brain adj2 accident*).tw. 39. ((brain* or cerebral or lacunar) adj2 infarct*).tw. 40. peripheral arter* disease*.tw. 41. aortic*.tw. 42. (arterial adj occlus*).tw. 43. infarct*.tw. 44. or/16‐43 45. 15 and 44 46. random$.tw. 47. factorial$.tw. 48. crossover$.tw. 49. cross over$.tw. 50. cross‐over$.tw. 51. placebo$.tw. 52. (doubl$ adj blind$).tw. 53. (singl$ adj blind$).tw. 54. assign$.tw. 55. allocat$.tw. 56. volunteer$.tw. 57. crossover procedure/ 58. double blind procedure/ 59. randomized controlled trial/ 60. single blind procedure/ 61. 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 62. (animal/ or nonhuman/) not human/ 63. 61 not 62 64. 45 and 63
Web of Science
#5 #4 AND #3 #4 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*) #3 #2 AND #1 #2 TS=( cardio* or cardia* or heart* or coronary* or angina* or ventric* or myocard* or pericard* or isch?em* or emboli* or arrhythmi* or thrombo* or "atrial fibrillat*" or tachycardi* or endocardi* or "sick sinus" or stroke or strokes or cerebrovasc* or "cerebral vascular" or apoplexy or "brain accident*" or infarct* or "peripheral arter* disease*" or aortic* or "arterial occlus*") #1 TS=(sms or mms or "short messag*" or "text messag*" or texting or telemedicine* or "reminder text*" or "reminder system*" or "reminder messag*" or telehealth or "mobile health*" or " mobile phone*" or mhealth or telemonitor*)
Clinicaltrials.gov
Advanced search: study type: interventional studies conditions: cardiovascular interventions: text
WHO ICTRP
text AND cardio*
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dale 2015a.
| Methods |
Design: two‐arm, parallel RCT Setting: two large metropolitan hospitals, Auckland, New Zealand Recruitment period: over 10 months between 2013 and 2014 Length of intervention: 6 months Two‐arm, parallel, RCT |
|
| Participants |
Inclusion criteria: "Included participants were English‐speaking adults with a documented diagnosis of CHD (myocardial infarction, angina, or revascularization). [...] access to the Internet was a requirement. [...] phones were supplied for the duration of the study if necessary." Exclusion criteria: "Those with untreated ventricular tachycardia, severe heart failure, life‐threatening coexisting disease with life expectancy less than 1 year, and/or significant exercise limitations for reasons other than CHD were excluded." Randomised: n = 123, n = 61 intervention, n = 62 control group Number available for follow‐up: n = 57 intervention (n = 2 withdrew due to medical reasons, n = 2 withdrew due to being too busy), n = 59 control (n = 3 could not be contacted) Mean age in years (SD): 59.9 (11.1), intervention group: 59.0 (10.5), control group: 59.9 (11.8) Sex (% male): 81.3, intervention group: 79, control group: 84 |
|
| Interventions | "All participants received usual care, which included inpatient rehabilitation and encouragement to attend center‐based CR. Traditional CR offered at the hospital recruiting sites in this study consisted of one 1‐hour outpatient education program per week for 6 weeks at a hospital or community center covering a range of topics, including cardiovascular risk factors, lifestyle change, and psychosocial support. Patients also were encouraged to attend a 16‐session supervised exercise program at the participating hospital or outpatient center. Participants could take part in usual care CR from point of discharge to 6 months after their heart event." "All participants were telephoned at 3‐months postrandomization to collect primary outcome data. [...] At 6‐months postrandomization, participants were seen at a clinic or in a home setting for final follow‐up assessment." Intervention group: "In addition to usual care, the intervention group received a 24‐week mHealth program sent by automated daily text messages and access to a supporting website commencing within a week of the baseline assessment. [...] Messages were tailored to participants' name and preferred time of day to receive messages. From weeks 13 to 24, the frequency of messages decreased to 5 per week. Bidirectional messaging was used because participants were prompted to text in their weekly pedometer step counts and to ask questions or for feedback on other behaviors." Text type: automated, bidirectional Control group: usual care as describe above |
|
| Outcomes |
Primary outcome: adherence to recommended health guidelines measured as a binary variable using a self‐reported composite health behavior score based on the European Prospective Investigation into Cancer (EPIC)‐Norfolk Prospective Population Study at 6 months (smoking status, physical activity, alcohol consumption, fruit and vegetable intake). Secondary outcomes: biomedical risk factors (systolic and diastolic blood pressure, lipid profile, weight, BMI, waist‐hip‐ratio) and subsequent CHD risk probability, medication adherence was measured using the Morisky 8‐item Medication Adherence Questionnaire, serious adverse events, hospital anxiety, hospital depression, overall self‐efficacy, overall illness threat, engagement in the intervention. Medication adherence: at 6 months: intervention group 7.3 (0.9), control group 6.8 (1.2), adjusted mean difference 0.58, 95% CI 0.19 to 0.97 Blood pressure (mmHg) mean (SD): systolic: intervention group: 131 (17) at baseline, 136 (20) at 6 months, control group: 129 (26) at baseline, 135 (16) at 6 months, adjusted mean difference at 6 months 0.09 (‐6.43 to 6.61); diastolic: intervention group: 78 (11) at baseline, 79 (11) at 6 months, control group: 75 (11) at baseline, 79 (10) at 6 months, adjusted mean difference at 6 months ‐0.24 (‐3.86 to 3.38) LDL cholesterol (mmol/L) mean (SD): intervention group: 2.7 (1.3) at baseline, 1.7 (0.6) at 6 months, control group: 2.4 (1.0) at baseline, 1.9 (0.8) at 6 months, adjusted mean difference at 6 months: ‐0.25 (‐0.49 to 0.01) Serious adverse events: n = 8 intervention group, n = 5 control group ("although none were study related") |
|
| Notes | Funding: Government body (National Institute for Health Innovation, the University of Auckland) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization sequence was computer generated by a statistician independent to the project using a block size of 6" |
| Allocation concealment (selection bias) | Low risk | "allocation was concealed in sequentially numbered, opaque, sealed envelopes. Participant enrolment and assignment to the intervention were completed by a trained research assistant" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the nature of the intervention, participants and outcome assessors were not blinded to their treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Because of the nature of the intervention, participants and outcome assessors were not blinded to their treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned in protocol |
| Other bias | Low risk | Funding from government body |
Fang 2016.
| Methods |
Design: three‐arm, parallel RCT (arm 1: SMS, arm 2: SMS + Micro letter, arm 3: phone) Setting: Chengdu City, China Recruitment period: over 10 months in 2013 Length of intervention: 6 months |
|
| Participants |
Inclusion criteria: adult patients with CAD treated in the General Medicine Department at West China Hospital. All patients had chronic stable angina consistent with criteria of the Chinese Medical Association of Cardiovascular Disease Guide. Exclusion criteria: (1) nonconformance with the diagnostic standards for chronic stable angina established by the Chinese Medical Association of Cardiovascular Epidemiology, (2) history of mental illness, (3) infection, fever, operation, serious heart failure, respiratory failure or acute stroke in the prior month and (4) inability to use a mobile phone that accepts SMS. Randomised: n = 280, arm 1: 95, arm 2: 92, arm 3: 93 Number available for follow‐up: n = 271. Nine withdrew for either unwillingness to complete (6) personal issues (3) arm 1 n = 4, arm 2 n = 2, arm 3 n = 3 Mean age in years (SD): arm 1 = 53.73 (7.20), arm 2 = 53.69 (7.74), arm 3 = 53.50 (7.62) Sex (% male): arm 1 = 70.33 arm 2 = 67.78, arm 3 = 67.78 Disease duration (average years): arm 1 = 3.02 years, arm 2 = 2.98, arm 3 = 2.94 |
|
| Interventions | All patients received initial questionnaires at the hospital. The SMS group received medication reminders and educational materials via SMS. The SMS + Micro Letter group received medication reminders via SMS and educational materials via ML. We built a public ML platform, from which we regularly released CAD‐related information, including the hazards and methods of preventing hyperlipidaemia, the role, scope, usage, method of use, and side effects of lipid‐lowering drugs and other related information. Patients in the SMS + ML group had open access to all information on the ML platform. The phone group received a telephone call once a month to remind them of their medication schedule and upcoming appointments. After six months, we compared statin prescription adherence among the three groups. Text type: Not stated |
|
| Outcomes |
Primary outcome: adherence to statin medication using the Morisky Medication Adherence Scale (MMAS) Medication adherence: at baseline: Arm 1 2.88 (0.71), Arm 2 2.86 (0.71), Arm 3 2.86 (0.87), at six months: Arm 1 (SMS only compared to phone) OR 0.40 (0.18, 0.63) Arm 2 (SMS + ML compared to phone) OR 0.07 (0.03, 0.15) Serious adverse events: Not discussed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed but given nature of intervention unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Unclear risk | Only one outcome, but no protocol |
| Other bias | Unclear risk | None discussed |
Kamal 2015.
| Methods |
Design: two‐arm, parallel RCT Setting: Karachi, Pakistan Recruitment period: not stated Length of intervention: 2 months |
|
| Participants |
Inclusion criteria: age greater than 18 years old; history of stroke(s) confirmed by neuroimaging at the time of the episode; more than 1 month since last episode of stroke; use of at least two drugs such as (but not limited to) antiplatelets, statins, anti‐hypertensives to control risk factors of stroke; modified Rankin Score of 3 or less (so that they are able to operate mobile phones); possession of a personal cell phone that the patient has access to at all times. In the case of patients who do not own or are unable to use mobile phones, they must have a caregiver available at all times who possesses a cell phone; ability to receive, comprehend and reply to an SMS in English, Nastaleeq Urdu (local Urdu script) or Roman Urdu. In the case of patients who themselves are unable to receive, comprehend or reply to an SMS, they must have caregivers available at all times who could perform the above mentioned tasks Exclusion criteria: biological impairment in reading or responding to SMS in the caregiver such as (but not limited to) loss of vision, visual field cuts, aphasia in case the patient himself/herself is supposed to receive SMS; Diagnosed organ dysfunction or malignancy such as hepatic, renal or malignancy; plans to travel outside the country inside the two months following enrolment Randomised: n = 200. Intervention = 100, control = 100 Number available for follow‐up: n = 162. Intervention arm: 17 lost to follow‐up: 10 unwilling to come, 2 sick, 3 out of station 2 discontinued intervention. Control arm: 21 lost to follow‐up: 17 unwilling to come, 4 out of station Mean age in years (SD): Control: 57.6 (1.3), intervention: 56 (1.5) Sex (% male): 67.5 % male (64% in control 71% in intervention) Disease duration: at least one month |
|
| Interventions |
Intervention: In addition to the usual care, intervention group received automated SMS reminders customised to their individual prescription. The participants were required to respond to the SMS stating if they have taken their medicines. Moreover, twice weekly health information SMS were also sent to the intervention group. Health information SMS were customised according to medical and drug profile of every patient by the research team. Control: patients received the usual standard of care provided at the centre for stroke patients. This primarily consisted of regular follow‐up visits (as advised by their neurologist) with their stroke neurologist. In general, these were at 1, 3, 5,9,12 months after a stroke. Each patient was provided with a telephone number that could be used to reach the stroke team in case of an emergency and each patient was also reminded of their clinic appointments 1–2 days prior via SMS and/or phone Text type: automated‐ two‐way "The messages were designed in a weekly schedule at preset days of the week for total 8 weeks e.g., Wednesday and Saturday week 1 for patient X. The timings were decided according to the prescription so that health messages do not collide with the reminder messages for that day. Usually 5 pm was found feasible for most participants. These messages did not ask for a reply. These health information SMS were codified by Michie’s Taxonomy of Behavioural Change for repeatability" |
|
| Outcomes |
Primary outcome: Change in medication adherence after 2 months using the Morisky Medication Adherence Scale (MMAS) Secondary Outcome: Change in blood pressure, acceptability of SMS Medication adherence: at baseline: Control 6.6 (0.17) Intervention 6.6 (0.16) at two months: Control 6.7 (1.32) Intervention 7.4 (0.93) Adjusted mean difference (adjusted for baseline, number of pills, dosing frequency, age, gender, employment status, education, use of alarms) 0.54 (95% CI 0.22, 0.85) Blood pressure The mean diastolic blood pressure in the intervention group was 2.6 mmHg (95 % CI; −5.5 to 0.15) lower compared to the usual care group Serious adverse events: Not discussed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomised computer‐generated sequence. The staff who randomised and those who assessed and those who delivered the intervention were separate. |
| Allocation concealment (selection bias) | Low risk | Concealed in white envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed but based on intervention high risk |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only mention is that "The staff who randomized and those who assessed and those who delivered the intervention were separate". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported on. Blood pressure not mentioned in protocol, but acceptability and patient satisfaction were. |
| Other bias | Unclear risk | Nothing discussed |
Khonsari 2015.
| Methods |
Design: prospective, parallel, two‐arm Setting: tertiary teaching hospital In Kuala Lumpar, Malaysia Recruitment period: 23 January 2013 to 23 February 2013 Length of intervention: 8 weeks |
|
| Participants |
Inclusion criteria: ACS Exclusion criteria: no cell phone to receive text messages; were not discharged during the specified study timeline or were discharged to a care facility or transferred to another health care institution; were illiterate or unable to read text messages; were not available for the two‐month period of the study (including being unavailable by phone and/or travelling out of the country); or had been diagnosed with cognitive impairment so that the informed consent process might be incomprehensible. Randomised: n = 62, n = 31 intervention group, n = 31 control group Number available for follow‐up: n = 31 intervention group, n = 29 control (n = 2 death) Mean age in years: intervention (56), control (59) Sex (% male): intervention (87.1), control (83.9) |
|
| Interventions |
Intervention group: The participants randomised to the intervention group received text‐message reminders based on the following template before every medication intake, starting the day after discharge: ‘‘[Mr/Ms] [Patient’s Name], please take [Medication Quantity] tablet of [Medication Name] at [Time]’’. When the course of medication was completed (patients were given a 30‐day dosage), a message was sent to remind the patients to come to the hospital and have their prescribed cardiac medications refilled. The SMS reminder service was continued until two months after discharge. The system is a web‐based software where all tasks are handled automatically. Reminders were generated and sent to each participant in the intervention group before every cardiac medication intake in an 8‐week programme. The researcher also followed up with the participants in the SMS group via telephone calls once per two weeks during the study to reassure the delivery of text messages, to enquire whether any emergency readmission was needed as well as to show up for their appointments. Text type: Automated, one‐way Control group: Usual care for ACS post‐discharge including cardiac rehabilitation and follow‐up appointments with the cardiologist, usually occurring at six or eight weeks following discharge. |
|
| Outcomes |
Primary outcome: medication adherence, measured with eight‐item Morisky Medication Adherence Scale (MMAS‐8‐item) Secondary outcomes: NYHA classification, death, hospital readmission rates, patient's perception on the automated short message service Medication adherence: intervention (64.5% (n = 20) high adherence; 16.1% (n = 5) low adherence); control (12.9% (n = 4) high adherence; 58.1% (n = 18) low adherence); risk of being low adherent among the control group is 4.09 times greater than the intervention group (RR = 4.09, 95%CI 1.82 to 9.18) Death: intervention (n = 0); control (n = 2) due to ACS complication |
|
| Notes | Funding: "This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the nature of the intervention, it was impossible to blind either the subjects or the researchers to the study group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "To prevent potential bias in the results of the study, all participants were visited by cardiologists and cardiac rehabilitation specialists who were unaware of the study group assignment to assess the participants' heart function status based on the New York Heart Association Functional Classification (NYHA) at the endpoint of the study". However, NYHA class was not an outcome of this review and no blinding of outcome assessors was done in relation to the other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "intention‐to‐treat analysis was performed assuming that patients who were missing were categorised under the low medication adherence level as well as the last classification of heart functional status in the patient's most recent document." |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol found to compare planned with reported outcomes. |
| Other bias | Low risk | No specific funding |
Pandey 2014.
| Methods |
Design: prospective, parallel, two‐arm Setting: cardiac rehabilitation facility, country not reported Recruitment period: not reported Length of intervention: 12 months |
|
| Participants |
Inclusion criteria: recently discharged after MI who were receiving care at a single cardiac rehabilitation facility; had to be on a once daily regimen of aspirin, a beta‐blocker, an ACE or ARB and a statin Exclusion criteria: patients without cell phones and those unable to provide informed consent in English were excluded. Randomised: n = 34, not separately reported for intervention and control group Number available for follow‐up: n = 1 dropout of control group, no reason Mean age in years: total (63 in Pandey 2014, 64 in Pandey 2015), not separately reported for intervention and control group Sex (% male): total (59% in Pandey 2014 2014, 64% in Pandey 2015), not separately reported for intervention and control group |
|
| Interventions |
Intervention group: daily text message reminders at the times they were to take their prescribed medication; "Please take your morning medication now" and indicated which medication they should take at that time Text Type: automated Control group: usual care |
|
| Outcomes |
Primary outcome: medication adherence 12 months after randomisation assessed with self‐reported logs Medication adherence: intervention (month 1 = 98%, month 12 = 90%); control (month 1 = 92%, month 12 = 70%) |
|
| Notes |
Funding: not reported Publication: Published abstracts only (two different abstracts published with some differing information. Authors contacted on multiple occasions to clarify data, but have not responded). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details reported but we made the assumption that given the nature of the intervention it was impossible to blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in the control group dropped out and therefore did not have follow‐up data." |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol found to compare planned with reported outcomes. |
| Other bias | High risk | Abstract publication only, no contact with authors possible, funding source unclear, differing details in two abstracts |
Park 2014a.
| Methods |
Design: prospective, parallel, three‐arm Setting: non‐profit community hospital, Northern California, USA Recruitment period: April 2012 to March 2013 Length of intervention: 30 days |
|
| Participants |
Inclusion criteria: ≥21 years of age, hospitalised for non‐ST elevation MI, ST elevation MI or PCI, prescribed an antiplatelet medication [thienopyridine class of ADP receptor inhibitors and/or a cycloozygenase inhibitor (i.e. aspirin), prescribed a statin medication (HMG‐CoA reductase inhibitors), owned a mobile phone with text messaging capability and were able to speak, read, and understand English Exclusion criteria: cognitive impairment that limited ability to understand and complete questionnaires, and inability to operate a mobile phone Randomised: n = 90 total, n = 30 intervention group 1, n = 30 intervention group 2, n = 30 control group Number available for follow‐up: n = 84, n = 28 intervention group 1 (n = 2 lost to follow‐up, withdrew due to illness), n = 28 intervention group 2 (n = 2 lost to follow‐up, of which n = 1 withdrew due to busy schedule and n = 1 withdrew due to illness), n = 28 control group (n = 2 lost to follow = up, of which n = 1 due to privacy request and n = 1 was unable to contact) Mean age in years (SD): 59.2 total, 58.2 (10.6) intervention group 1, 58.3 (8.5) intervention group 2, 61.1 (9.1) control group Sex (% male): 76 total, 76.7 intervention group 1, 66.7 intervention group 2, 83.3 control group |
|
| Interventions |
Intervention group 1: text messages for medication reminders and health education; The medication reminders were two‐way, requiring patients to respond back to confirm receipt. They were delivered, twice daily, at times selected by the patients that correlated with their medication schedule). An example of a medication reminder was, ‘‘John, take Plavix 75 mg at 9:00 AM. Respond with 1.’’ Intervention group 2: text messages for health education; health education messages were one‐way educational health messages on cardiovascular risk reduction on Monday, Wednesday, and Friday at 2 PM. Text type: sent from a customisable program through CareSpeak Communications ‘‘mobile Health manager’’ platform (New Jersey), two‐way Control group: no text messages For the purpose of the review, the two intervention arms were compared ‐ intervention group 1 (intervention), intervention group 2 (control). |
|
| Outcomes |
Primary outcome: medication adherence, measured by Medication Event Monitoring System (opening of he two electronic pill bottles provided a time‐stamp corresponding with medication self‐administration), responses to messages and MMAS‐8, a self‐report measure completed at baseline and follow‐up. Secondary outcomes: feasibility and patient satisfaction, assessed by successful execution of the intervention, patient participation, and by the Mobile Phone use Questionnaire (developed for this study) Medication adherence: MEMS, per cent (SD) doses taken on schedule in % (SD) (antiplatelets): Intervention group (analysed n = 24): 86.2 (15.4), control group (analysed n = 19): 85.7 (18.2) MEMS, per cent (SD) doses taken on schedule in % (SD) (statins): Intervention group (analysed n = 24): 84.1 (19.4), control group (analysed n = 20): 79.7 (19.3) responses to messages, mean response rate in % (SD) (antiplatelets): M = 90.2 (9) responses to messages, mean response rate in % (SD) (statins): M = 83.4 (15.8) MMAS‐8 score (SD): intervention group (analysed n = 28): baseline 6.20 (1.66), follow‐up 6.43 (1.22), control group (analysed n = 28): baseline 5.85 (2.10), follow‐up 6.73 (1.49) |
|
| Notes | Funding: research materials from not‐for profit organisations, for‐profit organisation provided use of the mobile Health manager platform | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was generated by random allocation sequence using blocks of six that was prepared by a biostatistician." |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes"; "The PI assigned patients to their groups by distributing envelopes in consecutive, numbered order." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to the nature of the study design, the PI and patients could not be blinded to the intervention once group assignment was determined." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss‐to follow‐up equal in both groups included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | No trial registry entry or published protocol available (confirmed by author via email) |
| Other bias | High risk | For‐profit organisation provided use of the mobile Health manager platform |
Quilici 2013.
| Methods |
Design: prospective, parallel, two‐arm Setting: not reported Recruitment period: Length of intervention: 1 month/30 days |
|
| Participants |
Inclusion criteria: patients were considered eligible to enter the study if they undergone coronary stenting for ACS with good in‐hospital aspirin response defined by arachidonic acid induced platelet aggregation (AA‐Ag) lower than 30%. Participants needed to own a mobile phone with ability to communicate via short message service (SMS). Patients were discharged with a prescription of aspirin 75 mg and clopidogrel and were provided with educational sessions highlighting the importance of patient adherence to physicians' recommendations. Patients, randomised to SMS, received for one month a daily personalised SMS reminding aspirin intake, with different formulation every day. Exclusion criteria: not reported Randomised: n = 521 total, n = 262 intervention group, n = 259 control group Number available for follow‐up: n = 250 intervention group (n = 12 withdrew, no reasons), n = 249 control group (n = 10 withdrew, no reasons) Mean age in years (SD): 64 (14) intervention group, 64 (10) control group Sex (% male): 78% intervention group, 75.1 control group |
|
| Interventions |
Intervention group: patients received daily personalised SMS reminding of aspirin intake, with different formulation every day. Text Type: personalised computer‐generated Control group: standard care |
|
| Outcomes |
Primary outcome: aspirin adherence, measured by self‐report (oral and paper questionnaire) and platelet function testing, good adherence was defined as more than 95% of prescribed doses in the past 30 days. Medication adherence: self‐report ‐ adherence (%): 96.4 (in text), 97.2 (in fig 2) in intervention group, 93.6 (in text), 92.8 (in table 2) in control group platelet testing ‐ adherence (%): 94.8 in intervention group, 88.8 in control group (OR for non‐adherence: 0.43, 95% CI 0.22 to 0.86) Adverse events: 20/41 non‐adherent participants stopped the medication "because of side effects, mainly bleeding". |
|
| Notes |
Funding: not reported Publication: Published letter to the editor, outcome data for self‐reported non‐adherence differs between text and Figure 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details reported but we made the assumption that given the nature of the intervention it was impossible to blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up similar in both groups |
| Selective reporting (reporting bias) | High risk | Minimal data, no trial protocol found |
| Other bias | High risk | Ooutcome data for self‐reported non‐adherence differ between text and Figure 2 |
ACE: angiotensin‐converting enzyme ACS: acute coronary syndrome ADP: Adenosine diphosphate ARBs: angiotensin receptor blockers BMI: body mass index CAD: coronary artery disease CHD: coronary heart disease CI: confidence interval CR: cardiac rehabilitation LDL: low‐density lipoprotein MI: myocardial infarction PCI: percutaneous coronary intervention RCT: randomised controlled trial RR: risk ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12611000388910 | Wrong outcomes |
| ACTRN12611001196932 | Wrong intervention |
| Anonymous 1998 | Wrong study design |
| Anonymous 2008 | Wrong intervention |
| Antypas 2014 | Wrong outcome |
| Bekelman 2013 | Wrong intervention |
| Bendelac 2014 | Wrong intervention |
| Blasco 2012 | Wrong outcome |
| Bobrow 2016 | Wrong patient population (primary prevention) |
| Boroumand 2016 | Wrong outcome |
| Bove 2010 | Wrong intervention |
| Brath 2013 | Wrong patient population |
| Buis 2015 | Wrong study design |
| Capomolla 2005 | wrong intervention |
| Dale 2015c | Wrong outcome |
| de Jongh 2012 | Systematic review |
| Ferrante 2010 | Wrong intervention |
| Frederix 2015 | Wrong outcome |
| Fruhwald 2009 | Wrong intervention |
| Gill 2013 | Wrong intervention |
| Goldstein 2014 | Intervention is an App, not an SMS |
| Golshahi 2015 | Wrong patient population (primary prevention) |
| Hickey 2016 | Wrong outcome |
| Karanam 2012 | Wrong intervention |
| Karhula 2015 | Wrong intervention |
| Kashem 2006 | Wrong intervention |
| Kashem 2008 | Wrong intervention |
| Kiselev 2011 | Wrong study design |
| Kulshreshtha 2010 | Wrong intervention |
| Lambert‐Kerzner 2012 | Wrong intervention |
| Lauffenburger 2016 | Not a RCT |
| Liew 2009 | Wrong outcome (non‐attendance) |
| Lounsbury 2015 | Wrong study design |
| Maddison 2015 | Wrong outcomes |
| Mortara 2006 | Wrong intervention |
| NCT01752192 | Wrong intervention |
| NCT02377960 | Patient population hypertensive patients |
| Owolabi 2014 | Wrong outcomes |
| Patel 2013 | Wrong patient population |
| Patnaik 2014 | Wrong patient population (primary prevention) |
| Petrella 2014 | Wrong intervention |
| Piette 2015 | Wrong patient population |
| Raiman 2013 | Less than 50% of patients were for secondary prevention. Contacted authors, but unable to provide data on subgroup |
| Sahu 2014a | Systematic review |
| Saywell 2012 | Wrong intervention |
| Scalvini 2004 | Wrong intervention |
| Schiff 2010 | Editorial |
| Seto 2011 | Wrong intervention |
| Seto 2012 | Wrong intervention |
| Snoek 2016 | Wrong intervention |
| Tulder 2014 | Wrong intervention |
| Varleta 2014 | Wrong patient population |
| Varnfield 2012 | Wrong intervention |
| Varnfield 2014 | Wrong outcomes |
| Vodopivec‐Jamsek 2012 | Systematic review |
| Walters 2012 | Wrong outcomes |
| Wolf 2016 | Wrong intervention |
| Yoo 2009 | Wrong patient population |
| Yu 2015 | Wrong intervention |
| Yudi 2016 | Wrong intervention |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000422426.
| Trial name or title | Text4Heart Partnership |
| Methods |
Design: Prospective, parallel RCT Setting: Auckland, New Zealand |
| Participants |
Expected: 330 Inclusion criteria: Aged 18‐80, A documented diagnosis of an acute coronary syndrome (including myocardial infarction [MI], unstable angina) or percutaneous coronary revascularisation procedure, are 18 years or older, eligible for cardiac rehabilitation Exclusion criteria: Untreated ventricular tachycardia, severe heart failure, life threatening co‐existing disease with life expectancy < 1 year, and significant exercise limitations other than cardiovascular disease. |
| Interventions |
Intervention:Each participant in the intervention group will receive at minimum the basic heart health CR program, consisting of 5 messages per week for 6 months. The general heart health messages provide overall advice and support on undertaking lifestyle change, including, taking medication, being physically active, eating healthy, and reducing alcohol consumption. One message per week on each topic is delivered for the entire six months. Control group: Participants in the control arm will be offered the standard outpatient CR programme provided by each hospital, which involves support and education provision to discharged patients, with supervised exercise offered at all three participating hospitals for those wishing to participate (Phase 2 CR usually of 6‐12 weeks duration). During Phase 3, participants are encouraged to continue with their lifestyle changes and join a cardiac club. Heart Guide Aotearoa is also offered at the discretion of cardiac nurses. Given the proven effectiveness of CR, it would be unethical not to offer usual CR to all participants; therefore the Intervention arm participants will be advised that they are able to access the usual CR programme in addition to the mHealth intervention, if they wish to do so. |
| Outcomes |
Primary outcome: Proportion of participants adhering to medication at 24 weeks. The medication adherence measure in this trial will be prescription record‐assessed adherence, defined as: a dispensed medication ratio of 80% for each of the classes of medications consistent with guideline recommended therapy (e.g., antiplatelet, statin, and blood pressure lowering therapy, ACE‐inhibitor and or a beta blocker). Secondary outcomes: Adherence to recommended lifestyle behaviours, self‐report medication adherence, patient engagement, cost‐effectiveness |
| Starting date | 18/04/2016 |
| Contact information | Ralph Madison National Institure for Health Innovation, The Unviersity of Auckland Private Bag 92019, Wellesley Street, Auckland, 1001 |
| Notes |
Chow 2015.
| Trial name or title | Tobacco, EXercise and dieT MEssages (TEXT ME): The effect of a semi‐personalised lifestyle reminder text message intervention on cardiovascular risk factors in patients with cardiovascular disease and those who are at high risk of cardiovascular disease. |
| Methods |
Design: prospective, parallel Setting: large tertiary referral centre and university teaching hospital in Sydney, Australia Follow‐up: six months |
| Participants |
Inclusion criteria: older than 18 years, had documented CHD, and were able to provide informed consent. CHD defined as documented prior MI, coronary artery bypass graft surgery, PCI or 50% or greater stenosis in at least 1 major epicardial vessel on coronary angiography Exclusion criteria: did not have an active mobile phone or sufficient English language proficiency to read text messages Randomized: 710 randomised, n = 352 intervention group, n = 358 control group Number available for follow‐up: n = 319 analysed in intervention group (n = 7 requested to stop, n = 4 died during intervention period, n = 2 unable to contact at 6 months follow‐up, n = 20 excluded due to missing LDL‐C measures at baseline, 6 months or both), n = 333 analysed in control group (n = 3 unable to contact at 6 months, n = 1 died prior to 6 months follow‐up, n = 21 excluded due to missing LDL‐C measures at baseline, 6 months or both). Mean age in years (SD): 59 (41–75) Sex (% male): |
| Interventions | Both groups received 3 study management text messages providing them with their allocation assignment, study contact details, and a reminder prior to the follow‐up appointment. Intervention group: Participants received, in addition to usual care, the 6‐month prevention program of approximately 96 messages.Four text messages per week about medicines, general health information, diet, physical activity and smoking cessation (where applicable). Text messages were semi‐personalised (addressed to participant's preferred name). Each message was sent on 4 to 5 randomly selected weekdays and arrived at random times of the day during working hours. The message management program selected messages for each participant at random from a bank of messages. Participants were told not to respond to messages. Control group: Control participants received usual care, which generally included community follow‐up with the majority referred to inpatient cardiac rehabilitation, as determined by their usual physicians. |
| Outcomes |
Primary outcome: low‐density lipoprotein cholesterol (LDL‐C) ‐ fasting blood sample, level at 6 months, analysed by local laboratories Secondary outcomes: systolic blood pressure (measured using electronic device), body mass index (BMI), physical activity (Global Physical Activity Questionnaire), smoking status, heart rate (measured using electronic device), waist circumference, proportions of patients taking |
| Starting date | |
| Contact information | A/Prof Clara Chow, cchow@georgeinstitute.org.au |
| Notes | As it was not clear from paper, we emailed authors who confirmed that all participants received texts about adherence to medications. The authors also stated that medication adherence data are planned to be published as a sub‐analysis. Trial registry number: ACTRN12611000161921 |
NCT01642355.
| Trial name or title | Investigation of Motivational interviewing and Prevention consults to Achieve Cardiovascular Targets (IMPACT) |
| Methods | Design: Randomised controlled trial |
| Participants |
Estimated enrolment: 400 Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Study conducted: USA No Intervention: Usual Care: includes physician assistant and/or nurse‐based medical and lifestyle recommendations in consultation with cardiac catheterisation attending or patient's clinical cardiologist to potentially improve the patient's medical and lifestyle regimen. Relevant educational material is routinely distributed to patients. Active Comparator: Prevention consult in addition to usual care, patients will receive a prevention consult by a prevention fellow and attending following their intervention. The consult will include guideline‐based medical recommendations for optimisation of the patient's medical regimen targeting dyslipidaemia, hypertension and diabetes. In addition, each patient will be educated on the cardiovascular disease process and given detailed lifestyle recommendations on physical activity, improved nutrition, smoking cessation and medication adherence. Active Comparator: Consult & Behavioural Intervention In addition to usual care and prevention consult (as detailed above), patients will receive a full motivational intervention program by a trained motivational coach and text messages over 6 months. |
| Outcomes |
Primary Outcome Measures: Reduction of Non‐HDL cholesterol [ Time Frame: 6 months ]
Secondary Outcome Measures: Lipids [ Time Frame: 6 months ] ‐ LDL‐C, HDL, triglycerides Metabolic risk factors [ Time Frame: 6 months ] ‐ weight, BMI, HbA1C, abdominal circumference Physical Activity [ Time Frame: 6 months ] ‐ Yale Physical Activity assessment (Part 2) Nutrition [ Time Frame: 6 months ] ‐ Northwest Lipid Research Clinic (NWLRC) Fat Intake Score and fruit and vegetable assessment questions Medication Adherence [ Time Frame: 6 months ] ‐ Morisky‐4 medication adherence survey Optimal medical regimen [ Time Frame: 6 months ] ‐ assessment of lipid‐lowering and cardiovascular medication regimen Quality of life [ Time Frame: 6 months ] ‐ Euro Qual 5D survey Smoking cessation [ Time Frame: 6 months ] ‐ Patient‐based Assessment and Counseling for Physical Activity and Nutrition (PACE) smoking assessment Cardiovascular risk [ Time Frame: 6 months ] ‐ cardiovascular risk assessment score (i.e. Framingham) Cardiovascular events [ Time Frame: 5 years ] ‐ cardiovascular events and hospitalisations reported by phone call follow‐up to 5 years |
| Starting date | June 2012 |
| Contact information | Ramsha Jabbar, Ramsha.Jabbar@nyumc.org Eugenia Gianos, eugenia.gianos@nyumc.org |
| Notes | This study has been completed but no results have been identified with the search. |
NCT02336919.
| Trial name or title | The use of texting messaging to improve the hospital‐community transition and prevent readmission in patients with cardiovascular disease (Txt2Prevent) |
| Methods | Randomised efficacy study with parallel assignment Masking: Single‐blind (Investigator) Study location: Canada |
| Participants |
Estimated enrolment: 75 Inclusion criteria:
|
| Interventions | In addition to usual discharge treatment, the intervention arm will receive instructions and information for acute coronary syndrome patients as well as the Txt2Prevent text‐messaging program. The program will include a variety of topics such as standard follow‐up care reminders as well as general self‐management and healthy living texts. There will be two streams, one for current/recent smokers and one for non‐smokers. Texts will be sent out every 1‐3 days for 60 days. All participants in the same stream will receive the same texts in the same order. |
| Outcomes | Primary Outcome: Self‐management Secondary Outcomes: Medication adherence, health‐related quality of life, hospital readmissions, mortality |
| Starting date | September 2015 |
| Contact information | Contact: Scott Lear 604‐682‐2344 ext 62778 SLear@providencehealth.bc.ca Contact: Emily Ross, 604‐682‐2344 ext 64874 SLear@providencehealth.bc.ca |
| Notes |
NCT02354040.
| Trial name or title | TalkingRx |
| Methods | Randomised parallel arm study |
| Participants |
Estimated enrolment: 200 Inclusion criteria:
|
| Interventions | Study conducted in Pakistan. Assigned to receive Health Literacy and Reminder Updates via the IT‐based intervention Talking Rx, in addition to Usual Care for patients in the intervention group. The physician‐written prescription for antiplatelets and statins will be transferred on an OMR sheet and will be scanned. The information on the prescription (dose, name of the medication, duration, route or any other special instruction) will be sent to the patients via a text and a voice SMS (in Urdu language). The patients also receive an individualised code that helps them request for repeated reminders for their medication timings. However, a weekly medication reminder SMS will be sent to the patients in the intervention arm. |
| Outcomes |
Primary outcome: Medication adherence Secondary outcome: Health literacy |
| Starting date | March 2015 |
| Contact information | Dr. Ayeesha Kamran Kamal, Aga Khan University, Karachi, Pakistan |
| Notes | The study has been completed but no study results were available at the time of search. |
NCT02783287.
| Trial name or title | The impact of text messaging on medication adherence and exercise regimen among post‐myocardial infarction patients |
| Methods |
Design: Parallel randomised control trial Setting: Cambridge Cardiac Rehabilitation in Ontario, Canada |
| Participants | 84 participants aged 18 years and older Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | 2 trials: medication adherence and exercise adherence Intervention Text message reminder for medication adherence Patients randomised to this arm receive one text message per day (at the scheduled time) reminding them to take their medication. control: Usual care for medication adherence. |
| Outcomes | Primary Outcomes: Medication adherence (Time Frame: 12 months), Exercise frequency (Time Frame: 12 months) Secondary Outcomes: Exercise duration (Time Frame: 12 months), Full medication adherence (Time Frame: 12 months) |
| Starting date | January 2012 |
| Contact information | Niteesh K Choudhry, MD, PhD Brigham and Women's hospital |
| Notes | This study has been completed but no results were available at the time of search. This study was sponsored by Brigham and Women's Hospital, Boston, Massachusetts. |
NCT02883842.
| Trial name or title | China PEACE 3: Cardiovascular Health And Texting‐Diabetes Mellitus (CHAT‐DM) Study |
| Methods |
Design: Parallel RCT Setting: Fuwai Hospital Beijing, China |
| Participants | Patients 19‐90 years Inclusion Criteria: Participants with coronary artery disease defined as history of myocardial infarction and percutaneous coronary intervention (PCI), history of diabetes, capability to read and send text messages Exclusion Criteria: assumed poor adherence, do not have an active mobile phone |
| Interventions |
Intervention: Patients will receive regular semi‐personalised text messages for 12 months. Each participants will receive 6 text messages per week, which will be sent at random times of the day (9.00 am, 12 noon, 4.00 pm). They will receive one general messages, one hypertension message, one glucose control message, one lifestyle message, one medication adherence message and one physical activity message per week. Control: Participants in the control group will receive 2 thank‐you messages per month and undertake routine clinical practice. |
| Outcomes | Primary Outcomes: Change in glucose level measured by HbA1C level (Time Frame: 6 months; 12 months ) Secondary Outcomes: Change in BMI according to national standards (Time Frame: 6 months; 12 months), Change in systolic blood pressure obtained in office during each interview (Time Frame: 6 months; 12 months), Change in level of physical activity measured via International Physical Activity Questionnaire (IPAQ) scale (Time Frame: 6 months; 12 months ), Change in medication adherence measured via Morisky scale (Time Frame: 6 months; 12 months) |
| Starting date | August 2016 |
| Contact information | Xin Zheng, MD, PhD xin.zheng@fwoxford.org |
| Notes | Sponsor: China National Center for Cardiovascular Diseases |
NCT02888769.
| Trial name or title | Cardiovascular Health And Texting (CHAT) Study |
| Methods | Design: Parallel RCT |
| Participants | Patients 19‐90 years Inclusion Criteria: Participants with CAD defined as history of MI and percutaneous coronary intervention (PCI), capability to read and send text messages Exclusion Criteria: History of diabetes, assumed poor adherence, do not have an active mobile phone |
| Interventions |
Intervention: Patients will receive regular semi‐personalised text messages for 12 months. Each participants will receive 6 text messages per week, which will be sent at random times of the day (9.00am, 12noon, 4.00pm). Non‐smokers will receive two general messages, two hypertension messages, one medication adherence message and one physical activity message per week. Smokers will receive one general message, two hypertension messages, one medication adherence message, one physical activity message and one smoking cessation message per week. Control: Participants in the control group will receive 2 thank‐you messages per month and undertake routine clinical practice. |
| Outcomes | Primary Outcome: Change in systolic blood pressure obtained in office during each interview (Time Frame: Baseline; 6months; 12 months) Secondary Outcomes: Change in level of physical activity measured via International Physical Activity Questionnaire (IPAQ) scale (Time Frame: Baseline; 6months; 12 months), Change in medication adherence measured via Morisky scale (Time Frame: Baseline; 6months; 12 months), Change in proportion of non‐smokers (Time Frame: Baseline; 6months; 12 months) |
| Starting date | August 2016 |
| Contact information | Xin Zheng, MD, PhD xin.zheng@fwoxford.org |
| Notes | Sponsor: China National Center for Cardiovascular Diseases |
ACE: angiotensin‐converting enzyme ARBs: angiotensin receptor blockers BMI: body mass index CAD: coronary artery disease CHD: coronary heart disease CR: cardiac rehabilitation HDL‐C: high‐density lipoprotein LDL‐C: low‐density lipoprotein cholesterol MI: myocardial infarctionPCI: percutaneous coronary intervention RCT: randomised controlled trial
Differences between protocol and review
Due to the heterogeneity between the studies with respect to participants, methods and outcome measures, we did not pool the results in a meta‐analysis but describe the results in narrative form.
Contributions of authors
The first (AJA) and second (NM) author contributed equally to the review.
AJA: screening, data extraction, writing of review, 'Risk of bias' assessment. NM: searches, screening, data extraction, 'Risk of bias' assessment, 'Summary of findings' table with GRADE, writing of review. JM: 'Rrisk of bias' tables. CT: 'Risk of bias' tables. OO: Screening, data extraction. CF: Reviewed manuscript, advised on text messaging interventions NS: screening, extraction and consulting on design and methods. JPC: arbitration of disagreement, consulting on design and methods. PP: proposal, writing, arbitration of disagreement, consulting on design and methods.
Sources of support
Internal sources
No sources of support supplied
External sources
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
AJA: nothing to declare. NM: nothing to declare. JM: nothing to declare. CT: has collaborated with Bristol Myers Squibb and Pfizer to provide expert testimony and lectures. These have been unrelated to the subject matter of this review. OO: nothing to declare. CF: I am a co‐applicant on a global health MRC UK grant and have worked on the development of interventions for Colombia and the UK on the topic of SMS as intervention to improve adherence to medications in secondary prevention of cardiovascular disease. NS: nothing to declare. JPC: I am a co‐applicant in two grants (one from UK and one from Colombia) in the topic of SMS as intervention to improve adherence to medications in secondary prevention of cardiovascular disease. PP: I am the principal investigator for a study developing and piloting an mHealth intervention to increase adherence for cardiovascular secondary prevention interventions.
New
References
References to studies included in this review
Dale 2015a {published data only}
- ACTRN12613000901707. Text4Heart: A text message and internet‐based comprehensive cardiac rehabilitation intervention. http://www.anzctr.org.au/ACTRN12613000901707.aspx (accessed 20 January 2016).
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self‐management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials [Electronic Resource] 2014;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self‐management: results from the Text4Heart randomized controlled trial. Journal of Medical Internet Research 2015;17(10):e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fang 2016 {published data only}
- Fang R, Li X. Electronic messaging support service programs improve adherence to lipid‐lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. Journal of Clinical Nursing 2016;25(5‐6):664‐71. [DOI] [PubMed] [Google Scholar]
Kamal 2015 {published data only}
- Kamal AK, Shaik QN, Pasha O, Azam I, Islam M, Memon AA, et al. Improving medication adherence in stroke patients through Short Text Messages (SMS4Stroke)‐study protocol for a randomized, controlled trial. BMC Neurology 2015;15:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AK, Shaikh Q, Pasha O, Azam I, Islam M, Memon AA, et al. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored short messaging service (SMS)‐SMS4Stroke study. BMC Neurolology 2015;15:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khonsari 2015 {published data only}
- Khonsari S, Subramanian P, Chinna K, Latif LA, Ling L W, Gholami O. Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. European Journal of Cardiovascular Nursing 2015;14(2):170‐9. [DOI] [PubMed] [Google Scholar]
Pandey 2014 {published data only}
- Pandey A, Choudhry N. An m‐health application of daily text messages to address forgetfulness as contributor to non‐adherence to medications post‐myocardial infarction. Circulation Conference: American Heart Association 2015;132:A18641. [Google Scholar]
- Pandey AK, Choudhry N. Text message reminders to address medication non‐adherence in post‐MI patients: A one year intervention study. Canadian Journal of Cardiology 2014;1:S179. [Google Scholar]
Park 2014a {published data only}
- Park LG, Howie‐Esquivel J, Chung M, Dracup K. A text messaging intervention improves medication adherence for patients with coronary heart disease: A randomized controlled trial. Circulation 2013;128:A15249. [DOI] [PubMed] [Google Scholar]
- Park LG, Howie‐Esquivel J, Chung ML, Dracup K. A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Education & Counseling 2014;94(2):261‐8. [DOI] [PubMed] [Google Scholar]
- Park LG, Howie‐Esquivel J, Whooley MA, Dracup K. Psychosocial factors and medication adherence among patients with coronary heart disease: A text messaging intervention. European Journal of Cardiovascular Nursing 2015;14(3):264‐73. [DOI] [PubMed] [Google Scholar]
Quilici 2013 {published data only}
- Quilici J, Fugon L, Beguin S, Morange PE, Bonnet JL, Alessi MC, et al. Effect of motivational mobile phone short message service on aspirin adherence after coronary stenting for acute coronary syndrome. International Journal of Cardiology 2013;168(1):568‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12611000388910 {published data only}
- ACTRN12611000388910. The utility of mobile phone in the prevention of coronary heart disease, through lifestyle modification: a prospective, randomised, controlled clinical trial. http://www.anzctr.org.au/ACTRN12611000388910.aspx (20 January 2016).
ACTRN12611001196932 {published data only}
- ACTRN12611001196932. Using telephone and text‐messaging to improve an integrated cardiac and diabetes self‐management program: A cross cultural international collaborative project. http://www.anzctr.org.au/ACTRN12611001196932.aspx (accessed 20 January 2016).
Anonymous 1998 {published data only}
- Anonymous. Telemedicine device encourages patients to take medications. Telemedicine & Virtual Reality 1998;3(9):103. [PubMed] [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Program brings hypertensive patients within BP ranges. Disease Management Advisor 2008;14(1):11‐2, 1. [PubMed] [Google Scholar]
Antypas 2014 {published data only}
- Antypas K, Wangberg SC. An Internet‐ and mobile‐based tailored intervention to enhance maintenance of physical activity after cardiac rehabilitation: short‐term results of a randomized controlled trial. Journal of Medical Internet Research 2016;16:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bekelman 2013 {published data only}
- Bekelman DB, Plomondon ME, Sullivan MD, Nelson K, Hattler B, McBryde C, et al. Patient‐centered disease management (PCDM) for heart failure: Study protocol for a randomised controlled trial. BMC Cardiovascular Disorders 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendelac 2014 {published data only}
- Bendelac H, Pathak A, Molinier L, Ruidavets JB, Mayere A, Berry M, et al. Optimization of ambulatory monitoring of patients with heart failure using telecardiology (OSICAT). European Research in Telemedicine 2014; Vol. 3, issue 4:161‐7.
Blasco 2012 {published data only}
- Blasco A, Carmona M, Fernández‐Lozano I, Salvador CH, Pascual M, Sagredo PG, et al. Evaluation of a telemedicine service for the secondary prevention of coronary artery disease. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(1):25‐31. [DOI] [PubMed] [Google Scholar]
Bobrow 2016 {published data only}
- Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu LM, Brannan T, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS‐Text Adherence Support [StAR]): a single‐blind, randomized trial. Circulation 2016;133(3):592‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boroumand 2016 {published data only}
- Boroumand S, Moeini M. The effect of a text message and telephone follow‐up program on cardiac self‐efficacy of patients with coronary artery disease: A randomized controlled trial. Iranian Journal of Nursing and Midwifery Research 2016;21(2):171‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bove 2010 {published data only}
- Bove AA. Using modern communications to manage chronic heart disease. American Heart Hospital Journal 2010;8(1):25‐8. [DOI] [PubMed] [Google Scholar]
Brath 2013 {published data only}
- Brath H, Morak J, Kastenbauer T, Modre‐Osprian R, Strohner‐Kastenbauer H, Schwarz M, et al. Mobile health (mHealth) based medication adherence measurement ‐ a pilot trial using electronic blisters in diabetes patients. British Journal of Clinical Pharmacology 2013;76 Suppl 1:47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buis 2015 {published data only}
- Buis LR, Artinian NT, Schwiebert L, Yarandi H, Levy PD. Text messaging to improve hypertension medication adherence in African Americans: BPMED intervention development and study protocol. JMIR Research Protocols 2015;4(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01465217. Text messaging to improve hypertension medication adherence in African Americans. https://ClinicalTrials.gov/show/NCT01465217 (accessed 20 January 2016).
Capomolla 2005 {published data only}
- Capomolla S, Pinna G, Maestri R, Ferrari M, Ceresa M. The telemonitoring service [Italian] [Il servizio di telemonitoraggoio]. Monaldi Archives for Chest Disease [Archivio Monaldi per le Malattie del Torace] 2005;64:135‐6. [PubMed] [Google Scholar]
Dale 2015c {published data only}
- Dale L, Whittaker R, Dixon R, Stewart R, Jiang Y, Carter K, et al. Acceptability of a mobile health exercise‐based cardiac rehabilitation intervention: a randomized trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2015;35(5):312‐9. [DOI] [PubMed] [Google Scholar]
de Jongh 2012 {published data only}
- Jongh T, Gurol‐Urganci I, Vodopivec‐Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self‐management of long‐term illnesses. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007459.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrante 2010 {published data only}
- Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, et al. Long‐term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow‐up. Journal of the American College of Cardiology 2010;56(5):372‐8. [DOI] [PubMed] [Google Scholar]
Frederix 2015 {published data only}
- Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Medium‐term effectiveness of a comprehensive internet‐based and patient‐specific telerehabilitation program with text messaging support for cardiac patients: randomized control trial. Journal of Medical Internet Research 2015;17(7):e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fruhwald 2009 {published data only}
- Fruhwald FM, Scherr D, Kastner P, Kollmann A, Schreier G. Telemonitoring using mobile phones reduces the event rate after recent acute heart failure. Results of the MOBIle TELemonitoring in heart failure patients study (MOBITEL). European Heart Journal 2009;30:911‐2. [Google Scholar]
Gill 2013 {published data only}
- Gill DP, Stuckey MI, Shapiro S, Sabourin KJ, Hubbard RA, Petrella RJ. A randomized clinical trial of mhealth supported exercise intervention in patients with metabolic syndrome. Journal of Diabetes 2013;5:80. [Google Scholar]
Goldstein 2014 {published data only}
- Goldstein CM, Gathright EC, Dolansky MA, Gunstad J, Sterns A, Redle JD, et al. Randomized controlled feasibility trial of two telemedicine medication reminder systems for older adults with heart failure. Journal of Telemedicine & Telecare 2014;20(6):293‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golshahi 2015 {published data only}
- Golshahi J, Ahmadzadeh H, Sadeghi M, Mohammadifard N, Pourmoghaddas A. Effect of self‐care education on lifestyle modification, medication adherence and blood pressure in hypertensive adults: randomized controlled clinical trial. Advanced Biomedical Research 2015;4:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hickey 2016 {published data only}
- Hickey KT, Hauser NR, Valente LE, Riga TC, Frulla AP, Masterson Creber R, et al. A single‐center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovascular Disorders 2016;16:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karanam 2012 {published data only}
- Karanam C, Dayanand S, Dang S, Cobian S, Gomez‐Marin O, Mallon S, et al. Outcomes from a mobile‐phone study for heart failure in an ethnically diverse County Hospital. Journal of the American Geriatrics Society 2012;60:S221. [Google Scholar]
Karhula 2015 {published data only}
- Karhula T, Vuorinen AL, Raapysjarvi K, Pakanen M, Itkonen P, Tepponen M, et al. Telemonitoring and mobile phone‐based health coaching among Finnish diabetic and heart disease patients: randomized controlled trial. Journal of Medical Internet Research 2015;17(6):e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kashem 2006 {published data only}
- Kashem A, Droogan MT, Santamore WP, Wald JW, Marble JF, Cross RC, et al. Web‐based internet telemedicine management of patients with heart failure. Telemedicine Journal & E‐Health 2006;12(4):439‐47. [DOI] [PubMed] [Google Scholar]
Kashem 2008 {published data only}
- Kashem A, Droogan MT, Santamore WP, Wald JW, Bove AA. Managing heart failure care using an internet‐based telemedicine system. Journal of Cardiac Failure 2008;14(2):121‐6. [DOI] [PubMed] [Google Scholar]
Kiselev 2011 {published data only}
- Kiselev AR, Shvarts VA, Posnenkova OM, Gridnev VI, Dovgalevskii PIa, Oshchepkova EV, et al. [Outpatient prophylaxis and treatment of arterial hypertension with application of mobile telephone systems and Internet techniques] [Article in Russian]. Terapevticheskii Arkhiv 2011;83(4):46‐52. [PubMed] [Google Scholar]
Kulshreshtha 2010 {published data only}
- Kulshreshtha A, Kvedar JC, Goyal A, Halpern EF, Watson AJ. Use of remote monitoring to improve outcomes in patients with heart failure: A pilot trial. International Journal of Telemedicine and Applications 2010;2010:870959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert‐Kerzner 2012 {published data only}
- Lambert‐Kerzner A, Giacco EJ, Fahdi IE, Bryson CL, Melnyk SD, Bosworth HB, et al. Patient‐centered adherence intervention after acute coronary syndrome hospitalization. Circulation. Cardiovascular Quality & Outcomes 2012;5(4):571‐6. [DOI] [PubMed] [Google Scholar]
Lauffenburger 2016 {published data only}
- Lauffenburger JC, Choudhry NK. Text messaging and patient engagement in an increasingly mobile world. Circulation 2016;133(6):555‐6. [DOI] [PubMed] [Google Scholar]
Liew 2009 {published data only}
- Liew SM, Tong SF, Lee VKM, Ng CJ, Leong KC, Teng CL. Text messaging reminders to reduce non‐attendance in chronic disease follow‐up: A clinical trial. British Journal of General Practice 2009;59(569):916‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lounsbury 2015 {published data only}
- Lounsbury P, Elokda AS, Gylten D, Arena R, Clarke W, Gordon EEI. Text‐messaging program improves outcomes in outpatient cardiovascular rehabilitation. IJC Heart and Vasculature 2015;7:170‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddison 2015 {published data only}
- Maddison R, Pfaeffli L, Whittaker R, Stewart R, Kerr A, Jiang Y, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. European Journal of Preventive Cardiology 2015;22(6):701‐9. [DOI] [PubMed] [Google Scholar]
Mortara 2006 {published data only}
- Mortara A, Pinna GD, Capomolla S, Johnson P, Rovere MT, Ponikowski P, et al. A new telemonitoring system in heart failure. Preliminary data from the multi‐country randomized study HHH (Home or Hospital in Heart failure). European Heart Journal 2006;27:345. [Google Scholar]
NCT01752192 {published data only}
- NCT01752192. Teledi@Log ‐ Tele‐rehabilitation of heart patients. https://ClinicalTrials.gov/show/NCT01752192 (accessed 20 January 2016).
NCT02377960 {published data only}
- NCT02377960. Check and support ‐ enhancing the treatment of hypertension in outpatient care, a multicenter study. https://ClinicalTrials.gov/show/NCT02377960 (accessed 20 January 2016).
Owolabi 2014 {published data only}
- Owolabi MO, Akinyemi RO, Gebregziabher M, Olaniyan O, Salako BL, Arulogun O, et al. Randomized controlled trial of a multipronged intervention to improve blood pressure control among stroke survivors in Nigeria. International Journal of Stroke 2014;9(8):1109‐16. [DOI] [PubMed] [Google Scholar]
Patel 2013 {published data only}
- Patel S, Jacobus‐Kantor L, Marshall L, Ritchie C, Kaplinski M, Khurana PS, et al. Mobilizing your medications: an automated medication reminder application for mobile phones and hypertension medication adherence in a high‐risk urban population. Journal of Diabetes Science & Technology 2013;7(3):630‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patnaik 2014 {published data only}
- Patnaik L, Joshi A, Sahu T. Mobile based intervention for reduction of coronary heart disease risk factors among patients with diabetes mellitus attending a tertiary care hospital of India. Journal of Cardiovascular Disease Research 2014;5(4):28‐36. [Google Scholar]
Petrella 2014 {published data only}
- Petrella RJ, Stuckey MI, Shapiro S, Gill DP. Mobile health, exercise and metabolic risk: a randomized controlled trial. BMC Public Health 2014;14:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piette 2015 {published data only}
- Piette JD, Striplin D, Marinec N, Chen J, Trivedi RB, Aron DC, et al. A mobile health intervention supporting heart failure patients and their informal caregivers: a randomized comparative effectiveness trial. Journal of Medical Internet Research 2015;17(6):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raiman 2013 {published data only}
- Raiman L, Wald D, Bestwick J. Interact investigation of text message reminders on adherence to cardiac treatment‐pilot study. Circulation 2013;127(12):Meeting Abstracts. [Google Scholar]
- Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ. Randomised trial of text messaging on adherence to cardiovascular preventive treatment (INTERACT trial). PLoS ONE 2014;9:e114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahu 2014a {published data only}
- Sahu M, Grover A, Joshi A. Role of mobile phone technology in health education in Asian and African countries: a systematic review. International Journal of Electronic Healthcare 2014;7(4):269‐86. [DOI] [PubMed] [Google Scholar]
Saywell 2012 {published data only}
- Saywell N, Vandal AC, Brown P, Hanger HC, Hale L, Mudge S, et al. Telerehabilitation to improve outcomes for people with stroke: study protocol for a randomised controlled trial. Trials [Electronic Resource] 2012;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scalvini 2004 {published data only}
Schiff 2010 {published data only}
- Schiff A. Telemonitoring‐‐or better follow‐up?. Lancet 2010;376(9754):1737; author reply 1737‐8. [DOI] [PubMed] [Google Scholar]
Seto 2011 {published data only}
- Seto E, Leonard KJ, Cafazzo JA, Masino C, Barnsley J, Ross HJ. Mobile phone‐based remote patient monitoring improves heart failure management and outcomes: A randomized controlled trial. Journal of the American College of Cardiology 2011;1:E1260. [Google Scholar]
Seto 2012 {published data only}
- Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone‐based telemonitoring for heart failure management: a randomized controlled trial. Journal of Medical Internet Research 2012;14(1):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snoek 2016 {published data only}
- Snoek JA, Meindersma EP, Prins LF, Van't Hof AW, Hopman MT, Boer MJ, et al. Rationale and design of a randomised clinical trial for an extended cardiac rehabilitation programme using telemonitoring: the TeleCaRe study. BMC Cardiovascular Disorders 2016;16(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulder 2014 {published data only}
Varleta 2014 {published data only}
- Varleta P, Akel C, Acevedo M, Salinas C, Pino J, Garcia A, et al. Mobile phone text messaging improves antihypertensive drug adherence in the community. Circulation 2014;130:A18144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varnfield 2012 {published data only}
- Varnfield M, Karunanithi M, Ding H, Honeyman E, Arnold D, Keightley A, et al. Technology based home‐care model improves outcomes of uptake, adherence and health in cardiac rehabilitation. European Heart Journal 2012;33:445. [Google Scholar]
Varnfield 2014 {published data only}
- Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H. Smartphone‐based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100:1770‐9. [DOI] [PubMed] [Google Scholar]
Vodopivec‐Jamsek 2012 {published data only}
- Vodopivec‐Jamsek V, Jongh T, Gurol‐Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007457.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2012 {published data only}
- Walters D, Varnfield M, Karunanithi M, Ding H, Honeyman E, Arnold D, et al. Technology based home‐care model improves outcomes of uptake, adherence and health in cardiac rehabilitation. Heart Lung and Circulation 2012;21:S315. [Google Scholar]
Wolf 2016 {published data only}
- Wolf A, Fors A, Ulin K, Thorn J, Swedberg K, Ekman I. An ehealth diary and symptom‐tracking tool combined with person‐centered care for improving self‐efficacy after a diagnosis of acute coronary syndrome: a substudy of a randomized controlled trial. Journal of Medical Internet Research 2016;18(2):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoo 2009 {published data only}
- Yoo HJ, Kim SM, Baik SH, Choi DS, Choi KM. A ubiquitous chronic disease care system using cellular phones and the internet. Journal of Diabetes 2009;1:A198. [DOI] [PubMed] [Google Scholar]
Yu 2015 {published data only}
- Yu M, Chair SY, Chan CW, Choi KC. A health education booklet and telephone follow‐ups can improve medication adherence, health‐related quality of life, and psychological status of patients with heart failure. Heart & Lung 2015;44(5):400‐7. [DOI] [PubMed] [Google Scholar]
Yudi 2016 {published data only}
- Yudi MB, Clark DJ, Tsang D, Jelinek M, Kalten K, Joshi S, et al. SMARTphone‐based, early cardiac REHABilitation in patients with acute coronary syndromes [SMART‐REHAB Trial]: a randomized controlled trial protocol. BMC Cardiovascular Disorders 2016;60(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000422426 {published data only}
- ACTRN12616000422426. Text4Heart Partnership: a text messaging program to enhance self‐management of cardiovascular disease [Text messaging to enhance self‐management of cardiovascular disease]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370398 (first received 23 March 2016).
Chow 2015 {published data only}
- ACTRN12611000161921. Tobacco, EXercise and dieT MEssages (TEXT ME): The effect of semi‐personalised lifestyle reminder text message intervention on cardiovascular disease risk. http://www.anzctr.org.au/ACTRN12611000161921.aspx (accessed 20 January 2016).
- Anonymous. Erratum: Effect of lifestyle‐focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial (JAMA (2015) 314:12 (1255‐63)). JAMA 2016;315(10):1057. [DOI] [PubMed] [Google Scholar]
- Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle‐focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314(12):1255‐63. [DOI] [PubMed] [Google Scholar]
- Chow CK, Redfern J, Thiagalingam A, Jan S, Whittaker R, Hackett M, et al. Design and rationale of the tobacco, exercise and diet messages (TEXT ME) trial of a text message‐based intervention for ongoing prevention of cardiovascular disease in people with coronary disease: a randomised controlled trial protocol. BMJ Open 2012;2:e000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern J, Santo K, Coorey G, Thakkar J, Hackett M, Thiagalingam A, et al. Factors influencing engagement, perceived usefulness and behavioral mechanisms associated with a text message support program. PLOS One 2016;11(10):e0163929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar J, Redfern J, Keizer L, Thiagalingam A, Chow C. Patient perception of text message‐based intervention for secondary prevention of cardiovascular events. Heart Lung and Circulation 2013;22:S264. [Google Scholar]
- Thakkar J, Redfern J, Thiagalingam A, Chow CK. Patterns, predictors and effects of texting intervention on physical activity in CHD ‐ insights from the TEXT ME randomized clinical trial. European Journal of Preventive Cardiology 2016;23(17):1894‐902. [DOI] [PubMed] [Google Scholar]
NCT01642355 {published data only}
- Gianos E, Schoenthaler A, Mushailov M, Fisher EA, Berger JS. Rationale and design of the Investigation of Motivational interviewing and Prevention consults to Achieve Cardiovascular Targets (IMPACT) trial. American Heart Journal 2015;170(3):430‐7. [DOI] [PubMed] [Google Scholar]
- NCT01642355. Prevention trial to achieve cardiovascular targets. https://ClinicalTrials.gov/show/NCT01642355.
NCT02336919 {published data only}
- NCT02336919. The use of texting messaging to improve the hospital‐to‐community transition period in cardiovascular disease patients. http://clinicaltrials.gov/show/NCT02336919 (accessed 20 January 2016).
NCT02354040 {published data only}
- Kamal AK, Muqeet A, Farhat K, Khalid W, Jamil A, Gowani A, et al. Using a tailored health information technology‐ driven intervention to improve health literacy and medication adherence in a Pakistani population with vascular disease (Talking Rx): study protocol for a randomized controlled trial. Trials 2016;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02354040. Using a tailored health information technology driven intervention to improve health literacy and medication adherence. https://ClinicalTrials.gov/show/NCT02354040 (first received 29 January 2015).
NCT02783287 {published data only}
- NCT02783287. The impact of text messaging on medication adherence and exercise regimen among post‐myocardial infarction patients. https://clinicaltrials.gov/show/NCT02783287 (first received 7 January 2016).
NCT02883842 {published data only}
- NCT02883842. China PEACE 3: Cardiovascular Health And Texting‐Diabetes Mellitus (CHAT‐DM) Study [Effect of text messaging on risk factors control and medication adherence among patients with coronary heart disease and diabetes in China: Cardiovascular Health And Texting‐Diabetes Mellitus (CHAT‐DM) Trial]. https://clinicaltrials.gov/show/NCT02883842 (first received 24 August 2016).
NCT02888769 {published data only}
- NCT02888769. China PEACE 3: Cardiovascular Health And Texting (CHAT) Study [Effect of text messaging on risk factors control and medication adherence among coronary heart disease patients without diabetes in China: Cardiovascular Health And Texting (CHAT) Trial]. https://clinicaltrials.gov/show/NCT02888769 (first received 23 August 2016).
Additional references
Abroms 2015
- Abroms LC, Whittaker R, Free C, Mendel Van Alstyne J, Schindler‐Ruwisch JM. Developing and pretesting a text messaging program for health behavior change: recommended steps. JMIR mHealth uHealth 2015;3(4):e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Ganmi 2016
- Al‐Ganmi AH, Perry L, Gholizadeh L, Alotaibi AM. Cardiovascular medication adherence among patients with cardiac disease: a systematic review. Journal of Advanced Nursing 2016;5:5. [DOI] [PubMed] [Google Scholar]
Anglada‐Martinez 2015
- Anglada‐Martinez H, Riu‐Viladoms G, Martin‐Conde M, Rovira‐Illamola M, Sotoca‐Momblona JM, Codina‐Jane C. Does mHealth increase adherence to medication? Results of a systematic review. International Journal of Clinical Practice 2015;69(1):9‐32. [DOI] [PubMed] [Google Scholar]
Bandura 1997
- Bandura A. Self‐efficacy: The Exercise of Control. New York: W.H. Freeman and Company, 1997. [Google Scholar]
Chambless 1997
- Chambless L, Keil U, Dobson A, Mähönen M, Kuulasmaa K, Rajakangas AM, et al. Population versus clinical view of case fatality from acute coronary heart disease: results from the WHO MONICA Project 1985‐1990. Multinational MONitoring of Trends and Determinants in Cardiovascular Disease. Circulation 1997;96(11):3849‐59. [DOI] [PubMed] [Google Scholar]
Chow 2016
- Chow CK, Islam SM, Farmer A, Bobrow K, Maddision R, Whittaker R, et al. Text2PreventCVD: protocol for a systematic review and individual participant data meta‐analysis of text message‐based interventions for the prevention of cardiovascular diseases. BMJ Open 2016;6(10):e012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdhury 2013
- Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. European Heart Journal 2013;34(38):2940‐8. [DOI] [PubMed] [Google Scholar]
Dale 2014a
- Dale LP, Whittaker R, Eyles H, Ni Mhurchu C, Ball K, Smith N, et al. Cardiovascular disease self‐management: pilot testing of an mHealth healthy eating program. Journal of Personalized Medicine 2014;4(1):88‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2014b
- Dale LP, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Improving coronary heart disease self‐management using mobile technologies (Text4Heart): a randomised controlled trial protocol. Trials [Electronic Resource] 2014;15:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Devi 2015
- Devi BR, Syed‐Abdul S, Kumar A, Iqbal U, Nguyen PA, Li YC, et al. mHealth: An updated systematic review with a focus on HIV/AIDS and tuberculosis long term management using mobile phones. Comput Methods Programs Biomed 2015;122:257‐65. [DOI] [PubMed] [Google Scholar]
Douglas 2013
- Douglas N, Free C. 'Someone batting in my corner': Experiences of smoking‐cessation support via text message. British Journal of General Practice 2013;63(616):e768‐e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebbinghaus 1885
- Ebbinghaus H. Über das Gedächtnis. Leipzig: Dunker, 1885. [Google Scholar]
Ershad Sarabi 2016
- Ershad Sarabi R, Sadoughi F, Jamshidi Orak R, Bahaadinbeigy K. The effectiveness of mobile phone text messaging in improving medication adherence for patients with chronic diseases: a systematic review. Iranian Red Crescent Medical Journal 2016;18(5):e25183. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESC 2012
- European Society of Cardiology. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Heart Journal 2012;33:1635‐701. [DOI] [PubMed] [Google Scholar]
Free 2013
- Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile‐health technology‐based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Medicine 2013;10(1):e1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadkari 2012
- Gadkari AS, McHorney CA. Unintentional non‐adherence to chronic prescription medications: how unintentional is it really?. BMC Health Services Research 2012;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamine 2015
- Hamine S, Gerth‐Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. Journal of Medical Internet Research 2015;17(2):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICT 2014
- ICT. Key ICT indicators for developed and developing countries and the world. http://www.itu.int/en/ITU‐D/Statistics/Pages/stat/default.aspx (accessed 4 February 2015).
Kerr 2009
- Kerr AJ, Broad J, Wells S, Riddell T, Jackson R. Should the first priority in cardiovascular risk management be those with prior cardiovascular disease?. Heart 2009;95(2):125‐9. [DOI] [PubMed] [Google Scholar]
Kotseva 2010
- Kotseva K, Wood D, Backer G, Bacquer D, Pyörälä K, EUROASPIRE Study Group. EUROASPIRE III. Management of cardiovascular risk factors in asymptomatic high‐risk patients in general practice: cross‐sectional survey in 12 European countries. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17(5):530‐40. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lozano 2012
- Lozano R, Naghavi M, Foreman KT, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Misono 2010
- Misono AS, Cutrona SL, Choudhry NK, Fischer MA, Stedman MR, Liberman JN, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. American Journal of Managed Care 2010;16:SP82‐92. [PubMed] [Google Scholar]
Morisky 1986
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Medical Care 1986;24:67‐74. [DOI] [PubMed] [Google Scholar]
Nieuwlaat 2013
- Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease?. European Heart Journal 2013;34(17):1262‐9. [DOI] [PubMed] [Google Scholar]
Pandey 2015
- Pandey A, Choudhry N. Arest MI: Adherence effects of a comprehensive reminder system for post‐myocardial infarction secondary prevention. Journal of the American College of Cardiology 2015;1:A1384. [Google Scholar]
Park 2014b
- Park LG, Howie‐Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. Journal of Advanced Nursing 2014;70(9):1932‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sahu 2014b
- Sahu M, Grover A, Joshi A. Role of mobile phone technology in health education in Asian and African countries: a systematic review. International Journal of Electronic Healthcare 2014;7(4):269‐86. [DOI] [PubMed] [Google Scholar]
Sharma 2012
- Sharma P, Agarwal P. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. RHL commentary http://apps.who.int/rhl/hiv_aids/cd009756_sharmap_com/en/ (accessed 4 February 2015).
Smith 2011
- Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124(22):2458‐73. [DOI] [PubMed] [Google Scholar]
Thakkar 2016
- Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A, et al. Mobile phone text messaging for medication adherence in chronic disease: a meta‐analysis. JAMA International Medicine 2016;Published online:1 February 2016. [DOI] [PubMed] [Google Scholar]
Viswanathan 2012
- Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, et al. Interventions to improve adherence to selfadministered medications for chronic diseases in the United States: a systematic review. Annals of Internal Medicine 2012;157(11):785‐95. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. World Health Report 2002. Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization, 2002. [Google Scholar]
WHO 2003a
- World Health Organization. Prevention of recurrent heart attacks and strokes in low and middle income populations. Evidence‐based recommendations for policymakers and health professionals. Prevention of Recurrent Heart Attacks and Strokes in Low and Middle Income Populations: Evidence‐based Recommendations for Policy Makers and Health Professionals. Geneva: WHO, 2003. [Google Scholar]
WHO 2003b
- World Health Organization. Adherence to long‐term therapies: evidence for action. Chapter V ‐ Towards the Solution. World Health Organization, 2003:27‐38. [Google Scholar]
Worldbank 2012
- Mobile Phone Access Reaches Three Quarters of Planet's Population. http://www.worldbank.org/en/news/press‐release/2012/07/17/mobile‐phone‐access‐reaches‐three‐quarters‐planets‐population (accessed 12 February 2016).
Worldbank 2015
- Worldbank ‐ Data by country. http://data.worldbank.org/country (accessed 12 February 2016).
Yusuf 2011
- Yusuf S, Islam S, Chow CK, Rangarajan S, Daenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high income, middle‐income and low income countries (the PURE study): a prospective epidemiological survey. Lancet 2011;378:1231‐43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Adler 2015
- Adler AJ, Martin N, Mariani J, Tajer CD, Serrano NC, Casas JP, et al. Mobile phone text messaging to improve adherence to cardiovascular disease secondary prevention interventions. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011851] [DOI] [PMC free article] [PubMed] [Google Scholar]


